Abstract
STUDY QUESTION
Is the severity of menstrual cyclicity related to hyperinsulinemia and dysglycemia in women with hyperandrogenic polycystic ovary syndrome (PCOS)?
SUMMARY ANSWER
Hyperandrogenic PCOS women with amenorrhea, compared to those with oligomenorrhea or eumenorrhea, had a greater risk of post-challenge hyperinsulinemia, which may explain their higher prevalence of dysglycemia.
WHAT IS KNOWN ALREADY
PCOS is associated with metabolic dysregulation including insulin resistance (IR) and hyperinsulinemia, risk factors for type 2 diabetes mellitus (T2DM) and other vascular-metabolic morbidities. Although the severity of menstrual cyclicity is associated with IR in PCOS, it is unclear whether, and to what extent, it is related to hyperinsulinemia and glycemic abnormalities.
STUDY DESIGN, SIZE, DURATION
We prospectively compared the degree of menstrual cyclicity with the presence of dysglycemia (elevated 1-h plasma glucose ≥155 mg/dl; abnormal glucose tolerance [AGT], including prediabetes and T2DM; and AUC for glucose [G-AUC]) or dynamic state hyperinsulinemia (peak insulin levels either at 1 or 2 h of the oral glucose tolerance test (oGTT) and AUC for insulin [I-AUC]) in 333 hyperandrogenic PCOS women.
PARTICIPANTS/MATERIALS, SETTING, METHODS
In a tertiary care setting, hyperandrogenic PCOS participants with ovulatory eumenorrhea (Ov-Eumeno, n = 25), anovulatory eumenorrhea (Anov-Eumeno, n = 33), oligomenorrhea (Oligo, n = 150) and amenorrhea (Ameno, n = 125) underwent comprehensive phenotyping and a 2-h 75 g oGTT.
MAIN RESULTS AND THE ROLE OF CHANCE
Mean BMI was greater among Ameno women than among Oligo, Anov-Eumeno or Ov-Eumeno women. Adjusting for BMI, the Ameno group demonstrated higher mean 1- and 2-h insulin and glucose, peak insulin and I-AUC and G-AUC, and either had a higher, or tended toward having a higher, prevalence of elevated 1-h glucose level and prevalence of AGT than the Oligo, Anov-Eumeno or Ov-Eumeno groups. In logistic regression, adjusting for BMI, Ameno women were more likely to have: AGT than Oligo women (odds ratio [OR]: 2.3; 95% CI: 1.3 to 4.2); elevated 1-h glucose (OR: 10.2; CI: 1.3–79.7) than those with Ov-Eumeno; and both AGT (OR: 1.7; CI: 1.1–2.6) and elevated 1-h glucose (OR: 1.8; CI: 1.1–2.8) than those with Anov-Eumeno or Ov-Eumeno when combined. Race/ethnicity, age, waist-to-hip ratio, fasting insulin and glucose, and biochemical or clinical measures of hyperandrogenism were similar across the four menstrual categories.
LIMITATIONS, REASONS FOR CAUTION
Our study was limited by its cross-sectional nature and by studying women affected by PCOS as defined by the Androgen Excess & PCOS Society criteria (i.e. Rotterdam Phenotypes A, B and C) who were identified in the clinical setting. Consequently, extrapolation of the present data to other PCOS phenotypes (e.g. PCOS Phenotype D) should be made with caution.
WIDER IMPLICATIONS OF THE FINDINGS
In hyperandrogenic PCOS phenotypes, a history of amenorrhea, compared to oligomenorrhea or eumenorrhea, suggests a more severe cardiometabolic risk, including a higher degree of hyperinsulinemia and greater prevalence of glycemic abnormalities. These findings may assist in refining the treatment and screening guidelines for glycemic abnormalities in PCOS.
STUDY FUNDING/COMPETING INTEREST(S)
This work was supported in part by grants R01-DK073632 and R01-HD29364 from the NIH and an endowment of the Helping Hand of Los Angeles, Inc. (to R.A.). M.D.P. has no competing interests to declare. U.E. is an investor in Concentric Analgesics, Inc. R.A. serves as a consultant for Spruce Biosciences and Fortress Biotech and an advisor for Aurora Forge.
TRIAL REGISTRATION NUMBER
N/A.
Keywords: menstrual dysfunction, metabolic dysfunction, glucose intolerance, hyperinsulinemia, diabetes, 1-h glucose, PCOS
Introduction
Polycystic ovary syndrome (PCOS) is the most common endocrine-medical disorder in females, occurring in 10–15% of reproductive-aged women (Bozdag et al., 2016; Lizneva et al., 2016) and is associated with enhanced risk for metabolic and vascular dysfunction (Ezeh et al., 2018, 2019; Berni et al., 2021; Joham et al., 2021). Approximately 65–95% of PCOS women demonstrate insulin resistance (IR) and compensatory hyperinsulinemia (Dunaif et al., 1989; Ezeh et al., 2020b, 2021), which increase their risk of glycemic abnormalities, including prediabetes, type 2 diabetes mellitus (T2DM) (Legro et al., 1999; Ryu et al., 2021) and metabolic syndrome (Meyer et al., 2020), which confer increased risks for cardiovascular disease (Berni et al., 2021; Joham et al., 2021). Overall, PCOS has substantial health and economic costs (Riestenberg et al., 2021).
Women with PCOS have a 4-fold increased risk of T2DM, a population attributable risk of 19.4–28% (Rodgers et al., 2019), a younger disease onset and a more rapid conversion from prediabetes to T2DM (Ehrmann et al., 1999; Celik et al., 2014) compared to individuals without PCOS, buttressing the concept of PCOS as a prediabetic state (Dunaif et al., 1989; Ehrmann et al., 1999; Legro et al., 1999; American Diabetes Association, 2017). Compelling evidence indicates that early detection and intervention can delay, if not prevent, the transition from prediabetes to T2DM and reduce the diabetes-related disease burden (Tabák et al., 2012; American Diabetes Association, 2017). Thus, it is important to identify PCOS women at the greatest risk of glycemic abnormalities in order to optimize preventive and therapeutic strategies.
Compensatory hyperinsulinemia, characterized by high circulating insulin levels stemming from peripheral IR, is a key feature of PCOS (Barbieri et al., 1986; Dunaif et al., 1989; Moghetti et al., 1996; Ezeh et al., 2018, 2020b). In fact, the degree of hyperinsulinemia in PCOS is greater than that found in other IR disorders (e.g. T2DM), as pancreatic islet β-cell function is generally still robust, albeit often not fully normal (e.g. distemporal and delayed) (Dunaif and Finegood, 1996; Ehrmann et al., 2005). There is increasing evidence that postprandial hyperinsulinemia may be the root cause of many diseases, including obesity, coronary heart disease, T2DM and others (Rizza et al., 1985; Després et al., 1996; Abdul-Ghani and DeFronzo, 2021). Although the relationship of hyperinsulinemia to other morbidities in PCOS has been less well studied, it is well established that hyperandrogenism in PCOS is partly underpinned by hyperinsulinemia (Barbieri et al., 1986; Moghetti et al., 1996).
IR (Martin et al., 1992) and hyperinsulinemia (Haffner et al., 1986) predict future development of glycemic abnormalities. Interestingly, we have previously demonstrated an association between the severity of menstrual cyclicity and IR, estimated either at baseline (Brower et al., 2013) or dynamically (Ezeh et al., 2021), in PCOS. Furthermore, many epidemiological studies have associated women with a history of irregular menstruation with greater risks of T2DM, metabolic syndrome, coronary heart disease and premature mortality, compared to healthy women with regular menstrual cycles (Solomon et al., 2001; Wang et al., 2011, 2020a,b; Polotsky et al., 2012).
We hypothesize that the severity of menstrual dysfunction is associated with hyperinsulinemia and glycemic abnormalities and could potentially be used as a proxy for metabolic dysfunction in women with PCOS. To test this hypothesis, we studied a population of 333 hyperandrogenic PCOS women, recruited prospectively, who underwent a 75-g oral glucose tolerance test (oGTT), assessing both plasma insulin and glucose levels.
Materials and methods
Study population
Research participants included women with PCOS prospectively and consecutively recruited through advertisements, and the clinical and research practices of the University of Alabama at Birmingham (UAB) and the Center for Androgen-Related Disorders at Cedars Sinai Medical Center (CSMC), Los Angeles. Because we were interested in studying metabolic dysfunction, only adult PCOS female participants with hyperandrogenic PCOS diagnosed by the Androgen Excess & PCOS Society criteria (Azziz et al., 2006), equivalent to Phenotypes A, B and C of the Rotterdam criteria (Rotterdam ESHRE/ASRM-Sponsored PCOS consensus workshop group, 2004), were included. Study subjects demonstrated clinical and/or biochemical hyperandrogenism, including a modified Ferriman-Gallwey (mF-G) hirsutism score ≥6 and/or hyperandrogenemia (i.e. total testosterone [T], free T or dehydroepiandrosterone sulfate [DHEAS] above normal) while other known related endocrinopathies were excluded, as previously described (Knochenhauer et al., 1998; Azziz et al., 2004a,b). Only PCOS participants of African American, Hispanic White and non-Hispanic White ancestry were enrolled in this prospective cohort study, because of the small number of other ethnicities/races.
