Abstract
We tested the hypothesis that limb vascular conductance (LVC) would increase during the immediate recovery phase of dynamic exercise above, but not below, critical power (CP) indicating a threshold for muscular contraction-induced impedance of limb blood flow (LBF). CP (115 ± 26W) was determined in 7 men and 7 women who subsequently performed ∼5 minutes of near-supine cycling exercise both below and above CP. LVC demonstrated a greater increase during immediate recovery and remained significantly higher following exercise above, compared to below, CP (all p<0.001). Power output was associated with the immediate increases in LVC following exercise above, but not below, CP (p<0.001; r=0.85). Additionally, variance in percent LBF impedance was significantly lower above (CV: 10.7%), compared to below (CV: 53.2%), CP (p<0.01). CP appears to represent a threshold above which the characteristics of LBF impedance by muscular contraction become intensity-dependent. These data suggest a critical level of LBF impedance relative to contraction intensity exists and, once attained, may promote the progressive metabolic and neuromuscular responses known to occur above CP.
Keywords: Limb Blood Flow, Vascular Conductance, Critical Power, Contraction Impedance
1. Introduction
Skeletal muscle contractions are accompanied by increases in intramuscular pressure and therefore mechanical compression of blood vessels and increased vascular resistance (Barcroft and Dornhorst, 1949; Gaffney et al., 1990; Radegran and Saltin, 1998; Sadamoto et al., 1983; Sjogaard et al., 1986). During rhythmic exercise, blood flow is highest during the relaxation phase of the contraction-relaxation cycle when intramuscular pressure is low (Barcroft and Dornhorst, 1949; Lutjemeier et al., 2005; Radegran and Saltin, 1998; Robergs et al., 1997; Walloe and Wesche, 1988). Thus, altering relaxation time between contractions (i.e., duty-cycle or contraction-frequency) has a profound effect on muscle blood flow (Bentley et al., 2017; Broxterman et al., 2014; Caldwell et al., 2018; Hoelting et al., 2001). Indeed, Broxterman et al. demonstrated that an increase in duty cycle (i.e., reduced relaxation time) attenuated the blood flow response to exhaustive rhythmic handgrip exercise (Broxterman et al., 2014). Additionally, the time to task failure (Tlim), and therefore the asymptote of the hyperbolic power-duration relationship (i.e., critical power; CP), was significantly reduced (Broxterman et al., 2014). While the oxygen delivery dependency of CP is well documented (Dekerle et al., 2012; Moritani et al., 1981; Vanhatalo et al., 2010), the influence of muscular contraction on limb vascular conductance (LVC) during exercise above versus below CP remains unknown.
CP is an important physiological threshold (Poole et al., 2016) that differentiates between steady-state and progressive metabolic (Jones et al., 2008; Poole et al., 1988) and neuromuscular (Burnley et al., 2012) responses to exercise. Recently, our laboratory has demonstrated that limb blood flow (LBF) and microvascular oxygen extraction responses to isometric handgrip exercise above critical force (CF; the isometric analog of CP) are distinct from those below CF. Specifically, while steady-state responses were demonstrated below CF, LBF and microvascular oxygen delivery appeared to reach task-specific physiological maximums above CF suggesting intensity-dependent limitations (Hammer et al., 2020b). Additionally, threshold-like (i.e., biphasic) responses in microvascular oxygen delivery have been observed during incremental exercise with apparent limitations (i.e., submaximal plateaus) existing at higher intensities (Boushel et al., 2002; Chin et al., 2011; Habazettl et al., 2010; Hammer et al., 2018; Koga et al., 2014). These findings are consistent with those of Lutjemeier et al. who experimentally described the effect of muscular contraction on LBF as being positive and neutral at low and moderate work rates, respectively, but negative (i.e., blood flow impedance) at higher intensities (Lutjemeier et al., 2005). These observations have wide-reaching implications for muscular performance and fatigue development yet remain to be contextualized within the CP framework of exercise tolerance.
The aim of this study was to determine if CP represents a threshold for muscular contraction-induced impedance of LBF during dynamic locomotor-muscle exercise. Specifically, we compared LVC during near-supine cycling to changes in LVC immediately following exercise termination. Under the assumption that blood vessel diameter during immediate recovery reflects the level of vasodilation during exercise but without the influence of muscular contractions, changes in LVC subsequent to the near-instantaneous cessation of muscular contractions have been previously used to inversely reflect the influence of contraction on LBF during exercise (Barcroft and Dornhorst, 1949; Broxterman et al., 2014; Lutjemeier et al., 2005; Tschakovsky et al., 2004). We hypothesized that LVC would increase during the immediate recovery phase of dynamic exercise above, but not below, CP. If confirmed, we would interpret these findings to indicate that CP represents a threshold for muscular contraction-induced impedance of LBF during dynamic locomotor-muscle exercise.
2. Methods
2.1. Ethical approval
All experimental procedures were approved by the Institutional Review Board for Research Involving Human Subjects at Kansas State University (#9954) and conformed to the standards set forth by the Declaration of Helsinki apart from database registration. Subjects were informed of all testing procedures and potential risks of participation before providing written, informed consent.
2.2. Subjects
Seven men and seven women (mean ± SD; 23 ± 3 yr, 172 ± 8 cm, 70.7 ± 16.3 kg) volunteered to participate in this study. It has been demonstrated that muscle blood flow during exercise is not modulated by phases of the menstrual cycle (Limberg et al., 2010; Smith et al., 2017), therefore no attempt was made to control for menstrual cycle phase in the women. Each subject completed a medical health history evaluation to confirm the absence of any known cardiovascular, pulmonary, or metabolic disease. Subjects were instructed to abstain from vigorous physical activity 12 hours prior and caffeine and food consumption 2 hours prior to their scheduled testing times. A minimum of 24 hours was mandated between each test.
2.3. Experimental design
Subjects were familiarized with all testing procedures and equipment prior to any experimental testing. All exercise tests were performed on a modified near-supine (10-degree head-up tilt) cycle ergometer (Lode, Groningen, The Netherlands). A relatively slow pedal cadence (45 rpm) and short crank-arm length (16 cm) were utilized during all cycling tests to reduce the frequency and magnitude of hip-angle fluctuations, respectively, and facilitate high-quality Doppler ultrasound signals of the femoral artery. Pedal position was adjusted for each subject to achieve a slight knee bend at bottom dead center and was utilized on all subsequent test days to minimize day-to-day biomechanical variability. Additionally, shoulder supports were installed to minimize extraneous muscular work related to body stabilization. Tlim was defined as an inability to maintain a pedal cadence above 40 rpm for > 5 s despite strong verbal encouragement for each exercise test performed until exhaustion.
Subjects first performed a 10 Watt·min−1 incremental ramp cycling test until exhaustion to determine peak power output (Ppeak). Ppeak was determined as the greatest power-output achieved during the incremental ramp test. Subjects next performed a minimum of three constant power-output cycling tests on separate days. Ppeak was utilized to determine power-outputs for the constant-power cycling tests that would elicit exhaustion between ∼2–15 minutes. The Tlim of each test was recorded and linear regression of the 1/Tlim versus power-output relationship for each subject was used to determine CP (the power-output intercept). Goodness-of-fit criteria were established a priori to ensure accurate determination of CP. If standard error of the power-output intercept (i.e., CP) was greater than 10% of CP, a fourth constant power-output test was performed in an attempt to improve the intercept confidence interval. Finally, subjects performed ∼5-minute constant power-output tests at 10% below and 10% above CP in random order. The 10% was chosen to ensure the respective power-outputs were confidently below and above CP according to the goodness-of-fit criteria. A 20-Watt warm-up was performed for 1 minute immediately preceding the transition to the below and above CP power-outputs to allow a trained sonographer to identify the movement patterns of the femoral artery. Following confirmation of high-quality Doppler signal from the trained sonographer, a countdown was commenced in synchrony with the subject’s pedal cadence such that at exercise cessation the right leg would be straight and relaxed. Exercise was terminated between 4.5 and 5.5 minutes for all below- and above-CP tests.
