Abstract
Background
The large and diverse Coffeeae alliance clade of subfamily Ixoroideae (Rubiaceae) consists of 10 tribes, > 90 genera, and > 2000 species. Previous molecular phylogenetics using limited numbers of markers were often unable to fully resolve the phylogenetic relationships at tribal and generic levels. Also, the structural variations of plastomes (PSVs) within the Coffeeae alliance tribes have been poorly investigated in previous studies. To fully understand the phylogenetic relationships and PSVs within the clade, highly reliable and sufficient sampling with superior next-generation analysis techniques is required. In this study, 71 plastomes (40 newly sequenced and assembled and the rest from the GenBank) were comparatively analyzed to decipher the PSVs and resolve the phylogenetic relationships of the Coffeeae alliance using four molecular data matrices.
Results
All plastomes are typically quadripartite with the size ranging from 153,055 to 155,908 bp and contained 111 unique genes. The inverted repeat (IR) regions experienced multiple contraction and expansion; five repeat types were detected but the most abundant was SSR. The size of the Coffeeae alliance clade plastomes and its elements are affected by the IR boundary shifts and the repeat types. However, the emerging PSVs had no taxonomic and phylogenetic implications. Eight highly divergent regions were identified within the plastome regions ndhF, ccsA, ndhD, ndhA, ndhH, ycf1, rps16-trnQ-UUG, and psbM-trnD. These highly variable regions may be potential molecular markers for further species delimitation and population genetic analyses for the clade. Our plastome phylogenomic analyses yielded a well-resolved phylogeny tree with well-support at the tribal and generic levels within the Coffeeae alliance.
Conclusions
Plastome data could be indispensable in resolving the phylogenetic relationships of the Coffeeae alliance tribes. Therefore, this study provides deep insights into the PSVs and phylogenetic relationships of the Coffeeae alliance and the Rubiaceae family as a whole.
Supplementary Information
The online version contains supplementary material available at 10.1186/s12870-022-03480-5.
Keywords: Coffeeae alliance, Phylogenomic, Plastome, Plastome structural variations (PSVs), Rubiaceae
Introduction
With over 13,000 species belonging to 611 genera and 65 tribes, Rubiaceae, commonly known as coffee, madder, or bedstraw family, is the fourth-largest angiosperm family after Asteraceae, Orchidaceae and Fabaceae [1]. Rubiaceae is cosmopolitan, occupies all the tropical and subtropical ecosystems [2]. The family occupies wide range of ecological niches from moist evergreen tropical lowland forests to deserts with altitudes 4000 m above sea level [3]. Also, the family is mostly characterized as tropical trees/shrubs and rarely annual/perennial herbs [4]; hence, often referred to as one of the most biomass-rich and species-rich woody plant families [3]. Despite the eminence of previous studies on the taxonomy of Rubiaceae, still the number of traditional subfamilies that should be recognized is yet to be fully substantiated [5, 6]. However, the recent classification [6] has suggested three subfamilies Rubioideae, Cinchonoideae, and Ixoroideae.
Subfamily Ixoroideae, the largest subfamily has the highest species richness which contain ca. 4000 species in 189 genera and 26 recognized tribes [7, 8]. The Ixoroideae is morphologically characterized by secondary pollen presentation, entire interpetiolar stipules, distorted aestivation, and various succulent fruits [9]. It also includes many well-known economically important genera used for horticultural e.g., Gardenia J.Ellis and Ixora L. [10] and medicine—e.g., Ixora., Gardenia and beverages Coffea L. [11]. Broadly, two informal alliances are recognized within the Ixoroideae: the Vanguerieae alliance (8 tribes, > 37 genera and ca. 1200 species) and the Coffeeae alliance (10 tribes, > 90 genera and > 2000 species) [7, 8]. The Coffeeae alliance is an important radiation of the subfamily Ixoroideae and previous taxonomic classification was based on morphological features such as secondary pollen presentation, total interpetiolar stipules, and contorted aestivation [5, 6]. Fruit characters, number of seeds per carpel, and the number of ovules are of low taxonomic use because of high variations within the group [10]. Interestingly, handful of previous studies have made efforts to delimit the species and clarify the phylogenetic relationships using morphological and molecular data at different taxonomic levels [5, 6].
Previous molecular phylogenetic studies based on plastid (matK, ndhF, rbcL, rps16 intron, trnS-G, trnT-F, rbcL, and atpB-rbcL) and nuclear DNA data (nrITS) have shed light on the evolutionary circumstance of the Coffeeae alliance [7, 8]. In most reports, some tribes, such as Airospermeae, Alberteae, Augusteae, Bertiereae, Coffeeae, Octotropideae, and Pavetteae, have been described as monophyletic while the polyphyletic status of tribe Gardenieae remained obscured. Additionally, the clade support was generally low indicating many unknown relationships and internal resolution. Furthermore, several genera such as Burchellia R.Br., Didymosalpinx Keay, Mantalania Capuron ex J.-F.Lero and Monosalpinx N.Hallé nested within the tribe failed to appear to be related to any of the described tribes [7, 8]. In addition, Previous studies have also described multiple genera relative to the tribe Gardenieae e.g., such as Adenorandia Vermoesen, Aidiopsis Tirveng, Alleizettella Pit, Fosbergia Tirveng & Sastre, Himalrandia T. Yamaz, Pseudaidia Tirveng, Pseudomantalania J.-F.Leroy, and Sulitia Merr. Hence, urgent taxonomic reassessment via molecular phylogeny is required to understand the pattern of evolutionary relationships [5, 8]. On the other hand, in the study by Mouly et al., a revised tribal description is given for the Gardenieae by using plastid loci. The Alibertia and Sherbournia groups are recognized at the tribal level as extended Cordiereae and Sherbournieae [8]. Therefore, further studies are needed to confirm the two tribes. Overall, molecular phylogenetic studies of the Coffeeae alliance using few markers (plastid regions and nrITS) have often failed to resolve the phylogenetic relationships at tribal/generic levels and yield a well-supported tree [7, 8, 10].
In recent years, the emergence of Next Generation Sequencing (NGS) technology has improved the availability of genome data, including the plastome, to address phylogenetic relationships and understand the potential roles of the plastome structural variations (PSVs) in different plant lineages. Plastome has a quadripartite structure consisting of a large single copy (LSC) region, a small single copy (SSC) region, and a pair of inverted repeat (IR) regions [12, 13]. The PSVs, including the IR contraction and expansion, gene loss and duplication, pseudogenization, and inversions have been substantiated in many angiosperm families, e.g., Geraniaceae [14], Leguminosae [15, 16], and Rubiaceae [1, 11, 17]. A recent study has explored the PSVs within the Coffeeae alliance tribes of subfamily Ixoroideae and revealed frequent inversions, gene duplications, gene loss/pseudogenization, IR expansion/contraction, and loss of one IR in the plastomes [11]. Also, previous phylogenetic studies using few plastid loci, nuclear data and a limited number of species could not fully substantiate the tribe-level phylogenetic relationships of the Coffeeae alliance tribes [1, 11, 17]. For instance, the sister relationships of the tribes Cordierinae and Octotropideae, as well as that of the tribes Gardenieae with Pavetteae and Sherbournieae were unresolved. Thus, studying further plastomes of the representative species can provide deep insights into the PSVs alongside their phylogenetic implications and clarify the prominent phylogenetic relationships of the Coffeeae alliance.
In this study, 71 (40 newly sequenced and assembled and the remainder from the Gene bank) plastome data. We aim to explore the PSVs and reconstruct the phylogenetic tree of the Coffeeae alliance using four data matrices: coding sequences (CDs), noncoding sequences (NCDs), protein-coding genes (PCGs), and complete plastome (CP) sequences. The following key challenges were systematically explored: (1) investigation of structural variation and screening of promising marker(s) from the plastome data to provide a way forward.; (2) Identification of PSV traits and taxonomic implications; (3) Establishment of a well-supported phylogenetic framework from the partitioning schemes of the plastome data, (4) Examination of the relationships of the Coffeeae alliance tribe and comparison with previous gene fragment studies of plastid phylogenomics. The results of this study will provide insights into the plastome evolution and phylogenomics of the Coffeeae alliance tribes.