Study exclusion criteria included pregnancy or lactation state, other endocrine disorders including known pre-existing T2DM, which precluded the performance of an oGTT, inability to assess menstruation or ovulation status (e.g. prior hysterectomy, bilateral oophorectomy, vaginal agenesis, or postmenopausal or premenarcheal state), or use of any hormonal medication (including oral contraceptives, insulin-sensitizing agents, anti-diabetic medications, antiandrogens or glucocorticoids) within 3 months preceding the evaluation. All subjects had normal thyroid-stimulating hormone, 17-hydroxyprogesterone and prolactin levels, as previously described (Knochenhauer et al., 1998; Azziz et al., 2004a,b). Screening for Cushing’s syndrome and androgen-secreting neoplasms was performed if clinically indicated.
Ethical approval
The study was reviewed and approved by the Institutional Review Boards of CSMC and UAB. All subjects were fully informed about the study and provided written informed consent before study entry.
Protocol
All subjects completed a uniform questionnaire providing information regarding age, race and menstrual history, which were further reviewed at consultation. Participants with PCOS were grouped according to the interval between episodes of vaginal bleeding (Treloar et al., 1967; Brower et al., 2013; Ezeh et al., 2021). As previously described, women with cycle lengths <26 days were considered to have polymenorrhea (Poly); those with 26–34 days bleeding intervals were considered eumenorrheic (Eumeno) and their ovulatory function assessed by measuring a menstrual cycle Days 22–24 progesterone (P4) level; eumenorrheic women with a P4 <4 ng/ml were considered anovulatory (i.e. Anov-Eumeno), whereas the remainder were considered to be ovulatory (i.e. Ov-Eumeno) (Wathen et al., 1984; Brower et al., 2013; Ezeh et al., 2021). PCOS women with 35 days to 3 months bleeding intervals were classified as oligomenorrheic (i.e. 35 days to 6 weeks [Early-Oligo] and 6 weeks to 3 months [Late-Oligo]), and those with cycles >3 months were classed as amenorrheic (Ameno) (Brower et al., 2013; Ezeh et al., 2021).
We should note that continuing controversy has surrounded the definition of normal versus abnormal menstruation, with some investigators defining normal menstrual length as a 21–35 days interval (Steiner et al., 2001), while others define it as 26–31 days (Solomon et al., 2001; Wang et al., 2020a,b) or 25–45 days (Gaete et al., 2010). In turn, the FIGO Menstrual Disorders Committee has standardized the classification of Abnormal Uterine Bleeding and set the definition of normal menstrual length/frequency as a 24–38 days interval (Munro et al., 2018). Considering these controversies, in the present study, as well as in our prior studies (Brower et al., 2013; Ezeh et al., 2021), we have defined a normal menstrual interval as being 26–34 days and defined polymenorrhea as a menstrual cycle <26 days in length in order to provide continuity to our definition of menstrual cyclicity.
All subjects underwent a history and physical examination, as previously described (Knochenhauer et al., 1998; Azziz et al., 2004a,b). In addition to height, weight and mF-G score, waist circumference was measured at the narrowest portion of the torso approximately midway between the lower costal margin and the iliac crest, and the hip circumference was measured over the widest portion of the gluteal and greater trochanteric region. The BMI and waist-to-hip ratio (WHR) were then calculated. Polycystic ovarian morphology was assessed by either transvaginal ultrasonography or abdominal ultrasonography for those patients not tolerating or nondesirous of transvaginal ultrasonography (Philips EnVisor Ultrasound System, with 6.26 MHz endovaginal transducer) and was defined per 2003 Rotterdam criteria contemporary with recruitment, including at least one ovary containing 12 or more ovarian follicles measuring 2–9 mm and/or an ovarian volume of more than 10 cm3 (Rotterdam ESHRE/ASRM-Sponsored PCOS consensus workshop group, 2004; Brower et al., 2013).
Metabolic assessment
After an overnight fast, blood samples were obtained on Days 3–8 of a spontaneous or a Prometrium® (Solvay Pharmaceuticals, Marietta, GA, USA)-induced withdrawal bleed (i.e. the follicular phase) for measurement of circulating total T, free T and DHEAS, as well as insulin and glucose levels (Woods et al., 2002). All subjects also underwent a standard 2-h 75-g oGTT (American Diabetes Association, 2017) and plasma insulin and glucose levels were determined at 0 min, 1 and 2 h. In accordance with the American Diabetes Association guidelines, glucose tolerance was classified as follows: (i) normal glucose tolerance, by a fasting glucose of <100 mg/dl and/or a 2-h glucose <140 mg/dl; (ii) prediabetes, by a fasting glucose between 100 and 125 mg/dl (impaired fasting glucose) or a 2-h glucose between 140 and 200 mg/dl (impaired glucose tolerance); and (iii) T2DM, by a fasting glucose ≥126 mg/dl or a 2-h glucose ≥200 mg/dl (American Diabetes Association, 2017). An elevated 1-h glucose was defined as ≥155 mg/dl during the oGTT, reported to be a robust predictor of future risk for T2DM (Cubeddu and Hoffmann, 2010; Bianchi et al., 2013; Peddinti et al., 2019; Jagannathan et al., 2020).
Insulin data from the oGTT were used to calculate peak insulin, defined as the highest insulin levels reached during the oGTT (either at 1 or 2 h) (Jagannathan et al., 2020). The dynamic insulin and glucose responses were quantified by calculating the AUC for insulin (I-AUC) and glucose (G-AUC) during the oGTT, using the standard mathematical trapezoidal method (American Diabetes Association, 2017; Jagannathan et al., 2020).
Hormonal and biochemical analysis
Total T was measured using a high-turbulence liquid chromatography–tandem mass spectrometry (LC-MS/MS), and free T was determined by equilibrium dialysis (Quest Diagnostics, San Juan Capistrano, CA, USA), as previously described (Salameh et al., 2014) in 282 participants. In 51 consecutive participants, total T was measured using a high-quality radioimmunoassay (RIA) method after serum extraction and chromatography, and sex hormone-binding globulin (SHBG) activity was measured by diffusion equilibrium dialysis, using Sephadex G-25 (Sigma-Aldrich Corp., St. Louis, MO, USA), and [3H]T as the ligand and the free T was calculated, as previously described (Azziz et al., 1995; Boots et al., 1998); the results were converted to LC-MS/MS values as previously reported, as these methods of assessing total T and free T are highly correlated (Salameh et al., 2014; Ezeh et al., 2019). Serum DHEAS and P4 were measured by direct RIA, using commercially available kits (DHEAS and P4 from Diagnostic Products Corp., Los Angeles, CA), as previously described (Knochenhauer et al., 1998). Plasma insulin was assayed by chemiluminescence (ADVIA Centaur chemiluminescent immunoassay system; Siemens Healthcare, Deerfield, IN, USA) and plasma glucose was measured using the hexokinase/glucose-6-phosphate dehydrogenase method (Roche Applied Sciences). Except for plasma glucose, samples were batched at regular intervals for analysis to minimize the impact of interassay variability. The intra-assay and interassay variations for total T, SHBG, DHEAS, A4, prolactin, thyroid-stimulating hormone, 17-hydroxyprogesterone and P4 have been previously reported and did not exceed 10% (Knochenhauer et al., 1998; Salameh et al., 2014).
Statistical analysis
The primary outcome for hyperinsulinemia was the peak insulin level, and for dysglycemia, it was the prevalence of abnormal glucose tolerance (AGT), including prediabetes and T2DM. Secondary outcomes included mean I-AUC and G-AUC, and proportion of subjects with elevated 1-h glucose ≥155 mg/dl. The Shapiro–Wilks W-test was used to determine whether continuous variables were normally distributed. All continuous variables, except for the mF-G score, reasonably followed a parametric distribution on the original or log scales.
For the analysis of menstrual dysfunction, participants with PCOS were divided into four groups (Ov-Eumeno, Anov-Eumeno, Oligo and Ameno). Mean intergroup differences were evaluated using a one-way ANOVA (with Tukey’s post hoc multiple-comparison test) for normally distributed continuous variables or the Kruskal–Wallis test for mF-G score. Differences in frequency were computed with the χ2 test with Yates correction or Fisher’s exact test as appropriate. Differences in mean glucose and insulin levels, and prevalence of glycemic abnormalities were adjusted for BMI, using linear regression and logistic regression analysis, respectively. Because of the same study design, patient phenotyping and laboratory analysis by the same team, and similar populations in the cohorts, data from UAB and CSMC cohorts were pooled for analysis. Continuous variables were expressed as mean ± standard error or geometrical mean (range) if log transformed and categorical variables were expressed as count (percent) unless otherwise stated. A two-sided P < 0.05 was considered statistically significant. All statistical analyses were conducted using the Stats Direct statistics software package, version 3.2.10 2020 (Cheshire, UK).
