Abstract
Sexual development in malaria parasites involves multiple signal transduction pathways mediated by reversible protein phosphorylation. Here, we functionally characterized a protein phosphatase, Ser/Thr protein phosphatase 5 (PbPP5), during sexual development of the rodent malaria parasite Plasmodium berghei. The recombinant protein phosphatase domain displayed obvious protein phosphatase activity and was sensitive to PP1/PP2A inhibitors including cantharidic acid (IC50=122.2 nM), cantharidin (IC50=74.3 nM), endothall (IC50=365.5 nM) and okadaic acid (IC50=1.3 nM). PbPP5 was expressed in both blood stages and ookinetes with more prominent expression during sexual development. PbPP5 was localized in the cytoplasm of the parasite and highly concentrated beneath the parasite plasma membrane in free merozoites and ookinetes. Targeted deletion of the pbpp5 gene had no influence on asexual blood-stage parasite multiplication or the survival curve of the infected hosts. However, male gamete formation and fertility were severely affected, resulting in almost complete blockade of ookinete conversion and oocyst development in the Δpbpp5 lines. This sexual development defect was rescued by crossing Δpbpp5 with the female defective Δpbs47 parasite line, but not with the male defective Δpbs48/45 line, thus confirming the essential function of the pbpp5 gene in male gamete fertility. Furthermore, the aforementioned PP1/PP2A inhibitors all had inhibitory effects on exflagellation of male gametocytes and ookinete conversion. In particular, endothall, a selective inhibitor of PP2A, completely blocked exflagellation and ookinete conversion at ~548.3 nM. This study elucidated an essential function of PbPP5 during male gamete development and fertility.
Keywords: Protein phosphatase, Phosphorylation, Male gametocyte, Fertility, Malaria
Graphical Abstract
1. Introduction
Despite decades of effort to control malaria, it remains a global health burden, especially for the tropical and subtropical countries. The 2017 World Malaria Report showed that there were approximately 216 million malaria cases in 2016, resulting in an estimated 445,000 deaths (WHO, 2018). Current malaria control efforts rely heavily on vector-based approaches such as insecticide-treated nets and indoor residual sprays, and effective artemisinin-based combination therapy (ACT) (Bhatt et al., 2015). However, the emergence and spread of insecticide-resistant vectors and artemisinin-resistant Plasmodium falciparum parasites have compromised the effectiveness of these control measures. Integrated and novel approaches targeting vulnerable steps in the life cycle of the malaria parasites are needed. Transmission of malaria from the vertebrate host to the mosquito vector is dependent on the formation of gametocytes, a process termed gametocytogenesis (Liu et al., 2011).Strategies to interfere with these developmental processes by using gametocytocidal drugs and transmission blocking vaccines are actively pursued. Currently, transmission blocking vaccines are still in development (Wu et al., 2015). Most of the commonly used antimalarial drugs only target asexual stages and have no effect on gametocyte stages (Kiszewski, 2011; Cui et al., 2015). Only the 8-aminoquinoline drug primaquine is effective on gametocytes, and to interrupt Plasmodium falciparum transmission a single dose of primaquine (0.25 mg/kg) is recommended by the World Health Organization (WHO) to be added to the standard ACT (Graves et al., 2018). However, there is still concern about the hemolytic risk of primaquine in people with glucose-6-phosphate dehydrogenase deficiency. Thus, there is an urgent need for new transmission blocking drugs, and it is hoped that better understanding of the transmission stages will yield novel drug targets.
Transmission of malaria to a susceptible mosquito is initiated when she ingests the arrested sexual precursor stage, the gametocyte, from the vertebrate host. Inside the mosquito midgut, a drop in temperature, change in pH, and mosquito factors such as xanthurenic acid induce the gametocyte to undergo gametogenesis to form male and female gametes (Billker et al., 1997). Microgametes then fertilize macrogametes to form zygotes, which transform into motile and invasive ookinetes. To orchestrate these form changes, the parasites utilize multiple signaling pathways, which involve reversible protein phosphorylation through kinases and phosphatases (Billker et al., 2004; Doerig et al., 2008; McRobert et al., 2008; Miliu et al., 2017). It is thus conceivable that a better understanding of kinases and phosphatases involved in sexual development of the parasites will help identify viable targets for transmission blocking drug discovery (Doerig and Meijer, 2007; Lucet et al., 2012).
