Abstract
All bacteria display surface-exposed glycans that can play an important role in their interaction with the host and in select cases mimic the glycans found on host cells, an event called molecular or glycan mimicry. In this review, we highlight the key bacteria that display human glycan mimicry and provide an overview of the involved glycan structures. We also discuss the general trends and outstanding questions associated with human glycan mimicry by bacteria. Finally, we provide an overview of several techniques that have emerged from the discipline of chemical glycobiology, which can aid in the study of the composition, variability, interaction and functional role of these mimicking glycans.
Keywords: bacteria, carbohydrates, chemical biology, glycans, molecular mimicry
Introduction
Bacterial glycans play an important role in host–microbe interactions (Poole et al. 2018). Glycans at the bacterial cell surface are often the first molecules to interact with the environment and can mimic glycan structures of the host. The existence of identical molecular structures on the host and a microbe is referred to as molecular or glycan mimicry (Damian 1964). Bacteria may exploit glycan mimics to hijack host biology to establish infection, but the glycan mimicry may also inadvertently induce abnormal immune responses causing serious auto-immune pathology. Bacterial surface glycans that have been found to display glycan mimicry with host glycan structures include capsular polysaccharides (CPS) and glycolipids, such as lipopolysaccharide (LPS) and lipooligosaccharides (LOS). These bacterial surface glycans mimic certain classes of eukaryotic glycans for which an overview is presented in Figure 1. The bacteria that will be discussed in this review are grouped per class of eukaryotic glycans they are mimicking. A detailed overview of the bacterial glycan structures that display glycan mimicry are depicted in Supplementary Table SI.
Fig. 1.
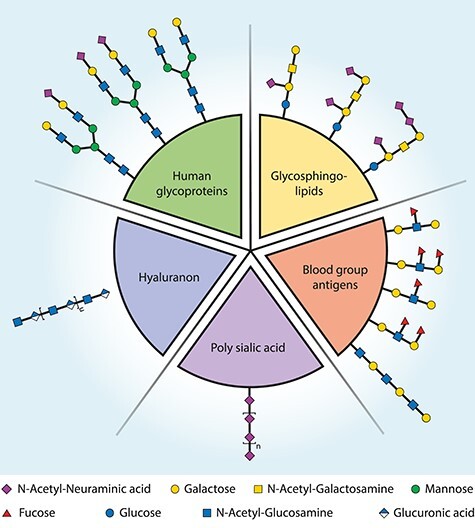
Bacterial glycans can mimic the glycans of the (human) host. Here, the targets of glycan mimicry are grouped for several human glycan types. Bacterial glycans that resemble these and are thus molecular mimics can be found in Supplementary Table SI.
The concept of bacterial glycan mimicry and its effects on host immunity has been subject of a few excellent reviews (Mandrell and Apicella 1993; Moran et al. 1996). Here, we will focus on an updated discussion of research into the glycan structures and interacting proteins involved in bacterial host glycan mimicry and its impact on host biology. We have selected 11 bacterial species that display glycan mimicry. First, we will discuss the bacterial glycan structures involved [using a graphical notation according to the current symbol nomenclature (Varki et al. 2015), Supplementary Table SI], their interaction with host receptors (see Figure 2 for an overview) and their role in bacterial pathogenesis. In a second part of the review, we will highlight the potential of recent advances in the field of chemical glycobiology and make a case for their use in future study of bacterial glycan mimicry. Of note, microbe-host mimicry is not limited to glycans and also not all bacterial glycans on microbes that interact with their host are involved in mimicry. However, discussion of other forms of molecular mimicry (e.g., protein mimicry) (Carolin 2019), microbial glycan interactions (Poole et al. 2018) and the nature of unique microbial glycans (Dube et al. 2011; Tra and Dube 2014; Imperiali 2019) is beyond the scope of the current review.
Fig. 2.
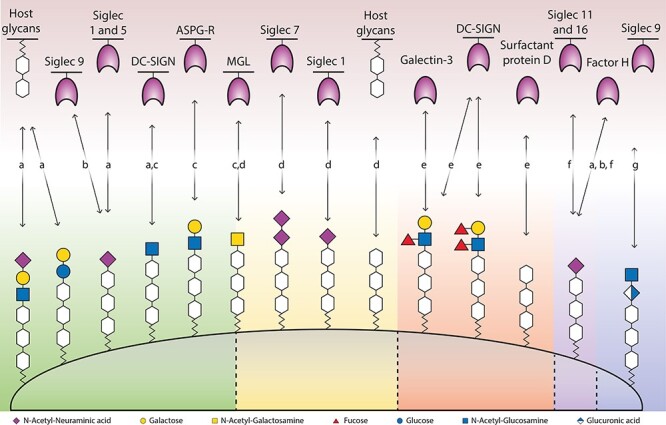
Interactions between the terminal surface glycan structures of mimicking bacteria and the host’s glycan binding proteins or glycans. The bacteria are indicated by the following letters: (A) N. meningitidis, (B) Group B streptococcus, (C) N. gonorrhoeae, (D) C. jejuni, (E) H. pylori, (F) E. coli and (G) Group A streptococcus.
Bacterial pathogens that display glycan mimicry
Glycosphingolipids
Campylobacter jejuni
One of the most well-studied forms of glycan mimicry involves the bacterial surface glycans of the food-borne pathogen Campylobacter jejuni. Infection by C. jejuni is a common cause of human gastroenteritis and in rare cases can lead to Guillain–Barré syndrome (GBS). In case of GBS, the immune system produces antibodies against the infecting microbe’s glycans that cross-react with similar glycan structures on the host’s nerves (Van Den Berg et al. 2014). GBS can cause acute flaccid paralysis and is classified in different subtypes based on further clinical features (Jasti et al. 2016). Although infections from many different bacteria and viruses could potentially result in GBS (Jasti et al. 2016), C. jejuni is the most frequent preceding infection, especially for the GBS subtypes, acute motor axonal neuropathy and Miller Fisher syndrome (MFS) (Yu et al. 2006; Jasti et al. 2016). Campylobacter jejuni infections are frequent, but only an estimated 1 per 1000 infections results in GBS, which suggests that additional factors play a role in developing GBS (Van Den Berg et al. 2014; Jasti et al. 2016). For C. jejuni, it is well documented that some forms of LOS mimic the gangliosides found on nerves (Moran and Prendergast 2001). Cross-reactive antibodies against different LOS epitopes and gangliosides can be found in patient sera (Prendergast and Moran 2000). In addition, the link between the glycan mimicry of gangliosides and the development of GBS was demonstrated in rabbits (Yuki et al. 2004). The animals were administered GM1-like LOS from a clinical isolate, upon which they developed GBS (Yuki et al. 2004). Campylobacter coli and Brucella melitensis might also have GM1-like structures, because they reacted with an anti-ganglioside antibody (Yu et al. 2006), but it remains unknown whether they can cause GBS (Van Belkum et al. 2009). Although the evidence for a link between glycan mimicry and onset of GBS is strong, it also does not appear to be a prerequisite. For instance, Godschalk et al. (2007) used capillary electrophoresis MS to determine the LOS of C. jejuni strains from patients with Enteritis, GBS and MFS. Although a correlation was found between C. jejuni strains that had mimicking LOS and the diseases GBS and MFS, there were also C. jejuni strains that lacked this type of LOS and did give rise to disease (Godschalk et al. 2007). This suggests that other still unknown factors might play a role in developingGBS.
Variants of C. jejuni LOS can bind to several host receptors and although less well studied, also to host glycans (Figure 2) (Day et al. 2015). The Siglec 7 receptor, which occurs on monocyte-derived macrophages, Natural Killer and dendritic cells, is bound by strains associated with GBS and MFS through disialylated LOS and not monosialylated LOS (Avril et al. 2006; Heikema, Jacobs, et al. 2013). LOS that contains a terminal GalNAc can bind to the Macrophage Galactose-type lectin (MGL, CLEC10A and CD301) on immature dendritic cells (van Sorge et al. 2009). LOS structures mimicking Gd1a, GM1b and GM3 gangliosides show binding to both murine (Heikema et al. 2010) and human (Heikema, Koning, et al. 2013) sialoadhesin (Sn, Siglec-1 and CD169) that is dependent on terminal α2,3-linked N-acetylneuraminic acid (α2,3-Neu5Ac). The binding of LOS to Sn leads to increased uptake by the macrophages and increased IL-6 production but does not facilitate bacterial survival as lysosomal degradation still occurs. The exact role of Sn binding remains unclear, but Sn-mediated LOS ingestion by antigen presenting cells might facilitate the production of auto-antibodies (Heikema, Koning, et al. 2013).
The key role of the sialic acid family, mainly in the form of Neu5Ac, in glycan mimicry by C. jejuni LOS has also been confirmed by genetic studies. For example, mutations of either heptosyltransferase, which leads to truncated LOS (Perera et al. 2007), or Neu5Ac synthetase (Xiang et al. 2006) both abolish LOS sialylation and mimicry. The sialyltransferase CstII is responsible for the sialyation of LOS and can create either α2,3- or both α2,3- and α2,8-linkages, depending on only a single point mutation (Gilbert et al. 2002; Godschalk et al. 2007). In vitro studies of C. jejuni with sialylated LOS show that it is more invasive yet less adherent (Louwen et al. 2008). Additionally, sialylated LOS facilitates translocation across Caco-2 cells, yet intracellular survival depends on the number of translocated bacteria and not on LOS structure (Louwen et al. 2012). Most studies on LOS sialylation are in vitro, so the exact in vivo role remains to be further elucidated. However, a patient study from Finland indicated that LOS sialylation might only have a limited contribution to in vivo invasiveness (Ellström et al. 2014). Only 23% of the C. jejuni isolates had the genetic potential to sialylate the LOS (Ellström et al. 2014).
Besides the genetic potential for mimicry by C. jejuni, the exact LOS composition in a culture is also dependent on growth conditions. For example, the model strain C. jejuni 11 168 produces LOS with 90% of glycan structures mimicking GM1 if grown at 37°C, which drops to 50% if grown at 42°C (Day et al. 2012). Day et al. (2012) suggest that the heterogeneity of LOS could contribute to population fitness and hypothesized that the different LOS structures form multiple targets for auto-antibodies.
