Abstract
Beyond conventional risk factors for cardiovascular disease, women face an additional burden of sex-specific risk factors. Key stages of a woman’s reproductive history may influence or reveal short- and long-term cardiometabolic and cardiovascular trajectories. Early and late menarche, polycystic ovary syndrome, infertility, adverse pregnancy outcomes (e.g., hypertensive disorders of pregnancy, gestational diabetes, preterm delivery, and intrauterine growth restriction), and absence of breastfeeding are all associated with increased future cardiovascular disease risk. The menopause transition additionally represents a period of accelerated cardiovascular disease risk, with timing (e.g., premature menopause), mechanism, and symptoms of menopause, as well as treatment of menopause symptoms, each contributing to this risk. Differences in conventional cardiovascular disease risk factors appear to explain some, but not all, of the observed associations between reproductive history and later-life cardiovascular disease; further research is needed to elucidate hormonal effects and unique sex-specific disease mechanisms. A history of reproductive risk factors represents an opportunity for comprehensive risk factor screening, refinement of cardiovascular disease risk assessment, and implementation of primordial and primary prevention to optimize long-term cardiometabolic health in women.
Keywords: Cardiovascular Disease, Pregnancy, Risk Factors, Women, Sex, Gender
Introduction
Cardiovascular disease (CVD) is the leading cause of death in women and men in the U.S. and worldwide.1 Women and men share conventional CVD risk factors, albeit with important sex differences,2, 3 but mounting evidence has identified unique sex-specific risk factors related to reproductive and pregnancy history in women. These sex-specific risk factors are increasingly recognized in cardiovascular and obstetrical society guidelines, with premature age of menopause and adverse pregnancy outcomes (APOs) in particular now codified as risk-enhancing factors for atherosclerotic CVD (ASCVD).4–7
In this review, we summarize the current evidence for associations and mechanistic links between a woman’s reproductive history and CVD risk, spanning menarche to menopause, infertility, and pregnancy (Figure 1). We review how reproductive history might be further incorporated in clinical practice and highlight important unanswered questions and directions for future research.
Figure 1. Key reproductive exposures associated with future risk of cardiovascular disease in women.
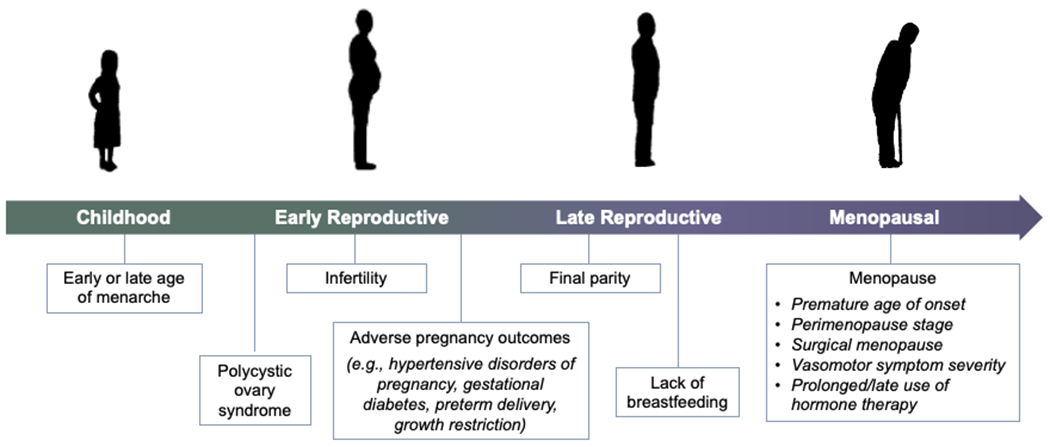
Key stages of a woman’s reproductive history may influence or reveal short- and long-term cardiometabolic and cardiovascular trajectories. Early and late menarche, polycystic ovary syndrome, infertility, adverse pregnancy outcomes (e.g., hypertensive disorders of pregnancy, gestational diabetes, preterm delivery, and intrauterine growth restriction), and absence of breastfeeding may all be adversely associated with a woman’s cardiovascular risk. In addition, the menopause transition represents a period of accelerated cardiovascular disease risk, with timing (e.g., premature menopause), mechanism, and symptoms of menopause, as well as treatment of menopause symptoms, each modifying these risks.
Age of Menarche and CVD Risk
Menarche is the onset of the first menstrual period and often considered the central event of female puberty. The average age of menarche is approximately 12 years in the U.S. Early menarche is typically defined as occurring before age 12 years, but some researchers have defined it as occurring before 10-11 years of age. While genetic factors play a role, potentially modifiable factors such as lower birthweight, increased weight fluctuation during childhood, and especially higher childhood body mass index (BMI) may increase the likelihood of early menarche.8 In parallel with rising rates of obesity in children, the mean age of menarche in the U.S. has decreased over the past 20 years from 12.1 to 11.9 years.9 Black and Hispanic women are disproportionately likely to undergo early menarche.10
Early menarche is associated with future obesity and metabolic syndrome in adulthood. Whether this is independent of pre-menarchal and adolescent BMI values is unclear. In the U.K. EPIC-Norfolk study of 15,807 women, history of early menarche was associated with greater odds of having hypertension, diabetes, obesity (BMI ≥30 kg/m2), and central obesity (waist circumference ≥80 cm) later in life.11 Other studies have linked early menarche with greater risk of future metabolic syndrome and its individual components, including dyslipidemia and hypertension.12
Early menarche has been associated with a 15-30% higher risk of future CVD11, 13, 14 independent of sociodemographic factors.11, 14 Data further suggest the relationship between age of menarche and future CVD may be U-shaped, with increased risk also noted for late menarche (after age 17).13–15 The association of early menarche with future CVD may be stronger in higher-risk women. Among women with suspected ischemia undergoing coronary angiography in the Women’s Ischemic Syndrome Evaluation study (mean baseline age 58 years), a history of menarche ≤10 years of age was associated with >4-fold risk of a subsequent major adverse cardiovascular event over 6-year follow-up (hazard ratio [HR] 4.53, 95% CI 2.13-9.63) in adjusted analysis; menarche ≥15 years of age was also associated with CVD risk (HR 2.58, 95% CI 1.28-5.21).15
Mechanisms linking early menarche to increased CVD risk later in life are incompletely understood. Given the strong association between elevated childhood BMI and early menarche, early menarche may reflect genetic or lifestyle factors, including excess calorie consumption and reduced physical activity. Higher leptin levels, which are associated with increased adiposity, are associated with earlier onset of menarche.16 This suggests dysregulation of adipokines during puberty may reflect negative metabolic influence that increases a woman’s CVD risk later in life.16 Early vs. normal or late menarche is associated with unfavorable levels of insulin, glucose, blood pressure (BP), and body fat among adolescent girls.17
Polycystic Ovary Syndrome and CVD Risk
Polycystic ovary syndrome (PCOS), which affects 5-13% of women, is characterized by amenorrhea or oligomenorrhea, hyperandrogenism and its associated clinical features (e.g., hirsutism, acne), and polycystic ovaries.18 PCOS is associated with an adverse cardiometabolic profile. Women with PCOS have a higher prevalence of elevated BMI, dyslipidemia, hypertension, and insulin resistance and deficiencies in insulin secretion19 compared with similarly-aged women without PCOS.18, 20 The most common lipid abnormalities noted in PCOS are elevated low-density lipoprotein cholesterol (LDL-C), elevated triglycerides, and decreased high-density lipoprotein cholesterol (HDL-C) levels.21 Among women with PCOS, insulin resistance is seen in approximately two-thirds,22 and 7.5-10% have established type 2 diabetes (T2D).23 Women with PCOS have a higher risk of developing T2D independent of BMI.24 In addition, a recent meta-analysis confirmed an association between PCOS and incident hypertension (pooled RR 1.70, 95% CI 1.43-2.07), although risk attributable to PCOS was greater among women of reproductive vs. post-menopausal age.25 PCOS has also been associated with higher levels of inflammatory markers such as C-reactive protein (CRP) as well as thrombotic markers such as homocysteine.26, 27
Women with PCOS have greater carotid intima-media thickness (CIMT) and coronary artery calcium (CAC), even after adjusting or matching for BMI vs. women without PCOS.28–30 The relationship between PCOS and clinical CVD is well established, but data are conflicting regarding whether excess cardiovascular risk conferred by PCOS is independent of other conventional risk factors. Some studies,31, 32 but not all,33, 34 have reported increased CVD risk among women with PCOS after accounting for adiposity. A recent meta-analysis found that PCOS was associated with a 30% higher risk of overall CVD (OR 1.30, 95% CI 1.09-1.56), including both coronary heart disease (CHD, OR 1.44, 95% CI 1.13-1.84) and stroke (OR 1.36, 95% CI 1.09-1.70).35 A recent Danish registry found 19% increased risk of incident CVD (adjusted HR 1.19, 95% CI 1.07-1.33) for women with PCOS, but this association was not seen in women after age 50 years.36 Women with PCOS are also approximately 3-fold more likely to develop APOs, including gestational diabetes mellitus (GDM) and preeclampsia, which in turn predict increased CVD risk.37
Contraception and CVD Risk
Multiple effective contraceptive options are available, including long-acting reversible contraception (LARC, levonorgestrel-releasing and copper intrauterine devices [IUDs] and subdermal implants), injectable depot medroxyprogesterone acetate, combined oral contraceptive pills (OCPs) that contain a combination of estrogen and progestins, progestin-only pills, the transdermal contraceptive patch (“the patch”), and the vaginal ring (NuvaRing) (Figure 2). Of these, LARCs are preferred given high efficacy and safety, especially in women with established CVD or high CVD risk, where avoidance of unplanned pregnancy is key.38, 39 As copper IUDs are associated with increased menstrual bleeding, hormonal IUDs may be preferred in women taking antiplatelet therapy or anticoagulation.39
Figure 2. Effectiveness and select cardiovascular considerations of commonly used contraceptive methods.
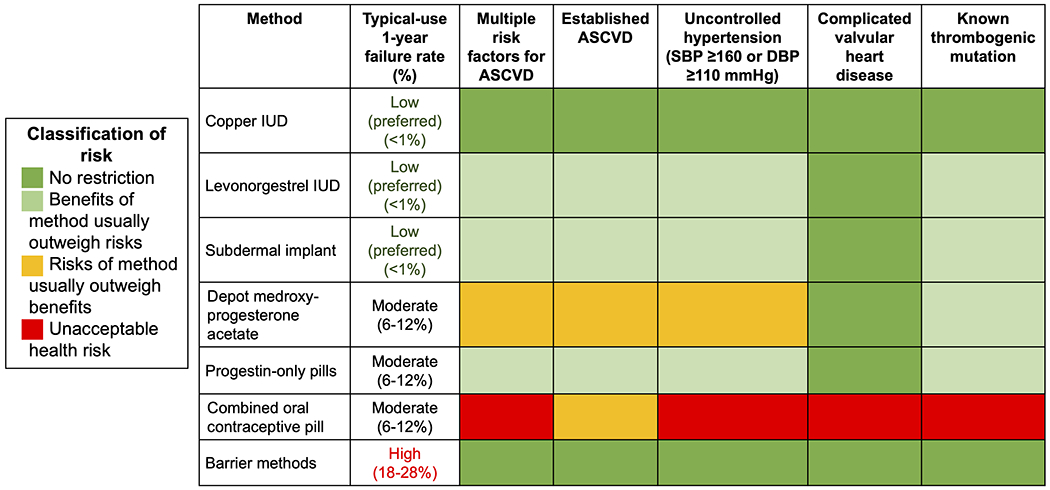
Contraceptive failures rates are from Lindley et al. (39). Complicated valvular heart disease refers to endocarditis and valvular lesions associated with atrial fibrillation or pulmonary hypertension. For additional cardiovascular considerations, refer to the U.S. Medical Eligibility Criteria for Contraceptive Use (38).
OCPs are the most prescribed contraceptive method in the U.S. and are used by approximately 25% of women of reproductive age. Safety concerns that arise from their use are largely related to estrogen content.38, 39 The metabolism of the synthetic estrogens can cause blood pressure to rise, and normotensive women taking OCPs can have up to a 7-8 mmHg increase in BP.40 Within the first year of use, risk of venous thromboembolism (VTE) is increased among OCP users (3-9/10,000 woman-years) compared with nonusers (1-5/10,000 woman-years).41 Similar to elevations in BP, this increased VTE risk is attributed to the synthetic estrogens within the pills. Progestins in OCPs have evolved over time to reduce, but not erase, this VTE risk. The risk of myocardial infarction (MI) or ischemic stroke also appears to be higher in women using OCPs, especially those formulations with higher levels (≥50 μg) of estrogen.42 The hormonal makeup of these pills has evolved, with newer generations of OCPs containing less synthetic ethinyl estradiol (≤30 μg) than older generation pills, reducing the CVD risk associated with newer pills; older data may therefore overestimate contemporary OCP-associated risks.
Cardiovascular risk factors should be considered when prescribing OCPs. Women with uncontrolled hypertension (BP ≥160/100 mmHg) should not use OCPs, and they are also not recommended in women with systolic BP 140-159 mmHg and diastolic BP 90-99 mmHg or in women over the age of 35 years with controlled hypertension. Smoking is a relative contraindication with OCPs given that concurrent use is associated with a 10-fold increased risk of MI and 3-fold increased risk of stroke.43 OCPs are not recommended in women at increased risk of VTE, which includes but is not limited to women in the early postpartum period, ≥35 years old who use tobacco, and those with thrombophilia.39 The U.S. Medical Eligibility Criteria for Contraceptive Use, in addition, cautions against the use of OCPs in other groups of women, including women with a history of ischemic heart disease, migraine with aura, or complicated valvular heart disease or certain congenital heart disease conditions.38 LARCs and progestin-only options are recommended for these patients.38, 39 Although barrier methods are safe from a cardiovascular perspective, use of barrier methods in isolation is discouraged given their poor efficacy. An update to the U.S. Medical Eligibility Criteria is expected in 2022.
Infertility, Fertility Treatment, and CVD Risk
High-quality data on the relationship between infertility (defined by the World Health Organization as inability to achieve pregnancy after ≥12 months of unprotected intercourse), fertility treatment, and cardiovascular risk are limited. Women with infertility, even prior to conception, have a higher prevalence of cardiovascular risk factors,44–46 suggesting that shared risk factors may underlie both infertility and CVD. Higher rates of diabetes and chronic hypertension45 and obesity46 are seen in both women with infertility and CVD as well as shared comorbidities, e.g., PCOS as discussed above.
Use of assisted reproductive technology (ART) itself is also associated with increased CVD risk, although causality is unclear. High-quality studies to clarify these relationships are challenging due partly to marked disparities in access to ART and variability in ART practice patterns. ART use is associated with significantly higher rates of APOs such as hypertensive disorders of pregnancy (HDP) and GDM,44 conditions which in turn are associated with increased future CVD. A systematic review of 47 studies showed that in-vitro fertilization in particular is associated with HDPs.47 Data regarding the longer-term associations of ART use with long-term cardiovascular health, however, are both limited and mixed.44, 48 One cohort study of >6,000 women found no increased risk after successful ART, suggesting ART itself may not be a causal risk factor.44 Further research is needed to clarify whether ART is a marker for subclinical risk at the time of fertility treatment or whether it contributes causally to CVD risk.
Pregnancy
Hypertensive Disorders of Pregnancy and CVD Risk
HDPs include pre-pregnancy chronic hypertension, gestational hypertension, preeclampsia/eclampsia, and preeclampsia superimposed on chronic hypertension.49 Preeclampsia is a pregnancy-specific hypertensive disorder and multisystemic syndrome of inflammation, oxidative and endoplasm reticulum stress, and angiogenic dysfunction.50 Early preeclampsia pathophysiology includes abnormal trophoblast invasion of the endometrium, which leads to incomplete spiral artery remodeling and, ultimately, placental ischemia, although researchers and clinicians suspect that the diverse presentations of preeclampsia likely reflect a spectrum of pathophysiology.51 Our understanding of preeclampsia subtypes, at present, is rather crude and based largely on timing of onset during pregnancy (early vs. late), presence of severe features, or association with intrauterine growth restriction (IUGR). Each of these features may have different long-term implications.
