Abstract
Antiviral drugs have gained much more attention in recent years due to severe acute respiratory syndrome coronavirus-2 (SARS-CoV-2) infection and many drug candidates are currently under investigation in order to end pandemic. Molnupiravir, a prodrug of the synthetic nucleoside derivative N4-hydroxycytidine, is one of the promising candidates for SARS-CoV-2 treatment. In this study, a RP-HPLC method was developed for the determination of Molnupiravir and applied for in vitro permeability studies of self-emulsifying drug delivery system (SEDDS) formulations using Caco-2 cell line. Discovery® HS C18 Column (75 ×4.6 mm, 3 µm) was used at 30 °C. Isocratic elution was performed with ACN:water (20:80 v/v) mixture. The flow rate was 0.5 mL/min and UV detection was at 240 nm. Molnupiravir eluted within 5 min. Molnupiravir was exposed to thermal, photolytic, hydrolytic, and oxidative stress conditions. Peak homogeneity data of Molnupiravir in the stressed samples peak obtained using photodiode array detector, in the stressed sample chromatograms, demonstrated the specificity of the method for their estimation in presence of degradants. The developed method was validated according to the International Council for Harmonisation (ICH) guidelines and found to be linear within the range 0.1–60.0 μg/mL. The method was simple, rapid, selective, sensitive, accurate, precise, robust and rugged. Thus, it was applied successfully for permeability quantitation of Molnupiravir in nanoformulations. The apparent permeability of Molnupiravir in SEDDS formulations, which have droplet size under 350 nm, was calculated as 3.20 ± 0.44 × 10−6 cm/s.
Keywords: Molnupiravir, RP-HPLC, COVID-19, Optimization, Validation, Permeability
Abbreviations: SARS-CoV-2, severe acute respiratory syndrome coronavirus-2; MLP, Molnupiravir; SEDDS, Self-emulsifying drug delivery system; EMA, European Medicines Agency; ICH, International Council for Harmonisation; LOQ, Limit of quantitation; LOD, Limit of dedection
1. Introduction
Since the announcement of the pandemic caused by severe acute respiratory syndrome coronavirus-2 (SARS-CoV-2) in March 2020, approximately 300 million confirmed cases and over 5 million death has been reported globally by World Health Organization, and it has been still causing serious public health problems [1]. There have been over 9 billion COVID-19 vaccine doses administrated worldwide, and there still has been a seek for effective therapy options to control the pandemic. Here, the antiviral agents could be one of the treatments of coronavirus disease, especially for high-risk patient groups.
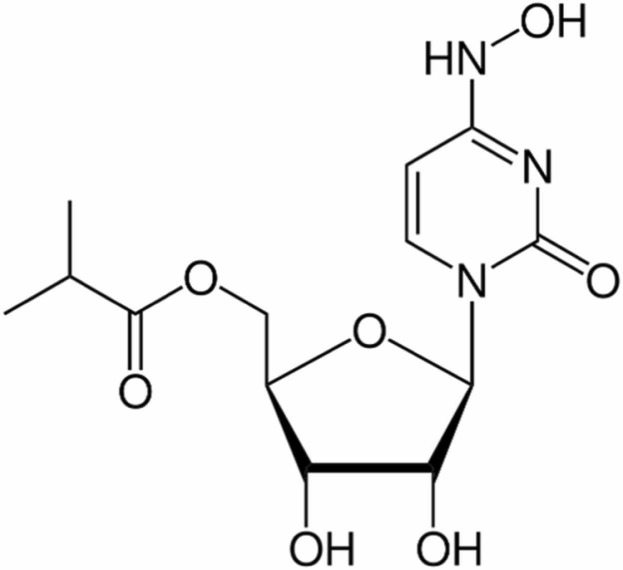
Molnupiravir (MLP, Fig. 1) is an oral broad-spectrum antiviral agent originally designed for the treatment of Alphavirus infections and now has been used for the treatment of COVID-19 disease caused by the nasopharyngeal SARS-CoV-2 infectious virus [2]. An emergency use authorization has been issued on December 2021 for MLP by the U.S. Food and Drug Administration (FDA) for the treatment of mild-to-moderate COVID-19 in adults with positive results of direct SARS-CoV-2 viral testing and who are at high risk for progression to severe COVID-19 and European Medicines Agency (EMA) has issued advice on the use of MLP in adults with the increased risk of developing severe COVID-19 on November 2021. MLP, a prodrug of the synthetic nucleoside derivative N4-hydroxycytidine, has been shown to be directly effective in reducing viral RNA and SARS-CoV-2 virus and is known to have favorable safety and tolerability profile. It shows its antiviral effect by adding transcription errors in viral RNA replication. In pharmacokinetic profiling studies, the oral bioavailability of MLP in ferrets and non-human primates was found to be sufficient, and it was also found to be effective in preventing viral transmission in animal studies. If ongoing clinical trials result as expected, MLP is considered to become an important tool to counter the effects of the COVID-19 pandemic [3], [4], [5].
