Abstract
Campylobacter species are one of the leading causes of gastroenteritis in humans. This review reports on the prevalence and antibiotic resistance data of Campylobacter spp. isolated from humans and food-producing animals in West Africa. A systematic search was carried out in five databases for original articles published between January 2000 and July 2021. Among 791 studies found, 38 original articles from seven (41%) out of the 17 countries in West Africa met the inclusion criteria. For studies conducted in food-producing animals, the overall pooled prevalence of Campylobacter spp. was 34% (95% CI: 25–45). The MDR prevalence was 59% (95% CI: 29–84) and half (50%, 13/26) of the animal studies had samples collected from the market. The human studies recorded a lower pooled prevalence of Campylobacter spp. (10%, 95% CI: 6–17), but a considerably higher rate of MDR prevalence (91%; 95% CI: 67–98). The majority (85%, 11/13) of the human studies took place in a hospital. Campylobacter jejuni and Campylobacter coli were the most common species isolated from both animals and humans. Our findings suggest that Campylobacter spp. is highly prevalent in West Africa. Therefore, improved farm hygiene and ‘One Health’ surveillance systems are needed to reduce transmission.
Keywords: campylobacteriosis, Campylobacter, pooled prevalence, food-producing animals, antibiotic resistance, West Africa
1. Introduction
Animals are natural reservoirs for Campylobacter spp. [1], which are among the leading causes of bacterial gastroenteritis in humans, worldwide [2]. Human Campylobacter infection is mainly acquired by the consumption of undercooked poultry, livestock, or by direct contact with animals [1]. A significant proportion of the population in Africa keeps livestock and/or poultry [3]. However, these animals are often reared and slaughtered under poor hygienic and sanitary conditions [4] and high frequencies of Campylobacter have been reported in animal husbandry. For example, in Nigeria and Côte d’Ivoire, Campylobacter were isolated from 82% [5] and 81% [6] of fecal samples from poultry, respectively. There is sufficient evidence that Campylobacter found in retail poultry eventually lead to infections in humans [7].
In humans, the species Campylobacter jejuni and coli are mainly associated with campylobacteriosis followed by Campylobacter lari [8]. Dogs and cats are also known to harbor pathogenic Campylobacter species which cause infections in humans [9]. Although Campylobacter infections are typically self-limiting, in immunocompromised individuals post-infection complications such as reactive arthritis (painful inflammation of the joints) and Guillain-Barré syndrome (neurological disorders) might occur [10]. Additionally, Campylobacter species resistant to commonly used antibiotics are on the increase in Sub-Saharan Africa [11] and worldwide [12]. While information on Campylobacter spp. from industrialized countries is broadly available [1], only few meta-analyses have been performed on Campylobacter prevalence studies from Africa [8,13,14].
This systematic review and meta-analysis reports on the prevalence and antibiotic resistance data of Campylobacter spp. in humans and food-producing animals in West Africa from 1 January 2000 to 31 July 2021. To the best of our knowledge, this is the first meta-analysis from West Africa conducted on Campylobacter in both animals and humans in the last 21 years.
2. Results
2.1. Literature Search
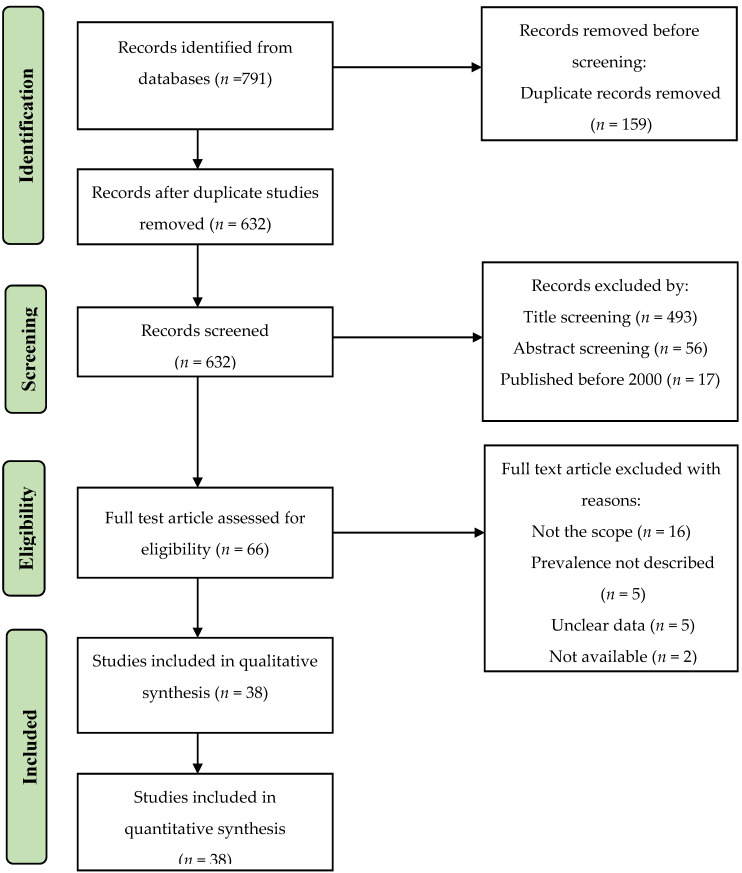
A total of 791 studies were initially identified across Medline (via PubMed), Directory of Open Access Journals (DOAJ), Google Scholar, African Index Medicus and the African Journal Online (AJOL) database. After the removal of duplicate articles, 632 unique articles remained, out of which, 66 articles fulfilled the eligibility criteria for full-text review. Twenty-eight of the full-text articles were excluded due to the following reasons: 16 were not within the scope of this review, five studies did not provide Campylobacter prevalence, another five had unclear data, and two were unavailable. Finally, 38 original research articles describing Campylobacter prevalence in West African countries were found to be eligible for further analysis. Figure 1 shows a flowchart of the article selection process. Out of the 38 eligible studies, 26 were conducted in food-producing animals [5,6,15,16,17,18,19,20,21,22,23,24,25,26,27,28,29,30,31,32,33,34,35,36,37,38]. One out of the 26 animal studies also had prevalence data on humans [38], thus 13 human studies were included [38,39,40,41,42,43,44,45,46,47,48,49,50]. Four studies conducted in humans were case-control studies and reported Campylobacter frequencies in both diarrhea and non-diarrhea patients [39,41,43,48]. One study each was conducted among pig farmers [38] and patients with urinary tract infections (UTIs) [46].
Figure 1.
Flow diagram of the article selection process.
2.2. Number of Campylobacter Prevalence Studies Conducted
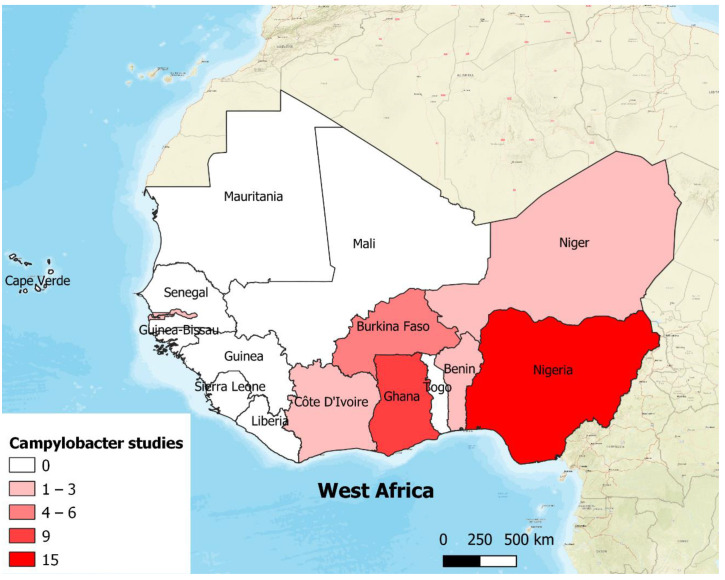
Campylobacter spp. prevalence data were available from seven (Nigeria, Ghana, Burkina Faso, Côte d’Ivoire, Benin, Niger and The Gambia) out of the 17 countries in West Africa (Figure 2). Approximately 39% (n/N = 15/38) of the 38 studies identified in this review were conducted in Nigeria followed by studies conducted in Ghana (24%, n/N = 9/38). The majority (87%, n/N = 33/38) of the studies were conducted between 2011 and 2021.
