Abstract
In a previous paper we have shown that ε-(phenoxyalkanecarboxylyl)-l-Lys conjugates are potent inhibitors of amino acid transport systems and that it is possible to modulate the uptake inhibition by hydrophobic or hydrophilic additions in the 4-position of the aromatic ring (J.F. Chollet, C. Delétage, M. Faucher, L. Miginiac, J.L. Bonnemain [1997] Biochem Biophys Acta 1336: 331–341). In this report we demonstrate that ε-(2,4-dichlorophenoxyacetyl)-l-Lys (2,4D-Lys), one of the largest molecules of the series and one of the most potent inhibitors, is a highly permeant conjugate. Uptake of 2,4D-Lys by broad bean (Vicia faba) leaf discs is mediated by an active carrier system (Km1 = 0.2 mm; Vmax1 = 2.4 nmol cm−2 h−1 at pH 5.0) complemented by an important diffusive component. Among the compounds tested (neutral, basic, and acidic amino acids, auxin, glutathione, and sugars), only the aromatic amino acids clearly compete with 2,4D-Lys. The conjugate accumulates in the vein network, is exported toward the growing organs, and exhibits a distribution pattern different from that of the herbicide moiety. However, over time 2,4D-Lys progressively splits into 2,4D and lysine. Analyses by high-performance liquid chromatography and liquid scintillation spectrometry of the phloem sap collected from the castor bean system, used as a systemy test, indicate decreasing capacities of 2,4D, 2,4D-Lys, and glyphosate, respectively, to move from the epidermis cell wall to the sieve element. Our results show that it is possible to design synthesis of large-size xenobiotics (approximately 350 D) with a lipophilic pole, exhibiting high mobility within the vascular system.
We have recently synthesized a new type of molecule by conjugation of an α-amino acid and a pesticide, the latter being a xenobiotic with a triazole motif or an auxinic herbicide. The structures of these molecules are fundamentally different from those of the in vivo conjugates. In the case of the in vivo formation of an auxin-amino acid conjugate, the hormone is covalently bonded by an amidic bond between its carboxyl group and the α-amine function of the amino acid. By contrast, our synthetic conjugates are distinguished by their free amino acid function (Dufaud et al., 1994; Chollet et al., 1997). We showed first that some of these xenobiotics with a free α-amino acid function markedly inhibited Thr uptake by leaf tissues when applied at 1 mm conjugate against 1 mm amino acid. Thr uptake by minor veins was especially affected, contrarily to Suc loading, which remained unchanged (Dufaud et al., 1994). By contrast, other conjugates had only marginal or no effect on Thr uptake under these experimental conditions.
It was consequently necessary to analyze the structure-activity relationship of this kind of conjugate (Chollet et al., 1997). Phenoxyalkanecarboxylic acids were chosen as xenobiotics because of the wide variety of products available. This allowed the synthesis of derivatives varying by the position of the amine and carboxyl functions, their octanol/water partition coefficient (Kow), and their size. First, it was shown that as for the α-amino acid analogs (Li and Bush, 1992), stereochemistry is an important parameter in conjugate recognition. Second, hydrophobic or hydrophilic functions in the 4-position of the aromatic ring modify the biological activity of conjugates in terms of amino acid uptake inhibition. Chlorinated conjugates are highly potent inhibitors of neutral and acidic amino acid uptake at acidic pH and, to a lesser extent, of basic amino acid uptake. By contrast, some hydrophilic conjugates significantly inhibit amino acid influx only when they are present in concentrations 5 to 10 times greater. Third, for a similar Kow the increase in size of derivatives may strengthen inhibitory activity. It appears that the amino acid transport systems, which include a large number of transporters (Fisher et al., 1998), are capable of recognizing a wide range of conjugates of various sizes and Kows (Chollet et al., 1997).
Molecular recognition does not necessarily mean that conjugates with an α-amino acid function are taken up in plant cells via a specific carrier system of the plasma membrane, especially in the case of the large-sized chlorinated derivatives (>300 D). As a general rule the phloem mobility of pesticides is related to their physico-chemical properties and to plant parameters, especially phloem sap velocity and pH (Tyree et al., 1979; Kleier, 1988; Bromilow et al., 1991; Kleier et al., 1998 and refs. therein). In brief, most are acidic (carboxylic) in nature (e.g. auxinic herbicides) or esters, i.e. acid precursors (e.g. phenoxypropionic acid derivatives). Weakly acidic compounds (3 < pKa < 7) are, at least in part, in nonionic form in the phloem apoplast (pH of approximately 5). Once in the phloem sap (pH of approximately 8), the anionic form is trapped because of the membrane permeation difference between the nonionic and the anionic forms, and can be translocated at distance. No clear role was established for carrier involvement in the phloem transport of xenobiotics until the end of the 1980s (Bromilow et al., 1991). Only three compounds are well known to be transported by plasmalemma carriers: phosphite (Barchietto et al., 1989; Leconte, 1989) and glyphosate (Denis and Delrot, 1993) are handled by the phosphate carrier system, and paraquat is translocated by a diamine carrier (Hart et al., 1992). The common features of these molecules are that they are hydrophilic and of rather small Mr.
In this work we have examined whether larger-size xenobiotics, including lipophilic groups (such as various herbicides and fungicides), could be translocated by a plant carrier system. The chlorinated conjugate we chose was ε-(2, 4-dichlorophenoxyacetyl)-L-Lys (2, 4D-Lys; Fig. 1) for the following reasons: It is one of the largest molecules previously tested (349.2 D; Chollet et al., 1997); it is one of the most efficient inhibitors of the amino acid transport systems (Chollet et al., 1997); it can be obtained in a straightforward manner with satisfactory yield compared with other conjugates, thus allowing access to radiolabeled material; and in the event of phloem transport of this conjugate, its distribution in the whole plant could be compared with that of the available radiolabeled xenobiotic moiety (2, 4D). Furthermore, previous data (Dufaud et al., 1994) suggest that 2,4D-Lys is stable or poorly hydrolyzed in the apoplast.
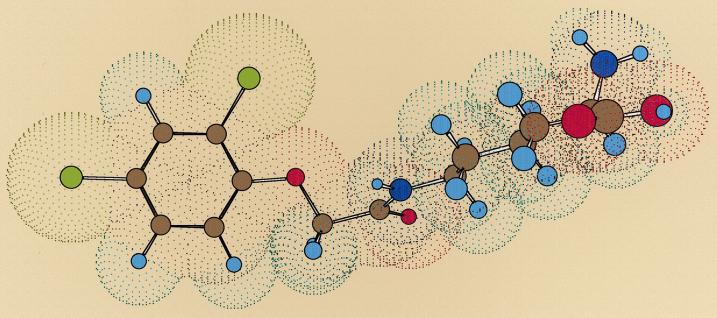
Figure 1.
Three-dimensional structure of 2,4D-Lys conjugate (Mr 349.2 D; Kow, −1.05 for zwitterionic form, predicted by the ACD/LogD program) obtained using Chem3D Pro, energy minimization with PM3 parameters, and simulating presence of water. Atoms are denoted by spheres in the following colors: carbon in gray, hydrogen in cyan, chlorine in green, oxygen in red, and nitrogen in blue.
RESULTS
Time Course Experiments and Localization of Uptake
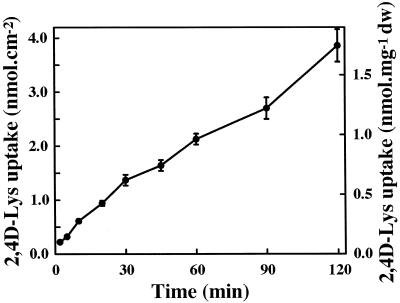
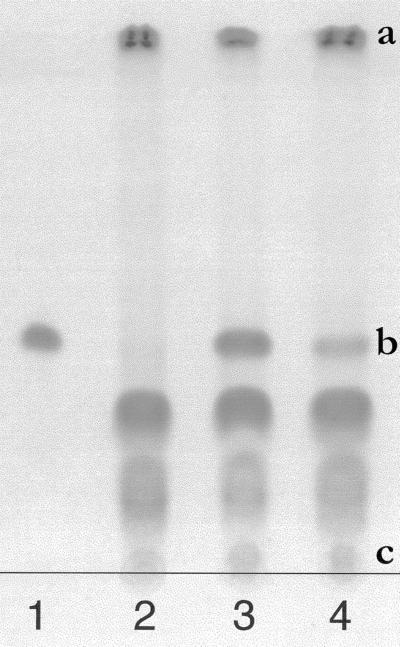
When broad bean (Vicia faba) foliar discs were incubated in the presence of 0.1 mm 2,4D-[3H]Lys, label uptake was roughly linear for at least 2 h (Fig. 2). This suggests that the labeled molecules enter the leaf cells and are not simply adsorbed on the cell walls and plasma membrane. Taking into account the water content of the leaf tissues (91% of fresh material) under our culture conditions, the concentration of label in the cells was equal to that of the incubation medium after 1 h. Qualitative approaches indicated that the conjugate was not immediately hydrolyzed after uptake (Fig. 3).
