Abstract
Study Objectives:
There is a paucity of data on the association between day-to-day variation in sleep pattern and all-cause mortality. We aimed to investigate whether day-to-day variation in sleep duration and onset of sleep are associated with cardiovascular and all-cause mortality.
Methods:
We used data belonging to 388 unique patients from the Midlife in the United States 2 Biomarker study (2004–2009). Information on sleep onset, duration, and sleep-wake cycles was collected for 7 consecutive days using the Actiwatch device. Sleep irregularity was assessed using mean and standard deviations in sleep duration and time of onset of sleep over 7 days. Cox proportional regression analysis and the Fine and Gray subdistribution method were used with all-cause and cardiovascular mortality, respectively.
Results:
Over a median of 8.6 years of follow-up, 37 patients died, including 10 deaths resulting from cardiovascular causes. There was no statistically significant increase in cardiovascular mortality with variation in sleep duration in the highest vs the lowest tertile (hazard ratio, 4.00; 0.45–35.48; P = .21). However, increased all-cause mortality was seen in the highest vs the lowest tertile (hazard ratio, 3.99; 1.33–11.94; P = .01). Multivariable model adjusting for confounders had higher all-cause mortality with increased sleep duration variation in the highest vs the lowest tertile: hazard ratio, 4.85; 1.52–15.49; P < .01).
Conclusions:
Day-to-day variation in sleep duration is associated with increased all-cause mortality but not cardiovascular mortality after adjusting for mean sleep duration, inflammation, diabetes, age, body mass index, renal function, and blood pressure. Irregularity in the onset of sleep is not associated with all-cause mortality or cardiovascular mortality.
Citation:
Katamreddy A, Uppal D, Ramani G, et al. Day-to-day variation in sleep duration is associated with increased all-cause mortality. J Clin Sleep Med. 2022;18(3):921–926.
Keywords: sleep irregularity, all-cause mortality, cardiovascular disease mortality
BRIEF SUMMARY
Current Knowledge/Study Rationale: There are few studies using objective sleep data to determine whether day-to-day variation in sleep is associated with increased cardiovascular mortality or all-cause mortality.
Study Impact: In a metavariable model, day-to-day variation in sleep duration is associated with increased all-cause mortality but not cardiovascular mortality.
INTRODUCTION
Sleep is essential for health and well-being.1 Sleep plays an important role in the regulation of a number of physiologic responses including appetite, endothelial function, neurohumoral balance, vascular tone, blood pressure, and glucose control.2–5 The importance of sleep duration is widely recognized in various epidemiological studies.6 The American College of Cardiology and the American Heart Association have endorsed the importance of sleep in their preventive guidelines.7 Both more sleep and less sleep have been associated with increased cardiometabolic risk in multiple observational studies.6 In addition, there is an increasing body of evidence showing that certain disruptions in circadian rhythm are associated with an increased risk of atherosclerosis and coronary artery disease.8 Further, circadian rhythm disruption is connected with higher blood pressure, glucose intolerance, dyslipidemia, and obesity; all of these risk factors are associated with increased cardiovascular risk.9 Variation in day-to-day sleep may represent this chronic sleep disruption and has been shown to be prevalent in the general population. A number of factors in modern society negatively influence the quality and quantity of sleep; these factors include the use of artificial light, the use of cell phones and other portable media devices especially immediately before falling asleep, and increased work hours and shift work.10 Day-to-day variation in sleep is not well studied and has been recognized as a potential risk factor only recently.8 Questionnaire-based studies on sleep are prone to recall bias. There are few studies using objective sleep data. We aimed to determine whether objective actigraphy-measured day-to-day variation in sleep was associated with increased cardiovascular mortality or all-cause mortality independently with a long duration of follow-up.
