Abstract
Derivatives of (2-aminopropyl)indole (API) and (2-aminopropyl)benzofuran (APB) are new psychoactive substances which produce stimulant effects in vivo. (2-Aminopropyl)benzo[β]thiophene (APBT) is a novel sulfur-based analog of API and APB that has not been pharmacologically characterized. In the current study, we assessed the pharmacological effects of six APBT positional isomers in vitro, and three of these isomers (3-APBT, 5-APBT, and 6-APBT) were subjected to further investigations in vivo. Uptake inhibition and efflux assays in human transporter-transfected HEK293 cells and in rat brain synaptosomes revealed that APBTs inhibit monoamine reuptake and induce transporter-mediated substrate release. Despite being nonselective transporter releasers like MDMA, the APBT compounds failed to produce locomotor stimulation in C57BL/6J mice. Interestingly, 3-APBT, 5-APBT, and 6-APBT were full agonists at 5-HT2 receptor subtypes as determined by calcium mobilization assays and induced the head-twitch response in C57BL/6J mice, suggesting psychedelic-like activity. Compared to their APB counterparts, ABPT compounds demonstrated that replacing the oxygen atom with sulfur results in enhanced releasing potency at the serotonin transporter and more potent and efficacious activity at 5-HT2 receptors, which fundamentally changed the in vitro and in vivo profile of APBT isomers in the present studies. Overall, our data suggest that APBT isomers may exhibit psychedelic and/or entactogenic effects in humans, with minimal psychomotor stimulation. Whether this unique pharmacological profile of APBT isomers translates into potential therapeutic potential, for instance as candidates for drug-assisted psychotherapy, warrants further investigation.
Subject terms: Cellular neuroscience, Neurochemistry
Introduction
Naturally occurring tryptamines include serotonin (5-hydroxytyptamine; 5-HT), melatonin (N-acetyl-5-methoxytryptamine), N,N-dimethyltryptamine (DMT), and psilocybin. DMT and psilocybin are known to produce powerful psychedelic-like effects (e.g., hallucinations), and many structurally related synthetic tryptamine derivatives are used recreationally [1–4]. Most psychoactive tryptamines interact with monoaminergic receptors and transporters that can lead to untoward effects [5], which underscores the importance of thorough pharmacological profiling for this class of substances. As an historical example, the synthesis of α-methyltryptamine, also known as 3-(2-aminopropyl)indole (3-API), (Fig. 1A) was first published in 1947 [6], and the substance was marketed as an antidepressant in the former Soviet Union in the 1960s. However, it was withdrawn from clinical use after a short period of time due to adverse effects, including psychedelic-like effects such as altered perception (e.g., hallucinations) and mood [7, 8]. Due to its hallucinogenic properties, 3-API is used recreationally since the 1990s, but the drug is also associated with acute mental health disturbances and seizures [2, 7]. Studies in rat brain synaptosomes not only revealed that 3-API inhibits monoamine uptake by serotonin, dopamine, and norepinephrine transporters (SERT, DAT, and NET), but also that it potently induces transporter-mediated efflux [9], and therefore exhibits characteristics reminiscent of amphetamine-type psychostimulants [10].
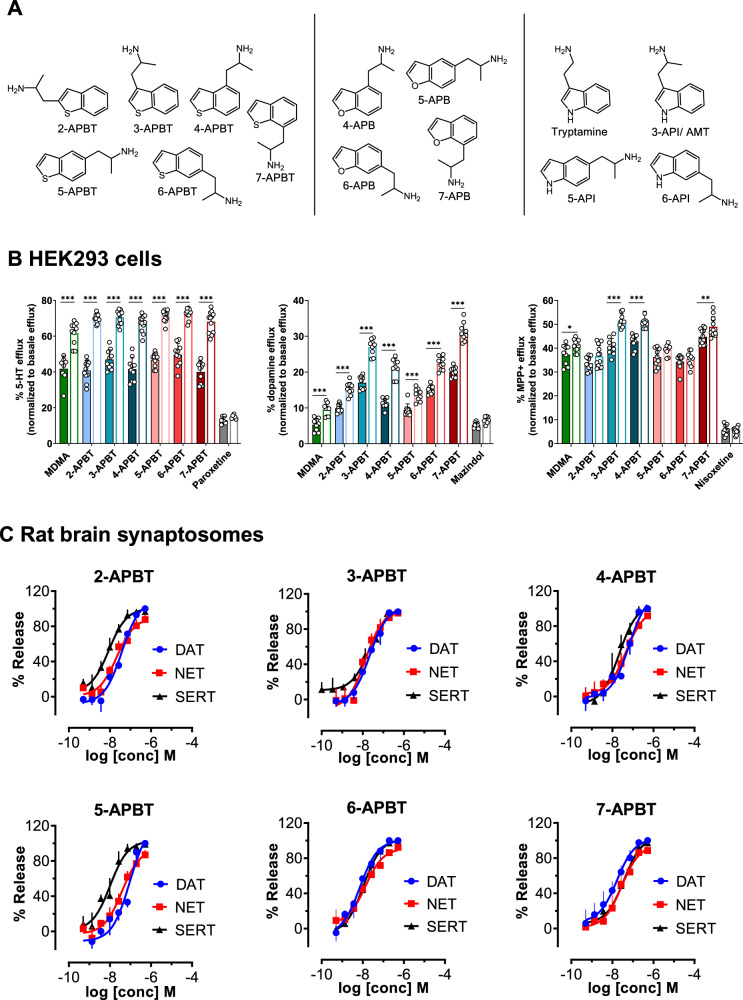
Fig. 1. Chemical structures of APBT isomers and chemically related compounds and effects of APBT isomers on transporter-mediated efflux in HEK293 cells and in rat brain synaptosomes.
A Chemical structures of (2-aminopropyl)benzo[β]thiophens and examples of the 5-(2-aminopropyl)indoles 3-API, 5-API, and 6-API and the (2-aminopropyl)benzofuran isomers 4-APB, 5-APB, 6-APB, and 7-APB. B Transporter-mediated efflux in HEK293 cells expressing MATs. Monoamine release was induced by 10 μM compound in the absence (filled bars) or presence (empty bars) of 10 μM monensin after preloading the transporter-transfected cells with the respective radiolabeled substrate. Transporter blockers (paroxetine, mazindol, and nisoxetine for hSERT, hDAT, and hNET, respectively) were used to assess nonspecific efflux. Data are expressed as mean ± SD from at least three experiments performed in triplicate. Data were analyzed by two-way ANOVA followed by Sidak’s multiple comparison test (***P < 0.001, **P < 0.01, *P < 0.05 when compared to the corresponding condition without monensin). C Transporter-mediated efflux in rat brain synaptosomes. Data are mean ± SD expressed as a % of maximal release response for three experiments performed in triplicate.
In contrast to 3-API, psychedelic-like effects have rarely been reported for the positional isomers 5-(2-aminopropyl)indole (5-API) and 6-(2-aminopropyl)indole (6-API), which originated from pharmaceutical industry research [11]. The recreational use of 5-API is associated with severe sympathomimetic adverse effects and has contributed to several fatalities in Europe [12–16]. 5-API and 6-API are substrates of SERT, DAT, and NET in rat brain synaptosomes, potently inducing substrate release [17]. The position of the alkylamine side chain strongly impacts the selectivity of API isomers at DAT vs. SERT, resulting in eightfold higher DAT selectivity for 5-API and eightfold higher SERT selectivity for 6-API [17]. In human transporter-transfected cells, 5-API also potently inhibits monoamine transport at SERT and DAT [18]; in addition, 5-API acts as a releaser of dopamine and serotonin. However, in contrast to studies in rat brain synaptosomes, 5-API was reported to strongly inhibit norepinephrine uptake without inducing significant norepinephrine efflux in human NET-transfected cells [18]. In addition to its interactions with monoamine transporters (MATs), 5-API inhibits human monoamine oxidase A (MAO-A) [19]. Furthermore, 5-API binds to adrenergic and serotonergic receptors, and partially activates serotonergic 5-HT2A and 5-HT2B receptors [18]. The extant literature illustrates the complex pharmacology of API positional isomers.
The structurally related (2-aminopropyl)benzofurans (APBs) share the heterocyclic core ring structure found in API analogs but with bioisosteric replacement of the nitrogen by an oxygen atom. Pharmacological studies with 4-APB, 5-APB, 6-APB, and 7-APB revealed potent norepinephrine uptake inhibition combined with a more pronounced 5-HT vs. dopamine uptake inhibition in human transporter-transfected cells [20]. In rat brain synaptosomes, 5-APB and 6-APB were found to be nonselective monoamine releasers, serving as efficacious substrates for SERT, DAT, and NET [21]. Furthermore, 4-APB, 5-APB, 6-APB, and 7-APB are strong monoamine releasers that also interact with various monoaminergic receptors and the trace amine-associated receptor 1 [20].
