Abstract
In this study, the vaccination coverage, serological sampling and infection rate of sheep and goats were evaluated as predictors for the modeling of human brucellosis in Greece. The human brucellosis disease frequency per local regional unit (RU) varied significantly (RR90) among consecutive years. The notification rate was higher (p < 0.001) in the RUs with implementation of vaccination in sheep and goats (vaccination zone—VZ) with a median of 1.4 (IQR 0.0–3.1) compared with the RUs of the eradication zone (EZ) with a median of 0.0 (IQR 0.0–0.0). In VZ, the increased frequency of human cases was associated with delayed vaccine administration (estimate: 0.14 (0.04; 0.29), p = 0.03) and higher vaccination coverage of the animals (estimate: −0.349 (−0.72; −0.07), p < 0.01). However, the flock sampling rate was highly heterogenous among RUs (IQR 10.56–52.93), and inconsistent within RUs throughout the period of the study 2013–2017 (p = 0.001), limiting the reliable estimation of the infection rate in livestock and the design of an integrated One Health model for human disease.
Keywords: brucellosis, Brucella melitensis, vaccination, eradication, livestock
1. Introduction
Brucellosis is a debilitating zoonosis with worldwide occurrence [1]. Brucella melitensis is responsible for the vast majority of human cases [2]. Sheep and goats represent the natural hosts of B. melitensis [3,4]. The infection is transmitted to humans mainly by direct contact with animals or by consumption of milk products [5]. The disease is endemic in countries around the Mediterranean basin [6]. Greece presents the highest toll in human cases among European countries [7], with more than 90% of Brucella spp. isolates from clinical specimens identified as B. melitensis [8].
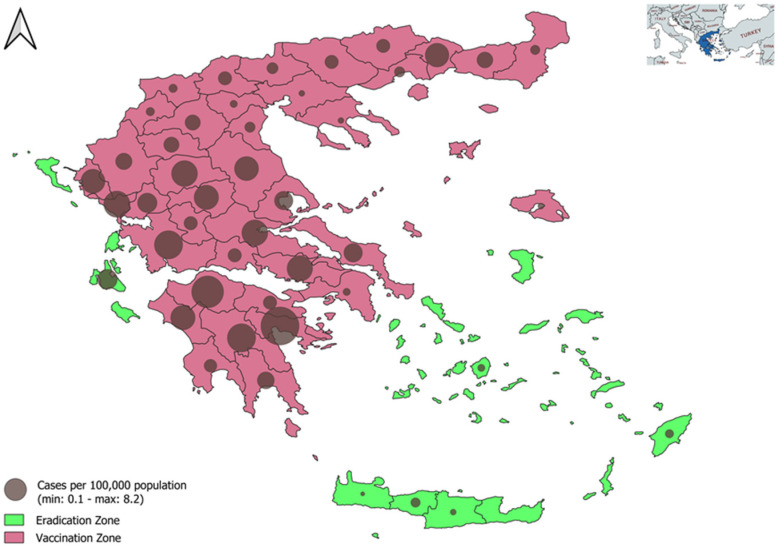
Since 1977, the basic concept for controlling and eradicating brucellosis in Greece has always been vaccination of herds combined with testing and slaughtering of seropositive animals. The State Veterinary Services from the Ministry of Rural Development & Food and the local Veterinary Services have implemented a control and eradication policy: in the vaccination zone (VZ), female animals are vaccinated, and males are blood-sampled, whereas in the eradication zone (EZ), a test-and-slaughter policy is applied (Figure 1). The transportation of animals from the VZ to the EZ is legally prohibited and in the VZ zone only vaccinated animals are allowed to move from one local Regional Unit (RU) to another. The VZ comprises the mainland and a few islands and has a population of 9,532,504 inhabitants (88.6% of the country’s population), whereas the EZ includes the main body of the islands with a population of 1,228,437 (11.4%) [9].
Figure 1.
Mean annual brucellosis cases per 100,000 of human population, at local level (Regional Units), Greece, 2013–2017.
In the VZ zone, sheep and goats are vaccinated by the conjunctival administration of the attenuated live vaccine strain, Rev. 1, once in their life. The vaccine is administered only to females, ideally at the age of 3–6 months. The test-and-slaughter scheme is applied in the EZ and is based on the regular sampling and serological testing of all animals over the age of six months. Positive herds are identified by serology tests (Rose Bengal Test and Complement Fixation Test for screening and confirmation, respectively). Infected animals are slaughtered separately from healthy animals, taking all necessary biosecurity measures [10]. Unfortunately, this scheme did not result in eradication due to various reasons, and brucellosis remains endemic in livestock in Greece [11].
