To the Editor:
Pulmonary rehabilitation (PR) for adults with chronic obstructive pulmonary disease (COPD) results in substantial improvement in dyspnea and functional exercise tolerance, and it is also associated with reduction in exacerbations and improved survival rates (1). Despite these proven benefits, only a minority of patients with COPD are able to access PR. In 2012, only 3.7% of Medicare beneficiaries used PR (2). Even after hospitalization for an exacerbation, only 1.9% received PR within 6 months of discharge (3). PR is traditionally delivered at centers equipped with exercise tools. A major barrier to access is the limited number of PR centers within a reasonable distance from patients’ homes (4–6). Indeed, even a distance of 10 miles lowers the odds of participating in PR by approximately half (3). The limited access to center-based PR—especially in light of the increase in PR-center closures because of poor reimbursement and the ongoing coronavirus disease (COVID-19) pandemic—provides an impetus to find alternative avenues for administration of PR.
The benchmark for PR is a supervised, center-based program wherein higher-intensity exercise can be achieved compared with non–center-based interventions with minimal equipment. Only a few studies have evaluated alternative avenues for delivery of PR. Although a recent Cochrane Review suggested telerehabilitation is equivalent to center-based PR, the studies included were heterogeneous and included those with home visits or exercise equipment available mostly at centers (7). The results of studies comparing home-based with center-based PR are inconclusive but generally suggest equivalence for short- and long-term improvements in clinical status (8–11). With improvement in access to technology, we aimed to test whether remotely delivered PR, using minimal equipment via a live video telehealth intervention, can achieve clinical improvements similar to those attained in center-based PR for stable COPD.
Methods
We enrolled adults with COPD from the pulmonary clinic at a single quaternary care academic hospital to receive real-time video PR from August 2018 to June 2020. Only those patients who were unable to access center-based PR because of distance or insurance coverage were offered the telehealth PR intervention, and physician preference did not influence the ability to enroll in telehealth PR. Patients were included irrespective of severity of airflow obstruction. Exclusion criteria included unstable arrhythmias; congestive heart failure with left ventricular ejection fraction at less than 25%; oxygen requirement greater than 5 L/min at rest; or other comorbidities that precluded participation in exercise, including dementia and physical infirmities. All telehealth participants provided written informed consent. Participants were not randomized. Telehealth PR participants who completed the prescribed exercise intervention (minimum 20 sessions) and had postintervention assessments were retrospectively group matched 1:3 to get a similar distribution of forced expiratory volume in 1 second (FEV1) with patients who previously received center-based PR at the same institution. The study was approved by the University of Alabama Institutional Review Board.
The live, interactive video telehealth PR intervention was provided via center-delivered or patient-owned smartphones using a Health Insurance Portability and Accountability Act–compliant application. The video PR intervention was constructed to mimic all components of center-based PR but with minimal equipment (12). An initial 6-minute-walk distance (6MWD) was performed at the center, and a tailored exercise regimen was prescribed by an exercise physiologist based on functional capacity. A foot peddler was provided for aerobic exercise and stretch bands for resistance training. Thirty-six sessions of 45–60 minutes were administered over 12 weeks, and sessions included a combination of stretching, resistance training, and 20 minutes of aerobic exercises targeted to achieve heart rates between 60 and 80% of the maximum recorded on the baseline 6-minute-walk test. Sessions also included breathing exercises such as pursed-lips breathing, paced breathing, diaphragmatic muscle strength training, and basic yoga exercises. The video sessions also incorporated education on disease management, monitoring for exacerbations, smoking cessation, diet, and appropriate inhaler techniques. Safety assessments included blood pressure, heart rate, and pulse oximetry monitoring before and after exercise as well as intermittently during exercise. Telehealth PR was delivered to a maximum of four participants at a time.
Functional capacity was assessed pre- and post-intervention using the 6MWD, and dyspnea was assessed using The University of California, San Diego Shortness of Breath Questionnaire (SOBQ). We compared the change in 6MWD and SOBQ separately in telehealth PR and center-based PR using the paired t test and compared between-group differences using the independent t test. All analyses were performed using Statistical Package for the Social Sciences (SPSS 22.0), and a two-tailed α of 0.05 was deemed statistically significant.
