Graphical abstract

Keywords: Deep learning, Protozoan Parasite Dataset, Microscopy, Malaria, Plasmodium, Trypanosome, Babesia, Toxoplasma, Leishmania, Trichomonad, Evaluation metrics
Abstract
The infectious and parasitic diseases represent a major threat to public health and are among the main causes of morbidity and mortality. The complex and divergent life cycles of parasites present major difficulties associated with the diagnosis of these organisms by microscopic examination. Deep learning has shown extraordinary performance in biomedical image analysis including various parasites diagnosis in the past few years. Here we summarize advances of deep learning in the field of protozoan parasites microscopic examination, focusing on publicly available microscopic image datasets of protozoan parasites. In the end, we summarize the challenges and future trends, which deep learning faces in protozoan parasite diagnosis.
1. Introduction
Parasitic diseases have far-reaching consequences on public health globally. According to World Health Organization’s Global Health Estimates in 2020 [1], infectious and parasitic diseases are among the main causes of mortality in Africa. There are three main classes of parasites that can cause disease in humans: protozoa, helminths, and ectoparasites. Most parasitic protozoan in humans range less than 50 μm in size [2]. Due to the smaller size, protozoan parasites present a greater challenge to diagnosis from microscopic examination. Best known disease-causing protozoan parasites include Plasmodium, Trypanosome, Babesia, Toxoplasma, Leishmania, and Trichomonad. Malaria is caused by Plasmodium, which destroys the red blood cells, thus causing fatal disease. According to the World Health Organization (WHO) , malaria deaths has increased to an estimated 627,000 (±5%) due to disruptions during the COVID-19 pandemic [3]. Trypanosome is the causative agent of trypanosomiasis, which threatens the lives of people in sub-Saharan African countries and is transmitted by the tsetse fly [4]. Chagas Disease is a tropical parasitic disease in Latin America caused by Trypanosoma cruzi (T. cruzi) that leads to approximately 14,000 annual deaths [5]. Babesiosis, a malaria-like parasitic disease, affects not only a wide range of domestic and wild animals, but occasionally also people [6]. In the past 20–30 years, the United States has reported increasing numbers of blood transfusion-transmitted cases of Babesia, making Babesia infections a major public health problem [7]. Toxoplasma is the causative agent of toxoplasmosis that infects over a third of the world‘s population [8]. Toxoplasma can reach various parts of the body via the bloodstream, leading to a decreased immunity and various symptoms by destroying the brain, heart, and fundus of the eyes [9]. Most infected people have either no symptoms or mild symptoms, but Toxoplasma gondii (T. gondii) infection during pregnancy can cause severe nerve damage and even fetal death [10]. Leishmania causes leishmaniasis, which is a neglected tropical disease and transmitted by the female phlebotomine sandflies, affecting more than 700,000 people every year [11]. Trichomonad parasites in the genitourinary system, intestines and oral cavity cause human diseases, collectively referred to as trichomoniasis [12]. Trichomoniasis is highly epidemic in women, can cause poor perinatal outcomes and infertility [13]. Each year approximately 3.1 million women are infected with Trichomonas vaginalis in United States [14].
Mass drug administration programs have significantly reduced the disease burden [15]. To administer the correct drugs and to avoid drug abuse requires first a reliable and efficient diagnosis. Microscopy is one of the most commonly used methods for the diagnosing of the parasitic diseases [16], [17]. However, microscopy-based parasite identification and quantification is challenging, time-consuming and labor-intensive, requires microscope and well-trained researchers [18], [19], [20]. Parasite examination in microscopic images is challenging for human experts because of the variations and uncertainties in the shape, density and staining color of the parasites [21]. Human infections caused by parasites usually occur in developing and undeveloped countries, which often suffer from a lack of good medical conditions and well-trained microscope operators [22]. Another disadvantage of the microscopic examination of some parasites is the relatively low sensitivity, particularly at low parasite levels [20]. With the increase of computing power and data availability, artificial intelligence has been successfully employed in many applications, such as face recognition, natural language processing and biomedical image analysis [23], [24], [64]. Biomedical image analysis refers to the characterization, recognition and understanding of biomedical images, with the aid of the prior knowledge in large amount of data and the efficient extraction of data features [25]. As a subfield of machine learning, deep learning uses multi-layer neural networks to extract and characterize the features of input data, which can be divided into supervised, weakly supervised and unsupervised models. As shown in Fig. 1, the main difference between supervised and unsupervised learning is whether the data provided to the model is labeled. Labeled data is usually difficult to obtain, thus requiring labeling personnel, special equipment and additional expenses. Relatively speaking, unlabeled data is cheap and easy to obtain. Deep learning has been applied in the life and health sciences with the aim to improve the accuracy and efficiency of clinical diagnosis [26], and to discover novel biomarkers. From the perspective of biomedical image datasets, neural network with convolutional neural network (CNN) can extract the features of the images of these diseases [27], [28]. Furthermore, deep learning has played an important role in the analysis of a wide variety of microorganisms, including bacteria, fungi, parasites, and viruses [29].
Fig. 1.
Deep learning schemes of supervised learning (A) and unsupervised learning (B) in parasite detection. The main difference between supervised learning and unsupervised learning is whether the training dataset is labeled. Datasets should be clean and representative with balanced number of categories and statistically independent. After multiple epochs of training, the loss function converge to the optimal value. Then the best performing model can be used for predicting the infected and uninfected samples from the testing dataset.
