Abstract
Parents of 30 school-age children with Down syndrome participated in a small-scale randomized clinical trial of a behavioral sleep treatment designed specifically for children with Down syndrome. The aim was to improve child sleep, child daytime behavior problems, caregiver sleep, and caregiver stress. The intervention spanned 5–8 weeks, and assessments occurred pre-treatment, immediately post-treatment, and three months post-treatment using a double-blinded design. Both the active treatment and a treatment-as-usual attention-controlled comparison group showed improvements in actigraphy and parent-report measures of child sleep, parent-reported child internalizing behaviors, and actigraphy measures of parent-sleep. The behavioral sleep treatment did not yield significantly different outcomes than a treatment-as-usual approach supplemented with non-sleep-specific behavioral or education sessions. Possible interpretations of study findings are discussed.
Keywords: Down syndrome, trisomy 21, sleep, randomized behavioral intervention, parental stress, children
Down syndrome (DS) is a prevalent chromosomal disorder and the most common genetic cause of intellectual disability, affecting 1 in 707 live births (Mai et al., 2019). Sleep is a primary concern among individuals with DS. Because 1/3 – 2/3 have obstructive sleep apnea (OSA), pediatric guidelines recommend that all children with DS receive polysomnography (PSG) by age 4 years (Bull, 2011). Beyond OSA, most (52–69%) children with DS also experience behavioral sleep problems (Epstein et al., 1992; Marcus et al., 1991; Rosen et al., 2011; Shott et al., 2006; Stebbens et al., 1991). Common behavioral sleep concerns include: bedtime resistance, sleep onset delay, sleep anxiety, night waking, and problematic sleep onset associations (e.g., inability to fall asleep without a parent present) (Carter, 2009; Stores & Stores, 1996). While substantial research has focused on treatment of OSA, behavioral sleep problems, which result mainly from a failure to learn satisfactory sleep habits (Stores & Wiggs, 2001), are often overlooked in the care of individuals with DS.
Poor sleep has the potential to exacerbate existing DS-related deficits. Indeed, in the general population, behavioral sleep problems are associated with poor attention, impulse control, cognitive abilities (e.g., poor learning/memory), and behavior regulation (Beebe, 2011; Dewald, 2010; Fallone, 2002; Paavonen, Porkka-Heiskanen, et al., 2009; Paavonen, Raikkonen, et al., 2009; Steenari, 2003). Furthermore, there are adverse associations with parental and family functioning (Beebe, 2006; Dahl, 2006; Sadeh & Gruber, 2002; Sadeh & Raviv, 2000) and a causal relationship with attentional functioning in experimental sleep manipulation studies (Beebe, 2011). Though less extensively documented, comparable associations involving sleep problems have been found among children with intellectual disability (Malow et al., 2006; Montgomery et al., 2004; Richdale & Wiggs, 2005; Stores & Wiggs, 2001). Among school-age children with DS, disturbed sleep has been associated with both the child’s daily functioning (irritability and over-activity) and parental well-being (Stores, 1993; Stores et al., 1998). Disturbed sleep is also reported to be associated with parental report of inhibitory control, shifting and working memory, and of teacher report of inhibitory control (Esbensen & Hoffman, 2018). Finally, sleep duration is associated with parental reports of increased inattention and hyperactivity (Esbensen, Hoffman, Beebe, et al., 2018).
Despite the association between disrupted sleep and poor daytime functioning, there are limited empirically supported sleep treatment options available to parents and clinicians. Pharmaceutical sleep treatments have limited safety and efficacy data in this population, are often associated with side effects, and parents are understandably reluctant to use them (Pillar et al., 2000). Moreover, even after children with OSA are successfully treated with airway therapy, behavioral sleep problems often persist (Merrell, 2007; Rosen et al., 2011; Shott, 2006).
In healthy children, behavioral sleep problems have responded well to behavioral sleep treatment (BST) (Byars et al., 2011; Kuhn & Elliott, 2003). Similarly, parent training programs and randomized controlled trials have demonstrated that BST can reduce severe bedtime and sleep problems in children with autism spectrum disorders (ASD) (Johnson et al., 2013; Malow et al., 2014; Reed et al., 2009; Weiskop S, 2005). In both children with ASD and those who are typically developing, treatment that improves sleep has also led to improved daytime functioning (Dahl et al., 1991; Johnson et al., 2013; Wright et al., 2011), helping to spur the publication of empirically-supported behavioral sleep screening and treatment guidance (Dahl et al., 1991; Johnson et al., 2013; Malow et al., 2012; Meltzer, 2010; Reed et al., 2009; Tikotzky, 2010; Wright et al., 2011).
In contrast, there is limited empirically supported guidance for treating behavioral sleep disturbances in children with DS. Despite promising findings, the few studies that have treated sleep among children with DS have targeted only very young children (Stores & Stores, 2004), relied on a case study design (Thackeray & Richdale, 2002), or failed to document objective improvements in sleep (Wiggs & Stores, 1998). The very few prior randomized studies on sleep problems have used some, but not all the CONSORT requirements for randomized clinical trials (blinding, equipoise of comparison group), limiting the ability to evaluate methods or intensity of treatment (Stores & Stores, 2004; Wiggs & Stores, 1998). The lack of empirically-supported treatment options for behavioral sleep problems in children with DS contributes to under-treatment of these problems by health care providers, which can result in worse behavioral outcomes and increased family stress (Esbensen et al., 2016; Wiggs & France, 2000; Wiggs & Stores, 1996). A critical next step in improving outcomes for children with DS is to develop and test an intervention for these prevalent and disruptive behavioral sleep problems.
The DS behavioral phenotype must be considered when developing a sleep intervention. As children with DS often have difficulty with changes in routine, BST for children with DS must place greater emphasis on extinction bursts and need for consistency (McGuire & Chicoine, 2006). Children with DS also have difficulty with transitions, so a successful intervention in DS will likely need to emphasize structured routine and transition cues (McGuire & Chicoine, 2006). Children with DS are often described as stubborn, impulsive, and frequently noncompliant, and refusal behavior may contribute to sleep disturbances (McGuire & Chicoine, 2006). Thus, a BST will need to place greater emphasis on addressing these behaviors in the bedtime routine. Children with DS tend to have a relative weakness in language abilities, which can be accommodated through an emphasis of visual schedules, social stories, and other visual supports (Dykens et al., 2000; Yang et al., 2014). Given the high rate of comorbid OSA, a BST must also consider atypical sleep positions common in children with OSA (e.g., sleeping upright or with neck hyperextension to ease breathing during sleep). Finally, while BST in ASD and other populations typically target preschool or young children with a focus on early intervention of sleep problems, sleep disturbances often persist to school-age among children with DS. Thus, BST targeting older children with DS needs to make accommodations for differences in school schedules and adjust for changing sleep schedules and expectations in adolescents.
Given the need for evidence-based behavioral sleep intervention for children with DS, this study evaluated a small-scale randomized behavioral clinical trial of a BST designed specifically for children with DS. The aim of the BST was to directly target child sleep across multiple sessions, with expectations that improved sleep would be associated with secondary improvement in child daytime behavior problems, caregiver sleep, and caregiver stress. To establish equipoise, the comparison group controlled for intensity of treatment by adding non-sleep-related educational content pertinent to DS to the very limited sleep education often provided by pediatricians. First, we hypothesized that BST would improve child sleep duration and sleep quality more than in an attention-controlled comparison group that included treatment-as-usual content. Secondarily, we hypothesized that children receiving BST would demonstrate greater reductions in behavioral problems than the comparison group. Third, we hypothesized that BST would improve parental sleep and decrease parental stress more than the comparison group.
Method
Design
The design of the study included two interventions (BST, control) and three measurement points (pre-test, post-test, and 3-month follow-up). A double-blinded randomized behavioral clinical trial was employed, with testing examiners blinded to intervention allocation. All study activities were conducted at the medical center and approved and overseen by the Institutional Review Board and registered at clinicaltrials.gov (NCT02996175). The CONSORT check list for the trial is available in the Appendix.
