Abstract
Background
Focal segmental glomerulosclerosis (FSGS) can be separated into primary, genetic or secondary causes. Primary disease results in nephrotic syndrome while genetic and secondary forms may be associated with asymptomatic proteinuria or with nephrotic syndrome. Overall only about 20% of patients with FSGS experience a partial or complete remission of nephrotic syndrome with treatment. FSGS progresses to kidney failure in about half of the cases. This is an update of a review first published in 2008.
Objectives
To assess the benefits and harms of immunosuppressive and non‐immunosuppressive treatment regimens in adults with FSGS.
Search methods
We searched the Cochrane Kidney and Transplant Register of Studies to 21 June 2021 through contact with the Information Specialist using search terms relevant to this review. Studies in the Register are identified through searches of CENTRAL, MEDLINE, and EMBASE, conference proceedings, the International Clinical Trials Register (ICTRP) Search Portal and ClinicalTrials.gov.
Selection criteria
Randomised controlled trials (RCTs) and quasi‐RCTs of any intervention for FSGS in adults were included. Studies comparing different types, routes, frequencies, and duration of immunosuppressive agents and non‐immunosuppressive agents were assessed.
Data collection and analysis
At least two authors independently assessed study quality and extracted data. Statistical analyses were performed using the random‐effects model and results were expressed as a risk ratio (RR) for dichotomous outcomes, or mean difference (MD) for continuous data with 95% confidence intervals (CI). Confidence in the evidence was assessed using the Grading of Recommendations Assessment, Development and Evaluation (GRADE) approach.
Main results
Fifteen studies (560 participants) were included. No studies specifically evaluating corticosteroids compared with placebo or supportive therapy were identified. Studies evaluated participants with steroid‐resistant FSGS. Five studies (240 participants) compared cyclosporin with or without prednisone with different comparators (no specific treatment, prednisone, methylprednisolone, mycophenolate mofetil (MMF), dexamethasone). Three small studies compared monoclonal antibodies (adalimumab, fresolimumab) with other agents or placebo. Six single small studies compared rituximab with tacrolimus, cyclosporin plus valsartan with cyclosporin alone, MMF with prednisone, chlorambucil plus methylprednisolone and prednisone with no specific treatment, different regimens of dexamethasone and CCX140‐B (an antagonist of the chemokine receptor CCR2) with placebo. The final study (109 participants) compared sparsentan, a dual inhibitor of endothelin Type A receptor and of the angiotensin II Type 1 receptor, with irbesartan. In the risk of bias assessment, seven and five studies were at low risk of bias for sequence generation and allocation concealment, respectively. Four studies were at low risk of performance bias and 14 studies were at low risk of detection bias. Thirteen, six and five studies were at low risk of attrition bias, reporting bias and other bias, respectively.
Of five studies evaluating cyclosporin, four could be included in our meta‐analyses (231 participants). Cyclosporin with or without prednisone compared with different comparators may increase the likelihood of complete remission (RR 2.31, 95% CI 1.13 to 4.73; I² = 1%; low certainty evidence) and of complete or partial remission (RR 1.64, 95% CI 1.10 to 2.44; I² = 19%) but not of partial remission (RR 1.36, 95% CI 0.78 to 2.39, I² = 22%). In Individual studies, cyclosporin with prednisone versus prednisone may increase the likelihood of partial (49 participants: RR 7.96, 95% CI 1.09 to 58.15) or complete or partial remission (49 participants: RR 8.85, 95% CI 1.22 to 63.92) but not of complete remission. The remaining individual comparisons may make little or no difference to the likelihood of complete remission, partial remission or complete or partial remission compared with no treatment, methylprednisolone, MMF, or dexamethasone. Individual study data and combined data showed that cyclosporin may make little or no difference to the outcomes of chronic kidney disease or kidney failure. It is uncertain whether cyclosporin compared with these comparators in individual or combined analyses makes any difference to the outcomes of hypertension or infection.
MMF compared with prednisone may make little or no difference to the likelihood of complete remission (33 participants: RR 1.05, 95% CI 0.58 to 1.88; low certainty evidence), partial remission, complete or partial remission, glomerular filtration rate, or infection. It is uncertain whether other interventions make any difference to outcomes as the certainty of the evidence is very low. It is uncertain whether sparsentan reduces proteinuria to a greater extent than irbesartan.
Authors' conclusions
No RCTs, which evaluated corticosteroids, were identified although the KDIGO guidelines recommend corticosteroids as the first treatment for adults with FSGS. The studies identified included participants with steroid‐resistant FSGS. Treatment with cyclosporin for at least six months was more likely to achieve complete remission of proteinuria compared with other treatments but there was considerable imprecision due to few studies and small participant numbers. In future studies of existing or new interventions, the investigators must clearly define the populations included in the study to provide appropriate recommendations for patients with primary, genetic or secondary FSGS.
Plain language summary
Immunosuppressive treatment for focal segmental glomerulosclerosis in adults
What is the issue?
Nephrotic syndrome is a condition where the kidneys leak protein from the blood into the urine. Focal segmental glomerulosclerosis (FSGS) defined on kidney biopsy is an uncommon cause of nephrotic syndrome disease but it progresses to kidney failure in about half of all cases. It can be divided into three groups ‐ primary FSGS (thought to be due to a factor circulating in the blood that damages the kidneys), genetic (secondary to mutations in one or more genes), and secondary to other causes, including certain infections. Treatments aim to reduce the amount of protein in the urine completely or partly to increase the time before kidney failure develops.
What did we do?
We looked at all randomised controlled trials (RCTs) which examined therapy with prednisone or other agents which affect the immune system such as cyclosporin and mycophenolate mofetil and other agents with or without steroid therapy.
What did we find?
We found 15 studies involving 553 participants. In five studies cyclosporin was compared with different treatments. Combining four studies (231 participants) showed that cyclosporin was more effective than these other treatments in achieving complete remission of nephrotic syndrome. The studies were too small and lasted for too short a time to determine if cyclosporin reduced the risk of kidney failure. Nine small studies examined different medicines that suppress the body's immune system. None of these treatments reduced the amount of protein in the urine.
Conclusions
We found limited information that cyclosporin may reduce the amount of protein in the urine in some people with FSGS but the data are uncertain because the studies enrolled too few participants. We need new agents for the treatment of FSGS with nephrotic syndrome to prevent kidney failure.
Summary of findings
Summary of findings 1. Cyclosporin versus different comparators for focal segmental glomerulosclerosis in adults.
| Cyclosporin versus different comparators for focal segmental glomerulosclerosis (FSGS) in adults | |||||
| Patient or population: adults with FSGS Setting: nephrology departments Intervention: cyclosporin with or without prednisone Comparison: different comparators (supportive treatment, prednisone, methylprednisolone, MMF, dexamethasone) | |||||
| Outcomes | Anticipated absolute effects* (95% CI) | Relative effect (95% CI) | No. of participants (studies) | Certainty of the evidence (GRADE) | |
| Risk with different comparators | Risk with Cyclosporin | ||||
| Complete remission of proteinuria Follow‐up: 6 to 12 months |
73 per 1000 | 168 per 1000 (82 to 344) | RR 2.31 (1.13 to 4.73) | 231 (4) | ⊕⊕⊝⊝ LOW 1 |
| Partial remission of proteinuria Follow‐up: 6 to 12 months |
218 per 1000 | 297 per 1000 (170 to 521) | RR 1.36 (0.78 to 2.39) | 231 (4) | ⊕⊕⊝⊝ LOW 1 |
| Complete or partial remission Follow‐up: 6 to 12 months |
291 per 1000 | 477 per 1000 (320 to 710) | RR 1.64 (1.10 to 2.44) | 231 (4) | ⊕⊕⊝⊝ LOW 1 |
| Chronic kidney disease Follow‐up: 6 to 12 months |
209 per 1000 | 174 per 1000 (73 to 410) | RR 0.83 (0.35 to 1.96) | 231 (4) | ⊕⊕⊝⊝ LOW 1 |
| Kidney failure Follow‐up: 6 to 12 months |
145 per 1000 | 80 per 1000 (22 to 291) | RR 0.55 (0.15 to 2.00) | 231 (4) | ⊕⊕⊝⊝ LOW 1 |
| Adverse effects: hypertension Follow‐up: 6 to 12 months |
Data not pooled** | Data not pooled | ‐‐ | 187 (2) | ⊕⊝⊝⊝ VERY LOW 1 2 |
| Adverse effects: infection Follow‐up: 6 to 12 months |
Data not pooled | Dat not pooled | ‐‐ | 157 (2) | ⊕⊕⊝⊝ LOW 1 |
| *The risk in the intervention group (and its 95% CI) is based on the assumed risk in the comparison group and the relative effect of the intervention (and its 95% CI). ** Adverse effects were not pooled due to the inconsistency between the studies CI: Confidence interval; RR: Risk ratio | |||||
| GRADE Working Group grades of evidence High certainty: We are very confident that the true effect lies close to that of the estimate of the effect Moderate certainty: We are moderately confident in the effect estimate: The true effect is likely to be close to the estimate of the effect, but there is a possibility that it is substantially different Low certainty: Our confidence in the effect estimate is limited: The true effect may be substantially different from the estimate of the effect Very low certainty: We have very little confidence in the effect estimate: The true effect is likely to be substantially different from the estimate of effect | |||||
1 Downgraded 2 levels due to serious imprecision due to small number of studies with few participants
2 Donwgraded 1 level due to inconsistency between two studies in this analysis
Background
Description of the condition
Focal segmental glomerulosclerosis (FSGS) is associated with asymptomatic proteinuria or nephrotic syndrome. It progresses to kidney failure in about half of the cases. It is now recognised that the FSGS pattern is associated with several different aetiologies (De Vriese 2018; Shabaka 2020) with the primary target of the damaging agent being the podocyte resulting in podocyte loss. Currently, FSGS is classified into primary (also known as idiopathic), genetic and secondary forms. The primary form is considered to be caused by circulating factors, which damage podocytes leading to increased glomerular permeability so that protein leaks into the urine. Mutations in several different genes result in nephrotic syndrome associated with FSGS. The incidence of FSGS is higher in Africans and African‐Americans associated with a higher incidence of the APOL‐1 genotype (Kopp 2011). The secondary forms of FSGS include maladaptive forms (secondary to glomerular hyperfiltration associated with obesity or nephron loss), virus‐associated or medication‐associated FSGS. There is considerable overlap in the clinical and pathological features of these different forms of FSGS. Typically, primary FSGS is associated clinically with nephrotic syndrome and pathologically with ≥ 80% foot process effacement in glomeruli on electron microscopy while genetic and secondary forms of FSGS are more likely to present with isolated proteinuria and with < 80% foot process effacement (De Vriese 2018; Shabaka 2020).
FSGS is a rare kidney disease with an annual incidence of 0.2 to 1.8 cases/100,000 individuals (Chao 2020) but the disease can appear at any age (Bohle 1986). The initial histological lesion on kidney biopsy is seen in some but not all glomeruli (focal) and involves part of a glomerulus (segmental). It develops first in the juxtamedullary glomeruli and progresses to involve a greater number and portion of the glomerular tufts. Because of sampling difficulties on kidney biopsies, FSGS lesions in a few glomeruli may be missed initially and the condition is mislabelled as minimal change disease (MCD).
Description of the intervention
Corticosteroids are recommended as the first line of treatment in primary FSGS (KDIGO 2012; KDIGO 2021). However, the response of adults to corticosteroids is much lower when compared to children (Meyrier 1999). Although the efficacy of corticosteroids has not been evaluated in randomised controlled trials (RCTs), the initial treatment of FSGS in adults is considered to be prednisone at a dose of 0.5 to 2.0 mg/kg/day for four to six months before declaring the patient to be steroid resistant (KDIGO 2012; KDIGO 2021). Complete remission predicts a good long‐term outcome without relapses or progression to kidney failure. Those patients not receiving any treatment, or failing to respond to treatment, had a high risk of developing chronic kidney disease (CKD) (Burgess 1999; Shabaka 2020).
Corticosteroid resistance or steroid dependency in participants with primary FSGS justify the trial of other therapeutic agents including calcineurin inhibitors (CNI) (Cattran 1999). Steroid‐dependent patients are more likely to experience remission than steroid‐resistant patients. Approximately 40% of patients with primary FSGS have sustained remission of nephrotic syndrome while maintained on CNIs. However, relapses are common when CNIs are ceased. The maximum cumulative rate of complete remission is usually achieved by six months. Mycophenolate mofetil (MMF) has also been investigated in steroid‐resistant FSGS (FSGS‐CT 2011).
Novel treatments, which have been or are being trialled in patients with steroid‐ and CNI‐resistant FSGS, include monoclonal antibodies (rituximab, adalimumab, abatacept), adrenocorticotropic hormone (ACTH) and plasmapheresis. In addition, sparsentan, which is a dual endothelin and angiotensin receptor blocker (ARB), may reduce proteinuria in patients with FSGS and nephrotic syndrome (DUET 2017).
How the intervention might work
The interventions currently used in adult patients with primary FSGS are immunosuppressive agents, monoclonal antibodies, ACTH and plasmapheresis. Their use is based on the presumption that the primary form of FSGS is caused by circulating factors produced by immune mechanisms (De Vriese 2018; Shabaka 2020) and that suppression or removal of these factors will lead to remission of the nephrotic syndrome. In addition, reduction of proteinuria per se slows the progression to kidney failure (Troost 2021; Troyanov 2005) so angiotensin‐converting enzyme inhibitors (ACEi) and ARB are recommended for all patients with nephrotic syndrome to reduce proteinuria.
Why it is important to do this review
This review aimed to assess the efficacy of any treatment for adult patients with FSGS. It included studies, particularly older studies, which may have included participants with genetic or secondary forms of FSGS as well as primary FSGS.
This review was first published in 2008 and included five studies evaluating immunosuppressive agents. Since then a better understanding of the types of FSGS has become available with clearer definitions of primary FSGS, genetic FSGS and secondary forms of FSGS. In addition, several novel agents have been trialled in patients with primary and genetic FSGS so it is important to review these additional studies and determine any benefits of newer treatments.
Objectives
To assess the benefits and harms of immunosuppressive and non‐immunosuppressive treatment regimens in adults with FSGS.
Methods
Criteria for considering studies for this review
Types of studies
RCTs and quasi‐RCTs (RCTs in which allocation to treatment was obtained by alternation, use of alternate medical records, date of birth or other predictable methods) which examined the effects of different agents in the treatment of FSGS in adults were included.
Types of participants
Adults (aged ≥ 16 years) with biopsy‐proven FSGS were included.
Types of interventions
Corticosteroids including prednisone, methylprednisolone and dexamethasone
CNIs (cyclosporin, tacrolimus) either alone or in combination with corticosteroids
Alkylating agents (cyclophosphamide, chlorambucil) either alone or in combination with corticosteroids
Antimetabolites (azathioprine, MMF) either alone or in combination with corticosteroids
Anti‐CD20 monoclonal antibodies (rituximab, ofatumumab)
Novel medications (including fresolimumab, abatacept, adalimumab, antagonists of CCR2, a chemokine receptor)
Plasmapheresis or immunoadsorption, either alone or in combination with immunomodulatory/immunosuppressive drug therapy.
Types of outcome measures
Primary outcomes
-
Complete or partial remission of proteinuria. Complete remission and partial remission were defined according to the definitions used by the study authors. KDIGO 2021 used the following definitions for complete or partial remission:
Complete remission: reduction in urine protein to < 0.3 g/day or urinary protein‐creatinine ratio (UPCR) < 300 mg/g (< 30 mg/mmol), stable serum creatinine (SCr) and serum albumin > 3.5 g/dL (>35 g/L)
Partial remission: reduction in urine protein to 0.3 to 3.5 g/day or UPCR 300 to 3500 mg/g (30 to 350 mg/mmol) and a decrease > 50% from baseline.
Secondary outcomes
Occurrence of relapse in participants with complete remission
Kidney function defined by estimated (e) glomerular filtration rate (GFR), doubling of SCr, requirement for dialysis and transplantation
Adverse effects of therapy (infection, drug‐induced diabetes mellitus, malignancy)
Side effects associated with nephrotic syndrome (infection, thromboembolic events, hospitalisation)
Search methods for identification of studies
Electronic searches
We searched the Cochrane Kidney and Transplant Register of Studies up to 21 June 2021 through contact with the Information Specialist using search terms relevant to this review. The Specialised Register contains studies identified from the following sources:
Monthly searches of the Cochrane Central Register of Controlled Trials (CENTRAL)
Weekly searches of MEDLINE OVID SP
Handsearching of kidney‐related journals and the proceedings of major kidney conferences
Searching of the current year of EMBASE OVID SP
Weekly current awareness alerts for selected kidney and transplant journals
Searches of the International Clinical Trials Register (ICTRP) Search Portal and ClinicalTrials.gov.