Association of HDPs with CVD:
HDPs are significant sex-specific risk factors for both short- and long-term maternal CVD. During the peripartum period, the odds of stroke,52 MI,52 cardiomyopathy52, 53 and spontaneous coronary artery dissection (SCAD)54 are significantly increased for women with vs. without a history of HDP. It is also well recognized that such CVD risk extends well into later life.55–57 Meta-analyses and subsequent large prospective cohort studies have established a two-fold risk of CVD among women with a history of gestational hypertension55, 57 or preeclampsia55–57; research suggests the risk is especially high following preeclampsia with severe features,57 onset prior to 34 weeks’ gestation,8 term preeclampsia with small-for-gestational age (SGA) infant, or recurrent HDP in >1 pregnancy.58
HDPs are associated with future development of chronic hypertension and diverse cardiovascular conditions (Table 1). Furthermore, women with a history of HDPs tend to have earlier-onset CVD, suggesting that HDPs are associated with accelerated cardiovascular aging.59, 60 Hypertension, diabetes and hyperlipidemia are diagnosed up to 10 years earlier and sub-clinical markers of vascular damage are significantly more prevalent among women with a history of HDP compared to women without.61–64 Earlier-onset valvular heart disease has also been demonstrated.62 Beyond the cardiovascular system, women with HDPs have been found to have >2-fold risk of death due to infectious, respiratory, and nervous system disease.65
Table 1.
Long-term cardiovascular risks associated with hypertensive disorders of pregnancy.
| Maternal Outcome | Study Design | Events | Effect estimate (95% CI) |
|---|---|---|---|
| Hypertension (≥140/90 mm Hg) | |||
|
| |||
| Hypertensive pregnancy disorder | Prospective cohort | 566 | aHR 2.3 (1.9-2.8)66 |
| Retrospective cohort | - | aHR 2.8 (2.5-3.1)67 | |
| Preeclampsia | Meta-analysis | - | OR 3.9 (3.1-5.0)68 |
| Meta-analysis | - | RR 3.1 (2.5-3.9)69 | |
| Meta-analysis | - | RR 3.7 (2.7-5.1)70 | |
| Prospective cohort | 4,259 | aHR 4.5 (4.3-4.6)56 | |
| Prospective cohort | 1,922 | aHR 2.2 (2.1-2.3)60 | |
| Gestational hypertension | Prospective cohort | 979 | aHR 2.8 (2.6-3.0)60 |
|
| |||
| Cardiovascular disease ‡ | |||
|
| |||
| Hypertensive pregnancy disorder | Prospective cohort | 25,606 | aHR 1.7 (1.6-1.8)56 |
| Prospective cohort | 145 | aHR 1.6 (1.3-1.9)55 | |
| Prospective cohort | 49 | aHR 2.3 (2.0-2.7)71 | |
| Gestational hypertension | Meta-analysis | - | OR 1.7 (1.3-2.2)57 |
| Prospective cohort | 54 | aHR 1.4 (1.1-1.9)55* | |
| Preeclampsia | Meta-analysis | - | OR 1.7 (2.5-3.0)57 |
| Prospective cohort | 861 | aHR 1.7 (1.6-1.8)56 | |
| Prospective cohort | 91 | aHR 1.7 (1.3-2.1)55 | |
| Preeclampsia with severe features | Meta-analysis | - | OR 2.7 (2.5-3.0)57 |
| Early onset preeclampsia† | Meta-analysis | - | OR 5.6 (1.5-21.4)68 |
| Retrospective cohort | - | aHR 4.9 (3.0-7.8)72 | |
|
| |||
| Coronary heart disease | |||
|
| |||
| Hypertensive pregnancy disorder | Prospective cohort | 1,954 | aHR 1.8 (1.3-2.6)62 |
| Retrospective cohort | 1,001 | aHR 2.2 (2.0-2.3)73, 74 | |
| Prospective cohort | 102 | aHR 1.7 (1.3-2.3)66 | |
| Prospective cohort | 54 | aHR 1.9 (1.4-2.5)55 | |
| Retrospective cohort | - | aHR 2.2 (2.0-3.8)67 | |
| Preeclampsia | Meta-analysis | - | RR 2.5 (1.4-4.4)75 |
| Prospective cohort | 664 | aHR 1.7 (1.5-1.8)56 | |
| Prospective cohort | 35 | aHR 2.1 (1.5-3.0)55 | |
|
| |||
| All stroke | |||
|
| |||
| Hypertensive pregnancy disorder | Retrospective cohort | 273 | aHR 1.9 (1.6-2.2)74 |
| Prospective cohort | 262 | aHR 1.8 (1.6-2.1)56 | |
| Prospective cohort | 130 | aHR 1.9 (1.3-2.6)66 | |
| Retrospective cohort | - | aHR 1.9 (1.4-2.7)67 | |
| Preeclampsia | Meta-analysis | - | RR 1.8 (1.3-2.6)75 |
| Prospective cohort | 93 | aHR 1.9 (1.5-2.4)56 | |
| Prospective cohort | 46 | aHR 1.5 (1.1-2.1)55 | |
|
| |||
| Ischemic hemorrhage | Prospective cohort | 123 | aHR 1.7 (1.4-2.1)56 |
|
| |||
| Intracerebral hemorrhage | Prospective cohort | 43 | aHR 1.7 (1.2-2.4)56 |
|
| |||
| Subarachnoid hemorrhage | Prospective cohort | 91 | aHR 2.0 (1.6-2.5)56 |
|
| |||
| Heart failure | |||
|
| |||
| Hypertensive pregnancy disorder | Prospective cohort | 1,300 | aHR 1.7 (1.0-2.6)62 |
| Retrospective cohort | 480 | aHR 4.2 (3.7-4.8)73 | |
| Prospective cohort | 145 | aHR 1.5 (1.3-1.9)56 | |
| Retrospective cohort | 66 | aHR 1.8 (1.4-2.4)76 | |
| Prospective cohort | 55 | aHR 2.7 (1.6-4.6)66 | |
| Preeclampsia | Meta-analysis | - | RR 4.2 (2.1-8.4)75 |
| Prospective cohort | 67 | aHR 2.1 (1.6-2.8)56 | |
| Retrospective cohort | 51 | aHR 2.0 (1.5-2.7)76 | |
| Prospective cohort | 13 | aHR 2.0 (1.1-3.7)55 | |
|
| |||
| Atrial Fibrillation | |||
|
| |||
| Hypertensive pregnancy disorder | Prospective cohort | 529 | aHR 1.4 (1.1-1.6)66 |
| Preeclampsia | Prospective cohort | 86 | aHR 1.7 (1.4-2.2)56 |
|
| |||
| Vascular dementia | |||
|
| |||
| Gestational hypertension | Retrospective cohort | 654 | aHR 3.0 (2.1-4.3)77 |
| Preeclampsia | Retrospective cohort | 654 | aHR 2.4 (1.8-3.2)77 |
| Prospective cohort | 14 | aHR 2.2 (1.2-4.0)78 | |
|
| |||
| Chronic kidney disease | |||
|
| |||
| Hypertensive pregnancy disorder | Prospective cohort | 1,279 | aHR 4.3 (3.8-4.8)71 |
| Gestational hypertension | Meta-analysis | - | RR 1.5 (1.1-2.0)79 |
| Preeclampsia | Meta-analysis | - | RR 2.3 (1.5-3.5)79 |
|
| |||
| End stage kidney disease | |||
|
| |||
| Gestational hypertension | Meta-analysis | - | RR 3.6 (2.3-5.7)79 |
| Preeclampsia | Meta-analysis | - | RR 6.6 (2.7-14.8)79 |
Chronic hypertension was included as a CVD endpoint in this study.
Early preeclampsia = <34 weeks’ gestation
Cardiovascular disease included ischemic or hypertensive heart disease, or stroke
Hypertensive disorder or pregnancy was a combined endpoint of chronic hypertension, gestational hypertension, preeclampsia, preeclampsia superimposed on chronic hypertension and eclampsia.
Different studies adjusted for different variables. Comparison groups were women who had normotensive pregnancies.
BMI = body mass index, SBP = systolic blood pressure, DBP = diastolic blood pressure, HR = hazard ratio, OR = odds ratio, RR = risk ratio, a = adjusted.
Mechanistic links to CVD:
HDPs and vascular disease share common mechanisms which may lead to both the development of preeclampsia and CVD during a woman’s life course (Figure 3). The development of HDP in pregnancy has been termed a “failed stress test” that identifies women at higher risk of CVD later in life. Alternatively, or in addition, preeclampsia may causally induce long-term metabolic and vascular abnormalities that increase overall risk for CVD later in life. The extent of vascular dysfunction during pregnancy likely depends on a woman’s genetic predisposition and interactions with pre-existing cardiovascular risk factors. Research demonstrates that the association between HDPs and future vascular disease persists but is attenuated when adjusted for pre-pregnancy CVD risk factors.56, 77 Causal mediation analyses further suggest that only approximately two-thirds of HDP-associated CVD risk is mediated via traditional CVD risk factors, chiefly development of chronic hypertension.55, 62 Pathways to specific CVD outcomes likely vary across different HDP phenotypes. It has been postulated that accelerated cellular senescence may represent one mechanism of accelerated cardiovascular aging in women with HDPs.80, 81
Figure 3. Putative mechanisms linking of hypertensive disorders of pregnancy to cardiovascular disease.
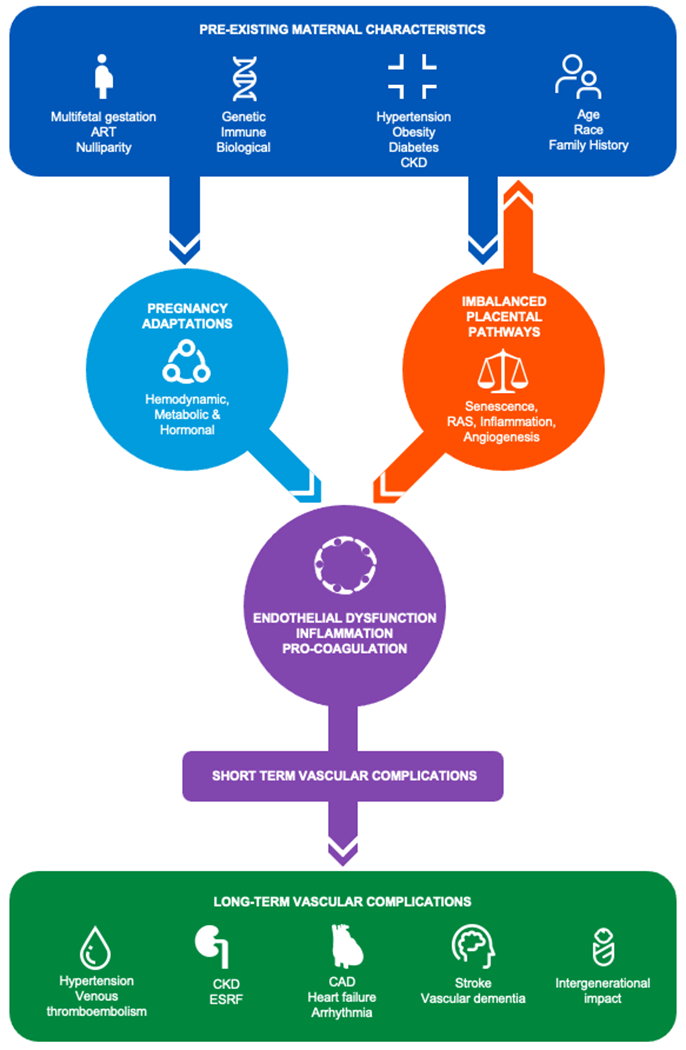
Hypertensive disorders of pregnancy are phenotypically heterogeneous, which likely reflects interactions between pre-existing maternal characteristics, genetics, and comorbidities (hypertension, obesity, diabetes, and chronic kidney disease), pregnancy-specific factors (nulliparity, multi-gestation and assisted reproduction), and an imbalance of placental biological pathways. Hypertensive disorders of pregnancy are linked to short-term vascular complications as well as earlier and increased risk of developing traditional cardiovascular disease risk factors and diverse cardiovascular conditions.
ART = assisted reproductive technology, CKD = chronic kidney disease, RAS = renin angiotensin system, ESRF = end stage renal failure, CAD = coronary artery disease
There appears to be a strong genetic contribution to preeclampsia risk. Women whose first degree relatives had preeclampsia have a 2-3.5-fold risk of developing preeclampsia.82, 83 Recent data demonstrate that women with HDPs have elevated polygenic risk of hypertension and obesity (Table 2), implying that HDPs signify latent genetic cardiometabolic risk.84–86 In addition, the fetal FLT1 gene, whose product (soluble fms-like tyrosine kinase receptor 1) is a causal preeclampsia biomarker, has been associated with development of maternal preeclampsia.86
Table 2.
Evidence for genetic associations of hypertensive disorders of pregnancy with chronic hypertension and obesity.
| Study | Sample | Main findings: Blood pressure | Main findings: Body mass index |
|---|---|---|---|
| Honigberg et al. 202085 | 2,772 cases with HDPs, 211,593 parous controls (UK Biobank) | ● OR for HDPs per SD of systolic BP PRS: 1.22 (95% CI 1.17-1.27) ○ Gestational hypertension: 1.24 (95% CI 1.13-1.35) ○ Preeclampsia: 1.19 (95% CI 1.08-1.31) ● OR for HDPs per SD of diastolic BP PRS: 1.22 (95% CI 1.17-1.26) ● rs7692387-A (GUCY1A3 intron variant) had disproportionately large protective effect against HDPs in comparison with BP effect |
● OR for HDPs per SD of BMI PRS: 1.06 (95% CI 1.02-1.10) ● BP and BMI PRS independent and additive when jointly modeled |
| Steinthorsdottir et al. 202086 | 5,181 cases with preeclampsia, 61,426 parous controls (3 European and 2 Central Asian cohorts) 1,532 cases with gestational hypertension, 50,943 parous controls (Icelandic cohort) |
● OR for preeclampsia per SD of hypertension PRS: 1.26 (95% CI 1.22-1.31) ● OR for gestational hypertension per SD of hypertension PRS: 1.48 (95% CI 1.04-1.57) |
● OR for preeclampsia per SD of BMI PRS: 1.07 (95% CI 1.02-1.13) ○ After further adjustment for hypertension PRS: 1.04 (95% CI 0.99-1.09) ● OR for gestational hypertension per SD of BMI PRS: 1.12 (95% CI 1.06-1.17) ○ After further adjustment for hypertension PRS: 1.06 (95% CI 1.01-1.12) |
| Gray et al. 202184 | 498 cases with preeclampsia, 1,864 parous controls (5 U.S. sites; all with European ancestry) | ● OR for preeclampsia per diastolic BP risk allele: 1.11 (95% CI 1.01-1.21) ○ Early-onset (<34 w) preeclampsia: 1.30 (95% CI 1.08-1.56) |
● OR for preeclampsia per BMI risk allele: 1.10 (95% CI 1.00-1.20) |
HDPs = hypertensive disorders of pregnancy; OR = odds ratio; SD = standard deviation; CI = confidence interval; BP = blood pressure; PRS = polygenic risk score; BMI = body mass index
Gestational Diabetes and CVD Risk
GDM results when pancreatic beta-cells incompletely respond to the physiologic and placental-mediated insulin resistance that characterizes pregnancy.87 Approximately one third of women who develop GDM will develop T2D in the future, corresponding to a 7-fold greater risk compared to women without GDM.88 International diabetes associations recommend glucose screening starting within the first 1-6 months postpartum and lifelong screening every one to three years in women who develop GDM.89–91 In women who develop GDM, subclinical risk factors (e.g., suboptimal glycemic control, dyslipidemia, CRP, adiponectin) may be evident prior to pregnancy and early in the first trimester.92
Women who develop GDM have a 2-fold risk of CAC at midlife independent of progression to prediabetes or T2D93 and 1.5- to 2-fold risk of cardiovascular events94; CVD event risk appears greater if there is progression to T2D.94,82 Similar to the relationship between impaired gestational glucose tolerance and risk of subsequent T2D, there appears to be a dose-dependent relationship between degree of glucose impairment during pregnancy and risk of subsequent CVD.95 These relationships have been consistently demonstrated across databases, countries, and ethnic groups.95
Preterm Delivery and CVD Risk
The association between preterm delivery (PTD) and future maternal CVD risk is well established.96, 97 The underlying mechanisms, however, remain unclear. PTD is broadly defined as a live birth prior to 37 weeks gestation and further delineated based on gestational age (late preterm [34-36 weeks], moderately preterm [32-36 weeks], and very preterm [<32 weeks]). In the U.S., Black women experience the highest rate of PTD at 29% higher than the national average (Figure 4) and have nearly 3-fold risk of delivering a very preterm baby (3.1% vs 1.2% in White women).98
Figure 4. Rate of very preterm delivery (<32 weeks gestational), U.S. average between 2017-2019.
