Abstract
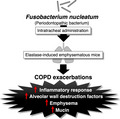
Exacerbation of chronic obstructive pulmonary disease (COPD) is associated with disease progression and increased mortality. Periodontal disease is a risk factor for exacerbation of COPD, but little is known about the role of periodontopathic bacteria in this process. Here, we investigated the effects of intratracheal administration of Fusobacterium nucleatum, a periodontopathic bacteria species, on COPD exacerbation in elastase‐induced emphysematous mice. The administration of F. nucleatum to elastase‐treated mice enhanced inflammatory responses, production of alveolar wall destruction factors, progression of emphysema, and recruitment of mucin, all of which are symptoms observed in patients with COPD exacerbation. Hence, we propose that F. nucleatum may play a role in exacerbation of COPD.
Keywords: chronic obstructive pulmonary disease, COPD exacerbations, emphysema, Fusobacterium nucleatum, periodontal disease
Periodontal disease is a risk factor for chronic obstructive pulmonary disease (COPD) exacerbations. We found that intratracheally administered Fusobacterium nucleatum, a periodontopathic bacterium that increases in saliva when periodontal disease advances, to elastase‐induced emphysematous mice enhances (i) inflammatory responses, (ii) alveolar wall destruction factor production, (iii) emphysema progression, and (iv) mucin hypersecretion. These are known symptoms observed in patients with COPD exacerbations.
Abbreviations
- AB/PAS
Alcian blue/Periodic acid‐Schiff
- BALF
bronchoalveolar lavage fluid
- BHI
brain‐heart infusion
- CFU
colony‐forming units
- COPD
chronic obstructive pulmonary disease
- Lm
mean linear intercept
- MMP
matrix metalloproteinase
- MUC5AC
mucin 5AC
- PBS
phosphate‐buffered saline
Chronic obstructive pulmonary disease (COPD), the third leading cause of mortality worldwide, is characterized by emphysema and limitation of the airflow, symptoms that are likely result from chronic inflammation in the lung periphery [1, 2]. COPD is also characterized by progression, which occurs by the repetition of COPD exacerbations [3]. Repeated exacerbations of COPD, which is frequently caused by bacterial or viral infection [4], are associated with worsened lung function and increased risk of death [3]. COPD exacerbation leads to increased infiltration of inflammatory cells [5, 6] and production of proinflammatory cytokines [7, 8] and proteases, such as matrix metalloproteinases (MMPs) [9], in the lungs of patients. Repeated COPD exacerbations accelerate the progression of emphysema [10] and induce hypersecretion of mucus [11]. Therefore, effective prevention of COPD exacerbations has important implications for clinical management and public health.
Periodontal disease, one of the most prevalent diseases worldwide, is an inflammatory reaction induced by infection by bacteria, such as Porphyromonas gingivalis and Fusobacterium nucleatum. It results in the destruction of the periodontium, including the periodontal bone, leading to tooth loss [12, 13]. Recently, several studies have found that periodontal disease is a risk factor for several systemic diseases, such as pneumonia, diabetes, and atherosclerosis [12, 14]. Epidemiological evidence indicates that periodontal disease is also associated with exacerbations of COPD [15, 16]. A systematic review indicated that periodontal treatment in patients with COPD and periodontal disease is associated with reduction in the frequency of COPD exacerbation and a slower rate of decline of lung function [17]. The European Federation of Periodontology and the American Academy of Periodontology have proposed that aspiration of dental plaque and/or hematogenous dissemination of inflammatory mediators from periodontal pockets underlie the worsening of COPD inflammatory status [18]. However, the details of the relationship between periodontal disease and COPD are not fully understood.
Fusobacterium nucleatum, a Gram‐negative anaerobe, is abundant in the oral cavity and has been implicated in various forms of periodontal disease, including a mild reversible form of gingivitis and advanced irreversible forms of periodontitis, including chronic periodontitis [19]. Recent reports have indicated that F. nucleatum is associated with several systemic diseases, including respiratory diseases such as pneumonia [19] and COPD [20], colorectal cancer [21], and gastric cancer [22]. In patients with severe COPD, the number of members of the genus Fusobacterium was found to be increased in bronchoalveolar lavage fluid (BALF) [23] and sputum [24]. Previously, we demonstrated that heat‐killed F. nucleatum strongly induces proinflammatory cytokines in primary respiratory epithelial cells and respiratory epithelial cell lines [25, 26]. Therefore, it is quite possible that tracheal aspiration of F. nucleatum plays a role in COPD exacerbations. Despite its importance, the relationship between oral bacteria, including F. nucleatum, and COPD exacerbation remains unexamined, and it is not clear whether this relationship is causal. In this study, we investigated the effects of intratracheal administration of heat‐killed F. nucleatum on the exacerbation of COPD in elastase‐induced emphysematous mice. Because F. nucleatum, an anaerobic bacterium, is considered to be incapable of survival in the respiratory tract, we used heat‐killed F. nucleatum. We found that administration of F. nucleatum to elastase‐treated mice enhanced the inflammatory responses, production of alveolar wall destruction factors, recruitment of mucin, and progression of emphysema.
Materials and methods
Preparation of bacteria
Fusobacterium nucleatum ATCC 25586 was cultured in brain–heart infusion (BHI) broth supplemented with 5 µg·mL−1 hemin and 0.5 µg·mL−1 menadione. The bacterial cell culture was incubated at 37 °C for 24–72 h in an anaerobic chamber (TE‐HERANAEROBOX, Hirasawa, Tokyo, Japan) under aerobic conditions of 10% CO2, 10% H2, and 80% N2. The bacterial cells were centrifuged at 7000 g for 10 min at 4 °C, and the pellet was resuspended in phosphate‐buffered saline (PBS). The bacterial cell density was adjusted to 1.0 × 1010 colony‐forming units (CFU)·mL−1. The bacterial suspension was heat‐killed at 60 °C for 1 h and then stored at −80 °C until use.
Mice
Eight‐week‐old male C57BL/6J mice were purchased from CLEA Japan (Tokyo, Japan). All mice had free access to food and water and were kept in temperature‐controlled room (23 °C) on reverse 12/12 h light/dark cycle. All experimental protocols and procedures were performed in compliance with the Nihon University Rules concerning Animal Care and Use. All animal experiments were approved by the Nihon University Animal Care and Use Committee (AP18DEN031). The mice were euthanized by CO2 asphyxiation before harvesting lung specimens or BALF.
Elastase‐induced emphysema mouse model
Mice under isoflurane anesthesia were intratracheally injected with 5 U of porcine pancreatic elastase (Elastin Products, Owensville, MO, USA). PBS‐treated age‐matched mice were used as controls. Three weeks after elastase or PBS injection, the mice under isoflurane anesthesia were intratracheally administered either 1 × 108 CFU of F. nucleatum suspended in 50 µL of PBS or PBS alone every day for 7 days.
