Abstract
The primary cilium is a nonmotile microtubule-based organelle in most vertebrate cell types. The primary cilium plays a critical role in tissue development and homeostasis by sensing and transducing various signaling pathways. Ciliary proteins such as intraflagellar transport (IFT) proteins as well as ciliary motor proteins, kinesin and dynein, comprise a bidirectional intraflagellar transport system needed for cilia formation and function. Mutations in ciliary proteins that lead to loss or dysfunction of primary cilia cause ciliopathies such as Jeune syndrome and Ellis–van Creveld syndrome and cause abnormalities in tooth development. These diseases exhibit severe skeletal and craniofacial dysplasia, highlighting the significance of primary cilia in skeletal development. Cilia are necessary for the propagation of hedgehog, transforming growth factor β, platelet-derived growth factor, and fibroblast growth factor signaling during osteogenesis and chondrogenesis. Ablation of ciliary proteins such as IFT80 or IFT20 blocks cilia formation, which inhibits osteoblast differentiation, osteoblast polarity, and alignment and reduces bone formation. Similarly, cilia facilitate chondrocyte differentiation and production of a cartilage matrix. Cilia also play a key role in mechanosensing and are needed for increased bone formation in response to mechanical forces.
Keywords: ciliary proteins, chondrocytes, osteoblasts, tooth development, skeletal development, fracture
Introduction
Cilia are highly conserved microtubule-based organelles projecting from the apical surface of most types of cells. In contrast to motile cilia, primary cilia serve as an essential sensory organelle to sense and transduce extracellular signaling and thereby regulate cell behavior (Mitchison and Valente 2017). A cilium has different beat patterns in response to extracellular signaling, which is governed by the ultrastructure of the cilium and coordination of motor proteins in the cilium (Ishikawa et al. 2021). More than 600 proteins are estimated to reside in the cilium. These proteins are synthesized in the cytosol and then transported to the cilium via a 2-way transport system named intraflagellar transport (IFT). The IFT system consists of IFT complex B (IFT-B) proteins, which are powered by kinesin II motor proteins to mediate anterograde trafficking from the ciliary base to the tip of the cilium, and IFT complex A (IFT-A) proteins, which are powered by dynein motor proteins to regulate retrograde transport from the ciliary axoneme to the cell body. The IFT-B complex is composed of 16 IFT proteins, including IFT22/25/27/46/52/70/74/81/88 in the core and IFT172/80/57/20 in the periphery. The IFT-A complex includes 6 proteins, including IFT144/140/139/122/121/43 (reviewed in Ishikawa and Marshall 2017; Nakayama and Katoh 2018). The IFT system is also responsible for the assembly and maintenance of the primary cilium, as mutations in IFT-A proteins commonly result in short and wide cilia with bulb-like structures at their tips, and mutations in most IFT-B proteins lead to absent or shortened cilia (Nakayama and Katoh 2018).
The significance of primary cilia and IFT proteins in bone is highlighted by a group of disorders termed ciliopathies with cilia loss and/or malfunction caused by mutations of ciliary proteins. Some ciliopathies display severe skeletal and craniofacial dysplasia, such as Ellis–van Creveld syndrome, Jeune asphyxiating thoracic dystrophy, and short-rib polydactyly syndrome, showing short bones, narrow chest with short ribs, and polydactyly (Schock and Brugmann 2017; Wheatley 2018). The linkage between cilia dysfunction and skeletal and craniofacial osseous defects demonstrates the importance of primary cilia in regulating the function of osteoblasts and chondrocytes. This review summarizes the recent findings of primary cilia and IFT proteins in bone, cartilage and craniofacial development. We also describe the mechanistic regulation of cilia in the hedgehog (Hh), transforming growth factor β (TGF-β), platelet-derived growth factor (PDGF), and fibroblast growth factor (FGF) signaling pathways in osteogenesis and chondrogenesis. Moreover, recent findings that the FOXO1 transcription factor in diabetic conditions downregulates ciliary protein expression and cilia formation in chondrocytes and osteoblasts provide new insight into how diabetes may affect bone.
Primary Cilia Regulate Osteoblasts and Bone Formation
The primary cilium has been reported to participate in osteoblast alignment and polarization, as well as osteoblast differentiation and bone formation (Izu et al. 2011). We recently reported that ciliary proteins such as IFT80, IFT88, KIF3A, and EVC play essential roles in osteoblast differentiation and function through various pathway(s) (Yuan and Yang 2016). Ablation of IFT80 in osteoblast precursor cells using OSX-cre transgenic mice causes significant growth retardation and osteopenia due to impaired osteoblast differentiation. Loss of IFT80 blocks cilia-dependent canonical Hh-Gli (hedgehog-glioma–associated oncogene) signaling and overactivates cilia-independent noncanonical Hh-Gαi-RhoA pathways that inhibit osteoblast differentiation (Yuan et al. 2016) (Fig. 2A). In addition to the regulation of osteoblast differentiation, ciliary protein(s) also regulate cell polarity and alignment. A recent study showed that deletion of IFT20 in osteoprogenitors results in the disruption of osteoblast polarity, compromised osteoblast/osteocyte alignment, and disorganized collagen fibril formation. Mechanistically, IFT20 interacts with the ceramide–protein kinase Cζ (PKCζ) complex located in the cilium to promote PKCζ phosphorylation and induce the apical localization of β-catenin in osteoblasts (Fig. 1). In addition, deletion of IFT20 disrupts the ceramide–pPKCζ–β-catenin signaling axis, leading to inhibition of osteoblast formation, loss of normal polarity, and alignment of osteoblasts/osteocytes in bone, which ultimately interferes with normal collagen fiber formation and arrangement with diminished bone strength and stiffness (Lim et al. 2020).
