Abstract
B-cell non-Hodgkin lymphoma (B-NHL) encompasses multiple clinically and phenotypically distinct subtypes of malignancy with unique molecular etiologies. Common subtypes of B-NHL, such as diffuse large B-cell lymphoma, have been comprehensively interrogated at the genomic level, but rarer subtypes, such as mantle cell lymphoma, remain less extensively characterized. Furthermore, multiple B-NHL subtypes have thus far not been comprehensively compared using the same methodology to identify conserved or subtype-specific patterns of genomic alterations. Here, we employed a large targeted hybrid-capture sequencing approach encompassing 380 genes to interrogate the genomic landscapes of 685 B-NHL tumors at high depth, including diffuse large B-cell lymphoma, mantle cell lymphoma, follicular lymphoma, and Burkitt lymphoma. We identified conserved hallmarks of B-NHL that were deregulated in the majority of tumors from each subtype, including frequent genetic deregulation of the ubiquitin proteasome system. In addition, we identified subtype-specific patterns of genetic alterations, including clusters of co-occurring mutations and DNA copy number alterations. The cumulative burden of mutations within a single cluster were more discriminatory of B-NHL subtypes than individual mutations, implicating likely patterns of genetic cooperation that contribute to disease etiology. We therefore provide the first cross-sectional analysis of mutations and DNA copy number alterations across major B-NHL subtypes and a framework of co-occurring genetic alterations that deregulate genetic hallmarks and likely cooperate in lymphomagenesis.
Introduction
Non-Hodgkin lymphomas (NHL) are a heterogeneous group of lymphoid malignancies that predominantly arise from mature B cells (B-NHL). Although mature B-cell neoplasms encompass 38 unique diagnostic subtypes, over 85% of cases fall within only seven histologies.1,2 Recent next-generation sequencing studies have shed light onto the key driver mutations in many of these B-NHL subtypes; for example, large studies of diffuse large B-cell lymphoma (DLBCL) have led to proposed genomic subtypes that have unique etiologies.3-5 However, many less common NHL subtypes such as mantle cell lymphoma (MCL) have not been as extensively characterized.6,7 Furthermore, until recently3,4 genetic alterations have been considered in a binary fashion as either driver events, which directly promote disease genesis or progression, or passenger events, which have little or no impact on disease biology. In contrast to this principle, most B-NHL do not result from a single dominant driver but instead result from the serial acquisition of genetic alterations that cooperate in lymphomagenesis. 8 The genetic context of each mutation likely determines its oncogenic potential, and groups of mutations should therefore be considered collectively rather than as singular events. For example, the ‘C5’ and ‘MCD’ clusters identified in DLBCL by Chapuy et al. and Schmitz et al., respectively, are characterized by the co-occurrence of CD79B and MYD88 mutations.3,4 In animal models, the Myd88 L252P mutation (equivalent to human L265P) was found to promote downregulation of surface IgM and a phenotype resembling B-cell anergy.9 However, this effect could be rescued by the Cd79b mutation, showing that these co-occurring mutations cooperate.9 The characterization of other significantly co-occurring genetic alterations are therefore likely to reveal additional important cooperative relationships. We approached this challenge by performing genomic profiling of 685 B-NHL across different subtypes. Through this cross-sectional analysis we characterized genomic hallmarks of B-NHL and sets of significantly co-associated events that likely represent subtype-specific cooperating genetic alterations. This study therefore provides new insight into how co-occurring clusters of genetic alterations may contribute to molecularly and phenotypically distinct subtypes of B-NHL.
Methods
An overview of our approach is shown in Online Supplementary Figure S1. For detailed methods, please refer to the Online Supplementary Information.
Tumor DNA samples
We collected DNA from 685 B-NHL tumors, including 199 FL, 196 MCL, 148 DLBCL, 107 BL, 21 high-grade B-cell lymphoma not otherwise specified (HGBL-NOS), and 14 high-grade B-cell lymphoma with MYC, BCL2 and/or BCL6 rearrangement (DHL) (Online Supplementary Table S1). All samples were archival and deidentified. The study was approved by the institutional review board of the University of Nebraska Medical Center (203-15-EP) and performed in accordance with the Declaration of Helsinki. A total of 462 samples were obtained from the University of Nebraska Medical Center, and were prioritized for inclusion in this study if they had previously undergone pathology review and been interrogated by Affymetrix U133 Plus 2.0 gene expression microarrays10-12 (n=284). An additional series of 223 formalin-fixed paraffin-embedded tumors were provided by other centers. Samples were de-identified and accompanied by the patients’ diagnosis from the medical records, plus overall survival time and status when available. Medical record diagnosis was used in all cases except for those with fluorescence in situ hybridization (FISH) showing translocations in MYC and BCL2 and/or BCL6, which were amended to DHL. Sequencing results for a subset of 52 BL tumors were described previously.13 All MCL samples were either positive for CCND1 translocation by FISH or positive for CCND1 protein expression by immunohistochemistry, depending on the diagnostic practices of the contributing institution.
Next-generation sequencing
A total of 500-1000 ng of genomic DNA was sonicated using a Covaris S2 Ultrasonicator, and libraries prepared using KAPA Hyper Prep Kits (Roche) with TruSeq Adapters (Bioo Scientific) and a maximum of eight cycles of polymerase chain reaction (average of 4 cycles). Libraries were qualified by TapeStation 4200, quantified by Qubit and 10- to 12-plexed for hybrid capture. Each multiplexed library was enriched using our custom LymphoSeq panel encompassing the full coding sequences of 380 genes that were determined to be somatically mutated in B-cell lymphoma (Online Supplementary Table S2, Online Supplementary Methods), as well as tiling recurrent translocation breakpoints. Enrichments were amplified with four to eight cycles of polymerase chain reaction and sequenced on a single lane of an Illumina HiSeq 4000 with 100PE reads in high-output mode at the Hudson Alpha Institute for Biotechnology or the MD Anderson Sequencing and Microarray Facility. Variants were called using our previously validated ensemble approach,13,14 germline polymorphisms were filtered using dbSNP annotation and the EXAC dataset containing 60,706 healthy individuals,15 and significantly mutated genes were defined by MutSig2CV.16 Copy number alterations (CNA) identified by CopyWriteR,17 which was validated using three FL tumors with matched Affymetrix 250K SNP array (Online Supplementary Figure S2), and significant DNA CNA were determined by GISTIC2.18 Translocations were called using FACTERA,19 which we previously validated against MYC translocation status determined by FISH.20 Mutation and CNA data are publicly viewable through cBioPortal: https://www.cbioportal. org/study/summary?id=mbn_mdacc_2013. Matched gene expression microarray data are available through the Gene Expression Omnibus, accession GSE132929. For further details, refer to the Online Supplementary Methods.
