Abstract
Background
Antiplatelet agents are widely used to prevent cardiovascular events. The risks and benefits of antiplatelet agents may be different in people with chronic kidney disease (CKD) for whom occlusive atherosclerotic events are less prevalent, and bleeding hazards might be increased. This is an update of a review first published in 2013.
Objectives
To evaluate the benefits and harms of antiplatelet agents in people with any form of CKD, including those with CKD not receiving renal replacement therapy, patients receiving any form of dialysis, and kidney transplant recipients.
Search methods
We searched the Cochrane Kidney and Transplant Register of Studies up to 13 July 2021 through contact with the Information Specialist using search terms relevant to this review. Studies in the Register are identified through searches of CENTRAL, MEDLINE, and EMBASE, conference proceedings, the International Clinical Trials Register (ICTRP) Search Portal and ClinicalTrials.gov.
Selection criteria
We selected randomised controlled trials of any antiplatelet agents versus placebo or no treatment, or direct head‐to‐head antiplatelet agent studies in people with CKD. Studies were included if they enrolled participants with CKD, or included people in broader at‐risk populations in which data for subgroups with CKD could be disaggregated.
Data collection and analysis
Four authors independently extracted data from primary study reports and any available supplementary information for study population, interventions, outcomes, and risks of bias. Risk ratios (RR) and 95% confidence intervals (CI) were calculated from numbers of events and numbers of participants at risk which were extracted from each included study. The reported RRs were extracted where crude event rates were not provided. Data were pooled using the random‐effects model. Confidence in the evidence was assessed using the Grading of Recommendations Assessment, Development and Evaluation (GRADE) approach.
Main results
We included 113 studies, enrolling 51,959 participants; 90 studies (40,597 CKD participants) compared an antiplatelet agent with placebo or no treatment, and 29 studies (11,805 CKD participants) directly compared one antiplatelet agent with another. Fifty‐six new studies were added to this 2021 update. Seven studies originally excluded from the 2013 review were included, although they had a follow‐up lower than two months.
Random sequence generation and allocation concealment were at low risk of bias in 16 and 22 studies, respectively. Sixty‐four studies reported low‐risk methods for blinding of participants and investigators; outcome assessment was blinded in 41 studies. Forty‐one studies were at low risk of attrition bias, 50 studies were at low risk of selective reporting bias, and 57 studies were at low risk of other potential sources of bias.
Compared to placebo or no treatment, antiplatelet agents probably reduces myocardial infarction (18 studies, 15,289 participants: RR 0.88, 95% CI 0.79 to 0.99, I² = 0%; moderate certainty). Antiplatelet agents has uncertain effects on fatal or nonfatal stroke (12 studies, 10.382 participants: RR 1.01, 95% CI 0.64 to 1.59, I² = 37%; very low certainty) and may have little or no effect on death from any cause (35 studies, 18,241 participants: RR 0.94, 95 % CI 0.84 to 1.06, I² = 14%; low certainty). Antiplatelet therapy probably increases major bleeding in people with CKD and those treated with haemodialysis (HD) (29 studies, 16,194 participants: RR 1.35, 95% CI 1.10 to 1.65, I² = 12%; moderate certainty). In addition, antiplatelet therapy may increase minor bleeding in people with CKD and those treated with HD (21 studies, 13,218 participants: RR 1.55, 95% CI 1.27 to 1.90, I² = 58%; low certainty). Antiplatelet treatment may reduce early dialysis vascular access thrombosis (8 studies, 1525 participants) RR 0.52, 95% CI 0.38 to 0.70; low certainty). Antiplatelet agents may reduce doubling of serum creatinine in CKD (3 studies, 217 participants: RR 0.39, 95% CI 0.17 to 0.86, I² = 8%; low certainty). The treatment effects of antiplatelet agents on stroke, cardiovascular death, kidney failure, kidney transplant graft loss, transplant rejection, creatinine clearance, proteinuria, dialysis access failure, loss of primary unassisted patency, failure to attain suitability for dialysis, need of intervention and cardiovascular hospitalisation were uncertain. Limited data were available for direct head‐to‐head comparisons of antiplatelet drugs, including prasugrel, ticagrelor, different doses of clopidogrel, abciximab, defibrotide, sarpogrelate and beraprost.
Authors' conclusions
Antiplatelet agents probably reduced myocardial infarction and increased major bleeding, but do not appear to reduce all‐cause and cardiovascular death among people with CKD and those treated with dialysis. The treatment effects of antiplatelet agents compared with each other are uncertain.
Plain language summary
Are anti‐blood clotting drugs beneficial for people with chronic kidney disease?
What is the issue? People with chronic kidney disease (CKD) have an increased risk of heart disease that can block the blood supply to the heart or brain causing a heart attack or stroke. Drugs that prevent blood clots from forming (antiplatelet agents) can prevent deaths caused by clots in arteries in the general adult population. However, there may be fewer benefits for people who have CKD, because blood clots in arteries is a less common cause of death or reason to be admitted to hospital compared with heart failure or sudden death in these people. People with CKD also have an increased tendency for bleeding due to changes in how the blood clots. Antiplatelet agents may therefore be more hazardous when CKD is present.
What did we do?
This updated review evaluated the benefits and harms of antiplatelet agents to prevent cardiovascular disease and death, and the impact on dialysis vascular access (fistula or graft) function in people who have CKD. We identified 90 studies comparing antiplatelet agents with placebo or no treatment and 29 studies directly comparing one antiplatelet agent with another.
What did we find? Antiplatelet agents probably prevent heart attacks, but do not clearly reduce death or stroke. Treatment with these agents may increase the risk of major and minor bleeding. Clotting of dialysis access was prevented with antiplatelet agents.
Conclusions The benefits of antiplatelet agents for people with CKD is probably limited to the prevention of a heart attack. The treatment does not appear to prevent stroke or death and probably incurs excess serious bleeding that may require hospital admission or transfusion.
Summary of findings
Summary of findings 1. Antiplatelet agents versus control for chronic kidney disease.
| Antiplatelet agents versus control for chronic kidney disease | |||||
|
Patient or population: people with chronic kidney disease (predialysis (GFR 15 to 60 mL/min/1.73 m²), HD, PD, transplant recipients) Settings: all settings involving people with any stage of CKD Intervention: antiplatelet agents (abciximab, aspirin, beraprost sodium, cilostazol, clopidogrel, dypiridamole, eptidifibatide, pentoxifylline, picotamide, prasugrel, prostacyclin, sarpogrelate, sulphinpyrazone, ticlopidine, tirofiban, alone or in combination) Comparison: placebo or no treatment | |||||
| Outcomes | Anticipated absolute effects* (95% CI) | Relative effect (95% CI) | No. of participants (RCTs) | Certainty of the evidence (GRADE) | |
| Risk with control | Risk with antiplatelet agents | ||||
|
Fatal or nonfatal myocardial infarction Follow‐up: 3 to 61.2 months (median 12 months) |
All patients (predialysis, dialysis, transplant recipients) | RR 0.88 (0.79 to 0.99) | 15,289 (18) |
moderate 1 ⊕⊕⊕⊝ |
|
| 70 per 1,000 | 8 fewer per 1,000 (1 to 15 fewer) | ||||
| CKD patients (GFR 15 to 60 mL/min/1.73 m²) | RR 0.85 (0.74 to 0.99) | 11,912 (11) |
moderate 1 ⊕⊕⊕⊝ |
||
| 85 per 1,000 | 13 fewer per 1,000 (1 to 22 fewer) | ||||
| HD patients | RR 0.83 (0.49 to 1.41) | 2929 (6) |
moderate 1 ⊕⊕⊕⊝ |
||
| 20 per 1,000 | 3 fewer per 1,000 (10 fewer to 8 more) | ||||
|
Fatal or nonfatal stroke Follow‐up: 3 to 61.2 months (median 12 months) |
All patients (predialysis, dialysis, transplant recipients) | RR 1.01 (0.64 to 1.59) | 10,382 (12) |
very low 1,2,3 ⊕⊝⊝⊝ |
|
| 20 per 1,000 | 0 per 1,000 (7 fewer to 12 more) | ||||
| CKD patients (GFR 15 to 60 mL/min/1.73 m²) | RR 1.06 (0.64 to 1.74) | 7062 (5) |
very low 1,2,3 ⊕⊝⊝⊝ |
||
| 25 per 1,000 | 2 more per 1,000 (9 fewer to 19 more) | ||||
| HD patients | RR 0.62 (0.15 to 2.60) | 2872 (6) |
very low 1,2,3 ⊕⊝⊝⊝ |
||
| 10 per 1,000 | 4 fewer per 1,000 (8 fewer to 16 more) | ||||
|
Death (any cause) Follow‐up: 0.9 to 88.2 months (median 12 months) |
All patients (predialysis, dialysis, transplant recipients) | RR 0.94 (0.84 to 1.06) | 18,241 (35) |
low 1,2 ⊕⊕⊝⊝ |
|
| 74 per 1,000 | 4 fewer per 1,000 (12 fewer to 4 more) | ||||
| CKD patients (GFR 15 to 60 mL/min/1.73 m²) | RR 0.97 (0.81 to 1.16) | 13,234 (19) |
low 1,2 ⊕⊕⊝⊝ |
||
| 72 per 1,000 | 2 fewer per 1,000 (14 fewer to 12 more) | ||||
| HD patients | RR 0.86 (0.72 to 1.03) | 4523 (14) |
low 1,2 ⊕⊕⊝⊝ |
||
| 87 per 1,000 | 12 fewer per 1,000 (24 fewer to 3 more) | ||||
|
Cardiovascular death Follow‐up: 0.9 to 88.2 months (median 12 months) |
All patients (predialysis, dialysis, transplant recipients) | RR 0.87 (0.65 to 1.15) | 9606 (21) |
very low 1,2,3 ⊕⊝⊝⊝ |
|
| 36 per 1,000 | 5 fewer per 1,000 (13 fewer to 5 more) | ||||
| CKD patients (GFR 15 to 60 mL/min/1.73 m²) | RR 0.98 (0.60 to 1.59) | 6525 (10) |
very low 1,2,3 ⊕⊝⊝⊝ |
||
| 37 per 1,000 | 1 fewer per 1,000 (15 fewer to 22 more) | ||||
| HD patients | RR 0.71 (0.47 to 1.09) | 2597 (9) |
very low 1,2,3 ⊕⊝⊝⊝ |
||
| 38 per 1,000 | 11 fewer per 1,000 (20 fewer to 3 more) | ||||
|
Major bleeding Follow‐up: 0.7 to 61.2 months (median 6 months) |
All patients (predialysis, dialysis, transplant recipients) | RR 1.35 (1.10 to 1.65) | 16,194 (29) |
moderate 1 ⊕⊕⊕⊝ |
|
| 29 per 1,000 | 10 more per 1,000 (3 to 19 more) | ||||
| CKD patients (GFR 15 to 60 mL/min/1.73 m²) | RR 1.51 (1.15 to 1.98) | 11591 (12) |
moderate 1 ⊕⊕⊕⊝ |
||
| 35 per 1,000 | 18 more per 1,000 (5 to 34 more) | ||||
| HD patients | RR 0.90 (0.53 to 1.55) | 4119 (15) |
moderate 1 ⊕⊕⊕⊝ |
||
| 13 per 1,000 | 1 fewer per 1,000 (6 fewer to 7 more) | ||||
|
Minor bleeding Follow‐up: 0.5 to 84 months (median 6 months) |
All patients (predialysis, dialysis, transplant recipients) | RR 1.55 (1.27 to 1.90) | 13,218 (21) |
low 1,3 ⊕⊕⊝⊝ |
|
| 92 per 1,000 | 51 more per 1,000 (25 to 83 more) | ||||
| CKD patients (GFR 15 to 60 mL/min/1.73 m²) | RR 1.48 (1.20 to 1.83) | 11,530 (12) |
low 1,3 ⊕⊕⊝⊝ |
||
| 103 per 1,000 | 50 more per 1,000 (21 to 86 more) | ||||
| HD patients | RR 1.87 (0.65 to 5.40) | 1240 (8) |
low 1,3 ⊕⊕⊝⊝ |
||
| 8 per 1,000 | 7 per 1,000 (3 fewer to 35 more) | ||||
|
Early access thrombosis (before 8 weeks) Follow‐up: 0.9 to 12 months (median 1.4 months) |
HD patients | RR 0.52 (0.38 to 0.70) | 1525 (8) |
low 1,4 ⊕⊕⊝⊝ |
|
| 200 per 1,000 | 6 fewer per 1,000 (60 to 124 fewer) | ||||
| *The risk in the intervention group (and its 95% CI is based on the assumed risk in the comparison group and the relative effect of the intervention (and its 95% CI). CI: confidence interval; RR: risk ratio; CKD: chronic kidney disease; GFR: glomerular filtration rate; HD: haemodialysis; OIS: Optimal Information Size | |||||
| GRADE Working Group grades of evidence High certainty: we are very confident that the true effect lies close to that of the estimate of the effect. Moderate certainty: we are moderately confident in the effect estimate: the true effect is likely to be close to the estimate of the effect, but there is a possibility that it is substantially different. Low certainty: our confidence in the effect estimate is limited: the true effect may be substantially different from the estimate of the effect. Very low certainty: we have very little confidence in the effect estimate: the true effect is likely to be substantially different from the estimate of effect. | |||||
1 Evidence certainty was downgraded by one level due to study limitations. Some or all studies were not blinded (participants and/or investigators)
2 Evidence certainty was downgraded by one level due to imprecision
3 Evidence certainty was downgraded by one level due to moderate between‐study heterogeneity
4 Evidence certainty was downgraded by one level due to imprecision (OIS criteria)
Background
Description of the condition
Cardiovascular disease is the leading cause of morbidity and death among people at all stages of chronic kidney disease (CKD) (Casas 2005; Keith 2004; Mann 2001; Norris 2006; Sarnak 2003; Weiner 2004a; Weiner 2004b) including kidney transplant recipients (Aakhus 1999; ANZDATA 2019; Kasiske 2000; Ojo 2000; USRDS 2010). Compared with the general population, the risk of cardiovascular disease is increased two‐fold in people with the early stages of CKD (Go 2004) and 30‐ to 50‐fold in people who need dialysis (de Jager 2009; Fort 2005) in whom it accounts for half of all deaths (Collins 2003). Population representative surveys in Australia (AusDiab 2003) and the USA (NHANES 2010) have shown that CKD (defined as proteinuria or reduction of glomerular filtration rate (GFR) below 60 mL/min/1.73 m²) affects approximately 16% of the adult population. With the increasing prevalence of some of the known risk factors for CKD, including hypertension, obesity and diabetes (Fields 2004; Koren‐Morag 2006; Mokdad 2003), the burden of CKD and its complications are projected to increase and to contribute significantly to global healthcare expenditure.
Description of the intervention
Excessive platelet activation occurs in CKD, even in the early stages of the disease. Specifically, the expression of P‐selectin, glycoprotein 53 and activated fibrinogen receptor‐1 on the platelet surface membrane is significantly increased in CKD patients. In ESRD, these abnormalities are more pronounced and may lead to access site thrombosis. Platelet activation is heavily implicated in the prothrombotic state observed in CKD patients, and oral antiplatelet agents have been extensively used in these patients (Alexopoulos 2011).
Dipyridamole is a phosphodiesterase inhibitor that reversibly inhibits platelet activation and aggregation by increasing adenosine levels and inhibiting cAMP‐phosphodiesterase (Hung 2014).
The antithrombotic action of aspirin is due to inhibition of platelet function by acetylation of the platelet cyclooxygenase (COX) at the functionally important amino acid serine529. This prevents the access of the arachidonic acid to the catalytic site of the enzyme at tyrosine385 and results in irreversible inhibition of platelet‐dependent thromboxane formation (Schror 1997).
P2Y12 is a G‐protein–coupled receptor that elicits specific intracellular responses to ADP resulting in the activation of the glycoprotein IIb/IIIa receptor. Active metabolites of thienopyridines (ticlopidine and clopidogrel) irreversibly bind to the ADP binding site and thereby prevent intracellular signalling and ADP‐induced platelet aggregation. P2Y12 antagonists, such as ticagrelor and prasugrel, inhibit adenosine reuptake in erythrocytes and other cells. The latter effect has been attributed to improved platelet inhibition and coronary blood flow and reduced infarct size (Gurbel 2019). Cilostazol, a selective reversible phosphodiesterase type III inhibitor, has antiplatelet effects due to subsequent increases in cyclic adenosine monophosphate within platelets. The potential to achieve platelet inhibition with minimal risk of bleeding might be explained by an endothelium‐targeted antithrombotic therapy, that is, reduction of partially activated platelets by improved endothelial function (Woo 2011).
Glycoprotein IIb/IIIa inhibitors (abciximab, eptifibatide and tirofiban) administered parenterally interfere with platelet activity at the final common pathway of platelet‐induced thrombosis, showing a much greater antiplatelet activity than aspirin with or without clopidogrel at normal doses. In addition to preventing platelet aggregation, GP IIb/IIIa antagonism has the ability to induce the dissolution of platelet‐rich clots by disrupting fibrinogen platelet interaction (Stangal 2010).
Sulfinpyrazone appears to interfere with the adhesion of platelets to subendothelial structures and atherosclerotic plaques (Oelz 1979).
How the intervention might work
Antiplatelet agents prevent arterial occlusion from thrombus via direct prevention of platelet aggregation. Currently available data suggest antiplatelet agents might be beneficial in patients with CKD for primary (ATT 2002; HOT 1993; Ruilope 2001) and secondary (Berger 2003; McCullough 2002) prevention of cardiovascular events. Antiplatelet agents may have beneficial effects on the kidney, possibly reducing proteinuria and protecting kidney function in people with glomerulonephritis (Taji 2006; Zäuner 1994), and improving graft function in kidney transplant recipients (Bonomini 1986; Frascà 1986). However, some have reported that the efficacy of antiplatelet agents in CKD might be lower than for other high cardiovascular risk populations (Best 2008). Despite this, the Kidney Disease Outcomes Quality Initiative guideline program (KDOQI) has supported the use of aspirin for the primary prevention of cardiovascular disease in CKD. Antiplatelet agents appear to have a modest effect on the preservation of arteriovenous fistula patency (Dember 2005). Their use for fistula preservation and as part of a multifactorial intervention strategy for patients with CKD is advocated by guideline groups (CARI 2000; UK Renal Association 2010).
Why it is important to do this review
The previous meta‐analyses did not clearly assess the benefits and harms of antiplatelet agents in people with CKD, including those undergoing dialysis (haemodialysis (HD) and peritoneal dialysis (PD)) and transplant recipients, and recently new studies have been performed in this area in contrast to the general population, people with CKD have a different profile of causes for major cardiovascular events, including a greater preponderance for arrhythmia and congestive heart failure (Amann 2003; Curtis 2005; Dikow 2005; Foley 1995; Remppis 2008), altered pharmacokinetics (Mosenkis 2004; Scheen 2008) and impaired haemostasis (Kaw 2006; Remuzzi 1988; Wattanakit 2008; Zwaginga 1991). Compared with people who do not have CKD, these factors might expose the CKD population to a different spectrum of risk and benefit from antiplatelet agents.
Objectives
To evaluate the benefits and harms of antiplatelet agents in people with any form of CKD, including those with CKD not receiving kidney replacement therapy, patients receiving any form of dialysis, and kidney transplant recipients.
Methods
Criteria for considering studies for this review
Types of studies
All randomised controlled trials (RCTs) and quasi‐RCTs (RCTs in which allocation to treatment was obtained by alternation, use of alternate medical records, date of birth or other predictable methods) of antiplatelet agents in people with CKD were included.
Types of participants
Participants with CKD, including those who needed kidney replacement therapy (dialysis), had a functioning kidney transplant, or whose kidney function was impaired (defined as a reduced GFR < 60 mL/min/1.73 m²), the presence of other markers of kidney damage such as proteinuria (KDOQI stages 1 to 5), or an elevated serum creatinine (SCr) level (SCr >120 μmol/L). Data from subgroups of participants with CKD within studies with broader inclusion criteria (e.g. people from the general population, people with diabetes, people with cardiovascular disease) were also included.
Types of interventions
Interventions included any antiplatelet agent. Agents could be administered at any dose or route of administration and compared with placebo, no treatment, different dose of the same or different antiplatelet agents, different administration regimens of the same or a different antiplatelet agent, or different combinations of antiplatelet agents. Antiplatelet agents included, but were not limited to:
Acetylsalicylic acid (aspirin)
Adenosine reuptake inhibitors (dipyridamole)
Adenosine diphosphate receptor inhibitors (ticlopidine and clopidogrel)
Phosphodiesterase 3 inhibitors (cilostazol)
P2Y₁₂ antagonists (prasugrel, ticagrelor, cangrelor, elinogrel)
Glycoprotein IIb/IIIa inhibitors (abciximab, eptifibatide, tirofiban, defibrotide)
Sulphinpyrazone.
We excluded studies comparing antiplatelet agents to anticoagulants.
Types of outcome measures
Primary outcomes
Myocardial infarction (MI) (nonfatal or fatal)
Stroke (nonfatal or fatal)
Death (any cause)
Cardiovascular death
Bleeding‐related death
Major bleeding
Minor bleeding
Haemorrhagic stroke
Kidney failure (previously referred to as end‐stage kidney disease (ESKD))
Kidney transplant graft loss
Transplant rejection
Dialysis vascular outcomes (failure, early thrombosis, loss of unassisted patency, failure to attain suitability for dialysis, and need for access intervention)
Hospitalisation
Treatment withdrawal.
Secondary outcomes
SCr
Proteinuria.
Search methods for identification of studies
A systematic and comprehensive literature search was carried out to identify eligible RCTs. There was no language restriction.
Electronic searches
We searched the Cochrane Kidney and Transplant Register of Studies up to 13 July 2021 through contact with the Information Specialist using search terms relevant to this review. The Register contains studies identified from the following sources:
Monthly searches of the Cochrane Central Register of Controlled Trials (CENTRAL)
Weekly searches of MEDLINE OVID SP
Searches of kidney and transplant journals, and the proceedings and abstracts from major kidney and transplant conferences
Searching of the current year of EMBASE OVID SP
Weekly current awareness alerts for selected kidney and transplant journals
Searches of the International Clinical Trials Register (ICTRP) Search Portal and ClinicalTrials.gov.
Studies contained in the Register are identified through searches of CENTRAL, MEDLINE, and EMBASE based on the scope of Cochrane Kidney and Transplant. Details of search strategies, as well as a list of handsearched journals, conference proceedings and current awareness alerts, are available on the Cochrane Kidney and Transplant website.
See Appendix 1 for search terms used in strategies for this review.
Searching other resources
Reference lists of review articles, relevant studies and clinical practice guidelines.
Contacting relevant individuals/organisations seeking information about unpublished or incomplete studies.
Grey literature sources (e.g. abstracts, dissertations and theses), in addition to those already included in the Cochrane Kidney and Transplant Register of Studies, were searched.
Data collection and analysis
Selection of studies
All RCTs enrolling participants with CKD were considered as well as studies in broader populations in which outcome data for subgroups with CKD could be disaggregated. Based on the search strategy described, we identified titles and abstracts that were potentially relevant to this systematic review. Four independent authors screened the titles and abstracts and selected those that met the inclusion criteria. Discrepancies in selection were resolved by discussion or by the review of an experienced arbitrator. Studies reported in non‐English language journals were translated before assessment.
Data extraction and management
Four authors independently read the full text of extracted articles and included studies that met the inclusion criteria. Where more than one publication of one study existed, reports were grouped together and the publication with the most complete data was used in the analyses.
The same independent authors used standardised data forms to extract data on:
Study design
Participants: baseline characteristics including age, sex, race, diabetic status (proportion with diabetes), hypertension status (proportion with hypertension), smoking status (proportion of smokers), visceral obesity (proportion with visceral obesity as defined by authors), previous cardiovascular events (proportion with existing cardiovascular disease), and stage of CKD (dialysis, predialysis, transplant)
Interventions and comparisons: antiplatelet agent, dose and route of administration, duration of treatment
Outcomes: as listed in Types of outcome measures.
Assessment of risk of bias in included studies
The following items were assessed independently by two authors using the risk of bias assessment tool (Higgins 2020) (see Appendix 2).
Was there adequate sequence generation (selection bias)?
Was allocation adequately concealed (selection bias)?
-
Was knowledge of the allocated interventions adequately prevented during the study?
Participants and personnel (performance bias)
Outcome assessors (detection bias)
Were incomplete outcome data adequately addressed (attrition bias)?
Are reports of the study free of suggestion of selective outcome reporting (reporting bias)?
Was the study apparently free of other problems that could put it at risk of bias?
Measures of treatment effect
For dichotomous outcomes (e.g. such as death, cardiovascular events), results were expressed as risk ratio (RR) with 95% confidence intervals (CI). Where continuous scales of measurement were used to assess the effects of treatment (e.g. creatinine clearance (CrCl), GFR, SCr, proteinuria), the mean difference (MD) and its 95% CI was used. The final results are presented in International System (SI) units. When crude event data were not reported by investigators, available reported risk estimates and their 95% CIs were included in meta‐analyses.
Unit of analysis issues
The unit of analysis was each participant recruited into the studies.
For cross‐over studies, we looked for reporting of paired data in order to estimate within‐user differences. Where no such data were provided, we used data from the first period only in the absence of washout periods to avoid the carry‐over effect.
For studies with more than two arms, we treated each pair of arms as a separate pairwise comparison.
Dealing with missing data
Where possible, data for each outcome of interest were evaluated, regardless of whether the analysis was based on intention‐to‐treat. In particular, dropout rates were investigated and reported in detail, including dropout due to discontinuation of study drug, treatment failure, death, withdrawal of consent, or loss to follow‐up. Corresponding authors of all large studies with broader inclusion were contacted to obtain data for the subgroup of CKD (Higgins 2020).
Assessment of heterogeneity
We first assessed the heterogeneity by visual inspection of the forest plot. We then quantified statistical heterogeneity using the I² statistic, which describes the percentage of total variation across studies that is due to heterogeneity rather than sampling error (Higgins 2003). A guide to the interpretation of I² values was as follows:
0% to 40%: might not be important
30% to 60%: may represent moderate heterogeneity
50% to 90%: may represent substantial heterogeneity
75% to 100%: considerable heterogeneity.
The importance of the observed value of I² depends on the magnitude and direction of treatment effects and the strength of evidence for heterogeneity (e.g. P‐value from the Chi² test, or a confidence interval for I²) (Higgins 2020).
Assessment of reporting biases
We evaluated asymmetries in the inverted funnel plots (i.e. for systematic differences in the effect sizes between more precise and less precise studies). There are many potential explanations for why an inverted funnel plot may be asymmetric, including chance, heterogeneity, publication and reporting bias (Higgins 2020). Insufficient data were available to evaluate the robustness of the results according to publication, namely, publication as a full manuscript in a peer‐reviewed journal versus studies published as abstracts/text/letters/editorials and publication.
Data synthesis
Data were pooled using the random‐effects model. The GRADE approach developed by Grades of Recommendation, Assessment, Development and Evaluation Working Group (GRADE Working Group) was used for evaluating the quality of evidence for outcomes to be reported. Based on the GRADE approach, the quality of a body of evidence, in terms of the extent to which one can be confident that an estimate of effect or association is close to the quantity of specific interest, was defined.
Subgroup analysis and investigation of heterogeneity
Heterogeneity was explored using subgroup analyses according to the following parameters (where sufficient numbers of studies were available):
-
Population characteristics
Stage of CKD (pre‐dialysis, dialysis, transplant)
Presence or absence of comorbidities (diabetes, hypertension, dyslipidaemia, smoking, obesity, family history of cardiovascular disease, baseline cardiovascular disease); percentage of patients with these comorbidities in each study
Age
Sex
Mean systolic blood pressure (SBP) (< 140 mm Hg versus ≥ 140 mm Hg)
Ethnicity (proportion white)
Presence or absence of previous cardiovascular events (e.g. primary versus secondary prevention)
Time on dialysis (< 3 years versus ≥ 3 years) and modalities of dialysis (HD versus PD)
Time with a functioning transplant (< 3 years versus ≥ 3 years)
-
Intervention characteristics
Types, doses and route of administration of the antiplatelet agents
Duration of intervention (< 6 months, 6 to 12 months, > 12 months).
Sensitivity analysis
Sensitivity analyses were undertaken to explore the robustness of findings to key decisions in the review process. We assessed the risks of death (any cause and cardiovascular death), nonfatal and fatal MI, and major bleeding only including studies with adequate allocation concealment, or at low risk of bias due to completeness of follow‐up. Insufficient data were available to perform indirect comparisons of antiplatelet agent versus antiplatelet agent (Song 2003).
Summary of findings and assessment of the certainty of the evidence
We presented the main results of the review in 'Summary of findings' tables. These tables present key information concerning the quality of the evidence, the magnitude of the effects of the interventions examined, and the sum of the available data for the main outcomes (Schunemann 2020a). The 'Summary of findings' tables also includes an overall grading of the evidence related to each of the main outcomes using the GRADE (Grades of Recommendation, Assessment, Development and Evaluation) approach (GRADE 2008; GRADE 2011a). The GRADE approach defines the quality of a body of evidence as to the extent to which one can be confident that an estimate of effect or association is close to the true quantity of specific interest. The quality of a body of evidence involves consideration of within‐trial risk of bias (methodological quality), directness of evidence, heterogeneity, the precision of effect estimates, and the risk of publication bias (Schunemann 2020b). We presented the following outcomes in the 'Summary of findings' tables:
MI (fatal or nonfatal)
Stroke (fatal or nonfatal)
Death (any cause)
Cardiovascular death
Major bleeding
Minor bleeding
Early access thrombosis
Results
Description of studies
Results of the search
Search results are shown in Figure 1. For this 2021 review update, we screened 407 titles and abstracts identified by the updated search. After full‐text assessment 98 new studies were identified. Fifty‐six new studies (152 reports) were included, 17 (82 reports) were excluded, and 25 ongoing studies were identified. We also identified 233 new reports of 20 existing included studies and 22 new reports of six excluded studies.
1.
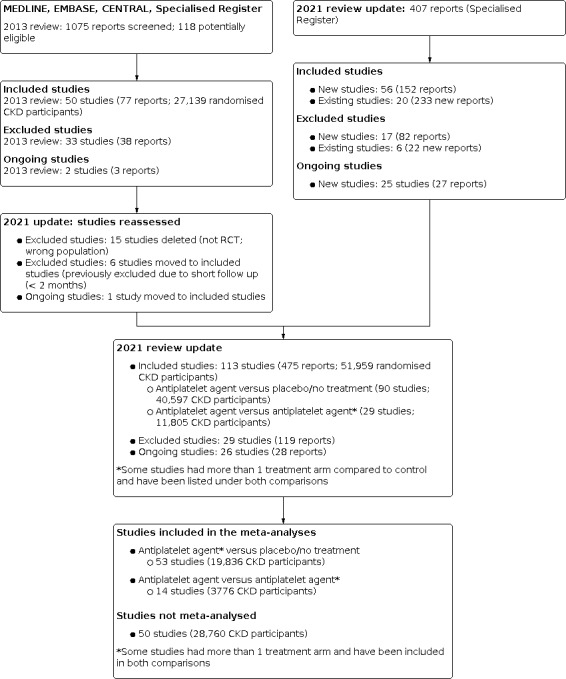
Study flow diagram; study identification and selection process.
We reclassified six previously excluded studies as included studies (Dmoszynska‐Giannopoulou 1990; Kamper 1997; Movchan 2001; RESIST 2008; Rubin 1982; Salter 1984), and one ongoing study has now been included (FAVOURED 2009).
For this 2011 update, 113 studies (475 reports, 51,959 CKD participants, Figure 1) were included, 29 studies were excluded, and there are 26 ongoing studies.
Included studies
The overall characteristics of the included studies are provided in the Characteristics of included studies. Information for three studies (1238 participants: Creek 1990; Ell 1982; Middleton 1992) including two internal study reports (Creek 1990; Middleton 1992) were only available in a previously published meta‐analysis of antiplatelet agents (ATT 2002). For three studies (103 participants), the most complete data were provided in published conference proceedings (Dodd 1980; Gonzalez 1995; Taber 1992), and for one study (NCT01252056), information about study characteristics and endpoint data was extracted from www.clinicaltrials.gov.
Studies compared antiplatelet agents with placebo or no treatment, or another antiplatelet agent; several studies compared two or more antiplatelet agents.
Ninety studies (40,597 CKD participants) compared an antiplatelet agent to placebo or no treatment (AASER 2017; Abacilar 2015; Abdul‐Rahman 2007; Anderson 1974; Andrassy 1974; ATACAS 2008; CASSIOPEIR 2014; Chan 1987; CHANCE 2013; CHARISMA 2006; Cheng 1998a; Christopher 1987; CREDO 2005; Creek 1990; CURE 2000; Dember 2005; Dixon 2005; Dmoszynska‐Giannopoulou 1990; Dodd 1980; Donadio 1984; EARLY ACS 2005; Ell 1982; EPIC 1994; EPILOG 1997; EPISTENT 1998; ETDRS 1992; FAVOURED 2009; Fiskerstrand 1985; Frascà 1997; Gaede 2003; Ghorbani 2009; Ghorbani 2013; Giustina 1998; GLOBAL LEADERS 2018; Goicoechea 2012; Gonzalez 1995; Gröntoft 1985; Gröntoft 1998; Guo 1998; Hansen 2000; Harter 1979; HOT 1993; IMPACT II 1997; Jiao 2013; JPAD 2008; Kaegi 1974; Kamper 1997; Kaufman 2003; Khajehdehi 2002; Kobayashi 1980; Kontessis 1993; Kooistra 1994; Koyama 1990; Michie 1977; Middleton 1992; Milutinovic 1993; Movchan 2001; Mozafar 2013; Mozafar 2018; Nakamura 2001d; Nakamura 2002b; NCT01252056; Nyberg 1984; PEGASUS‐TIMI 54 2014; Pierucci 1989; PLATO 2009; PREDIAN 2011; PRISM‐PLUS 1998; PURSUIT 1997; Quarto Di Palo 1991; RAPPORT 1998; Reams 1985; RESIST 2008; Rouzrokh 2010; Rubin 1982; Salter 1984; Schulze 1990; Sreedhara 1994; Steiness 2018; STOP 1995; Storck 1996; Taber 1992; Tang 2014; Tayebi 2018; TRA 2P‐TIMI 50 2009; TRACER 2013; UK‐HARP‐I 2005; Watanabe 2011b; Weseley 1982; Yuto 2012; Zäuner 1994)
Twenty‐nine studies (11,805 CKD participants) compared an antiplatelet agent to a second antiplatelet agent (Alexopoulos 2011; CASSIOPEIR 2014; CILON‐T 2010; Dash 2013; EUCLID 2017; Frascà 1986; Hidaka 2013; J‐PADD 2014; Kauffmann 1980; Khajehdehi 2002; Liang 2015; Movchan 2001; Ogawa 2008; OPT‐CKD 2018; Ota 1996; PIANO‐2 CKD 2011; PIANO‐3 2015; PIANO‐6 2017; PLATO 2009; RESIST 2008; Schnepp 2000; Sreedhara 1994; Taber 1992; TARGET 2000; Teng 2018; TRITON‐TIMI 38 2006; Waseda 2016; Xydakis 2004; Yang 2016b).
Antiplatelet versus placebo or no treatment studies
Ninety studies comparing an antiplatelet to placebo or no treatment were published between 1974 and 2018. The number of CKD participants ranged from 6 to 4983 participants (median 85 participants) and the mean age of the participants ranged from 29 to 73.4 years. The duration of study follow‐up ranged from 48 hours to 88.2 months (median six months).
Forty‐nine studies were conducted in people with CKD not yet requiring dialysis (37,013 participants: AASER 2017; ATACAS 2008; CASSIOPEIR 2014; Chan 1987; CHANCE 2013; CHARISMA 2006; Cheng 1998a; Christopher 1987; CREDO 2005; CURE 2000; Donadio 1984; EARLY ACS 2005; EPIC 1994; EPILOG 1997; EPISTENT 1998; ETDRS 1992; Frascà 1997; Gaede 2003; Giustina 1998; GLOBAL LEADERS 2018; Goicoechea 2012; Gonzalez 1995; Guo 1998; Hansen 2000; HOT 1993; IMPACT II 1997; Jiao 2013; JPAD 2008; Khajehdehi 2002; Kontessis 1993; Koyama 1990; Movchan 2001; Nakamura 2001d; NCT01252056; Nyberg 1984; PEGASUS‐TIMI 54 2014; Pierucci 1989; PREDIAN 2011; PRISM‐PLUS 1998; PURSUIT 1997; RAPPORT 1998; RESIST 2008; Steiness 2018 Tang 2014; TRA 2P‐TIMI 50 2009; TRACER 2013; Watanabe 2011b; Zäuner 1994).
Thirty‐two studies enrolled HD patients (5097 participants: Abacilar 2015; Abdul‐Rahman 2007; Andrassy 1974; Creek 1990; Dember 2005; Dixon 2005; Dmoszynska‐Giannopoulou 1990; Dodd 1980; Ell 1982; Fiskerstrand 1985; Ghorbani 2009; Ghorbani 2013; Gröntoft 1985; Harter 1979; Kaegi 1974; Kamper 1997; Kaufman 2003; Kobayashi 1980; Kooistra 1994; Michie 1977; Middleton 1992; Milutinovic 1993; Mozafar 2013; Mozafar 2018; Nakamura 2002b; Rouzrokh 2010; Salter 1984; Sreedhara 1994; STOP 1995; Taber 1992; Tayebi 2018; Yuto 2012).
Three studies were in patients treated with PD (40 participants: Reams 1985; Rubin 1982; Weseley 1982).
Four studies enrolled kidney transplant recipients (141 participants: Anderson 1974; Quarto Di Palo 1991; Schulze 1990; Storck 1996).
Two studies enrolled participants with earlier stages of CKD and those treated with HD (673 participants: FAVOURED 2009; Gröntoft 1998)
In one study (UK‐HARP‐I 2005; 448 participants), participants included those with earlier stages of CKD, transplant recipients and participants treated with dialysis (both HD and PD).
In the 90 studies that compared an antiplatelet agent with placebo or no treatment, the interventions included:
-
Acetylsalicylic acid
Aspirin (16 studies, 6140 participants: AASER 2017; Abdul‐Rahman 2007; Andrassy 1974; ATACAS 2008; ETDRS 1992; FAVOURED 2009; Gaede 2003; Guo 1998; Hansen 2000; Harter 1979; HOT 1993; JPAD 2008; Kooistra 1994; Mozafar 2013; Storck 1996; UK‐HARP‐I 2005)
Aspirin plus dextran (1 study, 45 participants; Taber 1992)
-
Adenosine reuptake inhibitors
Dilazep dihydrochloride (2 studies, 62 participants: Nakamura 2001d; Nakamura 2002b)
Dipyridamole (7 studies, 615 participants: Anderson 1974; Koyama 1990; Movchan 2001; Reams 1985; Rubin 1982; Schulze 1990; Weseley 1982)
Dipyridamole plus aspirin (11 studies, 2004 participants: Chan 1987; Christopher 1987; Dixon 2005; Donadio 1984; Gonzalez 1995; Khajehdehi 2002; Middleton 1992; Salter 1984; Sreedhara 1994; Tayebi 2018; Zäuner 1994)
Dypiridamole or aspirin (1 study, 501 participants: Rouzrokh 2010)
-
Adenosine diphosphate receptor inhibitors
Clopidogrel (7 studies, 7931 participants: CHANCE 2013; CHARISMA 2006; CREDO 2005; CURE 2000; Dember 2005; Ghorbani 2009; Mozafar 2018)
Clopidogrel and aspirin (1 study, 200 participants: Kaufman 2003)
Clopidogrel and prostacyclin (1 study, 96 participants: Abacilar 2015)
Ticlopidine (12 studies, 986 participants: Cheng 1998a; Creek 1990; Dodd 1980; Ell 1982; Fiskerstrand 1985; Ghorbani 2013; Gröntoft 1985; Gröntoft 1998; Kamper 1997; Kobayashi 1980; Milutinovic 1993; Nyberg 1984)
-
Haemorrhagic agents
Pentoxifylline (2 studies, 260 participants: Goicoechea 2012; PREDIAN 2011)
-
PAR‐1 antagonist
Vorapaxar (1 study, 4983 participants: TRA 2P‐TIMI 50 2009)
-
Phosphodiesterase 3 inhibitors
Cilostazol (3 studies, 483 participants: Jiao 2013; NCT01252056; Tang 2014)
Beraprost sodium (1 study, 892 participants: CASSIOPEIR 2014)
-
P2Y₁₂ antagonists
Ticagrelor (1 study, 4849 participants: PEGASUS‐TIMI 54 2014)
Ticagrelor plus aspirin then ticagrelor alone (1 study, 838 participants: GLOBAL LEADERS 2018)
-
Glycoprotein IIb/IIIa inhibitors
Abciximab (5 studies, 1537 participants: EPIC 1994; EPILOG 1997; EPISTENT 1998; RAPPORT 1998; RESIST 2008)
Tirofiban (1 study, 611 participants: PRISM‐PLUS 1998)
Eptifibatide (3 studies, 5065 participants: EARLY ACS 2005; IMPACT II 1997; PURSUIT 1997)
-
Other
Defibrotide (1 study, 20 participants: Frascà 1997)
Picotamide (3 studies, 901 participants: Giustina 1998; Quarto Di Palo 1991; STOP 1995)
Sarpogrelate (2 studies, 132 participants: Watanabe 2011b; Yuto 2012)
Sulphinpyrazone (3 studies, 108 participants: Dmoszynska‐Giannopoulou 1990; Kaegi 1974; Michie 1977)
Sulphonamide derivative (1 study, 6 participants: Pierucci 1989)
Thromboxane synthetase inhibitor (1 study, 15 participants: Kontessis 1993)
SER150 (novel anti‐thromboxane) (1 study, 72 participants: Steiness 2018)
Vorapaxar (1 study, 1477 participants: TRACER 2013)
Vascular access studies
We identified 31 studies that reported dialysis vascular access endpoints in 6449 participants (Abacilar 2015; Abdul‐Rahman 2007; Anderson 1974; Andrassy 1974; Creek 1990; Dember 2005; Dixon 2005; Dodd 1980; Ell 1982; FAVOURED 2009; Fiskerstrand 1985; Ghorbani 2009; Ghorbani 2013; Gröntoft 1985; Gröntoft 1998; Harter 1979; Kaegi 1974; Kaufman 2003; Kobayashi 1980; Kooistra 1994; Michie 1977; Middleton 1992; Milutinovic 1993; Mozafar 2013; Mozafar 2018; Rouzrokh 2010; Sreedhara 1994; STOP 1995; Taber 1992; Tayebi 2018; Yuto 2012). Generally, these studies were small; only five studies included more than 500 participants (Dember 2005; Dixon 2005; Middleton 1992; Rouzrokh 2010; STOP 1995), and sixteen studies enrolled fewer than 100 participants (Abacilar 2015; Abdul‐Rahman 2007; Anderson 1974; Andrassy 1974; Ell 1982; Fiskerstrand 1985; Ghorbani 2009; Ghorbani 2013; Gröntoft 1985; Harter 1979; Kaegi 1974; Michie 1977; Milutinovic 1993; Taber 1992; Tayebi 2018 Yuto 2012).
Ticlopidine was most the commonly administered (9 studies, 884 participants: Creek 1990; Dodd 1980; Ell 1982; Fiskerstrand 1985; Ghorbani 2013; Gröntoft 1985; Gröntoft 1998; Kobayashi 1980; Milutinovic 1993), followed by aspirin (6 studies, 917 participants: Abdul‐Rahman 2007; Andrassy 1974; FAVOURED 2009; Harter 1979; Kooistra 1994; Mozafar 2013). The combination of dipyridamole and aspirin was prescribed to 1720 participants in four studies (Dixon 2005; Middleton 1992; Sreedhara 1994; Tayebi 2018), three studies evaluated clopidogrel (1070 participants: Dember 2005; Ghorbani 2009; Mozafar 2018), two studies evaluated sulphinpyrazone (78 participants: Kaegi 1974; Michie 1977), and single studies assessed dipyridamole (27 participants: Anderson 1974), picotamide (832 participants: STOP 1995) and sarpogrelate (79 participants: Yuto 2012). One study each assessed the combination of clopidogrel and aspirin (200 participants: Kaufman 2003), the combination of dextran and aspirin (45 participants: Taber 1992), the combination of clopidogrel and prostacyclin (96 participants: Abacilar 2015), and the combination of aspirin or dipyridamole (501 participants: Rouzrokh 2010). The duration of the intervention varied from one month to 61,2 months, with a median of five months.
Studies evaluated whether treatment maintained patency of an arteriovenous fistula (10 studies, 1765 participants: Abacilar 2015; Andrassy 1974; Dember 2005; Fiskerstrand 1985; Ghorbani 2009; Ghorbani 2013; Gröntoft 1985; Gröntoft 1998; Kooistra 1994; Yuto 2012), shunt or graft (5 studies, 1063 participants: Dixon 2005; Harter 1979; Kaegi 1974; Kaufman 2003; Sreedhara 1994), fistula or graft (1 study, 16 participants: Michie 1977), or central venous catheter (1 study, 58 participants: Abdul‐Rahman 2007).
Antiplatelet versus antiplatelet studies
Thirty‐four studies comparing an antiplatelet drug with a second antiplatelet drug in people with CKD were published between 1980 and 2018. The number of CKD participants ranged from 6 to 4983 participants (median 85 participants) and the mean age of participants ranged from 33 to 74.4 years. The duration of follow‐up ranged from 2 days to 48 months (median four months).
Twelve studies were conducted in people with CKD not yet requiring dialysis (10,958 participants: CASSIOPEIR 2014; CILON‐T 2010; Dash 2013; EUCLID 2017; Khajehdehi 2002; Liang 2015; Movchan 2001; Ogawa 2008; OPT‐CKD 2018; PLATO 2009; TARGET 2000; TRITON‐TIMI 38 2006).
Thirteen studies evaluated treatment in people on HD (786 participants: Alexopoulos 2011; Hidaka 2013; J‐PADD 2014; Ota 1996; PIANO‐2 CKD 2011; PIANO‐3 2015; PIANO‐6 2017; Schnepp 2000; Sreedhara 1994; Teng 2018: Waseda 2016; Xydakis 2004; Yang 2016b).
Two studies enrolled kidney transplant recipients (122 participants: Frascà 1986; Kauffmann 1980).
In the studies that compared an antiplatelet with another antiplatelet, interventions included:
-
Acetylsalicylic acid
Aspirin versus clopidogrel (3 studies, 202 participants: Dash 2013; Xydakis 2004; Yang 2016b)
Aspirin versus clopidogrel versus ticlopidine (1 study, 30 participants: Schnepp 2000)
Aspirin versus sarpogrelate (1 study, 40 participants: Ogawa 2008)
-
Adenosine reuptake inhibitors
Dypiridamole versus aspirin (2 studies, 97 participants: Kauffmann 1980; Sreedhara 1994)
Dypiridamole versus defibrotide (1 study, 80 participants: Frascà 1986)
Dypiridamole versus aspirin versus dypiridamole plus aspirin versus placebo (1 study, 76 participants: Khajehdehi 2002)
Dypiridamole versus pentoxifylline (1 study, 40 participants: Movchan 2001)
-
Adenosine diphosphate receptor inhibitors
Clopidogrel versus cilostazol (1 study, 74 participants: PIANO‐2 CKD 2011)
Clopidogrel plus cilostazol versus clopidogrel (1 study, 184 participants: CILON‐T 2010)
Clopidogrel versus ticagrelor (2 studies, 3701 participants: EUCLID 2017; PIANO‐6 2017)
Low‐dose clopidogrel versus high‐dose clopidogrel (1 study, 370 participants: Liang 2015)
Ticlopidine versus satigrel (1 study, 224 participants: Ota 1996)
-
Phosphodiesterase 3 inhibitors
Cilostazol versus beraprost sodium (1 study, 72 participants: J‐PADD 2014)
Cilostazol versus sarpogrelate (1 study, 35 participants: Hidaka 2013)
-
P2Y12 antagonists
Ticagrelor versus clopidogrel (3 studies, 3322 participants: OPT‐CKD 2018; PIANO‐3 2015; PLATO 2009)
Ticagrerol pre‐dialysis versus ticagrelor post‐dialysis (1 study, 14 participants: Teng 2018)
Prasugrel versus clopidogrel (3 studies, 1544 participants: Alexopoulos 2011; TRITON‐TIMI 38 2006; Waseda 2016)
-
Glycoprotein IIb/IIIa inhibitor
Abciximab versus tirofiban (1 study, 790 participants: TARGET 2000)
-
Other
Low versus high‐dose beraprost sodium (1 study, 600 participants: CASSIOPEIR 2014)
Excluded studies
For this update, we reassessed all previously excluded studies. We deleted 15 studies (not randomised or wrong population) and reclassified six studies as included studies; these were previously excluded due to less than two months of follow‐up. For the 2021 search, we excluded 17 new studies (82 reports) and identified 22 new reports of 6 already excluded studies. In total, we have excluded 29 studies (119 reports).
Three studies were the wrong study design (Caravaca 1995a; Yang 2014a; Yeh 2017)
Eleven studies enrolled the wrong population (Bang 1994; EXCITE 2000; POISE‐2 2013; PRODIGY 2010; RAS‐CAD 2009; REPLACE‐2 2003; SPS3 2018; TRILOGY ACS 2010; Woo 1987; Wu 2018a; Zimmerman 1983).
Nine studies used the wrong intervention (Coli 2006; Foroughinia 2017; Lee 1997; NITER 2005; Perkovic 2004; STENO‐2 1999; Swan 1995a; Yoshikawa 1999; Zhang 2009a)
Six studies used the wrong comparator (AVERROES 2010; Changjiang 2015; Gorter 1998; Lindsay 1972; Sakai 1991; Zibari 1995).
See Characteristics of excluded studies.
Ongoing studies
Twenty‐six studies (27 reports) have yet to be completed (A‐CLOSE 2019; ALTIC 2016; ALTIC‐2 2018; ATTACK 2018; ChiCTR1900021393; IRCT2013012412256N1; IRCT2013100114333N8; IRCT20171023036953N1; LEDA 2017; Lemos Cerqueira 2018; NCT00272831; NCT01198379; NCT01743014; NCT02394145; NCT02459288; NCT03039205; NCT03150667; NCT03649711; Park 2010; PRASTO‐III 2018; SERENADE 2015; SONATA 2013; TROUPER 2020; TWILIGHT 2016; UMIN000003891; VA PTXRx 2018).
Risk of bias in included studies
The risk of bias in the included studies is summarised in Figure 2.
2.

Risk of bias graph: review authors' judgements about each risk of bias item presented as percentages across all included studies
Allocation
Random sequence generation
Methods for generating the random sequence were deemed to be at low risk of bias in 16 studies (AASER 2017; Alexopoulos 2011; ATACAS 2008CASSIOPEIR 2014; CHANCE 2013; CREDO 2005; Dash 2013; EUCLID 2017; FAVOURED 2009; Goicoechea 2012; JPAD 2008; Mozafar 2018; PIANO‐2 CKD 2011; PIANO‐3 2015; PIANO‐6 2017; UK‐HARP‐I 2005), at high risk of bias in three studies (Guo 1998; Kauffmann 1980; Rubin 1982), and unclear in 94 studies.
Allocation concealment
Allocation concealment was judged to be a low risk of bias in 22 studies (Anderson 1974; ATACAS 2008; CASSIOPEIR 2014; CHARISMA 2006; CURE 2000; Dixon 2005; EARLY ACS 2005; EPIC 1994; EPILOG 1997; EPISTENT 1998; EUCLID 2017; FAVOURED 2009; Ghorbani 2009; Ghorbani 2013; Giustina 1998; HOT 1993; Kaufman 2003; PEGASUS‐TIMI 54 2014; PURSUIT 1997; TARGET 2000; TRA 2P‐TIMI 50 2009; TRACER 2013), and unclear in 91 studies.
Blinding
Performance bias
Sixty‐four studies were blinded and considered to be at low risk of bias for performance bias (Abacilar 2015; Abdul‐Rahman 2007; Anderson 1974; Andrassy 1974; ATACAS 2008; CASSIOPEIR 2014; CHANCE 2013; CHARISMA 2006; Christopher 1987; CREDO 2005; CURE 2000; Dember 2005; Dixon 2005; Dodd 1980; Donadio 1984; EARLY ACS 2005; EPIC 1994; EPILOG 1997; EPISTENT 1998; ETDRS 1992; FAVOURED 2009; Fiskerstrand 1985; Gaede 2003; Ghorbani 2009; Ghorbani 2013; Giustina 1998; Gröntoft 1985; Gröntoft 1998; Guo 1998; Hansen 2000; Harter 1979; HOT 1993; IMPACT II 1997; Kaegi 1974; Kauffmann 1980; Kaufman 2003; Kobayashi 1980; Kontessis 1993; Kooistra 1994; Koyama 1990; Michie 1977; Milutinovic 1993; Mozafar 2013; Nyberg 1984; Ota 1996; PEGASUS‐TIMI 54 2014; Pierucci 1989; PLATO 2009; PRISM‐PLUS 1998; PURSUIT 1997; Quarto Di Palo 1991; RAPPORT 1998; Reams 1985; RESIST 2008; Rubin 1982; Salter 1984; Sreedhara 1994; STOP 1995; TARGET 2000; Tayebi 2018; TRA 2P‐TIMI 50 2009; TRACER 2013; TRITON‐TIMI 38 2006; Weseley 1982). One study was judged to have unclear risk of bias (EUCLID 2017) and 48 studies were not blinded and were considered at high risk of performance bias.
Detection bias
Blinding of outcome assessment was judged to be at low risk of bias for 41 studies (AASER 2017; ATACAS 2008; CASSIOPEIR 2014; Chan 1987; CHANCE 2013; Cheng 1998a; Christopher 1987; CILON‐T 2010; CURE 2000; Dash 2013; EPIC 1994; EPILOG 1997; EPISTENT 1998; ETDRS 1992; EUCLID 2017; HOT 1993; IMPACT II 1997; Jiao 2013; JPAD 2008; Kontessis 1993; Koyama 1990; Movchan 2001; Nakamura 2001d; Nakamura 2002b; Ogawa 2008; PEGASUS‐TIMI 54 2014; PIANO‐2 CKD 2011; PREDIAN 2011; PRISM‐PLUS 1998; PURSUIT 1997; RAPPORT 1998; Rubin 1982; Schnepp 2000; Storck 1996; TRA 2P‐TIMI 50 2009; TRACER 2013; TRITON‐TIMI 38 2006; Waseda 2016; Weseley 1982; Xydakis 2004; Zäuner 1994). Seventy‐two studies were considered at high risk of detection bias.
Incomplete outcome data
Follow‐up data was complete and judged to be at low risk of bias for 41 studies (AASER 2017; Abacilar 2015; Abdul‐Rahman 2007; Alexopoulos 2011; Andrassy 1974; CASSIOPEIR 2014; CHARISMA 2006; CREDO 2005; CURE 2000; Dember 2005; Dixon 2005; EPIC 1994; EPILOG 1997; EPISTENT 1998; ETDRS 1992; Gaede 2003; Ghorbani 2013; Goicoechea 2012; Gröntoft 1998; Hansen 2000; Hidaka 2013: HOT 1993; JPAD 2008; Kamper 1997; Kaufman 2003; Khajehdehi 2002; Kobayashi 1980; Liang 2015; Nyberg 1984; OPT‐CKD 2018; Ota 1996; PEGASUS‐TIMI 54 2014; PLATO 2009; Quarto Di Palo 1991; RAPPORT 1998; Reams 1985; Storck 1996; Tang 2014; TRACER 2013; TRITON‐TIMI 38 2006; Zäuner 1994), incomplete, and judged to be at high risk of bias for 24 studies (Chan 1987; Cheng 1998a; Dash 2013; Donadio 1984; EUCLID 2017; Fiskerstrand 1985; Frascà 1986; Ghorbani 2009; Giustina 1998; Gonzalez 1995; Gröntoft 1985; Harter 1979; J‐PADD 2014; Kaegi 1974; Kooistra 1994; Michie 1977; PIANO‐3 2015; PIANO‐6 2017; Rouzrokh 2010; Sreedhara 1994; Steiness 2018; TRA 2P‐TIMI 50 2009; UK‐HARP‐I 2005; Yang 2016b) and unclear in 48 studies.
Selective reporting
Fifty studies reported expected and clinically‐relevant outcomes and were deemed to be at low risk of bias (AASER 2017; Abacilar 2015; Alexopoulos 2011; ATACAS 2008; CASSIOPEIR 2014; CHANCE 2013; CHARISMA 2006; CREDO 2005; Creek 1990; CURE 2000; Dember 2005; Dixon 2005; EARLY ACS 2005; Ell 1982; EPIC 1994; EPILOG 1997; EPISTENT 1998; ETDRS 1992; FAVOURED 2009; Frascà 1986; Ghorbani 2009; Ghorbani 2013; Gröntoft 1998; Harter 1979; HOT 1993; IMPACT II 1997; JPAD 2008; J‐PADD 2014; Kaegi 1974; Kaufman 2003; Kooistra 1994; Liang 2015; Michie 1977; Middleton 1992; Nyberg 1984; OPT‐CKD 2018; Ota 1996; PEGASUS‐TIMI 54 2014; PLATO 2009; PRISM‐PLUS 1998; PURSUIT 1997; RAPPORT 1998; Sreedhara 1994; STOP 1995; TARGET 2000; TRA 2P‐TIMI 50 2009; TRACER 2013; TRITON‐TIMI 38 2006; UK‐HARP‐I 2005; Yang 2016b), and 63 studies did not report patient‐centred outcomes of bleeding, cardiovascular events, adverse events, or death and were judged to be at high risk of bias.
Other potential sources of bias
Fifty‐seven studies appeared to be free from other sources of bias (AASER 2017; Abacilar 2015; Abdul‐Rahman 2007, Alexopoulos 2011; Anderson 1974; CASSIOPEIR 2014; Chan 1987; CHANCE 2013; CURE 2000; Dash 2013; Dixon 2005; EARLY ACS 2005; EPISTENT 1998; ETDRS 1992; Frascà 1986; Frascà 1997; Ghorbani 2009; Ghorbani 2013; Giustina 1998; Goicoechea 2012; Gröntoft 1985; Hansen 2000; Hidaka 2013; Jiao 2013; JPAD 2008; J‐PADD 2014; Kaegi 1974; Kauffmann 1980; Khajehdehi 2002; Kobayashi 1980; Kontessis 1993; Kooistra 1994; Liang 2015; Michie 1977; Milutinovic 1993; Mozafar 2013; Mozafar 2018; Nakamura 2001d; Nakamura 2002b; Nyberg 1984; Ogawa 2008; PIANO‐2 CKD 2011; PIANO‐3 2015; PIANO‐6 2017; Quarto Di Palo 1991; RESIST 2008; Rouzrokh 2010; Rubin 1982; Salter 1984; Schulze 1990; Storck 1996; Tang 2014; TARGET 2000; Tayebi 2018; TRITON‐TIMI 38 2006; Yang 2016b; Zäuner 1994), 33 studies reported other sources of bias and were judged to be at high risk (Andrassy 1974; CHARISMA 2006; Cheng 1998a; CREDO 2005; Creek 1990; Dember 2005; Ell 1982; EPIC 1994; EPILOG 1997; FAVOURED 2009; Fiskerstrand 1985; Gaede 2003; GLOBAL LEADERS 2018; Gröntoft 1998; Guo 1998; Harter 1979; HOT 1993; IMPACT II 1997; Kamper 1997; Kaufman 2003; Middleton 1992; OPT‐CKD 2018; PEGASUS‐TIMI 54 2014; PLATO 2009; PRISM‐PLUS 1998; PURSUIT 1997; RAPPORT 1998; Sreedhara 1994; STOP 1995; Teng 2018; TRA 2P‐TIMI 50 2009; TRACER 2013; UK‐HARP‐I 2005), and risk of bias was judged to be unclear in 23 studies.
Effects of interventions
See: Table 1
Antiplatelet agents versus control
Fatal or nonfatal myocardial infarction
Antiplatelet agents probably reduced the risk of fatal or nonfatal MI in people with CKD (Analysis 1.1 (18 studies, 15,289 participants): RR 0.88, 95% CI 0.79 to 0.99; I² = 0%; moderate certainty evidence). The evidence was downgraded for risk of bias.
1.1. Analysis.

Comparison 1: Antiplatelet agents versus control, Outcome 1: Fatal or nonfatal myocardial infarction
Fatal or nonfatal stroke
It is uncertain whether antiplatelet agents made any difference to fatal or nonfatal stroke in people with CKD (Analysis 1.2 (12 studies, 10,382 participants): RR 1.01, 95% CI 0.64 to 1.59; I² = 37%; very low certainty evidence). The evidence was downgraded for risk of bias, inconsistency and imprecision. There was moderate heterogeneity observed between studies. Antiplatelet agents were used both for primary and secondary prevention.
1.2. Analysis.

Comparison 1: Antiplatelet agents versus control, Outcome 2: Fatal or nonfatal stroke
Death (any cause)
Antiplatelet agents may have little or no effect on death (any cause) in people with CKD (Analysis 1.3 (35 studies, 18,241 participants): RR 0.94, 95% CI 0.84 to 1.06; I² = 14%; low certainty evidence). The evidence was downgraded for risk of bias and imprecision.
1.3. Analysis.

Comparison 1: Antiplatelet agents versus control, Outcome 3: Death (any cause)
Haemorrhagic stroke
Antiplatelet agents had uncertain effects on haemorrhagic stroke in people with CKD (Analysis 1.4 (9 studies, 6844 participants): RR 1.22, 95% CI 0.69 to 2.17; I² = 0%; low certainty evidence). The evidence was downgraded for risk of bias and imprecision.
1.4. Analysis.

Comparison 1: Antiplatelet agents versus control, Outcome 4: Haemorrhagic stroke
Cardiovascular death
It is uncertain whether antiplatelet agents made any difference to cardiovascular death in people with CKD (Analysis 1.5 (21 studies, 9606 participants): RR 0.87, 95% CI 0.65 to 1.15; I² = 32%; very low certainty evidence). The evidence was downgraded for risk of bias, imprecision, and inconsistency. There was moderate heterogeneity observed between studies.
1.5. Analysis.

Comparison 1: Antiplatelet agents versus control, Outcome 5: Cardiovascular death
Fatal bleeding
It is uncertain whether antiplatelet agents made any difference to fatal bleeding in people with CKD (Analysis 1.6 (21 studies, 7629 participants): RR 1.39, 95% CI 0.10 to 19.48; I² = 30%; very low certainty evidence). The evidence was downgraded for Risk of Bias, imprecision, and inconsistency. There was moderate heterogeneity observed between studies.
1.6. Analysis.

Comparison 1: Antiplatelet agents versus control, Outcome 6: Fatal bleeding
Major bleeding
Major bleeding events included: retroperitoneal; intra‐articular; intra‐ocular, intracranial or intracerebral haemorrhage; gastrointestinal bleeding; bleeding that was fatal, life‐threatening, disabling or required transfusion; corrective surgery or hospitalisation, with or without a fall in haemoglobin (Hb) level of at least 2 g/dL; or melena.
Antiplatelet agents probably increased major bleeding in people CKD (Analysis 1.7 (29 studies, 16,194 participants): RR 1.35, 95% CI 1.10 to 1.65; I² = 12%; moderate certainty evidence). The evidence was downgraded for risk of bias.
1.7. Analysis.

Comparison 1: Antiplatelet agents versus control, Outcome 7: Major bleeding
Minor bleeding
Minor bleeding events were described as follows: not serious or significant; epistaxis; ecchymoses or bruising; blood loss and a drop of more than 10% points in the HCT or of 3 g/dL or more in the Hb concentration; not requiring transfusion; hospitalisation; and event‐related study visit; bleeding from cannulation sites, or haematuria.
Antiplatelet agents may increase the risk of minor bleeding in people with CKD (Analysis 1.8 (21 studies, 13218 participants): RR 1.55, 95% CI 1.27 to 1.90; I² = 58%; low certainty evidence). The was heterogeneity was moderate. The evidence was downgraded for risk of bias and inconsistency.
1.8. Analysis.

Comparison 1: Antiplatelet agents versus control, Outcome 8: Minor bleeding
Kidney failure (end‐stage kidney disease)
Antiplatelet agents may have little or no effect on kidney failure (Analysis 1.9 (11 studies, 1722 participants): RR 0.89, 95% CI 0.70 to 1.14; I² = 23%; low certainty evidence). The evidence was downgraded for risk of bias and imprecision. There was low heterogeneity observed between the studies.
1.9. Analysis.

Comparison 1: Antiplatelet agents versus control, Outcome 9: Kidney failure
Doubling of serum creatinine
Antiplatelet agents may reduce doubling of SCr in people with CKD (Analysis 1.10 (3 studies, 217 participants): RR 0.39, 95% CI 0.17 to 0.86; I² = 0%; low certainty evidence). The evidence was downgraded for risk of bias and imprecision.
1.10. Analysis.

Comparison 1: Antiplatelet agents versus control, Outcome 10: Doubling of serum creatinine
Kidney transplant graft loss
Antiplatelet agents had uncertain effects on kidney transplant graft loss (Analysis 1.11 (2 studies, 91 participants): RR 1.08, 95% CI 0.58 to 2.01; I² = 0%; low certainty evidence). The evidence was downgraded for risk of bias and imprecision.
1.11. Analysis.

Comparison 1: Antiplatelet agents versus control, Outcome 11: Kidney transplant graft loss
Transplant rejection
Antiplatelet agents may have little or no effect on kidney transplant rejection (Analysis 1.12 (2 studies, 97 participants): RR 0.95, 95% CI 0.77 to 1.19; low certainty evidence). The evidence was downgraded for risk of bias and imprecision.
1.12. Analysis.

Comparison 1: Antiplatelet agents versus control, Outcome 12: Transplant rejection
Creatinine clearance
It is uncertain whether antiplatelet agents made any difference to CrCl in people with CKD (Analysis 1.13 (3 studies, 90 participants): MD ‐5.46 mL/min, 95% CI ‐12.33 to 1.41; I² = 38%; very low certainty evidence). The evidence was downgraded for risk of bias, inconsistency and imprecision. There was moderate heterogeneity observed between studies.
1.13. Analysis.

Comparison 1: Antiplatelet agents versus control, Outcome 13: Creatinine clearance
Proteinuria
It is uncertain whether antiplatelet agents made any difference to proteinuria in people with CKD (Analysis 1.14 (3 studies, 80 participants): MD ‐0.74 g/day, 95% CI ‐1.35 to ‐0.13; very low certainty evidence) with substantial heterogeneity in the analysis (I² = 94%) which was as a result of Zäuner 1994; however, there was no difference in the direction of the effect when this study was removed from the meta‐analysis (MD ‐0.14 g/day, 95% CI ‐0.20 to ‐0.08). The evidence was downgraded for risk of bias, inconsistency, and optimal information size not met.
1.14. Analysis.

Comparison 1: Antiplatelet agents versus control, Outcome 14: Proteinuria
Dialysis access failure (thrombosis or loss of patency)
For all access types, it is uncertain whether antiplatelet agents made any difference in reducing the risk of HD access failure (Analysis 1.15 (17 studies, 2847 participants): RR 0.62, 95% CI 0.50 to 0.78; I² = 46%; very low certainty evidence). The evidence was downgraded for risk of bias, indirectness, and inconsistency. There was moderate heterogeneity in this analysis which we explored using subgroup analysis by access type. In these analyses, it is uncertain whether antiplatelet agents (aspirin, sarpogrelate, ticlopidine, or clopidogrel with or without prostacyclin) made any difference in reducing the risk of fistula thrombosis or patency failure by 50% (Analysis 1.15.1 (10 studies, 1741 participants): RR 0.50, 95% CI 0.36 to 0.69; I² = 17%; very low certainty evidence), or shunt or graft failure (Analysis 1.15.2 (5 studies, 1052 participants): RR 0.80, 95% CI 0.62 to 1.03; I² = 49%; very low certainty evidence). It is uncertain whether antiplatelet agents made any difference to fistula or graft, or central venous catheter thrombosis (Analysis 1.15.3 (1 study, 16 participants): RR 0.50, 95% 0.06 to 4.47; very low certainty evidence) (Analysis 1.15.4 (1 study, 38 participants); 0.44, 95% CI 0.16 to 1.20; very low certainty evidence) respectively. Overall, there was no evidence of subgroup interaction based on access type across all types, suggesting the specific vascular access (fistula, graft, shunt, or central venous catheter) (P = 0.13%) was not an effect modifier for the treatment effects observed and indicating the overall effect estimate was the most appropriate.
1.15. Analysis.

Comparison 1: Antiplatelet agents versus control, Outcome 15: Dialysis access failure (thrombosis or loss of patency)
Early access failure (within eight weeks of access creation)
Antiplatelet agents may reduce early dialysis vascular access thrombosis (Analysis 1.16 (8 studies, 1525 participants): RR 0.52, 95% CI 0.38 to 0.70; I²= 8%; low certainty evidence). The evidence was downgraded for risk of bias and optimal information size not met.
1.16. Analysis.

Comparison 1: Antiplatelet agents versus control, Outcome 16: Early access thrombosis (before 8 weeks)
Loss of primary unassisted patency
Two studies (Dixon 2005; Michie 1977) reported a loss of unassisted patency with Dixon 2005 providing 99% of the events. Antiplatelet agents may have little or no effect on reduction of loss of unassisted patency (Analysis 1.17 (2 studies, 665 participants): RR 0.95, 95% 0.89 to 1.03; I² = 0%; low certainty evidence). The evidence was downgraded for risk of bias and imprecision.
1.17. Analysis.