2. Microscopic examination of protozoan parasites
Due to its high speed, accuracy, flexibility and low cost [19], [30], deep learning has been widely applied in various microscopic image analyses, including microscopic examination of protozoan parasites. This analysis includes detection, segmentation, tracking and classification. Detection is used to obtain the distribution and location of individual parasites, clusters and hosts [32], [33]. Classification is used for multi-parasites identification in the case of mixed infections [35], [34]. Segmentation is used to observe the edges of parasites morphologically [36]. Tracking is a unique task for the dynamic observation of motile parasites [37]. The detection model represented by You Only Look Once (YOLO) series has been improved to detect parasites [38]. CNN-based models are usually used for parasite classification [39]. U-Net-based model is widely used for segmentation [36], [40]. In clinical scenarios of these algorithms, researchers use methods such as transfer learning [19], [34] and GAN-based model [19] to develop weakly supervised learning and unsupervised learning [41], [30], which reduce the volume of annotation needed. Notably, smartphones play an important role in developing parasite diagnosis algorithms, thus greatly reducing the need for well-trained microscope operators [38].
2.1. Malaria microscopic image analysis
Plasmodium infects humans through mosquito bites. The malaria parasite Plasmodium goes through two life stages in the human host, a clinically silent liver stage followed by a blood stage with clinical manifestation of the malaria disease [42]. Therefore, the examination of the blood smear is essential for the diagnosis of malaria. [43] developed a two-stage method for detecting and counting red blood cells (RBCs) in thin blood smear microscopy images. In the first stage, U-Net is used to superpixel or cell-cluster segmentation. Then, Faster R-CNN is used to detect small cell objects in a connected component cluster. Due to the foreground cell-cluster masks from U-Net adaptively guide the detection stage, this work achieved higher true positive and lower false alarm rates. Inspired by residual and dense networks and the combined attention mechanism, [44] proposed a miniature and effective CNN named Attentive Dense Circular Net (ADCN) for classifying Red Blood Cells in Malaria. It is worth noting that ADCN combines the attention mechanism, which can make CNN focus on critical factors. It has a patient-level accuracy of 97.47% in infected RBCs classification task. Although great achievements have been made in the accuracy of the above algorithms, their application in most of the developing and undeveloped countries with high incidence of Malaria diseases remains difficult, due to their inconvenience, complexity and need for data annotation.
Supervised learning usually requires extensive annotation and is therefore time-consuming and laborious, microscopic image analysis of parasites requires progress in the development of weakly supervised and unsupervised learning strategies. Unlike other weakly supervised algorithms detecting specific objects by using image-level labels, [41] proposed Multiple Objects Features Fusion (MOFF), which is based on the fusion of the convolutional features of multiple objects. This study successfully diagnosed malaria in one hundred high resolution thick blood film (TBF) fields of view by using sample-level labels associated with multiple image fields. In evaluation of automated malaria diagnosis in blood films, MOFF outperforms the existing supervised detection in TBF dataset. [30] proposed a graph convolutional network (GCN) based model to recognize various stages of malaria parasites in blood smear images. This model based on unsupervised learning is the first application of GCN on recognition of malaria parasite image. It successfully recognizes multiple stages of malaria parasites with a patch-level accuracy of 95.4 ± 0.05% in the P. vivax larger-scale dataset without any image label, which consists of 13,780 testing images with an equal quantity of infected and uninfected images. However, this method has only been evaluated on multiple stages of Plasmodium, and its recognition performance for other multi-stage parasites has not been validated.
In addition, other studies have tried to solve the scarcity of highly skilled microscopists in undeveloped countries and remote areas of developing countries by focusing on the diagnosis of malaria using smartphones [38], [45], [46]. [46] could be among the first to apply deep learning to detect parasites in thick smears and run on smartphones. [45] developed an Android smartphone application for the automated malaria parasites detection method based on CNN architecture. Smartphone is attached to the eyepiece of microscope to obtain the target image. Images are caught with 100x magnification in RGB color space with pixel resolution of 3024 × 4032. Then, Iterative Global Minimum Screening (IGMS) is used to find the parasite candidates. Finally, the classification and detection of Plasmodium cells is achieved by running the customized CNN, and the parasite counting results are recorded in the patient database and displayed in the user interface of the mobile-phone applications. [38] reached the state-of-the-art in the detection of small objects such as Plasmodium by optimizing YOLO3 and YOLO4. YOLO3 and YOLO4 are state-of-art detectors with simple network structure and great generalization capability, but their drawback is that they are not optimized for detecting small objects. [38] modified the architecture of YOLO3 and YOLO4 to increase their applicability in detecting malaria parasites images captured by smartphone camera over the microscope eyepiece. In this study, feature scales were increased and detection layers were added to enhance capability and speed of detecting small objects. They applied the optimized models to detect malaria parasites from 608 × 608 images, which were captured by smartphone over microscope eyepiece with 1000x magnification. This YOLO based architecture models were shown to have obvious advantages, including high performance of mean average precision (mAP), compared to existing models such as single shot detector (SSD) and two-stage detector Faster R-CNN for P. falciparum detection [38].
