Abstract
Study Objectives:
In some patients, it is difficult to correctly nosologically classify daytime sleepiness. Clinical manifestations may be nonspecific; on the basis of objective measures it is possible to determine the current severity of sleepiness, but they do not always allow accurate diagnosis. It is especially difficult to distinguish between idiopathic hypersomnia (IH) and hypersomnia associated with a psychiatric disorder (PSY).
Methods:
To find significant differences between the IH and PSY groups, we included 67 patients (IH, n = 15; PSY, n = 52) in the study, focusing on differences in self-reported symptoms, evaluating current depressive symptoms using the Beck Depression Inventory-II score and personality traits measured by the Temperament and Character Inventory. All of the patients underwent polysomnography, the Multiple Sleep Latency Test, and ad libitum sleep monitoring.
Results:
The patients with IH showed greater difficulty than those in the PSY group with waking up in the morning (P < .001) and complained of memory (P = .04) and attention deficit (P = .006). They also showed higher total sleep time (P < .001) and sleep efficiency (P = .007) and a shorter mean sleep latency on the Multiple Sleep Latency Test (P < .001). Nevertheless, the IH and PSY groups did not differ in Beck Depression Inventory scores or personality characteristics.
Conclusions:
IH is a syndrome in which depression/external life stressors and personality characteristics also play a role. Patients with IH may benefit from the cooperation of sleep specialists with psychotherapists/psychiatrists.
Citation:
Bušková J, Novák T, Miletínová E, et al. Self-reported symptoms and objective measures in idiopathic hypersomnia and hypersomnia associated with psychiatric disorders: a prospective cross-sectional study. J Clin Sleep Med. 2022;18(3):713–720.
Keywords: idiopathic hypersomnia, hypersomnia associated with psychiatric disorder, depressivity, personality traits
BRIEF SUMMARY
Current Knowledge/Study Rationale: Determining the boundary between idiopathic hypersomnia and hypersomnia associated with psychiatric disorders is very difficult in some patients. Because excessive daytime sleepiness is a truly debilitating symptom, assessing this boundary as accurately as possible is essential for effective treatment.
Study Impact: Our data showed a substantial overlap in self-reported features and objective sleep measures between patients with idiopathic hypersomnia and hypersomnia associated with psychiatric disorders: We detected a significant overlap in the current depression scores and premorbid personality traits with only a few self-reported and objective distinguishing parameters. Patients with IH may benefit from close cooperation between sleep specialists and psychologists/psychiatrists.
INTRODUCTION
Daytime sleepiness is a common sleep complaint among the general population.1–3 In many patients, it is not difficult to classify it nosologically. Medical conditions, sleep disorders, medications, substance use, insufficient sleep duration, or their combination may account for a patient’s sleepiness.4,5 However, in some patients there is an overlap of clinical symptoms/polysomnographic findings and it is difficult to distinguish individual nosological units.6 One of the challenges is the boundary between idiopathic hypersomnia (IH) and hypersomnia associated with psychiatric disorders (PSY).7,8
IH refers to a condition in which excessive sleepiness has no obvious explanation, unreliable objective measures, and no convincing biological markers. Clinically, it is characterized by prolonged nighttime sleep or repeated long unrefreshing daytime naps and sometimes sleep inertia. However, patients’ heterogeneity is so substantial that it has even led to a split within a previous International Classification of Sleep Disorders, second edition8 classification into 2 different subgroups. Similar diagnostic uncertainty exists in PSY. The symptoms widely differ among patients. They report excessive nocturnal sleep that is not restorative, they do not feel rested in the morning, and they experience excessive napping. In some specific situations, distinguishing these 2 conditions is extremely challenging: (1) differentiation of IH from less-severe forms of depression (dysthymia, atypical depression), (2) identifying situations in which hypersomnia predicts the onset of depressive episodes or persists many years after episodes (binding may be very loose), (3) mood changes are consequent to difficulty adapting to IH, and (4) personality traits or adjustment to major life events contributes to sleepiness.
Because excessive chronic sleepiness is a very debilitating symptom, we believe that reliable and highly precise assessment is necessary for effective treatment. In this article we aim, in addition to detailed clinical assessment and a Multiple Sleep Latency Test (MSLT)/polysomnographic investigation, to compare the acute level of depression and personality profiles in patients with IH and PSY.
METHODS
Participants
We prospectively selected patients with self-reported daytime sleepiness (Epworth Sleepiness Scale score > 10) among patients referred to our sleep disorders center (in a tertiary care university hospital) between 2017 and 2020. The patients were included in the study after they met the following inclusion criteria: (1) complaining of excessive daytime sleepiness occurring daily for ≥ 3 months; (2) no somatic, neurologic, or sleep disorder that manifested itself by sleepiness (sleep-disordered breathing with an apnea-hypopnea index > 5 events/h and periodic limb movements index > 5 events/h; (3) no multiple sleep-onset rapid eye movement periods during an MSLT; (4) no cataplexy; and (5) no drug or substance use history. None of the patients used psychostimulant medications at the time of the investigation. Patients taking antidepressants who could not withdraw from the medication for 2 weeks before the investigation were excluded.