Derivatives of (2-aminopropyl)benzo[β]thiophene (APBT) are sulfur-based analogs of APIs and APBs that have recently been synthesized and analytically characterized [22]. However, the pharmacological profile of these APBTs has not yet been investigated. APBTs share the heterocyclic core ring structure also found in API and APB analogs but with bioisosteric replacement of the nitrogen (APIs) or oxygen (APBs) by a sulfur atom (Fig. 1A). The pharmacological profile of APBTs may vary depending on the location and composition of side chains or the presence of ring substituents. The impact of side chain substitution has previously been described for the amphetamine scaffold, which essentially alters the potency and affinity of the resulting derivatives at different monoaminergic targets [23].
Previous studies with APBTs include a patent issued to Smith Kline & French Laboratories in 1960, which reported various central nervous effects [24]. In studies using mitochondrial suspensions from rat brain, 3-APBT inhibited MAO-A but had no effect on MAO-B [25]. The current study aimed to assess the pharmacological profile of six APBT positional isomers 2-, 3-, 4-, 5-, 6-, and 7-APBT (Fig. 1A). Based on the structural similarities to their indole and benzofuran counterparts, the APBT isomers featured in the present study are hypothesized to modulate monoaminergic neurotransmission similar to other stimulants such as 5-API/6-API or 5-APB/6-APB by interacting with MATs and possibly monoaminergic receptors.
Material and methods
Experimental procedures for cell culture, uptake inhibition, batch release, and FRET experiments in transporter-transfected human embryonic kidney 293 (HEK293) cells were performed as described previously [26–28]. Uptake inhibition experiments in rat brain synaptosomes have been reported earlier [17] and were conducted as described. All experiments using animal tissue were performed according to the ARRIVE guidelines. Receptor binding affinity and activation potency assays were performed as described in previous literature [29–31]. In vivo behavioral experiments using male C57/BL6J mice, including assessment of locomotor activity and temperature changes as well as head-twitch response (HTR) post ABPT administration, were performed as described previously [17, 32–34] or as described in the supplemental methods. All in vivo behavioral procedures were approved by the Animal Care and Use Committee of the NIDA, IRP. More detailed information on all experimental procedures, including in silico docking simulations, release experiments in rat brain synaptosomes, details of statistical analyses, and all reagents used, can be found in the supplemental file.
Results
Benzothiophene isomers inhibit transporter-mediated uptake in HEK293 cells
To assess whether the six APBTs interact with hSERT, hDAT, or hNET, we performed uptake inhibition experiments in transporter-transfected HEK293 cells at the respective MAT. All six isomers were fully efficacious inhibitors of 5-HT uptake with IC50 values ranging from 0.79 to 3.87 µM. IC50 values obtained for dopamine uptake inhibition ranged from 0.90 to 7.62 µM. Similar to the values obtained for hSERT and hDAT, IC50 values for MPP+ uptake inhibition at hNET were between 0.53 and 1.75 µM (Fig. S1A, Table 1). The APBTs were more potent inhibitors at hSERT vs. hDAT except for 7-APBT. In contrast, only 2-APBT and 5-APBT inhibited hSERT more potently than hNET. Compared to MDMA, all six isomers were more potent inhibitors at hSERT, hDAT, and hNET. Importantly, different side chain positions attached to the benzothiophene heterocycle did not dramatically affect inhibition potency at the different MATs. IC50 values for inhibition of GABA uptake at hGAT1 for 5-APBT, 6-APBT, and 7-APBT were weak (>1 mM) (Fig. S2, Table S1).
Table 1.
Inhibition of transporter-mediated uptake by APBT isomers compared to MDMA in HEK293 cells and rat synaptosomes.
| HEK293 cells | ||||||
|---|---|---|---|---|---|---|
| SERT IC50 [nM] (95% CI) | DAT IC50 [nM] (95% CI) | NET IC50 [nM] (95% CI) | DAT/SERT ratio | DAT/NET ratio | NET/SERT ratio | |
| 2-APBT | 786.8 (663.8–932.5) | 1344 (1010–1788) | 924.0 (766.4–1114) | 0.59 | 0.69 | 0.85 |
| 3-APBT | 1871 (1571–2229) | 3258 (2398–4427) | 536.4 (413.5–695.8) | 0.57 | 0.16 | 3.49 |
| 4-APBT | 2824 (2069–3854) | 7617 (5753–10,086) | 1295 (1048–1602) | 0.37 | 0.17 | 2.18 |
| 5-APBT | 1263 (1043–1530) | 2101 (1553–2843) | 1748 (1245–2453) | 0.60 | 0.83 | 0.72 |
| 6-APBT | 796.7 (678.2–935.8) | 903.9 (688.2–1187) | 655.2 (534.2–803.5) | 0.88 | 0.72 | 1.22 |
| 7-APBT | 3872 (3068–4886) | 992.4 (803.0–1226) | 527.7 (417.3–667.3) | 3.90 | 0.53 | 7.34 |
| MDMA | 4531 (3314–6195) | 19030 (14,090–25,700) | 4570 (2930–7128) | 0.24 | 0.24 | 0.99 |
| Rat brain synaptosomes | ||||||
| SERT IC50 [nM] (95% CI) | DAT IC50 [nM] (95% CI) | NET IC50 [nM] (95% CI) | DAT/SERT ratio | DAT/NET ratio | NET/SERT ratio | |
| 2-APBT | 32.9 (19.9–54.5) | 322.3 (186.7–556.5) | 267.0 (201.0–354.7) | 0.10 | 0.83 | 0.12 |
| 3-APBT | 91.2 (61.6–134.8) | 371.8 (264.3–523.0) | 247.0 (182.1–335.1) | 0.24 | 0.67 | 0.37 |
| 4-APBT | 152.3 (95.3–243.3) | 917.0 (776.2–1083.3) | 486.3 (351.9–672.1) | 0.17 | 0.53 | 0.31 |
| 5-APBT | 46.5 (32.2–67.2) | 430.9 (334.0–555.9) | 295.2 (216.7–402.3) | 0.11 | 0.69 | 0.16 |
| 6-APBT | 66.3 (43.9–100.1) | 160.6 (136.6–188.7) | 198.1 (138.6–283.2) | 0.41 | 1.23 | 0.33 |
| 7-APBT | 184.2 (126.1–269.1) | 212.8 (145.3–311.6) | 291.5 (230.4–368.8) | 0.86 | 1.37 | 0.63 |
| MDMA | 471.9 (251.9–884.1) | 1826 (1270–2626) | 1107 (852.8–1437) | 0.26 | 0.61 | 0.43 |
IC50 values are given as mean and 95% confidence intervals obtained from nonlinear curve fits obtained from at least three independent experiments, performed in triplicate (data shown in Fig. S1A, B). DAT/SERT ratio is expressed as 1/(DAT IC50) divided by 1/(SERT IC50). DAT/NET ratio is expressed as 1/(DAT IC50) divided by 1/(NET IC50). NET/SERT ratio is expressed as 1/(NET IC50) divided by 1/(SERT IC50).
Benzothiophene isomers inhibit transporter-mediated uptake in rat brain synaptosomes
The uptake inhibition activity of the APBTs was tested in rat brain synaptosomes in order to examine the effects in a native tissue preparation containing plasma membrane transporters in situ. As shown in Fig. S1B and Table 1, all six isomers were potent inhibitors of [3H]5-HT uptake in rat brain synaptosomes with IC50 values ranging from 32.9 to 184.2 nM. Likewise, all tested APBTs were fully efficacious inhibitors of [3H]dopamine (IC50 values between 161 and 917 nM) and [3H]norepinephrine (IC50 values between 198 and 486 nM) uptake in rat brain synaptosomes (Table 1). In general, the APBTs were nonselective in their effects on uptake inhibition, although 2-APBT and 5-APBT displayed some preference for rSERT. Compared to MDMA, all six isomers were more potent inhibitors of any tested neurotransmitter uptake in rat brain synaptosomes.
Benzothiophenes evoke transporter-mediated release in HEK293 cells
To distinguish between monoamine uptake blockers and substrate-type releasers, the effect of the APBTs on monoamine reverse transport was investigated. Release assays in cells were performed at a single concentration of 10 µM for each isomer with the same MAT-expressing cell lines used for uptake inhibition experiments. MDMA (10 µM) was used as a reference substance for comparison. All tested isomers were releasers at SERT, DAT, and NET (Fig. 1B). APBT-mediated release at SERT was similar for all tested isomers and slightly stronger compared to the effect of MDMA at the same concentration. Likewise, all tested isomers induced release at DAT comparable to MDMA, whereas 3-APBT and 7-APBT stimulated release at DAT the most. At NET, 3-APBT, 4-APBT, and 7-APBT were stronger releasers than MDMA, whereas 2-APBT, 5-APBT, and 6-APBT-induced efflux in a similar or slightly lower manner. At SERT and DAT, the Na+/H+-ionophore monensin significantly enhanced efflux for all six isomers (Fig. 1B). At NET, monensin significantly increased the efflux of the stronger norepinephrine releasers 3-APBT, 4-APBT, and 7-APBT as well as of MDMA, but not of the remaining isomers (Fig. 1B).
