Abstract
Smoking burdens are greatest among underserved patients. Lung cancer screening (LCS) reduces mortality among individuals at risk for smoking-associated lung cancer. Although LCS programs must offer smoking cessation support, the interventions that best promote cessation among underserved patients in this setting are unknown. This stakeholder-engaged, pragmatic randomized clinical trial will compare the effectiveness of four interventions promoting smoking cessation among underserved patients referred for LCS. By using an additive study design, all four arms provide standard “ask–advise–refer” care. Arm 2 adds free or subsidized pharmacologic cessation aids, arm 3 adds financial incentives up to $600 for cessation, and arm 4 adds a mobile device–delivered episodic future thinking tool to promote attention to long-term health goals. We hypothesize that smoking abstinence rates will be higher with the addition of each intervention when compared with arm 1. We will enroll 3,200 adults with LCS orders at four U.S. health systems. Eligible patients include those who smoke at least one cigarette daily and self-identify as a member of an underserved group (i.e., is Black or Latinx, is a rural resident, completed a high school education or less, and/or has a household income <200% of the federal poverty line). The primary outcome is biochemically confirmed smoking abstinence sustained through 6 months. Secondary outcomes include abstinence sustained through 12 months, other smoking-related clinical outcomes, and patient-reported outcomes. This pragmatic randomized clinical trial will identify the most effective smoking cessation strategies that LCS programs can implement to reduce smoking burdens affecting underserved populations.
Clinical trial registered with clinicaltrials.gov (NCT 04798664). Date of registration: March 12, 2021. Date of trial launch: May 17, 2021.
Keywords: smoking cessation, tobacco cessation, vulnerable populations, pragmatic clinical trial, lung cancer screening
Smoking remains the leading cause of preventable morbidity and mortality worldwide, causing more than 5 million deaths annually, including 500,000 in the United States (1). The National Lung Screening Trial found that annual lung cancer screening (LCS) among patients aged 55–74 years who are current or former heavy smokers (⩾30 pack-years) reduces mortality due to lung cancer (2). Thus, annual LCS became widely recommended by national guidelines and adopted by payers (3, 4). In 2020, a U.S. Preventive Services Task Force simulated optimal screening strategies and concluded that expanding screening eligibility to patients aged 50–80 years with at least 20 pack-years of smoking history would increase the benefit and reduce existing disparities in lung cancer mortality by race, ethnicity, and sex compared with using the prior minimum age of 55 and a minimum smoking history of 30 pack-years (5).
LCS provides a unique opportunity to engage the more than 5 million eligible Americans who continue to smoke tobacco (6, 7). The prevalence of smoking among individuals presenting for LCS is considerably higher than among those in the community, with 48–70% of those undergoing LCS actively smoking (8). Patients receiving abnormal results from their LCS stop smoking at higher rates than those with normal LCS results and those among the general population (9). Those who quit smoking during the period of LCS eligibility gain an average of 4 life-years, which is more than the number of life-years gained through early lung cancer detection (10). Therefore, smoking cessation interventions are a required core component of LCS as per national guidelines (3, 11) and the Centers for Medicare and Medicaid Services (12).
Embedding smoking cessation interventions within LCS programs may also mitigate the disproportionate impact of smoking on patients who are Black or Latinx, live in rural communities, and/or have a low socioeconomic status (SES) (13–15). These underserved populations (by which we mean historically underresourced or disenfranchised populations) (16) are overrepresented among patients eligible for LCS yet are less likely to successfully quit (17–19). Therefore, providing systematic smoking cessation support during LCS may begin to reduce health inequities attributable to tobacco use (20, 21).
Despite these compelling reasons to embed intensive smoking cessation services within LCS programs, numerous studies, systematic reviews, and consensus statements have noted the uncertainty regarding which interventions best promote cessation among patients undergoing LCS (4, 11, 12, 22, 23). A common approach is “ask–advise–refer” (AAR), whereby patients are asked if they smoke, advised of the benefits of cessation, and referred to local cessation resources (23). Yet few patients achieve cessation when exposed to the AAR approach (24). Because information alone is often ineffective in modifying health behaviors, interventions are needed that target specific barriers to successful smoking cessation, such as physiologic craving, the lack of an impetus to initiate the quitting process, and the human tendency to be present biased, neglecting the future health benefits resulting from cessation (Figure 1). We designed the Healthy Lungs trial to compare the effectiveness of combinations of interventions that target these barriers with the effectiveness of the AAR approach for promoting sustained smoking abstinence among underserved patients referred for LCS.
Figure 1.
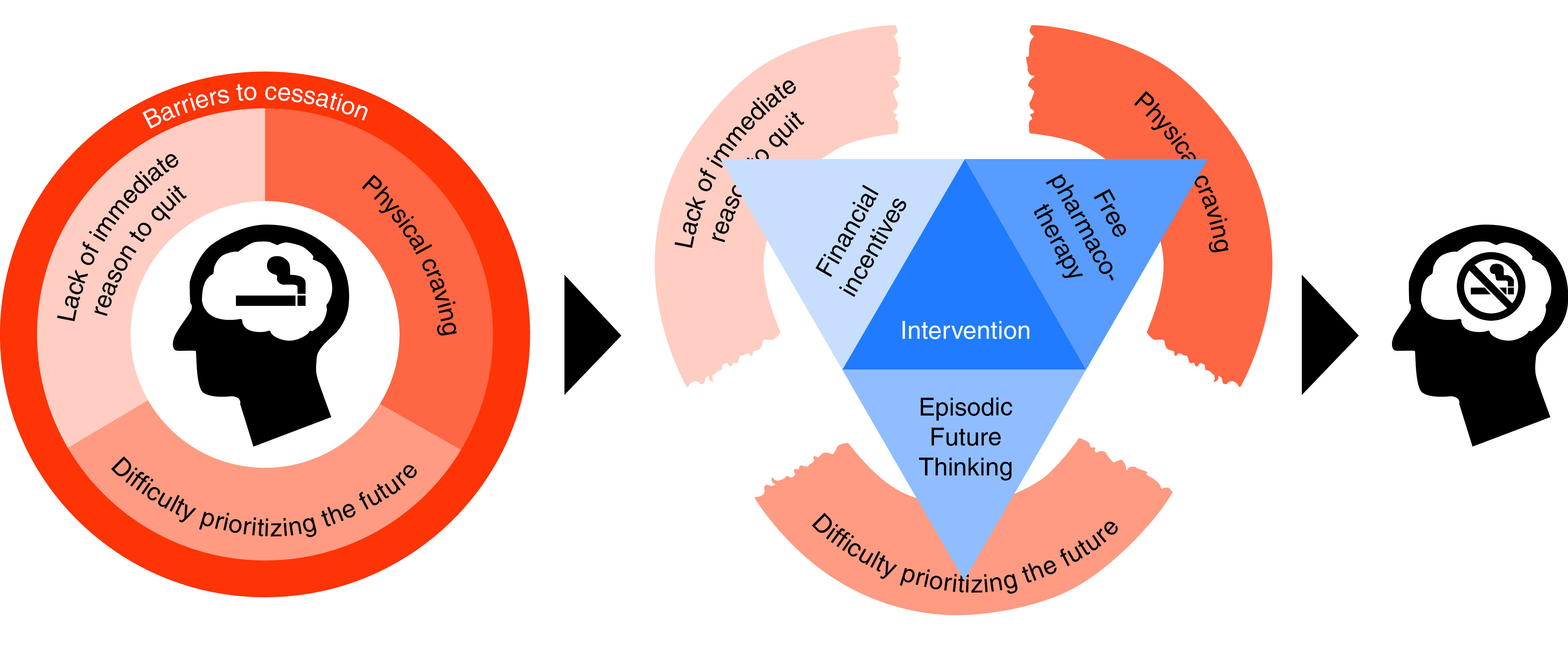
Conceptual model.
Methods
Design and Overview
We are conducting a stakeholder-engaged, pragmatic, four-arm randomized clinical trial (RCT) among 3,200 underserved patients who smoke tobacco at the time of LCS referral. The trial was designed to be highly pragmatic by using the PRagmatic-Explanatory Continuum Indicator Summary 2 criteria (PRECIS-2) (Figure 2; see also Table E1 in the online supplement) (25, 26). Patients will be assigned to one of four trial arms, including one, two, three, or four smoking cessation interventions. All arms will provide AAR care. Arm 2 will add free or subsidized U.S. Food and Drug Administration (FDA)-approved pharmacologic cessation aids, arm 3 will add financial incentives to stop smoking, and arm 4 will add an episodic future thinking (EFT) tool to prompt patients to think about their future health. Participants will be offered enrollment in the program after LCS referral and will be randomized once their eligibility based on self-identified sociodemographic characteristics has been confirmed. The primary outcome is sustained, biologically confirmed smoking abstinence, requiring negative results from biochemical evaluations of nicotine metabolites at 2 weeks, 3 months, and 6 months. Relapse and other outcomes will be assessed at 12 months (clinicaltrials.gov identifier NCT 04798664; date of registration: March 12, 2021; date of trial launch: May 17, 2021) (Figure 3).