To estimate power analysis, sample size was assessed for hyperinsulinemia using peak insulin levels as the endpoint. Based on our previous studies of the association of menstrual dysfunction and IR (Brower et al., 2013; Ezeh et al., 2021), a power analysis with a pooled standard deviation of 1.49, with an 80% power and an α = 0.05, based on unpaired t testing, indicated that a sample size of 35 participants per group was sufficient to detect a mean difference of 35% change in mean peak insulin levels between Ameno and Oligo PCOS. Additionally, we used the prevalence of T2DM as the endpoint to estimate statistical power for assessing differences in dysglycemia. As there is no previous study comparing the prevalence of T2DM by menstrual categories, we considered a difference of 8% in the prevalence of T2DM between Ameno and Oligo PCOS as being clinically significant. This difference is similar or less than that observed when comparing the prevalence of T2DM between PCOS and control subjects (Ehrmann et al., 1999). Using this assumption, we estimated that a sample size of 100 patients in each group would give the study 80% power and an α = 0.05.
Results
A total of 387 consecutive PCOS participants underwent screening for enrollment, of whom 44 (11.3%) participants were excluded because they did not meet the requirements of the study (26 were Asian Americans, 6 Native Hawaiian/Pacific Islander/other Americans and 5 multiracial; and 7 subjects had unclear menstrual history as the only problem), while 10 (2.6%) participants with polymenorrhea (Poly; cycle length <26 days) were also excluded due to the small size of this subgroup, leaving 333 (86.1%) PCOS participants for study. Of the 333 participants finally enrolled during this prospective cross-sectional study, 25 (7.5%) were Ov-Eumeno, 33 (9.9%) were Anov-Eumeno, 150 (45.1%) were Oligo and 125 were (37.5%) Ameno.
Baseline features
The basic demographic, anthropometric and endocrine characteristics of the 333 participants enrolled in the study are shown in Table I. The majority of the participants were of non-Hispanic White ethnicity. Mean age, biochemical and clinical measures of hyperandrogenism (i.e. mF-G score, and free and total T and DHEAS) were similar across the four menstrual categories, except for a slightly greater mean mF-G score among Anov-Eumeno compared to Oligo (Table I). The prevalence of hirsutism was also similar between groups. Based on current international guidelines for defining hirsutism (i.e. mF-G score ≥ 4) (Teede et al., 2018), the percent of hirsutism was similar among the groups: Ameno (76.4%), Oligo (76.9%), Anov-Eumeno (87.9%) and Ov-Eumeno (73.9%). Similar results were obtained when the cutoff mF-G score ≥6 was used to determine the prevalence of hirsutism (as used for study recruitment criteria, as discussed in the Materials and methods section above): Ameno (61.8%), Oligo (60.8%), Anov-Eumeno (78.1%) and Ov-Eumeno (56.5%). Mean BMI was similar between Oligo and Eumeno but was greater among Ameno than Oligo or Ov-Eumeno and tended toward being greater than Anov-Eumeno (Table I). Therefore, in subsequent analyses, the outcome variables were adjusted for BMI.
Table II.
Differences in metabolic characteristics by menstrual cyclicity for hyperandrogenic PCOS.
| Variables | Menstrual cyclicity |
P-value between groups (adjusted for BMI) |
||||||||
|---|---|---|---|---|---|---|---|---|---|---|
| Ovulatory PCOS |
Ovulatory dysfunction PCOS |
Ovulatory dysfunction PCOS |
Ovulatory PCOS vs Ovulatory dysfunction PCOS |
|||||||
| Ov- Eumeno | Anov- Eumeno | Oligo (n = 150) | Ameno (n = 125) | Anov- Eumeno vs Oligo | Oligo vs Ameno | Anov- Eumeno vs Ameno | Ov-Eumeno vs Anov- Eumeno | Ov- Eumen vs Oligo | Ov- Eumeno vs Ameno | |
| (n = 25) | (n = 33) | |||||||||
| Plasma glucose levels (mg/dl) | ||||||||||
| Fasting glucose | 85.5 ± 2.7 | 87.8 ± 2.4 | 86.8 ± 1.1 | 88.4 ± 1.3 | 0.626 | 0.811 | 0.711 | 0.554 | 0.766 | 0.786 |
| 1-hour glucose | 104.7 ± 5.5 | 123.1 ± 8.2 | 123.8 ± 3.6 | 143.7 ± 5.2 | 0.948 | 0.072 | 0.389 | 0.074 | 0.033 | 0.018 |
| 2-hour glucose | 88.7 ± 2.8 | 106.5 ± 7.3 | 103.7 ± 3.0 | 129.2 ± 7.7 | 0.602 | 0.010 | 0.272 | 0.052 | 0.052 | 0.055 |
| G-AUC (mg/dl/120 min) | 11 136.3 ± 528.2 | 13 136.4 ± 756.4 | 13 121.4 ± 312.2 | 15, 135.4 ± 501. | 0.815 | 0.016 | 0.267 | 0.079 | 0.043 | 0.015 |
| Plasma insulin levels (µIU/ml) | ||||||||||
| Fasting insulin | 11.3 ± 2.6 | 11.3 ± 1.3 | 14.9 ± 1.4 | 26.5 ± 6.1 | 0.399 | 0.133 | 0.371 | 0.403 | 0.158 | 0.068 |
| 1-h insulin | 80.9 ± 25.7 | 102.7 ± 15.2 | 92.0 ± 7.2 | 167.3 ± 14.4 | 0.309 | <0.001 | 0.256 | 0.031 | 0.172 | 0.007 |
| 2-h insulin | 49.1 ± 12.3 | 76.2 ± 13.4 | 66.6 ± 5.6 | 138.6 ± 13.4 | 0.260 | <0.001 | 0.225 | 0.005 | 0.027 | <0.001 |
| Peak insulin | 85.2 ± 25.9 | 110.1 ± 16.1 | 102.3 ± 7. | 181.4 ± 14.5 | 0.341 | 0.001 | 0.230 | 0.009 | 0.012 | 0.037 |
| I-AUC (mg/dl/120 min) | 6654.0 ± 1875.9 | 8575.2 ± 1251.8 | 7964.6 ± 578.5 | 14 899.6 ± 1261.0 | 0.342 | <0.001 | 0.184 | 0.018 | 0.404 | 0.033 |
| Glycemic abnormalities—no. (%) | ||||||||||
| Abnormal glucose tolerance | 2 (8.0) | 5 (15.2) | 23 (15.3) | 38 (30.4) | 0.896 | 0.007 | 0.143 | 0.448 | 0.453 | 0.066 |
| Prediabetes | 2 (8.0) | 4 (12.1) | 15 (10.0) | 23 (18.4) | 0.736 | 0.089 | 0.494 | 0.620 | 0.767 | 0.303 |
| Type 2 Diabetes | 0 (0) | 1 (3.0) | 8 (5.3) | 15 (12.0) | 0.626 | 0.092 | 0.210 | 0.995 | 0.987 | 0.986 |
| Elevated 1-hour PG* | 1 (4.0) | 6 (18.2) | 33 (22.0) | 46 (36.8) | 0.697 | 0.139 | 0.185 | 0.150 | 0.080 | 0.027 |
1-h plasma glucose (PG) ≥155 mg/dl during oGTT.
Ameno, amenorrhea; Anov-Eumeno, anovulatory eumenorrhea; G-AUC, glucose AUC; I-AUC, AUC for insulin; Oligo, oligomenorrhea; Ov-Eumeno, ovulatory eumenorrhea; PCOS, polycystic ovary syndrome.
P values in italics and bold are considered statistically significant.
Table I.
Differences in anthropometric and endocrine features by menstrual cyclicity for hyperandrogenic polycystic ovary syndrome (PCOS).
| Variables | Menstrual cyclicity |
P-value between groups (adjusted for BMI) |
||||||||
|---|---|---|---|---|---|---|---|---|---|---|
| Ovulatory PCOS |
Ovulatory dysfunction PCOS |
Ovulatory dysfunction PCOS |
Ovulatory PCOS vs Ovulatory dysfunction PCOS |
|||||||
| Ov- Eumeno | Anov- Eumeno | Oligo (n = 150) | Ameno (n = 125) | Anov- Eumeno vs Oligo | Oligo. vs Ameno | Anov- Eumeno vs Ameno | Ov-Eumeno vs Anov- Eumeno | Ov- Eumeno vs Oligo | Ov- Eumeno vs Ameno | |
| (n = 25) | (n = 33) | |||||||||
| Demographics | ||||||||||
| Age (years) | 29.4 ± 1.2 | 26.2 ± 1.4 | 28.0 ± 1.4 | 27.5 ± 0.7 | 0.125 | 0.926 | 0.926 | 0.123 | 0.530 | 0.126 |
| BMI (kg/m2)* | 30.2 ± 1.2 | 29.9 ± 1.8 | 30.7 ± 0.8 | 34.2 ± 1.0 | NA | 0.005 | 0.082 | NA | NA | 0.011 |
| WHR | 0.83 ± 0.02 | 0.84 ± 0.02 | 0.83 ± 0.01 | 0.86 ± 0.01 | 0.071 | 0.810 | 0.810 | 0.671 | 0.899 | 0.461 |
| Race/ethnicity—no./total no. (%) | ||||||||||
| African American | 3 (12) | 5.0 (15.2) | 12 (8.0) | 15 (12.0) | NA | NA | NA | NA | NA | NA |
| Hispanic White | 1 (4) | 6.0 (18.2) | 29 (19.3) | 22 (17.6) | NA | NA | NA | NA | NA | NA |
| Non-Hispanic White | 21 (84) | 22 (66.7) | 108 (72.0) | 88 (70.4) | NA | NA | NA | NA | NA | NA |
| Androgen measures | ||||||||||
| mF-G (Hirsutism) score | 7.5 ± 1.1 | 9.8 ± 1.0 | 7.0 ± 0.37 | 7.7 ± 0.5 | 0.006 | 0.517 | 0.075 | 0.147 | 0.565 | 0.762 |
| Free T (pmol/l) | 13.64 ± 2.67 | 14.9 ± 1.8 | 22.4 ± 2.9 | 23.0 ± 4.3 | 0.374 | 0.414 | 0.211 | 0.743 | 0.180 | 0.164 |
| Total T (nmol/L) | 1.92 ± 2.0.31 | 1.5 ± 0.2 | 1.6 ± 0.1 | 1.4 ± 0.1 | 0.556 | 0.854 | 0.624 | 0.158 | 0.390 | 0.241 |
| DHEAS (mol/l) | 7.10 ± 0.76 | 6.63 ± 0.60 | 6.97 ± 0.32 | 6.3 ± 0.4 | 0.592 | 0.235 | 0.949 | 0.994 | 0.850 | 0.847 |
Ameno, amenorrhea; Anov-Eumeno, anovulatory eumenorrhea; DHEAS, dehydroepiandrosterone sulfate; mF-G, modified Ferriman-Gallwey hirsutism score; N/A, not applicable; Oligo, oligomenorrhea; Ov-Eumeno, ovulatory eumenorrhea; PCOS, polycystic ovary syndrome; T, testosterone; WHR, waist to hip ratio. P values in italics and bold are considered statistically significant.
