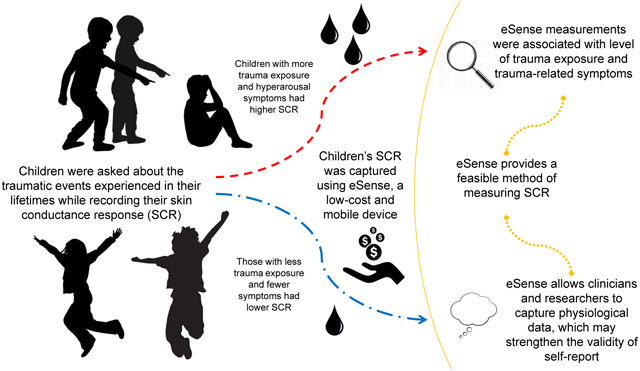
Abstract
Although many children experience trauma, few receive diagnoses and subsequent care despite experiencing trauma-related sequelae. At age nine (M = 9.11), children (N = 62; female = 46.4%) who predominantly identified as Black (78.7%) were enrolled in this first study examining how skin conductance as captured by mobile technology, eSense, related to children’s traumatic experiences and trauma-related symptoms. Skin conductance measures were associated with degree of trauma exposure and PTSD hyperarousal symptoms. These findings suggest that physiological responses in addition to self-report measures may be easily used to assess children’s trauma exposure and symptoms. Given eSense’s ease-of-use, this technology could assist clinics and research institutions assess children’s trauma-related needs.
Keywords: assessment/diagnosis, child/adolescent, computer/internet technology, cost-effectiveness, ethnicity/race, life events/stress, PTSD/Posttraumatic Stress Disorder, trauma
Graphical Abstract
Introduction
Traumatic experiences are common for children and adolescents. Recent studies indicate that approximately 60% of children experience at least one traumatic event before the age of eighteen, and many will experience more (McLaughlin et al., 2013; Tedeschi & Billick, 2017). Although trauma exposure is prevalent, few children are diagnosed with posttraumatic stress disorder (PTSD). Recent literature has suggested that only 9% of children receive a PTSD diagnosis (Garza & Jovanovic, 2017; McLaughlin et al., 2013). This discrepancy between prevalence of trauma exposure and PTSD diagnoses may indicate that many children are resilient, while others may be still struggling with trauma-related symptoms such as heightened physiological responses even without meeting full DSM-5 criteria (Gray et al., 2018). Reliable and effective methods of capturing heightened responses would allow professionals working with youth to better assess the symptomatology of children exposed to trauma to address those who are struggling with trauma-related symptoms without meeting the criteria of a PTSD diagnosis (Crockett, Gill, Cashwell, & Myers, 2017; Gray et al., 2018).
Self-reported PTSD symptoms in both children and adults are often subjective (Cody et al., 2017; Russo & Fingerhut, 2017); adding objective methods can assist in assessment of symptom severity. . Objective measures, such as the assessment of the physiological response associated with pediatric PTSD is emerging as a field that may improve diagnostic accuracy (Gray et al., 2018). Therefore, self-report in conjunction with psychophysiological measures could provide additional information and a more complete evaluation of symptoms. For example, Jenness and colleagues (2019) demonstrated that high vagal tone was associated with less severe PTSD symptoms among abused youth. A study of children admitted to the emergency room found that those with higher heart rates at the time of admission had greater PTSD symptoms both six-months and twelve-months after admission (Kirsh et al., 2011). Continued identification of biomarkers that identify youth with PTSD symptoms could lead to more targeted and nuanced treatments.
Skin conductance level (SCL) may contribute important information to assessment of pediatric PTSD as it is a direct index of sympathetic nervous system (SNS) activation (Gamwell et al, 2015). Recent studies have contextualized associations between psychopathology and skin conductance as an expression of physiological arousal and fear response. Skin conductance response (SCR) is typically defined as a change in SCL evoked by arousing stimuli, such as conditioned threat cues, affective images, or emotional scripts. As a marker of sympathetic arousal, SCR is increased with both negatively and positively valanced pictures, and is associated with general distress as well as specific trauma memories (Lang et al., 1988). Work in adults with PTSD has used script-driven imagery methods and shown that trauma-related scripts are more potent in evoking SCR compared to more general distress (Pitman et al., 1987). The research in children with trauma exposure has been relatively limited with respect to skin conductance measures, and most work has focused on fear conditioning paradigms, where children learn to associate a threat cue with an aversive outcome. Children who have experienced maltreatment may display a unique, blunted SCR to threat cues during fear conditioning (McLaughlin et al., 2016). Further, intrusive PTSD symptoms in boys and fear of repeated trauma and self-blame in girls may also be correlated with SCR (Gamwell et al., 2015). Beyond identification of trauma-related symptoms, heightened SCR may be a risk factor for pediatric psychopathology broadly. After controlling for confounding variables, SCR during observation of a simulated argument predicted internalizing problems for children in general, and externalizing and cognitive problems for girls in particular (El-Sheikh et al., 2008). These findings demonstrate the potential for SCR as a tool for distinguishing psychopathology and emerging symptoms within children and adolescents.
However, these prior studies have employed large, expensive equipment to measure skin conductance, and required significant training of research staff to administer and interpret the paradigms. This equipment is also burdensome for participants who undergo lengthy procedures within a specific location, typically a research laboratory. Recent technological advancements have created novel techniques for measuring SCL through a low-cost, mobile skin conductance recording app on a tablet. This technology has been validated with gold standard Biopac system equipment, and shown that SCR (i.e. change from baseline SL to the maximum SCL during a standard trauma interview) predicted PTSD symptoms within adult populations (Hinrichs et al., 2017). Hinrichs and colleagues (2017) found that the magnitude of SCR acquired using this mobile technology during a standardized trauma interview was greater in those with PTSD compared to those with trauma but without PTSD. They also identified a positive association between SCR and severity of PTSD symptoms. A later study using the same technology examined the potential of SCR collected immediately after trauma exposure to predict a chronic PTSD disease trajectory (Hinrichs et al., 2019). SCR collected with the app in the Emergency Department was the strongest predictor of an individual being classified to the chronic symptom trajectory over the following 12 months, over and above other known risk factors, such as childhood trauma exposure. SCR may reflect underlying neuroendocrinology of stress responses, such as the sympathetic-adrenal-medullary (SAM) axis, which may be dysregulated by early life stress (Heim et al., 2019). The SAM axis produces epinephrine from the adrenal glands, generating a rapid “fight-or-flight” SNS activation that includes increased SCR (Ulrich-Lai & Herman, 2009); trauma exposure and other adversity in childhood can lead to long-term alterations in sympathetic arousal that increase risk for PTSD (Heim & Nemeroff, 2009). Thus, measuring skin conductance in children with trauma exposure can help with early identification of at-risk individuals.