2.2. Other microscopic image analyses of protozoan parasites
In the absence of treatment, T. gondii infection is fatal to human infants and people with the weakened immunity, such as AIDS patients [47]. [19] presented a transfer learning-based method for T. gondii microscopic image recognition without any data annotation. The connection between micro and macro objects in this study established by embedding fuzzy C-means cluster into CycleGAN. The algorithm achieved patch-level accuracy of 93.1% and 94.0% in the detection of T. gondii microscopic image dataset with magnification of 400x and 1000x, respectively. [40] applied U-net to segment T. cruzi and detected T. cruzi amstigotes nests from histopathological images, thus decreasing the workload of pathologists. The proposed model achieved a segmentation accuracy of 99.19% and Jaccard index of 49.3%, which outperform the results of SVM [40]. However, this research also showed some drawbacks of U-net, such as the network performance can be improved by increasing the number of images and variability, whose result is heavily dependent on the input data. [36] proposed FCN-based system and developed U-net method to segment and classify Leishmania into promastigotes, amastigotes and adhered parasites. In order to overcome labor-intensive diagnostic procedures and false negative scores caused by straightforward nature of sample preparation, [4] proposed a ResNet18 based method to detect T. cruzi automatically in unstained microscope images. This method can overcome sensitivity limitations related to microscopic examination. [48] proposed an efficient artificial neural network named MCellNet to detect Giardia and Cryptosporidium from drinking water. In this study, 346 frames per second were captured and processed by the system of MCellNet combined with imaging flow cytometry to increase the high-throughput and efficiency of deep learning. This model achieved a classification accuracy of greater than 99.6%, but could not handle the data that model did not see. [49] automatically and quantitatively analyzed the Babesia-infected Erythrocytes by applying DenseNet, thus avoiding the quantitative analysis error generated by the manual microscopy. Integrated gradient used as an interpretability tool of model revealed that cell boundaries, precipitate and rouleaux formations are the direct factors of false positives.
In clinical diagnosis scenarios, it is very important to identify the parasite species and presence of potentially mixed infections [35]. The multi-parasite algorithm has better practicability and generalization. [37] built a cost-effective mobile instrument to quickly detect the moving parasites of optically dense bodily fluids. The CNN model was used to automatically detect the signals created by motile Trypanosomes. This study showed that this instrument can detect other motile parasites by imaging Trichomonads. [32] proposed a deep-learning approach of geometry-aware and applied geometric-feature spectrum to detect host nucleus, Toxoplasma, Trypanosome, and Babesia by ExtremeNet based architecture without misdiagnosis. Different from ordinary unsupervised learning, [34] proposed a deep cycle transfer learning (DCTL) method for parasite detection. DCTL can avoid tedious data annotation by transferring macro shape knowledge from human experts to replace micro shape knowledge of parasites. DCTL successfully uses the shape information of macro objects that are similar with parasites' shape such as ring, pear and banana, to detect Plasmodium, Babesia and Toxoplasma. The drawback of DCTL is that highly depend on shape information of macro objects.
3. Parasite datasets and metric
To facilitate the development of deep learning approaches to the diagnostic of parasites, we summarize and describe the publicly available protozoan parasite datasets in Table1. As shown in the Table 1, most of them are Plasmodium datasets, because malaria diagnosis still remains the main focus of deep learning based parasite research [21]. Studies containing datasets of other parasites, such as Toxoplasma, Babesia, Leishmania, Trypanosome and Trichomonad [19], [29], [34], are precious because there are only few publicly available datasets of these parasites. Most of the datasets are used for the detection and classification with only few datasets focusing on the segmentation [50].
Table 1.
Publicly available microscopic image datasets of protozoan parasites.
| Reference | Parasite-Image Number | Descriptions | DownloadLink |
|---|---|---|---|
| [51] | Plasmodium falciparum-13,799. | The dataset contains a total of 27,558 cell images with equal instances of parasitized and uninfected cells. | https://ceb.nlm.nih.gov/repositories/malaria-datasets/ |
| [50] | Four species of Malaria parasites: Plasmodium Falciparum-22, Malariae-37, Ovale-29, Vivax-46. | The dataset comprises four species of Malaria parasites. For each species, there are four distinct stages of life, namely Ring stage, Trophozoite stage, Schizont stage and Gametocyte stage. | https://github.com/andrealoddo/MP-IDB-The-Malaria-Parasite-Image-Database-for-Image-Processing-and-Analysis.gi |
