Abstract
Mitochondria play essential roles in cellular energetics, biosynthesis, and signaling transduction. Dysfunctional mitochondria have been implicated in different diseases such as obesity, diabetes, cardiovascular disease, nonalcoholic fatty liver disease, neurodegenerative disease, and cancer. Mitochondrial homeostasis is controlled by a triad of mitochondrial biogenesis, dynamics (fusion and fission), and autophagy (mitophagy). Studies have underscored FoxO transcription factors as key mitochondrial regulators. Specifically, FoxOs regulate mitochondrial biogenesis by dampening NRF1-Tfam and c-Myc-Tfam cascades directly, and inhibiting NAD-Sirt1-Pgc1α cascade indirectly by inducing Hmox1 or repressing Fxn and Urod. In addition, FoxOs mediate mitochondrial fusion (via Mfn1 and Mfn2) and fission (via Drp1, Fis1, and MIEF2), during which FoxOs elicit regulatory mechanisms at transcriptional, posttranscriptional (e.g. via miR-484/Fis1), and posttranslational (e.g. via Bnip3-calcineurin mediated Drp1 dephosphorylation) levels. Furthermore, FoxOs control mitochondrial autophagy in the stages of autophagosome formation and maturation (e.g. initiation, nucleation, and elongation), mitochondria connected to and engulfed by autophagosome (e.g. via PINK1 and Bnip3 pathways), and autophagosome-lysosome fusion to form autolysosome for cargo degradation (e.g. via Tfeb and cathepsin proteins). This article provides an up-to-date view of FoxOs regulating mitochondrial homeostasis and discusses the potential of targeting FoxOs for therapeutics.
Keywords: autophagy, FoxO, fusion and fission, homeostasis, mitochondrial biogenesis, mitophagy
Introduction
Mitochondrial homeostasis is essential to normal cell and tissue functions. Most known about mitochondria is the primary role in oxidative phosphorylation (OXPHOS) that produces energy molecule (i.e. ATP), underscoring mitochondria as the powerhouse in the cell [1–3]. Mitochondrial metabolism also produces intermediates or metabolites that serve as the chemical building blocks for biosynthesis (e.g. the synthesis of nucleotides, glucose, fatty acids, cholesterol, amino acids, and heme) [1,4]. In addition, mitochondria may release signaling molecules (e.g. reactive oxygen species, cytochrome C, and mitokines) that mediate intracellular and exocellular communications in homeostasis and stress [2,5–10]. As such, mitochondrial defects or dysfunction has been implicated in various human diseases including obesity, diabetes, cardiovascular disease, nonalcoholic fatty liver disease, neurodegenerative disease, and cancer [1,4,11–13].
Mitochondrial homeostasis is maintained primarily via a triad of mitochondrial biogenesis, mitochondrial dynamics (i.e. fusion and fission), and mitochondrial autophagy or mitophagy (i.e. autophagic removal of mitochondria) (Figure 1) [1,6,7,14–18]. Studies have shown that the family of peroxisome proliferator-activated receptor (PPAR)-γ coactivator 1 (Pgc1) interact with energy sensors (e.g. AMPK and Sirt1) among others to switch on mitochondrial biogenesis via mitochondrial transcription factor A (Tfam) [7,12,19]. Mitochondrial network is controlled by dynamic processes of fusion, fission, and remodeling that involve mitofusin 1 (Mfn1), Mfn2, optic atrophy protein 1(OPA1), dynamin-related protein 1 (Drp1), and mitochondrial fission protein 1 (Fis1) [6,17]. Mitochondrial dynamics not only regulates the morphology of but also facilitates content exchange among these organelles (including mitochondrial DNA), thereby keeping mitochondrial integrity in check [17]. Unilateral loss of fusion or fission dysregulates mitochondrial function and mitochondrial signaling pathways that mediate cell pluripotency, division, differentiation, senescence, and apoptosis [6,17]. Augmented fission promotes mitochondrial segregation and mitophagy by producing mitochondrial fragments of appropriate size for autophagosomes to engulf (non-selective mitochondrial autophagy) [6,16,17]. In addition, the dynamics proteins Drp1 and Mfn2 also participate in PINK1–Parkin mediated selective mitochondrial autophagy [6,16,17]. For instance, PINK1-mediated phosphorylation of Mfn2 facilitates Mfn2–parkin interaction, which promotes mitochondrial protein ubiquitination and recruitment of autophagosomes through the adaptor protein LC3 [6,16,20].
Figure 1. A schematic view of the triad in mitochondrial homeostasis.
Finely tuned mitochondrial biogenesis, dynamics (fusion and fission), and mitophagy contribute to the homeostasis of these organelles. Studies have established key regulators of mitochondrial biogenesis (e.g. Pgc1, NRF1, and Tfam), mitochondrial fusion (e.g. Mfn1, Mfn2, and OPA1), fission (e.g. Drp1 and Fis1), and mitophagy (e.g. PINK1/Parkin, FUNDC1, and Tfeb).
The family of forkhead box class O (FoxO) transcription factors include FoxO1, FoxO3, FoxO4, and FoxO6. FoxOs regulate genes that are involved in various pathways such as metabolic regulation, cell and tissue homeostasis, and immunity [21–25]. FoxO activities are controlled by a nuclear localization signal (NLS) domain, a nuclear export sequence (NES) domain, a DNA-binding (i.e. forkhead box) domain (DBD), and a C-terminal transactivation domain [21,24,26]. Emerging evidence suggests that FoxOs may localize to mitochondria and bind to mitochondrial DNA, and further studies are needed to define the role of FoxO in regulating mitochondrial genes [27,28]. Regardless, FoxO transcription factors regulate the expression of nuclear genes that mediate mitochondrial biogenesis, dynamics, and mitophagy, underscoring FoxOs as the key regulators of mitochondrial homeostasis [29–40]. This article discusses the mechanisms or pathways by which FoxOs control mitochondrial homeostasis.
Foxo transcription factors in mitochondrial biogenesis
FoxO proteins undergo posttranslational modifications (e.g. phosphorylation and acetylation) in response to external stimuli such as stress or altered nutrient or cellular signaling [24,26,41]. For instance, insulin signaling may silence FoxOs via protein kinase B (or Akt)-mediated phosphorylation, which controls glucose production in the liver and protein homeostasis in skeletal muscle [41–43]. Obese or diabetic individuals who are insulin resistant show metabolic derangements and mitochondrial dysfunction [44–47]. While it is under debate whether mitochondrial deficiency or dysfunction leads to insulin resistance [48], studies have shown that insulin sensitivity is essential to mitochondrial homeostasis by finely tuning FoxO activity [3,36,44,46,49,50].
Mitochondrial biogenesis requires Pgc1α, a transcription coactivator that can be activated by the NAD dependent deacetylase Sirt1, to trigger the cascade of NRF1-Tfam [7,12,19,29]. In line with the notion that insulin promotes mitochondrial biogenesis [46], insulin resistance activates FoxO1 and reduces mitochondrial content or compromises mitochondrial integrity [29,49,50]. Mitochondrial OXPHOS relies on a series of redox reactions (e.g. the oxidation of NADH into NAD) through respiratory chain complexes I-IV that build up an electrochemical gradient (i.e. mitochondrial membrane potential) to drive ATP production through complex V (ATP synthase) [29,51,52]. Activation of FoxO1 in the liver up-regulates heme oxygenase 1 (Hmox1), which is located in inner mitochondrial membrane and catabolizes mitochondrial heme [29,53], the essential cofactors for redox enzymes on the electron transport chain (ETC), thereby compromising the integrity and function of ETC (Figure 2A) [29,54]. Although the subcellular location of biliverdin reductase is arguable and under investigation [53,55,56], there is evidence showing that biliverdin reductase may partner with Hmox1 in inner mitochondrial membrane to facilitate heme breakdown (by Hmox1) into biliverdin and then into bilirubin (by biliverdin reductase), thereby interfering with ETC and mitochondrial respiration [53,56]. The ETC deficiency results in a lower NAD/NADH ratio and dampens the NAD-dependent deacetylase Sirt1. As a result, Pgc1α is deactivated by high level of acetylation, which inhibits the NRF1-Tfam cascade and reduces mitochondrial biogenesis [29] (Figure 2A). In contrast, overexpression of a constitutively active Pgc1α (i.e. R13-Pgc1α that contains 13 lysine-to-arginine substitutions to mimic Pgc1α activation by deacetylation) restores mitochondrial content, suggesting that deactivation of Sirt1-Pgc1α cascade accounts for FoxO1 induced suppression of mitochondrial biogenesis [29]. Suppression of Pgc1α and mitochondrial biogenesis by FoxO1 was also observed in renal tubular epithelial cells, where FoxO1 keeps CREB from forming CREB-CBP-P300 complex, thereby down-regulating Ppargc1 (the gene encoding Pgc1α) [40]. Recent studies show that FoxO1 down-regulates NRF1-Tfam and suppresses mitochondrial biogenesis, which may account for glucagon-mediated mitochondrial alteration [50]. In addition, glucagon induces ETC deficiency through FoxO1-dependent down-regulation of Fxn and Urod, the genes involved in heme biosynthesis (Figure 2A) [50]. Interestingly, glucagon stimulates fatty acid oxidation (FAO) regardless of ETC defects in hepatocytes [50,57], and long-term exposure to high glucagon level can impair fatty acid oxidation activity [50]. The increased FAO by glucagon is attributed to inositol triphosphate receptor 1 (INSP3R1) [57]. A glutamine-dependent reductive carboxylation pathway may account for sustained FAO during ETC impairment [58], but it warrants further studies to determine whether such a mechanism underlies glucagon-induced FAO and ETC defects.
