Abstract
Background
Whereas effusive–constrictive pericarditis (ECP) can rarely occur in coronavirus disease 2019 (COVID-19), to date no cases of ECP related to severe acute respiratory syndrome coronavirus 2 (SARS-CoV2) vaccine have been documented.
Case summary
A 59-year-old Caucasian man presented to our emergency department with ECP. Symptoms occurred shortly after the second dose of BNT162b2 (Comirnaty) vaccine. No other aetiological causes were identified. Guidelines-directed therapy for acute pericarditis was implemented, with clinical benefit.
Discussion
Systemic inflammatory response to COVID-19 can rarely trigger pericarditis. In our case, a strong temporal relationship between the second dose of BNT162b2 vaccine and symptoms occurrence was documented, indicating a possible rare adverse reaction to the vaccine, similarly to natural infection. Further research is needed to confirm a causal relationship.
Keywords: Effusive–constrictive pericarditis, Vaccine, Case report, BNT162b2, COVID-19, SARS-CoV2
Learning points.
Pericarditis in coronavirus disease 2019 (COVID-19) has been documented. Effusive–constrictive pericarditis in COVID-19 can rarely occur.
It is possible that BNT162b2 vaccine induces pericarditis similarly to natural infection. Further research is needed.
Introduction
Whereas effusive–constrictive pericarditis (ECP) can rarely occur in COVID-19, to date no cases of ECP related to the SARS-CoV2 vaccine have been documented. We report a case of ECP occurring shortly after administration of nucleoside modified RNA vaccine (BNT162b2) in a 59-year-old man with no history of cardiovascular disease.
Timeline
| Day 0 | A 59-year-old patient was administered the second dose of the BNT162b2 (Comirnaty) vaccine. |
| Day 5 | Patient presented to the emergency room (ER) with aggravating chest pain and dyspnoea. |
| Day 5 | An echocardiogram showed effusive–constrictive pericarditis. Treatment with ibuprofen and colchicine was started. |
| Day 12 | Cardiac magnetic resonance imaging confirmed echocardiographic findings. Autoimmune diseases and tuberculosis were ruled out. |
| Day 17 | Inflammatory markers decreased significantly. Symptoms resolved. Patient was discharged on ibuprofen and colchicine. |
| One week follow-up | Control echocardiography showed a reduction of pericardial effusion but the persistence of constrictive physiology. Inflammatory markers did not return to normal. Patient was started on prednisone and will be followed up closely. |
Case presentation
A 59-year-old Caucasian man with a history of type 2 diabetes mellitus accessed the emergency department reporting sharp stabbing pain to the right hemithorax, worsened by inbreathing and supine position, and aggravating exertional dyspnoea. The symptoms occurred about 5 days after the second dose of the BNT162b2 (Comirnaty) vaccine. Medical history was negative for SARS-CoV2 infection.
Physical examination was significant for muffled heart sounds, pulsus paradoxus, and decreased breath sounds at the lung bases. Electrocardiogram was not significant, showing only non-specific ST-T wave changes.
Blood tests showed increased inflammatory markers (C reactive protein (CRP) 120 mg/L; normal range between 0.3 mg/L and 10 mg/L). Two cardiac troponin assessments (at 0 h and 3 h) were within normal limits. Two nasopharyngeal swabs for SARS-CoV2 (at 0 h and 24 h) were negative. Chest X-ray showed mild bilateral pleural effusion and no radiological signs of pneumonia. Computed tomography (CT) pulmonary angiography was normal.