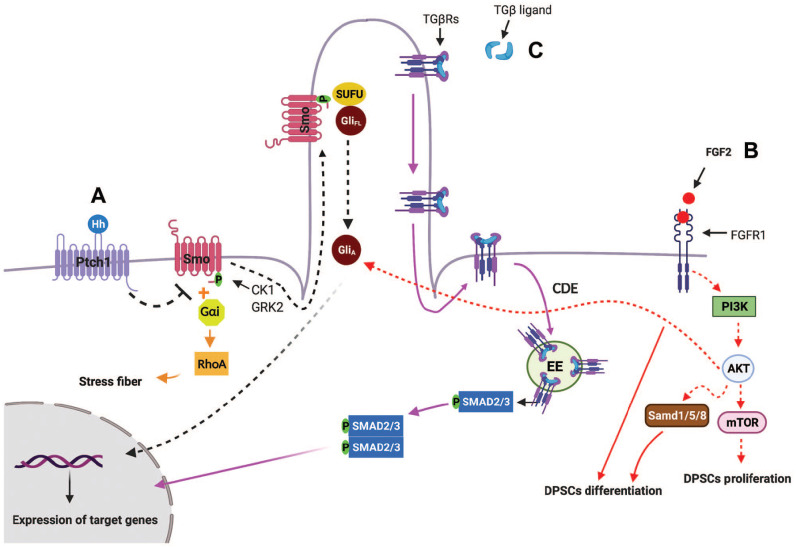
Figure 2.
Schematic and simplified representation of primary cilia-mediated HH, TGF-β, PDGFRα, and FGF signaling pathways. Hedgehog (Hh) signaling. (A) 1. Canonical Hh pathway. In the absence of Hh, Ptch1 prevents the ciliary localization and activation of SMO. When Hh ligand binds to Ptch1, Smo phosphorylates by casein kinase 1 (CK1) and G protein–coupled receptor kinase 2 (GRK2) and moves into primary cilia. Sufu-Gli full length (GliFL) also accumulate in primary cilia, where Smo disassociates Sufu-GliFL, resulting in activation of Gli (GliA). GliA then translocases into nucleus and induces the expression of Hh target genes (black arrows). 2. Noncanonical Hh pathway. One of the noncanonical Hh signaling that is independent of primary cilia is Hh-RhoA signaling, in which Smo-coupled with Gαi proteins activates small GTPase RhoA, which regulates actin stress fiber (orange arrows). (B) Crosstalk among primary cilia, FGF2-FGFR1, Hh, and PI3K-AKT signaling: binding of FGF2 ligands to FGFR1 activates PI3K-AKT-mTOR, resulting in dental pulp stem cell (DPSC) proliferation. In addition, FGF2/FGFR1 activate BMP2 signaling and also induce AKT activation, which aid Hh signaling, resulting in DPSC differentiation (red arrows). (C) TGF-β signaling: in the presence of TGF-β, TGβR I and II translocate from the ciliary membrane to the ciliary pocket through the clathrin-dependent endocytosis (CDE) process, which will result in phosphorylation of SMAD transcription factors 2/3 (SMAD2/3) in early endosomes (EEs) at the ciliary base. Then, the SMAD2/3 complex translocates into the nucleus and activates transcription of TGF-β target genes (purple arrows).
Figure 1.
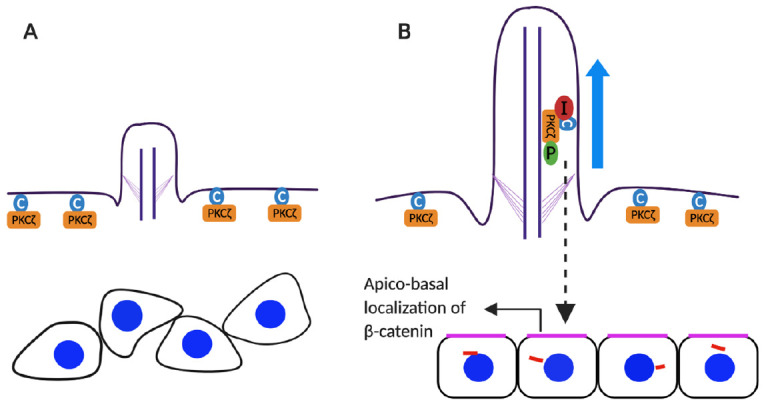
Schematic model for the osteoblast apicobasal polarity and alignment. (A) In the absence of IF20 cilia do not form and protein kinase Cz (PKCz; orange rectangular) and ceramide (blue circle) remain localized in plasma membranes. (B) In the presence of IFT20 (red circle), primary cilia form and IFT20 interacts with the ceramide-PKCz complex, which promotes phosphorylation (green circle) of PKCz in primary cilia. This leads to apical localization of b-catenin in osteoblasts and polarization.
In contrast to IFT complex–B proteins, the role of IFT complex–A proteins in osteoblasts is less well defined. Compared to IFT-B genes, whose mutation leads to the disruption of Hh signal transduction, IFT-A genes often display enhanced Hh signaling. For example, an N-ethyl-N-nitrosourea–derived mouse mutant with a hypomorphic missense mutation in the IFT144 gene impairs ciliogenesis and causes defects in limb outgrowth, disrupted somatic patterning of ribs, and facial/palatal clefting defects. Mechanistically, they found that mutation of IFT144 exhibits ligand-independent expansion of Hh signaling and defects in Shh/GREM1/FGF interactions, particularly in the limbs and facial prominences (Ashe et al. 2012). Recently, another IFT-A protein, IFT140, has been reported to play an important role in endochondral bone development (Zhang, Zhang et al. 2019). IFT140 is highly expressed in differentiated chondrocytes and preosteoblasts during primary ossification, and its expression gradually decreases with age. Lineage-specific ablation of IFT140 in osteoblast precursors and their progeny causes significant growth retardation and osteopenia with dwarfism phenotypes and enhances age-associated bone loss (Tao et al. 2019). These studies indicate that IFT complex A proteins play essential roles in bone development.