| [29] | Plasmodium-843, Toxoplasma-6691, Babesia-1,173, Leishmania-2,701, Trypanosome-2,385, Trichomonad-10,134. | The dataset contains 34,298 microscopic images of multiple parasites and host cells (Red blood cell, and Leukocyte) under 400x or 1000x magnification. | https://data.mendeley.com/datasets/38jtn4nzs6/3 |
| [32] | Toxoplasma-261, Trypanosome-480, Babesia-567. | The dataset contains three types of parasites acquired by a bright-field light microscope with 1000x magnification. | https://github.com/jiangdat/GFS-ExtremeNe |
| [34] | Plasmodium-5,758, Toxoplasma-5,741, Babesia-5,878. | The DCTL dataset contains 17,377 cropped image patches of Plasmodium, Toxoplasma, and Babesia and 6,981 Erythrocytes with 1000x magnification. | https://github.com/senli2018/DCTL |
| [19] | Toxoplasma gondii-8,156 with 400x, 6,969 with 1000x. | This FCGAN dataset also includes 4,979 host cell images at 400x and 8,023 host cell images at 1000x magnification. | https://github.com/senli2018/FCGAN/ |
| [30] | Plasmodium-15,927, Babesia-1,100. | This dataset contains totally 23,463 microscopic images of multi-stage Plasmodium, Babesia and host cells (RBCs and Leukocytes) under 1000x magnification. | https://github.com/senli2018/DTGCN_2021 |
| [61], [62] | Plasmodium falciparum-2,703. | Annotated image dataset with bounding boxes of 50,255 parasites. The dataset also contains 1,182 thick blood smear images with bounding boxes of 7,245 parasites. | https://air.ug/microscopy/ |
| [63], [21] | Plasmodium vivax-1,364. | The dataset contains images coming from 2 classes of uninfected cells (RBCs and leukocytes) and 4 classes of parasitized cells (Gametocytes, Rings, Trophozoites, and Schizonts) by a 1000x magnification. | https://bbbc.broadinstitute.org/BBBC041 |
| [41] | Plasmodium-TBF (155 Negative, 144 Positive) and BFS (69 Negative, 72 Positive). | Images of Thick Blood Film ( TBF) and Blood Film Smear (BFS) were captured with custom built brightfield microscope. | TBF:https://rdr.ucl.ac.uk/articles/dataset/Giemsa_Stained_Thick_Blood_Films_for_Clinical_Microscopy_Malaria_Diagnosis_with_Deep_Neural_Networks_Dataset_/12173568 BFS:https://rdr.ucl.ac.uk/articles/dataset/Digitized_Thin_Blood_Films_for_Sickle_Cell_Disease_Detection/12407567 |
| [43] | Plasmodium falciparum- Polygon set: 165, Point set: 800 images. | The RBCNet dataset contains Polygon set and Point set. Polygon set, cell outlines annotated by polygons for network training (165 images from 33 patients). Point set, annotated by placing a dot on each cell, for network evaluation (800 images from 160 patients). | https://github.com/nlm-malaria/RBCNet |
| [31], [38] | Plasmodium falciparum-948. | The dataset contains 1,182 thick blood smear slides with 1000x magnification, including 948 malaria-infected images with 7,628 P. falciparum parasites and 234 normal images with artifacts. | http://air.ug/downloads/plasmodium-phonecamera.zip |
3.1. Representative dataset
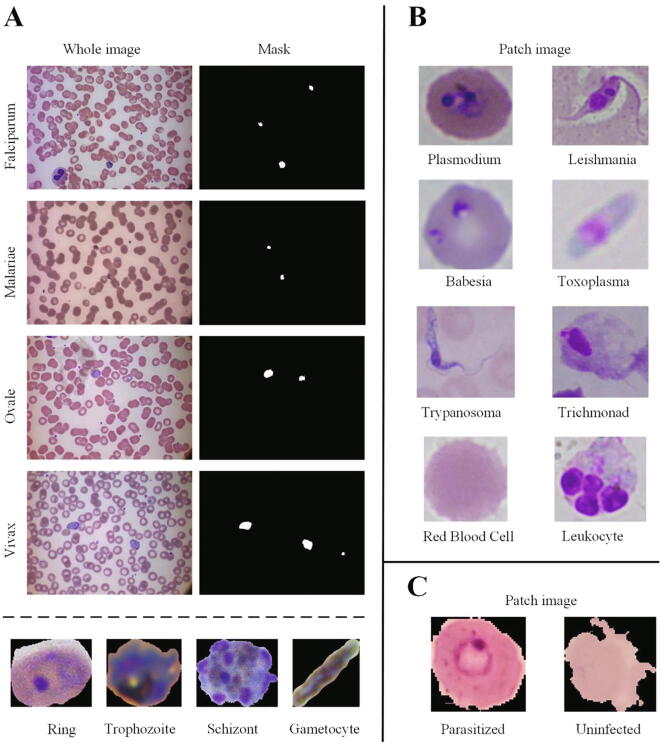
The detailed parameters of representative datasets are displayed in Table 1. Three representative datasets were displayed in Fig. 2. The malaria parasites dataset [50] is a representative dataset, which harbors four species of malaria parasites, namely Falciparum, Malariae, Ovale and Vivax. For each species, there are four distinct stages of life, namely Ring stage, Trophozoite stage, Schizont stage and Gametocyte stage. Each original image has corresponding mask provided and labeled by expert pathologists. In addition, the Plasmodium dataset produced by the National Library of Medicine in the United States of America [51] has attracted the attention of many researchers [39], [52], [44], [53]. This dataset was collected from the Chittagong Medical College Hospital, Bangladesh, and includes the Giemsa stains of 150P. falciparum-infected patients and 50 healthy patients. The uninfected cases did not contain infected cells but contained other interferences, such as staining interferences or dust impurities. These images were obtained by imaging of thin blood smear slides on a traditional optical microscope using the mobile phone's built-in camera. The focus of the available public datasets on the Plasmodium poses a difficulty for researchers who want to study other parasites. [29] collected 6 types of protozoan parasites' images, and made them publicly available. Images in this dataset are collected using a bright-field light microscope with either 400x or 1000x magnification. 34,298 microscopic images of multiple parasites and host cells were obtained, including Plasmodium, Toxoplasma, Babesia, Leishmania, Trypanosome, Trichomonad, Red blood cells and Leukocytes. It is worth noting that the current publicly available Leishmania datasets are very rare, and the flagellated promastigote stage present only in the midgut of insect vectors (sandflies) [54]. The images of Leishmania collected and presented here with promastigote morphology [29] might be not useful for medical diagnostic, but this dataset is still very useful for the study the life cycle of the Leishmania parasite, and would be also useful for deep learning training and model development.
Fig. 2.