Figure 2. FoxO transcription factors regulate mitochondrial biogenesis.
(A) In the liver, FoxO1 may induce Hmox1, Fxn, and Urod, which disrupt mitochondrial ETC and NAD/NADH ratio, thereby suppressing NAD-dependent Sirt1-Pgc1α-NRF1-Tfam pathway in mitochondrial biogenesis. Glucagon activated FoxO1 represses NRF1 and accounts for reduced mitochondrial biogenesis in the liver. (B) In contrast with the liver, FoxO1 induces Cyb5r3 and maintains ETC activity and NAD/NADH ratio in the pancreas. It is unclear but of interest whether the FoxO1–Cyb5r3 axis regulates mitochondrial biogenesis via the known NAD-dependent Sirt1-Pgc1α-NRF1-Tfam pathway (indicated by question marks). (C) In the heart, FoxO1 activation due to diabetes causes mitochondrial abnormality by dysregulating PDK4 and CPT1 via a to-be-defined mechanism (indicated by question marks). (D) In cancer cells, FoxO3 suppresses mitochondrial biogenesis and function by inhibiting c-Myc/Tfam signaling cascade.
In line with the role of FoxO1–Hmox1 axis in dysregulating mitochondrial and metabolic homeostasis, Hmox1 has been associated with metaflammation and insulin resistance in mouse and man [59]. Hmox1 may also contribute to hyperglycemia through catabolism of heme and release of excessive free ferrous in hepatocytes, which activates FoxO1 via NF-kB mediated phosphorylation at Ser273(FoxO1) and induces gluconeogenic gene in mice [60]. The Hmox1 → Fe2+ → NF-kB → FoxO1 cascade might serve as an adaptive mechanism of selective clearance of dysfunctional mitochondria in the liver given the essential role of FoxO1 in mitochondrial autophagy (discussed in detail below). In adipose tissue and human adipocytes, Hmox1 is associated with iron excess-induced dysfunction and impaired glucose uptake and respiratory capacity [61]. Of note, induction of Hmox1 in specific immune cells may exert protective function via antioxidant and anti-inflammatory reactions, underlining cell type- or tissue-dependent roles of FoxO1 or Hmox1 [26,54]. To this end, activation of FoxO1 in pancreas was shown to promote pancreatic β-cells function and insulin secretion [62,63]. In β-cells FoxO1 can directly bind to the promoter of Cyb5r3 and transactivates the gene to encode mitochondrial membrane-bound cytochrome b5 reductase 3, the enzyme that mediates mitochondrial electron transport (Figure 2B) [63]. Ablation of FoxO1 or Cyb5r3 dysregulates mitochondrial function and NAD/NADH ratio and causes secretory granule abnormalities [63]. Nevertheless, it is unclear whether ablation of FoxO1 or Cyb5r3 impairs mitochondrial biogenesis via the known NAD-dependent Sirt1-Pgc1α pathways. In diabetic cardiomyocyte, FoxO1 induced mitochondrial alteration is associated with elevation of PDK4 and CPT1, shifting substrate from glucose to fatty acid and causing cardiac dysfunction (Figure 2C) [49]. Suppression of FoxO1 activity with a selective inhibitor (AS1842856) ameliorates mitochondrial and cardiac abnormality [49]. Of note, mitochondrial biogenesis in skeletal muscle or myoblasts may undergo a Pgc1α independent pathway in response to exercise or high flux of oxidative substrates (e.g. pyruvate) [64,65]. Although the Pgc1α independent pathway remains to be defined, it is of interest for future studies to determine whether and how FoxOs regulate mitochondrial biogenesis in skeletal muscle.
As another member of the FoxO family, FoxO3 plays an inhibitory role in mitochondrial biogenesis like FoxO1 [38,50]. Activation of FoxO3 results in down-regulation of mitochondrial DNA copy number, lower expression of mitochondrial proteins and mitochondrial respiratory activity in cancer cells [38]. In addition, FoxO3 induces PDK4 and reduces mitochondrial oxygen consumption rates as observed for FoxO1 [38,49]. Intriguingly, FoxO3 induced suppression of mitochondrial biogenesis appears to be independent from Pgc1 family and NRF1; instead, it depends on the inhibition of c-Myc, a transcription factor that regulates nuclear encoded mitochondrial genes by directly binding to the promoter of Tfam (Figure 2D) [38].
Overall, FoxO1 and FoxO3 appear to serve as a suppressor of mitochondrial biogenesis. In pancreas, FoxO1 was found to up-regulate mitochondrial protein and maintain mitochondrial function, and the role in mitochondrial biogenesis remains to be defined. The role of FoxO4 and FoxO6 in mitochondrial regulation is largely unexplored. During oxidative stress FoxO4 binds to the promoter of SOD2 gene and induces expression of manganese superoxide dismutase, an antioxidant enzyme located within the mitochondrial matrix [66]. FoxO4 may also interact with p53 to induce apoptosis that involves mitochondria and caspase-dependent pathway [67,68]. FoxO6 activation was associated with redox homeostasis in kidney tissues from calorie restriction rats [69]. However, in colorectal cancer cells FoxO6 seems to increase glycolysis and suppresses mitochondrial respiration, and the regulatory mechanism remains to be defined [70]. Further studies of FoxO4 and FoxO6 in this respect are desirable and critical to paint a whole picture of FoxO transcription factors in mitochondrial regulation.
FoxO transcription factors in mitochondrial dynamics
Mitochondria undergo constant fusion and fission, and the balance of these dynamic processes is essential to mitochondrial hemostasis. Mitochondrial fusion is controlled by Mfn1, Mfn2 and Opa1 while mitochondrial fission is controlled by Drp1 and Fis1 among other regulators [6,17]. In overnutrition conditions (e.g. obesity), activation of FoxO1 leads to deformed mitochondria in the liver of insulin resistant mice [29,71] or glucagon treated mice [50], which is associated with dysregulated fusion (e.g. up-regulation of Mfn1 and Mfn2) and fission (e.g. down-regulation of Drp1 and Fis1) proteins (Figure 3A). Lower ATP production is reported for the deformed mitochondria compared with normal mitochondria [29]. Ablation of FoxO1 normalizes mitochondrial morphology and ATP production [29], suggesting that FoxO1 plays a central role in mitochondrial dynamics [29,50,71]. Interestingly, undernutrition conditions (e.g. nutrient depletion or starvation) activate cAMP-PKA pathway that leads to inhibitory phosphorylation of Drp1 and mitochondrial elongation, which serves as an important mechanism to sustain cell viability by preventing mitochondria from autophagic degradation and maintaining mitochondrial ATP production [72]. Given FoxO1 is also activated during fasting state [42], it will be of interest to investigate whether FoxO1 participates in undernutrition induced changes in mitochondrial dynamics. In addition, studies of mice lacking estrogen receptor α (ERα) in brown adipose tissue revealed a role of mtDNA polymerase γ (Polg1) in increased mitochondrial fission via Drp1 [73]. Because ERα is a potent inhibitor of FoxO1 via Akt -mediated phosphorylation [74,75], future studies are warranted to examine whether FoxO1 mediates ERα regulation of mitochondrial dynamics (Figure 3B).