Figure 2.
Number of included Campylobacter prevalence studies conducted by countries in West Africa between 2000 and 2021.
2.3. Subgroup Analysis of Campylobacter Studies in Animals
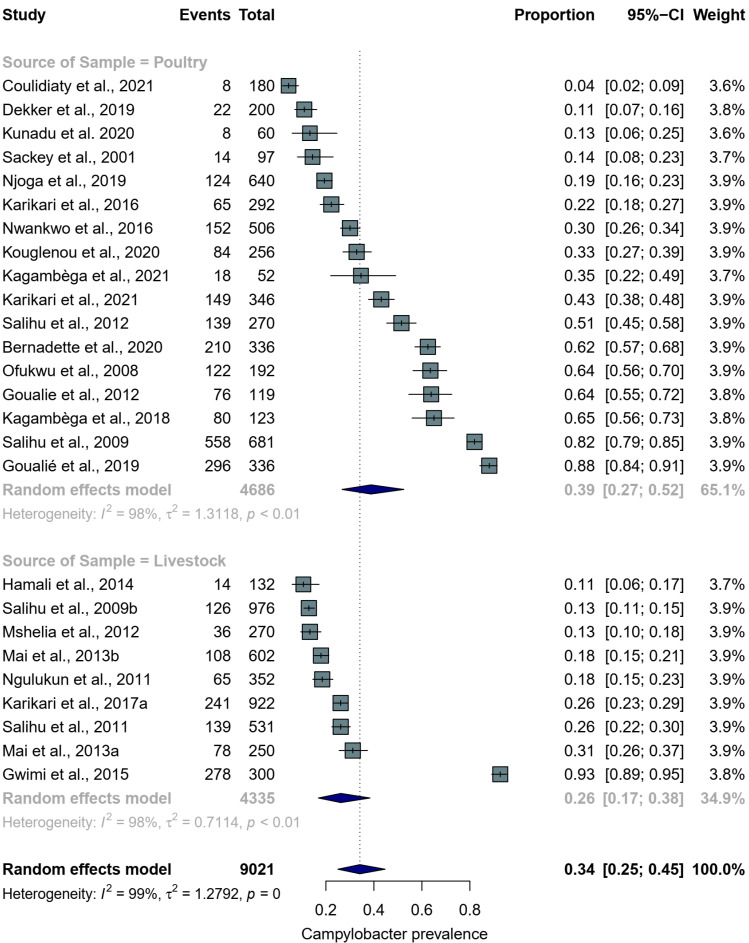
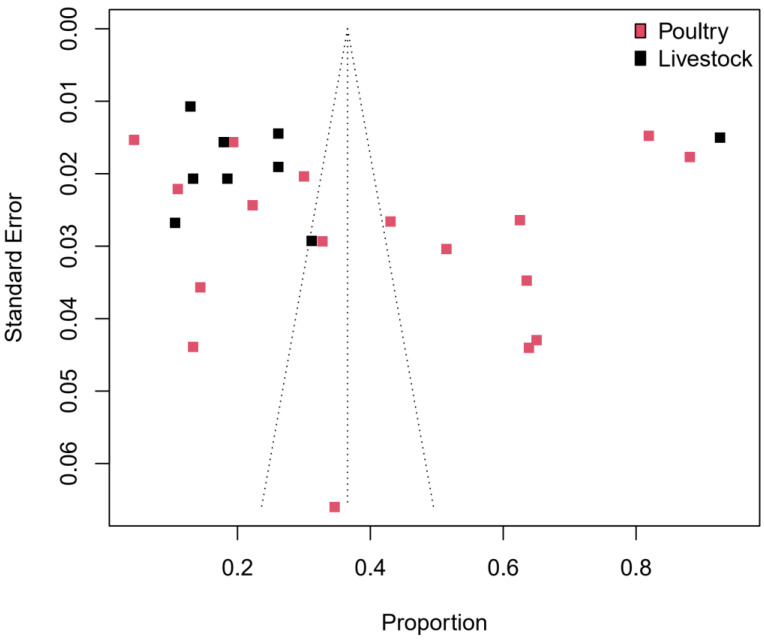
Figure 3 shows a forest plot with individual, subgroup and overall pooled prevalence estimates of Campylobacter spp. from the 26 animal studies with a total sample size of 9021. The individual prevalence estimates ranged widely, from 4% to 88% in poultry and 11% to 93% in livestock. For the subgroup analysis, poultry recorded a higher pooled prevalence (39%, 95% CI: 27–52) than livestock (26%, 95% CI: 17–38). The overall random-effects pooled prevalence of Campylobacter spp. isolated from poultry and livestock samples from West Africa was 34% (95% CI: 25–45) with a very high level of heterogeneity (I2 = 99%). Figure 4 shows a funnel plot with asymmetric distribution of Campylobacter studies conducted in food-producing animals in West Africa. Only three [22,23,37] out of the 26 food-producing animal studies lie within the triangular region, where 95% of the studies are expected to lie. The poultry studies show high variability in the prevalence rates, irrespective of the study sample size. A similar result is observed in the livestock studies; apart from one livestock study [38] that recorded the highest prevalence (93%), all the other seven studies had a prevalence of ≤31% and standard errors of ≤0.03.
Figure 3.
Forest plot showing Campylobacter prevalence from poultry and livestock studies from West Africa between 2000 and 2021. The light blue squares represent individual study weight in the meta-analysis and the black lines within the square reflect the 95% CI. The navy blue diamonds represent the results for random effects models, the left and right endpoints of which are the lower and upper bounds of the 95% CI, respectively.
Figure 4.
Funnel plot with 95% confidence limits showing the prevalence of Campylobacter species in poultry and livestock in West Africa.
Table 1 shows subgroup analysis of Campylobacter studies conducted in food-producing animals in West Africa. Three studies from Côte d’Ivoire [6,26,28] recorded the highest country-level pooled prevalence of 74% (95% CI: 52–88) but their combined sample size of 791 (weight = 11.6%) was the third largest. Nigeria, on the other hand, recorded the highest number of studies included [5,19,21,25,27,30,31,32,33,34,36,37,38] with a total sample size of 5702 (weight = 50.5%) and a pooled Campylobacter prevalence of 37% (95% CI: 25–51). There were six studies from Ghana [16,17,18,20,24,35] with a sample size of 1917 (weight = 22.8%) and pooled prevalence of 21% (95% CI: 14–30). About 43% (n/N = 13/30) of the animal studies collected their samples from markets [6,16,17,18,20,21,22,23,26,28,29] and in these studies, the highest pooled Campylobacter prevalence was observed (37%, 95% CI: 23–52). Most of the samples used were carcasses (43%, n/N = 13/30), followed by rectal swabs (23%, n/N = 7/30) and feces (23%, n/N = 7/30). Five studies conducted in animals combined culture and PCR diagnostic methods for the detection of Campylobacter [6,22,26,28,34] and this diagnostic method recorded the highest pooled isolation rate of 54% (95% CI: 28–78). The majority (62%, n/N = 16/26) of the studies used both culture and biochemical methods for strain identification, which recorded a pooled prevalence of 32% (95% CI: 21–47). Out of the 26 Campylobacter studies conducted in animals, 25 (96%) reported data on the various Campylobacter species isolated. C. jejuni (88%, n/N = 22/25) and C. coli (68%, n/N = 17/25) were the most reported Campylobacter spp. with a pooled prevalence of 52% (95% CI: 42–63) and 30% (95% CI: 22–40), respectively.
Table 1.