Based on these criteria, we included a total of 67 patients in the study, 15 with a diagnosis of IH (n = 15; 9 women/6 men; mean age 41.4 ± 10.6 years) and 52 with a diagnosis of PSY (n = 52; 38 women/14 men; mean age 36.6 ± 11.5 years). All of the participants were given actigraphs for 14 days to exclude behaviorally induced insufficient sleep syndrome and circadian disorders. They were instructed to keep a regular sleep-wake rhythm without any excess use of alcohol or drug abuse during the 2 weeks preceding investigation in the sleep laboratory. All of the patients underwent a neurologic and psychiatric examination upon their arrival to the sleep center. They were asked to fulfill the Epworth Sleepiness Scale for self-assessment of the likelihood of dozing or falling asleep in 8 hypothetical daytime situations9 and the Beck Depression Inventory (BDI-II)10 and the Temperament and Character Inventory (TCI)11 when they felt rested.
The BDI-II is a 21-item inventory for measuring a self-reported level of depressive symptoms. The answers are rated on a 4-point Likert scale from 0 (not present) to 3 (severe), with a total score ranging between 0 and 63 points. A higher score indicates a higher level of depressive symptoms. The psychiatric diagnosis was based on the Diagnostic and Statistical Manual of Mental Disorders, fourth edition criteria, confirmed using the Mini-International Neuropsychiatric Interview.12 However, because in some patients there was no clear association between psychiatric illness and sleepiness and the BDI-II carries a risk of false-positive results because of shared symptoms of IH and depression (fatigue, concentration difficulties, memory impairment, weight change), a psychological examination was indicated. We added a psychological examination in some patients in the following situations: (1) the patient was unable to respond or they gave a vague time link between sleepiness and the onset of psychiatric illness, (2) the patient described a major life event but was unable to comment on whether the sleepiness could be related to it, (3) the patient denied psychiatric symptoms but the clinical suspicion persisted, and (4) the clinical examination suggested a suspicion of a personality contribution to the presentation of sleep problems.
The TCI provides comprehensive insight into human personality with respect to genetic traits, learning, self-concept, interaction of personality dimensions with development, and environmental factors. The Cloninger’s personality model has 2 basic components: temperament and character. Temperament refers to automatic emotional responses to an experience. Temperament dimensions (novelty-seeking, harm avoidance, reward dependence, and persistence) are thought to be series of genetically independent traits that are both moderately inheritable and stable throughout life regardless of culture or social learning. Character traits (self-directedness, cooperativeness, and self-transcendence) refer to individual differences in self-object relationships, which develop in a stage-like manner as a result of interactions among temperament, family environment, and individual life experience. Character traits are believed to be more culturally inherited than temperament traits, which are not influenced by sociocultural learning.
Our PSY group consisted of patients whose sleepiness clearly occurred in association with concurrent psychiatric disease validated by diagnostic interview. We applied strict criteria to patients in the IH group: Patients were unaware of the temporal relationship of the onset of sleepiness with a significant life event, denied psychiatric symptoms, viewed their poor mood as a consequence of excessive sleepiness, and were not treated for psychiatric illness. Generally, they met both of the IH conditions (mean sleep latency [MSL] ≤ 8 minutes and total sleep time [TST] during 24 hours confirmed polysomnographically ≥ 660 minutes). In 1 patient, the TST was shorter but the patient met the other criteria for IH, and considering that the current classification admits adherence to only 1 of the objective measures, we also included this individual in the IH group.8 The study protocol was approved by the local ethical committee, and written informed consent was obtained.
Objective measures
All of our patients underwent a hypersomnia protocol that included video-polysomnography consisting of 19-channel electroencephalography; electrooculography; electromyography of the bilateral mentalis muscle, the bilateral flexor digitorum superficialis muscle, and the bilateral tibialis anterior muscle; electrocardiography; airflow, thoracic and abdominal respiratory effort; oxygen saturation; and microphone and synchronized video monitoring between 10 pm and 6 am according to the recommendation of the American Academy of Sleep Medicine (The AASM Manual for the Scoring of Sleep and Associated Events: Rules, Terminology and Technical Specifications, Version 2.0).13 Sleep stages, arousals, periodic leg movements, and respiratory events were scored visually according to standard criteria.13 Respiratory and electromyogram sensors were removed during the MSLT and the consecutive 24-hour sleep monitoring. MSLT measurements took place in agreement with the standard protocol at 8 am, 10 am, 12 am, 2 pm, and 4 pm and were terminated after 20 minutes if no sleep occurred and after 15 minutes asleep if sleep occurred.13 After the test, the participants received dinner at 7 pm, and they were free to determine when they wanted to go to sleep with the light switched off in the evening. They continued with long-term video-polysomnography (24-hour monitoring) to elicit the maximum spontaneous amount of sleep under relaxed, quiet, but not totally abnormal conditions, and any sleep episode, whether at night or during the day, was never interrupted by the technicians.
Statistics
To compare demographic variables, self-reported and objective sleep measures, self-assessment of depression, and personality characteristics between IH and PSY, an unpaired t test with Welch’s correction, a Mann-Whitney U test, and a Fisher exact test were used depending on data type and distribution. The TCI raw score for each dimension was transformed to a standardized z score from the normative data for Czech population [11] and lower (z score < –1), normal (z > –1 to < 1), and higher (z > 1) dimensional score levels were defined. A logistic regression model with the diagnostic group as a dependent variable, score levels in respective TCI dimensions as a categorical predictor (with normal level as a reference), and age and BDI score as covariates were applied. All of the analyses were performed using Stata Statistical Software 15.0 (StataCorp LLC, College Station, Texas).