Results
Identification of significantly mutated genes and structural alterations
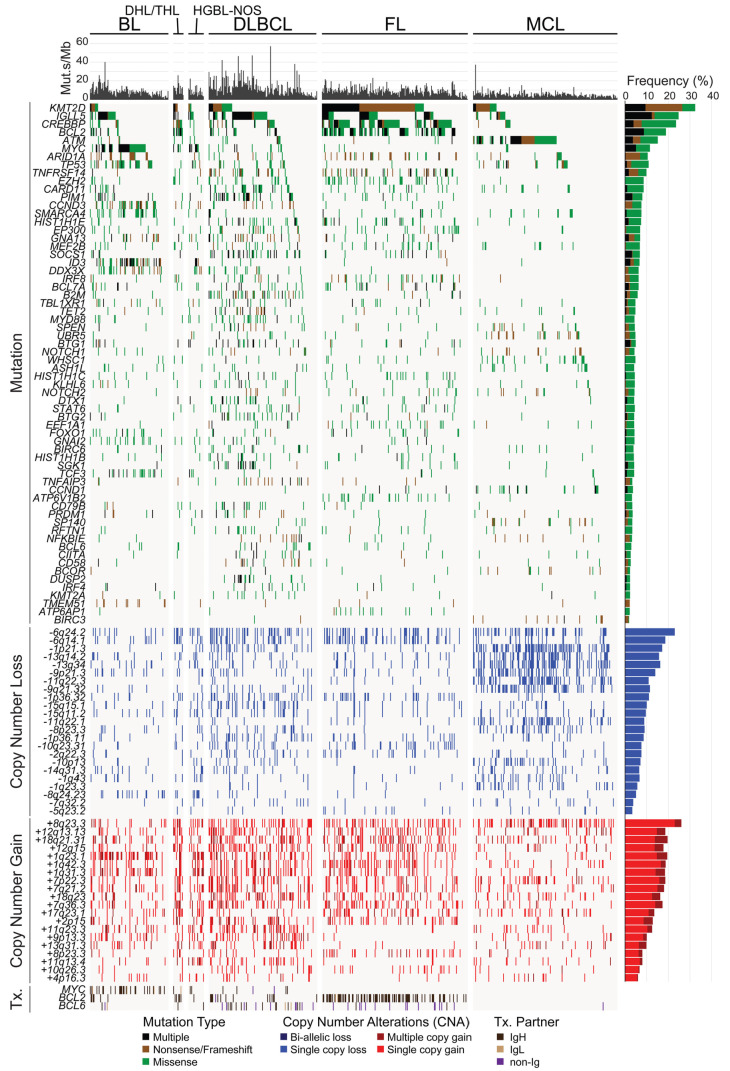
We used a 380-gene custom targeted sequencing approach, LymphoSeq, to interrogate the genomes of 685 mature B-NHL, sequencing to an average depth of 578X (minimum, 101X; maximum, 1785X) (Online Supplementary Table S1) for a total yield of 1.81 Tbp. Somatic nucleotide variants and small insertions/deletions were identified using an ensemble approach that we have previously validated14 (Online Supplementary Table S3) and significantly mutated genes were identified using MutSig2CV (Online Supplementary Table S4). Matched germline DNA was available from purified T cells of 20 tumors (11 FL and 9 MCL) and sequenced to validate the filtering of germline variants; 0/632 variants called within these tumors were identified in the matched germline samples, which indicates that the filtering of germline variants was effective. Genes that were significantly mutated in the full cohort or in any one of the four subtypes with more than 100 tumors (BL, DLBCL, FL, and MCL) were included, as well as frequently mutated genes that are targets of activation-induced cytidine deaminase (AID) (Figure 1, Online Supplementary Table S5). Predictably, the frequency of AID-associated mutations was higher among germinal center-derived lymphomas (BL, DLBCL, FL), but also accounted for 7.6% of all coding and non-coding mutations in MCL (Online Supplementary Table S6). The mutational burden calculated from our targeted region correlated significantly with that from the whole exome (Online Supplementary Figure S3A) and was significantly higher in DLBCL and other high-grade tumors than in FL and MCL (Figure 1, Online Supplementary Figure S3B).
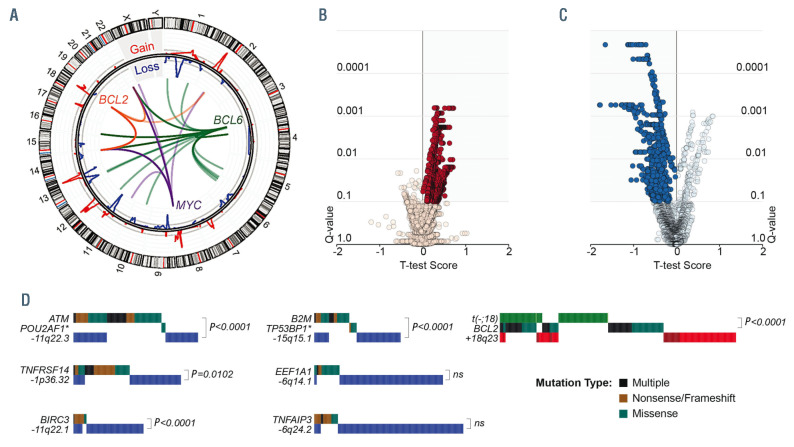
The hybrid capture probes utilized in our design also targeted recurrent breakpoint regions in the immunoglobulin heavy- and light-chain loci, and recurrent breakpoints in or near the BCL2, MYC and BCL6 genes, and translocations were called using a method that detects discordantly mapped reads19 (Figures 1 and 2A). Our prior validation of this approach in cases with matched FISH data for MYC showed that it is 100% specific, but only ~40% sensitive for translocation detection.13 This limit of sensitivity likely varies for different genes depending on how well the breakpoints are clustered into hotspots that are targeted by our capture probes. Nonetheless, we observed a significantly higher fraction of BCL6 translocations (57% [27/47]) partnered to non-immunoglobulin loci (e.g., CIITA, RHOH, EIF4A2, and ST6GAL1) (Online Supplementary Table S7) compared to BCL2 (1% [1/114]) and MYC (5% [2/38]) translocations (Figure 2A; Fisher P-value <0.001). These were more frequent in FL (88% [15/17] of BCL6 translocations) than in DLBCL (39% [9/23] of BCL6 translocations), presumably because the two immunoglobulin loci in FL are either translocated with the BCL2 gene or functioning in immunoglobulin expression.21 We also employed off-target reads to detect DNA CNA in a manner akin to low-pass whole genome sequencing, identified significant peaks of copy gain and losses using GISTIC218 (Figures 1 and 2A, Online Supplementary Figure S4, Online Supplementary Tables S8 and S9), and defined the likely targets of these CNA by integrative analysis of matched gene expression profiling data from 290 tumors (Figure 2B, C, Online Supplementary Figure S4, Online Supplementary Tables S10 and S11). This identified known CNA targets, including but not limited to deletion of TNFAIP3 (6q24.2),22 ATM (11q22.3),23 B2M (15q15.5),24 and PTEN (10q23.21),25 and copy gain of REL and BCL11A (2p15), and TCF4 (18q23).26 In addition, we identified novel targets such as deletion of IBTK (6q14.1), UBE3A (11q22.1) and FBXO25 (8p23.3), and copy gain of ATF7 (12q13.13), UCHL5 (1q31.3), and KMT2A (11q23.3). Importantly, the frequency of DNA CNA in the target genes identified by next-generation sequencing-based analysis significantly correlated with those derived from single nucleotide polymorphism microarray-based measurements in independent cohorts of BL, DLBCL, FL and MCL tumors from previously published studies6,20,26-30 (Online Supplementary Figure S5), providing validation for the accuracy of this approach. The CNA peaks, defined as the smallest and most statistically significant region, included multiple genes that were significantly mutated (Figure 2D) as well as other genes for which we detected mutations at lower frequencies that were not significant by MutSig2CV (POU2AF1, TP53BP1, FAS, PTEN). Deletions of ATM, B2M, BIRC3 and TNFRSF14 significantly co-associated with mutations of these genes, suggesting that these are complementary mechanisms contributing to biallelic inactivation.