Participants
Parents of children with DS were recruited through a pediatric medical center, a DS specialty clinic, and through newsletters distributed by the local DS association. Children eligible for the study trial were 6–17 years old with a documented diagnosis of DS and English as their primary language at home. Children had a nonverbal mental age of at least 36 months based on parental report and were on stable dosages of any current medication or ongoing treatment for the duration of the study to limit behavioral changes due to other interventions. Children were required to have at least one sleep disturbance, defined as the presence of one or more nights a week of bedtime resistance, delayed sleep onset, problematic sleep onset associations, nighttime awakenings, or premature morning awakenings, based on parental report. These criteria are consistent with those used in previous studies evaluating BST in other populations (Johnson et al., 2013). Children were excluded from the study if they had limited language abilities or sensory impairments (deafness or blindness) that would preclude valid administration of study measures, or if they had a psychotropic medication change within the last two months. Children were not excluded if they were receiving treatment for other sleep problems (i.e., medication, CPAP) or had comorbid OSA. Instead, these factors were used in block randomization.
Demographic and clinical characteristics, including block randomization variables, of the sample are summarized in Table 1. Children with DS averaged 10.69 years of age (SD = 3.22) and were primarily male (66.7%) and Caucasian (80.0%). Respondents were primarily mothers (96%), with one father participating. Parents reported on their child’s current medical diagnoses and surgery/intervention history.
Table 1.
Pre-Test Demographic and Clinical Characteristics by Group.
| BST (n=16) | CON (n=14) | Group differences | ||||||
|---|---|---|---|---|---|---|---|---|
|
|
||||||||
| M | SD | M | SD | t | p | |||
|
| ||||||||
| Age | 9.90 | 3.14 | 11.49 | 3.22 | −1.29 | .21 | ||
| Full scale IQ (KBIT2) | 41.94 | 2.77 | 42.57 | 3.52 | −.55 | .59 | ||
| Adaptive behavior (SIB-R) | 51.53 | 21.50 | 32.85 | 17.71 | 2.49 | .02* | ||
| n | % | n | % | χ2 | p | |||
|
|
||||||||
| Gender (males) | 11 | 68.8% | 9 | 64.3% | 0.07 | .80 | ||
| Race (Caucasian) | 13 | 81.2% | 11 | 78.6% | 1.24 | .54 | ||
| Diagnoses | ||||||||
| ADHD | 3 | 18.8% | 4 | 28.6% | 0.40 | .53 | ||
| CHD | 5 | 31.2% | 4 | 28.6% | 0.03 | .87 | ||
| Sleep disorder | 6 | 37.5% | 5 | 41.7% | 0.05 | .82 | ||
| Interventions | ||||||||
| Sleep study | 12 | 75.0% | 10 | 71.4% | 0.05 | .82 | ||
| Tonsillectomy | 10 | 62.5% | 9 | 64.3% | 0.01 | .92 | ||
| Adenoidectomy | 11 | 68.8% | 9 | 64.3% | 0.07 | .80 | ||
| CPAP/BiPAP | 3 | 18.8% | 2 | 14.3% | 0.11 | .74 | ||
| Medication | 10 | 62.5% | 12 | 85.7% | 2.06 | .15 | ||
p < .05
Behavioral Sleep Treatment
BST was a 5-session manualized behaviorally based parent training intervention, implemented through hourly weekly meetings, completed over a span of 5–8 weeks (manual available from first author). The BST was adapted from a manualized sleep intervention that has been used successfully to treat children with ASD and sleep disturbances (Johnson et al., 2013). Adaptations of the ASD protocol for DS included addressing the impact of poor sleep on daily behaviors, and greater emphasis on visual supports, planned ignoring and extinction within sessions. Session 1 (Function of Behavior) introduced the treatment program, provided standard of care information for common sleep problems in DS, basic principles of behavioral approach to understanding and managing sleep problems, and healthy sleep hygiene. Session 2 (Prevention) provided general information on healthy sleep hygiene, preventative strategies, and visual supports, including an itemized visual schedule for the family to use for the duration of the study. Session 3 (Reinforcement & Stimulus Control) provided general information on and definitions of reinforcement and stimulus control procedures, and specific information for bedtime struggles, promoting independent sleep, night waking, and early waking. This session also included a discussion of barriers to independent sleep (i.e., problematic sleep onset associations). Session 4 (Planned Ignoring and Extinction) provided procedures for managing delayed sleep onset and implementing planned ignoring. Lastly, session 5 (Maintenance) focused on providing feedback on implementation of behavioral sleep treatments and strategies for managing sleep hygiene in the future. Key behavioral interventions included reinforcement contingency, extinction, planned ignoring, graduated approach and extinction, withdrawal, stimulus control, faded bedtime, and adopting healthy sleep hygiene. These interventions had been successfully used to treat common pediatric sleep disorders, and among children with developmental disabilities during individually tailored treatments (Richdale & Wiggs, 2005). Each session provided direct instruction, vignettes, activity sheets and modeling to promote skill acquisition by parents. Parents were provided with data collection assignments to encourage practicing of skills.
Comparison Group
The comparison intervention (CON) included 5 individually administered didactic sessions, comprised of one session of standard of care sleep treatment (session 2) enhanced with four educational sessions that address topics of direct relevance to children with DS. The duration of sessions and therapeutic relationship mirrored that provided in the BST. Session 1 (DS Phenotype) introduced the general education program and reviewed the neurology of individuals with DS. Session 2 (Function of Behavior) provided standard of care information on common sleep problems in DS, basic principles of behavioral approach as it related to sleep problems, and healthy sleep hygiene, general information on healthy sleep hygiene, preventative strategies, and visual supports. Session 3 (Clinical Evaluations) provided information on understanding and interpreting results from clinical evaluations (non-specific to sleep). Session 4 (IEP and Transition Planning) provided information on educational planning, expectations, and transition planning. Session 5 (Lifespan Services and Resources) provided information on lifespan development, advocacy and support services available, and feedback on current concerns and methods for obtaining services to manage concerns.
Measures
Cognition and Adaptive Behavior.
Child cognitive ability was measured using the Kaufman Brief Intelligence Test, 2nd Edition (KBIT-II) during baseline. The KBIT-II is a brief measure of cognitive ability appropriate for individuals aged 4–90 years (Kaufman, 2004). The Scales of Independent Behavior-Revised (SIB-R) rates children’s adaptive daily living skills and yields a standard score in four domains (motor skills, social interaction/communication skills, personal living skills, and community living skills) and an overall Broad Independence score (Bruininks et al., 1996). Both the KBIT-II and SIB-R are recommended for use in children with DS (Edgin et al., 2010).
Child Sleep.
Child sleep was assessed using actigraphy and parent-report. Children wore a Micro-mini Motionlogger Actigraph (Ambulatory Monitoring, Inc.), which is a battery-operated device that closely resembles a watch and objectively infers sleep based on movement patterns. The actigraph was placed on the non-dominant wrist of the participant 30 minutes before bedtime and removed from the wrist 30 minutes after rising in the morning. Parents completed a companion sleep diary for the child’s sleep which was used to assess for gross inconsistencies with actigraphy sleep and wake time estimates that might signal an artifact (e.g., unit malfunction or removal). Movement data were processed using a validated sleep scoring algorithm (Micro-Mini Motionlogger Instruction Manual, 2000; Sadeh et al., 1994). Specific actigraph measures of sleep used for the current analyses included: (1) Sleep Duration: sleep period, the time from when the child fell asleep to when the child woke up, ignoring waking times within that period; and (2) Sleep Quality: sleep efficiency, defined as the percent of the sleep period that the child spent in sleep; and (3) WASO: Waking after sleep onset, the total minutes spent awake during the sleep period. Sleep indices were determined for each night children wore the actigraph, then averaged across the week to obtain more stable indices for analyses. In the BST group, children wore actigraphs an average of 6.71 nights at pre-test (SD = 0.61, range 5–7), 6.57 nights at post-test (SD = 0.75, range 5–7), and 5.92 nights at follow-up (SD = 1.14, range 3–7). In the CON group, children wore actigraphs an average of 6.46 nights at pre-test (SD = 0.96, range 4–7), 6.46 nights at post-test (SD = 1.12, range 4–7), and 6.85 nights at follow-up (SD = 0.36, range 6–7).