RESULTS
A total of 67 patients with an Epworth Sleepiness Scale score > 10 passing the inclusion criteria (47 women, of whom 7 were postmenopausal, and 20 men) completed the study protocol. We divided them into 2 groups according to the criteria for IH or PSY. The IH group consisted of 15 patients (9 women and 6 men, mean age 41.4 ± 10.6 years), and the PSY group included 52 patients (38 women and 14 men; Fisher exact test, P = .35; mean age 36.6 ± 11.5 years; Welch’s t test, t = 1.51, P = .15). Forty-five of the patients with PSY were diagnosed with major depressive disorder (11 currently depressed and 34 in remission), 2 with attention-deficit hyperactivity disorder, 2 with bipolar II disorder, 2 with dissociative disorder, and 1 with posttraumatic stress disorder. All psychoactive medications including escitalopram (8 patients), sertraline (5 patients), citalopram (2 patients), paroxetine (1 patient), venlafaxine (6 patients), trazodone (3 patients), quetiapine (2 patients), lamotrigine (2 patients), and hydroxyzine (1 patient) were discontinued within 2–6 weeks before the examination. The mean onset of sleepiness was estimated in the IH group to be 19.6 ± 8.2 years and in the PSY group to be 23.1 ± 16.2 years (t = 0.81, P = .43).
Most of the self-reported symptoms did not differ between groups. For daytime naps, the referred duration was 30 minutes (2–3 times a day) to 4 continuous hours in both groups of patients. Patients reported that the sleepiness could be overcome by sustained physical activity (sport, walking, moving, going outside, nonstop activity, cooking), bright light, cold air, heavy stress duty (“if I really have to”), and having company. For awakenings, the most powerful or helping conditions that assisted with waking up in the morning were a combination of alarm clocks (67.2% [10/15 patients with IH vs 35/52 patients with PSY, Fisher exact test, P = 1.0]), bright light (52.2% [9/15 vs 26/52, P = .6]), a person who helps including children (44.8% [13/15 vs 17/52, P < .001]), motivation or duty (58.2% [8/15 vs 31/52, P = .8]), stress or duty (67.2% [9/15 vs 36/52, P = .5]), hunger (26.9% [3/15 vs 15/52, P = .7]), a cold shower (11.9% [2/15 vs 6/52, P = 1.0]), and the need to use the bathroom (14.9% [2/15 vs 8/52, P = 1.0]). Self-reported sleepiness was characterized widely by recurrent periods of an irrepressible need to sleep, an unrefreshing prolonged main sleep period, and sleep inertia. Somatic symptoms that patients presented included fatigue (100%), temperature dysregulation (32.8% [7/15 vs 15/52, P = .2]), tension-type headache/migraine (67.2% [9/15 vs 36/52, P = .5]), frequent respiratory infections/allergy (56.7% [8/15 vs 30/52, P = .8]), digestive problems (32.8% [3/15 vs 19/52, P = .4]), vasovagal syncopes (25.4% [3/15 vs 14/52, P = .7]), and thyroid dysfunction successfully treated (17.9% [2/15 vs 10/52, P = .7]). Cognitive problems were also reported by both groups, including memory problems and attention deficit (62.7% [13/15 vs 29/52, P = .04]), maximal concentration duration < 1 hour (40.3% [11/15 vs 16/52, P = .006]), and problems that were low but that patients were not able to estimate (25.4% [8/15 vs 9/52, P = .01]).
The BDI-II score did not significantly differ between the groups (IH: 18.1 ± 12.3, minimum–maximum: 2–42; PSY: 18.9 ± 12.7, minimum–maximum: 0–56; t = –0.21, P = .83), and the number of patients who assessed themselves as moderately to severely depressed (BDI-II ≥ 20) was also comparable (IH 47%, PSY 48%; P = 1.00).
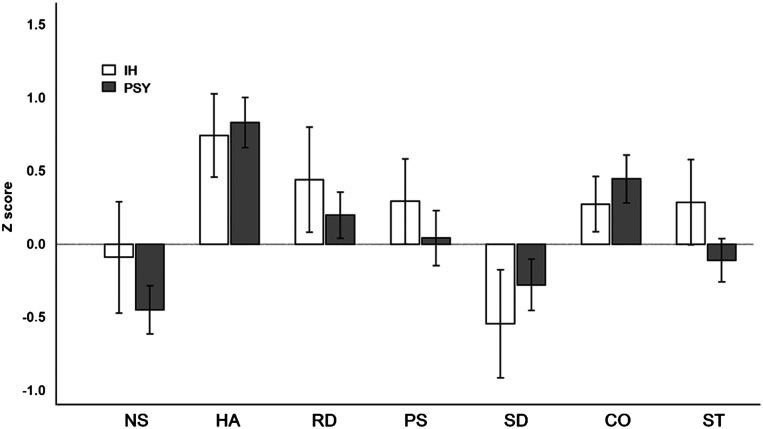
When comparing temperament and character traits (TCI), we failed to find any significant contrast between the groups (the mean difference of the z score ranged from 0.08 ± 1.66 for harm avoidance to 0.36 ± 1.53 in the case of self-transcendence; Figure 1) or an association of categorized TCI dimensions (higher, normal, lower) with diagnostic status after controlling for age, sex, and BDI-II score in a logistic regression model (χ2 [13] = 17.1, P = .19, pseudo R2McF = 0.16).
Figure 1. Comparison of IH and PSY groups according to z scores in the TCI.
Error bars indicate a standard error of the mean. CO = cooperativeness, HA = harm avoidance, IH = idiopathic hypersomnia, NS = novelty-seeking, PS = persistence, PSY = hypersomnia associated with psychiatric disorder, RD = reward dependence, SD = self-directedness, ST = self-transcendence.
