Abstract
Black-White inequities in cardiovascular health (CVH) pose a significant public health challenge, with these disparities also varying geographically across the US. There remains limited evidence of the impact of social determinants of health on these inequities. Using a national population-based cohort from the REasons for Geographic and Racial Differences in Stroke study, we assessed the spatial heterogeneity in Black-White differences in CVH and determined the extent to which individual- and neighborhood-level characteristics explain these inequities. We utilized a Bayesian hierarchical statistical framework to fit spatially varying coefficient models. Results showed overall and spatially varying inequities, where Black participants had significantly poorer CVH. The maps of the state level random effects also highlighted how inequities vary. The evidence produced in this study further highlights the importance of multilevel approaches – at the individual- and neighborhood-levels – that need to be in place to address these geographic and racial differences in CVH.
Keywords: Cardiovascular health, Individual- and neighborhood-level characteristics, Racial inequities, Residential segregation, Spatial heterogeneity
INTRODUCTION
Health disparities in the United States (US) exist by ethnicity, race, geography and socioeconomic status, where the overall cost of these health inequities is around $1.24 trillion [1]. With cardiovascular disease (CVD) being the leading cause of death in the US [2], the associated racial/ethnic inequities produce enormous health and economic burdens for the country. Black-White differences, in particular, continue to remain a significant public health challenge [3–5]. In a report by the Centers for Disease Control and Prevention, CVDs were estimated to explain 32% of the mortality difference between Black and White men and 43% of the difference between Black and White women in 2009 [6]. Recent evidence suggests that non-Hispanic Blacks have higher incidence of coronary heart disease, heart failure, stroke and overall CVD mortality as compared to non-Hispanic Whites [7, 8].
Cardiovascular health (CVH), as defined by the American Heart Association (AHA) is based on the following three concepts: (1) the power of primordial prevention; (2) the evidence that CVD and its risk factors often develop early in life; and (3) the appropriate balance between population-level approaches for health promotion and disease prevention and individualized high-risk approaches [9]. An important component of the CVH approach is the measurement of ideal CVH, which is defined as the presence of both ideal health behaviors (nonsmoking; body mass index [BMI] <25 kg/m2; physical activity at goal levels; and consumption of a diet consistent with current guideline recommendations) and ideal health factors (untreated total cholesterol <200 mg/dL, untreated blood pressure <120/<80 mm Hg, and fasting blood glucose <100 mg/dL) – often referred to as Life’s Simple 7 [10]. Prior work has shown that ideal CVH is associated with lower risks of myocardial infarction, stroke, and vascular death [11], better cognitive performance [12, 13], lower cancer incidence [14], and improvement in a number of other major health outcomes[15–19]. Recent research, using the Global Burden of Disease methodology, examined the burden of CVD among residents of the US and found that a large proportion of CVD is attributable to (in decreasing order of contribution) dietary risks, high systolic blood pressure, high body mass index, high total cholesterol level, high fasting plasma glucose level, tobacco smoking, and low levels of physical activity [20] – all factors defined by the AHA as contributing to overall CVH. Similar to CVD, there are noted CVH inequities; Blacks and Hispanics tend to have fewer metrics (health behaviors and health factors) at ideal levels than do Whites or other races [21].
The presence of geographic disparities, particularly in stroke mortality, has been documented since 1940 [22]. Historically, the southeastern US’ well-known Stroke Belt (which includes North Carolina, South Carolina, Georgia, Tennessee, Mississippi, Alabama, Louisiana, and Arkansas) has an overall average stroke mortality around 30% higher than the rest of the US; stroke mortality is around 40% higher in the Stroke Buckle (North Carolina, South Carolina, and Georgia) [23]. Racial inequities in CVH have also been shown to vary spatially. Using data from the REasons for Geographic and Racial Differences in Stroke (REGARDS) cohort, a recent study found significant spatial patterning in the racial differences in CVH, even beyond the well-known Stroke Belt and Stroke Buckle [24] – with moderate to large disparities noted in the Great Lakes region, portions of the Northeast, and along the West coast. Another study using data from the Multi-Ethnic Study of Atherosclerosis (MESA) cohort also documented geographic heterogeneities in Black-White disparities in CVH within and across 5 regions in the US - with initial models showing decreased odds of optimal CVH for Blacks that ranged from 60% reduced odds in Los Angeles, California, up to 70% reduced odds at the other cities/regions examined (Forsyth County, North Carolina; New York City, New York; Baltimore, Maryland; and Chicago, Illinois) [25].
Individual- and neighborhood-level characteristics have been shown to influence CVH and racial/ethnic inequities in CVH as well. One study found that neighborhood-level characteristics, such as favorable food stores, physical activity resources, walking/physical activity environment, and neighborhood socioeconomic status, were associated with higher odds of having an ideal CVH score [26]. Another study found that adjustment for neighborhood context slightly reduced but did not completely explain racial/ethnic differences in CVH [27]. Although informative, both studies assumed that racial inequities in CVH are invariant across geography. In prior work, we examined the contribution of individual and neighborhood-level characteristics to spatially varying Black-White differences in CVH within the MESA cohort and found that the racial differences persisted after accounting for these multi-level characteristics [25]. However, a limitation of these analyses is the limited geographic representation of the study sample which was originally sampled from only 6 study sites.
In this paper, we use data from the national REGARDS study to explore the spatially varying Black-White differences in CVH across the US. We build on previous research that found significant spatial patterning in the racial differences in CVH in this cohort [24], and examine the extent to which social determinants of health (i.e., individual- and neighborhood-level characteristics) explain these race differences as well as the spatial variation in these inequities. Given the AHA’s 2020 Impact Goals were to improve the CVH of all Americans by 20% while reducing deaths attributable to CVDs and stroke by 20% [28], it’s imperative to map and measure CVH across the US and examine how social determinants of health explain racial/ethnic differences and geographic variations in these differences.
METHODS
Data
REGARDS is a national, population-based, longitudinal study of 30,239 Black and White adults aged ≥ 45 years. The overall objective of REGARDS is to determine the causes for the excess stroke mortality in the Southeastern US and among Blacks. Participants were recruited between 2003–2007 by mail, then underwent an extensive telephone interview, during which data on stroke risk factors, sociodemographic, lifestyle, and psychosocial characteristics were collected. Written informed consent, physical and physiological measures, and fasting blood samples were collected during a subsequent in-home visit. Participants are followed via telephone at 6-month intervals for identification of stroke events and myocardial infarctions. The sample for these analyses included all REGARDS participants for whom geocoding was successful, and outcome as well as individual- and neighborhood-level measures were available – at baseline. Details of the REGARDS study objectives and design are provided elsewhere [29]. REGARDS study participants come from each state in the 48 contiguous US, with participants per state ranging from a low of 6 participants residing in Vermont to a high of 2,216 participants in South Carolina.
Measurements
Outcome
We utilized the AHA’s definition of CVH, where ideal CVH is defined as the presence of both ideal health behaviors (nonsmoking; BMI < 25 kg/m2; physical activity at goal levels; and pursuit of a diet consistent with current guideline recommendations) and ideal health factors (untreated total cholesterol <200 mg/dL; untreated blood pressure <120/<80 mm Hg; and fasting blood glucose <100 mg/dL). Smoking status was self-reported by participants and characterized as never, former, or current smokers; BMI was computed via clinically measured height and weight during the in-home study visit. Physical activity was self-reported by participants, who answered the following question “How many times per week do you engage in intense physical activity, enough to work up a sweat?”; participants answered the question with response options either none, 1 to 3 times per week, and 4 or more times per week. Diet was measured using a self-administered Block 98 Food Frequency Questionnaire [30], and food intake from the previous year was recalled. Cholesterol and fasting blood glucose were measured using blood samples collected during the in-home study visit. Blood pressure was also assessed during the in-home study visit, and the average of two readings was used. Self-reported medication use was considered for the use of antihypertensive, glucose-lowering, and lipid-lowering medications.
Each health behavior and health factor metric were assigned a score of 1, 2, or 3 to denote the metric as poor, intermediate, or ideal, respectively (see Table S1 in the Supplementary Material). The total CVH score, our outcome of interest, was computed for each participant as the sum of the health behavior and health factor metrics and ranges from 7 to 21 – higher total scores were indicative of better cardiovascular health. In secondary analyses we examined the total health behavior scores and the total health factor scores separately. The total health behavior score ranges from 4 to 12, since this was based on the four health behavior metrics (smoking; BMI; physical activity; and diet); the total health factor score ranges from 3 to 9, since this was based on the three health factor metrics (cholesterol; blood pressure; and blood glucose). Higher total health behavior and higher total health factor scores were indicative of better cardiovascular health.