Comparison 1: Antiplatelet agents versus control, Outcome 17: Loss of primary unassisted patency
Failure to attain access suitability of dialysis (maturation)
The definitions of access suitability included: the ability to use the fistula for dialysis with two needles and maintain a blood flow rate ≥ 300 mL/min during eight of 12 dialysis sessions occurring during a 30 day suitability ascertainment period (Dember 2005); failure to use graft by week 12 in patients with a catheter for access (Dixon 2005); fistula ceased to function (Gröntoft 1985); permanent shunt thrombosis (Harter 1979); and failure to develop adequate flow (Michie 1977). It is uncertain whether antiplatelet agents made any difference in the reduction of failure to attain access suitability (Analysis 1.18 (5 studies, 1503 participants): RR 0.63, 95% CI 0.34 to 1.15; I² = 59%, very low certainty evidence). The evidence was downgraded for risk of bias, inconsistency and imprecision. There was moderate heterogeneity potentially due to the differences in definitions of access suitability.
1.18. Analysis.

Comparison 1: Antiplatelet agents versus control, Outcome 18: Failure to attain suitability for dialysis
Need for intervention to attain patency or assist maturation
The need for the intervention to attain patency or assist maturation was described: as surgical revision (FAVOURED 2009: Kaegi 1974); thrombectomy (Abacilar 2015; Michie 1977); percutaneous intervention to restore patency or promote maturation (Dember 2005); or angioplasty (Dixon 2005).
Antiplatelet agents may have little or no effect on the reduction of the risk for the need for the intervention to attain patency or assist maturation in people treated with HD (Analysis 1.19 (6 studies, 2067 participants): RR 0.87, 95% CI 0.72 to 1.05; I² = 0%: low certainty evidence). The evidence was downgraded for risk of bias and imprecision.
1.19. Analysis.

Comparison 1: Antiplatelet agents versus control, Outcome 19: Need for intervention to attain patency or assist maturation
All‐cause hospitalisation
Antiplatelet agents may have little or no effect on all‐cause hospitalisation in people treated with HD (Analysis 1.20 (3 studies, 3535 participants): RR 0.97, 95% CI 0.87 to 1.10; I² = 0%; low certainty evidence). The evidence was downgraded for risk of bias and imprecision.
1.20. Analysis.

Comparison 1: Antiplatelet agents versus control, Outcome 20: Hospitalisation (any cause)
Cardiovascular hospitalisation
It is uncertain whether antiplatelet agents made any difference in cardiovascular hospitalisation in CKD and HD (Analysis 1.21 (3 studies, 3535 participants): RR 0.93, 95% CI 0.76 to 1.14; I² = 46%; very low certainty evidence). The evidence was downgraded for Risk of Bias, inconsistency and imprecision. There was moderate heterogeneity potentially due to differences in the adjudication of the outcome.
1.21. Analysis.

Comparison 1: Antiplatelet agents versus control, Outcome 21: Cardiovascular hospitalisation
Treatment withdrawal
Antiplatelet agents may have little or no effect on withdrawal from treatment compared with placebo or no treatment in CKD and HD (Analysis 1.22 (15 studies, 2669 participants): RR 0.97, 95% CI 0.83 to 1.14; I² = 0%; low certainty evidence). The evidence was downgraded for risk of bias and imprecision.
1.22. Analysis.

Comparison 1: Antiplatelet agents versus control, Outcome 22: Treatment withdrawal
Prasugrel versus clopidogrel
TRITON‐TIMI 38 2006 compared prasugrel plus aspirin with clopidogrel plus aspirin and provided data for 1490 people with CKD during a median follow‐up of 14.5 months. Data were not available for fatal or nonfatal stroke, haemorrhagic stroke, fatal bleeding, kidney failure, doubling of SCr, kidney transplant graft loss, transplant rejection, CrCl, proteinuria, dialysis access failure, early access thrombosis, loss of primary unassisted patency, failure to attain suitability for dialysis, need for intervention to attain patency or assist maturation, all‐cause hospitalisation, cardiovascular hospitalisation, and treatment withdrawal.
Fatal or nonfatal myocardial infarction
TRITON‐TIMI 38 2006 reported no difference between prasugrel plus aspirin compared to clopidogrel plus aspirin on fatal or nonfatal MI (Analysis 2.1 (1 study, 1490 participants): RR 0.78, 95% CI 0.58 to 1.05). Since not all participants experienced MI before treatment allocation, antiplatelet agents were used both for primary and secondary prevention.
2.1. Analysis.

Comparison 2: Prasugrel versus clopidogrel, Outcome 1: Fatal or nonfatal myocardial infarction
Death (any cause)
TRITON‐TIMI 38 2006 reported no difference between prasugrel plus aspirin compared to clopidogrel plus aspirin death (any cause) (Analysis 2.2 (1 study, 1490 participants): RR 0.81, 95% CI 0.56 to 1.18).
2.2. Analysis.

Comparison 2: Prasugrel versus clopidogrel, Outcome 2: Death (any cause)
Cardiovascular death
TRITON‐TIMI 38 2006 reported no difference between prasugrel plus aspirin compared to clopidogrel plus aspirin on cardiovascular death (Analysis 2.3 (1 study, 1469 participants): RR 1.35, 95% CI 0.87 to 2.10).
2.3. Analysis.

Comparison 2: Prasugrel versus clopidogrel, Outcome 3: Cardiovascular death
Major bleeding
Major bleeding was defined according to the Thrombolysis In Myocardial Infarction (TIMI) criteria for major bleeding (intracranial haemorrhage, clinically evident bleeding including imaging and a drop in the Hb of ≥5 g/dL). TRITON‐TIMI 38 2006 reported no difference between prasugrel plus aspirin compared to clopidogrel plus aspirin on major bleeding (Analysis 2.4 (1 study, 1475 participants): RR 1.49, 95% CI 0.83 to 2.66).
2.4. Analysis.

Comparison 2: Prasugrel versus clopidogrel, Outcome 4: Major bleeding
Minor bleeding
Minor bleeding was defined as clinically evident bleeding including imaging and a fall in the Hb of between 3 and 5 g/dL. TRITON‐TIMI 38 2006 reported no difference between prasugrel plus aspirin compared to clopidogrel plus aspirin on minor bleeding (Analysis 2.5 (1 study, 1469 participants): RR 1.35, 95% CI 0.87 to 2.10).
2.5. Analysis.

Comparison 2: Prasugrel versus clopidogrel, Outcome 5: Minor bleeding
Ticagrelor versus clopidogrel
Three studies (OPT‐CKD 2018; PIANO‐3 2015; PIANO‐6 2017) compared ticagrelor with or without aspirin with clopidogrel alone or in combination with aspirin. Data were not available for haemorrhagic stroke, kidney failure, doubling of SCr, kidney transplant graft loss, transplant rejection, CrCl, proteinuria, dialysis access failure, early access thrombosis, loss of primary unassisted patency, failure to attain suitability for dialysis, need for intervention to attain patency or assist maturation, all‐cause hospitalisation, and cardiovascular hospitalisation.
Fatal or nonfatal myocardial infarction
OPT‐CKD 2018 reported no difference between ticagrelor compared to clopidogrel on fatal or nonfatal MI in CKD during 30 days follow‐up (Analysis 3.1 (1 study, 60 participants): RR 3.00, 95% CI 0.13 to 70.83). Since not all participants experienced MI before treatment allocation, antiplatelet agents were used both for primary and secondary prevention.
3.1. Analysis.

Comparison 3: Ticagrelor versus clopidogrel, Outcome 1: Fatal or nonfatal myocardial infarction
Fatal or nonfatal stroke
OPT‐CKD 2018 reported no difference between ticagrelor compared to clopidogrel on fatal or nonfatal MI in CKD during 30 days follow‐up (Analysis 3.2 (1 study, 60 participants): RR 3.00, 95% CI 0.13 to 70.83). Since it was not reported if all participants experienced a stroke before treatment allocation, it was not clear if antiplatelet agents were used either for primary or secondary prevention.
3.2. Analysis.

Comparison 3: Ticagrelor versus clopidogrel, Outcome 2: Fatal or nonfatal stroke
Death (any cause)
OPT‐CKD 2018 and PIANO‐6 2017 reported the effect of ticagrelor with clopidogrel while PIANO‐3 2015 reported the effect of ticagrelor plus aspirin with clopidogrel plus aspirin between 14 to 30 days follow‐up. Antiplatelet agents had uncertain effects on death (any cause) in CKD and HD (Analysis 3.3 (3 studies, 137 participants): RR 2.00, 95% CI 0.19 to 20.90; very low certainty evidence). The evidence was downgraded for risk of bias and imprecision.
3.3. Analysis.

Comparison 3: Ticagrelor versus clopidogrel, Outcome 3: Death (any cause)
Cardiovascular death
OPT‐CKD 2018 and PIANO‐6 2017 reported the effect of ticagrelor with clopidogrel while PIANO‐3 2015 reported the effect of ticagrelor plus aspirin with clopidogrel plus aspirin between 14 to 30 days follow‐up. Antiplatelet agents had uncertain effects on cardiovascular death in CKD and HD (Analysis 3.4 (3 studies, 137 participants): RR 5.00, 95% CI 0.25 to 99.59; low certainty evidence). The evidence was downgraded for risk of bias and imprecision.
3.4. Analysis.

Comparison 3: Ticagrelor versus clopidogrel, Outcome 4: Cardiovascular death
Fatal bleeding
PIANO‐3 2015 reported the effect of ticagrelor plus aspirin with clopidogrel plus aspirin and PIANO‐6 2017 reported the effect of ticagrelor with clopidogrel in HD during 14 days follow‐up. No fatal bleeding events were reported in either study (Analysis 3.5; 2 studies, 77 participants).
3.5. Analysis.

Comparison 3: Ticagrelor versus clopidogrel, Outcome 5: Fatal bleeding
Major bleeding
Major bleeding was assessed using the Bleeding Academic Research Consortium (BARC) (OPT‐CKD 2018) or according to the PLATO criteria (PIANO‐3 2015). OPT‐CKD 2018 reported the effect of ticagrelor with clopidogrel during 30 days follow‐up, while PIANO‐3 2015 reported the effect of ticagrelor plus aspirin with clopidogrel plus aspirin during 14 days follow‐up. Antiplatelet agents had uncertain effects on major bleeding in CKD and HD (Analysis 3.6 (2 studies, 85 participants): RR 0.33, 95% CI 0.01 to 7.87; low certainty evidence). The evidence was downgraded for risk of bias and imprecision.
3.6. Analysis.

Comparison 3: Ticagrelor versus clopidogrel, Outcome 6: Major bleeding
Minor bleeding
Minor bleeding was assessed using the Bleeding Academic Research Consortium (BARC). PIANO‐6 2017 reported no difference between ticagrelor compared to clopidogrel on minor bleeding in HD during 14 days follow‐up (Analysis 3.7 (1 study, 52 participants): RR 1.06, 95% CI 0.10 to 10.90).
3.7. Analysis.

Comparison 3: Ticagrelor versus clopidogrel, Outcome 7: Minor bleeding
Treatment withdrawal
PIANO‐6 2017 reported no difference between ticagrelor compared to clopidogrel on treatment withdrawal in HD during 14 days follow‐up (Analysis 3.8 (1 study, 52 participants): RR 1.59, 95% CI 0.18 to 14.19).
3.8. Analysis.

Comparison 3: Ticagrelor versus clopidogrel, Outcome 8: Treatment withdrawal
Clopidogrel (low‐dose) versus clopidogrel (high‐dose)
Liang 2015, which compared a low‐ versus high‐dose clopidogrel, provided data for 370 people with CKD during 30 days follow‐up. Data were not available for fatal or nonfatal MI, fatal or nonfatal stroke, death (any cause), fatal bleeding, major bleeding, minor bleeding, kidney failure, doubling of SCr, kidney transplant graft loss, transplant rejection, CrCl, proteinuria, dialysis access failure, early access thrombosis, loss of primary unassisted patency, failure to attain suitability for dialysis, need for intervention to attain patency or assist maturation, all‐cause hospitalisation, cardiovascular hospitalisation, and treatment withdrawal.
Haemorrhagic stroke
Liang 2015 reported no haemorrhagic stroke events with either low‐dose or high‐dose clopidogrel (Analysis 4.1 (1 study, 370 participants)).
4.1. Analysis.

Comparison 4: Clopidogrel (low dose) versus clopidogrel (high dose), Outcome 1: Haemorragic stroke
Cardiovascular death
Liang 2015 reported no difference between low‐ versus high‐dose clopidogrel on cardiovascular death (Analysis 4.2 (1 study, 370 participants): RR 4.04, 95% CI 0.46 to 35.83).
4.2. Analysis.

Comparison 4: Clopidogrel (low dose) versus clopidogrel (high dose), Outcome 2: Cardiovascular death
Abciximab versus tirofiban
TARGET 2000 compared abciximab plus aspirin with tirofiban plus aspirin and provided unpublished data for 790 people with CKD between 6 and 12 months follow‐up. Data were not available for fatal or nonfatal stroke, haemorrhagic stroke, cardiovascular death, fatal bleeding, major bleeding, minor bleeding, kidney failure, doubling of SCr, kidney transplant graft loss, transplant rejection, CrCl, proteinuria, dialysis access failure, early access thrombosis, loss of primary unassisted patency, failure to attain suitability for dialysis, need for intervention to attain patency or assist maturation, all‐cause hospitalisation, cardiovascular hospitalisation, and treatment withdrawal.
Fatal or nonfatal myocardial infarction
TARGET 2000 reported abciximab plus aspirin may decrease fatal or nonfatal MI compared to tirofiban plus aspirin during 6 months follow‐up (Analysis 5.1 (1 study, 790 participants): RR 2.33, 95% CI 1.57 to 3.45). Since not all participants experienced MI before treatment allocation, antiplatelet agents were used both for primary and secondary prevention.
5.1. Analysis.

Comparison 5: Abciximab versus tirofiban, Outcome 1: Fatal or nonfatal myocardial infarction
Death (any cause)
TARGET 2000 reported no difference between abciximab plus aspirin compared to tirofiban plus aspirin on death (any cause) during 12 months follow‐up (Analysis 5.2 (1 study, 790 participants): RR 1.73, 95% CI 0.92 to 3.23).
5.2. Analysis.

Comparison 5: Abciximab versus tirofiban, Outcome 2: Death (any cause)
Defibrotide versus dypiridamole
Frascà 1986 compared defibrotide with dypiridamole and provided data for 80 people that received a kidney transplant during 4 years of follow‐up. Data were not available for fatal or nonfatal MI, fatal or nonfatal stroke, haemorrhagic stroke, major bleeding, minor bleeding, kidney failure, doubling of SCr, transplant rejection, CrCl, proteinuria, dialysis access failure, early access thrombosis, loss of primary unassisted patency, failure to attain suitability for dialysis, need for intervention to attain patency or assist maturation, all‐cause hospitalisation, cardiovascular hospitalisation, and treatment withdrawal.
Death (any cause)
Frascà 1986 reported no difference between defibrotide compared to dypiridamole on death (any cause) (Analysis 6.1 (1 study, 76 participants): RR 0.30, 95% CI 0.01 to 7.16).
6.1. Analysis.

Comparison 6: Defibrotide versus dypiridamole, Outcome 1: Death (any cause)
Cardiovascular death
Frascà 1986 reported no difference between defibrotide compared to dypiridamole on cardiovascular death (Analysis 6.2 (1 study, 76 participants): RR 0.30, 95% CI 0.01 to 7.16).
6.2. Analysis.

Comparison 6: Defibrotide versus dypiridamole, Outcome 2: Cardiovascular death
Fatal bleeding
Frascà 1986 reported no fatal bleeding events with either defibrotide or dypiridamole (Analysis 6.3 (1 study, 76 participants)).
6.3. Analysis.

Comparison 6: Defibrotide versus dypiridamole, Outcome 3: Fatal bleeding
Kidney transplant graft loss
Frascà 1986 reported no difference between defibrotide compared to dypiridamole on kidney transplant graft loss (Analysis 6.4 (1 study, 76 participants): RR 0.13, 95% CI 0.02 to 1.00).
6.4. Analysis.

Comparison 6: Defibrotide versus dypiridamole, Outcome 4: Kidney transplant graft loss
Cilostazol versus sarpogrelate
Hidaka 2013 compared cilostazol with sarpogrelate and provided data for 35 people undergoing HD during 24 weeks follow‐up. Data were not available for fatal or nonfatal MI, fatal or nonfatal stroke, death (any cause), haemorrhagic stroke, cardiovascular death, fatal bleeding, minor bleeding, kidney failure, doubling of SCr, kidney transplant graft loss, transplant rejection, CrCl, proteinuria, dialysis access failure, early access thrombosis, loss of primary unassisted patency, failure to attain suitability for dialysis, need for intervention to attain patency or assist maturation, all‐cause hospitalisation, cardiovascular hospitalisation and treatment withdrawal.
Major bleeding
Hidaka 2013 reported no major bleeding events with either cilostazol or sarpogrelate (Analysis 7.1 (1 study, 35 participants)).
7.1. Analysis.

Comparison 7: Cilostazol versus sarpogrelate, Outcome 1: Major bleeding
Beraprost versus cilostazol or sarpogrelate
J‐PADD 2014, which compared beraprost with cilostazol or sarpogrelate, provided data for 72 people undergoing HD during 24 weeks follow‐up. Data were not available for haemorrhagic stroke, major bleeding, minor bleeding, kidney failure, doubling of SCr, kidney transplant graft loss, transplant rejection, CrCl, proteinuria, dialysis access failure, early access thrombosis, loss of primary unassisted patency, failure to attain suitability for dialysis, need for intervention to attain patency or assist maturation, all‐cause hospitalisation, cardiovascular hospitalisation, and treatment withdrawal.
Fatal or nonfatal myocardial infarction
J‐PADD 2014 reported no fatal or nonfatal MI events with beraprost, cilostazol or sarpogrelate (Analysis 8.1 (1 study, 68 participants)). The treatment was performed for secondary prevention of MI.
8.1. Analysis.

Comparison 8: Beraprost versus cilostazol or sarpogrelate, Outcome 1: Fatal or nonfatal myocardial infarction
Fatal or nonfatal stroke
J‐PADD 2014 reported no difference between beraprost compared to cilostazol or sarpogrelate on fatal or nonfatal stroke (Analysis 8.2 (1 study, 68 participants): RR 0.19, 95% CI 0.01 to 3.79). The treatment was performed for secondary prevention of stroke.
8.2. Analysis.

Comparison 8: Beraprost versus cilostazol or sarpogrelate, Outcome 2: Fatal or nonfatal stroke
Death (any cause)
J‐PADD 2014 reported no difference between beraprost compared to cilostazol or sarpogrelate on death (any cause) (Analysis 8.3 (1 study, 68 participants): RR 0.94, 95% CI 0.06 to 14.47).
8.3. Analysis.

Comparison 8: Beraprost versus cilostazol or sarpogrelate, Outcome 3: Death (any cause)
Cardiovascular death
J‐PADD 2014 reported no difference between beraprost compared to cilostazol or sarpogrelate on cardiovascular death (Analysis 8.4 (1 study, 68 participants): RR 0.94, 95% CI 0.06 to 14.47).
8.4. Analysis.

Comparison 8: Beraprost versus cilostazol or sarpogrelate, Outcome 4: Cardiovascular death
Fatal bleeding
J‐PADD 2014 reported no fatal bleeding events with beraprost, cilostazol or sarpogrelate (Analysis 8.5 (1 study, 68 participants)).
8.5. Analysis.

Comparison 8: Beraprost versus cilostazol or sarpogrelate, Outcome 5: Fatal bleeding
Sensitivity and subgroups analyses
Antiplatelet agents versus placebo
Fatal or nonfatal myocardial infarction
Since not all participants experienced MI before treatment allocation, antiplatelet agents could be used for primary and secondary prevention. Five studies (Creek 1990; Ell 1982; Kaufman 2003; STOP 1995; UK‐HARP‐I 2005) reported insufficient information to assess if the intervention was performed either for primary or secondary prevention. These studies were not included in the subgroup analyses for primary/secondary prevention against MI. Four studies (Dember 2005; Dixon 2005; ETDRS 1992; HOT 1993) prescribed antiplatelet agents both for primary and secondary prevention. Since data were not reported separately for patients with or without previous MI, it was not possible to include these studies in the subgroup analyses for primary/secondary prevention against MI.
Subgroup analysis for primary prevention against myocardial infarction ‐ stratified by stage of CKD
There were no studies that assessed the intervention for primary prevention against MI, and subgroup analyses were not performed.
Subgroup analysis for secondary prevention against myocardial infarction ‐ stratified by stage of CKD
Antiplatelet agents may have little or no effect on MI for secondary prevention in CKD (Analysis 9.1 (8 studies, 7270 participants): RR 0.93, 95% CI 0.81 to 1.06: I² = 0%; low certainty evidence). The evidence was downgraded for risk of bias and imprecision. However, a small number of studies contributed data to predialysis and no data were available for dialysis and transplant, meaning that the analysis may not be able to detect subgroup differences within different stages of CKD.
9.1. Analysis.

Comparison 9: Primary/secondary prevention for fatal/non fatal myocardial infarction (subgroup analysis), Outcome 1: Secondary prevention
Sensitivity analysis for fatal or nonfatal myocardial infarction ‐ stratified by adequate allocation concealment
Considering only studies with adequate allocation concealment, antiplatelet agents may reduce the risk of fatal or nonfatal MI in CKD (Analysis 10.1.1 (8 studies, 10,459 participants): RR 0.80, 95% CI 0.65 to 0.98; I² = 31%; low certainty evidence). The evidence was downgraded for risk of bias and inconsistency. There was moderate heterogeneity.
10.1. Analysis.

Comparison 10: Sensitivity analysis (adequate allocation concealment), Outcome 1: Fatal or nonfatal myocardial infarction
Sensitivity analysis for fatal or nonfatal myocardial infarction ‐ stratified by a low risk of attrition bias
Considering only studies with low risk of attrition, antiplatelet agents probably reduce the risk of fatal or nonfatal MI in CKD (Analysis 11.1.1 (11 studies, 9387 participants): RR 0.75, 95% CI 0.62 to 0.90; I² = 0%; moderate certainty evidence). The evidence was downgraded for risk of bias.
11.1. Analysis.

Comparison 11: Sensitivity analysis (low risk of attrition), Outcome 1: Fatal or nonfatal myocardial infarction
Fatal or nonfatal stroke
Five studies (Creek 1990; Ell 1982; Kaufman 2003; STOP 1995; UK‐HARP‐I 2005) reported insufficient information to assess if the intervention was performed either for primary or secondary prevention. These studies were not included in the subgroup analyses for primary/secondary prevention against stroke. Four studies (Dember 2005; Dixon 2005; ETDRS 1992; HOT 1993) prescribed antiplatelet agents both for primary and secondary prevention. Since data were not reported separately for patients with or without previous stroke, it was not possible to include these studies in the subgroup analyses for primary/secondary prevention against stroke.
Subgroup analysis for stroke ‐ stratified by stage of CKD
It is uncertain whether antiplatelet agents made any difference in stroke for secondary prevention in CKD (Analysis 12.1 (11 studies, 9544 participants): RR 1.00, 95% CI 0.58 to 1.72: I² = 43%; very low certainty evidence). The evidence was downgraded for risk of bias, inconsistency, and imprecision. The test for subgroup differences indicates that there is no statistically significant subgroup effect (P = 0.63), suggesting that different stages of CKD do not modify the effect of antiplatelet agents on the risk of stroke.
12.1. Analysis.

Comparison 12: Stroke (subgroup analysis), Outcome 1: Stage of CKD
Subgroup analysis for stroke ‐ stratified by diabetes
The test for subgroup differences indicates that there is no statistically significant subgroup effect (P = 0.59), suggesting that diabetes does not modify the effect of antiplatelet agents on the risk of stroke (Analysis 12.2 (6 studies, 4368 participants): RR 1.49, 95% CI 0.68 to 3.25; I² = 40%; very low certainty evidence). The evidence was downgraded for risk of bias, inconsistency and imprecision. The pooled effect estimate for studies with < 50% of diabetic patients favoured antiplatelet agents (Analysis 12.2.1 (3 studies, 1525 participants): RR 0.96, 95% CI 0.15 to 6.03; I² = 22%), while the pooled effects for studies where at least 50% of participants had diabetes (Analysis 12.2.2 (3 studies, 2843 participants): RR 1.70, 95% CI 0.64 to 4.49; I² = 64%) and favoured control.
12.2. Analysis.

Comparison 12: Stroke (subgroup analysis), Outcome 2: Diabetes
Subgroup analysis for stroke ‐ stratified by males
The test for subgroup differences indicates that there is no statistically significant subgroup effect (P = 0.34), suggesting that gender does not modify the effect of antiplatelet agents on the risk of stroke (Analysis 12.3 (7 studies, 7987 participants): RR 1.19, 95% CI 0.68 to 2.07; I² = 43%; very low certainty evidence). The evidence was downgraded for risk of bias, inconsistency and imprecision. However, a different number of studies and participants contributed data to the studies with less than 50% of males subgroup compared to the studies with at least 50% of males subgroup, meaning that the analysis may not be able to detect subgroup differences.
12.3. Analysis.

Comparison 12: Stroke (subgroup analysis), Outcome 3: Sex
Subgroup analysis for stroke ‐ stratified by duration of intervention
The test for subgroup differences indicates that there is no statistically significant subgroup effect (P = 0.15), suggesting that duration of intervention does not modify the effect of antiplatelet agents on the risk of stroke (Analysis 12.4 (11 studies, 9544 participants): RR 1.00, 95% CI 0.58 to 1.72; I² = 43%; very low certainty evidence). The evidence was downgraded for risk of bias, inconsistency and imprecision. However, a smaller number of studies and/or participants contributed data to the duration of intervention lower than 6 months and between 6 and 12 months subgroups than to the duration of the treatment greater than 12 months subgroup, meaning that the analysis may not be able to detect subgroup differences.
12.4. Analysis.

Comparison 12: Stroke (subgroup analysis), Outcome 4: Duration of intervention
Death from any cause
Sensitivity analysis for death (any cause) ‐ stratified by adequate allocation concealment
Considering only studies with adequate allocation concealment, it is uncertain whether antiplatelet agents made any difference to death (any cause) in CKD (Analysis 10.2 (10 studies, 11,443 participants): RR 1.00, 95% CI 0.83 to 1.22; I² = 37%; very low certainty evidence). The evidence was downgraded for risk of bias, inconsistency and imprecision. The heterogeneity was moderate.
10.2. Analysis.

Comparison 10: Sensitivity analysis (adequate allocation concealment), Outcome 2: Death (any cause)
Sensitivity analysis for death (any cause) ‐ stratified by a low risk of attrition bias
Considering only studies with low risk of attrition, it is uncertain whether antiplatelet agents made any difference to death (any cause) in CKD and HD (Analysis 11.2 (19 studies, 10,966 participants): RR 0.99, 95% CI 0.82 to 1.20; I² = 30%; very low certainty evidence). The evidence was downgraded for risk of bias, inconsistency and imprecision. The heterogeneity was moderate.
11.2. Analysis.

Comparison 11: Sensitivity analysis (low risk of attrition), Outcome 2: Death (any cause)
Cardiovascular death
Sensitivity analysis for cardiovascular death ‐ stratified by adequate allocation concealment
Considering only studies with adequate allocation concealment, it is uncertain whether antiplatelet agents made any difference to cardiovascular death mortality in CKD (Analysis 10.3 (2 studies, 5628 participants): RR 1.08, 95% CI 0.48 to 2.44; I² = 85%; very low certainty evidence). The evidence was downgraded for risk of bias, inconsistency and imprecision. There was substantial heterogeneity.
10.3. Analysis.

Comparison 10: Sensitivity analysis (adequate allocation concealment), Outcome 3: Cardiovascular death
Sensitivity analysis for cardiovascular death ‐ stratified by a low risk of attrition bias
Considering only studies with low risk of attrition, it is uncertain whether antiplatelet agents made any difference to cardiovascular death mortality in CKD, HD and transplant recipients (Analysis 11.3 (11 studies, 6872 participants): RR 0.94, 95% CI 0.60 to 1.47; I² = 66%; very low certainty evidence). The evidence was downgraded for risk of bias, inconsistency and imprecision. There was moderate heterogeneity.
11.3. Analysis.

Comparison 11: Sensitivity analysis (low risk of attrition), Outcome 3: Cardiovascular death
Major bleeding
Sensitivity analysis for major bleeding ‐ stratified by adequate allocation concealment
Considering only studies with adequate allocation concealment, antiplatelet agents may increase major bleeding in CKD (Analysis 10.4 (9 studies, 10,360 participants): RR 1.53, 95% CI 1.07 to 2.20; I² = 52%; low certainty evidence). The evidence was downgraded for risk of bias and inconsistency. There was moderate heterogeneity.
10.4. Analysis.

Comparison 10: Sensitivity analysis (adequate allocation concealment), Outcome 4: Major bleeding
Sensitivity analysis for major bleeding ‐ stratified by a low risk of attrition bias
Considering only studies with low risk of attrition, antiplatelet agents probably increased major bleeding in CKD and HD (Analysis 11.4 (17 studies, 9549 participants): RR 1.62, 95% CI 1.19 to 2.20; I² = 15%; moderate certainty evidence). The evidence was downgraded for risk of bias. There was low heterogeneity.
11.4. Analysis.

Comparison 11: Sensitivity analysis (low risk of attrition), Outcome 4: Major bleeding
Minor bleeding
Subgroup analysis for minor bleeding ‐ stratified by stage of CKD
The test for subgroup differences indicates that there is no statistically significant subgroup effect (P = 0.16), suggesting that different stages of CKD do not modify the effect of antiplatelet agents on the risk of minor bleeding (Analysis 13.1). However, a smaller number of studies and participants contributed data to both predialysis, dialysis and transplant and HD subgroups than to the CKD subgroup, meaning that the analysis may not be able to detect subgroup differences.
13.1. Analysis.

Comparison 13: Minor bleeding (subgroup analysis), Outcome 1: Stage of CKD
Subgroup analysis for minor bleeding ‐ stratified by diabetes
The test for subgroup differences indicates that there is no statistically significant subgroup effect (P = 0.08), suggesting that diabetes does not modify the effect of antiplatelet agents on the risk of minor bleeding (Analysis 13.2).
13.2. Analysis.

Comparison 13: Minor bleeding (subgroup analysis), Outcome 2: Diabetes
Subgroup analysis for minor bleeding ‐ stratified by sex
The test for subgroup differences indicates that there is no statistically significant subgroup effect (P = 0.42), suggesting that gender does not modify the effect of antiplatelet agents on the risk of minor bleeding (Analysis 13.3).
13.3. Analysis.

Comparison 13: Minor bleeding (subgroup analysis), Outcome 3: Sex
Subgroup analysis for minor bleeding ‐ stratified by duration of intervention
The test for subgroup differences indicates that there is no statistically significant subgroup effect (P = 0.74), suggesting that duration of intervention does not modify the effect of antiplatelet agents on the risk of minor bleeding (Analysis 13.4). However, a smaller number of studies and participants contributed data to the duration of intervention lower than 6 months and greater than 12 months subgroups than to the duration of the treatment between 6 and 12 months subgroup, meaning that the analysis may not be able to detect subgroup differences.
13.4. Analysis.

Comparison 13: Minor bleeding (subgroup analysis), Outcome 4: Duration of intervention
Dialysis access failure
Subgroup analysis for dialysis access failure ‐ stratified by stage of CKD
Subgroup analyses based on the stage of CKD were not possible due to insufficient numbers of studies.
Subgroup analysis for dialysis access failure ‐ stratified by diabetes
The test for subgroup differences indicates that there is no statistically significant subgroup effect (P = 0.77), suggesting that diabetes does not modify the effect of antiplatelet agents on dialysis access failure (Analysis 14.1).
14.1. Analysis.

Comparison 14: Dialysis access failure (subgroup analysis), Outcome 1: Diabetes
Subgroup analysis for dialysis access failure ‐ stratified by male
The test for subgroup differences indicates that there is no statistically significant subgroup effect (P = 0.34), suggesting that gender does not modify the effect of antiplatelet agents on dialysis access failure (Analysis 14.2).
14.2. Analysis.

Comparison 14: Dialysis access failure (subgroup analysis), Outcome 2: Sex
Subgroup analysis for dialysis access failure ‐ stratified by duration of intervention
The test for subgroup differences suggests that there is a statistically significant subgroup effect (P = 0.001), meaning that duration of intervention significantly modifies the effect of antiplatelet agents on dialysis access failure (Analysis 14.3 (17 studies, 2847 participants): RR 0.62, 95% CI 0.50 to 0.78; I² = 46%; low certainty evidence). The evidence was downgraded for risk of bias and inconsistency. There was moderate heterogeneity. A sufficient number of studies and participants were not included in each subgroup, so the covariate distribution could be a concern for this subgroup analysis. Both the pooled effect estimate for the duration of the intervention less than 6 months (Analysis 14.3.1 (11 studies, 1705 participants): RR 0.55, 95% CI 0.44 to 0.70; I² = 0%; low certainty evidence), between 6 and 12 months (Analysis 14.3.2 (4 studies, 386 participants): RR 0.59, 95% CI 0.37 to 0.96; I² = 58%; very low certainty evidence) and greater than 12 months (Analysis 14.3.3 (2 studies, 756 participants): RR 0.94, 95% CI 0.79 to 1.11; I² = 0%; very low certainty evidence) favoured antiplatelet agents. There was substantial unexplained heterogeneity between the studies and the validity of the treatment effect estimated for each subgroup was uncertain, as individual study results were inconsistent.
14.3. Analysis.

Comparison 14: Dialysis access failure (subgroup analysis), Outcome 3: Duration of intervention
Failure to attain access suitability of dialysis
Subgroup analysis for failure to attain access suitability of dialysis ‐ stratified by stage of CKD
Subgroup analyses based on the stage of CKD were not possible due to insufficient numbers of studies.
Subgroup analysis for failure to attain access suitability of dialysis ‐ stratified by diabetes
Subgroup analyses based on diabetes were not possible due to insufficient numbers of studies.
Subgroup analysis for failure to attain access suitability of dialysis ‐ stratified by male
Subgroup analyses based on the prevalence of males were not possible due to insufficient numbers of studies.
Subgroup analysis for failure to attain access suitability of dialysis ‐ stratified by duration of intervention
The test for subgroup differences indicates that there is no statistically significant subgroup effect (P = 0.75), suggesting that duration of intervention does not modify the effect of antiplatelet agents on the failure to attain access suitability of dialysis (Analysis 15.1). However, a smaller number of studies and participants contributed data to the duration of intervention greater than 12 months subgroup than to the duration of the treatment less than 6 months subgroup, meaning that the analysis may not be able to detect subgroup differences.
15.1. Analysis.

Comparison 15: Failure to attain suitability for dialysis (subgroup analysis), Outcome 1: Duration of intervention
Antiplatelet agents versus antiplatelet agents
Sensitivity and subgroup analyses were not possible when comparing one antiplatelet with another antiplatelet due to the insufficient number of available studies.
Discussion
Summary of main results
This updated review indicated that antiplatelet agents (acetylsalicylic acid, adenosine diphosphate receptor inhibitors, adenosine reuptake inhibitors, glycoprotein IIb/IIIa inhibitors, picotamide, or sulphinpyrazone) probably prevents fatal or nonfatal MI in people with CKD. Antiplatelet treatment probably increases major bleeding (including bleeding events that result in hospital admission, transfusion, or disability) and may increase minor bleeding in people with CKD. There is insufficient available evidence to define clearly the role of antiplatelet treatment in primary prevention (preventing cardiovascular events in people without existing cardiovascular disease) in those with CKD. Few studies reported the efficacy of antiplatelet therapies for secondary prevention against MI or stroke in CKD, and sparse or no data were available for dialysis and transplant recipients.
Antiplatelet agents started around the time of vascular access surgery may reduce early vascular access thrombosis or patency failure, but there was insufficient evidence to show that antiplatelet therapy improves dialysis access maturation, access suitability for dialysis or reduces the need for intervention to attain patency. Overall, the effect of antiplatelet agents on the prevention of kidney failure in people with CKD, kidney transplant loss, or transplant rejection is uncertain.
Direct comparisons of antiplatelet agents are limited to a few studies in which data for the subgroup of participants with CKD, HD and kidney transplant have been recently reported or provided. Currently, there are scant data to recommend that one antiplatelet agent is more efficacious than another in any clinical setting (primary prevention or secondary prevention), particularly for people with acute coronary syndromes or those undergoing percutaneous coronary interventions who frequently have coexistent CKD.
Overall completeness and applicability of evidence
While the analyses included data obtained from a comprehensive search and unpublished data from numerous investigators, particularly for cardiovascular events, the data were incomplete in several areas. Firstly, data for transplant recipients were limited and provided by smaller and older studies, published between 1974 and 1996. A study of aspirin included transplant recipients in addition to individuals with CKD and those requiring dialysis (UK‐HARP‐I 2005) but data for the transplant subgroup (133 participants) were not available and would have provided very few events for relevant clinical outcomes. Outcome data for kidney transplant recipients were restricted generally to transplant function or rejection in two studies, and information about major cardiovascular events was scarce. Further, only Frascà 1986 showed a head‐to‐head comparison of antiplatelet agents (glycoprotein IIb/IIIa inhibitor versus adenosine reuptake inhibitor) in kidney transplant recipients and further research is needed in these populations. Secondly, very few or no data for cardiovascular death were available in studies of glycoprotein IIb/IIIa inhibitors administered in addition to standard therapy, low dose versus high dose clopidogrel and cilostazol versus sarpogrelate in patients with CKD or undergoing HD.
Quality of the evidence
Although this review found consistent effect estimates for important clinical outcomes (MI and bleeding) in analyses that include approximately 16,000 people with CKD and between 500 to 1000 events, our conclusions must be considered more cautiously due to several potential limitations in the available data. Studies with zero events in both arms could not be analysed because they did not yield information on both the magnitude and direction of the relative treatment effects.
Study limitations
In this updated review, selective reporting of outcomes may reduce the strength of our conclusions. Data for MI in smaller studies with smaller treatment benefits were absent because these (less precise) studies did not systematically report cardiovascular events. Accordingly, selective outcome reporting reduced the reliability of this treatment effect (13% reduction) in both magnitude and direction, although the effect of bias could not be determined in the absence of all data for this outcome. The small proportion of studies reporting vascular access outcomes including approximately 6500 participants reduced the strength of evidence for antiplatelet agents on vascular access function and maturation. Only 50% of such studies reported access failure or thrombosis, and only 15% reported on maturation and suitability for dialysis outcomes in these people. Overall, some studies did not report adequate blinding, allocation concealment or random sequence generation, although sensitivity analyses did not find differences in treatment effects when analyses were restricted to studies of higher methodological quality, because lower quality studies tended to be smaller and contributed fewer events to analyses. In addition, the number of major bleeding events in studies of dual antiplatelet agents was insufficient to determine in indirect evidence whether the bleeding risk was increased with dual antiplatelet agents compared with monotherapy. Data from studies that directly compared two antiplatelet agents against a single antiplatelet agent were rarely reported.
Consistency of results
Our major findings were that antiplatelet agents probably reduce MI, probably increase major bleeding, and may increase minor bleeding in CKD and HD. More than one‐third of studies reported death (any cause) in over 17,000 participants and showed no treatment effect in the majority of studies. The null result of antiplatelet agents on death (any cause) was due both to the lack of effect on aspirin on non‐cardiovascular causes of death and to the competing non‐atherosclerotic cardiovascular causes. Only CHARISMA 2006, which compared clopidogrel and aspirin versus aspirin alone in people with diabetic kidney disease, showed that there were more deaths amongst participants allocated to clopidogrel, although the reasons for this finding remain unclear. Similarly, in analyses for cardiovascular death that included 21 studies and nearly 10,000 participants, only CHARISMA 2006 had a 95% CI that did not include '1' suggesting the null effect of antiplatelet agents on cause‐specific death is robust. There was also very low heterogeneity in the summary estimate for MI, although only 18/90 potentially eligible studies reported this outcome. Approximately one‐third of placebo/no treatment studies reported major bleeding events with a consistent risk across all contributing studies of over 16,000 participants and nearly 600 events. The highly variable definitions of major bleeding in the included studies, together with the relative lack of specific reporting on intracranial haemorrhage, reduced the ability to weigh the relative benefits of treatment (reducing MI) with the comprehensive potential risks of harm for people with CKD and HD. The risks of minor bleeding varied among studies beyond chance alone and subgroup analyses, which included analyses for age, gender, pre‐existing comorbidities or time on dialysis, did not reduce the reliability of the effect estimate identified for this outcome.
Directness of evidence
There were 27 studies reporting direct comparisons, and 14 were meta‐analysed. . The small number of studies that directly compared different agents (prasugrel versus clopidogrel in one study; ticagrelor versus clopidogrel in three studies; different doses of clopidogrel in one study; abciximab versus tirofiban in one study; defibrotide versus dypiridamole in one study; sarpogrelate versus cilostazol in one study and beraprost versus cilostazol or sarpogrelate in one study) precluded indirect comparisons of the magnitude of the effect of each drug class (although such evidence is of lower quality than head‐to‐head comparisons of antiplatelet agents). Although we planned to identify whether a specific antiplatelet agent was particularly beneficial (or harmful) and if treatment effects varied with stage of CKD (dialysis versus earlier stages of CKD) using prespecified subgroup analyses, these analyses were not performed due to the small number of studies.
Precision
Effect estimates for major treatment benefits and harms (MI and major bleeding) had narrow confidence intervals, increasing their certainty and strengthening the evidence within the review for these clinical events. Several outcomes, however, included few participants and events, indicating the available evidence for benefits (and toxicities) of antiplatelet agents for these outcomes is of lower quality. These outcomes included death (any cause) and cardiovascular death, bleeding‐related death, fatal and nonfatal stroke, haemorrhagic stroke, kidney failure, transplant function and rejection, dialysis vascular access maturation, and hospitalisation. Effect estimates for direct antiplatelet versus antiplatelet comparisons were also very imprecise.
Potential biases in the review process
This review was carried out using standard Cochrane methods. Each step was completed independently by at least two authors including the selection of studies, data management, and risk of bias assessment, thus reducing the risks of errors in the identification of eligible studies and adjudication of evidence certainty. A highly sensitive search of the Cochrane Kidney Transplant specialised register was completed without language restriction in July 2021. The registry contains hand‐searched literature and conference proceedings, maximising the inclusion of grey literature in this review. We additionally requested data from authors. Some studies did not report key outcomes in a format available for meta‐analysis.
Potential biases in this review were related to the data available in the individual studies. First, there was heterogeneity in treatment interventions and comparisons; due to the small number of data observations and the different number of participants in each subgroup category, robust statistical estimates of heterogeneity could not be estimated. Second, we could not assess for potential reporting bias because most studies did not report key outcomes in a format available for meta‐analysis. Third, while most participants were on CKD (stage 1‐5) not requiring dialysis, there was wide variation in the definition of kidney disease for inclusion in eligible studies. Fourth, adverse event reporting in the available studies was infrequent and inconsistent. Finally, selective outcome reporting was a limitation across the included studies.
Visual inspection of funnel plots did not suggest any evidence of small study effects indicating possible publication bias for MI (Figure 3).
3.