Figure 2.
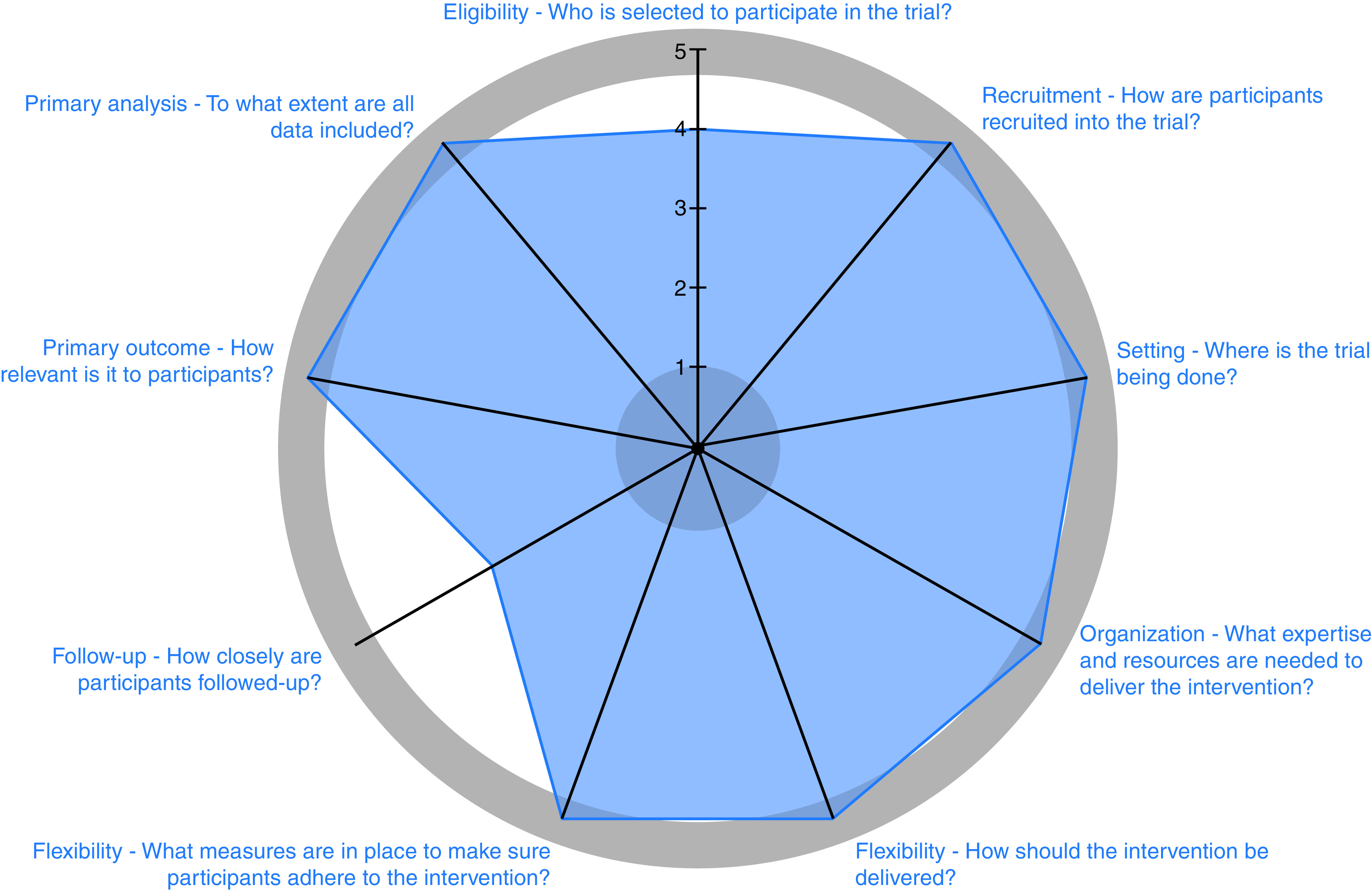
Pragmatism of the proposed trial based on PRagmatic–Explanatory Continuum Indicator Summary 2 criteria (PRECIS-2) (25, 26).
Figure 3.
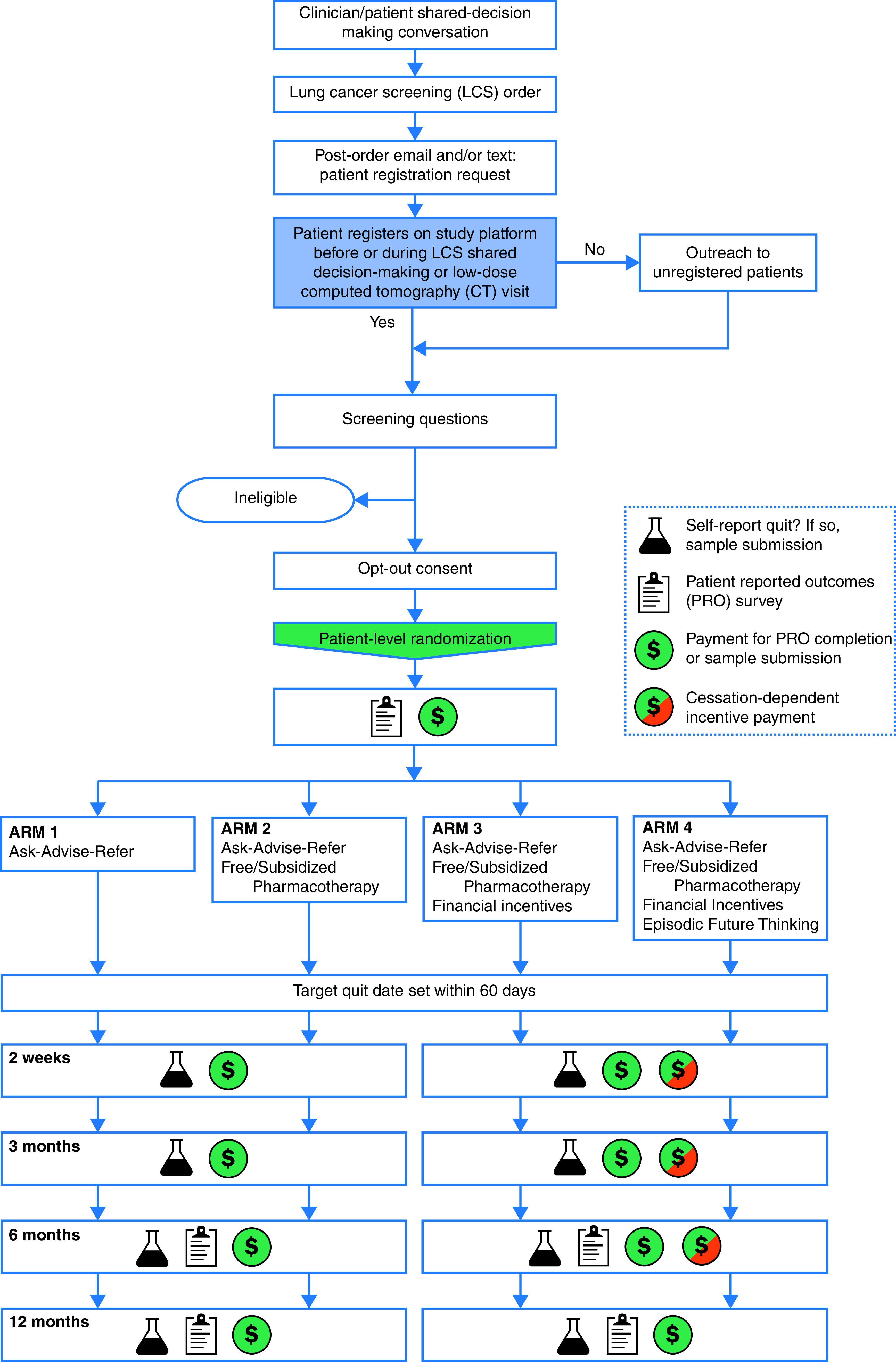
Study schema. CT = computed tomography; LCS = lung cancer screening; PRO = patient-reported outcomes.