Benzothiophenes evoke transporter-mediated release in rat brain synaptosomes
The dose-effect efflux curves for APBTs in rat brain synaptosomes are shown in Fig. 1C and the corresponding EC50 values are displayed in Table 2. All tested isomers were potent releasers of preloaded [3H]5-HT with EC50 values ranging from 8.9 to 36.9 nM, compared to 75.9 nM for MDMA. Similar EC50 values were found for [3H]MPP+ release at rDAT, which ranged from 7.2 to 92.8 nM, compared to 118.4 nM for MDMA. APBT-induced efflux of preloaded [3H]MPP+ at rNET occurred with EC50 values ranging from 13.4 to 46.2 nM, which is similar to the EC50 values at rSERT and rDAT. APBTs displayed more potent substrate-releasing properties at rNET in comparison to the effects of MDMA (EC50 113.7 nM). In agreement with the uptake inhibition findings from synaptosomes, the APBTs were generally nonselective in their releasing effects, with 2-APBT and 5-APBT showing some preference for rSERT.
Table 2.
Transporter-mediated efflux by APBT isomers in rat synaptosomes.
| Rat brain synaptosomes | ||||||
|---|---|---|---|---|---|---|
| SERT EC50 [nM] (95% CI) | DAT EC50 [nM] (95% CI) | NET EC50 [nM] (95% CI) | DAT/SERT | DAT/NET | NET/SERT | |
| ratio | ratio | ratio | ||||
| 2-APBT | 8.9 (5.6–14.2) | 38.6 (27.3–54.6) | 21.6 (13.6 –34.2) | 0.23 | 0.56 | 0.41 |
| 3-APBT | 21.9 (14.6–32.8) | 21.7 (16.0–29.6) | 13.4 (7.9 –22.8) | 1.00 | 0.62 | 1.62 |
| 4-APBT | 21.2 (12.7–35.2) | 66.6 (42.0–105.5) | 46.2 (28.9 –73.7) | 0.32 | 0.69 | 0.46 |
| 5-APBT | 10.3 (5.6–19.0) | 92.8 (48.4–178.1) | 38.4 (23.8 –62.2 | 0.11 | 0.41 | 0.27 |
| 6-APBT | 10.7 (7.5–15.4) | 7.2 (4.5–11.7) | 13.6 (9.1 –20.6) | 1.50 | 1.90 | 0.78 |
| 7-APBT | 36.9 (23.4–58.3) | 16.8 (9.7–28.9) | 28.5 (17.0 –47.8) | 2.20 | 1.70 | 1.29 |
| MDMA | 75.9 (41.3–139.2) | 118.4 (80.2–174.9) | 113.7 (69.3 –186.3) | 0.64 | 0.96 | 0.67 |
EC50 values are given as mean and 95% confidence intervals obtained from nonlinear curve fits obtained from at least three independent experiments, performed in triplicate (data shown in Fig. 1C). DAT/SERT ratio is expressed as 1/(DAT EC50) divided by 1/(SERT EC50). DAT/NET ratio is expressed as 1/(DAT EC50) divided by 1/(NET EC50). NET/SERT ratio is expressed as 1/(NET EC50) divided by 1/(SERT EC50).
Benzothiophenes induce the inward-facing conformation of SERT
To assess potential conformational changes induced by APBTs, intramolecular FRET was recorded in HEK293 cells stably expressing an hSERT construct with a fluorescence donor (CFP) and acceptor (YFP) attached to the N terminus and C terminus, respectively. This construct can be used to detect conformational changes of SERT exposed to its substrates. Hence, an accumulation of inward-facing SERT conformations, which results in decreased FRET, is an indicator for substrate-type substances [28]. All six isomers reduced the NFRET between 6 and 10%, greater than the effect produced by MDMA (3%), but less than the effect of PCA (19%) (Fig. S3A). The observed change in FRET indicates a conformational change to an inward-facing conformation [28], supporting the substrate-like behavior and stimulatory effects in in vitro efflux experiments, indicating that all tested isomers are substrates at SERT (Fig. S3A).
Benzothiophenes fit into the orthosteric binding site of MATs in silico
APBTs and the respective native substrates were docked in silico into the primary substrate binding pocket S1 of SERT, DAT, and NET. The MATs were in the ligand-binding competent outward-open conformation [35] in the presence of bound Na+ and Cl− ions. For each transporter and compound, 1000 poses with estimated docking energies were obtained to allow for achieving extensive sampling. Representative poses of highly populated clusters with low binding energies containing at least 100 docking poses are shown in Fig. S3B (right panel). The distribution of estimated binding energies of all docked poses is shown in Fig. S3B (left panel). Each APBT showed overlapping docking poses in hSERT, hDAT, and hNET with seemingly indistinguishable binding energies. Moreover, the poses overlapped with those of the respective native substrate, supporting the consistency of the docking results. Of note, the docking pose of dopamine at hDAT was identical to the binding conformation observed in the drosophila DAT crystal structure [36]. Docking energies of the APBTs ranged from −35 to −20 kJ/mol, which correspond to estimated Ki values ranging from 0.7 to 313 µM. The native substrates, which were used as controls, showed weaker affinity for the transporters. The indistinguishable binding energies of the tested isomers was consistent with our experimental and FRET microscopy data despite the differences in chemical structure.
Benzothiophenes bind to and activate 5-HT2 receptor subtypes
For receptor binding affinity and activation potency assays as well as for subsequent in vivo behavioral investigations, only 3-APBT, 5-APBT, and 6-APBT were included; these isomers correspond to the most frequently encountered substitution profile of benzofurans and aminopropyl indoles. The binding affinities and agonist potencies of 3-APBT, 5-APBT, and 6-APBT at 5-HT2 subtypes are listed in Table 3 and corresponding curves are shown in Fig. S4. All tested APBTs bound to the 5-HT2A and 5-HT2C receptors with affinities in the range of 196–461 nM. 3-APBT and 5-APBT bound to the 5-HT2B receptor with high nanomolar affinity (3-APBT: Ki = 5.88 nM; 5-APBT: Ki = 3.98 nM). The three APBT isomers were highly efficacious agonists at all three 5-HT2 subtypes, but they had at least an order of magnitude higher potency at the 5-HT2B receptor compared to the 5-HT2A and 5-HT2C subtypes.
Table 3.
Serotonin receptor binding affinities and activation potencies.
| 5-HT2A | 5-HT2B | 5-HT2C | ||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Ki ± SEM [nM] | EC50 [nM] | pEC50 ± SEM | Emax ± SEM [%] | Ki ± SEM [nM] | EC50 [nM] | pEC50 ± SEM | Emax ± SEM [%] | Ki ± SEM [nM] | EC50 [nM] | pEC50 ± SEM | Emax ± SEM [%] | |
| 5-HT | N.D. | 0.34 | 9.47 ± 0.03 | 100 | N.D. | 0.20 | 9.69 ± 0.03 | 100 | N.D. | 0.40 | 9.40 ± 0.03 | 100 |
| 3-APBT | 461 ± 50 | 44.4 | 7.35 ± 0.03 | 94.3 ± 1.1 | 5.88 ± 0.65 | 3.40 | 8.47 ± 0.02 | 89.2 ± 0.5 | 231 ± 52 | 25.4 | 7.60 ± 0.02 | 96.4 ± 0.7 |
| 5-APBT | 400 ± 76 | 14.4 | 7.84 ± 0.03 | 93.3 ± 0.8 | 3.98 ± 0.59 | 0.79 | 9.10 ± 0.02 | 94.2 ± 0.5 | 321 ± 74 | 21.6 | 7.67 ± 0.02 | 104.4 ± 0.8 |
| 6-APBT | 196 ± 29 | 6.69 | 8.18 ± 0.03 | 93.1 ± 0.4 | N.D. | 0.45 | 9.35 ± 0.02 | 94.2 ± 0.4 | 288 ± 104 | 10.0 | 8.00 ± 0.03 | 105.8 ± 0.9 |
Binding data were generated according to PDSP protocols. Ki values represent means ± SEM from three independent experiments performed in triplicate. Calcium flux data were collected in HEK T-Rex-293 inducible cell lines stably expressing either human 5-HT2A, human 5-HT2B, or human 5-HT2C receptors. Data represent EC50, pEC50, and Emax means ± SEM from three independent experiments performed in triplicate. Emax is defined as percent of 5-HT maximum response.