Two patients were found to be experiencing depressive feelings in a detailed psychological examination, even though the depressive symptoms were not evident at setup. In another 12 patients with IH, 2 different features were described: (1) sleepiness with a “personality component,” meaning accented personality features, immature personality, or even personality disorder; and (2) “stress response,” referring to past or acutely present excessive and long-term stress and a patient who no longer had sufficient skills and resources to manage it (a “more fragile personality”) and reactively developed excessive sleepiness as an escape from a perceived stressful and unmanageable situation. It is possible to assume that this response was a defense mechanism that ceased to be an adaptive strategy. Moreover, based on the results of the psychological examinations, the role of psychological processes in the sleep problems could not be excluded.
Objective parameters
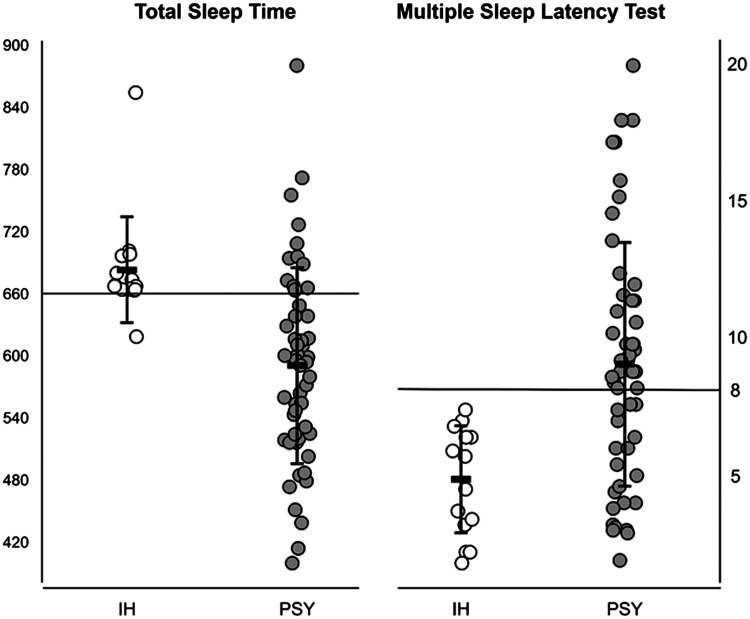
The polysomnographic results of ad libitum video-polysomnography are detailed in Table 1. We found significantly increased TST (P < .001) and sleep efficiency (P = .007) in the IH group compared to the PSY group. Similarly, the patients with IH showed a significantly shorter MSL on the MSLT (P < .001) in the same between-group comparison: The MSL on the MSLT was not in a pathological range in the PSY group (9.1 ± 4.5 minutes) in contrast to the IH group (4.9 ± 2.0 minutes), but the values were quite variable. A precise comparison of TST and MSL on the MSLT is shown in Figure 2.
Table 1.
Objective sleep parameters in patients with IH and PSY obtained on the MSLT and the second night in the sleep laboratory.
| IH | PSY | P Value | |
|---|---|---|---|
| Number | 15 | 52 | |
| Age (y) | 41.4 ± 10.6 | 36.6 ± 11.5 | .15a |
| Female/male | 9/6 | 38/14 | .35b |
| TST, min | 686.9 ± 64.8.0 | 569.9 ± 119.7 | < .001c |
| Sleep latency, min | 13.5 ± 9.0 | 14.8 ± 10.4 | .6a |
| REM sleep latency, min | 109.5 ± 33.1 | 113.8 ± 26.2 | .7a |
| Sleep efficiency, % | 92.9 ± 3.2 | 87.6 ± 6.4 | .007c |
| NREM sleep 1, % SPT | 5.7 ± 4.6 | 5.0 ± 4.3 | .6a |
| NREM sleep 2, % SPT | 49.4 ± 6.7 | 45.1 ± 6.9 | .04a |
| NREM sleep 3, % SPT | 19.3 ± 5.4 | 16.1 ± 5.1 | .06a |
| REM sleep, % SPT | 20.7 ± 5.0 | 19.9 ± 5.7 | .6 |
| WASO, % SPT | 8.8 ± 7.2 | 12.7 ± 9.3 | .1a |
| AHI, events/h | 1.2 ± 1.8 | 1.8 ± 0.4 | .2c |
| PLMI | 2.8 ± 2.0 | 3.9 ± 1.1 | .06c |
| MSLT mean latency, min | 4.9 ± 2.0 | 9.1 ± 4.5 | < .001c |
| MSLT, no SOREMPs (total number) | 6 | 4 |
aWelch’s t test. bFisher exact test. cMann-Whitney U test. AHI = apnea-hypopnea index, IH = idiopathic hypersomnia, MSLT = Multiple Sleep Latency Test, NREM = nonrapid eye movement, PLMI = periodic limb movement in sleep, PSY = hypersomnia associated with psychiatric disorder, REM = rapid eye movement, SOREMPs = sleep-onset REM periods, SPT = sleep period time, WASO = wake after sleep onset.
Figure 2. Comparison of IH and PSY groups according to TST (minutes) and MSL (minutes).
Mean and standard deviations are indicated. IH = idiopathic hypersomnia, MSL = mean sleep latency, PSY = hypersomnia associated with psychiatric disorder, TST = total sleep time.