Patient and Stakeholder Engagement
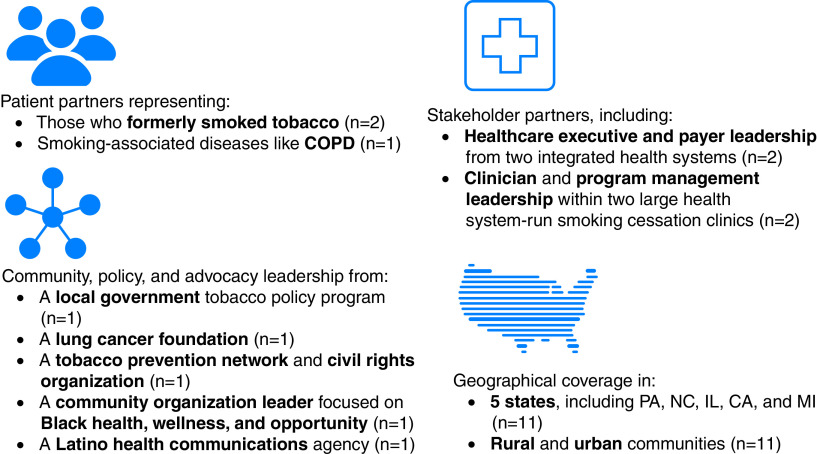
An 11-member Stakeholder Advisory Committee (SAC) was formed to ensure that the study’s aims reflect critical questions important to affected groups and that the study materials and design were appropriate for the target populations (Figure 4). The SAC co-developed, reviewed, and revised study materials and study procedures to improve responsiveness to patient and health system needs and reduce the burden on participants. They reviewed the statistical analysis plan, including proposed covariates and potential confounders, for face validity and suggested secondary and sensitivity analyses. During the analysis stage, SAC members will identify the findings of greatest interest to patients and stakeholders and help design figures that portray the core results in understandable ways. In addition, SAC partners will participate in manuscript preparation and result dissemination and implementation via media and internet platforms and through community organizations and health systems. A SAC Charter was developed to help guide responsibilities, reimbursement, andcommunication expectations. The SAC reviewed and approved the SAC Charter, research protocol, recruitment plans, and remuneration on June 14, 2019.
Figure 4.
Stakeholder Advisory Committee members. COPD = chronic obstructive pulmonary disease.
Study Setting
We are testing the interventions among patients referred for LCS at the University of Pennsylvania Health System (Penn), Geisinger Health System (Geisinger), Henry Ford Health System (Henry Ford), and Kaiser Permanente Southern California (KPSC). We selected these sites because each serves a core constituent of our underserved patient groups. Penn serves the Philadelphia Metro area, nearly half of Penn’s patients are Black, and many of Penn’s patients have a low SES (nearly 70% have a high school degree or less; over 25% live below the federal poverty line [FPL]). Lancaster General Health (LGH), a part of Penn, serves patients from both low-SES urban and low-SES rural residences in Pennsylvania (approximately 75% have a high school degree or less; approximately 30% live below the FPL), including a >35% Latinx population. Geisinger serves patients who are predominantly white (>90%) from rural residences in Pennsylvania, and more than half have a low SES (65% have a high school degree or less; approximately 15% live below the FPL). Henry Ford serves patients in the Detroit Metro area, >20% of their patients are Black, and approximately 70% have a low SES (>60% have a high school degree or less; approximately 40% live below the FPL). KPSC is an integrated health system that serves a population that is approximately 40% Latinx and also includes those who have a low SES (>60% have a high school degree or less; approximately 40% live below the FPL) (27).
Population
We aim to enroll 3,200 adult patients who have had an LCS order placed in the electronic health record by any clinician, smoke at least one cigarette daily (not including e-cigarettes) at the time of study eligibility screening, have access to a phone with text messaging, and are underserved, defined as having one or more of the following characteristics: Black or Latinx race or ethnicity; rural community residence based on self-report or, in the absence of self-reported data, residence within an area with a Rural–Urban Commuting Area code of >1 as determined by using the ZIP code; completion of a high school education or less; and/or a household income <200% of the FPL. For the first 2 months of the trial, we required that patients smoke at least five cigarettes per day to be eligible, consistent with our prior trials (28, 29). However, in light of more recent evidence that a quarter of Americans who smoke do so lightly (30), the recognition of the financial barriers to heavier smoking in our target sample, and the unanimous recommendation of our SAC, we have since modified the criterion to one cigarette per day or more.
All study-related activities will rely on a web-based platform, text messaging, and intermittent internet access for participation. To facilitate participation and inclusivity, our SAC community partners prepared comprehensive lists of locations with free internet access throughout each health system catchment, which will be provided to participants. For full program participation, patients will be required to participate in a minimum of six web-based activities over a 12-month study period (registration on study platform/eligibility screening/randomization/baseline survey; setting a target quit date [TQD]; and short assessments at 2 wk, 3 mo, 6 mo, and 12 mo) (Figure 3). All study-related activities will be offered in English and Spanish; patients fluent in neither language will be excluded. We anticipate that LCS orders will reflect national screening guidelines, but we will not exclude any adult patients for whom clinicians have ordered LCS (5, 31). Patients will receive all study-related payments through reloadable prepaid cards that can be used as debit cards or redeemed for cash at any bank (Figure 3).
Interventions
Patients will be randomized to one of four study arms. Each arm is additive and contains the interventions from the prior arm. All randomized patients will be asked to set a TQD within 60 days of enrollment, which was decided on the basis of SAC feedback. If no TQD is selected, a default TQD will be set to 60 days after enrollment. All study-related activities will be based on each patient’s TQD (Figure 3).
Arm 1: AAR
AAR is a minimum standard to promote smoking cessation during LCS (32). Clinicians may ask patients whether they smoke, advise them regarding the benefits of cessation if they are actively using tobacco, and refer patients who are using tobacco to local or national resources.
After enrollment, we will offer all patients information through the patient portal that includes national quit hotlines and local health system resources (e.g., smoking cessation clinics) they may choose to pursue. We will advise patients to speak with their clinician about these and other available resources. We will inform them that they will be sent text messages and/or e-mails on their TQD and at 2 weeks, 3 months, and 6 months to assess their smoking cessation status. Because usual care at KPSC includes no-cost provision of smoking cessation pharmacotherapy for all patients, no KPSC patients will be randomized to arm 1.
Arm 2: FDA-approved pharmacotherapy
Pharmacotherapy (e.g., nicotine replacement therapy [NRT], varenicline, and bupropion) can reduce nicotine withdrawal symptoms and decrease tobacco cravings. NRT is direct nicotine replacement. Varenicline is a partial neuronal α4β2 nicotinic receptor agonist, which prevents nicotine stimulation of the mesolimbic dopamine system associated with nicotine addiction. Bupropion is believed to enhance central nervous system noradrenergic and dopaminergic release (33–35). NRT, varenicline, and bupropion all improve smoking cessation rates compared with placebo and are recommended as first-line therapies for tobacco dependence (36). We do not offer e-cigarettes as part of this trial because they are not FDA-approved for smoking cessation.
Some insurers (e.g., KPSC) have already adopted Affordable Care Act guidance to cover all copays associated with smoking cessation aids for most of their members (37). However, many underserved patients do not receive such coverage (38, 39). Patients assigned to arms 2–4 may order NRT (i.e., patches, lozenges, or gum) directly from their patient portal at no cost for mail delivery, as in our recent trial, because these are available without prescriptions (29). Because of vendor limitations, some forms of NRT are not available (i.e., nasal sprays and oral inhalers). Patients who receive prescriptions from their clinicians for varenicline or bupropion will submit receipts through the patient portal for co-payment or out-of-pocket reimbursements up to $300 total. Educational materials about optimal pharmacotherapy use, co-developed with the SAC, will be available in the portal and will be mailed in a participant welcome packet.
Arm 3: financial incentives
Patients will be eligible to earn $100, $200, and $300 if they submit negative test results for nicotine metabolites at 2 weeks, 3 months, and 6 months after their TQD, respectively (Table E2). These are the identical financial incentives and schedule that have been shown to triple quit rates among similarly nonselected people who smoke who also have access to free cessation aids (29), and these incentives and this schedule have recently been demonstrated to be highly cost-effective from a societal perspective (40). This strategy is based in behavioral economic theory, including 1) providing substitute, proximal reinforcers of cessation in the form of incentives (41); 2) using large payments to reinforce target behaviors (42); and 3) providing the largest payment at the end of the intervention to offset the present bias of patients who smoke (43).