Agricultural professions and consumption of unpasteurized milk products are established risk factors for human brucellosis. Particularly, people having occupational contact with farm animals and their secretions or being involved in the processing of animal products, such as farmers, stockmen, shepherds, veterinarians, slaughtermen, and butchers, are at high risk of exposure to Brucella spp. [2,4]. However, despite the fact that sheep and goats are the reservoirs of the pathogen, specific markers have not been adequately described in livestock as predictors of human disease likelihood.
This study aimed primarily to assess the effect of infection rate, sampling intensity, and vaccination coverage of sheep and goats on the human brucellosis notification rate. Secondary objectives were to compare the frequency of human cases between VZ and EZ and to estimate the relative risk of brucellosis occurrence for RUs in Greece.
2. Results
2.1. Descriptive Results, Estimated Relative Risks and RR90
In Greece, in the period of the study (2013–2017), 621 human brucellosis cases were recorded and the notification rate per RU-year ranged from 0.00 to 12.3 with a median 0.7 (IQR 0.0–2.5). The notification rate in the VZ was significantly higher (p < 0.001), with a median 1.4 (IQR 0.0–3.1) compared with a median 0.0 (IQR 0.0–0.0) in the EZ (Figure 1).
The median age of cases was 47.0 years (IQR 32.3–58.0) and 69.6% were male. Among the cases, 77.6% (482/621) had a high-risk occupation or contact with farm animals.
A median of 85,219 (IQR 74,268–115,328) holdings and 14,502,751 (IQR 13,365,434–15,700,954) sheep and goats were recorded in Greece during 2013–2017. The average ratio of sheep to goats was 2.48 (SD = 0.10); however, data regarding the herd size and the composition of farmed species per holding were not available.
In the VZ, a median of 461 (IQR 294–804) unvaccinated holdings, 151,048 (IQR 118,370–240,229) unvaccinated sheep and goats and 6496 (IQR 2085–11,537) sheep and goats with delayed vaccination were identified annually, per RU, per 100,000 inhabitants (n = 195).
The rate of new infected flocks per RU and 100,000 inhabitants was higher in the VZ (mdn = 9, IQR 2–23) than in the EZ (mdn = 0, IQR 0–2) (U = 63.5, p = 0.00016) on an annual basis.
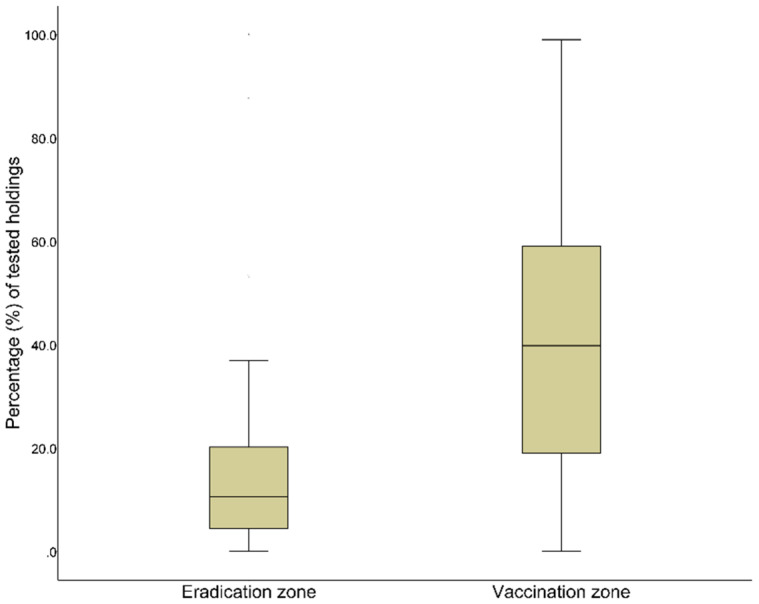
Among holdings, 22,899 (IQR 21,022–22,905) were annually tested for brucellosis. The sampling rates in all RUs of Greece throughout the study period varied from 0% to 100% with a median of 31.40% (IQR 10.56–52.93). The percentage of flock sampling per RU and year was significantly higher (p < 0.001) in the VZ, with a median of 39.8% (IQR 18.93–59.00) compared with 10.60% (IQR 4.35–20.23) in the EZ (Figure 2) (Supplementary Material: Table S1).
Figure 2.
Percentage of tested holdings per Regional Unit on annual basis in the vaccination (n = 195) and eradication zone (n = 60), Greece, 2013–2017.
