Abstract
Inflammasomes are multiprotein complexes with an important role in the innate immune response. Canonical activation of inflammasomes results in caspase-1 activation and maturation of cytokines interleukin-1β and -18. These cytokines can elicit their effects through receptor activation, both locally within a certain tissue and systemically. Animal models of kidney diseases have shown inflammasome involvement in inflammation, pyroptosis and fibrosis. In particular, the inflammasome component nucleotide-binding domain-like receptor family pyrin domain containing 3 (NLRP3) and related canonical mechanisms have been investigated. However, it has become increasingly clear that other inflammasome components are also of importance in kidney disease. Moreover, it is becoming obvious that the range of molecular interaction partners of inflammasome components in kidney diseases is wide. This review provides insights into these current areas of research, with special emphasis on the interaction of inflammasome components and redox signalling, endoplasmic reticulum stress, and mitochondrial function. We present our findings separately for acute kidney injury and chronic kidney disease. As we strictly divided the results into preclinical and clinical data, this review enables comparison of results from those complementary research specialities. However, it also reveals that knowledge gaps exist, especially in clinical acute kidney injury inflammasome research. Furthermore, patient comorbidities and treatments seem important drivers of inflammasome component alterations in human kidney disease.
Keywords: acute kidney injury, chronic kidney disease, kidney transplantation, inflammasome, redox signalling, endoplasmic reticulum stress, interleukin-18, interleukin-1β, NLRP3, AIM2, caspase-8
1. Introduction to Kidney Disease and Inflammation in Kidney Disease
The 2020 analysis of the Global Burden of Disease Study 1990–2017 estimated the global prevalence of chronic kidney disease (CKD) to be 9.1%, corresponding to 697.5 million cases [1,2]. The all-age global prevalence of CKD increased by 29.3% during this period due to aging of the population globally. The number of deaths at all ages attributable to CKD increased by 41.5%. While age-standardized CKD mortality did not change between 1990 and 2017, it declined by 41.3% for chronic obstructive pulmonary disease, 30.4% for cardiovascular disease, and 14.9% for cancer [1,2]. Therefore, new strategies for early detection and prevention of CKD and the development of more effective therapies are needed.
CKD can result from kidney injuries of any cause if the process of injury was of sufficient duration and/or intensity. It is defined by the presence of decreased kidney function or kidney damage for more than three months, while acute kidney injury (AKI) comprises multiple renal conditions associated with a sudden decrease in kidney function. Kidney disease refers to a heterogeneous group of disorders that impact on the function or structure of the kidney. It is important to note that even milder reductions in kidney function are associated with an increased risk for AKI, complications in other organ systems than the kidneys, and mortality [3].
Inflammatory and immunological processes are intimately linked to kidney disease. They contribute to the initiation of injury in AKI, for example during sepsis, but also play an important role for AKI’s extension and maintenance phase [4], and AKI-to-CKD transition [5]. In addition, inflammatory and immunological processes are involved in a wide range of CKD causes, like diabetes mellitus or systemic lupus erythematosus. Importantly, they also contribute to CKD progression, independent of the underlying cause [6]. Finally, CKD-related morbidity, such as CKD-attributable cardiovascular disease (CVD), and CKD mortality are related to chronic inflammation [7].
Inflammasomes and inflammasome components have an important role in infectious and non-infectious tissue injury [8]. This review discusses the involvement of inflammasome components in the pathophysiology and course of kidney diseases. Excellent reviews have been published on the topic, reporting inflammasome taxonomy and tissue expression pattern, molecular mechanisms of inflammasome activation and function, respective renal disease models, roles in human kidney disease, inflammasome gene mutations and polymorphisms, and inflammasome-related therapeutic options, such as the Refs. [9,10,11].
In this review, we focus on up-to-date information on inflammasome component-related molecular mechanisms that contribute to the pathogenesis of kidney diseases. We put special emphasis on the interrelations between inflammasome components and redox-signalling, mitochondria, and endoplasmic reticulum stress. Furthermore, we aggregate available clinical data on inflammasome component-related gene expression, protein, and protein activity analyses, to help the assessment of inflammasome relevance in human kidney disease. Genetic polymorphisms of inflammasome components are not covered.
2. Introduction to Inflammasome Components and Inflammasome Biology
The inflammasome structurally contains a sensory component, a mediator of caspase-associated recruitment domains (CARD), apoptosis-associated speck-like protein containing a CARD (ASC), and procaspase-1 [10]. The inflammasome sensors are classified as nucleotide-binding domain-like receptors (NLRs), missing in melanoma 2–like receptors (ALRs), and the newly discovered pyrin, based on structural criteria [12] (Table 1). Some members of the group, such as NLRP1, NLRP3, and NLRC4, have been identified as inflammasome-forming NLRs, while others, such as NLRP6 and NLRP12, are still classified as prospective inflammasome sensors [12]. Additional molecules that could trigger caspase-1 include NLRP2, NLRP7, NLRP9, a retinoic acid-inducible gene I (RIG-I), and interferon-inducible protein 16 (IFI16), although the molecular basis is less understood [13]. A comprehensive overview of inflammasome components, their functions and activators is given in the Supplementary Table S1 online. The NLRP3-inflammasome has been widely studied and characterized. The NLRP (receptor protein) has a NACHT configuration in the centre (known as the ‘nucleotide-binding oligomerization domain (NOD)’), an LRR domain (leucine-rich repeat) at the C terminus, along with a CARD domain/Pyrin Domain (PYD) at the amino-terminal end. The ASC protein (a mixture of PYD and CARD) may bind with NLRP3′s amino-terminal pyrin domain thus stimulating procaspase-1 [10]. NLRP3, ASC and procaspase-1 form a multiprotein inflammasome complex that activates caspase-1. Active caspase-1 converts pro-interleukin-1β (pro-IL-1β) and pro-interleukin-18 (IL-18) to mature IL-1β and IL-18 [14]. Production of mature IL-1β and IL-18 involves two distinct mechanisms: the production of pro-IL-1β and pro-IL-18 by nuclear factor κ-light-chain-enhancer of activated B cells (NF-κB) signalling (termed “signal 1”) and their cleavage by caspase-1 to generate mature IL-1β and IL-18 (termed “signal 2”) [14]. Signal 1 regulates the expression of numerous inflammasome components as well as the synthesis of “pro” forms of cytokines, even though the NLRP3 inflammasome complex is only important in signal 2. Pathogen-associated molecular patterns (PAMPs) and danger-associated molecular patterns (DAMPs) activate the toll-like receptor (TLR)-mediated NF-κB signalling to provide signal 1, whereas various metabolically and environmentally formed crystal particles and adenosine triphosphate (ATP) provide signal 2 for activating the inflammasome complex [15]. These stimuli that activate the inflammasome are different from the ones that provide signal 1. As a result, the inflammasome acts as a detector, and its induction is a frequent way for cells to react to cellular stress.
Table 1.
Proposed inflammasome components and molecular mechanisms related to their function and activation. The table gives the components most consistently reported in kidney disease to date.
| Inflammasome Component | Structure | Function | Activator | Reference |
|---|---|---|---|---|
| NLRP3 | Consists of three components-NLRP3 scaffold, PYCARD adaptor (ASC), which functions as a caspase-1 activator, and caspase-1. | Initiates an inflammatory form of cell death and triggers the release of proinflammatory IL-1β and IL-18. | Bacterial and viral nucleic acids, LPS, and damage-associated molecular patterns, such as ATP, uric acid, and amyloid β peptides. Ionic flux, mitochondrial dysfunction, and the production of reactive oxygen species, and lysosomal damage have been shown to trigger its activation. | [12,21,22] |
| NLRP6 | Composed of three domains. The N-terminus consists of a pyrin domain (PYD) and is considered the essential element for inflammasome assembly as it interacts with ASC. The NBD builds the central module of NLRP6 and is followed by the C-terminal LRR domain, which senses DAMPs and MAMPs. | Regulates the production of IL-18 | Bacterial products, bacterial acylated lipopeptides | [28,29,30] |
| NLRC4 | NLRC4 contains a common three-domain structure: An N-terminal homotypic interaction domain, a central nucleotide-binding domain, and a series of C-terminal LRRs. | The activation of NAIP proteins attracts and activates NLRC4, which in turn attracts caspase-1 either directly or indirectly through ASC, causing inflammatory responses | Bacterium’s type 3 secretion system (T3SS) and flagellin | [31,32] |
| NLRC5 | Largest NLR family member, consisting of 1 866 aa with C-terminal 27 LRRs. Sequence analysis suggests that NLRC5 is most similar to CIITA among NLR family members. | Regulation of MHC class I gene expression, inflammasome activation in response to bacterial infection through a mechanism involving heterodimerization with NLRP3. | Bacterial PAMPs and crystals | [33,34,35] |
| AIM2 | AIM2 consists of two domains connected through a long linker: an N-terminal PYD domain, and a C-terminal HIN-200 domain. HIN-200 region directly binds to DNA while the PYD region mediates protein-protein interaction. | Triggers the formation of inflammasomes that also contain ASC and caspase-1, and that induce the cleavage of caspase-1, the maturation ofIL-1β, IL-18, and pyroptosis. | dsDNA, exogenous DNA of bacteria (e.g., Listeria monocytogenes) and viruses (e.g., Papillomavirus), as well as endogenous DNA. | [36,37,38,39] |
| Pyrin | Pyrin is coded by the MEFV gene, and its mature form includes a PYD, two B-boxes, and a coiled-coil domain. | Causes various modifications (glycosylation, adenylation, ADP-ribosylation, etc.) of Rho GTPases, causing the rearrangement of the cytoskeleton and subsequent activation of pyrin inflammasomes. | Clostridium difficile TcdB, Clostridium botulinum C3, and Vibrio parahaemolyticus VopS proteins. | [40,41] |
| ASC | Two death domains (pyrin and CARD). ASC interacts with cell death executioners. | ASC is a central adaptor molecule of the inflammasome complex, activation of caspase-1, mediates the secretion ofIL-1β and IL-18. | Activation of NLRP3 protein recruits ASC. | [42,43] |
| Caspase-1 | Active Caspase-1 contains two heterodimers of p20 and p10. It contains a catalytic domain with an active site that spans both the p20 and p10 subunits, as well as a noncatalytic CARD. | Activated Caspase-1 proteolytically cleaves pro IL-1β and pro-IL-18 into their active forms, IL-1β and IL-18. The active cytokines lead to a downstream inflammatory response. It also cleaves Gasdermin D into its active form, which leads to pyroptosis. | Autoactivates when it is assembled into the filamentous inflammasome complex by autoproteolysis into the p10 and p20 subunits. | [44,45] |
NBD—Nucleotide-binding domain; LRR—leucine rich repeat; CARD—caspase activation and recruitment domains; PYCARD—PYD And CARD Domain Containing; CASP1—caspase-1; IL—interleukin; ASC—apoptosis-associated speck-like protein containing a CARD; DAMPs—damage-associated molecular patterns; MAMPs—microbe- or pathogen-associated molecular patterns; NLR—NOD-like receptor; NFκB—nuclear factor κ-light-chain-enhancer of activated B cells; MHC—major histocompatibility complex; NAIP—neuronal apoptosis inhibitory protein; CIITA—class II transactivator; AIM2—Absent In Melanoma 2; HIN—Hematopoietic expression, interferon-inducible nature, and nuclear localization; MEFV—mediterranean fever; CGAS—Cyclic GMP-AMP synthase; LPS—lipopolysaccharide; ATP—adenosine triphosphate, NLRC—NLR family CARD domain-containing protein.
Interestingly, NLRP3 signalling during chronic tissue remodelling does not necessarily involve the inflammasome complex. NLRP3 expression in epithelial cells affects tissue remodelling following chronic trauma in parallel with cell death signalling. For example, NLRP3 enhanced angiogenesis in mice, independent of IL-1β, during cutaneous wound healing [16]. Moreover, an NLRP3 augmented transforming growth factor β (TGFβ)/Smad signalling pathway independent of inflammasome leads to tubular-interstitial fibrosis during development of CKD [9,17]. Furthermore, similar to epithelial cells, it was found that an NLRP3-augmented TGFβ-Smad pathway in kidney fibroblasts independent of the inflammasome complex contributed to kidney fibrosis [18].
The diversity reported in NLRP3 activation highlights the inflammasome’s potential role as a detector of stress/injury in cells that operates as a unifying mechanism [19,20]. Three main mechanisms have been proposed to activate the NLRP3 inflammasome: cathepsin B release following lysosomal injury, potassium efflux, and phagocytosis [21,22]. Canonical inflammasome activation signalling involving caspase-1 has been extensively studied. The mechanisms involved in non-canonical inflammasome activation signalling via caspase-11 or other effectors, such as caspase-4, caspase-5, and caspase-8, are still incompletely understood [19,23].
In addition, post-translational protein modifications (PTMs) contribute to the regulation of inflammasome components’ activity and degradation. Such PTMs have been reported for inflammasomes’ sensory and ASC components, and include phosphorylation, ubiquitination, deubiquitination, S-nitrosylation, and SUMOylation [24,25]. Bruton tyrosine kinase interacts with both ASC and NLRP3 through kinase domains. This is thought to promote activation of the NLRP3 inflammasome. Therefore, the bruton tyrosine kinase inhibitor ibrutinib was recently tested in two kidney disease models, but the results for this substance were not promising [26]. While PTMs are frequently reported in kidney disease [27], the specific PTMs of inflammasome components in kidney disease have not been established yet, and further research is indispensable.
3. Inflammasome Components in AKI
3.1. Preclinical Data on Inflammasome Components in AKI
AKI is associated with a high incidence of cell death and the production of cellular debris. The most prominent cause of acute renal damage is acute tubular necrosis (ATN). ATN is linked to an inflammatory response comprising monocytes/macrophages and neutrophil infiltration, which exacerbates the kidney damage [46,47]. The evidence for the inflammasome’s function in acute renal disease is compelling. Deficiency of the inflammasome component, caspase-1, in animals provides resistance against AKI in several models such as cisplatin and ischemia-induced acute renal failure [48,49,50,51]. Moreover, inhibition of NLRP3 by hydroxychloroquine decreased NF-κB signalling, as well as cathepsin-B and L activities, and protected rodents from AKI [52]. The extracellular matrix (ECM) components biglycan and hyaluronan, as well as ATP acting via P2X7 receptors, activated the NLRP3 inflammasome [53,54]. The pathogenic roles of the NLRP3 inflammasome have been demonstrated in ischemia-reperfusion injury (IRI) [52,55,56,57], folic acid-induced AKI [58], rhabdomyolysis-induced kidney injury [59], and contrast-induced kidney injury [60]. During contrast-induced AKI, canonical NLRP3 inflammasome activation in local and migratory macrophages led to elevated IL-1β levels in mice, whereas Nlrp3-/- animals were protected [60]. Infection-induced AKI models have also been shown to activate canonical inflammasomes. In a caecal ligation puncture model (sepsis-induced AKI), NLRP3 deficiency and caspase-1 suppression reduced kidney injury, inflammation, and caspase-1 activation [61]. Furthermore, mice with caspase-1 deficiency were protected from endotoxemic AKI, hypotension, and mortality caused by LPS [62]. Neutralization of IL-1β and IL-18, on the other hand, was unable to reverse LPS-induced AKI, implying that the non-canonical inflammasome and pyroptosis play an important role [63]. Accordingly, the induction of pyroptosis has been related to caspase 11 upregulation, the non-canonical pyroptosis pathway, demonstrated in renal proximal tubular cells treated with LPS. The authors suggest that pyroptosis induction could be an early event in septic models [64]. Furthermore, Astragaloside-IV protected from cisplatin-induced AKI by promoting autophagy and inhibiting NF-kB signalling, thus lowering the expression of inflammasome components [65]. Interestingly, NLRP1 activation was elevated in cisplatin-induced AKI, likely upstream of caspase-1 activation [66]. In this model, the deletion of caspase 11 promotes the downregulation of IL-18 urine secretion, decreasing tubular damage, immune macrophage, and neutrophil infiltration, and attenuating renal dysfunction. On the other way, caspase 11 upregulation induces the cleavage of gasdermin D into gasdemin N to trigger pyroptosis [67]. In addition, the deletion of GASMDE, a member of the GASDM family, decreases cisplatin-induced damage by blocking pyroptosis and IL-1β release [68]. During the pathogenesis of uric acid-induced nephropathy, uric acid crystals activate the NLRP3 inflammasome, suggesting a novel pathomechanism of crystalline nephropathy [69]. The NLRP3 inflammasome complex must be activated for renal IL-17A to be produced, which is an essential proinflammatory cytokine in AKI [70]. The discovery of the underlying mechanisms could assist the therapeutic suppression of IL-17A in AKI. Further, Deplano et al. reported that P2X7R (P2X purinoceptor) deficiency in rats reduced the activation of NLRP3-inflammasome in macrophages, and also crescentic glomerular damage in experimental nephrotoxic nephritis coupled with crescentic glomerulonephritis [71]. Together, these data suggest that inflammasome components can be used as therapeutic targets for treatment of AKI.
Several of the signalling molecules involved in regulating programmed cell death also modulate inflammasome activation in a cell-intrinsic manner. Necroptosis is typically seen as a back-up that kicks in when apoptosis is prevented; pyroptosis is a fundamental cellular mechanism triggered by the inflammasome in response to a wide spectrum of PAMPs and DAMPs [72]. Activation of inflammatory caspases such as caspase-1, caspase-4, caspase-5, and caspase-11 leads to pyroptosis, which relies on gasdermin-D to produce plasma membrane pores [73]. Due to their limited potential to release IL-1β, the prevalence of pyroptosis in tubular epithelial cells (TECs) has been disputed [74]. Pyroptosis, characterised by elevated caspase-1 activation and IL-1β production, has been proposed to emerge in kidney tubular cells during renal IRI [75].
Mice lacking distinct inflammasome components were utilised to establish the inflammasome’s participation in several experimental models of renal damage, but the specific role of intrinsic renal cells in inflammasome activation remains unknown [76]. Some studies stated that TEC apoptosis and pyroptosis are the key drivers of contrast-induced AKI [77,78], others did not show TEC apoptosis [60,79], canonical inflammasome formation in TECs, or IL-1β release from TECs in response to contrast-induced AKI [60,80]. Necroptosis mediated NLRP3 inflammasome plays a key role in the pathogenesis of lupus nephritis [81] as well as the transition from AKI to CKD [82]. Emerging evidence indicates that several signalling mechanisms that had been assumed to be biochemically independent for a long time communicate with one another. Nevertheless, the impact of apoptotic and regulated necrosis signalling molecules on the inflammasome is inconsistent and depends on the cell type and cellular environment. As a result, we are still a long way from understanding how these chemicals lead to altered inflammasome expression across various settings, as well as why these cell death mechanisms have developed to participate in inflammasome activation.
3.1.1. Preclinical Data on Redox Signalling and Inflammasome Components in AKI
Inflammasome activation via reactive oxygen species (ROS) has been fully reported upstream of NLRP3 priming. The latter has been demonstrated by ROS inhibitors that blocked the priming steps of inflammasome [83,84]. Additionally, it has been hypothesized that DAMPs and PAMPs enhance ROS generation by interacting with pattern recognition receptors, inducing inflammasome activation. In IRI-induced AKI, the renal damage produces endogenous DAMPS, heat shock protein and ATP, which prompts ROS and activates inflammasome to cause tubular necrosis [55]. Moreover, in vitro, the treatment of human kidney proximal epithelial (HK-2) cells with hydrogen peroxide (H2O2), a ROS, activates NLRP3 [85].
Nicotinamide adenine dinucleotide phosphate (NADPH) oxidases (NOXs) and mitochondria are the principal ROS sources that induce inflammasome activation [86,87,88,89]. NOXs are a family of seven members, of which NOX1, NOX2, NOX4 and NOX5 are expressed in renal cells [90]. The production of radical anion superoxide (O2•−) by NOXs requires the assembly of p22- and glycoprotein 91 (gp91)-phox membrane subunits with the cytoplasmic subunits p47-, p67-phox, and ras-related C3 botulinum toxin substrate 1 (Rac1) [91]. In macrophages, using diphenyleneiodonium (DPI), a NOXs blocker, inhibits the proteolytic excision of caspase-1, impeding inflammasome activation [92]. The levels of NOXs are commonly elevated in AKI models, leading to ROS overproduction and then inflammasome activation [93]. NOXs activation also promotes the upregulation of redox signalling, such as NF-κB and activating protein-1 (AP-1) via mitogen-activated protein kinases (MAPK), which are involved in the priming steps of inflammasome activation [94]. Moreover, ROS induces NF-κB activation through degradation of its inhibitor, IκBα, releasing and permitting NF-κB translocation to the nucleus to induce the expression of inflammatory genes, including NLRP3 [95,96,97].
Liu et al. [98] showed that death-associated protein kinase (DAPK) was implicated in NLRP3 activation via ROS overproduction in an AKI model induced by paraquat (PQ) (Table 2) [98]. PQ is an herbicide used in agricultural production that produces ROS, involving cyclic reactions of reduction/oxidation, which ultimately activates NF-κB and DAPK. PQ also upregulates the phosphorylation of IκBα, which induces nuclear translocation of NF-κB, the activation of DAPK, and consequently, NLRP3 induction. The use of BAY, an inhibitor of NF-κB, attenuated this NLRP3 induction [98]. This group also showed that the DAPK inhibitor impedes caspase-1 activity, but it could not attenuate NLRP3, suggesting that DAPK protein is not required to priming steps of inflammasome but for activation. Moreover, Mahmoud et al. [99] reported that the activation of peroxisome proliferator-activated receptor-gamma (PPARγ) by ferulic acid mitigates inflammation through prevention of NLRP3 activation via NF-κB inhibition and nuclear factor erythroid 2-related factor 2 (Nrf2) activation in a methotrexate-induced nephrotoxicity model. In this model, the activation of Nrf2 and PPARγ prevents the upregulation of NF-κB, suggesting that the activation of these proteins decreases ROS, preventing NLRP3 priming. In summary, the generation of ROS in AKI models promotes NLRP3 priming by reducing antioxidant response and augmenting the inflammatory response that triggers NLRP3 assembly.
Table 2.
Redox-signalling pathways-induced inflammasome activation in AKI.
| AKI model | Redox-Signalling-Induced Inflammasome Activation | Effects | Reference |
|---|---|---|---|
| PQ-induced nephrotoxicity | ROS/NF-κB/DAPK/NLRP3 | PQ produces ROS, activating to NF-κB and DAPK that in turn activates NLRP3 | [98] |
| Methotrexate-induced nephrotoxicity | decreasedNrf2/ARE/HO-1 signalling and PPARγ | Methotrexate induces the decreasing of Nrf2 and PPARγ by promoting ROS, leading to antioxidant system decrease and lipid peroxidation | [99] |
| Ceftriaxone-induced urolithiasis | decreasedNrf2/HO-1 | Ceftriaxone promotes inflammation and oxidative stress by activating NLRP3 and inducing ROS production and decreasing the antioxidant system. | [100] |
NF-κB: nuclear factor κB; NLRP3: Nod-like receptor (NLR) family pyrin domain containing 3; Nrf2: nuclear factor erythroid 2-related factor 2; ROS: reactive oxygen species; PQ: paraquat; DAPK: death-associated; antioxidant response element (ARE); heme oxygenase 1 (HO-1); PPARγ: peroxisome proliferator-activated receptor-gamma; mitogen-activated protein kinases (MAPK).
3.1.2. Preclinical Data on Mitochondria and Inflammasome Components in AKI
Mitochondria are essential in the kidney because they produce the energy, in ATP form, required for tubular reabsorption, principally in the proximal tubules (PT) [101]. Mitochondrial disruption commonly leads to inflammasome activation through mitochondrial ROS (mtROS) production [102,103]. Following this, the use of antioxidants that target mitochondria like (2-(2,2,6,6-Tetramethylpiperidin-1-oxyl-4-ylamino)-2-oxoethyl)triphenylphosphonium chloride (mitoTEMPO), N-acetyl cysteine (NAC), and resveratrol can prevent inflammasome activation [89,103,104]. Additionally, the use of rotenone, an inhibitor of complex I, has been shown to inhibit NLPR3 by reducing mtROS and mitochondrial dysfunction [105]. Moreover, IL-22, an interleukin that reduces loss of membrane potential and avoids mtROS production, impeded NLRP3 activation in the acetaminophen-induced AKI model [102].
Mitochondrial proteins have been implicated in inflammasome activation via mtROS [89,106]. Excessive mtROS promotes NLRP3-thioredoxin-interacting protein (TXNIP) translocation from cytosol to the mitochondria, where TXNIP interacts with mitochondrial thioredoxin 2 (TRX2), inhibiting TRX2 activity. TXNIP is then released and directly binds to leucine-rich regions of NLRP3, leading to inflammasome formation [106]. During IRI, mtROS trigger the interaction between TXNIP and NLRP3 three days after reperfusion, promoting renal injury [89]. In addition, mitochondrial antiviral signalling protein (MAVS) is an adaptor protein involved in the relocalization and association of NLRP3 to the mitochondria, facilitating its oligomerization with caspase-1 and ASC [88].
Proteins participating in mitochondrial dynamics also activate the inflammasome. Mitochondrial dynamic comprises the balance between mitochondrial fission and fusion, which requires the recruitment of proteins to the mitochondria that carry out fission or fusion [107,108]. Moreover, under ROS production, the mitochondrial fission protein dynamic related protein 1 (DRP1) is recruited to the outer mitochondrial membrane to trigger fission. However, excessive mitochondrial fission impairs mitochondrial function, causing further mtROS production and inflammasome activation [109]. In accordance with this, Liu et al. [110] found that DRP-1 promotes NLRP3 activation during sepsis-induced AKI, triggered by LPS, highlighting the involvement of mitochondrial proteins in the promotion of inflammation via NLRP3. Supporting this, the use of mitochondrial division inhibitor 1 (Mdivi-1), a DRP1 inhibitor, decreases the inflammasome components (i.e., NLRP3, caspase-1, IL-1β and IL-18) [110]. Furthermore, the upregulation of DRP-1 leads to mitophagy induction, which is a negative regulator of inflammasome; but, defective mitophagy is commonly found in AKI, contributing to mtROS accumulation [111,112,113]. In line with this, in contrast-induced AKI, the contrast agent iohexol increases NLRP3 and malondialdehyde (MDA) (a biomarker of oxidative stress) levels and decreases the antioxidant activity of superoxide dismutase (SOD) and the protein levels of phosphatase and tensin homolog (PTEN)-induced putative kinase 1 (PINK1), Parkin and microtubule-associated proteins 1A/1B light chain 3B (LC3-II) [114]. Likewise, the autophagy inhibitor 3-methyladenine (3-MA) promotes inflammasome activation [113]. Furthermore, in the contrast-induced AKI model, the activation of mitophagy by PINK1/Parkin prevents NLRP3 activation, decreasing kidney injury by avoiding apoptosis in renal tubular epithelial cells [97]. Besides, the lowering of sirtuin 1 (SIRT1) and 3 (SIRT3), involved in mitophagy, promote inflammasome activation in AKI models [85,115,116]. SIRT1 and SIRT3 are NAD-dependent deacetylases that protect against mitochondrial dysfunction by avoiding mtROS, and their deregulation has been associated with mitophagy deregulation, leading to inflammasome activation [115,117]. Gao et al. [117] reported that in sepsis induced by caecal ligation and puncture (CLP), the blocking of mitophagy upregulates NLRP3, ASC, caspase-1 and IL-1β. In addition, the inhibition of SIRT1 with EX527 blocked Parkin translocation to the mitochondria, suggesting that SIRT1 is required for mitophagy activation, impeding inflammasome stimulation [80]. In the CLP model, a perforation in the cecum is realized, which permits fecal material release into the peritoneum, generating an exacerbated immune response, including NLRP3 inflammasome activation [118]. Therefore, mitophagy is required to avoid excessive NLRP3 activation in this model.
In addition to mitophagy, crosstalk between inflammasome and intrinsic apoptosis has been found in leucocytes, involving caspase-8 and caspase-1 [119,120]. In this context, in IRI-induced AKI, NLRP3 modulates a non-canonical caspase-8-activating platform at the mitochondria, required for epithelial cell death. Although caspase-8 activation via NLRP3 is independent of canonical NLRP3 activation, it involves ASC, suggesting that caspase-8 is a downstream protein of NLRP3 [80].
In summary, mitochondria are involved in inflammasome activation in AKI models, related to mtROS production. The latter activates the proteins TXNIP and MAVS, imported to the mitochondria to induce inflammasome assembly by interacting with NLRP3. On the other hand, mitophagy is considered an inflammasome inhibitor, since its blocking activates NLRP3 components.