Figure 2.
Time course of 0.1 mm 2,4D-Lys uptake by broad bean leaf discs at pH 5.0.
Figure 3.
TLC of broad bean leaf tissue extracts. The plate was developed by ninhydrin. 1, 6.9 nmol 2,4D-Lys; 2, leaf tissues (control); 3, leaf tissues + 6.9 nmol 2,4D-Lys; 4, discs incubated for 6 h in a 1 mm 2,4D-Lys solution and then rinsed three time for 2 min each; a, Chlorophylls; b, 2,4D-Lys RF; c, Lys RF.
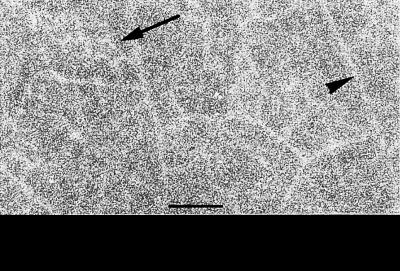
Autoradiographs of leaf tissues showed that labeled molecules were localized in mesophyll and veins (Fig. 4). The minor veins network was the most labeled tissue, suggesting a preferential uptake by veins. A similar picture was noted for amino acid uptake in broad bean foliar discs (Despeghel et al., 1980; Despeghel and Delrot, 1983; Chollet et al., 1997). By contrast, Suc is accumulated more intensively in the veins (Delrot and Bonnemain, 1981; Chollet et al., 1997), whereas iprodione, a permeant but non-systemic xenobiotic, remains excluded from the veins (El Ibaoui et al., 1986).
Figure 4.
Autoradiograph of broad bean leaf tissues after uptake of 2,4D-[3H]Lys. The tissues were incubated for 2 h in the presence of 0.5 mm 2,4D-[3H]Lys, rinsed, frozen, freeze dried, and then exposed for 45 d to hyperfilm βmax. The radioactivity appears in white. The background of the film is shown at the bottom of the figure. Arrow, Minor vein; arrowhead, middle vein. Scale bar = 1 mm.
Concentration Dependence of 2,4D-[3H]Lys Uptake
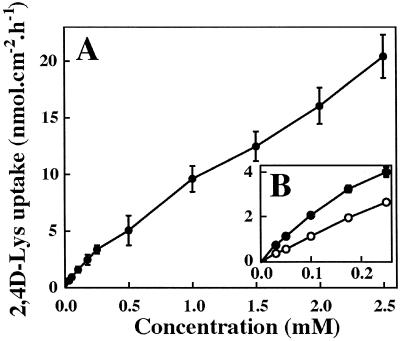
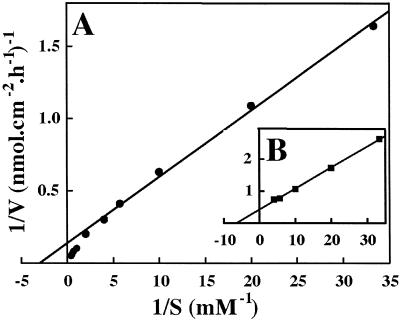
Uptake of 2,4D-[3H]Lys was measured after a 60-min incubation period (pH 5.0) for concentrations ranging from 0.03 to 2.5 mm. Higher concentrations were not used because of the limited water solubility of the conjugate. The data enabled a saturable component (between 0.03 and 0.25 or 0.5 mm, according to the sets) to be distinguished, suggesting that part of 2,4D-Lys uptake is carrier-mediated and a more or less linear component at higher concentrations (Fig. 5A). The apparent kinetic parameters deduced from Lineweaver-Burk plots (Fig. 6A) were Km1 = 0.3 mm and Vm1 = 7.1 nmol cm−2 h−1. Fifty micromolar carbonylcyanide m-chlorophenylhydrazone (CCCP) practically abolished the saturable component observed at the lowest concentrations (Fig. 5B). After subtracting the passive component from the total amount of 2,4D-Lys absorbed, the kinetic parameters of phase 1 (Fig. 6B) were Km1 = 0.16 mm and Vm1 = 2.4 nmol cm−2 h−1. Active uptake represents 53% of total influx at the lowest concentration (0.03 mm), 46% at 0.1 mm, and only 34% at 0.25 mm (Fig. 5B). In view of these results and the weak specific activity of labeled conjugate, we decided to operate at 0.1 mm concentration to study uptake properties of 2,4D-[3H]Lys by foliar tissues.
Figure 5.
Concentration dependence of 2,4D-Lys uptake (1-h incubation) by leaf tissues at pH 5.0. Each point was the mean of four sets of 12 discs (A) or 12 discs (B, complementary set). (●), Control; (○), 50 μm CCCP. Vertical bars were not shown when smaller than the symbols.
Figure 6.
Lineweaver-Burk plots of 2,4D-Lys uptake. A, Data from Figure 5A (●); B, data from Figure 5B after subtracting the CCCP-insensitive component; (▪), active component. Using Eadie-Hofstee plots, the points corresponding to the highest concentrations (1.5, 2.0, and 2.5 mm) parallel the vertical axis.
pH Dependence of 2,4D-Lys Uptake: Comparison with Lys and 2,4D
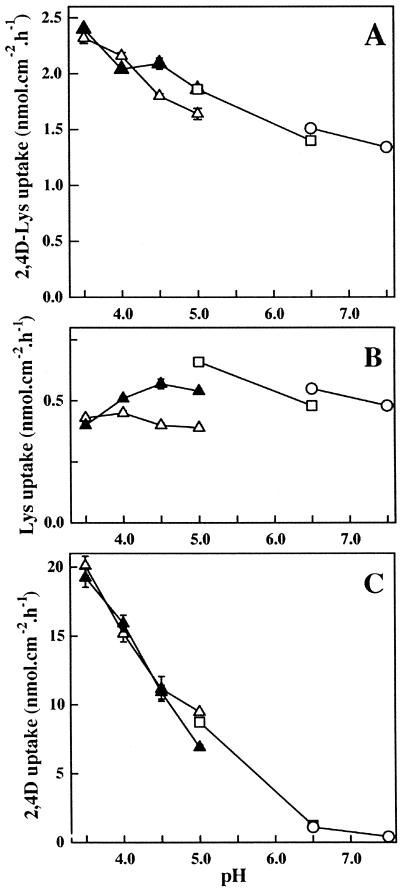
Uptake of 0.1 mm 2,4D-[3H]Lys was sensitive to pH changes in the incubation medium (Fig. 7A). Maximal uptake rates were found to occur in the acidic pH range (pH 3.5–4.0). Experiments performed using two different buffers at pH 6.5 (MES [2-(N-morpholino)ethanesulfonic acid] and HEPES [4-(2-hydroxyethyl)-1-piperazine-ethanesulfonic acid]) and at pH 5.0 (MES and citrate-phosphate) showed that the nature of the buffer had no clear effect on the conjugate uptake. However, at pH 5.0 2,4D-Lys influx was slightly (10%), but significantly, reduced in presence of citrate-phosphate buffer.
Figure 7.
pH-dependence of 0.1 mm 2,4D-Lys (A), 0.1 mm Lys (B), and 0.1 mm 2,4D (C) uptake by broad bean leaf tissues. Three different buffers were used: (▵) and (▴), 10 mm citrate, 20 mm disodium phosphate for pH 3.5 to 5.0; (□), 20 mm MES/NaOH for pH 5.0 and 6.5; and (○), 20 mm HEPES/NaOH for pH 6.5 and 7.5. (▵), standard medium; (▴) presence of 5 mm CaCl2 in the preincubation and the incubation solutions. Each point is the mean of 24 discs ± se. Vertical bars were not shown when smaller than the symbols.
