Abstract
Objective:
Oral postmenopausal hormone therapy (HT) has been shown to be associated with venous thromboembolism (VTE), but whether this association is modified by VTE-associated genetic susceptibility is unknown. We examined interactions between oral HT use and a genetic risk score (GRS) of VTE.
Method:
Eligible women were postmenopausal women who had data on oral HT use, VTE incidence between 1990 and 2012, and genetic data in the Nurses’ Health Study. We built a GRS aggregating 16 VTE-related genetic variants. We used Cox regression to estimate associations of HT use with incident VTE and assessed interactions between HT use and VTE GRS. We also estimated incidence of VTE between age 50 and 79 years for groups of women defined by HT use and VTE GRS.
Results:
We identified 432 incident VTE cases. Current HT users were at higher risk of VTE than never users (HR: 1.9, 95% CI: 1.5–2.6), with slightly higher risk for estrogen plus progestin HT than estrogen only (HR: 2.4 versus 1.9). The GRS was associated with VTE risk (HR comparing 4th quartile to 1st: 2.0, 95% CI: 1.2–3.4). We did not observe significant multiplicative interactions between HT use and GRS. The estimated VTE risk difference (per 10,000 person-years) comparing 50-year-old current HT users to never users was 22.5 for women in the highest GRS quartile and 9.8 for women in the lowest GRS quartile.
Conclusion:
The VTE GRS might inform clinical guidance regarding the balance of risks and benefits of HT use, especially among younger women.
Keywords: Oral postmenopausal hormone therapy, Genetic risk, Venous thromboembolism, Gene-Hormone interaction
Introduction
Oral hormone therapy (HT) was widely used among postmenopausal women in the late 20th century.1–3 However, use has declined by 70–80% since 2002, when the Women’s Health Initiative (WHI), a large double blinded randomized trial of combined estrogen plus progestin, reported health risks exceeding benefits.4–6 One of the reported excess harms associated with oral HT was the risk of venous thromboembolic disease (VTE), which has consistently been reported in observational studies as a two- to three-fold greater risk.7–10 Despite the health risks, some women are still prescribed oral HT (e.g., oral estrogen) to ameliorate or treat postmenopausal symptoms such as hot flashes or osteoporosis11; for example, 42 per 1,000 women aged ≥ 50 were prescribed oral estrogen in 2015.12 In 2017, the North American Menopause Society issued a HT position statement that different HT-related disease risks could be due to different characteristics of HT (e.g., type, duration of use, age at first use, and timing of initiation since menopause onset).13
VTE, including both pulmonary embolism (PE) and deep vein thrombosis (DVT), is a multifactorial disease associated with a combination of genetic and environmental risk factors.14 However, only a few studies have explored gene and environment (G-E) interactions for VTE with regard to HT use, and most of these were small case-control studies testing well-known candidate genes (e.g., Factor V Leiden, Factor II mutations15) or genetic polymorphisms associated with relevant mechanisms (e.g., NFE2L2, CYP genes for estrogen metabolism16,17). Although some of the studies observed greater risk of VTE in HT users carrying specific genetic mutations, it remains unknown whether overall genetic risk modifies the association of HT with VTE risk.
As a large number of genome-wide association studies (GWAS) have identified thousands of common genetic polymorphisms associated with human diseases, genetic risk scores (GRS) aggregating common risk variants have emerged as useful tools to estimate genetic predisposition or detect G-E interactions.18–20 For VTE, eight GWAS based on individuals of European descent have been published.21–28 GWAS-based GRS have been associated with a 40–80% greater risk of VTE per additional risk allele and 1.2~1.5-fold greater risk per one standard deviation increment.29–31 However, no quantitative study has assessed the interaction effect of the GRS with oral HT use in relation to the risk of VTE.
To untangle the complex interplay between oral HT use and genetic predisposition to VTE, we evaluated joint associations and multiplicative interactions between oral HT use and GRS in relation to VTE risk among postmenopausal women in a large prospective study. We also assessed the joint associations and interactions for PE risk (with or without diagnosed DVT) as a secondary outcome. To further examine age-specific impact of HT use and genetic risk on VTE, we assessed the disease incidence between age 50 and 79 years by oral HT use and GRS groups.
Methods
Study population
Our study included participants in a large prospective population-based cohort, the Nurses’ Health Study (NHS). Details of the cohort have been previously described.32 Briefly, the NHS began in 1976, recruiting 121,700 female registered nurses aged between 30 and 55 years in 11 U.S. states. Participants have completed mailed questionnaires biennially to update information on lifestyle and medical history. A follow-up rate between 85–90% has been maintained since inception. A subset of 32,826 women provided blood samples since 1989 and another subset of 29,684 women provided buccal cell samples since 2002. All cohort participants provided informed consent, and the study protocol was approved by the institutional review boards at the Brigham and Women’s Hospital and Harvard T.H. Chan School of Public Health.
Eligible participants for this study were postmenopausal women who reported their reproductive history (menopausal status, oral HT use, and history of hysterectomy), and had information on the presence or absence of incident VTE as well as genotype data available. We defined the baseline as 1990, which was the year in which blood samples were collected. We excluded participants who had a baseline history of VTE, breast cancer, or cardiovascular disease (myocardial infarction or angina), reported any form of current HT use at baseline or other routes (transdermal or vaginal) than oral HT administration during follow-up, had missing data on age or oral HT use, or returned only the baseline questionnaire. Since a majority of NHS participants (Approximately 95%) are of European descent, genotyping was performed in women with European ancestry. During the 22-year follow-up, we censored participants at death, loss to follow-up, or occurrence of the outcomes of interest (incident VTE and incident PE). We removed an individual’s contribution to person-time from a questionnaire cycle if the woman was pre-menopausal or had dubious menopausal status or missing menopausal status data during that questionnaire cycle. In result, 8,105 women were included to the analyses of this study.
Ascertainment of venous thromboembolism
In the NHS, all incident VTE (first event of either PE or DVT) cases were identified by self-report by cohort participants on mailed biennial questionnaires. For this analysis, we included cases identified between 1990 and 2012. The questionnaire asked about physician-diagnosed major diseases since the prior questionnaire, including PE (provoked or unprovoked) every two years. Consistent with standard NHS follow-up procedures, cohort participants reporting a PE without any previous cancer were asked to confirm self-reports and give consent for NHS investigators to access their medical records for confirmation. Details about PE cases have been described previously,33 briefly, 56% of included PE cases were confirmed by medical record, 18% were reconfirmed by the participant but record review was refused or unable to be completed, and the remainder had had a prior diagnosis of cancer and were not contacted regarding medical records. Identified DVT cases were based on self-report in response to a request for write-in reports of “other major illness” and not confirmed by medical record review. However, a previous validation study of 101 self-reported cases of DVT found that 94% of cases were confirmed by medical record review, 2% were probable, and only 4% were not confirmed.34 As a result, in the NHS cohort, identified VTE cases were either cases with PE event only or first event of PE (29%) or cases with DVT event or first event of DVT (71%).
