Abstract
Objectives
This study was conducted to evaluate the efficacy and safety of Cap. Torchnil & Tab. Febcin when given as add-on therapy to Covid19 positive patients with moderate disease.
Material and methods
Following written informed consent, patients were randomized to receive Cap. Torchnil & Tab. Febcin in addition to standard of care (SOC) [Add-on Group] or only SOC [SOC Group] for 14 days. Effect on clinical symptoms, WHO Clinical Assessment scale, hospital stay duration, time to Covid negative report, Sp02 levels and biomarkers was assessed during admission and relapse rate, if any, post discharge for 3 months.
Results
193 patients were screened and 150 completed the study, 77 in Add-on Group and 73 in SOC Group. Improvement in Covid related symptoms, WHO Assessment scale, time to covid negative report and duration of hospital stay was observed earlier in Add-on Group. Statistically significant fall in biomarker levels viz. CPK, D-dimer and IL-6 values at Day 14 and LDH levels at Days 7 & 14 was observed in Add-on Group. Improvement in Sp02 levels was also seen earlier in Add-on Group. Only 2 patients complained of acidity. Post discharge, 91 patients (49 from Add-on group and 42 from SOC group) came for physical visits. All these patients were clinically stable with no evidence of relapse.
Conclusion
The study results thus showed that Cap. Torchnil and Tab. Febcin were effective and safe when given as add-on therapy to SOC in the clinical management of patients with moderate Covid-19 disease.
Keywords: Covid-19, Cap. Torchnil, Tab. Febcin, Biomarkers, Oxygen requirements, Hospital stay
1. Introduction
Coronavirus disease (Covid-19) is an infectious disease caused by the SARS-CoV-2 virus. The novel virus was first identified in the Chinese city of Wuhan in December 2019 which then quickly spread around the World inspite of a lockdown. The World Health Organization (WHO) declared a Public Health Emergency of International Concern on 30 January 2020, and a pandemic on 11 March 2020 [1]. Since then, multiple variants of the virus have emerged and become dominant in many countries, with the Alpha, Beta, Gamma, Delta and Omicron variants being the most virulent [2]. As of 10 December 2021, 267,865,289 confirmed cases and 5,285,888 deaths have been reported from India [3].
The Covid-19 pandemic is unprecedented in several aspects and has challenged the health care system. Till date there is no specific treatment available for the disease. Covid-19 vaccines have been looked at to prevent disease severity [4,5]. However, the emergence of mutant and virulent strains of the virus can impose challenge to the efficaciousness of Covid-19 vaccines [6].
Different health systems are trying to find out new or repurposed drugs to treat Covid-19 infection [7]. D. Silveira et al. have reported that herbal medicines have better safety margin and enough efficacy to be used as adjuvant in Covid-19 [8]. Certain compounds like Curcumin have been postulated to be effective in Covid-19 treatment due to its antiviral and anti-inflammatory properties and it's action at different levels of the viral infection [9]. Vigorous efforts are also being taken by the Department of AYUSH to find an effective herbal Ayurvedic intervention for both prophylaxis and therapeutic management.
Cap. Torchnil and Tab. Febcin are 2 polyherbal proprietary medicines marketed by Dr. Palep's Medical Research Foundation. Cap. Torchnil has been in use for more than two decades to successfully treat recurrent pregnancy loss due TORCH infections and unknown etiology [10]. Cap. Torchnil has been shown to inhibit the HIV Reverse transcriptase (RT) enzyme and P24 antigen in vitro and the activity of the herbal constituents were comparable with that of AZT and Lamivudine [10]. Tab. Febcin contains ingredients which are known to possess anti-inflammatory, immunomodulatory, antioxidant and antimicrobial (bacteria & viruses) properties. In-silico docking studies of different ingredients of Cap. Torchnil & Tab. Febcin were carried out against the corona spike protein to evaluate their ability to inhibit the active proteins. The results showed that most of the ingredients bound to the spike proteins of Covid-19 thus indicating that they could be used as treatment against the Corona virus (data on file).
The present study was thus aimed to assess the efficacy and safety of these 2 AYUSH interventions (Cap. Torchnil + Tab. Febcin) when given as add-on therapy in the clinical management of Covid-19 positive patients with moderate Covid-19 disease.
2. Methods
2.1. Study design
This open-labeled, randomized, controlled, comparative, proof of concept, single center clinical trial was conducted after receiving Institutional Ethics Committee approval (IEC/135/2020). The study was registered in the Clinical Trial Registry of India – CTRI number REF/2021/09/047694. The study was conducted in compliance with the Indian Good Clinical Practice (GCP), guidelines, Declaration of Helsinki concerning medical research in humans (Revised Seoul, 2008) and ‘Ethical Guidelines for Biomedical Research on Human Subjects’ issued by Indian Council of Medical Research, 2017. The study was in compliance with updated CONSORT [2010] reporting guidelines.
Adult patients between 18 and 70 years of age, proven to be positive for SARS-CoV-2 infection, as confirmed by the RT-PCR test 48 h prior to the entry into the study and were admitted with moderate Covid-19 (as per MOFHW criteria) [11] for treatment at the hospital were enrolled after their written informed consent to participate in the study. Women were enrolled following a negative urine pregnancy test and only those patients who were able to consume the study drugs orally were enrolled.
Patients suffering from any severe, uncontrolled medical disorders viz. Diabetes mellitus, secondary Hypertension, liver and/or kidney disorders etc, those with any medical or surgical condition that would require immediate medical or surgical intervention, patients having immunocompromised status like HIV, Hepatitis, Tuberculosis and Cancer, those on oral steroid therapy and/or any kind of immunosuppressive therapy, pregnant and lactating women were excluded from the study.
