Abstract
BACKGROUND & AIMS:
Because inflammatory bowel disease is increasing worldwide and can lead to colitis-associated carcinoma (CAC), new interventions are needed. We have shown that spermine oxidase (SMOX), which generates spermidine (Spd), regulates colitis. Here we determined if Spd treatment reduces colitis and carcinogenesis.
METHODS:
SMOX was quantified in human colitis and associated dysplasia using RT-qPCR and immunohistochemistry. We used wild-type (WT) and Smox–/– C57BL/6 mice treated with dextran sulfate sodium (DSS) or azoxymethane (AOM)-DSS as models of colitis and CAC, respectively. Mice with epithelial-specific deletion of Apc were used as a model of sporadic colon cancer. Animals were supplemented or not with Spd in the drinking water. Colonic polyamines, inflammation, tumorigenesis, transcriptomes, and microbiomes were assessed.
RESULTS:
SMOX mRNA levels were decreased in human ulcerative colitis tissues and inversely correlated with disease activity, and SMOX protein was reduced in colitis-associated dysplasia. DSS colitis and AOM-DSS-induced dysplasia and tumorigenesis were worsened in Smox–/– versus WT mice and improved in both genotypes with Spd. Tumor development caused by Apc deletion was also reduced by Spd. Smox deletion and AOM-DSS treatment were both strongly associated with increased expression of alpha-defensins, which was reduced by Spd. A shift in the microbiome, with reduced abundance of Prevotella and increased Proteobacteria and Deferribacteres, occurred in Smox–/– mice and was reversed with Spd.
CONCLUSIONS:
Loss of SMOX is associated with exacerbated colitis and CAC, increased alpha-defensin expression, and dysbiosis of the microbiome. Spd supplementation reverses these phenotypes, indicating that it has potential as an adjunctive treatment for colitis and chemopreventive for colon carcinogenesis.
Keywords: Inflammatory bowel disease, Colitis-associated carcinogenesis, Polyamines, Spermidine, Alpha-defensins, Chemoprevention, Intestinal microbiota
Graphical Abstract

Introduction
Inflammatory bowel disease (IBD), which includes ulcerative colitis (UC) and Crohn’s disease (CD), is a major public health problem. The prevalence of IBD is increasing in the USA, with over 3 million Americans affected.1 IBD is also rising dramatically worldwide, especially in areas with the largest populations, such as Brazil, India, and China.2,3 Newer biologic therapies for IBD have been developed and extensively used, but they induce remission in only about half of patients, are expensive, and have various side effects. There is a substantial risk for patients with IBD of developing colitis-associated carcinoma (CAC),3 a subset of colorectal cancer (CRC), the 3rd most common cancer and 2nd greatest cause of cancer deaths worldwide.4 This leads to the need for frequent colonoscopic surveillance and progression to colectomy with discovery of dysplasia. Thus, strategies to dampen colitis and reduce the risk for neoplastic transformation may positively impact IBD patients.
Polyamines are ubiquitous molecules with many biological effects in microbes, plants, and animals.5 In mammalian cells, there are three polyamines: putrescine (Put) is generated by the rate-limiting enzyme ornithine decarboxylase (ODC) from L-ornithine and is converted sequentially to spermidine (Spd) and spermine (Spm) by Spd synthase and Spm synthase, respectively.5 The enzyme spermine oxidase (SMOX) catalyzes the back-conversion of Spm to Spd.6 We have reported that Spd concentration is regulated by SMOX in eukaryotic cells.7
The effect of polyamines on colon carcinogenesis remains a subject of debate. The traditional assumption is that polyamines mainly act to stimulate proliferation of cancer cells. This has mainly been shown in cell culture systems using the ODC inhibitor difluoromethylornithine (DFMO).8,9 Moreover, DFMO reduces tumorigenesis in the small intestine, but not in the colon, of ApcMin/+ mice.10 In combination with the NSAID sulindac, DFMO reduced the recurrence of adenomas in a population of individuals at high risk for sporadic adenomas,11 but DFMO plus acetylsalicylic acid did not reduce the recurrence of adenomatous polyps.12 No significant difference in adenoma count was observed between celecoxib versus celecoxib plus DFMO in patients with familial adenomatous polyposis.13 In addition, high dietary intake of polyamines was significantly correlated with a lower risk of CRC in a large study of postmenopausal women.14 Furthermore, the role of polyamines in CAC has not been investigated. Thus, in-depth and mechanistic studies related to the role of polyamines in colon tumorigenesis are needed.
We have reported that i) mice with deletion of Smox display low levels of Spd in the colon and exhibit more severe dextran sulfate sodium (DSS)-induced colitis than wild-type (WT) animals,7 and ii) the colonic concentration of Spd, but not Put or Spm, is inversely correlated with the severity of histopathology in WT mice.7 However, these observations do not prove that loss of SMOX activity aggravates colitis by reducing Spd or that Spd is beneficial. Therefore, we sought to directly address the therapeutic potential of Spd supplementation. We found that oral Spd treatment protects from DSS colitis, azoxymethane (AOM)-DSS model of CAC, and tumorigenesis associated with Apc deletion. Our transcriptomic and microbiome analyses indicate that Spd i) reduces expression of genes encoding for alpha-defensins (DEFAs), and ii) maintains a protective gut microbiota. Human translational relevance is supported by our findings that SMOX levels are decreased in IBD patients with UC, and further reduced in those with colitis-associated high-grade dysplasia (HGD), indicative of dysregulated polyamine metabolism in IBD and potential opportunities for intervention with Spd.
Methods
Ethics Statement
The ethics statements regarding human tissues and animal experiments are provided in the Supplementary Methods.
Animals Models and Spd Treatment
Age-matched (8–12 wk) C57BL/6 and C57BL/6 Smox–/– mice7,15,16 were house-bred in our animal facility and were fed 5L0D chow (LabDiet). C57BL/6 germ-free (GF) animals (6 wk) were purchased from Charles River. Mice were treated or not with the two antibiotics, enrofloxacin (0.575 mg/ml) and ampicillin (1 mg/ml), in the drinking water for 14 days; this combination has been recently reported to efficiently reduce the number of bacteria in the gut microbiota.17. The DSS colitis (10 days) and AOM-DSS CAC (56 days) models were performed on male mice and analyzed as described.18–20
We also used transgenic C57BL/6 mice with a tamoxifen-inducible disruption of Apc using the intestinal epithelial cell-specific, caudal type homeobox 2 (CDX2) Cre (CDX2P-CreERT2;Apcfl/fl),21,22 which recapitulates genetically-driven CRC associated with APC loss23. Male mice were i.p. injected or not with 4.5 mg/kg tamoxifen (TAM) dissolved in corn oil:ethanol (9:1, vol:vol) solution and sacrificed after 39 days.
For all these models, animals were treated or not with 14 mM Spd (Sigma) in the drinking water during the whole experiments. We chose this concentration by the following rationale: The regular 5L0D chow contains 330 ng/mg of Spd (Supplementary Figure 1); according to food and water consumption by mice,24 this corresponds to an intake of 8 μmol Spd/day, equivalent to 1.4 mM Spd in the drinking water; we thus used 14 mM Spd, representing a 10-fold increase in Spd intake per day.
Fecal Transplantation and Gut Microbiota Analysis
The cecal contents of 3 WT or 3 Smox–/– mice were harvested, pooled, and diluted in PBS (10 mg/ml); each GF mouse was gavaged with 100 μl of the WT or Smox–/– suspension. After 14 days, stools were collected, and these animals were subjected to DSS colitis. Details regarding the sequencing of the V4 region of the 16S rRNA gene of the intestinal microbiota are provided in the Supplementary Methods.
Histological Injury Scores
Swiss-rolled colons were fixed in formalin and embedded in paraffin, and 5 μm sections were stained with H&E and examined in a blinded manner by a gastrointestinal pathologist (M.K.W.). Histological injury score (0–40) and severity of dysplasia were determined as described.18–20
Immunostaining
The details regarding immunohistochemistry for SMOX and immunofluorescence for lysozyme C-1 (LYZ1) and DEFA5 are provided in the Supplementary Methods.