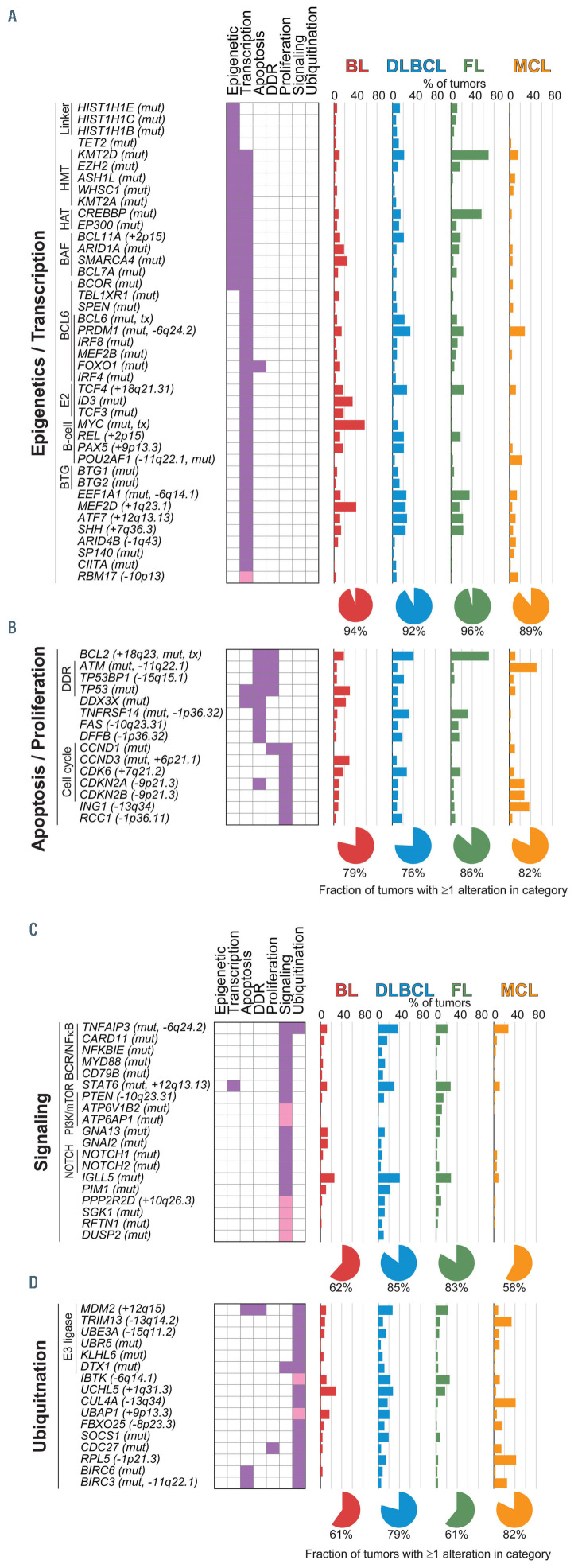
Conserved functional hallmarks of B-cell non-Hodgkin lymphoma
To understand key hallmarks that are deregulated by genetic alterations, we performed hypergeometric enrichment analysis of genes targeted by recurrent mutations and DNA CNA using DAVID31 (Online Supplementary Table S12). This revealed a significant enrichment of multiple overlapping gene sets that could be summarized into hallmark processes associated with epigenetics and transcription (Figure 3A), apoptosis and proliferation (Figure 3B), signaling (Figure 3C), and ubiquitination (Figure 3D). One or more genes within these hallmarks was altered in the majority (>50%) of tumors from each of the four major histologies included in this study. Genes annotated in epigenetic- associated gene sets were altered in 72%, 70%, 93% and 50% of BL, DLBCL, FL, and MCL, respectively, whereas genes annotated in transcription-associated gene sets were altered in 94%, 91%, 95% and 88% of BL, DLBCL, FL, and MCL, respectively. However, there is an extremely high degree of functional overlap between epigenetics and transcriptional regulation, as well as overlapping gene set annotations for many genes, leading us to consider these categories collectively as a single hallmark. Collectively, genes involved in epigenetics and transcription were mutated in 94% of BL, 92% of DLBCL, 96% of FL and 89% of MCL, and included those that encode proteins that catalyze post-translational modifications of histones (KMT2D, CREBBP, EZH2, EP300, WHSC1, ASHL1L, KMT2A), components of the BAF chromatin remodeling complex (ARID1A, SMARCA4, BCL7A, BCL11A), linker histones (HIST1H1E, HIST1H1C, HIST1H1B), and transcription factors (BCL6, IRF4, IRF8, TCF3, TCF4, MYC, REL, PAX5, POU2AF2, FOXO1, CIITA). Genes with a role in signaling included those involved in B-cell receptor signaling (CD79B, ID3, TCF3, TCF4, RFTN1), NFκB (TNFAIP3, CARD11, NFKBIE), NOTCH (NOTCH1, NOTCH2), JAK/STAT (SOCS1, STAT6), PI3K/mTOR (FOXO1, ATP6V1B2, APT6AP1) and G-protein signaling (GNA13, GNAI2). The CD79A and BCL10 genes were also mutated at a lower frequency that was not significant by MutSig2CV (Online Supplementary Figure S6A, B). Among these, the RFTN1 gene (Online Supplementary Figure S6C) is a novel recurrently mutated gene that was mutated in 7.4% of DLBCL and encodes a lipid raft protein that is critical for B-cell receptor signaling.32
Deregulation of the ubiquitin proteasome system is important in many cancers,33 but is not a well-defined hallmark of B-NHL. However, one or more genes with a role in regulating ubiquitination was genetically altered in 61% of BL, 79% of DLBCL, 61% of FL and 82% of MCL (Figure 3D). These included previously described genetic alterations such as amplification of MDM2,34 deletions of TNFAIP3,35 CUL4A,36 and RPL5,36 and mutations of KLHL6,37 DTX1,38 UBR5,39 SOCS1,40 and BIRC3.6 In addition, we identified novel targets such as recurrent deletions of IBTK, a negative regulator of Bruton tyrosine kinase,41 and somatic mutation of CDC27 in 14% of MCL, which encodes an E3 ligase for CCND1.42 Therefore, common hallmark processes are targeted by genetic alterations in the majority of major B-NHL subtypes, including genes with a role in regulating protein ubiquitination.