Three representative datasets of publicly available protozoan parasite for deep learning. A: Malaria dataset [50]. The dataset contains four species of Malaria parasites: Falciparum, Malariae, Ovale, Vivax in original whole images and cropped image patches. For each species, the parasites have four distinct stages of life including Ring stage, Trophozoite stage, Schizont stage and Gametocyte stage. The patch image of four stages are shown coming from the specie of Plasmodium Falciparum. Each original whole image has corresponding mask, provided and labeled by expert pathologists. B: Multiple protozoan parasites dataset [29]. This dataset contains six types of protozoan parasites (Plasmodium, Toxoplasma, Babesia, Leishmania, Trypanosome, Trichomonad) and two types of host cells (RBCs and Leukocyte) presented in the cropped image patches. C: Plasmodium falciparum dataset [51]. The dataset contains cropped image patches from parasitized and uninfected RBCs.
3.2. Evaluation metrics
To aid parasite classification, detection and segmentation of deep learning, we summarized the algorithms-related formulas for standard metrics in Table 2. The evaluation metrics of classification mainly include Accuracy, Precision, Recall, Specificity, F1 score and Confusion Matrix. The evaluation indicators of object detection mainly include precision-recall curve, average precision (AP), mean average precision (mAP), Intersection-over-Union (IoU), receiver operating characteristic curve (ROC curve) and area under curve (AUC). In order to measure the segmentation task, Pixel Accuracy (PA), Dice, volumetric overlap error (VOE) and average symmetric surface distance (ASD) are usually calculated in medical image analysis.
Table 2.
Standard evaluation metrics for parasite analysis based on deep learning. A is the predicted result and B is the Ground truth. TP: true positive, TN: true negative, FP: false positive, FN: false negative.
| Classification | |
| Detection | |
| Segmentation | |
4. Challenges and future trends
Deep learning assists in the analysis of parasites and their hosts. In this paper, we systematically and comprehensively summarized the development and the application of deep learning in microscopic examination of protozoan parasites. Furthermore, we also collected and described the available datasets and model evaluation metrics used in protozoan parasites.
4.1. Pros and cons
There is a number of methods used for protozoan parasite examination. For example, the main methods of malaria diagnosis include rapid diagnostic tests (RDTs) and microscopy [3]. Compared to microscopic examination by human experts, RDTs are quite convenient, effective and fast. However, RDTs is not always available and detectable in all regions with the largest disease burden [3]. According to world malaria report 2021, Nicaragua suffered undersupply of RDTs during a malaria outbreak [3]; between 2020 and 2021, 17 publications from 13 countries have recorded the pfhrp2/3 deletions, which made plasmodium undetectable by RDTs [3]. Hence, microscopic examination and other molecular or antigenic diagnostic tests are more complementing than replacing each other. Microscopic examination remains the gold standard for laboratory diagnosis [17], and deep learning represents promising technology for microscopic parasite examination [18]. Compared to parasite microscopic examination by the human experts, microscopic parasite diagnosis employing deep learning is fast, automatable and accurate, while traditional microscopic examination by human experts is usually tedious, time-consuming and labor-intensive, which can lead to misdiagnosis and missed diagnosis [55], [56]. Deep learning-based microscopic parasite diagnosis can save a lot of time for doctors, thus reducing the rate of misdiagnosis, missed diagnosis and drug abuse. Using deep learning-based microscopic parasite diagnosis clinicians can spend more time on advanced decision-making tasks. Besides, deep learning has advantages in multi-parasite diagnosis, multi-stage parasite recognition and parasite quantification in real-time, high-throughput and at low parasite density [32], [48], [57], [30].
Although deep learning has made progress in protozoan parasite diagnosis, several challenges remain. As our article highlights, dataset is an important factor restricting the wide application of deep learning for parasite diagnosis. Compared with other fields, the scarcity of publicly available datasets in the field of parasite diagnosis is more prominent, especially in other protozoan parasites other than Plasmodium. In some protozoan parasite examination studies, datasets are too small to give a convincing evaluation about model‘s performance. Therefore, collecting existing publicly available protozoan parasite datasets is very important in training model and distinguishing model‘s over-fitting, robustness and good generalization [40]. In supervised learning, the lack of labeled data is an important factor hindering the application of deep learning in parasitology. Collecting and labeling large datasets for training models is costly and time-consuming, especially in the pixel-level labeling for segmentation tasks [36], [40]. Moreover, some datasets currently use only parts of samples in the microscopy slide, while whole slide image needs to be analyzed [43], [45]. This requires additional time, computing resources and more robust models for small objects. Inference speed, model size and accuracy of model need to be compromised in clinics. Parameters of dataset, such as the different staining methods, preparation of samples, color differences, standards of images and digitization devices, need to be unified and be more specific to determine their impact on the performance of deep learning models [58].
To solve the limitation of available labeled datasets, some transfer learning-based methods have been created [19], [34]. These methods rely heavily on the selection of specific macro objects to match the parasites, and might be only effectively against specific types of protozoan parasites tested, such as Toxoplasma, Plasmodium and Babesia. These methods might not be able to apply to other parasites which might have no corresponding macro objects, such as Leishmania, Trichmonad and Trypanosoma. Although deep learning model performs well in the analysis of microscopic parasite images, deep learning is like an opaque black box, which lack convincing interpretability [59]. The complete interpretability of deep learning model would be conducive to the promotion of artificial intelligence in the protozoan parasites diagnosis. The deep learning model has a large number of hyperparameters which usually depends on empirical settings, such as learning rate, batch size and epochs, which are also unexplainable. Although there are currently some automated tuning tools such as Neural Network Intelligence and AutoML [60], the long training time and vast parameter verifications make those newly proposed state-of-the-art models require a large amount of time and computing resources.