Assessment of oral HT use and menopausal status
Since cohort inception in 1976, data on HT use has been updated every two years with biennial questionnaires that asked whether respondents had taken HT and, if so, for how long (number of months) since the previous questionnaire cycle. Participants have reported on HT type (estrogen alone or combined estrogen plus progestin) from 1978 and routes of HT administration later than that (oral and vaginal from 1982 and transdermal from 1988). The route of HT was predominantly oral and the formulations were estrogens with or without progestin (mostly medroxyprogesterone acetate)35 in the cohort. Participants also reported age at menopause and type of menopause (e.g., natural or surgical), which were shown to be valid when comparing to medical records.36 In this study, postmenopausal women were those who had reported natural menopause, hysterectomy with bilateral oophorectomy, or menopause because of whatever reasons such as radiation.
In this study, we used oral HT data collected from the 1990 baseline to 2010 and generated time-varying variables for oral HT use. Women were considered as current users if they reported current use of oral HT during the follow-up cycle, whereas past users were those who reported HT use at any time before but not at the current follow-up cycle. Cumulative duration of HT use was calculated by summing the duration of HT use (months) reported at each questionnaire cycle. Age at initiation was derived from age at the time in which participants reported the first HT use during follow-up, and initiation time of HT use since menopause onset was derived by using the calendar time of HT initiation and time of menopause. We defined ‘early initiation’ if women were aged < 60 years at HT initiation or had the first use within the first 10 years of menopause and ‘late initiation’ if women were aged ≥ 60 years at initiation and had the first use ≥ 10 years after menopause onset.37
Covariates
With the biennial follow-up questionnaires, we obtained information on age, height, weight, smoking status, aspirin use, parity, surgical history including hysterectomy and oophorectomy, and medical history including diabetes, hypertension, hypercholesterolemia, myocardial infarction (MI) or angina, and cancer. Height was reported based on the cohort baseline (1976) questionnaire. Information on weight, smoking status, and surgical and medical history was updated every 2 years. Using the height and weight, we derived body mass index (BMI) by dividing weight in kg by squared height in meters. Aspirin use was reported starting in 1980 and parity was assessed from 1976 to 1984 and in 1996. Physical activity, as measured in metabolic equivalent of tasks (METs) per week, was assessed in 1988 and every 4 years since then. Starting in 1980, alcohol use was measured with food frequency questionnaires every 4 years. If participants had missing data on any covariate, we carried forward responses from the prior questionnaire. If it remained missing in two questionnaire cycles (e.g., BMI, physical activity, and alcohol use), then we generated an indicator variable for missing data.
Genotyping and calculation of genetic risk score
Genotyping was carried out from 2006 through 2015 using five classes of genotyping arrays (Affymetrix 6.0, Illumina HumanHap, Illumina OmniExpress, OncoArray, and HumanCore Exome) for 18,499 women in the subsets of the NHS participants who provided either a blood or buccal cell sample (N= 14,856 and 3,643, respectively). Detailed information on selecting study participants, genotyping, quality control, and imputation have been described elsewhere.38 In brief, genotype data were imputed based on 1000 Genome Project Phase 3 version 5 haplotypes as reference, with the use of Minimac software (https://genome.sph.umich.edu/wiki/Minimac). Among imputed single nucleotide polymorphism (SNPs), we included SNPs with a minor allele frequency ≥ 5% and high imputation quality scores (> 0.8).
For the present study, we constructed a VTE GRS using 16 SNPs identified to be associated with VTE risk at a genome-wide significance threshold (p < 5.0×10−8) from several previously published GWASs of European descent populations (Supplementary Table 1).23–28 The 16 SNPs included were rs6025 (F5), rs1018827 (F5), rs6427196 (F5), rs16861990 (NME7), rs3756008 (F11), rs4253399 (F11), rs7659024 (FGA-FGG), rs6536024 (FGG-LRAT), rs2519093 (ABO), rs495828 (ABO), rs687621 (ABO), rs78707713 (TSPAN15), rs1799963 (F2), rs2288904 (SLC44A2), and rs6087685 (PROCR). The GRS was calculated by summing the number of risk alleles (alleles associated with increased risk of VTE in an additive genetic model) of each SNP weighted by SNP-specific effect sizes (log odds ratios) obtained from GWAS (i.e., GRS = β1×SNP1 + β2×SNP2 +…).39 Thus, a higher score indicated a higher genetic predisposition to VTE. We standardized the score with mean 0 and standard deviation (SD) 1 and categorized the score into three genetic risk groups, ‘low’ (the lowest quartile), ‘intermediate’ (the 2nd and 3rd quartiles), and ‘high’ (the highest quartile).
Statistical analysis
Study participants contributed person-time from the baseline (1990) until the date of death, loss to follow-up, disease diagnosis (VTE or PE), or the end of follow-up (June 1, 2012), whichever came first. To describe characteristics of our study population by oral HT use status, we tabulated frequencies and distributions standardized40 to the age distribution of the study population at midpoint of follow-up (2000) according to the three HT use status groups (i.e., never, past, and current users).
We used multivariable Cox proportional hazards models to estimate hazard ratios (HRs) and 95% confidence intervals (CIs) for VTE risk associated with several different measures of oral HT use such as HT use status (never, past, or current), HT type (estrogen only or estrogen plus progestin), duration of HT use (< 2 or ≥ 2 years), and HT initiation (early or late). To estimate comparable relative risks, we chose women who never used oral HT as a reference group for all analyses. Covariates included age (months, continuous), BMI (kg/m2, continuous), physical activity (METs per week, continuous), alcohol use (g/day, continuous), smoking (never, former, and current smoker), aspirin use (yes or no), parity (none, one, > one), and separate indicators for history of diabetes, hypertension, hypercholesterolemia, MI or angina, and cancer, and history of hysterectomy (with or without oophorectomy). The HT-related variables and covariates were updated at each questionnaire cycle as a time-varying variable. We also examined the associations of VTE risk with quartiles of GRS adjusting for the covariates.
We assessed the multivariable-adjusted associations with VTE in the joint categories of oral HT use and GRS. We also evaluated multiplicative interactions between oral HT use and GRS using a likelihood ratio test that compared the −2 log likelihood of two models with and without cross-product interaction terms. We repeated these analyses for PE, our secondary outcome, as well.
To compare risk differences of VTE by the oral HT use and GRS effects, we calculated incidence rates (IR) of VTE (range of age: 50–59, 60–69, and 70–79) by combining the estimated relative risks of oral HT use and GRS with age-specific average IRs of European-descent individuals in the U.S. population (Supplementary Table 2).41 Using these data, first we calculated the baseline incidence for the reference group (women with low GRS who never used oral HT) in each age group by dividing the age-specific IRs by the average relative risks (i.e., HRs) of all controls (who never had VTE events) in each age group in our study. Then, we calculated age-specific VTE incidences for each joint category of oral HT use and GRS by multiplying the baseline incidence by the relative risk corresponding to the category of the HT use and GRS. To further assess BMI effect on VTE incidences, we also calculated age-specific VTE incidences for each join category of oral HT use, GRS, and BMI. Additionally, we calculated the number needed to harm (NNH = 1/(IRexp – IRunexp)) as an absolute measure of how many individuals need to be exposed to oral hormone therapy to cause harm to one person.
All analyses were performed using SAS version 9.3 (SAS Institute Inc. Cary, NC, USA) and R version 3.6.3 (R code Team, 2020). A two-sided p-value of 0.05 was used to determine statistical significance.
