Abstract
Plant cells rely on plasmodesmata for intercellular transport of small signaling molecules as well as larger informational macromolecules such as proteins. A green fluorescent protein (GFP) reporter and low-pressure microprojectile bombardment were used to quantify the degree of symplastic continuity between cells of the leaf at different developmental stages and under different growth conditions. Plasmodesmata were observed to be closed to the transport of GFP or dilated to allow the traffic of GFP. In sink leaves, between 34% and 67% of the cells transport GFP (27 kD), and between 30% and 46% of the cells transport double GFP (54 kD). In leaves in transition transport was reduced; between 21% and 46% and between 2% and 9% of cells transport single and double GFP, respectively. Thus, leaf age dramatically affects the ability of cells to exchange proteins nonselectively. Further, the number of cells allowing GFP or double GFP movement was sensitive to growth conditions because greenhouse-grown plants exhibited higher diffusion rates than culture-grown plants. These studies reveal that leaf cell plasmodesmata are dynamic and do not have a set size exclusion limit. We also examined targeted movement of the movement protein of tobacco mosaic virus fused to GFP, P30::GFP. This 58-kD fusion protein localizes to plasmodesmata, consistently transits from up to 78% of transfected cells, and was not sensitive to developmental age or growth conditions. The relative number of cells containing dilated plasmodesmata varies between different species of tobacco, with Nicotiana clevelandii exhibiting greater diffusion of proteins than Nicotiana tabacum.
Plasmodesmata are passageways that span the cell wall between plant cells, providing a thoroughfare for symplastic communication. Plasmodesmata are delimited by membranes, plasma membrane externally, and internal modified endoplasmic reticulum (Robards, 1971; Tilney et al., 1991). The space between these membranes, the cytoplasmic annulus, is believed to be the main passageway for cell-to-cell transport (Gunning, 1976; Overall et al., 1982; Ding et al., 1992b). How transport through these channels is regulated and how molecules manipulate these channels to gain access to adjacent cells is unknown. Size exclusion limits (SEL) have been determined that reflect the size of molecules that freely transit this annulus (Tucker, 1982; Erwee and Goodwin, 1983; Goodwin, 1983; Kempers and Van Bel, 1997). In addition, treatments affecting the physiological state of the cell result in altered plasmodesmatal aperture. Plasmolysis, calcium influx, pressure differentials, or inositol triphosphate reduce connectivity, whereas actin-disrupting drugs, profilin, azide, or osmotic shock increase SEL (Tucker, 1988, 1990, 1993; Oparka and Prior, 1992; Cleland and Lucas, 1993; Cleland et al., 1994; White et al., 1994; Schultz, 1995; Ding et al., 1996).
The size of the plasmodesmata annulus is highly regulated and can vary from being closed to all molecules, being open to the passage of small metabolites, and being dilated, allowing the passage of large biomolecules (Crawford and Zambryski, 2000). In the shoot apical meristem, fluctuations between closed and open plasmodesmata have been observed, corresponding to times of developmental transitions (Rinne and van der Schoot, 1998; Gisel et al., 1999; van der Schoot and Rinne, 1999). Plasmodesmata can also be permanently closed as seen for mature stomata and epidermal cell files in the root (Duckett et al., 1994; Oparka et al., 1994). Dilated plasmodesmata occur in a developmentally controlled manner (Oparka et al., 1999; Crawford and Zambryski, 2000) and during the transit of targeted proteins, which results in concurrent “gating” of the channel (Wolf et al., 1989; Fujiwara et al., 1993; Waigmann et al., 1994). Although complex, it is clear that plasmodesmata are responsive to environmental conditions and likely facilitate nutritional flow, as well as regional and whole-plant coordination (McLean et al., 1997; Rinne and van der Schoot, 1998).
The ability of plasmodesmata to transport macromolecules provides a possible mechanism to control transcellular programs (for review, see Zambryski and Crawford, 2000). A small group of endogenous proteins exists that can transit between cells following microinjection, although whether manipulation of these passageways is a requirement for function of these proteins is still unknown (Lucas et al., 1995; Balachandran et al., 1997; Ishiwatari et al., 1998; Crawford and Zambryski, 1999; Xoconostle-Cazares et al., 1999). Function resulting from movement of an endogenous protein was recently demonstrated for the Arabidopsis transcription factor LEAFY, important for the transition to flowering and for floral organ identity (Sessions et al., 2000). When LEAFY is expressed only in the L1 layer of mutant plants, a complete restoration of the wild-type phenotype is observed. This restoration corresponds to movement of the protein, but not RNA, out of the L1 layer and throughout the shoot apical meristem (Sessions et al., 2000). Whether this movement is a requirement of LEAFY function normally is unknown.
Intercellular transit through plasmodesmata is an absolute required function for viral movement proteins. As plasmodesmata provide an impediment to viral local and systemic movement, viruses have evolved these proteins, capable of manipulating these channels, to facilitate entrance to neighboring cells. Mutations in movement proteins, which destroy the ability of these proteins to transit plasmodesmata, destroy the ability of the virus to infect neighboring cells (for review, see Carrington et al., 1996; Lazarowitz and Beachy, 1999).
The movement protein of tobacco mosaic virus (TMV), P30, traffics between cells, gates plasmodesmata allowing the movement in trans of large macromolecules not specified for such traffic, and associates with the cytoskeleton (Heinlein et al., 1995; McLean et al., 1995; Citovsky, 1999; Ding et al., 1999). P30 also binds directly to single-stranded nucleic acids (e.g. viral genomes), creating elongated protein/RNA complexes with dimensions compatible with plasmodesmatal pore size (Citovsky et al., 1990, 1992). In the context of viral infection, these combined functions of P30 result in transfer of the TMV RNA genome through plasmodesmata into neighboring and distant cells (Deom et al., 1987; Meshi et al., 1987). The ability of P30 to manipulate plasmodesmata to allow for such transport is most likely direct, as this protein localizes to plasmodesmata in infected and transgenic plants (Tomenius et al., 1987; Atkins et al., 1991; Ding et al., 1992a) and dramatically increases the SEL in cells in which it is present (Wolf et al., 1989; Deom et al., 1990; Waigmann et al., 1994; Oparka et al., 1997).
The speed at which P30 and analogous viral movement proteins alter plasmodesmata to move into adjacent cells indicates use of an endogenous pathway (Waigmann et al., 1994). Proteins that interact with plasmodesmata and induce their own efficient movement have been designated “targeted” plasmodesmata proteins (Crawford and Zambryski, 2000). This movement is distinct and supplements the non-targeted, diffusive mode of protein transit exemplified by large tracer proteins such as green fluorescent protein (GFP), which move from the phloem and between cells of the leaf blade (Imlau et al., 1999; Oparka et al., 1999; Crawford and Zambryski, 2000).
