Abstract
The Lyme disease-associated spirochete, Borrelia burgdorferi, is maintained in enzootic cycles involving Ixodes ticks and small mammals. Previous studies demonstrated that B. burgdorferi expresses outer surface protein A (OspA) but not OspC when residing in the midgut of unfed ticks. However, after ticks feed on blood, some spirochetes stop making OspA and express OspC. Our current work examined the timing and frequency of OspA and OspC expression by B. burgdorferi in infected Ixodes scapularis nymphs as they fed on uninfected mice and in uninfected I. scapularis larvae and nymphs as they first acquired spirochetes from infected mice. Smears of midguts from previously infected ticks were prepared at 12- or 24-h intervals following attachment through repletion at 96 h, and spirochetes were stained for immunofluorescence for detection of antibodies to OspA and OspC. As shown previously, prior to feeding spirochetes in nymphs expressed OspA but not OspC. During nymphal feeding, however, the proportion of spirochetes expressing OspA decreased, while spirochetes expressing OspC became detectable. In fact, spirochetes rapidly began to express OspC, with the greatest proportion of spirochetes having this protein at 48 h of attachment and then with the proportion decreasing significantly by the time that the ticks had completed feeding. In vitro cultivation of the spirochete at different temperatures showed OspC to be most abundant when the spirochetes were grown at 37°C. Yet, the synthesis of this protein waned with continuous passage at this temperature. Immunofluorescence staining of spirochetes in smears of midguts from larvae and nymphs still attached or having completed feeding on infected mice demonstrated that OspA but not OspC was produced by these spirochetes recently acquired from mice. Therefore, the temporal synthesis of OspC by spirochetes only in feeding ticks that were infected prior to the blood meal suggests that this surface protein is involved in transmission from tick to mammal but not from mammal to tick.
The spirochetal etiologic agent of Lyme borreliosis, Borrelia burgdorferi, is maintained in nature by alternating infections in tick and mammalian hosts, with transmission between them occurring during tick attachment and feeding. While the diversity of spirochetes, ticks, and mammals that maintain zoonotic foci in many regions of the world is appearing to be more complex, in the areas of the northern Midwest and northeastern United States where Lyme disease is highly endemic, the essential biologic factors for Lyme disease include B. burgdorferi sensu stricto, black-legged or deer ticks (Ixodes scapularis), white-footed mice (Peromyscus leucopus), whitetail deer (Odocoileus virginianus), and human beings. White-footed mice serve as both the reservoir for the bacterium and the host for larval and nymphal I. scapularis (13). Deer are the primary host for adult I. scapularis. Therefore, these animals are extremely important as a means of support of tick populations, although these large mammals play no significant part in perpetuating spirochetes. Given that these ticks typically feed only once in each stage, transstadial transmission of spirochetes in ticks, when the infection is maintained through the tick's molt, is essential for the maintenance of the bacterium in nature. However, transovarial transmission of spirochetes, when the infected female tick passes the infection on to the subsequent generation of larvae, is extremely rare, at least in North America (25, 34), and therefore is of no consequence for the maintenance of spirochetes. These various factors demonstrate that the primary chain of infection that perpetuates the spirochete is the passage of spirochetes to uninfected larvae by infected mice. The infected larvae then remain infected through the molt to the nymphal stage, and the infected nymphs pass the spirochetes back to mice during the next blood meal. Infected nymphs are also the primary vector for passage of the spirochete to humans when they inadvertently replace mice as hosts.
Alternating infection between ticks and mammals imposes two contrasting environments upon the spirochetes. Survival is complicated by the changes occurring in ticks during feeding, the digestion of blood, physiological changes associated with molting and quiescence, and immunological pressure mounted by mammals during infection in them. The plasticity displayed in some of the spirochete's outer surface proteins (Osps) during cultivation in vitro now appears to be indicative of adaptations by the spirochete to changing hosts and altered conditions within hosts (40). Previous studies in our laboratories (44) and elsewhere (9, 10, 12) have shown that two of the spirochete's Osps, OspA and OspC, are differentially expressed depending on whether the infected tick host has recently completed feeding on blood. In the study described in the present report, we examined the temporal presence of OspA and OspC on spirochetes in ticks during and after feeding, including ticks that were infected during a previous blood meal and ticks that acquired spirochetes for the first time.
MATERIALS AND METHODS
Spirochetal isolates and cultivation.
B. burgdorferi B31 and Sh-2-82 originated from I. scapularis ticks collected on Shelter Island, N.Y. (7, 41). B. burgdorferi 4A was cloned by limiting dilution from Sh-2-82. Spirochetes were routinely cultivated in BSK-II medium at 33°C. However, for one experiment incubations ranged from 28 to 41°C to examine the influence of temperature on the spirochetal synthesis of OspC.
Tick feeding and infections.
I. scapularis ticks were from infected and uninfected laboratory colonies maintained at both the Division of Vector-Borne Infectious Diseases, National Center for Infectious Diseases, Centers for Disease Control and Prevention, Fort Collins, Colo., and the Rocky Mountain Laboratories, National Institute of Allergy and Infectious Diseases, National Institutes of Health, Hamilton, Mont. The length of time required for larvae and nymphs to feed to repletion and drop off white-footed mice was determined by placing the ticks on mice in cages over pans of water at 1600 h of day 0. At 0800 and 1600 h of each successive day, the pans were examined and all engorged ticks were removed and counted. The ticks were fed in a room with 12 h of light and 12 h of dark; the light came on at 0700 h and went off at 1900 h.