Studies contained in the Register are identified through searches of CENTRAL, MEDLINE, and EMBASE based on the scope of Cochrane Kidney and Transplant. Details of search strategies, as well as a list of handsearched journals, conference proceedings and current awareness alerts, are available on the Cochrane Kidney and Transplant website under CKT Register of Studies.
See Appendix 1 for search terms used in strategies for this review.
Searching other resources
Reference lists of review articles, relevant studies, and clinical practice guidelines.
Letters seeking information about unpublished or incomplete studies to investigators known to be involved in previous studies.
Data collection and analysis
Selection of studies
The search strategy described was used to obtain titles and abstracts of studies that may be relevant to the review. The titles and abstracts were screened and irrelevant studies discarded, although studies and reviews that might include relevant data or information on studies were retained initially. Basic information was entered into a separate data sheet for each identified study. At least two authors independently assessed abstracts and, if necessary the full text, of these studies to determine which studies satisfy the inclusion criteria. Disagreements were resolved by discussion with a third reviewer.
Data extraction and management
Data extraction was carried out independently by at least two authors using standard data extraction forms. Disagreements were resolved in consultation with a third author. Studies reported in non‐English language journals were translated before assessment. Where more than one publication of one study existed, reports were grouped together and the publication with the most complete data was used in the analyses. Where relevant outcomes were only published in earlier versions these data were to be used.
Assessment of risk of bias in included studies
The following items were independently assessed by two authors using the risk of bias assessment tool (Higgins 2020) (see Appendix 2).
Was there adequate sequence generation (selection bias)?
Was allocation adequately concealed (selection bias)?
-
Was knowledge of the allocated interventions adequately prevented during the study?
Participants and personnel (performance bias)
Outcome assessors (detection bias)
Were incomplete outcome data adequately addressed (attrition bias)?
Are reports of the study free of suggestion of selective outcome reporting (reporting bias)?
Was the study apparently free of other problems that could put it at risk of bias?
Measures of treatment effect
For dichotomous outcomes (complete or partial remission, number with kidney failure, adverse effects) results were expressed as risk ratio (RR) with 95% confidence intervals (CI). Where continuous scales of measurement were used to assess the effects of treatment (GFR, SCr, urinary protein excretion) the mean difference (MD) was used, or the standardised mean difference (SMD) if different scales were used.
Unit of analysis issues
In cross‐over studies, data was to be used in analyses from the first part of the study before the cross over if separate data were available. However separate data were not available for the single included cross‐over study (Walker 1990) so the results from both parts of the studies were described in the text.
Dealing with missing data
Any further information required from the original author was requested by written correspondence (e.g. emailing corresponding author/s) and any relevant information obtained in this manner was included in the review. Evaluation of important numerical data such as screened, randomised patients, as well as intention‐to‐treat, as‐treated and per‐protocol population, were carefully performed. Attrition rates, for example, drop‐outs, losses to follow‐up and withdrawals were investigated. Issues of missing data and imputation methods (for example, last‐observation‐carried‐forward) were critically appraised (Higgins 2020).
Assessment of heterogeneity
We first assessed the heterogeneity by visual inspection of the forest plot. We then quantified statistical heterogeneity using the I² statistic, which describes the percentage of total variation across studies that is due to heterogeneity rather than sampling error (Higgins 2003). A guide to the interpretation of I² values was as follows.
0% to 40%: might not be important
30% to 60%: may represent moderate heterogeneity
50% to 90%: may represent substantial heterogeneity
75% to 100%: considerable heterogeneity.
The importance of the observed value of I² depends on the magnitude and direction of treatment effects and the strength of evidence for heterogeneity (e.g. P‐value from the Chi² test, or a CI for I²) (Higgins 2020).
Assessment of reporting biases
Funnel plots were planned to assess for the potential existence of small study bias (Higgins 2020) however, there were too few studies to do this.
Data synthesis
For dichotomous outcomes (kidney failure, remission, side effects) the RR with 95% CI were calculated and a summary point was estimated using the random‐effects model. Heterogeneity was analysed with an alpha of 0.1 used for statistical significance. For continuous outcomes (GFR, 24‐hour urinary protein excretion), outcomes were reported as MD with 95% CI using the random‐effects model.
Subgroup analysis and investigation of heterogeneity
The only intervention that was assessed in several studies was cyclosporin. Each study used a different comparator so each study was considered separately initially. Since the heterogeneity between studies for the outcomes of proteinuria reduction defined by I² levels of 0% to 22% might not be important, we included these studies in meta‐analyses.
Sensitivity analysis
Each study that evaluated cyclosporin, was assessed as an individual study and then an overall assessment was obtained. In the analyses of partial remission and combined partial and complete remission following CNI therapy, the removal of a single study (Cattran 1999) was investigated to assess whether the variability between studies was due to this single study. We were not able to perform other sensitivity analyses due to the small number of studies.
Summary of findings and assessment of the certainty of the evidence
We presented the main results of the review in 'Summary of findings' tables. These tables present key information concerning the quality of the evidence, the magnitude of the effects of the interventions examined, and the sum of the available data for the main outcomes (Schünemann 2020a). The 'Summary of findings' tables also includes an overall grading of the evidence related to each of the main outcomes using the GRADE (Grades of Recommendation, Assessment, Development and Evaluation) approach (GRADE 2008; GRADE 2011). The GRADE approach defines the quality of a body of evidence as to the extent to which one can be confident that an estimate of effect or association is close to the true quantity of specific interest. The quality of a body of evidence involves consideration of the within‐trial risk of bias (methodological quality), directness of evidence, heterogeneity, the precision of effect estimates and risk of publication bias (Schünemann 2020b). We presented the following outcomes in the 'Summary of findings' tables.
Complete remission
Partial remission
Complete and partial remission
CKD
Kidney failure
Adverse effects.
Results
Description of studies
Results of the search
The systematic literature search performed for the first version of this review published in 2008 identified four studies with four reports and 108 participants (Bhaumik 2002; Cattran 1999; Imbasciati 1980; Ponticelli 1993a).
For this update, we searched the Cochrane Kidney and Transplant Register of Studies up to 21 June 2021 and identified 73 new reports of 27 studies. Ten new studies (48 reports) were included (Cho 2019; Dasgupta 2020; DUET 2017; FONT I 2009; FONT II 2011; FSGS‐CT 2011; LUMINA‐1 2018; Quintaes 2000; Senthil Nayagam 2008; Vincenti 2017), five (five reports) were excluded (GloMY 2010; Liu 2016c; NCT01451489; Ren 2013; Trachtman 2011), and six ongoing studies were identified (ACTION 2018; DUPLEX 2019; NCT03298698; PODOCYTE 2017; Trachtman 2018; TURING 2019). Three studies are awaiting assessment (recently completed but no data available) (EudraCT2005‐004460‐22; NCT00801463; NCT00956059). We also identified nine new reports of existing included and excluded studies. One study previously excluded has been included in this update (Walker 1990).
A total of 15 studies (59 reports, 553 participants, Figure 1) were included, eight excluded, three are awaiting assessment, and there are six ongoing studies.
1.
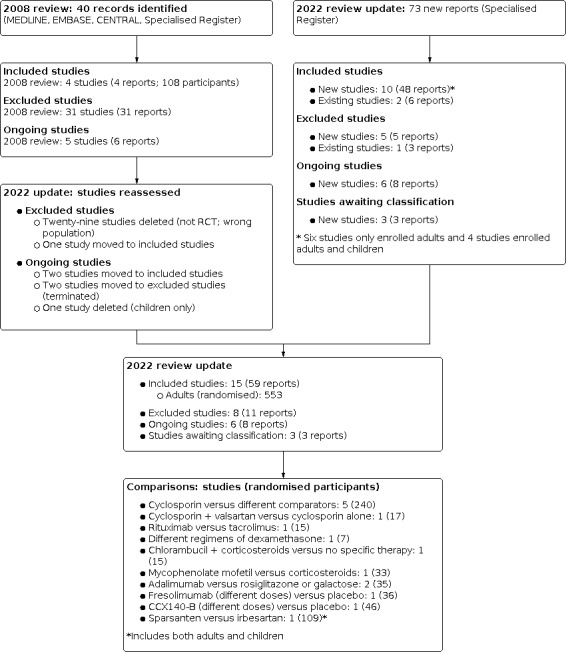
Flow diagram.
Included studies
This updated review included 15 studies (59 reports; 553 randomised participants) (see Figure 1).
No studies evaluating corticosteroids alone compared with placebo or no specific treatment were identified.
All studies enrolled participants with steroid‐resistant FSGS.
Ten studies reported that all included participants had nephrotic syndrome resistant to corticosteroids (Bhaumik 2002; Cattran 1999; Cho 2019; Dasgupta 2020; Imbasciati 1980; Ponticelli 1993a; Quintaes 2000; Senthil Nayagam 2008; Vincenti 2017; Walker 1990).
Five studies did not specifically report that they only included participants with nephrotic syndrome and used a definition of UPCR of > 1g/g for study entry so could have included participants with nephrotic range proteinuria without overt nephrotic syndrome (DUET 2017; FONT I 2009; FONT II 2011; FSGS‐CT 2011; LUMINA‐1 2018).
Serum albumin and urinary protein excretion at entry to each study are shown in Table 2.
1. Serum and urine protein levels at presentation of FSGS.
| Study name | Serum albumin | Urinary protein excretion | Included participants as reported by authors | ||
| Treatment group | Control group | Treatment group | Control group | ||
| Cyclosporin studies | |||||
| Bhaumik 2002 | ‐‐ | ‐‐ | ‐‐ | ‐‐ | All had nephrotic syndrome |
| Cattran 1999 | 3.1 ± 0.9 g/dL | 3.0 ± 0.9 g/dL | 6.9 g/24 hours | 8.7 g/24 hours | All had nephrotic syndrome |
| FSGS‐CT 2011 | 3.0 g/dL (IQR 2.3 to 3.7) | 2.7 g/dL (IQR 2‐3.5) | UPCR: 1 to 1.9 g/g in 20 UPCR: > 1.9 g/g in 47 |
UPCR: 1 to 1.9 g/g in 13 UPCR: > 1.9 g/g in 53 |
Did not specifically state that all included participants had nephrotic syndrome |
| Ponticelli 1993a | ‐‐ | ‐‐ | 167 ± 56 mg/m²/hour (4.0 g/day) |
116 ± 34 mg/m²/hour (2.78 g/day) | All had nephrotic syndrome |
| Walker 1990 | ‐‐ | ‐‐ | ‐‐ | ‐‐ | All had nephrotic syndrome |
| Other studies | |||||
| Cho 2019 | ‐‐ | ‐‐ | 9.6 (8.1 to 12.2) g/day (mean for all patients) | Not separated for groups | All had nephrotic syndrome |
| Dasgupta 2020 | 2.84 ± 0.58 g/dL | 2.54 ± 0.57 g/dL | 6.31 ± 2.27 g/24 hours | 7.01 ± 2.35 g/24 hours | All had nephrotic syndrome |
| DUET 2017 | ‐‐ | ‐‐ | UPCR: 3.61 (0.4‐18.7) g/g (median/range) | UPCR: 3.12 (0.9‐10.7) g/g (median/range) |
Did not specifically state that all included participants had nephrotic syndrome. Entry criteria required UPCR > 1g/g |
| FONT I 2009 | 2.1 ± 1 g/dL Adalimumab |
2.3 ± 1 g/dL Rosiglitazone |
UPCR: 15.9 ± 10.4 g/g Adalimumab |
UPCR: 5.5 ± 2.6 g/g Rosiglitazone |
Did not specifically state that all included participants had nephrotic syndrome. Entry criteria required UPCR > 1g/g |
| FONT II 2011 | 2.40 g/dL (IQR 2.10 ‐ 3.50) Adalimumab or galactose or standard therapy |
Data not separated for groups | UPCR: 4.93 g/g (IQR 3.3 to 11.5) | Data not separated for groups | Did not specifically state that all included participants had nephrotic syndrome. Entry criteria required UPCR > 1g/g |
| Imbasciati 1980 | ‐‐ | ‐‐ | ‐‐ | ‐‐ | All had nephrotic syndrome |
| LUMINA‐1 2018 | ‐‐ | ‐‐ | ‐‐ | ‐‐ | Did not specifically state that all included participants had nephrotic syndrome. Entry criteria required UPCR > 1g/g |
| Quintaes 2000 | 2.52 ± 1.3 g/L | 2.18 ± 1.0 g/L | 6.3 ± 2.7 g/24 hours | 10.83 ± 4.1 g/24 hours | All had nephrotic syndrome |
| Senthil Nayagam 2008 | ‐‐ | ‐‐ | UPCR: 4.68 ± 1.82 mg/mg (baseline for 17 FSGS and 11 MN) |
UPCR: 4.95 ± 1.65 mg/mg (baseline for 16 FSGS and 10 MN) |
All had nephrotic syndrome |
| Vincenti 2017 | ‐‐ | ‐‐ | UPCR: 5.92 (2.6,17.3) mg/mg UPCR: 6.46 (1.3, 15.9) mg/mg |
UPCR: 6.41 (2.2, 13.7) mg/mg | Did not specifically state that all included participants had nephrotic syndrome. Entry criteria required UPCR ≥ 3 mg/mg in > 1 urine specimen |
FSGS ‐ focal segmental glomerulosclerosis; IQR ‐ interquartile range; MN ‐ membranous nephropathy; UPCR ‐ urinary protein:creatinine ratio
Cyclosporin studies
-
Five studies evaluated the CNI, cyclosporin with or without prednisone, compared with other immunosuppressive agents or no specific treatment.
Ponticelli 1993a (19 participants) compared cyclosporin with supportive treatment with the primary outcome at 12 months.
Cattran 1999 (49 participants) compared cyclosporin plus prednisone with prednisone alone with the primary outcome at 6 months.
Bhaumik 2002 (25 participants) compared cyclosporin plus prednisone with IV methylprednisolone with the primary outcome at 6 months.
FSGS‐CT 2011 (138 participants)compared cyclosporin plus prednisone with MMF plus dexamethasone plus prednisone with the primary outcome at 12 months.
Walker 1990 (9 participants) compared cyclosporin with no specific therapy. This was a cross‐over study, did not provide numerical data for outcomes, and did not provide data separately for the first part of the study so it could not be included in meta‐analyses. This study did not specify whether cyclosporin was given with prednisone. The duration of follow‐up was unclear.
In three studies (Cattran 1999; Ponticelli 1993a; Walker 1990), participants either did not receive ACEi or ARBs or these were given only at the physician's discretion. Participants in the other studies received ACEi or ARBs (Bhaumik 2002; FSGS‐CT 2011).
Immunosuppressive agents
Quintaes 2000 (17 participants with nephrotic syndrome) compared cyclosporin and the ARB, valsartan, with cyclosporin. The primary outcome was complete or partial remission at six months. Only the treatment group received an ARB.
Dasgupta 2020) (15 participants with nephrotic syndrome) compared rituximab with tacrolimus. The primary outcome was complete or partial remission by 12 months. All participants received ACEi or ARB.
Cho 2019 (seven participants with nephrotic syndrome) compared two regimens of dexamethasone pulses. The primary outcome was complete or partial remission at 48 weeks. All participants received ACEi or ARB.
Imbasciati 1980 (15 participants with nephrotic syndrome) compared chlorambucil, methylprednisolone and prednisone with no specific treatment. The primary outcome was complete or partial remission at six months. It was unclear whether any participants received ACEi or ARB.
Senthil Nayagam 2008 (33 participants with nephrotic syndrome) compared MMF with prednisone. The primary outcome was complete or partial remission at six months. All participants received ACEi or ARBs.
Three studies randomising 68 participants (FONT I 2009; FONT II 2011; Vincenti 2017) compared monoclonal antibodies (adalimumab, fresolimumab) with other agents or placebo. All participants received ACEi or ARBs. FONT II 2011 could enrol participants with identified podocyte mutations as well as biopsy‐proven primary FSGS. In FONT I 2009 and FONT II 2011 the definition of proteinuria used was a UPCR ≥ 1g/g and the authors did not specify that study participants had nephrotic syndrome at study entry. The duration of follow up was 16 weeks for FONT I 2009, 26 weeks for FONT II 2011, and 16 weeks for Vincenti 2017.
LUMINA‐1 2018 (46 participants) compared different doses of CCX140‐B, which is an antagonist of the chemokine receptor CCR2, with placebo for 12 weeks. LUMINA‐1 2018 could enrol participants with identified podocyte mutations as well as biopsy‐proven primary FSGS. The definition of proteinuria using the UPCR was > 1g/g and the authors did not specify that study participants had nephrotic syndrome at study entry.
Other interventions
DUET 2017 (109 participants) compared sparsentan, a dual inhibitor of endothelin type A receptor and of the angiotensin II type 1 receptor, with irbesartan, an angiotensin II type 1 receptor inhibitor, for eight weeks. This study could enrol participants with identified podocyte mutations as well as biopsy‐proven primary FSGS. The definition of proteinuria using the UPCR was > 1g/g and the authors did not specify that study participants had nephrotic syndrome at study entry.
No studies were identified that evaluated plasmapheresis or immunoadsorption, either alone or in combination with immunomodulatory/immunosuppressive drug therapy.
Ongoing studies
ACTION 2018 will compare propagermanium (CCR2 receptor antagonist) with placebo in participants receiving irbesartan. Recruitment has been completed.
DUPLEX 2019 will compare sparsentan with irbesartan for two years. The expected completion date is 2022
NCT03298698 will compare rituximab with prednisone. The expected completion date is 2021
TURING 2019) will compare rituximab with placebo. The expected completion date is 2025
PODOCYTE 2017 will compare Acthar® Gel (ACTH) with placebo. The expected completion date is 2021
Trachtman 2018 will compare abatacept with placebo. Recruitment has been completed.
Studies awaiting classification
Three studies (EudraCT2005‐004460‐22; NCT00801463; NCT00956059) were identified, and are listed as awaiting classification. No results have been published, although the studies are likely to have been completed some years ago. Two studies provided no contact details, and no response from the contact person for the third study was received.
Excluded studies
Eight studies were excluded (Chan 2007; GloMY 2010; Heering 2004; Liu 2006; Liu 2016c; NCT01451489; Ren 2013; Trachtman 2011).
Heering 2004 was excluded because some participants in the control group were transferred to the treatment group and then analysed in both the treatment and control groups so that it was impossible to determine to which treatment a participant had responded.
Three studies (GloMY 2010; Liu 2006; NCT01451489) were terminated without results because of recruitment issues.
Chan 2007 had initially planned to recruit participants with FSGS as well as those with idiopathic membranous nephropathy (IMN) based on the information from the entry in ClinicalTrials.gov but the author confirmed via email that the study only enrolled participants with IMN.
Two studies (Liu 2016c; Ren 2013) were excluded because they included mixed populations and FSGS participants could not be separated.
Trachtman 2011 was excluded because it was unclear whether all included participants were randomised.
Risk of bias in included studies
Risk of bias assessments are summarised in Figure 2 and Figure 3.
2.