Results
During 157,043 person-years of follow-up, 432 incident VTE cases (including 178 incident PE cases) were identified in this study. Table 1 shows the age-standardized characteristics of study participants at midpoint (2000) of the follow-up (1990–2012) according to the three categories of oral HT use status. On average, participants were 65 years old, had BMI of 27 kg/m2, and were 51 years old at the onset of menopause. Women using HT in the past or currently have a higher proportion of hysterectomy (26% and 34% as compared to 18% for never users). When comparing characteristics of the full cohort (at the midpoint of the cohort follow-up [1994]) to those participants with genotype data, we did not observe substantial differences in the distributions between the two groups, indicating that selection bias for genotyping was unlikely (Supplementary Table 3).
Table 1.
Characteristics of Nurses’ Health Study participants with genetic data according to oral hormone therapy use at the midpoint (2000) of follow-up
| Oral hormone therapy use |
|||
|---|---|---|---|
| Never users (n = 3,038) |
Past users (n = 1,368) |
Current users (n = 2,496) |
|
| Age (year), mean (SD) | 64.7 (6.9) | 64.7 (6.9) | 64.7 (6.9) |
| BMIa (kg/m2), mean (SD) | 27.7 (5.8) | 27.1 (5.7) | 26.7 (5.2) |
| Physical activitya, MET-h/wk | 17.7 (21.0) | 17.2 (19.1) | 18.6 (23.1) |
| Alcohol intakea (g/day), mean (SD) | 4.9 (8.9) | 5.6 (9.7) | 5.5 (9.1) |
| Smokinga, % | |||
| Never | 45 | 44 | 45 |
| Former | 44 | 48 | 49 |
| Current | 10 | 8 | 6 |
| Current aspirin usea, (yes) % | 44 | 47 | 49 |
| Paritya, % | |||
| None | 5 | 4 | 4 |
| One | 6 | 6 | 6 |
| More than one | 89 | 90 | 90 |
| Prevalent diagnosisa | |||
| Diabetes, % | 9 | 7 | 6 |
| Hypertension, % | 31 | 34 | 36 |
| Hyperlipidemia, % | 35 | 38 | 34 |
| MI or angina, % | 1 | 2 | 2 |
| Cancer, % | 11 | 17 | 3 |
| Hysterectomya,b, % | 18 | 26 | 34 |
| Age at menopausea (year) mean (SD) | 50.9 (3.6) | 51.2 (3.5) | 51.2 (3.4) |
| Type of menopausea, % | |||
| Natural | 80 | 76 | 72 |
| Surgical | 12 | 16 | 22 |
| Radiation | 2 | 1 | 0 |
| Unknown | 6 | 7 | 6 |
| GRSa,c, % | |||
| Low (Quartile 1) | 26 | 25 | 27 |
| Intermediate (Quartile 2 and 3) | 51 | 51 | 49 |
| High (Quartile 4) | 23 | 25 | 24 |
SD, standard deviation; BMI, body mass index; MET, metabolic equivalent task; MI, myocardial infarction; GRS, genetic risk score.
All values of mean (SD) and percentages were standardized to the age distribution of study population.
Hysterectomy with or without oophorectomy.
GRS was standardized with mean = 0 and SD = 1.
We examined the associations between oral HT use and VTE risk (Table 2). Compared to never users, current HT users had 94% greater VTE risk adjusting for all confounders (HR = 1.94 [95% CI, 1.45–2.60]). In the multivariable-adjusted model, estrogen-only use was associated with 91% greater risk (HR = 1.91 [95% CI, 1.30–2.80]) of VTE and the use of estrogen plus progestin was associated with 2.4-fold greater risk of VTE as compared to the risk of never users (HR = 2.35 [95% CI, 1.62–3.40]). Initiation of HT at early age or soon after menopause onset was related to 38% greater risk of VTE in the multivariable-adjusted model. For PE, we found a similar pattern of associations with current HT use and HT type (Supplementary Table 4). We also found a greater risk of VTE with increasing GRS (e.g., HR = 2.24 [95% CI, 1.33–3.78] for 3rd quartile against 1st quartile of GRS). (Supplementary Figure 1).
Table 2.
Associations between oral HT categories and VTE risk
|
Cases/Person-years |
Age-adjusted | Multivariable-adjusted | |
|---|---|---|---|
| Oral HT | HRa (95% CI) | HRb (95% CI) | |
| HT use status | |||
| Never user | 168/67962 | 1.00 (Ref.) | 1.00 (Ref.) |
| Past user | 173/61340 | 0.93 (0.75–1.16) | 0.90 (0.72–1.12) |
| Current user | 91/27740 | 1.87 (1.41–2.48) | 1.94 (1.45–2.60) |
| HT type | |||
| Never user | 168/67962 | 1.00 (Ref.) | 1.00 (Ref.) |
| Estrogen onlyc | 42/10010 | 2.19 (1.54–3.11) | 1.91 (1.30–2.80) |
| Estrogen + Progestinc | 46/15205 | 1.90 (1.32–2.73) | 2.35 (1.62–3.40) |
| Duration of HT use | |||
| Never user | 168/67962 | 1.00 (Ref.) | 1.00 (Ref.) |
| < 2 years | 67/26405 | 1.03 (0.78–1.38) | 1.00 (0.75–1.33) |
| 2+ years | 197/62675 | 1.16 (0.94–1.44) | 1.13 (0.90–1.41) |
| HT initiationd | |||
| Never user | 168/67962 | 1.00 (Ref.) | 1.00 (Ref.) |
| Early initiation | 140/48007 | 1.40 (1.08–1.82) | 1.38 (1.06–1.81) |
| Late initiation | 124/41074 | 0.95 (0.75–1.21) | 0.91 (0.71–1.16) |
HT, hormone therapy; VTE, venous thromboembolism; HR, hazard ratio; CI, confidence interval.
Age-adjusted model: adjusting for age (in months).
Multivariable-adjusted model: adjusting for age (in months), physical activity (continuous and indicator for missing), alcohol use (continuous and indicator for missing), smoking (never, former, or current), current aspirin use (yes or no), parity (none, one, or more than one), prevalent diabetes, prevalent hypertension, prevalent hypercholesterolemia, prevalent MI/angina, prevalent cancer, hysterectomy (yes or no), and BMI (continuous and indicator for missing).
Only among current HT users.
We defined ‘early initiation’ if women were aged < 60 years at HT initiation or had the first use within the first 10 years of menopause and ‘late initiation’ if women were aged ≥ 60 years at initiation and had the first use ≥ 10 years after menopause onset.
Figure 1 presents the joint effects and interaction effects between oral HT use and GRS groups. The risk of VTE was higher with increasing GRS in most HT-related categories of women in the full cohort. In each category, the highest risk of VTE was found among women in the highest GRS quartile, together with current HT use (HR = 5.29 [95% CI, 3.04–9.22]), estrogen plus progestin use (HR = 7.67 [95% CI, 4.07–14.43]), 2+ years of HT use (HR = 3.11 [95% CI, 1.95–4.98]), or early initiation (HR = 4.01 [95% CI, 2.42–6.64]) (Figure 1; Supplementary Table 5). We also assessed multiplicative interactions between oral HT use and GRS, but none of the interactions were statistically significant (all P-interaction > 0.1) (Figure 1; Supplementary Table 5). For PE, we found there were similar patterns of joint effects and one significant interaction between HT type and GRS (P-interaction = 0.01) (Supplementary Table 5).
FIG. 1.