This novel mobile SCL collection method has not yet been used with trauma-exposed youth. However, a few recent studies support its feasibility and sensitivity to mental health outcomes. For example, a study protocol showed preliminary feasibility in collecting SCL data using the eSense app in Hong Kong youth (Gomez et al., 2018). Another recent study using eSense SCL data collection in youth with bowel disease found that SCR to a stressful experience moderated the relations between stressful life events and depression symptoms (Cushman et al., 2021). However, neither of these studies evaluated the association of mobile SCL data to trauma exposure and trauma-related symptoms in youth.
The current study builds on this prior research by examining associations between SCL measures, as assessed by this low-cost, mobile skin conductance recording method, and children’s reports of traumatic experiences and trauma-related distress. The study had three primary aims. First, examine the feasibility of mobile data collection in detecting changes in SCL of children during a trauma interview. Second, probe the association between SCL measures and the degree of trauma exposure in children. Third, assess whether SCL measures were associated with children’s PTSD hyperarousal symptoms. Hyperarousal symptoms were of particular interest as they have been associated with skin conductance in previous studies with children (El-Sheikh et al., 2008) and physiological responses in adults (Jovanovic et al., 2010). We hypothesized that children who reported experiencing more traumatic events would have greater changes in SCL (i.e. higher SCR), which would be also be associated with more severe hyperarousal symptoms.
Methods
Participants
Children (n = 62) were recruited from the metro Detroit area through in-person and online community recruitment to participate in a three-year longitudinal study. The average age of the participants at the time of data collection was nine years old (M = 9.11, SD = 0.37). Approximately half of the participants (46.4%) identified as female, and 61 participants provided self-identified race and ethnicity information. Of those, 78.7% identified as Black, 13.1% identified as White, and 8.2% identified as Other. A few of the participants identified as Hispanic or Latinx (4.9%), with the majority (95.1%) identifying as non-Hispanic. Participants included in the current study completed the baseline visit of the longitudinal study which is still ongoing. Inclusion criteria were age 9 at start of the study and being able and willing to participate, and the exclusion criteria were autism spectrum disorder and cognitive disability. Caregivers were required to demonstrate proof of guardianship of the child before enrollment, and provided written informed consent for themselves and their child. Children provided verbal assent. All study procedures were approved by the Wayne State University Institutional Review Board.
Assessments
Trauma exposure.
The Traumatic Events Screening Inventory-Child report (TESI-C) was administered verbally to child participants to determine the number of traumatic events experienced during the child’s lifetime (Ghosh-Ippen et al., 2002). The TESI-C is a semi-structured interview comprised of 24-items assessing exposure to a variety of traumatic experiences, including domestic and community violence, injuries, natural disasters, sexual abuse, and verbal abuse. If a participant endorsed a qualifying event, further assessment included detailed information about the event, such as age of the child when the event occurred, who was involved in the event, and severity of the event. In the current study, the total number of traumatic events experienced by the children were included in analyses. This total did not include events that children reported either witnessing or confronting.
PTSD Hyperarousal symptoms.
The University of California Los Angeles Post-Traumatic Stress Disorder Reaction Index (UCLA-RI) was administered verbally by trained research staff to child participants to assess trauma-related PTSD symptoms (Steinberg et al., 2004). The UCLA-RI has been used extensively across a variety of trauma types, age ranges, settings, and cultures. The UCLA-RI consists of 27 statements assessing frequency symptoms experienced within the last month using a five-point frequency scale (0 = None of the Time, 1 = Some of the time, 2 = Little of the time, 3 = Some of the time, 4 = Much of the time, 5 = Most of the time). Given the association between skin conductance and sympathetic arousal, this study primarily examined the hyperarousal symptoms reported by children on the cluster E total subscale. However, in order to test whether hyperarousal symptoms were uniquely associated with SCL beyond other PTSD symptoms, we controlled for scores on B, C, and D symptom clusters in a regression analysis.
Mobile SCL measurement
SCL was measured using the eSense skin conductance recording application (Mindfield Biosystems, Inc., Berlin, Germany) collected on an Apple iPad. Before conducting the interview, isotonic paste was applied to the child’s non-dominant index and middle fingers, and electrodes were attached to the fingers. The electrodes were then connected to the iPad using the audio connection input. eSense data were acquired at a sampling rate of 5Hz.
At the start of the session, a two-minute baseline SCL recording was collected during which the child participant was asked to sit quietly and remain calm. At the end of the baseline, the recording was stopped and the instructions for the TESI-C interview were read to the child. Then, eSense was re-started and SCL was continuously recorded during the interview. Any extraneous events that could impact the integrity of the data during the interview, such as loud noises or interruptions, were marked for later removal. Once the TESI-C was completed, the eSense recording was stopped, and the electrodes were removed from the child’s fingers.
This recording resulted in two data files: the baseline measurement and the trauma interview measurement, as in prior studies (Hinrichs 2017; 2019; Cushman et al., 2021). The two files were exported from the Apple iPad as .csv files for processing. Any artifacts in the data from extraneous events or disconnection of the electrodes from the skin were deleted. The last thirty seconds of the baseline recording was averaged to generate a baseline SCL value. The maximum SCL represented the peak value over the course of the TESI-C interview. As in prior work, the SCR for each participant was calculated by subtracting the average SCL value of the last thirty seconds of the baseline recording from the maximum SCL value during the trauma interview (Hinrichs et al., 2017; Cushman et al., 2021). In addition, to explore the participant’s sympathetic recovery by the end of the trauma interview, we derived a variable for SCL habituation, by subtracting the SCL during the last 30 seconds of the trauma interview from the maximum SCL. To calculate habituation time, the time of the maximum SCL value in the interview was subtracted from the time the interview ended. The skin conductance variables are listed in Table 1 along with the definitions.
