Abstract
Alcohol is the most commonly used drug among adolescents. Their decreased sensitivity to self-regulating cues to stop drinking coincides with an enhanced vulnerability to negative outcomes of excessive alcohol drinking . In adolescents, the hippocampus is one brain region that is particularly susceptible to alcohol-induced neurodegeneration. While cell death is causal, alcohol effects on adult neurogenesis also impact hippocampal structure and function. This review describes what little is known about adolescent-specific effects of alcohol on adult neurogenesis and its relationship to hippocampal integrity. For example, alcohol intoxication inhibits neurogenesis persistently in adolescents but produces aberrant neurogenesis after alcohol dependence. Little is known, however, about the role of adolescent-born neurons in hippocampal integrity or the mechanisms of these effects. Understanding the role of neurogenesis in adolescent alcohol use and misuse is critical to our understanding of adolescent susceptibility to alcohol pathology and increased likelihood of developing alcohol problems in adulthood.
Keywords: Adolescence, Alcohol, Alcoholism, Ethanol, Hippocampus, Neurogenesis, Neural stem cell
Adolescence is often defined by a propensity for risk-taking and reward-seeking behavior, such as alcohol use. Unique qualities of the adolescent brain make this transitional period between childhood and adulthood of especially high risk for the development of an alcohol use disorder (AUD; Nixon & McClain, 2010; Spear, 2018). Though the factors involved are numerous and complex, slow-to-mature behavioral control centers coupled with a low sensitivity for negative effects of alcohol intoxication manifest as ease to drink to excess, the key risk factor for developing an AUD (Bava et al., 2010). Unfortunately, adolescents are more sensitive to many of the consequences of excessive alcohol consumption. Adolescents diagnosed with an AUD show not only cognitive impairments, but also neurodegeneration with only a few years of consumption (Nagel, Schweinsburg, Phan, & Tapert, 2005). Indeed, the hippocampus, known for its role in learning and memory, appears to be a particular target of alcohol toxicity in the adolescent (White & Swartzwelder, 2004). The hippocampus is also one region that continues to generate and incorporate newly born neurons through adulthood, or adult neurogenesis, a process that is how well-accepted for its role in hippocampal integrity and function (Denoth-Lippuner & Jessberger, 2021; Imayoshi et al., 2008). Adolescent-specific effects of alcohol consumption on the various aspects of adult neurogenesis may underlie the adolescent’s susceptibility to hippocampal degeneration and dysfunction, although this nascent research area has many gaps in knowledge (Crews, Mdzinarishvili, Kim, He, & Nixon, 2006; Crews & Nixon, 2009; McClain, Hayes, Morris, & Nixon, 2011; McClain, Morris, Marshall, & Nixon, 2014; Morris, Eaves, Smith, & Nixon, 2010). As is explored in this chapter, binge patterns of alcohol consumption (including extreme binge drinking) in and around the adolescent period, coupled with the distinct vulnerability of the developing brain, leads to lasting effects on adult neurogenic processes and thus hippocampal integrity and function.
1. Alcohol use in adolescence
Adolescence, roughly defined as ages 10-19, is the time when most drug experimentation first occurs but also corresponds to a uniquely dynamic period of brain development and maturation (Jaworska & MacQueen, 2015; Sussman & Arnett, 2014). Alcohol is the most commonly used drug among 12-20 year olds, with 19% reporting drinking and 11% reporting binge drinking in the previous 30 days (SAMHSA, 2020). Binge drinking is a pattern of high alcohol consumption that raises the blood alcohol concentration (BAC) to 80 mg/dL or above (Alcoholism, 2012). Among college freshmen surveyed for drinking in the previous 2 weeks, 41% of men and 34% of women reported binge drinking, while 20% of men and 10% of women reported consuming two or three times the binge drinking threshold per occasion (White, Kraus, & Swartzwelder, 2006). Although adolescents may drink less often than adults, they consume a larger number of drinks each time they initiate (SAMHSA, 2020). For example, 18-24 year olds in the U.S. reported an average of 9.5 drinks per binge episode (Naimi, Nelson, & Brewer, 2010), which well exceeds the 4/5-drink threshold that defines adult binge drinking for women and men respectively (NIAAA, 2012). Furthermore, when compared to adults, adolescents require a fewer number of drinks to reach a 0.08 BAC, the binge drinking threshold, which is due in part to a smaller body size (Donovan, 2009). As such, rates of binge drinking in adolescents may be underestimated because these definitions are based on adult size and intake (Chung, Creswell, Bachrach, Clark, & Martin, 2018).
Adolescents are less sensitive to many of the acute effects of alcohol and specifically cues that aid in self-regulation of alcohol drinking. For example, adolescents are not as sensitive to the sedative and locomotor effects of alcohol (Little, Kuhn, Wilson, & Swartzwelder, 1996), and its suppressive activity on social interaction requires higher doses (Varlinskaya & Spear, 2002). Accordingly, in young rats, less sensitivity to the interoceptive effects of alcohol is suggested from findings that alcohol serves as a less effective cue in an operant drug discrimination task (Anderson & Spear, 2014). In conditioned taste aversion experiments, adolescents require higher ethanol doses than adults need to induce aversion (Anderson, Varlinskaya, & Spear, 2010; Vetter-O’Hagen, Varlinskaya, & Spear, 2009). Adolescents’ relative ease in reaching the same BAC coupled with low sensitivity to impairment could explain why human studies reveal not only binge drinking but drinking levels above and beyond a typical binge termed “high intensity drinking” or “extreme binge drinking” in this age group (Patrick et al., 2013; Patrick & Terry-McElrath, 2017). Adolescents also exhibit attenuated anxiogenic effects following alcohol withdrawal, though withdrawal severity is similar to adults when blood ethanol concentrations are similar (Doremus, Brunell, Varlinskaya, & Spear, 2003; Morris, Kelso, Liput, Marshall, & Nixon, 2010; Varlinskaya & Spear, 2004). Alarmingly, adolescent consumption can result in these insensitivities continuing into adulthood, in a process termed the “lock-in effect” (Spear & Swartzwelder, 2014). Taken together, the lack of sensitivity to these effects allows adolescents to ingest large amounts of ethanol with fewer aversive effects, increasing the likelihood of drinking to excess (Towner & Varlinskaya, 2020).
2. Enhanced susceptibility of adolescents to the consequences of excessive drinking
While adolescents are less susceptible to many of the negative effects of alcohol intoxication that typically serve to regulate their alcohol intake, they are more sensitive to many of the consequences of excessive alcohol drinking than adults. Heavy drinking damages the brain, a consequence of excessive consumption which is thought to contribute to developing an AUD (Crews & Nixon, 2009). Adolescents may be uniquely susceptible to alcohol induced brain damage compared to adults, especially in regions known to develop during adolescence (Crews, Braun, Hoplight, Switzer, & Knapp, 2000; Monti et al., 2005). MRI studies demonstrate that adolescent heavy drinkers experience widespread reductions in both white and gray matter, with most studies supporting decreased volume in certain subcortical and cortical regions (Feldstein Ewing et al., 2014; Squeglia et al., 2014). Such alcohol use can damage major white matter pathways (Bava et al., 2010), including limbic and cortical projection fibers (McQueeny et al., 2009). Alcohol can also blunt increases in white matter that typically occur over adolescent development, notably in regions critical for executive control (Luciana, Collins, Muetzel, & Lim, 2013; Vargas, Bengston, Gilpin, Whitcomb, & Richardson, 2014). The hippocampus, a predominantly gray matter region critical for learning and memory that also contributes to other addiction processes (Tannenholz, Jimenez, & Kheirbek, 2014), is reduced in volume in adolescents with an AUD compared to healthy teens (Nagel et al., 2005). Greater hippocampal volume loss is associated with adolescents who drank starting at younger ages (De Bellis et al., 2000) and who consumed more alcohol per drinking episode (De Bellis et al., 2005). This enhanced susceptibility to damage by those who drink at younger ages parallels their increased risk of developing an AUD (Grant & Dawson, 1997).
Neurotoxicity associated with heavy alcohol consumption during adolescence can have behavioral consequences that stretch into adulthood and therefore influence the perpetuation of alcohol problems as adults (Crews et al., 2019; Lees, Meredith, Kirkland, Bryant, & Squeglia, 2020). Animal studies indicate that adolescent alcohol exposure leads to increased susceptibility to stress in later life (Boutros et al., 2018; Torcaso, Asimes, Meagher, & Pak, 2017). Various aspects of executive functioning can be severely impaired as well, with adult rats exposed to alcohol in adolescence demonstrating persistent impairments in cognitive flexibility (Fernandez & Savage, 2017), reversal learning deficits (Coleman, He, Lee, Styner, & Crews, 2011), and increased resistance to extinction of ethanol-seeking behavior (Gass et al., 2014). Especially alarming are reports of increased impulsive behaviors in adult rats exposed to alcohol as adolescents (Ehlers, Criado, Wills, Liu, & Crews, 2011). In humans, positive correlations have been made between heavy alcohol consumption during adolescence and increased impulsivity later on (White et al., 2011). Thus, loss of executive control and increased impulsivity due to alcohol induced brain damage during adolescence likely increases the likelihood of developing an AUD (Crews & Boettiger, 2009).