Primary Cilia Regulate Cartilage Formation
During endochondral ossification, mesenchymal stem cells (MSCs) differentiate into chondrocytes to produce a cartilage template, which is eventually replaced by bone (Akkiraju and Nohe 2015). Although the significance of primary cilia in chondrogenesis has been reviewed previously (Yuan and Yang 2016), recent studies provide new evidence about the importance of primary cilia and ciliary proteins in cartilage pathology. Kitami et al. (2019) recently reported that IFT20 (one of the components of IFT complex B) plays important roles in the homeostasis of condylar cartilage. They found that IFT20 is highly expressed in the cis-Golgi of condylar cartilage. Deletion of IFT20 in condylar cartilage reduces chondrocyte proliferation and Golgi size and impairs ciliogenesis in the cells. Furthermore, loss of IFT20 in chondrocytes severely attenuates ciliary-mediated Hh signaling and significantly reduces the production levels of type X collagen. This study suggests that IFT20 is required for the maintenance of cartilaginous matrix in condylar cartilage by regulating Golgi and Hh signaling (Kitami et al. 2019). IFT46 is another component of the IFT-B complex. Park et al. (2016) reported that knockdown of IFT46 using morpholino microinjections results in a loss of primary cilia and defective craniofacial development. Their findings further suggest that IFT46 regulates ciliogenesis, neurogenesis, and craniofacial development by modulating Wnt/PCP and Shh signaling pathways (Park et al. 2016). In addition to IFT complex B components, IFT motor kinesin family proteins such as KIF3A and KIF7 have been reported to play an important role in chondrocyte proliferation and differentiation and cartilage development (reviewed by Yuan and Yang 2016). Recently, the role of KIF5B in chondrocytes has also been revealed. Similar to KIF3A and KIF7, deletion of KIF5B in chondrocytes impairs chondrocyte proliferation and differentiation. Notably, loss of KIF5B in chondrocytes leads to delayed and defective cytokinesis, which thereby causes incomplete cell rotation, disrupts proliferation and differentiation, and results in a disorganized growth plate (Gan et al. 2019). These findings demonstrate that KIF5B is a key modulator of cytokinesis in chondrocytes via maintenance of central spindle organization. KIF3B and KIF4 have been found to participate in cytokinesis and central spindle assembly (Gan et al. 2019). Whether other KIF proteins or IFT proteins also play role(s) in regulating cytokinesis needs to be further studied. Consistent with the aforementioned findings in craniofacial cartilage, primary cilia play an essential role in intervertebral disc cartilage development and function. Mice with ablation of IFT80 in chondrocytes show an intervertebral disc (IVD) degeneration phenotype with disrupted intervertebral disc structure, as well as disorganized and reduced growth plates and endplates in the IVD. Mechanistically, deletion of IFT80 significantly impairs ciliogenesis, which leads to increased chondrocyte apoptosis and decreased chondrocyte differentiation and function (Li et al. 2020). These findings provide a new understanding of ciliary protein regulation in cartilage hemostasis. Further studies need to investigate whether different IFTs or other ciliary proteins have distinct functions in the regulation of cartilage development and function and how these proteins regulate signaling pathway networks during cartilage development and function.
Primary Cilia in Mechanotransduction of Chondrocytes
One of the critical functions of the primary cilium in bone is to sense and transduce mechanical signals (Spasic and Jacobs 2017). Different types of mechanical stimulation can alter cilia length and function and thereby affect cell function. Primary cilia and IFT proteins have been found to regulate osteoblast and osteocyte mechanosensing, but few studies have focused on chondrocyte mechanosensory function (reviewed by Yuan and Yang 2016; Tao et al. 2020). Therefore, interest has been growing in discovering the role of primary cilia in chondrocytes. Subramanian et al. (2017) reported that low-intensity ultrasound can elongate and bend primary cilia in chondrocytes to transduce mechanical signals via activation of the MAPK/ERK pathway. Loss of cilia by knockdown of IFT88 inhibits activation of the MAPK/ERK pathway and expression of downstream cartilage matrix genes such as type II collagen (COL-II), type X collagen (COL-X), and BMP-2 (Deren et al. 2016). Moreover, primary cilia can transduce cyclic tensile strain in chondrocytes to inhibit matrix metalloproteinase 1 and 13 expression by activating transcriptional regulator–Cbp/p300 interacting transactivator with ED-rich tail-2 (CITED2). Loss of cilia in chondrocytes abolishes the tensile strain–induced transactivation of CITED2 (He et al. 2016). In addition to IFT proteins, polycystic kidney disease protein polycystin 1 (PC1) and the transient receptor potential channel polycystin 2 (PC2) are mechanosensory proteins. These proteins form a receptor ion–channel complex that localizes to primary cilia in chondrocytes and is essential for sensing physical strain by regulating the activation of transient receptor potential cation channel subfamily V member 4 (TRPV4) (Thompson et al. 2021). Despite these findings highlighting the role of primary cilia in chondrocyte mechanotransduction, the mechanism by which these proteins regulate mechanosensing in chondrocytes remains largely undefined.
Studies have shown that mechanical stimulation within normal limits plays an anti-inflammatory role in different tissues. In cartilage, physiological mechanical loading can inhibit IL-1β and other proinflammatory mediators, such as nitric oxide (NO) and prostaglandin E2 (PGE2), but how mechanical signaling reduces inflammatory responses is underdetermined. Interestingly, recent studies have shown that primary cilia are involved in the regulation of cartilage inflammation. Fu et al. (2019) reported that IL-1β stimulates primary cilia elongation, which promotes the downstream inflammatory response by increasing the release of inflammatory mediators NO and PGE2. However, mechanical loading or IFT88 deficiency blocks cilia elongation induced by IL-1β and inhibits the release of NO or PGE2 in chondrocytes. Mechanical stimulation activates histone deacetylase 6 (HDAC6) to block tubulin acetylation and polymerization to inhibit IL-1–induced primary cilia elongation and the release of NO/PGE (Fu et al. 2019). These findings demonstrate that primary cilia elongation can promote the inflammatory response. However, it remains unclear how mechanical loading inhibits inflammatory signaling and promotes HDAC6 to affect cilia.