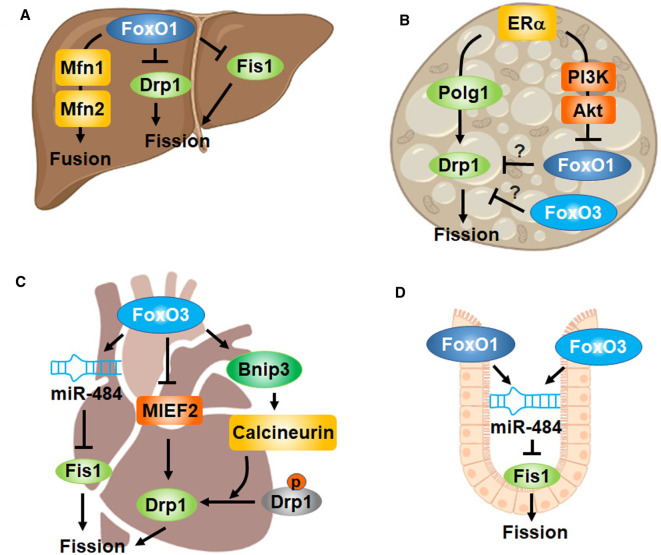
Figure 3. FoxO transcription factors regulate mitochondrial dynamics.
(A) In the liver or hepatocytes, FoxO1 up-regulates fusion proteins (Mfn1 and Mfn2) but down-regulates fission proteins (Drp1 and Fis1), leading to enlarged mitochondria. (B) Estrogen receptor (ERα) signaling induces Drp1 via Polg1 in brown adipocytes. It is known that ERα signaling deactivates FoxO via PI3K/Akt in adipose tissue and the liver, raising the question whether FoxOs may account for ERα induced mitochondrial fission (indicated by question marks). (C) FoxO3 inhibits mitochondrial fission by repressing MIEF2 or inducing miR-484, which are cardioprotective in doxorubicin (DOX)-induced mouse cardiotoxicity. Notably, FoxO3 was shown to stimulate mitochondrial fission via Bnip3-calcineurin mediated dephosphorylation (activation) of Drp1 in phenylephrine (PE)-stressed adult cardiomyocytes or heart from rats. The discrepancy may arise from different models of cardio stress induced by DOX vs PE. (D) In intestinal crypt-based columnar cells, FoxO1 and FoxO3 dampens mitochondrial fission by transactivating miR-484 that in turn silences Fis1.
FoxO3 is implicated in the regulation of mitochondrial dynamics, and the role appear to be multifaceted. In cardiomyocytes, FoxO3 inhibits mitochondrial fission by transactivating microRNA-484 (miR-484) expression [76]. FoxO3 induced miR-484 binds to the amino acid coding sequence of Fis1 mRNA and suppresses Fis1 protein expression and mitochondrial fission, which attenuates apoptosis and myocardial infarction in mice (Figure 3C) [76]. The cardioprotective function is also associated with FoxO3 repressing mitochondrial dynamics protein of 49kDa (MiD49 or MIEF2) by directly binding to the promoter of MIEF2 gene (Figure 3C) [35]. MIEF2 protein facilitates the recruitment of Drp1 to mitochondrial membrane, where Drp1 is polymerized and rings at constriction sites to promote mitochondrial fission [77]. Overexpression of FoxO3 in cardiomyocytes suppresses mitochondrial fission and apoptosis, protecting against chemotherapeutic drug doxorubicin-induced cardiotoxicity in mice [35]. Interestingly, FoxO3 was also shown to promote mitochondrial fission, apoptosis, and cardiac stress or heart failure by up-regulating BCL2/adenovirus E1B 19-kDa protein-interacting protein 3 (Bnip3) in rats [37]. Mechanistically, FoxO3 induced Bnip3 dysregulates calcium in the cytosolic and mitochondrial compartments. The increase in cytosolic calcium activates calcineurin, a phosphatase that activates Drp1 via dephosphorylation at Ser637(Drp1), thereby promoting Drp1-mediated mitochondrial fragmentation (Figure 3C) [37]. This discrepancy may arise from the different chemicals and resultant models of cardiotoxicity, i.e. phenylephrine-stressed adult cardiomyocytes versus doxorubicin-induced cardiotoxicity [35,37].
FoxO-regulation of mitochondrial dynamics plays a key role in stem cell proliferation and differentiation [32,78]. In line with FoxO dampening mitochondrial fission by transactivating miR-484 to silence Fis1 [76], ablation of FoxO1 and FoxO3 in mouse Lgr5+ intestinal stem cell or crypt-based columnar cells (CBC) promotes mitochondrial fission (Figure 3D) [32]. FoxO deficient CBC have lower mitochondrial respiration rates and undergo a metabolic transition from OXPHOS to glycolysis, which drives the differentiation of CBC into secretory Paneth cells and goblet cells [32,79]. Inhibition of mitochondrial fission by targeting Drp1 prevents the increase in secretory cell numbers [32]. Interestingly, the proliferation and differentiation of intestinal stem cells (ISC) in fruit flies requires a metabolic transition from glycolysis to OXPHOS [78]. Disruption of ETC complexes leads to up-regulated FoxO, which blocks the ISC commitment to enteroblast (EB), EB-to-absorptive enterocyte specification, and EB-to-secretory enteroendocrine cell specification [78]. The discrepancy may arise from cell type (e.g. Lgr5+ vs. Lgr5-intestinal stem cell) or species (e.g. mouse vs. fruit flies) dependent differences.
Taken together, activation of FoxOs may induce transcriptional, posttranscriptional (e.g. miR-484), and posttranslational (e.g. Drp1 dephosphorylation) changes that dysregulate mitochondrial fusion and fission. FoxOs seem to play multifaceted roles in mitochondrial fission depending on experimental models or species, and further studies are warranted to identify the underlying determinants of the multifaceted roles.
FoxO transcription factors in mitochondrial autophagy
Selective mitochondrial clearance by autophagy may undergo receptor (e.g. Bnip3, NIX, and FUNDC1) and adaptor (e.g. NBR1 and p62/SQSTM1) dependent pathways, which facilitate mitochondria being connected to and engulfed by autophagosome (Figure 4A) [15,16,80,81]. Receptor proteins contain a COOH-terminal transmembrane domain that connects with mitochondrial membrane and an NH2-terminal LC3-interacting region (LIR) motif that binds to lipidated LC3 and facilitates connecting mitochondria to autophagosome membrane [16]. Like receptor proteins, adaptor proteins contain an LIR motif. However, a transmembrane domain is absent from adaptor proteins; instead, a ubiquitin binding domain (UBD) is present to facilitate the connection to mitochondria through the binding to polyubiquitinated proteins located on mitochondrial outer membrane [15,16]. The ubiquitin-dependent mitophagy requires PTEN induced kinase 1 (PINK1), which is accumulated during stress conditions and recruits of Parkin to mitochondria to initiate ubiquitination of mitochondrial proteins. Parkin also participates in cargo sorting, budding of mitochondrial-derived vesicles, and matrix delivery to lysosomes for degradation [82,83]. Regardless of the differences discussed above, studies have revealed cross-talk existing between receptor-mediated pathways and adaptor-mediated ubiquitin-dependent pathways [15,81]. For instance, the mitophagy receptor protein Bnip3 interacts with PINK1 to promote PINK1 accumulation on the mitochondrial outer membrane, triggering the PINK1–Parkin mediated ubiquitin-dependent pathways [84,85]. On the other hand, Parkin induced ubiquitination of mitophagy receptor protein NIX promotes the recruitment of adaptor protein NBR1, initiating ubiquitin dependent pathway to mitochondrial clearance [86].
Figure 4. FoxO transcription factors regulate mitochondrial autophagy (mitophagy).
FoxOs mediate mitophagy in three aspects: (A) mitochondria connected to and engulfed by autophagosome via adaptor- and receptor-dependent pathways; (B) the formation and maturation of autophagosome (e.g. the initiation, nucleation, and elongation steps), and (C) fusion with lysosome to form autophagosome for cargo degradation.