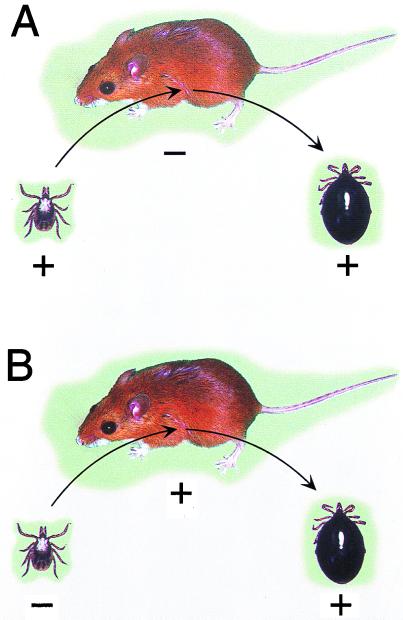
B. burgdorferi B31 (uncloned) was examined in ticks that had been infected at two times (Fig. 1). First, the nymphal ticks were infected previously as larvae on white mice or white-footed mice that had been infected by the bite of infected nymphs as described previously (31). These engorged larvae were allowed to molt and were then examined for spirochetes just prior to, during, and after feeding. Second, previously uninfected larvae and nymphs were examined during and after feeding on white-footed mice infected by tick bite. This allowed us to examine the spirochetes already present in ticks at the time of their feeding (Fig. 1A) and to compare them to the spirochetes that had only recently been acquired by ticks during the same blood meal (Fig. 1B).
FIG. 1.
These diagrams depict the two transmission events examined. (A) An infected nymph (+) feeding on an uninfected white-footed mouse (−). Prior to, during, and after feeding, the spirochetes in the ticks' midguts were examined for expression of OspA and OspC. (B) An uninfected nymph (−) feeding on an infected white-footed mouse (+). The spirochetes that were acquired during tick feeding and that were in the ticks' midguts were examined for expression of OspA and OspC.
Tick dissection and immunofluorescence staining of spirochetes.
Ticks were removed from mice at 12- or 24-h intervals after attachment or were collected from the water under the cages after they dropped off the mice. The midgut was removed from the ticks and was placed in a 10-μl drop of phosphate-buffered saline (PBS) on a glass microscope slide. The organ was teased apart and was smeared with microforceps while viewed with a dissecting microscope. The smears were allowed to dry at room temperature and were then fixed in acetone for 10 to 15 min just prior to antibody staining. Spirochetes in tick smears were examined by indirect fluorescent-antibody (IFA) or direct fluorescent-antibody (DFA) staining with one or two antibodies. These included a hyperimmune rabbit anti-B. burgdorferi Sh-2-82 antiserum (1:250 dilution) for detection of entire spirochetes, a hyperimmune rabbit anti-OspC antiserum (1:50 dilution) to OspC of B. burgdorferi Sh-2-82, mouse monoclonal antibody H5332 (1:25 dilution) to OspA (4), mouse monoclonal antibody H9724 to flagellin (3), and mouse monoclonal antibody B5 to OspC of B. burgdorferi B31 (26). Secondary antibodies were goat anti-rabbit immunoglobulin G (IgG; heavy and light chains) labeled with fluorescein isothiocyanate (FITC) and rhodamine isothiocyanate (RITC) and goat anti-mouse IgG (heavy and light chains) labeled with FITC and RITC (Kirkegaard & Perry Laboratories, Inc., Gaithersburg, Md.). DFA was performed with a 1:25 dilution of hyperimmune serum conjugated with FITC. The hyperimmune serum had been produced in a rabbit immunized with B. burgdorferi Guilford (32). Fixed smears of the infected tick tissues were immersed with antiserum, and the mixture was incubated at 37°C for 30 to 45 min, washed in PBS, dried, immersed with the appropriate FITC- or RITC-labeled secondary antibody, washed in PBS, dried, and covered with glycerol in PBS and a glass coverslip. For double labeling of the spirochetes, the same procedure was repeated with different primary and labeled secondary antibodies. Smears were examined with a Zeiss or a Nikon epifluorescence microscope at ×400 magnification with fluorescein or rhodamine emission filters to detect spirochetes and determine the presence of OspA or OspC. Total spirochetes were counted in selected larvae and nymphs at 24 to 96 h after attachment by viewing the entire smears with successive fields at ×400 magnification.
SDS-PAGE and immunoblot analysis.
The numbers of spirochetes examined were standardized by optical density determination. Whole-cell lysates of spirochetes were electrophoresed by sodium dodecyl sulfate-polyacrylamide gel electrophoresis (SDS-PAGE) in a 12.5% gel by using the buffer described by Laemmli (24) and a vertical gel electrophoresis system (Bethesda Research Laboratories-GIBCO, Gaithersburg, Md.). Proteins were visualized by staining with Coomassie brilliant blue. After electrophoresis, whole-cell lysates were blotted onto a nitrocellulose membrane with the buffer system described by Towbin et al. (46) and a Trans-Blot Cell (Bio-Rad Laboratories, Hercules, Calif.) by following the instructions of the manufacturer. The membrane was blocked overnight at room temperature with TSE-Tween (50 mM Tris [pH 7.4], 150 mM NaCl, 5 mM EDTA, 0.05% Tween 20) and was incubated with rabbit anti-OspC antiserum, and bound antibodies were detected by 125I-labeled protein A autoradiography.
RESULTS
Spirochetes producing OspA and OspC in ticks infected during a previous feeding.