In vivo behavioral studies in mice
Benzothiophene isomers fail to stimulate locomotor activity
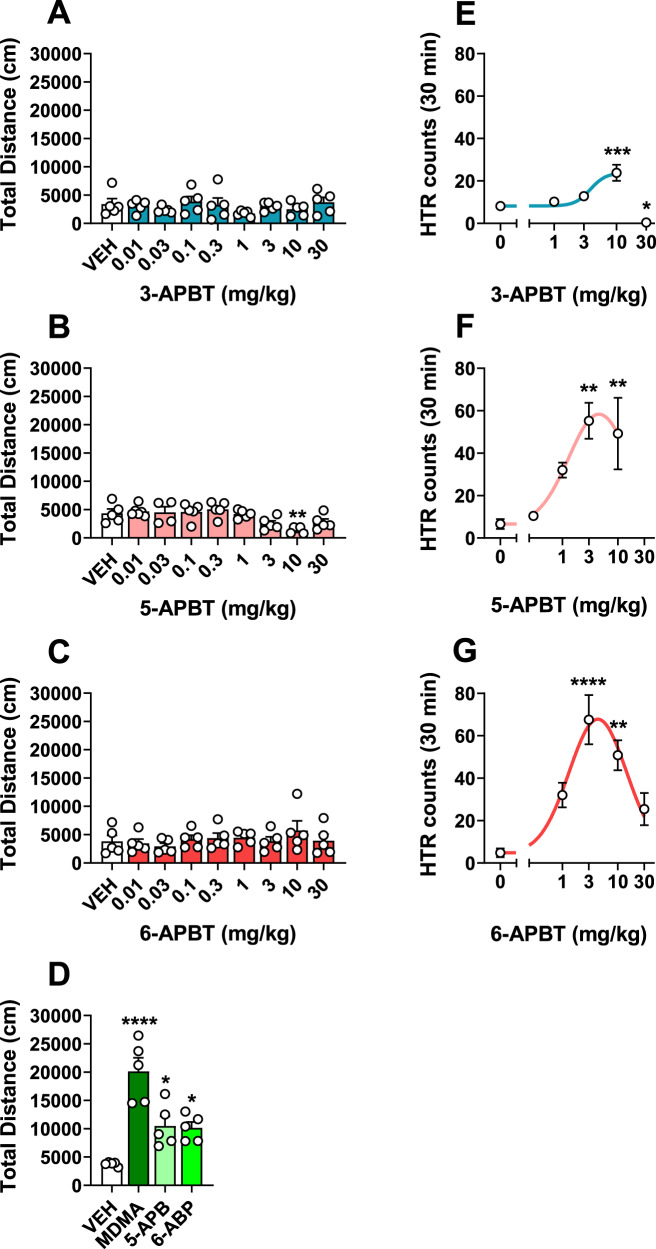
Treatment with 3-APBT or 6-APBT across a broad range of doses surprisingly did not increase locomotor activity compared to saline-treated control mice (Fig. 2A, C; F8,36 = 0.86 n.s., F8,36 = 0.93 n.s.). Similarly, 5-APBT did not increase locomotor activity at any dose tested and reduced the total distance traveled significantly (F8,36 = 4.62 P = 0.0006) at 10 mg/kg s.c. vs. vehicle controls (P < 0.05, Dunnett’s multiple comparisons test; Fig. 2B), but not at the highest dose. In contrast, 10 mg/kg s.c. 5-APB and 6-APB as well as 30 mg/kg s.c. MDMA all produced significant locomotor stimulation vs. saline controls (F3,16 = 18.69 P = 0.0006; *P < 0.05, Dunnett’s multiple comparisons test; Fig. 2D). Outside of increased rest time induced by 10 mg/kg 5-APBT, none of the APBT compounds at any doses tested differed from vehicle controls in number of movements (F8,36 = 0.29 n.s., F8,36 = 0.07 n.s., F8,36 = 0.70 n.s.), rest time (F8,36 = 0.56 n.s., F8,36 = 0.01 n.s., F8,36 = 0.75 n.s.), or stereotypy episodes (F8,36 = 0.70 n.s., F8,36 = 0.03 n.s., F8,36 = 0.48 n.s.) in locomotor experiments (Fig S5A–I). These data support that lack of locomotor activity by APBT compounds is not due to masking by stereotypies or differences in activity. Despite having no effect on locomotor activity, 3-APBT (F8,35 = 15.97 P < 0.0001), 5-APBT (F8,34 = 20.42 P < 0.0001), and 6-APBT (F8,35 = 28.92 P < 0.0001) significantly decreased core body temperature at the two highest doses vs. vehicle controls (P < 0.05, Dunnett’s multiple comparisons test; Fig. S6A–C), attesting to the bioavailability of the drugs at the doses tested. Comparatively, 10 mg/kg 5-APB or 6-APB also reduced core body temperature (F3,17 = 13.09 P = 0.0001; P < 0.05, Dunnett’s multiple comparisons test), but this effect was not observed in mice given 30 mg/kg s.c. MDMA (Fig. S6D).
Fig. 2. Effects of 3-APBT, 5-APBT, and 6-APBT on locomotor activity and HTR in mice.
A–D APBT-treated mice received s.c. injections of 0.01–30 mg/kg s.c. 3-APBT, 5-APBT, 6-APBT, or vehicle (saline 0.9%). Locomotor activity was assessed over 60 min following habituation and drug injections. MDMA (30 mg/kg), 5-APB (10 mg/kg), and 6-APB (10 mg/kg) were administered s.c. as positive controls that are known to produce locomotor stimulation in mice. Data are mean ± SEM for n = 5 mice per group and were compared via one-way ANOVA with Dunnett’s post hoc test comparing all groups to 0 mg/kg vehicle controls (****P < 0.0001, **P < 0.01, *P < 0.05). E–G Effect of 3-APBT, 5-APBT, and 6-APBT on the HTR in mice. Data are presented as group means ± SEM. ****P < 0.0001, ***P < 0.001, **P < 0.01, *P < 0.05 indicate significant difference from the vehicle control group (Dunnett’s test).
Benzothiophene isomers induce the head-twitch response (HTR)
The HTR serves as a measure of 5-HT2A activation in mice [37]. Given the potent agonist activity of 3-APBT, 5-APBT, and 6-APBT at the 5-HT2A receptor, HTR studies were conducted in C57BL/6J mice to determine whether those isomers can activate the receptor in vivo. As shown in Fig. 2E–G, 3-APBT (F(4,20) = 17.2, P < 0.0001), 5-APBT (F(4,20) = 6.40, P = 0.0017), and 6-APBT (F(4,20) = 10.23, P = 0.0001) induced the HTR with inverted U-shaped dose-response functions. The ED50 of 3-APBT was calculated by nonlinear regression as 3.70 (95% CI 1.97–6.96) mg/kg, which is equivalent to 16.2 µmol/kg. 5-APBT was more potent, inducing the HTR with ED50 = 1.05 (95% CI 0.51–2.13) mg/kg, which is equivalent to 4.60 µmol/kg. The ED50 for 6-APBT was 1.08 (95% CI 0.63–1.82) mg/kg, which equals 4.72 µmol/kg. In summary, 5-APBT and 6-APBT are equipotent, and both compounds have about four times higher potency than 3-APBT.
Discussion
The aim of the current study was to determine the pharmacological effects of six APBT isomers, as compared to structurally similar APIs and APBs reported in the literature. All six APBTs exhibited a similar ability to inhibit uptake at MATs and induced transporter-mediated substrate release. The in vitro results from HEK293 cells and rat brain synaptosomes showed that APBTs inhibit substrate uptake in a concentration-dependent manner at human and rat SERT, DAT, and NET, whereas they had no effect at human GAT1, a transporter from the same family that was included as a negative control. The different potencies of APBTs in HEK293 cells compared to rat brain synaptosomes may be based on the different species and tissue origin, as it has been observed for other stimulants [38]. Uptake inhibition potencies in HEK293 cells were in the low micromolar range, with potencies typically being higher at SERT than at DAT, which is comparable to the activity of the benzofurans [20]. In HEK293 cells, 5-APBT was nearly equipotent at SERT and DAT, similar to the nitrogen analog 5-API; however, 5-APBT was around 40-fold less potent at NET than its indole analog [18]. Overall, replacing the oxygen (APBs) or nitrogen (APIs) atoms with sulfur (APBTs) had no overt effect on the in vitro SERT uptake inhibition potency. However, the potency of NET inhibition decreased after replacing oxygen and nitrogen with sulfur, whereas the potency of DAT inhibition decreased when replacing nitrogen with sulfur [18, 20].
The DAT:SERT inhibition ratio (defined as [1/DAT IC50]/[1/SERT IC50]) can be used to predict the reinforcing potential and abuse liability of psychostimulant drugs, with higher DAT selectivity indicating greater propensity for addictive properties [39–44]. Entactogenic substances, such as MDMA, typically exhibit a DAT:SERT ratio of <1 and display lower abuse liability when compared to drugs like amphetamine and methamphetamine [43, 45, 46]. The observed DAT:SERT inhibition ratios of APBTs tested in rat brain synaptosomes and in HEK293 cells were generally <1, which suggests that their pharmacological effects are more MDMA-like rather than methamphetamine-like. The predominantly serotonergic properties of the tested APBTs observed in vitro may also indicate lower abuse liability, similar to what has been observed for MDMA [42, 47], which is a weak reinforcer in self-administration studies [48]. Furthermore, MDMA has been proven effective as an adjunct to psychotherapy in patients suffering from posttraumatic stress disorder and other neuropsychiatric diseases [49–59].
The APBTs were not only efficacious uptake inhibitors, but they also acted as potent transporter substrates by way of releasing preloaded substrate at human and rat SERT, DAT, and NET. Similar results have been reported for their benzofuran counterparts in transporter-transfected HEK293 cells [20, 60]. The sodium-proton ionophore monensin augmented the APBT-triggered outward transport of preloaded MAT substrates, a clear indication of the substrate activity of APBTs [10]. To further explore the substrate activity of the compounds, we performed FRET microscopy to examine the conformational equilibrium induced by the six isomers using a SERT-construct tagged at the N- and C-termini with CFP and YFP, respectively [61]. Indeed, similar to previous findings with known transporter substrates [62], the APBT isomers induced an inward-facing conformation of SERT, as seen with PCA and MDMA.