Fig. 1.
Chemical structure of MLP.
Pharmaceutical analyses are very important in the pharmaceutical industry because of the need for accurate and precise analytical methods to analyze drugs, impurities and drug metabolites in drug development processes. Permeability studies are one of the applications of pharmacokinetic studies and require the analysis of drug substances in the transport media to determine the permeability of targeted drugs [6]. According to the guidelines, permeability studies could be performed utilizing human pharmacokinetic studies. In addition to that validated and standardized in vitro systems capable of predicting the extent of drug absorption could also be applied [7], [8]. Among these in vitro models, Caco-2 cell monolayers are one of the most preferred cellular-based alternatives for drug transport evaluation due to the expression of a wide range of drug transporters [9]. However, variability of results generated in different laboratories due to inter-laboratory culturing differences, and the need for precise and validated analytical methods, especially for poorly water-soluble drug molecules, could be considered [9], [10], [11].
HPLC is one of the widely used analytical techniques in pharmaceutical analysis, as it enables precise, accurate, fast, and low-cost analysis [12]. In the literature, there is an LC-MS/MS method for the determination of MLP and its metabolite in human plasma and saliva [13]. However, there is no method reported for the quantification of MLP in pharmaceutical dosage forms. In this study, it was aimed to develop a rapid, selective, accurate, precise, and robust RP-HPLC method for the determination of MLP in transport samples. The developed method was fully validated according to the ICH guidelines [14]. The stability of MLP was evaluated and also a forced degradation procedure was applied under stress conditions for selectivity analysis. The application of the method to in vitro permeability studies was conducted using Caco-2 cell lines. After the characterization of SEDDS containing MLP, the quantification of the drug was conducted in transport media and apparent permeability of MLP formulation was determined.
2. Materials and methods
2.1. Chemicals and reagents
MLP was purchased from Optimus Drugs, India. The analytical standard of MLP and HPLC-grade acetonitrile (ACN) were purchased from Merck-Sigma Aldrich (Darmstadt, Germany). Milli-Q water was obtained from a Barnstead Nanopure™ system from Thermo. Labrafil® M1944C, Labrafac™ lipophile WL1349, Transcutol®HP were kind gift from Kura Chemicals, Turkey. Tween 80 was obtained from Sigma-Aldrich, Germany. All the solutions were prepared in Milli-Q water.
2.2. Apparatus and chromatographic conditions
HPLC analyses were performed on a Shimadzu UFLC system. The liquid chromatographic system was comprised of a solvent delivery system (Shimadzu LC-20AB) and a diode array detector (Shimadzu SPD-M20A). The autosampler (Shimadzu SIL-20AC) was used for sample injection and the column was kept in the oven (Shimadzu CTO-20AC). Chromatographic separations were carried on a Discovery® HS C18 Column (75 ×4.6 mm, 3 µm) via isocratic elution of ACN:water (20:80, v/v) mixture as mobile phase. The column temperature was set at 30 °C and the flow rate was 0.5 mL/min. The injection volume was 10 μL and 240 nm was selected as the detection wavelength for the diode array detector. Peak identity was confirmed by retention time comparison.
2.3. Data processing
Data acquisition and processing were done with the LabSolutions software (version 1.25, Shimadzu). The quantification of MLP in the samples was performed using calibration curves. Therefore, least squares regression was used to create the calibration curves by plotting MLP concentration to the peak areas of MLP. The statistical calculations were carried out using Microsoft Excel software.
2.4. Preparation of calibration standards
The standard stock solution of MLP (1000 μg/mL) was prepared in water. Calibration standard solutions (0.1, 0.5, 1.0, 2.0, 5.0, 10.0, 20.0, 30.0, 50.0, and 60.0 μg/mL) were prepared by diluting the stock solution in the mobile phase solution. The stock solution was kept at +4 °C where it is stable for at least one month. The standard solutions were daily prepared by diluting the stock solution with mobile phase mixture.
2.5. Preparation of formulations
The SEDDS were prepared using ternary phase diagrams. All excipients selected in formulation studies were suitable for oral administration. For the selection of the oil phase, the solubility of MLP was assessed using Labrafac™ lipophile WL1349 and Peceol™. For the preparation of SEDDS formulations, the oil phase; Labrafac™ lipophile WL1349 surfactant mixture; Labrafil® M1944C:Tween 80 (1:2), and cosolvent Transcutol®HP was used. Briefly, the aforementioned excipients were weighed in screw-capped borosilicate vials and the mixture was heated above the melting point of the solid excipients and stirred with a mechanical stirrer. MLP was added to the formulations by simply mixing until a homogenous mixture was obtained. All the formulations were kept for 24 h at ambient temperature to reach equilibrium before transport studies and diluted with transport media prior to the application [15], [16]. The formulations were assessed in terms of organoleptic properties, as well as droplet size and zeta potential values were obtained. The characterization studies were conducted using Malvern Nanosizer ZS 2000 (United Kingdom).