Figure 1.
Recurrently mutated genes in subtypes of B-cell non-Hodgkin lymphoma. An oncoplot shows significantly mutated genes, DNA copy number alterations (CNA) and translocations (Tx.) across our cohort of 685 B-cell non-Hodgkin lymphoma tumors. Mutation types and frequencies are summarized for each gene/CNA on the right, and the mutational burden for each case is shown at the top. DHL: double-hit lymphoma; THL: triple-hit lymphoma; HGBL-NOS: high-grade B-cell lymphoma not otherwise specified; BL: Burkitt lymphoma; DLBCL: diffuse large B-cell lymphoma; FL: follicular lymphoma; MCL: mantle cell lymphoma.
Figure 2.
Structural alterations in subtypes of B-cell non-Hodgkin lymphoma. (A) A circos plot shows translocations of MYC (purple), BCL2 (orange) and BCL6 (green) genes, and GISTIC tracks of DNA copy number gains (red) and losses (blue). (B, C) Volcano plots of integrative analysis results showing the changes in gene expression of genes within peaks of DNA copy number gain (B) or loss (C). A positive T-test score indicates increased expression in tumors with a given copy number alteration, and vice versa. Significantly expressed genes with the correct directionality are highlighted in the shaded areas. (D) Oncoplots show the overlap of structural alterations and mutations that target the same genes. P-values are derived from a Fisher exact test (ns: not significant).
Subtype-specific patterns of genetic alterations
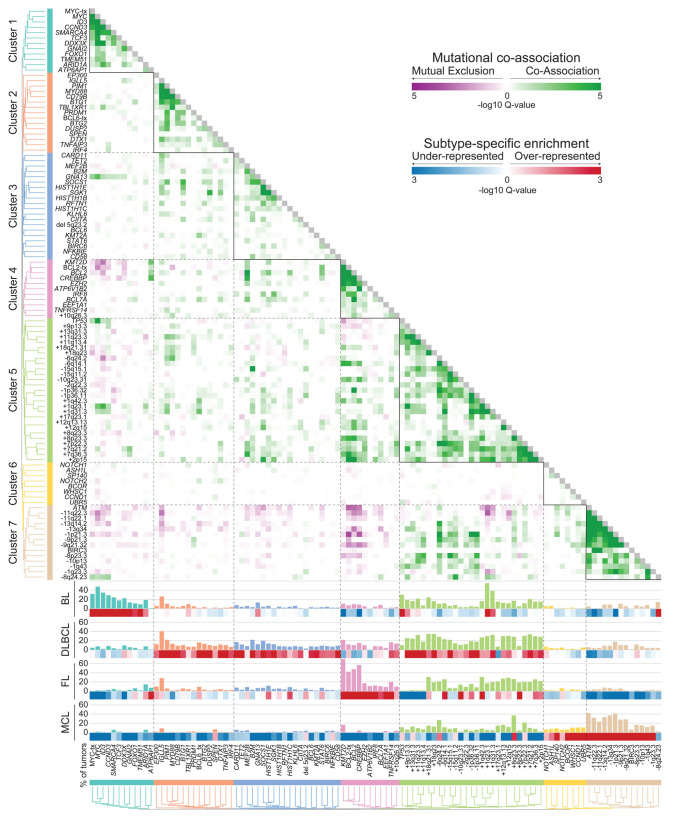
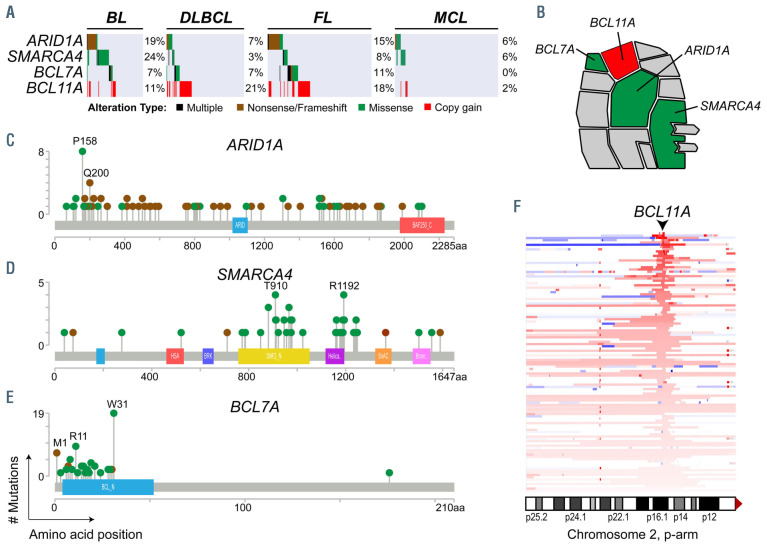
We formally tested the over- or under-representation of recurrent genetic alterations in each of the four subtypes with more than 100 samples (BL, DLBCL, FL, MCL), compared to all other tumors in the study (Figure 4, Online Supplementary Table S13). We observed some interesting patterns within hallmark characteristics that differ between subtypes. An illustrative example of this is the alternative avenues for BAF complex perturbation between different histologies (Figure 5). Specifically, mutations of the SMARCA4 (aka. BRG1) component of the ATPase module were significantly enriched in BL (24%) compared to other subtypes (4%, Q-value <0.001), while mutations of the BCL7A component of the ATPase module were significantly enriched in FL (11%) compared to other subtypes (4%, Qvalue= 0.007). In contrast, mutations of ARID1A were frequent in both BL (19%) and FL (15%), and DNA copy number gains of BCL11A were frequent in both DLBCL (28%) and FL (22%). The BAF complex is therefore a target of recurrent genetic alterations, as previously suggested,43 but the manner in which this complex is perturbed varies between B-NHL subtypes (Figure 5). Similar disease-specific patterns were also observed for signaling genes; for example, TCF3 and ID3 have important functions in normal germinal center B cells (GCB),44 but mutations of these genes are specifically enriched within BL and are rarely found in the other GCB-derived malignancies, DLBCL and FL. Similarly, the ATP6AP1 and ATP6V1B2 genes that function in mTOR signaling45,46 are specifically mutated in FL, and the DUSP2 gene which inactivates ERK1/247 and STAT348 is specifically mutated in DLBCL. The disease-specific patterns of genetic alterations therefore reveal subtle but important differences in how each subtype of B-NHL perturbs hallmark features.
