Abstract
Double-stranded RNA (dsRNA) of viral origin triggers two programs of the innate immunity in virus-infected cells. One is intended to decrease the rate of host cell protein synthesis and thus to prevent viral replication. This program is mediated by protein kinase R (PKR) and by RNase L and contributes, eventually, to the self-elimination of the infected cell via apoptosis. The second program is responsible for the production of antiviral (type I) interferons and other alarmone cytokines and serves the purpose of preparing naive cells for the viral invasion. This second program requires the survival of the infected cell and depends on the expression of antiapoptotic genes through the activation of the NF-κB transcription factor. The second program therefore relies on ongoing transcription and translation. It has been proposed that PKR plays an essential role in the activation of NF-κB by dsRNA. Here we present evidence that the dsRNA-induced NF-κB activity and the expression of beta interferon and inflammatory cytokines do not require either PKR or RNase L. Our results indicate, therefore, that the two dsRNA-activated programs are separate and can function independently of each other.
At the cellular level, the innate immune response to viruses relies on the execution of two apparently conflicting cellular programs: cell suicide (apoptosis) and survival. The first program is probably most efficient for viral infections that are initiated by a small number of infected cells at a local site of virus entry. In such case, it seems beneficial (for the organism) for this first population of infected cells to undergo a rapid process of self-elimination through apoptosis, thus preventing further infection. That this first line of antiviral defense is widely used is evident from the multitude of antiapoptotic strategies employed by viruses. Viral genomes encode a growing number of apoptosis-inhibiting proteins, such as the adenovirus E1B protein (45, 72), the baculovirus p35 protein (5, 44, 58), the cowpox virus CrmA protein (17, 63), the poxvirus and gammaherpesvirus v-FLIP proteins (66), and others (for a review, see reference 62). Genetic evidence from mice (48) (see below) demonstrates that inhibition of apoptosis by the virus is critical for the virulence of encephalomyocarditis virus (EMCV), a picornavirus that is lethal to infected mice.
A common viral intermediate that is recognized by specific cellular sensory systems to trigger apoptosis is viral double-stranded RNA (dsRNA). The best-characterized effect of dsRNA on cells is the inhibition of protein synthesis in host cells. The cellular dsRNA-detecting systems that are responsible for the translational inhibition in response to viral infection are the dsRNA-activated protein kinase (PKR) and the coupled 2-5 oligoadenylate synthase/RNase L system. PKR (39) is a dormant enzyme directly activated by binding of dsRNA (for recent reviews, see references 28 and 73). A major substrate of PKR is the α-subunit of the eukaryotic translation initiation factor 2 (eIF-2α) (22, 35). Phosphorylation of eIF-2α greatly reduces the rate of initiation of translation (50). The 2-5 oligoadenylate synthase/RNase L system is composed of a family of dsRNA-dependent enzymes known as 2′-5′ oligoadenylate synthetases (OAS) (7, 24, 43) and the dormant cytosolic RNase L (82). Upon dsRNA binding, OAS produce unusual second messengers, short 2′-5′-linked oligoadenylates (2-5A), which, in turn, specifically bind to and activate RNase L (41). Activated RNase L cleaves diverse RNA substrates, including 18S and 28S rRNA, thus inhibiting cellular protein synthesis (26, 51, 52, 74). Fibroblasts from mice nullizygous for both PKR and RNase L alleles are unable to inhibit protein synthesis when challenged with dsRNA (26), thus demonstrating that these two enzymes are both required and sufficient for the translational inhibition caused by dsRNA. Recently, both PKR (2, 3, 15, 20, 34, 53, 77) and RNase L (7–9, 13, 83, 85) have been found to mediate dsRNA-induced apoptosis. The mechanisms of involvement of PKR and RNase L in the dsRNA-triggered apoptosis are, however, poorly understood. Considering the role of both PKR and RNase L in inhibiting protein synthesis, one obvious candidate for a death-inducing signal is the impaired process of translation. A sustained inhibition of protein synthesis is sufficient to trigger apoptosis in cells in a way that is independent of the particular means of achieving translational inhibition (27). However, other (more direct) mechanisms of dsRNA-induced cell death, which are independent of the state of cellular translation, are very likely to exist.
The second dsRNA-initiated program for antiviral defense involves the production and secretion by the infected cells of alarmone cytokines, the best-studied examples of which are the alpha, beta, and omega interferons, [for reviews, see references 16 and 54]). The importance of these interferons for conferring viral resistance to naive cells has been demonstrated by the strong sensitivity to viral infections of mice lacking the common subunit of the alpha, beta, and omega interferon receptor (40). It is thought that these interferons exert their pleiotropic antiviral actions by preparing cells to interfere with multiple, virus-specific steps of the viral replication cycle, including viral entry, uncoating, transcription, RNA stability, maturation, assembly, and release (for a review, see reference 54). Interferons are also important for the ability of adaptive immunity to take over the innate immune response in combating the virus. For instance, mice lacking the interferon alpha, beta, and omega receptor are unable to mount a cytotoxic T-lymphocyte response to infection with lymphocytic choriomeningitis virus (40).
A crucial step in the virus-induced beta interferon production appears to be its transcriptional upregulation by viral dsRNA (for a review, see reference 37). The highly specific transcriptional induction of the beta interferon gene by viruses has been best studied for the human beta interferon gene promoter/enhancer region. This region contains a set of regulatory elements called positive regulatory domains (PRDI to PRDIV). PRDII, PRDI-III, and PRDIV bind the transcription factors NF-κB, IRF-1 (or IRF-3), and ATF-2/c-Jun, respectively (for a review, see reference 37). Importantly, NF-κB appears to be absolutely required for the virus-induced activation of the human beta interferon promoter (18, 64, 65).
For the second (interferon-dependent) program of innate antiviral immune response to be successful, the proapoptotic response of the infected cells must be suppressed, at least for the time required to complete the production and secretion of alarmone cytokines. Interestingly, genetic evidence strongly suggests that NF-κB not only plays an important role in the production of beta interferon but also is essential in suppressing virus-induced apoptosis. Mice engineered to lack the p50/NF-κB1 subunit of NF-κB (see below) are resistant to infection with EMCV (48, 49). This surprising result (which seems to contradict the important role of NF-κB in combating viral infections) is explained by the discovery that EMCV-infected cells from p50/NF-κB1-nullizygous mice (as well as from mice engineered to lack the other common subunit of NF-κB, p65/RelA [see below]) undergo very rapid apoptosis before the virus could reproduce (48). These results demonstrate the apoptosis-suppressing function of NF-κB in EMCV infection. The antiapoptotic role of NF-κB is thought to result from the NF-κB-dependent transcriptional activation of several apoptosis-inhibiting genes, such as the genes encoding the inhibitor-of-apoptosis proteins (IAPs; for a review, see reference 32) IAP-1, IAP-2, and X-linked IAP (X-IAP1) (12, 55, 70), Bcl-XL (10, 33, 68), and A1/Bfl1 (33, 57, 69, 86).
How is NF-κB activated in general and by viruses and dsRNA in particular? NF-κB is a collective name for a group of homo- and heterodimeric transcriptional regulators (activators or repressors) consisting in vertebrates, of the polypeptide products of the p50/p105(nfkb1), p52/p100(nfkb2), c-rel, relA, and relB genes (for reviews, see references 21 and 42). In mammalian cells, the most common NF-κB complex is the p50/NF-κB1–p65/RelA heterodimer, and it is this combination that is most commonly referred to as NF-κB proper (42). An essential role in the regulation of NF-κB is played by a family of inhibitory proteins, collectively termed IκBs (the family encompasses IκB-α, IκB-β, IκB-ɛ, and Bcl-3 [for a review, see reference 31]). In nonstimulated cells, IκBs sequester the p50/NF-κB1–p65/RelA heterodimer in the cytoplasm, thus preventing it from localizing in the nucleus and stimulating the transcription of NF-κB-dependent genes (for a review, see reference 31). With the notable exception of UV radiation, a potent NF-κB activator, most stimuli that activate NF-κB (including viruses and dsRNA) cause the phosphorylation of serine residues 32 and 36 in IκB-α (and of the corresponding serine residues in IκB-β). The phosphorylation of IκB causes its rapid polyubiquitinylation and degradation by the 26S proteasome, thus releasing NF-κB and allowing it to translocate to the nucleus (most extensively reviewed in references 30, 31, and 80). A multicomponent IκB kinase (IKK) complex has been purified, molecularly cloned, and found to consist of two homologous catalytic subunits (IKK1/α and IKK2/β) (14, 38, 81) and a noncatalytic subunit (IKKγ, also known as NEMO) (75). Genetic inactivation of IKK in mice demonstrated that IKK2/β and IKKγ (but not IKK1/α) are required for the IκB phosphorylation and subsequent NF-κB activation in response to most agents (25, 36, 46, 59, 61). Due to the lack of suitable targeted gene inactivation models, however, the modes of upstream regulation of IKK activity are currently completely unknown, even though several kinases have been proposed to act upstream of IKKs. For instance, in striking contrast to all experimental evidence concerning the role of PKR in triggering the protein synthesis-inhibiting and proapoptotic program of antiviral innate immunity, this kinase has been proposed to be a major mediator of virus- and dsRNA-induced activation of NF-κB. Using mouse embryonic fibroblasts (MEF) and 3T3-like fibroblast cell lines from one of the two published PKR genetic knockouts (76), several groups found these cells to be deficient in dsRNA-induced NF-κB activation, thus postulating an important role for this kinase in activating NF-κB in response to viruses (11, 19, 79).
We considered that this postulated role of PKR in dsRNA-induced NF-κB activation ultimately imposes the paradoxical situation that the same dsRNA-sensing molecule (PKR) would trigger the execution of the two opposing antiviral programs in the same cell: the program that attempts to eliminate the infected cell through translational inhibition and apoptosis and the program that attempts to suppress apoptosis through NF-κB activation. To resolve this paradox, we have employed a panel of primary MEF or 3T3-like cell lines from two independent successful attempts to inactivate the PKR gene in mice (1, 76), from the RNase L-deficient mice (83), and from mice with a double deficiency in both the PKR and the RNase L genes (26, 84). Our study demonstrates that neither PKR nor RNase L is required for the activation of NF-κB by dsRNA or EMCV. Furthermore, we found that the ability of dsRNA to stimulate the expression of beta interferon and of the inflammatory cytokines interleukin-6 (IL-6) and tumor necrosis factor alpha (TNF-α) was also independent of the presence of PKR. Thus, the “translational inhibition/pro-apoptotic program” and the “biosynthetic/anti-apoptotic program,” each triggered by viral dsRNA, appear to be mechanistically separate and to function independently of one another.