2.6. Transport studies
Caco-2 cell line (passage number 15–20) was used for the evaluation of permeability. Cells were seeded in 75 cm2 flasks using high glucose DMEM containing 4 mM L-glutamine and sodium pyruvate, 10% FBS, 0.5% penicillin (10,000 units) and streptomycin sulfate (10,000 μg/mL). The cells were cultured at 37 °C in an environment containing 5% CO2.
After reaching confluency, Caco-2 cells were seeded into the inserts at a concentration of 12 × 104 cells/mL (ThinCert™, 12 well, 1 μM pore size, transparent). Plates were incubated at 37 °C in humid air containing 5% CO2. The culture medium was changed every other day, and transport studies were carried out after 21 days when the cell monolayer reached confluency.
The integrity of the cell monolayer was assessed by measuring the transepithelial electrical resistance (TEER). TEER values of Caco-2 cell monolayers were measured by Millicell–ERS epithelial voltammetry. The cell monolayers with an electrical resistance of 500 Ω.cm2 and above were used for experiments.
At the end of 21 days, the medium in the apical and basolateral sections was collected and formulation containing 0.8 mg/mL MLP was applied using HBSS transport medium (without phenol red) containing 10 mM HEPES to the apical compartment. The samples were kept in the water bath at 37 °C for 2 h with shaking at 60 rpm. After 2 h, the media in the basolateral section was collected and stored at −20 °C for analysis (Fig. 1). The cell integrity was confirmed by measuring the electrical resistance of the cell monolayers after 2 h of incubation.
2.7. Forced degradation
2.7.1. Thermal degradation
100 μL of MLP standard stock solution was diluted to 1000 μL with water. Then the solution was kept at 80 °C for 2 h. The solution was cooled to room temperature, and then it was diluted with mobile phase to 20 μg/mL and injected into the RP-HPLC system.
2.7.2. Oxidative degradation
100 μL of MLP standard stock solution was diluted to 1000 μL by adding 3% H2O2. Then the solution was kept at 40 °C for 15 min. The solution was boiled at 100 °C for 15 min and then cooled to room temperature. The solutions were diluted with mobile phase to 20 μg/mL and injected into the RP-HPLC system.
2.7.3. Acid and alkali hydrolysis
100 μL of MLP standard stock solution was diluted to 1000 μL by adding 0.1 M HCI, or 0.1 M NaOH. Then the solution was kept at 40 °C for 2 h. These solutions were cooled at room temperature after they were neutralized with a suitable amount of HCI or NaOH. The solutions were diluted with mobile phase to 20 μg/mL and injected into the RP-HPLC system. MLP was exposed to different concentrations of NaOH solutions (0.01 and 0.1 M) at different temperatures and times (i- 40 °C for 2 h, ii- 40 °C for 15 min, iii- room temperature for 15 min, iv- room temperature for 2 min).
2.7.4. Photolytic degradation
100 μL of MLP standard stock solution was diluted to 1000 μL with water. The solution was kept under UV light (254 nm) combined with tungsten lamp for 24 h at room temperature. The solution was then diluted with mobile phase to 20 μg/mL and injected into the RP-HPLC system.
2.8. Analytical method validation
The RP-HPLC method was validated for selectivity, linearity, sensitivity, precision, accuracy, robustness, and ruggedness following the ICH guidelines [17].
2.8.1. Selectivity
The selectivity of the RP-HPLC method was investigated by comparing chromatograms obtained for blank transport medium and MLP spiked transport medium at limit of quantitation (LOQ) concentration (0.10 μg/mL). Moreover, matrix effects were tested for transport medium and standards. Therefore, MLP (1.0–50 μg/mL) was added to both blank solutions (HBSS transport medium without phenol red, containing 10 mM HEPES) and water to evaluate the matrix effect. The modified HBSS which is used in this particular study contains calcium (as calcium chloride), magnesium (as magnesium chloride and magnesium sulfate) and glucose (D-glucose) whereas it is formulated without phenol red and sodium pyruvate. The other inorganic salts in the formulation are potassium chloride, potassium phosphate monobasic, sodium bicarbonate, sodium chloride, and sodium phosphate dibasic anhydrous. In addition, 10 mM HEPES (N-2-hydroxyethylpiperazine-N-2-ethane sulfonic acid) was added to the HBSS medium as a zwitterionic organic chemical buffering agent. The samples were analyzed under the optimum analytical conditions.
2.8.2. Linearity
The linearity studies were carried out by analyzing calibration solutions containing different concentrations of MLP (0.1–60.0 μg/mL) using the proposed RP-HPLC method. The calibration plots were created by plotting the peak area of MLP against the concentrations with least-squares linear regression analysis.
2.8.3. Sensitivity
The sensitivity of the developed method was evaluated with limit of detection (LOD) and LOQ values based on signal-to-noise ratio at 3:1 and 10:1, respectively.