Moraxella catarrhalis
Moraxella catarrhalis is a nonmotile gram-negative pathogenic bacterium with an affinity for the human upper respiratory system. The LOS of serotype A, B and C was shown to have a common Gal-Gal-Glc motive, which can also be found on human glycolipids (Masoud et al. 1994; Holme et al. 1999; Schwingel et al. 2009). The interactions of the LOS with host biology remain to be studied. The glycan mimicry by this bacterium might aid in host colonization and invasion (Holme et al. 1999).
Blood group antigens
Helicobacter pylori
Helicobacter pylori is a gram-negative, microaerophilic, helically shaped bacterium. It is a gastric pathogen that persists for a lifetime in most human’s stomach (Appelmelk et al. 2000) and can lead to a variety of diseases, such as gastric and duodenal ulcers, chronic gastritis and mucosa-associated lymphoid tissue (Chmiela and Gonciarz 2017). This persistent colonization is associated with glycan mimicry by the O-antigen of H. pylori’s LPS of Lewis blood group antigens (Moran and Prendergast 2001; Bergman et al. 2006). A study that investigated the LPS composition of eight Helicobacter strains with reactivity toward antibodies directed against Lewis antigens found only H. pylori and H. mustelae displayed glycan mimicry, of which the latter is a pathogen that can be found in ferrets (Hynes et al. 2004). In an effort to understand the role of glycan mimicry in auto-immunity, Appelmelk et al. (1996) studied seven H. pylori strains for mimicry with antibodies against Lewis antigens and found cross reactivity between LPS and gastric tissue.
The glycan structure of H. pylori LPS is important for invasiveness (Leker et al. 2017), colonization (Logan et al. 2000) and antigenicity (Chmiela et al. 2014); details on the biosynthesis of LPS with Lewis antigens have been previously described (Li et al. 2016, 2017). Helicobacter pylori with truncated LPS, which lacks the O-antigen, colonized less compared with wildtype (Logan et al. 2000). Comparison of the glycan composition of two H. pylori strains with different virulence found that they differed in the monosaccharides rhamnose and mannose, yet the significance of this for invasiveness remains unclear (Leker et al. 2017). In relation to antigenicity, one study compared a weakly and highly antigenic form of LPS and the two LPS structures only differed in one GlcNAc residue (Chmiela et al. 2014), highlighting that a minor change in glycan structure can have direct impact on immunogenicity. Helicobacter pylori might persist in the host by balancing the population of T-helper-1 and -2-cells through phase variation and expression of Lewis antigens (Bergman et al. 2006). This balance might be achieved via Lewis x and Lewis y that bind to DC-SIGN (Figure 2), which causes a down-regulation in T-helper-1-cells (Bergman et al. 2006; Li et al. 2016). Besides their binding to DC-SIGN, Lewis antigens bind to several other receptors (Figure 2). Lewis x binds to Galectin-3 and is thought to contribute to adhesion (Li et al. 2016). Helicobacter pylori O-antigens with a lower degree of fucosylation preferentially bind to surfactant protein D and this is dependent on the phase-variable expression of the bacterial fucosyltransferase (Khamri et al. 2005; Chmiela et al. 2014; Li et al. 2016). For a more detailed discussion about the function of glycan mimicry for H. pylori, the reader is referred to a perspective by Bergman et al. (2006).
Polysialic acid
Escherichia coli
Escherichia coli is a gram-negative, facultative anaerobic, bacterium that is commonly found in the lower intestine. Its O-antigen and capsule can display glycan mimicry. The O-antigen is composed of 10–25 repeating units of two to seven monosaccharides (Stenutz and Weintraub 2006). Serotypes O86, O90, O127 and O128 are presumed to be glycan mimicking due to their resemblance to blood group antigens (Stenutz and Weintraub 2006). For more details, we refer to a comprehensive overview of O-antigens of E. coli (Stenutz and Weintraub 2006). The capsule of E. coli K1 and K92 consists of polysialic acid, which is either α2,8- or both α2,8- and α2,9-linked, respectively (Suerbaum et al. 1994), and mimics the polysialic acid found on neural cell adhesion molecules (Steenbergen et al. 1992). Contrary to vertebrates, where polysialylation is found on the nonreducing termini of sialylated N- and O-linked glycans of certain glycoproteins, bacterial polysialic acid structures are covalently linked to an oligo-KDO that is anchored to the plasma membrane (Lizak et al. 2017). The capsule of E. coli K1 was shown to bind both inhibitory Siglec 11 and activating Siglec 16 (Figure 2), which give opposite signals to the immune system (Schwarz et al. 2017). In addition to Siglec binding, the polysialic acid capsule possibly also recruits factor H, as described for Neisseria meningitidis (Figure 2) (Suerbaum et al. 1994).
Neisseria meningitidis serogroup B
Neisseria meningitidis is a gram-negative bacterium that can cause meningitis and sepsis. Its serogroup B strain has a polysialic acid capsule that mimics the polysialic acid that can be found on neural cell adhesion molecules, similar to E. coli (Steenbergen et al. 1992). Neisseria meningitidis serogroup C also has a polysialic acid capsule but is α2,9-linked instead of α2,8-linked like the capsule of serogroup B. Although this serogroup C, and the serogroups W-135 and Y contain Neu5Ac, these have not been described as involved in glycan mimicry. For a more detailed discussion about the glycan structures of Neisseria, the reader is referred to two excellent reviews (Mandrell and Apicella 1993; Mubaiwa, Semchenko, et al. 2017). A possible function of the glycan mimicry by N. meningitidis serogroup B’s polysialic acid capsule could be immune evasion through the possible recruitment of factor H (Figure 2), which suppresses the complement system, conferring serum-resistance (Jarvis and Vedros 1987). If there is less sialylation of the capsule, there is more C3 deposition on the bacterial cell surface (Jarvis and Vedros 1987). The binding of C3 on the cell surface is the first step in the complement pathway to form the membrane-attack complex that eventually can lead to cell death (Doorduijn et al. 2016). The deposited C3 can be removed by factor H, which is usually responsible for recognizing “self,” and it has been hypothesized that Neu5Ac could attract factor H (Jarvis and Vedros 1987).
Human glycoproteins
Neisseria meningitidis
The LOS of N. meningitidis contains a terminal structure, lacto-N-neotetraose, that is also found on human glycoproteins and glycosphingolipids (Tsai et al. 1998). Sialyltransferases from N. meningitidis sialylate its LOS, often with an α2,3-linkage, except for immunotype L1 in which its α2,6-linked Neu5Ac (Tsai et al. 1998, 2002). This Sialylated LOS has been shown to bind to Siglecs Sn or 5 (Figure 2) (Jones et al. 2003); 3-Sialyllactosamine or 3-siallylactose that is coupled to polyacrylamide probes has been shown to bind even more Siglecs. The fact that meningococcal LOS only binds two but has identical terminal structures shows that the underlying LOS glycan structure probably also plays a role in this. Of note is that Siglecs Sn or 5 do have a relaxed ligand specificity and can bind sialylated galactose connected through either an α2,3- α2,6- or α2,8- linkage (Jones et al. 2003). It has been hypothesized that binding of LOS to Siglecs could lead to the release of inhibitory signals or to uptake of the bacteria with a trojan horse-like effect (Jones et al. 2003). Truncated meningococcal LOS that instead contains a terminal GlcNAc (lgtB mutant) has been found to bind DC-SIGN, another important lectin for the immune system (Steeghs et al. 2006). However, because the lgtB mutant is not phase variable, it probably does not have an in vivo contribution to infection (Johswich 2017). Interestingly, a glycan array study identified 31 interactions between LOS of immunotype L3 and L8 and host glycans, including the high-affinity binding to the Thomsen–Friedenreich antigen (Mubaiwa, Hartley-Tassell, et al. 2017). The downstream effects of these glycan–glycan interactions on meningococcal infection remain to be investigated. In general, the glycan mimicry by meningococcal LOS is thought to be a form of immune evasion (Mandrell and Apicella 1993; Tsai et al. 1998; Mubaiwa, Semchenko, et al. 2017). LOS sialylation could for instance prevent the binding of antibodies of the classical pathway or inhibit parts of the complement pathway (Lewis et al. 2012). The mimicking LOS has many interactions with the host cells that might aid in immune evasion, but the detailed mechanism of immune evasion is not clearyet.
Neisseria gonorrhoeae
Neisseria gonorrhoeae is a gram-negative, diplococci bacteria that causes the sexually transmitted gonorrhea infection. Its LOS, similar to N. meningitidis, contains terminal lacto-N-neotetraose that mimics human glycoproteins and glycosphingolipids (Tong et al. 2002). Gonococcal LOS is phase-variable and can be sialylated, typically with an α2,3-linkage except for the PK LOS which contains a α2,6-linkage (Tong et al. 2002; Gulati et al. 2015). Phase-variable gonococcal LOS has multiple interactions with host cells (Figure 2) and can bind to C-type lectins MGL and DC-SIGN in case of a terminal N-acetylglucosamine or N-acetylgalactosamine, respectively (Van Vliet et al. 2009). Binding to DC-SIGN results in increased IL-10 production and binding to MGL skews the population to T helper 2 cells, the latter could be a form of immune evasion (Van Vliet et al. 2009).
Sialylation of LOS plays an important factor in the interaction of N. gonorrhoeae with its host. Upon invasion, only bacteria with low levels of sialylated LOS invade the host epithelial cells (van Putten 1993). In absence of sialylation, the exposed terminal lactosamine residues of the LOS bind to the asialoglycoprotein receptor (ASGP-R) (Harvey et al. 2001) that induces clathrin-dependent receptor-mediated endocytosis, thus facilitating invasion. Once inside the host cell, the bacteria can sialylate themselves by scavenging the host’s CMP-Neu5Ac, resulting in serum resistance (van Putten 1993). Several reasons have been postulated for why sialylation of gonococcal LOS contributes to immune evasion, including: recruitment of factor H, shielding of underlying immunogenic structures against antibodies and engagement of human inhibitory Siglecs (Landig et al. 2019). Finally, it is hypothesized that N. gonorrhoeae has evolved to specifically use Neu5Ac to commit to a human host and to avoid innate immunity, as the immune system would be triggered by LOS with N-glycolylneuraminic acid (Neu5Gc) (Landig et al. 2019). Notably, when incorporating different sialic acids on the gonococcal LOS, including Neu5Ac, Neu5Gc, Pseudaminic acid (Pse) and Legionaminic acid (Leg), it was found that both Neu5Ac and NeuGc confer high serum resistance (Gulati et al. 2015).