Pooled prevalence of Campylobacter spp. in animals stratified by subgroup variables.
| Variables | Included Studies | Sample Size | Pooled Prevalence (95% CI) | I2 (%) | p Value |
|---|---|---|---|---|---|
| Country | |||||
| Nigeria | 13 | 5702 | 34 (21–51) | 99 | <0.01 |
| Ghana | 6 | 1917 | 21(14–30) | 94 | <0.01 |
| Burkina Faso | 3 | 355 | 27 (5–73) | 98 | <0.01 |
| Cote d’Ivoire | 3 | 791 | 74 (52–88) | 97 | <0.01 |
| Benin | 1 | 256 | 33 (27–39) | - | - |
| Study setting a | |||||
| Market | 13 | 2367 | 37 (23–52) | 97 | <0.01 |
| Farm | 10 | 3955 | 31 (18–47) | 99 | <0.01 |
| Abattoir | 6 | 2670 | 33 (15–57) | 99 | <0.01 |
| Veterinary clinic | 1 | 473 | 11 (6–17) | - | - |
| Type of Sample a | |||||
| Carcasses | 13 | 3353 | 35 (21–53) | 98 | <0.01 |
| Rectal swab | 7 | 2930 | 33 (17–54) | 98 | <0.01 |
| Feces | 7 | 1719 | 32 (19–50) | 97 | <0.01 |
| Preputial scraping | 3 | 1122 | 20 (12–31) | 92 | <0.01 |
| Diagnostic method | |||||
| Culture and biochemistry | 16 | 5970 | 32 (21–47) | 99 | <0.01 |
| Culture and PCR | 5 | 1399 | 54 (28–78) | 98 | <0.01 |
| PCR only | 3 | 1106 | 22 (12–36) | 88 | <0.01 |
| Culture and latex agglutination | 1 | 346 | 43 (38–48) | - | - |
| Culture and MALDI-TOF MS | 1 | 200 | 11 (7–16) | - | - |
| Campylobacter species | |||||
| C. jejuni | 22 | 3075 | 52 (42–63) | 96 | <0.01 |
| C. coli | 17 | 2512 | 30 (22–40) | 95 | <0.01 |
| C. lari | 7 | 1420 | 12 (6–22) | 84 | <0.01 |
| C. fetus | 5 | 434 | 8 (1–46) | 93 | <0.01 |
| C. hyointestinalis | 4 | 505 | 4 (2–7) | 39 | 0.18 |
| C. jejuni subsp.doylei | 3 | 320 | 5 (1–21) | 80 | <0.01 |
| C. upsaliensis | 2 | 292 | 12 (2–49) | 89 | <0.01 |
| C. sputorum | 1 | 36 | 6 (1–20) | - | - |
I2-heterogeneity; a number of included studies is greater than 26 because three studies had data on two groups.
2.4. Subgroup Analysis of Campylobacter Studies in Humans
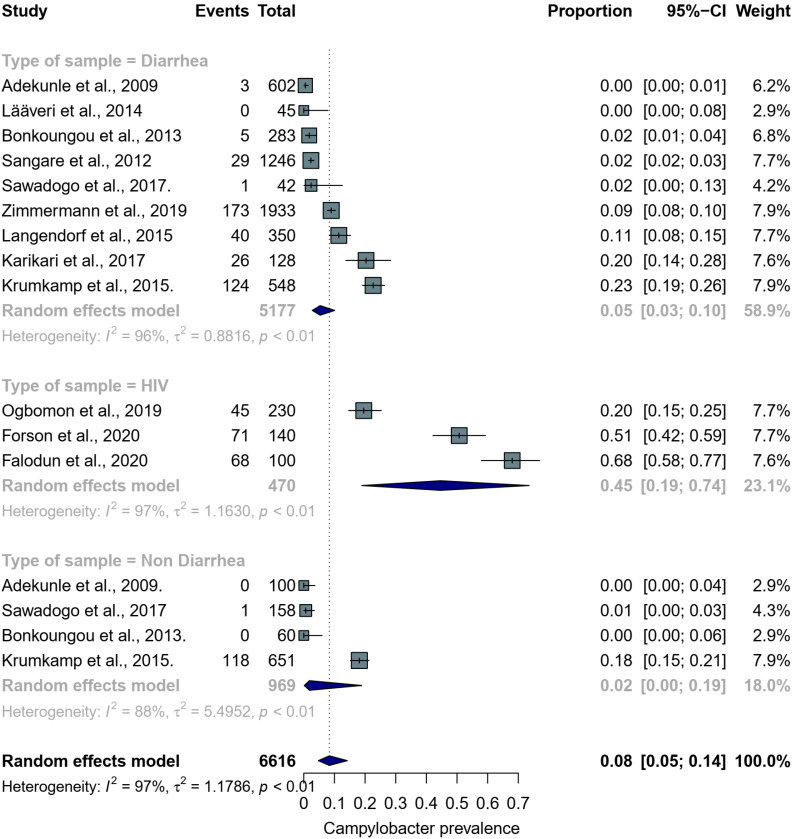
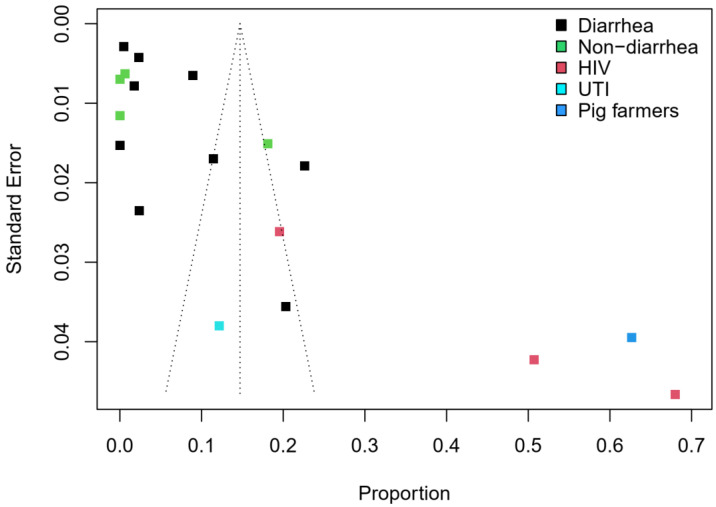
Thirteen articles on Campylobacter prevalence in humans were included, with a total sample size of 6840. The second highest prevalence was observed in a single study [38] conducted among pig farmers and their household members (63%, 95% CI: 54–70). One study [46] conducted among patients with UTI reported a prevalence of 12% (95% CI: 6–22). Figure 5 shows the forest plot of the remaining Campylobacter prevalence studies conducted in HIV, diarrhea and non-diarrhea patients. A high pooled prevalence of 45% (95% CI: 19–74) was estimated for HIV patients. The pooled prevalence for diarrhea patients (5%, 95% CI: 3–10) was higher than in non-diarrhea patients (2%, 95% CI: 0–19). The overall pooled estimate in humans was 10% (95% CI: 6–17) with a considerably high level of heterogeneity (I2 = 98%). Figure 6 shows a funnel plot with asymmetric distribution of subgroups of Campylobacter prevalence studies conducted in humans. The majority of studies (61%, n/N = 11/18) had low standard errors (≤0.02) and 83% (n/N = 15/18) recorded a prevalence of ≤20%. A study [50] conducted among HIV patients had the highest prevalence (68%) as well as the highest standard error (>0.04).
Figure 5.
Forest plot showing Campylobacter prevalence in HIV, diarrhea and non-diarrhea patients. The light blue squares represent individual study weight in the meta-analysis and the black lines within the square reflect the 95% CI. The navy blue diamonds represent the results for random effects models, the left and right endpoints of which are the lower and upper bounds of the 95% CI, respectively.
Figure 6.
Funnel plot with 95% confidence limits showing the prevalence of Campylobacter species in humans in West Africa.
Table 2 shows subgroup analysis of Campylobacter studies conducted in humans in West Africa. Most (85%, n/N = 11/13) of the studies were conducted in a hospital setting [39,41,42,43,44,45,46,47,48,49,50] and four (29%, n/N = 4/14) were diarrhea and non-diarrhea case-control studies [39,41,43,47]. The highest pooled Campylobacter prevalence (33%, 95% CI: 13–62) was estimated in adults (>15 years), while children less than five years had the lowest (4%, 95% CI: 2–8). A combination of culture and biochemistry diagnostic methods was used by 46% (n/N = 6/13) of the studies to isolate and identify the bacteria [38,42,45,46,48,50]. This method recorded the highest pooled prevalence estimate of 22% (95% CI: 7–51). Eight studies reported data on Campylobacter species [38,39,46,47,48,49,50], of which C. coli and C. jejuni were most common with a pooled prevalence of 47% (95% CI: 25–69) and 42% (95% CI 26–59), respectively.
Table 2.