Exposures
Individual-level characteristics
Individual-level characteristics included self-reported race/ethnicity (Black, non-Hispanic; White, non-Hispanic), age (years), and sex (male; female). Income (annual household) categories included the following: < $20,000; 20,000–34,999; 35,000–74,999; > $75,000; refused to answer. Education (number of years) was operationalized as no schooling (0 years) and as the midpoint of the following categories: grades 1–8 (4 years); grades 9–11 (10 years); complete high school/GED (12 years); some technical school/technical school certificate (13 years); some college (14 years); college graduate (16 years); and postgraduate or professional degree (18 years); refused/don’t know/not sure. Marital status (married; not married) was also considered. All individual-level characteristics have been utilized previously in examining CVH disparities, especially in observational cohort studies that focus on cardiovascular disease [24, 26].
Neighborhood-level characteristics
For each REGARDS participant, we characterized their neighborhood using the census tract (CT) of their residential address at baseline. While CTs for each REGARDS participant ranges in size, CTs are common units of analysis used in assessing the spatial patterning of various health outcomes [31, 32]. Similar to previous studies [25, 27] that examined neighborhood characteristics and their impact on CVH [25–27], we considered the following CT density measures of: (1) physical activity resources, (2) walkability, (3) favorable food stores, and (4) social engagement venues. Physical activity resources included physical activity venues where participants could engage in activities considered light, moderate, or vigorous, such as gyms. Walkability was captured by four broad domains: destination density (of walkable destinations for daily living), population density (based on population counts), rail train stop density (based on counts of all rail transit stops), and census block density (which is a proxy for intersection density) [33]. Favorable food stores included all chain supermarkets/supercenters and fruit and vegetable markets. Social engagement venues included a variety of destinations offering services, such as beauty shops and barbers, spas, libraries, museums and art galleries, strip clubs/gentlemen’s clubs, religious institutions, bars and night clubs not serving alcohol, professional/semi-professional sports and stadium entertainment venues, zoos, aquariums, and arboretum venues. All CT density measures were per land area of the census tract and were derived by the Retail Environment and Cardiovascular Disease (RECVD) study [34]. We also considered whether or not the REGARDS participant resided in the Stroke Belt.
Lastly, we used the index of concentration at the extremes (ICE) to quantify racialized economic segregation - a form of structural racism that has persisted across urban settings for decades [35]. This index quantifies the extent to which the residents of a neighborhood are concentrated in the top vs. bottom categories of variables that measure a specified dimension of privilege or deprivation [36, 37]. The ICE measure has been proven to be more sensitive to detecting inequities than other commonly used US poverty measures, and has been shown to be predictive of health using census tract level measures compared to city/town level measures [38]. ICE measures were obtained from the Public Health Geocoding Project Monograph [39] for every CT in the US, where the ICE measure ranged from −1 (low-income Black neighborhoods) to +1 (high-income White neighborhoods).
Because many of the neighborhood measures were operationalized on different scales, we standardized each measure based on the distribution in our sample to facilitate comparison and interpretation.
Final Analytic Dataset
The total REGARDS study initially included 30,239 participants. Figure 1 shows the various exclusions for our analysis to explore the spatial heterogeneity in Black-White differences in CVH. Specifically, we excluded participants due to data anomalies (N = 56), missing at least one CVH component (N = 12,285) – mostly due to the diet component, missing education information (N = 5), and missing address and thus latitude/longitude measures (N = 9), leaving a final analytic sample size of N = 17,884.
Figure 1.
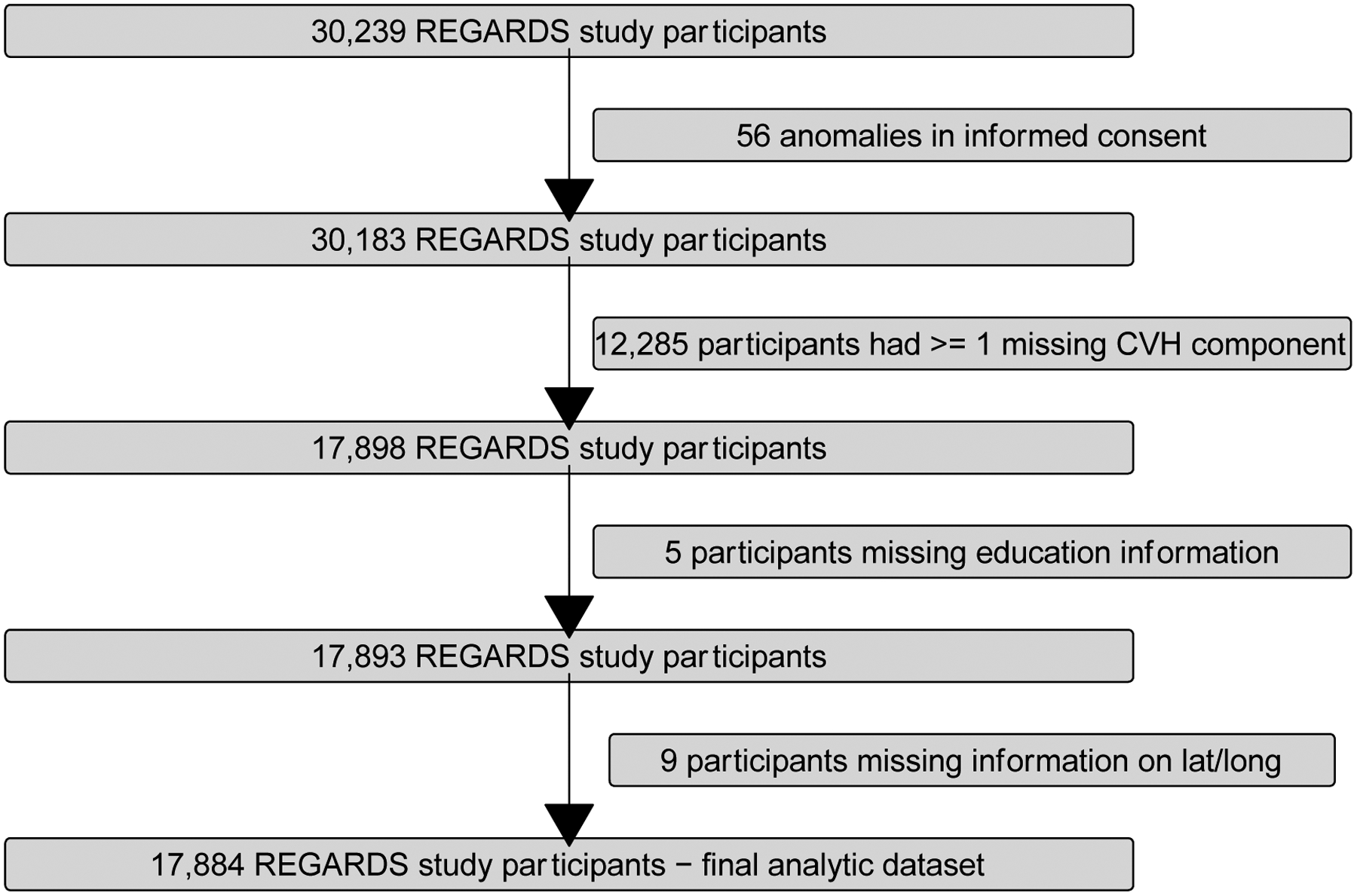
Flowchart displaying inclusion and exclusion criteria for the examination racial differences in CVH in the REGARDS Study 2003–2007.*
*REGARDS, Reasons for Geographic and Racial Differences in Stroke; CVH, cardiovascular health.
STATISTICAL ANALYSIS
We first examined descriptive statistics of the health behavior and health factor components of CVH as well as the individual- and neighborhood-level social determinants of health by race. Additionally, we compared all continuous and categorical measures between Black and White REGARDS participants using t-tests and chi-square tests, respectively.
We utilized a Bayesian hierarchical statistical framework to examine the spatial heterogeneity in CVH with a focus on estimating the global and spatially varying Black-White differences in CVH. Specifically, for each participant i(i = 1, …, N) residing in state j(j = 1, …, J), we assumed the following spatially varying coefficient model for total CVH score (Yij):
where β0 is the overall fixed intercept, quantifying the average CVH score, β1 is the fixed (global) effect of race, and σ2 represents the variance of the total CVH scores (i.e., residual error). The model also includes spatially unstructured (uj) and structured (vj) state level random effects (i.e., random intercepts). A convolution spatial prior, corresponding to the Besag-York-Mollie model [40], incorporates the combination of spatially unstructured random effects in addition to spatially structured random effects (vj). The spatially structured random effects assume a conditional autoregressive (CAR) prior such that , where wij is a weight that measures the strength of the relationship between neighboring states j and k and indicates the conditional variance of the CAR specification [41]. Neighboring states are defined based on sharing a common border. The term Raceij × δj captures the spatially varying Black-White differences at the state level and assumes a CAR prior similar to the spatially structured random effects vj. Each variance component in the above model is defined in terms of precision, such that, ; therefore, as precision increases, variance decreases. Also, we assume rather weakly-informative Gamma(1,0.5) priors for the hyperparameter precision terms, rather than the common prior of Gamma(1,0.0005) [42].