*Denotes that the p-value of BMI between groups is not adjusted.
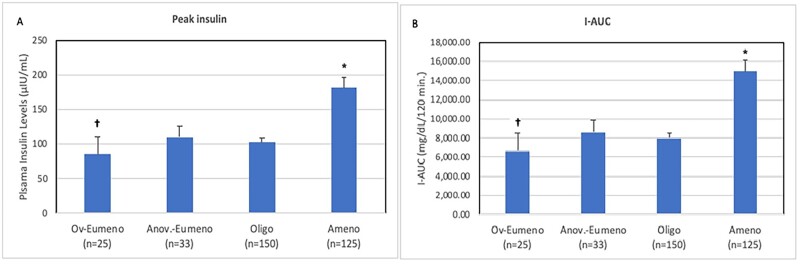
Insulin response to glucose challenge
Table II and Fig. 1 depict the insulin response among all the PCOS participants according to the four categories of menstrual dysfunction. At baseline, mean fasting insulin levels were similar across the menstrual groups. The degree of hyperinsulinemia in response to the glucose challenge (i.e. elevated mean 1- and 2-h insulin, peak insulin and I-AUC) was higher in Ameno vs. Oligo and Ov-Eumeno subjects, but not Anov-Eumeno (Table II and Fig. 1). These values were also higher for Anov-Eumeno versus Ov-Eumeno, but not Oligo patients. The 2 h and peak insulin levels were higher in Oligo versus Ov-Eumeno (Table II and Fig. 1). Separately, we also assessed the insulin response among all the PCOS participants, including PCOS women with Poly or those with Oligo categorized into Early-Oligo (bleeding intervals 35 days to 6 weeks) and Late-Oligo (bleeding intervals 6 weeks to 3 months) (Brower et al., 2013), as depicted in Supplementary Fig. S1 and Table SI.
Table III.
Odds ratio for association of menstrual cyclicity and glycemic abnormalities in hyperandrogenic PCOS.
| Ov-Eumeno vs Oligo or Ameno | ||||||
|---|---|---|---|---|---|---|
| Glycemic abnormalities | Ov-Eumeno vs Oligo |
Ov-Eumeno vs Ameno |
||||
| OR | 95% CI | Adjusted P-value | OR | 95% CI | Adjusted P-value | |
| Abnormal glucose tolerance | 0.75 | 0.35–1.60 | 0.453 | 0.24 | 0.05–1.10 | 0.066 |
| Prediabetes | 0.75 | 0.35–1.60 | 0.767 | 0.44 | 0.09–2.08 | 0.303 |
| Type 2 Diabetes | 0.001 | – | 0.987 | 4.85 | – | 0.986 |
| Elevated 1-h PG* | 0.40 | 0.14–1.11 | 0.080 | 10.20 | 1.31–79.74 | 0.027 |
| Anov-Eumeno vs Ov-Eumeno or Oligo | ||||||
|---|---|---|---|---|---|---|
| Glycemic abnormalities | Anov-Eumeno vs Ov-Eumeno |
Anov-Eumeno vs Oligo |
||||
| OR | 95% CI | Adjusted P-value | OR | 95% CI | Adjusted P-value | |
| Abnormal glucose tolerance | 0.80 | 0.45–1.43 | 0.448 | 0.93 | 0.32–2.72 | 0.896 |
| Prediabetes | 1.86 | 1.47–1.56 | 0.620 | 0.82 | 0.25–2.67 | 0.736 |
| Type 2 Diabetes | 0.006 | – | 0.995 | 1.70 | 0.20–14.46 | 0.626 |
| Elevated 1-h PG* | 0.58 | 0.28–1.22 | 0.150 | 1.23 | 0.43–3.55 | 0.697 |
| Ameno vs Anov-Eumeno or Oligo | ||||||
|---|---|---|---|---|---|---|
| Glycemic abnormalities | Ameno vs Anov-Eumeno |
Ameno vs Oligo |
||||
| OR | 95% CI | Adjusted P-value | OR | 95% CI | Adjusted P-value | |
| Abnormal glucose tolerance | 1.48 | 0.88–2.50 | 0.143 | 2.31 | 1.26–4.24 | 0.007 |
| Prediabetes | 1.23 | 0.68–2.21 | 0.494 | 1.88 | 0.91–3.87 | 0.089 |
| Type 2 Diabetes | 1.95 | 0.69–5.64 | 0.210 | 2.22 | 0.88–5.62 | 0.092 |
| Elevated 1-h PG* | 1.99 | 0.72–5.52 | 0.185 | 1.56 | 0.87–2.81 | 0.139 |
| Eumeno combined (Ov-Eumono and Anov-Eumeno) vs Ameno or Oligo | ||||||
|---|---|---|---|---|---|---|
| Glycemic abnormalities | Eumeno (Combined) vs Oligo |
Eumeno (Combined) vs Oligo |
||||
| OR | 95% CI | Adjusted P-value | OR | 95% CI | Adjusted P-value | |
| Abnormal glucose tolerance | 1.25 | 0.50–3.13 | 0.638 | 1.66 | 1.06–2.59 | 0.028 |
| Prediabetes | 0.97 | 0.35–2.66 | 0.949 | 1.33 | 0.81–2.19 | 0.259 |
| Type 2 Diabetes | 0.31 | 0.37–25.77 | 0.295 | 2.64 | 0.94–7.45 | 0.067 |
| Elevated 1-h PG* | 0.14 | 0.19–1.27 | 0.143 | 1.79 | 1.14–2.83 | 0.012 |
1-h plasma glucose (PG) ≥155 mg/dl during oGTT.
Ameno, amenorrhea; Anov-Eumeno, anovulatory eumenorrhea; Oligo, oligomenorrhea; OR, odds ratio; Ov-Eumeno, ovulatory eumenorrhea; PCOS, polycystic ovary syndrome.
P values in italics and bold are considered statistically significant.
Figure 1.
Differences in the degree of hyperinsulinemia in hyperandrogenic PCOS women according to severity of menstrual cyclicity. The degree of hyperinsulinemia, reflected by post-challenge peak insulin levels (A) and AUC for insulin [I-AUC] (B) according to four categories of menstrual cyclicity described as ovulatory eumenorrhea (Ov-Eumeno; bleeding intervals 26- to 34-day with ovulation confirmed by a menstrual cycle Days 22–24 progesterone [P4] level), anovulatory eumenorrhea (Anov-Eumeno; bleeding intervals 26- to 34-day with anovulation confirmed by a menstrual cycle Days 22–24 P4 level); oligomenorrhea (Oligo, bleeding intervals 35 days to 3 months) and amenorrhea (Ameno; bleeding intervals >3 months). Error bars represent SEM. Values have not been adjusted for BMI. *Denotes significantly higher degree of hyperinsulinemia in Ameno than in other menstrual categories. †Denotes significantly lower degree of hyperinsulinemia in Ov-Eumeno than in Anov-Eumeno, or Oligo. PCOS, polycystic ovary syndrome.
Glucose response to glucose challenge and prevalence of glycemic abnormalities
Post-challenge glucose responses according to the severity of their menstrual dysfunction are also depicted in Table II. At baseline, mean fasting glucose levels were similar across the four menstrual groups. The G-AUC was higher for Ameno versus Oligo or Ov-Eumeno subjects and higher for Oligo versus Ov-Eumeno. The mean 1-h glucose was higher in Ameno or Oligo versus Ov-Eumeno, and the 2-h glucose was higher for Ameno vs Oligo (Table II).
