Abstract
Background:
The vaginal microbiome has been associated with adverse pregnancy outcomes, but information on the impact of diet on microbiome composition is largely unexamined.
Objective:
To estimate the association between prenatal diet and vaginal microbiota composition overall and by race.
Methods:
We leveraged a racially diverse prenatal cohort of North Carolina women enrolled between 1995-2001 to conduct this analysis using cross-sectional data. Women completed food frequency questionnaires about diet in the previous 3 months and foods were categorized into subgroups: fruits, vegetables, nuts/seeds, whole grains, low-fat dairy, sweetened beverages, and red meat. We additionally assessed dietary vitamin D, fiber, and yogurt consumption. Stored vaginal swabs collected in mid-pregnancy were sequenced using 16S taxonomic profiling. Women were categorized into 3 groups based on predominance of species: Lactobacillus iners, Lactobacillus miscellaneous, and Bacterial Vaginosis (BV)-associated bacteria. Adjusted Poisson models with robust variance estimators were run to assess the risk of being in a specific vagitype compared to the referent. Race-stratified models (Black/White) were also run.
Results:
In this study of 634 women, higher consumption of dairy was associated with increased likelihood of membership in the L. crispatus group compared to the L. iners group in a dose-dependent manner (RR quartile 4 vs. 1: 2.01 [95% CI: 1.36, 2.95]). Increased intake of fruit, vitamin D, fiber, and yogurt was also associated with increased likelihood of membership in L. crispatus compared to L. iners, but only among black women. Statistical heterogeneity was only detected for fiber intake. There were no detected associations between any other food groups or risk of membership in the BV-group.
Conclusions:
Higher consumption of low-fat dairy was associated with increased likelihood of membership in a beneficial vagitype, potentially driven by probiotics.
Keywords: microbiota, diet, bacterial vaginosis, pregnancy
Introduction
The vaginal microbiome is a constellation of bacterial species whose balance helps maintain a woman’s health. Though women have unique patterns, evidence suggests that a shift towards anaerobic microbes coupled with a decrease in Lactobacillus species is associated with higher risk of adverse pregnancy outcomes like preterm birth and miscarriage.1-3 These studies examined associations with individual species or a diagnosis of Bacterial Vaginosis (BV), a dysbiotic vaginal state characterized by pH >4.5 and increased levels of anaerobic species including Gardnerella vaginalis, Atopobium vaginae, and Mycoplasma hominis.4 Up to 40% of preterm births are thought to be caused by intrauterine infection and subsequent inflammation, potentially resulting from pathogenic organisms gaining access to the amniotic cavity through the vagina.5,6 Bacteria of the Lactobacillus genus serve as physiological barriers to these pathogens by producing lactic acid and lowering vaginal pH.7 Lowered pH also helps promote “healthy” bacteria, thereby preventing colonization by pathologic organisms.8,9
Known influences on the vaginal microbiome include douching, sexual activity, race, and smoking.10 Effects of diet on vaginal microbiota remain largely unexamined. Previous studies have examined associations between diet and BV, but there are very few studies on diet and microbiome composition beyond a BV diagnosis, or studies examining specific dietary subgroups and vaginal microbiota. Prior literature found that BV risk increases with deficiencies in iron and vitamin D during pregnancy,11,12 as well as higher levels of dietary fat, glycemic load, and a diet consisting of low nutritional density foods.13,14
Despite a clear scientific gap in the relationship between diet and vaginal microbiota, many lay articles discuss the impact of foods on vaginal health.15,16 Frequently mentioned is the benefit of probiotic foods, most commonly yogurt, which contains active cultures. While yogurt is often discussed as an important food for maintaining vaginal health, there have been no scientific studies examining its effects, or dairy consumption generally, on the vaginal microbiome beyond a BV diagnosis.
Mounting literature suggests that there may be racial/ethnic differences in vaginal microbiome compositions.8,17 Dietary patterns, behavioral practices, and sociodemographic factors also differ by race/ethnicity.18,19 Additionally, it is well established that there are significant racial disparities in preterm birth such that black women are at substantially higher risk than White women.20,21 Because of these important racial differences, we examined the association between diet and microbiome by maternal race.
Disentangling diet from other underlying determinants of vaginal microbiome composition will better inform our understanding of modifiable routes to optimal vaginal microbial community patterns. Our study objective was to measure the association between diet and vaginal microbiome composition in a racially diverse prenatal cohort.
Methods
Cohort selection
The Pregnancy, Infection, and Nutrition (PIN) study enrolled pregnant women with singleton pregnancies in the central North Carolina region between August 1995 and February 2001. Women were recruited from prenatal care clinics at the University of North Carolina Hospitals, Wake County Human Services, and the Wake Area Health Education Center. Women were eligible if they were between 24- and 29-weeks’ gestation, had a singleton pregnancy, able to communicate in English, >16 years old, had phone access, and planned to deliver at the recruitment site. Of the 5,196 women eligible for participation, 3,163 were recruited successfully (61%).22
The PIN study was structured to enable a nested case-cohort design, such that a random subset of the cohort was identified at enrollment to serve as a reference population for nested studies that is representative of the exposure distribution in the larger cohort, referred to as “subcohort”. This random subcohort was sampled irrespective of pregnancy outcome. For the current study, we included women who were sampled into this nested subcohort (n = 855). Of these women, 723 had information on both dietary consumption and taxonomic profiling. We further excluded women who did not self-identify as Black or White (Figure 1).
Figure 1.
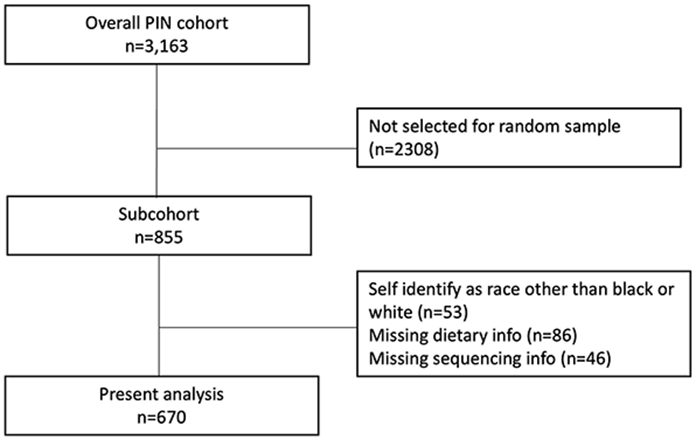
Flow chart diagramming selection from overall cohort to present analytic sample
Abbreviations: Pregnancy, Infection, and Nutrition (PIN)
Exposure
Women completed a self-administered Block food frequency questionnaire (FFQ) between 26- and 29-weeks’ gestation. The questionnaire was modified to reflect the previous 3-month period and to include “southern” foods.23 Women recorded both frequency of intake and portion sizes using a serving-size visual, allowing for the calculation of total servings consumed. Using relevant food items from the FFQ, we constructed 7 sub-categories of intake: fruits, vegetables, nuts/legumes, low-fat dairy, whole grains, red meats, and sweetened beverages, expressed in servings consumed per day. These categorizations, known as the DASH diet, reflect previously established components of a healthy diet.24,25 Studies have found that this diet is associated with reduced risk of pregnancy complications.26,27 Our objective was to understand the role that the DASH diet played as an upstream predictor of vaginal microbiome. We additionally examined fiber and vitamin D based on associations with the vaginal microbiome and BV in prior literature28,29 and yogurt due to its probiotic content.