Table 1:
Skin conductance variables derived from eSense and operational definitions. SCL=skin conductance level, SCR=skin conductance response
| eSense Variable | Definition |
|---|---|
| Baseline SCL | Average of last 30 seconds of SCL recording during quiet rest prior to interview, in μSiemens |
| Maximum SCL | Peak SCL during the trauma interview (TESI-C), in μSiemens |
| End SCL | Average of last 30 seconds of SCL recording of the trauma interview, in μSiemens |
| SCR | Maximum SCL during trauma interview minus Baseline SCL, in μSiemens |
| SCL Habituation | Maximum SCL during trauma interview minus End SCL, in μSiemens |
Data analysis plan
In order to first test whether SCL increased during the trauma interview, a repeated-measures ANOVA (RM-ANOVA) was performed with 3 levels: baseline SCL, maximum SCL during trauma, and SCL at the end of the trauma interview. Next, we tested associations between trauma exposure and hyperarousal with SCL measures (baseline, SCR, SCL habituation) using Pearson correlations. Finally, hierarchical regression analyses were performed to examine the extent to which the SCL measures were predicted by the self-reported trauma exposure and hyperarousal symptoms after controlling for demographic characteristics, such as sex, race, and age of the child. Baseline SCL was also included in the regression to account for individual differences in baseline driving changes in SCL. Data were analyzed using SPSS (v26 IBM Corp), and the α level was set to ≤ .05 for significance.
Results
Participant characteristics
Use of the eSense app was readily accepted by the study youth, as none had refused or discontinued the skin conductance recording. However, six participants had missing data due to experimenter error and were excluded from analyses (final sample n = 56; see Table 2). Although 5 of the 6 children with missing data self-identified as Black, there were no differences in proportion of available eSense SCL data (89.6% and 92.9% of Black and non-Black participants, respectively, χ2=.13, p=.72). Studies in adults have reported lower SCL levels in Black Americans compared to While Americans (Kredlow et al., 2017), therefore, we examined whether there were race related differences in the eSense SCL data. The baseline SCL did not differ between Black youth (M=4.89, SD=3.11 μS) and non-Black youth (M=4.67, SD=2.68 μS), p=.82. Most children reported experiencing at least one traumatic event (M = 1.43, SD = 1.23, range: 0-5.0) and an average PTSD total score on UCLA-RI of 26.21 (SD = 13.93, range: 2.0-59.0).
Table 2:
Participant demographic and clinical data.
| Variable | N = 56 |
|---|---|
| Age (SD) | 9.11 (.37) |
| Sex (%) | |
| Male | 30 (53.6%) |
| Female | 26 (46.4%) |
| Race (%) | |
| Self-identified as Black | 43 (76.8%) |
| Self-identified as White | 8 (14.3%) |
| Self-identified as Other | 5 (8.9%) |
| Ethnicity (%) | |
| Self-identified as Hispanic or Latinx | 3 (5.4%) |
| Not self-identified as Hispanic or Latinx | 53 (94.6%) |
| Trauma exposure (SD) | 1.43 (1.23) |
| PTSD Hyperarousal symptoms (SD) | 6.23 (4.66) |
| PTSD-RI Total Score (SD) | 26.21 (13.93) |
SCL to trauma interview
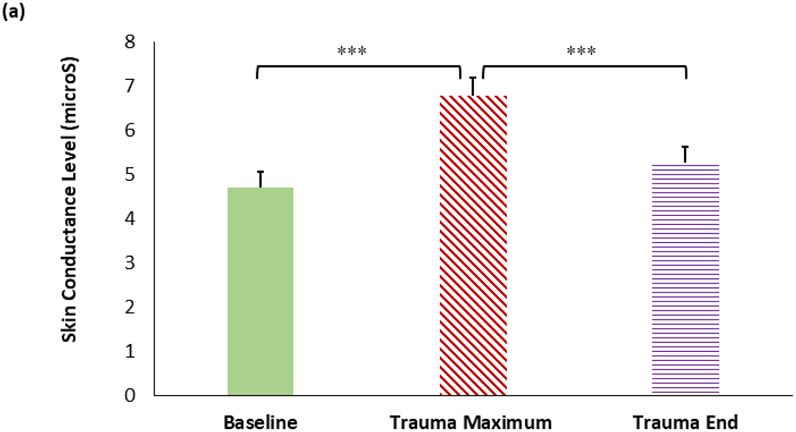
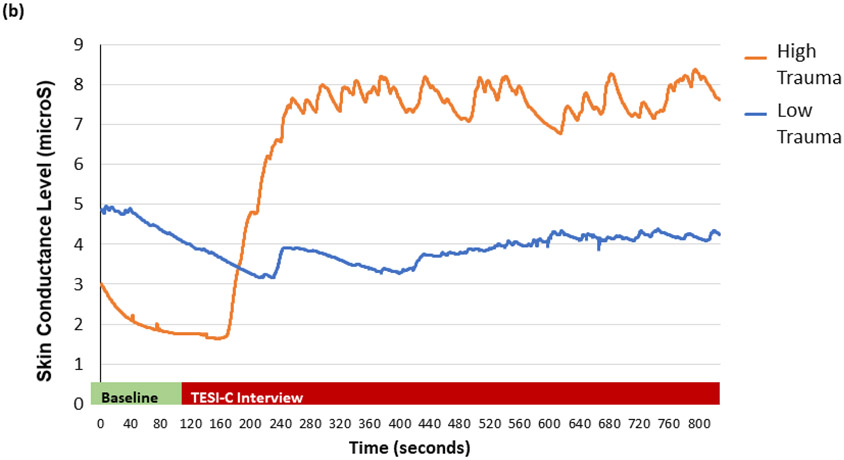
Results of the RM-ANOVA indicated that SCL changed across the recording segments (i.e. baseline, trauma maximum, trauma end), F(2,110)= 46.13, p < .001; see Figure 1a and 1b. Planned simple contrasts showed significant differences between timepoints. The maximum SCL value during the TESI-C interview was significantly higher than the baseline SCL value, F(1,55)= 68.08, p < .001. Further, there was a significant decrease in SCL from the maximum SCL value to the end SCL value, F(1,55) = 72.49, p < .001. These data show that the eSense SCL recording was highly sensitive to arousal elicited by the trauma interview and showed robust habituation.