FoxO transcription factors regulate an array of genes involved in autophagic regulation [23,24,87]. In the process of selective mitochondrial autophagy, FoxO proteins regulate both adaptor-mediated ubiquitin-dependent pathways and receptor-mediated pathways (Figure 4A). In mouse podocyte cells, FoxO1 induces PINK1 by directly binding to the promoter of PINK1 gene and stimulates PINK/Parkin dependent mitophagy, which protects against podocyte injury and ameliorates diabetic nephropathy progression [36]. In white adipocytes, ERα signaling induces a browning phenotype by deactivating PINK1/Parkin pathways [73], presumably because ERα suppresses FoxO1 by activating Akt [74,88]. Indeed, FoxO1 occupancy on PINK1 promoter is dampened by insulin sensitization that enhances Akt-mediated inhibition of FoxO1, whereas overexpression of constitutively active FoxO1 promotes PINK1-dependent mitophagy [89]. Conditional deletion of FoxO1 and FoxO3 in cardiomyocytes down-regulates PINK1 and significantly increases the infarct area in mice subjected to myocardial infarction (MI) or acute ischemia/reperfusion (I/R) injury [90]. Interestingly, inhibition of FoxO1 prevents renal I/R injury in mice [40]. In dopamine neurons, manganese increases FoxO3 nuclear retention and activates PINK1/Parkin cascade, which is associated with reduced cell viability [91]. Mechanistically, FoxO3 stimulates mitophagy by transactivating the expression of PINK1 gene (Figure 4A) [92]. Given manganese induced neurotoxicity accounts for the loss of dopamine neurons in Parkinson's disease (PD), future study of the FoxO3-mitophagy pathways may lead to new therapeutic options for PD [33,91].
In receptor dependent mitophagy, FoxO transcription factors control the expression Bnip3 and Bnip3L (Figure 4A) [33,37,39]. FoxO3 expression is elevated in heart failure, concurrent with up-regulation of Bnip3, mitophagy, and apoptosis in cardiomyocytes [39]. Knockdown or overexpression of FoxO3 in cardiomyocytes leads to down- or up-regulation of Bnip3, respectively [37,39]. Chromatin immunoprecipitation (ChIP) sequencing analysis suggests that FoxO3 directly binds to the promoters of Bnip3 and Bnip3L among other genes [33]. In adult neural stem and progenitor cells, ablation of FoxO3 reduces Bnip3 and Bnip3L expression and mitochondrial turnover but increases aggregate levels [33]. FoxO1 induction of Bnip3 was also observed in neurons lacking JNK [93] and in skeletal muscle [94]. Overexpression of Sirt1 deactivates FoxO1 and FoxO3 through deacetylation, thereby suppressing Bnip3 [94].
With the assistance of adaptor or receptor proteins, mitochondria are connected with and engulfed by autophagosomes (Figure 4A,B), which in turn fuse with lysosome to form autolysosomes for mitochondrial degradation (Figure 4C) [16]. FoxOs regulate not only adaptor- and receptor-dependent engulfing of mitochondria (as discussed above) but also gene expression that are involved in autophagosome [23,24] and lysosome regulation [31,95]. Specifically, FoxOs regulate genes involved in the stages of initiation (e.g. Ulk1 and Ulk2), nucleation (e.g. Becn1, Atg14, and Pi3kIII), elongation (e.g. Map1lc3b, Gabarapl, Atg4, Atg5, and Atg12), and fusion (e.g. Tfeb and Rab7) (Figure 4A), which has been discussed in recent reviews [23,24]. As a target of FoxOs, Tfeb regulates autophagosomal and lysosomal genes as well as the fusion of autophagosome with lysosome [96–98]. Tfeb activity is controlled by posttranslational modification, such as mTORC1 mediated phosphorylation that excludes Tfeb from the nucleus [99–101]. At transcriptional level, Tfeb gene was transactivated by FoxO1, which might account for mitophagy regulation and white-beige adipose tissue conversion [95]. FoxO1 induces Tfeb by directly binding to the promoter of Tfeb gene [95], and inhibition of FoxO1 down-regulates Tfeb and its target genes (e.g. CTSB, CTSD, CTSH, and CTSS) (Figure 4C) [31]. In aged T cells, FoxO1 deficiency increases cell mass and secretion of cytotoxic exosomes due to impairment of TFEB-mediated lysosomal activity and proteostasis [31].
Together, FoxOs induce mitophagy in three major aspects, (i) expression of autophagosome machinery proteins, (ii) expression of adaptor and receptor proteins that facilitate mitochondria connected to and engulfed by autophagosome, and (iii) expression of lysosome proteins essential to autolysosome formation and cargo degradation.
Conclusions
Mitochondrial quality is controlled through a triad of biogenesis, dynamics, and mitophagy, which underpins metabolic health and tissue homeostasis. Accumulated evidence has underscored FoxO transcription factors as the key regulators of mitochondrial homeostasis. FoxO activation may suppress mitochondrial biogenesis, dysregulate mitochondrial fusion and fission, and induces mitophagy through adaptor- and receptor-dependent pathways. Dysregulation of FoxOs is associated with mitochondrial alterations and metabolic derangements, and pharmacological modulation of FoxO activity has been one of the top candidates for drug discovery [21]. Regardless, caution should be exercised with the following complexity in order to develop effective therapeutics in the future: first, FoxOs may regulate mitochondria in a cell type- and tissue-dependent manner. For instance, inactivation of FoxO1 improves mitochondrial homeostasis in the liver [29,50,71] and kidney [40] but the opposite was observed in the pancreatic β-cells [62,63]. Likewise, ablation of FoxO in cardiomyocytes dampens PINK1/Parkin dependent mitophagy and increases cardiac ischemia/reperfusion (I/R) injury [90], while inhibition of FoxO1 prevents renal I/R injury in mice [40]. Secondly, the interplays among mitochondrial biogenesis, dynamics, and mitophagy may complicate the outcome of FoxO modulation. In addition to mediating mitochondrial dynamics, Mfn2 and Drp1 also regulate mitophagy by interacting with PINK1, Parkin, and Bnip3 in cardiomyocytes and dopamine neurons [20,37,102–104]. Moreover, Mfn2 also regulates Pgc1α-mediated mitochondrial adaptation in response to increased energy demand in skeletal muscle and brown adipose tissue [105]. As such, targeting FoxOs for mitochondrial biogenesis (e.g. via Pgc1α cascade) could impose undesired effects on mitochondrial dynamics and mitophagy, or vice versa. Future studies designed to precisely target FoxOs and mitochondrial alterations are critical for the development of effective therapeutics, such as selective organ targeting approaches and nanotechnology [106,107].
Abbreviations
- Akt or PKB
protein kinase B
- AMPK
AMP-activated protein kinase
- Atg12
autophagy related 12
- Atg14
autophagy related 14
- Atg4
autophagy related 4
- Atg5
autophagy related 5
- ATP
adenosine triphosphate
- Becn1
Beclin 1
- Bnip3
BCL2 interacting protein 3
- Bnip3L
BCL2 interacting protein 3 like
- CBC
crypt-based columnar cell
- ChIP
chromatin immunoprecipitation
- CPT1
carnitine palmitoyltransferase 1
- CREB
cAMP response element-binding protein
- CTSB
cathepsin B
- CTSD
cathepsin D
- CTSH
cathepsin H
- CTSS
cathepsin S
- Cyb5r3
cytochrome b5 reductase 3
- DBD
DNA-binding domain
- Drp1
dynamin-related protein 1
- EB
enteroblast
- ERα
estrogen receptor α
- ETC
electron transport chain
- FAO
fatty acid oxidation
- Fis1
fission protein 1
- FoxO
forkhead box class O
- FUNDC1
FUN14 Domain Containing 1
- Fxn
frataxin
- Hmox1
heme oxygenase 1
- I/R
ischemia/reperfusion
- ISC
intestinal stem cell
- LC3 or Map1lc3
microtubule-associated protein 1A/1B-light chain 3
- LIR
LC3-interacting region
- Mfn1
mitofusin 1
- Mfn2
mitofusin 2
- MI
myocardial infarction
- MIEF2 or MiD49
mitochondrial dynamics protein of 49 kDa
- miR-484
microRNA-484
- NBR1
neighbor of BRCA1 gene 1
- NES
nuclear export sequence
- NF-kB
nuclear factor-kB
- NIX
Nip3-like protein X
- NLS
nuclear localization signal
- NRF1
nuclear respiratory factor 1
- OPA1
optic atrophy protein 1
- OXPHOS
oxidative phosphorylation
- P53
tumor protein p53
- PD
Parkinson's disease
- PDK4
pyruvate dehydrogenase kinase 4
- Pgc1
peroxisome proliferator-activated receptor (PPAR)-γ coactivator 1
- Pi3kIII
the class III phosphatidylinositol 3-kinase
- PINK1
PTEN induced kinase 1
- Polg1
mitochondrial DNA polymerase γ
- Sirt1
Sirtuin 1
- SOD2
manganese superoxide dismutase
- Tfam
mitochondrial transcription factor A
- TFEB
transcription factor EB
- UBD
ubiquitin binding domain
- Ulk1
Unc-51 like autophagy activating kinase 1
- Ulk2
Unc-51 like autophagy activating kinase 2
- Urod
uroporphyrinogen decarboxylase
Competing Interests
The author declares that there are no competing interests associated with this manuscript.
