This systematic review and meta-analysis evaluates the use of bloodborne brain injury biomarkers for neurologic prognostication in patients with hypoxic ischemic brain injury after cardiac arrest.
Key Points
Question
What is the neuroprognostic utility of brain injury biomarkers in patients with a hypoxic ischemic brain injury following cardiac arrest?
Findings
In this systematic review and meta-analysis of more than 10 000 patients, summary receiver operating curve characteristic analyses indicated that neurofilament light had a higher area under the curve compared with neuron-specific enolase, serum calcium binding protein 100β, glial fibrillary acidic protein, tau, or ubiquitin carboxyl hydrolase L1.
Meaning
In this study, neurofilament light, which reflects white matter damage, had the highest accuracy to prognosticate unfavorable neurologic outcome in patients with a hypoxic ischemic brain injury following cardiac arrest.
Abstract
Importance
Brain injury biomarkers released into circulation from the injured neurovascular unit are important prognostic tools in patients with cardiac arrest who develop hypoxic ischemic brain injury (HIBI) after return of spontaneous circulation (ROSC).
Objective
To assess the neuroprognostic utility of bloodborne brain injury biomarkers in patients with cardiac arrest with HIBI.
Data Sources
Studies in electronic databases from inception to September 15, 2021. These databases included MEDLINE, Embase, Evidence-Based Medicine Reviews, CINAHL, Cochrane Database of Systematic Reviews, and the World Health Organization Global Health Library.
Study Selection
Articles included in this systmatic review and meta-analysis were independently assessed by 2 reviewers. We included studies that investigated neuron-specific enolase, S100 calcium-binding protein β, glial fibrillary acidic protein, neurofilament light, tau, or ubiquitin carboxyl hydrolase L1 in patients with cardiac arrest aged 18 years and older for neurologic prognostication. We excluded studies that did not (1) dichotomize neurologic outcome as favorable vs unfavorable, (2) specify the timing of blood sampling or outcome determination, or (3) report diagnostic test accuracy or biomarker concentration.
Data Extraction and Synthesis
Data on the study design, inclusion and exclusion criteria, brain biomarkers levels, diagnostic test accuracy, and neurologic outcome were recorded. This study was conducted in accordance with the Preferred Reporting Items for Systematic Reviews and Meta-Analyses (PRISMA) guideline.
Main Outcomes and Measures
Summary receiver operating characteristic curve analysis was used to calculate the area under the curve, sensitivity, specificity, and optimal thresholds for each biomarker. Risk of bias and concerns of applicability were assessed with the Quality Assessment of Diagnostic Accuracy Studies (QUADAS-2) tool.
Results
We identified 2953 studies, of which 86 studies with 10 567 patients (7777 men [73.6] and 2790 women [26.4]; pooled mean [SD] age, 62.8 [10.2] years) were included. Biomarker analysis at 48 hours after ROSC demonstrated that neurofilament light had the highest predictive value for unfavorable neurologic outcome, with an area under the curve of 0.92 (95% CI, 0.84-0.97). Subgroup analyses of patients treated with targeted temperature management and those who specifically had an out-of-hospital cardiac arrest showed similar results (targeted temperature management, 0.92 [95% CI, 0.86-0.95] and out-of-hospital cardiac arrest, 0.93 [95% CI, 0.86-0.97]).
Conclusions and Relevance
Neurofilament light, which reflects white matter damage and axonal injury, yielded the highest accuracy in predicting neurologic outcome in patients with HIBI at 48 hours after ROSC.
Trial Registration
PROSPERO Identifier: CRD42020157366
Introduction
Hypoxic ischemic brain injury (HIBI) is the primary determinant of outcome following cardiac arrest.1,2 Survivors of HIBI can experience a wide range of clinical outcomes along a spectrum encompassing full neurologic recovery, persistent vegetative states, or severe or mild disabilities,3,4,5,6 thereby making accurate prognostication imperative to inform health care teams and caregivers. Considerable efforts have been directed toward identifying accurate neurologic prognostication for patients with HIBI.7 Guidelines recommend using multiple modalities including clinical examination, electroencephalography, somatosensory-evoked potentials, and neuroimaging.8,9,10
Although these modalities have established roles, they require expert interpretation and resources.11 The advent of brain injury biomarkers provides clinicians with a generalizable prognostic tool,12 and biomarkers have demonstrated utility in HIBI prognostication.12 As the primary anatomical and functional interface between the vasculature, neurons, and glia, the neurovascular unit maintains neuronal homestasis.13 Brain injury biomarkers of neurovascular unit injury include neuron-specific enolase (NSE; neuron cell body), S100 calcium-binding protein β (S100β; glia), glial fibrillary acidic protein (GFAP; astrocytes), neurofilament light (Nf-L; axons), tau (axons), and ubiquitin carboxyl hydrolase L1 (UCH-L1; neuron cell body).12,14,15,16 Among these, NSE has a lengthy history of use in neurologic prognostication15; however, the remaining biomarkers have garnered substantial interest. We conducted a systematic review and meta-analysis investigating the use of brain injury biomarkers for neurologic prognostication of HIBI after cardiac arrest.
Methods
The protocol was registered and conducted in accordance with the Preferred Reporting Items for Systematic Reviews and Meta-analyses (PRISMA) reporting guideline17 and the Diagnostic Test Accuracy extension.18 Detailed methods are included in eAppendix 1 in the Supplement and are briefly described here.
We searched electronic databases from inception to September 15, 2021. Our search included the following terms: “biomarker,” “neuron specific enolase,” “NSE,” “S100 beta,” “S100 calcium binding protein,” “glial fibrillary protein,” “GFAP,” “neurofilament-light,” “Nf-L,” “tau,” “ubiquitin carboxyl hydrolase L1” and “UCH-L1,” “cardiac arrest,” “post cardiac arrest,” “hypoxic ischemic brain injury,” “anoxic brain injury,” “ROSC [return of sponanteous circulation],” and “return of spontaneous circulation.” eAppendix 1 and eAppendix 2 in the Supplement provide additional details.
Inclusion and Exclusion Criteria
We included studies that measured at least 1 of NSE, S100β, GFAP, Nf-L, tau, or UCH-L1 in blood to prognosticate neurologic outcome in patients with HIBI (aged ≥18 years) following cardiac arrest. We did not include studies investigating the diagnostic accuracy of biomarker concentrations in cerebrospinal fluid because cerebrospinal fluid diversion is not widely conducted in post–cardiac arrest management. Literature on other prognostic techniques, such as electroencephalography, somatosensory-evoked potentials, and neuroimaging, is available for the interested reader.19,20,21,22 Unfavorable vs favorable neurologic outcome scales included the Cerebral Performance Category, Glasgow Outcome Scale, modified Glasgow Outcome Scale, modified Rankin Scale, or survival (survivors vs nonsurvivors; eTable 5 in the Supplement includes a list of studies that used survival for outcome assessment). The neurologic outcome grading could be applied at intensive care unit discharge or thereafter. Both observational and interventional studies were included. We excluded studies in which patients had a neurologic cause of cardiac arrest, concomitant traumatic brain injury, intracranial hemorrhage, stroke, or pregnancy. Studies not in English were translated and included. Following our survey of the literature, we modified our inclusion criteria from that which was prespecified in our PROSPERO registration (eAppendix 1 in the Supplement). Additional exclusion criteria, the study selection process, and data extraction methods are detailed in eAppendix 1 in the Supplement. Study quality was assessed by 2 reviewers (R.L.H. and K.J.K.R.) using the Quality Assessment of Diagnostic Accuracy Studies (QUADAS-2) tool.23 Data on race and ethnicity were not collected because they were not consistently reported in the included studies.
