Abstract
Chronic kidney disease (CKD) is a major public health problem in low- and middle-income countries (LMICs). Although CKD prevalence has been rapidly increasing in LMICs, particularly in Asia, quantitative studies on the current epidemiology of CKD in this region are limited. This study aimed to identify the prevalence of CKD stages 3–5 in LMICs in Asia, by subregion, country economy classification, identification of CKD, traditional and non-traditional risk factors. A systematic review and meta-analysis of observational studies was conducted through a literature search of seven electronic databases and grey literature search published until November 2021. The Newcastle-Ottawa quality assessment scale (NOS) was used to assess the risk of bias of each study. A random-effects model was used to estimate pooled prevalence. The protocol is registered in the International Prospective Register of Systematic Reviews (PROSPERO CRD42019120519). Of 4,548 potentially relevant records, 110 studies with moderate and high quality were included with 4,760,147 subjects. The average prevalence (95% CI) of CKD stages 3–5 in 14 LMICs in Asia was 11.2% (9.3–13.2%). The prevalence of CKD stages 3–5 was varied among subregions and country economic classification. CKD prevalence was 8.6% (7.2–10.2%) in east Asia, 12.0% (7.7–17.0%) in south-east Asia, 13.1% (8.7–18.2%) in western Asia, and 13.5% (9.5–18.0%) in south Asia. CKD prevalence was 9.8% (8.3–11.5%) in upper-middle-income countries and 13.8% (9.9–18.3%) in lower-middle-income countries. Prevalence of CKD stage 3–5 in LMICs in Asia is comparable to global prevalence. High level of heterogeneity was observed. Study of factors and interventions that lead to the delay of CKD progression is needed.
Introduction
The global prevalence of chronic kidney disease (CKD) was estimated to be 13.4% in all five stages and 10.6% in stages 3–5 in a recent systematic review and meta-analysis [1]. CKD cases have been continuously increasing, with a 7% increase in end-stage renal disease (ESRD) observed worldwide [2]. The mean prevalence of CKD in high-income countries is approximately 8.6% in men and 9.6% in women [3]. In 2010, about 500 million people worldwide were afflicted with CKD, with 80% of those people living in low- and middle-income countries (LMICs) [3, 4]. A lower income has been identified to be a significant factor associated with CKD prevalence [5]. LMICs have undergone rapid urbanisation that has coincided with a growing number of people with chronic diseases such as diabetes and hypertension, which may lead to CKD. In Asia, 41 countries have been classified as LMICs by the World Bank [6, 7]. The prevalence of CKD in Asia varies widely [8]. Although several studies have assessed CKD prevalence in different countries in Asia, there are very few quantitative studies on the current epidemiology of CKD and its risk factors in this region. Therefore, the objectives of this study aimed to examine the prevalence of CKD stages 3–5 in LMICs in Asia.
Methods
Data sources and search strategy
The protocol was published in the PROSPERO (CRD42019120519). Observational studies published up to 30 November 2021 were searched from the following seven databases: PubMed/Medline, ScienceDirect, Embase, Scopus, Cochrane Library, Thai Library Integrated System and Thai Thesis Database. A search of ‘grey literature’ was also conducted through OpenSIGLE, conference proceedings and a manual search. Articles published in English or Thai were considered, using the following keywords: ‘prevalence’, ‘chronic kidney disease’ and ‘Asia’.
Selection criteria
Published studies that specified the prevalence of CKD stages 3–5 as an outcome were included. CKD stages 3–5 was defined as an estimated glomerular filtration rate (eGFR) <60 mL/min/1.73m2. Two investigators independently selected relevant studies based on the following inclusion criteria: (1) the observational studies, including cross-sectional study and cohort study; (2) the study participants aged 15 years and above; (3) the study participants included both males and females; (4) included at least 50 participants; (5) reported the prevalence of CKD stage 3–5 and ESRD; (6) the observed participants were non-dialysis-dependent CKD; and (7) CKD estimated by serum creatinine (Scr) or eGFR using the CKD-EPI creatinine equation (CKD-EPI), four-variable Modification of Diet in Renal Disease (MDRD), body surface area (BSA) or standardised Cockcroft-Gault (CG) equations. Studies were excluded based on the following criteria: (1) review articles, (2) not available in full-text version, (3) selected participants based on the absence or presence of kidney disease, (4) studied only in pregnant women, (5) studied only in children and (6) duplicate publications.
Data extraction
The citations of all searched articles were imported into Endnote X9 citation management software [9]. Two authors, PK and PS, screened the titles and abstracts of each study independently. Duplicate publications were removed. Full review and quality assessment were conducted for all full-text articles by two authors (PS and PK). Then, data were extracted from each study and entered into the standard data collection form [10, 11]. Data extraction was independently performed by the two authors (PS and PK) [10, 11]. Dissimilarities in data extraction were discussed until a consensus was reached. The following data were extracted: title of the article, first author’s surname, authors’ affiliations, publication year, study period, study design, country where the study was conducted, targeted population, sample size, inclusion criteria, exclusion criteria, diagnosis methods for CKD, number of cases, number of controls, percentage of males and females, number of observation CKD cases, and CKD risk factors [10–12]. The prevalence of CKD stages 3–5, odds ratios (OR), relative risk (RR), or the adjusted OR if available were extracted [10–12].
Quality assessment
Study quality and risk of bias were evaluated by the two authors (PS and PK). The Newcastle-Ottawa quality assessment scale (NOS) was used to assess the bias of each study. If any assessments were inconsistent, these were resolved through discussion to reach a consensus for each study [13–15]. The study characteristics were rated using seven and eight multiple choice questions across three areas [15, 16] including: (1) the selection of study participants (maximum of 4 stars in cohort studies and 5 stars in cross-sectional studies); (2) the comparability of groups by adjusting for first and second most appropriate factors (maximum of 2 stars); and (3) assessment of interested outcome in cohort and cross-sectional studies (maximum of 3 stars) [11, 13, 15, 16]. A total score of <3 was considered low quality, 4–6 was moderate and 7–10 was high quality.
Publication bias testing
The validity of systematic reviews and meta-analyses on publication bias was tested using an objective judgment funnel plot and Egger’s test [10, 17, 18]. When publication bias was present, the trim-and-fill method was performed to identify and correct it.
Statistical analysis
Meta-analyses were conducted using Stata version 14 statistical software [19]. Analyses of pooled estimates of the prevalence of CKD stages 3–5 were performed using inverse of the Freeman-Tukey double arcsine transformation for stabilising the variance prior to the estimation [20, 21] and presented as percentages with a 95% confidence interval (95% CI), I2, and p-value for heterogeneity. The prevalence of CKD stages 3–5 was presented using forest plots. Heterogeneity was assessed for the statistical significance using Cochran’s Q, I2, Tau2 and p-value <0.05. If I2 ≤50%, pooled prevalence was analysed using a fixed-effects model. If I2 >50%, pooled prevalence was analysed using a random-effects model [11, 22, 23].
Furthermore, sensitivity analysis was performed to repeat the random-effects of meta-analysis after the addition of a low-quality study on the pooled estimate of the prevalence of CKD stages 3–5 [17]. Subgroup analysis was performed by geographical subregion in Asia, economic group based on the World Bank, study design, measurement method of eGFR, quality of study, gender, proportion of hypertensive population, and proportion of diabetic population. Meta-regression identified the effect of study design on pooled OR of each risk factor on prevalence of CKD stage 3–5 in LMICs in Asia.[17].