By contrast, 0.1 mm [3H]Lys uptake was little dependent on the external pH and showed an apparent optimum at pH 5.0 with MES buffer (Fig. 7B). The citrate-phosphate buffer, which chelates calcium, reduced Lys uptake by 40% at this same pH value. Addition of CaCl2 (5 mm) to the incubation medium partially restored Lys uptake. It should be noted that the rate of 2,4D-[3H]Lys influx is 3- to 6-fold faster than [3H]-Lys uptake depending on the pH values.
[14C]2,4D uptake, which was weak at basic or near neutral pH values, increased exponentially at acidic pH (Fig. 7C). This is consistent with the reported uptake properties for this herbicide (Delrot and Bonnemain, 1991 and refs. therein).
Effect of p-Chloromercuribenzenesulfonic Acid (PCMBS) and Substances Affecting the Transmembrane Electrical and pH Gradients on the Uptake of 2,4D-Lys, Lys, and 2,4D
PCMBS, a non- or slowly permeant thiol reagent, reduced 0.1 mm 2,4D-[3H]Lys, 0.1 mm [3H]Lys, and 0.1 mm [14C]2,4D uptake of 22%, 33%, and 14%, respectively, when used at a concentration of 1 mm (Table I). Ten micromolar CCCP induced a 20% inhibition of 0.1 mm 2,4D-[3H]Lys uptake by leaf discs (Table I). However, it did not significantly affect 0.1 mm 2,4D-[3H]Lys uptake by mesophyll protoplasts (8.98 ± 0.10 nmol 10−6 protoplasts 30 min−1 in control, 8.17 ± 0.15 nmol 10−6 protoplasts 30 min−1 in treated sets, mean of three triplicates ± sd). This suggests that the active uptake of conjugate occurs mainly in the veins. Ten micromolar CCCP inhibited uptake of [3H]Lys and [14C]2,4D by foliar discs in the same proportions (approximately 35%; Table I). However, 100 mm KCl clearly inhibits Lys uptake while stimulating 2,4D uptake by 20% (Table I). This latter particularity may be due, at least in part, to the pH dependent uptake of 2,4D. Massive influx of K+ into the cell induced dramatic depolarization of the transmembrane potential difference (PD), thus stimulating the plasma membrane H+-ATPase activity. As a consequence, transmembrane ΔpH may increase, promoting 2,4D trapping (Delrot and Bonnemain, 1991). [3H] 2,4D-Lys uptake was less dependent to the transmembrane PD that the basic amino acid. The inhibitory effect of 100 mm KCl (about 10%) on the conjugate influx, not strictly significant in Table I (P = 0.07), was also observed in a complementary experiment (data not shown). It represented about 20% of the 50 μm CCCP-sensitive component.
Table I.
Effect of 1 mm PCMBS, 10 μm CCCP, and 100 mm KCl on 0.1 mm 2,4D-Lys, 0.1 mm lysine, and 0.1 mm 2,4D uptake by broad bean leaf discs
| Treatment | 2,4D-Lys | Lys | 2,4D | |||
|---|---|---|---|---|---|---|
| nmol cm−2 h−1 | % | nmol cm−2 h−1 | % | nmol cm−2 h−1 | % | |
| Control | 1.43 ± 0.05 | 100.0 | 0.64 ± 0.02 | 100.0 | 6.35 ± 0.28 | 100.0 |
| PCMBS | 1.11 ± 0.03*** | 77.6 | 0.43 ± 0.01*** | 67.2 | 5.49 ± 0.22* | 86.4 |
| CCCP | 1.17 ± 0.02*** | 81.8 | 0.42 ± 0.01*** | 65.6 | 4.10 ± 0.21*** | 64.6 |
| KCI | 1.30 ± 0.03 (NS) | 90.9 | 0.39 ± 0.01*** | 61.0 | 7.84 ± 0.39* | 123.5 |
Means ± se, n = 24. NS, Not significant. Asterisk indicates 0.05 > P > 0.01; triple asterisks indicate P < 0.001.
CCCP, which dissipates the proton motive force, is usually used at concentrations ranging from 10 to 50 μm. Depending on the tissue, 20 to 80 μm are required to abolish the active component of the transmembrane PD completely (Renault et al., 1989; Fleurat-Lessard et al., 1997). Fifty micromolar CCCP inhibited 0.1 mm [3H] 2,4D-Lys uptake by nearly 50% (46% in Fig. 5B; 48% in Table II). PCMBS is generally used at concentrations from 1 to 5 mm. Infiltration of broad bean leaf tissues with 2 mm PCMBS does not affect photosynthesis or the transmembrane PD for at least 3 h (Bourquin et al., 1990). At this latter concentration the thiol reagent reduced 0.1 mm 2,4D-Lys uptake by 38% (Table II). This represented about 75% of the 50 μm CCCP-sensitive component. One or 2 mm PCMBS added to 50 μm CCCP did not increase the inhibition induced by the protonophore alone (Table II). As a consequence, the decrease of 2,4D-Lys uptake observed in the presence of 1 or 2 mm PCMBS (Tables I and II) is not the result of an effect of this thiol reagent on phospholipid bilayer permeability. Its blocking effect on carriers—and especially on the system translocating the conjugate—cannot be seen when the transport systems are not operating due to a lack of energy.
Table II.
Effect of 50 μm CCCP, 2 mm PCMBS, and 50 μm CCCP + 1 or 2 mm PCMBS on 0.1 mm 2,4D-Lys uptake by broad bean leaf discs
| Treatment | nmol cm−2 h−1 | % |
|---|---|---|
| Control | 1.42 ± 0.23 | 100 |
| CCCP | 0.74 ± 0.15*** | 52.1 |
| PCMBS | 0.88 ± 0.14*** | 62 |
| CCCP + PCMBS (1 mm) | 0.72 ± 0.15*** | 50.7 |
| CCCP + PCMBS (2 mm) | 0.72 ± 0.16*** | 50.7 |
Means ± se, n = 18; triple asterisks indicate P < 0.001.
Effect of Various Substrates on 0.1 mm 2,4D-[3H]Lys Uptake
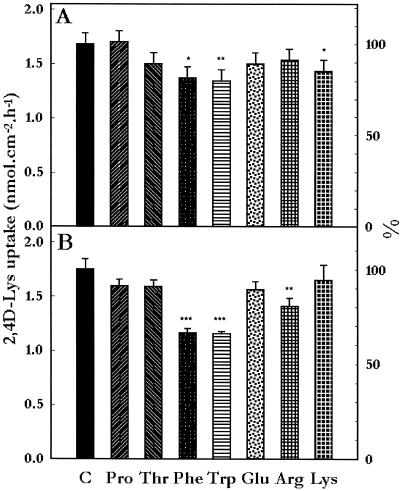
Several amino acids, a tripeptide (glutathione), a weak auxin (indole butyric acid), and two sugars (Glc and Suc) were tested against 0.1 mm 2,4D-[3H]Lys. At the concentration of 1 mm the most competitive substances were Trp and Phe (Fig. 8A). These two aromatic amino acids inhibit the absorption of conjugate (0.1 mm) by 20%. This represents about 40% of the 50 μm CCCP-sensitive uptake component (Fig. 5B).
Figure 8.
Effect of various amino acids at 1 mm (A) and at 10 mm (B) concentration on 0.1 mm 2,4D-Lys uptake by broad bean leaf discs at pH 5.0. It must be taken in mind that the active component of the conjugate influx is less than the one-half of the total uptake. Data are the mean of 24 discs ± se. Asterisk, 0.05 > P > 0.01; double asterisks, 0.01 > P > 0.001; triple asterisks, P < 0.001.
At 10 mm concentration Trp and Phe inhibited 0.1 mm 2,4D-Lys uptake by 35%, i.e. the major part (about 70%) of the CCCP-sensitive component. At a same concentration, glutathione (Table III) and other amino acids had only a marginal effect, except Arg, which reduced conjugate uptake by 20% (Fig. 8B). Glc and Suc, at 10 mm concentration, had no effect (Table III). This latter result indicates that the inhibition induced by the aromatic amino acids is not due to competition for the proton motive force.
Table III.
Effect of 10 mm sugars, 10 mm glutathione, and 1 mm IBA on 0.1 mm 2,4D-Lys uptake by broad bean leaf discs
| Treatment | nmol cm−2 h−1 | % |
|---|---|---|
| Control | 1.75 ± 0.12 | 100.0 |
| Glc | 1.73 ± 0.11 (NS) | 98.9 |
| Suc | 1.94 ± 0.10 (NS) | 110.9 |
| Control + dithiothreitol | 1.54 ± 0.13 | 100.0 |
| Glutathione + dithiothreitol | 1.35 ± 0.12 (NS) | 87.7 |
| Control + 0.5% EtOH | 1.58 ± 0.07 | 100.0 |
| Indole butyric acid + 0.5% EtOH | 1.82 ± 0.09 (NS) | 115.0 |
Mean ± se, n ≥ 12. NS, Not significant.