Statistical Analysis
Data pertaining to diagnostic test accuracy, such as sample size, outcome prevalence, threshold value used, sensitivity, specificity, area under the curve (AUC), and the number of true-positive, true-negative, false-positive, and false-negative results, were extracted from each available study at 24, 48, and 72 hours following cardiac arrest. The number of true-positive, true-negative, false-positive, and false-negative results were either extracted directly from included manuscripts or calculated from the provided sample size, outcome prevalence, sensitivity, and specificity at each threshold and time. Most included studies did not have a prespecified threshold and instead provided several thresholds to ascertain a particular specificity value (eg, 100% specificity), and consequently, there were no common thresholds. Lack of a common threshold precluded the ability to construct traditional bivariate models for overall sensitivity and specificity. Instead, we used a 2-stage random-effects model (including a linear mixed-effects model) to allow for the integration of multiple thresholds within each study and for each individual biomarker (diagmeta package in R statistical software [R Foundation for Statistical Computing]).24,25 Using this model, we were able to calculate a summary receiver operating characteristic (SROC) curve for each biomarker at 48 hours following cardiac arrest as our primary outcome. We chose to use 48 hours because neurologic prognostication typically occurs at least 24 hours after cardiac arrest, with different time recommendations for patients treated with targeted temperature management (TTM) vs those kept normothermic.26 Additionally, using the SROC curves, we estimated optimal thresholds for each biomarker for particular weights of specificity. Neurologic prognostication following cardiac arrest places a greater value on test specificity. As such, when ascertaining optimal thresholds via the Youden index, we weighted specificity at 75%, 80%, and 85%, with sensitivity weighted at 25%, 20%, and 15%, respectively. For each weight of specificity, an associated optimal threshold on the SROC curve was ascertained. As a prespecified subgroup analysis, we calculated SROC curves and respective optimal thresholds for each biomarker using data from patients who received TTM as well as specifically in patients with an out-of-hospital cardiac arrest (OHCA). Moreover, we also conducted a subanalysis excluding studies that assessed outcome at less than 3 months after cardiac arrest. Heterogeneity was assessed by clinical evaluation of each included study and by visual inspection of individual study ROC plots.25
Median concentration and spread (IQR or range) and/or mean (SD) for each biomarker at 0 hours, 24 hours, 48 hours, and 72 hours were extracted from available studies. We performed a meta-analysis of median concentration for each biomarker at each of the selected times using the quantile estimation method (metamedian package in R statistical software).27 We synthesized and presented median concentration owing to the highly skewed distribution of included brain biomarkers. Heterogeneity was assessed by clinical evaluation of included studies and via the I2 statistic, although traditional I2 thresholds are not applicable. In addition, we summarized overall study characteristics stratified by the biomarker assessed. We pooled mean age using a weighted fixed-effects model (meta package in R statistical software). Data that were duplicated across more than 1 publication were not duplicated within our analysis (eAppendix 3 in the Supplement). In studies that reported age as median (IQR), the mean (SD) was estimated using appropriate and validated methods.28,29 The number of studies and patients included in each statistical test and comparison is included in each figure. The variable number of studies and/or patients for each comparison is a reflection of differences in data collection or reporting between studies (eg, some studies collected biomarkers at only 1 point, while some studies reported ROC curve data but not group summary statistics such as median [IQR] for biomarker levels). All analyses were performed using R statistical software, version 4.0.4 (R Core Team).
Results
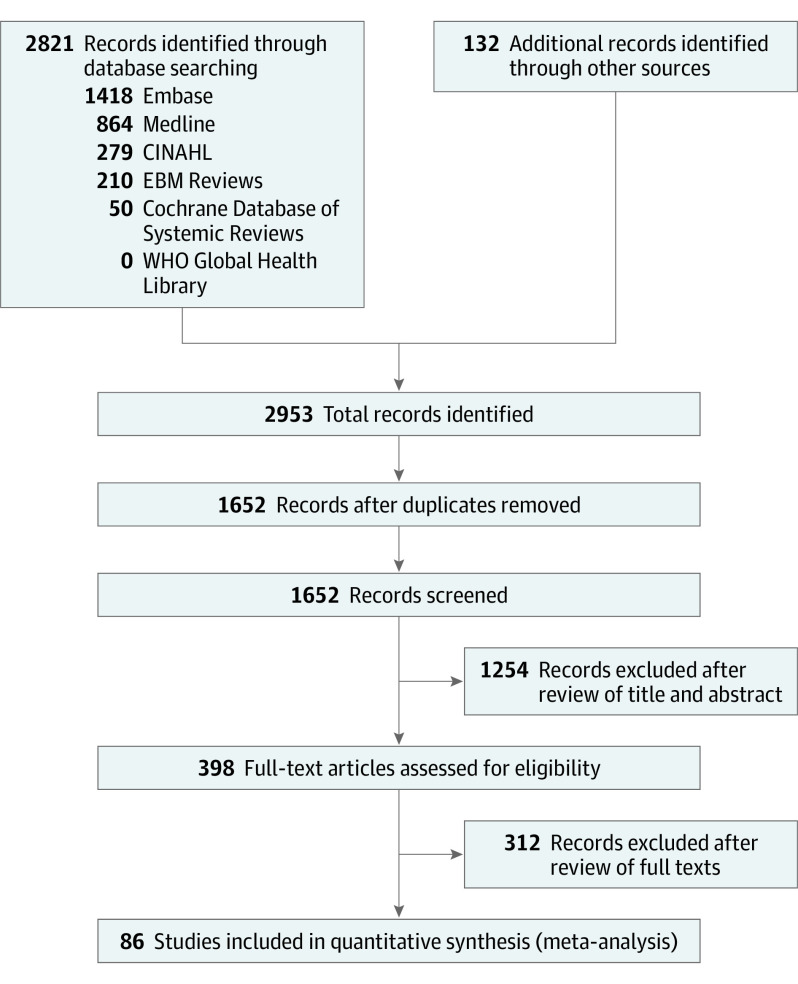
Our search strategy identified 2953 studies (Figure 1). After removal of duplicate works, 1652 studies remained. We excluded 1254 studies following title and abstract screening. We reviewed the full text of the remaining 398 studies, of which 86 studies were included (eTable 1 in the Supplement). The Table outlines summary statistics for all included studies and for the studies stratified by brain biomarker. Summary analyses included a total of 10 567 patients (7777 men [73.6%] and 2790 women [26.4%]). All studies were in the English language. In terms of design methods, 53 studies were prospective and 33 were retrospective. Pertaining to the brain biomarkers of interest, the following number of studies evaluated each biomarker in relation to prediction of neurologic outcome: (1) NSE, 72; (2) S100β, 29; (3) GFAP, 9; (4) Nf-L, 10; (5) tau, 6; and (6) UCH-L1, 4.