Enrolled patients were randomized to one of the 2 study groups (1:1 ratio) based on a computer-generated randomization table. Patients in the Add-on Group received Cap. Torchnil (1 capsule thrice daily) & Tab. Febcin (1 tab. four times daily) for 14 days. The dose and dosing regimen of these herbal medicines was based on the that approved for the treatment of TORCH infections. Only the duration of dosing was shortened to 14 days from 3 to 6 months based on Covid-19 disease pathogenesis. These medications were in addition to the standard of care (SOC) treatment given to all patients with moderate Covid-19 infection. Patients in the SOC Group received only Standard of Care treatment given to all patients with moderate infection, the details of which were as follows:
-
•
Symptomatic treatment (paracetamol, levocetrizine/other antihistaminics, Condy' s gargles).
-
•
Tab.azithromycin 500 mg OD × 3–5 days
-
•
Inj remdesivir – IV loading dose of 200 mg on day 1, followed by 100 mg daily IV for 4 days (total 5 days)
-
•
Tab. hydroxychloroquine 400 mg bd on Day 1, then 400 mg OD for 5 days (ECG to be monitored for QT prolongation, especially in the presence of other agents causing the same) (chloroquine 500 mg bd may be used if HCQs not available)
-
•
Tab Ivermectin 12 mg single dose
-
•
Antiemetics, proton pump inhibitors and probiotics as indicated
-
•
Supportive treatment as indicated
2.2. Drug details
The constituents of the study medications are as follows:
Each Capsule of Torchnil contains Glycyrrhiza glabra (Yashtimadhu), Tinospora cordifolia (Guduchi), Solanum indicus (Ringimul), Piper longum (Lendi Pimpli), Tribulis terrestris (Gokshuru), Clerodendrum serratum (Bharang-Mul), Punica granatum (Dadim), Vanda roxburghii (Rasna), Rubia cordifolia (Manjistha), Solanum xanthocarpum (Kantakari) and Andropogan muricatus (vala).
Each Tablet of Febcin contains Cyperus rotudus (Musta), Fumaria parviflora (Parpataka), Fagonia Arabica (Duralabha), Tinospora cordifolia (Guduchi), Gingiber officianalis (Shunthi), Cocculus hirsutus (Laghupatha), Glycerrhiza glabra (Yashtimadhu) and Andropogan muricatum (Vala/Usheer).
The details of the role of each plant as per Ayurvedic textbooks with their quantity in the study drugs along with the package insert of the study drugs is appended as Appendix 1.
The study medications were given only for 14 days, however the patients were monitored and followed up for a period of 3 months, i.e. 2 ½ months post stopping of treatment. Following discharge, the patients were requested to follow-up either physically or telephonically every 15 days till the end of the study. At every physical visit, the patients' vital signs and clinical examination was conducted and Sp02 levels were monitored. In case they had any symptoms suggestive of a repeat Covid infection, like fever, sore throat, cough, loss of taste and/or smell etc, an RT-PCR was done to rule out Covid 19 infection.
3. Objectives
3.1. Primary study objectives
To confirm the efficacy of 2 AYUSH interventions (Cap. Torchnil + Tab. Febcin) prescribed as add-on therapy in improving the clinical symptoms, reducing the duration to RT-PCR negative reports and confirming safety in patients with Covid-19 moderate disease.
3.2. Secondary study objectives
To confirm the efficacy of two AYUSH interventions (Cap. Torchnil + Tab. Febcin) when given add-on therapy in decreasing the need for oxygen supplementation and hospital stay in patients with Covid-19 moderate disease.
4. Statistical considerations
4.1. Sample size
Assuming a 15% difference between the AYUSH Interventions + SOC Group & the SOC alone group based on previous data wherein in vitro evaluation of anti-HIV reverse transcriptase activity of Cap. Torchnil, showed that one of the constituents, S. xanthocarpum (Kantakari) gave the highest inhibition of about 37%, followed by P. granatum (Pomegranate), –34% inhibition when compared to AZT (15%). Keeping the power at 80%, the significance level at 5% & the superiority margin (δ) at 7%, the sample size came to 140 completed cases with 70 cases in each group. Keeping the dropout rate at 10%, the sample size was estimated to 154 cases with 77 cases in each group.
4.2. Statistical analysis
Statistical analysis was done using ANOVA followed by posthoc tests for the parametric data and Kruskal Wallis test for non-parametric data. The level of significance for all analysis was taken as p<0.05.
5. Study outcomes
The study Outcome measures that were assessed during the study were as follows:
5.1. Primary outcomes
To assess the proportion of patients who became clinically asymptomatic, Covid RT-PCR test negative and those who worsened to severe stage of the disease during the hospital stay.
5.2. Secondary outcomes
To determine the changes in Covid associated serum biomarker levels, oxygen requirement from baseline to discharge and decrease in the duration of hospital stay.
5.3. Assessment parameters
The following parameters were assessed during the study:
-
1.
Clinical COVID19 related symptoms
-
2.
WHO Clinical assessment scale (from enrollment to discharge)
-
3.
RT-PCR reports
-
4.
Duration of hospital stay
-
5.
Biomarkers: CRP, CPK, IL6, LDH, Ferritin, D-dimer (from enrollment to discharge)
-
6.
Assessment of safety: hematological and biochemical reports and clinical adverse events.