Funnel plot of comparison of antiplatelet agents versus control for the outcome of fatal or nonfatal myocardial infarction
Agreements and disagreements with other studies or reviews
The updated results of this review expand the available evidence for people with CKD including data for 51,959 participants. An earlier collaborative systematic overview of 287 RCTs of an antiplatelet agent versus control (130,000 participants) or of one antiplatelet treatment versus another (77,000 participants) in people at high risk of cardiovascular disease (acute or previous vascular disease or other predisposing condition) included 2632 people requiring HD (ATT 2002). This review found that antiplatelet agents produced a 41% proportional reduction in serious vascular events in this population. However, only 99 vascular events and 46 major extracranial bleeds were available at the time of publication, limiting the reliability of the conclusions drawn (ATT 2002). Data for people with earlier stages of CKD were not available in this earlier review and have only recently become more available. Another systematic review of individual patient data for aspirin in the primary and secondary prevention of vascular disease did not provide specific analyses for individuals based on the presence of CKD (ATT 2009).
Notably, our systematic review (that finds that antiplatelet agents probably lower by 12% the risk of MI, increase major bleeding, may reduce death (any cause), and may increase minor bleeding) differs from these two previous studies. We suggest that the benefits of antiplatelet agents on cardiovascular events may be smaller in people with CKD compared with other populations at risk of cardiovascular events. The relatively reduced efficacy for antiplatelet agents on total death in CKD is potentially explained by the competing mechanisms for cardiovascular death in this population. Progressive kidney dysfunction is characterised by vascular stiffening and calcification, cardiomyopathy, hyperkalaemia, and sudden cardiac death, in addition to occlusive vascular disease. About half of cardiovascular deaths in both dialysis and transplant patients are caused by cardiac arrest and heart failure (ANZDATA 2019) for which the predominant pathogenetic mechanisms include hypertension, volume expansion, vascular calcification, and arrhythmia, rather than platelet aggregation and thrombosis. Therefore, while we find that antiplatelet agents prevent occlusive vascular events (MI) in CKD as expected, they have a lower overall effect on non‐thrombotic causes of death (both vascular and nonvascular). The results of the present review are consistent with the effects of antiplatelet agents in primary prevention of cardiovascular events, which reduce nonfatal MI by 20% but do not prevent stroke or vascular death with similar effects in men and women (ATT 2009). Notably, in that review, the authors concluded that aspirin may be of uncertain net value, because reducing occlusive events may not be outweighed by risks of major bleeding.
A previous meta‐analysis of medical adjuvant treatment to increase the patency of arteriovenous fistulae and grafts included placebo‐controlled studies of antiplatelet agents, low‐dose warfarin, or fish oil was published in 2008 (Osborn 2008). In that systematic review, antiplatelet agents were considered separately in analyses that combined access types (graft or fistula) and analyses included a maximum of only three studies and 41 events. Analyses in that review may have been insufficient to provide reliable estimates of the benefits or toxicity of antiplatelet agents on vascular access outcomes. Our review also differs from the second review of antiplatelet agents for the prevention of arteriovenous fistula thrombosis of 10 studies (approximately 2000 participants), as we considered the outcomes of suitability for dialysis or access maturation, summarised study risks of bias, and explored sources of heterogeneity within treatment effects (Coleman 2010).
Our review showed similar results compared with a recent meta‐analysis that provided data for approximately 28,000 CKD patients, where the risk of MI decreased, major and minor bleeding increased in the antiplatelet agent group compared with control (Su 2019). Moreover, the authors reported that the effects of antiplatelet agents on HD patients or kidney transplant patients were rarely or not reported.
Authors' conclusions
Implications for practice.
Overall evidence ratings and recommendations for antiplatelet agents to prevent cardiovascular and dialysis access outcomes in people with CKD using the GRADE system for grading evidence are summarised (GRADE 2011b). This updated systematic review has shown that antiplatelet agents in people with CKD and HD probably reduces the risk of MI, while the impact on death from any cause, cardiovascular death and stroke is uncertain or there is little evidence of impact from treatment. Treatment incurs major and minor bleeding that may impact the decision‐making process by patients and clinicians balancing the potential benefits and harms of therapy. Antiplatelet agents given at the time of access surgery may reduce thrombosis or failure of vascular access, but effects on dialysis vascular access suitability for dialysis and access maturation are uncertain. The relative benefits of treatment in kidney transplant recipients and with primary prevention strategies in CKD are insufficient to inform practice. Based on absolute risks of clinical outcomes, it might be expected that antiplatelet agents would prevent 13 people with CKD and 3 treated with HD for every 1000 people treated over 1 year (Table 1), while 18 people with CKD and 1 people treated with HD might experience a major bleeding event without strong evidence that treatment prevents death. This implies that the balance of benefits and harms for people with CKD and those treated with dialysis depends on treatment goals and the relative importance of reducing the risk of MI or avoiding a serious bleeding event.
Implications for research.
This review shows that there are little data for antiplatelet agents to prevent cardiovascular events in kidney transplant recipients with chronic or acute coronary artery disease. Further, adequately powered placebo‐controlled RCTs are required to determine whether antiplatelet agents provide primary prevention against cardiovascular disease in people with CKD, including kidney transplant recipients, compared with aspirin monotherapy. To inform decisions in clinical practice, powered RCTs on any antiplatelet therapy‐based cardiovascular study should include at least 2000 participants for each stage of CKD to meet the optimal information size criterion and evaluate adequately the confidence in the estimate of effect, with a relative risk reduction of 25% (GRADE 2011c). Specific head‐to‐head studies of antiplatelet agent regimens in individuals with all stages CKD and established atherosclerotic disease, acute coronary syndrome or undergoing percutaneous coronary intervention are required, particularly comparing thienopyridines (prasugrel or ticagrelor) or P2Y antagonists versus clopidogrel, different doses of clopidogrel, glycoprotein IIb/IIIa inhibitors versus another glycoprotein IIb/IIIa inhibitor or adenosine reuptake inhibitors, cilostazol versus sarpogrelate and beraprost sodium versus cilostazol or sarpogrelate. Studies should be designed to use standardised criteria to capture systematically all cardiovascular outcomes and major bleeding events in studies in which severe CKD is not an exclusion criterion. More information is required on the relative benefits of antiplatelet agents compared with other antiplatelet agents in people with CKD and the effects of therapy on cardiovascular mortality and bleeding. The role of antiplatelet agents as a primary prevention strategy to reduce death (any cause) and cardiovascular death in individuals with CKD, dialysis (HD and PD) and kidney transplant without existing cardiovascular disease appears to be a lower research priority.
Feedback
Feedback concerning conclusions, 9 May 2013
Summary
Dear Editor,
Thank you for a much needed review addressing the gaps in literature regarding the risks and benefits of antiplatelets in the chronic kidney disease (CKD) population. We thought the literature search was very thorough and well done. However, we came up with a few questions upon reading this review and felt that the stated conclusion "antiplatelets reduce myocardial infarction...including those with early stages of CKD who do not have clinically‐evident occlusive cardiovascular disease" may not be accurately reflected by the presented data.
Looking at the first primary outcome ‐ fatal and non‐fatal myocardial infarction (MI), it was unclear whether the population studied was addressing primary prevention, secondary prevention or acute treatment of MI as the included populations had different cardiovascular histories. Of the two studies that were given the most weight in the analysis (HOT Study 2010 and PURSUIT Study 1998), one investigated primary prevention of MI using ASA versus placebo, while the other investigated acute treatment of MI using eptifibatide + ASA + heparin compared to ASA + heparin. In the non‐CKD population, efficacy of antiplatelets is dependent on the indication (i.e. primary or secondary prophylaxis or treatment). Different antiplatelets also have different places in therapy.
We therefore feel that it may be inappropriate to pool these trials together as they were investigating different populations.
In this same analysis, there were also multiple interventions such as single antiplatelets versus placebo (HOT study 2010, Ell study 1982, Creek 1990, Dember 2008, STOP study 1995, UK‐HARP‐I study 2005, ETDRS 1992), dual antiplatelets versus placebo (Kaufman 2003), as well as dual antiplatelets versus single antiplatelet agents (CREDO study 2008, CHARISMA study 2009, EPILOG study 1997, EPIC study 1994, EPISTENT study 1998, Dixon study 2009, RAPPORT study 1998, PURSUIT study 1998, and IMPACT II 1997). With both placebo and antiplatelet in the "control" arms of one meta‐analysis, comparison groups and treatment groups are not clearly delineated from one another. As this was unclear, readers may be misled into believing that the effect is driven purely from antiplatelet compared to placebo, when this is not the case. Even pooling the data on the seven placebo‐controlled trials may be inappropriate as they were studied in different patient
populations and indications (e.g. primary prevention, non‐cardiovascular outcomes). Similarly, the "treatment arms" of the meta‐analysis contained one or more antiplatelet agents, which may have biased the result towards the treatment arm over single agent or placebo "control". This can also make it difficult to isolate the beneficial agent in the dual antiplatelet studies. Due to the differences in treatment arms and patient populations, we feel it would valuable to investigate the outcomes of these factors in separate analyses.
It should also be mentioned that the patients included in this review were derived as subgroups from larger studies with different baseline cardiovascular risk factors (e.g. diabetes, coronary artery disease, hypertension, etc). As a result, one cannot conclude that patients with only CKD, and no additional cardiovascular risk factors, would benefit from antiplatelet use to decrease cardiovascular outcomes such as fatal and non‐fatal MIs. Dixon 2009 and Dember 2008 were two studies enrolling haemodialysis patients with a primary outcome of AV graft patency or thrombosis; fatal and non‐fatal MIs were only reported as an adverse effect and could have been under‐reported in the study.
We commend the authors for assessing bias in the included trials and for performing a sensitivity analysis to explore the impact of the bias. We feel that with the relatively high percentage of unclear or high risk of biases that exist in the trials, it would have been beneficial for the authors to report on the results of their sensitivity analyses to clarify the role of the bias and to substantiate the reported results.
We feel that the author's conclusion "antiplatelet agents reduce myocardial infarction" may be too broad of a conclusion to be drawn based on the analysis that was performed looking at fatal and non‐fatal MI. As well, their specific reference to "patients with early stages of CKD who do not have a clinically‐evident occlusive cardiovascular disease" suggests this effect is shown in the CKD
population when using antiplatelets for primary prevention; however, this aspect was not separated out in their analysis. We feel that the pooling of studies with varying patient populations and treatments is not appropriate in helping clinicians determine whether antiplatelets provide any benefit for MI in patients with CKD. While we did not explore the other identified primary outcomes in this review, we wonder if similar concerns exist for not only the efficacy but also the safety outcomes. We would appreciate an investigation into single antiplatelet therapy versus placebo for various cardiovascular indications. We hope the authors will provide clarification and address these concerns in their future updates.
We look forward to hearing your response to our comments.
Sincerely,
Gloria Su, BSc. Pharm Wan‐Yun Polinna Tsai, BSc. Pharm Megan Harbin, BSc. Pharm Asal Taheri, BSc. Pharm Aaron M Tejani, BSc. Pharm, PharmD
Reply
Thanks for the constructive comments.
1. Primary versus secondary prevention versus acute treatment
We combined treatment estimates for all available studies comparing antiplatelet therapy (with or without standard therapy) versus placebo/no treatment (with or without standard therapy alone) to examine treatment effects, which is a standard starting point for meta‐analyses. For the outcome of fatal or nonfatal myocardial infarction, there was little or no heterogeneity in the treatment effects observed in all the available trials, suggesting that treatment estimates could be appropriately summarised into a single effect size.
While not necessary in the absence of significant heterogeneity, we explored for pre‐specified trial‐level variables that might have modified the treatment estimates that we observed. We specifically wished to know whether treatment effects differed for patients with existing cardiovascular disease compared to those without cardiovascular disease but this was not feasible due to as we found insufficient numbers of studies that were clearly primary prevention or secondary prevention studies. However, the lack of heterogeneity in the overall summary estimate suggests that antiplatelet agents have similar effects irrespective of the presence or absence of cardiovascular disease.
2. Multiple interventions:
Unlike the relative lack of primary versus secondary prevention trials, there was sufficient studies to explore any differences in treatment effects based on the class of antiplatelet used. While there were numerous different strategies for antiplatelet treatment in contributing trials, all the treatment interventions could be characterised by an antiplatelet agent in addition to standard care versus no treatment/placebo in addition to standard care. We have called this antiplatelet therapy versus control to acknowledge the heterogeneity of the intervention strategies used (rather than antiplatelet treatment versus placebo).
We used stratified analyses according to overall class of antiplatelet drug where possible but there was lack of power from available studies to understand fully all the various treatment effects for each individual antiplatelet regimen. An individual patient meta‐analysis would be needed to give a more fine‐grained understanding of the different interventions and their combinations in the CKD population.
3. Deriving patients from subgroups of larger studies:
Patients with CKD were evaluated in post‐hoc analyses of larger trials in broader populations. These included trials in populations with acute coronary syndromes requiring percutaneous coronary artery procedures, patients with hypertension and those with diabetes mellitus. Trials of treatment tended to use different interventions (glycoprotein IIb/IIIa inhibitors with or without clopidogrel) whereas trials of primary or secondary prevention did not use these agents, preventing useful stratified analyses for either class of agent or cardiovascular prevention in these trials. We have concluded that the lack of a priori assessment of glycoprotein IIb/IIIa inhibitors in people with CKD is an important limitation of the current evidence.
4. Potential under‐reporting of clinical outcomes
We agree that many trials were not designed to evaluate mortality and cardiovascular outcomes and that these events were reported in an ad hoc fashion (not prespecified) which may have underestimated their frequency. We include evaluation of this aspect of trials when considering whether they are at risk of bias due to selective reporting of expected outcomes.
5. Risks of bias
We did not specify risk of bias items as sources of heterogeneity we would explore in stratified analyses. In further updates of this review and if deemed appropriate and feasible, we will explore attrition bias and allocation concealment as potential sources of heterogeneity in subgroup or sensitivity analyses.
6. Conclusions
In conclusion, we thank Dr Su and others for constructive comments to this review. We agree that the review cannot provide high quality information about antiplatelet agents as primary prevention for cardiovascular disease in people with CKD. We acknowledge the limitations of studies in which adults with CKD were studied post hoc and which are heterogeneous for presence of cardiovascular disease and antiplatelet agent studied. We agree that clinical events may be under‐reported in available studies and will explore in future versions of this review the effects of risk of bias on the estimated treatment effects of antiplatelet treatment in CKD.
Contributors
Suetonia Palmer
Giovanni Strippoli
What's new
| Date | Event | Description |
|---|---|---|
| 18 December 2021 | New citation required but conclusions have not changed | New studies have not altered the previous conclusions |
| 18 December 2021 | New search has been performed | New studies incorporated |
History
Protocol first published: Issue 11, 2010 Review first published: Issue 2, 2013
| Date | Event | Description |
|---|---|---|
| 5 November 2019 | Amended | Search strategies updated |
| 24 November 2014 | Amended | Feedback and reply incorporated |
| 11 March 2013 | New citation required but conclusions have not changed | Minor amendment to abstract |
Acknowledgements
The authors are grateful to the following peer reviewers for their time and comments: Dr William G. Herrington (MRC Population Health Research Unit at the University of Oxford); Arlene C Crisostomo (Section of Nephrology, St Luke's Medical Center Quezon City, Philippines); and Swapnil Hiremath (University of Ottawa).
The authors would like to thank all study authors who responded to our queries about their studies. We received additional unpublished data from Drs James, Wiviott, Ferris, Lincoff, Balog, Wolski, Baigent, Kaufman, Topol, and Shao. We thank Hebatullah M. Abdulazeem, Cholpon Bolotbekovna and Liliya Eugenevna Ziganshina for helping us in the translation of foreign papers.
The authors would like to thank Lucia Di Micco, Vlado Perkovic and Sophia Zoungas who worked on the protocol and the first version of the review, and Fabio Pellegrini who worked on the first version of this review.
Appendices
Appendix 1. Electronic search strategies
| Database | Search terms |
| CENTRAL |
|
| MEDLINE |
|
| EMBASE |
|
Appendix 2. Risk of bias assessment tool
| Potential source of bias | Assessment criteria |
|
Random sequence generation Selection bias (biased allocation to interventions) due to inadequate generation of a randomised sequence |
Low risk of bias: Random number table; computer random number generator; coin tossing; shuffling cards or envelopes; throwing dice; drawing of lots; minimization (minimization may be implemented without a random element, and this is considered to be equivalent to being random). |
| High risk of bias: Sequence generated by odd or even date of birth; date (or day) of admission; sequence generated by hospital or clinic record number; allocation by judgement of the clinician; by preference of the participant; based on the results of a laboratory test or a series of tests; by availability of the intervention. | |
| Unclear: Insufficient information about the sequence generation process to permit judgement. | |
|
Allocation concealment Selection bias (biased allocation to interventions) due to inadequate concealment of allocations prior to assignment |
Low risk of bias: Randomisation method described that would not allow investigator/participant to know or influence intervention group before eligible participant entered in the study (e.g. central allocation, including telephone, web‐based, and pharmacy‐controlled, randomisation; sequentially numbered drug containers of identical appearance; sequentially numbered, opaque, sealed envelopes). |
| High risk of bias: Using an open random allocation schedule (e.g. a list of random numbers); assignment envelopes were used without appropriate safeguards (e.g. if envelopes were unsealed or non‐opaque or not sequentially numbered); alternation or rotation; date of birth; case record number; any other explicitly unconcealed procedure. | |
| Unclear: Randomisation stated but no information on method used is available. | |
|
Blinding of participants and personnel Performance bias due to knowledge of the allocated interventions by participants and personnel during the study |
Low risk of bias: No blinding or incomplete blinding, but the review authors judge that the outcome is not likely to be influenced by lack of blinding; blinding of participants and key study personnel ensured, and unlikely that the blinding could have been broken. |
| High risk of bias: No blinding or incomplete blinding, and the outcome is likely to be influenced by lack of blinding; blinding of key study participants and personnel attempted, but likely that the blinding could have been broken, and the outcome is likely to be influenced by lack of blinding. | |
| Unclear: Insufficient information to permit judgement | |
|
Blinding of outcome assessment Detection bias due to knowledge of the allocated interventions by outcome assessors. |
Low risk of bias: No blinding of outcome assessment, but the review authors judge that the outcome measurement is not likely to be influenced by lack of blinding; blinding of outcome assessment ensured, and unlikely that the blinding could have been broken. |
| High risk of bias: No blinding of outcome assessment, and the outcome measurement is likely to be influenced by lack of blinding; blinding of outcome assessment, but likely that the blinding could have been broken, and the outcome measurement is likely to be influenced by lack of blinding. | |
| Unclear: Insufficient information to permit judgement | |
|
Incomplete outcome data Attrition bias due to amount, nature or handling of incomplete outcome data. |
Low risk of bias: No missing outcome data; reasons for missing outcome data unlikely to be related to true outcome (for survival data, censoring unlikely to be introducing bias); missing outcome data balanced in numbers across intervention groups, with similar reasons for missing data across groups; for dichotomous outcome data, the proportion of missing outcomes compared with observed event risk not enough to have a clinically relevant impact on the intervention effect estimate; for continuous outcome data, plausible effect size (difference in means or standardized difference in means) among missing outcomes not enough to have a clinically relevant impact on observed effect size; missing data have been imputed using appropriate methods. |
| High risk of bias: Reason for missing outcome data likely to be related to true outcome, with either imbalance in numbers or reasons for missing data across intervention groups; for dichotomous outcome data, the proportion of missing outcomes compared with observed event risk enough to induce clinically relevant bias in intervention effect estimate; for continuous outcome data, plausible effect size (difference in means or standardized difference in means) among missing outcomes enough to induce clinically relevant bias in observed effect size; ‘as‐treated’ analysis done with substantial departure of the intervention received from that assigned at randomisation; potentially inappropriate application of simple imputation. | |
| Unclear: Insufficient information to permit judgement | |
|
Selective reporting Reporting bias due to selective outcome reporting |
Low risk of bias: The study protocol is available and all of the study’s pre‐specified (primary and secondary) outcomes that are of interest in the review have been reported in the pre‐specified way; the study protocol is not available but it is clear that the published reports include all expected outcomes, including those that were pre‐specified (convincing text of this nature may be uncommon). |
| High risk of bias: Not all of the study’s pre‐specified primary outcomes have been reported; one or more primary outcomes is reported using measurements, analysis methods or subsets of the data (e.g. subscales) that were not pre‐specified; one or more reported primary outcomes were not pre‐specified (unless clear justification for their reporting is provided, such as an unexpected adverse effect); one or more outcomes of interest in the review are reported incompletely so that they cannot be entered in a meta‐analysis; the study report fails to include results for a key outcome that would be expected to have been reported for such a study. | |
| Unclear: Insufficient information to permit judgement | |
|
Other bias Bias due to problems not covered elsewhere in the table |
Low risk of bias: The study appears to be free of other sources of bias. |
| High risk of bias: Had a potential source of bias related to the specific study design used; stopped early due to some data‐dependent process (including a formal‐stopping rule); had extreme baseline imbalance; has been claimed to have been fraudulent; had some other problem. | |
| Unclear: Insufficient information to assess whether an important risk of bias exists; insufficient rationale or evidence that an identified problem will introduce bias. |
Data and analyses
Comparison 1. Antiplatelet agents versus control.
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
|---|---|---|---|---|
| 1.1 Fatal or nonfatal myocardial infarction | 18 | 15289 | Risk Ratio (IV, Random, 95% CI) | 0.88 [0.79, 0.99] |
| 1.1.1 CKD | 11 | 11912 | Risk Ratio (IV, Random, 95% CI) | 0.85 [0.74, 0.99] |
| 1.1.2 HD | 6 | 2929 | Risk Ratio (IV, Random, 95% CI) | 0.83 [0.49, 1.41] |
| 1.1.3 CKD, dialysis and transplant | 1 | 448 | Risk Ratio (IV, Random, 95% CI) | 0.99 [0.06, 15.75] |
| 1.2 Fatal or nonfatal stroke | 12 | 10382 | Risk Ratio (IV, Random, 95% CI) | 1.01 [0.64, 1.59] |
| 1.2.1 CKD | 5 | 7062 | Risk Ratio (IV, Random, 95% CI) | 1.06 [0.64, 1.74] |
| 1.2.2 HD | 6 | 2872 | Risk Ratio (IV, Random, 95% CI) | 0.62 [0.15, 2.60] |
| 1.2.3 CKD, dialysis and transplant | 1 | 448 | Risk Ratio (IV, Random, 95% CI) | 2.97 [0.12, 72.60] |
| 1.3 Death (any cause) | 35 | 18241 | Risk Ratio (IV, Random, 95% CI) | 0.94 [0.84, 1.06] |
| 1.3.1 CKD | 19 | 13234 | Risk Ratio (IV, Random, 95% CI) | 0.97 [0.81, 1.16] |
| 1.3.2 HD | 14 | 4523 | Risk Ratio (IV, Random, 95% CI) | 0.86 [0.72, 1.03] |
| 1.3.3 Transplant | 1 | 36 | Risk Ratio (IV, Random, 95% CI) | Not estimable |
| 1.3.4 CKD, dialysis and transplant | 1 | 448 | Risk Ratio (IV, Random, 95% CI) | 0.99 [0.14, 6.97] |
| 1.4 Haemorrhagic stroke | 9 | 6844 | Risk Ratio (IV, Random, 95% CI) | 1.22 [0.69, 2.17] |
| 1.4.1 CKD | 7 | 6655 | Risk Ratio (IV, Random, 95% CI) | 1.22 [0.69, 2.17] |
| 1.4.2 HD | 2 | 189 | Risk Ratio (IV, Random, 95% CI) | Not estimable |
| 1.5 Cardiovascular death | 21 | 9606 | Risk Ratio (IV, Random, 95% CI) | 0.87 [0.65, 1.15] |
| 1.5.1 CKD | 10 | 6525 | Risk Ratio (IV, Random, 95% CI) | 0.98 [0.60, 1.59] |
| 1.5.2 HD | 9 | 2597 | Risk Ratio (IV, Random, 95% CI) | 0.71 [0.47, 1.09] |
| 1.5.3 Transplant | 1 | 36 | Risk Ratio (IV, Random, 95% CI) | Not estimable |
| 1.5.4 CKD, dialysis and transplant | 1 | 448 | Risk Ratio (IV, Random, 95% CI) | 0.99 [0.06, 15.75] |
| 1.6 Fatal bleeding | 21 | 7629 | Risk Ratio (IV, Random, 95% CI) | 1.39 [0.10, 19.48] |
| 1.6.1 CKD | 7 | 4539 | Risk Ratio (IV, Random, 95% CI) | Not estimable |
| 1.6.2 HD | 12 | 2606 | Risk Ratio (IV, Random, 95% CI) | 1.39 [0.10, 19.48] |
| 1.6.3 Transplant | 1 | 36 | Risk Ratio (IV, Random, 95% CI) | Not estimable |
| 1.6.4 CKD, dialysis and transplant | 1 | 448 | Risk Ratio (IV, Random, 95% CI) | Not estimable |
| 1.7 Major bleeding | 29 | 16194 | Risk Ratio (IV, Random, 95% CI) | 1.35 [1.10, 1.65] |
| 1.7.1 CKD | 12 | 11591 | Risk Ratio (IV, Random, 95% CI) | 1.51 [1.15, 1.98] |
| 1.7.2 HD | 15 | 4119 | Risk Ratio (IV, Random, 95% CI) | 0.90 [0.53, 1.55] |
| 1.7.3 Transplant | 1 | 36 | Risk Ratio (IV, Random, 95% CI) | Not estimable |
| 1.7.4 CKD, dialysis and transplant | 1 | 448 | Risk Ratio (IV, Random, 95% CI) | 0.66 [0.19, 2.31] |
| 1.8 Minor bleeding | 21 | 13218 | Risk Ratio (IV, Random, 95% CI) | 1.55 [1.27, 1.90] |
| 1.8.1 CKD | 12 | 11530 | Risk Ratio (IV, Random, 95% CI) | 1.48 [1.20, 1.83] |
| 1.8.2 HD | 8 | 1240 | Risk Ratio (IV, Random, 95% CI) | 1.87 [0.65, 5.40] |
| 1.8.3 CKD, dialysis and transplant | 1 | 448 | Risk Ratio (IV, Random, 95% CI) | 2.81 [1.49, 5.28] |
| 1.9 Kidney failure | 11 | 1722 | Risk Ratio (IV, Random, 95% CI) | 0.89 [0.70, 1.14] |
| 1.9.1 CKD | 8 | 1247 | Risk Ratio (IV, Random, 95% CI) | 0.80 [0.59, 1.08] |
| 1.9.2 Transplant | 2 | 100 | Risk Ratio (IV, Random, 95% CI) | 1.40 [0.73, 2.67] |
| 1.9.3 CKD, dialysis and transplant | 1 | 375 | Risk Ratio (IV, Random, 95% CI) | 1.17 [0.40, 3.42] |
| 1.10 Doubling of serum creatinine | 3 | 217 | Risk Ratio (IV, Random, 95% CI) | 0.39 [0.17, 0.86] |
| 1.10.1 CKD | 3 | 217 | Risk Ratio (IV, Random, 95% CI) | 0.39 [0.17, 0.86] |
| 1.11 Kidney transplant graft loss | 2 | 91 | Risk Ratio (IV, Random, 95% CI) | 1.08 [0.58, 2.01] |
| 1.12 Transplant rejection | 2 | 97 | Risk Ratio (IV, Random, 95% CI) | 0.95 [0.77, 1.19] |
| 1.13 Creatinine clearance | 3 | 90 | Mean Difference (IV, Random, 95% CI) | ‐5.46 [‐12.33, 1.41] |
| 1.13.1 CKD | 3 | 90 | Mean Difference (IV, Random, 95% CI) | ‐5.46 [‐12.33, 1.41] |
| 1.14 Proteinuria | 3 | 80 | Mean Difference (IV, Random, 95% CI) | ‐0.74 [‐1.35, ‐0.13] |
| 1.14.1 CKD | 3 | 80 | Mean Difference (IV, Random, 95% CI) | ‐0.74 [‐1.35, ‐0.13] |
| 1.15 Dialysis access failure (thrombosis or loss of patency) | 17 | 2847 | Risk Ratio (IV, Random, 95% CI) | 0.62 [0.50, 0.78] |
| 1.15.1 Fistula | 10 | 1741 | Risk Ratio (IV, Random, 95% CI) | 0.50 [0.36, 0.69] |
| 1.15.2 Shunt or graft | 5 | 1052 | Risk Ratio (IV, Random, 95% CI) | 0.80 [0.62, 1.03] |
| 1.15.3 Fistula or graft | 1 | 16 | Risk Ratio (IV, Random, 95% CI) | 0.50 [0.06, 4.47] |
| 1.15.4 Catheter | 1 | 38 | Risk Ratio (IV, Random, 95% CI) | 0.44 [0.16, 1.20] |
| 1.16 Early access thrombosis (before 8 weeks) | 8 | 1525 | Risk Ratio (IV, Random, 95% CI) | 0.52 [0.38, 0.70] |
| 1.17 Loss of primary unassisted patency | 2 | 665 | Risk Ratio (IV, Random, 95% CI) | 0.95 [0.89, 1.03] |
| 1.18 Failure to attain suitability for dialysis | 5 | 1503 | Risk Ratio (IV, Random, 95% CI) | 0.63 [0.34, 1.15] |
| 1.19 Need for intervention to attain patency or assist maturation | 6 | 2067 | Risk Ratio (IV, Random, 95% CI) | 0.87 [0.72, 1.05] |
| 1.20 Hospitalisation (any cause) | 3 | 3535 | Risk Ratio (IV, Random, 95% CI) | 0.97 [0.87, 1.10] |
| 1.20.1 CKD | 1 | 2009 | Risk Ratio (IV, Random, 95% CI) | 0.93 [0.72, 1.21] |
| 1.20.2 HD | 2 | 1526 | Risk Ratio (IV, Random, 95% CI) | 0.96 [0.78, 1.17] |
| 1.21 Cardiovascular hospitalisation | 3 | 3535 | Risk Ratio (IV, Random, 95% CI) | 0.93 [0.76, 1.14] |
| 1.21.1 CKD | 1 | 2009 | Risk Ratio (IV, Random, 95% CI) | 0.93 [0.72, 1.21] |
| 1.21.2 HD | 2 | 1526 | Risk Ratio (IV, Random, 95% CI) | 0.88 [0.58, 1.33] |
| 1.22 Treatment withdrawal | 15 | 2669 | Risk Ratio (IV, Random, 95% CI) | 0.97 [0.83, 1.14] |
| 1.22.1 CKD | 4 | 202 | Risk Ratio (IV, Random, 95% CI) | 0.64 [0.27, 1.55] |
| 1.22.2 HD | 8 | 1973 | Risk Ratio (IV, Random, 95% CI) | 0.99 [0.83, 1.19] |
| 1.22.3 PD | 1 | 10 | Risk Ratio (IV, Random, 95% CI) | Not estimable |
| 1.22.4 Transplant | 1 | 36 | Risk Ratio (IV, Random, 95% CI) | Not estimable |
| 1.22.5 CKD, dialysis and transplant | 1 | 448 | Risk Ratio (IV, Random, 95% CI) | 0.95 [0.66, 1.37] |
Comparison 2. Prasugrel versus clopidogrel.
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
|---|---|---|---|---|
| 2.1 Fatal or nonfatal myocardial infarction | 1 | Risk Ratio (IV, Random, 95% CI) | Totals not selected | |
| 2.2 Death (any cause) | 1 | Risk Ratio (IV, Random, 95% CI) | Totals not selected | |
| 2.3 Cardiovascular death | 1 | Risk Ratio (IV, Random, 95% CI) | Totals not selected | |
| 2.4 Major bleeding | 1 | Risk Ratio (IV, Random, 95% CI) | Totals not selected | |
| 2.5 Minor bleeding | 1 | Risk Ratio (IV, Random, 95% CI) | Totals not selected |
Comparison 3. Ticagrelor versus clopidogrel.
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
|---|---|---|---|---|
| 3.1 Fatal or nonfatal myocardial infarction | 1 | Risk Ratio (IV, Random, 95% CI) | Totals not selected | |
| 3.2 Fatal or nonfatal stroke | 1 | Risk Ratio (IV, Random, 95% CI) | Totals not selected | |
| 3.3 Death (any cause) | 3 | 137 | Risk Ratio (IV, Random, 95% CI) | 2.00 [0.19, 20.90] |
| 3.3.1 CKD | 1 | 60 | Risk Ratio (IV, Random, 95% CI) | 2.00 [0.19, 20.90] |
| 3.3.2 HD | 2 | 77 | Risk Ratio (IV, Random, 95% CI) | Not estimable |
| 3.4 Cardiovascular death | 3 | 137 | Risk Ratio (IV, Random, 95% CI) | 5.00 [0.25, 99.95] |
| 3.4.1 CKD | 1 | 60 | Risk Ratio (IV, Random, 95% CI) | 5.00 [0.25, 99.95] |
| 3.4.2 HD | 2 | 77 | Risk Ratio (IV, Random, 95% CI) | Not estimable |
| 3.5 Fatal bleeding | 2 | 77 | Risk Ratio (IV, Random, 95% CI) | Not estimable |
| 3.5.1 HD | 2 | 77 | Risk Ratio (IV, Random, 95% CI) | Not estimable |
| 3.6 Major bleeding | 2 | 85 | Risk Ratio (IV, Random, 95% CI) | 0.33 [0.01, 7.87] |
| 3.6.1 CKD | 1 | 60 | Risk Ratio (IV, Random, 95% CI) | 0.33 [0.01, 7.87] |
| 3.6.2 HD | 1 | 25 | Risk Ratio (IV, Random, 95% CI) | Not estimable |
| 3.7 Minor bleeding | 1 | Risk Ratio (IV, Random, 95% CI) | Totals not selected | |
| 3.8 Treatment withdrawal | 1 | Risk Ratio (IV, Random, 95% CI) | Totals not selected |
Comparison 4. Clopidogrel (low dose) versus clopidogrel (high dose).
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
|---|---|---|---|---|
| 4.1 Haemorragic stroke | 1 | Risk Ratio (IV, Random, 95% CI) | Totals not selected | |
| 4.2 Cardiovascular death | 1 | Risk Ratio (IV, Random, 95% CI) | Totals not selected |
Comparison 5. Abciximab versus tirofiban.
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
|---|---|---|---|---|
| 5.1 Fatal or nonfatal myocardial infarction | 1 | Risk Ratio (IV, Random, 95% CI) | Totals not selected | |
| 5.2 Death (any cause) | 1 | Risk Ratio (IV, Random, 95% CI) | Totals not selected |
Comparison 6. Defibrotide versus dypiridamole.
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
|---|---|---|---|---|
| 6.1 Death (any cause) | 1 | Risk Ratio (IV, Random, 95% CI) | Totals not selected | |
| 6.2 Cardiovascular death | 1 | Risk Ratio (IV, Random, 95% CI) | Totals not selected | |
| 6.3 Fatal bleeding | 1 | Risk Ratio (IV, Random, 95% CI) | Totals not selected | |
| 6.4 Kidney transplant graft loss | 1 | Risk Ratio (IV, Random, 95% CI) | Totals not selected |
Comparison 7. Cilostazol versus sarpogrelate.
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
|---|---|---|---|---|
| 7.1 Major bleeding | 1 | Risk Ratio (IV, Random, 95% CI) | Totals not selected |
Comparison 8. Beraprost versus cilostazol or sarpogrelate.
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
|---|---|---|---|---|
| 8.1 Fatal or nonfatal myocardial infarction | 1 | Risk Ratio (IV, Random, 95% CI) | Totals not selected | |
| 8.2 Fatal or nonfatal stroke | 1 | Risk Ratio (IV, Random, 95% CI) | Totals not selected | |
| 8.3 Death (any cause) | 1 | Risk Ratio (IV, Random, 95% CI) | Totals not selected | |
| 8.4 Cardiovascular death | 1 | Risk Ratio (IV, Random, 95% CI) | Totals not selected | |
| 8.5 Fatal bleeding | 1 | Risk Ratio (IV, Random, 95% CI) | Totals not selected |
Comparison 9. Primary/secondary prevention for fatal/non fatal myocardial infarction (subgroup analysis).
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
|---|---|---|---|---|
| 9.1 Secondary prevention | 8 | Risk Ratio (IV, Random, 95% CI) | Subtotals only | |
| 9.1.1 CKD | 8 | 7270 | Risk Ratio (IV, Random, 95% CI) | 0.93 [0.81, 1.06] |
Comparison 10. Sensitivity analysis (adequate allocation concealment).
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
|---|---|---|---|---|
| 10.1 Fatal or nonfatal myocardial infarction | 8 | 10459 | Risk Ratio (IV, Random, 95% CI) | 0.80 [0.65, 0.98] |
| 10.2 Death (any cause) | 10 | 11443 | Risk Ratio (IV, Random, 95% CI) | 1.00 [0.83, 1.22] |
| 10.3 Cardiovascular death | 2 | 5628 | Risk Ratio (IV, Random, 95% CI) | 1.08 [0.48, 2.44] |
| 10.4 Major bleeding | 9 | 10360 | Risk Ratio (IV, Random, 95% CI) | 1.53 [1.07, 2.20] |
Comparison 11. Sensitivity analysis (low risk of attrition).
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
|---|---|---|---|---|
| 11.1 Fatal or nonfatal myocardial infarction | 11 | 9387 | Risk Ratio (IV, Random, 95% CI) | 0.75 [0.62, 0.90] |
| 11.2 Death (any cause) | 19 | 10966 | Risk Ratio (IV, Random, 95% CI) | 0.99 [0.82, 1.20] |
| 11.3 Cardiovascular death | 11 | 6872 | Risk Ratio (IV, Random, 95% CI) | 0.94 [0.60, 1.47] |
| 11.4 Major bleeding | 17 | 9549 | Risk Ratio (IV, Random, 95% CI) | 1.62 [1.19, 2.20] |
Comparison 12. Stroke (subgroup analysis).
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
|---|---|---|---|---|
| 12.1 Stage of CKD | 11 | 9544 | Risk Ratio (IV, Random, 95% CI) | 1.00 [0.58, 1.72] |
| 12.1.1 HD | 6 | 2872 | Risk Ratio (IV, Random, 95% CI) | 0.62 [0.15, 2.60] |
| 12.1.2 CKD | 4 | 6224 | Risk Ratio (IV, Random, 95% CI) | 1.08 [0.57, 2.04] |
| 12.1.3 Predialysis, dialysis and transplant | 1 | 448 | Risk Ratio (IV, Random, 95% CI) | 2.97 [0.12, 72.60] |
| 12.2 Diabetes | 6 | 4368 | Risk Ratio (IV, Random, 95% CI) | 1.49 [0.68, 3.25] |
| 12.2.1 Less than 50% diabetic patients | 3 | 1525 | Risk Ratio (IV, Random, 95% CI) | 0.96 [0.15, 6.03] |
| 12.2.2 At least 50% diabetic patients | 3 | 2843 | Risk Ratio (IV, Random, 95% CI) | 1.70 [0.64, 4.49] |
| 12.3 Sex | 7 | 7987 | Risk Ratio (IV, Random, 95% CI) | 1.19 [0.68, 2.07] |
| 12.3.1 Less than 50% males | 2 | 4268 | Risk Ratio (IV, Random, 95% CI) | 0.85 [0.56, 1.28] |
| 12.3.2 At least 50% males | 5 | 3719 | Risk Ratio (IV, Random, 95% CI) | 1.44 [0.53, 3.95] |
| 12.4 Duration of intervention | 11 | 9544 | Risk Ratio (IV, Random, 95% CI) | 1.00 [0.58, 1.72] |
| 12.4.1 Less than 6 months | 3 | 1212 | Risk Ratio (IV, Random, 95% CI) | 1.98 [0.18, 21.73] |
| 12.4.2 Between 6 and 12 months | 4 | 1870 | Risk Ratio (IV, Random, 95% CI) | 0.37 [0.12, 1.12] |
| 12.4.3 More than 12 months | 4 | 6462 | Risk Ratio (IV, Random, 95% CI) | 1.24 [0.67, 2.32] |
Comparison 13. Minor bleeding (subgroup analysis).
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
|---|---|---|---|---|
| 13.1 Stage of CKD | 21 | 13218 | Risk Ratio (IV, Random, 95% CI) | 1.55 [1.27, 1.90] |
| 13.1.1 HD | 8 | 1240 | Risk Ratio (IV, Random, 95% CI) | 1.87 [0.65, 5.40] |
| 13.1.2 CKD | 12 | 11530 | Risk Ratio (IV, Random, 95% CI) | 1.48 [1.20, 1.83] |
| 13.1.3 Predialysis, dialysis and transplant | 1 | 448 | Risk Ratio (IV, Random, 95% CI) | 2.81 [1.49, 5.28] |
| 13.2 Diabetes | 5 | 3431 | Risk Ratio (IV, Random, 95% CI) | 1.87 [1.24, 2.83] |
| 13.2.1 Less than 50% diabetic patients | 3 | 1346 | Risk Ratio (IV, Random, 95% CI) | 2.81 [1.51, 5.21] |
| 13.2.2 At least 50% diabetic patients | 2 | 2085 | Risk Ratio (IV, Random, 95% CI) | 1.59 [1.37, 1.83] |
| 13.3 Sex | 11 | 7377 | Risk Ratio (IV, Random, 95% CI) | 1.80 [1.29, 2.51] |
| 13.3.1 Less than 50% males | 3 | 3802 | Risk Ratio (IV, Random, 95% CI) | 2.28 [1.29, 4.03] |
| 13.3.2 At least 50% males | 8 | 3575 | Risk Ratio (IV, Random, 95% CI) | 1.71 [1.12, 2.60] |
| 13.4 Duration of intervention | 21 | 13218 | Risk Ratio (IV, Random, 95% CI) | 1.55 [1.27, 1.90] |
| 13.4.1 Less than 6 months | 9 | 1316 | Risk Ratio (IV, Random, 95% CI) | 1.87 [0.65, 5.40] |
| 13.4.2 Between 6 and 12 months | 10 | 6274 | Risk Ratio (IV, Random, 95% CI) | 1.47 [1.11, 1.96] |
| 13.4.3 More than 12 months | 2 | 5628 | Risk Ratio (IV, Random, 95% CI) | 1.71 [1.28, 2.27] |
Comparison 14. Dialysis access failure (subgroup analysis).
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
|---|---|---|---|---|
| 14.1 Diabetes | 6 | 1942 | Risk Ratio (IV, Random, 95% CI) | 0.68 [0.49, 0.95] |
| 14.1.1 Less than 50% diabetic patients | 4 | 1197 | Risk Ratio (IV, Random, 95% CI) | 0.67 [0.45, 0.98] |
| 14.1.2 At least 50% diabetic patients | 2 | 745 | Risk Ratio (IV, Random, 95% CI) | 0.55 [0.16, 1.88] |
| 14.2 Sex | 12 | 2513 | Risk Ratio (IV, Random, 95% CI) | 0.65 [0.51, 0.82] |
| 14.2.1 Less than 50% males | 4 | 838 | Risk Ratio (IV, Random, 95% CI) | 0.75 [0.52, 1.09] |
| 14.2.2 At least 50% males | 8 | 1675 | Risk Ratio (IV, Random, 95% CI) | 0.60 [0.45, 0.79] |
| 14.3 Duration of intervention | 17 | 2847 | Risk Ratio (IV, Random, 95% CI) | 0.62 [0.50, 0.78] |
| 14.3.1 Less than 6 months | 11 | 1705 | Risk Ratio (IV, Random, 95% CI) | 0.55 [0.44, 0.70] |
| 14.3.2 Between 6 and 12 months | 4 | 386 | Risk Ratio (IV, Random, 95% CI) | 0.59 [0.37, 0.96] |
| 14.3.3 More than 12 months | 2 | 756 | Risk Ratio (IV, Random, 95% CI) | 0.94 [0.79, 1.11] |
Comparison 15. Failure to attain suitability for dialysis (subgroup analysis).
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
|---|---|---|---|---|
| 15.1 Duration of intervention | 5 | 1503 | Risk Ratio (IV, Random, 95% CI) | 0.63 [0.34, 1.15] |
| 15.1.1 Less than 6 months | 4 | 854 | Risk Ratio (IV, Random, 95% CI) | 0.64 [0.31, 1.30] |
| 15.1.2 More than 12 months | 1 | 649 | Risk Ratio (IV, Random, 95% CI) | 0.51 [0.16, 1.68] |
Characteristics of studies
Characteristics of included studies [ordered by study ID]
AASER 2017.
| Study characteristics | ||
| Methods |
|
|
| Participants |
|
|
| Interventions | Treatment group
Control group
Cointerventions
|
|
| Outcomes |
|
|
| Notes |
|
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Quote: "The randomisation list was generated by software that assigned the codes for all patients at each participating centre in order of enrolment." |
| Allocation concealment (selection bias) | Unclear risk | Insufficient information to permit judgement |
| Blinding of participants and personnel (performance bias) All outcomes | High risk | Open‐label study |
| Blinding of outcome assessment (detection bias) All outcomes | Low risk | Quote: "The same independent researcher, blinded as to the therapeutic group, adjudicated renal and cardiovascular events in clinical documentation." |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | 50/54 patients in aspirin group were included in analysis; 61/62 patients in control group were included in analysis |
| Selective reporting (reporting bias) | Low risk | Study endpoints included all critical outcomes that would be expected for a study of this type |
| Other bias | Low risk | No evidence of other sources of bias. Funder was likely to influence data analysis and study reporting or interpretation |
Abacilar 2015.
| Study characteristics | ||
| Methods |
|
|
| Participants |
|
|
| Interventions | Treatment group
Control group
Cointerventions
|
|
| Outcomes |
|
|
| Notes |
|
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Quote: "The randomisation was stratified according to the medical centre with a permuted block scheme, with a block size of four and an equal allocation." Comment: Insufficient information to permit judgement |
| Allocation concealment (selection bias) | Unclear risk | Insufficient information to permit judgement |
| Blinding of participants and personnel (performance bias) All outcomes | Low risk | Double‐blind study |
| Blinding of outcome assessment (detection bias) All outcomes | High risk | Quote: "Venous and arterial line diameters were calculated using sonography." Comment: Some outcomes may have been influenced by knowledge of treatment assignment |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | Quote: "None of the patients died or were lost to follow‐up." Comment: All participants were included in the analysis |
| Selective reporting (reporting bias) | Low risk | Study endpoints included all critical outcomes that would be expected for a study of this type |
| Other bias | Low risk | No evidence of other sources of bias |
Abdul‐Rahman 2007.
| Study characteristics | ||
| Methods |
|
|
| Participants |
|
|
| Interventions | Treatment group 1
Treatment group 2
Control group
Cointerventions
|
|
| Outcomes |
|
|
| Notes |
|
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Insufficient information to permit judgement |
| Allocation concealment (selection bias) | Unclear risk | Insufficient information to permit judgement |
| Blinding of participants and personnel (performance bias) All outcomes | Low risk | Double‐blind study |
| Blinding of outcome assessment (detection bias) All outcomes | High risk | Quote: "The presence of haemodialysis tunnelled central venous catheter thrombosis was determined by a staff member, who was blinded to treatment allocation." Comment: Although the researcher was blinded, outcome adjudication (bleeding) may have been influenced by knowledge of treatment assignment |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | No loss to follow‐up |
| Selective reporting (reporting bias) | High risk | Study endpoints did not include all critical outcomes (death, cardiovascular events) that would be expected for a study of this type |
| Other bias | Low risk | No evidence of other sources of bias |
Alexopoulos 2011.
| Study characteristics | ||
| Methods |
|
|
| Participants |
|
|
| Interventions | Treatment group 1
Treatment group 2
Cointerventions
|
|
| Outcomes |
|
|
| Notes |
|
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Quote: "Patients with HTPR (as defined below) were randomised (day 0) in a 1:1 ratio, by the use of computerized random number generation by an independent investigator." Comment: Computer‐generated random number is considered as low risk of bias |
| Allocation concealment (selection bias) | Unclear risk | Insufficient information to permit judgement |
| Blinding of participants and personnel (performance bias) All outcomes | High risk | Single‐blind study |
| Blinding of outcome assessment (detection bias) All outcomes | High risk | Quote: "Physicians and operators who performed platelet function testing were blinded as to the actual drug used, and an independent physician monitored bleeding and adverse event data." Comment: Independent physician monitored bleeding may have been influenced by knowledge of treatment assignment (not reported if the physician was blind) |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | All patients completed follow‐up |
| Selective reporting (reporting bias) | Low risk | Study endpoints included all critical outcomes that would be expected for a study of this type. Data reported for the first phase of the cross‐over RCT |
| Other bias | Low risk | No evidence of other sources of bias |
Anderson 1974.
| Study characteristics | ||
| Methods |
|
|
| Participants |
|
|
| Interventions | Treatment group
Control group
Cointerventions
|
|
| Outcomes |
|
|
| Notes |
|
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Insufficient information to permit judgement |
| Allocation concealment (selection bias) | Low risk | Quote: "Patients within each group were allocated at random by the hospital pharmacy." |
| Blinding of participants and personnel (performance bias) All outcomes | Low risk | Double‐blind study |
| Blinding of outcome assessment (detection bias) All outcomes | High risk | Not reported. Outcomes may have been influenced by knowledge of treatment allocation |
| Incomplete outcome data (attrition bias) All outcomes | Unclear risk | Insufficient information to permit judgement |
| Selective reporting (reporting bias) | High risk | Study endpoints did not include all critical outcomes that would be expected for a study of this type |
| Other bias | Low risk | No evidence of other sources of bias |
Andrassy 1974.
| Study characteristics | ||
| Methods |
|
|
| Participants |
|
|
| Interventions | Treatment group
Control group
Cointerventions
|
|
| Outcomes |
|
|
| Notes |
|
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Insufficient information to permit judgement |
| Allocation concealment (selection bias) | Unclear risk | Insufficient information to permit judgement |
| Blinding of participants and personnel (performance bias) All outcomes | Low risk | Double‐blind study |
| Blinding of outcome assessment (detection bias) All outcomes | High risk | Not reported. Outcome may have been influenced by knowledge of treatment allocation |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | All participants were accounted for in analysis |
| Selective reporting (reporting bias) | High risk | Study endpoints did not include all critical outcomes that would be expected for this type of study |
| Other bias | High risk | Imbalance of baseline characteristics |
ATACAS 2008.
| Study characteristics | ||
| Methods |
|
|
| Participants |
|
|
| Interventions | Treatment group
Control group
Cointerventions
|
|
| Outcomes |
|
|
| Notes |
|
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Quote: "Randomisation was performed with the use of a computer‐generated code that was accessed by means of an automated telephone voice recognition or Web‐based service. Treatment assignment was stratified according to study site with the use of permuted blocks." |
| Allocation concealment (selection bias) | Low risk | Quote: "Randomisation was performed with the use of a computer‐generated code that was accessed by means of an automated telephone voice recognition or Web‐based service." |
| Blinding of participants and personnel (performance bias) All outcomes | Low risk | Double‐blind study |
| Blinding of outcome assessment (detection bias) All outcomes | Low risk | Quote: "An adjudication committee whose members were unaware of the group assignments assessed all major study outcomes." |
| Incomplete outcome data (attrition bias) All outcomes | Unclear risk | Insufficient data on CKD patients to permit judgement |
| Selective reporting (reporting bias) | Low risk | Study reported expected outcomes for this type of study |
| Other bias | Unclear risk | Insufficient data on CKD patients to permit judgement. Funder was unlikely to influence data analysis and study reporting or interpretation |
CASSIOPEIR 2014.
| Study characteristics | ||
| Methods |
|
|
| Participants |
|
|
| Interventions | Treatment group 1
Treatment group 2
Control group
Cointerventions
|
|
| Outcomes |
|
|
| Notes |
|
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Quote: "After the run‐in period, patients were randomly assigned in a 1:1:1 ratio via a computer generated randomisation sequence" Comment: Computer‐generation is considered as low risk of bias |
| Allocation concealment (selection bias) | Low risk | Quote: "After the run‐in period, patients were randomly assigned in a 1:1:1 ratio via a computer generated randomisation sequence, with either an interactive voice or web‐response system" Comment: Interactive voice or web‐response system are considered as low risk of bias |
| Blinding of participants and personnel (performance bias) All outcomes | Low risk | Double‐blind study |
| Blinding of outcome assessment (detection bias) All outcomes | Low risk | Quote: "Prior to the start of the study, an Endpoint Judgment Committee (EJC) and Data and Safety Monitoring Board (DSMB) were established. The EJC, comprised of three members not involved directly in the study, examined the validity of dialysis introduction, renal transplantation, and cardiovascular events among the efficacy endpoints in each institution. Both the EJC and DSMB provided oversight of the study without breaking the blinded randomisation of the patients." Comment: Since an external and blinded committee assessed the outcomes, it is considered at low risk of bias |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | Quote: "After removing patients who did not provide the appropriate consent (n = 2), the safety population consisted of 890; patients 299 received TRK‐100STP 120 μg, 300 received TRK‐100STP 240 μg, and 291 received the placebo. After excluding patients who were missing data after receiving the study drug (n = 4) and who failed to meet the inclusion and exclusion criteria (n = 1), the full analysis set included 885 patients; 296 received TRK‐100STP 120 μg, 298 received TRK‐100STP 240 μg and 291 received the placebo." Comment: 296/299 in the treatment group 1, 298/301 in the treatment group 2 and 291/292 in the control group completed the intention to treat analysis, respectively (< 10% of lost to follow‐up without differences between groups) |
| Selective reporting (reporting bias) | Low risk | Outcomes reported according to published protocol. Study endpoints included all critical outcomes that would be expected for this type of study |
| Other bias | Low risk | No evidence of other sources of bias. Funder was unlikely to influence data analysis and study reporting or interpretation |
Chan 1987.
| Study characteristics | ||
| Methods |
|
|
| Participants |
|
|
| Interventions | Treatment group
Control group
Cointerventions
|
|
| Outcomes |
|
|
| Notes |
|
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Insufficient information to permit judgement |
| Allocation concealment (selection bias) | Unclear risk | Insufficient information to permit judgement |
| Blinding of participants and personnel (performance bias) All outcomes | High risk | Open‐label study |
| Blinding of outcome assessment (detection bias) All outcomes | Low risk | Not reported; outcomes were generally unlikely to be influenced by knowledge of treatment allocation |
| Incomplete outcome data (attrition bias) All outcomes | High risk | Overall, 14/52 did not complete follow‐up. Uncertain reasons |
| Selective reporting (reporting bias) | High risk | Study endpoints did not include all critical outcomes that would be expected for a study of this type |
| Other bias | Low risk | No evidence of other sources of bias |
CHANCE 2013.
| Study characteristics | ||
| Methods |
|
|
| Participants |
|
|
| Interventions | Treatment group
Control group
Cointerventions
|
|
| Outcomes |
|
|
| Notes |
|
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Quote: "The site investigator called into an automated system that randomly assigned a number corresponding to a medication kit stored at the study site, and the medication in the kit was administered to the patient." Comment: Automised system random number generator is considered as low risk of bias |
| Allocation concealment (selection bias) | Unclear risk | Insufficient information to permit judgement |
| Blinding of participants and personnel (performance bias) All outcomes | Low risk | Double‐blind study |
| Blinding of outcome assessment (detection bias) All outcomes | Low risk | Quote: "All reported efficacy and safety outcomes were confirmed by a central adjudication committee that was blinded to the study group assignments." |
| Incomplete outcome data (attrition bias) All outcomes | Unclear risk | Quote: "A total of 5170 eligible patients were enrolled at 114 medical centres in China. Among them, 5150 (99.61%) subjects with renal parameters and 90‐day outcome data were analysed in this study." Comment: Insufficient data on CKD patients to permit judgement |
| Selective reporting (reporting bias) | Low risk | Study endpoints included all critical outcomes that would be expected for a study of this type |
| Other bias | Low risk | No evidence of other sources of bias. Funder was unlikely to influence data analysis and study reporting or interpretation |
CHARISMA 2006.
| Study characteristics | ||
| Methods |
|
|
| Participants |
|
|
| Interventions | Treatment group
Control group
Cointerventions
|
|
| Outcomes |
|
|
| Notes |
|
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Insufficient information to permit judgement |
| Allocation concealment (selection bias) | Low risk | Quote: "Study‐drug assignment performed centrally by an interactive voice‐response system, on the basis of a pre‐established randomisation scheme, stratified according to site." |
| Blinding of participants and personnel (performance bias) All outcomes | Low risk | Double‐blind study |
| Blinding of outcome assessment (detection bias) All outcomes | High risk | Not reported. However, outcomes assessment may have been influenced by knowledge of treatment allocation |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | Quote: "All patients were followed until a common study end date based on the prespecified target of 1040 primary efficacy end points was reached." Comment: Attrition was considered as a low risk of bias |
| Selective reporting (reporting bias) | Low risk | The study protocol was available. Study endpoints included all critical outcomes that would be expected for a study of this type |
| Other bias | High risk | No evidence of other sources of bias. The role of funding was not reported |
Cheng 1998a.
| Study characteristics | ||
| Methods |
|
|
| Participants |
|
|
| Interventions | Treatment group
Control group 1
Control group 2
Cointerventions
|
|
| Outcomes |
|
|
| Notes |
|
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Insufficient information to permit judgement |
| Allocation concealment (selection bias) | Unclear risk | Insufficient information to permit judgement |
| Blinding of participants and personnel (performance bias) All outcomes | High risk | Not reported. As the treatments were physically different, it was likely that participants and/or investigators were aware of treatment allocation |
| Blinding of outcome assessment (detection bias) All outcomes | Low risk | Not reported. Outcome assessment was unlikely to be influenced by knowledge of treatment outcome |
| Incomplete outcome data (attrition bias) All outcomes | High risk | 1/17 (nadolol group), 3/15 (captopril group) and 1/20 (treatment group) participants in the three treatment groups withdrew prematurely (differences between groups) |
| Selective reporting (reporting bias) | High risk | Study endpoints did not include all critical outcomes that would be expected for a study of this type |
| Other bias | High risk | No evidence of other sources of bias. The role of Bristol‐Myer‐Squibb and Sanofi were not reported |
Christopher 1987.
| Study characteristics | ||
| Methods |
|
|
| Participants |
|
|
| Interventions | Treatment group
Control group
Cointerventions
|
|
| Outcomes |
|
|
| Notes |
|
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Insufficient information to permit judgement |
| Allocation concealment (selection bias) | Unclear risk | Insufficient information to permit judgement |
| Blinding of participants and personnel (performance bias) All outcomes | Low risk | Double‐blind study |
| Blinding of outcome assessment (detection bias) All outcomes | Low risk | Not reported. Outcomes were unlikely to be influenced by knowledge of treatment allocation |
| Incomplete outcome data (attrition bias) All outcomes | Unclear risk | Insufficient information to permit judgement |
| Selective reporting (reporting bias) | High risk | Study endpoints did not include all critical outcomes that would be expected for a study of this type |
| Other bias | Unclear risk | Insufficient information to permit judgement |
CILON‐T 2010.
| Study characteristics | ||
| Methods |
|
|
| Participants |
|
|
| Interventions | Treatment group 1
Treatment group 2
Cointerventions
|
|
| Outcomes |
|
|
| Notes |
|
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Insufficient information to permit judgement |
| Allocation concealment (selection bias) | Unclear risk | Insufficient information to permit judgement |
| Blinding of participants and personnel (performance bias) All outcomes | High risk | Not reported. As the treatments were physically different, it was likely that participants and investigators were aware of treatment allocation |
| Blinding of outcome assessment (detection bias) All outcomes | Low risk | Not reported. However, outcomes were unlikely to be influenced by knowledge of treatment allocation |
| Incomplete outcome data (attrition bias) All outcomes | Unclear risk | Insufficient information to permit judgement |
| Selective reporting (reporting bias) | High risk | Study endpoints did not include all critical outcomes that would be expected for a study of this type |
| Other bias | Unclear risk | Insufficient information to permit judgement |
CREDO 2005.
| Study characteristics | ||
| Methods |
|
|
| Participants |
|
|
| Interventions | Treatment group
Control group
Cointerventions
|
|
| Outcomes |
|
|
| Notes |
|
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Quote: "Patients were randomly assigned to groups using a prospective randomisation schedule. The randomisation was performed in blocks of two and stratified by centre." Comment: Random number is considered as low risk of bias |
| Allocation concealment (selection bias) | Unclear risk | Quote: "When a patient was ready to be randomised, the site dispensed a drug package that contained a unique 4‐digit random number; this number was entered on the case report form and provided an identifier of the treatment assigned." Comment: Insufficient information to permit judgement |
| Blinding of participants and personnel (performance bias) All outcomes | Low risk | Double‐blind study |
| Blinding of outcome assessment (detection bias) All outcomes | High risk | Not reported. However, outcomes assessment may have been influenced by knowledge of treatment allocation |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | All randomised patients included in intention‐to‐treat and safety analysis |
| Selective reporting (reporting bias) | Low risk | Study endpoints included all critical outcomes that would be expected for a study of this type |
| Other bias | High risk | Four authors were employees of the Pharmaceutical companies providing the grants |
Creek 1990.
| Study characteristics | ||
| Methods |
|
|
| Participants |
|
|
| Interventions | Treatment group
Control group
Cointerventions
|
|
| Outcomes |
|
|
| Notes |
|
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Insufficient information to permit judgement |
| Allocation concealment (selection bias) | Unclear risk | Insufficient information to permit judgement |
| Blinding of participants and personnel (performance bias) All outcomes | High risk | Not reported. As the treatments were physically different, it was likely that participants and investigators were aware of treatment allocation |
| Blinding of outcome assessment (detection bias) All outcomes | High risk | Not reported. However, outcomes assessment may have been influenced by knowledge of treatment allocation |
| Incomplete outcome data (attrition bias) All outcomes | Unclear risk | Insufficient information to permit judgement |
| Selective reporting (reporting bias) | Low risk | Study endpoints included all critical outcomes that would be expected for a study of this type |
| Other bias | High risk | Full study report not available |
CURE 2000.
| Study characteristics | ||
| Methods |
|
|
| Participants |
|
|
| Interventions | Treatment group
Control group
Cointerventions
|
|
| Outcomes |
|
|
| Notes |
|
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Quote: "Patients are randomised to either clopidogrel or placebo in CURE by a telephone call to a central, 24‐h, computerized randomisation service. Permuted block randomisation, stratified by clinical centre is used." Comment: Insufficient information to permit judgement |
| Allocation concealment (selection bias) | Low risk | Quote: "Patients are randomised to either clopidogrel or placebo in CURE by a telephone call to a central, 24‐h, computerized randomisation service located at the Canadian Cardiovascular Collaboration Project Office, McMaster University, Hamilton, Canada." |
| Blinding of participants and personnel (performance bias) All outcomes | Low risk | Double‐blind study |
| Blinding of outcome assessment (detection bias) All outcomes | Low risk | Quote: "All primary outcomes and major bleeding complications were determined by adjudicators who were blinded to treatment status." |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | 0.1% were lost to follow‐up |
| Selective reporting (reporting bias) | Low risk | Study endpoints included all critical outcomes that would be expected for a study of this type |
| Other bias | Low risk | No evidence of other sources of bias. Funder was unlikely to influence data analysis and study reporting or interpretation |
Dash 2013.
| Study characteristics | ||
| Methods |
|
|
| Participants |
|
|
| Interventions | Treatment group 1
Treatment group 2
Cointerventions
|
|
| Outcomes |
|
|
| Notes |
|
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Quote: "Randomization was done by using computer‐generated random list." Comment: Computer‐generated random list is considered as low risk of bias |
| Allocation concealment (selection bias) | Unclear risk | Insufficient information to permit judgement |
| Blinding of participants and personnel (performance bias) All outcomes | High risk | Open‐label study |
| Blinding of outcome assessment (detection bias) All outcomes | Low risk | Not reported. Outcomes were unlikely to be influenced by knowledge of treatment allocation |
| Incomplete outcome data (attrition bias) All outcomes | High risk | 9/80 participants did not complete follow‐up due to non‐compliance (3/40 in clopidogrel group and 6/40 in aspirin group; differences between groups) |
| Selective reporting (reporting bias) | High risk | Study did not report all expected outcomes for this type of study |
| Other bias | Low risk | No evidence of other sources of bias |
Dember 2005.
| Study characteristics | ||
| Methods |
|
|
| Participants |
|
|
| Interventions | Treatment group
Control group
Cointerventions
|
|
| Outcomes |
|
|
| Notes |
|
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Quote: "Computer‐generated permuted block randomisation with stratification by location of the fistula (forearm vs upper arm) and by centre." Comment: Insufficient information to permit judgement |
| Allocation concealment (selection bias) | Unclear risk | Insufficient information to permit judgement |
| Blinding of participants and personnel (performance bias) All outcomes | Low risk | Double‐blind study |
| Blinding of outcome assessment (detection bias) All outcomes | High risk | Independent determinations of the fistula patency were conducted by two independent observers in a random sub‐set. Hovewer, outcomes (QoL, adverse events, bleeding) may have been influenced by knowledge of treatment allocation |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | Quote: "Thirty‐seven participants (8.4%) in the clopidogrel group and 33 participants (7.6%) in the placebo group discontinued the study medication early. The reasons for early discontinuation of study medication did not differ between treatment groups." Comment: < 10% were lost to follow‐up. Missing outcome data balanced in numbers across groups, with similar reasons across groups |
| Selective reporting (reporting bias) | Low risk | The study protocol was available. Study endpoints included all critical outcomes that would be expected for a study of this type |
| Other bias | High risk | The study was terminated early by the Data Safety Monitoring Board based in the prespecified stopping rule for efficacy of the intervention of the primary endpoint. Funder was unlikely to influence data analysis and study reporting or interpretation |
Dixon 2005.
| Study characteristics | ||
| Methods |
|
|
| Participants |
|
|
| Interventions | Treatment group
Control group
Cointerventions
|
|
| Outcomes |
|
|
| Notes |
|
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Quote: "Randomization was stratified according to clinical centre and access location (forearm or alternative site) with the use of a random permuted‐block design." Comment: Insufficient information to permit judgement |
| Allocation concealment (selection bias) | Low risk | Quote: "Randomization is performed centrally via the Internet using a Web browser following verification of eligibility by the Data Coordinating Center (Cleveland Clinic)." |
| Blinding of participants and personnel (performance bias) All outcomes | Low risk | Double‐blind study |
| Blinding of outcome assessment (detection bias) All outcomes | High risk | Quote: "A sample of angiograms from each clinical centre was reviewed in a blinded manner to confirm that the indication for intervention was uniform across study sites." Comment: Some outcomes may have been influenced by knowledge of treatment allocation |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | All patients were analysed |
| Selective reporting (reporting bias) | Low risk | The study protocol was available. Study endpoints included all critical outcomes that would be expected for a study of this type |
| Other bias | Low risk | No evidence of other sources of bias. Funder did not influence data analysis and study reporting or interpretation |
Dmoszynska‐Giannopoulou 1990.
| Study characteristics | ||
| Methods |
|
|
| Participants |
|
|
| Interventions | Treatment group
Control group 1
Control group 2
Cointerventions
|
|
| Outcomes |
|
|
| Notes |
|
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Insufficient information to permit judgement |
| Allocation concealment (selection bias) | Unclear risk | Insufficient information to permit judgement |
| Blinding of participants and personnel (performance bias) All outcomes | High risk | Not reported. As the treatments were physically different, it was likely that participants and investigators were aware of treatment allocation |
| Blinding of outcome assessment (detection bias) All outcomes | High risk | Not reported. Outcomes were generally unlike to be influenced by knowledge of treatment allocation. However, bleeding time could be influenced by the knowledge of the treatment allocation |
| Incomplete outcome data (attrition bias) All outcomes | Unclear risk | Insufficient information to permit judgement |
| Selective reporting (reporting bias) | High risk | Study endpoints did not include all critical outcomes that would be expected for a study of this type |
| Other bias | Unclear risk | Insufficient information to permit judgement |
Dodd 1980.
| Study characteristics | ||
| Methods |
|
|
| Participants |
|
|
| Interventions | Treatment group
Control group
Cointerventions
|
|
| Outcomes |
|
|
| Notes |
|
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Insufficient information to permit judgement |
| Allocation concealment (selection bias) | Unclear risk | Insufficient information to permit judgement |
| Blinding of participants and personnel (performance bias) All outcomes | Low risk | Double‐blind study |
| Blinding of outcome assessment (detection bias) All outcomes | High risk | Not reported. Outcome assessment may have been influenced by knowledge of treatment allocation |