To assess the spatial heterogeneity in the Black-White differences in CVH, we fitted a series of sequential models. The first model considered only the racial differences in CVH, adjusted for age and sex (Model 1). We then considered the impact of other individual-level characteristics, including income, education and marital status (Model 2). Lastly, we additionally adjusted for the impact of neighborhood-level characteristics, including physical activity resources, walkability, favorable food stores, social engagement, living in the Stroke Belt, and the ICE measure based on race and income combined (Model 3). All three models included the unstructured and structured state level random intercepts, in addition to the random slope for race, which varied by state. To assess potential multicollinearity, we examined the variance inflation factor (VIF) for all individual- and neighborhood-level measures considered [43].
To compare the fit of the models, we used the deviance information criterion (DIC), where lower DIC values are indicative of improved model fit and efficiency. All models were implemented in R [44], via the R-INLA package [45] using the integrated nested Laplace approximation (INLA) method [46].
RESULTS
The Black-White differences in total CVH score, as well as its components, for REGARDS participants are presented in Figure 2. The proportion of White participants with ideal CVH scores was more than twice that for Black REGARDS participants (22 vs. 9%). When examining individual CVH components, Whites had greater proportions of ideal physical activity (33 vs. 26%), glucose (68 vs. 54%), blood pressure (24 vs. 10%), BMI (30 vs. 17%) and smoking status (87 vs. 80%). While almost no Black or White REGARDS participants had ideal diet, a greater proportion of White participants had intermediate levels (21 vs. 17%). Ideal cholesterol levels in Black REGARDS participants were higher than in White participants (37 vs. 33%). The full descriptive statistics of the cardiovascular health score components for all REGARDS participants and by race are presented in Supplementary Table S1.
Figure 2.
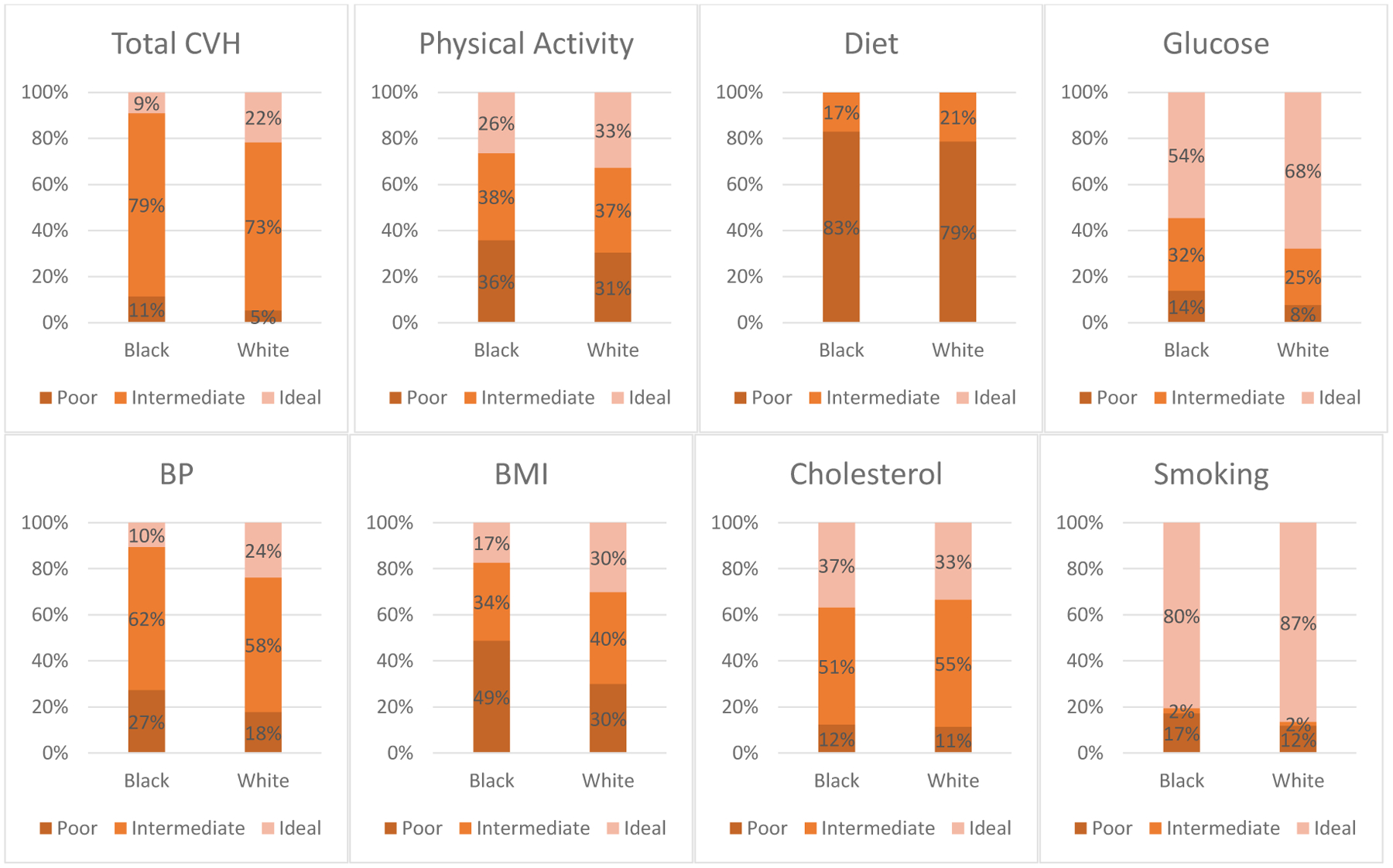
Black-White differences in total CVH score and components in REGARDS participants 2003–2007.*
*REGARDS, Reasons for Geographic and Racial Differences in Stroke; CVH, cardiovascular health; BP, blood pressure; BMI, body mass index.
Table 1 displays the descriptive statistics for the individual- and neighborhood-level characteristics for all REGARDS participants included in the analysis (N = 17,884), and also stratified by race. On average, participants were 64.6 years (SD = 9.2), female (56%), and White (68%), with 14 years of education. The majority of REGARDS participants were married (62%), and most fell into either the $20,000–$34,999 or the $35,000–$74,999 income categories (24 and 32%, respectively). Significant (p<0.05) differences were found between White and Black REGARDS participants, with respect to individual- and neighborhood-level characteristics. White REGARDS participants were significantly older, had a higher annual income, more years of education, and a higher proportion were married. Black REGARDS participants lived in neighborhoods that have higher densities of physical activity resources and favorable food stores, increased walkability, and more social engagement – possibly indicative of Black participants living in more urban environments compared to more rural environments. However, the mean ICE measure based on race and income for Black REGARDS participants was −0.3, indicating that they resided in neighborhoods that had a greater extreme concentration of Black people in the most economically deprived category. White REGARDS participants, on the other hand, lived in neighborhoods with an average ICE measure of close to 0. To assess multicollinearity between all individual- and neighborhood-level measures, we evaluated the VIF of each and all resulted in values less than 4 (Supplementary Table S2) - indicating little to no evidence of significant multicollinearity.
Table 1.
Individual- and neighborhood-level characteristics of REGARDS participants, overall and by race.*
| Characteristics | REGARDS Participants (N=17884) | White REGARDS Participants (N=12074) | Black REGARDS Participants (N=5810) |
|---|---|---|---|
| Individual Level | |||
| Age, mean (SD) | 64.6 (9.2) | 65.3 (9.3) | 63.3 (8.9) |
| Women, % | 10059 (56) | 6183 (51) | 3876 (67) |
| Race, % | |||
| White | 12074 (68) | 12074 (100) | 0 (0) |
| Black | 5810 (32) | 0 (0) | 5810 (100) |
| Education, mean (SD) | 14.0 (2.9) | 14.3 (2.8) | 13.4 (3.1) |
| Married, % | 11147 (62) | 8494 (70) | 2653 (46) |
| Income ($/year) | |||
| < $20,000 | 2690 (15) | 1294 (11) | 1396 (24) |
| $20,000–$34,999 | 4287 (24) | 2728 (23) | 1559 (27) |
| $35,000–$74,999 | 5676 (32) | 4033 (33) | 1643 (28) |
| $75,000 and above | 3170 (18) | 2587 (21) | 583 (10) |
| Refused | 2061 (11) | 1432 (12) | 629 (11) |
| Neighborhood Level | |||
| Physical activity resources, density, mean (SD) | 0.2 (0. 6) | 0.2 (0.5) | 0.3 (0.6) |
| Walkability, density, mean (SD) | −0.4 (1.6) | −0.6 (1.3) | 0.2 (2.1) |
| Favorable food stores, density, mean (SD) | 0.2 (0.5) | 0.1 (0.3) | 0.2 (0.7) |
| Social engagement, density, mean (SD) | 5.6 (8.0) | 4.1 (5.5) | 8.6 (11.0) |
| Index of concentration at the extremes, mean (SD)** | −0.1 (0.3) | 0.0 (0.2) | −0.3 (0.2) |
REGARDS, Reasons for Geographic and Racial Differences in Stroke; SD = standard deviation; All differences between continuous and categorical measures were statistically significant, p < 0.05.