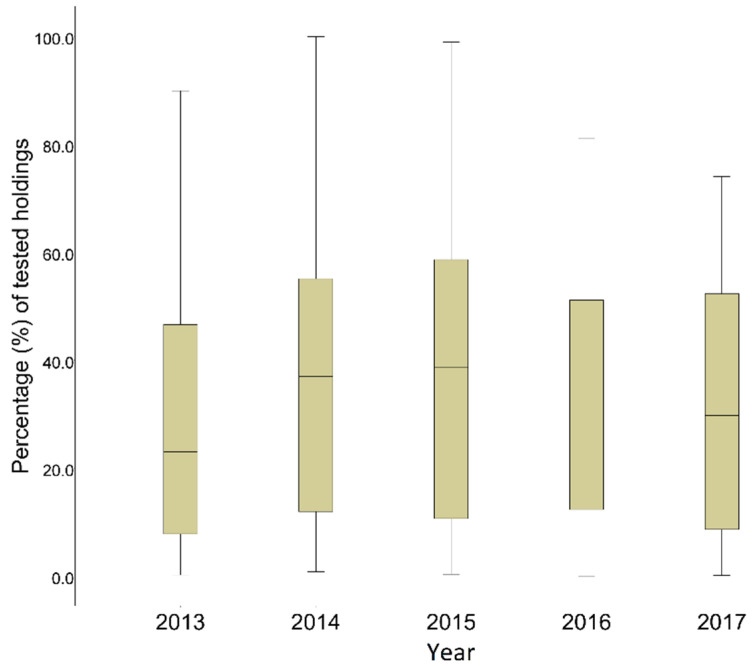
Nationwide sampling rates differed throughout the years, χ2(4) = 30.202, p < 0.0001 (Figure 3). Analysis, using Bayesian hierarchical Poisson models, revealed an increase in the sampling effort from 2013 to 2014 (Z = −3.828, p < 0.001) and to 2015 (Z = −3.374, p = 0.001) and a reduction in 2017 compared with 2015 (Z = −2.906, p = 0.004). A time effect during the years was also observed within RUs, Wilk’s lambda = 0.67, F (4, 46) = 5.659, p = 0.001, η2 = 0.33.
Figure 3.
Median values and interquartile ranges of the annual sampling rates, Greece (n = 51), 2013–2017.
The newly diagnosed holdings with brucellosis had a positive correlation with the flock sampling rate on an annual basis (rs(208) = 0.565, p< 0.001).
Estimated relative risks (with 95% Probability intervals—PrIs) by each RU and year can be found in the Supplementary Material (Table S2). There was significant variability (RR90) in the human notification rate between consecutive years, as it was 7.998 (3.521; 20.31), 24.9 (5.287; 92.52), 13.84 (4.945; 41.17), 14.24 (4.443; 46.23) and 15.13 (4.661; 48.62) in the years from 2013 to 2017, respectively.
2.2. Effect of the National Brucellosis Program Parameters on the Number of Human Cases
Among the different hierarchical Poisson models, the model ignoring the temporal trends had the best fit to the data. Thus, the number of human cases in the previous year does not affect the number of observed cases in the current year. For all RUs in Greece (Model 1, Table 1), the number of human cases is positively associated with the application of a vaccination program. For RUs of VZ (Model 2, Table 2), the number of human cases is positively associated with the number of animals vaccinated at an age greater than 6 months per 100,000 humans but negatively associated with the number of the remaining unvaccinated animals per 100,000 humans.
Table 1.
Parameter estimates (means and 95% probability intervals—PrIs) of the fitted model for all Regional Units in Greece (model 1).
| Variable | Estimate | p | |
|---|---|---|---|
| Intercept | −1.84 (−2.81; −0.95) | <0.01 | |
| Vaccination | No | Reference | |
| Yes | 2.31 (1.35; 3.32) | <0.01 | |
| Random-effect variance | 1.23 (0.88; 2.22) | <0.01 |
Table 2.
Parameter estimates (means and PrIs) of the fitted model for the Greek Regional Units where a vaccination program is applied (model 2).