Various mechanisms underlie the brain matter loss in the neurobiological and behavioral consequences of excessive alcohol consumption. In general, drinking to excess can cause neuronal death (Walker, Barnes, Zornetzer, Hunter, & Kubanis, 1980), with a variety of hypotheses on the mechanisms involved, as has been reviewed elsewhere (Cortez, Rodgers, Kosten, & Leasure, 2020; Crews & Nixon, 2009; Guerri & Pascual, 2019; Lees et al., 2020; Melbourne, Thompson, Peng, & Nixon, 2019). In adolescence, a combination of greater cell death after alcohol dependence (e.g. Crews et al., 2000) in combination with derangement in ongoing developmental processes (Giedd et al., 1999; Morris, Eaves, et al., 2010; Vargas et al., 2014) underlies the enhanced susceptibility to degeneration (Nixon & McClain, 2010). For regions such as the hippocampus, a particular target of alcohol effects (White & Swartzwelder, 2004), the adolescent-specific effects of alcohol on the processes of adult neurogenesis may underlie the susceptibility of this region to alcohol toxicity.
3. Adult neurogenesis
Adult neurogenesis is the mechanism by which new neurons are generated, which is now accepted to occur throughout the lifespan of an organism (Denoth-Lippuner & Jessberger, 2021; Gebara et al., 2016; Toda, Parylak, Linker, & Gage, 2019). The two best-accepted brain areas that contain niches conducive to neurogenesis throughout the life of an organism are the hippocampus and the lateral ventricles, specifically the subgranular zone of the dentate gyrus (SGZ) and the subventricular zone (SVZ), respectively (Altman & Das, 1965; Doetsch, Garcia-Verdugo, & Alvarez-Buylla, 1999; Gage, 2000; Gould, 1999). Adult neurogenesis has been observed within the hippocampus of all mammals, including humans (Boldrini et al., 2018; Eriksson et al., 1998; Spalding et al., 2013), with recent debate on the extent adult neurogenesis in humans generally resolved (Kempermann et al., 2018). While the role of SVZ neurogenesis in humans and mammals has been less clear, adult neurogenesis appears to play a key role in hippocampal structure and function (Clelland et al., 2009; Imayoshi et al., 2008; Sahay et al., 2011; Shors et al., 2001; for review see Denoth-Lippuner & Jessberger, 2021; Imayoshi et al., 2008; Kempermann, Song, & Gage, 2015; Olsufka, Peng, Newton, & Nixon, 2018; Snyder & Cameron, 2012; Snyder & Drew, 2020; Toda et al., 2019).
There are four major components to adult neurogenesis: 1) proliferation of stem or progenitor cells, 2) differentiation into a neuronal fate 3) migration, and 4) survival / integration into hippocampal circuitry. The initial birth of a new neuron within the SGZ, originates with what prior studies have identified as true neural stem cells (NSCs): type 1 radial glial-like cells that maintain stemness or the capacity to generate both newborn neurons but also astrocytes (Bonaguidi et al., 2011; Goncalves, Schafer, & Gage, 2016; Olsufka et al., 2018). These cells are identified by the presence of astrocytic markers such as glial fibrillary acidic protein (GFAP; Bonaguidi et al., 2011) and are typically found in a quiescent, or non-actively dividing state. Provocation by both internal or external stimuli can drive activation of NSCs, identified by the presence of the endogenous proliferation marker Ki67 (Gardella et al., 2002), which results in the asymmetrical division of NSCs and the creation of intermediate neural progenitor cells (NPCs; Seri, Garcia-Verdugo, McEwen, & Alvarez-Buylla, 2001). Fate-restricted differentiation of these NPCs ultimately results in the generation of newborn dentate gyrus granule cells that migrate through the granule cell layer and may ultimately integrate into hippocampal circuitry (Esposito et al., 2005; Kronenberg et al., 2003; Palmer, Takahashi, & Gage, 1997; Seri et al., 2001). While full hippocampal integration of these newborn neurons takes months (Laplagne et al., 2006), around three weeks post cell birth, axonal projections can be detected in the mossy fiber pathway to the CA3 region of the hippocampus (Hastings, Seth, Tanapat, Rydel, & Gould, 2002) and dendritic connections to the perforant path (Toda et al., 2019) . Furthermore, the expression of immature neuronal markers, such as doublecortin, PSA-NCAM, and NeuroD1, declines as mature neuronal markers, like NeuN and Prox1 begin to emerge (Bonfanti, 2006; Brown et al., 2003; Karalay et al., 2011; Lavado, Lagutin, Chow, Baker, & Oliver, 2010).
The specific role of newborn cells has been suggested through studies that examined the immediate early gene, c-Fos, in newborn neurons. In response to the hippocampal-dependent spatial learning and memory task, the Morris water maze, an increased activation and preferential recruitment of newborn neurons has been observed (Kee, Teixeira, Wang, & Frankland, 2007). Furthermore, behavioral studies have implicated hippocampal neurogenesis in cognitive flexibility (Burghardt, Park, Hen, & Fenton, 2012; Toda et al., 2019). For example, impaired spatial memory retention has been observed with inhibition of adult hippocampal neurogenesis (Imayoshi et al., 2008; Snyder, Hong, McDonald, & Wojtowicz, 2005), while increases in adult neurogenesis improved the ability to distinguish between two similar contexts, also known as pattern separation (Sahay et al., 2011). While these studies suggest the relevance of hippocampal neurogenesis to the structure and function of the hippocampus, factors or conditions that impact any aspect of the neurogenesis such as injury, sex, or age, similarly result in alterations to hippocampal-related behavior.
4. Alcohol and adult neurogenesis in adolescents
Differences between adolescents and adults
Neurogenesis, specifically newborn cell production, in the dentate gyrus peaks early after birth, around postnatal day (PND) 6 in rats and declines significantly throughout adolescence and into adulthood (Altman & Das, 1965; Schlessinger, Cowan, & Gottlieb, 1975; Snyder, 2019). Adult hippocampal neurogenesis is around 6-14% of its maximum between PND 20-30 (PND 28 a commonly accepted onset of adolescence) and 3% by PND 120 (Snyder, 2019). Furthermore, the number of proliferating cells in the dentate gyrus decreases significantly between PND 35 and PND 63-70 (Cameron & McKay, 2001). Thus, baseline adult neurogenesis and NPC proliferation differs for adolescent and adult animals. This difference forces comparisons to only be of relative percent change from age matched controls.
Adult neurogenesis has been shown consistently to be decreased by alcohol intoxication in adolescent rats in a variety of ethanol exposure models (Briones & Woods, 2013; Broadwater, Liu, Crews, & Spear, 2014; Crews et al., 2006; Ehlers, Oguz, Budin, Wills, & Crews, 2013), but also in adolescent mice (Lacaille et al., 2015) and rhesus monkeys (Taffe et al., 2010). Differences are seen in how the neurogenic niche is affected, indicating that the length of exposure, ethanol concentration, and timing of neurogenesis measurement are all important factors when considering the overall impact of ethanol on adult neurogenesis. In addition, each aspect of neurogenesis, proliferation, differentiation, migration, and integration (e.g. Figure 1) must be studied separately in order to understand how alcohol produces its effects. Starting with proliferation, acute exposures to ethanol dose-dependently decrease proliferation in both adult and adolescent rats (Crews et al., 2006; Nixon & Crews, 2002). While this effect appears to be exaggerated in adolescents, with larger decreases in proliferation from age matched controls compared to adults, methodological differences in the exogenous proliferation marker BrdU, muddy this interpretation (Crews et al., 2006; Nixon & Crews, 2002). Adults received two non-saturating (Cameron & McKay, 2001) 100mg/kg BrdU injections 45- and 105-minutes following ethanol while adolescents received one saturating 300mg/kg injection 30-minutes following ethanol. Notably, acute alcohol did not decrease proliferation in either adult or adolescent mice (Lacaille et al., 2015). Results in a binge model of alcohol dependence and AUD in which animals receive three daily intragastric gavages of ethanol for four days (PND 35-38 in adolescent rats; approximately PND 70 in adults), are quite different from acute effects. After ethanol dependence but notably while the animals are still intoxicated, overall decreases in both NPC proliferation and neurogenesis are observed (Morris, Eaves, et al., 2010; Nixon & Crews, 2002) but the decreases in proliferation are reportedly smaller in adolescents compared to adults following the last dose of ethanol (Morris, Eaves, et al., 2010; Nixon & Crews, 2004). Again, methodological differences in the exogenous marker, BrdU, and lack of direct comparisons cloud interpretations: the timing of the BrdU injections differ slightly between studies though the rats received the same (300 mg/kg) dose which could explain the differences. Thus, more work is needed and specifically direct comparisons and the inclusion of additional endogenous markers of proliferation in order to fully understand how ethanol produces its effects on the components of adult neurogenesis.