Results
Plastome Organization of the Coffeeae alliance tribes
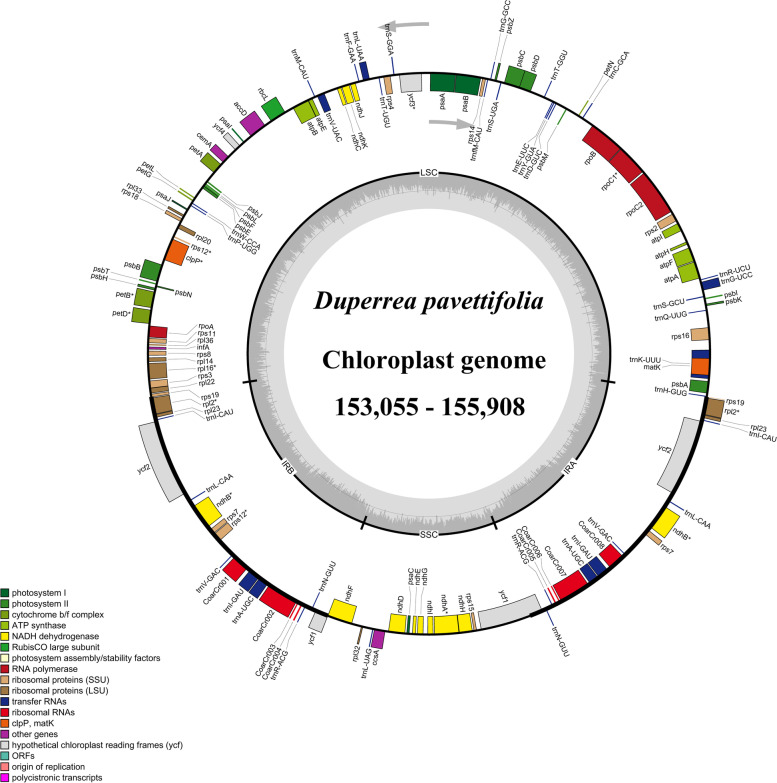
Mean coverage of the newly sequenced plastome ranged between 165.7 × (Pavetta barbertonensis Bremek. of Pavetteae) and 635 × (Rubovietnamia aristata Tirveng. of Gardenieae) (Table S1). The Plastomes of species from the Coffeeae alliance tribes exhibited a typical quadripartite structure (Fig. 1) except for Feretia aeruginescens Stapf of Octotropideae, in which one of the IRs is absent (Fig. S1). The plastome lengths varied from 129,434 bp in Feretia aeruginescens to 155,908 bp in Rothmannia manganjae (Hiern) Keay in Gardenieae. Likewise, there were apparent variations in the size of the plastome regions (Table S2); LSC from 83,586 bp (Pavetta abyssinica Frese, Pavetteae) to 85,556 bp (Diplospora dubia (Lindl.) Masam, Gardenieae), SSC from 15,216 bp (Diplospora dubia, Gardenieae) to 18,229 bp (Fosbergia shweliensis (J. Anthony) Tirveng. & Sastre, Gardenieae), and IR from 25,138 bp (Bertiera breviflora Hiern, Bertiereae) to 25,937 bp (Feretia aeruginescens).
Fig. 1.
Gene map of Duperrea pavettifolia plastome, representative species from the Coffeeae alliance clade. Genes inside the circles are transcribed clockwise, while genes outside the circles are transcribed counter-clockwise. The light grey inner circle and the dark grey correspond to the AT and GC content, respectively. Genes belonging to different functional groups are shown with different colors
Additionally, we observed a marginal difference in the GC content within the range of 36.1% (Feretia aeruginescens) to 37.8% (Rubovietnamia aristata) (Table S2). Typically, the observed plastomes contain 131 genes, 84 CDS, 37 tRNA, and 4rRNA, Exon 33, and Intron 15, but in the plastome of Feretia aeruginescens, 114 genes, 79 CDS, 37 tRNA, and 4 rRNA, Exon 30 and Intron 12 were detected (Table S2). Among the 11 genes with introns detected in our plastomes analyses, five had double introns (clpP, ndhF, rpl2, rps12, and ycf3), while six had single intron (ndhA, ndhB, petB, petD, rpl16, and rpoC1) (Table 1). In addition, the rps12 gene trans-spliced where one intron was shared between two exons, which was duplicated in the IR region. Also, the pseudogenization of ycf1 and rps19 genes was observed (Fig. S1).
Table 1.
Plastome content of Coffeeae alliance tribes
| Gene group | Gene |
|---|---|
| Subunit of acetyl-CoA | accD |
| Large subunit of ribosomal proteins | rpl2+,*, rpl14, rpl16+,#, rpl20, rpl23*, rpl32, rpl33, rpl36 |
| Small subunit of ribosomal proteins | rps2, rps3, rps4, rps7*, rps8, rps11, rps12++,*, rps14, rps15, rps16, rps18, rps19Ψ,# |
| Ribosomal RNA genes | rrn4.5*, rrn5, rrn16*, rrn23* |
| Transfer RNA genes | trnA-UGC*, trnC-GCA, trnD-GUC, trnE-UUC, trnF-GAA, trnfM-CAU, trnG-GCC, trnG-UCC, trnH-GUG, trnI-CAU*, trnI-GAU*, trnK-UUU, trnL-CAA*, trnL-UAA, trnL-UAG, trnM-CAU, trnN-GUU*, trnP-UGG, trnQ-UUG, trnR-ACG*, trnR-UCU, trnS-GCU, trnS-GGA, trnS-UGA, trnT-GGU, trnT-UGU, trnV-GAC*, trnV-UAC, trnW-CCA, trnY-GUA |
| DNA-dependent RNA polymerase | rpoA, rpoB, rpoC1+, rpoC2 |
| Photosystem I | psaA, psaB, psaC, psaI, psaJ |
| Photosystem II | petA, petB+, petD+, petG, petL, petN |
| Cytochrome b/f complex | psbA, psbB, psbC, psbD, psbE, psbF, psbH, psbI, psbJ, psbK, psbL, psbM, psbN, psbT, psbZ |
| ATP synthase | atpA, atpB, atpE, atpF, atpH, atpI |
| Maturase K | matK |
| Envelope membrane proteins | cemA |
| subunit of acetyl-CoA | accD |
| RubisCo large subunit | rbcL |
| Hypothetical reading frames | ycf1Ψ,#, ycf2*, ycf3++, ycf4 |
| Protease | clpP++ |
| NADH dehydrogenase | ndhA+, ndhB+,*, ndhC, ndhD, ndhE, ndhF++, ndhG, ndhH, ndhI, ndhJ, ndhK |
| c-type cytochrome | ccsA |
Note: +, single intron; ++, double introns; *, gene in the IR; Ψpseudogenes; #, duplicated
Plastome structural variations in the Coffeeae alliance tribes
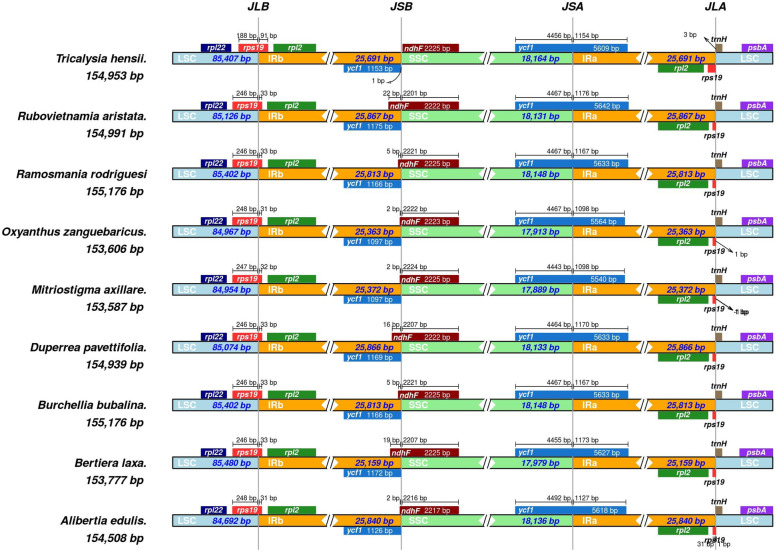
Extensive IR expansion and contraction were recorded among the Coffee alliance tribes (Fig. 2, Fig. S1). The LSC/IRB (JSB) junction, which is located within the rps19 gene led to the gene duplication in the IRA region with lengths extending from 27 bp in Diplospora dubia to 95 bp in Coffea canephora Pierre ex A.Froehner. Also, the observed IRB gene duplication of SSC/IRA (JSA) junction largely lies within the ycf1 gene ranging from 987 bp in Leptactina leopoldi Buettner to 1199 bp in Tarenna drummondii Bridson. Mainly, the SSC/IRB junction (JSB) is located between the duplicated Ψycf1 (in the IR) and ndhF genes (in the SSC) except in few species, where the IRB is partially expanded into the ndhF gene in the IR (1 to 22 bp) (Fig. 2). The LSC/IRA junction (JLA) was located between duplicated IRA boundary of Ψrps19 (in the IR) and trnH-GUG genes (in the LSC). However, the location of the trnH-GUG gene varied at the JLA border in different lengths (1 to 3 bp). No significant gene rearrangements were observed in the studied plastomes.
Fig. 2.