FRET and efflux measurements showed that APBTs are MAT substrates, which suggests a binding mode similar to the native substrate(s). Accordingly, the best poses for all APBTs were very similar to each other and fully consistent with the known binding mode of dopamine and various amphetamines as observed in complexes with drosophila DAT [36]. The best binding poses of the APBTs show that their positively charged amino group interacts with the conserved and functionally essential aspartate (D79 in hDAT, D98 in hSERT, D75 in hNET) in TM1, while the hydrophobic benzothiophene ring structure binds into the dominantly hydrophobic pocket between TM3 and TM8. The cumulative interactions of the hydrophobic benzothiophene ring with the hydrophobic pocket in the S1 are similar as all APBT poses occupy the same volume in the substrate binding site. They can tolerate small difference in their binding mode without a big change in affinity, because of the absence of orientation-defining hydrogen bonds. Importantly, these small adjustments allow for optimally positioning the amine nitrogen for all APBT isomers, which is important for substrate transport.
In rat brain synaptosomes, 5-API and 6-API have previously been shown to act as substrates at SERT, DAT, and NET [17] though 5-API was a more potent releaser at DAT compared to SERT with eightfold higher selectivity for DAT. In contrast, 5-APBT was more potent at releasing 5-HT in rat brain synaptosomes when compared to DAT, with a tenfold higher selectivity for SERT (Table 2). Overall, replacement of the oxygen or nitrogen with sulfur in the drug scaffold led to enhanced 5-HT releasing properties at SERT.
Since 3-APBT, 5-APBT, and 6-APBT potently induced efflux at DAT and NET in HEK293 cells and rat brain synaptosomes, we expected a corresponding increase in locomotor activity consistent with a typical psychostimulant profile. Surprisingly, in contrast to locomotor stimulant effects of 5-APB, 6-APB, and MDMA in the present and previous studies [21, 63–65], none of the tested APBTs produced locomotor stimulation, and 5-APBT was even observed to reduce the distance traveled at the higher dose of 10 mg/kg. Importantly, the compounds at various doses did not differ from vehicle controls in number of movements, rest time, or stereotypy episodes throughout the testing session supporting that the lack of locomotor activity is not due to competing behaviors (stereotypies) or differences in activity. Thus, APBTs are potent transporter releasers much like indoles, benzofurans, and MDMA, yet the APBTs do not induce locomotor stimulation. The lack of locomotor stimulation may be related to the potent activation of 5-HT2C receptors, which has been shown to decrease locomotor activity [66, 67]. Additionally, although interactions with the 5-HT1A receptor were not assessed, activation of the 5-HT1A receptor by the APBT isomers could potentially reduce locomotor stimulation, as has been demonstrated for selective and nonselective 5-HT1A agonists [68, 69]. Furthermore, potential antagonism at the histamine receptor H1 might depress locomotor activity [70, 71]. Interestingly, the benzofuran analogs 5-APB and 6-APB produce time- and dose-dependent stimulation of locomotor activity [63] and have been shown to bind to 5-HT1A, 5-HT2C, and H1 receptors, but with unknown activation potential [20]. Furthermore, the indole analog 5-API strongly induced locomotor activation, while 6-API did not, which correlated well with the corresponding dopamine releasing potencies in rat brain synaptosomes (5-API EC50 = 12.9 nM; 6-API EC50 = 164 nM) [17]. The DAT EC50 value of 5-API was around sevenfold lower than the corresponding value of 5-APBT, hence, pointed to a higher efficacy of 5-API to induce dopamine release. Importantly, 5-API displayed a higher DAT:SERT ratio than any APBT assessed in the current study [17]. Hence, potentially none of the APBTs features a DAT:SERT ratio that was sufficient to elicit substantial motor stimulation. 6-APBT, which had the highest efflux potency at DAT in rat brain synaptosomes, was also a very potent 5-HT releaser. Hence, locomotor stimulation by 6-APBT may have been depressed by its strong serotonergic effects [72–74], potential activation of H1 receptors, or other yet unidentified or unexamined downstream effects, which impact on monoamine release. Since compounds that increase locomotor activity are likely to be reinforcing [75] and the assessed APBTs lacked motor stimulatory effects and displayed predominantly low DAT:SERT ratios, these substances can be expected to have a low abuse potential [41, 42, 45, 46]. However, further behavioral assays are needed to confirm this anticipation.
The tested APBTs displayed high affinity for all tested 5-HT2 receptor subtypes and activated each receptor in the low nanomolar range. The 5-HT2A receptor is the primary target for LSD, psilocybin, and other hallucinogenic drugs in the brain [76, 77]. Other hallucinogenic drugs acting strongly at 5-HT2A include 3-API [78, 79], while, by contrast, 5-API displays a much lower potency and efficacy [18]. Hence, the interaction of APIs with the 5-HT2A receptor is dependent on the position of the alkylamine side chain on the indole ring. Although relevant data have not been reported for 3-APB, the 5-methoxy-substituted derivative 5-methoxy-3-(2-aminopropyl)benzofuran has high affinity for 5-HT2A sites [80]. In line with the activity of 5-API, both 5-APB and 6-APB have been shown to be active at 5-HT2A with relatively low potency and efficacy [20]. Given those previous findings, it is notable that 3-APBT, 5-APBT, and 6-APBT are highly efficacious 5-HT2A agonists. Hence, compared to APIs and APBs, the ability of APBTs to activate the 5-HT2A receptor does not depend on side chain position. Furthermore, the switch to the benzothiophene heterocyclic ring system led to increased binding affinity, activation potency, and efficacy at 5-HT2 receptors. HTR studies confirmed that 3-APBT, 5-APBT, and 6-APBT activate 5-HT2A in vivo, indicating those compounds may have psychedelic-like psychopharmacology (the HTR is used as a behavioral proxy in rodents for human psychedelic effects [33]). The investigated APBT isomers did not show a clear selectivity for either 5-HT2A or 5-HT2C. Previous studies showed that 5-HT2C agonists may act to mask behavioral effects induced by 5-HT2A receptor activation in rats [66, 81]. However, these agonists displayed much higher selectivity for 5-HT2C receptors vs. 5-HT2A receptors. For example, the 5-HT2 agonist Ro 60–0175, which does not induce the HTR, shows a 30-fold selectivity for the 5-HT2C receptor vs. the 5-HT2A receptor [82], unless administered in combination with a 5-HT2C-selective antagonist [83]. Additionally, the well characterized psychedelic 2,5-dimethoxy-4-methylamphetamine (DOM), an equipotent 5-HT2A and 5-HT2C receptor agonists, has been shown to induce the HTR [37, 84]. Hence, the investigated APBT isomers did not display enough 5-HT2C selectivity to mask the behavioral effects induced by 5-HT2A receptor activation. Like most psychedelics and various stimulants, including benzofuran and indole derivatives, the tested APBT isomers displayed relatively potent agonist activity at 5-HT2B receptors [85]. The activation of 5-HT2B receptors has, among others, been linked to cardiac valvulopathy [86, 87]. However, several studies concluded that long-term regular substance exposure is needed to induced this adverse effect. Hence, potential future medical application of APBTs is not excluded as cardiac valvulopathy is an unlikely adverse effect if these compounds are only sporadically used [88].
Further studies are needed to assess affinities at adrenergic, dopaminergic, and histaminergic receptors to evaluate possible systemic effects. Additionally, assessment of inhibitory effects on MAO, which has already been shown for 3-APBT and MAO-A [25], is needed to estimate the risk for drug–drug interactions. The ability of APBTs to simultaneously release 5-HT and potentially inhibit MAO-A and/or MAO-B could exacerbate the serotonergic effects of co-administered drugs or could potentially result in serotonin toxicity [89].
The potential psychedelic-like properties of these novel APBT isomers combined with their lack of locomotor stimulation and therefore anticipated low abuse potential raises interesting questions for further research regarding their potential development as medications for use in drug-assisted psychotherapy [90].
Conclusion
In summary, the APBT isomers investigated here inhibit monoamine uptake and stimulate substrate release, but without inducing locomotor activity typically seen with structurally related APIs, ABPs, and MDMA. Interestingly, the position of the aminopropyl side chain of APBTs had little influence on transporter selectivity. When compared to APBs and APIs, replacement of the ring oxygen or nitrogen with a sulfur atom as in the APBT isomers led to enhanced SERT releasing effects and 5-HT receptor activities that fundamentally changed the in vivo profile of the compounds in mice. The investigated APBTs may be expected to exhibit psychedelic and entactogenic effects combined with low abuse potential. Whether this pharmacological profile translates into potential therapeutic activity, for instance as candidates for drug-assisted psychotherapy, warrants further investigation.
Supplementary information
Acknowledgements
Receptor binding data were generously provided by the National Institute of Mental Health’s Psychoactive Drug Screening Program (NIMH PDSP), Contract No. HHSN-271–2008–00025-C. The NIMH PDSP is directed by Dr. Bryan Roth at the University of North Carolina at Chapel Hill and Project Officer Jamie Driscol at NIMH, Bethesda, MD, USA.