Figure 1:
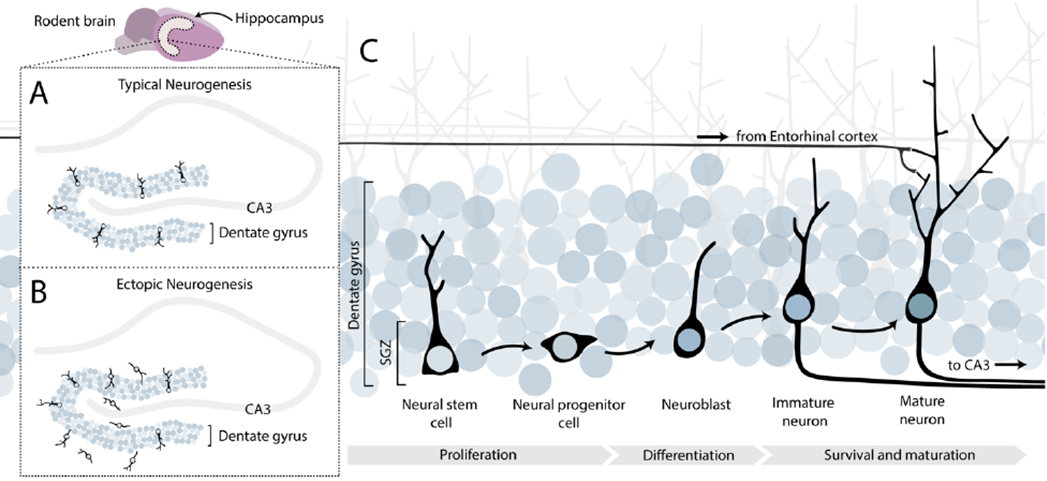
In the rodent brain adult neurogenesis occurs in at least two regions, one of which is the hippocampus. (A) As part of normal brain functioning, new neurons are born in the subgranular zone of the dentate gyrus of the hippocampus. (B) In adolescent rats, reactive neurogenesis following ethanol administration leads to increased abnormal “ectopic” migration of newborn neurons. (C) Adult neurogenesis is a multi-step process that occurs over a time-span of several weeks. First, neural stem cells proliferate and differentiate into neural progenitor cells, which themselves can proliferate and differentiate into neuroblasts. Some neuroblasts will gradually mature into adult neurons and migrate into the dentate gyrus granule cell layer. As newborn cells mature, they extend their axons to the CA3 pyramidal cells of the hippocampus, while their dendrites elongate and ramify to receive inputs from the entorhinal cortex. Alcohol can potentially affect any one or multiple steps in its overall effect on adult neurogenesis.
Indeed, the examination of the endogenous active cell cycle marker, Ki67 (Kee, Sivalingam, Boonstra, & Wojtowicz, 2002) revealed that ethanol may affect proliferation differently in adults versus adolescents after alcohol dependence. BrdU, which is taken up during S phase of the cell cycle, is decreased in both adults and adolescents (Morris, Eaves, et al., 2010). However, Ki67, which is expressed across all active division portions of the cell cycle, is not decreased in adolescents after the 4-day binge, while it is decreased in adult animals (Crews et al., 2006; McClain et al., 2011; Morris, Eaves, et al., 2010). This difference led to subsequent experiments which found that ethanol dependence shortens the cell cycle, and the S-phase specifically, of neural stem and progenitor cells in adolescence leading to an expansion of the neural progenitor cell pool (McClain et al., 2011). This difference appears to return to normal a week after binge exposure when adolescent rats show a reactive burst in hippocampal neurogenesis, as the proportion of cells in each phase of the cell cycle was not different between alcohol exposed and control animals.
The adolescent intermittent ethanol (AIE) model is another common model of ethanol exposure that has been used to study the effects of ethanol on adult neurogenesis. AIE exposure differs from the 4-day binge in that it does not induce physical dependence, produces transient, lower BECs (in the range of 150-190 mg/dL at PND 38, measured 1 hr after ethanol administration) and perhaps mimics adolescent weekend drinking. During AIE, animals typically receive ethanol 2-days on and 2-days off starting early in adolescence and ending in early adulthood. In this model, animals were sacrificed 3 days after the last ethanol dose (and 2 hrs after BrdU injection) and thus show no difference in proliferation consistent with alcohol’s inhibitory effect during intoxication (Nixon, 2006), but do display decreased numbers of stem and progenitor cells (Liu & Crews, 2017). One report found less neurogenesis one day following AIE (Vetreno & Crews, 2015) while another found no difference three days following AIE (Liu & Crews, 2017). After protracted abstinence, however, adolescent AIE animals have been shown consistently to have decreased proliferation and neurogenesis (Liu & Crews, 2017; Sakharkar et al., 2016; Swartzwelder et al., 2019; Vetreno & Crews, 2015, 2018). In agreement with AIE models, after chronic ethanol vapor exposure, NPC proliferation and neurogenesis as assessed by doublecortin and Ki67 immunoreactivity is also persistently decreased following two- and eight-weeks of abstinence (Ehlers, Liu, Wills, & Crews, 2013). This extended decrease of neurogenesis appears counter to that seen in the 4-day binge in which decreased neurogenesis is transient and returns to baseline after reactive increases in neurogenesis (Geil et al., 2014; Nickell et al., 2017). Differences in neurogenesis levels in protracted abstinence may reflect differences in exposure times or when neurogenesis is measured in these two models, but also a differential impact of the development of dependence and therefore withdrawal seizures on the components of neurogenesis. Long-term or repeated exposures to excessive alcohol may result in multiple cycles of reactive neurogenesis, resulting in depletion of the stem cell pool. Indeed, stem cell depletion from hyperactivation has been shown to occur in seizure models (Sierra et al., 2015). Thus a major gap in our understanding is how and why decreased neurogenesis persists into adulthood following some types of adolescent ethanol exposure, but not in all models.
Only two studies to date have directly compared adult and adolescent animals. One in mice utilized a single binge-like exposure of three IP injections in one day or a multiple binge-like exposure of three one day binges in five-day intervals (Lacaille et al., 2015). Though only one marker was used to assess cell proliferation (BrdU), results showed that adolescents with multiple binges had decreased proliferation while the single binge exposure adolescents and all adults did not differ from controls. The other study in rats utilized a 1 day on and 2 day off variant of AIE (Broadwater et al., 2014). Using this model, Broadwater et al. (2014) found that four weeks after the final dose of ethanol, animals did not differ in the number of proliferating cells in adolescence or adulthood, but intriguingly AIE exposed adolescents had significantly decreased neurogenesis as measured by doublecortin expression while adults did not. This study suggests that alcohol has long-term effects on the neurogenic niche that also impacts the integration and survival of newborn neurons (Zou & Crews, 2012).
Alcohol inhibition of neurogenesis impairs hippocampal integrity
Alterations to neurogenesis following adolescent drinking can have lasting impacts on the brain, and especially to hippocampal integrity and cognitive function. Studies using human subjects allow a glimpse into lasting structural changes that can occur after adolescent drinking. For example, Pfefferbaum at al. (2018) recruited 483 no- or low-drinking adolescents and conducted MRI scans at the beginning of the study and also at 1 and 2 years later. Subjects who had initiated heavy drinking during the course of the study showed rates of frontal cortical gray matter degeneration above and beyond subjects who were no- or low-drinkers. Additionally, hippocampal volume loss was greater in adolescents who started drinking at a younger age, and who consumed more alcohol each time they drank (De Bellis et al., 2000; De Bellis et al., 2005).
In mice, adolescent binge alcohol exposure led to reduced neurogenesis accompanied by impaired short-term memory, effects which were not observed in adult mice (Lacaille et al., 2015). In rats, adolescent alcohol exposure is linked to an increase in anhedonia and behavioral despair during withdrawal/abstinence along with reduced BrdU+ immunoreactivity in the dentate gyrus (Briones & Woods, 2013). However, administration of a brain-derived neurotrophic factor (BDNF) receptor agonist (TrkB) restored neurogenesis and ameliorated the depression-like symptoms. AIE exposed adolescent rats also display anxiety-like behavior (Loxton & Canales, 2017; Sakharkar et al., 2016), which coincides with histone deacetylase (HDAC) increases though decreases in histone H3 acetylation at the BDNF exon IV promoter in the hippocampus (Sakharkar et al., 2016). Treatment with the HDAC inhibitor trichostatin A reversed histone acetylation deficits and recovered the anxiety-like behavior, providing evidence of epigenetic disturbances to a pro-neurogenic trophic factor promoter following adolescent alcohol use. In a separate study, AIE impaired adult object recognition memory, and interestingly, memory for objects was positively correlated with doublecortin immunoreactivity in the both the dorsal and ventral dentate gyrus while latency to enter the center of the testing arena (thigmotaxis, a measure of anxiety-like behavior) was negatively correlated with ventral dentate gyrus doublecortin immunoreactivity (Vetreno & Crews, 2015). Taken together, these studies suggest how sensitive cognitive and emotional health is to the long-term anti-neurogenic effects of adolescent drinking.
Reactive neurogenesis after alcohol dependence
After the hippocampus has experienced an insult such as trauma (Dash, Mach, & Moore, 2001; Yu, Zhang, Liebl, & Kernie, 2008), seizure (Cho et al., 2015; Parent et al., 1997), ischemic stroke (Jin et al., 2006; Liu, Solway, Messing, & Sharp, 1998) or alcohol dependence (Geil et al., 2014; Hansson et al., 2010; Mandyam & Koob, 2012; Nawarawong, Nickell, Hopkins, Pauly, & Nixon, 2021; Nixon & Crews, 2004; Somkuwar et al., 2016), a phenomenon known as “reactive neurogenesis” occurs, during which there is a surge in progenitor cell proliferation followed by an increase in newborn neurons (Molowny, Nacher, & Lopez-Garcia, 1995). Although reactive neurogenesis has most often been studied in the context of injury, it has also been observed in response to naturally occurring apoptosis (Larson, Thatra, Lee, & Brenowitz, 2014), suggesting it plays an important part in homeostatic regulation of cell birth and death. Evidence of reactive neurogenesis has been observed in both adults and adolescent models of alcohol dependence which suggests that hippocampal damage or excitation upon withdrawal may be the fundamental cause of this striking event (Hansson et al., 2010; McClain et al., 2014; Nawarawong et al., 2021; Nixon & Crews, 2004; Somkuwar et al., 2016).