2.4. Measurements
2.4.1. Oxygen consumption ()
Breath-by-breath was measured continuously during the incremental ramp cycling test (Ultima CardiO2; Medical Graphics Corp., MN, USA) to characterize the aerobic capacity of each subject. The metabolic system was calibrated prior to each use according to the manufacturer’s instructions. Breath-by-breath were converted into 10s time-binned mean values and peak () was determined as the highest 10 s time-binned mean achieved during the incremental ramp test.
2.4.2. Limb blood flow (LBF)
A Doppler ultrasound system (LOGIQ S8; GE Medical Systems, Milwaukee, WI) equipped with a multi-frequency linear array transducer operating at 10 MHz was utilized to simultaneously measure common femoral artery diameter and peak blood velocity (Vpeak). Doppler ultrasound measurements were made during the below- and above-CP tests at baseline, during the final minute of exercise, and the first minute of early recovery. All measurements were made ∼ 3 cm proximal to the bifurcation of the deep- and superficial-femoral arteries to minimize the influence of bifurcation-induced turbulent flow. Doppler sample volume was set to the full width of the vessel and all Doppler velocity measurements were performed at a Doppler frequency of 4.0 MHz with an insonation angle < 60 degrees. Vpeak was defined as the time-averaged peak velocity across each complete cardiac-cycle at baseline and during early recovery. During exercise, Vpeak was defined as the time-averaged peak velocity during an 8 s (6 complete pedal revolutions) time-window immediately preceding the termination of the test. Vpeak was intentionally utilized over the time-averaged mean velocities (Vmean) as the parabolic-like profiles of arterial blood flow place Vpeak near the center of the vessel lumen and require less confidence in maintaining wall-to-wall sample volume (Ade et al., 2012). Subsequently, Vmean was calculated as Vpeak/2 which has previously been reported to be an accurate estimate (Ade et al., 2012). Common femoral artery vessel diameter was analyzed at a perpendicular angle along the central axis of the vessel during the r-wave of cardiac cycles at rest and early recovery from exercise. Cross-sectional area (CSA) of the vessel was then calculated as CSA = πr2. LBF was calculated as the product of CSA and Vmean at baseline, during the final 8s of exercise, and for 9 cardiac-cycles (3 cardiac-cycle bins: CC1–3, CC4–6, and CC7–9) in early recovery. LBF calculations during the final 8s of exercise were made using the femoral artery vessel diameter measurement taken during CC1–3 following the cessation of exercise.
2.4.3. Hemodynamic responses
Heart rate (HR) and beat-by-beat systolic, mean, and diastolic blood pressures (SBP, MAP, and DBP, respectively), and systemic vascular resistance (SVR) were measured continuously during the below- and above-CP tests via calibrated finger photoplethysmography (Finometer Pro; Finapress Medical Systems, Amsterdam, The Netherlands). Blood pressure measurements were made on the third middle phalanx of the left hand which was placed on a height-adjustable platform to ensure stability during the cycling protocols. All blood pressure and hemodynamic measurements were averaged over the same time-windows as Doppler ultrasound measurements. The rate pressure product (RPP) was calculated as (SBP×HR)/100 and used as an index of myocardial work (Gobel et al., 1978; Kitamura et al., 1972). Cardiac output () was calculated as the product of HR and stroke volume (SV), with SV calculated using the ModelFlow method (Finometer Pro; Finapress Medical Systems) after correction for age, sex, body mass, and stature (Bogert and van Lieshout, 2005). Blood pressure and LBF data were time-aligned and LVC was calculated as the quotient of LBF and MAP. Lastly, assuming that LVC at CC1–3 reflects the LVC during exercise without the influence of muscular contractions, the percent-impedance of LBF (%IMPLBF) at end-exercise was calculated as:
2.5. Statistical analysis
Data are expressed as means ± standard deviation. All statistical analyses were performed using SigmaStat (Systat Software; Point Richmond, CA). Linear regression was used to determine CP from the constant power-output cycling tests. Two-way repeated-measures ANOVAs were used to compare LBF, blood pressure, and hemodynamic measurements at baseline and end-exercise between below- and above-CP tests (time × intensity). Two-way repeated-measures ANOVAs were used to test for changes in LBF, MAP, and LVC throughout the early recovery phase following the below- and above-CP exercise tests (time × intensity). When a significant overall effect was detected, a Tukey’s post hoc analysis was performed to determine where significant differences existed. Paired t-tests were used to test for differences in both %IMPLBF at end-exercise and %-change in LVC during immediate recovery between below- and above-CP exercise tests. Linear regression was used to detect significant relationships between test power-output and LBF, %IMPLBF, and changes in LVC. Finally, a Levene’s test was used to test for differences in variance of LVC and %IMPLBF responses between below- and above-CP tests. Results were considered statistically significant when p < 0.05.
3. Results
3.1. Incremental ramp data and determination of CP
Ppeak and were 146 ± 30 W and 33.1 ± 7.2 mL/kg/min, respectively. CP was 115 ± 26 W (79.0 ± 8.4 %Ppeak). Standard error of the power-output intercept used to determine CP ranged from 0.39 – 6.27 %CP. Power-outputs below and above CP were 71.1 ± 7.6 and 86.9 ± 9.2 %Ppeak, respectively.
3.2. Hemodynamic responses to exercise
Hemodynamic values at baseline and end-exercise during the below- and above-CP tests are presented in Table 1. No significant differences were detected in any hemodynamic measurements at baseline between tests. HR increased significantly from baseline to end-exercise during both tests (both p < 0.001) but was significantly greater at end-exercise above CP compared to below CP (p < 0.001). SV increased significantly from baseline to end-exercise only below CP (p < 0.01) and no significant differences were detected in end-exercise SV between tests. increased significantly from baseline to end-exercise during both tests (both p < 0.001) but was significantly greater at end-exercise above CP compared to below CP (p < 0.01). SBP, DBP, and RPP increased significantly from baseline to end-exercise during both tests (all p < 0.001) but were each significantly greater at end-exercise above CP compared to below CP (all p < 0.05).
Table 1.
Hemodynamic responses to exercise below and above critical power (CP)
| Baseline | End-exercise | ||||
|---|---|---|---|---|---|
|
|
|||||
| Below CP | Above CP | Below CP | Above CP | ||
|
|
|||||
| HR | (bpm) | 68 ± 10 | 69 ± 11 | 131 ± 13 * | 151 ± 15 *† |
| SV | (mL) | 86.8 ± 9.0 | 90.0 ± 9.7 | 101 ± 15 * | 96.7 ± 15.7 |
| Q | (L·min−1) | 5.75 ± 0.9 | 6.10 ± 1.2 | 13.1 ± 2.3 * | 14.5 ± 3.1 *† |
| SBP | (mmHg) | 130 ± 6 | 130 ± 7 | 175 ± 18 * | 186 ± 14 *† |
| DBP | (mmHg) | 76.8 ± 6.1 | 75.2 ± 6.2 | 94.3 ± 9.2 * | 101 ± 12 *† |
| RPP | (bpm·mmHg·10−2) | 87.1 ± 13.5 | 89.2 ± 14.2 | 227 ± 28 * | 279 ± 33 *† |
| SVR | (dyne·s·cm−5·10−3) | 1.37 ± 0.25 | 1.31 ± 0.27 | 0.77 ± 0.15* | 0.76 ± 0.21* |
Values are means ± SD. HR, heart rate; SV, stroke volume;, cardiac output; SBP and DBP, systolic and diastolic blood pressures, respectively; RPP, rate pressure product; SVR, systemic vascular resistance.
Significantly different from baseline (p < 0.01).
Significantly greater than below CP (p < 0.05).