Author contributions
SDB, GD, and PVK synthesized the APBT compounds. DR, TL, MH, and KJ performed uptake inhibition and efflux experiments in HEK293 cells and analyzed the data. DW conducted uptake inhibition and efflux experiments in rat brain synaptosomes and analyzed the data with assistance of MHB. DL performed FRET microscopy and analyzed the data. DS implemented and analyzed docking simulations at human SERT, DAT and NET with the supervision of TS. JDM conducted 5-HT2 functional experiments and analyzed the data. GCG complemented in vivo locomotor activity and temperature assessment experiments in mice and analyzed the data. ALH conducted HTR experiments in mice and analyzed the data. DR, GCG, DL, DS, TS, SDB, MHB, ALH, and HHS wrote the manuscript with significant inputs from JDM, GD, and PVK.
Funding
This study was supported by grants from the Swiss National Science foundation (SNSF; grant No. P2BSP3_191740 to DR and grant No. P400PM_191032 to DL), the Austrian Science Fund/FWF (doc.fund DOC33 to HHS and grant P32017 to TS), the Intramural Research Program of the National Institute on Drug Abuse, National Institutes of Health (NIH grant DA 00522–13 to MHB), the National Institute on Drug Abuse (NIDA grant R01 DA 041336 to ALH), and the Veteran’s Administration VISN 22 Mental Illness Research, Education, and Clinical Center (ALH).
Competing interests
The authors declare no competing interests.
Footnotes
Publisher’s note Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary information
The online version contains supplementary material available at 10.1038/s41386-021-01221-0.
References
- 1.Shulgin AT, Shulgin A. Tihkal: the continuation. Berkeley, CA: Transform Press; 1997.
- 2.Araújo AM, Carvalho F, Bastos MDL, Guedes de Pinho P, Carvalho M. The hallucinogenic world of tryptamines: an updated review. Arch Toxicol. 2015;89:1151–73. doi: 10.1007/s00204-015-1513-x. [DOI] [PubMed] [Google Scholar]
- 3.Hill SL, Thomas SHL. Clinical toxicology of newer recreational drugs. Clin Toxicol. 2011;49:705–19. doi: 10.3109/15563650.2011.615318. [DOI] [PubMed] [Google Scholar]
- 4.Tittarelli R, Mannocchi G, Pantano F, Romolo FS. Recreational use, analysis and toxicity of tryptamines. Curr Neuropharmacol. 2015;13:26–46. doi: 10.2174/1570159X13666141210222409. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Luethi D, Liechti ME. Designer drugs: mechanism of action and adverse effects. Arch Toxicol. 2020;94:1085–133.. doi: 10.1007/s00204-020-02693-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Snyder BHR, Katz L. The alkylation of aliphatic nitro compounds with gramine. A new synthesis of derivatives of tryptamine1,2. J Am Chem Soc. 1947;69:3140–2. doi: 10.1021/ja01204a061. [DOI] [PubMed] [Google Scholar]
- 7.Kamour A, James D, Spears R, Cooper G, Lupton DJ, Eddleston M, et al. Patterns of presentation and clinical toxicity after reported use of alpha methyltryptamine in the United Kingdom. A report from the UK National Poisons Information Service. Clin Toxicol. 2014;52:192–7. doi: 10.3109/15563650.2014.885983. [DOI] [PubMed] [Google Scholar]
- 8.Wilcox J. Psychoactive properties of alpha-methyltryptamine: analysis from self reports of users. J Psychoact Drugs. 2012;44:274–6. doi: 10.1080/02791072.2012.704592. [DOI] [PubMed] [Google Scholar]
- 9.Nagai F, Nonaka R. Satoh Hisashi Kamimura K. The effects of non-medically used psychoactive drugs on monoamine neurotransmission in rat brain. Eur J Pharmacol. 2007;559:132–7. doi: 10.1016/j.ejphar.2006.11.075. [DOI] [PubMed] [Google Scholar]
- 10.Sitte HH, Freissmuth M. Amphetamines, new psychoactive drugs and the monoamine transporter cycle. Trends Pharmacol Sci. 2015;36:41–50. doi: 10.1016/j.tips.2014.11.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Hofmann A, Troxler F. Nouveaux dérivés de l’indole et leur préparation. France Patent FR1344579A (1962).
- 12.Bäckberg M, Beck O, Hultén P, Rosengren-Holmberg J, Helander A. Intoxications of the new psychoactive substance 5-(2-aminopropyl)indole (5-IT): a case series from the Swedish STRIDA project. Clin Toxicol. 2014;52:618–24. doi: 10.3109/15563650.2014.920088. [DOI] [PubMed] [Google Scholar]
- 13.Katselou M, Papoutsis I, Nikolaou P, Spiliopoulou C, Athanaselis S. 5-(2-aminopropyl)indole: a new player in the drama of ‘legal highs’ alerts the community. Drug Alcohol Rev. 2015;34:51–7. doi: 10.1111/dar.12136. [DOI] [PubMed] [Google Scholar]
- 14.Coppola M, Mondola R. A new stimulant of abuse: 5-(2-aminopropyl)indole. Am J Psychiatry. 2013;170:226. doi: 10.1176/appi.ajp.2012.12091168. [DOI] [PubMed] [Google Scholar]
- 15.Kronstrand R, Roman M, Dahlgren M, Thelander G, Wikström M, Druid H. A cluster of deaths involving 5-(2-aminopropyl)indole (5-IT) J Anal Toxicol. 2013;37:542–6. doi: 10.1093/jat/bkt058. [DOI] [PubMed] [Google Scholar]
- 16.Seetohul LN, Pounder DJ. Four fatalities involving 5-IT. J Anal Toxicol. 2013;37:447–51. doi: 10.1093/jat/bkt053. [DOI] [PubMed] [Google Scholar]
- 17.Marusich JA, Antonazzo KR, Blough BE, Brandt SD, Kavanagh PV, Partilla JS, et al. The new psychoactive substances 5-(2-aminopropyl)indole (5-IT) and 6-(2-aminopropyl)indole (6-IT) interact with monoamine transporters in brain tissue. Neuropharmacology. 2016;101:68–75. doi: 10.1016/j.neuropharm.2015.09.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Luethi D, Kolaczynska KE, Docci L, Krähenbühl S, Hoener MC, Liechti ME. Pharmacological profile of mephedrone analogs and related new psychoactive substances. Neuropharmacology. 2018;134:4–12. doi: 10.1016/j.neuropharm.2017.07.026. [DOI] [PubMed] [Google Scholar]
- 19.Herraiz T, Brandt SD. 5-(2-Aminopropyl)indole (5-IT): a psychoactive substance used for recreational purposes is an inhibitor of human monoamine oxidase (MAO) Drug Test Anal. 2014;6:607–13. doi: 10.1002/dta.1530. [DOI] [PubMed] [Google Scholar]
- 20.Rickli A, Kopf S, Hoener MC, Liechti ME. Pharmacological profile of novel psychoactive benzofurans. Br J Pharm. 2015;172:3412–25. doi: 10.1111/bph.13128. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Brandt SD, Walters HM, Partilla JS, Blough BE, Kavanagh PV, Baumann MH. The psychoactive aminoalkylbenzofuran derivatives, 5-APB and 6-APB, mimic the effects of 3,4-methylenedioxyamphetamine (MDA) on monoamine transmission in male rats. Psychopharmacology. 2020;237:3703–14.. doi: 10.1007/s00213-020-05648-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Brandt SD, Carlino L, Kavanagh PV, Westphal F, Dreiseitel W, Dowling W. Syntheses and analytical characterizations of novel (2-aminopropyl)benzo[b]thiophene (APBT) based stimulants. Drug Testing Anal. 2020;12:1109–25. doi: 10.1002/dta.2813. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Simmler LD, Liechti ME. Interactions of Cathinone NPS with Human Transporters and Receptors in Transfected Cells. In: Baumann MH, Glennon RA, Wiley JL, editors. Neuropharmacology of New Psychoactive Substances (NPS): The Science Behind the Headlines. Cham: Springer International Publishing; 2017. p. 49–72. [DOI] [PubMed]
- 24.Smith Kline and French Laboratories. Improvements in or relating to β-aminoalkylthiananaphthene and β-aminoalkylbenzofuran derivatives. Philadelphia, USA Patent. (1960).