Morphometric measurements of air space size
To measure the air space size, lung specimens were harvested early (1, 3, and 7 days) and late (42 days) after the last F. nucleatum administration and were fixed with 4% paraformaldehyde, embedded in paraffin, cut out as thin sections with 4 µm thickness, and stained with hematoxylin and eosin [27]. The linear intercepts of 100 alveoli were measured, and the mean linear intercept (Lm) was used as a morphometric parameter of emphysema [27, 28].
Collection and analysis of bronchoalveolar lavage fluid
At 1, 3, and 7 days after the last F. nucleatum administration, whole lungs were washed three times with 1 mL of PBS, and BALF was collected from each mouse. The number of cells in the BALF was determined using a hemocytometer. Cell differentials were counted on smears prepared using a Cytospin and stained with Giemsa stain (Muto Pure Chemicals, Tokyo, Japan). The number of cells in each inflammatory cell fraction found in each BALF was estimated by multiplying the total number of cells that had been counted with a hemocytometer by the ratio of each cell fraction per 100 cells in the smear.
Real‐time quantitative PCR
Total RNA was isolated from whole lungs using RNeasy Plus Mini Kits (Qiagen, Hiden, Germany) according to the manufacturer’s instructions. Synthesis of cDNA from total RNA was performed using PrimeScript RT Master Mix (Takara Bio, Shiga, Japan). The following primers were used in this study: Mmp12, forward 5′‐TGGTATTCAAGGAGATGCACATTT‐3′ and reverse 5′‐ GGTTTGTGCCTTGAAAACTTTTAGT‐3′; Tnf, forward 5′‐CTGTGCTCAGAGCTTTCAACAACTA‐3′ and reverse 5′‐TCCTTGATGGTGGTGCATGA‐3′; Il6, forward 5′‐GAGGATACCACTCCCAACAGACC‐3′ and reverse 5′‐AAGTGCATCATCGTTGTTCATACA‐3′; Cxcl1, forward 5′‐TGTGGGAGGCTGTGTTTGTA‐3′ and reverse 5’‐ACGAGACCAGGAGAAACAGG‐3′; Cxcl5, forward 5′‐GGTCCACAGTGCCCTACG‐3′ and reverse 5′‐GCGAGTGCATTCCGCTTA‐3′; Ccl2, forward 5′‐TTAAAAACCTGGATCGGAACCAA‐3′ and reverse 5′‐GCATTAGCTTCAGATTTACGGGT‐3′; Cxcl10, forward 5′‐ACCCAAGTGCTGCCGTCATT‐3′ and reverse 5′‐ATTCTCACTGGCCCGTCATC‐3′; Muc5ac, forward 5′‐CATGGAGGGGACCTGGAAAC‐3′ and reverse 5’‐ CCACACTGGGGTCACACTTC‐3′; Actb, forward 5′‐GGTCAGAAGGACTCCTATGTGG‐3′ and reverse 5′‐TGTCGTCCCAGTTGGTAACA‐3′. The amplification and detection of the cDNA was accomplished using a TP‐800 Thermal Cycler Dice Real‐Time System (Takara Bio) with TB Green Premix Ex Taq (Takara Bio).
Western Blotting
Lung tissue extracts and BALF supernatants were prepared, separated using SDS/PAGE, and transferred to PVDF membranes. The membranes were blocked with 2% BSA and incubated with primary antibodies overnight at 4 °C. The primary antibodies included anti‐MMP12 (1 : 1000; Proteintech, Rosemont, IL, USA), anti‐perforin (1 : 1000; Cell Signaling Technology, Danvers, MA, USA), and anti‐β‐actin antibodies (1 : 200; Santa Cruz Biotechnology, Santa Cruz, CA, USA). The membranes were washed with Tris‐buffered saline containing Tween 20 and incubated with secondary antibody for 1 h at room temperature. After washing, the membranes were treated with ECL prime detection reagent (Cytiva, Tokyo, Japan). The bands were visualized using a ChemiDoc XRS System (Bio‐Rad, Hercules, CA, USA).
Histological analysis
Lung specimens were harvested 7 days after the last administration of F. nucleatum, fixed with 4% paraformaldehyde, embedded in paraffin, sectioned, and mounted onto glass slides. To assess the mucin production and goblet cell counts, the slides were stained with Alcian blue/periodic acid‐Schiff (AB/PAS). Goblet cells were counted as described previously [29]. Briefly, two hundred cells were counted, and the number of goblet cells was divided by the total number of cells to calculate the percentage of goblet cells. One slide per animal, for seven animals per group, was assessed. For immunohistochemical analysis, the slides were incubated with anti‐mucin 5AC (MUC5AC) antibody (1 : 50; Abcam Plc, Cambridge, UK) overnight. The samples were then incubated with a secondary antibody for 30 min. The immunolabeling was visualized using DAB substrate, and the staining reaction was observed with a light microscope.
Statistical analysis
All data are expressed as mean ± SEM. The data were analyzed using one‐way ANOVA with Tukey’s post hoc analysis using kaleidagraph (Synergy Software, Reading, PA, USA). Differences were considered significant at P < 0.05.
Results
Recruitment of inflammatory cells into the bronchoalveolar space
To evaluate whether intratracheal administration of F. nucleatum caused a significant increase in the number of inflammatory cells in the elastase‐treated mice, we analyzed the number of inflammatory cells in the BALF from all groups one, three, and seven days after the final administration of F. nucleatum. At days 1 and 3, the total cell number, number of macrophages, and number of lymphocytes in the elastase‐treated mice dosed with F. nucleatum was significantly higher than in the control mice (Fig. 1A–C). One day after the administration, the accumulation of neutrophils in the BALF was significantly higher in the elastase‐treated mice given F. nucleatum than in the other mice (Fig. 1D). Seven days after administration, most of the inflammatory cells in the BALF had disappeared. These results suggest that the administration of F. nucleatum to the elastase‐treated mice produced augmented inflammatory responses, similar to those seen in patients with COPD exacerbations [5].
Fig. 1.
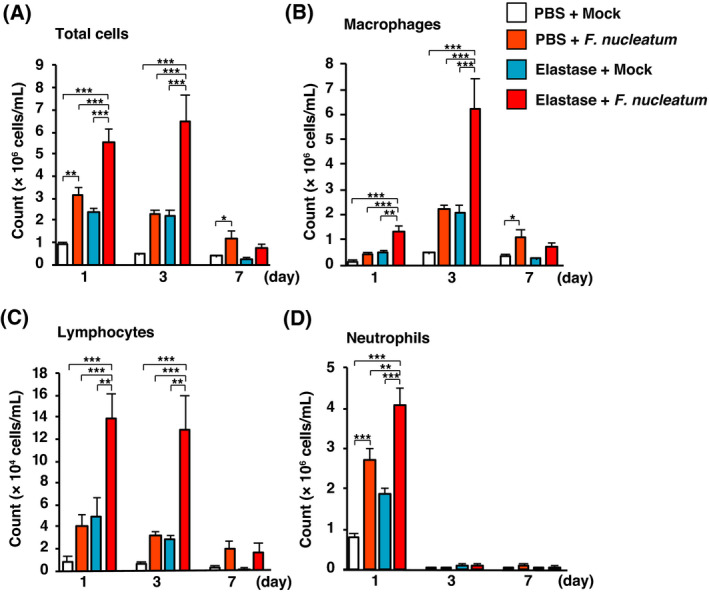
Elastase‐treated mice administered F. nucleatum showed altered BALF inflammatory cell profiles. BAL was performed on elastase‐ and PBS‐treated mice administered F. nucleatum or the vehicle (Mock). The numbers of total cells (A), macrophages (B), lymphocytes (C), and neutrophils (D) are shown for one, three, and seven days after the administration of F. nucleatum. The values are presented as the mean ± SEM, n = 7. *P < 0.05; **P < 0.01; ***P < 0.001. Statistical significance was evaluated using one‐way ANOVA with Tukey’s post hoc analysis (A–D).