3.1.3. Preclinical Data on Endoplasmic Reticulum Stress and Inflammasome Components in AKI
Endoplasmic reticulum-stress induces inflammasome activation by triggering unfolded protein response (UPR) [121]. The three pathways of ER stress have been implicated in inflammasome activation: pancreatic eukaryotic translation initiation factor 2a (eIF2a) kinase (PERK), inositol-requiring protein 1 (IRE1α) and activating transcription factor 6 (ATF6) by promoting caspase-1 activation and IL-1β maturation [122]. In vitro, the three signalling pathways induce UPR activation in cadmium-induced nephrotoxicity, leading to NLRP3 activation [123]. In addition, the activation of SIRT1 avoids these effects by generating the deacetylation of X-box binding protein-1 (XBP-1), a member of IRE-1α, into its spliced form XBP-1s, suggesting that SIRT1 is required to avoid inflammasome activation mediated by ER stress [123]. Guo et al. [124] reported that the use of taurine-conjugated ursodeoxycholic acid, a chemical chaperone that alleviates ER stress, prevents inflammasome activation in aldosterone-induced AKI. Moreover, resting NLRP3 localizes in the ER; meanwhile, ASC and NLRP3 localize in perinuclear membranes of ER and mitochondria under inflammasome activation [113,125]. These works suggest that ER is crucial in inflammasome activation. Indeed, Wang et al. [125] demonstrated that HK-2 cells stimulated with angiotensin II (Ang II) showed inflammasome activation and ER-stress induction and the pretreatment with 4-PBA, an ER-stress inhibitor, decreases ASC and NLRP3 [125]. It has been proposed that ER-stress-induced inflammasome activation is mediated by ROS overproduction. Following the latter, in rhabdomyolysis-induced AKI, the treatment with the antioxidant anisodamine decreases inositol-requiring enzyme-1α (IRE-1α), CCAAT/enhancer-binding protein (C/EBP) homologous protein (CHOP) and activating transcription factor 4 (ATF4), reducing NLRP3 inflammasome components (i.e., NLRP3, caspase-1, IL-1β and IL-18) [126], suggesting that ROS is implicated in ER-stress protein’s activation. The authors also showed that inflammasome activation induced via ER stress depends on the interaction between NLRP3 and TXNIP [126]. Thus, these results highlight that activation of the inflammasome by mitochondria and ER stress are intimately related in AKI models.
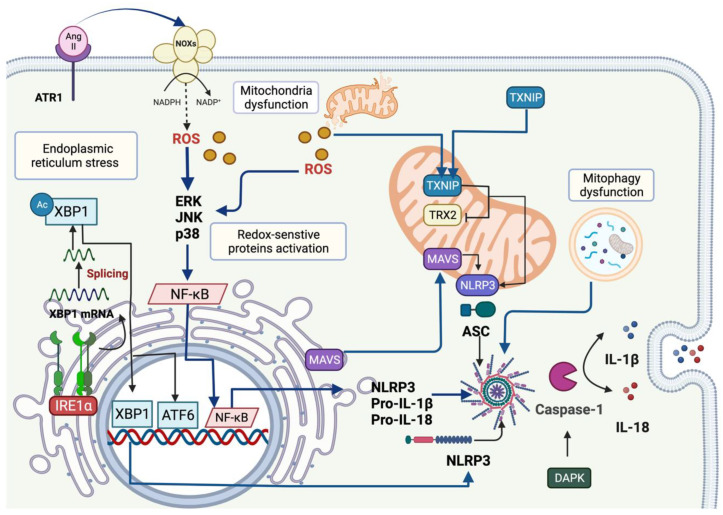
Figure 1 summarizes the mechanisms that induce NLRP3 inflammasome activation mediated by redox signalling, mitochondria and ER stress in renal epithelial cells of AKI models.
Figure 1.
Activation of NOD-like receptor (NLR) family pyrin domain containing 3 (NLRP3) inflammasome mediated by redox-signalling, mitochondria and ER-stress during acute kidney injury (AKI). The binding of angiotensin II (Ang II) to its receptor, the angiotensin receptor 1 (ATR1), triggers ROS overproduction by stimulating NADPH oxidases (NOXs) in the plasmatic membrane. The ROS produced by NOXs induces the activation of the redox-sensitive pathway, including extracellular signal-regulated kinase (ERK), c-Jun N-terminal kinases (JNK) and p38, proteins able to activate to nuclear factor κ-light-chain-enhancer of activated B cells (NF-κB). The latter promotes the transcription of target genes such as NLRP3, pro-interleukin 1 β (IL-1β) and pro-interleukin 18 (IL-18). Under mitochondrial dysfunction, ROS are also generated, named mitochondrial ROS (mtROS), which activates the priming steps of inflammasome mediated by the redox-sensitive proteins. Further, excessive mtROS leads to the translocation of thioredoxin-interacting protein (TXNIP) from the cytosol to the mitochondria. In mitochondria, TXNIP interacts with thioredoxin 2 (TRX2), inhibiting its activity, promoting the interaction with NLRP3 protein. Moreover, mitochondrial antiviral signalling protein (MAVS), an adaptor protein, is involved in NLRP3 localization in the mitochondria and participates in the inflammasome formation by promoting this interaction with apoptosis-associated speck-like protein containing a CARD (ASC). High levels of mtROS activate mitophagy, a negative regulator of inflammasome; however, in the case of AKI, mitophagy is dysfunctional, contributing to NLRP3 inflammasome activation. Another protein that activates inflammasome is death-associated protein kinase (DAPK), which activates caspase-1. On the other hand, the endoplasmic stress reticulum produces ROS and triggers unfolded protein response (UPR), recognized as an inductor of the inflammasome. Figure created using BioRender (Toronto, ON, Canada).
3.1.4. Preface to Clinical Data Analyses of Inflammasome Components in Kidney Disease
In 2010, it was shown that the gene expression of NLRP3 was significantly increased in nondiabetic patients with different causes of acute and chronic kidney diseases. These analyses included renal tissue samples from patients with IgA nephropathy and membranous glomerulonephritis, acute tubular necrosis and crescentic glomerulonephritis, and also hypertensive/vascular nephrosclerosis [127]. These data illustrate the potentially widespread involvement of inflammasome components in kidney disease. However, they also highlight the complexity in studying inflammasome components in human kidney disease, as hypertension, diabetes, and other comorbidities are frequent. Additional points of caution can be raised. As in other areas of complex regulations in kidney disease, the combined investigation of a set of meaningful molecular parameters is inevitable. Thorough analyses of gene expression, protein characteristics, and protein activity together enable a better understanding of molecular networks [128]. Ideally, in investigations of inflammasomes, this comprises the gene expression and protein amount of inflammasome-related components, post-translational modifications of the inflammasome sensory components, inflammasome assembly and proteolytic activity, and production of mature interleukins [11]. On the other hand, the spectrum of relevant molecular partners for inflammasome components is wide. It not only includes molecules involved in canonical and non-canonical inflammasome-related mechanisms, but also inflammasome-independent mechanisms of sensory inflammasome components, as repeatedly shown for NLRP3 [10,11]. In addition, intensive molecular links exist for different forms of regulated cell death [8,129] and a close and bi-directional relation to caspase-8 has been reported [11,130]. Caspase-8 was therefore included in our search of clinical data in relation to inflammasome components.
The current review, and a great number of original research articles, report analyses of IL-1β and IL-18 to propose an underlying action or activation of inflammasome components. The creation of functional IL-1β and IL-18 through canonical inflammasome activity has been described in the introduction, and caspase-8-mediated maturation of IL-1β through non-canonical inflammasome activation has been reported [11]. Besides, inflammasome-independent maturation of Il-1β and IL-18 occurs, for example, through neutrophil-derived serine proteases or proteases originating from microorganisms [131]. As a rule of thumb, inflammasome-dependent interleukin processing will be of importance if the involved inflammatory cells are of the monocytes/macrophages lineage. This brings us to the last critical issue of inflammasome component involvement in kidney disease. Interpretation of the data is compartment- and context-dependent. The context in this respect can be a comorbidity, like diabetes mellitus [132], or higher age [133], which themselves influence inflammasome components, and therefore need to be accounted for by matched control groups. The context also includes the state of uraemia in a patient with kidney disease. The uremic toxin indoxyl sulfate, for example, can decrease NLRP3 [134]. Thereby, the degree of renal function impairment, such as GFR, will interfere with the observed NLRP3 regulation in a given kidney disease, and needs to be accounted for in the analyses. The compartment in which inflammasome components are analysed is likewise important for the interpretation. Circulating immune cells are involved in the pathophysiology of several kidney diseases [135], but they are also influenced by uremic conditions [136] and contribute to CKD-dependent morbidity [7]. In the kidney, the mechanisms involving inflammasome components partially differ between resident and recruited immune cells on the one hand, and cells of tubules or glomeruli on the other [11]. In addition, inflammasome-related characteristics like mode of activation and expression level of the components change during the differentiation from monocytes to macrophages or dendritic cells [137,138]. Furthermore, even distant events and organ crosstalk need to be considered. A study of kidney function after liver transplantation suggested a relation between the IRI of the liver transplant and AKI of the organ recipient, involving liver and circulating IL-18 [139]. Not least, the interpretation of inflammasome components in urine requires consideration of several aspects. For example, IL-18 protein was reported to be expressed in intercalated cells from the late distale tubule to the collecting duct of the kidney [140] and tubular epithelial cells [141], and IL-18 protein expression was inducible in glomeruli in Lupus nephritis [142]. Currently, IL-18 is interpreted as a marker of tubule injury [143]. Increased concentrations in the urine could result from renal cell damage, leaderless secretion, or decreased or insufficient tubular reuptake after glomerular filtration, the latter in analogy to IL-2 [144]. Interleukin-18 can predict AKI, but it is not a marker for AKI severity or kidney recovery [145]. This suggests that IL-18 found in urine, mirrors regulated cellular processes rather than just passive loss due to cell damage. While IL-18 is mainly regarded as a proinflammatory cytokine, its role in homeostasis and protection of epithelial and mucosal surfaces has also been stressed [146].
Resulting from all the considerations above, we chose an inclusive approach for our analyses of inflammasome components and inflammasome substrates that have been reported in the clinical research of kidney disease.
3.2. Clinical Data on Inflammasome Components in AKI
Acute kidney injury refers to a sudden loss of kidney function. It can be defined and staged according to the KDIGO guidelines using serum creatinine increase within the prior seven days and/or the presence of oliguria [147]. AKI is a heterogeneous syndrome with a broad range of etiologies. Therefore, the following section summarizes the clinical data on inflammasome components according to the respective AKI cause. It should be noted that in clinical practice, pathophysiological mechanisms in AKI may overlap, thereby complicating the dissection of underlying molecular mechanisms [148].
3.2.1. Cardiac Surgery-Associated AKI (CSA-AKI)
The pathophysiology of CSA-AKI includes mechanisms related to IRI, significant alterations in hemodynamics, oxidative stress, and the activation of inflammatory processes [149]. The use of cardiopulmonary bypass (CPB) leads to the activation of proinflammatory pathways, the coagulation cascade and the complement system. No data on inflammasome components in human CSA-AKI have been published so far. A current trial (NCT04125069) is investigating the relation between preoperative mitochondrial dysfunction, which may contribute to inflammasome activation, and CSA-AKI. In preclinical studies on cardiac transplantation, IRI resulted in the release of RNA [150], and the release of mitochondrial DNA (mtDNA) into human plasma during cardiopulmonary bypass surgery has been reported [151]. Mitochondrial DNA, and especially oxidised mtDNA, which is formed in the presence of ROS, can activate the NLRP3 inflammasome and stimulate IL-1β secretion [152]. Additionally, double-stranded RNA, through interaction with the Toll-like receptor 3 (TLR3), resulted in NF-κB activation [153] and was suggested to activate the NLRP3 inflammasome and IL-1β secretion [150]. Furthermore, activation of NLRP3 by double-stranded RNA has been reported, involving caspase-8 scaffolding function [154]. Similarly, a recent study on CSA-AKI in children suggested an upregulation of TLR3 and NF-κB protein in peripheral blood mononuclear cells (PBMCs), and IL-1β protein in the according serum samples [155].
Increased urinary concentrations of IL-18 protein have been reported in CSA-AKI. The pattern of changes in urinary IL-18 concentration in CSA-AKI is heterogeneous, due to different timing of the sample collection in relation to the operation period/use of CPB, differences in patient populations, and probably also due to differences in patient management. The current data suggest that timing is an important aspect. Studies have so far described one protein concentration peak either approximately 0–6 h after operation [156,157] or 12–18 h after CPB [158,159]. Other studies reported two peaks (approximately 0–6 h and 12–18 h after operation) [160] or a broad peak at these points [161] in paediatric patients. The picture is complicated by the fact that non-AKI patients often show a similar pattern of urinary IL-18, so that the difference between AKI and non-AKI is quantitative, with lower IL-18 concentrations in non-AKI patients at one or several points in time [156,159,160,161,162]. A higher resolution of the temporal pattern of IL-18 concentrations, especially with respect to the start of rises in concentration, and a direct investigation of involved inflammasome components could promote the understanding of CSA-AKI pathogenesis.
3.2.2. Cardiorenal Syndromes (CRS)
Cardiorenal syndrome refers to the bidirectional nature of interactions between dysfunction of the heart and dysfunction of the kidneys, and has been classified into five types [163].
In CRS type 1, acute heart failure results in AKI. The underlying pathophysiology involves hemodynamic and non-hemodynamic mechanisms, including oxidative stress and inflammation [164]. Plasma from patients with CRS type 1, compared to healthy control plasma, induced higher caspase-8 activity in monocytes [165] and caspase-3, -8, -9 activity in renal tubular epithelial cells [166]. Plasma from patients with CRS type 5, where cardiac and renal dysfunction are secondary to the same systemic disorder, induced caspase-3, -8, -9 activity and cell death in renal tubular epithelial cells [167]. In CRS type 1, increased serum concentrations of IL-18 protein were reported [168] and increased plasma IL-1β in CRS type 5 [167]. Inflammasome-mediated non-canonical caspase-8 activation that contributes to IL-1β production has been described [169], but also, in mitochondrial apoptosis, the parallel induction of caspase-8 and the NLRP3 inflammasome, both contributing to IL-1β production [170]. Hence, the involvement of inflammasome components in the pathogenesis of CRS is possible, but has not been directly addressed so far in humans.
3.2.3. Sepsis-Associated AKI (S-AKI)
In S-AKI, the acute reduction in kidney function is associated with infection or sepsis. Several DAMPs that could be involved in human S-AKI have been described [171]. DAMPs contribute to inflammasome priming by increasing the gene expression of inflammasome components, as NLRP3, and inflammasome substrates, like pro-IL-1β [10,11]. This review did not identify direct analyses of inflammasome components in human S-AKI. Increased concentrations of serum IL-1β protein concentration in S-AKI have been reported [172,173]. On the other hand, elevations of IL-1β serum concentrations in human sepsis can be mild [174] or irregular [175], are dependent on the phase of septic state [176], and, in addition, the kidney has a role in IL-1β removal in sepsis as long as diuresis is preserved [177]. Recently, a study reported the association of urinary IL-18 protein concentrations with AKI progression in critically ill patients at the intensive care unit [178] and a meta-analysis of biomarkers in S-AKI reported a correlation of urinary IL-18 and the diagnosis of S-AKI [179].
Serum NLRP3 protein concentrations have been reported in septic patients [180]. The NLRP3 concentrations in this study were significantly higher in patients with septic shock compared to healthy controls, intensive care unit controls, and septic non-shock controls. Higher NLRP3 serum concentrations were associated with higher 30-day mortality. The relation to renal function, hence to S-AKI, was not specifically analysed. Thus, mechanisms of IL-1β and IL-18 elevations and inflammasome-related pathomechanisms in human S-AKI require further study.
3.2.4. Contrast-Induced AKI (CI-AKI)
In CI-AKI, a reduction in kidney function develops after administration of iodinated contrast material. The underlying pathogenesis is multifactorial. Upon injection of iodinated contrast, medullary ischemia and tubular cell toxicity can develop [60]. The generation of reactive oxygen species and the activation of subsequent signalling pathways, as well as the development of oxidative distress, resulting in inflammation and increased apoptosis, have frequently been reported and contribute to kidney function impairment [181].
The involvement of NLRP3 in CI-AKI was recently described [60]. The suggested mechanisms involve contrast-induced activation of the canonical NLRP3 inflammasome in renal macrophages and subsequent IL-1-dependent leukocyte recruitment. The clinical part of the study showed that urinary caspase-1protein was associated with leukocyturia. An increase of urinary IL-18 protein and the tubular injury marker kidney injury molecule-1 (KIM-1) was observed in the period of 12 to 24 h following contrast injection. Urinary IL-1β increase was not observed, and IL-18 and KIM-1 did not predict AKI within 7 days in this cohort. The timing seems to be important in the pathophysiology of CI-AKI. A study in a patient cohort undergoing coronary angiography, reported significant increases for urinary IL-18 and KIM-1 at an earlier time point (6 h post-intervention), which predicted CI-AKI within 2 days [182]. When analysed even earlier, at 2–4 h post-intervention, changes of plasma and urinary IL-18 and KIM-1 concentrations were not significantly different between patients with and without CI-AKI within 3–5 days [183]. Taken together, inflammasome components seem to be part of the multistep/multifactorial pathogenesis of CI-AKI. Further evaluation of the temporal pattern of the underlying pathomechanisms will inform research on the timing and duration of therapeutic and prophylactic measures for CI-AKI.
3.2.5. Further Data on the Involvement of Inflammasome Components in AKI
The gene expression of NLRP6 was reported in human whole-kidney samples [184]. Recently, it was shown in murine nephrotoxic AKI that NLRP6 reduced sterile inflammation and exerted nephroprotective effects [185]. This publication also reported NLRP6 protein expression in human kidney tubules. Based on the comparison of the renal immunohistochemistry between a patient with histologic acute tubular injury and clinical signs of renal function impairment and a healthy control subject, a clinical significance of tubular NLRP6 reduction in human AKI was suggested.
Another publication analysed inflammasome components in a patient with non-traumatic rhabdomyolysis-induced AKI [186]. The kidney biopsy showed tubular injury at the sites of myoglobin and uric acid deposits. Using quantitative immunohistochemistry, the authors showed associated tubular oxidative stress, inflammation and necroptosis. Adjacent to the sites of tubular injury, tubulointerstitial inflammatory infiltrates and increased staining for adaptor protein ASC and active caspase-1 were reported, pointing to inflammasome activation.
3.2.6. Acute Kidney Allograft Dysfunction
Immediate Post-Transplantation
Reperfusion injury or postischemic ATN is the most common cause of delayed graft function (DGF) in kidney transplantation. An involvement of inflammasome components in IRI in the human kidney has been suggested. In kidney cortical biopsy samples, an enrichment of the inflammasome pathway (NLRC4 gene) under reperfusion conditions compared to the preceding ischemic phase was reported [187]. Another study suggested inflammasome activation and subsequent recruitment of IFN gamma effector cells in DGF, and reported significant upregulation of NLRC4 gene expression in pre-transplant donor kidney biopsies [188]. It is interesting that IL-18 concentrations in kidney donor urine at kidney procurement did neither correlate with the severity of acute tubular injury at the time of procurement nor with the kidney recipient-estimated glomerular filtration rate (eGFR) at six months post-transplantation [189]. On the other hand, IL-18 concentrations in recipient urine were already considerably increased directly after the surgical procedure, and continued to be high until the second post-operative day. These IL-18 concentrations were significantly higher in patients with delayed graft function compared to patients with immediate graft function and with a predicted need for dialysis within the first week after transplantation, as well as the recovery of kidney graft function at three months [190]. Together, these data point to an involvement of inflammasome components during kidney ischemia and/or reperfusion. This could be in line with results from a recent study that tested the application of multipotent adult progenitor cells (MAPCs) to reduce IRI during normothermic machine perfusion of donor kidneys. Treatment with MAPCs resulted in improved surrogate parameters for kidney function and a downregulation of IL-1β protein in kidney perfusate [191]. Therefore, interventions to modulate inflammasome activation during IRI could reduce AKI in the immediate peri-transplantation period and may improve long-term kidney transplant function, but more detailed knowledge on the nature and temporal pattern of inflammasome components’ involvement is mandatory.
The risk for IRI and DGF is higher in the transplantation of extended criteria donor (ECD) kidneys, which comprises kidneys from donors of higher age and a history of reduced kidney function or hypertension (for more details, see [192,193]). The activation of inflammasome-related pathways in ECD kidneys has recently been addressed. A comparison between ECD and non-ECD perirenal adipose tissue samples, used as surrogate for the corresponding kidney transplant, showed an upregulation of gene expression in the NOD-like receptor and the NFκB-signalling pathways. Gene expression of IL-1β was significantly higher in ECD renal tissue [194]. Another study found a significantly increased gene expression of NLRP3, caspase-1 and IL-1β in ECD compared to non-ECD kidney biopsies obtained immediately before kidney implantation [195].
Early or Late Post-Transplantation
Kidney allograft dysfunction in the early post-transplantation period is frequently caused by acute rejection of the kidney transplant. Acute T-cell-mediated rejection (TCMR) is one of the principal histologic forms of acute rejection, and an involvement of inflammasome components has been discussed [196]. Microarray analyses in a large set of dysfunctional kidney allografts revealed an association of inflammasome activation with pure TCMR. Inflammasome-related gene transcripts with association to TCMR were caspase-1, IL-1β, and IL-18. AIM2 gene expression was highly increased compared to non-TCMR kidney biopsy samples, maybe reflecting interferon gamma-dependent induction in macrophages [197].
Viral infections can cause kidney allograft dysfunction. The DNA virus BK polyomavirus can reactivate under immunosuppression and result in BK polyomavirus-associated nephropathy in kidney transplant recipients. The pathogenesis comprises the upregulation of multiple inflammatory pathways with subsequent tubular injury, tubular atrophy, and fibrosis. An analysis comparing IL-18 in kidney biopsies between BK polyomavirus-associated nephropathy and TCMR reported significantly higher gene expression of IL-18 in BK polyomavirus-associated nephropathy. Additionally, a significantly higher IL-18 protein amount was reported, which in BK polyomavirus-associated nephropathy, could be localized to the tubular epithelium, while in TCMR, the IL-18 protein was mainly localized in CD163-positive macrophages [198]. An overview of inflammasome components involved in human AKI is given in Table 3 and Table 4.
Table 3.
Inflammasome component involvement in human AKI.
| Clinical Condition | Altered/Involved Inflammasome Component or Inflammasome Substrate | Investigated Tissue/Cells/Fluid | Reference |
|---|---|---|---|
| S-AKI | NLRP3 IL-1β IL-18 |
Serum Serum Urine |
[180] * [172,173] [178,179] |
| CSA-AKI | IL-1β IL-18 |
Serum Urine |
[155] [156,157,158,159,160,161,162] |
| CRS | IL-1β IL-18 |
Plasma Serum |
[167] [168] |
| CI-AKI | Caspase-1, IL-18 | Urine | [60] |
| Acute tubular injury | NLRP6 | Renal tubules | [185] |
| Rhabdomyolysis-induced AKI | ASC, active caspase-1 | Renal tubulointerstitium | [186] |
* Septic patients (~25% with AKI), but relation to renal function not investigated. S-AKI—Sepsis-associated acute kidney injury; CSA-AKI—Cardiac surgery-associated acute kidney injury; CRS—Cardiorenal syndromes; CI-AKI—Contrast-induced acute kidney injury, IL—interleukin; ASC—apoptosis-associated speck-like protein containing a caspase-associated recruitment domain; NLRP—NOD-like receptor family pyrin domain containing; NOD—nucleotide-binding oligomerization domain.
Table 4.
Inflammasome component involvement in acute loss of kidney function in transplanted kidneys.
| Clinical Condition | Altered/Involved Inflammasome Component or Inflammasome Substrate | Investigated Tissue/Cells/Fluid | Reference |
|---|---|---|---|
| Reperfusion injury | NLRC4 | Renal tissue | [187] |
| DGF | NLRC4 IL-18 |
Pretransplant donor renal tissue Transplant recipient urine day 0–2 post-transplantation |
[188] [190] |
| Extended vs. non-extended criteria donor kidneys | NOD-like receptor and NFκB signalling pathways, IL-1β NLRP3, caspase-1, IL-1β |
Perirenal donor adipose tissue Pretransplant donor renal tissue |
[194] [195] |
| Acute T cell-mediated rejection | AIM2, caspase-1, IL-1β, IL-18 IL-18 |
Renal tissue CD 163-positive macrophages in renal tissue |
[197] [198] |
| BK polyomavirus-associated nephropathy | IL-18 | Renal tubular epithelium | [198] |
NLRC—NLR family CARD domain-containing protein; IL—interleukin; NOD—nucleotide-binding oligomerization domain; NLRP—NOD-like receptor family pyrin domain containing; DGF—delayed graft function; NFκB—nuclear factor κ-light-chain-enhancer of activated B cells, AIM2—Absent in Melanoma 2; CD—cluster of differentiation; BK—abbreviation of the name of the first patient from whom the BK polyomavirus was isolated.
4. Inflammasome Components in CKD
4.1. Preclinical Data on Inflammasome Components in CKD
The NLRP3 inflammasome also contributes to the pathogenesis of CKD. In murine unilateral ureteric obstruction (UUO), IL-18 neutralization reduces kidney damage and fibrosis [199]. Additional biological factors, such as the ECM components biglycan and hyaluronan, stimulate NLRP3 inflammasome activation, thus aggravating CKD progression [200]. Biglycan-deficient animals are resistant to damage following UUO [53]. Additionally, NLRP3-deficient mice have less tubular damage and decreased renal inflammation when compared to wild-type controls upon UUO [127,201,202]. The NLRP3 inflammasome was implicated in the pathogenesis of multiple kidney diseases, such as adenine-induced tubulointerstitial nephritis [203], metabolic syndrome-associated nephropathy [204,205], hyperhomocysteinemia-induced glomerulosclerosis, proteinuria-induced tubular injury [206], angiotensin II-induced hypertensive kidney injury [207], and diabetic kidney disease (DKD) [208]. These studies demonstrated the pathological roles of NLRP3 in both inflammasome-dependent and inflammasome-independent manners during CKD. Lack of NLRP3 inflammasome and related genes results in lower kidney damage, cell death, inflammation, and fibrosis. Nevertheless, there are also still unresolved questions. For example, in UUO, blocking the renin-angiotensin system decreased NLRP3 inflammasome and boosted water channel AQP2 activity [209]. Vilaysane et al. found decreased damage in tubules, along with reduced inflammatory cell infiltration, and fibrosis in connection with a decline in caspase-1 expression, and also activated IL-1β and IL-18 upon UUO in kidney-specific, NLRP3-deficient mice [127]. The NLRP3 was found to augment the TGFβ/Smad pathway, which resulted in epithelial-mesenchymal transition and fibrosis [210]. In mice with selective elimination of NLRP3, ASC, or caspase-1, the inflammasome pathway was investigated, but no differences in glomerular disease were discovered [211]. Immune cells and resident glomerular cells, such as podocytes, endothelial cells, and mesangial cells can release IL-18 and IL-1β, which may increase the advancement of DKD [208]. Inflammasome molecules and pro-inflammatory cytokine expression levels in mice with diabetes were elevated compared to non-diabetic mice [208]. The extent of renal damage in diabetic mice was identical to wild-type mice when bone marrow from Nlrp3-/- and Caspase-1-/- mice was transplanted in db/db mice, and induction of the NLRP3 inflammasome originating from intrinsic renal cells worsened diabetic nephropathy (DN). Furthermore, hyperglycaemia activated NADPH oxidase leading to NLRP3 inflammasome activation in glomerular podocytes and subsequent podocyte damage. TXNIP antagonists and short hairpin RNA (shRNA) can stop inflammasomes from triggering DKD [212]. Therefore, decreasing NLRP3/caspase-1 production could be a potential therapy for DKD. The canonical NLRP3 inflammasome is linked to the pathogenesis of crystalline nephropathy [213]. NLRP3 inflammasomes are triggered by calcium oxalate crystals, which stimulate kidney dendritic cell activity. Subsequent, administration of IL-1β receptor inhibitors protected mice from obstructive nephropathy [214]. Furthermore, allopurinol reduced kidney inflammation by decreasing NLRP3-inflammasome activity [215]. Next, Tamm-Horsfall protein, secreted by tubular cells, serves as a platform for nanoparticles to form in diseased tubules. These nanoparticles operate as an endogenous warning signal, activating the NLRP3 inflammasome following macrophage phagocytosis [73]. The NLRP3-inflammasomes also contribute to the pathogenesis of lupus nephritis. Zhao and group discovered that decreasing NLRP3 recruitment and subsequent caspase-1 activity by blocking P2 × 7R, decreased glomerular damage in MRL/LPR mice with lupus nephritis, lowering serum anti-dsDNA antibody level, as well as IL-1β and IL-17 levels [216]. BAY 11-7082, a phosphorylated NF-κB inhibitor decreased macrophage invasion by inhibiting NLRP3-inflammasome activation resulting in decreased lupus nephritis and increased mice survival [217]. TLR7, TLR8, and TLR9 inhibitors suppress NF-κB signalling and significantly reduce the progression of glomerular damage and immune cells infiltration by decreasing the expression of renal IL-1β and NLRP3-inflammasome [203]. Subsequent studies utilizing bone marrow chimeras indicated that NLRP3 causes kidney damage and inflammation [218]. Additional treatments or medications that interfere with the signalling of the NLRP3-inflammasome could minimise or prevent tubular injury, including mitochondrial-targeted antioxidants, compound-K, neferine, allopurinol, and ghrelin [219,220,221,222]. Lu et al. found that NLRP3 gain of function mutation in myeloid cells had substantial abnormal glomerular hypercellularity and interstitial nephritis, resulting in serious albuminuria and focal segmental glomerulosclerosis, in a pristine-induced experimental lupus mouse model [223]. In lupus-prone NZM2328 mice, NLRP3 stimulation in non-myeloid cells, like podocytes, enhanced lupus nephritis progression [224]. These findings imply that NLRP3 inflammasome activation and subsequent IL-1β and IL-18 release are important drivers of lupus nephritis. Moreover, in MRL/LPR animals, inhibiting NLRP3 inflammasome activity indirectly by P2 × 7 suppression and NF-κB pathway suppressed lupus nephritis [216,217]. In oxalate-induced CKD, Knauf et al. discovered that mice fed an oxalate-rich diet showed higher NLRP3-inflammosome activation in their kidneys [218]. Mice lacking NLRP3 or ASC were reliably protected from oxalate-induced kidney fibrosis without compromising oxalate equilibrium, implying that NLRP3/ASC/caspase-1/IL-1β/IL-18 are vital factors involved in the pathophysiology of oxalate-induced CKD [18,218]. Furthermore, an IL-1β receptor inhibitor, Anakinra, treatment prevented oxalate-induced CKD in mice, implying that NLRP3 plays a role in crystalline nephropathies [18]. These findings suggest NLRP3 as a possible therapeutic candidate for type-II crystal-induced nephropathy. Likewise, pharmacological suppression of NLRP3 with CP-456,773 (also known as MCC950) and β-hydroxybutyrate rescued mice from calcium-oxalate and adenine crystal-induced kidney immune cell infiltration and interstitial fibrosis [18,225]. Additionally, animals treated with CP-456,773 were protected from adenine-induced CKD [225]. Nonetheless, CP-456,773 did not reduce NLRP3–NLRP3 or NLRP3–ASC associations, indicating an undiscovered upstream signalling mechanism that targets the NLPR3 inflammasome [226]. Notably, inhibiting NLRP3 with β-hydroxybutyrate causes a phenotypic change in macrophages, which helps to reduce kidney fibrosis [227].
