Abstract
Many flowering plants have evolved self-incompatibility (SI) systems to prevent inbreeding. In the Brassicaceae, SI is genetically controlled by a single polymorphic locus, termed the S-locus. Pollen rejection occurs when stigma and pollen share the same S-haplotype. Recognition of S-haplotype specificity has recently been shown to involve at least two S-locus genes, S-receptor kinase (SRK) and S-locus protein 11 or S-locus Cys-rich (SP11/SCR). SRK encodes a polymorphic membrane-spanning protein kinase, which is the sole female determinant of the S-haplotype specificity. SP11/SCR encodes a highly polymorphic Cys-rich small basic protein specifically expressed in the anther tapetum and in pollen. In cauliflower (B. oleracea), the gain-of-function approach has demonstrated that an allele of SP11/SCR encodes the male determinant of S-specificity. Here we examined the function of two alleles of SP11/SCR of B. rapa by the same approach and further established that SP11/SCR is the sole male determinant of SI in the genus Brassica sp. Our results also suggested that the 522-bp 5′-upstream region of the S9-SP11 gene used to drive the transgene contained all the regulatory elements required for the unique sporophytic/gametophytic expression observed for the native SP11 gene. Promoter deletion analyses suggested that the highly conserved 192-bp upstream region was sufficient for driving this unique expression. Furthermore, immunohistochemical analyses revealed that the protein product of the SP11 transgene was present in the tapetum and pollen, and that in pollen of late developmental stages, the SP11 protein was mainly localized in the pollen coat, a finding consistent with its expected biological role.
Self-incompatibility (SI) prevents self-fertilization and promotes out-crossing in hermaphrodite seed plants (Nettancourt, 1977). In most species the self/non-self recognition in SI is controlled by a single multi-allelic locus termed the S-locus. The S-locus is expected to contain at least two separate polymorphic genes, one determining the female and the other the male S-haplotype specificity. Numerous attempts have been made to identify these genes in several families that possess SI, e.g. Brassicaceae, Solanaceae, and Papaveraceae (McCubbin and Kao, 2000).
In the Brassicaceae, three highly polymorphic genes have been identified at the S-locus: SLG (for S-locus glycoprotein), SRK (for S-locus receptor kinase), and SP11/SCR (for S-locus protein 11 or S-locus Cys-rich). SLG encodes an abundant, secreted glycoprotein located in the cell wall of the papillar cell of the stigma (Takayama et al., 1987; Kandasamy et al., 1989). SRK encodes a membrane-anchored Ser/Thr protein kinase containing an extracellular domain, which shares extensive sequence similarity with SLG (Stein et al., 1991). SRK is expected to span the plasma membrane of the papillar cell. Earlier loss-of-function experiments using an antisense SLG gene demonstrated that SLG and/or SRK encoded the female determinant of SI; however, the precise role of each gene was not determined (Shiba et al., 1995, 2000). Recent gain-of-function experiments have provided conclusive evidence that SRK is the sole determinant of the S-haplotype specificity of the stigma (Takasaki et al., 2000). These experiments have also demonstrated that SLG is not required for the S-haplotype specificity, but may nonetheless play a role in enhancing the SI response. However, how this is accomplished is not yet known.
SP11/SCR is the third polymorphic gene at the S-locus to be discovered. SP11 was first identified as an anther-expressed S9-haplotype specific gene in an SLG/SRK flanking region of S9-haplotype of Brassica rapa (Suzuki et al., 1999), and a different allele of the same gene (but named SCR) was independently identified in the corresponding region of S8-haplotype of B. rapa (Schopfer et al., 1999). To date, 22 alleles of SP11/SCR have been identified in B. rapa, cauliflower, and oilseed rape, all of which encode proteins characteristic of novel pollen coat protein (PCP) family proteins (small, basic Cys-rich proteins; Bi et al., 2000; Schopfer et al., 1999; Suzuki et al., 1999; Takayama et al., 2000b; Watanabe et al., 2000). The fact that SP11/SCR determines the S-haplotype specificity of pollen was first demonstrated in cauliflower by a gain-of-function experiment (Schopfer et al., 1999). In this experiment, pollen of the transgenic plants carrying SCR6 transgene from the S6-haplotype of cauliflower was shown to acquire the S6-haplotype specificity. In B. rapa we also demonstrated the biological role of SP11/SCR using a pollination bioassay (Takayama et al., 2000b). In this bioassay, recombinant S9-SP11 (SP11 protein of the S9-haplotype) was shown to elicit an SI response in the papillar cells in an S-haplotype-specific manner (i.e. the response was observed only when the protein was applied to the papillar cells of the same S9-haplotype), resulting in the inhibition of cross-pollen hydration.
In this work we independently used the gain-of-function approach to further confirm the role of SP11/SCR in B. rapa. We report the results of the analyses of two lines of transgenic plants, one carrying S8-SP11 cDNA driven by the promoter of the S9-SP11 gene, and the other carrying S9-SP11 genomic DNA, including the promoter region. S8-SP11 and S9-SP11 transgenes were expressed at high levels in some of their respective transgenic plants and the pollen of these plants was shown to acquire the corresponding S-haplotype specificity. These results together with the previously reported transformation experiment in cauliflower conclusively establish that SP11/SCR is the sole male determinant of SI in the genus Brassica.