METHODS
Study design, participants, and data collection
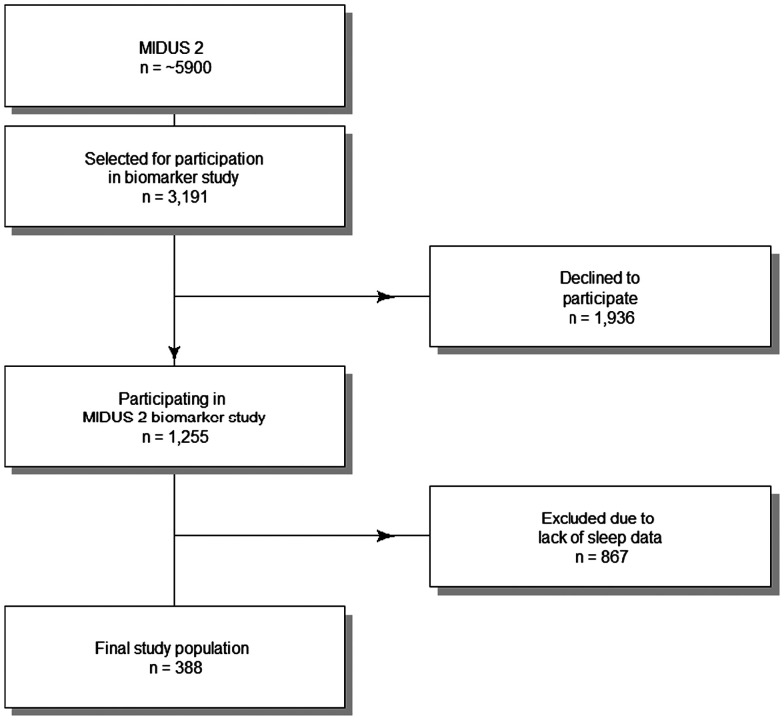
The Midlife in the United States 2 (MIDUS 2) biomarker study is a subset of MIDUS, a national longitudinal study of health and well-being. MIDUS started in 1994 with a national survey of > 7,000 Americans (aged 25 to 74 years). The participants from the original MIDUS study were then followed up in 2004–2006. An African American sample of participants was recruited in addition to the follow-up participants. This new cohort of nearly 5,900 participants was called MIDUS 2 sample. Of these patients, 388 had sleep data and were used in our study (2004–2009; Figure 1). More details about the study design, follow-up, and sample selection are available at www.midus.wisc.edu/ and https://www.icpsr.umich.edu/web/NACDA/studies/29282/versions/V9. Data were available as deidentified datasets for secondary analysis.11
Figure 1. Flowchart of patients included in the current study as a subset of the MIDUS 2 cohort.
MIDUS 2 = Midlife in the United States 2.
Sleep and other variables
The objective sleep data were measured using the Mini Mitter Actiwatch-64 activity monitor device (Mini Mitter/Respironics/Philips, Bend, OR). Information on sleep onset, duration, and sleep-wake cycles was collected for 7 consecutive days. Means and standard deviations (SDs) in sleep duration and time of onset of sleep over the 7 days were calculated to assess for sleep irregularity. Mortality data were available with a follow-up until December 2016. International Classification of Diseases, Tenth Revision codes (I10–I99) were used to identify all cardiovascular mortality including that occurring in the community.
Other variables included in the analysis included age, sex, diabetes, body mass index, mean sleep duration, high-density lipoprotein cholesterol and non-high-density lipoprotein cholesterol, systolic blood pressure, diastolic blood pressure, glomerular filtration rate, and C-reactive protein.
Statistical analysis
Sleep duration and sleep onset duration data available in minutes in the dataset were divided into tertiles because this division would be more clinically meaningful. Continuous variables were described as mean ± SD or median (interquartile range) and compared using the overall analysis of variance test between the various tertile groups. Categorical variables were compared using the chi-square test. Cox proportional hazard regression analysis was performed with all-cause mortality as the outcome. A Cox competing risk analysis for cardiovascular mortality was done using the Fine and Gray subdistribution hazard.12 Schoenfeld residuals were used to assess whether the Cox proportional hazard assumptions were fulfilled. Kaplan-Meier survival curves were plotted with all-cause mortality as the outcome. All statistical analysis was performed using R version 4.0.3 (R Foundation for Statistical Computing, Vienna, Austria).