Pooled prevalence of Campylobacter spp. in humans stratified by subgroup variables.
| Variables | Included Studies | Sample Size | Pooled Prevalence (95% CI) | I2 (%) | p Value |
|---|---|---|---|---|---|
| Country | |||||
| Nigeria | 4 | 1182 | 22 (5–58) | 98 | <0.01 |
| Ghana | 3 | 1576 | 27(13–36) | 97 | <0.01 |
| Burkina Faso | 3 | 1729 | 2 (2–3) | 0 | 0.45 |
| Benin | 1 | 45 | 1 (0–2) | - | - |
| Gambia | 1 | 1933 | 9 (8–10) | - | - |
| Niger | 1 | 350 | 11 (8–15) | - | - |
| Study setting | |||||
| Hospital | 11 | 6620 | 10 (5–18) | 98 | <0.01 |
| Community | 2 | 195 | 14 (0–96) | 92 | <0.01 |
| Study design | |||||
| Cross sectional | 8 | 2618 | 19 (7–42) | 99 | |
| Case control | 4 | 2264 | 2(0–19) | 96 | |
| Retrospective | 1 | 1933 | 9 (8–10) | - | - |
| Age range | |||||
| Adults only (>15) | 4 | 515 | 33 (13–62) | 96 | <0.01 |
| <13 years | 1 | 1234 | 20 (17–22) | - | - |
| <5 years | 4 | 3268 | 4 (2–8) | 93 | <0.01 |
| All ages | 4 | 1798 | 9 (1–47) | 99 | <0.01 |
| Diagnostic method | |||||
| Culture and biochemistry | 6 | 2278 | 22 (7–51) | 99 | <0.01 |
| PCR only | 4 | 3412 | 7 (3–15) | 97 | <0.01 |
| Culture and PCR | 3 | 1125 | 4 (0–63) | 99 | <0.01 |
| Campylobacter species | |||||
| C. coli | 7 | 397 | 47 (25–69) | 91 | <0.01 |
| C. jejuni | 6 | 565 | 42 (26–59) | 86 | <0.01 |
| C. lari | 3 | 249 | 12 (4–28) | 81 | <0.01 |
| C. upsaliensis | 3 | 243 | 11 (3–33) | 87 | <0.01 |
| C. fetus | 2 | 165 | 13 (9–20) | 0 | 0.61 |
| C. hyointestinalis | 2 | 139 | 6 (3–11) | 0 | 0.75 |
| C. jejuni subsp.doylei | 1 | 35 | 3 (0–18) | - | - |
I2-heterogeneity.
2.5. Antimicrobial Resistance Profile of Campylobacter Species
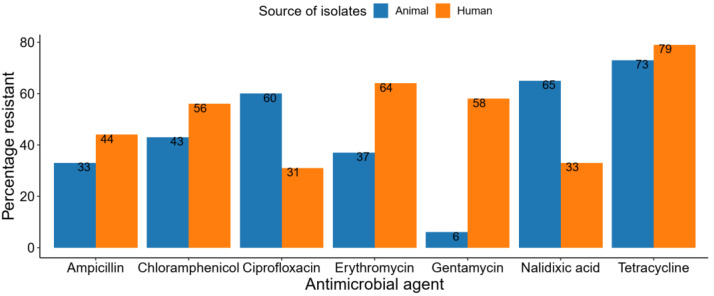
Apart from ciprofloxacin and nalidixic acid, a higher proportion of antibiotic resistance was observed in Campylobacter isolates from humans than from animals (Figure 7). The majority of Campylobacter isolates recovered from animals were susceptible to gentamicin (94%, n/N = 1183/1265) and those from humans to ciprofloxacin (69%, n/N = 175/255).
Figure 7.
The proportion of Campylobacter spp. resistant to commonly tested antibiotics.
Resistance to three or more antibiotics, multi-drug resistance (MDR), was reported in 10 studies, two studies in humans [46,50] and eight in animals [6,20,22,24,25,26,35,36]. The overall pooled prevalence estimate for AMR was 69% (95% CI: 40–88, I2 = 98%), 91% (95% CI: 67–98, I2 = 65%) in the human studies and 59% (95% CI: 29–84, I2 = 98%) in food-producing animals. Two studies tested 306 Campylobacter isolates against imipenem and observed 0% resistance [20,35]. The following virulence makers: cdtA, cdtB, cdtC, cadF [6,26]; antibiotic resistance genes: tet (O), blaOXA-61, aadE, and cmeB [49] and change in amino acid sequence of the gyrA gene [16] have been reported by some studies.
3. Discussion
This review shows that the majority (58.8%) of countries in West Africa has no published studies on Campylobacter prevalence that met our inclusion criteria. This finding is in agreement with other systematic reviews that also report low numbers of Campylobacter research in Africa [8,14]. The cumbersome procedures involved in isolating Campylobacter spp. makes it difficult for most low-income countries to conduct such studies. Nigeria (50%) and Ghana (23%) had the highest number of studies, probably because these countries have a higher socio-economic status in the region, hence they can afford well-equipped health and research facilities needed to conduct such research [51]. The number of studies published within 2011–2021 was far more than during the preceding decade. This could be because knowledge of new and advanced methods of detection (such as PCR) became available in recent years. Additionally, researchers are now becoming more aware of the burden of Campylobacter infections in humans and animals.
Poultry and livestock samples recorded the highest pooled prevalence of Campylobacter spp. The intestinal tract of poultry and livestock are frequently colonized in high numbers by Campylobacter spp., hence constituting a natural reservoir and an important source of transmission [1,52]. Studies conducted on Campylobacter spp. colonization in poultry and livestock are in agreement with the current findings [1,53]. This suggests that poultry and other animals are primary reservoirs responsible for Campylobacter infections in humans [54,55].
Our review shows that the pooled prevalence of Campylobacter infections in humans was 10% (95% CI: 6–17). Summarized findings from Ethiopia [56] and Sub-Saharan African countries [8] have reported a similar pooled prevalence in humans (9–10%). West Africa has just four published articles on Campylobacter infection in children under 5 years of age. Surprisingly, these studies recorded a pooled prevalence of 4%, lower than the 10% reported in a summary of results from Ethiopia [57]. The lower Campylobacter prevalence observed in children might be because most of the included studies were conducted in healthy (non-diarrhea) subjects. The low number of studies conducted within this age group shows that some populations in West Africa have not been investigated; hence, there is the need to conduct more studies in these populations. Our review reported high heterogeneity between studies, which could be due to differences in environmental conditions, socio-demographics, sociocultural factors and disease awareness levels. Additionally, the protocols used and the experience level of staff in isolating the bacteria could account for the differences in prevalence observed between studies.
Approximately 56% of the studies used culture and biochemical tests to identify Campylobacter spp. Other reviews conducted in Africa have also observed that this method is most common for identifying Campylobacter in the region [8,11]. However, the culture method has some limitations; environmental stress during sample transportation and processing can make some Campylobacter spp. viable but not culturable on media [58], this could lead to lower sensitivity [59]. In our review, the culture and biochemical method produced a high pooled prevalence of Campylobacter species. However, these findings must be interpreted with caution because the culture method has lower specificity compared to PCR-based methods. A lot of researchers in West Africa rely on the laborious and time-consuming culture method because their laboratories are not well equipped to use PCR in the diagnoses of Campylobacter. This could be a possible reason why fewer studies have been conducted.
C. jejuni and C. coli were isolated by 28 and 24 studies, respectively, making them the predominantly isolated Campylobacter species from both food-producing animals and humans. Other authors have reported similar observations [8,11]. The high numbers of virulence genes associated with C. jejuni and C. coli [60] possibly make researchers develop research questions focused on discovering more of these species. Additionally, the high prevalence of C. jejuni and C. coli observed in this study could be attributed to the culture and biochemical test method used for speciation, which is incapable of detecting the lesser-known Campylobacter spp. [61]. Campylobacter selective media containing antibiotics and higher incubation temperatures does inhibit the growth of some Campylobacter species such as C. upsaliensis and C. lari [11]. Nonetheless, it is well known that the two Campylobacter species most frequently associated with diarrhea in humans are C. jejuni and C. coli [62].
