Abstract
Background
Transient tachypnoea of the newborn (TTN) is characterised by tachypnoea and signs of respiratory distress. It is caused by delayed clearance of lung fluid at birth. TTN typically appears within the first two hours of life in term and late preterm newborns. Although it is usually a self‐limited condition, admission to a neonatal unit is frequently required for monitoring, the provision of respiratory support, and drugs administration. These interventions might reduce respiratory distress during TTN and enhance the clearance of lung liquid. The goals are reducing the effort required to breathe, improving respiratory distress, and potentially shortening the duration of tachypnoea. However, these interventions might be associated with harm in the infant.
Objectives
The aim of this overview was to evaluate the benefits and harms of different interventions used in the management of TTN.
Methods
We searched the Cochrane Database of Systematic Reviews on 14 July 2021 for ongoing and published Cochrane Reviews on the management of TTN in term (> 37 weeks' gestation) or late preterm (34 to 36 weeks' gestation) infants. We included all published Cochrane Reviews assessing the following categories of interventions administered within the first 48 hours of life: beta‐agonists (e.g. salbutamol and epinephrine), corticosteroids, diuretics, fluid restriction, and non‐invasive respiratory support. The reviews compared the above‐mentioned interventions to placebo, no treatment, or other interventions for the management of TTN. The primary outcomes of this overview were duration of tachypnoea and the need for mechanical ventilation. Two overview authors independently checked the eligibility of the reviews retrieved by the search and extracted data from the included reviews using a predefined data extraction form. Any disagreements were resolved by discussion with a third overview author. Two overview authors independently assessed the methodological quality of the included reviews using the AMSTAR 2 (A MeaSurement Tool to Assess systematic Reviews) tool. We used the GRADE approach to assess the certainty of evidence for effects of interventions for TTN management. As all of the included reviews reported summary of findings tables, we extracted the information already available and re‐graded the certainty of evidence of the two primary outcomes to ensure a homogeneous assessment. We provided a narrative summary of the methods and results of each of the included reviews and summarised this information using tables and figures.
Main results
We included six Cochrane Reviews, corresponding to 1134 infants enrolled in 18 trials, on the management of TTN in term and late preterm infants, assessing salbutamol (seven trials), epinephrine (one trial), budesonide (one trial), diuretics (two trials), fluid restriction (four trials), and non‐invasive respiratory support (three trials). The quality of the included reviews was high, with all of them fulfilling the critical domains of the AMSTAR 2. The certainty of the evidence was very low for the primary outcomes, due to the imprecision of the estimates (few, small included studies) and unclear or high risk of bias.
Salbutamol may reduce the duration of tachypnoea compared to placebo (mean difference (MD) −16.83 hours, 95% confidence interval (CI) −22.42 to −11.23, 2 studies, 120 infants, low certainty evidence). We did not identify any review that compared epinephrine or corticosteroids to placebo and reported on the duration of tachypnoea. However, one review reported on "trend of normalisation of respiratory rate", a similar outcome, and found no differences between epinephrine and placebo (effect size not reported). The evidence is very uncertain regarding the effect of diuretics compared to placebo (MD −1.28 hours, 95% CI −13.0 to 10.45, 2 studies, 100 infants, very low certainty evidence). We did not identify any review that compared fluid restriction to standard fluid rates and reported on the duration of tachypnoea. The evidence is very uncertain regarding the effect of continuous positive airway pressure (CPAP) compared to free‐flow oxygen therapy (MD −21.1 hours, 95% CI −22.9 to −19.3, 1 study, 64 infants, very low certainty evidence); the effect of nasal high‐frequency (oscillation) ventilation (NHFV) compared to CPAP (MD −4.53 hours, 95% CI −5.64 to −3.42, 1 study, 40 infants, very low certainty evidence); and the effect of nasal intermittent positive pressure ventilation (NIPPV) compared to CPAP on duration of tachypnoea (MD 4.30 hours, 95% CI −19.14 to 27.74, 1 study, 40 infants, very low certainty evidence).
Regarding the need for mechanical ventilation, the evidence is very uncertain for the effect of salbutamol compared to placebo (risk ratio (RR) 0.60, 95% CI 0.13 to 2.86, risk difference (RD) 10 fewer, 95% CI 50 fewer to 30 more per 1000, 3 studies, 254 infants, very low certainty evidence); the effect of epinephrine compared to placebo (RR 0.67, 95% CI 0.08 to 5.88, RD 70 fewer, 95% CI 460 fewer to 320 more per 1000, 1 study, 20 infants, very low certainty evidence); and the effect of corticosteroids compared to placebo (RR 0.52, 95% CI 0.05 to 5.38, RD 40 fewer, 95% CI 170 fewer to 90 more per 1000, 1 study, 49 infants, very low certainty evidence). We did not identify a review that compared diuretics to placebo and reported on the need for mechanical ventilation. The evidence is very uncertain regarding the effect of fluid restriction compared to standard fluid administration (RR 0.73, 95% CI 0.24 to 2.23, RD 20 fewer, 95% CI 70 fewer to 40 more per 1000, 3 studies, 242 infants, very low certainty evidence); the effect of CPAP compared to free‐flow oxygen (RR 0.30, 95% CI 0.01 to 6.99, RD 30 fewer, 95% CI 120 fewer to 50 more per 1000, 1 study, 64 infants, very low certainty evidence); the effect of NIPPV compared to CPAP (RR 4.00, 95% CI 0.49 to 32.72, RD 150 more, 95% CI 50 fewer to 350 more per 1000, 1 study, 40 infants, very low certainty evidence); and the effect of NHFV versus CPAP (effect not estimable, 1 study, 40 infants, very low certainty evidence).
Regarding our secondary outcomes, duration of hospital stay was the only outcome reported in all of the included reviews. One trial on fluid restriction reported a lower duration of hospitalisation in the restricted‐fluids group, but with very low certainty of evidence. The evidence was very uncertain for the effects on secondary outcomes for the other five reviews. Data on potential harms were scarce, as all of the trials were underpowered to detect possible increases in adverse events such as pneumothorax, arrhythmias, and electrolyte imbalances. No adverse effects were reported for salbutamol; however, this medication is known to carry a risk of tachycardia, tremor, and hypokalaemia in other settings.
Authors' conclusions
This overview summarises the evidence from six Cochrane Reviews of randomised trials regarding the effects of postnatal interventions in the management of TTN. Salbutamol may reduce the duration of tachypnoea slightly. We are uncertain as to whether salbutamol reduces the need for mechanical ventilation. We are uncertain whether epinephrine, corticosteroids, diuretics, fluid restriction, or non‐invasive respiratory support reduces the duration of tachypnoea and the need for mechanical ventilation, due to the extremely limited evidence available. Data on harms were lacking.
Plain language summary
Treatments to manage rapid breathing in babies (transient tachypnoea of the newborn)
Review question
Do drugs and other treatments in babies with abnormally rapid breathing (known as transient tachypnoea of the newborn) improve lung function and reduce the need for breathing support (i.e. mechanical ventilation) and/or duration of symptoms?
Background
Transient tachypnoea of the newborn is characterised by rapid breathing (more than 60 breaths per minute) and signs of respiratory distress (difficulty in breathing). It typically appears within the first two hours of life in babies born at or after 34 weeks' of pregnancy. Although transient tachypnoea of the newborn usually improves without treatment, it might be associated with wheezing in late childhood. This Cochrane overview reported and critically analysed the available evidence on the benefit and harms of different treatments for the management of transient tachypnoea of the newborn.
Study characteristics
We included six Cochrane Reviews. Four of them compared drugs (salbutamol, epinephrine, corticosteroid, and diuretics) with placebo, whilst the remaining two reviews assessed the effects of giving a lesser quantity of fluids and breathing (respiratory) support without insertion of a tube into the lungs. Salbutamol, epinephrine, and corticosteroids remove the excess fluid from the lungs, whereas a diuretic is a drug that promotes the secretion of lung fluid in the urine.
The evidence is up‐to‐date as of July 2021.
Results
Due to the very limited available evidence, we could not answer the question of our overview. Salbutamol may reduce the duration of rapid breathing compared to placebo. The studies on epinephrine and corticosteroids did not provide information on this outcome. The evidence is very uncertain for the effect of diuretics compared to placebo. The studies on giving a lesser quantity of fluids did not provide information on this outcome.
The evidence is very uncertain regarding the effect of different types of respiratory support without the insertion of a tube into the lungs compared to oxygen or compared to each other on the duration of rapid breathing. We are uncertain about the effects of salbutamol, epinephrine, and corticosteroids in reducing the need for mechanical ventilation (use of a machine to help the patient breathe where a tube is inserted into the lungs). The studies on diuretics did not provide information on this outcome. We are uncertain about the effects of respiratory support without insertion of a tube into the lungs, and giving a lesser quantity of fluids in reducing the need for mechanical ventilation.
Certainty of evidence
The certainty of the evidence was low for salbutamol for duration of rapid breathing, and very low for all other outcomes and treatments. The studies either did not report information that we could use or produced findings in which we have very little confidence. These studies were small and used methods likely to introduce errors in their results.
Background
Description of the condition
Transient tachypnoea of the newborn (TTN) is a respiratory disorder that occurs in full‐term (≥ 37 weeks' gestation) or late preterm (34 to 36 weeks' gestation) infants. TTN consists of tachypnoea, defined as respiratory rate above 60/min, that is self‐limiting and usually lasts up to a couple of days. Although the diagnosis is based on clinical symptoms and signs of respiratory distress (chest wall retractions, flaring of nostrils, grunting), chest X‐ray may support the differential diagnosis (Edwards 2013). TTN may be confused with the early stages of other, more significant respiratory conditions such as early‐onset pneumonia or respiratory distress syndrome, and to some extent is a diagnosis of exclusion.
TTN is caused by inadequate clearance of lung fluid after the transition from living in the womb to breathing air (Avery 1966; McGillick 2017). The pathophysiology may involve a dysregulation in the change from a secretive to an absorptive function of the lungs. TTN is associated with factors that hasten this transition, such as elective caesarean section and fast delivery (Cohen 1985; Hansen 2008). Risk factors include macrosomia, maternal diabetes, twin pregnancy, and family history of asthma (Edwards 2013; Liem 2007). TTN is the most common cause of respiratory distress amongst infants (Clark 2005), with an overall incidence around 0.5% to 2.8%, which may reach up to 30% in term infants delivered by elective caesarean section with its inherent lack of initiation of labour and subsequent changes in reabsorptive processes in the lungs (Hansen 2008; Hibbard 2010; Kumar 1996; Morrison 1995). Respiratory distress is a common reason for admission of term infants to neonatal units, and TTN accounts for approximately 10% of all the admissions of term infants to neonatal units (Edwards 2013).
Although TTN is a self‐limiting condition that usually leaves no sequelae, it may interfere with enteral feeding, require testing and close monitoring along with oxygen therapy, and be a cause of great concern for parents (Hack 1976). Studies have shown an association between TTN and asthma, bronchiolitis, and other wheezing syndromes later in life (Liem 2007), as well as persistent pulmonary hypertension in rare cases (Miller 1980; Tudehope 1979).
Description of the interventions
Beta‐agonists
β‐agonists are drugs that activate β‐adrenergic receptors. These receptors play a vital role in the switch from secretion to absorption that takes place in the foetal lung epithelium at birth. The surge of endogenous catecholamines during labour leads to lung fluid resorption through increased expression of epithelial sodium channels and increased activity of sodium‐potassium adenosine triphosphatase (Na+/K+–ATPase) (Barker 2002).
This is consistent with the observation that TTN is more common with caesarean sections, which might not induce this rise of adrenaline in the foetal circulation (Irestedt 1982). Given that deficiency of catecholamines is one of the causes of TTN, exogenous β‐agonists such as salbutamol, epinephrine, and norepinephrine may serve as potentially effective treatments (Hansen 2008). β‐agonists have been shown to reduce lung fluid (Perkins 2006; Sakuma 1997; Sartori 2002). These drugs are usually administered as inhalation therapy and intravenous injection/infusion.
