Abstract
Expression of six Hsp70s in spinach (Spinacia oleracea cv Longstanding Bloomsdale) leaves grown under isothermal conditions is regulated by a light/dark (L/D) mechanism distinctly different from the light-regulated mechanism for the chlorophyll a/b-binding protein (cab) or small subunit of ribulose-1,5-bisphosphate carboxylase oxygenase (rbcS). Subjecting entrained plants to two or three L/D cycles within a 24-h period resulted in an equal number of oscillations in expression for five out of six 70-kD heat shock proteins (Hsp70s). Three cycles appear to be the maximum, as shorter L/D treatments do not consistently increase the number of cycles in a 24-h period. The expression response of Hsp70s to L/D is overridden by heat shock. Protein disulfide isomerase, a second molecular chaperone of the endoplasmic reticulum, has an expression pattern in entrained plants that is similar to hsc70-2, the endoplasmic reticulum luminal Hsp70 binding protein. The parallel expression patterns for the various Hsp70s and protein disulfide isomerase indicate a likely general coordinate L/D regulation for molecular chaperones in plants. Multiple inductions in response to successive L/D treatments within a 24-h period in entrained plants for five of six Hsp70s support the conclusion that expression is not a consequence of circadian control, but instead is independently cued by non-circadian-mediated L/D signals where peak Hsp70 expression precedes the daily thermoperiod maximum.
The 70-kD heat shock proteins (Hsp70s) play a central role in cell biology and biochemistry (Bukau and Horwich, 1998; Mayer and Bukau, 1998). Cytosolic forms associate with nascent polypeptides as they emerge from the ribosome and prevent premature inappropriate folding prior to completion of polypeptide synthesis (Pfund et al., 1998). Following nascent polypeptide release from the ribosome, Hsp70s continue to interact with many proteins individually or in concert with cochaperones and/or regulatory proteins until folding and assembly are essentially completed (Nelson et al., 1992). For polypeptides destined for import into organelles, cytosolic Hsp70s are thought to function by helping organelle precursor proteins remain in an unfolded translocation-competent state (Sheffield et al., 1990). Once translocation is initiated, organellar Hsp70s in the chloroplast stroma, mitochondrial matrix, or endoplasmic reticulum (ER) lumen act as molecular ratchets (Schneider et al., 1994), and provide a driving force for polypeptide importation (Matlack et al., 1999; Voisine et al., 1999). Following polypeptide translocation, the organellar Hsp70s and their cochaperones participate in folding and assembly processes in the same manner as occurs in the cytosol (Kang et al., 1990; Sheffield et al., 1990).
Plant Hsp70s are regulated in at least three different ways. The regulation by heat shock is the best understood of the various modes of regulation controlling the expression of plant Hsp70s (Nover et al., 1996; Schöffl et al., 1998). In response to heat shock, many members of the Hsp70 family are coordinately induced or up-regulated, and equally coordinated is the autorepression response where expression is reduced while still under heat shock conditions (Li et al., 1999). Hsp70s are also under developmental control (Duck and Folk, 1994; DeRocher and Vierling, 1995; Dudley et al., 1997). Certain members of the Hsp70 family manifest differential expression patterns that vary with developmental stage or appear to be tissue- or cell type-specific, but beyond the spatial and temporal expression patterns, little is known about this form of regulation. However, one study has indicated a role for heat shock element sequences and heat shock transcription factor in developmentally regulated expression (Prändl and Schöffl, 1996). The third mode of regulation is known only for the alga Chlamydomonas, where several heat shock genes undergo an induction when dark-grown cells are shifted to light conditions. Two of the light-inducible heat shock genes encode Hsp70s, a cytosolic form, and a chloroplast stromal form (Müller et al., 1992; Drzymalla et al., 1996). Maximal induction occurs 1 to 2 h after exposure to light (von Gromoff et al., 1989). This light induction was shown to be mediated by a regulatory pathway that is independent of that operating during the heat shock response (Kropat et al., 1995), and recent evidence implicated chlorophyll biosynthetic intermediates as plastidic signals acting in the induction of Hsp70s in Chlamydomonas in response to light (Kropat et al., 1997).
Light is an important ecological, physiological, and biochemical factor for plants. Our laboratory recently demonstrated a form of light regulation of Hsp70s operating in plants that may or may not be different from the light induction described for Chlamydomonas, and that is distinct from heat shock or that which is developmentally programmed (Li et al., 2000). We observed that three Hsp70s in spinach (Spinacia oleracea cv Longstanding Bloomsdale) exhibit a diurnal expression pattern under isothermal conditions in mature fully expanded light/dark-grown leaves. During the light phase, RNA abundance reached a peak and was at a nadir during the dark phase. The daily oscillation in mRNA abundance was found to require light and dark environmental cues for continuance as induction ceased in constant light or dark. These earlier studies demonstrated that the cyclic expression pattern of Hsp70s in entrained plants continued for one subjective day cycle and then stopped when plants were placed in constant light at the beginning of the entrained dark-to-light transition (Li et al., 2000). When entrained plants were placed in constant dark at the beginning of the subjective light phase, no induction of expression occurred. The results suggested that the daily wax and wane of spinach leaf Hsp70 expression might not be under circadian clock control.
Strong evidence against circadian control of Hsp70 expression in spinach leaf tissue is presented here. It is shown that plants entrained to a single light/dark (L/D) cycle per 24-h period exhibit multiple inductions of expression when given successive L/D treatments within a 24-h period. The minimum period when induction can be stimulated by L/D treatments appears to be around 4 h for each phase. The purpose of this modulation of Hsp70 expression cued by L/D signals in plant photosynthetic tissue is discussed.