| Variable | Estimate | p |
|---|---|---|
| Intercept | 0.56 (0.17; 0.94) | <0.01 |
| Remaining unvaccinated female animals per 105 humans | −0.349 (−0.72; −0.07) | <0.01 |
| Female animals vaccinated at age > 6 mo per 105 humans | 0.14 (0.04; 0.29) | 0.03 |
| Random-effect variance | 1.23 (0.72; 2.20) | <0.01 |
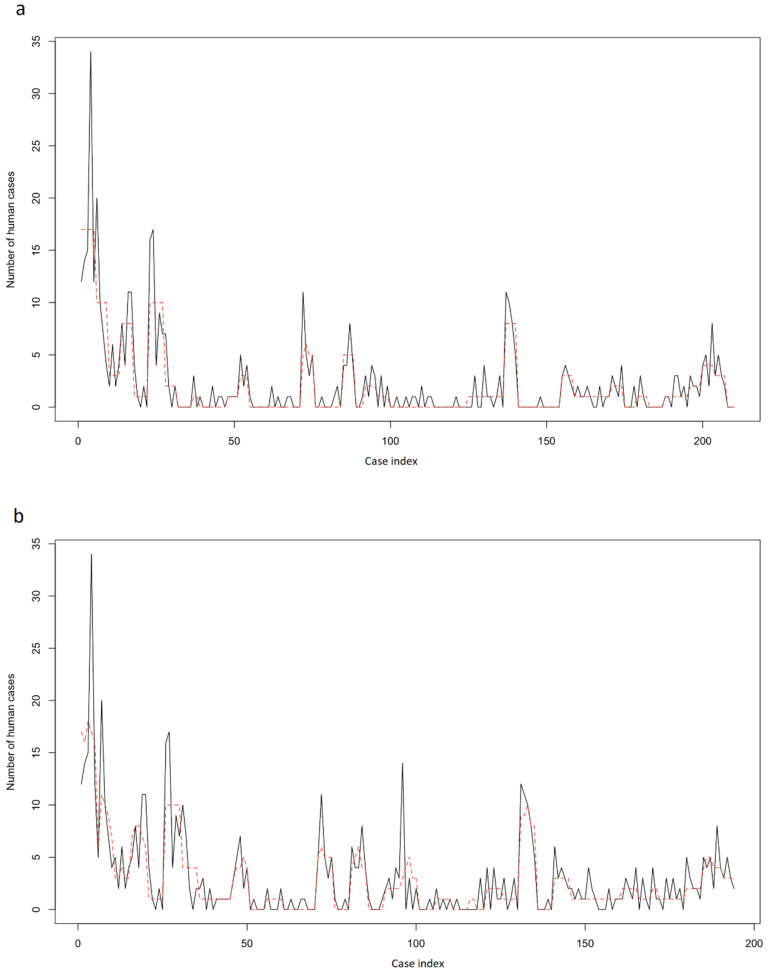
For both models 1 and 2, the plot of the predicted vs. the observed human brucellosis cases revealed an adequate fit of the final models to the data (Figure 4).
Figure 4.
Observed (black line) and predicted (red dotted line) human brucellosis cases (a) under Model 1 in all Regional Units and (b) under Model 2 in the Regional Units of the vaccination zone, Greece, 2013–2017.
3. Discussion
This study examined the effect of sheep and goats’ brucellosis status, including vaccination, testing and infection rate, on the frequency of human brucellosis cases.
The data revealed a significant variability in the reported incidence of human brucellosis among RUs but also a lack of a consistent trend during consecutive years within RUs (as depicted by the RR90). This means that a very diversified setting exists in relation to the occurrence of the disease and the previous number of human cases does not affect present or future counts. The result is that a temporal trend does not exist at RU or country level.
Regions where vaccination is implemented presented a higher frequency of human brucellosis cases compared to the test-and-slaughter policy areas, in accordance with previous reports [12]. It is conceivable that the increased infection rate of the livestock in the VZ zone may lead to greater risk of human exposure.
Within RUs that vaccinate, the vaccination of female sheep and goats during the optimal age of three to six months had a protective effect for human brucellosis. This is in accordance with previous reports indicating that vaccination past the optimal age of 3–6 months fails to provide optimal protection to the animals and may cause increased spillover of Brucella spp. to the environment and higher likelihood of human exposure [13]. Furthermore, vaccinating within the optimal age may reflect adherence to the program and vaccinations performed according to the organized schedule. Vaccination in due time may also indicate increased compliance of the stockholder and closer veterinary attendance of the animals. The latter may aid in the early recognition of clinically suspect animals and application of biosecurity measures, therefore reducing the risk of infection [14]. High-risk practices can be avoided by adhering to safety protocols. In particular, the illegal exchange of sires among herds may increase the flock-to-flock dissemination of B. melitensis, as has been observed with other pathogens [15]. To our knowledge, there are no previous reports acknowledging the protective effect of the timely livestock vaccination on human brucellosis.
The study also revealed a seemingly paradoxical finding: a positive association of the animal vaccination coverage and the human disease rate. Vaccination was expected to have a protective effect against human disease as gaps in the vaccination program hinder eradication and maintain the disease in livestock [16]. We theorize that the veterinary authorities intensified the vaccination as a response to the locally increased flock infection rate or due to the higher number of human cases in the area.