The fruit category included whole fruits only and excluded 100% fruit juices. Whole grain foods included whole wheat bread and high fiber cereals. The red meat category included pork and beef products. Sweetened beverages included soft drinks, Snapple, KoolAid, drinks with some juice (Sunny D), or the addition of sugar to coffee/tea. In addition, the FFQ was used to calculate dietary intake of various nutrients and specific food items expressed in nutrient specific units or grams/day.
Outcome
DNA extraction and sequencing
Self-collected vaginal swabs were obtained between 24- and 29 weeks’ gestation. Samples were collected using cotton swabs from the posterior vaginal apex. Frozen swabs were thawed on ice and processed using the PowerSoil DNA Isolation Kit from MO BIO Laboratories, Inc. as described by the Vaginal Microbiome Consortium at Virginia Commonwealth University.30 DNA samples were eluted with 100 μL buffer into tubes and quantitated using PicoGreen.
Extracted DNA was amplified with barcoded primers targeting the V1–V3 hypervariable regions of the bacterial 16S rRNA gene.31,32 Samples are multiplexed (384 samples/run) using a sample-specific dual-index strategy and sequenced using 2 x 300 b PE technology on Illumina MiSeq sequencers. The paired-end quality-aware raw sequence files (.fastq) are demultiplexed into sample-specific data using custom scripts. The raw paired-end sequencing data is subjected to merging and quality-filtering using MeFiT,33 our software package that invokes CASPER for merging paired-end sequences and quality filters them using their maximum expected error rate. Of the 1,077 samples, seven samples with <1,000 high-quality reads were excluded. High-quality sequences were then identified to species-level taxonomy using STIRRUPS with a 97% identity cutoff.1
Comprehensive 16S rRNA gene-based taxonomic survey yielded a mean count of 43,276 reads/sample with minimum and maximum counts of 1,824 and 186,784. Over 99.9% of the high-quality single reads generated overlapping pair-end reads. High-quality sequences were then assigned to the species-level taxonomic assignments for vaginal samples using STIRRUP,31 an analysis platform that employs the USEARCH algorithm34 combined with a curated 16S rRNA sequence database. Paired reads which did not align to the same reference sequence were discarded as chimeras.
Vagitype clusters
Because previous research has found that vaginal microbial community patterns cluster within groups,1,8 we applied the clustering algorithm of Fettweis et al.1 to the current cohort. Women were classified based on their most abundant species, if detected at >30%. If no species was detected >30% abundance, women were classified “no type.” We created vagitypes by combining women with similar dominant species. The BV-mix vagitype was characterized by species associated with BV (Gardnerella vaginalis, Atopobium vaginae, Lachnospiraceae BVAB1). The L. crispatus vagitype was characterized by the predominance of Lactobacillus species: Lactobacillus crispatus cluster, Lactobacillus gasseri cluster, Lactobacillus jensenii/fornicalis/psittaci, and Lactobacillus delbrueckii. Lastly, the Lactobacillus iners vagitype was characterized by the predominance of Lactobacillus iners, which is hypothesized as a transitional species between healthy and a BV-like microbiome. 36 women did not fall into the aforementioned vagitypes and were excluded from analyses due to heterogeneity.
Covariates
Trained staff members interviewed participants within two weeks of recruitment. Women were asked about demographics, sexual behaviors, and behaviors like smoking, alcohol and drug use. Maternal self-reported race/ethnicity was categorized into White, Black, or other. “Other” was excluded due to small sample size (n=45). Women completed a Life Experiences Survey35 in which they indicated if they experienced any of a listed 39 life events since the beginning of pregnancy, with the option to write in additional events. Examples of events on the questionnaire include job loss, illness/injury of family members, and relationship difficulties.
Statistical analysis
Demographic characteristics were assessed in a univariate analysis, and additionally stratified on vagitype assignment. We then examined the distribution of our exposures (food group and specific nutrients/food item) in the population and stratified on race and vagitype. For fruit, vegetables, nuts, red meat, and sweetened beverages, each component was divided into equal quartiles based on the distribution of daily servings in the entire population. The distributions of low-fat dairy and whole grains were right skewed, so quartiles were created by examining the distributions and selecting interpretable cut-points. Red meats and sweetened beverages were reverse coded so that the highest quartile represents the fewest servings. Thus, for all components, the 4th quartile indicates the most beneficial consumption, following current and previous scores of a good quality diet.36 Fiber was modeled in quartiles based on distributions in the total population. The distributions of dietary vitamin D and yogurt were right-skewed so exposure was modeled using tertiles. For yogurt, many women (n=332) reported no daily intake so tertiles were created using 0 grams/day as a referent, and the upper two tertiles created by halving the number of women who consumed any yogurt. A sensitivity analysis was conducted which excluded women with reported calorie intakes <2.5th percentile or >97.5th percentile.
Covariates were selected a priori using a Directed Acyclic Graph (eFigure 1). Poisson models with robust variance estimators were run to assess the risk of being in a specific vagitype compared to the referent. Due to sample size considerations, the referent was the L. iners vagitype. Each category of food was run in an independent model that adjusted for race (White, Black), parity (0, 1-2, 3+), maternal age (continuous), pre-pregnancy BMI (continuous), and maternal stress (continuous count of stressful events during pregnancy). Fiber, vitamin D, and yogurt were adjusted for the same covariates.
We additionally stratified analyses on race to examine race-specific associations, given the heterogeneity in both microbiome composition and diet. We considered p<0.1 evidence of statistically significant interaction.
Missing data
Our analysis restricted to those with both exposure and outcome data. Missing values for covariates selected for the final model were multiply imputed using chained equations. Prior to imputation, the only covariate in our minimally sufficient set with missing >5% was negative life events (nmissing = 148).
Ethics approval
All procedures were approved by the University of North Carolina’s Institutional Review Board.
Results
The largest vagitype was L. iners (n=308), followed by the L. crispatus-dominated vagitype (n=250), and the smallest retained vagitype was the BV-mix species (n=76). Most women in this population were aged 20-34, had normal pre-pregnancy BMI, and were non-smokers (Table 1).
Table 1.