To obtain subjective sleep data, parents completed the Children’s Sleep Habits Questionnaire (CSHQ), which is a 33-item sleep screening instrument for children and assesses major childhood medical and behavioral sleep disorders during a typical week (Owens et al., 2000). Items are rated on a 3-point ordinal scale from (1) rarely (0–1 time/week) to (3) usually (5–7 times/week). Although designed for use in pediatric populations under 10 years of age without intellectual disabilities, the CSHQ demonstrates strong psychometric properties and convergence in identifying behavioral sleep problems in school-age children with DS ages 6–17 years (Esbensen & Hoffman, 2017) and has demonstrated validity in other pediatric populations characterized by intellectual and developmental disabilities (Goldman et al., 2012; Richdale & Baker, 2014; Veatch et al., 2016). Four CSHQ subscales assessing sleep problems were used in the current analyses, specifically the subscales of Bedtime Resistance, Sleep Duration, Parasomnias, and Total Score. These subscales were selected to reflect difficulties with sleep hygiene, duration, quality of sleep, and overall sleep problems that are commonly addressed during BST.
Child Behavior Problems.
Two measures of maladaptive behavior were used to assess child behaviors, specifically the Achenbach Child Behavior Checklist (CBCL) and the Aberrant Behavior Checklist (ABC). The CBCL for children ages 6–18 years obtains parent ratings of 112 problem behaviors, in addition to descriptions of their child’s strengths and challenges (Achenbach & Rescorla, 2001). The CBCL assesses symptoms on the following subscales: Anxious/Depressed, Withdrawn/Depressed, Somatic Complaints, Social Problems, Thought Problems, Attention Problems, Rule-Breaking Behavior and Aggressive Behavior. An Internalizing Problems score is derived from symptoms on the Anxious/Depressed, Withdrawn/Depressed, and Somatic Complaints subscales, an Externalizing Problems score is derived from symptoms of Rule-Breaking Behavior and Aggressive Behavior, and a total score is available. Internal consistency and one-week test-retest reliability ranges from good to excellent for each of the subscales with typically-developing children (Achenbach & Rescorla, 2001). Although not initially designed for use with children who have developmental disabilities, internal consistency is moderate to high for all subscales with children who have intellectual disabilities or DS (Esbensen, Hoffman, Shaffer, et al., 2018; Jacola et al., 2014). Items are rated on a 3-point scale from (0) not true to (2) very true, and t-scores are created based on an age and gender normative sample. Current analyses were focused on the Internalizing and Externalizing t-scores for the CBCL.
The ABC is a 58-item rating scale of maladaptive behaviors for children and adults with intellectual or developmental disabilities (Aman et al., 1985a, 1985b). Subscales assess Irritability, Lethargy, Stereotypic Behaviors, Hyperactivity, and Inappropriate Speech. Items are rated on a 4-point ordinal scale from (0) not at all a problem to (3) the problem is severe in degree. Internal consistency is good to excellent, inter-rater reliability is moderate and retest reliability extremely high (Aman et al., 1985b). Current analyses were focused on the Irritability subscale of the ABC as it is commonly used in clinical trials in individuals with intellectual and developmental disability.
Parental Functioning.
Measures of parental functioning were focused on parental sleep and family quality of life. Parental sleep was objectively measured using their own actigraph, with the same procedures as described above. Parents in the BST group wore actigraphs an average of 6.60 nights at pre-test (SD = 0.63, range 5–7), 5.93 nights at post-test (SD = 1.38, range 3–7), and 5.73 nights at follow-up (SD = 1.33, range 3–7). Those in the CON group wore actigraphs an average of 6.64 nights at pre-test (SD = 0.49, range 6–7), 6.38 nights at post-test (SD = 1.12, range 4–7), and 6.53 nights at follow-up (SD = 0.66, range 5–7).
Parental stress was measured with the Family Impact Questionnaire (FIQ). The FIQ is a 50-item scale specially designed to measure the impact of a child with a developmental disability on the family in several domains, specifically how the child positively and negatively impacts parenting, social relationships, finances, and as applicable, siblings, and marriage (Donenberg & Baker, 1993). Items are rated on a 4-point ordinal scale from (0) not at all to (3) very much. Internal consistency is excellent (Baker, 2003). Current analyses focused on the Negative Feelings about Parenting and the Positive Feelings about Parenting subscales of the FIQ.
Fidelity.
At each session, fidelity of intervention delivery was documented (therapist fidelity: adherence of therapists to the manual; and parent adherence: parents’ compliance with homework and use of strategies in session), as well as parental engagement (therapist impression of parental participation). Criteria for adequate fidelity was pre-determined to be 90% for therapist fidelity, and 75% for parental adherence.
Therapist fidelity was rated by the therapist at each session on a 3-point ordinal scale to indicate if session goals were (0) not introduced or covered, (1) partially achieved, or (2) fully achieved. Each session had between three to five session goals aligned with the treatment manual. Ten percent of sessions were reviewed by a supervising psychologist to ensure adherence to manual, with 100% agreement.
Parent adherence was rated by the therapist at each session on a 3-point ordinal scale to indicate if parent met integrity goals of (0) not completed assignments or demonstrate skills in session, (1) partially completed assignments or responded correctly to a few of the session queries, or (2) completed all assignments or responded correctly to nearly all queries (incorrect response of less than 2). Each session had between three to five integrity goals reflecting parental adherence to homework, assignments, and understanding of concepts. Parental engagement was rated by the therapist on a 4-point ordinal scale of parent (0) does not “buy in” to concepts, (1) struggles during much of session to grasp concepts, (2) engaged and successful during majority of session, and (3) participates actively and positively throughout session.
Procedures
Participants were screened for eligibility by phone and scheduled for an initial consent and pre-test visit. Children and families meeting inclusion criteria were randomly assigned to either receive BST or CON by an unblinded study staff, using block random assignment that was stratified based on (1) child age (6–11 years, 12–17 years), (2) presence/absence of OSA, and (3) presence/absence of any existing sleep interventions. Pre-test measures of parent and child were collected by study staff blind to group assignment starting in August 2015. Parents participated in the intervention visits blind to study groups. Outcome measures were obtained a week following the last BST or CON intervention session (post-test), within 5–8 weeks of pre-test measures, by blinded study staff. Outcome measures were collected again at follow-up 12 weeks after the post-test visit, again by blinded study staff, with the last visit completed November 2017. At all study visits, teacher reports forms were obtained, when available. Recruitment stopped with expiration of grant funding.
Data Analysis
Demographic and clinical characteristics were compared across the two intervention groups using t-tests and chi-square test. Although 100% of participants were retained at post-test and follow-up, some measures were missing for some participants at some time points (at most 6.6%, see Table 2). Random effects linear regression using full information maximum likelihood estimation was used to model outcomes as a function of time (pre-test, post-test, follow-up), group (BST, CON), and time by group interaction. Analyses were conducted with intervention centered. Centering occurs by taking the mean of the intervention variable coded 0/1 and subtracting the mean from the 0/1 values, and it allows one to interpret the intercept as the overall sample mean at the study’s start. Treatment effects would be indicated by a significant Time x Group interaction effect. Because we used full information maximum likelihood estimation, we were able to include all available data from each participant, even if participants were missing data at a given time point (Shin et al., 2017). Effect sizes were calculated using f2, where 0.02 is a small effect, 0.15 a medium effect, and 0.35 a large effect (Cohen, 1992). For descriptive purposes, we also calculate dppc2 as a measure of effect size for group comparisons across pre-test to post-test, which is similar to Cohen’s d and a less biased and more precise estimate of population treatment effect (Morris, 2008). Effect size interpretations for dppc2 are similar to Cohen’s d, where 0.2 is a small effect, 0.5 is a medium effect, and 0.8 is a large effect.
Table 2.