3.3. LBF, MAP, and LVC responses during exercise and early recovery
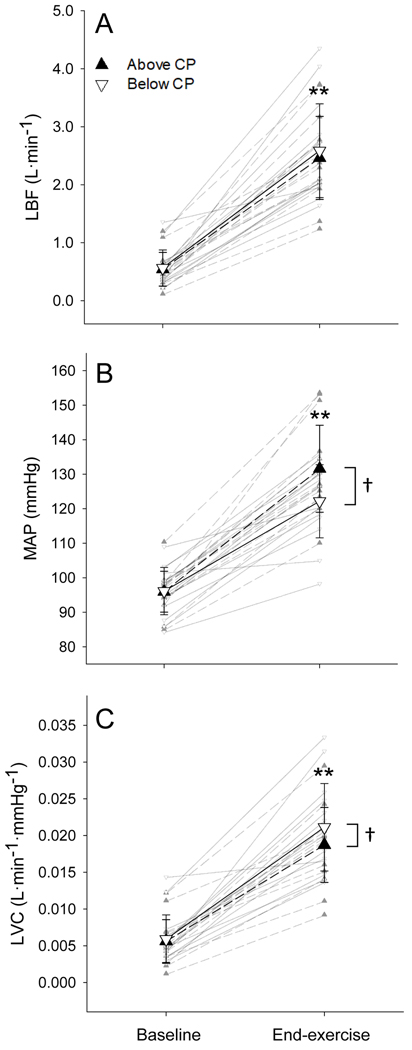
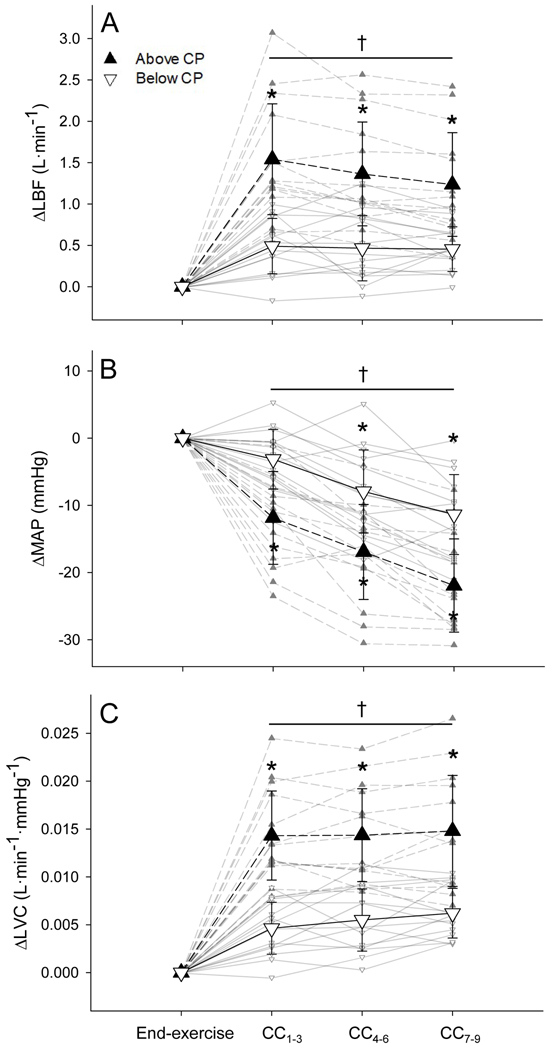
LBF was not different at baseline between below- and above-CP tests and increased significantly from baseline to end-exercise during both tests (both p < 0.001; Fig. 1A). LBF was not different at end-exercise between tests (Fig. 1A). LBF increased immediately during recovery (end-to-CC1–3) and remained elevated (CC4–6 and CC7–9) following both the below- and above-CP exercise tests (all p < 0.001; Fig. 2A). However, LBF demonstrated a greater immediate increase during recovery (end-to-CC1–3) and remained significantly higher (CC4–6 and CC7–9) following exercise above CP compared to below CP (all p < 0.001; Fig. 2A).
Figure 1. Cardiovascular responses to exercise.
The mean absolute values of limb blood flow (LBF; A), mean arterial pressure (MAP; B), and limb vascular conductance (LVC; C) at baseline and end-exercise below (▽) and above (▲) CP. Individual responses are presented in the background. * Significantly greater than baseline (p < 0.001). † Significant difference between below- and above-CP tests (p < 0.05).
Figure 2. Cardiovascular responses during early recovery from exercise.
The mean absolute change in limb blood flow (LBF; A), mean arterial pressure (MAP; B), and limb vascular conductance (LVC; C) from end-exercise to early recovery (cardiac cycles 1–3, 4–6, and 7–9; CC1–3, CC4–6, and CC7–9, respectively) following exercise below (▽) and above (▲) CP. Individual responses are presented in the background. * Significantly different from end-exercise (p < 0.001). † Significant difference between below- and above-CP tests (p < 0.001).
MAP was not different at baseline between below- and above-CP tests and increased significantly from baseline to end-exercise during both tests (both p < 0.001; Fig. 1B). MAP was significantly greater at end-exercise above CP compared to below CP (p < 0.01; Fig. 1B). Following exercise below CP, MAP remained unchanged during immediate recovery (end-to-CC1–3) but was significantly lower during CC4–6 and CC7–9 compared to end-exercise (both p < 0.001; Fig. 2B). Following exercise above CP, MAP decreased significantly during immediate recovery (end-to-CC1–3) and remained lower during CC4–6 and CC7–9 compared to end exercise (all p < 0.001; Fig. 2B). MAP was significantly lower during all stages of early recovery above CP compared to below CP (p < 0.001; Fig. 2B).
LVC was not different at baseline between tests and increased significantly from baseline to end-exercise during both tests (both p < 0.001; Fig. 1C). LVC was significantly lower at end-exercise above CP compared to below CP (p < 0.05; Fig. 1C). LVC increased immediately during recovery (end-to-CC1–3) and remained elevated (CC4–6 and CC7–9) following both the below- and above-CP exercise tests (all p < 0.001; Fig. 2C). However, LVC demonstrated a greater increase during immediate recovery (end-to-CC1–3) and remained significantly higher (CC4–6 and CC7–9) following exercise above CP compared to below CP (all p < 0.001; Fig. 2C).
