Abstract
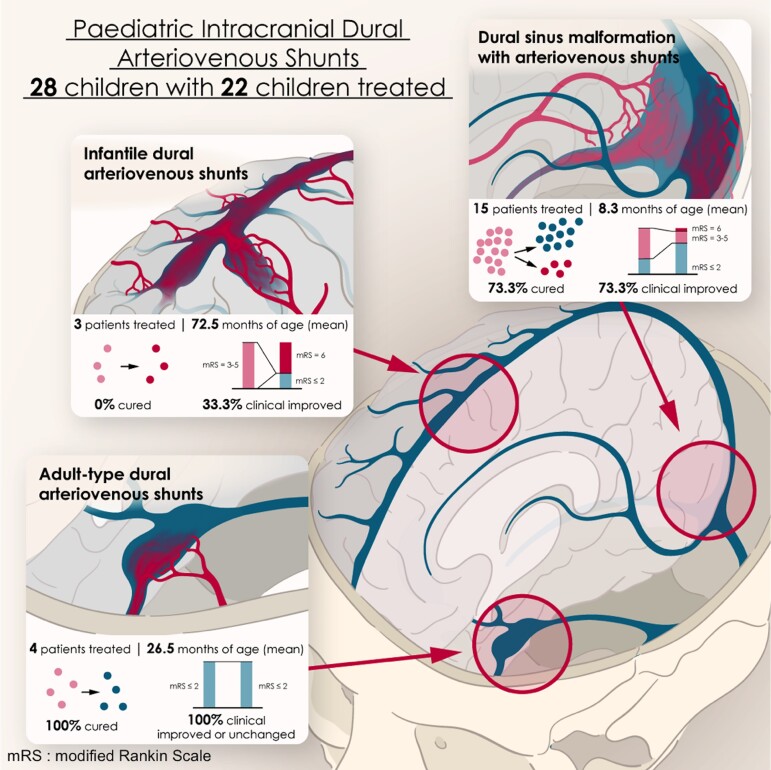
Paediatric intracranial dural arteriovenous shunts have clinical presentations and evolutions, with angiographic characteristics that differ from those described in adults. We report our experience concerning their therapeutic management, emphasizing the relevance of early diagnosis and appropriate treatment for satisfactory neurocognitive development. Using a prospective database, we reviewed the clinical and radiological data of all children with dural arteriovenous shunts managed between 2002 and 2020. Dural shunts were categorized into three types: dural sinus malformations with arteriovenous shunts; infantile dural arteriovenous shunts; and adult-type dural arteriovenous shunts. Therapeutic strategies and outcomes were analysed depending on lesional subtypes. Modified Rankin Scale for the paediatric population was assessed pre-treatment and at last follow-up. Twenty-eight patients [16 girls (57.1%); 12 boys (42.9%)] were included: 17 dural sinus malformation [10 boys (58.8%); seven girls (41.2%)], three infantile shunts [three girls (100%)], eight adult-type shunts [four girls (50%)]; four boys (50%)], with a mean age of 19.2 ± 36.6 months at presentation. Twelve (42.9%) had a modified Rankin Scale score of 0–2, four (14.3%) had a score of 3, three (10.7%) had a score of 4 and eight (28.6%) had a score of 5. Embolization was performed in 22 children [78.6%; 12 girls (54.5%); 10 boys (45.5%)]. Fifteen patients could be cured (68.2%): 11 dural sinus malformations (73.3%), four adult-type lesions (100%) but no infantile shunt. Mean post-treatment follow-up was 39.5 months (max. 139 months): 14 patients (63.6%) presented a modified Rankin Scale score of 0–2 and eight (36.4%) had a score ≥3. In the dural sinus malformation group, the modified Rankin Scale score was improved in 11 patients (73.3%) and unchanged in three (20%). Only one patient with infantile subtype (33.3%) improved clinically. In the adult-subtype group, all children (100%) improved. Of six untreated patients [four girls (66.7%); two boys (33.3%)], four with adult-subtype shunts showed uneventful evolutions, one with dural sinus malformation died, and therapeutic abortion was conducted in an antenatally diagnosed dural sinus malformation. Paediatric dural fistulas comprise different subtypes with variable clinical courses. Proper diagnosis is mandatory for optimal therapeutic strategies within appropriate therapeutic windows.
Keywords: vascular malformation, paediatrics, fistula, congenital, dural shunts
Smajda et al. report their experience in paediatric intracranial dural arteriovenous shunts, emphasizing the relevance of early diagnosis and appropriate treatment for satisfactory neurocognitive development. Three different subtypes have to be recognized as they request different therapeutic management.
Graphical Abstract
Graphical Abstract.
Introduction
Dural arteriovenous shunts (DAVSs) account for approximately 10% of intracranial arteriovenous shunts (AVSs) in the paediatric population.1,2 They present specific clinical courses and angiographic characteristics which differ from those seen in adults. Lasjaunias first described the various types of paediatric DAVSs and their complex natural history:3
dural sinus malformations (DSMs) are congenital diseases found either antenatally, in neonates or infants and characterized by a bloated aspect of the affected sinus with or without parietal shunts draining into it;
infantile/juvenile-type dural shunts (IDAVSs) are acquired high-flow low-pressure lesions with multiple AVSs draining into enlarged sinuses mostly found in older infants and young children;
adult-type dural shunts (ADAVSs) are acquired and architecturally similar to adult dural fistulas; they mostly affect the cavernous sinus area in older children.
Each of these lesions shows differences in their morphology, symptomatology and natural history; these features vary indeed according to the child’s age, the aspect and location of the lesion, the cerebral venous status and the type of shunt. Neonates may present with haemo- or hydrodynamic disorders with an audible intracranial bruit. Infants display similar symptoms but are also prone to present macrocephaly, dilated facial veins, seizures and developmental delay. Older children may suffer from neurocognitive delay, focal neurological deficits or haemorrhages. Each group, therefore, requires specific therapeutic considerations.
We will here present our experience in the diagnosis and management of paediatric DAVS and investigate potential determinants of patient outcomes. We will also emphasize the importance of adequate treatments in both timing and targets to allow satisfactory neurocognitive evolution.
Materials and methods
Patient population
The institutional ethics committee approved the study. In line with French regulations, the institutional review board waived the need for signed consents. Only parents’ approval was requested before any procedure.
A review was conducted on a prospectively collected database comprising clinical and radiological data of all DAVS children (<16 years) managed between 2002 and 2020 by the senior member of our group (GR). DSM without AVS was not included in this study. The clinical data collected were clinical signs, age at presentation, age at diagnosis, the time between first symptoms and treatment and post-therapeutic clinical evolution. Age at presentation was divided into five groups: prenatal, neonatal (0–30 days), infancy (30 days–2 years), early childhood (2–12 years) and teenage (13–16 years).
All baseline and follow-up MRI, computed tomography, magnetic resonance angiography (MRA) and digital subtraction angiography (DSA) imaging studies were assessed by two investigators. Each patient underwent a brain MRI before and after treatment.
Classification and description of arteriovenous shunts
DAVSs were categorized into the three usual groups: DSM with AVS, IDAVS and ADAVS.3 The lesions were further analysed according to their arterial supply and their venous drainage.
Particular attention was paid to the superficial and deep venous drainage of the brain, respectively, toward the cavernous plexus and the straight sinus (SS). The brain parenchyma was evaluated by MRI before and after treatment.
Treatment
Therapeutic managements were decided collectively with paediatric neurosurgeons, paediatric neurologists and interventional neuroradiologists and included medical and invasive treatments (embolization, ventricular shunts or third ventricle ventriculostomy) or a combination of modalities.
All patients had diagnostic angiographies followed during the same session by endovascular therapy (EVT) under general anaesthesia. The arterial and venous phases of both internal carotid and vertebral arteries were carefully studied. Treatment options included trans-arterial or transvenous DAVS embolization using cyanoacrylate synthetic glue (Glubran, GEM, Viareggio, Italy; Histoacryl, Braun, Melsungen, Germany) or detachable coils. Interventional procedures were usually staged with an interval between procedures of 1–12 months.
Adjuvant therapies by steroids were given after each embolization with glue, and anticoagulation was delivered when the venous drainages were markedly modified compared with pre-EVT.
We use the term ‘complete obliteration’ when no residual AVS was demonstrated on immediate post-treatment angiographies. We considered a child ‘cured’ when treatment was completed with obliteration on follow-up imaging.
Outcome evaluation and follow-up
All children were followed clinically and radiologically. An adapted modified Rankin Scale (mRS) score of neurological disability for paediatric patients with brain arteriovenous malformations (AVMs) was used for clinical evaluation.4 A good clinical outcome was defined as paediatric mRS ≤ 2 and a poor outcome as mRS ≥ 3.
Diagnostic control angiographies by either DSA and/or MRA were systematically planned between 6 months and 1 year after the last EVT. A patient was considered cured if no residual AVS could be assessed on these controls with, in the case of DSM, remodelling of the abnormal venous lake. Further follow-ups were planned by MRA every 3 years until the patient reached the age of 18. DSA was performed if there was any doubt about AVS recurrence.