MATERIALS AND METHODS
Chemicals.
Lipofectin reagent was from Gibco BRL/Life Technologies. pI-pC was from Midland Certified Reagent Co. and was stored at −20°C as a 10-mg/ml stock solution in double-distilled deionized water. The proteasome inhibitors benzyloxycarbonylleucyl-leucyl-leucine aldehyde (MG 132) and benzyloxycarbonyliso-leucyl-glutamyl(OtBu)-alanyl-leucine aldehyde (proteasome inhibitor I) were purchased both from Alexis Biochemicals and from Calbiochem, and there was no detectable difference in their activities. Recombinant human TNF-α was from R&D Systems. All radiochemicals were from DuPont NEN Research Products.
Cells.
All cells were maintained in Dulbecco's modified Eagle's medium (DMEM) supplemented with 10% calf serum (HyClone, Logan, Ut.). pkr+/+ (EX12) and pkr0/0(EX12) MEF have been described previously (1) and were referred to there as pkr+/+ and pkr0/0 cells. pkr+/+(EX2+3) and pkr0/0(EX2+3) MEF were described previously (76) and were also referred to there as pkr+/+ and pkr0/0 cells. The rnasel+/+/pkr+/+, rnasel−/−/pkr+/+, and rnasel−/−/pkr−/− 3T3-like fibroblasts were described previously (84) and are referred to here by the same names as in reference 26.
Lipofectin-mediated delivery of pI-pC.
The procedure for treatment of cells with pI-pC was the same as described in reference 26. For each milliliter of final volume of Lipofectin mixture, an initial concentrated mixture (containing Lipofectin and pI-pC) was prepared in one-quarter of the final volume (250 μl). To this end, 10 μl of Lipofectin (1 mg/ml) was added to serum- and antibiotic-free DMEM and mixed, and the desired amount of pI-pC was added (in a volume of 250 μl). This mixture was left for 10 min at room temperature. Finally, the remaining three-quarters of the final volume (750 μl) was added. Before the application of the Lipofectin–pI-pC mixtures, the cells were washed once with serum-free DMEM.
Preparation of cell lysates for immunoblot analysis.
To avoid any possible dephosphorylation or proteolytic degradation of the proteins of interest, the cells were typically harvested by aspirating the cell culture medium, scraping the cells directly on the tissue culture plate in 2× sodium dodecyl sulfate-polyacrylamide gel electrophoresis (SDS-PAGE) sample-loading buffer, and subjecting them to heat denaturation at 95°C for 5 min. Cell lysates were stored at −70°C.
Antibodies and immunoblot analyses.
The antibodies against IκB-β (C-20), phospho-(serine-32)–IκB-α (B-9), PKR (M-515 and D-20), and p65/RelA (C-20) and the blocking peptide solutions used in the experiment in Fig. 3 (p65/RelA C-20 peptide and MEKK1 C-22 peptide) were from Santa Cruz Biotechnologies. For the antibody blocking, 1 volume of the anti-p65/RelA antibody was preincubated with 5 volumes of the respective blocking peptide solution for 6 h at room temperature. The antibody against IκB-α (13996E) was from Pharmingen. The antibodies against the dually phosphorylated forms of JNK and p38α mitogen-activated protein (MAP) kinase were from New England BioLabs. The antibody against the phosphorylated form of eIF-2α was from Research Genetics. The electrophoretic separation of proteins in SDS-PAGE and electrotransfer to a polyvinylidene difluoride membrane (Millipore) were performed using standard procedures. Immunoprobing with specific antibodies and enhanced chemiluminescence detection (DuPont NEN Research Products) were performed as specified by the respective manufacturers.
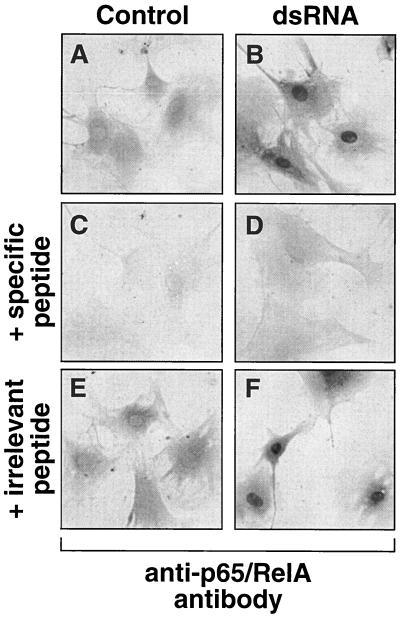
FIG. 3.
dsRNA-induced nuclear translocation of NFκB. pkr+/+ (EX12) MEF were treated with Lipofectin alone (A, C, and E) or with pI-pC (10 μg/ml) in the presence of Lipofectin (B, D, and F). At 3 h after the treatment, the cells were fixed and immunostained with an antibody recognizing the p65/RelA subunit of NFκB, as described in Materials and Methods. An irrelevant peptide (representing an epitope corresponding to the C-terminal domain of MEKK1) or a specific blocking peptide were used (as described in Materials and Methods) in panels E and F and in panels C and D, respectively.
Immunocytochemical staining of p65/RelA.
Cells were grown on Thermanox coverslips (Nunc). After the appropriate treatments, the cells were fixed in cold (−20°C) methanol for 5 min, dried, and stored at −20°C. Blocking was performed with 1.5% normal goat serum in PBS for 1 h followed by incubation with the primary antibody (anti-p65/RelA [C-20 from Santa Cruz] at a 1:800 dilution in PBS with 1.5% serum) for 1 h. After the cells were washed in PBS, incubation with secondary antibody was performed with biotinylated anti-rabbit immunoglobulin G (1:500 dilution in PBS with 1.5% serum) for 1 h, followed again by washing. Endogenous peroxidase activity was quenched with 2% hydrogen peroxide in PBS for 30 min. After being washed, the cells were incubated with VectaStain Elite ABC reagent (Vector Laboratories) for 1 h. Finally, the cells were washed, incubated with diaminobenzidine (Sigma) until the desired stain intensity developed, and rinsed with water. Photographs were taken using a CoolSnap digital camera mounted on a Zeiss microscope.
EMSA.
Nuclear extracts were prepared and used in electrophoretic mobility shift assays as described in references 4 and 56. Briefly, cells were collected by scraping in ice-cold PBS, sedimented, and resuspended in 100 μl of lysis buffer (10 mM HEPES [pH 7.9], 1 mM EDTA, 60 mM KCl, 0.5% Nonidet P-40 [NP-40], 1 mM dithiothreitol [DTT], protease inhibitor cocktail [Roche Molecular Biochemicals]). After 5 min on ice, nuclei were sedimented at 1,200 × g for 5 min. The supernatant was used as the cytoplasmic extract. The nuclei were washed with lysis buffer without NP-40 and suspended in 100 μl of nuclear buffer (250 mM Tris-HCl [pH 7.8], 60 mM KCl, 1 mM DTT, protease inhibitor cocktail). Nuclei were lysed by three cycles of freezing and thawing in liquid nitrogen and ice. The nuclear extracts were cleared by centrifugation at 13,000 × g for 15 min. EMSAs were done as described in reference 4: the binding reaction was performed in a volume of 20 μl with 5 μg of nuclear protein in a buffer containing 12 mM HEPES (pH 7.8), 62.5 mM Tris-HCl (pH 7.8), 60 mM KCl, 0.6 mM EDTA, 12% glycerol, 5 mM DTT, 2 μg of bovine serum albumin, and 1 μg of poly(dI-dC). 32P-radiolabeled consensus double-stranded NF-κB-binding oligonucleotide (5′-AGT TGA GGG GAC TTT CCC AGG C-3′) or the corresponding oligonucleotide with a single-base point mutation (5′-AGT TGA GGC GAC TTT CCC AGG C-3′) from Santa Cruz Biotechnologies were used as probes. For the competition experiments, a consensus double-stranded p53-binding oligonucleotide (5′-TAC AGA ACA TGT CTA AGC ATG CTG GGG-3′) from Santa Cruz Biotechnologies was used as a nonspecific competitor.
RNA isolation and Northern blot detection of mRNA.
Total cellular-RNA was isolated using TRIzol reagent (GIBCO BRL) as specified by the manufacturer. The multiprobe detection of beta interferon, IL-6, and TNF-α was performed using a RiboQuant RNase protection assay kit with a mCK-3b multiprobe template (Pharmingen) as specified by the manufacturer. A 10-μg portion of total RNA was used.
Determination of IL-6 production.
The production of IL-6 was determined quantitatively using the Quantikine M mouse IL-6 enzyme-linked immunosorbent assay (R&D Systems) as specified by the manufacturer and as described previously (26).
RESULTS
EMCV infection causes the proteolytic degradation of IκB-β in both pkr+/+ and pkr0/0 MEF.
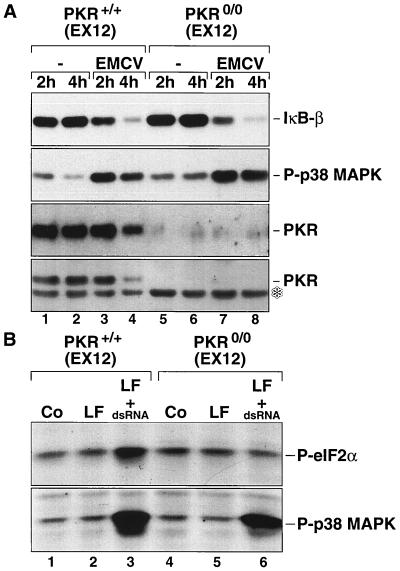
To investigate the possible role of PKR in virus-induced activation of NF-κB, we employed primary fibroblasts derived from wild-type (pkr+/+) or pkr0/0 mouse embryos established in the laboratory of one of us (1). Since the inactivation of PKR alleles in these mice was achieved through a homologous recombination event involving exon 12 of the PKR gene, these MEF are referred to hereafter as pkr+/+(EX12) and pkr0/0(EX12), respectively. Later, when MEF from a different PKR knockout (inactivating the PKR gene exons 2 and 3) (76) are used (see below), these cell will be referred to as pkr+/+(EX2+3) and pkr0/0(EX2+3), respectively. At 2 h after infection with EMCV, there was a detectable decrease in the steady-state levels of IκB-β both in the pkr+/+(EX12) and in the pkr0/0(EX12) MEF (Fig. 1A, lanes 3 and 7). Four hours after the infection, IκB-β levels in both the pkr+/+(EX12) and the pkr0/0(EX12) MEF were reduced to a minor fraction of those in the control cells (compare lanes 2 and 6 with lanes 4 and 8). The absence of PKR in the pkr0/0(EX12) MEF was confirmed using two independent PKR antisera (compare lanes 1 to 4 with lanes 5 to 8). At 4 h after the infection with EMCV, the levels of PKR in the wild-type MEF were reduced (lane 4), probably reflecting the overall inhibition of protein synthesis and the subsequent turnover of PKR protein in these cells.