The transformation experiments also revealed that the 522-bp 5′-upstream region of the S9-SP11 gene used to drive the S8-SP11 transgene contained all the regulatory elements required for the unique sporophytic/gametophytic expression pattern of SP11/SCR that we had previously observed by in situ hybridization (Takayama et al., 2000b). Furthermore, promoter-deletion analyses suggested that the 192-bp 5′-upstream region, which is highly conserved in all of the SP11/SCR alleles examined, defines the minimum promoter sequence necessary for driving this unique expression. Immunohistochemical analyses using an antibody for recombinant S8-SP11 protein showed that the protein product of the S8-SP11 transgene in transgenic plants was located in the tapetum and pollen, and was mainly localized in the pollen coat at late developmental stages, consistent with its expected biological role. This is the first demonstration that SP11/SCR protein is present on the surface of the pollen grain.
RESULTS
Promoter Sequences of SP11 Alleles
We have previously shown that the SP11 gene is specifically expressed in the tapetal cell of the anther at early developmental stages, as well as in the microspore at late developmental stages (Takayama et al., 2000b). To identify the promoter sequence elements required for this dual sporophytic/gametophytic expression we first compared the nucleotide sequences of the 5′-flanking region of five alleles of SP11, three from S9, S8, and S12 haplotypes of B. rapa (designated S9-SP11, S8-SP11, and S12-SP11, respectively) and two from S910 and SA14 haplotypes of oilseed rape (designated S910-SP11 and SA14-SP11, respectively). These sequences are highly conserved (69.4%–88.0% identity) in the SP11 promoter from −200 to −1 bp, but less conserved in the region upstream beyond −200 bp (Fig. 1). From database searches, no common repeat or palindromic sequences were found in the 5′-flanking region of these SP11 alleles.
Figure 1.
Alignment of the nucleotide sequences of the promoter region of S9-SP11, S8-SP11, S12-SP11, S910-SP11, and SA14-SP11. The open box represents the putative TATA box. The arrows indicate the 5′ endpoints of the truncated promoter constructs used in the promoter analysis. Identical and conserved sequences are indicated by asterisks and periods, respectively. The position of the translation start site is assigned +1.
Analyses of Promoter Deletions of SP11
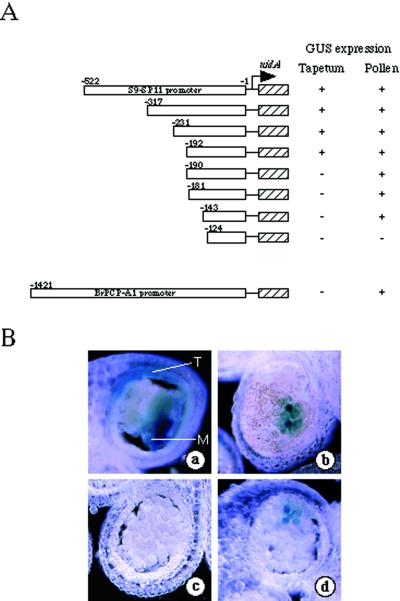
To identify and characterize cis-regulatory elements involved in promoter strength and specificity, transient expression analyses were performed using promoter deletion-β-glucuronidase (GUS) constructs. The GUS expression pattern for each of the constructs in the anther tapetum and pollen is presented in Figure 2A.
Figure 2.
Deletion analyses of the SP11 promoter region. A, SP11 promoter deletion constructs and summary of GUS staining results. Numbers denote the 5′ most positions of the truncated promoters relative to the translation initiation codon (ATG) of the S9-SP11 gene. The GUS expression of each promoter construct in the tapetum and pollen is represented by + (positive) or − (negative). B, Representative GUS staining results of transient promoter-gus fusion analyses. Cross-sections of anthers that had been bombarded with 317-bp SP11 promoter-gus (a), 143-bp SP11 promoter-gus (b), 124-bp SP11 promoter-gus (c), and 1,421-bp BrPCP-A1 promoter-gus (d). T and M represent tapetum and microspore, respectively.
The entire 522-bp S9-SP11 5′-flanking region drove high levels of GUS expression in anthers and pollen, but did not drive any expression in petals, sepals, or pistils (data not shown). Progressive deletions from the 5′ end to −317, −231, and −192 bp did not affect the promoter activity in tapetum or pollen (Fig. 2B, a). Further deletions removing the region between −190 to −143 bp resulted in GUS expression in pollen only (Fig. 2B, b). The smallest construct that contained the 5′-flanking region up to position −124 showed no detectable GUS expression in tapetum or pollen (Fig. 2B, c). In the control experiment, the 5′-flanking region of the BrPCP-A1 gene, which has previously been shown to be expressed gametophytically in pollen (Takayama et al., 2000b), exhibited GUS expression only in pollen (Fig. 2B, d). These results suggested that the 5′-flanking region up to −192 bp is sufficient to direct gene expression in tapetum and pollen, and that the region from −124 to 190 is involved in the specific expression in pollen.
Transformation of B. rapa Plants with S8-SP11 and S9-SP11 of B. rapa
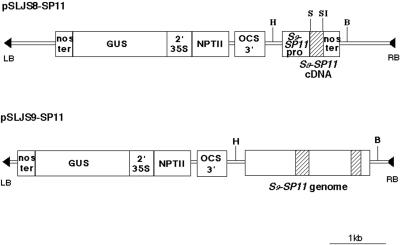
We constructed two transformation vectors, pSLJS8-SP11 and pSLJS9-SP11 (Fig. 3), to introduce S8-SP11 cDNA and S9-SP11 genomic DNA, respectively, into SI B. rapa cv Osome, a heterozygote of S52 and S60 haplotypes. Seven transgenic plants with the S8-SP11 transgene and 29 transgenic plants with the S9-SP11 transgene were obtained by using the Agrobacterium-mediated transformation procedure described previously (Shiba et al., 2000). These transgenics were morphologically indistinguishable from the wild-type cv Osome. We chose three plants (T66, T67, and T74) containing the S8-SP11 transgene and two plants (T161 and T254) containing the S9-SP11 transgene for further analyses, all of which strongly expressed the GUS marker gene (data not shown). One GUS-negative plant carrying the S8-SP11 transgene (T59) was used as a negative control. The presence of both SP11 transgenes was confirmed by PCR amplification of the SP11 gene and by DNA-blot analysis using a full-length S8-SP11 and S9-SP11 cDNA probe (data not shown).