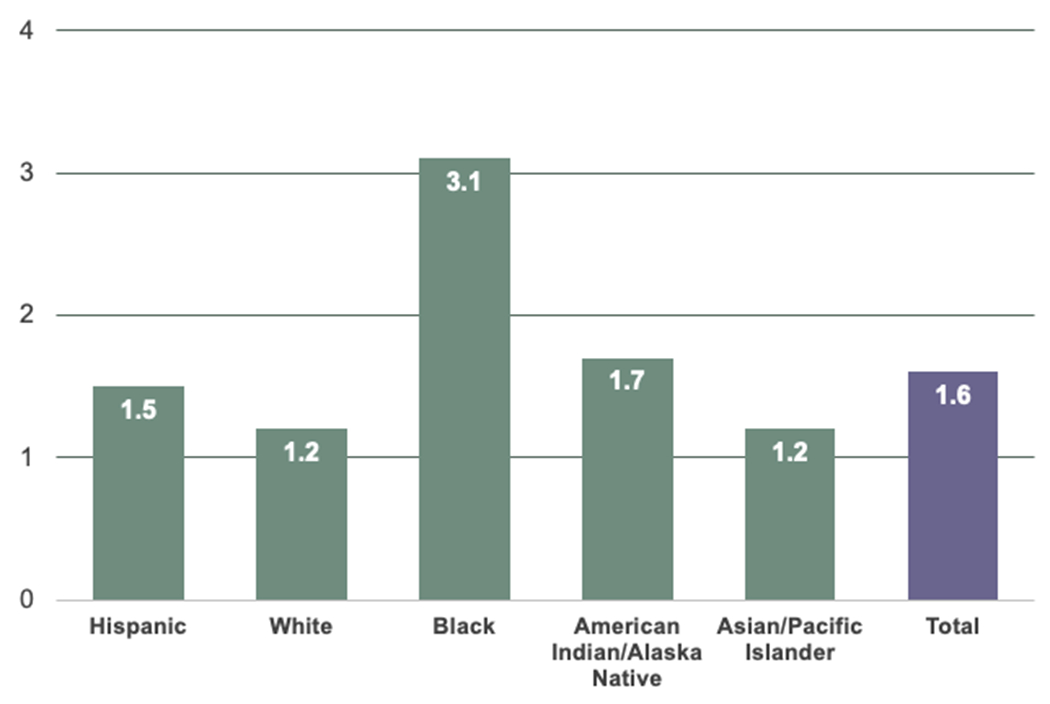
In the U.S., Black women experience the highest rate of preterm delivery at 29% higher than the national average.
All race categories exclude Hispanics. Very preterm is less than 32 weeks’ gestation.
Data source: National Center for Health Statistics, final natality data.
Whether PTD occurs spontaneously or due to medical necessity influences its relationship with future CVD risk.99 Compared with spontaneous term delivery, medically indicated PTD is associated with a higher risk of cardiovascular mortality (HR 3.7) than spontaneous PTD (HR 1.7).100 Women who experience medically indicated moderate-to-very-preterm delivery due to HDPs appear to be the highest risk group. Preeclampsia, however, only explains ~25% of the association between PTD and CVD.101 Although spontaneous and medically indicated PTD share many ASCVD risk factors, the most notable difference is in vascular function. The augmentation index, which indirectly measures smooth muscle vascular function, appears to be reduced in women with spontaneous PTD vs. medically indicated PTD. These findings have been reproduced in both the early antepartum as well as the early postpartum phase.102, 103
Infant Birth Weight, Fetal Growth, and CVD
Infant birth weight has been independently associated with maternal CVD risk. In the Women’s Health Initiative (WHI), delivery of a low-birth-weight infant was independently associated with increased maternal ASCVD risk after adjustment for conventional risk factors and other APOs (adjusted OR 1.25).104 Infant birth weight has also been shown to predict maternal life span.105
Many studies have examined the association SGA and IUGR, i.e., SGA associated with pathologic growth restriction, with CVD.106 Fetal growth has been correlated with subclinical markers of CVD including arterial stiffness and endothelial dysfunction.107, 108 Bonamy et al. observed a multiplicative interaction between gestational age and birth weight, with a 3-fold maternal CVD risk in women with preterm and SGA infants even after accounting for socioeconomic factors, smoking, and pregnancy-related complications.109 Growth restriction at term is also associated with increased risk of maternal CVD,110 and, to a lesser extent, with paternal CVD.105 This suggests that environmental and behavioral risk factors may influence both parents and infants.
Most cases of IUGR are thought to result from uteroplacental insufficiency due to poor implantation of the spiral arteries and subsequent increased placental vascular resistance, which has similarities to the early pathophysiology of preeclampsia. Preexisting hypertension and increasing maternal age have also been associated with growth restriction and may result from endothelial dysfunction that affects placental implantation. Low levels of insulin-like growth factor 1 are found in pregnancies complicated by IUGR and are also associated with increased risk of CVD suggesting a potential shared mechanism.111 Further research is needed to understand the complex interplay between environmental, behavioral and maternal vascular health and the association between birth weight, fetal growth, and CVD risk.
Other Adverse Pregnancy Outcomes and CVD Risk
Placental abruption has been associated with cardiovascular risk factors and CVD.112, 113 In a retrospective Dutch study, abruption was strongly associated with other concomitant APOs as well as with higher BMI, total cholesterol, and fasting blood glucose in women with isolated abruption without other APOs.112 A large retrospective cohort study similarly found a 1.7-fold risk of CVD (aHR 1.7, 95% CI 1.3-2.2) in women with placental abruption or infarction.113 Pregnancies with large-for-gestational age fetuses are also associated with increased CVD risk, likely mediated by associations with elevated BMI and diabetes.114, 115
Though there are numerous shared risk factors between miscarriage and CVD, including obesity and cigarette smoking, data show that miscarriage is independently associated with future CHD and MI after adjustment for conventional risk factors.116, 117 Greater number of miscarriages appears to associate with progressively higher CVD risk,117, 118 with one cohort study observing a >3-fold CVD risk associated with ≥3 miscarriages (HR 3.18, 95% CI 1.76-5.78).118 Mechanisms linking miscarriage to CVD remain unclear, although shared vascular, genetic, immunologic factors have been proposed.119
Breastfeeding and CVD Risk Reduction
Maternal breastfeeding is associated with a lower risk of later-life cardiometabolic and CVD independent of socioeconomic and lifestyle factors. A recent meta-analysis indicated that achieving ≥1 year of cumulative lactation was associated with 13% lower risk of chronic hypertension,120 with a dose-responsive relationship between duration of lactation and degree of risk reduction.121 An even stronger dose-dependent protective association has been observed between lactation and incident T2D.120, 122 Notably, associations were not explained by postpartum weight differences by breastfeeding status.121, 122
Similar associations have been observed for subclinical atherosclerosis and clinical CVD events.35 In the Study of Women’s Health Across the Nation (SWAN) Heart Study, women who did not breastfeed had elevated risks of aortic calcification (aOR 3.85, 95% CI 1.47-10.00) and CAC (aOR 2.78, 95% CI 1.05-7.14) compared to women who breastfed each child for ≥3 months, even after adjustment for demographic and lifestyle factors.123 In the Nurses’ Health Study, women with ≥2 years of cumulative breastfeeding vs. no breastfeeding had 23% (95% CI 6-38%) lower adjusted risk of CHD events.124 A recent analysis observed similar findings for cardiovascular hospitalization and mortality.125
A smaller number of studies have examined lactation and cardiometabolic risk factors specifically in women with APOs with mixed findings. In a study of 622 women with various APOs seen 6 months postpartum at a dedicated postpartum transitional clinic in Ontario, Canada, increased lactation duration was associated with higher HDL-C and lower fasting glucose, triglycerides, BMI, and risk of metabolic syndrome after adjustment for demographic factors and pre-pregnancy confounders.126 Finally, in CARDIA, longer duration of lactation was associated with lower risk of incident metabolic syndrome, with greater protection observed in women with vs. without GDM.127
Gestation is associated with accumulation of maternal visceral fat and insulin resistance.128 Given the observed associations of lactation with cardiometabolic traits human and animal studies, lactation has been hypothesized to reset maternal metabolism postpartum. Specific protective mechanisms, however, are incompletely understood. A recent analysis found that lactation duration was inversely associated with computed tomography imaging-derived measurements of pericardial adipose tissue and visceral adipose tissue volumes assessed at age 50, and that longitudinal weight changes mediated only ~20% of this relationship.129 Mouse studies suggest lactation-related hormones may preserve pancreatic beta cell function.130
Parity and CVD Risk
Most large cohort studies suggest a J-shaped relationship between parity and CVD, in which the highest risk is among nulliparous and grand multiparous (≥5 births) women.131 The Atherosclerosis Risk in Communities Study found that a history of ≥5 live births was associated with CHD risk and hospitalization for MI, independent of breastfeeding history.132 These studies also found that having a prior pregnancy and no live birth was associated with greater CHD and heart failure risk. Parikh et al., in the largest study to date of 1.3 million Swedish women, observed a J-shaped association between parity and CVD with a nadir of CVD risk in women with 2 births, HR 1.09 (95% CI 1.03-1.15) for women with 1 birth, and HR 1.47 (95% CI 1.37-1.57) in women with ≥5 births.133
Parity may be a proxy for socioeconomic and behavioral factors.134 In the British Women’s Heart and Health Study and British Regional Heart Study, a J-shaped association has been observed between number of children and CHD in both sexes, with the lowest prevalence among those with 2 children, suggesting a role of social and lifestyle factors. For those with ≥2 children, each additional child increased the age-adjusted odds of CHD by 30% in women vs. 12% for men, however, suggesting the possibility of additional biological effects in women.
Several putative biological mechanisms have also been proposed to explain this relationship. Repeated pregnancies may result in multiple exposures to the metabolic changes of pregnancy, including insulin resistance, impaired lipid metabolism, weight gain, inflammation, and oxidative stress that result in enduring vascular changes.135 Further research is needed to fully understand the biological and/or social mechanisms responsible for these associations.
Menopause
The Menopausal Transition and CVD Risk
Menopause is a critical reproductive aging event signifying the end of fertility. The menopause transition (MT) is a complex phase marked by dynamic changes in hormonal and menstrual bleeding patterns as well as multiple physiological and psychological symptoms.136 A recent American Heart Association scientific statement identifies the MT as a uniquely impactful period of time associated with acceleration in CVD risk.137 Longitudinal studies following women as they transition through menopause have contributed substantially to our understanding of how the MT is related to increased CVD risk in women.138
Lipids/lipoproteins:
During the MT, women experience adverse changes in several lipids/lipoproteins measures that have been linked to a greater risk of CVD thereafter. Increases in total cholesterol, LDL-C, and apolipoprotein B levels occur from 1 year before to 1 year after menopause, independent of chronologic aging.139 Menopause-related increases in LDL-C are independently associated with greater risk of carotid plaque in subsequent years.140 The association between the MT and HDL-C is complex.141 Recent longitudinal evaluation of multiple metrics of HDL142 demonstrates significant increases in HDL-C over the MT accompanied by changes in other HDL metrics (e.g., efflux capacity) consistent with a more atherogenic profile.143, 144 Associations of HDL subclasses with early markers of atherosclerosis vary by menopause stage, with higher small HDL-P, lower large HDL-P, and smaller HDL size associated with greater risk of CAC or density in late peri-menopause than in the pre/early peri-menopause stage.145 Notably, higher estradiol level during the 2 years post-menopause is associated with greater triglyceride content of HDL,145 a feature that has been linked to greater CVD risk.146 Thus, changes in HDL quality during the MT are affected by estradiol level and the timing relative to menopause. This could have clinical implications for timing of the initiation of menopausal hormone therapy (HT) and calls for a re-evaluation of the inclusion of HDL-C in risk prediction147 for midlife women in future studies.
Weight gain and body composition:
Women at midlife experience increases in their body weight, apparently unrelated to the MT itself.148 However, the MT influences where women store body fat. Most recently, using data from SWAN, a nonlinear increase in abdominal visceral adipose tissue was reported with acceleration starting at 2 years before menopause. This acceleration was independent of age, lifestyle factors and overall adiposity. Importantly, the study also showed that the reported menopause-related acceleration in visceral abdominal fat was related to a significant increase in internal CIMT independent of traditional risk factors and overall adiposity.149 During this period women also accumulate cardiovascular fat150 which has been associated with increased CVD risk.151,152
Vascular health measures:
The MT is associated with adverse vascular remodeling (changes in CIMT and adventitial diameter).138, 153 The MT is also linked to functional metrics of the vasculature.154–156 Endothelial function may begin to decline during perimenopause.154 Moreover, arterial stiffness, measured by carotid femoral pulse wave velocity, accelerates within 1 year of menopause independent of other risk factors.156 Whether changes in vascular health metrics during the MT predict later CVD is currently unknown.
Premature/Early Age of Menopause and CVD Risk
Women experience menopause at a median age of 50.0 years (interquartile range: 48.0-53.0 years).157 However, 7.3% of women experience menopause between the ages of 40-45 (early menopause) and 1.9% before the age of 40 (premature menopause).157 Black women experience early menopause more frequently than White or Hispanic women.158 In addition to reproductive aging, age at menopause is increasingly recognized as a marker of somatic aging and general health.159 Earlier age of menopause is consistently associated with greater risks of CHD, heart failure, CVD mortality, and/or all-cause mortality.160–162 Among 144,260 postmenopausal women (40-69 years old at enrollment in 2006-2010) from the UK Biobank followed through 2016, compared with women who experienced menopause after age 40, women with premature natural menopause (<40 years) had a 1.36-fold increase in risk (95% CI 1.19-1.56) for a broad CVD composite outcome independent of traditional risk factors and use of menopausal HT.160 Similarly, meta-analysis of 32 observational studies including 310,329 women showed a higher risk of overall (RR 1.50, 95% CI: 1.28-1.76) and fatal (RR 1.11, 95% CI: 1.03-1.20) CHD associated with age of menopause onset <45 vs. ≥45 years after adjustment for conventional CVD risk factors.163
The association of premature menopause and CVD risk may be bidirectional. Data from the Framingham Heart Study showed that worse cardiovascular risk factor profile before menopause is associated with earlier menopause, independent of factors known to impact menopause timing.164 Additionally, a first CVD event before age 35 is associated with 2-fold risk of early menopause.165
Exact mechanisms linking age of menopause with CVD risk are unclear. Recent research analyzing data from 11,495 postmenopausal women (aged 40-70) from the UK Biobank with whole exome sequences and 8,111 (aged 50-79) from the WHI with whole genome sequences assessed clonal hematopoiesis of indeterminate potential (CHIP), the age-related expansion of hematopoietic cells with leukemogenic mutation without detectable malignancy, as a potential mechanistic link between premature/early menopause and CVD.166 CHIP has been linked to accelerated atherosclerosis.167 Adjusting for potential confounders, premature menopause was independently associated with CHIP; this association was only evident in natural premature menopause but not in surgical premature menopause, supporting the notion that CHIP may predate premature menopause or that both conditions may share upstream risk factors. These findings suggest that history of premature menopause may help identify individuals for CHIP screening and surveillance to guide targeted CVD prevention.