6. Results
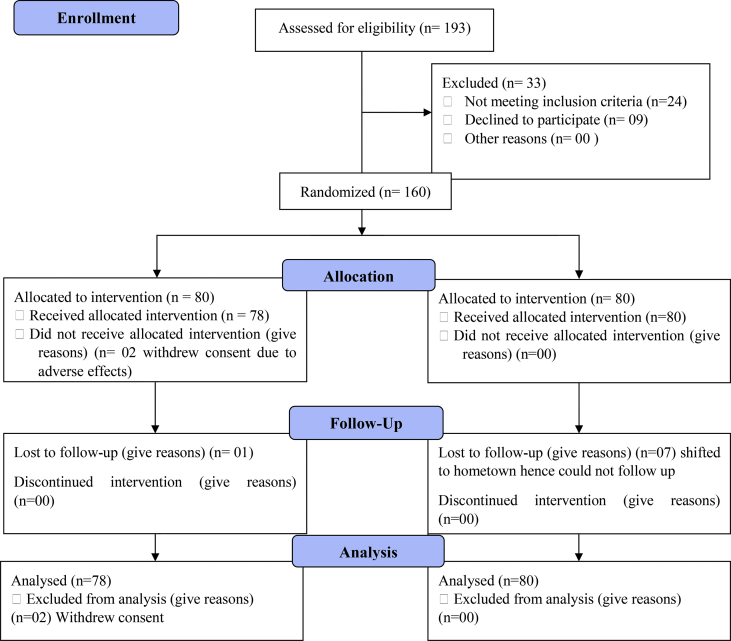
Following ethical approval, the study was conducted over a period of 9 months from October 2020 to June 2021. 193 patients with moderate Covid-19 disease were assessed for eligibility, of which 160 patients with moderate Covid-19 disease were enrolled in the study. 80 patients each were allocated to one of the 2 study groups. From the Add-on Group, 2 patients withdrew from the study while in hospital due to adverse effects of the study medications. Post discharge, 77 participants completed the study while 3 patients dropped out of the study. From the SOC Group, 73 participants completed the study and 7 participants dropped out of the study. Fig. 1 gives the study flowchart of the status of enrolled patients.
Fig. 1.
CONSORT 2010 flow chart.
6.1. Demographic & co-morbid conditions details
The demographic details of the study participants showed that the average age of the study patients ranged from 43 to 63 years and that there was a similar distribution of men and women in both the study groups. Majority of the study patients also had underlying medical conditions like controlled type 2 Diabetes mellitus, hypertension or a combination of both. The details are given in Table 1 below.
Table 1.
Demographic details & co-morbid medical conditions of the study patients.
| Add-on Group (n=80) | SOC Group (n=80) | |
|---|---|---|
| Age (in years) expressed as median [with range] | 53.5 [28–78] | 55 [31–72] |
| Gender n (%) | Males: 55 (74.03%) Females: 20 (25.97%) |
Males: 49 (63.64 %) Females: 28 (36.36 %) |
| No co-morbid conditions | 22 (27.5%) | 17 (21.25%) |
| Type 2 Diabetes mellitus (T2DM) | 32 (40%) | 35 (43.75%) |
| Hypertension (HT) | 21 (26.25%) | 25 (31.25%) |
| T2DM + HT | 17 (32.08%) | 21 (35%) |
| Bronchial asthma | 5 (6.25%) | 3 (3.75%) |
6.2. Change in Covid-19 related clinical symptoms
At admission, most patients clinically had symptoms like fever, sore throat, difficulty in breathing, dry cough, nasal discharge, headache, loss of taste and/or smell, generalized weakness and GIT symptoms. The change in these symptoms were assessed for a period of 3 months on days 1, 7, 14, 30, 45, 60, 75 and 90. Clinically the symptoms were assessed as “present” OR “absent” over the period of 3 months. As can be seen in Table 2 below, majority of the symptoms resolved or improved earlier in the Add-on Group as compared to the SOC Group. The quicker recovery was statistically significant with regard to symptoms of headache and dry cough.
Table 2.
Number of patients reporting a change in Covid-19 related clinical symptoms.
| Baseline | Day 7 | Day 14 | Day 30 | Day 45 | Day 60 | Day 75 | Day 90 | |
|---|---|---|---|---|---|---|---|---|
| Fever | ||||||||
| Add-on Group (n = 77) | 43 | 0∗∗ | 0∗∗ | 0∗∗ | 0∗∗ | 0∗∗ | 0∗∗ | 0∗∗ |
| SOC Group (n = 73) | 55 | 11∗∗ | 4∗∗ | 0∗∗ | 0∗∗ | 0∗∗ | 0∗∗ | 0∗∗ |
| Sore throat | ||||||||
| Add-on Group (n = 77) | 10 | 0∗∗ | 0∗∗ | 0∗∗ | 0∗∗ | 0∗∗ | 0∗∗ | 0∗∗ |
| SOC Group (n = 73) | 15 | 7 | 4∗ | 3∗∗ | 2∗∗ | 0∗∗ | 0∗∗ | 0∗∗ |
| Difficulty in breathing | ||||||||
| Add-on Group (n = 77) | 64 | 49 | 18∗∗,### | 10∗∗,@@@ | 5∗∗,### | 0∗∗,### | 0∗∗,### | 0∗∗,### |
| SOC Group (n = 73) | 70 | 49∗ | 19∗∗,### | 10∗∗,### | 7∗∗,### | 0∗∗,### | 0∗∗,### | 0∗∗,### |
| Dry cough | ||||||||
| Add-on Group (n = 77) | 52 | 24∗∗ | 9∗∗,## | 3∗∗,### | 0∗∗,### | 0∗∗,### | 0∗∗,### | 0∗∗,### |
| SOC Group (n = 73) | 56 | 37∗,$$ | 18∗∗,$ | 7∗∗ | 3∗∗ | 0∗∗ | 0∗∗ | 0∗∗ |
| Nasal discharge | ||||||||
| Add-on Group (n = 77) | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 |
| SOC Group (n = 73) | 6 | 2∗∗ | 0∗∗ | 0∗∗ | 0∗∗ | 0∗∗ | 0∗∗ | 0∗∗ |
| Headache | ||||||||
| Add-on Group (n = 77) | 40 | 24 | 20 | 18 | 15 | 12 | 7∗,# | 5∗,## |
| SOC Group (n = 73) | 45 | 40$$ | 32$ | 26∗∗ | 23∗∗ | 20∗∗ | 15∗∗ | 13∗∗,$ |
| Loss of taste | ||||||||
| Add-on Group (n = 77) | 9 | 2 | 0 | 0 | 0 | 0 | 0∗∗,### | 0∗∗,### |
| SOC Group (n = 73) | 12 | 4 | 2 | 0∗∗ | 0∗∗ | 0∗∗,### | 0∗∗,### | 0∗∗,### |
| Loss of smell | ||||||||
| Add-on Group (n = 77) | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 |
| SOC Group (n = 73) | 4 | 0∗ | 0∗ | 0∗ | 0∗ | 0∗ | 0∗ | 0∗ |
| Generalized weakness | ||||||||
| Add-on Group (n = 77) | 56 | 56 | 54 | 51 | 50 | 46 | 39 | 35∗,# |
| SOC Group (n = 73) | 58 | 55 | 52 | 43 | 40 | 38∗ | 35∗,## | 35∗,## |
| GIT symptoms | ||||||||
| Add-on Group (n = 77) | 21 | 11 | 5 | 0 | 0∗∗,### | 0∗∗,### | 0∗∗,### | 0∗∗,### |
| SOC Group (n = 73) | 25 | 18 | 11 | 7∗ | 5∗∗,# | 3∗∗,## | 0∗∗,### | 0∗∗,### |
∗p<0.01, ∗∗p<0.001 as compared to Day 1 using Kruskal Wallis test.