Nontypeable Haemophilus influenzae
Nontypeable Haemophilus influenzae (NTHi) is a gram-negative, facultatively anaerobic, opportunistic pathogen that can cause otitis media. The LOS of nontypeable H. influenzae is glycan mimicking when it contains a terminal N-acetyllactosamine, similar to Neisseria spp, or a lactose that is capped with a Neu5Ac (Phillips et al. 1992; Apicella 2012; Day et al. 2015; Kalograiaki et al. 2016). The structure of NTHi375 and RdKW20 LOS have been shown to bind galactose specific lectins through microarray technologies (Kalograiaki et al. 2016, 2018). There has also been a report that NTHi LOS might have a terminal KDO, illustrating LOS heterogeneity (Apicella et al. 2018). The interactions and role of these mimicking LOS in NTHi pathogenesis are not fully understood. Heise et al. (2018) used a probe to study the sialylation of the LOS of NTHi. Combined with a sialyltransferase inhibitor, they showed decreased serum resistance when the LOS was not sialylated and thus a possible function of this in glycan mimicry (Heise et al. 2018). The sialylation of NTHi does not lead to more factor H binding, but to less deposition of C3 on the bacterial surface (Figueira et al. 2007). Sialylated LOS might also mask the underlying glycans and thus prevent the binding of antibodies of the classical complement pathway (Jackson et al. 2019), a similar observation was made for Neu5Gc (Oerlemans et al. 2019). However, as an indication of the complexity of bacterial glycan mimicry in relation to the host immune system, it has also been shown that the uptake by NTHi of nonhuman Neu5Gc from the human diet can lead to the formation of anti-Neu5Gc antibodies that were shown to bind specifically to Neu5Gc-displaying NTHi and not to nonsialylated NTHi (Taylor et al. 2010).
Haemophilus ducreyi
Haemophilus ducreyi is a human pathogen that can cause chancroid. Similar to NTHi, the LOS can contain a terminal N-acetyllactosamine, which can also be sialylated (Melaugh et al. 1994, 1996; Schweda et al. 1994). Haemophilus ducreyi sialylates its LOS, by scavenging Neu5Ac from the environment since it does not have the necessary biosynthetic pathway to produce Neu5Ac (Goon et al. 2003; Wratil et al. 2016). The LOS of H. ducreyi has been shown to contribute to binding of human foreskin fibroblast and keratinocytes, among other components (Alfa and Degagne 1997; Gibson et al. 1997), yet it remains to be investigated whether this interaction depends on glycan mimicry. Although the LOS of H. ducreyi can be sialylated, this does not appear to function as a form of immune evasion, because the strain is virulent regardless of the sialylation (Spinola et al. 2012). Additionally, the bacterium is resistant against serum, so sialylation is not required for the recruitment of factor H. The sialylation of the LOS might have occurred to commit to a human host (Spinola et al. 2012).
Klebsiella pneumoniae
Klebsiella pneumoniae is a gram-negative, nonmotile, facultative anaerobic, rod-shaped bacterium that colonizes the human mucosal surfaces of the oropharynx and gastrointestinal tract. The capsule is an important virulence factor and may contain terminal Neu5Ac, as suggested for K. pneumonia showing the hypermucoviscosity phenotype (Lee et al. 2014), but the capsule structure has not been fully elucidated yet. At first glance, the bacteria seem to engage with inhibitory Siglec 9, but further studies are needed to confirm this binding (Lee et al. 2014). The sialylated capsule of K. pneumonia could prevent complement mediated phagocytosis or might attract complement factor H to deactivate C3 (Doorduijn et al. 2016) and would thus aid in immune evasion.
Group B Streptococcus
Within the genus of Streptococcus, a gram-positive, spherical bacteria, the group B Streptococcus (GBS, Streptococcus agalactiae) can be classified into 10 serotypes that all have a terminal α2,3-Neu5Ac. GBS serotype III dampens the host immune response (Carlin et al. 2009) by binding to Siglec-9 (Figure 2) (Carlin et al. 2007), an inhibitory receptor found on human neutrophils and platelets (Uchiyama et al. 2019). In case of platelets, Neu5Ac binds the inhibitory Siglec-9, which suppresses platelet activation, and is considered responsible for intrinsic resistance against microbial peptides (Uchiyama et al. 2019). Overall, the glycan mimicry of GBS could aid its survival (Carlin et al. 2009; Yamaguchi et al. 2016).
Hyaluronan
Group A Streptococcus
Group A Streptococcus (GAS, Streptococcus pyogenes) is a gram-positive, spherical bacteria that has a capsule containing hyaluranon, a glycan which can also be found in humans as part of the extracellular matrix (Mouw et al. 2014). GAS can bind to the human inhibitory receptor Siglec-9 (Figure 2) (Secundino et al. 2016). Although Siglec-9 is a sialic acid receptor, it was reported that the hyaluranon of GAS can also bind to this receptor. The interaction between Siglec-9 and GAS is specific for hyaluranon and is not observed for glycosaminoglycans such as heparan sulfate and chrondoitin sulfate (Secundino et al. 2016). As Siglec-9 binds both Group A and B Streptococcus (Carlin et al. 2007, 2009), it is involved in glycan mimicry by two bacteria with structurally different glycans that also each have distinct Siglec-9 binding sites (Secundino et al. 2016). The downstream effect of this interaction with inhibitory Siglec-9 and, in general, the effect on pathology by the glycan mimicry of GAS remains to be further studied.
General trends and outstanding questions in bacterial glycan mimicry
The previous sections provided a brief overview of the bacteria, glycans, lectins and interactions involved in glycan mimicry by bacteria. Although these encompass a very diverse set of glycan structures and bacterial species, some general trends can be observed. First, most glycan mimicking bacteria are gram-negative. Second, mostly facultative anaerobe mucosal pathogens display glycan mimicry. Third, Neu5Ac is a recurring monosaccharide involved in glycan mimicry. We will briefly discuss the third trend, because several published hypotheses have attempted to explain this observation.
The presence of Neu5Ac in bacterial glycoconjugates stands out and the reason for its occurrence in specific bacterial species has been the subject of much discussion, but the exact reason for its involvement in glycan mimicry by bacteria is unknown. A general hypothesis is that Neu5Ac is displayed by bacteria in glycan mimicry for its prominent terminal position on mammalian glycans and its charge. An associated question is if other terminal sialic acids found on bacteria, such as Pse and Leg, also have a function in glycan mimicry. Another hypothesis for the frequent involvement of Neu5Ac in glycan mimicry is their interaction with Siglecs. Many mimicking bacteria interact with inhibitory Siglecs and can thereby downregulate their host’s immune response (Crocker et al. 2007). In turn, their interaction with inhibitory Siglecs might have led to the evolution of activating Siglecs and perhaps also to the microbial glycan activated intelectins (Wesener et al. 2015; Schwarz et al. 2017). Varki (2006) has postulated that these type of interactions might be tied to the Red Queen effect, an evolutionary race between host and pathogen, which might explain the diversification of glycans. The engagement of Siglecs by bacterial glycans could also play a role in masking the bacteria as “self.” For instance, human Siglecs do not recognize the nonhuman Neu5Gc and this glycan has also not been found on bacteria (Crocker et al. 2007; Varki 2017). This suggests that bacteria have committed to using only Neu5Ac to avoid immune recognition (Padler-Karavani et al. 2008; Ng et al. 2019). These hypotheses and the possible role of sialic acids in glycan mimicry by bacteria are part of an ongoing discussion (Varki 2006, 2017; Varki and Gagneux 2012).
Besides the general trends mentioned, there are still many unanswered questions about glycan mimicry by bacteria, see Box 1 for the outstanding questions highlighted by us. A major outstanding question is: what is the function of glycan mimicry? For a few bacteria, their interactions and influence on the host’s immune system are known, with sialylated glycan mimicry playing a prominent role, but an unequivocal conclusion for the existence and function of glycan mimicry cannot yet be made. This and other outstanding questions provide ample opportunities for research. In the final part of the review, we highlight one specific discipline, chemical glycobiology, that we believe that will contribute significantly in studying glycan mimicry by bacteria.
Box 1: outstanding questions
-
▪
What are the minimal glycan structures required by specific bacteria to achieve functional glycan mimicry?
-
▪
Besides Neu5Ac, could other sialic acids such as Pse/Leg play a role in glycan mimicry? (Stenutz and Weintraub 2006; Lewis et al. 2009; Gulati et al. 2015)
-
▪
To what extent does sialic acid O-acetylation (Lewis et al. 2004, 2007; Houliston et al. 2006; Dzieciatkowska et al. 2007) occur on mimicking bacteria and does this O-acetylation affect glycan mimicry and its function?
-
▪
What is the exact location, lifetime and substrate specificity of the native sialyltransferases used by various bacteria to sialylate their outer glycans involved in glycan mimicry?
-
▪
Can human Galectins discriminate between self and nonself glycans, and thus act as a form of immunity? (Stowell et al. 2014; Arthur et al. 2015)
-
▪
Do commensal bacteria also use glycan mimicry to maintain themselves at the host gut microbiota interface which is continuously exposed to our immune system?
Chemical glycobiology techniques to study glycan mimicry
Human glycan mimicry by bacteria is a complex phenomenon that involves many interacting elements and thus needs to be studied with various approaches. Frequently used approaches are biochemistry and genetics (Ausubel 1987; Moran et al. 2009). Techniques from these fields can, for example, demonstrate the cross-reactivity between antibodies of the host and a microorganism or make mutations in glycosyltransferases and glycosidases involved in LPS/LOS biosynthesis to further dissect their function. Chemical glycobiology is a powerful approach that has emerged over the past two decades to study and understand glycans and their function, and carbohydrate-related proteins. The assembly of all glycans, also bacterial, is not template-driven and this poses unique challenges in studying glycobiology (Wen et al. 2018; Zol-Hanlon and Schumann 2020). Glycans are also generally more diverse in structure and often exert their function in a multivalent fashion as heterogeneous mixtures. This structural complexity offers an opportunity for a chemistry-based strategy to unravel the complex mechanisms at play in glycobiology. Indeed, the past two decades has seen the development of many techniques for the study of microbial glycobiology, in addition to the chemical techniques to study other bacterial components such as amino acids, lipids and peptidoglycan (Griffin and Hsieh-Wilson 2016; Zhang et al. 2020). In the following sections, we will highlight several of these chemical glycobiology techniques (Figure 3) and report on their application or speculate on their potential use to study bacterial mimicry of human glycans.
Fig. 3.