RESULTS
Baseline characteristics
Our analysis included a total of 388 participants. The mean age of the cohort was 54.56 ± 11.79 years; there were 230 women (59.3%), and the mean BMI was 30.56 ± 7.06 kg/m2. Over a median of 8.6 years of follow-up, 37 patients died, including 10 deaths because of cardiovascular mortality. Eighty-five (21.9%) patients had diabetes. Further, the study cohort was divided into tertiles based on the SD of the actigraphy-measured sleep duration for 7 consecutive days. Sleep duration SD tertile ranges were 11–41 minutes, 41–68 minutes, and 68–258 minutes in the lowest to the highest tertile, respectively. A higher tertile indicated more sleep variation. Participants in the higher tertiles were younger, with a mean age of 52.51 ± 11.59 years compared to those in the lowest tertile, whose mean age was 56.50 ± 11.02 years. Notably, the group of participants with higher sleep duration variability had a higher prevalence of diabetes (27.9%) compared to the lowest-variation group (14.7%; P = .03). The mean sleep duration was lower (353 ± 69 minutes) in the highest-tertile group compared to the lowest-tertile group (383 ± 57 minutes; P < .001). SDs for sleep onset were 5–57 minutes, 57–103 minutes, and 104–1,107 minutes in the lowest to the highest tertile, respectively. A higher tertile indicated more variation in sleep onset. Participants in the higher tertiles were younger, with a mean age of 52.16 ± 10.88 years compared to those in the lowest tertile, whose mean age was 57.26 ± 12.10 years. Notably, the group of participants with higher variability in sleep onset had a higher prevalence of diabetes (28.7%) compared to the lowest-variation group (15.5%; P = .04). The mean sleep duration was lower (339 ± 70 minutes) in the highest-tertile group compared to the lowest-tertile group (398 ± 51 minutes; P < .001; Table 1).
Table 1.
Baseline characteristics.
| Study Population (n = 388) | Sleep Duration SD (Tertiles)* | Sleep Timing SD (Tertiles)† | |||||||
|---|---|---|---|---|---|---|---|---|---|
| Lowest (n = 129) | Intermediate (n = 130) | Highest (n = 129) | P Value | Lowest (n = 129) | Intermediate (n = 130) | Highest (n = 129) | P Value | ||
| Age (y), mean (SD) | 54.56 (11.79) | 56.50 (11.02) | 54.68 (12.46) | 52.51 (11.59) | .024 | 57.26 (12.10) | 54.28 (11.87) | 52.16 (10.88) | .002 |
| Female, n (%) | 230 (59.3) | 78 (60.5) | 79 (60.8) | 73 (56.6) | .748 | 76 (58.9) | 84 (64.6) | 70 (54.3) | .236 |