Measurement of Polyamines
The concentrations of the three biogenic polyamines were determined by mass spectrometry as reported.25
Analysis of mRNA Expression
Gene expression was assessed by RNA sequencing (RNA-Seq) and by RT-real-time PCR. The procedures are described in the Supplementary Methods. Ingenuity Pathway Analysis (IPA) software (QIAGEN) was used for the functional interpretation of differentially expressed genes (DEGs) obtained from the RNA-Seq.
Statistics
The details about statistical analysis are provided in the Supplementary Methods.
Data Availability
RNA-Seq data can be accessed on the Gene Expression Omnibus repository using the accession number GSE158826. The 16S rRNA sequencing of the fecal microbiota has been deposited on the Sequence Read Archive website with the BioProject ID: PRJNA685397.
Results
Regulation of SMOX Expression in Human Colitis
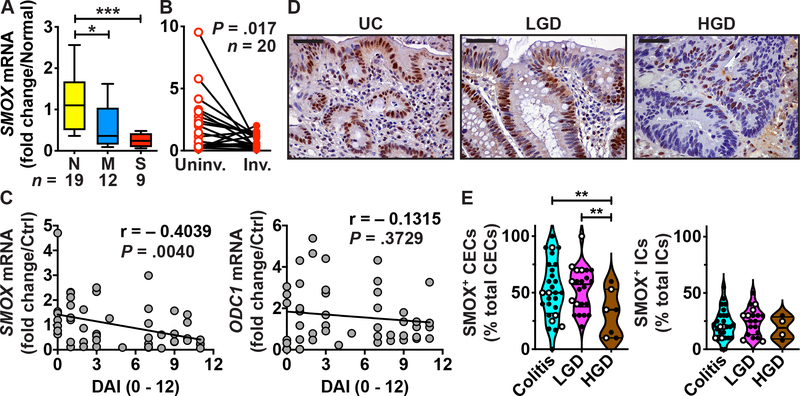
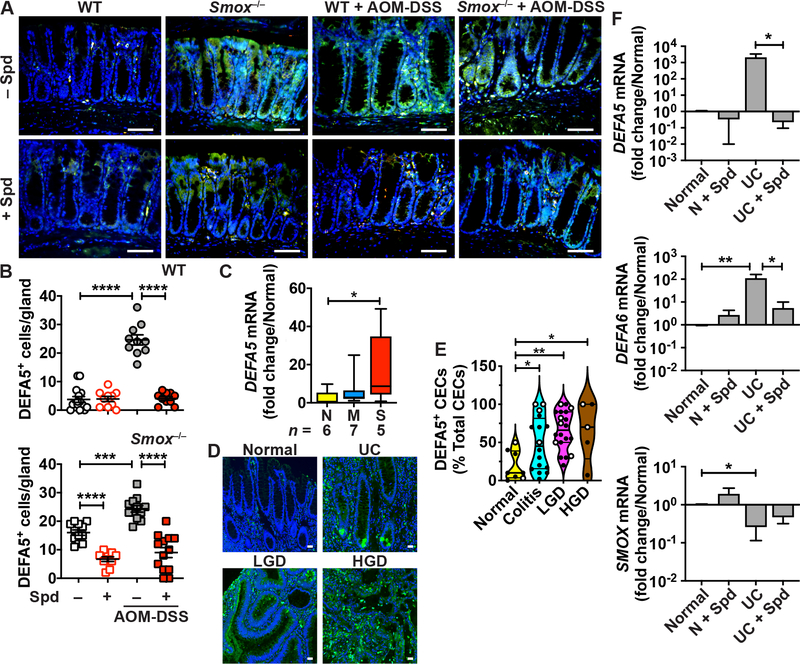
We assessed the clinical relevance of our finding that Smox deletion in mice worsens colitis7 by determining the levels of SMOX in patients with UC. Utilizing RNA samples from a previous study conducted at Vanderbilt University Medical Center (VUMC),26,27 we found that SMOX mRNA expression was reduced in colon tissues of patients with moderate and severe UC (Figure 1A) versus normal controls. In patients with left-sided UC, SMOX mRNA levels were low in involved areas compared to paired uninvolved proximal tissues (Figure 1B). Moreover, the expression of the SMOX gene was inversely correlated with the Mayo disease activity index (DAI) in UC patients (Figure 1C), suggesting that the lowest Spd content is associated with higher disease activity. In contrast, expression of ODC1, which encodes for ODC, was not associated with the DAI in the same patients, highlighting the specificity of our finding to SMOX mRNA (Figure 1C). Further, we immunostained an IBD tissue microarray (TMA) from VUMC18,19 for SMOX. We observed expression in colonic epithelial cells (CECs) and in lamina propria immune cells (ICs) from patients with UC, which was reduced in patients with HGD (Figure 1D). Quantification of the staining evidenced a significant reduction of SMOX level in CECs, but not in ICs, in patients with HGD compared to individuals with colitis or low-grade dysplasia (LGD; Figure 1E).
Figure 1.
SMOX expression in human colitis. (A-C) RNA was extracted from colon biopsies from normal (N) or UC patients with moderate (M) or severe (S) colitis (A), and from paired uninvolved and involved UC tissues (B). SMOX mRNA levels were determined by RT-real-time PCR. (C) Correlation between DAI and SMOX or ODC1 mRNA levels. This figure depicts the UC patients shown in (A) plus patients with quiescent (n = 15) and mild (n = 11) UC. (D-E) Tissues sections of a TMA were immunostained for SMOX. (D) Representative images of patients with UC (n = 28), LGD (n = 22), and HGD (n = 7); scale bar, 50 μm. (E) Quantification of SMOX+ epithelial cells among total CECs and SMOX+ ICs among all immune infiltrates. In E, solid circles are UC tissues and open circles are CD tissues. In A and E, *P < .05, **P < .01, ***P < .001 by one-way ANOVA and Tukey test; in B, the P values was obtained by Wilcoxon matched-pairs signed rank test; in C, the Pearson r and the P values were determined by simple linear regression.
These data suggest that patients with colitis and advanced dysplasia have loss of SMOX, needed to back-convert Spm to Spd.
Treatment with Spd Improves DSS Colitis
Because patients with colitis have low levels of SMOX in their colon (Figure 1) and Smox–/– mice exhibit exacerbated DSS colitis,7 we first tested the effect of Spd supplementation in this model. Upon DSS treatment, Put levels were increased in the colon of WT and Smox–/– mice compared to naïve animals (Figure 2A). In contrast, the concentration of Spd was enhanced only in WT mice after DSS treatment, and there was significant reduction of Spd levels in the colon of Smox–/– mice, treated or not with DSS, compared to WT animals (Figure 2A). The treatment of Smox-deficient mice with Spd led to a significant increase of Spd content in the colon; this was observed in control animals and in mice with colitis (Figure 2A). Together, these data indicate that Spd treatment restores colonic Spd concentration in mice with or without colitis, without increasing the concentration of the two other polyamines.
Figure 2.
Effect of Spd supplementation on DSS colitis. C57BL/6 WT and Smox–/– mice were treated or not with 4% DSS for 5 days and then kept for 5 more days on water alone. Spd was added to the water or to the DSS solution during all the experiments. (A) Colonic polyamine levels measured by LC-MS. (B) Body weights were measured daily and are depicted as percentage of initial body weight. (C) Colons were harvested, washed, and measured. (D-E) Histological injury scores (D) were determined from H&E staining performed on Swiss-rolls of the colon (E); the histologic injury scores of untreated WT and Smox–/– mice ± Spd were all 0 (not shown). Scale Bar, 50 μm. In A-D, *P < . 05, **P < .01, ***P < .001, ****P < .0001 by two-way ANOVA and Tukey test. In B, *P < .05 compared to Smox–/– + DSS + Spd.