The 69% AMR recorded by this review shows that Campylobacter’s resistance to commonly used antibiotics is widespread in both humans and animals. Among the most tested antibiotics, Campylobacter were found to be highly resistant to tetracycline, nalidixic acid, ciprofloxacin, erythromycin, chloramphenicol, ampicillin and gentamycin. Ciprofloxacin resistance rate in animal isolates was higher than humans, even though it is not approved for use in veterinary medicine. Consistent with our findings, 88% of E. coli isolated from poultry farms in Ibadan, Nigeria were resistant to ciprofloxacin [63], suggesting that its use in poultry and livestock farming may be on the increase. We also observed high erythromycin resistance in human isolates compared to animals. The high erythromycin resistance observed in humans could be explained by the overuse of azithromycin due to its low risk of side effects [64]. The high antimicrobial-resistant rate observed in our study agrees with findings from similar studies conducted in both low and middle-income countries [65] and high-income countries [66] showing an increasing trend of antibiotic resistance in Campylobacter spp. The increasing trend might also be due to the extensive use of antimicrobials in animal farming for growth promotion and prophylaxis [67] and the indiscriminate use in humans [68]. Carbapenem resistance is on the increase in Gram-negative bacteria [69]; however, our review observed that all isolates tested against imipenem were susceptible. The high rate of Campylobacter susceptibility observed against imipenem might be attributed to it not being authorized for use in animal husbandry [70].
To lower the high Campylobacter prevalence and antimicrobial resistance observed in this review, we recommend the appropriate use of antibiotics in human and veterinary medicine, improved hygiene and sanitation practices and the implementation of biosecurity measures in farms [65]. If possible, antimicrobial susceptibility testing should be performed before the administration of antibiotics to humans. Since the virulence and pathogenicity of Campylobacter is affected by the genetic variants, we recommend the use of molecular diagnostic methods in addition or as a replacement to the widely used culture method, in order to accurately diagnose infections and to determine the real Campylobacter burden [60]. Furthermore, strong commitment from policymakers is needed to implement ‘One Health’ surveillance systems.
Our systematic review and meta-analysis have few potential limitations. The search strategy was limited to only articles published in English, there might be articles published in other languages that were not considered. The analyses were not uniformly spread since data was absent from majority of the countries and most of the studies were conducted between 2011 and 2021. Another limitation of our review is that majority of the studies used culture methods, which is not the preferred method for reporting Campylobacter prevalence. We recorded high heterogeneity because studies conducted in different countries and under different conditions were pooled together. Since we only found data from less than half of the countries in West Africa, our findings may not be generalizable to the entire region.
4. Materials and Methods
4.1. Study Design and Systematic Review Protocol
The protocol of this review is registered at PROSPERO with registration number: CRD 42021260515. This study was conducted according to the Preferred Reporting Items for Systematic Reviews and Meta-Analyses (PRISMA) guidelines [71]. The UN macro-geographical definition of West Africa (https://unstats.un.org/unsd/methodology/m49/ (accessed on 29 September 2021) was used to define West African countries included in this review, namely: Benin, Burkina Faso, Cape Verde, Côte d’Ivoire, Gambia, Ghana, Guinea, Guinea-Bissau, Liberia, Mali, Mauritania, Niger, Nigeria, Saint Helena, Senegal, Sierra Leone and Togo.
4.2. Selection Criteria and Literature Search Strategies
A systematic search for original articles covering West African countries and published between January 2000 and July 2021 was conducted using the following databases: Medline (via PubMed), Directory of Open Access Journals (DOAJ), Google Scholar, African Index Medicus and the African Journal Online (AJOL) database. The systematic search of these databases was performed using the search terms listed in the Supplement File S1. EKP screened the titles and abstracts of all recovered articles. Articles were eligible for full-text review when: (i) they contained data from a West African country, (ii) they were published between January 2000 to July 2021, and (iii) they were written in English. During the full-text review, two authors (EKP, SA) independently assessed the articles to determine if each one met the inclusion criteria. An article was included if it contained primary data, was conducted in food-producing animals and/or humans, and Campylobacter prevalence was reported or can be calculated from available information. Articles whose full texts could not be accessed and those with inconsistent results, overlapping or duplicate data were excluded. Additionally, articles that did not report on the age of study participants, type of samples collected and laboratory diagnostic method used were excluded. In case of any disagreement in the review process, a third reviewer (LAO) was available to give a decisive opinion on any unresolved issues.
4.3. Data Extraction
For each included original full-length study article, we extracted data on the first author, year of publication, name of the country where the study was conducted, type of food-producing animals sampled, age of human participants, type of samples collected, sample size, study design and study setting. We also collected data on the laboratory diagnostic methods used, Campylobacter prevalence, Campylobacter spp. isolated, antimicrobials tested and antibiotic resistance.
4.4. Risk of Bias Assessment
Conventional funnel plots show inaccurate results when assessing publication bias in systematic reviews on prevalence studies [72]. This is because of the unequal and small sample sizes, high prevalence diversity due to study design differences and zero prevalence which may be recorded in studies. Therefore, we decided to include all studies that met the final inclusion criteria without assessing the risk of publication bias. Nonetheless, funnel plots were plotted to indicate the across-study biases and between-study heterogeneity.
4.5. Data Analysis
In studies that did not explicitly report Campylobacter prevalence, but reported the number of positives and the total number of samples collected, the prevalence was calculated as the fraction of both terms. The Meta (version 5.0-1, R Core Team, Vienna, Austria) package, in R software (version 4.1.1, R Core Team, Vienna, Austria) [73], was used to calculate pooled prevalence estimates using a random-effects model [74]. The pooled prevalence with a 95% confidence interval (CI) was presented using forest plots and tables. The heterogeneity of study prevalence estimates was evaluated by computing the inverse variance index (I2) statistic. Heterogeneity was considered to be high when I2, which describes the percentage of total variation between studies that is attributable to prevalence differences rather than chance, was above 75%.
Subgroup analyses were used to investigate potential associations with the prevalence estimates. The potential sources of heterogeneity were investigated considering the year of publication, the country where sampling occurred, study setting, sample type, age of human participants, laboratory diagnostic method used and types of Campylobacter species isolated. For human studies, the subgroup analysis also included patients with and without diarrhea, HIV and urinary tract infections (UTI). The proportion of Campylobacter spp. that were resistant to commonly tested antibiotics was calculated for both food-producing animal and human studies. The ggplot2 package, in the R (version 4.1.1) statistical environment, was used to plot a bar chart to illustrate the proportion of resistant Campylobacter in humans and animals. QGIS software (version 3.18.3, QGIS Development Team, Zurich, Switzerland) [75] was used to draw a map to show the number of Campylobacter prevalence studies across West Africa.
5. Conclusions
Research articles on Campylobacter prevalence were not available from 59% of countries in West Africa. Countries in West Africa should be supported to have well-equipped laboratories for Campylobacter research. To curb the high Campylobacter prevalence and resistance observed in this review, routine diagnosis, appropriate use of antibiotics, improved hygienic practices and ‘One Health’ surveillance systems should be implemented. Furthermore, strong commitment from policymakers and societal actions are needed to improve farm hygiene and antimicrobial usage in food-producing animals and humans.
Supplementary Materials
The following supporting information can be downloaded at: https://www.mdpi.com/article/10.3390/pathogens11020140/s1. File S1: Search Strategy.
Author Contributions
Conceptualization, E.K.P., L.A.O.; methodology, D.D., R.K., C.W.A., S.A., M.L., O.M.-A., R.O.P. and E.K.P.; validation, R.K., L.A.O., K.O.D., C.W.A. and D.D.; formal analysis, E.K.P. and R.K.; data curation, S.A., M.L. and C.W.A. original draft preparation, E.K.P.; writing—review and editing, E.K.P., D.D., R.K., L.A.O., K.O.D., R.O.P. and O.M.-A.; supervision, L.A.O., K.O.D., R.O.P. and D.D.; funding acquisition, D.D. and J.M. All authors have read and agreed to the published version of the manuscript.
Funding
This work was funded by the German Research Foundation (DFG; project number 380545990).
Institutional Review Board Statement
Not applicable.
Informed Consent Statement
Not applicable.
Data Availability Statement
The data presented in this study are available on request from the corresponding author.
Conflicts of Interest
The authors declare no conflict of interest.
Footnotes
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations.