| Diabetes, n (%) | 85 (21.9) | 19 (14.7) | 30 (23.1) | 36 (27.9) | .035 | 20 (15.5) | 28 (21.5) | 37 (28.7) | .038 |
| Mean sleep duration (min), mean (SD) | 371.35 (64.74) | 383.56 (57.43) | 376.76 (62.87) | 353.68 (69.97) | < .001 | 398.67 (51.38) | 376.19 (57.04) | 339.15 (70.23) | < .001 |
| HDL cholesterol (mg/dL), median [IQR] | 52.00 [42.00–64.00] | 54.00 [44.00–65.00] | 49.50 [40.00–61.75] | 51.00 [42.00–65.00] | .094 | 54.00 [45.00–64.00] | 52.00 [40.00–64.75] | 48.00 [42.00–61.00] | .349 |
| Non-HDL cholesterol (mg/dL), mean (SD) | 129.25 (39.84) | 131.19 (37.18) | 124.96 (38.25) | 131.63 (43.75) | .322 | 124.64 (33.55) | 127.67 (36.20) | 135.45 (47.86) | .08 |
| Body mass index (kg/m2), median [IQR] | 29.52 [25.60–33.83] | 28.91 [25.12–33.11] | 29.48 [25.86–33.36] | 30.77 [26.11–35.64] | .088 | 28.45 [25.26–31.88] | 29.80 [25.63–34.55] | 30.86 [26.35–36.09] | .009 |
| Systolic blood pressure (mm Hg), mean (SD) | 131.79 (17.04) | 133.31 (16.62) | 129.92 (18.40) | 132.15 (15.95) | .267 | 131.66 (16.99) | 132.00 (16.73) | 131.71 (17.52) | .985 |
| Diastolic blood pressure (mm Hg), mean (SD) | 76.97 (10.56) | 76.39 (10.25) | 76.46 (11.18) | 78.05 (10.19) | .359 | 75.98 (10.19) | 76.19 (9.92) | 78.73 (11.37) | .066 |
| Glomerular filtration rate (mL/min), mean (SD) | 118.71 (39.23) | 117.65 (33.23) | 116.05 (38.73) | 122.46 (44.88) | .393 | 112.81 (36.46) | 114.69 (36.12) | 128.66 (43.10) | .002 |
| C-reactive protein (µg/mL), median [IQR] | 1.62 [0.76–3.91] | 1.31 [0.65–2.92] | 1.85 [0.79–4.27] | 1.96 [0.89–4.60] | .058 | 1.22 [0.60–3.04] | 1.82 [0.75–3.91] | 2.19 [0.96–4.60] | .002 |
*Sleep duration SD (tertiles) in minutes: lowest tertile = 11–41 minutes; intermediate tertile = 41–68 minutes; highest tertile = 68–258 minutes. †Sleep timing SD (tertiles) in minutes: lowest tertile = 5–57 minutes; intermediate tertile = 57–103 minutes; highest tertile = 104–1,107 minutes. HDL = high-density lipoprotein, IQR = interquartile range, SD = standard deviation.
Sleep duration SD and mortality
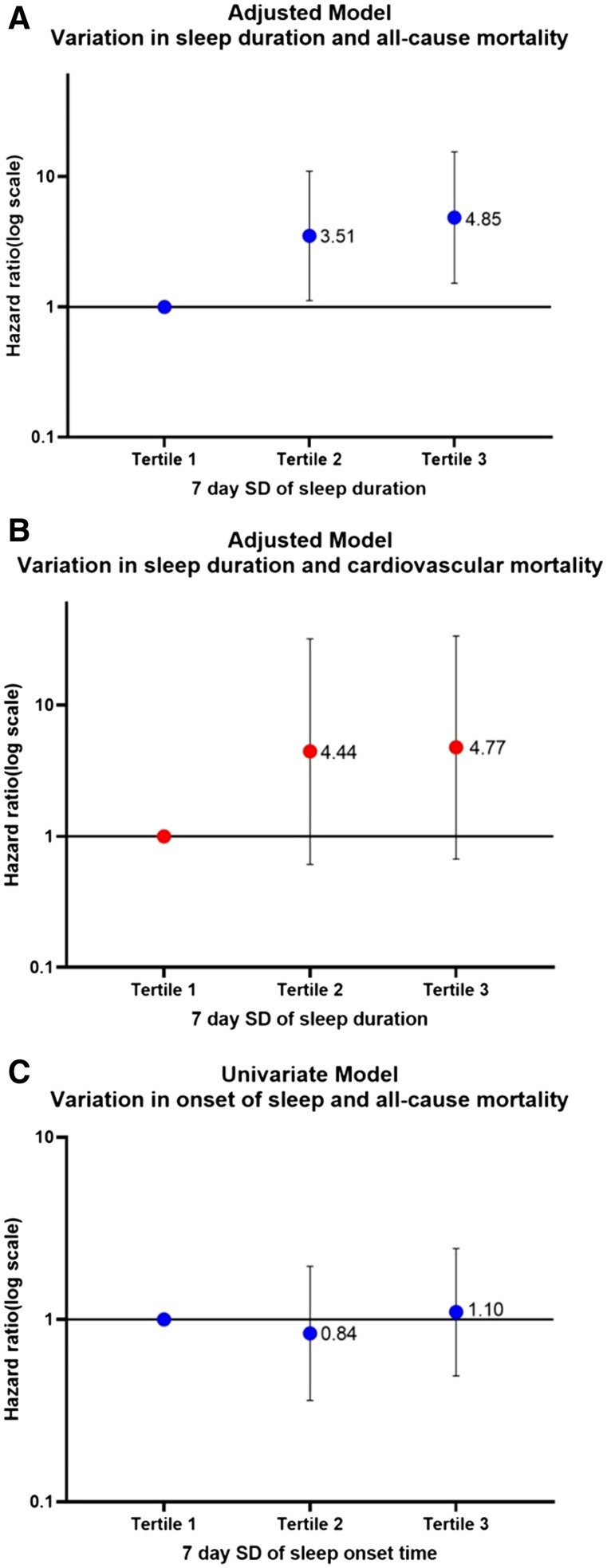
The SD of 7-day sleep duration in the highest vs the lowest tertile was associated with increased all-cause mortality (hazard ratio, 3.99; 1.33–11.94; P < .01) on univariate analysis. After adjusting for age, sex, body mass index, diabetes, mean sleep duration, high-density lipoprotein and non-high-density lipoprotein cholesterol, systolic and diastolic blood pressures, renal function, and C-reactive protein, we found that day-to-day variation in sleep duration was independently associated with increased all-cause mortality (adjusted hazard ratio, 4.85; 1.52–15.49; P < .01). Sleep variation was not associated with cardiovascular mortality in the highest vs the lowest tertile (hazard ratio, 4.00; 0.45–35.48; P = .21; Table 2 and Figure 2).