WT and Smox–/– mice began losing weight on day 4 after starting DSS (Figure 2B). However, there was more body weight loss in Smox–/– mice compared to WT animals (Figure 2B). Remarkably, the weight loss was ameliorated in Smox–/– mice treated with Spd (Figure 2B). In addition, DSS-induced colon shortening was significantly improved in WT and Smox–/– mice that were given Spd (Figure 2C). Further, histological injury was significantly increased in Smox–/– mice compared to WT animals (Figure 2D); and histological damage was reduced in both WT and Smox–/– mice receiving Spd (Figure 2D), with less colonic inflammation, epithelial damage, and crypt loss (Figure 2E).
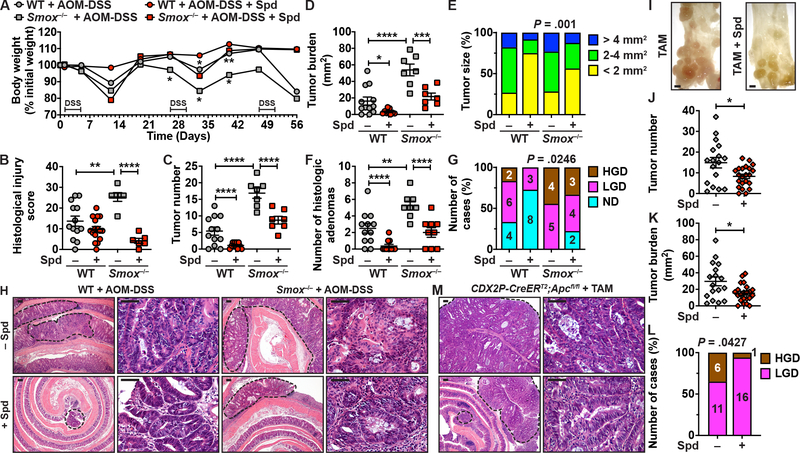
Smox Deletion Worsens CAC, which is Reversed with Spd
Since Spd protected WT and Smox-deficient mice from DSS colitis, we next determined whether it could have a protective effect on development of CAC using the AOM-DSS model.18–20 There was more body weight loss (Figure 3A) and histologic colitis (Figure 3B) in Smox–/– mice compared to WT animals, and these parameters were improved with Spd treatment. Moreover, we found a significant increase in the number of tumors (Figure 3C), total tumor burden per colon (Figure 3D), tumor size (Figure 3E), and the number of histologic adenomas (Figure 3F) in mice lacking Smox compared to WT animals. Of importance, all four of these parameters were ameliorated in both WT and Smox–/– mice receiving Spd (Figs. 3C–3F). Histologic assessment further revealed that in WT mice, 50% and 17% treated with AOM-DSS alone had LGD and HGD, respectively, while those treated with Spd had no HGD (Figure 3G); in Smox–/– mice, 100% had dysplasia, with 44% HGD, which was reduced to 77% dysplasia and 33% HGD with Spd supplementation (Figure 3G). The lower magnification hematoxylin and eosin (H&E) photomicrographs showed that there were no tumors in control mice (Supplementary Figure 2) and that the number and the size of the tumors was reduced in Spd-treated WT and Smox–/– mice (Figure 3H). Images at higher magnification showed HGD with marked reduction of interglandular stroma, complex irregularity of glands with a cribriform pattern and back-to-back glands, and loss of nuclear polarity with respect to the basement membrane in WT and Smox–/– mice (Figure 3H). In contrast, the images from animals treated with Spd exhibit improvement in histology with LGD (Figure 3H).
Figure 3.
Effect of Spd treatment on colon carcinogenesis. (A-H) WT and Smox–/– mice were treated with AOM-DSS and were given Spd throughout the experiments. Body weights were measured weekly and are depicted as percentage of initial body weight (A). *P < .05 and **P < .01 compared to mice from the same genotype not treated with Spd. After 56 days, colons were removed and histological injury score (B), tumor number (C), tumor burden (D), tumor size (E), number of histologic adenomas (F), and frequency of LGD and HGD (G) were determined; ND, no dysplasia. Panel H depicts representative images of H&E staining. Tumors are surrounded by dotted lines. High-power photomicrographs show HGD in WT and Smox–/– mice treated with AOM-DSS and LGD in those animals that were given Spd. The scale bars on the low-power and high-power photomicrographs correspond to 100 and 50 μm, respectively. (I-M) CDX2P-CreERT2;Apcfl/fl mice were treated with TAM and were given Spd or not. After 39 days, tumor number (I-J) and tumor burden (K) were determined in the mid and distal colon; scale bar in I, 1 mm. The frequency of LGD and HGD was determined by histologic assessment (L). Panel M shows representative images of H&E staining of the colon of TAM-treated animals. For all the panels with symbols, *P < .05, **P < .01, ***P < .001, ****P < .0001 by two-way ANOVA and Tukey test (A-D and F) or Student’s t test (J, K). We also used Chi-Square (E and G) and Fisher’s exact test (L).
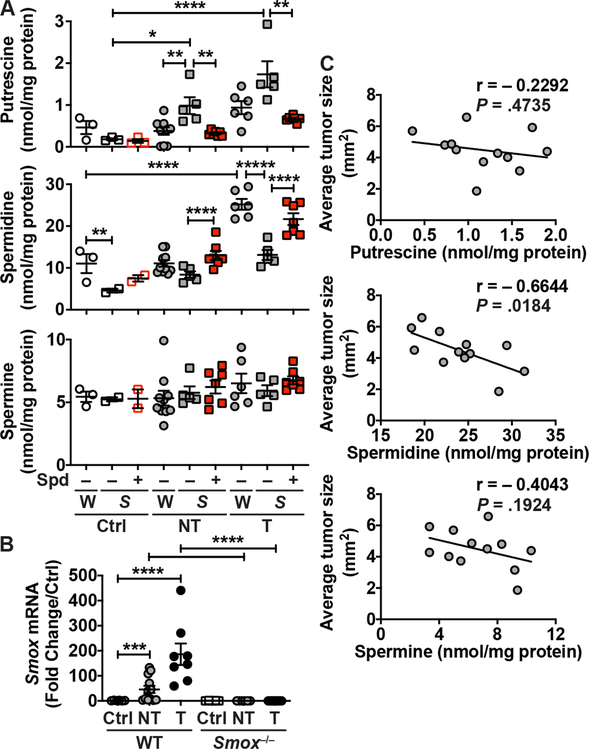
We then analyzed the concentration of the three polyamines in CAC tissues. Because WT animals treated with Spd had very few tumors, it was not possible to perform this analysis in this group. In this 56-day model, Put concentration was not affected by AOM-DSS treatment in WT mice, but was significantly augmented by 5.4- and 9.4-fold in the non-tumor and tumor tissues of Smox–/– mice, respectively (Figure 4A). In addition, the levels of Put in Smox-deficient mice were reduced with Spd treatment (Figure 4A). Conversely, Spd was significantly increased by ~ 2.3-fold in the tumors of WT mice compared to control animals (Figure 4A). This was consistent with the enhanced expression of Smox mRNA in the tumors of WT mice at day 56 (Figure 4B); note that the Smox gene was not expressed in control mice and in non-tumor and tumor tissues from Smox–/– mice. The deletion of Smox resulted in a significant reduction of Spd concentration in the tumors compared to WT animals, and this was reversed by Spd supplementation (Figure 4A). Spm level was not affected by AOM-DSS treatment nor by Spd supplementation in either genotype (Figure 4A). To gain more insight into the role of polyamines in colon carcinogenesis, we correlated the size of the tumors in WT mice with the concentration of each polyamine. We found that Spd level in the colon was inversely correlated with tumor size in the AOM-DSS model (Figure 4C); thus, more Spd was associated with decreased tumorigenesis (Figure 4C). There were no significant associations between the concentration of Put or Spm with tumor development (Figure 4C).