Spontaneous vs. Surgical Menopause and CVD Risk
Menopause can occur as spontaneously or as the result of medications (e.g., chemotherapy) or surgery. Both the mechanism and timing of menopause affect the cardiovascular system differently. Surgical menopause is defined as bilateral salpingo-oophorectomy (BSO), which results in the abrupt loss of endogenous ovarian hormones. Large observational studies suggest that BSO prior to the age of natural menopause is associated with an increased CVD risk and mortality.168 In the abovementioned UK Biobank study, surgical premature menopause was associated with an 87% increased risk of composite CVD (HR 1.87, 95% CI 1.36-2.58) while natural premature menopause was associated with a 36% increased risk (HR 1.36, 95% CI 1.19-1.59); for CHD specifically, HRs were 2.52 (95% CI 1.48-4.29) and 1.39 (95% CI 1.06-1.82) for surgical and natural premature menopause, respectively.160 Observational data from both the Nurses’ Health Study and the Danish Nurses Cohort Study found that HT use was associated with attenuated CVD risk after surgical premature menopause.169, 170
Vasomotor Symptoms and CVD Risk
More than 70% of women report vasomotor symptoms (VMS) at some point during midlife. VMS, including hot flashes and night sweats, are the cardinal symptoms of the MT, though are not isolated to this transition period.171, 172 There appears to be a dose-dependent relationship between frequency of VMS and CVD risk factors. Women with VMS have a worse overall cardiovascular risk profile.173 Longitudinal data from the SWAN showed correlation between VMS presence and LDL-C, apolipoprotein B, triglycerides, HDL-C and apolipoprotein A-1 levels, adjusting for CVD risk factors.174 VMS are also linked to future hypertension,175 insulin resistance,174 and diabetes, independent of obesity.176
VMS are correlated with worse metrics of subclinical CVD including increased CIMT, endothelial dysfunction, and arterial calcification. Women with vs. without hot flashes have reduced flow-mediated dilation and greater aortic calcification, independent of CVD risk factors and estradiol.177 Women who experience hot flashes also have higher CIMT than those without.178 This relationship appears to be influenced by timing of symptoms, with women who experience VMS earlier in the MT having higher mean and maximal CIMT than those with consistently low frequency of VMS across the MT.179
VMS are associated with the development of clinical CVD events. A large meta-analysis reported a 28% increased risk of CVD after adjusting for traditional risk factors.163 In SWAN, frequent and persistent VMS were associated with increased risk of later CVD events.180 A prospective cohort study of 11,725 women (aged 45-50 at baseline) followed for 14 years reported 2-fold increased odds of CHD in women with frequent VMS relative to women without symptoms.176 Other studies show that frequency of VMS is not the only marker of CVD risk. One study showed a stronger association between night sweats and CHD development than with hot flashes.181 Severity of VMS is also associated with increased risk of CVD.182
Exogenous Hormone Therapy and CVD Risk
Prior to randomized controlled trials (RCTs), observational and animal models consistently reported a 40-50% reduction in CVD in women receiving HT compared to nonusers.183, 184 The belief that HT prevents CVD dramatically changed, however, after unexpected results emerged from 2 large double-blind RCTs. The WHI (primary prevention trial) and the Heart and Estrogen/progestin Replacement Study (HERS, secondary prevention trial) of menopausal HT both concluded that there was no benefit of HT on CVD risk.185, 186 Furthermore, the WHI study found increased CVD events in older women randomized to HT, while the HERS trial found increased CVD events during the first year of HT use compared to placebo.
Subsequent analyses of the WHI study identified that those receiving HT in the age group 50-59 years or within 10 years of entering menopause did not have excess CVD risk. A 2015 Cochrane review of RCT data also found less CHD in women who started HT less than 10 years after menopause (RR 0.52, 95% CI 0.29-0.96).187 In a WHI substudy, women who were within 5 years of their final menstrual period also had significantly lower mean CAC when randomized to HT vs placebo after 7 years of follow up.188 The “timing hypothesis” suggests there is a critical window in which the risks versus benefits of HT and CVD change, whereby women who start HT closer to the age of menopause may have a neutral or possibly reduction in risk of CVD, while women further from menopause (>10 years) and starting HT may have an increased risk of CVD.189
Two contemporary RCTs evaluated CIMT and CAC in healthy women who had recently entered menopause to study the timing hypothesis. The Kronos Early Estrogen Prevention Study randomized women within 3-years of their final menstrual period to oral conjugated estrogen, transdermal estradiol patch, or placebo, with cyclic oral progesterone. After 4 years, there was no difference in CIMT between HT versus placebo groups.190 The Early versus Late Intervention Trial with Estradiol compared oral estradiol with vaginal progesterone gel vs. placebo in women within six years (early cohort, median age 55.4 years) or >10 years since menopause (late cohort, median age 63.6 years). After 5 years, the early cohort of women randomized to oral estradiol had lower progression of subclinical atherosclerosis as measured by CIMT (p=0.008).191 These results showed that using HT at the time of menopause did not increase subclinical atherosclerosis progression. While no direct comparison RCTs have examined the appropriate estrogen dose or method of delivery in HT, studies suggest that transdermal estradiols pose lower VTE and stroke risk than oral estrogens.192, 193 Current HT position statements recommend starting HT in women with premature menopause and continuing through the average age of natural menopause.194
While the risks and benefits of exogenous HT on cardiovascular health have been extensively studied over the past 4 decades, current consensus is that HT is appropriate for treatment of VMS in women who are otherwise healthy, within 10 years of menopause, and under age 60 years. However, the decision to pursue HT should still take into consideration a woman’s individual CVD risk through a shared decision-making model (Figure 5). Future studies should focus on HT dose, formulation, route of delivery, and duration of use with respect to CVD risk.
Figure 5. Contemporary approach to prescribing hormone therapy and hormone therapy risk assessment.
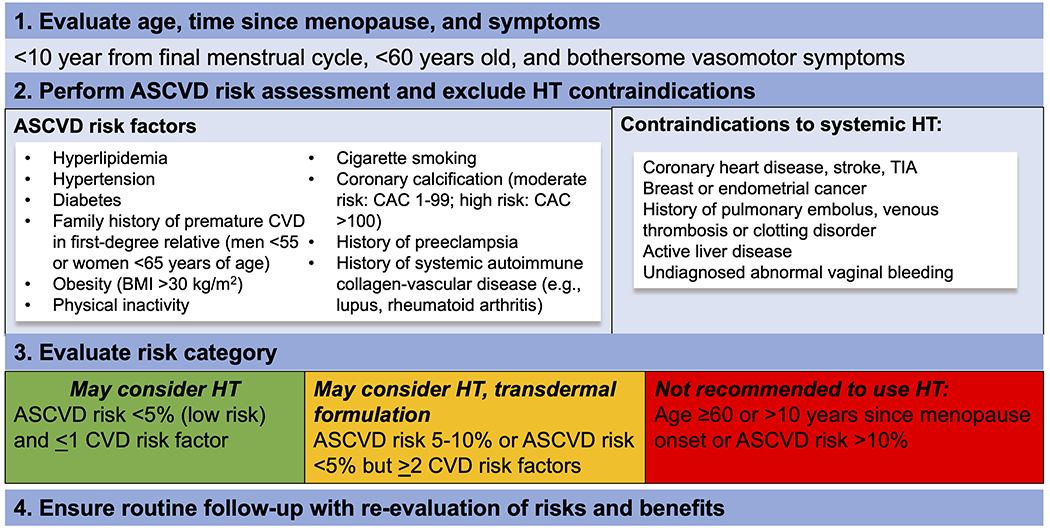
Hormone therapy is appropriate for treatment of vasomotor symptoms in women who are otherwise healthy at the time of menopause, within 10 years of menopause, and under age 60 years. However, the decision to prescribe hormone therapy should still consider a woman’s individual cardiovascular disease risk factors and employ a shared decision-making approach.
Incorporating Reproductive History in Clinical Practice
Sex-specific risk factors represent an important component of comprehensive CVD risk assessment in women. Women with PCOS, for instance, should be screened for glucose intolerance, dyslipidemia, and hypertension.195, 196 As noted above, a history of premature menopause and APOs are now considered risk-enhancing factors to refine ASCVD risk assessment and guide statin prescription among women aged 40-75 years with borderline (5-<7.5%) or intermediate (7.5-<20%) calculated 10-year ASCVD risk using conventional risk calculators, e.g., the Pooled Cohort Equations.4,5 Consensus statements recommend close monitoring for CVD risk factors in women during the first year postpartum following APOs.197 Though guidelines recommend aspirin use for the prevention of preeclampsia in persons at risk,198 data are unclear regarding the long-term benefit of aspirin use for cardiovascular prevention in women with a history of HDP.198 A recent AHA statement on menopause and CVD recommends an aggressive prevention-based approach for women during the menopause transition to decrease the probability of a future CVD event.137 For women whose CVD risk may be uncertain (e.g., borderline/intermediate calculated 10-year CVD risk), monitoring for subclinical development of CVD including CAC measurement can be considered to identify those who might benefit from preventive pharmacotherapies.199, 200 Overall, a history of pregnancy-associated and other reproductive risk factors for CVD represent an opportunity for primordial and primary prevention.
Appropriate risk assessment and prevention of CVD in women remains a challenge.201, 202 One study found that only 42% cardiologists felt “extremely well prepared” to assess cardiovascular risk in their female patients, and only 22% reported using guideline-directed prevention guidelines in treating female patients.203 This is an especially important issue in the U.S. given the considerable burden of CVD risk factors among women, with pronounced racial/ethnic disparities.1, 204, 205
Additional work is needed to better educate women about their risk of CVD such that they can become more active in their own care. Recent data show that only 45% of U.S. women recognize that CVD is the leading cause of death among women,203 and fewer than half of Canadian women recognized common symptoms of and risk factors for CVD.206 Minority women in particular continue to significantly underrecognize their risk of CVD.203 Awareness of CVD risk factors and symptoms has been shown to improve patient engagement with healthful behaviors and medication adherence.207, 208
With many elements of cardiovascular health to be discussed in a time-limited clinical encounter, efficiently incorporating reproductive history in clinical practice can be challenging. Formal clinical tools have been developed to help with this integration. A toolkit recommended by the American College of Obstetrics and Gynecology provides an algorithm to help in screening and identifying women who may be at increased CVD risk based on pre-pregnancy or pregnancy history.7, 209 Enhanced CVD risk factor screening can also be facilitated by standardized pre-visit patient questionnaires and patient note templates to prompt reflection on and discussion of risk factors.202 Written educational materials about cardiovascular health may efficiently supplement clinical encounters.
Postpartum transitional clinics for women with APOs have emerged as a promising strategy for identifying women at elevated risk for CVD and facilitating the transition to longitudinal primary care.210, 211 Many structural barriers exist to universal access to such clinics, however, including inconsistent access to healthcare insurance particularly among high-risk women, lack of social support for maternity leave, and logistical challenges to accessing medical appointments with a young infant. In addition, bundled payments for obstetrical care represent a potential financial disincentive for systems to introduce transitional clinics. Many patients are now turning to wearable consumer medical technologies, such as mobile phone applications (“apps”) and remote BP monitoring, to become more actively engaged in their own care; these results can be integrated into clinic visits to help facilitate patient-centered lifestyle recommendations.202, 212, 213 Reliance on wearables, however, risks exacerbating existing racial and socioeconomic disparities among those without access to these new technologies. Finally, all fellows-in-training should receive education regarding the importance of reproductive history-taking as part of their core educational curriculum,214, 215 and women’s cardiovascular health topics should be featured more prominently at professional society conferences such that all providers may feel better informed about the unique aspects of caring for women with CVD.
Unanswered Questions and Future Directions
Despite considerable advances in our understanding of sex-specific risk factors for CVD, many unanswered questions remain (Table 3). Ongoing research is needed to elucidate the pathophysiology of sex-specific CVD risk factors and their mechanistic links to later-life CVD to inform precision prevention strategies. Whether prevention strategies tailored to specific subsets of women can alter long-term cardiometabolic risk trajectories will be a critically important area of future clinical investigation. Including more women, and in particular women of child-bearing age who may be either pregnant or breastfeeding, as well as women transitioning through menopause,137 in clinical trials remains an important component of efforts to address these knowledge gaps, as does shifting the priorities of trial leaders, pharmaceutical companies, and regulatory bodies to recognize the value of this inclusion. Capturing reproductive history in clinical trials is critical to generate evidence about the effects of therapies on specific subpopulations of women. To date, incorporation of sex-specific risk factors to refine CVD risk assessment has not demonstrated clinically meaningful risk reclassification over the intermediate term in relatively low-risk populations.216, 217 However, ongoing efforts to develop sex-specific risk calculators over longer time horizons (e.g. 30-year or lifetime risk) and in diverse populations remain important, as traditional risk models underestimate CVD risk in women with reproductive risk factors.216 Increased clinician and patient awareness of sex-specific CVD risk factors and implementation of evidence-based prevention strategies are critical to improving the cardiovascular health of women.
Table 3.
Unanswered questions and key areas for future research on sex-specific cardiovascular disease risk factors in women.
|
Menarche and fertility ● Do unique hormonal or epigenetic mechanisms link early and late menarche and polycystic ovary syndrome with future CVD risk? ● Are assisted reproductive technologies causally linked to CVD or simply a signal for underlying infertility and associated shared risk factors with CVD? |
|
Pregnancy and adverse pregnancy outcomes ● What are the shared and unique pathophysiologic pathways underlying the various adverse pregnancy outcomes? How do long-term cardiometabolic implications differ across different adverse pregnancy outcomes and their subtypes? ● What are the key novel mechanisms and pathways linking adverse pregnancy outcomes with later-life CVD risk? ● Are associations between parity and CVD risk driven by socio-behavioral or biological effects (or both)? ● What is the protective mechanism of breastfeeding against CVD, and can this be leveraged therapeutically? |
|
Menopause ● Do cardiometabolic changes during the menopause transition independently predict future CVD? ● What are the mechanisms linking premature age of menopause and vasomotor symptoms with CVD? ● What are the relationships among clonal hematopoiesis of indeterminate potential, premature menopause, and CVD? ● Is there a role for exogenous estradiol in cardiometabolic and lipid profile improvement in subsets of postmenopausal women? |
|
Overarching questions for future research ● Can reproductive history prompt targeted cardiometabolic risk-reduction approaches for primordial and primary prevention? ● Can mechanistic insights into reproductive exposures yield novel preventive strategies and therapeutics? ● How do associations between reproductive history and CVD differ across racial/ethnic groups? ● Can sex-specific cardiovascular risk prediction models effectively incorporate reproductive history to refine risk prediction? ● How can we better educate clinicians about the role of reproductive history in CVD risk? |
CVD = cardiovascular disease
Funding:
Dr. Michos is supported by the Amato Fund for Women’s Cardiovascular Health research at Johns Hopkins University. Dr. Shufelt is supported by the U.S. National Institute for Child Health and Human Development (R01HD106096-01). Dr. Minissian is supported by the National Institutes of Nursing Research (1K99NR018679-01, F31NR015725) and CTSI support UL1TR000124 and UL1TR001881-01 and philanthropic support by Beta Chi Chapter. Dr. Quesada is supported by the U.S. National Heart, Lung, and Blood Institute (K23HL151867). Dr. Garovic is supported by the NHLBI (R01HL136348). Dr. El Khoudary is supported by the U.S. National Institute on Aging (R01AG058690) and the NHLBI (R01HL143010).