Figure 3.
Schematic maps of the SP11 transgenes. The coding regions of the SP11 genes are indicated by hatched boxes. S9-SP11 pro, S9-SP11 promoter; Nos ter, nopaline synthase terminator; 2′35S, divergently transcribed 2′ and cauliflower mosaic virus 35S promoters; Npt II, neomycin phosphotransferase gene; OCS3, octopine synthase 3′ end; RB and LB, right and left borders of the T-DNA, respectively; H, HindIII restriction digest site; S, SmaI site; SI, SacI site; B, BamHI site.
Expression Analyses of S8-SP11 and S9-SP11 Transgenes in Transgenic Plants
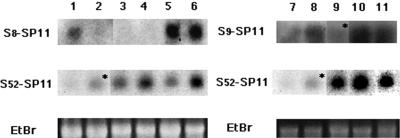
To confirm expression of the transgenes in the transgenic plants we performed RNA gel-blot analyses (Fig. 4). All three GUS-positive S8-SP11 transgenic lines, T66, T67, and T74, produced S8-SP11 mRNA, whereas the GUS-negative control line, T59, did not. The expression levels of the S8-SP11 mRNA in the former three transgenic lines were comparable with that in S8-homozygote. Both GUS-positive S9-SP11 transgenic lines, T161 and T254, produced similar amounts of S9-SP11 mRNA, as did the S9-homozygote. We also detected endogenous S52-SP11 mRNA in these transgenic plants, confirming the absence of cosuppression between the endogenous S52-SP11 gene and both SP11 transgenes.
Figure 4.
RNA-blot analysis of transcript levels of the endogenous S52-SP11 gene and the S8-SP11 and S9-SP11 transgenes. Blots containing total RNA from anthers at stage 7 (see “Materials and Methods”) were hybridized with an S8-SP11 probe (lanes 1–6, top), an S9-SP11 probe (lanes 7–11, top), and an S52-SP11 probe (middle). Bottom, The ethidium bromide staining of rRNA. Lanes 1 through 11 contain anther RNA isolated from a B. rapa S8 homozygote (lanes 1 and 7); an S9 homozygote (lanes 2 and 8); an S52S60 heterozygote (recipient of the transgenes, lanes 3 and 9); S8-SP11 transformants T66 and T74 (lanes 5 and 6, respectively); T59, a GUS-negative S8-SP11 transformant (lane 4); and S9-SP11 transformants T161 and T254 (lanes 10 and 11, respectively). The asterisks indicate cross-hybriding bands due to high sequence similarity between S9-SP11 and S52-SP11 (Takayama et al., 2000b).
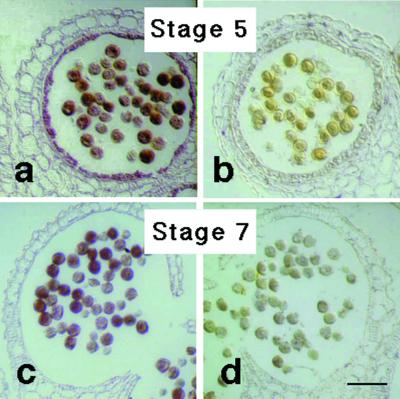
To confirm the expression of the SP11 transgene products at protein level we performed immunohistochemical analyses utilizing antibodies produced against the recombinant S8-SP11 protein. In the S8-SP11 transgenic line T66, strong signals for S8-SP11 protein were detected in the tapetal cells of anther and in the pollen grains (Fig. 5, a and c). In control experiments no signal was detected in these organs of the wild-type plant throughout their developmental stages (Fig. 5, b and d).
Figure 5.
Immunolocalization of S8-SP11. Anther sections derived from stage 5 and stage 7 flower buds (see “Materials and Methods”) were immunostained with the anti-S8-SP11 antibody. a, T66 line (S52 S60 /S8-SP11) at stage 5; b, wild type (S52 S60) at stage 5; c, T66 line at stage 7; d, wild type at stage 7. Scale bars = 50 μm.
Localization of the SP11 Protein in the Pollen Grains of Transgenic Plants
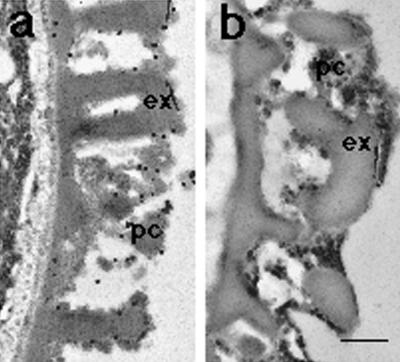
The localization of the SP11 protein in pollen grains at late developmental stages was further confirmed by immunoelectron-microscopic analyses. When sections of pollen grains of the S8-SP11 transgenic line T66 were treated with the anti-S8-SP11 antibody and gold-conjugated anti rabbit IgG, the gold particles were mainly observed on the surface of pollen, especially in the pollen coat (Fig. 6a). In a control experiment pollen sections of the host strain (S52S60) showed very little labeling (Fig. 6b). Treatment of these sections with preimmune serum also produced very little labeling (data not shown).