DISCUSSION
Delineating IH from PSY may be one of the most difficult diagnostic challenges faced in the practice of sleep medicine. We found a significant overlap between patients with IH and PSY, which was evident in the assessment of the patients’ self-reports and in the objective MSLT/polysomnographic parameters. We found that neither acute depressive feelings nor personality traits were decisive for IH. Regarding the self-reported description of sleepiness, patients with IH convincingly more often needed the help of another person to get up and reported more memory and concentration disorders. We found a wide variability and between-group overlap in total sleep duration with the possibility of ad libitum sleep and in MSL on the MSLT. However, it is likely that increased sleep efficiency may be a suitable auxiliary parameter for distinguishing between the 2 groups.
Clinical symptoms
Our results showed an overlap of most clinical symptoms in the 2 nosological units. The heterogeneity of IH symptoms has been confirmed by several studies. The “classic IH phenotype” with nonimperative sleepiness, long unrefreshing naps, prolonged nighttime sleep, difficult awakening with sleep drunkenness, and prominent mood disturbances was found in only 29% of the patients, whereas 32% had clinical features similar to narcolepsy, ie irresistible sleepiness, short and refreshing naps, and few problems with awakening without cataplexy or any abnormality of rapid eye movement sleep. Another 39% had “intermediate” clinical characteristics.14 Sleep drunkenness was found only in 21% of patients with IH14 and in 10%–30% of patients with narcolepsy and in patients with other disorders associated with sleepiness.14,15 Other symptoms of IH (fatigue, attention/memory, vegetative symptoms) have been repeatedly detected and seem to be unspecific.16,17 Similar nonspecific symptoms descriptions have been found in patients with PSY.1
Nevertheless, we found more prominent difficulties with morning awakening in patients with IH; the need for help from another person/children appeared significantly more often, as did reporting memory/concentration difficulties. Because of the significant difference between the IH and PSY groups, this trend may be a specific manifestation of IH that reflects difficulty with sustained attention for long periods of time18 rather than a reflection of a nonspecific vigilance decrement resulting from sleepiness itself19 or cognitive fatigability previously described, eg, in chronic fatigue syndrome.20
Interestingly, several studies of IH have claimed possible etiologies to be viral illness, head trauma, and primary mood disorder;14 another study reported an important personal event before the IH onset, such as the death of a loved one, divorce, the end of military service, or the end of a high level of sports practice in half of 63 patients with IH,16 indicating that external factors may in certain circumstances trigger or aggravate pre-existing excessive sleepiness (if hypersomnia is excluded because of somatic disease or a mental disorder). Our results are in line with these observations, defining some patients with IH with sleepiness in response to past or present extensive stress without the ability or resources to cope with it.
Sleepiness and depressivity
We expected a close relationship between excessive sleepiness and the actual depressivity score based on previous studies, which have shown self-reported sleepiness and excessive sleep duration to be associated with increased odds of depression;21 this close relationship has also been supported by the results of a number of epidemiological studies.22–24 Nevertheless, our results with an overlapping range of depressivity scores in the IH and PSY groups confirm the previously mentioned complex and bidirectional association between sleepiness and depressive symptoms.1,2,7 We agree with the statement that the presence or absence of reported comorbid depression in IH did not meaningfully change the expression of hypersomnia symptoms.25 Therefore, it is possible to claim that patients with different depressivity scores may have the same sleep phenotypes. We still lack an understanding of this interrelationship. Mood and sleepiness may not fluctuate in parallel, but sleepiness may be present many years before mood disturbance and persist long after a depressive episode.26,27 The mechanism by which sleepiness contributes to the development of depressive symptoms is not clear. On the other hand, sleepiness may present a nonspecific response to chronic stress or illness, lifetime difficulties, or trauma. That is the reason it is so important to view sleepiness as a separate clinical entity comorbid to mood disorders such as major depressive disorder, bipolar depression, seasonal affective disorder, atypical depression, or dysthymia and work with it using psychotherapy.
Personality traits
According to the personality traits, the PSY group was defined with 92% sensitivity, which was not observed in the IH group. However, in the z scores of both groups (Figure 2), we found corresponding deflections; in particular, higher harm avoidance (reflects the tendency to be motivated by a desire to avoid aversive experiences), reward dependence (the tendency to respond markedly to signals of reward, particularly to verbal signals of sentiment, social approval, and support), and persistence (expresses the preservation of resistance to frustration behavior). On the other hand, low self-directedness (a measure of self-concepts about oneself, self-acceptance, and the ability to direct one’s own life according to personal goals and values) was evident in both groups.
The importance of the psychological factors has also been shown in psychological investigations defining the role of accented personality features or maladaptive strategies as a precursor of depression. They may indicate subsyndromal forms of depression that have been distinguished as variants of personality or emotionality, implying that personality assessment may allow the reliable measurement of the risk of mood disorders.28 Personality features and stressors may then lead to certain problems including excessive sleepiness, but they are not the only reason for it.
MSLT/polysomnographic findings
It is generally accepted that there is no objective evidence supporting the statement that patients with mood disorder have either an abnormal MSL on the MSLT or that they objectively extend nocturnal sleep.1 However, 1 older study described a subgroup of younger depressed patients with abnormal MSLTs and prolonged nighttime sleep,29 and another stated that 10% of patients with excessive sleepiness secondary to mood disorder had an MSL < 5 minutes on the MSLT.30 A more recent systematic review and meta-analysis of MSLT findings also disproved this belief.31 The review showed that approximately 25% of patients with psychiatric hypersomnolence had an MSL < 8 minutes and that there were also patients with an MSL < 5 minutes on the MSLT. Our results confirmed short latencies on the MSLT in patients with psychiatric disorders. Furthermore, we found that almost 1 in 3 of these patients had an MSL < 8 minutes. In fact, the MSLT can vary within a very wide range.