3.4. Immediate changes in LVC, %IMPLBF, and power-output
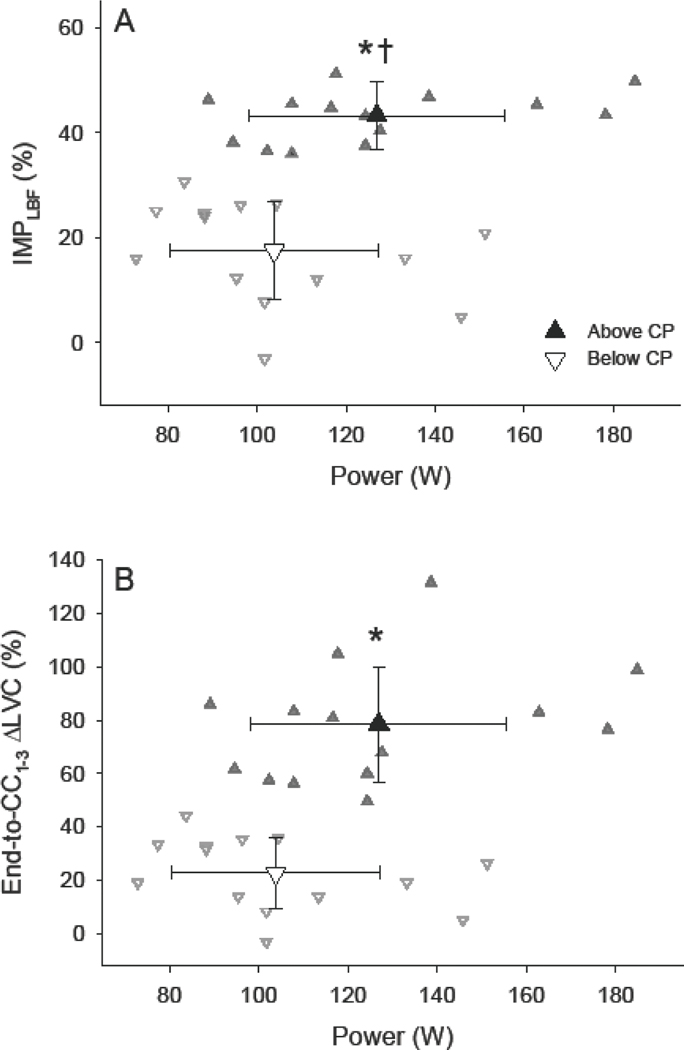
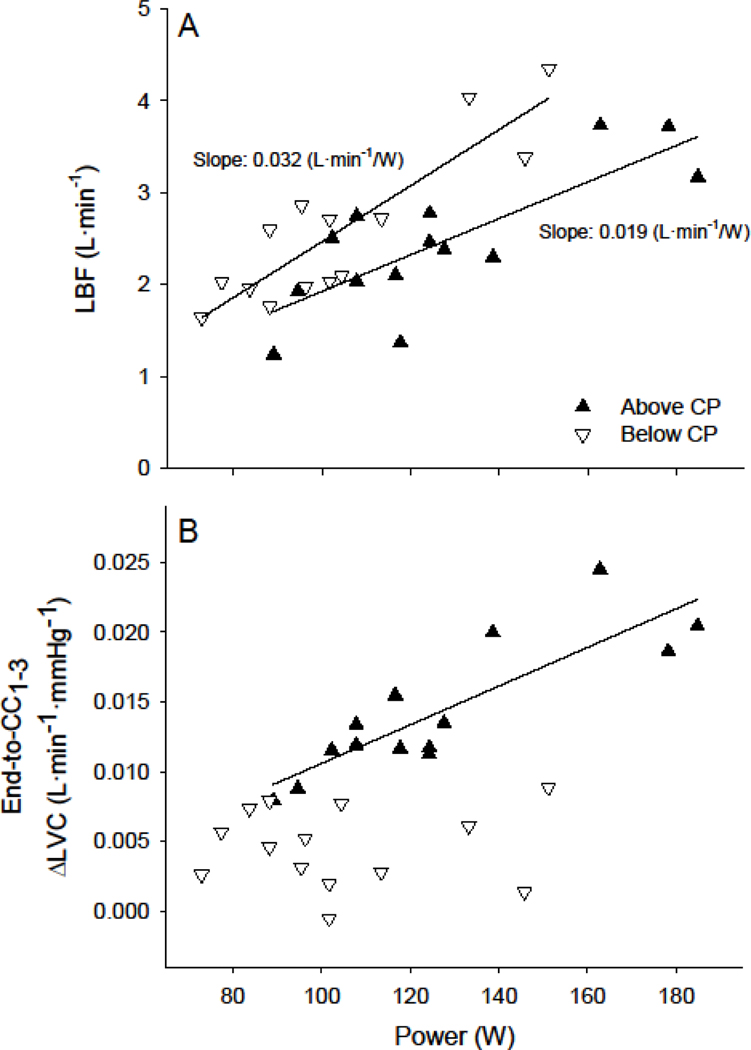
%IMPLBF at end-exercise (Fig. 3A) and %ΔLVC during immediate recovery (Fig. 3B) were significantly greater above, compared to below, CP (both p < 0.001). Neither %IMPLBF at end-exercise (Fig. 3A) nor %ΔLVC during immediate recovery (Fig. 3B) were significantly associated with power-output below or above CP. Variance in %ΔLVC during immediate recovery was not different between below- and above-CP tests, however variance in %IMPLBF was significantly lower above (CV: 10.7%), compared to below (CV: 53.2%), CP (p < 0.01). Cycling power-output was not associated with the absolute increases in LVC immediately following exercise (end-to-CC1–3) below CP (Fig. 4B). However, power-output was significantly associated with the absolute increases in LVC immediately following exercise (end-to-CC1–3) above CP (p < 0.001; r = 0.85; Fig. 4B). No relationships were detected between individual subject CPs and the percent-difference between immediate changes in LVC (end-to-CC1–3) during the below- or above-CP tests (Fig. 5).
Figure 3. Percent impedance of limb blood flow and percent change in limb vascular conductance during immediate recovery as a function of power-output.
The mean percent impedance of limb blood flow (%IMPLBF; A) and percent change in limb vascular conductance (ΔLVC; B) from end-exercise to immediate recovery (cardiac cycles 1–3; CC1–3,) following exercise below (▽) and above (▲) CP. Individual responses are presented in the background. * Significantly greater than below CP (p < 0.001). † Significant difference in variance between below- and above-CP tests (p < 0.01).
Figure 4. Limb blood flow and changes in limb vascular conductance during immediate recovery as a function of power-output.
End-exercise limb blood flow (LBF; A) and the absolute change in limb vascular conductance (ΔLVC; B) during immediate recovery (cardiac cycles 1–3; CC1–3) from exercise below (▽) and above (▲) CP as a function of test power-output for each subject. Note 1) the >30% reduction in slope of the LBF/W relationship above, compared to below, CP and 2) the significant relationship between ΔLVC and power-output above, but not below, CP (p < 0.001; r = 0.85).
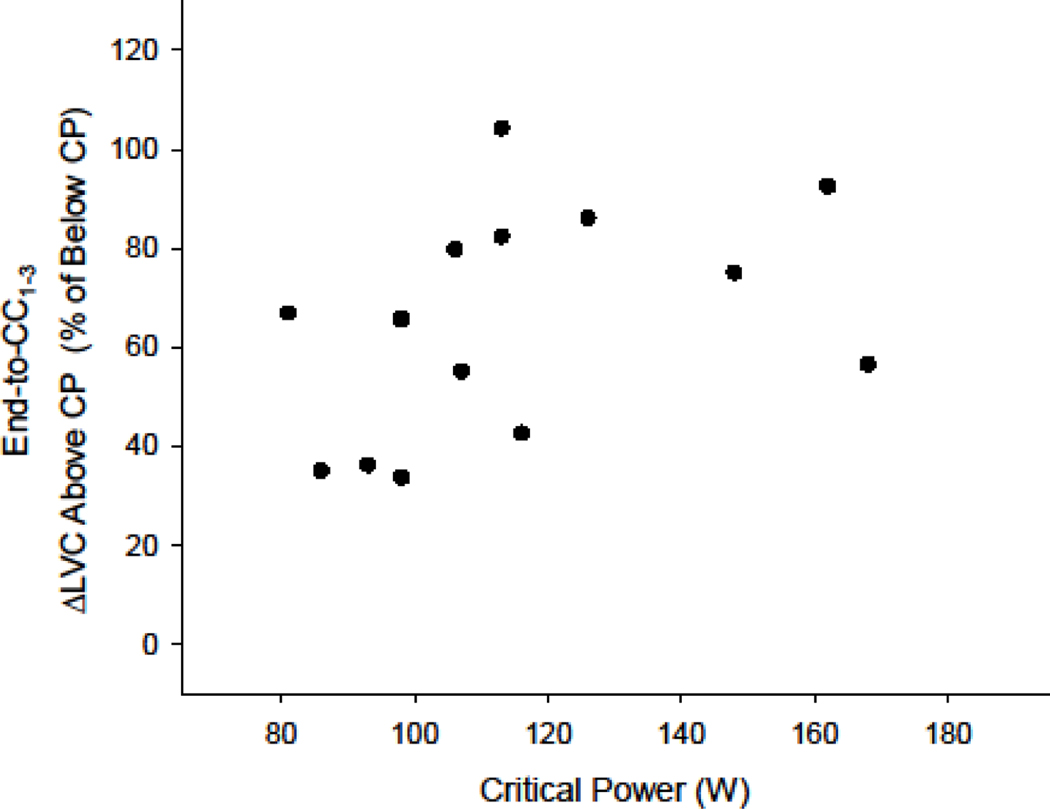
Figure 5. Relationship between critical power and below-versus-above critical power differences in limb vascular conductance changes during immediate recovery.
Individual critical powers (CPs) and changes in limb vascular conductance (ΔLVC) during immediate recovery (CC1–3) above CP as a percent greater than the below-CP response. Note no significant relationship suggesting individual differences in CP (and therefore below-to-above CP power-output differences) do not explain the relationship between absolute ΔLVC and power-output above-CP in Fig. 4B.