2.8.4. Precision and accuracy
Intra-day and inter-day precision and accuracy were estimated by analyzing three replicates containing MLP at four different concentration levels (LOQ, 1.0, 10.0, and 50.0 μg/mL) on the same day and on six consecutive days, respectively. Relative standard deviation (RSD %) for precision and relative error (RE) for accuracy was calculated at each concentration level. In addition, the accuracy of the developed method was also examined with recovery studies.
2.8.5. Robustness
The robustness of analytical methods is checked by examining the effect of small deliberate changes in experimental conditions on the analysis results. In this study, an experimental design was applied with the simultaneous change of factors to determine the robustness of the RP-HPLC method. Small changes were made in ACN percentage of the mobile phase (19–21%), flow rate (0.495–0.505 mL/min) and column temperature (29–31 °C). The results were compared statistically.
2.8.6. Ruggedness
The ruggedness of the developed method was achieved by analyzing the MLP standard solution (20.0 µg/mL) by two different analysts (Analyst 1 and Analyst 2) under optimum conditions. The results were compared statistically.
2.8.7. Stability
The stability of the method was investigated using 20 μg/mL standard solution of MLP for different conditions such as short-term (room temperature and +4 °C for 24 h), autosampler (for 6 h), long term (+4 °C for 1 month). The results were compared with the analysis results of a freshly prepared standard solution of MLP.
3. Results and discussion
3.1. Method optimization
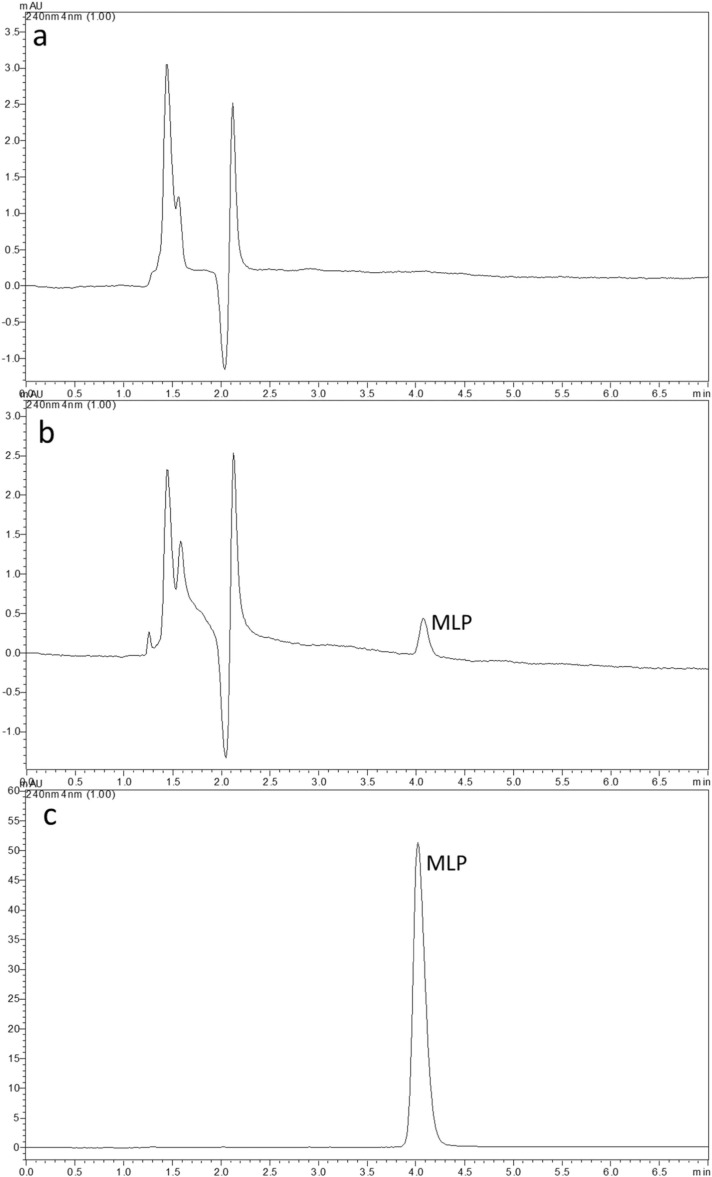
During the method development, analysis conditions were optimized considering the physicochemical properties of MLP. Various sizes of C18 columns, organic phase-type (ACN and methanol), mobile phase organic phase percentage (10–90%), and flow rate 0.4–1.0 mL/min were investigated in order to optimize the chromatographic parameters. In conclusion, a C18 column (75 × 4.6 mm, 3 µm) was selected as stationary phase due to short retention time and symmetric peaks for MLP. It was decided to use an isocratic elution with mixture of ACN:water (20:80, v/v) at 30 °C and 0.5 mL/min flow rate to perform the analyses. The UV spectrum of MLP in the mobile phase showed a max at 240 nm and there were not any interferences that might come from matrix components (Supplementary Fig. 1). The total run time was 7 min and the retention time of MLP was 4.1 min ( Fig. 3).
Fig. 3.
