Abstract
Background:
Randomized clinical trials offer the highest quality data for modifying clinical practice. Results of a phase III randomized trial of non-myeloablative transplantation for adults with high-risk hematologic malignancies with two umbilical cord blood (UCB) units (n=183) or HLA-haploidentical relative bone marrow (Haplo-BM) (n=154) revealed 2-year progression-free survival (PFS) of 41% and 35% after Haplo-BM and two-unit UCB transplantation, respectively (p=0.41); overall survival was 57% and 46%, respectively (p=0.04), BMT CTN 1101; NCT01597778.
Objectives:
We sought to examine the generalizability of BMT CTN 1101 to a contemporaneous cohort beyond the trial’s pre-specified 2-year outcomes. All transplantation occurred between June 2012 and June 2018 in the United States. We hypothesized that the results of a rigorous phase III randomized trial will be generalizable. Changes in graft selection for HLA-haploidentical relative transplantation during the trial period allowed comparison of outcomes after transplantation with Haplo-BM to those after haploidentical peripheral blood (Haplo-PB).
Study Design:
The trial’s broad eligibility criteria were applied to the data source of the Center for International Blood and Marrow Transplant Research to select non-trial subjects. Extended follow up of trial subjects was obtained from this data source. Three separate analyses were performed: 1) trial subjects beyond the trial’s 2-year endpoint 2) comparison of trial subjects to a contemporaneous cohort of non-trial subjects (195 two-unit UCB, 358 Haplo-BM, 403 Haplo-PB) and 3) comparison of non-trial subjects by donor and graft type. Multivariate analyses were performed using Cox proportional hazards models for comparison of outcomes by treatment groups.
Results:
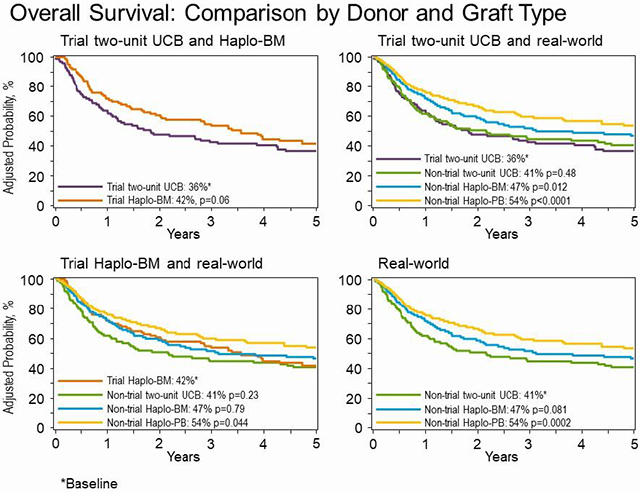
With longer follow up of the trial cohorts, 5-year PFS (37% versus 29%, p=0.08) and overall survival (42% versus 36%, p=0.06) were not significantly different between treatment groups. We then compared the trial results to comparable real-world transplantations. Five-year overall survival after trial and non-trial two-unit UCB (36% versus 41%, p=0.48) and trial and non-trial Haplo-BM (42% versus 47%, p=0.80) transplantation were not different confirming generalizability. The randomized trial did not accrue as planned and therefore lacked the statistical power to detect a 15% difference in progression-free survival. With substantially larger numbers of non-trial Haplo-BM transplantations, 5-year survival was higher after non-trial Haplo-BM compared to trial two-unit UCB (47% versus 36%, p=0.012). Non-trial patients who received Haplo-PB transplantation had higher 5-year survival (54%) compared to trial (HR 0.76, p=0.044) and non-trial (HR 0.78, p=0.026) Haplo-BM. Similarly, survival was higher after Haplo-PB compared to trial (HR 0.57, p<0.0001) and non-trial UCB (HR 0.63, p=0.0002).
Conclusion:
When considering alternative donor low intensity conditioning regimen transplantation, a haploidentical relative is preferred. Further, PB is the preferred graft.
Keywords: Cord blood, HLA-haploidentical, non-myeloablative regimen, leukemia, lymphoma, survival
Graphical Abstract
INTRODUCTION
Randomized clinical trials (RCT) with their unbiased allocation of subjects to treatment arms offer the highest quality data for modifying clinical practice.1 A key metric of an RCT is its generalizability, i.e., whether the results are confirmed among patients who meet the trial’s eligibility criteria and treated as per the clinical trial during the trial period. Generalizability of these trials to the general population meet an important quality indicator specified by the CONSORT Statement.2 The Blood and Marrow Transplant Clinical Trials Network (BMT CTN) published the results of a phase III randomized trial of non-myeloablative conditioning and transplantation of either two umbilical cord blood (UCB) units or HLA-haploidentical bone marrow (Haplo-BM) for adults with high-risk hematologic malignancies (BMT CTN 1101; NCT01597778).3 That trial,3 which was open from June 2012 through June 2018, enrolled 368 patients and 325 patients received their assigned treatment. Analysis by intent-to-treat did not show a significant difference in 2-year progression-free survival between treatment arms (41% and 35% after Haplo-BM and UCB transplantation, respectively, p=0.409).3 However, the trial did not complete accrual which may have impacted its ability to detect a significant difference. Differences were seen in pre-specified secondary endpoints. Two-year non-relapse mortality was lower (11% vs. 18%, p=0.039) and overall survival was higher (57% vs. 46%, p=0.037) after Haplo-BM.3 The 2-year incidence of relapse/progression did not differ between treatment groups (p=0.968).3
A review of two-unit UCB and Haplo-BM transplants that met the broad eligibility criteria for the randomized trial and its conditioning regimen and graft-versus-host disease (GVHD) prophylaxis reported to the Center for International Blood and Marrow Transplant Research (CIBMTR) during the trial period identified 195 two-unit UCB, 358 Haplo-BM and 403 Haplo-peripheral blood (PB) transplantations in the United States. In this report, we compare the results of BMT CTN 1101 to contemporaneous cohorts of patients transplanted in the United States. We also compared outcomes between non-trial contemporaneous two-unit UCB and non-trial Haplo-BM and Haplo-PB during the trial period.