Clusters of co-associated genomic alterations in subtypes of B-cell non-Hodgkin lymphoma
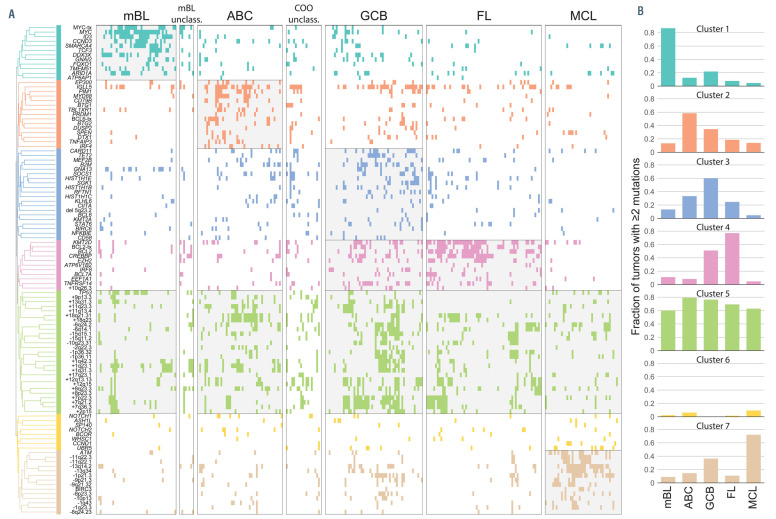
We next defined how each genetic alteration co-associated with or mutually excluded other genetic alterations by pairwise assessments using false-discovery rate (FDR)-corrected Fisher tests (Online Supplementary Table S14). A matrix of the transformed FDR Q-values (-logQ) was used for unsupervised hierarchical clustering to identify clusters of co-associated genetic alterations. Together with patterns of disease-specificity, unsupervised clustering revealed clear groupings of co-associated events for BL, DLBCL, FL and MCL (Figure 4). We identified a single cluster of significantly co-associated genetic alterations that was specifically enriched in BL (Cluster 1), including mutations and translocations of MYC, and mutations of CCND3, SMARCA4, TCF3 and ID3 which have been previously reported in BL.4 A single cluster was significantly enriched in MCL (Cluster 7), with a high frequency of ATM mutations and deletions, as well as other DNA CNA. Other mutations that were not significantly co-associated were also enriched in MCL (Cluster 6), such as those in WHSC1, NOTCH1, NOTCH2, BCOR and UBR5, although statistical assessment of coassociation may be hampered in this context by the low frequencies of mutations within these genes. A single cluster was also enriched in FL (Cluster 4), with a high prevalence of KMT2D, BCL2, CREBBP, EZH2 and TNFRSF14 mutations and BCL2 translocations. The genes within Cluster 4 also significantly overlapped with the previously reported C3, EZB and BCL2 clusters from prior whole exome sequencing studies of DLBCL3,49,50 (Fisher test P-values: P=0.0006, P=0.0148 and P=0.0173, respectively). Two clusters (Clusters 2 and 3) were enriched in DLBCL, with lower frequencies of mutations in a larger number of genes, in line with the genetic heterogeneity of this disease.3,4 Cluster 2 includes co-associated genetic alterations that overlapped with the previously described C5, MCD, and MYD88 clusters3,4 (Fisher P-values: P=0.0004, P=0.0002 and P=0.0007, respectively) including CD79B, MYD88 and TBL1XR1 mutations. Genes within Cluster 3 overlapped in a statistically significant manner with those in the previously described C4 and SOCS1/SGK1 clusters (Fisher P-values: P=0.0002 and P=0.0074, respectively), including SGK1, TET2, SOCS1 and histone H1 genes. We also identified a cluster consisting of TP53 mutations and multiple CNA (Cluster 5) similar to the genetically complex C2/A53 subtype reported in DLBCL;3,49 however, the overlap of features within these clusters could not be formally assessed due to differing annotations. The CNA captured in this cluster were variably represented across B-NHL subtypes, but were most frequent in DLBCL. B-NHL subtypes therefore harbor characteristic clusters of co-associated genetic alterations that likely cooperate in disease etiology.
Figure 3.
Functional enrichment of targets of somatic mutations and DNA copy number alterations. Genes targeted by somatic mutation and/or DNA copy number alteration were evaluated for enrichment in curated gene sets, and significant gene sets subsequently grouped according to overlapping gene set membership and functional similarity. In addition to genes assigned by DAVID (purple), some genes were manually curated into hallmark processes by literature review of their function (pink). Enriched gene sets could be summarized into four major hallmark processes, including (A) epigenetic and transcriptional control of gene expression, (B) regulation of apoptosis and proliferation, (C) regulation of signaling pathway activity, and (D) regulation of protein ubiquitination. The frequency of each genetic alteration is shown for each of the four major histologies included in this study, and the fraction of tumors in each histology bearing genetic alterations of one or more of the genes is summarized by a pie graph at the bottom for each hallmark. BL: Burkitt lymphoma; DLBCL: diffuse large B-cell lymphoma; FL: follicular lymphoma; MCL: mantle cell lymphoma; HMT, histone methyltransferase. HAT, histone acetyltransferase. DDR, DNA damage response. BCR, B-cell receptor.
Combinations of genetic alterations define molecular subtypes of B-cell non-Hodgkin lymphoma
Our data have revealed statistical enrichment of individual genetic alterations in subtypes of B-NHL, and pairwise relationships between different genetic alterations that define clusters of subtype-specific events. To validate and expand upon these observations we leveraged gene expression microarray data from 284 tumors that underwent pathology review and were profiled as part of prior studies. 10-12 We utilized BL, DHL, HGBL-NOS and DLBCL tumors to perform classification into molecularly-defined BL (mBL) and non-mBL using a Bayesian classifier with previously described marker genes,51 and subclassified nonmBL into activated B-cell (ABC)-like and GCB-like subtypes as we have described previously26 (Online Supplementary Figure S7). We evaluated the frequency of cumulative (≥2) genetic alterations within each cluster among mBL, ABClike DLBCL, GCB-like DLBCL, FL and MCL (Figure 6). This showed that Cluster 1 genetic alterations that were individually enriched in BL are cumulatively acquired in mBL, with 87% of tumors having ≥2 of these alterations compared to only 22% of GCB-like DLBCL. Similarly, Cluster 4 and Cluster 7 alterations were cumulatively acquired in 77% and 72% of molecularly-annotated FL and MCL, respectively. Cluster 4 mutations were also cumulatively acquired in 51% of GCB-like DLBCL, likely capturing the C3/EZB/BCL2 subtype that has genetic similarities to FL.3,4,50 Furthermore, Cluster 2 and Cluster 4 alterations were cumulatively acquired in 58% of ABC-like DLBCL and 60% of GCB-like DLBCL, respectively, further supporting their respective overlap with the C5/MCD/MYD88 and C4/ST2 subtypes of DLBCL. CNA within Cluster 5 were cumulatively acquired at high but variable frequencies in all of the subtypes, but showed subtype-specific patterns within this cluster such as higher frequencies of 18q21 and 18q23 gains in ABC-like DLBCL, and higher frequencies of chromosome 7 gains in GCB-like DLBCL and FL. B-NHL tumors therefore cumulatively acquire co-associated sets of genetic alterations in a manner that is characteristically associated with histologically- and molecularly-defined subsets of disease.