β‐agonists are associated with adverse effects such as tachycardia, other arrhythmias, and vasoconstriction leading to hypertension. Dosage, type of drug, and route of administration (inhalation, intravenous, oral) are factors that might affect these risks; for instance, epinephrine seems to have greater effects on systemic circulation as compared with salbutamol (Becker 1983).
Postnatal corticosteroids
Plasma cortisol is important for the development of correct epithelial sodium channel (ENaC) expression in the lung epithelia (Barker 2002), and exogenous corticosteroids might accelerate lung maturity when this process does not develop naturally.
Antenatal administration of corticosteroids reduces the risk of TTN in near‐term foetuses (Saccone 2016). This treatment has also been shown to reduce the risk of respiratory distress (Roberts 2017). However, the harms of postnatal corticosteroid treatment may outweigh the benefits given that TTN is such a benign disorder. Moreover, a large proportion of women would be exposed to this prophylactic measure when only a few of them would have a newborn with TTN, thus leading to overtreatment (Saccone 2016).
Many types of corticosteroids, such as betamethasone, hydrocortisone, budesonide, and dexamethasone, may be used. Routes of administration include inhalation, infusion, injection, and dermal and enteral routes.
Possible harms associated with corticosteroid treatment include neonatal hypoglycaemia, gastrointestinal bleeding, infection, and impaired neurological development, although evidence for the last is limited to studies in very preterm infants (Linsell 2016; Saccone 2016; Stark 2001).
Diuretics
Diuretics are drugs that affect fluid regulation, usually through inhibition of electrolyte reabsorption in the kidney, which leads to increased loss of salts and fluids. Diuretics that have been used to treat patients with TTN include furosemide and bumetanide, both of which inhibit chlorine/sodium channels in the looping nephron.
Furosemide has been shown to be an effective treatment for other pathologies involving lung fluids in adults, such as lung oedema after myocardial infarction (Biddle 1979). In neonatology, diuretics are often used for management of chronic lung disease in newborns who are born extremely preterm, although this approach is not supported by appropriate evidence. Besides providing a diuretic effect, furosemide has been shown to confer beneficial direct effects in the newborn lung, although this benefit appears to be temporary (Bland 1978).
Adverse effects associated with furosemide include dehydration, hypotension, hypochloraemia, hypokalaemia, hyponatraemia, and kidney injury (Spino 1978). Toxicity with chronic furosemide in newborns has been reported, including risk for the development of intrarenal calcifications in infants with low birth weight (Pacifici 2013).
Fluid restriction
Restricting the amount of fluid available to the newborn seems a reasonable, feasible, and cheap intervention, as TTN is caused by an increased amount of fluids in the lungs. Mild fluid restriction has been reported to be safe for term and late preterm neonates with TTN (Dehdashtian 2014; Stroustrup 2012).
Fluid intake of the infant is calculated as a combination of intravenous and enteral fluid intake. Normally, babies receive about 60 to 80 mL/kg/d of fluids on the first day of life, and on each day following that day, the amount is increased by about 20 mL until a dosage of about 150 mL/kg/d is reached. Fluid restriction as practised in the cited study means that the infant receives 40 to 60 mL/kg/d at first, and after that, the same ratio of increase is applied (Stroustrup 2012).
Adverse effects associated with fluid restriction include electrolyte imbalance, dehydration, and jaundice (Stroustrup 2012). Fluid restriction might limit the possibility of breastfeeding or parenteral nutrition, which can cause stress for both parents and healthcare personnel and discomfort for the baby.
Non‐invasive respiratory support
Non‐invasive respiratory support includes any respiratory support with no endotracheal or tracheostomy tube. This approach decreases the risk of damage to larynx and trachea, preserves newborn laryngeal function (adduction of the vocal cords during expiration), and may reduce the risk of nosocomial infection. Non‐invasive respiratory support is used to decrease respiratory distress and discomfort in the tachypnoeic infant (Alexiou 2016; Cordero 1997).
The type of respiratory support used depends mainly on the severity of TTN and on the availability of equipment in the neonatal unit. Modes of ventilation include:
high‐flow nasal cannula (HFNC), a nasal cannula that provides heated and humidified gas at a flow of 1 litre/min or higher (Wilkinson 2016);
continuous positive airway pressure (CPAP), a system that delivers continuous positive pressure that prevents collapse of respiratory ducts and alveoli. It can be used with a variety of systems, including face mask, nasal mask, or prongs (De Paoli 2003);
nasal intermittent positive‐pressure ventilation (NIPPV), a system by which nasal mask or prongs are used for delivery. It is similar to CPAP, but adds on inflations to a peak pressure. Some machines can synchronise, to some extent, with the patient's own attempts at breathing (Lemyre 2017); and
nasal high‐frequency (oscillation) ventilation (NHFV), a newer form of respiratory support that has been suggested to be effective for quicker clearing of carbon dioxide (Fischer 2015).
Long exposure to positive pressures can lead to barotrauma and volume injury, which are seen primarily in preterm infants (Zielinska 2014). However, the lungs of late preterm and full‐term infants are likely to be less vulnerable.
How the intervention might work
Given that the main issue in TTN is the delayed clearance of lung fluid by the interstitial tissue, the aim of most treatments is to remove this excess fluid from the alveoli. Drugs such as catecholamines and corticosteroids facilitate the natural absorption of lung fluid by increasing the number of epithelial sodium channels in the alveolar cells. Diuretics eliminate salt via urine and therefore lead to more osmolar blood, which in turn draws water from the interstitium into the vascular system. Diuretics such as furosemide have also been shown to have a local effect in improving pulmonary dynamics directly. Fluid restriction seems to be the most straightforward approach and can help with fluid regulation through increased clearance of free water from the lung. Respiratory support is seen as a way to support the child through a period of impaired breathing and as a triggering event by which to clear fluid from the lungs. Animal studies suggest that oxygen itself might lead to increased clearance of lung fluid through increased expression of certain membrane channels (Barker 2002). Oxygen is provided mainly to support the infant whilst lung fluid is cleared via normal physiological mechanisms.
Why it is important to do this overview
Although TTN usually resolves within 48 hours, the infant may benefit from neonatal care with respiratory support provided during this time. This intensive care might be traumatic for parents, can limit parent‐child bonding, and might delay the first breastfeeding (Dehdashtian 2018). In addition, unnecessary and ineffective treatments for TTN could cause harm through adverse effects. Neonatal care with respiratory support is resource and personnel intensive. Shortening of treatment time in TTN can save resources, especially nowadays, when caesarean sections are becoming more common in some countries (Stroustrup 2012).
Several factors are associated with an increased incidence of TTN, including caesarean section, macrosomia (birth weight greater than two standard deviations for gestational age), maternal diabetes, family history of asthma, and twin pregnancy (Hansen 2008). Because these prenatal risk factors are widespread, most cases of TTN occur in level 1 neonatal units, where resources for immediate respiratory support may be suboptimal and expertise for its use might be lower. It would therefore be advantageous to identify effective and safe interventions that can be applied in this setting, which would improve the management of TTN and subsequently reduce the need for intensive care with or without transfer to a level 3 neonatal intensive care unit.
Objectives
The aim of this overview was to evaluate the benefits and harms of different interventions used in the management of TTN.
Methods
Criteria for considering reviews for inclusion
Types of studies
We included all published Cochrane Reviews on the management of TTN.
Types of participants
We included Cochrane Reviews evaluating infants who were born at term (> 37 weeks' gestation) or late preterm (34 to 36 weeks' gestation).
TTN is usually defined as a respiratory rate above 60 breaths/min, and might have characteristics of respiratory distress such as grunting, flaring, and chest wall retraction. Other causes of respiratory distress, such as pneumonia and pneumothorax, must not be present (Reuter 2014). We also included reviews mentioning slightly different definitions of TTN if it is clear that the studies were describing the same condition.
Types of interventions
We assessed the following categories of interventions: β‐agonists (e.g. salbutamol and epinephrine), corticosteroids, diuretics, fluid restriction, and non‐invasive respiratory support (e.g. CPAP, NHFV, NIPPV) (see Description of the interventions).
Interventions must have been started within the first 48 hours of life, as onset of TTN occurs within the very first hours of life.
Given that this is an overview of systematic reviews and not a review of primary studies, we expected there to be a large number of possible comparisons amongst these interventions. We thus did not specify in advance the comparisons to be included. After the analysis of the retrieved reviews, we compared the above‐mentioned interventions to:
placebo;
no treatment/intervention; or
other interventions for the management of TTN.
Types of outcome measures
Primary outcomes
Duration (hours) of tachypnoea.
Need for mechanical ventilation (yes/no).
Secondary outcomes
Time (hours) to established breastfeeding or bottle‐feeding.
Duration (hours) of hospital stay.
Clinical assessment of respiratory distress as quantified by Silverman or Downes score > 6 (indicative of impending respiratory failure) (yes/no) 24 and 48 hours after study entry (Silverman 1956; Wood 1972).
Neonatal mortality (within 28 days of life).
Any adverse events as reported in the included reviews, such as: pneumothorax (for non‐invasive respiratory support), tachycardia (for epinephrine and salbutamol), hyperglycaemia (for corticosteroids), gastrointestinal bleed (for corticosteroids), dehydration (for fluid restriction and diuretics), or electrolyte disturbances (for fluid restriction, β‐agonists, and diuretics).
Search methods for identification of reviews
We searched the Cochrane Database of Systematic Reviews (2021, Issue 7) in the Cochrane Library on 14 July 2021 for ongoing and published Cochrane Reviews on the management of TTN (see Appendix 1).
Data collection and analysis
We followed the methods reported in Chapter 22 of the Cochrane Handbook for Systematic Reviews of Interventions (Higgins 2019). Cochrane overviews of reviews do not aim to repeat the searches, assessment of eligibility, assessment of risk of bias, or meta‐analyses from the included reviews. We therefore summarised evidence from the included systematic reviews of the effects of interventions.
Selection of reviews
Two overview authors (MB and OR) independently checked the eligibility of the reviews retrieved by the search and confirmed their inclusion. Any disagreements were resolved through discussion.
Data extraction and management
Two overview authors (KOH and RB) independently extracted data from the included reviews using a predefined data extraction form. Any disagreements were resolved through discussion with a third overview author (MGC).
We extracted the following information from each review.
Review title and authors.
Objective and research question.
Date of publication.
Number of included trials.
Number of participants.
Interventions and comparisons.
Outcomes.
Effect measurements for the primary and secondary outcomes of this overview.
GRADE tables by which to judge the certainty of the evidence.
Strengths and limitations of the included reviews.
We analysed reports of the studies included in each review to retrieve details (e.g. gestational age) needed for this overview only if they were not available from the included systematic reviews.
Assessment of methodological quality of included reviews
Two overview authors (KOH and RB) independently assessed the methodological quality of the included reviews using the AMSTAR 2 (A MeaSurement Tool to Assess systematic Reviews) measurement tool (Shea 2017). This instrument has good inter‐rater agreement, test‐retest reliability, and face and construct validity (Shea 2017). Specifically, the tool addresses the following questions.
Did the research questions and inclusion criteria for the review include the components of PICO (Population, Intervention, Comparison, Outcome)?
Did the report of the review contain an explicit statement that review methods were established before conduct of the review?
Did the report justify any significant deviations from the protocol?*
Did review authors explain their selection of study designs for inclusion in the review?
Did review authors use a comprehensive literature search strategy?*
Did review authors perform study selection in duplicate?
Did review authors perform data extraction in duplicate?
Did review authors provide a list of excluded studies and justify exclusions?*
Did review authors describe the included studies in adequate detail?
Did review authors use a satisfactory technique for assessing risk of bias (RoB) in individual studies that were included in the review?*
Did review authors report on sources of funding for studies included in the review?
If meta‐analysis was performed, did review authors use appropriate methods for statistical combination of results?*
If meta‐analysis was performed, did review authors assess the potential impact of RoB of individual studies on results of the meta‐analysis or other evidence synthesis?
Did review authors account for RoB in individual studies when interpreting/discussing results of the review?*
Did review authors provide a satisfactory explanation for, and discussion of, any heterogeneity observed in results of the review?