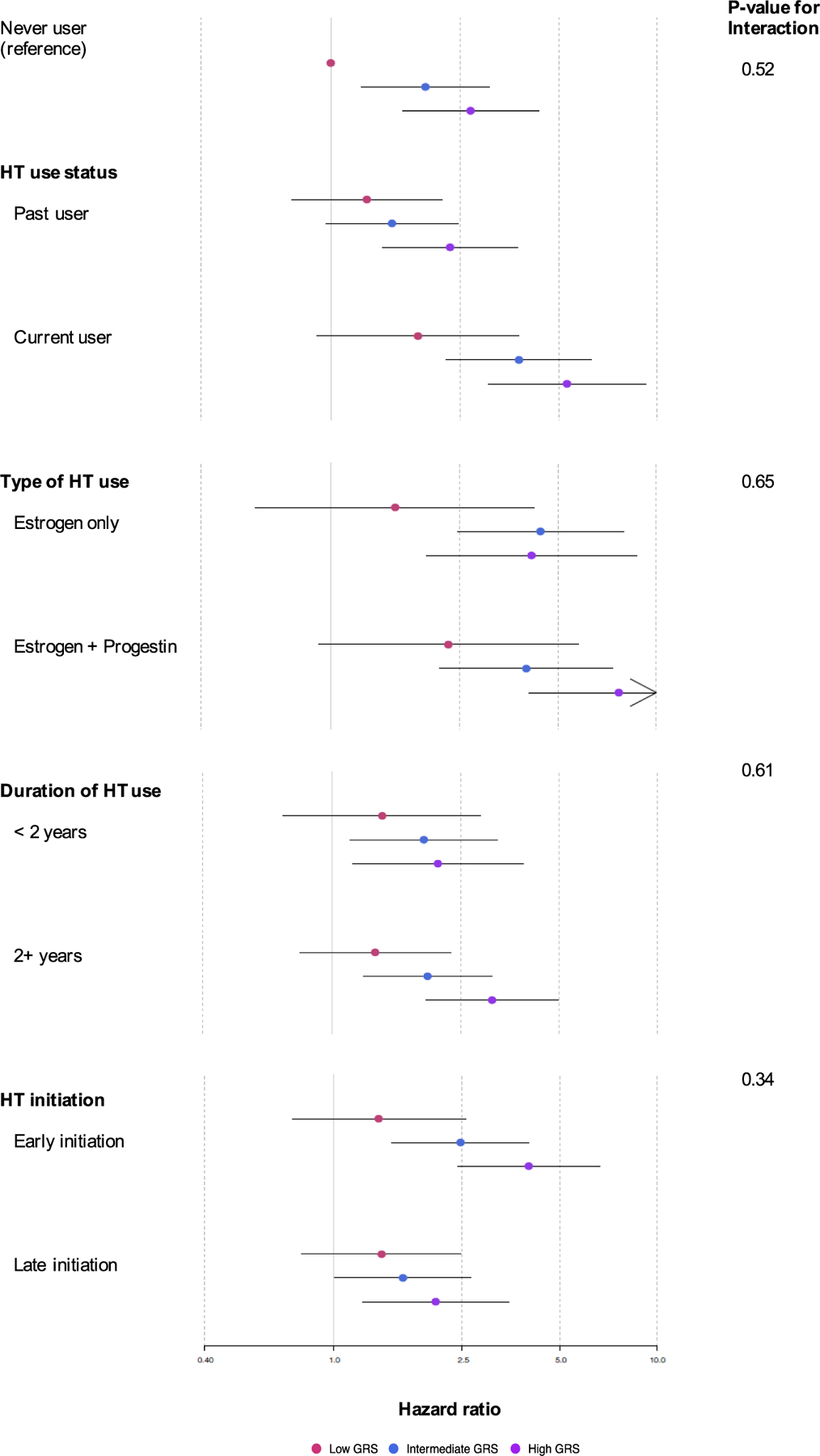
Multivariable-adjusted relative risk of VTE according to joint categories of oral hormone therapy use and GRS. All analyses were adjusted for age (in months), BMI (continuous and indicator for missing; excluded for the stratified analysis for BMI groups), physical activity (continuous and indicator for missing), alcohol use (continuous and indicator for missing), smoking, (never, former, or current), current aspirin use (yes or no), parity (none, one, or more than one), prevalent diabetes, prevalent hypertension, prevalent hypercholesterolemia, prevalent MI/angina, prevalent cancer, and hysterectomy (yes or no). Dots indicate the adjusted relative risk (RR) of VTE by joint categories of oral hormone therapy use and GRS. In each HT category, the red dot refers to the RR of women in the low GRS group, the blue dot refers to the RR of women in the intermediate GRS group, and the purple dot refers to the RR of women in the high GRS group. The black line including the dot shows the 95% CI of the RR. The reference group (never user) was used for comparing to all the subgroups of oral HT use status, type of oral HT use, duration of HT use, and HT initiation. BMI, body mass index; CI, confidence interval; GRS, genetic risk score; HT, hormone therapy; MI, myocardial infarction; VTE, venous thromboembolism.
VTE, venous thromboembolism; GRS, genetic risk score; HT, hormone therapy; CI, confidence interval.
Furthermore, we evaluated the incidence rates of VTE (per 10,000 person-years) between 50–79 years of age by oral HT use and GRS groups (Figure 2; Supplementary Table 6). Across all age groups (50–59, 60–69, and 70–79), the absolute risk of VTE was highest in older women (age 70–79) with a high GRS who used HT currently, estrogen only, for ≥ 2 years, or initiated HT early (incidence of VTE = 171.0, 164.3, 110.0, and 130.0, respectively). As compared to never HT users with a low GRS (incidence of VTE = 11.5, 21.7, and 40.1 for 50–59 years, 60–69 years, and 70–79 years, respectively), the risk differences (RDs) (per 10,000 person-years) of women with a high GRS and current HT use were 37.4 in those aged between 50 and 59 years (incidence = 48.9), 71.0 in those aged 60–69 years (incidence = 92.7), and 130.9 in those aged 70–79 years (incidence = 171.0). Such increasing RDs with age were also found in women who had a high GRS as well as used estrogen only (41.1, 77.7, and 131.9), used HT for ≥ 2 years (19.8, 37.5, and 68.3), or had early initiation of HT (25.4, 48.2, and 89.2) (Supplementary Table 6). The RDs for joint effects of HT-related characteristics (i.e., current use, estrogen only, using HT ≥ 2 years, and early initiation of HT) and high GRS were greater than the sum of separate RDs for each effect of HT use and a high GRS, suggesting supra-additive interaction effects of HT use and GRS on VTE risk (e.g., RD for women age 50–59 years with current use of HT and high GRS = 37.5; RD for current HT use only = 9.8; RD for high GRS only = 14.9). Moreover, these supra-additive interaction effects appeared larger with age (e.g., differences in incidence between joint effects of current HT use and high GRS and the sum of the individual effects = 12.8 for age 50–59, 24.2 for 60–69, and 44.6 for 70–79). We also found that the positive gene-hormone interaction effects increased further with higher BMI (Supplementary Table 7).
FIG. 2.