Table 2.
Cox proportion regression for all-cause mortality and Fine and Gray subhazard regression for cardiovascular mortality with variation in sleep duration and onset.
| All-Cause Mortality | Cardiovascular Mortality | |||
|---|---|---|---|---|
| HR (95% CI) | P Value | HR (95% CI) | P Value | |
| Unadjusted models | ||||
| SD (min) of sleep duration* over 7 days | ||||
| Lowest | Ref | Ref | ||
| Intermediate | 3.63 (1.19–10.99) | .02 | 5.11 (0.59–43.57) | .14 |
| Highest | 3.99 (1.33–11.94) | .01 | 4.00 (0.45–35.48) | .21 |
| SD (min) of time of sleep onset† over 7 days | ||||
| Lowest | Ref | Ref | ||
| Intermediate | 0.84 (0.36–1.96) | .69 | 1.32 (0.29–5.86) | .72 |
| Highest | 1.1 (0.49–2.45) | .81 | 0.98 (0.19–4.90) | .98 |
| Fully adjusted models | ||||
| SD (min) of sleep duration over 7 days | ||||
| Lowest | Ref | Ref | ||
| Intermediate | 3.51 (1.12–10.99) | .03 | 4.44 (0.61–31.98) | .14 |
| Highest | 4.85 (1.52–15.49) | < .01 | 4.77 (0.67–33.70) | .12 |
| Age (y) | 1.11 (1.07–1.17) | < .01 | 1.12 (1.06–1.18) | < .01 |
| Male | 1.53 (0.59–3.95) | .38 | 3.91 (0.56–27.27) | .17 |
| No diabetes | 1.07 (0.49–2.36) | .86 | 2.07 (0.39–10.83) | .39 |
| Mean sleep duration (min) | 0.99 (0.99–1.00) | .18 | 1.00 (0.99–1.01) | .94 |
| Body mass index (kg/m2) | 0.99 (0.92–1.06) | .72 | 1.01 (0.86–1.19) | .89 |
| Non-HDL cholesterol (mg/dL) | 0.99 (0.99–1.01) | .88 | 0.98 (0.95–1.01) | .23 |
| HDL cholesterol (mg/dL) | 0.98 (0.95–1.01) | .19 | 0.99 (0.94–1.05) | .84 |
| Systolic blood pressure (mm Hg) | 1.01 (0.98–1.03) | .64 | 0.99 (0.93–1.06) | .84 |
| Diastolic blood pressure (mm Hg) | 0.99 (0.94–1.04) | .71 | 0.99 (0.87–1.12) | .89 |
| Glomerular filtration rate (mL/min) | 1.00 (0.99–1.01) | .78 | 0.99 (0.96–1.02) | .61 |
| C-reactive protein (µg/mL) | 1.02 (0.98–1.06) | .25 | 1.01 (0.93–1.12) | .69 |
*Sleep duration SD (tertiles) in minutes: lowest tertile = 11–41 minutes; intermediate tertile = 41–68 minutes; highest tertile = 68–258 minutes. †Sleep timing SD (tertiles) in minutes: lowest tertile = 5–57 minutes; intermediate tertile = 57–103 minutes; highest tertile = 104–1,107 minutes. CI = confidence interval; HDL = high-density lipoprotein, HR = hazard ratio, Ref = reference, SD = standard deviation.