METHODS
Patients
Data were obtained from the CIBMTR, a working group of transplant centers that submit data on standardized reporting forms with patients followed longitudinally. Included in the current analyses are patients transplanted on BMT CTN 1101 (N=183 two-unit UCB and 154 Haplo-BM) and contemporaneous cohorts of patients (N=195 two-unit UCB, N=358 Haplo-BM and N=403 Haplo-PB) who met the eligibility criteria for BMT CTN 1101 but were not enrolled on the trial (Supplemental Figure 1). Eligible patients were aged 18 to 70 years and had high-risk acute leukemia (first complete remission that is not considered favorable risk and second or subsequent complete remission), biphenotypic leukemia in first or subsequent complete remission, Hodgkin or non-Hodgkin in lymphoma in complete or partial remission (failed at least 1 prior regimen of multi-agent chemotherapy and ineligible for autologous transplantation by treating physician), performance score 70-100 and adequate organ function. The transplant conditioning regimen for two-unit UCB transplants was cyclophosphamide 50 mg/kg, fludarabine 200 mg/m2, TBI 200 cGy and GVHD prophylaxis was cyclosporine and mycophenolate mofetil. UCB recipients were allowed to receive TBI 300 cGy (single fraction in lieu of 200 cGy) if they had not received cytotoxic chemotherapy within 3 months of enrollment or an auto HSCT within 24 months of enrollment (N=18). The transplant conditioning regimen for Haplo-BM or Haplo-PB transplants was cyclophosphamide 29 mg/kg, fludarabine 150 mg/m2, TBI 200 cGy and GVHD prophylaxis was 120 mg/kg cyclophosphamide, tacrolimus and mycophenolate mofetil. Patients enrolled on BMT CTN 1101 were transplanted at 33 centers in the United States. The contemporaneous cohorts were transplanted at 91 centers in the United States including 32 centers that enrolled patients on BMT CTN 1101. The contemporaneous cohorts were also transplanted between June 2012 through June 2018. Patients provided written informed consent and the Institutional Review Board of the National Marrow Donor Program study approved the study. The decision to enroll on the trial or the decision to offer trial-regimen for the non-trial cohort was at the discretion of the transplant centers and their treating physicians and patients.
Outcomes
Progression-free survival was the primary end point and was defined as survival without relapse or progression. Progression or relapse was defined as progressive disease or recurrence after a complete remission; death without relapse or progression was the competing risk. Non-relapse mortality was defined as death from any cause without relapse or progression; relapse or progression was the competing risk. Surviving patients were censored at last follow-up and death from any cause was considered an event.
Statistical Analysis
The characteristics of patients and their disease enrolled on BMT CTN 1101 and contemporaneous two-unit UCB, Haplo-BM and Haplo-PB transplants are shown in Table 1. Characteristics were compared using the Chi-square statistic for categorical variables and the Wilcoxon test for continuous variables. Three separate analyses were performed: 1) patients on BMT CTN 1101 as they were treated were followed beyond the trial’s 2-year endpoint 2) comparison of patients on BMT CTN 1101 to a contemporaneous cohort (non-trial two-unit UCB, Haplo-BM and Haplo-PB transplants during the trial period but were not enrolled on the trial) and 3) comparison of non-trial two-unit UCB to non-trial Haplo-BM and Haplo-PB transplants. Multivariate analyses were performed using Cox proportional hazards models for comparison of outcomes by treatment groups.4 Multivariate models were adjusted for age, sex, race, performance score, HCT co-morbidity score, cytomegalovirus serostatus, disease and disease risk index. A stepwise model building approach was adopted, and variables that attained a p-value ≤0.05 were retained in the final model with the exception of the variable for treatment group (donor type) which was held in the final model regardless of its level of significance. The probabilities of progression-free survival, non-relapse mortality, relapse and overall survival were calculated from the final Cox model.5 An effect of transplant center effect on survival was tested using the frailty and random effect model.6,7 All p-values are two-sided and analyses were done using SAS version 9·4 (Cary, NC).
Table 1.
Patient and disease characteristics of trial and non-trial participants by donor type
| Characteristic | Trial two-unit UCB | Non-trial two-unit UCB | Trial Haplo-BM | Non-trial Haplo-BM | Non-trial Haplo-PE |
|---|---|---|---|---|---|
| Number | 183 | 195 | 154 | 358 | 403 |
| Sex | |||||
| Male | 92 (50) | 105 (54) | 91 (59) | 194 (54) | 209 (52) |
| Female | 91 (50) | 90 (46) | 63 (41) | 164 (46) | 194 (48) |
| Age- median (min-max) | 58 (20-71) | 58 (19-70) | 60 (20-71) | 56 (18-70) | 58 (18-70 |
| Race | |||||
| White | 140 (77) | 141 (72) | 108 (70) | 253 (71) | 284 (70) |