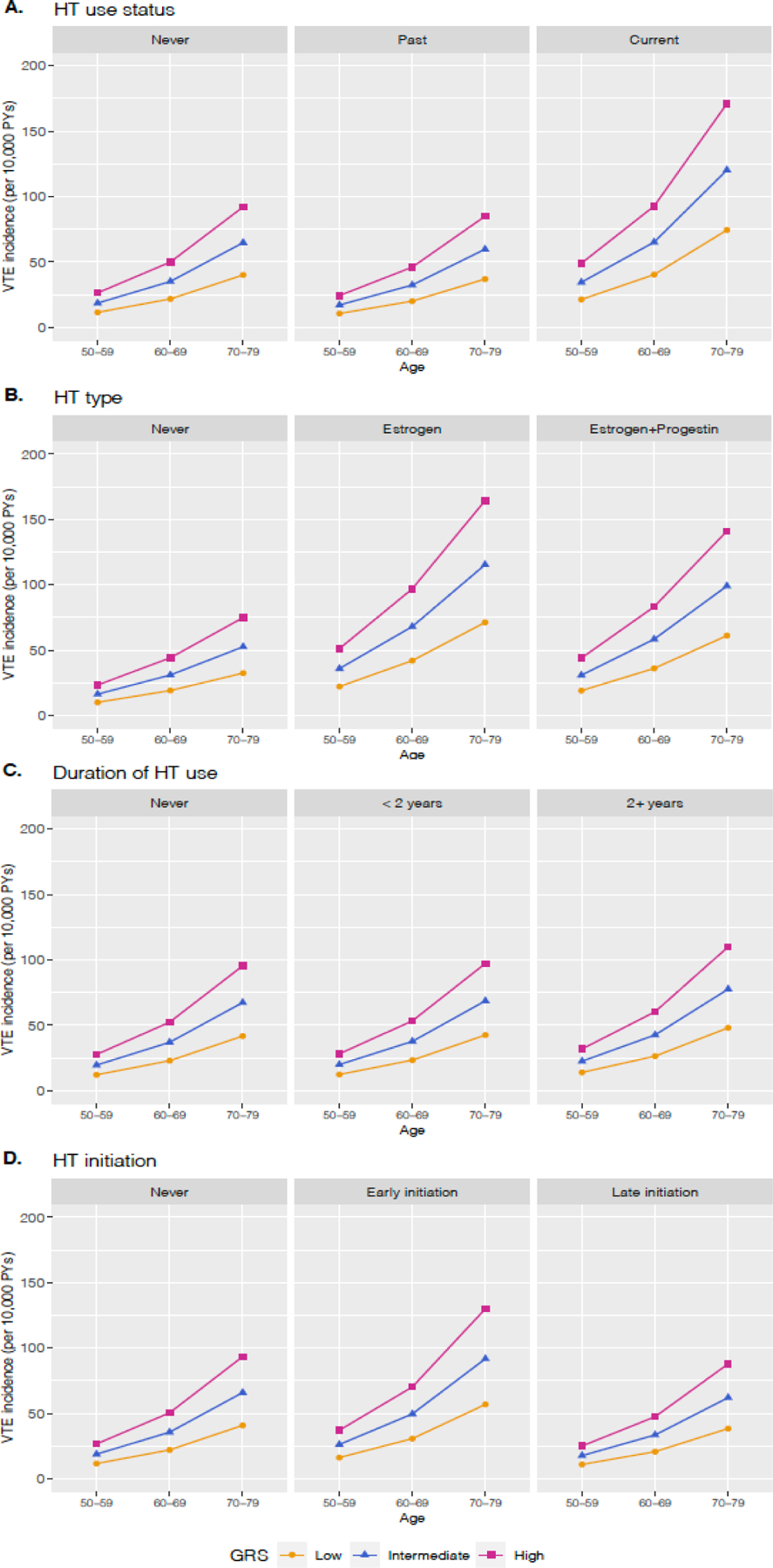
Incidence rates of VTE per 10,000 person-years between ages 50 and 79 by oral hormone therapy use and genetic risk groups. Age- and GRSspecific VTE incidences were presented by (A) oral HT use status, (B) type of HT use, (C) duration of HT use, and (D) HT initiation. Each line represents different levels of GRS; the yellow line for the low GRS, the blue line for the intermediate GRS, and the red line for the high GRS. BMI, body mass index; GRS, genetic risk score; HT, hormone therapy; VTE, venous thromboembolism.
VTE, venous thromboembolism; HT, hormone therapy; PY, person-years; GRS, genetic risk score.
These RDs (per 10,000 person-years) result in different numbers needed to harm across different GRS levels. For example, GRS-specific NNH comparing current HT users to never HT users (divided by 10,000) were 0.102 among women aged 50–59 years with a low GRS and 0.0444 among women aged 50–59 years with a high GRS. In other words, on average, one additional VTE event would be observed among 1,020 50-year-old women in the low GRS category taking hormone therapy, while one additional VTE event would be observed among 444 50-year-old women in the high GRS category.
Discussion
In our study, using a large prospective cohort of U.S. women, we assessed the main effects of oral HT use and its joint effects with a GWAS-based VTE GRS on relative risk and absolute risk of VTE. Our findings suggest that there are supra-additive interactions between the VTE GRS and current use of HT, using estrogen only or estrogen plus progestin, longer duration of HT use (≥ 2 years), and early HT initiation on the absolute risk of VTE, which means that women in those categories of HT use who also have a higher genetic risk of VTE may have excess risk of the disease beyond the sum of the excess risks of women with only one of the two.
In the main effect analyses, we observed that greater risks of VTE were associated with current use and types of oral HT, which is consistent with prior evidence on the unfavorable effects of current HT use and type (e.g., oral estrogen with or without progestin) on VTE risk.42–47 We did not find an association between duration of HT use and VTE, in contrast to several observational studies have found a greater incidence of acute VTE events in the first 1 or 2 years after initiating HT.48,49 Also, while our study found a higher risk of VTE associated with early initiation of HT use as compared to the risk of non-HT users, growing evidence supports a “hormone-timing hypothesis” that oral HT could have modest effects or even favorable effects on vascular diseases if young postmenopausal women (aged < 60 years) initiate HT use soon after menopause (e.g., < 5 or 6 years).50–53 For example, a recent randomized trial including 643 healthy postmenopausal women found that women who initiated HT (oral estrogen with vaginal progestin) within 6 years after menopause had less progression of atherosclerosis than those who initiated 10 or more years after menopause.54 However, the hypothesis is as yet unsettled for VTE.55
We found elevated risks of VTE due to joint effects of oral HT use and the VTE GRS. Although departures from a multiplicative odds ratio model between HT use and VTE GRS were not statistically significant, we observed supra-additive interactions where the joint effects on absolute VTE risk were larger than the sum of effects of each factor on the risk. To date no study has evaluated GRS-hormone interactions on VTE risk; to confirm the interaction effects, it will require more studies that examine GRS-HT interactions on both additive and multiplicative scales in the future.
Previously, gene-hormone studies for VTE have assessed interactions using a few candidate genes. Three case-control studies evaluated the joint effects of oral HT (estrogen with or without progestin) with high-risk mutations in candidate genes (e.g., F5, Prothrombin, MTHFR, Factor XIII, and PAI-1) on VTE risk.56–58 These studies found carriers of the F5 Leiden mutation (rs6025), one of risk variants in our GRS, had an excess risk of VTE associated with HT (range of OR = 6.7–25.5), though the interaction effects were not directly evaluated. We therefore examined the interaction between the SNP, rs6025, and oral HT (use status, type, duration, and early initiation) in the risk of VTE. However, no statistically significant multiplicative interaction was found even at a nominal significance level (all p-interactions ≥ 0.16) (Supplemental Table 8).