In DKD, both immune and non-immune cells of the glomerulus released IL-1β and IL-18, which exacerbated DN [228]. Additionally, renal podocytes and endothelial cells have higher expression of NLRP3 and caspase-1, suggesting its involvement in the pathogenesis of DKD [208]. Moreover, genetic and pharmacological suppression of the NLRP3-inflammasome together with IL-1β protected mice from DKD [208,228]. Several studies also showed that allopurinol, quercetin, and saxagliptin, through reducing the NLRP3/ASC inflammasome, protected mice from DN [40,228,229,230]. Yuan et al. discovered that a lack of acid ceramidase (AC) boosted the stimulation of the NLRP3-inflammasome and the secretion of exosomes, which in turn boosted the production of IL-1β in diabetic mice [231]. Dihydroquercetin effectively protects against DN by reducing ROS-induced NLRP3-inflammasome activation [219]. Shahzad et al. conducted bone marrow transplant studies with control, Nlrp3-/-, and caspase1-/- mice and discovered that the NLRP3 inflammasome in native renal cells plays a substantial role in the evolution of DKD [208]. Notably, it is yet unknown if active caspase-1 causes pyroptosis in podocytes during DKD. Furthermore, the NLRP3 inflammasome is known to be expressed by tubular epithelial cells in the kidney [232]. As a result, the role of NLRP3 in tubules in the progression of renal interstitial fibrosis in DKD remains unknown and deserves additional investigations. The expression of the NLRP3-inflammasome in the kidney has been linked to salt-sensitive hypertension [18,225]. MCC950, an NLRP3 antagonist, lowered blood pressure and fibrosis, reduced renal inflammation, and protected against kidney failure [218]. Kidney inflammation caused by the NLRP3 inflammasome has also been linked to the advancement of IgA nephropathy. In mice, both genetic deficit and pharmacological suppression of NLRP3 employing shRNA in a preventive or therapeutic way significantly slowed the development of IgA nephropathy [233]. IL-1β receptor inhibitors additionally stopped the course of pre-existing IgA nephropathy in mice [234]. These findings imply that targeting NLRP3-inflammasome can be a promising therapeutic strategy for IgA nephropathy. It is still unclear whether NLRP3′s inflammasome-independent actions are involved in the pathogenesis of IgA nephropathy.
Besides, ASC-deficient mice were protected from hypertensive nephrosclerosis [235], and mice lacking NLRP3 were protected from hyperhomocysteinemia-induced glomerulopathy [236] and Western diet-induced nephropathy [205]. Together, inflammasome components play a crucial role in various forms of CKD, and are therefore putative therapeutic targets for CKD.
4.1.1. Preclinical Data on Redox Signalling and Inflammasome Components in CKD
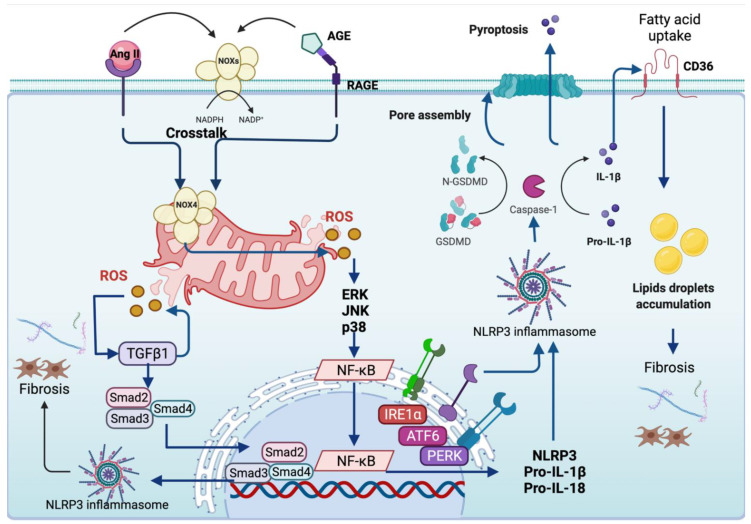
Diabetic nephropathy (DN), the principal cause of end-stage kidney disease (ESKD) worldwide, is the most common CKD associated with inflammasome activation [237]. DN is characterized by ROS overproduction, glomerular barrier expansion and thickness, cell proliferation, and tubular damage that lead to fibrosis development [238]. Concerning ROS, it induces the activation of inflammasome in DN. For instance, high glucose levels upregulate toll-like receptor 4 (TLR4), which in turn produces ROS, stimulating NLRP3, ASC, and caspase-1 activity [239]. Moreover, Ang II contributes to DN by inducing glomerular mesangial expansion, membrane basement thickening, and extracellular matrix deposition, and it has been implicated in NLRP3 activation [240]. In line with this, human mesangial cells exposed to Ang II showed upregulation of NLRP3 protein, caspase-1, and ASC, leading to NLRP3 complex activation [241]. The upregulation of Ang II prompts the stimulation of NOXs via angiotensin receptor 1 (ATR1), causing ROS overproduction and driving NLRP3 activation via ROS. In agreement with the latter, podocytes and glomeruli showed the priming of NLRP3 through ROS generated via NOXs, leading to inflammation, podocytes injury and glomerular sclerosis [86]. Moreover, in the deoxycorticosterone acetate (DOCA)/salt-induced hypertension model, the levels of the 32-residue hormone peptide ELABELA are decreased, promoting the activation of NOXs that generates ROS, and subsequently, NLRP3 inflammasome activation in the renal medulla and renal cortex (Table 5) [242]. In the immunoglobulin A (IgA)-induced nephropathy model, the levels of p47-phox are elevated, leading to ROS production and oxidative stress [115]. Moreover, in this model, the Nrf2 pathway and its target antioxidant enzymes (i.e., heme oxygenase (HO-1), NADPH dehydrogenase quinone 1 (NQO-1), GPx, glutathione S-transferase (GST), and glutamate-cysteine ligase (GCL)] are downregulated, increasing even more oxidative stress [229]. In consequence, the levels of inflammasome components are upregulated, resulting in the production of IL-18 and IL-1β [115]. Supporting the latter, the treatments that activate Nrf2 reduce O2•− and inhibit NF-κB and NLRP3 activation [243,244]. Furthermore, the inhibition of NOXs by apocynin (APO) and DPI and gp91-phox silencing attenuates caspase-1, suggesting that NOXs are implicated in NLRP3 activation [86]. In this model, the guanine nucleotide exchange factor Vav2 was involved in NOXs assembly and activation, demonstrated by Vav2 silencing, which prevents NLRP3 activation and podocyte injury [245]. Additionally, ATR1 knockout mice stimulated with Ang II do not show inflammasome activation [246].
Table 5.
Redox-signalling pathway-induced inflammasome activation in CKD.
| CKD Model | Redox-Signalling-Induced Inflammasome Activation | Effects | Reference |
|---|---|---|---|
| IgA-induced nephropathy | NF-κB/NLRP3 | The activation of NF-κB induces NLRP3 activation, required for progression promoted by IgA | [244] |
| IgA-induced nephropathy | decreasedNrf2/NLPR3 | IgA deactivates Nrf2, increasing ROS and oxidative stress, inducing NLRP3 activation | [243] |
| DOCA/salt-induced hypertension | NOXs/ROS/NLRP3 | The decreasing of ELABELA activates the NOXs/ROS/NLRP3 pathway | [242] |
| Ang II-induced DN | Ang II/ROS/NF-κB/NLRP3 | Ang II induces proliferation and the biomarker expressions FN, Col IV and CTGF, inducing fibrosis. | [241] |
IgA: immunoglobulin A; DOCA: deoxycorticosterone acetate; NOXs: NADPH oxidases; STZ: streptozotocin; Nrf2: nuclear factor erythroid 2-related factor 2; NF-κB: nuclear factor-κB; NLRP3: nucleotide-binding and oligomerization domain-like receptor family pyrin domain-containing 3; Ang II: angiotensin II.
In CKD models, NOX4 is the principal isoform activated due to Ang II upregulation [247]. NOX4 is highly expressed in cellular membranes like mitochondria and ER, and essentially produces H2O2 through histidine groups in its E-loop that permits the rapid dismutation of O2•− to H2O2 [248,249]. Besides Ang II, the binding of advanced glycation end products (AGES) to their receptor, named the receptor for advanced glycation end products (RAGE), induces NOX4 upregulation in DN, which generates ROS overproduction [250]. In DN, the overproduction of ROS by AGES induces pyroptosis by activating NLRP3 and gasdermin (GSDM); the latter is an executor protein functioning as a caspase-1 substrate implicated in pore formation during pyroptosis [251]. The treatment with Tangshen formula, a Chinese herbal medicine, avoids pyroptosis by inhibiting ROS overproduction under high glucose levels, suggesting that AGES-induced ROS participates in inflammasome-mediated pyroptosis in DN [251].
In streptozotocin (STZ)-induced DN, the upregulation of NOX4 stimulates MAPK signalling by inducing the phosphorylation of p38 [252,253]. In accordance with the latter, the downregulation of NOX4 by small-interfering RNA and inhibition of NOX4 activity by NOX4 Inhibitor, GK-136901, lessens high glucose-induced NOXs-derived ROS generation and p38 activation [254]. The activation of p38 promotes NF-κB activation to induce the secretion of IL-1β and IL-18 expression mediated by NLRP3 activation [252]. Thus, the priming steps of NLRP3 involving ROS are present in CKD. In line with this, albumin overload induces the synthesis of chemokines such as ‘regulated upon activation, normal T cell expressed and presumably secreted’ (RANTES) and monocyte chemoattracted protein (MCP-1) through NF-κB, triggering the recruitment of monocytes and T cells mediated by the secretion IL-1β and IL-18. The latter attracts neutrophils and molecules that further generate ROS accumulation [241]. Albumin is considered a DAMP able to trigger inflammasome priming because the levels of NLRP3 are albumin dose-dependent [255]. Concerning NF-κB, several authors have suggested a crosstalk between NF-κB and Nrf2, where NF-κB activation prompts Nrf2 inhibition, leading to the transcription of inflammatory genes and preventing antioxidant gene response [256,257]. In line with this, the activation of Nrf2 and its targets genes such as HO-1 and NOQ1 ameliorates kidney damage by decreasing the p65 NF-κB subunit along with the priming of NLRP3 [258,259]. In contrast, Nrf2 deletion does not inhibit NLRP3, TXNIP, IL-1β, Il-18 and cleaved caspase-1 upregulation, suggesting that Nrf2 is needed to avoid inflammasome priming [259]. Moreover, the enhancement of Nrf2 levels decreases apoptosis, attributed to NLPR3 inflammasome blocking [260]. Another protein that activates NLRP3 is never in mitosis gene A (NIMA)-related kinase 7 (NEK7) [261]. NEK7 participates in NLRP3 complex assembly, acting as a selective regulator of NLRP3 [262]. In this sense, the in vitro model uric acid (UA)-induced urolithiasis shows that UA causes ROS overproduction, which activates NEK7, triggering NLRP3 complex assembly [261].
4.1.2. Preclinical Data on Mitochondria and Inflammasome Components in CKD
Several authors have suggested that Ang II is implicated in maintaining mitochondrial homeostasis in CKD; however, the upregulation of Ang II during pathological conditions like DN causes mitochondrial dysfunction [263,264]. The latter is due to the stimulation of NOXs by Ang II, which induces crosstalk with mitochondria, causing mtROS overproduction and inducing mitochondrial damage [263]. AGES also generates mtROS by establishing mitochondrial crosstalk [247]. Mitochondrial ROS are implicated in tubular damage, either activating or deactivating redox-sensitive proteins. For instance, high levels of albumin generate mtROS, leading to activation of NLRP3 priming via stimulation of NF-κB [255]. In DN, mtROS also induces inflammasome priming steps by inducing the expression of TXNIP, decreasing TRX levels and promoting NLRP3/IL-1β and TGFβ1 expression [265]. In addition, the treatment of HK-2 cells with mitochondrial-targeting antioxidants coenzyme (mitoQ) impeded the dissociation of TRX from TXNIP and blocked the interaction between NLRP3 and TXNIP, highlighting the involvement of mtROS in priming NLPR3 activation [265]. The silencing of TXNIP by RNA interference triggers NLRP3 downregulation and inhibits IL-18 and IL-1β release in high-glucose environments [266].
The activation of inflammasomes has been related to fibrosis development because mtROS induces upregulation of the fibrosis master TGFβ1 [267]. In DN, the knockout of the NLPR3 gene decreases fibrosis by reducing fibronectin, collagen I and collagen IV along with downregulation of TGFβ1, Smad2, 3, and connective tissue growth factor (CTGF), improving tubulointerstitial fibrosis and preventing glomerular basement membrane thickness [268]. The mechanism involved in fibrosis inhibition is NLRP3 inflammasome-dependent, suggesting that NLRP3 activation is needed to mediate fibrosis in DN [268]. Furthermore, the activation of TGFβ1 leads to epithelial-mesenchymal transition (EMT), and NLRP3 has been implicated in this process because NLRP3 promotes the upregulation of α-SMA, E-cadherin expression and the induction of myofibroblasts [269]. In this context, Song et al. [270] reported that NLRP3 silencing avoids EMT under high glucose levels by blocking TGFβ1. Additionally, the authors demonstrated that in vitro, the treatment of HK-2 cells with TGFβ1 generates ROS and EMT. Interestingly, the use of NAC decreases both, suggesting that TGFβ1 generates ROS. According to the latter, mtROS are generated through TGFβ1 by establishing crosstalk with mitochondria in CKD models like UUO [271].
Several authors have attributed that fibrosis induction via NLRP3 is independent of inflammasome activation [17]. For instance, hypoxia induces NLRP3 upregulation independent of ASC, caspase-1, and IL-1β [87]. Consequently, NLRP3 relocalizes in the mitochondria and colocalizes with MAVS, causing ROS production and mitochondrial membrane depolarization without inducing inflammasome activation [87]. The NLRP3 interaction with MAVS induces tubulointerstitial fibrosis, emphasizing the independent mechanisms of NLRP3-induced fibrosis through MAVS [87].
Previous reports in DN showed that the increase of triglycerides, inducing lipotoxicity and later kidney damage is related to fibrosis onset [272]. In this regard, the activation of NLRP3 also promotes lipid accumulation, as was recently demonstrated by Wu et al. [273]. These authors reported that diabetic mice increased lipid deposition via IL-1β/ROS/NF-κB p65, triggering NLRP3 priming, which induces the upregulation of sterol regulatory element-binding protein 1 (SREBP1) and 2 (SREBP2). The latter proteins are involved in lipid biosynthesis. Moreover, the ATP-binding cassette transporter (ABCA1), a protein implicated in the transport of phospholipids, cholesterol, and other metabolites from cells to lipid-depleted HDL apolipoproteins, decreases [273,274]. The knockout of NLPR3 avoids inflammation and fibrosis by reducing lipid deposition, suggesting the involvement of NLPR3 to induce lipid accumulation [273]. Moreover, lipid deposition via inflammasome activation has been attributed to the upregulation of IL-1β, which stimulates the cluster of differentiation 36 (CD36) to mediate fatty acid uptake [275,276].
4.1.3. Preclinical Data on Endoplasmic Reticulum Stress and Inflammasome Components in CKD
ER stress is essential in the development of CKD [277]. The latter is sustained since the blocking of ER stress prevents fibrosis in several CKD models [277,278]. In UUO, the chemical chaperon sodium 4-phenylbutyrate (4-PBA) decreases GRP78, CHOP, and ATF4 levels. Moreover, 4-PBA restores the spliced XBP1, ameliorating UPR in the obstructed kidney, and decreases fibrosis by downregulating TGFβ1 and its targets α-SMA and CTGF, reducing tubulointerstitial fibrosis [278]. ER stress also promotes fibrosis by activating NLRP3 inflammasome [277]. In line with this, 14 days after obstruction, ER stress activates NLRP3 to induce the upregulation of fibrosis markers α-SMA and collagen I. The activation of NLRP3 mediated by ER stress was demonstrated because the use of ghrelin, a brain peptide that acts against renal injuries, could decrease fibrosis by inhibiting NLRP3 activation and ER stress [279]. Furthermore, it has recently been reported that endothelin (ET) promotes inflammasome NLRP3 activation [280]. ET1 and ET2 belong to ET family members, which form the pathway involving ET-1 itself, endothelin-converting enzymes (ECEs) that activate ET-1, and the endothelin A receptor (ETA), and they are commonly activated in CKD, inducing cell growth, vasoconstriction, and inflammation. In vitro, HK-2 cells treated with ET-1 and ET-2 at different doses (50–200 nM) activated the UPR through upregulation of IRE1α, cleaved ATF6 and phosphorylated elF2α, along with NLPRP3, ASC and cleaved caspase-1 to both ET-1 as ET-2, suggesting that the ET pathway might prompt NLRP3 via ER stress [281]. The latter is supported because the use of phosphoramidon, an ECE inhibitor, attenuates ER stress, and later, NLRP3 priming by triggering mitophagy. Together, these results suggest that ER stress is an NLRP3 activator in CKD models.
In Figure 2, we summarized the mechanisms that induce NLRP3 inflammasome activation, which include redox signalling, mitochondria, and ER stress in CKD models.
Figure 2.
Activation of NOD-like receptor (NLR) family pyrin domain containing 3 (NLRP3) inflammasome mediated by redox-signaling, mitochondria, and ER-stress during chronic kidney disease (CKD). The stimulation of NOXs by angiotensin II (Ang II) and the advanced glycation end products (AGE) generate crosstalk with the mitochondria, inducing ROS overproduction. These mechanisms are mediated by the interaction of Ang II and AGE with their receptors: angiotensin receptor 1 (ATR1) and AGE receptor (RAGE). ROS overproduction triggers activation of extracellular signal-regulated kinase (ERK), c-Jun N-terminal kinases (JNK) and p38, which activate nuclear factor κ-light-chain-enhancer of activated B cells (NF-κB), promoting the transcription of NLRP3, interleukin 1 β (IL-1β), and interleukin 18 (IL-18) to start inflammasome activation. Moreover, ROS induces transforming growth factor-β 1 (TGFβ1) activation, triggering the formation of the Smad complex, composed of Smad2, 3, and 4, which is translocated to the nucleus. In addition, TGFβ1 activation also generates ROS, promoting NLRP3 activation to induce fibrosis development. IL-1β also leads to fibrosis by causing lipid droplets accumulation, mediated by inducing the upregulation of cluster of differentiation 36 (CD36), a receptor implicated in fatty acid uptake. Thus, fatty acid uptake triggers lipid droplet formation, inducing lipotoxicity and later promoting fibrosis. Endoplasmic reticulum stress also activates NLRP3 by generating ROS. NLRP3 inflammasome induces pyroptosis in which caspase-1 cleaves pro-IL-1β to form IL-1β, the active form, and cleaves gasdermin (GSDMD) proteins to produce an N-terminal domain (GSDMD-N). Thus, GSDMD-N can oligomerize and form pores in the plasmatic membrane, triggering increased osmotic pressure, followed by cell swelling and bursting. Figure created using BioRender (Toronto, ON, Canada).
4.2. Clinical Data on Inflammasome Components in CKD
Chronic kidney disease (CKD) refers to a heterogeneous group of kidney disorders and is characterized by alterations in kidney structure or function for more than three months with implications for health. According to the KDIGO guidelines, CKD is classified based on the cause, estimated glomerular filtration rate (eGFR) category, and albuminuria category [282]. Therefore, the following section summarizes the clinical data on inflammasome components according to the cause of CKD, CKD severity (e.g., kidney failure), albuminuria, and CKD-related implications for health (e.g., CKD-attributable CVD).
It should be noted that some of the pathophysiological mechanisms described for CKD, such as in Lupus nephritis or IgA nephropathy, could also be subsumed under AKI, as the clinical course can be acute in several of the described entities.
4.2.1. Albuminuria
Albuminuria is the pathological occurrence of albumin in the urine. It is a marker of CKD, but it also contributes to CKD progression. Inflammasome components were proposed to mediate albumin-induced tubulointerstitial inflammation. In kidney biopsies, NLRP3 and IL-18 protein staining increased with worsening proteinuria and correlated with inflammatory cell infiltration of the renal interstitium [283]. Furthermore, children with proteinuria and diverse underlying kidney diseases showed significantly higher NLRP3 protein expression compared to healthy control subjects, and a significant correlation between tubular NLRP3 expression and severity of proteinuria [206]. In line with this finding, epithelial caspase-1, IL-1β and IL-18 protein in proximal tubules were significantly increased with more severe proteinuria in primary and secondary glomerular disease [284]. Both results from preclinical studies and the described inflammasome-related protein changes in the proximal tubule, the site of megalin-tubulin mediated albumin reabsorption, suggest a possible involvement of inflammasome components in albumin-induced renal tubular injury and CKD progression. However, a clear dissection of such albumin-related vs. underlying kidney disease-related mechanisms is still missing.
4.2.2. Nephrotic Syndrome
Nephrotic syndrome can be the result of a number of kidney diseases, and is characterized by severe proteinuria (>3.5 g per day), hypoalbuminemia, hyperlipidaemia, and oedema. Inflammasome involvement has been suggested. In peripheral blood mononuclear cells (PBMCs) from patients with glucocorticoid-resistant nephrotic syndrome, NLRP3 protein co-localized with ASC protein, which could indicate inflammasome activation [285]. In this study, methylation of the NLRP3 gene promotor, which should result in decreased NLRP3 gene expression, was significantly higher in PBMCs from patients with glucocorticoid-sensitive nephrotic syndrome. Interestingly, in kidney biopsies from children with non-minimal change nephrotic syndrome, IL-1β protein was increased compared to control samples, while in minimal-change nephrotic syndrome, which is mostly glucocorticoid-sensitive, such an IL-1β protein elevation in the kidney was not observed [286].
4.2.3. Diabetic Kidney Disease (DKD)
Diabetic kidney disease refers to the presence of albuminuria and/or decreased eGFR in patients with diabetes mellitus. Classically, diabetic glomerulopathy is characterized by thickening of the glomerular basement membrane, mesangial proliferation, and podocytes loss, but other glomerular lesions and tubulointerstitial disease are found in DKD. The role of inflammasome components in DKD has gained much attention. Interleukins 18 and 1β have been investigated in the blood and urine of patients with DKD.
In agreement with the inflammatory aspects in type 2 diabetes mellitus [287], relations between renal dysfunction and inflammasome components in blood were described. The serum IL-18 protein concentrations increased with progressive DKD and correlated negatively with eGFR [288]. Serum IL-18 concentrations were significantly higher compared to healthy controls and correlated with markers of podocyte and proximal tubule dysfunction [289]. Of note, the increase of serum IL-18 seemed to precede both eGFR decrease and urine IL-18 increase. In agreement with this observation, serum IL-18 protein was associated with progression of nephropathy in normoalbuminuric patients with diabetes mellitus type 2 [290]. In type 2 diabetes PBMCs, gene expression of NLRP3 and caspase-1 was higher in DKD than in non-DKD [291]. Caspase-1 gene expression was significantly and inversely associated with eGFR in this study. In the urine, significantly higher IL-18 concentrations were found in type 2 diabetes mellitus and DKD compared to healthy controls, and IL-18 correlated with markers of podocyte and proximal tubule dysfunction [289]. Here, the rise in urinary IL-18 was most prominent in patients with reduced eGFR. In accordance with this, in a study of longstanding (≥50 years) type 1 diabetes, urine IL-18 correlated negatively with GFR [292]. These diabetic patients with and without DKD had lower IL-18 concentrations than a healthy control group. The authors concluded that these selected patients who survived longstanding diabetes with only mild kidney impairment had a low inflammatory profile, which might have been advantageous. Interestingly, in a population of type 2 diabetic patients with normal or high GFR, urine IL-18 was not different from healthy controls even if macroalbuminuria was present [293].
In kidney biopsy analyses, increased glomerular and tubulointerstitial IL-1β protein was found in DKD compared to non-kidney disease control subjects [294], and IL-18 protein showed a stronger expression in tubular cells of diabetic patients compared to patients with minimal change nephrotic syndrome [141]. Furthermore, kidney biopsy gene expression analysis identified IL-1β as a key regulator upstream of inflammation-related gene expression, which correlated with GFR 10 years after the kidney biopsy [295]. Significantly higher NLRP3 protein was demonstrated in DKD compared to healthy renal tissue [296]. A higher NLRP3 protein amount compared to non-diabetic and non-DKD controls was also shown in DKD glomeruli (endothelial cells and podocytes) [208]. Herein, NLRP3 colocalized with cleaved caspase-1. In these patients, serum IL-1β was increased in albuminuric diabetis mellitus and correlated with albuminuria. Moreover, significantly increased NLRP3, ASC, and caspase-1 protein was found in podocytes of DKD compared to healthy renal tissue [297]. Another study showed higher renal tubular epithelial cell NLRP3 and IL-1β protein in DKD, significantly higher urinary IL-1β and IL-18, and suggested reduced optineurin-mediated mitophagy as an underlying mechanism [298]. Preclinical data on the inflammasome involvement in kidney disease point to the importance of ROS and mitochondria. In DKD, the renal NLRP3 protein amount was significantly higher compared to kidney samples from patients without diabetes [299]. In this investigation, NLRP3 protein correlated positively with tubulointerstitial damage, glomerular damage, urine protein excretion, and dihydroethidium-detectable ROS generation. Furthermore, NLRP3 protein and the presumably inflammasome activating protein TXNIP were both significantly elevated in DKD kidney biopsies [300]. In addition to NLRP3, significantly higher NLRC4 protein content in renal tubules and interstitium was also reported in DKD compared to normal renal tissue [301]. This was paralleled by an increased tissue infiltration with CD69-positive macrophages. In line with this, NLRC4 was suggested to be increased in DKD compared to healthy renal tissue, together with a decreased protein amount of the mitophagy regulators PINK1 and parkin [302].
Investigations of inflammasome component-related pathomechanisms in DKD, especially in type 2 diabetic patients, are complicated by existing comorbidities. The presence of a metabolic syndrome—or components hereof, like hypertension or obesity—is a feature in several of the above-presented studies. This makes it more complex to draw disease-specific conclusions. We also already highlighted the increased renal tissue gene expression of NLRP3 in hypertensive/vascular nephrosclerosis in the absence of diabetes [127]. Besides, the AIM2 protein amount was significantly higher in kidney samples from hypertensive nephrosclerosis and DN [303]. In this analysis, healthy renal tissue showed primarily glomerular AIM2 protein, with some staining of tubules and interstitial leukocytes. In the CKD renal tissue samples, the glomerular AIM2 protein amount did not change, but it increased in tubular epithelial cells and leukocytes in inflammatory tubulointerstitial lesions.
Furthermore, preclinical data indicate a relation between cellular lipid accumulation and inflammasome components. In kidney biopsies from obese patients with various kidney diseases, proximal tubule cells showed phospholipid accumulation and impaired autophagy that could result in inflammasome activation [304]. Bariatric surgery to obtain weight reduction in obese patients with CKD resulted, besides the improvement of all metabolic syndrome components, in a decrease of proteinuria and a correlated decrease in serum IL-1β concentrations [305].