On the other hand, note that patients with IH may have MSLT latencies longer than 8 or even 10 minutes,14,32–34 and the MSLT is known to have poor test-retest reliability in people with noncataplectic hypersomnia disorder,6,35,36 often resulting in a change of diagnosis. Because the results are so variable, it seems that the MSLT may determine the severity of daytime sleepiness but not estimate the diagnosis.
Similar uncertainty prevails regarding polysomnography measures. Considerably prolonged sleep time is not specific only for IH but may be seen in several psychiatric disorders including major depressive disorder.37,38 Similarly, a characteristic feature of atypical depression is extended night sleep or daytime napping that totals at least 10 hours of sleep,39 which is very close to IH.8 In patients with depression, in addition to greater sleep duration, sleep efficiency similar to that of healthy control patients (> 85%, which is a common cutoff used to define sleep disturbance,38,40,41) has also been repeatedly documented. Our results are congruent with these studies—we found sleep efficiency in our patients with psychiatric disorders to be in the normal range. Therefore, our study does not support the view that PSY is primarily characterized by low sleep efficiency and only increased time in bed.
The video-polysomnography findings of IH included a short sleep latency, sleep efficiency > 90%, and an increased amount of delta sleep; such findings are believed to be nonspecific14,34,42 because the same pattern can be seen in behaviorally induced insufficient sleep syndrome. However, our results confirmed a higher sleep efficiency in IH, although actigraphy was not indicative of social jet lag corresponding to insufficient sleep syndrome and these patients were unequivocally excluded. Meta-analysis on nocturnal sleep architecture in IH has shown higher sleep efficiency compared to that in healthy young adults in 6 out of 10 studies.43 So far, the largest published study on 75 patients with IH showed a sleep efficiency > 90% (90.9 ± 6,3%).33 These results confirm that IH may be seen as a disorder of hypoarousal.44
We claim a convincing overlap regarding the 2 main diagnostic criteria for IH (MSL ≤ 8 minutes or TST ≥ 660 minutes); the first was met by 36.5% and the second one by 25.0% of the patients with PSY. If we had chosen milder criteria, then the overlap would have been even more profound. Therefore, the performed examinations indicate the current burden of sleepiness rather than allowing a nosological classification on that basis.
Our results indicate a multifactorial etiology of IH, in the development of which personality traits and external life stressors may also be involved.14 These patients would probably benefit from additional psychosomatic treatment including pharmacotherapy and phototherapy and from the collaboration between neurologists and mental health experts on diagnosis and treatment. Some clinical features of IH deserve psychotherapy (regulation of emotions, stress management). Nevertheless, for many patients the diagnosis of IH is more acceptable than a psychiatric diagnosis.
A limitation of the study may be the small number of patients with IH, but the diagnosis is rare and we applied strict inclusion criteria, which, on the other hand, may be an advantage of the study. Another limitation may be the use of the BDI-II questionnaire for the group of patients with excessive sleepiness. We are aware of the shared symptoms between IH and depression (fatigue, concentration difficulties, memory impairment, weight change). To achieve greater certainty, our patients also underwent a structured clinical interview, which remains essential for conducting an optimal evaluation of depressive symptoms because of the risk of false-positive results. None of the patients’ medical histories led to a suspicion of drug or alcohol abuse, but this was not objectified by a blood or urine drug screen. Various psychiatric illnesses may also be a limitation; however, heterogeneity may be an advantage because other studies of IH have assessed depression only using the BDI-II, without the view of a clinical specialist in psychiatry to exclude other potential illnesses.
CONCLUSIONS
Recent studies describe a substantial overlap in the self-reported features and objective sleep measures between patients with IH and those with PSY. We detected a significant overlap in the current depression scores and premorbid personality traits with only a few self-reported and objective distinguishing parameters. We believe that IH should be seen as a multifactorial entity. The management of IH requires close collaboration between sleep specialists and psychologists/psychiatrists.
DISCLOSURE STATEMENT
All authors have read and approved the manuscript. Work for this study was performed at the National Institute of Mental Health, Klecany, Czech Republic. This study was supported by Charles University under the grant Progress Q35 and 260533/SVV/2020 and grant number NU20-04-00088 of the Ministry of Health of the Czech Republic. The authors report no conflicts of interest.