Index of concentration at the extremes, support: −1 (low-income Black neighborhoods) to +1 (high-income White neighborhoods)
Table S3 in the Supplementary Material shows the DIC values for examining the relationship between total CVH scores and race, individual-, andneighborhood-level characteristics for the various spatially varying coefficient model settings considered for: (1) total CVH score, (2) total health behavior score, and (3) total health factor score. For all three CVH outcomes, there was a noticeable and significant decrease in DIC when comparing Model 1 with only race, age, and sex, to the models that incorporated both the individual- and neighborhood-level characteristics – with Model 3 having the smallest DIC for total CVH score, total health behavior score, and total health factor score. Given the importance of considering both individual- and neighborhood-level characteristics, coupled with objective evidence from the DIC model fit statistics, we will focus on Model 3 for the remainder of the paper.
Table 2 shows the mean differences (fixed effects) in total CVH score, in addition to the random effects, for Models 1, 2, and 3. The mean difference in CVH score between Black participants and White participants is attenuated but significantly persists after additional adjustment for both individual- and neighborhood-level characteristics; the percent change is on the order of 44% (comparing Model 1 to Model 3). Specifically, in the adjusted model (Model 3), all of the individual-level characteristics have a significant impact on total CVH score. Most notably, Black REGARDS participants have, on average, significantly lower total CVH scores compared to White participants (β=−0.54; 95% credible interval: −0.65, −0.44). REGARDS participants who are male also have lower total CVH scores than females, while older participants in the study surprisingly have higher total CVH scores (perhaps due to a survivor effect). Additionally, higher income and more education are associated with higher total CVH scores for REGARDS participants. Married participants have higher total CVH scores, compared to single participants. For the neighborhood-level characteristics, only the ICE measure based on race and income combined significantly impacts total CVH scores. Particularly, there was a positive association between ICE and total CVH, such that those participants living in neighborhoods with higher concentrations of White people in the most economically privileged setting have higher total CVH scores on average (β=0.15; 95% credible interval: 0.11, 0.19).
Table 2.
Mean differences in total CVH score associated with race and individual- and neighborhood-level covariates and variances of random components for all REGARDS participants.*
| Model 1a | Model 2b | Model 3c | ||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Mean | SD | 2.5% | 97.5% | Mean | SD | 2.5% | 97.5% | Mean | SD | 2.5% | 97.5% | |
| Fixed Effects | ||||||||||||
| Individual-Level | ||||||||||||
| Race (black vs. White) | −0.97 | 0.05 | −1.06 | −0.87 | −0.71 | 0.05 | −0.81 | −0.61 | −0.54 | 0.05 | −0.65 | −0.44 |
| Age (years) | 0.01 | 0.02 | −0.02 | 0.04 | 0.12 | 0.02 | 0.09 | 0.15 | 0.12 | 0.02 | 0.09 | 0.15 |
| Sex (male vs. female) | 0.09 | 0.03 | 0.03 | 0.15 | −0.10 | 0.03 | −0.16 | −0.04 | −0.09 | 0.03 | −0.15 | −0.03 |
| Income (reference: < $20,000/year) | ||||||||||||
| $20,000–34,999 | 0.23 | 0.05 | 0.13 | 0.33 | 0.22 | 0.05 | 0.12 | 0.32 | ||||
| $35,000–74,999 | 0.49 | 0.05 | 0.39 | 0.60 | 0.46 | 0.05 | 0.36 | 0.56 | ||||
| > $75,000 | 0.83 | 0.06 | 0.70 | 0.95 | 0.76 | 0.06 | 0.64 | 0.88 | ||||
| Refused | 0.52 | 0.06 | 0.41 | 0.64 | 0.50 | 0.06 | 0.38 | 0.61 | ||||
| Education (# of years) | 0.28 | 0.02 | 0.24 | 0.31 | 0.27 | 0.02 | 0.23 | 0.30 | ||||
| Marital status (yes vs. no) | 0.19 | 0.04 | 0.12 | 0.26 | 0.18 | 0.04 | 0.11 | 0.25 | ||||
| Neighborhood-Lev e l | ||||||||||||
| Physical activity resources density | 0.00 | 0.02 | −0.03 | 0.04 | ||||||||
| Walkability density | 0.01 | 0.03 | −0.04 | 0.06 | ||||||||
| Favorable food stores density | 0.02 | 0.02 | −0.01 | 0.05 | ||||||||
| Social engagement density | −0.02 | 0.03 | −0.07 | 0.04 | ||||||||
| Living in the Stroke Belt (yes vs. no) | 0.07 | 0.16 | −0.24 | 0.39 | ||||||||
| Index of concentration at the extremes | 0.15 | 0.02 | 0.11 | 0.19 | ||||||||
| Random Effects | ||||||||||||
| Precision for the total CVH scores | 0.24 | 0.00 | 0.24 | 0.25 | 0.26 | 0.00 | 0.25 | 0.26 | 0.26 | 0.00 | 0.25 | 0.26 |
| Precision for unstructured state-level random effects | 13.45 | 4.08 | 6.87 | 22.75 | 14.26 | 4.26 | 7.53 | 24.06 | 14.02 | 4.28 | 7.50 | 24.13 |
| Precision for structured state-level random effects | 8.68 | 3.42 | 3.81 | 17.04 | 9.39 | 3.55 | 4.10 | 17.87 | 9.40 | 3.59 | 4.13 | 17.99 |
| Precision for structured state-level varying race coefficients | 11.31 | 3.81 | 5.34 | 20.14 | 12.09 | 4.02 | 5.90 | 21.51 | 12.45 | 4.14 | 6.05 | 22.12 |
REGARDS, Reasons for Geographic and Racial Differences in Stroke; CVH, cardiovascular health; SD = standard deviation; 2.5% and 97.5% are the lower and upper 95% credible interval limits, respectively
Model 1 includes race + age + sex
Model 2 is Model 1 + income + education + marital status
Model 3 is Model 2 + physical activity resources + walkability + favorable food stores + social engagement + living in the Stroke Belt + index of concentration at the extremes
The precision terms for the random effects are also presented in Table 2, where larger precision terms imply smaller variance or heterogeneity. While there were no noticeable gains in residual precision of the total CVH scores from Model 1 to Model 3 (as indicated by τ = 0.24 in Model 1 compared to τ = 0.26 in Model 3), there were some noted larger precision terms (indicative of smaller heterogeneity) comparing Models 1, 2, and 3 when focusing on the state level random effects. Particularly, the unstructured random effects’ precision component becomes larger when comparing Models 1 and 3 (τu = 13.45 in Model 1 vs. τu = 14.02 in Model 3), indicating that unstructured spatial heterogeneity decreases, although slightly, once individual- and neighborhood-level characteristics are considered in predicting total CVH. Similar patterns are observed for the structured random effects’ precision components (τv and τδ), which, again, speaks to the individual- and neighborhood-level characteristics explaining a small amount of the structured spatial heterogeneity overall and in the spatially varying Black-White differences in total CVH for the REGARDS participants.
The mean differences in total health behavior and total health factor scores are presented separately in Table 3 for the fully adjusted model (Model 3) that includes both individual- and neighborhood-level characteristics. Supplementary Tables S4 and S5 display the mean differences for Models 1, 2, and 3 for each of the total health behavior and total health factor scores, respectively, including the random effects. Similar patterns of attenuation were observed for the mean differences in total health behavior and total health factors scores with additional adjustment for both individual- and neighborhood-level characteristics such that percent change was on the order of 54% and 33% (comparing Model 1 to Model 3), respectively. Black REGARDS participants have, on average, significantly lower total health behavior scores (β=−0.25; 95% credible interval: −0.33, −0.17) and lower total health factor scores (β=−0.29; 95% credible interval: −0.36, −0.22) compared to White participants. While all of the individual-level characteristics were significantly associated with total health behavior (with the exception of sex) and total health factor scores (with the exception of marital status), there is one key distinction in these associations. Older REGARDS participants, compared to younger participants, have higher total health behavior scores (β=0.23; 95% credible interval: 0.21, 0.26) – indicative of ideal health behavior; whereas older REGARDS participants have lower total health factor scores (β=−0.08; 95% credible interval: −0.12, −0.04) – indicative of poorer health factors. The ICE measure based on race and income combined was significant when looking at both total health behavior scores and total health factor scores. Similar to total CVH scores, there was a positive association between ICE and total health behavior scores and separately total health factor scores – such that, those participants living in neighborhoods with higher concentrations of White people in the most economically privileged setting have, on average, higher total health behavior scores (β=0.11; 95% credible interval: 0.08, 0.14) and higher total health factor scores (β=0.04; 95% credible interval: 0.02, 0.06).
Table 3.