Several inhibitory effects of sodium-glucose cotransporter 2 (SGLT2) inhibitors on inflammasome activity in diabetes mellitus with cardiovascular disease or DKD have been suggested [306,307]. Further studies will indicate whether SGLT2 inhibitors reduce inflammasome-mediated pathophysiological mechanisms.
4.2.4. Immunoglobulin A (IgA) Nephropathy (IgAN)
Immunoglobulin A nephropathy is the most common cause for primary glomerulonephritis worldwide. Current understanding of the pathogenesis is based on a “multi-hit hypothesis”, comprising occurrence of galactose-deficient IgA1 (Gd-IgA1), autoantibody formation, and immune complex deposition in the glomerular mesangium [308]. The clinical manifestations of IgAN show pronounced variability and are probably influenced by both genetic and environmental factors. Inflammasome components have been investigated in that respect. Significantly more NLRP3 protein was found in IgAN kidney biopsy samples than in healthy control renal tissue. While the protein was restricted to the tubular epithelium in healthy renal tissue, IgAN samples showed stronger tubular, but also glomerular NLRP3 protein expression [309]. As fibrotic areas no longer showed NLRP3 staining, the NLRP3 gene transcripts were inversely related to higher risks for patient and CKD prognosis in progressive IgAN. Another study also reported significantly more NLRP3 protein in IgAN kidney samples compared to normal renal tissue, and confirmed the more pronounced localization in tubules compared to glomeruli [310]. Interestingly, this study reported a significantly higher glomerular NLRP3 amount in the case that proteinuria was higher than 3.5 g/day, while tubular NLRP3 was significantly higher if eGFR was below 60 mL/min/1.73 m2. Additionally, in these IgAN patients, NLRP3 co-localized with Gd-IgA1 and the podocyte marker podocalyxin, suggesting Gd-IgA1-related induction of inflammasome expression in podocytes. In a patient population with immunoglobulin A vasculitis (Henoch-Schönlein pupura), urinary IL-1β was associated with nephritis occurrence [311].
4.2.5. Lupus Nephritis (LN)
Systemic lupus erythematosus (SLE) is a chronic autoimmune disorder. Practically any organ system can be impaired, and LN is SLE’s renal manifestation. Diverse inflammatory pathways are involved in the organ manifestations of SLE [312]. Therefore, a longstanding interest in the involved cytokines exists, and IL-1 and IL-18 have been the focus for some time in LN [313]. In LN classes IV and V, protein amounts of active caspase-1 and IL-1β in tubular cells were suggested to be higher, compared to normal renal tissue [224]. Furthermore, the authors showed that in podocytes, active caspase-1 and IL-1β protein was only found in LN IV and V, not in normal renal tissue. The same study also investigated podocytes in urine. In accordance with the biopsy results, these urinary podocytes showed active caspase-1 and IL-1β protein in urine from patients with active LN, but not in urine from healthy controls. Other researchers, who also reported increased IL-1β in LN, localized it to renal monocytes/macrophages. In the glomeruli and interstitium of LN class IV, more IL-1β protein was found compared to other glomerulonephritides [314]. Dual-staining in this analysis suggested infiltrating monocytes/macrophages as the main cytokine source. In line with this, intracellular IL-1β protein was found in immunologically active monocyte-derived macrophages in the urine of LN patients [315]. Since according monocyte-derived macrophages were also found in the tubulointerstitium of LN kidney samples, a relevance for tubulointerstitial injury in LN was suggested. In addition, a significantly higher IL-18 protein amount was reported in infiltrating mononuclear cells in LN class IV, compared to class III and V glomeruli [316]. In this study, the serum IL-18 protein concentration in LN IV was significantly higher compared to healthy control sera and correlated positively with disease activity. In relation to the results above, an investigation of PBMCs from patients with SLE and LN is interesting. Compared to healthy controls, PBMCs in SLE had significantly higher caspase-1, IL-1β and IL-18 gene and protein expression [317]. This was paralleled by significantly increased serum IL-1β and IL-18 protein concentrations. In contrast, these SLE PBMCs had significantly lower NLRP3-activating NEK7, NLRP3, and ASC gene expression, as well as significantly lower NEK7 and NLRP3 protein amounts. In PBMCs from patients with LN, the NEK7, NLRP3 and ASC gene and protein expression were even significantly lower compared to patients without LN, and caspase-1, IL-1β, and IL-18 gene and protein expression were even higher. These results could indicate a negative feedback mechanism in PBMCs that diminishes the amount of some NLRP3-related components in the case of higher IL-1β and/or IL-18 concentrations, but confirmation is required.
In LN renal tissue, on the other hand, NLRP3 gene expression was shown to be higher than in normal control renal tissue [127]. Analyses in whole blood from patients with a median SLE disease duration of eight years did not show a difference for SLE and healthy control IL-18 gene expression. However, within the SLE patient group, IL-18 mRNA correlated positively with disease activity and was upregulated during severe flares [318]. Analyses of circulating cytokine concentrations mainly support the notion of inflammasome involvement in LN. Patients with SLE without immunosuppressive therapy had higher serum IL-1β protein concentrations compared to healthy controls [319]. In this patient collective, IL-1β concentrations in LN were nominally even higher than in non-LN SLE. In contrast, a patient population with a majority receiving immunosuppressive treatment showed no IL-1β elevation compared to healthy controls, and no association between IL-1β and renal impairment [320]. Still, serum IL-18 protein concentration in these patients was significantly associated with active LN. In children with SLE, serum IL-18 protein concentration was significantly higher compared to healthy controls, and correlated to LN activity [321]. Serum IL-18 at 6 months post-treatment predicted renal outcomes in these paediatric patients. In agreement with the results above and a possible involvement of inflammasome components in LN pathogenesis, a serum protein pattern analysis showed the usefulness of serum soluble IL-18 receptor 1 and caspase-8 for the prediction of active LN [322].
Taken together, regulation of inflammasome components is involved in LN pathophysiology. However, the exact method of inflammasome activation and the nature of the involved inflammasome sensory components, and particularly the role of circulating IL-18, requires further study in LN and SLE.
4.2.6. Autosomal Dominant Polycystic Kidney Disease (ADPKD)
Autosomal dominant polycystic kidney disease is a hereditary disease that can lead to CKD. Patients presenting with kidney cysts can also have cysts found in other organs, such as the liver or pancreas. Several mechanisms in tubular epithelial cells have been suggested to promote cyst growth, including reduction of functional polycystins, dysregulated cell proliferation, and apoptosis [323]. Apoptosis in ADPKD was described as an early event that was found mainly in normal-appearing tubules and small cysts, probably triggered by an extrinsic factor. Caspase-8 and caspase-3 protein seemed to be involved in the apoptotic pathway [324]. As multiple interconnections between caspase-8 and inflammasome action and activation have been described [8,325], inflammasome components are worth being investigated in ADPKD pathogenesis. In addition, cardiovascular events are a major cause of death in patients with ADPKD, and hypertension and overweight are risk factors for CKD progression in these patients. Inflammasome components are also of interest in this respect. Patients with ADPKD and metabolic syndrome had a more rapid loss of GFR than ADPKD patients without metabolic syndrome [326]. In these ADPKD patients with metabolic syndrome, IL-1β plasma protein was significantly higher compared to non-ADPKD, non-diabetic patients with comparable renal function. In addition, IL-1β gene expression in the whole blood of ADPKD patients was associated with hypertension [327]. This is in line with a study suggesting an association between serum NLRP3 protein concentration and endothelial dysfunction in ADPKD [328].
4.2.7. Uric Acid Kidney Diseases
Three types of kidney disease can result from the renal deposition of either urate crystals or uric acid: uric acid nephrolithiasis, which is a urinary tract stone disease; acute uric acid nephropathy, which results in AKI; and chronic urate nephropathy. Molecular mechanisms of crystal-related kidney injury have been reviewed elsewhere [69]. Here we present data from patients with CKD diagnosed with urate nephropathy (UAN). Serum NLRP3 mRNA concentrations, IL-1β, and IL-18 protein concentrations were reported as significantly higher in UAN compared to healthy controls [329]. In a patient population with gout, IL-1β protein concentrations in serum were significantly higher compared to healthy controls, and patients with UAN had significantly more IL-1β than patients with gout but without renal damage [330].
In another investigation, the NLRP3, ASC, caspase-1 gene and protein expression in PBMCs, and the IL-1β and IL-18 protein concentrations in plasma of patients with hyperuricemia and patients with UAN were significantly higher compared to healthy controls [331]. In these analyses, the plasma IL-1β concentrations and the PBMC caspase-1 and ASC gene and protein expression were significantly higher in UAN compared to hyperuricemia patients. The PBMC gene and protein expression for NLRP3 and the plasma IL-18 protein concentration, in contrast, were not. This could suggest negative feedback or regulation of NLRP3 in PBMCs through IL-1β or uremia.
4.2.8. Anti-Neutrophil Cytoplasmic Antibody (ANCA)—Associated Vasculitis (AAV)
Anti-neutrophil cytoplasmic antibody-associated vasculitis is a small-vessel vasculitis that is associated with the presence of ANCA specific for myeloperoxidase (MPO) or proteinase 3 (PR3). Renal involvement is present in the majority of patients with AAV. Inflammasome components have been investigated in AAV kidney biopsies. One study investigated specifically tubulointerstitial injury in MPO-AAV patients diagnosed with glomerulonephritis [332]. In these patients, NLRP3 and IL-1β gene expression correlated positively with the severity of tubulointerstitial injury. NLRP3 protein was detected in infiltrating macrophages, while NLRP3 protein staining was minimal in glomeruli. Another study (also mainly MPO) in AAV reported increased protein amounts of NOD2, NLRP3 and NLRC5 in both tubulointerstitium and glomeruli compared to healthy control renal tissue [333]. Protein staining of NOD2, NLRP3 and NLRC5 was mainly seen in podocytes and infiltrating monocytes/macrophages. NOD2 and NLRC5 staining was stronger in more severe (crescentic) glomerulonephritis.
4.2.9. End-Stage Kidney Disease (ESKD)
Once a patient with CKD reaches an eGFR < 15 mL/min/1.73 m2, signs and symptoms related to the accumulation of fluid and uremic retention solutes occur. In this end-stage of kidney disease, patients require renal replacement therapy with peritoneal dialysis (PD), hemodialysis (HD), or kidney transplantation to continue life. Chronic inflammation contributes to the progression of kidney disease to ESKD [6,334]. Inflammation in ESKD is likely multifactorial and contributes to a uremic phenotype with CVD, osteoporosis, and frailty [335]. Oxidative distress contributes to the pathogenesis of CVD in ESKD [336], and CKD stage-dependent alterations of antioxidant enzymes have been reported [337,338,339].
Inflammasome components were investigated in ESKD under several aspects. A study of neointimal hyperplasia in low-flow arteriovenous fistulas from HD patients showed a significantly higher NLRP3 protein amount compared to control vessels from a patient population matched with respect to age, diabetes and hypertension [340]. The increased amount of NLRP3 was primarily localized to neointimal vascular smooth muscle cells. Caspase-1/IL-1β, as well as Smad2/3 signalling, were activated in the arteriovenous fistulas. In an investigation of serum caspase-1 protein concentrations, significantly more circulating caspase-1 was found in ESKD compared to age-matched healthy control subjects [341]. Of note, caspase-1 protein was significantly higher in ESKD without renal replacement therapy compared to both HD and PD treatment.
In PBMCs from patients with ESKD and HD therapy, gene expression of NLRP3, caspase-1, IL-1β and IL-18 was significantly higher compared to healthy controls [342]. Co-localization of NLRP3/ASC and mitochondria in HD patients was described. Interestingly, in this study, a significantly higher protein amount in HD was only reported for caspase-1 and IL-1β/-18. Another study in HD patients could be in line with these results. Herein, the plasma IL-1β protein concentrations were significantly higher compared to a non-CKD control group that included diabetic and hypertensive subjects [343]. Nevertheless, the PBMCs from these HD patients did not show a secretion of caspase-1 and IL-1β in response to canonical inflammasome stimulation. This was attributed to a low protein expression of inflammasome components, including NLRP3, due to increased concentrations of the uremic toxin indoxyl sulfate. Such a reduction of NLRP3-related components or -activity in PBMCs from ESKD in comparison to a non-healthy control group without renal function impairment would also be in agreement with other investigations. In a study that compared hemodialysis patients to age- and diabetes-matched hypertensive patients, the HD patients had a significantly lower frequency of caspase-1 protein positive monocytes and a lower IL-1β protein secretion from PBMCs [344]. This could result from high indoxyl sulfate concentrations and/or negative feedback due to high IL-1β serum concentrations in HD patients.
The interleukins-1β and 18 belong to the group of uremic retention solutes. The serum concentration of IL-1β protein is reported as ~1.5 times higher in uraemia compared to normal, and IL-18 concentration as ~1.4 times higher (The European Uremic Toxins (EUTox) Database, available online at www.uremic-toxins.org, accessed on 12 November 2021). Interleukin 1β has been classified as belonging to uremic toxins with the second-highest evidence score for toxicity [345]. Per definition is the retention of uremic solutes directly or indirectly attributable to the reduction in renal clearance [346]. However, not only passive retention, but also upregulated production via caspase-1 has been suggested [347]. A considerable body of research investigated associations between interleukins and clinical conditions in ESKD, irrespective of the mechanisms underlying a certain serum interleukin concentration. Higher concentrations of IL-1β predicted hypotensive episodes during the hemodialysis procedure in ESKD [348]. Circulating protein concentrations of IL-18 were higher in HD patients with protein-energy wasting compared to HD patients without this condition [349] and predicted major adverse cardiovascular events [350], cardiocerebral vascular events [351], and, in children, left ventricular hypertrophy [352] among ESKD patients with HD therapy. In post-myocardial infarction patients with moderate CKD and increased high-sensitivity C-reactive protein concentrations, the inhibition of IL-1β with the monoclonal antibody canakinumab reduced major adverse cardiovascular events [353].
Taken together, the inflammasome substrates IL-1β and IL-18 are increased in advanced and end-stage kidney disease and are relevant to CKD-related morbidities. Both activated production and retention occur. Therefore, meaningful investigations of the relation between inflammasome components and uraemia/ESKD require a comparison to patients with less advanced CKD stages with the same underlying kidney disease, and/or patients matched for age and co-morbidities.
An overview of the alterations or involvement of inflammasome components or inflammasome substrates in human CKD is given in Table 6.
Table 6.
Inflammasome component involvement in CKD.
| Clinical Condition | Altered/Involved Inflammasome Component or Inflammasome Substrate | Investigated Tissue/Cells/Fluid | Reference |
|---|---|---|---|
| Albuminuria | NLRP3 NLRP3, IL-18 Caspase-1, IL-1β, IL-18 |
Renal tubules Renal interstitium Proximale renal tubule epithelium |
[206] [283] [284] |
| Nephrotic syndrome | NLRP3, ASC IL-1β |
PBMCs in glucocorticoid-resistant nephrotic syndrome Renal tissue in non-minimal change nephrotic syndrome |
[285] [286] |
| DKD |
IL-1β IL-18 IL-18 NLRP3, caspase-1 IL-1β IL-18 NLRP3 NLRP3, cleaved caspase-1 NLRP3, ASC, caspase-1 NLRP3, IL-1β NLRC4 AIM2 |
Serum Serum Urine PBMCs Renal tissue Glomerulum, tubulointerstitium Renal tubules Renal tissue Glomerular endothelial cells, podocytes Renal podocytes Renal tubule epithelial cells Renal tubules, renal interstitium Renal tubule epithelial cells, leukocytes in tubulointerstitial lesions |
[208] [288,289,290] [289,292] [291] [295] [294] [141] [296,299,300] [208] [297] [298] [301,302] [303] |
| DKD comorbidities Hypertension Metabolic syndrome |
NLRP3 AIM2 IL-1β |
Renal tissue Renal tubule epithelial cells, leukocytes in tubulointerstitial lesions Serum |
[127] [303] [305] |
| IgAN Nephritis in immunoglobulin A vasculitis |
NLRP3 IL-1β |
Renal tubules and glomeruli Urine |
[309,310] [311] |
| LN |
IL-1β IL-18 IL-18 NLRP3, ASC, caspase-1, IL-1β, IL-18 IL-1β IL-18 Active caspase-1, IL-1β NLRP3 |
Serum Serum Whole blood PBMCs Urinary macrophages Renal monocytes-macrophages Infiltrating mononuclear cells in renal glomeruli Renal tubules and podocytes Renal tissue |
[317,319] [316,317,320,321] [318] [317] [315] [314] [316] [224] [127] |
| ADPKD | IL-1β NLRP3 |
Whole blood Serum |
[327] [328] |
| UAN |
NLRP3, IL-1β, IL-18 IL-1β ASC, caspase-1 |
Serum Serum, plasma PBMCs |
[329] [330,331] [331] |
| AAV | NLRP3, IL-1β NOD2, NLRP3, NLRC5 |
Renal tissue, mainly infiltrating macrophages Renal tissue, podocytes and infiltrating monocytes-macrophages |
[332] [333] |
| ESKD |
IL-1β IL-18 Caspase-1 NLRP3, caspase-1, IL-1β, IL-18 NLRP3, caspase-1, IL-1β |
Plasma Serum Serum PBMCs Neointimal VSMCs of low-flow arteriovenous fistulas |
[343,348] [349,350,351,352,353] [341,347] [342] [340] |
NLRC—NLR family CARD domain-containing protein; IL—interleukin; NOD—nucleotide-binding oligomerization domain; NLRP—NOD-like receptor family pyrin domain containing; AIM2—Absent In Melanoma 2; DKD—diabetic kidney disease; PBMC—peripheral blood mononuclear cells; ASC—apoptosis-associated speck-like protein containing a caspase-associated recruitment domain; IgAN - Immunoglobulin A nephropathy; LN—Lupus nephritis; ADPKD—Autosomal dominant polycystic kidney disease; UAN—Urate nephropathy; AAV—Anti-neutrophil cytoplasmic antibody-associated vasculitis, ESKD—End-stage kidney disease, VSMC—vascular smooth muscle cell.
5. Summary and Perspectives
In summary, we demonstrated the considerable diversity of the inflammasome components and their interactions with various molecular partners involved in kidney disease pathophysiology.
In preclinical AKI models, canonical and non-canonical NLRP3 effects have consistently been reported, but NLRP1 could be involved, too. Reactive oxygen species contribute to inflammasome priming and activation, while mitochondria and ER stress both contribute to the activation of NLRP3 inflammasomes. Involvement of inflammasome components in AKI has been described in monocytes-macrophages, but also in renal epithelial cells. Furthermore, crosstalk between inflammasome components and cell death pathways involving caspase-8 was reported, both in leukocytes and epithelial cells in AKI models. Clinical data are consistent with an involvement of inflammasome components in AKI, but data are still sparse. In line with the systemic nature of AKI and the organ-crosstalk involved, alterations of IL-1β and IL-18, but also NLRP3 concentrations have been reported in blood plasma/serum. A protective role for NLRP6 was suggested in acute tubular injury, while NLRC4, NLRP3, and AIM2 were involved in acute loss of kidney function in transplanted kidneys. Urinary IL-18 concentrations have been under intense investigation as a biomarker in AKI. Our review identified the importance of the temporal pattern of IL-18 concentration changes that could be helpful to determine the optimal timing of inflammasome-targeting therapies in AKI.
In preclinical CKD models, involvement of NLRP3 and inflammasome substrates IL-1β and IL-18 have been reported for CKD pathogenesis. Alterations of inflammasome components were localized to immune cells, resident glomerular cells, and endothelial cells. Furthermore, a multitude of mechanisms related to redox signalling, mitochondria and ER stress are known, that contribute to NLRP3 priming and activation. Clinical data clearly point to an involvement of inflammasome components in CKD; related to CKD causes, but also CKD progression and CKD-induced morbidities, like CVD. Besides the sensory component NLRP3, alterations for AIM2, NLRC4 and NLRC5 were also reported. As several of the causes underlying CKD are of systemic nature, as in diabetes mellitus or SLE, and since advanced CKD affects the entire body, alterations of inflammasome components and substrates in CKD are not only found in renal tissue, but also in circulating and infiltrating immune cells, in whole blood, and blood plasma/serum.
Finally, we identified differential regulation of inflammasome components that require consideration in research on the pathogenesis of human CKD. First, frequent comorbidities as hypertension and metabolic syndrome, but also treatments, exert effects on inflammasome components; hence, control groups should be matched accordingly. Second, PBMC reduction or unexpected low amounts of NLRP3 or caspase-1 were observed in some patient populations with LN, UAN, and ESKD. This could result from increased concentrations of uremic toxins, as shown for high indoxyl sulfate concentrations, or represent a negative feedback regulation of inflammasome components in circulating immune cells that needs further study.
Acknowledgments
A.K.A.-R. is a Ph.D. student from Posgrado en Ciencias Biológicas at the Universidad Nacional Autónoma de México and is a recipient of a scholarship from CONACyT, Mexico (CVU 818062). We gratefully acknowledge to the postdoctoral grant programme from the Consejo Nacional de Ciencia y Tecnología (CONACyT) for the postdoctoral fellow position of A.C.-G. A.S. (Anjali Srivastava) acknowledges the research fellowship from the Council of Scientific and Industrial Research (CSIR), India.
Supplementary Materials
The following supporting information can be downloaded at: https://www.mdpi.com/article/10.3390/antiox11020246/s1.
Author Contributions
Conceptualization, A.S. (Alexandra Scholze); methodology, A.S. (Anjali Srivastava), S.R.M., A.K.A.-R., A.C.-G., J.P.-C. and A.S. (Alexandra Scholze); writing—original draft preparation, A.S. (Anjali Srivastava), S.R.M., A.K.A.-R., A.C.-G., J.P.-C. and A.S. (Alexandra Scholze); writing—review and editing, A.S. (Anjali Srivastava), S.R.M., A.K.A.-R., A.C.-G., J.P.-C. and A.S (Alexandra Scholze). All authors have read and agreed to the published version of the manuscript.
Funding
This research was funded by Consejo Nacional de Ciencia y Tecnología (CONACyT; grant number: A1-S-7495) and by Dirección General de Asuntos del Personal Académico (DGAPA; grant number: IN200922).
Conflicts of Interest
S.R.M. is now an employee for AstraZeneca (Biopharmaceuticals R&D, Cambridge, UK). All of this work was performed at CSIR-CDRI, Lucknow, India. No funding or support was received from AstraZeneca.
Footnotes
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations.