Primary Cilia in Mechanotransduction of Osteoblasts
Primary cilia have been identified as critical mechanosensors in osteoblasts, both in vitro and in vivo, through regulating multiple pathways, such as the expression of cyclooxygenase 2 (COX-2) and osteopontin (OPN), PGE2 secretion, production of cAMP, and calcium signaling (reviewed by Yuan and Yang 2016). A recent study showed that osteochondroprogenitor cells in the periosteum sense and respond to mechanical stimuli. Mechanical stimulation, such as fluid shear stress, upregulates the expression of osteoblastic genes and osteoblast differentiation only when primary cilia are present (Moore et al. 2018). When cilia are absent by deletion of IFT88, the osteogenic response from mechanical stimulation is essentially lost (Moore et al. 2018). Similarly, mechanical stimulation enhances bone marrow cell migration to the bone surface and enhances their commitment to the osteogenic lineage along with increased mineral apposition and bone formation (Chen et al. 2016; Moore et al. 2019). Disruption of cilia by deletion of KIF3A in osteoblast lineage cells blocks mechanical loading–induced bone formation (Chen et al. 2016; Moore et al. 2019). Although these studies show the significance of primary cilia as mechanosensory organelles in bone, the impact of mechanical stimulation on cilia function and the underlying signaling regulation remain largely undefined. One recent study reported that mechanical stimulation by a pulsed electromagnetic field (PEMF) can promote bone formation by upregulating bone morphogenic protein receptor II (BMPRII) expression at the base of cilia and activating BMP-Smad1/5/8 signaling, which is required for primary cilia. When IFT88 is ablated to disrupt cilia formation in osteoblasts, PEMF-induced upregulation of BMPRII and its ciliary localization are abolished, and BMP-Smad1/5/8 signaling is subsequently inactivated (Xie et al. 2016). These findings highlight that primary cilia and ciliary proteins are involved in regulating BMP-Smad1/5/8 signaling during mechanical transduction.
Primary Cilia in Craniofacial Development
The significance of primary cilia in craniofacial development is highlighted by ciliopathies showing craniofacial abnormalities, including cleft lip/plate, hypertelorism/hypotelorism, micrognathia, and craniosynostosis (reviewed in Zaghloul and Brugmann 2011; Schock and Brugmann 2017). The most extensively studied ciliary gene in palate, maxilla, and mandible development is IFT88, which is a core protein in IFT complex B. Neural crest cells are major cells that contribute to craniofacial skeleton development. The Wnt1-Cre transgenic line has been frequently used in craniofacial development studies because it expresses cre recombinase to delete the target gene in neural crest cells (Adameyko and Fried 2016). Wnt1-Cre;Ift88fl/fl conditional knockout (cKO) mice with loss of IFT88 in cranial neural crest (CNC) lineage cells die at birth due to severe craniofacial defects in midline fusion of the face and formation of the palatal shelf. Loss of IFT88 disrupts ciliogenesis and reduces neural crest cell proliferation in the palatal shelf during palatogenesis (Tian et al. 2017). Furthermore, IFT88-mediated ciliary defects in the CNC-derived mesenchyme result in downregulation of Shh signaling but upregulation of Wnt signaling during palatogenesis. In addition to palatal cleft defects, Wnt1-Cre;Ift88fl/fl mice also exhibit ectopic excess bone formation in the maxillary process and ectopic apoptosis in the maxillary process at an early stage of development (Watanabe et al. 2019). Most recently, Kitamura et al. (2020) found that Hh signaling was absent in most of the mesenchyme from the anterior to the posterior region of the Wnt1-Cre;Ift88fl/fl mandible, suggesting that cilia loss–mediated disruption of Hh signaling may be a key aspect of defective craniofacial development. Compared to IFT88, deletion of IFT20 in neural crest cells in Wnt1-Cre;IFT20 f/f mice also disrupts ciliogenesis. Interestingly, loss of IFT20 disrupts PDGF signaling, causing reduced osteoblast proliferation and decreased bone mineralization, as well as severe craniofacial malformation, including hypertelorism; abnormal expansion of the facial midline, hypoplastic mandibular, and maxillary bones; and lack of palatal shelves and tongue (Noda et al. 2016). Moreover, loss of IFT20 in CNC-derived mesenchymal cells disrupts the biosynthesis of collagen (a key element in bone mineralization) by increasing the FKBP65 chaperone level, resulting in poor-quality bone collagen, given that the FKBP65 chaperone regulates lysyl hydroxylase 2 activity, which plays an essential role in the collagen cross-linking process. In addition, IFT20 deletion also reduces the amount of collagen production due to dysfunction in endoplasmic reticulum to Golgi trafficking of collagen (Yamaguchi et al. 2020). Taken together, these studies highlight the important functions of primary cilia in craniofacial development through regulating different signaling pathways.
Primary Cilia in Tooth Development
During tooth formation, dental pulp stem cells (DPSCs) differentiate into odontoblasts and produce dentin. Primary cilia are present in the tooth germ and oral tissues. Mutation of ciliary proteins often causes ciliopathies with numerous facial defects and defective odontogenesis (reviewed by Hampl et al. 2017). Our lab recently found ciliary IFT80 expression in DPSCs during tooth development. Primary cilia in DPSCs are elongated during odontogenesis by regulating fibroblast growth factor 2 (FGF2)–FGF receptor 1 (FGFR1) signaling, thereby inducing actin cytoskeleton fiber rearrangement, which is a crucial determinant of cell shape and eventually regulates cell differentiation and proliferation. Deletion of IFT80 in DPSCs inhibits odontogenic differentiation by disrupting cilia formation and inhibiting FGF receptor expression and FGF signaling, Hh signaling, and their crosstalk (Yuan, Liu et al. 2019) (Fig. 2B). In addition, we found that primary cilia facilitate the polarization of odontoblasts during tooth formation, which is a fundamental step for tubular structure formation of dentin. Moreover, mice with deletion of IFT80 in odontoblasts also exhibit reduced DPSC proliferation in the cervical loop, impaired molar root development with shorter molar roots, and less mineralized dentin and delayed incisor eruption. We further found that decreased proliferation is associated with a reduction in FGFR1 expression and accumulation in the basal region of primary cilia, which disrupts FGF2-FGFR1-PI3K-AKT signaling in IFT80-deficient DPSCs (Yuan, Cao et al. 2019). In addition to IFT80, IFT140 has also been shown to be highly expressed during odontoblast differentiation and to play a significant role in tooth development. Mice with IFT140 ablation in odontoblasts (OSX-cre;IFT140 f/f ) display short molar roots with thinner dentin and slower reparative dentin formation in a tooth drilling model. Further research showed that loss of IFT140 in odontoblasts also disrupts primary cilia formation and Shh signaling transduction, resulting in impaired odontoblast differentiation and disrupted dentin deposition (Li et al. 2018). Thus, these findings demonstrate the important role of FGF and Hh signaling in primary cilia regulation of tooth development. Future studies should address whether these ciliary proteins have cilia-independent functions and whether other critical signaling pathways are involved in cilia regulation in odontoblast differentiation and tooth development. A summary of the role of primary cilia in bone, cartilage, teeth, and craniofacial development is provided in the Table.