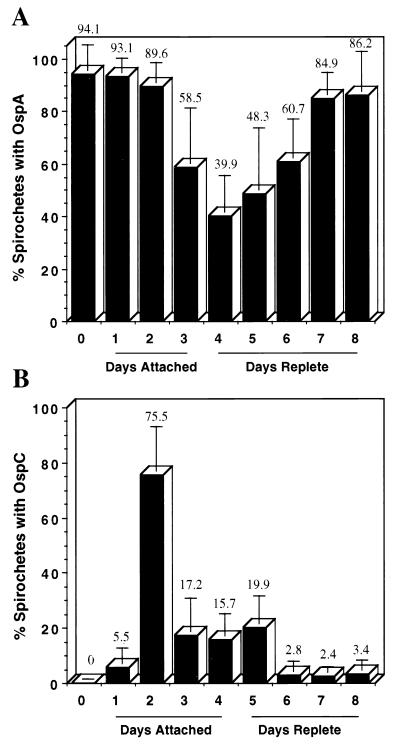
To determine the proportion of spirochetes with OspA in ticks, smears of the midgut from 6 to 12 nymphal I. scapularis ticks infected previously as larvae were examined by double fluorescence staining at 24-h intervals, beginning just prior to attachment and continuing through feeding and detachment. The midguts from a total of 77 infected nymphs were examined from 0 to 192 h (8 days) after attachment, and spirochetes were stained with the rabbit anti-B. burgdorferi antiserum plus anti-rabbit RITC and mouse anti-OspA monoclonal antibody plus anti-mouse FITC. During each time period, 152 to 458 spirochetes were examined, for a total of 2,475 spirochetes for all intervals. While nearly all spirochetes had OspA just prior to tick attachment to mice, the proportion dropped to a low of just under 40% at 96 h after attachment and increased to 86% at 192 h (8 days), when the last examination was done (Fig. 2A). This demonstrates that during tick feeding, the proportion of B. burgdorferi spirochetes with OspA decreased dramatically but increased again after ticks had engorged and dropped off the mice.
FIG. 2.
Proportion of B. burgdorferi expressing OspA or OspC in previously infected nymphal I. scapularis ticks during attachment and feeding (as depicted in Fig. 1A). In the two separate experiments, the midguts from unfed (day 0), attached (days attached), and fully engorged (days replete) nymphal I. scapularis ticks infected prior to feeding were examined by double immunofluorescence staining to determine the percentage of spirochetes expressing OspA (A) or OspC (B). The number above each column is the mean percentage for all spirochetes examined on that sampling day. The vertical error bar represents 1 standard deviation of the mean. As shown, the spirochetes in the tick midgut displayed dramatic temporal regulation of both proteins associated with tick attachment and feeding.
To determine when spirochetes synthesize OspC in ticks, a preliminary experiment was performed. The midguts from a total of 72 nymphal I. scapularis ticks infected previously as larvae were examined from 0 to 168 h (7 days, 8 to 10 ticks per day) after attachment by staining with the rabbit anti-OspC antiserum and anti-rabbit RITC. No spirochetes were detected just prior to or at 12 h after tick attachment to mice. However, by 24 h spirochetes were detected by staining for OspC, and the number was highest at 48 h after attachment, decreasing significantly as tick feeding proceeded. We then performed a second experiment to determine the proportion of spirochetes containing OspC prior to, during, and after tick attachment. A new series of infected nymphs was examined at 24-h intervals by using a monoclonal antibody to OspC (B5) and a rabbit FITC-labeled serum hyperimmune to the entire spirochete. The midguts from 82 nymphs were dissected on 9 successive days (8 to 10 ticks per day), and 2,382 spirochetes were examined for OspC. Again, no spirochetes were found to have OspC before attachment, but by 48 h, 75% of the spirochetes were positive for the protein, followed by a drop during the ensuing days (Fig. 2B). These two data sets confirmed our previous observation that during tick feeding, B. burgdorferi up-regulates OspC (44), yet they demonstrated that the presence of this protein is short-lived and is most prevalent before the ticks have completed feeding. No nymphs completed feeding within 2 days of attachment, and those ticks removed from mice during the first 48 h had in their midguts relatively little fresh blood detectable by microscopy. The majority of nymphs (59% of 580 nymphs) completed feeding during day 4 of attachment, although a few required up to 7 days to fully engorge and drop from the mice.
Spirochetes producing OspA or OspC following their acquisition by ticks during feeding.
To determine whether OspA was present on spirochetes in ticks that had just acquired them during feeding, uninfected nymphs were allowed to attach to white-footed mice that had previously been infected by tick bite. Ticks were removed at 24 and 48 h after attachment by dislodging them with small forceps and pulling them off the mice. The midguts from 16 nymphs were prepared as described above and were stained with the mouse anti-OspA monoclonal antibody, followed by staining with anti-mouse FITC. The entire smear from each midgut was examined, and all spirochetes were counted. Although little or no blood was detected by microscopy in the midguts of these nymphs, 94% (15 of 16) of these ticks were already infected. Staining for only OspA detected 3,407 spirochetes in these nymphs early in the course of feeding, with the number increasing during the second 24 h of attachment (Fig. 3). The midguts from larvae were also examined for spirochetes by staining for OspA after the ticks had completed feeding at 72 h (six ticks) and 96 h (two ticks). At 72 h, the mean number of spirochetes detected was 280 per larva (range, 103 to 578), while at 96 h, 285 and 1,175 spirochetes were seen in the two ticks, respectively.
FIG. 3.
The rapid acquisition of B. burgdorferi by nymphal I. scapularis ticks during attachment and early feeding on infected mice and the early presence of OspA (as depicted in Fig. 1B). The midguts from nymphal ticks were examined from ticks pulled off mice at 24 h (n = 11 ticks) and 48 h (n = 5 ticks) after attachment. At 24 h, 10 of 11 ticks were already infected, while at 48 h, all 5 ticks were infected. The number of spirochetes was determined by IFA with anti-OspA monoclonal antibody H5332. The mean number of spirochetes detected in all ticks at both times is shown above the column, and the vertical error bar represents 1 standard deviation of the mean.
The spirochetes were clearly expressing OspA in both larval and nymphal ticks that had recently been infected, including after only 24 h of tick attachment. However, any spirochetes that did not have this protein would not be detected. Therefore, we took smears of midguts from five ticks that were pulled at 24 h and that had previously been stained for OspA, gently rinsed the smears, and stained the spirochetes for flagellin with the mouse monoclonal antibody H9724, followed by staining with anti-mouse FITC. Since there is no evidence that the amount of flagellin varies, staining for this protein should detect spirochetes that may have been missed when staining for OspA was done. The entire smears were examined again, and all spirochetes were counted; the total was 236, whereas the total was 275 when the spirochetes were counted the first time. In all smears the number of spirochetes was less than that in the previous count. Thus, demonstration that spirochetes did not express OspA was not possible by this technique. To directly examine the proportion of spirochetes containing OspA, the double staining of spirochetes in the midguts of two larvae was performed immediately after they dropped off the mice at 72 h; 159 and 245 spirochetes that expressed OspA were observed, respectively, while no spirochetes that lacked this protein were detectable.