4. Discussion
4.1. Major Findings
This study demonstrated that CP represents a threshold for intensity-dependent characteristics of muscular contraction-induced impedance of LBF during dynamic locomotor-muscle exercise. Consistent with our hypothesis, LVC increased significantly during the immediate recovery phase of dynamic exercise above CP (Fig. 2C) suggesting impedance of LBF by muscular contractions. Indeed, LVC was significantly lower during exercise above, compared to below, CP (Fig. 1C). However, in contrast to our hypothesis, LVC also increased significantly, albeit less, during the immediate recovery phase of dynamic exercise below CP (Fig. 2C). These findings do not support the presence of a hardline threshold for LBF impedance at CP. However, the degree of LBF impedance during exercise was significantly greater above, compared to below, CP (Fig. 3). Further, intensity-dependent changes in LVC during immediate recovery existed exclusively above CP (Fig. 4B). Importantly, these intensity-dependent characteristics were not a result of differences in CP among subjects (Fig. 5). Together, these findings suggest that CP represents a threshold above which muscular contraction intensity becomes a significant determinant of LBF impedance during exercise.
4.2. LBF impedance is not exclusive to exercise above CP
During early recovery, LVC increased below CP suggesting some degree of LBF impedance by muscular contractions (Fig. 2C). This is in direct contrast with our hypothesis that LBF impedance would become manifest exclusively above CP. Lutjemeier et al. demonstrated a net impedance effect of muscular contractions on LBF during knee-extension exercise at ∼60–75% of Ppeak (Lutjemeier et al., 2005). The current study determined CP to be 79.0% of Ppeak and the power-output 10% below CP to be 71.1% of Ppeak on average. Given this, our data suggesting the presence of LBF impedance below CP, although in contrast to our hypothesis, is consistent with previous findings (Lutjemeier et al., 2005). While increases in LVC during immediate recovery (Fig. 2C and 3B) and %IMPLBF at end-exercise (Fig. 3A) were significantly greater above CP, data from this study cannot support the existence of a hardline threshold at CP. However, the determinants of absolute LBF impedance during exercise above CP appear to be distinct from those below CP (Fig. 4B).
Remarkably, LVC was significantly lower at end-exercise above, compared to below, CP (Fig. 1C). This finding supports the notion that increased impedance of LBF above CP resulting from increased contraction forces is not fully counterbalanced by increases in local vasodilation or blood pressure. In addition, the slope of the relationship between LBF during exercise and power output was markedly reduced above, compared to below CP (Fig. 4A) providing further evidence for increases in LBF impedance. The absolute magnitude of LBF impedance during exercise (indicated by the absolute change in LVC during immediate recovery) appears to be determined by exercise power-output exclusively above CP (Fig. 4B; solid triangles). Specifically, despite a relatively wide range of test power-outputs (73–151W) across subjects, the absolute magnitude of LBF impedance during exercise was not associated with contraction intensity below CP (Fig. 4B; open triangles). In contrast, subjects with higher CPs, and thus higher above-CP contraction intensities, were characterized with higher levels of absolute LBF impedance above CP (Fig. 4B; solid triangles). Per our study design, individuals with higher CPs were asked to perform exercise across a wider absolute range of power-outputs (±10% of CP). Therefore, it is important to note that the difference in the percent-increase in LVC during immediate recovery from below-to-above CP was not associated with individual subject CPs (Fig. 5). Thus, absolute changes in power-outputs within subjects from below-to-above CP were unlikely the root cause of this association. Considering that contraction-induced retrograde arterial flow reaches a maximum at very light contraction intensities (Hoelting et al., 2001), any power-output associations are likely dependent on the magnitude of inter-contraction hyperemia (i.e., during the relaxation phase). Together, these data suggest that absolute LBF impedance is dependent, at least in part, on muscular contraction intensity above, but not below, CP. Further, the capacity to rapidly perfuse skeletal muscle during the relaxation phase may play a significant role in determining CP and severe-intensity exercise tolerance (Broxterman et al., 2014).
4.3. Implications for skeletal muscle fatigue and exercise tolerance
Although the intrinsic coupling of CP to oxygen delivery is well known (Broxterman et al., 2015; Dekerle et al., 2012; Jones et al., 2010; Moritani et al., 1981; Poole et al., 2016; Vanhatalo et al., 2010), this is the first study to evaluate the magnitude of LBF impedance above versus below this exercise threshold. The greater limitations imposed on oxygen delivery above CP during this study (Fig. 2 and 3) would suggest a diminished relative contribution of aerobic metabolism to energy production and thus greater reliance on finite anaerobic energy sources. Exercise tolerance above CP has been associated with the rate of anaerobic energy store utilization (Miura et al., 1999; Miura et al., 2000; Smith et al., 1998), concomitant accumulation of metabolic byproducts (Burnley et al., 2010; Jones et al., 2008; Vanhatalo et al., 2010), and progressive loss of skeletal muscle efficiency (Vanhatalo et al., 2011). Indeed, intensity-independent critical levels of metabolite accumulation (e.g., inorganic phosphate and hydrogen ions) (Burnley et al., 2010; Vanhatalo et al., 2010) and peripheral skeletal muscle fatigue (Burnley et al., 2012; Hammer et al., 2020a) appear to exist above CP and, once attained, limit exercise tolerance. These findings, combined with those of this study, suggest that LBF impedance by skeletal muscle contraction contributes toward the development of metabolite-induced skeletal muscle fatigue more above, compared to below, CP.
The present study offers novel insight to the contraction-dependency characteristics of CP (Barker et al., 2006; Broxterman et al., 2014; Hill et al., 1995; Hoelting et al., 2001; McNaughton and Thomas, 1996). Specifically, the observation that %IMPLBF above CP demonstrated a significantly lower degree of variance across subjects (CV: 10.7%) compared to below CP (CV: 53.2%) (Fig. 3A) suggests that a specific level of LBF impedance, relative to oxygen demand, might be ‘tolerated’ before precipitating the well-documented above-CP exercise responses (i.e., progressive metabolite and fatigue accumulation) (Burnley et al., 2012; Jones et al., 2008). This interpretation is consistent with previous findings suggesting LBF reaches a similar end-exercise value, despite increases in contraction force, above critical force (CF; the isometric analog of CP) (Hammer et al., 2020b). Combined with a greater reliance on low-oxidative muscle fibers (Copp et al., 2010), limitations to the overall LBF response above CP would further complicate the matching of oxygen delivery to oxygen demand during exercise and promote the utilization of anaerobic energy stores that are associated with the development of peripheral fatigue (Jones et al., 2008).
4.4. Implications for blood flow heterogeneity
It is important to highlight that this study utilized bulk LBF measurements of a major conduit artery (i.e., common femoral) to assess changes in LVC and provided no index for microvascular oxygen delivery or distribution. As such, we can only speculate as to the impact of bulk LBF impedance on the heterogeneity of microvascular oxygen delivery known to exist during exercise (Heinonen et al., 2015; Koga et al., 2014; Vogiatzis et al., 2015). Increases in blood flow from below-to-above critical power are distributed primarily to low-oxidative muscle fibers (Copp et al., 2010). Additionally, differences in blood flow among fiber-types have been attributed to variances in both local vasodilator and vasoconstrictor sensitivities and the degree of sympathetically controlled vasoconstriction (Behnke et al., 2011; Laughlin et al., 2012). Given that increases in exercise intensity rely on the recruitment of additional motor-units (Chin et al., 2011; Hammer et al., 2018; Okushima et al., 2020), fiber-type differences in vascular control may lead to heterogeneity in the physiological consequences of increased blood flow impedance.