RESULTS
Heat Shock Induction
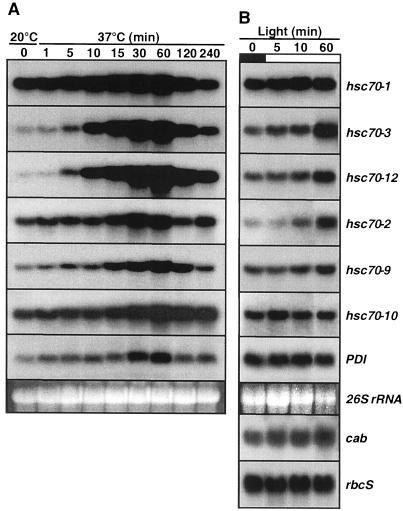
The kinetics of heat shock induction and autorepression for six Hsp70 genes and protein disulfide isomerase (PDI) were examined beginning 6 h after the start of the light phase to demonstrate responsiveness to heat shock at this stage of the photoperiod. In plants grown at constant 20°C, the six members of the Hsp70 family showed diverse induction responses upon exposure to 37°C air temperature (Fig. 1A). Strong induction was observed for two cytosolic members, hsc70-3 and hsc70-12, moderate up-regulation was observed for the ER luminal hsc70-2 and the chloroplast stromal hsc70-9, whereas only a slight increase in transcript abundance was observed for the major cytosolic member hsc70-1 and the mitochondrial matrix member hsc70-10 (Table I). Overall transcript abundance reached maximal levels within 1 h. Induction was detected within 5 min of exposure to the heat shock for hsc70-3 and hsc70-12, and by 15 min increased transcript levels could be detected for hsc70-1, hsc70-2, hsc70-9, and hsc70-10. For comparison, the response of PDI (a molecular chaperone of the lumen of the ER) was also studied. PDI showed moderate induction at 60 min of heat shock exposure (Fig. 1A; Table I). Four members of the family, hsc70-1, hsc70-2, hsc70-9, and hsc70-10, were amply expressed in non-stressed leaf tissue, and overall showed lesser magnitudes of induction than two members (hsc70-3 and hsc70-12) expressed at lower levels under non-stressed conditions at the beginning of heat shock.
Figure 1.
Heat shock, autorepression, and light response for six Hsp70s in spinach leaf tissue. A, Plants were grown at a constant 20°C with a 12-h/12-h L/D environment in a controlled environment cabinet. Plant temperature did not vary more than 1°C to 2°C between the light and dark phases during growth prior to the heat shock treatment. Heat shock was administered in the middle of the light phase after 6 h of light exposure by transfer to a second controlled environment cabinet set at 37°C. Heat shock was given in the light with the intensity unchanged from that of the control conditions. B, Plants were entrained to a 12-h/12-h L/D cycle at 20°C. Samples were harvested at the indicated times following the beginning of the entrained light phase. White and black bars indicate light and dark phases, respectively. Ethidium bromide-stained 26S rRNA was used as a loading control for each gel. Representative gels from two separate experiments are shown for referral. Film exposure times ranged from 6 h (hsc70-1) for highly abundant mRNA to 6 d (PDI) for lower abundance mRNA.
Table I.
Comparison of mRNA abundance change in leaf tissue during heat shock or light exposure in entrained plants
| Gene | Time at 37°C
|
Light Exposure
|
||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| 0 | 5 | 10 | 60 | 120 | 240 | 0 | 5 | 10 | 60 | 120 | 240 | |
| min | ||||||||||||
| hsc70-1 | 1 | 2 | 2 | 2 | 2 | 1 | 1 | 1 | 1 | 1 | 2 | 2 |
| hsc70-3 | 1 | 4 | 16 | 32 | 18 | 9 | 1 | 2 | 2 | 6 | 6 | 5 |
| hsc70-12 | 1 | 11 | 36 | 90 | 60 | 33 | 1 | 1 | 1 | 3 | 3 | 6 |
| hsc70-2 | 1 | 3 | 3 | 6 | 3 | 2 | 1 | 1 | 2 | 3 | 3 | 4 |
| hsc70-9 | 1 | 3 | 4 | 12 | 8 | 3 | 1 | 1 | 1 | 2 | 3 | 3 |
| hsc70-10 | 1 | 2 | 2 | 3 | 3 | 2 | 1 | 1 | 1 | 1 | 1 | 2 |
| PDI | 1 | 3 | 3 | 8 | 3 | 3 | 1 | 1 | 1 | 1 | 1 | 1 |
| Chlorophyll a/b-binding protein (cab) | – | – | – | – | – | – | 1 | 1 | 1 | 2 | 2 | 2 |
| Small subunit of RUBISCO (rbcS) | – | – | – | – | – | – | 1 | 1 | 1 | 1 | 1 | 1 |
Each value is the mean of two independent blots calculated as the abundance relative to time zero.
Exposure to the heat shock conditions for up to 4 h in the light revealed the classic autorepression response of heat shock gene expression even though the temperature remained at 37°C. The mRNAs for all genes examined, including PDI, were at lower levels 2 to 4 h after the onset of heat shock (Fig. 1A; Table I). The degree of autorepression was greatest for those genes that showed the strongest induction (note hsc70-3, hsc70-12, and hsc70-9).
Light Induction
Plants entrained to a 12-h/12-h L/D regime were sampled during the first 60 min of light exposure at the beginning of the light phase to assess the kinetics of light induction (Fig. 1B; Table I). Increased transcript abundance in response to light was much less dramatic than for heat shock. At 60 min, hsp70-3, hsp70-12, and hsc70-2 showed increased mRNA levels over the pre-lights-on level. These results compared favorably with the modest increase in the chlorophyll a/b-binding protein (cab) mRNA during the 1st h of illumination, but contrast with the inductive kinetics and magnitude in response to heat shock (Fig. 1A; Table I). The mRNA abundance for the small subunit of RUBISCO (rbcS) and PDI was not altered during the 1st h of the light phase.
L/D Responses
mRNA abundance was examined in response to a L/D treatment for plants entrained to the 12-h/12-h L/D regime prior to sampling. Figure 2 (column a) shows that all genes examined had mRNA levels that were highest during the light phase and lowest during the dark phase of the daily cycle. As expected, the mRNAs for two components of the photosynthetic apparatus, cab, and rbcS, also exhibited light-regulated variation in abundance with highest levels during the light phase. In a similar manner, the mRNA level for PDI was highest during the light phase and lowest in the dark.