Demographics of study population stratified on sequencing vagitype, N (%) or mean (std)
| Overall (n=634) |
L. iners vagitype (n=308) |
L. crispatus vagitype (n=250) |
BV-mix vagitype (n=76) |
|
|---|---|---|---|---|
| Maternal age (years) | ||||
| <20 | 122 (19.2) | 78 (25.3) | 30 (12.0) | 14 (18.4) |
| 20-34 | 437 (68.9) | 208 (67.5) | 182 (72.8) | 47 (61.8) |
| 35+ | 75 (11.8) | 22 (7.1) | 38 (15.2) | 15 (19.7) |
| Mean (SD) | 26.1 (6.1) | 24.6 (5.6) | 27.8 (6.1) | 26.6 (6.9) |
| Race | ||||
| White | 370 (58.4) | 152 (49.4) | 176 (70.4) | 42 (55.3) |
| Black | 264 (41.6) | 156 (50.6) | 74 (29.6) | 34 (44.7) |
| Maternal education (years) | ||||
| <12 | 135 (21.3) | 84 (27.3) | 30 (12.0) | 21 (27.6) |
| 12 | 198 (31.2) | 108 (35.1) | 65 (26.0) | 25 (32.9) |
| 13-15 | 136 (21.5) | 73 (23.7) | 53 (21.2) | 10 (13.2) |
| 16+ | 165 (26.0) | 43 (14.0) | 102 (40.8) | 20 (26.3) |
| Pre-pregnancy BMI (kg/m2) | ||||
| <18.5 | 33 (5.2) | 17 (5.5) | 10 (4.0) | 5 (6.6) |
| 18.5 – 24.9 | 300 (47.3) | 137 (44.5) | 133 (53.2) | 30 (39.5) |
| 25.0 – 29.9 | 125 (19.7) | 60 (19.5) | 49 (19.6) | 16 (21.1) |
| ≥30 | 176 (27.8) | 94 (30.5) | 58 (23.2) | 25 (32.9) |
| Mean (SD) | 26.4 (7.5) | 26.7 (7.5) | 25.8 (7.5) | 27.2 (7.1) |
| Parity | ||||
| 0 | 289 (45.6) | 142 (46.1) | 125 (50.0) | 23 (30.3) |
| 1-2 | 295 (46.5) | 141 (45.8) | 112 (44.8) | 41 (53.9) |
| >2 | 50 (7.9) | 25 (8.1) | 13 (5.2) | 12 (15.8) |
| Smoked months 1-6 | ||||
| Any | 154 (24.3) | 83 (27.0) | 52 (20.8) | 19 (25.0) |
| None | 480 (75.7) | 225 (73.0) | 198 (79.2) | 57 (75.0) |
| Negative life events | 3.8 (3.2) | 3.9 (3.1) | 3.5 (3.1) | 3.9 (3.4) |
| Income, % of 1996 poverty level | ||||
| <50 | 77 (12.1) | 48 (15.6) | 19 (7.6) | 11 (14.5) |
| 50-99 | 128 (20.2) | 78 (25.3) | 33 (13.2) | 17 (22.4) |
| 100-199 | 184 (29.0) | 101 (32.7) | 57 (22.8) | 25 (32.0) |
| ≥200 | 245 (38.6) | 81 (26.3) | 141 (56.4) | 23 (30.2) |
Of the food groups examined, women consumed the fewest daily servings of nuts/legumes, red meat, and whole grains (mean servings/day: 0.59, 0.69, 0.72, respectively) (Table 2). On average, Black women consumed more servings of fruits, vegetables, nuts, red meat, and sweetened beverages than White women. Black women also had more servings of dietary fiber (median grams/day: 22.8 vs. 19.2). White women consumed considerably more low-fat dairy and yogurt than Black women (median yogurt grams/day: 8.16 vs. 0).
Table 2.
Distribution of servings per day of diet components and selected nutrients and foods
| Median (25th, 75th) | ||||||||
|---|---|---|---|---|---|---|---|---|
| Min- max |
Mean (sd) | Total population (n=634) |
White women (n=370) |
Black women (n=264) |
L. iners vagitype (n=308) |
L. crispatus vagitype (n=250) |
BV-mix vagitype (n=76) |
|
| Fruit | 0 – 22.9 | 2.52 (2.8) | 1.8 (0.8, 3.2) | 1.5 (0.7, 2.8) | 2.3 (0.9, 4.4) | 1.5 (0.7, 3.1) | 2.0 (1.0, 3.4) | 1.5 (0.7, 2.9) |
| Veg | 0 – 15.9 | 2.45 (2.3) | 1.8 (0.9, 3.2) | 1.7 (0.9, 3.0) | 1.9 (1.0, 3.8) | 1.7 (0.8, 2.9) | 1.9 (1.1, 3.7) | 1.5 (0.9, 2.7) |
| Nuts | 0 – 6.81 | 0.59 (0.7) | 0.4 (0.2, 0.7) | 0.4 (0.2, 0.6) | 0.4 (0.2, 0.9) | 0.4 (0.2, 0.8) | 0.4 (0.2, 0.7) | 0.4 (0.2, 0.8) |
| Whole grains | 0 – 22.2 | 0.72 (1.3) | 0.4 (0.1, 1.0) | 0.5 (0.1, 1.1) | 0.3 (0, 0.8) | 0.3 (0, 0.8) | 0.5 (0.1, 1.2) | 0.4 (0, 1.1) |
| Low-fat dairy | 0 – 21.9 | 2.05 (2.8) | 1.0 (0, 3.0) | 1.5 (0.2, 3.9) | 0.3 (0, 1.4) | 0.4 (0, 2.1) | 1.7 (0.3, 4.0) | 0.7 (0, 2.8) |
| Red meat | 0 – 4.00 | 0.69 (0.6) | 0.5 (0.3, 0.9) | 0.4 (0.2, 0.7) | 0.8 (0.4, 1.2) | 0.6 (0.3, 1.0) | 0.4 (0.3, 0.8) | 0.5 (0.3, 1.0) |
| Sweetened beverages | 0 – 33.3 | 2.48 (4.1) | 1.2 (0.2, 3.0) | 0.9 (0.1, 2.5) | 1.2 (0.3, 3.7) | 1.2 (0.3, 3.3) | 0.7 (0.1, 2.1) | 1.3 (0.3, 4.1) |
| Selected nutrients/food | ||||||||
| Yogurt (grams/day) | 0 – 682 | 33.3 (69.7) | 0 (0, 32.4) | 8.16 (0, 48.4) | 0 (0, 16.1) | 0 (0, 30.3) | 8.16 (0, 56.8) | 0 (0, 32.4) |
| Dietary vit D (micrograms/day) | 2.07 – 801 | 55.2 (111) | 15.4 (10.7, 26.2) | 15.2 (10.0, 28.3) | 16.4 (11.5, 25.1) | 14.7 (10.5, 22.9) | 16.6 (11.0, 33.7) | 14.8 (11.0, 24.5) |
| Dietary fiber (grams/day) | 2.42 – 111 | 23.7 (13.7) | 20.1 (14.7, 29.6) | 19.2 (14.0, 25.8) | 22.8 (16.4, 35.3) | 19.5 (14.1, 27.1) | 20.5 (15.7, 31.2) | 20.6 (15.7, 32.3) |
Women in the L. crispatus vagitype reported more servings of low-fat dairy, yogurt, and dietary vitamin D than women in the other two vagitypes. When specifically examining yogurt consumption, women in the L. crispatus vagitype reported a median consumption of 9.4 grams/day, as compared to 0 grams/day in the L. iners and BV-mix vagitypes. Women in L. crispatus group consumed less red meat and sweetened beverages.