The relatively widespread range of %IMPLBF during exercise below CP suggests a lesser role for intramuscular pressures in the physiological responses to steady-state exercise (Fig. 3A). In contrast, ∼40% relative impedance to LBF was experienced by all subjects above CP. The heterogeneity of this impedance among individual muscles or muscle regions remains to be elucidated. However, it is possible that fiber-type specific mechanisms of flow regulation are differentially limited above, compared to below, CP at this level of %IMPLBF. For example, shear-stress mediated nitric oxide (NO) production is thought to play a major role in distributing blood flow to highly-oxidative muscles below CP while neuronal NO synthase appears to preferentially augment vascular conductance in highly-glycolytic muscles above CP (Copp et al., 2013). Additionally, neuronal NO synthase is thought to play a major role in duty cycle- and intensity-dependent inhibition of sympathetic vasoconstriction (i.e., sympatholysis) (Caldwell et al., 2018; Thomas et al., 2003; Tschakovsky et al., 2002). Considering the inherent dependency on blood flow, it seems reasonable to speculate that shear-stress mediated regulation of vascular conductance would be largely impacted by LBF impedance. Thus, highly oxidative regions may be particularly susceptible to increases in intramuscular pressures, resulting in oxygen delivery and oxygen demand mismatching and perhaps the augmented metabolite accumulation (Jones et al., 2008), contractile inefficiency (Vanhatalo et al., 2011), and response (Poole et al., 1988) observed above CP.
4.4. Experimental considerations
This study measured the impedance of LBF during dynamic locomotor-muscle exercise as opposed to simple single-joint exercises such as handgrip or knee-extension. To accomplish this, high-quality Doppler ultrasound images of the common femoral artery were required. Unlike gold-standard techniques for measuring LBF (e.g., thermodilution), movement of the target vessel relative to the Doppler transducer can influence measured blood velocity values by altering the angle of insonation (Gliemann et al., 2018). To improve our ability to maintain a consistent Doppler angle and attempt to minimize these effects, near-supine exercise, a relatively slow pedal cadence, and shortened pedal rotation were intentionally utilized. The findings from this study should be interpreted while considering these known limitations of the Doppler ultrasound technique and with this specific exercise modality in mind.
In the present study, Ppeak, , and CP were considerably lower than values expected during traditional upright cycling (Ade et al., 2013; Goulding et al., 2017). Additional physiological factors (or altered contributions from already existing factors) may influence the LBF responses below versus above CP at higher absolute intensities. During supine exercise, LBF (MacDonald et al., 1998) and (Koga et al., 1999; MacDonald et al., 1998) demonstrate slowed on-kinetic responses to exercise compared to upright exercise. To avoid the influence of posture during the on-transient phase and to ensure that changes in LBF and MAP during early recovery would reflect the influence of contractions during steady-state exercise, end-exercise measurements were made between 4.5- and 5.5-minutes. LBF is determined by downstream vascular resistance and driving pressure. During supine exercise, driving pressure into the legs is reduced by a decreased gravitational effect (i.e., reduced hydrostatic column). Therefore, downstream vascular resistance would have a proportionally greater influence on LBF and the influence of muscle contraction on LBF may have been exacerbated during this study. In addition, interpreting changes in LVC during recovery from exercise as evidence for muscle contraction induced impedance of LBF required the assumption of an unchanged vessel diameter. Therefore, the possibility of small changes in femoral artery diameter during immediate recovery should be considered when interpreting these data.
Reductions in contraction frequency have been demonstrated to elevate the LBF response to exercise (Hoelting et al., 2001) and increase CP (but not the metabolic rate at which it occurs) (Barker et al., 2006) during upright exercise. While the influence of contraction frequency on LBF and CP was not investigated in the present study, it is possible that a relatively slow pedal cadence augmented the LBF response through greater relaxation time between pedal strokes and thus reduced the net influence of muscular contractions. However, a slow pedal cadence combined with reductions in mechanical advantage (i.e., decreased crank-arm length) and posture associated increases in muscle activation (Egana et al., 2013) may have increased the net influence of muscular contractions on LBF during this study compared to traditional cycling exercise. Indeed, the contraction characteristics of this study (contraction frequency and range-of-motion) were more similar to those of stair-climbing (Heller et al., 2001; Holsgaard-Larsen et al., 2011). Therefore, our findings are generalizable to activities of daily living, particularly in populations whose CP is at a low absolute work rate (e.g., aging, heart failure, and pulmonary disease) (Neder et al., 2000a, b; van der Vaart et al., 2014). However, while our findings suggest that muscular contraction-induced LBF impedance may play a substantial role in determining CP in young and healthy individuals, it is important to consider that other factors (i.e., dyspnea, central cardiac limitations, microvascular dysfunction) may become proportionally more limiting in older individuals or those with chronic disease.
5. Conclusions
This is the first study that provides LBF measurements during locomotor-muscle exercise below and above the CP threshold in humans. This study demonstrated that muscular contraction-induced impedance to LBF was significantly greater above, compared to below, CP. Additionally, CP appears to represent a threshold above which the characteristics of LBF impedance by muscular contraction become intensity-dependent. Remarkably, while it remains to be elucidated if greater LBF impedance occurs with further increases in exercise intensity, a comparatively consistent degree of relative LBF impedance was demonstrated above CP. This evidence suggests a critical level of LBF limitation relative to contraction intensity exists and, once attained, may promote the progressive metabolic and neuromuscular responses known to occur above CP. Finally, future studies should aim to uncover differences in the interactions between energetic demand and the concomitant mechanical consequences associated with higher-intensity muscular contractions across the CP threshold.
Highlights.
Critical power is an important exercise threshold above which limb blood flow and microvascular oxygen delivery appear to reach task-specific physiological maximums.
We measured limb blood flow and mean arterial pressure to determine if critical power represents a threshold for muscular contraction-induced impedance of limb blood flow during dynamic locomotor-muscle exercise.
Limb vascular conductance demonstrated a greater increase during immediate recovery and remained significantly higher following exercise above, compared to below, critical power.
The influence of muscular contraction on limb vascular conductance was intensity dependent and the level of relative blood flow impedance was consistent above, but not below, critical power.
Critical power appears to represent an intensity threshold above which a critical level of limb blood flow impedance relative to contraction intensity is elicited and, once attained, may promote progressive metabolic and neuromuscular responses during exercise.
Acknowledgments
Author contributions
This work was completed at Kansas State University. SMH, STH, AMA, KDD, JRS, TJB, and CJA conceived and designed this study. SMH, STH, SKP, AMA, VGT, ZJW, KDD, JRS, TJB, and CJA acquired, analyzed, and interpreted the data. SMH prepared the first draft of the manuscript. STH, SKP, AMA, VGT, KDD, JRS, TJB, and CJA revised it critically for important intellectual content. All authors have read and approved the final version of this manuscript and agree to be accountable for all aspects of the work in ensuring that questions related to the accuracy or integrity of any part of the work are appropriately investigated and resolved. All persons designated as authors qualify for authorship, and all those who qualify for authorship are listed.
Funding
This work was supported by National Institutes of Health awards: T32 HL 07111 to SMH and JRS; K12 HD065987 to JRS
Footnotes
Additional Information
Data Availability
The data that support the findings of this study are available on request from the corresponding author.
Competing interests
The authors report no competing interests for this work.