If they performed quantitative synthesis, did review authors carry out an adequate investigation of publication bias (small‐study bias) and discuss its likely impact on the results of the review?*
Did review authors report any potential conflicts of interest, including any funding received for conducting the review?
Possible responses to each question are 'yes' and 'no'. A 'partial yes' response is applicable in some instances (Shea 2017). We provided a rationale for judgements for each AMSTAR item. Seven of 16 domains (marked with an * in the list above) are defined as critical because they can 'critically' affect the validity of a review. We did not report a summary score, as recommended by the developers of AMSTAR 2 (Shea 2017), but considered at higher methodological quality only those reviews fulfilling all the seven critical domains. However, we planned to consider the potential impact of an inadequate rating for each item.
Certainty of the body of evidence in included reviews
We assessed the certainty of evidence for the effects of interventions for TTN management using the GRADE approach, which considers the following criteria: study limitations (i.e. risk of bias), consistency of effect, imprecision, indirectness, and publication bias (Guyatt 2011). We planned to prepare de novo summary of findings tables for primary outcomes when they were not available in the included reviews, or if the review PICO did not fully match that of the overview. As all of the included reviews reported the summary of findings tables, we extracted the information already available and re‐graded the certainty of evidence for the two primary outcomes to ensure a homogeneous assessment. We planned to discuss potential discrepancies with original reviews.
Data synthesis
We provided a narrative summary of the methods and results of each of the included reviews and summarised this information using tables and figures (e.g. characteristics of included reviews, summary of quality of evidence within individual systematic reviews, AMSTAR 2 evaluation).
For the overview's primary and secondary outcomes, we extracted the effect estimates and 95% confidence intervals (CIs) from the meta‐analyses conducted by authors of the systematic reviews. In particular, we reported mean differences (MD) for the outcome duration of tachypnoea, and risk ratio (RR) and risk difference (RD) for the outcome need for mechanical ventilation.
A table on outcomes shows comparisons, numbers of participants and studies, measures of effect with 95% CIs, I², and certainty of evidence (GRADE). We classified interventions that are effective for the primary outcomes of this review and those that are not, according to effect estimates and 95% CIs as reported in the meta‐analyses conducted by authors of the systematic reviews. We summarised data on primary outcomes in summary of findings tables, as described in Chapter 11 of the Cochrane Handbook for Systematic Reviews of Interventions (Higgins 2019).
We did not pool data derived from different reviews in meta‐analyses, as we expected substantial heterogeneity, and we did not draw inferences about the comparative effectiveness of interventions considered by different reviews (i.e. avoid any ranking that would require network meta‐analysis). However, for future updates, if data permit, we may perform indirect comparisons of interventions across reviews for the primary outcomes.
We planned to report on the following subgroups.
Birth weight: ≤ 2500 g or > 2500 g.
Gestational age: term (> 37 weeks' gestation) or late preterm (34 to 36 weeks' gestation).
Antenatal steroid exposure.
However, the low number of included studies precluded these subgroup analyses.
Results
Description of included reviews
Our search (July 2021) identified eight Cochrane Reviews and three Cochrane Review protocols (Figure 1). We included six completed Cochrane Reviews on the management of TTN in term and late preterm infants in the overview (Bruschettini 2020; Gupta 2021; Kassab 2015; Moresco 2016; Moresco 2020; Moresco 2021). We excluded two Cochrane Reviews because they assessed antenatal interventions. We excluded three Cochrane Review protocols for the following reasons: 1) assessed a condition other than TTN; 2) the Cochrane Review protocol has been withdrawn; and 3) the Cochrane Review protocol was the protocol for this overview.
1.
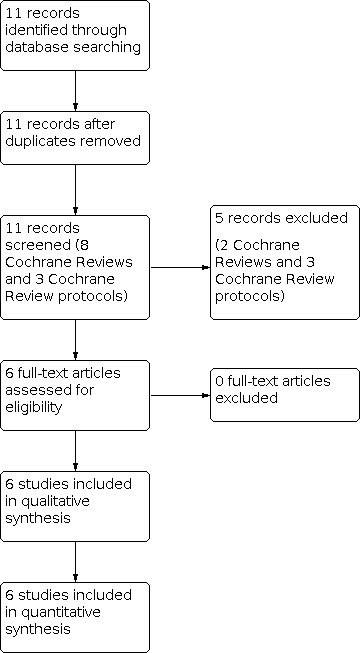
Study flow diagram.
Moresco 2021 included seven trials on the use of salbutamol as an intervention for TTN. These trials included a total of 498 newborns. They looked at nebulised salbutamol as compared to no treatment, placebo, or other treatments.
Moresco 2016 looked at epinephrine as a potential intervention against TTN, including only one trial (20 infants), which investigated nebulised epinephrine versus placebo.
Bruschettini 2020 included one study with 49 infants. They looked at the effect and safety of inhaled budesonide (a type of corticosteroids) in the clinical situation.
Kassab 2015 sought to determine if the use of diuretics could shorten hospital stay and length of oxygen therapy. They included two trials with a total of 100 participants.
Gupta 2021 included four studies with 317 participants. The author aimed to compare different rates of fluid restriction versus standard rate as treatment for TTN.
Moresco 2020 aimed to compare different types of respiratory support with no treatment/free‐flow oxygen, and when possible to also assess these different types of respiratory support in head‐to‐head comparisons. They included three studies, reaching a total of 150 infants.
The main characteristics of the included reviews are presented in Table 1.
1. Characteristics of included reviews.
| Numberofincludedtrials | Totalnumberofparticipants | Interventionsandcomparisons | Outcomes‐primary | Outcomes‐secondary | |
| Salbutamolfortransienttachypnoeaofthenewborn (Moresco 2021) | 7 (Armangil 2011; Babaei 2019; Kim 2014; Malakian 2018; Mohammadzadeh 2017; Monzoy‐Ventre 2015; Mussavi 2017) |
498 infants | Salbutamol compared to placebo or no treatment or other treatment by any route of administration |
|
|
| Epinephrinefortransienttachypnoeaofthenewborn (Moresco 2016) | 1 (Kao 2008) |
20 infants | Epinephrine compared to placebo or no treatment or any other drugs in the first 3 days of life, except salbutamol, by any route of administration |
|
|
| Postnatalcorticosteroidsfortransienttachypnoeaofthenewborn (Bruschettini 2020) | 1 (Vaisbourd 2017) |
49 infants | Corticosteroids compared to no treatment or placebo, head‐to‐head comparison of different corticosteroids, both inhaled and systemic |
|
|
| Diureticsfortransienttachypnoeaofthenewborn (Kassab 2015) | 2 (Karabayir 2006; Wiswell 1985) |
100 infants | Any diuretics compared with placebo or no therapy during the first week of life |
|
|
| Fluidrestrictioninthemanagementoftransienttachypnoeaofthenewborn (Gupta 2021) | 4 (Akbarian Rad 2018; Eghbalian 2018; Sardar 2020; Stroustrup 2012) |
317 infants | Restricted fluid therapy as defined by less than 90% of standard amount for at least 24 hours, by any route (oral, intravenous) compared to standard fluid therapy |
|
|
| Non‐invasiverespiratorysupport in themanagementoftransienttachypnoeaofthenewborn (Moresco 2020) | 3 (Demirel 2013; Dumas 2011; Osman 2019) |
150 infants | Any non‐invasive respiratory support versus supplemental oxygen or no treatment, as well as any non‐invasive respiratory support versus other types of non‐invasive respiratory support |
|
|
bpm: beats per minute; CPAP: continuous positive airway pressure; IQ: intelligence quotient; SD: standard deviation
Methodological quality of included reviews
The AMSTAR 2 assessment of the quality of the included reviews is presented in Table 2. Overall, the certainty of the included reviews was high, with all of them fulfilling the critical domains of the AMSTAR 2.
2. Quality of the included systematic reviews (AMSTAR 2).
| AMSTAR2question | Investigated review | |||||
|
Salbutamol (Moresco 2021) |
Epinephrine (Moresco 2016) |
Corticosteroids (Bruschettini 2020) |
Diuretics (Kassab 2015) |
Fluidrestriction (Gupta 2021) |
Non‐invasiverespiratorysupport (Moresco 2020) | |
| 1. Did the research questions and inclusion criteria for the review include the components of PICO? | Yes | Yes | Yes | Yes | Yes | Yes |
| 2. Did the report of the review contain an explicit statement that the review methods were established prior to the conduct of the review, and did the report justify any significant deviations from the protocol?* | Yes | Yes | Yes | Yes | Yes | Yes |
| 3. Did review authors explain their selection of the study designs for inclusion in the review? | No | No | No | No | No | No |
| 4. Did review authors use a comprehensive literature search strategy?* | Yes | Yes | Yes | Yes | Yes | Yes |
| 5. Did review authors perform study selection in duplicate? | Yes | Yes | Yes | Yes | Yes | Yes |
| 6. Did review authors perform data extraction in duplicate? | Yes | Yes | Yes | Yes | Yes | Yes |
| 7. Did review authors provide a list of excluded studies and justify the exclusions?* | No (none of the other identified studies was potentially eligible) | No (none of the other identified studies was potentially eligible) | No (none of the other identified studies was potentially eligible) | No (none of the other identified studies was potentially eligible) | Yes | No (none of the other identified studies was potentially eligible) |
| 8. Did review authors describe the included studies in adequate detail? | Yes | Yes | Yes | Yes | Yes | Yes |
| 9. Did review authors use a satisfactory technique for assessing the risk of bias in individual studies that were included in the review?* | Yes | Yes | Yes | Yes | Yes | Yes |
| 10. Did review authors report on the sources of funding for the studies included in the review? | No | No | No | No | No | No |
| 11. If meta‐analysis was performed, did review authors use appropriate methods for statistical combination of results?* | Yes | Meta‐analysis was not performed. | Meta‐analysis was not performed. | Yes | Yes | Meta‐analysis was not performed. |
| 12. If meta‐analysis was performed, did review authors assess the potential impact of risk of bias in individual studies on the results of the meta‐analysis or other evidence synthesis? | Yes | Meta‐analysis was not performed. | Meta‐analysis was not performed. | Yes | Yes | Meta‐analysis was not performed. |
| 13. Did review authors account for risk of bias in individual studies when interpreting/discussing the results of the review?* | Yes | Yes | Yes | Yes | Yes | Yes |
| 14. Did review authors provide a satisfactory explanation for, and discussion of, any heterogeneity observed in the results of the review? | Yes | Meta‐analysis was not performed. | Meta‐analysis was not performed. | Yes | Yes | Meta‐analysis was not performed. |
| 15. If they performed quantitative synthesis, did review authors carry out an adequate investigation of publication bias (small‐study bias) and discuss its likely impact on the results of the review?* | Funnel plot was not performed (fewer than 10 trials included). | Not applicable | Not applicable | Funnel plot was not performed (fewer than 10 trials included). | Funnel plot was not performed (fewer than 10 trials included). | Not applicable |
| 16. Did review authors report any potential conflicts of interest, including any funding they received for conducting the review? | Yes | Yes | Yes | Yes | Yes | Yes |
Risk of bias in the included trials is assessed in Table 3. The certainty of the evidence for the primary outcomes of this overview is summarised in Table 4.