Risk of bias graph: review authors' judgements about each risk of bias item presented as percentages across all included studies.
3.

Risk of bias summary: review authors' judgements about each risk of bias item for each included study.
Allocation
Random sequence generation was at low risk of bias in seven studies (Cattran 1999; Cho 2019; Dasgupta 2020; DUET 2017; FSGS‐CT 2011; LUMINA‐1 2018; Ponticelli 1993a) and at unclear risk of bias in the remaining eight studies.
Allocation concealment was at low risk of bias in five studies (Cattran 1999; DUET 2017; FSGS‐CT 2011; LUMINA‐1 2018; Ponticelli 1993a) and at unclear risk of bias in the remaining 10 studies.
Blinding
Performance bias was at low risk in four studies (DUET 2017; FSGS‐CT 2011; LUMINA‐1 2018; Vincenti 2017) and at high risk in the remaining 11 studies.
Detection bias was considered to be at low risk of bias in 13 studies as the outcome was laboratory‐based and unlikely to be influenced by lack of blinding. Detection bias was at high risk of bias in two studies (Bhaumik 2002; Senthil Nayagam 2008).
Incomplete outcome data
Incomplete outcome data reporting (attrition bias) was at low risk in 12 studies. One study (Cho 2019) was at high risk of attrition bias and two studies (LUMINA‐1 2018; Walker 1990) were at unclear risk of attrition bias.
Selective reporting
Reporting bias was considered to be at low risk in six studies (Cattran 1999; Dasgupta 2020; FONT I 2009; FONT II 2011; FSGS‐CT 2011; Senthil Nayagam 2008); at high risk in six studies (DUET 2017; LUMINA‐1 2018; Ponticelli 1993a; Quintaes 2000; Vincenti 2017; Walker 1990) and at unclear risk of bias in three studies (Bhaumik 2002; Cho 2019; Imbasciati 1980)
Other potential sources of bias
Five studies were at low risk of bias as they were funded by government sources (Cho 2019; Dasgupta 2020; FONT I 2009; FONT II 2011; FSGS‐CT 2011). Five studies were considered to be at high risk of bias as they were funded by commercial organisations (DUET 2017; LUMINA‐1 2018; Ponticelli 1993a; Senthil Nayagam 2008; Vincenti 2017. In the remaining five studies the source of funding was not reported.
Effects of interventions
See: Table 1
Cyclosporin versus corticosteroids with/without other immunosuppressive agents
Five studies compared cyclosporin with no treatment or different comparators (240 randomised/231 meta‐analysed).
Cyclosporin versus supportive treatment (Ponticelli 1993a)
Cyclosporin plus prednisone versus prednisone alone (Cattran 1999)
Cyclosporin plus prednisone versus IV methylprednisolone (Bhaumik 2002)
Cyclosporin plus prednisone versus MMF plus dexamethasone (FSGS‐CT 2011)
Cyclosporin versus no specific therapy (Walker 1990). This was a cross‐over study (9 participants), which did not provide numerical data for outcomes and did not provide data separately for the first part of the study so it could not be included in meta‐analyses.
Complete remission of proteinuria
Four studies could be included in this meta‐analysis (Bhaumik 2002; Cattran 1999; FSGS‐CT 2011; Ponticelli 1993a).
-
Individual studies found that cyclosporin with or without prednisone may make little or no difference to the likelihood of complete remission at 6 to 12 months compared with:
supportive treatment (Analysis 1.1.1 (1 study, 19 participants): RR 4.55, 95% CI 0.25 to 83.70);
prednisone (Analysis 1.1.2 (1 study, 49 participants): RR 2.67, 95% CI 0.11 to 62.42);
IV methylprednisolone (Analysis 1.1.3 (1 study, 25 participants): RR 2.31, 95% CI 0.55 to 9.74);
MMF plus dexamethasone (Analysis 1.1.4 (1 study, 138 participants): RR 2.14, 95% CI 0.87 to 5.24).
When these four studies were combined, cyclosporin compared with different comparators may increase the likelihood of complete remission (Analysis 1.1 (4 studies, 231 participants): RR 2.31, 95% CI 1.13 to 4.73; I² = 0%; low certainty evidence), Despite the different comparators, there was no heterogeneity between studies and the test for subgroups did not indicate any differences between studies. (Table 1).
1.1. Analysis.

Comparison 1: Cyclosporin versus different comparators, Outcome 1: Complete remission of proteinuria
Partial remission of proteinuria
Four studies could be included in this meta‐analysis (Bhaumik 2002; Cattran 1999; FSGS‐CT 2011; Ponticelli 1993a).
Cattran 1999 found that cyclosporin with prednisone compared with prednisone alone may increase the likelihood of partial remission at six months (Analysis 1.2.2 (1 study, 49 participants): RR 7.96, 95% CI 1.09 to 58.15).
-
The other three individual studies found that cyclosporin with or without prednisone may make little or no difference at 6 to 12 months to the likelihood of partial remission compared with:
supportive treatment (Analysis 1.2.1 (1 study, 19 participants): RR 1.20, 95% CI 0.36 to 3.97);
IV methylprednisolone (Analysis 1.2.3 (1 study, 25 participants): RR 1.38, 95% CI 0.51 to 3.74);
MMF plus dexamethasone (Analysis 1.2.4 (1 study, 138 participants): RR 1.09, 95% CI 0.61 to 1.93).
When the four studies were combined, cyclosporin compared with other agents may make little or no difference to the likelihood of partial remission (Analysis 1.2 (4 studies, 231 participants): RR 1.36, 95% CI 0.78 to 2.39; I² = 22%; low certainty evidence). Despite the different comparators, there was little heterogeneity (I² = 22%) and the test for subgroups did not indicate differences between studies. Heterogeneity between studies in these analyses was eliminated by the removal of Cattran 1999.
1.2. Analysis.

Comparison 1: Cyclosporin versus different comparators, Outcome 2: Partial remission of proteinuria
Complete or partial remission
Four studies could be included in this meta‐analysis (Bhaumik 2002; Cattran 1999; FSGS‐CT 2011; Ponticelli 1993a).
Cattran 1999 found that cyclosporin plus prednisone compared with prednisone alone may increase the likelihood of complete and partial remission at six months (Analysis 1.3.2 (1 study, 49 participants): RR 8.85, 95% CI 1.22 to 63.92).
-
The other three individual studies found that cyclosporin with or without prednisone may make little or no difference at 6 to 12 months to the likelihood of complete remission compared with:
supportive therapy (Analysis 1.3.1 (1 study, 19 participants): RR 1.80, 95% CI 0.63 to 5.16);
IV methylprednisolone (Analysis 1.3.3 (1 study, 25 participants): RR 1.69, 95% CI 0.92 to 3.12);
MMF plus dexamethasone (Analysis 1.3.4 (1 study, 138 participants): RR 1.38, 95% CI 0.90 to 2.10).
When the four studies were combined, cyclosporin compared with other agents may increase the likelihood of complete or partial remission at 6 to 12 months (Analysis 1.3 (4 studies, 231 participants): RR 1.64, 95% CI 1.10 to 2.44; I² = 19%; low certainty evidence). Despite the different comparators, there was little heterogeneity (I² = 19%) and the test for subgroups did not indicate differences between studies. Heterogeneity between studies in these analyses was eliminated by the removal of Cattran 1999.
1.3. Analysis.

Comparison 1: Cyclosporin versus different comparators, Outcome 3: Complete or partial remission
Chronic kidney disease or kidney failure
Four studies could be included in this meta‐analysis (Bhaumik 2002; Cattran 1999; FSGS‐CT 2011; Ponticelli 1993a).
Individual study data showed that cyclosporin may make little or no difference to the outcomes of CKD or kidney failure (Analysis 1.4; Analysis 1.5).
When the studies were combined, cyclosporin compared with other agents may make little or no difference to the outcomes of CKD (Analysis 1.4 (4 studies, 231 participants): RR 0.83, 95% CI 0.35 to 1.96; I² = 47%) or kidney failure (Analysis 1.5 (4 studies, 231 participants): RR 0.55, 95% CI 0.15 to 2.00; I² = 45%).
1.4. Analysis.

Comparison 1: Cyclosporin versus different comparators, Outcome 4: Chronic kidney disease
1.5. Analysis.

Comparison 1: Cyclosporin versus different comparators, Outcome 5: Kidney failure
Adverse effects
Individual study data showed that it is uncertain whether cyclosporin compared with other agents makes any difference to adverse effects of hypertension (Analysis 1.6), infections (Analysis 1.7), hospitalisations (Analysis 1.8) or gastrointestinal adverse effects (Analysis 1.9). When studies were combined, it remained uncertain whether cyclosporin compared with other agents makes any difference to these adverse effects (very low certainty evidence).
1.6. Analysis.

Comparison 1: Cyclosporin versus different comparators, Outcome 6: Adverse effects: hypertension
1.7. Analysis.

Comparison 1: Cyclosporin versus different comparators, Outcome 7: Adverse effects: infection
1.8. Analysis.

Comparison 1: Cyclosporin versus different comparators, Outcome 8: Adverse effects: total hospitalisations
1.9. Analysis.

Comparison 1: Cyclosporin versus different comparators, Outcome 9: Adverse effects: GI disturbances
Overall data were downgraded for serious imprecision due to few studies with small numbers of participants (Table 1).
Cyclosporin plus valsartan versus cyclosporin alone
Quintaes 2000 compared cyclosporin plus valsartan with cyclosporin alone (17 randomised/meta‐analysed participants).
It is uncertain whether cyclosporin plus valsartan compared with cyclosporin alone increases the numbers with complete (Analysis 2.1.1 (1 study, 17 participants): RR 4.50, 95% CI 0.25 to 81.76) or partial remission (Analysis 2.1.2 (1 study, 17 participants): RR 1.19, 95% CI 0.37 to 3.76) at six months because the certainty of the evidence was very low.
Cyclosporin plus valsartan compared with cyclosporin alone may make little or no difference to the change in the urine protein excretion (Analysis 2.2.2 (1 study, 17 participants): MD 1.72 g/L, 95% CI ‐1.45 to 4.89), may increase the serum albumin (Analysis 2.3.1 (1 study, 17 participants): MD 0.93, 95% CI 0.12 to 1.74) but may have little or no effect on SCr (Analysis 2.3.2 (1 study, 17 participants): MD ‐0.19 µmol/L, 95% CI ‐0.81 to 0.43).
Adverse effects were not reported.
2.1. Analysis.

Comparison 2: Cyclosporin plus valsartan versus cyclosporin alone, Outcome 1: Remission
2.2. Analysis.

Comparison 2: Cyclosporin plus valsartan versus cyclosporin alone, Outcome 2: Protein excretion at 6 months
2.3. Analysis.

Comparison 2: Cyclosporin plus valsartan versus cyclosporin alone, Outcome 3: Biochemical outcomes at 6 months
Chlorambucil plus prednisone versus no specific treatment
Imbasciati 1980 compared chlorambucil plus prednisone with no specific treatment (15 randomised/analysed participants).
It is uncertain whether chlorambucil plus prednisone compared with no specific treatment increases the likelihood of complete remission (Analysis 3.1.1 (1 study, 15 participants): RR 1.75, 95% CI 0.20 to 15.41), partial remission (Analysis 3.1.2 (1 study, 15 participants): RR 2.63, 95% CI 0.35 to 19.85), complete or partial remission (Analysis 3.1.3 (1 study, 15 participants): RR 2.19, 95% CI 0.60 to 7.93), or prevents doubling of SCr (Analysis 3.1.4 (1 study, 15 participants): RR 0.30, 95% CI 0.01 to 6.29) at six months (very low certainty evidence).
Adverse effects were not reported.
3.1. Analysis.

Comparison 3: Chlorambucil plus prednisone versus no specific treatment, Outcome 1: Kidney outcomes
Mycophenolate mofetil versus with prednisone
Senthil Nayagam 2008 compared MMF with prednisone (33 randomised/analysed participants).
MMF compared with prednisone may make little or no difference to the likelihood of complete remission (Analysis 4.1.1 (1 study, 33 participants): RR 1.05, 95% CI 0.58 to 1.88), partial remission (Analysis 4.1.2 (1 study, 33 participants): RR 0.94, 95% CI 0.15 to 5.91), or complete or partial remission (Analysis 4.1.3 (1 study, 33 participants): RR 1.03, 95% CI 0.65 to 1.61) by 6 months (low certainty evidence).
MMF compared with prednisone may make little or no difference to the risk of infection (Analysis 4.2.1) or to GFR (Analysis 4.3.1) (low certainty evidence).
4.1. Analysis.

Comparison 4: Mycophenolate mofetil versus prednisone, Outcome 1: Kidney outcomes
4.2. Analysis.

Comparison 4: Mycophenolate mofetil versus prednisone, Outcome 2: Adverse effects
4.3. Analysis.

Comparison 4: Mycophenolate mofetil versus prednisone, Outcome 3: GFR
Rituximab versus tacrolimus
Dasgupta 2020 compared rituximab with tacrolimus (15 randomised/analysed participants).
It is uncertain whether rituximab compared with tacrolimus makes any difference to the likelihood of complete remission (Analysis 6.1.1 (1 study, 15 participants): RR 0.67, 95% CI 0.09 to 4.89), partial remission (Analysis 6.1.2 (1 study, 15 participants): RR 2.00, 95% CI 0.83 to 4.81), or complete or partial remission (Analysis 6.1.3 (1 study, 15 participants): RR 1.34, 95% CI 0.84 to 2.15) at 12 months (very low certainty evidence).
It is uncertain whether rituximab compared with tacrolimus makes any difference to the number relapsing within 12 months (Analysis 6.2 (1 study, 12 participants): RR 0.93, 95% CI 0.24 to 3.68; very low certainty of the evidence).
It is uncertain whether rituximab compared with tacrolimus makes any difference to adverse effects of hypertension (Analysis 6.3.1), infection (Analysis 6.3.2), diabetes (Analysis 6.3.3), and doubling of SCr (Analysis 6.3.4) (very low certainty of the evidence).
6.1. Analysis.

Comparison 6: Rituximab versus tacrolimus, Outcome 1: Remission of proteinuria by 12 months
6.2. Analysis.

Comparison 6: Rituximab versus tacrolimus, Outcome 2: Relapse by 12 months
6.3. Analysis.

Comparison 6: Rituximab versus tacrolimus, Outcome 3: Adverse effects
Different dose regimens of dexamethasone
Cho 2019 compared 2‐weekly with 4‐weekly regimens of dexamethasone (7 randomised/analysed participants).
Neither dexamethasone using the same total dose but administered as two doses every two weeks or dexamethasone administered in four doses every four weeks increased the number of participants with partial remission by 48 weeks.
It is uncertain whether different regimens of dexamethasone increase the likelihood of partial remission (Analysis 5.1 (1 study, 7 participants): RR 0.75, 95% CI 0.07 to 7.73), alter GFR (Analysis 5.2 (1 study, 7 participants): MD ‐13.00 mL/min, 95% CI ‐40.53 to 14.53), or alter 24‐hour urinary protein excretion (Analysis 5.3 (1 study, 7 participants): MD ‐2.60 g/24 hours, 95% CI ‐8.07 to 2.87) (very low certainty of this evidence).
It is uncertain whether different dose regimens of dexamethasone increase the likelihood of serious adverse effects (Analysis 5.4.1), mood swings (Analysis 5.4.2) or infections (Analysis 5.4.3) (very low certainty of this evidence).
5.1. Analysis.

Comparison 5: Dexamethasone: 2 weekly versus 4 weekly, Outcome 1: Partial remission at 48 weeks
5.2. Analysis.

Comparison 5: Dexamethasone: 2 weekly versus 4 weekly, Outcome 2: GFR
5.3. Analysis.

Comparison 5: Dexamethasone: 2 weekly versus 4 weekly, Outcome 3: 24‐hour urine protein excretion
5.4. Analysis.

Comparison 5: Dexamethasone: 2 weekly versus 4 weekly, Outcome 4: Adverse effects
Fresolimumab versus placebo
Vincenti 2017 compared 2 doses of fresolimumab (1 mg and 4 mg) with placebo (36 randomised/analysed participants).
It is uncertain whether 1 mg fresolimumab compared with placebo improves the likelihood of partial remission by 16 weeks (Analysis 7.1.1 (1 study 24 participants): RR 3.67, 95% CI 0.19 to 69.01; very low certainty of this evidence).
Administration of 4 mg of fresolimumab compared with placebo resulted in no partial remissions in either group.
The study reported no treatment‐emergent serious adverse effects were considered to be related to the study medication.
7.1. Analysis.

Comparison 7: Fresolimumab versus placebo, Outcome 1: Partial remission at 16 weeks
Sparsentan versus irbesartan
DUET 2017 compared sparsentan with irbesartan (109 randomised/96 analysed participants).
Sparsentan compared with irbesartan may make little or no difference at eight weeks to the likelihood of partial remission using the FSGS partial remission endpoint defined as UPCR ≤ 1.5 g/g and > 40% reduction in UPCR (Analysis 8.1 (1 study, 96 participants): RR 3.00, 95% CI 0.95 to 9.44; low certainty evidence).
However, the study reported that there was a greater reduction in proteinuria at eight weeks in all sparsentan treated participants (‐45%; 95% CI ‐52.7% to ‐35.7%) compared with irbesartan treated participants (‐18.5%; 95% CI ‐34.6% to 1.7%).
Sparsentan compared with irbesartan may result in little or no difference in treatment‐related adverse effects (Analysis 8.2.1) or the need to cease medications because of adverse effects (Analysis 8.2.2) by eight weeks.
The study reported that higher doses of sparsentan (400 mg, 800 mg) had a greater antihypertensive effect than irbesartan and eGFR remained stable in both groups.
Compared with irbesartan, sparsentan‐treated participants reported more hypotension, dizziness, oedema, and gastrointestinal adverse effects. Irbesartan‐treated participants reported more fatigue, nasal congestion and hyperkalaemia.
8.1. Analysis.

Comparison 8: Sparsentan versus irbesartan, Outcome 1: Partial remission at 8 weeks
8.2. Analysis.