| Black or African American | 27 (15) | 25 (13) | 28 (18) | 52 (15) | 89 (22) |
| Other | 13 (7) | 23 (12) | 15 (10) | 30 (8) | 15 (4) |
| Not Reported | 3 (2) | 6 (3) | 3 (2) | 23 (6) | 15 (4) |
| Performance score | |||||
| 90-100 | 125 (68) | 124 (64) | 97 (63) | 258 (72) | 208 (52) |
| 70-80 | 58 (32) | 71 (36) | 57 (37) | 100 (28) | 195 (48) |
| Cytomegalovirus serostatus | |||||
| Negative | 78 (43) | 68 (35) | 63 (41) | 144 (40) | 135 (33) |
| Positive | 104 (57) | 127(65) | 91 (59) | 212(59) | 263 (65) |
| Not Reported | 1 (1) | 0 (0) | 0 (0) | 2 (1) | 5 (1) |
| Co-morbidity score | |||||
| ≤2 | 110 (60) | 87 (45) | 91 (59) | 210(59) | 208 (52) |
| >3 | 73 (40) | 108 (55) | 63 (41) | 145 (41) | 190 (47) |
| Not Reported | 0(0) | 0(0) | 0(0) | 3(1) | 5(1) |
| Disease | |||||
| Acute lymphoblastic leukemia | 31 (17) | 21 (11) | 26 (17) | 66 (18) | 53 (13) |
| Acute myeloid leukemia | 96 (52) | 130 (67) | 86 (56) | 157 (44) | 220 (55) |
| Biphenotypic leukemia | 1 (1) | 3 (2) | 5 (3) | 3 (1) | 3 (1) |
| Hodgkin lymphoma | 10 (5) | 8 (4) | 8 (5) | 37 (10) | 29 (7) |
| Large cell lymphoma | 21 (11) | 12 (6) | 16 (10) | 40 (11) | 34 (8) |
| Follicular lymphoma | 8 (4) | 5 (3) | 3 (2) | 11 (3) | 17 (4) |
| T-Cell lymphoma | 3 (2) | 11 (6) | 1 (1) | 20 (6) | 29 (7) |
| Mantle cell lymphoma | 6 (3) | 3 (2) | 3 (2) | 11 (3) | 10 (2) |
| Other lymphoma | 7 (4) | 2 (1) | 6 (4) | 13 (4) | 8 (2) |
| Disease Status | |||||
| Leukemia | |||||
| 1st complete remission | 95 (52) | 114 (58) | 98 (64) | 179 (50) | 211 (52) |
| 2nd complete remission | 33 (18) | 39 (20) | 18 (12) | 43 (12) | 56 (14) |
| 3rd complete remission | — | 1 (1) | 1 (1) | 4 (1) | 9 (2) |
| Lymphoma | |||||
| Complete remission | 24 (13) | 25 (13) | 13 (8) | 73 (20) | 63 (16) |
| Partial remission | 23 (13) | 11 (6) | 21 (14) | 48 (13) | 47 (12) |
| Follicular Lymphoma | 8 (4) | 5 (3) | 3 (2) | 11 (3) | 17 (4) |
| Disease risk index | |||||
| Low | 24 (13) | 18 (9) | 12 (8) | 43 (12) | 54 (13) |
| Intermediate | 123 (67) | 161 (83) | 107 (69) | 248 (69) | 271 (67) |
| High/ Very High | 36 (20) | 16 (8) | 35 (24) | 67 (19) | 78 (19) |
| Follow-up, median (range), months | 48 (12-80) | 58 (12-79) | 47 (7-76) | 48 (8-97) | 36 (6-74) |
RESULTS
Patient and Disease Characteristics
Patient and disease characteristics for the trial and non-trial patients are shown in Table 1. Racial and ethnic diversity between trial and non-trial patients enrolled were similar and mirror the racial and ethnic diversity of the U.S. population. Trial and non-trial patients received the trial specified conditioning regimen and graft-versus-host disease prophylaxis specific to umbilical cord blood or haploidentical transplants. The characteristics of two-unit UCB trial and non-trial UCB patients were similar except that, non-trial patients were more likely to have co-morbidity score ≥3 (40% versus 55%, p=0.003), more likely to have acute myeloid leukemia (52% versus 67%, p=0.0004) and intermediate disease risk index (67% versus 83%, p=0.010). Non-trial two-unit UCB transplantations had a longer median follow-up of 58 months compared to 48 months for two-unit trial transplantations. The characteristics of Haplo-BM trial and non-trial haplo-BM recipients were similar except, non-trial recipients were more likely to have performance score 90 or 100 (63% versus 72%, p=0.041) and less likely to have acute myeloid leukemia (56% versus 44%, p=0.008). Also included in the current analysis are patients who received PB grafts from a haploidentical relative (Haplo-PB) since the use of PB grafts gained widespread acceptance during the trial period. The characteristics of Haplo-BM trial and non-trial haplo-PB recipients were similar except haplo-PB recipients were less likely to have performance score 90 or 100 (63% versus 52%, p=0.016). The median follow-ups of haplo-BM trial and non-trial transplantations were 47 and 48 months, respectively. The median follow-up of Haplo-PB transplantations was 36 months.
Extended follow-up of clinical trial participants
Table 2, Figure 1A-D shows the comparison of two-unit UCB to Haplo-BM for trial participants. Progression-free and overall survival did not differ between transplantation of two-unit UCB compared to Haplo-BM although there was a trend favoring Haplo-BM. However, median progression-free and overall survival after Haplo-BM transplants (20 months, 95% CI 12 – 29 months and 43 months, 95% CI 25 – 62 months, respectively) were twice the median progression-free and overall survival after two-unit UCB transplants (10 months, 95% CI 7 – 13 months and 22 months, 95% CI 15 – 36 months, respectively, Figure 1 C and D). Other factors associated with death, relapse or progression were age ≥50 years, hematopoietic comorbidity score ≥3, transplantation in 3rd complete remission and follicular lymphoma. Consistent with the primary results of the trial, the risk of non-relapse mortality was higher after transplantation of two-unit UCB compared to Haplo-BM (HR 2.69, 95% CI 1.43-5.04, p=0.002). Relapse/progression risks did not differ by donor type.
