Abstract
Over the past decade, scientific evidence for the properties, functions, and beneficial effects of probiotics for humans has continued to accumulate. Interest in the use of probiotics for humans has increased tremendously. Among various microorganisms, probiotics using bacteria have been widely studied and commercialized, and, among them, Lactobacillus is representative. This genus contains about 300 species of bacteria (recently differentiated into 23 genera) and countless strains have been reported. They improved a wide range of diseases including liver disease, gastrointestinal diseases, respiratory diseases, and autoimmune diseases. Here, we intend to discuss in depth the genus Lactobacillus as a representative probiotic for chronic liver diseases.
Keywords: probiotics, liver disease, Lactobacillus
1. Introduction
The definition of probiotics has been changed constantly. The most recent definition is “live microorganisms, which when consumed in adequate amounts, confer a health effect on the host” [1]. Because of the properties of probiotics, many studies have been conducted on their effects on various diseases. For the safety of using probiotics, various guidelines have been established, including determining antibiotic resistance/susceptibility patterns [2]. Probiotics are already being used to treat or prevent human diseases, conditions, and syndromes. They have also been shown to have a positive effect on neuroinflammation and pain, as well as seasonal disease infections. They have a multifaceted effect, including a protective role in the gut. They compete with pathogens to produce direct antimicrobial effects and indirectly enhance intestinal barrier function [3]. They also modulate the host’s local and systemic mucosal immune systems and induce inhibitors of proinflammatory cytokine production [4,5]. Many mechanisms are affected depending on the strain specificity, even in the same species [6].
Many Lactobacillus have a long history of use in food. This is because Lactobacillus is a lactic acid-producing bacterium and has “generally recognized as safe” status. Currently, there is growing interest in its use as a dietary supplement for humans and animals [7]. Bacteria belonging to the genus are found in the oral cavity, intestines, and vagina [8,9,10]. Lactobacillus spp. could improve conditions such as gastrointestinal diseases, allergies, and liver disease through various mechanisms, such as producing metabolites that can directly inhibit pathogens, exhibiting immunomodulatory effects, and changing the intestinal microbiota [5,11,12,13]. Microorganisms in the gut are also known to affect liver disease. This is because the venous system of the portal circulation defines the gut−liver axis, and there is a close anatomical and functional interaction between the gastrointestinal tract and the liver [14]. The disease-improving effects of various probiotics have been confirmed in non-alcoholic and alcoholic liver disease.
This review will highlight the effects Lactobacillus has on various diseases, especially liver diseases. Finally, as probiotics, we provide insight into how Lactobacillus spp. works in various diseases, particularly liver disease.
2. Probiotics
Gut microbiome alteration using fecal material is an ancient practice. However, the application of specific strain-based methods is only five decades old. In 1965, the first probiotics definition emerged, which only referred to the bacterial products at that time and those known to promote the growth of other groups of bacteria [15]. Later, in 1989, living microbes were also included in probiotics; however, they were only linked with nutritional health. The newest definition of probiotics includes living microbes that can be ingested and produce beneficial health effects that are not only limited to nutritional outcomes. However, all of the above definitions indicate that probiotics produce beneficial health effects through various mechanisms such as improving the eubiosis, alleviating intestinal health, and strengthening the immune system [16]. Initially, probiotics were limited to Lactobacillus and Saccharomyces genera and presented positive preventive outcomes against Clostridium difficile infections [17].
The human microbiota, known for their intra-site changeable relationship, for instance parietal microbiota (microbes living in mucus layer and/or in intestinal wall), are closely related with luminal microbiota (microbes living in digested food and/or transit stool). Interestingly, microbiota composition is very dynamic and person specific, which can be influenced by diet, probiotic intake, intestinal environment, and other host-dependent factors that create some transiently new bacterial stains [18].
The close relationship between the microbiota and immune system deepens the understanding about microbial component involvement in energy homeostasis and glucose and lipid metabolism [19]. It is identified as a key regulatory mechanism that has is involved in the establishment and progression of various metabolic diseases by compromising the gut barrier function through changing the gut microbiota composition [16]. Moreover, particular types of alteration in the gut microbiota composition can lead to higher T cells accumulation in the gut of high-fat diet consuming obese individuals, and increases the obesity dependent mortality rate [20].
In addition, the gut microbiota is crucial for the regulation of cognitive functions. Multiple numbers of human and animal trials represent the pivotal role of the gut microbiota in the development of cognitive functions, regulation of emotions, and in making the person to person communication by powering the neuronal-immune system, which can help in neuronal cell differentiation, synaptic plasticity, and axonal development [21].
In contrast, gut microbial compositional impairment has been related to numerous psychiatric disorders like depression, autism, alcohol-related encephalopathy, and other disorders. For example, the depressed patients’ gut microbiota is less diverse, which can possibly help to increase the proinflammatory status and cortisol level, and alter the tryptophan metabolism. Moreover, the fecal microbiota transplantation from these depressed patients into the microbiota depleted animal model mimics the depression associated with pathophysiological and behavioral characteristics similar to patients [22]. These interesting findings about a close association between the gut and brain open a new avenue of probiotics-based psychopathological intervention via modulating the gut composition. In 1910, an improvement in depression related psychopathology was observed with the supplementation of Lactobacillus [16]. Likewise, supplementation with L. fermentum and/or L. plantarum also showed positive and beneficial health effects in hospitalized patients, and reduced the colonization of nosocomial multi-drug resistance bacterial strains such as Pseudomonas aeruginosa, Acinetobacter baumannii, or Candida albicans [23,24].
Surprisingly, probiotic’s health boosting effects are not limited to their strain level, they are extended to their metabolites; for an example, supernatant collected from probiotics liquid culture are also capable of limiting the growth as well as resistance gene transmission of carbapenemase-producing and extended-spectrum β-lactamase-carrying Enterobacteriaceae [25]. The microbiota act in a multidirectional manner at the same time by limiting the expression of the virulence factor-related genes and enhancing the expression level of commensalism associated genes. These multidirectional functionalities of the microbiota can be regulated by various mechanisms: microbiota produced bioactive molecules, antiaging, boosting the immune system, by influencing the adnexal development, strengthening the sensory functions, etc. [26]. Additionally, local skin application of probiotics bacterial strains L. acidophilus, L. bulgaricus, and/or L. plantarum improve skin health and reduce acne through controlling the skin colonization of Cutibacterium acnes [27,28]. In addition, the gut microbiota has a positive influence on drug metabolism, minimizing therapeutic side effects and hepatic health [29]. The lysosomal enzyme β-glucuronidases released by Bacteroides vulgatus, Escherichia coli, and Clostridium ramosum re-activate irinotecan from its inactive state as glucuronide, which is excreted via the bile duct with bile acid directly into the gastrointestinal tract in its toxic form, and is able to cause severe digestive damage [30,31]. The mechanisms of these various probiotics have been demonstrated through preclinical and clinical trials, and among them, Lactobacillus is one of the most actively studied among probiotics.
3. Mechanisms and Applications of Lactobacillus as a Probiotic
There are 315 species belonging to the genus Lactobacillus (recently reclassified into 23 genera), and many of these bacteria have been reported as probiotics. L. acidophilus, L. casei, L. johnsonii, L. reuteri, and L. rhamnosus, which belong to Lactobacillus, are actively studied as probiotics. Most of them are resistant to gastric acid and have good adhesion to intestinal cells. For this reason, they have been applied to modulate many diseases, including gastrointestinal diseases (Table 1).
Table 1.
Use of Lactobacillus as probiotics for various diseases.
| Classification of Diseases | Disease or Pathogen | Subject | Probiotics | Outcomes | Ref. |
|---|---|---|---|---|---|
| Gastrointestinal diseases | C. difficile | Human | L. paracasei F19 | Reduced the population of C. difficile, which can cause diarrhea and enteritis. | [32] |
| Acute watery diarrhea | Human | L. rhamnosus GG | Effective in reducing the frequency and duration of diarrhea in patients with different concentrations of the bacterium (1010 and 1012). | [33] | |
| Ulcerative colitis | Human | L. rhamnosus GG | Effective and safe for maintaining remission in patients with ulcerative colitis. | [34] | |
| Functional bowel disorders | Human | L. acidophilus NCFM (combined with another bacterium) | Improved symptoms of bloating. | [35] | |
| Colitis | Mouse | L. acidophilus, L. bulgaricus, L. casei, L. plantarum (combined with other bacteria) | Improved dextran sulfate sodium induced colitis. | [36] | |
| Allergy | Allergic sensitization | Mouse | VSL#3 | Reduced systemic and local anaphylactic symptoms by oral challenge with the sensitizing allergen Shrimp Tropomyosin. | [37] |
| Atopic dermatitis | Human | L. salivarius LS01 | Improved in scoring atopic dermatitis and itch values from baseline. | [38] | |
| Perennial allergic rhinitis | Human | L. acidophilus L-92 | Alleviated the symptoms. | [39] | |
| Allergic rhinitis | Human | L. paracasei KW3110 | Reduction of nasal symptoms and the serum level of eosinophil cationic protein and improvement of quality-of-life scores when pollen scattering was low. | [13] | |
| Food allergy (peanut) | Mouse |
L. salivarius HMI001, L. casei Shirota |
Partial protection in a mouse peanut allergy model. | [40] | |
| Respiratory diseases | Gastrointestinal and respiratory tract infections | Human | L. rhamnosus GG | Reduced risk of upper respiratory tract infections, respiratory tract infections, and number of days with respiratory symptoms. | [11] |
| Diarrhea and respiratory tract infection | Human | L. reuteri DSM 17938 | Reduced the frequency and duration of diarrhea and respiratory infections, and consequently reduced costs for the community. | [41] | |
| Pneumococcal respiratory infection | Mouse | L. casei CRL 431 | Accelerated the recovery of the innate immune system. | [42] | |
| Chronic asthma | Mouse | L. rhamnosus NutRes1 | Reduced lung resistance in a mouse model of chronic asthma to a similar extent to budesonide treatment. | [43] | |
| Chronic obstructive pulmonary disease | Mouse | L. rhamnosus | Regulates pro- and anti-inflammatory cytokines balance in human bronchial epithelial cells and alleviates pulmonary inflammatory responses. | [44] | |
| Neurological and psychiatric diseases | Neurological and psychiatric diseases | Mouse | L. rhamnosus JB-1 | Reduced stress-induced corticosterone and anxiety- and depression-related behaviors. | [45] |
| Neurological and psychiatric diseases | Human/Rat | L. helveticus R0052 (combined with another bacterium) | Anxiolytic-like activity in rats, beneficial psychological effects in healthy humans. | [46] | |
| Neurological and psychiatric diseases | Mouse | L. casei, L. acidophilus, L. reuteri (combined with other bacteria) (IRT5) | Suppressed experimental autoimmune encephalomyelitis. | [47] | |