Figure 1. Preferred Reporting Items for Systematic Reviews and Meta-analyses Flow Diagram.
This figure depicts the search strategy, records identified, as well as article exclusion following screening for eligibility.
Table. Patient Demographics for All Included Studies and Each Specific Biomarkera.
| Variable | No. (%) | ||||||
|---|---|---|---|---|---|---|---|
| All studies | NSE | S100β | GFAP | Nf-L | Tau | UCH-L1 | |
| Included studies, No. | 86 | 72 | 29 | 9 | 10 | 6 | 4 |
| Study patients | |||||||
| Total No. | 10 567 | 9880 | 3420 | 1055 | 1231 | 883 | 757 |
| Men | 7777 (73.6) | 7319 (74.1) | 2425 (70.9) | 803 (76.1) | 963 (78.2) | 698 (79.0) | 605 (79.9) |
| Women | 2790 (26.4) | 2561 (25.9) | 995 (20.1) | 252 (23.9) | 268 (21.8) | 185 (21.0) | 152 (20.1) |
| Age, mean (SD), y | 62.8 (10.2) | 63.3 (11.1) | 64.4 (9.1) | 64.1 (12.7) | 63.0 (12.6) | 63.5 (13.0) | 63.7 (12.3) |
| OHCA | 8629 (89.6) | 7952 (88.7) | 2958 (87.5) | 936 (88.7) | 1191 (97.9) | 860 (97.4) | 754 (99.6) |
| Cardiac origin | 4802 (69.4) | 4352 (69.0) | 993 (64.3) | 827 (91.9) | 919 (88.0) | 162 (83.5) | 727 (96.0) |
| Witnessed | 4104 (76.9) | 3869 (77.8) | 939 (68.0) | 776 (83.1) | 254 (77.7) | 49 (79.0) | 649 (45.1) |
| Shockable ECG | 5081 (52.5) | 4814 (53.4) | 1690 (51.9) | 705 (66.8) | 247 (63.7) | 100 (51.5) | 569 (39.5) |
| Bystander CPR | 3426 (57.4) | 3281 (59.0) | 907 (53.7) | 610 (69.2) | 745 (70.0) | 116 (67.4) | 524 (36.4) |
| TTM | 8070 (86.8) | 7736 (88.1) | 2381 (80.4) | 970 (91.9) | 967 (98.4) | 802 (90.1) | 751 (99.2) |
| Favorable outcome | 4733 (44.8) | 4466 (45.2) | 1499 (43.8) | 508 (48.2) | 645 (52.4) | 442 (50.1) | 373 (49.3) |
| Outcome scale | |||||||
| CPC | 67 (77.9) | 57 (79.2) | 20 (69.0) | 7 (77.8) | 8 (80.0) | 6 (100) | 4 (100) |
| GOS | 7 (8.1) | 7 (9.7) | 4 (13.8) | 1 (11.1) | 0 | 0 | 0 |
| mGOS | 2 (2.3) | 1 (1.4) | 0 | 1 (11.1) | 1 (10.0) | 0 | 0 |
| Survival | 10 (11.6) | 7 (9.7) | 5 (17.2) | 0 | 1 (10.0) | 0 | 0 |
| mRS | 0 | 0 | 0 | 0 | 0 | 0 | 0 |
Abbreviations: CPC, cerebral performance category; CPR, cardiopulmonary resuscitation; ECG, electrocardiogram; GFAP, glial fibrillary acidic protein; GOS, Glasgow Outcome Scale; mGOS, Modified Glasgow Outcome Scale; mRS, modified Rankin Scale; Nf-L, neurofilament light; NSE, neuron-specific enolase; OHCA, out-of-hospital cardiac arrest; S100β, S100 calcium-binding protein β; TTM, targeted temperature management; UCH-L1, ubiquitin carboxyl hydrolase L1.
Not every study reported demographic data, such as OHCA or whether the arrest was of cardiac origin; therefore, the percentage values are based on the total sample size of only the studies that reported on each specific demographic factor. Furthermore, if multiple publications were included in the meta-analyses from the same trial (eg, TTM), their data were added to the relevant biomarker columns but were not duplicated within any single column (eg, total sample size did not duplicate patients from the same trial but different publications).
The summary patient demographics of studies included are presented in the Table. The pooled mean (SD) age of all patients included was 62.8 (10.2) years. Notably, 44 studies included only patients with OHCA, 1 study included only patients with in-hospital cardiac arrest (IHCA), and 31 included both. The remaining 10 studies did not specify OHCA or IHCA. Sixty-eight studies specified patients undergoing TTM. Among these, 40 studies exclusively targeted 33 °C, 3 studies exclusively targeted 36 °C, and 9 studies targeted either 33 °C or 36 °C. Sixteen studies did not specify temperature targets.
Summary receiver operating characteristic curves for each biomarker at 48 hours following cardiac arrest are displayed in Figure 2. Neurofilament light and tau exhibited the highest AUCs of 0.92 (95% CI, 0.84-0.97) and 0.89 (95% CI, 0.71-0.97), respectively. Using specificity weighting of 85%, 80%, and 75%, the optimal Nf-L thresholds generated were 1361 pg/mL (sensitivity, 0.68; specificity, 0.98), 1034 pg/mL (sensitivity, 0.71; specificity, 0.97) and 827 pg/mL (sensitivity, 0.74; specificity, 0.96), respectively. Neuron-specific enolase exhibited an AUC of 0.84 (95% CI, 0.77-0.91). Using specificity weighting of 85%, 80%, and 75%, the optimal NSE thresholds generated were 64.1 μg/L (sensitivity, 0.46; specificity 0.97), 53.7 μg/L (sensitivity, 0.52; specificity, 0.96), and 46.7 μg/L (sensitivity, 0.57; specificity, 0.96), respectively. The summary AUC, specificity-weighting thresholds with associated sensitivity, and specificity for S100β, GFAP, tau, and UCH-L1 are shown in Figure 2. The remaining SROC curves and associated AUC values for 24 and 72 hours following ROSC are shown in eFigures 1 and 2 in the Supplement.
Figure 2. Receiver Operating Characteristic Curves for the Diagnostic Accuracy of Brain Biomarkers for Predicting Unfavorable Outcome.