Funding
The Figures were created with BioRender. This work was supported in part by the American Heart Association (18TPA34230082 to Z.C.), and the USDA National Institute of Food and Agriculture (1020373 to Z.C).
Open Access Statement
Open access for this article was enabled by the participation of University of Florida in an all-inclusive Read & Publish pilot with Portland Press and the Biochemical Society under a transformative agreement with Otto Harrasowitz.
References
- 1.Suomalainen, A. and Battersby, B.J. (2018) Mitochondrial diseases: the contribution of organelle stress responses to pathology. Nat. Rev. Mol. Cell Biol. 19, 77–92 10.1038/nrm.2017.66 [DOI] [PubMed] [Google Scholar]
- 2.McBride, H.M., Neuspiel, M. and Wasiak, S. (2006) Mitochondria: more than just a powerhouse. Curr. Biol. 16, R551–R560 10.1016/j.cub.2006.06.054 [DOI] [PubMed] [Google Scholar]
- 3.Cheng, Z., Tseng, Y. and White, M.F. (2010) Insulin signaling meets mitochondria in metabolism. Trends Endocrinol. Metab. 21, 589–598 10.1016/j.tem.2010.06.005 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Spinelli, J.B. and Haigis, M.C. (2018) The multifaceted contributions of mitochondria to cellular metabolism. Nat. Cell Biol. 20, 745–754 10.1038/s41556-018-0124-1 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Cheng, Z. and Ristow, M. (2013) Mitochondria and metabolic homeostasis. Antioxid. Redox Signal. 19, 240–242 10.1089/ars.2013.5255 [DOI] [PubMed] [Google Scholar]
- 6.Giacomello, M., Pyakurel, A., Glytsou, C. and Scorrano, L. (2020) The cell biology of mitochondrial membrane dynamics. Nat. Rev. Mol. Cell Biol. 21, 204–224 10.1038/s41580-020-0210-7 [DOI] [PubMed] [Google Scholar]
- 7.Quiros, P.M., Mottis, A. and Auwerx, J. (2016) Mitonuclear communication in homeostasis and stress. Nat. Rev. Mol. Cell Biol. 17, 213–226 10.1038/nrm.2016.23 [DOI] [PubMed] [Google Scholar]
- 8.Chandel, N.S. (2015) Evolution of mitochondria as signaling organelles. Cell Metab. 22, 204–206 10.1016/j.cmet.2015.05.013 [DOI] [PubMed] [Google Scholar]
- 9.Chandel, N.S. (2014) Mitochondria as signaling organelles. BMC Biol. 12, 34 10.1186/1741-7007-12-34 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Chakrabarty, R.P. and Chandel, N.S. (2021) Mitochondria as signaling organelles control mammalian stem cell fate. Cell Stem Cell 28, 394–408 10.1016/j.stem.2021.02.011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Cheng, Z., Zheng, L. and Almeida, F.A. (2018) Epigenetic reprogramming in metabolic disorders: nutritional factors and beyond. J. Nutr. Biochem. 54, 1–10 10.1016/j.jnutbio.2017.10.004 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Cheng, Z., Schmelz, E.M., Liu, D. and Hulver, M.W. (2014) Targeting mitochondrial alterations to prevent type 2 diabetes-Evidence from studies of dietary redox-active compounds. Mol. Nutr. Food Res. 58, 1739–1749 10.1002/mnfr.201300747 [DOI] [PubMed] [Google Scholar]
- 13.Cheng, Z. and Almeida, F.A. (2014) Mitochondrial alteration in type 2 diabetes and obesity: an epigenetic link. Cell Cycle 13, 890–897 10.4161/cc.28189 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Ploumi, C., Daskalaki, I. and Tavernarakis, N. (2017) Mitochondrial biogenesis and clearance: a balancing act. FEBS J. 284, 183–195 10.1111/febs.13820 [DOI] [PubMed] [Google Scholar]
- 15.Palikaras, K., Lionaki, E. and Tavernarakis, N. (2018) Mechanisms of mitophagy in cellular homeostasis, physiology and pathology. Nat. Cell Biol. 20, 1013–1022 10.1038/s41556-018-0176-2 [DOI] [PubMed] [Google Scholar]
- 16.Gustafsson, A.B. and Dorn, G.W. (2019) Evolving and expanding the roles of mitophagy as a homeostatic and pathogenic process. Physiol. Rev. 99, 853–892 10.1152/physrev.00005.2018 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Chan, D.C. (2020) Mitochondrial dynamics and its involvement in disease. Annu. Rev. Pathol. 15, 235–259 10.1146/annurev-pathmechdis-012419-032711 [DOI] [PubMed] [Google Scholar]
- 18.Romanello, V. and Sandri, M. (2021) The connection between the dynamic remodeling of the mitochondrial network and the regulation of muscle mass. Cell. Mol. Life Sci. 78, 1305–1328 10.1007/s00018-020-03662-0 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Piccinin, E., Villani, G. and Moschetta, A. (2019) Metabolic aspects in NAFLD, NASH and hepatocellular carcinoma: the role of PGC1 coactivators. Nat. Rev. Gastroenterol. Hepatol. 16, 160–174 10.1038/s41575-018-0089-3 [DOI] [PubMed] [Google Scholar]
- 20.Chen, Y. and Dorn, G.W. (2013) PINK1-Phosphorylated mitofusin 2 is a parkin receptor for culling damaged mitochondria. Science 340, 471–475 10.1126/science.1231031 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Calissi, G., Lam, E.W. and Link, W. (2021) Therapeutic strategies targeting FOXO transcription factors. Nat. Rev. Drug Discov. 20, 21–38 10.1038/s41573-020-0088-2 [DOI] [PubMed] [Google Scholar]
- 22.Martins, R., Lithgow, G.J. and Link, W. (2016) Long live FOXO: unraveling the role of FOXO proteins in aging and longevity. Aging Cell 15, 196–207 10.1111/acel.12427 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Webb, A.E. and Brunet, A. (2014) FOXO transcription factors: key regulators of cellular quality control. Trends Biochem. Sci. 39, 159–169 10.1016/j.tibs.2014.02.003 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Cheng, Z. (2019) The foxO-autophagy axis in health and disease. Trends Endocrinol. Metab. 30, 658–671 10.1016/j.tem.2019.07.009 [DOI] [PubMed] [Google Scholar]
- 25.Liu, L. and Cheng, Z. (2018) Forkhead box O (FoxO) transcription factors in autophagy, metabolic Health, and tissue Homeostasis. In Autophagy in Health and Disease- Potential Therapeutic Approaches (Turksen, K., ed.), pp. 47–69, Springer Nature, Switzerland AG [Google Scholar]
- 26.Cheng, Z. and White, M.F. (2011) Targeting forkhead box O1 from the concept to metabolic diseases: lessons from mouse models. Antioxid. Redox Signal. 14, 649–661 10.1089/ars.2010.3370 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Caballero-Caballero, A., Engel, T., Martinez-Villarreal, J., Sanz-Rodriguez, A., Chang, P., Dunleavy, M.et al. (2013) Mitochondrial localization of the Forkhead box class O transcription factor FOXO3a in brain. J. Neurochem. 124, 749–756 10.1111/jnc.12133 [DOI] [PubMed] [Google Scholar]
- 28.Lettieri-Barbato, D., Ioannilli, L., Aquilano, K., Ciccarone, F., Rosina, M. and Ciriolo, M.R. (2019) Foxo1 localizes to mitochondria of adipose tissue and is affected by nutrient stress. Metabolism 95, 84–92 10.1016/j.metabol.2019.04.006 [DOI] [PubMed] [Google Scholar]
- 29.Cheng, Z., Guo, S., Copps, K., Dong, X., Kollipara, R., Rodgers, J.T.et al. (2009) Foxo1 integrates insulin signaling with mitochondrial function in the liver. Nat. Med. 15, 1307–1311 10.1038/nm.2049 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Ludikhuize, M.C. and Colman, M.J.R. (2021) Metabolic regulation of stem cells and differentiation: a Forkhead Box O transcription factor perspective. Antioxid. Redox Signal. 34, 1004–1024 10.1089/ars.2020.8126 [DOI] [PubMed] [Google Scholar]