| Autoimmune myasthenia gravis | Rat | IRT5 | Prevented the development of experimental autoimmune myasthenia gravis. | [48] | |
| Autism spectrum disorder | Human | L. acidophilus Rosell-11 | Reduced D-arabinitol level and D-/L-arabinitol ratio in urine and improved concentration and carrying out orders. | [49] | |
| Genito-Urinary tract infections | Bacterial vaginosis | Mouse | L. johnsonii HY7042 | Inhibited myeloperoxidase activity in vaginal tissue and reduced viable numbers of Gardnerella vaginalis. | [12] |
| Bacterial vaginosis | Human | L. rhamnosus BMX 54 | Reduced recurrence rate and reduced pH. | [50] | |
| Urinary tract infections | Human | L. crispatus CTV-05 | Reduced recurrence. | [51] | |
| Metabolic syndrome | Type 1 diabetes | Rat | L johnsonii N6.2 | Mitigated the development of type 1 diabetes. | [52] |
| Type 2 diabetes mellitus | Human |
L. reuteri ADR-1, L. reuteri ADR-3 |
Beneficial effect on patients. | [53] | |
| Obesity | Mouse | L. gasseri BNR17 | Decreased leptin and insulin levels in serum and showed anti-obesity effects. | [54] | |
| Cardiovascular disease | Rat |
L. plantarum DMDL 9010 |
Decreased serum and total liver cholesterol and triglyceride and enhanced fecal excretion of bile acids. | [55] | |
| Oral diseases | Gingivitis | Human |
L. reuteri ATCC 55730, L. reuteri ATCC PTA 5289 |
Decreased bleeding on probing and gingival crevicular fluid during chewing gums containing probiotics. | [56] |
| Periodontitis | Human |
L. reuteri DSM 17938, L. reuteri ATCC PTA 5289 |
Improved clinical parameters and reduced abundance of pathogenic bacterium. | [57] | |
| Dental caries | Human | L. rhamnosus GG | Reduced the risk of caries and lowered mutans Streptococcus counts. | [58] | |
| Halitosis | Human | L. salivarius WB21 | Decreased an organoleptic test and BOP. | [59] | |
| Oral candidiasis | In vitro |
L. fermentum 20.4, L. paracasei 28.4, L. rhamnosus 5.2 |
Inhibited biofilms of Candida albicans. | [60] | |
| Autoimmune diseases | Rheumatoid arthritis | Rat | L. casei | Suppressed collagen-induced arthritis and reduced destruction of cartilage tissue, paw swelling, and lymphocyte infiltration. | [61] |
| Systemic lupus erythematosus | Mouse | L. fermentum CECT5716 | Reduced activity of lupus disease. | [62] | |
| Inflammatory bowel disease | Mouse |
L. paracasei 1602, L. reuteri 6798 |
Reduced intestinal inflammation Helicobacter hepaticus-challenged IL-10-deficient mice. | [63] | |
| Others | Osteoporosis | Mouse |
L. acidophilus ATCC 4356 |
Increased bones’ mineral density and heterogeneity and enhanced trabecular and cortical bone microstructure. | [64] |
| Tumor cells | In vitro | L. plantarum 70810 | Inhibited the proliferation of tumor cells. | [65] | |
| Vaccine adjuvant | Human | L. rhamnosus GG | Had a protective titer 28-day-after vaccination. | [66] |
3.1. Mode of Action as a Probiotic
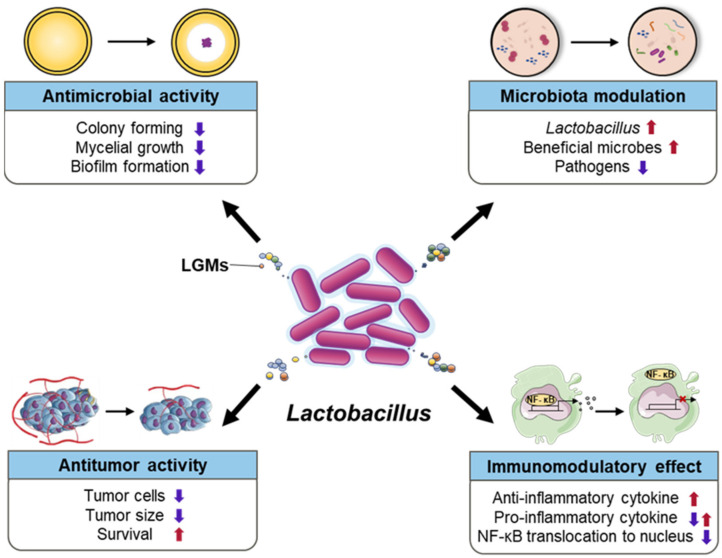
The disease alleviation effect was confirmed by preclinical and clinical trials using various species and strains belonging to the genus Lactobacillus. As they are effective in various diseases, various mechanisms are being elucidated. Representatively, antimicrobial activity, immunomodulatory effects, microbiota modulation, metabolites, and antitumor activity have been noted as their mechanisms of action (Figure 1).
Figure 1.
Various disease control mechanisms of the genus Lactobacillus. LGMs indicate Lactobacillus generated molecules.
Many Lactobacillus strains inhibit the growth of pathogens with antimicrobial activity [60,67]. L. paracasei 28.4, L. fermentum 20.4, and L. rhamnosus showed antimicrobial activity against C. albicans, an opportunistic pathogenic yeast. When these Lactobacillus were co-incubated with C. albicans, the mycelial growth of C. albicans was delayed and biofilm formation was inhibited. In addition, the expression of biofilm-specific genes was reduced in the pathogen [60]. In addition, 12 Lactobacillus strains exhibited an antagonistic activity against pathogenic microorganisms, C. albicans (ATCC 44831), Enterococcus faecium (ATCC 51558), Enterobacter cloacae, E. coli (ATCC 29181), Helicobacter pylori (ATCC 43579), Listeria monocytogenes, Propionibacterium acnes (ATCC 6919), Shigella sonnei (ATCC 25931), Staphylococcus epidermidis (ATCC 12228), and Vibrio parahaemolyticus, and the activity was strain dependent [67]. This inhibitory effect on pathogenic microorganisms is related to various bacteriocins, through metabolites produced by Lactobacillus spp. [68,69].
Lactobacillus could also improve the disease through immunomodulatory effects. L. rhamnosus ATCC 53103 increased the respiratory burst activity of blood cells. In addition, this strain significantly increased the serum-mediated killing of E. coli and serum immunoglobulin levels [70]. In mice, L. acidophilus LAFTI L10 and L. paracasei LAFTI L26 increased the number of immunoglobulin A (IgA), interleukin-10 (IL-10), and interferon-gamma (IFN-γ) cytokine producing cells in the small intestine. In addition, the secretion of anti-inflammatory cytokines (IL-10) and proinflammatory cytokines (IFN-γ) was increased in the systemic immune response [71]. Similarly, in a mouse peanut allergy model, L. salivarius HMI001 and L. casei Shirota (LCS) showed a partial protective effect with a high IL-10/IL-12 ratio and with high IFN-γ and IL-12, respectively [40].
In human study, L. paracasei F19 significantly increased its own population while decreasing the population of the pathogen Clostridium difficile in fecal samples [32]. This supported that Lactobacillus could modulate the microbiota; therefore, microbiota modulation by probiotics is being studied as one of the mechanisms to alleviate various diseases. Lactobacillus attenuated the progression of obesity-induced non-alcoholic fatty liver disease by regulating the microbiome [72]. In addition, the vaginal microbiota of nonpregnant sexually active women with diagnoses of bacterial vaginosis receiving L. rhamnosus BMX 54 was different from that of the untreated group. In the receiving BMX 54 group, the recurrence rate of bacterial vaginosis was also significantly reduced [50].
The immune response could also lead to antitumor activity. When L. casei was orally administered, human papillomavirus type 16 E7 protein (HPV 16 E7) specific serum IgG and mucosal IgA production were enhanced. Systemic and local cellular immunity increased, as demonstrated by the increased IFN-γ in the supernatants of vaginal lymphocytes and increased counts of IFN-γ and lymphocyte-secreting cells among splenocytes. In an E7-based mouse tumor model, L. casei reduced the tumor size and increased survival [73]. A novel exopolysaccharide (EPS) from L. plantarum 70810, a metabolite, also exhibited antitumor activity. In vitro, cell bound EPS, a novel EPS of this strain, inhibited the proliferation of HepG-2, BHC-823, and HT-29 tumor cells [65].
As shown here, disease inhibition by Lactobacillus spp. is not limited to one mechanism, but is highly likely to occur due to a series of actions of various mechanisms. In addition, their disease alleviating effect and related mechanisms might vary depending on the bacterial strains, not just the bacterial species level.
3.2. Properties as a Probiotic against Various Diseases
Lactobacillus is applicable to a wide range of diseases, such as gastrointestinal diseases, allergies, respiratory diseases, neurological and psychiatric diseases, liver diseases, genito-urinary infections, metabolic syndrome, cardiovascular diseases, obesity, cancer, oral disease, and vaccine adjuvants (Table 1).
There are some cases in which Lactobacillus has been applied to treat gastrointestinal diseases. Lactobacillus can reduce the abundance of the gastrointestinal microbiota. L. paracasei F19 was administered to children and elderly subjects twice a day for 12 weeks. The population of C. difficile was decreased in the fecal samples of the treated subjects [32]. C. difficile is a bacterium that produces toxins that cause diarrhea and enteritis [74]. Similarly, in 559 patients with acute watery diarrhea (AWD), the frequency and duration of diarrhea in the group receiving L. rhamnosus GG (LGG) along with an oral rehydration solution were significantly reduced compared with those in the control group receiving oral rehydration solution only [33]. This strain is safe and effective for maintaining remission in ulcerative colitis as well as in AWD [34].
In clinical practice, other bacteria are used together with Lactobacillus. Bifidobacterium lactis Bi-07 along with L. acidophilus NCFM was administered to 60 patients with functional bowel disorders (probiotics, n = 31; placebo, n = 29). Significantly improved bloating severity was observed in patients treated with the probiotics compared with the placebo controls [35]. The probiotic VSL#3, a mixture of more diverse bacteria, including L. acidophilus, L. casei, L. bulgaricus, and L. plantarum, alleviated dextran sulfate sodium-induced colitis in weanling rats. VSL#3 also contained Streptococcus thermophilus and three Bifidobacterium species (e.g., longum, infantis, and breve) [36]. Moreover, VSL#3 reduced both systemic and local anaphylaxis induced by the sensitizing allergen shrimp tropomyosin through oral treatment [37].
Other Lactobacillus species have also been shown to alleviate various allergy-related diseases, including atopic dermatitis, rhinitis, and food allergies. Probiotic treatment using L. salivarius LS01 was performed for children aged 0 to 11 years with atopic dermatitis (n = 43). Patients receiving this treatment had significant improvements in atopic dermatitis (SCORAD) scores and itch values from the baseline clinical parameters [38]. In patients with perennial allergic rhinitis (n = 49) who received milk fermented with L. acidophilus strain L-92, their nasal symptom-medication score was significantly improved compared to the placebo group (n = 24). In addition, swelling and color scores of nasal mucosae were clearly decreased [39]. Similar results were observed in Japanese cedar pollinosis patients who received L. paracasei strain KW3110 daily (n = 69). Japanese cedar pollinosis is known to be an important cause of allergic rhinitis. Patients who received KW3110 daily had significantly decreased nasal symptoms and serum levels of eosinophil cationic protein, and also increased quality of life scores compared with the placebo controls (n = 69). However, it was only effective when pollen scattering was low, and the effect was limited during the peak period of pollen scattering [13]. In addition, Lactobacillus alleviated food-induced allergies. Its prophylactic potential was investigated in a peanut sensitization model using L. salivarius HMI001 and LCS strains. Both strains showed partial protection in a mouse peanut allergy model [40].
Lactobacillus also had an inhibitory effect on respiratory infection. The group was randomly divided into the placebo group (n = 142) who consumed post-pasteurized fermented milk products without LGG, and the LGG group (n = 139) who consumed 100 mL of milk fermented with LGG. Compared to the placebo group, the LGG group significantly reduced their risk of upper respiratory tract infections. In addition, the number of days of respiratory symptoms was also significantly reduced [11]. L. reuteri DSM17938 also showed similar effects as LGG. The group that received the strain (n = 168) and a placebo group (n = 168) were administered the treatment daily for 3 months, after which they were followed up without supplementation for 3 months. The bacteria-treated group had a significantly decreased frequency and duration of diarrhea and respiratory infections at both 3 and 6 months than the placebo group [41]. Another species of Lactobacillus, L. casei CRL 431, showed an improvement effect on pneumoniae respiratory infection. The strain reduced the time required for a normal immune response from 21 days to 7 days, had effective pathogen clearance, and significantly reduced lung damage [42]. Lactobacillus also alleviated chronic asthma in a mouse model. In mice orally administered L. rhamnosus NutRes1, basal lung resistance was significantly increased compared to the control group that was orally administered PBS [43]. This species also attenuates cigarette smoke-induced chronic obstructive pulmonary disease (COPD). In the COPD group, which was orally administered L. rhamnosus three times per week, the influx of inflammatory cells into the airways was inhibited compared to that in the COPD group that did not receive L. rhamnosus. It was involved with various immune responses [44].