Long-Distance Transport and Metabolism
Thirty minutes after the deposition of 2,4D-[3H]Lys, [3H]Lys, or [14C]2,4D on leaves 4 and 5 (Fig. 9), labeled molecules were transported throughout the broad bean plants. However, the distribution of labeling was shown to be dependent on the nature of each of the molecules applied on these two exporting leaves (Table IV). In the case of 2,4D-[3H]Lys deposition, the transport of labeled molecules toward the roots (root basis and root apical part) was greater than for the two other sets ([3H]Lys and [14C]2,4D depositions). In a similar manner, transport to the expanding internode 9 was more pronounced, as was the label penetration in mature leaves L3 and L6. This triple specificity suggests that the 2,4D-[3H]Lys conjugate moves mainly without hydrolysis during this short period. After 6 h of transport, 2,4D still showed a lesser capacity to penetrate to the roots and the young apical bud than the label from the other two substances. After 24 h, the herbicide was shown to accumulate in the young internodes, which elongated rapidly and curved (Fig. 10). The same phenomenon was observed, but to a lesser extent, when 2,4D-[3H]Lys was applied to leaves L4 and L5 (Fig. 10). This suggests than the conjugate is progressively hydrolyzed, releasing 2,4D. Further experiments confirmed that this was indeed the case. Foliar discs were incubated for 1 h in a 1 mm 2,4D-Lys solution. The foliar apoplast was then washed three times for 2 min each with buffer and the discs were placed for 5 or 23 h in the dark in an atmosphere saturated with water. Extraction after these times in boiling ethanol provides, respectively, 1.19 ± 0.18 and 2.52 ± 0.50 nmol cm−2 (means ± sd, n = 4 sets of 12 discs) of72,4D (HPLC analysis), whereas the quantity of 2,4D-Lys absorbed was 9.6 ± 1.1 nmol cm−2 (means ± se, n = 12). Using this method the 2,4D-Lys amount could not be estimated by HPLC analysis because of interference with other extracted polar compounds.
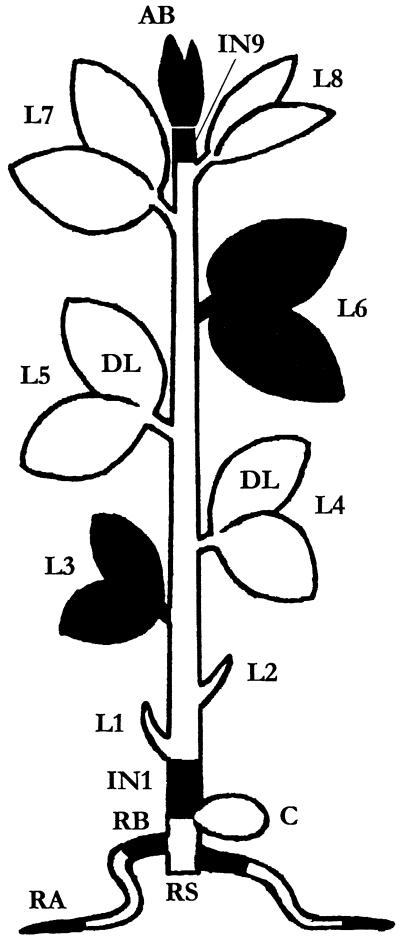
Figure 9.
Long-distance transport of labeled molecules from the donor leaves (DL) L4 and L5. The organs selected (in black) for label distribution from 2,4D-[3H]Lys, [3H]Lys, and [14C]2,4D were the apical bud (AB), internode 9 (IN9), leaves 6 (L6) and 3 (L3), internode 1 (IN1), the basis (RB), and the apical part (RA) of the secondary roots. RS, Root system.
Table IV.
Distribution of labeled molecules in broad bean tissues after deposition of 0.4 μmol [3H]Lys, [14C]2,4D, or 2,4D-[3H]Lys on each of the mature leaves L4 and L5
| Tissues | 30
min
|
6 h
|
24 h
|
||||||
|---|---|---|---|---|---|---|---|---|---|
| Lys | 2,4D | 2,4D-Lys | Lys | 2,4D | 2,4D-Lys | Lys | 2,4D | 2,4D-Lys | |
| AB | 1.0 ± 0.6 | 0.8 ± 0.7 | 1.2 ± 0.9 | 59.9 ± 4.7 | 2.9 ± 1.1 | 74.1 ± 18.0 | 132.8 ± 10.4 | 115.8 ± 57.4 | 124.5 ± 20.2 |
| IN 9 | 1.1 ± 1.0 | 1.3 ± 0.4 | 9.7 ± 7.2 | 51.3 ± 8.4 | 10.4 ± 3.8 | 48.0 ± 12.0 | 125.9 ± 18.5 | 265.4 ± 126.8 | 174.0 ± 33.1 |
| L6 | 2.4 ± 1.1 | 3.7 ± 2.1 | 9.2 ± 4.3 | 1.5 ± 1.5 | 0.8 ± 0.1 | 5.2 ± 1.1 | 4.1 ± 1.0 | 4.4 ± 3.5 | 9.0 ± 1.4 |
| L3 | 2.6 ± 0.4 | 0.6 ± 0.3 | 6.9 ± 4.6 | 4.1 ± 1.8 | 5.0 ± 4.5 | 3.8 ± 1.1 | 2.6 ± 0.8 | 1.1 ± 0.8 | 3.3 ± 1.1 |
| IN 1 | 0.6 ± 0.1 | 4.4 ± 4.3 | 1.9 ± 1.9 | 6.9 ± 0.6 | 9.8 ± 4.2 | 7.1 ± 3.0 | 7.4 ± 0.8 | 96.7 ± 40.7 | 33.5 ± 23.5 |
| RB | 0.6 ± 0.3 | 0.5 ± 0.4 | 4.7 ± 3.2 | 6.4 ± 1.2 | 1.0 ± 0.3 | 10.4 ± 1.1 | 8.7 ± 1.7 | 20.9 ± 9.5 | 18.2 ± 2.5 |
| RA | 0.7 ± 0.1 | 0.6 ± 0.5 | 6.1 ± 2.7 | 6.8 ± 1.5 | 1.5 ± 1.1 | 9.3 ± 2.2 | 19.5 ± 2.3 | 10.4 ± 6.3 | 35.6 ± 6.6 |
The harvest of material takes place 30 min, 6 h, or 24 h after deposition. Results are expressed in nanomoles per gram of dry wt. Mean of three triplicates ± sd. AB, Apical bud; IN1, internode 1; IN 9, internode 9; L3, leaf 3; L6, leaf 6; RA, root apex; RB, root basis (see Fig. 9). The dry wt. of AB, IN9, L6, L3, IN1, RB, and RA was, respectively, 30.6 ± 2.0, 9.2 ± 1.0, 164.7 ± 5.6, 93.3 ± 23.2, 44.5 ± 2.9, 30.9 ± 1.7, and 40.6 ± 3.8 mg (means ± se, n = 27, set 30 min).
Figure 10.
Broad bean plant reaction 24 h after the deposition of 0.4 μmol of Lys (B), 2,4D-Lys (C), and 2,4D (D) on each of the second and third bifoliate leaves (L4 and L5). A, Control.