Means and Standard Deviations for Outcomes at Each Occasion.
| Behavioral Sleep Treatment | Control | d ppc2 a | |||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
|
|
|||||||||||||||||||
| Pre-Test | Post-Test | Follow-up | Pre-Test | Post-Test | Follow-up | ||||||||||||||
|
|
|||||||||||||||||||
| n | M | SD | n | M | SD | n | M | SD | n | M | SD | n | M | SD | n | M | SD | ||
|
| |||||||||||||||||||
| Child Actigraphy | |||||||||||||||||||
| TST | 14 | 490.8 | 51.5 | 16 | 487.2 | 53.5 | 15 | 489.6 | 79.1 | 14 | 447.4 | 75.6 | 13 | 466.5 | 55.7 | 14 | 456.9 | 68.7 | −0.32 |
| Sleep Efficiency | 14 | 85.2 | 6.9 | 16 | 85.3 | 6.3 | 15 | 86.8 | 6.9 | 14 | 77.4 | 14.0 | 13 | 81.2 | 10.6 | 14 | 81.0 | 12.8 | −0.31 |
| WASO | 14 | 64.1 | 30.4 | 16 | 57.7 | 26.1 | 15 | 51.8 | 29.1 | 14 | 99.2 | 66.1 | 13 | 92.1 | 74.5 | 14 | 82.4 | 59.1 | 0.01 |
| Child Sleep (CSHQ) | |||||||||||||||||||
| Bedtime Resistance | 16 | 10.2 | 3.2 | 16 | 8.9 | 3.1 | 16 | 8.4 | 2.9 | 14 | 9.7 | 3.4 | 14 | 8.9 | 3.3 | 14 | 8.1 | 3.5 | −0.15 |
| Sleep Duration | 16 | 4.6 | 1.8 | 16 | 4.9 | 1.6 | 16 | 4.4 | 1.6 | 14 | 3.9 | 1.4 | 14 | 4.2 | 1.5 | 14 | 4.0 | 1.0 | 0.05 |
| Parasomnias | 16 | 9.0 | 1.4 | 16 | 8.7 | 1.1 | 16 | 8.5 | 1.4 | 14 | 9.2 | 1.6 | 14 | 9.3 | 1.4 | 14 | 8.3 | 0.8 | −0.25 |
| Total Score | 16 | 49.4 | 6.6 | 16 | 46.8 | 6.5 | 16 | 46.2 | 6.4 | 14 | 47.1 | 6.8 | 14 | 48.2 | 6.5 | 14 | 45.0 | 6.2 | −0.5 |
| Child Behaviors | |||||||||||||||||||
| ABC Irritability | 16 | 5.9 | 6.5 | 16 | 6.4 | 5.7 | 16 | 4.9 | 5.6 | 14 | 6.8 | 8.2 | 14 | 6.8 | 6.4 | 14 | 4.8 | 5.6 | 0.08 |
| CBCL Internalize | 16 | 50.2 | 9.7 | 16 | 47.6 | 10.8 | 16 | 48.7 | 8.8 | 13 | 54.0 | 8.5 | 14 | 52.1 | 8.5 | 14 | 49.5 | 9.1 | −0.08 |
| CBCL Externalize | 16 | 56.0 | 8.1 | 16 | 55.1 | 8.3 | 16 | 54.6 | 8.4 | 13 | 55.2 | 10.4 | 14 | 53.7 | 10.1 | 14 | 53.0 | 8.0 | 0.06 |
| Parent Actigraphy | |||||||||||||||||||
| TST | 16 | 433.2 | 78.3 | 16 | 448.5 | 59.9 | 15 | 465.9 | 69.2 | 14 | 390.9 | 45.9 | 13 | 406.7 | 44.1 | 13 | 414.7 | 82.3 | −0.02 |
| Sleep Efficiency | 16 | 89.3 | 9.0 | 16 | 92.5 | 4.6 | 15 | 93.1 | 3.6 | 14 | 88.7 | 6.0 | 13 | 88.3 | 8.3 | 13 | 88.7 | 11.0 | 0.47 |
| WASO | 16 | 26.7 | 30.2 | 16 | 17.0 | 16.1 | 15 | 19.2 | 15.2 | 14 | 33.1 | 37.4 | 13 | 35.6 | 38.5 | 13 | 36.8 | 34.2 | −0.42 |
| Parent Stress | |||||||||||||||||||
| FIQ Positive Parent | 16 | 21.8 | 5.7 | 16 | 21.6 | 4.8 | 15 | 19.8 | 5.0 | 14 | 20.6 | 5.7 | 14 | 19.9 | 5.1 | 14 | 22.2 | 4.9 | 0.09 |
| FIQ Negative Impact | 16 | 39.7 | 11.6 | 16 | 37.2 | 10.7 | 15 | 39.1 | 12.9 | 14 | 39.6 | 10.8 | 14 | 39.1 | 9.6 | 14 | 38.7 | 10.2 | −0.17 |
Effect size for the mean pre-test post-test difference.
Although not the focus of this manuscript’s analyses, several additional outcome variables were included in the larger clinical trial, including additional measures of actigraphy, parent- and teacher-reports of executive functioning, and teacher reports of child behavior. No Group or Time x Group interaction effects were noted. Data are available from the first author.
Results
Pre-Test Characteristics
Table 1 presents group differences on pre-test demographic variables and clinical characteristics. There were no significant differences between groups on age, cognitive skills, gender, race, comorbid diagnoses, medical/surgical sleep interventions, or medication. There was a significant group difference in adaptive behavior skills as measured by the SIB-R (t = 2.49, p = .02) with participants randomized to receive BST reported as higher functioning than participants receiving CON.
Fidelity
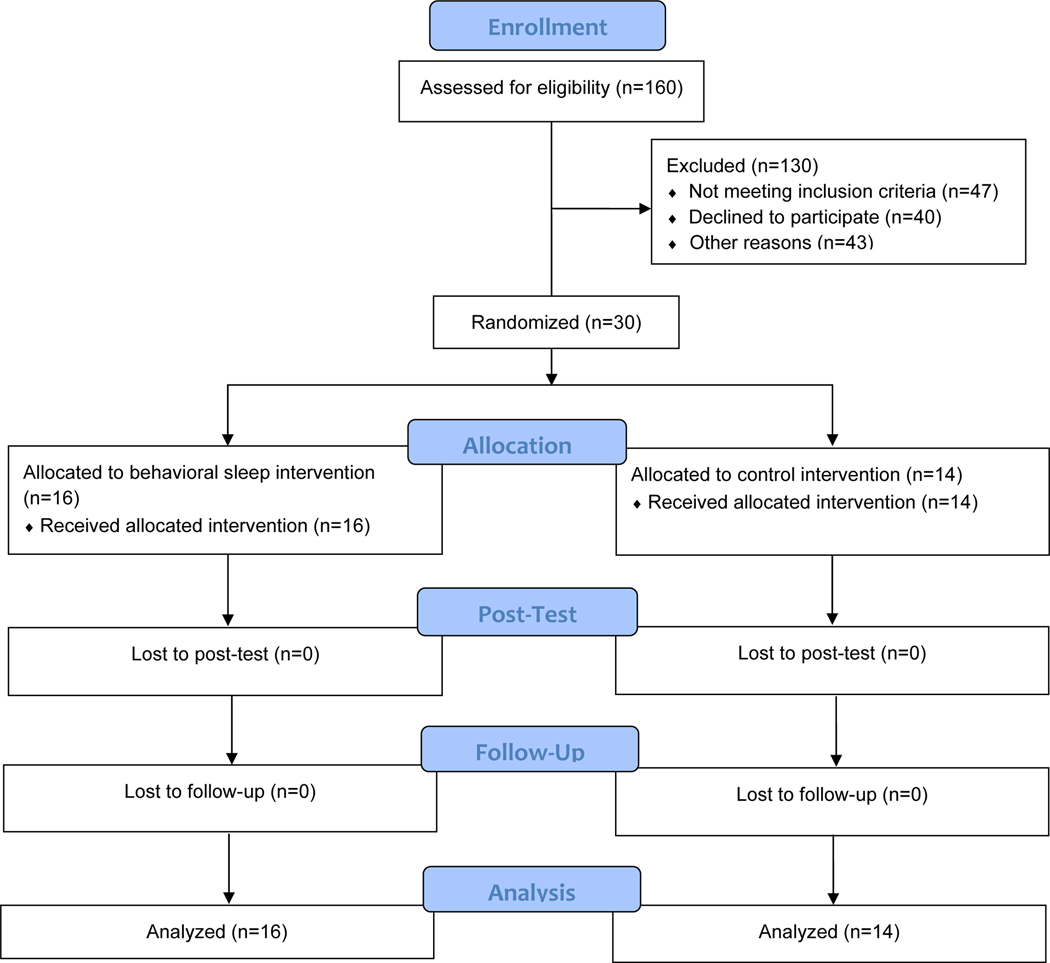
Participants (parent and child) attended all study visits with 0% drop out (see CONSORT diagram in Figure 1). Parents attended 100% of therapy sessions for both BST and CON. All therapy sessions across BST and CON met the criteria for therapist fidelity, with average group fidelity being very high (BST 99.9%, CON 100%). All therapy sessions across BST and CON met criteria for parental adherence, with average group fidelity being high (BST 96.2%, CON 92.1%). Parental engagement was also high, with average scores across sessions all above 2, reflecting parents engaged and successful during the majority of the session (BST M = 2.9, SD = 0.2; CON M = 2.7, SD = 0.3).