3. Risk of bias of studies included in the reviews.
| Review | Primary studies in the review | Risk of bias domains | ||||||
| Randomsequencegeneration (selection bias) | Allocationconcealment(selectionbias) |
Blindingofparticipantsandpersonnel(performancebias) Alloutcomes |
Blindingofoutcomeassessment(detectionbias) Alloutcomes |
Incompleteoutcomedata(attritionbias) Alloutcomes |
Selectivereporting(reportingbias) | Otherbias | ||
| Salbutamolfortransienttachypnoeaofthenewborn (Moresco 2021) | Armangil 2011 | Highrisk | Unclear risk | Low risk | Low risk | Unclear risk | Unclear risk | Low risk |
| Babaei 2019 | Low risk | Unclear risk | Unclear risk | Unclear risk | Low risk | Unclear risk | Low risk | |
| Kim 2014 | Highrisk | Unclear risk | Low risk | Unclear risk | Low risk | Unclear risk | Low risk | |
| Malakian 2018 | Low risk | Unclear risk | Low risk | Low risk | Low risk | Highrisk | Low risk | |
| Mohammadzadeh 2017 | Unclear risk | Unclear risk | Low risk | Unclear risk | Low risk | Low risk | Low risk | |
| Monzoy‐Ventre 2015 | Unclear risk | Unclear risk | Unclear risk | Unclear risk | Low risk | Unclear risk | Low risk | |
| Mussavi 2017 | Low risk | Unclear risk | Low risk | Unclear risk | Low risk | Highrisk | Low risk | |
| Epinephrinefortransienttachypnoeaofthenewborn (Moresco 2016) | Kao 2008 | Unclear risk | Unclear risk | Low risk | Unclear risk | Low risk | Unclear risk | Low risk |
| Postnatalcorticosteroidsfortransienttachypnoeaofthenewborn (Bruschettini 2020) | Vaisbourd 2017 | Unclear risk | Low risk | Low risk | Low risk | Low risk | Low risk | Low risk |
| Diureticsfortransienttachypnoeaofthenewborn (Kassab 2015) | Karabayir 2006 | Unclear risk | Unclear risk | Low risk | Low risk | Highrisk | Unclear risk | Unclear risk |
| Wiswell 1985 | Low risk | Low risk | Unclear risk | Unclear risk | Unclear risk | Unclear risk | Unclear risk | |
| Fluidrestrictioninthemanagementoftransienttachypnoeaofthenewborn (Gupta 2021) | Akbarian Rad 2018 | Highrisk | Highrisk | Highrisk | Low risk | Low risk | Low risk | Low risk |
| Eghbalian 2018 | Low risk | Unclear risk | Low risk | Unclear risk | Low risk | Low risk | Low risk | |
| Sardar 2020 | Low risk | Low risk | Highrisk | Highrisk | Low risk | Low risk | Low risk | |
| Stroustrup 2012 | Highrisk | Highrisk | Highrisk | Highrisk | Low risk | Low risk | Low risk | |
| Non‐invasiverespiratorysupportinthemanagementoftransienttachypnoeaofthenewborn (Moresco 2020) | Demirel 2013 | Low risk | Low risk | Highrisk | Unclear risk | Low risk | Low risk | Low risk |
| Dumas 2011 | Low risk | Low risk | Highrisk | Unclear risk | Low risk | Low risk | Low risk | |
| Osman 2019 | Low risk | Unclear risk | Highrisk | Unclear risk | Low risk | Highrisk | Low risk | |
4. Summary of findings table (by outcome).
| InterventionsforthemanagementofTTN | |||||||
|
Patientorpopulation: term and late preterm infants with TTN Interventions: salbutamol, epinephrine, postnatal corticosteroids, diuretics, non‐invasive respiratory support Comparison: no intervention or placebo for all outcomes except fluid restriction (standard fluid rates) and non‐invasive respiratory support (either free‐flow oxygen or NCPAP) (see below) | |||||||
| Outcomes | Interventions | Anticipatedabsoluteeffects* (95% CI) | Relative effect (95% CI) | № of participants (studies) | Certainty of the evidence (GRADE) | Comments | |
| Riskwithnotreatment | Riskwithtreatment | ||||||
| Duration of tachypnoea | Salbutamol | Range in the control arm was 31 to 34 hours. | Mean duration in the intervention arm was 16.83 hours less (22.42 less to 11.23 less). | MD −16.83 (−22.42 to −11.23) | 120 (2 RCTs) | ⨁ ⨁ ◯◯ LOW 1 | Salbutamol may reduce the duration of tachypnoea compared to placebo. |
| Epinephrine | ‐ | ‐ | ‐ | ‐ | ‐ | The review that compared epinephrine to placebo did not report on the duration of tachypnoea. However, the review reported on "trend of normalisation of respiratory rate", a similar outcome, and found no differences between epinephrine and placebo. | |
| Corticosteroids | ‐ | ‐ | ‐ | ‐ | ‐ | The review that compared corticosteroids to placebo did not report on the duration of tachypnoea. | |
| Diuretics | Range in the control arm was 51 to 57 hours. |
Mean duration in the intervention arm was 1.28 hour less (13.00 less to 10.45 more). | MD −1.28 (−13.0 to 10.45) | 100 (2 RCTs) | ⨁◯◯◯ VERY LOW 2 | The evidence is very uncertain regarding the effect of diuretics on duration of tachypnoea compared to placebo. | |
| Fluidrestriction | ‐ | ‐ | ‐ | ‐ | ‐ | The review that compared fluid restriction to standard fluid rates did not report on the duration of tachypnoea. | |
|
Non‐invasiverespiratorysupport (CPAP vs free‐flow oxygen) |
Mean duration in the control arm was 9 hours. | Mean duration in the intervention arm was 21.1 hours less (22.9 less to 19.3 less). | MD −21.1 (−22.9 to −19.3) | 64 (1 RCT) | ⨁◯◯◯ VERY LOW 3 | The evidence is very uncertain regarding the effect of CPAP on duration of tachypnoea compared to free‐flow oxygen. | |
|
Non‐invasiverespiratorysupport (NHFV vs CPAP) |
Mean duration in the control arm was 2 hours. |
Mean duration in the intervention arm was 4.53 hours less (5.64 less to 3.42 more). | MD −4.53 (−5.64 to ‐3.42) | 40 (1 RCT) | ⨁◯◯◯ VERY LOW 2 | The evidence is very uncertain regarding the effect of NHFV on duration of tachypnoea compared to CPAP. | |
|
Non‐invasiverespiratorysupport (NIPPV vs CPAP) |
Mean duration in the control arm was 68 hours. |
Mean duration in the intervention arm was 4 more (19 less to 28 more). | MD 4.30 (−19.14 to 27.74) | 40 (1 RCT) | ⨁◯◯◯ VERY LOW 2 | The evidence is very uncertain regarding the effect of NIPPV on duration of tachypnoea compared to CPAP. | |
| Need for mechanical ventilation | Salbutamol | 25 per 1000 | 15 per 1000 |
RR 0.60 (0.13 to 2.86), RD 10 fewer (50 fewer to 30 more per 1000) |
254 (3 RCTs) |
⨁◯◯◯ VERY LOW 2 | The evidence is very uncertain regarding the effect of salbutamol on need for mechanical ventilation compared to placebo. |
| Epinephrine | 200 per 1000 | 133 per 1000 |
RR 0.67 (0.08 to 5.88), RD 70 fewer (460 fewer to 320 more per 1000) |
20 (1 RCT) | ⨁◯◯◯ VERY LOW 2 | The evidence is very uncertain regarding the effect of epinephrine on need for mechanical ventilation compared to placebo. | |
| Corticosteroids | 80 per 1000 | 42 per 1000 |
RR 0.52 (0.05 to 5.38), RD 40 fewer (170 fewer to 90 more per 1000) |
49 (1 RCT) | ⨁◯◯◯ VERY LOW 2 | The evidence is very uncertain regarding the effect of corticosteroids on need for mechanical ventilation compared to placebo. | |
| Diuretics | ‐ | ‐ | ‐ | ‐ | ‐ | The review that compared diuretics to placebo did not report on the need for mechanical ventilation. | |
| Fluidrestriction | 57 per 1000 | 42 per 1000 |
RR 0.73 (0.24 to 2.23), RD 20 fewer (70 fewer to 40 more per 1000) |
242 (3 RCTs) | ⨁◯◯◯ VERY LOW 2 | The evidence is very uncertain regarding the effect of fluid restriction on need for mechanical ventilation compared to standard fluid rates. | |
|
Non‐invasiverespiratorysupport (CPAP vs free‐flow oxygen) |
33 per 1000 | 0 events in treatment group (n = 34) |
RR 0.30 (0.01 to 6.99), RD 30 fewer (120 fewer to 50 more per 1000) |
64 (1 RCT) | ⨁◯◯◯ VERY LOW 2 | The evidence is very uncertain regarding the effect of CPAP on need for mechanical ventilation compared to free‐flow oxygen. | |
|
Non‐invasiverespiratorysupport (NIPPV vs CPAP) |
50 per 1000 | 200 per 1000 |
RR 4.00 (0.49 to 32.72), RD 150 more (50 fewer to 350 more per 1000) |
40 (1 RCT) | ⨁◯◯◯ VERY LOW 2 | The evidence is very uncertain regarding the effect of NIPPV on need for mechanical ventilation compared to CPAP. | |
| Non‐invasiverespiratorysupport (NHFV vs CPAP) | 0 events in control group (n = 20) | 0 events in treatment group (n = 20) | Not estimable | 40 (1 RCT) | ⨁◯◯◯ VERY LOW 2 | The evidence is very uncertain regarding the effect of NHFV on need for mechanical ventilation compared to CPAP. | |
| CI: confidence interval; CPAP: continuous positive airway pressure; MD: mean difference; NCPAP: nasal continuous positive airway pressure; NHFV: nasal high frequency ventilation; NIPPV: nasal intermittent positive pressure ventilation; RCT: randomised controlled trial; RD: risk difference; RR: risk ratio; TTN: transient tachypnoea of the newborn | |||||||
|
GRADEWorkingGroupgradesofevidence Highcertainty: We are very confident that the true effect lies close to that of the estimate of the effect. Moderatecertainty: We are moderately confident in the effect estimate: the true effect is likely to be close to the estimate of the effect, but there is a possibility that it is substantially different. Lowcertainty: Our confidence in the effect estimate is limited: the true effect may be substantially different from the estimate of the effect. Verylowcertainty: We have very little confidence in the effect estimate: the true effect is likely to be substantially different from the estimate of effect. | |||||||
1Downgraded by one level for serious imprecision (due to low sample size) and one level for study limitations (risk of bias). 2Downgraded by two levels for very serious imprecision (due to low sample size and wide confidence intervals) and one level for serious study limitations (risk of bias). 3Downgraded by two levels for very serious imprecision (due to low sample size; only one small study) and one level for serious study limitations (risk of bias).
Effect of interventions
The comparator was placebo in the reviews on salbutamol, epinephrine, corticosteroids, and diuretics (Bruschettini 2020; Kassab 2015; Moresco 2016; Moresco 2021), and standard fluid rate in the review on fluid restriction (Gupta 2021). In the review on respiratory support, the comparator was either free‐flow oxygen or CPAP (Moresco 2020).
Primary outcomes
Duration of tachypnoea (hours)
Salbutamol: compared to placebo, salbutamol may reduce the duration of tachypnoea (mean difference (MD) −16.83 hours, 95% confidence interval (CI) −22.42 to −11.23, 2 studies, 120 infants (Babaei 2019; Kim 2014)) in the Moresco 2021 review. The certainty of the evidence was low, downgraded by one level for imprecision (due to low sample size) and one level for study limitations (risk of bias).
Epinephrine: the Moresco 2016 review investigated epinephrine, but this outcome was not reported in the included trial (Kao 2008). However, the review reported on "trend of normalisation of respiratory rate", a similar outcome, and found no differences between epinephrine and placebo (effect size not reported, only P > 0.75).
Corticosteroids: the review and the included trial did not report on this outcome (Bruschettini 2020).
Diuretics: the evidence regarding the effect of diuretics is very uncertain (MD −1.28 hours, 95% CI −13.0 to 10.45, 2 studies, 100 infants (Kassab 2015)). The certainty of the evidence was very low, downgraded by two levels for imprecision (due to low sample size and wide confidence intervals) and one level for study limitations (risk of bias).
Fluid restriction: the review and the included trials did not report on this outcome (Gupta 2021).
-
Respiratory support:
The evidence is very uncertain regarding the effect of CPAP compared to free‐flow oxygen on duration of tachypnoea (MD −21.1 hours, 95% CI −22.9 to −19.3, 1 study, 64 infants) (Osman 2019).
The evidence is very uncertain regarding the effect of NHFV compared to CPAP on duration of tachypnoea (MD −4.53 hours, 95% CI −5.64 to −3.42, 1 study, 40 infants) (Dumas 2011).