4.2. Pay more attention to biological view
Deep learning-based protozoan parasite researches should not only focus on improving model accuracy, but also pay more attention to the biological view. The complex and divergent life cycles of the protozoan parasites make lots of difficulties associated with the diagnosis of these organisms by deep learning. Therefore, deep learning-based protozoan parasite researches need to consider the different growth stages and distinct morphological characteristics of the parasites. Protozoan parasites accomplish their life cycle in different hosts where they adopt completely different morphological stages, which needs to be considered for accurate parasite microscopic examination. Besides Plasmodium [30], it is also true for other protozoan parasites such as T.gondii and Leishmania. T.gondii has three infective stages: tachyzoite, bradyzoite and sporozoite [10]. However, Plasmodium is perhaps the simplest case because it infects non-nucleated cells (RBCs) during blood stage. This is not the case for all protozoan parasites, care must be taken before generalizing the benefits of such deep learning approaches to other protozoa and in the context of medical decision making. Presenting and distinguishing their different morphological stages and divergent life cycles can help us to better understand the difficulties associated with the diagnosis of these organisms by microscopic examination and deep learning.
Funding
The work was supported by the Shenzhen Science and Technology Innovation Commission (Shenzhen Basic Research Project No. JCYJ20180306172131515), the Fundamental Research Funds for the Central Universities (HIT.NSRIF.2020064) and Shenzhen Science and Technology Program the university stable support program (20200821222112001).
CRediT authorship contribution statement
Chi Zhang: Formal analysis, Writing – original draft, Visualization, Implementation. Hao Jiang: Methodology, Writing – original draft, Implementation. Hanlin Jiang: Formal analysis. Hui Xi: Formal analysis. Baodong Chen: Writing – review & editing. Yubing Liu: Writing – review & editing. Mario Juhas: Writing – review & editing. Junyi Li: Writing – review & editing, Funding acquisition. Yang Zhang: Conceptualization, Supervision, Resources, Funding acquisition, Writing – review & editing.
Declaration of Competing Interest
The authors declare that they have no known competing financial interests or personal relationships that could have appeared to influence the work reported in this paper.
Contributor Information
Junyi Li, Email: lijunyi@hit.edu.cn.
Yang Zhang, Email: zhangyang07@hit.edu.cn.
References
- 1.Dyer O. African malaria deaths set to dwarf covid-19 fatalities as pandemic hits control efforts, WHO warns. BMJ. 2020 doi: 10.1136/bmj.m4711. [DOI] [PubMed] [Google Scholar]
- 2.Yaeger R.G. Medical Microbiology. 4th edition. University of Texas Medical Branch at Galveston; Galveston TX: 1996. Protozoa: structure, classification, growth, and development. [PubMed] [Google Scholar]
- 3.World malaria report 2021. World Health Organization; 2021. [Google Scholar]
- 4.Jung T., et al. Automatic detection of Trypanosomosis in thick blood smears using image pre-processing and deep learning. Intelligent Human Computer Interaction. 2020 [Google Scholar]
- 5.Sáez-Alquezar A., et al. Geographical origin of chronic Chagas disease patients in Brazil impacts the performance of commercial tests for anti-T. cruzi IgG. Memórias do Instituto Oswaldo Cruz. 2021;116 [Google Scholar]
- 6.Wormser G.P., P, et al. The Clinical Assessment, Treatment, and Prevention of Lyme Disease, Human Granulocytic Anaplasmosis, and Babesiosis: Clinical Practice Guidelines by the Infectious Diseases Society of America. Clinical infectious diseases : an official publication of the Infectious Diseases Society of America. 2006;43:1089–1134. doi: 10.1086/508667. [DOI] [PubMed] [Google Scholar]
- 7.Renard I., et al. Treatment of Human Babesiosis: Then and Now. Pathogens. 2021;10(9):1120. doi: 10.3390/pathogens10091120. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Wohlfert E.A., et al. Brains and Brawn: Toxoplasma Infections of the Central Nervous System and Skeletal Muscle. Trends in parasitology. 2017;33:519–531. doi: 10.1016/j.pt.2017.04.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Fisch D., et al. Human immunity to Toxoplasma gondii. PLoS pathogens. 2019;15(12) doi: 10.1371/journal.ppat.1008097. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Attias M.a, et al. The life-cycle of Toxoplasma gondii reviewed using animations. Parasites & Vectors. 2020;13(1):1–13. doi: 10.1186/s13071-020-04445-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Tomiotto-Pellissier Fernanda, et al. Macrophage Polarization in Leishmaniasis: broadening horizons. Frontiers in immunology. 2018 doi: 10.3389/fimmu.2018.02529. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Honigberg, B. M. "Trichomonads parasitic in humans." (1991): 405.