Arm 4: EFT
Patients who smoke tend to be “present biased” with a propensity to discount future benefits (e.g., greater health after smoking cessation) in favor of immediate satisfaction (e.g., the pleasures of smoking and avoidance of the discomfort of nicotine withdrawal) (44, 45). By contrast, cancer screening is an event that naturally motivates people to adopt a future orientation (44, 46) as they contemplate how their future health may be affected by tobacco use (20, 47). EFT as an intervention is an evidence-based, personalized behavioral tool that promotes prospective thinking about longer-term rewards (48). EFT interventions have been used successfully to promote behavior change in a variety of contexts, such as obesity, alcohol use disorder, and tobacco dependence (49–51). EFT prompts individuals to engage in thinking about delayed rewards (e.g., the benefits of smoking cessation) to overcome a focus on immediate rewards (e.g., the relief from a nicotine craving) through two mechanisms: the process of generating personalized future positive event “cues” about positive future experiences (see the online supplement for a sample cue) and then the delivery of these personalized cues by text message at regular intervals and on demand to promote future orientation as needed (e.g., when craving cigarettes) (49–51). EFT may work in a complementary fashion with financial incentives, with incentives providing an immediate, extrinsic motivation to quit and EFT helping to sustain abstinence by enhancing people’s intrinsic motivations to quit. EFT has previously proven effective among patients living in poverty (52).
Patients will be able to view introductory videos, co-developed and narrated by SAC members, on the study platform to orient them to personalizing and using the EFT tool (see the online supplement for links to the participant-facing explanatory videos designed for the study). This information will also be provided in the mailed participant welcome packet. When first engaging with EFT, patients will generate three EFT cues that are positive, specific, vivid descriptions of events that they are looking forward to and that are enhanced by smoking cessation. Over the 6-month intervention period, the mobile study platform will regularly prompt patients to focus on delayed rewards from smoking cessation through scheduled and on-demand text messages with their personalized EFT cues. Patients may update their cues at any time during the study period. Patients will receive cues from the TQD through the end of the intervention period unless they ask to stop receiving cues sooner. Patients who create three cues will be paid $20 and classified as having engaged with the intervention for the purposes of analysis (Table E2).
Recruitment
Recruitment began in May of 2021 at Penn, and a staggered roll-out across Penn radiology clinics was used to enable quality assessments of all study-related procedures. Recruitment will begin at the other health systems in serial fashion over the ensuing 4 months. On the basis of annual screening volumes from each health system and estimates of the proportions of screened patients who will be eligible, we anticipate that recruitment will continue for 32 months to enroll the target of 3,200 patients. Consistent with the goals of a large, scalable, pragmatic RCT to assess the real-world effectiveness of interventions, recruitment will occur via a three-pronged approach that will not rely on on-site research personnel (Table E3). Patients will receive financial remuneration for study-related tasks and will be sent up to five reminder text message and/or e-mail reminders at each study milestone to promote retention. Because of the pragmatic nature of this trial, retention efforts delivered outside of automated means are incompatible with the goals of this study. Patients recruited after shared decision-making visits who were referred to cessation resources or prescribed cessation pharmacotherapy remain eligible for study participation. Patients who have an LCS referral or order but do not schedule an appointment within 120 days will also be contacted for study enrollment via a text message, e-mail, and/or letter. There is a centralized study telephone hotline staffed with English- and Spanish-speaking research staff to answer questions and assist in enrollment and study participation.
Eligible patients may opt out of study participation after reviewing study-related materials, including an optional review of a detailed frequently-asked-questions document describing all elements of the trial (29, 53). Such opt-out consent is advocated for in pragmatic RCTs that are testing methods for improving the delivery of interventions within health systems (54, 55). Our SAC co-developed the approach to opt-out consent for this study, and our prior work has demonstrated that it is acceptable to underserved patients (56, 57). Details of the study arms will not be disclosed to potential study participants to avoid feelings of resentment or deprivation due to arm assignment (29, 58).
Patient Remuneration for Study-related Tasks
The 12-month maximal remuneration schedule for surveys, biochemical samples, and financial incentives is shown in Table E2. All enrolled patients will be asked to complete 6- and 12-month surveys, regardless of their self-reported smoking abstinence, and will be compensated $40 for each. At the 2-week and 3-, 6-, and 12-month time points, patients will report whether they are actively smoking and will be compensated $5 for completing this short assessment. Patients who self-report abstinence will be paid $45 for submitting a sample at an outpatient laboratory or $15 for submitting a sample by using a mobile service that collects samples at home through 6 months; these payments will be increased to $95 and $35, respectively, at 12 months to promote long-term retention.
Randomization
Patients will be randomized individually to one of the four study arms by using random number generation within the mobile study platform, stratified by health system. At Penn, Geisinger, and Henry Ford, patients will be assigned equally to each of the four arms. Although LGH is part of Penn, randomization will occur separately because LGH has a separate LCS program and a separate electronic health record system. Patients enrolled at KPSC will only be assigned to arms 2–4, each with a 33% probability, as KPSC provides smoking cessation aids to all patients at no additional cost, making arm 2 the KPSC minimum standard of care, as no patients will receive AAR (arm 1) alone.
Outcomes
Our primary outcome is sustained abstinence for 6 months, requiring self-reported smoking cessation followed by biochemical confirmation at 2 weeks, 3 months, and 6 months (Table E4), as supported by professional societies and stakeholders, including our SAC, and used in our prior RCTs (4, 28, 29, 59). To allow for a recommended grace period after the TQD (60), all patients will be allowed a “second chance” if they do not demonstrate abstinence at 2 weeks. From that date, patients will be given an additional 2 weeks to quit and demonstrate abstinence. Patients who do not complete study-related tasks, including survey completion and confirmatory biochemical specimen submission, will be classified as not having sustained abstinence (28, 29).
Secondary outcomes, co-developed with the SAC, include point-prevalent biochemically confirmed quit rates at 2 weeks, 3 months, and 12 months; point-prevalent self-reported quit rates at 2 weeks, 3 months, 6 months, and 12 months; point-prevalent self-reported other tobacco use at 2 weeks, 3 months, 6 months, and 12 months; and patient-reported outcomes, including motivation to quit (61), self-efficacy to quit smoking (62), perceived barriers to cessation (63), temporal discounting (64), and health-related quality of life (65) (Table 1).
Table 1.
Secondary patient-reported outcomes
| Patient-reported Outcome | Measure | Description |
|---|---|---|
| Motivation to quit | Stages of change (78) | Validated 1-item measure to assess a patient’s self-reported motivation to quit |
| Self-efficacy to quit smoking | Situational measure of self-efficacy (62, 79) | Validated 10-item measure to assess how certain a patient is that they can avoid smoking under various situational circumstances |
| Perceived barriers to cessation | Challenges to stopping smoking scale (63) | Validated 21-item measure to assess a patient’s perceived barriers to smoking cessation. It contains two subscales: intrinsic factors (physical, psychologic, or cognitive aspects of quitting) and extrinsic factors (social or environmental aspects of quitting) |
| Temporal discounting | Five-trial adjusting delay task (64) | Validated 5-item measure to assess a patient’s temporal discounting toward delayed rewards |
| Health-related quality of life | EuroQol-5 dimensions (80) | Validated 25-item measure used to assess a patient’s perceived health-related quality of life across the domains of mobility, self-care, usual activities, pain/discomfort, and anxiety/depression |
Definition of abbreviation: EuroQol = European Quality of Life Scale.
Analytic Plan
Sample size and power
On the basis of our previous trials and the target population, we anticipate a 6-month smoking abstinence rate of 2.5% with AAR (arm 1). Although higher rates were observed in the National Lung Screening Trial usual-care arm (10), that trial used self-reporting to determine smoking status and enrolled motivated patients, in contrast to the unselected population to be enrolled in this pragmatic trial. For example, patients may enroll in the trial who are not interested in cessation (e.g., they may not select a TQD, and a default TQD will be assigned to them). On the other hand, the rate of 2.5% is higher than the 1% observed in the usual-care arm of our prior pragmatic trial of smoking cessation (29), accounting for the greater propensity of patients undergoing LCS to quit. We wish to detect absolute differences of ⩾5% in smoking abstinence rates between arm 1 and any arm among arms 2–4 or between any two arms among arms 2–4 (six total contrasts). This difference is clinically important, given the dramatic health benefits of smoking cessation and the low rates of success of available interventions, and is identical to that used to determine power in our recent pragmatic RCTs (28, 29). We project enrollment of 470 patients in arm 1 and 910 patients per arm in arms 2–4 (as no patients from KPSC will be randomized to arm 1), for a total of 3,200 patients. This sample and distribution will yield >80% power to detect a difference of 5% in each of the six contrasts of interest while using the Proschan method of comparing arms 2–4 with arm 1 by using a P value of 0.05 divided by 3 and comparing arms 2–4 with one another by using a P value of 0.05 divided by 3, preserving the familywise type I error rate of 5% (66). We will classify patients lost to follow-up as having continued smoking.