Results
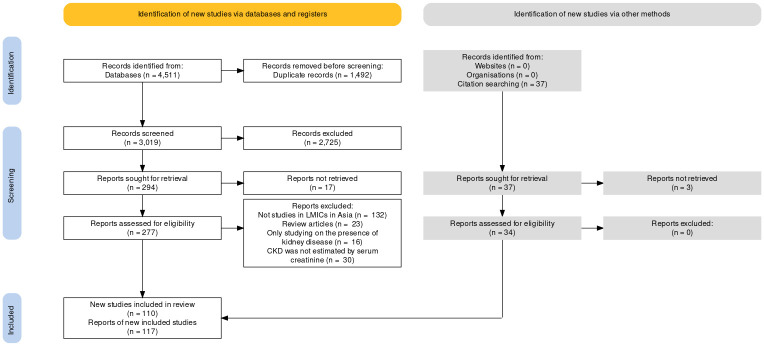
The initial search retrieved all 4,511 records from the databases. After screening the titles and abstracts, 2,725 records were excluded as nonrelevant based on the selection criteria. A full-text review of 277 studies was conducted, yielding 76 that met the criteria. In addition, 34 studies from citation search were included. Of these, 110 studies (76 database and 34 citation searching), 117 reports were included to estimate the pooled prevalence of CKD stages 3–5 (Fig 1).
Fig 1. PRISMA flow chart for identifying relevant studies.
Characteristics of the included studies
All 110 included studies were published in English and conducted between 2004 and 2021. A total of 4,760,147subjects was included in the meta-analysis of pooled estimate prevalence of CKD stages 3–5. The number of participants ranged from 100 in an Indian cross-sectional study [24] to 3,091,379 in a Chinese cross-sectional study [25]. The study populations’ age ranged from 15 to 95 years. There were 100 cross-sectional studies (61 high- and 39 moderate-quality studies) and 10 cohort studies (three high- and seven moderate-quality studies). China had the largest number of population samples in 47 studies. Most population lived in east Asia and upper-middle income countries. They were classified as CKD patients by the CKD-EPI method for the measurement of eGFR. Most observed studies had low proportions of hypertensive patients and high proportions of diabetes patients in study populations [26, 27] (Table 1). The other characteristics of the included studies are presented in S4 Appendix.
Table 1. Characteristic of studies included in the meta-analysis.
| Study characteristics | Number of participants | Number of reports (N = 117) | % |
|---|---|---|---|
| Geographical subregion in Asia | |||
| East Asia | 4,272,462 | 48 | 41.0 |
| South Asia | 311,067 | 41 | 35.1 |
| Southeast Asia | 99,159 | 17 | 14.5 |
| Western Asia | 77,459 | 11 | 9.4 |
| Economic group (classified by the World Bank) | |||
| Upper-middle income countries | 4,415,867 | 69 | 59.0 |
| Lower-middle income countries | 321,682 | 45 | 38.5 |
| Low income countries | 22,598 | 3 | 2.5 |
| Study design | |||
| Cross-sectional study | 4,715,186 | 108 | 92.3 |
| Cohort study | 44,961 | 9 | 7.7 |
| Methods for measurement of eGFR | |||
| CKD-EPI | 4,119,935 | 46 | 39.3 |
| MDRD 186 | 133,764 | 36 | 30.8 |
| eMDRD 175 | 442,723 | 29 | 24.8 |
| Cockcroft-Gault | 457 | 2 | 1.7 |
| Not reported | 63,268 | 4 | 3.4 |
| Quality of study | |||
| High-quality study | 1,102,079 | 67 | 57.3 |
| Moderate-quality study | 3,658,068 | 50 | 42.7 |
| Proportions of hypertension patients in study populations [26] | |||