To determine whether OspC was present on spirochetes in ticks that had just acquired them during feeding, uninfected larvae were first allowed to feed on white-footed mice infected previously by tick bite. Ticks were allowed to feed to repletion and at 72 h were collected from the water beneath the cages after they dropped off the mice. The midguts from 13 larvae were prepared as described above and were stained with the mouse anti-OspA monoclonal antibody and the rabbit anti-OspC antiserum. Eight of the smears were secondarily stained with anti-mouse FITC and anti-rabbit RITC, while five smears were secondarily stained with anti-mouse RITC and anti-rabbit FITC. The entire smear of each midgut was examined and showed that 12 of 13 larvae (92%) had become infected. In each positive smear, 50 to 463 spirochetes were examined by using both emission filters. Regardless of the combination of antibodies and fluorescent labels, spirochetes were readily observed when they were stained for OspA, but no spirochetes were seen when staining with the anti-OspC antiserum was done. This was strikingly different than what we observed for spirochetes in engorged ticks that had been infected prior to the blood meal.
Influence of temperature and continuous in vitro growth on presence of OspC.
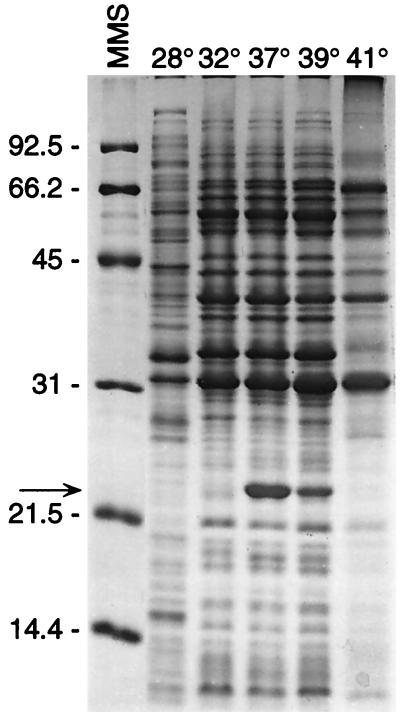
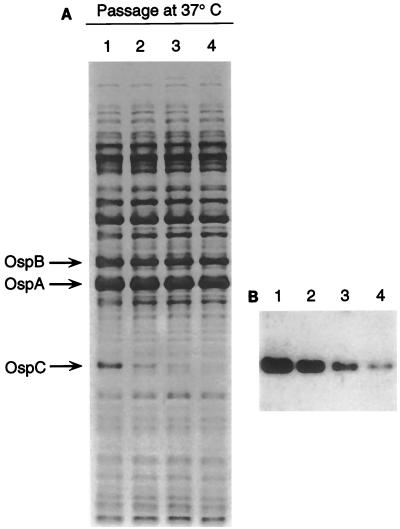
We extended our earlier work, which demonstrated that OspC of B. burgdorferi B31 and JD-1 isolated from ticks was up-regulated at 37°C (44), by examining a cloned isolate (isolate 4A) of Sh-2-82 grown at five different temperatures ranging from 28 to 41°C. The optical densities determined for each culture demonstrated that spirochetes grew fastest at 37°C (data not shown). Additionally, electrophoretic profiles of whole-cell lysates demonstrated that the amount of OspC present was greatest in spirochetes grown at this temperature (Fig. 4). At 41°C, the spirochetes did not survive beyond 24 h and many of the proteins appeared to have been degraded. Continued growth of B. burgdorferi B31 at 37°C also demonstrated that immediately after being elevated to this temperature, the amount of OspC decreased significantly during each of four successive passages (Fig. 5) and paralleled, although more slowly, what we had observed as described above for the proportion of spirochetes that expressed OspC during the feeding of infected ticks.
FIG. 4.
Influence of different growth temperatures on the in vitro synthesis of OspC by B. burgdorferi. Spirochetes were grown in BSK-II medium to the stationary phase at each of the indicated temperatures. Whole-cell lysates of the spirochetes were examined by SDS-PAGE with proteins stained with Coomassie brilliant blue. Molecular mass standards (MMS) are shown at the left (in kilodaltons). OspC, shown to the right of the arrow, is most abundant in the culture grown at 37°C.
FIG. 5.
Influence of continued in vitro cultivation of B. burgdorferi at 37°C on the presence of OspC. Spirochetes were grown to the stationary phase in BSK-II medium and were passaged three consecutive times. Whole-cell lysates of the spirochetes from each passage were examined by SDS-PAGE with proteins stained with Coomassie brilliant blue (A). Immunoblot analysis with rabbit anti-OspC antiserum (B) confirmed that the protein down-regulated during passage was OspC.
DISCUSSION
Previously, we and others demonstrated that spirochetes in the midgut of unfed ticks produce OspA (4, 11, 44), yet despite its immunogenicity (14), humans and other animals infected with B. burgdorferi by tick bite only rarely produce antibodies to this protein following infection (20, 22, 44). This suggested that OspA may be down-regulated when spirochetes are transmitted from ticks to mammals, and in fact, several additional lines of evidence support this hypothesis. We first noticed that some spirochetes in the midguts of engorged ticks do not express OspA (44), while other investigators have since observed the down-regulation of OspA in infected ticks during feeding (11) and the apparent lack of OspA on spirochetes having disseminated from the midgut to the tick's salivary glands (11). Obonyo et al. (28) recently demonstrated that the amount of OspA relative to the amount of flagellin decreased in two isolates of B. burgdorferi when the spirochetes were cocultivated with tick cells at 37°C compared to the amounts when the spirochetes and tick cells were cocultivated at 31°C. Barthold et al. (5) also demonstrated very nicely that mice immunized with OspA were not protected from infection when they were challenged with implants of skin from infected mice, suggesting that these “host-adapted” spirochetes no longer produced OspA. Montgomery et al. (27) demonstrated that at 30 days after cultured spirochetes were inoculated into mice, OspA could not be detected by either reverse transcriptase PCR or immunofluorescence staining of the few spirochetes recovered by peritoneal lavage. Two studies have demonstrated through the use of reverse transcriptase PCR and spirochetes grown in mouse-implanted dialysis membrane chambers that OspA is down-regulated by Lyme disease-associated spirochetes during mammalian infection (2, 15). In our present study, we also demonstrated that B. burgdorferi already present in nymphal I. scapularis ticks prior to tick feeding down-regulated OspA during and shortly after the ticks had fed. The lowest proportion of spirochetes expressing OspA occurred during days 3 and 4 (72 and 96 h after attachment), which corresponded to the time when approximately 90% of nymphs rapidly engorged and completed feeding.