Table 2:
Non-relapse mortality, relapse/progression, progression-free survival and overall survival by donor type: extended follow-up of trial participants
| Events/Evaluable | Hazard ratio (95% confidence interval) |
p-value | 5-year probability (95% confidence interval) |
|
|---|---|---|---|---|
| Non-relapse mortality | ||||
| Trial Haplo-BM | 13/154 | 1.00 | 10% (5 - 15) | |
| Trial two-unit UCB | 40/183 | 2.69(1.43 – 5.04) | 0.002 | 24% (18 – 31) |
| Relapse/progression | ||||
| Trial Haplo-BM | 81/154 | 1.00 | 53% (45 – 61) | |
| Trial two-unit UCB | 88/183 | 1.04 (0.77 – 1.41) | 0.81 | 50% (43 – 58) |
| Progression-free survival | ||||
| Trial Haplo-BM | 94/154 | 1.00 | 37% (29 – 45) | |
| Trial two-unit UCB | 128/183 | 1.27 (0.97 – 1.66) | 0.08 | 29% (22 – 36) |
| Overall survival | ||||
| Trial Haplo-BM | 78/154 | 1.00 | 42% (33 – 51) | |
| Trial two-unit UCB | 108/183 | 1.32 (0.98 – 1.77) | 0.06 | 36% (29 – 44) |
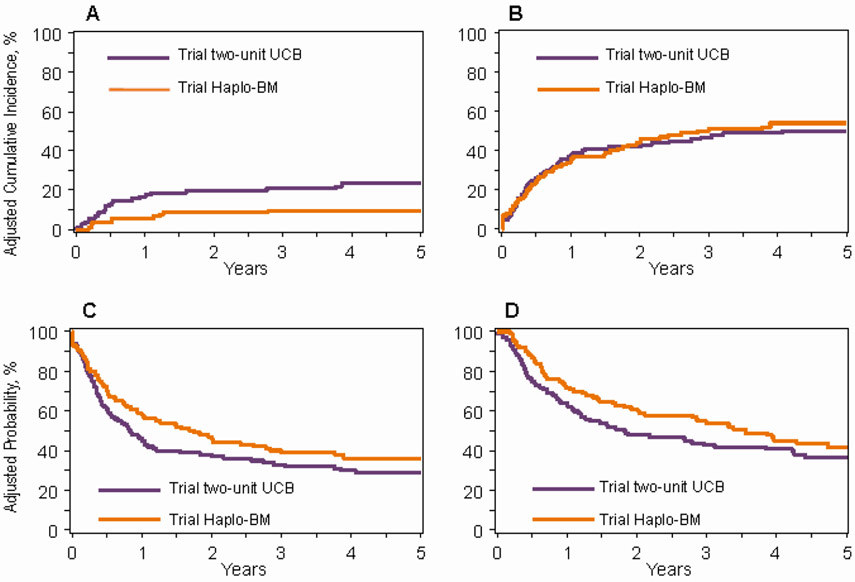
Figure 1: Trial two-unit UCB and trial Haplo-BM transplantation.
1A: Non-relapse mortality after two-unit UCB (24%, 9% CI 18-31) and Haplo-BM (10%, 95% CI 5-15) transplants
1B: Relapse/progression after two-unit UCB (50%, 9% CI 43-58) and Haplo-BM (53%, 95% CI 45-61) transplants
1C: Progression-free survival after two-unit UCB (29%, 9% CI 22-35) and Haplo-BM (37%, 95% CI 29-45) transplants
1D: Overall survival after two-unit UCB (36%, 9% CI 29-44) and Haplo-BM (42%, 95% CI 33-51) transplants
Comparing real-world versus clinical trial data
Trial two-unit UCB
Outcomes of patients on trial who received two-unit UCB transplantation compared to non-trial two-unit UCB, non-trial Haplo-BM and non-trial Haplo-PB transplantations are presented in Table 3, Figure 2A-D. Compared to trial patients receiving two-unit UCB transplantations, there were no differences in risk for non-relapse mortality, relapse/progression, progression-free and overall survival for non-trial patients receiving two-unit UCB transplantations. Consistent with the trial’s primary results, non-relapse mortality was higher and progression-free and overall survival were lower for trial patients transplanted on the two-unit UCB arm compared to non-trial patients receiving Haplo-BM and Haplo-PB transplantations. Additionally, relapse was higher for trial patients receiving two-unit UCB compared to non-trial Haplo-PB transplantations.
Table 3:
Non-relapse mortality, relapse/progression, progression-free survival and overall survival by donor type: Real world data versus trial two-unit UCB
| Events/Evaluable | Hazard ratio (95% confidence interval) |
p-value | 5-year probability (95% confidence interval) |
|
|---|---|---|---|---|
| Non-relapse mortality | ||||
| Trial two-unit UCB | 40/183 | 1.00 | 24% (18 – 31) | |
| Non-trial Two-unit UCB | 42/194 | 0.94 (0.61 – 1.47) | 0.80 | 23% (17 – 30) |
| Non-trial Haplo-BM | 53/358 | 0.60 (0.40 – 0.92) | 0.017 | 19% (14 – 25) |
| Non-trial Haplo-PB | 64/358 | 0.56 (0.38 – 0.84) | 0.005 | 18% (13 – 23) |
| Relapse/progression | ||||
| Trial two-unit UCB | 88/183 | 1.00 | 50% (43 – 58) | |
| Non-trial Two-unit UCB | 88/194 | 0.88 (0.65 – 1.18) | 0.37 | 47% (39 – 54) |
| Non-trial Haplo-BM | 164/358 | 0.88 (0.68 – 1.14) | 0.33 | 53% (46 – 59) |
| Non-trial Haplo-PB | 144/401 | 0.63 (0.49 – 0.83) | 0.001 | 42% (36 – 48) |
| Progression-free survival | ||||
| Trial two-unit UCB | 128/183 | 1.00 | 29% (22 – 36) | |
| Non-trial Two-unit UCB | 130/194 | 0.89 (0.70– 1.14) | 0.36 | 33% (26 – 40) |
| Non-trial Haplo-BM | 217/358 | 0.80 (0.64 – 0.99) | 0.044 | 32% (26 – 38) |
| Non-trial Haplo-PB | 208/401 | 0.62 (0.50 – 0.77) | <0.0001 | 41% (34 – 47) |
| Overall survival | ||||
| Trial two-unit UCB | 108/183 | 1.00 | 36% (29 – 44) | |
| Non-trial Two-unit UCB | 112/194 | 0.91 (0.69 – 1.19) | 0.48 | 41% (34 – 48) |
| Non-trial Haplo-BM | 171/358 | 0.74 (0.56 – 0.93) | 0.012 | 47% (41 – 53) |
| Non-trial Haplo-PB | 161/403 | 0.57 (0.45 – 0.74) | <0.0001 | 54% (47 – 60) |
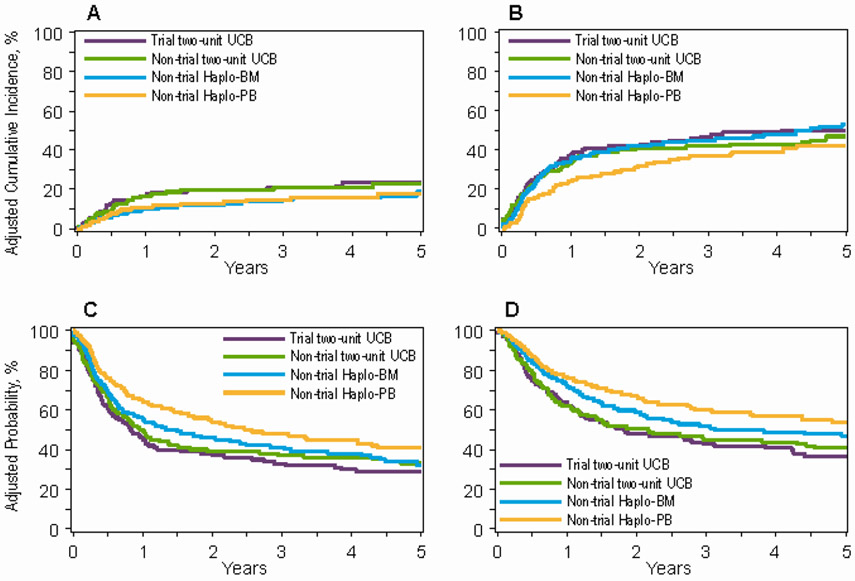
Figure 2: Trial two-unit UCB and real-world transplantation.