In adjusted models, we found a dose-dependent relationship between quartiles of low-fat dairy servings and risk of membership in the L. crispatus vagitype compared to the L. iners vagitype (Table 3). Women in the highest quartile were more likely to be classified into the L. crispatus vagitype than women in the lowest quartile (RR 1.58, 95% confidence interval [CI]: 1.17, 2.15). Associations were slightly stronger among Black women although not statistically heterogeneous by race. We also detected beneficial effects of higher fruit consumption, wherein women who were in the top quartiles of consumption were 1.39 and 1.34 times as likely to belong to L. crispatus vagitype (CIs: 1.05, 1.83; 1.00, 1.80). For consumption of vegetables, whole grains, and sweetened beverages, being in the 4th quartile (i.e., highest consumption of vegetables and whole grains and lowest consumption of sweetened beverages) was suggestively associated with increased membership in the L. crispatus vagitype. We found no associations between diet and likelihood of membership in the BV-mix vagitype. Sample sizes and cutpoints for each quartile are displayed in eTable 1.
Table 3.
Adjusted risk ratios (95% confidence intervals) for risk of being in select vagitype by quartile of daily servings of dietary components
|
L. crispatus vs. L. iners |
L. crispatus vs. L. iners, White women |
L. crispatus vs. L. iners, Black women |
P for interaction |
BV-mix vs. L. iners |
BV mix vs. L. iners, White women |
BV-mix vs. L. iners, Black women |
P for interaction |
|
|---|---|---|---|---|---|---|---|---|
| Fruit | ||||||||
| 2 vs. 1 | 1.25 (0.93, 1.67) | 1.22 (0.89, 1.68) | 1.25 (0.66, 2.37) | 1.10 (0.64, 1.90) | 0.97 (0.50, 1.88) | 1.16 (0.41, 3.27) | ||
| 3 vs. 1 | 1.39 (1.05, 1.83) | 1.40 (1.03, 1.90) | 1.24 (0.66, 2.34) | 1.11 (0.62, 1.96) | 0.88 (0.42, 1.86) | 1.41 (0.55, 3.61) | ||
| 4 vs. 1 | 1.34 (1.00, 1.80) | 1.15 (0.82, 1.62) | 1.77 (1.01, 3.11) | 0.22 | 1.10 (0.63, 1.91) | 0.48 (0.17, 1.32) | 1.98 (0.86, 4.57) | 0.10 |
| Vegetables | ||||||||
| 2 vs. 1 | 1.14 (0.87, 1.49) | 1.24 (0.92, 1.66) | 0.87 (0.49, 1.56) | 1.18 (0.69, 2.02) | 1.35 (0.66, 2.73) | 0.94 (0.42, 2.15) | ||
| 3 vs. 1 | 1.01 (0.77, 1.34) | 1.12 (0.83, 1.53) | 0.73 (0.50, 1.35) | 1.02 (0.58, 1.77) | 0.95 (0.44, 2.05) | 1.05 (0.48, 2.34) | ||
| 4 vs. 1 | 1.22 (0.94, 1.59) | 1.19 (0.88, 1.60) | 1.24 (0.75, 2.03) | 0.30 | 0.78 (0.42, 1.47) | 0.83 (0.36, 1.93) | 0.67 (0.27, 1.67) | 0.84 |
| Nuts | ||||||||
| 2 vs. 1 | 0.96 (0.77, 1.21) | 0.95 (0.74, 1.21) | 1.07 (0.61, 1.88) | 1.11 (0.61, 2.04) | 1.61 (0.68, 3.81) | 0.81 (0.31, 2.10) | ||
| 3 vs. 1 | 0.90 (0.71, 1.14) | 0.99 (0.78, 1.25) | 0.74 (0.40, 1.38) | 1.17 (0.63, 2.14) | 1.38 (0.53, 3.59) | 1.21 (0.56, 2.58) | ||
| 4 vs. 1 | 0.91 (0.70, 1.19) | 0.78 (0.56, 1.10) | 1.16 (0.70, 1.92) | 0.17 | 0.90 (0.48, 1.69) | 1.46 (0.60, 3.56) | 0.63 (0.25, 1.62) | 0.45 |
| Whole grains | ||||||||
| 2 vs. 1 | 1.16 (0.79, 1.40) | 1.09 (0.79, 1.51) | 0.93 (0.55, 1.57) | 0.86 (0.49, 1.49) | 0.87 (0.38, 1.97) | 0.76 (0.34, 1.72) | ||
| 3 vs. 1 | 1.01 (0.73, 1.39) | 0.85 (0.59, 1.23) | 1.35 (0.76, 2.39) | 0.88 (0.44, 1.76) | 0.42 (0.14, 1.29) | 1.80 (0.80, 4.08) | ||
| 4 vs. 1 | 1.31 (0.99, 1.73) | 1.20 (0.87, 1.67) | 1.44 (0.87, 2.40) | 0.26 | 1.35 (0.76, 2.39) | 1.03 (0.42, 2.53) | 1.32 (0.56, 3.11) | 0.16 |
| Low-fat dairy | ||||||||
| 2 vs. 1 | 1.32 (0.96, 1.83) | 1.26 (0.85, 1.88) | 1.43 (0.83, 2.47) | 0.77 (0.43, 1.36) | 1.14 (0.49, 2.65) | 0.55 (0.24, 1.23) | ||
| 3 vs. 1 | 1.47 (1.07, 2.00) | 1.30 (0.90, 1.89) | 1.70 (0.99, 2.93) | 1.00 (0.58, 1.73) | 1.44 (0.64, 3.25) | 0.61 (0.24, 1.53) | ||
| 4 vs. 1 | 1.58 (1.17, 2.15) | 1.43 (1.01, 2.05) | 1.89 (1.01, 3.55) | 0.85 | 1.22 (0.69, 2.17) | 1.35 (0.58, 3.15) | 1.48 (0.64, 3.40) | 0.31 |
| Red meat | ||||||||
| 2 vs. 1 | 1.02 (0.75, 1.38) | 1.42 (0.88, 2.30) | 0.76 (0.47, 1.24) | 0.86 (0.49, 1.53) | 1.07 (0.43, 2.67) | 0.78 (0.35, 1.75) | ||
| 3 vs. 1 | 1.12 (0.83, 1.51) | 1.45 (0.91, 2.32) | 0.92 (0.56, 1.52) | 1.25 (0.72, 2.15) | 1.45 (0.62, 3.40) | 1.12 (0.50, 2.59) | ||
| 4 vs. 1 | 1.03 (0.75, 1.40) | 1.32 (0.83, 2.12) | 0.82 (0.44, 1.54) | 0.28 | 0.86 (0.45, 1.64) | 0.88 (0.34, 2.32) | 0.95 (0.34, 2.60) | 0.92 |
| Sweetened beverages | ||||||||
| 2 vs. 1 | 1.13 (0.85, 1.51) | 1.14 (0.81, 1.60) | 1.11 (0.66, 1.85) | 0.92 (0.54, 1.57) | 0.92 (0.46, 1.85) | 0.94 (0.40, 2.22) | ||
| 3 vs. 1 | 1.18 (0.89, 1.57) | 1.26 (0.91, 1.75) | 0.98 (0.57, 1.71) | 0.85 (0.48, 1.52) | 0.73 (0.34, 1.57) | 0.92 (0.41, 2.07) | ||
| 4 vs. 1 | 1.23 (0.93, 1.61) | 1.21 (0.89, 1.66) | 1.26 (0.73, 2.18) | 0.77 | 0.87 (0.49, 1.54) | 0.62 (0.30, 1.27) | 1.37 (0.57, 3.29) | 0.58 |
All models adjusted for maternal age, parity, pre-pregnancy BMI, and number of stressful life events. Models for total population additionally adjusted for race.