Results
Of 59 patients referred for telehealth PR, 11 decided not to enroll and 11 did not complete at least 24 sessions. Of the 37 who completed PR, 32 participants who completed telehealth PR and had full follow-up assessments were group matched with 96 patients who completed center-based PR. Telehealth and center-based PR had similar distribution of ages (65 ± 9 vs. 67 ± 8 years, respectively), sex (50% vs. 57% female), race (22% vs. 22% Black participants), lung function impairment (FEV1 1.14 ± 0.59 vs. 1.13 ± 0.52 L), home oxygen use (56% vs. 52%), and comorbid congestive heart failure (6% vs. 15%) (Table 1). Baseline 6MWD was worse in telehealth PR compared with center-based PR (248 ± 102 vs. 298 ± 92 m; P = 0.01). The attrition rate for telehealth PR was 22.9% (11 out of 48).
Table 1.
Baseline characteristics of participants in video telehealth and center-based PR
| Center-based PR (n = 96) |
Video Telehealth PR (n = 32) |
|
|---|---|---|
| Age, yr | 67.0 (8.8) | 64.8 (9.0) |
| Females, n (%) | 55 (57) | 16 (50) |
| African Americans, n (%) | 21 (22) | 7 (22) |
| Body mass index, kg/m2 | 28.4 (7.5) | 31.2 (10.4) |
| Smoking status, current, n (%) | 8 (8) | 5 (16) |
| Smoking status, former, n (%) | 84 (88) | 26 (81) |
| Pack-years of smoking | 50.0 (31.7) | 48.7 (39.5) |
| FEV1, L | 1.1 (0.5) | 1.1 (0.6) |
| FEV1/FVC | 0.49 (0.17) | 0.47 (0.15) |
| Domiciliary oxygen use, n (%) | 50 (52) | 18 (56) |
| Hypertension, n (%) | 67 (70) | 18 (56) |
| Diabetes mellitus, n (%) | 27 (28) | 4 (13) |
| Depression, n (%) | 21 (22) | 6 (19) |
| Congestive heart failure, n (%) | 14 (15) | 2 (6) |
| Home oxygen use, n (%) | 50 (52) | 18 (56) |
| Charlson comorbidity index | 4.5 (1.8) | 3.6 (1.5) |
| Baseline 6MWD, m | 297.5 (92.2) | 280.0 (101.8) |
| Baseline SOBQ, units | 59.8 (20.0) | 66.4 (19.3) |
Definition of abbreviations: 6MWD = 6-minute-walk distance; FEV1 = forced expiratory volume in 1 second; FVC = forced vital capacity; PR = pulmonary rehabilitation; SOBQ = The University of California, San Diego Shortness of Breath Questionnaire.
All values are expressed as mean (standard deviation) unless otherwise indicated.
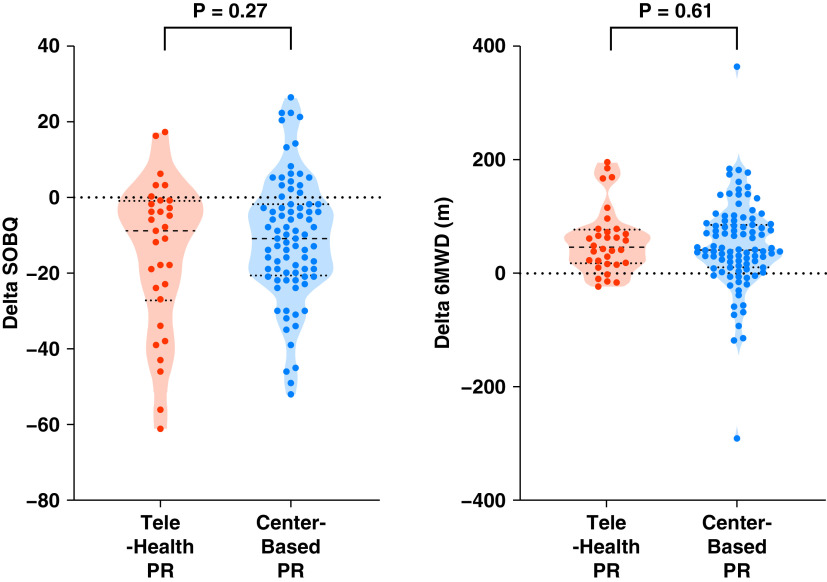
Participation in center-based PR was associated with clinically important improvements in SOBQ (−11.1 ± 16.2; P < 0.001) and 6MWD (47.3 ± 78.7 m; P < 0.001). Telehealth PR was also associated with clinically important improvements in SOBQ (−15.1 ± 19.9; P < 0.001) and 6MWD (55.0 ± 56.9 m; P < 0.001). The percentage of patients who achieved minimal clinically important differences of 5 units for SOBQ and 26 m for 6MWD were similar between telehealth and center-based PR, 19 (61.3%) versus 54 (65.1%) and 21 (65.6%) versus 63 (66.3%), respectively. There were no significant differences in change in SOBQ between the two groups (4.0; 95% confidence interval, −3.2 to 11.2; P = 0.27) and 6MWD (7.7 m; 95% confidence interval, −37.6 to 22.2; P = 0.61) (Figure 1). No adverse events were reported with telehealth PR.