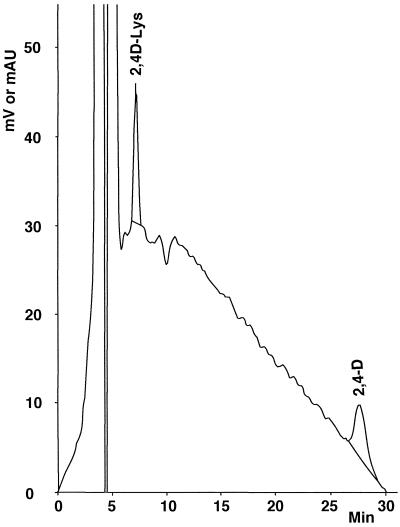
We used the castor bean system to check the phloem mobility of 2,4D-Lys. This system enables phloem-mobile molecules (2,4D and glyphosate) to be distinguished from non phloem-mobile molecules (fenpiclonil; Table V), and it may therefore be employed as a test for systemy. Thus, it was confirmed that the 2,4D-Lys conjugate is indeed phloem-mobile and also that it becomes metabolized over time, since we also found 2,4D in the phloem sap (Fig. 11; Table V). However, the observed 2,4D/2,4D-Lys ratio (1.3) is probably over-estimated. In this regard peptidases liberated from sectioned tissues may hydrolyze the conjugate during the formation of phloem sap droplets. Under our experimental conditions the concentration of 2,4D-Lys is almost double in the phloem sap than in the incubation medium of the cotyledons. This result is halfway between that of 2,4D (7-fold) and that of glyphosate (0.2-fold) under the same conditions (Table V). Complementary experiments showed that systemy data are dependent on environmental conditions. Thus, when castor bean plants grew at room temperature (21 ± 2°C), the 2,4D-Lys concentration in phloem sap was 0.12 ± 0.04 mm (means ± se, n = 10), with a smaller 2,4D/2,4D-Lys ratio (0.81).
Table V.
Ricinus phloem sap analysis after soaking cotyledons in a solution containing various xenobiotics
| Xenobiotics | Incubation Medium | Phloem Sap |
|---|---|---|
| mm | ||
| 2,4-D | 0.10 | 0.70 ± 0.01 |
| Glyphosate | 0.10 | 0.02 ± 0.00 |
| Fenpiclonil | 0.10 | ND |
| 2,4D-Lys | 0.10 | 0.19 ± 0.04* |
Phloem sap collection started 2 h after the beginning of soaking and lasted for 2 h. Means ± se, n = 10. ND, Not detected. Asterisk indicates that phloem sap contains 2,4D at the concentration of 0.25 ± 0.04 mm.
Figure 11.
HPLC analysis of Ricinus phloem sap after dilution with pure water (1 + 9, v/v). Injection of a 20-μL aliquot. For details, see Table V.
The castor bean system was also used to determine whether 2,4D-Lys is an ambimobile xenobiotic, as suggested by the presence of label in the broad bean mature leaves L3 and L6 after a 30-min transport of 2,4D-Lys (Table IV). Under our experimental conditions 2,4D-Lys was present in the castor bean vascular sap at a low concentration (6.5 ± 4.5 μm, means ± sd, n = 5 sets of three to five plants), i.e. about one-thirtieth of that found in the phloem sap (Table V). By contrast, no 2,4D was detected.
DISCUSSION
2,4D-Lys Uptake Does Not Need Previous Cleavage in the Apoplast, But the Conjugate Is Progressively Hydrolyzed to Lys and 2,4D
Several enzymes involved in the hydrolysis of natural auxin conjugates and oligopeptides have been characterized in the last few years (Bartel and Fink, 1995; Martin and Slovin, 2000). In view of the large specificity of some of these enzyme systems, the metabolic fate of 2,4D-Lys needed some investigation. The relative stability of 2,4D-Lys in the apoplast has been previously suggested (Dufaud et al., 1994). It was shown that 0.1 mm 2,4D inhibits in, a non-specific manner, Thr and Suc uptake by leaf tissues after 1 h of incubation. However, under the same conditions, 2.5 mm 2,4D-Lys dramatically inhibits Thr uptake without affecting the influx of Suc. This result indicates that the conjugate is stable in the apoplast or is not sufficiently hydrolyzed to affect Suc uptake during this incubation period. In a similar manner, uptake properties of 2,4D-[3H]Lys show that the conjugate is taken up in an essentially unchanged form, at least in short-term experiments (1 h). In particular, the influx rate of 2,4D-[3H]Lys is 3- to 6-fold higher than that of [3H]Lys, depending on the pH of the incubation medium. Furthermore, the pH sensitivity of uptake clearly differs for the two compounds (Fig. 7, A and B).
However, the auxinic effect observed 24 h after deposition of 2,4D-Lys on mature leaves (Fig. 10) suggests that the conjugate is progressively hydrolyzed within the tissues to Lys and 2,4D. Analysis of castor bean phloem sap points out the ability of 2,4D-Lys to cross membranes and to enter the phloem and it clearly shows that plants possess an enzymatic system able to hydrolyze this atypical conjugate.
2,4D-Lys Uptake Involves Two Components, an Active Carrier-Mediated System and Diffusion
Analysis of the concentration-dependence of 2,4D-Lys uptake by leaf discs (Figs. 5 and 6) and the inhibitory effects of PCMBS (Table II) and some competitors demonstrate that part of this 350-D chlorinated conjugate influx is carrier-mediated. Furthermore, the inhibitory effect of CCCP (Fig. 5B; Table II), a protonophore that decreases the two components of the proton motive force, is consistent with the involvement of an active uptake system.
However, compared with natural substrate uptake by the same material (broad bean leaf discs), 2,4D-Lys influx exhibits two particularities. The first concerns diffusion, which clearly becomes the main component of the influx for external concentrations higher than 0.1 mm. The importance of the diffusive component may be related to the structure of this polarized molecule (Fig. 1), which exhibits a highly lipophilic pole. By contrast, for 1 mm Suc, the CCCP-insensitive uptake by leaf tissues represents only 20% of the total uptake (Delrot and Bonnemain, 1981). Similar data were reported for Gly and Thr uptake (Despeghel et al., 1980; Despeghel and Delrot, 1983). The second particularity concerns the relatively high affinity of the transport system that manipulates 2,4D-Lys. The apparent Km value at pH 5.0 (about 0.2 mm) is 10- to 20-fold lower than the Km1 values reported for Suc, Gly, and Thr uptake in similar pH conditions (Despeghel et al., 1980; Delrot and Bonnemain, 1981; Despeghel and Delrot, 1983).
Like amino acids (Despeghel et al., 1980; Despeghel and Delrot, 1983), 2,4D-Lys penetrates into mesophyll and vascular tissues with a preferential accumulation in the vein network. By contrast, the dipeptide Gly-Gly seems weakly loaded in the minor veins (Jamaï et al., 1994). Furthermore, our data suggest that uptake of 2,4D-Lys from 0.1 mm solution is weakly, but significantly, decreased in the presence of the citrate-phosphate buffer (Fig. 8A). However, considering the active component of the conjugate influx, the inhibition noted at pH 5.0 reaches about 20%. This decrease, presumably due to Ca2+ chelation by citrate, is completely reversed by the addition of 5 mm CaCl2 in the incubation medium (Fig. 8A). The dependence of amino acid uptake on the buffers employed has been reported on in the past and it has been noted that neutral amino acid accumulation is affected by the citrate-phosphate buffer (Otsiogo-Oyabi and Roblin, 1985), as well as by typical chelating agents like EGTA and EDTA (Rickauer and Tanner, 1986). Basic amino acid accumulation is also strongly inhibited by Ca2+ deficiency (Fig. 8B). By contrast, buffers and Ca2+ additions do not affect 2,4D uptake.
The pH dependence of 2,4D-Lys uptake is similar to that of γ-glutamyl-cysteinyl-Gly (GSH; Jamaï et al., 1996) and rather similar to that of neutral and acidic amino acids (Despeghel et al., 1980; Despeghel and Delrot, 1983; Chollet et al., 1997), but differs from that of Gly-Gly, which is optimal at pH 6.0 (Jamaï et al., 1994).
Among Various Tested Substrates, Only Aromatic Amino Acids Clearly Inhibit 2,4D-Lys Uptake
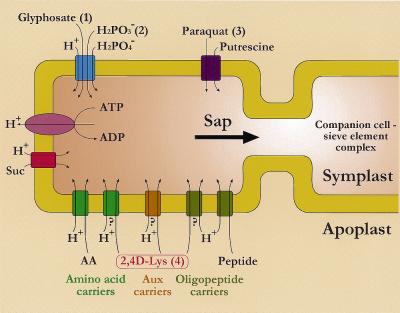
In the 1980s, physiological data suggested that amino acid transport across the plasma membrane was mediated by a small number of distinct transport systems (Kinraide and Etherton, 1980; Wyse and Komor, 1984). This would mean that practically all neutral amino acids are taken up by a single transport system exhibiting a very wide specificity. Later, transport studies with isolated membrane vesicles suggested that at least four amino acid transport systems, two neutral, one acidic, and one basic, are present in the plasma membrane (Li and Bush, 1990, 1991). In the last few years molecular approaches have permitted these initial results to be improved and complemented. A large number of amino acid carrier genes have been identified in Arabidopsis (Chen and Bush, 1997; Fisher et al., 1998 and refs. therein). Based on sequence homology, these transporters are classified into two superfamilies, the amino acid-polyamine-choline facilitators and the amino acid transporters. The amino acid-polyamine-choline facilitators are subdivided into two families, the cationic amino acid transporters and the γ-aminobutyrate permease-related family. The ATF superfamily is comprised of the Lys and His transporters (LHTs), the Pro transporters, the putative auxin transporters (AUXs), and the amino acid permeases (AAPs), which exhibit a wide specificity for amino acids. The latter are classified as neutral and acidic amino acid transporters or as general amino acid transporters. Carriers from the oligopeptide transport systems must also be taken into consideration (Fig. 12).