Administration of F. nucleatum enhances cytokine mRNA levels in elastase‐treated mice
We investigated whether the intratracheal administration of F. nucleatum affected the mRNA expression of proinflammatory cytokines and chemokines, including Tnf, Il6, Cxcl1, Cxcl5, Ccl2, and Cxcl10, in the lungs of elastase‐treated mice. The level of Tnf mRNA was significantly higher in lungs from elastase‐treated mice given F. nucleatum than in the other groups one and seven days after the last administration of F. nucleatum (Fig. 2A). The levels of Il6, Cxcl1, Cxcl5, Ccl2, and Cxcl10 mRNA increased following F. nucleatum administration to both elastase‐ and PBS‐treated mice (Fig. 2B–F). These results suggest that F. nucleatum induced inflammatory responses in the mouse lungs.
Fig. 2.
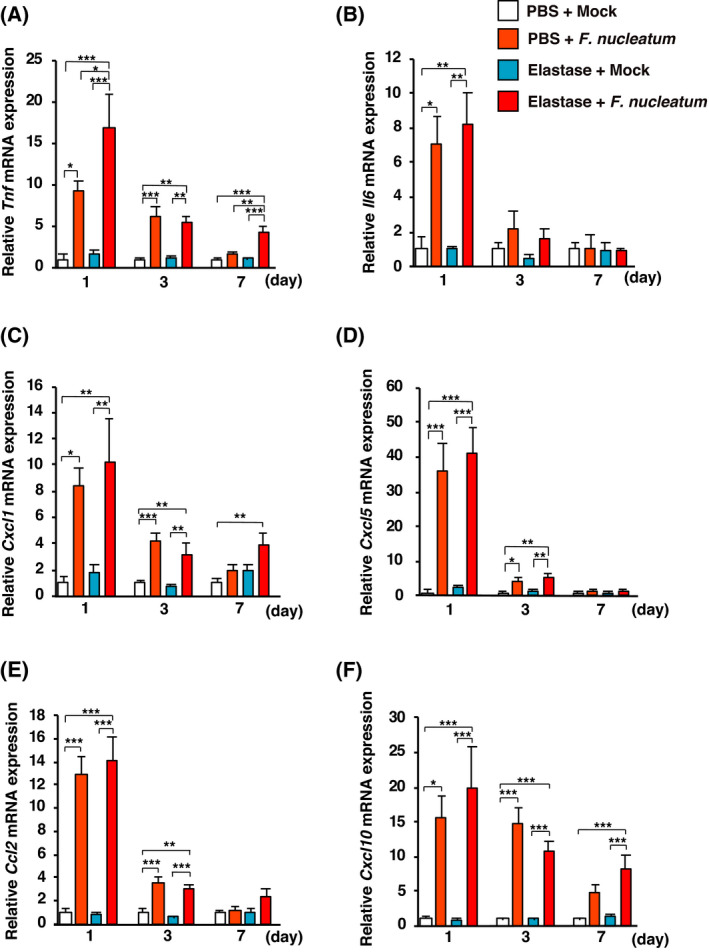
Effects of F. nucleatum on the expression of proinflammatory cytokine genes in lungs of elastase‐treated mice. Elastase‐ and PBS‐treated mice were administered F. nucleatum or the vehicle (Mock). The lungs were harvested at 1, 3, or 7 days after the administration of F. nucleatum. (A) Tnf, (B) Il6, (C) Cxcl1, (D) Cxcl5, (E) Ccl2, and (F) Cxcl10 mRNA expression in the lungs was determined using real‐time RT‐PCR. The values are presented as the mean ± SEM, n = 7. *P < 0.05; **P < 0.01; ***P < 0.001. Statistical significance was evaluated using one‐way ANOVA with Tukey’s post hoc analysis (A–F).
F. nucleatum administration enhanced MMP12 and perforin in elastase‐treated mice
MMP12 degrades the alveolar wall matrix [30] and perforin could induce apoptosis of the alveolar wall [31]. We therefore examined whether intratracheal administration of F. nucleatum affected the expression of MMP12 and perforin in lungs from elastase‐treated mice. Mmp12 mRNA expression was elevated 1, 3, and 7 days after the last F. nucleatum administration in elastase‐treated mice (Fig. 3A). The levels of MMP12 protein were also increased in the lungs of elastase‐ and PBS‐treated mice administered F. nucleatum (Fig. 3B). We evaluated the secretion of perforin in BALF supernatant 3 days after the administration of F. nucleatum. The secretion of perforin was increased by the administration of F. nucleatum to both elastase‐ and PBS‐treated mice (Fig. 3C). These data suggest that F. nucleatum can induce the expression of factors that contribute to alveolar wall destruction.
Fig. 3.