Our study has some limitations. First, due to biennial exposure, transient hormone therapy use may not have been captured. As thromboembolic events are likely to occur within one year of initiation of HT,7,59 it is possible that there were women who started and stopped HT within a single questionnaire cycle because of acute events. Moreover, although the majority of incident PE events were confirmed via medical records, the remaining thromboembolic events (i.e., DVT events) as well as risk factors were self-reported. Because we used risk factor data collected prior to diagnosis in our analyses, differential recall bias is unlikely; however, non-differential misclassification in exposures and outcomes may bias risk estimates in this study. Third, there is the potential of survival bias, as blood samples of cohort participants were collected 14 years after cohort inception. In addition, since cases of venous thromboembolism and other complex diseases were oversampled for genotyping38, our study participants might not be representative of the whole cohort, although we did not observe any substantial differences between our study participants and the full cohort participants (Supplementary Table 1). Fifth, to estimate absolute risk of VTE, we used age-specific incidence rates of VTE that were not sex-specific. However, this should not bias our inferences for the risk in women given that overall disease risk is not different between men and women in the general population.60 Sixth, due to limited numbers of participants using different HT administrations or formulations, this study did not evaluate interactions with other characteristics of HT such as routes of administration (e.g., transdermal therapy, which appears to be associated with fewer thromboembolic events than oral estrogen-based HT, likely similar to the community-based risk for thrombophilic events)46 or oral estrogen doses (e.g., ≤ 0.625 mg or > 0.625 mg). Furthermore, we couldn’t incorporate information on family history of VTE, which would help our assessment of genetic risk of VTE, as well as details of occupation history, any hospitalization, other medical conditions and medication use (e.g., aspirin dose) because of lack of the data. Our GRS included 16 GWAS-significant variants, though we acknowledge that there are more genetic variants known to increase the risk of VTE. Future analyses that include more variants in a GRS may be worthwhile. Lastly, we examined the gene-hormone interactions in middle-aged female nurse participants of European ancestry only; thus, our findings may have limited generalizability to women with other occupations or other races or ethnicities.
Despite the limitations, strengths of the current study include its setting in a well-defined, large prospective cohort of U.S. women and comprehensive inspection of the interaction effects between a GWAS-based VTE GRS and characteristics of HT (use status, type, duration, and initiation time) on VTE risk. Because we used a GRS that aggregated effects of genome-wide significant genetic variants associated with VTE risk, we were able to boost our power to detect gene-hormone interactions as compared to previous studies testing interactions of single mutations and hormone use. Also, the prospective cohort design enabled us to adjust for time-varying effects of confounders that have been collected in a uniform manner during 22 years of follow-up.
To reduce the risk of VTE among postmenopausal women considering HT, it is important to identify a subgroup of women genetically susceptible to VTE and to understand the characteristics of HT that synergistically increase VTE risk. In that context, our results suggest that genetically susceptible postmenopausal women may have an excess risk due to the joint effects of genetic variants with current HT use, use of oral estrogen with or without progestin, long duration of HT use, or early initiation of HT, and that excess risk may be differential by age. These results contribute evidence for personalized recommendations regarding HT use in postmenopausal women and point to the potential utility of genetic information in clinical decision-making and evaluating the benefit-risk profile for HT. However, to confirm this finding and make it generalizable to women with non-European ancestries, replication in large prospective studies that include women with diverse backgrounds should be a priority.
Conclusions
In conclusion, the estimated difference in VTE risk between current and never HT users was larger in young postmenopausal women with the highest genetic risk than in young postmenopausal women with the lowest genetic risk, suggesting that the VTE GRS could inform clinical decisions regarding HT use after menopause.
Supplementary Material
Acknowledgement:
We would like to thank the participants and study staff of the Nurses’ Health Study for their valuable contribution.
Sources of funding: This research was supported by the Nurses’ Health Study (NHS) cohort infrastructure grant (UM1 CA186107), NHS blood grant (R01 CA49449), and NHS cardiovascular grant (R01 HL034594). The content is solely the responsibility of the authors and does not necessarily represent the official views of the National Institutes of Health. And NHLBI grant T32-HL098048 supported L.B. Harrington and K.A. Hagan’s time on this project.
Footnotes
Financial disclosures/conflicts of interest: Linda B. Harrington is a full-time employee at Kaiser Permanente Washington Health Research Institute whose current research portfolio at the Kaiser Permanente Washington Health Research Institute is supported by grants from the NIH and FDA Sentinel. Shilpa N. Bhupathiraju is a scientific advisor for LayerIV for work unrelated to this manuscript. Christopher Kabrhel receives funding from Diagnostica Stago, Grifols, and consulting to Boston Scientific; he has received past funding from Janssen. The other authors have nothing to disclose.
References
- 1.Kennedy DL, Baum C, Forbes MB. Noncontraceptive estrogens and progestins: use patterns over time. Obstet Gynecol 1985;65(3):441–446. [PubMed] [Google Scholar]
- 2.Hemminki E, Kennedy DL, Baum C, McKinlay SM. Prescribing of noncontraceptive estrogens and progestins in the United States, 1974–86. Am J Public Health 1988;78(11):1479–1481. DOI: 10.2105/ajph.78.11.1479. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Wysowski DK, Golden L, Burke L. Use of menopausal estrogens and medroxyprogesterone in the United States, 1982–1992. Obstet Gynecol 1995;85(1):6–10. DOI: 10.1016/0029-7844(94)00339-f. [DOI] [PubMed] [Google Scholar]
- 4.Rossouw JE, Anderson GL, Prentice RL, et al. Risks and benefits of estrogen plus progestin in healthy postmenopausal women: principal results From the Women’s Health Initiative randomized controlled trial. JAMA 2002;288(3):321–333. DOI: 10.1001/jama.288.3.321. [DOI] [PubMed] [Google Scholar]
- 5.Hersh AL, Stefanick ML, Stafford RS. National use of postmenopausal hormone therapy: annual trends and response to recent evidence. JAMA 2004;291(1):47–53. DOI: 10.1001/jama.291.1.47. [DOI] [PubMed] [Google Scholar]