References
- 1.Plishka M., Sargeant J.M., Greer A.L., Hookey S., Winder C. The Prevalence of Campylobacter in Live Cattle, Turkey, Chicken, and Swine in the United States and Canada: A Systematic Review and Meta-Analysis. Foodborne Pathog. Dis. 2021;18:230–242. doi: 10.1089/fpd.2020.2834. [DOI] [PubMed] [Google Scholar]
- 2.Galanis E. Campylobacter and Bacterial Gastroenteritis. CMAJ. 2007;177:570–571. doi: 10.1503/cmaj.070660. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Molina-Flores B., Manzano-Baena P., Coulibaly M.D. The Role of Livestock in Food Security, Poverty Reduction and Wealth Creation in West Africa. FAO; Rome, Italy: 2020. [Google Scholar]
- 4.Ngure F., Gelli A., Becquey E., Ganaba R., Headey D., Huybregts L., Pedehombga A., Sanou A., Traore A., Zongo F., et al. Exposure to Livestock Feces and Water Quality, Sanitation, and Hygiene (WASH) Conditions among Caregivers and Young Children: Formative Research in Rural Burkina Faso. Am. J. Trop. Med. Hyg. 2019;100:998–1004. doi: 10.4269/ajtmh.18-0333. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Salihu M.D., Junaidu A.U., Magaji A.A., Abubakar M.B., Adamu A.Y., Yakubu A.S. Prevalence of Campylobacter in Poultry Meat in Sokoto, North-Western Nigeria. J. Public Health Epidemiol. 2009;1:41–45. [Google Scholar]
- 6.Goualié B.G., Akpa E.E., Kakou-N’Gazoa S.E., Ouattara H.G., Niamke S.L., Dosso M. Antimicrobial Resistance and Virulence Associated Genes in Campylobacter Jejuni Isolated from Chicken in Côte d’Ivoire. J. Infect. Dev. Ctries. 2019;13:671–677. doi: 10.3855/jidc.11355. [DOI] [PubMed] [Google Scholar]
- 7.Hodges L.M., Carrillo C.D., Upham J.P., Borza A., Eisebraun M., Kenwell R., Mutschall S.K., Haldane D., Schleihauf E., Taboada E.N. A Strain Comparison of Campylobacter Isolated from Retail Poultry and Human Clinical Cases in Atlantic Canada. PLoS ONE. 2019;14:e0215928. doi: 10.1371/journal.pone.0215928. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Hlashwayo D.F., Sigaúque B., Noormahomed E.V., Afonso S.M.S., Mandomando I.M., Bila C.G. A Systematic Review and Meta-Analysis Reveal That Campylobacter Spp. and Antibiotic Resistance Are Widespread in Humans in Sub-Saharan Africa. PLoS ONE. 2021;16:e0245951. doi: 10.1371/journal.pone.0245951. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Thépault A., Rose V., Queguiner M., Chemaly M., Rivoal K. Dogs and Cats: Reservoirs for Highly Diverse Campylobacter Jejuni and a Potential Source of Human Exposure. Animals. 2020;10:838. doi: 10.3390/ani10050838. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Endtz H.P. Hunter’s Tropical Medicine and Emerging Infectious Diseases. Elsevier; Amsterdam, The Netherlands: 2020. Campylobacter Infections; pp. 507–511. [Google Scholar]
- 11.Gahamanyi N., Mboera L.E.G., Matee M.I., Mutangana D., Komba E.V.G. Prevalence, Risk Factors, and Antimicrobial Resistance Profiles of Thermophilic Campylobacter Species in Humans and Animals in Sub-Saharan Africa: A Systematic Review. Int. J. Microbiol. 2020;2020:2092478. doi: 10.1155/2020/2092478. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Tang Y., Fang L., Xu C., Zhang Q. Antibiotic Resistance Trends and Mechanisms in the Foodborne Pathogen, Campylobacter. Anim. Health Res. Rev. 2017;18:87–98. doi: 10.1017/S1466252317000135. [DOI] [PubMed] [Google Scholar]
- 13.Thomas K.M., de Glanville W.A., Barker G.C., Benschop J., Buza J.J., Cleaveland S., Davis M.A., French N.P., Mmbaga B.T., Prinsen G., et al. Prevalence of Campylobacter and Salmonella in African Food Animals and Meat: A Systematic Review and Meta-Analysis. Int. J. Food Microbiol. 2020;315:108382. doi: 10.1016/j.ijfoodmicro.2019.108382. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Hlashwayo D.F., Sigaúque B., Bila C.G. Epidemiology and Antimicrobial Resistance of Campylobacter Spp. in Animals in Sub-Saharan Africa: A Systematic Review. Heliyon. 2020;6:e03537. doi: 10.1016/j.heliyon.2020.e03537. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Coulidiaty A.G.V., Sanou A., Houngbedji C.A., Djibougou D.A., Dicko A., Kobo G., Bonfoh B. Prevalence and Sensitivity to Antibiotics of Campylobacter Spp. in Chicken, Farmers and Soil in Bobo-Dioulasso, Burkina Faso. PAMJ-One Health. 2021;4:8. [Google Scholar]
- 16.Dekker D., Eibach D., Boahen K.G., Akenten C.W., Pfeifer Y., Zautner A.E., May J. Fluoroquinolone-Resistant Salmonella Enterica, Campylobacter Spp., and Arcobacter Butzleri from Local and Imported Poultry Meat in Kumasi, Ghana. Foodborne Pathog. Dis. 2019;16:352–358. doi: 10.1089/fpd.2018.2562. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Kunadu A.P.H., Otwey R.Y., Mosi L. Microbiological Quality and Salmonella Prevalence, Serovar Distribution and Antimicrobial Resistance Associated with Informal Raw Chicken Processing in Accra, Ghana. Food Control. 2020;118:107440. doi: 10.1016/j.foodcont.2020.107440. [DOI] [Google Scholar]
- 18.Sackey B.A., Mensah P., Collison E., Sakyi-Dawson E. Campylobacter, Salmonella, Shigella and Escherichia Coli in Live and Dressed Poultry from Metropolitan Accra. Int. J. Food Microbiol. 2001;71:21–28. doi: 10.1016/S0168-1605(01)00595-5. [DOI] [PubMed] [Google Scholar]
- 19.Njoga E.O., Nwankwo I.O., Ugwunwarua J.C. Epidemiology of Thermotolerant Campylobacter Infection in Poultry in Nsukka Agricultural Zone, Nigeria. Int. J. One Health. 2019;5:92–98. doi: 10.14202/IJOH.2019.92-98. [DOI] [Google Scholar]
- 20.Karikari A.B., Obiri-Danso K., Frimpong E.H., Krogfelt K.A. Multidrug Resistant Campylobacter in Faecal and Carcasses of Commercially Produced Poultry. Afr. J. Microbiol. Res. 2017;11:271–277. [Google Scholar]
- 21.Nwankwo I.O., Faleke O.O., Salihu M.D., Magaji A.A., Musa U., Garba J. Epidemiology of Campylobacter Species in Poultry and Humans in the Four Agricultural Zones of Sokoto State, Nigeria. J. Public Health Epidemiol. 2016;8:184–190. [Google Scholar]
- 22.Kouglenou S.D., Agbankpe A.J., Dougnon V., Djeuda A.D., Deguenon E., Hidjo M., Baba-Moussa L., Bankole H. Prevalence and Susceptibility to Antibiotics from Campylobacter Jejuni and Campylobacter Coli Isolated from Chicken Meat in Southern Benin, West Africa. BMC Res. Notes. 2020;13:305. doi: 10.1186/s13104-020-05150-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Kagambèga A., Thibodeau A., Soro D.K., Barro N., Fravalo P. Detection of Campylobacter Sp. from Poultry Feces in Ouagadougou, Burkina Faso. Food Nutr. Sci. 2021;12:107–114. doi: 10.4236/fns.2021.122009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Karikari A.B., Saba C.K.S., Kpordze S.W. Biotyping of Multidrug Resistant Campylobacter Jejuni from Poultry and Humans in Northern Region of Ghana. Res. Sq. 2020;11:18. doi: 10.21203/rs.3.rs-38356/v1. [DOI] [Google Scholar]
- 25.Salihu M.D., Junaidu A.U., Magaji A.A., Yakubu Y. Prevalence and Antimicrobial Resistance of Thermophilic Campylobacter Isolates from Commercial Broiler Flocks in Sokoto, Nigeria. Res. J. Vet. Sci. 2012;5:51–58. doi: 10.3923/rjvs.2012.51.58. [DOI] [Google Scholar]