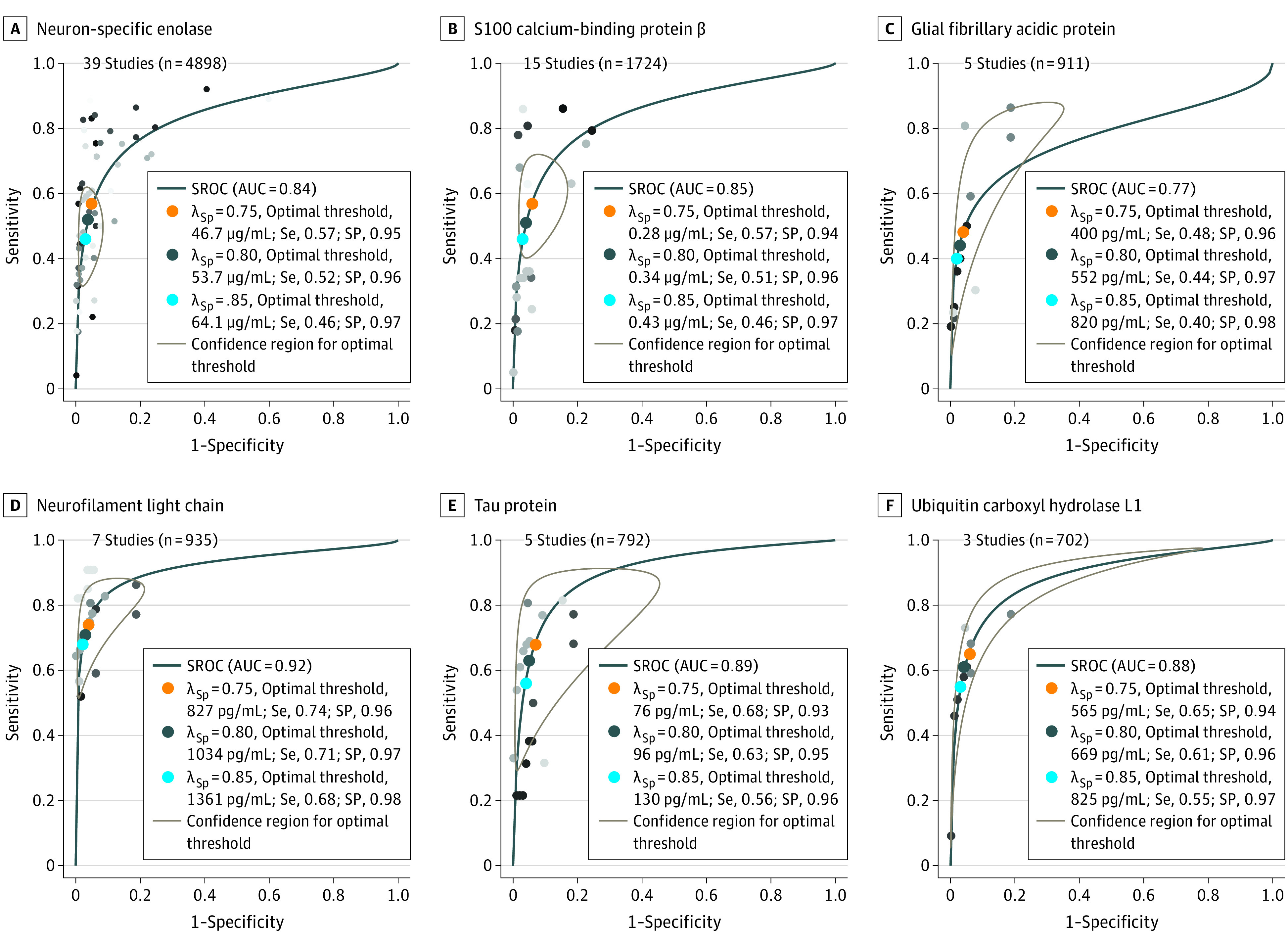
Summary receiver operating characteristic (SROC) curves and their confidence intervals for each biomarker at 48 hours following return of spontaneous circulation are presented. Each individual dot represents a unique study. We estimated optimal thresholds for each biomarker for particular weights of specificity. We weighted specificity at 75% (orange dots), 80% (gray dots), and 85% (blue dots) with sensitivity weighted 25%, 20%, and 15%, respectively. For each weight of specificity, an optimal threshold on the SROC curve was calculated and is reported in the figure for each variable. The SROC area under the curve for each biomarker are neuron-specific enolase, 0.84 (95% CI, 0.77-0.91); S100 calcium binding protein β, 0.85 (95% CI, 0.76-0.92); glial fibrillary acidic protein, 0.77 (95% CI, 0.59-0.91); neurofilament light chain, 0.92 (95% CI, 0.84-0.97); tau, 0.89 (95% CI, 0.71-0.97); and ubiquitin carboxyl hydrolase L1, 0.88 (95% CI, 0.52-0.99). Concentration threshold and corresponding sensitivity to achieve 95% and 100% specificity for each biomarker are presented in eTable 3 in the Supplement. λSp indicates the weighting of specificity at either 75%, 80%, or 85% (see statistical analysis for details); AUC, area under the curve; Se, sensitivity; SP, specificity.
Figure 3 displays the pooled medians for each brain biomarker longitudinally from admission, 24, 48, and 72 hours after ROSC in patients with favorable vs unfavorable neurologic outcome. For each brain biomarker, we performed a prespecified subgroup analysis of SROC curves for studies that specified the use of TTM (eFigure 3 in the Supplement) and calculated the pooled medians for these patients (eFigure 4 in the Supplement). We also performed a prespecified subgroup analysis of SROC curves for studies specifically including OHCA patients (eFigure 5 in the Supplement) and calculated the pooled medians for these patients (eFigure 6 in the Supplement). In addition, a subanalysis was performed on studies that assessed outcome at 3 months or greater from initial cardiac arrest (eTable 2 and eTable 4 in the Supplement). The individual AUCs for each brain biomarker did not appreciably differ compared with the overall results for the TTM, OHCA, or outcome determination at 3 months or later following cardiac arrest subanalyses. Neurofilament light continued to have the highest AUC in the TTM (0.92; 95% CI, 0.86-0.95), OHCA (0.93; 95% CI, 0.86-0.97), and outcome determination at 3 months or later following cardiac arrest subanalyses (0.92; 95% CI, 0.88-0.95).
Figure 3. Group Differences in Brain Biomarkers Between Patients With Favorable and Unfavorable Neurologic Outcome.