Figure 12.
Possible carriers involved in the uptake of xenobiotics by the phloem tissue (apoplastic loading); 1, from Denis and Delrot, 1993; 2, from Leconte, 1989; 3, from Hart et al., 1992; and 4, this work (hypotheses). Carrier-mediated uptake of glyphosate, paraquat, and 2,4D-Lys is complemented by a diffusive component.
As a consequence, the competitors we chose at the first step of our investigations were neutral amino acids (Pro, Thr, Phe, and Trp), basic (Lys and Arg) and acidic (Glu) amino acids and an auxin (IBA). GSH, a peptide with an α-amino acid function, was also tested. From our work there is no evidence that 2,4D-Lys uptake is mediated by an AUX carrier, as IBA does not compete with the conjugate (Table III). However, implication of an AUX carrier cannot be definitively discarded, taking in account the structural analogies between auxins and the conjugate.
In a similar manner, there is no evidence indicating a clear contribution of GSH transporters as well as Pro and basic amino acid transporters. Furthermore, our results show that although 2,4D-Lys acts as a potent competitor of Thr and Glu uptake (Chollet et al., 1997), the reverse is not true (Fig. 8). Among all the compounds we have tested, only Trp and Phe (Kow of approximately −1.4; Bromilow et al., 1991), which exhibit an hydrophobic side chain, clearly inhibit 2,4D-Lys uptake. It is known that these aromatic amino acids strongly compete for the Pro uptake mediated by several AAPs from Arabidopsis (Fisher et al., 1995) and by AAP2 from broad bean (Montamat et al., 1999). These initial results form a basis for studies using plant and yeast mutants to determine the nature of the carrier(s) involved in 2,4D-Lys uptake.
2,4D-Lys Is a Phloem-Mobile Conjugate
That a xenobiotic enters a cell does not prove ipso facto its capacity to penetrate into the phloem. Thus, iprodione, which is taken up by mesophyll cells, remains excluded from the vein network (El Ibaoui et al., 1986). Many pesticides, particularly fungicides, are only penetrating. Among phloem-mobile xenobiotics, three are translocated by identified carrier systems: phosphite, paraquat, and glyphosate (Barchietto et al., 1989; Leconte, 1989; Hart et al., 1992; Denis and Delrot, 1993; Fig. 12). Having demonstrated the capacity of 2,4D-Lys to penetrate foliar tissues it was advisable to explore whether this 350-D chlorinated xenobiotic was also phloem-mobile.
Complementary approaches conducted on broad bean plants, a species with a typical apoplastic phloem loading (Bourquin et al., 1990; Van Bel, 1993), and on Ricinus, a symplastic-apoplastic loader (Orlich and Komor, 1992), demonstrated that 2,4D-Lys was indeed phloem mobile (Table V). Under our experimental conditions 2,4D displayed the highest capacity of moving from the external medium to the sieve element followed by 2,4D-Lys and then glyphosate. The weak concentration of glyphosate found in phloem sap may be surprising in view of its well-known systemic properties (Delrot and Bonnemain, 1991 and refs. therein) and to the permeability of the epidermis cell wall to hydrophilic molecules at this stage of development (Schobert and Komor, 1989).
Short-term experiments (Table IV, 30 min) also show that the distribution pattern of 2,4D-Lys in the whole plant is different from that of 2,4D. In particular, the conjugate is more widely distributed to the apical part of roots than the herbicide. Furthermore, they suggest that 2,4D-Lys penetrates quickly in the non-donor mature leaves via the vascular sap. The presence of the conjugate in the castor bean vascular sap clearly indicates that part of the conjugate exported toward the roots is recycled upward via the vessels. The 2,4D-Lys concentration in the vascular sap is low compared with that found in the phloem sap, but the relative importance of the vascular and phloem sap fluxes must also be taken into consideration.
In conclusion, our results show that in addition to a few plasma membrane carriers that handle small-sized hydrophilic xenobiotics as previously reported (Fig. 12), others can translocate relatively large xenobiotics (approximately 350 D) with a lipophilic moiety. Moreover, 2,4D-Lys is phloem mobile and exhibits a distribution pattern in the whole plant different from that of 2,4D, especially greater efficiency in reaching the apical part of the roots. Its gradual scission underlines the broad spectrum activity of peptidases. It is well known that phloem-mobile and ambimobile fungicides do not exist as yet. They could control plant vascular diseases such as dying arm disease on grapevine. In this regard we are now studying the structure-phloem mobility relationships of various conjugates including fungicide derivatives.
MATERIALS AND METHODS
Synthetic Methods
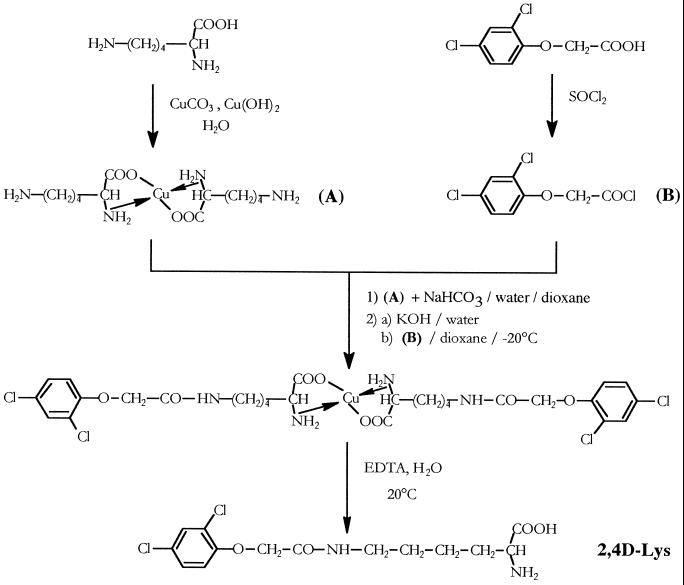
We have previously described the detailed synthesis of our conjugates (Chollet et al., 1997). In brief, three steps (Fig. 13) were required to make the linkage between the ε-amino group of Lys and the carboxyl group of 2,4D: selective blocking of the α-amino acid group of L-Lys with a copper salt; coupling of the ε-amino group of protected Lys with the phenoxyalkanecarboxylic acid chloride using the Schotten-Baumann reaction; and selective deprotection of the α-amino acid group with EDTA. The 2,4D-Lys conjugate was obtained as white crystals in an overall yield of around 50%. Further purification can be achieved by recrystallization in water.
Figure 13.
Reaction scheme for the synthesis of 2,4D-Lys.
Synthesis of 2,4D-[3H]Lys was performed in a similar manner by incorporating 5 mCi of L-[4,5–3H]Lys (2.7–3.7 TBq mmol−1, Amersham, France) to the medium at the time of the first step when blocking the α-amino acid group (Fig. 13). The specific activity of 2,4D-[3H]Lys obtained was 11 MBq mmol−1, except for experiments reported in Table II (39 MBq mmol−1).
Uptake Experiments
Plant Material
Broad bean (Vicia faba cv Aguadulce) plants were grown in a growth cabinet as described previously (Chollet et al., 1997). The experiments were performed on plants possessing four mature bifoliate leaves.