Chromatograms obtained under optimum chromatographic conditions; a) blank for MLP b) MLP standard spiked matrix at LOQ (0.10 μg/mL) concentration c) MLP standard solution (20 µg/mL).
The system suitability of the developed RP-HPLC under optimum analysis conditions by injection of 10 μg/mL standard (n = 6) was evaluated in terms of retention time and peak area repeatability (RSD%), column efficiency (theoretical plate number, N), capacity factor (k′) and tailing factor parameters. The values obtained (RSD (retention time) = 0.17%, RSD (peak area) = 1.00%, N = 4685 ± 5.85, k′= 1.48 ± 0.001 and tailing factor= 1.24 ± 0.002) are within the specified limits [17], and it has been determined that the system is suitable for the analysis of MLP.
3.2. Analytical method validation
The RP-HPLC method was validated for selectivity, linearity, sensitivity, precision, accuracy, robustness, and ruggedness following the ICH guidelines [17].
3.2.1. Selectivity
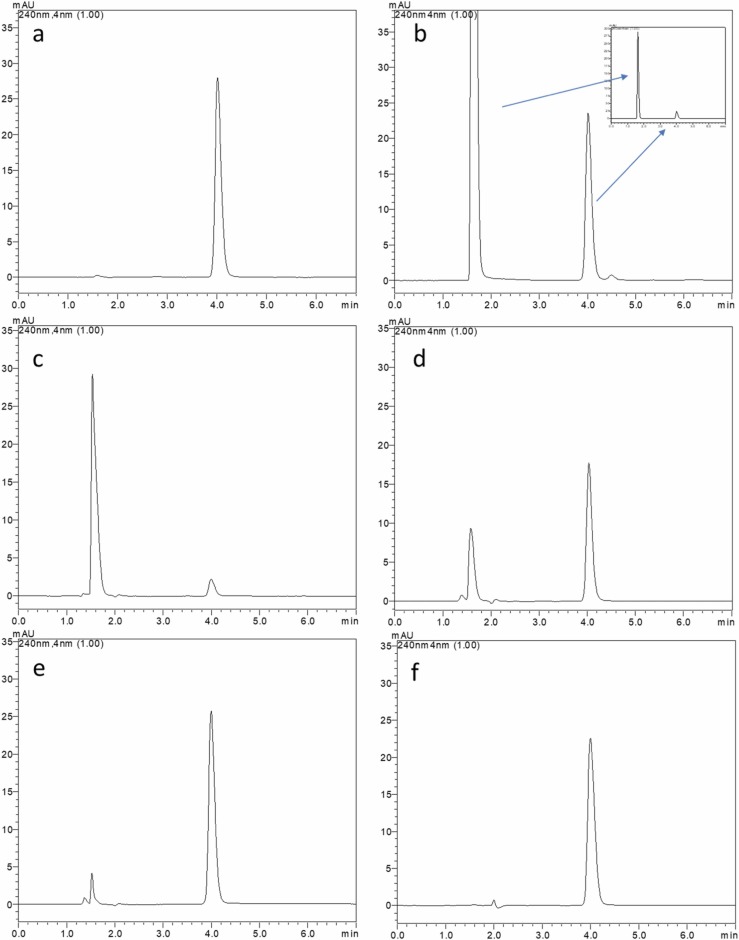
The chromatograms obtained in the selectivity study are given in Fig. 3. No distractive peaks were detected at the analyte retention time. This indicates that the developed method is selective for the analysis of MLP from transport samples. Moreover, forced degradation studies were performed to detect its degradation products. For this purpose, different stress conditions such as high temperature, oxidative degradation, acid-base hydrolysis, and irradiation with UV light were applied. The results obtained are presented in Table 1 and Fig. 2.
Table 1.
Forced degradation results of MLP.
| Conditions | Thermal degradation | Oxidative degradation | Acid hydrolysis | Alkali hydrolysis | Photolytic degradation |
|---|---|---|---|---|---|
| Temperature | 80 °C | 40 °C | 40 °C | 25 °C | 25 °C |
| Time | 2 h | 15 min | 2 h | 2 min | 24 h |
| Recovery (%) | 98.8 | 93.7 | 81.8 | 65.2 | 84.0 |
| Peak purity index | 0.9998 | 0.9997 | 0.9997 | 0.9908 | 0.9991 |
Fig. 2.
Forced degradation study of MLP; a) 80 °C for 2 h, b) 3% H2O2 at 40 °C for 15 min, c) 0.1 M NaOH at room temperature for 2 min, d) 0.01 M NaOH at room temperature for 2 min, e) 0.1 M HCI at 40 °C for 2 h, f) UV light at room temperature for 24 h.
The recovery was found to be 98.8% when exposed to high temperatures of the MLP standard solution. The recovery obtained as a result of oxidative degradation was found to be 93.7%.
When exposed to 0.01 M HCI solution at 40 °C for 2 h, it was observed that 81.8% recovery was obtained as a result of degradation (Table 1 and Fig. 2).