| ≤ 36.9% | 3,732,160 | 58 | 49.6 |
| > 36.9% | 494,622 | 43 | 36.7 |
| Not reported | 533,365 | 16 | 13.7 |
| Proportions of diabetes patients in study populations [27] | |||
| ≤ 8.1% | 3,588,668 | 32 | 27.4 |
| > 8.1% | 611,899 | 63 | 53.8 |
| Not reported | 559,580 | 22 | 18.8 |
* estimated prevalence calculated using random-effect models; eGFR, estimated glomerular filtration rate; CKD, chronic kidney disease; EPI, epidemiology collaboration equation; MDRD186, Modification of Diet in Renal Disease Study with constant factor of 186; eMDRD175, estimated Modification of Diet in Renal Disease Study with constant factor of 175
Quality assessment and publication bias testing
All 110 studies had a NOS score ≥4, as reported in S4 Appendix. Accordingly, 64 studies were considered high quality and 46 studies were moderate quality. Assessment of publication bias by funnel plot and Egger’s test of the logit prevalence of CKD stages 3–5 in LMICs in Asia showed no small-study effect (p = 0.137).
Prevalence
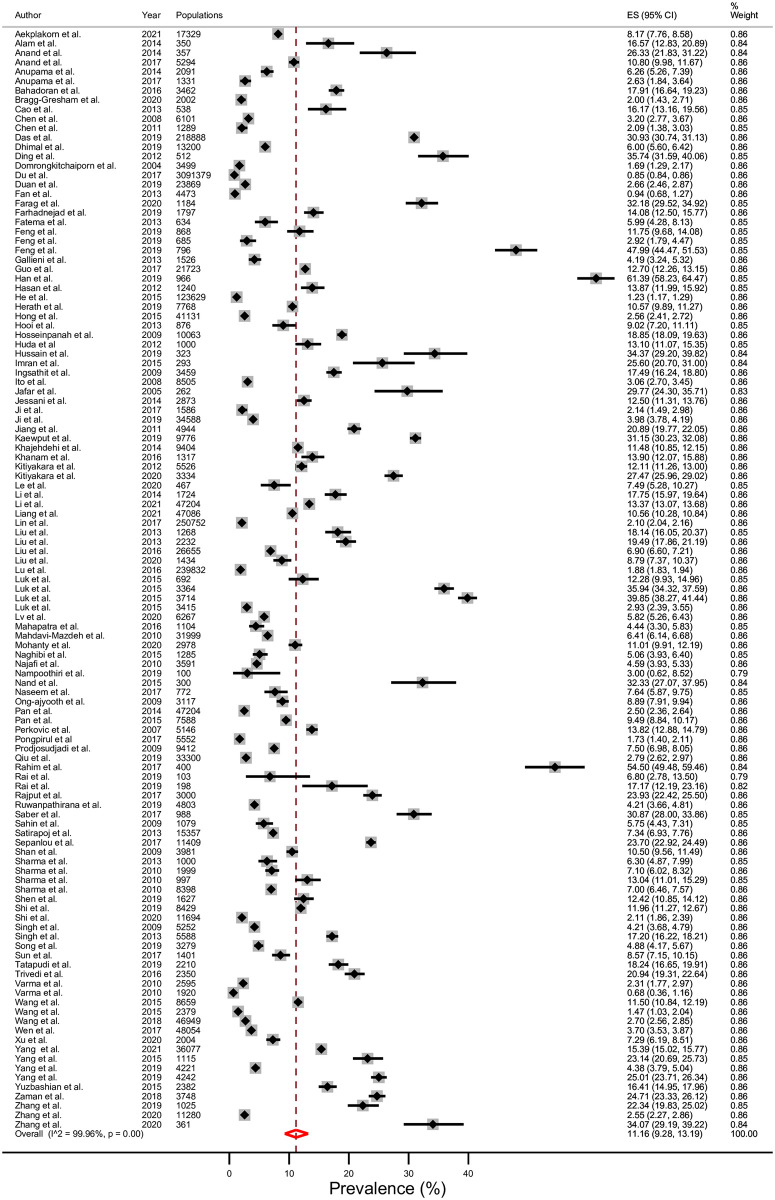
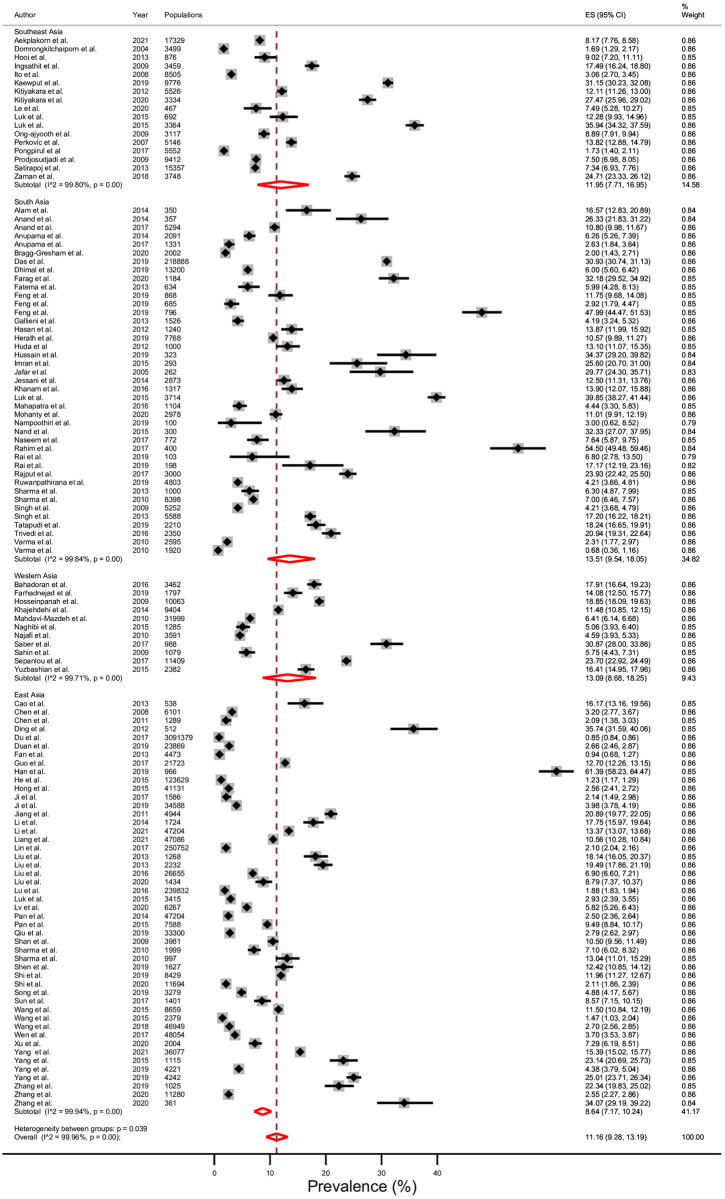
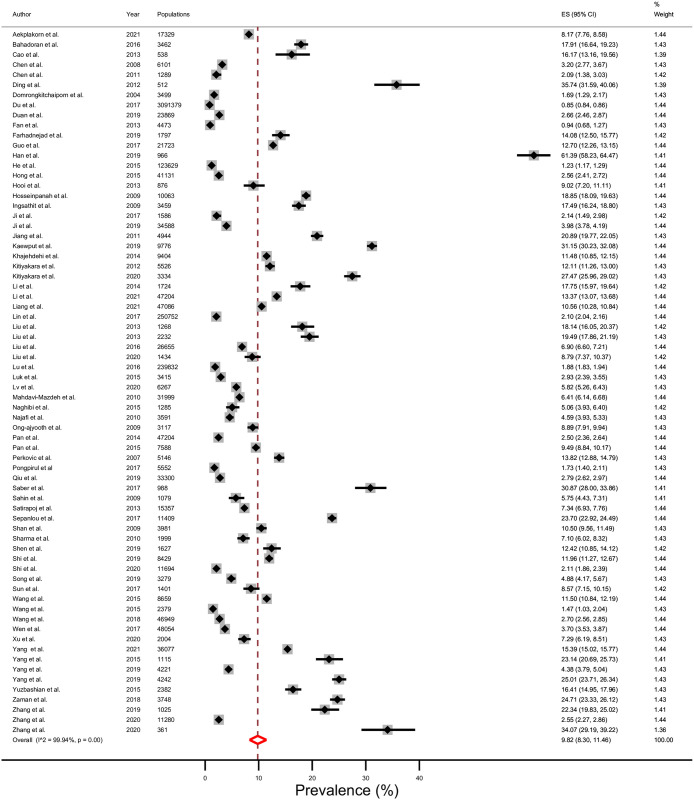
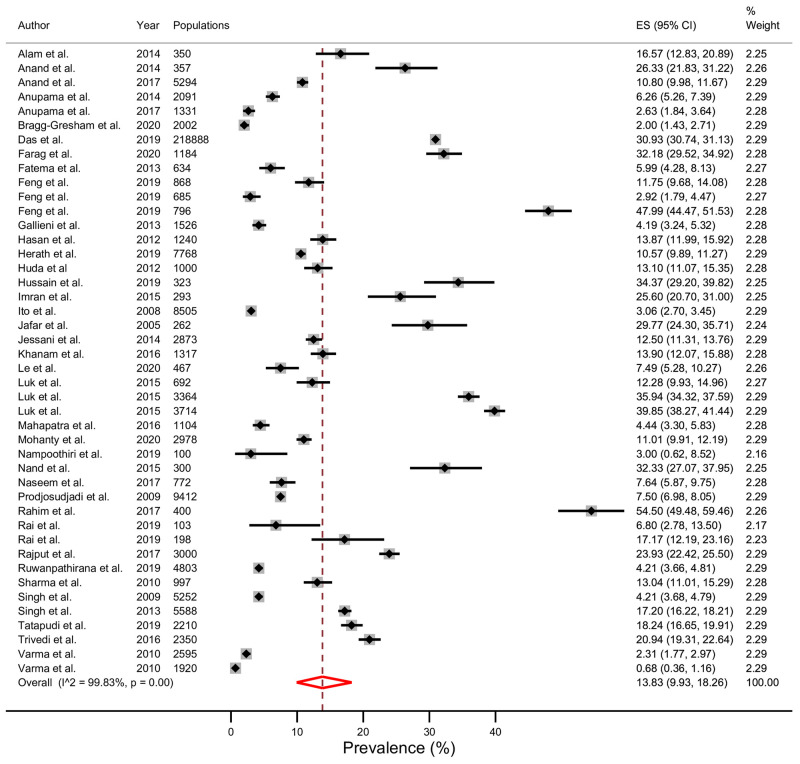
The average prevalence of CKD stages 3–5 in 14 LMICs in Asia was 11.2% (95% CI; 9.3–13.2%), Tau2 = 0.12, I2 = 99.96%, and p <0.001 (Fig 2). The prevalence of CKD stages 3–5 divided by geographical subregion in Asia (Fig 3) was 8.6% (7.2–10.2%) in east Asia, 12.0% (7.7–17.0%) in south-east Asia, 13.1% (8.7–18.2%) in western Asia, and 13.5% (9.5–18.0%) in south Asia. The prevalence of CKD stages 3–5 was 9.8% in upper-middle income countries (Fig 4); 13.8% in lower-middle income countries (Fig 5); and 6.4% in one low-income country. In addition, the rising mean prevalence of CKD stages 3–5 was observed in year 2011–2021 (12.4%) compared with 9.5% in the year before 2011(Table 2). In comparing with the global mean CKD stages 3–5 prevalence in the year 2015 [1] (Table 2), mean CKD prevalence in three countries; Thailand (12.4%), India (11.7%) and Malaysia (9.0%), was comparable to the global mean CKD stages 3–5 prevalence. The mean CKD prevalence in six countries; Philippines (35.9%), Bangladesh (19.8%), Sri Lanka (17.6%), Pakistan (14.3%), Iran (14.0%) and Mongolia (13.0%), was higher than global mean CKD stage 3–5 prevalence [1]. Conversely, mean CKD prevalence in five countries; China (8.6%), Indonesia (7.5%), Vietnam (7.1%), Nepal (6.4%) and Turkey (5.8%), was lower than global mean CKD stages 3–5 prevalence [1].
Fig 2. Prevalence of chronic kidney disease (CKD) stages 3–5 in low- and middle- income countries (LMICs) in Asia using a random-effects model.