Table.
Summary of the Studies Regarding the Role of Intraflagellar Transport (IFT) Proteins on Bone, Cartilage, Craniofacial, and Tooth Development.
| Ciliary Genes | Bone Phenotypes | References |
|---|---|---|
| IFT20 | Col1-CreERT;IFT20 f/f and OSX-Cre;IFT20 f/f mice exhibit reduced bone mass and strength. Deletion of IFT20 impairs osteoblast alignments and loss of osteoblast apicobasal polarity. | Lim et al. 2020 |
| IFT80 | OSX-Cre;IFT80 f/f mice display significant growth retardation and osteopenia phenotype. Mutations in IFT144 gene cause severe skeletal defects such as polydactyly, short ribs, small ribcage, short limbs, and craniofacial anomalies. | Yuan et al. 2016 |
| IFT144 | Ashe et al. 2012 | |
| IFT140 | IFT140 highly expresses in bone during bone development. IFT40 expression downregulates in the long bones of murine osteoporosis models (aging and ovariectomy). Osx-Cre;IFT140 f/f mice show dwarf phenotypes, such as short bone length and a significant decrease in trabecular bone mass/mineralization, with narrow and disorganized growth plate. |
Zhang, Zhang et al.
2019
Tao et al. 2019 |
| Ciliary Genes | Cartilage Phenotypes | References |
| IFT80 | IFT80 loss in type II collagen–positive cells increases apoptosis in intervertebral disc structure and decreases chondrogenic and hedgehog (Hh) signaling components markers, leading to early onset of intervertebral disc degeneration phenotype in the Col2-creERT;IFT80 f/f mice model. | Li et al. 2020 |
| KIF5B | The deficiency of KIF5B, a ubiquitously expressed motor protein for anterograde intracellular transport in the microtubule network, results in disorganized columnar structure in the growth plate of the long bone. The failure of cytokinesis in Kif5b null chondrocytes leads to incomplete cell rotation, disrupting proliferation and differentiation. | Gan et al. 2019 |
| IFT20 | IFT20 deletion in condylar cartilage using NG2-CreER mice results in reduced cell proliferation and decreased Golgi size in condylar cartilage, as well as severely attenuates ciliary-mediated Hh signaling, resulting in reduced production of type X collagen. | Kitami et al. 2019 |
| IFT46 | Loss of IFT46 caused craniofacial defects by interfering with cartilage formation. | Park et al. 2016 |
| Ciliary Genes | Craniofacial and Tooth Phenotypes | References |
| IFT88 | Wnt1-Cre;IFT88 f/f mice display disrupted ciliogenesis and reduced neural crest cell proliferation in the palatal shelf, resulting in severe craniofacial defects, including bilateral cleft lip and palate and tongue agenesis. |
Tian et al.
2017
Watanabe et al. 2019 Kitamura et al. 2020 |
| Maxillary process of Wnt1-Cre;IFT88 f/f mice shows ectopic Runx2 expression resulting in excess bone formation in the maxillary process at an early stage of development. | ||
| Mandibular bone of Wnt1-Cre;IFT88 f/f mice is slightly shortened with ectopic bone formation due to the cilia loss–mediated disruption of Hh signaling. | ||
| IFT20 | Ablation of IFT20 in craniofacial osteoblasts disrupts collagen biosynthesis by increasing the FKBP65 chaperone level, leading to craniofacial bone defects. Wnt1-Cre;IFT20 f/f mice demonstrate hypertelorism, frontonasal dysplasia with an opening in the metopic suture, abnormal expansion of the facial midline, and absence of the palatal process of the palatine and pterygoid bone. The maxillary and mandibular bones and palatal process of the maxilla in IFT20 KO mice are hypoplastic. |
Noda et al.