Figure 2. Modified forest plots.
(A) Adjusted model for variation in sleep duration and all-cause mortality. (B) Adjusted model for variation in sleep duration and cardiovascular mortality. (C) Univariate model for variation in onset of sleep and all-cause mortality. SD = standard deviation.
Sleep timing SD and mortality
The SD of 7-day sleep timing in the highest vs the lowest tertile was not associated with either cardiovascular mortality (hazard ratio, 0.98; 0.19–4.90; P =.98) or all-cause mortality (hazard ratio, 4.00; 0.45–35.48; P = .21; Table 2 and Figure 2).
DISCUSSION
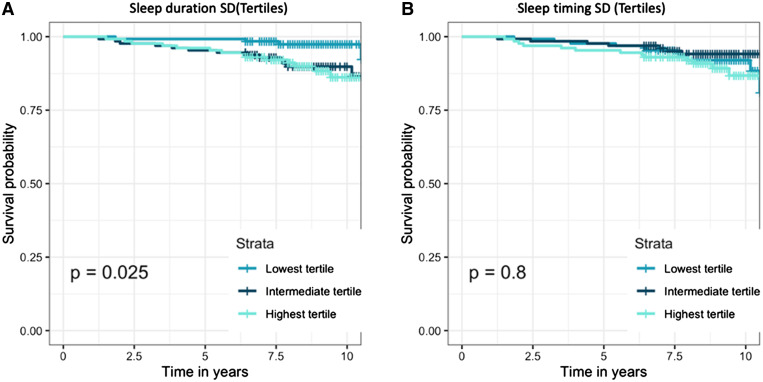
This is the first prospective analysis evaluating the association of sleep irregularity with all-cause mortality and cardiovascular mortality. We compared participants with regular sleep and irregular sleep from the community. Irregularity of sleep was assessed in terms of irregularity in the duration of sleep and irregularity in the timing of sleep. Our study results showed that irregular sleep is associated with a greater than 3-fold increase in all-cause mortality over a median follow-up of 8.6 years (Figure 3). In addition, after adjusting for various traditional cardiovascular risk factors, we found that there was increased all-cause mortality risk. Although there was a trend toward increased cardiovascular mortality with irregular sleep duration, there was no statistical significance. Irregularity in sleep timing was not associated with either increased cardiovascular mortality or all-cause mortality.
Figure 3. Kaplan-Meier plots for all-cause mortality with log-rank test P value inset.
(A) Grouped by sleep duration SD tertiles. (B) Grouped by sleep timing SD tertiles. SD = standard deviation.
Multiple observational studies have shown that poor sleep hygiene is associated with increased cardiometabolic risk.13–15 Disruptions in the circadian rhythm have been shown to be associated with glucose intolerance, risk of diabetes, hyperlipidemia, hypertension, and cardiovascular disease.16 All these risk factors are associated with increased mortality in multiple epidemiologic studies.17 Moreover, disruptions in the circadian rhythm observed in shift workers, pilots, and even those with social jet leg, defined as sleeping less on weekdays and catching up on weekends, suggest an increased cardiometabolic risk.18–20 Patients with irregular sleep patterns have significant metabolic derangement at baseline. Even after adjusting for these derangements, irregularity in sleep duration is associated with increased all-cause mortality. At a behavioral level, poor sleep hygiene is associated with irregular breakfast consumption and a change in the number of meals, which have been shown to be associated with a risk of obesity, diabetes, and increased mortality.21 In addition, changes in the neurohumoral balance and the transcription of various genes including the CLOCK gene are potential reasons for the increase in mortality seen with irregular sleep duration in our study.22
A recent study by Huang et al8 showed that sleep irregularity in both duration and timing is associated with increased cardiovascular disease risk. Our study endpoint is different, and we only evaluated for total mortality and mortality resulting from cardiovascular disease. In our study, there was a trend toward increased cardiovascular mortality; however, it was not statistically significant. Because of the wide confidence interval, it is likely that our findings resulted from the low sample size. This development is one of the major limitations of our study.