| Incomplete outcome data (attrition bias) All outcomes | Unclear risk | Insufficient information to permit judgement |
| Selective reporting (reporting bias) | High risk | Study did not report all outcomes expected in a study of this type |
| Other bias | Unclear risk | Insufficient information to permit judgement |
Donadio 1984.
| Study characteristics | ||
| Methods |
|
|
| Participants |
|
|
| Interventions | Treatment group
Control group
Cointerventions
|
|
| Outcomes |
|
|
| Notes |
|
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Insufficient information to permit judgement |
| Allocation concealment (selection bias) | Unclear risk | Quote: "Treatment was assigned randomly by our statistician in such a manner to achieve maximal balance between the two stratification factors." Comment: Insufficient information to permit judgement |
| Blinding of participants and personnel (performance bias) All outcomes | Low risk | Double‐blind study |
| Blinding of outcome assessment (detection bias) All outcomes | High risk | Not reported. Some outcomes were likely to be influenced by knowledge of treatment allocation |
| Incomplete outcome data (attrition bias) All outcomes | High risk | 10/50 participants (4/25 in the treatment group and 6/25 in the control group) not included in analyses |
| Selective reporting (reporting bias) | High risk | Study outcomes did not include critical outcomes expected for this type of study |
| Other bias | Unclear risk | Insufficient information to permit judgement |
EARLY ACS 2005.
| Study characteristics | ||
| Methods |
|
|
| Participants |
|
|
| Interventions | Treatment group 1 (early routine)
Treatment group 2 (delayed provisional)
Cointerventions
|
|
| Outcomes |
|
|
| Notes |
|
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Insufficient information to permit judgement |
| Allocation concealment (selection bias) | Low risk | Quote: "Randomization was managed through an interactive voice‐response system." |
| Blinding of participants and personnel (performance bias) All outcomes | Low risk | Double‐blind study |
| Blinding of outcome assessment (detection bias) All outcomes | High risk | Quote: "Stroke and all efficacy endpoints except death were adjudicated by an independent clinical events committee whose members were unaware of study group assignments. If classification of TIMI bleeding could not be determined by a programmed algorithm, blinded adjudication was performed." Comment: Outcome adjudication (adverse events and death) may have been influenced by knowledge of treatment assignment |
| Incomplete outcome data (attrition bias) All outcomes | Unclear risk | Overall, 8/4722 in treatment group and 20/4684 in control group did not complete follow‐up. Insufficient data on CKD patients |
| Selective reporting (reporting bias) | Low risk | Study endpoints included all critical outcomes that would be expected for a study of this type |
| Other bias | Low risk | No evidence of other sources of bias. Funder was unlikely to influence data analysis and study reporting or interpretation |
Ell 1982.
| Study characteristics | ||
| Methods |
|
|
| Participants |
|
|
| Interventions | Treatment group
Control group
Cointerventions
|
|
| Outcomes |
|
|
| Notes |
|
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Insufficient information to permit judgement |
| Allocation concealment (selection bias) | Unclear risk | Insufficient information to permit judgement |
| Blinding of participants and personnel (performance bias) All outcomes | High risk | Not reported. As the treatments were physically different, it was likely that participants and investigators were aware of treatment allocation |
| Blinding of outcome assessment (detection bias) All outcomes | High risk | Not reported. Outcome assessment may have been influenced by knowledge of treatment allocation |
| Incomplete outcome data (attrition bias) All outcomes | Unclear risk | Insufficient information to permit judgement |
| Selective reporting (reporting bias) | Low risk | Study outcomes included critical outcomes expected for this type of study |
| Other bias | High risk | Full study report not available |
EPIC 1994.
| Study characteristics | ||
| Methods |
|
|
| Participants |
|
|
| Interventions | Treatment group
Control group
Cointerventions
|
|
| Outcomes |
|
|
| Notes |
|
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Insufficient information to permit judgement |
| Allocation concealment (selection bias) | Low risk | Quote: "Central randomisation by telephone, and patients were stratified according to their study centre and where they having an acute evolving myocardial infarction." |
| Blinding of participants and personnel (performance bias) All outcomes | Low risk | Double‐blind study |
| Blinding of outcome assessment (detection bias) All outcomes | Low risk | Blinded clinical endpoints committee |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | Data available for all baseline participants (and deaths included in intention‐yo‐treat analysis) |
| Selective reporting (reporting bias) | Low risk | The study protocol was available. Study outcomes included critical outcomes expected for this type of study |
| Other bias | High risk | No evidence of other sources of bias. The role of the funder was not reported |
EPILOG 1997.
| Study characteristics | ||
| Methods |
|
|
| Participants |
|
|
| Interventions | Treatment group
Control group
Cointerventions
|
|
| Outcomes |
|
|
| Notes |
|
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Insufficient information to permit judgement |
| Allocation concealment (selection bias) | Low risk | Quote: "Patients were randomly assigned in a double‐blind fashion by means of a central telephone hot line to one of three treatment groups." |
| Blinding of participants and personnel (performance bias) All outcomes | Low risk | Double‐blind study |
| Blinding of outcome assessment (detection bias) All outcomes | Low risk | Quote: "End‐point classifications of a clinical‐events committee blinded to the study‐group assignment were used for the final analysis." |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | Loss to follow‐up < 10%, without differences between groups |
| Selective reporting (reporting bias) | Low risk | The study protocol was available. Study outcomes included critical outcomes expected for this type of study |
| Other bias | High risk | Sample size was smaller than planned and the study was terminated earlier because a prespecified stopping rule was met after the first interim analysis. The role of funding was not reported |
EPISTENT 1998.
| Study characteristics | ||
| Methods |
|
|
| Participants |
|
|
| Interventions | Treatment group 1
Treatment group 2
Control group
Cointerventions
|
|
| Outcomes |
|
|
| Notes |
|
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Insufficient information to permit judgement |
| Allocation concealment (selection bias) | Low risk | Quote: "We received the randomisation schedule by a telephone hotline." |
| Blinding of participants and personnel (performance bias) All outcomes | Low risk | Double‐blind study |
| Blinding of outcome assessment (detection bias) All outcomes | Low risk | Quote: "All endpoint events were assessed by a clinical events committee that was unaware of study‐group assignment." |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | Data provided for all patients |
| Selective reporting (reporting bias) | Low risk | The study protocol was available. Study outcomes included critical outcomes expected for this type of study |
| Other bias | Low risk | No evidence of other sources of bias. Funder did not influence data analysis and study reporting or interpretation |
ETDRS 1992.
| Study characteristics | ||
| Methods |
|
|
| Participants |
|
|
| Interventions | Treatment group
Control group
Cointerventions
|
|
| Outcomes |
|
|
| Notes |
|
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Quote: "The randomisation was designed to provide balance in the number of patients assigned to aspirin or placebo within each clinical centre." Comment: Insufficient information to permit judgement |
| Allocation concealment (selection bias) | Unclear risk | Insufficient information to permit judgement |
| Blinding of participants and personnel (performance bias) All outcomes | Low risk | Double‐blind study |
| Blinding of outcome assessment (detection bias) All outcomes | Low risk | Outcomes assessment was performed without knowledge of treatment assignment |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | Unpublished data only used. All participants were analysed |
| Selective reporting (reporting bias) | Low risk | Unpublished data only used. Study outcomes included critical outcomes expected for this type of study |
| Other bias | Low risk | Unpublished data only used. No evidence of other sources of bias. Funder was unlikely to influence data analysis and study reporting or interpretation |
EUCLID 2017.
| Study characteristics | ||
| Methods |
|
|
| Participants |
|
|
| Interventions | Treatment group
Control group
Cointerventions
|
|
| Outcomes |
|
|
| Notes |
|
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Quote from Hiatt 2017: "Randomization was performed with the use of an interactive voice‐response or Web‐response system." |
| Allocation concealment (selection bias) | Low risk | Quote from Hiatt 2017: "Randomization was performed with the use of an interactive voice‐response or Web‐response system." |
| Blinding of participants and personnel (performance bias) All outcomes | Unclear risk | Quote: "Double‐blind." Comment: Although author reported that the study used a double‐blind design, information about blinding of participants and investigators were not clearly stated |
| Blinding of outcome assessment (detection bias) All outcomes | Low risk | Quote from Hiatt 2017: "All primary efficacy and safety end points were adjudicated by an independent clinical events committee in a blinded fashion." |
| Incomplete outcome data (attrition bias) All outcomes | High risk | Insufficient information to permit judgement |
| Selective reporting (reporting bias) | High risk | Prespecified outcomes were reported. Clinically‐relevant outcomes that would be expected for this type of intervention were not reported |
| Other bias | Unclear risk | Quote from Hiatt 2017: "The Duke Clinical Research Institute held the clinical database and conducted all analyses for publication independent of the sponsor." Comment: Baseline characteristics were not reported for patients with CKD and diabetes. Funding did not influence analysis |
FAVOURED 2009.
| Study characteristics | ||
| Methods |
|
|
| Participants |
|
|
| Interventions | Treatment group
Control group
Cointerventions
|
|
| Outcomes |
|
|
| Notes |
|
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Quote: "Randomization was performed by a central, web‐based system (Flexetrials) using an adaptive minimization algorithm with study site and planned location of the AVF (upper vs lower arm) as minimization variables." Comment: Adaptive minimization algorithm is considered as low risk of bias |
| Allocation concealment (selection bias) | Low risk | Quote: "Randomization was performed by a central, web‐based system (Flexetrials)." |
| Blinding of participants and personnel (performance bias) All outcomes | Low risk | Double‐blind study |
| Blinding of outcome assessment (detection bias) All outcomes | High risk | Not reported. Outcome assessment may have been influenced by knowledge of treatment allocation |
| Incomplete outcome data (attrition bias) All outcomes | Unclear risk | Insufficient information to permit judgement |
| Selective reporting (reporting bias) | Low risk | Study outcomes included critical outcomes expected for this type of study |
| Other bias | High risk | Due to early cessation of recruitment, only the first interim analysis was performed after which the study continued as planned until terminated because of slower than anticipated accrual, funding issues, and lack of ongoing availability of trial medications. The role of the funder were not reported |
Fiskerstrand 1985.
| Study characteristics | ||
| Methods |
|
|
| Participants |
|
|
| Interventions | Treatment group
Control group
Cointerventions
|
|
| Outcomes |
|
|
| Notes |
|
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Insufficient information to permit judgement |
| Allocation concealment (selection bias) | Unclear risk | Insufficient information to permit judgement |
| Blinding of participants and personnel (performance bias) All outcomes | Low risk | Double‐blind study |
| Blinding of outcome assessment (detection bias) All outcomes | High risk | Not reported. Outcome adjudication may have been influenced by knowledge of treatment assignment |
| Incomplete outcome data (attrition bias) All outcomes | High risk | 3/18 patients did not complete the trial (1/10 in the placebo group and 2/8 in the treatment group) |
| Selective reporting (reporting bias) | High risk | Study outcomes did not include critical outcomes expected for this type of study |
| Other bias | High risk | Insufficient information to permit judgement. The role of the funder was not reported |
Frascà 1986.
| Study characteristics | ||
| Methods |
|
|
| Participants |
|
|
| Interventions | Treatment group
Control group
Cointerventions
|
|
| Outcomes |
|
|
| Notes |
|
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Insufficient information to permit judgement |
| Allocation concealment (selection bias) | Unclear risk | Insufficient information to permit judgement |
| Blinding of participants and personnel (performance bias) All outcomes | High risk | Not reported. As the treatments were physically different, it was likely that participants and investigators were aware of treatment allocation |
| Blinding of outcome assessment (detection bias) All outcomes | High risk | Not reported. Outcome adjudication may have been influenced by knowledge of treatment assignment |
| Incomplete outcome data (attrition bias) All outcomes | High risk | 0/40 patients in group A and 4/40 patients in group were not included in analysis (differences between groups) |
| Selective reporting (reporting bias) | Low risk | Study outcomes included critical outcomes expected for this type of study |
| Other bias | Low risk | No evidence of other sources of bias |
Frascà 1997.
| Study characteristics | ||
| Methods |
|
|
| Participants |
|
|
| Interventions | Treatment group
Control group
Cointerventions
|
|
| Outcomes |
|
|
| Notes |
|
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Insufficient information to permit judgement |
| Allocation concealment (selection bias) | Unclear risk | Insufficient information to permit judgement |
| Blinding of participants and personnel (performance bias) All outcomes | High risk | Not reported. As the treatments were physically different, it was likely that participants and investigators were aware of treatment allocation |
| Blinding of outcome assessment (detection bias) All outcomes | High risk | Not reported. Outcome adjudication may have been influenced by knowledge of treatment assignment |
| Incomplete outcome data (attrition bias) All outcomes | Unclear risk | Insufficient information to permit judgement |
| Selective reporting (reporting bias) | High risk | Study did not report all expected outcomes expected for a study of this type |
| Other bias | Low risk | No evidence of other sources of bias. Funder was unlikely to influence data analysis and study reporting or interpretation |
Gaede 2003.
| Study characteristics | ||
| Methods |
|
|
| Participants |
|
|
| Interventions | Treatment group
Control group
Cointerventions
|
|
| Outcomes |
|
|
| Notes |
|
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Quote: "The randomisation was individual with concealed, computer‐generated envelopes." Comment: Insufficient information to permit judgement |
| Allocation concealment (selection bias) | Unclear risk | Quote: "The randomisation was individual with concealed, computer‐generated envelopes." Comment: Insufficient information to permit judgement (not reported if envelopes were opaque and numbered) |
| Blinding of participants and personnel (performance bias) All outcomes | Low risk | Double‐blind study |
| Blinding of outcome assessment (detection bias) All outcomes | High risk | Not reported. Outcome adjudication (due to nature of outcomes) was generally unlikely to be influenced by knowledge of treatment allocation. However, bleeding may be influenced by the knowledge of the treatment assignment |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | Quote: "31 patients, who all gave informed consent, entered and completed the study." Comment: All participant completed the study |
| Selective reporting (reporting bias) | High risk | Study outcomes did not include all expected for this type of study. Data were not appropriately reported for a cross‐over RCT |
| Other bias | High risk | Analyses were not reported appropriately for cross‐over RCT design |
Ghorbani 2009.
| Study characteristics | ||
| Methods |
|
|
| Participants |
|
|
| Interventions | Treatment group
Control group
Cointerventions
|
|
| Outcomes |
|
|
| Notes |
|
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Quote: "The randomisation was stratified according to medical centre with a permuted block scheme, with a block size of four and equal allocation." Comment: Insufficient information to permit judgement |
| Allocation concealment (selection bias) | Low risk | Quote: "Randomization was performed centrally, by the coordinating centre." |
| Blinding of participants and personnel (performance bias) All outcomes | Low risk | Double‐blind study |
| Blinding of outcome assessment (detection bias) All outcomes | High risk | Quote: "Fistula failure was determined by a member of the team who was blinded to treatment allocation. [...] Assessment of the severity of bleeding episodes was performed by a panel blinded to the treatment assignment." Comment: Although the panel was blinded, some outcomes adjudication may have been influenced by knowledge of treatment assignment |
| Incomplete outcome data (attrition bias) All outcomes | High risk | 75/93 patients completed study (38 participants in clopidogrel group and 37 participants in placebo group). Limited information provided |
| Selective reporting (reporting bias) | Low risk | Study outcomes included critical outcomes expected for this type of study |
| Other bias | Low risk | No evidence of other sources of bias. Funder was unlikely to influence data analysis and study reporting or interpretation |
Ghorbani 2013.
| Study characteristics | ||
| Methods |
|
|
| Participants |
|
|
| Interventions | Treatment group
Control group
Cointerventions
|
|
| Outcomes |
|
|
| Notes |
|
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Quote: "The randomisation was stratified according to medical centre with a permuted block scheme, with a block size of four and equal allocation." Comment: Insufficient information to permit judgement |
| Allocation concealment (selection bias) | Low risk | Quote: "Randomization was performed centrally, by the coordinating centre." |
| Blinding of participants and personnel (performance bias) All outcomes | Low risk | Double‐blind study |
| Blinding of outcome assessment (detection bias) All outcomes | High risk | Assessment of the severity of bleeding episodes was performed by a panel blinded to the treatment assignments. Hovewer, outcome adjudication may have been influenced by knowledge of treatment assignment |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | No patients were lost to follow‐up |
| Selective reporting (reporting bias) | Low risk | Study outcomes included critical outcomes expected for this type of study |
| Other bias | Low risk | No evidence of other sources of bias. Funder was unlikely to influence data analysis and study reporting or interpretation |
Giustina 1998.
| Study characteristics | ||
| Methods |
|
|
| Participants |
|
|
| Interventions | Treatment group
Control group
Cointerventions
|
|
| Outcomes |
|
|
| Notes |
|
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Insufficient information to permit judgement |
| Allocation concealment (selection bias) | Low risk | Quote: "Central randomisation." |
| Blinding of participants and personnel (performance bias) All outcomes | Low risk | Double‐blind study |
| Blinding of outcome assessment (detection bias) All outcomes | High risk | Not reported. Outcomes were likely to be influenced by knowledge of treatment assignment |
| Incomplete outcome data (attrition bias) All outcomes | High risk | Quote: "An overall number of 33 patients were enrolled in the study. Three patients spontaneously withdrew from the study during the first 3 months of follow‐up due to lack of compliance. Two of these patients were in the placebo group, and the other was in the picotamide group." Comment: Although in total 9% were lost to follow‐up, there were some differences between groups |
| Selective reporting (reporting bias) | High risk | Study did not report all outcomes expected for this type of study |
| Other bias | Low risk | No evidence of other sources of bias. Funder was unlikely to influence data analysis and study reporting or interpretation |
GLOBAL LEADERS 2018.
| Study characteristics | ||
| Methods |
|
|
| Participants |
|
|
| Interventions | Treatment group
Control group
Cointerventions
|
|
| Outcomes |
|
|
| Notes |
|
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Sequence generation methods were not reported in sufficient detail to permit judgement |
| Allocation concealment (selection bias) | Unclear risk | Method of allocation concealment was not reported in sufficient detail to permit judgement |
| Blinding of participants and personnel (performance bias) All outcomes | High risk | Quote from Tomaniak 2020: "Open‐label" |
| Blinding of outcome assessment (detection bias) All outcomes | High risk | Objective and subjective outcomes were reported |
| Incomplete outcome data (attrition bias) All outcomes | Unclear risk | Quote from Gao 2020: "All the analyses were performed by the intention‐to‐treat principle." Comment: ITT analyses were performed however data on discontinuations were not clearly stated |
| Selective reporting (reporting bias) | High risk | Prespecified outcomes were reported. Clinically‐relevant outcomes that would be expected for this type of intervention were not reported |
| Other bias | High risk | Quote from Hiatt 2017: "The Duke Clinical Research Institute held the clinical database and conducted all analyses for publication independent of the sponsor." Comment: Baseline characteristics were not reported for patients with CKD and diabetes. Funding was likely to influence data analysis and interpretation |
Goicoechea 2012.
| Study characteristics | ||
| Methods |
|
|
| Participants |
|
|
| Interventions | Treatment group
Control group
Cointerventions
|
|
| Outcomes |
|
|
| Notes |
|
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Quote: "Patients were randomly assigned according to a computer‐generated list". Comment: Computer‐generation is considered as low risk of bias |
| Allocation concealment (selection bias) | Unclear risk | Insufficient information to permit judgement |
| Blinding of participants and personnel (performance bias) All outcomes | High risk | Single‐blind study |
| Blinding of outcome assessment (detection bias) All outcomes | High risk | Not reported. Outcomes were likely to be influenced by knowledge of treatment assignment |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | All participants were analysed |
| Selective reporting (reporting bias) | High risk | Study did not report all outcomes (bleeding event) expected for this type of study |
| Other bias | Low risk | No evidence of other sources of bias |
Gonzalez 1995.
| Study characteristics | ||
| Methods |
|
|
| Participants |
|
|
| Interventions | Treatment group
Control group
Cointerventions
|
|
| Outcomes |
|
|
| Notes |
|
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Insufficient information to permit judgement |
| Allocation concealment (selection bias) | Unclear risk | Insufficient information to permit judgement |
| Blinding of participants and personnel (performance bias) All outcomes | High risk | Not reported. As the treatments were physically different, it was likely that participants and investigators were aware of treatment allocation |
| Blinding of outcome assessment (detection bias) All outcomes | High risk | Not reported. Some outcomes were likely to be influenced by knowledge of treatment allocation |
| Incomplete outcome data (attrition bias) All outcomes | High risk | 23/58 patients dropped out of study for different causes (not clearly reported) |
| Selective reporting (reporting bias) | High risk | Study did not report expected outcomes for this type of study |
| Other bias | Unclear risk | Insufficient information to permit judgement |
Gröntoft 1985.
| Study characteristics | ||
| Methods |
|
|
| Participants |
|
|
| Interventions | Treatment group
Control group
Cointerventions
|
|
| Outcomes |
|
|
| Notes |
|
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Insufficient information to permit judgement |
| Allocation concealment (selection bias) | Unclear risk | Insufficient information to permit judgement |
| Blinding of participants and personnel (performance bias) All outcomes | Low risk | Double‐blind study |
| Blinding of outcome assessment (detection bias) All outcomes | High risk | Not reported. Outcome adjudication may likely to be influenced by knowledge of treatment allocation |
| Incomplete outcome data (attrition bias) All outcomes | High risk | 6/42 patients lost to follow‐up |
| Selective reporting (reporting bias) | High risk | Study did not report all outcomes expected for this type of study |
| Other bias | Low risk | No evidence of other sources of bias |
Gröntoft 1998.
| Study characteristics | ||
| Methods |
|
|
| Participants |
|
|
| Interventions | Treatment group
Control group
Cointerventions
|
|
| Outcomes |
|
|
| Notes |
|
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Insufficient information to permit judgement |
| Allocation concealment (selection bias) | Unclear risk | Insufficient information to permit judgement |
| Blinding of participants and personnel (performance bias) All outcomes | Low risk | Double‐blind study |
| Blinding of outcome assessment (detection bias) All outcomes | High risk | Not reported. Outcome adjudication was likely to be influenced by knowledge of treatment outcome |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | Quote: "In total, 258 patients were randomised to placebo or ticlopidine for 285 operations. Of the 285 randomised operations, 16 first entries (5P:11T) and 2 re‐entries (both T) were not evaluable, leaving 267 evaluable operations in 242 patients." Comment: 242/258 completed the study. Hoverer, outcome data related to death showed that there were 136 participants in the control group (124/136 completed the trial) and 131 participants in the treatment group (118/131 completed the trial). There were < 10% lost to follow‐up, with no differences between groups |
| Selective reporting (reporting bias) | Low risk | Study outcomes included critical outcomes expected for this type of study |
| Other bias | High risk | No evidence of other sources of bias. The role of funding was not reported |
Guo 1998.
| Study characteristics | ||
| Methods |
|
|
| Participants |
|
|
| Interventions | Treatment group
Control group
Cointerventions
|
|
| Outcomes |
|
|
| Notes |
|
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | High risk | Quote: "The assignment sequences (ASA / PL or PL / ASA) for the two patient groups A and B were alternatively randomised." Comment: Alternation is considered as a high risk of bias |
| Allocation concealment (selection bias) | Unclear risk | Insufficient information to permit judgement |
| Blinding of participants and personnel (performance bias) All outcomes | Low risk | Double‐blind study |
| Blinding of outcome assessment (detection bias) All outcomes | High risk | Not reported. Outcomes assessment may have been influenced by knowledge of treatment allocation |
| Incomplete outcome data (attrition bias) All outcomes | Unclear risk | Insufficient information to permit judgement |
| Selective reporting (reporting bias) | High risk | Study did not report expected outcomes for this type of study. Data were not appropriately reported for a cross‐over RCT |
| Other bias | High risk | Insufficient information to permit judgement. The role of funding was not reported |
Hansen 2000.
| Study characteristics | ||
| Methods |
|
|
| Participants |
|
|
| Interventions | Treatment group
Control group
Cointerventions
|
|
| Outcomes |
|
|
| Notes |
|
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Insufficient information to permit judgement |
| Allocation concealment (selection bias) | Unclear risk | Insufficient information to permit judgement |
| Blinding of participants and personnel (performance bias) All outcomes | Low risk | Double‐blind study |
| Blinding of outcome assessment (detection bias) All outcomes | High risk | Quote: "AER (enzyme‐linked immunosorbent assay), glomerular filtration rate (GFR) (plasma clearance of 51Cr‐EDTA), blood pressure (BP) (Hawksley), and HbA1c (by high‐performance liquid chromatography)." Comment: Outcomes generally were unlikely to be influenced by knowledge of treatment allocation. Adverse events may likely to be influenced by knowledge of treatment allocation |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | All randomised patients completed the study |
| Selective reporting (reporting bias) | High risk | Study did not report expected outcomes for this type of study. Data were not appropriately reported for a cross‐over RCT |
| Other bias | Low risk | No evidence of other sources of bias. Funder was unlikely to influence data analysis and study reporting or interpretation |
Harter 1979.
| Study characteristics | ||
| Methods |
|
|
| Participants |
|
|
| Interventions | Treatment group
Control group
Cointerventions
|
|
| Outcomes |
|
|
| Notes |
|
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Insufficient information to permit judgement |
| Allocation concealment (selection bias) | Unclear risk | Insufficient information to permit judgement |
| Blinding of participants and personnel (performance bias) All outcomes | Low risk | Double‐blind study |
| Blinding of outcome assessment (detection bias) All outcomes | High risk | Not reported. Outcome adjudication may have been influenced by knowledge of treatment allocation |
| Incomplete outcome data (attrition bias) All outcomes | High risk | High percentage of patients left the study with some differences between groups |
| Selective reporting (reporting bias) | Low risk | Study outcomes included critical outcomes expected for this type of study |
| Other bias | High risk | There were some differences in baseline characteristics between groups. Funder was unlikely to influence data analysis and study reporting or interpretation |
Hidaka 2013.
| Study characteristics | ||
| Methods |
|
|
| Participants |
|
|
| Interventions | Treatment group 1
Treatment group 2
Cointerventions
|
|
| Outcomes |
|
|
| Notes |
|
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Insufficient information to permit judgement |
| Allocation concealment (selection bias) | Unclear risk | Insufficient information to permit judgement |
| Blinding of participants and personnel (performance bias) All outcomes | High risk | Open‐label study |
| Blinding of outcome assessment (detection bias) All outcomes | High risk | Not reported. Outcome adjudication may have been influenced by knowledge of treatment allocation |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | All 35 patients completed the study |
| Selective reporting (reporting bias) | High risk | Study did not report expected outcomes for this type of study |
| Other bias | Low risk | No evidence of other sources of bias |
HOT 1993.
| Study characteristics | ||
| Methods |
|
|
| Participants |
* split into 2 groups based on eGFR: 45 to 59 and < 45 mL/min/1.73 m² |
|
| Interventions | Treatment group
Control group
Cointerventions
|
|
| Outcomes |
|
|
| Notes |
|
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Insufficient information to permit judgement |
| Allocation concealment (selection bias) | Low risk | Quote: "The randomisation was computer‐generated based on communications by fax between investigators and the Study Coordinating Centre at Östra Hospital, Göteborg, Sweden." |
| Blinding of participants and personnel (performance bias) All outcomes | Low risk | Double‐blind study |
| Blinding of outcome assessment (detection bias) All outcomes | Low risk | Quote: "An independent clinical event committee evaluated all events (masked)." |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | Lost to follow‐up < 10% with no differences between groups |
| Selective reporting (reporting bias) | Low risk | The study reported all outcomes expected for this type of study |
| Other bias | High risk | No evidence of other sources of bias. The role of funding was not reported |
IMPACT II 1997.
| Study characteristics | ||
| Methods |
|
|
| Participants |
|
|
| Interventions | Treatment group 1
Treatment group 2
Control group
Cointerventions
|
|
| Outcomes |
|
|
| Notes |
|
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Quote: "The allocation schedule was generated by computer". Comment: Insufficient information to permit judgement |
| Allocation concealment (selection bias) | Unclear risk | Insufficient information to permit judgement |
| Blinding of participants and personnel (performance bias) All outcomes | Low risk | Double‐blind study |
| Blinding of outcome assessment (detection bias) All outcomes | Low risk | All efficacy and safety events were adjudicated by consensus of the Clinical Events Committee from which treatment assignment was concealed during the trial |
| Incomplete outcome data (attrition bias) All outcomes | Unclear risk | Insufficient information to permit judgement |
| Selective reporting (reporting bias) | Low risk | The study reported all outcomes expected for this type of study |
| Other bias | High risk | No evidence of other sources of bias. The role of funding were not reported |
Jiao 2013.
| Study characteristics | ||
| Methods |
|
|
| Participants |
|
|
| Interventions | Treatment group
Control group
Cointerventions
|
|
| Outcomes |
|
|
| Notes |
|
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Insufficient information to permit judgement |
| Allocation concealment (selection bias) | Unclear risk | Insufficient information to permit judgement |
| Blinding of participants and personnel (performance bias) All outcomes | High risk | Not reported. As the treatments were physically different, it was likely that participants and investigators were aware of treatment allocation |
| Blinding of outcome assessment (detection bias) All outcomes | Low risk | Not reported. Outcome measurement adjudication unlikely to be influenced by knowledge of treatment allocation |
| Incomplete outcome data (attrition bias) All outcomes | Unclear risk | Insufficient information to permit judgement |
| Selective reporting (reporting bias) | High risk | Study did not report outcomes expected for a study of this type |
| Other bias | Low risk | No evidence of other sources of bias |
JPAD 2008.
| Study characteristics | ||
| Methods |
|
|
| Participants |
|
|
| Interventions | Treatment group
Control group
Cointerventions
|
|
| Outcomes |
|
|
| Notes |
|
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Quote: "The randomisation was performed as non stratified randomisation from a random number table." Comment: Random number table is considered as low risk of bias |
| Allocation concealment (selection bias) | Unclear risk | Quote: "The study centre prepared the sealed envelopes with random assignments and distributed them by mail to the physicians in charge at the study sites." Comment: Unclear whether envelopes were opaque and sequentially numbered |
| Blinding of participants and personnel (performance bias) All outcomes | High risk | Open‐label study |
| Blinding of outcome assessment (detection bias) All outcomes | Low risk | All potential primary end points, secondary end points, and adverse events were adjudicated by an independent committee on validation of data and events that was un‐aware of the group assignments |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | All participants who were randomised were included in the primary efficacy and safety analyses |
| Selective reporting (reporting bias) | Low risk | Study outcomes included critical outcomes expected for this type of study |
| Other bias | Low risk | No evidence of other sources of bias. Funder did not influence data analysis and study reporting or interpretation. Subgroup analysis (post‐hoc) |
J‐PADD 2014.
| Study characteristics | ||
| Methods |
|
|
| Participants |
|
|
| Interventions | Treatment group
Control group
Cointerventions
|
|
| Outcomes |
|
|
| Notes |
|
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Quote: "Randomization was conducted by permutated‐block randomisation method, where block size was 6 and allocation ratio was 1:1." Comment: Insufficient information to permit judgement |
| Allocation concealment (selection bias) | Unclear risk | Insufficient information to permit judgement |
| Blinding of participants and personnel (performance bias) All outcomes | High risk | Open‐label study |
| Blinding of outcome assessment (detection bias) All outcomes | High risk | Not reported. Outcome events were likely to be influenced by knowledge of treatment allocation |
| Incomplete outcome data (attrition bias) All outcomes | High risk | Quote: "In Group B, one patient did not receive medication and three patients received both cilostazol and sarpogrelate instead of sarpogrelate, in violation of protocol. Finally, patients qualifying for analysis numbered 68 patients including: 35 from Group A (n = 35) and 33 from Group B (n = 33; cilostazol 15, sarpogrelate 18)." Comment: 0/35 patients in treatment group and 4/37 patients in control group were not included in analysis |
| Selective reporting (reporting bias) | Low risk | Study outcomes included critical outcomes expected for this type of study |
| Other bias | Low risk | No evidence of other sources of bias. Funder was unlikely to influence data analysis and study reporting or interpretation |
Kaegi 1974.
| Study characteristics | ||
| Methods |
|
|
| Participants |
|
|
| Interventions | Treatment group
Control group
Cointerventions
|
|
| Outcomes |
|
|
| Notes |
|
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Quote: "This was a double blind crossover study, the allocation of the patients to treatment being made according to a prescribed randomised arrangement." Comment: Insufficient information to permit judgement |
| Allocation concealment (selection bias) | Unclear risk | Insufficient information to permit judgement |
| Blinding of participants and personnel (performance bias) All outcomes | Low risk | Double‐blind study |
| Blinding of outcome assessment (detection bias) All outcomes | High risk | Not reported. Outcome adjudication was likely to be influenced by any knowledge of treatment allocation |
| Incomplete outcome data (attrition bias) All outcomes | High risk | In the first phase, 52/62 completed the 6 months follow‐up; 24/30 in the treatment group and 28/32 in the control group completed the study; > 10% lost to follow‐up |
| Selective reporting (reporting bias) | Low risk | Study reported expected outcomes for a study of this type. Data were appropriately reported for a cross‐over RCT |
| Other bias | Low risk | No evidence of other sources of bias. Funder was unlikely to influence data analysis and study reporting or interpretation |
Kamper 1997.
| Study characteristics | ||
| Methods |
|
|
| Participants |
|
|
| Interventions | Treatment group
Control group
Cointerventions
|
|
| Outcomes |
|
|
| Notes |
|
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Insufficient information to permit judgement |
| Allocation concealment (selection bias) | Unclear risk | Insufficient information to permit judgement |
| Blinding of participants and personnel (performance bias) All outcomes | High risk | Open‐label study |
| Blinding of outcome assessment (detection bias) All outcomes | High risk | Not reported. Outcome adjudication (due to nature of outcomes) was generally unlikely to be influenced by knowledge of treatment allocation. However, bleeding time may be influenced by the knowledge of the treatment assignment |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | All patients completed the study |
| Selective reporting (reporting bias) | High risk | Study outcomes did not include all expected for this type of study |
| Other bias | High risk | Insufficient information to permit judgement. The role of funding was not reported |
Kauffmann 1980.
| Study characteristics | ||
| Methods |
|
|
| Participants |
|
|
| Interventions | Treatment group
Control group
Cointerventions
|
|
| Outcomes |
|
|
| Notes |
|
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | High risk | Quote: "Randomization was achieved by whether the last digit of the patients' hospital number was odd or even". Comment: This method is considered as high risk of bias |
| Allocation concealment (selection bias) | Unclear risk | Insufficient information to permit judgement |
| Blinding of participants and personnel (performance bias) All outcomes | Low risk | Open‐label study |
| Blinding of outcome assessment (detection bias) All outcomes | High risk | Not reported. Outcome adjudication likely to be influenced by knowledge of treatment assignment |
| Incomplete outcome data (attrition bias) All outcomes | Unclear risk | Insufficient information to permit judgement |
| Selective reporting (reporting bias) | High risk | Study did not report all outcomes that would be expected for a study of this type |
| Other bias | Low risk | No evidence of other sources of bias |
Kaufman 2003.
| Study characteristics | ||
| Methods |
|
|
| Participants |
|
|
| Interventions | Treatment group
Control group
Cointerventions
|
|
| Outcomes |
|
|
| Notes |
|
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Quote: "The randomisation was stratified according to medical centre with a permuted block scheme, with a block size of four and equal allocation." Comment: Insufficient information to permit judgement |
| Allocation concealment (selection bias) | Low risk | Quote: "Randomisation was performed centrally, by the coordinating centre." |
| Blinding of participants and personnel (performance bias) All outcomes | Low risk | Double‐blind study |
| Blinding of outcome assessment (detection bias) All outcomes | High risk | Quote: "Assessment of the severity of bleeding episodes was performed by a panel blinded to the treatment assignments." Comment: Although assessment of bleeding episode was performed in an objective way, adverse events were likely to be influenced by knowledge of treatment assignment |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | Quote: "Participants were censored at the time of death, kidney transplantation, transfer to peritoneal dialysis, loss to follow‐up monitoring, or withdrawal of consent. On the basis of intention‐to‐treat principles, all other participants for whom study medications were discontinued continued to be monitored according to the protocol." Comment: Reasons for exclusions listed and intention‐to‐treat analysis was performed |
| Selective reporting (reporting bias) | Low risk | Study reported expected outcomes for this type of study |
| Other bias | High risk | The study was terminated earlier because of a significantly increased bleeding risk in the active treatment arm. Sample size smaller than planned. The role of Sanofi was not reported |
Khajehdehi 2002.
| Study characteristics | ||
| Methods |
|
|
| Participants |
|
|
| Interventions | Treatment group 1
Treatment group 2
Treatment group 3
Control group
Cointerventions
|
|
| Outcomes |
|
|
| Notes |
|
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Insufficient information to permit judgement |
| Allocation concealment (selection bias) | Unclear risk | Insufficient information to permit judgement |
| Blinding of participants and personnel (performance bias) All outcomes | High risk | Not clear whether participants or trial personnel were blinded. A placebo is mentioned, but it is not clear whether this resulted in participants and personnel being unaware of treatment allocation. Hovewer, as the treatments were physically different, it was likely that participants and/or investigators were aware of treatment allocation |
| Blinding of outcome assessment (detection bias) All outcomes | High risk | Not reported. Outcome assessment could have been influenced by knowledge of treatment allocation |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | All participants were analysed |
| Selective reporting (reporting bias) | High risk | Study did not report all expected outcomes (cardiovascular disease) for this type of study |
| Other bias | Low risk | No evidence of other sources of bias |
Kobayashi 1980.
| Study characteristics | ||
| Methods |
|
|
| Participants |
|
|
| Interventions | Treatment group
Control group
Cointerventions
|
|
| Outcomes |
|
|
| Notes | Funding: not reported | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Insufficient information to permit judgement |
| Allocation concealment (selection bias) | Unclear risk | Insufficient information to permit judgement |
| Blinding of participants and personnel (performance bias) All outcomes | Low risk | Double‐blind study |
| Blinding of outcome assessment (detection bias) All outcomes | High risk | Not reported. Outcome adjudication may have been influenced by knowledge of treatment allocation |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | Quote: "Of 107 patients, 5 patients were excluded from analytical data because of offence against protocol (4 A‐V fistulas, 1 A‐V external shunt without shunt trouble), and 2 patients because they were dosed with the test drug only for 4 days. Consequently, the efficacy was evaluated on 100 patients (T47, P53)." Comment: 100/107 patients included in analysis with no differences between groups |
| Selective reporting (reporting bias) | High risk | Study did not report all expected outcomes (cardiovascular disease) for this type of study |
| Other bias | Low risk | No evidence of other sources of bias |
Kontessis 1993.
| Study characteristics | ||
| Methods |
|
|
| Participants |
*The available electronic copy of this paper was incomplete and study data in this review are incomplete as a result |
|
| Interventions | Treatment group
Control group
Cointerventions
In 7 additional patients, the effect of the thromboxane synthase inhibitor given as 400 mg twice/day was compared with that of the thromboxane synthase inhibitor given as 400 mg 3 times/day |
|
| Outcomes |
|
|
| Notes |
|
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Insufficient information to permit judgement |
| Allocation concealment (selection bias) | Unclear risk | Insufficient information to permit judgement |
| Blinding of participants and personnel (performance bias) All outcomes | Low risk | Double‐blind study |
| Blinding of outcome assessment (detection bias) All outcomes | Low risk | Not reported. Outcome adjudication was unlikely to be influenced by knowledge of treatment allocation |
| Incomplete outcome data (attrition bias) All outcomes | Unclear risk | Insufficient information to permit judgement |
| Selective reporting (reporting bias) | High risk | Study did not report all outcomes that would be expected for a study of this type. Data were not appropriately reported for a cross‐over RCT |
| Other bias | Low risk | No evidence of other sources of bias |
Kooistra 1994.
| Study characteristics | ||
| Methods |
|
|
| Participants |
|
|
| Interventions | Treatment group
Control group
Cointerventions
|
|
| Outcomes |
|
|
| Notes |
|
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Insufficient information to permit judgement |
| Allocation concealment (selection bias) | Unclear risk | Quote: "The patients were assigned at random to group A or B by the monitor (MH), who was not in charge of the medical care for the patients." Comment: Insufficient information to permit judgement |
| Blinding of participants and personnel (performance bias) All outcomes | Low risk | Double‐blind study |
| Blinding of outcome assessment (detection bias) All outcomes | High risk | Not reported. The outcome adjudication may have been influenced by knowledge of the treatment type |
| Incomplete outcome data (attrition bias) All outcomes | High risk | Quote: "One hundred and fifty‐three patients were included in this study. Of these, 16 were withdrawn for further evaluation because of proven non‐compliance to the ASA or placebo ingestion. From the remaining 137 patients, 68 had been randomised to group A (placebo‐ASA) and 69 to group B (ASA‐placebo). From the 68 group A patients, eight dropped out during the study. One patient, who suffered from chronic obstructive lung disease, died of progressive respiratory failure. Three patients received renal grafts, two patients had uncorrectable hypertension, and two stopped for unspecified personal reasons. From the 69 group B patients, 11 dropped out. Four patients died, one after a complicated hip fracture, one following a MI in the first week of the rHuEpo treatment, one from pulmonary embolism, and one from bacterial sepsis. Two patients received renal grafts, in one patient the Hct remained at target level after stopping rHuEpo administration, one proved to be a non‐responder, and three stopped for personal reasons. In cases of drop‐out, only data of completed study periods were used for evaluation." Comment: > 10% lost to follow‐up |
| Selective reporting (reporting bias) | Low risk | Study reported expected outcomes for this type of study. Data were appropriately reported for a cross‐over RCT |
| Other bias | Low risk | No evidence of other sources of bias. Funder was unlikely to influence data analysis and study reporting or interpretation |
Koyama 1990.
| Study characteristics | ||
| Methods |
|
|
| Participants |
|
|
| Interventions | Treatment group
Control group
Cointerventions
|
|
| Outcomes |
|
|
| Notes |
|
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Insufficient information to permit judgement |
| Allocation concealment (selection bias) | Unclear risk | Insufficient information to permit judgement |
| Blinding of participants and personnel (performance bias) All outcomes | Low risk | Double‐blind study |
| Blinding of outcome assessment (detection bias) All outcomes | Low risk | Not reported. Outcomes adjudication were unlikely to be influenced by knowledge of the treatment allocation |
| Incomplete outcome data (attrition bias) All outcomes | Unclear risk | Insufficient information to permit judgement |
| Selective reporting (reporting bias) | High risk | Study did not report all outcomes that would be expected for a study of this type |
| Other bias | Unclear risk | Insufficient information to permit judgement |
Liang 2015.
| Study characteristics | ||
| Methods |
|
|
| Participants |
|
|
| Interventions | Treatment group 1
Treatment group 2
Cointerventions
|
|
| Outcomes |
|
|
| Notes |
|
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Insufficient information to permit judgement |
| Allocation concealment (selection bias) | Unclear risk | Insufficient information to permit judgement |
| Blinding of participants and personnel (performance bias) All outcomes | High risk | Open‐label study |
| Blinding of outcome assessment (detection bias) All outcomes | High risk | Not reported. Outcomes adjudication were likely to be influenced by knowledge of the treatment allocation |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | No patient was lost to follow‐up |
| Selective reporting (reporting bias) | Low risk | Study reported expected outcomes for this type of study |
| Other bias | Low risk | No evidence of other sources of bias |
Michie 1977.
| Study characteristics | ||
| Methods |
|
|
| Participants |
|
|
| Interventions | Treatment group
Control group
Cointerventions
|
|
| Outcomes |
|
|
| Notes |
|
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Insufficient information to permit judgement |
| Allocation concealment (selection bias) | Unclear risk | Insufficient information to permit judgement |
| Blinding of participants and personnel (performance bias) All outcomes | Low risk | Double‐blind study |
| Blinding of outcome assessment (detection bias) All outcomes | High risk | Not reported. Outcome adjudication may have been influenced by knowledge of the treatment allocation |
| Incomplete outcome data (attrition bias) All outcomes | High risk | Data were not available for all patients |
| Selective reporting (reporting bias) | Low risk | Study reported expected outcomes for this type of study |
| Other bias | Low risk | No evidence of other sources of bias |
Middleton 1992.
| Study characteristics | ||
| Methods |
|
|
| Participants |
|
|
| Interventions | Treatment group
Control group
Cointerventions
|
|
| Outcomes |
|
|
| Notes |
|
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Insufficient information to permit judgement |
| Allocation concealment (selection bias) | Unclear risk | Insufficient information to permit judgement |
| Blinding of participants and personnel (performance bias) All outcomes | High risk | Not reported. As the treatments were physically different, it was likely that participants and investigators were aware of treatment allocation |
| Blinding of outcome assessment (detection bias) All outcomes | High risk | Not reported. Outcome adjudication may have been influenced by knowledge of the treatment allocation |
| Incomplete outcome data (attrition bias) All outcomes | Unclear risk | Insufficient information to permit judgement |
| Selective reporting (reporting bias) | Low risk | Study reported expected outcomes for this type of study |
| Other bias | High risk | Full study report not available |
Milutinovic 1993.
| Study characteristics | ||
| Methods |
|
|
| Participants |
|
|
| Interventions | Treatment group
Control group
Cointerventions
|
|
| Outcomes |
|
|
| Notes |
|
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Insufficient information to permit judgement |
| Allocation concealment (selection bias) | Unclear risk | Insufficient information to permit judgement |
| Blinding of participants and personnel (performance bias) All outcomes | Low risk | Double‐blind study |
| Blinding of outcome assessment (detection bias) All outcomes | High risk | Not reported. Outcomes may have been influenced by knowledge of treatment allocation |
| Incomplete outcome data (attrition bias) All outcomes | Unclear risk | Insufficient information to permit judgement |
| Selective reporting (reporting bias) | High risk | Study did not report all outcomes that would be expected for a study of this type. Data were not appropriately reported for a cross‐over RCT |
| Other bias | Low risk | No evidence of other sources of bias |
Movchan 2001.
| Study characteristics | ||
| Methods |
|
|
| Participants |
|
|
| Interventions | Treatment group 1
Treatment group 2
Control group
Cointerventions
|
|
| Outcomes |
|
|
| Notes |
|
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Insufficient information to permit judgement |
| Allocation concealment (selection bias) | Unclear risk | Insufficient information to permit judgement |
| Blinding of participants and personnel (performance bias) All outcomes | High risk | Open‐label study |
| Blinding of outcome assessment (detection bias) All outcomes | Low risk | Not reported. However, outcomes were unlikely to be influenced by knowledge of treatment allocation |
| Incomplete outcome data (attrition bias) All outcomes | Unclear risk | Insufficient information to permit judgement |
| Selective reporting (reporting bias) | High risk | Study did not report all outcomes that would be expected for a study of this type |
| Other bias | Unclear risk | Insufficient information to permit judgement |
Mozafar 2013.
| Study characteristics | ||
| Methods |
|
|
| Participants |
|
|
| Interventions | Treatment group
Control group
Cointerventions
|
|
| Outcomes |
|
|
| Notes |
|
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Insufficient information to permit judgement |
| Allocation concealment (selection bias) | Unclear risk | Insufficient information to permit judgement |
| Blinding of participants and personnel (performance bias) All outcomes | Low risk | Double‐blind study |
| Blinding of outcome assessment (detection bias) All outcomes | High risk | Not reported. Outcomes may have been influenced by knowledge of treatment allocation |
| Incomplete outcome data (attrition bias) All outcomes | Unclear risk | Insufficient information to permit judgement |
| Selective reporting (reporting bias) | High risk | Study did not report all outcomes that would be expected for a study of this type |
| Other bias | Low risk | No evidence of other sources of bias |
Mozafar 2018.
| Study characteristics | ||
| Methods |
|
|
| Participants |
|
|
| Interventions | Treatment group
Control group
Cointerventions
|
|
| Outcomes |
|
|
| Notes |
|
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Quote: "Randomization was carried out, using a computer‐generated table of random numbers at a ratio of 1:1." Comment: Random number is considered as low risk of bias |
| Allocation concealment (selection bias) | Unclear risk | Insufficient information to permit judgement |
| Blinding of participants and personnel (performance bias) All outcomes | High risk | Single‐blind study |
| Blinding of outcome assessment (detection bias) All outcomes | High risk | Not reported. Outcomes may have been influenced by knowledge of treatment allocation |
| Incomplete outcome data (attrition bias) All outcomes | Unclear risk | Insufficient information to permit judgement |
| Selective reporting (reporting bias) | High risk | Study did not report all outcomes that would be expected for a study of this type |
| Other bias | Low risk | No evidence of other sources of bias |
Nakamura 2001d.
| Study characteristics | ||
| Methods |
|
|
| Participants |
|
|
| Interventions | Treatment group
Control group
Cointerventions
|
|
| Outcomes |
|
|
| Notes |
|
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Insufficient information to permit judgement |
| Allocation concealment (selection bias) | Unclear risk | Insufficient information to permit judgement |
| Blinding of participants and personnel (performance bias) All outcomes | High risk | Not reported. As the treatments were physically different, it was likely that participants and investigators were aware of treatment allocation |
| Blinding of outcome assessment (detection bias) All outcomes | Low risk | Not reported. Outcomes adjudication were unlikely to be influenced by knowledge of the treatment allocation |
| Incomplete outcome data (attrition bias) All outcomes | Unclear risk | Insufficient information to permit judgement |
| Selective reporting (reporting bias) | High risk | Study outcomes did not include critical outcomes expected for this type of study |
| Other bias | Low risk | No evidence of other sources of bias |
Nakamura 2002b.
| Study characteristics | ||
| Methods |
|
|
| Participants |
|
|
| Interventions | Intervention group
Control group
Cointerventions
|
|
| Outcomes |
|
|
| Notes |
|
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Insufficient information to permit judgement |
| Allocation concealment (selection bias) | Unclear risk | Insufficient information to permit judgement |
| Blinding of participants and personnel (performance bias) All outcomes | High risk | Not reported. As the treatments were physically different, it was likely that participants and investigators were aware of treatment allocation |
| Blinding of outcome assessment (detection bias) All outcomes | Low risk | Not reported. Outcomes adjudication were unlikely to be influenced by knowledge of the treatment allocation |
| Incomplete outcome data (attrition bias) All outcomes | Unclear risk | Insufficient information to permit judgement |
| Selective reporting (reporting bias) | High risk | Study outcomes did not include critical outcomes expected for this type of study |
| Other bias | Low risk | No evidence of other sources of bias |
NCT01252056.
| Study characteristics | ||
| Methods |
|
|
| Participants |
|
|
| Interventions | Treatment group
Control group
Cointerventions
|
|
| Outcomes |
|
|
| Notes |
|
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Insufficient information to permit judgement |
| Allocation concealment (selection bias) | Unclear risk | Insufficient information to permit judgement |
| Blinding of participants and personnel (performance bias) All outcomes | High risk | Not reported. As the treatments were physically different, it was likely that participants and investigators were aware of treatment allocation |
| Blinding of outcome assessment (detection bias) All outcomes | High risk | Not reported. The outcome adjudication may have been influenced by knowledge of the treatment allocation |
| Incomplete outcome data (attrition bias) All outcomes | Unclear risk | Insufficient information to permit judgement |
| Selective reporting (reporting bias) | High risk | Study outcomes did not included critical outcomes expected for this type of study |
| Other bias | Unclear risk | Insufficient information to permit judgement (only available information is entry in www.clinicaltrials.gov). Funder was unlikely to influence data analysis and study reporting or interpretation |
Nyberg 1984.
| Study characteristics | ||
| Methods |
|
|
| Participants |
|
|
| Interventions | Treatment group
Control group
Cointerventions
|
|
| Outcomes |
|
|
| Notes |
|
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Insufficient information to permit judgement |
| Allocation concealment (selection bias) | Unclear risk | Insufficient information to permit judgement |
| Blinding of participants and personnel (performance bias) All outcomes | Low risk | Double‐blind study |
| Blinding of outcome assessment (detection bias) All outcomes | High risk | Not reported. The outcome adjudication may have been influenced by knowledge of the treatment allocation |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | 22/23 participants were included in analyses |
| Selective reporting (reporting bias) | Low risk | Study outcomes included critical outcomes expected for this type of study |
| Other bias | Low risk | No evidence of other sources of bias |
Ogawa 2008.
| Study characteristics | ||
| Methods |
|
|
| Participants |
|
|
| Interventions | Treatment group 1
Treatment group 2
Cointerventions
|
|
| Outcomes |
|
|
| Notes |
|
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Insufficient information to permit judgement |
| Allocation concealment (selection bias) | Unclear risk | Insufficient information to permit judgement |
| Blinding of participants and personnel (performance bias) All outcomes | High risk | Single‐blind study |
| Blinding of outcome assessment (detection bias) All outcomes | Low risk | Not reported. Outcomes adjudication were unlikely to be influenced by knowledge of the treatment allocation |
| Incomplete outcome data (attrition bias) All outcomes | Unclear risk | Insufficient information to permit judgement |
| Selective reporting (reporting bias) | High risk | Study outcomes did not include critical outcomes expected for this type of study |
| Other bias | Low risk | No evidence of other sources of bias |
OPT‐CKD 2018.
| Study characteristics | ||
| Methods |
|
|
| Participants |
|
|
| Interventions | Treatment group 1
Treatment group 2
Cointerventions
|
|
| Outcomes |
|
|
| Notes |
|
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Insufficient information to permit judgement |
| Allocation concealment (selection bias) | Unclear risk | Insufficient information to permit judgement |
| Blinding of participants and personnel (performance bias) All outcomes | High risk | Open‐label study |
| Blinding of outcome assessment (detection bias) All outcomes | High risk | Not reported. Some outcomes adjudication were likely to be influenced by knowledge of the treatment allocation |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | Overall, 57/60 patients included in outcome assessment (28/39 in the ticagrelor group and 29/30 in the clopidogrel group) |
| Selective reporting (reporting bias) | Low risk | Study outcomes included critical outcomes expected for this type of study |
| Other bias | High risk | No evidence of other sources of bias. The role of AstraZeneca was not reported |
Ota 1996.
| Study characteristics | ||
| Methods |
|
|
| Participants |
|
|
| Interventions | Treatment group 1
Treatment group 2
Cointerventions
|
|
| Outcomes |
|
|
| Notes |
|
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Insufficient information to permit judgement |
| Allocation concealment (selection bias) | Unclear risk | Insufficient information to permit judgement |
| Blinding of participants and personnel (performance bias) All outcomes | Low risk | Double‐blind study |
| Blinding of outcome assessment (detection bias) All outcomes | High risk | Quote: "Improvement and utility rate were assessed by steering committee". Comment: Some outcomes adjudication were likely to be influenced by knowledge of the treatment allocation (not reported if steering committee was blind) |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | Quote: "204 patients (106 in the group E and 98 in group T) were assessed for general improving rating, 223 (110 in the group E and 113 in group T) were assessed for overall safety rating, and 206 (106 and 100) were assessed for general utility rating" Comment: < 10% were lost to follow‐up with not differences between groups (only one patient in group E was completely excluded from the analysis) |
| Selective reporting (reporting bias) | Low risk | Study outcomes included critical outcomes expected for this type of study |
| Other bias | Unclear risk | Insufficient information to permit judgement |
PEGASUS‐TIMI 54 2014.
| Study characteristics | ||
| Methods |
|
|
| Participants |
|
|
| Interventions | Treatment group 1
Treatment group 2
Control group
Cointerventions
|
|
| Outcomes |
|
|
| Notes |
|
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Insufficient information to permit judgement |
| Allocation concealment (selection bias) | Low risk | Quote: "Randomization was performed using a central computerized telephone or web based system." |
| Blinding of participants and personnel (performance bias) All outcomes | Low risk | Double‐blind study |
| Blinding of outcome assessment (detection bias) All outcomes | Low risk | Quote: "Adjudication for each event is performed according to definitions in the PEGASUS‐TIMI 54 Clinical Endpoints Committee Charter (online Appendix B) by an independent, blinded, and trained Clinical Endpoints Committee with board certification in either Cardiology or Neurol‐ogy depending on the event type." |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | Ascertainment of the primary outcome was complete for 99.2% of the potential patient years of follow‐up |
| Selective reporting (reporting bias) | Low risk | Study reported all critical outcomes expected for a study of this type |
| Other bias | High risk | No evidence of other sources of bias. The role of funding was not reported |
PIANO‐2 CKD 2011.
| Study characteristics | ||
| Methods |
|
|
| Participants |
|
|
| Interventions | Treatment group 1
Treatment group 2
Treatment group 3
Cointerventions
|
|
| Outcomes |
|
|
| Notes |
|
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Quote: "CKD patients were randomly assigned using a computer‐generated randomisation sequence." |
| Allocation concealment (selection bias) | Unclear risk | Insufficient information to permit judgement |
| Blinding of participants and personnel (performance bias) All outcomes | High risk | Open‐label study |
| Blinding of outcome assessment (detection bias) All outcomes | Low risk | Outcomes adjudication were unlikely to be influenced by knowledge of the treatment allocation |
| Incomplete outcome data (attrition bias) All outcomes | Unclear risk | Insufficient information to permit judgement |
| Selective reporting (reporting bias) | High risk | Study did not report all critical outcomes expected for a study of this type |
| Other bias | Low risk | No evidence of other sources of bias. Funder did not influence data analysis and study reporting or interpretation |
PIANO‐3 2015.
| Study characteristics | ||
| Methods |
|
|
| Participants |
|
|
| Interventions | Treatment group 1
Treatment group 2
Cointerventions
|
|
| Outcomes |
|
|
| Notes |
|
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Quote: "Patients with HTPR were randomly assigned at a 1:1 ratio by an independent investigator to the clopidogrel or ticagrelor groups using computerized random‐number generation." Comment: Ramdon number is considered as low risk of bias |
| Allocation concealment (selection bias) | Unclear risk | Quote: "Patients with HTPR were randomly assigned by an independent investigator to the clopidogrel or ticagrelor groups." Comment: Insufficient information to permit judgement |
| Blinding of participants and personnel (performance bias) All outcomes | High risk | Open‐label study |
| Blinding of outcome assessment (detection bias) All outcomes | High risk | Not reported. Outcomes adjudication were likely to be influenced by knowledge of the treatment allocation |
| Incomplete outcome data (attrition bias) All outcomes | High risk | As reported in Figure 1, at the end of the first phase 4/13 in the clopidogrel group and 4/12 in the ticagrelor group did not complete the first phase of treatment (due to adverse event or non‐adherence)." |
| Selective reporting (reporting bias) | High risk | Study did not report all expected outcomes (cardiovascular disease) for a study of this type. Data were not appropriately reported for a cross‐over RCT |
| Other bias | Low risk | No evidence of other sources of bias. Funder did not influence data analysis and study reporting or interpretation |
PIANO‐6 2017.
| Study characteristics | ||
| Methods |
|
|
| Participants |
|
|
| Interventions | Treatment group 1
Treatment group 2
Treatment group 3
Cointerventions
|
|
| Outcomes |
|
|
| Notes |
|
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Quote: "An independent investigator randomised the patients in a 1:1:1 ratio to one of three treatment groups. The investigator employed a computerized random number generation method." Coment: Random number method is considered as a low risk of bias |
| Allocation concealment (selection bias) | Unclear risk | Quote: "An independent investigator randomised the patients in a 1:1:1 ratio." Comment: Insufficient information to permit judgement |
| Blinding of participants and personnel (performance bias) All outcomes | High risk | Quote: "The study was not conducted in a double‐blinded manner." |
| Blinding of outcome assessment (detection bias) All outcomes | High risk | Not reported. Outcomes adjudication were likely to be influenced by knowledge of the treatment allocation |