Figure 1:
SCL varies greatly according to interview timepoint. (a) Mean SCL during interview baseline, maximum SCL, and mean SCL at the end of interview (±1 SE), F(2,110)= 32.07, p < .001. (b) Example eSense SCL of a child with high and low trauma exposure during the TESI-C interview. ***: p < .001
Trauma exposure and SCR
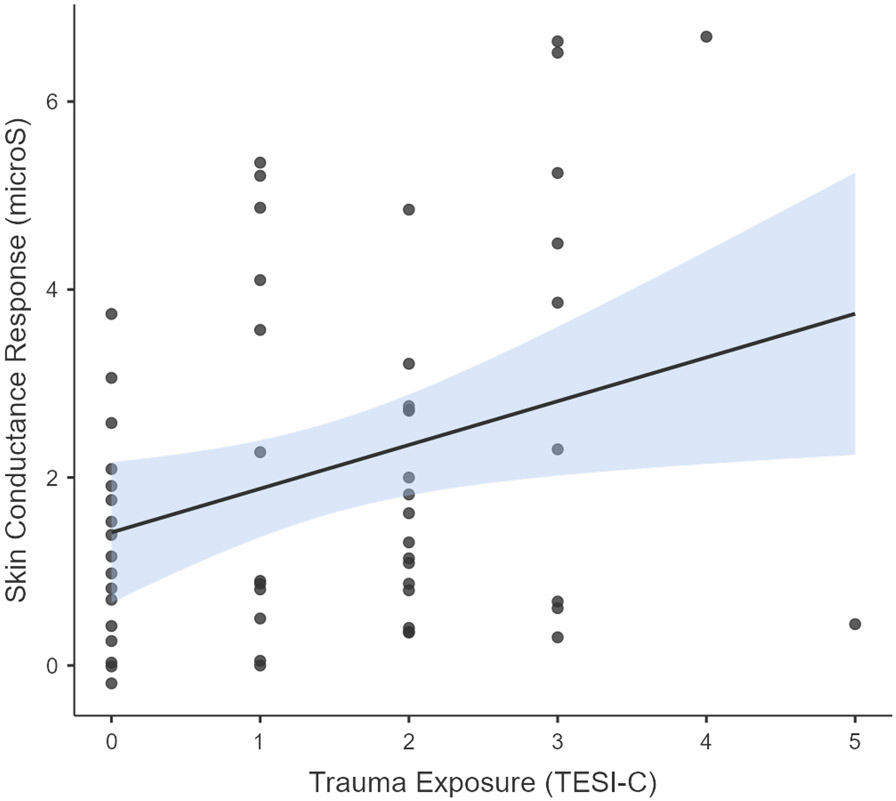
Degree of trauma exposure was significantly correlated with SCR (i.e. change in SCL from baseline to maximum SCL) during the TESI-C interview (r(55) = .30, p = .023, such that those who endorsed more traumatic events had greater SCR (see Figure 2a). A hierarchical linear regression was performed to predict SCR from sex, race, age, baseline SCL, and trauma exposure. The overall model predicting SCR, with demographic variables in Block 1, baseline SCL in Block 2, and trauma exposure in Block 3, was significant, F(5,55) = 2.87, R2 = .223, p = .024 (see Table 3a). Further, trauma exposure alone significantly predicted SCR after controlling for other variables, R2change = .129, F(1,50)change = 8.31, p = .006.
Figure 2:
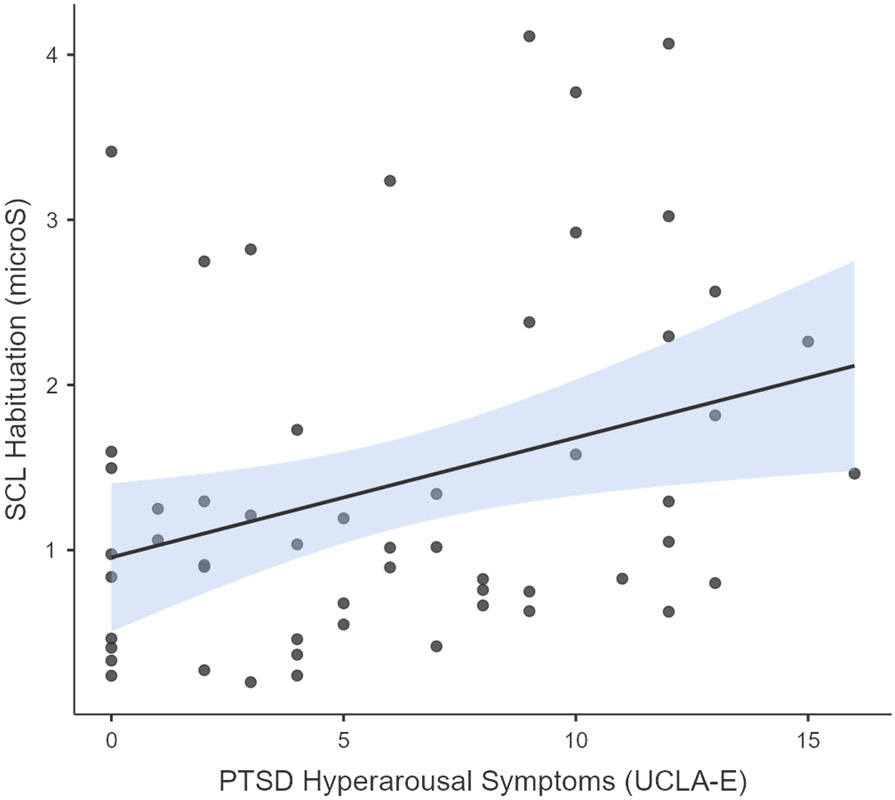
SCL relates to both trauma exposure and trauma-related symptoms. (a) SCR (trauma interview maximum minus baseline) was significantly correlated with the total number of traumatic events reported by children during the TESI-C interview, r(55) = .30, p = .023 (The jamovi project, 2021). (b) PTSD Hyperarousal symptoms as recorded on the UCLA-RI were significantly correlated with SCL habituation (trauma interview maximum minus trauma interview end) during the TESI-C interview, r(54) = .30, p = .016 (The jamovi project, 2021).