#p<0.05, ##p<0.01, ###p<0.001 as compared to Day 7 using Kruskal Wallis test.
@@@p < 0.001 as compared to Day 14 using Kruskal Wallis test.
$p<0.05, $$p<0.01 as compared to Add-on Group using unpaired t test with Welch correction.
6.3. WHO Clinical Assessment scale
As seen in Table 3, clinical improvement as per the WHO Clinical Assessment scale was seen in both the study groups, however the improvement was observed to be fast in in the Add-on Group (Day 7) as compared to the SOC Group with regard to decreased oxygen requirement and improvement in clinical symptoms although this difference was not found to be statistically significant.
Table 3.
Change in grading in the WHO clinical assessment scale.
| Day 0 | Day 3 | Day 7 | Day 10 | Day 14 | |
|---|---|---|---|---|---|
| Add-on Group (n = 78) | 4.04 ± 0.52 | 3.88 ± 0.46 | 3.35 ± 0.53 | 3.26 ± 0.47 | 3 |
| SOC Group (n = 80) | 4.04 ± 0.47 | 3.88 ± 0.46 | 3.74 ± 0.5 | 3.39 ± 0.52 | 3.42 ± 0.5 |
6.4. Changes in the vital signs monitored during hospitalization
The vital signs of the patients in both groups were monitored daily during their hospital stay till they were discharged. There was a significant improvement in the pulse rate at days 10 & 14 as compared to baseline and days 1, 3 & 7 in both groups. The pulse rate in the Add-on Group was significantly lower than the SOC Group at days 10 & 14.
A similar trend was observed with the respiratory rate and temperature readings in both groups. A significant improvement was recorded in the respiratory rate at day 7 as compared to baseline and day 3 in the Add-on Group whereas significant improvement in the respiratory rate was seen at Day 10 compared to baseline and days 3 & 7. The respiratory rate in the Add-on Group was significantly lower than the SOC Group at days 10 & 14.
With regard to the temperature, significant lowering of the temperature was seen at day 7 in the Add-on Group & day 10 in the SOC Group. The decrease in temperature was significant from day 3 to day 14 in the Add-on Group as compared to the SOC Group. The results are summarized in Table 4.
Table 4.
Change in the vital signs in both groups during hospitalization.
| Day 1 | Day 3 | Day 7 | Day 10 | Day 14 | |
|---|---|---|---|---|---|
| Pulse | |||||
| Add-on Group (n = 78) | 95.19 ± 7.02 | 92.94 ± 6.68 | 90.74 ± 6.23 | 88.00 ± 6.06∗∗∗,@@,φ | 83.28 ± 4.97∗∗∗,@@@,###,$$$,φφ |
| SOC Group (n = 80) | 96.48 ± 7.40 | 94.71 ± 5.58 | 92.95 ± 4.49φ | 90.33 ± 58.20∗∗∗,@@@ | 85.71 ± 5.57∗∗∗,@@@,###,$$$ |
| Respiratory Rate | |||||
| Add-on Group (n = 78) | 19.51 ± 2.08 | 19.12 ± 1.82 | 18.03 ± 1.8∗∗,@ | 15.65 ± 1.44∗∗∗,@@@,###,φ | 13.09 ± 1.07∗∗∗,@@@,###,$$$,φ |
| SOC Group (n = 80) | 19.53 ± 2.38 | 19.25 ± 1.83 | 18.15 ± 2.03 | 16.29 ± 1.82∗∗∗,@@@,### | 14.71 ± 1.79∗∗∗,@@@,###,$ |
| Temperature | |||||
| Add-on Group (n = 78) | 101.65 ± 1.79 | 99.90 ± 0.89∗∗,φφφ | 98.54 ± 0.19∗∗∗,@@@,φφφ | 98.51 ± 0.29∗∗∗,@@@,φφφ | 98.47 ± 0.32∗∗∗,@@@,φφφ |
| SOC Group (n = 80) | 101.93 ± 1.68 | 100.63 ± 1.69∗∗∗ | 99.51 ± 1.24∗∗∗,@@@ | 99.24 ± 1∗∗∗,@@@ | 98.86 ± 0.55∗∗∗,@@@ |
∗∗p<0.01, ∗∗∗p<0.001, as compared to Day 1 using Friedman test (nonparametric repeated measures ANOVA).