2A: Non-relapse mortality after trial two-unit UCB (24%, 9% CI 18-31), non-trial two-unit UCB (23%, 95% CI 17-30), non-trial Haplo-BM (19%, 95% CI 14-25) and non-trial Haplo-PB (18%, 95% CI 13-23) transplants
2B: Relapse/progression after trial two-unit UCB (50%, 9% CI 43-58), non-trial two-unit UCB (47%, 95% CI 39-54), non-trial Haplo-BM (53%, 95% CI 46-59) and non-trial Haplo-PB (42%, 95% CI 36-48) transplants
2C: Progression-free survival after trial two-unit UCB (29%, 9% CI 22-35), non-trial two-unit UCB (33%, 95% CI 26-40), non-trial Haplo-BM (32%, 95% CI 26-38) and non-trial Haplo-PB (41%, 95% CI 34-47) transplants
2D: Overall survival after trial two-unit UCB (36%, 9% CI 29-44), non-trial two-unit UCB (41%, 95% CI 34-48), non-trial Haplo-BM (47%, 95% CI 41-53) and non-trial Haplo-PB (54%, 95% CI 47-60) transplants
Trial Haplo-BM
Outcomes of patients on trial who received Haplo-BM transplantation compared to non-trial two-unit UCB, non-trial Haplo-BM and non-trial Haplo-PB transplantations are presented in Table 4, Figure 3A-D. Compared to patients on the trial Haplo-BM arm, non-relapse mortality was higher after non-trial two-unit UCB transplantation. However, there were no differences in relapse/progression, progression-free and overall survival. There were no differences in non-relapse mortality, relapse/progression, progression-free and overall survival between trial Haplo-BM and non-trial Haplo-BM transplantations. Non-trial patients who received Haplo-PB transplantation had lower relapse/progression risk and higher progression-free and overall survival compared to trial Haplo-BM. Risk for non-relapse mortality was not different between trial Haplo-BM and non-trial Haplo-PB.
Table 4.
NRM, Relapse/Progression, PFS, and OS by Donor Type: Real-World Data versus Trial Haplo-BM
| Parameter | Events/ Evaluable, n | HR(95% CI) | P Value | 5-yr Probability, % (95% CI) |
|---|---|---|---|---|
| NRM | ||||
| Trial Haplo-BM | 13/154 | 1.00 | 10(5-15) | |
| Nontrial 2-unit UCB | 42/194 | 2.54 (1.36-4.75) | .004 | 23 (17-30) |
| Nontrial Haplo-BM | 53/358 | 1.61 (0.88-3.03) | .12 | 19(14-25) |
| Nontrial Haplo-PB | 64/358 | 1.52(0.83-2.78) | .18 | 18(13-23) |
| Relapse/progression | ||||
| Haplo-BM trial | 81/154 | 1.00 | 53 (45-61) | |
| Nontrial 2-unit UCB | 88/194 | 0.91 (0.67-1.23) | .53 | 47 (39-54) |
| Nontrial Haplo-BM | 164/358 | 0.91 (0.70-1.19) | .50 | 53 (46-59) |
| Nontrial Haplo-PB | 144/401 | 0.66(0.50-0.86) | .003 | 42(36-48) |
| PFS | ||||
| Haplo-BM trial | 94/154 | 1.00 | 37 (29-45) | |
| Nontrial 2-unit UCB | 130/194 | 1.13 (0.87-1.48) | .36 | 33 (26-40) |
| Nontrial Haplo-BM | 217/358 | 1.01 (0.79-1.30) | .92 | 32 (26-38) |
| Nontrial Haplo-PB | 208/401 | 0.78(0.61-1.00) | .05 | 41 (34-47) |
| OS | ||||
| Haplo-BM trial | 78/154 | 1.00 | 42(33-51) | |
| Nontrial 2-unit UCB | 112/195 | 1.20 (0.89-1.60) | .23 | 41 (34-48) |
| Nontrial Haplo-BM | 171/358 | 0.96 (0.74-1.27) | .80 | 47 (41-53) |
| Nontrial Haplo-PB | 161/403 | 0.76 (0.57-0.99) | .044 | 54 (47-60) |
Significant values are in bold type.