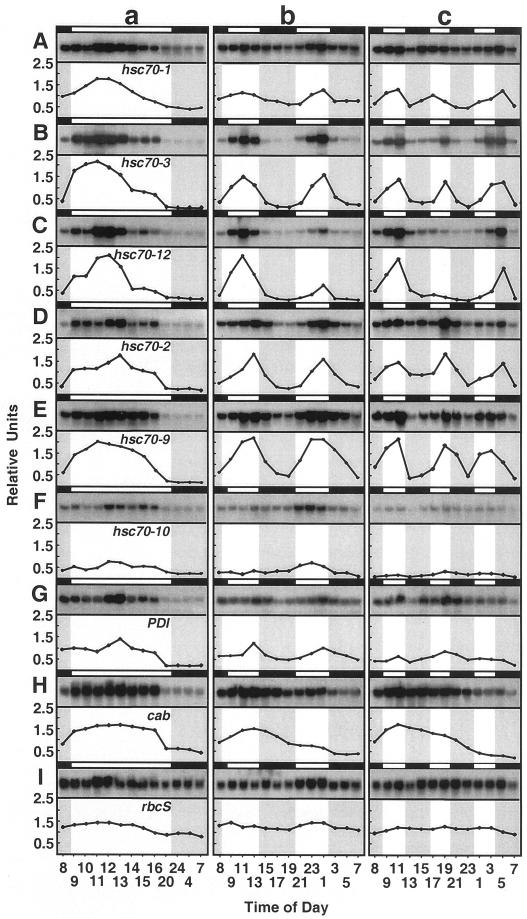
Figure 2.
RNA-blot analyses of the L/D cycling expression pattern for six Hsp70s, PDI, cab, and rbcS in spinach leaf tissue. The expression of hsc70-1 (A; cytosol), hsc70-3 (B; cytosol), hsc70-12 (C; cytosol), hsc70-2 (D; ER), hsc70-9 (E; chloroplast), hsc70-10 (F; mitochondria), PDI (G; ER), cab (H; chloroplast), and rbcS (I; chloroplast) genes in response to one (a), two (b), or three (c) L/D cycles in a single 24-h period is shown. Plants were grown at 20°C in a controlled environment cabinet and entrained with a 12-h/12-h L/D photoperiod (1 cycle, “a” column) or were transferred to a 6-h/6-h L/D photoperiod (2 cycles, “b” column) or to a 4-h/4-h L/D photoperiod (3 cycles, “c” column). Each cycling experiment was repeated two or three times with similar results. One representative autoradiogram is shown here. Time is expressed on a 24-h basis. White and black bars and unshaded and shaded areas indicate light and dark phases, respectively. The plots are the ratio of autoradiographic band density/rRNA gel band density, to normalize loading differences.
The pattern of induction in response to light varied from member to member. Peak expression for the Hsp70s occurred between 3 and 5 h after the start of the light period. Some showed strong induction early in the light phase followed by declining mRNA levels over the remainder of the light phase (hsc70-3), whereas others exhibited higher mRNA levels throughout most of the light phase (hsc70-2). In most cases the magnitude of induction was not similar to that elicited by heat shock (see Fig. 1A; Table I). For two members (see hsc70-3 and hsc70-12), the induction elicited by light had an appearance similar to the induction and autorepression of the heat shock response. However, this light induction occurred in the absence of a heat shock as the tissue temperature during the entire treatment duration varied no more than one to two degrees from the constant ambient air temperature. In total, these results are consistent with previous findings of L/D expression patterns for Hsp70s in spinach leaf tissue (Li et al., 2000).
Once the oscillating nature of expression for most of the members of the Hsp70 family in leaf tissue was unequivocally established, we wanted to know whether the pattern could be modified in entrained plants immediately upon change in the L/D regime. Plants entrained to a 12-h/12-h L/D regime were allowed to receive 6 h of light at the beginning of the subjective light phase followed by 6 h of dark so that the L/D treatment occurred twice in a 24-h period. The results are shown in Figure 2 (column b). As can be seen, five out of six Hsp70s exhibited two peaks in expression with lower levels of mRNA during the two 6-h dark phases. In the case of hsc70-1 (Fig. 2A, column b), the trough to peak induction was less than 3-fold, and less than that for a single L/D treatment in the 24-h period. In contrast, hsc70-3 exhibited two peaks each in the light phase, separated by a well-defined minimum. A third pattern of expression was exhibited by hsc70-12 where the first 6-h light phase produced a high amplitude induction that was strongly reduced in the second 6-h light phase. The mitochondrial member, hsc70-10, showed little or no induction during the first 6-h light phase, but did show an induction during the second 6-h light phase. In a similar manner, PDI showed two induction peaks of low amplitude. All of these patterns contrasted with that for the circadian, light-regulated cab. A single peak in mRNA level occurred that closely paralleled the timing of the expression pattern for a single L/D entrainment. Under a 12-h L/D entrainment per 24 h, expression of cab was highest from 1 to 8 h after the beginning of the light phase, and under the double L/D treatment per 24 h, expression was highest from 1 to 9 h after the beginning of the first subjective light phase. During the second light phase, the mRNA levels held steady or declined slightly.
When three L/D treatments were given within a 24-h period, several of the Hsp70s exhibited three oscillations of expression. A notable exception was hsc70-12 (Fig. 2C, column c), where the first peak in mRNA level was similar to that for the single or double L/D treatments per 24 h, but the second peak in expression occurred late in the 24-h period in the dark. Of the four Hsp70 members (hsc70-1, hsc70-3, hsc70-2, and hsc70-9) that exhibited three peaks of expression, the last peak was delayed and occurred in the dark for all but hsc70-9, indicating the onset of a disjunct of induction and repression resulting from inadequate time to allow the regulatory processes to reset for the next inductive response. The greater magnitude of dis-synchrony for hsc70-12 relative to the other Hsp70s suggests that perhaps the re-setting mechanism of hsc70-12 has a longer time requirement than for other Hsp70s. Again as for two L/D treatments per 24 h, the expression pattern of cab had approximately the same timing as for the single L/D treatment per 24 h, in keeping with the entrainment of its circadian-regulated expression.
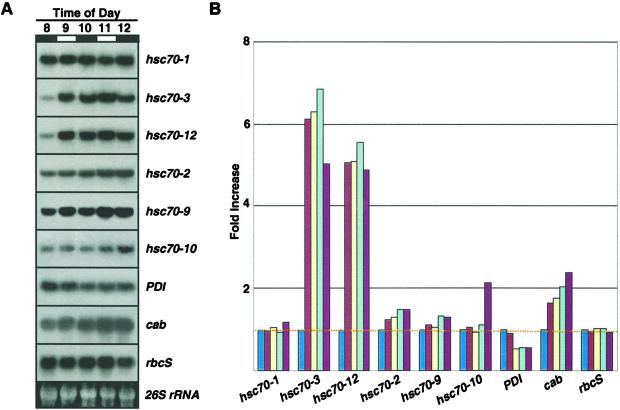
In a separate experiment, entrained plants at the beginning of the subjective light phase were given two L/D treatments where the light and dark phases were each 1 h in length, to see whether short-duration light exposure could cause induction and repression of the Hsp70s. Induction was observed for only hsc70-3 and hsc70-12 over the pre-lights-on level (Fig. 3). After the initial induction, there was little change in the mRNA levels for hsc70-3 and hsc70-12 over the next 3 h, indicating that the L/D treatments were too short to strongly influence mRNA abundance (Fig. 3B). The mRNA level for cab progressively increased from the pre-lights-on level, which was again consistent with its circadian regulation.