Fig 3. Prevalence of chronic kidney disease (CKD) stages 3–5 in low- and middle- income countries (LMICs) according to subregion of Asia using a random-effects model.
Fig 4. Prevalence of chronic kidney disease (CKD) stages 3–5 in upper-middle income countries using a random-effects model.
Fig 5. Prevalence of chronic kidney disease (CKD) stages 3–5 in lower-middle income countries using a random-effects model.
Table 2. Pooled estimate prevalence of chronic kidney disease (CKD) stages 3–5 in low- and middle-income countries (LMICs) by individual countries and in comparison with global mean CKD stages 3–5 prevalence 2015 [1] and study period using a random-effects model.
| Countries/ Study period | Number of reports | Total number of populations | Pooled prevalence a (95% CIb) | I2 | p-value |
|---|---|---|---|---|---|
| Lower than global CKD stage 3–5 prevalence (<9%) | |||||
| Turkey | 1 | 1,079 | 5.75 (4.43–7.31) | NR | NR |
| Nepal | 3 | 22,598 | 6.44 (5.67–7.25) | 76.51% | <0.001 |
| Vietnam | 3 | 9,664 | 7.08 (2.08–14.69) | 98.00% | <0.001 |
| Indonesia | 1 | 9,412 | 7.50 (6.98–8.05) | NR | NR |
| China | 47 | 4,271,465 | 8.56 (7.08–10.16) | 99.94% | <0.001 |
| Comparable to global CKD stage 3–5 prevalence (9–12%) | |||||
| Malaysia | 1 | 876 | 9.02 (7.20–11.11) | NR | NR |
| India | 21 | 45,163 | 11.73 (7.36–16.96) | 99.60% | <0.001 |
| Thailand | 11 | 75,843 | 12.42 (7.25–18.73) | 99.83% | <0.001 |
| Higher than global CKD stage 3–5 prevalence (>12%) | |||||
| Mongolia | 1 | 997 | 13.04 (11.01–15.29) | NR | NR |
| Iran | 10 | 76,380 | 13.96 (9.19–19.53) | 99.73% | <0.001 |
| Pakistan | 6 | 5,235 | 14.29 (8.14–21.82) | 97.52% | <0.001 |
| Sri Lanka | 3 | 13,367 | 17.63 (5.35–34.99) | 99.77% | <0.001 |
| Bangladesh | 8 | 224,704 | 19.77 (11.62–29.44) | 99.42% | <0.001 |
| Philippines | 1 | 3,364 | 35.94 (34.32–37.59) | NR | NR |
| Study period | |||||
| Year before 2011 | 49 | 496,636 | 9.49 (7.53–11.66) | 99.83% | <0.001 |
| Year 2011–2021 | 68 | 4,263,511 | 12.44 (9.70–15.46) | 99.97% | <0.001 |
a Pooled prevalence calculated using random-effect model;
b 95% confidence interval;
NR, not reported;
Effect of traditional risk factors and non-traditional risk factors on mean prevalence of chronic kidney disease (CKD) stages 3–5 in low- and middle-income countries (LMICs) in Asia
In 69 of 110 studies, the meta-analysis of CKD prevalence and covariates were analyzed. CKD was significantly associated with 10 traditional risk factors: elderly population (OR = 3.79), obese (OR = 1.33), hypertension (OR = 2.55), diabetes (OR = 2.25), hypertriglyceridemia (OR = 1.45), hypercholesterolemia (OR = 1.33), low level of high-density lipoprotein cholesterol (OR = 1.28), history of coronary heart disease (recalculated OR using trim-and-fill analysis for publication bias = 1.99), history of stroke (OR = 4.88) and history of cardiovascular disease (OR = 2.76). Furthermore, CKD was significantly associated with five non-traditional risk factors, including education level (OR = 2.01), hyperuricemia (OR = 2.74), anemia (OR = 2.80), family history of CKD (OR = 2.82) and nonsteroidal anti-inflammatory drugs (NSAIDs) use (OR = 1.97). On the contrary, this meta-analysis found the insignificant associations between CKD stages 3–5 and gender, marital status, lower body weight, dyslipidemia, high level of low-density lipoprotein cholesterol (LDLc), smoking status, alcohol consumption, physical activity, family history of hypertension, and CD4 cell count in HIV patients (Table 3).
Table 3. Pooled estimate odd ratio and meta-regression: The effect of traditional risk factors and non-traditional risk factors on prevalence of chronic kidney disease (CKD) stage 3–5 in low- and middle-income countries (LMICs) in Asia.
| Risk factors | Number of reports | Total number of populations | Pooled ORa (95% CIb) | I2, p-value | Univariable meta- regression by study design (p-value) |
|---|---|---|---|---|---|
| Traditional risk factors | |||||
| Elderly (age ≥60 years or <60 years) | 12 | 78,831 | 3.79 (2.02–7.13) | 99.50%, <0.001 | 0.922 |
| Male | 57 | 3,725,926 | 0.88 (0.71–1.09) | 99.10%,0.234 | 0.751 |
| Obese (BMI ≥25 or 18–25 kg/m2) | 29 | 3,373,809 | 1.33 (1.15–1.53) | 94.60%, <0.001 | 0.258 |
| Lower weight (BMI <18 or 18–25 kg/m2) | 16 | 200,483 | 1.00 (0.77–1.28) | 85.90%, 0.975 | 0.523 |
| Hypertension (yes or no) | 49 | 3,816,788 | 2.55 (2.00–3.25) | 99.20%, <0.001 | 0.881 |
| Diabetes (yes or no) | 40 | 3,754,121 | 2.25 (1.63–3.12) | 99.20%, <0.001 | 0.833 |
| Dyslipidemia (yes or no) | 10 | 111,060 | 1.91 (0.79–4.62) | 99.60%, 0.149 | 0.607 |
| Hypertriglyceridemia (yes or no) | 8 | 324,667 | 1.45 (1.24–1.71) | 89.0%, <0.001 | NR |
| Hypercholesterolemia (yes or no) | 6 | 315,127 | 1.33 (1.03–1.72) | 88.40%, 0.03 | NR |
| High LDLc (yes or no) | 6 | 346,250 | 2.06 (0.54–7.93) | 99.50%, 0.293 | NR |
| Low HDLc (yes or no) | 7 | 297,740 | 1.28 (1.06–1.55) | 86.8%, 0.009 | 0.650 |
| History of CHD (yes or no) | 10 | 156,885 | 1.99 (1.18–3.35)d | -, 0.01d | 0.955 |
| History of stroke (yes or no) | 4 | 78,983 | 4.88 (2.23–10.69) | 88.60%, <0.001 | NR |
| History of CVD (yes or no) | 6 | 124,508 | 2.76 (2.25–3.38) | 69.20%, <0.001 | 0.511 |
| Non-traditional risk factors | |||||
| Education (< high school or ≥ high school) | 18 | 452,300 | 2.01 (1.33–3.02) | 99.20%, 0.001 | 0.342 |
| Marital status (being unmarried or married) | 4 | 48,193 | 1.60 (0.87–2.97) | 96.10%, 0.134 | 0.875 |
| Hyperuricemia (yes or no) | 14 | 250,620 | 2.74 (1.40–5.36) | 99.60%, 0.003 | 0.552 |
| Anemia (yes or no) | 3 | 43,714 | 2.80 (2.55–3.08) | 0%, <0.001 | 0.486 |
| Smoking status (current smoker or non-smoker) | 32 | 583,337 | 0.83 (0.54–1.28) | 99.40%, 0.398 | 0.960 |
| Alcohol consumption (current drinker or non-drinker) | 19 | 498,173 | 0.83 (0.56–1.24) | 98.60%, 0.365 | 0.207 |
| Family history of HT (yes or no) | 3 | 6,842 | 2.23 (0.56–8.81) | 94.90%, 0.254 | NR |
| Family history of CKD (yes or no) | 2 | 6,128 | 2.82 (1.73–4.59) | 0%, <0.001 | NR |
| Physical activity (inactive or active) | 8 | 160,248 | 1.57 (0.97–2.53) | 99.10%, 0.064 | NR |
| NSAIDs use (yes or no) | 4 | 70,661 | 1.97 (1.48–2.61) | 56.60%, <0.001 | NR |
| CD4 cell countc (≥200 or <200 cells/ml) | 2 | 6,090 | 1.08 (0.74–1.58) | 41.10%, 0.679 | NR |
a OR calculated using random-effect model;
b 95% confidence interval,
c CD4 cell count only in HIV patients;
d the recalculated OR using trim-and-fill analysis with twelve adjusted studies;
CKD, chronic kidney disease; BMI, body mass index; CHD, coronary heart disease; CVD, cardiovascular disease; LDLc, low-density lipoprotein cholesterol; HDLc, high-density lipoprotein cholesterol; HT, hypertension; NSAIDs, non-steroidal anti-inflammatory drugs; NR, not reported;
Sensitivity analyses
Because the included studies were found to be the high or moderate quality, sensitivity analysis was not performed as planned. The mean prevalence of CKD stages 3–5 was 11.0% in high-quality studies and 11.4% in moderate-quality studies. The estimated prevalence of CKD stages 3–5 by study designs was 8.2% for a cohort study and 11.4% for a cross-sectional study. The mean prevalence of CKD stages 3–5 by methods for measurement of eGFR was 19.7% for CG, 10.3% for eMDRD175, 10.9% for CKD-EPI and 12.3% for MDRD186. CKD stage 3–5 prevalence was higher in the studies with high proportions of hypertensive patients in the study populations (14.1%) than those with low proportions (9.4%). Similarly, the prevalence of CKD stage 3–5 in the studies with high proportions of diabetic patients in study populations (14.1%) was higher than those with low proportions (6.4%) (P > 0.05; Table 4).