FIG. 1.
(A) EMCV-induced degradation of IκB-β. pkr+/+(EX12) and pkr0/0(EX12) MEF (∼2 × 106 cells) were infected, where indicated, with EMCV (100 PFU per cell) in 2 ml of serum-free medium for 1 h, after which time the excess virus was removed by extensive washing of the cells with serum-free medium. The cells were further incubated in serum-free medium. At 2 or 4 h after the removal of the extracellular virus, the mock-infected or infected cells were harvested and the cell lysates were processed for the immunoblot detection of IκB-β (top panel) as described in Materials and Methods. The membranes were stripped and reprobed consecutively with an anti-phosphorylated p38 MAP kinase antibody (second panel from top) and with the M-515 (third panel from top) and D-20 (bottom panel) PKR antisera. A nonspecific band recognized by the D-20 antibody is indicated by an asterisk. (B) Lack of PKR activity in the pkr0/0(EX12) MEF. pkr+/+(EX12) and pkr0/0(EX12) MEF were left untreated (Co) or were treated with Lipofectin (LF) alone or with pI-pC (10 μg/ml) in the presence of Lipofectin (LF + dsRNA). At 3 h after the treatments, the phosphorylation states of eIF-2α and p38α MAP kinase were assessed in immunoblot analyses.
dsRNA triggers the phosphorylation, polyubiquitinylation, and proteosome-mediated degradation of IκBs in both pkr+/+(EX12) and pkr0/0(EX12) MEF.
The degradation of IκB-β in EMCV-infected MEF was paralleled by the phosphorylation of the stress-activated protein kinases (SAPK) p38α MAP kinase (Fig. 1A, lanes 3, 4, 7, and 8), JNK1, and JNK2 (not shown). Previously, we reported that SAPK are potently activated by dsRNA (26). We set out, therefore, to investigate the specific role of dsRNA in virus-induced activation of NF-κB, independent of viral proteins that, in many cases, also modulate NF-κB activity. To achieve this goal, we used pI-pC, a synthetic dsRNA, which was delivered into cells via lipofection (see Materials and Methods). First, we set out to confirm that the deletion of exon 12 of PKR in the pkr0/0(EX12) MEF resulted in a complete abrogation of PKR activity. Treatment of pkr+/+(EX12) MEF with pI-pC (hereafter referred to as dsRNA) caused the phosphorylation of eIF-2α at serine-51 (Fig. 1B, lane 3). Identically treated pkr0/0(EX12) MEF failed to display the phosphorylation of eIF-2α at serine-51 (lane 6). In contrast to these results and in agreement with our previous findings (26), the p38α MAP kinase was phosphorylated in response to dsRNA both in the PKR-containing and in the PKR-deficient cells (lanes 3 and 6). We concluded, therefore, that the deletion of exon 12 of PKR in the pkr0/0(EX12) MEF has resulted indeed in a complete abrogation of PKR activity.
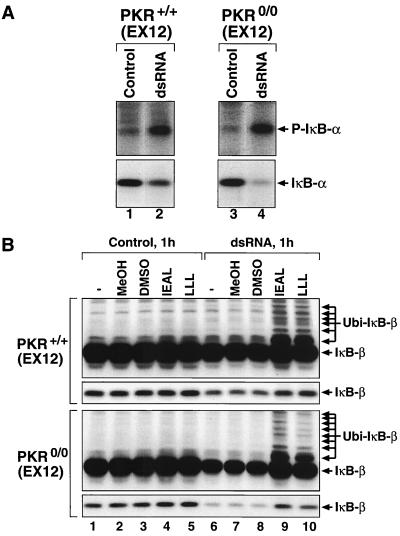
We next addressed the dsRNA-induced signaling to NF-κB. Treatment of either pkr+/+(EX12) or pkr0/0(EX12) MEF with pI-pC for 1 h resulted in a detectable increase in serine-32 phosphorylation of IκB-α as measured by immunoblot analysis using an antibody recognizing specifically only the serine-32-phosphorylated form of IκB-α (Fig. 2A, upper panels, lanes 2 and 4). Consistent with the role of IκB-α phosphorylation in its degradation by the ubiquitin-proteosome system (for a review, see reference 31), the levels of IκB-α were significantly reduced in dsRNA-treated cells (lower panels, lanes 2 and 4). To investigate whether IκB-β is also degraded by the ubiquitin-proteosome system in response to dsRNA, we employed two peptide proteosome inhibitors, benzyloxycarbonylleucyl-leucyl-leucine aldehyde (labeled LLL in Fig. 2B) and benzyloxycarbonyliso-leucyl-glutamyl(OtBu)-alanyl-leucine aldehyde (labeled IEAL in Fig. 2B). Treatment of either pkr+/+(EX12) or pkr0/0(EX12) MEF with dsRNA for 1 h led to a detectable reduction in the steady-state levels of IκB-β in both the pkr+/+ (EX12) and the pkr0/0(EX12) MEF (Fig. 2B, narrow panels, lane 6). Pretreatment of the cells with either of the two proteosome inhibitors prevented the dsRNA-induced degradation of IκB-β (narrow panels, lanes 9 and 10). Furthermore, in the presence of both dsRNA and the proteasome inhibitors, the cells accumulated multiple anti-IκB-β-immunoreactive bands with reduced mobility in SDS-PAGE (wide panels, lanes 9 and 10; note that the wide panels represent a longer film exposure of the same immunoblots presented in the narrow panels). The appearance of these novel forms of anti-IκB-β immunoreactivity with reduced mobility is consistent with unimpaired levels of dsRNA-induced polyubiquitinylation of IκB-β but a blocked polyubiquitinylation-induced degradation of IκB-β by the proteasome. Importantly, identical effects on the IκBs of these proteasome inhibitors were observed in cells stimulated with TNF-α, a well-established inducer of proteasome-mediated degradation of IκBs (reference 67 and data not shown). None of the dsRNA-induced effects on IκBs (phosphorylation, polyubiquitinylation, and proteasome-dependent degradation) appeared to require the presence of PKR. Neither single-stranded RNA (pI or pC), nor dsDNA (p[d(IC)]) had any effect on IκB-β or IκB-α (data not shown), indicating that the effects observed using pI-pC represent a bona fide dsRNA response. Despite the limited ability to compare the behavior of IκB-β and IκB-α in the same assay, the results shown in Fig. 2 favor the conclusion that both IκBs are addressed by dsRNA-induced signal transduction pathways in a similar manner.
FIG. 2.
dsRNA-triggered phosphorylation, polyubiquitinylation, and proteosome-mediated degradation of IκBs in both pkr+/+(EX12) and pkr0/0(EX12) MEF. (A) pkr+/+(EX12) and pkr0/0(EX12) MEF were treated with Lipofectin alone (lanes Control) or with pI-pC (10 μg/ml) in the presence of Lipofectin (lanes dsRNA). Note that the same procedure for Lipofectin treatment (with or without pI-pC) applies to all experiment presented in Fig. 2 to 9 and is described in Materials and Methods. At 1 h after the treatment, the cells were harvested and processed for immunoblot analysis of IκBα using either a phospho-(Ser32)-IκBα-specific antibody (upper panels) or an IκBα-specific antibody (lower panels). Treatment of cells with Lipofectin does not affect IκBβ or any other NFκB-related signaling pathway investigated in this work (data not shown). (B) pkr+/+(EX12) and pkr0/0(EX12) MEF were treated as in panel A, except that, where indicated, the cells were pretreated for 25 min with benzyloxycarbonyliso-leucyl-glutamyl(OtBu)-alanyl-leucine aldehyde (IEAL), benzyloxycarbonyl-leucyl-leucyl-leucine aldehyde (LLL), or the respective solvents for each of them, methanol (MeOH) or dimethyl sulfoxide (DMSO). IκBβ steady-state levels were monitored in an immunoblot analysis.
dsRNA-induced IκB degradation coincides with translocation of NF-κB to the nucleus, independent of the presence or the absence of PKR.
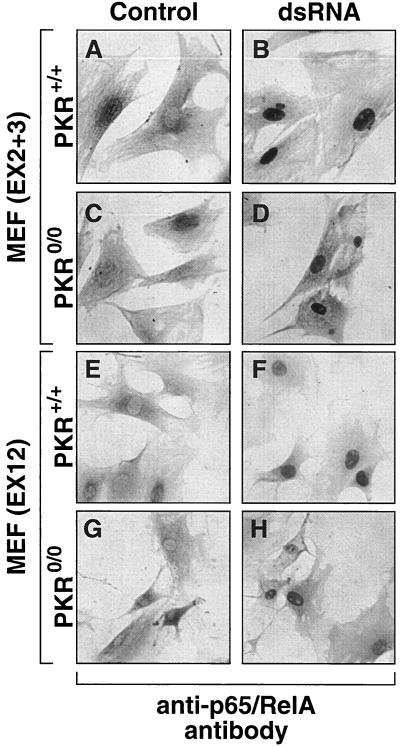
To investigate and determine conclusively if PKR may be required for a functional activation of NF-κB downstream of IκB phosphorylation and degradation, we employed pkr+/+(EX12) and pkr0/0(EX12) MEF and pkr+/+ (EX2+3) and pkr0/0(EX2+3) MEF (76). First, we demonstrated that in the pkr+/+(EX2+3) and pkr0/0(EX2+3) MEF, the Lipofectin-mediated delivery of dsRNA led to the degradation of IκB-α and IκB-β similarly to the effect of dsRNA on the pkr+/+(EX12) and pkr0/0(EX12) MEF [data not shown, but see also Fig. 8, demonstrating the dsRNA-induced IκB-β degradation in embryonic fibroblasts derived from pkr0/0(EX2+3) × rnasel−/− mice (84)]. We then performed immunocytochemical staining of control (Lipofectin-treated) and dsRNA-treated MEF (EX12 and EX2+3) by using an antibody recognizing the p65/RelA subunit of NF-κB. As shown in Fig. 3A, p65/RelA displayed a typical cytoplasmic distribution in the control pkr+/+(EX2+3) MEF. At 3 h after dsRNA treatment, a strong immunopositive signal appeared in the nucleus (Fig. 3B), indicative of induced nuclear translocation of NF-κB. Preincubation of the antibody with a p65/RelA peptide epitope abolished the immunocytochemical staining (Fig. 3C and D), whereas the preincubation with an irrelevant peptide epitope had no effect on the ability of the antibody to stain cells (Fig. 3E and 3F). An identical pattern of nuclear staining was detected after treatment of cells with TNF-α (data not shown). Thus, the method used in the experiment in Fig. 3 appeared to represent faithfully the translocation of p65/RelA following a signal that triggers IκB degradation. Using this method, we next demonstrated that, both in the pkr0/0(EX2+3) MEF and in the pkr0/0(EX12) MEF, dsRNA led to a nuclear translocation of p65/RelA in a manner identical to the ability of dsRNA to cause p65/RelA nuclear translocation in the respective wild-type cells (Fig. 4). Thus, the evidence obtained using MEF from two independent approaches to generate PKR-null mice demonstrates that PKR is not an essential kinase for the migration of NF-κB to the nucleus following dsRNA-induced IκB degradation.