- 25.Vallejos G, Fierro A, Rezende MC, Sepúlveda-Boza S, Reyes-Parada M. Heteroarylisopropylamines as MAO inhibitors. Bioorg Medicinal Chem. 2005;13:4450–7. doi: 10.1016/j.bmc.2005.04.045. [DOI] [PubMed] [Google Scholar]
- 26.Maier J, Rauter L, Rudin D, Niello M, Holy M, Schmid D, et al. α-PPP and its derivatives are selective partial releasers at the human norepinephrine transporter: a pharmacological characterization of interactions between pyrrolidinopropiophenones and high and low affinity monoamine transporters. Neuropharmacology. 2021;190:108570. doi: 10.1016/j.neuropharm.2021.108570. [DOI] [PubMed] [Google Scholar]
- 27.Mayer FP, Luf A, Nagy C, Holy M, Schmid R, Freissmuth M, et al. Application of a combined approach to identify new psychoactive street drugs and decipher their mechanisms at monoamine transporters. Curr Top Behav Neurosci. 2017;32:333–50.. doi: 10.1007/7854_2016_63. [DOI] [PubMed] [Google Scholar]
- 28.Schicker K, Uzelac Z, Gesmonde J, Bulling S, Stockner T, Freissmuth M, et al. Unifying concept of serotonin transporter-associated currents. J Biol Chem. 2012;287:438–45.. doi: 10.1074/jbc.M111.304261. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Brandt SD, Kavanagh PV, Twamley B, Westphal F, Elliott SP, Wallach J, et al. Return of the lysergamides. Part IV: analytical and pharmacological characterization of lysergic acid morpholide (LSM-775) Drug Test Anal. 2018;10:310–22. doi: 10.1002/dta.2222. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Besnard J, Ruda GF, Setola V, Abecassis K, Rodriguiz RM, Huang XP, et al. Automated design of ligands to polypharmacological profiles. Nature. 2012;492:215–20. doi: 10.1038/nature11691. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Klein AK, Chatha M, Laskowski LJ, Anderson EI, Brandt SD, Chapman SJ, et al. Investigation of the structure-activity relationships of psilocybin analogues. ACS Pharmacol Transl Sci. 2021;4:533–42.. doi: 10.1021/acsptsci.0c00176. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Halberstadt AL, Geyer MA. Characterization of the head-twitch response induced by hallucinogens in mice. Psychopharmacology. 2013;227:727–39. doi: 10.1007/s00213-013-3006-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Halberstadt AL, Chatha M, Klein AK, Wallach J, Brandt SD. Correlation between the potency of hallucinogens in the mouse head-twitch response assay and their behavioral and subjective effects in other species. Neuropharmacology. 2020;167:107933. doi: 10.1016/j.neuropharm.2019.107933. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Glatfelter GC, Walther D, Evans-Brown M, Baumann MH. Eutylone and its structural isomers interact with monoamine transporters and induce locomotor stimulation. ACS Chem Neurosci. 2021;12:1170–7. doi: 10.1021/acschemneuro.0c00797. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Hasenhuetl PS, Freissmuth M, Sandtner W. Electrogenic binding of intracellular cations defines a kinetic decision point in the transport cycle of the human serotonin transporter. The. J Biol Chem. 2016;291:25864–76.. doi: 10.1074/jbc.M116.753319. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Wang KH, Penmatsa A, Gouaux E. Neurotransmitter and psychostimulant recognition by the dopamine transporter. Nature. 2015;521:322–7. doi: 10.1038/nature14431. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Canal CE, Morgan D. Head-twitch response in rodents induced by the hallucinogen 2,5-dimethoxy-4-iodoamphetamine: a comprehensive history, a re-evaluation of mechanisms, and its utility as a model. Drug Test Anal. 2012;4:556–76. doi: 10.1002/dta.1333. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Ilic M, Holy M, Jaentsch K, Liechti ME, Lubec G, Baumann MH. Cell-Based Radiotracer Binding and Uptake Inhibition Assays: A Comparison of In Vitro Methods to Assess the Potency of Drugs That Target Monoamine Transporters. Front Pharmacol. 2020;11:673. doi: 10.3389/fphar.2020.00673. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Ritz MC, Lamb RJ, Goldberg SR, Kuhar MJ. Cocaine receptors on dopamine transporters are related to self-administration of cocaine. Science. 1987;237:1219–23. doi: 10.1126/science.2820058. [DOI] [PubMed] [Google Scholar]
- 40.Kuhar MJ, Ritz MC, Boja JW. The dopamine hypothesis of the reinforcing properties of cocaine. Trends Neurosci. 1991;14:299–302. doi: 10.1016/0166-2236(91)90141-g. [DOI] [PubMed] [Google Scholar]
- 41.Wee S, Woolverton WL. Self-administration of mixtures of fenfluramine and amphetamine by rhesus monkeys. Pharmacol Biochem, Behav. 2006;84:337–43. doi: 10.1016/j.pbb.2006.05.022. [DOI] [PubMed] [Google Scholar]
- 42.Wee S, Anderson KG, Baumann MH, Rothman RB, Blough BE, Woolverton WL. Relationship between the serotonergic activity and reinforcing effects of a series of amphetamine analogs. J Pharmacol Exp Therap. 2005;313:848–54. doi: 10.1124/jpet.104.080101. [DOI] [PubMed] [Google Scholar]
- 43.Baumann MH, Ayestas MA, Dersch CM, Brockington A, Rice KC, Rothman RB. Effects of phentermine and fenfluramine on extracellular dopamine and serotonin in rat nucleus accumbens: therapeutic implications. Synapse. 2000;36:102–13. doi: 10.1002/(SICI)1098-2396(200005)36:2<102::AID-SYN3>3.0.CO;2-#. [DOI] [PubMed] [Google Scholar]
- 44.Baumann MH, Ayestas MA, Jr., Partilla JS, Sink JR, Shulgin AT, Daley PF, et al. The designer methcathinone analogs, mephedrone and methylone, are substrates for monoamine transporters in brain tissue. Neuropsychopharmacology. 2012;37:1192–203. doi: 10.1038/npp.2011.304. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Suyama JA, Sakloth F, Kolanos R, Glennon RA, Lazenka MF, Negus SS, et al. Abuse-related neurochemical effects of para-substituted methcathinone analogs in rats: microdialysis studies of nucleus accumbens dopamine and serotonin. J Pharmacol Exp Therap. 2016;356:182–90. doi: 10.1124/jpet.115.229559. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Suyama JA, Banks ML, Negus SS. Effects of repeated treatment with methcathinone, mephedrone, and fenfluramine on intracranial self-stimulation in rats. Psychopharmacology. 2019;236:1057–66.. doi: 10.1007/s00213-018-5029-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Liechti M. Novel psychoactive substances (designer drugs): overview and pharmacology of modulators of monoamine signaling. Swiss Med Wkly. 2015;145:w14043. doi: 10.4414/smw.2015.14043. [DOI] [PubMed] [Google Scholar]
- 48.Cole JC, Sumnall HR. The pre-clinical behavioural pharmacology of 3,4-methylenedioxymethamphetamine (MDMA) Neurosci Biobehav Rev. 2003;27:199–217. doi: 10.1016/s0149-7634(03)00031-9. [DOI] [PubMed] [Google Scholar]
- 49.Amoroso T, Workman M. Treating posttraumatic stress disorder with MDMA-assisted psychotherapy: a preliminary meta-analysis and comparison to prolonged exposure therapy. J Psychopharmacology. 2016;30:595–600. doi: 10.1177/0269881116642542. [DOI] [PubMed] [Google Scholar]
- 50.Mithoefer MC, Wagner MT, Mithoefer AT, Jerome L, Doblin R. The safety and efficacy of {+/−}3,4-methylenedioxymethamphetamine-assisted psychotherapy in subjects with chronic, treatment-resistant posttraumatic stress disorder: the first randomized controlled pilot study. J Psychopharmacol. 2011;25:439–52.. doi: 10.1177/0269881110378371. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Mithoefer MC, Grob CS, Brewerton TD. Novel psychopharmacological therapies for psychiatric disorders: psilocybin and MDMA. Lancet Psychiatry. 2016;3:481–8. doi: 10.1016/S2215-0366(15)00576-3. [DOI] [PubMed] [Google Scholar]
- 52.Sessa B, Higbed L, O’Brien S, Durant C, Sakal C, Titheradge D, et al. First study of safety and tolerability of 3,4-methylenedioxymethamphetamine-assisted psychotherapy in patients with alcohol use disorder. J Psychopharmacol. 2021;35:375–83.. doi: 10.1177/0269881121991792. [DOI] [PubMed] [Google Scholar]
- 53.Monson CM, Wagner AC, Mithoefer AT, Liebman RE, Feduccia AA, Jerome L, et al. MDMA-facilitated cognitive-behavioural conjoint therapy for posttraumatic stress disorder: an uncontrolled trial. Eur J Psychotraumatol. 2020;11:1840123. doi: 10.1080/20008198.2020.1840123. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Wolfson PE, Andries J, Feduccia AA, Jerome L, Wang JB, Williams E, et al. MDMA-assisted psychotherapy for treatment of anxiety and other psychological distress related to life-threatening illnesses: a randomized pilot study. Sci Rep. 2020;10:20442. doi: 10.1038/s41598-020-75706-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Brewerton TD, Lafrance A, Mithoefer MC. The potential use of N-methyl-3,4-methylenedioxyamphetamine (MDMA) assisted psychotherapy in the treatment of eating disorders comorbid with PTSD. Med Hypotheses. 2021;146:110367. doi: 10.1016/j.mehy.2020.110367. [DOI] [PubMed] [Google Scholar]