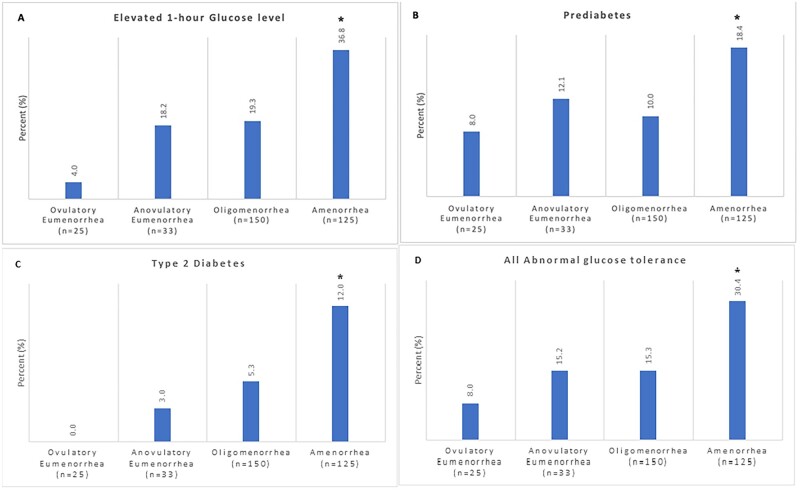
The prevalence of glycemic abnormalities according to the severity of menstrual dysfunction are also depicted in Table II and Fig. 2. Of the 333 participants analyzed, 265 (79.6%) had normal glucose tolerance, 86 (25.8%) had an elevated 1-h glucose (i.e. ≥155 mg/dl) and 68 (20.4%) had AGT, including 44 (13.2%) with prediabetes and 24 (7.2%) with T2DM. A significant trend was detected for hyperglycemia at 1 h, which was observed in 36.8% of PCOS participants with Ameno, 22.0% with Oligo, 18.2% with Anov-Eumeno and 4.0% with Ov-Eumeno (P = 0.001) (Fig. 2A). No significant difference in trend were detected in the prevalence of prediabetes (18.4%, 10.0%, 12.1% and 8.0% of Ameno, Oligo, Anov-Eumeno and Ov-Eumeno, respectively, P = 0.079) (Fig. 2B), although T2DM was detected in significantly more Ameno PCOS women (12.0%, 5.3%, 3.0% and 0% in Ameno, Oligo, Anov-Eumeno and Ov-Eumeno, respectively, P = 0.008) (Fig. 2C). Overall, the proportion of PCOS participants with any AGT was highest for Ameno and lowest for Ov-Eumeno patients (30.4%, 15.3%, 15.2% and 8.0% for Ameno, Oligo, Anov-Eumeno and Ov-Eumeno, respectively, P = 0.008) (Fig. 2D). In logistic regression, adjusting for BMI, PCOS Ameno patients were more likely to have: (i) an AGT than Oligo patients (odds ratio [OR]: 2.3; 95% CI: 1.3 to 4.2); (ii) an elevated 1-h glucose (OR: 10.2; CI: 1.3–79.7) than those with Ov-Eumeno; and (iii) both a higher frequency of AGT (OR: 1.7; CI: 1.1–2.6) and an elevated 1-h glucose (OR: 1.8; CI: 1.1–2.8) than those with Anov-Eumeno or Ov-Eumeno combined (Table III).
Figure 2.
Differences in the prevalence of glycemic abnormalities in hyperandrogenic PCOS women according to severity of menstrual cyclicity. The prevalence of glycemic abnormalities in PCOS subjects identified as having an elevated 1-h glucose level (i.e. ≥155 mg/dl) (A), prediabetes (B), Type 2 diabetes (C) and all abnormal glucose tolerance (AGT) combined (D) in Ov-Eumeno (n = 25), Anov-Eumeno (n = 33), Oligo (n = 150) and Ameno (n = 125) PCOS women is depicted. Analyses have been adjusted for BMI. *Denotes significantly higher or trend toward higher prevalence of glycemic abnormalities in Ameno than in other menstrual categories. PCOS, polycystic ovary syndrome.
Discussion
We previously reported that in oligo-ovulatory women with PCOS, the severity of menstrual dysfunction was associated with the presence of and degree of IR (Brower et al., 2013; Ezeh et al., 2021). In this prospective cohort study, we provide novel evidence demonstrating that the severity of menstrual cyclicity is also linked to hyperinsulinemia and glycemic abnormalities in women with hyperandrogenic PCOS (i.e. Rotterdam A, B and C phenotypes), a population shown to have a four-fold higher prevalence of T2DM and a more rapid onset of T2DM than PCOS women with a normoandrogenic phenotype (Persson et al., 2021).
Amenorrheic women with PCOS had greater degrees of hyperinsulinemia compared to those who were oligomenorrheic or eumenorrheic. Furthermore, and despite their higher degree of hyperinsulinemia, PCOS women with amenorrhea demonstrated a higher degree of dysglycemia, as indicated by their higher 1- and 2-h glucose levels, and G-AUC than those with oligomenorrhea or eumenorrhea. Hyperandrogenic PCOS women with amenorrhea, compared to those with oligomenorrhea or eumenorrhea, were also more likely to develop an elevated 1-h glucose (i.e. ≥ 155 mg/dl), which has been suggested to be a more robust predictor than fasting or 2-h glucose for the future development of metabolic syndrome, β-cell dysfunction, T2DM and coronary vascular disease (Cubeddu and Hoffmann, 2010; Bianchi et al., 2013; Peddinti et al., 2019; Jagannathan et al., 2020). These observations occurred regardless of age, racial/ethnic composition, WHR, degree of hyperandrogenism and even after adjustment for BMI, suggesting that the elevated cardiometabolic risk profile in PCOS participants with amenorrhea is more than can be explained by these cofounding factors. Overall, these data highlight the value of amenorrhea as a marker for the increased risk of hyperinsulinemia and dysglycemia in PCOS.
Notably, hyperandrogenic PCOS women with ovulatory eumenorrhea (Phenotype C) had less post-challenge hyperinsulinemia than anovulatory PCOS women (Phenotypes A/B), consistent with other studies demonstrating that PCOS women with the latter phenotype have higher risk of IR, fasting hyperinsulinemia and metabolic syndrome than those with other types of PCOS phenotypes (Moghetti et al., 2013). These data also affirm the need to evaluate ovulatory function in PCOS women with apparent eumenorrhea.
Our findings also indicate that PCOS women with the most severe form of oligo-ovulation (i.e. amenorrhea), and to some extent those with oligomenorrhea or anovulatory eumenorrhea, had higher degrees of hyperinsulinemia than those with ovulatory eumenorrhea, despite no significant differences in hyperandrogenism. Therefore, it appears that ovulation status (and secondarily, the severity of menstrual cyclicity) are linked more closely to the degree of hyperinsulinemia rather than hyperandrogenism. This hypothesis is supported by studies showing that chronic hyperinsulinemia disrupts ovarian follicular development (Thong et al., 2020), reports indicating that increased prevalence of PCOS in women with Type 1 diabetes is related to the ovarian impact of supraphysiologic doses of exogenous insulin not exposed to hepatic degradation (Thong et al., 2020; Łebkowska et al., 2021), and studies showing that reduction in hyperinsulinemia with lifestyle changes, bariatric surgery or insulin sensitizers enhance ovulation and restore menstrual cyclicity (Morley et al., 2017).
The potential mechanisms linking amenorrhea to glycemic abnormalities are not fully understood, and may reflect the pathogenic sequalae of hyperinsulinemia, given that hyperinsulinemia has been implicated in the development of IR, metabolic syndrome and many diseases including obesity, T2DM, coronary vascular disease and cancer (Rizza et al., 1985; Haffner et al., 1986; Martin et al., 1992; Després et al., 1996; Abdul-Ghani and DeFronzo, 2021), instead of hyperinsulinemia just representing mere compensation for IR (Kahn et al., 1993). Furthermore, studies in rodents indicate that chronic hyperinsulinemia uncouples insulin-mediated regulation of glucose transporter-4 and the Forkhead box protein O1 (FoXO1) transcription factor (Gonzalez et al., 2011) and impairs insulin-mediated suppression of circulating non-esterified free fatty acid levels (Koopmans et al., 1999) in adipocytes, leading to adipocyte IR. Furthermore, pharmacological suppression of hyperinsulinemia results in improvements in metabolic syndrome and increased insulin sensitivity, reduction in hyperglycemia (Templeman et al., 2017; Loves et al., 2018) and extension of lifespan (Templeman et al., 2017).
Additionally, hyperandrogenism is known to be underpinned, at least in part, by hyperinsulinemia, via its synergy with LH as a co-gonadotropin to augment ovarian androgen biosynthesis, increased adrenocorticotropic hormone-induced adrenal androgen production, and increased testosterone bioavailability through suppression of hepatic SHBG biosynthesis (Barbieri et al., 1986; Moghetti et al., 1996). This raises the possibility that further increases in hyperandrogenism may exacerbate IR and increase the risk of glycemic abnormalities. Indeed, several studies have suggested that hyperandrogenism contributes to IR in PCOS via adipocyte dysfunction including altered body composition (Ezeh et al., 2014), altered adipocyte morphology (O'Reilly et al., 2017; Dumesic et al., 2019) and non-esterified free fatty acids kinetics (Ezeh et al., 2019), and through inhibition of adipogenesis or/and lipolysis, and promotion of lipogenesis (Dumesic et al., 2019), increased visceral adiposity (O'Reilly et al., 2017; Dumesic et al., 2019) or an increase in adipose tissue IR (Ezeh et al., 2020a). However, our findings indicating that the degree of hyperinsulinemia, but not hyperandrogenism, tracked with the severity of menstrual dysfunction do not support the concept of hyperandrogenism-induced glycemic abnormalities. It should be noted that the evidence that hyperandrogenism contributes to glycemic abnormalities or metabolic risks of PCOS remains overall unresolved. Some longitudinal studies have observed no associations between hyperandrogenism and metabolic syndrome, T2DM, coronary vascular disease or stroke (Calderon-Margalit et al., 2010; Polotsky et al., 2014; LeBlanc et al., 2017; Kim et al., 2018).