It was determined that MLP was fully depredated when exposed to 0.1 M NaOH solution under different temperatures and times (i- 40 °C for 2 h, ii- 40 °C for 15 min, iii- room temperature for 15 min, iv- room temperature for 2 min). It was observed that almost all of MLP was degraded under these conditions and therefore the degradation study was continued with 0.01 N NaOH solution. Finally, when exposed to 0.01 M NaOH solution at room temperature for 2 min, it was observed that 65.2% recovery was obtained as a result of degradation. The recovery obtained as a result of photolytic degradation was found to be 84.0%. Our method was able to separate completely the degradation products from the intact MLP. The results obtained for MLP demonstrate the stability-indicating capability of the developed method. Therefore, the selectivity of the developed method has also been proven in the presence of degradation products (Fig. 2). The mass of major degradation products of MLP in different forced degradation conditions have been identified using high-resolution mass spectrometry (Supplementary Figs. 3 and 4). Extracted ion chromatograms were used to ß-d-N4-hydroxycytidine presences at 258 m/z in negative ionization mode and a peak at around one min was observed in all stress conditions (Supplementary Fig. 5). Its early retention time than MLP and high resolutions mass may confirm the presence of ß-d-N4-hydroxycytidine. A detailed study on the identification of degradation products of MLP would be a subject of interest in a separate study.
3.2.2. Linearity
The linearity of the calibration curves was determined over the concentration range of 0.1–60 μg/mL with a correlation coefficient value is 0.999 ± 0.002. The values (mean ± SE; n = 6) of the slope and intercept were 24,422 ± 786.4 and −7238.26 ± 3188.4, respectively. The RP-HPLC method showed an acceptable linearity range from 0.1 to 60 μg/mL for MLP.
3.2.3. Sensitivity
The LOD was 0.05 μg/mL and the LOQ was selected in this study was 0.10 μg/mL (Supplementary Fig. 2). The developed method was highly sensitive for estimating MLP in transport samples.
3.2.4. Precision and accuracy
The results of precision and accuracy are summarized in Table 2. The low RSD and RE values showed that the method was precise and accurate. In addition, the accuracy of the developed method was also examined with recovery studies. To evaluate the recovery of the method, the slope values of the curves of MLP prepared in sample blank matrix and water were compared and the recovery of the method was found as 100.63%.
Table 2.
Intra- and inter-day accuracy and precision of the developed method.
| Concentration (μg/mL) | Intra-day (n = 6) |
Inter-day (n = 6) |
||
|---|---|---|---|---|
| Accuracy (REa, %) | Precision (RSDb, %) | Accuracy (RE, %) | Precision (RSD, %) | |
| 0.1 | -2.02 | 1.89 | 1.78 | 1.90 |
| 1.0 | -1.98 | 1.07 | -1.32 | 2.06 |
| 10.0 | -1.90 | 0.62 | -0.77 | 1.91 |
| 50.0 | -0.38 | 0.92 | -0.13 | 0.96 |
RE, relative error.
RSD, relative standard deviation
3.2.5. Robustness
A nine-run fractional factor design with three experiments under optimized conditions was applied to determine whether a small deviation in experimental conditions produced a statistically significant variation in the obtained responses ( Table 3). The results of the analysis were statistically compared with ANOVA test and p values of regression coefficient and regression equation were calculated ( Table 4). There was no statistical difference between the results (p ≥ 0.05). Therefore, we can say that small changes do not have a statistically significant effect on the peak area ratio and the developed method is robust.
Table 3.
The parameters and their levels for robustness study.
| Parameters | Level |
||
|---|---|---|---|
| -1 | 0 | +1 | |
| ACN % | 19 | 20 | 21 |
| Flow rate (mL/min) | 0.495 | 0.500 | 0.505 |
| Temperature (°C) | 29 | 30 | 31 |
Table 4.
The experimental design and results for robustness study.
| Exp. no | ACN % | Flow rate (mL/min) | Temperature (°C) | Peak area (n = 3) |
|---|---|---|---|---|
| 1 | -1 | -1 | -1 | 377,505 |
| 2 | +1 | -1 | -1 | 381,399 |
| 3 | -1 | +1 | -1 | 365,307 |
| 4 | +1 | +1 | -1 | 378,373 |
| 5 | -1 | -1 | +1 | 365,453 |
| 6 | +1 | -1 | +1 | 411,689 |
| 7 | -1 | +1 | +1 | 358,608 |
| 8 | +1 | +1 | +1 | 380,554 |
| 9 | 0 | 0 | 0 | 411,043 |
| p values | 0.150 | 0.337 | 0.795 |
3.2.6. Ruggedness
The application of the same procedures by two different analysts demonstrated the ruggedness of the developed method. The analysis results were 19.28 ± 0.43 and 19.75 ± 0.07 (n = 6). The Student’s t-test was applied for statistical evaluation and it was found that there was no significant difference (p = 0.35) between them. Thus, the method was found rugged.