Primary analyses
We will use intention-to-treat analyses incorporating a generalized linear model with a logit link function to estimate the effect of each arm among arms 2–4 as compared with the effect of arm 1 to compare the overall effectiveness of the interventions in achieving sustained smoking abstinence among all randomized patients at 6 months. Analyses will include the prespecified baseline covariates (67), including age, sex, race, ethnicity, education, income, self-reported rurality or Rural–Urban Commuting Area code, the burden of chronic comorbid conditions, temporal discounting, the degree of nicotine dependence (68), the number of prior LCSs, and LCS results. We include the number of prior LCSs and LCS results because LCS has been demonstrated to be a “teachable moment,” and concerning results may further increase patients’ engagement with or interest in smoking cessation (20, 69). Analyses will also include fixed-effect terms for the health system, preventing confounding by system and adjusting variance estimates to account for potential similarities among patients within a given system.
Secondary analyses
We will perform similar analyses for sustained smoking abstinence at 12 months. We will use logistic and Poisson models to assess the point-prevalent and patient-reported secondary outcomes at the specified time points.
To test for heterogeneity of treatment effects, we will include terms for statistical interactions between a priori–selected patient characteristics (Table E5) and the contrast of each study arm in the foregoing primary analytic model. In addition, because the intention-to-treat approach will yield effect sizes that are influenced by the fact that some randomized patients will not use the interventions offered to them, we will compare interventions’ complier average causal effects (CACEs) by using a two-stage residual inclusion model (70) in which the randomization arm is used as an instrumental variable (71, 72). Unlike per-protocol analyses, CACE analyses use data on all randomized patients to provide unbiased estimates of the effects of using the interventions, after accounting for differences in uptake rates across arms.
Finally, we will conduct sensitivity analyses of the primary contrasts that include health system fixed effects but not patient-level covariates. We will follow all CONSORT (Consolidated Standards of Reporting Trials) reporting guidelines (73). Statisticians blinded to the trial arm will prepare reports describing all data by using appropriate summary statistics with estimates of variance and graphic representations of the distributions. We will compare distributions of the characteristics of enrolled patients with those of all patients identified as potentially eligible to quantify the trial’s external validity.
Ethics
Regulation
The trial has been approved by the Penn Institutional Review Board (IRB), which serves as the single IRB for the study, including the alteration to the informed-consent requirement (74).
Data Safety and Monitoring Board
To guide the safe and ethical conduct of this study, we assembled a Data Safety and Monitoring Board (DSMB). The DSMB consists of five individuals with relevant expertise who reviewed and approved the DSMB Charter, statistical analysis plan, research protocol, and plans for ongoing data and safety monitoring before the start of recruitment. The DSMB will evaluate patient risk versus benefit; make recommendations to ensure identified issues are appropriately addressed; and make recommendations about study progress, safety, or trial continuation on the basis of the futility of patient accrual. We have no prespecified adverse or serious adverse events that will be monitored during the study. All patient-, family-, and clinician-reported concerns arising during the study will be reported to the IRB and DSMB. The SAC also advised on the study design, including the informed-consent processes, compensation, and risk communication among underserved groups. For potential risks to trial patients, see the online supplement.
Discussion
Comparison with Other Trials
This trial will add novel knowledge to the findings of prior studies assessing smoking cessation interventions in the context of LCS. The largest smoking cessation intervention collaboration to date is the SCALE (Smoking Cessation within the Context of LCS) Collaboration. We aim to enroll a larger sample of participants than has been used in prior similar trials (3,200 patients vs. 1,650 patients in the largest SCALE trial). Our trial is the first using a pragmatic RCT study design to exclusively enroll underserved patients (100% underserved patients vs. 37% Black patients and 33% Latinx patients as the largest underserved populations in SCALE trials) for whom LCS has been ordered. Healthy Lungs is also the first study to incorporate additive interventions, including those demonstrated to promote smoking cessation among underserved populations, into smoking cessation trials within the context of LCS (Table 2).
Table 2.
Comparison of Healthy Lungs (present study) with the SCALE Collaboration trials
| Domain | Healthy Lungs | SCALE Trials (22) |
|---|---|---|
| Study design | • Pragmatic RCT | • Traditional RCTs |
| • Cluster RCTs | ||
| • Sequential multiple-assignment RCTs | ||
| • Factorial experimental designs (multiphase optimization strategy) | ||
| • Modeling of lung cancer screening outcomes | ||
| Sites (n) | • Four large health systems | • 1–26 health systems |
| Types of lung cancer screening facilities | • Academic clinics | • Academic clinics |
| • Community clinics | • Community clinics | |
| • Radiology sites | • Radiology sites | |
| • Veterans Health Administration clinics | ||
| Sample size | • 3,200 patients | • 616–1,650 patients |
| Population | • Black | • Black: 8–37% |
| • Latinx | • Latinx: 0–33% | |
| • Rural | ||
| • High school education or less | ||
| • Household income <200% of the federal poverty line | ||
| Intervention | • Arm 1: AAR | • Quitlines |
| • Arm 2: AAR + free or subsidized pharmacotherapy | • Pharmacotherapy | |
| • Arm 3: AAR + pharmacotherapy + financial incentives | • Tailored, individual smoking cessation counseling | |
| • Arm 4: AAR + pharmacotherapy + financial incentives + episodic future thinking | • Development of implementation toolkits for integrating evidence-based smoking cessation strategies into lung cancer screening | |
| • Digital resources (e.g., web-based cessation programs and text message-delivered cessation information) | ||
| • Gain vs. loss message framing of the effects of smoking cessation on health | ||
| Lung cancer screening eligible vs. completed | • Both | • Both: 1 study |
| • Eligible: 1 study | ||
| • Completed: 6 studies | ||
| Baseline vs. annual screen | • Both | • Baseline: 1 study |
| • Both: 7 studies |
Definition of abbreviations: AAR = ask–advise–refer; RCT = randomized clinical trial; SCALE = Smoking Cessation within the Context of Lung Cancer Screening.
Limitations of the Present Study
This highly pragmatic trial has certain limitations. First, a fundamental challenge of pragmatic trials is designing a flexible yet effective intervention adherence plan to enhance fidelity. Although co-investigators and SAC members have collaborated to develop methods that optimize uptake and use of the interventions, including a systematic approach of multifaceted patient reminders and compensation to encourage timely completion of surveys and biochemical sample collection, some participants will not engage in the offered interventions. However, the secondary CACE analysis will provide unbiased estimates of the specific efficacy of using the interventions among all patients who would use the interventions if assigned to receive them. Second, the underserved populations we seek to enroll may lack access to text message–capable phones or reliable internet access. Although our study relies on a web-based platform and text messaging for participation, we worked with the SAC to design the study to require only text message capabilities (i.e., rather than smartphone access) and intermittent internet access. To facilitate participation, our SAC community partners prepared comprehensive lists of locations with free internet access throughout each health system catchment, which will be provided to participants. In addition, over 95% of Americans have been shown to have a cellphone, with 85% having a smartphone (75). Third, patients who have primary languages other than English and Spanish will not be represented in our study. However, 92% of the U.S. population is fluent in English or Spanish (76). Fourth, this study does not seek to increase LCS, which is underutilized (77). Finally, we chose to use urine anabasine for a smoking abstinence biochemical confirmatory test for patients reporting NRT use rather than serum carboxyhemoglobin for participant ease and retention.
Potential Outcomes and Conclusions
The interventions being tested in this trial represent the first application of smoking cessation approaches specifically focused on and tailored to underserved populations for whom LCS has been ordered. Our pragmatic RCT among four large U.S. health systems is powered to detect differences in outcomes that are important to patients, clinicians, health systems, and payers. Most importantly, the interventions being tested are highly scalable, thereby facilitating widespread adoption if they are shown to be effective. The results of this trial will help health systems and payers choose smoking cessation interventions to integrate into LCS programs that maximally capitalize on the opportunity LCS presents to promote smoking cessation and reduce healthcare inequities.
Acknowledgments
Acknowledgment
The authors thank all of their Stakeholder Advisory Committee partners for their contributions and commitment to smoking cessation among underserved patients.