Figure 4.
Regulation of polyamine levels by Spd supplementation during experimental CAC. (A) Putrescine, spermidine, and spermine concentrations were measured by LC/MS in non-tumor (NT) and tumor (T) tissues from WT and Smox–/– mice ± AOM-DSS ± Spd. (B) Smox mRNA expression was assessed by RT-real-time PCR. In A and B, *P < .05, **P < .01, ***P < .001, ****P < .0001 by two-way ANOVA and Tukey test. (C) Correlation plots comparing the average tumor size of WT mice not receiving Spd supplementation to the corresponding polyamine concentration; this figure includes the animals depicted in A plus data from additional WT mice not treated with Spd. The Pearson r and the P values were determined by simple linear regression.
Therefore, Spd supplementation counteracted the development of CAC in WT mice and the exacerbated tumorigenesis observed with Smox deletion.
Spd Reduces Tumorigenesis Associated with Apc Loss
We then assessed whether Spd supplementation is protective in a model of sporadic colon cancer. The number of tumors (Figures 3I–J) and the tumor burden (Figure 3K) observed in TAM-treated CDX2P-CreERT2;Apcfl/fl mice were both significantly reduced by Spd supplementation. Further, the number of tumors with HGD was also reduced in mice treated with Spd (Figures 3L). Figure 3M shows a part of a large tumor with HGD in the TAM group, whereas smaller tumors with LGD were found in mice with Spd treatment. There were no tumors in animals that did not receive TAM, treated or not with Spd (Supplementary Figure 2).
Transcriptomic Regulation of DEFAs by Spd
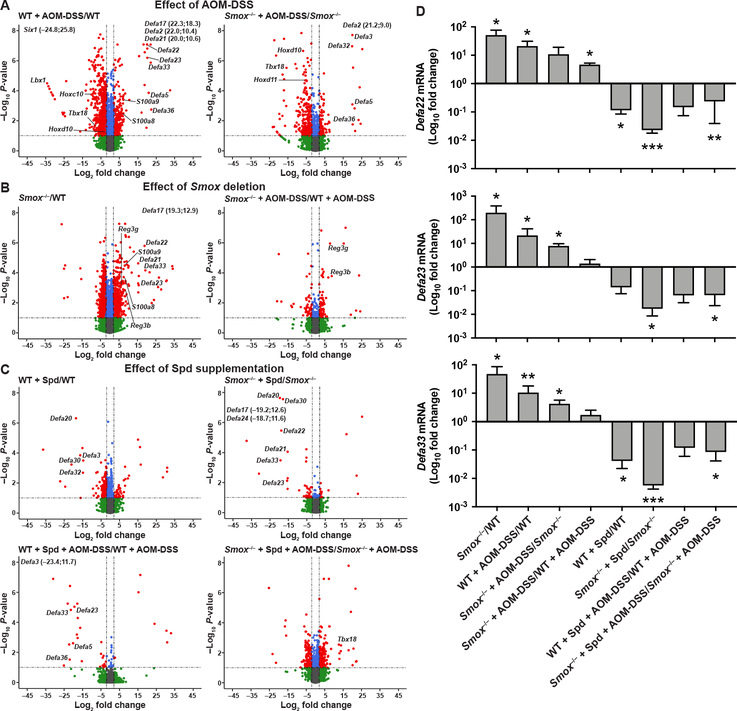
We then assessed by RNA-Seq the transcriptomic changes occurring in the colonic mucosa associated with AOM-DSS treatment, Smox deletion, and Spd supplementation. This analysis was focused on the non-tumor tissues because: i) our goal was to identify the effect of Smox deletion and Spd treatment on the molecular events potentially involved prior to tumorigenesis, and ii) there were very few tumors in mice that were given Spd, making analysis of tumors in the Spd-treated mice not possible. Overall, 31,023 sequences were identified, comprising 20,430 known mRNAs and 10,593 unknown sequences. For the analysis of the DEGs between groups, we focused on the genes upregulated or downregulated by 2–fold or more, and with a P < .05. The complete list of DEGs, including gene IDs, fold-changes, and P-values are listed in Supplementary Table 1. In WT mice receiving AOM-DSS, we found 392 and 1586 genes upregulated and downregulated, respectively. In Smox–/– mice + AOM-DSS, there were 114 genes upregulated and 271 downregulated compared to untreated animals. Strikingly, we observed that genes encoding for numerous DEFAs, such as Defa2, Defa5, Defa17, Defa20, Defa22, Defa23, Defa33, and Defa36, were the most induced in AOM-DSS-treated WT and Smox–/– mice (Figure 5A). Interestingly, S100a8 and S100a9, the two genes encoding for calprotectin, which plays a role in leukocyte recruitment and regulation of the immune response,28 were also stimulated in the colon during AOM-DSS treatment (Figure 5A). In addition, we observed that homeobox genes, such as Six1, Lbx1, and Hoxc10 for WT mice as well as Hoxd10, Hoxd11, and Tbx18 for Smox–/– mice, were downregulated in animals with AOM-DSS compared to control mice (Figure 5A).
Figure 5.
Transcriptomic changes in the colon regulated by Spd. RNA was extracted from non-tumor tissues from WT and Smox–/– mice ± AOM-DSS ± Spd after 56 days (n = 3 mice per group), and then sequenced. Paired comparisons were performed to study the effect of AOM-DSS treatment (A), Smox deletion (B), and Spd supplementation (C), and the corresponding volcano plots were generated. Red dots depict DEGs (more than 2-fold; P < .05). Genes with values outside the X- and/or Y-axis limits are given with their (x;y) coordinates. The full list of DEGs is shown in Supplementary Table 1. (D) The expression of Defa22, Defa23, and Defa33 was also assessed by RT-real-time PCR. *P < .05, **P < .01, ***P < .001 (Student’s t test) denote significant differences for each comparison presented in the X-axis labels.
Moreover, 622 genes were upregulated and 1197 were downregulated in the colon of naïve Smox–/– mice when compared to WT (Figure 5B). Surprisingly, the genes S100a8, S100a9, Defa17, Defa21, Defa22, Defa23, and Defa33, which were induced by AOM-DSS treatment (Figure 5A), were also upregulated in untreated Smox–/– mice (Figure 5B). The genes Reg3b and Reg3g that encode for bactericidal C-type lectins were also induced in mice lacking Smox (Figure 5B). When Smox–/– mice treated with AOM-DSS were compared to WT mice treated with AOM-DSS, 64 genes were upregulated, including Reg3b and Reg3g, and 55 were downregulated (Figure 5B).
We also assessed the effect of Spd on the transcriptome of the colon. The number of DEGs in response to Spd was relatively low in naïve WT animals (38 upregulated and 394 downregulated) and in Smox-deficient mice (12 upregulated and 28 downregulated). However, as shown in Figure 5C, Spd supplementation of WT and Smox–/– mice led to a significant decrease of many genes encoding for DEFAs. This effect of Spd was also observed in WT mice given AOM-DSS (Figure 5C).
To confirm the main findings with the genes encoding for DEFAs, we first performed real-time RT-PCR for the genes Defa22, Defa23, and Defa33 on the same RNA used for RNA-Seq. Similar expression patterns as detected in the RNA-Seq were observed by our semi-quantitative PCR analysis (Figure 5D). Then, we performed new experiments to further assess the unforeseen effect of Smox deletion and Spd supplementation. WT and Smox–/– mice were treated with 14 mM Spd for 14 days and RT-PCR was performed from isolated CECs. Again, we found that mRNA levels encoding DEFAs were enhanced in Smox-deficient mice and were reduced in those animals by Spd treatment (Supplementary Figure 3).