Comparison 8: Sparsentan versus irbesartan, Outcome 2: Adverse effects
Adalimumab versus rosiglitazone
FONT I 2009 compared adalimumab with rosiglitazone (19 randomised participants).
Four of nine participants had a 50% reduction in proteinuria with adalimumab by 16 weeks. One adverse effect was probably related to adalimumab.
Two of 10 participants had a 40% reduction in proteinuria with rosiglitazone by 16 weeks. Three adverse effects were probably related to rosiglitazone.
The data were not meta‐analysed as the reported outcome measures differed between the groups.
Adalimumab or galactose versus conservative treatment
FONT II 2011 compared adalimumab or galactose with conservative treatment (21 randomised/19 analysed participants).
None of six participants treated with adalimumab, 2/7 participants treated with galactose, and 2/6 participants in the control group achieved the primary outcome of preservation of GFR and > 50% reduction in proteinuria at 26 weeks.
None of six participants treated with adalimumab, 3/7 participants treated with galactose, and 2/6 participants in the control group had a > 50 % reduction in proteinuria at 26 weeks.
Three of six participants were treated with adalimumab, 4/7 participants were treated with galactose, and 5/6 participants in the control group had no deterioration in eGFR at 26 weeks.
CCX140‐B versus placebo
LUMINA‐1 2018 compared CCX140‐B with placebo (number randomised not available/46 analysed participants).
"CCX140 did not demonstrate a meaningful reduction in proteinuria relative to the control group after 12 weeks of blinded treatment". This information was obtained from the company's website (https://www.chemocentryx.com/pipeline/chronic-kidney-disease/).
Discussion
Summary of main results
In this update, we evaluated treatment outcomes in 15 studies with 553 randomised participants with FSGS.
Studies largely evaluated participants who had FSGS which was resistant to corticosteroids. Most studies included participants with nephrotic syndrome. Those studies which did not specifically say that the participants had nephrotic syndrome, only included participants with nephrotic range proteinuria.
No studies comparing corticosteroids with placebo or no treatment were identified.
When four studies with 231 analysed participants comparing cyclosporin with different comparators were combined in meta‐analyses, cyclosporin may increase the likelihood of complete remission and of complete or partial remission (low certainty evidence). Although there was considerable imprecision around this result due to few studies with few participants, there was no significant heterogeneity between studies (I²: 0% to 22%) and no differences on subgroup analyses (I²: 0% to 16%). The risk of CKD or kidney failure and of adverse effects did not differ (low certainty evidence) (Table 1).
In one study (33 analysed participants), MMF compared with prednisone alone may make little or no difference to the number with complete or partial remission, to GFR or to adverse effects (low certainty evidence).
In three small studies evaluating chlorambucil, MMF, or rituximab, it is uncertain whether these interventions made any difference to the number with complete or partial remission (very low certainty evidence).
In one study (7 analysed participants) of two regimens of dexamethasone administration, it is uncertain whether either regimen makes any difference to the likelihood of remission (very low certainty evidence).
In four small studies of novel therapies (fresolimumab (an anti‐TGF‐β antibody), adalimumab (anti‐TGF‐α antibody), rosiglitazone (an antidiabetic agent for type 2 diabetes mellitus in the thiazolidinedione group), galactose, and CCX140‐B (a CCR2 receptor antagonist)), it is uncertain whether these medications made any difference to the likelihood of remission (very low certainty evidence).
In one study (96 analysed participants), it is unclear whether sparsentan compared with irbesartan increases the number of participants with partial remission of proteinuria because different ways of assessing partial remission gave different results (low certainty evidence).
We identified six ongoing studies including two studies evaluating rituximab, one evaluating ACTH, one evaluating sparsentan in a long‐term study and two evaluating novel therapies (abatacept, a specific CD80 antagonist, and propagermanium, a CCR2 receptor antagonist).
Overall completeness and applicability of evidence
This systematic review identified only 15 studies, which evaluated different therapies in corticosteroid‐resistant FSGS. No studies were identified that evaluated corticosteroids compared with placebo or no treatment in FSGS. Thirteen studies only included participants with FSGS and nephrotic syndrome which would be consistent with primary FSGS, though genetic causes of FSGS could not be excluded since no studies reported on any genetic associations. Secondary FSGS isn't usually associated with nephrotic syndrome (De Vriese 2018; Shabaka 2020). In five studies (DUET 2017; FONT I 2009; FONT II 2011; FSGS‐CT 2011; LUMINA‐1 2018), the lower limit of the UPCR indicated that participants had nephrotic range proteinuria at entry but the authors did not specifically state that the participants had nephrotic syndrome at entry to the study, so these studies could have included participants with genetic or secondary causes of FSGS. FSGS‐CT 2011 included 38% African Americans. A follow‐up study showed that APOL‐1 variants were more common in this population than in the white population but this did not influence treatment responses to cyclosporin or to MMF (Kopp 2015). CNIs (cyclosporin, tacrolimus) are recommended as the first‐line treatment for primary FSGS, which is resistant to corticosteroids (KDIGO 2012; KDIGO 2021), as they have proved to be the most effective agents to date. This review identified four studies, which evaluated cyclosporin administered for at least six months. Although these studies used different comparators, there was no significant heterogeneity in the outcome of complete remission or combined complete and partial remission in the four studies. We chose to combine the data from these studies as well as show the data from individual studies. When the data were combined, cyclosporin increased the absolute number of participants who achieved complete or partial remission from 291 per 1000 to 477 per 1000 (95% CI 320 to 710) (Table 1). None of the studies which evaluated cyclosporin looked for genetic mutations which could cause FSGS, so a greater benefit of CNIs among participants without genetic mutations cannot be excluded. One study with only 15 participants compared rituximab to tacrolimus and found no difference but the results of the studies in progress (NCT03298698; TURING 2019) comparing these medications are required to determine the relative efficacies of these medications in FSGS. In one study, which enrolled patients with biopsy‐proven primary FSGS or an identified podocyte mutation, it was unclear whether there was a clinically important reduction in proteinuria in a short‐term study of sparsentan (a dual endothelin and ARB) compared with irbesartan as two different measures of partial remission gave contradictory results. Previous data have demonstrated that partial reduction in proteinuria is associated with better kidney survival than no reduction (Troost 2021; Troyanov 2005). A further study is evaluating sparsentan for longer‐term benefits over 108 weeks (DUPLEX 2019). The remaining seven studies evaluated a variety of interventions and identified no evidence of the benefits of the therapies. However, all studies included very few participants.
Quality of the evidence
Only five of 15 studies reported adequate allocation concealment while seven reported adequate sequence generation. Only four studies were at low risk of performance bias but the majority (15 studies) were at low risk of detection bias. The majority of studies were at low risk of attrition bias but fewer (six studies) were at low risk of reporting bias.
GRADE assessment was only reported in a summary of findings table for four of the five studies, which compared cyclosporin with another therapy. The outcomes for the number with complete remission, partial remission, complete or partial remission, and kidney failure were considered to be of low certainty evidence. It remained uncertain whether cyclosporin compared with other agents makes any difference to adverse effects because the certainty of the evidence was very low. Outcomes were downgraded for risk of bias issues and imprecision related to small numbers of included participants.
Potential biases in the review process
For this update, a comprehensive search of Cochrane Kidney and Transplant’s Specialised Register was performed, which reduced the likelihood that eligible published studies were omitted from the review. Eligible studies published after the last search date or published in congress proceedings not routinely searched could have been missed. Four studies were available only in abstract form (Bhaumik 2002; Imbasciati 1980; Quintaes 2000; Walker 1990) and for LUMINA‐1 2018, very limited results came from the pharmaceutical company's website.
The review was completed independently by three authors so that at least two authors participated in each step of the update. This limited the risk of errors in determining study eligibility, data extraction, risk of bias assessment and data synthesis. Only studies evaluating cyclosporin could be combined in meta‐analyses. The comparators varied between studies and could have altered the results of those studies though there was no significant heterogeneity in the meta‐analyses (Analysis 1.1). The outcomes particularly of adverse effects that could be included in meta‐analyses were limited by the poor reporting in the original publications.
Agreements and disagreements with other studies or reviews
This updated systematic review demonstrates the paucity of evidence from RCTs to inform the treatment of FSGS in adults. Although the guidelines on FSGS from KDIGO 2012 and KDIGO 2021 recommend that the initial treatment of FSGS in adults should be high dose corticosteroids given for a maximum of 16 weeks, this review did not identify any RCTs which evaluated corticosteroids alone compared with placebo or no treatment. For steroid‐resistant FSGS, the guideline from KDIGO 2012 and KDIGO 2021 recommend the use of CNI for at least six months. The updated review has provided some data from RCTs to support this KDIGO 2012 and KDIGO 2021 recommendation.
Authors' conclusions
Implications for practice.
This review update has identified five studies that evaluated cyclosporin compared with different comparators. Three of these studies were included in the 2008 review. A meta‐analysis of four studies in which cyclosporin was compared with different comparators, found that patients with FSGS treated with cyclosporin for at least six months were more likely to achieve complete remission or complete and partial remission. While there was considerable imprecision around these results because of small studies with small numbers of participants, there was no significant heterogeneity between studies. Therefore cyclosporin may be considered as first‐line treatment for steroid‐resistant FSGS.
In a single, short‐term study (DUET 2017) comparing sparsentan with irbesartan, it was unclear whether partial remission of proteinuria was more likely to occur with sparsentan as different measures of partial remission gave different results. A longer‐term study (DUPLEX 2019) comparing these interventions is now underway. Currently, patients with nephrotic syndrome are routinely treated with maximally tolerated doses of ACEi or ARB.
None of the other studies of immunosuppressive therapies identified an increased likelihood of complete or partial remission. The results of RCTs evaluating rituximab (NCT03298698; TURING 2019), ACTH gel (PODOCYTE 2017) and abatacept (Trachtman 2018) are awaited.
Implications for research.
FSGS is a rare condition in adult patients so the RCTs to date have generally involved too few participants for meaningful results. While there should be an RCT evaluating corticosteroids with placebo in participants with newly diagnosed primary FSGS, this is unlikely to be performed since longstanding recommendations based on observational studies suggest that corticosteroids should be tried first in such patients. Several novel therapies have been evaluated in very small studies and to date, none have shown evidence of improved outcomes for patients with FSGS. Since CNIs are accepted therapy for steroid‐resistant FSGS in adults, CNIs could be used in control groups of future RCTs evaluating rituximab and novel agents in FSGS.
Since FSGS resistance to CNIs is an uncommon condition, novel medications for its treatment could be tested in a small sample, sequential, multiple assignment RCTs (Chao 2020) rather than in traditional RCTs where the inability to recruit an adequate number of participants can lead to the study being abandoned.
What's new
| Date | Event | Description |
|---|---|---|
| 1 March 2022 | Amended | Peer reviewers added |
History
Protocol first published: Issue 3, 2001 Review first published: Issue 3, 2008
| Date | Event | Description |
|---|---|---|
| 10 January 2022 | New search has been performed | New studies and interventions included |
| 10 January 2022 | New citation required but conclusions have not changed | New studies included, however no change to conclusions |
| 17 May 2018 | Amended | Amended search strategies |
| 27 March 2008 | Amended | Converted to new review format. |
Acknowledgements
We would like to thank;
Andreas Pfaff, Hans‐Konrad Selbmann and Andrew Bagriy for their contribution to the original protocol of this review.
Peer reviewers: Richard J. Glassock, MD, FACP, FRCP, FASN; Professor Jonathan Barratt (University of Leicester, UK); Dr Giles Walters (Canberra Hospital, ANU Medical School)
Appendices
Appendix 1. Electronic search strategies
| Databases | Search Terms |
| CENTRAL |
|
| MEDLINE (OVID) |
|
| EMBASE (OVID) |
|
Appendix 2. Risk of bias assessment tool
| Potential source of bias | Assessment criteria |
|
Random sequence generation Selection bias (biased allocation to interventions) due to inadequate generation of a randomised sequence |
Low risk of bias: Random number table; computer random number generator; coin tossing; shuffling cards or envelopes; throwing dice; drawing of lots; minimization (minimization may be implemented without a random element, and this is considered to be equivalent to being random). |
| High risk of bias: Sequence generated by odd or even date of birth; date (or day) of admission; sequence generated by hospital or clinic record number; allocation by judgement of the clinician; by preference of the participant; based on the results of a laboratory test or a series of tests; by availability of the intervention. | |
| Unclear: Insufficient information about the sequence generation process to permit judgement. | |
|
Allocation concealment Selection bias (biased allocation to interventions) due to inadequate concealment of allocations prior to assignment |
Low risk of bias: Randomisation method described that would not allow investigator/participant to know or influence intervention group before eligible participant entered in the study (e.g. central allocation, including telephone, web‐based, and pharmacy‐controlled, randomisation; sequentially numbered drug containers of identical appearance; sequentially numbered, opaque, sealed envelopes). |
| High risk of bias: Using an open random allocation schedule (e.g. a list of random numbers); assignment envelopes were used without appropriate safeguards (e.g. if envelopes were unsealed or non‐opaque or not sequentially numbered); alternation or rotation; date of birth; case record number; any other explicitly unconcealed procedure. | |
| Unclear: Randomisation stated but no information on method used is available. | |
|
Blinding of participants and personnel Performance bias due to knowledge of the allocated interventions by participants and personnel during the study |
Low risk of bias: No blinding or incomplete blinding, but the review authors judge that the outcome is not likely to be influenced by lack of blinding; blinding of participants and key study personnel ensured, and unlikely that the blinding could have been broken. |
| High risk of bias: No blinding or incomplete blinding, and the outcome is likely to be influenced by lack of blinding; blinding of key study participants and personnel attempted, but likely that the blinding could have been broken, and the outcome is likely to be influenced by lack of blinding. | |
| Unclear: Insufficient information to permit judgement | |
|
Blinding of outcome assessment Detection bias due to knowledge of the allocated interventions by outcome assessors. |
Low risk of bias: No blinding of outcome assessment, but the review authors judge that the outcome measurement is not likely to be influenced by lack of blinding; blinding of outcome assessment ensured, and unlikely that the blinding could have been broken. |
| High risk of bias: No blinding of outcome assessment, and the outcome measurement is likely to be influenced by lack of blinding; blinding of outcome assessment, but likely that the blinding could have been broken, and the outcome measurement is likely to be influenced by lack of blinding. | |
| Unclear: Insufficient information to permit judgement | |
|
Incomplete outcome data Attrition bias due to amount, nature or handling of incomplete outcome data. |
Low risk of bias: No missing outcome data; reasons for missing outcome data unlikely to be related to true outcome (for survival data, censoring unlikely to be introducing bias); missing outcome data balanced in numbers across intervention groups, with similar reasons for missing data across groups; for dichotomous outcome data, the proportion of missing outcomes compared with observed event risk not enough to have a clinically relevant impact on the intervention effect estimate; for continuous outcome data, plausible effect size (difference in means or standardized difference in means) among missing outcomes not enough to have a clinically relevant impact on observed effect size; missing data have been imputed using appropriate methods. |