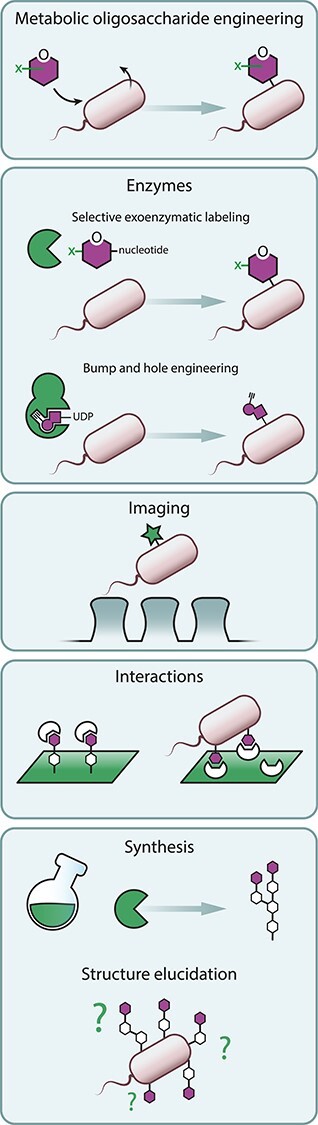
Chemical glycobiology techniques to study glycan mimicry on bacteria and theirhost.
Metabolic oligosaccharide engineering
Metabolic Oligosaccharide Engineering (MOE) is a technique that uses the cells’ own metabolic salvage pathway to incorporate an externally administered monosaccharide-based probe into glycoconjugates. These monosaccharide-based probes typically contain an unnatural functional group that can be covalently coupled through a click reaction to a reporter group, for example, biotin or a fluorophore, after incorporation into the bacterial glycans. It can serve as a technique to detect the presence of a specific monosaccharide in a complex glycoconjugates (e.g., LPS) in vivo or in vitro. Due to the possibility of attaching artificial groups to the labeled glycan, it can also be used to engineer the target glycoconjugate to investigate its structure–activity relationship or perturb an interaction with an associated lectin.
MOE has been performed with glycans that are unique for bacteria (Dube et al. 2011; Tra and Dube 2014), for example, bacillosamine in N-linked glycans of C. jejuni, Legionaminic acid (Leg) in Legionella Pneumophilia (Pons et al. 2014) and C. jejuni (Meng et al. 2021) and Pseudaminic acid (Pse) in C. jejuni, Pseudomonas aeruginosa, Acinetobacter baumannii and Vibrio vulnificus (Liu et al. 2009; Andolina et al. 2018). Two groups of unique bacterial glycans that are especially interesting to study glycan mimicry are: (1) monosaccharides that are frequently found on the core of the LOS, such as KDO and heptose, and (2) nonulosonic acids, such as the previously mentioned Pse and Leg, to dissect the role of these microbial sialic acids further (Tra and Dube 2014; Clark et al. 2016).
The extensive monosaccharide “toolbox” with different chemical reporters that has been developed for MOE in mammalian cells can be used to study the role of N-acetylneuraminic acid and other human monosaccharides in glycan mimicry by bacteria (Sminia et al. 2016; Moons et al. 2019).
MOE is often performed with monosaccharides that have a chemical reporter, which can reveal the incorporation into a potentially unknown glycoconjugate or the location of the labeled bacteria (see also imaging). A useful way to influence this process is to make use of inhibitors that can perturb the transferase enzymes of the glycan biosynthesis pathway. An illustrative example of such an approach was the study of LOS sialylation by NTHi which used a Neu5Ac probe in combination with a metabolic sialyltransferase inhibitor (Heise et al. 2018). By itself, the development of activity-based probes and (metabolic) inhibitors to perturb, detect and identify the glycosyl hydrolase and transferase enzymes involved in the processing of bacterial glycans is an important emerging research direction toward understanding bacterial glycobiology (Gloster and Vocadlo 2012; Wu et al. 2019; Luijkx et al. 2021).
MOE has two main drawbacks. One drawback is that the efficiency of MOE can vary a lot per bacterial species or strain, for instance bacteria can also degrade the probes for growth, which would require optimization per “tool” (Nischan and Kohler 2016). Second, a probe is processed through the bacteria’s metabolic pathway, which leads to incorporation into various types of glycoconjugates with limited control over this. An advantage of MOE is that the technique has been fairly well developed for microbiology and many of the monosaccharide probes and associated chemical reporters are commercially available. MOE provides exciting opportunities to study glycan mimicry and its function and possibly might also provide leads for future glycotherapies (Hudak and Bertozzi 2014; Tra and Dube 2014).
Enzymatic methods
Another approach to glycoengineer and thereby study the composition and function of complex glycoconjugates in a living organism is to make use of enzymes; here, we describe six of these techniques. First, the technique called selective exoenzymatic labeling, also called chemoenzymatic labeling, has been used to track N-glycans, identify human glycoproteins and introduce a large biomolecule (Mbua et al. 2013; Sun et al. 2016; Capicciotti et al. 2017). The technique has mostly been applied to mammalian cells thus far but can be used for glycoengineering of bacteria [unpublished data by our group]. A detailed review of the different enzymes available and their substrate scope has been reported (Lopez Aguilar et al. 2017).
A second engineering technique is to remove subclasses of N-glycans on mammalian cells by trimming them with the appropriate glycosidases and insert whole N-glycans with a chemical handle, an oxazoline (Tang et al. 2020). This technique could potentially homogenize the cell surface. This approach could also be used to homogenize the LOS of bacteria, although this would require extensive engineering of the enzymes and glycans involved.
A third technique studies the function of glycosyltransferases and their acceptors through bump-and-hole engineering, which employs a complementary set of modified enzymes and substrates that are not naturally present in a cell. Schumann et al. (2020) applied this to N-acetylgalactosaminyl transferases to profile the targets of this family of enzymes. This technique can be used to engineer the cell surface in a targeted way with glycans or clickable groups but also to scan for substrate specificity or labeling without the interference of other native bacterial transferases.
Most of the developed enzymatic glycoengineering techniques have only been applied to mammalian cells, but there are a few examples for bacteria. One study screened the substrate promiscuity of several enzymes involved in the biosynthesis of the heptasaccharide on the N-glycans of C. jejuni to introduce azides (Lukose et al. 2015). Attempts to label in vivo failed, because the labeled monosaccharide precursors were not converted to the corresponding nucleotide sugars (Lukose et al. 2015). In another study, the enzymatic synthesis of a glycolipid was developed to incorporate an arabinose onto the cell surface of mycobacterium (Calabretta et al. 2019). This strategy could, for example, also be applied to study the glycolipid portion of the LOS that is involved in mimicry.
The LOS of bacteria can also be glycoengineered through genetically introduced transferases (Mally et al. 2013). This approach was applied to engineer the O-antigen of E. coli and Salmonella enterica serovar Typhimurium (Mally et al. 2013). This technique can build a poly LacNAc unit, or a Lewis x motive, on a truncated lipid A core, and therefore, the LOS becomes glycan mimicking.
Imaging
In the past, labeled lectins or antibodies have often been used to indirectly detect and image certain glycans on the cell surface of bacteria. However, a powerful way to study glycan mimicry would be to directly image the actual glycans themselves. The Kasper group applied MOE to fluorescently label and track commensal bacteria in the gut via their cell surface glycans (Geva-Zatorsky et al. 2015). In another study, they labeled multiple glycoconjugates of the cell wall: peptidoglycan, LPS and CPS, with different fluorophores and tracked these bacteria (Hudak et al. 2017). Bacteria involved in glycan mimicry could also be labeled as such and visualized, for example, on their LOS or LPS (Wang et al. 2019; Heesterbeek et al. 2021). Imaging and spatiotemporal tracking of a specific glycan on a commensal or pathogenic bacterium in the presence of a mammalian cell model or even in vivo in an animal model (with for instance near-IR dyes) could provide valuable information about their location and their possible interaction partners.
Interactions
Glycan microarrays are an important technique to study bacterial glycans and their interactions. One approach to study glycan mimicry with microarrays is to investigate the binding of mimicking glycans to lectins of the innate immune system (Stowell et al. 2014). Another approach is to study the binding of unknown bacterial glycans to lectins on a microarray in order to glycophenotype the bacterial glycan coat (Semchenko et al. 2011, 2012; Tytgat and de Vos 2016). The presence of mimicking glycan structures on commensal bacteria might be elucidated with glycophenotyping. For a detailed overview of the application of microarrays to study both bacterial glycans and their interactions, we refer the reader to a recent review (Campanero-Rhodes et al. 2020).
In case the interactions between bacterial glycans and receptors are known, it might be possible to manipulate this interaction and steer it to a receptor of choice. In a recent study, Natural Killer cells were modified via MOE with a set of Neu5Ac’s that contained unnatural groups, of which some enhanced the binding to a tumor cell receptor that in turn led to increased tumor lysis (Wang et al. 2020). When studying a specific glycan-receptor interaction, multivalency effects play an important role and can be investigated with defined multimers of the bacterial glycans (Bernardi et al. 2013). In case of glycan mimicry by bacteria, a more fundamental application can be thought of where the binding to different receptors and the effect on the immune system can be studied, for example, the binding of activating or inhibiting Siglecs by N. gonorrhoeae (Landig et al. 2019). A set of molecules containing bio-orthogonal click groups is available to increase binding to certain Siglecs (Büll et al. 2017).
In case the in vivo interactions between bacterial glycans and receptors or binding partners are unknown, a photoreactive group, like a diazirine, might be installed on the bacterial glycans to photocrosslink nearby receptors (Nischan and Kohler 2016) and use proteomics to identify these binding partners.
Synthesis and structure elucidation
Progress to make glycans more accessible through automated glycan synthesis, both chemically and enzymatically, will speed up the study of glycobiology (Wen et al. 2018; Li, Yu, et al. 2020). Some examples that are of special interest for glycan mimicry are gangliosides and poly-LacNAc repeats that were synthesized through automated synthesis in combination with enzymes (Li et al. 2019) or the LOS of C. jejuni that was synthesized through chemoenzymatic synthesis (Li, Wolfert, et al. 2020).
Another fundamental approach in glycobiology is structure elucidation. This is typically performed through a range of techniques, such as monomer analysis, MS and NMR (Reuel et al. 2012). Mass spectrometry analysis of glycans can require a lot of expertise. Improvements can be made in purification before measuring, mass labels and annotation of the data (Pilobello and Mahal 2007).