| Incomplete outcome data (attrition bias) All outcomes | High risk | Quote: "Of 52 participants, four patients discontinued their drugs because of adverse events, as follows: one patient with BARC type 1 bleeding (gum bleeding) in the clopidogrel group; two patients with BARC type 1 and 2 bleeding (gum bleeding and arteriovenous fistula bleeding, respectively) in the standard‐dose ticagrelor group; and one patient with dyspnoea in the standard‐dose ticagrelor group. [...] A total of 52 patients underwent randomisation, and 48 completed the study protocol." Comment: 17/18 in the clopidogrel group, 18/21 in the standard‐dose ticagrelor group, and 13/13 in the low‐dose ticagrelor group completed the study. There were differences between groups (> 10% lost to follow‐up) |
| Selective reporting (reporting bias) | High risk | Study did not reported all expected outcomes (cardiovascular events) for a study of this type |
| Other bias | Low risk | No evidence of other sources of bias. Funder did not influence data analysis and study reporting or interpretation |
Pierucci 1989.
| Study characteristics | ||
| Methods |
|
|
| Participants |
|
|
| Interventions | Treatment group
Control group
Cointerventions
|
|
| Outcomes |
|
|
| Notes |
|
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Insufficient information to permit judgement |
| Allocation concealment (selection bias) | Unclear risk | Insufficient information to permit judgement |
| Blinding of participants and personnel (performance bias) All outcomes | Low risk | Double‐blind study |
| Blinding of outcome assessment (detection bias) All outcomes | High risk | Not reported. Outcomes adjudication were generally unlikely to be influenced by knowledge of the nature of the treatment allocation. However, bleeding time could be influenced by the knowledge of the treatment assignment |
| Incomplete outcome data (attrition bias) All outcomes | Unclear risk | Insufficient information to permit judgement |
| Selective reporting (reporting bias) | High risk | Study outcomes did not include critical outcomes expected for this type of study. Data were not appropriately reported for a cross‐over RCT |
| Other bias | Unclear risk | Insufficient information to permit judgement |
PLATO 2009.
| Study characteristics | ||
| Methods |
|
|
| Participants |
|
|
| Interventions | Treatment group 1
Treatment group 2
Cointerventions
|
|
| Outcomes |
|
|
| Notes |
|
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Quote: "Randomised 1:1 ratio using a randomisation schedule blocked by site." Comment: Insufficient information to permit judgement |
| Allocation concealment (selection bias) | Unclear risk | Insufficient information to permit judgement |
| Blinding of participants and personnel (performance bias) All outcomes | Low risk | Double‐blind study |
| Blinding of outcome assessment (detection bias) All outcomes | High risk | An independent central adjudication committee adjudicated all suspected primary and secondary efficacy end points as well as major and minor bleeding events. Hovewer, outcome adjudication (adverse events) may have been influenced by knowledge of treatment assignment |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | Vital status was available for all participants (except 5 participants that had missing vital status follow‐up) |
| Selective reporting (reporting bias) | Low risk | Study reported all critical outcomes expected for a study of this type |
| Other bias | High risk | FDA reporting identified a minimum of 106 participants without outcome data (instead of the 5 reported in the primary study report). There may have been an imbalance between study groups with significantly more patients allocated to ticagrelor that had incomplete vital status at follow‐up. Funder was unlikely to influence data analysis and study reporting or interpretation |
PREDIAN 2011.
| Study characteristics | ||
| Methods |
|
|
| Participants |
|
|
| Interventions | Treatment group
Control group
Cointerventions
|
|
| Outcomes |
|
|
| Notes |
|
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Sequence generation methods were not reported in sufficient detail to permit judgement |
| Allocation concealment (selection bias) | Unclear risk | Method of allocation concealment was not reported in sufficient detail to permit judgement |
| Blinding of participants and personnel (performance bias) All outcomes | High risk | Not reported. However, participants and/or investigators could be aware of treatment assigned |
| Blinding of outcome assessment (detection bias) All outcomes | Low risk | Only subjective outcomes were reported |
| Incomplete outcome data (attrition bias) All outcomes | Unclear risk | Quote: "Of the 169 patients in the PREDIAN trial, 166 (85 control group, 81 pentoxifylline group) who completed 1‐year follow‐up were included in this analysis." Comment: 166/169 participants completed the study (< 5% loss to follow‐up). However, no clear data were reported by the treatment group |
| Selective reporting (reporting bias) | High risk | Prespecified outcomes were reported. Clinically‐relevant outcomes that would be expected for this type of intervention were not reported |
| Other bias | Unclear risk | Quote: "The founders played no role in the study design, data collection and analysis, decision to publish, or preparation of the manuscript." Comment: Baseline characteristics were not reported. Funder did not influence data analyses and interpretation |
PRISM‐PLUS 1998.
| Study characteristics | ||
| Methods |
|
|
| Participants |
|
|
| Interventions | Treatment group 1
Treatment group 2
Control group
Cointerventions
|
|
| Outcomes |
|
|
| Notes |
|
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Insufficient information to permit judgement |
| Allocation concealment (selection bias) | Unclear risk | Quote: "Randomisation was performed locally by means of sealed envelopes." Comment: It was not clear if envelopes were opaque and numbered. Insufficient information to permit judgement |
| Blinding of participants and personnel (performance bias) All outcomes | Low risk | Double‐blind study |
| Blinding of outcome assessment (detection bias) All outcomes | Low risk | All events had been evaluated by the end‐points committee. The investigators remained blinded to treatment until after the six‐month visit |
| Incomplete outcome data (attrition bias) All outcomes | Unclear risk | Insufficient information to permit judgement |
| Selective reporting (reporting bias) | Low risk | Study reported all critical outcomes expected for a study of this type |
| Other bias | High risk | The tirofiban + placebo arm was terminated earlier than planned and not included in the final analysis due to excess death at seven days. An independent data and safety monitoring board reviewed unblinded data in two interim analyses |
PURSUIT 1997.
| Study characteristics | ||
| Methods |
|
|
| Participants |
|
|
| Interventions | Treatment group
Control group
Cointerventions
|
|
| Outcomes |
|
|
| Notes |
|
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Insufficient information to permit judgement |
| Allocation concealment (selection bias) | Low risk | Quote: "Randomisation was performed, in a double‐blind manner, by coordinating centres in the United States or the Netherlands." |
| Blinding of participants and personnel (performance bias) All outcomes | Low risk | Double‐blind study |
| Blinding of outcome assessment (detection bias) All outcomes | Low risk | Evaluated by a masked clinical events committee |
| Incomplete outcome data (attrition bias) All outcomes | Unclear risk | Insufficient information to permit judgement |
| Selective reporting (reporting bias) | Low risk | Study reported all critical outcomes expected for a study of this type |
| Other bias | High risk | Quote: "It was specified in the protocol that the study would be stopped in the lower‐dose group after the independent data safety and monitoring committee had conducted an interim review of safety data, provided the higher dose had an acceptable safety profile. After 3218 patients had been randomly assigned to treatment groups, the committee recommended dropping the lower dose." Comment: Percentage of discontinuation of study drug due to early discharge from hospital not balanced across groups. The role of funding was not reported |
Quarto Di Palo 1991.
| Study characteristics | ||
| Methods |
|
|
| Participants |
|
|
| Interventions | Treatment group
Control group
Cointerventions
|
|
| Outcomes |
|
|
| Notes |
|
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Insufficient information to permit judgement |
| Allocation concealment (selection bias) | Unclear risk | Insufficient information to permit judgement |
| Blinding of participants and personnel (performance bias) All outcomes | Low risk | Double‐blind study |
| Blinding of outcome assessment (detection bias) All outcomes | High risk | Not reported. The outcome adjudication may have been influenced by knowledge of the treatment assignment |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | Quote. "There was no rejections or major complications making it necessary to interrupt the trial." Comments: All participants completed the study |
| Selective reporting (reporting bias) | High risk | Study outcomes did not included critical outcomes expected for this type of study |
| Other bias | Low risk | No evidence of other sources of bias |
RAPPORT 1998.
| Study characteristics | ||
| Methods |
|
|
| Participants |
|
|
| Interventions | Treatment group
Control group
Cointerventions
|
|
| Outcomes |
|
|
| Notes |
|
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Insufficient information to permit judgement |
| Allocation concealment (selection bias) | Unclear risk | Insufficient information to permit judgement |
| Blinding of participants and personnel (performance bias) All outcomes | Low risk | Double‐blind study |
| Blinding of outcome assessment (detection bias) All outcomes | Low risk | Quote: "All clinical end points were independently adjudicated by a clinical events committee, who reviewed the case report forms, hospital records, and ECG and enzymatic data. All angiograms were reviewed by a central angiographic laboratory." |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | Low percentage of lost to follow‐up. Intention‐to‐treat analysis |
| Selective reporting (reporting bias) | Low risk | Study reported all critical outcomes expected for a study of this type |
| Other bias | High risk | No evidence of other sources of bias. The role of funding was not reported |
Reams 1985.
| Study characteristics | ||
| Methods |
|
|
| Participants |
|
|
| Interventions | Treatment group
Control group
Cointerventions
|
|
| Outcomes |
|
|
| Notes |
|
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Insufficient information to permit judgement |
| Allocation concealment (selection bias) | Unclear risk | Insufficient information to permit judgement |
| Blinding of participants and personnel (performance bias) All outcomes | Low risk | Double‐blind study |
| Blinding of outcome assessment (detection bias) All outcomes | High risk | Not reported. Some outcomes adjudication (adverse events) were likely to be influenced by knowledge of the treatment allocation |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | All participants completed the study |
| Selective reporting (reporting bias) | High risk | Study outcomes did not included critical outcomes expected for this type of study |
| Other bias | Unclear risk | Insufficient information to permit judgement |
RESIST 2008.
| Study characteristics | ||
| Methods |
|
|
| Participants |
|
|
| Interventions | Treatment group 1
Treatment group 2
Treatment group 3
Control group
Cointerventions
|
|
| Outcomes |
|
|
| Notes |
|
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Quote: "The 2x2 randomisation plan was generated from computer‐based pseudo random number generators with the following allocations: half to Angioguard and half to no Angioguard, and half to abciximab and half to placebo infusion. This yielded 4 groups: control, Angioguard only, abciximab only, and Angioguard with abciximab. Randomization was stratified by baseline creatinine >=1.6 mg/dL and enrolling centre." Comment: Insufficient information to permit judgement (pseudo random number) |
| Allocation concealment (selection bias) | Unclear risk | Insufficient information to permit judgement |
| Blinding of participants and personnel (performance bias) All outcomes | Low risk | Quote: "Double‐blind use of a platelet glycoprotein IIb/IIIa inhibitor." |
| Blinding of outcome assessment (detection bias) All outcomes | High risk | Some outcomes adjudication were likely to be influenced by knowledge of the treatment allocation |
| Incomplete outcome data (attrition bias) All outcomes | Unclear risk | Insufficient data on CKD patients to permit judgement |
| Selective reporting (reporting bias) | High risk | Study did not report all critical outcomes (cardiovascular events) expected for a study of this type |
| Other bias | Low risk | No evidence of other sources of bias. Funder did not influence data analysis and study reporting or interpretation |
Rouzrokh 2010.
| Study characteristics | ||
| Methods |
|
|
| Participants |
|
|
| Interventions | Treatment group 1
Treatment group 2
Control group
Cointerventions
|
|
| Outcomes |
|
|
| Notes |
|
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Insufficient information to permit judgement |
| Allocation concealment (selection bias) | Unclear risk | Insufficient information to permit judgement |
| Blinding of participants and personnel (performance bias) All outcomes | High risk | Not reported. As the treatments were physically different, it was likely that participants and investigators were aware of treatment allocation |
| Blinding of outcome assessment (detection bias) All outcomes | High risk | Not reported. Outcome adjudication may have been influenced by knowledge of the treatment allocation |
| Incomplete outcome data (attrition bias) All outcomes | High risk | Quote: "At least in six month period, 390 patients out of 501 (130 cases randomised in each group) remained and 111 patients were excluded, because they had failed to follow up, whose AVFs had failed within the first 72 h after the surgery or drugs discontinuity." Comment: > 10% lost of follow‐up |
| Selective reporting (reporting bias) | High risk | Study did not reported all expected outcomes for a study of this type |
| Other bias | Low risk | No evidence of other sources of bias |
Rubin 1982.
| Study characteristics | ||
| Methods |
|
|
| Participants |
|
|
| Interventions | Treatment group
Control group
Cointerventions
|
|
| Outcomes |
|
|
| Notes |
|
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | High risk | Quote: "The initial patient medication was determined by the flip of a coin. The next patient received the opposite to the first patient. The next patient's medication was chosen by coin flip, and so on." Comment: Flip of a coin is considered as a high risk of bias because it was used in alternate way |
| Allocation concealment (selection bias) | Unclear risk | Quote: "The study was balanced (by the pharmacist) so that five patients received the drug and five patients received the placebo during the first period." Comment: Insufficient information to permit judgement |
| Blinding of participants and personnel (performance bias) All outcomes | Low risk | Double‐blind study |
| Blinding of outcome assessment (detection bias) All outcomes | Low risk | Quote: "Peritoneal clearances of creatinine, urea, and inulin were calculated by multiplying the volume of dialysate effluent by the concentration of dialysate effluent and dividing this product by the plasma concentration multiplied by the time of the study exchanges. The plasma concentration used in the calculations was the average of values obtained at the start and close of the 8‐hr period. Sodium losses into dialysate were calculated by subtracting the amount infused from the amount in the dialysate effluent (concentration multiplied by effluent volume)." Comment: Outcomes were generally unlikely to be influenced by knowledge of treatment allocation |
| Incomplete outcome data (attrition bias) All outcomes | Unclear risk | Data related to the first period were not reported in sufficient detail to perform an adjudication |
| Selective reporting (reporting bias) | High risk | Study outcomes did not include all expected for this type of study. Data were not appropriately reported for a cross‐over RCT |
| Other bias | Low risk | No evidence of other sources of bias. Funder was unlikely to influence data analysis and study reporting or interpretation |
Salter 1984.
| Study characteristics | ||
| Methods |
|
|
| Participants |
|
|
| Interventions | Treatment group 1
Treatment group 2
Control group
Cointerventions
|
|
| Outcomes |
|
|
| Notes |
|
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Insufficient information to permit judgement |
| Allocation concealment (selection bias) | Unclear risk | Insufficient information to permit judgement |
| Blinding of participants and personnel (performance bias) All outcomes | Low risk | Double‐blind study |
| Blinding of outcome assessment (detection bias) All outcomes | High risk | Quote: "Platelet counts were made using 0.1% ammonium oxalate as a diluent under phase contrast microscopy. Plasma heparin concentrations were measured
chromo genically by the method of Teien, employing activated Factor X." Comment: Outcomes were generally unlikely to be influenced by knowledge of treatment allocation. However, adverse events may be influenced by the knowledge of the treatment allocation |
| Incomplete outcome data (attrition bias) All outcomes | Unclear risk | Insufficient information to permit judgement |
| Selective reporting (reporting bias) | High risk | Study outcomes did not include all expected for this type of study. Data were not appropriately reported for a cross‐over RCT |
| Other bias | Low risk | No evidence of other sources of bias. Funder was unlikely to influence data analysis and study reporting or interpretation |
Schnepp 2000.
| Study characteristics | ||
| Methods |
|
|
| Participants |
|
|
| Interventions | Treatment group 1
Treatment group 2
Control group
Cointerventions
|
|
| Outcomes |
|
|
| Notes |
|
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Insufficient information to permit judgement |
| Allocation concealment (selection bias) | Unclear risk | Insufficient information to permit judgement |
| Blinding of participants and personnel (performance bias) All outcomes | High risk | Not reported. As the treatments were physically different, it was likely that participants and investigators were aware of treatment allocation |
| Blinding of outcome assessment (detection bias) All outcomes | Low risk | Not reported. Outcomes adjudication were unlikely to be influenced by knowledge of the treatment allocation |
| Incomplete outcome data (attrition bias) All outcomes | Unclear risk | Insufficient information to permit judgement |
| Selective reporting (reporting bias) | High risk | Study outcomes did not include critical endpoints that might be expected for a study of this type |
| Other bias | Unclear risk | Insufficient information to permit judgement |
Schulze 1990.
| Study characteristics | ||
| Methods |
|
|
| Participants |
|
|
| Interventions | Treatment group
Control group
Cointerventions
|
|
| Outcomes |
|
|
| Notes |
|
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Insufficient information to permit judgement |
| Allocation concealment (selection bias) | Unclear risk | Insufficient information to permit judgement |
| Blinding of participants and personnel (performance bias) All outcomes | High risk | Not reported. As the treatments were physically different, it was likely that participants and investigators were aware of treatment allocation |
| Blinding of outcome assessment (detection bias) All outcomes | High risk | Not reported. The outcome adjudication may have been influenced by knowledge of the treatment allocation |
| Incomplete outcome data (attrition bias) All outcomes | Unclear risk | Insufficient information to permit judgement |
| Selective reporting (reporting bias) | High risk | Study outcomes did not include critical endpoints that might be expected for a study of this type |
| Other bias | Low risk | No evidence of other sources of bias |
Sreedhara 1994.
| Study characteristics | ||
| Methods |
|
|
| Participants |
|
|
| Interventions | Treatment group 1
Treatment group 2
Treatment group 3
Control group
Cointerventions
|
|
| Outcomes |
|
|
| Notes |
|
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Quote: "Randomization was done using a predetermined schedule." Comment: Insufficient information to permit judgement |
| Allocation concealment (selection bias) | Unclear risk | Insufficient information to permit judgement |
| Blinding of participants and personnel (performance bias) All outcomes | Low risk | Double‐blind study |
| Blinding of outcome assessment (detection bias) All outcomes | High risk | Quote: "Thrombosis was detected by the lack of blood flow by palpation and auscultation or the presence of thrombus detected during introduction of the dialysis needle into the graft." Comment: Some outcomes adjudication may have been influenced by knowledge of the treatment type due to the nature of the outcomes |
| Incomplete outcome data (attrition bias) All outcomes | High risk | Quote: "Eleven patients did not complete the protocol. Two of them were lost to follow‐up and the remaining were dropped from the study because of transplantation or patient refusal to continue. [...] Thirty‐four patients were discontinued from the study due to adverse events." Comment: Lost to follow‐up > 10% |
| Selective reporting (reporting bias) | Low risk | Study reported all critical outcomes expected for a study of this type |
| Other bias | High risk | No evidence of other sources of bias. The role of funding was not reported |
Steiness 2018.
| Study characteristics | ||
| Methods |
|
|
| Participants |
|
|
| Interventions | Treatment group 1
Treatment group 2
Control group
Cointerventions
|
|
| Outcomes |
|
|
| Notes |
|
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Sequence generation methods were not reported in sufficient detail to permit judgement |
| Allocation concealment (selection bias) | Unclear risk | Method of allocation concealment was not reported in sufficient detail to permit judgement |
| Blinding of participants and personnel (performance bias) All outcomes | Unclear risk | Quote: "Double‐blind." Comment: Although author reported that the study used a double‐blind design, information about blinding of participants and investigators were not clearly stated |
| Blinding of outcome assessment (detection bias) All outcomes | High risk | Objective and subjective outcomes were reported |
| Incomplete outcome data (attrition bias) All outcomes | High risk | Insufficient information to permit judgement |
| Selective reporting (reporting bias) | High risk | Prespecified outcomes were reported. Clinically‐relevant outcomes that would be expected for this type of intervention were not reported |
| Other bias | Unclear risk | Similar baseline characteristics were reported. Funding was not reported |
STOP 1995.
| Study characteristics | ||
| Methods |
|
|
| Participants |
|
|
| Interventions | Treatment group
Control group
Cointerventions
|
|
| Outcomes |
|
|
| Notes |
|
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Quote: "Assignment of the randomisation codes is organized in blocks and the patients are enrolled according to the sequential order designated for each centre." Comment: Insufficient information to permit judgement |
| Allocation concealment (selection bias) | Unclear risk | Quote: "The randomisation key relative to each individual patient is contained in a sealed envelope that must be opened in case of emergency." Comment: Not reported if envelopes were opaque and numbered |
| Blinding of participants and personnel (performance bias) All outcomes | Low risk | Double‐blind study |
| Blinding of outcome assessment (detection bias) All outcomes | High risk | Quote: "Members of the coordinating group take part in the steering committee of the study that validate outcomes events approve final results." Comment: It was not clear if these members of coordinating group were aware of treatment assigned. Outcome adjudication may have been influenced by knowledge of the treatment type due to the nature of the outcomes |
| Incomplete outcome data (attrition bias) All outcomes | Unclear risk | Insufficient information to permit judgement |
| Selective reporting (reporting bias) | Low risk | Study endpoints included critical outcomes for this type of study |
| Other bias | High risk | Baseline characteristics were not provided. The role of funding was not reported |
Storck 1996.
| Study characteristics | ||
| Methods |
|
|
| Participants |
|
|
| Interventions | Treatment group
Control group
Cointerventions
|
|
| Outcomes |
|
|
| Notes |
|
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Insufficient information to permit judgement |
| Allocation concealment (selection bias) | Unclear risk | Insufficient information to permit judgement |
| Blinding of participants and personnel (performance bias) All outcomes | High risk | Not reported. As the treatments were physically different, it was likely that participants and investigators were aware of treatment allocation |
| Blinding of outcome assessment (detection bias) All outcomes | Low risk | Not reported. However, outcomes were unlikely to be influenced by knowledge of treatment allocation |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | All patients completed the study |
| Selective reporting (reporting bias) | High risk | Study did not report all outcomes that would be expected for a study of this type |
| Other bias | Low risk | No evidence of other sources of bias |
Taber 1992.
| Study characteristics | ||
| Methods |
|
|
| Participants |
|
|
| Interventions | Treatment group 1
Treatment group 2
Treatment group 3
Control group
Cointerventions
|
|
| Outcomes |
|
|
| Notes |
|
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Insufficient information to permit judgement |
| Allocation concealment (selection bias) | Unclear risk | Insufficient information to permit judgement |
| Blinding of participants and personnel (performance bias) All outcomes | High risk | Not reported. As the treatments were physically different, it was likely that participants and investigators were aware of treatment allocation |
| Blinding of outcome assessment (detection bias) All outcomes | High risk | Not reported. Outcomes adjudication were likely to be influenced by knowledge of the treatment allocation |
| Incomplete outcome data (attrition bias) All outcomes | Unclear risk | Insufficient information to permit judgement |
| Selective reporting (reporting bias) | High risk | Study outcomes did not include critical endpoints that might be expected for a study of this type |
| Other bias | Unclear risk | Insufficient information to permit judgement |
Tang 2014.
| Study characteristics | ||
| Methods |
|
|
| Participants |
|
|
| Interventions | Treatment group
Control group
Cointerventions
|
|
| Outcomes |
|
|
| Notes |
|
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Insufficient information to permit judgement |
| Allocation concealment (selection bias) | Unclear risk | Insufficient information to permit judgement |
| Blinding of participants and personnel (performance bias) All outcomes | High risk | Single‐blind study |
| Blinding of outcome assessment (detection bias) All outcomes | High risk | Not reported. The outcome adjudication may have been influenced by knowledge of the treatment type due to the nature of the outcomes |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | 2/45 in treatment group and 1/45 in control group did not complete follow‐up and were not included in analysis |
| Selective reporting (reporting bias) | High risk | Study outcomes did not include critical endpoints (bleeding) that might be expected for a study of this type |
| Other bias | Low risk | No evidence of other sources of bias. Funder was unlikely to influence data analysis and study reporting or interpretation |
TARGET 2000.
| Study characteristics | ||
| Methods |
|
|
| Participants |
|
|
| Interventions | Treatment group 1
Treatment group 2
Cointerventions
|
|
| Outcomes |
|
|
| Notes |
|
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Quote: "Randomization was stratified according to the presence or absence of diabetes." Comment: Insufficient information to permit judgement |
| Allocation concealment (selection bias) | Low risk | Quote: "Patients who met the eligibility criteria were randomly assigned with the use of a central interactive system." |
| Blinding of participants and personnel (performance bias) All outcomes | Low risk | Double‐blind study |
| Blinding of outcome assessment (detection bias) All outcomes | High risk | Quote: "An independent Clinical Events Committee reviewed and adjudicated all investigators reported ischemics endpoints." Comment: Hovewer, some outcomes adjudication may have been influenced by knowledge of treatment assignment |
| Incomplete outcome data (attrition bias) All outcomes | Unclear risk | Overall, 499/5308 participants were excluded from analysis. However, data on CKD population were Insufficient information to permit judgement |
| Selective reporting (reporting bias) | Low risk | Study endpoints included critical outcomes for this type of study |
| Other bias | Low risk | No evidence of other sources of bias. Funder was unlikely to influence data analysis and study reporting or interpretation |
Tayebi 2018.
| Study characteristics | ||
| Methods |
|
|
| Participants |
|
|
| Interventions | Treatment group 1
Treatment group 2
Control group
Cointerventions
|
|
| Outcomes |
|
|
| Notes |
|
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Insufficient information to permit judgement |
| Allocation concealment (selection bias) | Unclear risk | Insufficient information to permit judgement |
| Blinding of participants and personnel (performance bias) All outcomes | Low risk | Double‐blind study |
| Blinding of outcome assessment (detection bias) All outcomes | High risk | Not reported. The outcome adjudication may have been influenced by knowledge of the treatment allocation |
| Incomplete outcome data (attrition bias) All outcomes | Unclear risk | Insufficient information to permit judgement |
| Selective reporting (reporting bias) | High risk | The study outcomes did not include those considered critical (cardiovascular events) to this type of study |
| Other bias | Low risk | No evidence of other sources of bias. Funder was unlikely to influence data analysis and study reporting or interpretation |
Teng 2018.
| Study characteristics | ||
| Methods |
|
|
| Participants |
|
|
| Interventions | Treatment group 1
Treatment group 2
Cointerventions
|
|
| Outcomes |
|
|
| Notes |
|
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Insufficient information to permit judgement |
| Allocation concealment (selection bias) | Unclear risk | Insufficient information to permit judgement |
| Blinding of participants and personnel (performance bias) All outcomes | High risk | Open‐label study |
| Blinding of outcome assessment (detection bias) All outcomes | High risk | Not reported. Outcomes were generally unlikely to be influenced by knowledge of treatment allocation but adverse events could be influenced by the knowledge of treatment allocation |
| Incomplete outcome data (attrition bias) All outcomes | Unclear risk | Quote: "Three haemodialysis subjects discontinued treatment (two who received the pre‐haemodialysis regimen first and one who received the post‐haemodialysis regimen first". Comment: However author reported that 3/14 patients discontinued, data were not reported for the fist phase. Insufficient information to permit judgement |
| Selective reporting (reporting bias) | High risk | The study outcomes did not include those considered critical to this type of study |
| Other bias | High risk | Funder was likely to influence data analysis and study reporting or interpretation |
TRA 2P‐TIMI 50 2009.
| Study characteristics | ||
| Methods |
|
|
| Participants |
|
|
| Interventions | Treatment group
Control group
Cointerventions
|
|
| Outcomes |
|
|
| Notes |
|
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Insufficient information to permit judgement |
| Allocation concealment (selection bias) | Low risk | Quote: "Central computerised system" |
| Blinding of participants and personnel (performance bias) All outcomes | Low risk | Double‐blind study |
| Blinding of outcome assessment (detection bias) All outcomes | Low risk | Comment: "All deaths, ischaemic and bleeding endpoints were adjudicated by a blinded Clinical Events Committee" |
| Incomplete outcome data (attrition bias) All outcomes | High risk | 2477/4983 reported outcomes data |
| Selective reporting (reporting bias) | Low risk | The study outcomes included those considered critical to this type of study |
| Other bias | High risk | Funder was likely to influence data analysis and study reporting or interpretation. After completion of enrolment and a median of 24 months of follow‐up, the data and safety monitoring board reported an excess of intracranial haemorrhage in patients with a history of stroke. The board recommended continuation of the trial in patients without a history of stroke |
TRACER 2013.
| Study characteristics | ||
| Methods |
|
|
| Participants |
|
|
| Interventions | Treatment group
Control group
Cointerventions
|
|
| Outcomes |
|
|
| Notes |
|
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Insufficient information to permit judgement |
| Allocation concealment (selection bias) | Low risk | Quote: "24‐hour automated voice‐response system." |
| Blinding of participants and personnel (performance bias) All outcomes | Low risk | Double‐blind study |
| Blinding of outcome assessment (detection bias) All outcomes | Low risk | A central clinical‐events committee, whose members were unaware of the study‐group assignments, assessed all suspected efficacy and bleeding events |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | Quote: "Overall, only 15 patients (0.1%) were lost to follow‐up." Comment: Lost to follow‐up < 10% |
| Selective reporting (reporting bias) | Low risk | The study reported all critical outcomes that might be expected for this type of study |
| Other bias | High risk | After an unplanned safety review on January 8 2011, the data and safety monitoring board recommended that the trial be stopped rather than continue as planned. The protocol‐defined target number of primary efficacy endpoints had been reached. Funder was unlikely to influence data analysis and study reporting or interpretation |
TRITON‐TIMI 38 2006.
| Study characteristics | ||
| Methods |
|
|
| Participants |
|
|
| Interventions | Treatment group 1
Treatment group 2
Cointerventions
|
|
| Outcomes |
|
|
| Notes |
|
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Insufficient information to permit judgement |
| Allocation concealment (selection bias) | Unclear risk | Insufficient information to permit judgement |
| Blinding of participants and personnel (performance bias) All outcomes | Low risk | Double‐blind study |
| Blinding of outcome assessment (detection bias) All outcomes | Low risk | Quote: "All components of the primary, secondary, and key safety end points were adjudicated by members of an independent clinical events committee that was unaware of the group assignments." |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | Quote: "Overall, a total of 14 patients (0.1%) were lost to follow‐up." Comment: < 10% of lost to follow‐up |
| Selective reporting (reporting bias) | Low risk | The study reported all critical outcomes that might be expected for this type of study |
| Other bias | Low risk | No evidence of other sources of bias. Funder did not influence data analysis and study reporting or interpretation |
UK‐HARP‐I 2005.
| Study characteristics | ||
| Methods |
|
|
| Participants |
|
|
| Interventions | Treatment group 1
Treatment group 2
Treatment group 3
Control group
Cointerventions
|
|
| Outcomes |
|
|
| Notes |
|
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Quote: "Minimized randomisation was used to balance the treatment groups with respect to eligibility criteria and other major prognostic factors." Comment: Minimized randomisation is considered as low risk of bias |
| Allocation concealment (selection bias) | Unclear risk | Insufficient information to permit judgement |
| Blinding of participants and personnel (performance bias) All outcomes | High risk | Single‐blind study |
| Blinding of outcome assessment (detection bias) All outcomes | High risk | Not reported. The outcome adjudication may have been influenced by knowledge of the treatment allocation |
| Incomplete outcome data (attrition bias) All outcomes | High risk | Quote: "Of 448 randomised patients, 71 patients stopped both treatments (that is, simvastatin [or matching placebo] and aspirin [or matching placebo]), 19 patients (4%) stopped aspirin (or matching placebo) only, and 11 patients (2%) stopped simvastatin (or matching placebo) only. [...] Although there was no excess of patients stopping among those allocated to active aspirin compared with placebo aspirin overall (44 patients, aspirin versus 46 patients, placebo aspirin), allocation to aspirin therapy was associated with an excess of adverse effects resulting in treatment discontinuation (20 versus 5 patients)." Comment: > 10% loss to follow‐up |
| Selective reporting (reporting bias) | Low risk | The study reported all critical outcomes that might be expected for this type of study |
| Other bias | High risk | Premature discontinuation of study due to insufficient bleeding events. The role of funding was not reported |
Waseda 2016.
| Study characteristics | ||
| Methods |
|
|
| Participants |
|
|
| Interventions | Treatment group
Control group
Cointerventions
|
|
| Outcomes |
|
|
| Notes |
|
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Insufficient information to permit judgement |
| Allocation concealment (selection bias) | Unclear risk | Insufficient information to permit judgement |
| Blinding of participants and personnel (performance bias) All outcomes | High risk | Not reported. As the treatments were physically different, it was likely that participants and investigators were aware of treatment allocation |
| Blinding of outcome assessment (detection bias) All outcomes | Low risk | Not reported. Outcomes adjudication were unlikely to be influenced by knowledge of the treatment allocation |
| Incomplete outcome data (attrition bias) All outcomes | Unclear risk | Insufficient information to permit judgement |
| Selective reporting (reporting bias) | High risk | Study outcomes did not include critical endpoints that might be expected for a study of this type |
| Other bias | Unclear risk | Insufficient information to permit judgement |
Watanabe 2011b.
| Study characteristics | ||
| Methods |
|
|
| Participants |
|
|
| Interventions | Treatment group
Control group
Cointerventions
|
|
| Outcomes |
|
|
| Notes |
|
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Insufficient information to permit judgement |
| Allocation concealment (selection bias) | Unclear risk | Insufficient information to permit judgement |
| Blinding of participants and personnel (performance bias) All outcomes | High risk | Not reported. As the treatments were physically different, it was likely that participants and investigators were aware of treatment allocation |
| Blinding of outcome assessment (detection bias) All outcomes | High risk | Not reported. Some outcomes adjudication were likely to be influenced by knowledge of the treatment allocation |
| Incomplete outcome data (attrition bias) All outcomes | Unclear risk | Insufficient information to permit judgement |
| Selective reporting (reporting bias) | High risk | Study outcomes did not include critical endpoints that might be expected for a study of this type |
| Other bias | Unclear risk | Insufficient information to permit judgement |
Weseley 1982.
| Study characteristics | ||
| Methods |
|
|
| Participants |
|
|
| Interventions | Treatment group
Control group
Cointerventions
|
|
| Outcomes |
|
|
| Notes |
|
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Insufficient information to permit judgement |
| Allocation concealment (selection bias) | Unclear risk | Insufficient information to permit judgement |
| Blinding of participants and personnel (performance bias) All outcomes | Low risk | Double‐blind study |
| Blinding of outcome assessment (detection bias) All outcomes | Low risk | Not reported. Outcomes adjudication were unlikely to be influenced by knowledge of the treatment allocation |
| Incomplete outcome data (attrition bias) All outcomes | Unclear risk | Insufficient information to permit judgement |
| Selective reporting (reporting bias) | High risk | Study outcomes did not include critical endpoints that might be expected for a study of this type. Data were not appropriately reported for a cross‐over RCT |
| Other bias | Unclear risk | Insufficient information to permit judgement |
Xydakis 2004.
| Study characteristics | ||
| Methods |
|
|
| Participants |
|
|
| Interventions | Treatment group 1
Treatment group 2
Cointerventions
|
|
| Outcomes |
|
|
| Notes |
|
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Insufficient information to permit judgement |
| Allocation concealment (selection bias) | Unclear risk | Insufficient information to permit judgement |
| Blinding of participants and personnel (performance bias) All outcomes | High risk | Not reported. As the treatments were physically different, it was likely that participants and investigators were aware of treatment allocation |
| Blinding of outcome assessment (detection bias) All outcomes | Low risk | Not reported. Outcomes adjudication were unlikely to be influenced by knowledge of the treatment allocation |
| Incomplete outcome data (attrition bias) All outcomes | Unclear risk | Insufficient information to permit judgement |
| Selective reporting (reporting bias) | High risk | Study outcomes did not include critical endpoints that might be expected for a study of this type |
| Other bias | Unclear risk | Insufficient information to permit judgement |
Yang 2016b.
| Study characteristics | ||
| Methods |
|
|
| Participants |
|
|
| Interventions | Treatment group
Control group
Cointerventions
|
|
| Outcomes |
|
|
| Notes |
|
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Quote: "All anti‐positive patients were subdivided into three groups by randomly selecting ID numbers of patients using the statistic software CHISS. All anti‐negative patients were divided into three groups by the same method." Comment: Insufficient information to permit judgement |
| Allocation concealment (selection bias) | Unclear risk | Insufficient information to permit judgement |
| Blinding of participants and personnel (performance bias) All outcomes | High risk | Open‐label study |
| Blinding of outcome assessment (detection bias) All outcomes | High risk | Not reported. Outcomes adjudication were likely to be influenced by knowledge of the treatment allocation |
| Incomplete outcome data (attrition bias) All outcomes | High risk | Large proportion of patients did not complete evaluate or switched treatment groups |
| Selective reporting (reporting bias) | Low risk | Study reported all critical outcomes that might be expected for this type of study |
| Other bias | Low risk | No evidence of other sources of bias |
Yuto 2012.
| Study characteristics | ||
| Methods |
|
|
| Participants |
|
|
| Interventions | Treatment group
Control group
Cointerventions
|
|
| Outcomes |
|
|
| Notes |
|
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Insufficient information to permit judgement |
| Allocation concealment (selection bias) | Unclear risk | Insufficient information to permit judgement |
| Blinding of participants and personnel (performance bias) All outcomes | High risk | Open‐label study |
| Blinding of outcome assessment (detection bias) All outcomes | High risk | Not reported. Outcomes adjudication were generally unlikely to be influenced by knowledge of the nature of the treatment allocation. However, patency failure could be influenced by the knowledge of the treatment assignment |
| Incomplete outcome data (attrition bias) All outcomes | Unclear risk | Insufficient information to permit judgement |
| Selective reporting (reporting bias) | High risk | Study did not report many critical outcomes that would be expected for this type of study |
| Other bias | Unclear risk | Insufficient information to permit judgement |
Zäuner 1994.
| Study characteristics | ||
| Methods |
|
|
| Participants |
|
|
| Interventions | Treatment group
Control group
Cointerventions
|
|
| Outcomes |
|
|
| Notes |
|
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Insufficient information to permit judgement |
| Allocation concealment (selection bias) | Unclear risk | Insufficient information to permit judgement |
| Blinding of participants and personnel (performance bias) All outcomes | High risk | Not reported. As the treatments were physically different, it was likely that participants and investigators were aware of treatment allocation |
| Blinding of outcome assessment (detection bias) All outcomes | Low risk | Not reported. Outcomes adjudication were unlikely to be influenced by knowledge of the treatment allocation |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | No loss to follow‐up |
| Selective reporting (reporting bias) | High risk | Study did not report many critical outcomes that would be expected for this type of study |
| Other bias | Low risk | No evidence of other sources of bias |
ABI ‐ ankle‐brachial index; ACEi ‐ angiotensin‐converting enzyme inhibitors; ACR ‐ albumin/creatinine ratio; ADPKD ‐ autosomal dominant polycystic kidney disease; ALT ‐ alanine aminotransferase; ARB ‐ angiotensin receptor blocker; ASP ‐ aspartate aminotransferase; AV ‐ arteriovenous; AVF ‐ arteriovenous fistula; AZA ‐ azathioprine; BACE ‐ Bleeding Academy Research consortium; BMI ‐ body mass index; BP ‐ blood pressure; BUN ‐ blood urea nitrogen; CABG ‐ coronary artery bypass graft; CAD ‐ coronary artery disease; CKD ‐ chronic kidney disease; CrCl ‐ creatinine clearance; CRP ‐ C‐reactive protein; CSA ‐ cyclosporin; CVA ‐ cerebrovascular accident; CVC ‐ central venous catheter; DBP ‐ diastolic BP; DES ‐ drug‐eluting stents; DKD ‐ diabetic kidney disease; DM ‐ diabetes mellitus; ESKD ‐ end‐stage kidney disease; ECG ‐ electrocardiogram; EPO ‐ erythropoietin; ESR ‐ erythrocyte sedimentation rate; GI ‐ gastrointestinal; (e)GFR ‐ (estimated) glomerular filtration rate; Hb ‐ haemoglobin; HbA1c ‐ haemoglobin A1c; HCT ‐ haematocrit; HD ‐ haemodialysis; HIV ‐ human immunodeficiency virus; IgAN ‐ IgA nephropathy; INR ‐ international normalised ratio; IQR ‐ interquartile range; IV ‐ intravenous; KRT ‐ kidney replacement therapy; LDL ‐ low‐density lipoprotein; MACE ‐ major adverse cardiovascular events; MDRD‐4 ‐ four‐variable Modification of Diet in Renal Disease; M/F ‐ male/female; MAP ‐ mean arterial BP; MI ‐ myocardial infarction; MPGN ‐ membranoproliferative glomerulonephritis; NACE ‐ net adverse clinical events; NSAID ‐ non‐steroidal anti‐inflammatory drug; NYHA ‐ New York Heart Association; PCTA ‐ percutaneous transluminal coronary angioplasty; PCI ‐ percutaneous coronary intervention; PD ‐ peritoneal dialysis; POCE ‐ patient‐oriented composite endpoint; PTFE ‐ polytetrafluoroethylene; QoL ‐ quality of life; RBC ‐ red blood cell; RCT ‐ randomised controlled trial; rHuEPO ‐ recombinant human erythropoietin; SBP ‐ systolic BP; SCr ‐ serum creatinine; SD ‐ standard deviation; SEM ‐ standard error of the mean; SLE ‐ systemic lupus erythematosus; STEMI ‐ ST‐elevation MI; TIA ‐ transient ischaemic attack; TIMI ‐ thrombolysis in MI; TNF‐a ‐ tumour necrosis factor‐a; UACR ‐ urinary albumin/creatinine ratio; UAE ‐ urinary albumin excretion; ULN ‐ upper limit of normal; UTI ‐ urinary tract infection; WBC ‐ white blood cells
Characteristics of excluded studies [ordered by study ID]
| Study | Reason for exclusion |
|---|---|
| AVERROES 2010 | Wrong comparator: antiplatelet agent versus anticoagulant |
| Bang 1994 | Wrong population: IgAN patients with normal kidney function |
| Caravaca 1995a | Unclear study design: patients randomly assigned to antiplatelets or not, however 3 different antiplatelet agents were used and it was not reported how these were assigned |
| Changjiang 2015 | Wrong comparator: antiplatelet agent versus anticoagulant |
| Coli 2006 | Wrong intervention: early warfarin therapy after tunnelled cuffed catheter placement versus warfarin therapy after tunnelled cuffed catheter thrombosis or malfunction |
| EXCITE 2000 | Wrong population: CKD patients excluded |
| Foroughinia 2017 | Wrong intervention: omega‐3 supplements versus placebo |
| Gorter 1998 | Wrong comparator: antiplatelet agent versus anticoagulant |
| Lee 1997 | Wrong intervention: antiplatelet agent + anticoagulant versus control |
| Lindsay 1972 | Wrong comparator: antiplatelet agent versus pyrimido‐pyrimidine compound RA 233 |
| NITER 2005 | Wrong intervention and comparator: medical treatment (included antiplatelet agents) versus medical treatment + percutaneous transluminal renal artery stenting |
| Perkovic 2004 | Wrong intervention: targeted risk factor modification |
| POISE‐2 2013 | Wrong population: all patients undergoing elective and urgent/emergent noncardiac surgery |
| PRODIGY 2010 | Wrong population: all patients undergoing PCI |
| RAS‐CAD 2009 | Wrong population: patients with ischaemic heart disease undergoing cardiac catheterization |
| REPLACE‐2 2003 | Wrong population: patients undergoing PCI |
| Sakai 1991 | Wrong comparator: dipyridamole versus urokinase |
| SPS3 2018 | Wrong population: patients with impaired kidney function were excluded |
| STENO‐2 1999 | Wrong intervention: antiplatelet agent + multiple non‐antiplatelet agents versus control |
| Swan 1995a | Wrong intervention: diaspirin cross‐linked Hb versus placebo |
| TRILOGY ACS 2010 | Wrong population: all patients with acute coronary syndromes |
| Woo 1987 | Wrong population: IgAN patients with normal kidney function |
| Wu 2018a | Wrong population: all patients undergoing emergency PCI for MI; no CKD data available (author contacted) |
| Yang 2014a | Wrong study design: stratified according to PF4/H antibodies (positive or negative) then assigned to control or intervention; numbers per group not even so unsure if truly randomised |
| Yeh 2017 | Wrong study design: states it is quasi‐RCT (by days of the week), however numbers per group are very different indicating the recruitment could have been subverted and there was a very high dropout/lost to follow‐up |
| Yoshikawa 1999 | Wrong intervention and comparator: prednisolone, AZA, heparin‐warfarin, and dipyridamole versus heparin‐warfarin and dipyridamole |
| Zhang 2009a | Wrong intervention: platelet activation inhibitor (Lipo‐PGE1 or low‐dose heparin) versus control |
| Zibari 1995 | Wrong comparator: aspirin versus heparin |
| Zimmerman 1983 | Wrong population: MPGN patients with normal kidney function |
AZA ‐ azathioprine; CKD, chronic kidney disease; Hb ‐ haemoglobin; IgAN ‐ IgA nephropathy; MI ‐ myocardial infarction; MPGN ‐ membranoproliferative glomerulonephritis; PCI ‐ percutaneous coronary intervention; RCT ‐ randomised controlled trial
Characteristics of ongoing studies [ordered by study ID]
A‐CLOSE 2019.
| Study name | A randomized comparison of CLOpidogrel monotherapy versus extended dual‐antiplatelet therapy beyond 12 months after implantation of drug‐eluting StEnts in high‐risk lesions or patients; A‐CLOSE Trial |
| Methods |
|
| Participants |
|
| Interventions | Treatment group 1
Treatment group 2
Cointerventions
|
| Outcomes |
|
| Starting date | August 2019 |
| Contact information | Byeong‐Keuk Kim Phone: 82‐2‐2228‐8460 Email: mailto:kimbk%40yuhs.ac?subject=NCT03947229, 4‐2019‐0234, A Randomized Comparison of CLOpidogrel Monotherapy Versus Extended Dual‐antiplatelet Therapy Beyond 12 Months After Implantation of Drug‐eluting StEnts in High‐risk Lesions or Patients; A‐CLOSE Trial |
| Notes |
|
ALTIC 2016.
| Study name | A randomized, pharmacodynamic comparison of Low dose TIcagrelor to Clopidogrel in patients with prior myocardial infarction (ALTIC) |
| Methods |
|
| Participants |
|
| Interventions | Treatment group 1
Treatment group 2
Cointerventions
|
| Outcomes |
|
| Starting date | January 2017 |
| Contact information | Dimitrios Alexopoulos Phone: not reported Email: not reported |
| Notes |
|
ALTIC‐2 2018.
| Study name | Low dose ticagrelor versus low dose prasugrel in patients with prior myocardial infarction (ALTIC‐2) |
| Methods |
|
| Participants |
|
| Interventions | Treatment group 1
Treatment group 2
Cointerventions
|
| Outcomes |
|
| Starting date | January 2018 |
| Contact information | Dimitrios Alexopoulos Phone: not reported Email: not reported |
| Notes | No results posted |
ATTACK 2018.
| Study name | Aspirin to target arterial events in chronic kidney disease (ATTACK) protocol |
| Methods |
|
| Participants |
|
| Interventions | Treatment group
Control group
Cointerventions
|
| Outcomes |
|
| Starting date | September 2018 |
| Contact information | Hugh Gallagher Phone: not reported Email: hugh.gallagher1@nhs.net |
| Notes |
|
ChiCTR1900021393.
| Study name | Antiplatelet therapy for prevention of atherosclerosis in chronic kidney disease: a perspective, multi‐center randomized controlled trial |
| Methods |
|
| Participants |
|
| Interventions | Treatment group
Control group
Cointerventions
|
| Outcomes |
|
| Starting date | February 2018 |
| Contact information | Zhao Jinghong Phone: +86 13668007369 Email: zhaojh@tmmu.edu.cn |
| Notes |
|
IRCT2013012412256N1.
| Study name | Evaluation the effect of clopidogrel in prevention of access graft thrombosis in upper extremity in patients undergoing haemodialysis in Emam Reza’s Hospital ‐ Kermanshah, 2012‐2013 |
| Methods |
|
| Participants |
|
| Interventions | Treatment group
Control group
Cointerventions
|
| Outcomes |
|
| Starting date | June 2012 |
| Contact information | Bahman Alinejad Phone: +98 83 1427 6311 Email: dr.bh.alinejad@kums.ac.ir |
| Notes |
|
IRCT2013100114333N8.
| Study name | Study of effects use and without use of aspirin on Permcath function in dialysis patients |
| Methods |
|
| Participants |
|
| Interventions | Treatment group
Control group
Cointerventions
|
| Outcomes |
|
| Starting date | June 2017 |
| Contact information | Feizollah Foroughi Phone: +98 83 1821 4653 Email: fforoughi@kums.ac.ir |
| Notes |
|
IRCT20171023036953N1.
| Study name | The effect of cilostazol on the mean time of arteriovenous fistula maturation and its comparison to control group in patients with chronic renal failure referring to Emam Reza hospital of Mashhad University of Medical Sciences |
| Methods |
|
| Participants |
|
| Interventions | Treatment group
Control group
Cointerventions
|
| Outcomes |
|
| Starting date | December 2018 |
| Contact information | Contact name: not reported Phone: not reported Email: not reported |
| Notes |
|
LEDA 2017.
| Study name | Effect of aspirin on renal disease progression in patients with type 2 diabetes: A multicenter, double‐blind, placebo‐controlled, randomised trial. The renaL disEase progression by aspirin in Diabetic pAtients (LEDA) trial. Rationale and study design |
| Methods |
|
| Participants |
|
| Interventions | Treatment group
Control group
Cointerventions
|
| Outcomes |
|
| Starting date | January 2017 |
| Contact information | Francesco Violi Phone: +390649970893 Email: francesco.violi@uniroma1.it |
| Notes |
|
Lemos Cerqueira 2018.
| Study name | The use of aspirin to reduce the risk of thrombotic events in patients with end‐stage renal disease: protocol for a randomised controlled trial |
| Methods |
|
| Participants |
|
| Interventions | Treatment group
Control group
Cointerventions
|
| Outcomes |
|
| Starting date | Not reported |
| Contact information | Nathalie Monique Vandevelde Phone: 32026425589 Email: nathalie.vandervelde@wiv‐isp.be |
| Notes |
|
NCT00272831.
| Study name | The use of cilostazol in patients with diabetic nephropathy |
| Methods |
|
| Participants |
|
| Interventions | Treatment group
Control
Cointerventions
|
| Outcomes |
|
| Starting date | December 2005 |
| Contact information | Peter CY Tong Phone: not reported Email: not reported |
| Notes |
|
NCT01198379.
| Study name | Aspirin in the prevention of cardiovascular events in haemodialysis patients |
| Methods |
|
| Participants |
|
| Interventions | Treatment group
Control group
Cointerventions
|
| Outcomes |
|
| Starting date | February 2010 |
| Contact information | Ying‐Hwa Chen Phone: not reported Email: not reported |
| Notes |
|
NCT01743014.
| Study name | Ramipril and clopidogrel in oxidative stress, vascular inflammation and endothelial dysfunction in type 2 diabetes and diabetic nephropathy |
| Methods |
|
| Participants |
|
| Interventions | Treatment group
Control group
Cointerventions
|
| Outcomes |
|
| Starting date | July 2012 |
| Contact information | Fotios S Iliadis Phone: +306974960728 Email: iliadis@med.auth.gr |
| Notes |
|
NCT02394145.
| Study name | Genotype and platelet reactivity in patients on haemodialysis |
| Methods |
|
| Participants |
|
| Interventions | Treatment group 1
Treatment group 2
Cointerventions
|
| Outcomes |
|
| Starting date | September 2009 |
| Contact information | Weon Kim Phone: 82‐2‐958‐8170 Email: mylovekw@hanmail.net |
| Notes |
|
NCT02459288.
| Study name | Platelet resistance with ticagrelor or standard‐dose clopidogrel among CKD and ACS patients (APROVE‐CKD) |
| Methods |
|
| Participants |
|
| Interventions | Treatment group 1
Treatment group 2
Cointerventions
|
| Outcomes |
|
| Starting date | January 2014 |
| Contact information | Ping‐Yen Liu Phone: +88662353535 Email: larry@mail.ncku.edu.tw |
| Notes |
|
NCT03039205.
| Study name | Platelet aggregation in patients with coronary artery disease and kidney dysfunction taking clopidogrel or ticagrelor |
| Methods |
|
| Participants |
|
| Interventions | Treatment group 1
Treatment group 2
Cointerventions
|
| Outcomes |
|
| Starting date | November 2017 |
| Contact information | André Franci Phone: 551126615850 Email: not reported |
| Notes |
|
NCT03150667.
| Study name | Study comparing treatment effectiveness of guideline indicated APT for ACS in patients with CKD (CPRS‐CKD) |
| Methods |
|
| Participants |
|
| Interventions | Treatment group 1
Treatment group 2
Cointerventions
|
| Outcomes |
|
| Starting date | April 2017 |
| Contact information | Subhash Banerjee Phone: 214‐867‐1608 Email: subhash.banerjee@utsouthwestern.edu |
| Notes |
|
NCT03649711.
| Study name | Chronic kidney disease (CKD) platelet study |
| Methods |
|
| Participants |
|
| Interventions | Treatment group 1
Treatment group 2
Cointerventions
|
| Outcomes |
|
| Starting date | November 2018 |
| Contact information | Jain Nishank Phone: 501‐686‐5295 Email: njain2@uams.edu |
| Notes |
|
Park 2010.
| Study name | The prevention of contrast induced nephropathy by sarpogrelate in patients with chronic kidney disease: a study protocol for a prospective randomised controlled clinical trial |
| Methods |
|
| Participants |
|
| Interventions | Treatment group 1
Treatment group 2
Cointerventions
|
| Outcomes |
|
| Starting date | December 2009 |
| Contact information | Woo‐Young Chung Phone: not reported Email: wychung@paran.com |
| Notes |
|
PRASTO‐III 2018.
| Study name | PRASTRO‐III: a double‐blind study of CS‐747S versus clopidogrel sulfate in patients with thrombotic stroke having risk factors for stroke recurrence |
| Methods |
|
| Participants |
|
| Interventions | Treatment group 1
Treatment group 2
Cointerventions
|
| Outcomes |
|
| Starting date | June 2017 |
| Contact information | Contact name: not reported Phone: +81‐95‐819‐7200 Email: dsclinicaltrial@daiichisankyo.co.jp |
| Notes |
|
SERENADE 2015.
| Study name | Study design of the influence of SErotonin inhibition on patients with RENAl impairment or diabetes undergoing drug‐eluting stent implantation (SERENADE) study: A multicenter, open‐label, prospective, randomised study |
| Methods |
|
| Participants |
|
| Interventions | Treatment group
Control group
Cointerventions
|
| Outcomes |
|
| Starting date | April 2009 |
| Contact information | Dong‐Ju Choi Phone: not reported Email: djchoi@snubh.org |
| Notes |
|
SONATA 2013.
| Study name | Effect of sarpogrelate on the nephropathy in type 2 diabetes (SONATA Study) |
| Methods |
|
| Participants |
|
| Interventions | Treatment group
Control group
Cointerventions
|
| Outcomes |
|
| Starting date | February 2013 |
| Contact information | D.S Choi Phone: not reported Email: not reported |
| Notes |
|
TROUPER 2020.
| Study name | TicagRelor Or Clopidogrel in severe and terminal chronic kidney disease patients undergoing PERcutaneous coronary intervention for an acute coronary syndrome (TROUPER) |
| Methods |
|
| Participants |
|
| Interventions | Treatment group 1
Treatment group 2
Cointerventions
|
| Outcomes |
|
| Starting date | 28 October 2018 |
| Contact information | Laurent Bonello Phone: 330491968683 Email: laurent.bonello@ap‐hm.fr |
| Notes |
|
TWILIGHT 2016.
| Study name | TWILIGHT Study: The anti platelet therapy with both ticagrelor and aspirin for 3 months after coronary intervention followed by ticagrelor only for a year rather than both aspirin and ticagrelor is better in reducing the ischaemic events in high risk patients |
| Methods |
|
| Participants |
|
| Interventions | Treatment group 1
Treatment group 2
Cointerventions
|
| Outcomes |
|
| Starting date | August 2016 |
| Contact information | Upendra Kaul Phone: 011268250014243 Email: upendra.kaul@fortishealthcare.com |
| Notes |
|
UMIN000003891.
| Study name | Examination concerning utility and safety of cilostazol use in patients with PAD complicated to CKD |
| Methods |
|
| Participants |
|
| Interventions | Treatment group 1
Treatment group 2
Cointerventions
|
| Outcomes |
|
| Starting date | June 2017 |
| Contact information | Yukio Yuzawa Phone: 052‐744‐5502 Email: |
| Notes |
|
VA PTXRx 2018.
| Study name | Pentoxifylline in diabetic kidney disease |
| Methods |
|
| Participants |
|
| Interventions | Treatment group
Control group
Cointerventions
|
| Outcomes |
|
| Starting date | November 2019 |
| Contact information | Leehey D.J Phone: not reported Email: not reported |
| Notes |
|
ABI ‐ ankle‐brachial index; ACEi ‐ angiotensin‐converting enzyme inhibitors; ACS ‐ acute coronary syndrome; AIDS ‐ acquired immune deficiency syndrome; AKI ‐ acute kidney injury; ALT ‐ alanine aminotransferase; ARB ‐ angiotensin receptor blocker; AV ‐ arteriovenous; AVF ‐ arteriovenous fistula; BARC ‐ Bleeding Academy Research consortium; BP ‐ blood pressure; CAD ‐ coronary artery disease; CKD ‐ chronic kidney disease; CrCl ‐ creatinine clearance; CRP ‐ C‐reactive protein; CSA ‐ cyclosporin; CVA ‐ cerebrovascular accident; CYP3A4 ‐ cytochrome P450 3A4; DES ‐ drug‐eluting stent; DM ‐ diabetes mellitus; DBP ‐ diastolic BP; DKD ‐ diabetic kidney disease; ECG ‐ electrocardiogram; ESKD ‐ end‐stage kidney disease; FBS ‐ fasting blood glucose; (e)GFR ‐ (estimated) glomerular filtration rate; GI ‐ gastrointestinal; HbA1c ‐ haemoglobin A1c; HCT ‐ hematocrit; HD ‐ haemodialysis; HDL ‐ high‐density lipoprotein; HIV ‐ human immunodeficiency virus; HRQoL ‐ health‐related quality of life; LDL ‐ low‐density lipoprotein; MACE ‐ major adverse cardiac events; MDRD ‐ Modification of Diet in Renal Disease; MI ‐ myocardial infarction; NYHA ‐ New York Heart Association; PAD ‐ peripheral artery disease; PCI ‐ percutaneous coronary intervention; PD ‐ peritoneal dialysis; RCT ‐ randomised controlled trial; SBP ‐ systolic BP; SCr ‐ serum creatinine; STEMI ‐ ST‐elevation myocardial infarction; TIA ‐ transient ischaemic attack; UACR ‐ urinary albumin/creatinine ratio; ULN ‐ upper limit of normal; UPCR ‐ urinary protein/creatinine ratio; WCC ‐ white cell count
Differences between protocol and review
We included studies of antiplatelet agents of fewer than two months follow‐up, even if they did not provide outcome data for vascular access outcomes.
Contributions of authors
Draft the protocol: MR, SP
Study selection: NP, VS, MR, SP
Extract data from studies: NP, VS, MR, SP
Enter data into RevMan: NP, VS, MR, SP
Carry out the analysis: NP, VS, MR, SP
Interpret the analysis: NP, VS, MR, SP, JC, VP, SZ, AW, MJ, GFMS
Draft the final review: NP, VS, MR, SP, JC, VP, SZ, AW, MJ, GFMS
Disagreement resolution: GFMS
Update the review: NP, SP, GFMS
Sources of support
Internal sources
No sources of support provided
External sources
-
Suetonia Palmer, New Zealand
Don and Lorraine Jacquot Fellowship; Amgen Dompe ‐ Consorzio Mario Negri Sud Fellowship
Declarations of interest
Patrizia Natale has declared they have no conflict of interest
Suetonia C Palmer has declared they have no conflict of interest
Valeria M Saglimbene has declared they have no conflict of interest
Marinella Ruospo has declared they have no conflict of interest
Mona Razavian has declared they have no conflict of interest
Jonathan C Craig has declared they have no conflict of interest
Meg J Jardine is supported by a Medical Research Future Fund Next Generation Clinical Researchers Program Career Development Fellowship; is responsible for research projects that have received unrestricted funding from Gambro, Baxter, CSL, Amgen, Eli Lilly, and MSD; has served on advisory boards sponsored by Akebia, Astra Zeneca, Baxter, Boehringer Ingelheim, Merck and Vifor; serves on Steering Committee for trials sponsored by Janssen and CSL; spoken at scientific meetings sponsored by Janssen, Amgen, Roche and Vifor; with any consultancy, honoraria or travel support paid to her institution
Angela C Webster has declared they have no conflict of interest
Giovanni FM Strippoli has declared they have no conflict of interest
Editorial contributions
Sign‐off Editor (final editorial decision): Dr Elisabeth Hodson
New search for studies and content updated (no change to conclusions)
References
References to studies included in this review
AASER 2017 {published data only}
- Goicoechea M, Vinuesa MS, Quiroga B, Verdalles U, Morales E, De Sequera P, et al.Aspirin treatment in primary cardiovascular prevention and renal disease progression in CKD patients: a randomized clinical trials (AASER study) [abstract no: FR-PO1065]. Journal of the American Society of Nephrology 2017;28(Abstract Suppl):B5. [EMBASE: 633704620] [Google Scholar]
- Goicoechea M, Vinuesa SG, Quiroga B, Verde E, Bernis C, Morales E, et al.Aspirin for primary prevention of cardiovascular disease and renal disease progression in chronic kidney disease patients: a multicenter randomized clinical trial (AASER Study). Cardiovascular Drugs & Therapy 2018;32(3):255-63. [MEDLINE: ] [DOI] [PubMed] [Google Scholar]
- Goicoechea M, Sanchez-Nino MD, Ortiz A, Vinuesa MS, Quiroga B, Morales E, et al.Low dose aspirin increases 15-epi-lipoxin a4 levels in CKD patients [abstract no: SA-PO186]. Journal of the American Society of Nephrology 2017;28(Abstract Suppl):726. [EMBASE: 633701100] [Google Scholar]
- Goicoechea M, Sanchez-Nino MD, Ortiz A, Garcia de Vinuesa S, Quiroga B, Bernis C, et al.Low dose aspirin increases 15-epi-lipoxin A4 levels in diabetic chronic kidney disease patients. Prostaglandins Leukotrienes & Essential Fatty Acids 2017;125:8-13. [MEDLINE: ] [DOI] [PubMed] [Google Scholar]
Abacilar 2015 {published data only}
- Abacilar AF, Atalay H, Dogan OF.Oral prostacycline analog and clopidogrel combination provides early maturation and long-term survival after arteriovenous fistula creation: a randomized controlled study. Indian Journal of Nephrology 2015;25(3):136-42. [MEDLINE: ] [DOI] [PMC free article] [PubMed] [Google Scholar]
Abdul‐Rahman 2007 {published data only}
- Abdul-Rahman IS, Al-Howaish AK.Warfarin versus aspirin in preventing tunneled hemodialysis catheter thrombosis: a prospective randomized study. Hong Kong Journal of Nephrology 2007;9(1):23-30. [EMBASE: 2007269768] [Google Scholar]
Alexopoulos 2011 {published data only}
- Alexopoulos D, Panagiotou A, Xanthopoulou I, Komninakis D, Kassimis G, Davlouros P, et al.Antiplatelet effects of prasugrel vs. double clopidogrel in patients on hemodialysis and with high on-treatment platelet reactivity. Journal of Thrombosis & Haemostasis 2011;9(12):2379-85. [MEDLINE: ] [DOI] [PubMed] [Google Scholar]
- Xanthopoulou I, Panagiotou A, Komninakis D, Davlouros P, Kassimis G, Fourtounas K, et al.Prasugrel versus double clopidpogrel maintenance dose in patients on chronic haemodialysis and high on clopidogrel platelet reactivity [abstract no: P2438]. European Heart Journal 2011;32(Suppl 1):417. [EMBASE: 70534608] [Google Scholar]
Anderson 1974 {published data only}
- Anderson M, Dewar P, Fleming LB, Hacking PM, Morley AR, Murray S, et al.A controlled trial of dipyridamole in human renal transplantation and an assessment of platelet function studies in rejection. Clinical Nephrology 1974;2(3):93-9. [MEDLINE: ] [PubMed] [Google Scholar]
Andrassy 1974 {published data only}
- Andrassy K, Malluche H, Bornefeld H, Comberg M, Ritz E, Jesdinsky H, et al.Prevention of p.o. clotting of av. cimino fistulae with acetylsalicyl acid: results of a prospective double blind study. Klinische Wochenschrift 1974;52(7):348-9. [MEDLINE: ] [DOI] [PubMed] [Google Scholar]
ATACAS 2008 {published data only}
- Di Franco A, Gaudino M, Girardi LN.Considerations about the Aspirin and Tranexamic Acid for Coronary Artery Surgery (ATACAS) trial. Journal of Thoracic Disease 2016;8(7):E599. [MEDLINE: ] [DOI] [PMC free article] [PubMed] [Google Scholar]
- Myles PS, Smith J, Knight J, Cooper DJ, Silbert B, McNeil J, et al.Aspirin and Tranexamic Acid for Coronary Artery Surgery (ATACAS) trial: rationale and design. American Heart Journal 2008;155(2):224-30. [MEDLINE: ] [DOI] [PubMed] [Google Scholar]
- Myles PS, Smith JA, Forbes A, Silbert B, Jayarajah M, Painter T, et al.Stopping vs. continuing aspirin before coronary artery surgery. New England Journal of Medicine 2016;374(8):728-37. [MEDLINE: ] [DOI] [PubMed] [Google Scholar]
- Myles PS, Smith JA, Forbes A, Silbert B, Jayarajah M, Painter T, et al.Tranexamic acid in patients undergoing coronary-artery surgery [Erratum for: N Engl J Med. 2017 Jan 12;376(2):136-148; PMID: 27774838]. New England Journal of Medicine 2017;376(2):136-48. [MEDLINE: ] [DOI] [PubMed] [Google Scholar]
- Myles PS, Smith JA, Kasza J, Silbert B, Jayarajah M, Painter T, et al.Aspirin in coronary artery surgery: 1-year results of the Aspirin and Tranexamic Acid for Coronary Artery Surgery trial. Journal of Thoracic & Cardiovascular Surgery 2019;157(2):633-40. [MEDLINE: ] [DOI] [PubMed] [Google Scholar]
- Myles PS, Smith JA, Kasza J, Silbert B, Jayarajah M, Painter T, et al.Tranexamic acid in coronary artery surgery: one-year results of the Aspirin and Tranexamic Acid for Coronary Artery Surgery (ATACAS) trial. Journal of Thoracic & Cardiovascular Surgery 2019;157(2):644-52.e9. [MEDLINE: ] [DOI] [PubMed] [Google Scholar]
CASSIOPEIR 2014 {published data only}
- Fujita T, Yu X, Kim S, Nakamoto H, Origasa H, Kurumatani H, et al.Effects of sustained-release beraprost sodium in patients with primary glomerular disease or nephrosclerosis: the CASSIOPEIR study [abstract no: SA-PO1096]. Journal of the American Society of Nephrology 2015;26(Abstract Suppl):4B. [CENTRAL: CN-01657821] [Google Scholar]