A positive effect of Lactobacillus on neurological and psychiatric diseases has been reported. When mice were chronically treated with L. rhamnosus JB-1, region-dependent alterations in GABAB1b mRNA were induced in the brain with increases in cortical regions and concomitant reductions in expression in the hippocampus, amygdala, and locus coeruleus compared to the controls. This bacterial strain, importantly, reduced stress-induced corticosterone and anxiety- and depression-related behaviors [45]. Psychotropic-like properties were shown in rats and healthy human volunteers fed L. helveticus R0052 combined with another bacterial strain (B. longum R0715). An anxiolytic-like activity was demonstrated when probiotics were administered daily for 2 weeks to anti-anxiety agents in a selection rat model. In addition, when these probiotics were administered to healthy clinical trial volunteers for 30 days, psychological distress was alleviated with statistically significant differences compared to baseline in the Hopkins symptom checklist, hospital anxiety and depression scale, and coping checklist [46]. The Probiotic IRT5 containing L. casei, L. acidophilus, and L. reuteri, which are known to have a positive effect against various diseases, reduced the incidence of experimental autoimmune encephalomyelitis (EAE). IRT5 also contained B. bifidum and S. thermophilus. Treatment with IRT5 before disease induction significantly inhibited the occurrence of EAE, and treatment with IRT5 for ongoing EAE delayed disease onset [47]. The probiotics also exhibited a protective effect against experimental autoimmune myasthenia gravis (EAMG). Oral administration of IRT5 probiotics five times per week significantly reduced symptoms of EAMG, such as weight loss, body tremors, and grip strength [48]. Another probiotic including L. acidophilus Rosell-11 affected arabinitol levels and behavior in autistic children. Autistic children who received the probiotic twice a day for 2 months had improved concentration and a better ability to follow instructions. In addition, the ratio of D-arabinitol to D-/L-arabinitol, which was high in autistic children, decreased in the urine [49].
Lactobacillus is also known for its inhibitory effect on genitourinary infections. The oral and intravaginal administration of L. johnsonii HY7042 to mice, induced to have vaginosis with Gardnerella vaginalis, had an inhibited myeloperoxidase activity in vaginal tissues, and a reduced population of G. vaginalis [12]. A positive effect of Lactobacillus on vaginosis was also observed in human experiments. Patients receiving L. rhamnosus BMX 54 (n = 125) had a significantly lower recurrence rate than subjects receiving only antibiotic treatment (n = 125). In addition, patients who received continuous supplementation during follow-up showed a significant decrease in pH compared to other subjects [50]. L. crispatus CTV-05 was effective at preventing urinary tract infection (UTI). After antibiotic treatment, the recurrence rate of UTIs was reduced from 27% in patients receiving placebo to 15% in patients receiving CTV-05. High levels of vaginal colonization of this strain throughout follow-up were associated with recurrent UTIs [51].
Probiotics have also been reported to have a disease-alleviating effect on metabolic syndromes such as diabetes and obesity. The incidence of diabetes was decreased in rats receiving L. johnsonii N6.2 daily [52]. When two bacterial strains, ADR-1 and ADR-3, of L. rhamnosus were orally administered to patients with type 2 diabetes mellitus (T2DM), significant reductions in HbA1c and serum cholesterol were observed in the ADR-1 group (n = 22) compared to the placebo group (n = 22). There was no significant difference in HbA1c serum levels in the heat-killed ADR-3 intake group (n = 24), however, the systolic and mean blood pressures were significantly decreased after 6 months of treatment with probiotics [53]. Metabolic syndrome is closely related to obesity. Mice receiving L. gasseri BNR17 along with a high-sucrose-diet had reduced body weight and reduced white adipose tissue weight. In addition, the mRNA level of fatty acid oxidation-related genes was significantly higher and that of fatty acid synthesis-related genes was lower than that of the high-sucrose-diet group [54]. Some species of Lactobacillus reduce the risk factors associated with cardiovascular disease. The serum total cholesterol, low-density lipoprotein cholesterol content levels, and atherosclerosis index were significantly decreased in rats fed L. plantarum DMDL9010. In addition, when morphological and pathological changes in the liver were observed, it was found to have a protective effect against hepatocellular steatosis. Decreasing of hepatic cholesterol and triglyceride levels and increasing of fecal excretion of bile acids were also observed [55].
A positive therapeutic effect of Lactobacillus is also observed in various oral diseases. The effect of L. reuteri ATCC 55730 and ATCC PTA5289 was confirmed in the group that received chewing gum containing probiotics and one placebo gum (n = 15), the group that received two gums containing each strain (n = 14), and the group that received two placebo gums (n = 13) for 2 weeks. Bleeding on probing (BOP) and gingival crevicular fluid were significantly improved in the two groups that received chewing gums containing probiotics compared to the placebo group [56].
Lactobacillus is known to suppress diseases through immune responses to various autoimmune diseases. In rats fed L. casei, collagen-induced arthritis was suppressed, and cartilage tissue destruction, paw swelling, and lymphocyte infiltration were reduced [61]. In mice, L. fermentum CECT5716 decreased the activity of lupus disease, blood pressure, cardiac and renal hypertrophy, and splenomegaly. This strain also reduced elevated T, B, regulatory T cells, T helper cells in mesenteric lymph nodes, and plasma lipopolysaccharide (LPS) levels [62]. Mice fed L. paracasei 1602 and L. reuteri 6798 showed reduced intestinal inflammation in IL-10-deficient mice compared with animals co-colonized with Helicobacter hepaticus at similar levels. In addition, proinflammatory colonic cytokine levels were reduced in the Lactobacillus spp. treated group [63].
Lactobacillus has been suggested to have a therapeutic effect against osteoporosis and tumors and to act as a vaccine adjuvant. In L. acidophilus-administrated ovariectomized mice, the bone mineral density and heterogeneity were increased and both the trabecular and cortical bone microstructure were enhanced [64]. Cell-bound EPS of L. plantarum 70810 was tested in vitro. The proliferation of HepG-2, BGC-823, and especially HT-29 tumor cells was significantly inhibited [65]. In a randomized double-blind placebo-controlled trial, LGG showed a protective titer of 1.84, a 95% confidence interval 1.04–3.22, and a p value of 0.048 compared to the control group 28 days after vaccination [66].
4. Effect of Lactobacillus in Liver Disease
The use of Lactobacillus as probiotics for liver diseases, NAFLD or ALD, are summarized in Table 2.
Table 2.
Use of Lactobacillus as probiotics for liver diseases.
| Subject | Disease | Treatment | Main Effect | Ref. |
|---|---|---|---|---|
| Animal | NAFLD | L. gasseri SBT2055 | (↓): body weight, pro-inflammatory (CCL2, CCR2, TNF), LPS (↑): intestinal barrier function, permeability |
[75] |
| L. rhamnosus GG | (↓): ALT, liver inflammation (IL-8R, IL-1β) and steatosis, LPS, TNF-α (↑): chREBP, FAS, ACC1 total numbers of the distal small intestinal microbiota, major tight junction proteins (occludin and claudin-1) |
[76] | ||
| L. plantarum CQPC03 | (↓): hepatic tissue damage, hepatic triglyceride, total cholesterol, IL-6, IL-1β, TNF-α, interferon-γ (↑): HDL-C, IL-4, IL-10, SOD, GSH-Px, lipoprotein lipase |
[77] | ||
| L. plantarum NCU116 | (↓): liver enzymes, bilirubin, IL-6, TNF-α, IL-10, oxidative stress, fat accumulation in the liver, lipogenesis, LPS (↑): fatty acid oxidation |
[78] | ||
|
L. casei pWQH01, L. plantarum AR113 |
(↓): Body weight, total cholesterol, atherogenic index, small heterodimer partner, farnesoid X receptor (↑): cholesterol 7α-hydroxylase, liver X receptor, lipoprotein receptor |
[79] | ||
| ALD |
L. acidophilus KLDS1, L. plantarum KLDS1.0344 |
(↓): liver enzymes, LPS, oxidative stress, inflammation, lipid accumulation (↑): intestinal epithelial permeability |
[80] | |
|
L. rhamnosus R0011, L. acidophilus R0052 |
(↓): TNF-α, IL-1β, IL-6, TLR4 expression, IL-10 | [81] | ||
| L. rhamnosus GG | (↓): TNF-α, CYP2E1, LPS, phosphorylation of p38 MAP kinase, nuclear factor erythroid 2-related factor 2 expression | [82] | ||
| L. reuteri | (↓): liver enzymes, lipid accumulation, inflammation, LPS, (↑): ZO-1, linoleic acid, arachidonic acid |
[83] | ||
| Fibrosis | L. rhamnosus GG | (↓): Hepatic bile acid, liver inflammation, liver injury, hepatic cholesterol 7α-hydroxylase (↑): expression of serum and ileum fibroblast growth factor 15 |
[84] | |
|
L. paracasei, L. casei |
(↓): inflammation, TNF-α, TGF-β1, α-SMA proteins, Col1a1, Acta2, Timp1, TGF-β | [85] | ||
| Cirrhosis | L. salivarius LI01 | (↓): Serum endotoxin, bacterial translocations, TNF-α, IL-6, IL-17A, TLR2, TLR9, TLR5, liver enzymes (↑): Intestinal barrier |
[86] | |
| HCC | L. rhamnosus GG | (↓): IL-17, recruitment of Th17 from gut to tumor sites, tumor progression (↑): IL-10, antitumor function |
[87] | |
| L. acidophilus | (↓): oncogene, MiR-122, oncomir, tumor suppressor gene (↑): tumor suppressor gene |
[88] | ||
| L. acidophilus LA14 | (↓): liver enzymes, bile acid, histological injury to the gut and liver, inflammatory cytokines | [89] | ||
| L. acidophilus ATCC 4356 | (↓): liver enzymes, IL-17, TGF-β1 | [90] | ||
| Human | NAFLD | L. rhamnosus GG | (↓): BMI, ALT, TNF-α, alanine aminotransferase, antipeptidoglycan-polysaccharide antibodies | [91] |
| NAFLD/NASH | L. acidophilus | (↓): liver enzymes, dyspepsia | [92] | |
| NASH | L. reuteri + inulin | (↓): Body weight, waist circumference, BMI | [93] | |
| ALD | L. casei | (↓): TG, LDL-C, liver enzymes, TNF-α, IL-1β, IL-6, intestinal flora imbalance (↑): Amount of Lactobacillus and Bifidobacterium in the intestinal flora, improve lipid metabolism |
[94] | |
| Cirrhosis | L. rhamnosus GG | (↓): endotoxemia, dysbiosis, TNF-α | [95] |
(↓) indicates a decrease in condition; (↑) indicates an increase in condition; NAFLD: non-alcoholic fatty liver disease; ALD: alcoholic liver disease; HCC: hepatocellular carcinoma; CCL2: chemokine ligand 2; CCR2: C-C chemokine receptor type 2; TNF: tumor necrosis factor; LPS: lipopolysaccharide; IL-8R: interleukin-8 receptor; IL: interleukin; chREBP: Carbohydrate response element binding protein; ACC1: acetyl-CoA carboxylase 1; LDL-C: low-density lipoprotein-cholesterol; SOD: superoxide dismutase; GSH-Px: glutathione peroxidase; ALT: alanine aminotransferase; TLR: toll-like receptor; CYP2E1: cytochrome P4502E1; TG: triglyceride; αSMA: α-smooth muscle actin; Col1a1: collagen type 1 alpha 1; Timp1: metallopeptidase inhibitor 1; TGF-β: transforming growth factor-beta.