References
- 1.Carney E.F. The impact of chronic kidney disease on global health. Nat. Rev. Nephrol. 2020;16:251. doi: 10.1038/s41581-020-0268-7. [DOI] [PubMed] [Google Scholar]
- 2.Bikbov B., Purcell C.A., Levey A.S., Smith M., Abdoli A., Abebe M., Adebayo O.M., Afarideh M., Agarwal S.K., Agudelo-Botero M., et al. Global, regional, and national burden of chronic kidney disease, 1990–2017: A systematic analysis for the Global Burden of Disease Study 2017. Lancet. 2020;395:709–733. doi: 10.1016/S0140-6736(20)30045-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Levey A.S., Levin A., Kellum J.A. Definition and Classification of Kidney Diseases. Am. J. Kidney Dis. 2013;61:686–688. doi: 10.1053/j.ajkd.2013.03.003. [DOI] [PubMed] [Google Scholar]
- 4.Rosner M.H., Okusa M.D. Pathogenesis and Etiology of Ischemic Acute Tubular Necrosis. In: Motwani S., editor. UpToDate. UpToDate; Waltham, MA, USA: [(accessed on 4 October 2021)]. Available online: https://www.uptodate.com/contents/7228. [Google Scholar]
- 5.Jiang M., Bai M., Lei J., Xie Y., Xu S., Jia Z., Zhang A. Mitochondrial dysfunction and the AKI-to-CKD transition. Am. J. Physiol. Physiol. 2020;319:F1105–F1116. doi: 10.1152/ajprenal.00285.2020. [DOI] [PubMed] [Google Scholar]
- 6.Stenvinkel P., Chertow G.M., Devarajan P., Levin A., Andreoli S.P., Bangalore S., Warady B.A. Chronic Inflammation in Chronic Kidney Disease Progression: Role of Nrf2. Kidney Int. Rep. 2021;6:1775–1787. doi: 10.1016/j.ekir.2021.04.023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Ebert T., Neytchev O., Witasp A., Kublickiene K., Stenvinkel P., Shiels P.G. Inflammation and Oxidative Stress in Chronic Kidney Disease and Dialysis Patients. Antioxid. Redox Signal. 2021;35:1426–1448. doi: 10.1089/ars.2020.8184. [DOI] [PubMed] [Google Scholar]
- 8.Lee K.-H., Kang T.-B. The Molecular Links between Cell Death and Inflammasome. Cells. 2019;8:1057. doi: 10.3390/cells8091057. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Lorenz G., Darisipudi M.N., Anders H.-J. Canonical and non-canonical effects of the NLRP3 inflammasome in kidney inflammation and fibrosis. Nephrol. Dial. Transplant. 2013;29:41–48. doi: 10.1093/ndt/gft332. [DOI] [PubMed] [Google Scholar]
- 10.Mulay S.R. Multifactorial functions of the inflammasome component NLRP3 in pathogenesis of chronic kidney diseases. Kidney Int. 2019;96:58–66. doi: 10.1016/j.kint.2019.01.014. [DOI] [PubMed] [Google Scholar]
- 11.Komada T., Muruve D.A. The role of inflammasomes in kidney disease. Nat. Rev. Nephrol. 2019;15:501–520. doi: 10.1038/s41581-019-0158-z. [DOI] [PubMed] [Google Scholar]
- 12.Sharma D., Kanneganti T.-D. The cell biology of inflammasomes: Mechanisms of inflammasome activation and regulation. J. Cell Biol. 2016;213:617–629. doi: 10.1083/jcb.201602089. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Rathinam V.A., Fitzgerald K.A. Inflammasome Complexes: Emerging Mechanisms and Effector Functions. Cell. 2016;165:792–800. doi: 10.1016/j.cell.2016.03.046. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Anders H.-J., Muruve D.A. The Inflammasomes in Kidney Disease. J. Am. Soc. Nephrol. 2011;22:1007–1018. doi: 10.1681/ASN.2010080798. [DOI] [PubMed] [Google Scholar]
- 15.Mangan M.S.J., Olhava E.J., Roush W.R., Seidel H.M., Glick G.D., Latz E. Targeting the NLRP3 inflammasome in inflammatory diseases. Nat. Rev. Drug Discov. 2018;17:588–606. doi: 10.1038/nrd.2018.97. [DOI] [PubMed] [Google Scholar]
- 16.Weinheimer-Haus E.M., Mirza R.E., Koh T.J. Nod-Like Receptor Protein-3 Inflammasome Plays an Important Role during Early Stages of Wound Healing. PLoS ONE. 2015;10:e0119106. doi: 10.1371/journal.pone.0119106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Bracey N.A., Gershkovich B., Chun J., Vilaysane A., Meijndert H.C., Wright J.R., Jr., Fedak P., Beck P.L., Muruve D.A., Duff H.J. Mitochondrial NLRP3 Protein Induces Reactive Oxygen Species to Promote Smad Protein Signaling and Fibrosis Independent from the Inflammasome. J. Biol. Chem. 2014;289:19571–19584. doi: 10.1074/jbc.M114.550624. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Anders H.-J., Suarez-Alvarez B., Grigorescu M., Foresto-Neto O., Steiger S., Desai J., Marschner J.A., Honarpisheh M., Shi C., Jordan J., et al. The macrophage phenotype and inflammasome component NLRP3 contributes to nephrocalcinosis-related chronic kidney disease independent from IL-1–mediated tissue injury. Kidney Int. 2017;93:656–669. doi: 10.1016/j.kint.2017.09.022. [DOI] [PubMed] [Google Scholar]
- 19.Zheng D., Liwinski T., Elinav E. Inflammasome activation and regulation: Toward a better understanding of complex mechanisms. Cell Discov. 2020;6:36. doi: 10.1038/s41421-020-0167-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Christgen S., Place D.E., Kanneganti T.-D. Toward targeting inflammasomes: Insights into their regulation and activation. Cell Res. 2020;30:315–327. doi: 10.1038/s41422-020-0295-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.He Y., Hara H., Núñez G. Mechanism and Regulation of NLRP3 Inflammasome Activation. Trends Biochem. Sci. 2016;41:1012–1021. doi: 10.1016/j.tibs.2016.09.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Hoseini Z., Sepahvand F., Rashidi B., Sahebkar A., Masoudifar A., Mirzaei H. NLRP3 inflammasome: Its regulation and involvement in atherosclerosis. J. Cell. Physiol. 2017;233:2116–2132. doi: 10.1002/jcp.25930. [DOI] [PubMed] [Google Scholar]
- 23.Kelley N., Jeltema D., Duan Y., He Y. The NLRP3 Inflammasome: An Overview of Mechanisms of Activation and Regulation. Int. J. Mol. Sci. 2019;20:3328. doi: 10.3390/ijms20133328. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Yang J., Liu Z., Xiao T.S. Post-translational regulation of inflammasomes. Cell. Mol. Immunol. 2016;14:65–79. doi: 10.1038/cmi.2016.29. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Zangiabadi S., Abdul-Sater A.A. Regulation of the NLRP3 Inflammasome by Posttranslational Modifications. J. Immunol. 2022;208:286–292. doi: 10.4049/jimmunol.2100734. [DOI] [PubMed] [Google Scholar]
- 26.Belliere J., Casemayou A., Colliou E., El Hachem H., Kounde C., Piedrafita A., Feuillet G., Schanstra J.P., Faguer S. Ibrutinib does not prevent kidney fibrosis following acute and chronic injury. Sci. Rep. 2021;11:11985. doi: 10.1038/s41598-021-91491-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Gajjala P.R., Fliser D., Speer T., Jankowski V., Jankowski J. Emerging role of post-translational modifications in chronic kidney disease and cardiovascular disease. Nephrol. Dial. Transplant. 2015;30:1814–1824. doi: 10.1093/ndt/gfv048. [DOI] [PubMed] [Google Scholar]
- 28.Seregin S.S., Golovchenko N., Schaf B., Chen J., Eaton K.A., Chen G.Y. NLRP6 function in inflammatory monocytes reduces susceptibility to chemically induced intestinal injury. Mucosal Immunol. 2016;10:434–445. doi: 10.1038/mi.2016.55. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Shen C., Lu A., Xie W.J., Ruan J., Negro R., Egelman E.H., Fu T.-M., Wu H. Molecular mechanism for NLRP6 inflammasome assembly and activation. Proc. Natl. Acad. Sci. USA. 2019;116:2052–2057. doi: 10.1073/pnas.1817221116. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Lamkanfi M., Dixit V.M. Mechanisms and Functions of Inflammasomes. Cell. 2014;157:1013–1022. doi: 10.1016/j.cell.2014.04.007. [DOI] [PubMed] [Google Scholar]
- 31.Krakauer T. Inflammasomes, Autophagy, and Cell Death: The Trinity of Innate Host Defense against Intracellular Bacteria. Mediat. Inflamm. 2019;2019:2471215. doi: 10.1155/2019/2471215. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Zhao Y., Shao F. The NAIP-NLRC4 inflammasome in innate immune detection of bacterial flagellin and type III secretion apparatus. Immunol. Rev. 2015;265:85–102. doi: 10.1111/imr.12293. [DOI] [PubMed] [Google Scholar]
- 33.Davis B.K., Roberts R.A., Huang M.T., Willingham S.B., Conti B.J., Brickey W.J., Barker B.R., Kwan M., Taxman D.J., Accavitti-Loper M.-A., et al. Cutting Edge: NLRC5-Dependent Activation of the Inflammasome. J. Immunol. 2011;186:1333–1337. doi: 10.4049/jimmunol.1003111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Meissner T.B., Li A., Kobayashi K.S. NLRC5: A newly discovered MHC class I transactivator (CITA) Microbes Infect. 2012;14:477–484. doi: 10.1016/j.micinf.2011.12.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Zhao Y., Shao F. NLRC5: A NOD-like receptor protein with many faces in immune regulation. Cell Res. 2012;22:1099–1101. doi: 10.1038/cr.2012.83. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Gray E.E., Winship D., Snyder J.M., Child S.J., Geballe A.P., Stetson D.B. The AIM2-like Receptors Are Dispensable for the Interferon Response to Intracellular DNA. Immunity. 2016;45:255–266. doi: 10.1016/j.immuni.2016.06.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Wang P., Ye Z., Deng J., Siu K., Gao W., Chaudhary V., Cheng Y., Fung S., Yuen K., Ho T., et al. Inhibition of AIM 2 inflammasome activation by a novel transcript isoform of IFI 16. EMBO Rep. 2018;19:e45737. doi: 10.15252/embr.201845737. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Yuan B., Zhou X.-M., You Z.-Q., Xu W.-D., Fan J.-M., Chen S.-J., Han Y.-L., Wu Q., Zhang X. Inhibition of AIM2 inflammasome activation alleviates GSDMD-induced pyroptosis in early brain injury after subarachnoid haemorrhage. Cell Death Dis. 2020;11:76. doi: 10.1038/s41419-020-2248-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Ru H., Ni X., Zhao L., Crowley C., Ding W., Hung L.-W., Shaw N., Cheng G., Liu Z.-J. Structural basis for termination of AIM2-mediated signaling by p202. Cell Res. 2013;23:855–858. doi: 10.1038/cr.2013.52. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Kim M.L., Chae J.J., Park Y.H., De Nardo D., Stirzaker R.A., Ko H.-J., Tye H., Cengia L.H., DiRago L., Metcalf D., et al. Aberrant actin depolymerization triggers the pyrin inflammasome and autoinflammatory disease that is dependent on IL-18, not IL-1β. J. Exp. Med. 2015;212:927–938. doi: 10.1084/jem.20142384. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Xu H., Yang J., Gao W., Li L., Li P., Zhang L., Gong Y.-N., Peng X., Xi J.J., Chen S., et al. Innate immune sensing of bacterial modifications of Rho GTPases by the Pyrin inflammasome. Nature. 2014;513:237–241. doi: 10.1038/nature13449. [DOI] [PubMed] [Google Scholar]
- 42.Yang Y., Wang H., Kouadir M., Song H., Shi F. Recent advances in the mechanisms of NLRP3 inflammasome activation and its inhibitors. Cell Death Dis. 2019;10:128. doi: 10.1038/s41419-019-1413-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Proell M., Gerlic M., Mace P., Reed J.C., Riedl S.J. The CARD plays a critical role in ASC foci formation and inflammasome signalling. Biochem. J. 2013;449:613–621. doi: 10.1042/BJ20121198. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Lu A., Li Y., Schmidt F.I., Yin Q., Chen S., Fu T.-M., Tong A.B., Ploegh H.L., Mao Y., Wu H. Molecular basis of caspase-1 polymerization and its inhibition by a new capping mechanism. Nat. Struct. Mol. Biol. 2016;23:416–425. doi: 10.1038/nsmb.3199. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Vince J.E., Silke J. The intersection of cell death and inflammasome activation. Cell. Mol. Life Sci. 2016;73:2349–2367. doi: 10.1007/s00018-016-2205-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Bonventre J.V., Zuk A. Ischemic acute renal failure: An inflammatory disease? Kidney Int. 2004;66:480–485. doi: 10.1111/j.1523-1755.2004.761_2.x. [DOI] [PubMed] [Google Scholar]
- 47.Maier S., Emmanuilidis K., Entleutner M., Zantl N., Werner M., Pfeffer K., Heidecke C.-D. Massive chemokine transcription in acute renal failure due to polymicrobial sepsis. Shock. 2000;14:187–192. doi: 10.1097/00024382-200014020-00019. [DOI] [PubMed] [Google Scholar]
- 48.Linkermann A., Chen G., Dong G., Kunzendorf U., Krautwald S., Dong Z. Regulated Cell Death in AKI. J. Am. Soc. Nephrol. 2014;25:2689–2701. doi: 10.1681/ASN.2014030262. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.McSweeney K., Gadanec L., Qaradakhi T., Ali B., Zulli A., Apostolopoulos V. Mechanisms of Cisplatin-Induced Acute Kidney Injury: Pathological Mechanisms, Pharmacological Interventions, and Genetic Mitigations. Cancers. 2021;13:1572. doi: 10.3390/cancers13071572. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Basile D.P., Anderson M.D., Sutton T.A. Pathophysiology of Acute Kidney Injury. Compr. Physiol. 2012;2:1303–1353. doi: 10.1002/cphy.c110041. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Gao L., Zhong X., Jin J., Li J., Meng X.-M. Potential targeted therapy and diagnosis based on novel insight into growth factors, receptors, and downstream effectors in acute kidney injury and acute kidney injury-chronic kidney disease progression. Signal Transduct. Target. Ther. 2020;5:9. doi: 10.1038/s41392-020-0106-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Tang T.-T., Lv L.-L., Pan M.-M., Wen Y., Wang B., Li Z.-L., Wu M., Wang F.-M., Crowley S.D., Liu B.-C. Hydroxychloroquine attenuates renal ischemia/reperfusion injury by inhibiting cathepsin mediated NLRP3 inflammasome activation. Cell Death Dis. 2018;9:351. doi: 10.1038/s41419-018-0378-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Babelova A., Moreth K., Tsalastra-Greul W., Zeng-Brouwers J., Eickelberg O., Young M.F., Bruckner P., Pfeilschifter J., Schaefer R.M., Gröne H.-J., et al. Biglycan, a Danger Signal That Activates the NLRP3 Inflammasome via Toll-like and P2X Receptors. J. Biol. Chem. 2009;284:24035–24048. doi: 10.1074/jbc.M109.014266. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Yamasaki K., Muto J., Taylor K.R., Cogen A.L., Audish D., Bertin J., Grant E.P., Coyle A.J., Misaghi A., Hoffman H.M., et al. NLRP3/Cryopyrin Is Necessary for Interleukin-1β (IL-1β) Release in Response to Hyaluronan, an Endogenous Trigger of Inflammation in Response to Injury. J. Biol. Chem. 2009;284:12762–12771. doi: 10.1074/jbc.M806084200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Iyer S.S., Pulskens W.P., Sadler J.J., Butter L.M., Teske G.J., Ulland T.K., Eisenbarth S.C., Florquin S., Flavell R.A., Leemans J.C., et al. Necrotic cells trigger a sterile inflammatory response through the Nlrp3 inflammasome. Proc. Natl. Acad. Sci. USA. 2009;106:20388–20393. doi: 10.1073/pnas.0908698106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Nazir S., Gadi I., Al-Dabet M.M., Elwakiel A., Kohli S., Ghosh S., Manoharan J., Ranjan S., Bock F., Braun-Dullaeus R.C., et al. Cytoprotective activated protein C averts Nlrp3 inflammasome–induced ischemia-reperfusion injury via mTORC1 inhibition. Blood. 2017;130:2664–2677. doi: 10.1182/blood-2017-05-782102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Shigeoka A.A., Mueller J.L., Kambo A., Mathison J.C., King A.J., Hall W.F., da Silva Correia J., Ulevitch R.J., Hoffman H.M., McKay D.B. An Inflammasome-Independent Role for Epithelial-Expressed Nlrp3 in Renal Ischemia-Reperfusion Injury. J. Immunol. 2010;185:6277–6285. doi: 10.4049/jimmunol.1002330. Erratum in: J. Immunol. 2011, 186, 1880. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Subramanian N., Natarajan K., Clatworthy M.R., Wang Z., Germain R.N. The Adaptor MAVS Promotes NLRP3 Mitochondrial Localization and Inflammasome Activation. Cell. 2013;153:348–361. doi: 10.1016/j.cell.2013.02.054. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Komada T., Usui F., Kawashima A., Kimura H., Karasawa T., Inoue Y., Kobayashi M., Mizushina Y., Kasahara T., Taniguchi S., et al. Role of NLRP3 Inflammasomes for Rhabdomyolysis-induced Acute Kidney Injury. Sci. Rep. 2015;5:10901. doi: 10.1038/srep10901. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Lau A., Chung H., Komada T., Platnich J., Sandall C.F., Choudhury S.R., Chun J., Naumenko V., Surewaard B.G., Nelson M.C., et al. Renal immune surveillance and dipeptidase-1 contribute to contrast-induced acute kidney injury. J. Clin. Investig. 2018;128:2894–2913. doi: 10.1172/JCI96640. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Cao Y., Fei D., Chen M., Sun M., Xu J., Kang K., Jiang L., Zhao M. Role of the nucleotide-binding domain-like receptor protein 3 inflammasome in acute kidney injury. FEBS J. 2015;282:3799–3807. doi: 10.1111/febs.13379. [DOI] [PubMed] [Google Scholar]
- 62.Wang W., Faubel S., Ljubanović D., Mitra A., Falk S.A., Kim J., Tao Y., Soloviev A., Reznikov L.L., Dinarello C.A., et al. Endotoxemic acute renal failure is attenuated in caspase-1-deficient mice. Am. J. Physiol. Physiol. 2005;288:F997–F1004. doi: 10.1152/ajprenal.00130.2004. [DOI] [PubMed] [Google Scholar]
- 63.Lee D., Faubel S., Edelstein C. Cytokines in acute kidney injury (AKI) Clin. Nephrol. 2011;76:165–173. doi: 10.5414/CN106921. [DOI] [PubMed] [Google Scholar]
- 64.Ye Z., Zhang L., Li R., Dong W., Liu S., Li Z., Liang H., Wang L., Shi W., Malik A.B., et al. Caspase-11 Mediates Pyroptosis of Tubular Epithelial Cells and Septic Acute Kidney Injury. Kidney Blood Press. Res. 2019;44:465–478. doi: 10.1159/000499685. [DOI] [PubMed] [Google Scholar]
- 65.Qu X., Gao H., Tao L., Zhang Y., Zhai J., Sun J., Song Y., Zhang S. Astragaloside IV protects against cisplatin-induced liver and kidney injury via autophagy-mediated inhibition of NLRP3 in rats. J. Toxicol. Sci. 2019;44:167–175. doi: 10.2131/jts.44.167. [DOI] [PubMed] [Google Scholar]
- 66.Herzog C., Yang C., Holmes A., Kaushal G.P. zVAD-fmk prevents cisplatin-induced cleavage of autophagy proteins but impairs autophagic flux and worsens renal function. Am. J. Physiol. Physiol. 2012;303:F1239–F1250. doi: 10.1152/ajprenal.00659.2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Miao N., Yin F., Xie H., Wang Y., Xu Y., Shen Y., Xu D., Yin J., Wang B., Zhou Z., et al. The cleavage of gasdermin D by caspase-11 promotes tubular epithelial cell pyroptosis and urinary IL-18 excretion in acute kidney injury. Kidney Int. 2019;96:1105–1120. doi: 10.1016/j.kint.2019.04.035. [DOI] [PubMed] [Google Scholar]
- 68.Xia W., Li Y., Wu M., Jin Q., Wang Q., Li S., Huang S., Zhang A., Zhang Y., Jia Z. Gasdermin E deficiency attenuates acute kidney injury by inhibiting pyroptosis and inflammation. Cell Death Dis. 2021;12:139. doi: 10.1038/s41419-021-03431-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Mulay S.R., Evan A., Anders H.-J. Molecular mechanisms of crystal-related kidney inflammation and injury. Implications for cholesterol embolism, crystalline nephropathies and kidney stone disease. Nephrol. Dial. Transplant. 2013;29:507–514. doi: 10.1093/ndt/gft248. [DOI] [PubMed] [Google Scholar]
- 70.Chan A.J., Alikhan M.A., Odobasic D., Gan P.Y., Khouri M.B., Steinmetz O.M., Mansell A.S., Kitching A.R., Holdsworth S.R., Summers S.A. Innate IL-17A–Producing Leukocytes Promote Acute Kidney Injury via Inflammasome and Toll-Like Receptor Activation. Am. J. Pathol. 2014;184:1411–1418. doi: 10.1016/j.ajpath.2014.01.023. [DOI] [PubMed] [Google Scholar]
- 71.Deplano S., Cook H.T., Russell R., Franchi L., Schneiter S., Bhangal G., Unwin R.J., Pusey C.D., Tam F.W.K., Behmoaras J. P2X7 receptor-mediated Nlrp3-inflammasome activation is a genetic determinant of macrophage-dependent crescentic glomerulonephritis. J. Leukoc. Biol. 2013;93:127–134. doi: 10.1189/jlb.0612284. [DOI] [PubMed] [Google Scholar]
- 72.Sanz A.B., Sanchez-Niño M.D., Izquierdo M.C., Gonzalez-Espinoza L., Ucero A., Poveda J., Ruiz-Andres O., Ruiz-Ortega M., Selgas R., Egido J., et al. Macrophages and Recently Identified Forms of Cell Death. Int. Rev. Immunol. 2013;33:9–22. doi: 10.3109/08830185.2013.771183. [DOI] [PubMed] [Google Scholar]
- 73.Darisipudi M.N., Thomasova D., Mulay S.R., Brech D., Noessner E., Liapis H., Anders H.-J. Uromodulin Triggers IL-1β–Dependent Innate Immunity via the NLRP3 Inflammasome. J. Am. Soc. Nephrol. 2012;23:1783–1789. doi: 10.1681/ASN.2012040338. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Sanchez-Niño M.D., Sanz A.B., Ortiz A. Uromodulin, Inflammasomes, and Pyroptosis. J. Am. Soc. Nephrol. 2012;23:1761–1763. doi: 10.1681/ASN.2012090942. [DOI] [PubMed] [Google Scholar]
- 75.Yang J.-R., Yao F.-H., Zhang J.-G., Ji Z.-Y., Li K.-L., Zhan J., Tong Y.-N., Lin L.-R., He Y.-N. Ischemia-reperfusion induces renal tubule pyroptosis via the CHOP-caspase-11 pathway. Am. J. Physiol. Physiol. 2014;306:F75–F84. doi: 10.1152/ajprenal.00117.2013. [DOI] [PubMed] [Google Scholar]
- 76.Anders H.-J. Of Inflammasomes and Alarmins: IL-1β and IL-1α in Kidney Disease. J. Am. Soc. Nephrol. 2016;27:2564–2575. doi: 10.1681/ASN.2016020177. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Zhang Z., Shao X., Jiang N., Mou S., Gu L., Li S., Lin Q., He Y., Zhang M., Zhou W., et al. Caspase-11-mediated tubular epithelial pyroptosis underlies contrast-induced acute kidney injury. Cell Death Dis. 2018;9:983. doi: 10.1038/s41419-018-1023-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Shen J., Wang L., Jiang N., Mou S., Zhang M., Gu L., Shao X., Wang Q., Qi C., Li S., et al. NLRP3 inflammasome mediates contrast media-induced acute kidney injury by regulating cell apoptosis. Sci. Rep. 2016;6:34682. doi: 10.1038/srep34682. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Linkermann A., Heller J.-O., Prókai Á., Weinberg J.M., De Zen F., Himmerkus N., Szabó A.J., Bräsen J.H., Kunzendorf U., Krautwald S. The RIP1-Kinase Inhibitor Necrostatin-1 Prevents Osmotic Nephrosis and Contrast-Induced AKI in Mice. J. Am. Soc. Nephrol. 2013;24:1545–1557. doi: 10.1681/ASN.2012121169. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Chung H., Vilaysane A., Lau A., Stahl M., Morampudi V., Bondzi-Simpson A., Platnich J., Bracey N.A., French M.-C., Beck P.L., et al. NLRP3 regulates a non-canonical platform for caspase-8 activation during epithelial cell apoptosis. Cell Death Differ. 2016;23:1331–1346. doi: 10.1038/cdd.2016.14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Guo C., Fu R., Zhou M., Wang S., Huang Y., Hu H., Zhao J., Gaskin F., Yang N., Fu S.M. Pathogenesis of lupus nephritis: RIP3 dependent necroptosis and NLRP3 inflammasome activation. J. Autoimmun. 2019;103:102286. doi: 10.1016/j.jaut.2019.05.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Chen H., Fang Y., Wu J., Chen H., Zou Z., Zhang X., Shao J., Xu Y. RIPK3-MLKL-mediated necroinflammation contributes to AKI progression to CKD. Cell Death Dis. 2018;9:878. doi: 10.1038/s41419-018-0936-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Nakahira K., Haspel J.A., Rathinam V.A., Lee S.-J., Dolinay T., Lam H.C., Englert J.A., Rabinovitch M., Cernadas M., Kim H.P., et al. Autophagy proteins regulate innate immune responses by inhibiting the release of mitochondrial DNA mediated by the NALP3 inflammasome. Nat. Immunol. 2011;12:222–230. doi: 10.1038/ni.1980. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Bauernfeind F., Bartok E., Rieger A., Franchi L., Núñez G., Hornung V. Cutting Edge: Reactive Oxygen Species Inhibitors Block Priming, but Not Activation, of the NLRP3 Inflammasome. J. Immunol. 2011;187:613–617. doi: 10.4049/jimmunol.1100613. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Zhao W.-Y., Zhang L., Sui M.-X., Zhu Y.-H., Zeng L. Protective effects of sirtuin 3 in a murine model of sepsis-induced acute kidney injury. Sci. Rep. 2016;6:33201. doi: 10.1038/srep33201. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Abais J.M., Zhang C., Xia M., Liu Q., Gehr T.W., Boini K.M., Li P.-L. NADPH Oxidase-Mediated Triggering of Inflammasome Activation in Mouse Podocytes and Glomeruli During Hyperhomocysteinemia. Antioxid. Redox Signal. 2013;18:1537–1548. doi: 10.1089/ars.2012.4666. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Kim S.-M., Kim Y.G., Kim D.-J., Park S.H., Jeong K.-H., Lee Y.H., Lim S.J., Lee S.-H., Moon J.-Y. Inflammasome-Independent Role of NLRP3 Mediates Mitochondrial Regulation in Renal Injury. Front. Immunol. 2018;9:2563. doi: 10.3389/fimmu.2018.02563. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Mills E.L., Kelly B., O’Neill L.A.J. Mitochondria are the powerhouses of immunity. Nat Immunol. 2017;18:488–498. doi: 10.1038/ni.3704. [DOI] [PubMed] [Google Scholar]
- 89.Wen Y., Liu Y.-R., Tang T.-T., Pan M.-M., Xu S.-C., Ma K.-L., Lv L.-L., Liu H., Liu B.-C. mROS-TXNIP axis activates NLRP3 inflammasome to mediate renal injury during ischemic AKI. Int. J. Biochem. Cell Biol. 2018;98:43–53. doi: 10.1016/j.biocel.2018.02.015. [DOI] [PubMed] [Google Scholar]
- 90.Bedard K., Krause K.-H. The NOX Family of ROS-Generating NADPH Oxidases: Physiology and Pathophysiology. Physiol. Rev. 2007;87:245–313. doi: 10.1152/physrev.00044.2005. [DOI] [PubMed] [Google Scholar]
- 91.Lambeth J.D. NOX enzymes and the biology of reactive oxygen. Nat. Rev. Immunol. 2004;4:181–189. doi: 10.1038/nri1312. [DOI] [PubMed] [Google Scholar]
- 92.Cruz C.M., Rinna A., Forman H.J., Ventura A.L.M., Persechini P., Ojcius D.M. ATP Activates a Reactive Oxygen Species-dependent Oxidative Stress Response and Secretion of Proinflammatory Cytokines in Macrophages. J. Biol. Chem. 2007;282:2871–2879. doi: 10.1074/jbc.M608083200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93.Aparicio-Trejo O.E., Reyes-Fermín L.M., Briones-Herrera A., Tapia E., León-Contreras J.C., Hernández-Pando R., Sanchez-Lozada L.-G., Pedraza-Chaverri J. Protective effects of N-acetyl-cysteine in mitochondria bioenergetics, oxidative stress, dynamics and S-glutathionylation alterations in acute kidney damage induced by folic acid. Free. Radic. Biol. Med. 2018;130:379–396. doi: 10.1016/j.freeradbiomed.2018.11.005. [DOI] [PubMed] [Google Scholar]
- 94.Bauernfeind F.G., Horvath G., Stutz A., Alnemri E.S., Macdonald K.L., Speert D.P., Fernandes-Alnemri T., Wu J., Monks B.G., Fitzgerald K., et al. Cutting Edge: NF-κB Activating Pattern Recognition and Cytokine Receptors License NLRP3 Inflammasome Activation by Regulating NLRP3 Expression. J. Immunol. 2009;183:787–791. doi: 10.4049/jimmunol.0901363. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95.Nishi T., Shimizu N., Hiramoto M., Sato I., Yamaguchi Y., Hasegawa M., Aizawa S., Tanaka H., Kataoka K., Watanabe H., et al. Spatial Redox Regulation of a Critical Cysteine Residue of NF-κB in Vivo. J. Biol. Chem. 2002;277:44548–44556. doi: 10.1074/jbc.M202970200. [DOI] [PubMed] [Google Scholar]