Statistical analysis
Continuous data were presented as means ± SDs or median (interquartile range), categorized data as counts and percentages. χ2 and Fisher’s exact testing was applied for statistical comparisons of categorized data. To assess the risk factors of poor prognosis (mRS > 2), a univariate analysis was performed. A P-value < 0.05 was considered statistically significant. All analyses were performed using SAS software, version 9.3 (SAS Institute, Cary, NC, USA)
Data availability
Anonymized data will be shared on request.
Results
Demographic information
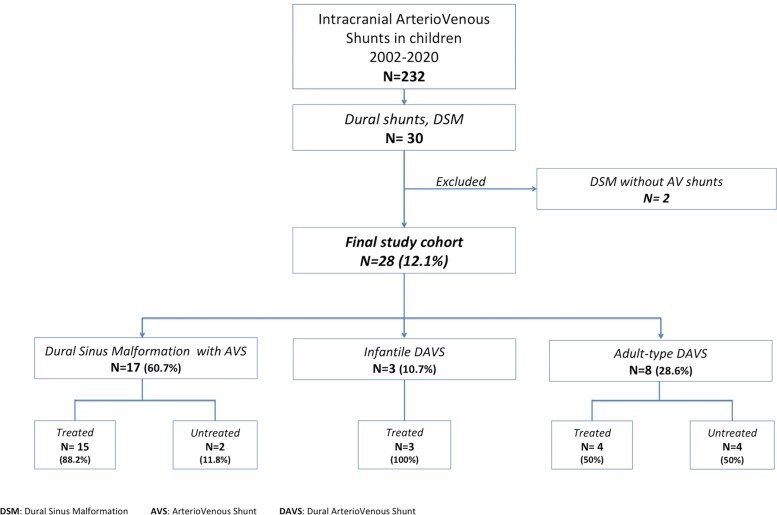
Thirty children with intracranial dural vascular malformations were referred between 2002 and 2020. Two patients with DSM without AVS were excluded from this study leaving a total of 28 DAVS [16 girls (57.1%) and 12 boys (42.9%)]: 17 DSMs with AVS [60.7%—10 boys (58.8%) and seven girls (41.2%)], three IDAVSs [10.7%—three girls (100%)] and eight ADAVSs [28.6%—six girls (75%) and two boys (25%)]. A flow chart is displayed in Fig. 1. The mean age at presentation (first symptoms) was 19.2 ± 36.6 months (range: 0–167) (Table 1 and Supplementary Fig. 1).
Figure 1.
Flowchart for study cohort selection. DSM, dural sinus malformation; AVS, arteriovenous shunt; DAVS, dural arteriovenous shunt.
Table 1.
Baseline data of the patients
| Baseline data | All patients (n = 28) | DSM (n = 17) | IDAVS (n = 3) | ADAVS (n = 8) |
|---|---|---|---|---|
| Males | 12 (42.9) | 10 (58.8) | 0 | 2 (25) |
| Age first symptoms (months) | 5 (0–167) | 4.5 (0–31) | 48 (2.5–167) | 26,5 (9–44) |
| Age diagnosis (months) | 14.5 (0–167) | 5 (0–31) | 48 (2.5–167) | 82 (12–127) |
| Age first consultation (months) | 19.5 (0–170) | 11 (0–72) | 54 (15–170) | 56.5 (13–114) |
| Presentation | ||||
| Antenatal | 2 (7.1) | 2 (11.8) | 0 | 0 |
| Seizure | 6 (21.4) | 4 (23.5) | 2 (66.7) | 0 |
| Hydrocephalus | 3 (10.7) | 3 (17.6) | 0 | 0 |
| Macrocrany | 11 (39.3) | 10 (58.8) | 1 (33.3) | 0 |
| Dilated facial veins | 9 (32.1) | 8 (47.1) | 0 | 1 (12.5) |
| Systemic repercussions (CHF, respiratory distress) | 4 (14.3) | 4 (23.5) | 0 | 0 |
| Developmental delay | 10 (35.7) | 9 (52.9) | 1 (33.3) | 0 |
| Intracranial hypertension | 2 (7.1) | 2 (11.8) | 0 | 0 |
| Focal neurologic deficit | 8 (28.6) | 6 (35.3) | 2 (66.7) | 0 |
| Exophtalmia | 3 (10.7) | 0 | 1 (33.3) | 2 (25) |
| Oculomotor palsy | 1 (3.6) | 1 (5.9) | 0 | 0 |
| Chemosis | 3 (10.7) | 1 (5.9) | 0 | 2 (25) |
| Bleeding | 2 (7.1) | 1 (5.9) | 1 (33.3) | 0 |
| Secondary bleeding | 2 (7.1) | 2 (11.8) | 0 | 0 |
| Incidental | 6 (21.4) | 0 | 0 | 6 (75) |
| Associated | ||||
| Facial lymphangioma | 3 (10.7) | 3 (17.6) | 0 | 0 |
| Facial AVM | 1 (3.6) | 0 | 1 (33.3) | 0 |
| Cardiac anomaly | 1 (3.6) | 1 (5.9) | 0 | 0 |
| Cavernoma | 2 (7.1) | 1 (5.9) | 1 (33.3) | 0 |
| DVA | 4 (14.3) | 3 (17.6) | 1 (33.3) | 0 |
| VGAM | 4 (14.3) | 0 | 0 | 4 (50) |
| Capillary malformations | 1 (3.6) | 1 (5.9) | 0 | 0 |
Data are presented as n(%). Mean ± SD or median (Q1–Q3).
DSM, dural sinus malformation; IDAVS, infantile dural arteriovenous shunt; ADAVS, adult-type dural arteriovenous shunt; CHF, congestive heart failure; AVM, arteriovenous malformation; DVA, developmental venous anomaly; VGAM, Vein of Galen aneurysmal malformation.
Clinical presentation
In the DSM group, the patients’ mean age at presentation was 8.3 ± 9.1 months (range: 0–31) but was at first consultation 16.6 ± 18.9 months (range: 0–72). The antenatal diagnosis was made in two patients (11.8%). Therapeutic abortion was decided by the parents in one antenatally diagnosed DSM. Macrocrania was seen at admission in 11 patients (64.7%), eight patients had dilated facial veins (47.1%). Ten children suffered from neurocognitive delay or behavioural disorders (58.8%), four from cardiac failure or overload (23.5%), four had seizures (23.5%) and two presented intracranial hypertension (11.8%). Intraventricular haemorrhage occurred in one patient (3.6%). At admission, five patients exhibited an mRS of 0–2 (29.4%), two mRS of 3 (11.8%), two mRS of 4 (11.8%) and seven mRS of 5 (41.2%). The median mRS was 4.
Ipsilateral forehead lymphatic malformations were associated with the DSM in three patients, a developmental venous anomaly (DVA) in three children, a cavernoma in one boy, moderate pulmonary valve stenosis in one patient and cutaneous capillary malformations in one child. Genetic testing was performed on that patient: a capillary malformation-arteriovenous malformation (CM-AVM) Type 1 related to a mutation of RASA1 gene (c.2707C > T) was diagnosed.
In the IDAVS group, the mean age at presentation was 72.5 ± 84.9 months (range: 2.5–167), and at first consultation, it was 79.7 ± 80.6 months (range: 15–170). One patient (33%) bled in the diencephalon and suffered from status epilepticus. Another patient presented with seizures and neurocognitive delay, in addition to atypical skin lesions, multiple intracranial cavernomas and DVA. Genetic testing for cerebral familial cavernous malformations and CM-AVM (Type 1 and 2) syndrome was negative. Macrocrania and vision loss due to chronic intracranial hypertension were seen in the third patient, along with an associated facial AVM on the ipsilateral side. At admission, one patient had an mRS of 3 (33.3%), one mRS of 4 (33.3%) and the remaining mRS of 5 (33.3%). The median mRS was 4.
In the ADAVS group, the mean age at presentation was 26.5 ± 24.7 months (range: 9–44), and at first consultation, it was 53.1 ± 34.7 months (range: 13–114). Two patients had (para)cavernous DAVS with unilateral homolateral chemosis and exophthalmia without oculomotor palsy (mRS of 1). Six patients with previously embolized Vein of Galen aneurysmal malformations (VGAMs) showed on control angiographies incidental new dural shunts located separately from the VGAMs. The mean delay to the last DSA was 20 months (range: 4–29). For all but one, dural shunts developed on sinuses that had thrombosed at one moment during management. At admission, five patients had an mRS of 0–2, and the remaining one mRS of 4. The median mRS was 1.
Baseline patients’ data are displayed in Table 1.
Angiographic characteristics
Selective angiography properly defined the vascular anatomy of each DAVS subtype (Table 2).
Table 2.