Discussion
By performing cross-sectional genomic profiling of a large cohort of tumors, we have developed a resource of genes and functional hallmarks that are recurrently targeted by genetic alterations in B-NHL, and have shown that the cumulative acquisition of combinations of genetic alterations are characteristic of histological and molecular subtypes of disease. Some of the functional hallmarks that we identified have been previously appreciated, with a few exceptions. For example, the mutation of genes with roles in epigenetic and transcriptional control of gene expression are known to be a hallmark of FL52 and we observed that 96% of FL tumors possessed mutations in one or more of the genes in this category. However, mutations within these genes were also observed in the majority of BL, DLBCL and MCL tumors, highlighting the conservation of this functional hallmark across B-NHL subtypes. There are subtype-specific patterns of chromatin-modifying gene alterations, such as those that we highlighted for BAF complex mutations, but we suggest that the genetic deregulation of epigenetic and transcriptional control of gene expression should be considered a general hallmark of BNHL. In addition, we suggest that the deregulation of the ubiquitin proteasome system is a hallmark of B-NHL that requires further investigation. Mutations in genes such as KLHL637 and UBR539 have recently been shown to play an important role in B-cell lymphoma, while the roles of other frequently mutated genes such as DTX1 and SOCS1 have not yet been functionally dissected. Furthermore, while the nature of AID-driven mutations in genes such as DTX1 and SOCS1 remain to be defined, other genes that are recurrently mutated by AID such as BCL7A53 and linker histone genes54 have been shown to play driving roles in lymphomagenesis. Genetic deregulation of the ubiquitin proteasome system has the potential to influence the activity or abundance of a range of substrate proteins, and represents a current gap in our knowledge of B-NHL etiology.
Figure 4.
Subtype-specific clusters of co-occurring genetic alterations. The frequency (bar graph) and over- or under-representation (blue to red scale) of mutations and structural alterations is shown on the left for Burkitt lymphoma, diffuse large B-cell lymphoma, follicular lymphoma and mantle cell lymphoma. The correlation matrix of co-associated (green) and mutually exclusive (purple) relationships was clustered to identify seven groups of co-occurring genetic alterations that were predominantly over-represented in a single subtype of B-cell non-Hodgkin lymphoma. BL: Burkitt lymphoma; DLBCL: diffuse large B-cell lymphoma; FL: follicular lymphoma; MCL: mantle cell lymphoma
Figure 5.
Subtype-specific patterns of BAF complex mutations. (A) An oncoplot shows the frequency of genetic alterations in genes that encode components of the BAF complex. (B) A schematic of the BAF complex shows recurrently mutated genes, ARID1A, SMARCA4 and BCL7A, and the BCL11A gene that is targeted by 2p15 DNA copy number gains. (C-E) Lollipop plots show the distribution of mutations in the BAF components ARID1A (C), SMARCA4 (D), and BCL7A (E). (F) A heatplot shows the location of chromosome 2p DNA copy number gains (red) ordered from highest DNA copy number (top) to lowest (bottom, copy number = 2.2). The BCL11A gene is in the peak focal copy gain. BL: Burkitt lymphoma; DLBCL: diffuse large B-cell lymphoma; FL: follicular lymphoma; MCL: mantle cell lymphoma.
The role of cooperative interactions between co-occurring genetic alterations is also an emerging field that requires further investigation. These interactions are not uncommon in cancer,55 and have been recently highlighted in DLBCL,3,4 but our data show that they are pervasive and characteristic features of the B-NHL genetic landscape. Cooperation between co-associated genetic alterations identified in this study requires formal validation in cell lines and/or animal models. However, there are many instances in which co-occurring genetic alterations that we observed have already been shown to cooperate in lymphomagenesis. In addition to the aforementioned example of MYD88 and CD79B mutations, transgenic mouse models of Ezh2 activating mutations or conditional deletion of Crebbp or Kmt2d have shown that these events are not alone sufficient for lymphomagenesis.56-61 We and others have observed a co-association between mutation of these genes and BCL2 translocations,14,62 and the addition of a Bcl2 transgene to these murine models indeed promoted lymphoma at a significantly higher rate than that observed with the Bcl2 transgene alone.56-61 These genetic alterations are therefore significantly more lymphomagenic in combination than they are alone, which provides proof of principle that a cooperative relationship exists between these cooccurring genetic alterations. Future studies focusing on other co-occurring mutations, such as MYC translocation and SMARCA4 mutation in BL, CREBBP and KMT2D mutation in FL, TCF4 copy gain and MYD88 mutation in DLBCL, and ATM mutation and RPL5 deletion in MCL, should therefore be performed to further explore these concepts and define their underlying functional relationship. We suggest that combinations of genetic alterations are likely to recapitulate the biology of B-NHL more accurately than are single gene models, and may reveal contextually different functional roles of genetic alterations depending on the co-occurring events.
Figure 6.
Cumulative acquisition of co-occurring genetic alterations. (A) An oncoplot shows the presence or absence of genetic alterations according to their clusters of co-association in molecularly-defined Burkitt lymphoma, activated Bcell- like diffuse large B-cell lymphoma (DLBCL), germinal center B-cell-like DLBCL, follicular lymphoma and mantle cell lymphoma with available gene expression microarray data. Shading shows histological or molecular subtypes with ≥50% of tumors bearing ≥2 genetic alterations within a given cluster. (B) Bar plots shows the frequency of tumors with ≥2 genetic alterations from each cluster. mBL: molecularly-defined Burkitt lymphoma; ABC: activated B-cell-like DLBCL; COO: cell of origin; GCB: germinal center B-cell-like DLBCL; FL: follicular lymphoma; MCL: mantle cell lymphoma.
The caveats regarding this study include the targeted nature of the LymphoSeq platform which may preclude consideration of a subset of important genes, the lack of germline DNA for the majority of samples that may lead to a small number of germ-line variants being falsely assigned as somatic, and the sample size for any given histological subtype being below that required to identify genes that are mutated at low frequency. Nonetheless, these data represent the first broad cross-sectional analysis of multiple histological and molecular subtypes of B-NHL using the same methodology and provide a framework of functional hallmarks and co-occurring genetic alterations that are enriched within these subtypes of B-NHL. These functional hallmarks are genetically perturbed in the majority of B-NHL, but our cross-sectional approach enabled us to elucidate subtype-specific preferences for genetic alterations within each functional hallmark. Furthermore, the subtype-specific clusters of co-occurring genetic alterations likely represent cooperative interactions that underpin the biology of different subtypes of B-NHL. These combinations identify opportunities for moving from single-allele to multiallele designs in cell line or animal models to better understand the molecular etiology of B-NHL subtypes. Together, these hallmarks and clusters of co-associated genetic alterations represent processes that are potentially druggable with targeted therapies,63-66 but that are likely influenced in a non-binary fashion by different combinations of genetic alterations. Deciphering the relationships between complex sets of genetic alterations and targetable dependencies will be a next step towards developing new rationally targeted therapeutic strategies in B-NHL.
Supplementary Material
Funding Statement
Funding: This research was supported by NCI R01CA201380 (MRG), the Nebraska Department of Health and Human Services (LB506 2016-17; MRG), and NCI cancer center support grants to the University of Texas MD Anderson Cancer Center (P30 CA016672) and the Fred & Pamela Buffet Cancer Center (P30 CA036727). HY is supported by a Fellow award from the Leukemia and Lymphoma Society. MRG is supported by a Scholar award from the Leukemia and Lymphoma Society and an Andrew Sabin Family Foundation Fellow award.