Here a quantitative low-pressure biolistic assay was used to examine the effect of leaf age, plant growth conditions, and species on these two modes of protein movement through plasmodesmata. Non-targeted transport was affected significantly by all these conditions. In contrast, targeted protein movement, characterized by the TMV P30 protein, was unaffected by the conditions tested. Thus, targeted proteins such as TMV P30 are able to manipulate plasmodesmata irrespective of their physiologically determined aperture.
RESULTS
Below we assay three different conditions, leaf age, plant growth conditions, and plant species, for their affects on targeted intercellular traffic and non-targeted diffusion of proteins via plasmodesmata. Low-pressure microprojectile bombardment of plasmid DNA into epidermal cells of intact plants allows the subsequently expressed protein to be quantitatively analyzed for cell-to-cell movement potential (Crawford and Zambryski, 2000). When intercellular movement occurred, the number of cells moved into was also determined (Table I). Percent movement is reported as the number of transfected cells that allow movement into adjacent cells out of the total number of transfected cells.
Table I.
Protein movement through plasmodesmata reflects leaf age and physiology
| Protein | Tissue |
N.
tabacum in Vitro
|
N. tabacum
Soil
|
N. clevlandii Soil
|
||||||
|---|---|---|---|---|---|---|---|---|---|---|
| Movementa | No. cells analyzed | No. cells movedb | Movementa | No. cells analyzed | No. cells movedb | Movementa | No. cells analyzed | No. cells movedb | ||
| % | % | % | ||||||||
| rsGFP | Region A | 34 | 172 | 15 (±8) | 49 | 94 | 12 (±6) | 67 | 117 | 14 (±11) |
| Region B | 21 | 218 | 7 (±3) | 33 | 217 | 9 (±6) | 46 | 149 | 5 (±2) | |
| 2×rsGFP | Region A | 30 | 93 | 6 (±1) | 38 | 74 | 7 (±2) | 53 | 141 | 7 (±2) |
| Region B | 2 | 154 | 4 (±1) | 2.5 | 56 | 2 (±1) | 9 | 103 | 4 (±1) | |
| P30∷rsGFP | Region A | 76 | 104 | 7 (±3) | 72 | 81 | 9 (±6) | 78 | 130 | 12 (±7) |
| Region B | 52 | 85 | 5 (±4) | 63 | 64 | 9 (±3) | 50 | 128 | 11 (±9) | |
Percentage of movement is the no. of transfected cells permitting protein movement out of the total no. of transfected cells analyzed. Reported as an average between experiments recorded 1 d post-bombardment.
No. cells moved is a average of the no. of cells trafficked to beyond the transfected cell 1 d post-bombardment (sd).
Non-Targeted Protein Movement Is Restricted with Leaf Age
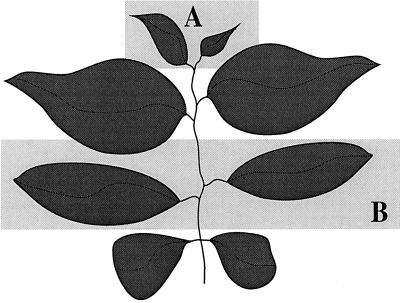
To understand parameters that affect the non-selective intercellular movement of macromolecules we examined the traffic of the heterologous protein, GFP. Following introduction into single cells, GFP (27kD), was able to move from 21% to 67% of transfected cells in young Nicotiana plants, depending on the conditions and plant type assayed (Table I). As Oparka et al. (1999) observed a developmental difference for the non-targeted movement of GFP in sink versus source leaves, we used our quantitative assay to score movement in reference to leaf age, which correlates with photosynthetic capacity. In this study the two smallest visible leaves on the plant were scored as region A, whereas the largest leaves were scored as region B (Fig. 1).
Figure 1.
Schematic representation of regions A and B of plants analyzed for protein movement
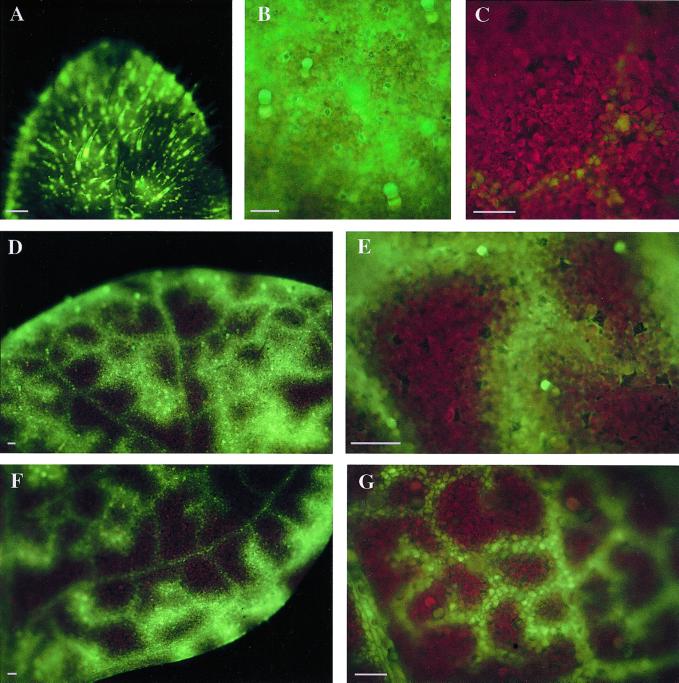
By carboxyfluorescein (CF; approximately 400 D) loading (Fig. 2) the leaves of region A are sinks and the leaves of region B are transition leaves (Roberts et al., 1997). Figure 2A shows a low magnification of a sink leaf (region A). A high magnification (Fig. 2B) view shows symplastic coupling between the cells in this sink leaf. For comparison, in striking contrast a source leaf (leaves below region B, Fig. 1) shows CF movement is limited to the vein; protein movement in source leaves is not the focus of the present study. Figure 2, D and F, shows the tip and mid-blade regions of a region B leaf in transition from sink to source. Figure 2, E and G, shows high and low magnification of symplastic unloading in transition leaves. Rather than compare sink with source leaves, which have limited symplastic trafficking, we chose to compare two types of leaves with different degrees of active symplastic connectivity; hence we used transition leaves. As large numbers of cells (50–200) were transfected and assessed for movement for any particular condition, we quantitatively assess the plasmodesmata transport in these two types of leaves. The experiments below assess cells for plasmodesmata in the dilated state, permitting macromolecular transport of proteins.
Figure 2.