Mean differences in total health behavior scores (left) and total health factor scores (right) scores associated with race and individual- and neighborhood-level covariates and variances of random components for all REGARDS participants.*
| Total Health Behavior Score | Mean | SD | 2.5% | 97.5% | Total Health Factor Score | Mean | SD | 2.5% | 97.5% |
|---|---|---|---|---|---|---|---|---|---|
| Fixed Effects | Fixed Effects | ||||||||
| Individual-Level | Individual-Level | ||||||||
| Race (black vs. White) | −0.25 | 0.04 | −0.33 | −0.17 | Race (black vs. White) | −0.29 | 0.04 | −0.36 | −0.22 |
| Age (years) | 0.23 | 0.01 | 0.21 | 0.26 | Age (years) | −0.11 | 0.01 | −0.13 | −0.09 |
| Sex (male vs. female) | −0.01 | 0.02 | −0.06 | 0.03 | Sex (male vs. female) | −0.08 | 0.02 | −0.12 | −0.04 |
| Income (reference: < $20,000/year) | Income (reference: < $20,000/year) | ||||||||
| $20,000–34,999 | 0.16 | 0.04 | 0.09 | 0.23 | $20,000–34,999 | 0.06 | 0.03 | 0.00 | 0.12 |
| $35,000–74,999 | 0.29 | 0.04 | 0.22 | 0.37 | $35,000–74,999 | 0.17 | 0.03 | 0.11 | 0.23 |
| > $75,000 | 0.48 | 0.04 | 0.40 | 0.57 | > $75,000 | 0.28 | 0.04 | 0.21 | 0.35 |
| Refused | 0.35 | 0.04 | 0.26 | 0.43 | Refused | 0.15 | 0.04 | 0.08 | 0.22 |
| Education (# of years) | 0.17 | 0.01 | 0.14 | 0.19 | Education (# of years) | 0.10 | 0.01 | 0.08 | 0.12 |
| Marital status (yes vs. no) | 0.15 | 0.03 | 0.10 | 0.20 | Marital status (yes vs. no) | 0.02 | 0.02 | −0.02 | 0.07 |
| Neighborhood-Level | Neighborhood-Level | ||||||||
| Physical activity resources density | 0.00 | 0.01 | −0.03 | 0.02 | Physical activity resources density | 0.01 | 0.01 | −0.02 | 0.03 |
| Walkability density | 0.01 | 0.02 | −0.03 | 0.04 | Walkability density | 0.01 | 0.02 | −0.03 | 0.04 |
| Favorable food stores density | 0.02 | 0.01 | −0.01 | 0.04 | Favorable food stores density | 0.00 | 0.01 | −0.02 | 0.02 |
| Social engagement density | 0.00 | 0.02 | −0.04 | 0.03 | Social engagement density | −0.01 | 0.02 | −0.04 | 0.02 |
| Living in the Stroke Belt (yes vs. no) | 0.10 | 0.14 | −0.18 | 0.37 | Living in the Stroke Belt (yes vs. no) | −0.01 | 0.13 | −0.26 | 0.25 |
| Index of concentration at the extremes | 0.11 | 0.01 | 0.08 | 0.14 | Index of concentration at the extremes | 0.04 | 0.01 | 0.02 | 0.06 |
| Random Effects | Random Effects | ||||||||
| Precision for the total health behavior scores | 0.52 | 0.01 | 0.51 | 0.53 | Precision for the total health factor scores | 0.74 | 0.01 | 0.72 | 0.75 |
| Precision for unstructured state-level random effects | 17.25 | 4.81 | 9.36 | 28.13 | Precision for unstructured state-level random effects | 19.40 | 5.13 | 10.95 | 30.97 |
| Precision for structured state-level random effects | 11.22 | 3.93 | 5.17 | 20.43 | Precision for structured state-level random effects | 11.86 | 4.04 | 5.79 | 21.49 |
| Precision for structured race-varying state-level random effects | 15.00 | 4.75 | 7.92 | 26.38 | Precision for structured race-varying state-level random effects | 15.72 | 4.66 | 8.32 | 26.45 |
REGARDS, Reasons for Geographic and Racial Differences in Stroke; CVH, cardiovascular health; SD = standard deviation; 2.5% and 97.5% are the lower and upper 95% credible interval limits, respectively
To further visualize the spatial heterogeneity in the Black-White differences in total CVH scores, Figure 3 displays a map of the structured spatially varying random components of the race coefficients (δj) for Model 3. Overall, as shown by the global effect of race in Model 3 (β=−0.54; 95% credible interval: −0.65, −0.44), Black REGARDS participants have lower total CVH scores compared to White participants. However, the map suggests that race differences in CVH vary across the US, at the state level. Negative random components of the race coefficient (darker red states), signifying larger racial differences in total CVH scores, are noticeable in parts of the Northeast, Midwest, and the Southeast. Specifically, large negative random race coefficients (large race differences denoted by dark red states) are observed in Maine, New Hampshire, Vermont and Michigan. States like Mississippi, Louisiana and Alabama fall within the Stroke Belt region and have noted lower random effect values as well, where states like Nebraska and South Dakota have similar smaller random effect values – again, signifying larger racial differences in total CVH scores. There were positive random coefficient values (light red states) indicating smaller race differences in states like Georgia, Arizona, Utah, and Colorado.
Figure 3. Map of state level random effects from the fully adjusted spatially varying random coefficient (race) model for total CVH scores for all REGARDS participants, United States (US).*.
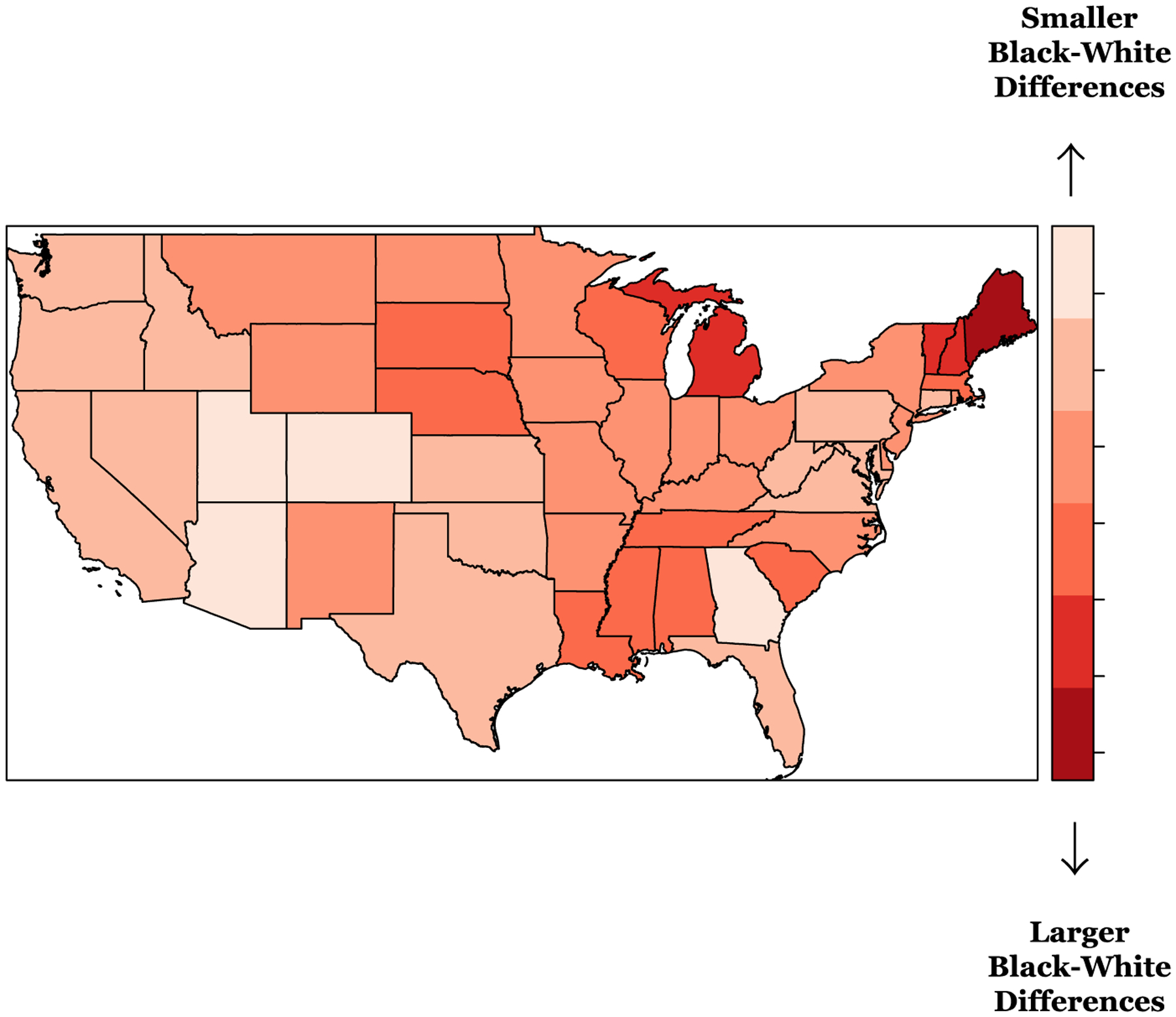
* REGARDS, Reasons for Geographic and Racial Differences in Stroke; CVH, cardiovascular health.