Abbreviations and Acronyms
- APOs
Adverse pregnancy outcomes
- ART
Assisted reproductive technology
- ASCVD
Atherosclerotic cardiovascular disease
- BSO
Bilateral salpingo-oophorectomy
- BP
Blood pressure
- BMI
Body mass index
- CRP
C-reactive protein
- CVD
Cardiovascular disease
- CIMT
Carotid intima-media thickness
- CHIP
Clonal hematopoiesis of indeterminate potential
- CAC
Coronary artery calcium
- CHD
Coronary heart disease
- GDM
Gestational diabetes mellitus
- HDL-C
High-density lipoprotein cholesterol
- HT
Hormone therapy
- HDPs
Hypertensive disorders of pregnancy
- IUD
Intrauterine device
- IUGR
Intrauterine growth restriction
- LDL-C
Low-density lipoprotein cholesterol
- MT
Menopause transition
- MI
Myocardial infarction
- OCPs
Oral contraceptive pills
- PCOS
Polycystic ovary syndrome
- PTD
Preterm delivery
- SGA
Small-for-gestational age
- SCAD
Spontaneous coronary artery dissection
- SWAN
Study of Women Across the Nation
- T2D
Type 2 diabetes
- VMS
Vasomotor symptoms
- VTE
Venous thromboembolism
- WHI
Women’s Health Initiative
Footnotes
Disclosures: Dr. Michos reports advisory boards for Novo Nordisk, Novartis, Amarin, Astra Zeneca, Bayer, and Esperion. Dr. Shufelt is President and a board member of the North American Menopause Society (NAMS). Dr. Minissian has served as a Consultant for Amgen and has received honoraria from the following organizations: 2021 National Lipid Association; North American Menopause Society, MJH Life Sciences, LLC, 2018-2019 Amgen, NACCME, LLC Co-Chair for CME; 2018-2019 Vox Media; 5/2019 Medtelligence; Minneapolis Heart, Primed, Good Samaritan Hospital, Cardiometabolic Health Congress, American Heart Association, Preventive Cardiovascular Nurses Association, American College of Cardiology. Dr. El Khoudary is a board member of the NAMS. The other authors report no disclosures.
References
- 1.Virani SS, Alonso A, Aparicio HJ, Benjamin EJ, Bittencourt MS, Callaway CW, Carson AP, Chamberlain AM, Cheng S, Delling FN, et al. Heart disease and stroke statistics-2021 update: A report from the american heart association. Circulation. 2021;143:e254–e743 [DOI] [PubMed] [Google Scholar]
- 2.Garcia M, Mulvagh SL, Merz CN, Buring JE, Manson JE. Cardiovascular disease in women: Clinical perspectives. Circ Res. 2016;118:1273–1293 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.O’Kelly AC, Honigberg MC. Sex differences in cardiovascular disease and unique pregnancy-associated risk factors in women. Current Treatment Options Cardiovascular Medicine. 2020;22 [Google Scholar]
- 4.Grundy SM, Stone NJ, Bailey AL, Beam C, Birtcher KK, Blumenthal RS, Bruan LT, de Ferranti S, Faiella-Tommasino J, Forman DE, et al. 2018 aha/acc/aacvpr/aapa/abc/acpm/ada/ags/apha/aspc/nla/pcna guideline on the management of blood cholesterol: A report of the american college of cardiology/american heart association task force on clinical practice guidelines. Circulation. 2019;139:e1082–e1143 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Arnett DK, Blumenthal RS, Albert MA, Buroker AB, Goldberger ZD, Hahn EJ, Himmelfarb CD, Khera A, Lloyd-Jones D, McEvoy JW, et al. 2019 acc/aha guideline on the primary prevention of cardiovascular disease: A report of the american college of cardiology/american heart association task force on clinical practice guidelines. Circulation. 2019;140:e596–e646 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Regitz-Zagrosek V, Roos-Hesselink JW, Bauersachs J, Blomström-Lundqvist C, Cífková R, De Bonis M, Iung B, Johnson MR, Kintscher U, Kranke P, et al. 2018 esc guidelines for the management of cardiovascular diseases during pregnancy. Eur Heart J. 2018;39:3165–3241 [DOI] [PubMed] [Google Scholar]
- 7.Acog practice bulletin no. 212 summary: Pregnancy and heart disease. Obstet Gynecol. 2019;133:1067–1072 [DOI] [PubMed] [Google Scholar]
- 8.Juul F, Chang VW, Brar P, Parekh N. Birth weight, early life weight gain and age at menarche: A systematic review of longitudinal studies. Obes Rev. 2017;18:1272–1288 [DOI] [PubMed] [Google Scholar]
- 9.Martinez GM. Trends and patterns in menarche in the united states: 1995 through 2013-2017. Natl Health Stat Report. 2020:1–12 [PubMed] [Google Scholar]
- 10.Biro FM, Pajak A, Wolff MS, Pinney SM, Windham GC, Galvez MP, Greenspan LC, Kushi LH, Teitelbaum SL. Age of menarche in a longitudinal us cohort. J Pediatr Adolesc Gynecol. 2018;31:339–345 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Lakshman R, Forouhi NG, Sharp SJ, Luben R, Bingham SA, Khaw KT, Wareham NJ, Ong KK. Early age at menarche associated with cardiovascular disease and mortality. J Clin Endocrinol Metab. 2009;94:4953–4960 [DOI] [PubMed] [Google Scholar]
- 12.Bubach S, De Mola CL, Hardy R, Dreyfus J, Santos AC, Horta BL. Early menarche and blood pressure in adulthood: Systematic review and meta-analysis. J Public Health (Oxf). 2018;40:476–484 [DOI] [PubMed] [Google Scholar]
- 13.Peters SA, Woodward M. Women’s reproductive factors and incident cardiovascular disease in the uk biobank. Heart. 2018;104:1069–1075 [DOI] [PubMed] [Google Scholar]
- 14.Canoy D, Beral V, Balkwill A, Wright FL, Kroll ME, Reeves GK, Green J, Carins BJ, Million Women Study Collaborators. Age at menarche and risks of coronary heart and other vascular diseases in a large uk cohort. Circulation. 2015;131:237–244 [DOI] [PubMed] [Google Scholar]
- 15.Lee JJ, Cook-Wiens G, Johnson BD, Braunstein GD, Berga SL, Stanczyk FZ, Pepine CJ, Bairey Merz CN, Shufelt CL. Age at menarche and risk of cardiovascular disease outcomes: Findings from the national heart lung and blood institute-sponsored women’s ischemia syndrome evaluation. J Am Heart Assoc. 2019;8:e012406. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Matkovic V, Ilich JZ, Skugor M, Badenhop NE, Goel P, Clairmont A, Klisovic D, Nahhas RW, Landoll JD. Leptin is inversely related to age at menarche in human females. J Clin Endocrinol Metab. 1997;82:3239–3245 [DOI] [PubMed] [Google Scholar]
- 17.Remsberg KE, Demerath EW, Schubert CM, Chumlea WC, Sun SS, Siervogel RM. Early menarche and the development of cardiovascular disease risk factors in adolescent girls: The fels longitudinal study. J Clin Endocrinol Metab. 2005;90:2718–2724 [DOI] [PubMed] [Google Scholar]
- 18.Osibogun O, Ogunmoroti O, Michos ED. Polycystic ovary syndrome and cardiometabolic risk: Opportunities for cardiovascular disease prevention. Trends Cardiovasc Med. 2020;30:399–404 [DOI] [PubMed] [Google Scholar]
- 19.Dunaif A Insulin resistance and the polycystic ovary syndrome: Mechanism and implications for pathogenesis. Endocr Rev. 1997;18:774–800 [DOI] [PubMed] [Google Scholar]
- 20.Chen MJ, Yang WS, Yang JH, Chen CL, Ho HN, Yang YS. Relationship between androgen levels and blood pressure in young women with polycystic ovary syndrome. Hypertension. 2007;49:1442–1447 [DOI] [PubMed] [Google Scholar]
- 21.Wild RA, Rizzo M, Clifton S, Carmina E. Lipid levels in polycystic ovary syndrome: Systematic review and meta-analysis. Fertil Steril. 2011;95:1073–1079.e1071-1011 [DOI] [PubMed] [Google Scholar]
- 22.DeUgarte CM, Bartolucci AA, Azziz R. Prevalence of insulin resistance in the polycystic ovary syndrome using the homeostasis model assessment. Fertil Steril. 2005;83:1454–1460 [DOI] [PubMed] [Google Scholar]
- 23.Salley KE, Wickham EP, Cheang KI, Essah PA, Karjane NW, Nestler JE. Glucose intolerance in polycystic ovary syndrome--a position statement of the androgen excess society. J Clin Endocrinol Metab. 2007;92:4546–4556 [DOI] [PubMed] [Google Scholar]
- 24.Wang ET, Calderon-Margalit R, Cedars MI, Daviglus ML, Merkin SS, Schreiner PJ, Sternfeld B, Wellons M, Schwartz SM, Lewis CE, et al. Polycystic ovary syndrome and risk for long-term diabetes and dyslipidemia. Obstet Gynecol. 2011;117:6–13 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Amiri M, Ramezani Tehrani F, Behboudi-Gandevani S, Bidhendi-Yarandi R, Carmina E. Risk of hypertension in women with polycystic ovary syndrome: A systematic review, meta-analysis and meta-regression. Reprod Biol Endocrinol. 2020;18:23. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Mak W, Dokras A. Polycystic ovarian syndrome and the risk of cardiovascular disease and thrombosis. Semin Thromb Hemost. 2009;35:613–620 [DOI] [PubMed] [Google Scholar]
- 27.Boulman N, Levy Y, Leiba R, Shachar S, Linn R, Zinder O, Blumenfeld Z. Increased c-reactive protein levels in the polycystic ovary syndrome: A marker of cardiovascular disease. J Clin Endocrinol Metab. 2004;89:2160–2165 [DOI] [PubMed] [Google Scholar]
- 28.Calderon-Margalit R, Siscovick D, Merkin SS, Wang E, Daviglus ML, Schreiner PJ, Sternfeld B, Williams OD, Lewis CE, Azziz R, et al. Prospective association of polycystic ovary syndrome with coronary artery calcification and carotid-intima-media thickness: The coronary artery risk development in young adults women’s study. Arterioscler Thromb Vasc Biol. 2014;34:2688–2694 [DOI] [PubMed] [Google Scholar]
- 29.Christian RC, Dumesic DA, Behrenbeck T, Oberg AL, Sheedy PF 2nd, Fitzpatrick LA. Prevalence and predictors of coronary artery calcification in women with polycystic ovary syndrome. J Clin Endocrinol Metab. 2003;88:2562–2568 [DOI] [PubMed] [Google Scholar]
- 30.Meyer ML, Malek AM, Wild RA, Korytkowski MT, Talbott EO. Carotid artery intima-media thickness in polycystic ovary syndrome: A systematic review and meta-analysis. Hum Reprod Update. 2012;18:112–126 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Zhou Y, Wang X, Jiang Y, Ma H, Chen L, Lai C, Peng C, He C, Sun C. Association between polycystic ovary syndrome and the risk of stroke and all-cause mortality: Insights from a meta-analysis. Gynecol Endocrinol. 2017;33:904–910 [DOI] [PubMed] [Google Scholar]
- 32.Glintborg D, Rubin KH, Nybo M, Abrahamsen B, Andersen M. Cardiovascular disease in a nationwide population of danish women with polycystic ovary syndrome. Cardiovasc Diabetol. 2018;17:37. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Jacewicz-Święcka M, Kowalska I. Polycystic ovary syndrome and the risk of cardiometabolic complications in longitudinal studies. Diabetes Metab Res Rev. 2018;34:e3054. [DOI] [PubMed] [Google Scholar]
- 34.Iftikhar S, Collazo-Clavell ML, Roger VL, St Sauver J, Brown RD Jr., Cha S, Rhodes DJ. Risk of cardiovascular events in patients with polycystic ovary syndrome. The Netherlands journal of medicine. 2012;70:74–80 [PMC free article] [PubMed] [Google Scholar]
- 35.Okoth K, Chandan JS, Marshall T, Thangaratinam S, Thomas GN, Nirantharakumar K, Adderley NJ. Association between the reproductive health of young women and cardiovascular disease in later life: Umbrella review. BMJ. 2020;371:m3502. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Oliver-Williams C, Vassard D, Pinborg A, Schmidt L. Polycystic ovary syndrome as a novel risk factor for atrial fibrillation: Results from a national danish registry cohort study. Eur J Prev Cardiol. 2020 [DOI] [PubMed] [Google Scholar]
- 37.Boomsma CM, Eijkemans MJ, Hughes EG, Visser GH, Fauser BC, Macklon NS. A meta-analysis of pregnancy outcomes in women with polycystic ovary syndrome. Hum Reprod Update. 2006;12:673–683 [DOI] [PubMed] [Google Scholar]
- 38.Tepper NK, Krashin JW, Curtis KM, Cox S, Whiteman MK. Update to cdc’s u.S. Medical eligibility criteria for contraceptive use, 2016: Revised recommendations for the use of hormonal contraception among women at high risk for hiv infection. MMWR. Morbidity and mortality weekly report. 2017;66:990–994 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Lindley KJ, Bairey Merz CN, Davis MB, Madden T, Park K, Bello NA, American College of Cardiology Cardiovascular Disease in Women Committee and the Cardio-Obstetrics Work Group. Contraception and reproductive planning for women with cardiovascular disease: Jacc focus seminar 5/5. J Am Coll Cardiol. 2021;77:1823–1834 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Shufelt C, LeVee A. Hormonal contraception in women with hypertension. JAMA. 2020;324:1451–1452 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Lidegaard Ø, Nielsen LH, Skovlund CW, Skjeldestad FE, Løkkegaard E. Risk of venous thromboembolism from use of oral contraceptives containing different progestogens and oestrogen doses: Danish cohort study, 2001-9. BMJ. 2011;343:d6423. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Roach RE, Helmerhorst FM, Lijfering WM, Stijnen T, Algra A, Dekkers OM. Combined oral contraceptives: The risk of myocardial infarction and ischemic stroke. Cochrane Database Syst Rev. 2015;2015:Cd011054. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Kaminski P, Szpotanska-Sikorska M, Wielgos M. Cardiovascular risk and the use of oral contraceptives. Neuro Endocrinol Lett. 2013;34:587–589 [PubMed] [Google Scholar]
- 44.Udell JA, Lu H, Redelmeier DA. Long-term cardiovascular risk in women prescribed fertility therapy. J Am Coll Cardiol. 2013;62:1704–1712 [DOI] [PubMed] [Google Scholar]
- 45.Luke B, Gopal D, Cabral H, Stern JE, Diop H. Adverse pregnancy, birth, and infant outcomes in twins: Effects of maternal fertility status and infant gender combinations; the massachusetts outcomes study of assisted reproductive technology. Am J Obstet Gynecol. 2017;217:330 e331–330 e315 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Mahalingaiah S, Sun F, Cheng JJ, Chow ET, Lunetta KL, Murabito JM. Cardiovascular risk factors among women with self-reported infertility. Fertil Res Pract. 2017;3:7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Thomopoulos C, Tsioufis C, Michalopoulou H, Makris T, Papademetriou V, Stefanadis C. Assisted reproductive technology and pregnancy-related hypertensive complications: A systematic review. J Hum Hypertens. 2013;27:148–157 [DOI] [PubMed] [Google Scholar]
- 48.Dayan N, Filion KB, Okano M, Kilmartin C, Reinblatt S, Landry T, Basso O, Udell JA. Cardiovascular risk following fertility therapy: Systematic review and meta-analysis. J Am Coll Cardiol. 2017;70:1203–1213 [DOI] [PubMed] [Google Scholar]
- 49.Roberts JM, August PA, Bakris G, Barton JR, Bernstein IM, Druzin ML, Gaiser RR, Granger JR, Jeyabalan A, Johnson DD, et al. Hypertension in pregnancy. Report of the american college of obstetricians and gynecologists’ task force on hypertension in pregnancy. Obstetrics & Gynecology. 2013;122:1122–1131 [DOI] [PubMed] [Google Scholar]
- 50.Burton GJ, Redman CW, Roberts JM, Moffett A. Pre-eclampsia: Pathophysiology and clinical implications. BMJ. 2019;366:l2381. [DOI] [PubMed] [Google Scholar]
- 51.Roberts JM, Rich-Edwards JW, McElrath TF, Garmire L, Myatt L. Subtypes of preeclampsia: Recognition and determining clinical usefulness. Hypertension. 2021;77:1430–1441 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Wu P, Chew-Graham CA, Maas AH, Chappell LC, Potts JE, Gulati M, Jordan KP, Mamas MA. Temporal changes in hypertensive disorders of pregnancy and impact on cardiovascular and obstetric outcomes. The American Journal of Cardiology. 2020;125:1508–1516 [DOI] [PubMed] [Google Scholar]
- 53.Afana M, Brinjikji W, Kao D, Jackson E, Maddox T, Childers D, Eagle KA, Davis MB. Characteristics and in-hospital outcomes of peripartum cardiomyopathy diagnosed during delivery in the united states from the nationwide inpatient sample (nis) database. Journal of Cardiac Failure. 2016;22:512–519 [DOI] [PubMed] [Google Scholar]