Figure 3.
RNA-blot analyses of rapid L/D cycling. Entrained plants were subjected to two rounds of L/D exposure beginning at the start of the subjective light phase (8 am). A, The light and dark phases were 1 h each and are indicated by white and black bars, respectively. B, Quantitative analysis of RNA blots shows transcript abundance relative to pre-lights-on 8 am sample. The blue, red, yellow, light-blue, and dark-red bars represent time points 8, 9, 10, 11, and 12, respectively. The 26S rRNA band was used to adjust values for equal loading.
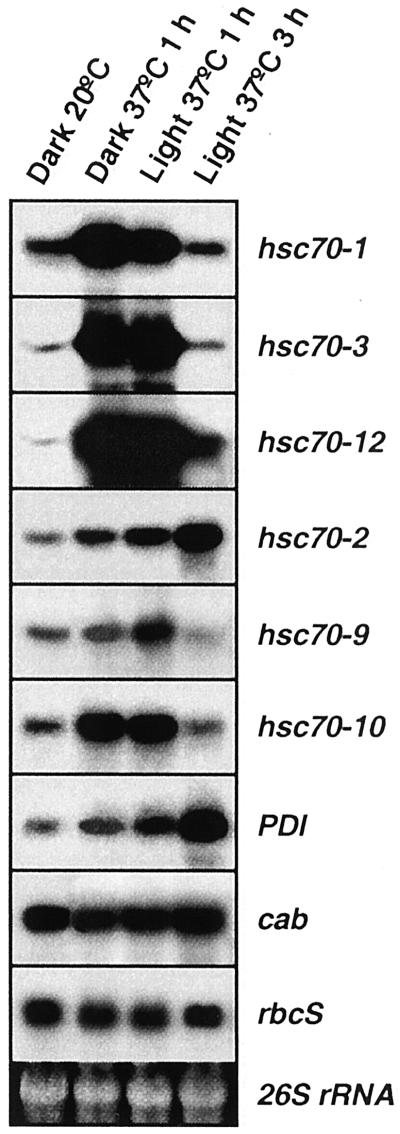
Heat Shock Overrides Isothermal L/D Expression Pattern
When entrained plants were given a heat shock in light or dark at the beginning of the light phase or 6 h into the light phase, induction for hsc70-1, hsc70-3, hsc70-12, hsc70-2, hsc70-9, and hsc70-10 was observed irrespective of light exposure duration (Fig. 4; the 6-h results not shown). It was previously established that isothermal oscillation does not occur without light exposure of entrained plants (Li et al., 2000). In contrast, heat shock in the dark (Fig. 4) or in the light (Figs. 1 and 4) promotes strong induction, demonstrating that heat shock overrides the light-regulated expression pattern. Heat shock did not influence cab or rbcS mRNA abundance. When heat shock conditions were maintained for 3 h at the beginning of the light phase, autorepression occurred for all Hsp70s except hsc70-2 and PDI, exactly as observed at later stages of the light phase. The autorepression early in the light phase compared favorably with that in the middle of the light phase (Fig. 1A). No evidence for heat shock-related autorepression for cab or rbcS was observed.
Figure 4.
RNA-blot analyses of the heat shock response of leaf tissue in light or dark. Plants were grown at 20°C with 12-h/12-h L/D. At the beginning of the subjective light phase heat shock was given for 1 h in the dark at 37°C, 1 h in the light at 37°C, and for 3 h in the light at 37°C. The 26S rRNA band was used to determine loading.
Thermotolerance Oscillation
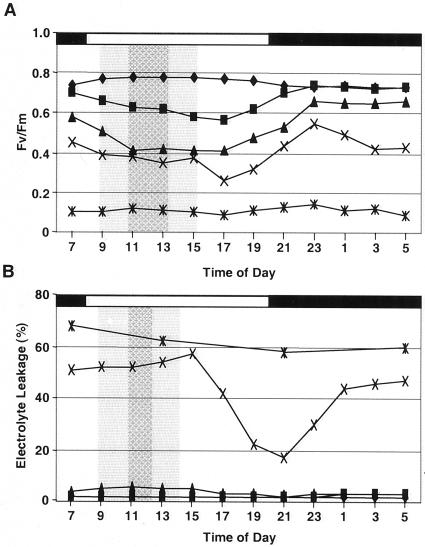
Two independent measures of relative heat injury, chlorophyll a fluorescence and membrane electrolyte leakage, were made over the course of 24 h for plants grown at isothermal temperatures to determine whether the light-regulated expression of Hsp70s was associated with daily changes in thermotolerance. The Fv/Fm ratio in response to heat stress showed a decline with increasing temperature at all time points and during the light phase for each temperature reaching lowest levels toward the end of the light phase (Fig. 5A). Highest Fv/Fm values occurred during the dark phase. This difference was not attributable to whether the plants were returned to light or dark immediately after heat treatment, because all plants were placed under low light conditions for the first 12 h after heat treatment. Therefore, the profiles show a clear daily variation in sensitivity of photosynthetic electron transport processes to heat stress. Electrolyte leakage was very low for plants exposed to ambient, 42°C and 43°C, but high for plants exposed to 45°C (Fig. 5B). All plants from the 45°C treatment were dead 7 d after heat stress. Plants subjected to 44°C showed a daily variation in thermotolerance with lowest electrolyte leakage at the end of the light phase and at the beginning of the dark phase. Highest sensitivity to the heat stress occurred during the first half of the light phase, prior to and during induction of peak Hsp70 mRNA abundance.
Figure 5.
Diurnal variation of leaf thermotolerance. The aerial portion of plants was given a 10-min heat treatment by immersion in water at the indicated temperature. A, Chlorophyll a fluorescence was measured 2 d after heat stress; B, electrolyte leakage was determined 7 d after heat stress. Temperature treatments were 25°C, ♦; 42°C, ▪; 43°C, ▴; 44°C, X; 45°C, ∗. The light and dark portions of the diurnal cycle are indicated with the white and black bar at the top. Intervals of elevated and peak expression of the chloroplast stromal hsc70-9 (A) and the cytosolic hsc70-3 (B) are indicated by the shaded and hatched areas, respectively.