3.2.7. Stability
The stability of MLP was investigated under different conditions (short, autosampler, and long term). Under these conditions, MLP recovery values were found to 100.6% ± 0.08 for the short term, 98.3% ± 0.11 for autosampler, and 101.5% ± 0.23 for long term. The results showed that MLP was at least stable up to 24 h at room temperature, 6 h in autosampler, and 1 month at −20 °C.
3.3. The apparent permeability of MLP
SEDDS formulations containing MLP was characterized and the results were summarized in Table 5. The droplet size under 350 nm was obtained for transport studies. The zeta potential of formulations was found to be close to zero as expected due to the use of nonionic surfactants.
Table 5.
The characteristics of prepared SEDDS formulations used in transport studies.
| Droplet size (nm) | Polydispersity index | Zeta potential (mV) | Apparent permeability (cm/s) | |
|---|---|---|---|---|
| Blank formulations | 249.9 ± 2.9 | 0.218 ± 0.016 | -1.83 ± 0.07 | – |
| MLP containing formulations | 315.7 ± 8.9 | 0.233 ± 0.003 | 0.46 ± 0.04 | 3.20 × 10−6 ± 0.44 × 10−6 |
At the end of 21 days of culture, the formulation containing 0.8 mg/mL MLP was applied using HBSS transport medium (without phenol red) containing 10 mM HEPES to the apical compartment and RP-HPLC analysis was performed using samples from the basolateral compartment. After the RP-HPLC analysis, the apparent permeability coefficient (Papp) of the applied formulation was calculated as 3.20 ± 0.44 × 10−6 cm/s. (n = 4) [18]. Although these findings indicate the low permeability compared to Metoprolol tartrate, which is used as a reference for permeability classification [19], the effect of the formulation on the permeability of MLP should be assessed by comparing the permeability of MLP in solution and formulation with further evaluation [19], [20], [21].
4. Conclusion
In the present study, a simple, fast, and stable RP-HPLC method for the determination of MLP in transport medium was developed and validated for the first time. The developed method was selective, linear, sensitive, accurate, precise, robust, rugged, and could be used in permeability studies. The results obtained from stress testing show the method is stability-indicating and capable of determining MLP in presence of its degradation products, which indicates the selectivity of the method. In addition, the fact that the mobile phase used does not contain any buffer solutions or ion-pairing agents allows for a simple, fast, and low-cost analysis, which makes the developed method advantageous. Considering all these features, it is understood that the developed RP-HPLC method is more applicable for the routine analysis of MLP compared to the LC-MS/MS method proposed by Amara et al. [13]. Moreover, the availability of the regular HPLC systems compared to LC-MS systems makes our study much more applicable for routine analysis.
Although cell culture-based models offer numerous advantages to determine the permeability across the intestinal barriers, there are still disadvantages due to the biologic nature of the cell culture models including culturing conditions, culture media complexity, as well as solubility properties of the drug molecule. Thus, the use of precise analytical tools is crucial for reproducible results. The researchers working on permeability studies could easily apply the proposed RP-HPLC method on their investigations. The developed RP-HPLC method can be easily adapted for the analysis of MLP in different matrices due to all these advantages.
Declaration of Competing Interest
The authors declare that they have no known competing financial interests or personal relationships that could have appeared to influence the work reported in this paper.
Footnotes
Supplementary data associated with this article can be found in the online version at doi:10.1016/j.jpba.2022.114693.
Appendix A. Supplementary material
Supplementary material
.
References
- 1.2022. 〈https://covid19.who.int/measures〉. (Accessed 10 Jaunary 2022).
- 2.Painter G.R., Natchus M.G., Cohen O., Holman W., Painter W.P. Developing a direct acting, orally available antiviral agent in a pandemic: the evolution of molnupiravir as a potential treatment for COVID-19. Curr. Opin. Virol. 2021 doi: 10.1016/j.coviro.2021.06.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Toots M., Yoon J.-J., Cox R.M., Hart M., Sticher Z.M., Makhsous N., Plesker R., Barrena A.H., Reddy P.G., Mitchell D.G. Characterization of orally efficacious influenza drug with high resistance barrier in ferrets and human airway epithelia. Sci. Transl. Med. 2019;11(515) doi: 10.1126/scitranslmed.aax5866. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Gordon C.J., Tchesnokov E.P., Schinazi R.F., Götte M. Molnupiravir promotes SARS-CoV-2 mutagenesis via the RNA template. J. Biol. Chem. 2021;297(1) doi: 10.1016/j.jbc.2021.100770. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Painter W.P., Holman W., Bush J.A., Almazedi F., Malik H., Eraut N.C., Morin M.J., Szewczyk L.J., Painter G.R. Human safety, tolerability, and pharmacokinetics of molnupiravir, a novel broad-spectrum oral antiviral agent with activity against SARS-CoV-2. Antimicrob. Agents Chemother. 2021;65(5) doi: 10.1128/AAC.02428-20. e02428-20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Soyseven M., Kaynak M.S., Çelebier M., Aboul-Enein H.Y., Arli G. Development of a RP-HPLC method for simultaneous determination of reference markers used for in-situ rat intestinal permeability studies. J. Chromatogr. B. 2020;1147 doi: 10.1016/j.jchromb.2020.122150. [DOI] [PubMed] [Google Scholar]
- 7.EMA, ICH M9 Guideline on Biopharmaceutics Classification System-based Biowaivers, in: C.f.M.P.f.H. Use (Ed.), 2020. [DOI] [PubMed]
- 8.FDA, Waiver of In Vivo Bioavailability and Bioequivalence Studies for Immediate-Release Solid Oral Dosage Forms Based on a Biopharmaceutics Classification System, in: U.S.D.o.H.a.H.S.F.a.D.A.C.f.D.E.a.R. (CDER) (Ed.), 2017.