- 96.Pineda-Molina E., Klatt P., Vázquez J., Marina A., de Lacoba M.G., Pérez-Sala D., Lamas S. Glutathionylation of the p50 Subunit of NF-κB: A Mechanism for Redox-Induced Inhibition of DNA Binding. Biochemistry. 2001;40:14134–14142. doi: 10.1021/bi011459o. [DOI] [PubMed] [Google Scholar]
- 97.Qanungo S., Starke D.W., Pai H.V., Mieyal J.J., Nieminen A.-L. Glutathione Supplementation Potentiates Hypoxic Apoptosis by S-Glutathionylation of p65-NFκB. J. Biol. Chem. 2007;282:18427–18436. doi: 10.1074/jbc.M610934200. [DOI] [PubMed] [Google Scholar]
- 98.Liu Z., Wang X., Wang Y., Zhao M. NLRP3 inflammasome activation regulated by NF-κB and DAPK contributed to paraquat-induced acute kidney injury. Immunol. Res. 2017;65:687–698. doi: 10.1007/s12026-017-8901-7. [DOI] [PubMed] [Google Scholar]
- 99.Mahmoud A.M., Hussein O., El-Twab S.M.A., Hozayen W.G. Ferulic acid protects against methotrexate nephrotoxicityviaactivation of Nrf2/ARE/HO-1 signaling and PPARγ, and suppression of NF-κB/NLRP3 inflammasome axis. Food Funct. 2019;10:4593–4607. doi: 10.1039/C9FO00114J. [DOI] [PubMed] [Google Scholar]
- 100.Yifan Z., Benxiang N., Zheng X., Luwei X., Liuhua Z., Yuzheng G., Ruipeng J. Ceftriaxone Calcium Crystals Induce Acute Kidney Injury by NLRP3-Mediated Inflammation and Oxidative Stress Injury. Oxidative Med. Cell. Longev. 2020;2020:6428498. doi: 10.1155/2020/6428498. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 101.Yang S.-K., Han Y.-C., He J.-R., Yang M., Zhang W., Zhan M., Li A.-M., Li L., Song N., Liu Y.-T., et al. Mitochondria targeted peptide SS-31 prevent on cisplatin-induced acute kidney injury via regulating mitochondrial ROS-NLRP3 pathway. Biomed. Pharmacother. 2020;130:110521. doi: 10.1016/j.biopha.2020.110521. [DOI] [PubMed] [Google Scholar]
- 102.Bhargava P., Schnellmann R.G. Mitochondrial energetics in the kidney. Nat. Rev. Nephrol. 2017;13:629–646. doi: 10.1038/nrneph.2017.107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 103.Shen Y., Jin X., Chen W., Gao C., Bian Q., Fan J., Luan J., Cao Z., Guo Z., Gu Y., et al. Interleukin-22 ameliorated acetaminophen-induced kidney injury by inhibiting mitochondrial dysfunction and inflammatory responses. Appl. Microbiol. Biotechnol. 2020;104:5889–5898. doi: 10.1007/s00253-020-10638-4. [DOI] [PubMed] [Google Scholar]
- 104.Chang Y.-P., Ka S.-M., Hsu W.-H., Chen A., Chao L.K., Lin C.-C., Hsieh C.-C., Chen M.-C., Chiu H.-W., Ho C.-L., et al. Resveratrol inhibits NLRP3 inflammasome activation by preserving mitochondrial integrity and augmenting autophagy. J. Cell. Physiol. 2014;230:1567–1579. doi: 10.1002/jcp.24903. [DOI] [PubMed] [Google Scholar]
- 105.Ding W., Xu C., Wang B., Zhang M. Rotenone Attenuates Renal Injury in Aldosterone-Infused Rats by Inhibiting Oxidative Stress, Mitochondrial Dysfunction, and Inflammasome Activation. Med. Sci. Monit. 2015;21:3136–3143. doi: 10.12659/MSM.895945. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 106.Zhou R., Tardivel A., Thorens B., Choi I., Tschopp J. Thioredoxin-interacting protein links oxidative stress to inflammasome activation. Nat. Immunol. 2010;11:136–140. doi: 10.1038/ni.1831. [DOI] [PubMed] [Google Scholar]
- 107.Brooks C., Wei Q., Cho S.-G., Dong Z. Regulation of mitochondrial dynamics in acute kidney injury in cell culture and rodent models. J. Clin. Investig. 2009;119:1275–1285. doi: 10.1172/JCI37829. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 108.Zhan M., Brooks C., Liu F., Sun L., Dong Z. Mitochondrial dynamics: Regulatory mechanisms and emerging role in renal pathophysiology. Kidney Int. 2013;83:568–581. doi: 10.1038/ki.2012.441. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 109.Dai W., Jiang L. Dysregulated Mitochondrial Dynamics and Metabolism in Obesity, Diabetes, and Cancer. Front. Endocrinol. 2019;10:570. doi: 10.3389/fendo.2019.00570. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 110.Liu R., Wang S.-C., Li M., Ma X.-H., Jia X.-N., Bu Y., Sun L., Yu K.-J. An Inhibitor of DRP1 (Mdivi-1) Alleviates LPS-Induced Septic AKI by Inhibiting NLRP3 Inflammasome Activation. BioMed Res. Int. 2020;2020:2398420. doi: 10.1155/2020/2398420. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 111.Briones-Herrera A., Ramírez-Camacho I., Zazueta C., Tapia E., Pedraza-Chaverri J. Altered proximal tubule fatty acid utilization, mitophagy, fission and supercomplexes arrangement in experimental Fanconi syndrome are ameliorated by sulforaphane-induced mitochondrial biogenesis. Free Radic. Biol. Med. 2020;153:54–70. doi: 10.1016/j.freeradbiomed.2020.04.010. [DOI] [PubMed] [Google Scholar]
- 112.Reyes-Fermín L.M., Avila-Rojas S.H., Aparicio-Trejo O.E., Tapia E., Rivero I., Pedraza-Chaverri J. The Protective Effect of Alpha-Mangostin against Cisplatin-Induced Cell Death in LLC-PK1 Cells is Associated to Mitochondrial Function Preservation. Antioxidants. 2019;8:133. doi: 10.3390/antiox8050133. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 113.Zhou R., Yazdi A.S., Menu P., Tschopp J. A role for mitochondria in NLRP3 inflammasome activation. Nature. 2011;469:221–225. doi: 10.1038/nature09663. [DOI] [PubMed] [Google Scholar]
- 114.Lin Q., Li S., Jiang N., Shao X., Zhang M., Jin H., Zhang Z., Shen J., Zhou Y., Zhou W., et al. PINK1-parkin pathway of mitophagy protects against contrast-induced acute kidney injury via decreasing mitochondrial ROS and NLRP3 inflammasome activation. Redox Biol. 2019;26:101254. doi: 10.1016/j.redox.2019.101254. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 115.Wu C., Hua K., Yang S., Tsai Y., Yang S., Hsieh C., Wu C., Chang J., Arbiser J.L., Chang C., et al. Tris DBA ameliorates IgA nephropathy by blunting the activating signal of NLRP3 inflammasome through SIRT1- and SIRT3-mediated autophagy induction. J. Cell. Mol. Med. 2020;24:13609–13622. doi: 10.1111/jcmm.15663. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 116.He Q., Li Z., Wang Y., Hou Y., Li L., Zhao J. Resveratrol alleviates cerebral ischemia/reperfusion injury in rats by inhibiting NLRP3 inflammasome activation through Sirt1-dependent autophagy induction. Int. Immunopharmacol. 2017;50:208–215. doi: 10.1016/j.intimp.2017.06.029. [DOI] [PubMed] [Google Scholar]
- 117.Gao Y., Dai X., Li Y., Li G., Lin X., Ai C., Cao Y., Li T., Lin B. Role of Parkin-mediated mitophagy in the protective effect of polydatin in sepsis-induced acute kidney injury. J. Transl. Med. 2020;18:114. doi: 10.1186/s12967-020-02283-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 118.Toscano M.G., Ganea D., Gamero A.M. Cecal Ligation Puncture Procedure. J. Vis. Exp. 2011:e2860. doi: 10.3791/2860. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 119.Kantari C., Walczak H. Caspase-8 and Bid: Caught in the act between death receptors and mitochondria. Biochim. Biophys. Acta. 2011;1813:558–563. doi: 10.1016/j.bbamcr.2011.01.026. [DOI] [PubMed] [Google Scholar]
- 120.Knodler L.A., Crowley S.M., Sham H.P., Yang H., Wrande M., Ma C., Ernst R., Steele-Mortimer O., Celli J., Vallance B.A. Noncanonical Inflammasome Activation of Caspase-4/Caspase-11 Mediates Epithelial Defenses against Enteric Bacterial Pathogens. Cell Host Microbe. 2014;16:249–256. doi: 10.1016/j.chom.2014.07.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 121.Lerner A.G., Upton J.-P., Praveen P., Ghosh R., Nakagawa Y., Igbaria A., Shen S., Nguyen V., Backes B.J., Heiman M., et al. IRE1α Induces Thioredoxin-Interacting Protein to Activate the NLRP3 Inflammasome and Promote Programmed Cell Death under Irremediable ER Stress. Cell Metab. 2012;16:250–264. doi: 10.1016/j.cmet.2012.07.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 122.Maekawa H., Inagi R. Stress Signal Network between Hypoxia and ER Stress in Chronic Kidney Disease. Front. Physiol. 2017;8:74. doi: 10.3389/fphys.2017.00074. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 123.Chou X., Ding F., Zhang X., Ding X., Gao H., Wu Q. Sirtuin-1 ameliorates cadmium-induced endoplasmic reticulum stress and pyroptosis through XBP-1s deacetylation in human renal tubular epithelial cells. Arch. Toxicol. 2019;93:965–986. doi: 10.1007/s00204-019-02415-8. [DOI] [PubMed] [Google Scholar]
- 124.Guo H., Li H., Ling L., Gu Y., Ding W. Endoplasmic Reticulum Chaperon Tauroursodeoxycholic Acid Attenuates Aldosterone-Infused Renal Injury. Mediat. Inflamm. 2016;2016:4387031. doi: 10.1155/2016/4387031. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 125.Wang J., Wen Y., Lv L.-L., Liu H., Tang R.-N., Ma K.-L., Liu B.-C. Involvement of endoplasmic reticulum stress in angiotensin II-induced NLRP3 inflammasome activation in human renal proximal tubular cells in vitro. Acta Pharmacol. Sin. 2015;36:821–830. doi: 10.1038/aps.2015.21. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 126.Yuan X., Zheng Y., Chen C., Wang C. Anisodamine inhibits endoplasmic reticulum stress-associated TXNIP/NLRP3 inflammasome activation in rhabdomyolysis-induced acute kidney injury. Apoptosis. 2017;22:1524–1531. doi: 10.1007/s10495-017-1414-y. [DOI] [PubMed] [Google Scholar]
- 127.Vilaysane A., Chun J., Seamone M.E., Wang W., Chin R., Hirota S., Li Y., Clark S.A., Tschopp J., Trpkov K., et al. The NLRP3 Inflammasome Promotes Renal Inflammation and Contributes to CKD. J. Am. Soc. Nephrol. 2010;21:1732–1744. doi: 10.1681/ASN.2010020143. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 128.Juul-Nielsen C., Shen J., Stenvinkel P., Scholze A. Systematic review of the nuclear factor erythroid 2-related factor 2 (NRF2) system in human chronic kidney disease: Alterations, interventions and relation to morbidity. Nephrol. Dial. Transplant. 2021 doi: 10.1093/ndt/gfab031. [DOI] [PubMed] [Google Scholar]
- 129.Sarhan M., Von Mässenhausen A., Hugo C., Oberbauer R., Linkermann A. Immunological consequences of kidney cell death. Cell Death Dis. 2018;9:114. doi: 10.1038/s41419-017-0057-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 130.Han J.-H., Park J., Kang T.-B., Lee K.-H. Regulation of Caspase-8 Activity at the Crossroads of Pro-Inflammation and Anti-Inflammation. Int. J. Mol. Sci. 2021;22:3318. doi: 10.3390/ijms22073318. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 131.Netea M.G., van de Veerdonk F.L., van der Meer J.W., Dinarello C.A., Joosten L.A. Inflammasome-Independent Regulation of IL-1-Family Cytokines. Annu. Rev. Immunol. 2015;33:49–77. doi: 10.1146/annurev-immunol-032414-112306. [DOI] [PubMed] [Google Scholar]
- 132.Gora I.M., Ciechanowska A., Ladyzynski P. NLRP3 Inflammasome at the Interface of Inflammation, Endothelial Dysfunction, and Type 2 Diabetes. Cells. 2021;10:314. doi: 10.3390/cells10020314. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 133.Furman D., Chang J., Lartigue L., Bolen C.R., Haddad F., Gaudilliere B., Ganio E.A., Fragiadakis G.K., Spitzer M., Douchet I., et al. Expression of specific inflammasome gene modules stratifies older individuals into two extreme clinical and immunological states. Nat. Med. 2017;23:174–184. doi: 10.1038/nm.4267. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 134.Wakamatsu T., Yamamoto S., Ito T., Sato Y., Matsuo K., Takahashi Y., Kaneko Y., Goto S., Kazama J.J., Gejyo F., et al. Indoxyl Sulfate Promotes Macrophage IL-1β Production by Activating Aryl Hydrocarbon Receptor/NF-κ/MAPK Cascades, but the NLRP3 inflammasome Was Not Activated. Toxins. 2018;10:124. doi: 10.3390/toxins10030124. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 135.Kitching A.R., Hickey M.J. Immune cell behaviour and dynamics in the kidney—Insights from in vivo imaging. Nat. Rev. Nephrol. 2021;18:22–37. doi: 10.1038/s41581-021-00481-9. [DOI] [PubMed] [Google Scholar]
- 136.Cohen G. Immune Dysfunction in Uremia 2020. Toxins. 2020;12:439. doi: 10.3390/toxins12070439. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 137.Awad F., Assrawi E., Jumeau C., Georgin-Lavialle S., Cobret L., Duquesnoy P., Piterboth W., Thomas L., Stankovic-Stojanovic K., Louvrier C., et al. Impact of human monocyte and macrophage polarization on NLR expression and NLRP3 inflammasome activation. PLoS ONE. 2017;12:e0175336. doi: 10.1371/journal.pone.0175336. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 138.Netea M.G., Nold-Petry C.A., Nold M.F., Joosten L.A.B., Opitz B., van der Meer J.H.M., van de Veerdonk F.L., Ferwerda G., Heinhuis B., Devesa I., et al. Differential requirement for the activation of the inflammasome for processing and release of IL-1β in monocytes and macrophages. Blood. 2009;113:2324–2335. doi: 10.1182/blood-2008-03-146720. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 139.Pulitano C., Ho P., Verran D., Sandroussi C., Joseph D., Bowen D.G., Mccaughan G., Crawford M., Shackel N. Molecular profiling of postreperfusion milieu determines acute kidney injury after liver transplantation: A prospective study. Liver Transplant. 2018;24:922–931. doi: 10.1002/lt.25178. [DOI] [PubMed] [Google Scholar]
- 140.Gauer S., Sichler O., Obermüller N., Holzmann Y., Kiss E., Sobkowiak E., Pfeilschifter J., Geiger H., Mühl H., Hauser I. IL-18 is expressed in the intercalated cell of human kidney. Kidney Int. 2007;72:1081–1087. doi: 10.1038/sj.ki.5002473. [DOI] [PubMed] [Google Scholar]
- 141.Miyauchi K., Takiyama Y., Honjyo J., Tateno M., Haneda M. Upregulated IL-18 expression in type 2 diabetic subjects with nephropathy: TGF-β1 enhanced IL-18 expression in human renal proximal tubular epithelial cells. Diabetes Res. Clin. Pract. 2009;83:190–199. doi: 10.1016/j.diabres.2008.11.018. [DOI] [PubMed] [Google Scholar]
- 142.Hu D., Liu X., Chen S., Bao C. Expressions of IL-18 and its binding protein in peripheral blood leukocytes and kidney tissues of lupus nephritis patients. Clin. Rheumatol. 2010;29:717–721. doi: 10.1007/s10067-010-1386-6. [DOI] [PubMed] [Google Scholar]
- 143.Ix J.H., Shlipak M.G. The Promise of Tubule Biomarkers in Kidney Disease: A Review. Am. J. Kidney Dis. 2021;78:719–727. doi: 10.1053/j.ajkd.2021.03.026. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 144.Konrad M., Hemstreet G., Hersh E.M., Mansell P.W., Mertelsmann R., Kolitz J.E., Bradley E.C. Pharmacokinetics of recombinant interleukin 2 in humans. Cancer Res. 1990;50:2009–2017. [PubMed] [Google Scholar]
- 145.Ostermann M., Zarbock A., Goldstein S., Kashani K., Macedo E., Murugan R., Bell M., Forni L., Guzzi L., Joannidis M., et al. Recommendations on Acute Kidney Injury Biomarkers From the Acute Disease Quality Initiative Consensus Conference. JAMA Netw. Open. 2020;3:e2019209. doi: 10.1001/jamanetworkopen.2020.19209. [DOI] [PubMed] [Google Scholar]
- 146.Novick D., Kim S., Kaplanski G., Dinarello C.A. Interleukin-18, more than a Th1 cytokine. Semin. Immunol. 2013;25:439–448. doi: 10.1016/j.smim.2013.10.014. [DOI] [PubMed] [Google Scholar]
- 147.Kidney Disease: Improving Global Outcomes (KDIGO) Acute Kidney Injury Work Group KDIGO Clinical Practice Guideline for Acute Kidney Injury. Kidney Inter. Suppl. 2012;2:c179–c184. [Google Scholar]
- 148.Pickkers P., Darmon M., Hoste E., Joannidis M., Legrand M., Ostermann M., Prowle J.R., Schneider A., Schetz M. Acute kidney injury in the critically ill: An updated review on pathophysiology and management. Intensiv. Care Med. 2021;47:835–850. doi: 10.1007/s00134-021-06454-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 149.Massoth C., Zarbock A., Meersch M. Acute Kidney Injury in Cardiac Surgery. Crit. Care Clin. 2021;37:267–278. doi: 10.1016/j.ccc.2020.11.009. [DOI] [PubMed] [Google Scholar]
- 150.Gollmann-Tepeköylü C., Graber M., Pölzl L., Naegele F., Moling R., Esser H., Summerer B., Mellitzer V., Ebner S., Hirsch J., et al. Toll-like receptor 3 mediates ischaemia/reperfusion injury after cardiac transplantation. Eur. J. Cardio-Thorac. Surg. 2020;57:826–835. doi: 10.1093/ejcts/ezz383. [DOI] [PubMed] [Google Scholar]
- 151.Sandler N., Kaczmarek E., Itagaki K., Zheng Y., Otterbein L., Khabbaz K., Liu D., Senthilnathan V., Gruen R.L., Hauser C.J. Mitochondrial DAMPs Are Released During Cardiopulmonary Bypass Surgery and Are Associated With Postoperative Atrial Fibrillation. Hear. Lung Circ. 2018;27:122–129. doi: 10.1016/j.hlc.2017.02.014. [DOI] [PubMed] [Google Scholar]
- 152.Riley J.S., Tait S.W. Mitochondrial DNA in inflammation and immunity. EMBO Rep. 2020;21:e49799. doi: 10.15252/embr.201949799. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 153.Alexopoulou L., Holt A.C., Medzhitov R., Flavell R.A. Recognition of double-stranded RNA and activation of NF-κB by Toll-like receptor 3. Nature. 2001;413:732–738. doi: 10.1038/35099560. [DOI] [PubMed] [Google Scholar]
- 154.Kang S., Fernandes-Alnemri T., Rogers C., Mayes L., Wang Y., Dillon C.P., Roback L., Kaiser W.J., Oberst A., Sagara J., et al. Caspase-8 scaffolding function and MLKL regulate NLRP3 inflammasome activation downstream of TLR3. Nat. Commun. 2015;6:7515. doi: 10.1038/ncomms8515. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 155.Xie Y., Jiang W., Cao J., Xie H. Dexmedetomidine attenuates acute kidney injury in children undergoing congenital heart surgery with cardiopulmonary bypass by inhibiting the TLR3/NF-κB signaling pathway. Am. J. Transl. Res. 2021;13:2763–2773. [PMC free article] [PubMed] [Google Scholar]
- 156.Xin C., Yulong X., Yu C., Changchun C., Feng Z., Xinwei M. Urine Neutrophil Gelatinase-Associated Lipocalin and Interleukin-18 Predict Acute Kidney Injury after Cardiac Surgery. Ren. Fail. 2008;30:904–913. doi: 10.1080/08860220802359089. [DOI] [PubMed] [Google Scholar]
- 157.Wang C., Zhang J., Han J., Yang Q., Liu J., Liang B. The level of urinary IL-18 in acute kidney injury after cardiopulmonary bypass. Exp. Ther. Med. 2017;14:6047–6051. doi: 10.3892/etm.2017.5317. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 158.Parikh C., Mishra J., Thiessen-Philbrook H., Dursun B., Ma Q., Kelly C., Dent C., Devarajan P., Edelstein C. Urinary IL-18 is an early predictive biomarker of acute kidney injury after cardiac surgery. Kidney Int. 2006;70:199–203. doi: 10.1038/sj.ki.5001527. [DOI] [PubMed] [Google Scholar]
- 159.Parikh C.R., Coca S., Thiessen-Philbrook H., Shlipak M.G., Koyner J.L., Wang Z., Edelstein C.L., Devarajan P., Patel U.D., Zappitelli M., et al. Postoperative Biomarkers Predict Acute Kidney Injury and Poor Outcomes after Adult Cardiac Surgery. J. Am. Soc. Nephrol. 2011;22:1748–1757. doi: 10.1681/ASN.2010121302. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 160.Parikh C.R., Devarajan P., Zappitelli M., Sint K., Thiessen-Philbrook H., Li S., Kim R.W., Koyner J.L., Coca S., Edelstein C.L., et al. Postoperative Biomarkers Predict Acute Kidney Injury and Poor Outcomes after Pediatric Cardiac Surgery. J. Am. Soc. Nephrol. 2011;22:1737–1747. doi: 10.1681/ASN.2010111163. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 161.Dong L., Ma Q., Bennett M., Devarajan P. Urinary biomarkers of cell cycle arrest are delayed predictors of acute kidney injury after pediatric cardiopulmonary bypass. Pediatr. Nephrol. 2017;32:2351–2360. doi: 10.1007/s00467-017-3748-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 162.Silverton N.A., Hall I.E., Melendez N.P., Harris B., Harley J.S., Parry S.R., Lofgren L.R., Stoddard G.J., Hoareau G.L., Kuck K. Intraoperative Urinary Biomarkers and Acute Kidney Injury After Cardiac Surgery. J. Cardiothorac. Vasc. Anesth. 2021;35:1691–1700. doi: 10.1053/j.jvca.2020.12.026. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 163.Ronco C., Haapio M., House A.A., Anavekar N., Bellomo R. Cardiorenal syndrome. J. Am. Coll. Cardiol. 2008;52:1527–1539. doi: 10.1016/j.jacc.2008.07.051. [DOI] [PubMed] [Google Scholar]
- 164.Delgado-Valero B., Cachofeiro V., Martínez-Martínez E. Fibrosis, the Bad Actor in Cardiorenal Syndromes: Mechanisms Involved. Cells. 2021;10:1824. doi: 10.3390/cells10071824. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 165.Virzì G.M., Torregrossa R., Cruz D.N., Chionh C.Y., De Cal M., Soni S.S., Dominici M., Vescovo G., Rosner M.H., Ronco C. Cardiorenal Syndrome Type 1 May Be Immunologically Mediated: A Pilot Evaluation of Monocyte Apoptosis. Cardiorenal Med. 2012;2:33–42. doi: 10.1159/000335499. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 166.Pastori S., Virzì G.M., Brocca A., De Cal M., Cantaluppi V., Castellani C., Fedrigo M., Thiene G., Valente M., Angelini A., et al. Cardiorenal Syndrome Type 1: Activation of Dual Apoptotic Pathways. Cardiorenal Med. 2015;5:306–315. doi: 10.1159/000438831. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 167.Brocca A., Virzì G.M., Pasqualin C., Pastori S., Marcante S., De Cal M., Ronco C. Cardiorenal Syndrome Type 5: In Vitro Cytotoxicity Effects on Renal Tubular Cells and Inflammatory Profile. Anal. Cell. Pathol. 2015;2015:1–7. doi: 10.1155/2015/469461. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 168.Pastori S., Virz㬠G.M., Brocca A., De Cal M., Clementi A., Vescovo G., Ronco C. Cardiorenal Syndrome Type 1: A Defective Regulation of Monocyte Apoptosis Induced by Proinflammatory and Proapoptotic Factors. Cardiorenal Med. 2015;5:105–115. doi: 10.1159/000371898. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 169.Man S.M., Tourlomousis P., Hopkins L., Monie T., Fitzgerald K., Bryant C.E. Salmonella Infection Induces Recruitment of Caspase-8 to the Inflammasome to Modulate IL-1β Production. J. Immunol. 2013;191:5239–5246. doi: 10.4049/jimmunol.1301581. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 170.Vince J.E., De Nardo D., Gao W., Vince A.J., Hall C., McArthur K., Simpson D., Vijayaraj S., Lindqvist L.M., Bouillet P., et al. The Mitochondrial Apoptotic Effectors BAX/BAK Activate Caspase-3 and -7 to Trigger NLRP3 Inflammasome and Caspase-8 Driven IL-1β Activation. Cell Rep. 2018;25:2339–2353.e4. doi: 10.1016/j.celrep.2018.10.103. [DOI] [PubMed] [Google Scholar]
- 171.Ludes P.-O., de Roquetaillade C., Chousterman B.G., Pottecher J., Mebazaa A. Role of Damage-Associated Molecular Patterns in Septic Acute Kidney Injury, from Injury to Recovery. Front. Immunol. 2021;12:429. doi: 10.3389/fimmu.2021.606622. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 172.Zheng C., Wu D., Shi S., Wang L. miR-34b-5p promotes renal cell inflammation and apoptosis by inhibiting aquaporin-2 in sepsis-induced acute kidney injury. Ren. Fail. 2021;43:291–301. doi: 10.1080/0886022X.2021.1871922. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 173.Shi L., Zhang Y., Xia Y., Li C., Song Z., Zhu J. MiR-150-5p protects against septic acute kidney injury via repressing the MEKK3/JNK pathway. Cell. Signal. 2021;86:110101. doi: 10.1016/j.cellsig.2021.110101. [DOI] [PubMed] [Google Scholar]
- 174.Girardin E., Grau G., Dayer J.-M., Roux-Lombard P., Lambert P.-H., the J5 Study Group* Tumor Necrosis Factor and Interleuktn-1 in the Serum of Children with Severe Infectious Purpura. N. Engl. J. Med. 1988;319:397–400. doi: 10.1056/NEJM198808183190703. [DOI] [PubMed] [Google Scholar]
- 175.Damas P., Reuter A., Gysen P., Demonty J., Lamy M., Franchimont P. Tumor necrosis factor and interleukin-1 serum levels during severe sepsis in humans. Crit. Care Med. 1989;17:975–978. doi: 10.1097/00003246-198910000-00001. [DOI] [PubMed] [Google Scholar]
- 176.Van Deuren M. Kinetics of tumour necrosis factor-alpha, soluble tumour necrosis factor receptors, interleukin 1-beta and its receptor antagonist during serious infections. Eur. J. Clin. Microbiol. Infect. Dis. 1994;13:S12–S16. doi: 10.1007/BF02390680. [DOI] [PubMed] [Google Scholar]
- 177.Graziani G., Bordone G., Bellato V., Finazzi S., Angelini C., Badalamenti S. Gruppo di Studio Trattamenti depurativi in area critica of the Italian Society of Nephrology. Role of the kidney in plasma cytokine removal in sepsis syndrome: A pilot study. J. Nephrol. 2006;19:176–182. [PubMed] [Google Scholar]
- 178.Duff S., Irwin R., Cote J.M., Redahan L., A McMahon B., Marsh B., Nichol A., Holden S., Doran P., Murray P.T. Urinary biomarkers predict progression and adverse outcomes of acute kidney injury in critical illness. Nephrol. Dial. Transplant. 2021 doi: 10.1093/ndt/gfab263. [DOI] [PubMed] [Google Scholar]
- 179.Xie Y., Huang P., Zhang J., Tian R., Jin W., Xie H., Du J., Zhou Z., Wang R. Biomarkers for the diagnosis of sepsis-associated acute kidney injury: Systematic review and meta-analysis. Ann. Palliat. Med. 2021;10:4159–4173. doi: 10.21037/apm-20-1855. [DOI] [PubMed] [Google Scholar]
- 180.Huang W., Wang X., Xie F., Zhang H., Liu D. Serum NLRP3: A biomarker for identifying high-risk septic patients. Cytokine. 2021;149:155725. doi: 10.1016/j.cyto.2021.155725. [DOI] [PubMed] [Google Scholar]
- 181.Kusirisin P., Chattipakorn S.C., Chattipakorn N. Contrast-induced nephropathy and oxidative stress: Mechanistic insights for better interventional approaches. J. Transl. Med. 2020;18:400. doi: 10.1186/s12967-020-02574-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 182.Wybraniec M.T., Chudek J., Msc M.B., Mizia-Stec K. Prediction of contrast-induced acute kidney injury by early post-procedural analysis of urinary biomarkers and intra-renal Doppler flow indices in patients undergoing coronary angiography. J. Interv. Cardiol. 2017;30:465–472. doi: 10.1111/joic.12404. [DOI] [PubMed] [Google Scholar]
- 183.Liu C., Mor M.K., Palevsky P.M., Kaufman J.S., Philbrook H.T., Weisbord S.D., Parikh C.R. Postangiography Increases in Serum Creatinine and Biomarkers of Injury and Repair. Clin. J. Am. Soc. Nephrol. 2020;15:1240–1250. doi: 10.2215/CJN.15931219. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 184.Lech M., Avila-Ferrufino A., Skuginna V., Susanti E., Anders H.-J. Quantitative expression of RIG-like helicase, NOD-like receptor and inflammasome-related mRNAs in humans and mice. Int. Immunol. 2010;22:717–728. doi: 10.1093/intimm/dxq058. [DOI] [PubMed] [Google Scholar]