The increased human brucellosis in areas where vaccination is more intensively implemented could theoretically be explained by the higher likelihood of accidental exposure to vaccine strains. The live attenuated B. melitensis vaccine (Rev. 1) may cause disease to humans indistinguishable from the one caused by the wild strains [17,18]. The inadequate use of personal protective equipment and the negligence to implement safe practices during high-risk activities such as vaccination, attending parturitions, handling aborted fetuses, and other obstetric procedures, increase the chance of infection [14]. In general, studies on the incidence of vaccine-induced human brucellosis, especially for the Rev. 1 vaccine, are scarce. Identification of wild or vaccine Brucella strains from clinical specimens is not routinely performed in Greece [19]; however, previous reports indicate that the vaccine strain can be held accountable for only a small proportion of human cases (0.65%, N = 308) [8]. A causative relation of human infection with the procedure of vaccinating the animals cannot be established based on the existing data.
Flock sampling was unexpectedly more intense in continental Greece than in the islands, even though a test-and-slaughter scheme is applied in the latter. Nationwide, sampling was highly variable among RUs, with a significant proportion of herds remaining untested. Our data indicated, as expected, that a higher flock sampling rate identified more infections. The weak and inconsistent sampling rates not only reduced the usefulness of infection data for modelling purposes, but also precluded the identification of recently infected herds. Undiagnosed new infections entail greater risk for humans than flocks with previously established infection due to a lack of awareness for biosecurity measures. Finally, a Bayesian model adequately fit human disease with delayed vaccination and vaccination coverage of the livestock. However, core predictors of the animal infection rate should be included in a cause-and-effect model. Good quality animal and human data are needed to explore the causalities among livestock and human brucellosis.
The underreporting from health settings across different RUs should also be considered in an integrated model of human disease. A nosocomial non-reporting rate of 24.1% to 35.0% of human brucellosis cases has been previously described in Greece [20].
Based on the findings of this study, the epidemiologic situation of human and animal brucellosis in Greece could be improved by implementing vaccination in due time and strengthening the serological screening; this may require allocating adequate resources to the local veterinary services and reassessing the efficacy of the existing brucellosis control and eradication program.
4. Materials and Methods
4.1. Data
Detailed data for brucellosis in sheep and goats for the years 2013 to 2017, stratified by RU and year, were obtained from the Department of Zoonoses of the Ministry of Rural Development & Food. The examined variables are shown in Table 3.
Table 3.
Sheep and goats’ husbandry and brucellosis status data investigated for association with human brucellosis.
| Variable | Definition |
|---|---|
| “New positive holdings” a | Only newly diagnosed with brucellosis holdings in a particular year |
| “Unvaccinated animals” a | Female sheep and goats remaining unvaccinated |
| “Unvaccinated holdings” a | Farms with sheep and/or goats, in which no vaccination was implemented in the animals eligible for vaccination |
| “Animals with delayed vaccination” a | Female sheep and goats that received vaccination after the optimal age (3–6 months old) |
| “Percentage of tested holdings” | Percentage of holdings tested serologically |
a per Regional Unit, year, and 100,000 of human population.
Human population data were extracted from the national census of 2011 (9 Hellenic Statistical Authority 2011).
Human brucellosis cases were defined according to European Commission decision 2018/945 requiring a combination of symptoms including fever, and laboratory findings (isolation of Brucella spp. by culture from a clinical specimen, and/or positive serological result with Standard Agglutination Test or Complement Fixation or ELISA, and/or detection of Brucella spp. DNA in a clinical specimen). Brucellosis is a mandatorily notified disease in Greece and cases are reported in the Greek National Public Health Organization (NPHO). Brucellosis notification rate represented the new human cases per RU, year, and 100,000 inhabitants and was derived from the regular data sharing between NPHO and the Department of Zoonoses of the Ministry of Rural Development & Food, and from the public database, available at the website of NPHO.
4.2. Bayesian Estimation of the Expected Number of Human Cases and the RU-Specific Relative Risk
Bayesian hierarchical Poisson models were used for the analysis.
We assume that the number of brucellosis cases in each of the ith RU follows a Poisson distribution:
where is the expected number of cases in each RU. Subsequently, a normal random effects prior is specified for :
with
is the mean log relative risk, the random effects term for each RU and the between RU variance, which captures the amount of extra-Poisson variation in the data. The can be considered as a latent variable, capturing the effects of unknown or unmeasured covariates that operate at the RU level. The relative risk () in each of the RUs compared to the expected risk based on the average count of brucellosis cases in the whole country is calculated by:
Finally, the expected number of brucellosis cases per 10,000 population for each RU is calculated as:
with being the replicated counts for each RU under the fitted model and being the total population for each RU.