- 54.Tweet MS, Hayes SN, Codsi E, Gulati R, Rose CH, Best PJM. Spontaneous coronary artery dissection associated with pregnancy. Journal of the American College of Cardiology. 2017;70:426–435 [DOI] [PubMed] [Google Scholar]
- 55.Haug EB, Horn J, Markovitz AR, Fraser A, Klykken B, Dalen H, Vatten LJ, Romundstad PR, Rich-Edwards JW, Asvold BO. Association of conventional cardiovascular risk factors with cardiovascular disease after hypertensive disorders of pregnancy: Analysis of the nord-trøndelag health study. JAMA Cardiol. 2019 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Leon LJ, McCarthy FP, Direk K, Gonzalez-Izquierdo A, Prieto-Merino D, Casas JP, Chappell L. Preeclampsia and cardiovascular disease in a large uk pregnancy cohort of linked electronic health records: A caliber study. Circulation. 2019;140:1050–1060 [DOI] [PubMed] [Google Scholar]
- 57.Grandi SM, Filion KB, Yoon S, Ayele HT, Doyle CM, Hutcheon JA, Smith GN, Gore GC, Ray JG, Neremberg K, et al. Cardiovascular disease-related morbidity and mortality in women with a history of pregnancy complications. Circulation. 2019;139:1069–1079 [DOI] [PubMed] [Google Scholar]
- 58.Riise HKR, Sulo G, Tell GS, Igland J, Nygård O, Iversen AC, Daltveit AK. Association between gestational hypertension and risk of cardiovascular disease among 617 589 norwegian women. J Am Heart Assoc. 2018;7 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Haug EB, Horn J, Markovitz AR, Fraser A, Vatten LJ, Macdonald-Wallis C, Tilling K, Romundstad PR, Rich-Edwards JW, Asvold BO. Life course trajectories of cardiovascular risk factors in women with and without hypertensive disorders in first pregnancy: The hunt study in norway. J Am Heart Assoc. 2018;7:e009250. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Stuart JJ, Tanz LJ, Cook NR, Spiegelman D, Missmer SA, Rimm EB, Rexrode KM, Mukamal KJ, Rich-Edwards JW. Hypertensive disorders of pregnancy and 10-year cardiovascular risk prediction. J Am Coll Cardiol. 2018;72:1252–1263 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Grand’Maison S, Pilote L, Okano M, Landry T, Dayan N. Markers of vascular dysfunction after hypertensive disorders of pregnancy: A systematic review and meta-analysis. Hypertension. 2016;68:1447–1458 [DOI] [PubMed] [Google Scholar]
- 62.Honigberg MC, Zekavat SM, Aragam K, Klarin D, Bhatt DL, Scott NS, Peloso GM, Natarajan P. Long-term cardiovascular risk in women with hypertension during pregnancy. J Am Coll Cardiol. 2019 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Garovic VD, Milic NM, Weissgerber TL, Mielke MM, Bailey KR, Lahr B, Jayachandran M, White WM, Hodis HN, Miller VM. Carotid artery intima-media thickness and subclinical atherosclerosis in women with remote histories of preeclampsia: Results from a rochester epidemiology project-based study and meta-analysis. Mayo Clinic Proceedings. 2017;92:1328–1340 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Stuart JJ, Tanz LJ, Missmer SA, Rimm EB, Spiegelman D, James-Todd TM, Rich-Edwards JW. Hypertensive disorders of pregnancy and maternal cardiovascular disease risk factor development: An observational cohort study. Ann Intern Med. 2018;169:224–232 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Wang YX, Arvizu M, Rich-Edwards JW, Wang L, Rosner B, Stuart JJ, Rexrode KM, Chavarro JE. Hypertensive disorders of pregnancy and subsequent risk of premature mortality. J Am Coll Cardiol. 2021;77:1302–1312 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Garovic VD, White WM, Vaughan L, Saiki M, Parashuram S, Garcia-Valencia O, Weissgerber TL, Milic N, Weaver A, Mielke MM. Incidence and long-term outcomes of hypertensive disorders of pregnancy. J Am Coll Cardiol. 2020;75:2323–2334 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Tooher J, Thornton C, Makris A, Ogle R, Korda A, Hennessy A. All hypertensive disorders of pregnancy increase the risk of future cardiovascular disease. Hypertension. 2017;70:798–803 [DOI] [PubMed] [Google Scholar]
- 68.Dall’Asta A, D’Antonio F, Saccone G, Buca D, Mastantuoni E, Liberati M, Flacco ME, Frusca T, Ghi T. Cardiovascular events following pregnancies complicated by preeclampsia with emphasis on the comparison between early and late onset forms: A systematic review and meta-analysis. Ultrasound in Obstetrics & Gynecology.n/a [DOI] [PubMed] [Google Scholar]
- 69.Brown MC, Best KE, Pearce MS, Waugh J, Robson SC, Bell R. Cardiovascular disease risk in women with pre-eclampsia: Systematic review and meta-analysis. Eur J Epidemiol. 2013;28:1–19 [DOI] [PubMed] [Google Scholar]
- 70.Bellamy L, Casas JP, Hingorani AD, Williams DJ. Pre-eclampsia and risk of cardiovascular disease and cancer in later life: Systematic review and meta-analysis. BMJ. 2007;335:974. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Wu MY, Hu PJ, Wong CS, Chen TT, Hsueh JY, Lin YF, Tu YK. Long-term clinical outcome of major adverse vascular events after hypertensive disorders of pregnancy. Obstet Gynecol. 2021;137:285–293 [DOI] [PubMed] [Google Scholar]
- 72.Arnott C, Nelson M, Alfaro Ramirez M, Hyett J, Gale M, Henry A, Celermajer DS, Taylor L, Woodward M. Maternal cardiovascular risk after hypertensive disorder of pregnancy. Heart. 2020 [DOI] [PubMed] [Google Scholar]
- 73.Malek AM, Wilson DA, Turan TN, Mateus J, Lackland DT, Hunt KJ. Incident heart failure within the first and fifth year after delivery among women with hypertensive disorders of pregnancy and prepregnancy hypertension in a diverse population. J Am Heart Assoc. 2021:e021616. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Malek AM, Wilson DA, Turan TN, Mateus J, Lackland DT, Hunt KJ. Maternal coronary heart disease, stroke, and mortality within 1, 3, and 5 years of delivery among women with hypertensive disorders of pregnancy and pre-pregnancy hypertension. J Am Heart Assoc. 2021;10:e018155. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Wu P, Haththotuwa R, Kwok CS, Babu A, Kotronias RA, Rushton C, Zaman A, Fryer AA, Kadam U, Chew-Graham CA, et al. Preeclampsia and future cardiovascular health: A systematic review and meta-analysis. Circ Cardiovasc Qual Outcomes. 2017;10 [DOI] [PubMed] [Google Scholar]
- 76.Honigberg MC, Riise HKR, Daltveit AK, Tell GS, Sulo G, Igland J, Klungsoyr K, Scott NS, Wood MJ, Natarajan P, Rich-Edwards JW. Heart failure in women with hypertensive disorders of pregnancy: Insights from the cardiovascular disease in norway project. Hypertension. 2020;76:1506–1513 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Andolf E, Bladh M, Möller L, Sydsjö G. Prior placental bed disorders and later dementia: A retrospective swedish register-based cohort study. BJOG: An International Journal of Obstetrics & Gynaecology.n/a [DOI] [PubMed] [Google Scholar]
- 78.Ss Basit, Jcs Wohlfahrt, Boyd HAsr. Pre-eclampsia and risk of dementia later in life: Nationwide cohort study. BMJ October. 2018;20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Barrett PMMB, Mfphmi McCarthy FPMB, Kublickiene KMB, et al. Adverse pregnancy outcomes and long-term maternal kidney disease: A systematic review and meta-analysis. JAMA Network Open. 2020;3:e1920964- [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Suvakov S, Cubro H, White WM, Butler Tobah YS, Weissgerber TL, Jordan KL, Zhu XY, Woolard JR, Chebib FT, Milic NM, et al. Targeting senescence improves angiogenic potential of adipose-derived mesenchymal stem cells in patients with preeclampsia. Biol Sex Differ. 2019;10:49. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Suvakov S, Ghamrawi R, Cubro H, Tu H, White WM, Tobah YSB, Milic NM, Grande JP, Cunningham JM, Chebib FT, et al. Epigenetic and senescence markers indicate an accelerated ageing-like state in women with preeclamptic pregnancies. EBioMedicine. 2021;70:103536. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Skjaerven R, Vatten LJ, Wilcox AJ, Rønning T, Irgens LM, Lie RT. Recurrence of pre-eclampsia across generations: Exploring fetal and maternal genetic components in a population based cohort. Bmj. 2005;331:877. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Serrano NC, Quintero-Lesmes DC, Dudbridge F, Leon LJ, Hingorani AD, Williams DJ, Casas JP. Family history of pre-eclampsia and cardiovascular disease as risk factors for pre-eclampsia: The genpe case-control study. Hypertens Pregnancy. 2020;39:56–63 [DOI] [PubMed] [Google Scholar]
- 84.Gray KJ, Kovacheva VP, Mirzakhani H, Bjonnes AC, Almoguera B, Wilson ML, Ingles SA, Lockwood CJ, Hakonarsaon H, McElrath TF, et al. Risk of pre-eclampsia in patients with a maternal genetic predisposition to common medical conditions: A case-control study. BJOG. 2021;128:55–65 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Honigberg MC, Chaffin M, Aragam K, Bhatt DL, Wood MJ, Sarma AA, Scott NS, Peloso GM, Natarajan P. Genetic variation in cardiometabolic traits and medication targets and the risk of hypertensive disorders of pregnancy. Circulation. 2020;142:711–713 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Steinthorsdottir V, McGinnis R, Williams NO, Stefansdottir L, Thorleifsson G, Shooter S, Fadista J, Sigurdsson JK, Auro KM, Berezina G, et al. Genetic predisposition to hypertension is associated with preeclampsia in european and central asian women. Nat Commun. 2020;11:5976. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Buchanan TA. Pancreatic b-cell defects in gestational diabetes: Implications for the pathogenesis and prevention of type 2 diabetes. J Clin Endocrinol Metab. 2001;86:989–993 [DOI] [PubMed] [Google Scholar]
- 88.Bellamy L, Casas JP, Hingorani AD, Williams D. Type 2 diabetes mellitus after gestational diabetes: A systematic review and meta-analysis. Lancet. 2009;373:1773–1779 [DOI] [PubMed] [Google Scholar]
- 89.14. Management of diabetes in pregnancy: Standards of medical care in diabetes-2021. Diabetes Care. 2021;44:S200–s210 [DOI] [PubMed] [Google Scholar]
- 90.Kramer CK, Swaminathan B, Hanley AJ, Connelly PW, Sermer M, Zinman B, Retnakaran R. Each degree of glucose intolerance in pregnancy predicts distinct trajectories of β-cell function, insulin sensitivity, and glycemia in the first 3 years postpartum. Diabetes Care. 2014;37:3262–3269 [DOI] [PubMed] [Google Scholar]
- 91.Hiersch L, Shah BR, Berger H, Geary M, McDonald SD, Murray-Davis B, Halperin I, Retnakaran R, Barrett J, Melamed N, et al. Oral glucose tolerance test results in pregnancy can be used to individualize the risk of future maternal type 2 diabetes mellitus in women with gestational diabetes mellitus. Diabetes Care. 2021;44:1860–1867 [DOI] [PubMed] [Google Scholar]
- 92.Retnakaran R The insulin-like growth factor axis: A new player in gestational diabetes mellitus? Diabetes. 2016;65:3246–3248 [DOI] [PubMed] [Google Scholar]
- 93.Gunderson EP, Sun B, Catov JM, Carnethon M, Lewis CE, Allen NB, Sidney S, Wellons M, Rana JS, Hou L, et al. Gestational diabetes history and glucose tolerance after pregnancy associated with coronary artery calcium in women during midlife: The cardia study. Circulation. 2021;143:974–987 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94.Kramer CK, Campbell S, Retnakaran R. Gestational diabetes and the risk of cardiovascular disease in women: A systematic review and meta-analysis. Diabetologia. 2019;62:905–914 [DOI] [PubMed] [Google Scholar]
- 95.Retnakaran R Hyperglycemia in pregnancy and its implications for a woman’s future risk of cardiovascular disease. Diabetes Res Clin Pract. 2018;145:193–199 [DOI] [PubMed] [Google Scholar]
- 96.Crump C, Sundquist J, Howell EA, McLaughlin MA, Stroustrup A, Sundquist K. Pre-term delivery and risk of ischemic heart disease in women. J Am Coll Cardiol. 2020;76:57–67 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 97.Tanz LJ, Stuart JJ, Williams PL, Rimm EB, Missmer SA, Rexrode KM, Mukamal KJ, Rich-Edwards JW. Preterm delivery and maternal cardiovascular disease in young and middle-aged adult women. Circulation. 2017;135:578–589 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 98.Smilowitz NR, Maduro GA Jr., Lobach IV, Chen Y, Reynolds HR. Adverse trends in ischemic heart disease mortality among young new yorkers, particularly young black women. PLoS One. 2016;11:e0149015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 99.Bavineni M, Wassenaar TM, Agnihotri K, Ussery DW, Luscher TF, Mehta JL. Mechanisms linking preterm birth to onset of cardiovascular disease later in adulthood. Eur Heart J. 2019;40:1107–1112 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 100.Rich-Edwards JW, Klungsoyr K, Wilcox AJ, Skjaerven R. Duration of pregnancy, even at term, predicts long-term risk of coronary heart disease and stroke mortality in women: A population-based study. Am J Obstet Gynecol. 2015;213:518.e511–518 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 101.Auger N, Potter BJ, He S, Healy-Profitos J, Schnitzer ME, Paradis G. Maternal cardiovascular disease 3 decades after preterm birth: Longitudinal cohort study of pregnancy vascular disorders. Hypertension. 2020;75:788–795 [DOI] [PubMed] [Google Scholar]
- 102.Khalil A, Elkhouli M, Garcia-Mandujano R, Chiriac R, Nicolaides KH. Maternal hemodynamics at 11-13 weeks of gestation and preterm birth. Ultrasound Obstet Gynecol. 2012;40:35–39 [DOI] [PubMed] [Google Scholar]
- 103.Minissian MB, Kilpatrick S, Shufelt CL, Eastwood JA, Robbins W, Sharma KJ, Bolton LB, Brecht M-L, Wei J, Cook-Wiens G, et al. Vascular function and serum lipids in women with spontaneous preterm delivery and term controls. J Womens Health (Larchmt). 2019;28:1522–1528 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 104.Sondergaard MM, Hlatky MA, Stefanick ML, Vittinghoff E, Nah G, Allison M, Gemmill A, Van Horn L, Park K, Salmoirago-Blotcher E, et al. Association of adverse pregnancy outcomes with risk of atherosclerotic cardiovascular disease in postmenopausal women. JAMA Cardiol. 2020;5:1390–1398 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 105.Davey Smith G, Hypponen E, Power C, Lawlor DA. Offspring birth weight and parental mortality: Prospective observational study and meta-analysis. Am J Epidemiol. 2007;166:160–169 [DOI] [PubMed] [Google Scholar]
- 106.McCowan LM, Figueras F, Anderson NH. Evidence-based national guidelines for the management of suspected fetal growth restriction: Comparison, consensus, and controversy. Am J Obstet Gynecol. 2018;218:S855–S868 [DOI] [PubMed] [Google Scholar]
- 107.Khan F MG, Macleod M, Belch JJ. Relationship between maternal arterial wave reflection, microvascular function and fetal growth in normal pregnancy. Microcirculation. 2010;17:608–614 [DOI] [PubMed] [Google Scholar]
- 108.Stergiotou I, Bijnens B, Cruz-Lemini M, Figueras F, Gratacos E, Crispi F. Maternal subclinical vascular changes in fetal growth restriction with and without pre-eclampsia. Ultrasound Obstet Gynecol. 2015;46:706–712 [DOI] [PubMed] [Google Scholar]
- 109.Bonamy AK, Parikh NI, Cnattingius S, Ludvigsson JF, Ingelsson E. Birth characteristics and subsequent risks of maternal cardiovascular disease: Effects of gestational age and fetal growth. Circulation. 2011;124:2839–2846 [DOI] [PubMed] [Google Scholar]