Table 4. Subgroup analysis of the pooled prevalence of CKD stage 3–5 in low- and middle-income countries in Asia.
| Subgroup | Pooled prevalence (95% CI) | I2, p-value | p-value for difference |
|---|---|---|---|
| Geographical subregion in Asia | |||
| East Asia | 8.64 (7.17–10.24) | 99.94%, <0.001 | 0.039 |
| Southeast Asia | 11.95 (7.71–16.95) | 99.80%, <0.001 | |
| Western Asia | 13.09 (8.68–18.25) | 99.71%, <0.001 | |
| South Asia | 13.51 (9.54–18.05) | 99.84%, <0.001 | |
| Economic group (classified by the World Bank) | |||
| Upper-middle income countries | 9.82 (8.30–11.46) | 99.94%, <0.001 | <0.001 |
| Lower-middle income countries | 13.83 9.93–18.26) | 99.83%, <0.001 | |
| Low income countries | 6.44 (5.67–7.25) | N/A, <0.001 | |
| Study design | |||
| Cross-sectional study | 11.42 (9.45–13.55) | 99.97%, <0.001 | 0.378 |
| Cohort study | 8.24 (3.06–15.61) | 99.82%, <0.001 | |
| Methods for measurement of eGFR | |||
| CKD-EPI | 10.87 (7.76–14.43) | 99.98%, <0.001 | <0.001 |
| MDRD 186 | 12.27 (9.33–15.55) | 99.66%, <0.001 | |
| eMDRD 175 | 10.33 (7.70–13.30) | 99.88%, <0.001 | |
| Cockcroft-Gault | 19.69 (16.14–23.49) | N/A, <0.001 | |
| Not reported | 10.20 (6.63–14.43) | 99.31%, <0.001 | |
| Quality of study | |||
| High-quality study | 10.98 (8.24–14.07) | 99.96%, <0.001 | 0.836 |
| Moderate-quality study | 11.37 (9.24–13.70) | 99.92%, <0.001 | |
| Gender of study populations | |||
| Male | 9.09 (7.08–11.31) | 99.87%, <0.001 | 0.563 |
| Female | 9.98 (7.94–12.23) | 99.84%, <0.001 | |
| Proportions of hypertension patients in study populations | |||
| ≤ 36.9% | 9.37 (7.63–11.27) | 99.92%, <0.001 | 0.055 |
| > 36.9% | 14.07 (10.72–17.79) | 99.88%, <0.001 | |
| Not reported | 10.48 (3.95–19.64) | 99.98%, <0.001 | |
| Proportions of diabetes patients in study populations | |||
| ≤ 8.1% | 6.41 (4.82–8.21) | 99.91%, <0.001 | <0.001 |
| > 8.1% | 14.11 (11.65–16.77) | 99.86%, <0.001 | |
| Not reported | 10.95 (5.11–18.64) | 99.98%, <0.001 | |
* estimated prevalence calculated using random-effect models; eGFR, estimated glomerular filtration rate; CKD, chronic kidney disease; EPI, epidemiology collaboration equation; MDRD186, Modification of Diet in Renal Disease Study with constant factor of 186; eMDRD175, estimated Modification of Diet in Renal Disease Study with constant factor of 175
Discussion
CKD was rated as the 16th highest cause for loss of life in the year 2017 [28], leading to poor health quality and very high costs of medical care [29]. Most people with CKD in stages 3–5 were undiagnosed and presented to seek care in the last stage [30]. This is the first systematic review and meta-analysis to assess the prevalence of CKD stages 3–5 and its associated risk factors in LMICs in Asia. The present study found that the current prevalence of CKD stages 3–5 in LMICs in Asia is 11.2% (95% CI; 9.3–13.2%), comprising 117 populations in LMICs across Asia. High CKD prevalence could lead to high treatment costs and a great macroeconomic burden [31]. Although, CKD commonly affects patients and families with insufficient resources [32], there are limited studies on the burden of CKD, especially in LMICs in Asia.
The estimated prevalence of CKD stages 3–5 in LMICs in Asia was found in this study resembles the global CKD stages 3–5 prevalence of 10.6% [1], and approximately 1 in every 10 people was affected by CKD (range from 0.7% to 61.4%). The prevalence of CKD stages 3–5 reported in this study was higher in south Asia (13.5%) and western Asia (13.1%) than those in south-east Asia (12.0%) and east Asia (8.6%). There was no relevant study from central Asia included in this meta-analysis. According to this study’s findings, the global prevalence of CKD stages 3–5 [1] is comparable to that reported in Thailand, India and Malaysia. The prevalence of CKD stages 3–5 in six countries (Philippines, Sri Lanka, Pakistan, Iran, Bangladesh and Mongolia) is higher than the global prevalence [1]. Conversely, lower levels of prevalence are observed in China, Indonesia, Vietnam, Nepal and Turkey, when compared with the global prevalence of CKD stages 3–5 [1]. Furthermore, this study found a wide range of estimated prevalence of CKD stages 3–5, which might be due to the different number of participants and high heterogeneity of their characteristics, in which Tau2 0.12 and I2 99.96% indicate high heterogeneity. Variations in CKD prevalence estimates appear to depend on many factors, such as target population, sample size and participation rate [3, 4, 33, 34]. The rising trend of global burden of CKD was reported from 1990 to 2019 due to hypertension [35]. This study found that the prevalence of CKD stages 3–5 in year 2011–2021 (12.4%) was higher than the years before 2011 (9.5%) in LMICs in Asia. Although CKD stage 3–5 prevalence in the year 2011–2021 was determined from larger populations (4.2 million in 68 reports) than the year before 2011 (0.5 million in 49 reports). The CKD stages 3–5 prevalence seemed to be higher using CG than MDRD186, CKD-EPI and eMDRD175. The differences in CKD stages 3–5 prevalence across studies in LMIC groups might be influenced by inadequately characterised data for CKD stage 3–5, disorganized data collection methods, inconsistent methods to identify kidney dysfunction, and used of different creatinine-based eGFR equations [4, 36, 37]. When countries with different economic classifications were compared, the prevalence of CKD stages 3–5 in lower-middle income countries was higher than upper-middle income countries. However, the comparison of the CKD stages 3–5 prevalence in low-income country and other groups must be interpreted carefully because CKD prevalence was available from only three studies. In LMICs, the country’s economy might affect the CKD stages 3–5 prevalence estimation due to differences in accessibility and quality of the CKD screening program from different health care systems when compared with high-income countries [3, 4, 33]. Previous studies have shown that individuals with a lower income were more likely to present CKD progression, which may be due to an inadequate diet, unhealthy lifestyle and poor access to health information and quality healthcare services [5, 38–40].