Model includes intercept + race + age + sex + income + education + marital status + physical activity resources + walkability + favorable food stores + social engagement + living in the Stroke Belt + index of concentration at the extremes + race-varying state-level random effects
Figure 4 shows the spatial heterogeneity in the Black-White differences in total health behavior scores (left) and total health factor scores (right). For both outcomes, as indicated by the global effect of race (βtotal health behavior scores = −0.25; 95% credible interval: −0.33, −0.17; βtotal health factor scores=−0.29; 95% credible interval: −0.36, −0.22), Black REGARDS participants have lower total health behavior and total health factor scores than White participants. However, the maps show noticeable variation in random race coefficients (δj). For the total health behavior scores, the state level random component for the race coefficient is negative (dark red states) in Mississippi, Vermont and New Hampshire - indicating larger race differences. There were positive random coefficient values (light red states) indicating smaller race differences in many states in the Midwest like Arizona, Utah, and Colorado, in addition to a number of other surrounding states like Texas and Oklahoma. Georgia, similar to the results from the total CVH score corresponding maps, also showed smaller race differences. For the total health factor scores, the random race coefficients are also negative (dark red states) in Maine, as well a number of states in the Southeast, Midwest, and the West - indicating larger race differences. There were positive random coefficient values (light red states) indicating smaller race differences in Florida, Georgia, Kansas, and states in the Northeast like Pennsylvania, New Jersey, Maryland and the District of Columbia.
Figure 4. Map of state level random effects from the fully adjusted spatially varying random coefficient (race) models for total health behavior scores (left) and total health factor scores (right) for all REGARDS participants, United States (US).*.
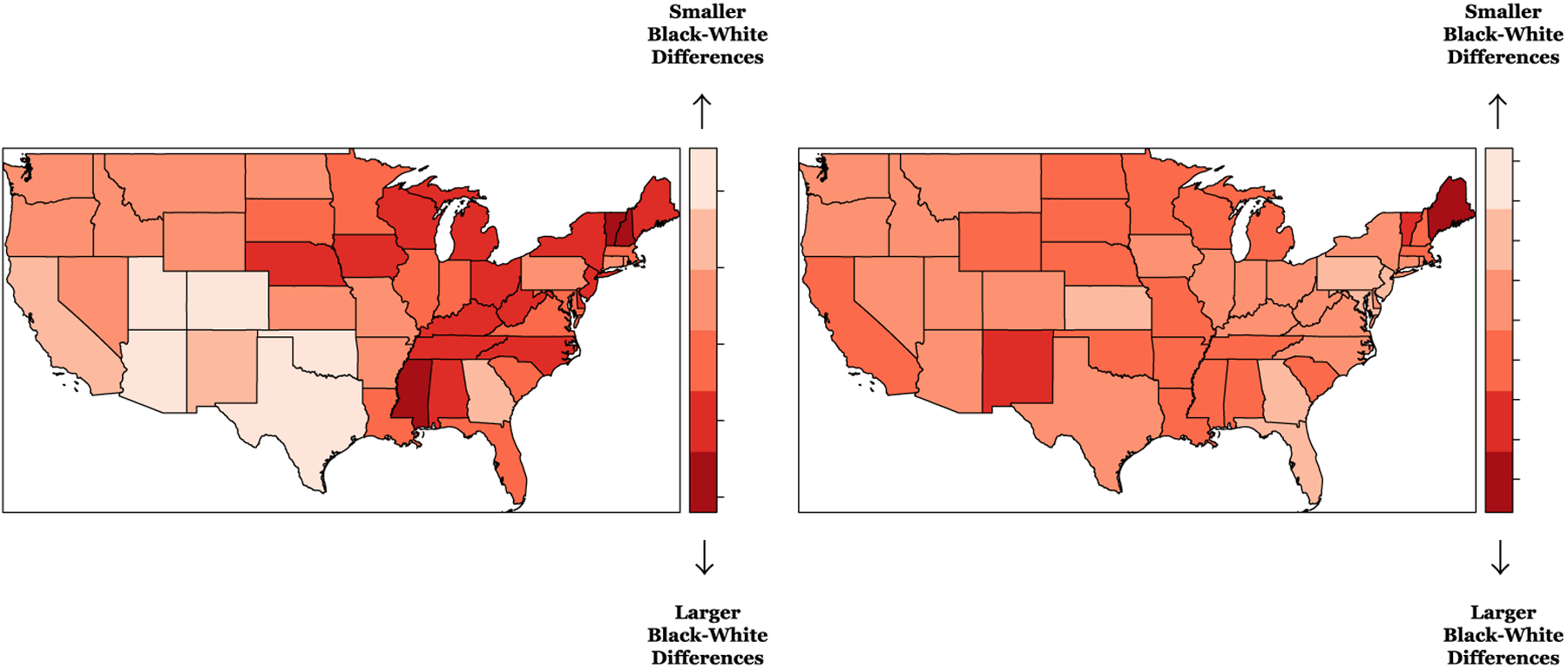
*REGARDS, Reasons for Geographic and Racial Differences in Stroke
Models include intercept + race + age + sex + income + education + marital status + physical activity resources + walkability + favorable food stores + social engagement + living in the Stroke Belt + index of concentration at the extremes + race-varying state-level random effects
DISCUSSION
We examined how spatially varying Black-White inequities in CVH change when individual- and neighborhood-level characteristics are considered. We found significant racial differences, in that Black participants consistently had lower CVH scores than White participants, indicative of poorer CVH, and these differences persisted even after adjustment for individual- and neighborhood-level characteristics. Notably, adjustment for the individual- and neighborhood-level characteristics resulted in an attenuation of Black-White differences in CVH. Among the neighborhood-level characteristics examined, residential segregation had a significant impact on CVH. We found that REGARDS participants living in census tracts with high concentrations of Black, low-income residents had significantly lower total CVH scores – which also was evident for total health behavior scores and total health factor scores.
Neither individual- nor neighborhood-level characteristics significantly explained the spatial heterogeneity in the racial differences as indicated by no substantial changes in the variance components of the random race coefficients across the various models considered. Our findings further extended previous research that focused on exploring the spatial patterning in racial differences in CVH within the REGARDS cohort [24]. This previous study aimed to answer the following questions: (1) How much spatial heterogeneity exists in the racial differences in CVH?, and (2) Is the spatial heterogeneity in the racial differences significantly explained by living in the Stroke Belt? And, while this earlier study found significant spatial patterning in these racial differences, even beyond the well-known Stroke Belt and Stroke Buckle regions of the US, this study did not consider how spatially varying Black-White inequities in CVH change when individual- and neighborhood-level characteristics are considered – which was evident from the results of our study.
Our maps showed state level variation in race differences in CVH scores even after individual- and neighborhood-level factors were considered, highlighting the patterning of the Black-White differences in CVH across the US for total CVH scores, as well as for the total health behavior and total health factor scores separately. Areas in the Northeast, Midwest, and the Southeast (states like Maine, Michigan, and Mississippi, respectively) showed significantly larger differences in CVH scores between Black and White REGARDS participants. And, while these larger differences were noted, smaller differences, although still significant, were found in pockets of the Southeast (Georgia) as well as the Midwest (Utah and Colorado), even after adjusting for the individual- and neighborhood-level characteristics. These geographic based disparities are likely a function of differences in rural and urban environments alike. Not only do these environments include the obvious behavioral, psychosocial, and cultural factors that contribute towards overall health, but also speak to a lack of access to health promoting resources, as well as limited access to quality healthcare.
Our results are consistent with previous studies that have examined the intersection of cardiovascular health and place with a focus on Black-White differences. Particularly, an earlier REGARDS study utilized heat maps of hypertension, diabetes mellitus, and smoking across the US, which are all individual CVH components, rather than the total CVH score defined by the AHA, and found significant differences between Black participants and White participants [47]. Another study based on the MESA cohort assessed the spatial heterogeneity in Black-White differences in optimal CVH and the impact of individual- and neighborhood-level characteristics [25]. However, the MESA study focused on variability in the Black-White differences in CVH across five regions of the US only and did not include the impact of residential segregation. Similar to these other studies, our findings found significant variation in Black-White CVH disparities. And, while our study found that individual-level characteristics were significantly associated with CVH, neighborhood-level factors, such as residential segregation, also were significantly linked to CVH. These social determinants of health and how they are associated with Black-White CVH disparities underscore the importance in where people are born, live, learn, work, play and worship.