References
- 1.Swerdlow SH, Campo E, Pileri SA, et al. The 2016 revision of the World Health Organization classification of lymphoid neoplasms. Blood. 2016;127(20):2375-2390. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Armitage JO, Gascoyne RD, Lunning MA, Cavalli F. Non-Hodgkin lymphoma. Lancet. 2017;390(10091):298-310. [DOI] [PubMed] [Google Scholar]
- 3.Chapuy B, Stewart C, Dunford AJ, et al. Molecular subtypes of diffuse large B cell lymphoma are associated with distinct pathogenic mechanisms and outcomes. Nat Med. 2018;24(5):679-690. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Schmitz R, Wright GW, Huang DW, et al. Genetics and pathogenesis of diffuse large B-cell lymphoma. N Engl J Med. 2018;378(15):1396-1407. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Reddy A, Zhang J, Davis NS, et al. Genetic and functional drivers of diffuse large B cell lymphoma. Cell. 2017;171(2):481-494. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Bea S, Valdes-Mas R, Navarro A, et al. Landscape of somatic mutations and clonal evolution in mantle cell lymphoma. Proc Natl Acad Sci U S A. 2013;110(45):18250-18255. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Zhang J, Jima D, Moffitt AB, et al. The genomic landscape of mantle cell lymphoma is related to the epigenetically determined chromatin state of normal B cells. Blood. 2014;123(19):2988-2996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Green MR, Alizadeh AA. Common progenitor cells in mature B-cell malignancies: implications for therapy. Curr Opin Hematol. 2014;21(4):333-340. [DOI] [PubMed] [Google Scholar]
- 9.Wang JQ, Jeelall YS, Humburg P, et al. Synergistic cooperation and crosstalk between MYD88L265P and mutations that dysregulate CD79B and surface IgM. J Exp Med. 2017;214(9):2759-2776. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Lenz G, Wright G, Dave SS, et al. Stromal gene signatures in large-B-cell lymphomas. N Engl J Med. 2008;359(22):2313-2323. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Dave SS, Fu K, Wright GW, et al. Molecular diagnosis of Burkitt's lymphoma. N Engl J Med. 2006;354(23):2431-2442. [DOI] [PubMed] [Google Scholar]
- 12.Iqbal J, Shen Y, Liu Y, et al. Genome-wide miRNA profiling of mantle cell lymphoma reveals a distinct subgroup with poor prognosis. Blood. 2012;119(21):4939-4948. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Bouska A, Bi C, Lone W, et al. Adult high grade B-cell lymphoma with Burkitt lymphoma signature: genomic features and potential therapeutic targets. Blood. 2017;130(16):1819-1831. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Green MR, Kihira S, Liu CL, et al. Mutations in early follicular lymphoma progenitors are associated with suppressed antigen presentation. Proc Natl Acad Sci U S A. 2015;112(10):E1116-1125. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Lek M, Karczewski KJ, Minikel EV, et al. Analysis of protein-coding genetic variation in 60,706 humans. Nature. 2016;536(7616):285-291. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Lawrence MS, Stojanov P, Polak P, et al. Mutational heterogeneity in cancer and the search for new cancer-associated genes. Nature. 2013;499(7457):214-218. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Kuilman T, Velds A, Kemper K, et al. CopywriteR: DNA copy number detection from off-target sequence data. Genome Biol. 2015;16(1):49. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Mermel CH, Schumacher SE, Hill B, Meyerson ML, Beroukhim R, Getz G. GISTIC2.0 facilitates sensitive and confident localization of the targets of focal somatic copy-number alteration in human cancers. Genome Biol. 2011;12(4):R41. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Newman AM, Bratman SV, Stehr H, et al. FACTERA: a practical method for the discovery of genomic rearrangements at breakpoint resolution. Bioinformatics. 2014;30(23):3390-3393. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Bouska A, Bi C, Lone W, et al. Adult highgrade B-cell lymphoma with Burkitt lymphoma signature: genomic features and potential therapeutic targets. Blood. 2017;130(16):1819-1831. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Akasaka T, Lossos IS, Levy R. BCL6 gene translocation in follicular lymphoma: a harbinger of eventual transformation to diffuse aggressive lymphoma. Blood. 2003;102(4): 1443-1448. [DOI] [PubMed] [Google Scholar]
- 22.Kato M, Sanada M, Kato I, et al. Frequent inactivation of A20 in B-cell lymphomas. Nature. 2009;459(7247):712-716. [DOI] [PubMed] [Google Scholar]
- 23.Greiner TC, Dasgupta C, Ho VV, et al. Mutation and genomic deletion status of ataxia telangiectasia mutated (ATM) and p53 confer specific gene expression profiles in mantle cell lymphoma. Proc Natl Acad Sci U S A. 2006;103(7):2352-2357. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Challa-Malladi M, Lieu YK, Califano O, et al. Combined genetic inactivation of beta2- Microglobulin and CD58 reveals frequent escape from immune recognition in diffuse large B cell lymphoma. Cancer Cell. 2011;20(6):728-740. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Pfeifer M, Grau M, Lenze D, et al. PTEN loss defines a PI3K/AKT pathway-dependent germinal center subtype of diffuse large B-cell lymphoma. Proc Natl Acad Sci U S A. 2013;110(30):12420-12425. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Jain N, Hartert K, Tadros S, et al. Targetable genetic alterations of TCF4 (E2-2) drive immunoglobulin expression in the activated B-cell subtype of diffuse large B-cell lymphoma. Sci Transl Med. 2019;11(497): eeav5599. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Kim D, Fiske BP, Birsoy K, et al. SHMT2 drives glioma cell survival in ischaemia but imposes a dependence on glycine clearance. Nature. 2015;520(7547):363-367. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Rosenwald A, Wright G, Wiestner A, et al. The proliferation gene expression signature is a quantitative integrator of oncogenic events that predicts survival in mantle cell lymphoma. Cancer Cell. 2003;3(2):185-197. [DOI] [PubMed] [Google Scholar]
- 29.Salaverria I, Royo C, Carvajal-Cuenca A, et al. CCND2 rearrangements are the most frequent genetic events in cyclin D1(-) mantle cell lymphoma. Blood. 2013;121(8): 1394-1402. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Green MR, Vicente-Duenas C, Romero-Camarero I, et al. Transient expression of Bcl6 is sufficient for oncogenic function and induction of mature B-cell lymphoma. Nat Commun. 2014;5:3904. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Huang DW, Sherman BT, Lempicki RA. Systematic and integrative analysis of large gene lists using DAVID bioinformatics resources. Nat Protoc. 2009;4(1):44-57. [DOI] [PubMed] [Google Scholar]