- 185.Valiño-Rivas L., Cuarental L., Nuñez G., Sanz A.B., Ortiz A., Sanchez-Niño M.D. Loss of NLRP6 expression increases the severity of acute kidney injury. Nephrol. Dial. Transplant. 2019;35:587–598. doi: 10.1093/ndt/gfz169. [DOI] [PubMed] [Google Scholar]
- 186.Grivei A., Giuliani K.T., Wang X., Ungerer J., Francis L., Hepburn K., John G.T., Gois P.F., Kassianos A.J., Healy H. Oxidative stress and inflammasome activation in human rhabdomyolysis-induced acute kidney injury. Free Radic. Biol. Med. 2020;160:690–695. doi: 10.1016/j.freeradbiomed.2020.09.011. [DOI] [PubMed] [Google Scholar]
- 187.Park M., Kwon C.H., Ha H.K., Han M., Song S.H. RNA-Seq identifies condition-specific biological signatures of ischemia-reperfusion injury in the human kidney. BMC Nephrol. 2020;21:398. doi: 10.1186/s12882-020-02025-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 188.McGuinness D., Mohammed S., Monaghan L., Wilson P.A., Kingsmore D., Shapter O., Stevenson K.S., Coley S.M., Devey L., Kirkpatrick R.B., et al. A molecular signature for delayed graft function. Aging Cell. 2018;17:e12825. doi: 10.1111/acel.12825. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 189.Moledina D., Hall I.E., Thiessen-Philbrook H., Reese P.P., Weng F.L., Schröppel B., Doshi M.D., Wilson F., Coca S., Parikh C.R. Performance of Serum Creatinine and Kidney Injury Biomarkers for Diagnosing Histologic Acute Tubular Injury. Am. J. Kidney Dis. 2017;70:807–816. doi: 10.1053/j.ajkd.2017.06.031. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 190.Hall I.E., Yarlagadda S.G., Coca S., Wang Z., Doshi M., Devarajan P., Han W.K., Marcus R.J., Parikh C.R. IL-18 and Urinary NGAL Predict Dialysis and Graft Recovery after Kidney Transplantation. J. Am. Soc. Nephrol. 2009;21:189–197. doi: 10.1681/ASN.2009030264. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 191.Thompson E.R., Bates L., Ibrahim I.K., Sewpaul A., Stenberg B., McNeill A., Figueiredo R., Girdlestone T., Wilkins G.C., Wang L., et al. Novel delivery of cellular therapy to reduce ischemia reperfusion injury in kidney transplantation. Am. J. Transplant. 2020;21:1402–1414. doi: 10.1111/ajt.16100. [DOI] [PubMed] [Google Scholar]
- 192.Lehner L.J., Kleinsteuber A., Halleck F., Khadzhynov D., Schrezenmeier E., Duerr M., Eckardt K.-U., Budde K., Staeck O. Assessment of the Kidney Donor Profile Index in a European cohort. Nephrol. Dial. Transplant. 2018;33:1465–1472. doi: 10.1093/ndt/gfy030. [DOI] [PubMed] [Google Scholar]
- 193.Zens T.J., Danobeitia J.S., Leverson G., Chlebeck P.J., Zitur L., Redfield R.R., D’Alessandro A.M., Odorico S., Kaufman D.B., Fernandez L.A. The impact of kidney donor profile index on delayed graft function and transplant outcomes: A single-center analysis. Clin. Transplant. 2018;32:e13190. doi: 10.1111/ctr.13190. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 194.Boissier R., François P., Tellier B.G., Meunier M., Lyonnet L., Simoncini S., Magalon J., Legris T., Arnaud L., Giraudo L., et al. Perirenal Adipose Tissue Displays an Age-Dependent Inflammatory Signature Associated with Early Graft Dysfunction of Marginal Kidney Transplants. Front. Immunol. 2020;11:445. doi: 10.3389/fimmu.2020.00445. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 195.Florim G.M., Caldas H.C., Gonçalves N.N., Bueno G.O., Baptista M.A., Fernandes-Charpiot I.M., Abbud-Filho M. Activation of HMGB1–TLR4 Pathway and Inflammasome Contribute to Enhanced Inflammatory Response in Extended Criteria and Kidneys with KDPI ≥85% Transplantation. 2020;104:724–730. doi: 10.1097/TP.0000000000003048. [DOI] [PubMed] [Google Scholar]
- 196.Asgari E., Le Friec G., Yamamoto H., Perucha E., Sacks S., Koehl J., Cook H.T., Kemper C. C3a modulates IL-1β secretion in human monocytes by regulating ATP efflux and subsequent NLRP3 inflammasome activation. Blood. 2013;122:3473–3481. doi: 10.1182/blood-2013-05-502229. [DOI] [PubMed] [Google Scholar]
- 197.Venner J., Famulski K.S., Badr D., Hidalgo L.G., Chang J., Halloran P. Molecular Landscape of T Cell-Mediated Rejection in Human Kidney Transplants: Prominence of CTLA4 and PD Ligands. Arab. Archaeol. Epigr. 2014;14:2565–2576. doi: 10.1111/ajt.12946. [DOI] [PubMed] [Google Scholar]
- 198.Stokman G., Kers J., Yapici Ü., Hoelbeek J.J., Claessen N., de Boer O.J., Netea M.G., Hilbrands L., Bemelman F.J., Berge I.J.T., et al. Predominant Tubular Interleukin-18 Expression in Polyomavirus-Associated Nephropathy. Transplantation. 2016;100:e88–e95. doi: 10.1097/TP.0000000000001086. [DOI] [PubMed] [Google Scholar]
- 199.Liang H., Xu F., Zhang T., Huang J., Guan Q., Wang H., Huang Q. Inhibition of IL-18 reduces renal fibrosis after ischemia-reperfusion. Biomed. Pharmacother. 2018;106:879–889. doi: 10.1016/j.biopha.2018.07.031. [DOI] [PubMed] [Google Scholar]
- 200.Theocharis A.D., Manou D., Karamanos N.K. The extracellular matrix as a multitasking player in disease. FEBS J. 2019;286:2830–2869. doi: 10.1111/febs.14818. [DOI] [PubMed] [Google Scholar]
- 201.Pulskens W.P., Butter L.M., Teske G.J., Claessen N., Dessing M.C., Flavell R.A., Sutterwala F.S., Florquin S., Leemans J.C. Nlrp3 Prevents Early Renal Interstitial Edema and Vascular Permeability in Unilateral Ureteral Obstruction. PLoS ONE. 2014;9:e85775. doi: 10.1371/journal.pone.0085775. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 202.Komada T., Usui F., Shirasuna K., Kawashima A., Kimura H., Karasawa T., Nishimura S., Sagara J., Noda T., Taniguchi S., et al. ASC in Renal Collecting Duct Epithelial Cells Contributes to Inflammation and Injury after Unilateral Ureteral Obstruction. Am. J. Pathol. 2014;184:1287–1298. doi: 10.1016/j.ajpath.2014.01.014. [DOI] [PubMed] [Google Scholar]
- 203.Corrêa-Costa M., Braga T.T., Semedo P., Hayashida C.Y., Bechara L.R.G., Elias R.M., Barreto C.R., Silva-Cunha C., Hyane M.I., Gonçalves G.M., et al. Pivotal Role of Toll-Like Receptors 2 and 4, Its Adaptor Molecule MyD88, and Inflammasome Complex in Experimental Tubule-Interstitial Nephritis. PLoS ONE. 2011;6:e29004. doi: 10.1371/journal.pone.0029004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 204.Solini A., Menini S., Rossi C., Ricci C., Santini E., Fantauzzi C.B., Iacobini C., Pugliese G. The purinergic 2X7receptor participates in renal inflammation and injury induced by high-fat diet: Possible role of NLRP3 inflammasome activation. J. Pathol. 2013;231:342–353. doi: 10.1002/path.4237. [DOI] [PubMed] [Google Scholar]
- 205.Bakker P.J., Butter L.M., Kors L., Teske G.J., Aten J., Sutterwala F.S., Florquin S., Leemans J.C. Nlrp3 is a key modulator of diet-induced nephropathy and renal cholesterol accumulation. Kidney Int. 2014;85:1112–1122. doi: 10.1038/ki.2013.503. [DOI] [PubMed] [Google Scholar]
- 206.Zhuang Y., Ding G., Zhao M., Bai M., Yang L., Ni J., Wang R., Jia Z., Huang S., Zhang A. NLRP3 Inflammasome Mediates Albumin-induced Renal Tubular Injury through Impaired Mitochondrial Function. J. Biol. Chem. 2014;289:25101–25111. doi: 10.1074/jbc.M114.578260. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 207.Wen Y., Liu Y., Tang T., Lv L., Liu H., Ma K., Liu B. NLRP3 inflammasome activation is involved in Ang II-induced kidney damage via mitochondrial dysfunction. Oncotarget. 2016;7:54290–54302. doi: 10.18632/oncotarget.11091. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 208.Shahzad K., Bock F., Dong W., Wang H., Kopf S., Kohli S., Al-Dabet M.M., Ranjan S., Wolter J., Wacker C., et al. Nlrp3-inflammasome activation in non-myeloid-derived cells aggravates diabetic nephropathy. Kidney Int. 2015;87:74–84. doi: 10.1038/ki.2014.271. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 209.Wang W., Luo R., Lin Y., Wang F., Zheng P., Levi M., Yang T., Li C. Aliskiren restores renal AQP2 expression during unilateral ureteral obstruction by inhibiting the inflammasome. Am. J. Physiol. Physiol. 2015;308:F910–F922. doi: 10.1152/ajprenal.00649.2014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 210.Wang W., Wang X., Chun J., Vilaysane A., Clark S., French G., Bracey N.A., Trpkov K., Bonni S., Duff H., et al. Inflammasome-Independent NLRP3 Augments TGF-β Signaling in Kidney Epithelium. J. Immunol. 2012;190:1239–1249. doi: 10.4049/jimmunol.1201959. [DOI] [PubMed] [Google Scholar]
- 211.Lichtnekert J., Kulkarni O.P., Mulay S.R., Rupanagudi K.V., Ryu M., Allam R., Vielhauer V., Muruve D., Lindenmeyer M.T., Cohen C.D., et al. Anti-GBM Glomerulonephritis Involves IL-1 but Is Independent of NLRP3/ASC Inflammasome-Mediated Activation of Caspase-1. PLoS ONE. 2011;6:e26778. doi: 10.1371/journal.pone.0026778. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 212.Gao P., He F.-F., Tang H., Lei C.-T., Chen S., Meng X.-F., Su H., Zhang C. NADPH Oxidase-Induced NALP3 Inflammasome Activation Is Driven by Thioredoxin-Interacting Protein Which Contributes to Podocyte Injury in Hyperglycemia. J. Diabetes Res. 2015;2015:504761. doi: 10.1155/2015/504761. Erratum in: J. Diabetes Res. 2016, 2016, 1213892. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 213.Mulay S.R., Anders H.-J. Crystal nephropathies: Mechanisms of crystal-induced kidney injury. Nat. Rev. Nephrol. 2017;13:226–240. doi: 10.1038/nrneph.2017.10. [DOI] [PubMed] [Google Scholar]
- 214.Mulay S.R., Kulkarni O.P., Rupanagudi K.V., Migliorini A., Darisipudi M.N., Vilaysane A., Muruve D., Shi Y., Munro F., Liapis H., et al. Calcium oxalate crystals induce renal inflammation by NLRP3-mediated IL-1β secretion. J. Clin. Investig. 2012;123:236–246. doi: 10.1172/JCI63679. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 215.Petreski T., Piko N., Ekart R., Hojs R., Bevc S. Review on Inflammation Markers in Chronic Kidney Disease. Biomedicines. 2021;9:182. doi: 10.3390/biomedicines9020182. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 216.Zhao J., Wang H., Dai C., Wang H., Zhang H., Huang Y., Wang S., Gaskin F., Yang N., Fu S.M. P2X7Blockade Attenuates Murine Lupus Nephritis by Inhibiting Activation of the NLRP3/ASC/Caspase 1 Pathway. Arthritis Care Res. 2013;65:3176–3185. doi: 10.1002/art.38174. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 217.Zhao J., Zhang H., Huang Y., Wang H., Wang S., Zhao C., Liang Y., Yang N. Bay11-7082 attenuates murine lupus nephritis via inhibiting NLRP3 inflammasome and NF-κB activation. Int. Immunopharmacol. 2013;17:116–122. doi: 10.1016/j.intimp.2013.05.027. [DOI] [PubMed] [Google Scholar]
- 218.Knauf F., Asplin J.R., Granja I., Schmidt I.M., Moeckel G., David R.J., Flavell R.A., Aronson P.S. NALP3-mediated inflammation is a principal cause of progressive renal failure in oxalate nephropathy. Kidney Int. 2013;84:895–901. doi: 10.1038/ki.2013.207. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 219.Ding T., Wang S., Zhang X., Zai W., Fan J., Chen W., Bian Q., Luan J., Shen Y., Zhang Y., et al. Kidney protection effects of dihydroquercetin on diabetic nephropathy through suppressing ROS and NLRP3 inflammasome. Phytomedicine. 2018;41:45–53. doi: 10.1016/j.phymed.2018.01.026. [DOI] [PubMed] [Google Scholar]
- 220.Foresto-Neto O., Ávila V.F., Arias S.C.A., Zambom F.F.F., Rempel L.C.T., Faustino V.D., Machado F.G., Malheiros D.M.A.C., Abensur H., Câmara N.O.S., et al. NLRP3 inflammasome inhibition ameliorates tubulointerstitial injury in the remnant kidney model. Lab. Investig. 2018;98:773–782. doi: 10.1038/s41374-018-0029-4. [DOI] [PubMed] [Google Scholar]
- 221.Hsu W.-H., Hua K.-F., Tuan L.-H., Tsai Y.-L., Chu L.J., Lee Y.-C., Wong W.-T., Lee S.-L., Lai J.-H., Chu C.-L., et al. Compound K inhibits priming and mitochondria-associated activating signals of NLRP3 inflammasome in renal tubulointerstitial lesions. Nephrol. Dial. Transplant. 2019;35:74–85. doi: 10.1093/ndt/gfz073. [DOI] [PubMed] [Google Scholar]
- 222.Ling L., Yang M., Ding W., Gu Y. Ghrelin attenuates UUO-induced renal fibrosis via attenuation of Nlrp3 inflammasome and endoplasmic reticulum stress. Am. J. Transl. Res. 2019;11:131–141. [PMC free article] [PubMed] [Google Scholar]
- 223.Lu A., Li H., Niu J., Wu S., Xue G., Yao X., Guo Q., Wan N., Abliz P., Yang G., et al. Hyperactivation of the NLRP3 Inflammasome in Myeloid Cells Leads to Severe Organ Damage in Experimental Lupus. J. Immunol. 2016;198:1119–1129. doi: 10.4049/jimmunol.1600659. [DOI] [PubMed] [Google Scholar]
- 224.Fu R., Guo C., Wang S., Huang Y., Jin O., Hu H., Chen J., Xu B., Zhou M., Zhao J., et al. Podocyte Activation of NLRP3 Inflammasomes Contributes to the Development of Proteinuria in Lupus Nephritis. Arthritis Rheumatol. 2017;69:1636–1646. doi: 10.1002/art.40155. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 225.Ludwig-Portugall I., Bartok E., Dhana E., Evers B.D., Primiano M.J., Hall J.P., Franklin B.S., Knolle P.A., Hornung V., Hartmann G., et al. An NLRP3-specific inflammasome inhibitor attenuates crystal-induced kidney fibrosis in mice. Kidney Int. 2016;90:525–539. doi: 10.1016/j.kint.2016.03.035. [DOI] [PubMed] [Google Scholar]
- 226.Coll R., Robertson A., Chae J.J., Higgins S.C., Muñoz-Planillo R., Inserra M.C., Vetter I., Dungan L.S., Monks B.G., Stutz A., et al. A small-molecule inhibitor of the NLRP3 inflammasome for the treatment of inflammatory diseases. Nat. Med. 2015;21:248–255. doi: 10.1038/nm.3806. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 227.Youm Y.-H., Nguyen K.Y., Grant R.W., Goldberg E.L., Bodogai M., Kim D., D’Agostino D., Planavsky N., Lupfer C., Kanneganti T.-D., et al. The ketone metabolite β-hydroxybutyrate blocks NLRP3 inflammasome–mediated inflammatory disease. Nat. Med. 2015;21:263–269. doi: 10.1038/nm.3804. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 228.Wu M., Han W., Song S., Du Y., Liu C., Chen N., Wu H., Shi Y., Duan H. NLRP3 deficiency ameliorates renal inflammation and fibrosis in diabetic mice. Mol. Cell. Endocrinol. 2018;478:115–125. doi: 10.1016/j.mce.2018.08.002. [DOI] [PubMed] [Google Scholar]
- 229.Wang C., Pan Y., Zhang Q.-Y., Wang F.-M., Kong L.-D. Quercetin and Allopurinol Ameliorate Kidney Injury in STZ-Treated Rats with Regulation of Renal NLRP3 Inflammasome Activation and Lipid Accumulation. PLoS ONE. 2012;7:e38285. doi: 10.1371/journal.pone.0038285. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 230.Birnbaum Y., Bajaj M., Qian J., Ye Y. Dipeptidyl peptidase-4 inhibition by Saxagliptin prevents inflammation and renal injury by targeting the Nlrp3/ASC inflammasome. BMJ Open Diabetes Res. Care. 2016;4:e000227. doi: 10.1136/bmjdrc-2016-000227. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 231.Yuan X., Bhat O.M., Lohner H., Zhang Y., Li P.-L. Endothelial acid ceramidase in exosome-mediated release of NLRP3 inflammasome products during hyperglycemia: Evidence from endothelium-specific deletion of Asah1 gene. Biochim. Biophys. Acta (BBA) Mol. Cell Biol. Lipids. 2019;1864:158532. doi: 10.1016/j.bbalip.2019.158532. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 232.Franke E.I., Vanderbrink B.A., Hile K.L., Zhang H., Cain A., Matsui F., Meldrum K.K. Renal IL-18 Production Is Macrophage Independent During Obstructive Injury. PLoS ONE. 2012;7:e47417. doi: 10.1371/journal.pone.0047417. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 233.Tsai Y.-L., Hua K.-F., Chen A., Wei C.-W., Chen W.-S., Wu C.-Y., Chu C.-L., Yu Y.-L., Lo C.-W., Ka S.-M. NLRP3 inflammasome: Pathogenic role and potential therapeutic target for IgA nephropathy. Sci. Rep. 2017;7:srep41123. doi: 10.1038/srep41123. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 234.Chen A., Lai-Fa S., Wei-Yuan C., Shin-Chang T., Deh-Ming C., San-Chi L., Fu-Gong L., Wei-Hwa L. Interleukin-1 receptor antagonist modulates the progression of a spontaneously occurring IgA nephropathy in mice. Am. J. Kidney Dis. 1997;30:693–702. doi: 10.1016/S0272-6386(97)90495-9. [DOI] [PubMed] [Google Scholar]
- 235.Krishnan S.M., Dowling J., Ling Y.H., Diep H., Chan C.T., Ferens D., Kett M.M., Pinar A., Samuel C.S., Vinh A., et al. Inflammasome activity is essential for one kidney/deoxycorticosterone acetate/salt-induced hypertension in mice. Br. J. Pharmacol. 2015;173:752–765. doi: 10.1111/bph.13230. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 236.Xia M., Conley S.M., Li G., Li P.-L., Boini K.M. Inhibition of Hyperhomocysteinemia-Induced Inflammasome Activation and Glomerular Sclerosis by NLRP3 Gene Deletion. Cell. Physiol. Biochem. 2014;34:829–841. doi: 10.1159/000363046. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 237.Chan G., Tang S.C.-W. Current practices in the management of diabetic nephropathy. J. R. Coll. Physicians Edinb. 2013;43:330–333. doi: 10.4997/JRCPE.2013.413. [DOI] [PubMed] [Google Scholar]
- 238.Anders H.-J., Huber T.B., Isermann B., Schiffer M. CKD in diabetes: Diabetic kidney disease versus nondiabetic kidney disease. Nat. Rev. Nephrol. 2018;14:361–377. doi: 10.1038/s41581-018-0001-y. [DOI] [PubMed] [Google Scholar]
- 239.Liu Y., Xu Z., Ma F., Jia Y., Wang G. Knockdown of TLR4 attenuates high glucose-induced podocyte injury via the NALP3/ASC/Caspase-1 signaling pathway. Biomed. Pharmacother. 2018;107:1393–1401. doi: 10.1016/j.biopha.2018.08.134. [DOI] [PubMed] [Google Scholar]
- 240.Leehey D.J., Singh A.K., Alavi N., Singh R. Role of angiotensin II in diabetic nephropathy. Kidney Int. 2000;58:S93–S98. doi: 10.1046/j.1523-1755.2000.07715.x. [DOI] [PubMed] [Google Scholar]
- 241.Yoon J.J., Lee H.K., Kim H.Y., Han B.H., Lee H.S., Lee Y.J., Kang D.G. Sauchinone Protects Renal Mesangial Cell Dysfunction against Angiotensin II by Improving Renal Fibrosis and Inflammation. Int. J. Mol. Sci. 2020;21:7003. doi: 10.3390/ijms21197003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 242.Chen Z., Wu C., Liu Y., Li H., Zhu Y., Huang C., Lin H., Qiao Q., Huang M., Zhu Q., et al. ELABELA attenuates deoxycorticosterone acetate/salt-induced hypertension and renal injury by inhibition of NADPH oxidase/ROS/NLRP3 inflammasome pathway. Cell Death Dis. 2020;11:698. doi: 10.1038/s41419-020-02912-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 243.Yang S.-M., Ka S.-M., Hua K.-F., Wu T.-H., Chuang Y.-P., Lin Y.-W., Yang F.-L., Wu S.-H., Yang S.-S., Lin S.-H., et al. Antroquinonol mitigates an accelerated and progressive IgA nephropathy model in mice by activating the Nrf2 pathway and inhibiting T cells and NLRP3 inflammasome. Free Radic. Biol. Med. 2013;61:285–297. doi: 10.1016/j.freeradbiomed.2013.03.024. [DOI] [PubMed] [Google Scholar]
- 244.Hua K.-F., Yang S.-M., Kao T.-Y., Chang J.-M., Chen H.-L., Tsai Y.-J., Chen A., Yang S.-S., Chao L.K., Ka S.-M. Osthole Mitigates Progressive IgA Nephropathy by Inhibiting Reactive Oxygen Species Generation and NF-κB/NLRP3 Pathway. PLoS ONE. 2013;8:e77794. doi: 10.1371/journal.pone.0077794. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 245.Conley S.M., Abais-Battad J.M., Yuan X., Zhang Q., Boini K.M., Li P.-L. Contribution of guanine nucleotide exchange factor Vav2 to NLRP3 inflammasome activation in mouse podocytes during hyperhomocysteinemia. Free. Radic. Biol. Med. 2017;106:236–244. doi: 10.1016/j.freeradbiomed.2017.02.027. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 246.Ekholm M., Kahan T. The Impact of the Renin-Angiotensin-Aldosterone System on Inflammation, Coagulation, and Atherothrombotic Complications, and to Aggravated COVID-19. Front Pharmacol. 2021;12:640185. doi: 10.3389/fphar.2021.640185. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 247.Aranda-Rivera A., Cruz-Gregorio A., Aparicio-Trejo O., Pedraza-Chaverri J. Mitochondrial Redox Signaling and Oxidative Stress in Kidney Diseases. Biomolecules. 2021;11:1144. doi: 10.3390/biom11081144. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 248.Babelova A., Avaniadi D., Jung O., Fork C., Beckmann J., Kosowski J., Weissmann N., Anilkumar N., Shah A.M., Schaefer L., et al. Role of Nox4 in murine models of kidney disease. Free Radic. Biol. Med. 2012;53:842–853. doi: 10.1016/j.freeradbiomed.2012.06.027. [DOI] [PubMed] [Google Scholar]
- 249.Serrander L., Cartier L., Bedard K., Banfi B., Lardy B., Plastre O., Sienkiewicz A., Fórró L., Schlegel W., Krause K.-H. NOX4 activity is determined by mRNA levels and reveals a unique pattern of ROS generation. Biochem. J. 2007;406:105–114. doi: 10.1042/BJ20061903. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 250.Hallan S., Sharma K. The Role of Mitochondria in Diabetic Kidney Disease. Curr. Diabetes Rep. 2016;16:61. doi: 10.1007/s11892-016-0748-0. [DOI] [PubMed] [Google Scholar]
- 251.Li N., Zhao T., Cao Y., Zhang H., Peng L., Wang Y., Zhou X., Wang Q., Li J., Yan M., et al. Tangshen Formula Attenuates Diabetic Kidney Injury by Imparting Anti-pyroptotic Effects via the TXNIP-NLRP3-GSDMD Axis. Front. Pharmacol. 2021;11:623489. doi: 10.3389/fphar.2020.623489. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 252.Zhu Y., Zhu C., Yang H., Deng J., Fan D. Protective effect of ginsenoside Rg5 against kidney injury via inhibition of NLRP3 inflammasome activation and the MAPK signaling pathway in high-fat diet/streptozotocin-induced diabetic mice. Pharmacol. Res. 2020;155:104746. doi: 10.1016/j.phrs.2020.104746. [DOI] [PubMed] [Google Scholar]
- 253.Zhang L., Jing M., Liu Q. Crocin alleviates the inflammation and oxidative stress responses associated with diabetic nephropathy in rats via NLRP3 inflammasomes. Life Sci. 2021;278:119542. doi: 10.1016/j.lfs.2021.119542. [DOI] [PubMed] [Google Scholar]
- 254.Sedeek M., Callera G., Montezano A., Gutsol A., Heitz F., Szyndralewiez C., Page P., Kennedy C.R.J., Burns K.D., Touyz R.M., et al. Critical role of Nox4-based NADPH oxidase in glucose-induced oxidative stress in the kidney: Implications in type 2 diabetic nephropathy. Am. J. Physiol. Physiol. 2010;299:F1348–F1358. doi: 10.1152/ajprenal.00028.2010. [DOI] [PubMed] [Google Scholar]
- 255.Zhuang Y., Yasinta M., Hu C., Zhao M., Ding G., Bai M., Yang L., Ni J., Wang R., Jia Z., et al. Mitochondrial dysfunction confers albumin-induced NLRP3 inflammasome activation and renal tubular injury. Am. J. Physiol. Renal. Physiol. 2015;308:F857–F866. doi: 10.1152/ajprenal.00203.2014. [DOI] [PubMed] [Google Scholar]
- 256.Lv D., Zhou Q., Xia Y., You X., Zhao Z., Li Y., Zou H. The Association between Oxidative Stress Alleviation via Sulforaphane-Induced Nrf2-HO-1/NQO-1 Signaling Pathway Activation and Chronic Renal Allograft Dysfunction Improvement. Kidney Blood Press. Res. 2018;43:191–205. doi: 10.1159/000487501. [DOI] [PubMed] [Google Scholar]
- 257.Li Z., Guo H., Li J., Ma T., Zhou S., Zhang Z., Miao L., Cai L. Sulforaphane prevents type 2 diabetes-induced nephropathy via AMPK-mediated activation of lipid metabolic pathways and Nrf2 antioxidative function. Clin. Sci. 2020;134:2469–2487. doi: 10.1042/CS20191088. [DOI] [PubMed] [Google Scholar]
- 258.Zhou X., Liu Z., Ying K., Wang H., Liu P., Ji X., Chi T., Zou L., Wang S., He Z. WJ-39, an Aldose Reductase Inhibitor, Ameliorates Renal Lesions in Diabetic Nephropathy by Activating Nrf2 Signaling. Oxidative Med. Cell. Longev. 2020;2020:7950457. doi: 10.1155/2020/7950457. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 259.Du L., Wang J., Chen Y., Li X., Wang L., Li Y., Jin X., Gu X., Hao M., Zhu X., et al. Novel Biphenyl Diester Derivative AB-38b Inhibits NLRP3 Inflammasome through Nrf2 Activation in Diabetic Nephropathy. Cell Biol. Toxicol. 2020;36:243–260. doi: 10.1007/s10565-019-09501-8. [DOI] [PubMed] [Google Scholar]
- 260.Yang S.-M., Ka S.-M., Wu H.-L., Yeh Y.-C., Kuo C.-H., Hua K.-F., Shi G.-Y., Hung Y.-J., Hsiao F.-C., Yang S.-S., et al. Thrombomodulin Domain 1 Ameliorates Diabetic Nephropathy in Mice via Anti-NF-ΚB/NLRP3 Inflammasome-Mediated Inflammation, Enhancement of NRF2 Antioxidant Activity and Inhibition of Apoptosis. Diabetologia. 2014;57:424–434. doi: 10.1007/s00125-013-3115-6. [DOI] [PubMed] [Google Scholar]
- 261.Li D., Wang L., Ou J., Wang C., Zhou J., Lu L., Wu Y., Gao J. Reactive Oxygen Species Induced by Uric Acid Promote NRK-52E Cell Apoptosis through the NEK7-NLRP3 Signaling Pathway. Mol. Med. Rep. 2021;24:729. doi: 10.3892/mmr.2021.12368. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 262.Liu G., Chen X., Wang Q., Yuan L. NEK7: A Potential Therapy Target for NLRP3-Related Diseases. Biosci. Trends. 2020;14:74–82. doi: 10.5582/bst.2020.01029. [DOI] [PubMed] [Google Scholar]
- 263.Daiber A. Redox Signaling (Cross-Talk) from and to Mitochondria Involves Mitochondrial Pores and Reactive Oxygen Species. Biochim. Biophys. Acta (BBA) Bioenerg. 2010;1797:897–906. doi: 10.1016/j.bbabio.2010.01.032. [DOI] [PubMed] [Google Scholar]
- 264.Wenzel P., Mollnau H., Oelze M., Schulz E., Wickramanayake J.M.D., Müller J., Schuhmacher S., Hortmann M., Baldus S., Gori T., et al. First Evidence for a Crosstalk between Mitochondrial and NADPH Oxidase-Derived Reactive Oxygen Species in Nitroglycerin-Triggered Vascular Dysfunction. Antioxid. Redox Signal. 2008;10:1435–1447. doi: 10.1089/ars.2007.1969. [DOI] [PubMed] [Google Scholar]
- 265.Han Y., Xu X., Tang C., Gao P., Chen X., Xiong X., Yang M., Yang S., Zhu X., Yuan S., et al. Corrigendum to ‘Reactive oxygen species promote tubular injury in diabetic nephropathy: The role of the mitochondrial ros-txnip-nlrp3 biological axis’ [Redox Biology 16 (2018) 32–46] Redox Biol. 2019;24:101216. doi: 10.1016/j.redox.2019.101216. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 266.Gu C., Liu S., Wang H., Dou H. Role of the Thioredoxin Interacting Protein in Diabetic Nephropathy and the Mechanism of Regulating NOD-like Receptor Protein 3 Inflammatory Corpuscle. Int. J. Mol Med. 2019;43:2440–2450. doi: 10.3892/ijmm.2019.4163. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 267.Romero C.A., Remor A., Latini A., De Paul A.L., Torres A.I., Mukdsi J.H. Uric Acid Activates NRLP3 Inflammasome in an In-Vivo Model of Epithelial to Mesenchymal Transition in the Kidney. J. Mol. Hist. 2017;48:209–218. doi: 10.1007/s10735-017-9720-9. [DOI] [PubMed] [Google Scholar]