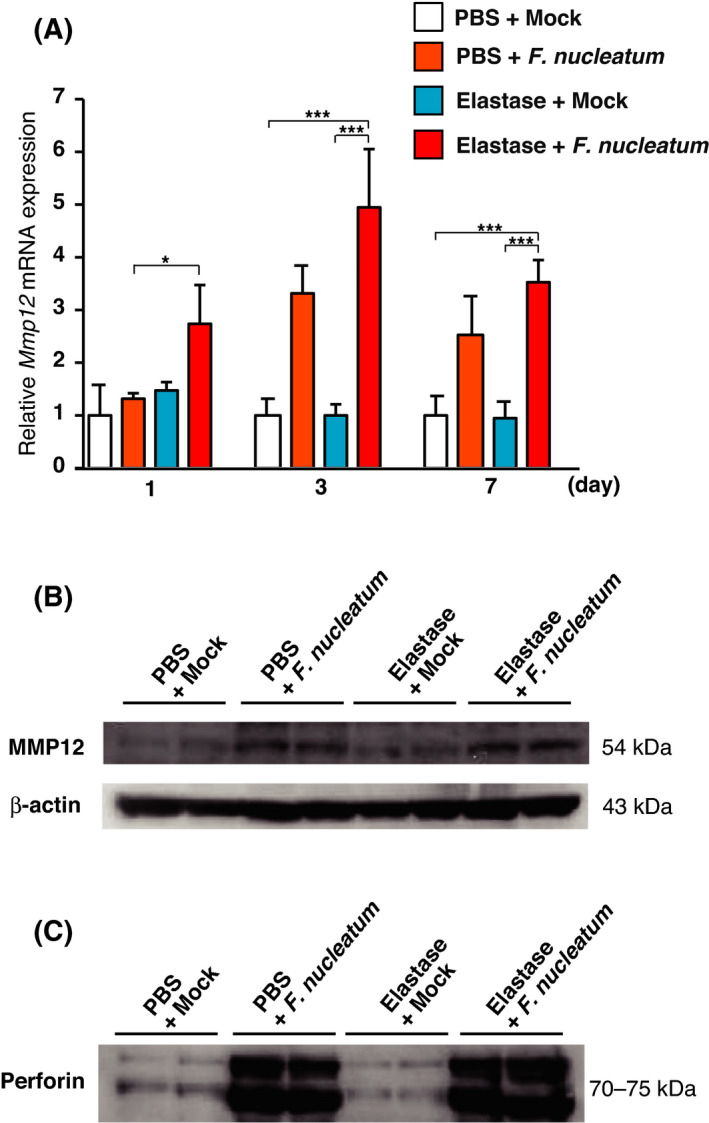
Effects of F. nucleatum on the production of MMP12 and perforin in the lungs of elastase‐treated mice. Elastase‐ and PBS‐treated mice were administered either F. nucleatum or the vehicle (Mock). (A) The lungs were harvested at one, three, or seven days after the administration of F. nucleatum. Mmp12 mRNA expression in the lungs was determined using real‐time RT‐PCR. The values are presented as the mean ± SEM, n = 7. *P < 0.05; ***P < 0.001. Statistical significance was evaluated using one‐way ANOVA with Tukey’s post hoc analysis. (B) The lungs were harvested three days after the administration of F. nucleatum. MMP12 protein expression was detected using western blotting of lung lysates. (C) BALF was harvested three days after exposure to F. nucleatum. Perforin protein expression was detected using western blotting of BALF.
Progression of emphysema was accelerated by F. nucleatum in elastase‐treated mice
To investigate whether F. nucleatum contributes to the progression of emphysema in elastase‐treated mice, we assessed the Lm, the mean of the inner diameter of the pulmonary alveoli, and lung specimens 1, 3, 7, and 42 days after the last intratracheal administration of F. nucleatum (Fig. 4A). Noticeably, early (1, 3, and 7 days) and late (42 days) harvested lung specimens putatively have a similar pattern of F. nucleatum enhanced elastase‐induced alveolar enlargement. Figure 4B shows micrographs of representative lung sections taken from 42 days after the last intratracheal administration of F. nucleatum or Mock. We observed obvious enlargement of alveoli in elastase‐treated mice administered F. nucleatum. These results indicate that stimulation originated from F. nucleatum accelerated the progression of emphysema in the elastase‐treated mice, as is seen in patients with repeated COPD exacerbations [10].
Fig. 4.
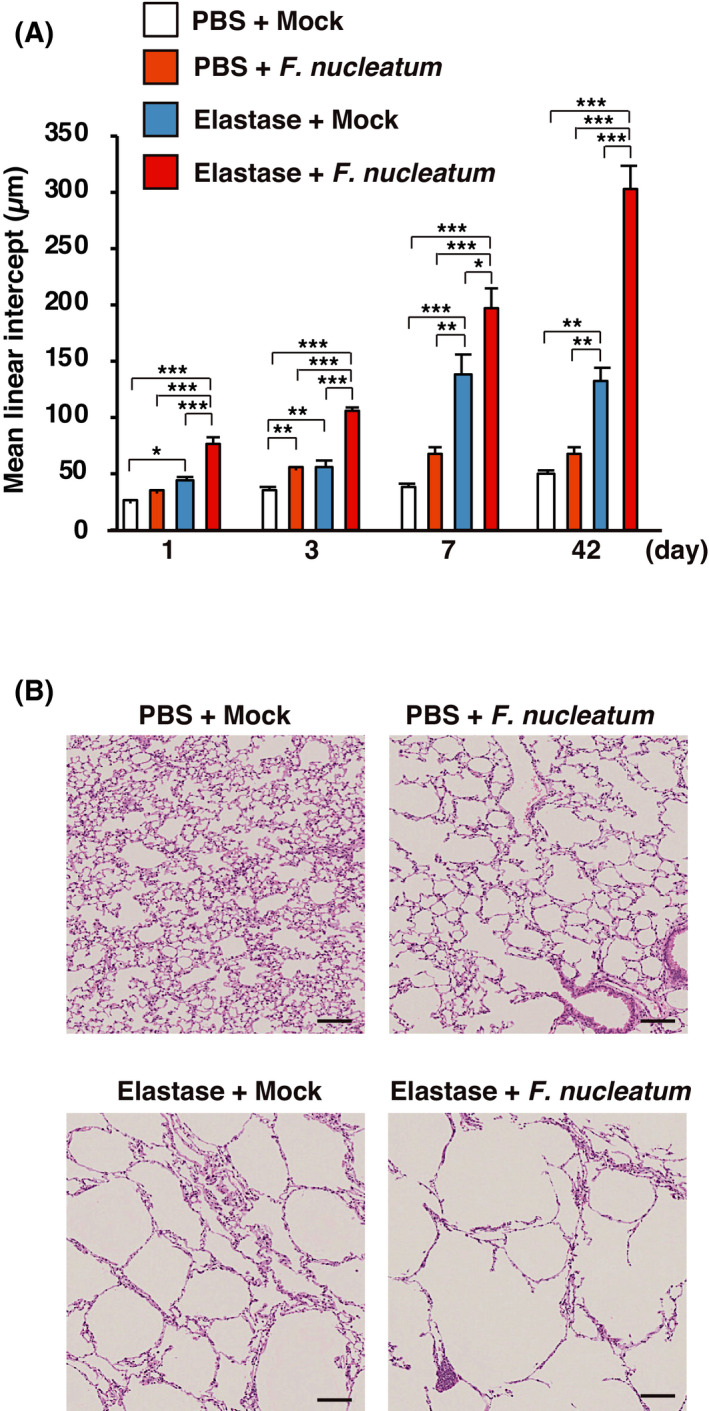
F. nucleatum enhanced elastase‐induced alveolar enlargement in elastase‐treated mice. Lung morphology was determined in elastase‐ and PBS‐treated mice at days 1, 3, 7, and 42 after administration of F. nucleatum or the vehicle (Mock). (A) Mean linear intercept (Lm) was measured on random sections of the lung (n = 7). The data are shown as means ± SEM. *P < 0.05; **P < 0.01; ***P < 0.001. Statistical significance was evaluated using one‐way ANOVA with Tukey’s post hoc analysis. (B) Representative lung sections at 42 days after F. nucleatum or the vehicle administration stained with hematoxylin and eosin. Scale bar = 100 µm.