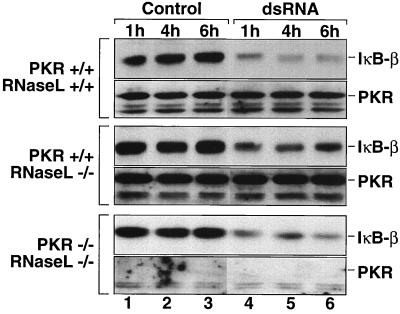
FIG. 8.
dsRNA-induced degradation of IκB-β in cells deficient in RNase L or both RNase L and PKR. 3T3-like fibroblast cell lines with pkr+/+/rnasel+/+, pkr+/+/rnasel−/−, and pkr−/−/rnasel−/− genotypes were treated with Lipofectin alone (lanes Control) or with pI-pC (10 μg/ml) in the presence of Lipofectin (lanes dsRNA). At the indicated times after treatment, the cells were harvested and the steady-state levels of IκB-β were assessed as in the experiment in Fig. 1A. The membranes were stripped and reprobed with an anti-PKR antibody (D-20).
FIG. 4.
dsRNA-induced nuclear translocation of NFκB independent of the presence or the absence of PKR. pkr+/+(EX2+3), pkr0/0(EX2+3), pkr+/+(EX12), or pkr0/0(EX12) MEF were treated with Lipofectin alone (A, C, E, and G) or with pI-pC (10 μg/ml) in the presence of Lipofectin (B, D, F, and H). At 3 h after the treatment, the cells were fixed and immunostained with an antibody recognizing the p65/RelA subunit of NFκB as in Fig. 3.
PKR deficiency does not affect the specific DNA-binding activity of NF-κB.
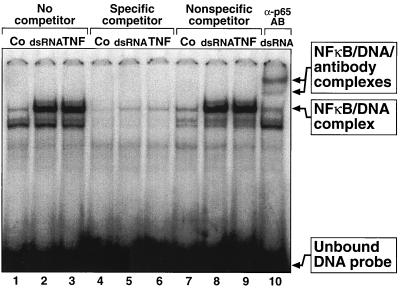
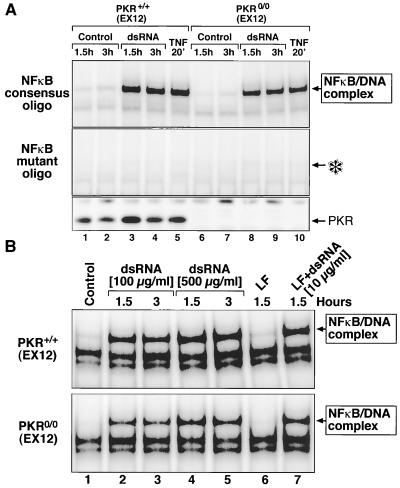
Since a fraction of PKR has been found in the nucleus (29), it was not unreasonable to investigate whether PKR might be involved in modulating NF-κB activity at the level of the DNA-binding ability of this transcription factor. Nuclear extracts from wild-type MEF (EX12) treated with either dsRNA or TNF-α displayed a prominent DNA-binding activity in EMSA using an NF-κB-specific oligonucleotide probe (Fig. 5, lanes 1 to 3). This DNA-binding activity was successfully competed by a 100-fold molar excess of the same unlabeled probe (lanes 4 to 6) but was not competed by a 100-fold molar excess of an irrelevant oligonucleotide (lanes 7 to 9). Furthermore, the DNA-binding activity was supershifted when the nuclear extracts were preincubated with the anti-p65/RelA antibody (lane 10), thus leading to the positive identification of NF-κB in the retarded protein-oligonucleotide complex. Therefore, the EMSA appeared to be a suitable assay to study the DNA-binding activity of NF-κB. As shown in Fig. 6A, treatment of either pkr+/+(EX12) or pkr0/0(EX12) MEF with dsRNA led to the appearance in the nuclear extracts of NF-κB with similar DNA-binding activity that did not require the presence of PKR (Fig. 6A, compare lanes 3 and 4 with lanes 8 and 9). Furthermore, cells treated with TNF-α displayed a similar DNA-binding activity that was independent of PKR (lanes 5 and 10). With either dsRNA or TNF-α treatment, the induced DNA-binding activity failed to form on an oligonucleotide probe with a single nucleotide substitution (Fig. 6A, second panel from the bottom), further demonstrating that NF-κB is the major (and possibly the only) DNA-binding activity in the complex. The corresponding cytosolic extracts demonstrated the presence of PKR only in the pkr+/+(EX12) but not in the pkr0/0(EX12) MEF (bottom panel).
FIG. 5.
dsRNA- and TNF-α-induced specific DNA-binding activity of NFκB. pkr+/+(EX12) MEF were treated with Lipofectin alone (lanes Co), with pI-pC (10 μg/ml) in the presence of Lipofectin (lanes dsRNA), or with TNF-α (lanes TNF). At 3 h after either Lipofectin or dsRNA treatments or 20 min after the TNF-α treatment, the cells were harvested and nuclear extracts were prepared as described in Materials and Methods. EMSAs were performed as described in Materials and Methods. Where indicated, a 100-fold molar excess of either the specific NF-κB-binding nonlabeled oligonucleotide (specific competitor) or a p53-binding nonlabeled oligonucleotide (nonspecific competitor) was added to the binding-reaction mixtures for 10 min before the addition of the specific 32P-labeled NF-κB-binding oligonucleotide. In the last lane, the undiluted anti-p65/RelA (C-20 from Santa Cruz) antibody was added in 1/10 of the final reaction volume for 10 min before the addition of the specific 32P-labeled NF-κB-binding oligonucleotide. Addition of several irrelevant antibodies did not interfere with the specific binding of NF-κB to DNA, demonstrating the specificity of the anti-p65/RelA antibody-induced supershift (data not shown).
FIG. 6.
dsRNA-induced specific DNA-binding activity of NFκB independent of the presence or the absence of PKR. (A) pkr+/+(EX12) or pkr0/0(EX12) MEF were treated with Lipofectin alone (lanes Control), with pI-pC (10 μg/ml) in the presence of Lipofectin (lanes dsRNA), or with TNF-α (lanes TNF). At the indicated times after the treatment, the cells were harvested and EMSAs were performed as in the experiment in Fig. 5. A specific 32P-labeled NF-κB-binding oligonucleotide (top panel) or 32P-labeled oligonucleotide bearing a single-base substitution (Santa Cruz) (middle panel) was used. The asterisk depicts the position in the middle panel that corresponds to the position of the NF-κB-DNA complex in the upper panel. The corresponding cytosolic extracts (see Materials and Methods) were used in an immunoblot procedure to demonstrate the absence of PKR in the pkr0/0(EX12) MEF (bottom panel). (B) pkr+/+(EX12) or pkr0/0(EX12) MEF were left untreated (lanes Control) or were treated with pI-pC (100 or 500 μg/ml) in the absence of Lipofectin (lanes dsRNA), with Lipofectin alone (lane LF), or with pI-pC (10 μg/ml) in the presence of Lipofectin (lane LF+dsRNA). At the indicated times after the treatment, the cells were harvested and EMSAs were performed as in the experiment in Fig. 5.
The results presented in Fig. 2 to 6A contrast with the findings of others (11, 76, 79), who reported that fibroblasts deficient in PKR failed to respond to dsRNA with NF-κB activation. To investigate whether the different modes of dsRNA delivery used by us and by others are responsible for these differences, we compared the responses of MEF either to 10 μg of pI-pC per ml in the presence of Lipofectin or to 100 or 500 μg of pI-pC per ml without Lipofectin (as used by Zamanian-Daryoush et al. 79). Using the EMSA, we observed that both in the presence and in the absence of Lipofectin, dsRNA was able to induce the NF-κB DNA-binding activity in the PKR-containing and in the PKR-deficient cells (Fig. 6B).
The dsRNA-induced accumulation of the mRNA for beta interferon occurs in the absence of PKR.