ABBREVIATIONS
- BDI-II
Beck Depression Inventory II
- IH
idiopathic hypersomnia
- MSL
mean sleep latency
- MSLT
Multiple Sleep Latency Test
- PSY
hypersomnia associated with a psychiatric disorder
- TCI
Temperament and Character Inventory
- TST
total sleep time
REFERENCES
- 1. Dauvilliers Y , Lopez R , Ohayon M , Bayard S . Hypersomnia and depressive symptoms: methodological and clinical aspects . BMC Med. 2013. ; 11 ( 1 ): 78 . [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Lopez R , Barateau L , Evangelista E , Dauvilliers Y . Depression and hypersomnia: a complex association . Sleep Med Clin. 2017. ; 12 ( 3 ): 395 – 405 . [DOI] [PubMed] [Google Scholar]
- 3. Fernandez-Mendoza J , Vgontzas AN , Kritikou I , Calhoun SL , Liao D , Bixler EO . Natural history of excessive daytime sleepiness: role of obesity, weight loss, depression, and sleep propensity . Sleep. 2015. ; 38 ( 3 ): 351 – 360 . [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Barateau L , Lopez R , Franchi JA , Dauvilliers Y . Hypersomnolence, hypersomnia, and mood disorders . Curr Psychiatry Rep. 2017. ; 19 ( 2 ): 13 . [DOI] [PubMed] [Google Scholar]
- 5. Berkowski JA , Shelgikar AV . Disorders of excessive daytime sleepiness including narcolepsy and idiopathic hypersomnia . Sleep Med Clin. 2016. ; 11 ( 3 ): 365 – 378 . [DOI] [PubMed] [Google Scholar]
- 6. Trotti LM , Staab BA , Rye DB . Test-retest reliability of the Multiple Sleep Latency Test in narcolepsy without cataplexy and idiopathic hypersomnia . J Clin Sleep Med. 2013. ; 9 ( 8 ): 789 – 795 . [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Plante DT . Hypersomnia in mood disorders: a rapidly changing landscape . Curr Sleep Med Rep. 2015. ; 1 ( 2 ): 122 – 130 . [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. American Academy of Sleep Medicine . International Classification of Sleep Disorders. 3rd ed. Darien, IL: : American Academy of Sleep Medicine; ; 2014. . [Google Scholar]
- 9. Johns MW . A new method for measuring daytime sleepiness: the Epworth Sleepiness Scale . Sleep. 1991. ; 14 ( 6 ): 540 – 545 . [DOI] [PubMed] [Google Scholar]
- 10. Beck A , Steer R , Brown G . Manual for the BDI-II. San Antonio, TX: : Psychological Corporation; ; 1996. . [Google Scholar]
- 11. Preiss M , Kucharová J , Novák T , Stepánková H . The temperament and character inventory-revised (TCI-R): psychometric characteristics of the Czech version . Psychiatr Danub. 2007. ; 19 ( 1-2 ): 27 – 34 . [PubMed] [Google Scholar]
- 12. Sheehan DV , Lecrubier Y , Sheehan KH , et al . The Mini-International Neuropsychiatric Interview (M.I.N.I.): the development and validation of a structured diagnostic psychiatric interview for DSM-IV and ICD-10 . J Clin Psychiatry. 1998. ; 59 ( Suppl 20 ): 22 – 33 . [PubMed] [Google Scholar]
- 13. Berry RB , Brooks R , Gamaldo CE , et al. ; for the American Academy of Sleep Medicine . The AASM Manual for the Scoring of Sleep and Associated Events: Rules, Terminology and Technical Specifications. Version 2.0. Darien, IL: : American Academy of Sleep Medicine; ; 2012. . [Google Scholar]
- 14. Bassetti C , Aldrich MS . Idiopathic hypersomnia. A series of 42 patients . Brain. 1997. ; 120 ( 8 ): 1423 – 1435 . [DOI] [PubMed] [Google Scholar]
- 15. Roth B . Narcolepsy and hypersomnia: review and classification of 642 personally observed cases . Schweiz Arch Neurol Neurochir Psychiatr. 1976. ; 119 ( 1 ): 31 – 41 . [PubMed] [Google Scholar]
- 16. Vernet C , Leu-Semenescu S , Buzare MA , Arnulf I . Subjective symptoms in idiopathic hypersomnia: beyond excessive sleepiness . J Sleep Res. 2010. ; 19 ( 4 ): 525 – 534 . [DOI] [PubMed] [Google Scholar]
- 17. Bruck D , Parkes JD . A comparison of idiopathic hypersomnia and narcolepsy-cataplexy using self report measures and sleep diary data . J Neurol Neurosurg Psychiatry. 1996. ; 60 ( 5 ): 576 – 578 . [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Ramm M , Boentert M , Lojewsky N , Jafarpour A , Young P , Heidbreder A . Disease-specific attention impairment in disorders of chronic excessive daytime sleepiness . Sleep Med. 2019. ; 53 : 133 – 140 . [DOI] [PubMed] [Google Scholar]
- 19. Thomann J , Baumann CR , Landolt HP , Werth E . Psychomotor vigilance task demonstrates impaired vigilance in disorders with excessive daytime sleepiness . J Clin Sleep Med. 2014. ; 10 ( 9 ): 1019 – 1024 . [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Capuron L , Welberg L , Heim C , et al . Cognitive dysfunction relates to subjective report of mental fatigue in patients with chronic fatigue syndrome . Neuropsychopharmacology. 2006. ; 31 ( 8 ): 1777 – 1784 . [DOI] [PubMed] [Google Scholar]
- 21. Plante DT , Finn LA , Hagen EW , Mignot E , Peppard PE . Subjective and objective measures of hypersomnolence demonstrate divergent associations with depression among participants in the Wisconsin Sleep Cohort Study . J Clin Sleep Med. 2016. ; 12 ( 4 ): 571 – 578 . [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Tanaka H , Sasazawa Y , Suzuki S , Nakazawa M , Koyama H . Health status and lifestyle factors as predictors of depression in middle-aged and elderly Japanese adults: a seven-year follow-up of the Komo-Ise cohort study . BMC Psychiatry. 2011. ; 11 ( 1 ): 20 . [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. van Mill JG , Hoogendijk WJ , Vogelzangs N , van Dyck R , Penninx BW . Insomnia and sleep duration in a large cohort of patients with major depressive disorder and anxiety disorders . J Clin Psychiatry. 2010. ; 71 ( 3 ): 239 – 246 . [DOI] [PubMed] [Google Scholar]