Reactive neurogenesis can be beneficial to recovery or aberrant, depending on the condition, and perhaps the degree of hippocampal activation incurred during insult. For example, seizures appear to initially abolish neurogenesis by biasing neural stem cells to differentiate into astrocytes, while sub-seizure levels of excitation enhance both new neuron and new astrocyte formation (Sierra et al., 2015). With similar alcohol withdrawal seizures following the 4-day binge, there is a reactive increase of neurogenesis in abstinence for both adult and adolescent animals (McClain et al., 2011; Nickell et al., 2017; Nixon & Crews, 2004). Reactive proliferation first occurs peaking seven days following the last dose of ethanol (Hayes et al., 2018; Nixon, Kim, Potts, He, & Crews, 2008) with similar two-fold increases in actively proliferating progenitor cells (BrdU+/Sox2+ co-labeled cells) at T7 in both adults and adolescents (Hayes et al., 2018; Nickell et al., 2017). Also by T7, the percentage of cycling cells in each phase of the cell cycle are similar for 4-day binge alcohol exposed versus controls in adults versus adolescents. Qualitatively, however, there could be age differences in the percentage of cells in each phase with adults having 60% of actively dividing cells estimated to be in G1 while adolescents have only 40%. This may not be surprising given the expected age-related decline in neurogenesis that occurs (Altman & Das, 1965). Although withdrawal severity and specifically seizure severity based on overt behavior appears to be similar between adults and adolescents, the driver of reactive neurogenesis may differ between the age groups. In adolescents, ethanol shortens the cell cycle during ethanol dependence (McClain et al., 2011) possibly as a means of amplifying type 2 progenitor cells (Nickell et al., 2017). In adults, however, reactive proliferation at T7 results from activating type 1 radial glial-like NSC out of quiescence (Hayes et al., 2018). This type-1 NSC activation is not observed in adolescents at T7, though other time points have not been examined as exhaustively as they have in adults (Nickell et al., 2017). The extent of activated type 1 NSCs has implications for exhaustion of the stem cell pool, potentially explaining long term decreases in NSC proliferation or adult neurogenesis (Sierra et al., 2015).
Fourteen days after the last dose of ethanol, these reactive increases in NPC and/or NSC proliferation in the SGZ at T7 subsequently produce increased adult neurogenesis as measured by either doublecortin or NeuroD1 expression at T14 in adolescents and adults (Hayes et al., 2018; McClain et al., 2014; Nickell et al., 2017). The two-fold increase in newborn neurons holds through to T35 (BrdU pulse at T7, sacrificed at T35 “chase”) with a remarkably similar rate of neuronal differentiation (~82-84%) in both groups (McClain et al., 2014; Nixon & Crews, 2004). These similarities are for cells found in the SGZ and inner 1/3 of the granule cell layer where newborn cells are expected. As such, this is where the similarities end: adolescent rats have a striking pattern of ectopically expressed BrdU (T7), doublecortin (T14) and then Prox1 (T14), the transcription factor expressed in granule neurons (Karalay et al., 2011). Interestingly, ectopic neurogenesis was observed essentially only in those rats that experienced high withdrawal (McClain et al., 2014). Ectopic neurogenesis has not been observed in adults, male, or female (Nawarawong, submitted) in the 4-day binge model nor any other alcohol model to our knowledge.
Results to date in adult animals have indicated that adult neurogenesis is a reparative process in adult rats (Nickell, Thompson, Pauly, & Nixon, 2020), however perhaps not in adult mice (Cuartero et al., 2019; Golub et al., 2015; Lee et al., 2019). In adolescents, reactive neurogenesis leads to increased ectopic migration of newborn cells in those rats with greater withdrawal severity (McClain et al., 2014), but this effect is not observed in adults (Nixon & Crews, 2004). Specifically, in adolescents, severe ethanol withdrawal was linked to an increase in Prox1+ cells in the hilus of the dentate gyrus (McClain et al., 2014); similarly, pilocarpine-induced seizures elicit ectopic Prox1+ cells in the hilus (Parent, Elliott, Pleasure, Barbaro, & Lowenstein, 2006) suggesting that for alcohol withdrawal, seizure is the key to this phenomenon. At the same time, seizures are linked to ectopic neural stem cells, aberrant neurogenesis, and pathogenesis in models of seizure (Cho et al., 2015; Parent et al., 1997). There is evidence that these hilar-ectopic cells can travel to and integrate into the hippocampal CA3 cell layer while still morphologically and electrophysiologically retaining the properties of a granule cell (Scharfman, Goodman, & Sollas, 2000), which is in line with recent work implicating a derangement in newborn neuron integration in alcohol withdrawal seizures (Lee et al., 2019). As such, it is possible that aberrant neurogenesis that occurs with alcohol dependence may be responsible for the long-term consequences of adolescent drinking.
5. Conclusions
There are multiple ways that alcohol exposure can potentially impact adult neurogenesis with discoveries in this relatively nascent field still being made on descriptive but important questions of effects on cell proliferation, expression of newborn neuron markers and new cell survival. Thus, even less is known about how alcohol affects this process in the adolescent brain. Major gaps in our knowledge also remain for understanding the role of adult neurogenesis in hippocampal pathology in alcohol dependence, specifically the contribution of newborn cells not only to normal hippocampal structure and function but also the recovery process from the damaging effects of excess alcohol consumption. The presence of ectopic newborn cells after binge exposure may explain the greater damage to the hippocampus in adolescents (McClain et al., 2014). Finally, no mechanistic studies have yet probed the role of these cells in damage or recovery processes in adolescents. Of the existing studies, it does sem clear that adolescent exposure has a more detrimental impact on adult neurogenesis than in adults. Whether that effect is through persistent impairment in newborn neuron production as is observed in AIE models (e.g. (Broadwater et al., 2014) or through aberrant neurogenesis after dependence (McClain et al., 2014), either or both mechanisms together have implications on long term hippocampal structure and function. Understanding the cellular signaling mechanisms unique to adolescents is important to our overall understanding of the harms of adolescent drinking.
Abbreviations
- AIE
Adolescent Intermittent Ethanol
- AUD
Alcohol Use Disorder
- BAC
Blood Alcohol Concentration
- BDNF
Brain-Derived Neurotrophic Factor
- GFAP
Glial Fibrillary Acidic Protein
- HDAC
Histone Deacetylase
- NPC
Neural Progenitor Cell
- NSC
Neural Stem Cell
- PND
Postnatal Day
- PFC
Prefrontal Cortex
- SGZ
Subgranular Zone
- SVZ
Subventricular Zone
References
- SAMHSA, Substance Abuse Mental Health Services Administration (2020). Key substance use and mental health indicators in the United States: Results from the 2019 National Survey on Drug Use and Health (HHS Publication No. PEP20-07-01-001, NSDUH Series H-55). Rockville, MD: Center for Behavioral Health Statistics and Quality, Substance Abuse and Mental Health Services Administration.(Retrieved from https://www.samhsa.gov/data/). [Google Scholar]
- Altman J, & Das GD (1965). Autoradiographic and histological evidence of postnatal hippocampal neurogenesis in rats. Journal of Comparative Neurology, 124(3), 319–335. 10.1002/cne.901240303 [DOI] [PubMed] [Google Scholar]
- Anderson RI, & Spear LP (2014). Age differences in ethanol discrimination: acquisition and ethanol dose generalization curves following multiple training conditions in adolescent and adult rats. Alcoholism: Clinical & Experimental Research, 38(1), 186–194. 10.1111/acer.12255 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Anderson RI, Varlinskaya EI, & Spear LP (2010). Ethanol-induced conditioned taste aversion in male sprague-dawley rats: impact of age and stress. Alcoholism: Clinical & Experimental Research, 34(12), 2106–2115. 10.1111/j.1530-0277.2010.01307.x [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bava S, Thayer R, Jacobus J, Ward M, Jernigan TL, & Tapert SF (2010). Longitudinal characterization of white matter maturation during adolescence. Brain Research, 1327, 38–46. 10.1016/j.brainres.2010.02.066 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Boldrini M, Fulmore CA, Tartt AN, Simeon LR, Pavlova I, Poposka V, … Mann JJ (2018). Human Hippocampal Neurogenesis Persists throughout Aging. Cell Stem Cell, 22(4), 589–599 e585. 10.1016/j.stem.2018.03.015 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bonaguidi MA, Wheeler MA, Shapiro JS, Stadel RP, Sun GJ, Ming GL, & Song H (2011). In vivo clonal analysis reveals self-renewing and multipotent adult neural stem cell characteristics. Cell, 145(7), 1142–1155. 10.1016/j.cell.2011.05.024 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bonfanti L (2006). PSA-NCAM in mammalian structural plasticity and neurogenesis. Progress in Neurobiology, 80(3), 129–164. 10.1016/j.pneurobio.2006.08.003 [DOI] [PubMed] [Google Scholar]