- 56.Vermetten E, Yehuda R. MDMA-assisted psychotherapy for posttraumatic stress disorder: A promising novel approach to treatment. Neuropsychopharmacology. 2020;45:231–2. doi: 10.1038/s41386-019-0482-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Heifets BD, Salgado JS, Taylor MD, Hoerbelt P, Cardozo Pinto DF, Steinberg EE, et al. Distinct neural mechanisms for the prosocial and rewarding properties of MDMA. Sci Transl Med. 2019;11:eaaw6435. doi: 10.1126/scitranslmed.aaw6435. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Mitchell JM, Bogenschutz M, Lilienstein A, Harrison C, Kleiman S, Parker-Guilbert K, et al. MDMA-assisted therapy for severe PTSD: a randomized, double-blind, placebo-controlled phase 3 study. Nat Med. 2021;27:1025–33. doi: 10.1038/s41591-021-01336-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Mullard A. MDMA scores PTSD success in a landmark phase III trial. Nat Rev Drug Discov. 2021;20:414. [DOI] [PubMed]
- 60.Eshleman AJ, Nagarajan S, Wolfrum KM, Reed JF, Swanson TL, Nilsen A, et al. Structure-activity relationships of bath salt components: substituted cathinones and benzofurans at biogenic amine transporters. Psychopharmacology. 2019;236:939–52.. doi: 10.1007/s00213-018-5059-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Just H, Sitte HH, Schmid JA, Freissmuth M, Kudlacek O. Identification of an additional interaction domain in transmembrane domains 11 and 12 that supports oligomer formation in the human serotonin transporter. J Biol Chem. 2004;279:6650–7. doi: 10.1074/jbc.M306092200. [DOI] [PubMed] [Google Scholar]
- 62.Sandtner W, Stockner T, Hasenhuetl PS, Partilla JS, Seddik A, Zhang YW, et al. Binding mode selection determines the action of ecstasy homologs at monoamine transporters. Mol Pharmacol. 2016;89:165–75. doi: 10.1124/mol.115.101394. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Dolan SB, Forster MJ, Gatch MB. Discriminative stimulus and locomotor effects of para-substituted and benzofuran analogs of amphetamine. Drug Alcohol Depend. 2017;180:39–45. doi: 10.1016/j.drugalcdep.2017.07.041. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Gatch MB, Dolan SB, Forster MJ. Locomotor and discriminative stimulus effects of four novel hallucinogens in rodents. Behav Pharmacol. 2017;28:375–85.. doi: 10.1097/FBP.0000000000000309. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Gatch MB, Dolan SB, Forster MJ. Locomotor activity and discriminative stimulus effects of five novel synthetic cathinone analogs in mice and rats. Drug Alcohol Depend. 2019;199:50–8. doi: 10.1016/j.drugalcdep.2019.02.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Halberstadt AL, van der Heijden I, Ruderman MA, Risbrough VB, Gingrich JA, Geyer MA, et al. 5-HT(2A) and 5-HT(2C) receptors exert opposing effects on locomotor activity in mice. Neuropsychopharmacology. 2009;34:1958–67. doi: 10.1038/npp.2009.29. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Howell LL, Cunningham KA. Serotonin 5-HT2 receptor interactions with dopamine function: implications for therapeutics in cocaine use disorder. Pharm Rev. 2015;67:176–97. doi: 10.1124/pr.114.009514. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Halberstadt AL, Koedood L, Powell SB, Geyer MA. Differential contributions of serotonin receptors to the behavioral effects of indoleamine hallucinogens in mice. J Psychopharmacology. 2011;25:1548–61. doi: 10.1177/0269881110388326. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.van den Buuse M, Ruimschotel E, Martin S, Risbrough VB, Halberstadt AL. Enhanced effects of amphetamine but reduced effects of the hallucinogen, 5-MeO-DMT, on locomotor activity in 5-HT(1A) receptor knockout mice: implications for schizophrenia. Neuropharmacology. 2011;61:209–16. doi: 10.1016/j.neuropharm.2011.04.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Tokunaga S, Takeda Y, Shinomiya K, Hirase M, Kamei C. Effects of some H1-antagonists on the sleep-wake cycle in sleep-disturbed rats. J Pharmacol Sci. 2007;103:201–06.. doi: 10.1254/jphs.fp0061173. [DOI] [PubMed] [Google Scholar]
- 71.Inoue I, Yanai K, Kitamura D, Taniuchi I, Kobayashi T, Niimura K, et al. Impaired locomotor activity and exploratory behavior in mice lacking histamine H1 receptors. Proc Natl Acad Sci USA. 1996;93:13316–20. doi: 10.1073/pnas.93.23.13316. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Howell LL, Czoty PW, Byrd LD. Pharmacological interactions between serotonin and dopamine on behavior in the squirrel monkey. Psychopharmacology. 1997;131:40–8. doi: 10.1007/s002130050263. [DOI] [PubMed] [Google Scholar]
- 73.Prisco S, Pagannone S, Esposito E. Serotonin-dopamine interaction in the rat ventral tegmental area: an electrophysiological study in vivo. J Pharmacol Exp Therap. 1994;271:83–90. [PubMed] [Google Scholar]
- 74.Howell LL, Byrd LD. Serotonergic modulation of the behavioral effects of cocaine in the squirrel monkey. J Pharmacol Exp Therap. 1995;275:1551–9. [PubMed] [Google Scholar]
- 75.Wise RA, Bozarth MA. A psychomotor stimulant theory of addiction. Psychol Rev. 1987;94:469–92. [PubMed] [Google Scholar]
- 76.Nichols DE. Psychedelics. Pharmacol Rev. 2016;68:264. doi: 10.1124/pr.115.011478. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Halberstadt AL. Recent advances in the neuropsychopharmacology of serotonergic hallucinogens. Behav Brain Res. 2015;277:99–120. doi: 10.1016/j.bbr.2014.07.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Hollister LE, Prusmack JJ, Paulsen JA, Rosenquist N. Comparison of three Psychotropic Drugs (Psilocybin, JB-329, and IT-290) in Volunteer Subjects. J Nerv Ment Dis. 1960;131:428–34. doi: 10.1097/00005053-196011000-00007. [DOI] [PubMed] [Google Scholar]
- 79.Murphree HB, Dippy RH, Jenney EH, Pfeiffer CC. Effects in normal man of α-methyltryptamine and α-ethyltryptamine. Clin Pharmacol Therap. 1961;2:722–26. doi: 10.1002/cpt196126722. [DOI] [PubMed] [Google Scholar]
- 80.Tomaszewski Z, Johnson MP, Huang X, Nichols DE. Benzofuran bioisosteres of hallucinogenic tryptamines. J Med Chem. 1992;35:2061–4. doi: 10.1021/jm00089a017. [DOI] [PubMed] [Google Scholar]
- 81.Fantegrossi WE, Simoneau J, Cohen MS, Zimmerman SM, Henson CM, Rice KC, et al. Interaction of 5-HT2A and 5-HT2C receptors in R(-)−2,5-dimethoxy-4-iodoamphetamine-elicited head twitch behavior in mice. J Pharmacol Exp Therap. 2010;335:728–34. doi: 10.1124/jpet.110.172247. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Martin JR, Bös M, Jenck F, Moreau J, Mutel V, Sleight AJ, et al. 5-HT2C receptor agonists: pharmacological characteristics and therapeutic potential. J Pharmacol Exp Therap. 1998;286:913–24. [PubMed] [Google Scholar]
- 83.Vickers SP, Easton N, Malcolm CS, Allen NH, Porter RH, Bickerdike MJ, et al. Modulation of 5-HT(2A) receptor-mediated head-twitch behaviour in the rat by 5-HT(2C) receptor agonists. Pharmacol Biochem Behav. 2001;69:643–52. doi: 10.1016/s0091-3057(01)00552-4. [DOI] [PubMed] [Google Scholar]
- 84.Eshleman AJ, Wolfrum KM, Reed JF, Kim SO, Johnson RA, Janowsky A. Neurochemical pharmacology of psychoactive substituted N-benzylphenethylamines: High potency agonists at 5-HT(2A) receptors. Biochem Pharmacol. 2018;158:27–34. doi: 10.1016/j.bcp.2018.09.024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Luethi D, Liechti ME. Drugs of Abuse Affecting 5-HT2B Receptors. In: Maroteaux L, Monassier L, editors. 5-HT2B Receptors: From Molecular Biology to Clinical Applications. Cham: Springer International Publishing; 2021. p. 277–89.
- 86.Roth BL. Drugs and valvular heart disease. N. Engl J Med. 2007;356:6–9. doi: 10.1056/NEJMp068265. [DOI] [PubMed] [Google Scholar]
- 87.Rothman RB, Baumann MH. Serotonergic drugs and valvular heart disease. Expert Opin Drug Saf. 2009;8:317–29. doi: 10.1517/14740330902931524. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Droogmans S, Cosyns B, D’Haenen H, Creeten E, Weytjens C, Franken PR, et al. Possible association between 3,4-methylenedioxymethamphetamine abuse and valvular heart disease. Am J Cardiol. 2007;100:1442–5. doi: 10.1016/j.amjcard.2007.06.045. [DOI] [PubMed] [Google Scholar]
- 89.Rudin D, Liechti ME, Luethi D. Molecular and clinical aspects of potential neurotoxicity induced by new psychoactive stimulants and psychedelics. Exp Neurol. 2021;343:113778. doi: 10.1016/j.expneurol.2021.113778. [DOI] [PubMed] [Google Scholar]
- 90.Garcia-Romeu A, Kersgaard B, Addy PH. Clinical applications of hallucinogens: a review. Exp Clin Psychopharmacol. 2016;24:229–68. doi: 10.1037/pha0000084. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.