The evidence is very uncertain regarding the effect of nasal NIPPV compared to CPAP on duration of tachypnoea (MD 4.30 hours, 95% CI −19.14 to 27.74, 1 study, 40 infants) (Demirel 2013)
The certainty of the evidence for both comparisons was very low, downgraded by two levels for imprecision (due to low sample size; only one small study) and one level for study limitations (risk of bias).
Need for mechanical ventilation (i.e. invasive respiratory support)
Salbutamol: the evidence is very uncertain regarding the effect of salbutamol compared to placebo (risk ratio (RR) 0.6, 95% CI 0.13 to 2.86, risk difference (RD) 10 fewer, 95% CI 50 fewer to 30 more per 1000, 3 studies, 254 infants (Malakian 2018; Monzoy‐Ventre 2015; Mussavi 2017)) in the Moresco 2021 review. The certainty of the evidence was very low, downgraded by two levels for imprecision (due to low sample size and wide confidence intervals) and one level for study limitations (risk of bias).
Epinephrine: the evidence is very uncertain regarding the effect of epinephrine versus placebo (RR 0.67, 95% CI 0.08 to 5.88, RD 70 fewer, 95% CI 460 fewer to 320 more per 1000, 1 study, 20 infants) in the Moresco 2016 review. The certainty of the evidence was very low, downgraded by two levels for imprecision (due to low sample size and wide confidence intervals) and one level for study limitations (risk of bias).
Corticosteroids: the evidence is very uncertain regarding the effect of corticosteroids compared to placebo (RR 0.52, 95% CI 0.05 to 5.38, RD 40 fewer, 95% CI 170 fewer to 90 more per 1000, 1 study, 49 infants) in the Bruschettini 2020 review. The certainty of the evidence was very low: downgraded by two levels for imprecision (due to low sample size and wide confidence intervals) and one level for study limitations (risk of bias).
Diuretics: this outcome was investigated by the review but not reported in the included trials (Kassab 2015).
Fluid restriction: the evidence is very uncertain regarding the effect of fluid restriction compared to standard fluid rate (RR 0.73, 95% CI 0.24 to 2.23, RD 20 fewer, 95% CI 70 fewer to 40 more per 1000, 3 studies, 242 infants (Akbarian Rad 2018; Sardar 2020; Stroustrup 2012)) in the Gupta 2021 review. The certainty of the evidence was very low, downgraded by two levels for imprecision (due to low sample size and wide confidence intervals) and one level for study limitations (risk of bias).
-
Respiratory support:
The evidence is very uncertain regarding the effect of CPAP compared to free‐flow oxygen (RR 0.30, 95% CI 0.01 to 6.99, RD 30 fewer, 95% CI 120 fewer to 50 more per 1000, 1 study, 64 infants) in the Moresco 2020 review.
The evidence is very uncertain regarding the effect of NIPPV compared to CPAP (RR 4.00, 95% CI 0.49 to 32.72, RD 150 more, 95% CI 50 fewer to 350 more per 1000, 1 study, 40 infants) in the Moresco 2020 review.
The evidence is very uncertain regarding the effect of NHFV versus CPAP (effect not estimable, 1 study, 40 infants) (Demirel 2013).
The certainty of the evidence was very low for both comparisons, downgraded by two levels for imprecision (due to low sample size and wide confidence intervals) and one level for study limitations (risk of bias).
Secondary outcomes
Time to established breastfeeding or bottle‐feeding (hours)
Salbutamol: the evidence is very uncertain regarding the effect of salbutamol compared to placebo (MD −28.49 hours, 95% CI −38.14 to 18.84, 2 studies, 188 infants (Kim 2014; Malakian 2018)) in the Moresco 2021 review. The certainty of the evidence was very low, downgraded by two levels for imprecision (due to low sample size and wide confidence intervals) and one level for study limitations (risk of bias).
Epinephrine: the evidence is very uncertain regarding the effect of epinephrine compared to placebo (MD −11.1 hours, 95% CI −51.37 to 29.17, 1 study, 20 infants (Moresco 2016)). The certainty of the evidence was very low, downgraded by two levels for imprecision (due to low sample size and wide confidence intervals) and one level for study limitations (risk of bias).
Corticosteroids: the evidence is very uncertain regarding the effect of corticosteroids compared to placebo (MD 2.40 hours, 95% CI −35.51 to 40.3, 1 study, 49 infants (Vaisbourd 2017)). The certainty of the evidence was very low, downgraded by two levels for imprecision (due to low sample size and wide confidence intervals) and one level for study limitations (risk of bias).
Diuretics: the review and the included trial did not report on this outcome (Kassab 2015).
Fluid restriction: the review and the included trials did not report on this outcome (Gupta 2021).
Respiratory support: this outcome was investigated by the review but not reported in the trials (Moresco 2020).
Duration (hours) of hospital stay
Salbutamol: five studies reported on duration of hospital stay. The evidence is very uncertain regarding the effect of salbutamol compared to placebo (MD −1.48 days, 95% CI −1.8 to 1.16, 4 studies, 338 infants) (Babaei 2019; Kim 2014; Malakian 2018; Mohammadzadeh 2017). The certainty of the evidence was very low, downgraded by two levels for imprecision (due to low sample size and wide confidence intervals) and one level for study limitations (risk of bias). The fifth study, Armangil 2011, reported that salbutamol reduced median hospital stay compared to placebo, four days versus six days. These data were not pooled in the analyses because only the median was reported (Moresco 2021).
Epinephrine: the evidence is very uncertain regarding the effect of epinephrine compared to placebo: median time of 73.6 hours with epinephrine and 63.4 with placebo, interquartile ranges not reported, 1 study, 20 infants (Moresco 2016). The certainty of the evidence was very low, downgraded by two levels for imprecision (due to low sample size and wide confidence intervals) and one level for study limitations (risk of bias).
Corticosteroids: the evidence is very uncertain regarding the effect of corticosteroids compared to placebo (MD of 2.6 days, 95% CI −6.43 to 1.23, 1 study, 49 infants (Bruschettini 2020)). The certainty of the evidence was very low, downgraded by two levels for imprecision (due to low sample size and wide confidence intervals) and one level for study limitations (risk of bias).
Diuretics: the evidence is very uncertain regarding the effect of diuretics compared to placebo (MD −4.95 hours, 95% CI −18.54 to 8.64, 2 studies, 100 infants (Kassab 2015)). The certainty of the evidence was very low, downgraded by two levels for imprecision (due to low sample size and wide confidence intervals) and one level for study limitations (risk of bias).
Fluid restriction: the evidence is very uncertain regarding the effect of restricted fluids compared to standard fluid rate (MD −0.92 days, 95% CI −1.53 to −0.31 days, 1 study (Eghbalian 2018)). The certainty of the evidence was very low, downgraded by two levels for imprecision (due to low sample size and wide confidence intervals) and one level for study limitations (risk of bias).
-
Respiratory support:
The evidence is very uncertain regarding the effect of CPAP compared to free‐flow oxygen (MD −0.8 days, 95% CI −1.65 to 0.05, 1 study, 64 infants (Osman 2019)).
The evidence is very uncertain regarding the effect of NIPPV compared to CPAP (MD 0.8 days, 95% CI −0.64 to 2.24, 1 study, 40 infants (Demirel 2013)).
The certainty of the evidence was very low for both comparisons, downgraded by two levels for imprecision (due to low sample size and wide confidence intervals) and one level for study limitations (risk of bias).
Clinical assessment of respiratory distress as quantified by Silverman or Downes score > 6 (indicative of impending respiratory failure) (yes/no), 24 and 48 hours after study entry
Salbutamol, epinephrine, diuretics, fluid restriction: the reviews and the included trials did not report on this outcome (Kassab 2015; Moresco 2016; Moresco 2021).
Corticosteroids: Vaisbourd 2017 did not report on the outcome as specified in our overview. However, they used their own modified TTN score, which found no differences between control and treatment groups.
Respiratory support: this outcome was investigated by the review but not reported on in the trials (Moresco 2020).
Neonatal mortality (within 28 days of life)
Salbutamol, epinephrine, corticosteroids, diuretics, fluid restriction: the reviews and the included trials did not report on this outcome (Bruschettini 2020; Gupta 2021; Kassab 2015; Moresco 2016; Moresco 2020; Moresco 2021).
Respiratory support: the evidence is very uncertain regarding the effect of CPAP compared to free‐flow oxygen (RR 0.30, 95% CI 0.01 to 6.99, RD 30 fewer, 95% CI 120 fewer to 50 more per 1000, 1 study, 64 infants (Osman 2019)). The certainty of the evidence was very low, downgraded by two levels for imprecision (due to low sample size and wide confidence intervals) and one level for study limitations (risk of bias).
Any adverse events as reported in the included review
Salbutamol: Kim 2014 monitored for tachycardia and arrhythmias; no cases were reported (Moresco 2021). The certainty of the evidence was very low, downgraded by two levels for imprecision (due to low sample size and wide confidence intervals) and one level for study limitations (risk of bias).
Epinephrine: no adverse events such as tachycardia (> 200 beats per minute (bpm)), arrhythmia, or hypertension was reported. The authors did, however, observe some transient increases in heart rate and blood pressure in the treatment group compared to the placebo group, max 194 bpm) (Moresco 2016). One of the newborns in the epinephrine group in Kao 2008 developed hypoglycaemia requiring intravenous glucose therapy. The certainty of the evidence was very low, downgraded by two levels for imprecision (due to low sample size and wide confidence intervals) and one level for study limitations (risk of bias).
Corticosteroids: no adverse effects were reported in the included study (Bruschettini 2020). The certainty of the evidence was very low, downgraded by two levels for imprecision (due to low sample size and wide confidence intervals) and one level for study limitations (risk of bias).
Diuretics: neither of the two trials found electrolyte imbalances in sodium or potassium levels. Both trials reported an increased weight loss in the first 24 hours in the treatment group compared to the placebo group; however, at discharge there was no difference (Kassab 2015). The certainty of the evidence was very low, downgraded by two levels for imprecision (due to low sample size and wide confidence intervals) and one level for study limitations (risk of bias).
-
Fluid restriction:
Hypernatraemia: the evidence is very uncertain regarding the effect of fluid restriction compared to standard fluid rate (RR 4.00, 95% CI 0.46 to 34.54, RD 60 more, 95% CI 20 fewer to 140 more per 1000, 1 study, 100 infants (Sardar 2020)). The certainty of the evidence was very low, downgraded by two levels for imprecision (due to low sample size and wide confidence intervals) and one level for study limitations (risk of bias).
Hyperbilirubinaemia requiring phototherapy: the evidence is very uncertain regarding the effect of fluid restriction compared to standard fluid rate (RR 1.09, 95% CI 0.79 to 1.48, RD 40 more, 95% CI 110 fewer to 180 more per 1000, 2 studies, 156 infants (Sardar 2020; Stroustrup 2012)). The certainty of the evidence was very low, downgraded by two levels for imprecision (due to low sample size and wide confidence intervals) and one level for study limitations (risk of bias).
Incidence of hypoglycaemia: the evidence is very uncertain regarding the effect of fluid restriction compared to standard fluid rate (RR 1.00, 95% CI 0.15 to 6.82, RD 0, 95% CI 50 fewer to 50 more per 1000, 2 studies, 164 infants (Sardar 2020; Stroustrup 2012)). The certainty of the evidence was very low, downgraded by two levels for imprecision (due to low sample size and wide confidence intervals) and one level for study limitations (risk of bias).
Respiratory support: Demirel 2013 reported on several potential adverse events, and found pneumothorax (n = 2), pneumonia (n = 7), intubation (n = 5), inotrope requirement (n = 2), and feeding intolerance (n = 10). The certainty of the evidence was very low, downgraded by two levels for imprecision (due to low sample size and wide confidence intervals) and one level for study limitations (risk of bias).