- Boutros N, Der-Avakian A, Kesby JP, Lee S, Markou A, & Semenova S (2018). Effects of adolescent alcohol exposure on stress-induced reward deficits, brain CRF, monoamines and glutamate in adult rats. Psychopharmacology, 235(3), 737–747. 10.1007/s00213-017-4789-0 [DOI] [PubMed] [Google Scholar]
- Briones TL, & Woods J (2013). Chronic binge-like alcohol consumption in adolescence causes depression-like symptoms possibly mediated by the effects of BDNF on neurogenesis. Neuroscience, 254, 324–334. 10.1016/j.neuroscience.2013.09.031 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Broadwater MA, Liu W, Crews FT, & Spear LP (2014). Persistent loss of hippocampal neurogenesis and increased cell death following adolescent, but not adult, chronic ethanol exposure. Developmental Neuroscience, 36(3-4), 297–305. 10.1159/000362874 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brown JP, Couillard-Despres S, Cooper-Kuhn CM, Winkler J, Aigner L, & Kuhn HG (2003). Transient expression of doublecortin during adult neurogenesis. Journal of Comparative Neurology, 467(1), 1–10. 10.1002/cne.10874 [DOI] [PubMed] [Google Scholar]
- Burghardt NS, Park EH, Hen R, & Fenton AA (2012). Adult-born hippocampal neurons promote cognitive flexibility in mice. Hippocampus, 22(9), 1795–1808. 10.1002/hipo.22013 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cameron HA, & McKay RD (2001). Adult neurogenesis produces a large pool of new granule cells in the dentate gyrus. Journal of Comparative Neurology, 435(4), 406–417. 10.1002/cne.1040 [DOI] [PubMed] [Google Scholar]
- Cho KO, Lybrand ZR, Ito N, Brulet R, Tafacory F, Zhang L, … Hsieh J (2015). Aberrant hippocampal neurogenesis contributes to epilepsy and associated cognitive decline. Nature Communications, 6, 6606. 10.1038/ncomms7606 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chung T, Creswell KG, Bachrach R, Clark DB, & Martin CS (2018). Adolescent Binge Drinking. Alcohol Research & Health, 39(1), 5–15. https://pubmed.ncbi.nlm.nih.gov/30557142/ [PMC free article] [PubMed] [Google Scholar]
- Clelland CD, Choi M, Romberg C, Clemenson GD Jr., Fragniere A, Tyers P, … Bussey TJ (2009). A functional role for adult hippocampal neurogenesis in spatial pattern separation. Science, 325(5937), 210–213. 10.1126/science.1173215 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Coleman LG Jr., He J, Lee J, Styner M, & Crews FT (2011). Adolescent binge drinking alters adult brain neurotransmitter gene expression, behavior, brain regional volumes, and neurochemistry in mice. Alcoholism: Clinical & Experimental Research, 35(4), 671–688. 10.1111/j.1530-0277.2010.01385.x [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cortez I, Rodgers SP, Kosten TA, & Leasure JL (2020). Sex and Age Effects on Neurobehavioral Toxicity Induced by Binge Alcohol. Brain Plasticity, 6(1), 5–25. 10.3233/bpl-190094 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Crews FT, & Boettiger CA (2009). Impulsivity, frontal lobes and risk for addiction. Pharmacology, Biochemistry, & Behavior, 93(3), 237–247. 10.1016/j.pbb.2009.04.018 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Crews FT, Braun CJ, Hoplight B, Switzer RC 3rd, & Knapp DJ (2000). Binge ethanol consumption causes differential brain damage in young adolescent rats compared with adult rats. Alcoholism: Clinical & Experimental Research, 24(11), 1712–1723. 10.1111/j.1530-0277.2000.tb01973.x [DOI] [PubMed] [Google Scholar]
- Crews FT, Mdzinarishvili A, Kim D, He J, & Nixon K (2006). Neurogenesis in adolescent brain is potently inhibited by ethanol. Neuroscience, 137(2), 437–445. 10.1016/j.neuroscience.2005.08.090 [DOI] [PubMed] [Google Scholar]
- Crews FT, & Nixon K (2009). Mechanisms of neurodegeneration and regeneration in alcoholism. Alcohol & Alcoholism, 44(2), 115–127. 10.1093/alcalc/agn079 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Crews FT, Robinson DL, Chandler LJ, Ehlers CL, Mulholland PJ, Pandey SC, … Vetreno RP (2019). Mechanisms of Persistent Neurobiological Changes Following Adolescent Alcohol Exposure: NADIA Consortium Findings. Alcoholism: Clinical & Experimental Research, 43(9), 1806–1822. 10.1111/acer.14154 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cuartero MI, de la Parra J, Perez-Ruiz A, Bravo-Ferrer I, Duran-Laforet V, Garcia-Culebras A, … Moro MA (2019). Abolition of aberrant neurogenesis ameliorates cognitive impairment after stroke in mice. Journal of Clinical Investigation, 129(4), 1536–1550. 10.1172/JCI120412 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dash PK, Mach SA, & Moore AN (2001). Enhanced neurogenesis in the rodent hippocampus following traumatic brain injury. Journal of Neuroscience Research, 63(4), 313–319. [DOI] [PubMed] [Google Scholar]
- De Bellis MD, Clark DB, Beers SR, Soloff PH, Boring AM, Hall J, … Keshavan MS (2000). Hippocampal volume in adolescent-onset alcohol use disorders. American Journal of Psychiatry, 157(5), 737–744. 10.1176/appi.ajp.157.5.737 [DOI] [PubMed] [Google Scholar]
- De Bellis MD, Narasimhan A, Thatcher DL, Keshavan MS, Soloff P, & Clark DB (2005). Prefrontal cortex, thalamus, and cerebellar volumes in adolescents and young adults with adolescent-onset alcohol use disorders and comorbid mental disorders. Alcoholism: Clinical & Experimental Research, 29(9), 1590–1600. 10.1097/01.alc.0000179368.87886.76 [DOI] [PubMed] [Google Scholar]
- Denoth-Lippuner A, & Jessberger S (2021). Formation and integration of new neurons in the adult hippocampus. Nature Reviews Neuroscience, 22(4), 223–236. 10.1038/s41583-021-00433-z [DOI] [PubMed] [Google Scholar]
- Doetsch F, Garcia-Verdugo JM, & Alvarez-Buylla A (1999). Regeneration of a germinal layer in the adult mammalian brain. Proceedings of the National Academies of Science, 96(20), 11619–11624. 10.1073/pnas.96.20.11619 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Donovan JE (2009). Estimated blood alcohol concentrations for child and adolescent drinking and their implications for screening instruments. Pediatrics, 123(6), e975–981. 10.1542/peds.2008-0027 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Doremus TL, Brunell SC, Varlinskaya EI, & Spear LP (2003). Anxiogenic effects during withdrawal from acute ethanol in adolescent and adult rats. Pharmacology, Biochemistry, & Behavior, 75(2), 411–418. 10.1016/s0091-3057(03)00134-5 [DOI] [PubMed] [Google Scholar]
- Ehlers CL, Criado JR, Wills DN, Liu W, & Crews FT (2011). Periadolescent ethanol exposure reduces adult forebrain ChAT+IR neurons: correlation with behavioral pathology. Neuroscience, 199, 333–345. https://dx.doi.org/10.1016%2Fj.neuroscience.2011.10.011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ehlers CL, Liu W, Wills DN, & Crews FT (2013). Periadolescent ethanol vapor exposure persistently reduces measures of hippocampal neurogenesis that are associated with behavioral outcomes in adulthood. Neuroscience, 244, 1–15. 10.1016/j.neuroscience.2013.03.058 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ehlers CL, Oguz I, Budin F, Wills DN, & Crews FT (2013). Peri-adolescent ethanol vapor exposure produces reductions in hippocampal volume that are correlated with deficits in prepulse inhibition of the startle. Alcoholism: Clinical & Experimental Research, 37(9), 1466–1475. 10.1111/acer.12125 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Eriksson PS, Perfilieva E, Bjork-Eriksson T, Alborn AM, Nordborg C, Peterson DA, & Gage FH (1998). Neurogenesis in the adult human hippocampus. Nature Medicine, 4(11), 1313–1317. 10.1038/3305 [DOI] [PubMed] [Google Scholar]
- Esposito MS, Piatti VC, Laplagne DA, Morgenstern NA, Ferrari CC, Pitossi FJ, & Schinder AF (2005). Neuronal differentiation in the adult hippocampus recapitulates embryonic development. Journal of Neuroscience, 25(44), 10074–10086. 10.1523/jneurosci.3114-05.2005 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fernandez GM, & Savage LM (2017). Adolescent binge ethanol exposure alters specific forebrain cholinergic cell populations and leads to selective functional deficits in the prefrontal cortex. Neuroscience, 361, 129–143. 10.1016/j.neuroscience.2017.08.013 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gage FH (2000). Mammalian neural stem cells. Science, 287(5457), 1433–1438. 10.1126/science.287.5457.1433 [DOI] [PubMed] [Google Scholar]