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- Ade CJ, Broxterman RM, Barstow TJ, 2013. Effects of body posture and exercise training on cardiorespiratory responses to exercise. Respir Physiol Neurobiol 188, 39–48. [DOI] [PubMed] [Google Scholar]
- Ade CJ, Broxterman RM, Wong BJ, Barstow TJ, 2012. Anterograde and retrograde blood velocity profiles in the intact human cardiovascular system. Experimental physiology 97, 849–860. [DOI] [PubMed] [Google Scholar]
- Barcroft H, Dornhorst AC, 1949. The Blood Flow through the Human Calf during Rhythmic Exercise. J Physiol-London 109, 402-&. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Barker T, Poole DC, Noble ML, Barstow TJ, 2006. Human critical power-oxygen uptake relationship at different pedalling frequencies. Exp Physiol 91, 621–632. [DOI] [PubMed] [Google Scholar]
- Behnke BJ, Armstrong RB, Delp MD, 2011. Adrenergic control of vascular resistance varies in muscles composed of different fiber types: influence of the vascular endothelium. Am J Physiol-Reg I 301, R783–R790. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bentley RF, Poitras VJ, Hong T, Tschakovsky ME, 2017. Characteristics and effectiveness of vasodilatory and pressor compensation for reduced relaxation time during rhythmic forearm contractions. Experimental Physiology 102, 621–634. [DOI] [PubMed] [Google Scholar]
- Bogert LWJ, van Lieshout JJ, 2005. Non-invasive pulsatile arterial pressure and stroke volume changes from the human finger. Experimental Physiology 90, 437–446. [DOI] [PubMed] [Google Scholar]
- Boushel R, Langberg H, Gemmer C, Olesen J, Crameri R, Scheede C, Sander M, Kjaer M, 2002. Combined inhibition of nitric oxide and prostaglandins reduces human skeletal muscle blood flow during exercise. J Physiol 543, 691–698. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Broxterman RM, Ade CJ, Craig JC, Wilcox SL, Schlup SJ, Barstow TJ, 2015. Influence of blood flow occlusion on muscle oxygenation characteristics and the parameters of the power-duration relationship. Journal of applied physiology (Bethesda, Md. : 1985) 118, 880–889. [DOI] [PubMed] [Google Scholar]
- Broxterman RM, Ade CJ, Wilcox SL, Schlup SJ, Craig JC, Barstow TJ, 2014. Influence of duty cycle on the power-duration relationship: observations and potential mechanisms. Respir Physiol Neurobiol 192, 102–111. [DOI] [PubMed] [Google Scholar]
- Burnley M, Vanhatalo A, Fulford J, Jones AM, 2010. Similar metabolic perturbations during all-out and constant force exhaustive exercise in humans: a 31P magnetic resonance spectroscopy study. Experimental Physiology 95, 798–807. [DOI] [PubMed] [Google Scholar]
- Burnley M, Vanhatalo A, Jones AM, 2012. Distinct profiles of neuromuscular fatigue during muscle contractions below and above the critical torque in humans. J Appl Physiol 113, 215–223. [DOI] [PubMed] [Google Scholar]
- Caldwell JT, Sutterfield SL, Post HK, Lovoy GM, Banister HR, Hammer SM, Ade CJ, 2018. Vasoconstrictor responsiveness through alterations in relaxation time and metabolic rate during rhythmic handgrip contractions. Physiol Rep 6, e13933. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chin LM, Kowalchuk JM, Barstow TJ, Kondo N, Amano T, Shiojiri T, Koga S, 2011. The relationship between muscle deoxygenation and activation in different muscles of the quadriceps during cycle ramp exercise. Journal of applied physiology (Bethesda, Md. : 1985) 111, 1259–1265. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Copp SW, Hirai DM, Musch TI, Poole DC, 2010. Critical speed in the rat: implications for hindlimb muscle blood flow distribution and fibre recruitment. J Physiol-London 588, 5077–5087. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Copp SW, Holdsworth CT, Ferguson SK, Hirai DM, Poole DC, Musch TI, 2013. Muscle fibre-type dependence of neuronal nitric oxide synthase-mediated vascular control in the rat during high speed treadmill running. J Physiol-London 591, 2885–2896. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dekerle J, Mucci P, Carter H, 2012. Influence of moderate hypoxia on tolerance to high-intensity exercise. Eur J Appl Physiol 112, 327–335. [DOI] [PubMed] [Google Scholar]
- Egana M, Columb D, O’Donnell S, 2013. Effect of low recumbent angle on cycling performance, fatigue, and V O(2) kinetics. Med Sci Sports Exerc 45, 663–673. [DOI] [PubMed] [Google Scholar]
- Gaffney FA, Sjogaard G, Saltin B, 1990. Cardiovascular and Metabolic Responses to Static Contraction in Man. Acta Physiologica Scandinavica 138, 249–258. [DOI] [PubMed] [Google Scholar]
- Gliemann L, Mortensen SP, Hellsten Y, 2018. Methods for the determination of skeletal muscle blood flow: development, strengths and limitations. Eur J Appl Physiol 118, 1081–1094. [DOI] [PubMed] [Google Scholar]
- Gobel FL, Norstrom LA, Nelson RR, Jorgensen CR, Wang Y, 1978. The rate-pressure product as an index of myocardial oxygen consumption during exercise in patients with angina pectoris. Circulation 57, 549–556. [DOI] [PubMed] [Google Scholar]
- Goulding RP, Roche DM, Marwood S, 2017. Prior exercise speeds pulmonary oxygen uptake kinetics and increases critical power during supine but not upright cycling. Exp Physiol 102, 1158–1176. [DOI] [PubMed] [Google Scholar]
- Habazettl H, Athanasopoulos D, Kuebler WM, Wagner H, Roussos C, Wagner PD, Ungruhe J, Zakynthinos S, Vogiatzis I, 2010. Near-infrared spectroscopy and indocyanine green derived blood flow index for noninvasive measurement of muscle perfusion during exercise. Journal of applied physiology (Bethesda, Md. : 1985) 108, 962–967. [DOI] [PubMed] [Google Scholar]
- Hammer SM, Alexander AM, Didier KD, Barstow TJ, 2020a. Influence of blood flow occlusion on muscular recruitment and fatigue during maximal-effort small muscle-mass exercise. J Physiol. [DOI] [PubMed] [Google Scholar]
- Hammer SM, Alexander AM, Didier KD, Huckaby LM, Barstow TJ, 2020b. Limb blood flow and muscle oxygenation responses during handgrip exercise above vs. below critical force. Microvascular Research. [DOI] [PubMed] [Google Scholar]
- Hammer SM, Alexander AM, Didier KD, Smith JR, Caldwell JT, Sutterfield SL, Ade CJ, Barstow TJ, 2018. The noninvasive simultaneous measurement of tissue oxygenation and microvascular hemodynamics during incremental handgrip exercise. J Appl Physiol 124, 604–614. [DOI] [PubMed] [Google Scholar]
- Heinonen I, Koga S, Kalliokoski KK, Musch TI, Poole DC, 2015. Heterogeneity of Muscle Blood Flow and Metabolism: Influence of Exercise, Aging, and Disease States. Exerc Sport Sci Rev 43, 117–124. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Heller MO, Bergmann G, Deuretzbacher G, Durselen L, Pohl M, Claes L, Haas NP, Duda GN, 2001. Musculo-skeletal loading conditions at the hip during walking and stair climbing. J Biomech 34, 883–893. [DOI] [PubMed] [Google Scholar]
- Hill DW, Smith JC, Leuschel JL, Chasteen SD, Miller SA, 1995. Effect of Pedal Cadence on Parameters of the Hyperbolic Power - Time Relationship. Int J Sports Med 16, 82–87. [DOI] [PubMed] [Google Scholar]
- Hoelting BD, Scheuermann BW, Barstow TJ, 2001. Effect of contraction frequency on leg blood flow during knee extension exercise in humans. J Appl Physiol (1985) 91, 671–679. [DOI] [PubMed] [Google Scholar]
- Holsgaard-Larsen A, Caserotti P, Puggaard L, Aagaard P, 2011. Stair-ascent performance in elderly women: effect of explosive strength training. J Aging Phys Act 19, 117–136. [DOI] [PubMed] [Google Scholar]