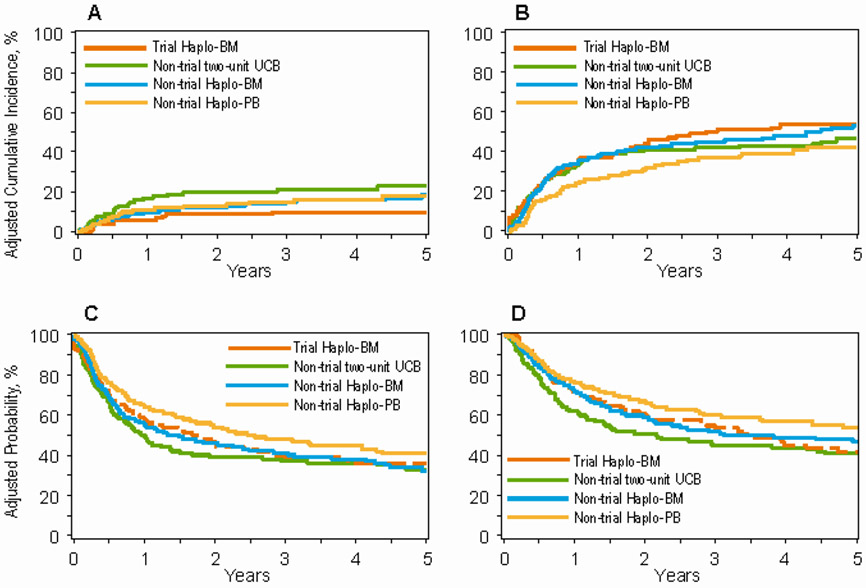
Figure 3: Trial two-unit UCB and real-world transplantation.
3A: Non-relapse mortality after trial Haplo-BM (10%, 95% CI 5-15), non-trial two-unit UCB (23%, 95% CI 17-30), non-trial Haplo-BM (19%, 95% CI 14-25) and non-trial Haplo-PB (18%, 95% CI 13-23) transplants
3B: Relapse/progression after trial Haplo-BM (53%, 95% CI 45-61), non-trial two-unit UCB (47%, 95% CI 39-54), non-trial Haplo-BM (53%, 95% CI 46-59) and non-trial Haplo-PB (42%, 95% CI 36-48) transplants
3C: Progression-free survival after trial Haplo-BM (37%, 95% CI 29-45), non-trial two-unit UCB (33%, 95% CI 26-40), non-trial Haplo-BM (32%, 95% CI 26-38) and non-trial Haplo-PB (41%, 95% CI 34-47) transplants
3D: Overall survival after trial Haplo-BM (42%, 95% CI 33-51), non-trial two-unit UCB (41%, 95% CI 34-48), non-trial Haplo-BM (47%, 95% CI 41-53) and non-trial Haplo-PB (54%, 95% CI 47-60) transplants
Non-trial two-unit UCB, non-trial Haplo-BM and non-trial Haplo-PB
The generalizability of the results of the phase III randomized trial was examined in a comparison of non-trial transplantations during the trial period. Our observations were consistent with the results of the trial (Table 2). Compared to non-trial two-unit UCB transplantation non-relapse mortality was lower with non-trial Haplo-BM (HR 0.64, 95% CI 0.42 – 0.96, p=0.033) but risks for relapse/progression (HR 1.01, 95% CI 0.84 – 1.29, p=0.97), progression-free (HR 0.89, 95% CI 0.72 – 1.11, p=0.31) and overall survival (HR 0.81, 95% CI 0.63 – 1.03, p=0.08) were not different between treatment groups. Comparison of non-trial two-unit UCB to Haplo-PB transplantation showed lower risks for non-relapse mortality (HR 0.60, 95% CI 0.40 – 0.88, p=0.011) and relapse/progression (HR 0.72, 95% CI 0.56 – 0.94, p=0.018) and higher progression-free (HR 0.69, 95% CI 0.55 – 0.86, p=0.001) and overall survival (HR 0.63, 95% CI 0.50–0.81, p=0.0002) after Haplo-PB transplantation. Comparison of non-trial Haplo-BM to Haplo-PB transplantation did not a difference in non-relapse mortality (HR 0.93, 95% CI 0.65 – 1.35, p=0.71). However, compared to Haplo-BM, relapse/progression (HR 0.72, 95% CI 0.57 – 0.90, p=0.004) was lower and progression-free (HR 0.76, 95% CI 0.64 – 0.93, p=0.009) and overall survival (HR 0.78, 95% CI 0.63 – 0.97, p=0.027) were higher after Haplo-PB transplantation.
Other factors associated with transplant outcomes
In studying the effect of donor type on transplantation outcomes we also examined for patient and disease characteristics that may be associated with outcomes. Patients aged ≥50 years (HR 2.41, 95% CI 1.69 – 3.43, p<0.0001), hematopoietic co-morbidity score ≥3 (HR 1.79, 95% CI 1.36 – 2.37, p<0.0001) and transplantation in 3rd complete remission (HR 2.39, 95% CI 1.68 – 3.39, p<0.0001) were associated with higher risk for non-relapse mortality. Relapse was higher for patients with leukemia transplanted in 2nd (HR 1.26, 95% CI 1.01 – 1.57, p=0.041) and 3rd (HR 2.72, 95% CI 1.48 – 4.98, p=0.001) complete remission compared to those transplanted in 1st complete remission. Patients with lymphoma in complete remission (HR 0.52, 95% CI 0.38 – 0.70, p<0.0001) and with follicular lymphoma (HR 0.19, 95% CI 0.08 – 0.47, p=0.0003) had lower risk for relapse compared to those transplanted in 1st complete remission for leukemia. Progression-free survival was lower for patients aged ≥50 years (HR 1.25, 95% CI 1.07 – 1.48, p=0.0061), hematopoietic co-morbidity score ≥3 (HR 1.21, 95% CI 1.05 – 1.39, p=0.010) and transplantation in 3rd complete remission (HR 2.91, 95% CI 1.64 – 5.16, p=0.0002). Patients with follicular lymphoma had higher progression-free survival compared to those transplanted in 1st complete remission for leukemia (HR 0.43, 95% CI 0.25 – 0.73, p=0.002). Similarly, overall survival was lower for patients aged ≥50 years (HR 1.57, 95% CI 1.30 – 1.88, p<0.0001), hematopoietic co-morbidity score ≥3 (HR 1.38, 95% CI 1.18 – 1.62, p<0.0001) and transplantation in 3rd complete remission (HR 3.02, 95% CI 1.62 – 5.64, p=0.0005). Patients with follicular lymphoma had higher overall survival compared to those transplanted in 1st complete remission for leukemia (HR 0.45, 95% CI 0.25 – 0.82, p=0.009).