| High risk of bias: Reason for missing outcome data likely to be related to true outcome, with either imbalance in numbers or reasons for missing data across intervention groups; for dichotomous outcome data, the proportion of missing outcomes compared with observed event risk enough to induce clinically relevant bias in intervention effect estimate; for continuous outcome data, plausible effect size (difference in means or standardized difference in means) among missing outcomes enough to induce clinically relevant bias in observed effect size; ‘as‐treated’ analysis done with substantial departure of the intervention received from that assigned at randomisation; potentially inappropriate application of simple imputation. | |
| Unclear: Insufficient information to permit judgement | |
|
Selective reporting Reporting bias due to selective outcome reporting |
Low risk of bias: The study protocol is available and all of the study’s pre‐specified (primary and secondary) outcomes that are of interest in the review have been reported in the pre‐specified way; the study protocol is not available but it is clear that the published reports include all expected outcomes, including those that were pre‐specified (convincing text of this nature may be uncommon). |
| High risk of bias: Not all of the study’s pre‐specified primary outcomes have been reported; one or more primary outcomes is reported using measurements, analysis methods or subsets of the data (e.g. subscales) that were not pre‐specified; one or more reported primary outcomes were not pre‐specified (unless clear justification for their reporting is provided, such as an unexpected adverse effect); one or more outcomes of interest in the review are reported incompletely so that they cannot be entered in a meta‐analysis; the study report fails to include results for a key outcome that would be expected to have been reported for such a study. | |
| Unclear: Insufficient information to permit judgement | |
|
Other bias Bias due to problems not covered elsewhere in the table |
Low risk of bias: The study appears to be free of other sources of bias. |
| High risk of bias: Had a potential source of bias related to the specific study design used; stopped early due to some data‐dependent process (including a formal‐stopping rule); had extreme baseline imbalance; has been claimed to have been fraudulent; had some other problem. | |
| Unclear: Insufficient information to assess whether an important risk of bias exists; insufficient rationale or evidence that an identified problem will introduce bias. |
Data and analyses
Comparison 1. Cyclosporin versus different comparators.
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
|---|---|---|---|---|
| 1.1 Complete remission of proteinuria | 4 | 231 | Risk Ratio (M‐H, Random, 95% CI) | 2.31 [1.13, 4.73] |
| 1.1.1 Cyclosporin versus supportive treatment at 12 months | 1 | 19 | Risk Ratio (M‐H, Random, 95% CI) | 4.55 [0.25, 83.70] |
| 1.1.2 Cyclosporin + prednisone versus prednisone at 6 months | 1 | 49 | Risk Ratio (M‐H, Random, 95% CI) | 2.67 [0.11, 62.42] |
| 1.1.3 Cyclosporin + prednisone versus IV methylprednisolone at 6 months | 1 | 25 | Risk Ratio (M‐H, Random, 95% CI) | 2.31 [0.55, 9.74] |
| 1.1.4 Cyclosporin + prednisone versus mycophenolate mofetil + dexamethasone + prednisone at 12 months | 1 | 138 | Risk Ratio (M‐H, Random, 95% CI) | 2.14 [0.87, 5.24] |
| 1.2 Partial remission of proteinuria | 4 | 231 | Risk Ratio (M‐H, Random, 95% CI) | 1.36 [0.78, 2.39] |
| 1.2.1 Cyclosporin versus no treatment | 1 | 19 | Risk Ratio (M‐H, Random, 95% CI) | 1.20 [0.36, 3.97] |
| 1.2.2 Cyclosporin + prednisone versus prednisone | 1 | 49 | Risk Ratio (M‐H, Random, 95% CI) | 7.96 [1.09, 58.15] |
| 1.2.3 Cyclosporin + prednisone versus IV methylprednisolone | 1 | 25 | Risk Ratio (M‐H, Random, 95% CI) | 1.38 [0.51, 3.74] |
| 1.2.4 Cyclosporin + prednisone versus mycophenolate + dexamethasone + prednisone | 1 | 138 | Risk Ratio (M‐H, Random, 95% CI) | 1.09 [0.61, 1.93] |
| 1.3 Complete or partial remission | 4 | 231 | Risk Ratio (M‐H, Random, 95% CI) | 1.64 [1.10, 2.44] |
| 1.3.1 Cyclosporin versus supportive treatment | 1 | 19 | Risk Ratio (M‐H, Random, 95% CI) | 1.80 [0.63, 5.16] |
| 1.3.2 Cyclosporin + prednisone versus prednisone | 1 | 49 | Risk Ratio (M‐H, Random, 95% CI) | 8.85 [1.22, 63.92] |
| 1.3.3 Cyclosporin + prednisone versus IV methylprednisolone | 1 | 25 | Risk Ratio (M‐H, Random, 95% CI) | 1.69 [0.92, 3.12] |
| 1.3.4 Cyclosporin + prednisone versus mycophenolate + dexamethasone + prednisone | 1 | 138 | Risk Ratio (M‐H, Random, 95% CI) | 1.38 [0.90, 2.10] |
| 1.4 Chronic kidney disease | 4 | 231 | Risk Ratio (M‐H, Random, 95% CI) | 0.83 [0.35, 1.96] |
| 1.4.1 Cyclosporin versus supportive treatment | 1 | 19 | Risk Ratio (M‐H, Random, 95% CI) | 0.30 [0.04, 2.39] |
| 1.4.2 Cyclosporin + prednisone versus prednisone | 1 | 49 | Risk Ratio (M‐H, Random, 95% CI) | 1.18 [0.72, 1.94] |
| 1.4.3 Cyclosporin + prednisone versus IV methylprednisolone | 1 | 25 | Risk Ratio (M‐H, Random, 95% CI) | 0.31 [0.08, 1.24] |
| 1.4.4 Cyclosporin + prednisone versus mycophenolate mofetil + dexamethasone + prednisone | 1 | 138 | Risk Ratio (M‐H, Random, 95% CI) | 2.29 [0.46, 11.41] |
| 1.5 Kidney failure | 4 | 231 | Risk Ratio (M‐H, Random, 95% CI) | 0.55 [0.15, 2.00] |
| 1.5.1 Cyclosporin versus supportive treatment | 1 | 19 | Risk Ratio (M‐H, Random, 95% CI) | 0.30 [0.01, 6.62] |
| 1.5.2 Cyclosporin + prednisone versus prednisone | 1 | 49 | Risk Ratio (M‐H, Random, 95% CI) | 0.35 [0.13, 0.98] |
| 1.5.3 Cyclosporin + prednisone versus IV methylprednisolone | 1 | 25 | Risk Ratio (M‐H, Random, 95% CI) | 0.23 [0.03, 1.79] |
| 1.5.4 Cyclosporin + prednisone versus mycophenolate mofetil + dexamethasone + prednisone | 1 | 138 | Risk Ratio (M‐H, Random, 95% CI) | 4.58 [0.55, 38.22] |
| 1.6 Adverse effects: hypertension | 2 | Risk Ratio (M‐H, Random, 95% CI) | Totals not selected | |
| 1.6.1 Cyclosporin + prednisone versus prednisone | 1 | Risk Ratio (M‐H, Random, 95% CI) | Totals not selected | |
| 1.6.2 Cyclosporin + prednisone versus mycophenolate mofetil + dexamethasone + prednisone | 1 | Risk Ratio (M‐H, Random, 95% CI) | Totals not selected | |
| 1.7 Adverse effects: infection | 2 | Risk Ratio (M‐H, Random, 95% CI) | Totals not selected | |
| 1.7.1 Cyclosporin versus supportive treatment | 1 | Risk Ratio (M‐H, Random, 95% CI) | Totals not selected | |
| 1.7.2 Cyclosporin + prednisone versus mycophenolate mofetil + dexamethasone + prednisone | 1 | Risk Ratio (M‐H, Random, 95% CI) | Totals not selected | |
| 1.8 Adverse effects: total hospitalisations | 2 | Risk Ratio (M‐H, Random, 95% CI) | Totals not selected | |
| 1.8.1 Cyclosporin + prednisone versus IV methylprednisolone | 1 | Risk Ratio (M‐H, Random, 95% CI) | Totals not selected | |
| 1.8.2 Cyclosporin + prednisone versus mycophenolate mofetil + dexamethasone + prednisone | 1 | Risk Ratio (M‐H, Random, 95% CI) | Totals not selected | |
| 1.9 Adverse effects: GI disturbances | 2 | Risk Ratio (M‐H, Random, 95% CI) | Totals not selected | |
| 1.9.1 Cyclosporin + prednisone versus prednisone | 1 | Risk Ratio (M‐H, Random, 95% CI) | Totals not selected | |
| 1.9.2 Cyclosporin + prednisone versus mycophenolate mofetil + dexamethasone + prednisone | 1 | Risk Ratio (M‐H, Random, 95% CI) | Totals not selected |
Comparison 2. Cyclosporin plus valsartan versus cyclosporin alone.
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
|---|---|---|---|---|
| 2.1 Remission | 1 | 34 | Risk Ratio (M‐H, Random, 95% CI) | 1.42 [0.49, 4.16] |
| 2.1.1 Complete remission at 6 months | 1 | 17 | Risk Ratio (M‐H, Random, 95% CI) | 4.50 [0.25, 81.76] |
| 2.1.2 Partial remission at 6 months | 1 | 17 | Risk Ratio (M‐H, Random, 95% CI) | 1.19 [0.37, 3.76] |
| 2.2 Protein excretion at 6 months | 1 | Mean Difference (IV, Random, 95% CI) | Totals not selected | |
| 2.3 Biochemical outcomes at 6 months | 1 | Mean Difference (IV, Random, 95% CI) | Totals not selected | |
| 2.3.1 Serum albumin g/dL | 1 | Mean Difference (IV, Random, 95% CI) | Totals not selected | |
| 2.3.2 Serum creatinine µmol/L | 1 | Mean Difference (IV, Random, 95% CI) | Totals not selected |
Comparison 3. Chlorambucil plus prednisone versus no specific treatment.
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
|---|---|---|---|---|
| 3.1 Kidney outcomes | 1 | Risk Ratio (M‐H, Random, 95% CI) | Totals not selected | |
| 3.1.1 Complete remission at 6 months | 1 | Risk Ratio (M‐H, Random, 95% CI) | Totals not selected | |
| 3.1.2 Partial remission at 6 months | 1 | Risk Ratio (M‐H, Random, 95% CI) | Totals not selected | |
| 3.1.3 Complete or partial remission at 6 months | 1 | Risk Ratio (M‐H, Random, 95% CI) | Totals not selected | |
| 3.1.4 Doubling of serum creatinine at 6 months | 1 | Risk Ratio (M‐H, Random, 95% CI) | Totals not selected |
Comparison 4. Mycophenolate mofetil versus prednisone.
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
|---|---|---|---|---|
| 4.1 Kidney outcomes | 1 | Risk Ratio (M‐H, Random, 95% CI) | Totals not selected | |
| 4.1.1 Complete remission at 6 months | 1 | Risk Ratio (M‐H, Random, 95% CI) | Totals not selected | |
| 4.1.2 Partial remission at 6 months | 1 | Risk Ratio (M‐H, Random, 95% CI) | Totals not selected | |
| 4.1.3 Complete or partial remission at 6 months | 1 | Risk Ratio (M‐H, Random, 95% CI) | Totals not selected | |
| 4.2 Adverse effects | 1 | Risk Ratio (M‐H, Random, 95% CI) | Totals not selected | |
| 4.2.1 Infection | 1 | Risk Ratio (M‐H, Random, 95% CI) | Totals not selected | |
| 4.3 GFR | 1 | Mean Difference (IV, Random, 95% CI) | Totals not selected | |
| 4.3.1 MMF versus prednisolone | 1 | Mean Difference (IV, Random, 95% CI) | Totals not selected |
Comparison 5. Dexamethasone: 2 weekly versus 4 weekly.
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
|---|---|---|---|---|
| 5.1 Partial remission at 48 weeks | 1 | Risk Ratio (M‐H, Random, 95% CI) | Totals not selected | |
| 5.2 GFR | 1 | Mean Difference (IV, Random, 95% CI) | Totals not selected | |
| 5.3 24‐hour urine protein excretion | 1 | Mean Difference (IV, Random, 95% CI) | Totals not selected | |
| 5.4 Adverse effects | 1 | Risk Ratio (M‐H, Random, 95% CI) | Totals not selected | |
| 5.4.1 serious adverse effects | 1 | Risk Ratio (M‐H, Random, 95% CI) | Totals not selected | |
| 5.4.2 Mood swings | 1 | Risk Ratio (M‐H, Random, 95% CI) | Totals not selected | |
| 5.4.3 Infection | 1 | Risk Ratio (M‐H, Random, 95% CI) | Totals not selected |
Comparison 6. Rituximab versus tacrolimus.
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
|---|---|---|---|---|
| 6.1 Remission of proteinuria by 12 months | 1 | Risk Ratio (M‐H, Random, 95% CI) | Totals not selected | |
| 6.1.1 Complete remission at 12 months | 1 | Risk Ratio (M‐H, Random, 95% CI) | Totals not selected | |
| 6.1.2 Partial remission at 12 months | 1 | Risk Ratio (M‐H, Random, 95% CI) | Totals not selected | |
| 6.1.3 Complete or partial remission at 12 months | 1 | Risk Ratio (M‐H, Random, 95% CI) | Totals not selected | |
| 6.2 Relapse by 12 months | 1 | Risk Ratio (M‐H, Random, 95% CI) | Totals not selected | |
| 6.3 Adverse effects | 1 | Risk Ratio (M‐H, Random, 95% CI) | Totals not selected | |
| 6.3.1 Worsening hypertension | 1 | Risk Ratio (M‐H, Random, 95% CI) | Totals not selected | |
| 6.3.2 Infection | 1 | Risk Ratio (M‐H, Random, 95% CI) | Totals not selected | |
| 6.3.3 Diabetes | 1 | Risk Ratio (M‐H, Random, 95% CI) | Totals not selected | |
| 6.3.4 Doubling of serum creatinine | 1 | Risk Ratio (M‐H, Random, 95% CI) | Totals not selected |
Comparison 7. Fresolimumab versus placebo.
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
|---|---|---|---|---|
| 7.1 Partial remission at 16 weeks | 1 | Risk Ratio (M‐H, Random, 95% CI) | Totals not selected | |
| 7.1.1 Fresolimumab 1 mg versus placebo | 1 | Risk Ratio (M‐H, Random, 95% CI) | Totals not selected | |
| 7.1.2 Fresolimumab 4 mg versus placebo | 1 | Risk Ratio (M‐H, Random, 95% CI) | Totals not selected |
Comparison 8. Sparsentan versus irbesartan.
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
|---|---|---|---|---|
| 8.1 Partial remission at 8 weeks | 1 | Risk Ratio (M‐H, Random, 95% CI) | Totals not selected | |
| 8.2 Adverse effects | 1 | Risk Ratio (M‐H, Random, 95% CI) | Totals not selected | |
| 8.2.1 Drug‐related adverse effects | 1 | Risk Ratio (M‐H, Random, 95% CI) | Totals not selected | |
| 8.2.2 Need to cease medication because of adverse effects | 1 | Risk Ratio (M‐H, Random, 95% CI) | Totals not selected |
Characteristics of studies
Characteristics of included studies [ordered by study ID]
Bhaumik 2002.
| Study characteristics | ||
| Methods |
|
|
| Participants |
|
|
| Interventions | Treatment group
Control group
Co‐interventions
|
|
| Outcomes |
|
|
| Notes |
|
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Method not specified: '... were randomised for two treatment options...' |
| Allocation concealment (selection bias) | Unclear risk | Method not specified: '... were randomised for two treatment options...' |
| Blinding of participants and personnel (performance bias) All outcomes | High risk | Open‐label study |
| Blinding of outcome assessment (detection bias) All outcomes | High risk | Open‐label study. While the laboratory measure is unlikely to be influenced by lack of blinding, the time point of outcome assessment is not defined and is susceptible to bias in an open‐label study |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | All patients were accounted for |
| Selective reporting (reporting bias) | Unclear risk | Pre‐specified outcomes not reported in available abstract |
| Other bias | Unclear risk | Funding source not reported; full manuscript was not published; trial not registered and no published protocol available |
Cattran 1999.
| Study characteristics | ||
| Methods |
|
|
| Participants |
|
|
| Interventions | Treatment group
Control group
Co‐interventions
|
|
| Outcomes |
Early stop points of study medication
|
|
| Notes |
|
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Quote: "Randomization was performed by the clinical coordinating center from a table of random numbers and was stratified by center in blocks of two to ensure a balance between groups" |
| Allocation concealment (selection bias) | Low risk | Central randomisation Quote: "Randomization was performed by the clinical coordinating center from a table of random numbers and was stratified by center in blocks of two to ensure a balance between groups" |
| Blinding of participants and personnel (performance bias) All outcomes | High risk | Quote: "The patients were masked in regards to active versus placebo assignment, but the physicians were not, for safety reasons and because the end points were objective and measured centrally by a lab masked to patient designation" |
| Blinding of outcome assessment (detection bias) All outcomes | Low risk | Quote: "The patients were masked in regards to active versus placebo assignment, but the physicians were not for safety reasons and because the end points were objective and measured centrally by a lab masked to patient designation" |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | All participants accounted for; therapy discontinued only as per pre‐specified stopping rules |
| Selective reporting (reporting bias) | Low risk | Expected outcomes reported |
| Other bias | Unclear risk | Supported by the Kidney Foundation of Canada and Norvatis Canada Ltd |
Cho 2019.
| Study characteristics | ||
| Methods |
|
|
| Participants |
|
|
| Interventions | Treatment group 1
Treatment group 2
Co‐interventions
|
|
| Outcomes |
Definitions
|
|
| Notes |
|
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Quotes: "Randomised/parallel assignment." "... subjects were randomised to 2 doses every 2 weeks or 4 doses every 4 weeks"; "Subjects were randomised (1: 1) using a stratified block design to 2 doses every 2 weeks versus 4 doses every 4 weeks for both periods" |
| Allocation concealment (selection bias) | Unclear risk | Quotes: "Randomised/parallel assignment. ." "...subjects were randomised to 2 doses every 2 weeks or 4 doses every 4 weeks"; "Subjects were randomised (1: 1) using a stratified block design to 2 doses every 2 weeks versus 4 doses every 4 weeks for both periods"; No other information provided |
| Blinding of participants and personnel (performance bias) All outcomes | High risk | Open‐label study |
| Blinding of outcome assessment (detection bias) All outcomes | Low risk | Remission confirmed by 24‐hour urine collection for protein. Laboratory measure unlikely to be influenced by lack of blinding |
| Incomplete outcome data (attrition bias) All outcomes | High risk | Only 8 participants enrolled; one of 8 participants randomised (to treatment group 2) withdrew before taking medication and was excluded |
| Selective reporting (reporting bias) | Unclear risk | All pre‐specified outcomes were reported but not always reported separately for RCT and for another non‐randomised study |
| Other bias | Low risk | National Institute of Diabetes and Digestive and Kidney Disease, Intramural Research Program (ZO1‐DK04312), NIH Clinical Center Pharmacy |
Dasgupta 2020.
| Study characteristics | ||
| Methods |
|
|
| Participants |
|
|
| Interventions | Treatment group 1
Treatment group 2
|
|
| Outcomes |
|
|
| Notes |
|
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Quote: "...were randomised using random numbers table in 2:1 distribution to receive tacrolimus or rituximab" |
| Allocation concealment (selection bias) | Unclear risk | No information provided on whether participant allocation to treatment groups was concealed |
| Blinding of participants and personnel (performance bias) All outcomes | High risk | Open‐label study |
| Blinding of outcome assessment (detection bias) All outcomes | Low risk | End point of proteinuria determined by UPCR measured in a laboratory and so unlikely to be influenced by lack of blinding |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | All participants completed the study |
| Selective reporting (reporting bias) | Low risk | Reported expected outcomes |
| Other bias | Low risk | No funding obtained for study |
DUET 2017.
| Study characteristics | ||
| Methods |
|
|
| Participants |
|
|
| Interventions | Treatment group
Control group (ARB)
Co‐interventions
During a subsequent open‐label, phase, sparsentan was given for 144 weeks to both groups |