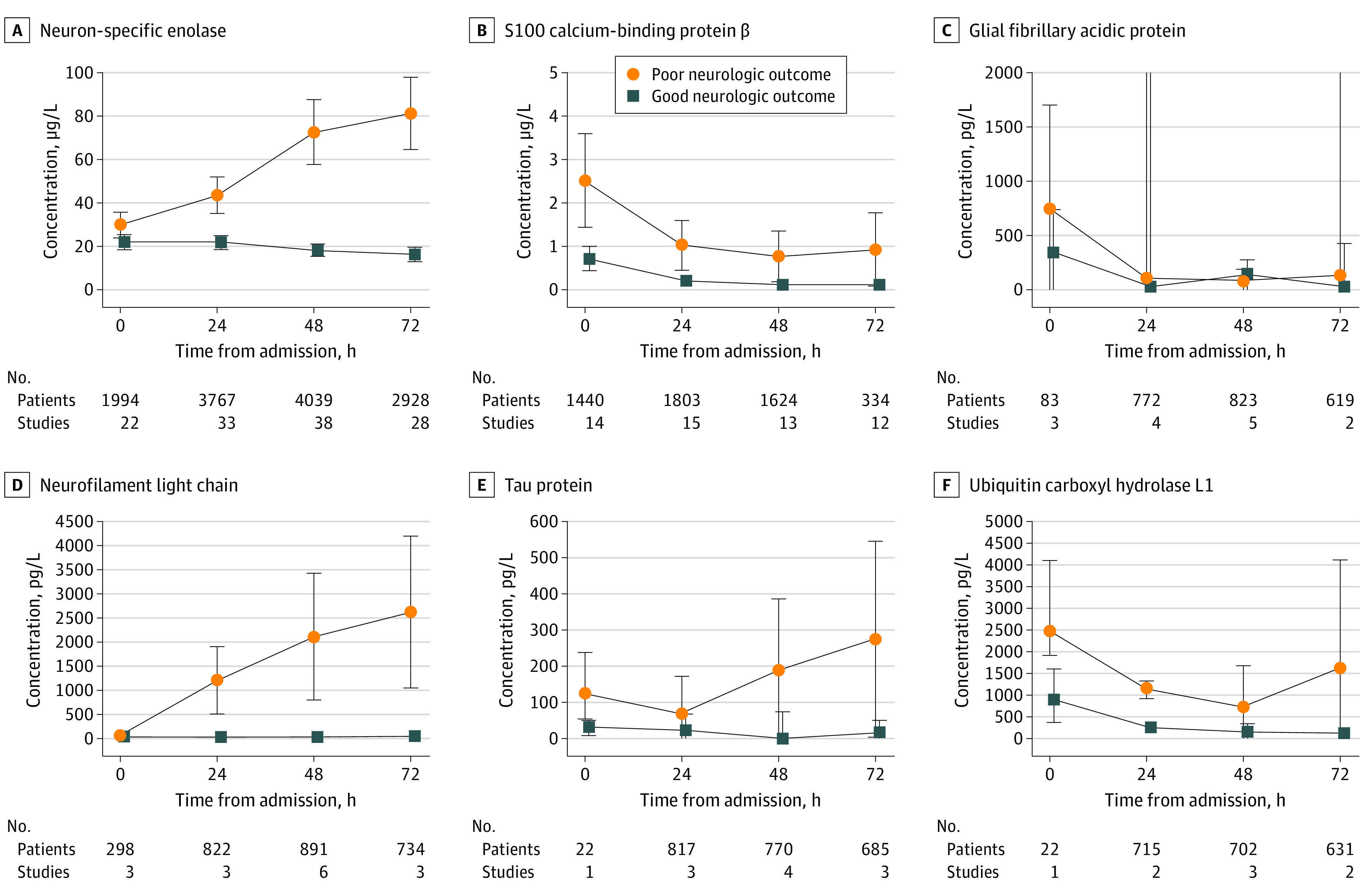
The median concentration and spread (IQR) are reported in patients with good (blue squares) and poor (orange circles) outcome at 0, 24, 48, and 72 hours following admission. The number of patients and studies included in the determination of the median and interquartile range for each point is noted within each graph.
The results from the QUADAS-2 assessment of risk of bias and concerns of applicability are detailed in eAppendix 4 and summarized in eFigure 7 in the Supplement. Briefly, risk of bias was high in 54% of studies relative to patient selection and 44% of studies relative to flow and timing (interval between the measurement of biomarkers and the assessment of neurologic outcome). In 51% of studies it was unclear whether the assessment of neurologic outcome was conducted without the knowledge of the biomarker results. Overall, the concerns of applicability were low in 92% of studies for patient selection, 92% of studies for the index test, and 94% of studies for the reference standard.
Discussion
The results suggest that Nf-L, followed by tau, have greater diagnostic accuracy in predicting favorable vs unfavorable neurologic outcomes compared with NSE, S100β, GFAP, and UCH-L1. We found that the diagnostic accuracy of NSE for unfavorable outcome appears to be less robust than previously reported.30 In addition, we establish a range of optimal thresholds for each biomarker to prognosticate neurologic outcome based on different weights of desired specificity. The use of brain injury biomarkers has emerged as a valuable tool for neurologic prognostication of HIBI (Figure 4). Although not used in isolation, NSE is included in guidelines.9,10,31 Given the general paucity of studies involving more novel brain biomarkers, they are not yet included in guidelines10 despite evidence they (ie, Nf-L) hold greater sensitivity to diagnose unfavorable neurologic outcome than traditional methods.32
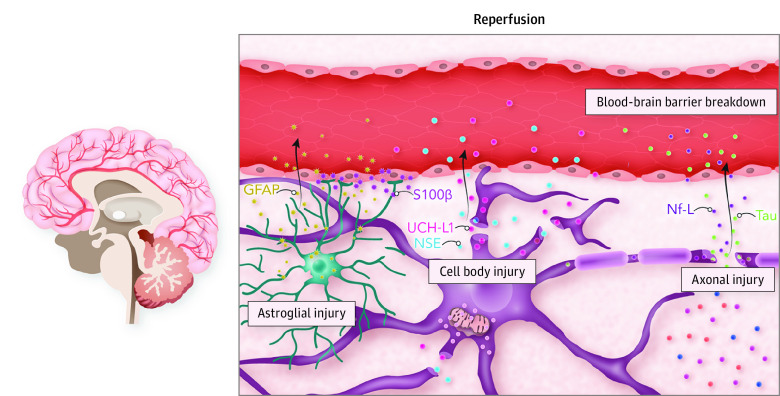
Figure 4. The Neurovascular Unit and Brain Injury Biomarker Release.
The neurovascular unit represents the principal anatomical and functional unit of the brain parenchyma where complex interplay occurs among neurons, the cerebral microvasculature, and surrounding glial cells to maintain homeostasis. The microvasculature is composed of the blood-brain barrier, which is partially formed by adjoining projections from astrocytes. Surrounding neuron cell bodies give rise to myelinated axons, which conduct signal transduction and facilitate communication with distinct anatomical locations in the brain. Following return of spontaneous circulation, ischemia-reperfusion injury pathophysiology occurs, and widespread injury across the neurovascular unit is reflected in the release of brain injury biomarkers into the bloodstream, which is facilitated by blood-brain barrier breakdown. Biomarkers reflecting astrocyte injury include glial fibrillary acidic protein (GFAP) and serum 100 calcium-binding protein β (S100β). Neuron cell body injury is reflected by release of neuron-specific enolase (NSE) and ubiquitin carboxyl hydrolase L1 (UCH-L1). In addition, axonal injury is reflected by release of neurofilament light (Nf-L) and tau. As such, the relative concentrations of the various biomarkers seen in the bloodstream can allude to signatures of damage to the neurovascular unit and its specific components.
Increasingly, subcortical white matter is recognized as a major anatomical site of HIBI associated with unfavorable neurologic outcome.33 Velley et al34 demonstrated that white matter injury on magnetic resonance imaging was the strongest predictor of unfavorable neurologic outcome. Our results corroborate these findings in that Nf-L, a biomarker reflective of axonal injury, was the most accurate in determining unfavorable neurologic outcome. Tau, also reflecting axonal injury, had greater diagnostic accuracy compared with NSE or S100β. Tau was less diagnostically accurate compared with Nf-L, which may be explained by extracerebral sources during global ischemia-reperfusion. That both Nf-L and tau demonstrated the greatest prognostic accuracy is in keeping with the observation35 that the difference in cerebral Nf-L and tau release between patients with HIBI with secondary brain hypoxia and those with normal brain oxygenation is greater than the difference observed with other biomarkers (eg, NSE, GFAP, and UCH-L1).35 In other words, Nf-L and tau appear to be the most sensitive markers of secondary brain hypoxia in patients with HIBI following cardiac arrest.35 Although these results are promising, and the included studies are of overall strong methodologic design, they are tempered by the relative paucity of prospective studies investigating Nf-L. Prospective and blinded studies with standardized bio-specimen collection as well as analytical methods are needed in this field.