The 75-g oGTT is the gold standard for the screening, diagnosis and management of glycemic abnormalities, including prediabetes and T2DM (American Diabetes Association, 2017; Jagannathan et al., 2020). Indeed, many expert organizations recommend routine screening of women with PCOS using a 2-h oGTT (e.g. Endocrine Society, American Association of Clinical Endocrinologists, American College of Endocrinology, and Androgen Excess and PCOS Society, and the International PCOS Guidelines) (Legro et al., 2013; Teede et al., 2018; Goodman et al., 2015), recognizing the limitations of simpler screening tests such as fasting glucose or HbA1C levels in PCOS (Legro et al., 1999; Lerchbaum et al., 2013). Nonetheless, for many regions of the world and for many patients with PCOS, obtaining an oGTT is not as easy as these guidelines would suggest. Identification of PCOS women at greatest risk of glycemic abnormalities, potentially using a readily obtainable marker, such as the degree of clinically evident menstrual dysfunction, may help refine the indications for oGTT in PCOS and maximize the efficiency of prevention strategies.
The strengths of our study include the use of well-phenotyped PCOS participants, its prospective design, and the unique classification of PCOS phenotypes according to the degree of menstrual dysfunction. Our study was limited by its cross-sectional nature, and concerns that the number of subjects in some phenotypic subsets were small (e.g. those with ovulatory or anovulatory eumenorrhea). We also excluded non-hyperandrogenic PCOS (i.e. Rotterdam phenotype D) from our study. The use of a 5- or 9-point 3-h oGTT instead of a 3-point 2-h oGTT could also have enabled simultaneous measurements of plasma C-peptide levels to potentially obtain a more robust assessment of β-cell function and determination of insulin clearance and insulin sensitivity. There is also the potential for measurement bias, since in 51 participants total T was measured using a high-quality RIA method, while in the remaining total T was assessed using LC-MS/MS, and the number of subjects assessed using LC-MS/MS was significantly greater in Oligo and lower in Ameno subjects (data not shown). However, our previous studies indicate a very good correlation between circulating total and free T levels determined by either method (Salameh et al., 2014; Ezeh et al., 2019). Furthermore, in the current study, the distribution of demographic parameters and androgen measures were similar across the four menstrual categories for subjects recruited at either at UAB or CSMC (data not shown). Finally, while our data support the use of the degree of menstrual dysfunction as a marker of metabolic dysfunction, we should note that this observation does not indicate causality.
In conclusion, a history of amenorrhea is associated with greater degrees of post-challenge hyperinsulinemia in all ethnic/racial groups studied (women of African American, White Hispanic and non-White Hispanic ancestry), compared to those with oligomenorrhea or eumenorrhea (either ovulatory or anovulatory), which may explain their greater prevalence of glycemic abnormalities. Our data suggest that menstrual history, relatively non-invasive and easy to obtain information, can be a simple but clinically important marker of metabolic dysfunction risk. Furthermore, our data also suggests that if a screening oGTT is to be done in a PCOS patient, then insulin levels should also be assessed during the test (e.g. at 0, 1 and 2 h) to determine her degree of hyperinsulinemia. These findings can potentially be used to refine the guidelines for the screening of PCOS women for metabolic dysfunction and risk.
Supplementary data
Supplementary data are available at Human Reproduction online.
Data availability
The data underlying this article will be shared upon reasonable request to the corresponding author.
Supplementary Material
Acknowledgements
The authors thank Marita Pall and Ruchi Mathur for assistance in phenotyping the participants.
Authors’ roles
U.E. and R.A. designed the study, identified and phenotyped the participants, researched the data, contributed to the discussion, wrote the manuscript, and reviewed and edited the manuscript. M.D.P. assisted in phenotyping the participants, obtained study samples, researched the data and reviewed the manuscript. U.E. also performed additional statistical analyses. R.A. is the guarantor of this work and, as such, takes responsibility for the data integrity and accuracy of the data analysis.
Funding
This work was supported in part by grants R01-DK073632 and R01-HD29364 from the NIH and an endowment of the Helping Hand of Los Angeles, Inc. (to R.A.). M.D.P. has no competing interests to declare. U.E. is an investor in Concentric Analgesics, Inc. R.A. serves as a consultant for Spruce Biosciences and Fortress Biotech, and Aurora Forge; serves on the Medical/Scientific Advisory Board of PCOS Challenge, and CARES Foundation; and received honoraria from Virtual Int’l Congress on the future of Women’s Health-PCOS and King Abdulaziz University in 2021.
Conflict of interest
M.D.P. has no competing interests to declare. U.E. is an investor in Concentric Analgesics, Inc. R.A. serves as a consultant for Spruce Biosciences and Fortress Biotech, and Aurora Forge; serves on the Medical/Scientific Advisory Board of PCOS Challenge, and CARES Foundation; and received honoraria from Virtual Int’l Congress on the future of Women’s Health-PCOS and King Abdulaziz University in 2021.
References
- Abdul-Ghani M, DeFronzo RA.. Insulin resistance and hyperinsulinemia: the egg and the chicken. J Clin Endocrinol Metab 2021;106:e1897–e1899. [DOI] [PMC free article] [PubMed] [Google Scholar]
- American Diabetes Association. 2. Classification and diagnosis of diabetes. Diabetes Care 2017;40(Suppl 1):S11–S24. [DOI] [PubMed] [Google Scholar]
- Azziz R, Bradley EL Jr, Potter HD, Parker CR Jr, Boots LR.. Chronic hyperinsulinemia and the adrenal androgen response to acute corticotropin-(1-24) stimulation in hyperandrogenic women. Am J Obstet Gynecol 1995;172:1251–1256. [DOI] [PubMed] [Google Scholar]
- Azziz R, Carmina E, Dewailly D, Diamanti-Kandarakis E, Escobar-Morreale HF, Futterweit W, Janssen OE, Legro RS, Norman RJ, Taylor AE. et al. ; Androgen Excess Society. Positions statement: criteria for defining polycystic ovary syndrome as a predominantly hyperandrogenic syndrome: an Androgen Excess Society guideline. J Clin Endocrinol Metab 2006;91:4237–4245. [DOI] [PubMed] [Google Scholar]
- Azziz R, Sanchez LA, Knochenhauer ES, Moran C, Lazenby J, Stephens KC, Taylor K, Boots LR.. Androgen excess in women: experience with over 1000 consecutive patients. J Clin Endocrinol Metab 2004a;89:453–462. [DOI] [PubMed] [Google Scholar]
- Azziz R, Woods KS, Reyna R, Key TJ, Knochenhauer ES, Yildiz BO.. The prevalence and features of the polycystic ovary syndrome in an unselected population. J Clin Endocrinol Metab 2004b;89:2745–2749. [DOI] [PubMed] [Google Scholar]
- Barbieri R, Makris A, Randall R, Daniels G, Kistner R, Ryan K.. Insulin stimulates androgen accumulation in incubations of ovarian stroma obtained from women with hyperandrogenism. J Clin Endocrinol Metab 1986;62:904–910. [DOI] [PubMed] [Google Scholar]
- Berni TR, Morgan CL, Rees DA.. Women with polycystic ovary syndrome have an increased risk of major cardiovascular events: a population study. J Clin Endocrinol Metab 2021;106:e3369–e3380. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bianchi C, Miccoli R, Trombetta M, Giorgino F, Frontoni S, Faloia E, Marchesini G, Dolci MA, Cavalot F, Cavallo G. et al. ; GENFIEV Investigators. Elevated 1-hour postload plasma glucose levels identify subjects with normal glucose tolerance but impaired β-cell function, insulin resistance, and worse cardiovascular risk profile: the GENFIEV study. J Clin Endocrinol Metab 2013;98:2100–2105. [DOI] [PubMed] [Google Scholar]
- Boots LR, Potter S, Potter D, Azziz R.. Measurement of total serum testosterone levels using commercially available kits: high degree of between-kit variability. Fertil Steril 1998;69:286–292. [DOI] [PubMed] [Google Scholar]
- Bozdag G, Mumusoglu S, Zengin D, Karabulut E, Yildiz BO.. The prevalence and phenotypic features of polycystic ovary syndrome: a systematic review and meta-analysis. Hum Reprod 2016;31:2841–2855. [DOI] [PubMed] [Google Scholar]