DISCUSSION
The main findings of the current analyses were two-fold. First, follow-up of trial participants beyond 2-years post-transplantation confirmed higher non-relapse mortality but without a clear advantage for progression-free and overall survival after two-unit UCB transplantation compared Haplo-BM. A marginal survival advantage with Haplo-BM transplantation that was observed 2-years after randomization did not persist with longer follow-up. The clinical trial did not complete accrual as planned.3 Consequently, the trial cohort was not powered to detect the expected 10% in progression-free survival. An absolute 8% difference in 5-year progression-free survival and overlapping confidence intervals as a result of the limited sample size yielded a significance level of 0.08. Nonetheless, median progression-free and overall survival after trial Haplo-BM transplants was two-fold greater than trial two-unit UCB transplants which is significant clinically. Second, a comparison of outcomes of trial and non-trial participants and another comparison amongst the non-trial participants demonstrated generalizability of the findings of the clinical trial. Comparison of trial two-unit UCB to non-trial Haplo-BM demonstrated higher progression-free and overall survival 5-years after Haplo-BM transplantation. The inclusion of 358 non-trial recipients of haplo-BM transplants has statistical power to detect a clinically meaningful difference of 10%. A comparison of trial Haplo-BM to non-trial two-unit UCB did not show a significant difference in progression-free or overall survival. A lack of significant difference is explained by the relatively modest numbers of non-trial two-unit UCB (N=195) and trial Haplo-BM recipients (N=154). The clinical trial did not complete accrual as planned3 and UCB transplants have declined substantially in the real-world limiting sample size.8 It is of interest that during the study period the proportion of patients on trial who received two-unit UCB was approximately half of all two-unit UCB transplants in the U.S. for the trial’s inclusion criteria. On the other hand, only a third of recipients of Haplo-BM transplants were on trial. We could not find a significant effect of transplant center on progression-free or overall survival for either donor type. It is noteworthy that the ethnic diversity of the trial and non-trial participants was an accurate reflection of ethnic diversity within the general U.S. population which makes this study truly representative. This is contrary to other reports that have observed fewer racial/ethnic minority representation on trials9 and likely due to the inclusion of patients from all ethnicities in both the trial and non-trial cohorts who do not have HLA-matched donors.
During the course of the trial there was increasing use of PB for Haplo-transplantation.8 A comparison of outcomes of trial two-unit UCB to non-trial Haplo-PB confirmed higher non-relapse mortality and lower progression-free and overall survival after both trial and non-trial two-unit UCB transplants. A comparison of trial Haplo-BM to non-trial Haplo-PB also showed an advantage for progression-free and overall survival after Haplo-PB that is mediated by lower relapse risks associated with Haplo-PB transplantation. Lower risk of relapse after Haplo-PB transplantation is a consistent observation, but to our knowledge this is the first study to demonstrate a survival advantage with Haplo-PB.10,11 An examination of 5-year overall chronic GVHD after transplantation of trial and non-trial Haplo-BM (23% and 22%) and non-trial Haplo-PB (43%) showed higher rates after transplantation of PB. However, an examination of moderate and severe chronic GVHD comparing trial Haplo-BM (p=0.45) and non-trial Haplo-BM (p=0.45) to Haplo-PB transplants did not show differences. Similarly, chronic GVHD severity did not differ between trial-UCB (p=0.43) and non-trial Haplo-PB transplants. An examination of quality of life of surviving patients is beyond the scope of our current study.
For reduced intensity conditioning transplantations, recent literature favors an HLA-matched unrelated donor for myeloid malignancy, the most common indication for transplantation in the current analyses.12 When such a donor is not available is there an optimal alternative donor? The trial cohort and its contemporaneous cohort which is representative of the real-world favor Haplo-BM or -PB over two-unit UCB transplantation. Patients in the current analyses had predominantly acute leukemia and within this disease type, acute myeloid leukemia. A separate study that compared UCB to Haplo-BM for lymphoma also confirmed higher PFS and OS after Haplo-BM with reduced intensity conditioning regimens.13 Taken together, an appropriate hierarchy for selection of an alternative donor for transplantation using reduced intensity conditioning could be a haploidentical relative, and when such a donor is not available, one or two-unit UCB.
Our study has strengths and limitations. A strength of the current analyses was our ability to perform a careful comparison of outcomes after two-unit UCB and Haplo-BM transplantations of patients who were treated on trial and treated off trial at centers (32 of 33) that participated in the trial and 59 centers that did not participate in the trial. Trial and real-word patients were broadly similar except for performance score, co-morbidity score and disease type which were carefully considered in all comparisons. Comparison of outcomes by donor type of real-world transplantations (i.e., a comparison of the 3 non-trial groups) further extend and confirm generalizability of the findings of the trial. We used a consistent data source and trial/non-trial transplant conditioning regimens and graft versus host disease prophylaxis regimens were the same. An added advantage was our ability to perform a comparison of trial versus non-trial outcomes of a graft type (PB) that was not standard of care when the trial was designed. Our observations extend and confirm our confidence that treatment effects for this trial translate to the real-world setting and can be extrapolated to future patients who meet the requirements for this trial. Use of two-unit UCB has extended access to transplantation especially for minorities14,15 but higher non-relapse mortality after UCB transplantation remains an obstacle. Strategies to overcome the cell dose barrier for UCB transplantation has not led to lower non-relapse mortality or higher survival despite faster hematopoietic recovery.16,17 We applied the trial’s inclusion and exclusion criteria to select the non-trial cohort and restricted to the same conditioning regimen and graft versus host disease prophylaxis regimens but there are unknown and unmeasured factors that may influence transplantation outcomes. Although we could not identify differences in outcome by transplant center, choice of one donor type over another for non-trial patients may have been influenced by patient, physician or center practice, a possible bias that was not addressed in our analyses.