4.1. Non-Alcoholic Fatty Liver Disease
Non-alcohol fatty liver diseases (NAFLD) is the most common cause of chronic liver disease, however no definite treatment has been described thus far [96]. Probiotics have been proven in many studies to have anti-obesity effects. Based on the fact that many NAFLD patients are obese or overweight [97], it has been suggested that probiotics could be a new treatment for NAFLD [98]. The intake of Lactobacillus, the most commonly used probiotic, not only suppresses obesity caused by HFD, but also improves inflammation and regulation of the intestinal microbiome and increases the protective effect on the intestine [75,76].
The occurrence of NAFLD is associated with a disorder in liver lipid metabolism. Changes in lipid metabolism mainly cause fatty acid accumulation [99], and increase triglyceride accumulation in the liver [100]. Treatment with L. plantarum CQPC03 restored liver function and oxidative stress in mice and reduced fat accumulation in the liver. In addition to regulating lipid metabolism in the liver, it also alleviates inflammation by increasing the levels of interleukins IL-10 and IL-4, and decreasing the levels of proinflammatory factors, including IL-6, IL-1β, TNF-α, and IFN-γ [77]. Additionally, endotoxin levels and proinflammatory cytokines were significantly reduced, and the microbiota was controlled in the colon [78]. In mice fed a high-fat diet (HFD), the Lactobacillus strain can inhibit liver HMG-CoA reductase and make ferulic acid, which can promote the excretion of acidic sterol, showing that it is effective against NAFLD [101,102]. In addition, studies have shown that increased cholesterol accumulation contributes to liver damage, worsening NAFLD [103]. The group of mice treated with LGG upregulates the expression of cholesterol 7α-hydroxylase (CYP7A1), low density lipoprotein receptor (LDLR), and liver X receptor (LXR) genes, but downregulates the expression of small heterodimer partner (SHP) and farnesoid X receptor (FXR) [79]. These results indicate that it can inhibit cholesterol absorption and promote cholesterol transport. Lactobacillus suggests that bile salt hydrolase activity is a potential mechanism through which probiotics decrease cholesterol [104].
Over the past decade, many clinical trials have been performed to study the therapeutic effects of probiotics in NAFLD patients. When LGG was administered to obese children with NAFLD for 8 weeks, BMI decreased and alanine transaminase (ALT), TNF-α, and antipeptidoglycan-polysaccharide antibodies were significantly reduced [91]. In a study in which L. acidophilus was administered three times daily to adult NAFLD patients for 1 month, aminotransferase (AST) and ALT were significantly decreased. This showed that Lactobacillus helped improve the inflammatory condition of the patient’s liver [92]. As a result of a study in which L. reuteri and inulin were administered to NASH patients for 3 months, body weight, waist circumference, and BMI were reduced, and liver inflammation was improved [93]. The overall results indicate that Lactobacillus may be a promising therapeutic strategy for NAFLD (Table 2).
4.2. Alcoholic Liver Disease
Worldwide, alcoholic liver disease has the highest mortality rate among liver-related diseases. Alcoholic liver disease (ALD) is caused by bacterial translocation and LPS release due to intestinal barrier dysfunction, and intestinal-derived LPS plays a key role in increased liver inflammation and hepatic steatosis [105,106]. Probiotics alter the composition of the gut microbiota, reducing endotoxemia, bacterial translocation, dysbiosis, and consequently the development of ALD [107].
Several studies have found that animal models of ALD and ALD patients have abnormally very high LPS levels and increased intestinal permeability to alcohol-induced endotoxin [108]. In an animal model of chronic ALD, a Lactobacillus mixture (L. acidophilus KLDS1 and L. plantarum KLDS1.0344) decreased the serum LPS and improved the intestinal tight junction. It also inhibited inflammation, lipid accumulation, and oxidative stress through the gut−liver axis by regulating TLR4/NF-kB [80]. The effects of Lactobacillus on the gut−liver axis in ALD were evaluated by administering probiotics (L. rhamnosus R0011 and L. acidophilus R0052) together to ALD-induced mice inducted by intraperitoneal injection of ethanol and LPS. Consequently, Lactobacillus regulated alcohol-induced TLR4 overexpression and decreased proinflammatory cytokines (TNF-α, IL-1β, and IL-6) and ALT levels [81]. LGG treatment reduced alcohol-induced liver inflammation by attenuating TNF-α production through the inhibition of TLR4- and TLR5-mediated endotoxin activation [82]. In the ALD animal model modeled by Gao-binge, the group administered with L. reuteri had alleviated inflammatory cell infiltration and lipid accumulation. In addition, AST, ALT, triglyceride (TG), and total cholesterol (TCH) levels were also decreased [83].
In human study, supplementation of patients with alcoholic liver injury with L. casei significantly decreased their serum TG and LDL-C. The amounts of Lactobacillus and Bifidobacterium were increased compared to those in the control group; consequently, gut microbiota disorders were controlled, and lipid metabolism was improved [94]. Many studies have shown that Lactobacillus improves ALD. However, more research is needed to explain the mechanism behind this.
4.3. Liver Fibrosis and Cirrhosis
Liver fibrosis occurs when the tissue in the liver is damaged or inflamed and does not work properly [109]. There are many causes of liver fibrosis, including hepatitis virus, alcohol consumption, and bile acid accumulation [110]. When liver fibrosis progresses throughout, it becomes liver cirrhosis.
Hepatic accumulation of bile acids (BAs) plays a key role in the pathogenesis of cholestasis-induced liver injury, and an excess of cytotoxic BAs in the liver can lead to liver fibrosis and cirrhosis [111]. LGG supplementation increases the intestinal FXR-FGF-15 signaling pathway-mediated inhibition of BA de novo synthesis to reduce hepatic BA and enhance BA excretion to prevent excessive bile acid induced liver injury and fibrosis in mice [84]. In another study, ccl4 injection with L. salivarius LI01 reduced liver inflammatory responses, including TNF-α, INOS-2, TGF-β, IL-17A, and IL-6, and decreased AST and ALT. As a result, it relieved hepatocellular damage and showed anti-inflammatory effects [86]. In addition, Lactobacillus reduced the expression of fibrosis-related genes (Timp1, TGF-β1, Col1α1, and Acta2), and intestinal barrier function was also improved by enhancing the expression of tight junction protein [85,86]. In patients, LGG is tolerated in liver cirrhosis patients and is involved in the reduction of endotoxin and dysbiosis [95]. In another study, in the case of cirrhosis patients administered a capsule of probiotics containing L. acidophilus and L. bulgaricus, no significant effect was found, but ammonia levels were reduced in patients with ammonia levels above the normal baseline [112]. The effect of Lactobacillus needs further investigation in a larger cohort.
4.4. Hepatocellular Carcinoma
Hepatocellular carcinoma (HCC) is a hepatocyte cancer and mainly occurs in patients with viral infection, alcohol-induced cirrhosis, or non-alcohol associated steatohepatitis (NASH) [113,114]. Probiotics are food supplements containing microbes for human consumption and may be applied as biotherapeutic agents because they have a beneficial effect on health due to desirable changes in the intestinal microbial balance. While general cancer treatment methods have many side effects, biological treatments such as probiotic intake do not have side effects [115].
In a mouse model of hepatocellular carcinoma, the administration of LGG has been shown to reduce tumor progression [87]. In an animal study using azoxymethane (AOM), which is a carcinogen for mouse colon and liver cells, L. acidophilus downregulated the oncogenes (Bcl-w and KRAS) and up-regulated PTEN, a tumor suppressor gene, compared to the control group. Therefore, it helped control cancer progression [88]. L. acidophilus LA14 reduces liver damage caused by D-GaLN (D-galactosamine) by alleviating upregulated ROCK2 (which promotes hepatocellular carcinoma), FBLM1 (which promotes cancer progression), and COL12A1, a collagen type XII α1 chain. This means that LA14 prevents hepatocellular carcinoma during liver injury [89]. When hepatocellular carcinoma-induced rats treated with diethylnitrosamine (DEN) and gamma radiation (IR) were treated with EPS produced by L. acidophilus ATCC 4356, serum ALT and γ-GT activities were alleviated. Additionally, MDA, IL-17, and TGF-β1 were also ameliorated, and they showed a preventive effect on HCC through the regulation of the inflammation-related TLR2/STAT-3/P38-MAPK pathway [90]. In vitro, Lactobacillus strains (L. acidophilus HM1, L. buchneri FD2, and L. fermentum HM3) also showed a strong inhibitory activity against liver cancer HepG2 cells and showed selectivity for the apoptosis of cancer cells compared to normal cells [116].
The cell wall components of L. acidophilus and L. casei act as anticancer substances [117], and L. plantarum 70810 EPS prevents the proliferation of hepatocellular carcinoma cell lines [65]. Accordingly, Lactobacillus may be a prospective probiotic for the prevention and treatment of HCC, but further clinical studies are needed.
5. Perspective
Still, there is no definitive treatment method for liver disease. Many studies are being conducted to improve liver disease by using Lactobacillus as well as several probiotics to reduce hepatic steatosis and inflammation and control the microbiome. More research is needed on how probiotics improve liver disease.
Biological treatments, such as probiotics, currently have no side effects and are emerging as microbial drug candidates. However, because each gut microbiome is different, it is important to elucidate the mechanisms by which probiotics affect liver disease, and further studies are needed.
Probiotics can be applied as biotherapeutics because they have beneficial effects on health through changes in microbial balance. For probiotics, lactic acid bacteria such as Bifidobacterium and Lactobacillus are widely used as microorganisms. Among them, the genus Lactobacillus is one of the most studied microbial genera, and many of them have completed whole genome sequence analysis and are still in progress. There are many animal studies that Lactobacillus improves liver disease. Based on these, many clinical trials are currently underway, and further studies in a larger cohort study needed (Table 3).
Table 3.
Clinical trials currently in progress with Lactobacillus.
| Status | Disease | Study Title | Interventions | Identifier |
|---|---|---|---|---|
| Recruiting | NAFLD | Role of probiotics in Treatment of pediatric NAFLD patients by assessing with fibroscan |
|
NCT04671186 |
| Unknown | NAFLD | Probiotics in the treatment of NAFLD | • Dietary supplement: probiotic L. acidophilus 10⁹, B. lactis 10⁹. |
NCT02764047 |
| Unknown | ALD | Effect of probiotics on gut-liver axis of alcoholic hepatitis | • Drug: Probiotics (Lacidofil®) 7 days of cultured L. rhamnosus R0011/L. acidophilus R0052 (120 mg/day) • Drug: Placebo |
NCT02335632 |
| Recruiting | ALD | Alcoholic liver disease and the gut microbiome | • Drug: VSL#3 112.5 Capsule A commercial probiotic mixture consisting of Four strains of L. (L. casei, L. acidophilus, L. delbrueckii subspecies bulgaricus, and L. plantarum), three strains of Bifidobacterium, and one strain of Streptococcus. • Other: Placebo |
NCT05007470 |
| Suspended | Acute alcoholic hepatitis | Novel therapies in moderately severe acute alcoholic hepatitis |
|
NCT01922895 |
| Active, not recruiting | ALD fibrosis cirrhosis |
Profermin®: prevention of progression in alcoholic liver disease by modulating dysbiotic microbiota | • Profermin Plus® Based on fermented oats, L. plantarum 299v, lecithin and barley malt. |
NCT03863730 |
| Completed | Cirrhosis | Influence of probiotics on infections in cirrhosis | • Wonclove-849 (L. brevis W63, L. salivarius W24, L. casei W56, L. acidophilus W37, Lactococcus lactis W19, Lactococcus lactis, B. bifidum W23, B. lactis W52) | NCT01607528 |
| Not yet recruiting | cirrhosis hepatocellular carcinoma |
Probiotics in the Prevention of Hepatocellular carcinoma in cirrhosis | • Probiotics contains L. casei, L. plantarum, Streptococcus faecalis, and B. brevis | NCT03853928 |
| Completed | Fibrosis Cirrhosis hepatocellular carcinoma |
Influence of probiotics administration before liver resection in liver disease | • Active substance mixture of lactic 10% B. lactis LA 303, 10% L. acidophilus LA 201, LA 40% L. plantarum 301, 20% L. salivarius LA 302, LA 20% B. lactis 304 Dosage: 10 × 109 probiotic/capsule | NCT02021253 |
NAFLD, non-alcoholic fatty liver disease; ALD, alcoholic liver disease.