- Gardella S, Andrei C, Ferrera D, Lotti LV, Torrisi MR, Bianchi ME, & Rubartelli A (2002). The nuclear protein HMGB1 is secreted by monocytes via a non-classical, vesicle-mediated secretory pathway. EMBO Rep, 3(10), 995–1001. 10.1093/embo-reports/kvf198 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gass JT, Glen WB Jr., McGonigal JT, Trantham-Davidson H, Lopez MF, Randall PK, … Chandler LJ (2014). Adolescent alcohol exposure reduces behavioral flexibility, promotes disinhibition, and increases resistance to extinction of ethanol self-administration in adulthood. Neuropsychopharmacology, 39(11), 2570–2583. 10.1038/npp.2014.109 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gebara E, Bonaguidi MA, Beckervordersandforth R, Sultan S, Udry F, Gijs PJ, … Toni N (2016). Heterogeneity of Radial Glia-Like Cells in the Adult Hippocampus. Stem Cells, 34(4), 997–1010. 10.1002/stem.2266 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Geil CR, Hayes DM, McClain JA, Liput DJ, Marshall SA, Chen KY, & Nixon K (2014). Alcohol and adult hippocampal neurogenesis: promiscuous drug, wanton effects. Progress in Neuropsychopharmacology & Biological Psychiatry, 54, 103–113. 10.1016/j.pnpbp.2014.05.003 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Giedd JN, Blumenthal J, Jeffries NO, Castellanos FX, Liu H, Zijdenbos A, … Rapoport JL (1999). Brain development during childhood and adolescence: a longitudinal MRI study. Nature Neuroscience, 2(10), 861–863. 10.1038/13158 [DOI] [PubMed] [Google Scholar]
- Golub HM, Zhou QG, Zucker H, McMullen MR, Kokiko-Cochran ON, Ro EJ, … Suh H (2015). Chronic Alcohol Exposure is Associated with Decreased Neurogenesis, Aberrant Integration of Newborn Neurons, and Cognitive Dysfunction in Female Mice. Alcoholism: Clinical & Experimental Research, 39(10), 1967–1977. 10.1111/acer.12843 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Goncalves JT, Schafer ST, & Gage FH (2016). Adult Neurogenesis in the Hippocampus: From Stem Cells to Behavior. Cell, 167(4), 897–914. 10.1016/j.cell.2016.10.021 [DOI] [PubMed] [Google Scholar]
- Gould E (1999). Serotonin and hippocampal neurogenesis. Neuropsychopharmacology, 21(2 Suppl), 46S–51S. 10.1016/S0893-133X(99)00045-7 [DOI] [PubMed] [Google Scholar]
- Grant BF, & Dawson DA (1997). Age at onset of alcohol use and its association with DSM-IV alcohol abuse and dependence: results from the National Longitudinal Alcohol Epidemiologic Survey. Journal of Substance Abuse, 9, 103–110. 10.1016/s0899-3289(97)90009-2 [DOI] [PubMed] [Google Scholar]
- Guerri C, & Pascual M (2019). Impact of neuroimmune activation induced by alcohol or drug abuse on adolescent brain development. International Journal of Developmental Neuroscience, 77, 89–98. 10.1016/j.ijdevneu.2018.11.006 [DOI] [PubMed] [Google Scholar]
- Hansson AC, Nixon K, Rimondini R, Damadzic R, Sommer WH, Eskay R, … Heilig M (2010). Long-term suppression of forebrain neurogenesis and loss of neuronal progenitor cells following prolonged alcohol dependence in rats. International Journal of Neuropsychopharmacology, 13(5), 583–593. 10.1017/s1461145710000246 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hastings NB, Seth MI, Tanapat P, Rydel TA, & Gould E (2002). Granule neurons generated during development extend divergent axon collaterals to hippocampal area CA3. Journal of Comparative Neurology, 452(4), 324–333. 10.1002/cne.10386 [DOI] [PubMed] [Google Scholar]
- Hayes DM, Nickell CG, Chen KY, McClain JA, Heath MM, Deeny MA, & Nixon K (2018). Activation of neural stem cells from quiescence drives reactive hippocampal neurogenesis after alcohol dependence. Neuropharmacology, 133, 276–288. 10.1016/j.neuropharm.2018.01.032 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Imayoshi I, Sakamoto M, Ohtsuka T, Takao K, Miyakawa T, Yamaguchi M, … Kageyama R (2008). Roles of continuous neurogenesis in the structural and functional integrity of the adult forebrain. Nature Neuroscience, 11(10), 1153–1161. 10.1038/nn.2185 [DOI] [PubMed] [Google Scholar]
- Jaworska N, & MacQueen G (2015). Adolescence as a unique developmental period. Journal of Psychiatry & Neuroscience, 40(5), 291. https://dx.doi.org/10.1503%2Fjpn.150268 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jin K, Wang X, Xie L, Mao XO, Zhu W, Wang Y, … Greenberg DA (2006). Evidence for stroke-induced neurogenesis in the human brain. Proceedings of the National Academies of Science, 103(35), 13198–13202. 10.1073/pnas.0603512103 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Karalay O, Doberauer K, Vadodaria KC, Knobloch M, Berti L, Miquelajauregui A, … Jessberger S (2011). Prospero-related homeobox 1 gene (Prox1) is regulated by canonical Wnt signaling and has a stage-specific role in adult hippocampal neurogenesis. Proceedings of the National Academies of Science, 108(14), 5807–5812. 10.1073/pnas.1013456108 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kee N, Sivalingam S, Boonstra R, & Wojtowicz JM (2002). The utility of Ki-67 and BrdU as proliferative markers of adult neurogenesis. Journal of Neuroscience Methods, 115(1), 97–105. 10.1016/s0165-0270(02)00007-9 [DOI] [PubMed] [Google Scholar]
- Kee N, Teixeira CM, Wang AH, & Frankland PW (2007). Imaging activation of adult-generated granule cells in spatial memory. Nature Protocols, 2(12), 3033–3044. 10.1038/nprot.2007.415 [DOI] [PubMed] [Google Scholar]
- Kempermann G, Gage FH, Aigner L, Song H, Curtis MA, Thuret S, … Frisen J (2018). Human Adult Neurogenesis: Evidence and Remaining Questions. Cell Stem Cell, 23(1), 25–30. 10.1016/j.stem.2018.04.004 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kempermann G, Song H, & Gage FH (2015). Neurogenesis in the Adult Hippocampus. Cold Spring Harbor Perspectives in Biology, 7(9), a018812. 10.1101/cshperspect.a018812 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kronenberg G, Reuter K, Steiner B, Brandt MD, Jessberger S, Yamaguchi M, & Kempermann G (2003). Subpopulations of proliferating cells of the adult hippocampus respond differently to physiologic neurogenic stimuli. Journal of Comparative Neurology, 467(4), 455–463. 10.1002/cne.10945 [DOI] [PubMed] [Google Scholar]
- Lacaille H, Duterte-Boucher D, Liot D, Vaudry H, Naassila M, & Vaudry D (2015). Comparison of the deleterious effects of binge drinking-like alcohol exposure in adolescent and adult mice. Journal of Neurochemistry, 132(6), 629–641. 10.1111/jnc.13020 [DOI] [PubMed] [Google Scholar]
- Laplagne DA, Esposito MS, Piatti VC, Morgenstern NA, Zhao C, van Praag H, … Schinder AF (2006). Functional convergence of neurons generated in the developing and adult hippocampus. Public Library of Science Biology, 4(12), e409. 10.1371/journal.pbio.0040409 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Larson TA, Thatra NM, Lee BH, & Brenowitz EA (2014). Reactive neurogenesis in response to naturally occurring apoptosis in an adult brain. Journal of Neuroscience, 34(39), 13066–13076. 10.1523/JNEUROSCI.3316-13.2014 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lavado A, Lagutin OV, Chow LM, Baker SJ, & Oliver G (2010). Prox1 is required for granule cell maturation and intermediate progenitor maintenance during brain neurogenesis. Public Library of Science Biology, 8(8), e1000460. 10.1371/journal.pbio.1000460 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lee D, Krishnan B, Zhang H, Park HR, Ro EJ, Jung YN, & Suh H (2019). Activity of hippocampal adult-born neurons regulates alcohol withdrawal seizures. Journal of Clinical Investigation Insight, 4(19). 10.1172/jci.insight.128770 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lees B, Meredith LR, Kirkland AE, Bryant BE, & Squeglia LM (2020). Effect of alcohol use on the adolescent brain and behavior. Pharmacology, Biochemistry, & Behavior, 192, 172906. https://dx.doi.org/10.1016%2Fj.pbb.2020.172906 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Little PJ, Kuhn CM, Wilson WA, & Swartzwelder HS (1996). Differential effects of ethanol in adolescent and adult rats. Alcoholism: Clinical & Experimental Research, 20(8), 1346–1351. 10.1111/j.1530-0277.1996.tb01133.x [DOI] [PubMed] [Google Scholar]