- 6.Sprague BL, Trentham-Dietz A, Cronin KA. A sustained decline in postmenopausal hormone use: results from the National Health and Nutrition Examination Survey, 1999–2010. Obstet Gynecol 2012;120(3):595–603. DOI: 10.1097/AOG.0b013e318265df42. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Daly E, Vessey MP, Hawkins MM, Carson JL, Gough P, Marsh S. Risk of venous thromboembolism in users of hormone replacement therapy. Lancet 1996;348(9033):977–980. DOI: 10.1016/S0140-6736(96)07113-9. [DOI] [PubMed] [Google Scholar]
- 8.Canonico M, Plu-Bureau G, Lowe GD, Scarabin PY. Hormone replacement therapy and risk of venous thromboembolism in postmenopausal women: systematic review and meta-analysis. BMJ 2008;336(7655):1227–1231. DOI: 10.1136/bmj.39555.441944.BE. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Roach RE, Lijfering WM, Helmerhorst FM, Cannegieter SC, Rosendaal FR, van Hylckama Vlieg A. The risk of venous thrombosis in women over 50 years old using oral contraception or postmenopausal hormone therapy. J Thromb Haemost 2013;11(1):124–131. DOI: 10.1111/jth.12060. [DOI] [PubMed] [Google Scholar]
- 10.Bhupathiraju SN, Grodstein F, Rosner BA, et al. Hormone Therapy Use and Risk of Chronic Disease in the Nurses’ Health Study: A Comparative Analysis With the Women’s Health Initiative. Am J Epidemiol 2017;186(6):696–708. DOI: 10.1093/aje/kwx131. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Rymer J, Wilson R, Ballard K. Making decisions about hormone replacement therapy. BMJ 2003;326(7384):322–326. DOI: 10.1136/bmj.326.7384.322. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Weissfeld JL, Liu W, Woods C, et al. Trends in oral and vaginally administered estrogen use among US women 50 years of age or older with commercial health insurance. Menopause 2018;25(6):611–614. DOI: 10.1097/GME.0000000000001054. [DOI] [PubMed] [Google Scholar]
- 13.The NHTPSAP. The 2017 hormone therapy position statement of The North American Menopause Society. Menopause 2017;24(7):728–753 10.1097/GME.0000000000000921. [DOI] [PubMed] [Google Scholar]
- 14.Rosendaal FR. Venous thrombosis: a multicausal disease. Lancet 1999;353(9159):1167–1173. DOI: 10.1097/GME.0000000000000921. [DOI] [PubMed] [Google Scholar]
- 15.Rosendaal FR, Vessey M, Rumley A, et al. Hormonal replacement therapy, prothrombotic mutations and the risk of venous thrombosis. Br J Haematol 2002;116(4):851–854. DOI: 10.1046/j.0007-1048.2002.03356.x. [DOI] [PubMed] [Google Scholar]
- 16.Bouligand J, Cabaret O, Canonico M, et al. Effect of NFE2L2 genetic polymorphism on the association between oral estrogen therapy and the risk of venous thromboembolism in postmenopausal women. Clin Pharmacol Ther 2011;89(1):60–64. DOI: 10.1038/clpt.2010.241. [DOI] [PubMed] [Google Scholar]
- 17.Canonico M, Bouaziz E, Carcaillon L, et al. Synergism between oral estrogen therapy and cytochrome P450 3A5*1 allele on the risk of venous thromboembolism among postmenopausal women. J Clin Endocrinol Metab 2008;93(8):3082–3087. DOI: 10.1210/jc.2008-0450. [DOI] [PubMed] [Google Scholar]
- 18.Walter S, Mejia-Guevara I, Estrada K, Liu SY, Glymour MM. Association of a Genetic Risk Score With Body Mass Index Across Different Birth Cohorts. JAMA 2016;316(1):63–69. DOI: 10.1001/jama.2016.8729. [DOI] [PubMed] [Google Scholar]
- 19.Khera AV, Emdin CA, Drake I, et al. Genetic Risk, Adherence to a Healthy Lifestyle, and Coronary Disease. N Engl J Med 2016;375(24):2349–2358. DOI: 10.1056/NEJMoa1605086. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Torkamani A, Wineinger NE, Topol EJ. The personal and clinical utility of polygenic risk scores. Nat Rev Genet 2018;19(9):581–590. DOI: 10.1038/s41576-018-0018-x. [DOI] [PubMed] [Google Scholar]
- 21.Morange PE, Bezemer I, Saut N, et al. A follow-up study of a genome-wide association scan identifies a susceptibility locus for venous thrombosis on chromosome 6p24.1. Am J Hum Genet 2010;86(4):592–595. DOI: 10.1016/j.ajhg.2010.02.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Tregouet DA, Heath S, Saut N, et al. Common susceptibility alleles are unlikely to contribute as strongly as the FV and ABO loci to VTE risk: results from a GWAS approach. Blood 2009;113(21):5298–5303. DOI: 10.1182/blood-2008-11-190389. [DOI] [PubMed] [Google Scholar]
- 23.Germain M, Saut N, Greliche N, et al. Genetics of venous thrombosis: insights from a new genome wide association study. PLoS One 2011;6(9):e25581. DOI: 10.1371/journal.pone.0025581. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Heit JA, Armasu SM, Asmann YW, et al. A genome-wide association study of venous thromboembolism identifies risk variants in chromosomes 1q24.2 and 9q. J Thromb Haemost 2012;10(8):1521–1531. DOI: 10.1111/j.1538-7836.2012.04810.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Tang W, Teichert M, Chasman DI, et al. A genome-wide association study for venous thromboembolism: the extended cohorts for heart and aging research in genomic epidemiology (CHARGE) consortium. Genet Epidemiol 2013;37(5):512–521. DOI: 10.1002/gepi.21731. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Germain M, Chasman DI, de Haan H, et al. Meta-analysis of 65,734 individuals identifies TSPAN15 and SLC44A2 as two susceptibility loci for venous thromboembolism. Am J Hum Genet 2015;96(4):532–542. DOI: 10.1016/j.ajhg.2015.01.019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Hinds DA, Buil A, Ziemek D, et al. Genome-wide association analysis of self-reported events in 6135 individuals and 252 827 controls identifies 8 loci associated with thrombosis. Hum Mol Genet 2016;25(9):1867–1874. DOI: 10.1093/hmg/ddw0137. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Klarin D, Emdin CA, Natarajan P, Conrad MF, Consortium I, Kathiresan S. Genetic Analysis of Venous Thromboembolism in UK Biobank Identifies the ZFPM2 Locus and Implicates Obesity as a Causal Risk Factor. Circ Cardiovasc Genet 2017;10(2). DOI: 10.1161/CIRCGENETICS.116.001643. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Wassel CL, Rasmussen-Torvik LJ, Callas PW, et al. A genetic risk score comprising known venous thromboembolism loci is associated with chronic venous disease in a multi-ethnic cohort. Thromb Res 2015;136(5):966–973. DOI: 10.1016/j.thromres.2015.09.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Crous-Bou M, De Vivo I, Camargo CA Jr., et al. Interactions of established risk factors and a GWAS-based genetic risk score on the risk of venous thromboembolism. Thromb Haemost 2016;116(4):705–713. DOI: 10.1160/TH16-02-0172. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Kim J, Kraft P, Hagan KA, Harrington LB, Lindstroem S, Kabrhel C. Interaction of a genetic risk score with physical activity, physical inactivity, and body mass index in relation to venous thromboembolism risk. Genet Epidemiol 2018;42(4):354–365. DOI: 10.1002/gepi.22118. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Colditz GA, Hankinson SE. The Nurses’ Health Study: lifestyle and health among women. Nat Rev Cancer 2005;5(5):388–396. DOI: 10.1038/nrc1608. [DOI] [PubMed] [Google Scholar]