Discussion
Summary of main results
We included six Cochrane Reviews on the management of TTN in term and late preterm infants, corresponding to 1134 infants enrolled in 18 trials. The number of trials included in each review ranged from seven to one: the review on salbutamol included seven trials, fluid restriction: four trials, non‐invasive respiratory support: three trials, diuretics: two trials, budesonide: one trial, epinephrine: one trial. These extremely limited data hindered us from drawing any meaningful conclusions on the benefit and harms of any of the treatments of interest. The certainty of the evidence was very low for the primary outcomes of this overview.
The duration of tachypnoea might be reduced in the salbutamol group compared to the placebo group; however, the certainty of evidence was low. The trials included in the reviews on epinephrine, corticosteroids, and fluid restriction did not report this outcome. The evidence is very uncertain regarding the effect of the following comparisons: diuretics compared to placebo, CPAP compared to free‐flow oxygen, NHFV compared to CPAP, and nasal NIPPV compared to CPAP.
Similarly, the certainty of evidence was very low for the outcome need for mechanical ventilation for salbutamol, epinephrine, corticosteroids, non‐invasive respiratory support, and fluid restriction. The trials included in the reviews on diuretics did not report this outcome. As need for mechanical ventilation is a rare complication of TTN, a higher number of participants is required to reach some evidence.
Duration of hospital stay was the only outcome reported in all included reviews; one trial on fluid restriction found that the duration of hospitalisation may be reduced in the restricted‐fluids group, but the certainty of the evidence was very low.
Most of the reviews and their included trials considered harms associated with treatments. No relevant adverse events were reported. However, this result should be interpreted with caution, as the trials did not have enough statistical power (due to small sample sizes) to find possible increases in harms such as pneumothorax, arrhythmias, and electrolyte imbalances. No adverse effects were reported for salbutamol, although this medication is known to carry a risk of tachycardia, tremor, and hypokalaemia in other settings.
Overall completeness and applicability of evidence
We found reviews for all of our prespecified interventions. For β‐agonists, two separate reviews were included, one on salbutamol and one on epinephrine. The use of the six interventions described in this overview may vary significantly in different countries, and specific guidelines for the management of TTN are lacking (Alhassen 2021). All of the included reviews investigated the same population: term or late preterm infants with TTN, which was defined consistently across the reviews. Most of the reviews had similar outcomes as the main ones defined in our overview, though not all reviews defined duration of tachypnoea as a primary outcome. Mortality, one of our secondary outcomes, was not addressed in any review, likely because TTN is a non‐fatal condition and self‐limiting within a couple of days. However, in low‐income countries with limited resources for neonatal care, the clinical course of TTN might lead to significant clinical worsening and potentially to death. In addition, the clinical score for TTN severity was not used in any of the identified trials, despite its clinical relevance and the possibility to monitor the effectiveness of the treatment. The external validity of our overview is limited, mainly because of the few studies that have been published and their limitations in study design. The treatments that are not supported by any evidence should not be used clinically until more research has proven their efficacy and safety unless the systematic evaluation of high‐quality data from observational studies should indicate differently. Of note, identifying effective therapy to improve the clinical course of TTN would benefit newborn infants delivered in hospitals without a higher level of intensive care and decrease the need for neonatal transport.
Quality of the evidence
The quality of the included reviews was high, with all of them fulfilling the critical domains of the AMSTAR 2. The certainty of the evidence was low for duration of tachypnoea for salbutamol, and very low for all other outcomes due to few included studies and risk of bias.
When reviewing the results of the assessment of methodological quality (AMSTAR 2), two of the findings were of special interest. On question 3 ('Did review authors explain their selection of the study designs for inclusion in the review?'), it is notable that none of the reviews provided a rationale for excluding observational studies. This may be because of praxis – when writing systematic reviews of interventions, it is standard to include only randomised trials. However, in this field where there are very few randomised trials available, one might have considered including other study designs as well, such as observational studies, though this approach is known to increase sources of heterogeneity and bias due to confounders or selection of infants into the study (Reeves 2021). On question 10 ('Did review authors report on the sources of funding for the studies included in the review?'), the answer was 'no' for all reviews. This is unfortunate since full disclosure of any funding is important to ensure that no economic incentive might have introduced bias (Lundh 2017). The certainty of the evidence for all outcomes and treatments was assessed as very low according to the GRADE approach (Table 4), except for duration of tachypnoea for salbutamol, which was assessed as low certainty (downgraded by one level due to serious concern for imprecision and one level for study limitations). All other interventions/outcomes were downgraded by two levels due to very serious concern for imprecision since the numbers of participants were low and confidence of intervals often wide, and one level for study limitations. The collected evidence was also downgraded due to unclear risk of bias, most frequently related to unclear reporting of allocation and blinding. No inconsistency was identified in the studies on diuretics: the same drug (i.e. furosemide) was administered at the same dose.
Potential biases in the overview process
We are confident that this overview is a comprehensive summary of all currently available Cochrane Reviews on TTN. We did not apply any date restrictions to the search. Four of the six included reviews were published in the last two years. At least two overview authors independently assessed reviews for inclusion, carried out data extraction and quality assessment, and assessed the certainty of evidence using the GRADE approach. A potential source of bias is related to the fact that four overview authors are authors of several of the included reviews; as prespecified in our protocol, two other overview authors (KOH and RB), who were not authors of these reviews, carried out data extraction and quality assessment for this review in order to minimise intellectual bias.
Agreements and disagreements with other studies or reviews
Our findings are in line with the review by Buchiboyina and colleagues, which identified limited evidence supporting the use of salbutamol (Buchiboyina 2017). However, they did not assess non‐invasive respiratory support, where we could report a reduction the duration of tachypnoea without adverse events, though the certainty of evidence was very low.
Authors' conclusions
Implications for practice.
This overview summarises the evidence from six Cochrane Reviews of randomised trials regarding the effects of postnatal interventions in the management of transient tachypnoea of the newborn (TTN). Salbutamol may reduce the duration of tachypnoea slightly; we are uncertain as to whether salbutamol reduces the need for mechanical ventilation. We are uncertain as to whether epinephrine, corticosteroids, diuretics, fluid restriction, or non‐invasive respiratory support reduces the duration of tachypnoea and need for mechanical ventilation, due to the extremely limited evidence available. Data on harms were lacking.
Implications for research.
Large, multicentre randomised trials are needed to achieve an optimal information size to assess both the benefits and harms of the described interventions. Reporting of the outcome data should include the assessment of the clinical course of TTN. The use of multiple interventions, such as non‐invasive respiratory support and salbutamol in the same infants, might be investigated as well.
History
Protocol first published: Issue 3, 2020
Acknowledgements
We would like to thank Cochrane Neonatal for supporting the development of this overview of reviews: Colleen Ovelman and Jane Cracknell, former Managing Editors; Michelle Fiander, Information Specialist; and Roger Soll and Bill McGuire, Co‐ordinating Editors, for their valuable advice and support. We also thank Maria Björklund (Library and ICT services, Lund University) for designing the literature searches.
The following people conducted the editorial process for this article.
Sign‐off Editor (final editorial decision): Robert Boyle, Imperial College London
Managing Editor (selected peer reviewers, collated peer‐reviewer comments, provided editorial guidance to authors, edited the article): Lara Kahale, Cochrane Evidence Production and Methods Directorate
Editorial Assistant (conducted editorial policy checks and supported editorial team): Leticia Rodrigues, Cochrane Evidence Production and Methods Directorate
Copy Editor (copy‐editing and production): Lisa Winer, Cochrane Copy Edit Support
Peer reviewers (provided comments and made editorial suggestion): Georg M Schmölzer, University of Alberta (clinical reviewer), Manal Kassab, Jordan University of Science and Technology (clinical reviewer), Payam Vali, University of California (clinical reviewer), Rachel Richardson, Associate Editor, Cochrane (methods), Robin Featherstone, Cochrane Evidence Production and Methods Directorate (search).
One additional peer reviewer provided consumer peer review but chose not to be publicly acknowledged.
Appendices
Appendix 1. Search strategy
Cochrane Database of Systematic Reviews, (2021, Issue 7) via Cochrane Library (Wiley)
Search date: 14 July 2021
#1 infant or infants or infant's or "infant s" or infantile or infancy or newborn* or "new born" or "new borns" or "newly born" or neonat* or baby* or babies or premature or prematures or prematurity or preterm or preterms or "pre term" or premies or "low birth weight" or "low birthweight" or VLBW or LBW or ELBW or NICU 94794 #2 MeSH descriptor: [Infant, Newborn] explode all trees 16573 #3 #1 OR #2 94794 #4 MeSH descriptor: [Transient Tachypnea of the Newborn] explode all trees 41 #5 ((transient* or transitor*) NEAR/2 Tachypn*):ti,ab,kw 178 #6 #4 OR #5 178 #7 #3 AND #6 in Cochrane Reviews 8
Differences between protocol and review
We made the following changes to the published protocol (Bruschettini 2020).
The objective and research question for each included review is not reported in the tables, as it is considered redundant.
Contributions of authors
MB, LM, OR, and MGC reviewed the literature and wrote the review.
KOH and RB carried out data extraction and quality assessment for the review.
Sources of support
Internal sources
-
Institute for Clinical Sciences, Lund University, Lund, Sweden
MB and OR are employed by this organisation.
-
Istituto Giannina Gaslini, Genoa, Italy
MGC is employed by this organisation.
-
Pediatric and Neonatology Unit, Ospedale San Paolo, Savona, Italy
LM is employed by this organisation.
External sources
-
Vermont Oxford Network, USA
Cochrane Neonatal Reviews are produced with support from Vermont Oxford Network, a worldwide collaboration of health professionals dedicated to providing evidence‐based care of the highest quality for newborn infants and their families.
-
Region Skåne, Skåne University Hospital, Lund University and Region Västra Götaland, Sweden, Sweden
Cochrane Sweden is supported from Region Skåne, Skåne University Hospital Lund University and Region Västra Götaland.
Declarations of interest
Four overview authors are authors of several of the included reviews: MB of five reviews (Bruschettini 2020; Gupta 2021; Moresco 2016; Moresco 2020; Moresco 2021); MGC and LM of five reviews (Bruschettini 2020; Moresco 2016; Moresco 2020; Moresco 2021); and OR of two reviews (Bruschettini 2020; Moresco 2020). As prespecified in our protocol, two other overview authors (KOH and RB) carried out data extraction and quality assessment for these reviews in order to minimise intellectual bias.
MB has no other interests to declare.
KOH has no interests to declare.
OR has no other interests to declare.
RB has no interests to declare.
MGC has no other interests to declare.
LM has no other interests to declare.