Figure 6.
Immunoelectron microscopy of pollen grains. Immunogold localization of pollen grains treated with the anti-S8-SP11 antibody and 20-nm gold-conjugated anti-rabbit IgG. a, T66 line (S52S60/ S8-SP11); b, wild-type plant. ex, Exine; pc, pollen coating. Scale bar = 1 μm.
Examination of SI Phenotypes of Transgenic Plants
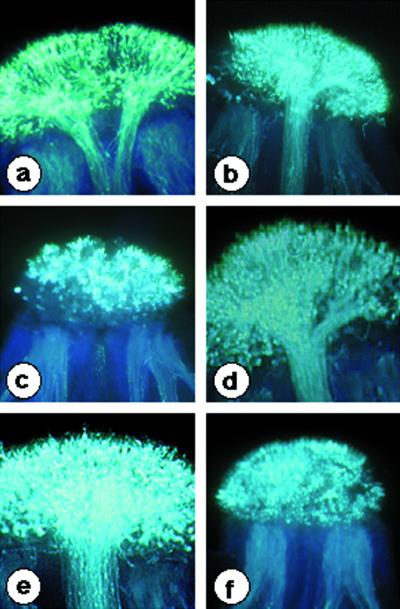
To investigate the effect of the introduced SP11 transgenes on the SI phenotype of the transgenic plants, pollination tests were reciprocally performed with three lines of B. rapa; S8 homozygotes, S9 homozygotes, and S52S60 heterozygotes (host strain). Pollen germination and pollen tube penetration in the style were examined under UV-fluorescence microscopy following staining with aniline blue. Pollen from all three lines of S8-SP11 transgenic plants (T66, T67, and T74) and two lines of S9-SP11 transgenic plants (T161 and T254) was incompatible with the stigma of the S52S60 host strain. In addition, pollen from the three lines of S8-SP11 transgenic lines was incompatible with the stigma of S8 homozygotes, whereas the wild-type host pollen was fully compatible with S8 stigmas (Fig. 7, c and a). The pollen of these S8-SP11 transgenic plants was compatible with an unrelated S haplotype, S9 (Fig. 7d), indicating that the incompatibility observed with S8 homozygotes did not result from a reduced viability of pollen. For the two lines of S9-SP11 transgenic plants, their pollen was incompatible with S9 stigmas (Fig. 7f), yet compatible with S8 stigmas (Fig. 7e). No phenotypic alterations were observed in the stigmas of all these S8-SP11 and S9-SP11 transgenic plants (data not shown).
Figure 7.
Representative results of pollination tests. Photographs were obtained by UV fluorescence microscopy. a, Cross-pollination of an S8S8 stigma with wild-type pollen (S52S60); b, cross-pollination of an S9S9 stigma with wild-type pollen; c, cross-pollination of an S8S8 stigma with transgenic pollen from T66 (S52S60 /S8-SP11); d, cross-pollination of an S9S9 stigma with transgenic pollen from T66; e, cross-pollination of an S8S8 stigma with transgenic pollen from T161 (S52S60 /S9-SP11); f, cross-pollination of an S9S9 stigma with transgenic pollen from T161.
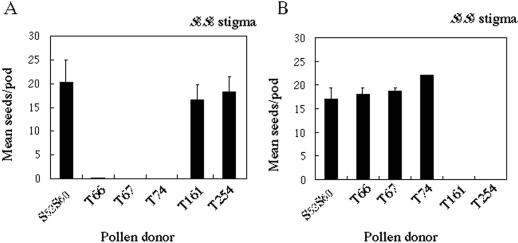
To ensure that the pollen behavior observed in the above pollination tests reflected the plant incompatibility phenotype, we examined the ability of these plants to produce seeds following reciprocal crosses. When pollen from the three S8-SP11-expressing transgenic lines was used to pollinate S8 stigmas, no seeds were produced (Fig. 8A); however, silique and ample seeds were produced when pollen from these three transgenic lines was used to pollinate S9 stigmas (Fig. 8B). Moreover, the stigma phenotype of the S8-SP11 transgenic plants was the same as that of the wild-type host plants (data not shown). For the two S9-SP11-expressing lines, no seeds were produced when their pollen was used to pollinate S9 stigma (Fig. 8B) and again, silique and ample seeds were produced when their pollen was used to pollinate S8 stigmas (Fig. 8A). These results demonstrate that SP11 is the determinant of the S-haplotype specificity of pollen in SI recognition.
Figure 8.
Seed set analyses. Data represent the mean number of seeds per pod after cross-pollination. A, S8S8 pistils pollinated with transgenic pollen; B, S9 S9 pistils pollinated with transgenic pollen. T66, T67, and T74, S52S60 /S8-SP11; T161 and T254, S52S60 /S9-SP11. Error bars indicate ±se.
DISCUSSION
In this report we introduced two SP11 transgenes, S8-SP11 and S9-SP11, into SI B. rapa S52S60 heterozygotes to examine the effect of the transgenes on SI behavior. Pollen from S8-SP11 and S9-SP11 transformants was shown to acquire the S8- and the S9-haplotype specificity, respectively. Our results, together with the previously reported SCR gene transformation in cauliflower (Schopfer et al., 1999), have definitively established that SP11/SCR is the sole male determinant of SI in the genus Brassica.