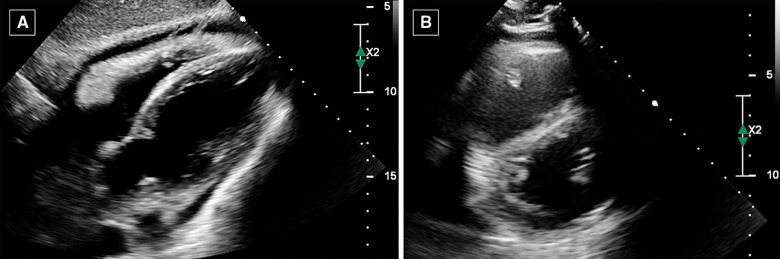
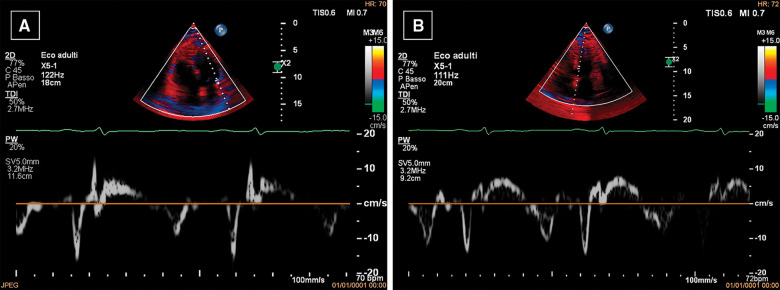
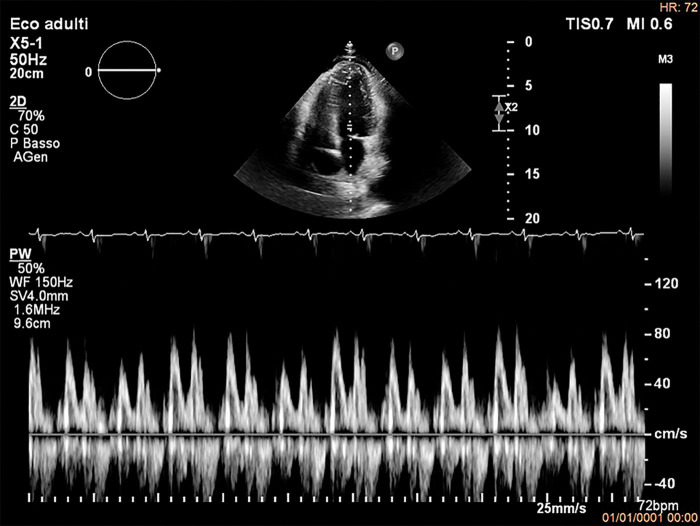
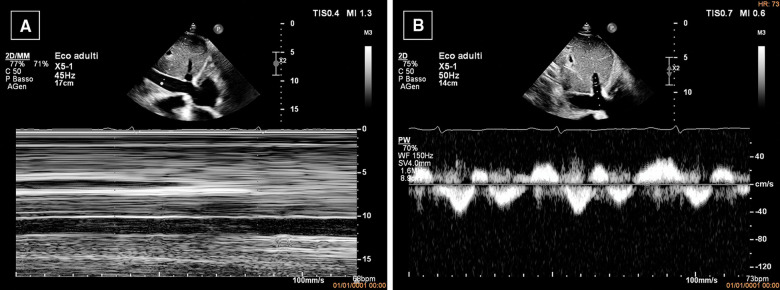
Due to the characteristics of the pain, acute pericarditis was suspected and an echocardiography was performed, with findings suggestive of ECP. In particular, the exam showed mild circumferential pericardial effusion (0.8 cm max in front of the right ventricle) with fibrinous stranding and pericardial thickening (Figure 1A) and annulus reversus (Figure 2). Respirophasic septal shift (Figure 1C and Video 1), septal bounce (Supplementary material online, Video S1), inspiratory decrease in mitral E velocity > 25%, and reduced E wave deceleration time (Figure 3) were also present, while cardiac chambers collapse was absent. Finally, the inferior vena cava plethora with prominent flow reversal in expiration was detected (Figure 4).
Figure 1.
Echocardiography. (A) Sub-costal view showing mild pericardial effusion with fibrinous stranding and pericardial thickening. The findings are suggestive for effusive–constrictive pericarditis. (B) Parasternal short-axis view showing D-shaped left ventricle, due to ventricular interdependence.
Figure 2.
Echocardiography. Lateral pulsed wave tissue Doppler imaging (PW-TDI) e′ values (9 cm/s) (A) are lower than septal PW-TDI e′ values (11 cm/s) (B), as opposed to normal. This finding is known as ‘annulus paradoxus’ and is caused by tethering of the lateral wall by the thickened pericardium.
Figure 3.
Echocardiography. PW Doppler of mitral inflow showing inspiratory decrease in mitral E velocity > 25% which reflects respiratory variation in ventricular filling. Reduced E wave deceleration time is also present. These are typical findings for constrictive physiology.
Figure 4.
Echocardiography. (A) M-mode evaluation shows dilated, non-collapsing inferior vena cava. (B) PW Doppler of hepatic vein showing flow reversal, which is prominent in expiration (arrow). The combination of ventricular septal shift, annulus paradoxus and prominent expiratory flow reversal in hepatic veins is specific for diagnosis of constrictive pericarditis.
Therefore, the patient was admitted to the cardiology ward and therapy with two 0.5 mg colchicine doses a day (1 mg daily) and three 600 mg ibuprofen doses a day (1800 mg daily) were started, with progressive reduction of inflammatory markers (CRP at time of discharge 16 mg/L) and complete resolution of the symptoms.