New
References
References to included reviews
Bruschettini 2020
- Bruschettini M, Moresco L, Calevo MG, Romantsik O.Postnatal corticosteroids for transient tachypnoea of the newborn. Cochrane Database of Systematic Reviews 2020, Issue 3. Art. No: CD013222. [DOI: 10.1002/14651858.CD013222.pub2] [PMID: ] [DOI] [PMC free article] [PubMed] [Google Scholar]
Gupta 2021
- Gupta N, Bruschettini M, Chawla D.Fluid restriction in the management of transient tachypnea of the newborn. Cochrane Database of Systematic Reviews 2021, Issue 2. Art. No: CD011466. [DOI: 10.1002/14651858.CD011466.pub2] [PMID: ] [DOI] [PMC free article] [PubMed] [Google Scholar]
Kassab 2015
- Kassab M, Khriesat WM, Anabrees J.Diuretics for transient tachypnoea of the newborn. Cochrane Database of Systematic Reviews 2015, Issue 11. Art. No: CD003064. [DOI: 10.1002/14651858.CD003064.pub3] [PMID: ] [DOI] [PMC free article] [PubMed] [Google Scholar]
Moresco 2016
- Moresco L, Calevo MG, Baldi F, Cohen A, Bruschettini M.Epinephrine for transient tachypnea of the newborn. Cochrane Database of Systematic Reviews 2016, Issue 5. Art. No: CD011877. [DOI: 10.1002/14651858.CD011877.pub2] [PMID: ] [DOI] [PMC free article] [PubMed] [Google Scholar]
Moresco 2020
- Moresco L, Romantsik O, Calevo MG, Bruschettini M.Non-invasive respiratory support for the management of transient tachypnea of the newborn. Cochrane Database of Systematic Reviews 2020, Issue 4. Art. No: CD013231. [DOI: 10.1002/14651858.CD013231.pub2] [PMID: ] [DOI] [PMC free article] [PubMed] [Google Scholar]
Moresco 2021
- Moresco L, Bruschettini M, Macchi M, Calevo MG.Salbutamol for transient tachypnea of the newborn. Cochrane Database of Systematic Reviews 2021, Issue 2. Art. No: CD011878. [DOI: 10.1002/14651858.CD011878.pub3] [PMID: ] [DOI] [PMC free article] [PubMed] [Google Scholar]
Additional references
Akbarian Rad 2018
- Akbarian Rad Z, Gorji Rad M, Haghshenas M.Effects of restrictive fluid management in transient tachypnea in neonates. Iranian Journal of Neonatology 2018;9(4):47-52. [DOI: 10.22038/ijn.2018.27719.1371] [DOI] [Google Scholar]
Alexiou 2016
- Alexiou S, Panitch HB.Physiology of non-invasive respiratory support. Seminars in Fetal & Neonatal Medicine 2016;21(3):174-80. [DOI: 10.1016/j.siny.2016.02.007] [PMID: ] [DOI] [PubMed] [Google Scholar]
Alhassen 2021
- Alhassen Z, Vali P, Guglani L, Lakshminrusimha S, Ryan RM.Recent advances in pathophysiology and management of transient tachypnea of newborn. Journal of Perinatology 2021;41(1):6-16. [DOI: 10.1038/s41372-020-0757-3] [PMID: ] [DOI] [PMC free article] [PubMed] [Google Scholar]
Armangil 2011
- Armangil D, Yurdakök M, Korkmaz A, Yiğit S, Tekinalp G.Inhaled beta-2 agonist salbutamol for the treatment of transient tachypnea of the newborn. Journal of Pediatrics 2011;159(3):398-403.e1. [DOI: 10.1016/j.jpeds.2011.02.028] [PMID: 21481414] [DOI] [PubMed] [Google Scholar]
Avery 1966
- Avery ME, Gatewood OB, Brumley G.Transient tachypnea of newborn. Possible delayed resorption of fluid at birth. American Journal of Diseases of Children 1966;111(4):380-5. [DOI: 10.1001/archpedi.1966.02090070078010] [PMID: ] [DOI] [PubMed] [Google Scholar]
Babaei 2019
- Babaei H, Dabiri S, Pirkashani LM, Mohsenpour H.Effects of salbutamol on the treatment of transient tachypnea of the newborn. Iranian Journal of Neonatology 2019;10(1):42-9. [DOI: 10.22038/IJN.2018.31294.1430] [DOI] [Google Scholar]
Barker 2002
- Barker PM, Olver RE.Invited review: clearance of lung liquid during the perinatal period. Journal of Applied Physiology 2002;93(4):1542-8. [DOI: 10.1152/japplphysiol.00092.2002] [PMID: ] [DOI] [PubMed] [Google Scholar]
Becker 1983
- Becker AB, Nelson NA, Simons FE.Inhaled salbutamol (albuterol) vs injected epinephrine in the treatment of acute asthma in children. Journal of Pediatrics 1983;102(3):465-9. [DOI: 10.1016/s0022-3476(83)80679-9] [PMID: ] [DOI] [PubMed] [Google Scholar]
Biddle 1979
- Biddle TL, Yu PN.Effect of furosemide on hemodynamics and lung water in acute pulmonary edema secondary to myocardial infarction. American Journal of Cardiology 1979;43(1):86-90. [DOI: 10.1016/0002-9149(79)90049-3] [PMID: ] [DOI] [PubMed] [Google Scholar]
Bland 1978
- Bland RD, McMillan DD, Bressack MA.Decreased pulmonary transvascular fluid filtration in awake newborn lambs after intravenous furosemide. Journal of Clinical Investigation 1978;62(3):601-9. [DOI: 10.1172/JCI109166] [PMID: ] [DOI] [PMC free article] [PubMed] [Google Scholar]
Buchiboyina 2017
- Buchiboyina A, Jasani B, Deshmukh M, Patole S.Strategies for managing transient tachypnoea of the newborn - a systematic review. Journal of Maternal-Fetal & Neonatal Medicine 2017;30(13):1524-32. [DOI: 10.1080/14767058.2016.1193143] [PMID: ] [DOI] [PubMed] [Google Scholar]
Clark 2005
- Clark RH.The epidemiology of respiratory failure in neonates born at an estimated gestational age of 34 weeks or more. Journal of Perinatology 2005;25(4):251-7. [DOI: 10.1038/sj.jp.7211242] [PMID: ] [DOI] [PubMed] [Google Scholar]
Cohen 1985
- Cohen M, Carson BS.Respiratory morbidity benefit of awaiting onset of labor after elective cesarean section. Obstetrics & Gynecology 1985;65(6):818-24. [PMID: ] [PubMed] [Google Scholar]
Cordero 1997
- Cordero L, Ayers LW, Davis K.Neonatal airway colonization with gram-negative bacilli: association with severity of bronchopulmonary dysplasia. Pediatric Infectious Disease Journal 1997;16(1):18-23. [DOI: 10.1097/00006454-199701000-00005] [PMID: ] [DOI] [PubMed] [Google Scholar]
Dehdashtian 2014
- Dehdashtian M, Aramesh MR, Melekian A, Aletayeb MH, Ghaemmaghami A.Restricted versus standard maintenance fluid volume in management of transient tachypnea of newborn: a clinical trial. Iranian Journal of Pediatrics 2014;24(5):575-80. [PMID: ] [PMC free article] [PubMed] [Google Scholar]
Dehdashtian 2018
- Dehdashtian M, Aletayeb M, Malakian A, Aramesh MR, Malvandi H.Clinical course in infants diagnosed with transient tachypnea of newborn: a clinical trial assessing the role of conservative versus conventional management. Journal of the Chinese Medical Association 2018;81(2):183-6. [DOI: 10.1016/j.jcma.2017.06.016] [PMID: ] [DOI] [PubMed] [Google Scholar]
Demirel 2013
- Demirel G, Uras N, Celik IH, Canpolat FE, Dilmen U.Nasal intermittent mandatory ventilation versus nasal continuous positive airway pressure for transient tachypnea of newborn: a randomized, prospective study. Journal of Maternal-Fetal & Neonatal Medicine 2013;26(11):1099-102. [DOI: 10.3109/14767058.2013.766707] [13339468] [PMID: ] [DOI] [PubMed] [Google Scholar]
De Paoli 2003
- De Paoli AG, Morley C, Davis PG.Nasal CPAP for neonates: what do we know in 2003? Archives of Disease in Childhood. Fetal and Neonatal Edition 2003;88(3):F168-72. [DOI: 10.1136/fn.88.3.f168] [PMID: ] [DOI] [PMC free article] [PubMed] [Google Scholar]
Dumas 2011
- Dumas De La Roque E, Bertrand C, Tandonnet O, Rebola M, Roquand E, Renesme L, et al.Nasal high frequency percussive ventilation versus nasal continuous positive airway pressure in transient tachypnea of the newborn: a pilot randomized controlled trial (NCT00556738). Pediatric Pulmonology 2011;46(3):218-23. [DOI: 10.1002/ppul.21354] [13339470] [PMID: ] [DOI] [PubMed] [Google Scholar]
Edwards 2013
- Edwards MO, Kotecha SJ, Kotecha S.Respiratory distress of the term newborn infant. Paediatric Respiratory Reviews 2013;14(1):29-36; quiz 36-7. [DOI: 10.1016/j.prrv.2012.02.002] [PMID: ] [DOI] [PubMed] [Google Scholar]
Eghbalian 2018
- Eghbalian F, Sabzehei M, Emamzadeh N, Shokouhi M, Basiri B, Faradmal J, et al.Comparison of restricted fluid volume with standard fluid volume in management of transient tachypnea of the newborns: a randomized controlled trial. International Journal of Pediatrics 2018;6(9):8289-96. [DOI: 10.22038/ijp.2018.30462.2677] [DOI] [Google Scholar]
Fischer 2015
- Fischer HS, Bohlin K, Buhrer C, Schmalisch G, Cremer M, Reiss I, et al.Nasal high-frequency oscillation ventilation in neonates: a survey in five European countries. European Journal of Pediatrics 2015;174(4):465-71. [DOI: 10.1007/s00431-014-2419-y] [PMID: ] [DOI] [PubMed] [Google Scholar]
Guyatt 2011
- Guyatt G, Oxman AD, Akl EA, Kunz R, Vist G, Brozek J, et al.GRADE guidelines: 1. Introduction - GRADE evidence profiles and summary of findings tables. Journal of Clinical Epidemiology 2011;64(4):383-94. [DOI: 10.1016/j.jclinepi.2010.04.026] [PMID: ] [DOI] [PubMed] [Google Scholar]
Hack 1976
- Hack M, Fanaroff AA, Klaus MH, Mendelawitz BD, Merkatz IR.Neonatal respiratory distress following elective delivery. A preventable disease? American Journal of Obstetrics and Gynecology 1976;126(1):43-7. [DOI: 10.1016/0002-9378(76)90462-2] [PMID: ] [DOI] [PubMed] [Google Scholar]
Hansen 2008
- Hansen AK, Wisborg K, Uldbjerg N, Henriksen TB.Risk of respiratory morbidity in term infants delivered by elective caesarean section: cohort study. BMJ 2008;336(7635):85-7. [DOI: 10.1136/bmj.39405.539282.BE] [PMID: ] [DOI] [PMC free article] [PubMed] [Google Scholar]
Hibbard 2010
- Hibbard JU, Wilkins I, Sun L, Gregory K, Haberman S, Hoffman M, et al.Respiratory morbidity in late preterm births. JAMA 2010;304(4):419-25. [DOI: 10.1001/jama.2010.1015] [PMID: ] [DOI] [PMC free article] [PubMed] [Google Scholar]
Higgins 2019
- Higgins JPT, Thomas J, Chandler J, Cumpston M, Li T, Page MJ, Welch VA, editor(s).Cochrane Handbook for Systematic Reviews of Interventions version 6.0 (updated July 2019). Cochrane, 2019. Available from training.cochrane.org/handbook/archive/v6.