The synthesis of OspC by spirochetes in ticks and mammals, however, appears to be the opposite of the synthesis of OspA, although the former is less studied. We observed previously that while spirochetes did not produce OspC in unfed ticks, an undetermined proportion of spirochetes in ticks had synthesized this protein when the spirochetes were examined immediately following tick engorgement (44). This observation has since been confirmed by Coleman et al. (9). Fingerle et al. (17) examined 472 field-collected, unfed I. ricinus ticks in Germany and detected spirochetes in only 1 tick by IFA staining with an anti-OspC monoclonal antibody, supporting our observations concerning the lack of expression of OspC by spirochetes in unfed ticks. Yet, the early antibody response to OspC in humans and mice following spirochetal infection by tick bite, unlike that which is lacking for OspA, supports the hypothesis that OspC is expressed by spirochetes in mammals, at least during the initial infection following their delivery in tick saliva (1, 18, 44). In our present study, we observed that the presence of OspC on B. burgdorferi in ticks already infected prior to feeding was short-lived and peaked rapidly after only 2 days of nymphal attachment, before these ticks had begun to ingest significant amounts of blood. After 48 h, the proportion of spirochetes with OspC decreased quickly during the next several days. This ephemeral existence of OspC on spirochetes in the tick midgut during the later stage of tick feeding suggests that this Osp is required for the successful dissemination and transmission of these bacteria from the ticks to mammals.
When we examined spirochetes in ticks that had just acquired them by feeding on infected mice, differences in the presence of OspA and OspC compared to the situation described above were observed. First, Lyme disease-associated spirochetes were rapidly acquired by nymphal ticks during the first 24 h of attachment, even before any significant amounts of blood were detectable by light microscopy of the dissected midguts. The acquisition of spirochetes by larval ticks during their first 24 to 48 h of attachment on infected mice has been observed previously (10, 30; Y. Nakayama and A. Spielman, Letter, J. Infect. Dis. 160:166–167, 1989). Our immunofluorescence staining of these spirochetes for OspA demonstrated that essentially all spirochetes in ticks that had attached for no more than 24 h contained this protein, confirming the work of de Silva et al. (10). Given that OspA is not usually produced by spirochetes in mice, OspA must be up-regulated just before or very soon after spirochete transfer from mice to ticks. Second, OspC was not detected on spirochetes that had recently transferred from mice to either engorged larvae or nymphs. Therefore, the stimuli for spirochetes to produce OspC in the midguts of engorged ticks apparently differ, depending on whether these bacteria are infecting ticks or mammals prior to tick feeding. For spirochetes infecting ticks prior to the blood meal, tick attachment and feeding result in dramatic increases in temperature, nutrient levels, and spirochetal density (33); under these conditions spirochetes briefly produce OspC. The synthesis of OspC is influenced by a change in temperature when the spirochetes are grown in vitro (44, 45), spirochete density (12), growth phase (37), and changes in environmental pH (8). Host serum complement may also be involved by selectively lysing spirochetes that are expressing different outer surface proteins (23). Ryan et al. (39) have also demonstrated alternating antigenic profiles, including different forms of OspC, for one strain of B. burgdorferi as it cycled between ticks and mice. For spirochetes that infect mice but that transfer to ticks, feeding brings spirochetes into the blood meal but with no increase in temperature; under these conditions spirochetes do not produce OspC. We believe that this difference also supports our hypothesis that OspC is essential for the spirochete's transmission from tick to mammal but not from mammal to tick. The relapsing fever-associated spirochete, Borrelia hermsii, also produces an OspC homolog, Vmp33, at the time when it is transmitted from tick to mammal but not when it is transferred from mammal to tick (42). This difference in spirochetal synthesis of OspC at different junctures during its chain of infection may also be an adaptation that is used to escape the immunological attack. Expression of OspC during transmission from mouse to tick could be lethal for the spirochetes as they become exposed to the mouse's anti-OspC antibodies in the tick's midgut, similar to the borreliacidal effect of anti-OspA antibodies produced experimentally through immunization (16).