A useful summary metric for the variability among RUs, in a hierarchical setting, is the difference in the attained risk of brucellosis occurrence between the RUs with the higher and lower relative risk. If and denote the relative risk of brucellosis in the RU ranked at the 5th and 95th percentile, respectively, then is the ratio of the relative risks for brucellosis between the top and bottom 5% RUs.
4.3. Bayesian Assessment of the Effect of the Parameters of the National Brucellosis Program on the Number of Human Brucellosis Cases
As previously, let the number of human brucellosis cases for each of the years (with i = 2013, …, 2017) in each of the RUs follow a Poisson distribution:
The logarithm for the mean is modelled as,
where X is the design matrix and encodes all known information about the independent variables and is the matrix for the coefficients corresponding to each of the p independent variables. We further considered two additional variations of this model in order to assess whether a calendar trend exists (i.e., the number of human cases in the previous year affects the number of observed cases in the current year). The first variation assumed a first order autoregressive structure (AR1):
with
The second variation assumed a Normal random walk (RW1) prior for the coefficients:
Non-informative prior distributions were specified for and the precision parameter . The autocorrelation parameter was given a Uniform (−1,1) prior.
4.4. Selection of Independent Variables
We modelled the data in two steps and two separate sets of candidate variables were offered to each model. For the first model (Model 1), a set of variables that was recorded in all RUs was considered. These were: (i) vaccination (coded as yes or no for each RU), (ii) the total number of infected holdings per 100,000 citizens in the RU, (iii) the number of newly infected holdings per 100,000 citizens in the RU and (iv) the percent of tested holdings in the RU. The second model (Model 2) was relevant to the subset of data where a vaccination program was active. The variables offered to this model were (i) the remaining unvaccinated sheep and goats per 100,000 citizens in the RU, (ii) the remaining unvaccinated holdings per 100,000 citizens in the RU and (iii) the number of sheep and goats that were vaccinated after the age of 6 months (i.e., the optimal vaccination age is 3–6 months). Each of the two sets was offered independently to all models described in the previous section, and a backward selection procedure was repeated until all remaining variables were significant at p < 0.05. Bayesian p-values were calculated using the step function in OpenBUGS [21].
4.5. Model Selection Criteria and Model Goodness-of-Fit Tests, Selection of the Final Model and Assessment of the Adequate Fit to the Data
Selection of the best model was based on the comparison of the deviance information criterion (DIC), which is a global measure of comparative model fit [22]. The model with the lowest DIC was chosen. Yet, DIC and other similar indices are measures of comparative model fit and indicate which model best fits the data at hand. However, they do not assess whether the best model has an adequate fit to the data. This can be evaluated by the use of goodness-of-fit tests, which are based on the comparison between the observed data and the predictions under the model [23].
4.6. Convergence Diagnostics and Software
The convergence check for the MCMC chain was based on a combination of checks because convergence diagnostics of the MCMC chain are not foolproof. Therefore, a combination of the Raftery and Lewis method, the Gelman–Rubin diagnostic [24,25] autocorrelation checks and visual inspection of the trace plots and summary statistics was used as recommended by [26]. Parameter estimates were based on analytical summaries of 49,500 iterations of three chains after a burn-in phase of 500 iterations. This was adequate because the Raftery and Lewis method suggested that analytical summaries of 45,000 iterations after a burn-in of 15 iterations were needed. All checks suggested that convergence occurred and autocorrelations dropped off quickly. Models were run in the freeware program OpenBUGS [21] and the code is available as upon request from PK.
5. Conclusions
Delayed vaccination of sheep and goats, past the optimal age of 3–6 months, was associated with a higher human brucellosis disease rate.
A positive association of livestock vaccination with human brucellosis was explained as the result of an enhanced veterinary response to increased animal or human infection.
Strengthening of veterinary sampling is crucial for a reliable estimation of infection rate and the development of integrated models of human brucellosis.
Supplementary Materials
The following supporting information can be downloaded at: https://doi.org/10.5281/zenodo.5806444, accessed on 27 December 2021, Table S1: Brucellosis in Humans, Sheep and Goat Farms, Table S2: Relative risks (with 95% Probability intervals—PrIs) by Regional Unit (RU) and year.
Author Contributions
Conceptualization, G.D. and A.K.; Data curation, G.D., A.K. and P.K.; Formal analysis, P.K.; Funding acquisition, C.B.; Methodology, G.D., A.K. and P.K.; Project administration, C.B.; Supervision, M.L. and C.B.; Validation, P.K., M.L. and C.B.; Visualization, G.D.; Writing—original draft, G.D., A.K., P.K. and M.L.; Writing—review and editing, G.D., A.K., M.L., P.K. and C.B. All authors have read and agreed to the published version of the manuscript.