2016
Yamaguchi et al. 2020 |
| IFT80 | OSX-Cre;IFT80 f/f mice exhibit impaired molar root development with less mineralized dentin and delayed incisor eruption. IFT80 regulates dental pulp stem cell proliferation and differentiation through FGF2-FGFR1 and Hh/BMP2 signaling. | Yuan, Cao et al. 2019 |
| IFT140 | OSX-Cre;IFT140 f/f mice demonstrated disrupted Shh signaling with impaired odontoblast differentiation, resulting in short molar root with thinner dentin and slower rate of dentin formation. | Li et al. 2018 |
Primary Cilia in Fracture Healing
Fracture healing is a well-orchestrated regenerative process that involves sequential cellular events, beginning with hematoma formation and an inflammatory response followed by recruitment of mesenchymal/progenitor cells and formation of cartilaginous callus, which is replaced by hard callus and then undergoes remodeling (Bragdon et al. 2015). IFT80 expression is highly upregulated during fracture healing. Mice with conditional deletion of IFT80 in chondrocytes have a clear decrease in callus size with less bone and cartilage formation (Liu et al. 2020). In addition, IFT80 deletion in chondrocytes downregulates the expression of proangiogenic factors, including VEGF, PDGF, and angiopoietin, and inhibits fracture callus vascularization. Mechanistically, loss of IFT80 in chondrocytes dramatically reduces chondrocyte proliferation, cilia assembly, and chondrogenic gene expression and differentiation by downregulating the expression of TGF-βI and TGF-βR and the phosphorylation of Smad2/3 in the fracture callus (Liu et al. 2020) (Fig. 2C). Moreover, we investigated the role of IFT80 and primary cilia in osteoblasts using an OSX-cre;IFT80 f/f mouse model and found a significant reduction in bone density with reduced mechanical strength in fracture calluses of IFT80 cKO mice (manuscript under revision). Most recently, Moore et al. 2021 studied the role of primary cilia in mesenchymal stem cells during fracture healing by using a Prx1-cre transgenic line. Prx1-cre;IFT88 f/f mice display a significant delay in fracture healing with persistent cartilaginous nodules and lack of newly formed bone in fracture calluses. Interestingly, they found that Hh signaling is downregulated during the initial stages of repair but upregulated in the periosteum during the later phase of fracture healing. Furthermore, the results from lineage tracing of the prx+ cells showed that the increased Hh signaling did not correlate with Prx1-expressing cells and their progeny in the callus and new bone tissue. Thus, these results suggest that loss of cilia nonautonomously regulates Hh signaling. Another interesting finding is that deletion of IFT88 leads to an enlarged callus and upregulates chondrogenesis and angiogenesis accompanied by increased expression levels of chondrogenic and vascular markers in IFT88-deficient mice compared to controls. They also found that deletion of IFT88 downregulates the expression of Wnt targets (Axin2, Lef1, Tcf1). Thus, inhibition of Wnt signaling likely results in increased chondrogenesis and angiogenesis. These results demonstrate that primary cilia are involved in the regulation of different signaling pathways in the early and late stages of fracture healing. Further studies need to address how primary cilia control different types of cells and regulate Hh, Wnt, and other signaling pathways to affect different stages of cell processes and fracture healing.
Primary Cilia, Fracture Healing, and FOXO1
FOXO1 is the most abundant and studied forkhead box-O family member found in bone. It plays a significant role in cellular processes such as apoptosis, oxidative stress, and proliferation. FOXO1 positively regulates osteoblast differentiation and modulates bone remodeling to preserve skeletal homeostasis (Siqueira et al. 2011). In fracture healing, FOXO1 induces chondrocytes to express VEGFA and enhance angiogenesis (Zhang, Feinberg et al. 2019). It is also required for regulating the production of cartilage during endochondral formation and the transition from cartilage to bone later in fracture healing (Ding et al. 2021). FOXO1 plays an opposite role in pathologic conditions, such as diabetes, which is characterized by high levels of glucose or advanced glycation end products (AGEs) (Xiao and Graves 2015). For example, diabetes caused significantly enhanced cartilage resorption and impaired subsequent bone formation and mechanical strength, which was rescued by deletion of FOXO1 in chondrocytes (Alharbi et al. 2018; Lu et al. 2019). Mechanistically, diabetes causes FOXO1 to shift from binding with the promoter regions of growth factors such as TGF-β and VEGF to binding with the promoter regions of proinflammatory and proapoptotic genes, thereby reducing the expression of growth factors and enhancing inflammation and apoptosis under diabetic conditions (Wang and Graves 2020). Similar events occur during fracture healing, where diabetes causes a shift from anabolic to catabolic events that are FOXO1 dependent and rescued by FOXO1 deletion. Interestingly, our most recent findings showed that diabetes causes a loss of primary cilia in chondrocytes and osteoblasts. Deletion of FOXO1 in chondrocytes rescues diabetes-impaired cilia formation and defective cartilage production during fracture healing (manuscript in preparation). Our data further indicate that FOXO1 in a diabetic environment suppresses ciliary gene expression to substantially reduce cilia formation and impair the fracture healing process. This finding provides new insight into how diabetic fracture healing may be impaired by FOXO1-suppressed cilia formation.
Concluding Remarks
Great progress has been recently made in understanding the role of both IFT-B and IFT-A complex proteins and primary cilia in the regulation of bone cell behaviors as well as fracture healing and craniofacial and tooth development, as reviewed in this article. Currently, most studies have shown the cilia-dependent functions of ciliary proteins, and little is known about the distinct effects of the different ciliary proteins. In addition, it is largely unknown how ciliary proteins are transcriptionally regulated and how these proteins are involved in cilia-independent pathways. Thus, more investigation to address this unknown question would be interesting. Moreover, although enormous progress has been made in understanding the mechanism by which primary cilia and IFT proteins regulate, little is defined about therapeutic options for ciliopathy-related skeletal and craniofacial disorders. Modulation of ciliogenesis by screening proteins or compounds that can promote or inhibit primary cilia formation and elongation is needed for further study. Moreover, gene therapies have been shown to be a viable treatment option for olfactory ciliopathies (Uytingco et al. 2019); however, whether gene therapies are translatable for the treatment of skeletal ciliopathies is largely unclear. Future studies should assess different treatment modalities, such as gene therapy with ectopic expression of ciliary and related regulatory genes, compounds, and recombinant proteins in skeletal and craniofacial ciliopathies to develop suitable treatment strategies.
Author Contributions
Z. Chinipardaz, D. Graves, S. Yang, contributed to conception, design, data acquisition, analysis, and interpretation, drafted and critically revised the manuscript; M. Liu, contributed to conception and data acquisition, drafted the manuscript. All authors gave final approval and agree to be accountable for all aspects of the work.
Footnotes
Declaration of Conflicting Interests: The authors declared no potential conflicts of interest with respect to the research, authorship, and/or publication of this article.
Funding: The authors disclosed receipt of the following financial support for the research, authorship, and/or publication of this article: The authors acknowledge support from the National Institutes of Health, National Institute of Dental and Craniofacial Research under Award Numbers DE023105 to S. Yang and R01DE019108 to D.T. Graves.