Figure 1.
Change in dyspnea and functional capacity with telehealth and center-based pulmonary rehabilitation (PR). Violin plots show difference before and after PR for SOBQ and 6MWD, with horizontal lines indicating median and interquartile range. The outlines illustrate the proportion of data concentrated at each value. 6MWD = 6-minute-walk distance; SOBQ = The University of California, San Diego Shortness of Breath Questionnaire.
Discussion
We demonstrated that a video telehealth PR confers benefits similar to those observed in center-based PR and is an effective alternative that may overcome some of the barriers to access noted with center-based PR.
Poor access to PR and capacity constraints have driven a search for alternate avenues for delivery of PR. Given the concerns about the benefits derived from lower-intensity exercise protocols at home, several studies have examined the impact of home-based PR in comparison with center-based PR. Maltais and colleagues showed that an 8-week, home-based PR intervention was associated with similar improvement in dyspnea as center-based PR but was limited by the need for an initial 4-week, center-based education program and the provision of a cycle ergometer for home use (8). Holland and colleagues showed that an unsupervised home exercise program with minimal equipment was associated with clinically important improvement at 8 weeks, but comparisons were limited by the lack of expected improvement in the center-based PR arm (10). Horton and colleagues showed that an unsupervised, 7-week, home-based PR intervention resulted in similar shuttle walk test distances (13). The improvement in dyspnea was also not different but favored the center-based program. The attrition rate compared favorably with the 48% attrition rate with center-based PR at our center (6). These data suggest that perhaps more direct supervision can bridge the gap between unsupervised home-based and supervised center-based PR. Indeed, Hansen and colleagues found no difference in 6MWD between telehealth PR and center-based PR at completion and at 22 weeks (11).
These telehealth interventions are, however, currently not reimbursed by insurance in the United States, primarily because of a lack of studies demonstrating benefits. We add to the literature by demonstrating that a supervised video telehealth PR intervention is safe and is associated with clinical improvements comparable to those with center-based PR.
Our study has a few limitations. Although we group matched individuals by lung function impairment, participants were not randomized. To compare the benefits accrued from telehealth PR and center-based PR, we included completers only. This case-control design, however, results in a per-protocol analysis that enables direct comparison of the interventions. These results should be confirmed with a noninferiority randomized controlled trial.
Footnotes
Supported by the National Heart, Lung, and Blood Institute grants (R01 HL151421 and UG3HL155806) and the National Institutes of Health (K23HL133438).
Author Contributions: S.P.B. had full access to all data in the study and takes responsibility for the integrity of the data and the accuracy of the data analysis. S.P.B. conceived and designed the study, drafted the manuscript, and managed the statistical analysis. All authors were involved in the acquisition, analysis, or interpretation of data; critical revision of the manuscript for important intellectual content; and study supervision.
Author disclosures are available with the text of this letter at www.atsjournals.org.