Lastly, we assessed the expression of DEFA5 and LYZ1, a marker of myeloid and Paneth cells, by immunofluorescence. DEFA5 was present in colonic mononuclear myeloid cells of WT mice (Figure 6A). The expression of DEFA5 was enhanced in the crypt and surface epithelium of naive Smox–/– mice and in both WT and Smox–/– mice treated with AOM-DSS (Figure 6A). Note that both DEFA5+LYZ1+ and DEFA5+LYZ1– CECs were increased in Smox-deficient-mice. Overall, we also found a marked reduction of DEFA5 immunostaining in CECs in animals supplemented with Spd (Figure 6B). Similarly, with DSS colitis, DEFA5 was more abundant in CECs of Smox–/– mice than in WT mice, and this was reduced with Spd supplementation (Supplementary Figure 4A). In the ileum, where Paneth cells are located, the double staining for DEFA5 and LYZ1 was essentially found at the base of the glands of WT and Smox–/– mice, with no difference between the genotypes (Supplementary Figure 4B). When mice were treated with DSS, we observed increased DEFA5 staining in the upper part of the glands, and more intense staining of DEFA5+LYZ1+ cells in Smox–/– mice (Supplementary Figure 4B). There were less DEFA5+ cells in the ileum of DSS-treated mice that were given Spd (Supplementary Figure 4B).
Figure 6.
DEFA expression in murine and human colitis. (A) Representative photomicrographs of DEFA5 (green) and LYZ1 (red) immunostaining in the colon of WT and Smox–/– mice ± AOM-DSS ± Spd after 56 days. Merged colors are depicted in yellow. The nuclei stained with DAPI are shown in blue. Scale bars, 50 μm. (B) Quantification of epithelial staining shown in A. Each dot represents one gland, and the staining was quantified from multiple glands from n = 3 mice per group. (C) RNA was extracted from colon biopsies from normal (N) or UC patients with moderate (M) or severe (S) colitis and DEFA5 mRNA levels were measured by RT-real-time PCR. (D) Tissues sections of a TMA were immunostained for DEFA5 (green) and the representative images of normal tissues or colon from patients with UC, LGD, and HGD are shown; scale bar, 50 μm. (E) Quantification of DEFA5+ epithelial cells among total CECs; solid circles are UC tissues and open circles are CD tissues. (F) Human colonoids from normal (n = 2) or UC patients (n = 2) were cultured 48 h with or without 10 mM Spd; RNA was extracted and the expression of DEFA5, DEFA6, and SMOX was determined; each bar represents the mean ± SEM of three experiments performed with each colonoid line. In the panels with statistics, *P < .05, **P < .01, ***P < .001, and ****P < .0001 by one-way (C, E) or two-way (B, F) ANOVA and Tukey test.
In conclusion, our data indicate that numerous genes encoding for DEFAs are: i) increased in the colon of Smox–/– mice, which exhibit a low level of Spd in their colon, compared to WT; ii) enhanced in WT mice in response to DSS and AOM-DSS, and further induced in Smox–/– mice; iii) reduced in both WT and Smox–/– genotypes with Spd supplementation.
Spd Regulates Pathways Related to Immunity and Carcinogenesis
To further define the functions and metabolic pathways regulated by Smox deletion and Spd supplementation, we analyzed the DEGs of each single comparison derived from the RNA-Seq data using IPA. The complete data are shown in Supplementary Table 2. The conditions with the highest number of pathways predicted to be significantly affected (–2 > z-score > 2) were Smox–/–/WT (218/500), WT + AOM-DSS/WT (88/500), and Smox–/– + Spd + AOM-DSS/Smox–/– + AOM-DSS (56/500). When we focused on the functions related to cell metabolism, immune pathways, and carcinogenesis, we found that the categories linked to recruitment, migration, and activation/response of leukocytes, such as macrophages and neutrophils, were significantly increased in the colon of Smox–/– mice compared to WT (Supplementary Figure 5A). In addition, the categories related to morbidity or mortality, cancer, carcinoma, and malignant solid tumor were also enhanced by Smox deletion (Supplementary Figure 5A). In contrast, cellular functions, including formation of intercellular junctions, formation of plasma membrane, formation of cellular protrusions, organization of cytoplasm, or organization of cytoskeleton were reduced in the colon of mice lacking Smox (Supplementary Figure 5A). The treatment with Spd of AOM-DSS-treated Smox–/– mice led to a dampening of the pathways related to immune cell recruitment and activation (Supplementary Figure 5B).
These data further indicate that Smox deficiency is associated with a pro-inflammatory and pro-carcinogenic state in the colon, and that Spd reduces this immune dysregulation.
DEFAs in Patients with Colitis
The expression of the DEFA5 gene was enhanced in the colon of patients with severe colitis compared to normal individuals (Figure 6C). Further, using the VUMC TMA26,27, we observed that DEFA5 protein was significantly more abundant in CECs of patients with colitis, LGD, and HGD (Figures 6D–E). Notably, colonoids isolated from patients with UC had reduced levels of SMOX and higher levels of DEFA5 and DEFA6 transcripts compared to cells from normal areas from the same patients (Figure 6F). Finally, Spd supplementation reduced DEFA5 and DEFA6 mRNA expression in UC-derived organoids (Figure 6F).
Spd Affects the Composition of the Gut Microbiota
Because the deletion of Smox led to the increase of antimicrobial DEFA expression, we reasoned that this could lead to dysbiosis of the intestinal microbiota prior to induction of colitis or CAC. We thus determined the composition of the fecal microbiota of WT and Smox–/– mice treated or not with 14 mM Spd for 14 days. The total number of bacteria in the gut was not significantly affected by Smox deletion or Spd supplementation (Figure 7A). We then assessed the microbiome by sequencing the V4 region of the 16S rRNA gene. The beta-diversity indicated that fecal samples of each group were clustered together, and there was a significant, distinct clustering of the four groups (Figure 7B). In addition, we found increased diversity in the microbiome of Smox–/– mice compared to WT animals (Figure 7C). The treatment of WT mice with Spd enhanced the microbial diversity, whereas Spd supplementation of Smox-deficient mice reduced it significantly (Figure 7C).
Figure 7.
Effect of Smox deletion and Spd supplementation on the fecal microbiome. DNA was extracted from colonic feces of WT and Smox–/– mice treated or not with Spd for 14 days. (A) Total number of bacteria, determined by real-time PCR. (B) Principal coordinate analysis plot, based on the unweighted UniFrac metric; P was determined by PERMANOVA. (C) Alpha diversity, evaluated by the inverse Simpson index. (D-E) Gut bacterial community composition at phylum (D) and genus (E) levels, expressed as a ratio to the total community. *P < .05 and **P < .01. All P-values are provided in Supplementary Table 3.
The fecal microbiota of C57BL/6 mice fed with the regular 5L0D diet was dominated by the Bacteroidetes phylum (Figure 7D), as we reported.29 This was also observed in Smox–/– mice and in animals treated with Spd (Figure 7D). However, the relative abundance of the Proteobacteria and Deferribacteres phyla was increased in Smox–/– mice and was reduced with Spd supplementation (Figure 7D). Further, we observed that bacteria belonging to the Prevotella and Bacteroides genus were dominant in WT mice, whereas Smox–/– mice exhibited a significant increase of the prevalence of bacteria from the Porphyromonadaceae and Lachnospiraceae family, and a marked reduction of the Prevotella genus and Prevotellaceae (Figure 7E). WT and Smox–/– mice treated with Spd exhibited a similar microbiome at the genus levels, characterized by the prevalence of Prevotella, Bacteroides, and Porphyromonadaceae (Figure 7E).
To further verify whether SMOX influences the gut microbiome, we transplanted C57BL/6 GF mice with the cecal contents of WT or Smox–/– mice. We confirmed with a new set of mice that Smox deletion was associated with the diminution of the prevalence of Prevotella and an increase of bacteria from the Porphyromonadaceae and Lachnospiraceae family (Supplementary Figure 6A). Strikingly, the abundance of Prevotella was restored in gnotobiotic mice transplanted with the Smox–/– gut microbiota, and mice associated with WT or Smox–/– microbiota exhibited a similar microbiome (Supplementary Figure 6A). As expected, we did not observe a worsening of the DSS colitis in gnotobiotic WT mice harboring the Smox–/– microbiota (Supplementary Figure 6B).