Administration of F. nucleatum enhanced mucin expression in elastase‐treated mice
Goblet cell metaplasia contributes to the hypersecretion of mucus in the airway, which has been associated with accelerated decline in lung function and progression of COPD [11, 32]. Therefore, we examined whether the intratracheal administration of F. nucleatum affects goblet cell metaplasia and the expression of MUC5AC, the major respiratory mucin [33], in the lungs of elastase‐treated mice. As shown in Fig. 5A, goblet cell metaplasia was observed in mice administered F. nucleatum. In particular, the quantification of AB/PAS staining confirmed that the number of goblet cells was significantly increased in elastase‐treated mice administered F. nucleatum (Fig. 5B). We found that the expression of Muc5ac mRNA was increased in the lungs of elastase‐treated mice administered F. nucleatum compared with the other groups (Fig. 5C). As shown using immunohistochemical staining for MUC5AC (Fig 5D), the MUC5AC protein accumulated along the epithelial cells of the airway of elastase‐treated mice administered F. nucleatum compared with PBS‐treated mice administered F. nucleatum. These results imply that F. nucleatum was associated with the mucus hypersecretion through goblet cell metaplasia and expression of MUC5AC.
Fig. 5.
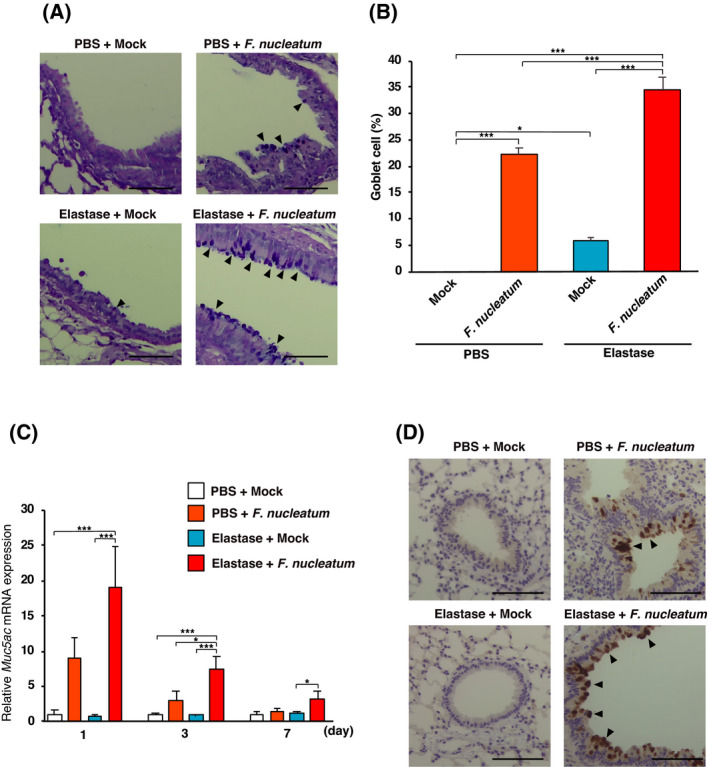
Effects of F. nucleatum on goblet cell metaplasia and MUC5AC expression in lungs of elastase‐treated mice. Elastase‐ and PBS‐treated mice were administered either F. nucleatum or the vehicle (Mock). The lungs were harvested at 1, 2, or 7 days after the administration of F. nucleatum. (A) AB/PAS‐stained bronchi from elastase‐ and PBS‐treated mice seven days after the administration of either F. nucleatum or vehicle (Mock). Scale bar = 100 µm. (B) Percentage of goblet cells seven days after the administration of F. nucleatum. The values are presented as the mean ± SEM, n = 7. *P < 0.05; ***P < 0.001. (C) Muc5ac mRNA expression in the lungs determined using real‐time RT‐PCR. The values are presented as the mean ± SEM, n = 7. **P < 0.01; ***P < 0.001. (D) Immunohistochemistry for MUC5AC on bronchi from elastase‐ and PBS‐treated mice 7 days after the administration of F. nucleatum or Mock. Scale bar = 100 µm. Statistical significance was evaluated using one‐way ANOVA with Tukey’s post hoc analysis (B and C).
Discussion
Periodontal disease has been shown to exacerbate pulmonary disease, including COPD [16, 34, 35]. Various mechanisms that produce this effect have been proposed, including the aspiration of oral bacteria [36]. COPD and periodontal disease share a similar mechanism of tissue destruction, characterized by inflammatory responses and release of proteolytic enzymes, resulting in the destruction of the pulmonary alveoli or the periodontal tissues [37, 38]. Periodontal pathogens, including F. nucleatum, are probably associated with the exacerbation of COPD [19, 20, 39]. However, little is known about how periodontal pathogens affect COPD exacerbations in vivo. In this study, we attempted to elucidate the effect of F. nucleatum on mice with elastase‐induced emphysema. The intratracheal administration of F. nucleatum to the lungs of elastase‐treated mice exacerbated inflammatory responses, leading to increased numbers of inflammatory cells and increased levels of proinflammatory cytokines. The administration of F. nucleatum enhanced the production of factors that contribute to alveolar wall destruction and accelerated the progression of emphysema. F. nucleatum also induced the hypersecretion of mucus in the airways. These observations suggest that F. nucleatum plays a role in the exacerbation of COPD.
One and three days after the administration of F. nucleatum, the number of total cells in the BALF was significantly higher in elastase‐treated mice administered F. nucleatum than in any of the other treatment groups. Elastase‐treated mice experienced a strong recruitment of neutrophils, macrophages, and lymphocytes to the lung in response to F. nucleatum. The severity of COPD is related to the degree to which the lung tissue is infiltrated by inflammatory cells such as neutrophils, macrophages, and lymphocytes [40]. Neutrophils have the capacity to induce tissue damage through the release of serine proteases such as elastase [41, 42]. Alveolar macrophages from COPD patients release more inflammatory mediators, such as TNF‐α and IL‐6, than macrophages from control subjects, and these inflammatory mediators play an important role in the pathophysiology of COPD [42]. There is an increase in the number of T lymphocytes in the lung parenchyma and airways of patients with COPD [41, 42] and increased release of perforin from CD8+ T lymphocytes [42]. These observations, together with our findings, suggest that F. nucleatum‐induced recruitment of inflammatory cells plays an essential role in the exacerbation of COPD.
Cytokines and chemokines play critical roles in many pathobiological processes in COPD, including the infiltration of inflammatory cells into lung tissue, exacerbation, and emphysema [7, 43, 44]. Inflammatory cytokines induced by pneumonia‐related bacteria, such as Haemophilus influenzae and Streptococcus pneumoniae, are important in COPD exacerbation [4, 42, 43], and TNF‐α and IL‐6 levels are increased in sputum during COPD exacerbations [7, 44]. The current study indicated that the administration of F. nucleatum induced the expression of the Tnf and Il6 genes in elastase‐treated mouse lungs. Our previous studies demonstrated that F. nucleatum is a potent stimulator of TNF‐α and IL‐6 in human respiratory epithelial cells [25, 26, 45]. In the current study, although the expression of chemokine genes in the lungs of elastase‐treated mice administered F. nucleatum was not significantly different from that in the lungs of PBS‐treated mice administered F. nucleatum, F. nucleatum caused a significant increase in the number of inflammatory cells in the BALF, which were enhanced in the lungs of elastase‐treated mice. This finding suggests that elastase‐treated lungs lead to a more active cellular entry compared with PBS‐treated lungs. Hence, it appears that the F. nucleatum‐induced recruitment of cytokines and chemokines plays an important role in the exacerbation of COPD.