CF loading. CF was loaded into the phloem through a cut that severs the root system. B through E, Images of leaves after a 30-min loading period; A and G, after a 10-min loading. The leaves analyzed in this study and subsequent figures are represented by A, B, and D through G. A is a low magnification view of loading in a sink leaf; the densely spaced trichome hairs illustrate that the leaf is unexpanded. B shows symplastic coupling between the cells in this sink leaf. For comparison, C shows minimal loading in a source leaf (leaves below region B, Fig. 1). D and F show the tip and mid-blade regions of a leaf in transition (from sink to source). E and G show high (30 min) and low (10 min) symplastic unloading in transition leaves. A, Scale bar = 1 mm; B through G, scale bar = 200 μm.
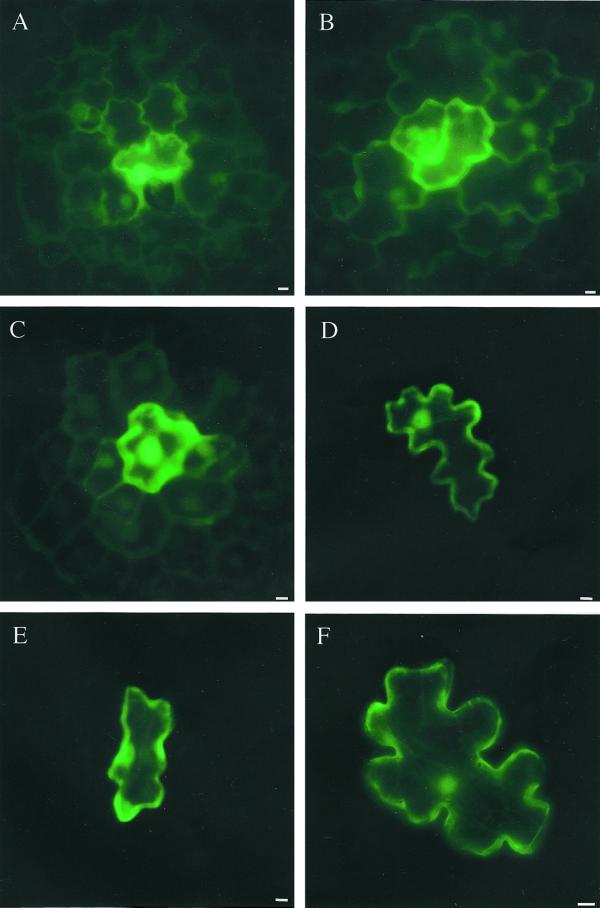
In all cases leaf age affected plasmodesmatal dilation, as more cells exhibited protein movement in region A than in region B (Fig. 3; Table I). This developmental restriction is most dramatically illustrated if cells are transfected with DNA encoding double GFP, 2×GFP (54 kD; Fig. 3, C and D). The percentage of cells permitting diffusion of 2×GFP through region A leaf cells (30%–53%) was comparable with GFP (34%–67%; compare Fig. 3, C with A), but was drastically reduced in region B leaves (2%–9%; Fig. 3D; Table I). Single GFP (27 kD) movement was only slightly reduced in region B cells (21%–46%; Fig. 3B; Table I). 2×GFP movement was completely inhibited (0%) in the first true leaves on the plant, older than those scored as region B. In addition, the distance traveled (no. of cells away) from the transfected cell was less for 2×GFP. Furthermore, triple GFP, 3×GFP (81 kD) was unable to move through plasmodesmata, irrespective of leaf age (Fig. 3, E and F). That a small portion of 3×GFP is seen in the nucleus is perhaps unexpected, based on Mr. However, 3×GFP in its narrowest dimension is 3 nm (longest dimension of approximately 12 nm) and is compatible with that of the nuclear pore (10 nm; Talcott and Moore, 1999). Thus, 3×GFP molecules may enter the nucleus if the GFP units are arranged in a linear conformation. This movement into the nucleus is unlikely to result from cleavage to smaller GFP forms because 3×GFP is never seen to move from transfected cells, even in sink leaves.
Figure 3.
Non-targeted diffusion through plasmodesmata. All images were captured 16 to 20 h post-bombardment using a CCD camera and epifluorescent microscope equipped with a fluorescein isothiocyanate filter set. Scale bars = 10 μm. A, GFP expression in sink tissue (region A) of N. tabacum. B, GFP expression in transition leaf (region B) of N. tabacum. C, Localization and spread of 2×GFP is similar to GFP in sink leaf. D, 2×GFP is often restricted to the transfected cell as seen here in a transition leaf. E, 3×GFP does not leave the transfected cell in sink tissues. F, 3×GFP movement is limited also in transition leaves.
The number of cells reached by non-targeted proteins was greater in sink leaves, potentially a reflection of the smaller cell size and implying that the distance traveled may be solely dependent on the conditions that affect diffusion (Table I). Therefore, the non-targeted flux of proteins between cells is dependent on leaf age. In further support of this conclusion, leaves just above region B contain more cells with dilated plasmodesmata exhibiting protein movement (for example, 4%–20% movement of 2×sGFP), and leaves below region B exhibit no movement (0%) of 2×sGFP (K.M. Crawford and P. Zambryski, unpublished data).
Non-Targeted Protein Movement Varies with Physiological State
The prevalence of cells exhibiting diffusion of non-targeted proteins varied with growth conditions. The movement of GFP and 2×GFP in Nicotiana tabacum plants grown in soil in the greenhouse was more prevalent than when plants were grown in culture containers in a growth chamber (Table I). More diffusion of GFP was observed in greenhouse-grown than in culture-grown plants in region A leaves, 49% versus 34%, and in region B leaves, 33% versus 21% (Table I). In a similar manner, a greater number of cells allowed intercellular diffusion of 2×GFP in greenhouse compared with cultured plants (Table I). Thus, the physiological conditions induced by greenhouse growth resulted in a greater number of dilated plasmodesmata, and thus was more conducive to the passage of non-targeted proteins. Physiology of the plant is, in addition to age, a regulator of the extent of cell-to-cell interchange of proteins.
The number of cells reached by non-targeted protein diffusion was, however, unaffected by growth conditions (Table I). Thus, we are detecting the frequency of cells with dilated plasmodesmata versus a change in the extent of movement. This further suggests that cells with dilated plasmodesmata are not singular, but rather exist as groups. Culture-grown and greenhouse-grown plants were identical in terms of age (32-d-old) and leaf number (6–7 true leaves). However, the size of greenhouse-grown leaves was much larger; region B leaves in culture are about 3 to 4 cm long, whereas they were about 8 to 10 cm long in the greenhouse. This difference likely reflects the greater expansion that can occur outside the restraints of a culture container, as well as the beneficial growth conditions afforded by natural light. The high humidity in the closed containers of cultured plants also potentially contributes to their moderately decreased plasmodesmata function. These studies highlight the known sensitivity of plants to their environment for a new parameter, plasmodesmata function.