Finally, we found that the increased colonic expression of DEFA5 in Smox–/– mice compared to WT was still observed when animals were pre-treated with an antibiotic cocktail (Supplementary Figure 7). Spd supplementation decreased DEFA5 levels in Smox–/– mice that were given antibiotics or not (Supplementary Figure 7). These data indicate that Spd regulates DEFA level in the colon through a mechanism independent of the gut microbiota.
Discussion
Our study presents compelling evidence that supplementation with the polyamine Spd protects against experimental colitis and CAC in mice. We found that deletion of the Smox gene, which results in Spd depletion in the colon, worsens AOM-DSS-induced carcinogenesis in addition to DSS-induced colitis, and that the exacerbated disease is reduced by Spd treatment. Mechanistically, we demonstrate that the low Spd level in Smox–/– mice is associated with increased expression of DEFAs in CECs and with a switch in the composition of the gut microbiota. Strikingly, Spd treatment reduces DEFA levels and restores the normal microbiome.
Spd supplementation has anti-aging, neurological, and cardiovascular benefits, with no apparent risks of developing cancer in long-term use in humans or mice.30–32 These recent studies and our current findings suggest that the concept that polyamines support carcinogenesis should be amended. First, cancer development is a dynamic pathophysiological process involving multiple stages ranging from initiation, promotion, malignant progression, to metastasis. In accordance with our present data, epidemiological data have linked increased dietary intake of Spd to reduced CRC prevalence.14 However, it has been shown that the growth of established tumor cells requires polyamines and that ODC inhibition with DFMO, in combination or not with other agents, reduces growth of tumors in anaplastic glioma,33 prostate cancer,34 nonmelanoma skin cancer,35 and colorectal adenomas.11 This dichotomous role of polyamines is supported by findings that i) DFMO reduces the proliferation of colonic tumors rather than primary intestinal epithelial cells,36 ii) the sensitivity to DFMO varies by cell line and does not correlate with polyamine content,37 and iii) transgenic mice overexpressing ODC do not develop cancer spontaneously, but exhibit increased tumorigenesis after induction with carcinogens.38,39 Moreover, we show here that Spd treatment also reduced development of tumors in a model of sporadic CRC. We propose that Spd protects from the initiation of neoplasia, but that once tumors are established, polyamines may sustain their growth.
Second, the role of polyamines, and more specifically of Spd, needs to be recognized as cell- and tissue-specific. In the gastrointestinal tract, high levels of SMOX activity have been associated with development of gastric cancer;40,41 in contrast, we found herein that SMOX reduces tumorigenesis in the colon and that Spd protects against CAC. Similarly, Spd treatment prevents development of hepatocellular carcinoma induced by diethylnitrosamine.42 Third, the etiology of carcinogenesis should be taken into consideration. We found that Smox deletion is deleterious in a model of colon cancer initiated by the association of a carcinogen and DSS that induces chronic inflammation through epithelial injury. Accordingly, we have shown that DFMO worsens colitis in mice,43 further indicating that polyamines are required to stimulate mucosal repair and/or limit inflammation. In contrast, in two models of bacterial infection leading to cancer, gastric cancer induced by Helicobacter pylori and Bacteroides fragilis-induced colon tumorigenesis in ApcMin/+ mice, it has been found that SMOX activity supports carcinogenesis by causing inflammation and oxidative DNA damage.16,40,41,44 Supporting the concept that polyamines are deleterious in infection, we have also described that Smox deletion reduced colonic inflammation during Citrobacter rodentium colitis and that all three polyamines were positively correlated with the injury score in this infection model.7 Thus, in the same way as other pluripotent metabolites with reactive and/or signaling properties, such as nitric oxide or prostaglandins, the role of polyamines in carcinogenesis remains complex, but the present experimental evidence that Spd supplementation is protective in the AOM-DSS model represents a critical step toward the use of Spd as chemopreventive agent for CAC.
It is desirable to understand the cellular mechanism by which a dietary supplement to prevent CAC may benefit patients. The colonic transcriptomic analysis revealed that several genes encoding for DEFAs were upregulated in the colon of mice with Smox deletion and were downregulated in WT and Smox–/– mice treated with Spd, indicating that Spd reduces their expression. Importantly, the Defa genes were regulated by Smox deletion and Spd treatment in naïve mice, before initiation of tumorigenesis by AOM-DSS. This suggests that the effect of Spd on colitis is mediated by DEFAs and not that the differential regulation of Defa genes is the result of the varied inflammatory response observed in WT and Smox–/– mice ± Spd. The number of DEFA5+ cells was markedly increased by Smox deficiency and in response to AOM-DSS, and was reduced by Spd treatment, confirming the transcriptomic data. Supporting our finding that DEFAs are potential key elements in colon inflammation/carcinogenesis, it has been shown that that mice treated with human DEFA1 exhibited a worsening of DSS colitis45 and tumor development in rats treated with AOM is associated with an upregulation of Defa5.46 Moreover, mice with deletion of type I IFN receptor in intestinal epithelial cells exhibited expansion of cells secreting DEFAs and increased tumorigenesis.47 Further, the gene DEFA5 has been identified by RNA-Seq as a marker for cancer development in IBD patients48,49, supporting our finding that DEFA5 mRNA and DEFA5 protein are more abundant in the colon of patients with colitis compared to normal individuals. In addition, DEFA6 is highly expressed in CRC-derived cell lines and in colon biopsies from CRC patients.50 Gene silencing of DEFA6 impairs proliferation, migration, and invasion of CRC cell lines,50 indicating a pro-oncogenic role of DEFAs. Of note, we demonstrated that UC-derived colonoids exhibit less SMOX and more DEFA5 and DEFA6 transcripts compared to normal colonoids, and that Spd treatment dampens DEFA5 and DEFA6 expression. Since we also found that Smox deletion and Spd supplementation affect DEFA5 expression independently of the presence of the gut microbiota, we suggest that Spd is a direct regulator of DEFAs. The involvement of DEFAs in the AOM-DSS-induced CAC model and the anti-oncogenic role of Spd represents an unrecognized mechanism of carcinogenesis regulation that may have critical implications for human health.
In addition to the role of DEFAs in colitis and CAC, these antimicrobial effectors can also affect the composition of the microbiota51,52 without affecting the total bacterial numbers.52 We found that Smox deletion led to a dysregulation of the gut microbiome, characterized by reduced abundance of Prevotella, which have been shown to be susceptible to human beta-defensins,53 and an increase of Porphyromonadaceae and Lachnospiraceae. Notably, there was no change in the relative abundance of the genus Bacteroides, consistent with the fact that DEFAs have no effect on these bacteria.54 The dysbiosis in Smox–/– mice was reversed towards the WT microbiome in animals supplemented with Spd, demonstrating that this polyamine has a key role in affecting the composition of the microbiota and is essential for its resilience. Importantly, decreased abundance of Prevotella55 and enrichment in Proteobacteria56,57 and Deferribacteres58 have been associated with colitis. Thus, the impact of Spd on the microbiome may play a role in the development of IBD and CAC.
In summary, we demonstrate that Spd is a key polyamine for colon homeostasis by dampening DEFA expression in CECs and maintaining a normal gut microbiota. This is of particular importance since our data also indicate that expression of SMOX, which is the main source of Spd in the colon, is reduced in patients with colitis and that tissues from patients with precancerous lesions exhibit the lowest level of SMOX in CECs. The reduced expression of SMOX in patients with chronic colitis could increase the risk for developing CAC. In this context, Spd supplementation, through a Spd-enriched diet that has been shown to be safe and well-tolerated in mice and humans59 could represent a cost-effective and rational alternative therapy for colitis and chemopreventive for CAC, and potentially CRC in general.