Most literature in this field has focused on NSE and S100β.15 Wang et al30 conducted a systematic review and meta-analysis that concluded that NSE and S100β had high specificity in predicting adverse neurologic outcome in HIBI. Although the analysis was well executed, it is limited from the nature in which brain biomarker studies are conducted. There is a lack of common concentration thresholds, and consequently many, if not all, studies report multiple values of specificity and/or sensitivity corresponding to varying thresholds and times that are often chosen a posteriori to satisfy an acceptable level of specificity. Although statistically sound, the methods are dependent on a single pair of specificity and/or sensitivity from each study that must be selected at a particular threshold and point, resulting in most of the data being omitted.24,25,36 Further, the models used are unable to provide optimal concentration thresholds.24,25,36 Pooled sensitivities and specificities provide clinicians with no information regarding which threshold or point is preferred, limiting their clinical utility. The results of the present study indicate that both NSE and S100β had lower pooled AUC and specificity. This is not surprising because the analysis by Wang et al30 used a single pair of specificity and/or sensitivity with the greatest specificity for each study, which may bias the result toward greater specificity.24,25,36 Our analysis used all reported values of specificity and sensitivity stratified by time. The discrepancy in pooled AUC and specificity may also be attributable to additional studies included in our review published since 2019 and the inclusion of studies that evaluated neurologic outcome up to 6 months following cardiac arrest. Conversely, Wang et al30 included only studies that assessed neurologic outcome up to 1 month after cardiac arrest. Notably, HIBI survivors may continue to make clinical improvements in the months after hospital admission.37
Mechanistically, concern over extraneuronal release of NSE from red blood cells, small cell lung carcinoma, and neuroendocrine tumors may limit its interpretation.15 S100 calcium-binding protein β, with its short serum half-life (2 hours), presents a challenge to use beyond the 24-hour period, in addition to concerns related to its extracerebral sources (eg, cardiomyocytes).14,38 Ubiquitin carboxyl hydrolase L1 similarly has a short half-life. Conversely, the interpretation of serum Nf-L levels is not associated with these limitations and was demonstrated to possess greater prognostic accuracy than NSE and S100β (Figure 2; eTable 3 in the Supplement).
Three additional considerations for using biomarkers for neurologic prognostication are (1) the timing at which biomarkers are drawn, (2) the impact of TTM, and (3) arrest etiology (eg, presumed cardiac cause). In Figure 3, we provide a longitudinal trajectory of median concentration for each biomarker stratified by patients with an unfavorable and favorable neurologic outcome. Based on the associated interquartile ranges, it appears that Nf-L and NSE concentrations were higher among patients with unfavorable neurologic outcome at all included times. S100 calcium-binding protein β, GFAP, and UCH-L1 showed greater variability and more overlap between patients with favorable and unfavorable outcome (Figure 3). This may be owing to smaller number of patients studied for these biomarkers and differences in absolute biomarker concentrations that may be attributable to varying analytical platforms. Variability may also be associated with extracerebral sources of these biomarkers and half-life duration (as described previously).38 Moreover, in some instances GFAP may reflect astroglial activation rather than irreversible cell injury.38 To provide insight into the role of TTM in neurologic prognostication, we conducted a prespecified subgroup analysis investigating the diagnostic accuracy of brain biomarkers in studies that stipulated the use of TTM (eFigure 3 in the Supplement). In this subgroup, the SROCs and corresponding AUCs for each biomarker remained consistent with the primary analysis. Most studies included evaluated patients following OHCA stemming from shockable rhythms (ie, cardiac origin; approximately 90% of patients; Table), thereby limiting the extrapolation of the results to both IHCA or cardiac arrest stemming from noncardiac causes, both of which are associated with worse outcome. The OHCA subanalysis demonstrated findings similar to those of the primary analysis, yet future studies must clarify the accuracy of these biomarkers in predicting adverse clinical outcomes in a more generalized population of patients following cardiac arrest.
Limitations
We present novel and clinically relevant data pertaining to SROC curves, optimal thresholds, and median concentrations for all included patients and subgroups. However, our study is not without limitations. First, several included studies were of poor quality, as evaluated using the QUADAS-2 tool. Second, the use of 2-stage random-effects models for meta-analyses is a nascent concept not yet extensively validated. Third, there was substantial heterogeneity in the studies included. Fourth, this review has not addressed all biomarkers that have been investigated for neurologic prognostication (eg, creatine kinase isoenzyme BB39) but rather focused on those commonly discussed in guideline statements (ie, NSE and S100β) as well as newly emerging biomarkers (ie, Nf-L, tau, GFAP, and UCH-L1) that show imminent promise for clinical use. Fifth, withdrawal of life-sustaining therapies is a major modality of death in HIBI, and although the assumption is that the severity of neurologic injury is a factor in the decision of whether to proceed with palliative measures, this link is imperfect. In this regard, not all studies included in the meta-analyses specified major extracranial comorbid conditions that could be factors in the decision to withdraw life-sustaining therapies. Future studies investigating the accuracy of brain injury biomarkers in predicting clinical outcome need to incorporate standardized prognostication algorithms. In addition, uniformity pertaining to biospecimen collection, timing, and the analytical platform used was limited. Currently, there are no specified recommended standards for these critical methodologic variables, and developing uniform approaches are essential in future work.
Conclusions
Neurofilament light, reflecting white matter damage, was associated with the highest accuracy to prognosticate unfavorable neurologic outcome compared with NSE, S-100β, GFAP, tau, and UCH-L1 in HIBI. Future work must be conducted in a prospective manner with standardized bio-specimen collection methods, timing, and analytical platforms.
eAppendix 1. Detailed Methods
eAppendix 2. Search Strategy for MEDLINE
eAppendix 3. Outline of Data Inclusion for Studies With Duplicated Data
eAppendix 4. QUADAS-2 Results
eFigure 1. Receiver Operator Characteristic Curves for the Diagnostic Accuracy of Brain Biomarkers Post ROSC for Predicting Unfavorable Outcome at 24 hours.
eFigure 2. Receiver Operator Characteristic Curves for the Diagnostic Accuracy of Brain Biomarkers Post ROSC for Predicting Unfavorable Outcome at 72 hours.
eFigure 3. Receiver Operator Characteristic Curves for the Diagnostic Accuracy of Brain Biomarkers for Predicting Unfavorable outcome at 48 hours in patients that underwent targeted temperature management.
eFigure 4. Group Differences in Brain Biomarkers Between Patients That Underwent TTM with Favorable and Unfavorable Neurological Outcome.