@@p<0.01, @@@p<0.001, as compared to Day 3 using Friedman test (nonparametric repeated measures ANOVA).
###p<0.001 as compared to Day 7 using Friedman test (nonparametric repeated measures ANOVA).
$$$p<0.001 as compared to Day 10 using Friedman test (nonparametric repeated measures ANOVA).
φp<0.05, φφp<0.01, φφφp<0.001 as compared to SOC Group using unpaired t test with Welch correction.
6.5. Number of days wherein patients became covid negative
It was observed that patients who received the study medications became Covid negative earlier than those on SOC treatment. The mean duration for the RT-PCR negative report (COVID negative status) was 9.51 ± 1.55 days in the Add-on Group as compared to 10.89 ± 1.74 days for the SOC Group.
None of the study patients had a worsening of their clinical condition during the hospital stay and there were no deaths reported during the study period.
6.6. Effect on serum biomarkers levels
As seen from Table 5, an improvement in the serum biomarker levels was seen in both groups with a greater fall observed in the Add-on group. The fall in CPK, D-dimer and IL-6 values at Day 14 was statistically significant as compared to the SOC Group whereas the fall in LDH levels was statistically significant at Days 7 & 14 in the Add-on group.
Table 5.
Effect of study medications on serum biomarkers levels.
| Sr. no. | Day 0 | Day 7 | Day 14 | |
|---|---|---|---|---|
| CRP (mg/L) | ||||
| 1 | Add-on group (n = 78) | 40.65 ± 21.85 | 38.98 ± 25.55 | 37.76 ± 21.16 |
| 2 | SOC Group (n = 80) | 40.74 ± 24.29 | 39.31 ± 22.35 | 40.71 ± 22.41 |
| CPK (U/L) | ||||
| 1 | Add-on group (n = 78) | 71.65 ± 49.57 | 68.75 ± 41.75 | 57.23 ± 38.75*,**,# |
| 2 | SOC Group (n = 80) | 72.24 ± 43.71 | 68.98 ± 43.29 | 70.60 ± 42.91 |
| LDH (U/L) | ||||
| 1 | Add-on group (n = 78) | 769.61 ± 336.12 | 688.54 ± 250.50*,@ | 666.68 ± 227.91**,# |
| 2 | SOC Group (n = 80) | 776.98 ± 349.37 | 827.53 ± 316.90 | 767.56 ± 285.35 |
| Ferritin (ng/ml) | ||||
| 1 | Add-on group (n = 78) | 542.52 ± 269.62 | 463.66 ± 242.28** | 454.54 ± 242.56** |
| 2 | SOC Group (n = 80) | 547.47 ± 328.45 | 499.93 ± 279.56 | 457.06 ± 278.51* |
| D-Dimer (μg/ml) | ||||
| 1 | Add-on group (n = 78) | 394.76 ± 343.21 | 325.77 ± 295.81** | 253.48 ± 242.09**,## |
| 2 | SOC Group (n = 80) | 444.95 ± 295.13 | 399.57 ± 266.08 | 355.18 ± 245.53* |
| IL-6 (pg/ml) | ||||
| 1 | Add-on group (n = 78) | 118.64 ± 166.70 | 115.33 ± 169.68 | 85.18 ± 123.02*,## |
| 2 | SOC Group (n = 80) | 212.10 ± 263.47 | 168.46 ± 234.66 | 157.73 ± 189.64* |
*p<0.01 as compared to Day 7; **p<0.01 as compared to Day 1 (Within group).
#p<0.01; ##p<0.001 as compared to Day 14 of SOC Group using Unpaired t test with Welch correction.
@p<0.001 as compared to Day 7 of SOC Group using unpaired t test with Welch correction.
6.7. Change in the requirement for supplemental oxygen
It was observed that the average percentage of oxygen in the blood (Sp02) and oxygen requirements of the study patients improved during their hospital stay in both groups with the improvement seen earlier in the Add-on group though not statistically significant. The results are presented in Table 6.
Table 6.
Requirement of supplemental oxygen in both study groups.
| Baseline | Day 1 | Day 2 | Day 3 | Day 4 | Day 5 | Day 6 | Day 7 | Day 10 | Day 14 | |
|---|---|---|---|---|---|---|---|---|---|---|
| Add-on group (n = 78) | ||||||||||
| Average SpO2 | 91.36 ± 1.38 | 95.94 ± 2.18 | 96.52 ± 2.06 | 96.73 ± 1.73 | 96.75 ± 1.02 | 96.94 ± 1.22 | 97.08 ± 1.35 | 97.17 ± 1.01 | 97.25 ± 0.92 | 97.45 ± 0.94 |
| O2 requirement (L/min) | 7.90 ± 3.98 | 7.43 ± 4.32 | 6.53 ± 3.69 | 5.35 ± 2.98 | 5 ± 3.10 | 4.78 ± 3.20 | 3.69 ± 2.88 | 2.53 ± 2.66 | 1.64 ± 2.48 | 0 |
| SOC Group (n = 80) | ||||||||||
| Average SpO2 | 91.43 ± 1.27 | 94.30 ± 1.05 | 95.50 ± 1.50 | 96.14 ± 1.07 | 96.38 ± 1.60 | 96.50 ± 1.85 | 96.62 ± 1.37 | 96.67 ± 1.22 | 96.82 ± 1.24 | 96.96 ± 1.12 |
| O2 requirement (L/min) | 7.93 ± 3.79 | 7.55 ± 4.21 | 6.91 ± 3.51 | 6.57 ± 3.50 | 6.47 ± 3.43 | 6.05 ± 3.21 | 5.53 ± 3.57 | 4.95 ± 3.52 | 2.55 ± 3.81 | 1.16 ± 1.51 |
6.8. Mean duration of hospital stay
Patients in the Add-on group recovered faster and were discharged earlier as compared to the SOC Group, though the difference was not statistically significant. The mean duration of hospital stay for the Add-on group was 11.4 days as compared to the SOC Group (13.4 days).