Summary
Bacteria are covered by glycans. In the cases described here, and possibly even more, bacteria display glycans that mimic glycans found in their human or animal host. This glycan mimicry is often found for gram-negative and mucosal pathogens. Bacterial glycans are important for many interactions that could possibly result in infection. For bacterial glycans mimicking their host, an increasing number of interaction partners have been identified, yet their effects beyond glycan recognition by host receptors are not always clear. In general, the overall function of glycan mimicry by bacteria remains to be studied. The techniques developed for chemical glycobiology could aid in identifying bacteria that display glycan mimicry and elucidate the function of glycan mimicry.
Supplementary Material
Acknowledgements
Space limitations necessitated restrictions in the selection of literature to review, so we apologize to researchers whose work was not cited here. We acknowledge Jos van Putten for his invaluable input and useful comments during the preparation of this manuscript.
Contributor Information
Hanna de Jong, Department of Chemical Biology and Drug Discovery, Utrecht Institute for Pharmaceutical Sciences, and Bijvoet Center for Biomedical Research, Utrecht University, Universiteitsweg 99, Utrecht 3584 CG, The Netherlands; Department of Biomolecular Health Sciences, Utrecht University, Yalelaan 1, Utrecht 3584 CL, The Netherlands.
Marc M S M Wösten, Department of Biomolecular Health Sciences, Utrecht University, Yalelaan 1, Utrecht 3584 CL, The Netherlands.
Tom Wennekes, Department of Chemical Biology and Drug Discovery, Utrecht Institute for Pharmaceutical Sciences, and Bijvoet Center for Biomedical Research, Utrecht University, Universiteitsweg 99, Utrecht 3584 CG, The Netherlands.
Funding
Netherlands Foundation for Scientific Research (NWO) via a VIDI grant (723.014.005 to TW); BBOL grant (737.016.013).
References
- Alfa MJ, Degagne P. 1997. Attachment of Haemophilus ducreyi to human foreskin fibroblasts involves LOS and fibronectin. Microb Pathog. 22:39–46. [DOI] [PubMed] [Google Scholar]
- Andolina G, Wei R, Liu H, Zhang Q, Yang X, Cao H, Chen S, Yan A, Li XD, Li X. 2018. Metabolic labeling of pseudaminic acid-containing glycans on bacterial surfaces. ACS Chem Biol. 13:3030–3037. [DOI] [PubMed] [Google Scholar]
- Apicella MA. 2012. Nontypeable Haemophilus influenzae: The role of N-acetyl-5-neuraminic acid in biology. Front Cell Infect Microbiol. 2:19. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Apicella MA, Coffin J, Ketterer M, Post DMB, Day CJ, Jen FE, Jennings MP. 2018. Nontypeable Haemophilus influenzae lipooligosaccharide expresses a terminal ketodeoxyoctanoate in vivo, which can be used as a target for bactericidal antibody. Mol Microbiol. 9:e01401–e01418. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Appelmelk BJ, Monteiro MA, Martin SL, Moran AP, Vandenbroucke-Grauls CMJE. 2000. Why Helicobacter pylori has Lewis antigens. Trends Microbiol. 8:565–570. [DOI] [PubMed] [Google Scholar]
- Appelmelk BJ, Simoons-Smit I, Negrini R, Moran AP, Aspinall GO, Forte JG, De Vries T, Quan H, Verboom T, Maaskant JJ, et al. 1996. Potential role of molecular mimicry between Helicobacter pylori lipopolysaccharide and host Lewis blood group antigens in autoimmunity. Infect Immun. 64:2031–2040. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Arthur CM, Patel SR, Mener A, Kamili NA, Fasano RM, Meyer E, Winkler AM, Sola-Visner M, Josephson CD, Stowell SR. 2015. Innate immunity against molecular mimicry: Examining galectin-mediated antimicrobial activity. Bio Essays. 37:1327–1337. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ausubel FM. 1987. Current Protocols in Molecular Biology. New York: Greene Publishing Associates. [Google Scholar]
- Avril T, Wagner ER, Willison HJ, Crocker PR. 2006. Sialic acid-binding immunoglobulin-like lectin 7 mediates selective recognition of sialylated glycans expressed on Campylobacter jejuni lipooligosaccharides. Infect Immun. 74:4133–4141. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bergman M, Del Prete G, Kooyk Y, Appelmelk B. 2006. Helicobacter pylori phase variation, immune modulation and gastric autoimmunity. Nat Rev Microbiol. 4:151–159. [DOI] [PubMed] [Google Scholar]
- Bernardi A, Jiménez-Barbero J, Casnati A, De Castro C, Darbre T, Fieschi F, Finne J, Funken H, Jaeger KE, Lahmann M, et al. 2013. Multivalent glycoconjugates as anti-pathogenic agents. Chem Soc Rev. 42:4709–4727. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Büll C, Heise T, Hilten N, Pijnenborg JFA, Bloemendal VRLJ, Gerrits L, Kers-Rebel ED, Ritschel T, Brok MH, Adema GJ, et al. 2017. Steering Siglec–sialic acid interactions on living cells using bioorthogonal chemistry. Angew Chemie - Int Ed. 56:3309–3313. [DOI] [PubMed] [Google Scholar]
- Calabretta P, Hodges HL, Kraft MB, Marando V, Kiessling LL. 2019. Bacterial cell wall modification with a glycolipid substrate. J Am Chem Soc. 141:9262–9272. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Campanero-Rhodes MA, Palma AS, Menéndez M, Solís D. 2020. Microarray strategies for exploring bacterial surface glycans and their interactions with glycan-binding proteins. Front Microbiol. 10:2909. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Capicciotti CJ, Zong C, Sheikh MO, Sun T, Wells L, Boons GJ. 2017. Cell-surface glyco-engineering by exogenous enzymatic transfer using a bifunctional CMP-Neu5Ac derivative. J Am Chem Soc. 139:13342–13348. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Carlin AF, Lewis AL, Varki A, Nizet V. 2007. Group B streptococcal capsular sialic acids interact with siglecs (immunoglobulin-like lectins) on human leukocytes. J Bacteriol. 189:1231–1237. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Carlin AF, Uchiyama S, Chang YC, Lewis AL, Nizet V, Varki A. 2009. Molecular mimicry of host sialylated glycans allows a bacterial pathogen to engage neutrophil Siglec-9 and dampen the innate immune response. Blood. 113:3333–3336. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Carolin FA. 2019. Molecular host mimicry and manipulation in bacterial symbionts. FEMS Microbiol Lett. 366:fnz038. [DOI] [PubMed] [Google Scholar]
- Chmiela M, Gonciarz W. 2017. Molecular mimicry in Helicobacter pylori infections. World J Gastroenterol. 23:3964–3977. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chmiela M, Miszczyk E, Rudnicka K. 2014. Structural modifications of Helicobacter pylori lipopolysaccharide: An idea for how to live in peace. World J Gastroenterol. 20:9882–9897. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Clark EL, Emmadi M, Krupp KL, Podilapu AR, Helble JD, Kulkarni SS, Dube DH. 2016. Development of rare bacterial monosaccharide analogs for metabolic glycan labeling in pathogenic bacteria. ACS Chem Biol. 11:3365–3373. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Crocker PR, Paulson JC, Varki A. 2007. Siglecs and their roles in the immune system. Nat Rev Immunol. 7:255–266. [DOI] [PubMed] [Google Scholar]
- Damian RT. 1964. Molecular mimicry: Antigen sharing by parasite and host and its consequences. Am Nat. 98:129–149. [Google Scholar]
- Day CJ, Semchenko EA, Korolik V. 2012. Glycoconjugates play a key role in Campylobacter jejuni infection: Interactions between host and pathogen. Front Cell Infect Microbiol. 2:9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Day CJ, Tran EN, Semchenko EA, Tram G, Hartley-Tassell LE, Ng PSK, King RM, Ulanovsky R, McAtamney S, Apicella MA, et al. 2015. Glycan:Glycan interactions: High affinity biomolecular interactions that can mediate binding of pathogenic bacteria to host cells. Proc Natl Acad Sci. 112:E7266–E7275. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Doorduijn DJ, Rooijakkers SHM, Van Schaik W, Bardoel BW. 2016. Complement resistance mechanisms of Klebsiella pneumoniae. Immunobiology. 221:1102–1109. [DOI] [PubMed] [Google Scholar]
- Dube DH, Champasa K, Wang B. 2011. Chemical tools to discover and target bacterial glycoproteins. Chem Commun. 47:87–101. [DOI] [PubMed] [Google Scholar]
- Dzieciatkowska M, Brochu D, Van Belkum A, Heikema AP, Yuki N, Houliston RS, Richards JC, Gilbert M, Li J. 2007. Mass spectrometric analysis of intact lipooligosaccharide: Direct evidence for O-acetylated sialic acids and discovery of O-linked glycine expressed by Campylobacter jejuni. Biochemistry. 46:14704–14714. [DOI] [PubMed] [Google Scholar]
- Ellström P, Feodoroff B, Hänninen ML, Rautelin H. 2014. Lipooligosaccharide locus class of Campylobacter jejuni: Sialylation is not needed for invasive infection. Clin Microbiol Infect. 20:524–529. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Figueira MA, Ram S, Goldstein R, Hood DW, Moxon ER, Pelton SI. 2007. Role of complement in defense of the middle ear revealed by restoring the virulence of nontypeable Haemophilus influenzae siaB mutants. Infect Immun. 75:325–333. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Geva-Zatorsky N, Alvarez D, Hudak JE, Reading NC, Erturk-Hasdemir D, Dasgupta S, Von Andrian UH, Kasper DL. 2015. In vivo imaging and tracking of host-microbiota interactions via metabolic labeling of gut anaerobic bacteria. Nat Med. 21:1091–1100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gibson BW, Campagnari AA, Melaugh W, Phillips NJ, Apicella MA, Grass S, Wang J, Palmer KL, Munson RS. 1997. Characterization of a transposon Tn 916 -generated mutant of Haemophilus ducreyi 35000 defective in lipooligosaccharide biosynthesis. J Bacteriol. 179:5062–5071. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gilbert M, Karwaski MF, Bernatchez S, Young NM, Taboada E, Michniewicz J, Cunningham AM, Wakarchuk WW. 2002. The genetic bases for the variation in the lipo-oligosaccharide of the mucosal pathogen, Campylobacter jejuni. Biosynthesis of sialylated ganglioside mimics in the core oligosaccharide. J Biol Chem. 277:327–337. [DOI] [PubMed] [Google Scholar]