We found some evidence that dietary vitamin D intake was associated with membership in the L crispatus vagitype, more so among Black women (Table 4). Overall, women with the highest dietary vitamin D intake were 1.21 times as likely (95% CI: 0.98 1.50) to belong to L. crispatus vagitype compared to the L. iners vagitype. In multivariable models, yogurt intake was suggestively associated with vagitype overall, driven by the association among Black women (RR: 1.54, 95% CI: 0.99, 2.42). The relationship between dietary fiber intake and vagitype was heterogeneous by race (p = 0.06); associations were generally null for White women and an increased likelihood of belonging to L. crispatus vagitype was only seen in the highest quartiles of intake for Black women (RR: 2.10, 95% CI: 1.19, 3.72). There was statistically significant heterogeneity noted in the association between yogurt intake and membership in BV-mix vagitype, but no individual associations were non-null. Sample sizes in each tertile were notably low and imbalanced in the BV-mix vs. L. iners comparison (eTable 2). Results were similar when restricting our sample to those with energy intakes between 2.5th percentile and 97.5th percentile of the population (eTables 3 and 4).
Table 4.
Adjusted risk ratios (95% confidence intervals) for risk of being in select vagitype for dietary vitamin D, yogurt, and fiber quantiles
| L. crispatus vs. L. iners | BV-mix vs. L. iners | |||||
|---|---|---|---|---|---|---|
| Total population | White women | Black women | Total population | White women | Black women | |
| Dietary vitamin D | ||||||
| 2 vs. 1 | 1.14 (0.91, 1.44) | 1.15 (0.90, 1.48) | 1.11 (0.66, 1.88) | 1.08 (0.68, 1.76) | 0.99 (0.52, 1.89) | 1.16 (0.56, 2.44) |
| 3 vs. 1 | 1.21 (0.98, 1.50) | 1.13 (0.90, 1.42) | 1.43 (0.87, 2.33) | 1.20 (0.73, 1.97) | 1.13 (0.60, 2.13) | 1.20 (0.54, 2.65) |
| P for interaction | 0.48 | 0.94 | ||||
| Yogurt | ||||||
| 2 vs. 1 | 1.05 (0.84, 1.33) | 0.90 (0.70, 1.16) | 1.36 (0.86, 2.17) | 0.75 (0.44, 1.29) | 0.97 (0.50, 1.87) | 0.18 (0.03, 1.33) |
| 3 vs. 1 | 1.18 (0.95, 1.46) | 1.00 (0.80, 1.26) | 1.54 (0.99, 2.42) | 0.97 (0.67, 1.68) | 0.86 (0.42, 1.77) | 1.11 (0.51, 2.43) |
| P for interaction | 0.15 | 0.05 | ||||
| Fiber | ||||||
| 2 vs. 1 | 1.15 (0.88, 1.51) | 1.13 (0.85, 1.50) | 1.07 (0.52, 2.20) | 1.07 (0.59, 1.95) | 0.93 (0.45, 1.93) | 1.52 (0.54, 4.30) |
| 3 vs. 1 | 1.06 (0.80, 1.40) | 0.98 (0.73, 1.32) | 1.16 (0.59, 2.30) | 1.06 (0.59, 1.88) | 0.79 (0.38, 1.64) | 1.53 (0.58, 3.99) |
| 4 vs. 1 | 1.42 (1.09, 1.86) | 1.13 (0.82, 1.55) | 2.10 (1.19, 3.72) | 1.49 (0.87, 2.55) | 0.99 (0.49, 1.98) | 2.39 (0.97, 5.89) |
| P for interaction | 0.06 | 0.45 | ||||
All models adjusted for maternal age, parity, pre-pregnancy BMI, and impact of life events. Models for total population additionally adjusted for race.
Comment
Principal findings
We leveraged a diverse cohort of pregnant women to investigate the relationship between dietary intake and vaginal microbial community patterns, allowing for potential racial heterogeneity. Higher consumption of low-fat dairy and fruit were associated with membership in the L. crispatus cluster. While associations were somewhat stronger among Black women, overall effects were not heterogeneous by race. Similarly, higher vitamin D, yogurt, and fiber intake was associated with membership in the more favorable L. crispatus vagitype, with somewhat stronger associations among Black women, although associations were only significantly heterogenous for fiber.
Strengths of the study
Our study had many strengths. We used a valid and reliable FFQ to capture food intake; moreover, our specific FFQ was modified to reflect local foods to better capture women’s complete intake. Compared to studying individual food items, examining dietary subgroups offers multiple benefits: it reduces the number of comparisons made to avoid statistically spurious associations, provides effects that are sufficiently large to detect, and allows for synergistic effects of nutrients within food groups. Additionally, our population was large and racially diverse, allowing us to meaningfully investigate population-specific differences in associations. Lastly, our study is the first to consider dietary intake in relation to microbiome composition, not just a diagnosis of BV. This improved resolution of vaginal microbial community types better reflects current research linking vagitypes with risk for adverse pregnancy outcomes. Vaginal microbial community patterns dominated by L. crispatus have been associated with lower risk of preterm birth,1,37-39 which may be due to beneficial effects on cervical health.40 Identifying factors potentially amenable to intervention/modification, including diet, may help reduce adverse pregnancy outcomes.