References
- 1. McCarthy B, Casey D, Devane D, Murphy K, Murphy E, Lacasse Y. Pulmonary rehabilitation for chronic obstructive pulmonary disease. Cochrane Database Syst Rev . 2015;2:CD003793. doi: 10.1002/14651858.CD003793.pub3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Nishi SPE, Zhang W, Kuo YF, Sharma G. Pulmonary rehabilitation utilization in older adults with chronic obstructive pulmonary disease, 2003 to 2012. J Cardiopulm Rehabil Prev . 2016;36:375–382. doi: 10.1097/HCR.0000000000000194. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Spitzer KA, Stefan MS, Priya A, Pack QR, Pekow PS, Lagu T, et al. Participation in pulmonary rehabilitation after hospitalization for chronic obstructive pulmonary disease among medicare beneficiaries. Ann Am Thorac Soc . 2019;16:99–106. doi: 10.1513/AnnalsATS.201805-332OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Bhatt SP.It’s time to rehabilitate pulmonary rehabilitation Ann Am Thorac Soc 20191655–57.. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Spitzer KA, Stefan MS, Priya A, Pack QR, Pekow PS, Lagu T, et al. A geographic analysis of racial disparities in use of pulmonary rehabilitation after hospitalization for COPD exacerbation. Chest . 2020;157:1130–1137. doi: 10.1016/j.chest.2019.11.044. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Brown AT, Hitchcock J, Schumann C, Wells JM, Dransfield MT, Bhatt SP. Determinants of successful completion of pulmonary rehabilitation in COPD. Int J Chron Obstruct Pulmon Dis . 2016;11:391–397. doi: 10.2147/COPD.S100254. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Cox NS, Dal Corso S, Hansen H, McDonald CF, Hill CJ, Zanaboni P, et al. Telerehabilitation for chronic respiratory disease. Cochrane Database Syst Rev . 2021;1:CD013040. doi: 10.1002/14651858.CD013040.pub2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Maltais F, Bourbeau J, Shapiro S, Lacasse Y, Perrault H, Baltzan M, et al. Chronic Obstructive Pulmonary Disease Axis of Respiratory Health Network, Fonds de recherche en santé du Québec Effects of home-based pulmonary rehabilitation in patients with chronic obstructive pulmonary disease: a randomized trial. Ann Intern Med . 2008;149:869–878. doi: 10.7326/0003-4819-149-12-200812160-00006. [DOI] [PubMed] [Google Scholar]
- 9. Mendes de Oliveira JC, Studart Leitão Filho FS, Malosa Sampaio LM, Negrinho de Oliveira AC, Hirata RP, Costa D, et al. Outpatient vs. home-based pulmonary rehabilitation in COPD: a randomized controlled trial. Multidiscip Respir Med . 2010;5:401–408. doi: 10.1186/2049-6958-5-6-401. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Holland AE, Mahal A, Hill CJ, Lee AL, Burge AT, Cox NS, et al. Home-based rehabilitation for COPD using minimal resources: a randomised, controlled equivalence trial. Thorax . 2017;72:57–65. doi: 10.1136/thoraxjnl-2016-208514. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Hansen H, Bieler T, Beyer N, Kallemose T, Wilcke JT, Østergaard LM, et al. Supervised pulmonary tele-rehabilitation versus pulmonary rehabilitation in severe COPD: a randomised multicentre trial. Thorax . 2020;75:413–421. doi: 10.1136/thoraxjnl-2019-214246. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Bhatt SP, Patel SB, Anderson EM, Baugh D, Givens T, Schumann C, et al. Video telehealth pulmonary rehabilitation intervention in chronic obstructive pulmonary disease reduces 30-day readmissions. Am J Respir Crit Care Med . 2019;200:511–513. doi: 10.1164/rccm.201902-0314LE. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Horton EJ, Mitchell KE, Johnson-Warrington V, Apps LD, Sewell L, Morgan M, et al. Comparison of a structured home-based rehabilitation programme with conventional supervised pulmonary rehabilitation: a randomised non-inferiority trial. Thorax . 2018;73:29–36. doi: 10.1136/thoraxjnl-2016-208506. [DOI] [PubMed] [Google Scholar]