Observed negligible ndhF genes shift of IRB/SSC boundaries of representative chloroplast genomes of the Coffeeae alliance tribes. The IR, LSC, and IR border regions of chloroplast genomes for Coffeeae alliance tribe are indicated. Numbers below and above the gene indicate a gap between gene ends and boundary site in base lengths (bp). The genes are denoted by colored boxes
Identification of sequence divergence
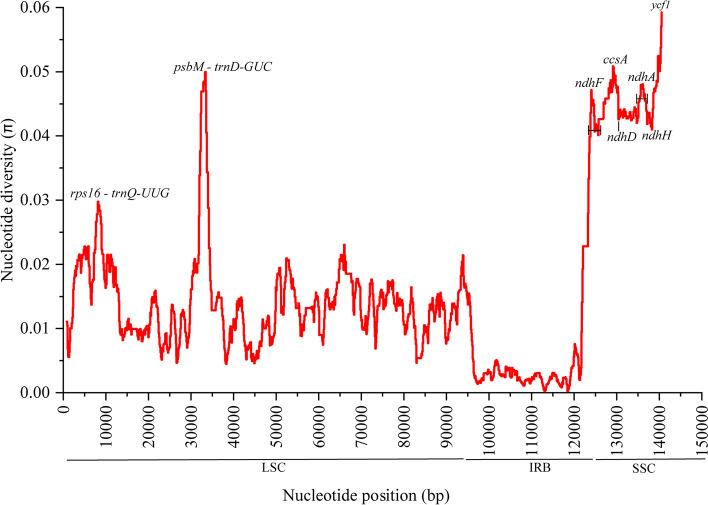
It is noteworthy that the mutation and parsimony-informative site (PIS) were different in the observed plastome. A relatively large number of variable regions (mutation hotspots) were identified, where a high rate of nucleotide substitutions may occur. These include two intragenic regions and six gene regions with an informative parsimony site between 0.02977 (rps16 - trnQ-UUG) and 0.05927 (ycf1) (Fig. 3). In addition, the informative sites in the IR region was lower than the SC regions (Fig. 3).
Fig. 3.
Sliding-window analysis of nucleotide variability (Pi) across the sampled 62 plastome sequences of the Coffeeae alliance including the single copy of IR
Codon usage and repeat analyses in the tribes of the Coffeeae alliance
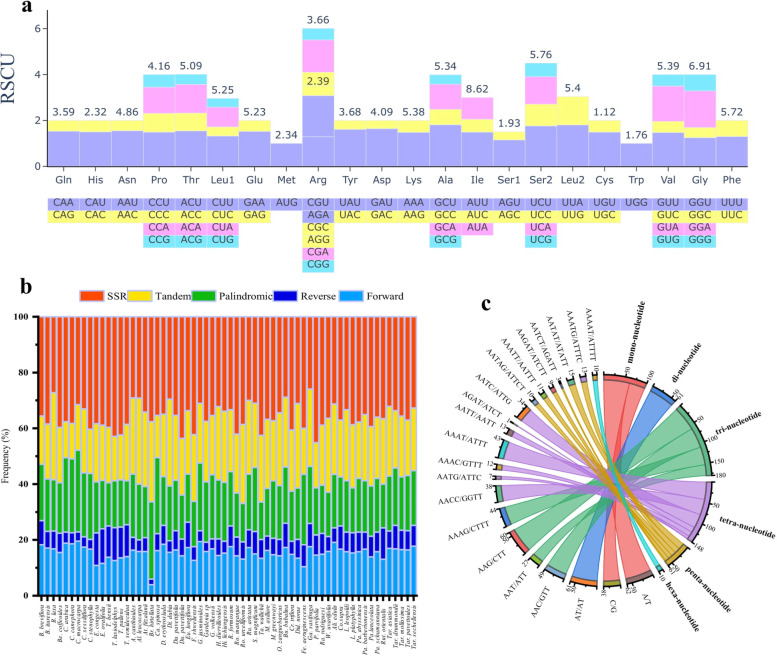
Codon usage and amino acid abundances in the tribes of the Coffeeae alliance were similar (Fig. 4a, Fig. S2). The total sequence of encoding protein genes ranged between 74,682 bp in Empogona ovalifolia (Coffeeae) to 79,249 bp in Rothmannia manganjae (Gardenieae). For the protein-coding genes, codons ranged from 22,573 bp in Feretia aeruginescens (Octotropideae) to 26,304 bp in Atractocarpus fitzalanii (F.Muell.) Puttock (Gardenieae) (Fig. S2). The most abundant amino acid, isoleucine Codon usage, ranged from 5.8 to 8.75% and the least abundant amino acid, cysteine Codon usage, averaged from 1.1 to 1.17% in Coffeeae alliance tribes (Fig. S2). The majority of amino acid codons have a bias, although codons AU(T)G and U(T)GG, which encode methionine (Met) and tryptophan (Trp) respectively, both show no codon preferences (RSCU = 1.00). Additionally, most types of preferred synonymous codons (RSCU > 1.00) possessed C- or U-ending codons (Fig. S2). The percentage Effective Number of Codon (Enc) and Codon Bias Index (CBI) ranged from 55.01 to 55.24% and 0.20 to 0.25%, respectively (Table S5).
Fig. 4.
Codon and repeat analyses of the 62 sampled plastomes from the Coffeeae alliance. a Codon usage biases of the plastome of Alibertia edulis, a representative species from the Coffeeae alliance clade. The values at the top of each stack indicate the frequency of use of each amino acid, while the bars (colour-coded) represent the relative values of synonymous codon usage for each codon. b The percentage distribution of five types of repeats. c Chord diagram showing the number and type of SSRs
We recorded five repeat types within the studied plastomes and their percentage distribution was strikingly different (Table S4). We recorded five repeat types within the studied plastomes and their percentage distribution was strikingly different Forward repeats from 4 to 20%, Reverse repeats from 2 to 12%, Palindromic repeats from 13 to 29%, Tandem repeats from 15 to 31% and SSR repeats ranging from 29 to 45% (Table S4). In addition, of the total frequency of the repeats in the sampled group, the Dispersed repeats accounted for approximately 19% (of which 19% were palindromic, 15% Forward, and 7% Reverse), Tandem repeats for 23%, and SSR for 36% (Fig. 4b).
Microsatellite analyses of the Coffeeae alliance tribes identified a total of 2588 SSRs (Table S4). Our result showed a variable distribution within the tribe ranging from 60 in Himalrandia lichiangensis (W.W.Sm.) Tirveng. (Gardenieae) to 28 in Polyshpaeria parvifolia (Octotropideae) (Fig. 4b). Of the six SSR motif types, penta-nucleotide (n = 7) was the most common while only one hexa-nucleotide was recorded. The analyses also revealed that the mononucleotide (A/T) motif was present in all the plastomes contrasting the tetra-nucleotide (AGAT/ATCT) motif that was only found in Mitriostigma axillare Hochst. (Fig. 4c). The A/T motifs accounted for more than 19%, and the other motif types occurred in less than 10% (Fig. 4c). In addition, mononucleotide repeats accounted for (71%) and hexanucleotides were the least common (Table S4).
Phylogenetic relationships of the Coffeeae alliance tribes
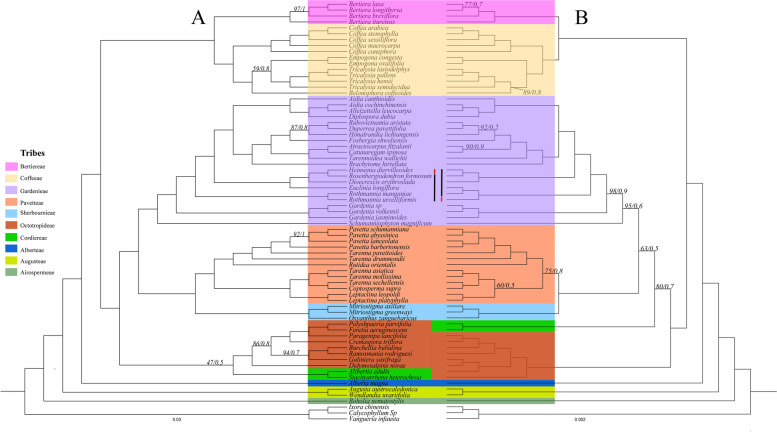
The phylogenies inferred from four data matrices using two tree methods (ML and BI) yielded nearly similar topologies. Our plastid phylogenomic analyses supported the monophyly relationships of the Coffeeae alliance tribes and resolved relationships of all the deep nodes, with the strong support values (ML =100%, BI =1.0, Fig. 5). However, few subclades, namely the tribes Cordiereae + Octotropideae (ML =47%, BI = 0.5, Fig. 5a) and Belonophora — Empogona of the tribe Coffeeae (ML =59%, BI =0.8, Fig. 5a) are weakly supported. Also, the analyses presented well-supported sister relationships of the tribe Airospermaeae to the remaining Coffeeae alliance tribes. The tribe Augusteae formed a sister relationship with the tribe Albertieae, as a sister tribe to the lineages consisting of tribes Octotropideae and Cordiereae.