Funding
The APC was funded by the Special Account for Research Grants of the University of Thessaly, Giannitson & Lahana, Tsalapata Complex, Volos 38334, Greece VAT: EL090071277.
Institutional Review Board Statement
Ethical review and approval were waived for this study, due to the exclusive use of aggregate and non-identifiable surveillance data of statistic nature which do not qualify as personal data according to Greek Law 2472/1997 article 2, Law 4624/2019 and the currently in force Regulation (EU) 2016/679 General Data Protection Regulation.
Informed Consent Statement
Patient consent was waived due to the exclusive use of aggregate and non-identifiable data of statistic nature that cannot be associated with physical persons.
Data Availability Statement
Data are publicly available at https://doi.org/10.5281/zenodo.5806444, accessed on 27 December 2021.
Conflicts of Interest
The authors declare no conflict of interest. The funders had no role in the design of the study; in the collection, analyses, or interpretation of data; in the writing of the manuscript, or in the decision to publish the results.
Footnotes
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations.
References
- 1.Gwida M., Al Dahouk S., Melzer F., Rösler U., Neubauer H., Tomaso H. Brucellosis–Regionally Emerging Zoonotic Disease? Croat. Med. J. 2010;51:289–295. doi: 10.3325/cmj.2010.51.289. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Corbel M.J., Elberg S.S., Cosivi O. Brucellosis in Humans and Animals. World Health Organization; Geneva, Switzerland: Food and Agriculture Organization of the United Nations; Rome, Italy: World Organization for Animal Health; Paris, France: 2006. [Google Scholar]
- 3.Blasco J.M., Molina-Flores B. Control and Eradication of Brucella melitensis Infection in Sheep and Goats. Veter.-Clin. North Am. Food Anim. Pr. 2011;27:95–104. doi: 10.1016/j.cvfa.2010.10.003. [DOI] [PubMed] [Google Scholar]
- 4.Aparicio D.D. Epidemiology of brucellosis in domestic animals caused by Brucella melitensis, Brucella suis and Brucella abortus. Rev. Sci. Tech. l’OIE. 2013;32:53–60. doi: 10.20506/rst.32.1.2187. [DOI] [PubMed] [Google Scholar]
- 5.Atluri V.L., Xavier M.N., de Jong M.F., den Hartigh A.B., Tsolis R.M. Interactions of the Human Pathogenic Brucella Species with Their Hosts. Annu. Rev. Microbiol. 2011;65:523–541. doi: 10.1146/annurev-micro-090110-102905. [DOI] [PubMed] [Google Scholar]
- 6.Moreno E. Retrospective and prospective perspectives on zoonotic brucellosis. Front. Microbiol. 2014;5:213. doi: 10.3389/fmicb.2014.00213. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Authority E.F.S., Prevention E.C.F.D. Control, The European Union summary report on trends and sources of zoonoses, zoonotic agents and food-borne outbreaks in 2014. EFSA J. 2015;13:4329. [Google Scholar]
- 8.Papaparaskevas J., Kandili A., Pantazatou A., Gartzonika C., Charalampakis N., Stathi A., Tarpatzi K., Refene E., Priftis A., Mageropoulou A., et al. Infections due to the Brucella melitensis REV-1 vaccine strain in Greece, 2001–2015; Proceedings of the 26th ECCMID; Amsterdam, The Netherlands. 9–12 April 2016; [(accessed on 24 August 2021)]. Available online: https://www.escmid.org/escmid_publications/escmid_elibrary/material/?mid=46811. [Google Scholar]
- 9.Hellenic Statistical Authority, 2011 Population-Housing Census. [(accessed on 26 August 2021)]. Available online: http://www.statistics.gr/2011-census-pop-hous.