- 9.Buckley S.T., Fischer S.M., Fricker G., Brandl M. In vitro models to evaluate the permeability of poorly soluble drug entities: challenges and perspectives. Eur. J. Pharm. Sci. Off. J. Eur. Fed. Pharm. Sci. 2012;45(3):235–250. doi: 10.1016/j.ejps.2011.12.007. [DOI] [PubMed] [Google Scholar]
- 10.Artursson P., Palm K., Luthman K. Caco-2 monolayers in experimental and theoretical predictions of drug transport. Adv. Drug Deliv. Rev. 2001;46(1–3):27–43. doi: 10.1016/s0169-409x(00)00128-9. [DOI] [PubMed] [Google Scholar]
- 11.Sambuy Y., De Angelis I., Ranaldi G., Scarino M.L., Stammati A., Zucco F. The Caco-2 cell line as a model of the intestinal barrier: influence of cell and culture-related factors on Caco-2 cell functional characteristics. Cell Biol. Toxicol. 2005;21(1):1–26. doi: 10.1007/s10565-005-0085-6. [DOI] [PubMed] [Google Scholar]
- 12.O. McPolin, An introduction to HPLC for pharmaceutical analysis, Lulu.com, Mourine Training Services, Northern, Ireland, UK, 2009.
- 13.Amara A., Penchala S.D., Else L., Hale C., FitzGerald R., Walker L., Lyons R., Fletcher T., Khoo S. The development and validation of a novel LC-MS/MS method for the simultaneous quantification of Molnupiravir and its metabolite ß-d-N4-hydroxycytidine in human plasma and saliva. J. Pharm. Biomed. Anal. 2021;206 doi: 10.1016/j.jpba.2021.114356. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.I.H.T. Guideline, Validation of Analytical Procedures: Text and Methodology, Q2 (R1) 1(20) (2005), 5.
- 15.Timur S.S., Yoyen-Ermis D., Esendagli G., Yonat S., Horzum U., Esendagli G., Gursoy R.N. Efficacy of a novel LyP-1-containing self-microemulsifying drug delivery system (SMEDDS) for active targeting to breast cancer. Eur. J. Pharm. Biopharm. Off. J. Arb. Pharm. Verfahr. 2019;136:138–146. doi: 10.1016/j.ejpb.2019.01.017. [DOI] [PubMed] [Google Scholar]
- 16.Gursoy R.N., Benita S. Self-emulsifying drug delivery systems (SEDDS) for improved oral delivery of lipophilic drugs. Biomed. Pharmacother. = Biomed. Pharmacother. 2004;58(3):173–182. doi: 10.1016/j.biopha.2004.02.001. [DOI] [PubMed] [Google Scholar]
- 17.ICH Harmonised Tripartite Guideline, Validationof analytical procedures: text and methodology, Q2 (R1) 1(20) (2005), 5.
- 18.Zur M., Gasparini M., Wolk O., Amidon G.L., Dahan A. The low/high BCS permeability class boundary: physicochemical comparison of metoprolol and labetalol. Mol. Pharm. 2014;11(5):1707–1714. doi: 10.1021/mp500152y. [DOI] [PubMed] [Google Scholar]
- 19.Berginc K., Sibinovska N., Zakelj S., Trontelj J., Legen I. Biopharmaceutical classification of desloratadine - not all drugs are classified the easy way. Acta Pharm. 2020;70(2):131–144. doi: 10.2478/acph-2020-0006. [DOI] [PubMed] [Google Scholar]
- 20.Kim J.S., Mitchell S., Kijek P., Tsume Y., Hilfinger J., Amidon G.L. The suitability of an in situ perfusion model for permeability determinations: utility for BCS class I biowaiver requests. Mol. Pharm. 2006;3(6):686–694. doi: 10.1021/mp060042f. [DOI] [PubMed] [Google Scholar]
- 21.Incecayir T., Tsume Y., Amidon G.L. Comparison of the permeability of metoprolol and labetalol in rat, mouse, and Caco-2 cells: use as a reference standard for BCS classification. Mol. Pharm. 2013;10(3):958–966. doi: 10.1021/mp300410n. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Supplementary material