DISCUSSION
This study conclusively demonstrates that a daily oscillation in expression is a general characteristic for many members of the Hsp70 family in spinach photosynthetic tissue under isothermal conditions. This light-regulated expression pattern is distinct from the heat shock response and it represents a mode of regulation unique to plants. The daily modulation of expression of Hsp70s is similar to that for genes that encode components of the photosynthetic apparatus (Kloppstech, 1985; Giuliano et al., 1988).
The Hsp70 expression pattern in spinach also closely parallels that for the nuclear-encoded CPN60α and CPN60β genes of stromal-localized chaperonins in Arabidopsis (Pilgrim and McClung, 1993). Oscillation of mRNA abundance for CPN60α and CPN60β was evident in entrained plants, but not in plants transferred to continuous light or continuous dark conditions. In a similar manner, previous findings for three members of the Hsp70 family demonstrated that induction/repression was not maintained under constant light or dark conditions (Li et al., 2000). The lack of continued oscillation suggested that expression of the three Hsp70s was not under the control of the circadian clock.
In the present study, entrained plants given multiple L/D treatments in a 24-h period showed multiple oscillations of expression that frequently, but not always, corresponded with the L/D phases. This pattern contrasted sharply with that of cab, a clock-controlled, light-regulated gene (Apel, 1979; Kloppstech, 1985; Piechulla and Gruissem, 1987; Giuliano et al., 1988; Anderson and Kay, 1995; Kolar et al., 1995; Millar et al., 1995; Piechulla, 1999), where the entrained expression was not dramatically altered by imposition of multiple L/D treatments within a 24-h period. Together, the lack of induction under constant conditions and the immediate and flexible ability to adjust expression in response to a modified L/D treatment period for many Hsp70s is robust evidence that Hsp70s in spinach leaf tissue are light regulated independently of the circadian clock. The one exception in the multiple cycling experiments is hsc70-12. In the 6:6 treatment (Fig. 2C, column b) there are two peaks that occur in the light, but the second peak is much smaller than the first. Such a pattern may reflect circadian gating over simple light inducibility. The 4:4 treatment (Fig. 2C, column c) lends support to this interpretation where two expression peaks were observed instead of three. The delay in the third peak relative to the onset of the third light period is suggestive of an underlying circadian component. Together, these results suggest that oscillation of hsc70-12 mRNA is not simply a direct response to light, as it appears to be for the other Hsp70s, but may also involve circadian regulation. A recent microarray-based study found that the expression of Hsc70-3 of Arabidopsis appeared to be under the control of the circadian clock, whereas Hsc70-1 and mtHsc70-1 did not exhibit a clock-controlled expression pattern (Harmer et al., 2000).
The present findings sharply contrast with that for the prokaryote Synechocystis where studies (Rensing and Monnerjahn, 1996) have shown cyclic expression of DnaK in constant light, and a DnaK promoter/luciferase reporter construct has demonstrated an oscillation of expression with a period of 22 h under continuous light (Aoki et al., 1995). In both studies, the data strongly support a circadian regulation of DnaK in Synechocystis.
Compared with the heat shock response, light induction of Hsp70s in leaf tissue is slow. Although heat shock induction occurs within minutes for many members of the Hsp70 family, light induction requires anywhere from 1 to 4 h. When the L/D treatment is reduced to 4 h for each phase, inducible expression continues for many of the Hsp70s, but becomes asynchronous particularly for hsc70-12 where the last peak is delayed occurring in the dark. Although this could reflect circadian gating, an alternative interpretation could be that the regulatory mechanism may possess a minimum “lights-on” and/or a “lights-off” requirement to reset for the next oscillation of expression. The fact that a 1-h L/D treatment did not result in RNA abundance oscillation further supports this view (Fig. 3). This result is similar to findings with cab for plants grown in continuous light where a single dark phase of 3 to 9 h was sufficient to re-synchronize the circadian rhythm (Riesselmann and Piechulla, 1990) and initiate high amplitude cycling. Just as a dark phase is important in cab cycling, apparently, light and dark cues (Li et al., 2000) of sufficient duration are necessary for oscillating expression of Hsp70s.
What is the purpose for the light-regulated expression pattern of Hsp70s in leaf tissue? Two hypotheses are under consideration. The first is the “demand for molecular chaperone function.” Plant metabolic activities are linked to photosynthesis in leaf tissue (Heldt, 1997). The expression of numerous genes for components of the photosynthetic apparatus (Tobin and Silverthorne, 1985) and a host of other genes involved in non-photosynthetic metabolic activities are activated during the day (Heldt, 1997). This results in an increase in total protein synthesis and greater protein biogenesis during this part of the daily cycle (Li et al., 2000). If not already present in excess, additional molecular chaperone capacity would be needed during the day when photosynthetic activity is high. In support of this hypothesis is the similar light-regulated oscillation of a second chaperone class, that of CPN60α and CPN60β in Arabidopsis (Pilgrim and McClung, 1993), and the demonstration by Schroda et al. (1999) that the stromal Hsp70 of Chlamydomonas participates in photoprotection and repair of photosystem II from photoinhibition. By use of over- and underexpression mutants, Schroda et al., 1999 showed that higher levels of Hsp70B improved photoprotection and enhanced restoration of photosystem II function. Implicit in their results would be a molecular chaperone function for Hsp70B in the stabilization of photosystem II components or during repair that required de novo synthesis, folding, and assembly of replacement polypeptides that were irreversibly damaged.
The second hypothesis relates to “Hsps and diurnal variation of thermotolerance.” Several studies have shown that plant thermotolerance varies diurnally with greatest thermotolerance toward the afternoon and the lowest thermotolerance prior to dawn (Laude, 1939; Kappen and Lösch, 1984; Rikin, 1992; Colombo et al., 1995). The observed timing of oscillation of Hsp70s here and in our previous study (Li et al., 2000) is consistent with higher RNA levels preceding the diurnal maximum thermotolerance which occurs near the end of the light phase.