Anatomic and angiographic characteristics
| Total (n = 28) | DSM (n = 17) | IDAVS (n = 3) | ADAVS (n = 8) | |
|---|---|---|---|---|
| Locationa | ||||
| Torcular (+− SSS or transverse sinuses) | 11 (39.3) | 10 (58.8) | 0 | 1 (12.5) |
| SSS | 6 (21.4) | 2 (11.8) | 1 (33.3) | 3 (37.5) |
| Lateral (transverse/sigmoïd) | 7 (25) | 3b (17.6) | 0 | 4 (50) |
| Tentorial | 1 (3.6) | 1 (5.9) | 0 | 0 |
| Cavernous/paracavernous | 2 (7.1) | 0 | 0 | 2 (25) |
| Sphenoparietal | 3 (10.7) | 1 (5.9) | 2 (66.7) | 0 |
| Angiographic features | ||||
| Mean arterial feeders (min–max) | 4,5 (1–8) | 5,5 (1–8) | 5,3 (4–7) | 2,4 (1–5) |
| >1 DAVS | 5 (17.9) | 0 | 3 (100) | 2 (25) |
| Venous sinus dilatation | 17 (60.7) | 17 (100) | 0 | 0 |
| Jugular bulbs steno-occlusive disease | 18 (64.3) | 11 (64.7) | 2 (66.7) | 5 (62.5) |
| Unilateral | 5 (17.9) | 3 (17.6) | 1 (33.3) | 1 (12.5) |
| Bilateral | 13 (46.4) | 8 (47.1) | 1 (33.3) | 4 (50) |
| Thrombosed sinus | 12 (42.9) | 5 (29.4) | 1 (33.3) | 6 (75) |
| Cavernous capture | 24 (85.7) | 13 (76.5) | 3 (100) | 8 (100) |
| SS unseen on the venous phase | 11 (39.3) | 10 (58.8) | 1 (33.3) | 0 |
| Cortical venous reflux | 16 (57.1) | 11 (64.7) | 3 (100) | 2 (25) |
| Deep venous reflux | 12 (42.9) | 9 (52.9) | 2 (66.7) | 1 (12.5) |
Data are presented as n(%).
DSM, dural sinus malformation; IDAVS, infantile dural arteriovenous shunt; ADAVS, adult-type dural arteriovenous shunt; SS, straight sinus; SSS, superior sagittal sinus; DAVS, dural arteriovenous shunt.
Two patients presenting 2 DAVSs on different sinuses.
DSM with AVS of single-hole subtype.
DSM (17 patients) were separated into two entities in agreement with Lasjaunias:3,5
DSM with giant sinus lakes and multiple AVS were found in 13 patients [76.5%—eight boys (61.5%) and five girls (38.5%)]. Ten lesions affected the torcular (58.8%), two the posterior SSS segment (11.8%) and one a medial tentorial sinus (5.9%). The shunts were vascularized by five to eight dural arteries; pial arteries participated in the vascularization in four cases (23.5%). Sigmoid-jugular stenoses/occlusions were observed bilaterally in seven patients (41.2%) and unilaterally in three (17.6%). Two DSMs were partially thrombosed (11.8%), and three transverse sinuses were occluded (17.6%). Cortical venous reflux was visible in 11 children (64.7%), with a deep venous system involvement in eight of them (47%) (Fig. 2).
Single-hole fistulas were found in four patients [23.5%—two boys (50%) and two girls (50%)]: three on the sigmoid sinus (17.7%) and one on the SSS (5.9%). One or two dural arteries vascularized the shunt. Ipsilateral occlusion of the sigmoid-jugular junction was found in all sigmoid DAVSs (17.6%). The contralateral sigmoid sinus was stenosed in two patients (11.8%) and occluded in one child (5.9%). No sinus stenosis was observed in the SSS shunt. Deep venous reflux was noted in all three sigmoid sinus DAVSs (17.6%) (Fig. 3).
Figure 2.
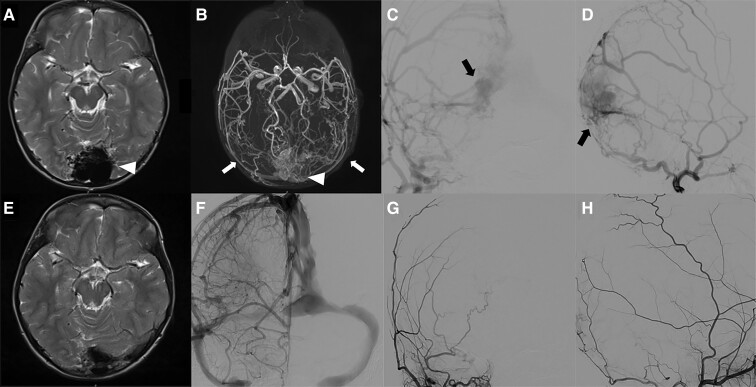
Illustrative case. Midline DSM involving the torcular in a 3-year-old child presenting chronic headaches and severe behavioural disorders with anorexia requiring gastrostomy. MRI showed (A, axial T2) a bloated torcular (white arrowhead). MRA (B, TOF sequence) diagnosed an arterialization of the torcular (white arrowhead) vascularized by both middle meningeal arteries (white arrows). Multiple shunts (black arrows) in the malformed torcular were confirmed by a six-vessel angiography with super-selective injections of both external carotid artery (ECA) branches (AP and lateral views of the right ECA (C and D); left ECA not shown). Staged trans-arterial EVT obtained complete obliteration of the AVS. Control MRI (E, axial T2) and MRA (not shown) showed remodelling of the torcular without any parenchymal damage. Control angiography of both internal and ECA branches confirmed the obliteration of the DSM and the remodelling of the torcular (F, right internal carotid injection AP view; right maxillary artery in AP (G), and lateral (H) views). The patient improved clinically with complete resolution of the headaches and behavioural disorders.
Figure 3.
Illustrative case. Single-hole fistula subtype DSM involving the left sigmoid sinus in a 21-month-old infant with seizures, right hemiparesis and neurocognitive delay. Since the infant was referred from a foreign country, there was a time interval of 4 months between the diagnosis in the home country and the treatment in our institution. MRI (A, axial view, FLAIR sequences), showed a bloated aspect of the left sigmoid sinus (white arrowhead) with thrombosis of the right transverse sinus (white arrow) confirmed on T1 post-gadolinium MRI (B, axial view) white arrow. Congestion of infratentorial veins was diagnosed, associated with pathological enhancement of the pons (asterisk). Angiography diagnosed a single-hole fistula (double white arrows) vascularized mainly by the mastoid branch of the left occipital artery (C, lateral view). Because of proximal and distal occlusion of the sinus, the high-flow shunt was responsible for reflux in the Labbe vein (black arrow) and thus poor ipsilateral hemispheric venous drainage (D, venous phase of the left ICA, long white arrow). The patient was cured after trans-arterial EVT with glue. MRI realized a few months after treatment (E, axial T2) demonstrated, however, global brain atrophy related to the long-lasting consequences of the venous congestion. Axial T1 post-gadolinium (F) showed regression of the pathological enhancement of the pons and disappearance of the posterior fossa congested veins. A selective occipital artery control angiography failed to show any residual shunts (G). The venous phase of the left ICA (H) normalized.
IDAVSs (all three girls) were angiographically characterized by multifocal high-flow shunts vascularized by four to seven arteries (including pial feeders in one patient) and draining into enlarged sinuses (SSS: one patient; sphenoparietal sinus and SSS: one patient; bilateral sphenoparietal sinuses: one patient). Two children had ipsilateral sigmoid stenosis (66.7%), one of them also with a contralateral sigmoid sinus occlusion. Cortical venous reflux was seen in all cases, among which two refluxed in the deep venous system (66.7%) (Fig. 4).
Figure 4.
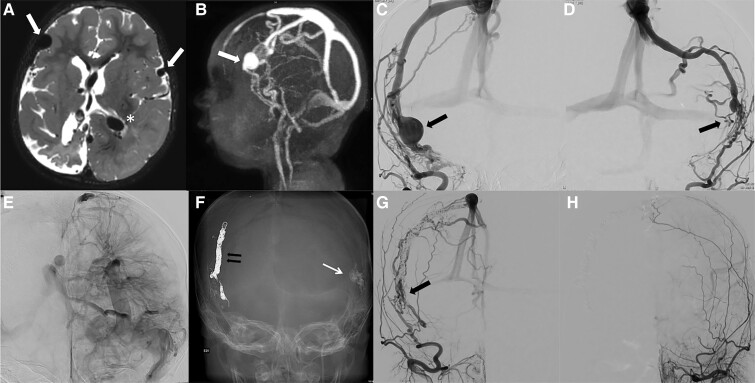
Illustrative case. Infantile DVASs in a 1-year-old child with seizures and neurocognitive delay. MRI (T2 axial view, A) and MRA (B, lateral view) showed atrophic changes in the right cerebral hemisphere and dilated vascular structures suspected of correspondence to arterialized veins (white arrow) bilaterally in the fronto-insular area. A DVA was suspected in the left hemisphere (asterisk). Angiography of the right (C, AP view) and left (D, AP view) external carotid arteries diagnosed bilateral dural arteriovenous shunts (black arrows) located at the level of the convexity accessory sinuses. A deep complex DVA of the left cerebral hemisphere (E, left internal carotid artery, AP view) was confirmed by left ICA angiography. Both dural shunts were embolized: trans-arterially with glue on the left side (long arrow) and combining trans-arterial and transvenous approaches with glue and coils for the right side (F, skull X-ray, AP view), which allowed complete obliteration of the shunts. A 6-month control angiography of the right (G, AP view) and left (H, AP view) external carotid arteries demonstrated, however, recurrence of the right shunt (black arrow) but stable complete obliteration of the left shunt. The patient was planned for a complementary embolization that occluded the right shunt; angiography confirmed, however, the occurrence of other dural shunts, whilst MRI showed development of intracerebral cavernomas (not shown).