Table 3:
Trauma exposure and trauma-related symptoms predict SCL after controlling for other variables. (a) Regression analysis summary for trauma exposure predicting SCR after controlling for the effects of sex, race, age, and baseline SCL. (b) Regression analysis summary for hyperarousal symptoms predicting SC habituation after controlling for the effects of sex, race, age, and baseline SCL as well as trauma exposure and total PTSD symptom severity.
| (a) Dependent variable: SCR (Maximum SCL during trauma minus baseline SCL) | ||||
|---|---|---|---|---|
| Predictor Variable | B | SE B | β | p |
| Sex | .098 | .492 | .026 | .843 |
| Race | .160 | .574 | .036 | .782 |
| Age | −1.709 | .695 | −.331 | .017 |
| Baseline SCL | −.121 | .084 | −.184 | .155 |
| Trauma exposure | .593 | .206 | .388 | .006 |
| (b) Dependent variable: SCL Habituation (Maximum SCL during trauma minus SCL at end of trauma interview) | ||||
| Predictor Variable | B | SE B | β | p |
| Sex | −.036 | .299 | −.017 | .904 |
| Race | .139 | .362 | .054 | .703 |
| Age | −.458 | .393 | −.172 | .251 |
| Baseline SCL | .090 | .050 | .261 | .079 |
| Trauma exposure | .064 | .128 | .073 | .623 |
| PTSD clusters B, C, and D symptom severity | −.031 | .018 | −.319 | .102 |
| PTSD Hyperarousal symptoms (cluster E) | .133 | .044 | .537 | .005 |
Hyperarousal symptoms and SCL habituation
PTSD hyperarousal symptoms on the UCLA-RI were significantly correlated with magnitude of SCL habituation (r(54) = .30, p = .016), such that those with higher hyperarousal symptoms exhibited greater SCL habituation (see Figure 2b). Children with higher hyperarousal symptoms also had longer interviews (r(54) = .45, p < .001). However, the length of the interview was not correlated with the number of traumatic events experienced, or with SCL habituation (r = .24, p = .08; r = .21, p = .13 respectively). In addition, hyperarousal symptoms were not correlated with the maximum SCL value (r = .17, p = .20).
A hierarchical linear regression was performed to predict SCL habituation from demographic variables (Block 1), baseline SCL (Block 2), and hyperarousal symptoms (Block 3). The overall model was significant, F(5,54)= 2.99, R2 = .234, p = .020, and hyperarousal symptoms alone significantly predicted greater SCL habituation after controlling for other variables, R2change = .079, F(1,49)change = 5.03, p = .030. Because SCL habituation (i.e. maximum SCL during interview minus SCL at the end of the interview) was highly correlated with the maximum SCL (r(54) = .58, p < .001), we repeated the above hierarchical regression analysis while adding maximum SCL in Block 2 in order to assess whether the association between hyperarousal symptoms and habituation was accounted for by the maximum SCL. This model was significant, F(6,54)= 6.23, R2 = .438, p < .001, and hyperarousal symptoms still contributed significant variance in habituation after controlling for the maximum SCL value, R2change = .051, F(1,48)change = 4.31, p = .043.
A second hierarchical regression to test the unique association with hyperarousal symptoms was performed to control for the effect of the demographic variables in Block 1 and baseline SCL in Block 2, as well as trauma exposure and the sum of clusters B, C, and D PTSD symptom severity in Block 3. Hyperarousal symptoms (cluster E) were entered in Block 4, and the overall model again explained a significant percentage of the variance in SCL habituation (R2 = .328), F(7,46) = 2.72, p = .022 (see Table 3b). Additionally, hyperarousal symptoms uniquely predicted SCL habituation after controlling for the effect of the other variables and symptom clusters, R2change = .156, F(1,39)change = 9.03, p = .005.
Discussion
The current study found that SCL robustly increased during a trauma interview as compared to baseline SCL in children, using a mobile skin conductance recording app. The magnitude of the change in SCL (i.e. the SCR) elicited by the trauma interview was positively associated with the level of trauma exposure reported by the child. In addition, there was a significant positive correlation between SCL habituation and PTSD hyperarousal symptoms. SCR has been correlated with trauma-related symptoms in previous studies of adults (Orr et al., 1997a; Pineles et al., 2013; Pitman et al., 1987; Shalev et al., 1993), and a small number of studies have used eSense technology to record SCR in adults (Hinrichs et al., 2017; Hinrichs et al., 2019) and youth (Cushman et al 2021). However, this is the first study to our knowledge to use eSense to measure SCR in children with respect to trauma exposure. This study did not include a control condition to examine whether SCL would be similarly modulated by any distressing stimulus, therefore, it is unclear whether this measure of sympathetic arousal was specific to trauma memories. Studies using script-driven imagery in adults suggest that trauma-related imagery is more arousing than generally stressful events (Pitman et al., 1987); since the goal of the current study was primarily to establish feasibility and utility of this mobile measurement we wanted to use a low-burden approach. We found that this method 1) was very feasible, as data collection was completed on an iPad and averaged 15 minutes with over 90% of usable data; and 2) provided data supporting associations between skin conductance, trauma exposure and symptoms. These findings demonstrate that a low-cost, mobile measurement can capture increased sympathetic activity in traumatized children, potentially identifying those a higher risk for later psychopathology. Given that the majority of children with trauma exposure do not develop full criteria for PTSD, early identification of vulnerable individuals could focus intervention resources where they are most needed.
Another strength of the study is the inclusion of a primarily Black American children (76.8%). Lower levels of SCR have often resulted in Black Americans being classified as nonresponders and excluded from psychophysiological studies (Kredlow at el., 2017). Such demographic biases in physiological measurement potentially related to skin tone (Nelson et al., 2020) have resulted in a historical underrepresentation of non-White samples at the intersection of technology and health. However, disproportionally higher rates of trauma exposure in Black urban youth underscore the need for representation of this population in research studies of biomarkers of PTSD. This study provides useful preliminary data supporting validity of a new mobile skin conductance measurement app in a sample of predominantly Black children, although further research is minority populations is needed.