- Nakamoto H, Fujita T, Origasa H, Isono M, Kurumatani H, Okada K, et al.A multinational phase IIb/III trial of beraprost sodium, an orally active prostacyclin analogue, in patients with primary glomerular disease or nephrosclerosis (CASSIOPEIR trial), rationale and study design. BMC Nephrology 2014;15:153. [MEDLINE: ] [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nakamoto H, Yu XQ, Kim S, Origasa H, Zheng H, Chen J, et al.Effects of sustained-release beraprost in patients with primary glomerular disease or nephrosclerosis: CASSIOPEIR study results. Therapeutic Apheresis & Dialysis 2020;24(1):42-55. [MEDLINE: ] [DOI] [PubMed] [Google Scholar]
Chan 1987 {published data only}
- Chan MK, Kwan SY, Chan KW, Yeung CK.Controlled trial of antiplatelet agents in mesangial IgA glomerulonephritis. American Journal of Kidney Diseases 1987;9(5):417-21. [MEDLINE: ] [DOI] [PubMed] [Google Scholar]
CHANCE 2013 {published data only}
- Jing J, Meng X, Zhao X, Liu L, Wang A, Pan Y, et al.Dual antiplatelet therapy in transient ischemic attack and minor stroke with different infarction patterns: subgroup analysis of the CHANCE randomized clinical trial. JAMA Neurology 2018;75(6):711-9. [MEDLINE: ] [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li J, Wang Y, Lin J, Wang D, Wang A, Zhao X, et al.Soluble CD40L is a useful marker to predict future strokes in patients with minor stroke and transient ischemic attack. Stroke 2015;46(7):1990-2. [MEDLINE: ] [DOI] [PubMed] [Google Scholar]
- Li J, Zhao X, Meng X, Lin J, Liu L, Wang C, et al.High-sensitive C-reactive protein predicts recurrent stroke and poor functional outcome: subanalysis of the clopidogrel in high-risk patients with acute nondisabling cerebrovascular events trial. Stroke 2016;47(8):2025-30. [MEDLINE: ] [DOI] [PubMed] [Google Scholar]
- Lin Y, Wang A, Li J, Lin J, Wang D, Meng X, et al.Impact of glycemic control on efficacy of clopidogrel in transient ischemic attack or minor stroke patients with CYP2C19 genetic variants. Stroke 2017;48(4):998-1004. [MEDLINE: ] [DOI] [PubMed] [Google Scholar]
- Liu L, Wong KS, Leng X, Pu Y, Wang Y, Jing J, et al.Dual antiplatelet therapy in stroke and ICAS: subgroup analysis of CHANCE. Neurology 2015;85(13):1154-62. [MEDLINE: ] [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li Z, Wang Y, Zhao X, Liu L, Wang D, Wang C, et al.Treatment effect of clopidogrel plus aspirin within 12 hours of acute minor stroke or transient ischemic attack. Journal of the American Heart Association 2016;5(3):e003038. [MEDLINE: ] [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ma Y, Liu Y, Xu J, Wang Y, Wang Y, Du F.Effect of dual antiplatelet on recurrent stroke in minor stroke or TIA depends on bodyweight. Therapeutics & Clinical Risk Management 2018;14:861-70. [MEDLINE: ] [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pan Y, Cai X, Jing J, Meng X, Li H, Wang Y, et al.Stress hyperglycemia and prognosis of minor ischemic stroke and transient ischemic attack: the CHANCE study (Clopidogrel in High-Risk Patients With Acute Nondisabling Cerebrovascular Events). Stroke 2017;48(11):3006-11. [MEDLINE: ] [DOI] [PubMed] [Google Scholar]
- Pan Y, Jing J, Chen W, Meng X, Li H, Zhao X, et al.Risks and benefits of clopidogrel-aspirin in minor stroke or TIA: time course analysis of CHANCE.[Erratum in: Neurology. 2019 Aug 13;93(7):322; PMID: 31405943]. Neurology 2017;88(20):1906-11. [MEDLINE: ] [DOI] [PubMed] [Google Scholar]
- Pan Y, Jing J, Li H, Wang Y, Wang Y, He Y, et al.Abnormal glucose regulation increases stroke risk in minor ischemic stroke or TIA. Neurology 2016;87(15):1551-6. [MEDLINE: ] [DOI] [PubMed] [Google Scholar]
- Pan Y, Meng X, Jing J, Li H, Zhao X, Liu L, et al.Association of multiple infarctions and ICAS with outcomes of minor stroke and TIA. Neurology 2017;88(11):1081-8. [MEDLINE: ] [DOI] [PubMed] [Google Scholar]
- Wang A, Li S, Zhang N, Dai L, Zuo Y, Wang Y, et al.Oxidized low-density lipoprotein to high-density lipoprotein ratio predicts recurrent stroke in minor stroke or transient ischemic attack. Stroke 2018;49(11):2637-42. [MEDLINE: ] [DOI] [PubMed] [Google Scholar]
- Wang A, Xu J, Chen G, Wang D, Johnston SC, Meng X, et al.Oxidized low-density lipoprotein predicts recurrent stroke in patients with minor stroke or TIA. Neurology 2018;91(10):e947-55. [MEDLINE: ] [DOI] [PubMed] [Google Scholar]
- Wang D, Gui L, Dong Y, Li H, Li S, Zheng H, et al.Dual antiplatelet therapy may increase the risk of non-intracranial haemorrhage in patients with minor strokes: a subgroup analysis of the CHANCE trial. Stroke & Vascular Neurology 2016;1(2):29-36. [MEDLINE: ] [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wangqin R, Wang X, Wang Y, Xian Y, Zhao X, Liu L, et al.Risk factors associated with 90-day recurrent stroke in patients on dual antiplatelet therapy for minor stroke or high-risk TIA: a subgroup analysis of the CHANCE trial. Stroke & Vascular Neurology 2017;2(4):176-83. [MEDLINE: ] [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang Y, Pan Y, Zhao X, Li H, Wang D, Johnston SC, et al.Clopidogrel with aspirin in acute minor stroke or transient ischemic attack (CHANCE) trial: one-year outcomes. Circulation 2015;132(1):40-6. [MEDLINE: ] [DOI] [PubMed] [Google Scholar]
- Wang Y, Wang Y, Zhao X, Liu L, Wang D, Wang C, et al.Clopidogrel with aspirin in acute minor stroke or transient ischemic attack. New England Journal of Medicine 2013;369(1):11-9. [MEDLINE: ] [DOI] [PubMed] [Google Scholar]
- Wang Y, Zhao X, Lin J, Li H, Johnston SC, Lin Y, et al.Association between CYP2C19 loss-of-function allele status and efficacy of clopidogrel for risk reduction among patients with minor stroke or transient ischemic attack. JAMA 2016;316(1):70-8. [MEDLINE: ] [DOI] [PubMed] [Google Scholar]
- Wu Y, Zhou Y, Pan Y, Zhao X, Liu L, Wang D, et al.Impact of CYP2C19 polymorphism in prognosis of minor stroke or TIA patients with declined eGFR on dual antiplatelet therapy: CHANCE substudy. Pharmacogenomics Journal 2018;18(6):713-20. [MEDLINE: ] [DOI] [PubMed] [Google Scholar]
- Zhou Y, Pan Y, Wu Y, Zhao X, Li H, Wang D, et al.Effect of estimated glomerular filtration rate decline on the efficacy and safety of clopidogrel with aspirin in minor stroke or transient ischemic attack: CHANCE substudy (Clopidogrel in high-risk patients with acute nondisabling cerebrovascular events). Stroke 2016;47(11):2791-6. [MEDLINE: ] [DOI] [PubMed] [Google Scholar]
- Zhu B, Liu H, Pan Y, Jing J, Li H, Zhao X, et al.Elevated neutrophil and presence of intracranial artery stenosis increase the risk of recurrent stroke. Stroke 2018;49(10):2294-300. [MEDLINE: ] [DOI] [PubMed] [Google Scholar]
CHARISMA 2006 {published and unpublished data}
- Bangalore S, Bhatt DL, Steg PG, Weber MA, Boden WE, Hamm CW, et al.beta-blockers and cardiovascular events in patients with and without myocardial infarction: post hoc analysis from the CHARISMA trial. Circulation: Cardiovascular Quality & Outcomes 2014;7(6):872-81. [MEDLINE: ] [DOI] [PubMed] [Google Scholar]
- Berger PB, Bhatt DL, Fuster V, Steg PG, Fox KA, Shao M, et al.Bleeding complications with dual antiplatelet therapy among patients with stable vascular disease or risk factors for vascular disease: results from the Clopidogrel for High Atherothrombotic Risk and Ischemic Stabilization, Management, and Avoidance (CHARISMA) trial. Circulation 2010;121(23):2575-83. [MEDLINE: ] [DOI] [PubMed] [Google Scholar]
- Bhatt DL, Flather MD, Hacke W, Berger PB, Black HR, Boden WE, et al.Patients with prior myocardial infarction, stroke, or symptomatic peripheral arterial disease in the CHARISMA trial. Journal of the American College of Cardiology 2007;49(19):1982-8. [MEDLINE: ] [DOI] [PubMed] [Google Scholar]
- Bhatt DL, Fox KA, Hacke W, Berger PB, Black HR, Boden WE, et al.A global view of atherothrombosis: baseline characteristics in the Clopidogrel for High Atherothrombotic Risk and Ischemic Stabilization, Management, and Avoidance (CHARISMA) trial [Erratum in: Am Heart J. 2006 Jan;151(1):247]. American Heart Journal 2005;150(3):401. [MEDLINE: ] [DOI] [PubMed] [Google Scholar]
- Bhatt DL, Fox KA, Hacke W, Berger PB, Black HR, Boden WE, et al.Clopidogrel and aspirin versus aspirin alone for the prevention of atherothrombotic events. New England Journal of Medicine 2006;354(16):1706-17. [MEDLINE: ] [DOI] [PubMed] [Google Scholar]
- Dasgupta A, Steinhubl SR, Bhatt DL, Berger PB, Shao M, Mak KH, et al.Clinical outcomes of patients with diabetic nephropathy randomized to clopidogrel plus aspirin versus aspirin alone (a post hoc analysis of the clopidogrel for high atherothrombotic risk and ischemic stabilization, management, and avoidance [CHARISMA] trial). American Journal of Cardiology 2009;103(10):1359-63. [MEDLINE: 19427428] [DOI] [PubMed] [Google Scholar]
- Eikelboom JW, Hankey GJ, Thom J, Bhatt DL, Steg PG, Montalescot G, et al.Incomplete inhibition of thromboxane biosynthesis by acetylsalicylic acid: determinants and effect on cardiovascular risk. Circulation 2008;118(17):1705-12. [MEDLINE: ] [DOI] [PubMed] [Google Scholar]
- Mak KH, Bhatt DL, Shao M, Haffner SM, Hamm CW, Hankey GJ, et al.The influence of body mass index on mortality and bleeding among patients with or at high-risk of atherothrombotic disease. European Heart Journal 2009;30(7):857-65. [MEDLINE: ] [DOI] [PubMed] [Google Scholar]
- Steinhubl SR, Bhatt DL, Brennan DM, Montalescot G, Hankey GJ, Eikelboom JW, et al.Aspirin to prevent cardiovascular disease: the association of aspirin dose and clopidogrel with thrombosis and bleeding [Summary for patients in Ann Intern Med. 2009 Mar 17;150(6):I-22; PMID: 19293067]. Annals of Internal Medicine 2009;150(6):379-86. [MEDLINE: ] [DOI] [PubMed] [Google Scholar]
- Wang TH, Bhatt DL, Fox KA, Steinhubl SR, Brennan DM, Hacke W, et al.An analysis of mortality rates with dual-antiplatelet therapy in the primary prevention population of the CHARISMA trial. European Heart Journal 2007;28(18):2200-7. [MEDLINE: ] [DOI] [PubMed] [Google Scholar]
Cheng 1998a {published data only}
- Cheng IK, Fang GX, Wong MC, Ji YL, Chan KW, Yeung HW.A randomized prospective comparison of nadolol, captopril with or without ticlopidine on disease progression in IgA nephropathy. Nephrology 1998;4(1-2):19-26. [EMBASE: 28282953] [Google Scholar]
- Cheng IK, Fang GX, Wong MC, Ji YL, Yeung H.A randomised prospective comparison of nadolol, captopril with or without ticlopidine on disease progression in IgA glomerulonephritis (IgAN) [abstract]. In: 12th International Congress of Nephrology; 1993 Jun 13-18; Jerusalem, Israel. 1993:45. [CENTRAL: CN-00550664]
Christopher 1987 {published data only}
- Christopher TG, Samels K.A study of aspirin and dipyridamole in slowing the progression of diabetic glomerulosclerosis [abstract]. Kidney International 1987;31(1):194. [Google Scholar]
- Christopher TG, Samels K.A study of the treatment effect of aspirin and dipyridamole in diabetic glomerulosclerosis [abstract]. In: 10th International Congress of Nephrology; 1987 Jul 26-31; London, UK. 1987:56.
CILON‐T 2010 {published data only}
- Lee MH, Suh JW, Lee SP, Park KW, Lee HY, Kang HJ, et al.The effect of cilostazol on the antiplatelet efficacy of patients with moderate renal dysfunction: post-hoc analysis of CILON-T (Influence of cilostazol based triple antiplatelet therapy on ischemic complication after drug eluting stent implantation) trial [abstract no: TCT-495]. Journal of the American College of Cardiology 2011;58(20 Suppl 1):B134-5. [EMBASE: 70581865] [Google Scholar]
- Lee SP, Suh JW, Park KW, Lee HY, Kang HJ, Koo BK, et al.Study design and rationale of 'Influence of Cilostazol-based triple anti-platelet therapy on ischemic complication after drug-eluting stent implantation (CILON-T)' study: a multicenter randomized trial evaluating the efficacy of cilostazol on ischemic vascular complications after drug-eluting stent implantation for coronary heart disease. Trials [Electronic Resource] 2010;11:87. [MEDLINE: ] [DOI] [PMC free article] [PubMed] [Google Scholar]
- Suh JW, Lee SP, Park KW, Lee HY, Kang HJ, Koo BK, et al.Multicenter randomized trial evaluating the efficacy of cilostazol on ischemic vascular complications after drug-eluting stent implantation for coronary heart disease: results of the CILON-T (influence of CILostazol-based triple antiplatelet therapy ON ischemic complication after drug-eluting stenT implantation) trial. Journal of the American College of Cardiology 2011;57(3):280-9. [MEDLINE: ] [DOI] [PubMed] [Google Scholar]
CREDO 2005 {published and unpublished data}
- Aronow HD, Steinhubl SR, Brennan DM, Berger PB, Topol EJ, CREDO Investigators.Bleeding risk associated with 1 year of dual antiplatelet therapy after percutaneous coronary intervention: insights from the Clopidogrel for the Reduction of Events During Observation (CREDO) trial. American Heart Journal 2009;157(2):369-74. [MEDLINE: ] [DOI] [PubMed] [Google Scholar]
- Beinart SC, Kolm P, Veledar E, Zhang Z, Mahoney EM, Bouin O, et al.Long-term cost effectiveness of early and sustained dual oral antiplatelet therapy with clopidogrel given for up to one year after percutaneous coronary intervention results: from the Clopidogrel for the Reduction of Events During Observation (CREDO) trial. Journal of the American College of Cardiology 2005;46(5):761-9. [MEDLINE: ] [DOI] [PubMed] [Google Scholar]
- Best PJ, Steinhubl SR, Berger PB, Dasgupta A, Brennan DM, Szczech LA, et al.The efficacy and safety of short- and long-term dual antiplatelet therapy in patients with mild or moderate chronic kidney disease: results from the Clopidogrel for the Reduction of Events During Observation (CREDO) trial. American Heart Journal 2008;155(4):687-93. [MEDLINE: ] [DOI] [PubMed] [Google Scholar]
- Brener SJ, Steinhubl SR, Berger PB, Brennan DM, Topol EJ, CREDO Investigators.Prolonged dual antiplatelet therapy after percutaneous coronary intervention reduces ischemic events without affecting the need for repeat revascularization: insights from the CREDO trial. Journal of Invasive Cardiology 2007;19(7):287-90. [MEDLINE: ] [PubMed] [Google Scholar]
- Ringborg A, Lindgren P, Jonsson B.The cost-effectiveness of dual oral antiplatelet therapy following percutaneous coronary intervention: a Swedish analysis of the CREDO trial. European Journal of Health Economics 2005;6(4):354-56, 358-62. [MEDLINE: ] [DOI] [PubMed] [Google Scholar]
- Steinhubl SR, Berger PB, Mann JT 3rd, Fry ET, DeLago A, Wilmer C, et al.Early and sustained dual oral antiplatelet therapy following percutaneous coronary intervention: a randomized controlled trial [Erratum in: JAMA. 2003 Feb 26;289(8):987]. JAMA 2002;288(19):2411-20. [MEDLINE: ] [DOI] [PubMed] [Google Scholar]
Creek 1990 {published data only}
- Creek R.Ticlopidine. Patency of haemodialysis access sites. Guildford Sanofi Winthrop. Internal report 1990.
CURE 2000 {published data only (unpublished sought but not used)}
- Berger PB, Steinhubl S.Clinical implications of percutaneous coronary intervention-clopidogrel in unstable angina to prevent recurrent events (PCI-CURE) study: a US perspective. Circulation 2002;106(17):2284-7. [MEDLINE: ] [DOI] [PubMed] [Google Scholar]
- Gerschutz GP, Bhatt DL, Clopidogrel in Unstable Angina to Prevent Recurrent Events study.The Clopidogrel in Unstable Angina to Prevent Recurrent Events (CURE) study: to what extent should the results be generalizable? American Heart Journal 2003;145(4):595-601. [MEDLINE: ] [DOI] [PubMed] [Google Scholar]
- Keltai M, Tonelli M, Mann JF, Sitkei E, Lewis BS, Hawken S, et al.Renal function and outcomes in acute coronary syndrome: impact of clopidogrel. European Journal of Cardiovascular Prevention & Rehabilitation 2007;14(2):312-8. [MEDLINE: ] [DOI] [PubMed] [Google Scholar]
- Lewis BS, Mehta SR, Fox KA, Halon DA, Zhao F, Peters RJ, et al.Benefit of clopidogrel according to timing of percutaneous coronary intervention in patients with acute coronary syndromes: further results from the Clopidogrel in Unstable angina to prevent Recurrent Events (CURE) study. American Heart Journal 2005;150(6):1177-84. [MEDLINE: ] [DOI] [PubMed] [Google Scholar]
- Mehta SR, Yusuf S, Peters RJ, Bertrand ME, Lewis BS, Natarajan MK, et al.Effects of pretreatment with clopidogrel and aspirin followed by long-term therapy in patients undergoing percutaneous coronary intervention: the PCI-CURE study. Lancet 2001;358(9281):527-33. [MEDLINE: ] [DOI] [PubMed] [Google Scholar]
- Mehta SR, Yusuf S, Clopidogrel in Unstable angina to prevent Recurrent Events (CURE) Study Investigators.The Clopidogrel in Unstable angina to prevent Recurrent Events (CURE) trial programme; rationale, design and baseline characteristics including a meta-analysis of the effects of thienopyridines in vascular disease. European Heart Journal 2000;21(24):2033-41. [MEDLINE: ] [DOI] [PubMed] [Google Scholar]
- Peters RJ, Mehta SR, Fox KA, Zhao F, Lewis BS, Kopecky SL, et al.Effects of aspirin dose when used alone or in combination with clopidogrel in patients with acute coronary syndromes: observations from the Clopidogrel in Unstable angina to prevent Recurrent Events (CURE) study. Circulation 2003;108(14):1682-7. [MEDLINE: ] [DOI] [PubMed] [Google Scholar]
- Yusuf S, Zhao F, Mehta SR, Chrolavicius S, Tognoni G, Fox KK, et al.Effects of clopidogrel in addition to aspirin in patients with acute coronary syndromes without ST-segment elevation [Erratum in: N Engl J Med 2001 Dec 6;345(23):1716; Erratum in: N Engl J Med 2001 Nov 15;345(20):1506]. New England Journal of Medicine 2001;345(7):494-502. [MEDLINE: ] [DOI] [PubMed] [Google Scholar]
Dash 2013 {published data only}
- Dash A, Maiti R, Bandakkanavar TK, Bhaskar A, Prakash J, Pandey BL.Prophylactic add-on antiplatelet therapy in chronic kidney disease with type 2 diabetes mellitus: comparison between clopidogrel and low-dose aspirin. International Journal of Preventive Medicine 2013;4(8):902-10. [MEDLINE: ] [PMC free article] [PubMed] [Google Scholar]
Dember 2005 {published data only}
- Dember L, Allon M, Delmez J, Dixon B, Greenberg A, Himmelfarb J, et al.Dialysis access consortium (DAC) fistula trial: progress report and baseline characteristics [abstract no: SA-PO940]. Journal of the American Society of Nephrology 2003;14(Nov):506A. [CENTRAL: CN-00550593] [Google Scholar]
- Dember LM, Beck GJ, Allon M, Delmez JA, Dixon BS, Greenberg A, et al.Effect of clopidogrel on early failure of arteriovenous fistulas for hemodialysis: a randomized controlled trial. JAMA 2008;299(18):2164-71. [MEDLINE: ] [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dember LM, Kaufman JS, Beck GJ, Dixon BS, Gassman JJ, Greene T, et al.Design of the Dialysis Access Consortium (DAC) clopidogrel prevention of early AV fistula thrombosis trial. Clinical Trials 2005;2(5):413-22. [MEDLINE: ] [DOI] [PubMed] [Google Scholar]
- Dember LM, Kaufman JS, Beck GJ, Dixon BS, Gassman JJ, Greene T, et al.Dialysis access consortium (DAC) trial design: clopidogrel prevention of early AV fistula thrombosis [abstract no: F-PO827]. Journal of the American Society of Nephrology 2002;13(Program & Abstracts):229A. [CENTRAL: CN-00445067] [Google Scholar]
Dixon 2005 {published data only}
- Allon M, Zhang L, Maya ID, Bray MS, Fernandez JR, Dialysis Access Consortium.Association of factor V gene polymorphism with arteriovenous graft failure. American Journal of Kidney Diseases 2012;59(5):682-8. [MEDLINE: ] [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dixon B, Beck G, Meyers C, Kusek J, Feldman H, DAC Study Group.Effect of aspirin (ASA) on primary unassisted graft patency in the Dialysis Access Consortium (DAC) graft trial [abstract no: TH-PO660]. Journal of the American Society of Nephrology 2008;19(Abstract Issue):256A. [CENTRAL: CN-00716048] [Google Scholar]
- Dixon BS, Allon M, Delmez J, Dember L, Greenberg A, Himmelfarb J, et al.Dialysis access consortium (DAC) graft trial: progress report and baseline characteristics [abstract no: SA-PO939]. Journal of the American Society of Nephrology 2003;14(Nov):506A. [CENTRAL: CN-00583294] [Google Scholar]
- Dixon BS, Beck GJ, Dember LM, Depner TA, Gassman JJ, Greene T, et al.Design of the Dialysis Access Consortium (DAC) aggrenox prevention of access stenosis trial. Clinical Trials 2005;2(5):400-12. [MEDLINE: ] [DOI] [PubMed] [Google Scholar]
- Dixon BS, Beck GJ, Dember LM, Vazquez MA, Greenberg A, Delmez JA, et al.Use of aspirin associates with longer primary patency of hemodialysis grafts. Journal of the American Society of Nephrology 2011;22(4):773-81. [MEDLINE: ] [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dixon BS, Beck GJ, Vazquez MA, Greenberg A, Delmez JA, Allon M, et al.Dialysis Access Consortium (DAC) trial design: sustained-release dipyridamole plus aspirin (D/A) to prevent graft failure [abstract no: F-PO841]. Journal of the American Society of Nephrology 2002;10(Abstract Issue):232A. [CENTRAL: CN-00677737] [Google Scholar]
- Dixon BS, Beck GJ, Vazquez MA, Greenberg A, Delmez JA, Allon M, et al.Effect of dipyridamole plus aspirin on hemodialysis graft patency. New England Journal of Medicine 2009;360(21):2191-201. [MEDLINE: ] [DOI] [PMC free article] [PubMed] [Google Scholar]
- Farber A, Hu B, Dember L, Beck G, Dixon B, Kusek J.Patency of forearm and upper arm hemodialysis arteriovenous grafts: does configuration or location matter? [abstract]. Journal of Vascular Surgery 2013;57(5 Suppl 1):31-2S. [EMBASE: 71055757] [DOI] [PubMed] [Google Scholar]
- Farber A, Tan TW, Hu B, Dember LM, Beck GJ, Dixon BS, et al.The effect of location and configuration on forearm and upper arm hemodialysis arteriovenous grafts. Journal of Vascular Surgery 2015;62(5):1258-64. [MEDLINE: ] [DOI] [PubMed] [Google Scholar]
- Nee R, Parker AL, Little DJ, Yuan CM, Himmelfarb J, Lowe SR, et al.Cost-effectiveness of antiplatelet therapy to prolong primary patency of hemodialysis graft. Clinical Nephrology 2014;81(1):38-51. [MEDLINE: ] [DOI] [PubMed] [Google Scholar]
Dmoszynska‐Giannopoulou 1990 {published data only}
- Dmoszynska-Giannopoulou A, Janicka L, Sokolowska B, Ksiazek A, Orlowska G, Janicki K.The effect of sulphinpyrazone and alpha-tocopherol on platelet activation and function in haemodialysed patients. International Urology & Nephrology 1990;22(6):561-6. [MEDLINE: ] [DOI] [PubMed] [Google Scholar]
Dodd 1980 {published data only}
- Dodd NJ, Turney JH, Weston MJ.Ticlopidine preserves vascular access for haemodialysis [abstract no: 326F]. In: Proceedings of the VI International Congress of Mediterranean League Against Thrombosis; 1980 Oct 25-26; Montecarlo, Monaco. 1980.
Donadio 1984 {published data only}
- Donadio JV, Anderson CF, Mitchell JC, Holley KE, Ilstrup DM, Fuster V.Membranoproliferative glomerulonephritis (MPGN): a prospective clinical trial of platelet inhibitor therapy [abstract]. Kidney International 1983;23(1):121. [CENTRAL: CN-00602010] [DOI] [PubMed] [Google Scholar]
- Donadio JV Jr, Anderson CF, Mitchell JC 3rd, Holley KH, Ilstrup DM, Fuster V, et al.Membranoproliferative glomerulonephritis. A prospective clinical trial of platelet-inhibitor therapy. New England Journal of Medicine 1984;310(22):1421-6. [MEDLINE: ] [DOI] [PubMed] [Google Scholar]
EARLY ACS 2005 {published data only}
- Bagai A, White JA, Lokhnygina Y, Giugliano RP, Van de Werf F, Montalescot G, et al.Routine early eptifibatide versus delayed provisional use at percutaneous coronary intervention in high-risk non-ST-segment elevation acute coronary syndromes patients: an analysis from the Early Glycoprotein IIb/IIIa Inhibition in Non-ST-Segment Elevation Acute Coronary Syndrome trial. American Heart Journal 2013;166(3):466-73. [MEDLINE: ] [DOI] [PubMed] [Google Scholar]
- De Ferrari GM, Van de Werf F, Armstrong P, Bode C, Lewis BS, Tricoci P, et al.Contrast induced nephropathy predicts later mortality among patients with non-ST-segment elevation acute coronary syndrome undergoing PCI: a sub-analysis from the EARLY ACS study [abstract no: P3820]. European Heart Journal 2011;32(Suppl 1):657. [EMBASE: 70535519] [Google Scholar]
- Ezekowitz JA, Bakal JA, Westerhout CM, Giugliano RP, White H, Keltai M, et al.The relationship between meteorological conditions and index acute coronary events in a global clinical trial. International Journal of Cardiology 2013;168(3):2315-21. [MEDLINE: ] [DOI] [PubMed] [Google Scholar]
- Farhan S, Clare RM, Jarai R, Giugliano RP, Lokhnygina Y, Harrington RA, et al.Fasting glucose, NT-proBNP, treatment with eptifibatide, and outcomes in non-ST-segment elevation acute coronary syndromes: an analysis from EARLY ACS. International Journal of Cardiology 2017;232:264-70. [MEDLINE: ] [DOI] [PubMed] [Google Scholar]
- Farhan S, Clare RM, Jarai R, Newby LK, Morrow D, Giugliano RP, et al.Fasting glucose, NT-proBNP, treatment with glycoprotein IIB/IIIA inhibitors, and outcomes in non-st-segment elevation acute coronary syndromes: an analysis from early ACS [abstract no: A14618]. Circulation 2013;128(22 Suppl 1). [EMBASE: 71340424] [Google Scholar]
- Giugliano RP, Newby LK, Harrington RA, Gibson CM, Van de Werf F, Armstrong P, et al.The early glycoprotein IIb/IIIa inhibition in non-ST-segment elevation acute coronary syndrome (EARLY ACS) trial: a randomized placebo-controlled trial evaluating the clinical benefits of early front-loaded eptifibatide in the treatment of patients with non-ST-segment elevation acute coronary syndrome--study design and rationale. American Heart Journal 2005;149(6):994-1002. [MEDLINE: ] [DOI] [PubMed] [Google Scholar]
- Giugliano RP, White JA, Bode C, Armstrong PW, Montalescot G, Lewis BS, et al.Early versus delayed, provisional eptifibatide in acute coronary syndromes. New England Journal of Medicine 2009;360(21):2176-90. [MEDLINE: ] [DOI] [PubMed] [Google Scholar]
- Hess CN, Schulte PJ, Newby LK, Steg PG, Dalby AJ, Schweiger MJ, et al.Duration of eptifibatide infusion after percutaneous coronary intervention and outcomes among high-risk patients with non-ST-segment elevation acute coronary syndrome: insights from EARLY ACS. European Heart Journal: Acute Cardiovascular Care 2013;2(3):246-55. [MEDLINE: ] [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kaul P, Tanguay JF, Newby LK, Hochman JS, Westerhout CM, Califf RM, et al.Association between bleeding and mortality among women and men with high-risk acute coronary syndromes: insights from the Early versus Delayed, Provisional Eptifibatide in Acute Coronary Syndromes (EARLY ACS) trial. American Heart Journal 2013;166(4):723-8. [MEDLINE: ] [DOI] [PubMed] [Google Scholar]
- Klutstein MW, Westerhout CM, Armstrong PW, Giugliano RP, Lewis BS, Gibson CM, et al.Radial versus femoral access, bleeding and ischemic events in patients with non-ST-segment elevation acute coronary syndrome managed with an invasive strategy. American Heart Journal 2013;165(4):583-90. [MEDLINE: ] [DOI] [PubMed] [Google Scholar]
- Kunadian V, Giugliano RP, Newby LK, Zorkun C, Guo J, Bagai A, et al.Angiographic outcomes with early eptifibatide therapy in non-ST-segment elevation acute coronary syndrome (from the EARLY ACS Trial). American Journal of Cardiology 2014;113(8):1297-305. [MEDLINE: ] [DOI] [PubMed] [Google Scholar]
- Lopes RD, White JA, Tricoci P, White HD, Armstrong PW, Braunwald E, et al.Age, treatment, and outcomes in high-risk non-ST-segment elevation acute coronary syndrome patients: insights from the EARLY ACS trial. International Journal of Cardiology 2013;167(6):2580-7. [MEDLINE: ] [DOI] [PubMed] [Google Scholar]
- Melloni C, James SK, White JA, Giugliano RP, Harrington RA, Huber K, et al.Safety and efficacy of adjusted-dose eptifibatide in patients with acute coronary syndromes and reduced renal function. American Heart Journal 2011;162(5):884-92. [MEDLINE: ] [DOI] [PubMed] [Google Scholar]
- Piccini JP, White JA, Mehta RH, Lokhnygina Y, Al-Khatib SM, Tricoci P, et al.Sustained ventricular tachycardia and ventricular fibrillation complicating non-ST-segment-elevation acute coronary syndromes. Circulation 2012;126(1):41-9. [MEDLINE: ] [DOI] [PubMed] [Google Scholar]
- Pride YB, Mohanavelu S, Giugliano RP, Newby LK, Zorkun C, Kunadian V, et al.Association between angiographic complications during percutaneous coronary intervention and clinical outcomes among patients with acute coronary syndrome: an early ACS angiographic substudy [abstract no: A10394]. Circulation 2011;124(21 Suppl 1). [EMBASE: 70620664] [DOI] [PubMed] [Google Scholar]
- Pride YB, Mohanavelu S, Zorkun C, Kunadian V, Giugliano RP, Newby LK, et al.Association between angiographic complications and clinical outcomes among patients with acute coronary syndrome undergoing percutaneous coronary intervention: an EARLY ACS (Early Glycoprotein IIb/IIIa Inhibition in Non-ST-Segment Elevation Acute Coronary Syndrome) angiographic substudy. Jacc: Cardiovascular Interventions 2012;5(9):927-35. [MEDLINE: ] [DOI] [PubMed] [Google Scholar]
- Roe MT, White JA, Kaul P, Tricoci P, Lokhnygina Y, Miller CD, et al.Regional patterns of use of a medical management strategy for patients with non-ST-segment elevation acute coronary syndromes: insights from the EARLY ACS Trial. Circulation. Cardiovascular Quality & Outcomes 2012;5(2):205-13. [MEDLINE: ] [DOI] [PubMed] [Google Scholar]
- Tanguay JF, Newby LK, Hochman J, Westerhout CM, Califf RM, Gibson CM, et al.Sex differences in high-risk acute coronary syndromes: Insights from early-ACS [abstract]. Journal of the American College of Cardiology 2010;55(10 Suppl 1):A120.E1122. [EMBASE: 70096317] [Google Scholar]
- Toleva O, Westerhout CM, Senaratne M, Bode C, Lindroos M, Ardissino D, et al.Association of hub and spoke practice patterns with coronary intervention and outcomes in non ST elevation acute coronary syndromes (NSTE ACS): Insights from the early glycoprotein IIb/IIIa inhibition in NSTE ACS (early-ACS) trial [abstract]. Journal of the American College of Cardiology 2011;57(14 Suppl 1):E1101. [EMBASE: 70400778] [Google Scholar]
- Toleva O, Westerhout CM, Senaratne MP, Bode C, Lindroos M, Sulimov VA, et al.Practice patterns and clinical outcomes among non-ST-segment elevation acute coronary syndrome (NSTE-ACS) patients presenting to primary and tertiary hospitals: insights from the EARLY glycoprotein IIb/IIIa inhibition in NSTE-ACS (EARLY-ACS) trial. Catheterization & Cardiovascular Interventions 2014;84(6):934-42. [MEDLINE: ] [DOI] [PubMed] [Google Scholar]
- Wang T, White JA, Giugliano RP, Tricoci P, Harrington RA, Montalescot G, et al.Upfront clopidogrel use and the efficacy and safety of early eptifibatide use in patients with acute coronary syndrome: An analysis from the early versus delayed provisional eptifibatide in acute coronary syndromes (early ACS) trial [abstract]. Journal of the American College of Cardiology 2010;55(10 Suppl 1):A47.E451. [EMBASE: 70095646] [Google Scholar]
- Wang TY, White JA, Tricoci P, Giugliano RP, Zeymer U, Harrington RA, et al.Upstream clopidogrel use and the efficacy and safety of early eptifibatide treatment in patients with acute coronary syndrome: an analysis from the Early Glycoprotein IIb/IIIa Inhibition in Patients with Non-ST-Segment Elevation Acute Coronary Syndrome (EARLY ACS) trial. Circulation 2011;123(7):722-30. [MEDLINE: ] [DOI] [PubMed] [Google Scholar]
Ell 1982 {published and unpublished data}
- Ell S, Mihindukulasuriya JC, O'Brien JR, Polak A, Vernham G.Ticlopidine in the prevention of blockage of fistulae and shunts [abstract no: 332]. Haemostasis 1982;12:180. [Google Scholar]
EPIC 1994 {published data only}
- Aguirre FV, Topol EJ, Anderson KM, Kleiman NS, Weisman HF, FitzPatrick SE, et al.Benefit within patient subgroups receiving c7e3 fab (abciximab) during percutaneous coronary revascularization: subgroup analysis from the EPIC trial. Journal of Invasive Cardiology 1996;8 Suppl B:21-9B. [MEDLINE: ] [PubMed] [Google Scholar]
- Aguirre FV, Topol EJ, Ferguson JJ, Anderson K, Blankenship JC, Heuser RR, et al.Bleeding complications with the chimeric antibody to platelet glycoprotein IIb/IIIa integrin in patients undergoing percutaneous coronary intervention. EPIC Investigators. Circulation 1995;91(12):2882-90. [MEDLINE: ] [DOI] [PubMed] [Google Scholar]
- Berkowitz SD, Sane DC, Sigmon KN, Shavender JH, Harrington RA, Tcheng JE, et al.Occurrence and clinical significance of thrombocytopenia in a population undergoing high-risk percutaneous coronary revascularization. Evaluation of c7E3 for the Prevention of Ischemic Complications (EPIC) Study Group. Journal of the American College of Cardiology 1998;32(2):311-9. [MEDLINE: ] [DOI] [PubMed] [Google Scholar]
- Blankenship JC, Hellkamp AS, Aguirre FV, Demko SL, Topol EJ, Califf RM.Vascular access site complications after percutaneous coronary intervention with abciximab in the Evaluation of c7E3 for the Prevention of Ischemic Complications (EPIC) trial. American Journal of Cardiology 1998;81(1):36-40. [MEDLINE: ] [DOI] [PubMed] [Google Scholar]
- Boehrer JD, Kereiakes DJ, Navetta FI, Califf RM, Topol EJ.Effects of profound platelet inhibition with c7E3 before coronary angioplasty on complications of coronary bypass surgery. EPIC Investigators. Evaluation Prevention of Ischemic Complications. American Journal of Cardiology 1994;74(11):1166-70. [MEDLINE: ] [DOI] [PubMed] [Google Scholar]
- Califf RM, Lincoff AM, Tcheng JE, Topol EJ.An overview of the results of the EPIC trial. European Heart Journal 1995;16 Suppl L:43-9. [MEDLINE: ] [DOI] [PubMed] [Google Scholar]
- EPIC Investigators.Use of a monoclonal antibody directed against the platelet glycoprotein IIb/IIIa receptor in high-risk coronary angioplasty. New England Journal of Medicine 1994;330(14):956-61. [MEDLINE: ] [DOI] [PubMed] [Google Scholar]
- Khan MM, Ellis SG, Aguirre FV, Weisman HF, Wildermann NM, Califf RM, et al.Does intracoronary thrombus influence the outcome of high risk percutaneous transluminal coronary angioplasty? Clinical and angiographic outcomes in a large multicenter trial. EPIC Investigators. Evaluation of IIb/IIIa Platelet Receptor Antagonist 7E3 in Preventing Ischemic Complications. Journal of the American College of Cardiology 1998;31(1):31-6. [MEDLINE: ] [DOI] [PubMed] [Google Scholar]
- Lefkovits J, Blankenship JC, Anderson KM, Stoner GL, Talley JD, Worley SJ, et al.Increased risk of non-Q wave myocardial infarction after directional atherectomy is platelet dependent: evidence from the EPIC trial. Evaluation of c7E3 for the Prevention of Ischemic Complications. Journal of the American College of Cardiology 1996;28(4):849-55. [MEDLINE: ] [DOI] [PubMed] [Google Scholar]
- Lefkovits J, Ivanhoe RJ, Califf RM, Bergelson BA, Anderson KM, Stoner GL, et al.Effects of platelet glycoprotein IIb/IIIa receptor blockade by a chimeric monoclonal antibody (abciximab) on acute and six-month outcomes after percutaneous transluminal coronary angioplasty for acute myocardial infarction. EPIC investigators. American Journal of Cardiology 1996;77(12):1045-51. [MEDLINE: ] [DOI] [PubMed] [Google Scholar]
- Lincoff AM, Califf RM, Anderson KM, Weisman HF, Aguirre FV, Kleiman NS, et al.Evidence for prevention of death and myocardial infarction with platelet membrane glycoprotein IIb/IIIa receptor blockade by abciximab (c7E3 Fab) among patients with unstable angina undergoing percutaneous coronary revascularization. EPIC Investigators. Evaluation of 7E3 in Preventing Ischemic Complications. Journal of the American College of Cardiology 1997;30(1):149-56. [MEDLINE: ] [DOI] [PubMed] [Google Scholar]
- Mak KH, Challapalli R, Eisenberg MJ, Anderson KM, Califf RM, Topol EJ.Effect of platelet glycoprotein IIb/IIIa receptor inhibition on distal embolization during percutaneous revascularization of aortocoronary saphenous vein grafts. EPIC Investigators. Evaluation of IIb/IIIa platelet receptor antagonist 7E3 in Preventing Ischemic Complications. American Journal of Cardiology 1997;80(8):985-8. [MEDLINE: ] [DOI] [PubMed] [Google Scholar]
- Mark DB, Talley JD, Topol EJ, Bowman L, Lam LC, Anderson KM, et al.Economic assessment of platelet glycoprotein IIb/IIIa inhibition for prevention of ischemic complications of high-risk coronary angioplasty. EPIC Investigators. Circulation 1996;94(4):629-35. [MEDLINE: ] [DOI] [PubMed] [Google Scholar]
- Moliterno DJ, Califf RM, Aguirre FV, Anderson K, Sigmon KN, Weisman HF, et al.Effect of platelet glycoprotein IIb/IIIa integrin blockade on activated clotting time during percutaneous transluminal coronary angioplasty or directional atherectomy (the EPIC trial). Evaluation of c7E3 Fab in the Prevention of Ischemic Complications trial. American Journal of Cardiology 1995;75(8):559-62. [MEDLINE: ] [DOI] [PubMed] [Google Scholar]
- Narins CR, Miller DP, Califf RM, Topol EJ.The relationship between periprocedural myocardial infarction and subsequent target vessel revascularization following percutaneous coronary revascularization: insights from the EPIC trial. Evaluation of IIb/IIIa platelet receptor antagonist 7E3 in Preventing Ischemic Complications. Journal of the American College of Cardiology 1999;33(3):647-53. [MEDLINE: ] [DOI] [PubMed] [Google Scholar]
- Thel MC, Califf RM, Tcheng JE, Sigmon KN, Lincoff AM, Topol EJ, et al.Clinical risk factors for ischemic complications after percutaneous coronary interventions: results from the EPIC trial. The EPIC Investigators. American Heart Journal 1999;137(2):264-73. [MEDLINE: ] [DOI] [PubMed] [Google Scholar]
- Topol EJ, Califf RM, Weisman HF, Ellis SG, Tcheng JE, Worley S, et al.Randomised trial of coronary intervention with antibody against platelet IIb/IIIa integrin for reduction of clinical restenosis: results at six months. The EPIC Investigators. Lancet 1994;343(8902):881-6. [MEDLINE: ] [DOI] [PubMed] [Google Scholar]
- Topol EJ, Ferguson JJ, Weisman HF, Tcheng JE, Ellis SG, Kleiman NS, et al.Long-term protection from myocardial ischemic events in a randomized trial of brief integrin beta3 blockade with percutaneous coronary intervention. EPIC Investigator Group. Evaluation of Platelet IIb/IIIa Inhibition for Prevention of Ischemic Complication. JAMA 1997;278(6):479-84. [MEDLINE: ] [DOI] [PubMed] [Google Scholar]
- Hout BA, Bowman L, Zelinger DJ, Simoons ML.Costs and effects in therapy for acute coronary syndromes: the case of abciximab in high-risk patients undergoing percutaneous transluminal coronary angioplasty in the EPIC study. Evaluation of 7E3 for the Prevention of Ischemic Complications. American Heart Journal 1998;135(4):S98-106. [MEDLINE: ] [DOI] [PubMed] [Google Scholar]
- Hout BA, Bowman L, Zelinger DJ, Simoons ML.Costs and effects in therapy for acute coronary syndromes: the case of abciximab in high-risk patients undergoing percutaneous transluminal coronary angioplasty in the EPIC study. Evaluation of 7E3 for the Prevention of Ischemic Complications. European Heart Journal 1998;19 Suppl D:D59-66. [MEDLINE: ] [PubMed] [Google Scholar]
EPILOG 1997 {unpublished data only}
- EPILOG Investigators.Platelet glycoprotein IIb/IIIa receptor blockade and low-dose heparin during percutaneous coronary revascularization. New England Journal of Medicine 1997;336(24):1689-96. [MEDLINE: ] [DOI] [PubMed] [Google Scholar]
- Ghaffari S, Kereiakes DJ, Lincoff AM, Kelly TA, Timmis GC, Kleiman NS, et al.Platelet glycoprotein IIb/IIIa receptor blockade with abciximab reduces ischemic complications in patients undergoing directional coronary atherectomy. EPILOG Investigators. Evaluation of PTCA to Improve Long-term Outcome by c7E3 GP IIb/IIIa Receptor Blockade. American Journal of Cardiology 1998;82(1):7-12. [MEDLINE: ] [DOI] [PubMed] [Google Scholar]
- Kereiakes DJ, Lincoff AM, Miller DP, Tcheng JE, Cabot CF, Anderson KM, et al.Abciximab therapy and unplanned coronary stent deployment: favorable effects on stent use, clinical outcomes, and bleeding complications. EPILOG Trial Investigators. Circulation 1998;97(9):857-64. [MEDLINE: ] [DOI] [PubMed] [Google Scholar]
- Kleiman NS, Lincoff AM, Kereiakes DJ, Miller DP, Aguirre FV, Anderson KM, et al.Diabetes mellitus, glycoprotein IIb/IIIa blockade, and heparin: evidence for a complex interaction in a multicenter trial. EPILOG Investigators. Circulation 1998;97(19):1912-20. [MEDLINE: ] [DOI] [PubMed] [Google Scholar]
- Lincoff AM, Mark DB, Tcheng JE, Califf RM, Bala MV, Anderson KM, et al.Economic assessment of platelet glycoprotein IIb/IIIa receptor blockade with abciximab and low-dose heparin during percutaneous coronary revascularization: results from the EPILOG randomized trial. Evaluation in PTCA to Improve Long-term Outcome with abciximab GP IIb/IIIa blockade. Circulation 2000;102(24):2923-9. [MEDLINE: ] [DOI] [PubMed] [Google Scholar]
- Lincoff AM, Tcheng JE, Califf RM, Kereiakes DJ, Kelly TA, Timmis GC, et al.Sustained suppression of ischemic complications of coronary intervention by platelet GP IIb/IIIa blockade with abciximab. One-year outcome in the EPILOG trial. Circulation 1999;99(15):1951-8. [MEDLINE: ] [DOI] [PubMed] [Google Scholar]
EPISTENT 1998 {unpublished data only}
- Cho L, Marso SP, Bhatt DL, Topol EJ.Optimizing percutaneous coronary revascularization in diabetic women: analysis from the EPISTENT trial. Journal of Womens Health & Gender-Based Medicine 2000;9(7):741-6. [MEDLINE: ] [DOI] [PubMed] [Google Scholar]
- De Servi S.Ticlopidine pretreatment before coronary stenting is associated with sustained decrease in adverse events. Data from the Evaluation of Platelets IIb/IIIa Inhibitor for Stenting (EPISTENT) trial [Il pretrattamento con ticlopidina nello stent coronarico e associato a significativa riduzione di eventi avversi. Dati dal trial EPISTENT]. Italian Heart Journal Supplement 2001;2(7):805-6. [MEDLINE: ] [PubMed] [Google Scholar]
- EPISTENT Investigators.Randomised placebo-controlled and balloon-angioplasty-controlled trial to assess safety of coronary stenting with use of platelet glycoprotein-IIb/IIIa blockade. Lancet 1998;352(9122):87-92. [MEDLINE: ] [DOI] [PubMed] [Google Scholar]
- Islam MA, Blankenship JC, Balog C, Iliadis EA, Lincoff AM, Tcheng JE, et al.Effect of abciximab on angiographic complications during percutaneous coronary stenting in the Evaluation of Platelet IIb/IIIa Inhibition in Stenting Trial (EPISTENT). American Journal of Cardiology 2002;90(9):916-21. [MEDLINE: ] [DOI] [PubMed] [Google Scholar]
- Lincoff AM.Potent complementary clinical benefit of abciximab and stenting during percutaneous coronary revascularization in patients with diabetes mellitus: results of the EPISTENT trial. American Heart Journal 2000;139(2 Pt 2):S46-52. [MEDLINE: ] [DOI] [PubMed] [Google Scholar]
- Marso SP, Lincoff AM, Ellis SG, Bhatt DL, Tanguay JF, Kleiman NS, et al.Optimizing the percutaneous interventional outcomes for patients with diabetes mellitus: results of the EPISTENT (Evaluation of platelet IIb/IIIa inhibitor for stenting trial) diabetic substudy. Circulation 1999;100(25):2477-84. [MEDLINE: ] [DOI] [PubMed] [Google Scholar]
- Steinhubl SR, Ellis SG, Wolski K, Lincoff AM, Topol EJ.Ticlopidine pretreatment before coronary stenting is associated with sustained decrease in adverse cardiac events: data from the Evaluation of Platelet IIb/IIIa Inhibitor for Stenting (EPISTENT) Trial. Circulation 2001;103(10):1403-9. [MEDLINE: ] [DOI] [PubMed] [Google Scholar]
- Steinhubl SR, Tan WA, Foody JM, Topol EJ.Incidence and clinical course of thrombotic thrombocytopenic purpura due to ticlopidine following coronary stenting. EPISTENT Investigators. Evaluation of Platelet IIb/IIIa Inhibitor for Stenting. JAMA 1999;281(9):806-10. [MEDLINE: ] [DOI] [PubMed] [Google Scholar]
- Topol EJ, Mark DB, Lincoff AM, Cohen E, Burton J, Kleiman N, et al.Outcomes at 1 year and economic implications of platelet glycoprotein IIb/IIIa blockade in patients undergoing coronary stenting: results from a multicentre randomised trial. EPISTENT Investigators. Evaluation of Platelet IIb/IIIa Inhibitor for Stenting.[Erratum in: Lancet 2000 Mar 25;355(9209):1104]. Lancet 1999;354(9195):2019-24. [MEDLINE: ] [DOI] [PubMed] [Google Scholar]
- Zwart-van Rijkom JE, Hout BA.Cost-efficacy in interventional cardiology; results from the EPISTENT study. Evaluation of Platelet IIb/IIIa Inhibitor For Stenting Trial. European Heart Journal 2001;22(16):1476-84. [MEDLINE: ] [DOI] [PubMed] [Google Scholar]
ETDRS 1992 {unpublished data only}
- Aspirin effects on mortality and morbidity in patients with diabetes mellitus. Early Treatment Diabetic Retinopathy Study report 14. ETDRS Investigators. JAMA 1992;268(10):1292-300. [MEDLINE: ] [DOI] [PubMed] [Google Scholar]
EUCLID 2017 {published data only}
- Baumgartner I, Norgren L, Fowkes FG, Mulder H, Patel MR, Berger JS, et al.Cardiovascular outcomes after lower extremity endovascular or surgical revascularization: the EUCLID trial. Journal of the American College of Cardiology 2018;72(14):1563-72. [MEDLINE: ] [DOI] [PubMed] [Google Scholar]
- Berger JS, Abramson BL, Lopes RD, Heizer G, Rockhold FW, Baumgartner I, et al.Ticagrelor versus clopidogrel in patients with symptomatic peripheral artery disease and prior coronary artery disease: Insights from the EUCLID trial. Vascular Medicine 2018;23(6):523-30. [MEDLINE: ] [DOI] [PubMed] [Google Scholar]
- Haine A, Kavanagh S, Berger JS, Hess CN, Norgren L, Fowkes FG, et al.Sex-specific risks of major cardiovascular and limb events in patients with symptomatic peripheral artery disease. Journal of the American College of Cardiology 2020;75(6):608-17. [MEDLINE: ] [DOI] [PubMed] [Google Scholar]
- Hess CN, Huang Z, Patel MR, Baumgartner I, Berger JS, Blomster JI, et al.Acute limb ischemia in peripheral artery disease. Circulation 2019;140(7):556-65. [MEDLINE: ] [DOI] [PubMed] [Google Scholar]
- Hiatt WR, Fowkes FG, Heizer G, Berger JS, Baumgartner I, Held P, et al.Ticagrelor versus clopidogrel in symptomatic peripheral artery disease. New England Journal of Medicine 2017;376(1):32-40. [MEDLINE: ] [DOI] [PubMed] [Google Scholar]
- Hopley CW, Kavanagh S, Patel MR, Ostrom C, Baumgartner I, Berger JS, et al.Chronic kidney disease and risk for cardiovascular and limb outcomes in patients with symptomatic peripheral artery disease: the EUCLID trial. Vascular Medicine 2019;24(5):422-30. [MEDLINE: ] [DOI] [PubMed] [Google Scholar]
- Jones WS, Baumgartner I, Hiatt WR, Heizer G, Conte MS, White CJ, et al.Ticagrelor compared with clopidogrel in patients with prior lower extremity revascularization for peripheral artery disease. Circulation 2017;135(3):241-50. [MEDLINE: ] [DOI] [PubMed] [Google Scholar]
- Kolls BJ, Sapp S, Rockhold FW, Jordan JD, Dombrowski KE, Fowkes FG, et al.Stroke in patients with peripheral artery disease. Stroke 2019;50(6):1356-63. [MEDLINE: ] [DOI] [PubMed] [Google Scholar]
- Low Wang CC, Blomster JI, Heizer G, Berger JS, Baumgartner I, Fowkes FG, et al.Cardiovascular and limb outcomes in patients with diabetes and peripheral artery disease: the EUCLID trial. Journal of the American College of Cardiology 2018;72(25):3274-84. [MEDLINE: ] [DOI] [PubMed] [Google Scholar]
- Norgren L, Patel MR, Hiatt WR, Wojdyla DM, Fowkes FG, Baumgartner I, et al.Outcomes of patients with critical limb ischaemia in the EUCLID trial. European Journal of Vascular & Endovascular Surgery 2018;55(1):109-17. [MEDLINE: ] [DOI] [PubMed] [Google Scholar]
- Olivier CB, Mulder H, Hiatt WR, Jones WS, Fowkes FG, Rockhold FW, et al.Incidence, characteristics, and outcomes of myocardial infarction in patients with peripheral artery disease: insights from the EUCLID trial. JAMA Cardiology 2019;4(1):7-15. [MEDLINE: ] [DOI] [PMC free article] [PubMed] [Google Scholar]
FAVOURED 2009 {published data only}
- Badve S, Hawley CM, Irish AB, Paul-Brent P.High rate of screening failure in the FAVOURED study [abstract no: PUB345]. Journal of the American Society of Nephrology 2009;20(Abstract Suppl):905A. [Google Scholar]
- Irish A, Dogra G, Mori T, Beller E, Heritier S, Hawley C, et al.Preventing AVF thrombosis: the rationale and design of the Omega-3 fatty acids (Fish Oils) and Aspirin in Vascular access OUtcomes in REnal Disease (FAVOURED) study. BMC Nephrology 2009;10:1. [MEDLINE: ] [DOI] [PMC free article] [PubMed] [Google Scholar]
- Irish A, Viecelli A, Hawley C, Hooi L, Pascoe E, Paul-Brent P, et al.Effect of fish oil and aspirin on arteriovenous fistula failure in haemodialysis-a randomized controlled trial [abstract]. Nephrology 2016;21(Suppl 2):47. [EMBASE: 612312804] [Google Scholar]
- Irish A, FAVOURED Study Group.High rate of screening failure in the FAVOURED study [abstract no: 169]. Nephrology 2009;14(Suppl 1):A45. [CENTRAL: CN-00756870] [Google Scholar]
- Irish A.Baseline characteristics of the patients participating in the FAVOURED trial [abstract no: 009]. Nephrology 2014;19(Suppl 4):19. [EMBASE: 71587790] [Google Scholar]
- Irish A.Baseline characteristics of the patients participating in the FAVOURED trial [abstract no: 034]. Nephrology 2010;15(Suppl 4):35. [EMBASE: 70467038] [Google Scholar]
- Irish AB, Viecelli AK, Hawley CM, Hooi LS, Pascoe EM, Paul-Brent PA, et al.Effect of fish oil supplementation and aspirin use on arteriovenous fistula failure in patients requiring hemodialysis: a randomized clinical trial. JAMA Internal Medicine 2017;177(2):184-93. [MEDLINE: ] [DOI] [PubMed] [Google Scholar]
- Irish AB.The omega-3 fatty acids (fish oils) and aspirin in vascular access outcomes in renal disease (FAVOURED) study: a randomised placebo-controlled trial [abstract no: HI-OR08]. Journal of the American Society of Nephrology 2015;26(Abstract Suppl):B2-3. [CENTRAL: CN-01658143] [Google Scholar]
- Viecelli A, Pascoe E, Hawley C, Polkinghorne K, Mori T, Johnson D, et al.Effects of fish oil supplementation and aspirin use on arteriovenous fistula patency, need for interventions and dialysis suitability in patients requiring haemodialysis-post hoc analysis of the FAVOURED study [abstract no: MO049]. Nephrology Dialysis Transplantation 2017;32(Suppl 3):iii65. [EMBASE: 617291475] [Google Scholar]
- Viecelli A, Pascoe E, Hawley C, Polkinghorne K, Mori T, Johnson D, et al.Effects of fish oil supplementation and aspirin use on the need for arteriovenous fistula interventions and central venous catheters in patients requiring haemodialysis [abstract]. Nephrology 2018;23(Suppl 3):56. [EMBASE: 623841152] [Google Scholar]
- Viecelli A, Pascoe E, Polkinghorne K, Darssan D, Mori T, Hawley C, et al.Regional differences in arteriovenous fistula failure observed in the FAVOURED trial [abstract no: SP593]. Nephrology Dialysis Transplantation 2017;32(Suppl 3):iii335. [EMBASE: 617291178] [Google Scholar]
- Viecelli A, Pascoe E, Polkinghorne K, Mori T, Hawley C, Johnson D, et al.Effects of fish oil supplementation and aspirin use on need for arteriovenous fistula interventions and central venous catheters in patients requiring haemodialysis [abstract no: FP555]. Nephrology Dialysis Transplantation 2018;33(Suppl 1):i227. [EMBASE: 622605265] [Google Scholar]
- Viecelli A, Pascoe E, Polkinghorne K, Paul-Brent P, Darssan D, Hooi L, et al.A comparison of arteriovenous fistula failure between Malaysian and Australian and New Zealand participants enrolled in the FAVOURED trial [abstract]. Nephrology 2016;21(Suppl 2):115-6. [EMBASE: 612312707] [Google Scholar]
- Viecelli AK, Pascoe E, Polkinghorne KR, Hawley C, Paul-Brent PA, Badve SV, et al.The Omega-3 fatty acids (Fish Oils) and Aspirin in Vascular access OUtcomes in REnal Disease (FAVOURED) study: the updated final trial protocol and rationale of post-initiation trial modifications. BMC Nephrology 2015;16:89. [MEDLINE: ] [DOI] [PMC free article] [PubMed] [Google Scholar]
- Viecelli AK, Pascoe EM, Hawley CM, Polkinghorne KR, Mori TA, Johnson DW, et al.Effects of fish oil supplementation and aspirin use on arteriovenous fistula patency, need for interventions and dialysis suitability in patients requiring haemodialysis - Post hoc analysis of the FAVOURED study [abstract]. Nephrology 2017;22(Suppl 3):12. [EMBASE: 618236182] [Google Scholar]
- Viecelli AK, Pascoe EM, Polkinghorne KR, Hawley CM, Paul-Brent PA, Badve SV, et al.Baseline characteristics of the omega-3 fatty acids (Fish oils) and Aspirin in Vascular access OUtcomes in REnal Disease (FAVOURED) study. Nephrology 2016;21(3):217-28. [MEDLINE: ] [DOI] [PubMed] [Google Scholar]
- Viecelli AK, Pascoe EM, Polkinghorne KR, Hawley CM, Paul-Brent PA, Badve SV, et al.Updates on baseline characteristics of the omega-3 fatty acids (Fish oils) and Aspirin in Vascular access Outcomes in Renal Disease (FAVOURED) study. Nephrology 2017;22(10):823-4. [MEDLINE: ] [DOI] [PubMed] [Google Scholar]
- Viecelli AK, Polkinghorne KR, Pascoe EM, Paul-Brent PA, Hawley CM, Badve SV, et al.Fish oil and aspirin effects on arteriovenous fistula function: Secondary outcomes of the randomised omega-3 fatty acids (Fish oils) and Aspirin in Vascular access OUtcomes in REnal Disease (FAVOURED) trial. PLoS ONE [Electronic Resource] 2019;14(3):e0213274. [MEDLINE: ] [DOI] [PMC free article] [PubMed] [Google Scholar]
Fiskerstrand 1985 {published data only}
- Fiskerstrand CE, Thompson IW, Burnet ME, Williams P, Anderton JL.Double-blind randomized trial of the effect of ticlopidine in arteriovenous fistulas for hemodialysis. Artificial Organs 1985;9(1):61-3. [MEDLINE: ] [DOI] [PubMed] [Google Scholar]
Frascà 1986 {published data only}
- Bonomini V, Vangelista A, Stefoni S, Scolari MP, Frascà GM, Raimondi C.Use of defibrotide in renal transplantation in man. Haemostasis 1986;16 Suppl 1:48-50. [MEDLINE: ] [DOI] [PubMed] [Google Scholar]
- Frascà GM, Vangelista A, Raimondi C, Bonomini V.Prevention of vascular graft lesions in renal transplant recipients with a new antithrombotic agent (defibrotide): a controlled study. Life Support Systems 1986;4(3):231-7. [MEDLINE: ] [PubMed] [Google Scholar]
Frascà 1997 {published data only}
- Frascà GM, Cianciolo G.A clinical trial with defibrotide in IgA nephritis (IgA-GN) with impaired renal function [abstract]. Nephrology Dialysis Transplantation 1995;10(6):968. [CENTRAL: CN-00261099] [Google Scholar]
- Frascà GM, Martello M, Canova C, Isola E, Vangelista A, Bonomini V.Defibrotide treatment and disease progression in patients with IgA nephropathy and impaired renal function at diagnosis. Clinical Drug Investigation 1997;13(4):185-91. [EMBASE: 27192802] [Google Scholar]
- Frascà GM, Martello M, Sestigiani E, Canova C, Vangelista A, Bonomini V.Effects of defibrotide treatment in patients with IgA nephropathy and reduced renal function. Nephrology Dialysis Transplantation 1996;11(2):392-3. [MEDLINE: ] [DOI] [PubMed] [Google Scholar]
Gaede 2003 {published data only}
- Gaede P, Hansen HP, Parving HH, Pedersen O.Impact of low-dose acetylsalicylic acid on kidney function in type 2 diabetic patients with elevated urinary albumin excretion rate. Nephrology Dialysis Transplantation 2003;18(3):539-42. [MEDLINE: ] [DOI] [PubMed] [Google Scholar]
- Gaede P, Parving H, Pedersen O.Lack of impact of low-dose acetylsalisyic acid (ASA) on albuminuria in type 2 diabetic patients [abstract no: A0660]. Journal of the American Society of Nephrology 1999;10(Program & Abstracts):128A. [CENTRAL: CN-00583369] [Google Scholar]
Ghorbani 2009 {published data only}
- Ghorbani A, Aalamshah M, Shahbazian H, Ehsanpour A, Aref A.Randomized controlled trial of clopidogrel to prevent primary arteriovenous fistula failure in hemodialysis patients. Indian Journal of Nephrology 2009;19(2):57-61. [MEDLINE: ] [DOI] [PMC free article] [PubMed] [Google Scholar]
Ghorbani 2013 {published data only}
- Ghorbani A, Jasemi-Zergani F.Ticlopidine to prevent primary arteriovenous fistula failure in hemodialysis patients; a randomized controlled trial. Journal of Renal Injury Prevention 2013;2(3):109-11. [MEDLINE: ] [DOI] [PMC free article] [PubMed] [Google Scholar]
Giustina 1998 {published data only}
- Giustina A, Perini P, Desenzani P, Bossoni S, Ianniello P, Milani M, et al.Long-term treatment with the dual antithromboxane agent picotamide decreases microalbuminuria in normotensive type 2 diabetic patients. Diabetes 1998;47(3):423-30. [MEDLINE: ] [DOI] [PubMed] [Google Scholar]
GLOBAL LEADERS 2018 {published data only}
- Gao C, Tomaniak M, Takahashi K, Kawashima H, Wang R, Hara H, et al.Ticagrelor monotherapy in patients with concomitant diabetes mellitus and chronic kidney disease: a post hoc analysis of the GLOBAL LEADERS trial. Cardiovascular Diabetology 2020;19(1):179. [MEDLINE: ] [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tomaniak M, Chichareon P, Klimczak-Tomaniak D, Takahashi K, Kogame N, Modolo R, et al.Impact of renal function on clinical outcomes after PCI in ACS and stable CAD patients treated with ticagrelor: a prespecified analysis of the GLOBAL LEADERS randomized clinical trial. Clinical Research in Cardiology 2020;109(7):930-43. [MEDLINE: ] [DOI] [PubMed] [Google Scholar]
- Vranckx P, Valgimigli M, Juni P, Hamm C, Steg PG, Heg D, et al.Ticagrelor plus aspirin for 1 month, followed by ticagrelor monotherapy for 23 months vs aspirin plus clopidogrel or ticagrelor for 12 months, followed by aspirin monotherapy for 12 months after implantation of a drug-eluting stent: a multicentre, open-label, randomised superiority trial. Lancet 2018;392(10151):940-9. [MEDLINE: ] [DOI] [PubMed] [Google Scholar]
Goicoechea 2012 {published data only}
- Morales AM, Goicoechea M, Verde E, Carbayo J, Barbieri D, Delgado A, et al.Pentoxifylline, progression of chronic kidney disease (CKD) and cardiovascular mortality: long-term follow-up of a randomized clinical trial. Journal of Nephrology 2019;32(4):581-7. [MEDLINE: ] [DOI] [PubMed] [Google Scholar]
- Goicoechea M, Garcia de Vinuesa S, Quiroga B, Verdalles U, Barraca D, Yuste C, et al.Effects of pentoxifylline on inflammatory parameters in chronic kidney disease patients: a randomized trial. Journal of Nephrology 2012;25(6):969-75. [MEDLINE: ] [DOI] [PubMed] [Google Scholar]
Gonzalez 1995 {published data only}
- Gonzalez MT, Castelao AM, Valles M, Cruzado JM, Mauri JM.Platelet antiaggregants (PA) could decrease the rate of progression of chronic renal failure (CRF) in diabetic patients (DP) treated previously with angiotensin converting enzyme inhibitors (ACEi) [abstract]. In: ISN XIII International Congress of Nephrology; 1995 Jul 2-6; Madrid, Spain. 1995:200. [CENTRAL: CN-00509215]
Gröntoft 1985 {published data only}
- Gröntoft KC, Mulec H, Gutierrez A, Olander R.Thromboprophylactic effect of ticlopidine in arteriovenous fistulas for haemodialysis. Scandinavian Journal of Urology & Nephrology 1985;19(1):55-7. [MEDLINE: ] [DOI] [PubMed] [Google Scholar]
Gröntoft 1998 {published data only}
- Gröntoft K, Larsson R, Mulec H, Weiss LG, Dickinson JP.Ticlopidine in fistula surgery: double-blind comparison against placebo on the rate of early occlusion of arterio-venous fistulae [abstract]. In: 12th International Congress of Nephrology; 1993 Jun 13-18; Jerusalem, Israel. 1993:398. [CENTRAL: CN-00601921]
- Gröntoft KC, Larsson R, Mulec H, Weiss LG, Dickinson JP.Effects of ticlopidine in AV-fistula surgery in uremia. Fistula Study Group. Scandinavian Journal of Urology & Nephrology 1998;32(4):276-83. [MEDLINE: ] [DOI] [PubMed] [Google Scholar]
Guo 1998 {published data only}
- Guo Z, Hasbach J, Koschinsky T.Effect of acetylsalicylic acid on renal function of Type 1 diabetic patients with microalbuminuria [Wirkung von Azetylsalizylsaure auf die Nierenfunktion bei Typ-1- Diabetikern mit Mikroalbuminurie. Placebokontrollierte Crossover-Pilotstudie]. Diabetes und Stoffwechsel 1998;7(2):41-7. [EMBASE: 28146337] [Google Scholar]
Hansen 2000 {published data only}
- Hansen HP, Gaede PH, Jensen BR, Parving HH.Lack of impact of low-dose acetylsalicylic acid on kidney function in type 1 diabetic patients with microalbuminuria. Diabetes Care 2000;23(12):1742-5. [MEDLINE: ] [DOI] [PubMed] [Google Scholar]
Harter 1979 {published data only}
- Harter HR, Burch JW, Majerus PW, Stanford N, Delmez JA, Anderson CB, et al.Prevention of thrombosis in patients on hemodialysis by low-dose aspirin. New England Journal of Medicine 1979;301(11):577-9. [MEDLINE: ] [DOI] [PubMed] [Google Scholar]
Hidaka 2013 {published data only}
- Hidaka S, Kobayashi S, Iwagami M, Isshiki R, Tsutsumi D, Mochida Y, et al.Sarpogrelate hydrochloride, a selective 5-HT(2A) receptor antagonist, improves skin perfusion pressure of the lower extremities in hemodialysis patients with peripheral arterial disease. Renal Failure 2013;35(1):43-8. [MEDLINE: ] [DOI] [PubMed] [Google Scholar]
HOT 1993 {published and unpublished data}
- Hansson L, Zanchetti A, Carruthers SG, Dahlöf B, Elmfeldt D, Julius S, et al.Effects of intensive blood-pressure lowering and low-dose aspirin in patients with hypertension: principal results of the Hypertension Optimal Treatment (HOT) randomised trial. HOT Study Group. Lancet 1998;351(9118):1755-62. [MEDLINE: ] [DOI] [PubMed] [Google Scholar]
- Hansson L, Zanchetti A.The Hypertension Optimal Treatment (HOT) Study: 24-month data on blood pressure and tolerability. Blood Pressure 1997;6(5):313-7. [MEDLINE: ] [DOI] [PubMed] [Google Scholar]
- Hansson L, Zanchetti A.The Hypertension Optimal Treatment (HOT) Study--patient characteristics: randomization, risk profiles, and early blood pressure results. Blood Pressure 1994;3(5):322-7. [MEDLINE: ] [DOI] [PubMed] [Google Scholar]
- Hansson L.The Hypertension Optimal Treatment study and the importance of lowering blood pressure. Journal of Hypertension - Supplement 1999;17(1):S9-13. [MEDLINE: ] [PubMed] [Google Scholar]
- Jardine M, Ninomiya T, Cass A, Turnbull F, Gallagher M, Zoungas S, et al.Aspirin benefit increases as eGFR declines: results from a randomised controlled trial in a hypertensive population [abstract no: 086]. Nephrology 2010;15(Suppl 4):49. [EMBASE: 70467090] [Google Scholar]
- Jardine MJ, Ninomiya T, Perkovic V, Cass A, Turnbull F, Gallagher MP, et al.Aspirin is beneficial in hypertensive patients with chronic kidney disease: a post-hoc subgroup analysis of a randomized controlled trial. Journal of the American College of Cardiology 2010;56(12):956-65. [MEDLINE: ] [DOI] [PubMed] [Google Scholar]
- Jones DW, Miller ME, Wofford MR, Anderson DC Jr, Cameron ME, Willoughby DL, et al.The effect of weight loss intervention on antihypertensive medication requirements in the hypertension Optimal Treatment (HOT) study. American Journal of Hypertension 1999;12(12 Pt 1-2):1175-80. [MEDLINE: ] [DOI] [PubMed] [Google Scholar]
- Jonsson B, Hansson L, Stalhammar NO.Health economics in the Hypertension Optimal Treatment (HOT) study: costs and cost-effectiveness of intensive blood pressure lowering and low-dose aspirin in patients with hypertension. Journal of Internal Medicine 2003;253(4):472-80. [MEDLINE: ] [DOI] [PubMed] [Google Scholar]
- Kjeldsen SE, Hedner T, Jamerson K, Julius S, Haley WE, Zabalgoitia M, et al.Hypertension optimal treatment (HOT) study: home blood pressure in treated hypertensive subjects. Hypertension 1998;31(4):1014-20. [MEDLINE: ] [DOI] [PubMed] [Google Scholar]
- Kjeldsen SE, Kolloch RE, Leonetti G, Mallion JM, Zanchetti A, Elmfeldt D, et al.Influence of gender and age on preventing cardiovascular disease by antihypertensive treatment and acetylsalicylic acid. The HOT study. Hypertension Optimal Treatment. Journal of Hypertension 2000;18(5):629-42. [MEDLINE: ] [DOI] [PubMed] [Google Scholar]
- Kolloch RE, Rahn KH.The Hypertension Optimal Treatment (HOT) study: results of 12-month treatment related to age [Die 'Hyperyension Optimal Treatment' (HOT)-Studie: Behandlungsergebnisse nach Zwolfmonatiger Therapie in Abhangigkeit vom Alter]. Deutsche Medizinische Wochenschrift 1998;123(1-2):1-5. [MEDLINE: ] [DOI] [PubMed] [Google Scholar]
- Leonetti G, Zanchetti A.Principal results of hypertension optimal treatment (HOT) study and their clinical impact. HOT cooperative group. Clinical Hemorheology & Microcirculation 1999;21(3-4):217-24. [MEDLINE: ] [PubMed] [Google Scholar]
- Lithell H, Berglund L.Validation of an oscillometric blood pressure measuring device: a substudy of the HOT Study. Hypertension Optimal Treatment. Blood Pressure 1998;7(3):149-52. [MEDLINE: ] [DOI] [PubMed] [Google Scholar]
- Mancia G, Omboni S, Parati G, Clement DL, Haley WE, Rahman SN, et al.Twenty-four hour ambulatory blood pressure in the Hypertension Optimal Treatment (HOT) study. Journal of Hypertension 2001;19(10):1755-63. [MEDLINE: ] [DOI] [PubMed] [Google Scholar]
- Ruilope LM, Salvetti A, Jamerson K, Hansson L, Warnold I, Wedel H, et al.Renal function and intensive lowering of blood pressure in hypertensive participants of the hypertension optimal treatment (HOT) study. Journal of the American Society of Nephrology 2001;12(2):218-25. [MEDLINE: ] [DOI] [PubMed] [Google Scholar]
- Struijker-Boudier H, Safar M, Bortel L.Effects of individual risk factors on the incidence of cardiovascular event in the treated hypertensive patients of the Hypertension Optimal Treatment Study. Journal of Hypertension 2001;19(11):2105-6. [MEDLINE: ] [DOI] [PubMed] [Google Scholar]
- The Hypertension Optimal Treatment Study (the HOT Study). Blood Pressure 1993;2(1):62-8. [MEDLINE: ] [DOI] [PubMed] [Google Scholar]
- Waeber B, Leonetti G, Kolloch R, McInnes GT.Compliance with aspirin or placebo in the Hypertension Optimal Treatment (HOT) study. Journal of Hypertension 1999;17(7):1041-5. [MEDLINE: ] [DOI] [PubMed] [Google Scholar]
- Zanchetti A, Hansson L, Dahlof B, Elmfeldt D, Kjeldsen S, Kolloch R, et al.Effects of individual risk factors on the incidence of cardiovascular events in the treated hypertensive patients of the Hypertension Optimal Treatment Study. HOT Study Group. Journal of Hypertension 2001;19(6):1149-59. [MEDLINE: ] [DOI] [PubMed] [Google Scholar]
- Zanchetti A, Hansson L, Menard J, Leonetti G, Rahn KH, Warnold I, et al.Risk assessment and treatment benefit in intensively treated hypertensive patients of the hypertension Optimal Treatment (HOT) study. Journal of Hypertension 2001;19(4):819-25. [MEDLINE: ] [DOI] [PubMed] [Google Scholar]
IMPACT II 1997 {unpublished data only}
- Blankenship JC, Sigmon KN, Pieper KS, O'Shea C, Tardiff BE, Tcheng JE, et al.Effect of eptifibatide on angiographic complications during percutaneous coronary intervention in the IMPACT--(Integrilin to Minimize Platelet Aggregation and Coronary Thrombosis) II Trial. American Journal of Cardiology 2001;88(9):969-73. [MEDLINE: ] [DOI] [PubMed] [Google Scholar]
- Gilchrist IC, Gardner LH, Muhlestein JB, Arnold AM, Lincoff AM, Califf RM, et al.Effect of institutional volume and academic status on outcomes of coronary interventions: the IMPACT-II experience. American Heart Journal 1999;138(5 Pt 1):976-82. [MEDLINE: ] [DOI] [PubMed] [Google Scholar]
- Mandak JS, Blankenship JC, Gardner LH, Berkowitz SD, Aguirre FV, Sigmon KN, et al.Modifiable risk factors for vascular access site complications in the IMPACT II trial of angioplasty with versus without eptifibatide. Integrilin to Minimize Platelet Aggregation and Coronary Thrombosis. Journal of the American College of Cardiology 1998;31(7):1518-24. [MEDLINE: ] [DOI] [PubMed] [Google Scholar]
- Randomised placebo-controlled trial of effect of eptifibatide on complications of percutaneous coronary intervention: IMPACT-II. Integrilin to Minimise Platelet Aggregation and Coronary Thrombosis-II. Lancet 1997;349(9063):1422-8. [MEDLINE: ] [PubMed] [Google Scholar]
- Tardiff BE, Califf RM, Tcheng JE, Lincoff AM, Sigmon KN, Harrington RA, et al.Clinical outcomes after detection of elevated cardiac enzymes in patients undergoing percutaneous intervention. IMPACT-II Investigators. Integrilin (eptifibatide) to Minimize Platelet Aggregation and Coronary Thrombosis-II. Journal of the American College of Cardiology 1999;33(1):88-96. [MEDLINE: ] [DOI] [PubMed] [Google Scholar]
- Thel MC, Califf RM, Tardiff BE, Gardner LH, Sigmon KN, Lincoff AM, et al.Timing of and risk factors for myocardial ischemic events after percutaneous coronary intervention (IMPACT-II). Integrilin to Minimize Platelet Aggregation and Coronary Thrombosis. American Journal of Cardiology 2000;85(4):427-34. [MEDLINE: ] [DOI] [PubMed] [Google Scholar]
Jiao 2013 {published data only}
- Jiao XM, Jiao XJ, Zhang XG, Xu XP, Wu JX, Yao L, et al.Cilostazol reduces microalbuminuria in type 2 diabetic nephropathy. Chinese Medical Journal 2013;126(22):4395-6. [MEDLINE: ] [PubMed] [Google Scholar]
JPAD 2008 {published data only}
- Ogawa H, Nakayama M, Morimoto T, Uemura S, Kanauchi M, Doi N, et al.Low-dose aspirin for primary prevention of atherosclerotic events in patients with type 2 diabetes: a randomized controlled trial.[Erratum in: JAMA. 2009 May 13;301(18):1882], [Erratum in: JAMA. 2012 Nov 14;308(18):1861]. JAMA 2008;300(18):2134-41. [MEDLINE: ] [DOI] [PubMed] [Google Scholar]
- Okada S, Morimoto T, Ogawa H, Sakuma M, Soejima H, Nakayama M, et al.Effect of low-dose aspirin on primary prevention of cardiovascular events in Japanese diabetic patients at high risk. Circulation Journal 2013;77(12):3023-8. [MEDLINE: ] [DOI] [PubMed] [Google Scholar]
- Okada S, Morimoto T, Ogawa H, Sakuma M, Soejima H, Nakayama M, et al.Is long-term low-dose aspirin therapy associated with renal dysfunction in patients with type 2 diabetes? JPAD2 cohort study. PLoS ONE [Electronic Resource] 2016;11(1):e0147635. [MEDLINE: ] [DOI] [PMC free article] [PubMed] [Google Scholar]
- Okada S, Morimoto T, Ogawa H, Sakuma M, Soejima H, Ohtorii M, et al.Long-term use of low-dose aspirin develops proteinuria in patients with diabetes: A reanalysis of JPAD study [abstract no: 10863]. Circulation 2013;128(22 Suppl 1). [EMBASE: 71337722] [Google Scholar]
- Saito Y, Morimoto T, Ogawa H, Nakayama M, Uemura S, Doi N, et al.Low-dose aspirin therapy in patients with type 2 diabetes and reduced glomerular filtration rate: subanalysis from the JPAD trial. Diabetes Care 2011;34(2):280-5. [MEDLINE: ] [DOI] [PMC free article] [PubMed] [Google Scholar]
J‐PADD 2014 {published data only}
- Ohtake T, Sato M, Nakazawa R, Kondoh M, Kobayashi S.Effect of beraprost sodium (PGI2 analogue) on peripheral arterial disease (PAD) in patients on hemodialysis: result from a multicenter randomized prospective interventional study [abstract no: Su528]. NDT Plus 2010;3(Suppl 3):iii487. [EMBASE: 70484748] [Google Scholar]
- Ohtake T, Sato M, Nakazawa R, Kondoh M, Miyaji T, Moriya H, et al.Randomized pilot trial between prostaglandin I2 analog and anti-platelet drugs on peripheral arterial disease in hemodialysis patients. Therapeutic Apheresis & Dialysis 2014;18(1):1-8. [MEDLINE: ] [DOI] [PubMed] [Google Scholar]
Kaegi 1974 {published data only}
- Kaegi A, Pineo GF, Shimizu A, Trivedi H, Hirsh J, Gent M.Arteriovenous-shunt thrombosis. Prevention by sulfinpyrazone. New England Journal of Medicine 1974;290(6):304-6. [MEDLINE: ] [DOI] [PubMed] [Google Scholar]
- Kaegi A, Pineo GF, Shimizu A, Trivedi H, Hirsh J, Gent M.The role of sulfinpyrazone in the prevention of arterio-venous shunt thrombosis. Circulation 1975;52(3):497-9. [MEDLINE: ] [DOI] [PubMed] [Google Scholar]
Kamper 1997 {published data only}
- Kamper AM, Lins RL, Zachee P, Van Bergen S, Hosten S, Daelemans R.Safety of combining ticlopidine with nadroparin in the routine treatment of chronic hemodialysis patients. Nephron 1997;77(4):484-5. [MEDLINE: ] [DOI] [PubMed] [Google Scholar]
Kauffmann 1980 {published data only}
- Kauffmann HM, Adams MB, Hebert LA, Walczak PM.Platelet inhibitors in human renal homotransplantation: randomized comparison of aspirin versus dipyridamole. Transplantation Proceedings 1980;12(2):311-4. [MEDLINE: 6771905 ] [PubMed] [Google Scholar]
Kaufman 2003 {published and unpublished data}
- Chang JJ, Concato J, Wells CK, Crowley ST.Impact of adherence to clinical guidelines on mortality in hemodialysis patients [abstract no: SA-PO384]. Journal of the American Society of Nephrology 2004;15(Oct):386A. [CENTRAL: CN-00583156] [Google Scholar]
- Kaufman JS, O'Connor TZ, Zhang JH, Cronin RE, Fiore LD, Ganz MB, et al.Randomized controlled trial of clopidogrel plus aspirin to prevent hemodialysis access graft thrombosis. Journal of the American Society of Nephrology 2003;14(9):2313-21. [MEDLINE: ] [DOI] [PubMed] [Google Scholar]
- Kaufman JS, O’Connor TZ, Zhang JH, Cronin RE, Fiore LD, Ganz MB, et al.Combination aspirin plus clopidogrel in the prevention of hemodialysis access graft thrombosis [abstract no: A1495]. Journal of the American Society of Nephrology 2001;12(Program & Abstracts):291A. [DOI] [PubMed] [Google Scholar]
Khajehdehi 2002 {published data only}