Targeted Protein Movement Is Not Sensitive to Leaf Age or Physiology
GFP is an exogenous protein whose pattern of movement suggests diffusion, as a gradient of tracer, from the transfected cell. This type of movement we have designated non-targeted, as it does not localize to plasmodesmata; GFP is absent from defined regions within the cell wall, suggesting no direct interaction with plasmodesmata (Fig. 3). In contrast, GFP fusion to a viral movement protein such as TMV P30 marks its transit between cells with fluorescent punctae in the cell wall (see below). Further, although initially the transfected cell can be identified by bright fluorescence, within hours the fluorescence becomes equalized with adjacent cells, indicating a more active transport (Crawford and Zambryski, 2000). This latter protein movement is designated “targeted” as puncta represent protein localized to plasmodesmata (Padgett et al., 1996).
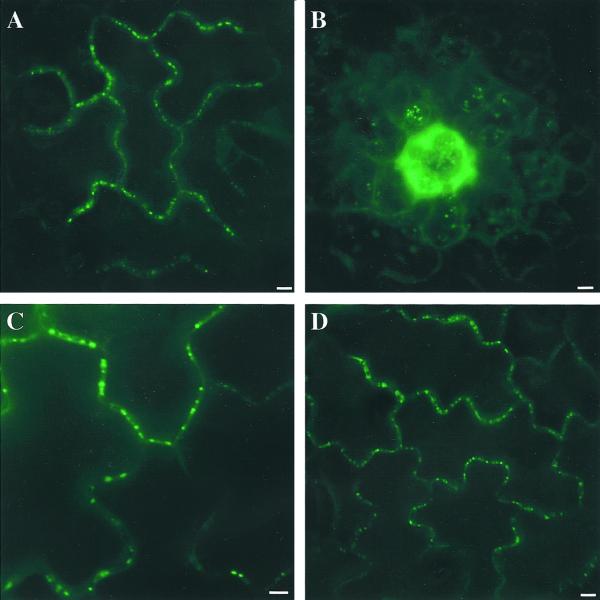
Although non-targeted GFP movement is sensitive to the developmental age of the leaf and growth conditions, targeted movement of P30::GFP was only weakly influenced by the developmental age of the leaf, as the number of cells allowing its intercellular transit was only slightly greater in young sink (region A) versus transitioning leaves (region B; Fig. 4; Table I). P30::GFP (57 kD) is similar in size to 2×GFP (54 kD), yet P30::GFP trafficked much more efficiently between cells of sink (72%–78%) and transition leaves (50%–63%). The movement of P30::GFP is 1.5-fold greater in region A and 26-fold greater in region B than 2×GFP (Table I). The efficiency of targeted transport is further illustrated in that P30::GFP moved from more transfected cells in region B (52%–63%) than the much smaller GFP in region A (34%–49%), a region of higher connectivity. That the number of cells exhibiting targeted movement is higher than for non-targeted movement implies that P30 is capable of moving through plasmodesmata in cells that do not allow non-targeted movement. The data reveal that targeted movement is not affected in the same way by plasmodesmatal aperture as non-targeted protein diffusion, and that targeted proteins can overcome physiological limitations on this aperture.
Figure 4.
P30::GFP is targeted to punctae in the cell wall in region A and region B leaves. All images were captured using a CCD camera and epifluorescent microscope equipped with a fluorescein isothiocyanate filter set. Scale bars are 10 μm A, P30::GFP moves efficiently and localizes to cell wall punctae in sink tissue of cultured N. tabacum. B, P30::GFP moves frequently to adjacent cells in transition leaves. Fluorescent punctae of P30::GFP are seen here in underlying mesophyll cells of cultured N. tabacum (focused through overlying epidermal cells). Mesophyll cells are smaller and more oval-shaped than puzzle-shaped cells of epidermis seen in A, C, and D. C, P30::GFP punctae in the cell wall of a sink leaf of a soil-grown N. tabacum. D, P30::GFP localizes to punctae in transitioning leaves of soil-grown N. tabacum.
The pattern of targeted movement of P30::GFP is distinct from that of non-targeted. P30::GFP localizes to points in the cell wall (compare Figs. 3 and 4, A, C, and D), and likely directly interacts with plasmodesmata structural components, enabling its proficient transport to neighboring cells. The number of N. tabacum cells reached by P30::GFP was not much affected by growth conditions (Table I). Targeted protein transport of P30::GFP did not result in a larger foci of epidermal cells than non-targeted movement (Table I). However, that P30::GFP (but not GFP) was detected often in underlying mesophyll cells (Fig. 4B) suggests that it can move farther. Thus, the mechanism that results in targeted transport of protein is unrestrained by the parameters that restrict non-targeted protein movement through plasmodesmata.
Non-Targeted Plasmodesmatal Movement Differs between Species
As plants differ in their ability to support viral infections, we also assessed movement of non-targeted and targeted proteins in a frequent experimental host, Nicotiana clevelandii. N. clevelandii plants were grown under greenhouse conditions identical to those used for N. tabacum above. The percentage of cells allowing plasmodesmatal transit of non-targeted proteins in N. clevelandii was greater than for N. tabacum. The parameters affecting protein diffusion were, however, the same as detected with N. tabacum. A significant number of transfected N. clevelandii cells allowed GFP diffusion irrespective of leaf age (Table I) and passage of 2×GFP occurred frequently in sink tissues, but was severely restricted in older leaves (Table I). Targeted transport of P30::GFP in N. clevelandii was comparable with that observed in N. tabacum (Table I). The number of cells into which non-targeted protein trafficked was equivalent between these two species (Table I). Overall, non-targeted protein diffusion in N. clevelandii was more prevalent, likely representing a greater number of cells with dilated plasmodesmata at any given time than in N. tabacum. Movement in an additional tobacco species, N. benthamiana, was also investigated and was found to exhibit even greater non-targeted movement than N. tabacum or N. clevelandii (data not shown); this enhanced capacity for non-targeted movement is reflective of its ability to act as a permissive host for different types of plant viruses. In contrast, targeted transport was similar in all species examined, again indicating that targeted protein movement is controlled by different parameters than non-targeted movement.
DISCUSSION
Here we illustrate that the extent of non-targeted intercellular exchange of proteins is controlled by the presence of dilated plasmodesmata and that the number of cells exhibiting plasmodesmata dilation is determined by developmental age and growth conditions. Proteins are capable of diffusing intercellularly if dilated plasmodesmata are present and certain criteria are met, as non-targeted protein flux is also sensitive to size and subcellular localization (Fig. 2; Crawford and Zambryski, 2000). It is interesting that the length of a molecule may be of importance in traversing plasmodesmata, as the GFP fusions utilized here could potentially have similar width dimensions, with increasing length restricting their capacity for movement. Non-targeted protein diffusion through plasmodesmata is dependent on the developmental age of a leaf, as sink leaves showed greater non-targeted movement than leaves in transition, even under varying growth conditions. In leaves transitioning to source there is a reduction in the number of cells exhibiting dilated plasmodesmata, which limits GFP and disrupts 2×GFP and 3×GFP diffusion.