There are several additional advantages to using eSense in research and clinical settings. Foremost, the recording software is a mobile application that can be administered to participants in any location with minimal preparation. The other materials required for administration of eSense are similarly low-cost and time-effective. In contrast to other data acquisition devices that have been used in previous studies of physiological response (Glover et al., 2011; Rothbaum et al., 2014), eSense can be used and interpreted with little training by research and clinical staff. Prior work has validated the eSense data with gold standard psychophysiological measurements, i.e. a Biopac MP150 system (Hinrichs et al., 2017). This app enables assessment of physiological response in a variety of settings, which can facilitate translational work between research and clinical organizations to enhance patient treatment (Crockett et al., 2017).
Although previous studies associated SCR as recorded by eSense with PTSD symptoms in adults (Hinrichs et al., 2017; Hinrichs et al., 2019), our current study of 9-year-old children found that SCR was predicted by a child’s reported degree of trauma exposure, rather than PTSD severity. A high degree of trauma exposure during childhood could lead to more severe PTSD symptoms or a PTSD diagnosis as the children mature into adolescence and adulthood (McLaughlin et al., 2013). It is possible that our sample of children was too young (i.e. age 9) to adequately capture total PTSD severity, and that higher symptoms may emerge in time. Notably, even though this was a trauma-exposed sample, the average UCLA-RI PTSD score was 26.21, well below the DSM-5 cutoff of 35 (Kaplow et al., 2020). Future work will determine whether the SCR could predict development of future symptoms in a prospective longitudinal study.
While we did not replicate the adult findings with SCR (i.e. SCL change from baseline in response to trauma interview) and total PTSD symptoms, this study is the first to find that SCL habituation (i.e. change from maximum during trauma interview to the end of the interview) was associated with hyperarousal PTSD symptoms. This positive association between SCL habituation and hyperarousal was unexpected, as we predicted that higher hyperarousal would impair the ability to habituate (Jovanovic et al., 2009; Orr et al., 1997; Pole, 2007). Notably, SCL habituation was related to the maximum SCL during the interview, so it is possible that hyperarousal symptoms were related to peak response as well. However, we did not find that symptoms were directly correlated with maximum SCL and further, when we controlled for this variable, hyperarousal accounted for SCL habituation independently of the maximum. On the other hand, higher hyperarousal symptoms were associated with longer interviews independent of the overall number of traumas experienced, suggesting that more symptomatic children were more distressed and talked more during the interview. It is possible that the longer duration allowed for the increased SCL habituation.
Although most research in trauma populations has found that PTSD is associated with increased physiological reactivity and less habituation (Pole, 2007), there have been a few notable exceptions. A previous study of adult women who survived sexual assault found blunted SCR and faster SCL habituation in women with high dissociation, suggesting over-suppression of the sympathetic nervous system (Griffin et al., 1997). A study of children with maltreatment found that abused children showed a blunted SCR to a fear conditioning paradigm, and these children also exhibited higher rates of externalizing problems (McLaughlin et al., 2016). In conjunction with the findings involving externalizing symptoms, SCR in children has been associated with externalizing problems including hyperactivity (El-Sheikh et al., 2005). Hyperarousal symptoms have been uniquely associated with dysregulated psychophysiological responses to safety signals (Jovanovic et al., 2010). Taken together, these studies suggest that trauma exposure leads to over-regulation of the autonomic system as a potential coping mechanism, which could be observed as increased SCL habitation during the trauma interview.
Although this study introduces a novel mobile technique to capture SCR in children with trauma exposure, replication studies with larger samples will better assess the reliability of this method and whether it predicts long-term trauma-related psychopathology. Further, longitudinal studies could reveal whether these patterns of response change over time and if they are associated with other biological changes such as pubertal development. These findings would then indicate the applicability of eSense within clinical settings and its viability as a potential biomarker in pediatric cohorts. Subjective reports of symptoms (Pitman et al., 2012) complemented with a reliable and objective method of assessing trauma-related distress would not only assist in the identification of children who may be most in need of intervention, but it would also assist in the creation of more individualized treatment plans (Miller et al., 2016). Barriers associated with data collection of physiological response have precluded community-based organizations and clinical settings from widespread use of these methods. The emergence and validation of novel technology for psychophysiological assessment could improve its dissemination.
Highlights.
A low-cost, user-friendly device can reliably record changes in skin conductance
Children’s trauma exposure and symptoms predict physiological responses
Clinicians can use physiology to provide further potential insight from self-report alone
Skin conductance allows medical staff to identify children suffering from trauma
Acknowledgements:
This study was funded by support from the National Institute of Mental Health (R01-MH111682).
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Conflict of Interest Statement
Charis N. Wiltshire has no conflicts of interest to report.
Cassandra P. Wanna has no conflicts of interest to report.
Anaïs F. Stenson has no conflicts of interest to report.
Sean T. Minton has no conflicts of interest to report.
Mariam H. Reda has no conflicts of interest to report.
William M. Davie has no conflicts of interest to report.
Rebecca Hinrichs has no conflicts of interest to report.
Sterling Winters has no conflicts of interest to report.
John M. France has no conflicts of interest to report.
Tanja Jovanovic has no conflicts of interest to report.