Ten ADAVSs were found in eight patients [six girls (75%) and two boys (25%)]. The architecture of ADAVS was simpler. The two (para-)cavernous lesions were vascularized by a single cavernous MMA branch, drained into the cavernous sinus and in the superior ophthalmic vein (Fig. 5). The six other shunts occurred after thrombosis of the involved sinus in the aftermath of VGAM treatment: three affected the SSS, four affected the transverse sinuses and one affected the torcular. They were vascularized by one to five dural arteries. Cortical venous reflux was identified in two cases (20%), with a deep venous system involvement in one (10%).
Figure 5.
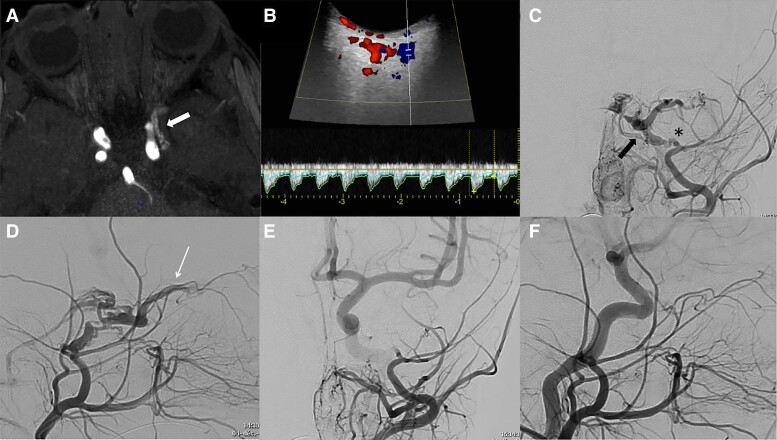
Illustrative case. Adult-type DAVS in a 1-year-old child presenting a left chemosis with exophtalmia (without oculomotor palsy) for 3 months. Arterialization of the left cavernous sinus (white arrow) was suspected on MRA (A, axial TOF); Doppler ultrasonography (B) showed an arterialization of the superior ophthalmic vein. The diagnosis of dural cavernous arteriovenous fistula (black arrow) was confirmed by ECA angiography (C and D, AP and lateral views), the shunt being vascularized by the cavernous branch of the middle meningeal artery (asterisk) and responsible for reflux in the superior ophthalmic vein (long arrow) and the inferior petrosal sinus. The patient was cured after trans-arterial EVT with glue and improved clinically with complete resolution of the symptoms. Control angiography failed to show any residual shunt (Left maxillary angiography in AP (E) and lateral (F) views).
Treatment
Six patients were not treated (four girls and two boys): one antenatal DSM because of therapeutic abortion, one sigmoid sinus single-hole DSM in an infant with initial severe neurological status (mRS of 5) and four post-VGAM treatment ADAVS that were not considered at risk because of slow flow drainage into a patent sinus with no compromised brain drainage and no pial reflux. The latter patients remain under MRI/MRA controls.
Twenty-two patients [12 girls (54.5%) and 10 boys (45.5%)] were treated by staged EVT [68 procedures (range: 1–7 per patient)]. Mean age at first EVT was 28.0 ± 36.7 months (range: 0.5–167).
Endoscopic third ventricle ventriculostomy or ventricular shunting was performed in three (13.6%) and nine (40.9%) patients, respectively, because of hydrocephalus.
Treatment modalities and results are displayed in Supplementary Table 1.
EVT angiographic results
Complete angiographic DAVS obliteration was obtained in 19 of 22 patients (86.4%): 13 DSMs, two IDAVSs and four ADAVSs. Three patients are at present partially treated, one of whom (IDAVS) is scheduled for further treatment while two DSMs were lost to follow-up.
In the DSM group, 15 patients [88%—nine boys (60%) and six girls (40%)] were treated. The mean age at first EVT was 14.5 ± 10.3 months (range: 0.5–51). The mean time between first symptoms and treatment was 5.1 months (range: 0–20). Overall, 52 endovascular procedures were performed including four transvenous (7.7%) and three combined trans-arterial/transvenous approaches (5.7%). Four transvenous occlusions of SS were performed to suppress significant deep venous reflux as previously published.6 Complete obliteration was achieved in 13 cases (86.7%). Three complications occurred: one asymptomatic gluing of a micro-catheter due to a misinterpretation of the flow velocity giving rise to this technical mistake without any clinical consequence, one posterior fossa haemorrhagic venous infarct occurring 72 h post-EVT for a complex tentorial sinus DSM, which was managed by posterior fossa craniotomy and anticoagulation with final good recovery (mRS of 1) and one death a few hours after the EVT.
In the IDAVS group, all three patients were treated (a total of 10 EVT procedures, range: 1–5 per patient). The mean age at first EVT was 76 ± 80.3 months (range: 15–167), and the mean time between first symptom and treatment was 7.2 months (range: 3–12.5). Complete angiographic obliteration was obtained in two patients (66.6%), although fatal outcomes occurred in both.
In the ADAVS group, four patients [three girls (75%) and one boy (25%)] were treated (the two with cavernous shunts and two with post-VGAM DAVSs with retrograde pial reflux); overall five EVT (range: 1–2) were performed. The mean age at first EVT was 39.2 ± 23.3 months (range: 15–68), and the mean time between first symptom and treatment was 1.5 months (range: 0–4). Complete obliteration was achieved in all patients (100%) without complications.
The mean angiographic follow-up post-EVT was 22.6 months. MRA was obtained in 16 of 19 patients (84.2%) and DSA in 11 of 19 patients (57.9%). In one patient with initially complete DSM obliteration, MRA at 18 months after last DSA showed doubt for a small residual shunt; DSA is further scheduled. For all other patients, no residual shunt on angiographic follow-up was noted (clinical cure rate of 68.2%—15 of 22).
In the ADAVS as in the DSM subgroups, apart from the dubious patient mentioned previously, we did not observe (as of the writing of this article) any recurrence of AVS in patients considered cured. MRA follow-ups remain nevertheless planned till children reach the age of 18.
In the IDAVS subgroup, no complete cure could be achieved as recurrences were observed as early as on the first angiographic control at 4–6 months of the last EVT.
Clinical outcomes
The mean clinical follow-up was 50.4 months. All four untreated ADAVS patients remained stable (unchanged mRS of 0–2); the untreated DSM patient with melting brain syndrome died.
At last follow-up, 14 among the 22 treated patients (63.6%) had an mRS ≤ 2 and eight (36.4%) had an mRS score ≥ 3 (Supplementary Fig. 2). Neurological improvement was obtained in 18 patients (81.8%). Complete clinical cure was obtained in 15 of 22 patients (68.2%): 11 DSMs (73.3%), four ADAVSs (100%) but no IDAVS.
In the DSM group, among the 15 treated patients, the mRS improved in 11 patients (73.3%) and remained unchanged in three (20%), with a slight clinical deterioration (mRS of 1, unchanged) observed in one due to peri-procedural thrombotic complication (described previously). Nine (60%) patients had a good outcome (mRS ≤ 2), five (33.3%) a poor outcome (mRS of 3–5) and one patient (6.7%) died.
In the IDAVS group, one patient (33.3%) had a good outcome (mRS ≤ 2) with improved mRS, whereas two died.
In the ADAVS group, all four patients (100%) had a good outcome (mRS ≤ 2). The mRS was improved for two symptomatic ones (50%) and unchanged for the others (50%).
Death occurred in 3 of 22 patients after EVT (13.6%). One neonate with a single-hole subtype DSM located on the SSS was embolized with coils and glue. Despite the angiographic cure of the lesion, the baby died of rapidly deteriorating cardiac failure. One 6-year-old IDAVS girl with an associated large facial AVM made a peri-procedural cardiac arrest due to massive pulmonary embolism of glue that occurred during the management of the facial AVM at the end of the session that had cured the IDAVS.7 One 14-year-old girl with complex IDAVS of the SSS and severe bilateral venous reflux revealed by intracranial haemorrhage, and secondary severe status epilepticus was sent to us too late after failed treatment of the IDAVS by her referring team. She could be anatomically cured by coils and glue during one session but angiographic controls showed the absence of intracranial perfusion on both carotid and vertebral arteries related to acute increase of intracranial pressure by venous thrombosis. Despite anticoagulation, the patient developed intractable elevated intracranial pressures and died.