Figure 1.
CONSORT flow diagram
Treatment
Mean scores and standard deviations for each outcome measure at each study visit (pre-test, post-test, follow-up) are presented by group in Table 2. For descriptive purposes, Table 2 also includes dppc2 as a measure of effect size for group comparisons across pre-test to post-test. Findings from regression models for each outcome measure predicted by Time, Intervention Centered, and Time by Intervention Centered are presented in Table 3. Across all of the models, the effect size (f2) for the treatment effect (time by intervention centered) ranged from 0 (negligible effect) to 0.21 (a medium effect). The mean effect size was 0.02 (SD = 0.03) and the median was 0.01. Space constraints prevent us from depicting all effect sizes. They are available upon request.
Table 3.
Regression Models for Each Outcome Measure by Time, Intervention Centered, and Time by Intervention Centered (−/+ 95% Confidence Intervals in Parentheses).
| Intercept | Time (β) | Intervention (β) | Time X Intervention (β) | |
|---|---|---|---|---|
|
| ||||
| Child Actigraphy | ||||
| TST | 471.91 (449.83/493.90) | 1.58 (−9.46/12.63) | 40.75 (−3.34/84.85) | −5.95 (−28.01/16.11) |
| Sleep Efficiency | 81.51 (78.16/84.93) | 1.24 (−0.29/2.77) | 7.23 (0.45/14.01)* | −1.08 (−4.13/1.97) |
| WASO | 80.61 (63.57/97.65) | −7.06 (−13.50/−0.61)* | −37.46 (−71.57/−3.35)* | 2.57 (−10.30/15.44) |
| Child Sleep (CSHQ) | ||||
| Bedtime Resistance | 9.91 (8.83/11.01) | −0.88 (−1.33/−0.44)*** | 0.40 (−1.79/2.59) | −0.12 (−1.01/0.78) |
| Sleep Duration | 4.41 (3.88/4.89) | −0.02 (−0.23/0.20) | 0.70 (−0.32/1.71) | −0.10 (−0.53/0.34) |
| Parasomnias | 9.21 (8.73/9.61) | −0.35 (−0.58/−0.12)*** | −0.41 (−1.30/0.47) | 0.21 (−0.25/0.67) |
| Total Score | 48.51 (46.30/50.69) | −1.35 (−2.25/−0.45)*** | 1.20 (−3.21/5.60) | −0.52 (−2.33/1.29) |
| Child Behaviors | ||||
| ABC Irritability | 6.61 (4.48/8.80) | −0.72 (−1.49/0.06) | −0.90 (−5.23/3.43) | 0.53 (−1.02/2.09) |
| CBCL Internalize | 51.81 (48.61/54.92) | −1.49 (−2.68/−0.30)* | −4.57 (−10.90/1.76) | 1.51 (−0.88/3.90) |
| CBCL Externalize | 55.31 (52.31/58.39) | −0.78 (−1.68/0.11) | 1.29 (−4.81/7.38) | 0.14 (−1.66/1.93) |
| Parent Actigraphy | ||||
| TST | 414.61 (393.14/436.03) | 13.55 (1.34/25.76)* | 41.05 (−1.94/84.05) | 3.11 (−21.37/27.59) |
| Sleep Efficiency | 89.21 (86.81/91.65) | 0.85 (−0.77/2.46) | 1.12 (−3.73/5.98) | 2.07 (−1.15/5.30) |
| WASO | 28.41 (19.09/37.79) | −0.31 (−5.49/4.87) | −7.82 (−26.56/10.93) | −7.68 (−18.07/2.71) |
| Parent Stress | ||||
| FIQ Positive Parent | 21.11 (19.29/22.86) | 0.01 (−0.71/0.72) | 1.86 (−1.72/5.44) | −1.53 (−2.96/−0.10)* |
| FIQ Negative Impact | 39.31 (35.52/43.10) | −0.56 (−1.70/0.59) | −0.45 (−8.05/7.15) | −0.24 (−2.53/2.06) |
p < .05,
p < .001
Child Sleep.
Across pre-test, post-test, and follow-up time points, children in the BST group showed better sleep quality on average, as indexed by main effects for actigraphy-determined sleep efficiency (β = 7.23, p = .04) and WASO (β = −.37.47, p = .04). Also, both groups demonstrated time-related reductions in minutes in WASO (β = −7.06, p = .04). However, change over time did not significantly differ across the two groups. Contrary to predictions, participants receiving BST did not clearly have greater improvement in actigraphy measures of child sleep in comparison to participants receiving CON.
Similarly, participants receiving BST also showed no greater improvements in parent-report measures of child sleep than did participants receiving CON. However, both groups showed reductions over time in parental reports of bedtime resistance (β = −0.88, p < .0001, parasomnias (β = −0.35, p = .003), and the total score on the CSHQ (β = −1.35, p = .003).
Child Behavior Problems.
Participants receiving BST demonstrated no greater change over time in parent-report measures of child behavior problems than did participants receiving CON. However, all participants demonstrated reductions in parental reports of internalizing behavior problems on the CBCL (β = −1.49, p = .02, f2 = .026).
Parental Functioning.
Parents in the BST group did not have significantly greater change in parents’ actigraphy measures than did those in the CON group. However, parents in both groups improved their total sleep time across the study duration (β = 13.54, p = .03, f2 = .001).
Participants receiving BST did not have significantly greater change in parent-report measures of family stress over time than did those receiving CON. However, there was a significant interaction between time and intervention for ratings of positive feelings about parenting on the FIQ (β = −1.53, p = .04, f2 = .070). The participants receiving CON reported increased positive feeling about parenting whereas those receiving BST had no change on this measure.
Discussion
The current study evaluated a small-scale randomized behavioral clinical trial of a BST designed specifically for children with DS. Findings did not support our hypotheses that BST would improve child sleep or secondary outcome measures (child behavior, parent sleep, parental stress) more than the attention-controlled comparison group. However, the BST and the comparison groups both improved across actigraphy and parent-report measures of child sleep, parent-reported child internalizing behaviors, and actigraphy measures of parent-sleep. These findings have at least three different interpretations.
First, both conditions could be effective at improving some outcomes of child sleep, child behavior, and parent sleep. Over the course of the study and across both conditions, onset of child sleep improved on average by 14 minutes, and parent total sleep time by 26 minutes. Both included a session that reviewed basic sleep management, with BST augmented with evidence-based treatments for sleep in children with developmental disabilities (Johnson et al., 2013) and the comparison group augmented with an equivalent number of non-sleep-related educational sessions. Thus, the basic sleep management session might be sufficient to drive improvements in sleep outcomes over time for both groups, consistent with findings from case studies and sleep interventions with younger children with DS (Stores & Stores, 2004; Thackeray & Richdale, 2002). The equivalent findings across treatment groups are also consistent with evaluations of different sleep therapies in the general pediatric population, where there is limited evidence to support one therapy over another, or to guide frequency and duration of treatment (Byars & Simon, 2014). The additional behavioral strategies and knowledge imparted by subsequent sessions, though different in specific content, may also have similar and broad benefits for parent-child interactions. These findings would extend upon prior intervention studies that reported improved child and parent sleep on subjective measures, but not objective measures (Wiggs & Stores, 1998). Another potential contributor to similar benefit across groups was that the same therapist – trained to provide generally supportive guidance, albeit differing in specific focus – was used for both treatment groups, and frequently was listed as a strength of the treatment sessions in the parent satisfaction survey completed following the completion of the study. The attention from the therapist in both groups could have contributed to benefits from both treatment protocols.