CRediT Author Statement
Ms. Wiltshire: Conceptualization, Methodology, Data Curation, Project administration, Writing - Original Draft, Writing - Review & Editing, Visualization
Ms. Wanna: Conceptualization, Methodology, Formal analysis, Data Curation, Writing - Original Draft, Writing - Review & Editing, Visualization
Dr. Stenson: Conceptualization, Methodology, Writing - Review & Editing
Mr. Minton: Data Curation, Writing - Review & Editing
Ms. Reda: Data Curation, Writing - Review & Editing
Mr. Davie: Data Curation, Writing - Review & Editing
Ms. Hinrichs: Conceptualization, Methodology, Project administration, Writing - Review & Editing, Supervision
Mr. Winters: Methodology, Project administration, Writing - Review & Editing, Supervision
Mr. France: Investigation, Data Curation, Writing - Review & Editing
Dr. Jovanovic: Conceptualization, Methodology, Investigation, Writing - Review & Editing, Supervision, Visualization, Resources, Funding acquisition
Works Cited
- Cody MW, Jones JM, Woodward MJ, Simmons CA, & Beck JG (2017). Correspondence between self-report measures and clinician assessments of psychopathology in female intimate partner violence survivors. Journal of Interpersonal Violence, 32(10), 1501–1523. doi: 10.1177/0886260515589566 [DOI] [PubMed] [Google Scholar]
- Crockett JE, Gill DL, Cashwell TH, & Myers JE (2017). Integrating non-technological and technological peripheral biofeedback in counseling. Journal of Mental Health Counseling, 39(2), 163–179. doi: 10.17744/mehc.39.2.06 [DOI] [Google Scholar]
- Cushman GK, Shih S, Stolz MG, Hinrichs RC, Jovanovic T, Lee JL, Kugathasan S, & Reed B (2021). Stressful life events, depression, and the moderating role of psychophysiological reactivity in patients with pediatric inflammatory bowel disease. Journal of psychosomatic research, 141. doi: 10.1016/j.jpsychores.2020.110323 [DOI] [PMC free article] [PubMed] [Google Scholar]
- El- Sheikh M (2005). The role of emotional responses and physiological reactivity in the marital conflict–child functioning link. Journal of Child Psychology and Psychiatry, 46(11), 1191–1199. doi: 10.1111/j.1469-7610.2005.00418.x [DOI] [PubMed] [Google Scholar]
- El-Sheikh M, Erath SA, Buckhalt JA, Granger DA, & Mize J (2008). Cortisol and children’s adjustment: The moderating role of sympathetic nervous system activity. Journal of Abnormal Child Psychology, 36(4), 601–611. doi: 10.1007/s10802-007-9204-6 [DOI] [PubMed] [Google Scholar]
- Gamwell K, Nylocks M, Cross D, Bradley B, Norrholm SD, & Jovanovic T (2015). Fear conditioned responses and PTSD symptoms in children: Sex differences in fear-related symptoms. Developmental Psychobiology, 57(7), 799–808. doi: 10.1002/dev.21313 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Garza K, & Tanja J (2017). Impact of gender on child and adolescent PTSD. Current Psychiatry Reports, 19(11). doi: 10.1007/s11920-017-0830-6 [DOI] [PubMed] [Google Scholar]
- Ghosh-Ippen C, Ford J, Racusin R, Acker M, Bosquet K, Rogers C, & Edwards J (2002). Trauma events screening inventory-parent report revised. San Francisco: The Child Trauma Research Project of the Early Trauma Network and The National Center for PTSD Dartmouth Child Trauma Research Group. [Google Scholar]
- Glover EM, Phifer JE, Crain DF, et al. (2011). Tools for translational neuroscience: PTSD is associated with heightened fear responses using acoustic startle but not skin conductance measures. Depression and Anxiety, 28(12), 1058–1066. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gomez IN, Lai CY, Chan CC, & Tsang HW (2018). The role of ethnicity and environment in the regulation of response to sensory stimulus in children: Protocol and pilot findings of a neurophysiological study. JMIR Research Protocols, 7(1), e7. doi: 10.2196/resprot.8157 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gray SAO, Lipschutz RS, & Scheeringa MS (2018). Young children’s physiological reactivity during memory recall: Associations with posttraumatic stress and parent physiological synchrony. Journal of Abnormal Child Psychology, 46(4), 871–880. doi: 10.1007/s10802-017-0326-1 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Griffin MG, Resick PA, & Mechanic MB (1997). Objective assessment of peritraumatic dissociation: Psychophysiological indicators. American Journal of Psychiatry, 154(8), 1081–1088. doi: 10.1176/ajp.154.8.1081 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Harris PA, Taylor R, Thielke R, Payne J, Gonzalez N, & Conde JG (2009). Research electronic data capture (REDCap)—A metadata-driven methodology and workflow process for providing translational research informatics support. Journal of Biomedical Informatics, 42(2),377–381. doi: 10.1016/j.jbi.2008.08.010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Heim C, & Nemeroff CB (2009). Neurobiology of posttraumatic stress disorder. CNS spectrums, 14(1 Suppl 1), 13–24. doi: 10.1177/107839039500100607 [DOI] [PubMed] [Google Scholar]
- Heim CM, Entringer S, & Buss C (2019). Translating basic research knowledge on the biological embedding of early-life stress into novel approaches for the developmental programming of lifelong health. Psychoneuroendocrinology, 105, 123–137. doi: 10.1016/j.psyneuen.2018.12.011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hinrichs R, van Rooij SJH, Michopoulos V, Schultebraucks K, Winters S, Maples-Keller J, Rothbaum AO, Stevens JS, Galatzer-Levy I, Rothbaum BO, Ressler KJ, & Jovanovic T (2019). Increased Skin Conductance Response in the Immediate Aftermath of Trauma Predicts PTSD Risk. Chronic Stress, 3, 1–11. doi: 10.1177/2470547019844441 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hinrichs R, Michopoulos V, Winters S, Rothbaum AO, Rothbaum BO, Ressler KJ, & Jovanovic T (2017). Mobile assessment of heightened skin conductance in posttraumatic stress disorder. Depression and Anxiety, 34(6), 502–507. doi: 10.1002/da.22610 [DOI] [PMC free article] [PubMed] [Google Scholar]
- The jamovi project (2021). jamovi (Version 1.6) [Computer Software]. Retrieved from https://www.jamovi.org