Our previous in situ hybridization analysis has revealed that SP11/SCR is expressed in the tapetal cells at early developmental stages and in the microspores at late stages (Takayama et al., 2000b). This sporophytic/gametophytic expression pattern is quite unique to SP11/SCR when compared with genes encoding other PCP family of proteins, PCP-A and PCP-B classes (Doughty et al., 1998, 2000; Takayama et al., 2000a), and can explain why SI phenotype of pollen is sporophytically determined in Brassica sp. In this study we have analyzed the 5′-upstream sequences of five alleles of the SP11 gene from B. rapa or oilseed rape. Alignment of these sequences suggested that the immediate upstream region within 200 bp of the coding region is highly conserved; these sequences share 69.4% to 88.0% identity with one another. However, no common repeat or palindromic sequence that could potentially play a role in regulation of gene expression was identified within this region. The region beyond 200 bp upstream of the coding region is not conserved among these alleles. This region does not appear to have any conserved sequence element either. In the promoter region of the PCP1 gene, the first reported PCP-A class gene from cauliflower, two direct repeats (TTTTAGATTATAAA) and a putative pollen-specific element (CTTAAATTAGA), were identified (Stanchev et al., 1996). However, these sequences were not found in the 5′-flanking region of the SP11 gene.
Although no known regulatory element was found in the promoter region of the SP11 gene, the analysis of the S8-SP11 gene transformants indicated that the 522 bp of the S9-SP11 promoter region used to drive the transgene is sufficient to drive proper SP11 gene expression. Thus, this region must contain all the regulatory elements required for the unique expression of the SP11 gene. Transient expression analyses using truncated SP11 promoter-gus fusions allowed us to examine the active elements involved in this promoter region. Our results revealed that the region around −192 bp contains the element(s) required for GUS expression in the tapetum, and that the region between −124 and −143 represents the minimal promoter region for pollen expression. Tissue-specific expression is thought to be the result of combinatorial regulation by specific sets of regions present in the promoter. For example, the tapetum and pollen expression of Bcp1 was controlled by different cis-acting elements that act in conjunction with common cis-acting elements to confer the expression in the tapetum or pollen (Xu et al., 1993). Our results also suggest that the sporophytic and gametophytic expression patterns of the SP11/SCR gene are controlled by different cis-regulatory elements.
Immunolocalization analyses of S8-SP11 protein revealed that this protein was specifically localized to the pollen coat when pollen grains reached the trinucleate stage. The pollen coat is the outermost layer of a pollen grain and makes the initial contact with the stigma surface during sexual reproduction. Therefore, the localization of SP11 is consistent with its biological role as an S-determinant. Although the pollen coating has been considered to be derived principally from the tapetal cells that line the anther locule, the origin of SP11 protein in the pollen coat (whether derived from the tapetal cells and/or the pollen grain) remains unclear (Doughty et al., 1998). Further transformation experiments introducing the SP11 gene under the control of a pollen-specific or tapetum-specific promoter will likely provide the answer to this question.
MATERIALS AND METHODS
Plant Material
Brassica rapa (syn. campestris) S8 and S9 homozygotes were established from spontaneous populations at Oguni in Japan as previously described (Hinata and Nishio, 1978). B. rapa cv Osome (Takii Seed Co., Kyoto), a commercial F1 hybrid of the S52S60 haplotype (Takasaki et al., 1997), was used as the recipient of transgenes. S8, S9, and S52 belong to class I S-haplotypes and S60 belongs to class II S-haplotypes (Hatakeyama et al., 1998; Takasaki et al., 2000). The dominance/recessive relationships among S8, S9, S52, and S60 are as follows. S52 is codominant with S9 and dominant over S8 and S60 in the stigma; S9 is dominant over S8 and S60 in the stigma; S8 is dominant over S60 in the stigma; S52 is codominant with S9 and S8 and is dominant over S60 in pollen; S9 and S8 are dominant over S60 in pollen. Agrobacterium tumefaciens strain EHA105 was a kind gift of Prof. Atsuhiko Sinmyo (Nara Institute of Science and Technology, Ikoma, Japan).
Transient Expression Vectors and Promoter Deletion Constructs
A cassette containing the gus coding region followed by the nopaline synthase polyadenylation signal from pBI221 (CLONTECH, Palo Alto, CA) was used to construct promoter-gus fusions. The 522-bp S9-SP11 5′-flanking region was obtained from a P1-derived artificial chromosome clone, E89 (Suzuki et al., 1997), and the 1,421-bp fragment containing the 5′-flanking region of the BrPCP-A1 gene (Takayama et al., 2000b) was isolated from genomic DNA of B. rapa S12 homozygote. These fragments were re-amplified by PCR using specific primers designed to add HindIII and BamHI restriction sites to the 5′ and 3′ ends, respectively (for the amplification of the 5′-flanking region of the S9-SP11 gene: sense primer 5′-GAAGCTTGGTACATTAACTATGTCT-3′, antisense primer 5′-GGATCCGCGAGTCAACGAGATTAACGGGTC-3′; for the amplification of 5′-flanking region of the BrPCP-A1 gene: sense primer 5′-GAAGCTTCTACACTAGATCAATGGCAA-3′, antisense primer 5′-CCCGGGAACCGTGTTTTTCATCTTAG-3′). The amplified fragments were subcloned into pBI221 to yield the 522-bp SP11 promoter-gus and 1,421-bp PCP-A1 promoter-gus constructs, respectively.
A series of deletion constructs was generated from the 522-bp SP11 promoter-gus construct by Exonuclease III and religation, following the protocol supplied with the kilo-sequence deletion kit (Takara, Shiga, Japan). All seven constructs were sequenced to confirm the absence of any possible mutation introduced during PCR. All plasmid DNA was prepared using the Plasmid Midi kit (Qiagen, Valencia, CA), following the manufacturer's instructions.