- 32.Saeki K, Miura Y, Aki D, Kurosaki T, Yoshimura A. The B cell-specific major raft protein, Raftlin, is necessary for the integrity of lipid raft and BCR signal transduction. EMBO J. 2003;22(12):3015-3026. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Senft D, Qi J, Ronai ZA. Ubiquitin ligases in oncogenic transformation and cancer therapy. Nat Rev Cancer. 2018;18(2):69-88. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Monti S, Chapuy B, Takeyama K, et al. Integrative analysis reveals an outcomeassociated and targetable pattern of p53 and cell cycle deregulation in diffuse large B cell lymphoma. Cancer Cell. 2012;22(3): 359-372. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Honma K, Tsuzuki S, Nakagawa M, et al. TNFAIP3/A20 functions as a novel tumor suppressor gene in several subtypes of non- Hodgkin lymphomas. Blood. 2009;114(12): 2467-2475. [DOI] [PubMed] [Google Scholar]
- 36.Hartmann EM, Campo E, Wright G, et al. Pathway discovery in mantle cell lymphoma by integrated analysis of high-resolution gene expression and copy number profiling. Blood. 2010;116(6):953-961. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Choi J, Lee K, Ingvarsdottir K, et al. Loss of KLHL6 promotes diffuse large B-cell lymphoma growth and survival by stabilizing the mRNA decay factor roquin2. Nat Cell Biol. 2018;20(5):586-596. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Meriranta L, Pasanen A, Louhimo R, et al. Deltex-1 mutations predict poor survival in diffuse large B-cell lymphoma. Haematologica. 2017;102(5):e195-e198. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Swenson SA, Gilbreath TJ, Vahle H, et al. UBR5 HECT domain mutations identified in mantle cell lymphoma control maturation of B cells. Blood. 2020;136(3):299-312. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Mottok A, Renne C, Seifert M, et al. Inactivating SOCS1 mutations are caused by aberrant somatic hypermutation and restricted to a subset of B-cell lymphoma entities. Blood. 2009;114(20):4503-4506. [DOI] [PubMed] [Google Scholar]
- 41.Liu W, Quinto I, Chen X, et al. Direct inhibition of Bruton's tyrosine kinase by IBtk, a Btk-binding protein. Nat Immunol. 2001;2(10):939-946. [DOI] [PubMed] [Google Scholar]
- 42.Pawar SA, Sarkar TR, Balamurugan K, et al. C/EBP{delta} targets cyclin D1 for proteasome- mediated degradation via induction of CDC27/APC3 expression. Proc Natl Acad Sci U S A. 2010;107(20):9210-9215. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Krysiak K, Gomez F, White BS, et al. Recurrent somatic mutations affecting Bcell receptor signaling pathway genes in follicular lymphoma. Blood. 2017;129(4): 473-483. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Gloury R, Zotos D, Zuidscherwoude M, et al. Dynamic changes in Id3 and E-protein activity orchestrate germinal center and plasma cell development. J Exp Med. 2016;213(6):1095-1111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Okosun J, Wolfson RL, Wang J, et al. Recurrent mTORC1-activating RRAGC mutations in follicular lymphoma. Nat Genet. 2016;48(2):183-188. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Wang F, Gatica D, Ying ZX, et al. Follicular lymphoma-associated mutations in vacuolar ATPase ATP6V1B2 activate autophagic flux and mTOR. J Clin Invest. 2019;130: 1626-1640. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Hu J, Li L, Chen H, et al. MiR-361-3p regulates ERK1/2-induced EMT via DUSP2 mRNA degradation in pancreatic ductal adenocarcinoma. Cell Death Dis. 2018;9(8):807. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Lu D, Liu L, Ji X, et al. The phosphatase DUSP2 controls the activity of the transcription activator STAT3 and regulates TH17 differentiation. Nat Immunol. 2015;16(12):1263-1273. [DOI] [PubMed] [Google Scholar]
- 49.Wright GW, Huang DW, Phelan JD, et al. A probabilistic classification tool for genetic subtypes of diffuse large B cell lymphoma with therapeutic implications. Cancer Cell. 2020;37(4):551-568. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Lacy SE, Barrans SL, Beer PA, et al. Targeted sequencing in DLBCL, molecular subtypes, and outcomes: a Haematological Malignancy Research Network report. Blood. 2020;135(20):1759-1771. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Hummel M, Bentink S, Berger H, et al. A biologic definition of Burkitt's lymphoma from transcriptional and genomic profiling. N Engl J Med. 2006;354(23):2419-2430. [DOI] [PubMed] [Google Scholar]
- 52.Green MR. Chromatin modifying gene mutations in follicular lymphoma. Blood. 2018;131(6):595-604. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Balinas-Gavira C, Rodriguez MI, Andrades A, et al. Frequent mutations in the aminoterminal domain of BCL7A impair its tumor suppressor role in DLBCL. Leukemia. 2020;34(10):2722-2735. [DOI] [PubMed] [Google Scholar]
- 54.Yusufova N, Kloetgen A, Teater M, et al. Histone H1 loss drives lymphoma by disrupting 3D chromatin architecture. Nature. 2021;589(7841):299-305. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Ashworth A, Lord CJ, Reis-Filho JS. Genetic interactions in cancer progression and treatment. Cell. 2011;145(1):30-38. [DOI] [PubMed] [Google Scholar]
- 56.Beguelin W, Popovic R, Teater M, et al. EZH2 is required for germinal center formation and somatic EZH2 mutations promote lymphoid transformation. Cancer Cell. 2013;23(5):677-692. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Garcia-Ramirez I, Tadros S, Gonzalez-Herrero I, et al. Crebbp loss cooperates with Bcl2 over-expression to promote lymphoma in mice. Blood. 2017;129(19):2645-2656. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Zhang J, Vlasevska S, Wells VA, et al. The Crebbp acetyltransferase is a haploinsufficient tumor suppressor in B cell lymphoma. Cancer Discov. 2017;7(3):322-337. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Jiang Y, Ortega-Molina A, Geng H, et al. CREBBP inactivation promotes the development of HDAC3-dependent lymphomas. Cancer Discov. 2017;7(1):38-53. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Zhang J, Dominguez-Sola D, Hussein S, et al. Disruption of KMT2D perturbs germinal center B cell development and promotes lymphomagenesis. Nat Med. 2015;21(10): 1190-1198. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Ortega-Molina A, Boss IW, Canela A, et al. The histone lysine methyltransferase KMT2D sustains a gene expression program that represses B cell lymphoma development. Nat Med. 2015;21(10):1199-1208. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Morin RD, Mendez-Lago M, Mungall AJ, et al. Frequent mutation of histone-modifying genes in non-Hodgkin lymphoma. Nature. 2011;476(7360):298-303. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Sermer D, Pasqualucci L, Wendel HG, Melnick A, Younes A. Emerging epigeneticmodulating therapies in lymphoma. Nat Rev Clin Oncol. 2019;16(8):494-507. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Shen M, Schmitt S, Buac D, Dou QP. Targeting the ubiquitin-proteasome system for cancer therapy. Expert Opin Ther Targets. 2013;17(9):1091-1108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Merino D, Kelly GL, Lessene G, Wei AH, Roberts AW, Strasser A. BH3-mimetic drugs: blazing the trail for new cancer medicines. Cancer Cell. 2018;34(6):879-891. [DOI] [PubMed] [Google Scholar]
- 66.Roschewski M, Staudt LM, Wilson WH. Diffuse large B-cell lymphoma-treatment approaches in the molecular era. Nat Rev Clin Oncol. 2014;11(1):12-23. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.