Here both leaf regions assayed contain a population of dilated plasmodesmata, illustrated by GFP movement. The size of that population and the extent of dilation, however, appears greater in young versus older leaves, illustrated by lower levels of 2×GFP movement in region B. The modification of primary plasmodesmata to branched secondary plasmodesmata during the sink/source transition potentially leads to greater regulation of plasmodesmatal aperture, restricting non-targeted intercellular flux of proteins.
In addition to the down-regulation of plasmodesmatal aperture with leaf age and hence development, the regulation of plasmodesmata is also revealed to be dependent on growth condition. Plants of the same age with presumably the same type and number of plasmodesmata, but grown in different conditions show clear differences in their ability to allow intercellular diffusion of proteins. That greenhouse-grown plants have a higher percentage of cells with dilated plasmodesmata indicates the well-known fact that plants are environmentally responsive and must alter plasmodesmata accordingly. It is likely that several parameters work concurrently in controlling plasmodesmatal aperture, including temperature, light duration and quantity, and nutritional state. Differences in plasmodesmatal transport, dependent on the physiological condition, may reflect alterations to accommodate photosynthetic rates, although we cannot exclude that heightened transport of environmentally induced macromolecules could alter the aperture of plasmodesmata in trans. Certain environmental conditions may evoke a tighter regulation of non-targeted protein exchange, but not affect targeted proteins, which are able to directly interact with plasmodesmata to achieve their own transport. Thus, plasmodesmata likely are altered in form and function with developmental age, but remain highly responsive and likely fluctuate between conformations as required by environment.
It is notable that the targeted transport of TMV movement protein was unaffected by the conditions observed to limit non-targeted movement. P30::GFP movement was unaffected by leaf age or physiology. P30::GFP movement was extremely efficient and appeared to actively access adjacent cells in all conditions tested. It is remarkable that nearly all leaf cells (up to 78%) allow targeted movement, whereas non-targeted diffusion is restricted by size, leaf age, and growth conditions. In only roughly 20% of leaf cells did there appear to be a restriction on targeted movement of P30::GFP. These cells may be closed to all intercellular movement or their plasmodesmata may be in a state that prohibits manipulation by a targeted protein. These results further demonstrate that targeted transport is active, and that this form of movement is unique in its capacity to proficiently manipulate plasmodesmatal status to promote protein transit.
The present study extends the observations of Oparka et al. (1999). These authors tracked carbon import patterns and correlated restriction of GFP diffusion in source leaves with loss of photosynthetic influx. Changes in plasmodesmatal morphology from simple, linear, to more complex branched plasmodesmata suggested that plasmodesmatal structural changes are responsible for decreased connectivity in source leaves (Oparka et al., 1999). Specialized transport required for modification or implementation of a developmental or physiological program often is mirrored by the formation of secondary plasmodesmata (Ding et al., 1992a; Evert et al., 1996; Rinne and Van Der Schoot, 1998; Oparka et al., 1999; van der Schoot and Rinne, 1999; Ormenese et al., 2000). Here we perform a quantitative study to show that the restriction on non-targeted movement also occurs as leaves undergo the transition process, thus comparing two types of leaves that exhibit different degrees of active symplastic trafficking. Oparka et al. (1999) compare sink with source, a region with highly reduced symplastic transport. Further, we directly compare two similarly sized proteins for non-targeted (2×GFP, 58 kD) and targeted movement (P30-GFP, 57 kD) to demonstrate that although size matters, a targeted protein can supersede size limitations.
Our studies here (see also Crawford and Zambryski, 2000) have predominantly used plants grown in culture containers. As we assess movement quantitatively we early on realized that greenhouse-grown plants varied in their capacity to support intercellular movement of macromolecules. Thus, we used cultured plants to have a reproducible source of plant material to assess plasmodesmata function; these culture plants give highly consistent results, allowing us to compare their trafficking potential with plants grown under different conditions.
Further, we use very low pressure biolistic bombardment (60 psi) to minimize stress during transfection. In a recent report by Itaya et al. (2000), several different plants were tested (Nicotiana, Arabidopsis, cucumber, and tomato) for non-targeted movement of GFP using two different pressures for delivery, low (150–200) and high (1,000 psi). It is interesting that these authors observed much higher movement with their low pressure system, as previously discussed (Crawford and Zambryski, 2000). In fact, at higher pressures, Itaya et al. (2000) do not observe movement even in some sink leaves, whereas our own work, as well as that of Oparka et al. (1999), consistently see movement in sink tissues (using low pressure bombardment). Thus, pressure used during delivery significantly effects intercellular movement potential. Also, Itaya et al. (2000) often use detached leaves, which we have found to contain plasmodesmata that allow less non-targeted GFP movement, again indicating the sensitivity of plasmodesmata to stress.
That plasmodesmata can fluctuate in aperture as a function of growth conditions implies they are highly dynamic. Thus, to directly compare data from different research groups requires information on exact leaf (or other tissue) size, physiological and developmental status, and conditions of growth. As there is increasing evidence for endogenous protein movement, more and more studies will be performed to directly assess their interaction with plasmodesmata components. The present data are a first step to underscore the importance of reproducible plant growth conditions, as well as non-stressful experimental manipulations, to assess plasmodesmata functionality.
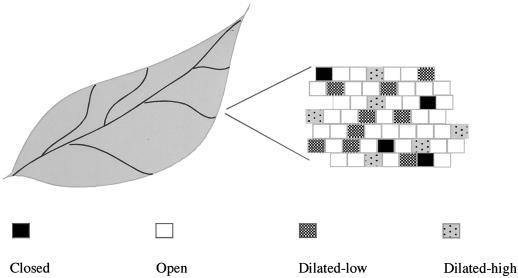
Figure 5 illustrates that the cells of the leaf contain plasmodesmata that can fluctuate in aperture. Plasmodesmatal dilation is regulated and dynamic, and cells of the leaf are highly heterogeneous with regard to plasmodesmata aperture. Some cells likely contain plasmodesmata closed to all intercellular traffic and do not transport even low Mr tracers. We have not examined closed plasmodesmata in this study, but we expect that they exist based on other analyses in the root and apical meristem (Duckett et al., 1994; Oparka et al., 1994; Rinne and Van Der Schoot, 1998; Gisel et al., 1999). A large number of cells allow low-weight tracers to traffic and contain open plasmodesmata (Crawford and Zambryski, 2000).