In addition, this study found 15 risk factors (10 traditional and five non-traditional risk factors) that are significantly associated with CKD stages 3–5 in LMICs in Asia. People had an increased risk of CKD stages 3–5 if they were elderly, had less than high school education, suffered from obesity, hypertension, diabetes, hypertriglyceridemia, hypercholesterolaemia, low levels of HDLc, hyperuricaemia, anemia, history of CHD, history of stroke, history of CVD, family history of CKD, and NSAIDs medication use. Previous studies that identified major risk factors for CKD included only the factors of age, education, obesity, diabetes, hypertension, stroke, hyperuricaemia and a family history of CKD [3, 5, 41–47]. Individuals with obesity and unhealthy metabolic syndrome had a high risk of CKD [46, 48]. In particular, this study found an association between non-traditional risk factors unrelated to diabetes, hypertension or the other traditional causes of CKD and uncertain etiology (CKDu). In this meta-analysis study, five risk factors were found to be significantly associated with CKDu, including education lower than high school, hyperuricemia, anemia, history of CKD among family members and NSAIDs medication use. In addition, there were two individual studies reported other CKDu risk factors, such as environmental factors (home location in the Indonesia study) [49] and occupational factors (agriculture in the Sri Lanka study) [50]. Thus, CKD is associated with a plurality of risk factors in traditional risk factors and non-traditional risk factors, in which a few studies were conducted. Similarly, previous systematic reviews reported that CKD non-traditional etiology (CKDnT) or CKD of uncertain (CKDu) was associated with the parent history of CKD and farmers exposed to agrochemicals, heavy metals, hard water, dehydration and heat-stress [51, 52]. Besides, there was significant association between NSAIDs medication use and CKD pooled from four cross-sectional surveys (three studies reported a significant association and one study reported an insignificant association), similarly to the previous meta-analysis reported that high-dose NSAID medication exposure would increase the risk of CKD [10]. Also, further research is required to explain the association between NSAIDs exposure and CKD stage 3–5. On the other hand, this meta-analysis reported no significant statistical association between CKD stages 3–5 and some risk factors, including gender, dyslipidemia, high LDLc level, smoking status, alcohol consumption and physical activity. Conversely, most studies found gender and smoking to be independent risk factors for CKD stages 3–5 [3, 41, 46, 53]. Two previous meta-analyses reported that gender and alcohol consumption were not associated with CKD [54, 55]. Besides, the insignificant association between CKD and dyslipidemia, low LDLc level and physical activity were pooled amongst the diversely individual study results. The meta-regression in this study showed no statistical effect of different study designs on associated risk factors and CKD. Some risk factors were analyzed using a small number of studies (<10 studies), including marital status, hypertriglyceridemia, hypercholesterolaemia, high LDLc, low HDLc, anemia, history of stroke, history of CVD, family history of CKD, family history of HT, physical activity, NSAIDs use, and CD4 cell count in HIV patients. Most studies were cross-sectional prevalence surveys in various targeted-populations and did not aim to determine the disease-related risk factors.
This meta-analysis consisted of individual studies with residual confounding and undetermined sources of bias. Unfortunately, these publication biases were difficult to quantify [3]. However, this study attempted to minimise the risk of bias with various methods [56]. This systematic review considered studies with a broad publication date and included articles that were published in more than one language (English and Thai) [57]. We also included unpublished literature identified from manual searches and conferences. All study selection processes were independently investigated by two reviewers. Observational studies were included through a homogeneous protocol regarding the identification of CKD stage 3–5 using Scr or eGFR for kidney function estimations [58]. Duplicate publications were excluded by reaching a consensus between two reviewers according to the criteria for comparing reports. The pooled prevalence identified in this study was estimated from studies with moderate to high quality. No discovered publication bias distorted the result of the estimated prevalence of CKD by the funnel plot and Egger’s test [59].
This study dealt with several limitations. First, the language of publications was restricted to only English and Thai, although grey literature and studies from relevant conferences were included. Second, there was high heterogeneity in the estimated prevalence across populations of different sizes due to differences in individual study criteria, including variations in the methods used to measure eGFR and the disparities of targeted-population, as well as the difference of national policies of each country, political commitment that drives Universal Health Coverage where access to health care is high, healthcare and information systems with limited resources to address the findings of chronic kidney disease prevalence. However, the author did make an effort to robust the included study quality by using NOS quality assessments and they were found to be moderate or high quality. In conclusion, this meta-analysis presented the current epidemiology of CKD stages 3–5 in LMICs in Asia. The findings of this meta-analysis might be of use in CKD diagnostics and aetiology research. In addition, the results might be used in future strategy planning to improve early detection, slow the progression and prevent CKD stages 3–5 in LMICs in Asia.
Conclusion
This meta-analysis reported a prevalence of CKD stages 3–5 of 11.2% in LMICs in Asia, with the highest prevalence of 13.5% observed in countries in south Asia and the lowest prevalence of 8.6% among countries in east Asia. The estimated prevalence of CKD stages 3–5 in LMICs in Asia varied among individual countries and subregions.
Supporting information
(DOCX)
(DOCX)
(PDF)
(PDF)
(PDF)
(PDF)
(PDF)
(PDF)
Data Availability
All relevant data are within the paper and its Supporting information files.
Funding Statement
Pongpan Suriyong received grant from the Graduate School of Chiang Mai University; Penkarn Kanjanarat received grant from the Division of Research Administration, Academic Services and International Relations, Faculty of Pharmacy, Chiang Mai University. This work was partially supported by the Pharmacoepidemiology and Statistics Research Center (PESRC) through the Chiang Mai University, Thailand. The funders had no role in study design, data collection and analysis, decision to publish, or preparation of the manuscript.
References
- 1.Hill NR, Fatoba ST, Oke JL, Hirst JA, O’Callaghan CA, Lasserson DS, et al. Global prevalence of chronic kidney disease–a systematic review and meta-analysis. PloS one. 2016;11(7):e0158765. doi: 10.1371/journal.pone.0158765 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Ylenia I, Janet S, Francesco G, Andrea F, Antonio S, Daniele Ugo T, et al. Association of Individual Non-Steroidal Anti-Inflammatory Drugs and Chronic Kidney Disease: A Population-Based Case Control Study. PLOS ONE [Internet]. 2015; 4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Mills KT, Xu Y, Zhang W, Bundy JD, Chen C-S, Kelly TN, et al. A systematic analysis of worldwide population-based data on the global burden of chronic kidney disease in 2010. Kidney International. 2015;88(5):950–7. doi: 10.1038/ki.2015.230 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Stanifer JW, Muiru A, Jafar TH, Patel UD. Chronic kidney disease in low- and middle-income countries. Nephrol Dial Transplant. 2016;31(6):868–74. Epub 2016/05/25. doi: 10.1093/ndt/gfv466 . [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Zeng X, Liu J, Tao S, Hong HG, Li Y, Fu P. Associations between socioeconomic status and chronic kidney disease: a meta-analysis. J Epidemiol Community Health. 2018;72(4):270–9. doi: 10.1136/jech-2017-209815 [DOI] [PubMed] [Google Scholar]
- 6.COUNTRIES-ofthe-WORLD.COM. List of countries in Asia 2020 [20 July 2020]. https://www.countries-ofthe-world.com/.