eFigure 5. Receiver Operator Characteristic Curves for the Diagnostic Accuracy of Brain Biomarkers for Predicting Unfavorable outcome at 48 hours in patients that had an out-of-hospital cardiac arrest.
eFigure 6. Group Differences in Brain Biomarkers Between Patients That Had an Out-of-Hospital Cardiac Arrest with Favorable and Unfavorable Neurological Outcome.
eFigure 7. QUADAS-2 assessment for risk of bias and concerns of applicability
eTable 1. Study Characteristics.
eTable 2. Receiver Operator Characteristic Curve Analysis for the Diagnostic Accuracy of Brain Biomarkers for Predicting Unfavorable outcome at 48 hours in patients where outcome was determined ≥3 months following cardiac arrest.
eTable 3. Concentration Thresholds for Each Biomarker.
eTable 4. Studies Including Outcome Determination at Times Other Than Hospital Discharge and 6 Months.
eTable 5. Studies Including Survival vs Death as Outcome.
eReferences
References
- 1.Lemiale V, Dumas F, Mongardon N, et al. Intensive care unit mortality after cardiac arrest: the relative contribution of shock and brain injury in a large cohort. Intensive Care Med. 2013;39(11):1972-1980. doi: 10.1007/s00134-013-3043-4 [DOI] [PubMed] [Google Scholar]
- 2.Laver S, Farrow C, Turner D, Nolan J. Mode of death after admission to an intensive care unit following cardiac arrest. Intensive Care Med. 2004;30(11):2126-2128. doi: 10.1007/s00134-004-2425-z [DOI] [PubMed] [Google Scholar]
- 3.Yan S, Gan Y, Jiang N, et al. The global survival rate among adult out-of-hospital cardiac arrest patients who received cardiopulmonary resuscitation: a systematic review and meta-analysis. Crit Care. 2020;24(1):61. doi: 10.1186/s13054-020-2773-2 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Andersen LW, Holmberg MJ, Berg KM, Donnino MW, Granfeldt A. In-hospital cardiac arrest: a review. JAMA. 2019;321(12):1200-1210. doi: 10.1001/jama.2019.1696 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Graf J, Mühlhoff C, Doig GS, et al. Health care costs, long-term survival, and quality of life following intensive care unit admission after cardiac arrest. Crit Care. 2008;12(4):R92. doi: 10.1186/cc6963 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Efendijev I, Folger D, Raj R, et al. Outcomes and healthcare-associated costs one year after intensive care-treated cardiac arrest. Resuscitation. 2018;131(October):128-134. doi: 10.1016/j.resuscitation.2018.06.028 [DOI] [PubMed] [Google Scholar]
- 7.Oddo M, Friberg H. Neuroprognostication after cardiac arrest in the light of targeted temperature management. Curr Opin Crit Care. 2017;23(3):244-250. doi: 10.1097/MCC.0000000000000406 [DOI] [PubMed] [Google Scholar]
- 8.Nolan JP, Soar J, Cariou A, et al. ; European Resuscitation Council; European Society of Intensive Care Medicine . European Resuscitation Council and European Society of Intensive Care Medicine 2015 guidelines for post-resuscitation care. Intensive Care Med. 2015;41(12):2039-2056. doi: 10.1007/s00134-015-4051-3 [DOI] [PubMed] [Google Scholar]
- 9.Callaway CW, Donnino MW, Fink EL, et al. Part 8: post-cardiac arrest care: 2015 American Heart Association guidelines update for cardiopulmonary resuscitation and emergency cardiovascular care. Circulation. 2015;132(18)(suppl 2):S465-S482. doi: 10.1161/CIR.0000000000000262 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Rossetti AO, Rabinstein AA, Oddo M. Neurological prognostication of outcome in patients in coma after cardiac arrest. Lancet Neurol. 2016;15(6):597-609. doi: 10.1016/S1474-4422(16)00015-6 [DOI] [PubMed] [Google Scholar]
- 11.Geocadin RG, Callaway CW, Fink EL, et al. ; American Heart Association Emergency Cardiovascular Care Committee . Standards for studies of neurological prognostication in comatose survivors of cardiac arrest: a scientific statement from the American Heart Association. Circulation. 2019;140(9):e517-e542. doi: 10.1161/CIR.0000000000000702 [DOI] [PubMed] [Google Scholar]
- 12.Diaz-Arrastia R, Shahim P, Sandsmark DK. Molecular biomarkers in the neurological ICU: is there a role? Curr Opin Crit Care. 2020;26(2):103-108. doi: 10.1097/MCC.0000000000000703 [DOI] [PubMed] [Google Scholar]
- 13.Iadecola C. The neurovascular unit coming of age: a journey through neurovascular coupling in health and disease. Neuron. 2017;96(1):17-42. doi: 10.1016/j.neuron.2017.07.030 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Stammet P. Blood biomarkers of hypoxic-ischemic brain injury after cardiac arrest. Semin Neurol. 2017;37(1):75-80. doi: 10.1055/s-0036-1593858 [DOI] [PubMed] [Google Scholar]
- 15.Sandroni C, D’Arrigo S, Nolan JP. Prognostication after cardiac arrest. Crit Care. 2018;22(1):150. doi: 10.1186/s13054-018-2060-7 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Liu H, Povysheva N, Rose ME, et al. Role of UCHL1 in axonal injury and functional recovery after cerebral ischemia. Proc Natl Acad Sci U S A. 2019;116(10):4643-4650. doi: 10.1073/pnas.1821282116 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Moher D, Liberati A, Tetzlaff J, et al. ; PRISMA Group . Preferred reporting items for systematic reviews and meta-analyses: the PRISMA statement. BMJ. 2009;339(July):b2535. doi: 10.1136/bmj.b2535 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.McInnes MDF, Moher D, Thombs BD, et al. ; and the PRISMA-DTA Group . Preferred Reporting Items for a Systematic Review and Meta-analysis of Diagnostic Test Accuracy Studies: the PRISMA-DTA statement. JAMA. 2018;319(4):388-396. doi: 10.1001/jama.2017.19163 [DOI] [PubMed] [Google Scholar]
- 19.Sandroni C, Cronberg T, Sekhon M. Brain injury after cardiac arrest: pathophysiology, treatment, and prognosis. Intensive Care Med. 2021;47(12):1393-1414. doi: 10.1007/s00134-021-06548-2 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Bongiovanni F, Romagnosi F, Barbella G, et al. Standardized EEG analysis to reduce the uncertainty of outcome prognostication after cardiac arrest. Intensive Care Med. 2020;46(5):963-972. doi: 10.1007/s00134-019-05921-6 [DOI] [PubMed] [Google Scholar]
- 21.Cloostermans MC, van Meulen FB, Eertman CJ, Hom HW, van Putten MJAM. Continuous electroencephalography monitoring for early prediction of neurological outcome in postanoxic patients after cardiac arrest: a prospective cohort study. Crit Care Med. 2012;40(10):2867-2875. doi: 10.1097/CCM.0b013e31825b94f0 [DOI] [PubMed] [Google Scholar]