The most obvious environmental cue and difference presented to spirochetes mixing with blood as ticks complete their feeding is temperature. Again, spirochetes that infect ticks prior to their feeding produce OspC as the ticks attach and warm to near 37°C, whereas spirochetes already at this elevated temperature in mice do not produce OspC as they move from the mouse to the engorged tick. Disregarding the immune status of the mouse upon which the ticks are feeding, the conditions for spirochetes within the midguts of engorged ticks should be identical except for their thermal histories (recent or prolonged exposure to 37°C). While increasing the temperature of recently isolated spirochetes in culture to 37°C appears to be optimal for the production of OspC, the synthesis of this protein is not sustained with continued growth at this temperature. Other workers have also observed this same phenomenon (21), although it led them to conclude that such an observation is contrary to our previous and present work, demonstrating that spirochetes in previously infected ticks produce OspC during tick warming and feeding. However, we believe that these observations are consistent with our hypothesis that OspC is required for transmission of spirochetes from tick to mouse and that an increase in temperature is a critical signal for this event. The lack of a sustained presence of OspC during continued cultivation of B. burgdorferi at 37°C does not conflict with the up-regulation of this protein by spirochetes in ticks when the temperature increases during feeding. Rather, this decrease in OspC during continuous growth of spirochetes at 37°C parallels, although more slowly, what we have observed in the present study when previously infected ticks feed. While the magnitude of the shutdown of OspC with continuous growth in vitro at 37°C may vary among strains of the spirochete, we believe that this response is suggestive of what happens following the transmission of spirochetes from ticks to mammals. The early immunological response of mice and humans to OspC following infection by tick bite also suggests that this protein is present on spirochetes as they enter these hosts (18, 29, 44). These observations have stimulated some workers to consider OspC an additional immunogen for Lyme disease vaccine development. Yet, the subsequent waning of the titer of antibody to this protein while infection continues, as can be seen in our previous work concerning the antibody response of white-footed mice to OspC (P24) (43), also suggests that this protein is not produced later in the infection. Anti-OspC antibodies are protective (6, 19, 35, 36), are borreliacidal (38), and are produced early during infection (18, 29, 38). Therefore, persistent infections in mammals could be impeded if the spirochetes did not down-regulate this surface protein soon after establishing infections in these hosts. Contrary to this notion, however, are the reports that anti-OspC immune serum is therapeutic for established infections in mice (47) and that OspC is constitutively expressed in mice (48). If this is true, then spirochetes must rapidly down-regulate and lose this protein soon after they are acquired by ticks.
We have focused our attention on only OspA and OspC, although Lyme disease-associated spirochetes have the genetic potential to produce many putative surface proteins. While the biological functions of these surface proteins are unknown, the temporal regulation of OspA and OspC appears to be significant for the spirochete to successfully alternate between ticks and mammals. Questions remain, such as the following: when is OspA turned on as the spirochetes transfer from mice to ticks; how long is OspC produced after the spirochetes are transmitted to mice; and how would the genetic inactivation of ospA and ospC affect the infectious cycle and transmission by ticks? The information now at hand, however, suggests that OspA provides the spirochete with an outer membrane that allows it to reside in ticks and that is antigenically distinct from the outer membrane of spirochetes that infect mammals. This switch may allow spirochetes in the midguts of ticks to be less vulnerable to immunological attack by antibodies present in the blood meal when ticks feed on mice with antibodies to other surface components of the spirochete. OspA may also function as a midgut adhesion, restricting the spirochetes to this organ as they first enter ticks, keeping them there until ticks feed again and are capable of transmission. The down-regulation of OspA at this time would allow the spirochetes to escape the midgut and disseminate to the salivary glands. In contrast, OspC is only briefly produced in ticks during the entire chain of infection and is correlated with either the dissemination from the tick midgut, infection of the tick's salivary glands, leading to their transmission via saliva, and/or the early colonization of the vertebrate host.
ACKNOWLEDGMENTS
We thank Marc Dolan and Paul Policastro for help in rearing ticks, Merry Schrumpf for technical assistance, Lamine Mbow for the monoclonal antibody B5, Gary Hettrick for graphic arts, and Jim Battisti and Centers for Disease Control and Prevention reviewers for reading the manuscript.
REFERENCES