Irregularity in sleep timing was not associated with either increased cardiovascular mortality or all-cause mortality in our cohort. This is an interesting finding and differs from the observations in the study by Huang et al8 that showed increased cardiovascular disease risk with sleep irregularity. Even though our study endpoints are different, this discrepancy in findings needs to be evaluated further.
There are a number of limitations that need to be noted. First, the sample size was small to modest, and the study should be viewed as hypothesis-generating. Pooled cohort studies need to be performed to further evaluate the importance of sleep irregularity. Second, other sleep disorders such as sleep-disordered breathing were not included because of the low sample size. Third, although we used 7-day actigraphy recordings to measure day-to-day variation in sleep, they may not represent chronic circadian rhythm irregularity. Furthermore, morning-ness/evening-ness data were not available. Finally, other ways to assess sleep irregularity, such as the sleep irregularity index23 and social jet lag,24 were not evaluated in the current study.
CONCLUSIONS
Day-to-day variation in sleep duration is associated with increased all-cause mortality but not cardiovascular mortality after adjusting for mean sleep duration, inflammation, diabetes, age, body mass index, renal function, and blood pressure. Irregularity in the onset of sleep is not associated with all-cause mortality or cardiovascular mortality. There is increasing body of evidence regarding the importance of sleep duration and regularity in health and disease. Therefore, this topic should become a part of preventive disease discussions in primary care clinics and preventive cardiology clinics.
DISCLOSURE STATEMENT
The authors report no conflicts of interest. All authors have seen and approved this manuscript.
ACKNOWLEDGMENTS
The authors acknowledge the Inter-University Consortium for Political and Social Research and the MIDUS investigators and institutions for collecting the data and making them available for analysis.
ABBREVIATIONS
- MIDUS
Midlife in the United States
- SD
standard deviation
REFERENCES
- 1. Mukherjee S , Patel SR , Kales SN , et al. ; American Thoracic Society Ad Hoc Committee on Healthy Sleep . An official American Thoracic Society statement: the importance of healthy sleep. Recommendations and future priorities . Am J Respir Crit Care Med. 2015. ; 191 ( 12 ): 1450 – 1458 . [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Lin J , Jiang Y , Wang G , et al . Associations of short sleep duration with appetite-regulating hormones and adipokines: a systematic review and meta-analysis . Obes Rev. 2020. ; 21 ( 11 ): e13051 . [DOI] [PubMed] [Google Scholar]
- 3. Taheri S , Lin L , Austin D , Young T , Mignot E . Short sleep duration is associated with reduced leptin, elevated ghrelin, and increased body mass index . PLoS Med. 2004. ; 1 ( 3 ): e62 . [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Van Laake LW , Lüscher TF , Young ME . The circadian clock in cardiovascular regulation and disease: lessons from the Nobel Prize in Physiology or Medicine 2017 . Eur Heart J. 2018. ; 39 ( 24 ): 2326 – 2329 . [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Degaute JP , van de Borne P , Linkowski P , Van Cauter E . Quantitative analysis of the 24-hour blood pressure and heart rate patterns in young men . Hypertension. 1991. ; 18 ( 2 ): 199 – 210 . [DOI] [PubMed] [Google Scholar]
- 6. Consensus Conference Panel ; Watson NF , Badr MS , Belenky G , et al. Joint consensus statement of the American Academy of Sleep Medicine and Sleep Research Society on the recommended amount of sleep for a healthy adult: methodology and discussion . Sleep. 2015. ; 38 ( 8 ): 1161 – 1183 . [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Arnett DK , Blumenthal RS , Albert MA , et al . 2019 ACC/AHA guideline on the primary prevention of cardiovascular disease: a report of the American College of Cardiology/American Heart Association Task Force on Clinical Practice Guidelines . Circulation. 2019. ; 140 ( 11 ): e596 – e646 . [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Huang T , Mariani S , Redline S . Sleep irregularity and risk of cardiovascular events: the Multi-Ethnic Study of Atherosclerosis . J Am Coll Cardiol. 2020. ; 75 ( 9 ): 991 – 999 . [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Gangwisch JE . Epidemiological evidence for the links between sleep, circadian rhythms and metabolism . Obes Rev. 2009. ; 10 ( Suppl 2 ): 37 – 45 . [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Oldenburg O , Spiesshoefer J . Impact of lifestyle on sleep: can we alter cardiovascular risk? J Am Coll Cardiol. 2020. ; 75 ( 9 ): 1000 – 1002 . [DOI] [PubMed] [Google Scholar]
- 11. Ryff CD , Seeman T , Weinstein M . Midlife in the United States (MIDUS 2): Biomarker Project, 2004-2009 (ICPSR 29282). https://www.icpsr.umich.edu/web/NACDA/studies/29282 . Accessed September 22, 2021.