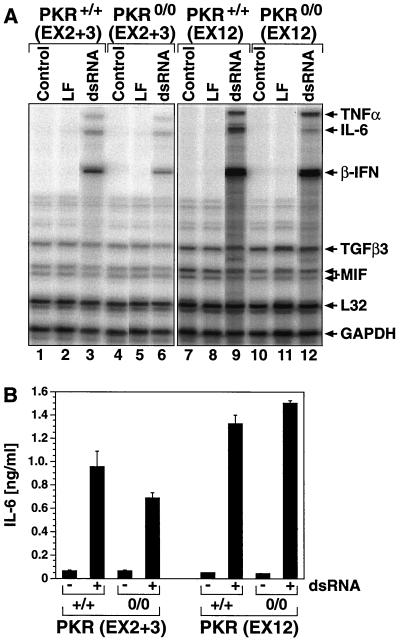
Previously, Yang et al. (76) and Chu et al. (11) found that dsRNA (applied without the aid of a lipophilic internalization vehicle) was severely impaired in its ability to induce beta interferon expression in the pkr0/0(EX2+3) MEF (76) or in a pkr0/0(EX2+3) 3T3-like fibroblast cell line (11). We investigated whether PKR might be required for the dsRNA-induced expression of beta interferon when dsRNA was delivered with the aid of Lipofectin. Treatment with dsRNA for 4 h led to the accumulation of the mRNA for beta interferon (as measured in an RNase protection assay) in the pkr+/+(EX2+3), pkr0/0(EX2+3), pkr+/+(EX12), and pkr0/0(EX12) MEF (Fig. 7A, lanes 3, 6, 9, and 12). Determination of the actual fold activation was impossible due to the undetectable levels of beta interferon mRNA expression in the control cells (lanes 1, 4, 7, and 10). Similar to beta interferon, the mRNAs for two inflammatory cytokines, IL-6 and TNF-α, were also induced by dsRNA in the pkr+/+(EX2+3), pkr0/0(EX2+3), pkr+/+(EX12), and pkr0/0(EX12) MEF (lanes 3, 6, 9, and 12). In contrast, the mRNAs encoding the cytokines transforming growth factor β3 (TGF-β3) and migration inhibitory factor (MIF) were expressed constitutively in the MEF and were not induced by dsRNA, demonstrating the specificity of the dsRNA response (lanes 3, 6, 9, and 12). None of these mRNAs was induced by Lipofectin alone (lanes 2, 5, 8, and 11). We concluded, therefore, that PKR was not required for the dsRNA-induced expression of beta interferon mRNA or of IL-6 and TNF-α mRNAs. PKR was also not required for the dsRNA-induced accumulation of the mRNA for the NF-κB-dependent antiapoptotic gene iap-2 (data not shown).
FIG. 7.
Expression of dsRNA-induced genes independent of the presence or absence of PKR. (A) MEF with the indicated genotype were left untreated (lanes Control) or were treated with Lipofectin alone (lanes LF) or with pI-pC (10 μg/ml) in the presence of Lipofectin (lanes dsRNA). At 4 h later, the cells were harvested, total RNA was prepared, and the steady-state levels of expression of multiple cytokines were assessed in a multiprobe RNase protection assay as described in Materials and Methods. The levels of the mRNAs for the ribosomal protein L32 and the housekeeping gene glyceraldehyde-3-phosphate dehydrogenase (GAPDH) were used as controls for RNA amount and loading. TGFβ3, transforming growth factor β3; MIF, migration inhibitory factor. (B) MEF with the indicated genotype were treated with Lipofectin alone (−) or with pI-pC (10 μg/ml; dsRNA) in the presence of Lipofectin (+). At 24 h later, the presence of IL-6 in the conditioned culture medium was determined as described in Materials and Methods. Error bars represent standard deviation from experimental points in triplicate.
PKR contributes, in mouse fibroblasts, about 50% of the overall inhibition of translation in response to dsRNA (the other 50% being contributed by the 2-5 oligoadenylate synthase/RNase L system) (26). It was therefore reasonable to speculate that activated PKR may negatively affect gene expression via its inhibitory action on protein synthesis. To address this question, we employed a highly sensitive enzyme-linked immunosorbent assay for the detection of IL-6 and determined whether the presence or absence of PKR affects the expression and/or secretion of this cytokine. As shown in Fig. 7B, the levels of IL-6 in the culture medium were markedly elevated 24 h after the treatment with dsRNA in the pkr+/+(EX2+3), pkr0/0(EX2+3), pkr+/+(EX12), and pkr0/0(EX12) MEF. We were unable, therefore, to discern a clear pattern of PKR involvement in the production of IL-6 in response to dsRNA.
The dsRNA-induced activation of NF-κB does not require RNase L.
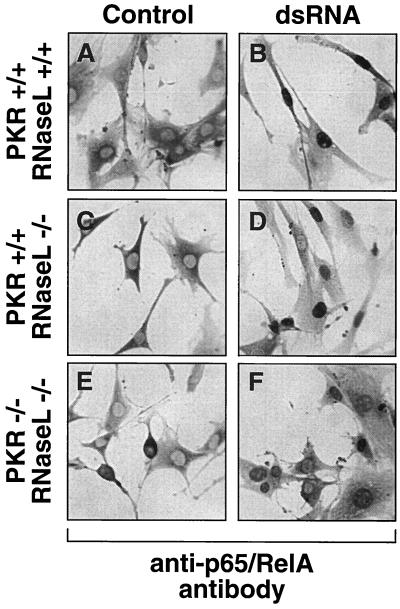
The OAS/2-5A/RNase L system is a dsRNA-activated signal transduction cascade that parallels the PKR/eIF-2α cascade (54). In mouse fibroblasts, PKR and RNase L are solely required and sufficient for the dsRNA-induced inhibition of translation, since cells with a combined deficiency in both genes fail to inhibit translation when challenged with dsRNA (26). We considered the possibility that RNase L might be involved in mediating the dsRNA-induced signaling to NF-κB. To test this hypothesis, we employed 3T3-like fibroblast cell lines with pkr+/+/rnasel+/+, pkr+/+/rnasel−/−, and pkr−/−/rnasel−/− genotypes (84). Both the cells with a single RNase L deficiency and the cells with a combined PKR and RNase L deficiency displayed dsRNA-induced degradation of IκB-β in a manner similar to the wild-type cells (Fig. 8, compare lanes 1 to 3 with lanes 4 to 6). Furthermore, in each cell line, NF-κB translocated to the nucleus in response to dsRNA treatment (Fig. 9). We concluded, therefore, that neither PKR nor RNase L is a critical component in mediating the dsRNA-induced signaling to NF-κB.
FIG. 9.
dsRNA-induced nuclear translocation of NFκB in cells deficient in RNase L or both RNase L and PKR. 3T3-like fibroblast cell lines with pkr+/+/rnasel+/+, pkr+/+/rnasel−/−, and pkr−/−/rnasel−/− genotypes were treated with Lipofectin alone (Control) or with pI-pC (10 μg/ml) in the presence of Lipofectin (dsRNA). At 3 h after treatment, the cells were fixed and immunostained with an antibody recognizing the p65/RelA subunit of NFκB as in the experiment in Fig. 3.
DISCUSSION
The most important result of this study is the demonstration of a novel response of cells to viral dsRNA that is independent of the dsRNA-activated PKR. Surprisingly, we found that this response includes the dsRNA-induced activation of NF-κB (Fig. 2 to 6, 8, and 9) and the production of beta (“fibroblast”) interferon (Fig. 7A), two critical components of innate immunity that were previously described by others to depend, in fibroblasts, on the presence of PKR (11, 76, 79). We argue that the activation of NF-κB by dsRNA does not require PKR because the following critical steps of activation of this transcription factor by dsRNA were found to be unimpaired in cells lacking PKR: (i) the phosphorylation, polyubiquitinylation, and degradation of IκB (Fig. 2); (ii) the nuclear translocation of NF-κB (Fig. 4); (iii) the specific DNA-binding activity of NF-κB (Fig. 6); and (iv) the induction of NF-κB-dependent genes, such as the genes for beta interferon, IL-6, TNF-α, and IAP-2 (Fig. 7 and data not shown) (for an extended list of established NF-κB-dependent genes, see reference 42 and references therein).
Evidence for the existence of previously unsuspected novel cellular sensors for dsRNA and their implication in the anti-viral response and postviral immunopathic diseases.
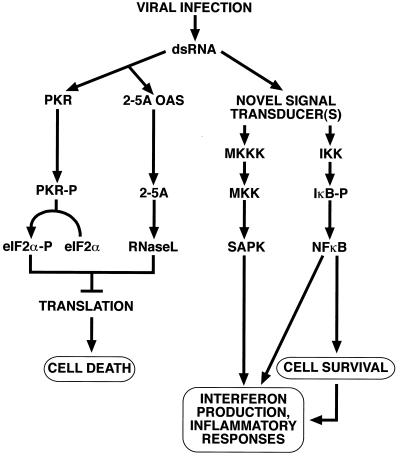
Figure 10 summarizes the model we propose for the ability of viral dsRNA to trigger both pro- and antiapoptotic cellular programs. The apoptotic program requires the activities of PKR and RNase L (see below). Based on our findings that the antiapoptotic program of innate antiviral immunity (which proceeds through the activation of NF-κB) is independent of the presence of PKR and RNase L, we postulate the existence of novel, yet to be identified sensors for dsRNA that are different from PKR and, probably, OAS. It is possible that the same novel dsRNA-sensing machinery also mediates the virus-induced activation of the SAPK (i.e., JNK and p38 MAP kinase), since the dsRNA-induced activation of SAPK can also proceed in the absence of PKR and RNase L (26). The activation of NF-κB is thought to mediate cell survival in response to viral infection (48). The combined activation of SAPK and NF-κB, in turn, is probably involved in the production of alpha, beta, and omega interferons and other alarmones, including inflammatory mediators such as IL-6 and TNF-α. Concerning the role of inflammatory cytokines induced by dsRNA, we hypothesize that the PKR-independent, dsRNA-induced activation of the SAPK- and NF-κB-dependent signal transduction pathways (which lead to the expression of inflammatory mediators [reference 26 and this study]) may be an important contributor not only to the acute inflammation but also to the chronic inflammation caused by persistent viral infections. Enteroviruses (such as coxsackievirus, poliovirus, echovirus, EMCV, and other members of the picornavirus family; for a review, see reference 47) are known to cause debilitating and long-lasting postviral immunopathic muscle diseases. For instance, the coxsackievirus-induced mouse model of inflammatory myopathy is associated with the presence in the affected muscle of persistent dsRNA viral sequences (60). Wessely et al. (71) have used transgenic expression of both the plus and minus strands of a replication-restricted coxsackievirus genome in the mouse heart to provide experimental evidence that coxsackievirus dsRNA can cause dilated cardiomyopathy in the absence of infectious viral progeny. Heart failure due to dilated cardiomyopathy accounts for ∼45% of the cardiac transplantations performed in the United States (23). An investigation of the involvement of dsRNA-induced SAPK- and NF-κB-dependent signal transduction pathways in virus-induced inflammatory myopathies may therefore have important therapeutic consequences.
FIG. 10.
Model for the diverse actions of viral dsRNA at the cellular level. See explanations in the text.
Is there an irreconcilable difference between our finding that PKR is not required for the dsRNA-induced signaling to NF-κB and interferon production and the results of others who have reached the opposite conclusion?