- Jenness JL, Miller AB, Rosen ML, & McLaughlin KA (2019). Extinction learning as a potential mechanism linking high vagal tone with lower PTSD symptoms among abused youth. Journal of Abnormal Child Psychology, 47(4), 659–670. doi: 10.1007/s10802-018-0464-0 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jovanovic T, Norrholm SD, Sakoman AJ, Esterajher S, & Kozarić-Kovačić D (2009). Altered resting psychophysiology and startle response in Croatian combat veterans with PTSD. International Journal of Psychophysiology, 71(3), 264–268. doi: 10.1016/j.ijpsycho.2008.10.007 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jovanovic T, Norrholm SD, Blanding NQ, Davis M, Duncan E, Bradley B, & Ressler KJ (2010). Impaired fear inhibition is a biomarker of PTSD but not depression. Depression and Anxiety, 27(3), 244–251. doi: 10.1002/da.20663 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kaplow JB, Rolon-Arroyo B, Layne CM, Rooney E, Oosterhoff B, Hill R, Steinberg AM, Lotterman J, Gallagher K, & Pynoos RS (2020). Validation of the UCLA PTSD Reaction Index for DSM-5: A developmentally informed assessment tool for youth. Journal of the American Academy of Child and Adolescent Psychiatry, 59(1), 186–194. https://doi-org.proxy.lib.wayne.edu/10.1016/j.jaac.2018.10.019 [DOI] [PubMed] [Google Scholar]
- Kirsch V, Wilhelm FH, & Goldbeck L (2011). Psychophysiological characteristics of PTSD in children and adolescents: A review of the literature. Journal of Traumatic Stress, 24(2), 146–154. doi: 10.1002/jts.20620 [DOI] [PubMed] [Google Scholar]
- Kredlow AM, Pineles SL, Inslicht SS, Marin MF, Milad MR, Otto MW, & Orr SP (2017). Assessment of skin conductance in African American and Non-African American participants in studies of conditioned fear. Psychophysiology. 54(11): 1741–1754. doi: 10.1111/psyp.12909. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lang PJ, Bradley MM, Cuthbert BN. Emotion, motivation, and anxiety: brain mechanisms and psychophysiology. Biological Psychiatry, 1998. 44: p. 1248–1263. [DOI] [PubMed] [Google Scholar]
- McLaughlin KA, Koenen KC, Hill ED, Petukhova M, Sampson NA, Zaslavsky AM, & Kessler RC (2013). Trauma exposure and posttraumatic stress disorder in a national sample of adolescents. Journal of the American Academy of Child & Adolescent Psychiatry, 52(8), 815–830.e14. doi: 10.1016/j.jaac.2013.05.011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- McLaughlin KA, Sheridan MA, Gold AL, Duys A, Lambert HK, Peverill M, Heleniak C, Shechner T, Wojcieszak Z, & Pine DS (2016). Maltreatment exposure, brain structure, and fear conditioning in children and adolescents. Neuropsychopharmacology, 41(8), 1956–1964. doi: 10.1038/npp.2015.365 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Miller GA, Rockstroh BS, Hamilton HK, & Yee CM (2016). Psychophysiology as a core strategy in RDoC. Psychophysiology, 53(3), 410–414. doi: 10.1111/psyp.12581 [DOI] [PubMed] [Google Scholar]
- Nelson BW, Low CA, Jacobson N, Areán P, Torous J, & Allen NB (2020). Guidelines for wrist-worn consumer wearable assessment of heart rate in biobehavioral research. NPJ Digit Med, 3, 90. doi: 10.1038/s41746-020-0297-4 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Orr SP, Lasko NB, Metzger LJ, et al. (1997a). Psychophysiologic assessment of PTSD in adult females sexually abused during childhood. Annals of New York Academy Science, 821, 491–493. doi: 10.1111/j.1749-6632.1997.tb48313.x [DOI] [PubMed] [Google Scholar]
- Orr SP, Solomon Z, Peri T, Pitman RK, & Shalev AY (1997b). Physiologic responses to loud tones in Israeli veterans of the 1973 Yom Kippur war. Biological Psychiatry, 41(3), 319–326. doi: 10.1016/S0006-3223(95)00671-0 [DOI] [PubMed] [Google Scholar]
- Pineles SL, Suvak MK, Liverant GI, et al. (2013). Psychophysiologic reactivity, subjective distress, and their associations with PTSD diagnosis. The Journal of Abnormal Psychology, 122(3), 635–644. doi: 10.1037/a0033942 [DOI] [PubMed] [Google Scholar]
- Pitman RK, Orr SP, Forgue DF, et al. (1987). Psychophysiologic assessment of posttraumatic stress disorder imagery in Vietnam combat veterans. Archives of General Psychiatry, 44(11), 970–975. doi: 10.1001/archpsyc.1987.01800230050009 [DOI] [PubMed] [Google Scholar]
- Pitman RK, Rasmusson AM, Koenen KC, Shin LM, Orr SP, Gilbertson MW, Milad MR, & Liberzon I (2012). Biological studies of post-traumatic stress disorder. Nature Reviews Neuroscience, 13(11), 769–787. doi: 10.1038/nrn3339 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pole N (2007). The psychophysiology of posttraumatic stress disorder: A meta-analysis. Psychological Bulletin, 133(5), 725–746. doi: 10.1037/0033-2909.133.5.725 [DOI] [PubMed] [Google Scholar]
- Rothbaum BO, Kearns MC, Reiser E, et al. (2014). Early intervention following trauma may mitigate genetic risk for PTSD in civilians: A pilot prospective emergency department study. Journal of Clinical Psychiatry, 75(12), 1380–1387. doi: 10.4088/JCP.13m08715 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Russo AC, & Fingerhut EC (2017). Consistency of self-reported neurocognitive symptoms, post-traumatic stress disorder symptoms, and concussive events from end of first deployment to veteran health administration comprehensive traumatic brain injury evaluation by Operations Enduring Freedom/Iraqi Freedom/New Dawn Veterans. Archives of Clinical Neuropsychology, 32, 184–197. doi: 10.1093/arclin/acw093 [DOI] [PubMed] [Google Scholar]
- Shalev AY, Orr SP, & Pitman RK (1993). Psychophysiologic assessment of traumatic imagery in Israeli civilian patients with posttraumatic stress disorder. American Journal of Psychiatry, 150(4), 620–624. [DOI] [PubMed] [Google Scholar]
- Steinberg A, Brymer M, Decker K, & Pynoos R (2004). The University of California at Los Angeles post-traumatic stress disorder reaction index. Current Psychiatry Reports, 6(2),96–100. doi: 10.1007/s11920-004-0048-2 [DOI] [PubMed] [Google Scholar]
- Tedeschi FK, & Billick SB (2017). Pediatric PTSD: Clinical, forensic, and diagnostic understanding. Journal of the American Academy of Psychiatry and the Law Online, 45(2), 161. [PubMed] [Google Scholar]
- Ulrich-Lai YM, & Herman JP (2009). Neural regulation of endocrine and autonomic stress responses. In Nature Reviews Neuroscience, 10(6), 397–409. doi: 10.1038/nrn2647 [DOI] [PMC free article] [PubMed] [Google Scholar]