- Gloster TM, Vocadlo DJ. 2012. Developing inhibitors of glycan processing enzymes as tools for enabling glycobiology. Nat Chem Biol. 8:683–694. [DOI] [PubMed] [Google Scholar]
- Godschalk PCR, Kuijf ML, Li J, St. Michael F, Ang CW, Jacobs BC, Karwaski MF, Brochu D, Moterassed A, Endtz HP, et al. 2007. Structural characterization of Campylobacter jejuni lipooligosaccharide outer cores associated with Guillain-Barré and Miller Fisher syndromes. Infect Immun. 75:1245–1254. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Goon S, Schilling B, Tullius MV, Gibson BW, Bertozzi CR. 2003. Metabolic incorporation of unnatural sialic acids into Haemophilus ducreyi lipooligosaccharides. Proc Natl Acad Sci U S A. 100:3089–3094. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Griffin ME, Hsieh-Wilson LC. 2016. Glycan engineering for cell and developmental biology. Cell Chem Biol. 23:108–121. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gulati S, Schoenhofen IC, Whitfield DM, Cox AD, Li J, St. Michael F, Vinogradov EV, Stupak J, Zheng B, Ohnishi M, et al. 2015. Utilizing CMP-sialic acid analogs to unravel Neisseria Gonorrhoeae lipooligosaccharide-mediated complement resistance and design novel therapeutics. PLoS Pathog. 11:e1005290. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Harvey HA, Jennings MP, Campbell CA, Williams R, Apicella MA. 2001. Receptor-mediated endocytosis of Neisseria gonorrhoeae into primary human urethral epithelial cells: The role of the asialoglycoprotein receptor. Mol Microbiol. 42:659–672. [DOI] [PubMed] [Google Scholar]
- Heesterbeek DAC, Muts RM, Hensbergen VP, Saint AP, Wennekes T, Bardoel BW, Sorge NM, Rooijakkers SHM. 2021. Outer membrane permeabilization by the membrane attack complex sensitizes gram-negative bacteria to antimicrobial proteins in serum and phagocytes. PLoS Pathog. 17:e1009227. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Heikema AP, Bergman MP, Richards H, Crocker PR, Gilbert M, Samsom JN, Van Wamel WJB, Endtz HP, Van Belkum A. 2010. Characterization of the specific interaction between sialoadhesin and sialylated Campylobacter jejuni lipooligosaccharides. Infect Immun. 78:3237–3246. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Heikema AP, Jacobs BC, Horst-Kreft D, Huizinga R, Kuijf ML, Endtz HP, Samsom JN, Wamel WJB. 2013. Siglec-7 specifically recognizes Campylobacter jejuni strains associated with oculomotor weakness in Guillain-Barré syndrome and Miller Fisher syndrome. Clin Microbiol Infect. 19:E106–E112. [DOI] [PubMed] [Google Scholar]
- Heikema AP, Koning RI, Rico SD d S, Rempel H, Jacobs BC, Endtz HP, WJB v W, Samsom JN. 2013. Enhanced, sialoadhesin-dependent uptake of guillain-barré syndrome-associated Campylobacter jejuni strains by human macrophages. Infect Immun. 81:2095–2103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Heise T, Langereis JD, Rossing E, Jonge MI, Adema GJ, Büll C, Boltje TJ. 2018. Selective inhibition of sialic acid-based molecular mimicry in Haemophilus influenzae abrogates serum resistance. Cell Chem Biol. 25:1279–1285.e8. [DOI] [PubMed] [Google Scholar]
- Holme T, Rahman M, Jansson PE, Widmalm G. 1999. The lipopolysaccharide of Moraxella catarrhalis. Structural relationships and antigenic properties. Eur J Biochem. 265:524–529. [DOI] [PubMed] [Google Scholar]
- Houliston RS, Endtz HP, Yuki N, Li J, Jarrell HC, Koga M, Van Belkum A, Karwaski MF, Wakarchuk WW, Gilbert M. 2006. Identification of a sialate O-acetyltransferase from Campylobacter jejuni: Demonstration of direct transfer to the C-9 position of terminal α-2,8-linked sialic acid. J Biol Chem. 281:11480–11486. [DOI] [PubMed] [Google Scholar]
- Hudak JE, Alvarez D, Skelly A, Von Andrian UH, Kasper DL. 2017. Illuminating vital surface molecules of symbionts in health and disease. Nat Microbiol. 2:17099. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hudak JE, Bertozzi CR. 2014. Glycotherapy: New advances inspire a reemergence of glycans in medicine. Chem Biol. 21:16–37. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hynes SO, Ferris JA, Szponar B, Wadström T, Fox JG, O’Rourke J, Larsson L, Yaquian E, Ljungh Å, Clyne M, et al. 2004. Comparative chemical and biological characterization of the lipopolysaccharides of gastric and enterohepatic Helicobacters. Helicobacter. 9:313–323. [DOI] [PubMed] [Google Scholar]
- Imperiali B. 2019. Bacterial carbohydrate diversity — A brave new world. Curr Opin Chem Biol. 53:1–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jackson MD, Wong SM, Akerley BJ. 2019. Underlying glycans determine the ability of sialylated lipooligosaccharide to protect Nontypeable Haemophilus influenzae from serum IgM and complement. Infect Immun. 87:e00456–e00419. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jarvis GA, Vedros NA. 1987. Sialic acid of group B Neisseria meningitidis regulates alternative complement pathway activation. Infect Immun. 55:174–180. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jasti AK, Selmi C, Sarmiento-Monroy JC, Vega DA, Anaya JM, Gershwin ME. 2016. Guillain-Barré syndrome: Causes, immunopathogenic mechanisms and treatment. Expert Rev Clin Immunol. 12:1175–1189. [DOI] [PubMed] [Google Scholar]
- Johswich K. 2017. Innate immune recognition and inflammation in Neisseria meningitidis infection. Pathog Dis. 75:ftx022. [DOI] [PubMed] [Google Scholar]
- Jones C, Virji M, Crocker PR. 2003. Recognition of sialylated meningococcal lipopolysaccharide by siglecs expressed on myeloid cells leads to enhanced bacterial uptake. Mol Microbiol. 49:1213–1225. [DOI] [PubMed] [Google Scholar]
- Kalograiaki I, Euba B, Fernández-Alonso M d C, Proverbio D, St. Geme JW, Aastrup T, Garmendia J, Cañada FJ, Solís D. 2018. Differential recognition of Haemophilus influenzae whole bacterial cells and isolated lipooligosaccharides by galactose-specific lectins. Sci Rep. 8:16292. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kalograiaki I, Euba B, Proverbio D, Campanero-Rhodes MA, Aastrup T, Garmendia J, Solís D. 2016. Combined bacteria microarray and quartz crystal microbalance approach for exploring glycosignatures of Nontypeable Haemophilus influenzae and recognition by host lectins. Anal Chem. 88:5950–5957. [DOI] [PubMed] [Google Scholar]
- Khamri W, Moran AP, Worku ML, Karim QN, Walker MM, Annuk H, Ferris JA, Appelmelk BJ, Eggleton P, Reid KBM, et al. 2005. Variations in Helicobacter pylori lipopolysaccharide to evade the innate immune component surfactant protein D. Infect Immun. 73:7677–7686. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Landig CS, Hazel A, Kellman BP, Fong JJ, Schwarz F, Agarwal S, Varki N, Massari P, Lewis NE, Ram S, et al. 2019. Evolution of the exclusively human pathogen Neisseria gonorrhoeae: Human-specific engagement of immunoregulatory Siglecs. Evol Appl. 12:337–349. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lee CH, Chang CC, Liu JW, Chen RF, Yang KD. 2014. Sialic acid involved in hypermucoviscosity phenotype of Klebsiella pneumoniae and associated with resistance to neutrophil phagocytosis. Virulence. 5:673–679. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Leker K, Lozano-Pope I, Bandyopadhyay K, Choudhury BP, Obonyo M. 2017. Comparison of lipopolysaccharides composition of two different strains of Helicobacter pylori. BMC Microbiol. 17:226. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lewis AL, Cao H, Patel SK, Diaz S, Ryan W, Carlin AF, Thon V, Lewis WG, Varki A, Chen X, et al. 2007. NeuA sialic acid O-acetylesterase activity modulates O-acetylation of capsular polysaccharide in group B Streptococcus. J Biol Chem. 282:27562–27571. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lewis AL, Desa N, Hansen EE, Knirel YA, Gordon JI, Gagneux P, Nizet V, Varki A. 2009. Innovations in host and microbial sialic acid biosynthesis revealed by phylogenomic prediction of nonulosonic acid structure. Proc Natl Acad Sci U S A. 106:13552–13557. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lewis AL, Nizet V, Varki A. 2004. Discovery and characterization of sialic acid O-acetylation in group B Streptococcus. Proc Natl Acad Sci U S A. 101:11123–11128. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lewis LA, Carter M, Ram S. 2012. The relative roles of factor H binding protein, Neisserial surface protein A, and lipooligosaccharide sialylation in regulation of the alternative pathway of complement on Meningococci. J Immunol. 188:5063–5072. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li H, Liao T, Debowski AW, Tang H, Nilsson HO, Stubbs KA, Marshall BJ, Benghezal M. 2016. Lipopolysaccharide structure and biosynthesis in Helicobacter pylori. Helicobacter. 21:445–461. [DOI] [PubMed] [Google Scholar]