Predictors of poor neurological outcome
Six factors were found significantly associated with poor clinical outcome (mRS ≥ 3) in treated patients (Supplementary Table 2): initial poor clinical condition (mRS ≥ 3; P = 0.0055); spontaneous haemorrhage (P = 0.01); seizures (P = 0.04); hydrovenous disorders (P = 0.04); white matter calcifications (P = 0.01); brain atrophy (P = 0.04).
Midline lesion location (SSS and torcular) and venous reflux were not statistically associated with poor outcomes (P = 1 and P = 0.19).
IDAVS had poorer outcomes than ADAVS or DSM. Furthermore, longer delays between the first symptoms and the moment of treatment also induced poor neurological evolution. Regardless of the subtype, better outcomes were obtained if treatment was more rapidly performed after diagnosis (7.1 versus 4.5 months, respectively).
Discussion
Cerebral DAVSs observed in children have distinct clinical and pathological patterns from those observed in adults. Therefore, the usual descriptions and adult classifications of DAVS have no value in children as they do not reflect their particular clinical course.3,8,9 The results reported in the literature attest to the complexity of the pathology, the often unfavourable spontaneous evolution and difficult management.3,5 Barbosa et al.5 reported 14 untreated DSMs: only five had a good outcome (35.7%). In a seven-patient cohort including two DSMs and five ADAVSs, Walcott et al.10 reported an 85.7% complete obliteration rate, with good outcomes in all. Hetts1 described 21 children treated with 38% complete obliteration, 70% good outcomes, 19% complications and 27% mortality rates. Unfavourable evolution occurred in 50% of DSM, in 83.3% of IDAVS but favourable outcomes in all but one ADAVS who died from unrelated leukaemia.
In our series, EVTs cured 68.2% of all DAVS patients with a clinical complication rate of 18.2% including mortality of 13.6%. Eighteen of our treated patients (81.8%) improved clinically, with good outcomes (mRS ≤ 2) obtained in 14 of them (63.6%). These global data have to be nuanced depending on the clinical and radiological characteristics of each lesion type for a better understanding of the challenge they represent.
Dural sinus malformations
DSM are congenital lesions that may be discovered antenatally by ultrasound or MRI in view of a bloated aspect of the affected sinus.5,11,12 As described by Okudera, major morphological modifications of the dural sinuses start around 4 months of gestation: the transverse sinuses enlarge from their lateral borders on each side, and this ballooning progresses medially to reach the primitive torcular around 5–6 months of gestation, extending also into the posterior portion of the superior petrosal sinuses and the superior sagittal sinus which is initially not a single channel but a network of plexuses. At the age of 7 foetal months, enlargement of the sinuses stops, and they regain a relatively even aspect.13 A DSM is thus considered to be a real congenital malformation formed between the fourth and fifth months of intrauterine life: the remodelling seems not to occur properly, and the sinus remains expanded for reasons which are currently unclear.13 It could be hypothesized that a part of the initial plexuses or one of the intra-sinusal venous pouches described by Okudera do not remodel or regress normally but continue to grow independently. The biological mechanisms regulating the growth and development of dural sinuses are mainly related to those of the dura mater.14 Cerebral venogenesis requires paracrine bone morphogenetic protein signalling from skull pre-osteoblasts and dura. Loss of this signalling induces cerebral vein malformations highlighting the role of cellular interactions in the venous development independently of arterial influences.14 Because of the initial absence of the cavernous plexus, the venous circulation related to the marked flow from the rapidly growing hemispheres is mainly directed towards the posterior sinuses. Thus, they play the role of a blood collector as they also drain the cerebellum and the structures of the vault. The venous turbulences and the reduction of flow in that structure after 7 months of gestation induce its spontaneous thrombosis and regression.15
AVSs are not systematically associated to DSMs, but they influence the natural history of the disease as DSMs without shunts evolve more favourably than those with AVSs.2,16 The factors underlying AVS formation are unknown. Two hypotheses are evoked in the literature. Yang et al.17,18 postulated that every DSM is at some point associated with arterialization; owing to the low level of flow, intrauterine spontaneous thrombosis of the shunts occurs with the persistence of a certain degree of ballooning of the sinus. It has also been emphasized that dural sinus thrombosis plays a role in AVS formation:19 because spontaneous thrombosis belongs to the potential evolution of the disease, and this phenomenon occurring in utero could promote AVS formation. Both theories are attractive at first sight but leave unanswered questions. Indeed, there is a discrepancy between the rarity of DSM with AVSs and the potential systematic primary clotting of the DSMs; on the other hand, DSMs with AVSs are not always those preceded by thrombosis. There is thus in our opinion no compelling evidence of clots preceding DAVS formation systematically.17,18
Other mechanisms, either genetic or epigenetic, could, therefore, be involved in the pathogenesis of these lesions. Associations between cervicofacial venous malformations, dural venous sinus abnormalities and cavernomas have been reported and described to correspond to a metameric ‘insult’.5,20–23 A somatic mutation is here considered to induce venous abnormalities before neural crest cell migration. Three torcular DSM of our series were associated with facial ipsilateral lymphatic malformations, two of these also with deep DVAs and one with a posterior fossa DVA and cavernoma, arguing for a common venous metameric origin. Such associations were not seen in DSM sparing the torcular, hypothesizing different underlying biological mechanisms. One patient with a DSM affecting the SSS presented a CM-AVM Type 1 related to RASA 1 mutation.24
In the light of these considerations and of the observations in our series, an identical origin of all DSM with AVS seems debatable: only 11.8% of our cases presented with a partial thrombosis of the malformed sinus, all located at the torcular. The majority of such posterior lesions in our series presented without clots. Sigmoid sinus malformations are also associated with a bloated aspect of the affected sinus: this latter does not present any intra-sinusal clotted portions but usually shows real occlusions of the ipsilateral jugular bulb even frequently associated with contralateral sigmoid sinus stenoses or occlusions that do not present any shunts. The origin of such dispositions could be related to the dysmaturation of the sigmoid-jugular junction or venous high-flow angiopathy, both potentially induced by the shunt. 13 Furthermore, AVSs located in the torcular area were usually of lower flow velocity than those located in other malformative areas (superior sagittal sinus or sigmoid sinus): if the former could be close to those seen in the usual DAVS in adults (considered to be induced by angiogenesis on thrombosis19), the latter were real single-hole fistulas similar to those seen in mural Vein of Galen AVMs. These findings could point to different pathogenetic origins at various stages of development. The development and architecture of the different types of DSMs may potentially also be influenced by the embryological origin (neural crest or mesoderm) of the dura localization.25
The clinical presentation of DSMs depends on their location, the existence of associated AVS, the status of the venous outlets, the drainage of the normal brain and potential venous reflux.5 DSM without AVSs (even diagnosed antenatally) are reported to spontaneously remodel with favourable clinical outcomes.5,17,18,26 DSM with AVS, can be diagnosed antenatally (on foetal Doppler and confirmed on MRA) and have a poorer prognosis. Severe neurological morbidity or death have been reported in 41% of these DSM cases, which is why the distinction between these two entities must be made.1,1,13,17,18,27,28
The combination of AVS, sinus stenosis and venous reflux results in cerebral congestion and hydrovenous disorders responsible for diffuse or focal brain damage, cortical/subependymal atrophy, hydrocephaly and haemorrhages. White matter calcifications are markers of chronic venous ischaemia. Because of their midline location and absence of alternate drainage routes, DSMs involving the torcular are particularly prone to parenchymal injury in the territory of the deep venous system as reflux in the SS is frequently associated. This feature may also be seen in lateralized lesions in cases of associated contralateral sigmoid stenosis or thrombosis, potentially increasing the risk of venous reflux.5,29,30 Such lesions can rapidly induce devastating irreversible lesions as seen also in our series.3,5 In these circumstances, we have recommended transvenous coiling of the SS to suppress the reflux in the deep territory and avoid these damages.6
DSMs with AVS are aggressive lesions requiring prompt treatment.17,18 If the lesion is discovered antenatally by ultrasound, we recommend completing the assessment by MRI and MRA to analyse both lesion and brain. The poor prognostic factors identified in the literature include venous pouch arterialization, ventriculomegaly (considered to be related to hydrovenous disorders) and neuroparenchymal damage.12 A better outcome seems to be correlated to the spontaneous decrease of the size of the venous lake on subsequent controls and intra-saccular thrombi, signs of a favourable regression of the DSM. For severe brain damage or melting brain syndrome, therapeutic abortion can be envisaged.
In our series, severe neonatal heart failure requiring EVT was rare and occurred only twice (11.7%).5,31 Both babies presented a DSM of the SSS with high-flow fistulous shunts. For all other cases, the mean age at which diagnosis of the DSM was made was 8.3 months. Macrocrania was frequently detected (58.8%). Neurological symptoms were variable consisting in seizures, developmental delay or behavioural disorders and more rarely intracranial hypertension. Haemorrhage as a primary event was uncommon in our series (5.9%) in accordance with previously published data.1
We recommend an MRI and MRA as soon as clinical signs suspect intracranial vascular malformations. If a DSM is diagnosed antenatally, these examinations should be performed rapidly after birth to determine the lesion and the aspect of the brain. We took into consideration several factors for our therapeutic strategy: lesion location, torcular involvement, DSM connections with normal veins and (deep) venous reflux. The anatomical cure of the DSM was not the primary goal to reach, at least in the first intention. Treatment was mainly meant to preserve brain maturation and obtain normal neurological development. A stepwise EVT based on angioarchitecture was usually performed, designed to preserve cerebral venous drainage, progressively occlude the various AVS and allow proper sinus remodelling. The choice of the right moment to perform the treatments (optimal therapeutic window) and their sequence over time are crucial to obtain favourable clinical evolutions.