- 31.Jin, J., Li, X.Y., Hu, B., Kim, C., Cao, W.Q., Zhang, H.M.et al. (2020) FOXO1 deficiency impairs proteostasis in aged T cells. Sci. Adv. 6, eaba1808 10.1126/sciadv.aba1808 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Ludikhuize, M.C., Meerlo, M., Gallego, M.P., Xanthakis, D., Julia, M.B., Nguyen, N.T.B.et al. (2020) Mitochondria define intestinal stem cell differentiation downstream of a FOXO/Notch axis. Cell Metab. 32, 889–900.e7 10.1016/j.cmet.2020.10.005 [DOI] [PubMed] [Google Scholar]
- 33.Audesse, A.J., Dhakal, S., Hassell, L.A., Gardell, Z., Nemtsova, Y. and Webb, A.E. (2019) FOXO3 directly regulates an autophagy network to functionally regulate proteostasis in adult neural stem cells. PLoS Genet. 15, e1008097 10.1371/journal.pgen.1008097 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Kim, S. and Koh, H. (2017) Role of FOXO transcription factors in crosstalk between mitochondria and the nucleus. J. Bioenerg. Biomembr. 49, 335–341 10.1007/s10863-017-9705-0 [DOI] [PubMed] [Google Scholar]
- 35.Zhou, L.Y., Li, R.B., Liu, C.Y., Sun, T., Aung, L.H.H., Chen, C.et al. (2017) Foxo3a inhibits mitochondrial fission and protects against doxorubicin-induced cardiotoxicity by suppressing MIEF2. Free Radic. Biol. Med. 104, 360–370 10.1016/j.freeradbiomed.2017.01.037 [DOI] [PubMed] [Google Scholar]
- 36.Li, W., Du, M.M., Wang, Q.Z., Ma, X.J., Wu, L.N., Guo, F.et al. (2017) Foxo1 promotes mitophagy in the podocytes of diabetic male mice via the PINK1/Parkin pathway. Endocrinology 158, 2155–2167 10.1210/en.2016-1970 [DOI] [PubMed] [Google Scholar]
- 37.Chaanine, A.H., Kohlbrenner, E., Gamb, S.I., Guenzel, A.J., Klaus, K., Fayyaz, A.U.et al. (2016) FOXO3a regulates BNIP3 and modulates mitochondrial calcium, dynamics, and function in cardiac stress. Am. J. Physiol. 311, H1540–H1559 10.1152/ajpheart.00549.2016 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Ferber, E.C., Peck, B., Delpuech, O., Bell, G.P., East, P. and Schulze, A. (2012) FOXO3a regulates reactive oxygen metabolism by inhibiting mitochondrial gene expression. Cell Death Differ. 19, 968–979 10.1038/cdd.2011.179 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Chaanine, A.H., Jeong, D., Liang, L., Chemaly, E.R., Fish, K., Gordon, R.E.et al. (2012) JNK modulates FOXO3a for the expression of the mitochondrial death and mitophagy marker BNIP3 in pathological hypertrophy and in heart failure. Cell Death Dis. 3, 265 10.1038/cddis.2012.5 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Wang, D., Wang, Y.Q., Zou, X.T., Shi, Y.D., Liu, Q., Huyan, T.R.et al. (2020) FOXO1 inhibition prevents renal ischemia-reperfusion injury via cAMP-response element binding protein/PPAR-gamma coactivator-1 alpha-mediated mitochondrial biogenesis. Br. J. Pharmacol. 177, 432–448 10.1111/bph.14878 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Cheng, Z. (2015) Foxo1: mute for a tuned metabolism? Trends Endocrinol. Metab. 26, 402–403 10.1016/j.tem.2015.06.006 [DOI] [PubMed] [Google Scholar]
- 42.Cheng, Z. and White, M.F. (2012) The AKTion in non-canonical insulin signaling. Nat. Med. 18, 351–353 10.1038/nm.2694 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.James, H.A., O'Neill, B.T. and Nair, K.S. (2017) Insulin regulation of proteostasis and clinical implications. Cell Metab. 26, 310–323 10.1016/j.cmet.2017.06.010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Ruegsegger, G.N., Vanderboom, P.M., Dasari, S., Klaus, K.A., Kabiraj, P., McCarthy, C.B.et al. (2019) Exercise and metformin counteract altered mitochondrial function in the insulin-resistant brain. JCI Insight 4, e130681 10.1172/jci.insight.130681 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Heinonen, S., Buzkova, J., Muniandy, M., Kaksonen, R., Ollikainen, M., Ismail, K.et al. (2015) Impaired mitochondrial biogenesis in adipose tissue in acquired obesity. Diabetes 64, 3135–3145 10.2337/db14-1937 [DOI] [PubMed] [Google Scholar]
- 46.Ruegsegger, G.N., Creo, A.L., Cortes, T.M., Dasari, S. and Nair, K.S. (2018) Altered mitochondrial function in insulin-deficient and insulin-resistant states. J. Clin. Invest. 128, 3671–3681 10.1172/JCI120843 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Zheng, L.D., Linarelli, L.E., Liu, L., Wall, S.S., Greenawald, M.H., Seidel, R.W.et al. (2015) Insulin resistance is associated with epigenetic and genetic regulation of mitochondrial DNA in obese humans. Clin. Epigenetics 7, 60 10.1186/s13148-015-0093-1 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Morrow, R.M., Picard, M., Derbeneva, O., Leipzig, J., McManus, M.J., Gouspillou, G.et al. (2017) Mitochondrial energy deficiency leads to hyperproliferation of skeletal muscle mitochondria and enhanced insulin sensitivity. Proc. Natl Acad. Sci. U.S.A. 114, 2705–2710 10.1073/pnas.1700997114 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Yan, D., Cai, Y., Luo, J.R., Liu, J.J., Li, X., Ying, F.et al. (2020) FOXO1 contributes to diabetic cardiomyopathy via inducing imbalanced oxidative metabolism in type 1 diabetes. J. Cell. Mol. Med. 24, 7850–7861 10.1111/jcmm.15418 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Yang, W.B., Yan, H., Pan, Q., Shen, J.Z., Zhou, F.H., Wu, C.D.et al. (2019) Glucagon regulates hepatic mitochondrial function and biogenesis through FOXO1. J. Endocrinol. 241, 265–278 10.1530/JOE-19-0081 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Chaban, Y., Boekema, E.J. and Dudkina, N.V. (2014) Structures of mitochondrial oxidative phosphorylation supercomplexes and mechanisms for their stabilisation. Biochim. Biophys Acta 1837, 418–426 10.1016/j.bbabio.2013.10.004 [DOI] [PubMed] [Google Scholar]
- 52.Signes, A. and Fernandez-Vizarra, E. (2018) Assembly of mammalian oxidative phosphorylation complexes I-V and supercomplexes. Essays Biochem. 62, 255–270 10.1042/EBC20170098 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Converso, D.P., Taille, C., Carreras, M.C., Jaitovich, A., Poderoso, J.J. and Boczkowski, J. (2006) HO-1 is located in liver mitochondria and modulates mitochondrial heme content and metabolism. FASEB J. 20, 1236–1238 10.1096/fj.05-4204fje [DOI] [PubMed] [Google Scholar]
- 54.Campbell, N.K., Fitzgerald, H.K. and Dunne, A. (2021) Regulation of inflammation by the antioxidant haem oxygenase 1. Nat. Rev. Immunol. 21, 411–425 10.1038/s41577-020-00491-x [DOI] [PubMed] [Google Scholar]
- 55.Shum, M., Shintre, C.A., Althoff, T., Gutierrez, V., Segawa, M., Saxberg, A.D.et al. (2021) ABCB10 exports mitochondrial biliverdin, driving metabolic maladaptation in obesity. Sci. Transl. Med. 13, eabd1869 10.1126/scitranslmed.abd1869 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Mustafa, M.G., Cowger, M.L. and King, T.E. (1969) Effects of bilirubin on mitochondrial reactions. J. Biol. Chem. 244, 6403–6414 10.1016/S0021-9258(18)63479-9 [DOI] [PubMed] [Google Scholar]