- Li H, Yang T, Liao T, Debowski AW, Nilsson HO, Haslam SM, Dell A, Stubbs KA, Marshall BJ, Benghezal M. 2017. The redefinition of Helicobacter pylori lipopolysaccharide O-antigen and core-oligosaccharide domains. PLoS Pathog. 4:175–178. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li R, Yu H, Chen X. 2020. Recent progress in chemical synthesis of bacterial surface glycans. Curr Opin Chem Biol. 58:121–136. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li T, Liu L, Wei N, Yang J-Y, Chapla DG, Moremen KW, Boons G-J. 2019. An automated platform for the enzyme-mediated assembly of complex oligosaccharides. Nat Chem. 11:229–236. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li T, Wolfert MA, Wei N, Huizinga R, Jacobs BC, Boons GJ. 2020. Chemoenzymatic synthesis of Campylobacter jejuni lipo-oligosaccharide core domains to examine Guillain-Barré syndrome serum antibody specificities. J Am Chem Soc. 142:19611–19621. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu F, Aubry AJ, Schoenhofen IC, Logan SM, Tanner ME. 2009. The engineering of bacteria bearing azido-pseudaminic acid-modified flagella. Chem Bio Chem. 10:1317–1320. [DOI] [PubMed] [Google Scholar]
- Lizak C, Worrall LJ, Baumann L, Pfleiderer MM, Volkers G, Sun T, Sim L, Wakarchuk W, Withers SG, Strynadka NCJ. 2017. X-ray crystallographic structure of a bacterial polysialyltransferase provides insight into the biosynthesis of capsular polysialic acid. Sci Rep. 7:5842. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Logan SM, Conlan JW, Monteiro MA, Wakarchuk WW, Altman E. 2000. Functional genomics of Helicobacter pylori: Identification of a β-1,4 galactosyltransferase and generation of mutants with altered lipopolysaccharide. Mol Microbiol. 35:1156–1167. [DOI] [PubMed] [Google Scholar]
- Lopez Aguilar A, Briard JG, Yang L, Ovryn B, Macauley MS, Wu P. 2017. Tools for studying glycans: Recent advances in chemoenzymatic glycan labeling. ACS Chem Biol. 12:611–621. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Louwen R, Heikema A, Van Belkum A, Ott A, Gilbert M, Ang W, Endtz HP, Bergman MP, Nieuwenhuis EE. 2008. The sialylated lipooligosaccharide outer core in Campylobacter jejuni is an important determinant for epithelial cell invasion. Infect Immun. 76:4431–4438. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Louwen R, Nieuwenhuis EES, Marrewijk L, Horst-Kreft D, Ruiter L, Heikema AP, Wamel WJB, Wagenaar JA, Endtz HP, Samsom J, et al. 2012. Campylobacter jejuni translocation across intestinal epithelial cells is facilitated by ganglioside-like lipooligosaccharide structures. Infect Immun. 80:3307–3318. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Luijkx YMCA, Jongkees S, Strijbis K, Wennekes T. 2021. Development of a 1,2-difluorofucoside activity-based probe for profiling GH29 fucosidases. Org Biomol Chem. 19:2968–2977. [DOI] [PubMed] [Google Scholar]
- Lukose V, Whitworth G, Guan Z, Imperiali B. 2015. Chemoenzymatic assembly of bacterial glycoconjugates for site-specific orthogonal labeling. J Am Chem Soc. 137:12446–12449. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mally M, Fontana C, Leibundgut-Landmann S, Laacisse L, Fan YY, Widmalm G, Aebi M. 2013. Glycoengineering of host mimicking type-2 LacNAc polymers and Lewis X antigens on bacterial cell surfaces. Mol Microbiol. 87:112–131. [DOI] [PubMed] [Google Scholar]
- Mandrell RE, Apicella MA. 1993. Lipo-oligosaccharides (LOS) of mucosal pathogens: Molecular mimicry and host-modification of LOS. Immunobiology. 187:382–402. [DOI] [PubMed] [Google Scholar]
- Masoud H, Perry MB, Brisson J-R, Uhrin D, Richards JC. 1994. Structural elucidation of the backbone oligosaccharide from the lipopolysaccharide of Moraxella catarrhalis serotype A. Can J Chem. 72:1466–1477. [Google Scholar]
- Mbua NE, Li X, Flanagan-Steet HR, Meng L, Aoki K, Moremen KW, Wolfert MA, Steet R, Boons GJ. 2013. Selective exo-enzymatic labeling of N-glycans on the surface of living cells by recombinant ST6Gal I. Angew Chemie - Int Ed. 52:13012–13015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Melaugh W, Campagnari AA, Gibson BW. 1996. The lipooligosaccharides of Haemophilus ducreyi are highly sialylated. J Bacteriol. 178:564–570. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Melaugh W, Phillips NJ, Campagnari AA, Tullius MV, Gibson BW. 1994. Structure of the major oligosaccharide from the lipooligosaccharide of Haemophilus ducreyi strain 35000 and evidence for additional glycoforms. Biochemistry. 33:13070–13078. [DOI] [PubMed] [Google Scholar]
- Meng X, Boons G-J, Wösten M, Wennekes T. 2021. Metabolic Labeling of Legionaminic acid in Flagellin Glycosylation of Campylobacter jejuni Identifies Maf4 as a Putative Legionaminyl Transferase. Angew Chem Int Ed. 10.1002/anie.202107181. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Moons SJ, Adema GJ, Derks MT, Boltje TJ, Büll C. 2019. Sialic acid glycoengineering using N-acetylmannosamine and sialic acid analogs. Glycobiology. 29:433–445. [DOI] [PubMed] [Google Scholar]
- Moran AP, Holst O, Brennan PJ, Itzstein M. 2009. Microbial Glycobiology: Structures, Relevance and Applications. 1st ed. Amsterdam: Academic Press/Elsevier. [Google Scholar]
- Moran AP, Prendergast MM. 2001, 2001. Molecular mimicry in Campylobacter jejuni and Helicobacter pylori lipopolysaccharides: Contribution of gastrointestinal infections to autoimmunity. J Autoimmun. 16:241–256. [DOI] [PubMed] [Google Scholar]
- Moran AP, Prendergast MM, Appelmelk BJ. 1996. Molecular mimicry of host structures by bacterial lipopolysaccharides and its contribution to disease. FEMS Immunol Med Microbiol. 16:105–115. [DOI] [PubMed] [Google Scholar]
- Mouw JK, Ou G, Weaver VM. 2014. Extracellular matrix assembly: A multiscale deconstruction. Nat Rev Mol Cell Biol. 15:771–785. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mubaiwa TD, Hartley-Tassell LE, Semchenko EA, Jen FEC, Srikhanta YN, Day CJ, Jennings MP, Seib KL. 2017. The glycointeractome of serogroup B Neisseria meningitidis strain MC58. Sci Rep. 7:5693. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mubaiwa TD, Semchenko EA, Hartley-Tassell LE, Day CJ, Jennings MP, Seib KL. 2017. The sweet side of the pathogenic Neisseria: The role of glycan interactions in colonisation and disease. Pathog Dis. 75:ftx063. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ng PSK, Day CJ, Atack JM, Hartley-Tassell LE, Winter LE, Marshanski T, Padler-Karavani V, Varki A, Barenkamp SJ, Apicella MA, et al. 2019. Nontypeable Haemophilus influenzae has evolved preferential use of N-acetylneuraminic acid as a host adaptation. MBio. 10:1–11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nischan N, Kohler JJ. 2016. Advances in cell surface glycoengineering reveal biological function. Glycobiology. 26:789–796. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Oerlemans MMP, Moons SJ, Heming JJA, Boltje TJ, De Jonge MI, Langereis JD. 2019. Uptake of sialic acid by nontypeable Haemophilus influenzae increases complement resistance through decreasing IgM-dependent complement activation. Infect Immun. 87:e00077–e00019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Padler-Karavani V, Yu H, Cao H, Chokhawala H, Karp F, Varki N, Chen X, Varki A. 2008. Diversity in specificity, abundance, and composition of anti-Neu5Gc antibodies in normal humans: Potential implications for disease. Glycobiology. 18:818–830. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Perera VN, Nachamkin I, Ung H, Patterson JH, McConville MJ, Coloe PJ, Fry BN. 2007. Molecular mimicry in Campylobacter jejuni: Role of the lipo-oligosaccharide core oligosaccharide in inducing anti-ganglioside antibodies. FEMS Immunol Med Microbiol. 50:27–36. [DOI] [PubMed] [Google Scholar]
- Phillips NJ, Apicella MA, Griffiss JML, Gibson BW. 1992. Structural characterization of the cell surface lipooligosaccharides from a Nontypable strain of Haemophilus influenzae. Biochemistry. 31:4515–4526. [DOI] [PubMed] [Google Scholar]
- Pilobello KT, Mahal LK. 2007. Deciphering the glycocode: The complexity and analytical challenge of glycomics. Curr Opin Chem Biol. 11:300–305. [DOI] [PubMed] [Google Scholar]
- Pons JM, Dumont A, Sautejeau G, Fugier E, Baron A, Dukan S, Vauzeilles B. 2014. Identification of living Legionella pneumophila using species-specific metabolic lipopolysaccharide labeling. Angew Chemie - Int Ed. 53:1275–1278. [DOI] [PubMed] [Google Scholar]
- Poole J, Day CJ, Itzstein M, Paton JC, Jennings MP. 2018. Glycointeractions in bacterial pathogenesis. Nat Rev Microbiol. 16:440–452. [DOI] [PubMed] [Google Scholar]
- Prendergast MM, Moran AP. 2000. Lipopolysaccharides in the development of the Guillain-Barré syndrome and Miller Fisher syndrome forms of acute inflammatory peripheral neuropathies. J Endotoxin Res. 6:341–359. [PubMed] [Google Scholar]
- Putten JP. 1993. Phase variation of lipopolysaccharide directs interconversion of invasive and immuno-resistant phenotypes of Neisseria gonorrhoeae. EMBO J. 12:4043–4051. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Reuel NF, Mu B, Zhang J, Hinckley A, Strano MS. 2012. Nanoengineered glycan sensors enabling native glycoprofiling for medicinal applications: Towards profiling glycoproteins without labeling or liberation steps. Chem Soc Rev. 41:5744–5779. [DOI] [PubMed] [Google Scholar]
- Schumann B, SAl M, Wisnovsky SP, Debets MF, Agbay AJ, Fernandez D, LJS W, Lin L, Choi J, Fox DM, et al. 2020. Bump-and-hole engineering identifies specific substrates of glycosyltransferases in living cells. Mol Cell. 78:824–834.e15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schwarz F, Landig CS, Siddiqui S, Secundino I, Olson J, Varki N, Nizet V, Varki A. 2017. Paired Siglec receptors generate opposite inflammatory responses to a human-specific pathogen. EMBO J. 36:751–760. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schweda EKH, Sundström AC, Eriksson LM, Jonasson JA, Lindberg AA. 1994. Structural studies of the cell envelope lipopolysaccharides from Haemophilus ducreyi strains ITM 2665 and ITM 4747. J Biol Chem. 269:12040–12048. [PubMed] [Google Scholar]