Figure 5.
Different plasmodesmata states within the leaf. We detect different frequencies of movement with the various probes used to assess plasmodesmata aperture. Some transfected cells do not exhibit GFP movement; thus, their plasmodesmata are completely closed or only open to small molecules. Closed plasmodesmata would not allow any transport, and could be permanently (as in stomata) or temporarily sealed (as has been reported in the shoot apical meristem; Rinne and Van Der Schoot, 1998; Gisel et al., 1999; van der Schoot and Rinne, 1999). Open plasmodesmata would allow for the exchange of nutrients and small dyes (i.e. sugars, CF, and 8-hydroxypyrene 1,3,6 trisulfonic acid). Other cells of the leaf have dilated plasmodesmata of varying apertures that allow for macromolecular trafficking through plasmodesmata. One population of cells allows GFP diffusion, implying their plasmodesmata are dilated to a sufficient degree to allow this 27-kD molecule to transit. A smaller population of transfected cells have plasmodesmata that are dilated to a higher degree, as they allow 2×GFP (54 kD) transit. These results suggest that the leaf is a mosaic where cells exist with plasmodesmata in varying states of distention and that dilated plasmodesmata do not have a single-set aperture.
The present study uncovers additional populations of cells that contain dilated plasmodesmata, allowing for the transit of macromolecules between cells. Dilation can be low (permitting GFP movement) or high (permitting double-sized GFP movement). The population size of cells with a dilated-high plasmodesmatal aperture is less than that for those with a dilated-low aperture. It is remarkable that the cells of the leaf are so heterogeneous with respect to plasmodesmatal aperture. Thus, leaf cells do not have a single-set size exclusion limit. Developmental age of the leaf and additionally, plant growth conditions, influence the number of cells capable of non-targeted movement. In contrast, plasmodesmatal targeted proteins specifically and efficiently manipulate plasmodesmata to achieve their own cell-to-cell movement independent of cell physiology, growth conditions, or species of plant tested.
MATERIALS AND METHODS
Plant Material
Nicotiana tabacum cv Samsun (nn) plants were grown in magenta culture containers (Carolina Biological Supply Co., Burlington, NC) in a growth chamber under a day/night regime of 16 h of light at 22°C and 8 h of dark at 19°C, 43% relative humidity; plants in closed containers are likely at higher humidity. All plants were grown one per pot. Cultured plants were grown in a Murashige and Skoog-based medium containing Murashig and Skoog salts (Gibco-BRL, Cleveland), 60g/L Suc, 1× vitamins, and 0.8% (w/v) agar. Soil-grown N. tabacum cv Samsun and Nicotiana clevelandii were grown in the greenhouse (25°C, day length of 10–12 h). All plants used were 31- to 34-d-old and had six to seven true leaves. Intact plants were bombarded in situ in the evening, were returned to their growth conditions immediately following bombardment, and were analyzed following 16 to 20 h.
Sink-Source Tracer
CF (60 μg/mL in distilled, deionized water, pH 6.3) was used as a tracer of phloem translocation by severing the roots of whole culture-grown plants and placing them in tube of dye solution. Plants were loaded for 10 to 30 min and were then visualized.
Microprojectile Bombardment
Microprojectile bombardment was performed as in Crawford and Zambryski (2000).
Microscopic Analysis
Wet mounts of detached leaves were made at the time of analysis for immediate viewing. Cells were analyzed using an Axiphot epifluoresecence microscope (Zeiss, Jena, Germany) equipped with a charge-coupled device (CCD) camera (Princeton Instruments, Trenton, NJ) and Chroma GFP filter set (470/40 LP495 525/50). Images were captured using IPlab software (Scanalytics, Vienna, VA). Quantitative analysis was done with the epifluorescence microscope, which allows for efficient scanning and the monitoring of low fluorescence-emitting cells.
Plasmid Constructs
Plasmids were constructed as noted in Crawford and Zambryski (2000). All constructs utilized a GFP designed to be red-shifted (Davis and Vierstra, 1998). pRTL2-3×GFP was created by first constructing GFP with a NdeI at the start site and an NcoI at the 3′ end of the coding region. This construct, pRTL2GFP3 N, was then digested with NcoI and BamHI and was ligated with a NcoI/BamHI fragment from pRTL2-2×GFP, containing both GFP open reading frames. All constructs result in transcription from a cauliflower mosaic virus 35S promoter.
ACKNOWLEDGMENTS
We thank Drs. Steve Ruzin and Denise Schichnes of the College of Natural Resources Biological Imaging Facility for their generous assistance with microscopy.
Footnotes
This work was supported by the National Institutes of Health (grant no. GM45244).
LITERATURE CITED
- Atkins D, Hull R, Wells B, Roberts K, Moore P, Beachy RN. The tobacco mosaic virus 30K movement protein in transgenic tobacco plants is localized to plasmodesmata. J Gen Virol. 1991;72:209–211. doi: 10.1099/0022-1317-72-1-209. [DOI] [PubMed] [Google Scholar]
- Balachandran S, Xiang Y, Schobert C, Thompson GA, Lucas WJ. Phloem sap proteins from Cucurbita maxima and Ricinus communis have the capacity to traffic cell to cell through plasmodesmata. Proc Natl Acad Sci USA. 1997;94:14150–14155. doi: 10.1073/pnas.94.25.14150. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Carrington JC, Kasschau KD, Mahajan SK, Schaad MC. Cell-to-cell and long-distance transport of viruses in plants. Plant Cell. 1996;8:1669–1681. doi: 10.1105/tpc.8.10.1669. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Citovsky V. Tobacco mosaic virus: a pioneer of cell-to-cell movement. Phil Trans R Soc London B Biol Sci. 1999;354:637–643. doi: 10.1098/rstb.1999.0415. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Citovsky V, Knorr D, Schuster G, Zambryski P. The P30 movement protein of tobacco mosaic virus is a single-strand nucleic acid binding protein. Cell. 1990;60:637–647. doi: 10.1016/0092-8674(90)90667-4. [DOI] [PubMed] [Google Scholar]