- Brower M, Brennan K, Pall M, Azziz R.. The severity of menstrual dysfunction as a predictor of insulin resistance in PCOS. J Clin Endocrinol Metab 2013;98:E1967–E1971. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Calderon-Margalit R, Schwartz SM, Wellons MF, Lewis CE, Daviglus ML, Schreiner PJ, Sternfeld B, Williams OD, Lewis CE, Azziz R. et al. Prospective association of serum androgens and sex hormone-binding globulin with subclinical cardiovascular disease in young adult women: the “Coronary Artery Risk Development in Young Adults” women's study. J Clin Endocrinol Metab 2010;95:4424–4431. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Celik C, Tasdemir N, Abali R, Bastu E, Yilmaz M.. Progression to impaired glucose tolerance or type 2 diabetes mellitus in polycystic ovary syndrome: a controlled follow-up study. Fertil Steril 2014;101:1123–1128.e1. [DOI] [PubMed] [Google Scholar]
- Cubeddu LX, Hoffmann IS.. One-hour postload plasma glucose levels, a predictor of additional risk for diabetes: prevalence, mechanisms, and associated cardiovascular and metabolic risk factors in Hispanics. Metab Syndr Relat Disord 2010;8:395–402. [DOI] [PubMed] [Google Scholar]
- Després J-P, Lamarche B, Mauriège P, Cantin B, Dagenais GR, Moorjani S, Lupien PJ.. Hyperinsulinemia as an independent risk factor for ischemic heart disease. N Engl J Med 1996;334:952–958. [DOI] [PubMed] [Google Scholar]
- Dumesic DA, Phan JD, Leung KL, Grogan TR, Ding X, Li X, Hoyos LR, Abbott DH, Chazenbalk GD.. Adipose insulin resistance in normal-weight women with polycystic ovary syndrome. J Clin Endocrinol Metab 2019;104:2171–2183. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dunaif A, Finegood DT.. Beta-cell dysfunction independent of obesity and glucose intolerance in the polycystic ovary syndrome. J Clin Endocrinol Metab 1996;81:942–947. [DOI] [PubMed] [Google Scholar]
- Dunaif A, Segal KR, Futterweit W, Dobrjansky A.. Profound peripheral insulin resistance, independent of obesity, in polycystic ovary syndrome. Diabetes 1989;38:1165–1174. [DOI] [PubMed] [Google Scholar]
- Ehrmann DA, Barnes RB, Rosenfield RL, Cavaghan MK, Imperial J.. Prevalence of impaired glucose tolerance and diabetes in women with polycystic ovary syndrome. Diabetes Care 1999;22:141–146. [DOI] [PubMed] [Google Scholar]
- Ehrmann DA, Kasza K, Azziz R, Legro RS, Ghazzi MN; PCOS/Troglitazone Study Group. Effects of race and family history of type 2 diabetes on metabolic status of women with polycystic ovary syndrome. J Clin Endocrinol Metab 2005;90:66–71. [DOI] [PubMed] [Google Scholar]
- Ezeh U, Arzumanyan Z, Lizneva D, Mathur R, Chen YH, Boston RC, Chen YI, Azziz R.. Alterations in plasma non-esterified fatty acid (NEFA) kinetics and relationship with insulin resistance in polycystic ovary syndrome. Hum Reprod 2019;34:335–344. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ezeh U, Chen IY, Chen YH, Azziz R.. Adipocyte insulin resistance in PCOS: relationship with GLUT-4 expression and whole-body glucose disposal and β-cell function. J Clin Endocrinol Metab 2020a;105:e2408–e2420. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ezeh U, Ezeh C, Pisarska MD, Azziz R.. Menstrual dysfunction in polycystic ovary syndrome: association with dynamic state insulin resistance rather than hyperandrogenism. Fertil Steril 2021;115:1557–1568. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ezeh U, Huang A, Landay M, Azziz R.. Long-term response of hirsutism and other hyperandrogenic symptoms to combination therapy in polycystic ovary syndrome. J Womens Health (Larchmt) 2018;27:892–902. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ezeh U, Ida Chen YD, Azziz R.. Racial and ethnic differences in the metabolic response of polycystic ovary syndrome. Clin Endocrinol (Oxf) 2020b;93:163–172. [DOI] [PubMed] [Google Scholar]
- Ezeh U, Pall M, Mathur R, Azziz R.. Association of fat to lean mass ratio with metabolic dysfunction in women with polycystic ovary syndrome. Hum Reprod 2014;29:1508–1517. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gaete X, Vivanco M, Eyzaguirre FC, López P, Rhumie HK, Unanue N, Codner E.. Menstrual cycle irregularities and their relationship with HbA1c and insulin dose in adolescents with type 1 diabetes mellitus. Fertil Steril 2010;94:1822–1826. [DOI] [PubMed] [Google Scholar]
- Gonzalez E, Flier E, Molle D, Accili D, McGraw TE.. Hyperinsulinemia leads to uncoupled insulin regulation of the GLUT4 glucose transporter and the FoxO1 transcription factor. Proc Natl Acad Sci USA 2011;108:10162–10167. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Goodman NF, Cobin RH, Futterweit W, Glueck JS, Legro RS, Carmina E.. American Association of Clinical Endocrinologists, American College of Endocrinology, and Androgen Excess and PCOS Society disease state clinical review: guide to the best practices in the evaluation and treatment of polycystic ovary syndrome—part 2. Endocr Pract 2015;21:1415–1426. [DOI] [PubMed] [Google Scholar]
- Haffner SM, Stern MP, Hazuda HP, Pugh JA, Patterson JK.. Hyperinsulinemia in a population at high risk for non-insulin-dependent diabetes mellitus. N Engl J Med 1986;315:220–224. [DOI] [PubMed] [Google Scholar]
- Jagannathan R, Neves JS, Dorcely B, Chung ST, Tamura K, Rhee M, Bergman M.. The oral glucose tolerance test: 100 years later. Diabetes Metab Syndr Obes 2020;13:3787–3805. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Joham AE, Kakoly NS, Teede HJ, Earnest A.. Incidence and predictors of hypertension in a cohort of Australian women with and without polycystic ovary syndrome. J Clin Endocrinol Metab 2021;106:1585–1593. [DOI] [PubMed] [Google Scholar]
- Kahn SE, Prigeon RL, McCulloch DK, Boyko EJ, Bergman RN, Schwartz MW, Neifing JL, Ward WK, Beard JC, Palmer JP.. Quantification of the relationship between insulin sensitivity and beta-cell function in human subjects. Evidence for a hyperbolic function. Diabetes 1993;42:1663–1672. [DOI] [PubMed] [Google Scholar]
- Kim C, Aroda VR, Goldberg RB, Younes N, Edelstein SL, Carrion-Petersen M, Ehrmann DA; Diabetes Prevention Program Outcomes Study Group. Androgens, irregular menses, and risk of diabetes and coronary artery calcification in the diabetes prevention program. J Clin Endocrinol Metab 2018;103:486–496. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Knochenhauer ES, Key TJ, Kahsar-Miller M, Waggoner W, Bots LR, Azziz R.. Prevalence of polycystic ovary syndrome in unselected black and white women of the southeastern United States: a prospective study. J Clin Endocrinol Metab 1998;83:3078–3082. [DOI] [PubMed] [Google Scholar]
- Koopmans SJ, Kushwaha RS, DeFronzo RA.. Chronic physiologic hyperinsulinemia impairs suppression of plasma free fatty acids and increases de novo lipogenesis but does not cause dyslipidemia in conscious normal rats. Metabolism 1999;48:330–337. [DOI] [PubMed] [Google Scholar]
- Łebkowska A, Adamska A, Krentowska A, Uruska A, Rogowicz-Frontczak A, Araszkiewicz A, Ożegowska K, Hryniewicka J, Leśniewska M, Wender-Ożegowska E. et al. The influence of prepubertal onset of type 1 diabetes and age of menarche on polycystic ovary syndrome diagnosis. J Clin Endocrinol Metab 2021;106:1811–1820. [DOI] [PubMed] [Google Scholar]
- LeBlanc ES, Kapphahn K, Hedlin H, Desai M, Parikh NI, Liu S, Parker DR, Anderson M, Aroda V, Sullivan S. et al. Reproductive history and risk of type 2 diabetes mellitus in postmenopausal women: findings from the Women's Health Initiative. Menopause 2017;24:64–72. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Legro RS, Arslanian SA, Ehrmann DA, Hoeger KM, Murad MH, Pasquali R, Welt CK; Endocrine Society. Diagnosis and treatment of polycystic ovary syndrome: an Endocrine Society clinical practice guideline. J Clin Endocrinol Metab 2013;98:4565–4592. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Legro RS, Kunselman AR, Dodson WC, Dunaif A.. Prevalence and predictors of risk for type 2 diabetes mellitus and impaired glucose tolerance in polycystic ovary syndrome: a prospective, controlled study in 254 affected women. J Clin Endocrinol Metab 1999;84:165–169. [DOI] [PubMed] [Google Scholar]
- Lerchbaum E, Schwetz V, Giuliani A, Obermayer-Pietsch B.. Assessment of glucose metabolism in polycystic ovary syndrome: HbA1c or fasting glucose compared with the oral glucose tolerance test as a screening method. Hum Reprod 2013;28:2537–2544. [DOI] [PubMed] [Google Scholar]