Emphysema, destruction of the alveolar wall, is a major pathological feature of the lungs of individuals with COPD [3]. Emphysema progression is accelerated by exacerbations in patients with COPD [10]. In the current study, the emphysema in elastase‐treated mice administered with F. nucleatum progressed to a greater extent than in mice treated with F. nucleatum alone or those pretreated with elastase alone. Moreover and quite surprisingly, the progression significantly occurred as early as day 1 which suggests that if continuous aspiration is occurring among COPD patients with periodontal disease, COPD can be exacerbated quite easily in these patients due to aspiration of saliva which has high F. nucleatum levels. Administration of F. nucleatum induced the expression of the alveolar wall destruction factors MMP12 and perforin in mice. MMP12, which is secreted by macrophages, and perforin, which is secreted by CD8+ T lymphocytes, are believed to promote the degradation of the alveolar wall, contributing to the development of emphysematous lesions [30, 31, 46]. Our observations, together with previous findings, suggest that F. nucleatum has the potential to increase the progression of emphysema by inducing the release of MMP12 and perforin in patients with COPD.
Mucus hypersecretion in patients with COPD has been associated with compromising lung function, contributing to the severity of the disease, and with increased mortality [47, 48]. The administration of F. nucleatum increased goblet cell metaplasia and MUC5AC expression, which were more strongly enhanced in elastase‐treated mice. MUC5AC is partially induced by TNF‐α in the epithelial cells of the airway [11, 49]. The expression of Tnf was significantly higher in elastase‐treated mice administered F. nucleatum than in PBS‐treated mice administered F. nucleatum. TNF‐α expression may therefore contribute to F. nucleatum‐induced MUC5AC expression. Supernatants from F. nucleatum cultures promote the upregulation of MUC5AC expression in human airway epithelial cell lines [50]. Our previous study showed that MUC5AC expression was induced by P. gingivalis gingipain in human airway epithelial cell lines and primary cells [51]. These observations, together with our findings, suggest that major periodontal pathogens are potent inducers of the expression of mucin in patients with COPD.
The involvement of periodontal disease in COPD has been suggested by the results of many recent studies. Poor periodontal health is associated with COPD occurrence [15, 52], COPD exacerbation [16, 53]. Periodontal treatment in COPD patients with chronic periodontitis reduces the frequency of COPD exacerbations [54, 55]. The levels of antibody against F. nucleatum are markedly elevated in the sputum of patients with COPD exacerbations [20], and the number of bacteria of the genus Fusobacterium is higher in the sputum of patients with severe COPD [24]. In patients with COPD, an abnormal swallowing reflex is associated with the frequency of COPD exacerbation [56, 57]. The salivary concentration of F. nucleatum is increased in patients with periodontal diseases [58]. Consequently, aspiration of the saliva of periodontal disease, including F. nucleatum, may play a role in the induction of COPD exacerbations. It is therefore tempting to speculate that healthy periodontal conditions and good oral hygiene may prevent or reduce COPD exacerbations.
The major limitation of our study was that we used only heat‐killed whole bacterial cells for the investigation of the effects of F. nucleatum on elastase‐treated mice. Our findings suggest that because infection was not necessarily required for the exacerbations, these are caused by a virulence factor from F. nucleatum. F. nucleatum possesses virulence factors including adhesins, LPS, serine protease, and butyrate [19, 59]. In particular, fusobacterial adhesins, such as FadA, Fap2, and RadD, facilitate adhesion and invasion to host cells, and the triggering of host immune responses [19, 59]. These findings combined with our observations may provide a new insight for COPD as a non‐infectious disease which can be exacerbated by stimulation of F. nucleatum aspirated into the lower respiratory tracts. In addition, the amount of F. nucleatum that we used in the present study should be estimated to be adequate when referring to the facts that F. nucleatum is present in saliva at 8 × 104–3 × 107 CFU·mL−1 in individuals with healthy periodontium and that the number of F. nucleatum in saliva of patients with periodontal disease is 100–1000 times higher than that of those with healthy periodontium [58, 60]. Although we were able to link F. nucleatum with COPD exacerbations, further studies that focus on F. nucleatum virulence factors are needed to identify the details of the mechanism of F. nucleatum‐induced COPD exacerbation.
Periodontal disease and COPD are spreading worldwide. COPD is associated with the development of a range of diseases, such as ischemic heart disease, diabetes, osteoporosis, and lung cancer [3, 61]. Although periodontal disease has recently been implicated as a risk factor for COPD exacerbations, the causal relationship remains poorly understood. This study provides evidence suggesting that F. nucleatum, as one of the key periodontopathic bacteria that are aspirated into the lung, could cause exacerbation of COPD. Periodontal treatment is effective in reducing the frequency of COPD exacerbation in patients with COPD and periodontal disease [17], and it is therefore considered that aspirated periodontopathic bacteria may be a risk factor for COPD exacerbation.
Conflict of interest
The authors declare no conflict of interest.
Author contributions
RS and NK designed the experiments, analyzed the data, and wrote the paper. TK and YY contributed to the discussion, analyzed data, and reviewed drafts of the manuscript. KI was responsible for the study concept and design, analysis of results and manuscript writing. All authors have read and agreed to the published version of the manuscript.
Acknowledgements
This work was supported by JSPS KAKENHI Grant Number JP21K10265, Sato Fund of Nihon University School of Dentistry, Uemura Fund of Nihon University School of Dentistry, Grant from Dental Research Center, Nihon University School of Dentistry, and Nihon University Multidisciplinary Research Grant for 2021–2022.
Edited by Guangbiao Zhou
Contributor Information
Noriaki Kamio, Email: kamio.noriaki@nihon-u.ac.jp.
Kenichi Imai, Email: imai.kenichi@nihon-u.ac.jp.
Data accessibility
The data that support the findings of this study are available from the corresponding author upon reasonable request.