- Citovsky V, Wong ML, Shaw AL, Prasad BV, Zambryski P. Visualization and characterization of tobacco mosaic virus movement protein binding to single-stranded nucleic acids. Plant Cell. 1992;4:397–411. doi: 10.1105/tpc.4.4.397. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cleland RE, Fujiwara T, Lucas WJ. Plasmodesmal-mediated cell-to-cell transport in wheat roots is modulated by anaerobic stress. Protoplasma. 1994;178:81–85. doi: 10.1007/BF01404123. [DOI] [PubMed] [Google Scholar]
- Cleland RE, Lucas WJ. ATP regulation of the plasmodesmal size exclusion limit in wheat roots. Plant Physiol. 1993;102:22. [Google Scholar]
- Crawford K, Zambryski P. Characterization of two forms of plant intercellular protein movement: subcellular address controls cell-to-cell transportability. Curr Biol. 2000;10:1032–1040. [Google Scholar]
- Crawford KM, Zambryski PC. Phloem transport: are you chaperoned? Curr Biol. 1999;9:R281–R285. doi: 10.1016/s0960-9822(99)80179-1. [DOI] [PubMed] [Google Scholar]
- Davis SJ, Vierstra RD. Soluble, highly fluorescent variants of green fluorescent protein (GFP) for use in higher plants. Plant Mol Biol. 1998;36:521–528. doi: 10.1023/a:1005991617182. [DOI] [PubMed] [Google Scholar]
- Deom CM, Oliver MJ, Beachy RN. The 30-kilodalton gene product of tobacco mosaic virus potentiates virus movement. Science. 1987;1987:389–394. doi: 10.1126/science.237.4813.389. [DOI] [PubMed] [Google Scholar]
- Deom CM, Schubert KR, Wolf S, Holt CA, Lucas WJ, Beachy RN. Molecular characterization and biological function of the movement protein of tobacco mosaic virus in transgenic plants. Proc Natl Acad Sci USA. 1990;87:3284–3288. doi: 10.1073/pnas.87.9.3284. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ding B, Haudenshield JS, Hull RJ, Wolf S, Beachy RN, Lucas WJ. Secondary plasmodesmata are specific sites of localization of the tobacco mosaic virus movement protein in transgenic tobacco plants. Plant Cell. 1992a;4:915–928. doi: 10.1105/tpc.4.8.915. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ding B, Itaya A, Woo Y. Plasmodesmata and cell-to-cell communication in plants. Int Rev Cytol. 1999;190:251–316. [Google Scholar]
- Ding B, Kwon M-O, Warnberg L. Evidence that actin filaments are involved in controlling the permeability of plasmodesmata in tobacco mesophyll. Plant J. 1996;10:157–164. [Google Scholar]
- Ding B, Turgeon R, Parthasarathy MV. Substructure of freeze-substituted plasmodesmata. Protoplasma. 1992b;169:28–41. [Google Scholar]
- Duckett CM, Oparka KJ, Prior DAM, Dolan L, Roberts K. Dye-coupling in the root epidermis of Arabidopsis is progressively reduced during development. Development. 1994;120:3247–3255. [Google Scholar]
- Erwee MG, Goodwin PB. Characterization of the Egeria densa Planch. leaf symplast: inhibition of the intercellular movement of fluorescent probes by group II ions. Planta. 1983;158:320–328. doi: 10.1007/BF00397334. [DOI] [PubMed] [Google Scholar]
- Evert RF, Russin WA, Bosabalidis AM. Anatomical and ultrastructural changes associated with sink-to-source transition in developing maize leaves. Int J Plant Sci. 1996;157:247–261. [Google Scholar]
- Fujiwara T, Giesman-Cookmeyer D, Ding B, Lommel SA, Lucas WJ. Cell-to-cell trafficking of macromolecules through plasmodesmata potentiated by the red clover necrotic mosaic virus movement protein. Plant Cell. 1993;5:1783–1794. doi: 10.1105/tpc.5.12.1783. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gisel A, Barella S, Hempel FD, Zambryski PC. Temporal and spatial regulation of symplastic trafficking during development in Arabidopsis thaliana apices. Development. 1999;126:1879–1889. doi: 10.1242/dev.126.9.1879. [DOI] [PubMed] [Google Scholar]
- Goodwin PB. Molecular size limit for movement in the symplast of the Elodea leaf. Planta. 1983;157:124–130. doi: 10.1007/BF00393645. [DOI] [PubMed] [Google Scholar]
- Gunning BES. Intercellular Communication in Plants: Studies on Plasmodesmata. Berlin: Springer-Verlag; 1976. pp. 1–12. [Google Scholar]
- Heinlein M, Epel BL, Padgett HS, Beachy RN. Interaction of tobamovirus movement proteins with the plant cytoskeleton. Science. 1995;270:1983–1985. doi: 10.1126/science.270.5244.1983. [DOI] [PubMed] [Google Scholar]
- Imlau A, Truernit E, Sauer N. Cell-to-cell and long-distance trafficking of the green fluorescent protein in the phloem and symplastic unloading of the protein into sink tissues. Plant Cell. 1999;11:309–322. doi: 10.1105/tpc.11.3.309. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ishiwatari Y, Fujiwara T, McFarland KC, Nemoto K, Hayashi H, Chino M, Lucas WJ. Rice phloem thioredoxin h has the capacity to mediate its own cell-to-cell transport through plasmodesmata. Planta. 1998;205:12–22. doi: 10.1007/s004250050291. [DOI] [PubMed] [Google Scholar]
- Itaya A, Liang G, Woo Y-M, Nelson RS, Ding B. Non-specific intercellular protein trafficking probed by green-fluorescent protein in plants. Protoplasma. 2000;213:165–175. [Google Scholar]
- Kempers R, Van Bel AJE. Symplastic connections between sieve element and companion cell in the stem phloem of Vicia faba L. have a molecular exclusion limit of at least 10 kDa. Planta. 1997;201:195–201. [Google Scholar]
- Lazarowitz SG, Beachy RN. Viral movement proteins as probes for intracellular and intercellular trafficking in plants. Plant Cell. 1999;11:535–548. doi: 10.1105/tpc.11.4.535. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lucas WJ, Bouche-Pillon S, Jackson DP, Nguyen L, Baker L, Ding B, Hake S. Selective trafficking of KNOTTED1 homeodomain protein and its mRNA through plasmodesmata. Science. 1995;270:1980–1983. doi: 10.1126/science.270.5244.1980. [DOI] [PubMed] [Google Scholar]