- Khajehdehi P, Roozbeh J, Mostafavi H.A comparative randomized and placebo-controlled short-term trial of aspirin and dipyridamole for overt type-2 diabetic nephropathy. Scandinavian Journal of Urology & Nephrology 2002;36(2):145-8. [MEDLINE: ] [DOI] [PubMed] [Google Scholar]
Kobayashi 1980 {published data only}
- Kobayashi K, Maeda K, Koshikawa S, Kawaguchi Y, Shimizu N, Naito C.Antithrombotic therapy with ticlopidine in chronic renal failure patients on maintenance hemodialysis: a multicenter collaborative double blind study. Thrombosis Research 1980;20(2):255-61. [MEDLINE: ] [DOI] [PubMed] [Google Scholar]
Kontessis 1993 {published data only}
- Kontessis PS, Jones SL, Barrow SE, Stratton PD, Alessandrini P, De Cosmo S, et al.Effect of selective inhibition of thromboxane synthesis on renal function in diabetic nephropathy. Journal of Laboratory & Clinical Medicine 1993;121(3):415-23. [MEDLINE: ] [PubMed] [Google Scholar]
Kooistra 1994 {published data only}
- Kooistra MP, Es A, Marx JJ, Hertsig M, Struyvenberg A.Effects of low dose aspirin on thrombovascular accidents during treatment with rHuEpo: a multicentre, controlled, cross-over study [abstract]. Nephrology Dialysis Transplantation 1993;8:277. [CENTRAL: CN-00260772] [DOI] [PubMed] [Google Scholar]
- Kooistra MP, Es A, Marx JJ, Hertsig ML, Struyvenberg A.Low-dose aspirin does not prevent thrombovascular accidents in low-risk haemodialysis patients during treatment with recombinant human erythropoietin. Nephrology Dialysis Transplantation 1994;9(8):1115-20. [MEDLINE: ] [DOI] [PubMed] [Google Scholar]
Koyama 1990 {published data only}
- Koyama A, Narita M, Tojo S.Therapeutic effects of dipyridamole on primary glomerulonephritis [abstract]. In: 11th International Congress of Nephrology; 1990 Jul 15-20; Tokyo, Japan. 1990:12. [CENTRAL: CN-00446186]
Liang 2015 {published data only}
- Liang J, Wang Z, Shi D, Liu Y, Zhao Y, Han H, et al.High clopidogrel dose in patients with chronic kidney disease having clopidogrel resistance after percutaneous coronary intervention. Angiology 2015;66(4):319-25. [MEDLINE: ] [DOI] [PubMed] [Google Scholar]
Michie 1977 {published data only}
- Michie DD, Wombolt DG.Use of sulfinpyrazone to prevent thrombus formation in arteriovenous fistulas and bovine grafts of patients on chronic hemodialysis. Current Therapeutic Research - Clinical & Experimental 1977;22(1 II):196-204. [EMBASE: 8152617] [Google Scholar]
Middleton 1992 {published data only}
- Middleton DA, Deichsel G.The prophylaxis of thrombosis in new arteriovenous dialysis shunts in the arm by low-dose acetylsalicylic acid and dipyridamole. Boehringer Ingelheim. Internal report 1992.
Milutinovic 1993 {published data only}
- Milutinovic S, Gasparovic V, Milutinovic E, Buturovic-Ponikvar J.Ticlopidine improves dialysis clearance of solutes in uremic patients by reducing blood clotting in dialyser fibers. International Journal of Artificial Organs 1993;16(5):249-52. [MEDLINE: ] [PubMed] [Google Scholar]
Movchan 2001 {published data only}
- Movchan EA, Chuprova AV, Tov NL, Vol'vich NV.Desaggregation therapy of acute glomerulonephritis [Dezagregatsionnaia terapiia ostrogo glomerulonefrita]. Klinicheskaia Meditsina 2001;79(12):44-7. [MEDLINE: ] [PubMed] [Google Scholar]
Mozafar 2013 {published data only}
- Mozafar M, Samsami M, Sobhiyeh MR, Jabbehdari S, Fallah ZM.Effectiveness of aspirin on double lumen permanent catheter efficacy in ESRD. Nephrourology Monthly 2013;5(2):762-5. [MEDLINE: ] [DOI] [PMC free article] [PubMed] [Google Scholar]
Mozafar 2018 {published data only}
- Mozafar M, Alborzi M, Moradi A.Effects of clopidogrel on longevity of permanent double-lumen catheter patency in dialysis patients: A single-blind placebo-controlled clinical trial. Nephro-Urology Monthly 2018;10(2):e58135. [EMBASE: 622226387] [Google Scholar]
Nakamura 2001d {published data only}
- Nakamura T, Ushiyama C, Takahashi Y, Tanaka A, Shimada N, Ebihara I, et al.Effect of dilazep dihydrochloride on urinary albumin excretion in patients with autosomal dominant polycystic kidney disease. Nephron 2001;88(1):80-2. [MEDLINE: ] [DOI] [PubMed] [Google Scholar]
Nakamura 2002b {published data only}
- Nakamura T, Ushiyama C, Osada S, Ugai K, Takahashi Y, Tanaka A, et al.Effect of dilazep dihydrochloride on serum cardiac troponin T levels in hemodialysis patients. Kidney & Blood Pressure Research 2002;25(1):50-4. [MEDLINE: ] [DOI] [PubMed] [Google Scholar]
NCT01252056 {published data only}
- Guo X.A clinical study to evaluate the efficacy and safety of cilostazol and probucol in combination on patients with diabetic nephropathy. www.clinicaltrials.gov/show/nct01252056 (first received 2 December 2010).
Nyberg 1984 {published data only}
- Nyberg G, Larsson O, Westberg NG, Aurell M, Jagenburg R, Blohme G.A platelet aggregation inhibitor--ticlopidine--in diabetic nephropathy: a randomized double blind study. Clinical Nephrology 1984;21(3):184-7. [MEDLINE: ] [PubMed] [Google Scholar]
Ogawa 2008 {published data only}
- Ogawa S, Mori T, Nako K, Ishizuka T, Ito S.Reduced albuminuria with sarpogrelate is accompanied by a decrease in monocyte chemoattractant protein-1 levels in type 2 diabetes. Clinical Journal of the American Society of Nephrology: CJASN 2008;3(2):362-8. [MEDLINE: ] [DOI] [PMC free article] [PubMed] [Google Scholar]
OPT‐CKD 2018 {published data only}
- Han Y.Comparison of the pharmacodynamics and pharmacokinetics of ticagrelor versus clopidogrel in patients with chronic kidney disease and non-ST-elevation acute coronary syndromes (OPT-CKD trial) [abstract no: TCT-483]. Journal of the American College of Cardiology 2017;70(18 Suppl 1):B200. [EMBASE: 619771602] [Google Scholar]
- Wang H, Qi J, Li Y, Tang Y, Li C, Li J, et al.Pharmacodynamics and pharmacokinetics of ticagrelor vs. clopidogrel in patients with acute coronary syndromes and chronic kidney disease. British Journal of Clinical Pharmacology 2018;84(1):88-96. [MEDLINE: ] [DOI] [PMC free article] [PubMed] [Google Scholar]
Ota 1996 {published data only}
- Ota K, Teraoka S, Akiwasa T, Mimura N, Hirasawa Y, Sakai T, et al.Clinical efficacy of E5510 (satigrel), an antiplatelet agent, for preventing blood clotting in the extracorporeal circuit during hemodialysis (3) - A double-blind comparison with ticlopidine hydrochloride. Rinsho Hyoka 1996;24(1):9-38. [Google Scholar]
PEGASUS‐TIMI 54 2014 {published data only}
- Bonaca MP, Bhatt DL, Braunwald E, Cohen M, Steg PG, Storey RF, et al.Design and rationale for the Prevention of Cardiovascular Events in Patients With Prior Heart Attack Using Ticagrelor Compared to Placebo on a Background of Aspirin-Thrombolysis in Myocardial Infarction 54 (PEGASUS-TIMI 54) trial. American Heart Journal 2014;167(4):437-44. [MEDLINE: ] [DOI] [PubMed] [Google Scholar]
- Bonaca MP, Bhatt DL, Cohen M, Steg PG, Storey RF, Jensen EC, et al.Long-term use of ticagrelor in patients with prior myocardial infarction. New England Journal of Medicine 2015;372(19):1791-800. [MEDLINE: ] [DOI] [PubMed] [Google Scholar]
- Bonaca MP, Goto S, Bhatt DL, Steg PG, Storey RF, Cohen M, et al.Prevention of stroke with ticagrelor in patients with prior myocardial infarction: Insights from PEGASUS-TIMI 54 (Prevention of Cardiovascular Events in Patients With Prior Heart Attack Using Ticagrelor Compared to Placebo on a Background of Aspirin-Thrombolysis in Myocardial Infarction 54). Circulation 2016;134(12):861-71. [MEDLINE: ] [DOI] [PubMed] [Google Scholar]
- Dellborg M, Bonaca MP, Storey RF, Steg PG, Im KA, Cohen M, et al.Efficacy and safety with ticagrelor in patients with prior myocardial infarction in the approved European label: Insights from PEGASUS-TIMI 54 [abstract]. European Heart Journal 2017;38(Suppl 1):794-5. [EMBASE: 621235545] [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lozano I, Rondan J, Vegas JM, Segovia E.Cost-effectiveness of long-term ticagrelor in patients with prior myocardial infarction: analysis by subgroups. Journal of the American College of Cardiology 2018;71(1):107-8. [MEDLINE: ] [DOI] [PubMed] [Google Scholar]
- Magnani G, Sabatine MS, Bhatt DL, Choen M, Steg G, Storey R, et al.Efficacy and safety of ticagrelor for long-term secondary prevention of atherothrombotic events in relation to renal function: Insights from the PEGASUS-TIMI 54 trial [abstract]. European Heart Journal 2015;36(Suppl 1):520. [EMBASE: 72020712] [DOI] [PubMed] [Google Scholar]
- Magnani G, Storey RF, Steg G, Bhatt DL, Cohen M, Kuder J, et al.Efficacy and safety of ticagrelor for long-term secondary prevention of atherothrombotic events in relation to renal function: insights from the PEGASUS-TIMI 54 trial. European Heart Journal 2016;37(4):400-8. [MEDLINE: ] [DOI] [PubMed] [Google Scholar]
- Magnuson EA, Bonaca MP, Bhatt DL, Cohen M, Steg PG, Storey RF, et al.Reply: cost-effectiveness of long-term ticagrelor in patients with prior myocardial infarction: analysis by subgroups. Journal of the American College of Cardiology 2018;71(1):108. [MEDLINE: ] [DOI] [PubMed] [Google Scholar]
- Magnuson EA, Li H, Wang K, Vilain K, Shafiq A, Bonaca MP, et al.Cost-effectiveness of long-term ticagrelor in patients with prior myocardial infarction: results from the PEGASUS-TIMI 54 trial. Journal of the American College of Cardiology 2017;70(5):527-38. [MEDLINE: ] [DOI] [PubMed] [Google Scholar]
- Murphy SA, Bonaca MP, Goto S, Bhatt DL, Steg PG, Storey RF, et al.Reduction in total cardiovascular events with long-term use of ticagrelor in patients with prior myocardial infarction in the PEGASUS-TIMI 54 trial [abstract no: A17121]. Circulation 2015;132(Suppl 3). [EMBASE: 72179983] [Google Scholar]
PIANO‐2 CKD 2011 {published data only}
- Kim W, Kang WY, Woo JS.Objectives: The purpose of this study was to determine the functional impact of cilostazol in patients with chronic kidney disease (CKD) undergoing hemodialysis [abstract no: TCT-497]. Journal of the American College of Cardiology 2011;58(20 Suppl 1):B135. [EMBASE: 70581866] [Google Scholar]
- Woo JS, Kim W, Ha SJ, Jeong KH, Lee TW, Ihm CG, et al.A comparison of clopidogrel responsiveness in patients with chronic renal failure: results of the adjunctive cilostazol versus high maintenance dose clopidogrel (PIANO) study [abstract no: AS-252]. American Journal of Cardiology 2011;107(8 Suppl 1):100-1A. [EMBASE: 70401953] [Google Scholar]
- Woo JS, Kim W, Lee SR, Jung KH, Kim WS, Lew JH, et al.Platelet reactivity in patients with chronic kidney disease receiving adjunctive cilostazol compared with a high-maintenance dose of clopidogrel: results of the effect of platelet inhibition according to clopidogrel dose in patients with chronic kidney disease (PIANO-2 CKD) randomized study. American Heart Journal 2011;162(6):1018-25. [MEDLINE: ] [DOI] [PubMed] [Google Scholar]
PIANO‐3 2015 {published data only}
- Jeong KH, Cho JH, Woo JS, Kim JB, Kim WS, Lee TW, et al.Platelet reactivity after receiving clopidogrel compared with ticagrelor in patients with kidney failure treated with hemodialysis: a randomized crossover study. American Journal of Kidney Diseases 2015;65(6):916-24. [MEDLINE: ] [DOI] [PubMed] [Google Scholar]
- Jeong KH, Lee TW, Kim JS, Lee SY, Lee SH, Moon JY, et al.Platelet reactivity in patients with end stage renal disease receiving clopidogrel compared with ticagrelor: a randomized crossover study [abstract no: FP610]. Nephrology Dialysis Transplantation 2015;30(Suppl 3):iii276. [EMBASE: 72207028] [Google Scholar]
- Woo JS, Kim W, Kim HS, Hwang SJ, Kim JB, Kim SJ, et al.Randomized assessment of the onset and offset of the antiplatelet effects of ticagrelor versus clopidogrel in patients with chronic kidney disease performing hemodialysis [abstract]. European Heart Journal 2014;35(Suppl 1):7-8. [EMBASE: 71646762] [Google Scholar]
PIANO‐6 2017 {published data only}
- Kim JS, Lee TW, Ihm C, Lee SH, Kim SY, Lee SY, et al.Relationship of ticagrelor dose and platelet reactivity in patients with end stage renal disease on hemodialysis [abstract no: FR-PO727]. Journal of the American Society of Nephrology 2015;26(Abstract Suppl):529A. [Google Scholar]
- Kim JS, Woo JS, Kim JB, Kim WS, Lee TW, Kim KS, et al.The pharmacodynamics of low and standard doses of ticagrelor in patients with end stage renal disease on hemodialysis. International Journal of Cardiology 2017;238:110-6. [MEDLINE: ] [DOI] [PubMed] [Google Scholar]
- Kim W, Woo JS, Kim WS, Kim JB, Kim HO, Kim JM.Pharmacodynamics of low and standard doses of ticagrelor in patients with end stage renal disease on hemodialysis [abstract]. European Heart Journal 2016;37(Suppl 1):636. [EMBASE: 612284362] [Google Scholar]
Pierucci 1989 {published data only}
- Pierucci A, Simonetti BM, Pecci G, Feriozzi S, Mavrikakis G, Cinotti GA, et al.Low dose aspirin in patients with lupus nephritis [abstract]. Kidney International 1988;33(1):281. [CENTRAL: CN-00583819] [Google Scholar]
- Pierucci A, Simonetti BM, Pecci G, Mavrikakis G, Feriozzi S, Cinotti GA, et al.Acute effects of a thromboxane receptor antagonist on renal function in patients with lupus nephritis [abstract]. Kidney International 1987;31(1):283. [CENTRAL: CN-00724956] [Google Scholar]
- Pierucci A, Simonetti BM, Pecci G, Mavrikakis G, Feriozzi S, Cinotti GA, et al.Improvement of renal function with selective thromboxane antagonism in lupus nephritis. New England Journal of Medicine 1989;320(7):421-5. [MEDLINE: ] [DOI] [PubMed] [Google Scholar]
PLATO 2009 {published data only}
- Akerblom A, Wallentin L, Larsson A, Siegbahn A, Becker RC, Budaj A, et al.Cystatin C- and creatinine-based estimates of renal function and their value for risk prediction in patients with acute coronary syndrome: results from the PLATelet Inhibition and Patient Outcomes (PLATO) study. Clinical Chemistry 2013;59(9):1369-75. [MEDLINE: ] [DOI] [PubMed] [Google Scholar]
- Akerblom A, Wallentin L, Siegbahn A, Becker RC, Budaj A, Horrow J, et al.Outcome and causes of renal deterioration evaluated by serial cystatin C measurements in acute coronary syndrome patients -- results from the PLATelet inhibition and patient Outcomes (PLATO) study. American Heart Journal 2012;164(5):728-34. [MEDLINE: ] [DOI] [PubMed] [Google Scholar]
- Andell P, James SK, Cannon CP, Cyr DD, Himmelmann A, Husted S, et al.Ticagrelor versus clopidogrel in patients with acute coronary syndromes and chronic obstructive pulmonary disease: an analysis from the Platelet Inhibition and Patient Outcomes (PLATO) trial. Journal of the American Heart Association 2015;4(10):e002490. [MEDLINE: ] [DOI] [PMC free article] [PubMed] [Google Scholar]
- Armstrong PW, Siha H, Fu Y, Westerhout CM, Steg PG, James SK, et al.ST-elevation acute coronary syndromes in the Platelet Inhibition and Patient Outcomes (PLATO) trial: insights from the ECG substudy. Circulation 2012;125(3):514-21. [MEDLINE: ] [DOI] [PubMed] [Google Scholar]
- Armstrong PW, Westerhout CM, Fu Y, Harrington RA, Storey RF, Katus H, et al.Quantitative ST-depression in acute coronary syndromes: the PLATO electrocardiographic substudy. American Journal of Medicine 2013;126(8):723-9. [MEDLINE: ] [DOI] [PubMed] [Google Scholar]
- Becker RC, Bassand JP, Budaj A, Wojdyla DM, James SK, Cornel JH, et al.Bleeding complications with the P2Y12 receptor antagonists clopidogrel and ticagrelor in the PLATelet inhibition and patient Outcomes (PLATO) trial [Erratum in: Eur Heart J. 2012 Nov;33(21):2750]. European Heart Journal 2011;32(23):2933-44. [MEDLINE: ] [DOI] [PubMed] [Google Scholar]
- Bellavia A, Wallentin L, Orsini N, James SK, Cannon CP, Himmelmann A, et al.Time-based measures of treatment effect: reassessment of ticagrelor and clopidogrel from the PLATO trial. Open Heart 2017;4(2):e000557. [MEDLINE: ] [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brilakis ES, Held C, Meier B, Cools F, Claeys MJ, Cornel JH, et al.Effect of ticagrelor on the outcomes of patients with prior coronary artery bypass graft surgery: insights from the PLATelet inhibition and patient outcomes (PLATO) trial. American Heart Journal 2013;166(3):474-80. [MEDLINE: ] [DOI] [PubMed] [Google Scholar]
- Bui AH, Cannon CP, Steg PG, Storey RF, Husted S, Guo J, et al.Relationship between early and late nonsustained ventricular tachycardia and cardiovascular death in patients with acute coronary syndrome in the Platelet Inhibition and Patient Outcomes (PLATO) trial. Circulation: Arrhythmia and Electrophysiology 2016;9(2):e002951. [MEDLINE: ] [DOI] [PubMed] [Google Scholar]
- Capodanno D, Calvi V, Tamburino C.Effect size of ticagrelor over clopidogrel in the Platelet Inhibition and Patient Outcomes (PLATO) trial: from statistics to clinical judgment. Journal of Cardiovascular Medicine 2012;13(2):162-3. [MEDLINE: ] [DOI] [PubMed] [Google Scholar]
- Cornel JH, Becker RC, Goodman SG, Husted S, Katus H, Santoso A, et al.Prior smoking status, clinical outcomes, and the comparison of ticagrelor with clopidogrel in acute coronary syndromes-insights from the PLATelet inhibition and patient Outcomes (PLATO) trial. American Heart Journal 2012;164(3):334-42. [MEDLINE: ] [DOI] [PubMed] [Google Scholar]
- Cowper PA, Pan W, Anstrom KJ, Kaul P, Wallentin L, Davidson-Ray L, et al.Economic analysis of ticagrelor therapy from a U.S. perspective: results from the PLATO study. Journal of the American College of Cardiology 2015;65(5):465-76. [MEDLINE: ] [DOI] [PubMed] [Google Scholar]
- DiNicolantonio JJ, D'Ascenzo F, Tomek A, Chatterjee S, Niazi AK, Biondi-Zoccai G.Clopidogrel is safer than ticagrelor in regard to bleeds: a closer look at the PLATO trial. International Journal of Cardiology 2013;168(3):1739-44. [MEDLINE: ] [DOI] [PubMed] [Google Scholar]
- DiNicolantonio JJ, Tomek A.Inactivations, deletions, non-adjudications, and downgrades of clinical endpoints on ticagrelor: serious concerns over the reliability of the PLATO trial. International Journal of Cardiology 2013;168(4):4076-80. [MEDLINE: ] [DOI] [PubMed] [Google Scholar]
- DiNicolantonio JJ, Tomek A.Misrepresentation of vital status follow-up: challenging the integrity of the PLATO trial and the claimed mortality benefit of ticagrelor versus clopidogrel. International Journal of Cardiology 2013;169(2):145-6. [MEDLINE: ] [DOI] [PubMed] [Google Scholar]
- Ducrocq G, Schulte PJ, Becker RC, Cannon CP, Harrington RA, Held C, et al.Association of spontaneous and procedure-related bleeds with short- and long-term mortality after acute coronary syndromes: an analysis from the PLATO trial. Eurointervention 2015;11(7):737-45. [MEDLINE: ] [DOI] [PubMed] [Google Scholar]
- Franchi F, James SK, Ghukasyan LT, Budaj AJ, Cornel JH, Katus HA, et al.Impact of diabetes mellitus and chronic kidney disease on cardiovascular outcomes and platelet P2Y12 receptor antagonist effects in patients with acute coronary syndromes: insights from the PLATO trial. Journal of the American Heart Association 2019;8(6):e011139. [MEDLINE: ] [DOI] [PMC free article] [PubMed] [Google Scholar]
- Giannitsis E, Wallentin L, James SK, Bertilsson M, Siegbahn A, Storey RF, et al.Outcomes after planned invasive or conservative treatment strategy in patients with non-ST-elevation acute coronary syndrome and a normal value of high sensitivity troponin at randomisation: a Platelet Inhibition and Patient Outcomes (PLATO) trial biomarker substudy. European Heart Journal: Acute Cardiovascular Care 2017;6(6):500-10. [MEDLINE: ] [DOI] [PubMed] [Google Scholar]
- Hagstrom E, James SK, Bertilsson M, Becker RC, Himmelmann A, Husted S, et al.Growth differentiation factor-15 level predicts major bleeding and cardiovascular events in patients with acute coronary syndromes: results from the PLATO study. European Heart Journal 2016;37(16):1325-33. [MEDLINE: ] [DOI] [PubMed] [Google Scholar]
- Held C, Asenblad N, Bassand JP, Becker RC, Cannon CP, Claeys MJ, et al.Ticagrelor versus clopidogrel in patients with acute coronary syndromes undergoing coronary artery bypass surgery: results from the PLATO (Platelet Inhibition and Patient Outcomes) trial. Journal of the American College of Cardiology 2011;57(6):672-84. [MEDLINE: ] [DOI] [PubMed] [Google Scholar]
- Husted S, James S, Becker RC, Horrow J, Katus H, Storey RF, et al.Ticagrelor versus clopidogrel in elderly patients with acute coronary syndromes: a substudy from the prospective randomized PLATelet inhibition and patient Outcomes (PLATO) trial. Circulation. Cardiovascular Quality & Outcomes 2012;5(5):680-8. [MEDLINE: ] [DOI] [PubMed] [Google Scholar]
- Husted S, James SK, Bach RG, Becker RC, Budaj A, Heras M, et al.The efficacy of ticagrelor is maintained in women with acute coronary syndromes participating in the prospective, randomized, PLATelet inhibition and patient Outcomes (PLATO) trial. European Heart Journal 2014;35(23):1541-50. [MEDLINE: ] [DOI] [PMC free article] [PubMed] [Google Scholar]
- James S, Akerblom A, Cannon CP, Emanuelsson H, Husted S, Katus H, et al.Comparison of ticagrelor, the first reversible oral P2Y(12) receptor antagonist, with clopidogrel in patients with acute coronary syndromes: Rationale, design, and baseline characteristics of the PLATelet inhibition and patient Outcomes (PLATO) trial. American Heart Journal 2009;157(4):599-605. [MEDLINE: ] [DOI] [PubMed] [Google Scholar]
- James S, Angiolillo DJ, Cornel JH, Erlinge D, Husted S, Kontny F, et al.Ticagrelor vs. clopidogrel in patients with acute coronary syndromes and diabetes: a substudy from the PLATelet inhibition and patient Outcomes (PLATO) trial. European Heart Journal 2010;31(24):3006-16. [MEDLINE: ] [DOI] [PMC free article] [PubMed] [Google Scholar]
- James S, Budaj A, Aylward P, Buck KK, Cannon CP, Cornel JH, et al.Ticagrelor versus clopidogrel in acute coronary syndromes in relation to renal function: results from the Platelet Inhibition and Patient Outcomes (PLATO) trial. Circulation 2010;122(11):1056-67. [MEDLINE: ] [DOI] [PubMed] [Google Scholar]
- James S, Budaj A, Aylward P, Buck KK, Cannon CP, Cornel JH, et al.Ticagrelor versus clopidogrel in acute coronary syndromes in relation to renal function: results from the Platelet Inhibition and Patient Outcomes (PLATO) Trial. Circulation 2010;122(11):1056-67. [MEDLINE: ] [DOI] [PubMed] [Google Scholar]
- James SK, Roe MT, Cannon CP, Cornel JH, Horrow J, Husted S, et al.Ticagrelor versus clopidogrel in patients with acute coronary syndromes intended for non-invasive management: substudy from prospective randomised PLATelet inhibition and patient Outcomes (PLATO) trial. BMJ 2011;342:d3527. [MEDLINE: ] [DOI] [PMC free article] [PubMed] [Google Scholar]
- Johansson A, Eriksson N, Becker RC, Storey RF, Himmelmann A, Hagstrom E, et al.NLRC4 inflammasome is an important regulator of interleukin-18 levels in patients with acute coronary syndromes: genome-wide association study in the PLATelet inhibition and patient Outcomes Trial (PLATO). Circulation. Cardiovascular Genetics 2015;8(3):498-506. [MEDLINE: ] [DOI] [PubMed] [Google Scholar]
- Kang HJ, Clare RM, Gao R, Held C, Himmelmann A, James SK, et al.Ticagrelor versus clopidogrel in Asian patients with acute coronary syndrome: A retrospective analysis from the Platelet Inhibition and Patient Outcomes (PLATO) Trial. American Heart Journal 2015;169(6):899-905. [MEDLINE: ] [DOI] [PubMed] [Google Scholar]
- Kholaif N, Zheng Y, Jagasia P, Himmelmann A, James SK, Steg PG, et al.Baseline Q waves and time from symptom onset to ST-segment elevation myocardial infarction: insights from PLATO on the influence of sex. American Journal of Medicine 2015;128(8):914-9. [MEDLINE: ] [DOI] [PubMed] [Google Scholar]
- Kohli P, Wallentin L, Reyes E, Horrow J, Husted S, Angiolillo DJ, et al.Reduction in first and recurrent cardiovascular events with ticagrelor compared with clopidogrel in the PLATO Study. Circulation 2013;127(6):673-80. [MEDLINE: ] [DOI] [PubMed] [Google Scholar]
- Kontny F, Ueland T, Aukrust P, Michelsen AE, Becker RC, Bertilsson M, et al.Pentraxin 3 (PTX3) predict adverse outcome in acute coronary syndromes-a PLATO biomarker substudy [abstract]. European Heart Journal 2014;35(Suppl 1):319. [EMBASE: 71647923] [Google Scholar]
- Kotsia A, Brilakis ES, Held C, Cannon C, Steg GP, Meier B, et al.Extent of coronary artery disease and outcomes after ticagrelor administration in patients with an acute coronary syndrome: Insights from the PLATelet inhibition and patient Outcomes (PLATO) trial. American Heart Journal 2014;168(1):68-75. [MEDLINE: ] [DOI] [PubMed] [Google Scholar]
- Kunadian V, James SK, Wojdyla DM, Zorkun C, Wu J, Storey RF, et al.Angiographic outcomes in the PLATO Trial (Platelet Inhibition and Patient Outcomes). Jacc: Cardiovascular Interventions 2013;6(7):671-83. [MEDLINE: ] [DOI] [PubMed] [Google Scholar]
- Levin LA, Wallentin L, Bernfort L, Andersson D, Storey RF, Bergstrom G, et al.Health-related quality of life of ticagrelor versus clopidogrel in patients with acute coronary syndromes-results from the PLATO trial. Value in Health 2013;16(4):574-80. [MEDLINE: ] [DOI] [PubMed] [Google Scholar]
- Lindholm D, Varenhorst C, Cannon CP, Harrington RA, Himmelmann A, Maya J, et al.Ticagrelor vs. clopidogrel in patients with non-ST-elevation acute coronary syndrome with or without revascularization: results from the PLATO trial. European Heart Journal 2014;35(31):2083-93. [MEDLINE: ] [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mahaffey KW, Held C, Wojdyla DM, James SK, Katus HA, Husted S, et al.Ticagrelor effects on myocardial infarction and the impact of event adjudication in the PLATO (Platelet Inhibition and Patient Outcomes) trial. Journal of the American College of Cardiology 2014;63(15):1493-9. [MEDLINE: ] [DOI] [PubMed] [Google Scholar]
- Mahaffey KW, Wojdyla DM, Carroll K, Becker RC, Storey RF, Angiolillo DJ, et al.Ticagrelor compared with clopidogrel by geographic region in the Platelet Inhibition and Patient Outcomes (PLATO) trial. Circulation 2011;124(5):544-54. [MEDLINE: ] [DOI] [PubMed] [Google Scholar]
- Nikolic E, Janzon M, Hauch O, Wallentin L, Henriksson M, PLATO Health Economic Substudy Group.Cost-effectiveness of treating acute coronary syndrome patients with ticagrelor for 12 months: results from the PLATO study. European Heart Journal 2013;34(3):220-8. [MEDLINE: ] [DOI] [PubMed] [Google Scholar]
- Ohman EM, Roe MT.Explaining the unexpected: insights from the PLATelet inhibition and clinical Outcomes (PLATO) trial comparing ticagrelor and clopidogrel. Editorial on Serebruany "Viewpoint: Paradoxical excess mortality in the PLATO trial should be independently verified" (Thromb Haemost 2011; 105.5). Thrombosis & Haemostasis 2011;105(5):763-5. [MEDLINE: ] [DOI] [PubMed] [Google Scholar]
- Parker WA, Eriksson N, Becker RC, Voora D, Akerblom A, Himmelmann A, et al.Equilibrative nucleoside transporter 1 gene polymorphisms and clinical outcomes following acute coronary syndromes: findings from the PLATelet inhibition and patient Outcomes (PLATO) study. Platelets 2019;30(5):579-88. [MEDLINE: ] [DOI] [PubMed] [Google Scholar]
- Patel MR, Becker RC, Wojdyla DM, Emanuelsson H, Hiatt WR, Horrow J, et al.Cardiovascular events in acute coronary syndrome patients with peripheral arterial disease treated with ticagrelor compared with clopidogrel: Data from the PLATO Trial. European Journal of Preventive Cardiology 2015;22(6):734-42. [MEDLINE: ] [DOI] [PubMed] [Google Scholar]
- Pollack CV Jr, Davoudi F, Diercks DB, Becker RC, James SK, Lim ST, et al.Relative efficacy and safety of ticagelor vs clopidogrel as a function of time to invasive management in non-ST-segment elevation acute coronary syndrome in the PLATO trial. Clinical Cardiology 2017;40(6):390-8. [MEDLINE: ] [DOI] [PMC free article] [PubMed] [Google Scholar]
- Roguin A, Musallam A.Letter by Roguin and Musallam regarding article, "Stent thrombosis with ticagrelor versus clopidogrel in patients with acute coronary syndromes: an analysis from the prospective, randomized PLATO trial". Circulation 2014;129(19):e493. [MEDLINE: ] [DOI] [PubMed] [Google Scholar]
- Scirica BM, Bansilal S, Davoudi F, Armstrong PW, Clare RM, Schulte PJ, et al.Safety of ticagrelor in patients with baseline conduction abnormalities: A PLATO (Study of Platelet Inhibition and Patient Outcomes) analysis. American Heart Journal 2018;202:54-60. [MEDLINE: ] [DOI] [PubMed] [Google Scholar]
- Scirica BM, Cannon CP, Emanuelsson H, Michelson EL, Harrington RA, Husted S, et al.The incidence of bradyarrhythmias and clinical bradyarrhythmic events in patients with acute coronary syndromes treated with ticagrelor or clopidogrel in the PLATO (Platelet Inhibition and Patient Outcomes) trial: results of the continuous electrocardiographic assessment substudy. Journal of the American College of Cardiology 2011;57(19):1908-16. [MEDLINE: ] [DOI] [PubMed] [Google Scholar]
- Serebruany VL, Fortmann SD, Cherepanov V, Litvinov O, Kim MH, Marciniak TA.Excess ticagrelor mortality in the food and drug administration adverse event reporting system: time to recount PLATO trial deaths. American Journal of Medicine 2017;130(6):e245-6. [MEDLINE: ] [DOI] [PubMed] [Google Scholar]
- Serebruany VL.Angiographic outcomes contradict platelet data in the PLATO trial: confusion over official trial substudies. Cardiology 2014;127(3):190-5. [MEDLINE: ] [DOI] [PubMed] [Google Scholar]
- Serebruany VL.Discrepancies in the primary PLATO trial publication and the FDA reviews. International Journal of Cardiology 2014;172(1):8-10. [MEDLINE: ] [DOI] [PubMed] [Google Scholar]
- Serebruany VL.Peripheral vascular outcomes in the PLATO trial: update from the FDA ticagrelor complete response review. American Journal of Therapeutics 2012;19(2):160-1. [MEDLINE: ] [DOI] [PubMed] [Google Scholar]
- Serebruany VL.The FDA outlook of events reporting after ticagrelor or clopidogrel in the PLATO Trial: impact of sponsor censoring dates, drug discontinuation, and withdrawal of consent. Cardiology 2011;120(3):169-71. [MEDLINE: ] [DOI] [PubMed] [Google Scholar]
- Shimada YJ, Bansilal S, Wiviott SD, Becker RC, Harrington RA, Himmelmann A, et al.Impact of glycoprotein IIb/IIIa inhibitors on the efficacy and safety of ticagrelor compared with clopidogrel in patients with acute coronary syndromes: analysis from the Platelet Inhibition and Patient Outcomes (PLATO) Trial. American Heart Journal 2016;177:1-8. [MEDLINE: ] [DOI] [PubMed] [Google Scholar]
- Siha H, Das D, Fu Y, Zheng Y, Westerhout CM, Storey RF, et al.Baseline Q waves as a prognostic modulator in patients with ST-segment elevation: insights from the PLATO trial. CMAJ Canadian Medical Association Journal 2012;184(10):1135-42. [MEDLINE: ] [DOI] [PMC free article] [PubMed] [Google Scholar]
- Steg PG, Harrington RA, Emanuelsson H, Katus HA, Mahaffey KW, Meier B, et al.Response to letter regarding article, "Stent thrombosis with ticagrelor versus clopidogrel in patients with acute coronary syndromes: an analysis from the prospective, randomized PLATO trial". Circulation 2014;129(19):e494-5. [MEDLINE: ] [DOI] [PubMed] [Google Scholar]
- Steg PG, Harrington RA, Emanuelsson H, Katus HA, Mahaffey KW, Meier B, et al.Stent thrombosis with ticagrelor versus clopidogrel in patients with acute coronary syndromes: an analysis from the prospective, randomized PLATO trial. Circulation 2013;128(10):1055-65. [MEDLINE: ] [DOI] [PubMed] [Google Scholar]
- Steg PG, James S, Harrington RA, Ardissino D, Becker RC, Cannon CP, et al.Ticagrelor versus clopidogrel in patients with ST-elevation acute coronary syndromes intended for reperfusion with primary percutaneous coronary intervention: a Platelet Inhibition and Patient Outcomes (PLATO) trial subgroup analysis. Circulation 2010;122(21):2131-41. [MEDLINE: ] [DOI] [PubMed] [Google Scholar]
- Storey RF, Angiolillo DJ, Patil SB, Desai B, Ecob R, Husted S, et al.Inhibitory effects of ticagrelor compared with clopidogrel on platelet function in patients with acute coronary syndromes: the PLATO (PLATelet inhibition and patient Outcomes) PLATELET substudy. Journal of the American College of Cardiology 2010;56(18):1456-62. [MEDLINE: ] [DOI] [PubMed] [Google Scholar]
- Storey RF, Ardissino D, Vignali L, Cairns R, Becker RC, Cannon CP, et al.Ischaemic events and stent thrombosis following planned discontinuation of study treatment with ticagrelor or clopidogrel in the PLATO study. Thrombosis & Haemostasis 2018;118(2):427-9. [MEDLINE: ] [DOI] [PubMed] [Google Scholar]
- Storey RF, Becker RC, Harrington RA, Husted S, James SK, Cools F, et al.Characterization of dyspnoea in PLATO study patients treated with ticagrelor or clopidogrel and its association with clinical outcomes. European Heart Journal 2011;32(23):2945-53. [MEDLINE: ] [DOI] [PubMed] [Google Scholar]
- Storey RF, Becker RC, Harrington RA, Husted S, James SK, Cools F, et al.Pulmonary function in patients with acute coronary syndrome treated with ticagrelor or clopidogrel (from the Platelet Inhibition and Patient Outcomes [PLATO] pulmonary function substudy). American Journal of Cardiology 2011;108(11):1542-6. [MEDLINE: ] [DOI] [PubMed] [Google Scholar]
- Storey RF, James SK, Siegbahn A, Varenhorst C, Held C, Ycas J, et al.Lower mortality following pulmonary adverse events and sepsis with ticagrelor compared to clopidogrel in the PLATO study. Platelets 2014;25(7):517-25. [MEDLINE: ] [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sumaya W, Wallentin L, James SK, Siegbahn A, Gabrysch K, Bertilsson M, et al.Fibrin clot properties independently predict adverse clinical outcome following acute coronary syndrome: a PLATO substudy. European Heart Journal 2018;39(13):1078-85. [MEDLINE: ] [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ueland T, Akerblom A, Ghukasyan T, Michelsen AE, Aukrust P, Becker RC, et al.Osteoprotegerin is associated with major bleeding but not with cardiovascular outcomes in patients with acute coronary syndromes: insights from the PLATO (Platelet Inhibition and Patient Outcomes) trial. Journal of the American Heart Association 2018;7(2):e007009. [MEDLINE: ] [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ueland T, Michelsen A, Aukrust P, Becker R, Bertilsson M, James SK, et al.Chemokine (CXC motif) ligand 16 (CXCL16) and osteoprotegerin (OPG) as predictors of outcome in patients with acute coronary syndromes (ACS) a PLATO biomarker substudy [abstract]. European Heart Journal 2014;35(Suppl 1):486. [EMBASE: 71648554] [Google Scholar]
- Wallentin L, Becker RC, Budaj A, Cannon CP, Emanuelsson H, Held C, et al.Ticagrelor versus clopidogrel in patients with acute coronary syndromes. New England Journal of Medicine 2009;361(11):1045-57. [MEDLINE: ] [DOI] [PubMed] [Google Scholar]
- Wallentin L, Becker RC, Cannon CP, Held C, Himmelmann A, Husted S, et al.No misrepresentation of vital status follow-up in PLATO: predefined analyses guarantee the integrity of the benefits of ticagrelor over clopidogrel in the PLATO trial: Commentary on: DiNicolantonio JJ, Tomek A, Misrepresentation of vital status follow-up: challenging the integrity of the PLATO trial and the claimed mortality benefit of ticagrelor versus clopidogrel, International Journal of Cardiology, 2013 Serebruany VL. Discrepancies in the primary PLATO trial publication and the FDA reviews, International Journal of Cardiology, 2014. International Journal of Cardiology 2014;176(1):300-2. [MEDLINE: ] [DOI] [PubMed] [Google Scholar]
- Wallentin L, Becker RC, Cannon CP, Held C, Himmelmann A, Husted S, et al.Review of the accumulated PLATO documentation supports reliable and consistent superiority of ticagrelor over clopidogrel in patients with acute coronary syndrome: Commentary on: DiNicolantonio JJ, Tomek A, Inactivations, deletions, non-adjudications, and downgrades of clinical endpoints on ticagrelor: serious concerns over the reliability of the PLATO trial, International Journal of Cardiology, 2013. International Journal of Cardiology 2014;170(3):e59-62. [MEDLINE: ] [DOI] [PubMed] [Google Scholar]
- Wallentin L, Becker RC, James SK, Harrington RA.The PLATO trial reveals further opportunities to improve outcomes in patients with acute coronary syndrome. Editorial on Serebruany. "Viewpoint: Paradoxical excess mortality in the PLATO trial should be independently verified" (Thromb Haemost 2011; 105.5). Thrombosis & Haemostasis 2011;105(5):760-2. [MEDLINE: ] [DOI] [PubMed] [Google Scholar]
- Wallentin L, James S, Storey RF, Armstrong M, Barratt BJ, Horrow J, et al.Effect of CYP2C19 and ABCB1 single nucleotide polymorphisms on outcomes of treatment with ticagrelor versus clopidogrel for acute coronary syndromes: a genetic substudy of the PLATO trial. Lancet 2010;376(9749):1320-8. [MEDLINE: ] [DOI] [PubMed] [Google Scholar]
- Wallentin L, Lindholm D, Siegbahn A, Wernroth L, Becker RC, Cannon CP, et al.Biomarkers in relation to the effects of ticagrelor in comparison with clopidogrel in non-ST-elevation acute coronary syndrome patients managed with or without in-hospital revascularization: a substudy from the Prospective Randomized Platelet Inhibition and Patient Outcomes (PLATO) trial. Circulation 2014;129(3):293-303. [MEDLINE: ] [DOI] [PubMed] [Google Scholar]
PREDIAN 2011 {published data only}
- Navarro-Gonzalez JF, Sanchez-Nino MD, Donate-Correa J, Martin-Nunez E, Ferri C, Perez-Delgado N, et al.Effects of pentoxifylline on soluble klotho concentrations and renal tubular cell expression in diabetic kidney disease. Diabetes Care 2018;41(8):1817-20. [MEDLINE: ] [DOI] [PubMed] [Google Scholar]
PRISM‐PLUS 1998 {published data only (unpublished sought but not used)}
- Huynh T, Nasmith J, Luong TM, Bernier M, Pharand C, Xue-Qiao Z, et al.Complementary prognostic values of ST segment deviation and Thrombolysis In Myocardial Infarction (TIMI) risk score in non-ST elevation acute coronary syndromes: insights from the Platelet Receptor Inhibition in Ischemic Syndrome Management in Patients Limited by Unstable Signs and Symptoms (PRISM-PLUS) study. Canadian Journal of Cardiology 2009 Dec;25(12):e417-21. [MEDLINE: ] [DOI] [PMC free article] [PubMed] [Google Scholar]
- Huynh T, Piazza N, DiBattiste PM, Snapinn SM, Wan Y, Pharand C, et al.Analysis of bleeding complications associated with glycoprotein IIb/IIIa receptors blockade in patients with high-risk acute coronary syndromes: insights from the PRISM-PLUS study. International Journal of Cardiology 2005;100(1):73-8. [MEDLINE: ] [DOI] [PubMed] [Google Scholar]
- Huynh T, Theroux P, Snapinn S, Wan Y, PRISM-PLUS Investigators.Effect of platelet glycoprotein IIb/IIIa receptor blockade with tirofiban on adverse cardiac events in women with unstable angina/non-ST-elevation myocardial infarction (PRISM-PLUS Study). American Heart Journal 2003;146(4):668-73. [MEDLINE: ] [DOI] [PubMed] [Google Scholar]
- Januzzi JL Jr, Snapinn SM, DiBattiste PM, Jang IK, Theroux P.Benefits and safety of tirofiban among acute coronary syndrome patients with mild to moderate renal insufficiency: results from the Platelet Receptor Inhibition in Ischemic Syndrome Management in Patients Limited by Unstable Signs and Symptoms (PRISM-PLUS) trial. Circulation 2002;105(20):2361-6. [MEDLINE: ] [DOI] [PubMed] [Google Scholar]
- Mega JL, Morrow DA, Sabatine MS, Zhao XQ, Snapinn SM, DiBattiste PM, et al.Correlation between the TIMI risk score and high-risk angiographic findings in non-ST-elevation acute coronary syndromes: observations from the Platelet Receptor Inhibition in Ischemic Syndrome Management in Patients Limited by Unstable Signs and Symptoms (PRISM-PLUS) trial. American Heart Journal 2005;149(5):846-50. [MEDLINE: ] [DOI] [PubMed] [Google Scholar]
- Morrow DA, Sabatine MS, Antman EM, Cannon CP, Braunwald E, Theroux P.Usefulness of tirofiban among patients treated without percutaneous coronary intervention (TIMI high risk patients in PRISM-PLUS). American Journal of Cardiology 2004;94(6):774-6. [MEDLINE: ] [DOI] [PubMed] [Google Scholar]
- Platelet Receptor Inhibition in Ischemic Syndrome Management in Patients Limited by Unstable Signs and Symptoms (PRISM-PLUS) Study Investigators.Inhibition of the platelet glycoprotein IIb/IIIa receptor with tirofiban in unstable angina and non-Q-wave myocardial infarction. New England Journal of Medicine 1998;338(21):1488-97. [MEDLINE: ] [DOI] [PubMed] [Google Scholar]
- Servoss SJ, Wan Y, Snapinn SM, DiBattiste PM, Zhao XQ, Theroux P, et al.Tirofiban therapy for patients with acute coronary syndromes and prior coronary artery bypass grafting in the PRISM-PLUS trial. American Journal of Cardiology 2004;93(7):843-7. [MEDLINE: ] [DOI] [PubMed] [Google Scholar]
- Szucs TD, Meyer BJ, Kiowski W.Economic assessment of tirofiban in the management of acute coronary syndromes in the hospital setting: an analysis based on the PRISM PLUS trial. European Heart Journal 1999;20(17):1253-60. [MEDLINE: ] [DOI] [PubMed] [Google Scholar]
- Theroux P, Alexander J, Pharand C, Barr E, Snapinn S, Ghannam AF, et al.Glycoprotein IIb/IIIa receptor blockade improves outcomes in diabetic patients presenting with unstable angina/non-ST-elevation myocardial infarction: results from the Platelet Receptor Inhibition in Ischemic Syndrome Management in Patients Limited by Unstable Signs and Symptoms (PRISM-PLUS) study. Circulation 2000;102(20):2466-72. [MEDLINE: ] [DOI] [PubMed] [Google Scholar]
- Theroux P, Alexander J Jr, Dupuis J, Pesant Y, Gervais P, Grandmont D, et al.Upstream use of tirofiban in patients admitted for an acute coronary syndrome in hospitals with or without facilities for invasive management. PRISM-PLUS Investigators. American Journal of Cardiology 2001;87(4):375-80. [MEDLINE: ] [DOI] [PubMed] [Google Scholar]
- Zhao XQ, Theroux P, Snapinn SM, Sax FL.Intracoronary thrombus and platelet glycoprotein IIb/IIIa receptor blockade with tirofiban in unstable angina or non-Q-wave myocardial infarction. Angiographic results from the PRISM-PLUS trial (Platelet receptor inhibition for ischemic syndrome management in patients limited by unstable signs and symptoms). PRISM-PLUS Investigators. Circulation 1999;100(15):1609-15. [MEDLINE: ] [DOI] [PubMed] [Google Scholar]
PURSUIT 1997 {unpublished data only}
- Akkerhuis KM, Deckers JW, Boersma E, Harrington RA, Stepinska J, Mahaffey KW, et al.Geographic variability in outcomes within an international trial of glycoprotein IIb/IIIa inhibition in patients with acute coronary syndromes. Results from PURSUIT. European Heart Journal 2000;21(5):371-81. [MEDLINE: ] [DOI] [PubMed] [Google Scholar]
- Akkerhuis KM, Maas AC, Klootwijk PA, Krucoff MW, Meij S, Califf RM, et al.Recurrent ischemia during continuous 12-lead ECG-ischemia monitoring in patients with acute coronary syndromes treated with eptifibatide: relation with death and myocardial infarction. PURSUIT ECG-Ischemia Monitoring Substudy Investigators. Platelet glycoprotein IIb/IIIa in Unstable angina: Receptor Suppression Using Integrilin Therapy. Journal of Electrocardiology 2000;33(2):127-36. [MEDLINE: ] [DOI] [PubMed] [Google Scholar]
- Alexander JH, Harrington RA, Tuttle RH, Berdan LG, Lincoff AM, Deckers JW, et al.Prior aspirin use predicts worse outcomes in patients with non-ST-elevation acute coronary syndromes. PURSUIT Investigators. Platelet IIb/IIIa in Unstable angina: Receptor Suppression Using Integrilin Therapy. American Journal of Cardiology 1999;83(8):1147-51. [MEDLINE: ] [DOI] [PubMed] [Google Scholar]
- Boersma E, Pieper KS, Steyerberg EW, Wilcox RG, Chang WC, Lee KL, et al.Predictors of outcome in patients with acute coronary syndromes without persistent ST-segment elevation. Results from an international trial of 9461 patients. The PURSUIT Investigators. Circulation 2000;101(22):2557-67. [MEDLINE: ] [DOI] [PubMed] [Google Scholar]
- Brown RE, Henderson RA, Koster D, Hutton J, Simoons ML.Cost effectiveness of eptifibatide in acute coronary syndromes; an economic analysis of Western European patients enrolled in the PURSUIT trial. The Platelet IIa/IIb in unstable Angina: Receptor Suppression Using Integrilin Therapy. European Heart Journal 2002;23(1):50-8. [MEDLINE: ] [DOI] [PubMed] [Google Scholar]
- Chang WC, Harrington RA, Simoons ML, Califf RM, Lincoff AM, Armstrong PW, et al.Does eptifibatide confer a greater benefit to patients with unstable angina than with non-ST segment elevation myocardial infarction? Insights from the PURSUIT Trial. European Heart Journal 2002;23(14):1102-11. [MEDLINE: ] [DOI] [PubMed] [Google Scholar]
- Dyke CM, Bhatia D, Lorenz TJ, Marso SP, Tardiff BE, Hogeboom C, et al.Immediate coronary artery bypass surgery after platelet inhibition with eptifibatide: results from PURSUIT. Platelet Glycoprotein IIb/IIIa in Unstable Angina: Receptor Suppression Using Integrelin Therapy. Annals of Thoracic Surgery 2000;70(3):866-71. [MEDLINE: ] [DOI] [PubMed] [Google Scholar]
- Greenbaum AB, Harrington RA, Hudson MP, MacAulay CM, Wilcox RG, Simoons ML, et al.Therapeutic value of eptifibatide at community hospitals transferring patients to tertiary referral centers early after admission for acute coronary syndromes. PURSUIT Investigators. Journal of the American College of Cardiology 2001;37(2):492-8. [MEDLINE: ] [DOI] [PubMed] [Google Scholar]
- Harrington RA.Design and methodology of the PURSUIT trial: evaluating eptifibatide for acute ischemic coronary syndromes. Platelet Glycoprotein IIb-IIIa in Unstable Angina: Receptor Suppression Using Integrilin Therapy. American Journal of Cardiology 1997;80(4A):34-8B. [MEDLINE: ] [DOI] [PubMed] [Google Scholar]
- Hasdai D, Holmes DR Jr, Criger DA, Topol EJ, Califf RM, Wilcox RG, et al.Cigarette smoking status and outcome among patients with acute coronary syndromes without persistent ST-segment elevation: effect of inhibition of platelet glycoprotein IIb/IIIa with eptifibatide. The PURSUIT trial investigators. American Heart Journal 2000;139(3):454-60. [MEDLINE: ] [DOI] [PubMed] [Google Scholar]
- Kleiman NS, Lincoff AM, Flaker GC, Pieper KS, Wilcox RG, Berdan LG, et al.Early percutaneous coronary intervention, platelet inhibition with eptifibatide, and clinical outcomes in patients with acute coronary syndromes. PURSUIT Investigators. Circulation 2000;101(7):751-7. [MEDLINE: ] [DOI] [PubMed] [Google Scholar]
- Labinaz M, Kaul P, Harrington RA, Chang WC, Kleiman NS, Simoons ML, et al.Six-month outcomes of percutaneous coronary balloon angioplasty in acute coronary syndromes: Results from the PURSUIT trial. Canadian Journal of Cardiology 2004;20(8):773-8. [MEDLINE: ] [PubMed] [Google Scholar]
- Labinaz M, Kilaru R, Pieper K, Marso SP, Kitt MM, Simoons ML, et al.Outcomes of patients with acute coronary syndromes and prior coronary artery bypass grafting: results from the platelet glycoprotein IIb/IIIa in unstable angina: receptor suppression using integrilin therapy (PURSUIT) trial. Circulation 2002;105(3):322-7. [MEDLINE: ] [DOI] [PubMed] [Google Scholar]
- Lauer MA, Houghtaling PL, Peterson JG, Granger CB, Bhatt DL, Sapp SK, et al.Attenuation of rebound ischemia after discontinuation of heparin therapy by glycoprotein IIb/IIIa inhibition with eptifibatide in patients with acute coronary syndromes: observations from the platelet IIb/IIIa in unstable angina: receptor suppression using integrilin therapy (PURSUIT) trial. Circulation 2001;104(23):2772-7. [MEDLINE: ] [DOI] [PubMed] [Google Scholar]
- Lincoff AM, Harrington RA, Califf RM, Hochman JS, Guerci AD, Ohman EM, et al.Management of patients with acute coronary syndromes in the United States by platelet glycoprotein IIb/IIIa inhibition. Insights from the platelet glycoprotein IIb/IIIa in unstable angina: receptor suppression using integrilin therapy (PURSUIT) trial. Circulation 2000;102(10):1093-100. [MEDLINE: ] [DOI] [PubMed] [Google Scholar]
- Mahaffey KW, Harrington RA, Akkerhuis M, Kleiman NS, Berdan LG, Crenshaw BS, et al.Systematic adjudication of myocardial infarction end-points in an international clinical trial. Current Controlled Trials in Cardiovascular Medicine 2001;2(4):180-6. [MEDLINE: ] [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mahaffey KW, Harrington RA, Simoons ML, Granger CB, Graffagnino C, Alberts MJ, et al.Stroke in patients with acute coronary syndromes: incidence and outcomes in the platelet glycoprotein IIb/IIIa in unstable angina. Receptor suppression using integrilin therapy (PURSUIT) trial. The PURSUIT Investigators. Circulation 1999;99(18):2371-7. [MEDLINE: ] [DOI] [PubMed] [Google Scholar]
- Mark DB, Harrington RA, Lincoff AM, Califf RM, Nelson CL, Tsiatis AA, et al.Cost-effectiveness of platelet glycoprotein IIb/IIIa inhibition with eptifibatide in patients with non-ST-elevation acute coronary syndromes. Circulation 2000;101(4):366-71. [MEDLINE: ] [DOI] [PubMed] [Google Scholar]
- Marso SP, Bhatt DL, Roe MT, Houghtaling PL, Labinaz M, Kleiman NS, et al.Enhanced efficacy of eptifibatide administration in patients with acute coronary syndrome requiring in-hospital coronary artery bypass grafting. PURSUIT Investigators. Circulation 2000;102(24):2952-8. [MEDLINE: ] [DOI] [PubMed] [Google Scholar]
- McClure MW, Berkowitz SD, Sparapani R, Tuttle R, Kleiman NS, Berdan LG, et al.Clinical significance of thrombocytopenia during a non-ST-elevation acute coronary syndrome. The platelet glycoprotein IIb/IIIa in unstable angina: receptor suppression using integrilin therapy (PURSUIT) trial experience. Circulation 1999;99(22):2892-900. [MEDLINE: ] [DOI] [PubMed] [Google Scholar]
- Peterson JG, Topol EJ, Roe MT, Sapp SK, Lincoff AM, Deckers JW, et al.Prognostic importance of concomitant heparin with eptifibatide in acute coronary syndromes. PURSUIT Investigators. Platelet Glycoprotein IIb/IIIa in Unstable Angina: Receptor Suppression Using Integrilin Therapy. American Journal of Cardiology 2001;87(5):532-6. [MEDLINE: ] [DOI] [PubMed] [Google Scholar]
- PURSUIT Trial Investigators.Inhibition of platelet glycoprotein IIb/IIIa with eptifibatide in patients with acute coronary syndromes. New England Journal of Medicine 1998;339(7):436-43. [MEDLINE: ] [DOI] [PubMed] [Google Scholar]
- Roe MT, Harrington RA, Prosper DM, Pieper KS, Bhatt DL, Lincoff AM, et al.Clinical and therapeutic profile of patients presenting with acute coronary syndromes who do not have significant coronary artery disease.The Platelet Glycoprotein IIb/IIIa in Unstable Angina: Receptor Suppression Using Integrilin Therapy (PURSUIT) Trial Investigators. Circulation 2000;102(10):1101-6. [MEDLINE: ] [DOI] [PubMed] [Google Scholar]
- Ronner E, Boersma E, Akkerhuis KM, Harrington RA, Lincoff AM, Deckers JW, et al.Patients with acute coronary syndromes without persistent ST elevation undergoing percutaneous coronary intervention benefit most from early intervention with protection by a glycoprotein IIb/IIIa receptor blocker. European Heart Journal 2002;23(3):239-46. [MEDLINE: ] [DOI] [PubMed] [Google Scholar]
- Ronner E, Boersma E, Laarman GJ, Somsen GA, Harrington RA, Deckers JW, et al.Early angioplasty in acute coronary syndromes without persistent ST-segment elevation improves outcome but increases the need for six-month repeat revascularization: an analysis of the PURSUIT Trial. Platelet glycoprotein IIB/IIIA in Unstable angina: Receptor Suppression Using Integrilin Therapy. Journal of the American College of Cardiology 2002;39(12):1924-9. [MEDLINE: ] [DOI] [PubMed] [Google Scholar]
- Srichai MB, Jaber WA, Prior DL, Marso SP, Houghtaling PL, Menon V, et al.Evaluating the benefits of glycoprotein IIb/IIIa inhibitors in heart failure at baseline in acute coronary syndromes. American Heart Journal 2004;147(1):84-90. [MEDLINE: ] [DOI] [PubMed] [Google Scholar]
Quarto Di Palo 1991 {published data only}
- Quarto Di Palo F, Elli A, Rivolta R, Parenti M, Palazzi P, Zanussi C.Prevention of chronic cyclosporine nephrotoxicity in renal transplantation by picotamide. Transplantation Proceedings 1991;23(1 Pt 2):969-71. [MEDLINE: ] [PubMed] [Google Scholar]
RAPPORT 1998 {published and unpublished data}
- Brener SJ, Barr LA, Burchenal JE, Katz S, George BS, Jones AA, et al.Randomized, placebo-controlled trial of platelet glycoprotein IIb/IIIa blockade with primary angioplasty for acute myocardial infarction. ReoPro and Primary PTCA Organization and Randomized Trial (RAPPORT) Investigators. Circulation 1998;98(8):734-41. [MEDLINE: ] [DOI] [PubMed] [Google Scholar]
- Brener SJ, Barr LA, Burchenal JE, Wolski KE, Effron MB, Topol EJ.Effect of abciximab on the pattern of reperfusion in patients with acute myocardial infarction treated with primary angioplasty. RAPPORT investigators. ReoPro And Primary PTCA Organization and Randomized Trial. American Journal of Cardiology 1999;84(6):728-30. [MEDLINE: ] [DOI] [PubMed] [Google Scholar]
Reams 1985 {published data only}
- Reams GP, Young M, Sorkin M, Twardowski Z, Gloor H, Moore H, et al.Effects of dipyridamole on peritoneal clearances. Uremia Investigation 1985;9(1):27-33. [MEDLINE: ] [DOI] [PubMed] [Google Scholar]
RESIST 2008 {published data only}
- Cooper CJ, Haller ST, Colyer W, Steffes M, Burket MW, Thomas WJ, et al.Embolic protection and platelet inhibition during renal artery stenting. Circulation 2008;117(21):2752-60. [MEDLINE: ] [DOI] [PubMed] [Google Scholar]
- He W, Che J, Zhan D, Dawso T, Kanjwa S, Halle S, et al.Time dependant changes in systolic blood pressure after renal artery stenting: role of stenosis severity [abstract no: 14283]. Circulation 2012;126(21 Suppl 1). [EMBASE: 70958797] [Google Scholar]
- Kanjwal K, Cooper CJ, Virmani R, Haller S, Shapiro JI, Burket MW, et al.Predictors of embolization during protected renal artery angioplasty and stenting: Role of antiplatelet therapy. Catheterization & Cardiovascular Interventions 2010;76(1):16-23. [MEDLINE: ] [DOI] [PubMed] [Google Scholar]
- Kanjwal K, Haller S, Steffes M, Virmani R, Shapiro JI, Burket MW, et al.Complete versus partial distal embolic protection during renal artery stenting. Catheterization & Cardiovascular Interventions 2009;73(6):725-30. [MEDLINE: ] [DOI] [PubMed] [Google Scholar]
- Tian J, Haller S, Periyasamy S, Brewster P, Zhang H, Adlakha S, et al.Renal ischemia regulates marinobufagenin release in humans. Hypertension 2010;56(5):914-9. [MEDLINE: ] [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yu H, Zhang D, Haller S, Kanjwal K, Colyer W, Brewster P, et al.Determinants of renal function in patients with renal artery stenosis. Vascular Medicine 2011;16(5):331-8. [MEDLINE: ] [DOI] [PubMed] [Google Scholar]
Rouzrokh 2010 {published data only}
- Rouzrokh M, Abbasi MR, Mirshemirani AR, Sobhiyeh MR.The effect of antiplatelet drugs on the patency rate of arterio-venous fistulae in hemodialysis patients. Iranian Journal of Pharmaceutical Research 2010;9(4):451-7. [EMBASE: 2011024056] [PMC free article] [PubMed] [Google Scholar]
Rubin 1982 {published data only}
- Rubin J, Adair C, Barnes T, Bower JD.Augmentation of peritoneal clearance by dipyridamole. Kidney International 1982;22(6):658-61. [MEDLINE: ] [DOI] [PubMed] [Google Scholar]
Salter 1984 {published data only}
- Salter MC, Crow MJ, Donaldson DR, Roberts TG, Rajah SM, Davison AM.Prevention of platelet deposition and thrombus formation on hemodialysis membranes: a double-blind randomized trial of aspirin and dipyridamole. Artificial Organs 1984;8(1):57-61. [MEDLINE: ] [DOI] [PubMed] [Google Scholar]
Schnepp 2000 {published data only}
- Schnepp M, Teichler S, Markau S, Rettkowski O, Priesack J, Deuber HJ, et al.Platelet function analysis during therapy with clopidogrel in endstage renal disease [abstract]. In: 37th Congress. European Renal Association. European Dialysis and Transplantation Association; 2000 Sept 17-20; Nice, France. 2000:191. [CENTRAL: CN-00461687]
Schulze 1990 {published data only}
- Schulze R, Langkopf B, Sziegoleit W.The effect of dipyridamole on the results of allogenic kidney transplantation [Der Einfluss von Dipyridamol auf die Ergebnisse der allogenen Nieren-transplantation]. Zeitschrift für Urologie und Nephrologie 1990;83(5):255-9. [MEDLINE: ] [PubMed] [Google Scholar]
Sreedhara 1994 {published data only}
- Sreedhara R, Himmelfarb J, Lazarus JM, Hakim RM.Antiplatelet therapy in expanded polytetrafluoroethylene (EPTFE) graft thrombosis: results of a randomized double blind study [abstract no: 9P]. Journal of the American Society of Nephrology 1993;4(Program & Abstracts):388. [CENTRAL: CN-00485981] [Google Scholar]
- Sreedhara R, Himmelfarb J, Lazarus JM, Hakim RM.Anti-platelet therapy in graft thrombosis: results of a prospective, randomized, double blind study. Kidney International 1994;45(5):1477-83. [MEDLINE: ] [DOI] [PubMed] [Google Scholar]
Steiness 2018 {published data only}
- Steiness E, Brun N, Skarsfeldt T, Derwahl KM.Low dose anti-thromboxane reduces urinary albumin in patients with diabetic kidney disease [abstract no: SA-PO148]. Journal of the American Society of Nephrology 2018;29:773. [EMBASE: 633731990] [Google Scholar]
STOP 1995 {published and unpublished data}
- Mileti M, De Petri G, Bacchi M, Ogliari V, Pecchini F, Bufano G, et al.A trial to evaluate the efficacy of picotamide in preventing thrombotic occlusion of the vascular access in hemodialysis patients. Journal of Nephrology 1995;8(2):167-72. [EMBASE: 25209311] [Google Scholar]
Storck 1996 {published data only}
- Storck M, Schilling M, Mickley V, Techt B, Abendroth D.Influence of systemic cyclooxygenase inhibition with single-dose aspisol on kinetics of arachidonic acid metabolites in the venous effluate of transplanted kidney grafts in humans. Transplantation Proceedings 1996;28(1):312-3. [MEDLINE: ] [PubMed] [Google Scholar]
Taber 1992 {published data only}
- Taber T, Maikranz P, Haag B, Dilley R, Gaylord G.Hemodialysis vascular graft stenosis may be altered by low-molecular weight dextran (LMD), but not by aspirin (ASA) [abstract]. Journal of the American Society of Nephrology 1992;3(3):397. [CENTRAL: CN-00858239] [Google Scholar]
Tang 2014 {published data only}
- Tang WH, Lin FH, Lee CH, Kuo FC, Hsieh CH, Hsiao FC, et al.Cilostazol effectively attenuates deterioration of albuminuria in patients with type 2 diabetes: a randomized, placebo-controlled trial. Endocrine 2014;45(2):293-301. [MEDLINE: ] [DOI] [PubMed] [Google Scholar]
TARGET 2000 {published and unpublished data}
- Berger PB, Best PJ, Topol EJ, White J, DiBattiste PM, Chan AW, et al.The relation of renal function to ischemic and bleeding outcomes with 2 different glycoprotein IIb/IIIa inhibitors: the do Tirofiban and ReoPro Give Similar Efficacy Outcome (TARGET) trial. American Heart Journal 2005;149(5):869-75. [MEDLINE: ] [DOI] [PubMed] [Google Scholar]
- Kalyanasundaram A, Blankenship JC, Berger P, Herrmann H, McClure R, Moliterno D.Thrombus predicts ischemic complications during percutaneous coronary intervention in saphenous vein grafts: results from TARGET (do Tirofiban and ReoPro give similar efficacy trial?). Catheterization & Cardiovascular Interventions 2007;69(5):623-9. [MEDLINE: ] [DOI] [PubMed] [Google Scholar]
- Moliterno DJ, Topol EJ.A direct comparison of tirofiban and abciximab during percutaneous coronary revascularization and stent placement: rationale and design of the TARGET study. American Heart Journal 2000;140(5):722-6. [MEDLINE: ] [DOI] [PubMed] [Google Scholar]
- Moliterno DJ, Yakubov SJ, DiBattiste PM, Herrmann HC, Stone GW, Macaya C, et al.Outcomes at 6 months for the direct comparison of tirofiban and abciximab during percutaneous coronary revascularisation with stent placement: the TARGET follow-up study. Lancet 2002;360(9330):355-60. [MEDLINE: ] [DOI] [PubMed] [Google Scholar]
- Mukherjee D, Topol EJ, Bertrand ME, Kristensen SD, Herrmann HC, Neumann FJ, et al.Mortality at 1 year for the direct comparison of tirofiban and abciximab during percutaneous coronary revascularization: do tirofiban and ReoPro give similar efficacy outcomes at trial 1-year follow-up. European Heart Journal 2005;26(23):2524-8. [MEDLINE: ] [DOI] [PubMed] [Google Scholar]
- Roffi M, Moliterno DJ, Meier B, Powers ER, Grines CL, DiBattiste PM, et al.Impact of different platelet glycoprotein IIb/IIIa receptor inhibitors among diabetic patients undergoing percutaneous coronary intervention: Do Tirofiban and ReoPro Give Similar Efficacy Outcomes Trial (TARGET) 1-year follow-up. Circulation 2002;105(23):2730-6. [MEDLINE: ] [DOI] [PubMed] [Google Scholar]
- Topol EJ, Moliterno DJ, Herrmann HC, Powers ER, Grines CL, Cohen DJ, et al.Comparison of two platelet glycoprotein IIb/IIIa inhibitors, tirofiban and abciximab, for the prevention of ischemic events with percutaneous coronary revascularization. New England Journal of Medicine 2001;344(25):1888-94. [MEDLINE: ] [DOI] [PubMed] [Google Scholar]
Tayebi 2018 {published data only}
- Tayebi P, Kazemzadeh G, Banihashem A, Ravari H.Effect of low dose aspirin and dipyridamole on primary patency of arteriovenous grafts in hemodialysis patients: a randomized double-blind placebo-controlled trial. Electronic Physician [Electronic Resource] 2018;10(1):6135-9. [MEDLINE: ] [DOI] [PMC free article] [PubMed] [Google Scholar]
Teng 2018 {published data only}
- Teng R, Muldowney S, Zhao Y, Berg JK, Lu J, Khan ND.Pharmacokinetics and pharmacodynamics of ticagrelor in subjects on hemodialysis and subjects with normal renal function. European Journal of Clinical Pharmacology 2018;74(9):1141-8. [MEDLINE: ] [DOI] [PMC free article] [PubMed] [Google Scholar]
TRA 2P‐TIMI 50 2009 {published data only}
- Correa S, Bonaca MP, Scirica BM, Murphy SA, Goodrich EL, Morrow DA, et al.Efficacy and safety of more potent antiplatelet therapy with vorapaxar in patients with impaired renal function. Journal of Thrombosis & Thrombolysis 2019;47(3):353-60. [MEDLINE: ] [DOI] [PubMed] [Google Scholar]
- Gaviria SC, Braunwald E, Bonaca MP, Murphy SA, Goodrich EL, Morrow DA, et al.The efficacy and safety of more potent antiplatelet therapy with vorapaxar in patients with impaired renal function: insights from the TRA 2P-TIMI 50 trial [abstract no: 15480]. Circulation 2017;136(Suppl 1). [EMBASE: 619986693] [Google Scholar]
- Morrow DA, Braunwald E, Bonaca MP, Ameriso SF, Dalby AJ, Fish MP, et al.Vorapaxar in the secondary prevention of atherothrombotic events. New England Journal of Medicine 2012;366(15):1404-13. [MEDLINE: ] [DOI] [PubMed] [Google Scholar]
- Morrow DA, Scirica BM, Fox KA, Berman G, Strony J, Veltri E, et al.Evaluation of a novel antiplatelet agent for secondary prevention in patients with a history of atherosclerotic disease: design and rationale for the Thrombin-Receptor Antagonist in Secondary Prevention of Atherothrombotic Ischemic Events (TRA 2 degrees P)-TIMI 50 trial. American Heart Journal 2009;158(3):335-41. [MEDLINE: ] [DOI] [PubMed] [Google Scholar]
TRACER 2013 {published data only}
- Cornel JH, Tricoci P, Horton J, Moliterno D, Wallentin L, Armstrong P, et al.Effects of glycoprotein IIB/IIIA inhibitors in combination with vorapaxar, a platelet thrombin-receptor antagonist, among patients with non-St-segment elevation acute coronary syndromes: insights from the TRACER trial [abstract]. Journal of the American College of Cardiology 2013;61(10 Suppl 1):E102. [EMBASE: 71019466] [Google Scholar]
- Mahaffey KW, Pieper K, Vranckx P, Tricoci P, Van de Werf F, Held C, et al.Chronic kidney disease is associated with worse outcomes in ACS patients: results from the TRACER trial [abstract]. European Heart Journal 2014;35(Suppl 1):687. [EMBASE: 71649310] [Google Scholar]
TRITON‐TIMI 38 2006 {unpublished data only}
- Abaci A.The use of prasugrel in STEMI and NSTEMI: TRITON TIMI 38 study and subgroup analyses [Prasugrelin ST yukselmeli ve yukselmesiz miyokart enfarktusunde kullanimi: TRITON TIMI 38 caIismasi ve alt grup sonuclari]. Turk Kardiyoloji Dernegi Arsivi 2015;43 Suppl 2:1-6. [MEDLINE: ] [PubMed] [Google Scholar]
- Antman EM, Wiviott SD, Murphy SA, Voitk J, Hasin Y, Widimsky P, et al.Early and late benefits of prasugrel in patients with acute coronary syndromes undergoing percutaneous coronary intervention: a TRITON-TIMI 38 (TRial to Assess Improvement in Therapeutic Outcomes by Optimizing Platelet InhibitioN with Prasugrel-Thrombolysis In Myocardial Infarction) analysis. Journal of the American College of Cardiology 2008;51(21):2028-33. [MEDLINE: ] [DOI] [PubMed] [Google Scholar]
- Bonaca MP, Wiviott SD, Braunwald E, Murphy SA, Ruff CT, Antman EM, et al.American College of Cardiology/American Heart Association/European Society of Cardiology/World Heart Federation universal definition of myocardial infarction classification system and the risk of cardiovascular death: observations from the TRITON-TIMI 38 trial (Trial to Assess Improvement in Therapeutic Outcomes by Optimizing Platelet Inhibition With Prasugrel-Thrombolysis in Myocardial Infarction 38). Circulation 2012;125(4):577-83. [MEDLINE: ] [DOI] [PubMed] [Google Scholar]
- Capodanno D, Tamburino C.Cyphering the statistical and clinical significance of prasugrel in the TRITON-TIMI 38 trial. International Journal of Cardiology 2011;146(2):242-3. [MEDLINE: ] [DOI] [PubMed] [Google Scholar]
- Damman P, Winter RJ, Wallentin L, Fox KA.Letter by Damman et al regarding articles, "Long-term cardiovascular mortality after procedure-related or spontaneous myocardial infarction in patients with non-ST-segment elevation acute coronary syndrome: a collaborative analysis of individual patient data from the FRISC II, ICTUS, and RITA-3 Trials (FIR)" and "American College of Cardiology/American Heart Association/European Society of Cardiology/World Heart Federation universal definition of myocardial infarction classification system and the risk of cardiovascular death: observations from the TRITON-TIMI 38 Trial (Trial to Assess Improvement in Therapeutic Outcomes by Optimizing Platelet Inhibition With Prasugrel-Thrombolysis in Myocardial Infarction 38)". Circulation 2012;126(9):e136-7. [MEDLINE: ] [DOI] [PubMed] [Google Scholar]
- De Servi S, Goedicke J, Schirmer A, Widimsky P.Clinical outcomes for prasugrel versus clopidogrel in patients with unstable angina or non-ST-elevation myocardial infarction: an analysis from the TRITON-TIMI 38 trial. European Heart Journal: Acute Cardiovascular Care 2014;3(4):363-72. [MEDLINE: ] [DOI] [PubMed] [Google Scholar]
- Goodnough LT, Smith PK, Levy JH, Poston RS, Short MA, Weerakkody GJ, et al.Transfusion outcomes in patients undergoing coronary artery bypass grafting treated with prasugrel or clopidogrel: TRITON-TIMI 38 retrospective data analysis. Journal of Thoracic & Cardiovascular Surgery 2013;145(4):1077-82. [MEDLINE: ] [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hochholzer W, Wiviott SD, Antman EM, Contant CF, Guo J, Giugliano RP, et al.Predictors of bleeding and time dependence of association of bleeding with mortality: insights from the Trial to Assess Improvement in Therapeutic Outcomes by Optimizing Platelet Inhibition With Prasugrel--Thrombolysis in Myocardial Infarction 38 (TRITON-TIMI 38). Circulation 2011;123(23):2681-9. [MEDLINE: ] [DOI] [PubMed] [Google Scholar]
- Kohli P, Udell JA, Murphy SA, Cannon CP, Antman EM, Braunwald E, et al.Discharge aspirin dose and clinical outcomes in patients with acute coronary syndromes treated with prasugrel versus clopidogrel: an analysis from the TRITON-TIMI 38 study (trial to assess improvement in therapeutic outcomes by optimizing platelet inhibition with prasugrel-thrombolysis in myocardial infarction 38). Journal of the American College of Cardiology 2014;63(3):225-32. [MEDLINE: ] [DOI] [PubMed] [Google Scholar]
- Mahoney EM, Wang K, Arnold SV, Proskorovsky I, Wiviott S, Antman E, et al.Cost-effectiveness of prasugrel versus clopidogrel in patients with acute coronary syndromes and planned percutaneous coronary intervention: results from the trial to assess improvement in therapeutic outcomes by optimizing platelet inhibition with Prasugrel-Thrombolysis in Myocardial Infarction TRITON-TIMI 38. Circulation 2010;121(1):71-9. [MEDLINE: ] [DOI] [PubMed] [Google Scholar]
- Mariani M, Mariani G, De Servi S.Efficacy and safety of prasugrel compared with clopidogrel in patients with acute coronary syndromes: results of TRITON-TIMI 38 trials. Expert Review of Cardiovascular Therapy 2009;7(1):17-23. [MEDLINE: ] [DOI] [PubMed] [Google Scholar]
- Mega JL, Close SL, Wiviott SD, Shen L, Walker JR, Simon T, et al.Genetic variants in ABCB1 and CYP2C19 and cardiovascular outcomes after treatment with clopidogrel and prasugrel in the TRITON-TIMI 38 trial: a pharmacogenetic analysis. Lancet 2010;376(9749):1312-9. [MEDLINE: ] [DOI] [PMC free article] [PubMed] [Google Scholar]
- Michelson AD, Frelinger AL 3rd, Braunwald E, Downey WE, Angiolillo DJ, Xenopoulos NP, et al.Pharmacodynamic assessment of platelet inhibition by prasugrel vs. clopidogrel in the TRITON-TIMI 38 trial. European Heart Journal 2009;30(14):1753-63. [MEDLINE: ] [DOI] [PubMed] [Google Scholar]
- Mogabgab O, Wiviott SD, Cannon CP, Sloan S, Sabatine MS, Antman EM, et al.Circadian variation of stent thrombosis and the effect of more robust platelet inhibition: a post hoc analysis of the TRITON-TIMI 38 trial. Journal of Cardiovascular Pharmacology & Therapeutics 2013;18(6):555-9. [MEDLINE: ] [DOI] [PubMed] [Google Scholar]
- Montalescot G, Wiviott SD, Braunwald E, Murphy SA, Gibson CM, McCabe CH, et al.Prasugrel compared with clopidogrel in patients undergoing percutaneous coronary intervention for ST-elevation myocardial infarction (TRITON-TIMI 38): double-blind, randomised controlled trial. Lancet 2009;373(9665):723-31. [MEDLINE: ] [DOI] [PubMed] [Google Scholar]
- Murphy SA, Antman EM, Wiviott SD, Weerakkody G, Morocutti G, Huber K, et al.Reduction in recurrent cardiovascular events with prasugrel compared with clopidogrel in patients with acute coronary syndromes from the TRITON-TIMI 38 trial. European Heart Journal 2008;29(20):2473-9. [MEDLINE: ] [DOI] [PMC free article] [PubMed] [Google Scholar]
- O'Donoghue M, Antman EM, Braunwald E, Murphy SA, Steg PG, Finkelstein A, et al.The efficacy and safety of prasugrel with and without a glycoprotein IIb/IIIa inhibitor in patients with acute coronary syndromes undergoing percutaneous intervention: a TRITON-TIMI 38 (Trial to Assess Improvement in Therapeutic Outcomes by Optimizing Platelet Inhibition With Prasugrel-Thrombolysis In Myocardial Infarction 38) analysis. Journal of the American College of Cardiology 2009;54(8):678-85. [MEDLINE: ] [DOI] [PubMed] [Google Scholar]
- Ojeifo O, Wiviott SD, Antman EM, Murphy SA, Udell JA, Bates ER, et al.Concomitant administration of clopidogrel with statins or calcium-channel blockers: insights from the TRITON-TIMI 38 (trial to assess improvement in therapeutic outcomes by optimizing platelet inhibition with prasugrel-thrombolysis in myocardial infarction 38). Jacc: Cardiovascular Interventions 2013;6(2):1275-81. [MEDLINE: ] [DOI] [PubMed] [Google Scholar]
- Pakhomov IM.Comparison of effects of prasugrel and clopidogrel in patients with acute coronary syndrome, subjected to percutaneous coronary intervention: TRITON-TIMI 38 trial. Kardiologiia 2010;50(6):63-7. [MEDLINE: ] [PubMed] [Google Scholar]
- Pride YB, Tung P, Mohanavelu S, Zorkun C, Wiviott SD, Antman EM, et al.Angiographic and clinical outcomes among patients with acute coronary syndromes presenting with isolated anterior ST-segment depression: a TRITON-TIMI 38 (Trial to Assess Improvement in Therapeutic Outcomes by Optimizing Platelet Inhibition With Prasugrel-Thrombolysis In Myocardial Infarction 38) substudy. Jacc: Cardiovascular Interventions 2010;3(8):306-11. [MEDLINE: ] [DOI] [PubMed] [Google Scholar]
- Riesmeyer JS, Salazar DE, Weerakkody GJ, Ni L, Wrishko RE, Ernest CS 2nd, et al.Relationship between exposure to prasugrel active metabolite and clinical outcomes in the TRITON-TIMI 38 substudy. Journal of Clinical Pharmacology 2012;52(6):789-97. [MEDLINE: ] [DOI] [PubMed] [Google Scholar]
- Serebruany VL.Letter by Serebruany regarding article, "Cost-effectiveness of prasugrel versus clopidogrel in patients with acute coronary syndromes and planned percutaneous coronary intervention: results from the Trial to Assess Improvement in Therapeutic Outcomes by Optimizing Platelet Inhibition with Prasugrel-Thrombolysis in Myocardial Infarction TRITON-TIMI 38". Circulation 2010;122(8):e436. [MEDLINE: ] [DOI] [PubMed] [Google Scholar]
- Smith PK, Goodnough LT, Levy JH, Poston RS, Short MA, Weerakkody GJ, et al.Mortality benefit with prasugrel in the TRITON-TIMI 38 coronary artery bypass grafting cohort: risk-adjusted retrospective data analysis. Journal of the American College of Cardiology 2012;60(5):388-96. [MEDLINE: ] [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sorich MJ, Vitry A, Ward MB, Horowitz JD, McKinnon RA.Prasugrel vs. clopidogrel for cytochrome P450 2C19-genotyped subgroups: integration of the TRITON-TIMI 38 trial data. Journal of Thrombosis & Haemostasis 2010;8(8):1678-84. [MEDLINE: ] [DOI] [PubMed] [Google Scholar]