Fig. 5.
The reconstructed phylogenetic tree of the Coffeeae alliance tribes using the plastome data. a CP and NCDS. b CDS and PG. Colored boxes indicate tribe classification. The thick black line represents the topological differences within the tribe Gardenieae. Support values (BS/PP) either < 100 or < 1 are shown above the branches
The tribe Cordiereae has different phylogenetic positions within the entire clade of the Coffeeae alliance from the data matrices, especially the data from the CDs and NCDs compared to other data matrices (Fig. 5). Within the Coffeeae alliance clade, the phylogenetic position of the tribe Octotropideae in the CP and NCDs trees with weak support values was attributed to a sister relationship with the Corderieae (Fig. 5a), whereas the monophyletic relationship of this tribe is well-supported in the trees reconstructed from the PG and CDs (Fig. 5b). The analyses supported the sister relationships of the tribes Sherbournieae-Pavetteae clade, sister to the monophyletic tribe Gardenieae with high support values (ML = 100%/BI = 1 Fig. 5b). In addition, our analyses strongly supported the relationships of the tribes Coffeeae and Berticereae. Our data further revealed the topological differences at genera and species levels, which can easily be observed from Heinsenia diervilleoides K.Schum. located in different positions according to the molecular data matrices (Fig. 5).
Discussion
Evolutionary pattern of PSVs in the Coffeeae alliance tribes
Plastomes structural variations in the subfamily Ixoroideae have previously been documented [11]. Moreover our comprehensive sampling of the plastomes from the Coffeeae alliance tribe further enhances the understanding of its plastome evolutionary history. From our analyses, no gene loss was detected hence; there are marginal differences between the sizes of studied plastomes in the Coffeeae alliance tribe. Contrary to our results, loss of the trnH-GUG gene has been reported in Tarenna grevei (Drake) Homolle of the subfamily Ixoroideae [11]. Previous reports have revealed the potential implications of gene loss (single or multiple) in the plastome size variations. It has been suggested in previous studies that gene losses in the plastomes are attributed to the rate of sequence evolution, gene transfer into the nucleus, or substitution of a plastid gene product with a nuclear-encoded protein [18, 19].
In our study, we suspected two pseudogenes (rps19 and ycf1), based on the presence of premature stop codons and their short length. This is synapomorphy to the Coffeeae alliance tribes (Fig. S1). The observation is consistent with the previous studies where the pseudogenization of rps19 and ycf1 genes have been detected in many angiosperms [11, 20–22]. Pseudogenization is an evolutionary process in plastid genomes, which might occur during gene transfer to the nucleus, substitution, and nuclear-encoded protein for a plastid gene product source of DNA barcodes [23–25]. Our observation on the trans-spliced rps12 gene (exon 1 and exons 2) is congruent with the previous report [26]. During genetic evolution events, it has been proposed that exons, which are the gene fragments encoding for protein structural domains are impacted by reorganization into other genes [27]. Additionally, as a result of RNA splicing for rps12 exon 1 with transcripts from other genes, and polypeptide variations in the plastome have been reported [27]. Therefore, it could be suggested that similar events can explain the trans-splicing events of rps12 gene in the plastomes members of the Coffeeae alliance tribes.
The observed IR loss and occurrences of multiple IR contraction/expansion in the studied plastomes agrees with a previous study of the subfamily Ixoroideae [11]. In our investigation, IR contraction/expansion varies at the boundaries, and this character is synapomorphy of the Coffeeae alliance (Fig. S1). For instance, the loss of one IRs or expansion in Feretia aeruginescens species resulted in a larger IR size. The evolution of plant lineages is a dynamic process IR contraction/expansion at borders is the lineage with plastome genomes and plays an essential role in revealing the evolution events [28–30]. The terminal IR gene adjacent to the SSC region is highly conserved, however, there were variations at the IR/LSC boundary [31–33]. The rate of gene conversions during cell division/evolution and high concentration of short repeats (AT-rich) are the widely accepted explanation of the IR boundary shifts among several angiosperm lineages [34, 35]. In our study, the ndhF gene independently shifts at IRB/SSC border of the Coffeeae alliance plastomes (Fig. 2). Other previous studies have also revealed that unique boundary shifts of the IR and SSC regions are correlated with the transformations of ndhF and ycf1 genes [35–37]. The IR boundary shifts are widely accepted mechanisms for the plastomes rearrangements, which contributes to the structural diversification in plastomes [35].
Plastome sequencing has been used to infer relationships at all taxonomic levels, for example, to reveal relationships at the lowest level in land plants [36]. Two plastid protein coding genes, rbcL and matK, and the psbA-trnH intergenic spacer, have been recommended as universal plastid DNA barcodes for land plants [37]. Seven promising plastid DNA barcode (matK, accD, rps19, ndhF, ndhD, ccsA and ycf1) have been consistently consider the most variable regions for different plant [38–41]. It was important to determine whether these loci could be used as potential molecular markers to establish relationships between species and populations of Coffeeae allinese tribes. Our results showed that the IRs had negligible sequence divergence compared to the SC regions, which also occurred in higher plants and possibly due to copy correction between IR sequences by gene conversion [42] .
Codon usage has shown that plastomes evolve due to a high frequency of privileged mutations in the coding sequences in the evolution of species [43]. The RSCU values are affected by the mutation factor that affects the codon usage pattern. Thus, Relative synonymous codon usage with value < 1 indicates that codons are used less frequently, > 1 represents codons that are used more frequently, and value =1 indicates no bias [44]. We found identical trend in the pattern in which the amino acids were encoded for the 62 species (Fig. S2). The most abundant amino acid, isoleucine, and the trend are consistent with other angiosperm plastomes, where leucine and isoleucine were the most abundant codons [43, 45]. The Effective Number of Codons (ENc), which ranges from 20 (one codon per amino acid) to 61 (equal use of synonymous codons) [46], and the Codon Bias Index (CBI), which ranges from 0, no bias, to 1, equal use of all synonymous codons [47]. In our study, the values for ENc (55 to 55.2) and CBI (0.20 to 0.251) differed insignificantly among species of Coffeeae alliance consistence with other report [48, 49].
The high degree of polymorphism and genome-wide distribution, such as significant variation in motifs and with several repeats, can be studied using SSRs analyses. Especially in determining genetic diversity and population genetic issues such as gene flow, ancestry, and population structure [50]. From the comparative analysis of five repeats in the Coffeeae alliance tribe, SSRs are predominantly mononucleotide (A/T) motifs. A similar trend of SSR distribution was also confirmed in the chloroplast genome of other angiosperms [43]. Other downstream analyses, repeats in a system with various rearrangements, deletions, additions, and large inversions within the cp genome are well known [51]. The percentage distributions of Tandem repeats were abundant from our analyses (Fig. 4b). Several studies have shown that a high number of repeats positively correlates with the PSV or plastome size of angiosperms. Noteworthy, a high number of Tandem repeats are important for structural reorganization of the plastome and a wide range of IR expansions/contractions [52].
Phylogenetic relationship of Coffeeae alliance tribes
Our results confirm that ML and BI analyses with multiple genes partitioned models (CDs, NCDs, PG, and CP) could resolve the phylogeny relationships of the Coffeeae alliance tribes (Fig. 5). The most recent phylogenetic studies have reported improved phylogenetic resolutions of the Coffeeae alliance, but with only a limited number of representatives [1, 11, 17]. Again, our study punctuated the importance high taxonomic sampling for phylogenetic relationships as we included more than half of the total genus to achieve deep phylogenetic resolution of the Coffeeae alliance tribes. The phylogenetic relationships among key lineages, genera, and tribes were resolved with strong support values (Fig. 5) and are consistent with previous studies [8, 11, 17, 53]. Our multi-locus analyses showed that the monophyletic Airospermeae is the first diverged lineage followed by Augusteae, Alberteae, Cordiereae, Octotropideae, Sherbournieae, Pavetteae, Gardenieae, Coffeeae, and Bertiereae (Fig. 5) coinciding with recent findings [1]. Our phylogenetic analyses for all the datasets agree with previous analysis based using CDS, and combined CDS + non CDS on the phylogenetic position of the tribe Augusteae (ML = 100%/BI = 1) with full support non CDS [1].