The nomenclature of the genus Lactobacillus has been recently changed, therefore, the 315 species of Lactobacillus, as mentioned above, were divided into different 23 genera [118]. Lactobacillus mentioned in this review followed the nomenclature before it changed, therefore the correct nomenclature of each species is summarized in Table 4. The species of Lactobacillus not mentioned in Table 4 are to keep the original name.
Table 4.
Correct nomenclature of Lactobacillus spp.
| Old Nomenclature | Correct Nomenclature | Reference |
|---|---|---|
| L. casei | Lacticaseibacillus casei | [118] |
| L. fermentum | Limosilactobacillus fermentum | |
| L. reuteri | Limosilactobacillus reuteri | |
| L. rhamnosus | Lacticaseibacillus rhamnosus | |
| L. plantarum | Lactiplantibacillus plantarum | |
| L. paracasei | Lacticaseibacillus paracasei | |
| L. salivarius | Ligilactobacillus salivarius |
6. Conclusions
Preclinical clinical and clinical studies have provided evidence for the various disease-alleviation effects of probiotics. Lactobacillus, particularly, has improved effects on a wide range of diseases with mechanisms. In this review, the disease improvement mechanisms and various disease suppression effects of Lactobacillus, especially on liver disease, were discussed in detail. As summarized in Figure 1, Lactobacillus positively contributes to various diseases with antimicrobial activity, microbiota modulation, antitumor activity, and immunomodulatory effects. However, not all mechanisms for all diseases have been elucidated yet. Even in the same species, the difference in disease alleviation effect depending on the strain is one of the causes of this difficulty. In addition, it is considered that more developed technologies are needed to elucidate the mechanisms of bacteria used for all probiotics, as well as Lactobacillus. As mentioned above, probiotics are emerging as microbial medicine candidates. It could be more helpful, especially, for research where the medicines have not yet been developed, such as liver disease. Therefore, understanding the bacterial strains and the development of technologies with cohort studies will facilitate the use of Lactobacillus in the treatment of various diseases.
Author Contributions
Conceptualization, J.-J.J., H.J.P., M.G.C. and K.T.S.; methodology, J.-J.J., H.J.P. and M.G.C.; resources, J.-J.J., H.J.P., M.G.C., E.P., S.-M.W., R.G., H.G., Y.A.G., S.P.S., S.B.L., G.H.K., M.K.J., B.H.M., J.Y.H., J.A.E., S.J.Y., M.R.C. and D.J.K.; data curation, J.-J.J., H.J.P. and M.G.C.; writing—original draft preparation, J.-J.J., H.J.P. and M.G.C.; writing—review and editing, K.T.S.; visualization, K.T.S.; supervision, K.T.S.; project administration, K.T.S. and D.J.K.; funding acquisition, K.T.S. and D.J.K. All authors have read and agreed to the published version of the manuscript.
Funding
This research was supported by Hallym University Research Fund, the Basic Science Research Program through the National Research Foundation of Korea funded by the Ministry of Education, Science and Technology (NRF-2018M3A9F3020956, NRF-2019R1I1A3A01060447, NRF-2020R1I1A3073530, and NRF-2020R1A6A1A03043026).
Institutional Review Board Statement
Not applicable.
Informed Consent Statement
Not applicable.
Data Availability Statement
Data are contained within the article.
Conflicts of Interest
The authors declare no conflict of interest.
Footnotes
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations.
References
- 1.Guarner F., Schaafsma G. Probiotics. Int. J. Food Microbiol. 1998;39:237–238. doi: 10.1016/S0168-1605(97)00136-0. [DOI] [PubMed] [Google Scholar]
- 2.Hotel A.C.P., Cordoba A. Health and nutritional properties of probiotics in food including powder milk with live lactic acid bacteria. Prevention. 2001;5:1–10. [Google Scholar]
- 3.Ng S., Hart A., Kamm M., Stagg A., Knight S.C. Mechanisms of action of probiotics: Recent advances. Inflamm. Bowel Dis. 2009;15:300–310. doi: 10.1002/ibd.20602. [DOI] [PubMed] [Google Scholar]
- 4.Hörmannsperger G., Haller D. Molecular crosstalk of probiotic bacteria with the intestinal immune system: Clinical relevance in the context of inflammatory bowel disease. Int. J. Med. Microbiol. 2010;300:63–73. doi: 10.1016/j.ijmm.2009.08.006. [DOI] [PubMed] [Google Scholar]
- 5.Kwon H.-K., Lee C.-G., So J.-S., Chae C.-S., Hwang J.-S., Sahoo A., Nam J.H., Rhee J.H., Hwang K.-C., Im S.-H. Generation of regulatory dendritic cells and CD4+ Foxp3+ T cells by probiotics administration suppresses immune disorders. Proc. Natl. Acad. Sci. USA. 2010;107:2159–2164. doi: 10.1073/pnas.0904055107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Campana R., van Hemert S., Baffone W. Strain-specific probiotic properties of lactic acid bacteria and their interference with human intestinal pathogens invasion. Gut Pathog. 2017;9:12. doi: 10.1186/s13099-017-0162-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Hammes P. The genera lactobacillus and carnobacterium. Prokyatyotes. 1992:1535–1594. [Google Scholar]
- 8.Sookkhee S., Chulasiri M., Prachyabrued W. Lactic acid bacteria from healthy oral cavity of Thai volunteers: Inhibition of oral pathogens. J. Appl. Microbiol. 2001;90:172–179. doi: 10.1046/j.1365-2672.2001.01229.x. [DOI] [PubMed] [Google Scholar]
- 9.Buck L.M., Gilliland S. Comparisons of freshly isolated strains of Lactobacillus acidophilus of human intestinal origin for ability to assimilate cholesterol during growth. J. Dairy Sci. 1994;77:2925–2933. doi: 10.3168/jds.S0022-0302(94)77233-7. [DOI] [PubMed] [Google Scholar]
- 10.Kaewnopparat S., Dangmanee N., Kaewnopparat N., Srichana T., Chulasiri M., Settharaksa S. In vitro probiotic properties of Lactobacillus fermentum SK5 isolated from vagina of a healthy woman. Anaerobe. 2013;22:6–13. doi: 10.1016/j.anaerobe.2013.04.009. [DOI] [PubMed] [Google Scholar]
- 11.Hojsak I., Snovak N., Abdović S., Szajewska H., Mišak Z., Kolaček S. Lactobacillus GG in the prevention of gastrointestinal and respiratory tract infections in children who attend day care centers: A randomized, double-blind, placebo-controlled trial. Clin. Nutr. 2010;29:312–316. doi: 10.1016/j.clnu.2009.09.008. [DOI] [PubMed] [Google Scholar]
- 12.Joo H.-M., Hyun Y.-J., Myoung K.-S., Ahn Y.-T., Lee J.-H., Huh C.-S., Han M.J., Kim D.-H. Lactobacillus johnsonii HY7042 ameliorates Gardnerella vaginalis-induced vaginosis by killing Gardnerella vaginalis and inhibiting NF-κB activation. Int. Immunopharmacol. 2011;11:1758–1765. doi: 10.1016/j.intimp.2011.07.002. [DOI] [PubMed] [Google Scholar]
- 13.Yonekura S., Okamoto Y., Okawa T., Hisamitsu M., Chazono H., Kobayashi K., Sakurai D., Horiguchi S., Hanazawa T. Effects of daily intake of Lactobacillus paracasei strain KW3110 on Japanese cedar pollinosis. Allergy Asthma Proc. 2009;30:397–405. doi: 10.2500/aap.2009.30.3256. [DOI] [PubMed] [Google Scholar]
- 14.O’Hara S.P., Karlsen T.H., LaRusso N.F. Cholangiocytes and the environment in primary sclerosing cholangitis: Where is the link? Gut. 2017;66:1873–1877. doi: 10.1136/gutjnl-2017-314249. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Lee S.M., Donaldson G.P., Mikulski Z., Boyajian S., Ley K., Mazmanian S.K. Bacterial colonization factors control specificity and stability of the gut microbiota. Nature. 2013;501:426–429. doi: 10.1038/nature12447. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Wieërs G., Belkhir L., Enaud R., Leclercq S., Philippart de Foy J.-M., Dequenne I., de Timary P., Cani P.D. How probiotics affect the microbiota. Front. Cell. Infect. Microbiol. 2020;9:454. doi: 10.3389/fcimb.2019.00454. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Fuller R. A review: Probiotics in man and animals. J. Appl. Bacteriol. 1989;66:365–378. [PubMed] [Google Scholar]
- 18.Sonnenburg J.L. Genetic pot luck. Nature. 2010;464:837–838. doi: 10.1038/464837a. [DOI] [PubMed] [Google Scholar]
- 19.Cani P.D. Human gut microbiome: Hopes, threats and promises. Gut. 2018;67:1716–1725. doi: 10.1136/gutjnl-2018-316723. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Monte S.V., Caruana J.A., Ghanim H., Sia C.L., Korzeniewski K., Schentag J.J., Dandona P. Reduction in endotoxemia, oxidative and inflammatory stress, and insulin resistance after Roux-en-Y gastric bypass surgery in patients with morbid obesity and type 2 diabetes mellitus. Surgery. 2012;151:587–593. doi: 10.1016/j.surg.2011.09.038. [DOI] [PubMed] [Google Scholar]
- 21.Rutten N., Gorissen D., Eck A., Niers L., Vlieger A., Besseling-Van Der Vaart I., Budding A., Savelkoul P., Van der Ent C., Rijkers G. Long term development of gut microbiota composition in atopic children: Impact of probiotics. PloS ONE. 2015;10:e0137681. doi: 10.1371/journal.pone.0137681. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Kaushik M., Kumar S., Kapoor R.K., Gulati P. Integrons and antibiotic resistance genes in water-borne pathogens: Threat detection and risk assessment. J. Med. Microbiol. 2019;68:679–692. doi: 10.1099/jmm.0.000972. [DOI] [PubMed] [Google Scholar]
- 23.Singhi S.C., Kumar S. Probiotics in critically ill children. F1000Research. 2016;5:407. doi: 10.12688/f1000research.7630.1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Soltan Dallal M.M., Davoodabadi A., Abdi M., Hajiabdolbaghi M., Sharifi Yazdi M.K., Douraghi M., Tabatabaei Bafghi S.M. Inhibitory effect of Lactobacillus plantarum and Lb. fermentum isolated from the faeces of healthy infants against nonfermentative bacteria causing nosocomial infections. New Microbes New Infect. 2017;15:9–13. doi: 10.1016/j.nmni.2016.09.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Caballero S., Carter R., Ke X., Susac B., Leiner I.M., Kim G.J., Miller L., Ling L., Manova K., Pamer E.G. Distinct but Spatially Overlapping Intestinal Niches for Vancomycin-Resistant Enterococcus faecium and Carbapenem-Resistant Klebsiella pneumoniae. PLoS Pathog. 2015;11:e1005132. doi: 10.1371/journal.ppat.1005132. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Avershina E., Lundgård K., Sekelja M., Dotterud C., Storrø O., Øien T., Johnsen R., Rudi K. Transition from infant-to adult-like gut microbiota. Environ. Microbiol. 2016;18:2226–2236. doi: 10.1111/1462-2920.13248. [DOI] [PubMed] [Google Scholar]