- Udell JA, Braunwald E, Antman EM, Murphy SA, Montalescot G, Wiviott SD.Prasugrel versus clopidogrel in patients with ST-segment elevation myocardial infarction according to timing of percutaneous coronary intervention: a TRITON-TIMI 38 subgroup analysis (Trial to Assess Improvement in Therapeutic Outcomes by Optimizing Platelet Inhibition with Prasugrel-Thrombolysis In Myocardial Infarction 38).[Erratum in: JACC Cardiovasc Interv. 2014 Aug;7(8):946 Note: Antman, Elliot M [Corrected to Antman, Elliott M]]. Jacc: Cardiovascular Interventions 2014;7(6):604-12. [MEDLINE: ] [DOI] [PubMed] [Google Scholar]
- Wilcox R, Iqbal K, Costigan T, Lopez-Sendon J, Ramos Y, Widimsky P.An analysis of TRITON-TIMI 38, based on the 12 month recommended length of therapy in the European label for prasugrel. Current Medical Research & Opinion 2014;30(11):2193-205. [MEDLINE: ] [DOI] [PubMed] [Google Scholar]
- Wiviott SD, Antman EM, Gibson CM, Montalescot G, Riesmeyer J, Weerakkody G, et al.Evaluation of prasugrel compared with clopidogrel in patients with acute coronary syndromes: design and rationale for the TRial to assess Improvement in Therapeutic Outcomes by optimizing platelet InhibitioN with prasugrel Thrombolysis In Myocardial Infarction 38 (TRITON-TIMI 38). American Heart Journal 2006;152(4):627-35. [MEDLINE: ] [DOI] [PubMed] [Google Scholar]
- Wiviott SD, Braunwald E, McCabe CH, Horvath I, Keltai M, Herrman JP, et al.Intensive oral antiplatelet therapy for reduction of ischaemic events including stent thrombosis in patients with acute coronary syndromes treated with percutaneous coronary intervention and stenting in the TRITON-TIMI 38 trial: a subanalysis of a randomised trial. Lancet 2008;371(9621):1353-63. [MEDLINE: ] [DOI] [PubMed] [Google Scholar]
- Wiviott SD, Braunwald E, McCabe CH, Montalescot G, Ruzyllo W, Gottlieb S, et al.Prasugrel versus clopidogrel in patients with acute coronary syndromes. New England Journal of Medicine 2007;357(20):2001-15. [MEDLINE: ] [DOI] [PubMed] [Google Scholar]
- Wiviott SD, Desai N, Murphy SA, Musumeci G, Ragosta M, Antman EM, et al.Efficacy and safety of intensive antiplatelet therapy with prasugrel from TRITON-TIMI 38 in a core clinical cohort defined by worldwide regulatory agencies. American Journal of Cardiology 2011;108(7):905-11. [MEDLINE: ] [DOI] [PubMed] [Google Scholar]
- Wrishko RE, Ernest CS 2nd, Small DS, Li YG, Weerakkody GJ, Riesmeyer JR, et al.Population pharmacokinetic analyses to evaluate the influence of intrinsic and extrinsic factors on exposure of prasugrel active metabolite in TRITON-TIMI 38. Journal of Clinical Pharmacology 2009;49(8):984-98. [MEDLINE: ] [DOI] [PubMed] [Google Scholar]
UK‐HARP‐I 2005 {published and unpublished data}
- Baigent C, Landray M, Leaper C, Altmann P, Armitage J, Baxter A, et al.First United Kingdom Heart and Renal Protection (UK-HARP-I) study: biochemical efficacy and safety of simvastatin and safety of low-dose aspirin in chronic kidney disease. American Journal of Kidney Diseases 2005;45(3):473-84. [MEDLINE: ] [DOI] [PubMed] [Google Scholar]
- Baigent C, UK-HARP Steering Committee.Efficacy and safety of simvastatin and safety of low-dose aspirin among patients with chronic kidney disease: final results of the first UK-heart and renal protection (UK-HARP-I) study [abstract no: SA-PO851]. Journal of the American Society of Nephrology 2002;13(Program & Abstracts):437A. [CENTRAL: CN-00444305] [Google Scholar]
Waseda 2016 {published data only}
- Waseda K, Saka Y, Takashima H, Kurita A, Ando H, Sakurai S, et al.Effect of CYP2C19 genotype on inhibition of platelet aggregation in hemodialysis patients with coronary artery disease [abstract]. Circulation 2016;134(Suppl 1):A14237. [EMBASE: 619219587] [Google Scholar]
Watanabe 2011b {published data only}
- Watanabe H, Nakagawa K, Kakihana M.Long-term effects of sarpogrelate, a selective serotonin receptor antagonist, in diabetic patients with stable angina and chronic kidney disease [abstract no: 11204]. Circulation 2011;124(21 Suppl 1). [EMBASE: 70620962] [Google Scholar]
Weseley 1982 {published data only}
- Weseley S, Goodman A.The effect of dipyridamole (Di) on the peritoneal dialysis (PD) clearance of creatinine and urea - a double-blind study [abstract]. Kidney International 1982;21(1):182. [Google Scholar]
Xydakis 2004 {published data only}
- Xydakis D, Papadagiannakis A, Sfakianaki M, Vakouti E, Papachristoforou K.The combination of clopidogrel (CL) and acetylsalicylic acid (ASA) inhibits more effective the platelet activation in haemodialysis patients with acute coronary syndromes (ACS) and high C reactive protein [abstract no: MP370]. In: 41st Congress. European Renal Association. European Dialysis and Transplantation Association; 2004 May 15-18; Lisbon, Portugal. 2004:355. [CENTRAL: CN-00509568]
Yang 2016b {published data only}
- Yang MY, Han B, Xu Y, Tian LY, Zhang Y, Qiang YW.The effects of different dose of clopidogrel in elderly patients with unstable angina combining chronic kidney disease [abstract no: P12]. Journal of the American Geriatrics Society 2016;64(Suppl 2):S320. [EMBASE: 611887576] [Google Scholar]
Yuto 2012 {published data only}
- Yuto J, Ehara Y, Shibata K, Iwamoto T, Yasuda G, Yatsu K, et al.Effect of sarpogrelate on fistula patency of forearm arteriovenous anastomisis in uremic patients [abstract no: 276]. Kidney Research & Clinical Practice 2012;31(2):A86. [EMBASE: 70814985] [Google Scholar]
Zäuner 1994 {published data only}
- Zäuner I, Böhler J, Braun N, Grupp C, Heering P, Schollmeyer P.Effect of aspirin and dipyridamole on proteinuria in idiopathic membranoproliferative glomerulonephritis: a multicentre prospective clinical trial. Nephrology Dialysis Transplantation 1994;9(6):619-22. [MEDLINE: ] [DOI] [PubMed] [Google Scholar]
References to studies excluded from this review
AVERROES 2010 {published data only}
- Alexander W, Connolly S, Arnesen H.European Society of Cardiology: apixaban or aspirin in decreasing stroke risk (The AVERROES Trial) [abstract]. P and T 2010;35(10):580-1. [EMBASE: 359918604] [Google Scholar]
- Amin A, Deitelzweig S, Jing Y, Makenbaeva D, Wiederkehr D, Lin J, et al.Comparison of medical costs of patients with atrial fibrillation unsuitable for warfarin treatment with apixaban or aspirin based on AVERROES trial. Clinical & Applied Thrombosis/Hemostasis 2015;21(3):235-40. [MEDLINE: ] [DOI] [PubMed] [Google Scholar]
- Bhagirath VC, Eikelboom JW, Hirsh J, Coppens M, Ginsberg J, Vanassche T, et al.Apixaban- calibrated anti-FXa activity in relation to outcome events and clinical characteristics in patients with atrial fibrillation: results from the AVERROES trial. TH Open 2017;1(2):e139-45. [EMBASE: 624302177] [DOI] [PMC free article] [PubMed] [Google Scholar]
- Connolly SJ, Eikelboom J, Joyner C, Diener HC, Hart R, Golitsyn S, et al.Apixaban in patients with atrial fibrillation. New England Journal of Medicine 2011;364(9):806-17. [MEDLINE: ] [DOI] [PubMed] [Google Scholar]
- Coppens M, Synhorst D, Eikelboom JW, Yusuf S, Shestakovska O, Connolly SJ.Efficacy and safety of apixaban compared with aspirin in patients who previously tried but failed treatment with vitamin K antagonists: results from the AVERROES trial. European Heart Journal 2014;35(28):1856-63. [MEDLINE: ] [DOI] [PubMed] [Google Scholar]
- Diener HC, Eikelboom J, Connolly SJ, Joyner CD, Hart RG, Lip GY, et al.Apixaban versus aspirin in patients with atrial fibrillation and previous stroke or transient ischaemic attack: a predefined subgroup analysis from AVERROES, a randomised trial. Lancet Neurology 2012;11(3):225-31. [MEDLINE: ] [DOI] [PubMed] [Google Scholar]
- Diener HC, Yusuf S, Eikelboom J, O'Donnell MO, Connolly SJ.AVERROES: apixaban versus acetylsalicylic acid (ASA) to prevent strokes [abstract no: 127]. Stroke 2011;42(3):e81. [EMBASE: 70362332] [Google Scholar]
- Eikelboom J, Synhorst D, Wright R, Wang L, Afzal R, Yusuf S, et al.Efficacy and safety of apixaban compared with aspirin in patients with atrial fibrillation who previously used and discontinued warfarin therapy: A secondary analysis of the AVERROES trial [abstract]. European Heart Journal 2011;32(Suppl 1):465. [EMBASE: 70534798] [Google Scholar]
- Eikelboom JW, Connolly SJ, Gao P, Paolasso E, De Caterina R, Husted S, et al.Stroke risk and efficacy of apixaban in atrial fibrillation patients with moderate chronic kidney disease. Journal of Stroke & Cerebrovascular Diseases 2012;21(6):429-35. [MEDLINE: ] [DOI] [PubMed] [Google Scholar]
- Eikelboom JW, O'Donnell M, Yusuf S, Diaz R, Flaker G, Hart R, et al.Rationale and design of AVERROES: apixaban versus acetylsalicylic acid to prevent stroke in atrial fibrillation patients who have failed or are unsuitable for vitamin K antagonist treatment. American Heart Journal 2010;159(3):348-53. [MEDLINE: ] [DOI] [PubMed] [Google Scholar]
- Flaker GC, Eikelboom J, Connolly S, Yusuf S, Lip G, Hart R.Bleeding with aspirin and apixaban in patients unsuitable for vitamin K antagonist therapy: The AVERROES study [abstract]. Journal of the American College of Cardiology 2012;59(13 Suppl 1):E572. [EMBASE: 70714012] [Google Scholar]
- Flaker GC, Eikelboom JW, Shestakovska O, Connolly SJ, Kaatz S, Budaj A, et al.Bleeding during treatment with aspirin versus apixaban in patients with atrial fibrillation unsuitable for warfarin: the apixaban versus acetylsalicylic acid to prevent stroke in atrial fibrillation patients who have failed or are unsuitable for vitamin K antagonist treatment (AVERROES) trial. Stroke 2012;43(12):3291-7. [MEDLINE: ] [DOI] [PubMed] [Google Scholar]
- Hart RG, Eikelboom J, Yusuf S, Gao P, Paolasso E, De Caterina R, et al.Efficacy and safety of the novel oral factor Xa inhibitor apixaban in atrial fibrillation (AF) patients with chronic kidney disease (CKD): the AVERROES trial [abstract]. European Heart Journal 2011;32(Suppl 1):6. [EMBASE: 70533061] [Google Scholar]
- Hohnloser S, Yusuf S, Eikelboom J, Steg G, Atar D, Budaj A, et al.Apixaban in patients with atrial fibrillation and their risk for cardiovascular hospitalization: insights from the AVERROES trial [abstract]. European Heart Journal 2011;32(Suppl 1):671. [EMBASE: 70535570] [Google Scholar]
- Hohnloser SH, Shestakovska O, Eikelboom J, Franzosi MG, Tan RS, Zhu J, et al.The effects of apixaban on hospitalizations in patients with different types of atrial fibrillation: insights from the AVERROES trial. European Heart Journal 2013;34(35):2752-9. [MEDLINE: ] [DOI] [PubMed] [Google Scholar]
- Lip GY, Connolly S, Yusuf S, Shestakovska O, Flaker G, Hart R, et al.Modification of outcomes with aspirin or apixaban in relation to CHADS(2) and CHA(2)DS(2)-VASc scores in patients with atrial fibrillation: a secondary analysis of the AVERROES study. Circulation: Arrhythmia and Electrophysiology 2013;6(1):31-8. [MEDLINE: ] [DOI] [PubMed] [Google Scholar]
- Lip GY, Eikelboom J, Yusuf S, Shestakovska O, Hart RG, Connolly S, et al.Modification of outcomes with aspirin or apixaban in relation to female and male sex in patients with atrial fibrillation: a secondary analysis of the AVERROES study. Stroke 2014;45(7):2127-30. [MEDLINE: ] [DOI] [PubMed] [Google Scholar]
- Lip GY, Yusuf S, Eikelboom J, Flaker G, Hart R, Lanas-Zenetti F, et al.Impact of treatment with apixaban and aspirin in patients with atrial fibrillation in relation to the CHADS2 and CHA2DS2-vasc scores: the AVERROES study [abstract no: 15542]. Circulation 2011;124(21 Suppl 1). [EMBASE: 70618066] [Google Scholar]
- Ng KH, Shestakovska O, Connolly SJ, Eikelboom JW, Avezum A, Diaz R, et al.Efficacy and safety of apixaban compared with aspirin in the elderly: a subgroup analysis from the AVERROES trial. Age & Ageing 2016;45(1):77-83. [MEDLINE: ] [DOI] [PubMed] [Google Scholar]
Bang 1994 {published data only}
- Bang BK, Yang CW, Kim YS, Chang YS, Yoon YS.Effect of combination therapy of captopril and dipyridamole on proteinuria in patients with IgA nephropathy [abstract]. Journal of the American Society of Nephrology 1994;5(3):346. [CENTRAL: CN-00550477] [Google Scholar]
Caravaca 1995a {published data only}
- Caravaca F, Lopez-Minguez JR, Arrobas M, Cubero J, Pizarro JL, Cid MC, et al.Haemodynamic changes induced by the correction of anaemia by erythropoietin: role of antiplatelet therapy. Nephrology Dialysis Transplantation 1995;10(9):1720-4. [MEDLINE: ] [PubMed] [Google Scholar]
Changjiang 2015 {published data only}
- Changjiang H, Jian Q, Yuan Z, Liang Y, Puqing L, Xiaolong G.Tirofiban combined with fondaparinux for post-PCI treatment of patients with acute coronary syndrome and mild renal insufficiency. Cell Biochemistry & Biophysics 2015;73(3):603-7. [MEDLINE: ] [DOI] [PubMed] [Google Scholar]
Coli 2006 {published data only}
- Coli L, Donati G, Cianciolo G, Raimondi C, Comai G, Panicali L, et al.Anticoagulation therapy for the prevention of hemodialysis tunneled cuffed catheters (TCC) thrombosis. Journal of Vascular Access 2006;7(3):118-22. [MEDLINE: ] [DOI] [PubMed] [Google Scholar]
EXCITE 2000 {published data only}
- Brugts JJ, Mercado N, Hu S, Guarneri M, Price M, Schatz R, et al.Relation of periprocedural bleeding complications and long-term outcome in patients undergoing percutaneous coronary revascularization (from the Evaluation of Oral Xemilofiban in Controlling Thrombotic Events [EXCITE] Trial). American Journal of Cardiology 2009;103(7):917-22. [MEDLINE: ] [DOI] [PubMed] [Google Scholar]
- Mercado N, Brugts JJ, Ix JH, Shlipak MG, Dixon SR, Gersh BJ, et al.Usefulness of proteinuria as a prognostic marker of mortality and cardiovascular events among patients undergoing percutaneous coronary intervention (data from the Evaluation of Oral Xemilofiban in Controlling Thrombotic Events [EXCITE] trial). American Journal of Cardiology 2008;102(9):1151-5. [MEDLINE: ] [DOI] [PubMed] [Google Scholar]
- O'Neill WW, Serruys P, Knudtson M, Es GA, Timmis GC, Zwaan C, et al.Long-term treatment with a platelet glycoprotein-receptor antagonist after percutaneous coronary revascularization. EXCITE Trial Investigators. Evaluation of Oral Xemilofiban in Controlling Thrombotic Events. New England Journal of Medicine 2000;342(18):1316-24. [MEDLINE: ] [DOI] [PubMed] [Google Scholar]
Foroughinia 2017 {published data only}
- Foroughinia F, Foroozmehr M.Effect of pretreatment with omega-3 supplement on cardiac necrosis markers in chronic kidney disease patients undergoing elective percutaneous coronary intervention. Journal of Research in Pharmacy Practice 2017;6(2):94-9. [MEDLINE: ] [DOI] [PMC free article] [PubMed] [Google Scholar]
- Foroughinia F, Movahed Nouri B, Kojuri J, Ostovan MA.Impact of omega-3 supplementation on high sensitive C-reactive protein level and 30-day major adverse cardiac events after the implementation of coronary stent in patients with chronic kidney disease: a randomized clinical study. Advanced Pharmaceutical Bulletin 2018;8(3):471-8. [MEDLINE: ] [DOI] [PMC free article] [PubMed] [Google Scholar]
Gorter 1998 {published data only}
- Gorter JW.Preventive treatment of patients after non-disabling cerebral ischaemia of presumed arterial origin: Comparative randomized study with intensive anticoagulant therapy or aspirin treatment [Preventieve behandeling van patienten na niet-invaliderende cerebrale ischemie door vermoedelijk arteriele oorzaak: Vergelijkend, gerandomiseerd onderzoek met intensieve antistollingstherapie of behandeling met acetylsalicylzuur]. Nederlands Tijdschrift voor Geneeskunde 1998;142(6):306-12. [EMBASE: 28106209] [PubMed] [Google Scholar]
Lee 1997 {published data only}
- Lee G, Choong HL, Chian GSC, Woo KT.Stabilisation of elevated serum creatinine (CR) in dipyridamole (DYP) and warfarin (WAR) treated patients with IgA nephropathy (IGAN): a three year controlled trial [abstract]. In: 9th Asian Colloquium in Nephrology; 1992 May 17-21; Seoul, Korea. 1992:106. [CENTRAL: CN-00461145]
- Lee GS, Choong HL, Chiang GS, Woo KT.Three-year randomized controlled trial of dipyridamole and low-dose warfarin in patients with IgA nephropathy and renal impairment. Nephrology 1997;3(1):117-21. [EMBASE: 27161092] [Google Scholar]
- Lee GS, Woo KT, Lim CH.Controlled trial of dipyridamole and low-dose warfarin in patients with IgA nephritis with renal impairment. Clinical Nephrology 1989;31:276. [CENTRAL: CN-00740470] [PubMed] [Google Scholar]
Lindsay 1972 {published data only}
- Lindsay RM, Ferguson D, Prentice CR, Burton JA, McNicol GP.Reduction of thrombus formation on dialyser membranes by aspirin and RA 233. Lancet 1972;2(7790):1287-90. [MEDLINE: ] [DOI] [PubMed] [Google Scholar]
NITER 2005 {published data only}
- Scarpioni R, Michieletti E, Cristinelli L, Ugolotti U, Scolari F, Venturelli C, et al.Atherosclerotic renovascular disease: medical therapy versus medical therapy plus renal artery stenting in preventing renal failure progression: the rationale and study design of a prospective, multicenter and randomized trial (NITER). Journal of Nephrology 2005;18(4):423-8. [MEDLINE: ] [PubMed] [Google Scholar]
- Scarpioni R, Michieletti E, Pavone L, Gandolfi S, Ricardi M, Pecchini P, et al.Atherosclerotic renovascular disease (ARVD): medical therapy plus renal artery stenting (PTRS), compared with medical therapy alone, do not offer more chances in preventing cardio-vascular (CV) or renal events, preliminary results of a prospective, multicenter and randomized trial [abstract no: M074]. In: World Congress of Nephrology; 2009 May 22-26; Milan, Italy. 2009. [CENTRAL: CN-00841209]
- Siddiqui EU, Murphy TP, Naeem SS, Siddique A, McEnteggart GE, Scarpioni R.Interaction between albuminuria and treatment group outcomes for patients with renal artery stenosis: The NITER Study. Journal of Vascular & Interventional Radiology 2018;29(7):966-70. [MEDLINE: ] [DOI] [PubMed] [Google Scholar]
Perkovic 2004 {published data only}
- Perkovic V, Nicholls KM, Foreman A, Becker GJ.A randomised controlled trial of cardiovascular risk factor modification in end stage renal failure [abstract no: P62]. Nephrology 2004;9(Suppl 1):A16. [CENTRAL: CN-00509410] [Google Scholar]
- Perkovic V, Nicholls KM, Foreman A, Walker RG, Becker GJ.A randomised controlled trial of cardiovascular risk factor modification in end stage kidney disease [abstract no: SA-PO348]. Journal of the American Society of Nephrology 2004;15(Oct):377-8A. [CENTRAL: CN-00644351] [Google Scholar]
POISE‐2 2013 {published data only}
- Chludzinski A, Irani C, Mascha EJ, Kurz A, Devereaux PJ, Sessler DI.Protocol understanding and anxiety in perioperative clinical trial patients approached for consent on the day of surgery. Mayo Clinic Proceedings 2013;88(5):446-54. [MEDLINE: ] [DOI] [PubMed] [Google Scholar]
- Devereaux PJ, POISE-2 Investigators.Rationale and design of the PeriOperative ISchemic Evaluation-2 (POISE-2) trial: an international 2 x 2 factorial randomized controlled trial of acetyl-salicylic acid vs. placebo and clonidine vs. placebo in patients undergoing noncardiac surgery. American Heart Journal 2014;167(6):804-9. [MEDLINE: ] [DOI] [PubMed] [Google Scholar]
- Garg AX, Kurz A, Sessler DI, Cuerden M, Robinson A, Mrkobrada M, et al.Aspirin and clonidine in non-cardiac surgery: acute kidney injury substudy protocol of the Perioperative Ischaemic Evaluation (POISE) 2 randomised controlled trial. BMJ Open 2014;4(2):e004886. [MEDLINE: ] [DOI] [PMC free article] [PubMed] [Google Scholar]
- Garg AX, Kurz A, Sessler DI, Cuerden M, Robinson A, Mrkobrada M, et al.Perioperative aspirin and clonidine and risk of acute kidney injury: a randomized clinical trial. JAMA 2014;312(21):2254-64. [MEDLINE: ] [DOI] [PubMed] [Google Scholar]
PRODIGY 2010 {published data only}
- Campo G, Punzetti S, Malagu M, Ferrari R, Valgimigli M.Two-year outcomes after first- or second-generation drug-eluting stent implantation in patients with in-stent restenosis. A PRODIGY trial substudy. International Journal of Cardiology 2014;173(2):343-5. [MEDLINE: ] [DOI] [PubMed] [Google Scholar]
- Campo G, Tebaldi M, Vranckx P, Biscaglia S, Tumscitz C, Ferrari R, et al.Short- versus long-term duration of dual antiplatelet therapy in patients treated for in-stent restenosis: a PRODIGY trial substudy (Prolonging Dual Antiplatelet Treatment After Grading Stent-Induced Intimal Hyperplasia). Journal of the American College of Cardiology 2014;63(6):506-12. [MEDLINE: ] [DOI] [PubMed] [Google Scholar]
- Costa F, Adamo M, Ariotti S, Ferrante G, Navarese EP, Leonardi S, et al.Left main or proximal left anterior descending coronary artery disease location identifies high-risk patients deriving potentially greater benefit from prolonged dual antiplatelet therapy duration. Eurointervention 2016;11(11):e1222-30. [MEDLINE: ] [DOI] [PubMed] [Google Scholar]
- Costa F, Vranckx P, Leonardi S, Moscarella E, Ando G, Calabro P, et al.Impact of clinical presentation on ischaemic and bleeding outcomes in patients receiving 6- or 24-month duration of dual-antiplatelet therapy after stent implantation: a pre-specified analysis from the PRODIGY (Prolonging Dual-Antiplatelet Treatment After Grading Stent-Induced Intimal Hyperplasia) trial. European Heart Journal 2015;36(20):1242-51. [MEDLINE: ] [DOI] [PubMed] [Google Scholar]
- Crimi G, Leonardi S, Costa F, Adamo M, Ariotti S, Valgimigli M.Role of stent type and of duration of dual antiplatelet therapy in patients with chronic kidney disease undergoing percutaneous coronary interventions. Is bare metal stent implantation still a justifiable choice? A post-hoc analysis of the all comer PRODIGY trial. International Journal of Cardiology 2016;212:110-7. [MEDLINE: ] [DOI] [PubMed] [Google Scholar]
- Franzone A, Piccolo R, Gargiulo G, Ariotti S, Marino M, Santucci A, et al.Prolonged vs short duration of dual antiplatelet therapy after percutaneous coronary intervention in patients with or without peripheral arterial disease: a subgroup analysis of the PRODIGY randomized clinical trial. JAMA Cardiology 2016;1(7):795-803. [MEDLINE: ] [DOI] [PubMed] [Google Scholar]
- Gargiulo G, Ariotti S, Santucci A, Piccolo R, Baldo A, Franzone A, et al.Impact of sex on 2-year clinical outcomes in patients treated with 6-month or 24-month dual-antiplatelet therapy duration: a pre-specified analysis from the PRODIGY trial. Jacc: Cardiovascular Interventions 2016;9(17):1780-9. [MEDLINE: ] [DOI] [PubMed] [Google Scholar]
- Gargiulo G, Costa F, Ariotti S, Biscaglia S, Campo G, Esposito G, et al.Impact of proton pump inhibitors on clinical outcomes in patients treated with a 6- or 24-month dual-antiplatelet therapy duration: Insights from the PROlonging Dual-antiplatelet treatment after Grading stent-induced Intimal hyperplasia studY trial. American Heart Journal 2016;174:95-102. [MEDLINE: ] [DOI] [PubMed] [Google Scholar]
- Gargiulo G, Santucci A, Piccolo R, Franzone A, Ariotti S, Baldo A, et al.Impact of chronic kidney disease on 2-year clinical outcomes in patients treated with 6-month or 24-month DAPT duration: an analysis from the PRODIGY trial. Catheterization & Cardiovascular Interventions 2017;90(4):E73-84. [MEDLINE: ] [DOI] [PubMed] [Google Scholar]
- Santucci A, Gargiulo G, Ariotti S, Piccolo R, Franzone A, Magnani G, et al.Impact of chronic kidney disease on two-year clinical outcomes in patients treated with a six-month or 24-month DAPT duration: insights from the PRODIGY trial [abstract]. Eurointervention 2016:104. [EMBASE: 611934574] [Google Scholar]
- Valgimigli M, Borghesi M, Tebaldi M, Vranckx P, Parrinello G, Ferrari R, et al.Should duration of dual antiplatelet therapy depend on the type and/or potency of implanted stent? A pre-specified analysis from the PROlonging Dual antiplatelet treatment after Grading stent-induced Intimal hyperplasia studY (PRODIGY). European Heart Journal 2013;34(12):909-19. [MEDLINE: ] [DOI] [PubMed] [Google Scholar]
- Valgimigli M, Campo G, Monti M, Vranckx P, Percoco G, Tumscitz C, et al.Short- versus long-term duration of dual-antiplatelet therapy after coronary stenting: a randomized multicenter trial. Circulation 2012;125(16):2015-26. [MEDLINE: ] [DOI] [PubMed] [Google Scholar]
- Valgimigli M, Campo G, Percoco G, Monti M, Ferrari F, Tumscitz C, et al.Randomized comparison of 6- versus 24-month clopidogrel therapy after balancing anti-intimal hyperplasia stent potency in all-comer patients undergoing percutaneous coronary intervention Design and rationale for the PROlonging Dual-antiplatelet treatment after Grading stent-induced Intimal hyperplasia study (PRODIGY). American Heart Journal 2010;160(5):804-11. [MEDLINE: ] [DOI] [PubMed] [Google Scholar]
- Valgimigli M, Tebaldi M, Borghesi M, Vranckx P, Campo G, Tumscitz C, et al.Two-year outcomes after first- or second-generation drug-eluting or bare-metal stent implantation in all-comer patients undergoing percutaneous coronary intervention: a pre-specified analysis from the PRODIGY study (PROlonging Dual Antiplatelet Treatment After Grading stent-induced Intimal hyperplasia studY). Jacc: Cardiovascular Interventions 2014;7(1):20-8. [MEDLINE: ] [DOI] [PubMed] [Google Scholar]
RAS‐CAD 2009 {published data only}
- Marcantoni C, Zanoli L, Rastelli S, Tripepi G, Matalone M, Di Landro D, et al.Stenting of renal artery stenosis in coronary artery disease (RAS-CAD) study: a prospective, randomized trial. Journal of Nephrology 2009;22(1):13-6. [MEDLINE: ] [PubMed] [Google Scholar]
- Marcantoni C, Zanoli L, Rastelli S, Tripepi G, Matalone M, Mangiafico S, et al.Effect of renal artery stenting on left ventricular mass: a randomized clinical trial. American Journal of Kidney Diseases 2012;60(1):39-46. [MEDLINE: ] [DOI] [PubMed] [Google Scholar]
REPLACE‐2 2003 {published data only}
- Blankenship JC, Haldis T, Feit F, Hu T, Kleiman NS, Topol EJ, et al.Angiographic adverse events, creatine kinase-MB elevation, and ischemic end points complicating percutaneous coronary intervention (a REPLACE-2 substudy). American Journal of Cardiology 2006;97(11):1591-6. [MEDLINE: ] [DOI] [PubMed] [Google Scholar]
- Chacko M, Lincoff AM, Wolski KE, Cohen DJ, Bittl JA, Lansky AJ, et al.Ischemic and bleeding outcomes in women treated with bivalirudin during percutaneous coronary intervention: a subgroup analysis of the Randomized Evaluation in PCI Linking Angiomax to Reduced Clinical Events (REPLACE)-2 trial. American Heart Journal 2006;151(5):1032-7. [MEDLINE: ] [DOI] [PubMed] [Google Scholar]
- Chew DP, Lincoff AM, Gurm H, Wolski K, Cohen DJ, Henry T, et al.Bivalirudin versus heparin and glycoprotein IIb/IIIa inhibition among patients with renal impairment undergoing percutaneous coronary intervention (a subanalysis of the REPLACE-2 trial). American Journal of Cardiology 2005;95(5):581-5. [MEDLINE: ] [DOI] [PubMed] [Google Scholar]
- Cohen DJ, Lincoff AM, Lavelle TA, Chen HL, Bakhai A, Berezin RH, et al.Economic evaluation of bivalirudin with provisional glycoprotein IIB/IIIA inhibition versus heparin with routine glycoprotein IIB/IIIA inhibition for percutaneous coronary intervention: results from the REPLACE-2 trial. Journal of the American College of Cardiology 2004;44(9):1792-800. [MEDLINE: ] [DOI] [PubMed] [Google Scholar]
- Exaire JE, Butman SM, Ebrahimi R, Kleiman NS, Harrington RA, Schweiger MJ, et al.Provisional glycoprotein IIb/IIIa blockade in a randomized investigation of bivalirudin versus heparin plus planned glycoprotein IIb/IIIa inhibition during percutaneous coronary intervention: predictors and outcome in the Randomized Evaluation in Percutaneous coronary intervention Linking Angiomax to Reduced Clinical Events (REPLACE)-2 trial. American Heart Journal 2006;152(1):157-63. [MEDLINE: ] [DOI] [PubMed] [Google Scholar]
- Feit F, Voeltz MD, Attubato MJ, Lincoff AM, Chew DP, Bittl JA, et al.Predictors and impact of major hemorrhage on mortality following percutaneous coronary intervention from the REPLACE-2 Trial. American Journal of Cardiology 2007;100(9):1364-9. [MEDLINE: ] [DOI] [PubMed] [Google Scholar]
- Gibson CM, Ten Y, Murphy SA, Ciaglo LN, Southard MC, Lincoff AM, et al.Association of prerandomization anticoagulant switching with bleeding in the setting of percutaneous coronary intervention (A REPLACE-2 analysis). American Journal of Cardiology 2007;99(12):1687-90. [MEDLINE: ] [DOI] [PubMed] [Google Scholar]
- Gurm HS, Sarembock IJ, Kereiakes DJ, Young JJ, Harrington RA, Kleiman N, et al.Use of bivalirudin during percutaneous coronary intervention in patients with diabetes mellitus: an analysis from the randomized evaluation in percutaneous coronary intervention linking angiomax to reduced clinical events (REPLACE)-2 trial. Journal of the American College of Cardiology 2005;45(12):1932-8. [MEDLINE: ] [DOI] [PubMed] [Google Scholar]
- Lincoff AM, Bittl JA, Harrington RA, Feit F, Kleiman NS, Jackman JD, et al.Bivalirudin and provisional glycoprotein IIb/IIIa blockade compared with heparin and planned glycoprotein IIb/IIIa blockade during percutaneous coronary intervention: REPLACE-2 randomized trial.[Erratum in: JAMA. 2003 Apr 2;289(13):1638]. JAMA 2003;289(7):853-63. [MEDLINE: ] [DOI] [PubMed] [Google Scholar]
- Lincoff AM, Kleiman NS, Kereiakes DJ, Feit F, Bittl JA, Jackman JD, et al.Long-term efficacy of bivalirudin and provisional glycoprotein IIb/IIIa blockade vs heparin and planned glycoprotein IIb/IIIa blockade during percutaneous coronary revascularization: REPLACE-2 randomized trial.[Erratum in: JAMA. 2006 Jul 5;296(1):46]. JAMA 2004;292(6):696-703. [MEDLINE: ] [DOI] [PubMed] [Google Scholar]
- Maroo A, Lincoff AM.Bivalirudin in PCI: an overview of the REPLACE-2 trial. Seminars in Thrombosis & Hemostasis 2004;30(3):329-36. [MEDLINE: ] [DOI] [PubMed] [Google Scholar]
- Rajagopal V, Lincoff AM, Cohen DJ, Gurm HS, Hu T, Desmet WJ, et al.Outcomes of patients with acute coronary syndromes who are treated with bivalirudin during percutaneous coronary intervention: an analysis from the Randomized Evaluation in PCI Linking Angiomax to Reduced Clinical Events (REPLACE-2) trial. American Heart Journal 2006;152(1):149-54. [MEDLINE: ] [DOI] [PubMed] [Google Scholar]
- Saw J, Lincoff AM, DeSmet W, Betriu A, Rutsch W, Wilcox RG, et al.Lack of clopidogrel pretreatment effect on the relative efficacy of bivalirudin with provisional glycoprotein IIb/IIIa blockade compared to heparin with routine glycoprotein IIb/IIIa blockade: a REPLACE-2 substudy. Journal of the American College of Cardiology 2004;44(6):1194-9. [MEDLINE: ] [DOI] [PubMed] [Google Scholar]
Sakai 1991 {published data only}
- Sakai H, Watanabe S, Inoue I, Tanaka K, Yagame M, Machimura H, et al.Effect of urokinase on preservation of renal function in patients with diabetic nephropathy. Journal of Diabetic Complications 1991;5(2-3):95-7. [MEDLINE: ] [DOI] [PubMed] [Google Scholar]
SPS3 2018 {published data only}
- Ikeme JC, Pergola PE, Scherzer R, Shlipak MG, Benavente OR, Peralta CA.Post hoc analyses of randomized clinical trial for the effect of clopidogrel added to aspirin on kidney function. Clinical Journal of the American Society of Nephrology: CJASN 2017;12(7):1040-7. [MEDLINE: ] [DOI] [PMC free article] [PubMed] [Google Scholar]
STENO‐2 1999 {published data only}
- Gaede P, Lund-Andersen H, Parving HH, Pedersen O.Effect of a multifactorial intervention on mortality in type 2 diabetes. New England Journal of Medicine 2008;358(6):580-91. [MEDLINE: ] [DOI] [PubMed] [Google Scholar]
- Gaede P, Parving H, Pedersen O.Multifactorial intervention in patients with type 2 diabetes: long-term effects on mortality and vascular complications [abstract no: SA-FC042]. Journal of the American Society of Nephrology 2007;18(Abstracts):43A. [CENTRAL: CN-00740461] [Google Scholar]
- Gaede P, Valentine WJ, Palmer AJ, Tucker DM, Lammert M, Parving HH, et al.Cost-effectiveness of intensified versus conventional multifactorial intervention in type 2 diabetes: results and projections from the Steno-2 study. Diabetes Care 2008;31(8):1510-5. [MEDLINE: ] [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gaede P, Vedel P, Larsen N, Jensen G, Parving H, Pedersen O.The Steno-2 study: intensified multifactorial intervention reduces the risk of cardiovascular disease in patients with type 2 diabetes and microalbuminuria [abstract no: 181]. In: 38th Annual Meeting of the European Association for the Study of Diabetes (EASD); 2002 Sept 1-5; Budapest, Hungary. 2002. [CENTRAL: CN-01912459]
- Gaede P, Vedel P, Larsen N, Jensen G, Parving HH, Pedersen O.The STENO-2 study: intensified multifactorial intervention reduces the risk of cardiovascular disease in patients with type 2 diabetes and microalbuminuria [abstract no: F-FC031]. Journal of the American Society of Nephrology 2002;13(September, Program & Abstracts):72A. [CENTRAL: CN-00445410] [Google Scholar]
- Gaede P, Vedel P, Larsen N, Jensen GV, Parving HH, Pedersen O.Multifactorial intervention and cardiovascular disease in patients with type 2 diabetes. New England Journal of Medicine 2003;348(5):383-93. [MEDLINE: ] [DOI] [PubMed] [Google Scholar]
- Gaede P, Vedel P, Obel J, Parving HH, Pedersen O.Intensive multifactorial intervention in NIDDM patients with persistent microalbuminuria [abstract no: A0561]. Journal of the American Society of Nephrology 1996;7(9):1357. [CENTRAL: CN-00445411] [Google Scholar]
- Gaede P, Vedel P, Parving HH, Pedersen O.Elevated levels of plasma von Willebrand factor and the risk of macro- and microvascular disease in type 2 diabetic patients with microalbuminuria. Nephrology Dialysis Transplantation 2001;16(10):2028-33. [MEDLINE: ] [DOI] [PubMed] [Google Scholar]
- Gaede P, Vedel P, Parving HH, Pedersen O.Intensified multifactorial intervention in patients with type 2 diabetes mellitus and microalbuminuria: the Steno type 2 randomised study. Lancet 1999;353(9153):617-22. [MEDLINE: ] [DOI] [PubMed] [Google Scholar]
- Gaede PH, Jepsen PV, Parving HH, Pedersen OB.Intensified multifactorial intervention in patients with type 2 diabetes mellitus and microalbuminuria: the Steno-2 study [Intensiveret multifaktoriel intervention hos patienter med type 2-diabetes mellitus og mikroalbuminuri: Steno-2-studiet]. Ugeskrift for Laeger 1999;161(30):4277-85. [MEDLINE: ] [PubMed] [Google Scholar]
- Oellgaard J, Gaede P, Rossing P, Persson F, Parving HH, Pedersen O.Intensified multifactorial intervention in type 2 diabetics with microalbuminuria leads to long-term renal benefits.[Erratum in: Kidney Int. 2017 May;91(5):1257; PMID: 28407880]. Kidney International 2017;91(4):982-8. [MEDLINE: ] [DOI] [PubMed] [Google Scholar]
Swan 1995a {published data only}
- Halstenson CE, Swan SK, Collins AJ, Ellefson J, Parr K, Blue J, et al.Pharmacologic profile of diaspirin crosslinked hemoglobin (DCLHB) in hemodialysis (HD) patients [abstract no: 84P]. Journal of the American Society of Nephrology 1994;5(3):451. [CENTRAL: CN-00583893] [Google Scholar]
- Swan SK, Halstenson CE, Collins AJ, Colburn WA, Blue J, Przybelski RJ.Pharmacologic profile of diaspirin cross-linked hemoglobin in hemodialysis patients. American Journal of Kidney Diseases 1995;26(6):918-23. [MEDLINE: ] [DOI] [PubMed] [Google Scholar]
- Swan SK, Halstenson CE, Collins AJ, Ellefson J, Parr K, Blue J, et al.Pharmacodynamic and pharmacokinetic parameters of diaspirin cross-linked hemoglobin (DCLHB) in hemodialysis (HD) patients [abstract]. In: ISN XIII International Congress of Nephrology; 1995 Jul 2-6; Madrid, Spain. 1995:559. [CENTRAL: CN-00509497]
TRILOGY ACS 2010 {published data only}
- Bakal JA, Roe MT, Ohman EM, Goodman SG, Fox KA, Zheng Y, et al.Applying novel methods to assess clinical outcomes: insights from the TRILOGY ACS trial. European Heart Journal 2015;36(6):385-92A. [MEDLINE: ] [DOI] [PubMed] [Google Scholar]
- Chin CT, Neely B, Magnus OE, Armstrong PW, Corbalan R, White HD, et al.Time-varying effects of prasugrel versus clopidogrel on the long-term risks of stroke after acute coronary syndromes: results from the TRILOGY ACS trial. Stroke 2016;47(4):1135-9. [MEDLINE: ] [DOI] [PubMed] [Google Scholar]
- Chin CT, Roe MT, Fox KA, Prabhakaran D, Marshall DA, Petitjean H, et al.Study design and rationale of a comparison of prasugrel and clopidogrel in medically managed patients with unstable angina/non-ST-segment elevation myocardial infarction: the TaRgeted platelet Inhibition to cLarify the Optimal strateGy to medicallY manage Acute Coronary Syndromes (TRILOGY ACS) trial. American Heart Journal 2010;160(1):16-22. [MEDLINE: ] [DOI] [PubMed] [Google Scholar]
- Cornel JH, Ohman EM, Neely B, Clemmensen P, Sritara P, Zamoryakhin D, et al.Impact of smoking status on platelet function and clinical outcomes with prasugrel vs. clopidogrel in patients with acute coronary syndromes managed without revascularization: insights from the TRILOGY ACS trial.[Erratum in: Am Heart J. 2014 Oct;168(4):605]. American Heart Journal 2014;168(1):76-87. [MEDLINE: ] [DOI] [PubMed] [Google Scholar]
- Cornel JH, Tricoci P, Horton J, Moliterno D, Wallentin L, Armstrong P, et al.Effects of glycoprotein IIB/IIIA inhibitors in combination with vorapaxar, a platelet thrombin-receptor antagonist, among patients with non-St-segment elevation acute coronary syndromes: Insights from the TRACER trial [abstract]. Journal of the American College of Cardiology 2013;61(10 Suppl 1):E102. [EMBASE: 71019466] [Google Scholar]
- Gurbel PA, Erlinge D, Ohman EM, Neely B, Neely M, Goodman SG, et al.Platelet function during extended prasugrel and clopidogrel therapy for patients with ACS treated without revascularization: the TRILOGY ACS platelet function substudy. JAMA 2012;308(17):1785-94. [MEDLINE: ] [DOI] [PubMed] [Google Scholar]
- Hagstrom E, Roe MT, Hafley G, Neely ML, Sidhu MS, Winters KJ, et al.Association between very low levels of high-density lipoprotein cholesterol and long-term outcomes of patients with acute coronary syndrome treated without revascularization: insights from the TRILOGY ACS trial. Clinical Cardiology 2016;39(6):329-37. [MEDLINE: ] [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hinohara TT, Roe MT, White HD, Fox KA, Bhatt DL, Hamm C, et al.Outcomes of patients receiving downstream revascularization after initial medical management for non-st-segment elevation acute coronary syndromes (from the TRILOGY ACS trial). American Journal of Cardiology 2018;122(8):1322-9. [MEDLINE: ] [DOI] [PubMed] [Google Scholar]
- Jackson LR 2nd, Piccini JP, Cyr DD, Roe MT, Neely ML, Martinez F, et al.Dual antiplatelet therapy and outcomes in patients with atrial fibrillation and acute coronary syndromes managed medically without revascularization: insights from the TRILOGY ACS trial. Clinical Cardiology 2016;39(9):497-506. [MEDLINE: ] [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kaul P, Ohman EM, Knight JD, Anstrom KJ, Roe MT, Boden WE, et al.Health-related quality of life outcomes with prasugrel among medically managed non-ST-segment elevation acute coronary syndrome patients: Insights from the Targeted Platelet Inhibition to Clarify the Optimal Strategy to Medically Manage Acute Coronary Syndromes (TRILOGY ACS) trial. American Heart Journal 2016;178:55-64. [MEDLINE: ] [DOI] [PubMed] [Google Scholar]
- Lopes RD, Leonardi S, Neely B, Neely ML, Ohman EM, Ardissino D, et al.Spontaneous MI after non-st-segment elevation acute coronary syndrome managed without revascularization: the TRILOGY ACS trial. Journal of the American College of Cardiology 2016;67(11):1289-97. [MEDLINE: ] [DOI] [PubMed] [Google Scholar]
- Melloni C, Cornel JH, Hafley G, Neely ML, Clemmensen P, Zamoryakhin D, et al.Impact of chronic kidney disease on long-term ischemic and bleeding outcomes in medically managed patients with acute coronary syndromes: Insights from the TRILOGY ACS Trial. European Heart Journal: Acute Cardiovascular Care 2016;5(6):443-54. [MEDLINE: ] [DOI] [PubMed] [Google Scholar]
- White HD, Westerhout CM, Alexander KP, Roe MT, Winters KJ, Cyr DD, et al.Frailty is associated with worse outcomes in non-ST-segment elevation acute coronary syndromes: Insights from the TaRgeted platelet Inhibition to cLarify the Optimal strateGy to medicallY manage Acute Coronary Syndromes (TRILOGY ACS) trial. European Heart Journal: Acute Cardiovascular Care 2016;5(3):231-42. [MEDLINE: ] [DOI] [PubMed] [Google Scholar]
- Wiviott SD, White HD, Ohman EM, Fox KA, Armstrong PW, Prabhakaran D, et al.Prasugrel versus clopidogrel for patients with unstable angina or non-ST-segment elevation myocardial infarction with or without angiography: a secondary, prespecified analysis of the TRILOGY ACS trial.[Erratum in: Lancet. 2013 Aug 31;382(9894):768]. Lancet 2013;382(9892):605-13. [MEDLINE: ] [DOI] [PubMed] [Google Scholar]
- Yan AT, Roe MT, Neely M, Cyr DD, White H, Fox KA, et al.Early discontinuation of prasugrel or clopidogrel in acute coronary syndromes: insights from the TRILOGY ACS trial. Coronary Artery Disease 2018;29(6):469-76. [MEDLINE: ] [DOI] [PubMed] [Google Scholar]
Woo 1987 {published data only}
- Woo KT, Chiang GS, Lim CH.Follow-up renal biopsies in IgA nephritic patients on triple therapy. Clinical Nephrology 1987;28(6):304-5. [MEDLINE: ] [PubMed] [Google Scholar]
- Woo KT, Chiang GS, Yap HK, Lim CH.Controlled therapeutic trial of IgA nephritis with follow-up renal biopsies. Annals of the Academy of Medicine, Singapore 1988;17(2):226-31. [MEDLINE: ] [PubMed] [Google Scholar]
- Woo KT, Edmondson RP, Yap HK, Wu AY, Chiang GS, Lee EJ, et al.Effects of triple therapy on the progression of mesangial proliferative glomerulonephritis. Clinical Nephrology 1987;27(2):56-64. [MEDLINE: ] [PubMed] [Google Scholar]
- Woo KT, Lee GS, Lau YK, Chiang GS, Lim CH.Effects of triple therapy in IgA nephritis: a follow-up study 5 years later. Clinical Nephrology 1991;36(2):60-6. [MEDLINE: ] [PubMed] [Google Scholar]
- Woo KT, Lee GSL, Lau YK, Chiang GSC, Lim CH.Anti platelet therapy in IgA nephritis [abstract]. In: 11th International Congress of Nephrology; 1990 Jul 15-20; Tokyo, Japan. 1990:13. [CENTRAL: CN-00448412]
Wu 2018a {published data only}
- Wu HB, Tian HP, Wang XC, Bai SR, Li XN, Zhang LN, et al.Clinical efficacy of ticagrelor in patients undergoing emergency intervention for acute myocardial infarction and its impact on platelet aggregation rate. American Journal of Translational Research 2018;10(7):2175-83. [MEDLINE: ] [PMC free article] [PubMed] [Google Scholar]
Yang 2014a {published data only}
- Yang Y, Kong D, Wang C, Chen G, Shan F, Qi K, et al.Inhibition of platelet activation could decrease thrombotic events in hemodialysis PF4/H antibody-positive patients. Renal Failure 2014;36(6):870-6. [MEDLINE: ] [DOI] [PubMed] [Google Scholar]
Yeh 2017 {published data only}
- Yeh CH, Huang TS, Wang YC, Huang PF, Huang TY, Chen TP, et al.Initiation of antiplatelet medication after surgical thrombectomy jeopardized arteriovenous graft longevity. Journal of Vascular Access 2017;18(3):207-13. [MEDLINE: ] [DOI] [PubMed] [Google Scholar]
Yoshikawa 1999 {published data only}
- Ito H, Yoshikawa N.Prospective multicenter controlled therapeutic trial in IgA nephropathy in Japanese children: a preliminary report [abstract no: S-I-2]. Pediatric Nephrology 1992;6(6):C208. [CENTRAL: CN-01658022] [Google Scholar]
- Kamei K, Nakanishi K, Ito S, Saito M, Sako M, Ishikura K, et al.Long-term results of a randomized controlled trial in childhood IgA nephropathy. Clinical Journal of the American Society of Nephrology: CJASN 2011;6(6):1301-07. [MEDLINE: ] [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yoshikawa N, Ito H, Sakai T, Takekoshi Y, Honda M, Awazu M, et al.A controlled trial of combined therapy for newly diagnosed severe childhood IgA nephropathy. The Japanese Pediatric IgA Nephropathy Treatment Study Group. Journal of the American Society of Nephrology 1999;10(1):101-9. [MEDLINE: ] [DOI] [PubMed] [Google Scholar]
- Yoshikawa N, Ito H.Combined therapy with prednisolone, azathioprine, heparin-warfarin, and dipyridamole for paediatric patients with severe IgA nephropathy - is it relevant for adult patients? Nephrology Dialysis Transplantation 1999;14(5):1097-9. [MEDLINE: ] [DOI] [PubMed] [Google Scholar]
- Yoshikawa N, Ito H.Corticosteroids and immunosuppressive drugs [abstract no: S8.2]. Pediatric Nephrology 2001;16(8):C31. [CENTRAL: CN-00448482] [Google Scholar]
- Yoshikawa N, Itoh H, Japanese Pediatric IgA Nephropathy Treatment Study Group.A controlled trial of prednisolone (P), azathioprine (A), heparin-warfarin (H-W) and dipyridamole (D) in newly diagnosed severe childhood IgA nephropathy (IGAN) [abstract no: A0779]. Journal of the American Society of Nephrology 1996;7(9):1401. [CENTRAL: CN-00583182] [DOI] [PubMed] [Google Scholar]
Zhang 2009a {published data only}
- Zhang Y, Zhang XD, Ma LL, Guan DL.Relationship between platelet activation and acute rejection after renal transplantation. Transplantation Proceedings 2009;41(5):1547-51. [MEDLINE: ] [DOI] [PubMed] [Google Scholar]
Zibari 1995 {published data only}
- Zibari GB, Gadallah MF, Landreneau MD, McMillian R, Bridges R, Costley K, et al.The efficacy and complications of aspirin versus heparin in postoperative prophylaxis against thrombosis in newly placed hemodialysis access [abstract no: 195]. Journal of the American Society of Nephrology 1995;6(3):507. [CENTRAL: CN-00486594] [Google Scholar]
Zimmerman 1983 {published data only}
- Zimmerman SW, Moorthy AV, Dreher WH, Friedman A, Varanasi U.Prospective trial of warfarin and dipyridamole in patients with membranoproliferative glomerulonephritis. American Journal of Medicine 1983;75(6):920-7. [MEDLINE: ] [DOI] [PubMed] [Google Scholar]
References to ongoing studies
A‐CLOSE 2019 {published data only}
- Kim BK.A randomized comparison of CLOpidogrel monotherapy versus extended dual-antiplatelet therapy beyond 12 months after implantation of drug-eluting StEnts in high-risk lesions or patients; A-CLOSE trial. www.cris.nih.go.kr/cris/search/detailSearch.do/18621 (first received 30 April 2019).
ALTIC 2016 {published data only}
- Alexopoulos D.A randomized, pharmacodynamic comparison of low dose ticagrelor to clopidogrel in patients with prior myocardial infarction (ALTIC). www.clinicaltrials.gov/show/nct02663713 (first received 26 January 2016).
ALTIC‐2 2018 {published data only}
- Alexopoulos D.Low dose ticagrelor versus low dose prasugrel in patients with prior myocardial infarction (ALTIC-2) [A randomized, pharmacodynamic comparison of low dose ticagrelor (60mg Bid) to low dose prasugrel (5mg od) in patients with prior myocardial infarction]. www.clinicaltrials.gov/show/nct03387826 (first received 2 January 2018 ).
- EudraCT2016-004959-80.A randomized, pharmacodynamic comparison of ticagrelor 60mg bid vs prasugrel 5mg in patients with prior myocardial infarction. www.clinicaltrialsregister.eu/ctr-search/trial/2016-004959-80/GR (first received 14 June 2017).
ATTACK 2018 {published data only}40920200
- Gallagher H, Roderick P.Aspirin to target arterial events in chronic kidney disease (ATTACK) protocol final version 1.1. www.njl-admin.nihr.ac.uk/document/download/2035436 2018.
ChiCTR1900021393 {published data only}
- Zhao J.Antiplatelet therapy for prevention of atherosclerosis in chronic kidney disease: a perspective, multi-center randomized controlled trial [Antiplatelet prophylaxis for atherosclerosis in chronic kidney disease: a multicenter, randomized, pacebo-controlled trial]. www.chictr.org.cn/showproj.aspx?proj=34865 (first received 18 February 2019).
IRCT2013012412256N1 {published data only}
- IRCT2013012412256N1.Evaluation the effect of clopidogrel in prevention of access graft thrombosis in upper extrimity in patients undergoing hemodialysis in Emam Reza's Hospital - Kermanshah,2012-2013. www.en.irct.ir/trial/12352 (first received 10 May 2013).
IRCT2013100114333N8 {published data only}
- IRCT2013100114333N8.Study of aspirin effects on Permcath function in dialysis patients [Study of effects use and without use of aspirin on Permcath function in dialysis patients]. www.en.irct.ir/trial/13944 (first received 4 October 2013).
IRCT20171023036953N1 {published data only}
- IRCT20171023036953N1.The effect of Cilostazol on the mean time of arteriovenous fistula maturation and its comparison to control group in patients with chronic renal failure referring to Emam Reza hospital of Mashhad University of Medical Sciences. www.en.irct.ir/trial/27466 (first received 18 December 2017).
LEDA 2017 {published data only}
- Violi F, Targher G, Vestri A, Carnevale R, Averna M, Farcomeni A, et al.Effect of aspirin on renal disease progression in patients with type 2 diabetes: A multicenter, double-blind, placebo-controlled, randomized trial. The renaL disEase progression by aspirin in diabetic pAtients (LEDA) trial. Rationale and study design. American Heart Journal 2017;189:120-7. [MEDLINE: ] [DOI] [PubMed] [Google Scholar]
Lemos Cerqueira 2018 {published data only}
- Lemos Cerqueira T, Fartolino Guerrero A, Perez Fermin CK, Wang R, Balbino EE, Breeze JL, et al.The use of aspirin to reduce the risk of thrombotic events in patients with end-stage renal disease: protocol for a randomized controlled trial. JMIR Research Protocols 2018;7(8):e10516. [MEDLINE: ] [DOI] [PMC free article] [PubMed] [Google Scholar]
NCT00272831 {published data only}
- Tong PC.The use of cilostazol in patients with diabetic nephropathy [A randomised, double-blind, placebo-controlled study of cilostazol 100 mg twice daily in the treatment of diabetic nephropathy in Hong Kong Chinese]. www.clinicaltrials.gov/show/nct00272831 (first received 9 January 2006).
NCT01198379 {published data only}
- Tarng DC.Aspirin in the prevention of cardiovascular events in hemodialysis patients [Efficacy of monitoring of aspirin responsiveness in the prevention of cardiovascular events and decrease in bleeding complications in patients with end-stage kidney disease undergoing hemodialysis]. www.clinicaltrials.gov/ct2/show/NCT01198379 (first received 10 September 2010).
NCT01743014 {published data only}
- Bougatsa VF.Ramipril and clopidogrel in oxidative stress, vascular inflammation and endothelial dysfunction in type 2 diabetes and diabetic nephropathy [A prospective, randomized, two period, with an intermediate wash out period, cross-over study to compare the effects of either combined therapy with ramipril and clopidogrel or ramipril monotherapy on oxidative stress, vascular inflammation and endothelial dysfunction in patients with type 2 diabetes and diabetic nephropathy]. www.clinicaltrials.gov/show/nct01743014 (first received 6 December 2012).
NCT02394145 {published data only}
- Kim W, Woo JS.Genotype and platelet reactivity in patients on hemodialysis [The relationship between genotype and platelet reactivity in patients treated with ticagrelor versus clopidogrel: PIANO genotype study]. www.clinicaltrials.gov/show/nct02394145 (first received 20 March 2015).
NCT02459288 {published data only}
- Liu PY.Platelet resistance with ticagrelor or standard-dose clopidogrel among CKD and ACS patients (APPROVE-SCKD) [A comParison on Platelet Resistance with ticagrelor or standard-dose clopidogrel study among SeVerE Chronic Kidney Disease/end-stage-renal-disease patients with recent acute coronary syndrome]. www.clinicaltrials.gov/show/nct02459288 (first received 2 June 2015).
NCT03039205 {published data only}
- Nicolau JC.Platelet aggregation in patients with coronary artery disease and kidney dysfunction taking clopidogrel or ticagrelor [Evaluation of platelet aggregation and adenosine levels in patients with coronary artery disease and chronic kidney dysfunction taking dual antiplatelet therapy with aspirin and clopidogrel or ticagrelor]. www.clinicaltrials.gov/show/nct03039205 (first received 1 February 2017).
NCT03150667 {published data only}
- Banerjee S, Baskar A.Study comparing treatment effectiveness of guideline indicated APT for ACS in patients with CKD (CPRS-SKD) [Pragmatic randomized controlled trial comparing treatment effectiveness of guideline indicated anti-platelet therapy for acute coronary syndrome in patients with chronic kidney disease]. www.clinicaltrials.gov/show/nct03150667 (first received 12 May 2017).
NCT03649711 {published data only}
- Nishank J.Chronic kidney disease (CKD) platelet study [A mechanistic study in patients with non-dialysis chronic kidney disease to investigate altered platelet response to antiplatelet therapy (CKD-Platelet study)]. www.clinicaltrials.gov/show/nct03649711 (first received 28 August 2018).
Park 2010 {published data only}
- Ki YJ, Kwon SA, Kim HL, Seo JB, Chung WY.The prevention of contrast induced nephropathy by sarpogrelate: a prospective randomized controlled clinical trial. Journal of Korean Medical Science 2019;34(40):e261. [MEDLINE: ] [DOI] [PMC free article] [PubMed] [Google Scholar]
- Park K, Chung WY, Seo JB, Kim SH, Zo JH, Kim MA, et al.The prevention of contrast induced nephropathy by sarpogrelate in patients with chronic kidney disease: a study protocol for a prospective randomized controlled clinical trial. Trials [Electronic Resource] 2010;11:122. [MEDLINE: ] [DOI] [PMC free article] [PubMed] [Google Scholar]
PRASTO‐III 2018 {published data only}
- JapicCTI-184141.PRASTRO-III: a double-blind study of CS-747S versus clopidogrel sulfate in patients with thrombotic stroke having risk factors for stroke recurrence. www.clinicaltrials.jp/cti-user/trial/ShowDirect jsp?japicId=JapicCTI-184141 (first received 25 October 2018).
SERENADE 2015 {published data only}
- Lee SA, Suh JW, Park JJ, Yoon CH, Cho YS, Youn TJ, et al.Study design of the influence of SErotonin inhibition on patients with RENAl impairment or diabetes undergoing drug-eluting stent implantation (SERENADE) study: A multicenter, open-label, prospective, randomized study. Contemporary Clinical Trials 2015;43:20-4. [MEDLINE: ] [DOI] [PubMed] [Google Scholar]
SONATA 2013 {published data only}
- Choi DS.Effect of sarpogrelate on the nephropathy in type 2 diabetes (SONATA study). www.clinicaltrials.gov/show/NCT01869881 (first received June 5 2013).
TROUPER 2020 {published data only}
- Laine M, Lemesle G, Burtey S, Cayla G, Range G, Quaino G, et al.TicagRelor Or Clopidogrel in severe or terminal chronic kidney patients Undergoing PERcutaneous coronary intervention for acute coronary syndrome: The TROUPER trial. American Heart Journal 2020;225:19-26. [MEDLINE: ] [DOI] [PubMed] [Google Scholar]
TWILIGHT 2016 {published data only}
- Pandit N, Parakh N, Gupta S.TWILIGHT Study The anti platelet therapy with both ticagrelor and aspirin for 3 months after coronary intervention followed by ticagrelor only for a year rather than both aspirin and ticagrelor is better in reducing the ischemic events in high risk patients. www.ctri.nic.in/Clinicaltrials/pdf_generate php?trialid=12839&EncHid=&modid=&compid=%27,%2712839det%27 (first received 13 July 2016).
UMIN000003891 {published data only}
- UMIN000003891.Examination concerning utility and safety of cilostazol use in patients with PAD conplicated to CKD. www.upload.umin.ac.jp/cgi-open-bin/ctr_e/ctr_view cgi?recptno=R000003304 (first received 15 July 2010).
VA PTXRx 2018 {published data only}
- Leehey DJ, Carlson K, Reda D, Polzin L, Clise CE, Paine T, et al.Pentoxifylline in diabetic kidney disease: the VA pentoxifylline in diabetic kidney disease PTXRX study [abstract no: PUB074]. Journal of the American Society of Nephrology 2019;30(Abstract Suppl):1092. [EMBASE: 633771821] [Google Scholar]
- Leehey DJ, Craig I, Reda D, Carlson K, Conner TA, Agarwal R, et al.Design of pentoxifylline in diabetic kidney disease (VA PTXRx) [abstract no: SA-PO154]. Journal of the American Society of Nephrology 2018;29(Abstract Suppl):775. [EMBASE: 633732689] [Google Scholar]
Additional references
Aakhus 1999
- Aakhus S, Dahl K, Wideroe TE.Cardiovascular morbidity and risk factors in renal transplant patients. Nephrology Dialysis Transplantation 1999;14(3):648-54. [MEDLINE: ] [DOI] [PubMed] [Google Scholar]
Amann 2003
- Amann K, Tyralla K, Gross ML, Eifert T, Adamczak M, Ritz E.Special characteristics of atherosclerosis in chronic renal failure. Clinical Nephrology 2003;60 Suppl 1:S13-21. [MEDLINE: ] [PubMed] [Google Scholar]
ANZDATA 2019
- Australia & New Zealand Dialysis & Transplant Registry.The 42nd Annual Report 2019 (Data to 2018). www.anzdata.org.au/?s=annual+report&data-group=anzdata (accessed 24 October 2021).
ATT 2002
- Antithrombotic Trialists' Collaboration.Collaborative meta-analysis of randomised trials of antiplatelet therapy for prevention of death, myocardial infarction, and stroke in high risk patients.[Erratum in: BMJ 2002 Jan 19;324(7330):141]. BMJ 2002;324(7329):71-86. [MEDLINE: ] [DOI] [PMC free article] [PubMed] [Google Scholar]
ATT 2009
- Antithrombotic Trialists' (ATT) Collaboration, Baigent C, Blackwell L, Collins R, Emberson J, Godwin J, et al.Aspirin in the primary and secondary prevention of vascular disease: collaborative meta-analysis of individual participant data from randomised trials. Lancet 2009;373(9678):1849-60. [MEDLINE: ] [DOI] [PMC free article] [PubMed] [Google Scholar]
AusDiab 2003
- Chadban SJ, Briganti EM, Kerr PG, Dunstan DW, Welborn TA, Zimmet PZ, et al.Prevalence of kidney damage in Australian adults: The AusDiab kidney study. Journal of the American Society of Nephrology 2006;14(7 Suppl 2):S131-8. [MEDLINE: ] [DOI] [PubMed] [Google Scholar]
Berger 2003
- Berger AK, Duval S, Krumholz HM.Aspirin, beta-blocker, and angiotensin-converting enzyme inhibitor therapy in patients with end-stage renal disease and an acute myocardial infarction. Journal of the American College of Cardiology 2003;42(2):201-8. [MEDLINE: ] [DOI] [PubMed] [Google Scholar]
Best 2008
- Best PJ, Steinhubl SR, Berger PB, Dasgupta A, Brennan DM, Szczech LA, et al.The efficacy and safety of short- and long-term dual antiplatelet therapy in patients with mild or moderate chronic kidney disease: results from the Clopidogrel for the Reduction of Events During Observation (CREDO) trial. American Heart Journal 2008;155(4):687-93. [MEDLINE: ] [DOI] [PubMed] [Google Scholar]
Bonomini 1986
- Bonomini V, Vangelista A, Stefoni S, Scolari MP, Frasca GM, Raimondi C.Use of defibrotide in renal transplantation in man. Haemostasis 1986;16 Suppl 1:48-50. [MEDLINE: ] [DOI] [PubMed] [Google Scholar]
CARI 2000
- Caring for Australasians with Renal Impairment (CARI).Vascular access. www.cariguidelines.org/guidelines/dialysis/vascular-access/ (accessed 24 October 2021).
Casas 2005
- Casas JP, Chua W, Loukogeorgakis S, Vallance P, Smeeth L, Hingorani AD, et al.Effect of inhibitors of the renin-angiotensin system and other antihypertensive drugs on renal outcomes: systematic review and meta-analysis. Lancet 2005;366(9502):2026-33. [MEDLINE: ] [DOI] [PubMed] [Google Scholar]
Coleman 2010
- Coleman CI, Tuttle LA, Teevan C, Baker WL, White CM, Reinhart KM.Antiplatelet agents for the prevention of arteriovenous fistula and graft thrombosis: a meta analysis. International Journal of Clinical Practice 2010;64(9):1239-44. [MEDLINE: ] [DOI] [PubMed] [Google Scholar]
Collins 2003
- Collins AJ.Cardiovascular mortality in end-stage renal disease. American Journal of the Medical Sciences 2003;325(4):163-7. [MEDLINE: ] [DOI] [PubMed] [Google Scholar]
Curtis 2005
- Curtis BM, Parfrey PS.Congestive heart failure in chronic kidney disease: disease-specific mechanisms of systolic and diastolic heart failure and management. Cardiology Clinics 2005;23(3):275-84. [MEDLINE: ] [DOI] [PubMed] [Google Scholar]
de Jager 2009
- Jager DJ, Grootendorst DC, Jager KJ, Dijk PC, Tomas LM, Ansell D, et al.Cardiovascular and noncardiovascular mortality among patients starting dialysis. JAMA 2009;302(16):1782-9. [MEDLINE: ] [DOI] [PubMed] [Google Scholar]
Dikow 2005
- Dikow R, Zeier M, Ritz E.Pathophysiology of cardiovascular disease and renal failure. Cardiology Clinics 2005;23(3):311-7. [MEDLINE: ] [DOI] [PubMed] [Google Scholar]
Fields 2004
- Fields LE, Burt VL, Cutler JA, Hughes J, Roccella EJ, Sorlie P.The burden of adult hypertension in the United States 1999 to 2000: a rising tide. Hypertension 2004;44(4):398-404. [MEDLINE: ] [DOI] [PubMed] [Google Scholar]
Foley 1995
- Foley RN, Parfrey PS, Harnett JD, Kent GM, Martin CJ, Murray DC, et al.Clinical and echocardiographic disease in patients starting end-stage renal disease therapy. Kidney International 1995;47(1):186-92. [MEDLINE: ] [DOI] [PubMed] [Google Scholar]
Fort 2005
- Fort J.Chronic renal failure: a cardiovascular risk factor. Kidney International - Supplement 2005;99:S25-9. [MEDLINE: ] [DOI] [PubMed] [Google Scholar]
Go 2004
- Go AS, Chertow GM, Fan D, McCulloch CE, Hsu CY.Chronic kidney disease and the risks of death, cardiovascular events, and hospitalization.[Erratum in: N Engl J Med. 2008;18(4):4]. New England Journal of Medicine 2004;351(13):1296-305. [MEDLINE: ] [DOI] [PubMed] [Google Scholar]
GRADE 2008
- Guyatt GH, Oxman AD, Vist GE, Kunz R, Falck-Ytter Y, Alonso-Coello P, et al.GRADE: an emerging consensus on rating quality of evidence and strength of recommendations. BMJ 2008;336(7650):924-6. [MEDLINE: ] [DOI] [PMC free article] [PubMed] [Google Scholar]
GRADE 2011a
- Guyatt G, Oxman AD, Akl EA, Kunz R, Vist G, Brozek J, et al.GRADE guidelines: 1. Introduction-GRADE evidence profiles and summary of findings tables. Journal of Clinical Epidemiology 2011;64(4):383-94. [MEDLINE: ] [DOI] [PubMed] [Google Scholar]
GRADE 2011b
- GRADE Working Group.Grading of Recommendations Assessment, Development and Evaluation (GRADE). www.gradeworkinggroup.org (last accessed 24 October 2021).
GRADE 2011c
- Guyatt GH, Oxman AD, Kunz R, Brozek J, Alonso-Coello P, Rind D, et al.GRADE guidelines 6. Rating the quality of evidence--imprecision. Journal of Clinical Epidemiology 2011;64(12):1283-93. [MEDLINE: ] [DOI] [PubMed] [Google Scholar]
Gurbel 2019
- Gurbel PA, Fox KA, Tantry US, Ten Cate H, Weitz JI.Combination antiplatelet and oral anticoagulant therapy in patients with coronary and peripheral artery disease. Circulation 2019;139(18):2170-85. [MEDLINE: ] [DOI] [PubMed] [Google Scholar]
Higgins 2003
- Higgins JP, Thompson SG, Deeks JJ, Altman DG.Measuring inconsistency in meta-analyses. BMJ 2003;327(7414):557-60. [MEDLINE: ] [DOI] [PMC free article] [PubMed] [Google Scholar]
Higgins 2020
- Higgins JP, Thomas J, Chandler J, Cumpston M, Li T, Page MJ, Welch VA (editors).Cochrane Handbook for Systematic Reviewsof Interventions version 6.1 (updated September 2020). Cochrane, 2020. Available from www.training.cochrane.org/handbook.
Hung 2014
- Hung CC, Yang ML, Lin MY, Lin HY, Lim LM, Kuo HT, et al.Dipyridamole treatment is associated with improved renal outcome and patient survival in advanced chronic kidney disease. Kaohsiung Journal of Medical Science 2014;30(12):599-607. [MEDLINE: ] [DOI] [PubMed] [Google Scholar]
Kasiske 2000
- Kasiske BL.Cardiovascular disease after renal transplantation. Seminars in Nephrology 2000;20(2):176-87. [MEDLINE: ] [PubMed] [Google Scholar]
Kaw 2006
- Kaw D, Malhotra D.Platelet dysfunction and end-stage renal disease. Seminars in Dialysis 2006;19(4):317-22. [MEDLINE: ] [DOI] [PubMed] [Google Scholar]
Keith 2004
- Keith DS, Nichols GA, Gullion CM, Brown JB, Smith DH.Longitudinal follow-up and outcomes among a population with chronic kidney disease in a large managed care organization. Archives of Internal Medicine 2004;164(6):659-63. [MEDLINE: ] [DOI] [PubMed] [Google Scholar]
Koren‐Morag 2006
- Koren-Morag N, Goldbourt U, Tanne D.Renal dysfunction and risk of ischemic stroke or TIA in patients with cardiovascular disease. Neurology 2006;67(2):224-8. [MEDLINE: ] [DOI] [PubMed] [Google Scholar]
Mann 2001
- Mann JF, Gerstein HC, Pogue J, Bosch J, Yusuf S.Renal insufficiency as a predictor of cardiovascular outcomes and the impact of ramipril: the HOPE randomized trial. Annals of Internal Medicine 2001;134(8):629-36. [MEDLINE: ] [DOI] [PubMed] [Google Scholar]
McCullough 2002
- McCullough PA, Sandberg KR, Borzak S, Hudson MP, Garg M, Manley HJ.Benefits of aspirin and beta-blockade after myocardial infarction in patients with chronic kidney disease. American Heart Journal 2002;144(2):226-32. [MEDLINE: ] [DOI] [PubMed] [Google Scholar]
Mokdad 2003
- Mokdad AH, Ford ES, Bowman BA, Dietz WH, Vinicor F, Bales VS, et al.Prevalence of obesity, diabetes, and obesity-related health risk factors, 2001. JAMA 2003;289(1):76-9. [MEDLINE: ] [DOI] [PubMed] [Google Scholar]
Mosenkis 2004
- Mosenkis A, Berns JS.Use of low molecular weight heparins and glycoprotein IIb/IIIa inhibitors in patients with chronic kidney disease. Seminars in Dialysis 2004;17(5):411-5. [MEDLINE: ] [DOI] [PubMed] [Google Scholar]
NHANES 2010
- National Health and Nutrition Examination Survey. www.cdc.gov/nchs/nhanes.htm (accessed 24 October 2021).
Norris 2006
- Norris K, Bourgoigne J, Gassman J, Hebert L, Middleton J, Phillips RA, et al.Cardiovascular outcomes in the African American Study of Kidney Disease and Hypertension (AASK) Trial. American Journal of Kidney Diseases 2006;48(5):739-51. [MEDLINE: ] [DOI] [PubMed] [Google Scholar]
Oelz 1979
- Oelz O.Action mechanism and clinical indications for thrombocyte aggregation inhibitors [Wirkungsmechanismus und klinische Indikationen der Thrombozytenaggregationshemmer]. Schweizerische Medizinische Wochenschrift. Journal Suisse de Medecine 1979;109(10):348-53. [MEDLINE: ] [PubMed] [Google Scholar]
Ojo 2000
- Ojo AO, Hanson JA, Wolfe RA, Leichtman AB, Agodoa LY, Port FK.Long-term survival in renal transplant recipients with graft function. Kidney International 2000;57(1):307-13. [MEDLINE: ] [DOI] [PubMed] [Google Scholar]
Osborn 2008
- Osborn G, Escofet X, Da Silva A.Medical adjuvant treatment to increase patency of arteriovenous fistulae and grafts. Cochrane Database of Systematic Reviews 2008, Issue 4. Art. No: CD002786. [DOI: 10.1002/14651858.CD002786.pub2] [DOI] [PubMed] [Google Scholar]
Remppis 2008
- Remppis A, Ritz E.Cardiac problems in the dialysis patient: beyond coronary disease. Seminars in Dialysis 2008;21(4):319-25. [MEDLINE: ] [DOI] [PubMed] [Google Scholar]
Remuzzi 1988
- Remuzzi G.Bleeding in renal failure. Lancet 1988;1(8596):1205-8. [MEDLINE: ] [DOI] [PubMed] [Google Scholar]
Ruilope 2001
- Ruilope LM, Salvetti A, Jamerson K, Hansson L, Warnold I, Wedel H, et al.Renal function and intensive lowering of blood pressure in hypertensive participants of the hypertension optimal treatment (HOT) study. Journal of the American Society of Nephrology 2001;12(2):218-25. [MEDLINE: ] [DOI] [PubMed] [Google Scholar]
Sarnak 2003
- Sarnak MJ, Levey AS, Schoolwerth AC, Coresh J, Culleton B, Hamm LL, et al.Kidney disease as a risk factor for development of cardiovascular disease: a statement from the American Heart Association Councils on Kidney in Cardiovascular Disease, High Blood Pressure Research, Clinical Cardiology, and Epidemiology and Prevention. Hypertension 2003;42(5):1050-65. [MEDLINE: ] [DOI] [PubMed] [Google Scholar]
Scheen 2008
- Scheen AJ.Medications in the kidney. Acta Clinica Belgica 2008;63(2):76-80. [MEDLINE: ] [DOI] [PubMed] [Google Scholar]
Schror 1997
- Schrör K.Aspirin and platelets: the antiplatelet action of aspirin and its role in thrombosis treatment and prophylaxis. Seminars in Thrombosis & Hemostasis 1997;23(4):349-56. [MEDLINE: ] [DOI] [PubMed] [Google Scholar]
Schunemann 2020a
- Schünemann HJ, Higgins JP, Vist GE, Glasziou P, Akl EA, Skoetz N, et al.Chapter 14: Completing ‘Summary of findings’ tables and grading the certainty of the evidence. In: Higgins JPT, Thomas J, Chandler J, Cumpston M, Li T, Page MJ, Welch VA (editors). Cochrane Handbook for Systematic Reviews of Interventions version 6.1 (updated September 2020). Cochrane, 2020. www.training.cochrane.org/handbook.
Schunemann 2020b
- Schünemann HJ, Vist GE, Higgins JP, Santesso N, Deeks JJ, Glasziou P, et al.Chapter 15: Interpreting results and drawing conclusions. In: Higgins JPT, Thomas J, Chandler J, Cumpston M, Li T, Page MJ, Welch VA (editors). Cochrane Handbook for Systematic Reviews of Interventions version 6.1 (updated September 2020). Cochrane, 2020. Available from www.training.cochrane.org/handbook.
Song 2003
- Song F, Altman DG, Glenny AM, Deeks JJ.Validity of indirect comparison for estimating efficacy of competing interventions: empirical evidence from published meta-analyses. BMJ 2003;326(7387):472. [MEDLINE: ] [DOI] [PMC free article] [PubMed] [Google Scholar]
Stangal 2010
- Stangl PA, Lewis S.Review of currently available GP IIb/IIIa inhibitors and their role in peripheral vascular interventions. Seminars in Interventional Radiology 2010;27(4):412-21. [MEDLINE: ] [DOI] [PMC free article] [PubMed] [Google Scholar]
Su 2019
- Su X, Yan B, Wang L, LV J, Cheng H, Chen Y.Effect of antiplatelet therapy on cardiovascular and kidney outcomes in patients with chronic kidney disease: a systematic review and meta-analysis. BMC Nephrology 2019;20(1):309. [MEDLINE: ] [DOI] [PMC free article] [PubMed] [Google Scholar]
Taji 2006
- Taji Y, Kuwahara T, Shikata S, Morimoto T.Meta-analysis of antiplatelet therapy for IgA nephropathy. Clinical & Experimental Nephrology 2006;10(4):268-73. [MEDLINE: ] [DOI] [PubMed] [Google Scholar]
UK Renal Association 2010
- UK Renal Association.Cardiovascular disease in CKD. www.renal.org/Clinical/GuidelinesSection/CardiovascularDiseaseInCKD.aspx#Rationale3 (accessed 24 October 2021).
USRDS 2010
- US Renal Data System.USRDS 2010 Annual Data Report. Atlas of Chronic Kidney Disease and End-Stage Renal Disease in the United States, National Institutes of Health, National Institute of Diabetes and Digestive and Kidney Diseases, Bethesda, MD. www.usrds.org/annual-data-report/previous-adrs/ (accessed 24 October 2021).
Wattanakit 2008
- Wattanakit K, Cushman M, Stehman-Breen C, Heckbert SR, Folsom AR.Chronic kidney disease increases risk for venous thromboembolism. Journal of the American Society of Nephrology 2008;19(1):135-40. [MEDLINE: ] [DOI] [PMC free article] [PubMed] [Google Scholar]
Weiner 2004a
- Weiner DE, Tighiouart H, Amin MG, Stark PC, MacLeod B, Griffith JL, et al.Chronic kidney disease as a risk factor for cardiovascular disease and all-cause mortality: a pooled analysis of community-based studies. Journal of the American Society of Nephrology 2004;15(5):1307-15. [MEDLINE: ] [DOI] [PubMed] [Google Scholar]
Weiner 2004b
- Weiner DE, Tighiouart H, Stark PC, Amin MG, MacLeod B, Griffith JL, et al.Kidney disease as a risk factor for recurrent cardiovascular disease and mortality. American Journal of Kidney Diseases 2004;44(2):198-206. [MEDLINE: ] [DOI] [PubMed] [Google Scholar]
Woo 2011
- Woo JS, Kim W, Lee SR, Jung KH, Kim WS, Lew JH, et al.Platelet reactivity in patients with chronic kidney disease receiving adjunctive cilostazol compared with a high-maintenance dose of clopidogrel: results of the effect of platelet inhibition according to clopidogrel dose in patients with chronic kidney disease (PIANO-2 CKD) randomized study. American Heart Journal 2011;162(6):1018-25. [MEDLINE: ] [DOI] [PubMed] [Google Scholar]
Zwaginga 1991
- Zwaginga JJ, IJsseldijk MJ, Groot P G, Vos J, Bos Kuil RL, Sixma JJ.Defects in platelet adhesion and aggregate formation in uremic bleeding disorder can be attributed to factors in plasma. Arteriosclerosis & Thrombosis 1991;11(3):733-44. [MEDLINE: ] [DOI] [PubMed] [Google Scholar]
References to other published versions of this review
Palmer 2012
- Palmer SC, Di Micco L, Razavian M, Craig JC, Ravani P, Perkovic V, et al.Antiplatelet therapy to prevent hemodialysis vascular access failure: systematic review and meta-analysis. American Journal of Kidney Diseases 2013;61(1):112-22. [MEDLINE: ] [DOI] [PubMed] [Google Scholar]
Palmer 2013
- Palmer SC, Di Micco L, Razavian M, Craig JC, Perkovic V, Pellegrini F, et al.Antiplatelet agents for chronic kidney disease. Cochrane Database of Systematic Reviews 2013, Issue 4. Art. No: CD008834. [DOI: 10.1002/14651858.CD008834.pub3] [DOI] [PubMed] [Google Scholar]
Razavian 2010
- Razavian M, Di Micco L, Palmer SC, Craig JC, Perkovic V, Zoungas S, et al.Antiplatelet agents for chronic kidney disease. Cochrane Database of Systematic Reviews 2010, Issue 11. Art. No: CD008834. [DOI: 10.1002/14651858.CD008834] [DOI] [PubMed] [Google Scholar]