References
- Adameyko I, Fried K. 2016. The nervous system orchestrates and integrates craniofacial development: a review. Front Physiol. 7:49. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Akkiraju H, Nohe A. 2015. Role of chondrocytes in cartilage formation, progression of osteoarthritis and cartilage regeneration. J Dev Biol. 3(4):177–192. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Alharbi MA, Zhang C, Lu C, Milovanova TN, Yi L, Ryu JD, Jiao H, Dong G, O’Connor JP, Graves DT. 2018. FOXO1 deletion reverses the effect of diabetic-induced impaired fracture healing. Diabetes. 67(12):2682–2694. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ashe A, Butterfield NC, Town L, Courtney AD, Cooper AN, Ferguson C, Barry R, Olsson F, Liem KF, Parton RG, et al. 2012. Mutations in mouse Ift144 model the craniofacial, limb and rib defects in skeletal ciliopathies. Hum Mol Genet. 21(8):1808–1823. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bragdon B, Lybrand K, Gerstenfeld L. 2015. Overview of biological mechanisms and applications of three murine models of bone repair: closed fracture with intramedullary fixation, distraction osteogenesis, and marrow ablation by reaming. Curr Protoc Mouse Biol. 5(1):21–34. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen JC, Hoey DA, Chua M, Bellon R, Jacobs CR. 2016. Mechanical signals promote osteogenic fate through a primary cilia-mediated mechanism. FASEB J. 30(4):1504–1511. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Deren ME, Yang X, Guan Y, Chen Q. 2016. Biological and chemical removal of primary cilia affects mechanical activation of chondrogenesis markers in chondroprogenitors and hypertrophic chondrocytes. Int J Mol Sci. 17(2):188. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ding Z, Qiu M, Alharbi MA, Huang T, Pei X, Milovanova TN, Jiao H, Lu C, Liu M, Qin L, et al. 2021. FOXO1 expression in chondrocytes modulates cartilage production and removal in fracture healing. Bone. 148:115905. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fu S, Thompson CL, Ali A, Wang W, Chapple JP, Mitchison HM, Beales PL, Wann AKT, Knight MM. 2019. Mechanical loading inhibits cartilage inflammatory signalling via an HDAC6 and IFT-dependent mechanism regulating primary cilia elongation. Osteoarthr Cartil. 27(7):1064–1074. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gan H, Xue W, Gao Y, Zhu G, Chan D, Cheah KSE, Huang J. 2019. KIF5B modulates central spindle organization in late-stage cytokinesis in chondrocytes. Cell Biosci. 9:85. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hampl M, Cela P, Szabo-Rogers HL, Bosakova MK, Dosedelova H, Krejci P, Buchtova M. 2017. Role of primary cilia in odontogenesis. J Dent Res. 96(9):965–974. [DOI] [PMC free article] [PubMed] [Google Scholar]
- He Z, Leong DJ, Zhuo Z, Majeska RJ, Cardoso L, Spray DC, Goldring MB, Cobelli NJ, Sun HB. 2016. Strain-induced mechanotransduction through primary cilia, extracellular ATP, purinergic calcium signaling, and ERK1/2 transactivates CITED2 and downregulates MMP-1 and MMP-13 gene expression in chondrocytes. Osteoarthr Cartil. 24(5):892–901. [DOI] [PubMed] [Google Scholar]
- Ishikawa H, Marshall WF. 2017. Intraflagellar transport and ciliary dynamics. Cold Spring Harb Perspect Biol. 9(3):a021998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ishikawa T, Ueno H, Omori T, Kikuchi K. 2021. Cilia and centrosomes: ultrastructural and mechanical perspectives. Semin Cell Dev Biol. 110:61–69. [DOI] [PubMed] [Google Scholar]
- Izu Y, Sun M, Zwolanek D, Veit G, Williams V, Cha B, Jepsen KJ, Koch M, Birk DE. 2011. Type XII collagen regulates osteoblast polarity and communication during bone formation. J Cell Biol. 193(6):1115–1130. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kitami M, Yamaguchi H, Ebina M, Kaku M, Chen D, Komatsu Y. 2019. IFT20 is required for the maintenance of cartilaginous matrix in condylar cartilage. Biochem Biophys Res Commun. 509(1):222–226. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kitamura A, Kawasaki M, Kawasaki K, Meguro F, Yamada A, Nagai T, Kodama Y, Trakanant S, Sharpe PT, Maeda T, et al. 2020. Ift88 is involved in mandibular development. J Anat. 236(2):317–324. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li G, Liu M, Zhang S, Wan H, Zhang Q, Yue R, Yan X, Wang X, Wang Z, Sun Y. 2018. Essential role of IFT140 in promoting dentinogenesis. J Dent Res. 97(4):423–431. [DOI] [PubMed] [Google Scholar]
- Li X, Shuting Yang, Han L, Mao K, Shuying Yang. 2020. Ciliary IFT80 is essential for intervertebral disc development and maintenance. FASEB J. 34(5):6741–6756. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lim J, Li X, Yuan X, Yang S, Han L, Yang S. 2020. Primary cilia control cell alignment and patterning in bone development via ceramide-PKCζ-β-catenin signaling. Commun Biol. 3(1):45. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu M, Alharbi M, Graves D, Yang S. 2020. IFT80 is required for fracture healing through controlling the regulation of TGF-β signaling in chondrocyte differentiation and function. J Bone Miner Res. 35(3):571–582. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lu Y, Alharbi M, Zhang C, O’Connor JP, Graves DT. 2019. Deletion of FOXO1 in chondrocytes rescues the effect of diabetes on mechanical strength in fracture healing. Bone. 123:159–167. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mitchison HM, Valente EM. 2017. Motile and non-motile cilia in human pathology: from function to phenotypes. J Pathol. 241(2):294–309. [DOI] [PubMed] [Google Scholar]