References
- 1. Celli BR, Wedzicha JA. Update on clinical aspects of chronic obstructive pulmonary disease. N Engl J Med. 2019;381:1257–66. [DOI] [PubMed] [Google Scholar]
- 2. Hogg JC. Pathophysiology of airflow limitation in chronic obstructive pulmonary disease. Lancet. 2004;364:709–21. [DOI] [PubMed] [Google Scholar]
- 3. Decramer M, Janssens W, Miravitlles M. Chronic obstructive pulmonary disease. Lancet. 2012;379:1341–51. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Sethi S, Murphy TF. Infection in the pathogenesis and course of chronic obstructive pulmonary disease. N Engl J Med. 2008;359:2355–65. [DOI] [PubMed] [Google Scholar]
- 5. Fujimoto K, Yasuo M, Urushibata K, Hanaoka M, Koizumi T, Kubo K. Airway inflammation during stable and acutely exacerbated chronic obstructive pulmonary disease. Eur Respir J. 2005;25:640–6. [DOI] [PubMed] [Google Scholar]
- 6. Papi A, Bellettato CM, Braccioni F, Romagnoli M, Casolari P, Caramori G, et al. Infections and airway inflammation in chronic obstructive pulmonary disease severe exacerbations. Am J Respir Crit Care Med. 2006;173:1114–21. [DOI] [PubMed] [Google Scholar]
- 7. Caramori G, Adcock IM, Di Stefano A, Chung KF. Cytokine inhibition in the treatment of COPD. Int J Chron Obstruct Pulmon Dis. 2014;9:397–412. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Henrot P, Prevel R, Berger P, Dupin I. Chemokines in COPD: from implication to therapeutic use. Int J Mol Sci. 2019;20;2785. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Ilumets H, Rytila PH, Sovijarvi AR, Tervahartiala T, Myllarniemi M, Sorsa TA, et al. Transient elevation of neutrophil proteinases in induced sputum during COPD exacerbation. Scand J Clin Lab Invest. 2008;68:618–23. [DOI] [PubMed] [Google Scholar]
- 10. Tanabe N, Muro S, Hirai T, Oguma T, Terada K, Marumo S, et al. Impact of exacerbations on emphysema progression in chronic obstructive pulmonary disease. Am J Respir Crit Care Med. 2011;183:1653–9. [DOI] [PubMed] [Google Scholar]
- 11. Li J, Ye Z. The potential role and regulatory mechanisms of MUC5AC in chronic obstructive pulmonary disease. Molecules. 2020;25:4437. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Pihlstrom BL, Michalowicz BS, Johnson NW. Periodontal diseases. Lancet. 2005;366:1809–20. [DOI] [PubMed] [Google Scholar]
- 13. Hajishengallis G. Periodontitis: from microbial immune subversion to systemic inflammation. Nat Rev Immunol. 2015;15:30–44. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Takahashi Y, Watanabe N, Kamio N, Kobayashi R, Iinuma T, Imai K. Aspiration of periodontopathic bacteria due to poor oral hygiene potentially contributes to the aggravation of COVID‐19. J Oral Sci. 2020;63:1–3. [DOI] [PubMed] [Google Scholar]
- 15. Zeng XT, Tu ML, Liu DY, Zheng D, Zhang J, Leng W. Periodontal disease and risk of chronic obstructive pulmonary disease: a meta‐analysis of observational studies. PLoS One. 2012;7:e46508. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Liu Z, Zhang W, Zhang J, Zhou X, Zhang L, Song Y, et al. Oral hygiene, periodontal health and chronic obstructive pulmonary disease exacerbations. J Clin Periodontol. 2012;39:45–52. [DOI] [PubMed] [Google Scholar]
- 17. Apessos I, Voulgaris A, Agrafiotis M, Andreadis D, Steiropoulos P. Effect of periodontal therapy on COPD outcomes: a systematic review. BMC Pulm Med. 2021;21:92. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Linden GJ, Herzberg MC, Working Group 4 of the Joint EFP/AAP Workshop . Periodontitis and systemic diseases: a record of discussions of working group 4 of the Joint EFP/AAP Workshop on Periodontitis and Systemic Diseases. J Clin Periodontol. 2013;40(Suppl 14):S20–3. [DOI] [PubMed] [Google Scholar]
- 19. Han YW. Fusobacterium nucleatum: a commensal‐turned pathogen. Curr Opin Microbiol. 2015;23:141–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Brook I, Frazier EH. Immune response to Fusobacterium nucleatum and Prevotella intermedia in the sputum of patients with acute exacerbation of chronic bronchitis. Chest. 2003;124:832–3. [DOI] [PubMed] [Google Scholar]
- 21. Yachida S, Mizutani S, Shiroma H, Shiba S, Nakajima T, Sakamoto T, et al. Metagenomic and metabolomic analyses reveal distinct stage‐specific phenotypes of the gut microbiota in colorectal cancer. Nat Med. 2019;25:968–76. [DOI] [PubMed] [Google Scholar]
- 22. Erawijantari PP, Mizutani S, Shiroma H, Shiba S, Nakajima T, Sakamoto T, et al. Influence of gastrectomy for gastric cancer treatment on faecal microbiome and metabolome profiles. Gut. 2020;69:1404–15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Pragman AA, Kim HB, Reilly CS, Wendt C, Isaacson RE. The lung microbiome in moderate and severe chronic obstructive pulmonary disease. PLoS One. 2012;7:e47305. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Lee SW, Kuan CS, Wu LS, Weng JT. Metagenome and metatranscriptome profiling of moderate and severe COPD sputum in Taiwanese Han Males. PLoS One. 2016;11:e0159066. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Koike R, Cueno ME, Nodomi K, Tamura M, Kamio N, Tanaka H, et al. Heat‐killed Fusobacterium nucleatum triggers varying heme‐related inflammatory and stress responses depending on primary human respiratory epithelial cell type. Molecules. 2020;25:3839. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Hayata M, Watanabe N, Tamura M, Kamio N, Tanaka H, Nodomi K, et al. The periodontopathic bacterium Fusobacterium nucleatum induced proinflammatory cytokine production by human respiratory epithelial cell lines and in the lower respiratory organs in mice. Cell Physiol Biochem. 2019;53:49–61. [DOI] [PubMed] [Google Scholar]
- 27. Takahashi S, Ishii M, Namkoong H, Hegab AE, Asami T, Yagi K, et al. Pneumococcal infection aggravates elastase‐induced emphysema via matrix metalloproteinase 12 overexpression. J Infect Dis. 2016;213:1018–30. [DOI] [PubMed] [Google Scholar]
- 28. Takahashi S, Nakamura H, Seki M, Shiraishi Y, Yamamoto M, Furuuchi M, et al. Reversal of elastase‐induced pulmonary emphysema and promotion of alveolar epithelial cell proliferation by simvastatin in mice. Am J Physiol Lung Cell Mol Physiol. 2008;294:L882–90. [DOI] [PubMed] [Google Scholar]
- 29. Ridzuan N, Zakaria N, Widera D, Sheard J, Morimoto M, Kiyokawa H, et al. Human umbilical cord mesenchymal stem cell‐derived extracellular vesicles ameliorate airway inflammation in a rat model of chronic obstructive pulmonary disease (COPD). Stem Cell Res Ther. 2021;12:54. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Churg A, Zhou S, Wright JL. Series "matrix metalloproteinases in lung health and disease": matrix metalloproteinases in COPD. Eur Respir J. 2012;39:197–209. [DOI] [PubMed] [Google Scholar]