|
| Outcomes |
|
|
| Notes |
|
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | At week 0, a computer‐generated randomisation sequence, via an interactive Web response system, used to randomise patients (3:1) to receive sparsentan or irbesartan |
| Allocation concealment (selection bias) | Low risk | At week 0, a computer‐generated randomisation sequence, via an interactive Web response system, used to randomise patients (3:1) to receive sparsentan or irbesartan |
| Blinding of participants and personnel (performance bias) All outcomes | Low risk | Both medications were encapsulated in grey gelatin capsules Quote: "Investigators, participants, caregivers, and the study sponsor were blinded to treatment allocations until database extraction and unblinding at the completion of the 8‐week, double‐blind treatment period." |
| Blinding of outcome assessment (detection bias) All outcomes | Low risk | Both medications were encapsulated in grey gelatin capsules Quote: "Investigators, participants, caregivers, and the study sponsor were blinded to treatment allocations until database extraction and unblinding at the completion of the 8‐week, double‐blind treatment period." |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | 94% completed the double‐blind period and data on the primary outcome was reported on 96/109 (88%) |
| Selective reporting (reporting bias) | High risk | Primary outcome of change in proteinuria could not be included in meta‐analysis. Additional analysis (not pre‐specified) and adverse effects could be included in meta‐analyses |
| Other bias | High risk | Trial organised by Retrophin Inc. (San Diego, CA) |
FONT I 2009.
| Study characteristics | ||
| Methods |
|
|
| Participants |
|
|
| Interventions | Treatment group 1
Treatment group 2
Co‐interventions
|
|
| Outcomes |
|
|
| Notes |
|
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Patients were randomised to receive adalimumab or rosiglitazone. No other information provided |
| Allocation concealment (selection bias) | Unclear risk | Patients were randomised to receive adalimumab or rosiglitazone. No other information provided |
| Blinding of participants and personnel (performance bias) All outcomes | High risk | Open‐label study |
| Blinding of outcome assessment (detection bias) All outcomes | Low risk | Primary outcomes were laboratory‐based and unlikely to be influenced by lack of blinding |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | 9% did not complete the study; 1/11 did not complete rosiglitazone arm; 1/10 did not complete adalimumab arm |
| Selective reporting (reporting bias) | Low risk | Expected outcomes reported |
| Other bias | Low risk | This work was supported by grants from the NIH–NIDDK (5R21‐DK070341), and the GCRC program of the Division of Research Resources, NIH RR00046 (UNC) and NIH RR018535 (North Shore Long Island Jewish Health System) |
FONT II 2011.
| Study characteristics | ||
| Methods |
|
|
| Participants |
|
|
| Interventions | Treatment group 1
Treatment group 2
Control group
Co‐interventions in all participants
|
|
| Outcomes |
|
|
| Notes |
|
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | FONT II is a phase II open‐label RCT. No other information provided |
| Allocation concealment (selection bias) | Unclear risk | FONT II is a phase II open‐label RCT. No other information provided |
| Blinding of participants and personnel (performance bias) All outcomes | High risk | Open‐label study |
| Blinding of outcome assessment (detection bias) All outcomes | Low risk | Primary outcomes are laboratory‐based and unlikely to be influenced by lack of blinding |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | All participants accounted for in analyses |
| Selective reporting (reporting bias) | Low risk | Expected outcomes reported |
| Other bias | Low risk | Funding from the National Institutes of Health—National Institute of Diabetes, Digestive, and Kidney Diseases, grant DK70341 (HT). Abbott Laboratories provided adalimumab for use in the project. Supported by NephCure Kidney International |
FSGS‐CT 2011.
| Study characteristics | ||
| Methods |
|
|
| Participants |
|
|
| Interventions | Treatment group 1
Treatment group 2
Co‐interventions
Additional antihypertensive therapies were not restricted by study protocol |
|
| Outcomes |
|
|
| Notes |
|
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Randomisation schedules using randomly permuted blocks of random sizes were prepared by the Data Coordinating centre stratified by eGFR, race |
| Allocation concealment (selection bias) | Low risk | Study investigators were blinded to randomised schedules |
| Blinding of participants and personnel (performance bias) All outcomes | Low risk | Open‐label study; lack of blinding could influence patient management differently between treatment groups |
| Blinding of outcome assessment (detection bias) All outcomes | Low risk | Study investigators were blinded to results of interim analyses done for the Data and Safety Monitoring Board Laboratory values for primary outcomes and some secondary outcomes are unlikely to be influenced by lack of blinding |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | Minimal participants were lost to follow‐up/did not attend assessments (< 1%); all patients included in outcome measurement |
| Selective reporting (reporting bias) | Low risk | All expected outcomes (remission, relapse, adverse effects) were reported |
| Other bias | Low risk | NIH funded |
Imbasciati 1980.
| Study characteristics | ||
| Methods |
|
|
| Participants |
|
|
| Interventions | Treatment group
Control group
Co‐interventions
|
|
| Outcomes |
|
|
| Notes |
|
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Quote: "Patients ... were randomly allocated to treatment or control groups" |
| Allocation concealment (selection bias) | Unclear risk | Quote: "Patients ... were randomly allocated to treatment or control groups" |
| Blinding of participants and personnel (performance bias) All outcomes | High risk | Open‐label study |
| Blinding of outcome assessment (detection bias) All outcomes | Low risk | Remission confirmed by 24‐hour urine protein. Laboratory investigation and unlikely to be influenced by lack of blinding |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | All patients accounted for |
| Selective reporting (reporting bias) | Unclear risk | No information on adverse effects and limited information on other outcomes |
| Other bias | Unclear risk | Insufficient information to permit judgement |
LUMINA‐1 2018.
| Study characteristics | ||
| Methods |
|
|
| Participants |
|
|
| Interventions | Treatment group 1
Treatment group 2
Treatment group 3
Control group
Co‐interventions
|
|
| Outcomes |
|
|
| Notes |
|
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Randomised, placebo‐controlled, phase 2 |
| Allocation concealment (selection bias) | Low risk | Randomised, placebo‐controlled, phase 2 |
| Blinding of participants and personnel (performance bias) All outcomes | Low risk | Triple (participant, care provider, investigator) |
| Blinding of outcome assessment (detection bias) All outcomes | Low risk | Triple (participant, care provider, investigator) |
| Incomplete outcome data (attrition bias) All outcomes | Unclear risk | Incomplete information |
| Selective reporting (reporting bias) | High risk | No data reported |
| Other bias | High risk | Study supported by Chemocentryx |
Ponticelli 1993a.
| Study characteristics | ||
| Methods |
|
|
| Participants |
|
|
| Interventions | Treatment group
Control group
Co‐interventions
The following therapies were not allowed:
There was no dietary protein restriction |
|
| Outcomes |
|
|
| Notes |
|
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Quotes: "This study was an open, randomised trial". "The indication for the therapy was contained in sealed, completely opaque envelopes numbered in sequence according to a table of random numbers" |
| Allocation concealment (selection bias) | Low risk | Quotes:"This study was an open, randomised trial". "The indication for the therapy was contained in sealed, completely opaque envelopes numbered in sequence according to a table of random numbers" |
| Blinding of participants and personnel (performance bias) All outcomes | High risk | Open‐label study |
| Blinding of outcome assessment (detection bias) All outcomes | Low risk | Most outcomes were based on objective laboratory measures, which were unlikely to be influenced by lack of blinding |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | 4 of 41 patients were excluded from analyses. However, all 4 losses were within the first 45 days from randomisation. One patient randomised to CSA was wrongly included/randomised, and three children randomised to the control group did not follow up as scheduled Further, patients who did not complete treatment were included in the analysis according to the intention‐to‐treat principle |
| Selective reporting (reporting bias) | High risk | Expected outcomes reported but data on adults could not be separated from data in children |
| Other bias | High risk | Study supported in part (drug, organization, meeting) by Sandoz P.F., Milano. Italy |
Quintaes 2000.
| Study characteristics | ||
| Methods |
|
|
| Participants |
|
|
| Interventions | Treatment group
Control group
Co‐interventions
|
|
| Outcomes |
|
|
| Notes |
|
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Insufficient information to permit judgement |
| Allocation concealment (selection bias) | Unclear risk | Insufficient information to permit judgement |
| Blinding of participants and personnel (performance bias) All outcomes | High risk | Open‐label study |
| Blinding of outcome assessment (detection bias) All outcomes | Low risk | Outcome was laboratory‐based and unlikely to be influenced by lack of blinding |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | All participants accounted for |
| Selective reporting (reporting bias) | High risk | No report on adverse effects |
| Other bias | Unclear risk | Insufficient information to permit judgement |
Senthil Nayagam 2008.
| Study characteristics | ||
| Methods |
|
|
| Participants |
|
|
| Interventions | Treatment group
Control group
Co‐interventions
|
|
| Outcomes |
Definitions
|
|
| Notes |
|
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Quotes: "This was a randomised open‐label study". "Treatment allocation was on the basis of minimization, using the following parameters: (MN or FSGS), sex and eGFR. Minimization is a valid alternative to randomization, and ensures uniformity between the two groups with respect to the characteristics used in the allocation process" |
| Allocation concealment (selection bias) | Unclear risk | Quotes: "This was a randomised open‐label study". "Treatment allocation was on the basis of minimization, using the following parameters: (MN or FSGS), sex and eGFR. Minimization is a valid alternative to randomization, and ensures uniformity between the two groups with respect to the characteristics used in the allocation process" |
| Blinding of participants and personnel (performance bias) All outcomes | High risk | Open‐label study |
| Blinding of outcome assessment (detection bias) All outcomes | High risk | Primary outcomes were complete or partial remission as determined by UPCR. While the laboratory measure is unlikely to be influenced by lack of blinding, the time point of outcome assessment is not defined and is susceptible to bias in an open‐label study |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | All patients included in analyses |
| Selective reporting (reporting bias) | Low risk | Expected outcomes (remission, relapse, adverse effects, GFR) reported |
| Other bias | High risk | Quote: "This study was supported by a grant from M/s Panacea Biotec Ltd, New Delhi, India" |
Vincenti 2017.
| Study characteristics | ||
| Methods |
|
|
| Participants |
|
|
| Interventions | Treatment group 1
Treatment group 2
Control group
Co‐interventions
|
|
| Outcomes |
Exploratory efficacy endpoints
|
|
| Notes |
|
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Phase 2, multicentre, double‐blinded, parallel dosing, randomised trial. Stratified by race and previous CNI therapy. Randomised by 3:3:2 allocation. Method of sequence generation not reported Quote: "At day 1, eligible patients who met all inclusion and exclusion criteria were randomly assigned, stratified by race (black versus nonblack) and prior CNI therapy (yes, no), to 1 of 3 treatment groups in a 3:3:2 allocation" |
| Allocation concealment (selection bias) | Unclear risk | Allocation concealment not reported Quote: "At day 1, eligible patients who met all inclusion and exclusion criteria were randomly assigned, stratified by race (black versus nonblack) and prior CNI therapy (yes, no), to 1 of 3 treatment groups in a 3:3:2 allocation" |
| Blinding of participants and personnel (performance bias) All outcomes | Low risk | Investigators/patients/care givers were blinded to therapy groups Quote: "..with patients and investigators remaining blinded to treatment assignment." |
| Blinding of outcome assessment (detection bias) All outcomes | Low risk | Investigators/patients/care givers were blinded to therapy groups Quote: "..with patients and investigators remaining blinded to treatment assignment." |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | All participants accounted for; no loss to follow‐up (one lost to follow‐up in Fresolimumab 4 mg group beyond primary outcome) |
| Selective reporting (reporting bias) | High risk | Expected outcomes reported; composite primary outcome at day 252 and various exploratory endpoints (changes in serum lipids and albumin, serum and urinary biomarkers) were defined post hoc |
| Other bias | High risk | Sponsored by Sanofi; composite post hoc primary outcome of durable clinical response at day 252 was assessed after patients might have received other interventions during days 112 to 252; trial was stopped early |
Walker 1990.
| Study characteristics | ||
| Methods |
|
|
| Participants |
|
|
| Interventions | Treatment group
Control group
Co‐interventions
|
|
| Outcomes |
|
|
| Notes |
|
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Participants allocated "at random to treatment". Cross‐over study |
| Allocation concealment (selection bias) | Unclear risk | Participants allocated "at random to treatment". Cross‐over study |
| Blinding of participants and personnel (performance bias) All outcomes | High risk | Open‐label study |
| Blinding of outcome assessment (detection bias) All outcomes | Low risk | Outcome defined by 24‐hour urine protein excretion |
| Incomplete outcome data (attrition bias) All outcomes | Unclear risk | Abstract‐only publication. Unclear if all participants completed study |
| Selective reporting (reporting bias) | High risk | No report of adverse effects |
| Other bias | Unclear risk | Abstract‐only publication |
ACEi ‐ angiotensin‐converting enzyme inhibitor; ANC ‐ absolute neutrophil count; ARB ‐ aldosterone receptor blocker; AZA ‐ azathioprine; BMI ‐ body mass index; BP ‐ blood pressure; CKD‐EPI ‐ Chronic Kidney Disease Epidemiology Collaboration formula; CNI ‐ calcineurin inhibitor; CPA ‐ cyclophosphamide; CrCl ‐ creatinine clearance; CSA ‐ cyclosporin A; DM ‐ diabetes mellitus; eGFR ‐ estimated glomerular filtration rate; ESKD ‐ end‐stage kidney disease; FSGS ‐ focal segmental glomerulosclerosis; GI ‐ gastrointestinal; Hb ‐ haemoglobin; HBV ‐ hepatitis B virus; HBC hepatitis C virus; HCT ‐ haematocrit; HIV ‐ human immunodeficiency virus; HMG‐CoA ‐ hydroxy‐methylglutaryl coenzyme A; M/F ‐ male/female; MCD ‐ minimal change disease; MDRD ‐ modified diet in renal disease; MMF ‐ mycophenolate mofetil; MN ‐ membranous nephropathy; NSAIDS ‐ nonsteroidal anti‐inflammatory drugs; QoL ‐ quality of life; RCT ‐ randomised controlled trial; SC ‐ subcutaneous; SCr ‐ serum creatinine; SD ‐ standard deviation; SRNS ‐ steroid‐resistant nephrotic syndrome; TAC ‐ tacrolimus; UPCR ‐ urine protein:creatinine ratio
Characteristics of excluded studies [ordered by study ID]
| Study | Reason for exclusion |
|---|---|
| Chan 2007 | Wrong population: IMN. The authors had planned to include participants with FSGS or IMN according to the protocol (NCT00404833) However because of difficulty in recruitment of participants with FSGS, the study was not undertaken in participants with FSGS (Information from chief investigator Professor Daniel TM Chan) |
| GloMY 2010 | Terminated study: RCT comparing MMF with prednisolone in patients with FSGS terminated in 2012 because of insufficient enrolment |
| Heering 2004 | Said to be RCT but some participants in the control group (chlorambucil) moved into the experimental group (CSA). Some participants were analysed in both groups. Unclear whether remission of proteinuria in the control group occurred during control therapy or after transfer to experimental therapy |
| Liu 2006 | Terminated study: RCT comparing TAC in patients with FSGS terminated in 2012 because of insufficient enrolment |
| Liu 2016c | Wrong population: RCT but population included patients with several different types of glomerulonephritis and the results for FSGS cannot be separated |
| NCT01451489 | Terminated study: Study comparing TAC with CPA terminated due to inadequate recruitment. 70 patients enrolled of 130 estimated |
| Ren 2013 | Wrong population: RCT includes both patients with steroid‐sensitive and steroid‐resistant disease and the groups cannot be separated |
| Trachtman 2011 | Unclear methodology: phase 1 study of fresolimumab in participants with FSGS. While entry to 2 of 4 groups was randomised, unclear how participants were allocated to the remaining groups |
CPA ‐ cyclophosphamide; CSA ‐ cyclosporin; FSGS ‐ focal segmental glomerulosclerosis; IMN ‐ idiopathic membranous nephropathy; MMF ‐ mycophenolate mofetil; RCT ‐ randomised controlled trial; TAC ‐ tacrolimus
Characteristics of studies awaiting classification [ordered by study ID]
EudraCT2005‐004460‐22.
| Methods |
|
| Participants |
|
| Interventions | Treatment group
Control group
Co‐interventions
|
| Outcomes |
|
| Notes |
|
NCT00801463.
| Methods |
|
| Participants |
|
| Interventions | Treatment group 1
Treatment group 2
|
| Outcomes |
|
| Notes |
|
NCT00956059.
| Methods |
|
| Participants |
|
| Interventions | Treatment group