Plants use L/D cues to control developmental processes (Chory et al., 1996) and to initiate dormancy for over-wintering (Weiser, 1970). The photoreceptor for photoperiodic induction of dormancy is thought to be phytochrome (Williams et al., 1972). Light may be an equally reliable indicator of a daily need for enhanced chaperone function. Since the evidence presented here strongly argues against a role for circadian clock control in the oscillating pattern of Hsp70 expression, a likely first candidate in this light regulation is phytochrome given its multiplicity of roles in plants (Smith, 1995).
MATERIALS AND METHODS
Plant Material
Spinach (Spinacia oleracea cv Longstanding Bloomsdale) seedlings were grown from seed at a constant 20°C in a controlled environment cabinet for 1 month prior to experimentation. Irradiance, supplied by fluorescent and incandescent lamps, was about 400 μmol m−2 s−1. Plants were irrigated as needed to avoid the onset of water stress and were fertilized weekly with full-strength Hoagland solution. The photoperiod was 12 h with an abrupt change from dark to light and light to dark as previously described (Li et al., 1999). Experiments were initiated when seedlings had developed to the third or fourth true leaf stage. All experiments were repeated one or two times.
Heat Shock and Light Treatments
Plants were grown at 20°C/20°C with 12 h of light/12 h of dark as described above. Control samples were harvested at 8 am before lights-on and then plants were transferred to 37°C for heat stress with or without light. Additional sets of plants were transferred to 37°C at 2 pm after 6 h of light exposure. Samples were taken at specified times up to 4 h at 37°C.
L/D Treatments
Plants were acclimated and entrained at 20°C/20°C with a 12-h/12-h L/D regime prior to the first 8:00 am sampling. Lights came on immediately after the initial sampling. Entrained seedlings were given L/D treatments immediately following the end of the 12-h dark phase at 20°C. L/D was given for 6 h of light/6 h of dark (two cycles per day); for 4 h of light/4 h of dark (three cycles per day); or for 1 h of light/1 h of dark (the equivalent of 12 cycles per day). Samples were collected during light or dark at the indicated times. Samples harvested in the dark were prepared under illumination of a green safe light. Harvested leaf samples were flash frozen in liquid nitrogen and stored at −80°C.
RNA-Blot Analyses
Total RNA was extracted from spinach leaf and RNA blots were performed as previously described (Li et al., 1999). A total of 25 μg of total RNA per lane was electrophoresed in 1.2% (w/v) formaldehyde agarose gels. RNA gels were stained with ethidium bromide and photographed under UV illumination. Approximate equal loading was verified before proceeding with the transfer of the RNA to the hybridization membrane. RNA gels were pressure blotted onto Hybond-N nylon membrane (Amersham, Buckinghamshire, UK) and the RNA was UV cross-linked (1.2 × 105 μJ/cm2). Prehybridization of RNA blots was accomplished using 50% (w/v) formamide; 5× sodium chloride, sodium phosphate, and EDTA; 5× Denhardt's solution, 0.2% (w/v) SDS, and 10 μg/mL salmon sperm DNA at 42°C for 4 h. Randomly [α-32P]dCTP-labeled DNA was added to the prehybridization buffer and allowed to anneal for 16 h at 42°C. After hybridization, the RNA blots were washed one time each with 6, 2, 1, and 0.5× (0.15 m sodium chloride, 0.015 m sodium citrate) plus 0.2% (w/v) SDS for 15 min at room temperature, followed by repeated washing in 0.1× (sodium chloride, sodium citrate) plus 0.2% (w/v) SDS at 50°C to 65°C according to the level of probe radioactivity remaining on the blot. RNA blots were hybridized with random-primed-labeled probes prepared exclusively from spinach derived cDNA clones for cytosolic hsc70-1, -3, -12, ER luminal hsc70-2 (Guy and Li, 1998), chloroplast stromal hsc70-9, mitochondrial matrix hsp70-10, protein disulfide isomerase PDI (Li et al., 1999), the light-harvesting cab (Mason, 1989), and rbcS (provided by W. Martin, Technische Universität Braunschweig, Germany). Cross-hybridization of highly homologous members (Li et al., 1999) was minimized (less than 5%) by washing at the maximum stringency possible while still retaining enough signal to yield reasonable autoradiographic exposure times. After autoradiography the blots were stripped and reprobed two to three times. Relative RNA abundance was determined using Scion Image for Windows (Scion Corporation, Frederick, MD) to quantify the ribosomal RNA band density and the autoradiographic band density from digitized autoradiographs for the sequences as specified. Plots are the ratio of probe signal density/rRNA band density to normalize for loading differences.
Diurnal Thermotolerance
Plants were grown at 20°C to the third and fourth true leaf stage and were entrained as previously described (Li et al., 2000). At 2-h intervals, the aerial parts of plants were submerged for 10 min in a water bath at a temperature ranging from 41° to 46°C. After treatment, plants were placed under low constant light (180 μmol m−2 s−1) for at least 12 h before being returned to standard growth conditions. Chlorophyll fluorescence parameters were measured with the Plant Efficiency Analyser (Hansatech Instruments, UK) after a 10-min dark adaptation period 48 h after heat treatment. Readings were taken over a 5-s interval after exposure at 100% illumination level by high intensity light emitting diodes. Five replicates (first or second true leaf) were averaged for each time point. Variable fluorescence, Fv, was determined as the difference between the maximal fluorescence signal, Fm, and the initial darkness fluorescence level, Fo. Electrolyte leakage was determined 7 d after heat treatment. Three sets of five leaves for each treatment (first or second true leaves) were sampled for conductivity measurements. Electrolyte leakage was expressed as the mean percentage for the three replicates. Three time-point moving averages of the means, [Σ(Y(T) + Y(T + 2 h) + Y(T − 2 h))/3] where T is one time point, for Fv/Fm and electrolyte leakage percentages were manually calculated and plotted to better show diurnal thermotolerance trends.
ACKNOWLEDGMENTS
We thank J. Mason (Florigene) for the cab cDNA clone and W. Martin (Institut fur Genetik-Biozentrum, Technische Universitat Braunschweig) for the rbcS clone. We thank D. Haskell, H. McCleery, D.-Y. Sung, and C. Zhang for their constructive comments and discussion during the preparation of this manuscript, and one anonymous reviewer for helping us to better understand some of the experimental results.
Footnotes
This research was supported by the National Science Foundation (grant IBN 93–17540), by the U.S. Department of Agriculture, by the National Research Initiative, and by the Institute of Food and Agricultural Sciences (grant nos. 98051910 and 2000–00687). This is Florida Agricultural Experiment Station journal series no. R–08013.