Supplementary Material
Acknowledgments
Sequencing was performed by the Vanderbilt Technologies for Advanced Genomics (VANTAGE) core at VUMC.
Grant Support:
This work was funded by NIH grants R01DK128200, R01AT004821, R01CA190612, P01CA116087, and P01CA028842 (KTW); Veterans Affairs Merit Review grants I01BX001453 and I01CX002171 (KTW); Senior Research Award 703003 from the Crohn’s & Colitis Foundation (APG and KTW); a gift from CURE for IBD (KTW); the Thomas F Frist Sr. Endowment (KTW); and the Vanderbilt Center for Mucosal Inflammation and Cancer (KTW). LAC was supported by Veterans Affairs Career Development Award 1IK2BX002126 and VA Merit Review grant I01BX004366. YLL was supported by T32AI138932, and KMM was supported by T32CA009592. The use of the TMA was supported by the Vanderbilt Digestive Disease Research Center supported by NIH grant P30DK058404, and by P50CA236733. Polyamine measurements were supported by the Mass Spectrometry Core of P30DK058404. The NCI Cooperative Human Tissue Network is supported by grant UM1CA183727 (MKW).
Abbreviations used in this paper:
- AOM
azoxymethane
- CAC
colitis-associated carcinoma
- CD
Crohn’s disease
- CDX2
caudal type homeobox 2
- CECs
colonic epithelial cells
- CRC
colorectal cancer
- DEFAs
α-defensins
- DEGs
differentially expressed genes
- DFMO
difluoromethylornithine
- DSS
dextran sulfate sodium
- H&E
hematoxylin and eosin
- HGD
high-grade dysplasia
- IBD
inflammatory bowel disease
- ICs
Immune cells
- IPA
Ingenuity Pathway Analysis
- LGD
low-grade dysplasia
- ODC
ornithine decarboxylase
- Put
putrescine
- RNA-Seq
RNA sequencing
- SMOX
spermine oxidase
- Spd
spermidine
- Spm
spermine
- TAM
tamoxifen
- TMA
tissue microarray
- UC
ulcerative colitis
- VUMC
Vanderbilt University Medical Center
- WT
wild-type
Footnotes
Conflicts of interest
The authors disclose no conflicts.
References
- 1.Dahlhamer JM, Zammitti EP, Ward BW, et al. Prevalence of inflammatory bowel disease among adults aged >/=18 years - United States, 2015. Morb Mortal Wkly Rep 2016;65:1166–1169. [DOI] [PubMed] [Google Scholar]
- 2.Singh P, Ananthakrishnan A, Ahuja V. Pivot to Asia: inflammatory bowel disease burden. Intest Res 2017;15:138–141. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Terzic J, Grivennikov S, Karin E, et al. Inflammation and colon cancer. Gastroenterology 2010;138:2101–2114 e5. [DOI] [PubMed] [Google Scholar]
- 4.Mattiuzzi C, Sanchis-Gomar F, Lippi G. Concise update on colorectal cancer epidemiology. Ann Transl Med 2019;7:609. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Pegg AE. Functions of polyamines in mammals. J Biol Chem 2016;291:14904–14912. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Wang Y, Devereux W, Woster PM, et al. Cloning and characterization of a human polyamine oxidase that is inducible by polyamine analogue exposure. Cancer Res 2001;61:5370–5373. [PubMed] [Google Scholar]
- 7.Gobert AP, Al-Greene NT, Singh K, et al. Distinct immunomodulatory effects of spermine oxidase in colitis induced by epithelial injury or infection. Front Immunol 2018;9:1242. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Kingsnorth AN, Russell WE, McCann PP, et al. Effects of alpha-difluoromethylornithine and 5-fluorouracil on the proliferation of a human colon adenocarcinoma cell line. Cancer Res 1983;43:4035–4038. [PubMed] [Google Scholar]
- 9.Ray RM, McCormack SA, Johnson LR. Polyamine depletion arrests growth of IEC-6 and Caco-2 cells by different mechanisms. Am J Physiol Gastrointest Liver Physiol 2001;281:G37–G43. [DOI] [PubMed] [Google Scholar]
- 10.Erdman SH, Ignatenko NA, Powell MB, et al. APC-dependent changes in expression of genes influencing polyamine metabolism, and consequences for gastrointestinal carcinogenesis, in the Min mouse. Carcinogenesis 1999;20:1709–1713. [DOI] [PubMed] [Google Scholar]
- 11.Meyskens FL Jr., McLaren CE, Pelot D, et al. Difluoromethylornithine plus sulindac for the prevention of sporadic colorectal adenomas: a randomized placebo-controlled, double-blind trial. Cancer Prev Res (Phila) 2008;1:32–38. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Sinicrope FA, Velamala PR, Song L, et al. Efficacy of difluoromethylornithine and aspirin for treatment of adenomas and aberrant crypt foci in patients with prior advanced colorectal neoplasms. Cancer Prev Res (Phila) 2019;12:821–830. [DOI] [PubMed] [Google Scholar]
- 13.Lynch PM, Burke CA, Phillips R, et al. An international randomised trial of celecoxib versus celecoxib plus difluoromethylornithine in patients with familial adenomatous polyposis. Gut 2016;65:286–295. [DOI] [PubMed] [Google Scholar]
- 14.Vargas AJ, Ashbeck EL, Wertheim BC, et al. Dietary polyamine intake and colorectal cancer risk in postmenopausal women. Am J Clin Nutr 2015;102:411–419. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Zahedi K, Barone S, Destefano-Shields C, et al. Activation of endoplasmic reticulum stress response by enhanced polyamine catabolism is important in the mediation of cisplatin-induced acute kidney injury. PLoS One 2017;12:e0184570. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Sierra JC, Piazuelo MB, Luis PB, et al. Spermine oxidase mediates Helicobacter pylori-induced gastric inflammation, DNA damage, and carcinogenic signaling. Oncogene 2020;39:4465–4474. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Goulding DR, Myers PH, Dickerson AB, et al. Comparative efficacy of two types of antibiotic mixtures in gut flora depletion in female C57BL/6 mice. Comp Med 2021;71:203–209. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Hardbower DM, Coburn LA, Asim M, et al. EGFR-mediated macrophage activation promotes colitis-associated tumorigenesis. Oncogene 2017;36:3807–3819. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Singh K, Coburn LA, Asim M, et al. Ornithine decarboxylase in macrophages exacerbates colitis and promotes colitis-associated colon carcinogenesis by impairing M1 immune responses. Cancer Res 2018;78:4303–4315. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Coburn LA, Singh K, Asim M, et al. Loss of solute carrier family 7 member 2 exacerbates inflammation-associated colon tumorigenesis. Oncogene 2019;38:1067–1079. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Feng Y, Sentani K, Wiese A, et al. Sox9 induction, ectopic Paneth cells, and mitotic spindle axis defects in mouse colon adenomatous epithelium arising from conditional biallelic Apc inactivation. Am J Pathol 2013;183:493–503. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Triner D, Devenport SN, Ramakrishnan SK, et al. Neutrophils restrict tumor-associated microbiota to reduce growth and invasion of colon tumors in mice. Gastroenterology 2019;156:1467–1482. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Powell SM, Zilz N, Beazer-Barclay Y, et al. APC mutations occur early during colorectal tumorigenesis. Nature 1992;359:235–237. [DOI] [PubMed] [Google Scholar]
- 24.Bachmanov AA, Reed DR, Beauchamp GK, et al. Food intake, water intake, and drinking spout side preference of 28 mouse strains. Behav Genet 2002;32:435–443. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Hardbower DM, Asim M, Luis PB, et al. Ornithine decarboxylase regulates M1 macrophage activation and mucosal inflammation via histone modifications. Proc Natl Acad Sci U S A 2017;114:E751–E760. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Coburn LA, Horst SN, Chaturvedi R, et al. High-throughput multi-analyte Luminex profiling implicates eotaxin-1 in ulcerative colitis. PloS One 2013;8:e82300. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Coburn LA, Horst SN, Allaman MM, et al. L-arginine availability and metabolism is altered in ulcerative colitis. Inflamm Bowel Dis 2016;22:1847–1858. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Willers M, Ulas T, Vollger L, et al. S100A8 and S100A9 are important for postnatal development of gut microbiota and immune system in mice and infants. Gastroenterology 2020;159:2130–2145. [DOI] [PubMed] [Google Scholar]