A novel aspect of our work is the use of CVH, as opposed to the individual components of CVH or even simply the absence or presence of CVD. The use of a large, national cohort allowed for an assessment of geographic heterogeneity in race differences. We also included factors at both the individual- and neighborhood-level. Additionally, this research utilized both Bayesian and spatial statistical methods to fully assess the spatial heterogeneity and patterning in CVH inequities, allowing for the multi-level structure in the REGARDS data to be fully explored. The various neighborhood-level characteristics considered were diverse and included a number of measures (such as physical activity resources and walkability), as well as social constructs like residential segregation. Existing evidence ties residential segregation to overall cardiovascular disease [48], exposure to ambient air pollution [49], changes in blood pressure [50], obesity [51, 52], as well as mental health [53]. Most of these studies focus only on racial segregation. One cross-sectional study conducted in the Boston metropolitan area examined the use of the ICE measure jointly for income and race/ethnicity to analyze the risk of hypertension [36]. This study found even stronger associations observed for the ICE measures that compared concentrations of high-income White residents versus low-income residents of color and high-income White versus low-income Black residents – as opposed to only looking at the ICE measure for extreme concentrations of White compared to Black residents. Therefore, we believe our utilization of the ICE measure that is a function of both race and income is advantageous in fully exploring the joint impact of these domains on CVH disparities. The ICE measure utilized in this study, moves beyond the commonly used segregation measures by focusing on both the racial and economic segregation present in a neighborhood [36, 54].
In our analyses, racialized economic segregation significantly impacted total CVH scores – although the effect size was minimal when focusing on total health factor scores. This measure jointly examined the intersection of racial/ethnic and economic segregation – which has been shown to have strong associations with hypertension [36], premature mortality and diabetes [54], and cancer inequities [55]. This measure also captures multiple dimensions of social inequality and reflects decades of structural racism in the form policy-induced separation of individuals by both social class and race. Decades of systematic disinvestment in these neighborhood environments has led to a wide array of adverse exposures (e.g., limited access to health promoting resources and chronic stress) which may compromise CVH. Our findings highlighted the importance of residential segregation in CVH, such that living in neighborhoods with high concentrations of Black and low-income residents led all REGARDS participants to have poorer CVH. Thus, the racist and classist structures that currently reside in this country cannot be ignored when looking at health outcomes such as CVH.
As with any study, there are some limitations to this research. First, while our study has utilized a large, national population-based cohort study found in REGARDS, we recognize that the baseline data used was captured about two decades ago. Our findings, though, have been consistent with even recent research that has examined cardiovascular health related disparities between Blacks and Whites. Specifically, recent research that has made use of the All of Us Research Program data has shown that Black participants had a higher adjusted odds of CVD compared with White participants. The recently introduced All of Us Research Program [56] (launched in 2018) seeks to accelerate precision medicine research by acquiring and publicly sharing health data from 1 million Americans, with one of its goals to reduce health disparities across underrepresented groups. Unfortunately, their recent research findings continue to document the disparities present in these populations. Second, certain states and regions of the country had fewer REGARDS participants residing in those areas compared to other states and regions, particularly the Midwest and West. Because of that, measuring the spatially varying CVH inequities in these regions can be challenging; however, even with the sparsity of REGARDS participants in these states and regions, we still found significantly varying CVH inequities, with Black participants consistently having lower total CVH scores compared to White participants. This was likely due to the added benefit of utilizing a Bayesian spatial statistical framework, which allowed for spatial smoothing and borrowing of information across neighboring regions. Thirdly, unmeasured confounding likely plays a role in assessing these spatially varying inequities in CVH across the country. We also note that there was a significant amount of missing data from the original REGARDS cohort, where the diet-based measures contributed the most towards the missingness – especially for Black REGARDS participants. With any form of missing data, there is always the risk of the missing data potentially being informative; however, we did not explicitly model for this type of missing data patterning. Fourth, the use of census tract proxies may have resulted in measurement error in neighborhood attributes. Lastly, while residential segregation played a role in predicting CVH in this study, the historical context of residential segregation has not been fully captured in these analyses. Given the potential of historical racist and classist policies that have existed in this country for some time, it would be ideal to also measure the impact of both the historical and contemporary residential segregation landscape of this country – and how this influences spatially varying CVH racial inequities.
Our study has mapped and measured the Black-White differences in CVH in the US using the racially diverse REGARDS cohort and showed that both individual- and neighborhood-level characteristics play a role in these differences. Yet, spatial heterogeneity in Black-White differences persisted even after these factors were accounted for. Future studies that examine temporal changes in this spatial patterning may shed additional light on the drivers of these differences. The evidence produced in this current study further highlights how place matters, and is a reminder of the importance of multilevel, multisector systems that need to be in place to address these geographic and racial differences in CVH.
Supplementary Material
HIGHLIGHTS.
Cardiovascular health (CVH), defined by the American Heart Association, is examined
Spatial variation in CVH was explored using a large, national population-based cohort
Black-White inequities in CVH vary at the state level in the US
Individual- and neighborhood-level risk factors explained some of the spatial heterogeneity
Acknowledgements
Research reported in this publication was supported by the National Heart, Lung, And Blood Institute of the National Institutes of Health under Award Number K01HL133515. The content is solely the responsibility of the authors and does not necessarily represent the official views of the National Institutes of Health.
This research is also supported by the National Institute of Aging (grants 1R01AG049970, 3R01AG049970-04S1), Commonwealth Universal Research Enhancement (C.U.R.E) program funded by the Pennsylvania Department of Health - 2015 Formula award - SAP #4100072543, the Urban Health Collaborative at Drexel University, and the Built Environment and Health Research Group at Columbia University.
This research project is also supported by cooperative agreement U01 NS041588 co-funded by the National Institute of Neurological Disorders and Stroke (NINDS) and the National Institute on Aging (NIA), National Institutes of Health, Department of Health and Human Service. The content is solely the responsibility of the authors and does not necessarily represent the official views of the NINDS or the NIA. Representatives of the NINDS were involved in the review of the manuscript but were not directly involved in the collection, management, analysis or interpretation of the data. The authors thank the other investigators, the staff, and the participants of the REGARDS study for their valuable contributions. A full list of participating REGARDS investigators and institutions can be found at: https://www.uab.edu/soph/regardsstudy/
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Competing Interests
None.
References
- 1.Graham G, Disparities in cardiovascular disease risk in the United States. Current cardiology reviews, 2015. 11(3): p. 238–245. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Centers for Disease Control and Prevention, Underlying Cause of Death, 1999–2018. 2018: Atlanta, GA: Centers for Disease Control and Prevention. [Google Scholar]
- 3.Mensah GA, et al. , Reducing Cardiovascular Disparities Through Community-Engaged Implementation Research. Circulation research, 2018. 122(2): p. 213–230. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Mensah GA, et al. , State of disparities in cardiovascular health in the United States. Circulation, 2005. [DOI] [PubMed] [Google Scholar]
- 5.Williams MB, Mitchell F, and Thomson GE, Examining the health disparities research plan of the National Institutes of Health: unfinished business. 2006: National Academies Press. [PubMed] [Google Scholar]
- 6.Gillespie CD, Wigington C, and Hong Y, Coronary heart disease and stroke deaths-United States, 2009. MMWR Surveill Summ, 2013. 62(Suppl 3): p. 157–160. [PubMed] [Google Scholar]
- 7.Mozaffarian D, et al. , Executive summary: heart disease and stroke statistics—2016 update: a report from the American Heart Association. Circulation, 2016. 133(4): p. 447–454. [DOI] [PubMed] [Google Scholar]
- 8.Pearson-Stuttard J, et al. , Modeling future cardiovascular disease mortality in the United States: national trends and racial and ethnic disparities. Circulation, 2016. 133(10): p. 967–978. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Lloyd-Jones DM, et al. , Defining and setting national goals for cardiovascular health promotion and disease reduction the American Heart Association’s Strategic Impact Goal through 2020 and beyond. Circulation, 2010. 121(4): p. 586–613. [DOI] [PubMed] [Google Scholar]
- 10.Association”, A.H. My Life Check: Life’s Simple 7. 2021. [cited 2021 September 23]; Available from: https://www.heart.org/en/healthyliving/healthy-lifestyle/my-life-check--lifes-simple-7.