Despite the conflicting phylogenetic positions of the tribes Cordiereae and Octotropideae (Fig. 5), the sister relationship of both tribes is largely consistent with the recent phylogenetic analyses inferred based on the plastome data [1]. Herein, we revealed the tribes Pavetteae, Sherbournieae, and Gardenieae have monophyletic relationships, including most of the genera within the tribes with the highest support values (ML = 100%/BI = 1). In previous studies, analyses of CDS, combined with CDS + non CDS resulted in different topologies for the tribe Gardenieae, Sherbournieae and Pavetteae. In addition, the use of plastid data in the tribe Gardenieae poorly supported sister relationship with the tribes Sherbournieae and Pavetteae. However, the tribe Pavetteae showed an unsupported sister relationship to Sherbournieae, which contrasts with the tribes Sherbournieae and Gardenieae, that revealed polyphyletic based on plastid and nuclear data [1, 11, 17]. Our phylogenomic analyses have shown that the relationship of the tribe Pavetteae and tribe Sherbournieae is strongly supported (Fig. 5). Besides, this study strongly supports the sister relationship between the tribes Coffeeae and Berticereae, which agrees with the other studies [1, 11, 17, 54].
Previous studies using the morphological characters of the genera (Alleizettella Pit, Fosbergia Tirveng & Sastre and Himalrandia T. Yamaz) have a limited role in the classification of the tribe Gardenieae [8, 55]. Our phylogenomic analyses reveal a deeper phylogenetic resolution of the Coffeeae alliance tribe. We confirmed the phylogenetic placement of Fosbergia shweliensis, Himalrandia lichiangensis, and Duperrea pavettifolia (Kurz) Pit. Within the monophyletic tribe Gardenieae with strong support (ML = 87%/BI = 0.8; Fig. 5). Interestingly, the presence of morphological features such as terminal inflorescences and absent of Raphides seems to further reinforce the relationships of Duperrea pavettifolia and tribe Gardenieae, [8, 55]. In addition, previous phylogenetic studies of the Coffeeae alliance exploiting several plastid and nuclear markers have failed to resolve the relationships between several genera (e.g., Burchellia, Didymosalpinx, and Galiniera Delile) and their phylogenetic status with members of the tribes Sherbournieae and Cordiereae [5, 8]. Our finding moderately supported the close relationships of these genera with the tribe Octotropideae whereas the basal lineage, Didymosalpinx norae (Swynn.) Keay formed a sister relationship with the remaining genera of the tribes (Fig. 5). Thus, our study provides an improved understanding of the phylogenetic relationships in the Coffeeae alliance. Also, the study shows the potential of plastomes in resolving intertribal and intergeneric relationships within the Coffeeae alliance tribes. However, further phylogenetic studies of the tribes Cordiereae and Sherbournieae, perhaps integrating additional molecular data and morphological traits would fully clarify the evolutionary relationships of the Coffeeae alliance clade.
Conclusion
The study detected several PSVs which have occurred independently across the lineages but without clear taxonomic and phylogenetic implications. Rather, they played an important role in the structural restructuring of the plastome in the Coffeeae alliance tribe. Furthermore, we screened promising molecular markers in both the intragenic and coding regions which might be suitable for future phylogenetic studies for the Coffeeae alliance clade. We utilized the plastome data to present a well-resolved phylogenetic tree at the tribal and generic levels for the Coffeeae alliance tribes. Thus, the results obtained provide significant insight into the PSVs and phylogenetic relationships of the Coffeeae alliance and the Rubiaceae family and open a way for robust phylogenetic studies in future.
Materials and methods
Taxon sampling, DNA extraction, and genome sequencing
A total of 71 plastomes were used for this study, including 68 sequences from the alliance group of Coffeeae alliances (representing 44 genera), and three outgroups. Of these, 31 plastomes were obtained from GenBank (www.ncbi.nlm.nih.gov/genbank) and the remaining 40 were newly generated for this study (Table S1). For the newly sequenced taxa, total genomic DNA was extracted from silica-gel dried leaves using plant genomic DNA kit based on the manufacturer’s instructions. The genome skimming technique was used to obtain the plastome data using fragmented gDNA data; the library size 350 bp inserts were selected. Sequencing with 2 × 150-bp paired-end (PE) read was performed on the Illumina Hiseq 2500 platform at the Novogene Company (Beijing, China).
Plastomes assembly and annotation
The clean-up Raw and quality checks were performed using the NGS QC Tool Kit [56]. De novo assembly of the cleaned reads was performed in GetOrganelle with K-mer values of 21, 45, 65, 85 and 105 GetOrganelle, using the plastome of Coffea arabica L. (NC008535) as reference [57]. Subsequently, the assembled reads were visualized and filtered in Bandage v.0.80 [58] to generate a complete plastome. For incomplete plastomes, we filled the gaps between contigs with consensus sequences of raw reads mapped initially to the reference plastome to obtain the complete plastome. The number of mapped paired-end (PE) reads and the depth of coverage was determined by mapping the paired reads against the plastome using Bowtie2 [59] The locations of single copies (SC) and IR boundaries in the resequenced plastomes were determined using in Geneious v. 8.1.4 [60]. The find repeat function in Geneious was used to flank the IR regions. Then, the paired reads were reassigned to the assembled plastomes to validate the SC /IR regions with Bowtie2 [59]. Finally, we visualized the read stacks of the reassembled plastomes and compared the labeled SC/IR boundaries in Geneious. The new plastomes were annotated using the Dual Organellar Genome Annotator web interface (DOGMA) [61]. We manually checked the consistency of start/stop codons and intron/exon boundaries in Geneious. The Find ORFs function in Geneious was used to confirm protein-coding gene (PCG) annotations, while the tRNAscan-SE web service was used to determine tRNA genes [62]. The OrganellarGenomeDRAW web server was used to produce circular maps for the newly sequenced plastomes [63]. We also deposited the 40 newly assembled plastome sequences from the Coffeeae alliance and outgroup in GenBank (Table S1).
Plastome structural analyses
To investigate the patterns of plastome genomic evolution of the Coffeeae alliance clade, 62 annotated plastomes were compared and the length (bp) of the plastome and its elements (LSC, SSC, and IR), gene contents (number of genes), and GC contents were summarized (Table S2, Fig. S1). Comparative analyses of the SC/IR boundary shifts at four junctions (JLB-LSC/IRB, JSB-IRB/SSC, JSA-SSC/IRA, and JLA-IRA/LSC) of the sampled plastomes was conducted in Geneious and plotted in IRscope [60, 64]. We investigated the potential rearrangements in gene order (e.g., inversion, IR boundary shifts) for all the sampled species of the Coffeeae alliance using Coffea arabica as the reference. This analysis was performed in Mauve v.2.3.1 [65] using default settings: automatically calculate the seed weight (15) and Locally Collinear Blocks (LCBs) with the minimum LCB score of 30,000 [65].
Sequence divergence, codon usage and repeat analyses
To assess sequence divergence and examine highly variable regions in the plastome, nucleotide diversity (π) was calculated by sliding window analysis using DnaSP v6 [66] with a window size of 1000 bp and a step size of 100 bp. The level of codon usage bias was determined by analysing the Relative Synonymous Codon Usage RSCU [67];, Effective Number of codon ENc [46]; and the Codon Biased Index CBI [47]; for all PCGs, in DnaSP 6.10 [66]. The average RSCU and amino acid frequency values for each species were plotted using R-script (Fig. S2) [68].
We searched for three repeat types, compositions, and distributions within the sampled 62 plastomes from the Coffeeae alliance tribes. Tandem repeat distribution were identified using Tandem Repeats Finder v4.09 [69] with the following settings: alignment parameters match (2), mismatch (7), indels (7), minimum alignment score (80), and maximum period size (500). Disperse repeats (forward, reverse, complementary, and palindromic) were also detected via REPuter [70] with a minimum repeat size of 30 bp and a Hamming distance of 1 (Table S3). The simple sequence repeat (SSRs or microsatellites) motifs in the plastomes were searched using the Perl script using MISA v2.0 [71]. W set the minimum numbers (thresholds) of the SSRs for mono-, di-, tri-, tetra-, penta-, and hexanucleotides, to 10, 6, 5, 5, 5, and 5, respectively (Table S3).
Phylogenetic analyses
Phylogenetic analyses included a total of 71 plastomes data (68 ingroups and 3 outgroups). We performed individual gene alignment to overcome poor alignment challenges from the whole genome and generate different data matrices for the phylogenetic analyses. To achieve these, we extracted coding (CDs) and noncoding (NCDs) regions in Phylosuit v1.1.13 [68], followed by an individual gene alignments using MAFFT v7.450 [72] with LINSI algorithm and concatenation in Geneious [60]. To minimize the systematic error, we excluded NCDs loci with less than 80% taxon occupancy and alignment lengths less than 100 bp. The data matrices were generated to reconstruct the phylogenetic relationships of the Coffeeae alliances: CDs (84 genes), NCDs (122 loci), PG (78 genes), and CP (concatenated CDs and NCDs for all species). Substitution models of the four matrices were determined using Partition Finder v2.1.1 [73], and the best evolutionary models were selected using the Akaike Information Criterion (AIC) (Table S2). The phylogenetic relationships were reconstructed using Maximum Likelihood (ML) and Bayesian Inference (BI) tree methods. The ML analyses were performed in RAxML-HPC2 v.8.1 on XSEDE [74] with the GTRCAT substitution model and 1000 Bootstrap replicates. The BI analyses were inferred using MrBayes v.3.2.6 [75]. We estimating the Bayesian posterior probability (PP) with two independent Markov Chain Monte Carlo (MCMC) runs, via a single cold chain and three hot chains for 10,000,000 generations, while sampling the tree at every 1000 generations. We assessed the MCMC convergence of each parameter for every run based on sufficient effective sample size (ESS ≥200). The first 20% runs were discarded as burn-in using TRACER v.1.7 [76]. The resulting trees (ML and BI) are visualized and edited in Fig tree v.1. 3.1 [77].