Particle Bombardment
Developmental stages of anther were classified according to bud length as previously described (Takayama et al., 2000b). Immature buds at developmental stage 5 (bud length: 4–5 mm) from B. rapa S9 homozygote were cut transversely into approximately 1-mm sections. Thirty sections per plate (100 mm in diameter) were placed onto a filter paper (Toyo Roshi, Tokyo) immersed in sterilized water. Plasmid DNA (approximately 0.5 μg) was precipitated onto 1.0-μm gold particles (Bio-Rad, Hercules, CA) according the manufacturer's instructions. Bombardments were performed using a Biolistic particle acceleration device (PDS 1000/He, Bio-Rad). Three shots were performed per plate. One day after bombardment, the sections from each plate were incubated for 16 h at 37°C in 50 mm sodium phosphate buffer, pH 7.0, containing 1 mg mL−1 5-bromo-4-chloro-3-indolyl-β-d-glucuronic acid (Wako, Osaka). The samples were embedded in 2% (w/v) agar and then cut into 150-μm sections with a Microslicer (D. S. K., Osaka), and were observed by microscopy (Axiophot 2, Zeiss, Jena, Germany).
Construction of S8-SP11 and S9-SP11 Transgenes
The full length S8-SP11 cDNA (Takayama et al., 2000b) was amplified by PCR using SP11-specific primers 5′-CCCGGGATGAAATCGGCTGTTTATGC-3′ (underlined sequence indicating the incorporated SmaI site) and 5′-GAGCTC GATAGCATTTGCTAACAC-3′ (underlined sequence indicating the incorporated SacI site), and inserted into pGEM vector (Promega, Madison, WI). The chimeric gene, consisting of the 522-bp promoter region of S9-SP11, the coding region of S8-SP11 cDNA and the nopaline synthase transcription terminator, was inserted into binary vector, pSLJ1006 (Jones et al., 1992), to create pSLJS8-SP11. A 2.3-kb fragment of S9-SP11 genomic DNA was amplified by PCR using the E89 DNA (Suzuki et al., 1997) as a template and primers that had been designed based on the 5′-non-coding sequence of the S9-SP11 gene (5′-GAAGCTCCAGTACACCTGCTCAGTCATAGATG-3′, with the underlined sequence indicating the incorporated HindIII site) and the 3′-non-coding sequence of the S9-SP11 gene (5′-GAAGCTCGTTCACATGGATCAACATCTACCGG -3′, with the underlined sequence indicating the incorporated HindIII site). The DNA fragment obtained was digested with HindIII and inserted into the corresponding sites of binary vector, pSLJ1006 (Jones et al., 1992), to create pSLJS9-SP11.
Transformation
The plasmids pSLJS8-SP11 and pSLJS9-SP11 were electroporated into A. tumefaciens strain EHA105 (Hood et al., 1993). The hypocotyl transformation of B. rapa cv Osome with Agrobacterium harboring pSLJS8-SP11 or pSLJS9-SP11 and the subsequent regeneration of transgenic plants was performed as previously described (Shiba et al., 2000). To ascertain the presence of the SP11 transgenes, leaf pieces from the transgenic plants were examined for GUS staining using 5-bromo-4-chloro-3-indolyl-β-d-glucuronic acid and was analyzed by PCR amplification using SP11-specific primers.
RNA Gel-Blot Analyses
Total RNA was isolated from stage 7 anthers (bud length: 7–10 mm) using Isogen (Nippon Gene, Toyama, Japan). Fifteen micrograms of total RNA was electrophoresed on a 1.2% (w/v) agarose/formamide gel and transferred to a Gene-screen Plus membrane (DuPont/NEN, Boston). The membrane was hybridized at 60°C for 12 h with random-prime-32P-labeled S8-, S9-, and S52-SP11 gene probes specific for the coding region of the mature protein and the 3′-non-coding region. After hybridization, the membrane was washed in 0.1× SSPE, 2% (w/v) SDS at 60°C for 30 min and exposed on x-ray film (Amersham Pharmacia Biotech, Piscataway, NJ). Equal loading of total RNA was assessed by ethidium bromide staining of rRNA bands.
Immunocytochemistry
To produce recombinant S8-SP11 protein, the cDNA of mature S8-SP11 coding region was amplified using specific primers (5′-CGGAATTCCATCGAAGGTCGTCAAGAACTGGAAGCTAATCT-3′, with the underlined sequence indicating the incorporated EcoRI site, and 5′-GCTCGAGTCTAACACGATTTACAGTCACAG-3′, with the underlined sequence indicating the incorporated XhoI site), and inserted into pGEX-5X-3 vector (Amersham Pharmacia). This construct was transformed into Escherichia coli strain BL21. The induction and purification of the recombinant glutathione S-transferase (GST)-S8-SP11 fusion protein was performed according to the manufacturer's protocol. This fusion protein was used to immunize rabbits. To remove non-specific antibodies that might react with the GST-domain of the fusion protein, the crude serum was first absorbed with N-hydroxysuccinilamide-activated Sepharose resin (Amersham Pharmacia Biotech) to which the GST-S9-SP11 fusion protein had been covalently attached (Takayama et al., 2000b). The S8-SP11-specific antibody was then affinity purified on the same resin with the GST- S8-SP11 fusion protein also attached to it.