- 26.Bernadette G.G., Souleymane B., Laure K.M.-P. Antimicrobial Resistance and Virulence Factors of Campylobacter Coli Isolated from Chicken in Côte d’Ivoire. Annu. Res. Rev. Biol. 2020;35:86–92. doi: 10.9734/arrb/2020/v35i1130302. [DOI] [Google Scholar]
- 27.Ofukwu R.A., Okoh A.E.J., Akwuobu C.A. Prevalence of Campylobacter Jejuni in Duck Faeces around Drinking Water Sources in Makurdi, North-Central Nigeria. Sokoto J. Vet. Sci. 2008;7:26–30. [Google Scholar]
- 28.Goualié B.G., Ouattara H.G., Akpa E.E., Guessends N.K., Bakayoko S., Niamké S.L., Dosso M. Occurrence of Multidrug Resistance in Campylobacter from Ivorian Poultry and Analysis of Bacterial Response to Acid Shock. Food Sci. Biotechnol. 2014;23:1185–1191. doi: 10.1007/s10068-014-0162-9. [DOI] [Google Scholar]
- 29.Kagambèga A., Thibodeau A., Trinetta V., Soro D.K., Sama F.N., Bako É., Bouda C.S., Wereme N’Diaye A., Fravalo P., Barro N. Salmonella Spp. and Campylobacter Spp. in Poultry Feces and Carcasses in Ouagadougou, Burkina Faso. Food Sci. Nutr. 2018;6:1601–1606. doi: 10.1002/fsn3.725. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Hamali H., Fallah S., Joozani R.J., Zare P., Noorsaadat G. Detection of Campylobacter Spp. in Sheep Aborted Fetuses by PCR. Trends Life Sci. 2014;3:49–56. [Google Scholar]
- 31.Salihu M.D., Abdulkadir J.U., Oboegbulem S.I., Egwu G.O., Magaji A.A., Lawal M., Hassan Y. Isolation and Prevalence of Campylobacter Species in Cattle from Sokoto State, Nigeria. Vet. Ital. 2009;45:501–505. [PubMed] [Google Scholar]
- 32.Mshelia G.D., Amin J.D., Egwu G.O., Woldehiwet Z., Murray R.D. The Prevalence of Bovine Venereal Campylobacteriosis in Cattle Herds in the Lake Chad Basin of Nigeria. Trop. Anim. Health Prod. 2012;44:1487–1489. doi: 10.1007/s11250-012-0092-6. [DOI] [PubMed] [Google Scholar]
- 33.Mai H.M., Irons P.C., Kabir J., Thompson P.N. Prevalence of Bovine Genital Campylobacteriosis and Trichomonosis of Bulls in Northern Nigeria. Acta Vet. Scand. 2013;55:56. doi: 10.1186/1751-0147-55-56. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Ngulukun S.S., Oboegbulem S.I., Fagbamila I.O., Bertu W., Odugbo M.O. Prevalence and Molecular Characterization of Thermophilic Campylobacter Species Isolated from Cattle in Plateau State, Nigeria. Niger. Vet. J. 2011;32:349–356. [Google Scholar]
- 35.Karikari A.B., Obiri-Danso K., Frimpong E.H., Krogfelt K.A. Antibiotic Resistance of Campylobacter Recovered from Faeces and Carcasses of Healthy Livestock. Biomed Res. Int. 2017;2017:4091856. doi: 10.1155/2017/4091856. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Salihu M.D., Yakubu Y. Prevalence and Antibiotic Resistance of Thermophilic Campylobacter Spp. Isolates from Raw Beef, Mutton and Camel Meat in Sokoto, Nigeria. Res. Opin. Anim. Vet. Sci. 2011;1:401–405. [Google Scholar]
- 37.Mai H.M., Irons P.C., Kabir J., Thompson P.N. Herd-Level Risk Factors for Campylobacter Fetus Infection, Brucella Seropositivity and within-Herd Seroprevalence of Brucellosis in Cattle in Northern Nigeria. Prev. Vet. Med. 2013;111:256–267. doi: 10.1016/j.prevetmed.2013.05.016. [DOI] [PubMed] [Google Scholar]
- 38.Gwimi P.B., Faleke O.O., Salihu M.D., Magaji A.A., Abubakar M.B., Nwankwo I.O., Ibitoye E.B. Prevalence of Campylobacter Species in Fecal Samples of Pigs and Humans from Zuru Kebbi State, Nigeria. Int. J. One Health. 2015;1:1–5. doi: 10.14202/IJOH.2015.1-5. [DOI] [Google Scholar]
- 39.Adekunle O.C., Coker A.O., Kolawole D.O. Incidence, Isolation and Characterization of Campylobacter Species in Osogbo. Biol. Med. 2009;1:24–27. [Google Scholar]
- 40.Lääveri T., Pakkanen S.H., Antikainen J., Riutta J., Mero S., Kirveskari J., Kantele A. High Number of Diarrhoeal Co-Infections in Travellers to Benin, West Africa. BMC Infect. Dis. 2014;14:81. doi: 10.1186/1471-2334-14-81. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Bonkoungou I.J.O., Haukka K., Österblad M., Hakanen A.J., Traoré A.S., Barro N., Siitonen A. Bacterial and Viral Etiology of Childhood Diarrhea in Ouagadougou, Burkina Faso. BMC Pediatr. 2013;13:36. doi: 10.1186/1471-2431-13-36. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Sangaré L., Nikiéma A.K., Zimmermann S., Sanou I., Congo-Ouédraogo M., Diabaté A., Guissou P.I. Campylobacter Spp. Epidemiology and Antimicrobial Susceptibility in a Developing Country, Burkina Faso (West Africa) Afr. J. Clin. Exp. Microbiol. 2012;13:110–117. doi: 10.4314/ajcem.v13i2.9. [DOI] [Google Scholar]
- 43.Sawadogo S., Diarra B., BIsseye C., Compaore T.R., Djigma F.W., Ouermi D., Ouattara A.S., Simpore J. Molecular Diagnosis of Shigella, Salmonella and Campylobacter by Multiplex Real-Time PCR in Stool Culture Samples in Ouagadougou (Burkina Faso) Sudan J. Med. Sci. 2017;12:163. doi: 10.18502/sjms.v12i3.931. [DOI] [Google Scholar]
- 44.Zimmermann M., Kotloff K., Nasrin D., Roose A., Levine M.M., Rheingans R., Farag T., Walker D., Pecenka C. Household Costs of Diarrhea by Etiology in 7 Countries, the Global Enterics Mulitcenter Study (GEMS) Open Forum Infect. Dis. 2019;6:ofz150. doi: 10.1093/ofid/ofz150. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Langendorf C., Le Hello S., Moumouni A., Gouali M., Mamaty A.-A., Grais R.F., Weill F.-X., Page A.-L. Enteric Bacterial Pathogens in Children with Diarrhea in Niger: Diversity and Antimicrobial Resistance. PLoS ONE. 2015;10:e0120275. doi: 10.1371/journal.pone.0120275. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Karikari A.B., Obiri-Danso K., Frimpong E.H., Krogfelt K.A. Antibiotic Resistance in Campylobacter Isolated from Patients with Gastroenteritis in a Teaching Hospital in Ghana. Open J. Med. Microbiol. 2017;7:1–11. doi: 10.4236/ojmm.2017.71001. [DOI] [Google Scholar]
- 47.Krumkamp R., Sarpong N., Schwarz N.G., Adlkofer J., Loag W., Eibach D., Hagen R.M., Adu-Sarkodie Y., Tannich E., May J. Correction: Gastrointestinal Infections and Diarrheal Disease in Ghanaian Infants and Children: An Outpatient Case-Control Study. PLoS Negl. Trop. Dis. 2015;9:e0003728. doi: 10.1371/journal.pntd.0003728. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Ogbomon E.O., Whong C.M.Z., Doko M.H.I., Magaji S.N., Addai T.I., Orukotan Y.F. Prevalence of Campylobacter Spp. among Diarrhoeic HIV-Patients in Kaduna, Nigeria. Int. J. Appl. Microb. Biotech. Res. 2019;7:70–78. [Google Scholar]
- 49.Forson A.O., Adjei D.N., Olu-Taiwo M., Quarchie M.N., Asmah H.R. Characterization of Campylobacter Associated Gastric Enteritis among Patients with Human Immunodeficiency Virus (HIV) in a Hospital in Accra, Ghana. PLoS ONE. 2020;15:e0240242. doi: 10.1371/journal.pone.0240242. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Falodun O.I., Adesola E.A., Ademola E.A., Bakarey S.A. Faecal Carriage and Antibiotics Resistance Patterns of Campylobacter Species from HIV/AIDS Patients in Ibadan, Southwest Nigeria. South Asian J. Res. Microbiol. 2020;7:39–46. doi: 10.9734/sajrm/2020/v7i430181. [DOI] [Google Scholar]