- Lizneva D, Suturina L, Walker W, Brakta S, Gavrilova-Jordan L, Azziz R.. Criteria, prevalence, and phenotypes of polycystic ovary syndrome. Fertil Steril 2016;106:6–15. [DOI] [PubMed] [Google Scholar]
- Loves S, van Groningen L, Filius M, Mekking M, Brandon T, Tack CJ, Hermus A, de Boer H.. Effects of diazoxide-mediated insulin suppression on glucose and lipid metabolism in nondiabetic obese men. J Clin Endocr Metab 2018;103:2346–2353. [DOI] [PubMed] [Google Scholar]
- Martin BC, Warram JH, Krolewski AS, Soeldner JS, Kahn CR, Martin BC, Bergman RN.. Role of glucose and insulin resistance in development of type 2 diabetes mellitus: results of a 25-year follow-up study. Lancet 1992;340:925–929. [DOI] [PubMed] [Google Scholar]
- Meyer ML, Sotres-Alvarez D, Steiner AZ, Cousins L, Talavera GA, Cai J, Daviglus ML, Loehr LR.. Polycystic ovary syndrome signs and metabolic syndrome in premenopausal Hispanic/Latina women: the HCHS/SOL study. J Clin Endocrinol Metab 2020;105:e447–e456. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Moghetti P, Castello R, Negri C, Tosi F, Spiazzi GG, Brun E, Balducci R, Toscano V, Muggeo M.. Insulin infusion amplifies 17-alpha hydroxycorticosteroid intermediates response to adrenocorticotropin in hyperandrogenic women: apparent relative impairment of 17, 20-lyase activity. J Clin Endocrinol Metab 1996;81:881–886. [DOI] [PubMed] [Google Scholar]
- Moghetti P, Tosi F, Bonin C, Di Sarra D, Fiers T, Kaufman JM, Giagulli VA, Signori C, Zambotti F, Dall'Alda M. et al. Divergences in insulin resistance between the different phenotypes of the polycystic ovary syndrome. J Clin Endocrinol Metab 2013;98:E628–E637. [DOI] [PubMed] [Google Scholar]
- Morley LC, Tang T, Yasmin E, Norman RJ, Balen AH.. Insulin-sensitising drugs (metformin, rosiglitazone, pioglitazone, D-chiro-inositol) for women with polycystic ovary syndrome, oligo amenorrhoea and subfertility. Cochrane Database Syst Rev 2017;11:CD003053. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Munro MG, Critchley HOD, Fraser IS; FIGO Menstrual Disorders Committee. The two FIGO systems for normal and abnormal uterine bleeding symptoms and classification of causes of abnormal uterine bleeding in the reproductive years: 2018 revisions. Int J Gynaecol Obstet 2018;143:393–408. [DOI] [PubMed] [Google Scholar]
- O'Reilly MW, Kempegowda P, Walsh M, Taylor AE, Manolopoulos KN, Allwood JW, Semple RK, Hebenstreit D, Dunn WB, Tomlinson JW, Arlt W.. AKR1C3-mediated adipose androgen generation drives lipotoxicity in women with polycystic ovary syndrome. J Clin Endocrinol Metab 2017;102:3327–3339. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Peddinti G, Bergman M, Tuomi T, Groop L.. 1-hour post-OGTT glucose improves the early prediction of type 2 diabetes by clinical and metabolic markers. J Clin Endocrinol Metab 2019;104:1131–1140. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Persson S, Elenis E, Turkmen S, Kramer MS, Yong EL, Poromaa IS.. Higher risk of type 2 diabetes in women with hyperandrogenic polycystic ovary syndrome. Fertil Steril 2021;116:862–871. [DOI] [PubMed] [Google Scholar]
- Polotsky AJ, Allshouse AA, Crawford SL, Harlow SD, Khalil N, Kazlauskaite R, Santoro N, Legro RS.. Hyperandrogenic oligomenorrhea and metabolic risks across menopausal transition. J Clin Endocrinol Metab 2014;99:2120–2127. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Polotsky AJ, Allshouse A, Crawford SL, Harlow SD, Khalil N, Santoro N, Legro RS.. Relative contributions of oligomenorrhea and hyperandrogenemia to the risk of metabolic syndrome in midlife women. J Clin Endocrinol Metab 2012;97:E868–E877. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Riestenberg C, Jagasia A, Markovic D, Buyalos RP, Azziz R.. Health care-related economic burden of polycystic ovary syndrome in the United States: pregnancy-related and long-term health consequences. J Clin Endocrinol Metab 2021;doi:10.1210/clinem/dgab613. [DOI] [PubMed] [Google Scholar]
- Rizza RA, Mandarino LJ, Genest J, Baker BA, Gerich JE.. Production of insulin resistance by hyperinsulinemia in man. Diabetologia 1985;28:70–75. [DOI] [PubMed] [Google Scholar]
- Rodgers RJ, Avery JC, Moore VM, Davies MJ, Azziz R, Stener-Victorin E, Moran LJ, Robertson SA, Stepto NK, Norman RJ. et al. Complex diseases and co-morbidities: polycystic ovary syndrome and type 2 diabetes mellitus. Endocr Connect 2019;8:R71–R75. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rotterdam ESHRE/ASRM-Sponsored PCOS consensus workshop group. Revised 2003 consensus on diagnostic criteria and long-term health risks related to polycystic ovary syndrome (PCOS). Hum Reprod 2004;19:41. [DOI] [PubMed] [Google Scholar]
- Ryu KJ, Kim MS, Kim HK, Kim YJ, Yi KW, Shin JH, Hur JY, Kim T, Park H.. Risk of type 2 diabetes is increased in nonobese women with polycystic ovary syndrome: the National Health Insurance Service-National Sample Cohort Study. Fertil Steril 2021;115:1569–1575. [DOI] [PubMed] [Google Scholar]
- Salameh WA, Redor-Goldman MM, Clarke NJ, Mathur R, Azziz R, Reitz RE.. Specificity and predictive value of circulating testosterone assessed by tandem mass spectrometry for the diagnosis of polycystic ovary syndrome by the National Institutes of Health 1990 criteria. Fertil Steril 2014;101:1135–1141.e2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Solomon CG, Hu FB, Dunaif A, Rich-Edwards J, Willett WC, Hunter DJ, Colditz GA, Speizer FE, Manson JE.. Long or highly irregular menstrual cycles as a marker for risk of type 2 diabetes mellitus. JAMA 2001;286:2421–2426. [DOI] [PubMed] [Google Scholar]
- Steiner MJ, Hertz-Picciotto I, Taylor D, Schoenbach V, Wheeless A.. Retrospective vs. prospective coital frequency and menstrual cycle length in a contraceptive effectiveness trial. Ann Epidemiol 2001;11:428–433. [DOI] [PubMed] [Google Scholar]
- Tabák AG, Herder C, Rathmann W, Brunner EJ, Kivimäki M.. Prediabetes: a high-risk state for diabetes development. Lancet 2012;379:2279–2290. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Teede HJ, Misso ML, Costello MF, Dokras A, Laven J, Moran L, Piltonen T, Norman RJ; International PCOS Network. Recommendations from the international evidence-based guideline for the assessment and management of polycystic ovary syndrome. Fertil Steril 2018;110:364–379. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Templeman NM, Flibotte S, Chik JHL, Sinha S, Lim GE, Foster LJ, Nislow C, Johnson JD.. Reduced circulating insulin enhances insulin sensitivity in old mice and extends lifespan. Cell Rep 2017;20:451–463. [DOI] [PubMed] [Google Scholar]
- Thong EP, Codner E, Laven JSE, Teede H.. Diabetes: a metabolic and reproductive disorder in women. Lancet Diabetes Endocrinol 2020;8:134–149. [DOI] [PubMed] [Google Scholar]
- Treloar AE, Boynton RE, Behn BG, Brown BW.. Variation of the human menstrual cycle through reproductive life. Int J Fertil 1967;12:77–126. [PubMed] [Google Scholar]
- Wang ET, Calderon-Margalit R, Cedars MI, Daviglus ML, Merkin SS, Schreiner PJ, Sternfeld B, Wellons M, Schwartz SM, Lewis CE. et al. Polycystic ovary syndrome and risk for long-term diabetes and dyslipidemia. Obstet Gynecol 2011;117:6–13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang YX, Arvizu M, Rich-Edwards JW, Stuart JJ, Manson JE, Missmer SA, Pan A, Chavarro JE.. Menstrual cycle regularity and length across the reproductive lifespan and risk of premature mortality: prospective cohort study. BMJ 2020a;371:m3464. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang YX, Shan Z, Arvizu M, Pan A, Manson JE, Missmer SA, Sun Q, Chavarro JE.. Associations of menstrual cycle characteristics across the reproductive life span and lifestyle factors with risk of type 2 diabetes. JAMA Netw Open 2020b;3:e2027928. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wathen NC, Perry L, Lilford RJ, Chard T.. Interpretation of single progesterone measurement in diagnosis of anovulation and defective luteal phase: observations on analysis of the normal range. Br Med J (Clin Res Ed) 1984;288:7–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Woods KS, Reyna R, Azziz R.. Effect of oral micronized progesterone on androgen levels in women with polycystic ovary syndrome. Fertil Steril 2002;77:1125–1127. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
The data underlying this article will be shared upon reasonable request to the corresponding author.