Anthers at developmental stages 5 and 7 were collected and 10 μm of Paraplast (Sigma, St. Louis) sections were prepared as described by Doughty et al. (1998). Prior to incubation with the primary antibody, sections were blocked with 1% (w/v) non-fat dry milk (NFM) and 1% (v/v) goat serum in Tris-buffered saline (TBS) at 37°C for 30 min. The purified S8-SP11 antibody was diluted 1:100 in TBS supplemented with NFM and goat serum. Following incubation with the primary antibody at 37°C for 1 h, sections were washed with 0.05% (v/v) Tween 20 in TBS (TBST, pH 7.4). The secondary antibody, alkaline phosphatase-conjugated goat anti-rabbit IgG (Biocell Research Laboratories, Cardiff, UK), was diluted 1:100 in TBST. Sections were incubated with the secondary antibody at 37°C for 1 h, then washed in TBST and distilled water. Detection of the alkaline phosphatase activity was performed using 4-nitroblue tetrazolium chloride and 5-bromo-4-chloro-3-indolyl phosphate, according to the manufacturer's instructions (Sigma). In control experiments, the primary antibody was either omitted or replaced with preimmune rabbit serum.
Immunoelectron Microscopy
Immunoelectron microscopy was performed as described by Seo et al. (2000) except that anthers of the late developmental stage 7 were cut in halves and fixed with 4% (v/v) paraformaldehyde and 0.1% (v/v) glutaraldehyde in cacodylate buffer (pH 7.4) under vacuum. After dehydration, the tissues were embedded in Resin LR White (London Resin Co., London) at −20°C. Ultra-thin sections were incubated with the anti-S8-SP11 antibody (1:100 dilution in PBS-NFM) overnight at 4°C and then incubated with 20-nm (in diameter) gold-conjugated goat anti-rabbit IgG (dilution 1:100 in PBS buffer, Biocell Research Laboratories) for 1 h at 25°C. After immunolabeling, the sections were stained with uranyl acetate. As a control, specimens were incubated with preimmune rabbit serum. Samples were observed in a Hitachi transmission electron microscope (H-7100; Hitachi, Tokyo).
Pollination Assay
Fresh flowers collected at the day of anthesis were used for the pollination assay. Hand-pollinated pistils were cut at the peduncle, stood on 1% (w/v) solid agar, and kept at 20°C for 6 h. After fixation for 2 h in ethanol:acetic acid (3:1), the pistils were softened in 1 n NaOH at 60°C for 1.5 h, and stained with 0.01% (w/v) decolorized aniline blue for 2.5 h in 2% (w/v) K3PO4. Pistils were gently squashed onto a microscopic slide glass by placing the cover glass over the pistils. Samples were examined under a fluorescence microscope (Axiophot 2, Zeiss) with an excitation filter of 395 nm and an emission filter of 420 nm (Dumas and Knox, 1983).
ACKNOWLEDGMENTS
We thank Dr. K. Sakamoto (Takii Seed Company) and Dr. K. Hatakeyama (Research Institute of Seed Production) for the generous gift of B. rapa cv Osome. We acknowledge the technical help of T. Nakanishi, A. Arai, K. Fujii, H. Sato, K. Iwasaki, and T. Ueda.
Footnotes
This work was supported in part by Grants-in-Aid for Special Research on Priority Areas B (no. 11238025), Scientific Research B (nos. 0948015 and 11460056), and Scientific Research on Priority Areas (c; “Genome Biology”) from the Ministry of Education, Science, Sports and Culture, Japan.
LITERATURE CITED
- Bi Y-M, Brugière N, Cui Y, Goring DR, Rothstein SJ. transformation of Arabidopsis with a Brassica SLG/SRK region and ARC1 gene is not sufficient to transfer the self-incompatibility phenotype. Mol Gen Genet. 2000;263:648–654. doi: 10.1007/s004380051213. [DOI] [PubMed] [Google Scholar]
- Doughty J, Dixon S, Hiscock SH, Willis AC, Parkin IAP, Dickinson HG. PCP-A1, a defensin-like Brassica pollen coat protein that binds the S locus glycoprotein, is the product of gametophytic gene expression. Plant Cell. 1998;10:1333–1347. doi: 10.1105/tpc.10.8.1333. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Doughty J, Wong HY, Dickinson HG. Cysteine-rich pollen coat proteins (PCPs) and their interactions with stigmatic S (incompatibility) and S-related proteins in Brassica: putative roles in SI and pollination. Ann Bot. 2000;85:161–169. [Google Scholar]
- Dumas C, Knox RB. Callose and determination of pistil viability and incompatibility. Theor Appl Genet. 1983;67:1–10. doi: 10.1007/BF00303914. [DOI] [PubMed] [Google Scholar]
- Hatakeyama K, Watanabe M, Takasaki T, Ojima K, Hinata K. Dominance relationships between S-alleles in self-incompatible Brassica campestris L. Heredity. 1998;80:241–247. [Google Scholar]
- Hinata K, Nishio T. S-allele specificity of stigma proteins in Brassica oleracea and B. campestris. Heredity. 1978;41:93–100. [Google Scholar]
- Hood EE, Gelvin SB, Melchers LS, Hoekema A. New Agrobacterium helper plasmids for gene transfer to plants. Transgenic Res. 1993;2:208–218. [Google Scholar]