LITERATURE CITED
- Anderson SL, Kay SA. Functional dissection of circadian clock- and phytochrome-regulated transcription of the Arabidopsis CAB2 gene. Proc Natl Acad Sci USA. 1995;92:1500–1504. doi: 10.1073/pnas.92.5.1500. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Aoki S, Kondo T, Ishiura M. Circadian expression of the dnaK gene in the cyanobacterium Synechocystis sp. strain PCC 6803. J Bacteriol. 1995;177:5606–5611. doi: 10.1128/jb.177.19.5606-5611.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Apel K. Phytochrome-induced appearance of mRNA activity for the apoprotein of the light-harvesting chlorophyll a/b protein of barley (Hordeum vulgare) Eur J Biochem. 1979;97:183–188. doi: 10.1111/j.1432-1033.1979.tb13101.x. [DOI] [PubMed] [Google Scholar]
- Bukau B, Horwich AL. The Hsp70 and Hsp60 chaperone machines. Cell. 1998;92:351–366. doi: 10.1016/s0092-8674(00)80928-9. [DOI] [PubMed] [Google Scholar]
- Chory J, Chatterjee M, Cook RK, Elich T, Fankhauser C, Li J, Nagpal P, Neff M, Pepper A, Poole D. From seed germination to flowering, light controls plant development via the pigment phytochrome. Proc Natl Acad Sci USA. 1996;93:12066–12071. doi: 10.1073/pnas.93.22.12066. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Colombo SJ, Timmer VR, Colclough ML, Blumwald E. Diurnal variation in heat tolerance and heat shock protein expression in black spruce (Picea mariana) Can J For Res. 1995;25:369–375. [Google Scholar]
- DeRocher A, Vierling E. Cytoplasmic HSP70 homologues of pea: differential expression in vegetative and embryonic organs. Plant Mol Biol. 1995;27:441–456. doi: 10.1007/BF00019312. [DOI] [PubMed] [Google Scholar]
- Drzymalla C, Schroda M, Beck CF. Light-inducible gene HSP70B encodes a chloroplast-localized heat shock protein in Chlamydomonas reinhardtii. Plant Mol Biol. 1996;31:1185–1194. doi: 10.1007/BF00040835. [DOI] [PubMed] [Google Scholar]
- Duck NB, Folk WR. Hsp70 heat shock protein cognate is expressed and stored in developing tomato pollen. Plant Mol Biol. 1994;26:1031–1039. doi: 10.1007/BF00040686. [DOI] [PubMed] [Google Scholar]
- Dudley P, Wood CK, Pratt JR, Moore AL. Developmental regulation of the plant mitochondrial matrix located HSP70 chaperone and its role in protein import. FEBS Lett. 1997;417:321–324. doi: 10.1016/s0014-5793(97)01311-2. [DOI] [PubMed] [Google Scholar]
- Giuliano G, Hoffman NE, Ko K, Scolnik PA, Cashmore AR. A light-entrained circadian clock controls transcription of several plant genes. EMBO J. 1988;7:3635–3642. doi: 10.1002/j.1460-2075.1988.tb03244.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Guy C, Li QB. The organization and evolution of the spinach stress 70 molecular chaperone gene family. Plant Cell. 1998;10:539–556. doi: 10.1105/tpc.10.4.539. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Harmer SL, Hogenesch JB, Straume M, Chang H-S, Zhu T, Wang X, Kreps JA, Kay SA. Orchestrated transcription of key pathways in Arabidopsis by the circadian clock. Science. 2000;290:2110–2113. doi: 10.1126/science.290.5499.2110. [DOI] [PubMed] [Google Scholar]
- Heldt H-W. Plant Biochemistry and Molecular Biology. Oxford: Oxford University Press; 1997. [Google Scholar]
- Kang PJ, Ostermann J, Shilling J, Neupert W, Craig EA, Pfanner N. Requirement for hsp70 in the mitochondrial matrix for translocation and folding of precursor proteins. Nature. 1990;348:137–143. doi: 10.1038/348137a0. [DOI] [PubMed] [Google Scholar]
- Kappen L, Lösch R. Diurnal patterns of heat tolerance in relation to CAM [crassulacean acid metabolism, Aeonium haworthii, Aichryson laxum, a carbon 3 metabolic pathway plant] Z Pflanzenphysiol Bd. 1984;114:87–96. [Google Scholar]
- Kloppstech K. Diurnal and circadian rhythmicity in the expression of light-induced plant nuclear messenger RNAs. Planta. 1985;165:502–506. doi: 10.1007/BF00398095. [DOI] [PubMed] [Google Scholar]
- Kolar C, Ádám E, Schäfer E, Nagy F. Expression of tobacco genes for light-harvesting chlorophyll a/b-binding proteins of photosystem II is controlled by two circadian oscillators in a developmentally regulated fashion. Proc Natl Acad Sci USA. 1995;92:2174–2178. doi: 10.1073/pnas.92.6.2174. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kropat J, Oster U, Rüdiger W, Beck CF. Chlorophyll precursors are signals of chloroplast origin involved in light induction of nuclear heat-shock genes. Proc Natl Acad Sci USA. 1997;94:14168–14172. doi: 10.1073/pnas.94.25.14168. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kropat J, von Gromoff ED, Müller FW, Beck CF. Heat shock and light activation of a Chlamydomonas HSP70 gene are mediated by independent regulatory pathways. Mol Gen Genet. 1995;248:727–734. doi: 10.1007/BF02191713. [DOI] [PubMed] [Google Scholar]
- Laude HH. Diurnal cycle of heat resistance in plants. Science. 1939;89:556–557. doi: 10.1126/science.89.2320.556-a. [DOI] [PubMed] [Google Scholar]
- Li Q, Haskell D, Zhang C, Sung D-Y, Guy C. Diurnal regulation of Hsp70s in plant photosynthetic tissue. Plant J. 2000;21:373–378. doi: 10.1046/j.1365-313x.2000.00673.x. [DOI] [PubMed] [Google Scholar]
- Li QB, Haskell DW, Guy CL. Coordinate and non-coordinate expression of the stress 70 family and other molecular chaperones at high and low temperature in spinach and tomato. Plant Mol Biol. 1999;39:21–34. doi: 10.1023/a:1006100532501. [DOI] [PubMed] [Google Scholar]