- Schwingel JM, Edwards KJ, Cox AD, Masoud H, Richards JC, St. Michael F, Tekwe CD, Sethi S, Murphy TF, Campagnari AA. 2009. Use of Moraxella catarrhalis lipooligosaccharide mutants to identify specific oligosaccharide epitopes recognized by human serum antibodies. Infect Immun. 77:4548–4558. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Secundino I, Lizcano A, Roupé KM, Wang X, Cole JN, Olson J, Ali SR, Dahesh S, Amayreh LK, Henningham A, et al. 2016. Host and pathogen hyaluronan signal through human siglec-9 to suppress neutrophil activation. J Mol Med. 94:219–233. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Semchenko E, Moutin M, Korolik V, Tiralongo J, Day CJ. 2011. Lectin array analysis of purified lipooligosaccharide: A method for the determination of molecular mimicry. J Glycomics Lipidomics. 1:105. [Google Scholar]
- Semchenko EA, Day CJ, Moutin M, Wilson JC, Tiralongo J, Korolik V. 2012. Structural heterogeneity of terminal glycans in Campylobacter jejuni Lipooligosaccharides. PLoS One. 7:e40920. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sminia TJ, Zuilhof H, Wennekes T. 2016. Getting a grip on glycans: A current overview of the metabolic oligosaccharide engineering toolbox. Carbohydr Res. 435:121–141. [DOI] [PubMed] [Google Scholar]
- Spinola SM, Li W, Fortney KR, Janowicz DM, Zwickl B, Katz BP, Munson RS. 2012. Sialylation of lipooligosaccharides is dispensable for the virulence of Haemophilus ducreyi in humans. Infect Immun. 12:679–687. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Steeghs L, Vliet SJ, Uronen-Hansson H, Mourik A, Engering A, Sanchez-Hernandez M, Klein N, Callard R, Putten JPM, Ley P, et al. 2006. Neisseria meningitidis expressing IgtB lipopolysaccharide targets DC-SIGN and modulates dendritic cell function. Cell Microbiol. 8:316–325. [DOI] [PubMed] [Google Scholar]
- Steenbergen SM, Wrona TJ, Vimr ER. 1992. Functional analysis of the sialyltransferase complexes in Escherichia coli K1 and K92. J Bacteriol. 174:1099–1108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stenutz R, Weintraub A. 2006. The structures of Escherichia coli O-polysaccharide antigens. FEMS Microbiol Rev. 30:382–403. [DOI] [PubMed] [Google Scholar]
- Stowell SR, Arthur CM, Mcbride R, Berger O, Razi N, Heimburg-Molinaro J, Rodrigues LC, Gourdine JP, Noll AJ, Von Gunten S, et al. 2014. Microbial glycan microarrays define key features of host-microbial interactions. Nat Chem Biol. 10:470–476. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Suerbaum S, Friedrich S, Leying H, Opferkuch W. 1994. Expression of capsular polysaccacharide determines serum resistance in Escherichia coli K92. Zentralblatt fur Bakteriol. 281:146–157. [DOI] [PubMed] [Google Scholar]
- Sun T, Yu SH, Zhao P, Meng L, Moremen KW, Wells L, Steet R, Boons GJ. 2016. One-step selective exoenzymatic labeling (SEEL) strategy for the biotinylation and identification of glycoproteins of living cells. J Am Chem Soc. 138:11575–11582. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tang F, Zhou M, Qin K, Shi W, Yashinov A, Yang Y, Yang L, Guan D, Zhao L, Tang Y, et al. 2020. Selective N-glycan editing on living cell surfaces to probe glycoconjugate function. Nat Chem Biol. 16:766–775. [DOI] [PubMed] [Google Scholar]
- Taylor RE, Gregg CJ, Padler-Karavani V, Ghaderi D, Yu H, Huang S, Sorensen RU, Chen X, Inostroza J, Nizet V, et al. 2010. Novel mechanism for the generation of human xeno-autoantibodies against the nonhuman sialic acid N-glycolylneuraminic acid. J Exp Med. 207:1637–1646. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tong Y, Arking D, Ye S, Reinhold B, Reinhold V, Stein DC. 2002. Neisseria gonorrhoeae strain PID2 simultaneously expresses six chemically related lipooligosaccharide structures. Glycobiology. 12:523–533. [DOI] [PubMed] [Google Scholar]
- Tra VN, Dube DH. 2014. Glycans in pathogenic bacteria-potential for targeted covalent therapeutics and imaging agents. Chem Commun. 50:4659–4673. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tsai CM, Chen WH, Balakonis PA. 1998. Characterization of terminal NeuNAcα2-3Galβ1-4GlcNAc sequence in lipooligosaccharides of Neisseria meningitidis. Glycobiology. 8:359–365. [DOI] [PubMed] [Google Scholar]
- Tsai CM, Kao G, Zhu P. 2002. Influence of the length of the lipooligosaccharide α chain on its sialylation in Neisseria meningitidis. Infect Immun. 70:407–411. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tytgat HLP, Vos WM. 2016. Sugar coating the envelope: Glycoconjugates for microbe–host crosstalk. Trends Microbiol. 24:853–861. [DOI] [PubMed] [Google Scholar]
- Uchiyama S, Sun J, Fukahori K, Ando N, Wu M, Schwarz F, Siddiqui SS, Varki A, Marth JD, Nizet V. 2019. Dual actions of group B Streptococcus capsular sialic acid provide resistance to platelet-mediated antimicrobial killing. Proc Natl Acad Sci U S A. 116:7465–7470. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Van Belkum A, Jacobs B, Van Beek E, Louwen R, Van Rijs W, Debruyne L, Gilbert M, Li J, Jansz A, Mégraud F, et al. 2009. Can Campylobacter coli induce Guillain-Barré syndrome? Eur J Clin Microbiol Infect Dis. 28:557–560. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sorge NM, Bleumink NMC, Vliet SJ, Saeland E, Pol WL, Kooyk Y, Van Putten JPM. 2009. N-glycosylated proteins and distinct lipooligosaccharide glycoforms of Campylobacter jejuni target the human C-type lectin receptor MGL. Cell Microbiol. 11:1768–1781. [DOI] [PubMed] [Google Scholar]
- Van Den Berg B, Walgaard C, Drenthen J, Fokke C, Jacobs BC, Van Doorn PA. 2014. Guillain-Barré syndrome: Pathogenesis, diagnosis, treatment and prognosis. Nat Rev Neurol. 10:469–482. [DOI] [PubMed] [Google Scholar]
- Varki A. 2006. Nothing in glycobiology makes sense, except in the light of evolution. Cell. 126:841–845. [DOI] [PubMed] [Google Scholar]
- Varki A. 2017. Biological roles of glycans. Glycobiology. 27:3–49. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Varki A, Cummings RD, Aebi M, Packer NH, Seeberger PH, Esko JD, Stanley P, Hart G, Darvill A, Kinoshita T, et al. 2015. Symbol nomenclature for graphical representations of glycans. Glycobiology. 25:1323–1324. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Varki A, Gagneux P. 2012. Multifarious roles of sialic acids in immunity. Ann N Y Acad Sci. 1253:16–36. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Van Vliet SJ, Steeghs L, Bruijns SCM, Vaezirad MM, Blok CS, Arenas Busto JA, Deken M, Van Putten JPM, Van Kooyk Y. 2009. Variation of Neisseria gonorrhoeae lipooligosaccharide directs dendritic cell-induced T helper responses. PLoS Pathog. 5:e1000625. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang T-C, Cochet F, Facchini FA, Zaffaroni L, Serba C, Pascal S, Andraud C, Sala A, Di Lorenzo F, Maury O, et al. 2019. Synthesis of the new cyanine-labeled bacterial lipooligosaccharides for intracellular imaging and in vitro microscopy studies. Bioconjug Chem. 30:1649–1657. [DOI] [PubMed] [Google Scholar]
- Wang X, Lang S, Tian Y, Zhang J, Yan X, Fang Z, Weng J, Lu N, Wu X, Li T, et al. 2020. Glycoengineering of natural killer cells with CD22 ligands for enhanced anticancer immunotherapy. ACS Cent Sci. 6:382–389. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wen L, Edmunds G, Gibbons C, Zhang J, Gadi MR, Zhu H, Fang J, Liu X, Kong Y, Wang PG. 2018. Toward automated enzymatic synthesis of oligosaccharides. Chem Rev. 118:8151–8187. [DOI] [PubMed] [Google Scholar]
- Wesener DA, Wangkanont K, McBride R, Song X, Kraft MB, Hodges HL, Zarling LC, Splain RA, Smith DF, Cummings RD, et al. 2015. Recognition of microbial glycans by human intelectin-1. Nat Struct Mol Biol. 22:603–610. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wratil PR, Horstkorte R, Reutter W. 2016. Metabolic glycoengineering with N-acyl side chain modified mannosamines. Angew Chemie - Int Ed. 55:9482–9512. [DOI] [PubMed] [Google Scholar]
- Wu L, Armstrong Z, Schröder SP, Boer C, Artola M, Aerts JM, Overkleeft HS, Davies GJ. 2019. An overview of activity-based probes for glycosidases. Curr Opin Chem Biol. 53:25–36. [DOI] [PubMed] [Google Scholar]
- Xiang SL, Zhong M, Cai FC, Deng B, Zhang XP. 2006. The sialic acid residue is a crucial component of C. jejuni lipooligosaccharide ganglioside mimicry in the induction Guillain-Barré syndrome. J Neuroimmunol. 174:126–132. [DOI] [PubMed] [Google Scholar]
- Yamaguchi M, Hirose Y, Nakata M, Uchiyama S, Yamaguchi Y, Goto K, Sumitomo T, Lewis AL, Kawabata S, Nizet V. 2016. Evolutionary inactivation of a sialidase in group B streptococcus. Sci Rep. 6:28852. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yu RK, Usuki S, Ariga T. 2006. Ganglioside molecular mimicry and its pathological roles in Guillain-Barré syndrome and related diseases. Infect Immun. 74:6517–6527. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yuki N, Susuki K, Koga M, Nishimoto Y, Odaka M, Hirata K, Taguchi K, Miyatake T, Furukawa K, Kobata T, et al. 2004. Carbohydrate mimicry between human ganglioside GM1 and Campylobacter jejuni lipooligosaccharide causes Guillain-Barré syndrome. Proc Natl Acad Sci U S A. 101:11404–11409. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang Z, Wang Y-C, Yang X, Hang HC. 2020. Chemical reporters for exploring microbiology and microbiota mechanisms. Chem Bio Chem. 21:19–32. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zol-Hanlon MI, Schumann B. 2020. Open questions in chemical glycobiology. Commun Chem. 3:102. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