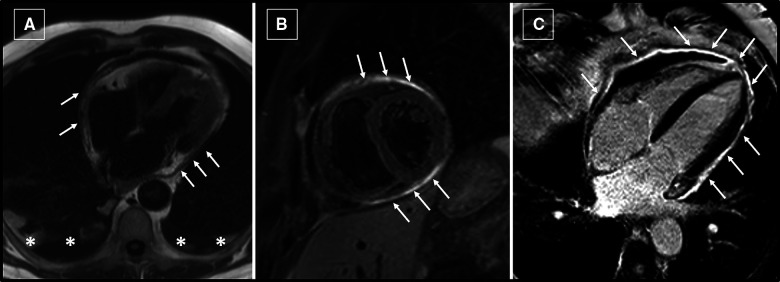
Cardiac magnetic resonance imaging showed pericardial thickening and oedema (Figure 5A and B), with intense late gadolinium enhancement of the pericardial layers (Figure 5C); a very mild pericardial effusion was detected (Video 2). Cine images showed septal bounce (Video 2) and inspiratory flattening of the inter-ventricular septum (Video 3), indicating ventricular interdependence and pericardial constriction.
Figure 5.
Cardiac magnetic resonance imaging. (A) Pericardial thickening (arrows) is evident on localizer images, as well as bilateral pleural effusion (asterisks). (B) Short-tau inversion-recovery T2-weighted image on short-axis orientation at mid-ventricular level. Hyperintense signal (arrows) of the pericardium indicates the presence of pericardial oedema. (C) Post-contrast image on four-chamber view shows intense enhancement of the pericardium (arrows).
No clear aetiology of the pericarditis was identified. In particular, viral tests for HIV, hepatitis C virus (HCV), Coxsackie, citomegalovirus (CMV), and Epstein-Barr virus (EBV) were negative for acute infections. Autoimmune diseases were ruled out, due to the absence of other suggestive symptoms and/or autoantibodies (ANA, ENA, and anti-dsDNA tested negative). Tuberculosis as well was deemed unlikely, since interferon gamma (INF-gamma) release assay was negative and radiological signs of pulmonary involvement were absent. Asbestosis was excluded, since there was no work-related exposure to asbestos or suggestive findings at chest CT scan. On the basis of the close temporal relation with the second shot of BNT162b2 vaccine, we hypothesized that the pericarditis might have been an adverse reaction to the drug, with a sub-acute evolution.
At 1-week follow-up the patient was completely asymptomatic, but CRP was still mildly elevated (18 mg/L) and control echocardiogram showed a reduction in the pericardial effusion, but persistence of constrictive physiology. Therefore, the patient was started on prednisone 25 mg daily and will be followed up closely in the next months for a possible therapeutical upgrade with IL1 receptor antagonists.1
Discussion
Effusive–constrictive pericarditis is a rare pericardial pathology, which combines clinical features of constrictive pericarditis and pericardial effusion, sometimes—as in our case—associated to pericardial inflammation. Effusive–constrictive pericarditis can require pericardiectomy and in some cases has a dire prognosis.2 Coronavirus disease 2019 can cause pericarditis,3 with mechanisms that are still unclear, but are possibly related to the systemic inflammatory response induced by the disease.4 No clear aetiology for ECP was identified in our patient. Notably, occurrence of ECP in COVID-19 disease has been documented.5 Although ECP is often deemed to have idiopathic origin, in this case there is a strong temporal relation between the second dose of BNT162b2 vaccine and symptoms occurrence. Nucleoside modified RNA vaccine BNT162b2 is remarkably effective (95% protection against COVID-19 after second dose) and safe: incidence of serious adverse events is very low.6 On the other hand, there are several reports of myocarditis and pericarditis after nucleoside modified RNA COVID vaccines in literature.6–13 Therefore, it is possible to speculate that in our patient the immune reaction against the spike protein of SARS-CoV2 might have caused ECP as a rare adverse reaction to the vaccine, with mechanisms that could be similar to natural infection.14 In particular, INF-gamma seems to have a role in post-vaccine pericarditis via mitogen-activated protein kinase (MAPK) and/or Janus kinase (JAK)/Stat pathways, which are key components of host defence to viral infection as well. Other potential mechanisms for cardiac involvement after mRNA based-vaccination include a molecular mimicry mechanism between viral spike protein and an unknown cardiac protein.15
In support of this hypothesis, the sudden onset of symptoms suggests that ECP presented acutely and constrictive physiology is not just a chronic collateral finding. However, it cannot be excluded that occurrence of pericarditis shortly after vaccine administration was just due to chance: correlation between ECP and vaccine in our case report is only presumptive. Guideline-directed therapy for acute pericarditis was implemented, with resolution of symptoms and sharp decrease of inflammatory markers. Nevertheless, the patient will need close echocardiographic and clinical follow-up.