6.9. Safety evaluation
Safety was monitored by clinical assessment and laboratory investigations. 2 patients from the Add-on group refused further participation in the study due to complaints of hyperacidity after starting the IP. No major changes were observed in the laboratory safety parameters in both the groups.
6.10. Follow up assessment
Following discharge, all the study patients were requested to follow up for a period of 2½ months to monitor their clinical condition and document any relapse or worsening of their Covid state. The patients followed up either physically or telephonically every 15 days till the end of the study. Of the 158 patients, 1 patient from the Add-on group and 7 patients from the SOC Group were lost to follow-up post discharge as they went to their native place and were uncontactable inspite of repeated attempts to call them. Of the 150 patients who followed up, 49 patients from the Add-on group came for physical visits while 28 patients preferred telephonic visits. In case of the SOC Group, 42 patients came for physical visits while 31 patients preferred telephonic visits. At every physical visit, the patients' vital signs and clinical examination was conducted and Sp02 levels were monitored, and RT-PCR done in case there were symptoms suggestive of a Covid infection.
All 91 patients who came for the physical visit were clinically stable with no worsening of their clinical condition and no signs or symptoms suggestive of a repeat Covid infection. Their vital signs and Sp02 levels were within the normal range in both groups although the decrease in the pulse and respiratory rate at days 75 & 90 was statistically significant in the Add-on group as compared to the SOC Group (refer Table 7).
Table 7.
Sp02 levels & vital signs in both study groups during the physical follow-up visits.
| Day 30 | Day 45 | Day 60 | Day 75 | Day 90 | |
|---|---|---|---|---|---|
| Sp02 | |||||
| Add-on group (n = 49) | 98.14 ± 0.94 | 98.37 ± 0.76 | 98.02 ± 1.11 | 98 ± 0.82 | 98.24 ± 0.8 |
| SOC Group (n = 42) | 97.83 ± 1.01 | 98.19 ± 0.77 | 97.98 ± 0.9 | 97.95 ± 0.85 | 98.19 ± 0.63 |
| Pulse rate | |||||
| Add-on group (n = 49) | 82.45 ± 2.39 | 81.59 ± 2.38 | 80.63 ± 3.44∗ | 79.63 ± 3.62∗∗,φφ | 78.33 ± 2.93∗∗∗,@@@,# φφ |
| SOC Group (n = 42) | 83.36 ± 3.66 | 82.48 ± 3.45 | 82 ± 3.88 | 81.69 ± 3.66 | 80.88 ± 3.39∗∗∗,# |
| Respiratory rate | |||||
| Add-on group (n = 49) | 12.76 ± 0.95 | 12.33 ± 0.63 | 11.71 ± 0.71∗∗,φφ | 11.49 ± 0.92∗∗∗,@,φ | 10.76 ± 0.88∗∗∗,@@@,##,$,φφφ |
| SOC Group (n = 42) | 12.90 ± 0.98 | 12.38 ± 0.70 | 12.05 ± 0.58∗ | 11.81 ± 0.55∗∗∗ | 11.48 ± 0.77∗∗∗,@@@ |
| Temperature | |||||
| Add-on group (n = 49) | 98.46 ± 0.32 | 98.4 ± 0.41∗∗ | 98.59 ± 0.03 | 98.58 ± 0.04 | 98.59 ± 0.04 |
| SOC Group (n = 42) | 98.67 ± 0.27φφφ | 98.45 ± 0.35 | 98.58 ± 0.05 | 98.58 ± 0.05 | 98.59 ± 0.05 |
∗p<0.05, ∗∗p<0.01,∗∗∗p<0.001 as compared to Day 30 using Friedman test (nonparametric repeated measures ANOVA).
@p<0.05, @@@p<0.001 as compared to Day 45 using Friedman test (nonparametric repeated measures ANOVA).
#p<0.05;##p<0.01 as compared to Day 60 using Friedman test (nonparametric repeated measures ANOVA).
$p<0.05 as compared to Day 75 using Friedman test (nonparametric repeated measures ANOVA).
φp<0.05, φφp<0.01, φφφp<0.001 as compared to SOC Group using unpaired t test with Welch correction.
7. Discussion
Medicines from natural sources have always been intriguing to researchers to develop novel drug candidates and/or molecules especially for the treatment of diseases that have no cure in modern medicine. Covid-19 caused by the novel coronavirus (SARS-CoV-2) is one such infectious disease for which no specific drug or vaccine is available, yet. Historically, natural products and herbal medicines have been used for the prevention of viral infections and generally show favorable efficacy and acceptable toxicity. Thus, since the Covid-19 pandemic outbreak, various herbal medicines have been used for the prevention and treatment of the infection [12].
In our study, we evaluated the effect of 2 polyherbal proprietary medicines, Cap. Torchnil and Tab. Febcin containing a combination of different medicinal plants for their efficacy in the clinical management of Covid-19 positive hospitalized patients with moderate disease in addition to standard of care. The effects of these 2 medications were compared to that of standard therapy offered to these patients.