Uptake in Leaf Discs
Leaf discs (1.13-cm2 surface) were obtained with a cork borer from the third and the fourth mature bifoliate leaves after the lower epidermis had been stripped off to facilitate exchange between the tissues and the incubation medium. They were then floated on a preincubation medium containing, except stated otherwise, 20 mm MES as buffer (pH 5.0), 250 mm mannitol, 0.5 mm CaCl2, and 0.25 mm MgCl2. After a 30-min preincubation period the discs were incubated in the same buffered solution containing, usually, 0.1 mm 2,4D-[3H]Lys with or without another product. Incubation was run under mild agitation on a reciprocal shaker at room temperature. At the end of incubation the disc apoplast was rinsed (three times for 2 min each) in a solution similar to the preincubation medium. Each disc was then digested in a mixture of perchloric acid (65% [w/w]; 25 μL), hydrogen peroxide (33% [w/w]; 50 μL), and Triton X-100 (1 g l−1; 50 μL). The radioactivity was counted by liquid scintillation spectrometry (Packard Tricarb 1900TR, Packard Instruments, Rungis, France). Furthermore, some discs were dry-ice frozen, lyophilized, and autoradiographed (hyperfilm βmax, Amersham).
Except for time dependence of 0.1 mm 2,4D-[3H]Lys uptake, the duration of incubation period was 1 h. In the case of kinetic measurement, the concentration range tested varied from 0.03 to 2.5 mm. To compare the influence of external pH on 0.1 mm 2,4D-[3H]Lys uptake, 0.1 mm [3H]Lys uptake, and [14C]2,4D uptake, the buffers used were sodium citrate (10 mm)/disodium phosphate (20 mm) from pH 3.5 to 5.0, 20 mm MES/NaOH for pH 5.0 and 6.5, and 20 mm HEPES/NaOH for pH 6.5 and 7.5. [14C]2,4D (0.44 GBq mmol−1) was supplied by Sigma (France).
The metabolic fate of molecules absorbed by foliar tissues was examined in a first approach by thin layer chromatography (TLC). Broad bean leaf discs were incubated for 6 h in a standard solution containing 1 mm 2,4D-Lys. The disc apoplasts were then rinsed (three time for 2 min each) and each disc was then put in 10 mL of boiling 80% (w/v) ethanol. After extraction, ethanol was evaporated under vacuum and the residue was dissolved in 1 mL of 80% (w/v) ethanol. A 100-μL portion was deposited on a TLC plate (Silica gel 60, Merck, Rahway, NJ), and migration was effected in butanol:acetic acid:water (8:2:2; v/v). After 3 h, the solvent front reached about 10 cm; the migration was stopped and the plate was developed using 0.2% (w/v) ninhydrin.
Uptake in Protoplasts
The third and fourth bifoliate broad bean leaves were used to prepare the protoplasts. After peeling the lower epidermis of the leaves, leaf pieces were cut and incubated for 90 min on a medium containing 500 mm mannitol, 0.25 mm MgCl2, 0.5 mm CaCl2, 0.2% (w/v) polyvinylpyrrolidone, 0.1% (w/v) bovine serum albumin, 2% (w/v) cellulase Onozuka R-10 (KALYS, Roubaix, France) 0.5% (w/v) pectolyase macerozyme R-10 (KALYS), and 20 mm MES/NaOH (pH 5.6). At the end of incubation the protoplast suspension was recovered and centrifuged for 9 min at 200g in a tube containing 1 mL of 100% (w/v) Percoll (Pharmacia Biotech, Piscataway, NJ), 500 mm mannitol, and 20 mm MES/NaOH (pH 5.5). The supernatant was removed and the protoplasts were mixed with the 100% (w/v) Percoll. Five milliliters of 25% (w/v) Percoll solution and 4 mL of 5% (w/v) Percoll solution were carefully added to this protoplast suspension and this gradient was centrifuged for 6 min at 200g. Only the protoplasts sedimenting at the 5%/25% (w/v) interface were used for the uptake experiments.
Uptake experiments were started by adding one volume of protoplast suspension to one volume of incubation medium containing 2,4D-[3H]Lys 0.2 mm with or without (control) CCCP 20 μm. At selected times 400-μL aliquots of the incubation medium were sampled and overlaid on 500 μL of silicone oil. Uptake experiments were terminated by sedimenting the protoplasts through the silicone oil at 2,000g for 1 min in a microfuge (M11, Beckman Instruments, Fullerton, CA). The tip of the tubes containing the protoplasts was cut and deposited in a scintillation vial with 4 mL of Ecolite (ICN, Pharmaceuticals France SA, Orsay, France). The radioactivity was measured by liquid- scintillation counting.
Long-Distance Transport
These experiments were performed on broad bean plants possessing four mature bifoliate leaves. After a gentle application of 1% (w/v) Tween 80 on the upper epidermis, 200 μL of 2,4D-[3H]Lys 2 mm, [3H]Lys 2 mm, or [14C]2,4D 2 mm were smeared on the second and third bifoliate leaves, i.e. 0.4 μmol on each leaf. The plants were harvested 30 min, 6 h, or 24 h after the labeled molecule deposit. Then they were dry-ice frozen, lyophilized, and the radioactivity of selected organs (Fig. 9) was counted by liquid-scintillation spectrometry.
Tests of Systemy
Plant Material
Seeds of castor bean (Ricinus communis cv Sanguineus) were obtained from Vilmorin (La Menitré, France). The seeds were placed in humid cotton for 48 h at 27°C ± 1°C prior to sowing in wet vermiculite. Seedlings were grown in a humid atmosphere (80% ± 5%) at 27°C ± 1°C.
Sap Collection and Analysis
Five days after sowing, the endosperm of seedlings was carefully removed without bending or crushing the cotyledons (Kallarackal et al., 1989). These organs were then incubated in buffer solution containing 20 mm MES (pH 5.0), 0.25 mm MgCl2, and 0.5 mm CaCl2 supplemented with 0.1 mm 2,4D-Lys, 0.1 mm 2,4D, 0.1 mm [14C]glyphosate, or 0.1 mm fenpiclonil. Two hours later the hypocotyl was severed in the hook region for phloem exudation. The phloem sap was collected from the upper part of hypocotyl for 2 h and was stored in ice until analysis. To reduce evaporation, the sap collection was performed in Plexiglas microcabinet that was laid out with wet filter paper.
The phloem sap was analyzed by liquid scintillation spectrometry ([14C]glyphosate) and by HPLC after dilution in pure water (2,4D-Lys, 2,4D, and fenpiclonil). We employed reversed phase chromatography using a Discovery C16 RP-amide column (Supelco, Bellefonte, PA) according to the procedure practiced in Table VI.
Table VI.
Chromatographic data for tested products
| Product | Gradient
|
Delivery | Detection UV | Retention Time | ||
|---|---|---|---|---|---|---|
| Time | Water + TFA 0.1% | CH3CN | ||||
| min | mL min−1 | nm | min | |||
| 2,4D | t = 0 | 50 | 50 | 0.8 | 202 | 11 |
| t = 15 | 50 | 50 | ||||
| 2,4D-Lys | t = 0 | 70 | 30 | 0.8 | 202 | 7 (30 min for 2,4D) |
| t = 35 | 60 | 40 | ||||
| t = 45 | 70 | 30 | ||||
| Fenpiclonil | t = 0 | 40 | 60 | 0.8 | 215 | 8.5 |
| t = 20 | 40 | 60 | ||||
On the other hand, vascular sap was collected for 45 min from the basal part of hypocotyl and was analyzed by HPLC without dilution.
ACKNOWLEDGMENTS
The authors acknowledge Prof. G. Bashiardes for his helpful assistance in reading the manuscript. We are grateful to Dr. G. Roblin and H. Kabli for advice and help.
Footnotes
This work was supported by Ciba-Geigy (Novartis; grant no. 780221), by the Conseil Interprofessionel du Vin de Bordeaux (grant nos. 19 674/19, 675/19, and 676/19), and by the Conseil Regional du Poitou-Charentes.