- 27.Bowe W.P., Logan A.C. Acne vulgaris, probiotics and the gut-brain-skin axis—Back to the future? Gut Pathog. 2011;3:1. doi: 10.1186/1757-4749-3-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Muizzuddin N., Maher W., Sullivan M., Schnittger S., Mammone T. Physiological effect of a probiotic on skin. J. Cosmet. Sci. 2012;63:385–395. [PubMed] [Google Scholar]
- 29.Sonnenburg J.L., Chen C.T.L., Gordon J.I. Genomic and metabolic studies of the impact of probiotics on a model gut symbiont and host. PLoS Biol. 2006;4:e413. doi: 10.1371/journal.pbio.0040413. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Vrieze A., Van Nood E., Holleman F., Salojärvi J., Kootte R.S., Bartelsman J.F., Dallinga–Thie G.M., Ackermans M.T., Serlie M.J., Oozeer R. Transfer of intestinal microbiota from lean donors increases insulin sensitivity in individuals with metabolic syndrome. Gastroenterology. 2012;143:913–916.e917. doi: 10.1053/j.gastro.2012.06.031. [DOI] [PubMed] [Google Scholar]
- 31.Gummesson A., Carlsson L.M., Storlien L.H., Bäckhed F., Lundin P., Löfgren L., Stenlöf K., Lam Y.Y., Fagerberg B., Carlsson B. Intestinal permeability is associated with visceral adiposity in healthy women. Obesity. 2011;19:2280–2282. doi: 10.1038/oby.2011.251. [DOI] [PubMed] [Google Scholar]
- 32.Sullivan Å., Bennet R., Viitanen M., Palmgren A.-C., Nord C.E. Influence of Lactobacillus F19 on intestinal microflora in children and elderly persons and impact on Helicobacter pylori infections. Microb. Ecol. Health Dis. 2002;14:17–21. doi: 10.1080/089106002760003305. [DOI] [Google Scholar]
- 33.Basu S., Paul D.K., Ganguly S., Chatterjee M., Chandra P.K. Efficacy of high-dose Lactobacillus rhamnosus GG in controlling acute watery diarrhea in Indian children: A randomized controlled trial. J. Clin. Gastroenterol. 2009;43:208–213. doi: 10.1097/MCG.0b013e31815a5780. [DOI] [PubMed] [Google Scholar]
- 34.Zocco M., Dal Verme L.Z., Cremonini F., Piscaglia A., Nista E., Candelli M., Novi M., Rigante D., Cazzato I., Ojetti V. Efficacy of Lactobacillus GG in maintaining remission of ulcerative colitis. Aliment. Pharmacol. Ther. 2006;23:1567–1574. doi: 10.1111/j.1365-2036.2006.02927.x. [DOI] [PubMed] [Google Scholar]
- 35.Ringel-Kulka T., Palsson O.S., Maier D., Carroll I., Galanko J.A., Leyer G., Ringel Y. Probiotic bacteria Lactobacillus acidophilus NCFM and Bifidobacterium lactis Bi-07 versus placebo for the symptoms of bloating in patients with functional bowel disorders: A double-blind study. J. Clin. Gastroenterol. 2011;45:518–525. doi: 10.1097/MCG.0b013e31820ca4d6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Fitzpatrick L.R., Hertzog K.L., Quatse A.L., Koltun W.A., Small J.S., Vrana K. Effects of the probiotic formulation VSL# 3 on colitis in weanling rats. J. Pediatric Gastroenterol. Nutr. 2007;44:561–570. doi: 10.1097/MPG.0b013e31803bda51. [DOI] [PubMed] [Google Scholar]
- 37.Di Felice G., Barletta B., Butteroni C., Corinti S., Tinghino R., Colombo P., Boirivant M. Use of probiotic bacteria for prevention and therapy of allergic diseases: Studies in mouse model of allergic sensitization. J. Clin. Gastroenterol. 2008;42:S130–S132. doi: 10.1097/MCG.0b013e318169c463. [DOI] [PubMed] [Google Scholar]
- 38.Niccoli A.A., Artesi A.L., Candio F., Ceccarelli S., Cozzali R., Ferraro L., Fiumana D., Mencacci M., Morlupo M., Pazzelli P. Preliminary results on clinical effects of probiotic Lactobacillus salivarius LS01 in children affected by atopic dermatitis. J. Clin. Gastroenterol. 2014;48:S34–S36. doi: 10.1097/MCG.0000000000000233. [DOI] [PubMed] [Google Scholar]
- 39.Ishida Y., Nakamura F., Kanzato H., Sawada D., Hirata H., Nishimura A., Kajimoto O., Fujiwara S. Clinical effects of Lactobacillus acidophilus strain L-92 on perennial allergic rhinitis: A double-blind, placebo-controlled study. J. Dairy Sci. 2005;88:527–533. doi: 10.3168/jds.S0022-0302(05)72714-4. [DOI] [PubMed] [Google Scholar]
- 40.Meijerink M., Wells J.M., Taverne N., de Zeeuw Brouwer M.-L., Hilhorst B., Venema K., van Bilsen J. Immunomodulatory effects of potential probiotics in a mouse peanut sensitization model. FEMS Immunol. Med. Microbiol. 2012;65:488–496. doi: 10.1111/j.1574-695X.2012.00981.x. [DOI] [PubMed] [Google Scholar]
- 41.Gutierrez-Castrellon P., Lopez-Velazquez G., Diaz-Garcia L., Jimenez-Gutierrez C., Mancilla-Ramirez J., Estevez-Jimenez J., Parra M. Diarrhea in preschool children and Lactobacillus reuteri: A randomized controlled trial. Pediatrics. 2014;133:e904–e909. doi: 10.1542/peds.2013-0652. [DOI] [PubMed] [Google Scholar]
- 42.Villena J., Racedo S., Aguero G., Bru E., Medina M., Alvarez S. Lactobacillus casei improves resistance to pneumococcal respiratory infection in malnourished mice. J. Nutr. 2005;135:1462–1469. doi: 10.1093/jn/135.6.1462. [DOI] [PubMed] [Google Scholar]
- 43.Sagar S., Morgan M.E., Chen S., Vos A.P., Garssen J., van Bergenhenegouwen J., Boon L., Georgiou N.A., Kraneveld A.D., Folkerts G. Bifidobacterium breve and Lactobacillus rhamnosus treatment is as effective as budesonide at reducing inflammation in a murine model for chronic asthma. Respir. Res. 2014;15:46. doi: 10.1186/1465-9921-15-46. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Carvalho J., Miranda M., Fialho A., Castro-Faria-Neto H., Anatriello E., Keller A., Aimbire F. Oral feeding with probiotic Lactobacillus rhamnosus attenuates cigarette smoke-induced COPD in C57Bl/6 mice: Relevance to inflammatory markers in human bronchial epithelial cells. PloS ONE. 2020;15:e0225560. doi: 10.1371/journal.pone.0225560. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Bravo J.A., Forsythe P., Chew M.V., Escaravage E., Savignac H.M., Dinan T.G., Bienenstock J., Cryan J.F. Ingestion of Lactobacillus strain regulates emotional behavior and central GABA receptor expression in a mouse via the vagus nerve. Proc. Natl. Acad. Sci. USA. 2011;108:16050–16055. doi: 10.1073/pnas.1102999108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Messaoudi M., Lalonde R., Violle N., Javelot H., Desor D., Nejdi A., Bisson J.-F., Rougeot C., Pichelin M., Cazaubiel M. Assessment of psychotropic-like properties of a probiotic formulation (Lactobacillus helveticus R0052 and Bifidobacterium longum R0175) in rats and human subjects. Br. J. Nutr. 2011;105:755–764. doi: 10.1017/S0007114510004319. [DOI] [PubMed] [Google Scholar]
- 47.Kwon H.-K., Kim G.-C., Kim Y., Hwang W., Jash A., Sahoo A., Kim J.-E., Nam J.H., Im S.-H. Amelioration of experimental autoimmune encephalomyelitis by probiotic mixture is mediated by a shift in T helper cell immune response. Clin. Immunol. 2013;146:217–227. doi: 10.1016/j.clim.2013.01.001. [DOI] [PubMed] [Google Scholar]
- 48.Chae C.-S., Kwon H.-K., Hwang J.-S., Kim J.-E., Im S.-H. Prophylactic effect of probiotics on the development of experimental autoimmune myasthenia gravis. PLoS ONE. 2012;7:e52119. doi: 10.1371/journal.pone.0052119. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Kałużna-Czaplińska J., Błaszczyk S. The level of arabinitol in autistic children after probiotic therapy. Nutrition. 2012;28:124–126. doi: 10.1016/j.nut.2011.08.002. [DOI] [PubMed] [Google Scholar]
- 50.Recine N., Palma E., Domenici L., Giorgini M., Imperiale L., Sassu C., Musella A., Marchetti C., Muzii L., Panici P.B. Restoring vaginal microbiota: Biological control of bacterial vaginosis. A prospective case–control study using Lactobacillus rhamnosus BMX 54 as adjuvant treatment against bacterial vaginosis. Arch. Gynecol. Obstet. 2016;293:101–107. doi: 10.1007/s00404-015-3810-2. [DOI] [PubMed] [Google Scholar]
- 51.Stapleton A.E., Au-Yeung M., Hooton T.M., Fredricks D.N., Roberts P.L., Czaja C.A., Yarova-Yarovaya Y., Fiedler T., Cox M., Stamm W.E. Randomized, placebo-controlled phase 2 trial of a Lactobacillus crispatus probiotic given intravaginally for prevention of recurrent urinary tract infection. Clin. Infect. Dis. 2011;52:1212–1217. doi: 10.1093/cid/cir183. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Valladares R., Sankar D., Li N., Williams E., Lai K.-K., Abdelgeliel A.S., Gonzalez C.F., Wasserfall C.H., Larkin III J., Schatz D. Lactobacillus johnsonii N6. 2 mitigates the development of type 1 diabetes in BB-DP rats. Plos ONE. 2010;5:e10507. doi: 10.1371/journal.pone.0010507. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Hsieh M.-C., Tsai W.-H., Jheng Y.-P., Su S.-L., Wang S.-Y., Lin C.-C., Chen Y.-H., Chang W.-W. The beneficial effects of Lactobacillus reuteri ADR-1 or ADR-3 consumption on type 2 diabetes mellitus: A randomized, double-blinded, placebo-controlled trial. Sci. Rep. 2018;8:16791. doi: 10.1038/s41598-018-35014-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Kang J.-H., Yun S.-I., Park M.-H., Park J.-H., Jeong S.-Y., Park H.-O. Anti-obesity effect of Lactobacillus gasseri BNR17 in high-sucrose diet-induced obese mice. PloS ONE. 2013;8:e54617. doi: 10.1371/journal.pone.0054617. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Liu D.M., Guo J., Zeng X.A., Sun D.W., Brennan C.S., Zhou Q.X., Zhou J.S. The probiotic role of Lactobacillus plantarum in reducing risks associated with cardiovascular disease. Int. J. Food Sci. Technol. 2017;52:127–136. doi: 10.1111/ijfs.13234. [DOI] [Google Scholar]