- 1.Aguero-Rosenfeld M E, Nowakowski J, Bittker S, Cooper D, Nadelman R B, Wormser G P. Evolution of the serologic response to Borrelia burgdorferi in treated patients with culture-confirmed erythema migrans. J Clin Microbiol. 1996;34:1–9. doi: 10.1128/jcm.34.1.1-9.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Akins D R, Bourell K W, Caimano M J, Norgard M V, Radolf J D. A new animal model for studying Lyme disease spirochetes in a mammalian host-adapted state. J Clin Invest. 1998;101:2240–2250. doi: 10.1172/JCI2325. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Barbour A G, Hayes S F, Heiland R A, Schrumpf M E, Tessier S L. A Borrelia-specific monoclonal antibody binds to a flagellar epitope. Infect Immun. 1986;52:549–554. doi: 10.1128/iai.52.2.549-554.1986. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Barbour A G, Tessier S L, Todd W J. Lyme disease spirochetes and ixodid tick spirochetes share a common surface antigenic determinant defined by a monoclonal antibody. Infect Immun. 1983;41:795–804. doi: 10.1128/iai.41.2.795-804.1983. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Barthold S W, Fikrig E, Bockenstedt L K, Persing D H. Circumvention of outer surface protein A immunity by host-adapted Borrelia burgdorferi. Infect Immun. 1995;63:2255–2261. doi: 10.1128/iai.63.6.2255-2261.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Bockenstedt L K, Hodzic E, Feng S, Bourrel K W, de Silva A, Montgomery R R, Fikrig E, Radolf J D, Barthold S W. Borrelia burgdorferi strain-specific OspC-mediated immunity in mice. Infect Immun. 1997;65:4661–4667. doi: 10.1128/iai.65.11.4661-4667.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Burgdorfer W, Barbour A G, Hayes S F, Benach J L, Grunwaldt E, Davis J P. Lyme disease—a tick-borne spirochetosis? Science. 1982;216:1317–1319. doi: 10.1126/science.7043737. [DOI] [PubMed] [Google Scholar]
- 8.Carroll J A, Garon C F, Schwan T G. Effects of environmental pH on membrane proteins in Borrelia burgdorferi. Infect Immun. 1999;67:3181–3187. doi: 10.1128/iai.67.7.3181-3187.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Coleman J L, Gebbia J A, Piesman J, Degen J L, Bugge T H, Benach J L. Plasminogen is required for efficient dissemination of B. burgdorferi in ticks and for enhancement of spirochetemia in mice. Cell. 1997;89:1111–1119. doi: 10.1016/s0092-8674(00)80298-6. [DOI] [PubMed] [Google Scholar]
- 10.de Silva A M, Fish D, Burkot T R, Zhang Y, Fikrig E. OspA antibodies inhibit the acquisition of Borrelia burgdorferi by Ixodes ticks. Infect Immun. 1997;65:3146–3150. doi: 10.1128/iai.65.8.3146-3150.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.de Silva A M, Telford III S R, Brunet L R, Barthold S W, Fikrig E. Borrelia burgdorferi OspA is an arthropod-specific transmission-blocking Lyme disease vaccine. J Exp Med. 1996;183:271–275. doi: 10.1084/jem.183.1.271. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.de Silva A M, Zeidner N S, Zhang Y, Dolan M C, Piesman J, Fikrig E. Influence of outer surface protein A antibody on Borrelia burgdorferi within feeding ticks. Infect Immun. 1999;67:30–35. doi: 10.1128/iai.67.1.30-35.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Donahue J G, Piesman J, Spielman A. Reservoir competence of white-footed mice for Lyme disease spirochetes. Am J Trop Med Hyg. 1987;36:92–96. doi: 10.4269/ajtmh.1987.36.92. [DOI] [PubMed] [Google Scholar]
- 14.Erdile L F, Brandt M-A, Warakomski D J, Westrack G J, Sadziene A, Barbour A G, Mays J P. Role of attached lipid in immunogenicity of Borrelia burgdorferi OspA. Infect Immun. 1993;61:81–90. doi: 10.1128/iai.61.1.81-90.1993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Fikrig E, Chen M, Barthold S W, Anguita J, Feng W, Telford III S R, Flavell R A. Borrelia burgdorferi erpT expression in the arthropod vector and murine host. Mol Microbiol. 1999;31:281–290. doi: 10.1046/j.1365-2958.1999.01171.x. [DOI] [PubMed] [Google Scholar]
- 16.Fikrig E, Telford III S R, Barthold S W, Kantor F S, Spielman A, Flavell R A. Elimination of Borrelia burgdorferi from vector ticks feeding on OspA-immunized mice. Proc Natl Acad Sci USA. 1992;89:5418–5421. doi: 10.1073/pnas.89.12.5418. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Fingerle V, Hauser U, Liegl G, Petko B, Preac-Mursic V, Wilske B. Expression of outer surface proteins A and C of Borrelia burgdorferi in Ixodes ricinus. J Clin Microbiol. 1995;33:1867–1869. doi: 10.1128/jcm.33.7.1867-1869.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Fung B P, McHugh G L, Leong J M, Steere A C. Humoral immune response to outer surface protein C of Borrelia burgdorferi in Lyme disease: role of the immunoglobulin M response in the serodiagnosis of early infection. Infect Immun. 1994;62:3213–3221. doi: 10.1128/iai.62.8.3213-3221.1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Gilmore R D, Kappel K J, Dolan M C, Burkot T R, Johnson B J B. Outer surface protein C (OspC), but not P39, is a protective immunogen against a tick-transmitted Borrelia burgdorferi challenge: evidence for a conformational protective epitope in OspC. Infect Immun. 1996;64:2234–2239. doi: 10.1128/iai.64.6.2234-2239.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Golde W T, Kappel K J, Dequesne G, Feron C, Plainchamp D, Capiau C, Lobet Y. Tick transmission of Borrelia burgdorferi to inbred strains of mice induces an antibody response to P39 but not to outer surface protein A. Infect Immun. 1994;62:2625–2627. doi: 10.1128/iai.62.6.2625-2627.1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Hu C M, Simon M, Kramer D, Gern L. Tick factors and in vitro cultivation influence the protein profile, antigenicity and pathogenicity of a cloned Borrelia garinii isolate from Ixodes ricinus hemolymph. Infection. 1996;24:251–257. doi: 10.1007/BF01781105. [DOI] [PubMed] [Google Scholar]
- 22.Kalish R A, Leong J M, Steere A C. Association of treatment-resistant chronic Lyme arthritis with HLA-DR4 and antibody reactivity to OspA and OspB of Borrelia burgdorferi. Infect Immun. 1993;61:2774–2779. doi: 10.1128/iai.61.7.2774-2779.1993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Kurtenbach K, Sewell H-S, Ogden N H, Randolph S E, Nuttall P A. Serum complement sensitivity as a key factor in Lyme disease ecology. Infect Immun. 1998;66:1248–1251. doi: 10.1128/iai.66.3.1248-1251.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Laemmli U K. Cleavage of structural proteins during the assembly of the head of bacteriophage T4. Nature (London) 1970;227:680–685. doi: 10.1038/227680a0. [DOI] [PubMed] [Google Scholar]
- 25.Magnarelli L A, Anderson J F, Fish D. Transovarial transmission of Borrelia burgdorferi in Ixodes dammini (Acari: Ixodidae) J Infect Dis. 1987;156:234–236. doi: 10.1093/infdis/156.1.234. [DOI] [PubMed] [Google Scholar]