- 11.Dong C, et al. , Ideal cardiovascular health predicts lower risks of myocardial infarction, stroke, and vascular death across whites, blacks and hispanics: the northern Manhattan Study. Circulation, 2012: p. CIRCULATIONAHA. 111.081083. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Crichton GE, et al. , Cardiovascular health and cognitive function: the Maine-Syracuse Longitudinal Study. PloS one, 2014. 9(3): p. e89317. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Thacker EL, et al. , The American Heart Association Life’s Simple 7 and Incident Cognitive Impairment: The REasons for Geographic And Racial Differences in Stroke (REGARDS) Study. Journal of the American Heart Association, 2014. 3(3): p. e000635. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Rasmussen-Torvik LJ, et al. , Ideal cardiovascular health is inversely associated with incident cancer: the Atherosclerosis Risk In Communities study. Circulation, 2013. 127(12): p. 1270. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Aaron KJ, et al. , Cardiovascular health and healthcare utilization and expenditures among Medicare beneficiaries: the REasons for Geographic And Racial Differences in Stroke (REGARDS) study. Journal of the American Heart Association: Cardiovascular and Cerebrovascular Disease, 2017. 6(2). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Allen NB, et al. , The association between cardiovascular health and health-related quality of life and health status measures among US adults: a cross-sectional study of the National Health and Nutrition Examination Surveys, 2001–2010. Health and quality of life outcomes, 2015. 13(1): p. 152. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.España-Romero V, et al. , A prospective study of ideal cardiovascular health and depressive symptoms. Psychosomatics, 2013. 54(6): p. 525–535. [DOI] [PubMed] [Google Scholar]
- 18.Kronish IM, et al. , Depressive symptoms and cardiovascular health by the American Heart Association’s definition in the Reasons for Geographic and Racial Differences in Stroke (REGARDS) study. PLoS One, 2012. 7(12): p. e52771. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Nasir K, Keeley B, and Krumholz HM, Living Longer in Good Cardiovascular Health: Prevention and Wellness Makes Economic Cents. 2017, Am Heart Assoc. [DOI] [PubMed] [Google Scholar]
- 20.Roth GA, et al. , The burden of cardiovascular diseases among US states, 1990–2016. JAMA cardiology, 2018. 3(5): p. 375–389. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Benjamin EJ, et al. , Heart disease and stroke Statistics-2019 update a report from the American Heart Association. Circulation, 2019. [DOI] [PubMed] [Google Scholar]
- 22.Howard G, et al. , Is the stroke belt disappearing? An analysis of racial, temporal, and age effects. Stroke, 1995. 26(7): p. 1153–1158. [DOI] [PubMed] [Google Scholar]
- 23.Lackland D, et al. , on behalf of the American Heart Association Stroke Council; Council on Cardiovascular and Stroke Nursing; Council on Quality of Care and Outcomes Research; Council on Functional Genomics and Translational Biology. Factors influencing the decline in stroke mortality: a statement from the American Heart Association/American Stroke Association. Stroke, 2014. 45: p. 315–353. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Tabb LP, et al. , Exploring the spatial patterning in racial differences in cardiovascular health between blacks and whites across the United States: the REGARDS study. Journal of the American Heart Association, 2020. 9(9): p. e016556. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Tabb LP, et al. , Assessing the spatial heterogeneity in black-white differences in optimal cardiovascular health and the impact of individual-and neighborhood-level risk factors: The Multi-Ethnic Study of Atherosclerosis (MESA). Spatial and Spatio-temporal Epidemiology, 2020. 33: p. 100332. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Unger E, et al. , Association of neighborhood characteristics with cardiovascular health in the Multi-Ethnic Study of Atherosclerosis. Circulation: Cardiovascular Quality and Outcomes, 2014. 7(4): p. 524–531. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Mujahid MS, et al. , Neighborhoods and racial/ethnic differences in ideal cardiovascular health (the Multi-Ethnic Study of Atherosclerosis). Health & place, 2017. 44: p. 61–69. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Lloyd-Jones DM, et al. , Defining and setting national goals for cardiovascular health promotion and disease reduction: the American Heart Association’s strategic Impact Goal through 2020 and beyond. Circulation, 2010. 121(4): p. 586–613. [DOI] [PubMed] [Google Scholar]
- 29.Howard VJ, et al. , The reasons for geographic and racial differences in stroke study: objectives and design. Neuroepidemiology, 2005. 25(3): p. 135–143. [DOI] [PubMed] [Google Scholar]
- 30.Block G, et al. , Validation of a self-administered diet history questionnaire using multiple diet records. Journal of clinical epidemiology, 1990. 43(12): p. 1327–1335. [DOI] [PubMed] [Google Scholar]
- 31.Chen JT, et al. , Mapping and measuring social disparities in premature mortality: the impact of census tract poverty within and across Boston neighborhoods, 1999–2001. Journal of Urban Health, 2006. 83(6): p. 1063–1084. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Tabb LP, Fillmore C, and Melly S, Location, location, location: assessing the spatial patterning between marijuana licenses, alcohol outlets and neighborhood characteristics within Washington state. Drug and alcohol dependence, 2018. 185: p. 214–218. [DOI] [PubMed] [Google Scholar]
- 33.Rundle AG, et al. , Development of a neighborhood walkability index for studying neighborhood physical activity contexts in communities across the US over the past three decades. Journal of urban health, 2019. 96(4): p. 583–590. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Hirsch JA, et al. , Business Data Categorization and Refinement for Application in Longitudinal Neighborhood Health Research: a Methodology. Journal of Urban Health, 2020: p. 1–14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Bailey ZD, et al. , Structural racism and health inequities in the USA: evidence and interventions. The Lancet, 2017. 389(10077): p. 1453–1463. [DOI] [PubMed] [Google Scholar]
- 36.Feldman JM, et al. , Spatial social polarisation: using the Index of Concentration at the Extremes jointly for income and race/ethnicity to analyse risk of hypertension. J Epidemiol Community Health, 2015. 69(12): p. 1199–1207. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Krieger N, et al. , Black carbon exposure, socioeconomic and racial/ethnic spatial polarization, and the Index of Concentration at the Extremes (ICE). Health & place, 2015. 34: p. 215–228. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Krieger N, et al. , Using the Index of Concentration at the Extremes at multiple geographical levels to monitor health inequities in an era of growing spatial social polarization: Massachusetts, USA (2010–14). International journal of epidemiology, 2018. 47(3): p. 788–819. [DOI] [PubMed] [Google Scholar]
- 39.Krieger N, Chen JT, and Waterman PD. The Public Health Disparities Geocoding Project Monograph. 2020. [cited 2020 September 2]; Available from: https://www.hsph.harvard.edu/thegeocodingproject/covid-19-resources/.
- 40.Besag J, York J, and Mollié A, Bayesian image restoration, with two applications in spatial statistics. Annals of the institute of statistical mathematics, 1991. 43(1): p. 1–20. [Google Scholar]
- 41.Bivand RS, et al. , Applied spatial data analysis with R. Vol. 747248717. 2008: Springer. [Google Scholar]
- 42.Carroll R, et al. , Comparing INLA and OpenBUGS for hierarchical Poisson modeling in disease mapping. Spatial and spatio-temporal epidemiology, 2015. 14: p. 45–54. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Chatterjee S and Hadi AS, Regression analysis by example. 2015: John Wiley & Sons. [Google Scholar]
- 44.R Core Team, R: A Language and Environment for Statistical Computing. 2020, R Foundation for Statistical Computing. [Google Scholar]
- 45.Rue H The R-INLA project. Available from: www.r-inla.org.
- 46.Rue H, Martino S, and Chopin N, Approximate Bayesian inference for latent Gaussian models by using integrated nested Laplace approximations. Journal of the royal statistical society: Series b (statistical methodology), 2009. 71(2): p. 319–392. [Google Scholar]
- 47.Loop MS, et al. , Heat Maps of Hypertension, Diabetes Mellitus, and Smoking in the Continental United States. Circulation: Cardiovascular Quality and Outcomes, 2017. 10(1): p. e003350. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Barber S, et al. , Neighborhood disadvantage, poor social conditions, and cardiovascular disease incidence among African American adults in the Jackson Heart Study. American journal of public health, 2016. 106(12): p. 2219–2226. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Jones MR, et al. , Race/ethnicity, residential segregation, and exposure to ambient air pollution: the Multi-Ethnic Study of Atherosclerosis (MESA). American journal of public health, 2014. 104(11): p. 2130–2137. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Kershaw KN, et al. , Association of changes in neighborhood-level racial residential segregation with changes in blood pressure among black adults: the CARDIA study. JAMA internal medicine, 2017. 177(7): p. 996–1002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Kershaw KN and Pender AE, Racial/ethnic residential segregation, obesity, and diabetes mellitus. Current diabetes reports, 2016. 16(11): p. 108. [DOI] [PubMed] [Google Scholar]
- 52.Pool LR, et al. , Longitudinal associations of neighborhood-level racial residential segregation with obesity among blacks. Epidemiology, 2018. 29(2): p. 207–214. [DOI] [PubMed] [Google Scholar]
- 53.Nobles CJ, et al. , Residential segregation and mental health among Latinos in a nationally representative survey. J Epidemiol Community Health, 2017. 71(4): p. 318–323. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Krieger N, et al. , Public health monitoring of privilege and deprivation with the index of concentration at the extremes. American journal of public health, 2016. 106(2): p. 256–263. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Krieger N, Singh N, and Waterman PD, Metrics for monitoring cancer inequities: residential segregation, the Index of Concentration at the Extremes (ICE), and breast cancer estrogen receptor status (USA, 1992–2012). Cancer Causes & Control, 2016. 27(9): p. 1139–1151. [DOI] [PubMed] [Google Scholar]
- 56.Investigators, A.o.U.R.P., The “All of Us” research program. New England Journal of Medicine, 2019. 381(7): p. 668–676. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