- 110.Smith GD, Whitley E, Gissler M, Hemminki E. Birth dimensions of offspring, premature birth, and the mortality of mothers. Lancet. 2000;356:2066–2067 [DOI] [PubMed] [Google Scholar]
- 111.Laughlin GA B-CE, Criqui MH, Kritz-Silverstein D. The prospective association of serum insulin-like growth factor i (igf-i) and igf-binding protein-1 levels with all cause and cardiovascular disease mortality in older adults: The rancho bernardo study. J Clin Endocrinol Metab. 2004;89:114–120 [DOI] [PubMed] [Google Scholar]
- 112.Veerbeek JH, Smit JG, Koster MP, Post Uiterweer ED, van Rijn BB, Koenen SV, Franx A. Maternal cardiovascular risk profile after placental abruption. Hypertension. 2013;61:1297–1301 [DOI] [PubMed] [Google Scholar]
- 113.Ray JG, Vermeulen MJ, Schull MJ, Redelmeier DA. Cardiovascular health after maternal placental syndromes (champs): Population-based retrospective cohort study. Lancet. 2005;366:1797–1803 [DOI] [PubMed] [Google Scholar]
- 114.Horn J, Haug EB, Markovitz AR, Fraser A, Vatten LJ, Romundstad PR, Rich-Edwards JW, Asvold BO. Life course trajectories of maternal cardiovascular risk factors according to offspring birthweight: The hunt study. Sci Rep. 2020;10:10436. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 115.Fraser A, Nelson SM, Macdonald-Wallis C, Cherry L, Butler E, Sattar N, et al. Associations of pregnancy complications with calculated cardiovascular disease risk and cardiovascular risk factors in middle age: The avon longitudinal study of parents and children. Circulation. 2012;125:1367–1380 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 116.Smith GC, Pell JP, Walsh D. Spontaneous loss of early pregnancy and risk of ischaemic heart disease in later life: Retrospective cohort study. BMJ. 2003;326:423–424 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 117.Oliver-Williams CT, Heydon EE, Smith GC, Wood AM. Miscarriage and future maternal cardiovascular disease: A systematic review and meta-analysis. Heart. 2013;99:1636–1644 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 118.Wagner Marise BS, Visser Jantien, Hannaford Philip C, Bloemenkamp Kitty WM. Association between miscarriage and cardiovascular disease in a scottish cohort. Heart. 2015;101:1954–1960 [DOI] [PubMed] [Google Scholar]
- 119.Ranthe MF, Andersen EA, Wohlfahrt J, Bundgaard H, Melbye M, Boyd HA. Pregnancy loss and later risk of atherosclerotic disease. Circulation. 2013;127:1775–1782 [DOI] [PubMed] [Google Scholar]
- 120.Rameez RM, Sadana D, Kaur S, Ahmed T, Patel J, Khan MS, Misbah S, Simonson MT, Riaz H, Ahmed HM. Association of maternal lactation with diabetes and hypertension: A systematic review and meta-analysis. JAMA Netw Open. 2019;2:e1913401. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 121.Schwarz EB, Ray RM, Stuebe AM, Allison MA, Ness RB, Freiberg MS, Cauley JA. Duration of lactation and risk factors for maternal cardiovascular disease. Obstet Gynecol. 2009;113:974–982 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 122.Gunderson EP, Lewis CE, Lin Y, Sorel M, Gross M, Sidney S, Jacobs DR Jr, Shikany JM, Quesenberry CP Jr. Lactation duration and progression to diabetes in women across the childbearing years: The 30-year cardia study. JAMA Intern Med. 2018;178:328–337 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 123.Schwarz EB, McClure CK, Tepper PG, Thurston R, Janssen I, Matthews KA, Sutton-Tyrrell K. Lactation and maternal measures of subclinical cardiovascular disease. Obstet Gynecol. 2010;115:41–48 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 124.Stuebe AM, Michels KB, Willett WC, Manson JE, Rexrode K, Rich-Edwards JW. Duration of lactation and incidence of myocardial infarction in middle to late adulthood. Am J Obstet Gynecol. 2009;200:138.e131–138 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 125.Nguyen B, Gale J, Nassar N, Bauman A, Joshy G, Ding D. Breastfeeding and cardiovascular disease hospitalization and mortality in parous women: Evidence from a large australian cohort study. J Am Heart Assoc. 2019;8:e011056. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 126.Yu J, Pudwell J, Dayan N, Smith GN. Postpartum breastfeeding and cardiovascular risk assessment in women following pregnancy complications. J Womens Health (Larchmt). 2020;29:627–635 [DOI] [PubMed] [Google Scholar]
- 127.Gunderson EP, Hedderson MM, Chiang V, Crites Y, Walton D, Azevedo RA, Fox G, Elmasian C, Young S, Salvador N, Lum M, et al. Lactation intensity and postpartum maternal glucose tolerance and insulin resistance in women with recent gdm: The swift cohort. Diabetes Care. 2012;35:50–56 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 128.Stuebe AM, Rich-Edwards JW. The reset hypothesis: Lactation and maternal metabolism. Am J Perinatol. 2009;26:81–88 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 129.Appiah D, Lewis CE, Jacobs DR, Shikany JM, Quesenberry CP, Gross M, Carr J, Sidney S, Gunderson EP. The association of lactation duration with visceral and pericardial fat volumes in parous women: The cardia study. J Clin Endocrinol Metab. 2021;106:1821–1831 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 130.Drynda R, Peters CJ, Jones PM, Bowe JE. The role of non-placental signals in the adaptation of islets to pregnancy. Horm Metab Res. 2015;47:64–71 [DOI] [PubMed] [Google Scholar]
- 131.Li W, Ruan W, Lu Z, Wang D. Parity and risk of maternal cardiovascular disease: A dose-response meta-analysis of cohort studies. Eur J Prev Cardiol. 2019;26:592–602 [DOI] [PubMed] [Google Scholar]
- 132.Oliver-Williams C VC, Loehr LR, Rosamond WD, Stuebe AM. The association between parity and subsequent cardiovascular disease in women: The atherosclerosis risk in communities study. J Womens Health (Larchmt). 2019;28:721–727 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 133.Parikh NI, Cnattingius S, Dickman PW, Mittleman MA, Ludvigsson JF, Ingelsson E. Parity and risk of later-life maternal cardiovascular disease. Am Heart J. 2010;159:215–221 e216 [DOI] [PubMed] [Google Scholar]
- 134.Lawlor DA, Emberson JR, Ebrahim S, Whincup PH, Wannamethee SG, Walker M, Smith GD, British Women’s Heart and Health Study, British Regional Heart Study. Is the association between parity and coronary heart disease due to biological effects of pregnancy or adverse lifestyle risk factors associated with child-rearing? Findings from the british women’s heart and health study and the british regional heart study. Circulation. 2003;107:1260–1264 [DOI] [PubMed] [Google Scholar]
- 135.Vladutiu CJ, Siega-Riz AM, Sotres-Alvarez D, Stuebe AM, Ni A, Tabb KM, Gallo LC, Potter JE, Heiss G. Parity and components of the metabolic syndrome among us hispanic/latina women: Results from the hispanic community health study/study of latinos. Circ Cardiovasc Qual Outcomes. 2016;9:S62–69 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 136.Harlow SD, Gass M, Hall JE, Lobo R, Maki P, Rebar RW, Sherman S, Sluss PM, de Villiers TJ, STRAW, et al. Executive summary of the stages of reproductive aging workshop + 10: Addressing the unfinished agenda of staging reproductive aging. J Clin Endocrinol Metab. 2012;97:1159–1168 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 137.El Khoudary SR, Aggarwal B, Beckie TM, Hodis HN, Johnson AE, Langer RD, Limacher MC, Manson JE, Stefanick ML, Allison MA, et al. Menopause transition and cardiovascular disease risk: Implications for timing of early prevention: A scientific statement from the american heart association. Circulation. 2020;142:e506–e532 [DOI] [PubMed] [Google Scholar]
- 138.Wildman RP, Colvin AB, Powell LH, Matthews KA, Everson-Rose SA, Hollenberg S, Johnston JM, Sutton-Tyrrell K. Associations of endogenous sex hormones with the vasculature in menopausal women: The study of women’s health across the nation (swan). Menopause. 2008;15:414–421 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 139.Matthews KA, Crawford SL, Chae CU, Everson-Rose SA, Sowers MF, Sternfeld B, Sutton-Tyrrell K. Are changes in cardiovascular disease risk factors in midlife women due to chronological aging or to the menopausal transition? J Am Coll Cardiol. 2009;54:2366–2373 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 140.Matthews KA, El Khoudary SR, Brooks MM, Derby CA, Harlow SD, Barinas-Mitchell EJ, Thurston RC. Lipid changes around the final menstrual period predict carotid subclinical disease in postmenopausal women. Stroke. 2017;48:70–76 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 141.El Khoudary SR. Hdl and the menopause. Curr Opin Lipidol. 2017;28:328–336 [DOI] [PubMed] [Google Scholar]
- 142.El Khoudary SR, Chen X, Nasr AN, Billheimer J, Brooks MM, McConnell D, Orchard TJ, Crawford SL, Matthews KA, Rader DJ. Hdl (high-density lipoprotein) subclasses, lipid content, and function trajectories across the menopause transition: Swan-hdl study. Arterioscler Thromb Vasc Biol. 2021;41:951–961 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 143.Otvos JD, Collins D, Freedman DS, Shalaurova I, Schaefer EJ, McNamara JR, Bloomfield HE, Robins SJ. Low-density lipoprotein and high-density lipoprotein particle subclasses predict coronary events and are favorably changed by gemfibrozil therapy in the veterans affairs high-density lipoprotein intervention trial. Circulation. 2006;113:1556–1563 [DOI] [PubMed] [Google Scholar]
- 144.Holmes MV, Millwood IY, Kartsonaki C, Hill MR, Bennett DA, Boxall R, Guo Y, Bian Z, Hu R, Walters R, et al. Lipids, lipoproteins, and metabolites and risk of myocardial infarction and stroke. J Am Coll Cardiol. 2018;71:620–632 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 145.El Khoudary SR, Nasr A, Billheimer J, Brooks MM, McConnell D, Crawford S, Orchard TJ, Rader DJ, Matthews KA. Associations of endogenous hormones with hdl novel metrics across the menopause transition: The swan hdl study. J Clin Endocrinol Metab. 2021 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 146.Agarwala AP, Rodrigues A, Risman M, McCoy M, Trindade K, Qu L, Cuchel M, Billheimer J, Rader DJ. High-density lipoprotein (hdl) phospholipid content and cholesterol efflux capacity are reduced in patients with very high hdl cholesterol and coronary disease. Arterioscler Thromb Vasc Biol. 2015;35:1515–1519 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 147.Goff DC Jr., Lloyd-Jones DM, Bennett G, Coady S, D’Agostino RB, Gibbons R, Greenland P, Lackland DT, Levy D, O’Donnell CJ, et al. 2013 acc/aha guideline on the assessment of cardiovascular risk: A report of the american college of cardiology/american heart association task force on practice guidelines. Circulation. 2014;129:S49–73 [DOI] [PubMed] [Google Scholar]
- 148.Davis SR, Castelo-Branco C, Chedraui P, Lumsden MA, Nappi RE, Shah D, Villaseca P, Writing Group of the International Menopause Society for World Menopause Day 2012. Understanding weight gain at menopause. Climacteric. 2012;15:419–429 [DOI] [PubMed] [Google Scholar]
- 149.Samargandy S, Matthews KA, Brooks MM, Barinas-Mitchell E, Magnani JW, Janssen I, Kazlauskaite R, El Khoudary SR. Abdominal visceral adipose tissue over the menopause transition and carotid atherosclerosis: The swan heart study. Menopause. 2021;28:626–633 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 150.El Khoudary SR, Shields KJ, Janssen I, Hanley C, Budoff MJ, Barinas-Mitchell E, Everson-Rose SA, Powell LH, Matthews KA. Cardiovascular fat, menopause, and sex hormones in women: The swan cardiovascular fat ancillary study. J Clin Endocrinol Metab. 2015;100:3304–3312 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 151.Eisenberg E, McElhinney PA, Commandeur F, Chen X, Cadet S, Goeller M, Razipour A, Gransar H, Cantu S, Miller RJH, et al. Deep learning-based quantification of epicardial adipose tissue volume and attenuation predicts major adverse cardiovascular events in asymptomatic subjects. Circ Cardiovasc Imaging. 2020;13:e009829. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 152.El Khoudary SR, Shields KJ, Janssen I, Budoff MJ, Everson-Rose SA, Powell LH, Matthews KA. Postmenopausal women with greater paracardial fat have more coronary artery calcification than premenopausal women: The study of women’s health across the nation (swan) cardiovascular fat ancillary study. J Am Heart Assoc. 2017;6 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 153.El Khoudary SR, Wildman RP, Matthews K, Thurston RC, Bromberger JT, Sutton-Tyrrell K. Progression rates of carotid intima-media thickness and adventitial diameter during the menopausal transition. Menopause. 2013;20:8–14 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 154.Moreau KL, Hildreth KL, Meditz AL, Deane KD, Kohrt WM. Endothelial function is impaired across the stages of the menopause transition in healthy women. J Clin Endocrinol Metab. 2012;97:4692–4700 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 155.Khan ZA, Janssen I, Mazzarelli JK, Powell LH, Dumasius A, Everson-Rose SA, Barinas-Mitchell E, Matthews K, El Khoudary SR, Weinstock PJ, et al. Serial studies in subclinical atherosclerosis during menopausal transition (from the study of women’s health across the nation). Am J Cardiol. 2018;122:1161–1168 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 156.Samargandy S, Matthews KA, Brooks MM, Barinas-Mitchell E, Magnani JW, Janssen I, Hollenberg SM, El Khoudary SR. Arterial stiffness accelerates within 1 year of the final menstrual period: The swan heart study. Arterioscler Thromb Vasc Biol. 2020;40:1001–1008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 157.Zhu D, Chung HF, Pandeya N, Dobson AJ, Cade JE, Greenwood DC, Crawford SL, Avis NE, Gold EB, Mitchell ES, et al. Relationships between intensity, duration, cumulative dose, and timing of smoking with age at menopause: A pooled analysis of individual data from 17 observational studies. PLoS Med. 2018;15:e1002704. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 158.Nowakowski ACH, Graves KY. Exploring black-white differences in the relationship between inflammation and timing of menopause. J Racial Ethn Health Disparities. 2017;4:410–417 [DOI] [PubMed] [Google Scholar]
- 159.El Khoudary SR. Age at menopause onset and risk of cardiovascular disease around the world. Maturitas. 2020;141:33–38 [DOI] [PubMed] [Google Scholar]
- 160.Honigberg MC, Zekavat SM, Aragam K, Finneran P, Klarin D, Bhatt DL, Januzzi JR. JL, Scott NS, Natarajan P. Association of premature natural and surgical menopause with incident cardiovascular disease. JAMA. 2019 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 161.Ley SH, Li Y, Tobias DK, Manson JE, Rosner B, Hu FB, Rexrode KM. Duration of reproductive life span, age at menarche, and age at menopause are associated with risk of cardiovascular disease in women. J Am Heart Assoc. 2017;6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 162.Muka T, Oliver-Williams C, Kunutsor S, Laven JS, Fauser BC, Chowdhury R, Kavousi M, Franco OH. ssociation of age at onset of menopause and time since onset of menopause with cardiovascular outcomes, intermediate vascular traits, and all-cause mortality: A systematic review and meta-analysis. JAMA Cardiol. 2016;1:767–776 [DOI] [PubMed] [Google Scholar]