- 57.Perry, R.J., Zhang, D.Y., Guerra, M.T., Brill, A.L., Goedeke, L., Nasiri, A.R.et al. (2020) Glucagon stimulates gluconeogenesis by INSP3R1-mediated hepatic lipolysis. Nature 579, 279–283 10.1038/s41586-020-2074-6 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Mullen, A.R., Wheaton, W.W., Jin, E.S., Chen, P.H., Sullivan, L.B., Cheng, T.et al. (2012) Reductive carboxylation supports growth in tumour cells with defective mitochondria. Nature 481, 385–388 10.1038/nature10642 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Jais, A., Einwallner, E., Sharif, O., Gossens, K., Lu, T.T.H., Soyal, S.M.et al. (2014) Heme oxygenase-1 drives metaflammation and insulin resistance in mouse and Man. Cell 158, 25–40 10.1016/j.cell.2014.04.043 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Liao, W., Yang, W.B., Shen, Z., Ai, W.Q., Pan, Q., Sun, Y.X.et al. (2021) Heme oxygenase-1 regulates ferrous iron and Foxo1 in control of hepatic gluconeogenesis. Diabetes 70, 696–709 10.2337/db20-0954 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Moreno-Navarrete, J.M., Ortega, F., Rodriguez, A., Latorre, J., Becerril, S., Sabater-Masdeu, M.et al. (2017) HMOX1 as a marker of iron excess-induced adipose tissue dysfunction, affecting glucose uptake and respiratory capacity in human adipocytes. Diabetologia 60, 915–926 10.1007/s00125-017-4228-0 [DOI] [PubMed] [Google Scholar]
- 62.Kim-Muller, J.Y., Kim, Y.J.R., Fan, J.S., Zhao, S.G., Banks, A.S., Prentki, M.et al. (2016) Foxo1 deacetylation decreases fatty acid oxidation in beta-cells and sustains insulin secretion in diabetes. J. Biol. Chem. 291, 10162–10172 10.1074/jbc.M115.705608 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Fan, J.S., Du, W., Kim-Muller, J.Y., Son, J., Kuo, T., Larrea, D.et al. (2020) Cyb5r3 links FoxO1-dependent mitochondrial dysfunction with beta-cell failure. Mol. Metab. 34, 97–111 10.1016/j.molmet.2019.12.008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Rowe, G.C., El-Khoury, R., Patten, I.S., Rustin, P. and Arany, Z. (2012) PGC-1 alpha is dispensable for exercise-induced mitochondrial biogenesis in skeletal muscle. PLoS ONE 7, e41817 10.1371/journal.pone.0041817 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Wilson, L., Yang, Q., Szustakowski, J.D., Gullicksen, P.S. and Halse, R. (2007) Pyruvate induces mitochondrial biogenesis by a PGC-1 alpha-independent mechanism. Am. J. Physiol. 292, C1599–C1605 10.1152/ajpcell.00428.2006 [DOI] [PubMed] [Google Scholar]
- 66.Araujo, J., Breuer, P., Dieringer, S., Krauss, S., Dorn, S., Zimmermann, K.et al. (2011) FOXO4-dependent upregulation of superoxide dismutase-2 in response to oxidative stress is impaired in spinocerebellar ataxia type 3. Hum. Mol. Genet. 20, 2928–2941 10.1093/hmg/ddr197 [DOI] [PubMed] [Google Scholar]
- 67.Le, H.H., Cinaroglu, S.S., Manalo, E.C., Ors, A., Gomes, M.M., Sahbaz, B.D.et al. (2021) Molecular modelling of the FOXO4-TP53 interaction to design senolytic peptides for the elimination of senescent cancer cells. Ebiomedicine 73, 103646 10.1016/j.ebiom.2021.103646 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Bourgeois, B. and Madl, T. (2018) Regulation of cellular senescence via the FOXO4-p53 axis. FEBS Lett. 592, 2083–2097 10.1002/1873-3468.13057 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Kim, D.H., Park, M.H., Chung, K.W., Kim, M.J., Jung, Y.R., Bae, H.R.et al. (2014) The essential role of FoxO6 phosphorylation in aging and calorie restriction. Age 36, 9679 10.1007/s11357-014-9679-3 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Li, Q.Y., Tang, H.N., Hu, F.F. and Qin, C.J. (2019) Silencing of FOXO6 inhibits the proliferation, invasion, and glycolysis in colorectal cancer cells. J. Cell. Biochem. 120, 3853–3860 10.1002/jcb.27667 [DOI] [PubMed] [Google Scholar]
- 71.O-Sullivan, I., Zhang, W., Wasserman, D.H., Liew, C.W., Liu, J., Paik, J.et al. (2015) Foxo1 integrates direct and indirect effects of insulin on hepatic glucose production and glucose utilization. Nat. Commun. 6, 7079 10.1038/ncomms8079 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Gomes, L.C., Di Benedetto, G. and Scorrano, L. (2011) During autophagy mitochondria elongate, are spared from degradation and sustain cell viability. Nat. Cell Biol. 13, 589–598 10.1038/ncb2220 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Zhou, Z.Q., Moore, T.M., Drew, B.G., Ribas, V., Wanagat, J., Civelek, M.et al. (2020) Estrogen receptor alpha controls metabolism in white and brown adipocytes by regulating Polg1 and mitochondrial remodeling. Sci. Transl. Med. 12, eaax8096 10.1126/scitranslmed.aax8096 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Yan, H., Yang, W., Zhou, F., Li, X., Pan, Q., Shen, Z.et al. (2019) Estrogen improves insulin sensitivity and suppresses gluconeogenesis via the transcription factor Foxo1. Diabetes 68, 291–304 10.2337/db18-0638 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Tao, Z., Shi, L., Parke, J., Zheng, L., Gu, W., Dong, X.C.et al. (2021) Sirt1 coordinates with ERalpha to regulate autophagy and adiposity. Cell Death Discov. 7, 53 10.1038/s41420-021-00438-8 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Wang, K., Long, B., Jiao, J.Q., Wang, J.X., Liu, J.P., Li, Q.et al. (2012) miR-484 regulates mitochondrial network through targeting Fis1. Nat. Commun. 3, 781 10.1038/ncomms1770 [DOI] [PubMed] [Google Scholar]
- 77.Kamerkar, S.C., Kraus, F., Sharpe, A.J., Pucadyil, T.J. and Ryan, M.T. (2018) Dynamin-related protein 1 has membrane constricting and severing abilities sufficient for mitochondrial and peroxisomal fission. Nat. Commun. 9, 5239 10.1038/s41467-018-07543-w [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Zhang, F., Pirooznia, M. and Xu, H. (2020) Mitochondria regulate intestinal stem cell proliferation and epithelial homeostasis through FOXO. Mol. Biol. Cell 31, 1538–1549 10.1091/mbc.E19-10-0560 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Rodriguez-Colman, M.J., Schewe, M., Meerlo, M., Stigter, E., Gerrits, J., Pras-Raves, M.et al. (2017) Interplay between metabolic identities in the intestinal crypt supports stem cell function. Nature 543, 424–427 10.1038/nature21673 [DOI] [PubMed] [Google Scholar]
- 80.Song, Y.J., Zhou, Y. and Zhou, X.M. (2020) The role of mitophagy in innate immune responses triggered by mitochondrial stress. Cell Commun. Signal. 18, 186 10.1186/s12964-020-00659-x [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Liu, L., Liao, X.D., Wu, H., Li, Y.J., Zhu, Y.S. and Chen, Q. (2020) Mitophagy and its contribution to metabolic and aging-associated disorders. Antioxid. Redox Signal. 32, 906–927 10.1089/ars.2019.8013 [DOI] [PubMed] [Google Scholar]