- 33.Harrington LB, Hagan KA, Mukamal KJ, et al. Alcohol consumption and the risk of incident pulmonary embolism in US women and men. J Thromb Haemost 2018;16(9):1753–1762. DOI: 10.1111/jth.14224. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Pun VC, Hart JE, Kabrhel C, Camargo CA, Jr., Baccarelli AA, Laden F. Prospective Study of Ambient Particulate Matter Exposure and Risk of Pulmonary Embolism in the Nurses’ Health Study Cohort. Environ Health Perspect 2015;123(12):1265–1270. DOI: 10.1289/ehp.1408927. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Bhupathiraju SN, Grodstein F, Stampfer MJ, Willett WC, Hu FB, Manson JE. Exogenous Hormone Use: Oral Contraceptives, Postmenopausal Hormone Therapy, and Health Outcomes in the Nurses’ Health Study. Am J Public Health 2016;106(9):1631–1637. DOI: 10.2105/AJPH.2016.303349. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Willett W, Stampfer MJ, Bain C, et al. Cigarette smoking, relative weight, and menopause. Am J Epidemiol 1983;117(6):651–658. DOI: 10.1093/oxfordjournals.aje.a113598. [DOI] [PubMed] [Google Scholar]
- 37.Oliver-Williams C, Glisic M, Shahzad S, et al. The route of administration, timing, duration and dose of postmenopausal hormone therapy and cardiovascular outcomes in women: a systematic review. Hum Reprod Update 2019;25(2):257–271. DOI: 10.1093/humupd/dmy039. [DOI] [PubMed] [Google Scholar]
- 38.Lindstrom S, Loomis S, Turman C, et al. A comprehensive survey of genetic variation in 20,691 subjects from four large cohorts. PLoS One 2017;12(3):e0173997. DOI: 10.1371/journal.pone.0173997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.International Consortium for Blood Pressure Genome-Wide Association S, Ehret GB, Munroe PB, et al. Genetic variants in novel pathways influence blood pressure and cardiovascular disease risk. Nature 2011;478(7367):103–109. DOI: 10.1038/nature10405. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Khalili H, Higuchi LM, Ananthakrishnan AN, et al. Hormone therapy increases risk of ulcerative colitis but not Crohn’s disease. Gastroenterology 2012;143(5):1199–1206. DOI: 10.1053/j.gastro.2012.07.096. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Puurunen MK, Gona PN, Larson MG, Murabito JM, Magnani JW, O’Donnell CJ. Epidemiology of venous thromboembolism in the Framingham Heart Study. Thromb Res 2016;145:27–33. DOI: 10.1016/j.thromres.2016.06.033. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Barsoum MK, Heit JA, Ashrani AA, Leibson CL, Petterson TM, Bailey KR. Is progestin an independent risk factor for incident venous thromboembolism? A population-based case-control study. Thromb Res 2010;126(5):373–378. DOI: 10.1016/j.thromres.2010.08.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Sweetland S, Beral V, Balkwill A, et al. Venous thromboembolism risk in relation to use of different types of postmenopausal hormone therapy in a large prospective study. J Thromb Haemost 2012;10(11):2277–2286. DOI: 10.1111/j.1538-7836.2012.04919.x. [DOI] [PubMed] [Google Scholar]
- 44.Bergendal A, Kieler H, Sundstrom A, Hirschberg AL, Kocoska-Maras L. Risk of venous thromboembolism associated with local and systemic use of hormone therapy in peri- and postmenopausal women and in relation to type and route of administration. Menopause 2016;23(6):593–599. DOI: 10.1097/GME.0000000000000611. [DOI] [PubMed] [Google Scholar]
- 45.Canonico M, Fournier A, Carcaillon L, et al. Postmenopausal hormone therapy and risk of idiopathic venous thromboembolism: results from the E3N cohort study. Arterioscler Thromb Vasc Biol 2010;30(2):340–345. DOI: 10.1161/ATVBAHA.109.196022. [DOI] [PubMed] [Google Scholar]
- 46.Vinogradova Y, Coupland C, Hippisley-Cox J. Use of hormone replacement therapy and risk of venous thromboembolism: nested case-control studies using the QResearch and CPRD databases. BMJ 2019;364:k4810. DOI: 10.1136/bmj.k4810. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Manson JE, Chlebowski RT, Stefanick ML, et al. Menopausal hormone therapy and health outcomes during the intervention and extended poststopping phases of the Women’s Health Initiative randomized trials. JAMA 2013;310(13):1353–1368. DOI: 10.1001/jama.2013.278040. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Renoux C, Dell’Aniello S, Brenner B, Suissa S. Bias from depletion of susceptibles: the example of hormone replacement therapy and the risk of venous thromboembolism. Pharmacoepidemiol Drug Saf 2017;26(5):554–560. DOI: 10.1002/pds.4197. [DOI] [PubMed] [Google Scholar]
- 49.Hoibraaten E, Abdelnoor M, Sandset PM. Hormone replacement therapy with estradiol and risk of venous thromboembolism--a population-based case-control study. Thromb Haemost 1999;82(4):1218–1221. [PubMed] [Google Scholar]
- 50.Salpeter SR, Walsh JM, Greyber E, Salpeter EE. Brief report: Coronary heart disease events associated with hormone therapy in younger and older women. A meta-analysis. J Gen Intern Med 2006;21(4):363–366. DOI: 10.1111/j.1525-1497.2006.00389.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Grodstein F, Manson JE, Stampfer MJ, Rexrode K. Postmenopausal hormone therapy and stroke: role of time since menopause and age at initiation of hormone therapy. Arch Intern Med 2008;168(8):861–866. DOI: 10.1001/archinte.168.8.861. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Rossouw JE, Prentice RL, Manson JE, et al. Postmenopausal hormone therapy and risk of cardiovascular disease by age and years since menopause. JAMA 2007;297(13):1465–1477. DOI: 10.1001/jama.297.13.1465. [DOI] [PubMed] [Google Scholar]
- 53.Schierbeck LL, Rejnmark L, Tofteng CL, et al. Effect of hormone replacement therapy on cardiovascular events in recently postmenopausal women: randomised trial. BMJ 2012;345:e6409. DOI: 10.1136/bmj.e6409. [DOI] [PubMed] [Google Scholar]
- 54.Hodis HN, Mack WJ, Henderson VW, et al. Vascular Effects of Early versus Late Postmenopausal Treatment with Estradiol. N Engl J Med 2016;374(13):1221–1231. DOI: 10.1056/NEJMoa1505241. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Prentice RL, Manson JE, Langer RD, et al. Benefits and risks of postmenopausal hormone therapy when it is initiated soon after menopause. Am J Epidemiol 2009;170(1):12–23. DOI: 10.1093/aje/kwp115. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Cushman M, Kuller LH, Prentice R, et al. Estrogen plus progestin and risk of venous thrombosis. JAMA 2004;292(13):1573–1580. DOI: 10.1001/jama.292.13.1573. [DOI] [PubMed] [Google Scholar]
- 57.Herrington DM, Vittinghoff E, Howard TD, et al. Factor V Leiden, hormone replacement therapy, and risk of venous thromboembolic events in women with coronary disease. Arterioscler Thromb Vasc Biol 2002;22(6):1012–1017. DOI: 10.1161/01.atv.0000018301.91721.94. [DOI] [PubMed] [Google Scholar]
- 58.Straczek C, Oger E, Yon de Jonage-Canonico MB, et al. Prothrombotic mutations, hormone therapy, and venous thromboembolism among postmenopausal women: impact of the route of estrogen administration. Circulation 2005;112(22):3495–3500. DOI: 10.1161/CIRCULATIONAHA.105.565556. [DOI] [PubMed] [Google Scholar]
- 59.Grodstein F, Clarkson TB, Manson JE. Understanding the divergent data on postmenopausal hormone therapy. N Engl J Med 2003;348(7):645–650. DOI: 10.1056/NEJMsb022365. [DOI] [PubMed] [Google Scholar]
- 60.Stein PD, Hull RD, Kayali F, Ghali WA, Alshab AK, Olson RE. Venous thromboembolism according to age: the impact of an aging population. Arch Intern Med 2004;164(20):2260–2265. DOI: 10.1001/archinte.164.20.2260. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