- 56.Twetman S., Derawi B., Keller M., Ekstrand K., Yucel-Lindberg T., Stecksen-Blicks C. Short-term effect of chewing gums containing probiotic Lactobacillus reuteri on the levels of inflammatory mediators in gingival crevicular fluid. Acta Odontol. Scand. 2009;67:19–24. doi: 10.1080/00016350802516170. [DOI] [PubMed] [Google Scholar]
- 57.Teughels W., Durukan A., Ozcelik O., Pauwels M., Quirynen M., Haytac M.C. Clinical and microbiological effects of Lactobacillus reuteri probiotics in the treatment of chronic periodontitis: A randomized placebo-controlled study. J. Clin. Periodontol. 2013;40:1025–1035. doi: 10.1111/jcpe.12155. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Näse L., Hatakka K., Savilahti E., Saxelin M., Pönkä A., Poussa T., Korpela R., Meurman J.H. Effect of long–term consumption of a probiotic bacterium, Lactobacillus rhamnosus GG, in milk on dental caries and caries risk in children. Caries Res. 2001;35:412–420. doi: 10.1159/000047484. [DOI] [PubMed] [Google Scholar]
- 59.Iwamoto T., Suzuki N., Tanabe K., Takeshita T., Hirofuji T. Effects of probiotic Lactobacillus salivarius WB21 on halitosis and oral health: An open-label pilot trial. Oral Surg. Oral Med. Oral Pathol. Oral Radiol. Endodontol. 2010;110:201–208. doi: 10.1016/j.tripleo.2010.03.032. [DOI] [PubMed] [Google Scholar]
- 60.Rossoni R.D., de Barros P.P., de Alvarenga J.A., Ribeiro F.d.C., Velloso M.d.S., Fuchs B.B., Mylonakis E., Jorge A.O.C., Junqueira J.C. Antifungal activity of clinical Lactobacillus strains against Candida albicans biofilms: Identification of potential probiotic candidates to prevent oral candidiasis. Biofouling. 2018;34:212–225. doi: 10.1080/08927014.2018.1425402. [DOI] [PubMed] [Google Scholar]
- 61.So J.-S., Kwon H.-K., Lee C.-G., Yi H.-J., Park J.-A., Lim S.-Y., Hwang K.-C., Jeon Y.H., Im S.-H. Lactobacillus casei suppresses experimental arthritis by down-regulating T helper 1 effector functions. Mol. Immunol. 2008;45:2690–2699. doi: 10.1016/j.molimm.2007.12.010. [DOI] [PubMed] [Google Scholar]
- 62.Toral M., Robles-Vera I., Romero M., de la Visitación N., Sánchez M., O’Valle F., Rodriguez-Nogales A., Gálvez J., Duarte J., Jiménez R. Lactobacillus fermentum CECT5716: A novel alternative for the prevention of vascular disorders in a mouse model of systemic lupus erythematosus. FASEB J. 2019;33:10005–10018. doi: 10.1096/fj.201900545RR. [DOI] [PubMed] [Google Scholar]
- 63.Peña J.A., Rogers A.B., Ge Z., Ng V., Li S.Y., Fox J.G., Versalovic J. Probiotic Lactobacillus spp. diminish Helicobacter hepaticus-induced inflammatory bowel disease in interleukin-10-deficient mice. Infect. Immun. 2005;73:912–920. doi: 10.1128/IAI.73.2.912-920.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Dar H.Y., Shukla P., Mishra P.K., Anupam R., Mondal R.K., Tomar G.B., Sharma V., Srivastava R.K. Lactobacillus acidophilus inhibits bone loss and increases bone heterogeneity in osteoporotic mice via modulating Treg-Th17 cell balance. Bone Rep. 2018;8:46–56. doi: 10.1016/j.bonr.2018.02.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Wang K., Li W., Rui X., Chen X., Jiang M., Dong M. Characterization of a novel exopolysaccharide with antitumor activity from Lactobacillus plantarum 70810. Int. J. Biol. Macromol. 2014;63:133–139. doi: 10.1016/j.ijbiomac.2013.10.036. [DOI] [PubMed] [Google Scholar]
- 66.Davidson L.E., Fiorino A.-M., Snydman D.R., Hibberd P.L. Lactobacillus GG as an immune adjuvant for live-attenuated influenza vaccine in healthy adults: A randomized double-blind placebo-controlled trial. Eur. J. Clin. Nutr. 2011;65:501–507. doi: 10.1038/ejcn.2010.289. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Shokryazdan P., Sieo C.C., Kalavathy R., Liang J.B., Alitheen N.B., Faseleh Jahromi M., Ho Y.W. Probiotic potential of Lactobacillus strains with antimicrobial activity against some human pathogenic strains. BioMed Res. Int. 2014;2014 doi: 10.1155/2014/927268. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Barefoot S.F., Klaenhammer T.R. Detection and activity of lactacin B, a bacteriocin produced by Lactobacillus acidophilus. Appl. Environ. Microbiol. 1983;45:1808–1815. doi: 10.1128/aem.45.6.1808-1815.1983. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Ogunbanwo S., Sanni A., Onilude A. Characterization of bacteriocin produced by Lactobacillus plantarum F1 and Lactobacillus brevis OG1. Afr. J. Biotechnol. 2003;2:219–227. [Google Scholar]
- 70.Nikoskelainen S., Ouwehand A.C., Bylund G., Salminen S., Lilius E.-M. Immune enhancement in rainbow trout (Oncorhynchus mykiss) by potential probiotic bacteria (Lactobacillus rhamnosus) Fish Shellfish Immunol. 2003;15:443–452. doi: 10.1016/S1050-4648(03)00023-8. [DOI] [PubMed] [Google Scholar]
- 71.Paturi G., Phillips M., Jones M., Kailasapathy K. Immune enhancing effects of Lactobacillus acidophilus LAFTI L10 and Lactobacillus paracasei LAFTI L26 in mice. Int. J. Food Microbiol. 2007;115:115–118. doi: 10.1016/j.ijfoodmicro.2006.10.007. [DOI] [PubMed] [Google Scholar]
- 72.Lee N.Y., Shin M.J., Youn G.S., Yoon S.J., Choi Y.R., Kim H.S., Gupta H., Han S.H., Kim B.K., Lee D.Y. Lactobacillus attenuates progression of nonalcoholic fatty liver disease by lowering cholesterol and steatosis. Clin. Mol. Hepatol. 2021;27:110. doi: 10.3350/cmh.2020.0125. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Poo H., Pyo H.M., Lee T.Y., Yoon S.W., Lee J.S., Kim C.J., Sung M.H., Lee S.H. Oral administration of human papillomavirus type 16 E7 displayed on Lactobacillus casei induces E7-specific antitumor effects in C57/BL6 mice. Int. J. Cancer. 2006;119:1702–1709. doi: 10.1002/ijc.22035. [DOI] [PubMed] [Google Scholar]
- 74.Aktories K., Schwan C., Jank T. Clostridium difficile toxin biology. Annu. Rev. Microbiol. 2017;71:281–307. doi: 10.1146/annurev-micro-090816-093458. [DOI] [PubMed] [Google Scholar]
- 75.Kawano M., Miyoshi M., Ogawa A., Sakai F., Kadooka Y. Lactobacillus gasseri SBT2055 inhibits adipose tissue inflammation and intestinal permeability in mice fed a high-fat diet. J. Nutr. Sci. 2016;5:e23. doi: 10.1017/jns.2016.12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Ritze Y., Bardos G., Claus A., Ehrmann V., Bergheim I., Schwiertz A., Bischoff S.C. Lactobacillus rhamnosus GG protects against non-alcoholic fatty liver disease in mice. PLoS ONE. 2014;9:e80169. doi: 10.1371/journal.pone.0080169. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Gan Y., Chen H., Zhou X.R., Chu L.L., Ran W.T., Tan F., Zhao X. Regulating effect of Lactobacillus plantarum CQPC03 on lipid metabolism in high-fat diet-induced obesity in mice. J. Food Biochem. 2020;44:e13495. doi: 10.1111/jfbc.13495. [DOI] [PubMed] [Google Scholar]
- 78.Li C., Nie S.P., Zhu K.X., Ding Q., Li C., Xiong T., Xie M.Y. Lactobacillus plantarum NCU116 improves liver function, oxidative stress and lipid metabolism in rats with high fat diet induced non-alcoholic fatty liver disease. Food Funct. 2014;5:3216–3223. doi: 10.1039/C4FO00549J. [DOI] [PubMed] [Google Scholar]
- 79.Wang G., Huang W., Xia Y., Xiong Z., Ai L. Cholesterol-lowering potentials of Lactobacillus strain overexpression of bile salt hydrolase on high cholesterol diet-induced hypercholesterolemic mice. Food Funct. 2019;10:1684–1695. doi: 10.1039/C8FO02181C. [DOI] [PubMed] [Google Scholar]
- 80.Li H., Shi J., Zhao L., Guan J., Liu F., Huo G., Li B. Lactobacillus plantarum KLDS1.0344 and Lactobacillus acidophilus KLDS1.0901 Mixture Prevents Chronic Alcoholic Liver Injury in Mice by Protecting the Intestinal Barrier and Regulating Gut Microbiota and Liver-Related Pathways. J. Agric. Food Chem. 2021;69:183–197. doi: 10.1021/acs.jafc.0c06346. [DOI] [PubMed] [Google Scholar]
- 81.Hong M., Kim S.W., Han S.H., Kim D.J., Suk K.T., Kim Y.S., Kim M.J., Kim M.Y., Baik S.K., Ham Y.L. Probiotics (Lactobacillus rhamnosus R0011 and acidophilus R0052) reduce the expression of toll-like receptor 4 in mice with alcoholic liver disease. PLoS ONE. 2015;10:e0117451. doi: 10.1371/journal.pone.0117451. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Wang Y., Liu Y., Kirpich I., Ma Z., Wang C., Zhang M., Suttles J., McClain C., Feng W. Lactobacillus rhamnosus GG reduces hepatic TNFalpha production and inflammation in chronic alcohol-induced liver injury. J. Nutr. Biochem. 2013;24:1609–1615. doi: 10.1016/j.jnutbio.2013.02.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Zheng T.X., Pu S.L., Tan P., Du Y.C., Qian B.L., Chen H., Fu W.G., Huang M.Z. Liver Metabolomics Reveals the Effect of Lactobacillus reuteri on Alcoholic Liver Disease. Front. Physiol. 2020;11:595382. doi: 10.3389/fphys.2020.595382. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Liu Y., Chen K., Li F., Gu Z., Liu Q., He L., Shao T., Song Q., Zhu F., Zhang L., et al. Probiotic Lactobacillus rhamnosus GG Prevents Liver Fibrosis Through Inhibiting Hepatic Bile Acid Synthesis and Enhancing Bile Acid Excretion in Mice. Hepatology. 2020;71:2050–2066. doi: 10.1002/hep.30975. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Jantararussamee C., Rodniem S., Taweechotipatr M., Showpittapornchai U., Pradidarcheep W. Hepatoprotective Effect of Probiotic Lactic Acid Bacteria on Thioacetamide-Induced Liver Fibrosis in Rats. Probiotics Antimicrob. Proteins. 2021;13:40–50. doi: 10.1007/s12602-020-09663-6. [DOI] [PubMed] [Google Scholar]