- 29.Singh K, Gobert AP, Coburn LA, et al. Dietary arginine regulates severity of experimental colitis and affects the colonic microbiome. Front Cell Infect Microbiol 2019;9:66. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Eisenberg T, Abdellatif M, Schroeder S, et al. Cardioprotection and lifespan extension by the natural polyamine spermidine. Nat Med 2016;22:1428–1438. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Madeo F, Eisenberg T, Pietrocola F, et al. Spermidine in health and disease. Science 2018;359:eaan2788. [DOI] [PubMed] [Google Scholar]
- 32.Kiechl S, Pechlaner R, Willeit P, et al. Higher spermidine intake is linked to lower mortality: a prospective population-based study. Am J Clin Nutr 2018;108:371–380. [DOI] [PubMed] [Google Scholar]
- 33.Levin VA, Chamberlain MC, Prados MD, et al. Phase I-II study of eflornithine and mitoguazone combined in the treatment of recurrent primary brain tumors. Cancer Treat Rep 1987;71:459–464. [PubMed] [Google Scholar]
- 34.Simoneau AR, Gerner EW, Nagle R, et al. The effect of difluoromethylornithine on decreasing prostate size and polyamines in men: results of a year-long phase IIb randomized placebo-controlled chemoprevention trial. Cancer Epidemiol Biomarkers Prev 2008;17:292–299. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Kreul SM, Havighurst T, Kim K, et al. A phase III skin cancer chemoprevention study of DFMO: long-term follow-up of skin cancer events and toxicity. Cancer Prev Res (Phila) 2012;5:1368–1374. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Tutton PJ, Barkla DH. Comparison of the effects of an ornithine decarboxylase inhibitor on the intestinal epithelium and on intestinal tumors. Cancer Res 1986;46:6091–6094. [PubMed] [Google Scholar]
- 37.Saydjari R, Alexander RW, Upp JR Jr., et al. Differential sensitivity of various human tumors to inhibition of polyamine biosynthesis in vivo. Int J Cancer 1991;47:44–48. [DOI] [PubMed] [Google Scholar]
- 38.Alhonen L, Halmekyto M, Kosma VM, et al. Life-long over-expression of ornithine decarboxylase (ODC) gene in transgenic mice does not lead to generally enhanced tumorigenesis or neuronal degeneration. Int J Cancer 1995;63:402–404. [DOI] [PubMed] [Google Scholar]
- 39.Chen Y, O’Brien T, Del Razo LM, et al. Tissue levels of arsenicals and skin tumor response following administration of monomethylarsonous acid and arsenite to K6/ODC mice. J Environ Pathol Toxicol Oncol 2008;27:43–52. [DOI] [PubMed] [Google Scholar]
- 40.Chaturvedi R, Asim M, Romero-Gallo J, et al. Spermine oxidase mediates the gastric cancer risk associated with Helicobacter pylori CagA. Gastroenterology 2011;141:1696–1708. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Chaturvedi R, de Sablet T, Asim M, et al. Increased Helicobacter pylori-associated gastric cancer risk in the Andean region of Colombia is mediated by spermine oxidase. Oncogene 2015;34:3429–3440. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Yue F, Li W, Zou J, et al. Spermidine prolongs lifespan and prevents liver fibrosis and hepatocellular carcinoma by activating MAP1S-mediated autophagy. Cancer Res 2017;77:2938–2951. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Gobert AP, Cheng Y, Akhtar M, et al. Protective role of arginase in a mouse model of colitis. J Immunol 2004;173:2109–2117. [DOI] [PubMed] [Google Scholar]
- 44.Goodwin AC, Destefano Shields CE, Wu S, et al. Polyamine catabolism contributes to enterotoxigenic Bacteroides fragilis-induced colon tumorigenesis. Proc Natl Acad Sci U S A 2011;108:15354–15359. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Hashimoto S, Uto H, Kanmura S, et al. Human neutrophil peptide-1 aggravates dextran sulfate sodium-induced colitis. Inflamm Bowel Dis 2012;18:667–675. [DOI] [PubMed] [Google Scholar]
- 46.Bousserouel S, Kauntz H, Gosse F, et al. Identification of gene expression profiles correlated to tumor progression in a preclinical model of colon carcinogenesis. Int J Oncol 2010;36:1485–1490. [DOI] [PubMed] [Google Scholar]
- 47.Tschurtschenthaler M, Wang J, Fricke C, et al. Type I interferon signalling in the intestinal epithelium affects Paneth cells, microbial ecology and epithelial regeneration. Gut 2014;63:1921–1931. [DOI] [PubMed] [Google Scholar]
- 48.Williams AD, Korolkova OY, Sakwe AM, et al. Human alpha defensin 5 is a candidate biomarker to delineate inflammatory bowel disease. PLoS One 2017;12:e0179710. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Taman H, Fenton CG, Hensel IV, et al. Transcriptomic landscape of treatment-naive ullcerative colitis. J Crohns Colitis 2018;12:327–336. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Jeong D, Kim H, Kim D, et al. Defensin alpha 6 (DEFA6) is a prognostic marker in colorectal cancer. Cancer Biomark 2019;24:485–495. [DOI] [PubMed] [Google Scholar]
- 51.Wehkamp J, Salzman NH, Porter E, et al. Reduced Paneth cell alpha-defensins in ileal Crohn’s disease. Proc Natl Acad Sci U S A 2005;102:18129–18134. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Salzman NH, Hung K, Haribhai D, et al. Enteric defensins are essential regulators of intestinal microbial ecology. Nat Immunol 2010;11:76–83. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Tang C, Kakuta S, Shimizu K, et al. Suppression of IL-17F, but not of IL-17A, provides protection against colitis by inducing Treg cells through modification of the intestinal microbiota. Nat Immunol 2018;19:755–765. [DOI] [PubMed] [Google Scholar]
- 54.Ou J, Liang S, Guo XK, et al. alpha-Defensins promote Bacteroides colonization on mucosal reservoir to prevent antibiotic-induced dysbiosis. Front Immunol 2020;11:2065. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Hirano A, Umeno J, Okamoto Y, et al. Comparison of the microbial community structure between inflamed and non-inflamed sites in patients with ulcerative colitis. J Gastroenterol Hepatol 2018;33:1590–1597. [DOI] [PubMed] [Google Scholar]
- 56.Frank DN, St Amand AL, Feldman RA, et al. Molecular-phylogenetic characterization of microbial community imbalances in human inflammatory bowel diseases. Proc Natl Acad Sci U S A 2007;104:13780–13785. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Mukhopadhya I, Hansen R, El-Omar EM, et al. IBD-what role do Proteobacteria play? Nat Rev Gastroenterol Hepatol 2012;9:219–230. [DOI] [PubMed] [Google Scholar]
- 58.Caruso R, Mathes T, Martens EC, et al. A specific gene-microbe interaction drives the development of Crohn’s disease-like colitis in mice. Sci Immunol 2019;4:eaaw4341. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Schwarz C, Stekovic S, Wirth M, et al. Safety and tolerability of spermidine supplementation in mice and older adults with subjective cognitive decline. Aging (Albany NY) 2018;10:19–33. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
RNA-Seq data can be accessed on the Gene Expression Omnibus repository using the accession number GSE158826. The 16S rRNA sequencing of the fecal microbiota has been deposited on the Sequence Read Archive website with the BioProject ID: PRJNA685397.