Stakeholder Advisory Committee Partners: Andrea Ferris, LUNGevity; George Fernandez, The Latino Connection; Amanda Holm, Tobacco Treatment Service, Center for Health Promotion and Disease Prevention, Henry Ford Health System; Sarah Evers-Casey, Comprehensive Smoking Treatment Program, University of Pennsylvania; Benjamin Broder, Quality and Clinical Analysis, Southern California Permanente Medical Group; Curt Hammock; Kathy Epps, The Urban League of Philadelphia; Karen Yacobucci, Henry Ford Cancer Institute; M. Regina Clanton; and Ryan Coffman, Tobacco Policy and Control Program, Philadelphia Department of Health.
Footnotes
A complete list of Stakeholder Advisory Committee Partners may be found before the beginning of the REFERENCES.
Supported by the Patient-centered Outcomes Research Institute (PCS-2018C1-11326). R.K. was also supported by the National Institutes of Health/National Heart, Lung, and Blood Institute grant K23 HL146894.
Author Contributions: Study design and protocol: A.V., D. Small, A.J.S.-S., V.L.M., B.A.B., M.C., M.K.G., M.H.I., M.A.F., C.C., C.M.N.-D., M.J.S., M.A.H., M.E.S., K.G.V., S.D.H., and J.L.H. Wrote and/or edited portions of the manuscript: R.K., A.V., D. Sheu, V.L.M., M.C., S.D.H., and J.L.H. Provided critical feedback and revisions of final manuscript: R.K., A.V., D. Small, A.J.S.-S., D. Sheu, V.L.M., B.A.B., M.C., S.F., J.K., M.K.G., M.H.I., B.C., M.A.F., C.C., K.K.B., C.M.N.-D., M.J.S., E.R.A., L.H.E., M.A.H., M.E.S., K.G.V., S.D.H., and J.L.H.
This article has an online supplement, which is accessible from this issue’s table of contents at www.atsjournals.org.
Author disclosures are available with the text of this article at www.atsjournals.org.
Contributor Information
the Stakeholder Advisory Committee:
Andrea Ferris, George Fernandez, Amanda Holm, Sarah Evers-Casey, Benjamin Broder, Curt Hammock, Karen Yacobucci, M. Regina Clanton, and Ryan Coffman
References
- 1. Rostron BL, Chang CM, Pechacek TF. Estimation of cigarette smoking-attributable morbidity in the United States. JAMA Intern Med . 2014;174:1922–1928. doi: 10.1001/jamainternmed.2014.5219. [DOI] [PubMed] [Google Scholar]
- 2. Aberle DR, Adams AM, Berg CD, Black WC, Clapp JD, Fagerstrom RM, et al. National Lung Screening Trial Research Team Reduced lung-cancer mortality with low-dose computed tomographic screening. N Engl J Med . 2011;365:395–409. doi: 10.1056/NEJMoa1102873. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Moyer VA, U.S. Preventive Services Task Force Screening for lung cancer: U.S. Preventive Services Task Force recommendation statement. Ann Intern Med . 2014;160:330–338. doi: 10.7326/M13-2771. [DOI] [PubMed] [Google Scholar]
- 4. Kathuria H, Detterbeck FC, Fathi JT, Fennig K, Gould MK, Jolicoeur DG, et al. Stakeholder research priorities for smoking cessation interventions within lung cancer screening programs: an official American Thoracic Society research statement. Am J Respir Crit Care Med . 2017;196:1202–1212. doi: 10.1164/rccm.201709-1858ST. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Krist AH, Davidson KW, Mangione CM, Barry MJ, Cabana M, Caughey AB, et al. U.S. Preventive Services Task Force Screening for lung cancer: US Preventive Services Task Force recommendation statement. JAMA . 2021;325:962–970. doi: 10.1001/jama.2021.1117. [DOI] [PubMed] [Google Scholar]
- 6. Humphrey LL, Deffebach M, Pappas M, Baumann C, Artis K, Mitchell JP, et al. Screening for lung cancer with low-dose computed tomography: a systematic review to update the US Preventive Services Task Force recommendation. Ann Intern Med . 2013;159:411–420. doi: 10.7326/0003-4819-159-6-201309170-00690. [DOI] [PubMed] [Google Scholar]
- 7. Ma J, Ward EM, Smith R, Jemal A. Annual number of lung cancer deaths potentially avertable by screening in the United States. Cancer . 2013;119:1381–1385. doi: 10.1002/cncr.27813. [DOI] [PubMed] [Google Scholar]
- 8. Steliga MA, Yang P. Integration of smoking cessation and lung cancer screening. Transl Lung Cancer Res . 2019;8:S88–S94. doi: 10.21037/tlcr.2019.04.02. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Tammemägi MC, Berg CD, Riley TL, Cunningham CR, Taylor KL. Impact of lung cancer screening results on smoking cessation. J Natl Cancer Inst . 2014;106:dju084. doi: 10.1093/jnci/dju084. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Tanner NT, Kanodra NM, Gebregziabher M, Payne E, Halbert CH, Warren GW, et al. The association between smoking abstinence and mortality in the National Lung Screening Trial. Am J Respir Crit Care Med . 2016;193:534–541. doi: 10.1164/rccm.201507-1420OC. [DOI] [PubMed] [Google Scholar]
- 11. Fucito LM, Czabafy S, Hendricks PS, Kotsen C, Richardson D, Toll BA, Association for the Treatment of Tobacco Use and Dependence/Society for Research on Nicotine and Tobacco Synergy Committee Pairing smoking-cessation services with lung cancer screening: a clinical guideline from the Association for the Treatment of Tobacco Use and Dependence and the Society for Research on Nicotine and Tobacco. Cancer . 2016;122:1150–1159. doi: 10.1002/cncr.29926. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Wiener RS, Gould MK, Arenberg DA, Au DH, Fennig K, Lamb CR, et al. ATS/ACCP Committee on Low-Dose CT Lung Cancer Screening in Clinical Practice An official American Thoracic Society/American College of Chest Physicians policy statement: implementation of low-dose computed tomography lung cancer screening programs in clinical practice. Am J Respir Crit Care Med . 2015;192:881–891. doi: 10.1164/rccm.201508-1671ST. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Trinidad DR, Pérez-Stable EJ, White MM, Emery SL, Messer K. A nationwide analysis of US racial/ethnic disparities in smoking behaviors, smoking cessation, and cessation-related factors. Am J Public Health . 2011;101:699–706. doi: 10.2105/AJPH.2010.191668. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Hill S, Amos A, Clifford D, Platt S. Impact of tobacco control interventions on socioeconomic inequalities in smoking: review of the evidence. Tob Control . 2014;23:e89–e97. doi: 10.1136/tobaccocontrol-2013-051110. [DOI] [PubMed] [Google Scholar]
- 15. Kaplan RC, Bangdiwala SI, Barnhart JM, Castañeda SF, Gellman MD, Lee DJ, et al. Smoking among U.S. Hispanic/Latino adults: the Hispanic community health study/study of Latinos. Am J Prev Med . 2014;46:496–506. doi: 10.1016/j.amepre.2014.01.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Health equity style guide for the COVID-19 response: principles and preferred terms for non-stigmatizing, bias-free language Atlanta, GA: Centers for Disease Control and Prevention; 2020. [updated 2020 Aug 11; accessed 2021 Apr 5]. Available from: https://ehe.jhu.edu/DEI/Health_Equity_Style_Guide_CDC_Reducing_Stigma.pdf [Google Scholar]
- 17. Bailey SR, Heintzman J, Jacob RL, Puro J, Marino M. Disparities in smoking cessation assistance in US primary care clinics. Am J Public Health . 2018;108:1082–1090. doi: 10.2105/AJPH.2018.304492. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Vijayaraghavan M, Benmarhnia T, Pierce JP, White MM, Kempster J, Shi Y, et al. Income disparities in smoking cessation and the diffusion of smoke-free homes among U.S. smokers: results from two longitudinal surveys. PLoS One . 2018;13:e0201467. doi: 10.1371/journal.pone.0201467. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Kulak JA, Cornelius ME, Fong GT, Giovino GA. Differences in quit attempts and cigarette smoking abstinence between Whites and African Americans in the United States: literature review and results from the International Tobacco Control US Survey. Nicotine Tob Res . 2016;18:S79–S87. doi: 10.1093/ntr/ntv228. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Taylor KL, Cox LS, Zincke N, Mehta L, McGuire C, Gelmann E. Lung cancer screening as a teachable moment for smoking cessation. Lung Cancer . 2007;56:125–134. doi: 10.1016/j.lungcan.2006.11.015. [DOI] [PubMed] [Google Scholar]