Of the 160 patients with Covid-19 infection of moderate severity included in the study, 150 completed the study duration. All these patients recovered from their illness while in hospital and were discharged with no deaths. Patients receiving the study medications showed a faster recovery with regard to their clinical symptoms with statistically significant improvement seen with regard to headache and dry cough. Symptoms such as fever and sore throat resolved within 7 days in the study group as compared to the Control group. This is possibly because of the anti-pyretic effects of Tab. Febcin which has been proven in a rat model wherein significant antipyretic action was observed as compared to paracetamol [13]. The mean SpO2% levels indicated an improvement from Day 1 onwards decreasing the requirement for oxygen from Day 10 in patients in the study group as compared to the Control group. With respect to the serum biomarker levels, an improvement in all the biomarker levels was seen in patients in the study group with a significant improvement seen for CPK, LDH, D-dimer & IL-6 levels, indicating anti-inflammatory effect of the study medications. The mean hospital stay duration was 2 days shorter in the treatment group (11.40 vs.13.44 days) and as was the number of days to a RT-PCR Covid-19 negative report (9.51 days for the Treatment group compared to 10.89 days in the Control group). All the above effects demonstrate that the study medications had anti-inflammatory, antipyretic and possibly antiviral effect and/or immunomodulatory effect promoting an earlier recovery in patients who consumed the medications. Both the study medications contain various medicinal plants that are known to have anti-inflammatory and immunomodulatory properties like Glycyrrhiza glabra, T. cordifolia, T. terrestris and C. serratum, plants like P. longum, S. indicus, Solanum xanthocarpus which are effective in respiratory illnesses and those with antipyretic properties like F. parviflora, Fagonia arabica, C. hirsutus and A. muricatum. [[14], [15], [16], [17], [18]].
In-silico studies have also shown that the two formulations when evaluated against the corona viral membrane protein for their inhibiting ability of the active spike protein showed that there was interaction between the different constituents with the different regions of the spike protein suggesting that the formulations would be functionally significant against the corona viral protein. (Unpublished data).
The results with these two herbal formulations are similar to those observed with other herbal drugs used to treat Covid-19 infection. AYUSH 64, which contains aqueous extracts of Alstonia scholaris (bark), Picrorhiza kurroa (rhizome), Swertia chirata (whole plant) and Caesalpinia crista was found to hasten recovery, reduce hospitalization and improve overall health when given to patients suffering from mild and moderate symptomatic Covid-19 along with SOC [19]. In silico docking studies to assess the potential mechanism of action of AYUSH-64 compounds against Severe Acute Respiratory Syndrome-Corona Virus (SARS-CoV-2) Main Protease (Mpro; PDB ID: 6LU7) demonstrated that of the 36 compounds of four ingredients of AYUSH-64 screened, 35 exhibited good binding energies to the main protease with the best affinity and interactions seen with Akuammicine N-Oxide (from A. scholaris) [20].
Clinical trials of different AYUSH medicines like Ashwagandha, Yashtimadhu, Guduchi, Pippali on patients, health workers, and those working in high-risk areas were also initiated by the Ministry of AYUSH. In silico network pharmacology and docking studies carried out to explore the immunomodulatory and anti SARS-CoV2 potential of phytoconstituents from Ashwagandha, Guduchi and Shatavari against the Spike protein, Main Protease and RNA dependent RNA polymerase of the virus. The results showed that these phytoconstituents possessed good affinity for the three targets, suggesting their application for the termination of viral life cycle [21].
Different ayurvedic proprietary medicines like ZINGIVIR-H, Aayudh Advance,/Purified aqueous extract of C. hirsutus (AQCH), a phytopharmaceutical, Amrta karuna syrup, Virulina®, Astha-15 were also shown to be effective in improving the clinical condition of Covid19 patients when given as add-on therapy through their immunomodulatory properties [22].
One of the limitations of our study was that the clinical symptoms were measured as ordinal data (Present/Absent) rather than using a VAS scale. This was done mainly to minimize the stress on the patients as initially many of them were on oxygen supplementation and also to restrict the length of contact between the patient and the study team member due to the Covid positive status of the patients.
8. Conclusion
Thus, the results of the randomized, comparative controlled clinical study assessing the efficacy of a combination of Cap. Torchnil and Tab. Febcin in patients with moderate Covid-19 disease showed that these two formulations were effective when given as add-on therapy to standard of care. The formulations hastened clinical recovery, viral clearance, decreased oxygen requirements and lowered inflammatory biomarker levels suggesting that they have a role in the clinical management of Covid-19 patients with moderate disease.
Source(s) of funding
Dr. Palep’s House of Health & Medical Research Foundation Pvt. Ltd, Mumbai.
Author contributions
RM: Conceptualization, Methodology/Study design, Validation, Formal analysis, Investigation, resources, Data curation, Writing – original draft, Writing – review and editing, Visualization, Supervision, Project administration. DK: Methodology/Study design, Formal analysis, Investigation, resources, Data curation, Writing – original draft, Writing – review and editing, Supervision, Project administration PP: Conceptualization, Methodology/Study design, Formal analysis, Investigation, resources, Data curation, Supervision, Project administration, GR: Conceptualization, Methodology/Study design, Formal analysis, Resources, Data curation, Supervision, Project administration, HSP: Conceptualization, Methodology/Study design, Resources, Writing – review and editing, Visualization, Supervision, Funding acquisition.
Conflict of interest
None.
Acknowledgment
The Authors wish to acknowledge the support given by Dr. Rumana Ansari and Dr. Prerna Hosmani for the recruitment and follow up of the study patients.
Footnotes
Peer review under responsibility of Transdisciplinary University, Bangalore.
Supplementary data to this article can be found online at https://doi.org/10.1016/j.jaim.2022.100559.
Appendix A. Supplementary data
The following is/are the supplementary data to this article:
References
- 1.Novel coronavirus (2019-nCoV) situation report 1 dated 21 January 2020, WHO. Available from: https://www.who.int/docs/default-source/coronaviruse/situation-reports/20200121-sitrep-1-2019-ncov.pdf; [accessed 13.12.2021].