Randomized clinical trials are unique in providing an unbiased comparison of treatments but are expensive and logistically challenging to conduct. It is sobering that ~60% of two-unit UCB transplants and ~80% of Haplo-BM non-trial transplants occurred at the 32 sites that were participating in BMT CTN 1101 implying reservations regarding the concept of randomization for donor assignment. Our findings of the trial’s generalizability to the real-world setting should offer confidence in utilizing transplant registries to explore outcomes of novel treatment strategies in transplantation. These data favoring haploidentical relative BM or PB transplantation for adults with high-risk hematologic malignancy may also facilitate changes in clinical practice with respect to selection of alternative donors. Donor availability may vary, and we recommend proceeding to transplantation with the most suitable donor who is available in a timely manner.
Supplementary Material
HIGHLIGHTS.
Confirmed generalizability of results of phase III randomized trial (BMT CTN 1101)
Haplo-relative is preferred to cord blood with low intensity conditioning regimen
Haplo-PB offers a survival advantage compared to Haplo-BM
ACKNOWLEDGEMENT
Support for this study was provided by grants U10HL069294 and U24HL138660 to the Blood and Marrow Transplant Clinical Trials Network from the National Heart, Lung and Blood Institute and National Cancer Institute. The Center for International Blood and Marrow Transplant Research is supported by U24-CA076518 from the National Cancer Institute, the National Heart, Lung and Blood Institute and the National Institute of Allergy and Infectious Diseases and contract HHSH234200637015C from the Health Resources and Services Administration/Department of Health and Human Services (HRSA/DHHS). The content is solely the responsibility of the authors and does not necessarily represent the official views of the National Institute of Health, HRSA/DHHS or any other agency of the US government.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
CONFLICT OF INTEREST DISCLOUSRE
M.R.G has received consulting fees from AbbVie, Agnios, Amgen, Astellas, Cardinal Health, Bristol-Myers Squibb/Celgene, Daiichi Sankyo, Gilead, Incyte, Karius, Merck, Pfizer, Premier, Stemline and Trovagene and research funding from Forma Therapeutics, Genentech/Roche, Incyte and Janssen. The remaining authors declare no competing financial interests.
REFERENCE
- 1.Sniderman AD, LaChapelle KJ, Rachon NA, et al. The necessity for clinical reasoning in the era of evidenced-based medicine. Mayo Clin Proc. 2013; 88:1108–1114. [DOI] [PubMed] [Google Scholar]
- 2.Moher D, Schulz KF, Altman DG. The CONSORT statement: revised recommendations for improving the quality of reports of parallel-group randomized trials. Ann Intern Med. 2001;134:657–662. [DOI] [PubMed] [Google Scholar]
- 3.Fuchs EJ, O'Donnell PV, Eapen M et al. Double unrelated umbilical cord blood vs HLA-haploidentical bone marrow transplantation: the BMT CTN 1101 trial. Blood. 2021; 21:137(3):420–428. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Cox DR. Regression models and life tables. J R Stat Soc. 1972; 34(4):187 – 217. [Google Scholar]
- 5.Zhang X, Loberiza FR, Klein JP, Zhang MJ. A SAS macro for estimation of direct adjusted survival curves based on a stratified Cox regression model. Comput Methods Programs Biomed. 2007;88(2):95–101. [DOI] [PubMed] [Google Scholar]
- 6.Andersen PK, Klein JP, Zhang MJ. Testing for centre effects in multi-centre survival studies: a Monte Carlo comparison of fixed and random effects tests. Med. 1999; 18(12):1489–1500. [DOI] [PubMed] [Google Scholar]
- 7.O’Quigley J, Stare J. Proportional hazards models with frailties and random effects. Statist Med. 2002; 21(21):3219–3233 [DOI] [PubMed] [Google Scholar]
- 8.D'Souza A, Fretham C, Lee SJ et al. Current use of and trends in hematopoietic cell transplantation in the United States. Biol Blood Marrow Transplant. 2020; 26(8):e177–e182. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Murthy VH, Krumholz HM, Gross CP. Participation in cancer clinical trials: race-, sex- and age-based disparities. JAMA. 2004; 291(22): 2720–2726 [DOI] [PubMed] [Google Scholar]
- 10.Bashey A, Zhang MJ, McCurdy SR et al. Mobilized peripheral blood stem cells versus unstimulated bone marrow as a graft source for T-cell-replete haploidentical donor transplantation using post-transplant cyclophosphamide. J Clin Olcol. 2017; 35(26):3002–3009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Ruggeri A, Labopin M, Bacigalupo A et al. Bone marrow versus mobilized peripheral blood stem cells in haploidentical transplants using posttransplantation cyclophosphamide. Cancer. 2018; 124(7):1428–1437. [DOI] [PubMed] [Google Scholar]
- 12.Gooptu M, Romee R, St Martin A et al. HLA haploidentical versus matched unrelated donor transplants with post-transplant cyclophosphamide-based prophylaxis. Blood. 2021; 138(3): 273–282. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Fatobene G, Rocha V, St Martin A et al. Nonmyeloablative alternative donor transplantation for Hodgkin and non-Hodgkin lymphoma: from the LWP-EBMT, Eurocord and CIBMTR. J Clin Oncol. 2020; 38(14):1518–1526 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Scaradavou A, Brunstein CG, Eapen M et al. Double unit grafts successfully extend the application of umbilical cord blood transplantation in adults with acute leukemia. Blood. 2013; 121(5):752–758. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Solomon SR, Martin AS, Zhang MJ et al. Optimal donor for African Americans with hematologic malignancy: HLA-haploidentical relative or umbilical cord blood transplant. Biol Blood Marrow Transplant. 2020; 26(10):1930–1936. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Horwitz ME, Wease S, Blackwell B et al. Phase I/II study of stem-cell transplantation using a single cord blood unit expanded ex vivo with nicotinamide. J Clin Oncol. 2019; 37(5):367–374. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Cohen S, Roy J, Lachance S et al. Hematopoietic stem cell transplantation using single UM171-expanded cord blood: a single-arm, phase 1-2 safety and feasibility study. Lancet Haematol. 2020; 7(2):134–e135 [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.