Limitations of the data
Our study has several limitations. The FFQ asked women to reflect on the past 3 months, however it was intended to reflect usual dietary patterns. The vaginal microbiota composition may shift over time and that these temporal shifts were not captured. However, previous data suggest that the vaginal microbiome is relatively stable across pregnancy, more so than in non-pregnant women.41,42 Furthermore, prior literature suggests that diet generally remains stable across pregnancy and thus risk of misclassification is mitigated.43 However, due to the cross-sectional nature of our study, we must be cautious about assuming causal relations between diet and vagitype. Additionally, we did not have information on vitamin D supplementation. Total vitamin D is comprised of dietary vitamin D, sunlight exposure, and supplementation; our study only examined dietary vitamin D and associations with total vitamin D may differ. Lastly, by limiting dairy to low-fat, not all dietary constituents of probiotics are captured. However, potential probiotic benefits of full-fat products must be balanced against a healthy diet and thus we opted to limit our assessment to healthy intake.
Interpretation
There have been few studies on diet and the vaginal microbiome and most examine nutrients rather than food groups. One previous study found no significant associations between sugar, fiber, protein, or fat intake and membership into one of five vagitypes using daily swabs from college-aged women, but they only examined 26 women.44 Additionally, most studies focused on general dysbiosis and BV as the outcome rather than community patterns. Some studies have found that suboptimal levels of vitamin D are associated with BV risk,11,13,45 while another found no association using season as a proxy for vitamin D.46 One study surveying 104 reproductive-aged women found that diets richer in fiber were associated with decreased BV risk; however, they could not isolate the source of fiber driving this association as results were null when examining fiber from beans, grains, and fruits/vegetables.29 In this same population, there were no associations between fat, protein, or carbohydrate intake and BV. Studies of probiotics, including trials, have examined preterm birth and/or BV but yielded mixed results.47-51 Results from randomized trials suggest that probiotic supplementation via oral pills during pregnancy had no effect on microbiome composition, although all three studies supplemented with different probiotics.50-52
Our findings that higher low-fat dairy, yogurt, and vitamin D consumption were associated with more favorable vagitype membership is consistent with our hypothesis. Yogurt has the highest volume of production among cultured dairy products in the U.S., and regulations stipulate that Steptococcus thermophilus and Lactobacillus bulgaricus be used in the starter culture in yogurt production.53 Additionally, Lactobacillus acidophilus, a taxonomically-similar species to L. crispatus, is often added.53 Lactobacillus species, including L. acidophilus, confer benefits by lowering the vaginal pH through production of lactic acid.8,54 The acidity promotes the establishment and growth of “healthy” bacteria and prevents colonization by pathologic organisms, serving as a barrier to infection.8,9 However, it is important to note that yogurt consumption in our population was low: women in the highest tertile of intake consumed >34 grams/day, approximately 20% of one recommended serving.
Although the relationship between yogurt intake and vaginal community pattern did not meet our criteria for heterogeneity, associations appeared to be largely driven by elevated effect estimates among Black women. The prevalence of lactose intolerance is higher in the Black community55 and yogurt is a dairy product often tolerated by those who are lactose intolerant due to its low lactose content.56 In our population, yogurt represented a slightly larger percentage of total dairy intake in Black women compared to White women. The association with fruit may be driven by mechanisms including antioxidants and prebiotics and/or improved immune function. It is well established that cranberries reduce risk of vaginal infections and a trial found that ascorbic acid pills can halve the risk of recurrent BV.57 Additionally, certain fruits such as watermelons and bananas contain prebiotics, specialized fibers that stimulate growth of beneficial bacteria.
Dietary vitamin D was associated with increased membership in the L. crispatus cluster among Black women with the highest intake. A trial of pregnant women in South Carolina found similar results: using plasma 25(OH)D levels, women with >40 ng/mL had vaginal microbiomes with greater abundances of Lactobacillus species compared to women with <30 ng/mL, with results stronger among women of African ancestry.28
Fortified milk and cereals are a major source of dietary vitamin D in the US. Therefore, our associations with dairy and fiber intake may be partly attributable to fortified foods. It should be noted, however, that dietary intake of vitamin D contributes only minimally to total circulating 25-hydroxyvitamin D.59 For light-skinned women, circulating levels are largely driven by sun exposure; however, among women with darker skin, higher levels of melanin reduce the skin’s ability to generate vitamin D from sunlight.60 Therefore, diet may represent a more important source of vitamin D among women with darker skin. Despite observing no significant differences in the effect of vitamin D consumption on vagitype by race, fiber intake was one of the few associations that was heterogeneous by race, which may be in part due to heterogeneous sources of fiber across populations. Total fiber levels reflect intake from various dietary components, including whole grains, fruits, and vegetables. There may be differences in predominant source of fiber across race and subsequent differences in the underlying nutrient contributions, potentially explaining the racial heterogeneity we observed.
Conclusions
In this cohort of pregnant women, we found that fruit, dairy, vitamin D, yogurt, and fiber intake was associated with the L. crispatus vagitype. While our results should be interpreted cautiously given the cross-sectional nature of our design, they may be used to generate hypotheses informing accessible and low risk intervention studies. Determining the relationship between dietary intake and vaginal health has important public health implications as well as substantial interest by the lay public and yet high-quality scientific evidence is lacking. More research is needed to disentangle these complex associations, ideally addressing the potential for longitudinal variability in vaginal microbial composition over time.
Supplementary Material
Synopsis:
Study question:
Is diet during pregnancy associated with composition of the vaginal microbiome, and do associations differ by maternal race?
What’s already known:
Diet is associated with Bacterial Vaginosis and there is lay literature suggesting a benefit of probiotics on vaginal health, but scientific studies on this question are limited.
What this study adds:
This study provides evidence that certain components of diet are associated with membership in more beneficial vagitype clusters, and that findings are stronger among Black women.
Funding:
This study was funded in part by NIH/NIMDH (R01MD011504) and NIH/NIEHS (P30ES010126). EMR was supported by T32ES007018. The Pregnancy, Infection, and Nutrition study was supported by Grants HD37584, HD39373, and DK61981. The General Clinic Research Center was supported by the National Institutes of Health General Clinical Research Centers program of the Division of Research Resources Grant RR00046.
Footnotes
Authors have no conflicts of interest or financial disclosures to report.