Glue has remained our first-choice embolic material every time the trans-arterial approach was considered: its manipulation is easy and fast; it can penetrate into the shunt if properly used and allows thrombosis and remodelling of the pathological compartment treated with good clinical outcomes. We deliberately chose not to use Onyx to reduce the length of the procedure in these babies.
Treatment is based on an ‘angioclinical semiology’ concept linking the symptoms with the DSM architecture. Reflux into cortical or deep veins has to be suppressed rapidly to avoid dramatic developments. If trans-arterial occlusion of the shunts is not sufficient to reduce the pathological flow and the reflux, targeted transvenous approaches should be considered with coiling of the SS if necessary 6. Furthermore, endoscopic third ventriculostomy should be proposed when embolization has failed to correctly master hydrocephaly.32,33
Early embolization is mandatory if the MRI/MRA findings as described previously are considered at risk for irreversible brain lesions and if the baby presents worrying symptoms.5,34
We believe that asymptomatic neonates with favourable angioarchitecture (slow flow shunts, absence of sinus stenosis or venous reflux) can be treated around 5–6 months of age. They will have to be followed up precisely in the meantime by monthly clinical examination and by MRI/MRA performed every 6–8 weeks. Any new abnormal clinical or radiological finding should lead to treatment. The decisional tree for management of DSM is summarized in Fig. 6. Anticoagulation will be given in cases of spontaneous rapid progression of thrombosis inducing growth of the DSM or after embolization when deleterious changes in the venous haemodynamics occur to avoid venous thrombosis that could have negative consequences.20
Figure 6.
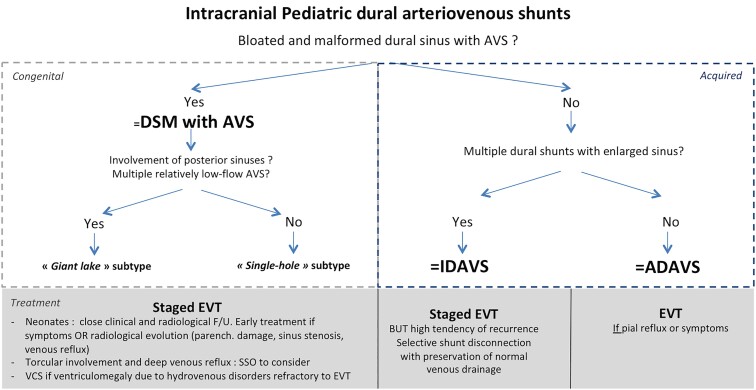
Decisional tree for the management of DAVS. AVS, arteriovenous shunt; DAVS, dural arteriovenous shunt; DSM, dural sinus malformation; IDAVS, infantile dural arteriovenous shunt; ADAVS, adult-type dural arteriovenous shunt.
This therapeutic strategy used in the 15 DSMs that we managed allowed us to cure 73.3% of patients, with a good clinical outcome in 60% of them. The poor outcomes (33%) were seen in children who already had a poor initial mRS before treatment related to installed brain damage. One neonate died after embolization because of refractory cardiac failure. Most patients (73.3%) improved clinically. These results can be advantageously compared with the reported series. Terada described 10 DAVF cases reported as DSM: eight patients underwent EVT, whereas one with spontaneous shunt occlusion was managed conservatively.28 A favourable outcome was obtained in 44% and unfavourable in 55% of patients. In Hetts’ series of 4 DSMs, two died and two attained their developmental milestones.1
Infantile dural arteriovenous shunts
IDAVSs are acquired multifocal, high-flow, low-pressure lesions, complex in their architecture and draining into enlarged sinuses. They are not observed antenatally and are revealed clinically at older infant ages.1,2,35,36 Small cortical pial AVSs close to the DAVS induced by a Venturi sump effect can be associated.2 Unlike congenital vascular malformations, these shunts display mature arterial and venous configurations, without persisting embryonic dispositions.
IDAVSs have an aggressive evolution, as de novo shunts appear over time even following initial angiographic obliteration.37,38 They are considered to be due to diffuse unregulated angiogenic activities for which the initial trigger is unknown.39 Two of the three IDAVS seen in our series (66.6%) presented associated lesions: multiple cavernomas and DVAs in one girl (without familial history) and a facial AVM in another girl. As for DSMs, the association of IDAVSs and DVAs argue for interrelated pathogenesis.36 Cerebral cavernomas associated to some DAVSs have rarely been reported in the paediatric population.10,40 This entity presents some similar points with the rare subgroup of multiple DAVSs described in the adult population (7.8% in Ha’s series), associated with poor clinical prognosis.41,42 Even if pathogenesis remains unclear, sinus thrombosis has been reported in a majority of the cases by these authors.
Clinical symptoms of IDAVS can be severe including haemorrhage, seizures and intracranial hypertension, related to the significant hemodynamic impairment induced. Lesion progression is indeed dictated by progressive venous outlet restriction and AVS recurrence.3 This latest feature associated with shunt multifocality made, in our experience, treatment particularly challenging and disappointing. Obliteration of IDAVS could be obtained in 66.7% of patients but with high mortality (66.7%). Good clinical outcome was achieved in only one child partially treated. This poor prognosis seems in line with what was previously described by Lasjaunias.31 Hetts reported six such IDAVS: two patients died, three had severe neurocognitive deficits and only one patient met developmental milestones (16.7%).35 Half of the IDAVS in Souza’s series had a poor outcome.35 It is difficult to make specific therapeutic guidelines as our series is too short. Our experience, however, guides us nowadays more towards a stepwise treatment with preservation of the normal venous drainages, suppression of the reflux into cortical veins and selective shunt disconnections by the trans-arterial route than to aggressive immediate cure of the IDAVS. The venous approach should be carefully balanced and only pathological sinuses/veins exclusively participating in the pathological drainage can be sacrificed in our opinion. Partial targeted repeated embolization could be, in most cases, a proper therapeutic option that could allow stabilization of the clinical status. Regular follow-ups using MRI–MRA could be emphasized, with diagnostic and therapeutic angiography sessions if it is felt that recurrence has occurred needing further treatment. This group of lesions points to the fact that embolization, although the only therapy available currently, is not the optimal way to handle IDAVS. A better understanding of their pathogenesis and targeted antiangiogenic therapies may potentially improve the outcome of these patients43.
Adult-type dural arteriovenous shunts
Paediatric ADAVS are similar to DAVS seen in adults regarding their angioarchitecture and clinical presentation. They develop within the sinus wall or ventral epidural space, mostly within the cavernous region or sigmoid sinus.39
In our series, two ADAVSs were located at the cavernous level, which is frequent in adults but very rare in children, with only few reported cases.44 One cavernous fistula in our series exhibited a partial venous outflow thrombosis as in some adult cavernous fistulas.45 Sinus thrombosis was also observed in the majority (83.6%) of ADAVS occurring secondarily in VGAM-treated patients, distant from the VGAM shunt itself as located on either transverse or superior sagittal sinuses and clearly different from the dural shunts that have been described at this level.46 Unlike IDAVS that results from diffuse unregulated angiogenic activity, ADAVSs are considered as a focal post-thrombotic sprouting angiogenic response.39,46–49
A few rare genetic factors linked with ADAVS have also been reported: PTEN hamartoma tumour syndromes [Cowden syndrome and Bannayan–Riley–Ruvalcaba syndrome (BRRS)], PHACE syndrome and neurofibromatosis Type 1.10,16,50–54 Hereditary haemorrhagic telangiectasia has exceptionally been reported with brain DAVF in adults.51,55–57 Cowden syndrome and BRRS are caused by germline mutations in tumour suppressor PTEN.58 Cowden syndrome is associated with hamartoma formation, whereas BRRS individuals are generally macrocephalic, with weight and height exceeding the 95th percentile.59 Vascular malformations described in these syndromes are mostly seen in the musculoskeletal and the central nervous system where they are usually dural.55 Moreover, cerebral venous anomaly and cavernous malformations are observed in the Cowden syndrome.60,61 The PTEN gene has a role in normal post-natal angiogenesis and in inhibition of vascular sprouting: a mutation will result in abnormal angiogenesis, explaining the high vascular malformation incidence.62 As mentioned by Walcott et al.10, these data suggest the interest of genetic screening for patients with associated vascular anomalies and as for DSM and IDAVS suggest the potential common physio-pathological factors shared with cavernoma and DVA.
Angiographic and clinical courses of this DAVS subtype share those of the adult population DAVS. Treatment is dictated by pial venous reflux and induced haemorrhagic risk, as well as ophthalmological symptoms in cavernous localization cases.