- 21. Reid JL, Hammond D, Boudreau C, Fong GT, Siahpush M, ITC Collaboration Socioeconomic disparities in quit intentions, quit attempts, and smoking abstinence among smokers in four Western countries: findings from the International Tobacco Control Four Country Survey. Nicotine Tob Res . 2010;12:S20–S33. doi: 10.1093/ntr/ntq051. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Joseph AM, Rothman AJ, Almirall D, Begnaud A, Chiles C, Cinciripini PM, et al. Lung cancer screening and smoking cessation clinical trials: SCALE (Smoking Cessation within the Context of Lung Cancer Screening) Collaboration. Am J Respir Crit Care Med. 2018;197:172–182. doi: 10.1164/rccm.201705-0909CI. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Slatore CG, Baumann C, Pappas M, Humphrey LL. Smoking behaviors among patients receiving computed tomography for lung cancer screening: systematic review in support of the U.S. Preventive Services Task Force. Ann Am Thorac Soc . 2014;11:619–627. doi: 10.1513/AnnalsATS.201312-460OC. [DOI] [PubMed] [Google Scholar]
- 24. Brain K, Carter B, Lifford KJ, Burke O, Devaraj A, Baldwin DR, et al. Impact of low-dose CT screening on smoking cessation among high-risk participants in the UK Lung Cancer Screening Trial. Thorax . 2017;72:912–918. doi: 10.1136/thoraxjnl-2016-209690. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Loudon K, Zwarenstein M, Sullivan F, Donnan P, Treweek S. Making clinical trials more relevant: improving and validating the PRECIS tool for matching trial design decisions to trial purpose. Trials . 2013;14:115. doi: 10.1186/1745-6215-14-115. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Thorpe KE, Zwarenstein M, Oxman AD, Treweek S, Furberg CD, Altman DG, et al. A Pragmatic-Explanatory Continuum Indicator Summary (PRECIS): a tool to help trial designers. J Clin Epidemiol. 2009;62:464–475. doi: 10.1016/j.jclinepi.2008.12.011. [DOI] [PubMed] [Google Scholar]
- 27.Overview of the United States Minneapolis, MN: Cedar Lake Ventures; 2018. [updated 2018 Sep 4; accessed 2021 Jun 30]. Available from: https://statisticalatlas.com/United-States/Overview [Google Scholar]
- 28.Halpern SD, French B, Small DS, Saulsgiver K, Harhay MO, Audrain-McGovern J, et al. Randomized trial of four financial-incentive programs for smoking cessation. N Engl J Med. 2015;372:2108–2117. doi: 10.1056/NEJMoa1414293. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Halpern SD, Harhay MO, Saulsgiver K, Brophy C, Troxel AB, Volpp KG. A pragmatic trial of e-cigarettes, incentives, and drugs for smoking cessation. N Engl J Med . 2018;378:2302–2310. doi: 10.1056/NEJMsa1715757. [DOI] [PubMed] [Google Scholar]
- 30. Jamal A, King BA, Neff LJ, Whitmill J, Babb SD, Graffunder CM. Current cigarette smoking among adults: United States, 2005-2015. MMWR Morb Mortal Wkly Rep . 2016;65:1205–1211. doi: 10.15585/mmwr.mm6544a2. [DOI] [PubMed] [Google Scholar]
- 31.Meza R, Jeon J, Toumazis I, ten Haaf K, Cao P, Bastani M, et al. Cancer Intervention and Surveillance Modeling Network (CISNET) Lung Cancer Working Group Evaluation of the benefits and harms of lung cancer screening with low-dose computed tomography: a collaborative modeling study for the US Preventive Services Task Force. Rockville, MD: Agency for Healthcare Research and Quality; 2020. AHRQ Publication No. 20-05266-EF-2. [Google Scholar]
- 32. Schroeder SA. What to do with a patient who smokes. JAMA . 2005;294:482–487. doi: 10.1001/jama.294.4.482. [DOI] [PubMed] [Google Scholar]
- 33. Coe JW, Brooks PR, Vetelino MG, Wirtz MC, Arnold EP, Huang J, et al. Varenicline: an α4β2 nicotinic receptor partial agonist for smoking cessation. J Med Chem . 2005;48:3474–3477. doi: 10.1021/jm050069n. [DOI] [PubMed] [Google Scholar]
- 34. Henningfield JE, Fant RV, Buchhalter AR, Stitzer ML. Pharmacotherapy for nicotine dependence. CA Cancer J Clin . 2005;55:281–299; quiz 322–323, 325. doi: 10.3322/canjclin.55.5.281. [DOI] [PubMed] [Google Scholar]
- 35. Wilkes S. The use of bupropion SR in cigarette smoking cessation. Int J Chron Obstruct Pulmon Dis . 2008;3:45–53. doi: 10.2147/copd.s1121. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36. Leone FT, Zhang Y, Evers-Casey S, Evins AE, Eakin MN, Fathi J, et al. Initiating pharmacologic treatment in tobacco-dependent adults: an official American Thoracic Society clinical practice guideline. Am J Respir Crit Care Med . 2020;202:e5–e31. doi: 10.1164/rccm.202005-1982ST. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37. Koh HK, Sebelius KG. Promoting prevention through the Affordable Care Act. N Engl J Med . 2010;363:1296–1299. doi: 10.1056/NEJMp1008560. [DOI] [PubMed] [Google Scholar]
- 38. Ku L, Bruen BK, Steinmetz E, Bysshe T. Medicaid tobacco cessation: big gaps remain in efforts to get smokers to quit. Health Aff (Millwood) . 2016;35:62–70. doi: 10.1377/hlthaff.2015.0756. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39. Sheffer CE, Anders M, Brackman SL, Steinberg MB, Barone C. Tobacco intervention practices of primary care physicians treating lower socioeconomic status patients. Am J Med Sci . 2012;343:388–396. doi: 10.1097/MAJ.0b013e3182302749. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40. Russell LB, Volpp KG, Kwong PL, Cosgriff BS, Harhay MO, Zhu J, et al. Cost-Effectiveness of Four Financial-Incentive Programs for Smoking Cessation. Ann Am Thorac Soc . 2021;18:1997–2006. doi: 10.1513/AnnalsATS.202012-1473OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Higgins ST, Bickel WK, Hughes JR. Influence of an alternative reinforcer on human cocaine self-administration. Life Sci. 1994;55:179–187. doi: 10.1016/0024-3205(94)00878-7. [DOI] [PubMed] [Google Scholar]
- 42. Silverman K, Chutuape MA, Bigelow GE, Stitzer ML. Voucher-based reinforcement of cocaine abstinence in treatment-resistant methadone patients: effects of reinforcement magnitude. Psychopharmacology (Berl) . 1999;146:128–138. doi: 10.1007/s002130051098. [DOI] [PubMed] [Google Scholar]
- 43. Audrain-McGovern J, Rodriguez D, Epstein LH, Cuevas J, Rodgers K, Wileyto EP. Does delay discounting play an etiological role in smoking or is it a consequence of smoking? Drug Alcohol Depend . 2009;103:99–106. doi: 10.1016/j.drugalcdep.2008.12.019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44. Hart JL, Pflug E, Madden V, Halpern SD. Thinking forward: future-oriented thinking among patients with tobacco-associated thoracic diseases and their surrogates. Am J Respir Crit Care Med . 2016;193:321–329. doi: 10.1164/rccm.201505-0882OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45. Bickel WK, Odum AL, Madden GJ. Impulsivity and cigarette smoking: delay discounting in current, never, and ex-smokers. Psychopharmacology (Berl) . 1999;146:447–454. doi: 10.1007/pl00005490. [DOI] [PubMed] [Google Scholar]
- 46. Gilbert DT, Wilson TD. Prospection: experiencing the future. Science . 2007;317:1351–1354. doi: 10.1126/science.1144161. [DOI] [PubMed] [Google Scholar]
- 47. Zeliadt SB, Heffner JL, Sayre G, Klein DE, Simons C, Williams J, et al. Attitudes and perceptions about smoking cessation in the context of lung cancer screening. JAMA Intern Med . 2015;175:1530–1537. doi: 10.1001/jamainternmed.2015.3558. [DOI] [PubMed] [Google Scholar]