- 13.Mielczarek E., et al. Trichomonas vaginalis: pathogenicity and potential role in human reproductive failure. Infection. 2016;44(4):447–458. doi: 10.1007/s15010-015-0860-0. [DOI] [PubMed] [Google Scholar]
- 14.Weatherhead J.E. Neglected Tropical Diseases-North America. Springer International Publishing; Cham: 2021. Neglected Tropical Diseases-North America; pp. 131–155. [Google Scholar]
- 15.Eisele T.P. Mass drug administration can be a valuable addition to the malaria elimination toolbox. Malaria journal. 2019 doi: 10.1186/s12936-019-2906-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Berzosa P., et al. Comparison of three diagnostic methods (microscopy, RDT, and PCR) for the detection of malaria parasites in representative samples from Equatorial Guinea. Malaria journal. 2018;17(1):1–12. doi: 10.1186/s12936-018-2481-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Dahal P., et al. Challenges in laboratory diagnosis of Malaria in a low resource country among cases of acute febrile illness at tertiary care hospital in eastern Nepal: Comparative study on Conventional Vs Molecular approach. Journal of Tropical Medicine. 2021 doi: 10.1155/2021/3811318. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Lebel P., et al. Label-free imaging and classification of live P. falciparum enables high performance parasitemia quantification without fixation or staining. PLoS computational biology. 2021;17(8) doi: 10.1371/journal.pcbi.1009257. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Li S., et al. Transfer learning for toxoplasma gondii recognition. Msystems. 2020;5(1):e00445–19. doi: 10.1128/mSystems.00445-19. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Tangpukdee N., et al. Malaria diagnosis: a brief review. The Korean journal of parasitology. 2009;47:93. doi: 10.3347/kjp.2009.47.2.93. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Hung J., et al. Applying Faster R-CNN for Object Detection on Malaria Images. Proceedings of the IEEE conference on computer vision and pattern recognition workshops. 2017 doi: 10.1109/cvprw.2017.112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Picot S., et al. Systematic review and meta-analysis of diagnostic accuracy of loop-mediated isothermal amplification (LAMP) methods compared with microscopy, polymerase chain reaction and rapid diagnostic tests for malaria diagnosis. International Journal of Infectious Diseases. 2020;98:408–419. doi: 10.1016/j.ijid.2020.07.009. [DOI] [PubMed] [Google Scholar]
- 23.Lee J.-G., et al. Deep learning in medical imaging: general overview. Korean journal of radiology. 2017;18(4):570–584. doi: 10.3348/kjr.2017.18.4.570. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Prevedello L.M., et al. Challenges related to artificial intelligence research in medical imaging and the importance of image analysis competitions. Radiology: Artificial Intelligence. 2019;1(1) doi: 10.1148/ryai.2019180031. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Wang S., et al. Artificial intelligence in lung cancer pathology image analysis. Cancers. 2019;11(11):1673. doi: 10.3390/cancers11111673. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Wu N., et al. Deep neural networks improve radiologists’ performance in breast cancer screening. IEEE transactions on medical imaging. 2019;39(4):1184–1194. doi: 10.1109/TMI.2019.2945514. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Lassau N., et al. Integrating deep learning CT-scan model, biological and clinical variables to predict severity of COVID-19 patients. Nature communications. 2021;(12.1 (2021):):1–11. doi: 10.1038/s41467-020-20657-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Srinidhi C.L., et al. Deep neural network models for computational histopathology: A survey. Med Image Analysis. 2021;67 doi: 10.1016/j.media.2020.101813. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Zhang Y., et al. Deep Learning for Imaging and Detection of Microorganisms. Trends in Microbiology. 2021;29:569–572. doi: 10.1016/j.tim.2021.01.006. [DOI] [PubMed] [Google Scholar]
- 30.Li S., et al. Multi-stage malaria parasite recognition by deep learning. GigaScience. 2021;10(6):giab040. doi: 10.1093/gigascience/giab040. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Quinn J.A., et al. Automated blood smear analysis for mobile malaria diagnosis. Mobile Point-of-Care Monitors and Diagnostic Device. Design. 2014 [Google Scholar]
- 32.Jiang H., et al. Geometry-aware cell detection with deep learning. Msystems. 2020;5(1):e00840–19. doi: 10.1128/mSystems.00840-19. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Umer M., et al. A novel stacked CNN for malarial parasite detection in thin blood smear images. IEEE Access. 2020;8:93782–93792. [Google Scholar]
- 34.Li S., et al. Parasitologist-level classification of apicomplexan parasites and host cell with deep cycle transfer learning (DCTL) Bioinformatics. 2020;36:4498–4505. doi: 10.1093/bioinformatics/btaa513. [DOI] [PubMed] [Google Scholar]
- 35.Poostchi M., et al. Image analysis and machine learning for detecting malaria. Translational Research. 2018:36–55. doi: 10.1016/j.trsl.2017.12.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Górriz M., et al. Leishmaniasis Parasite Segmentation and Classification Using Deep Learning. International Conference on Articulated Motion and Deformable Objects. 2018 [Google Scholar]
- 37.Zhang Y., et al. Motility-based label-free detection of parasites in bodily fluids using holographic speckle analysis and deep learning. Light: Science & Applications. 2018;7(1):1–18. doi: 10.1038/s41377-018-0110-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Abdurahman F., et al. Malaria parasite detection in thick blood smear microscopic images using modified YOLOV3 and YOLOV4 models. BMC bioinformatics. 2021;22(1):1–17. doi: 10.1186/s12859-021-04036-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Dang N., et al. International Conference on Innovative Computing and Communications. Springer; Singapore: 2020. Malaria Detection on Giemsa-Stained Blood Smears Using Deep Learning and Feature Extraction; pp. 789–803. [Google Scholar]