- 10.Tzani M., Katsiolis A. Program for the Control and Eradication of Brucellosis in Sheep and Goats in Greece. [(accessed on 13 March 2021)];HCDCP E-Bull. 2012 2:19–20. Available online: https://issuu.com/keelpno-hcdcp/docs/hcdcp_newsletter_april_2012. [Google Scholar]
- 11.Katsiolis A., Thanou O., Tzani Μ., Dile C., Korou L.M., Stournara A., Petridou E., Giadinis N. Investigation of the human resources needs for the effective and efficient implementation of the sheep and goat brucellosis program in Greece. Vet. J. Repub. Srp. (Banja Luka) 2018;18:270–296. doi: 10.7251/VETJEN1802270K. [DOI] [Google Scholar]
- 12.Fouskis I., Sandalakis V., Christidou A., Tsatsaris A., Tzanakis N., Tselentis Y., Psaroulaki A. The epidemiology of Brucellosis in Greece, 2007–2012: A ‘One Health’ approach. Trans. R. Soc. Trop. Med. Hyg. 2018;112:124–135. doi: 10.1093/trstmh/try031. [DOI] [PubMed] [Google Scholar]
- 13.Nicoletti P. brucellosis: Past, present and future. Prilozi. 2010;31:21–32. [PubMed] [Google Scholar]
- 14.Pereira C.R., De Almeida J.V.F.C., De Oliveira I.R.C., De Oliveira L.F., Pereira L.J., Zangerônimo M.G., Lage A.P., Dorneles E.M.S. Occupational exposure to Brucella spp.: A systematic review and meta-analysis. PLoS Neglected Trop. Dis. 2020;14:e0008164. doi: 10.1371/journal.pntd.0008164. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Wolf A., Prüfer T.L., Schoneberg C., Campe A., Runge M., Ganter M., Bauer B.U. Prevalence of Coxiella burnetii in German sheep flocks and evaluation of a novel approach to detect an infection via preputial swabs at herd-level. Epidemiol. Infect. 2020;148:e75. doi: 10.1017/S0950268820000679. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Roth F., Zinsstag J., Orkhon D., Chimed-Ochir G., Hutton G., Cosivi O., Carrin G., Otte J. Human health benefits from livestock vaccination for brucellosis: Case study. Bull. World Health Organ. 2004;81:867–876. [PMC free article] [PubMed] [Google Scholar]
- 17.Arapovic J., Špičić S., Duvnjak S., Ostojić M., Arapović M., Nikolić J. Cvetnić, Željko The first report of Brucella melitensis Rev.1 human brucellosis in Bosnia and Herzegovina. J. Infect. Dev. Ctries. 2020;14:232–235. doi: 10.3855/jidc.11949. [DOI] [PubMed] [Google Scholar]
- 18.Seyman D., Asik Z., Sepin-Ozen N., Berk H. Acute Brucellosis Following Accidental Exposure to Brucella melitensis Rev-1 Vaccine. West Indian Med. J. 2015;65:216–218. doi: 10.7727/wimj.2014.348. [DOI] [PubMed] [Google Scholar]
- 19.Christoforidou S., Boukouvala E., Zdragas A., Malissiova E., Sandalakis V., Psaroulaki A., Petridou E., Tsakos P., Ekateriniadou L., Hadjichristodoulou C. Novel diagnostic approach on the identification of Brucella melitensis Greek endemic strains-discrimination from the vaccine strain Rev.1 by PCR-RFLP assay. Veter-Med. Sci. 2018;4:172–182. doi: 10.1002/vms3.99. [DOI] [Google Scholar]
- 20.Dougas G., Mellou K., Kostoulas P., Billinis C., Georgakopoulou T., Tsiodras S. Brucellosis underreporting in Greece: Assessment based on aggregated laborato-ry data of culture-confirmed cases from public hospitals. Hippokratia. 2020;23:106–110. [PMC free article] [PubMed] [Google Scholar]
- 21.Speigelhalter D., Thomas D., Best N., Lunn D. OpenBUGS User Manual. 2010. [(accessed on 25 August 2021)]. Available online: https://www.mrc-bsu.cam.ac.uk/wp-content/uploads/2021/06/OpenBUGS_Manual.pdf.
- 22.Robert C., Ntzoufras I. Bayesian Modeling Using WinBUGS. Chance. 2012;25:60–61. doi: 10.1080/09332480.2012.685377. [DOI] [Google Scholar]
- 23.Gelman A., Hwang J., Vehtari A. Understanding predictive information criteria for Bayesian models. Stat. Comput. 2013;24:997–1016. doi: 10.1007/s11222-013-9416-2. [DOI] [Google Scholar]
- 24.Raftery A.E., Lewis S.M. [Practical Markov Chain Monte Carlo]: Comment: One Long Run with Diagnostics: Implementation Strategies for Markov Chain Monte Carlo. Stat. Sci. 1992;7:493–497. doi: 10.1214/ss/1177011143. [DOI] [Google Scholar]
- 25.Gelman A., Rubin D.B. Inference from Iterative Simulation Using Multiple Sequences. Stat. Sci. 1992;7:457–472. doi: 10.1214/ss/1177011136. [DOI] [Google Scholar]
- 26.Best N.G., Cowles M.K., Vines S.K. CODA Manual Version 0.30. Volume 46. MRC Biostatistics Unit; Cambridge, UK: 1995. pp. 2020–2027. [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
Data are publicly available at https://doi.org/10.5281/zenodo.5806444, accessed on 27 December 2021.