References
- 1.Fettweis JM, Serrano MG, Brooks JP, Edwards DJ, Girerd PH, Parikh HI, et al. The vaginal microbiome and preterm birth. Nature Medicine 2019;25:1012–1021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Hay PE, Lamont RF, Taylor-Robinson D, Morgan DJ, Ison C, Pearson J. Abnormal bacterial colonisation of the genital tract and subsequent preterm delivery and late miscarriage. BMJ 1994;308:295–298. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Ralph SG, Rutherford AJ, Wilson JD. Influence of bacterial vaginosis on conception and miscarriage in the first trimester: cohort study. BMJ 1999;319:220–223. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Onderdonk AB, Delaney ML, Fichorova RN. The Human Microbiome during Bacterial Vaginosis. Clinical Microbiology Reviews 2016;29:223–238. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Goldenberg RL, Culhane JF, Iams JD, Romero R. Epidemiology and causes of preterm birth. The Lancet 2008;371:75–84. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Goldenberg RL, Hauth JC, Andrews WW. Intrauterine infection and preterm delivery. The New England Journal of Medicine 2000;342:1500–1507. [DOI] [PubMed] [Google Scholar]
- 7.Brotman RM. Vaginal microbiome and sexually transmitted infections: an epidemiologic perspective. The Journal of Clinical Investigation 2011;121:4610–4617. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Ravel J, Gajer P, Abdo Z, Schneider GM, Koenig SSK, McCulle SL, et al. Vaginal microbiome of reproductive-age women. Proceedings of the National Academy of Sciences 2011;108:4680–4687. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Ma B, Forney LJ, Ravel J. Vaginal microbiome: rethinking health and disease. Annual Review of Microbiology 2012;66:371–389. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Lewis FMT, Bernstein KT, Aral SO. Vaginal Microbiome and Its Relationship to Behavior, Sexual Health, and Sexually Transmitted Diseases. Obstetrics and gynecology 2017;129:643–654. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Bodnar LM, Krohn MA, Simhan HN. Maternal vitamin D deficiency is associated with bacterial vaginosis in the first trimester of pregnancy. The Journal of Nutrition 2009;139:1157–1161. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Verstraelen H, Delanghe J, Roelens K, Blot S, Claeys G, Temmerman M. Subclinical iron deficiency is a strong predictor of bacterial vaginosis in early pregnancy. BMC infectious diseases 2005;5:55. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Neggers YH, Nansel TR, Andrews WW, Schwebke JR, Yu K, Goldenberg RL, et al. Dietary Intake of Selected Nutrients Affects Bacterial Vaginosis in Women. The Journal of Nutrition 2007;137:2128–2133. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Thoma ME, Klebanoff MA, Rovner AJ, Nansel TR, Neggers Y, Andrews WW, et al. Bacterial vaginosis is associated with variation in dietary indices. The Journal of Nutrition 2011;141:1698–1704. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Team L-S. The best (and not so great) foods for a healthy vaginal microbiome ∣ Life-Space Blog. https://www.lifespaceprobiotics.com/en/health-and-wellness-tips/womens-health-the-best-and-not-so-great-foods-for-a-healthy-vaginal-microbiome/ (last accessed July 2020). [Google Scholar]
- 16.Becco LB, FMCHC, Coach I a F-MH, Minneapolis health journalist in. How to Support Your Vaginal Microbiome. https://experiencelife.com/article/how-to-support-your-vaginal-microbiome/ (last accessed July 2020).
- 17.Fettweis JM, Brooks JP, Serrano MG, Sheth NU, Girerd PH, Edwards DJ, et al. Differences in vaginal microbiome in African American women versus women of European ancestry. Microbiology 2014;160:2272–2282. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Bahr PR. Race and nutrition: an investigation of Black-White differences in health-related nutritional behaviours. Sociology of Health & Illness 2007;29:831–856. [DOI] [PubMed] [Google Scholar]
- 19.Noia JD, Monica D, Cullen K, Perez-Escamilla R, Gray HL, Sikorskii A. Differences in Fruit and Vegetable Intake by Race/Ethnicity and by Hispanic Origin and Nativity Among Women in the Special Supplemental Nutrition Program for Women, Infants, and Children, 2015. Preventing Chronic Disease 2016;13:1–13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Manuck TA. Racial and ethnic differences in preterm birth: A complex, multifactorial problem. Seminars in perinatology 2017;41:511–518. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Culhane JF, Goldenberg RL. Racial disparities in preterm birth. Seminars in Perinatology 2011;35:234–239. [DOI] [PubMed] [Google Scholar]
- 22.Savitz DA, Dole N, Williams J, Thorp JM, McDonald T, Carter A, et al. Determinants of participation in an epidemiological study of preterm delivery. Paediatric and Perinatal Epidemiology 1999;13:114–125. [DOI] [PubMed] [Google Scholar]
- 23.Siega-Riz AM, Bodnar LM, Savitz DA. What are pregnant women eating? Nutrient and food group differences by race. American Journal of Obstetrics and Gynecology 2002;186:480–486. [DOI] [PubMed] [Google Scholar]
- 24.Azadbakht L, Mirmiran P, Esmaillzadeh A, Azizi T, Azizi F. Beneficial Effects of a Dietary Approaches to Stop Hypertension Eating Plan on Features of the Metabolic Syndrome. Diabetes Care 2005;28:2823–2831. [DOI] [PubMed] [Google Scholar]
- 25.Chiuve SE, Fung TT, Rimm EB, Hu FB, McCullough ML, Wang M, et al. Alternative Dietary Indices Both Strongly Predict Risk of Chronic Disease. The Journal of Nutrition 2012;142:1009–1018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Martin CL, Siega-Riz AM, Sotres-Alvarez D, Robinson WR, Daniels JL, Perrin EM, et al. Maternal dietary patterns are associated with lower levels of cardiometabolic markers during pregnancy. Paediatric and perinatal epidemiology 2016;30:246–255. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Asemi Z, Samimi M, Tabassi Z, Esmaillzadeh A. The effect of DASH diet on pregnancy outcomes in gestational diabetes: a randomized controlled clinical trial. European Journal of Clinical Nutrition 2014;68:490–495. [DOI] [PubMed] [Google Scholar]
- 28.Jefferson KK, Parikh HI, Garcia EM, Edwards DJ, Serrano MG, Hewison M, et al. Relationship between Vitamin D status and the vaginal microbiome during pregnancy. Journal of perinatology : official journal of the California Perinatal Association 2019;39:824–836. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Shivakoti R, Tuddenham S, Caulfield LE, Murphy C, Robinson C, Ravel J, et al. Dietary macronutrient intake and molecular-bacterial vaginosis: Role of fiber. Clinical Nutrition (Edinburgh, Scotland) 2020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Fettweis JM, Serrano MG, Girerd PH, Jefferson KK, Buck GA. A New Era of the Vaginal Microbiome: Advances Using Next-Generation Sequencing. Chemistry & Biodiversity 2012;9:965–976. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Fettweis JM, Serrano MG, Sheth NU, Mayer CM, Glascock AL, Brooks JP, et al. Species-level classification of the vaginal microbiome. BMC Genomics 2012;13:S17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Jean S, Huang B, Parikh HI, Edwards DJ, Brooks JP, Kumar NG, et al. Multi-omic Microbiome Profiles in the Female Reproductive Tract in Early Pregnancy. Infectious Microbes & Diseases 2019;1:49–60. [Google Scholar]