- 40.Sanchez-Patino N., et al. Convolutional Neural Networks for Chagas’ Parasite Detection in Histopathological Images. 2021 43rd Annual International Conference of the IEEE Engineering in Medicine & Biology Society (EMBC) 2021 doi: 10.1109/EMBC46164.2021.9629563. [DOI] [PubMed] [Google Scholar]
- 41.Manescu P., et al. A Weakly Supervised Deep Learning Approach for Detecting Malaria and Sickle Cells in Blood Films. Medical Image Computing and Computer-Assisted Intervention. 2020 [Google Scholar]
- 42.Holz L.E., et al. Protective immunity to liver‐stage malaria. Clinical & translational immunology. 2016;5(10) doi: 10.1038/cti.2016.60. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Kassim Y.M., et al. Clustering-Based Dual Deep Learning Architecture for Detecting Red Blood Cells in Malaria Diagnostic Smears. ieee journal of biomedical and health informatics. 2020;25:1735–1746. doi: 10.1109/JBHI.2020.3034863. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Quan Q., et al. An Effective Convolutional Neural Network for Classifying Red Blood Cells in Malaria Diseases. Interdisciplinary Sciences Computational Life Sciences. 2020;12:217–225. doi: 10.1007/s12539-020-00367-7. [DOI] [PubMed] [Google Scholar]
- 45.Yang F., et al. Deep Learning for Smartphone-Based Malaria Parasite Detection in Thick Blood Smears. IEEE journal of biomedical and health informatics. 2020;24:1427–1438. doi: 10.1109/JBHI.2019.2939121. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Yang F., et al. International workshop on machine learning in medical imaging. Springer; Cham: 2019. Smartphone-supported malaria diagnosis based on deep learning; pp. 73–80. [Google Scholar]
- 47.Manuel L., et al. Human toxoplasmosis in Mozambique: gaps in knowledge and research opportunities. Parasites Vectors. 2020 doi: 10.1186/s13071-020-04441-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Luo S., et al. Deep learning‐enabled imaging flow cytometry for high‐speed Cryptosporidium and Giardia detection. Cytometry Part A. 2021;99(11):1123–1133. doi: 10.1002/cyto.a.24321. [DOI] [PubMed] [Google Scholar]
- 49.Durant T., JS, et al. Applications of Digital Microscopy and Densely Connected Convolutional Neural Networks for Automated Quantification of Babesia-Infected Erythrocytes. Clinical chemistry. 2022;68(1):218–229. doi: 10.1093/clinchem/hvab237. [DOI] [PubMed] [Google Scholar]
- 50.Loddo A., et al. Sipaim–Miccai Biomedical Workshop. Springer; Cham: 2018. MP-IDB: the malaria parasite image database for image processing and analysis; pp. 57–65. [Google Scholar]
- 51.Rajaraman S., et al. Pre-trained convolutional neural networks as feature extractors toward improved malaria parasite detection in thin blood smear images. PeerJ. 2018;6:e4568. doi: 10.7717/peerj.4568. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.El Hachimi C., et al. International Conference on Advanced Intelligent Systems for Sustainable Development. Springer; Cham: 2019. Medical Use of Deep Learning: Malaria Testing Using Pre-trained ResNet; pp. 273–280. [Google Scholar]
- 53.Rajaraman S., et al. Performance evaluation of deep neural ensembles toward malaria parasite detection in thin-blood smear images. PeerJ. 2019;7:e6977. doi: 10.7717/peerj.6977. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.De Pablos L.M., et al. Developmental differentiation in Leishmania lifecycle progression: post-transcriptional control conducts the orchestra. Current opinion in microbiology. 2016;34:82–89. doi: 10.1016/j.mib.2016.08.004. [DOI] [PubMed] [Google Scholar]
- 55.Arsuaga M., et al. Misdiagnosis of babesiosis as malaria, Equatorial Guinea. Emerg Infect Disease. 2018 doi: 10.3201/eid2408.180180. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Ta-Tang, Thuy-Huong, et al. "Comparison of Three PCR-Based Methods to Detect Loa loa and Mansonella perstans in Long-Term Frozen Storage Dried Blood Spots." (2020). [DOI] [PubMed]
- 57.Holmström O., et al. A novel deep learning-based point-of-care diagnostic method for detecting Plasmodium falciparum with fluorescence digital microscopy. Plos one. 2020;15(11) doi: 10.1371/journal.pone.0242355. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Sanchez-Patiño N., et al. Convolutional Neural Networks for Chagas’ Parasite Detection in Histopathological Images. 2021 43rd Annual International Conference of the IEEE Engineering in Medicine & Biology Society (EMBC) 2021:2732–2735. doi: 10.1109/EMBC46164.2021.9629563. [DOI] [PubMed] [Google Scholar]
- 59.Thomas S., M., et al. Interpretable deep learning systems for multi-class segmentation and classification of non-melanoma skin cancer. Medical Image Analysis. 2021;68:101915. doi: 10.1016/j.media.2020.101915. [DOI] [PubMed] [Google Scholar]
- 60.Yao, Quanming, et al. "Taking human out of learning applications: A survey on automated machine learning." arXiv preprint arXiv:1810.13306 (2018).
- 61.Quinn J.A., et al. Automated blood smear analysis for mobile malaria diagnosis. Mobile Point-of-Care Monitors and Diagnostic Device Design. 2014;31:115. [Google Scholar]
- 62.Quinn J., A.,, et al. Deep convolutional neural networks for microscopy-based point of care diagnostics. Machine Learning for Healthcare Conference. 2016 [Google Scholar]
- 63.Ljosa, Vebjorn, et al. "Annotated high-throughput microscopy image sets for validation." Nature methods 9.7 (2012): 637–637. [DOI] [PMC free article] [PubMed]
- 64.Zare M., et al. A machine learning-based system for detecting leishmaniasis in microscopic images. BMC Infectious Diseases. 2022;22(1):1–6. doi: 10.1186/s12879-022-07029-7. [DOI] [PMC free article] [PubMed] [Google Scholar]