In our opinion, there is not an irreconcilable difference between our results and those of other workers (11, 76, 79). The best evidence for this is the indication (already contained in the work of Yang et al. 76) that PKR is not involved in mediating the innate immunity to viruses at the level of the organism. In that study, the authors found that injection of dsRNA peritoneally into either PKR-containing or PKR-deficient mice induced similar levels of beta interferon mRNA expression in the spleens of the mice of either genotype (76). However, we are currently unable to offer an explanation for the differences between our results and the results of Williams and coworkers and Karin and coworkers (11, 76, 79) in experiments performed in MEF in vitro.
Still, could PKR, under specific circumstances, play a role in the activation of NF-κB by viral dsRNA? Several recent reports indicate that this is possible. It has been discovered that when overexpressed in cells, PKR has the potential to activate IKK2/β (6, 11, 19). Interestingly, a kinase-deficient mutant of PKR retained the ability to activate IKK2/β upon overexpression, suggesting that in this case, PKR exerted a kinase-independent action whose nature is unknown (6, 11). It remains to be elucidated whether this phenomenon has biological relevance.
What is the major role of PKR (and of the OAS/RNase L system) in response to virus infections? Obviously, the most relevant answers to this question should come from the PKR and RNase L genetic knockouts. The most profound defects we observed in fibroblasts that are deficient in PKR, RNase L, or both PKR and RNase L were the reduced ability of dsRNA to inhibit translation (26) and to trigger apoptosis (our unpublished observations). These results alone establish both PKR and OAS/RNase L system as important mediators of the proapoptotic cellular program in response to virus infections. Additional confirmation of the proapoptotic role of PKR and RNase L comes from the attempts of several laboratories to establish cell lines overexpressing either of these two enzymes. Overexpression of either PKR or RNase L potentiates apoptosis in response to dsRNA (2, 3, 7, 9, 78). Interestingly, PKR-overexpressing cells are also more sensitive to the cytotoxic effects of TNF-α (78), an effect probably resulting from an increased sensitivity to apoptotic stimuli in the face of compromised sustainability of the process of protein synthesis. Is the PKR-mediated inhibition of protein synthesis per se a main cause of PKR-induced apoptosis? Some experimental evidence suggests that this may be the case. For instance, apoptosis induced by an overexpression of PKR could be counteracted by the concomitant overexpression of a nonphosphorylatable form of eIF-2α (20, 53). Furthermore, expression of a mutant form of eIF-2α that mimics phosphorylated eIF-2α was found to induce apoptosis by itself (53).
Finally, although the evidence provided in this study does not support a role for PKR in mediating the expression of NF-κB-dependent genes, our conclusions should not be interpreted as an attempt to rule out the possible participation of PKR in signal transduction to the nucleus. Further studies, especially those using powerful DNA array technologies, are likely to provide a conclusive answer in the near future. Furthermore, we have recently reported that the PKR- and RNase L-mediated inhibition of protein synthesis (but not PKR and RNase L per se) plays a critical role in the ability of dsRNA to trigger the activation of JNK (26). This establishes the interesting possibility that dsRNA-activated JNK may play a role in mediating dsRNA-induced apoptosis. This possibility is currently being investigated.
ACKNOWLEDGMENTS
We thank Olga Ryabinina, Thanh-Hoai Dinh, and Paul Spitz for excellent technical assistance. We thank Bryan Williams for the pkr+/+(EX2+3) and pkr0/0(EX2+3) MEF and Robert Silverman for the rnasel+/+|pkr+/+, rnasel−/−|pkr+/+, and rnasel−/−|pkr−/− 3T3-like fibroblasts and for the EMCV.
This work was supported by U.S. Public Health Service grants CA-39360 and ES-08456 to B.E.M. and by an N. L. Tartar Research Fund Fellowship to M.S.I. J.C.B. is supported by the National Cancer Institute of Canada and the Canadian Cancer Society.
REFERENCES
- 1.Abraham N, Stojdl D F, Duncan P I, Methot N, Ishii T, Dube M, Vanderhyden B C, Atkins H L, Gray D A, McBurney M W, Koromilas A E, Brown E G, Sonenberg N, Bell J C. Characterization of transgenic mice with targeted disruption of the catalytic domain of the double-stranded RNA-dependent protein kinase, PKR. J Biol Chem. 1999;274:5953–5962. doi: 10.1074/jbc.274.9.5953. [DOI] [PubMed] [Google Scholar]
- 2.Balachandran S, Kim C N, Yeh W C, Mak T W, Bhalla K, Barber G N. Activation of the dsRNA-dependent protein kinase, PKR, induces apoptosis through FADD-mediated death signaling. EMBO J. 1998;17:6888–6902. doi: 10.1093/emboj/17.23.6888. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Balachandran S, Roberts P C, Kipperman T, Bhalla K N, Compans R W, Archer D R, Barber G N. Alpha/beta interferons potentiate virus-induced apoptosis through activation of the FADD/caspase-8 death signaling pathway. J Virol. 2000;74:1513–1523. doi: 10.1128/jvi.74.3.1513-1523.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Bender K, Gottlicher M, Whiteside S, Rahmsdorf H J, Herrlich P. Sequential DNA damage-independent and -dependent activation of NF-kappaB by UV. EMBO J. 1998;17:5170–5181. doi: 10.1093/emboj/17.17.5170. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Birnbaum M J, Clem R J, Miller L K. An apoptosis-inhibiting gene from a nuclear polyhedrosis virus encoding a polypeptide with Cys/His sequence motifs. J Virol. 1994;68:2521–2528. doi: 10.1128/jvi.68.4.2521-2528.1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Bonnet M C, Weil R, Dam E, Hovanessian A G, Meurs E F. PKR stimulates NF-κB irrespective of its kinase function by interacting with the IκB kinase complex. Mol Cell Biol. 2000;20:4532–4542. doi: 10.1128/mcb.20.13.4532-4542.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Castelli J, Wood K A, Youle R J. The 2-5A system in viral infection and apoptosis. Biomed Pharmacother. 1998;52:386–390. doi: 10.1016/s0753-3322(99)80006-7. [DOI] [PubMed] [Google Scholar]
- 8.Castelli J C, Hassel B A, Maran A, Paranjape J, Hewitt J A, Li X L, Hsu Y T, Silverman R H, Youle R J. The role of 2′-5′ oligoadenylate-activated ribonuclease L in apoptosis. Cell Death Differ. 1998;5:313–320. doi: 10.1038/sj.cdd.4400352. [DOI] [PubMed] [Google Scholar]
- 9.Castelli J C, Hassel B A, Wood K A, Li X L, Amemiya K, Dalakas M C, Torrence P F, Youle R J. A study of the interferon antiviral mechanism: apoptosis activation by the 2-5A system. J Exp Med. 1997;186:967–972. doi: 10.1084/jem.186.6.967. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Chen C, Edelstein L C, Gelinas C. The Rel/NF-κB family directly activates expression of the apoptosis inhibitor Bcl-x(L) Mol Cell Biol. 2000;20:2687–2695. doi: 10.1128/mcb.20.8.2687-2695.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Chu W M, Ostertag D, Li Z W, Chang L, Chen Y, Hu Y, Williams B, Perrault J, Karin M. JNK2 and IKKbeta are required for activating the innate response to viral infection. Immunity. 1999;11:721–731. doi: 10.1016/s1074-7613(00)80146-6. [DOI] [PubMed] [Google Scholar]
- 12.Chu Z L, McKinsey T A, Liu L, Gentry J J, Malim M H, Ballard D W. Suppression of tumor necrosis factor-induced cell death by inhibitor of apoptosis c-IAP2 is under NF-kappaB control. Proc Natl Acad Sci USA. 1997;94:10057–10062. doi: 10.1073/pnas.94.19.10057. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Diaz-Guerra M, Rivas C, Esteban M. Activation of the IFN-inducible enzyme RNase L causes apoptosis of animal cells. Virology. 1997;236:354–363. doi: 10.1006/viro.1997.8719. [DOI] [PubMed] [Google Scholar]
- 14.DiDonato J A, Hayakawa M, Rothwarf D M, Zandi E, Karin M. A cytokine-responsive IkappaB kinase that activates the transcription factor NF-kappaB. Nature. 1997;388:548–554. doi: 10.1038/41493. [DOI] [PubMed] [Google Scholar]
- 15.Donze O, Dostie J, Sonenberg N. Regulatable expression of the interferon-induced double-stranded RNA dependent protein kinase PKR induces apoptosis and fas receptor expression. Virology. 1999;256:322–329. doi: 10.1006/viro.1999.9618. [DOI] [PubMed] [Google Scholar]
- 16.Foster G R, Finter N B. Are all type I human interferons equivalent? J Viral Hepatol. 1998;5:143–152. doi: 10.1046/j.1365-2893.1998.00103.x. [DOI] [PubMed] [Google Scholar]
- 17.Gagliardini V, Fernandez P A, Lee R K, Drexler H C, Rotello R J, Fishman M C, Yuan J. Prevention of vertebrate neuronal death by the crmA gene. Science. 1994;263:826–828. doi: 10.1126/science.8303301. [DOI] [PubMed] [Google Scholar]
- 18.Garoufalis E, Kwan I, Lin R, Mustafa A, Pepin N, Roulston A, Lacoste J, Hiscott J. Viral induction of the human beta interferon promoter: modulation of transcription by NF-kappa B/rel proteins and interferon regulatory factors. J Virol. 1994;68:4707–4715. doi: 10.1128/jvi.68.8.4707-4715.1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Gil J, Alcami J, Esteban M. Activation of NF-kappa B by the dsRNA-dependent protein kinase, PKR involves the I kappa B kinase complex. Oncogene. 2000;19:1369–1378. doi: 10.1038/sj.onc.1203448. [DOI] [PubMed] [Google Scholar]