- 82.McLelland, G.L., Soubannier, V., Chen, C.X., McBride, H.M. and Fon, E.A. (2014) Parkin and PINK1 function in a vesicular trafficking pathway regulating mitochondrial quality control. EMBO J. 33, 282–295 10.1002/embj.201385902 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Wang, X.L., Feng, S.T., Wang, Z.Z., Yuan, Y.H., Chen, N.H. and Zhang, Y. (2021) Parkin, an E3 ubiquitin ligase, plays an essential role in mitochondrial quality control in Parkinson's disease. Cell. Mol. Neurobiol. 41, 1395–1411 10.1007/s10571-020-00914-2 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Lee, Y., Lee, H.Y., Hanna, R.A. and Gustafsson, A.B. (2011) Mitochondrial autophagy by Bnip3 involves Drp1-mediated mitochondrial fission and recruitment of parkin in cardiac myocytes. Am. J. Physiol. 301, H1924–H1931 10.1152/ajpheart.00368.2011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Zhang, T.M., Xue, L., Li, L., Tang, C.Y., Wan, Z.Q., Wang, R.X.et al. (2016) BNIP3 protein suppresses PINK1 kinase proteolytic cleavage to promote mitophagy. J. Biol. Chem. 291, 21616–21629 10.1074/jbc.M116.733410 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Gao, F., Chen, D., Si, J.M., Hu, Q.S., Qin, Z.H., Fang, M.et al. (2015) The mitochondrial protein BNIP3L is the substrate of PARK2 and mediates mitophagy in PINK1/PARK2 pathway. Hum. Mol. Genet. 24, 2528–2538 10.1093/hmg/ddv017 [DOI] [PubMed] [Google Scholar]
- 87.Tao, Z., Aslam, H., Parke, J., Sanchez, M. and Cheng, Z. (2022) Mechanisms of autophagic responses to altered nutritional status. J. Nutr. Biochem., 108955 10.1016/j.jnutbio.2022.108955 [DOI] [PubMed] [Google Scholar]
- 88.Tao, Z., Zheng, L.D., Smith, C., Luo, J., Robinson, A., Almeida, F.A.et al. (2018) Estradiol signaling mediates gender difference in visceral adiposity via autophagy. Cell Death Dis. 9, 309 10.1038/s41419-018-0372-9 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.Bartolome, A., Garcia-Aguilar, A., Asahara, S.I., Kido, Y., Guillen, C., Pajvani, U.B.et al. (2017) MTORC1 regulates both general autophagy and mitophagy induction after oxidative phosphorylation uncoupling. Mol. Cell. Biol. 37, e00441-17 10.1128/MCB.00441-17 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90.Sengupta, A., Molkentin, J.D., Paik, J.H., DePinho, R.A. and Yutzey, K.E. (2011) Foxo transcription factors promote cardiomyocyte survival upon induction of oxidative stress. J. Biol. Chem. 286, 7468–7478 10.1074/jbc.M110.179242 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91.Song, D.M., Ma, J.X., Chen, L., Guo, C.X., Zhang, Y.Y., Chen, T.et al. (2017) FOXO3 promoted mitophagy via nuclear retention induced by manganese chloride in SH-SY5Y cells. Metallomics 9, 1251–1259 10.1039/C7MT00085E [DOI] [PubMed] [Google Scholar]
- 92.Mei, Y., Zhang, Y., Yamamoto, K., Xie, W., Mak, T.W. and You, H. (2009) FOXO3a-dependent regulation of Pink1 (Park6) mediates survival signaling in response to cytokine deprivation. Proc. Natl Acad. Sci. U.S.A. 106, 5153–5158 10.1073/pnas.0901104106 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93.Xu, P., Das, M., Reilly, J. and Davis, R.J. (2011) JNK regulates FoxO-dependent autophagy in neurons. Genes Dev. 25, 310–322 10.1101/gad.1984311 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94.Lee, D. and Goldberg, A.L. (2013) SIRT1 protein, by blocking the activities of transcription factors FoxO1 and FoxO3, inhibits muscle atrophy and promotes muscle growth. J. Biol. Chem. 288, 30515–30526 10.1074/jbc.M113.489716 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95.Liu, L., Tao, Z., Zheng, L.D., Brooke, J.P., Smith, C.M., Liu, D.et al. (2016) Foxo1 interacts with transcription factor EB and differentially regulates mitochondrial uncoupling proteins via autophagy in adipocytes. Cell Death Discov. 2, 16066 10.1038/cddiscovery.2016.66 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96.Settembre, C., Di Malta, C., Polito, V.A., Garcia Arencibia, M., Vetrini, F., Erdin, S.et al. (2011) TFEB links autophagy to lysosomal biogenesis. Science 332, 1429–1433 10.1126/science.1204592 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 97.Settembre, C. (2012) Transcription factor EB: a central regulator of both the autophagosome and lysosome (Reprinted from science, vol 332, pg 1429-1433, 2011). Hepatology 55, 1632–1634 10.1002/hep.25619 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 98.Zhao, Y.G., Codogno, P. and Zhang, H. (2021) Machinery, regulation and pathophysiological implications of autophagosome maturation. Nat. Rev. Mol. Cell Biol. 22, 733–750 10.1038/s41580-021-00392-4 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 99.Deleyto-Seldas, N. and Efeyan, A. (2021) The mTOR-autophagy axis and the control of metabolism. Front. Cell Dev. Biol. 9, 655731 10.3389/fcell.2021.655731 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 100.Roczniak-Ferguson, A., Petit, C.S., Froehlich, F., Qian, S., Ky, J., Angarola, B.et al. (2012) The transcription factor TFEB links mTORC1 signaling to transcriptional control of lysosome homeostasis. Sci. Signal. 5, ra42 10.1126/scisignal.2002790 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 101.Settembre, C., Zoncu, R., Medina, D.L., Vetrini, F., Erdin, S., Erdin, S.et al. (2012) A lysosome-to-nucleus signalling mechanism senses and regulates the lysosome via mTOR and TFEB. EMBO J. 31, 1095–1108 10.1038/emboj.2012.32 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 102.Wang, H.X., Song, P.P., Du, L., Tian, W.L., Yue, W., Liu, M.et al. (2011) Parkin ubiquitinates Drp1 for proteasome-dependent degradation implication of dysregulated mitochondrial dynamics in Parkinson disease. J. Biol. Chem. 286, 11649–11658 10.1074/jbc.M110.144238 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 103.Quinsay, M.N., Thomas, R.L., Lee, Y. and Gustafsson, A.B. (2010) Bnip3-mediated mitochondrial autophagy is independent of the mitochondrial permeability transition pore. Autophagy 6, 855–862 10.4161/auto.6.7.13005 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 104.Quinsay, M.N., Lee, Y., Rikka, S., Sayen, M.R., Molkentin, J.D., Gottlieb, R.A.et al. (2010) Bnip3 mediates permeabilization of mitochondria and release of cytochrome c via a novel mechanism. J. Mol. Cell. Cardiol. 48, 1146–1156 10.1016/j.yjmcc.2009.12.004 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 105.Soriano, F.X., Liesa, M., Bach, D., Chan, D.C., Palacin, M. and Zorzano, A. (2006) Evidence for a mitochondrial regulatory pathway defined by peroxisome proliferator-activated receptor-gamma coactivator-1 alpha, estrogen-related receptor-alpha, and mitofusin 2. Diabetes 55, 1783–1791 10.2337/db05-0509 [DOI] [PubMed] [Google Scholar]
- 106.Cheng, Q., Wei, T., Farbiak, L., Johnson, L.T., Dilliard, S.A. and Siegwart, D.J. (2020) Selective organ targeting (SORT) nanoparticles for tissue-specific mRNA delivery and CRISPR-Cas gene editing. Nat. Nanotechnol. 15, 313 10.1038/s41565-020-0669-6 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 107.Zhao, Z.M., Ukidve, A., Kim, J. and Mitragotri, S. (2020) Targeting strategies for tissue-specific drug delivery. Cell 181, 151–167 10.1016/j.cell.2020.02.001 [DOI] [PubMed] [Google Scholar]