- McLean BG, Hempel FD, Zambryski PC. Plant intercellular communication via plasmodesmata. Plant Cell. 1997;9:1043–1054. doi: 10.1105/tpc.9.7.1043. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McLean BG, Zupan J, Zambryski PC. Tobacco mosaic virus movement protein associates with the cytoskeleton in tobacco cells. Plant Cell. 1995;7:2101–2114. doi: 10.1105/tpc.7.12.2101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Meshi T, Watanabe Y, Saito T, Sugimoto A, Maeda T, Okada Y. Function of the 30-kD protein of tobacco mosaic virus: involvement in cell-to-cell movement and dispensability for replication. EMBO. 1987;6:2557–2563. doi: 10.1002/j.1460-2075.1987.tb02544.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Oparka KJ, Duckett CM, Prior DAM, Fisher DB. Real-time imaging of phloem unloading in the root tip of Arabidopsis. Plant J. 1994;6:759–766. [Google Scholar]
- Oparka KJ, Prior DAM. Direct evidence for pressure-generated closure of plasmodesmata. Plant J. 1992;2:741–750. [Google Scholar]
- Oparka KJ, Prior DAM, Santa Cruz S, Padgett HS, Beachy RN. Gating of epidermal plasmodesmata is restricted to the leading edge of expanding infection sites of tobacco mosaic virus (TMV) Plant J. 1997;12:781–789. doi: 10.1046/j.1365-313x.1997.12040781.x. [DOI] [PubMed] [Google Scholar]
- Oparka KJ, Roberts AG, Boevink P, Santa Cruz S, Roberts I, Pradel KS, Imlau A, Kotlizky G, Sauer N, Epel B. Simple, but not branched, plasmodesmata allow the nonspecific trafficking of proteins in developing tobacco leaves. Cell. 1999;97:743–754. doi: 10.1016/s0092-8674(00)80786-2. [DOI] [PubMed] [Google Scholar]
- Ormenese S, Havelange A, Deltour R, Bernier G. The frequency of plasmodesmata increases early in the whole shoot apical meristem of Sinapis alba L. during floral transition. Planta. 2000;211:370–375. doi: 10.1007/s004250000294. [DOI] [PubMed] [Google Scholar]
- Overall RL, Wolfe J, Gunning BES. Intercellular communication in Azolla roots: I. Ultrastructure of plasmodesmata. Protoplasma. 1982;111:134–150. [Google Scholar]
- Padgett HS, Epel BL, Kahn TW, Heinlein M, Watanabe Y, Beachy RN. Distribution of tobamovirus movement protein in infected cells and implications for cell-to-cell spread of infection. Plant J. 1996;10:1079–1088. doi: 10.1046/j.1365-313x.1996.10061079.x. [DOI] [PubMed] [Google Scholar]
- Rinne PLH, Van Der Schoot C. Symplasmic fields in the tunica of the shoot apical meristem coordinate morphogenetic events. Development. 1998;125:1477–1485. doi: 10.1242/dev.125.8.1477. [DOI] [PubMed] [Google Scholar]
- Robards AW. The ultrastructure of plasmodesmata. Protoplasma. 1971;72:315–323. [Google Scholar]
- Roberts AG, Santa Cruz S, Roberts IM, Prior DAM, Turgeon R, Oparka KJ. Phloem unloading in sink leaves of Nicotiana benthamiana: comparison of a fluorescent solute with a fluorescent virus. Plant Cell. 1997;9:1381–1396. doi: 10.1105/tpc.9.8.1381. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schultz A. Plasmodesmal widening accompanies the short-term increase in symplastic phloem unloading in pea root tips under osmotic stress. Protoplasma. 1995;188:22–37. [Google Scholar]
- Sessions A, Yanofsky MF, Weigel D. Cell-cell signaling and movement by the floral transcription factors LEAFY and APETALA1. Science. 2000;289:779–782. doi: 10.1126/science.289.5480.779. [DOI] [PubMed] [Google Scholar]
- Talcott B, Moore MS. Getting across the nuclear pore complex. Trends Cell Biol. 1999;9:312–318. doi: 10.1016/s0962-8924(99)01608-6. [DOI] [PubMed] [Google Scholar]
- Tilney LG, Cooke TJ, Connelly PS, Tilney MS. The structure of plasmodesmata as revealed by plasmolysis, detergent extraction, and protease digestion. J Cell Biol. 1991;112:739–747. doi: 10.1083/jcb.112.4.739. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tomenius K, Clapham D, Meshi T. Localization by immunogold cytochemistry of the virus-coded 30K protein in plasmodesmata of leaves infected with tobacco mosaic virus. Virology. 1987;160:363–371. doi: 10.1016/0042-6822(87)90007-9. [DOI] [PubMed] [Google Scholar]
- Tucker EB. Translocation in the staminal hairs of Setcreasea purpurea: I. A study of cell ultrastructure and cell-to-cell passage of molecular probes. Protoplasma. 1982;113:193–201. [Google Scholar]
- Tucker EB. Inositol biphosphate and inositol triphosphate inhibit cell-to-cell passage of carboxyfluorescein in staminal hairs of Setcreasea purpurea. Planta. 1988;174:358–363. doi: 10.1007/BF00959521. [DOI] [PubMed] [Google Scholar]
- Tucker EB. Calcium-loaded 1, 2-bis(2-aminophenoxy) ethane-N,N,N′,N′-tetraacetic acid blocks cell-to-cell diffusion of carboxyfluorescein in staminal hairs of Setcreasea purpurea. Planta. 1990;182:34–38. doi: 10.1007/BF00239980. [DOI] [PubMed] [Google Scholar]
- Tucker EB. Azide treatment enhances cell-to-cell diffusion in staminal hairs of Setcreasea purpurea. Protoplasma. 1993;174:45–49. [Google Scholar]
- van der Schoot C, Rinne P. Networks for shoot design. Trends Plant Sci. 1999;4:31–37. doi: 10.1016/s1360-1385(98)01362-4. [DOI] [PubMed] [Google Scholar]
- Waigmann E, Lucas WJ, Citovsky V, Zambryski P. Direct functional assay for tobacco mosaic virus cell-to-cell movement protein and identification of a domain involved in increasing plasmodesmal permeability. Proc Natl Acad Sci USA. 1994;91:1433–1437. doi: 10.1073/pnas.91.4.1433. [DOI] [PMC free article] [PubMed] [Google Scholar]
- White RG, Badelt K, Overall RL, Vesk M. Actin associated with plasmodesmata. Protoplasma. 1994;180:169–184. [Google Scholar]
- Wolf S, Deom CM, Beachy RN, Lucas WJ. Movement protein of tobacco mosaic virus modifies plasmodesmata size exclusion limit. Science. 1989;246:377–379. doi: 10.1126/science.246.4928.377. [DOI] [PubMed] [Google Scholar]
- Xoconostle-Cazares B, Xiang Y, Ruiz-Medrano R, Wang HL, Monzer J, Yoo BC, McFarland KC, Franceschi VR, Lucas WJ. Plant paralog to viral movement protein that potentiates transport of mRNA into the phloem. Science. 1999;283:94–98. doi: 10.1126/science.283.5398.94. [DOI] [PubMed] [Google Scholar]
- Zambryski PC, Crawford KM. Plasmodesmata: gatekeepers for cell-to-cell transport of developmental signals in plants. Annu Rev Cell Dev Biol. 2000;16:393–421. doi: 10.1146/annurev.cellbio.16.1.393. [DOI] [PubMed] [Google Scholar]