- Moore ER, Chen JC, Jacobs CR. 2019. Prx1-expressing progenitor primary cilia mediate bone formation in response to mechanical loading in mice. Stem Cells Int. 2019:3094154. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Moore ER, Mathews OA, Yao Y, Yang Y. 2021. Prx1-expressing cells contributing to fracture repair require primary cilia for complete healing in mice. Bone. 143:115738. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Moore ER, Zhu YX, Ryu HS, Jacobs CR. 2018. Periosteal progenitors contribute to load-induced bone formation in adult mice and require primary cilia to sense mechanical stimulation. Stem Cell Res Ther. 9(1):190. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nakayama K, Katoh Y. 2018. Ciliary protein trafficking mediated by IFT and BBSome complexes with the aid of kinesin-2 and dynein-2 motors.J Biochem. 163(3):155–164. [DOI] [PubMed] [Google Scholar]
- Noda K, Kitami M, Kitami K, Kaku M, Komatsu Y. 2016. Canonical and noncanonical intraflagellar transport regulates craniofacial skeletal development. Proc Natl Acad Sci U S A. 113(19):E2589–E2597. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Park I, Lee HK, Kim C, Ismail T, Kim YK, Park JW, Kwon OS, Kang BS, Lee DS, Park TJ, et al. 2016. IFT46 plays crucial roles in craniofacial and cilia development. Biochem Biophys Res Commun. 477(3):419–425. [DOI] [PubMed] [Google Scholar]
- Schock EN, Brugmann SA. 2017. Discovery, diagnosis, and etiology of craniofacial ciliopathies. Cold Spring Harb Perspect Biol. 9(9):a028258. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Siqueira MF, Flowers S, Bhattacharya R, Faibish D, Behl Y, Kotton DN, Gerstenfeld L, Moran E, Graves DT. 2011. FOXO1 modulates osteoblast differentiation. Bone. 48(5):1043–1051. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Spasic M, Jacobs CR. 2017. Primary cilia: cell and molecular mechanosensors directing whole tissue function. Semin Cell Dev Biol. 71:42–52. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Subramanian A, Budhiraja G, Sahu N. 2017. Chondrocyte primary cilium is mechanosensitive and responds to low-intensity-ultrasound by altering its length and orientation. Int J Biochem Cell Biol. 91(Pt A):60–64. [DOI] [PubMed] [Google Scholar]
- Tao D, Xue H, Zhang C, Li G, Sun Y. 2019. The role of IFT140 in osteogenesis of adult mice long bone. J Histochem Cytochem. 67(8):601–611. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tao F, Jiang T, Tao H, Cao H, Xiang W. 2020. Primary cilia: versatile regulator in cartilage development. Cell Prolif. 53(3):e12765. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Thompson CL, McFie M, Paul Chapple J, Beales P, Knight MM. 2021. Polycystin-2 is required for chondrocyte mechanotransduction and traffics to the primary cilium in response to mechanical stimulation. Int J Mol Sci. 22(9):4313. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tian H, Feng J, Li J, Ho TV, Yuan Y, Liu Y, Brindopke F, Figueiredo JC, Magee W, Sanchez-Lara PA, et al. 2017. Intraflagellar transport 88 (IFT88) is crucial for craniofacial development in mice and is a candidate gene for human cleft lip and palate. Hum Mol Genet. 26(5):860–872. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Uytingco CR, Green WW, Martens JR. 2019. Olfactory loss and dysfunction in ciliopathies: molecular mechanisms and potential therapies. Curr Med Chem. 26(17):3103–3119. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang Y, Graves DT. 2020. Keratinocyte function in normal and diabetic wounds and modulation by FOXO1. J Diabetes Res. 2020:3714704. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Watanabe M, Kawasaki M, Kawasaki K, Kitamura A, Nagai T, Kodama Y, Meguro F, Yamada A, Sharpe PT, Maeda T, et al. 2019. Ift88 limits bone formation in maxillary process through suppressing apoptosis. Arch Oral Biol. 101:43–50. [DOI] [PubMed] [Google Scholar]
- Wheatley DN. 2018. The primary cilium—once a “rudimentary” organelle that is now a ubiquitous sensory cellular structure involved in many pathological disorders. J Cell Commun Signal. 12(1):211–216. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Xiao E, Graves DT. 2015. Impact of diabetes on the protective role of FOXO1 in wound healing. J Dent Res. 94(8):1025–1026. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Xie YF, Shi WG, Zhou J, Gao YH, Li SF, Fang QQ, Wang MG, Ma HP, Wang JF, Xian CJ, et al. 2016. Pulsed electromagnetic fields stimulate osteogenic differentiation and maturation of osteoblasts by upregulating the expression of BMPRII localized at the base of primary cilium. Bone. 93:22–32. [DOI] [PubMed] [Google Scholar]
- Yamaguchi H, Terajima M, Kitami M, Wang J, He L, Saeki M, Yamauchi M, Komatsu Y. 2020. IFT20 is critical for collagen biosynthesis in craniofacial bone formation. Biochem Biophys Res Commun. 533(4):739–744. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yuan X, Cao J, He X, Serra R, Qu J, Cao X, Yang S. 2016. Ciliary IFT80 balances canonical versus non-canonical hedgehog signalling for osteoblast differentiation. Nat Commun. 7:11024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yuan X, Cao X, Yang S. 2019. IFT80 is required for stem cell proliferation, differentiation, and odontoblast polarization during tooth development. Cell Death Dis. 10(2):63. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yuan X, Liu M, Cao X, Yang S. 2019. Ciliary IFT80 regulates dental pulp stem cells differentiation by FGF/FGFR1 and Hh/BMP2 signaling. Int J Biol Sci. 15(10):2087–2099. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yuan X, Yang S. 2016. Primary cilia and intraflagellar transport proteins in bone and cartilage. J Dent Res. 95(12):1341–1349. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zaghloul NA, Brugmann SA. 2011. The emerging face of primary cilia. Genesis. 49(4):231–246. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang C, Feinberg D, Alharbi M, Ding Z, Lu C, O’Connor JP, Graves DT. 2019. Chondrocytes promote vascularization in fracture healing through a FOXO1-dependent mechanism. J Bone Miner Res. 34(3):547–556. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang C, Zhang S, Sun Y. 2019. Expression of IFT140 during bone development. J Histochem Cytochem. 67(10):723–734. [DOI] [PMC free article] [PubMed] [Google Scholar]