Lead author biography

My name is Giacomo Maria Viani, MD. After attending San Raffaele University in Italy, I became a specialist in cardiology in December 2019 at state university of Milan. Early on in residency, I developed a strong interest in cardiac imaging. Currently, I work at Fatebenefratelli Hospital in Milan.
Supplementary material
Supplementary material is available at European Heart Journal - Case Reports online.
Slide sets: A fully edited slide set detailing this case and suitable for local presentation is available online as Supplementary data.
Consent: The authors confirm that written consent for submission and publication of this case report including images and associated text has been obtained from the patient in line with COPE guidance.
Conflict of interest: A.B.: Institution received funding from Kiniksa Pharmaceuticals, Ltd. as an investigative site; unrestricted research grant from SOBI and ACARPIA; travel and accommodation for advisory committee from SOBI and Kiniksa.
Funding: Publication charges were funded by Università degli Studi di Milano.
Supplementary Material
References
- 1.Andreis A, Imazio M, Giustetto C, Brucato A, Adler Y, De Ferrari GM.. Anakinra for constrictive pericarditis associated with incessant or recurrent pericarditis. Heart 2020;106:1561–1565. [DOI] [PubMed] [Google Scholar]
- 2.Miranda WR, Oh JK.. Effusive-constrictive pericarditis. Cardiol Clin 2017;35:551–558. [DOI] [PubMed] [Google Scholar]
- 3.Su Y-B, Kuo M-J, Lin T-Y, Chien C-S, Yang Y-P, Chou S-J. et al. Cardiovascular manifestation and treatment in COVID-19. J Chin Med Assoc 2020;83:704–709. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Costela-Ruiz VJ, Illescas-Montes R, Puerta-Puerta JM, Ruiz C, Melguizo-Rodríguez L.. SARS-CoV-2 infection: the role of cytokines in COVID-19 disease. Cytokine Growth Factor Rev 2020;54:62–75. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Diaconu R, Popescu L, Voicu A, Donoiu I.. Subacute effusive-constrictive pericarditis in a patient with COVID-19. BMJ Case Rep 2021;14:e242443. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Polack FP, Thomas SJ, Kitchin N, Absalon J, Gurtman A, Lockhart S, et al. ; C4591001 Clinical Trial Group. Safety and efficacy of the BNT162b2 mRNA Covid-19 vaccine. N Engl J Med 2020;383:2603–2615. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Dionne A, Sperotto F, Chamberlain S, Baker AL, Powell AJ, Prakash A. et al. Association of myocarditis with BNT162b2 messenger RNA COVID-19 vaccine in a case series of children. JAMA Cardiol 2021;6:1446. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Kwan MYW, Chua GT, Chow CB, Tsao SSL, To KKW, Yuen KY. et al. mRNA COVID vaccine and myocarditis in adolescents. Hong Kong Med J 2021;27:326–327. [DOI] [PubMed] [Google Scholar]
- 9.Verma AK, Lavine KJ, Lin C-Y.. Myocarditis after Covid-19 mRNA vaccination. N Engl J Med 2021;385:1332–1334. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Diaz GA, Parsons GT, Gering SK, Meier AR, Hutchinson IV, Robicsek A.. Myocarditis and pericarditis after vaccination for COVID-19. JAMA 2021;326:1210–1212. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Das BB, Moskowitz WB, Taylor MB, Palmer A.. Myocarditis and pericarditis following mRNA COVID-19 vaccination: what do we know so far? Children (Basel) 2021;8:607. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Bozkurt B, Kamat I, Hotez PJ.. Myocarditis with COVID-19 mRNA vaccines. Circulation 2021;144:471–484. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Gargano JW, Wallace M, Hadler SC, Langley G, Su JR, Oster ME. et al. Use of mRNA COVID-19 vaccine after reports of myocarditis among vaccine recipients: update from the advisory committee on immunization practices - United States, June 2021. Morb Mortal Wkly Rep 2021;70:977–982. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Hajjo R, Sabbah DA, Bardaweel SK, Tropsha A.. Shedding the light on post-vaccine myocarditis and pericarditis in COVID-19 and non-COVID-19 vaccine recipients. Vaccines (Basel) 2021;9:1186. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Larson KF, Ammirati E, Adler ED, Cooper LT, Hong KN, Saponara G. et al. Myocarditis after BNT162b2 and mRNA-1273 vaccination. Circulation 2021;144:506–508. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.