- 31. Plataki M, Tzortzaki E, Rytila P, Demosthenes M, Koutsopoulos A, Siafakas NM. Apoptotic mechanisms in the pathogenesis of COPD. Int J Chron Obstruct Pulmon Dis. 2006;1:161–71. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Chung KF, Adcock IM. Multifaceted mechanisms in COPD: inflammation, immunity, and tissue repair and destruction. Eur Respir J. 2008;31:1334–56. [DOI] [PubMed] [Google Scholar]
- 33. Boucher RC. Muco‐obstructive lung diseases. N Engl J Med. 2019;380:1941–53. [DOI] [PubMed] [Google Scholar]
- 34. Peter KP, Mute BR, Doiphode SS, Bardapurkar SJ, Borkar MS, Raje DV. Association between periodontal disease and chronic obstructive pulmonary disease: a reality or just a dogma? J Periodontol. 2013;84:1717–23. [DOI] [PubMed] [Google Scholar]
- 35. Takeuchi K, Matsumoto K, Furuta M, Fukuyama S, Takeshita T, Ogata H, et al. Periodontal status and lung function decline in the community: the Hisayama study. Sci Rep. 2018;8:13354. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36. Linden GJ, Herzberg MC, Working Group 4 of the Joint EFP/AAP Workshop . Periodontitis and systemic diseases: a record of discussions of working group 4 of the Joint EFP/AAP Workshop on Periodontitis and Systemic Diseases. J Periodontol. 2013;84:S20–3. [DOI] [PubMed] [Google Scholar]
- 37. Mojon P. Oral health and respiratory infection. J can Dent Assoc. 2002;68:340–5. [PubMed] [Google Scholar]
- 38. Kelly N, El Karim I. Periodontitis may be associated with respiratory diseases such as asthma, COPD, and pneumonia. J Evid Based Dent Pract. 2020;20:101498. [DOI] [PubMed] [Google Scholar]
- 39. Scannapieco FA. Role of oral bacteria in respiratory infection. J Periodontol. 1999;70:793–802. [DOI] [PubMed] [Google Scholar]
- 40. Agusti A, Hogg JC. Update on the pathogenesis of chronic obstructive pulmonary disease. N Engl J Med. 2019;381:1248–56. [DOI] [PubMed] [Google Scholar]
- 41. Barnes PJ, Shapiro SD, Pauwels RA. Chronic obstructive pulmonary disease: molecular and cellular mechanisms. Eur Respir J. 2003;22:672–88. [DOI] [PubMed] [Google Scholar]
- 42. Barnes PJ. Cellular and molecular mechanisms of asthma and COPD. Clin Sci (Lond). 2017;131:1541–58. [DOI] [PubMed] [Google Scholar]
- 43. Caramori G, Casolari P, Barczyk A, Durham AL, Di Stefano A, Adcock I. COPD immunopathology. Semin Immunopathol. 2016;38:497–515. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44. Barnes PJ. The cytokine network in asthma and chronic obstructive pulmonary disease. J Clin Invest. 2008;118:3546–56. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45. Takahashi Y, Watanabe N, Kamio N, Yokoe S, Suzuki R, Sato S, et al. Expression of the SARS‐CoV‐2 receptor ACE2 and proinflammatory cytokines induced by the periodontopathic bacterium Fusobacterium nucleatum in human respiratory epithelial cells. Int J Mol Sci. 2021;22:1352. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46. Chrysofakis G, Tzanakis N, Kyriakoy D, Tsoumakidou M, Tsiligianni I, Klimathianaki M, et al. Perforin expression and cytotoxic activity of sputum CD8+ lymphocytes in patients with COPD. Chest. 2004;125:71–6. [DOI] [PubMed] [Google Scholar]
- 47. Kesimer M, Ford AA, Ceppe A, Radicioni G, Cao R, Davis CW, et al. Airway mucin concentration as a marker of chronic bronchitis. N Engl J Med. 2017;377:911–22. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48. Prescott E, Lange P, Vestbo J. Chronic mucus hypersecretion in COPD and death from pulmonary infection. Eur Respir J. 1995;8:1333–8. [DOI] [PubMed] [Google Scholar]
- 49. Lee SU, Lee S, Ro H, Choi JH, Ryu HW, Kim MO, et al. Piscroside C inhibits TNF‐alpha/NF‐kappaB pathway by the suppression of PKCdelta activity for TNF‐RSC formation in human airway epithelial cells. Phytomedicine. 2018;40:148–57. [DOI] [PubMed] [Google Scholar]
- 50. Nagaoka K, Yanagihara K, Harada Y, Yamada K, Migiyama Y, Morinaga Y, et al. Macrolides inhibit Fusobacterium nucleatum‐induced MUC5AC production in human airway epithelial cells. Antimicrob Agents Chemother. 2013;57:1844–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51. Miya C, Cueno ME, Suzuki R, Maruoka S, Gon Y, Kaneko T, et al. Porphyromonas gingivalis gingipains potentially affect MUC5AC gene expression and protein levels in respiratory epithelial cells. FEBS Open Bio. 2021;11:446–55. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52. Takeuchi K, Matsumoto K, Furuta M, Fukuyama S, Takeshita T, Ogata H, et al. Periodontitis is associated with chronic obstructive pulmonary disease. J Dent Res. 2019;98:534–40. [DOI] [PubMed] [Google Scholar]
- 53. Kelly N, Winning L, Irwin C, Lundy FT, Linden D, McGarvey L, et al. Periodontal status and chronic obstructive pulmonary disease (COPD) exacerbations: a systematic review. BMC Oral Health. 2021;21:425. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54. Kucukcoskun M, Baser U, Oztekin G, Kiyan E, Yalcin F. Initial periodontal treatment for prevention of chronic obstructive pulmonary disease exacerbations. J Periodontol. 2013;84:863–70. [DOI] [PubMed] [Google Scholar]
- 55. Zhou X, Han J, Liu Z, Song Y, Wang Z, Sun Z. Effects of periodontal treatment on lung function and exacerbation frequency in patients with chronic obstructive pulmonary disease and chronic periodontitis: a 2‐year pilot randomized controlled trial. J Clin Periodontol. 2014;41:564–72. [DOI] [PubMed] [Google Scholar]
- 56. Kobayashi S, Kubo H, Yanai M. Impairment of the swallowing reflex in exacerbations of COPD. Thorax. 2007;62:1017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57. Terada K, Muro S, Ohara T, Kudo M, Ogawa E, Hoshino Y, et al. Abnormal swallowing reflex and COPD exacerbations. Chest. 2010;137:326–32. [DOI] [PubMed] [Google Scholar]
- 58. Saygun I, Nizam N, Keskiner I, Bal V, Kubar A, Acikel C, et al. Salivary infectious agents and periodontal disease status. J Periodontal Res. 2011;46:235–9. [DOI] [PubMed] [Google Scholar]
- 59. de Andrade KQ, Almeida‐da‐Silva CLC, Coutinho‐Silva R. Immunological pathways triggered by Porphyromonas gingivalis and Fusobacterium nucleatum: therapeutic possibilities? Mediators Inflamm. 2019;2019:7241312. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60. Zhou X, Liu X, Li J, Aprecio RM, Zhang W, Li Y. Real‐time PCR quantification of six periodontal pathogens in saliva samples from healthy young adults. Clin Oral Investig. 2015;19:937–46. [DOI] [PubMed] [Google Scholar]
- 61. Smith MC, Wrobel JP. Epidemiology and clinical impact of major comorbidities in patients with COPD. Int J Chron Obstruct Pulmon Dis. 2014;9:871–88. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
The data that support the findings of this study are available from the corresponding author upon reasonable request.