- 20.Gil J, Alcami J, Esteban M. Induction of apoptosis by double-stranded-RNA-dependent protein kinase (PKR) involves the alpha subunit of eukaryotic translation initiation factor 2 and NF-κB. Mol Cell Biol. 1999;19:4653–4663. doi: 10.1128/mcb.19.7.4653. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Gilmore T D. The Rel/NF-kappaB signal transduction pathway: introduction. Oncogene. 1999;18:6842–6844. doi: 10.1038/sj.onc.1203237. [DOI] [PubMed] [Google Scholar]
- 22.Gross M, Knish W M, Kwan A. Rabbit reticulocyte double-stranded RNA-activated protein kinase and the hemin-controlled translational repressor phosphorylate the same Mr 1500 peptide of eukaryotic initiation factor 2 alpha. FEBS Lett. 1981;125:223–226. doi: 10.1016/0014-5793(81)80724-7. [DOI] [PubMed] [Google Scholar]
- 23.Hosenpud J D, Novick R J, Bennett L E, Keck B M, Fiol B, Daily O P. The Registry of the International Society for Heart and Lung Transplantation: thirteenth official report—1996. J Heart Lung Transplant. 1996;15:655–674. [PubMed] [Google Scholar]
- 24.Hovanessian A G. Interferon-induced and double-stranded RNA-activated enzymes: a specific protein kinase and 2′,5′-oligoadenylate synthetases. J Interferon Res. 1991;11:199–205. doi: 10.1089/jir.1991.11.199. [DOI] [PubMed] [Google Scholar]
- 25.Hu Y, Baud V, Delhase M, Zhang P, Deerinck T, Ellisman M, Johnson R, Karin M. Abnormal morphogenesis but intact IKK activation in mice lacking the IKKalpha subunit of IkappaB kinase. Science. 1999;284:316–320. doi: 10.1126/science.284.5412.316. [DOI] [PubMed] [Google Scholar]
- 26.Iordanov M S, Paranjape J M, Zhou A, Wong J, Williams B R, Meurs E F, Silverman R H, Magun B E. Activation of p38 mitogen-activated protein kinase and c-Jun NH2-terminal kinase by double-stranded RNA and encephalomyocarditis virus: involvement of RNase L, protein kinase R, and alternative pathways. Mol Cell Biol. 2000;20:617–627. doi: 10.1128/mcb.20.2.617-627.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Iordanov M S, Ryabinina O P, Wong J, Dinh T H, Newton D L, Rybak S M, Magun B E. Molecular determinants of apoptosis induced by the cytotoxic ribonuclease onconase: evidence for cytotoxic mechanisms different from inhibition of protein synthesis. Cancer Res. 2000;60:1983–1994. [PubMed] [Google Scholar]
- 28.Jagus R, Joshi B, Barber G N. PKR, apoptosis and cancer. Int J Biochem Cell Biol. 1999;31:123–138. doi: 10.1016/s1357-2725(98)00136-8. [DOI] [PubMed] [Google Scholar]
- 29.Jeffrey I W, Kadereit S, Meurs E F, Metzger T, Bachmann M, Schwemmle M, Hovanessian A G, Clemens M J. Nuclear localization of the interferon-inducible protein kinase PKR in human cells and transfected mouse cells. Exp Cell Res. 1995;218:17–27. doi: 10.1006/excr.1995.1126. [DOI] [PubMed] [Google Scholar]
- 30.Karin M. The beginning of the end: IkappaB kinase (IKK) and NF-kappaB activation. J Biol Chem. 1999;274:27339–27342. doi: 10.1074/jbc.274.39.27339. [DOI] [PubMed] [Google Scholar]
- 31.Karin M. How NF-kappaB is activated: the role of the IkappaB kinase (IKK) complex. Oncogene. 1999;18:6867–6874. doi: 10.1038/sj.onc.1203219. [DOI] [PubMed] [Google Scholar]
- 32.LaCasse E C, Baird S, Korneluk R G, MacKenzie A E. The inhibitors of apoptosis (IAPs) and their emerging role in cancer. Oncogene. 1998;17:3247–3259. doi: 10.1038/sj.onc.1202569. [DOI] [PubMed] [Google Scholar]
- 33.Lee H H, Dadgostar H, Cheng Q, Shu J, Cheng G. NF-kappaB-mediated up-regulation of Bcl-x and Bfl-1/A1 is required for CD40 survival signaling in B lymphocytes. Proc Natl Acad Sci USA. 1999;96:9136–9141. doi: 10.1073/pnas.96.16.9136. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Lee S B, Rodriguez D, Rodriguez J R, Esteban M. The apoptosis pathway triggered by the interferon-induced protein kinase PKR requires the third basic domain, initiates upstream of Bcl-2, and involves ICE-like proteases. Virology. 1997;231:81–88. doi: 10.1006/viro.1997.8494. [DOI] [PubMed] [Google Scholar]
- 35.Levin D H, Petryshyn R, London I M. Characterization of purified double-stranded RNA-activated eIF-2 alpha kinase from rabbit reticulocytes. J Biol Chem. 1981;256:7638–7641. [PubMed] [Google Scholar]
- 36.Li Z W, Chu W, Hu Y, Delhase M, Deerinck T, Ellisman M, Johnson R, Karin M. The IKKbeta subunit of IkappaB kinase (IKK) is essential for nuclear factor kappaB activation and prevention of apoptosis. J Exp Med. 1999;189:1839–1845. doi: 10.1084/jem.189.11.1839. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Maniatis T, Falvo J V, Kim T H, Kim T K, Lin C H, Parekh B S, Wathelet M G. Structure and function of the interferon-beta enhanceosome. Cold Spring Harbor Symp Quant Biol. 1998;63:609–620. doi: 10.1101/sqb.1998.63.609. [DOI] [PubMed] [Google Scholar]
- 38.Mercurio F, Zhu H, Murray B W, Shevchenko A, Bennett B L, Li J, Young D B, Barbosa M, Mann M, Manning A, Rao A. IKK-1 and IKK-2: cytokine-activated IkappaB kinases essential for NF-kappaB activation. Science. 1997;278:860–866. doi: 10.1126/science.278.5339.860. [DOI] [PubMed] [Google Scholar]
- 39.Meurs E, Chong K, Galabru J, Thomas N S, Kerr I M, Williams B R, Hovanessian A G. Molecular cloning and characterization of the human double-stranded RNA-activated protein kinase induced by interferon. Cell. 1990;62:379–390. doi: 10.1016/0092-8674(90)90374-n. [DOI] [PubMed] [Google Scholar]
- 40.Muller U, Steinhoff U, Reis L F, Hemmi S, Pavlovic J, Zinkernagel R M, Aguet M. Functional role of type I and type II interferons in antiviral defense. Science. 1994;264:1918–1921. doi: 10.1126/science.8009221. [DOI] [PubMed] [Google Scholar]
- 41.Nilsen T W, Wood D L, Baglioni C. 2′,5′-Oligo(A)-activated endoribonuclease. Tissue distribution and characterization with a binding assay. J Biol Chem. 1981;256:10751–10754. [PubMed] [Google Scholar]
- 42.Pahl H L. Activators and target genes of Rel/NF-kappaB transcription factors. Oncogene. 1999;18:6853–6866. doi: 10.1038/sj.onc.1203239. [DOI] [PubMed] [Google Scholar]
- 43.Player M R, Torrence P F. The 2-5A system: modulation of viral and cellular processes through acceleration of RNA degradation. Pharmacol Ther. 1998;78:55–113. doi: 10.1016/S0163-7258(97)00167-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Rabizadeh S, LaCount D J, Friesen P D, Bredesen D E. Expression of the baculovirus p35 gene inhibits mammalian neural cell death. J Neurochem. 1993;61:2318–2321. doi: 10.1111/j.1471-4159.1993.tb07477.x. [DOI] [PubMed] [Google Scholar]
- 45.Rao L, Debbas M, Sabbatini P, Hockenbery D, Korsmeyer S, White E. The adenovirus E1A proteins induce apoptosis, which is inhibited by the E1B 19-kDa and Bcl-2 proteins. Proc Natl Acad Sci USA. 1992;89:7742–7746. doi: 10.1073/pnas.89.16.7742. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Rudolph D, Yeh W C, Wakeham A, Rudolph B, Nallainathan D, Potter J, Elia A J, Mak T W. Severe liver degeneration and lack of NF-kappaB activation in NEMO/IKKgamma-deficient mice. Genes Dev. 2000;14:854–862. [PMC free article] [PubMed] [Google Scholar]
- 47.Rueckert R. Picornaviridae: the viruses and their replication. In: Fields B, Knipe D, Howley P, editors. Virology. 3rd ed. Philadelphia, Pa: Lippincott-Raven; 1996. pp. 477–522. [Google Scholar]
- 48.Schwarz E M, Badorff C, Hiura T S, Wessely R, Badorff A, Verma I M, Knowlton K U. NF-κB-mediated inhibition of apoptosis is required for encephalomyocarditis virus virulence: a mechanism of resistance in p50 knockout mice. J Virol. 1998;72:5654–5660. doi: 10.1128/jvi.72.7.5654-5660.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Sha W C, Liou H C, Tuomanen E I, Baltimore D. Targeted disruption of the p50 subunit of NF-kappa B leads to multifocal defects in immune responses. Cell. 1995;80:321–330. doi: 10.1016/0092-8674(95)90415-8. [DOI] [PubMed] [Google Scholar]
- 50.Siekierka J, Manne V, Ochoa S. Mechanism of translational control by partial phosphorylation of the alpha subunit of eukaryotic initiation factor 2. Proc Natl Acad Sci USA. 1984;81:352–356. doi: 10.1073/pnas.81.2.352. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Silverman R H, Cayley P J, Wreschner D H, Knight M, Gilbert C S, Brown R E, Kerr I M. 2-5A(pppA2′p5′A2′p5′A) in interferon-treated encephalomyocarditis virus-infected mouse L-cells. Tex Rep Biol Med. 1981;41:479–486. [PubMed] [Google Scholar]
- 52.Silverman R H, Skehel J J, James T C, Wreschner D H, Kerr I M. rRNA cleavage as an index of ppp(A2′p)nA activity in interferon-treated encephalomyocarditis virus-infected cells. J Virol. 1983;46:1051–1055. doi: 10.1128/jvi.46.3.1051-1055.1983. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Srivastava S P, Kumar K U, Kaufman R J. Phosphorylation of eukaryotic translation initiation factor 2 mediates apoptosis in response to activation of the double-stranded RNA-dependent protein kinase. J Biol Chem. 1998;273:2416–2423. doi: 10.1074/jbc.273.4.2416. [DOI] [PubMed] [Google Scholar]
- 54.Stark G R, Kerr I M, Williams B R, Silverman R H, Schreiber R D. How cells respond to interferons. Annu Rev Biochem. 1998;67:227–264. doi: 10.1146/annurev.biochem.67.1.227. [DOI] [PubMed] [Google Scholar]