- 26.Mbow M L, Gilmore R D J, Titus R G. An OspC-specific monoclonal antibody passively protects mice from tick-transmitted infection by Borrelia burgdorferi strain B31. Infect Immun. 1999;67:5470–5472. doi: 10.1128/iai.67.10.5470-5472.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Montgomery R R, Malawista S E, Feen K J M, Bockenstedt L K. Direct demonstration of antigenic substitution of Borrelia burgdorferi ex vivo: exploration of the paradox of the early immune response to outer surface proteins A and C in Lyme disease. J Exp Med. 1996;183:261–269. doi: 10.1084/jem.183.1.261. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Obonyo M, Munderloh U G, Fingerle V, Wilske B, Kurtti T J. Borrelia burgdorferi in tick cell culture modulates expression of outer surface proteins A and C in response to temperature. J Clin Microbiol. 1999;37:2137–2141. doi: 10.1128/jcm.37.7.2137-2141.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Padula S J, Sampieri A, Dias F, Szczepanski A, Ryan R W. Molecular characterization and expression of p23 (OspC) from a North American strain of Borrelia burgdorferi. Infect Immun. 1993;61:5097–5105. doi: 10.1128/iai.61.12.5097-5105.1993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Piesman J. Experimental acquisition of the Lyme disease spirochete, Borrelia burgdorferi, by larval Ixodes dammini (Acari: Ixodidae) during partial blood meals. J Med Entomol. 1991;28:259–262. doi: 10.1093/jmedent/28.2.259. [DOI] [PubMed] [Google Scholar]
- 31.Piesman J. Standard system for infecting ticks (Acari: Ixodidae) with the Lyme disease spirochete, Borrelia burgdorferi. J Med Entomol. 1993;30:199–203. doi: 10.1093/jmedent/30.1.199. [DOI] [PubMed] [Google Scholar]
- 32.Piesman J, Mather T N, Telford III S R, Spielman A. Concurrent Borrelia burgdorferi and Babesia microti infection in nymphal Ixodes dammini. J Clin Microbiol. 1986;24:446–447. doi: 10.1128/jcm.24.3.446-447.1986. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Piesman J, Oliver J R, Sinsky R J. Growth kinetics of the Lyme disease spirochete (Borrelia burgdorferi) in vector ticks (Ixodes dammini) Am J Trop Med Hyg. 1990;42:352–357. doi: 10.4269/ajtmh.1990.42.352. [DOI] [PubMed] [Google Scholar]
- 34.Piesman J F, Donahue J G, Mather T N, Spielman A. Transovarially acquired Lyme disease spirochetes (Borrelia burgdorferi) in field-collected larval Ixodes dammini (Acari: Ixodidae) J Clin Microbiol. 1986;24:446–447. doi: 10.1093/jmedent/23.2.219. [DOI] [PubMed] [Google Scholar]
- 35.Preac-Mursic V, Wilske B, Patsouris E, Jauris S, Will G, Soutschek E, Rainbardt S, Lehnert G, Klockmann U, Mehraein P. Active immunization with pC protein of Borrelia burgdorferi protects gerbils against B. burgdorferi infection. Infection. 1992;20:342–349. doi: 10.1007/BF01710681. [DOI] [PubMed] [Google Scholar]
- 36.Probert W S, LeFebvre R B. Protection of C3H/HeN mice from challenge with Borrelia burgdorferi through active immunization with OspA, OspB, or OspC, but not with OspD or the 83-kilodalton antigen. Infect Immun. 1994;62:1920–1926. doi: 10.1128/iai.62.5.1920-1926.1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Ramamoorthy R, Philipp M T. Differential expression of Borrelia burgdorferi proteins during growth in vitro. Infect Immun. 1998;66:5119–5124. doi: 10.1128/iai.66.11.5119-5124.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Rousselle J C, Callister S M, Schell R F, Lovrich S D, Jobe D A, Marks J A, Wieneke C A. Borreliacidal antibody production against outer surface protein C of Borrelia burgdorferi. J Infect Dis. 1998;178:733–741. doi: 10.1086/515382. [DOI] [PubMed] [Google Scholar]
- 39.Ryan J R, Levine J F, Apperson C S, Lubke L, Wirtz R A, Spears P A, Orndorff P E. An experimental chain of infection reveals that distinct Borrelia burgdorferi populations are selected in arthropod and mammalian hosts. Mol Microbiol. 1998;30:365–379. doi: 10.1046/j.1365-2958.1998.01071.x. [DOI] [PubMed] [Google Scholar]
- 40.Schwan T G. Ticks and Borrelia: model systems for investigating pathogen-arthropod interactions. Infect Agents Dis. 1996;5:167–181. [PubMed] [Google Scholar]
- 41.Schwan T G, Burgdorfer W, Garon C F. Changes in infectivity and plasmid profile of the Lyme disease spirochete, Borrelia burgdorferi, as a result of in vitro cultivation. Infect Immun. 1988;56:1831–1836. doi: 10.1128/iai.56.8.1831-1836.1988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Schwan T G, Hinnebusch B J. Bloodstream- versus tick-associated variants of a relapsing fever bacterium. Science. 1998;280:1938–1940. doi: 10.1126/science.280.5371.1938. [DOI] [PubMed] [Google Scholar]
- 43.Schwan T G, Kime K K, Schrumpf M E, Coe J E, Simpson W J. Antibody response in white-footed mice (Peromyscus leucopus) experimentally infected with the Lyme disease spirochete (Borrelia burgdorferi) Infect Immun. 1989;57:3445–3451. doi: 10.1128/iai.57.11.3445-3451.1989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Schwan T G, Piesman J, Golde W T, Dolan M C, Rosa P A. Induction of an outer surface protein on Borrelia burgdorferi during tick feeding. Proc Natl Acad Sci USA. 1995;92:2909–2913. doi: 10.1073/pnas.92.7.2909. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Stevenson B, Schwan T G, Rosa P A. Temperature-related differential expression of antigens in the Lyme disease spirochete, Borrelia burgdorferi. Infect Immun. 1995;63:4535–4539. doi: 10.1128/iai.63.11.4535-4539.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Towbin H, Staehelin T, Gordon J. Electrophoretic transfer of proteins from polyacrylamide gels to nitrocellulose sheets: procedure and some applications. Proc Natl Acad Sci USA. 1979;76:4350–4354. doi: 10.1073/pnas.76.9.4350. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Zhong W, Gern L, Stehle T, Museteanu C, Kramer M, Wallich R, Simon M M. Resolution of experimental and tick-borne Borrelia burgdorferi infection in mice by passive, but not active immunization using recombinant OspC. Eur J Immunol. 1999;29:946–957. doi: 10.1002/(SICI)1521-4141(199903)29:03<946::AID-IMMU946>3.0.CO;2-P. [DOI] [PubMed] [Google Scholar]
- 48.Zhong W, Stehle T, Museteanu C, Siebers A, Gern L, Kramer M, Wallich R, Simmon M M. Therapeutic passive vaccination against chronic Lyme disease in mice. Proc Natl Acad Sci USA. 1997;94:12533–12538. doi: 10.1073/pnas.94.23.12533. [DOI] [PMC free article] [PubMed] [Google Scholar]