- 12. Austin PC , Lee DS , Fine JP . Introduction to the analysis of survival data in the presence of competing risks . Circulation. 2016. ; 133 ( 6 ): 601 – 609 . [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Appelhans BM , Janssen I , Cursio JF , et al . Sleep duration and weight change in midlife women: the SWAN sleep study . Obesity (Silver Spring). 2013. ; 21 ( 1 ): 77 – 84 . [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Beccuti G , Pannain S . Sleep and obesity . Curr Opin Clin Nutr Metab Care. 2011. ; 14 ( 4 ): 402 – 412 . [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Beihl DA , Liese AD , Haffner SM . Sleep duration as a risk factor for incident type 2 diabetes in a multiethnic cohort . Ann Epidemiol. 2009. ; 19 ( 5 ): 351 – 357 . [DOI] [PubMed] [Google Scholar]
- 16. Voigt RM , Forsyth CB , Keshavarzian A . Circadian disruption: potential implications in inflammatory and metabolic diseases associated with alcohol . Alcohol Res. 2013. ; 35 ( 1 ): 87 – 96 . [PMC free article] [PubMed] [Google Scholar]
- 17. Rönnback M , Isomaa B , Fagerudd J , et al. ; Botnia Study Group . Complex relationship between blood pressure and mortality in type 2 diabetic patients: a follow-up of the Botnia Study . Hypertension. 2006. ; 47 ( 2 ): 168 – 173 . [DOI] [PubMed] [Google Scholar]
- 18. Wong PM , Hasler BP , Kamarck TW , Muldoon MF , Manuck SB . Social jetlag, chronotype, and cardiometabolic risk . J Clin Endocrinol Metab. 2015. ; 100 ( 12 ): 4612 – 4620 . [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Vyas MV , Garg AX , Iansavichus AV , et al . Shift work and vascular events: systematic review and meta-analysis . BMJ. 2012. ; 345 : e4800 . [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Kim JN , Lee BM . Risk factors, health risks, and risk management for aircraft personnel and frequent flyers . J Toxicol Environ Health B Crit Rev. 2007. ; 10 ( 3 ): 223 – 234 . [DOI] [PubMed] [Google Scholar]
- 21. Nakajima K . Unhealthy eating habits around sleep and sleep duration: to eat or fast? World J Diabetes. 2018. ; 9 ( 11 ): 190 – 194 . [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Trott AJ , Menet JS . Regulation of circadian clock transcriptional output by CLOCK:BMAL1 . PLoS Genet. 2018. ; 14 ( 1 ): e1007156 . [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Phillips AJK , Clerx WM , O’Brien CS , et al . Irregular sleep/wake patterns are associated with poorer academic performance and delayed circadian and sleep/wake timing . Sci Rep. 2017. ; 7 ( 1 ): 3216 . [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Goto Y , Fujiwara K , Sumi Y , Matsuo M , Kano M , Kadotani H . Work habit-related sleep debt: insights from factor identification analysis of actigraphy data . Front Public Health. 2021. ; 9 : 630640 . [DOI] [PMC free article] [PubMed] [Google Scholar]