- 163.Muka T, Oliver-Williams C, Colpani V, Kunutsor S, Chowdhury S, Chowdhury R, Kavousi M, Franco OH. Association of vasomotor and other menopausal symptoms with risk of cardiovascular disease: A systematic review and meta-analysis. PLoS One. 2016;11:e0157417. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 164.Kok HS, van Asselt KM, van der Schouw YT, van der Tweel I, Peeters PH, Wilson PW, Pearson PL, Grobbee DE. Heart disease risk determines menopausal age rather than the reverse. J Am Coll Cardiol. 2006;47:1976–1983 [DOI] [PubMed] [Google Scholar]
- 165.Zhu D, Chung H-F, Dobson AJ, Pandeya N, Giles GG, Bruinsma F, Brunner EJ, Kuh D, Hardy R, Avis NE, et al. Age at natural menopause and risk of incident cardiovascular disease: A pooled analysis of individual patient data. The Lancet. Public health. 2019;4:e553–e564 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 166.Honigberg MC, Zekavat SM, Niroula A, Griffin GK, Bick AG, Pirruccello JP, Nakao T, Whitsel EA, Farland LV, Laurie C, et al. Premature menopause, clonal hematopoiesis, and coronary artery disease in postmenopausal women. Circulation. 2021;143:410–423 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 167.Jaiswal S, Natarajan P, Silver AJ, Gibson CJ, Bick AG, Shvartz E, McConeky M, Gupta N, Garbeil S, Ardissino D, et al. Clonal hematopoiesis and risk of atherosclerotic cardiovascular disease. N Engl J Med. 2017;377:111–121 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 168.Rivera CM, Grossardt BR, Rhodes DJ, Brown RD Jr., Roger VL, Melton LJ 3rd, et al. Increased cardiovascular mortality after early bilateral oophorectomy. Menopause (New York, N.Y.). 2009;16:15–23 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 169.Løkkegaard E, Jovanovic Z, Heitmann BL, Keiding N, Ottesen B, Pedersen AT. The association between early menopause and risk of ischaemic heart disease: Influence of hormone therapy. Maturitas. 2006;53:226–233 [DOI] [PubMed] [Google Scholar]
- 170.Parker WH, Broder MS, Chang E, Feskanich D, Farquhar C, Liu Z, Shoupe D, Berek JS, Hankinson S, Manson JE. Ovarian conservation at the time of hysterectomy and long-term health outcomes in the nurses’ health study. Obstet Gynecol. 2009;113:1027–1037 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 171.Gold EB, Colvin A, Avis N, Bromberger J, Greendale GA, Powell L, Sternfeld B, Mattews K. Longitudinal analysis of the association between vasomotor symptoms and race/ethnicity across the menopausal transition: Study of women’s health across the nation. Am J Public Health. 2006;96:1226–1235 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 172.Avis NE, Crawford SL, Greendale G, Bromberger JT, Everson-Rose SA, Gold EB, Hess R, Joffee H, Kravitz HM, Tepper PG, et al. Duration of menopausal vasomotor symptoms over the menopause transition. JAMA Intern Med. 2015;175:531–539 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 173.Franco OH, Muka T, Colpani V, Kunutsor S, Chowdhury S, Chowdhury R, Kavousi M. Vasomotor symptoms in women and cardiovascular risk markers: Systematic review and meta-analysis. Maturitas. 2015;81:353–361 [DOI] [PubMed] [Google Scholar]
- 174.Thurston RC, El Khoudary SR, Sutton-Tyrrell K, Crandall CJ, Gold EB, Sternfeld B, Joffe H, Selzer F, Matthews KA. Vasomotor symptoms and lipid profiles in women transitioning through menopause. Obstet Gynecol. 2012;119:753–761 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 175.Jackson EA, El Khoudary SR, Crawford SL, Matthews K, Joffe H, Chae C, Thurston RC. Hot flash frequency and blood pressure: Data from the study of women’s health across the nation. J Womens Health (Larchmt). 2016;25:1204–1209 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 176.Herber-Gast GC, Mishra GD. Early severe vasomotor menopausal symptoms are associated with diabetes. Menopause. 2014;21:855–860 [DOI] [PubMed] [Google Scholar]
- 177.Thurston RC, Sutton-Tyrrell K, Everson-Rose SA, Hess R, Matthews KA. Hot flashes and subclinical cardiovascular disease: Findings from the study of women’s health across the nation heart study. Circulation. 2008;118:1234–1240 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 178.Thurston RC, Sutton-Tyrrell K, Everson-Rose SA, Hess R, Powell LH, Matthews KA. Hot flashes and carotid intima media thickness among midlife women. Menopause. 2011;18:352–358 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 179.Thurston RC, El Khoudary SR, Tepper PG, Jackson EA, Joffe H, Chen HY, et al. Trajectories of vasomotor symptoms and carotid intima media thickness in the study of women’s health across the nation. Stroke. 2016;47:12–17 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 180.Thurston RC, Aslanidou Vlachos HE, Derby CA, Jackson EA, Brooks MM, Matthews KA, Appendix, Harlow S, Sowers M, Finkelsetein J, et al. Menopausal vasomotor symptoms and risk of incident cardiovascular disease events in swan. J Am Heart Assoc. 2021;10:e017416. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 181.Gast GC, Pop VJ, Samsioe GN, Grobbee DE, Nilsson PM, Keyzer JJ, Wijnands-van Gent CJM, van der Schouw YT. Vasomotor menopausal symptoms are associated with increased risk of coronary heart disease. Menopause. 2011;18:146–151 [DOI] [PubMed] [Google Scholar]
- 182.Zhu D, Chung HF, Dobson AJ, Pandeya N, Anderson DJ, Kuh D, Hardy R, Brunner EJ, Avis NE, Gold EB, et al. Vasomotor menopausal symptoms and risk of cardiovascular disease: A pooled analysis of six prospective studies. Am J Obstet Gynecol. 2020;223:898.e891–898.e816 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 183.Grodstein F, Stampfer MJ, Manson JE, Colditz GA, Willett WC, Rosner B, Speizer FE, Hennekens CH. Postmenopausal estrogen and progestin use and the risk of cardiovascular disease. N Engl J Med. 1996;335:453–461 [DOI] [PubMed] [Google Scholar]
- 184.Grodstein F, Manson JE, Colditz GA, Willett WC, Speizer FE, Stampfer MJ. A prospective, observational study of postmenopausal hormone therapy and primary prevention of cardiovascular disease. Ann Intern Med. 2000;133:933–941 [DOI] [PubMed] [Google Scholar]
- 185.Rossouw JE, Anderson GL, Prentice RL, LaCroix AZ, Kooperberg C, Stefanick ML, Jackson RD, Beresford SAA, Howard BV, Johnson KC, et al. Risks and benefits of estrogen plus progestin in healthy postmenopausal women: Principal results from the women’s health initiative randomized controlled trial. Jama. 2002;288:321–333 [DOI] [PubMed] [Google Scholar]
- 186.Anderson GL, Limacher M, Assaf AR, Bassford T, Beresford SA, Black H, Bonds D, Brunner R, Brzyski R, Caan B, et al. Effects of conjugated equine estrogen in postmenopausal women with hysterectomy: The women’s health initiative randomized controlled trial. Jama. 2004;291:1701–1712 [DOI] [PubMed] [Google Scholar]
- 187.Boardman HM, Hartley L, Eisinga A, Main C, Roque i Figuls M, Bonfill Cosp X, Gabriel Sanchez R, Knight B. Hormone therapy for preventing cardiovascular disease in post-menopausal women. Cochrane Database Syst Rev. 2015:CD002229. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 188.Manson JE, Allison MA, Rossouw JE, Carr JJ, Langer RD, Hsia J, Kuller LH, Cochrane BB, Hunt JR, Ludlam SE, et al. Estrogen therapy and coronary-artery calcification. N Engl J Med. 2007;356:2591–2602 [DOI] [PubMed] [Google Scholar]
- 189.Manson JE, Aragaki AK, Bassuk SS, Chlebowski RT, Anderson GL, Rossouw JE, Howard BV, Thomson CA, Stefanick ML, Kaunitz AM, et al. Menopausal estrogen-alone therapy and health outcomes in women with and without bilateral oophorectomy: A randomized trial. Ann Intern Med. 2019;171:406–414 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 190.Harman SM, Black DM, Naftolin F, Brinton EA, Budoff MJ, Cedars MI, Hopkins PN, Lobo RA, Manson JE, Merriam GR, et al. Arterial imaging outcomes and cardiovascular risk factors in recently menopausal women: A randomized trial. Ann Intern Med. 2014;161:249–260 [DOI] [PubMed] [Google Scholar]
- 191.Hodis HN, Mack WJ, Henderson VW, Shoupe D, Budoff MJ, Hwang-Levine J, Li Y, Feng M, Dustin L, Kono N, et al. Vascular effects of early versus late postmenopausal treatment with estradiol. New England Journal of Medicine. 2016;374:1221–1231 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 192.Renoux C, Dell’aniello S, Garbe E, Suissa S. Transdermal and oral hormone replacement therapy and the risk of stroke: A nested case-control study. BMJ. 2010;340:c2519. [DOI] [PubMed] [Google Scholar]
- 193.Canonico M, Oger E, Plu-Bureau G, Conard J, Meyer G, Levesque H, Trillot N, Barrellier M-T, Wahl D, Emmerich K, et al. Hormone therapy and venous thromboembolism among postmenopausal women: Impact of the route of estrogen administration and progestogens: The esther study. Circulation. 2007;115:840–845 [DOI] [PubMed] [Google Scholar]
- 194.The 2017 hormone therapy position statement of the north american menopause society. Menopause. 2018;25:1362–1387 [DOI] [PubMed] [Google Scholar]
- 195.Acog practice bulletin no. 194: Polycystic ovary syndrome. Obstet Gynecol. 2018;131:e157–e171 [DOI] [PubMed] [Google Scholar]
- 196.Teede HJ, Misso ML, Costello MF, Dokras A, Laven J, Moran L, Piltonen T, Norman RJ. Recommendations from the international evidence-based guideline for the assessment and management of polycystic ovary syndrome. Hum Reprod. 2018;33:1602–1618 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 197.Parikh NI, Gonzalez JM, Anderson CAM, Judd SE, Rexrode KM, Hlatky MA, Gunderson EP, Stuart JJ, Vaidya D. Adverse pregnancy outcomes and cardiovascular disease risk: Unique opportunities for cardiovascular disease prevention in women: A scientific statement from the american heart association. Circulation. 2021;143:e902–e916 [DOI] [PubMed] [Google Scholar]
- 198.USPSTF, Davidson KW, Barry MJ, Mangione CM, Cabana M, Caughey AB, Davis EM, Donahue KE, Doubeni CA, Kubik M, et al. Aspirin use to prevent preeclampsia and related morbidity and mortality: Us preventive services task force recommendation statement. JAMA. 2021;326:1186–1191 [DOI] [PubMed] [Google Scholar]
- 199.Elder P, Sharma G, Gulati M, Michos ED. Identification of female-specific risk enhancers throughout the lifespan of women to improve cardiovascular disease prevention. Am J Prev Cardiol. 2020;2:100028. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 200.Kavousi M, Desai CS, Ayers C, Blumenthal RS, Budoff MJ, Mahabadi AA, Ikram MA, van der Lugt A, Hofman A, Erbel R, et al. Prevalence and prognostic implications of coronary artery calcification in low-risk women: A meta-analysis. JAMA. 2016;316:2126–2134 [DOI] [PubMed] [Google Scholar]
- 201.Shaw LJ, Pepine CJ, Xie J, Mehta PK, Morris AA, Dickert NW, Ferdinand KC, GUlati M, Reynolds H, Hayes SN, et al. Quality and equitable health care gaps for women: Attributions to sex differences in cardiovascular medicine. J Am Coll Cardiol. 2017;70:373–388 [DOI] [PubMed] [Google Scholar]
- 202.Brown HL, Warner JJ, Gianos E, Gulati M, Hill AJ, Hollier LM, Rosen SE, Rosser ML, Wenger NK. Promoting risk identification and reduction of cardiovascular disease in women through collaboration with obstetricians and gynecologists: A presidential advisory from the american heart association and the american college of obstetricians and gynecologists. Circulation. 2018;137:e843–e852 [DOI] [PubMed] [Google Scholar]
- 203.Bairey Merz CN, Andersen H, Sprague E, Burns A, Keida M, Walsh MN, Greenberger P, Campbell S, Pollin I, McCullough C, et al. Knowledge, attitudes, and beliefs regarding cardiovascular disease in women: The women’s heart alliance. J Am Coll Cardiol. 2017;70:123–132 [DOI] [PubMed] [Google Scholar]
- 204.Hus 2018 trend tables—table 26: Normal weight, overweight, and obesity among adults aged 20 and over, by selected characteristics: United states, selected years 1988-1994 through 2013–2016.
- 205.Benjamin EJ, Muntner P, Alonso A, Bittencourt MS, Callaway CW, Carson AP, Chamberlain AM, Chang AR, Chen S, Das SR, et al. Heart disease and stroke statistics-2019 update: A report from the american heart association. Circulation. 2019;139:e56–e528 [DOI] [PubMed] [Google Scholar]
- 206.McDonnell LA, Pipe AL, Westcott C, Perron S, Younger-Lewis D, Elias N, Nooyen J, Reid RD. Perceived vs actual knowledge and risk of heart disease in women: Findings from a canadian survey on heart health awareness, attitudes, and lifestyle. Can J Cardiol. 2014;30:827–834 [DOI] [PubMed] [Google Scholar]
- 207.Valle JA, Ho PM. Medication adherence in secondary prevention post-myocardial infarction. Curr Treat Options Cardiovasc Med. 2014;16:349. [DOI] [PubMed] [Google Scholar]
- 208.Spoletini I, Ferrari R, Rosano GMC. Living with stable angina: Patients’ pathway and needs in angina. J Cardiovasc Med (Hagerstown). 2020;21:377–382 [DOI] [PubMed] [Google Scholar]
- 209.Force CDiPT. Improving health care response to cardiovascular disease in pregnancy and postpartum. 2017
- 210.Cusimano MC, Pudwell J, Roddy M, Cho CK, Smith GN. The maternal health clinic: An initiative for cardiovascular risk identification in women with pregnancy-related complications. Am J Obstet Gynecol. 2014;210:438 e431–439 [DOI] [PubMed] [Google Scholar]
- 211.Jowell AR SA, Gulati M, Michos ED, Vaught AJ, Natarajan P, Powe CE, Honigberg MC. Interventions to mitigate risk of cardiovascular disease after adverse pregnancy outcomes: A review. JAMA Cardiol. 2021 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 212.Plante TB, O’Kelly AC, Urrea B, MacFarlane ZT, Blumenthal RS, Charleston J, Miller ER, Appel LJ, Martin SS. User experience of instant blood pressure: Exploring reasons for the popularity of an inaccurate mobile health app. NPJ Digit Med. 2018;1:31. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 213.Rich-Edwards JW, Stuart JJ, Skurnik G, Roche AT, Tsigas E, Fitzmaurice GM, Wilkins-Haug LE, Levkoff SE, Seeley EW. Randomized trial to reduce cardiovascular risk in women with recent preeclampsia. J Womens Health (Larchmt). 2019;28:1493–1504 [DOI] [PubMed] [Google Scholar]
- 214.Sharma G, Zakaria S, Michos ED, Bhatt AB, Lundberg GP, Florio KL, Vaught AJ, Ouyang P, Mehta L. Improving cardiovascular workforce competencies in cardio-obstetrics: Current challenges and future directions. J Am Heart Assoc. 2020;9:e015569. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 215.O’Kelly AC, Scott N, DeFaria Yeh D. Delivering coordinated cardio-obstetric care from preconception through postpartum. Cardiol Clin. 2021;39:163–173 [DOI] [PubMed] [Google Scholar]
- 216.Gladstone RA, Pudwell J, Nerenberg KA, Grover SA, Smith GN. Cardiovascular risk assessment and follow-up of women after hypertensive disorders of pregnancy:A prospective cohort study. J Obstet Gynaecol Can. 2019;41:1157–1167 e1151 [DOI] [PubMed] [Google Scholar]
- 217.Timpka S, Fraser A, Schyman T, Stuart JJ, Asvold BO, Mogren I, Franks PW, Rich-Edwards JW. The value of pregnancy complication history for 10-year cardiovascular disease risk prediction in middle-aged women. Eur J Epidemiol. 2018;33:1003–1010 [DOI] [PMC free article] [PubMed] [Google Scholar]