Irestedt 1982
- Irestedt L, Lagercrantz H, Hjemdahl P, Hagnevik K, Belfrage P.Fetal and maternal plasma catecholamine levels at elective cesarean section under general or epidural anesthesia versus vaginal delivery. American Journal of Obstetrics and Gynecology 1982;142(8):1004-10. [DOI: 10.1016/0002-9378(82)90783-9] [PMID: ] [DOI] [PubMed] [Google Scholar]
Kao 2008
- Kao B, Stewart de Ramirez SA, Belfort MB, Hansen A.Inhaled epinephrine for the treatment of transient tachypnea of the newborn. Journal of Perinatology 2008;28(3):205-10. [DOI: 10.1038/sj.jp.7211917] [PMID: ] [DOI] [PubMed] [Google Scholar]
Karabayir 2006
- Karabayir N, Kavuncuoglu S.Intravenous frusemide for transient tachypnoea of the newborn: a randomised controlled trial. Journal of Paediatrics and Child Health 2006;42(10):640-2. [DOI: 10.1111/j.1440-1754.2006.00942.x] [PMID: ] [DOI] [PubMed] [Google Scholar]
Kim 2014
- Kim MJ, Yoo JH, Jung JA, Byun SY.The effects of inhaled albuterol in transient tachypnea of the newborn. Allergy, Asthma & Immunology Research 2014;6(2):126-30. [DOI: 10.4168/aair.2014.6.2.126] [PMID: ] [DOI] [PMC free article] [PubMed] [Google Scholar]
Kumar 1996
- Kumar A, Bhat BV.Epidemiology of respiratory distress of newborns. Indian Journal of Pediatrics 1996;63(1):93-8. [DOI: 10.1007/BF02823875] [PMID: ] [DOI] [PubMed] [Google Scholar]
Lemyre 2017
- Lemyre B, Davis PG, De Paoli AG, Kirpalani H.Nasal intermittent positive pressure ventilation (NIPPV) versus nasal continuous positive airway pressure (NCPAP) for preterm neonates after extubation. Cochrane Database of Systematic Reviews 2017, Issue 2. Art. No: CD003212. [DOI: 10.1002/14651858.CD003212.pub3] [DOI] [PMC free article] [PubMed] [Google Scholar]
Liem 2007
- Liem JJ, Huq SI, Ekuma O, Becker AB, Kozyrskyj AL.Transient tachypnea of the newborn may be an early clinical manifestation of wheezing symptoms. Journal of Pediatrics 2007;151(1):29-33. [DOI: 10.1016/j.jpeds.2007.02.021] [PMID: ] [DOI] [PubMed] [Google Scholar]
Linsell 2016
- Linsell L, Malouf R, Morris J, Kurinczuk JJ, Marlow N.Prognostic factors for cerebral palsy and motor impairment in children born very preterm or very low birthweight: a systematic review. Developmental Medicine and Child Neurology 2016;58(6):554-69. [DOI: 10.1111/dmcn.12972] [PMID: ] [DOI] [PMC free article] [PubMed] [Google Scholar]
Lundh 2017
- Lundh A, Lexchin J, Mintzes B, Schroll JB, Bero L.Industry sponsorship and research outcome. Cochrane Database of Systematic Reviews 2017, Issue 2. Art. No: MR000033. [DOI: 10.1002/14651858.MR000033.pub3] [PMID: ] [DOI] [PMC free article] [PubMed] [Google Scholar]
Malakian 2018
- Malakian A, Dehdashtian M, Aramesh MR, Aletayeb MH, Heidari S.The effect of inhaled salbutamol on the outcomes of transient tachypnea of the newborn. Journal of the Chinese Medical Association 2018;81(11):990-7. [DOI: 10.1016/j.jcma.2018.01.015] [PMID: ] [DOI] [PubMed] [Google Scholar]
McGillick 2017
- McGillick EV, Lee K, Yamaoka S, Te Pas AB, Crossley KJ, Wallace MJ, et al.Elevated airway liquid volumes at birth: a potential cause of transient tachypnea of the newborn. Journal of Applied Physiology 2017;123(5):1204-13. [DOI: 10.1152/japplphysiol.00464.2017] [PMID: ] [DOI] [PubMed] [Google Scholar]
Miller 1980
- Miller LK, Calenoff L, Boehm JJ, Riedy MJ.Respiratory distress in the newborn. JAMA 1980;243(11):1176-9. [PMID: ] [PubMed] [Google Scholar]
Mohammadzadeh 2017
- Mohammadzadeh I, Akbarian-Rad Z, Heidari F, Zahedpasha Y, Haghshenas-Mojaveri M.The effect of inhaled salbutamol in transient of tachypnea of the newborn: a randomized clinical trial. Iranian Journal of Pediatrics 2017;27(5):e9633. [DOI: 10.5812/ijp.9633] [DOI] [Google Scholar]
Monzoy‐Ventre 2015
- Monzoy-Ventre MA, Rosas-Sumano AB, Hernández-Enríquez NP, Galicia-Flores L.Inhaled salbutamol in newborn infants with transient tachypnea [Salbutamol inhalado en los niños recién nacidos con taquipnea transitoria]. Revista Mexicana de Pediatria 2015;82(1):5-9. [Google Scholar]
Morrison 1995
- Morrison JJ, Rennie JM, Milton PJ.Neonatal respiratory morbidity and mode of delivery at term: influence of timing of elective caesarean section. British Journal of Obstetrics and Gynaecology 1995;102(2):101-6. [DOI: 10.1111/j.1471-0528.1995.tb09060.x] [PMID: ] [DOI] [PubMed] [Google Scholar]
Mussavi 2017
- Mussavi M, Asadollahi K, Kayvan M, Sadeghvand S.Effects of nebulized albuterol in transient tachypnea of the newborn a clinical trial. Iranian Journal of Pediatrics 2017;27(3):e8211. [DOI: 10.5812/ijp.8211] [DOI] [Google Scholar]
Osman 2019
- Osman AM, El-Farrash RA, Mohammed EH.Early rescue Neopuff for infants with transient tachypnea of newborn: a randomized controlled trial. Journal of Maternal-Fetal & Neonatal Medicine 2019;32(4):597-603. [DOI: 10.1080/14767058.2017.1387531] [PMID: ] [PMID: ] [DOI] [PubMed] [Google Scholar]
Pacifici 2013
- Pacifici GM.Clinical pharmacology of furosemide in neonates: a review. Pharmaceuticals (Basel, Switzerland) 2013;6(9):1094-129. [DOI: 10.3390/ph6091094] [PMID: ] [DOI] [PMC free article] [PubMed] [Google Scholar]
Perkins 2006
- Perkins GD, McAuley DF, Thickett DR, Gao F.The Beta-Agonist Lung Injury Trial (BALTI): a randomized placebo-controlled clinical trial. American Journal of Respiratory and Critical Care Medicine 2006;173(3):281-7. [DOI: 10.1164/rccm.200508-1302OC] [PMID: ] [DOI] [PubMed] [Google Scholar]
Reeves 2021
- Reeves BC, Deeks JJ, Higgins JPT, Shea B, Tugwell P, Wells GA.Chapter 24: Including non-randomized studies on intervention effects. In: Higgins JPT, Thomas J, Chandler J, Cumpston M, Li T, Page MJ, Welch VA, editor(s). Cochrane Handbook for Systematic Reviews of Interventions version 6.2 (updated February 2021). Cochrane, 2021. Available from training.cochrane.org/handbook.
Reuter 2014
- Reuter S, Moser C, Baack M.Respiratory distress in the newborn. Pediatrics in Review 2014;35(10):417-28; quiz 429. [DOI: 10.1542/pir.35-10-417] [PMID: ] [DOI] [PMC free article] [PubMed] [Google Scholar]
Roberts 2017
- Roberts D, Brown J, Medley N, Dalziel SR.Antenatal corticosteroids for accelerating fetal lung maturation for women at risk of preterm birth. Cochrane Database of Systematic Reviews 2017, Issue 3. Art. No: CD004454. [DOI: 10.1002/14651858.CD004454.pub3] [DOI] [PMC free article] [PubMed] [Google Scholar]
Saccone 2016
- Saccone G, Berghella V.Antenatal corticosteroids for maturity of term or near term fetuses: systematic review and meta-analysis of randomized controlled trials. BMJ (Clinical Research Ed.) 2016;355:i5044. [DOI: 10.1136/bmj.i5044] [PMID: ] [DOI] [PMC free article] [PubMed] [Google Scholar]
Sakuma 1997
- Sakuma T, Folkesson HG, Suzuki S, Okaniwa G, Fujimura S, Matthay MA.Beta-adrenergic agonist stimulated alveolar fluid clearance in ex vivo human and rat lungs. American Journal of Respiratory and Critical Care Medicine 1997;155(2):506-12. [DOI: 10.1164/ajrccm.155.2.9032186] [PMID: ] [DOI] [PubMed] [Google Scholar]
Sardar 2020
- Sardar S, Pal S, Mishra R.A randomized controlled trial of restricted versus standard fluid management in late preterm and term infants with transient tachypnea of the newborn. Journal of Neonatal-Perinatal Medicine 2020;13(4):477-87. [DOI: 10.3233/NPM-190400] [PMID: ] [DOI] [PubMed] [Google Scholar]
Sartori 2002
- Sartori C, Allemann Y, Duplain H, Lepori M, Egli M, Lipp E, et al.Salmeterol for the prevention of high-altitude pulmonary edema. New England Journal of Medicine 2002;346(21):1631-6. [DOI: 10.1056/NEJMoa013183] [PMID: ] [DOI] [PubMed] [Google Scholar]
Shea 2017
- Shea BJ, Reeves BC, Wells G, Thuku M, Hamel C, Moran J, et al.AMSTAR 2: a critical appraisal tool for systematic reviews that include randomised or non-randomised studies of healthcare interventions, or both. BMJ (Clinical Research Ed.) 2017;358:j4008. [DOI: 10.1136/bmj.j4008] [PMID: ] [DOI] [PMC free article] [PubMed] [Google Scholar]
Silverman 1956
- Silverman WA, Andersen DH.A controlled clinical trial of effects of water mist on obstructive respiratory signs, death rate and necropsy findings among premature infants. Pediatrics 1956;17(1):1-10. [PMID: ] [PubMed] [Google Scholar]
Spino 1978
- Spino M, Sellers EM, Kaplan HL, Stapleton C, MacLeod SM.Adverse biochemical and clinical consequences of furosemide administration. Canadian Medical Association Journal 1978;118(12):1513-8. [PMID: ] [PMC free article] [PubMed] [Google Scholar]
Stark 2001
- Stark AR, Carlo WA, Tyson JE, Papile LA, Wright LL, Shankaran S, et al, National Institute of Child Health and Human Development Research Network.Adverse effects of early dexamethasone treatment in extremely-low-birth-weight infants. National Institute of Child Health and Human Development Neonatal Research Network. New England Journal of Medicine 2001;344(2):95-101. [DOI: 10.1056/NEJM200101113440203] [PMID: ] [DOI] [PubMed] [Google Scholar]
Stroustrup 2012
- Stroustrup A, Trasande L, Holzman IR.Randomized controlled trial of restrictive fluid management in transient tachypnea of the newborn. Journal of Pediatrics 2012;160(1):38-43. [DOI: 10.1016/j.jpeds.2011.06.027] [PMID: ] [DOI] [PMC free article] [PubMed] [Google Scholar]
Tudehope 1979
- Tudehope DI, Smyth MH.Is "transient tachypnoea of the newborn" always a benign disease? Report of 6 babies requiring mechanical ventilation. Australian Paediatric Journal 1979;15(3):160-5. [DOI: 10.1111/j.1440-1754.1979.tb01215.x] [PMID: ] [DOI] [PubMed] [Google Scholar]
Vaisbourd 2017
- Vaisbourd Y, Abu-Raya B, Zangen S, Arnon S, Riskin A, Shoris I, et al.Inhaled corticosteroids in transient tachypnea of the newborn: a randomized, placebo-controlled study. Pediatric Pulmonology 2017;52(8):1043-50. [DOI: 10.1002/ppul.23756] [PMID: ] [DOI] [PubMed] [Google Scholar]
Wilkinson 2016
- Wilkinson D, Andersen C, O'Donnell CP, De Paoli AG, Manley BJ.High flow nasal cannula for respiratory support in preterm infants. Cochrane Database of Systematic Reviews 2016, Issue 2. Art. No: CD006405. [DOI: 10.1002/14651858.CD006405.pub3] [DOI] [PMC free article] [PubMed] [Google Scholar]
Wiswell 1985
- Wiswell TE, Rawlings MC, Smith MC, Goo ED.Effect of furosemide on the clinical course of transient tachypnea of the newborn. Pediatrics 1985;75(5):908-10. [PMID: ] [PubMed] [Google Scholar]
Wood 1972
- Wood DW, Downes JJ, Lecks HI.A clinical scoring system for the diagnosis of respiratory failure. Preliminary report on childhood status asthmaticus. American Journal of Diseases of Children 1972;123(3):227-8. [DOI: 10.1001/archpedi.1972.02110090097011] [PMID: ] [DOI] [PubMed] [Google Scholar]
Zielinska 2014
- Zielinska M, Zielinski S, Sniatkowska-Bartkowska A.Mechanical ventilation in children - problems and issues. Advances in Clinical and Experimental Medicine 2014;23(5):843-8. [DOI: 10.17219/acem/37264] [PMID: ] [DOI] [PubMed] [Google Scholar]
References to other published versions of this review
Bruschettini 2020
- Bruschettini M, Hassan KO, Romantsik O, Banzi R, Calevo MG, Moresco L.Interventions for the management of transient tachypnoea of the newborn ‐ an overview of systematic reviews. Cochrane Database of Systematic Reviews 2020, Issue 3. Art. No: CD013563. [DOI: 10.1002/14651858.CD013563] [DOI] [PMC free article] [PubMed] [Google Scholar]