- 22.Sandroni C, D’Arrigo S, Cacciola S, et al. Prediction of Poor Neurological Outcome in Comatose Survivors of Cardiac Arrest: A Systematic Review. Vol 46. Springer Berlin Heidelberg; 2020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Whiting PF, Rutjes AW, Westwood ME, et al. ; QUADAS-2 Group . QUADAS-2: a revised tool for the quality assessment of diagnostic accuracy studies. Ann Intern Med. 2011;155(8):529-536. doi: 10.7326/0003-4819-155-8-201110180-00009 [DOI] [PubMed] [Google Scholar]
- 24.Steinhauser S, Schumacher M, Rücker G. Modelling multiple thresholds in meta-analysis of diagnostic test accuracy studies. BMC Med Res Methodol. 2016;16(1):97. doi: 10.1186/s12874-016-0196-1 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Zapf A, Albert C, Frömke C, et al. Meta-analysis of diagnostic accuracy studies with multiple thresholds: comparison of different approaches. Biom J. 2021;63(4):699-711. doi: 10.1002/bimj.202000091 [DOI] [PubMed] [Google Scholar]
- 26.Sandroni C, Cariou A, Cavallaro F, et al. Prognostication in comatose survivors of cardiac arrest: an advisory statement from the European Resuscitation Council and the European Society of Intensive Care Medicine. Resuscitation. 2014;85(12):1779-1789. doi: 10.1016/j.resuscitation.2014.08.011 [DOI] [PubMed] [Google Scholar]
- 27.McGrath S, Sohn H, Steele R, Benedetti A. Meta-analysis of the difference of medians. Biom J. 2020;62(1):69-98. doi: 10.1002/bimj.201900036 [DOI] [PubMed] [Google Scholar]
- 28.Wan X, Wang W, Liu J, Tong T. Estimating the sample mean and standard deviation from the sample size, median, range and/or interquartile range. BMC Med Res Methodol. 2014;14(135):135. doi: 10.1186/1471-2288-14-135 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Higgins JPT, Li T, Deeks JJ. Chapter 6: choosing effect measures and computing estimates of effect. In: Higgins JPT, Thomas J, Chandler J, et al, eds. Cochrane Handbook for Systematic Reviews of Interventions, Version 6.0. Cochrane; July 2019. Accessed September 15, 2021. http://www.training.cochrane.org/handbook
- 30.Wang CH, Chang WT, Su KI, et al. Neuroprognostic accuracy of blood biomarkers for post-cardiac arrest patients: a systematic review and meta-analysis. Resuscitation. 2020;148(148):108-117. doi: 10.1016/j.resuscitation.2020.01.006 [DOI] [PubMed] [Google Scholar]
- 31.Nolan JP, Sandroni C, Böttiger BW, et al. European Resuscitation Council and European Society of Intensive Care Medicine Guidelines 2021: post-resuscitation care. Resuscitation. 2021;161(161):220-269. doi: 10.1016/j.resuscitation.2021.02.012 [DOI] [PubMed] [Google Scholar]
- 32.Moseby-Knappe M, Mattsson N, Nielsen N, et al. Serum neurofilament light chain for prognosis of outcome after cardiac arrest. JAMA Neurol. 2019;76(1):64-71. doi: 10.1001/jamaneurol.2018.3223 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Cronberg T. White matter is what matters after cardiac arrest. Lancet Neurol. 2018;17(4):291-292. doi: 10.1016/S1474-4422(18)30079-6 [DOI] [PubMed] [Google Scholar]
- 34.Velly L, Perlbarg V, Boulier T, et al. ; MRI-COMA Investigators . Use of brain diffusion tensor imaging for the prediction of long-term neurological outcomes in patients after cardiac arrest: a multicentre, international, prospective, observational, cohort study. Lancet Neurol. 2018;17(4):317-326. doi: 10.1016/S1474-4422(18)30027-9 [DOI] [PubMed] [Google Scholar]
- 35.Hoiland RL, Ainslie PN, Wellington CL, et al. Brain hypoxia is associated with neuroglial injury in humans post-cardiac arrest. Circ Res. 2021;129(5):583-597. doi: 10.1161/CIRCRESAHA.121.319157 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.McGrath S, Zhao X, Steele R, Thombs BD, Benedetti A; DEPRESsion Screening Data (DEPRESSD) Collaboration . Estimating the sample mean and standard deviation from commonly reported quantiles in meta-analysis. Stat Methods Med Res. 2020;29(9):962280219889080. doi: 10.1177/0962280219889080 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Terman SW, Hume B, Meurer WJ, Silbergleit R. Impact of presenting rhythm on short- and long-term neurologic outcome in comatose survivors of cardiac arrest treated with therapeutic hypothermia. Crit Care Med. 2014;42(10):2225-2234. doi: 10.1097/CCM.0000000000000506 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Moseby-Knappe M, Cronberg T. Blood biomarkers of brain injury after cardiac arrest: a dynamic field. Resuscitation. 2020;156:273-276. doi: 10.1016/j.resuscitation.2020.09.004 [DOI] [PubMed] [Google Scholar]
- 39.Sherman AL, Tirschwell DL, Micklesen PJ, Longstreth WT Jr, Robinson LR. Somatosensory potentials, CSF creatine kinase BB activity, and awakening after cardiac arrest. Neurology. 2000;54(4):889-894. doi: 10.1212/WNL.54.4.889 [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
eAppendix 1. Detailed Methods
eAppendix 2. Search Strategy for MEDLINE
eAppendix 3. Outline of Data Inclusion for Studies With Duplicated Data
eAppendix 4. QUADAS-2 Results
eFigure 1. Receiver Operator Characteristic Curves for the Diagnostic Accuracy of Brain Biomarkers Post ROSC for Predicting Unfavorable Outcome at 24 hours.
eFigure 2. Receiver Operator Characteristic Curves for the Diagnostic Accuracy of Brain Biomarkers Post ROSC for Predicting Unfavorable Outcome at 72 hours.
eFigure 3. Receiver Operator Characteristic Curves for the Diagnostic Accuracy of Brain Biomarkers for Predicting Unfavorable outcome at 48 hours in patients that underwent targeted temperature management.
eFigure 4. Group Differences in Brain Biomarkers Between Patients That Underwent TTM with Favorable and Unfavorable Neurological Outcome.
eFigure 5. Receiver Operator Characteristic Curves for the Diagnostic Accuracy of Brain Biomarkers for Predicting Unfavorable outcome at 48 hours in patients that had an out-of-hospital cardiac arrest.
eFigure 6. Group Differences in Brain Biomarkers Between Patients That Had an Out-of-Hospital Cardiac Arrest with Favorable and Unfavorable Neurological Outcome.
eFigure 7. QUADAS-2 assessment for risk of bias and concerns of applicability
eTable 1. Study Characteristics.
eTable 2. Receiver Operator Characteristic Curve Analysis for the Diagnostic Accuracy of Brain Biomarkers for Predicting Unfavorable outcome at 48 hours in patients where outcome was determined ≥3 months following cardiac arrest.
eTable 3. Concentration Thresholds for Each Biomarker.
eTable 4. Studies Including Outcome Determination at Times Other Than Hospital Discharge and 6 Months.
eTable 5. Studies Including Survival vs Death as Outcome.
eReferences