Supplementary Information
Additional file 1. The Mauve alignment of the 62 coffeeae alliance plastomes. The colliner blocks rearrangement of the accessible gene at junction of inverted repeat and small single copy. White colored blocks: protein coding gene, Black colored blocks: tRNA genes, Red coloured blocks: Rrna. For interpretation of references to the colors, see the web version of the article.
Additional file 2. The codon usage bias in all the protein-coding genes of the plastome of the 62 Coffeeae allinces tribes.
Additional file 3: Table S1. GenBank accession numbers and coverage of the newly sequenced species from the Coffeeae alliance studied.
Additional file 4: Table S2. Summary of plastomes for 63 species Coffeeae alliance tribes.
Additional file 5: Table S3. Summary of partitioning scheme for the ML tree and BI analysis.
Additional file 6: Table S4. Distributions of SSR in the Coffeeae alliance tribes.
Additional file 7: Table S5. Details of Relative Synonymous Codon Usage in plastome of Coffeeae alliance tribes.
Acknowledgments
We thank Dr. Romain Guyot from University de Montpellier, Colombia, for sharing data, Mr. Bashir Bolaji Timiyu from Wuhan Botanical Garden, CAS, China, Dr. Samaila S. Yaradua from King Abdulaziz University, Saudi Arabia, Dr. Endale Tamiru Basore from Zhejiang University, Hangzhou, Duncan Gichuki from Wuhan Botanical Garden, CAS, China and Vincent Wanga from Wuhan Botanical Garden, CAS, China for technical support during our project.
Authors’ contributions
Zhou Y., Hu G., and Wang Q. conceived the idea and supervised the project. Sara G., Wei N. performed the experiments. Wei N., Wu L., Oyebanji O., Zhou, Y., and Sara G. analyzed the data ands wrote the manuscript. All the authors discussed the results, commented, and revised them. The author(s) read and approved the final manuscript.
Funding
The authors acknowledge the financial support from the National Science Foundation of China (31961143026& 31800176) and the Sino-Africa Joint Research Center, CAS, China (Y323771W07&SAJC201322).
Availability of data and materials
All sequences used in for this study can be found in GenBank; the list of accession can be found in Supplementary Table S1.
Declarations
Ethics approval and consent to participate
Not applicable.
Consent for publication
Not applicable.
Competing interests
The authors declare no competing interests
Footnotes
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Sara Getachew Amenu and Neng Wei contributed equally to this work.
Contributor Information
Yadong Zhou, Email: zhouyd@wbgcas.cn.
Qingfeng Wang, Email: qfwang@wbgcas.cn.
References
- 1.Wikstrom N, Bremer B, Rydin C. Conflicting phylogenetic signals in genomic data of the coffee family (Rubiaceae) J Syst Evol. 2020;58(4):440–460. [Google Scholar]
- 2.Mendoza-Cifuentes H, Celis A, Tovar E, Gonzalez MA. Lintersemina (Rubiaceae: Condamineeae), a new and enigmatic genus from the Magdalena Medio region of Colombia. Phytotaxa. 2020;451(1):1–20. [Google Scholar]
- 3.Davis AP, Govaerts R, Bridson DM, Ruhsam M, Moat J, Brummitt NA, et al. A global assessment of distribution, diversity, endemism, and taxonomic effort in the Rubiaceae. Ann Mo Bot Gard. 2009;96(1):68–78. [Google Scholar]
- 4.Robbrecht E. Tropical woody Rubiaceae. Op Bot Belg. 1988;1(272):599–602. [Google Scholar]
- 5.Robbrecht E, Manen JF. The major evolutionary lineages of the coffee family (Rubiaceae, Angiosperms). Combined analysis (nDNA and cpDNA) to infer the position of Coptosapelta and Luculia, and supertree construction based on rbcL, rps16, trnL-trnF, and atpB-rbcL data. A new classification in two subfamilies, Cinchonoideae and Rubioideae. Syst Geogr Pl. 2006;76:85–146. [Google Scholar]
- 6.Bremer B, Eriksson T. Time tree of Rubiaceae: phylogeny and dating the family, subfamilies, and tribes. Int J Plant Sci. 2009;170(6):766–793. [Google Scholar]
- 7.Kainulainen K, Mouly A, Khodabandeh A, Bremer B. Molecular phylogenetic analysis of the tribe Alberteae (Rubiaceae), with description of a new genus, Razafimandimbisonia. Taxon. 2009;58(3):757–768. [Google Scholar]
- 8.Mouly A, Kainulainen K, Persson C, Davis AP, Wong KM, Razafimandimbison SG, Bremer B, et al. Phylogenetic structure and clade circumscriptions in the Gardenieae complex (Rubiaceae) Taxon. 2014;63(4):801–818. [Google Scholar]
- 9.Puff C, Robbrecht E, Buchner R, De Block P. A survey of secondary pollen presentation in the Rubiaceae. Opera Bot Belg. 1996;7:369–402. [Google Scholar]
- 10.Andreasen K, Bremer B. Combined phylogenetic analysis in the Rubiaceae-Ixoroideae: morphology, nuclear and chloroplast DNA data. Am J Bot. 2000;87(11):1731–1748. [PubMed] [Google Scholar]
- 11.Ly SN, Garavito A, De Block P, Asselman P, Guyeux C, Charr JC, Janssens S, Mouly A, Hamon P, Guyot R, et al. Chloroplast genomes of Rubiaceae: comparative genomics and molecular phylogeny in subfamily Ixoroideae. PLoS One. 2020;15(4):e0232295. doi: 10.1371/journal.pone.0232295. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Yaradua SS, Alzahrani DA. Complete chloroplast genome sequence of Justicia flava: comparative genome analysis and phylogenetic relationships among Acanthaceae. Biomed Res Int. 2019;2019:1–17. doi: 10.1155/2019/4370258. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Alzahrani DA, Albokhari EJ, Yaradua SS, Abba A. Comparative analysis of chloroplast genomes of four medicinal Capparaceae species: genome structures, phylogenetic relationships, and adaptive evolution. Plants. 2021;10(6):1229. doi: 10.3390/plants10061229. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Martin GE, Rousseau-Gueutin M, Cordonnier S, Lima O, Michon-Coudouel S, Naquin D, et al. The first complete chloroplast genome of the Genistoid legume Lupinus luteus: evidence for a novel major lineage-specific rearrangement and new insights regarding plastome evolution in the legume family. Ann Bot. 2014;113(7):1197–1210. doi: 10.1093/aob/mcu050. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Oyebanji O, Zhang R, Chen SY, Yi TS. New insights into the plastome evolution of the Millettioid/Phaseoloid Clade (Papilionoideae, Leguminosae) Front Plant Sci. 2020;11:151. doi: 10.3389/fpls.2020.00151. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Wang YH, Qu XJ, Chen SY, Li DZ, Yi TS. Plastomes of Mimosoideae: structural and size variation, sequence divergence, and phylogenetic implication. Tree Genet Genomes. 2017;13(2):41. [Google Scholar]
- 17.Antonelli A, Clarkson JJ, Kainulainen K, Maurin O, Brewer GE, Davis AP, et al. Settling a family feud: a high-level phylogenomic framework for the Gentianales based on 353 nuclear genes and partial plastomes. Am J Bot. 2021;108:1143–1165. doi: 10.1002/ajb2.1697. [DOI] [PubMed] [Google Scholar]
- 18.Slimp M, Williams LD, Hale H, Johnson MG. On the potential of angiosperms353 for population genomic studies. Appl Plant Sci. 2021;9(7):e11419. doi: 10.1002/aps3.11419. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Chu X, Li S, Wang S, Luo D, Luo H. Gene loss through pseudogenization contributes to the ecological diversification of a generalist Roseobacter lineage. ISME J. 2021;15(2):489–502. doi: 10.1038/s41396-020-00790-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Shrestha B, Gilbert LE, Ruhlman TA, Jansen RK. Rampant nuclear transfer and substitutions of plastid genes in Passiflora. Genome Biol Evol. 2020;12(8):1313–1329. doi: 10.1093/gbe/evaa123. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Qian J, Song J, Gao H, Zhu Y, Xu J, Pang X, et al. The complete chloroplast genome sequence of the medicinal plant Salvia miltiorrhiza. PloSOne. 2013;8:e57607. doi: 10.1371/journal.pone.0057607. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Curci PL, De Paola D, Danzi D, Vendramin GG, Sonnante G. Complete chloroplast genome of the multifunctional crop globe artichoke and comparison with other Asteraceae. PloSOne. 2015;10:e0120589. doi: 10.1371/journal.pone.0120589. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Menezes AP, Resende-Moreira LC, Buzatti RS, Nazareno AG, Carlsen M, Lobo FP, Kalapothakis et al. Chloroplast genomes of Byrsonima species (Malpighiaceae): comparative analysis and screening of high divergence sequences. Sci Rep 2018;8:1–12. [DOI] [PMC free article] [PubMed]