- 48. Lin H, Epstein LH. Living in the moment: effects of time perspective and emotional valence of episodic thinking on delay discounting. Behav Neurosci . 2014;128:12–19. doi: 10.1037/a0035705. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49. Athamneh LN, Stein MD, Lin EH, Stein JS, Mellis AM, Gatchalian KM, et al. Setting a goal could help you control: comparing the effect of health goal versus general episodic future thinking on health behaviors among cigarette smokers and obese individuals. Exp Clin Psychopharmacol . 2021;29:59–72. doi: 10.1037/pha0000351. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50. Stein JS, Tegge AN, Turner JK, Bickel WK. Episodic future thinking reduces delay discounting and cigarette demand: an investigation of the good-subject effect. J Behav Med . 2018;41:269–276. doi: 10.1007/s10865-017-9908-1. [DOI] [PubMed] [Google Scholar]
- 51. Patel H, Amlung M. Acute and extended exposure to episodic future thinking in a treatment seeking addiction sample: a pilot study. J Subst Abuse Treat . 2020;116:108046. doi: 10.1016/j.jsat.2020.108046. [DOI] [PubMed] [Google Scholar]
- 52. O’Donnell S, Daniel TO, Koroschetz J, Kilanowski C, Otminski A, Bickel WK, et al. Do process simulations during episodic future thinking enhance the reduction of delay discounting for middle income participants and those living in poverty? J Behav Decis Making . 2019;32:231–240. doi: 10.1002/bdm.2108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Asch DA, Ziolek TA, Mehta SJ. Misdirections in informed consent: impediments to health care innovation. N Engl J Med. 2017;377:1412–1414. doi: 10.1056/NEJMp1707991. [DOI] [PubMed] [Google Scholar]
- 54. Faden RR, Kass NE, Goodman SN, Pronovost P, Tunis S, Beauchamp TL. An ethics framework for a learning health care system: a departure from traditional research ethics and clinical ethics. Hastings Cent Rep . 2013;43:S16–S27. doi: 10.1002/hast.134. [DOI] [PubMed] [Google Scholar]
- 55. Gould MK, Smith-Bindman R, Kelly K, Altman DE, Barjaktarevic I, Creekmur B, et al. Methods for the Watch the Spot Trial: a pragmatic trial of more- versus less-intensive strategies for active surveillance of small pulmonary nodules. Ann Am Thorac Soc . 2019;16:1567–1576. doi: 10.1513/AnnalsATS.201903-268SD. [DOI] [PubMed] [Google Scholar]
- 56. Courtright KR, Halpern SD, Joffe S, Ellenberg SS, Karlawish J, Madden V, et al. Willingness to participate in pragmatic dialysis trials: the importance of physician decisional autonomy and consent approach. Trials . 2017;18:474. doi: 10.1186/s13063-017-2217-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Kraybill A, Dember LM, Joffe S, Karlawish J, Ellenberg SS, Madden V, et al. Patient and physician views about protocolized dialysis treatment in randomized trials and clinical care. AJOB Empir Bioeth. 2016;7:106–115. doi: 10.1080/23294515.2015.1111272. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58. Wendler D. What should be disclosed to research participants? Am J Bioeth . 2013;13:3–8. doi: 10.1080/15265161.2013.851578. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59. West R, Hajek P, Stead L, Stapleton J. Outcome criteria in smoking cessation trials: proposal for a common standard. Addiction . 2005;100:299–303. doi: 10.1111/j.1360-0443.2004.00995.x. [DOI] [PubMed] [Google Scholar]
- 60. Hughes JR, Keely JP, Niaura RS, Ossip-Klein DJ, Richmond RL, Swan GE. Measures of abstinence in clinical trials: issues and recommendations. Nicotine Tob Res . 2003;5:13–25. [PubMed] [Google Scholar]
- 61. Biener L, Abrams DB. The contemplation ladder: validation of a measure of readiness to consider smoking cessation. Health Psychol . 1991;10:360–365. doi: 10.1037//0278-6133.10.5.360. [DOI] [PubMed] [Google Scholar]
- 62. Lee S, Cappella JN, Lerman C, Strasser AA. Effects of smoking cues and argument strength of antismoking advertisements on former smokers’ self-efficacy, attitude, and intention to refrain from smoking. Nicotine Tob Res . 2013;15:527–533. doi: 10.1093/ntr/nts171. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63. Thomas D, Mackinnon AJ, Bonevski B, Abramson MJ, Taylor S, Poole SG, et al. Development and validation of a 21-item challenges to stopping smoking (CSS-21) scale. BMJ Open . 2016;6:e011265. doi: 10.1136/bmjopen-2016-011265. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64. Koffarnus MN, Bickel WK. A 5-trial adjusting delay discounting task: accurate discount rates in less than one minute. Exp Clin Psychopharmacol . 2014;22:222–228. doi: 10.1037/a0035973. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65. Herdman M, Gudex C, Lloyd A, Janssen M, Kind P, Parkin D, et al. Development and preliminary testing of the new five-level version of EQ-5D (EQ-5D-5L) Qual Life Res . 2011;20:1727–1736. doi: 10.1007/s11136-011-9903-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66. Proschan MA. A multiple comparison procedure for three- and four-armed controlled clinical trials. Stat Med . 1999;18:787–798. doi: 10.1002/(sici)1097-0258(19990415)18:7<787::aid-sim77>3.0.co;2-m. [DOI] [PubMed] [Google Scholar]
- 67. Permutt T. Do covariates change the estimand? Stat Biopharm Res . 2020;12:45–53. [Google Scholar]
- 68. Heatherton TF, Kozlowski LT, Frecker RC, Fagerström KO. The Fagerström test for nicotine dependence: a revision of the Fagerström Tolerance Questionnaire. Br J Addict . 1991;86:1119–1127. doi: 10.1111/j.1360-0443.1991.tb01879.x. [DOI] [PubMed] [Google Scholar]
- 69. Pistelli F, Aquilini F, Falaschi F, Puliti D, Ocello C, Lopes Pegna A, et al. moking cessation in the ITALUNG Lung Cancer Screening: what does “teachable moment” mean? Nicotine Tob Res . 2020;22:1484–1491. doi: 10.1093/ntr/ntz148. [DOI] [PubMed] [Google Scholar]
- 70. Cai B, Small DS, Have TR. Two-stage instrumental variable methods for estimating the causal odds ratio: analysis of bias. Stat Med . 2011;30:1809–1824. doi: 10.1002/sim.4241. [DOI] [PubMed] [Google Scholar]
- 71. Newhouse JP, McClellan M. Econometrics in outcomes research: the use of instrumental variables. Annu Rev Public Health . 1998;19:17–34. doi: 10.1146/annurev.publhealth.19.1.17. [DOI] [PubMed] [Google Scholar]
- 72. Angrist JD, Imbens GW, Rubin DB. Identification of causal effects using instrumental variables. J Am Stat Assoc . 1996;91:444–455. [Google Scholar]
- 73.EQUATOR Network. Enhancing the quality and transparency of health research Oxford, UK: University of Oxford; 2020. [accessed 2020 Oct 23]. Available from: https://www.equator-network.org/ [Google Scholar]
- 74. Largent EA, Halpern SD, Fernandez Lynch H. Waivers and alterations of research informed consent during the COVID-19 pandemic. Ann Intern Med . 2021;174:415–416. doi: 10.7326/M20-6993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Mobile fact sheet Washington, DC: Pew Research Center; 2021. [accessed 2021 Jun 1]. Available from: https://www.pewresearch.org/internet/fact-sheet/mobile/ [Google Scholar]
- 76.Detailed languages spoken at home and ability to speak English for the population 5 years and over: 2009–2013 Suitland, MD: U.S. Census Bureau; 2015. [accessed 2021 Feb 22]. Available from: https://www.census.gov/data/tables/2013/demo/2009-2013-lang-tables.html [Google Scholar]
- 77. Okereke IC, Nishi S, Zhou J, Goodwin JS. Trends in lung cancer screening in the United States, 2016-2017. J Thorac Dis . 2019;11:873–881. doi: 10.21037/jtd.2019.01.105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78. Fava JL, Velicer WF, Prochaska JO. Applying the transtheoretical model to a representative sample of smokers. Addict Behav . 1995;20:189–203. doi: 10.1016/0306-4603(94)00062-x. [DOI] [PubMed] [Google Scholar]
- 79. Cappella JN, Lerman C, Romantan A, Baruh L. News about genetics and smoking: priming, family smoking history, and news story believability on inferences of genetic susceptibility to tobacco addiction. Communic Res . 2005;32:478–502. [Google Scholar]
- 80. EuroQol Group. EuroQol: a new facility for the measurement of health-related quality of life. Health Policy . 1990;16:199–208. doi: 10.1016/0168-8510(90)90421-9. [DOI] [PubMed] [Google Scholar]