- Jones AM, Vanhatalo A, Burnley M, Morton RH, Poole DC, 2010. Critical power: implications for determination of V O2max and exercise tolerance. Med Sci Sports Exerc 42, 1876–1890. [DOI] [PubMed] [Google Scholar]
- Jones AM, Wilkerson DP, DiMenna F, Fulford J, Poole DC, 2008. Muscle metabolic responses to exercise above and below the “critical power” assessed using 31P-MRS. American journal of physiology. Regulatory, integrative and comparative physiology 294, R585–593. [DOI] [PubMed] [Google Scholar]
- Kitamura K, Jorgensen CR, Gobel FL, Taylor HL, Wang Y, 1972. Hemodynamic correlates of myocardial oxygen consumption during upright exercise. J Appl Physiol 32, 516–522. [DOI] [PubMed] [Google Scholar]
- Koga S, Rossiter HB, Heinonen I, Musch TI, Poole DC, 2014. Dynamic heterogeneity of exercising muscle blood flow and O2 utilization. Medicine and science in sports and exercise 46, 860–876. [DOI] [PubMed] [Google Scholar]
- Koga S, Shiojiri T, Shibasaki M, Kondo N, Fukuba Y, Barstow TJ, 1999. Kinetics of oxygen uptake during supine and upright heavy exercise. J Appl Physiol (1985) 87, 253–260. [DOI] [PubMed] [Google Scholar]
- Laughlin MH, Davis MJ, Secher NH, van Lieshout JJ, Arce-Esquivel AA, Simmons GH, Bender SB, Padilla J, Bache RJ, Merkus D, Duncker DJ, 2012. Peripheral Circulation. Compr Physiol 2, 321–447. [DOI] [PubMed] [Google Scholar]
- Limberg JK, Eldridge MW, Proctor LT, Sebranek JJ, Schrage WG, 2010. Alpha-adrenergic control of blood flow during exercise: effect of sex and menstrual phase. Journal of applied physiology (Bethesda, Md. : 1985) 109, 1360–1368. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lutjemeier BJ, Miura A, Scheuermann BW, Koga S, Townsend DK, Barstow TJ, 2005. Muscle contraction-blood flow interactions during upright knee extension exercise in humans. Journal of applied physiology (Bethesda, Md. : 1985) 98, 1575–1583. [DOI] [PubMed] [Google Scholar]
- MacDonald MJ, Shoemaker JK, Tschakovsky ME, Hughson RL, 1998. Alveolar oxygen uptake and femoral artery blood flow dynamics in upright and supine leg exercise in humans. J Appl Physiol (1985) 85, 1622–1628. [DOI] [PubMed] [Google Scholar]
- McNaughton L, Thomas D, 1996. Effects of differing pedalling speeds on the power-duration relationship of high intensity cycle ergometry. Int J Sports Med 17, 287–292. [DOI] [PubMed] [Google Scholar]
- Miura A, Kino F, Kajitani S, Sato H, Fukuba Y, 1999. The effect of oral creatine supplementation on the curvature constant parameter of the power-duration curve for cycle ergometry in humans. Jpn J Physiol 49, 169–174. [DOI] [PubMed] [Google Scholar]
- Miura A, Sato H, Sato H, Whipp BJ, Fukuba Y, 2000. The effect of glycogen depletion on the curvature constant parameter of the power-duration curve for cycle ergometry. Ergonomics 43, 133–141. [DOI] [PubMed] [Google Scholar]
- Moritani T, Nagata A, Devries HA, Muro M, 1981. Critical Power as a Measure of Physical Work Capacity and Anaerobic Threshold. Ergonomics 24, 339–350. [DOI] [PubMed] [Google Scholar]
- Neder JA, Jones PW, Nery LE, Whipp BJ, 2000a. Determinants of the exercise endurance capacity in patients with chronic obstructive pulmonary disease. The power-duration relationship. Am J Respir Crit Care Med 162, 497–504. [DOI] [PubMed] [Google Scholar]
- Neder JA, Jones PW, Nery LE, Whipp BJ, 2000b. The effect of age on the power/duration relationship and the intensity-domain limits in sedentary men. Eur J Appl Physiol 82, 326–332. [DOI] [PubMed] [Google Scholar]
- Okushima D, Poole DC, Barstow TJ, Kondo N, Chin LMK, Koga S, 2020. Effect of differential muscle activation patterns on muscle deoxygenation and microvascular haemoglobin regulation. Experimental Physiology 105, 531–541. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Poole DC, Burnley M, Vanhatalo A, Rossiter HB, Jones AM, 2016. Critical Power: An Important Fatigue Threshold in Exercise Physiology. Med Sci Sports Exerc 48, 2320–2334. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Poole DC, Ward SA, Gardner GW, Whipp BJ, 1988. Metabolic and respiratory profile of the upper limit for prolonged exercise in man. Ergonomics 31, 1265–1279. [DOI] [PubMed] [Google Scholar]
- Radegran G, Saltin B, 1998. Muscle blood flow at onset of dynamic exercise in humans. Am J Physiol-Heart C 274, H314–H322. [DOI] [PubMed] [Google Scholar]
- Robergs RA, Icenogle MV, Hudson TL, Greene ER, 1997. Temporal inhomogeneity in brachial artery blood flow during forearm exercise. Med Sci Sport Exer 29, 1021–1027. [DOI] [PubMed] [Google Scholar]
- Sadamoto T, Bondepetersen F, Suzuki Y, 1983. Skeletal-Muscle Tension, Flow, Pressure, and Emg during Sustained Isometric Contractions in Humans. Eur J Appl Physiol O 51, 395–408. [DOI] [PubMed] [Google Scholar]
- Sjogaard G, Kiens B, Jorgensen K, Saltin B, 1986. Intramuscular Pressure, Emg and Blood-Flow during Low-Level Prolonged Static Contraction in Man. Acta Physiologica Scandinavica 128, 475–484. [DOI] [PubMed] [Google Scholar]
- Smith JC, Stephens DP, Hall EL, Jackson AW, Earnest CP, 1998. Effect of oral creatine ingestion on parameters of the work rate-time relationship and time to exhaustion in high-intensity cycling. Eur J Appl Physiol Occup Physiol 77, 360–365. [DOI] [PubMed] [Google Scholar]
- Smith JR, Hageman KS, Harms CA, Poole DC, Musch TI, 2017. Respiratory muscle blood flow during exercise: Effects of sex and ovarian cycle. Journal of applied physiology (Bethesda, Md. : 1985) 122, 918–924. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Thomas GD, Shaul PW, Yuhanna IS, Froehner SC, Adams ME, 2003. Vasomodulation by skeletal muscle-derived nitric oxide requires alpha-syntrophin-mediated sarcolemmal localization of neuronal Nitric oxide synthase. Circ Res 92, 554–560. [DOI] [PubMed] [Google Scholar]
- Tschakovsky ME, Rogers AM, Pyke KE, Saunders NR, Glenn N, Lee SJ, Weissgerber T, Dwyer EM, 2004. Immediate exercise hyperemia in humans is contraction intensity dependent: evidence for rapid vasodilation. J Appl Physiol 96, 639–644. [DOI] [PubMed] [Google Scholar]
- Tschakovsky ME, Sujirattanawimol K, Ruble SB, Valic Z, Joyner MJ, 2002. Is sympathetic neural vasoconstriction blunted in the vascular bed of exercising human muscle? J Physiol-London 541, 623–635. [DOI] [PMC free article] [PubMed] [Google Scholar]
- van der Vaart H, Murgatroyd SR, Rossiter HB, Chen C, Casaburi R, Porszasz J, 2014. Selecting constant work rates for endurance testing in COPD: the role of the power-duration relationship. COPD 11, 267–276. [DOI] [PubMed] [Google Scholar]
- Vanhatalo A, Fulford J, DiMenna FJ, Jones AM, 2010. Influence of hyperoxia on muscle metabolic responses and the power-duration relationship during severe-intensity exercise in humans: a 31P magnetic resonance spectroscopy study. Experimental Physiology 95, 528–540. [DOI] [PubMed] [Google Scholar]
- Vanhatalo A, Poole DC, DiMenna FJ, Bailey SJ, Jones AM, 2011. Muscle fiber recruitment and the slow component of O-2 uptake: constant work rate vs. all-out sprint exercise. Am J Physiol-Reg I 300, R700–R707. [DOI] [PubMed] [Google Scholar]
- Vogiatzis I, Habazettl H, Louvaris Z, Andrianopoulos V, Wagner H, Zakynthinos S, Wagner PD, 2015. A method for assessing heterogeneity of blood flow and metabolism in exercising normal human muscle by near-infrared spectroscopy. Journal of applied physiology (Bethesda, Md. : 1985) 118, 783–793. [DOI] [PubMed] [Google Scholar]
- Walloe L, Wesche J, 1988. Time Course and Magnitude of Blood-Flow Changes in the Human Quadriceps Muscles during and Following Rhythmic Exercise. J Physiol-London 405, 257–273. [DOI] [PMC free article] [PubMed] [Google Scholar]