Second, to some degree, the changes over time observed in both groups could be artifacts. Although study design blinded parents to the purpose of the intervention and content of the two treatment groups, tracking of sleep diaries could have contributed to parents determining that the study was targeting sleep. Participants intuiting the purpose of the study could have led to the Hawthorne effect and bias in ratings improving over time (McCarney et al., 2007), although this cannot account for findings on objective actigraphy. Alternatively, since sleep problems were required for entry into the study, some degree of improvement may reflect regression to the mean, although it is noteworthy that behavioral sleep problems are quite common in this population.
Third, the study design may not be sensitive to detecting effects of treatment. Recruitment for this study was a challenge, which contributed to a small sample and low power. A sample with the observed variation within and across participants and with the size of the groups (14 and 16) has 80% power to detect an f2 effect size of ~0.60, many times the effects observed in this sample and twice the size of a “large” effect (Cohen, 1992). A much larger sample would be needed to detect a more modest cross-condition effect, holding all other things constant. As a further limitation, inclusion criteria required sleep problems to be reported at least one night per week and not a minimum threshold on any specific measure. These inclusion criteria may have contributed to measures not having sufficiently high scores at pre-test or being sensitive to detecting treatment change (Fogel, 2018). Further, the five-session intervention may not have provided enough time for families to implement the behavioral strategies in the BST.
Additional limitations contribute to the complexity of interpreting results of this pilot randomized clinical trial. In creating equipoise between treatment groups, no waitlist control group was included. Lack of a waitlist control group contributes to challenges in interpreting whether the interventions were effective or a result of regression to the mean. Also, although there were reductions in both groups on the parent-report of sleep problems, follow-up mean scores continued to be above clinical cut-offs, so at least some participants may not have experienced clinically significant improvements. Although teacher reports of behavior problems and executive functioning were obtained, those data were often missing, so they were not presented formally here. Despite these limitations, our study demonstrates that parents of children with DS and behavioral sleep problems can participate in behavioral treatments with high rates of engagement and attendance. No attrition was noted, demonstrating the potential for a highly invested and motivated population of parents to participate in future clinical trials. Feedback from parents regarding recommendations to improve treatment were to include transportation to treatment sessions or reduce transportation burden by using telemedicine given the weekly treatment sessions. Future trials are encouraged to use a true treatment-as-usual control group and larger and more diverse samples to test the effectiveness of behavioral sleep treatments for children with DS who have behavioral consequences from behavioral sleep disorders. Future trials are also encouraged to include blinded direct assessment of child functioning as well as teacher ratings.
Children with DS frequently experience behavioral sleep concerns that warrant evidence-based intervention options (Carter, 2009; Stores & Stores, 1996). This pilot randomized clinical trial provides preliminary support for two treatments that demonstrate improvement in child sleep, child behavior problems, and parent sleep. Although these findings are preliminary, they support the ongoing work necessary to support child sleep problems, and the down-stream effects of sleep problems (behavior problems, parental sleep), in children with DS.
Acknowledgments
This manuscript was prepared with support from the Eunice Kennedy Shriver National Institute of Child Health and Human Development of the National Institutes of Health (R21 HD082307, A. Esbensen, PI). The content is solely the responsibility of the authors and does not necessarily represent the official views of the National Institutes of Health. This research would not have been possible without the contributions of the participating families and the community support from the Down Syndrome Association of Greater Cincinnati.
Appendix. CONSORT 2010 checklist of information to include when reporting a randomised trial
| Section/Topic | Item No | Checklist item | Reported on page No |
|---|---|---|---|
|
| |||
| Title and abstract | |||
| 1a | Identification as a randomized trial in the title | 1 |
|
| 1b | Structured summary of trial design, methods, results, and conclusions | 2 |
|
| Introduction | |||
| Background and objectives | 2a | Scientific background and explanation of rationale | 3–6 |
| 2b | Specific objectives or hypotheses | 6–7 |
|
| Methods | |||
| Trial design | 3a | Description of trial design including allocation ratio | 7, 14–15 |
| 3b | Important changes to methods after trial commencement, with reasons | na |
|
| Participants | 4a | Eligibility criteria for participants | 7–8 |
| 4b | Settings and locations where the data were collected | 7 |
|
| Interventions | 5 | The interventions for each group with sufficient details to allow replication, including how and when they were actually administered | 8–10 |
| Outcomes | 6a | Completely defined pre-specified primary and secondary outcome measures, including how and when they were assessed | 6, 10–13 |
| 6b | Any changes to trial outcomes after the trial commenced, with reasons | na |
|
| Sample size | 7a | How sample size was determined | 7 |
| 7b | When applicable, explanation of any interim analyses and stopping guidelines | na |
|
| Randomization: | |||
| Sequence generation | 8a | Method used to generate the random allocation sequence | 14 |
| 8b | Type of randomization; details of any restriction (such as blocking and block size) | 14 |
|
| Allocation concealment mechanism | 9 | Mechanism used to implement the random allocation sequence (such as sequentially numbered containers), describing any steps taken to conceal the sequence until interventions were assigned | 14 |
| Implementation | 10 | Who generated the random allocation sequence, who enrolled participants, and who assigned participants to interventions | 14 |
| Blinding | 11a | If done, who was blinded after assignment to interventions (for example, participants, care providers, those assessing outcomes) and how | 14–15 |
| 11b | If relevant, description of the similarity of interventions | 8–10 |
|
| Statistical methods | 12a | Statistical methods used to compare groups for primary and secondary outcomes | 15 |
| 12b | Methods for additional analyses, such as subgroup analyses and adjusted analyses | na |
|
| Results | |||
| Participant flow | 13a | For each group, the numbers of participants who were randomly assigned, received intended treatment, and were analyzed for the primary outcome | 29 |
| 13b | For each group, losses and exclusions after randomization, together with reasons | 29 |
|
| Recruitment | 14a | Dates defining the periods of recruitment and follow-up | 14–15 |
| 14b | Why the trial ended or was stopped | 15 |
|
| Baseline data | 15 | A table showing baseline demographic and clinical characteristics for each group | 26 |
| Numbers analyzed | 16 | For each group, number of participants (denominator) included in each analysis and whether the analysis was by original assigned groups | 27 |
| Outcomes and estimation | 17a | For each primary and secondary outcome, results for each group, and the estimated effect size and its precision (such as 95% confidence interval) | 28 |
| 17b | For binary outcomes, presentation of both absolute and relative effect sizes is recommended | na |
|
| Ancillary analyses | 18 | Results of any other analyses performed, including subgroup analyses and adjusted analyses, distinguishing pre-specified from exploratory | na |
| Harms | 19 | All important harms or unintended effects in each group | na |
| Discussion | |||