In our series, the best outcomes were obtained in patients harbouring ADAVS, as also described by other authors.1,37 Treatment will be decided according to symptoms and architecture. The benign ADAVS with usual ante grade sinusal venous drainage discovered fortuitously after VGAM treatment, were not treated and have not exhibited unfavourable evolution so far. They will be followed up and managed according to their evolution. Among our treated patients, a complete obliteration rate of ADAVS was noted, with good clinical outcomes and no complications.
Conclusion
Paediatric DAVS comprise different lesion subtypes with variable clinical courses. Recognizing these lesions is mandatory to optimize therapeutic strategies: DSM with AVS patients requires early management and staged EVT achieves a good outcome in a significant proportion of patients. IDAVS still represent major challenges and are associated with a high morbi-mortality despite EVT. ADAVSs display a less aggressive evolution with treatment dictated by angio-architectural factors similar to those used in adults. The association with other vascular anomalies or malformations such as cavernomas, DVAs or facial AVMs suggests a common physio-pathological background that could be further investigated. A better understanding of the pathogenesis of these lesions is likely to improve their management.
Supplementary Material
Acknowledgements
The authors would like to thank Professor Laurent Guibaud and Dr Darren Orbach for the constructive discussions they had with them on the topic of DSM. They thank F. Salviat for statistics reviewing. We also thank Philipp Jordan (www.JordanGraphics.eu) for his help in drawing up the graphical abstract.
Abbreviations
- ADAVS =
adult-type dural arteriovenous shunt
- AVS =
arteriovenous shunt
- DAVS =
dural arteriovenous shunt
- DSM =
dural sinus malformation
- DVA =
developmental venous anomaly
- EVT =
endovascular therapy
- IDAVS =
infantile-type dural arteriovenous shunt
- mRS =
modified Rankin Scale
- SSS =
superior sagittal sinus
- SS =
straight sinus
- VGAM =
Vein of Galen aneurysmal malformation
Contributor Information
Stanislas J. Smajda, Department of Interventional Neuroradiology, Rothschild Foundation Hospital, Paris, France
Michael Söderman, Department of Neuroradiology, Karolinska University Hospital, Stockholm, Sweden; Department of Clinical Neuroscience, Karolinska Institute, Stockholm, Sweden.
Georg Dorfmüller, Department of Pediatric Neurosurgery, Rothschild Foundation Hospital, Paris, France.
Nathalie Dorison, Department of Pediatric Neurosurgery, Rothschild Foundation Hospital, Paris, France.
Marie-Claire Nghe, Department of Anesthesiology and Intensive Care, Rothschild Foundation Hospital, Paris, France.
Georges L. Rodesch, Department of Interventional Neuroradiology, Rothschild Foundation Hospital, Paris, France Department of Diagnostic and Interventional Neuroradiology, Hôpital Foch, Suresnes, France.
Funding
No funding was received towards this work.
Competing interests
The authors report no competing interests.
Supplementary material
Supplementary material is available at Brain Communications online.
References
- 1. Hetts SW, Moftakhar P, Maluste N, et al. Pediatric intracranial dural arteriovenous fistulas: Age-related differences in clinical features, angioarchitecture, and treatment outcomes. J Neurosurg Pediatr. 2016;18:602–610. [DOI] [PubMed] [Google Scholar]
- 2. Garcia-Monaco R, Rodesch G, Terbrugge K, Burrows P, Lasjaunias P. Multifocal dural arteriovenous shunts in children. Childs Nerv Syst. 1991;7:425–431. [DOI] [PubMed] [Google Scholar]
- 3. Lasjaunias P, Magufis G, Goulao A, et al. Anatomoclinical aspects of dural arteriovenous shunts in children. Review of 29 cases. Interv Neuroradiol. 1996;2:179–191. [DOI] [PubMed] [Google Scholar]
- 4. Sanchez-Mejia RO, Chennupati SK, Gupta N, Fullerton H, Young WL, Lawton MT. Superior outcomes in children compared with adults after microsurgical resection of brain arteriovenous malformations. J Neurosurg. 2006;105(2 Suppl):82–85. [DOI] [PubMed] [Google Scholar]
- 5. Barbosa M, Mahadevan J, Weon YC, et al. Dural Sinus Malformations (DSM) with Giant Lakes, in neonates and infants. Review of 30 consecutive cases. Interv Neuroradiol. 2003;9:407–424. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Smajda S, Soderman M, Dorfmuller G, Dorison N, Nghe MC, Rodesch G. Endovascular management of torcular dural sinus malformations in children: The role of straight sinus occlusion. J Neurointerv Surg. 2020;13:278–283. [DOI] [PubMed] [Google Scholar]
- 7. Rodesch G, Smajda S. Complications in pediatric interventional neuroradiology management. Reflections on a personal experience. Interv Neuroradiol. 2020;26:741–751. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Cognard C, Gobin YP, Pierot L, et al. Cerebral dural arteriovenous fistulas: Clinical and angiographic correlation with a revised classification of venous drainage. Radiology. 1995;194:671–680. [DOI] [PubMed] [Google Scholar]
- 9. Borden JA, Wu JK, Shucart WA. A proposed classification for spinal and cranial dural arteriovenous fistulous malformations and implications for treatment. J Neurosurg. 1995;82:166–179. [DOI] [PubMed] [Google Scholar]
- 10. Walcott BP, Smith ER, Scott RM, Orbach DB. Dural arteriovenous fistulae in pediatric patients: Associated conditions and treatment outcomes. J Neurointerv Surg. 2013;5:6–9. [DOI] [PubMed] [Google Scholar]
- 11. Komiyama M, Ishiguro T, Kitano S, Sakamoto H, Nakamura H. Serial antenatal sonographic observation of cerebral dural sinus malformation. AJNR Am J Neuroradiol. 2004;25:1446–1448. [PMC free article] [PubMed] [Google Scholar]
- 12. Rossi A, De Biasio P, Scarso E, et al. Prenatal MR imaging of dural sinus malformation: A case report. Prenat Diagn. 2006;26:11–16. [DOI] [PubMed] [Google Scholar]
- 13. Okudera T, Huang YP, Ohta T, et al. Development of posterior fossa dural sinuses, emissary veins, and jugular bulb: Morphological and radiologic study. AJNR Am J Neuroradiol. 1994;15:1871–1883. [PMC free article] [PubMed] [Google Scholar]
- 14. Tischfield MA, Robson CD, Gilette NM, et al. Cerebral vein malformations result from loss of Twist1 expression and BMP signaling from skull progenitor cells and dura. Dev Cell. 2017;42:445–461.e5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Streeter GL. The development of the venous sinuses of the dura mater in the human embryo. Am J Anat. 1915;18:145–178. [Google Scholar]
- 16. Hetts SW, Keenan K, Fullerton HJ, et al. Pediatric intracranial nongalenic pial arteriovenous fistulas: Clinical features, angioarchitecture, and outcomes. AJNR Am J Neuroradiol. 2012;33:1710–1719. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Yang E, Storey A, Olson HE, et al. Imaging features and prognostic factors in fetal and postnatal torcular dural sinus malformations, Part I: Review of experience at Boston Children’s Hospital. J Neurointerv Surg. 2018;10:467–470. [DOI] [PubMed] [Google Scholar]
- 18. Yang E, Storey A, Olson HE, et al. Imaging features and prognostic factors in fetal and postnatal torcular dural sinus malformations, Part II: Synthesis of the literature and patient management. J Neurointerv Surg. 2018;10:471–475. [DOI] [PubMed] [Google Scholar]
- 19. Herman JM, Spetzler RF, Bederson JB, Kurbat JM, Zabramski JM. Genesis of a dural arteriovenous malformation in a rat model. J Neurosurg. 1995;83:539–545. [DOI] [PubMed] [Google Scholar]
- 20. Brinjikji W, Mark IT, Silvera VM, Guerin JB. Cervicofacial venous malformations are associated with intracranial developmental venous anomalies and dural venous sinus abnormalities. AJNR Am J Neuroradiol. 2020;41:1209–1214. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Brinjikji W, Nicholson P, Hilditch CA, Krings T, Pereira V, Agid R. Cerebrofacial venous metameric syndrome—spectrum of imaging findings. Neuroradiology. 2020;62:417–425. [DOI] [PubMed] [Google Scholar]
- 22. Brinjikji W, Flemming KD, Lanzino G. De novo formation of a large cavernoma associated with a congenital torcular dural arteriovenous fistula: Case report. J Neurosurg Pediatr. 2017;19:567–570. [DOI] [PubMed] [Google Scholar]
- 23. Manjila S, Bazil T, Thomas M, Mani S, Kay M, Udayasankar U. A review of extraaxial developmental venous anomalies of the brain involving dural venous flow or sinuses: Persistent embryonic sinuses, sinus pericranii, venous varices or aneurysmal malformations, and enlarged emissary veins. Neurosurg Focus. 2018;45:E9. [DOI] [PubMed] [Google Scholar]