- 86.Shi D., Lv L., Fang D., Wu W., Hu C., Xu L., Chen Y., Guo J., Hu X., Li A., et al. Administration of Lactobacillus salivarius LI01 or Pediococcus pentosaceus LI05 prevents CCl4-induced liver cirrhosis by protecting the intestinal barrier in rats. Sci. Rep. 2017;7:6927. doi: 10.1038/s41598-017-07091-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Li J., Sung C.Y., Lee N., Ni Y., Pihlajamaki J., Panagiotou G., El-Nezami H. Probiotics modulated gut microbiota suppresses hepatocellular carcinoma growth in mice. Proc. Natl. Acad. Sci. USA. 2016;113:E1306–E1315. doi: 10.1073/pnas.1518189113. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Heydari Z., Rahaie M., Alizadeh A.M. Different anti-inflammatory effects of Lactobacillus acidophilus and Bifidobactrum bifidioum in hepatocellular carcinoma cancer mouse through impact on microRNAs and their target genes. J. Nutr. Intermed. Metab. 2019;16:100096. doi: 10.1016/j.jnim.2019.100096. [DOI] [Google Scholar]
- 89.Lv L., Yao C., Yan R., Jiang H., Wang Q., Wang K., Ren S., Jiang S., Xia J., Li S., et al. Lactobacillus acidophilus LA14 Alleviates Liver Injury. mSystems. 2021;6:e0038421. doi: 10.1128/mSystems.00384-21. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90.Khedr O.M.S., El-Sonbaty S.M., Moawed F.S.M., Kandil E.I., Abdel-Maksoud B.E. Lactobacillus acidophilus ATCC 4356 Exopolysaccharides Suppresses Mediators of Inflammation through the Inhibition of TLR2/STAT-3/P38-MAPK Pathway in DEN-Induced Hepatocarcinogenesis in Rats. Nutr. Cancer. 2021:1–11. doi: 10.1080/01635581.2021.1934490. [DOI] [PubMed] [Google Scholar]
- 91.Vajro P., Mandato C., Licenziati M.R., Franzese A., Vitale D.F., Lenta S., Caropreso M., Vallone G., Meli R. Effects of Lactobacillus rhamnosus strain GG in pediatric obesity-related liver disease. J. Pediatr. Gastroenterol. Nutr. 2011;52:740–743. doi: 10.1097/MPG.0b013e31821f9b85. [DOI] [PubMed] [Google Scholar]
- 92.Abdel Monem S.M. Probiotic Therapy in Patients with Nonalcoholic Steatohepatitis in Zagazig University Hospitals. Euroasian J. Hepatogastroenterol. 2017;7:101–106. doi: 10.5005/jp-journals-10018-1226. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93.Ferolla S.M., Couto C.A., Costa-Silva L., Armiliato G.N., Pereira C.A., Martins F.S., Ferrari Mde L., Vilela E.G., Torres H.O., Cunha A.S., et al. Beneficial Effect of Synbiotic Supplementation on Hepatic Steatosis and Anthropometric Parameters, But Not on Gut Permeability in a Population with Nonalcoholic Steatohepatitis. Nutrients. 2016;8:397. doi: 10.3390/nu8070397. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94.Li X., Liu Y., Guo X., Ma Y., Zhang H., Liang H. Effect of Lactobacillus casei on lipid metabolism and intestinal microflora in patients with alcoholic liver injury. Eur. J. Clin. Nutr. 2021;75:1227–1236. doi: 10.1038/s41430-020-00852-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95.Bajaj J.S., Heuman D.M., Hylemon P.B., Sanyal A.J., Puri P., Sterling R.K., Luketic V., Stravitz R.T., Siddiqui M.S., Fuchs M., et al. Randomised clinical trial: Lactobacillus GG modulates gut microbiome, metabolome and endotoxemia in patients with cirrhosis. Aliment. Pharm. 2014;39:1113–1125. doi: 10.1111/apt.12695. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96.Younossi Z., Tacke F., Arrese M., Chander Sharma B., Mostafa I., Bugianesi E., Wai-Sun Wong V., Yilmaz Y., George J., Fan J., et al. Global Perspectives on Nonalcoholic Fatty Liver Disease and Nonalcoholic Steatohepatitis. Hepatology. 2019;69:2672–2682. doi: 10.1002/hep.30251. [DOI] [PubMed] [Google Scholar]
- 97.Henao-Mejia J., Elinav E., Jin C., Hao L., Mehal W.Z., Strowig T., Thaiss C.A., Kau A.L., Eisenbarth S.C., Jurczak M.J., et al. Inflammasome-mediated dysbiosis regulates progression of NAFLD and obesity. Nature. 2012;482:179–185. doi: 10.1038/nature10809. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 98.Xin J., Zeng D., Wang H., Ni X., Yi D., Pan K., Jing B. Preventing non-alcoholic fatty liver disease through Lactobacillus johnsonii BS15 by attenuating inflammation and mitochondrial injury and improving gut environment in obese mice. Appl. Microbiol. Biotechnol. 2014;98:6817–6829. doi: 10.1007/s00253-014-5752-1. [DOI] [PubMed] [Google Scholar]
- 99.Tessari P., Coracina A., Cosma A., Tiengo A. Hepatic lipid metabolism and non-alcoholic fatty liver disease. Nutr. Metab. Cardiovasc. Dis. 2009;19:291–302. doi: 10.1016/j.numecd.2008.12.015. [DOI] [PubMed] [Google Scholar]
- 100.Labenz C., Prochaska J.H., Huber Y., Nagel M., Straub B.K., Wild P., Galle P.R., Schattenberg J.M. Cardiovascular Risk Categories in Patients With Nonalcoholic Fatty Liver Disease and the Role of Low-Density Lipoprotein Cholesterol. Hepatol. Commun. 2019;3:1472–1481. doi: 10.1002/hep4.1428. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 101.Ma Y., Chen K., Lv L., Wu S., Guo Z. Ferulic acid ameliorates nonalcoholic fatty liver disease and modulates the gut microbiota composition in high-fat diet fed ApoE−/− mice. Biomed. Pharmacother. 2019;113:108753. doi: 10.1016/j.biopha.2019.108753. [DOI] [PubMed] [Google Scholar]
- 102.Tomaro-Duchesneau C., Saha S., Malhotra M., Coussa-Charley M., Al-Salami H., Jones M.L., Labbé A., Prakash S. Lactobacillus fermentum NCIMB 5221 has a greater ferulic acid production compared to other ferulic acid esterase producing Lactobacilli. Int. J. Probiotics Prebiotics. 2012;7:23–32. [Google Scholar]
- 103.Malhotra P., Gill R.K., Saksena S., Alrefai W.A. Disturbances in Cholesterol Homeostasis and Non-alcoholic Fatty Liver Diseases. Front. Med. 2020;7:467. doi: 10.3389/fmed.2020.00467. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 104.Albano C., Morandi S., Silvetti T., Casiraghi M.C., Manini F., Brasca M. Lactic acid bacteria with cholesterol-lowering properties for dairy applications: In vitro and in situ activity. J. Dairy Sci. 2018;101:10807–10818. doi: 10.3168/jds.2018-15096. [DOI] [PubMed] [Google Scholar]
- 105.Wang Y., Kirpich I., Liu Y., Ma Z., Barve S., McClain C.J., Feng W. Lactobacillus rhamnosus GG treatment potentiates intestinal hypoxia-inducible factor, promotes intestinal integrity and ameliorates alcohol-induced liver injury. Am. J. Pathol. 2011;179:2866–2875. doi: 10.1016/j.ajpath.2011.08.039. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 106.Gu Z., Li F., Liu Y., Jiang M., Zhang L., He L., Wilkey D.W., Merchant M., Zhang X., Deng Z.B., et al. Exosome-Like Nanoparticles from Lactobacillus rhamnosusGG Protect Against Alcohol-Associated Liver Disease Through Intestinal Aryl Hydrocarbon Receptor in Mice. Hepatol. Commun. 2021;5:846–864. doi: 10.1002/hep4.1679. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 107.Hong M., Han D.H., Hong J., Kim D.J., Suk K.T. Are Probiotics Effective in Targeting Alcoholic Liver Diseases? Probiotics Antimicrob. Proteins. 2019;11:335–347. doi: 10.1007/s12602-018-9419-6. [DOI] [PubMed] [Google Scholar]
- 108.Parlesak A., Schafer C., Schutz T., Bode J.C., Bode C. Increased intestinal permeability to macromolecules and endotoxemia in patients with chronic alcohol abuse in different stages of alcohol-induced liver disease. J. Hepatol. 2000;32:742–747. doi: 10.1016/S0168-8278(00)80242-1. [DOI] [PubMed] [Google Scholar]
- 109.Bataller R., Brenner D.A. Liver fibrosis. J. Clin. Invest. 2005;115:209–218. doi: 10.1172/JCI24282. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 110.Friedman S.L. Molecular regulation of hepatic fibrosis, an integrated cellular response to tissue injury. J. Biol. Chem. 2000;275:2247–2250. doi: 10.1074/jbc.275.4.2247. [DOI] [PubMed] [Google Scholar]
- 111.Schaap F.G., Trauner M., Jansen P.L. Bile acid receptors as targets for drug development. Nat. Rev. Gastroenterol. Hepatol. 2014;11:55–67. doi: 10.1038/nrgastro.2013.151. [DOI] [PubMed] [Google Scholar]
- 112.Pereg D., Kotliroff A., Gadoth N., Hadary R., Lishner M., Kitay-Cohen Y. Probiotics for patients with compensated liver cirrhosis: A double-blind placebo-controlled study. Nutrition. 2011;27:177–181. doi: 10.1016/j.nut.2010.01.006. [DOI] [PubMed] [Google Scholar]
- 113.Ganne-Carrie N., Nahon P. Hepatocellular carcinoma in the setting of alcohol-related liver disease. J. Hepatol. 2019;70:284–293. doi: 10.1016/j.jhep.2018.10.008. [DOI] [PubMed] [Google Scholar]
- 114.Mahmud N., Fricker Z., Hubbard R.A., Ioannou G.N., Lewis J.D., Taddei T.H., Rothstein K.D., Serper M., Goldberg D.S., Kaplan D.E. Risk Prediction Models for Post-Operative Mortality in Patients with Cirrhosis. Hepatology. 2021;73:204–218. doi: 10.1002/hep.31558. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 115.Grajek W., Olejnik A., Sip A. Probiotics, prebiotics and antioxidants as functional foods. Acta Biochim. Pol. 2005;52:665–671. doi: 10.18388/abp.2005_3428. [DOI] [PubMed] [Google Scholar]
- 116.Shokryazdan P., Jahromi M.F., Liang J.B., Sieo C.C., Kalavathy R., Idrus Z., Ho Y.W. In Vitro Assessment of Bioactivities of Lactobacillus Strains as Potential Probiotics for Humans and Chickens. J. Food Sci. 2017;82:2734–2745. doi: 10.1111/1750-3841.13921. [DOI] [PubMed] [Google Scholar]
- 117.Desrouilleres K., Millette M., Bagheri L., Maherani B., Jamshidian M., Lacroix M. The synergistic effect of cell wall extracted from probiotic biomass containing Lactobacillus acidophilus CL1285, L. casei LBC80R, and L. rhamnosus CLR2 on the anticancer activity of cranberry juice-HPLC fractions. J. Food Biochem. 2020;44:e13195. doi: 10.1111/jfbc.13195. [DOI] [PubMed] [Google Scholar]
- 118.Zheng J., Wittouck S., Salvetti E., Franz C.M., Harris H., Mattarelli P., O’Toole P.W., Pot B., Vandamme P., Walter J. A taxonomic note on the genus Lactobacillus: Description of 23 novel genera, emended description of the genus Lactobacillus Beijerinck 1901, and union of Lactobacillaceae and Leuconostocaceae. Int. J. Syst. Evol. Microbiol. 2020;70:2782–2858. doi: 10.1099/ijsem.0.004107. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
Data are contained within the article.