- 268.Wang L., Wang H.L., Liu T.T., Lan H.Y. TGF-Beta as a Master Regulator of Diabetic Nephropathy. Int. J. Mol. Sci. 2021;22:7881. doi: 10.3390/ijms22157881. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 269.Wei J., Shi Y., Hou Y., Ren Y., Du C., Zhang L., Li Y., Duan H. Knockdown of Thioredoxin-Interacting Protein Ameliorates High Glucose-Induced Epithelial to Mesenchymal Transition in Renal Tubular Epithelial Cells. Cell. Signal. 2013;25:2788–2796. doi: 10.1016/j.cellsig.2013.09.009. [DOI] [PubMed] [Google Scholar]
- 270.Song S., Qiu D., Luo F., Wei J., Wu M., Wu H., Du C., Du Y., Ren Y., Chen N., et al. Knockdown of NLRP3 Alleviates High Glucose or TGFB1-Induced EMT in Human Renal Tubular Cells. J. Mol. Endocrinol. 2018;61:101–113. doi: 10.1530/JME-18-0069. [DOI] [PubMed] [Google Scholar]
- 271.Aranda-Rivera A.K., Cruz-Gregorio A., Aparicio-Trejo O.E., Ortega-Lozano A.J., Pedraza-Chaverri J. Redox Signaling Pathways in Unilateral Ureteral Obstruction (UUO)-Induced Renal Fibrosis. Free Radic. Biol. Med. 2021;172:65–81. doi: 10.1016/j.freeradbiomed.2021.05.034. [DOI] [PubMed] [Google Scholar]
- 272.Herman-Edelstein M., Scherzer P., Tobar A., Levi M., Gafter U. Altered Renal Lipid Metabolism and Renal Lipid Accumulation in Human Diabetic Nephropathy. J. Lipid Res. 2014;55:561–572. doi: 10.1194/jlr.P040501. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 273.Wu M., Yang Z., Zhang C., Shi Y., Han W., Song S., Mu L., Du C., Shi Y. Inhibition of NLRP3 Inflammasome Ameliorates Podocyte Damage by Suppressing Lipid Accumulation in Diabetic Nephropathy. Metabolism. 2021;118:154748. doi: 10.1016/j.metabol.2021.154748. [DOI] [PubMed] [Google Scholar]
- 274.Oram J.F., Heinecke J.W. ATP-Binding Cassette Transporter A1: A Cell Cholesterol Exporter That Protects against Cardiovascular Disease. Physiol. Rev. 2005;85:1343–1372. doi: 10.1152/physrev.00005.2005. [DOI] [PubMed] [Google Scholar]
- 275.Zhang G., Li Q., Wang L., Chen Y., Wang L., Zhang W. Interleukin-1β Enhances the Intracellular Accumulation of Cholesterol by up-Regulating the Expression of Low-Density Lipoprotein Receptor and 3-Hydroxy-3-Methylglutaryl Coenzyme A Reductase in Podocytes. Mol. Cell Biochem. 2011;346:197–204. doi: 10.1007/s11010-010-0605-4. [DOI] [PubMed] [Google Scholar]
- 276.Zhang Y., Ma K.L., Liu J., Wu Y., Hu Z.B., Liu L., Lu J., Zhang X.L., Liu B.C. Inflammatory Stress Exacerbates Lipid Accumulation and Podocyte Injuries in Diabetic Nephropathy. Acta Diabetol. 2015;52:1045–1056. doi: 10.1007/s00592-015-0753-9. [DOI] [PubMed] [Google Scholar]
- 277.Chang J.W., Kim H., Baek C.H., Lee R.B., Yang W.S., Lee S.K. Up-Regulation of SIRT1 Reduces Endoplasmic Reticulum Stress and Renal Fibrosis. Nephron. 2016;133:116–128. doi: 10.1159/000447067. [DOI] [PubMed] [Google Scholar]
- 278.Liu S.-H., Yang C.-C., Chan D.-C., Wu C.-T., Chen L.-P., Huang J.-W., Hung K.-Y., Chiang C.-K. Chemical Chaperon 4-Phenylbutyrate Protects against the Endoplasmic Reticulum Stress-Mediated Renal Fibrosis in vivo and in vitro. Oncotarget. 2016;7:22116–22127. doi: 10.18632/oncotarget.7904. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 279.Chong W.C., Shastri M.D., Peterson G.M., Patel R.P., Pathinayake P.S., Dua K., Hansbro N.G., Hsu A.C., Wark P.A., Shukla S.D., et al. The complex interplay between endoplasmic reticulum stress and the NLRP3 inflammasome: A potential therapeutic target for inflammatory disorders. Clin. Transl. Immunol. 2021;10:e1247. doi: 10.1002/cti2.1247. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 280.Yeager M.E., Belchenko D.D., Nguyen C.M., Colvin K.L., Ivy D.D., Stenmark K.R. Endothelin-1, the Unfolded Protein Response, and Persistent Inflammation: Role of Pulmonary Artery Smooth Muscle Cells. Am. J. Respir. Cell Mol. Biol. 2012;46:14–22. doi: 10.1165/rcmb.2010-0506OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 281.Hsu Y.-H., Zheng C.-M., Chou C.-L., Chen Y.-J., Lee Y.-H., Lin Y.-F., Chiu H.-W. Therapeutic Effect of Endothelin-Converting Enzyme Inhibitor on Chronic Kidney Disease through the Inhibition of Endoplasmic Reticulum Stress and the NLRP3 Inflammasome. Biomedicines. 2021;9:398. doi: 10.3390/biomedicines9040398. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 282.Kidney Disease: Improving Global Outcomes (KDIGO) CKD Work Group KDIGO 2012 Clinical Practice Guideline for the Evaluation and Management of Chronic Kidney Disease. Kidney Inter. 2013;3:1–150. [Google Scholar]
- 283.Liu D., Xu M., Ding L.-H., Lv L.-L., Liu H., Ma K.-L., Zhang A.-H., Crowley S.D., Liu B.-C. Activation of the Nlrp3 inflammasome by mitochondrial reactive oxygen species: A novel mechanism of albumin-induced tubulointerstitial inflammation. Int. J. Biochem. Cell Biol. 2014;57:7–19. doi: 10.1016/j.biocel.2014.09.018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 284.Fang L., Xie D., Wu X., Cao H., Su W., Yang J. Involvement of Endoplasmic Reticulum Stress in Albuminuria Induced Inflammasome Activation in Renal Proximal Tubular Cells. PLoS ONE. 2013;8:e72344. doi: 10.1371/journal.pone.0072344. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 285.Lucafò M., Granata S., Bonten E.J., McCorkle R., Stocco G., Caletti C., Selvestrel D., Cozzarolo A., Zou C., Cuzzoni E., et al. Hypomethylation of NLRP3 gene promoter discriminates glucocorticoid-resistant from glucocorticoid-sensitive idiopathic nephrotic syndrome patients. Clin. Transl. Sci. 2020;14:964–975. doi: 10.1111/cts.12961. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 286.Wang L., Li Q., Wang L., Li C., Yang H., Wang X., Tao H. The Role of Th17/IL-17 in the Pathogenesis of Primary Nephrotic Syndrome in Children. Kidney Blood Press. Res. 2013;37:332–345. doi: 10.1159/000350161. [DOI] [PubMed] [Google Scholar]
- 287.Donath M.Y., Shoelson S.E. Type 2 diabetes as an inflammatory disease. Nat. Rev. Immunol. 2011;11:98–107. doi: 10.1038/nri2925. [DOI] [PubMed] [Google Scholar]
- 288.Zhou Y., Ren J., Li P., Ma R., Zhou M., Zhang N., Kong X., Hu Z., Xiao X. Expression of Urokinase-type Plasminogen Activator Receptor and its Soluble Form in Type 2 Diabetic Kidney Disease. Arch. Med Res. 2019;50:249–256. doi: 10.1016/j.arcmed.2019.08.007. [DOI] [PubMed] [Google Scholar]
- 289.Milas O., Gadalean F., Vlad A., Dumitrascu V., Velciov S., Gluhovschi C., Bob F., Popescu R., Ursoniu S., Jianu D.C., et al. Pro-inflammatory cytokines are associated with podocyte damage and proximal tubular dysfunction in the early stage of diabetic kidney disease in type 2 diabetes mellitus patients. J. Diabetes Complicat. 2020;34:107479. doi: 10.1016/j.jdiacomp.2019.107479. [DOI] [PubMed] [Google Scholar]
- 290.Araki S., Haneda M., Koya D., Sugimoto T., Isshiki K., Chin-Kanasaki M., Uzu T., Kashiwagi A. Predictive impact of elevated serum level of IL-18 for early renal dysfunction in type 2 diabetes: An observational follow-up study. Diabetologia. 2007;50:867–873. doi: 10.1007/s00125-006-0586-8. [DOI] [PubMed] [Google Scholar]
- 291.Bhattacharjee C.K., Paine S.K., Mahanta J., Borphukan S., Borah P.K. Expression of inflammasome complex mRNA and its targeted microRNA in type 2 diabetes mellitus: A possible predictor of the severity of diabetic nephropathy. J. Diabetes. 2018;11:90–92. doi: 10.1111/1753-0407.12845. [DOI] [PubMed] [Google Scholar]
- 292.Ambinathan J.P.N., Sridhar V.S., Lytvyn Y., Lovblom L.E., Liu H., Bjornstad P., Perkins B.A., Lovshin J.A., Cherney D.Z. Relationships between inflammation, hemodynamic function and RAAS in longstanding type 1 diabetes and diabetic kidney disease. J. Diabetes Complicat. 2021;35:107880. doi: 10.1016/j.jdiacomp.2021.107880. [DOI] [PubMed] [Google Scholar]
- 293.Sueud T., Hadi N.R., Abdulameer R., Jamil D.A., Al-Aubaidy H.A. Assessing urinary levels of IL-18, NGAL and albumin creatinine ratio in patients with diabetic nephropathy. Diabetes Metab. Syndr. Clin. Res. Rev. 2018;13:564–568. doi: 10.1016/j.dsx.2018.11.022. [DOI] [PubMed] [Google Scholar]
- 294.Araújo L.S., Torquato B.G.S., Da Silva C.A., Monteiro M.L.G.D.R., Martins A.L.M.D.S., Da Silva M.V., Dos Reis M.A., Machado J.R. Renal expression of cytokines and chemokines in diabetic nephropathy. BMC Nephrol. 2020;21:308. doi: 10.1186/s12882-020-01960-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 295.Nair V., Komorowsky C.V., Weil E.J., Yee B., Hodgin J., Harder J.L., Godfrey B., Ju W., Boustany-Kari C.M., Schwarz M., et al. A molecular morphometric approach to diabetic kidney disease can link structure to function and outcome. Kidney Int. 2017;93:439–449. doi: 10.1016/j.kint.2017.08.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 296.Hou Y., Lin S., Qiu J., Sun W., Dong M., Xiang Y., Wang L., Du P. NLRP3 inflammasome negatively regulates podocyte autophagy in diabetic nephropathy. Biochem. Biophys. Res. Commun. 2019;521:791–798. doi: 10.1016/j.bbrc.2019.10.194. [DOI] [PubMed] [Google Scholar]
- 297.Gao P., Meng X.-F., Su H., He F.-F., Chen S., Tang H., Tian X.-J., Fan D., Wang Y.-M., Liu J.-S., et al. Thioredoxin-interacting protein mediates NALP3 inflammasome activation in podocytes during diabetic nephropathy. Biochim. Biophys. Acta. 2014;1843:2448–2460. doi: 10.1016/j.bbamcr.2014.07.001. [DOI] [PubMed] [Google Scholar]
- 298.Chen K., Feng L., Hu W., Chen J., Wang X., Wang L., He Y. Optineurin inhibits NLRP3 inflammasome activation by enhancing mitophagy of renal tubular cells in diabetic nephropathy. FASEB J. 2018;33:4571–4585. doi: 10.1096/fj.201801749RRR. [DOI] [PubMed] [Google Scholar]
- 299.Han Y., Xu X., Tang C., Gao P., Chen X., Xiong X., Yang M., Yang S., Zhu X., Yuan S., et al. Reactive oxygen species promote tubular injury in diabetic nephropathy: The role of the mitochondrial ros-txnip-nlrp3 biological axis. Redox Biol. 2018;16:32–46. doi: 10.1016/j.redox.2018.02.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 300.Wang J., Zhao S.-M. LncRNA-antisense non-coding RNA in the INK4 locus promotes pyroptosis via miR-497/thioredoxin-interacting protein axis in diabetic nephropathy. Life Sci. 2020;264:118728. doi: 10.1016/j.lfs.2020.118728. [DOI] [PubMed] [Google Scholar]
- 301.Yuan F., Kolb R., Pandey G., Li W., Sun L., Liu F., Sutterwala F.S., Liu Y., Zhang W. Involvement of the NLRC4-Inflammasome in Diabetic Nephropathy. PLoS ONE. 2016;11:e0164135. doi: 10.1371/journal.pone.0164135. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 302.Wang Y., Gou R., Yu L., Wang L., Yang Z., Guo Y., Tang L. Activation of the NLRC4 inflammasome in renal tubular epithelial cell injury in diabetic nephropathy. Exp. Ther. Med. 2021;22:814. doi: 10.3892/etm.2021.10246. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 303.Komada T., Chung H., Lau A., Platnich J., Beck P.L., Benediktsson H., Duff H.J., Jenne C.N., Muruve D.A. Macrophage Uptake of Necrotic Cell DNA Activates the AIM2 Inflammasome to Regulate a Proinflammatory Phenotype in CKD. J. Am. Soc. Nephrol. 2018;29:1165–1181. doi: 10.1681/ASN.2017080863. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 304.Yamamoto T., Takabatake Y., Takahashi A., Kimura T., Namba T., Matsuda J., Minami S., Kaimori J.-Y., Matsui I., Matsusaka T., et al. High-Fat Diet–Induced Lysosomal Dysfunction and Impaired Autophagic Flux Contribute to Lipotoxicity in the Kidney. J. Am. Soc. Nephrol. 2016;28:1534–1551. doi: 10.1681/ASN.2016070731. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 305.Morales E., Porrini E., Martin-Taboada M., Luis-Lima S., Vila-Bedmar R., de Pablos I.G., Gómez P., Rodríguez E., Torres L., Lanzón B., et al. Renoprotective role of bariatric surgery in patients with established chronic kidney disease. Clin. Kidney J. 2020;14:2037–2046. doi: 10.1093/ckj/sfaa266. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 306.Kim S.R., Lee S.-G., Kim S.H., Kim J.H., Choi E., Cho W., Rim J., Hwang I., Lee C.J., Lee M., et al. SGLT2 inhibition modulates NLRP3 inflammasome activity via ketones and insulin in diabetes with cardiovascular disease. Nat. Commun. 2020;11 doi: 10.1038/s41467-020-15983-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 307.Packer M. Role of Impaired Nutrient and Oxygen Deprivation Signaling and Deficient Autophagic Flux in Diabetic CKD Development: Implications for Understanding the Effects of Sodium-Glucose Cotransporter 2-Inhibitors. J. Am. Soc. Nephrol. 2020;31:907–919. doi: 10.1681/ASN.2020010010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 308.Pattrapornpisut P., Avila-Casado C., Reich H.N. IgA Nephropathy: Core Curriculum 2021. Am. J. Kidney Dis. 2021;78:429–441. doi: 10.1053/j.ajkd.2021.01.024. [DOI] [PubMed] [Google Scholar]
- 309.Chun J., Chung H., Wang X., Barry R., Taheri Z.M., Platnich J., Ahmed S.B., Trpkov K., Hemmelgarn B., Benediktsson H., et al. NLRP3 Localizes to the Tubular Epithelium in Human Kidney and Correlates with Outcome in IgA Nephropathy. Sci. Rep. 2016;6:24667. doi: 10.1038/srep24667. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 310.Peng W., Pei G.-Q., Tang Y., Tan L., Qin W. IgA1 deposition may induce NLRP3 expression and macrophage transdifferentiation of podocyte in IgA nephropathy. J. Transl. Med. 2019;17:406. doi: 10.1186/s12967-019-02157-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 311.Berthelot L., Jamin A., Viglietti D., Chemouny J.M., Ayari H., Pierre M., Housset P., Sauvaget V., Hurtado-Nedelec M., Vrtovsnik F., et al. Value of biomarkers for predicting immunoglobulin A vasculitis nephritis outcome in an adult prospective cohort. Nephrol. Dial. Transplant. 2017;33:1579–1590. doi: 10.1093/ndt/gfx300. [DOI] [PubMed] [Google Scholar]
- 312.Aringer M. Inflammatory markers in systemic lupus erythematosus. J. Autoimmun. 2019;110:102374. doi: 10.1016/j.jaut.2019.102374. [DOI] [PubMed] [Google Scholar]
- 313.Aringer M., Smolen J.S. Cytokine expression in lupus kidneys. Lupus. 2005;14:13–18. doi: 10.1191/0961203305lu2053oa. [DOI] [PubMed] [Google Scholar]
- 314.Takemura T., Yoshioka K., Murakami K., Akano N., Okada M., Aya N., Maki S. Cellular localization of inflammatory cytokines in human glomerulonephritis. Virchows Arch. 1994;424:459–464. doi: 10.1007/BF00191429. [DOI] [PubMed] [Google Scholar]
- 315.Kim J., Jeong J.H., Jung J., Jeon H., Lee S., Lim J.S., Go H., Oh J.S., Kim Y.-G., Lee C.-K., et al. Immunological characteristics and possible pathogenic role of urinary CD11c+ macrophages in lupus nephritis. Rheumatology. 2020;59:2135–2145. doi: 10.1093/rheumatology/keaa053. [DOI] [PubMed] [Google Scholar]
- 316.Sigdel K.R., Duan L., Wang Y., Hu W., Wang N., Sun Q., Liu Q., Liu X., Hou X., Cheng A., et al. Serum Cytokines Th1, Th2, and Th17 Expression Profiling in Active Lupus Nephritis-IV: From a Southern Chinese Han Population. Mediat. Inflamm. 2016;2016:4927530. doi: 10.1155/2016/4927530. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 317.Ma Z.-Z., Sun H.-S., Lv J.-C., Guo L., Yang Q.-R. Expression and clinical significance of the NEK7-NLRP3 inflammasome signaling pathway in patients with systemic lupus erythematosus. J. Inflamm. 2018;15:16. doi: 10.1186/s12950-018-0192-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 318.Miteva L.D., Manolova I.M., Ivanova M.G., Stoilov R.M., Stanilova S.A. High interleukin-18 and low FOXP3 mRNAs in peripheral blood of women with severe systemic lupus erythematosus: A cross-sectional study. Rheumatol. Int. 2020;40:727–735. doi: 10.1007/s00296-020-04542-3. [DOI] [PubMed] [Google Scholar]
- 319.Wu Q., Qin Y., Shi M., Yan L. Diagnostic significance of circulating miR-485-5p in patients with lupus nephritis and its predictive value evaluation for the clinical outcomes. J. Chin. Med. Assoc. 2021;84:491–497. doi: 10.1097/JCMA.0000000000000522. [DOI] [PubMed] [Google Scholar]
- 320.Mende R., Vincent F.B., Kandane-Rathnayake R., Koelmeyer R., Lin E., Chang J., Hoi A., Morand E.F., Harris J., Lang T. Analysis of Serum Interleukin (IL)-1β and IL-18 in Systemic Lupus Erythematosus. Front. Immunol. 2018;9:1250. doi: 10.3389/fimmu.2018.01250. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 321.Wu C.-Y., Yang H.-Y., Yao T.-C., Liu S.-H., Huang J.-L. Serum IL-18 as biomarker in predicting long-term renal outcome among pediatric-onset systemic lupus erythematosus patients. Medicine. 2016;95:e5037. doi: 10.1097/MD.0000000000005037. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 322.Petrackova A., Smrzova A., Gajdos P., Schubertova M., Schneiderova P., Kromer P., Snasel V., Skacelova M., Mrazek F., Zadrazil J., et al. Serum protein pattern associated with organ damage and lupus nephritis in systemic lupus erythematosus revealed by PEA immunoassay. Clin. Proteom. 2017;14:32. doi: 10.1186/s12014-017-9167-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 323.Pei Y., Watnick T. Autosomal Dominant Polycystic Kidney Disease (ADPKD): Genetics of the Disease and Mechanisms of Cyst Growth. In: Forman J.P., editor. UpToDate. UpToDate; Waltham, MA, USA: [(accessed on 10 October 2021)]. Available online: https://www.uptodate.com/contents/autosomal-dominant-polycystic-kidney-disease-adpkd-genetics-of-the-disease-and-mechanisms-of-cyst-growth. [Google Scholar]
- 324.Goilav B., Satlin L.M., Wilson P.D. Pathways of apoptosis in human autosomal recessive and autosomal dominant polycystic kidney diseases. Pediatr. Nephrol. 2008;23:1473–1482. doi: 10.1007/s00467-008-0851-9. [DOI] [PubMed] [Google Scholar]
- 325.Wang Y., Kanneganti T.-D. From pyroptosis, apoptosis and necroptosis to PANoptosis: A mechanistic compendium of programmed cell death pathways. Comput. Struct. Biotechnol. J. 2021;19:4641–4657. doi: 10.1016/j.csbj.2021.07.038. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 326.Kocyigit I., Ozturk F., Eroğlu E., Karaca Z., Kaynar A.S., Cetin M., Tokgoz B., Sipahioglu M.H., Bayramov R., Sen A., et al. Dysmetabolic markers predict outcomes in autosomal dominant polycystic kidney disease. Clin. Exp. Nephrol. 2019;23:1130–1140. doi: 10.1007/s10157-019-01748-z. [DOI] [PubMed] [Google Scholar]
- 327.Kocyigit I., Taheri S., Eroğlu E., Sener E.F., Zararsız G., Uzun I., Tufan E., Mehmetbeyoglu E., Bayram K.K., Sipahioglu M.H., et al. Systemic Succinate, Hypoxia-Inducible Factor-1 Alpha, and IL-1β Gene Expression in Autosomal Dominant Polycystic Kidney Disease with and without Hypertension. Cardiorenal Med. 2019;9:370–381. doi: 10.1159/000500478. [DOI] [PubMed] [Google Scholar]
- 328.Raptis V., Loutradis C., Boutou A.K., Faitatzidou D., Sioulis A., Ferro C.J., Papagianni A., Sarafidis P.A. Serum Copeptin, NLPR3, and suPAR Levels among Patients with Autosomal-Dominant Polycystic Kidney Disease with and without Impaired Renal Function. Cardiorenal Med. 2020;10:440–451. doi: 10.1159/000510834. [DOI] [PubMed] [Google Scholar]
- 329.Hu J., Wu H., Wang D., Yang Z., Dong J. LncRNA ANRIL promotes NLRP3 inflammasome activation in uric acid nephropathy through miR-122-5p/BRCC3 axis. Biochimie. 2019;157:102–110. doi: 10.1016/j.biochi.2018.10.011. [DOI] [PubMed] [Google Scholar]
- 330.Tang H., Zhao Y., Tan C., Liu Y. Significance of Serum Markers and Urinary Microalbumin in the Diagnosis of Early Renal Damage in Patients with Gout. Clin. Lab. 2021;67 doi: 10.7754/Clin.Lab.2020.200722. [DOI] [PubMed] [Google Scholar]
- 331.Zhang Y., Sui X., Xu Y., Gu F., Zhang A., Chen J. Association between Nod-like receptor protein 3 inflammasome and gouty nephropathy. Exp. Ther. Med. 2020;20:195–204. doi: 10.3892/etm.2020.8694. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 332.Ashiro M., Sasatomi Y., Watanabe R., Watanabe M., Miyake K., Abe Y., Yasuno T., Ito K., Ueki N., Hamauchi A., et al. IL-1β promotes tubulointerstitial injury in MPO-ANCA-associated glomerulonephritis. Clin. Nephrol. 2016;86:190–199. doi: 10.5414/CN108902. [DOI] [PubMed] [Google Scholar]
- 333.Wang L.-Y., Sun X.-J., Chen M., Zhao M.-H. The expression of NOD2, NLRP3 and NLRC5 and renal injury in anti-neutrophil cytoplasmic antibody-associated vasculitis. J. Transl. Med. 2019;17:197. doi: 10.1186/s12967-019-1949-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 334.Andrade-Oliveira V., Foresto-Neto O., Watanabe I.K.M., Zatz R., Câmara N.O.S. Inflammation in Renal Diseases: New and Old Players. Front. Pharmacol. 2019;10:1192. doi: 10.3389/fphar.2019.01192. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 335.Cobo G., Lindholm B., Stenvinkel P. Chronic inflammation in end-stage renal disease and dialysis. Nephrol. Dial. Transplant. 2018;33((Suppl. 3)):iii35–iii40. doi: 10.1093/ndt/gfy175. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 336.Pedraza-Chaverri J., Sanchez-Lozada L.-G., Osorio H., Tapia E., Scholze A. New Pathogenic Concepts and Therapeutic Approaches to Oxidative Stress in Chronic Kidney Disease. Oxidative Med. Cell. Longev. 2016;2016:1–21. doi: 10.1155/2016/6043601. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 337.Scholze A., Krueger K., Diedrich M., Räth C., Torges A., Jankowski V., Maier A., Thilo F., Zidek W., Tepel M. Superoxide dismutase type 1 in monocytes of chronic kidney disease patients. Amino Acids. 2010;41:427–438. doi: 10.1007/s00726-010-0763-4. [DOI] [PubMed] [Google Scholar]
- 338.Krueger K., Shen J., Maier A., Tepel M., Scholze A. Lower Superoxide Dismutase 2 (SOD2) Protein Content in Mononuclear Cells Is Associated with Better Survival in Patients with Hemodialysis Therapy. Oxidative Med. Cell. Longev. 2016;2016:7423249. doi: 10.1155/2016/7423249. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 339.Shen J., Rasmussen M., Dong Q.-R., Tepel M., Scholze A. Expression of the NRF2 Target Gene NQO1 Is Enhanced in Mononuclear Cells in Human Chronic Kidney Disease. Oxidative Med. Cell. Longev. 2017;2017:9091879. doi: 10.1155/2017/9091879. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 340.Ding X., Chen J., Wu C., Wang G., Zhou C., Chen S., Wang K., Zhang A., Ye P., Wu J., et al. Nucleotide-Binding Oligomerization Domain-Like Receptor Protein 3 Deficiency in Vascular Smooth Muscle Cells Prevents Arteriovenous Fistula Failure Despite Chronic Kidney Disease. J. Am. Heart Assoc. 2019;8:e011211. doi: 10.1161/JAHA.118.011211. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 341.Moser B., Roth G., Brunner M., Lilaj T., Deicher R., Wolner E., Kovarik J., Boltz-Nitulescu G., Vychytil A., Ankersmit H.J. Aberrant T cell activation and heightened apoptotic turnover in end-stage renal failure patients: A comparative evaluation between non-dialysis, haemodialysis, and peritoneal dialysis. Biochem. Biophys. Res. Commun. 2003;308:581–585. doi: 10.1016/S0006-291X(03)01389-5. [DOI] [PubMed] [Google Scholar]
- 342.Granata S., Masola V., Zoratti E., Scupoli M., Baruzzi A., Messa M., Sallustio F., Gesualdo L., Lupo A., Zaza G. NLRP3 Inflammasome Activation in Dialyzed Chronic Kidney Disease Patients. PLoS ONE. 2015;10:e0122272. doi: 10.1371/journal.pone.0122272. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 343.Ho L.-C., Wu T.-Y., Lin T.-M., Liou H.-H., Hung S.-Y. Indoxyl Sulfate Mediates the Low Inducibility of the NLRP3 Inflammasome in Hemodialysis Patients. Toxins. 2021;13:38. doi: 10.3390/toxins13010038. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 344.Ulrich C., Wildgrube S., Fiedler R., Seibert E., Kneser L., Fick S., Schäfer C., Markau S., Trojanowicz B., Girndt M. NLRP3 Inflammasome Activation in Hemodialysis and Hypertensive Patients with Intact Kidney Function. Toxins. 2020;12:675. doi: 10.3390/toxins12110675. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 345.Rosner M.H., Reis T., Husain-Syed F., Vanholder R., Hutchison C., Stenvinkel P., Blankestijn P.J., Cozzolino M., Juillard L., Kashani K., et al. Classification of Uremic Toxins and Their Role in Kidney Failure. Clin. J. Am. Soc. Nephrol. 2021;16:1918–1928. doi: 10.2215/CJN.02660221. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 346.Vanholder R. Uremic Toxins. In: Lam A.Q., editor. UpToDate. UpToDate; Waltham, MA, USA: [(accessed on 11 November 2021)]. Available online: https://www.uptodate.com/contents/uremic-toxins. [Google Scholar]
- 347.Li R., Sun Y., Johnson C., Zhou J., Wang L., Li Y.-F., Lu Y., Nanayakkara G., Fu H., Shao Y., et al. Uremic toxins are conditional danger- or homeostasis-associated molecular patterns. Front. Biosci. 2018;23:348–387. doi: 10.2741/4595. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 348.Yu J., Chen X., Li Y., Wang Y., Cao X., Liu Z., Shen B., Zou J., Ding X. Pro-inflammatory cytokines as potential predictors for intradialytic hypotension. Ren. Fail. 2021;43:198–205. doi: 10.1080/0886022X.2021.1871921. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 349.Bi X., Chu M., Ai H., Hu C., Ding W. Association of serum IL-18 with protein-energy wasting in end-stage renal disease patients on haemodialysis. Int. Urol. Nephrol. 2019;51:1271–1278. doi: 10.1007/s11255-019-02167-5. [DOI] [PubMed] [Google Scholar]
- 350.Wang S., Chen F., Yang S., Shi J. Interleukin-18. Int. Heart J. 2018;59:786–790. doi: 10.1536/ihj.17-154. [DOI] [PubMed] [Google Scholar]
- 351.Chang C.-H., Fan P.-C., Lin C.-Y., Yang C.-H., Chen Y.-T., Chang S.-W., Yang H.-Y., Jenq C.-C., Hung C.-C., Yang C.-W., et al. Elevation of Interleukin-18 Correlates with Cardiovascular, Cerebrovascular, and Peripheral Vascular Events. Medicine. 2015;94:e1836. doi: 10.1097/MD.0000000000001836. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 352.Badawy A., Nigm D.A., Ezzat G.M., Gamal Y. Interleukin 18 as a new inflammatory mediator in left ventricular hypertrophy in children with end-stage renal disease. Saudi J. Kidney Dis. Transplant. 2020;31:1206–1216. doi: 10.4103/1319-2442.308329. [DOI] [PubMed] [Google Scholar]
- 353.Ridker P.M., MacFadyen J.G., Glynn R.J., Koenig W., Libby P., Everett B.M., Lefkowitz M., Thuren T., Cornel J. Inhibition of Interleukin-1β by Canakinumab and Cardiovascular Outcomes in Patients with Chronic Kidney Disease. J. Am. Coll. Cardiol. 2018;71:2405–2414. doi: 10.1016/j.jacc.2018.03.490. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.