- 33.Parikh HI, Koparde VN, Bradley SP, Buck GA, Sheth NU. MeFiT: merging and filtering tool for illumina paired-end reads for 16S rRNA amplicon sequencing. BMC Bioinformatics 2016;17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Edgar RC. Search and clustering orders of magnitude faster than BLAST. Bioinformatics 2010;26:2460–2461. [DOI] [PubMed] [Google Scholar]
- 35.Sarason IG, Johnson JH, Siegel JM. Assessing the impact of life changes: Development of the Life Experiences Survey. Journal of Consulting and Clinical Psychology 1978;46:932–946. [DOI] [PubMed] [Google Scholar]
- 36.Schwingshackl L, Bogensberger B, Hoffmann G. Diet Quality as Assessed by the Healthy Eating Index, Alternate Healthy Eating Index, Dietary Approaches to Stop Hypertension Score, and Health Outcomes: An Updated Systematic Review and Meta-Analysis of Cohort Studies. Journal of the Academy of Nutrition and Dietetics 2018;118:74–100.e11. [DOI] [PubMed] [Google Scholar]
- 37.Stafford GP, Parker JL, Amabebe E, Kistler J, Reynolds S, Stern V, et al. Spontaneous Preterm Birth Is Associated with Differential Expression of Vaginal Metabolites by Lactobacilli-Dominated Microflora. Frontiers in Physiology 2017;8:615. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Brown RG, Marchesi JR, Lee YS, Smith A, Lehne B, Kindinger LM, et al. Vaginal dysbiosis increases risk of preterm fetal membrane rupture, neonatal sepsis and is exacerbated by erythromycin. BMC medicine 2018;16:9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Kindinger LM, Bennett PR, Lee YS, Marchesi JR, Smith A, Cacciatore S, et al. The interaction between vaginal microbiota, cervical length, and vaginal progesterone treatment for preterm birth risk. Microbiome 2017;5:6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Anton L, Sierra L-J, DeVine A, Barila G, Heiser L, Brown AG, et al. Common Cervicovaginal Microbial Supernatants Alter Cervical Epithelial Function: Mechanisms by Which Lactobacillus crispatus Contributes to Cervical Health. Frontiers in Microbiology 2018;9:2181. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Romero R, Hassan SS, Gajer P, Tarca AL, Fadrosh DW, Nikita L, et al. The composition and stability of the vaginal microbiota of normal pregnant women is different from that of non-pregnant women. Microbiome 2014;2:4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.DiGiulio DB, Callahan BJ, McMurdie PJ, Costello EK, Lyell DJ, Robaczewska A, et al. Temporal and spatial variation of the human microbiota during pregnancy. Proceedings of the National Academy of Sciences of the United States of America 2015;112:11060–11065. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Crozier SR, Robinson SM, Godfrey KM, Cooper C, Inskip HM. Women’s dietary patterns change little from before to during pregnancy. The Journal of Nutrition 2009;139:1956–1963. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Song SD, Acharya KD, Zhu JE, Deveney CM, Walther-Antonio MRS, Tetel MJ, et al. Daily Vaginal Microbiota Fluctuations Associated with Natural Hormonal Cycle, Contraceptives, Diet, and Exercise. mSphere 2020;5:1–14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Tohill BC, Heilig CM, Klein RS, Rompalo A, Cu-Uvin S, Piwoz EG, et al. Nutritional biomarkers associated with gynecological conditions among US women with or at risk of HIV infection. The American Journal of Clinical Nutrition 2007;85:1327–1334. [DOI] [PubMed] [Google Scholar]
- 46.Klebanoff MA, Turner AN. Bacterial vaginosis and season, a proxy for vitamin D status. Sexually Transmitted Diseases 2014;41:295–299. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Nordqvist M, Jacobsson B, Brantsæter A-L, Myhre R, Nilsson S, Sengpiel V. Timing of probiotic milk consumption during pregnancy and effects on the incidence of preeclampsia and preterm delivery: a prospective observational cohort study in Norway. BMJ open 2018;8:e018021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Jarde A, Lewis-Mikhael A-M, Moayyedi P, Stearns JC, Collins SM, Beyene J, et al. Pregnancy outcomes in women taking probiotics or prebiotics: a systematic review and meta-analysis. BMC Pregnancy and Childbirth 2018;18:14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Daskalakis GJ, Karambelas AK. Vaginal Probiotic Administration in the Management of Preterm Premature Rupture of Membranes. Fetal Diagnosis and Therapy 2017;42:92–98. [DOI] [PubMed] [Google Scholar]
- 50.Olsen P, Williamson M, Traynor V, Georgiou C. The impact of oral probiotics on vaginal Group B Streptococcal colonisation rates in pregnant women: A pilot randomised control study. Women and Birth: Journal of the Australian College of Midwives 2018;31:31–37. [DOI] [PubMed] [Google Scholar]
- 51.Husain S, Allotey J, Drymoussi Z, Wilks M, Fernandez-Felix BM, Whiley A, et al. Effects of oral probiotic supplements on vaginal microbiota during pregnancy: a randomised, double-blind, placebo-controlled trial with microbiome analysis. BJOG: an international journal of obstetrics and gynaecology 2020;127:275–284. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Gille C, Böer B, Marschal M, Urschitz MS, Heinecke V, Hund V, et al. Effect of probiotics on vaginal health in pregnancy. EFFPRO, a randomized controlled trial. American Journal of Obstetrics & Gynecology 2016;215:608.e1–608.e7. [DOI] [PubMed] [Google Scholar]
- 53.Aryana KJ, Olson DW. A 100-Year Review: Yogurt and other cultured dairy products. Journal of Dairy Science 2017;100:9987–10013. [DOI] [PubMed] [Google Scholar]
- 54.Hickey RJ, Zhou X, Pierson JD, Ravel J, Forney LJ. Understanding vaginal microbiome complexity from an ecological perspective. Translational Research: The Journal of Laboratory and Clinical Medicine 2012;160:267–282. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Suchy FJ, Brannon PM, Carpenter TO, Fernandez JR, Gilsanz V, Gould JB, et al. NIH consensus development conference statement: Lactose intolerance and health. NIH consensus and state-of-the-science statements 2010;27:1–27. [PubMed] [Google Scholar]
- 56.Yogurt in Nutrition. Lactose intolerance. https://www.yogurtinnutrition.com/tag/lactose-intolerance/ (last accessed January 2021).
- 57.Krasnopolsky VN, Prilepskaya VN, Polatti F, Zarochentseva NV, Bayramova GR, Caserini M, et al. Efficacy of Vitamin C Vaginal Tablets as Prophylaxis for Recurrent Bacterial Vaginosis: A Randomised, Double-Blind, Placebo-Controlled Clinical Trial. Journal of Clinical Medicine Research 2013;5:309–315. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Calvo MS, Whiting SJ, Barton CN. Vitamin D fortification in the United States and Canada: current status and data needs. Tshe American Journal of Clinical Nutrition 2004;80:1710S–6S. [DOI] [PubMed] [Google Scholar]
- 59.Nair R, Maseeh A. Vitamin D: The “sunshine” vitamin. Journal of Pharmacology & Pharmacotherapeutics 2012;3:118–126. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Institute of Medicine, Board of Food and Nutrition, Calcium C to RDRI for VD and. Dietary Reference Intakes for Calcium and Vitamin D. National Academies Press; 2011. [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