- 24.Jansen RK, Ruhlman TA. Plastid genomes of seed plants. In: Bock R, Knoop V, editors. Genomics of chloroplasts and mitochondria. Dordrecht: Springer Netherlands: Springer; 2012. pp. 103–126. [Google Scholar]
- 25.Williams AV, Boykin LM, Howell KA, Nevill PG, Small I. The complete sequence of the Acacia ligulata chloroplast genome reveals a highly divergent clpP1 gene. PloSOne. 2015;10(9):e0138367. doi: 10.1371/journal.pone.0138367. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Hildebrand M, Hallick RB, Passavant CW, Bourque DP. Trans-splicing in chloroplasts: the rps12 loci of Nicotianatabacum. Proc Natl Acad Sci. 1988;85(2):371–376. doi: 10.1073/pnas.85.2.372. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Sharp PA. On the origin of RNA splicing and introns. Cell. 1985;42:397–400. doi: 10.1016/0092-8674(85)90092-3. [DOI] [PubMed] [Google Scholar]
- 28.Zhu A, Guo W, Gupta S, Fan W, Mower JP. Evolutionary dynamics of the plastid inverted repeat: the effects of expansion, contraction, and loss on substitution rates. New Phytol. 2016;209(4):1747–1756. doi: 10.1111/nph.13743. [DOI] [PubMed] [Google Scholar]
- 29.Raubeson LA, Peery R, Chumley TW, Dziubek C, Fourcade HM, Boore JL, Jansen RK, et al. Comparative chloroplast genomics: analyses including new sequences from the angiosperms Nuphar advena and Ranunculus macranthus. BMC Genomics. 2007;8(1):1–27. doi: 10.1186/1471-2164-8-174. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Wei N, Pérez-Escobar OA, Musili PM, Huang WC, Yang JB, Hu AQ, et al. Plastome evolution in the hyperdiverse genus Euphorbia (Euphorbiaceae) using phylogenomic and comparative analyses: large-scale expansion and contraction of the inverted repeat region. Front Plant Sci. 2020;1555. [DOI] [PMC free article] [PubMed]
- 31.Wu C-S, Lai YT, Lin CP, Wang YN, Chaw SM. Evolution of reduced and compact chloroplast genomes (cpDNAs) in gnetophytes: selection toward a lower-cost strategy. Mol Phylogenet Evol. 2009;52(1):115–124. doi: 10.1016/j.ympev.2008.12.026. [DOI] [PubMed] [Google Scholar]
- 32.Plunkett GM, Downie SR. Expansion and contraction of the chloroplast inverted repeat in Apiaceae subfamily Apioideae. Syst Bot. 2000;25(4):648–667. [Google Scholar]
- 33.Guisinger MM, Kuehl JV, Boore JL, Jansen RK. Extreme reconfiguration of plastid genomes in the angiosperm family Geraniaceae: rearrangements, repeats, and codon usage. Mol Biol Evol. 2011;28(4):1543. doi: 10.1093/molbev/msq229. [DOI] [PubMed] [Google Scholar]
- 34.Zhang SD, Jin JJ, Chen SY, Chase MW, Soltis DE, Li HT, Yang JB, Li DZ, Yi TS, et al. Diversification of Rosaceae since the late cretaceous based on plastid phylogenomics. New Phytol. 2017;214(3):1355–1367. doi: 10.1111/nph.14461. [DOI] [PubMed] [Google Scholar]
- 35.Dugas DV, Hernandez D, Koenen EJ, Schwarz E, Straub S, Hughes CE, Jansen RK, Nageswara-Rao M, Staats M, Trujillo JT, et al. Mimosoid legume plastome evolution: IR expansion, tandem repeat expansions and accelerated rate of evolution in clpP. Sci Rep. 2015;5(1):1–13. doi: 10.1038/srep16958. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Shaw J, Shafer HL, Leonard OR, Kovach MJ, Schorr M, Morris AB. Chloroplast DNA sequence utility for the lowest phylogenetic and phylogeographic inferences in angiosperms: the tortoise and the hare IV. Am J Bot. 2014;101(11):1987–2004. doi: 10.3732/ajb.1400398. [DOI] [PubMed] [Google Scholar]
- 37.Bai HR, Oyebanji O, Zhang R, Yi TS. Plastid phylogenomic insights into the evolution of subfamily Dialioideae (Leguminosae) Plant Divers. 2021;43(1):27–34. doi: 10.1016/j.pld.2020.06.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Duchene D, Bromham L. Rates of molecular evolution and diversification in plants: chloroplast substitution rates correlate with species-richness in the Proteaceae. BMC Evol Biol. 2013;13(1):1–11. doi: 10.1186/1471-2148-13-65. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Ni L, Zhao Z, Xu H, Chen S, Dorje G. The complete chloroplast genome of Gentiana straminea (Gentianaceae), an endemic species to the Sino-Himalayan subregion. Gene. 2016;577(2):281–288. doi: 10.1016/j.gene.2015.12.005. [DOI] [PubMed] [Google Scholar]
- 40.Li Z, Long H, Zhang L, Liu Z, Cao H, Shi M, Tan X. The complete chloroplast genome sequence of tung tree (Vernicia fordii): organization and phylogenetic relationships with other angiosperms. Sci Rep. 2017;7(1):1–11. doi: 10.1038/s41598-017-02076-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Li Y, Jia L, Wang Z, Xing R, Chi X, Chen S, Gao Q. The complete chloroplast genome of Saxifraga sinomontana (Saxifragaceae) and comparative analysis with other Saxifragaceae species. Rev Bras Bot. 2019;42(4):601–611. [Google Scholar]
- 42.Khakhlova O, Bock R. Elimination of deleterious mutations in plastid genomes by gene conversion. Plant J. 2006;46(1):85–94. doi: 10.1111/j.1365-313X.2006.02673.x. [DOI] [PubMed] [Google Scholar]
- 43.Saina JK, Li Z-Z, Gichira AW, Liao Y-Y. The complete chloroplast genome sequence of tree of heaven (Ailanthus altissima (mill.)) (sapindales: Simaroubaceae), an important pantropical tree. Int J Mol Sci. 2018;19(4):929. doi: 10.3390/ijms19040929. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Barbhuiya PA, Uddin A, Chakraborty S. Analysis of compositional properties and codon usage bias of mitochondrial CYB gene in anura, urodela and gymnophiona. Gene. 2020;751:144762. doi: 10.1016/j.gene.2020.144762. [DOI] [PubMed] [Google Scholar]
- 45.Henriquez CL, Mehmood F, Carlsen MM, Islam M, Waheed MT, Poczai P, Croat TB, Ahmed I. Complete chloroplast genomes of Anthurium huixtlense and Pothos scandens (Pothoideae, Araceae): unique inverted repeat expansion and contraction affect rate of evolution. J Mol Evol. 2020;88(7):562–574. doi: 10.1007/s00239-020-09958-w. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Wright F. The effective number of codons used in a gene. Gene. 1990;87(1):23–29. doi: 10.1016/0378-1119(90)90491-9. [DOI] [PubMed] [Google Scholar]
- 47.Morton BR. Chloroplast DNA codon use: evidence for selection at the psbA locus based on tRNA availability. J Mol Evol. 1993;37(3):273–280. doi: 10.1007/BF00175504. [DOI] [PubMed] [Google Scholar]
- 48.Nie S, Yao S, Qin C, Li K, Liu X, Wang L, Song X, Wang S. Kinetics of AOX formation in chlorine dioxide bleaching of bagasse pulp. Bioresour. 2014;9(3):5604–5614. [Google Scholar]
- 49.Gichira AW, Avoga S, Li Z, Hu G, Wang Q, Chen J. Comparative genomics of 11 complete chloroplast genomes of Senecioneae (Asteraceae) species: DNA barcodes and phylogenetics. Bot Stud. 2019;60(1):17. doi: 10.1186/s40529-019-0265-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Li X, Yang Y, Henry RJ, Rossetto M, Wang Y, Chen S. Plant DNA barcoding: from gene to genome. Biol Rev. 2015;90(1):157–166. doi: 10.1111/brv.12104. [DOI] [PubMed] [Google Scholar]