LITERATURE CITED
- Barchietto T, Saindrenan P, Bompeix G. Characterization of phosphonate uptake in two Phytophthora spp. and its inhibition by phosphate. Arch Microbiol. 1989;151:54–58. [Google Scholar]
- Bartel B, Fink GR. ILR1, an amidohydrolase that releases active indole-3-acetic acid from conjugates. Science. 1995;268:1745–1748. doi: 10.1126/science.7792599. [DOI] [PubMed] [Google Scholar]
- Bourquin S, Bonnemain JL, Delrot S. Inhibition of loading of 14C assimilates by p-chloromercuribenzene-sulfonic acid: localization of the apoplastic pathway in Vicia faba. Plant Physiol. 1990;92:97–102. doi: 10.1104/pp.92.1.97. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bromilow RH, Chamberlain K, Evans AA. Molecular structure and properties of xenobiotics in relation to phloem translocation. In: Bonnemain JL, Delrot S, Lucas WJ, Dainty J, editors. Recent Advances in Phloem Transport and Assimilate Compartmentation. Nantes, France: Academic Press; 1991. pp. 336–340. [Google Scholar]
- Chen L, Bush DR. LHT1, a lysine- and histidine-specific amino acid transporter in Arabidopsis. Plant Physiol. 1997;115:1127–1134. doi: 10.1104/pp.115.3.1127. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chollet JF, Delétage C, Faucher M, Miginiac L, Bonnemain JL. Synthesis and structure-activity relationship of some pesticides with an α-amino acid function. Biochem Biophys Acta. 1997;1336:331–341. doi: 10.1016/s0304-4165(97)00041-x. [DOI] [PubMed] [Google Scholar]
- Delrot S, Bonnemain JL. Involvement of protons as a substrate for the sucrose carrier during phloem loading in Vicia faba leaves. Plant Physiol. 1981;67:560–564. doi: 10.1104/pp.67.3.560. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Delrot S, Bonnemain JL. Absorption et migration des herbicides. In: Scalla R, editor. Les Herbicides, Mode d'Action et Principes d'Utilisation. Paris: Institut National de la Recherche Agronomique; 1991. pp. 51–77. [Google Scholar]
- Denis MH, Delrot S. Carrier-mediated uptake of glyphosate in broad bean (Vicia faba) via a phosphate transporter. Physiol Plant. 1993;87:569–575. [Google Scholar]
- Despeghel JP, Delrot S. Energetics of amino acid uptake by Vicia faba leaf tissue. Plant Physiol. 1983;71:1–6. doi: 10.1104/pp.71.1.1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Despeghel JP, Delrot S, Bonnemain JL, Besson J. Etude du mécanisme de l'absorption des acides aminés par les tissus foliaires de Vicia faba L. CR Acad Sci (Paris) 1980;290:609–614. [Google Scholar]
- Dufaud A, Chollet JF, Rudelle J, Miginiac L, Bonnemain JL. Derivatives of pesticides with an α-amino acid function: synthesis and effect on threonine uptake. Pest Sci. 1994;41:297–304. [Google Scholar]
- El Ibaoui H, Delrot S, Besson J, Bonnemain JL. Uptake and release of a phloem-mobile (glyphosate) and a non-phloem-mobile (iprodione) xenobiotic by broad bean leaf tissues. Physiol Vég. 1986;24:431–442. [Google Scholar]
- Fisher WN, André B, Rentsch D, Krolkiewicz S, Tegeder M, Breitkreuz K, Frommer WB. Amino acid transport in plants. Trends Plant Sci. 1998;3:188–195. [Google Scholar]
- Fisher WN, Kwart M, Hummel S, Frommer WB. Substrate specificity and expression profile of amino acid transporters (AAPS) in Arabidopsis. J Biol Chem. 1995;270:16315–16320. doi: 10.1074/jbc.270.27.16315. [DOI] [PubMed] [Google Scholar]
- Fleurat-Lessard P, Bouché-Pillon S, Leloup C, Bonnemain JL. Distribution and activity of the plasma membrane H+-ATPase in Mimosa pudica L. in relation to ionic fluxes and leaf movements. Plant Physiol. 1997;113:747–754. doi: 10.1104/pp.113.3.747. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hart JJ, DiTomaso JM, Linscott DL, Kochian LV. Transport interactions between paraquat and polyamines in roots of intact maize seedlings. Plant Physiol. 1992;99:1400–1405. doi: 10.1104/pp.99.4.1400. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jamaï A, Chollet JF, Delrot S. Proton-peptide cotransport in broad bean leaf tissues. Plant Physiol. 1994;106:1023–1031. doi: 10.1104/pp.106.3.1023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jamaï A, Tommasini R, Martinoia E, Delrot S. Characterization of glutathione uptake in broad bean leaf protoplasts. Plant Physiol. 1996;111:1145–1152. doi: 10.1104/pp.111.4.1145. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kallarackal J, Orlich G, Schobert C, Komor E. Sucrose transport into phloem of Ricinus communis L. seedlings as measured by the analysis of sieve-tube sap. Planta. 1989;177:327–335. doi: 10.1007/BF00403590. [DOI] [PubMed] [Google Scholar]
- Kinraide TB, Etherton B. Electrical evidence for different mechanisms of uptake for basic, neutral and acidic amino acids in oat coleoptiles. Plant Physiol. 1980;65:1085–1089. doi: 10.1104/pp.65.6.1085. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kleier DA. Phloem mobility of xenobiotics: I. Mathematical model unifying the weak acid and intermediate permeability theories. Plant Physiol. 1988;86:803–810. doi: 10.1104/pp.86.3.803. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kleier DA, Grayson BT, Hsu FC. The phloem mobility of pesticides. Pest Outlook. 1998;9:26–30. [Google Scholar]
- Leconte F. Etude des propriétés du transport des métabolites du Phosétyl-Al et de l'Atrazine chez les plantes supérieures en relation avec l'aptitude à la systémie de ces molécules. PhD thesis. Poitiers, France: Université de Poitiers; 1989. [Google Scholar]
- Li ZC, Bush DR. ΔpH-dependent amino acid transport into plasma membrane vesicles isolated from sugar beet leaves: I. Evidence for carrier-mediated, electrogenic flux through multiple transport systems. Plant Physiol. 1990;94:268–277. doi: 10.1104/pp.94.1.268. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li ZC, Bush DR. ΔpH-dependent amino acid transport into plasma membrane vesicles isolated from sugar beet leaves: II. Evidence for multiple aliphatic, neutral amino acid symports. Plant Physiol. 1991;96:1338–1344. doi: 10.1104/pp.96.4.1338. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li ZC, Bush DR. Structural determinants in substrate recognition by proton-amino acid symports in plasma membrane vesicles isolated from sugar beet leaves. Arch Biochem Biophys. 1992;294:519–526. doi: 10.1016/0003-9861(92)90719-d. [DOI] [PubMed] [Google Scholar]
- Martin MN, Slovin JP. Purified γ-glutamyl transpeptidases from tomato exhibit high affinity for glutathione and glutathione S-conjugates. Plant Physiol. 2000;122:1417–1426. doi: 10.1104/pp.122.4.1417. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Montamat F, Maurousset L, Tegeder M, Frommer W, Delrot S. Cloning and expression of amino acid transporters from broad bean. Plant Mol Biol. 1999;41:259–268. doi: 10.1023/a:1006321221368. [DOI] [PubMed] [Google Scholar]
- Orlich G, Komor E. Phloem loading in Ricinus cotyledons: sucrose pathways via the mesophyll and the apoplasm. Planta. 1992;187:460–474. doi: 10.1007/BF00199964. [DOI] [PubMed] [Google Scholar]
- Otsiogo-Oyabi H, Roblin G. Effect of glycine on dark- and light-induced pulvinar movements and modifications of proton fluxes in the pulvinus of Mimosa pudica during glycine uptake. Planta. 1985;161:404–408. doi: 10.1007/BF00394570. [DOI] [PubMed] [Google Scholar]
- Renault S, Despeghel-Caussin C, Bonnemain JL, Delrot S. The proton electrochemical transmembrane gradients generated by the transfer cells of the haustorium of Polytrichum formosum and their use in the uptake of amino acids. Plant Physiol. 1989;90:913–920. doi: 10.1104/pp.90.3.913. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rickauer M, Tanner W. Effects of Ca2+ on amino acid transport and accumulation in roots of Phaseolus vulgaris. Plant Physiol. 1986;82:41–46. doi: 10.1104/pp.82.1.41. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schobert C, Komor E. The differential transport of amino acids into the phloem of Ricinus communis L. seedlings as shown by the analysis of sieve-tube sap. Planta. 1989;177:342–349. doi: 10.1007/BF00403592. [DOI] [PubMed] [Google Scholar]
- Tyree MT, Peterson CA, Edginton LV. A simple theory regarding ambimobility of xenobiontics with special reference to the nematicide, oxamyl. Plant Physiol. 1979;63:367–374. doi: 10.1104/pp.63.2.367. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Van Bel AJE. Strategies of phloem loading. Annu Rev Plant Physiol Plant Mol Biol. 1993;44:253–281. [Google Scholar]
- Wyse RE, Komor E. Mechanism of amino acid uptake by sugarcane suspension cells. Plant Physiol. 1984;76:865–870. doi: 10.1104/pp.76.4.865. [DOI] [PMC free article] [PubMed] [Google Scholar]