- 7.Current classification by income [Internet]. 2020 [cited 15 Jan 2021]. https://datahelpdesk.worldbank.org/knowledgebase/articles/906519-world-bank-country-and-lending-groups.
- 8.Khan YH, Mallhi TH, Sarriff A, Khan AH, Tanveer N. Prevalence of Chronic Kidney Disease in Asia: A Systematic Review of Population-Based Studies. Journal of the College of Physicians and Surgeons Pakistan. 2018;28(12):960–6. doi: 10.29271/jcpsp.2018.12.960 [DOI] [PubMed] [Google Scholar]
- 9.Hupe M. EndNote X9. Journal of Electronic Resources in Medical Libraries. 2019;16(3–4):117–9. [Google Scholar]
- 10.Nderitu P, Doos L, Jones PW, Davies SJ, Kadam UT. Non-steroidal anti-inflammatory drugs and chronic kidney disease progression: a systematic review. Family practice. 2013;30(3):247–55. Epub 2013/01/11. doi: 10.1093/fampra/cms086 . [DOI] [PubMed] [Google Scholar]
- 11.Ungprasert P, Cheungpasitporn W, Crowson CS, Matteson EL. Individual non-steroidal anti-inflammatory drugs and risk of acute kidney injury: A systematic review and meta-analysis of observational studies. European journal of internal medicine. 2015;26(4):285–91. Epub 2015/04/12. doi: 10.1016/j.ejim.2015.03.008 . [DOI] [PubMed] [Google Scholar]
- 12.Yaxley J, Litfin T. Non-steroidal anti-inflammatories and the development of analgesic nephropathy: a systematic review. Ren Fail. 2016;38(9):1328–34. Epub 2016/11/04. doi: 10.1080/0886022X.2016.1216708 . [DOI] [PubMed] [Google Scholar]
- 13.Berger I, Wu S, Masson P, Kelly PJ, Duthie FA, Whiteley W, et al. Cognition in chronic kidney disease: a systematic review and meta-analysis. BMC Medicine. 2016;14(1):206. doi: 10.1186/s12916-016-0745-9 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Hartling L, Hamm M, Milne A, Vandermeer B, Santaguida PL, Ansari M, et al. Validity and inter-rater reliability testing of quality assessment instruments. 2012. [PubMed] [Google Scholar]
- 15.Sanguankeo A, Upala S, Cheungpasitporn W, Ungprasert P, Knight EL. Effects of statins on renal outcome in chronic kidney disease patients: a systematic review and meta-analysis. PLoS One. 2015;10(7):e0132970. doi: 10.1371/journal.pone.0132970 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Margulis AV, Pladevall M, Riera-Guardia N, Varas-Lorenzo C, Hazell L, Berkman ND, et al. Quality assessment of observational studies in a drug-safety systematic review, comparison of two tools: the Newcastle–Ottawa scale and the RTI item bank. Clinical epidemiology. 2014;6:359. doi: 10.2147/CLEP.S66677 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Higgins JP, Thomas J, Chandler J, Cumpston M, Li T, Page MJ, et al. Cochrane handbook for systematic reviews of interventions: John Wiley & Sons; 2019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Song F, Parekh S, Hooper L, Loke YK, Ryder J, Sutton AJ, et al. Dissemination and publication of research findings: an updated review of related biases. Health technology assessment (Winchester, England). 2010;14(8):iii, ix–xi, 1–193. Epub 2010/02/26. doi: 10.3310/hta14080 . [DOI] [PubMed] [Google Scholar]
- 19.StataCorp L. Stata statistical software (version release 14). College Station, TX: Author. 2015. [Google Scholar]
- 20.Xu C. The Freeman–Tukey double arcsine transformation for the meta-analysis of proportions: Recent criticisms were seriously misleading. 2021. [DOI] [PubMed] [Google Scholar]
- 21.Nyaga VN, Arbyn M, Aerts MJAoPH. Metaprop: a Stata command to perform meta-analysis of binomial data. 2014;72(1):1–10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Borenstein M, Hedges L, Rothstein H. Meta-analysis: Fixed effect vs. random effects. Meta-analysis com. 2007. [DOI] [PubMed] [Google Scholar]
- 23.Rhee CM, Ahmadi SF, Kovesdy CP, Kalantar-Zadeh K. Low-protein diet for conservative management of chronic kidney disease: a systematic review and meta-analysis of controlled trials. Journal of cachexia, sarcopenia and muscle. 2018;9(2):235–45. doi: 10.1002/jcsm.12264 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Nampoothiri RV, Duseja A, Rathi M, Agrawal S, Sachdeva N, Mehta M, et al. Renal dysfunction in patients with nonalcoholic fatty liver disease is related to the presence of diabetes mellitus and severity of liver disease. Journal of clinical and experimental hepatology. 2019;9(1):22–8. doi: 10.1016/j.jceh.2017.12.005 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Du Y, Zhang S, Hu M, Wang Q, Shen H, Zhang Y, et al. Prevalence of chronic kidney disease markers: Evidence from a three-million married population with fertility desire in rural China. Sci Rep. 2017;7(1). doi: 10.1038/s41598-017-02355-2 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Müller-Nurasyid MJL. Worldwide trends in hypertension prevalence and progress in treatment and control from 1990 to 2019: a pooled analysis of 1201 population-representative studies with 104 million participants. 2021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Smolen J, Burmester G, Combeet B. NCD Risk Factor Collaboration (NCD-RisC). Worldwide trends in diabetes since 1980: a pooled analysis of 751 population-based studies with 4· 4 million participants. Lancet2016; 387: 1513–30—In this Article, Catherine Pelletier. 2016. [DOI] [PMC free article] [PubMed]
- 28.Collaborators G, Roth G, Abate D, Abate K, Abay S, Abbafati C, et al. Global, regional, and national age-sex-specific mortality for 282 causes of death in 195 countries and territories, 1980–2017: a systematic analysis for the Global Burden of Disease Study 2017. The Lancet. 2018;392(10159):1736–88. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Tonelli M, Riella M. Chronic kidney disease and the aging population. Brazilian Journal of Nephrology. 2014;36(1):1–5. [PubMed] [Google Scholar]
- 30.Anand S, Zheng Y, Montez-Rath ME, Jin W, Perico N, Carminati S, et al. Do attributes of persons with chronic kidney disease differ in low-and middle-income countries compared to high income countries? Evidence from population-based data in six countries. BMJ Global Health. 2017;4(2). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Nugent RA, Fathima SF, Feigl AB, Chyung D. The burden of chronic kidney disease on developing nations: a 21st century challenge in global health. Nephron Clinical Practice. 2011;118(3):c269–c77. doi: 10.1159/000321382 [DOI] [PubMed] [Google Scholar]
- 32.Silva RE, Baldim JL, Chagas-Paula DA, Soares MG, Lago JHG, Gonçalves RV, et al. Predictive metabolomic signatures of end-stage renal disease: A multivariate analysis of population-based data. Biochimie. 2018;152:14–30. doi: 10.1016/j.biochi.2018.06.009 [DOI] [PubMed] [Google Scholar]
- 33.Nicholas SB, Kalantar-Zadeh K, Norris KC. Socioeconomic disparities in chronic kidney disease. Advances in chronic kidney disease. 2015;22(1):6–15. doi: 10.1053/j.ackd.2014.07.002 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Ene-Iordache B, Perico N, Bikbov B, Carminati S, Remuzzi A, Perna A, et al. Chronic kidney disease and cardiovascular risk in six regions of the world (ISN-KDDC): a cross-sectional study. The Lancet Global Health. 2016;4(5):e307–e19. doi: 10.1016/S2214-109X(16)00071-1 [DOI] [PubMed] [Google Scholar]