- 24. Grillner P, Söderman M, Holmin S, Rodesch G. A spectrum of intracranial vascular high-flow arteriovenous shunts in RASA1 mutations. Childs Nerv Syst. 2016;32:709–715. [DOI] [PubMed] [Google Scholar]
- 25. Tanaka M. Embryological consideration of dural arteriovenous fistulas. Neurol Med Chir. 2016;56:544–551. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Robertson F. Torcular dural sinus malformation. J Neurointerv Surg. 2018;10:423. [DOI] [PubMed] [Google Scholar]
- 27. Merzoug V, Flunker S, Drissi C, et al. Dural sinus malformation (DSM) in fetuses. Diagnostic value of prenatal MRI and follow-up. Eur Radiol. 2008;18:692–699. [DOI] [PubMed] [Google Scholar]
- 28. Terada A, Komiyama M, Ishiguro T, Niimi Y, Oishi H. Nationwide survey of pediatric intracranial arteriovenous shunts in Japan: Japanese Pediatric Arteriovenous Shunts Study (JPAS). J Neurosurg Pediatr. 2018;22:550–558. [DOI] [PubMed] [Google Scholar]
- 29. Friedmann DR, Eubig J, McGill M, Babb JS, Pramanik BK, Lalwani AK. Development of the jugular bulb: A radiologic study. Otol Neurotol. 2011;32:1389–1395. [DOI] [PubMed] [Google Scholar]
- 30. Saliou G, Dirks P, Sacho RH, Chen L, terBrugge K, Krings T. Decreased superior sagittal sinus diameter and jugular bulb narrowing are associated with poor clinical outcome in vein of galen arteriovenous malformation. AJNR Am J Neuroradiol. 2016;37:1354–1358. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Berenstein A, ter Brugge KG, Lasjaunias P. Surgical neuroangiography: clinical and interventional aspects in children. Springer; 2006. [Google Scholar]
- 32. Liby P, Lomachinsky V, Petrak B, et al. Torcular dural sinus malformations: A single-center case series and a review of literature. Childs Nerv Syst. 2020;36:333–341. [DOI] [PubMed] [Google Scholar]
- 33. Zerah M, Garcia-Monaco R, Rodesch G, et al. Hydrodynamics in vein of Galen malformations. Childs Nerv Syst. 1992;8:111–117; discussion 117. [DOI] [PubMed] [Google Scholar]
- 34. Appaduray SP, King JA, Wray A, Lo P, Maixner W. Pediatric dural arteriovenous malformations. J Neurosurg Pediatr. 2014;14:16–22. [DOI] [PubMed] [Google Scholar]
- 35. Souza MPS, Willinsky RA, Terbrugge KG. Intracranial dural arteriovenous shunts in children. The toronto experience. Interv Neuroradiol. 2003;9:47–52. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36. Geibprasert S, Krings T, Pereira V, Lasjaunias P. Infantile dural arteriovenous shunt draining into a developmental venous anomaly. Interv Neuroradiol. 2007;13:67–74. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37. Yu J, Lv X, Li Y, Wu Z. Therapeutic progress in pediatric intracranial dural arteriovenous shunts: A review. Interv Neuroradiol. 2016;22:548–556. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38. Pan H-C, Sheehan J, Huang C-F, Yang D-Y. Two consecutive dural arteriovenous fistulae in a child: A case report of successful treatment with gamma knife radiosurgery. Childs Nerv Syst. 2007;23:1185–1190. [DOI] [PubMed] [Google Scholar]
- 39. Roccatagliata L, Bracard S, Holmin S, Soderman M, Rodesch G. Pediatric intracranial arteriovenous shunts: A global overview. Childs Nerv Syst. 2013;29:907–919. [DOI] [PubMed] [Google Scholar]
- 40. Chakravarthy H, Lin T-K, Chen Y-L, Wu Y-M, Yeh C-H, Wong H-F. De novo formation of cerebral cavernous malformation adjacent to existing developmental venous anomaly – an effect of change in venous pressure associated with management of a complex dural arterio-venous fistula. Neuroradiol J. 2016;29:458–464. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41. Ha SY, Kwon YS, Kim BM, Kim DI, Kim DJ. Clinical and angiographic characteristics of multiple dural arteriovenous shunts. AJNR Am J Neuroradiol. 2012;33:1691–1695. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42. Guo Y, Yu J, Zhao Y, Yu J. Progress in research on intracranial multiple dural arteriovenous fistulas. Biomed Rep. 2018;8:17–25. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43. Queisser A, Seront E, Boon LM, Vikkula M. Genetic basis and therapies for vascular anomalies. Circ Res. 2021;129:155–173. [DOI] [PubMed] [Google Scholar]
- 44. Livi F, Ndoro S, Caird J, Crimmins D. Indirect cavernous carotid fistula in a 12-year-old girl. J Surg Case Rep. 2016;2016:rjw095. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45. Robert T, Sylvestre P, Blanc R, et al. Thrombosis of venous outflows of the cavernous sinus: Possible aetiology of the cortical venous reflux in case of indirect carotid-cavernous fistulas. Acta Neurochir. 2017;159:835–843. [DOI] [PubMed] [Google Scholar]
- 46. Paramasivam S, Niimi Y, Meila D, Berenstein A. Dural arteriovenous shunt development in patients with vein of galen malformation. Interv Neuroradiol. 2014;20:781–790. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47. Bhogal P, Yeo LL, Henkes H, Krings T, Soderman M. The role of angiogenesis in dural arteriovenous fistulae: The story so far. Interv Neuroradiol. 2018;24:450–454. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48. Li Q, Zhang Q, Huang Q-H, et al. A pivotal role of the vascular endothelial growth factor signaling pathway in the formation of venous hypertension-induced dural arteriovenous fistulas. Mol Med Rep. 2014;9:1551–1558. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49. Zhu Y, Lawton MT, Du R, et al. Expression of hypoxia-inducible factor-1 and vascular endothelial growth factor in response to venous hypertension. Neurosurgery. 2006;59:687–696; discussion 687-696. [DOI] [PubMed] [Google Scholar]
- 50. Tan W-H, Baris HN, Burrows PE, et al. The spectrum of vascular anomalies in patients with PTEN mutations: Implications for diagnosis and management. J Med Genet. 2007;44:594–602. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51. Brinjikji W, Iyer VN, Sorenson T, Lanzino G. Cerebrovascular manifestations of hereditary hemorrhagic telangiectasia. Stroke. 2015;46:3329–3337. [DOI] [PubMed] [Google Scholar]
- 52. Srinivasa RN, Burrows PE. Dural arteriovenous malformation in a child with Bannayan-Riley-Ruvalcaba Syndrome. AJNR Am J Neuroradiol. 2006;27:1927–1929. [PMC free article] [PubMed] [Google Scholar]
- 53. Cohen JE, Gomori JM, Grigoriadis S, Spektor S, Rajz G. Dural arteriovenous fistula of the greater sphenoid wing region in neurofibromatosis Type 1. Pediatr Neurosurg. 2008;44:172–175. [DOI] [PubMed] [Google Scholar]
- 54. Wang H, Oh AK, Orbach DB. Arteriovenous shunting as a new feature of PHACES. Case report. J Neurosurg Pediatr. 2009;3:53–56. [DOI] [PubMed] [Google Scholar]
- 55. Burrows PE. Angioarchitecture of hereditary arteriovenous malformations. Semin Intervent Radiol. 2017;34:250–257. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56. Krings T, Kim H, Power S, et al. Neurovascular manifestations in hereditary hemorrhagic telangiectasia: Imaging features and genotype-phenotype correlations. AJNR Am J Neuroradiol. 2015;36:863–870. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57. Maher CO, Piepgras DG, Brown RD Jr, Friedman JA, Pollock BE. Cerebrovascular manifestations in 321 cases of hereditary hemorrhagic telangiectasia. Stroke. 2001;32:877–882. [DOI] [PubMed] [Google Scholar]
- 58. Hobert JA, Eng C. PTEN hamartoma tumor syndrome: An overview. Genet Med. 2009;11:687–694. [DOI] [PubMed] [Google Scholar]
- 59. Marsh DJ, Kum JB, Lunetta KL, et al. PTEN mutation spectrum and genotype-phenotype correlations in Bannayan-Riley-Ruvalcaba syndrome suggest a single entity with Cowden syndrome. Hum Mol Genet. 1999;8:1461–1472. [DOI] [PubMed] [Google Scholar]
- 60. Lok C, Viseux V, Avril MF, et al. Brain magnetic resonance imaging in patients with Cowden syndrome. Medicine. 2005;84:129–136. [DOI] [PubMed] [Google Scholar]
- 61. Dhamija R, Weindling SM, Porter AB, Hu LS, Wood CP, Hoxworth JM. Neuroimaging abnormalities in patients with Cowden syndrome: retrospective single-center study. Neurol Clin Pract. 2018;8:207–213. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62. Hamada K, Sasaki T, Koni PA, et al. The PTEN/PI3K pathway governs normal vascular development and tumor angiogenesis. Genes Dev. 2005;19:2054–2065. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
Anonymized data will be shared on request.