- 55.Stehlik C, de Martin R, Kumabashiri I, Schmid J A, Binder B R, Lipp J. Nuclear factor (NF)-kappaB-regulated X-chromosome-linked iap gene expression protects endothelial cells from tumor necrosis factor alpha-induced apoptosis. J Exp Med. 1998;188:211–216. doi: 10.1084/jem.188.1.211. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Stein B, Rahmsdorf H J, Steffen A, Litfin M, Herrlich P. UV-induced DNA damage is an intermediate step in UV-induced expression of human immunodeficiency virus type 1, collagenase, c-fos, and metallothionein. Mol Cell Biol. 1989;9:5169–5181. doi: 10.1128/mcb.9.11.5169. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Stroka D M, Badrichani A Z, Bach F H, Ferran C. Overexpression of A1, an NF-kappaB-inducible anti-apoptotic bcl gene, inhibits endothelial cell activation. Blood. 1999;93:3803–3810. [PubMed] [Google Scholar]
- 58.Sugimoto A, Friesen P D, Rothman J H. Baculovirus p35 prevents developmentally programmed cell death and rescues a ced-9 mutant in the nematode Caenorhabditis elegans. EMBO J. 1994;13:2023–2028. doi: 10.1002/j.1460-2075.1994.tb06475.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Takeda K, Takeuchi O, Tsujimura T, Itami S, Adachi O, Kawai T, Sanjo H, Yoshikawa K, Terada N, Akira S. Limb and skin abnormalities in mice lacking IKKalpha. Science. 1999;284:313–316. doi: 10.1126/science.284.5412.313. [DOI] [PubMed] [Google Scholar]
- 60.Tam P E, Messner R P. Molecular mechanisms of coxsackievirus persistence in chronic inflammatory myopathy: viral RNA persists through formation of a double-stranded complex without associated genomic mutations or evolution. J Virol. 1999;73:10113–10121. doi: 10.1128/jvi.73.12.10113-10121.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Tanaka M, Fuentes M E, Yamaguchi K, Durnin M H, Dalrymple S A, Hardy K L, Goeddel D V. Embryonic lethality, liver degeneration, and impaired NF-kappa B activation in IKK-beta-deficient mice. Immunity. 1999;10:421–429. doi: 10.1016/s1074-7613(00)80042-4. [DOI] [PubMed] [Google Scholar]
- 62.Teodoro J G, Branton P E. Regulation of apoptosis by viral gene products. J Virol. 1997;71:1739–1746. doi: 10.1128/jvi.71.3.1739-1746.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Tewari M, Dixit V M. Fas- and tumor necrosis factor-induced apoptosis is inhibited by the poxvirus crmA gene product. J Biol Chem. 1995;270:3255–3260. doi: 10.1074/jbc.270.7.3255. [DOI] [PubMed] [Google Scholar]
- 64.Thanos D, Maniatis T. The high mobility group protein HMG I(Y) is required for NF-kappa B-dependent virus induction of the human IFN-beta gene. Cell. 1992;71:777–789. doi: 10.1016/0092-8674(92)90554-p. [DOI] [PubMed] [Google Scholar]
- 65.Thanos D, Maniatis T. Identification of the rel family members required for virus induction of the human beta interferon gene. Mol Cell Biol. 1995;15:152–164. doi: 10.1128/mcb.15.1.152. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Thome M, Schneider P, Hofmann K, Fickenscher H, Meinl E, Neipel F, Mattmann C, Burns K, Bodmer J L, Schroter M, Scaffidi C, Krammer P H, Peter M E, Tschopp J. Viral FLICE-inhibitory proteins (FLIPs) prevent apoptosis induced by death receptors. Nature. 1997;386:517–521. doi: 10.1038/386517a0. [DOI] [PubMed] [Google Scholar]
- 67.Traenckner E B, Pahl H L, Henkel T, Schmidt K N, Wilk S, Baeuerle P A. Phosphorylation of human I kappa B-alpha on serines 32 and 36 controls I kappa B-alpha proteolysis and NF-kappa B activation in response to diverse stimuli. EMBO J. 1995;14:2876–2883. doi: 10.1002/j.1460-2075.1995.tb07287.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Tsukahara T, Kannagi M, Ohashi T, Kato H, Arai M, Nunez G, Iwanaga Y, Yamamoto N, Ohtani K, Nakamura M, Fujii M. Induction of Bcl-x(L) expression by human T-cell leukemia virus type 1 Tax through NF-kappaB in apoptosis-resistant T-cell transfectants with Tax. J Virol. 1999;73:7981–7987. doi: 10.1128/jvi.73.10.7981-7987.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Wang C Y, Guttridge D C, Mayo M W, Baldwin A S., Jr NF-kappaB induces expression of the Bcl-2 homologue A1/Bfl-1 to preferentially suppress chemotherapy-induced apoptosis. Mol Cell Biol. 1999;19:5923–5929. doi: 10.1128/mcb.19.9.5923. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Wang C Y, Mayo M W, Korneluk R G, Goeddel D V, Baldwin A S., Jr NF-kappaB antiapoptosis: induction of TRAF1 and TRAF2 and c-IAP1 and c-IAP2 to suppress caspase-8 activation. Science. 1998;281:1680–1683. doi: 10.1126/science.281.5383.1680. [DOI] [PubMed] [Google Scholar]
- 71.Wessely R, Klingel K, Santana L F, Dalton N, Hongo M, Jonathan Lederer W, Kandolf R, Knowlton K U. Transgenic expression of replication-restricted enteroviral genomes in heart muscle induces defective excitation-contraction coupling and dilated cardiomyopathy. J Clin Investig. 1998;102:1444–1453. doi: 10.1172/JCI1972. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.White E, Sabbatini P, Debbas M, Wold W S, Kusher D I, Gooding L R. The 19-kilodalton adenovirus E1B transforming protein inhibits programmed cell death and prevents cytolysis by tumor necrosis factor alpha. Mol Cell Biol. 1992;12:2570–2580. doi: 10.1128/mcb.12.6.2570. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Williams B R. PKR: a sentinel kinase for cellular stress. Oncogene. 1999;18:6112–6120. doi: 10.1038/sj.onc.1203127. [DOI] [PubMed] [Google Scholar]
- 74.Wreschner D H, James T C, Silverman R H, Kerr I M. Ribosomal RNA cleavage, nuclease activation and 2-5A(ppp(A2′p)nA) in interferon-treated cells. Nucleic Acids Res. 1981;9:1571–1581. doi: 10.1093/nar/9.7.1571. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Yamaoka S, Courtois G, Bessia C, Whiteside S T, Weil R, Agou F, Kirk H E, Kay R J, Israel A. Complementation cloning of NEMO, a component of the IkappaB kinase complex essential for NF-kappaB activation. Cell. 1998;93:1231–1240. doi: 10.1016/s0092-8674(00)81466-x. [DOI] [PubMed] [Google Scholar]
- 76.Yang Y L, Reis L F, Pavlovic J, Aguzzi A, Schafer R, Kumar A, Williams B R, Aguet M, Weissmann C. Deficient signaling in mice devoid of double-stranded RNA-dependent protein kinase. EMBO J. 1995;14:6095–6106. doi: 10.1002/j.1460-2075.1995.tb00300.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Yeung M C, Chang D L, Camantigue R E, Lau A S. Inhibitory role of the host apoptogenic gene PKR in the establishment of persistent infection by encephalomyocarditis virus in U937 cells. Proc Natl Acad Sci USA. 1999;96:11860–11860. doi: 10.1073/pnas.96.21.11860. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Yeung M C, Liu J, Lau A S. An essential role for the interferon-inducible, double-stranded RNA-activated protein kinase PKR in the tumor necrosis factor-induced apoptosis in U937 cells. Proc Natl Acad Sci USA. 1996;93:12451–12455. doi: 10.1073/pnas.93.22.12451. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Zamanian-Daryoush M, Mogensen T H, DiDonato J A, Williams B R. NF-κB activation by double-stranded-RNA-activated protein kinase (PKR) is mediated through NF-κB-inducing kinase and IκB kinase. Mol Cell Biol. 2000;20:1278–1290. doi: 10.1128/mcb.20.4.1278-1290.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Zandi E, Karin M. Bridging the gap: composition, regulation, and physiological function of the IκB kinase complex. Mol Cell Biol. 1999;19:4547–4551. doi: 10.1128/mcb.19.7.4547. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Zandi E, Rothwarf D M, Delhase M, Hayakawa M, Karin M. The IkappaB kinase complex (IKK) contains two kinase subunits, IKKalpha and IKKbeta, necessary for IkappaB phosphorylation and NF-kappaB activation. Cell. 1997;91:243–252. doi: 10.1016/s0092-8674(00)80406-7. [DOI] [PubMed] [Google Scholar]
- 82.Zhou A, Hassel B A, Silverman R H. Expression cloning of 2-5A-dependent RNAase: a uniquely regulated mediator of interferon action. Cell. 1993;72:753–765. doi: 10.1016/0092-8674(93)90403-d. [DOI] [PubMed] [Google Scholar]
- 83.Zhou A, Paranjape J, Brown T L, Nie H, Naik S, Dong B, Chang A, Trapp B, Fairchild R, Colmenares C, Silverman R H. Interferon action and apoptosis are defective in mice devoid of 2′,5′-oligoadenylate-dependent RNase L. EMBO J. 1997;16:6355–6363. doi: 10.1093/emboj/16.21.6355. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Zhou A, Paranjape J M, Der S D, Williams B R, Silverman R H. Interferon action in triply deficient mice reveals the existence of alternative antiviral pathways. Virology. 1999;258:435–440. doi: 10.1006/viro.1999.9738. [DOI] [PubMed] [Google Scholar]
- 85.Zhou A, Paranjape J M, Hassel B A, Nie H, Shah S, Galinski B, Silverman R H. Impact of RNase L overexpression on viral and cellular growth and death. J Interferon Cytokine Res. 1998;18:953–961. doi: 10.1089/jir.1998.18.953. [DOI] [PubMed] [Google Scholar]
- 86.Zong W X, Edelstein L C, Chen C, Bash J, Gelinas C. The prosurvival Bcl-2 homolog Bfl-1/A1 is a direct transcriptional target of NF-kappaB that blocks TNFalpha-induced apoptosis. Genes Dev. 1999;13:382–387. doi: 10.1101/gad.13.4.382. [DOI] [PMC free article] [PubMed] [Google Scholar]