- Liu J, Solway K, Messing RO, & Sharp FR (1998). Increased neurogenesis in the dentate gyrus after transient global ischemia in gerbils. Journal of Neuroscience, 18(19), 7768–7778. 10.1523/jneurosci.18-19-07768.1998 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu W, & Crews FT (2017). Persistent Decreases in Adult Subventricular and Hippocampal Neurogenesis Following Adolescent Intermittent Ethanol Exposure. Frontiers in Behavioral Neuroscience, 11, 151. 10.3389/fnbeh.2017.00151 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Loxton D, & Canales JJ (2017). Long-term cognitive, emotional and neurogenic alterations induced by alcohol and methamphetamine exposure in adolescent rats. Progress in Neuropsychopharmacology & Biological Psychiatry, 74, 1–8. 10.1016/j.pnpbp.2016.11.003 [DOI] [PubMed] [Google Scholar]
- Luciana M, Collins PF, Muetzel RL, & Lim KO (2013). Effects of alcohol use initiation on brain structure in typically developing adolescents. Americal Journal of Drug & Alcohol Abuse, 39(6), 345–355. 10.3109/00952990.2013.837057 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mandyam CD, & Koob GF (2012). The addicted brain craves new neurons: putative role for adult-born progenitors in promoting recovery. Trends in Neuroscience, 35(4), 250–260. 10.1016/j.tins.2011.12.005 [DOI] [PMC free article] [PubMed] [Google Scholar]
- McClain JA, Hayes DM, Morris SA, & Nixon K (2011). Adolescent binge alcohol exposure alters hippocampal progenitor cell proliferation in rats: effects on cell cycle kinetics. Journal of Comparative Neurology, 519(13), 2697–2710. https://dx.doi.org/10.1002%2Fcne.22647 [DOI] [PMC free article] [PubMed] [Google Scholar]
- McClain JA, Morris SA, Marshall SA, & Nixon K (2014). Ectopic hippocampal neurogenesis in adolescent male rats following alcohol dependence. Addiction Biology, 19(4), 687–699. 10.1111/adb.12075 [DOI] [PMC free article] [PubMed] [Google Scholar]
- McQueeny T, Schweinsburg BC, Schweinsburg AD, Jacobus J, Bava S, Frank LR & Tapert SF (2009). Altered white matter integrity in adolescent binge drinkers. Alcoholism: Clinical & Experimental Research, 33(7), 1278–1285. https://dx.doi.org/10.1111%2Fj.1530-0277.2009.00953.x [DOI] [PMC free article] [PubMed] [Google Scholar]
- Melbourne JK, Thompson KR, Peng H, & Nixon K (2019). Its complicated: The relationship between alcohol and microglia in the search for novel pharmacotherapeutic targets for alcohol use disorders. Progress in Molecular Biology & Translational Science, 167, 179–221. 10.1016/bs.pmbts.2019.06.011 [DOI] [PubMed] [Google Scholar]
- Molowny A, Nacher J, & Lopez-Garcia C (1995). Reactive neurogenesis during regeneration of the lesioned medial cerebral cortex of lizards. Neuroscience, 68(3), 823–836. 10.1016/0306-4522(95)00201-s [DOI] [PubMed] [Google Scholar]
- Monti PM, Miranda R Jr., Nixon K, Sher KJ, Swartzwelder HS, Tapert SF, … Crews FT (2005). Adolescence: booze, brains, and behavior. Alcoholism: Clinical & Experimental Research, 29(2), 207–220. 10.1097/01.alc.0000153551.11000.f3 [DOI] [PubMed] [Google Scholar]
- Morris SA, Eaves DW, Smith AR, & Nixon K (2010). Alcohol inhibition of neurogenesis: a mechanism of hippocampal neurodegeneration in an adolescent alcohol abuse model. Hippocampus, 20(5), 596–607. 10.1002/hipo.20665 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Morris SA, Kelso ML, Liput DJ, Marshall SA, & Nixon K (2010). Similar withdrawal severity in adolescents and adults in a rat model of alcohol dependence. Alcohol, 44(1), 89–98. https://dx.doi.org/10.1016%2Fj.alcohol.2009.10.017 [DOI] [PMC free article] [PubMed] [Google Scholar]
- NIAAA, National Institute of Alcohol Abuse and Alcoholism (2012). Drinking Levels Defined. Retrieved from https://www.niaaa.nih.gov/alcohol-health/overview-alcohol-consumption/moderate-binge-drinking. [Google Scholar]
- Nagel BJ, Schweinsburg AD, Phan V, & Tapert SF (2005). Reduced hippocampal volume among adolescents with alcohol use disorders without psychiatric comorbidity. Psychiatry Research, 139(3), 181–190. 10.1016/j.pscychresns.2005.05.008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Naimi TS, Nelson DE, & Brewer RD (2010). The intensity of binge alcohol consumption among US adults. American Journal of Preventive Medicine, 38(2), 201–207. 10.1016/j.amepre.2009.09.039 [DOI] [PubMed] [Google Scholar]
- Nawarawong NN, Nickell CG, Hopkins DM, Pauly JR, & Nixon K (2021). Functional Activation of Newborn Neurons Following Alcohol-Induced Reactive Neurogenesis. Brain Sciences, 11(4), 499. https://dx.doi.org/10.3390%2Fbrainsci11040499 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nickell CG, Thompson KR, Pauly JR, & Nixon K (2020). Recovery of Hippocampal-Dependent Learning Despite Blunting Reactive Adult Neurogenesis After Alcohol Dependence. Brain Plasticity, 6(1), 83–101. https://dx.doi.org/10.3233%2FBPL-200108 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nickell CRG, Peng H, Hayes DM, Chen KY, McClain JA, & Nixon K (2017). Type 2 Neural Progenitor Cell Activation Drives Reactive Neurogenesis after Binge-Like Alcohol Exposure in Adolescent Male Rats. Frontiers in Psychiatry, 8, 283. 10.3389/fpsyt.2017.00283 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nixon K (2006). Alcohol and adult neurogenesis: roles in neurodegeneration and recovery in chronic alcoholism. Hippocampus, 16(3), 287–295. 10.1002/hipo.20162 [DOI] [PubMed] [Google Scholar]
- Nixon K, & Crews FT (2002). Binge ethanol exposure decreases neurogenesis in adult rat hippocampus. Journal of Neurochemistry, 83(5), 1087–1093. 10.1046/j.1471-4159.2002.01214.x [DOI] [PubMed] [Google Scholar]
- Nixon K, & Crews FT (2004). Temporally specific burst in cell proliferation increases hippocampal neurogenesis in protracted abstinence from alcohol. Journal of Neuroscience, 24(43), 9714–9722. 10.1523/JNEUROSCI.3063-04.2004 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nixon K, Kim DH, Potts EN, He J, & Crews FT (2008). Distinct cell proliferation events during abstinence after alcohol dependence: microglia proliferation precedes neurogenesis. Neurobiology of Disease, 31(2), 218–229. 10.1016/j.nbd.2008.04.009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nixon K, & McClain JA (2010). Adolescence as a critical window for developing an alcohol use disorder: current findings in neuroscience. Current Opinion in Psychiatry, 23(3), 227–232. 10.1097/yco.0b013e32833864fe [DOI] [PMC free article] [PubMed] [Google Scholar]
- Olsufka R, Peng H, Newton J, & Nixon K (2018). Alcohol effects on adult neural stem cells – a novel mechanism of neurotoxicity in alcohol use disorders. In Rasmussen T (Ed.), Stem Cells in Toxicology and Teratology. New York: John Wiley and Sons. 10.1002/9781119283249.ch8 [DOI] [Google Scholar]
- Palmer TD, Takahashi J, & Gage FH (1997). The adult rat hippocampus contains primordial neural stem cells. Molecular & Cellular Neuroscience, 8(6), 389–404. 10.1006/mcne.1996.0595 [DOI] [PubMed] [Google Scholar]
- Parent JM, Elliott RC, Pleasure SJ, Barbaro NM, & Lowenstein DH (2006). Aberrant seizure-induced neurogenesis in experimental temporal lobe epilepsy. Annals of Neurology, 59(1), 81–91. 10.1002/ana.20699 [DOI] [PubMed] [Google Scholar]
- Parent JM, Yu TW, Leibowitz RT, Geschwind DH, Sloviter RS, & Lowenstein DH (1997). Dentate granule cell neurogenesis is increased by seizures and contributes to aberrant network reorganization in the adult rat hippocampus. Journal of Neuroscience, 17(10), 3727–3738. 10.1523/jneurosci.17-10-03727.1997 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Patrick ME, Schulenberg JE, Martz ME, Maggs JL, O’Malley PM, & Johnston LD (2013). Extreme binge drinking among 12th-grade students in the United States: prevalence and predictors. Journal of the American Medical Association Pediatrics, 167(11), 1019–1025. 10.1001/jamapediatrics.2013.2392 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Patrick ME, & Terry-McElrath YM (2017). High-intensity drinking by underage young adults in the United States. Addiction, 112(1), 82–93. 10.1111/add.13556 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pfefferbaum A, Kwon D, Brumback T, Thompson WK, Cummins K, Tapert SF, … Sullivan EV (2018). Altered Brain Developmental Trajectories in Adolescents After Initiating Drinking. American Journal of Psychiatry, 175(4), 370–380. 10.1176/appi.ajp.2017.17040469 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sahay A, Scobie KN, Hill AS, O’Carroll CM, Kheirbek MA, Burghardt NS, … Hen R (2011). Increasing adult hippocampal neurogenesis is sufficient to improve pattern separation. Nature, 472(7344), 466–470. 10.1038/nature09817 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sakharkar AJ, Vetreno RP, Zhang H, Kokare DM, Crews FT, & Pandey SC (2016). A role for histone acetylation mechanisms in adolescent alcohol exposure-induced deficits in hippocampal brain-derived neurotrophic factor expression and neurogenesis markers in adulthood. Brain Structure and Function, 221(9), 4691–4703. 10.1007/s00429-016-1196-y [DOI] [PMC free article] [PubMed] [Google Scholar]