Control group
|
| Outcomes |
|
| Notes |
|
ACEi ‐ angiotensin‐converting enzyme inhibitors; ALT ‐ alanine aminotransferase; AST ‐ aspartate aminotransferase; AZA ‐ azathioprine; CSA ‐ cyclosporin; DM ‐ diabetes mellitus; eGFR ‐ estimated glomerular filtration rate; FSGS ‐ focal segmental glomerulosclerosis; GI ‐ gastrointestinal; MMF ‐ mycophenolate mofetil; SCr ‐ serum creatinine
Characteristics of ongoing studies [ordered by study ID]
ACTION 2018.
| Study name | ACTION. Safety and effectiveness of propagermanium (CCR2 receptor antagonist) in focal segmental glomerulosclerosis participants receiving irbesartan (AT1R receptor antagonist) to test the hypothesis that simultaneous antagonism of the angiotensin II receptor type 1 (AT1R) and the chemokine receptor 2 (CCR2) is beneficial in patients with primary FSGS |
| Methods |
|
| Participants |
|
| Interventions | Group 1
Group 2
|
| Outcomes |
|
| Starting date | 8 November 2018 |
| Contact information | Dr Simon Roger, Gosford Research |
| Notes | Estimated completion date: June 2020. Confirmed that study completed but results not yet analysed. Information from Dr Simon Roger, chief investigator |
DUPLEX 2019.
| Study name | Study of sparsentan in patients with primary focal segmental glomerulosclerosis (FSGS) (DUPLEX) |
| Methods |
|
| Participants |
|
| Interventions | Intervention
Comparator
|
| Outcomes | Primary outcomes
Secondary outcomes
|
| Starting date | March 29, 2018 |
| Contact information | Study Director: Radko Komers, MD, PhD |
| Notes | Expected completion December 2022 |
NCT03298698.
| Study name | Randomised controlled trial to evaluate rituximab compared with high dose prednisone (standard therapy) in patients with minimal change disease or focal segmental glomerulosclerosis |
| Methods |
|
| Participants | 40 patients aged >18 years
|
| Interventions | Treatment group
Control group
|
| Outcomes | Primary outcome
Secondary outcomes
|
| Starting date | 22 August 2018 |
| Contact information | Jeroen Deegens, MD,PhD. Jeroen.Deegens@radboudumc.nl; +31243614761 |
| Notes | Estimated completion date 22 January 2021 |
PODOCYTE 2017.
| Study name | PODOCYTE: treatment of treatment resistant or treatment intolerant idiopathic focal and segmental glomerulosclerosis |
| Methods |
|
| Participants |
|
| Interventions | Treatment group
Control group
|
| Outcomes |
|
| Starting date | May 16, 2016. Estimated completion date June 2021 |
| Contact information | Susan Vanmeter, Mallinckrodt Pharmaceuticals, Ellicott City,MD; Brad Rovin, Ohio State University Wexner Medical Center, Columbus, Ohio |
| Notes | NCT02633046 |
Trachtman 2018.
| Study name | A Phase II randomised, placebo‐controlled, double‐blind, parallel arms with switchover, pilot study to evaluate the efficacy and safety of intravenous abatacept in treatment resistant nephrotic syndrome (focal segmental glomerulosclerosis/minimal change disease) |
| Methods |
|
| Participants |
|
| Interventions | Group 1
Group 2
Group 3
Group 4
Group 4
|
| Outcomes |
|
| Starting date | 1 March 2016. Actual completion date 28 January 2020. No results available |
| Contact information | Anna Greka: agreka@bwh.harvard.edu |
| Notes | 27 study sites. NCT02592798. Sponsor: Bristol‐Myers Squibb |
TURING 2019.
| Study name | TURING. The use of rituximab in the treatment of nephrotic glomerulonephritis |
| Methods |
|
| Participants |
|
| Interventions | Treatment group
Control group
|
| Outcomes | Primary outcomes
Secondary outcomes
|
| Starting date | 01/11/2018. Estimated completion date 30/12/2025 |
| Contact information | Dr Lisa Willcocks 01223 245151; Dr Megan Griffith 02083835272. Cambridge Clinical Trials Unit: add‐tr.turing@nhs.net |
| Notes | Eudrac 2018‐004611‐50; ISRCTN 16948923 |
ACEi ‐ angiotensin‐converting enzyme inhibitor; ARB ‐ angiotensin receptor blocker; BMI ‐ body mass index; CNI ‐ calcineurin inhibitor; DM ‐ diabetes mellitus; eGFR ‐ estimated glomerular filtration rate; FSGS ‐ focal segmental glomerulosclerosis; MCD ‐ minimal change disease; MMF ‐ mycophenolate mofetil; UPCR ‐ urinary protein:creatinine ratio
Differences between protocol and review
2021: Risk of bias assessment tool has replaced the quality assessment checklist (Braun 2001)
2021: GRADE summary of findings tables have been incorporated
2021: non‐immunosuppressive agents have been included in this update
Contributions of authors
Norbert Braun: Design of initial review published in 2008, literature survey, supervision of review process, writing the systematic review
Frank Schmutzler: Literature survey, data extraction, data analysis, writing the 2008 review
Annalisa Perna: Literature survey, data extraction, critical reading of the 2008 review
Catalina Lange: Literature survey, review of data and manuscript of the 2008 review
Giuseppe Remuzzi: Critical reading and commenting of the 2008 review
Narelle Willis: Literature survey, finalising the initial review in 2008
Aditi Sinha: Data extraction, critical reading of the 2021 update
Elisabeth Hodson: Data extraction, writing of the 2021 update
Tess Cooper: Data extraction, critical reading of the 2021 update
Sources of support
Internal sources
No sources of support provided
External sources
No sources of support provided
Declarations of interest
Elisabeth M Hodson has declared they have no conflict of interest
Aditi Sinha has declared they have no conflict of interest
Tess E Cooper has declared they have no conflict of interest
Edited (no change to conclusions)
References
References to studies included in this review
Bhaumik 2002 {published data only}
- Bhaumik SK, Majumdar A, Barman SC.Comparison of pulse methylprednisolone vs cyclosporin based therapy in steroid resistant focal segmental glomerulosclerosis [abstract]. Indian Journal of Nephrology 2002;12(4):190. [CENTRAL: CN-00460392] [Google Scholar]
Cattran 1999 {published data only}
- Cattran D, Greenwood C, Bernstein K, Hebert L, Pohl M, Maxwell D, et al.Results of a 6 month randomized controlled trial of cyclosporine (T) vs placebo (C) in adults with steroid resistant idiopathic focal segmental glomerulosclerosis (SR-FSGS) [abstract no: 1544]. Journal of the American Society of Nephrology 1995;6(3):415. [CENTRAL: CN-00483444] [Google Scholar]
- Cattran D, Greenwood C, Hebert L, Pohl M, Hunsicker L, Maxwell D, et al.Follow up results from a randomized controlled trial (RCT) of cyclosporine (T) vs placebo (C) in adults with steroid resistant idiopathic focal segmental glomerulosclerosis [abstract no: 1457]. Journal of the American Society of Nephrology 1995;6(3):414. [CENTRAL: CN-00483445] [Google Scholar]
- Cattran D, Greenwood C.Results of a randomized controlled trial (RCT) of cyclosporine (T) vs placebo (C) in adults with biopsy proven steroid resistant idiopathic focal segmental glomerulosclerosis (FSGS) [abstract]. In: ISN XIII International Congress of Nephrology; July 2-6 1995; Madrid, Spain. 1995:266. [CENTRAL: CN-00509124]
- Cattran D, Neogi T, Sharma R, McCarthy ET, Savin VJ.Serial estimates of serum permeability activity and clinical correlates in patients with native kidney focal segmental glomerulosclerosis. Journal of the American Society of Nephrology 2003;14(2):448-53. [MEDLINE: ] [DOI] [PubMed] [Google Scholar]
- Cattran DC, Appel GB, Hebert LA, Hunsicker LG, Pohl MA, Hoy WE, et al.A randomized trial of cyclosporine in patients with steroid-resistant focal segmental glomerulosclerosis. North America Nephrotic Syndrome Study Group. Kidney International 1999;56(6):2220-6. [MEDLINE: ] [DOI] [PubMed] [Google Scholar]
- Cattran DC, Wald R, Brenchley PE, Coupes B, North American Nephrotic Syndrome Group, Genes, Gender and Glomerulonephritis Group.Clinical correlates of serial urinary membrane attack complex estimates in patients with idiopathic membranous nephropathy. Clinical Nephrology 2003;60(1):7-12. [MEDLINE: ] [PubMed] [Google Scholar]
Cho 2019 {published data only}
- Cho ME, Branton MH, Smith DA, Bartlett L, Howard L, Reynolds JC, et al.Open-label clinical trials of oral pulse dexamethasone for adults with idiopathic nephrotic syndrome. American Journal of Nephrology 2019;49(5):377-85. [MEDLINE: ] [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cho ME, Smith D, Fervenza FC, Kopp JB.Pulse dexamethasone over 48 weeks for minimal change nephropathy and focal segmental glomerulosclerosis [abstract no: SA-PO997]. Journal of the American Society of Nephrology 2005;16(Abstracts):775A. [Google Scholar]
Dasgupta 2020 {published data only}
- Dasgupta S, Mondal R, Chakravarty K, Tiwari V, Sahu RK, Pal A, et al.Tacrolimus versus rituximab in adult onset steroid resistant nephrotic syndrome. Journal of the Indian Medical Association 2020;118(6):26-30. [EMBASE: 2004751282] [Google Scholar]
DUET 2017 {published data only}
- Gesualdo L, Lieberman K, Tesar V, Srivastava T, Komers R.Antiproteinuric effect of sparsentan, a dual angiotensin II and endothelin type a receptor antagonist, in patients with primary focal segmental glomerulosclerosis (FSGS): a subgroup analysis of the DUET trial [abstract no: TO042]. Nephrology Dialysis Transplantation 2017;32(Suppl 3):iii97. [EMBASE: 617290819] [Google Scholar]
- Hogan J, Diva U, Murphy E, Rosenberg N, Trachtman H, Komers R.Complete remission of proteinuria in patients with focal segmental glomerulosclerosis treated with sparsentan, a dual endothelin and angiotensin receptor antagonist, in the DUET trial [abstract no: SU-OR38]. Journal of the American Society of Nephrology 2020;31(Abstract Suppl):55. [EMBASE: 633699267] [Google Scholar]
- Hogan JJ, Derebail VK, Murphy E, Raguram PC, Pergola PE, Sanghani NS, et al.Long-term effects of sparsentan, a dual angiotensin and endothelin receptor antagonist in primary focal segmental glomerulosclerosis (FSGS): interim 84-week analysis of the DUET trial [abstract no: FR-OR087]. Journal of the American Society of Nephrology 2018;29(Abstract Suppl):61. [EMBASE: 633737399] [Google Scholar]
- Karol M, Pan-Zhou XR, Tuller SE, Komers R.Sparsentan pharmacokinetics and pharmacodynamics as the basis of dose selection for primary focal segmental glomerular sclerosis (FSGS) [abstract no: SA-PO635]. Journal of the American Society of Nephrology 2017;28(Abstract Suppl):843. [EMBASE: 633698888] [Google Scholar]
- Komers R, Gipson DS, Nelson P, Adler S, Srivastava T, Derebail VK, et al.Efficacy and safety of sparsentan compared with irbesartan in patients with primary focal segmental glomerulosclerosis: randomized, controlled trial design (DUET). Kidney International Reports 2017;2(4):654-64. [MEDLINE: ] [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lieberman K, Meyer KE, Murphy E, Srivastava T, Trachtman H, Komers R.DUET pediatric subset: a phase 2 study evaluating sparsentan in focal segmental glomerulosclerosis (FSGS) [abstract no: IPN11173-84]. Pediatric Nephrology 2019;34(10):2025-6. [Google Scholar]
- Tesar V, Trachtman H, Murphy E, Ferguson B, Komers R.No impact of newly initiated immunosuppressive therapy observed on long-term antiproteinuric effect of sparsentan in focal segmental glomerulosclerosis: interim 84-week analysis of the DUET trial [abstract no: SUN-037]. Kidney International Reports 2019;4(7 Suppl):S168-9. [EMBASE: 2002180135] [Google Scholar]
- Trachtman H, Hogan JJ, Ferguson B, Komers R.Impact of sparsentan on quality of life (QOL) in focal segmental glomerulosclerosis (FSGS) patients in DUET: an interim analysis [abstract no: TH-PO999]. Journal of the American Society of Nephrology 2019;30(Abstract Suppl):380. [EMBASE: 633770962] [Google Scholar]
- Trachtman H, Nelson P, Adler S, Campbell KN, Chaudhuri A, Derebail VK, et al.DUET: A phase 2 study evaluating the efficacy and safety of sparsentan in patients with FSGS. Journal of the American Society of Nephrology 2018;29(11):2745-54. [MEDLINE: ] [DOI] [PMC free article] [PubMed] [Google Scholar]
- Trachtman H, Nelson PJ, Komers R.Efficacy and safety of sparsentan, a dual angiotensin II (Ang II) and endothelin (ET) type A receptor antagonist in patients with focal segmental glomerulosclerosis (FSGS): a phase 2 trial (DUET) [abstract no: HI-OR06]. Journal of the American Society of Nephrology 2016;27(Abstract Suppl):2B. [Google Scholar]
- Trachtman H, Rychlik I, Haws R, Nester C, Fornoni A, Komers R.Newly administered immunosuppressive therapy (IST) has no impact on long-term antiproteinuric effect of sparsentan (SPAR), a dual angiotensin and endothelin receptor antagonist, in patients with primary focal segmental glomerulosclerosis (FSGS): interim analysis of the DUET trial [abstract no: FP129]. Nephrology Dialysis Transplantation 2018;33(Suppl 1):i20. [EMBASE: 622606410] [Google Scholar]
- Trachtman H, Rychlik I, Haws RM, Nester CM, Fornoni A, Komers R.Long-term effect of sparsentan (SPAR), a dual angiotensin and endothelin receptor antagonist, on proteinuria in patients with primary FSGS: interim analysis of the DUET trial [abstract no: FR-OR026]. Journal of the American Society of Nephrology 2017;28(Abstract Suppl):43-4. [EMBASE: 633700131] [Google Scholar]
FONT I 2009 {published data only}
- Gassman JJ, Trachtman H, Gipson D, Friedman A, Greene T, Vento S, et al.Implementing a second randomized trial enrolling mid-study treatment failures from the Focal Segmental Glomerular Sclerosis (FSGS) Clinical Trial [abstract no: 33]. Clinical Trials 2007;4(4):382. [CENTRAL: CN-00794067] [Google Scholar]
- Joy MS, Gipson DS, Dike M, Powell L, Thompson A, Vento S, et al.Phase I trial of rosiglitazone in FSGS: I. Report of the FONT Study Group. Clinical Journal of the American Society of Nephrology: CJASN 2009;4(1):39-47. [MEDLINE: ] [DOI] [PMC free article] [PubMed] [Google Scholar]
- Joy MS, Gipson DS, Powell L, MacHardy J, Jennette JC, Vento S, et al.Phase 1 trial of adalimumab in Focal Segmental Glomerulosclerosis (FSGS): II. Report of the FONT (Novel Therapies for Resistant FSGS) study group. American Journal of Kidney Diseases 2010;55(1):50-60. [MEDLINE: ] [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ternant D, Paintaud G, Trachtman H, Gipson DS, Joy MS.A possible influence of age on absorption and elimination of adalimumab in focal segmental glomerulosclerosis (FSGS). European Journal of Clinical Pharmacology 2016;72(2):253-5. [MEDLINE: ] [DOI] [PubMed] [Google Scholar]
- Trachtman H, Gipson D, Vento S, Thompson A, Dike M.Novel therapies in resistant FSGS: preliminary results [abstract no: 879 (P)]. Pediatric Nephrology 2007;22(9):1629. [CENTRAL: CN-00794725] [Google Scholar]
FONT II 2011 {published data only}
- Trachtman H, Vento S, Gipson D, Wickman L, Gassman J, Joy M, et al.Novel therapies for resistant focal segmental glomerulosclerosis (FONT) phase II clinical trial: study design. BMC Nephrology 2011;12:8. [MEDLINE: ] [DOI] [PMC free article] [PubMed] [Google Scholar]
- Trachtman H, Vento S, Herreshoff E, Radeva M, Gassman J, Stein DT, et al.Efficacy of galactose and adalimumab in patients with resistant focal segmental glomerulosclerosis: report of the FONT clinical trial group. BMC Nephrology 2015;16:111. [MEDLINE: ] [DOI] [PMC free article] [PubMed] [Google Scholar]
FSGS‐CT 2011 {published data only}
- D'Agati VD, Alster JM, Jennette JC, Thomas DB, Pullman J, Savino DA, et al.Association of histologic variants in FSGS clinical trial with presenting features and outcomes. Clinical Journal of the American Society of Nephrology: CJASN 2013;8(3):399-406. [MEDLINE: ] [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ferris M, Norwood V, Radeva M, Gassman JJ, Al-Uzri A, Askenazi D, et al.Patient recruitment into a multicenter randomized clinical trial for kidney disease: report of the Focal Segmental Glomerulosclerosis Clinical Trial (FSGS CT). Clinical and Translational Science 2013;6(1):13-20. [MEDLINE: ] [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gassman J, Fine R, Gipson D, Greene T, Hogg R, Kaskel F, et al.A preliminary report of the Focal Segmental Glomerulosclerosis Clinical Trial (FSGS-CT) [abstract no: F-PO1984]. Journal of the American Society of Nephrology 2008;19(Abstracts Issue):559A. [CENTRAL: CN-00790759] [Google Scholar]
- Gassman JJ, Trachtman H, Gipson D, Friedman A, Greene T, Vento S, et al.Implementing a second randomized trial enrolling mid-study treatment failures from the Focal Segmental Glomerular Sclerosis (FSGS) Clinical Trial [abstract no: 33]. Clinical Trials 2007;4(4):382. [CENTRAL: CN-00794067] [Google Scholar]
- Gipson DS, Radeva M, Fine R, Friedman A, Gassman J, Greene T et al.Focal Segmental Glomerulosclerosis Clinical Trial (FSGS-CT) study cohort [abstract no: TH-PO165]. Journal of the American Society of Nephrology 2009;20(Abstracts issue):147A. [CENTRAL: CN-00793840] [Google Scholar]
- Gipson DS, Trachtman H, Kaskel FJ, Greene TH, Radeva MK, Gassman JJ, et al.Clinical trial of focal segmental glomerulosclerosis in children and young adults. Kidney International 2011;80(8):868-78. [MEDLINE: ] [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gipson DS, Trachtman H, Kaskel FJ, Radeva MK, Gassman J, Greene TH, et al.Clinical trials treating focal segmental glomerulosclerosis should measure patient quality of life. Kidney International 2011;79(6):678-85. [MEDLINE: ] [DOI] [PMC free article] [PubMed] [Google Scholar]
- Greene T, Gassman J, Gipson D, Hogg R, Kaskel F, Moxey-Mims M, et al.Multicenter randomized clinical trial of FSGS in children and young adults [abstract no: F-PO1014]. Journal of the American Society of Nephrology 2005;16(Abstract Suppl):558A. [CENTRAL: CN-00794633] [Google Scholar]
- Hogg RJ, Friedman A, Greene T, Radeva M, Budisavljevic MN, Gassman J, et al.Renal function and proteinuria after successful immunosuppressive therapies in patients with FSGS. Clinical Journal of the American Society of Nephrology: CJASN 2013;8(2):211-8. [MEDLINE: ] [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kopp JB, Winkler CA, Zhao X, Radeva MK, Gassman JJ, D'Agati VD, et al.Clinical features and histology of apolipoprotein L1-associated nephropathy in the FSGS Clinical Trial. Journal of the American Journal of Nephrology 2015;26(6):1443-8. [MEDLINE: ] [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kopp JB, Zhao X, Winkler CA, Woroniecki R, Radeva M, Gassman JJ, et al.APOL1 variant-associated FSGS is more aggressive but manifests similar response to cyclosporine and mycophenolate compared to others forms of primary FSGS [abstract no: FR-OR050]. Journal of the American Society of Nephrology 2012;23(Abstract Suppl):41A. [Google Scholar]