- 51.Keller J, Rousseau-Gueutin M, Martin GE, Morice J, Boutte J, Coissac E, Ourari M, Aïnouche M, Salmon A, Cabello-Hurtado F. The evolutionary fate of the chloroplast and nuclear rps16 genes as revealed through the sequencing and comparative analyses of four novel legume chloroplast genomes from Lupinus. DNA Res. 2017;24(4):343–358. doi: 10.1093/dnares/dsx006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Cole LW, Guo W, Mower JP, Palmer JD. High and variable rates of repeat-mediated mitochondrial genome rearrangement in a genus of plants. Mol Biol Evol. 2018;35(11):2773–2785. doi: 10.1093/molbev/msy176. [DOI] [PubMed] [Google Scholar]
- 53.Kainulainen K, Razafimandimbison SG, Wikström N, Bremer B. Island hopping, long-distance dispersal and species radiation in the Western Indian Ocean: historical biogeography of the Coffeeae alliance (Rubiaceae) J Biogeogr. 2017;44(9):1966–1979. [Google Scholar]
- 54.Tosh J, Davis AP, Dessein S, De Block P, Huysmans S, Fay MF, Smets E, Robbrecht E. Phylogeny of Tricalysia (Rubiaceae) and its relationships with allied genera based on plastid DNA data: resurrection of the genus Empogona. Ann Mo Bot Gard. 2009;96:194–213. [Google Scholar]
- 55.Robbrecht E, Puff C. Survey of the Gardenieae and related tribes (Rubiaceae) Bot Jahrb Syst Pflanzengesch Pflanzengeogr. 1986;108:63–137. [Google Scholar]
- 56.Patel RK, Jain M. NGS QC Toolkit: a toolkit for quality control of next-generation sequencing data. PloSOne. 2012;7:e30619. doi: 10.1371/journal.pone.0030619. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Jin JJ, Yu W-B, Yang JB, Song Y, Depamphilis CW, Yi TS, Li DZ. GetOrganelle: a fast and versatile toolkit for accurate de-novo assembly of organelle genomes. Genome Biol. 2020;21(1):1–31. doi: 10.1186/s13059-020-02154-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Wick RR, Schultz MB, Zobel J, Holt KE. Bandage: interactive visualization of de novo genome assemblies. Bioinformatics. 2015;31(20):3350–3352. doi: 10.1093/bioinformatics/btv383. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Langmead B, Salzberg SL. Fast gapped-read alignment with bowtie 2. Nat Methods. 2012;9(4):357–359. doi: 10.1038/nmeth.1923. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Kearse M, Moir R, Wilson A, Stones-Havas S, Cheung M, Sturrock S, et al. Geneious basic: an integrated and extendable desktop software platform for the organization and analysis of sequence data. Bioinformatics. 2012;28(12):1647–1649. doi: 10.1093/bioinformatics/bts199. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Wyman SK, Jansen RK, Boore JL. Automatic annotation of organellar genomes with DOGMA. Bioinformatics. 2004;20(17):3252–3255. doi: 10.1093/bioinformatics/bth352. [DOI] [PubMed] [Google Scholar]
- 62.Schattner P, Brooks AN, Lowe TM. The tRNAscan-SE, snoscan and snoGPS web servers for the detection of tRNAs and snoRNAs. Nucleic Acids Res. 2005;33:W686–W689. doi: 10.1093/nar/gki366. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Lohse M, Drechsel O, Kahlau S, Bock R. OrganellarGenomeDRAW—a suite of tools for generating physical maps of plastid and mitochondrial genomes and visualizing expression data sets. Nucleic Acids Re. 2013;41(W1):W575–W581. doi: 10.1093/nar/gkt289. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Amiryousefi A, Hyvönen J, Poczai P. IRscope: an online program to visualize the junction sites of chloroplast genomes. Bioinformatics 2018; 34(17):3030–3031. [DOI] [PubMed]
- 65.Darling AC, Mau B, Blattner FR, Perna NT. Mauve: multiple alignment of conserved genomic sequence with rearrangements. Genome Res. 2004;14(7):1394–1403. doi: 10.1101/gr.2289704. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Rozas J, Ferrer-Mata A, Sánchez-DelBarrio JC, Guirao-Rico S, Librado P, Ramos-Onsins SE, Sánchez-Gracia A. DnaSP 6: DNA sequence polymorphism analysis of large data sets. Mol Biol Evol. 2017;34(12):3299–3302. doi: 10.1093/molbev/msx248. [DOI] [PubMed] [Google Scholar]
- 67.Sharp PM, Li W-H. The codon adaptation index-a measure of directional synonymous codon usage bias, and its potential applications. Nucleic Acids Res. 1987;15(3):1281–1295. doi: 10.1093/nar/15.3.1281. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Zhang D, Gao F, Jakovlić I, Zou H, Zhang J, Li WX, Wang GT. PhyloSuite: an integrated and scalable desktop platform for streamlined molecular sequence data management and evolutionary phylogenetics studies. Mol.Ecol.Resour. 2020;20(1):348–355. doi: 10.1111/1755-0998.13096. [DOI] [PubMed] [Google Scholar]
- 69.Benson G. Tandem repeats finder: a program to analyze DNA sequences. Nucleic Acids Res. 1999;27(2):573–580. doi: 10.1093/nar/27.2.573. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Kurtz S, Choudhuri JV, Ohlebusch E, Schleiermacher C, Stoye J, Giegerich R. REPuter: the manifold applications of repeat analysis on a genomic scale. Nucleic Acids Res. 2001;29(22):4633–4642. doi: 10.1093/nar/29.22.4633. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Thiel T, Michalek W, Varshney R, Graner A. Exploiting EST databases for the development and characterization of gene-derived SSR-markers in barley (Hordeum vulgare L.) Theor Appl Genet. 2003;106(3):411–422. doi: 10.1007/s00122-002-1031-0. [DOI] [PubMed] [Google Scholar]
- 72.Katoh K, Rozewicki J, Yamada KD. MAFFT online service: multiple sequence alignment, interactive sequence choice, and visualization. Brief Bioinform. 2019;20(4):1160–1166. doi: 10.1093/bib/bbx108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Lanfear R, Frandsen PB, Wright AM, Senfeld T, Calcott B. Partition finder 2: new methods for selecting partitioned models of evolution for molecular and morphological phylogenetic analyses. Mol Biol Evol. 2017;34(3):771–773. doi: 10.1093/molbev/msw260. [DOI] [PubMed] [Google Scholar]
- 74.Stamatakis A. RAxML-HPC2 v.8.1 on XSEDE: a tool for phylogenetic analysis and post-analysis of large phylogenies. Bioinformatics. 2014;30(9):1312–1313. doi: 10.1093/bioinformatics/btu033. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Ronquist F, Huelsenbeck JP. MrBayes 3: Bayesian phylogenetic inference under mixed models. Bioinformatics. 2003;19(12):1571–1574. doi: 10.1093/bioinformatics/btg180. [DOI] [PubMed] [Google Scholar]
- 76.Rambaut A, Drummond AJ, Xie D, Baele G, Suchard MA. Posterior summarization in Bayesian phylogenetics using Tracer 1.7. Syst Biol. 2018;67(5):901. doi: 10.1093/sysbio/syy032. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Rambaut A. Fig Tree version 1.3.1(2009). [computer program] http://tree.bio.ed.ac.uk.
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Additional file 1. The Mauve alignment of the 62 coffeeae alliance plastomes. The colliner blocks rearrangement of the accessible gene at junction of inverted repeat and small single copy. White colored blocks: protein coding gene, Black colored blocks: tRNA genes, Red coloured blocks: Rrna. For interpretation of references to the colors, see the web version of the article.
Additional file 2. The codon usage bias in all the protein-coding genes of the plastome of the 62 Coffeeae allinces tribes.
Additional file 3: Table S1. GenBank accession numbers and coverage of the newly sequenced species from the Coffeeae alliance studied.
Additional file 4: Table S2. Summary of plastomes for 63 species Coffeeae alliance tribes.
Additional file 5: Table S3. Summary of partitioning scheme for the ML tree and BI analysis.
Additional file 6: Table S4. Distributions of SSR in the Coffeeae alliance tribes.
Additional file 7: Table S5. Details of Relative Synonymous Codon Usage in plastome of Coffeeae alliance tribes.
Data Availability Statement
All sequences used in for this study can be found in GenBank; the list of accession can be found in Supplementary Table S1.