- Scharfman HE, Goodman JH, & Sollas AL (2000). Granule-like neurons at the hilar/CA3 border after status epilepticus and their synchrony with area CA3 pyramidal cells: functional implications of seizure-induced neurogenesis. Journal of Neuroscience, 20(16), 6144–6158. 10.1523/jneurosci.20-16-06144.2000 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schlessinger AR, Cowan WM, & Gottlieb DI (1975). An autoradiographic study of the time of origin and the pattern of granule cell migration in the dentate gyrus of the rat. Journal of Comparative Neurology, 159(2), 149–175. 10.1002/cne.901590202 [DOI] [PubMed] [Google Scholar]
- Seri B, Garcia-Verdugo JM, McEwen BS, & Alvarez-Buylla A (2001). Astrocytes give rise to new neurons in the adult mammalian hippocampus. Journal of Neuroscience, 21(18), 7153–7160. 10.1523/jneurosci.21-18-07153.2001 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shors TJ, Miesegaes G, Beylin A, Zhao M, Rydel T, & Gould E (2001). Neurogenesis in the adult is involved in the formation of trace memories. Nature, 410(6826), 372–376. 10.1038/35066584 [DOI] [PubMed] [Google Scholar]
- Sierra A, Martin-Suarez S, Valcarcel-Martin R, Pascual-Brazo J, Aelvoet SA, Abiega O, Encinas JM (2015). Neuronal hyperactivity accelerates depletion of neural stem cells and impairs hippocampal neurogenesis. Cell Stem Cell, 16(5), 488–503. 10.1016/j.stem.2015.04.003 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Snyder JS (2019). Recalibrating the Relevance of Adult Neurogenesis. Trends in Neuroscience, 42(3), 164–178. 10.1016/j.tins.2018.12.001 [DOI] [PubMed] [Google Scholar]
- Snyder JS, & Cameron HA (2012). Could adult hippocampal neurogenesis be relevant for human behavior? Behavioral Brain Research, 227(2), 384–390. 10.1016/j.bbr.2011.06.024 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Snyder JS, & Drew MR (2020). Functional neurogenesis over the years. Behavioral Brain Research, 382, 112470. 10.1016/j.bbr.2020.112470 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Snyder JS, Hong NS, McDonald RJ, & Wojtowicz JM (2005). A role for adult neurogenesis in spatial long-term memory. Neuroscience, 130(4), 843–852. 10.1016/j.neuroscience.2004.10.009 [DOI] [PubMed] [Google Scholar]
- Somkuwar SS, Fannon MJ, Staples MC, Zamora-Martinez ER, Navarro AI, Kim A,Mandyam CD (2016). Alcohol dependence-induced regulation of the proliferation and survival of adult brain progenitors is associated with altered BDNF-TrkB signaling. Brain Structure & Function, 221(9), 4319–4335. 10.1007/s00429-015-1163-z [DOI] [PMC free article] [PubMed] [Google Scholar]
- Spalding KL, Bergmann O, Alkass K, Bernard S, Salehpour M, Huttner HB, … Frisen J (2013). Dynamics of hippocampal neurogenesis in adult humans. Cell, 153(6), 1219–1227. 10.1016/j.cell.2013.05.002 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Spear LP (2018). Effects of adolescent alcohol consumption on the brain and behaviour. Nature Reviews Neuroscience, 19(4), 197–214. 10.1038/nrn.2018.10 [DOI] [PubMed] [Google Scholar]
- Spear LP, & Swartzwelder HS (2014). Adolescent alcohol exposure and persistence of adolescent-typical phenotypes into adulthood: a mini-review. Neuroscience & Biobehavioral Reviews, 45, 1–8. 10.1016/j.neubiorev.2014.04.012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sussman S, & Arnett JJ (2014). Emerging adulthood: developmental period facilitative of the addictions. Evaluation & the Health Professions, 37(2), 147–155. 10.1177/0163278714521812 [DOI] [PubMed] [Google Scholar]
- Swartzwelder HS, Healey KL, Liu W, Dubester K, Miller KM, & Crews FT (2019). Changes in Neuroimmune and Neuronal Death Markers after Adolescent Alcohol Exposure in Rats are Reversed by Donepezil. Scientific Reports, 9(1), 12110. 10.1038/s41598-019-47039-1 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Taffe MA, Kotzebue RW, Crean RD, Crawford EF, Edwards S, & Mandyam CD (2010). Long-lasting reduction in hippocampal neurogenesis by alcohol consumption in adolescent nonhuman primates. Proceedings of the National Academies of Science, 107(24), 11104–11109. 10.1073/pnas.0912810107 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tannenholz L, Jimenez JC, & Kheirbek MA (2014). Local and regional heterogeneity underlying hippocampal modulation of cognition and mood. Frontiers in Behavioral Neuroscience, 8, 147. 10.3389/fnbeh.2014.00147 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Toda T, Parylak SL, Linker SB, & Gage FH (2019). The role of adult hippocampal neurogenesis in brain health and disease. Molecular Psychiatry, 24(1), 67–87. 10.1038/s41380-018-0036-2 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Torcaso A, Asimes A, Meagher M, & Pak TR (2017). Adolescent binge alcohol exposure increases risk assessment behaviors in male Wistar rats after exposure to an acute psychological stressor in adulthood. Psychoneuroendocrinology, 76, 154–161. 10.1016/j.psyneuen.2016.11.032 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Towner TT, & Varlinskaya EI (2020). Adolescent Ethanol Exposure: Anxiety-Like Behavioral Alterations, Ethanol Intake, and Sensitivity. Frontiers in Behavioral Neuroscience, 14, 45. https://dx.doi.org/10.3389%2Ffnbeh.2020.00045 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vargas WM, Bengston L, Gilpin NW, Whitcomb BW, & Richardson HN (2014). Alcohol binge drinking during adolescence or dependence during adulthood reduces prefrontal myelin in male rats. Journal of Neuroscience, 34(44), 14777–14782. 10.1523/jneurosci.3189-13.2014 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Varlinskaya EI, & Spear LP (2002). Acute effects of ethanol on social behavior of adolescent and adult rats: role of familiarity of the test situation. Alcoholism: Clinical & Experimental Research, 26(10), 1502–1511. 10.1097/01.alc.0000034033.95701.e3 [DOI] [PubMed] [Google Scholar]
- Varlinskaya EI, & Spear LP (2004). Acute ethanol withdrawal (hangover) and social behavior in adolescent and adult male and female Sprague-Dawley rats. Alcoholism: Clinical & Experimental Research, 28(1), 40–50. 10.1097/01.alc.0000108655.51087.df [DOI] [PubMed] [Google Scholar]
- Vetreno RP, & Crews FT (2015). Binge ethanol exposure during adolescence leads to a persistent loss of neurogenesis in the dorsal and ventral hippocampus that is associated with impaired adult cognitive functioning. Frontiers in Neuroscience, 9, 35. https://dx.doi.org/10.3389%2Ffnins.2015.00035 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vetreno RP, & Crews FT (2018). Adolescent binge ethanol-induced loss of basal forebrain cholinergic neurons and neuroimmune activation are prevented by exercise and indomethacin. Public Library of Science One, 13(10), e0204500. 10.1371/journal.pone.0204500 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vetter-O’Hagen C, Varlinskaya E, & Spear L (2009). Sex differences in ethanol intake and sensitivity to aversive effects during adolescence and adulthood. Alcohol & Alcoholism, 44(6), 547–554. 10.1093/alcalc/agp048 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Walker DW, Barnes DE, Zornetzer SF, Hunter BE, & Kubanis P (1980). Neuronal loss in hippocampus induced by prolonged ethanol consumption in rats. Science, 209(4457), 711–713. 10.1126/science.7394532 [DOI] [PubMed] [Google Scholar]
- White AM, Kraus CL, & Swartzwelder H (2006). Many college freshmen drink at levels far beyond the binge threshold. Alcoholism: Clinical & Experimental Research, 30(6), 1006–1010. 10.1111/j.1530-0277.2006.00122.x [DOI] [PubMed] [Google Scholar]
- White AM, & Swartzwelder HS (2004). Hippocampal function during adolescence: a unique target of ethanol effects. Annals of the New York Academy of Science, 1021, 206–220. 10.1196/annals.1308.026 [DOI] [PubMed] [Google Scholar]
- White HR, Marmorstein NR, Crews FT, Bates ME, Mun EY, & Loeber R (2011). Associations between heavy drinking and changes in impulsive behavior among adolescent boys. Alcoholism: Clinical & Experimental Research, 35(2), 295–303. 10.1111/j.1530-0277.2010.01345.x [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yu TS, Zhang G, Liebl DJ, & Kernie SG (2008). Traumatic brain injury-induced hippocampal neurogenesis requires activation of early nestin-expressing progenitors. Journal of Neuroscience, 28(48), 12901–12912. 10.1523/jneurosci.4629-08.2008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zou J, & Crews FT (2012). Inflammasome-IL-1beta Signaling Mediates Ethanol Inhibition of Hippocampal Neurogenesis. Frontiers in Neuroscience, 6(77), 77. https://dx.doi.org/10.3389%2Ffnins.2012.00077 [DOI] [PMC free article] [PubMed] [Google Scholar]