| Limitations | 20 | Trial limitations, addressing sources of potential bias, imprecision, and, if relevant, multiplicity of analyses | 20 |
| Generalizability | 21 | Generalizability (external validity, applicability) of the trial findings | 20–21 |
| Interpretation | 22 | Interpretation consistent with results, balancing benefits and harms, and considering other relevant evidence | 18–21 |
| Other information | 7 | ||
| Registration | 23 | Registration number and name of trial registry | |
| Protocol | 24 | Where the full trial protocol can be accessed, if available | 8 |
| Funding | 25 | Sources of funding and other support, role of funders | 1 (author note) |
References
- Achenbach TM, & Rescorla L. (2001). ASEBA School-Age Forms & Profiles. Aseba. [Google Scholar]
- Aman MG, Singh NN, Stewart AW, & Field CJ (1985a). The Aberrant Behavior Checklist: A behavior rating scale for the assessment of treatment effects. American Journal of Mental Deficiency, 89, 485–491. [PubMed] [Google Scholar]
- Aman MG, Singh NN, Stewart AW, & Field CJ (1985b). Psychometric characteristics of the aberrant behavior checklist. American Journal of Mental Deficiency, 89, 492–502. [PubMed] [Google Scholar]
- Baker BL, McIntyre LL, Blacher J, Crnic K, Edelbrock C, & Low C. (2003). Pre‐school children with and without developmental delay: behaviour problems and parenting stress over time. Journal of Intellectual Disability Research, 47, 214–230. [DOI] [PubMed] [Google Scholar]
- Beebe D. (2006). Neurobehavioral effects of childhood sleep-disordered breathing (SDB): A comprehensive review. Sleep, 29, 1115–1134. [DOI] [PubMed] [Google Scholar]
- Beebe D. (2011). Cognitive, behavioral, and functional consequences of inadequate sleep in children and adolescents. Pediatric Clinics of North America, 58(3), 649. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bruininks RH, Woodcock R, Weatherman R, & Hill B. (1996). SIB-R: Scales of Independent Behavior-Revised. Riverside. [Google Scholar]
- Bull MJ (2011). Health supervision for children with Down syndrome. Pediatrics, 128, 393–406. [DOI] [PubMed] [Google Scholar]
- Byars K, Apiwattanasawee P, Leejakpai A, Tangchityongsiva S, & Simakajornboom N. (2011). Behavioral sleep disturbances in children clinically referred for evaluation of obstructive sleep apnea. Sleep medicine, 12, 163–169. [DOI] [PubMed] [Google Scholar]
- Byars K, & Simon S. (2014). Practice patterns and insomnia treatment outcomes from an evidence-based pediatric behavioral sleep medicine clinic. Clinical Practice in Pediatric Psychology, 2(3), 337–349. [Google Scholar]
- Carter M, McCaughey E, Annaz D, & Hill CM . (2009). Sleep problems in a Down syndrome population. Archives of Diseases in Childhood, 94, 308–310. [DOI] [PubMed] [Google Scholar]
- Cohen J. (1992). A power primer. Psychological bulletin, 112(1), 155–159. [DOI] [PubMed] [Google Scholar]
- Dahl R. (2006). Sleeplessness and aggression in youth. Journal of Adolescent Health, 38, 641–642. [DOI] [PubMed] [Google Scholar]
- Dahl R, Pelham W, & Wierson M. (1991). The role of sleep disturbances in attention deficit disorder symptoms: A case study. Journal of Pediatric Psychology, 16(2), 229–239. [DOI] [PubMed] [Google Scholar]
- Dewald FJ, Meijer AM, Oort FJ, Kerkhof GA, Bögels SM (2010). The influence of sleep quality, sleep duration and sleepiness on school performance in children and adolescents: A meta-analytic review. Sleep medicine reviews, 14, 179–189. [DOI] [PubMed] [Google Scholar]
- Donenberg G, & Baker BL (1993). The impact of young children with externalizing behaviors on their families. Journal of abnormal child psychology, 21(2), 179–198. [DOI] [PubMed] [Google Scholar]
- Dykens E, Hodapp R, & Finucane BM (2000). Genetics and mental retardation syndromes: A new look at behavior and interventions. Paul H Brookes Publishing. [Google Scholar]
- Edgin JO, Mason GM, Allman MJ, Capone GT, DeLeon I, Maslen C, Reeves RH, Sherman SL, & Nadel L. (2010). Development and validation of the Arizona Cognitive Test Battery for Down syndrome. Journal of neurodevelopmental disorders, 2, 149–164. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Epstein R, Pillar D, Tzichinsky O, Here P, & Lavie P. (1992). Sleep disturbances in children with Downs’ syndrome. Journal of Sleep Research, 1(Supplement 1), 68. [Google Scholar]
- Esbensen AJ, Beebe DW, Byars KC, & Hoffman EK (2016). Use of Sleep Evaluations and Treatments in Children with Down Syndrome. Journal of Developmental & Behavioral Pediatrics, 37(8), 629–636. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Esbensen AJ, & Hoffman E. (2017). Reliability of parent report measures of sleep in children with Down syndrome. Journal of Intellectual Disability Research, 61(3), 210–220. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Esbensen AJ, & Hoffman EK (2018). Impact of sleep on executive functioning in school‐age children with Down syndrome. Journal of Intellectual Disability Research, 62(6), 569–580. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Esbensen AJ, Hoffman EK, Beebe DW, Byars KC, & Epstein J. (2018). Links between sleep and daytime behaviour problems in children with Down syndrome. Journal of Intellectual Disability Research, 62(2), 115–125. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Esbensen AJ, Hoffman EK, Shaffer R, Chen E, Patel L, & Jacola L. (2018). Reliability of parent report measures of behaviour in children with Down syndrome. Journal of Intellectual Disability Research, 62(9), 785–797. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fallone G, Owens JA, & Deane J. . (2002). Sleepiness in children and adolescents: Clinical implications. Sleep medicine reviews, 6, 287–306. [DOI] [PubMed] [Google Scholar]
- Fogel DB (2018). Factors associated with clinical trials that fail and opportunities for improving the likelihood of success: a review. Contemporary Clinical Trials Communications, 11, 156–164. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Goldman SE, Bichell T, Surdyka K, & Malow B. (2012). Sleep in children and adolescents with Angelman syndrome: Association with parent sleep and stress. Journal of Intellectual Disability Research, 56(6), 600–608. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jacola LM, Hickey F, Howe SR, Esbensen A, & Shear PK (2014). Behavior and adaptive functioning in adolescents with Down syndrome: Specifying targets for intervention. Journal of Mental Health Research in Intellectual Disabilities, 7(4), 287–305. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Johnson CR, Turner KS, Foldes E, Brooks MM, Kronk R, & Wiggs L. (2013). Behavioral parent training to address sleep distrubances in young children with autism spectrum disorder: A pilot trial. Sleep medicine, 14, 995–1004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kaufman A. (2004). KBIT-2: Kaufman Brief Intelligence Test. Second Edition. In. Upper Saddle River, NJ: Pearson. [Google Scholar]
- Kuhn B, & Elliott A. (2003). Treatment efficacy in behavioral pediatric sleep medicine. Journal of Psychosomatic Research, 54(6), 587–597. [DOI] [PubMed] [Google Scholar]
- Mai CT, Isenburg JL, Canfield MA, Meyer RE, Correa A, Alverson CJ, Lupo PJ, Riehle‐Colarusso T, Cho SJ, & Aggarwal D. (2019). National population‐based estimates for major birth defects, 2010–2014. Birth Defects Research, 111(18), 1420–1435. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Malow BA, Adkins KW, Reynolds A, Weiss SK, Loh A, Fawkes D, Katz T, Goldman SE, Madduri N, & Hundley R. (2014). Parent-based sleep education for children with autism spectrum disorders. Journal of Autism and Developmental Disorders, 44(1), 216–228. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Malow BA, Byars K, Johnson K, Weiss S, Bernal P, Goldman SE, & Glaze DG (2012). A practice pathway for the identification, evaluation, and management of insomnia in children and adolescents with autism spectrum disorders. Pediatrics, 130(Supplemental 2), S106–S124. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Malow BA, Marzec ML, McGrew SG, Wang L, Henderson LM, & Stone WL (2006). Characterizing sleep in children with autism spectrum disorders: A multidimensional approach. Sleep, 29(12), 1563. [DOI] [PubMed] [Google Scholar]