- 51.Schroeder L.F., Amukele T. Medical Laboratories in Sub-Saharan Africa That Meet International Quality Standards. Am. J. Clin. Pathol. 2014;141:791–795. doi: 10.1309/AJCPQ5KTKAGSSCFN. [DOI] [PubMed] [Google Scholar]
- 52.Sahin O., Kassem I.I., Shen Z., Lin J., Rajashekara G., Zhang Q. Campylobacter in Poultry: Ecology and Potential Interventions. Avian Dis. 2015;59:185–200. doi: 10.1637/11072-032315-Review. [DOI] [PubMed] [Google Scholar]
- 53.Rukambile E., Sintchenko V., Muscatello G., Kock R., Alders R. Infection, Colonization and Shedding of Campylobacter and Salmonella in Animals and Their Contribution to Human Disease: A Review. Zoonoses Public Health. 2019;66:562–578. doi: 10.1111/zph.12611. [DOI] [PubMed] [Google Scholar]
- 54.Newell D.G., Mughini-Gras L., Kalupahana R.S., Wagenaar J.A. Campylobacter. Elsevier; Amsterdam, The Netherlands: 2017. Campylobacter Epidemiology—Sources and Routes of Transmission for Human Infection; pp. 85–110. [Google Scholar]
- 55.Aarestrup F.M., Wegener H.C., McDermott P.F. Campylobacter, Third Edition. American Society of Microbiology; Washington, DC, USA: 2008. Transmission of Antibiotic Resistance from Food Animals to Humans; pp. 645–665. [Google Scholar]
- 56.Zenebe T., Zegeye N., Eguale T. Prevalence of Campylobacter Species in Human, Animal and Food of Animal Origin and Their Antimicrobial Susceptibility in Ethiopia: A Systematic Review and Meta-Analysis. Ann. Clin. Microbiol. Antimicrob. 2020;19:61. doi: 10.1186/s12941-020-00405-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Diriba K., Awulachew E., Anja A. Prevalence and Associated Factor of Campylobacter Species among Less than 5-Year-Old Children in Ethiopia: A Systematic Review and Meta-Analysis. Eur. J. Med. Res. 2021;26:2. doi: 10.1186/s40001-020-00474-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Lv R., Wang K., Feng J., Heeney D.D., Liu D., Lu X. Detection and Quantification of Viable but Non-Culturable Campylobacter Jejuni. Front. Microbiol. 2019;10:2920. doi: 10.3389/fmicb.2019.02920. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Limmathurotsakul D., Jamsen K., Arayawichanont A., Simpson J.A., White L.J., Lee S.J., Wuthiekanun V., Chantratita N., Cheng A., Day N.P.J., et al. Defining the True Sensitivity of Culture for the Diagnosis of Melioidosis Using Bayesian Latent Class Models. PLoS ONE. 2010;5:e12485. doi: 10.1371/journal.pone.0012485. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Cantero G., Correa-Fiz F., Ronco T., Strube M., Cerdà-Cuéllar M., Pedersen K. Characterization of Campylobacter Jejuni and Campylobacter Coli Broiler Isolates by Whole-Genome Sequencing. Foodborne Pathog. Dis. 2018;15:145–152. doi: 10.1089/fpd.2017.2325. [DOI] [PubMed] [Google Scholar]
- 61.Steinbrueckner B., Haerter G., Pelz K., Kist M. Routine Identification of Campylobacter Jejuni and Campylobacter Coli from Human Stool Samples. FEMS Microbiol. Lett. 1999;179:227–232. doi: 10.1111/j.1574-6968.1999.tb08732.x. [DOI] [PubMed] [Google Scholar]
- 62.Sheppard S.K., Maiden M.C.J. The Evolution of Campylobacter Jejuni and Campylobacter Coli. Cold Spring Harb. Perspect. Biol. 2015;7:a018119. doi: 10.1101/cshperspect.a018119. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Ayandiran T.O., Falgenhauer L., Schmiedel J., Chakraborty T., Ayeni F.A. High resistance to tetracycline and ciprofloxacin in bacteria isolated from poultry farms in Ibadan, Nigeria. J. Infect. Dev. Ctries. 2018;12:462–470. doi: 10.3855/jidc.9862. [DOI] [PubMed] [Google Scholar]
- 64.Mougey E.B., Saunders M., Franciosi J.P., Gomez-Suarez R.A. Comparative Effectiveness of Intravenous Azithromycin versus Erythromycin Stimulating Antroduodenal Motility in Children. J. Pediatr. Gastroenterol. Nutr. 2022;74:25–32. doi: 10.1097/MPG.0000000000003271. [DOI] [PubMed] [Google Scholar]
- 65.Asuming-Bediako N., Parry-Hanson Kunadu A., Abraham S., Habib I. Campylobacter at the Human-Food Interface: The African Perspective. Pathogens. 2019;8:87. doi: 10.3390/pathogens8020087. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Yang Y., Feye K.M., Shi Z., Pavlidis H.O., Kogut M., Ashworth A.J., Ricke S.C. A Historical Review on Antibiotic Resistance of Foodborne Campylobacter. Front. Microbiol. 2019;10:1509. doi: 10.3389/fmicb.2019.01509. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Paintsil E.K., Ofori L.A., Akenten C.W., Fosu D., Ofori S., Lamshöft M., May J., Danso K.O., Krumkamp R., Dekker D. Antimicrobial Usage in Commercial and Domestic Poultry Farming in Two Communities in the Ashanti Region of Ghana. Antibiotics. 2021;10:800. doi: 10.3390/antibiotics10070800. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Dekker D., Wolters M., Mertens E., Boahen K.G., Krumkamp R., Eibach D., Schwarz N.G., Adu-Sarkodie Y., Rohde H., Christner M., et al. Antibiotic Resistance and Clonal Diversity of Invasive Staphylococcus Aureus in the Rural Ashanti Region, Ghana. BMC Infect. Dis. 2016;16:720. doi: 10.1186/s12879-016-2048-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Dong L.T., Espinoza H.V., Espinoza J.L. Emerging Superbugs: The Threat of Carbapenem Resistant Enterobacteriaceae. AIMS Microbiol. 2020;6:176–182. doi: 10.3934/microbiol.2020012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Sarkar D.J., Mukherjee I., Shakil N.A., Rana V.S., Kaushik P., Debnath S. Antibiotics in Agriculture: Use and Impact. Ind. J. Ethnophytopharm. 2018;4:4–19. [Google Scholar]
- 71.Page M.J., McKenzie J.E., Bossuyt P.M., Boutron I., Hoffmann T.C., Mulrow C.D., Shamseer L., Tetzlaff J.M., Akl E.A., Brennan S.E., et al. The PRISMA 2020 Statement: An Updated Guideline for Reporting Systematic Reviews. BMJ. 2021;372:n71. doi: 10.1136/bmj.n71. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Hunter J.P., Saratzis A., Sutton A.J., Boucher R.H., Sayers R.D., Bown M.J. In Meta-Analyses of Proportion Studies, Funnel Plots Were Found to Be an Inaccurate Method of Assessing Publication Bias. J. Clin. Epidemiol. 2014;67:897–903. doi: 10.1016/j.jclinepi.2014.03.003. [DOI] [PubMed] [Google Scholar]
- 73.R Core Team . R: A Language and Environment for Statistical Computing. R Foundation for Statistical Computing; Vienna, Austria: 2021. [Google Scholar]
- 74.Harrer M., Cuijpers P., Furukawa T.A., Ebert D.D. Doing Meta-Analysis with R: A Hands-On Guide. Chapmann & Hall/CRC Press; Boca Raton, FL, USA; London, UK: Chapmann & Hall/CRC Press; London, UK: 2021. [Google Scholar]
- 75.QGIS Development Team QGIS Geographic Information System. Open Source Geospatial Foundation Project. 2021. [(accessed on 15 November 2021)]. Available online: http://www.qgis.org.
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
The data presented in this study are available on request from the corresponding author.