- 35.Chen A, Zou M, Young CA, Zhu W, Chiu H-C, Jin G, et al. Disease burden of chronic kidney disease due to hypertension from 1990 to 2019: a global analysis. 2021;8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.van Blijderveen JC, Straus SM, Zietse R, Stricker BH, Sturkenboom MC, Verhamme KM. A population-based study on the prevalence and incidence of chronic kidney disease in the Netherlands. International urology and nephrology. 2014;46(3):583–92. doi: 10.1007/s11255-013-0563-3 [DOI] [PubMed] [Google Scholar]
- 37.Stanifer JW, Jing B, Tolan S, Helmke N, Mukerjee R, Naicker S, et al. The epidemiology of chronic kidney disease in sub-Saharan Africa: a systematic review and meta-analysis. The Lancet Global Health. 2014;2(3):e174–e81. doi: 10.1016/S2214-109X(14)70002-6 [DOI] [PubMed] [Google Scholar]
- 38.ElHafeez SA, Bolignano D, D’Arrigo G, Dounousi E, Tripepi G, Zoccali C. Prevalence and burden of chronic kidney disease among the general population and high-risk groups in Africa: a systematic review. BMJ open. 2018;8(1):e015069. doi: 10.1136/bmjopen-2016-015069 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Plantinga LC. Socio-economic impact in CKD. Néphrologie & thérapeutique. 2013;9(1):1–7. doi: 10.1016/j.nephro.2012.07.361 [DOI] [PubMed] [Google Scholar]
- 40.Vart P, Gansevoort RT, Crews DC, Reijneveld SA, Bültmann U. Mediators of the association between low socioeconomic status and chronic kidney disease in the United States. American journal of epidemiology. 2015;181(6):385–96. doi: 10.1093/aje/kwu316 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Travers K, Martin A, Khankhel Z, Boye KS, Lee LJ. Burden and management of chronic kidney disease in Japan: systematic review of the literature. International journal of nephrology and renovascular disease. 2013;6:1. doi: 10.2147/IJNRD.S30894 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Jha V, Garcia-Garcia G, Iseki K, Li Z, Naicker S, Plattner B, et al. Chronic kidney disease: global dimension and perspectives. The Lancet. 2013;382(9888):260–72. doi: 10.1016/S0140-6736(13)60687-X [DOI] [PubMed] [Google Scholar]
- 43.Xia X, Luo Q, Li B, Lin Z, Yu X, Huang F. Serum uric acid and mortality in chronic kidney disease: A systematic review and meta-analysis. Metabolism. 2016;65(9):1326–41. doi: 10.1016/j.metabol.2016.05.009 [DOI] [PubMed] [Google Scholar]
- 44.Vart P, Gansevoort RT, Joosten MM, Bültmann U, Reijneveld SA. Socioeconomic disparities in chronic kidney disease: a systematic review and meta-analysis. American journal of preventive medicine. 2015;48(5):580–92. doi: 10.1016/j.amepre.2014.11.004 [DOI] [PubMed] [Google Scholar]
- 45.Masson P, Webster AC, Hong M, Turner R, Lindley RI, Craig JC. Chronic kidney disease and the risk of stroke: a systematic review and meta-analysis. Nephrology Dialysis Transplantation. 2015;30(7):1162–9. doi: 10.1093/ndt/gfv009 [DOI] [PubMed] [Google Scholar]
- 46.Alizadeh S, Esmaeili H, Alizadeh M, Daneshzad E, Sharifi L, Radfar H, et al. Metabolic phenotypes of obese, overweight, and normal weight individuals and risk of chronic kidney disease: a systematic review and meta-analysis. Archives of endocrinology and metabolism. 2019;(AHEAD). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Garofalo C, Borrelli S, Minutolo R, Chiodini P, De Nicola L, Conte G. A systematic review and meta-analysis suggests obesity predicts onset of chronic kidney disease in the general population. Kidney international. 2017;91(5):1224–35. doi: 10.1016/j.kint.2016.12.013 [DOI] [PubMed] [Google Scholar]
- 48.Zhang J, Jiang H, Chen J. Combined effect of body mass index and metabolic status on the risk of prevalent and incident chronic kidney disease: a systematic review and meta-analysis. Oncotarget. 2017;8(22):35619. doi: 10.18632/oncotarget.10915 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Fitria L, Prihartono NA, Ramdhan DH, Wahyono TYM, Kongtip P, Woskie S. Environmental and occupational risk factors associated with chronic kidney disease of unknown etiology in West Javanese rice farmers, Indonesia. International Journal of Environmental Research and Public Health. 2020;17(12):4521. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Ruwanpathirana T, Senanayake S, Gunawardana N, Munasinghe A, Ginige S, Gamage D, et al. Prevalence and risk factors for impaired kidney function in the district of Anuradhapura, Sri Lanka: a cross-sectional population-representative survey in those at risk of chronic kidney disease of unknown aetiology. BMC public health. 2019;19(1):1–11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Lunyera J, Mohottige D, Von Isenburg M, Jeuland M, Patel UD, Stanifer JW. CKD of uncertain etiology: a systematic review. Clinical journal of the American Society of Nephrology. 2016;11(3):379–85. doi: 10.2215/CJN.07500715 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Chapman E, Haby MM, Illanes E, Sanchez-Viamonte J, Elias V, Reveiz L. Risk factors for chronic kidney disease of non-traditional causes: a systematic review. Revista Panamericana de Salud Pública. 2019;43. doi: 10.26633/RPSP.2019.35 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Xia J, Wang L, Ma Z, Zhong L, Wang Y, Gao Y, et al. Cigarette smoking and chronic kidney disease in the general population: a systematic review and meta-analysis of prospective cohort studies. Nephrology Dialysis Transplantation. 2017;32(3):475–87. doi: 10.1093/ndt/gfw452 [DOI] [PubMed] [Google Scholar]
- 54.Cheungpasitporn W, Thongprayoon C, Kittanamongkolchai W, Brabec BA, O’Corragain O, Edmonds P, et al. High alcohol consumption and the risk of renal damage: a systematic review and meta-analysis. QJM: An International Journal of Medicine. 2015;108(7):539–48. doi: 10.1093/qjmed/hcu247 [DOI] [PubMed] [Google Scholar]
- 55.Shen Y, Cai R, Sun J, Dong X, Huang R, Tian S, et al. Diabetes mellitus as a risk factor for incident chronic kidney disease and end-stage renal disease in women compared with men: a systematic review and meta-analysis. Springer; 2017. [DOI] [PubMed] [Google Scholar]
- 56.Knobloch K, Yoon U, Vogt PM. Preferred reporting items for systematic reviews and meta-analyses (PRISMA) statement and publication bias. Journal of Cranio-Maxillofacial Surgery. 2011;39(2):91–2. doi: 10.1016/j.jcms.2010.11.001 [DOI] [PubMed] [Google Scholar]
- 57.Egger M, Juni P, Bartlett C, Holenstein F, Sterne J. How important are comprehensive literature searches and the assessment of trial quality in systematic reviews? Empirical study. Health technology assessment (Winchester, England). 2003;7(1):1–76. [PubMed] [Google Scholar]
- 58.Levey AS, Becker C, Inker LA. Glomerular filtration rate and albuminuria for detection and staging of acute and chronic kidney disease in adults: a systematic review. Jama. 2015;313(8):837–46. doi: 10.1001/jama.2015.0602 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Godavitarne C, Robertson A, Ricketts DM, Rogers BA. Understanding and interpreting funnel plots for the clinician. British Journal of Hospital Medicine. 2018;79(10):578–83. doi: 10.12968/hmed.2018.79.10.578 [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
(DOCX)
(DOCX)
(PDF)
(PDF)
(PDF)
(PDF)
(PDF)
(PDF)
Data Availability Statement
All relevant data are within the paper and its Supporting information files.