- 24. Krueger PM , Friedman EM . Sleep duration in the United States: a cross-sectional population-based study . Am J Epidemiol. 2009. ; 169 ( 9 ): 1052 – 1063 . [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Trotti LM , Ong JC , Plante DT , Friederich Murray C , King R , Bliwise DL . Disease symptomatology and response to treatment in people with idiopathic hypersomnia: initial data from the Hypersomnia Foundation registry . Sleep Med. 2020. ; 75 : 343 – 349 . [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Kaplan KA , Harvey AG . Hypersomnia across mood disorders: a review and synthesis . Sleep Med Rev. 2009. ; 13 ( 4 ): 275 – 285 . [DOI] [PubMed] [Google Scholar]
- 27. Matza LS , Revicki DA , Davidson JR , Stewart JW . Depression with atypical features in the National Comorbidity Survey: classification, description, and consequences . Arch Gen Psychiatry. 2003. ; 60 ( 8 ): 817 – 826 . [DOI] [PubMed] [Google Scholar]
- 28. Cloninger CR , Bayon C , Svrakic DM . Measurement of temperament and character in mood disorders: a model of fundamental states as personality types . J Affect Disord. 1998. ; 51 ( 1 ): 21 – 32 . [DOI] [PubMed] [Google Scholar]
- 29. Billiard M , Dolenc L , Aldaz C , Ondze B , Besset A . Hypersomnia associated with mood disorders: a new perspective . J Psychosom Res. 1994. ; 38 ( Suppl 1 ): 41 – 47 . [DOI] [PubMed] [Google Scholar]
- 30. van den Hoed J , Kraemer H , Guilleminault C , et al . Disorders of excessive daytime somnolence: polygraphic and clinical data for 100 patients . Sleep. 1981. ; 4 ( 1 ): 23 – 37 . [DOI] [PubMed] [Google Scholar]
- 31. Plante DT . Sleep propensity in psychiatric hypersomnolence: a systematic review and meta-analysis of Multiple Sleep Latency Test findings . Sleep Med Rev. 2017. ; 31 : 48 – 57 . [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Billiard M , Dauvilliers Y . Idiopathic hypersomnia . Sleep Med Rev. 2001. ; 5 ( 5 ): 349 – 358 . [DOI] [PubMed] [Google Scholar]
- 33. Vernet C , Arnulf I . Idiopathic hypersomnia with and without long sleep time: a controlled series of 75 patients . Sleep. 2009. ; 32 ( 6 ): 753 – 759 . [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. Anderson KN , Pilsworth S , Sharples LD , Smith IE , Shneerson JM . Idiopathic hypersomnia: a study of 77 cases . Sleep. 2007. ; 30 ( 10 ): 1274 – 1281 . [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35. Lopez R , Doukkali A , Barateau L , et al . Test-retest reliability of the Multiple Sleep Latency Test in central disorders of hypersomnolence . Sleep. 2017. ; 40 ( 12 ): zsx164 . [DOI] [PubMed] [Google Scholar]
- 36. Ruoff C , Pizza F , Trotti LM , et al . The MSLT is repeatable in narcolepsy type 1 but not narcolepsy type 2: a retrospective patient study . J Clin Sleep Med. 2018. ; 14 ( 1 ): 65 – 74 . [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37. Plante DT , Cook JD , Goldstein MR . Objective measures of sleep duration and continuity in major depressive disorder with comorbid hypersomnolence: a primary investigation with contiguous systematic review and meta-analysis . J Sleep Res. 2017. ; 26 ( 3 ): 255 – 265 . [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38. Hawkins DR , Taub JM , Van de Castle RL . Extended sleep (hypersomnia) in young depressed patients . Am J Psychiatry. 1985. ; 142 ( 8 ): 905 – 910 . [DOI] [PubMed] [Google Scholar]
- 39. American Psychiatric Association . Diagnostic and Statistical Manual of Mental Disorders. 5th ed. Washington, DC: : American Psychiatric Association; ; 2013. . [Google Scholar]
- 40. Kofmel NC , Schmitt WJ , Hess CW , Gugger M , Mathis J . Sleepiness and performance is disproportionate in patients with non-organic hypersomnia in comparison to patients with narcolepsy and mild to moderate obstructive sleep apnoea . Neuropsychobiology. 2014. ; 70 ( 3 ): 189 – 194 . [DOI] [PubMed] [Google Scholar]
- 41. Nofzinger EA , Thase ME , Reynolds CF III , et al . Hypersomnia in bipolar depression: a comparison with narcolepsy using the Multiple Sleep Latency Test . Am J Psychiatry. 1991. ; 148 ( 9 ): 1177 – 1181 . [DOI] [PubMed] [Google Scholar]
- 42. Baker TL , Guilleminault C , Nino-Murcia G , Dement WC . Comparative polysomnographic study of narcolepsy and idiopathic central nervous system hypersomnia . Sleep. 1986. ; 9 ( 1 ): 232 – 242 . [DOI] [PubMed] [Google Scholar]
- 43. Plante DT . Nocturnal sleep architecture in idiopathic hypersomnia: a systematic review and meta-analysis . Sleep Med. 2018. ; 45 : 17 – 24 . [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44. Vgontzas AN , Bixler EO , Kales A , Criley C , Vela-Bueno A . Differences in nocturnal and daytime sleep between primary and psychiatric hypersomnia: diagnostic and treatment implications . Psychosom Med. 2000. ; 62 ( 2 ): 220 – 226 . [DOI] [PubMed] [Google Scholar]