- Jones JDG, Shlumukov L, Carland F, English J, Scofield SR, Bishop GJ, Harrison K. Effective vectors for transformation, expression of heterologous genes, and assaying transposon excision in transgenic plants. Transgenic Res. 1992;1:285–297. doi: 10.1007/BF02525170. [DOI] [PubMed] [Google Scholar]
- Kandasamy MK, Paolillo DJ, Faraday CD, Nasrallah JB, Nasrallah ME. The S-locus specific glycoproteins of Brassica accumulate in the cell wall of developing stigma papillae. Dev Biol. 1989;134:462–472. doi: 10.1016/0012-1606(89)90119-x. [DOI] [PubMed] [Google Scholar]
- McCubbin AG, Kao T-h. Molecular recognition and response in pollen and pistil interactions. Annu Rev Cell Dev Biol. 2000;16:333–364. doi: 10.1146/annurev.cellbio.16.1.333. [DOI] [PubMed] [Google Scholar]
- Nettancourt DD. Incompatibility in Angiosperms. Berlin: Springer-Verlag; 1977. pp. 1–230. [Google Scholar]
- Schopfer CR, Nasrallah ME, Nasrallah JB. The male determinant of self- incompatibility in Brassica. Science. 1999;286:1697–1700. doi: 10.1126/science.286.5445.1697. [DOI] [PubMed] [Google Scholar]
- Seo S, Okamoto M, Iwai T, Iwano M, Fukui K, Isogai A, Nakajima N, Ohashi Y. Reduced levels of chloroplast RtsH protein in tobacco mosaic virus-infected tobacco leaves accelerate the hypersensitive reaction. Plant Cell. 2000;12:917–932. doi: 10.1105/tpc.12.6.917. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shiba H, Hinata K, Suzuki A, Isogai A. Breakdown of self-incompatibility in Brassica by the antisense RNA of SLG gene. Proc Japan Acad. 1995;71:81–83. [Google Scholar]
- Shiba H, Kimura N, Takayama S, Hinata K, Suzuki A, Isogai A. Alteration of the self-incompatibility phenotype in Brassica by transformation of the antisense SLG gene. Biosci Biotechnol Biochem. 2000;64:1016–1024. doi: 10.1271/bbb.64.1016. [DOI] [PubMed] [Google Scholar]
- Stanchev BS, Doughty J, Scutt CP, Dickinson H, Croy RD. Cloning of PCP1, a member of a family of pollen coat protein (PCP) genes from Brassica oleracea encoding novel cysteine-rich proteins involved in pollen-stigma interactions. Plant J. 1996;10:303–313. doi: 10.1046/j.1365-313x.1996.10020303.x. [DOI] [PubMed] [Google Scholar]
- Stein JC, Howlett B, Boyes DC, Nasrallah ME, Nasrallah JB. Molecular cloning of a putative receptor protein kinase gene encoded at the self-incompatibility locus of Brassica oleracea. Proc Natl Acad Sci USA. 1991;88:8816–8820. doi: 10.1073/pnas.88.19.8816. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Suzuki G, Kai N, Hirose T, Nishio T, Takayama S, Isogai A, Watanabe M, Hinata K. Genomic organization of the S locus: identification and characterization of genes in SLG/SRK region of S 9 haplotype of Brassica campestris (syn. rapa) Genetics. 1999;153:391–400. doi: 10.1093/genetics/153.1.391. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Suzuki G, Watanabe M, Toriyama K, Isogai A, Hinata K. Direct cloning of the Brassica S locus by using a P1-derived artificial chromosome (PAC) vector. Gene. 1997;199:133–137. doi: 10.1016/s0378-1119(97)00358-2. [DOI] [PubMed] [Google Scholar]
- Takasaki T, Hatakeyama K, Ojima K, Watanabe M, Toriyama K, Hinata K. Factors influencing Agrobacterium-mediated transformation of Brassica rapa. L Breed Sci. 1997;47:127–134. [Google Scholar]
- Takasaki T, Hatakeyama K, Suzuki G, Watanabe M, Isogai A, Hinata K. The S receptor kinase determines self-incompatibility in Brassica stigma. Nature. 2000;403:913–916. doi: 10.1038/35002628. [DOI] [PubMed] [Google Scholar]
- Takayama S, Isogai A, Tsukamoto C, Ueda Y, Hinata K, Okazaki K, Suzuki A. Sequences of S-glycoproteins, products of the Brassica campestris self-incompatibility locus. Nature. 1987;318:263–267. [Google Scholar]
- Takayama S, Shiba H, Iwano M, Asano K, Hara M, Che F-S, Watanabe M, Hinata K, Isogai A. Isolation and characterization of pollen coat proteins of Brassica campestris that interact with S locus-related glycoprotein 1 involved in pollen-stigma adhesion. Proc Natl Acad Sci USA. 2000a;97:3765–3770. doi: 10.1073/pnas.040580797. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Takayama S, Shiba H, Iwano M, Shimosato H, Che F-S, Kai N, Watanabe M, Suzuki G, Hinata K, Isogai A. The pollen determinant of self-incompatibility in Brassica campestris. Proc Natl Acad Sci USA. 2000b;97:1920–1925. doi: 10.1073/pnas.040556397. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Watanabe M, Ito A, Takada Y, Ninomiya C, Kakizaki T, Takahata Y, Hatakeyama K, Hinata K, Suzuki G, Takasaki T. Highly divergent sequences of the pollen self-incompatibility (S) gene in class-I S haplotypes of Brassica campestris (syn. rapa) L. FEBS Lett. 2000;473:139–144. doi: 10.1016/s0014-5793(00)01514-3. [DOI] [PubMed] [Google Scholar]
- Xu H, Davies SP, Kwan BYH, O'Brien AP, Singh M, Knox RB. Haploid and diploid expression of a Brassica campestris anther-specific gene promoter in Arabidopsis and tobacco. Mol Gen Genet. 1993;239:58–65. doi: 10.1007/BF00281601. [DOI] [PubMed] [Google Scholar]