- Marcus C, Keens T, Bautista D, von Pechman W, & Ward S. (1991). Obstructive sleep apnea in children with Down syndrome. Pediatrics, 88, 132–139. [PubMed] [Google Scholar]
- McCarney R, Warner J, Iliffe S, Van Haselen R, Griffin M, & Fisher P. (2007). The Hawthorne Effect: A randomised, controlled trial. BMC Medical Research Methodology, 7(1), 30. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McGuire D, & Chicoine B. (2006). Mental Wellness in Adults with Down Syndrome. Woodbine. (Woodbine House Publishers) [Google Scholar]
- Meltzer LJ, & Mindell JA (2010). Clinical management of behavioral insomnia of childhood: treatment of bedtime problems and night wakings in young children. Behavioral sleep medicine, 8(3), 172–189. [DOI] [PubMed] [Google Scholar]
- Merrell JA, & Shott SR . (2007). OSAS in Down syndrome: T&A versus T&A plus lateral pharyngoplasty. International journal of pediatric otorhinolaryngology, 71, 1197–1203. [DOI] [PubMed] [Google Scholar]
- Micro-Mini Motionlogger Instruction Manual. (2000). Ambulatory Monitoring, Inc. [Google Scholar]
- Montgomery P, Stores G, & Wiggs L. (2004). The relative efficacy of two brief treatments for sleep problems in young learning disabled (mentally retarded) children: a randomised controlled trial. Archives of Disease in Childhood, 89, 125–130. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Morris SB (2008). Estimating effect sizes from pretest-posttest-control group designs. Organizational research methods, 11(2), 364–386. [Google Scholar]
- Owens JA, Spirito A, & McGuinn M. (2000). The Children’s Sleep Habits Questionnaire (CSHQ): psychometric properties of a survey instrument for school-aged children. Sleep, 23(8), 1043–1052. [PubMed] [Google Scholar]
- Paavonen E, Porkka-Heiskanen T, & Lahikainen AR (2009). Sleep quality, duration and behavioral symptoms among 5–6 year old children. European Child and Adolescent Psychiatry, 18, 747–754. [DOI] [PubMed] [Google Scholar]
- Paavonen E, Raikkonen K, Lahti J, Komsi N, Heinonen K, & Pesonen AK (2009). Short sleep duration and behavioral symptoms of attention-deficit/hyperactivity disorder in healthy 7- to 8-year old children. Pediatric, 123, e857–864. [DOI] [PubMed] [Google Scholar]
- Pillar G, Shahar E, Peled N, Ravid S, Lavie P, & Etzioni A. (2000). Melatonin improves sleep-wake patterns in psychomotor retarded children. Pediatric Neurology, 23(225–228). [DOI] [PubMed] [Google Scholar]
- Reed H, McGrew S, Artibee K, Surdkya K, Goldman S, Frank K, Wang L, & Malow B. (2009). Parent-based sleep education workshops in autism. Journal of Child Neurology, 24, 936–945. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Richdale AL, & Baker EK (2014). Sleep in individuals with an intellectual or developmental disability: Recent research reports. Current Developmental Disorders Reports, 1(2), 74–85. [Google Scholar]
- Richdale AL, & Wiggs L. (2005). Behavioral approaches to the treatment of sleep problems in children with developmental disorders: What is the state of the art? International Journal of Behavioral Consultation and Therapy, 1, 165–190. [Google Scholar]
- Rosen D, Lombardo A, Skotko B, & Davidson EJ (2011). Parental perceptions of sleep disturbances and sleep-disordered breathing in children with Down syndrome. Clinical Pediatrics, 50, 121–125. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sadeh A, & Gruber R, & Raviv A. . (2002). Sleep, Neurobehavioral Functioning, and Behavior Problems in School‐Age Children. Child development, 73, 405–417. [DOI] [PubMed] [Google Scholar]
- Sadeh A, & Raviv A, & Gruber R. . (2000). Sleep patterns and sleep disruptions in school-age children. Developmental Psychology, 36, 291–301. [DOI] [PubMed] [Google Scholar]
- Sadeh A, Sharkey KM, & Carskadon MA (1994). Activity-Based Sleep—Wake Identification: An Empirical Test of Methodological Issues. Sleep, 17(3), 201–207. [DOI] [PubMed] [Google Scholar]
- Shin T, Davison ML, & Long JD (2017). Maximum likelihood versus multiple imputation for missing data in small longitudinal samples with nonnormality. Psychological methods, 22(3), 426–449. [DOI] [PubMed] [Google Scholar]
- Shott S. (2006). Down syndrome: Common otolaryngologic manifestations. American Journal of Medical Genetics Part C: Seminars in Medical Genetics, 142C, 131–140. [DOI] [PubMed] [Google Scholar]
- Shott S, Amin R, Chini B, Heubi C, Hotze S, & Akers R. (2006). Obstructive sleep apnea: Should children with Down syndrome be tested? Archives of Otolaryngology Head Neck and Surgery, 132, 432–436. [DOI] [PubMed] [Google Scholar]
- Stebbens V, Dennis J, Samuels M, Croft C, & Southall D. (1991). Sleep related upper airway obstruction in a cohort with Down’s syndrome. Archives of Disease in Childhood, 66, 1333–1338. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Steenari MR, Vuontela V, Paavonen EJ, Carlson S, Fjalberg M, & Aronen E. . (2003). Working memory and sleep in 6- to 13- year old school children. Journal of the American Academy of Child and Adolescent Psychiatry, 42, 85–92. [DOI] [PubMed] [Google Scholar]
- Stores G, & Wiggs LE (2001). Sleep disturbance in children and adolescents with disorders of development: Its significance and management. In: Cambridge University Press. [Google Scholar]
- Stores R. (1993). A preliminary study of sleep disorders and daytime behaviour problems in children with Down’s syndrome. Down Syndrome Research and Practice, 1, 29–33. [Google Scholar]
- Stores R, & Stores G. (1996). Research on sleep problems and psychological function in children with Down syndrome: Implications for clinical practice and everyday care. Down Syndrome Research and Practice, 4, 110–112. [Google Scholar]
- Stores R, & Stores G. (2004). Evaluation of a group-administered instruction for parents to prevent or minimize sleep problems in young children with Down syndrome. Journal of Applied Research in Intellectual Disabilities, 17, 61–70. [Google Scholar]
- Stores R, Stores G, Fellows B, & Buckley S. (1998). A factor analysis of sleep problems and their psychological associations in children with Down’s syndrome. Journal of Applied Research in Intellectual Disability, 17, 345–354. [Google Scholar]
- Thackeray EJ, & Richdale AL (2002). The behavioral treatment of sleep difficulties in children with an intellectual disability. Behavioral Interventions, 17, 211–231. [Google Scholar]
- Tikotzky L, & Sadeh A. . (2010). The role of cognitive–behavioral therapy in behavioral childhood insomnia. Sleep medicine, 11(7), 686–691. [DOI] [PubMed] [Google Scholar]
- Veatch OJ, Reynolds A, Katz T, Weiss SK, Loh A, Wang L, & Malow BA (2016). Sleep in children with autism spectrum disorders: How are measures of parent report and actigraphy related and affected by sleep education? Behavioral sleep medicine, 14(6), 665–676. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Weiskop S RA, Matthews J. . (2005). Behavioral treatment to reduce sleep problems in children with autism or fragile X syndrome. Dev Med Child Neurol, 47, 94–104. [DOI] [PubMed] [Google Scholar]
- Wiggs L, & France K. (2000). Behavioural treatments for sleep problems in children and adolescents with physical illness, psychological problems, or intellectual disabilities. Sleep medicine reviews, 4, 299–314. [DOI] [PubMed] [Google Scholar]
- Wiggs L, & Stores G. (1996). Sleep problems in children with severe intellectual disabilities: What help is being provided? Journal of Applied Research in Intellectual Disabilities, 9, 160–165. [Google Scholar]
- Wiggs L, & Stores G. (1998). Behavioural treatment for sleep problems in children with severe learning disabilities and challenging daytime behaviour: effect on sleep patterns of mother and child. Journal of Sleep Research, 7, 119–126. [DOI] [PubMed] [Google Scholar]
- Wright B, Sims D, Smart S, Alwazeer A, Alderson-Day B, Allgar V, & Miles J. (2011). Melatonin versus placebo in children with autism spectrum conditions and severe sleep problems not amenable to behaviour management strategies: A randomised controlled crossover trial. Journal of Autism and Developmental Disorders, 41(2), 175–184. [DOI] [PubMed] [Google Scholar]
- Yang Y, Conners FA, & Merrill EC (2014). Visuo-spatial ability in individuals with Down syndrome: Is it really a strength? Research in developmental disabilities, 35(7), 1473–1500. [DOI] [PMC free article] [PubMed] [Google Scholar]