- Middleton JP, Yorgin P, Gipson D, Radeva M, Fine R, Friedman A et al.Prevalence of blood pressure (BP) control in the Focal Segmental Glomerulosclerosis Clinical Trial (FSGS-CT) study cohort [abstract no: TH-PO168]. Journal of the American Society of Nephrology 2009;20(Abstracts issue):148A. [CENTRAL: CN-00795224] [Google Scholar]
- Radeva M, McMahan J, Gassman J.Optimizing logistics under a three level administration system: coordination of the Focal Segmental Glomerular Sclerosis (FSGS) trial [abstract no: P63]. Clinical Trials 2007;4(4):417-8. [CENTRAL: CN-00794883] [Google Scholar]
- Radeva M, Trachtman H, Fine R, Friedman A, Gassman J, Gipson D, et al.Measuring quality of life (QOL) in children and young adults in the Focal Segmental Glomerulosclerosis Clinical Trial (FSGS-CT) [abstract no: P45]. Clinical Trials 2010;7(4):471. [EMBASE: 70462847] [Google Scholar]
- Sethna CB, Boone V, Kwok J, Jun D, Trachtman H.Adiponectin in children and young adults with focal segmental glomerulosclerosis. Pediatric Nephrology 2015;30(11):1977-85. [MEDLINE: ] [DOI] [PubMed] [Google Scholar]
- Trachtman H, Devarajan P, Bennet MR, Thurman JM, Radeva M, Gipson DS, et al.Neutrophil gelatinase-associated lipocalcin (NGAL) and alternate pathway of complement (APC) factors as biomarkers of response to treatment in patients with focal segmental glomerulosclerosis (FSGS) [abstract no: TH-PO368]. Journal of the American Society of Nephrology 2011;22(Abstract Suppl):197A. [Google Scholar]
- Trachtman H, Fine R, Friedman A, Grassman J, Gipson D, Greene T et al.Quality of life in children with focal segmental glomerulosclerosis (FSGS): baseline findings. Report of the FSGS-Clinical Trial (CT) [abstract no: TH-PO164]. Journal of the American Society of Nephrology 2009;20(Abstracts Issue):147A. [CENTRAL: CN-00795355] [Google Scholar]
- Troost JP, Trachtman H, Kaskel FJ, Friedman AL, Moxey-Mims MM, Gassman JJ, et al.Incremental reduction of proteinuria and kidney survival in FSGS [abstract no: TH-PO998]. Journal of the American Society of Nephrology 2019;30(Abstract Suppl):379-80. [EMBASE: 633770892] [Google Scholar]
- Troost JP, Trachtman H, Spino C, Kaskel FJ, Friedman A, Moxey MM, et al.Proteinuria reduction and kidney survival in focal segmental glomerulosclerosis. American Journal of Kidney Diseases 2021;77(2):216-25. [MEDLINE: ] [DOI] [PMC free article] [PubMed] [Google Scholar]
- Woroniecki R, Du Z, Radeva M, Gassman JJ, Gipson DS, Trachtman H, et al.Osteopontin (OPN) and transforming growth factor beta 1 (TGFb1) in steroid resistant primary focal segmental glomerulosclerosis (FSGS), FSGS-clinical trial (FSGS-CT) experience [abstract no: SA-PO390]. Journal of the American Society of Nephrology 2012;23(Abstract Suppl):727A. [Google Scholar]
Imbasciati 1980 {published data only}
- Imbasciati E, Cagnoli L, Case N, Pasquali S, Di Filippo G, Limido D, et al.Controlled study of treatment of steroids and chlorambucil, in alternate months, for membranous nephropathy and focal glomerulosclerosis. Preliminary evaluation of the results [Studio controllato di un trattamento a mesi alterni di steroidi e clorambucil nella nefropatia membranosa e nella glomerulosclerosi focale. Valutazione preliminare dei risultati]. Minerva Nefrologica 1980;27(4):571-5. [MEDLINE: ] [PubMed] [Google Scholar]
LUMINA‐1 2018 {published data only}
- Staehr P.A study of CCX140-B in subjects with FSGS [A randomized, double-blind, placebo-controlled dose-ranging study to evaluate the safety and efficacy of CCX140-B in subjects with focal segmental glomerulosclerosis (FSGS)]. www.clinicaltrials.gov/show/nct03536754 (first received 25 May 2018).
Ponticelli 1993a {published data only}
- Ponticelli C, Rizzoni G, Edefonti A, Altieri P, Rivolta E, Rinaldi S, et al.A randomized trial of cyclosporine in steroid-resistant idiopathic nephrotic syndrome. Kidney International 1993;43(6):1377-84. [MEDLINE: ] [DOI] [PubMed] [Google Scholar]
Quintaes 2000 {published data only}
- Quintaes PS, Barros RT, Noronha IL, Saldanha LB, Woronik V.FSGS: clinical response and urinary excretion of RGF-Beta after cyclosporin a treatment and angiotensin II blockade [abstract no: A0640]. Journal of the American Society of Nephrology 2001;12(Program & Abstracts):122A. [CENTRAL: CN-00447313] [Google Scholar]
- Quintaes PS, Barros RT, Woronik V, Sabbaga E, Machado MM.The association of cyclosporin and angiotensin II receptor antagonist - valsartan, in the treatment of patients with focal and segmental glomerulosclerosis [abstract no: A0518]. Journal of the American Society of Nephrology 2000;11(Sept):95-6A. [CENTRAL: CN-00796164] [Google Scholar]
Senthil Nayagam 2008 {published data only}
- Senthil Nagayam L, Ganguli A, Rathi M, Kohli HS, Gupta KL, Joshi K, et al.Mycophenolate mofetil or standard therapy for membranous nephropathy and focal segmental glomerulosclerosis: a pilot study. Nephrology Dialysis Transplantation 2008;23(6):1926-30. [MEDLINE: ] [DOI] [PubMed] [Google Scholar]
Vincenti 2017 {published data only}
- Tumlin JA, Vincenti F, Fervenza FC, Campbell K, Diaz Encarnacion MM, Praga M, et al.A phase 2, double-blind, randomized study of fresolimumab or placebo in patients with steroid-resistant primary focal segmental glomerulosclerosis [abstract no: SA-PO1095]. Journal of the American Society of Nephrology 2015;26(Abstract Suppl):B4. [CENTRAL: CN-01657786] [Google Scholar]
- Vincenti F, Fervenza FC, Campbell KN, Diaz M, Gesualdo L, Nelson P, et al.A phase 2, double-blind, placebo-controlled, randomized study of fresolimumab in patients with steroid-resistant primary focal segmental glomerulosclerosis. Kidney International Reports 2017;2(5):800-10. [MEDLINE: ] [DOI] [PMC free article] [PubMed] [Google Scholar]
Walker 1990 {published data only}
- Walker RG, Kincaid-Smith P.Cyclosporin A (Cy-A) treatment in focal and segmental hyalinosis and sclerosis (FSHS): a controlled trial [abstract no: 17]. Kidney International 1989;36(6):1162. [CENTRAL: CN-00509552] [Google Scholar]
- Walker RG, Kincaid-Smith P.The effect of treatment of corticosteroid-resistant idiopathic (primary) focal and segmental hyalinosis and sclerosis (focal glomerulosclerosis) with ciclosporin. Nephron 1990;54(2):117-21. [MEDLINE: ] [DOI] [PubMed] [Google Scholar]
References to studies excluded from this review
Chan 2007 {published data only}
- Chan TM, Lin AW, Tang SC, Qian JQ, Lam MF, Ho YW, et al.Prospective controlled study on mycophenolate mofetil and prednisolone in the treatment of membranous nephropathy with nephrotic syndrome. Nephrology 2007;12(6):576-81. [MEDLINE: ] [DOI] [PubMed] [Google Scholar]
GloMY 2010 {published data only}11937028
- Harper L.Randomised pilot trial of myfortic for the treatment of primary proteinuric glomerulonephritis. www.clinicaltrialsregister.eu/ctr-search/trial/2009-016003-26/GB (accessed 5 January 2022).
Heering 2004 {published data only}
- Heering P, Braun N, Müllejans R, Ivens K, Zäuner I, Fünfstück R, et al.Cyclosporine A and chlorambucil in the treatment of idiopathic focal segmental glomerulosclerosis. American Journal of Kidney Diseases 2004;43(1):10-8. [MEDLINE: ] [DOI] [PubMed] [Google Scholar]
- Heering P, Mullejans R, Braun N, Zauner I, Funfstuck R, Keller F, et al.Therapy of FSGS with nephrotic syndrome - 3 years follow up of a prospective randomised multicenter study [abstract]. Nephrology Dialysis Transplantation 1999;14(9):A98. [CENTRAL: CN-00509229] [Google Scholar]
- Heering P, Mullejans R, Braun N, Zauner I, Funfstuck R, Keller F, et al.Therapy of FSGS with nephrotic syndrome - 3 years follow up of a prospective randomised multicenter study [abstract no: A0416]. Journal of the American Society of Nephrology 1997;8(Program & Abstracts):87A. [CENTRAL: CN-00445685] [Google Scholar]
- Heering P, Mullejans R, Braun N, Zauner I, Schollmeyer P, Funfstuck R.Therapy of FSGS with nephrotic syndrome - 3 years follow up of a prospective randomized multicenter study [abstract]. Nephrology Dialysis Transplantation 1996;11(6):A105. [CENTRAL: CN-00261254] [Google Scholar]
Liu 2006 {published data only}
- Liu Z.Tacrolimus treatment of patients with idiopathic focal segmental glomerulosclerosis. www.clinicaltrials.gov/ct2/show/NCT00302536 (first received 14 March 2006). [CENTRAL: CN-00583312]
Liu 2016c {published data only}
- Liu Y, Qu X, Chen W, Zhang Y, Liu L.Efficacy of leflunomide combined with prednisone in the treatment of refractory nephrotic syndrome. Renal Failure 2016;38(10):1616-21. [MEDLINE: ] [DOI] [PubMed] [Google Scholar]
NCT01451489 {published data only}
- Liu ZH.The efficacy and safety of tacrolimus in treated refractory focal segmental glomerulosclerosis (FSGS) [A randomized, multicentre, prospective study on the tacrolimus (FK506) for focal segmental glomerulosclerosis]. www.clinicaltrials.gov/show/NCT01451489 (first received 13 October 2011).
Ren 2013 {published data only}
- Ren H, Shen P, Li X, Pan X, Zhang W, Chen N.Tacrolimus versus cyclophosphamide in steroid-dependent or steroid-resistant focal segmental glomerulosclerosis: a randomized controlled trial. American Journal of Nephrology 2013;37(1):84-90. [MEDLINE: ] [DOI] [PubMed] [Google Scholar]
Trachtman 2011 {published data only}
- Trachtman H, Fervenza FC, Gipson DS, Heering P, Jayne DR, Peters H, et al.A phase 1, single-dose study of fresolimumab, an anti-TGF-beta antibody, in treatment-resistant primary focal segmental glomerulosclerosis. Kidney International 2011;79(11):1236-43. [MEDLINE: ] [DOI] [PMC free article] [PubMed] [Google Scholar]
References to studies awaiting assessment
EudraCT2005‐004460‐22 {published data only}
- EudraCT2005-004460-22.Multicenter, pilot, open, randomized, parallel group study to evaluate the association of Neoral with ACE-inhibitors as compared to corticosteroids with ACE-inhibitors in the treatment of de novo nephrotic syndrome due to focal segmental glomerulosclerosis. www.clinicaltrialsregister.eu/ctr-search/search?query=eudract_number:2005-004460-22 (first received 12 May 2006).
NCT00801463 {published data only}
- Liu ZH.Prednisone plus Tripterygium wilfordii treatment of adult patients with idiopathic focal segmental glomerulosclerosis. www.clinicaltrials.gov/show/NCT00801463 (first received 3 December 2008).
NCT00956059 {published data only}
- Gui B.Therapeutic effect of low-dose prednisone combined with MMF and FK506 in focal segmental glomerulosclerosis. www.clinicaltrials.gov/show/NCT00956059 (first received 11 August 2009).
References to ongoing studies
ACTION 2018 {published data only}
- Roger S, Packham D, Shephard R, Power D.Action (AT1R and CCR2 targets for inflammatory nephrosis) phase 2a trial for focal segmental glomerulusclerosis [abstract]. Nephrology 2018;23(Suppl 3):78. [EMBASE: 623841909] [Google Scholar]
DUPLEX 2019 {published data only}
- Komers R, Diva U, Inrig JK, Loewen A, Trachtman H, Rote WE.Study design of the phase 3 sparsentan versus irbesartan (DUPLEX) study in patients with focal segmental glomerulosclerosis. Kidney International Reports 2020;5(4):494-502. [MEDLINE: ] [DOI] [PMC free article] [PubMed] [Google Scholar]
- Trachtman H, Diva U, Inrig J, Loewen A, Rote W, Komers R.Sparsentan for treatment of patients with focal segmental glomerulosclerosis (FSGS): design of the phase 3 DUPLEX study [abstract no: 373]. American Journal of Kidney Diseases 2019;73(5):740-1. [EMBASE: 2001804388] [Google Scholar]
NCT03298698 {published data only}
- Deegens HK, Wetzels JF.Efficacy of rituximab in comparison to continued corticosteroid treatment in idiopathic nephrotic syndrome [Efficacy of rituximab in comparison to continued corticosteroid treatment in idiopathic nephrotic syndrome unresponsive to 8 weeks of high dose prednisone]. www.ClinicalTrials.gov/show/NCT03298698 (first received 2 October 2017).
PODOCYTE 2017 {published data only}
- Tumlin JA, Rovin BH, Lafayette RA, Zhao E, Becker P, Patel L, et al.Treatment of proteinuria due to treatment resistant or treatment intolerant idiopathic focal segmental glomerulosclerosis: a 2 part prospective study of H.P.acthar gel ( PODOCYTE) [abstract no: PUB515]. Journal of the American Society of Nephrology 2017;28(Abstract Suppl):1095. [EMBASE: 633704574] [Google Scholar]
Trachtman 2018 {published data only}
- Trachtman H, Gipson DS, Somers M, Spino C, Adler S, Holzman L, et al.Randomized clinical trial design to assess abatacept in resistant nephrotic syndrome. Kidney International Reports 2018;3(1):115-21. [MEDLINE: ] [DOI] [PMC free article] [PubMed] [Google Scholar]
TURING 2019 {published data only}16948923
- Qian W, Machin A, Griffith M, Willcocks L.Analysis of duration of remission as an intention-to-treat analysis with application to the TURING trial [abstract no: P-194]. Trials 2019;20(Suppl 1):56. [EMBASE: 629759857] [Google Scholar]
- Willcocks L, Griffith M.TURING - the use of rituximab in the treatment of nephrotic glomerulonephritis - clinical trial protocol V2.0. https://njl-admin.nihr.ac.uk/document/download/2030929 (accessed 5 January 2022).
Additional references
Bohle 1986
- Bohle GH.Die Niere. Stuttgart: Schattauer, 1986. [Google Scholar]
Burgess 1999
- Burgess E.Management of focal segmental glomerulosclerosis: evidence based recommendations. Kidney International - Supplement 1999;70:S26-32. [MEDLINE: ] [DOI] [PubMed] [Google Scholar]
Chao 2020
- Chao YC, Trachtman T, Gipson GS, Spino C, Braun TM, Kidwell KM.Dynamic treatment regimens in small n, sequential, multiple assignment, randomized trials: an application in focal segmental glomerulosclerosis. Contemporary Clinical Trials 2020;92:105989. [MEDLINE: ] [DOI] [PMC free article] [PubMed] [Google Scholar]
De Vriese 2018
- De Vriese AN, Sethi S, Nath KA, Glassock RJ, Fervenza FC.Differentiating primary, genetic and secondary FSGS in adults: a clinicopathologic approach. Journal of the American Society of Nephrology 2018;29(3):759-74. [MEDLINE: ] [DOI] [PMC free article] [PubMed] [Google Scholar]
GRADE 2008
- Guyatt GH, Oxman AD, Vist GE, Kunz R, Falck-Ytter Y, Alonso-Coello P, et al.GRADE: an emerging consensus on rating quality of evidence and strength of recommendations. BMJ 2008;336(7650):924-6. [MEDLINE: ] [DOI] [PMC free article] [PubMed] [Google Scholar]
GRADE 2011
- Guyatt G, Oxman AD, Akl EA, Kunz R, Vist G, Brozek J, et al.GRADE guidelines: 1. Introduction-GRADE evidence profiles and summary of findings tables. Journal of Clinical Epidemiology 2011;64(4):383-94. [MEDLINE: ] [DOI] [PubMed] [Google Scholar]
Higgins 2003
- Higgins JP, Thompson SG, Deeks JJ, Altman DG.Measuring inconsistency in meta-analyses. BMJ 2003;327(7414):557-60. [MEDLINE: ] [DOI] [PMC free article] [PubMed] [Google Scholar]
Higgins 2020
- Higgins JP, Thomas J, Chandler J, Cumpston M, Li T, Page MJ, et al (editors).Cochrane Handbook for Systematic Reviews of Interventions version 6.1 (updated September 2020). Cochrane, 2020. Available from www.training.cochrane.org/handbook.
KDIGO 2012
- KDIGO Clinical Practice Guidelines for Glomerulonephritis. Kidney International Supplements 2012;2(2):139-274. [Google Scholar]
KDIGO 2021
- Kidney Disease: Improving Global Outcomes (KDIGO) Glomerular Diseases Work Group.KDIGO 2021 clinical practice guideline for the management of glomerular diseases. Kidney International 2021;100(Suppl 4):S1-276. [DOI] [PubMed] [Google Scholar]
Kopp 2011
- Kopp JB, Nelson GW, Sampath K, Johnson RC, Genovese G, An P, et al.APOL1 genetic variants in focal segmental glomerulosclerosis and HIV-associated nephropathy. Journal of the American Society of Nephrology 2011;22(11):2129-37. [MEDLINE: ] [DOI] [PMC free article] [PubMed] [Google Scholar]
Kopp 2015
- Kopp JB, Winkler CA, Zhao X, Radeva MK, Gassman JJ, D’Agati VD, et al.Clinical features and histology of apolipoprotein L1-associated nephropathy in the FSGS clinical trial. Journal of the American Society of Nephrology 2015;26(6):1443-8. [MEDLINE: ] [DOI] [PMC free article] [PubMed] [Google Scholar]
Meyrier 1999
- Meyrier A.Treatment of primary focal segmental glomerulosclerosis. NephrologyDialysis Transplantation 1999;14 Suppl 3:74-8. [MEDLINE: ] [DOI] [PubMed] [Google Scholar]
Schünemann 2020a
- Schünemann HJ, Higgins JP, Vist GE, Glasziou P, Akl EA, Skoetz N, et al.Chapter 14: Completing ‘Summary of findings’ tables and grading the certainty of the evidence. In: Higgins JPT, Thomas J, Chandler J, Cumpston M, Li T, Page MJ, Welch VA (editors). Cochrane Handbook for Systematic Reviews of Interventions version 6.1 (updated September 2020). Cochrane, 2020. Available from www.training.cochrane.org/handbook.
Schünemann 2020b
- Schünemann HJ, Vist GE, Higgins JP, Santesso N, Deeks JJ, Glasziou P, et al.Chapter 15: Interpreting results and drawing conclusions. In: Higgins JPT, Thomas J, Chandler J, Cumpston M, Li T, Page MJ, Welch VA (editors). Cochrane Handbook for Systematic Reviews of Interventions version 6.1 (updated September 2020). Cochrane, 2020. Available from www.training.cochrane.org/handbook.
Shabaka 2020
- Shabaka A, Tato Ribera A, Fernandez-Juarez G.Focal segmental glomerulosclerosis: state-of-the-art and clinical perspective. Nephron 2020;144(9):413-27. [MEDLINE: ] [DOI] [PubMed] [Google Scholar]
Troost 2021
- Troost JP, Trachtman H, Spino C, Kaskel FJ, Friedman A, Moxey-Mims MM, et al.Proteinuria reduction and kidney survival in focal and segmental glomerulosclerosis. American Journal of Kidney Diseases 2021;77(2):216-25. [MEDLINE: ] [DOI] [PMC free article] [PubMed] [Google Scholar]
Troyanov 2005
- Troyanov S, Wall CA, Miller JA, Scholey JW, Cattran DC, Toronto Glomerulonephritis Registry Group.Focal and segmental glomerulosclerosis: definition and relevance of a partial remission. Journal of the American Society of Nephrology 2005;16(4):1061-8. [MEDLINE: ] [DOI] [PubMed] [Google Scholar]
References to other published versions of this review
Braun 2001
- Braun N, Schmutzler F, Perna A, Pfaff A, Bagriy A, Remuzzi G.Immunosuppressive treatment for focal segmental glomerulosclerosis in adults with nephrotic syndrome. Cochrane Database of Systematic Reviews 2001, Issue 1. Art. No: CD003233. [DOI: 10.1002/14651858.CD003233] [DOI] [PMC free article] [PubMed] [Google Scholar]
Braun 2008
- Braun N, Schmutzler F, Lange C, Perna A, Remuzzi G, Willis NS.Immunosuppressive treatment for focal segmental glomerulosclerosis in adults. Cochrane Database of Systematic Reviews 2008, Issue 3. Art. No: CD003233. [DOI: 10.1002/14651858.CD003233.pub2] [DOI] [PMC free article] [PubMed] [Google Scholar]