- 2.Tracking SARS-CoV-2 variants. Available from: https://www.who.int/en/activities/tracking-SARS-CoV-2-variants/; [accessed 13.12.2021].
- 3.WHO coronavirus (COVID-19) dashboard. Available from: https://covid19.who.int/; [accessed 30.08.2021].
- 4.WHO COVID-19 vaccines. Available from: https://www.who.int/emergencies/diseases/novel-coronavirus-2019/covid-19-vaccines; [accessed 13.12.21].
- 5.COVID-19 advice for the public: getting vaccinated. Available from: https://www.who.int/emergencies/diseases/novel-coronavirus-2019/covid-19-vaccines/advice; [accessed 13.12.21].
- 6.Williams T.C., Burgers W.A. SARS-CoV-2 evolution and vaccines: cause for concern? Lancet Respir Med. 2021;9(4):333–335. doi: 10.1016/S2213-2600(21)00075-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Singh T.U., Parida S., Lingaraju M.C., Kesavan M., Kumar D., Singh R.K. Drug repurposing approach to fight COVID-19. Pharmacol Rep. 2020;72(6):1479–1508. doi: 10.1007/s43440-020-00155-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Silveira D., Prieto-Garcia J.M., Boylan F., Estrada O., Fonseca-Bazzo Y.M., Jamal C.M., et al. COVID-19: is there evidence for the use of herbal medicines as adjuvant symptomatic therapy? Front Pharmacol. 2020;11:581840. doi: 10.3389/fphar.2020.581840. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Zahedipour F., Hosseini S.A., Sathyapalan T., Majeed M., Jamialahmadi T., Al-Rasadi K., et al. Potential effects of curcumin in the treatment of COVID-19 infection. Phytother Res. 2020;34(11):2911–2920. doi: 10.1002/ptr.6738. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Palep H.S. Role of herbal immunomodulators & antioxidants in recurrent pregnancy loss. Bombay Hosp J. 2006;48(3):1–7. Available from: Bombay Hospital Journal – Original Article (bhj.org.in) accessed. [Google Scholar]
- 11.Clinical management protocol: COVID-19 government of India ministry of health and family welfare directorate general of health services (EMR Division) Version 3 dated 13.06.20. Available from: https://www.mohfw.gov.in/pdf/ClinicalManagementProtocolforCOVID19.pdf [accessed 10.12.21].
- 12.Ang L., Song E., Lee H.W., Lee M.S. Herbal medicine for the treatment of coronavirus disease 2019 (COVID-19): a systematic review and meta-analysis of randomized controlled trials. J Clin Med. 2020;9(5):1583. doi: 10.3390/jcm9051583. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Palep H.S., patil Swati, funde Snehal, phalak Ankur. Anti-pyretic and analgesic activity of herbal formulation: implications in managing COVID-19 fever. Int J Res Pharm Sci. 2020;11(SPL1):1873–1878. doi: 10.26452/ijrps.v11iSPL1.4525. [DOI] [Google Scholar]
- 14.Acharya Jadavaji Trikamji., editor. 5th ed. Verse 145. Chowkhambha Sanskrit Sansthan; Varanasi: 2001. p. 410. (Charaksamhita by Agnivesha, chikitsa sthan; Jwarachikitsa: Chapter 3). [Google Scholar]
- 15.Vaidya Harishastri Paradkar., editor. 1st ed. Verse 45–46. Krishnadas Academy; Varanasi: 2000. p. 552. (Astangahridayam by Vagbhat, chikitsa sthan; Jwarachikitsa: Chapter 1). [Google Scholar]
- 16.Gogte V.M. Bhartiya Vidya Bhavan; Mumbai: 2000. Ayurvedic pharmacology and therapeutic uses of medicinal plants (Dravyagunavignyan) [Google Scholar]
- 17.Chunekar K.C. 2nd ed. Chaukhambha Surbharati Academy; Varanasi: 2018. Bhavprakash Nighantu (Indian Materia Medica) [Google Scholar]
- 18.Acharya Jadavaji Trikamji., editor. 1st ed. Verse 63–69. Krishnadas Academy; Varanasi: 1998. pp. 394–395. (Sushrut Samhita by Sushrut with the Nibandhasangraha commentary of Dalhanacharya, Sharirsthana; Garbhnivyakarana). [Reprint 1998, Chapter 10] [Google Scholar]
- 19.Chopra A., Chavan-Gautam P., Tillu G., Saluja M., Borse S., Sarmukaddam S., et al. 2021. Coadministration of AYUSH 64 as an adjunct to standard of care in mild and moderate COVID-19 a randomised, controlled, multicentric clinical trial. Preprint available at: medRxiv. 06.12.21258345. last accessed. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Ram T.S., Munikumar M., Raju V.N., Devaraj P., Boiroju N.K., Hemalatha R., et al. In silico evaluation of the compounds of the ayurvedic drug, AYUSH-64, for the action against the SARS-CoV-2 main protease. J Ayurveda Integr Med. 2022;13(1):100413. doi: 10.1016/j.jaim.2021.02.004. last accessed. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Borse S., Joshi M., Saggam A., Bhat V., Walia S., Marathe A., et al. Ayurveda botanicals in COVID-19 management: an in silico multi-target approach. PLoS One. 2021;16(6) doi: 10.1371/journal.pone.0248479. accessed. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Ahmad S., Zahiruddin S., Parveen B., Basist P., Parveen A., Gaurav, et al. Indian medicinal plants and formulations and their potential against COVID19 – preclinical and clinical research. Front Pharmacol. 2021;11:578970. doi: 10.3389/fphar.2020.578970. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.