- Mason JG. Nucleotide sequence of a cDNA encoding the light-harvesting chlorophyll a/b-binding protein from spinach. Nucleic Acids Res. 1989;17:5387. doi: 10.1093/nar/17.13.5387. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Matlack KES, Misselwitz B, Plath K, Rapoport TA. BiP acts as a molecular ratchet during post-translational transport of prepro-alpha factor across the ER membrane. Cell. 1999;97:553–564. doi: 10.1016/s0092-8674(00)80767-9. [DOI] [PubMed] [Google Scholar]
- Mayer MP, Bukau B. Hsp70 chaperone systems: diversity of cellular functions and mechanisms of action. Biol Chem. 1998;379:261–268. [PubMed] [Google Scholar]
- Millar AJ, Carré IA, Strayer CA, Chua N-H, Kay SA. Circadian clock mutants in Arabidopsis identified by luciferase imaging. Science. 1995;267:1161–1163. doi: 10.1126/science.7855595. [DOI] [PubMed] [Google Scholar]
- Müller FW, Igloi GL, Beck CF. Structure of a gene encoding heat-shock protein HSP70 from the unicellular alga Chlamydomonas reinhardtii. Gene. 1992;111:165–173. doi: 10.1016/0378-1119(92)90684-h. [DOI] [PubMed] [Google Scholar]
- Nelson RJ, Ziegelhoffer T, Nicolet C, Werner-Washburne M, Craig EA. The translation machinery and 70 kD heat shock protein cooperate in protein synthesis. Cell. 1992;71:97–105. doi: 10.1016/0092-8674(92)90269-i. [DOI] [PubMed] [Google Scholar]
- Nover L, Scharf KD, Gagliardi D, Vergne P, Czarnecka-Verner E, Gurley WB. The Hsf world: classification and properties of plant heat stress transcription factors. Cell Stress Chaperones. 1996;1:215–223. doi: 10.1379/1466-1268(1996)001<0215:thwcap>2.3.co;2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pfund C, Lopez-Hoyo N, Ziegelhoffer T, Schilke BA, Lopez-Buesa P, Walter WA, Wiedmann M, Craig EA. The molecular chaperone Ssb from Saccharomyces cerevisiae is a component of the ribosome-nascent chain complex. EMBO J. 1998;17:3981–3991. doi: 10.1093/emboj/17.14.3981. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Piechulla B. Circadian expression of the light-harvesting complex protein genes in plants. Chronobiol Int. 1999;16:115–128. doi: 10.3109/07420529909019080. [DOI] [PubMed] [Google Scholar]
- Piechulla B, Gruissem W. Diurnal mRNA fluctuations of nuclear and plastid genes in developing tomato fruits. EMBO J. 1987;6:3593–3599. doi: 10.1002/j.1460-2075.1987.tb02690.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pilgrim MI, McClung CR. Differential involvement of the circadian clock in the expression of genes required for ribulose-1,5-bisphosphate carboxylase/oxygenase synthesis, assembly and activation in Arabidopsis thaliana. Plant Physiol. 1993;103:553–564. doi: 10.1104/pp.103.2.553. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Prändl R, Schöffl F. Heat shock elements are involved in heat shock promoter activation during tobacco seed maturation. Plant Mol Biol. 1996;31:157–162. doi: 10.1007/BF00020615. [DOI] [PubMed] [Google Scholar]
- Rensing L, Monnerjahn C. Heat shock proteins and circadian rhythms. Chronobiol Int. 1996;13:239–250. doi: 10.3109/07420529609020904. [DOI] [PubMed] [Google Scholar]
- Riesselmann S, Piechulla B. Effect of dark phases and temperature on the chlorophyll a/b-binding protein mRNA level oscillations in tomato seedlings. Plant Mol Biol. 1990;14:605–616. doi: 10.1007/BF00027506. [DOI] [PubMed] [Google Scholar]
- Rikin A. Circadian rhythm of heat resistance in cotton seedlings: synthesis of heat-shock proteins. Eur J Cell Biol. 1992;59:160–165. [PubMed] [Google Scholar]
- Schneider HC, Berthold J, Bauer MF, Dietmeier K, Guiard B, Brunner M, Neupert W. Mitochondrial Hsp70/MIM44 complex facilitates protein import. Nature. 1994;371:768–774. doi: 10.1038/371768a0. [DOI] [PubMed] [Google Scholar]
- Schöffl F, Prändl R, Reindl A. Regulation of the heat-shock response. Plant Physiol. 1998;117:1135–1141. doi: 10.1104/pp.117.4.1135. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schroda M, Vallon O, Wollman F-A, Beck C. A chloroplast-targeted heat shock protein 70 (HSP70) contributed to the photoprotection and repair of photosystem II during and after photoinhibition. Plant Cell. 1999;11:1165–1178. doi: 10.1105/tpc.11.6.1165. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sheffield WP, Shore GC, Randall SK. Mitochondrial precursor protein: effects of 70-kilodalton heat shock protein on polypeptide folding, aggregation, and import competence. J Biol Chem. 1990;265:11069–11076. [PubMed] [Google Scholar]
- Smith H. Physiological and ecological function within the phytochrome family. Annu Rev Plant Physiol Plant Mol Biol. 1995;46:289–315. [Google Scholar]
- Tobin EM, Silverthorne J. Light regulation of gene expression in higher plants. Annu Rev Plant Physiol. 1985;36:569–593. [Google Scholar]
- Voisine C, Craig EA, Zufall N, von Ahsen O, Pfanner N, Voos W. The protein import motor of mitochondria: unfolding and trapping of preproteins are distinct and separable functions of matrix Hsp70. Cell. 1999;97:565–574. doi: 10.1016/s0092-8674(00)80768-0. [DOI] [PubMed] [Google Scholar]
- von Gromoff ED, Treier U, Beck CF. Three light-inducible heat shock genes of Chlamydomonas reinhardtii. Mol Cell Biol. 1989;9:3911–3918. doi: 10.1128/mcb.9.9.3911. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Weiser CJ. Cold resistance and injury in woody plants. Science. 1970;169:1269–1278. doi: 10.1126/science.169.3952.1269. [DOI] [PubMed] [Google Scholar]
- Williams BJ, Jr, Pellett NE, Klein RM. Phytochrome control of growth cessation and initiation of cold acclimation in selected woody plants. Plant Physiol. 1972;50:262–265. doi: 10.1104/pp.50.2.262. [DOI] [PMC free article] [PubMed] [Google Scholar]