Malaria parasites encode approximately 85 putative protein kinases and 30 protein phosphatases (Ward et al., 2004; Wilkes and Doerig, 2008; Guttery et al., 2014). Advances in functional genomics tools allowed large-scale functional analyses of Plasmodium kinases and phosphatases. Studies in the human and rodent Plasmodium parasites revealed that approximately half of the protein kinases are essential for asexual blood stages, while a further 14 protein kinases are needed for sexual development (Tewari et al., 2010; Solyakov et al., 2011). Similarly, functional studies of the 30 predicted phosphatases of the Plasmodium berghei phosphatome showed that 14 of them are dispensable for asexual stages, among which six show essential functions during sexual development (Guttery et al., 2014). Specifically, among the metal-dependent protein phosphatase (PPM) members, ppm1 is essential for exflagellation of the microgametocytes, ppm2 deletion reduces macrogamete number and ookinete conversion, whereas ppm5 deletion severely affects oocyst development. Two unique phosphatase family members, the protein phosphatase containing kelch-like domains (ppkl) and the Shewannella-like protein phosphatase (shlp1), involve ookinete differentiation and oocyst development, respectively (Guttery et al., 2012b; Patzewitz et al., 2013). The Protein Tyrosine Phosphatases (PTP)-like A homologue is essential for sporogony, as its knockout produces similar numbers of oocysts to the wild type (WT) but no sporozoites.
Malaria parasites have homologues of all major human phosphoprotein phosphatase (PPP) subfamilies PP1 – PP7 (Kutuzov and Andreeva, 2008). Functional studies showed that all of them are indispensable for asexual blood stages (Guttery et al., 2014). Of the PPP subfamilies, PP5 differs from other phosphatases in possessing three N-terminal tetratricopeptide repeat (TPR) domains, which are auto-inhibited and responsible for protein-protein interactions. Being expressed in virtually all mammalian systems, PP5 interacts with a number of proteins through its TPR domains to function as a modulator in multiple signaling pathways (Becker et al., 1994; Chinkers, 1994; Hinds and Sanchez, 2008). In protozoan parasites, down-regulation of PP5 in Eimeria tenella leads to apoptosis in second generation merozoites (Zhou et al., 2013). Over-expression of PP5 in Trypanosoma brucei rendered the parasite more resistant to geldanamycin treatment, whereas its knockdown reduced cell growth (Jones et al., 2008). PP5 in P. falciparum shares a similar structure with other protozoan PP5 proteins and it interacts with Heat Shock Protein 90 (Hsp90) (Dobson et al., 2001; Lindenthal and Klinkert, 2002).Although its function is not known, PP5 is highly expressed in male gametocytes in P. berghei, suggesting an important role in sexual development (Guttery et al., 2014). Here, we attempted to elucidate the function of PbPP5 through in vitro enzyme studies and genetic manipulation, which identified an important role of PbPP5 in regulating male gamete fertilization.
2. Materials and methods
2.1. Sequence analysis
SMART software (http://smart.embl-heidelberg.de/) was used to analyze the conserved domains of PbPP5 (GenBank Accession number XP_673410.2) (Letunic and Bork, 2018), while TMHMM software (http://www.cbs.dtu.dk/services/TMHMM-2.0/) was used to analyze its transmembrane domain. Motif scan software (https://myhits.isb-sib.ch/cgi-bin/motif_scan) was used to analyze structural domains, and BLASTp (http://www.ncbi.nlm.nih.gov/BLAST/) was used to search for homologous proteins. Phylogenetic analysis of PbPP5 and known PPs in other species retrieved from GenBank were aligned using MUSCLE software (https://www.ebi.ac.uk/Tools/msa/muscle/). Subsequently, a phylogenetic tree was constructed using the neighbor-joining method in MEGA 7.0 (https://www.megasoftware.net/). The reliability of the tree was measured by bootstrap analysis with 1,000 replicates.
2.2. Experimental animals, parasites and mosquitoes
Six- to eight-week old female BALB/c mice (Beijing Animal Institute, China) and mosquitoes were infected with WT Plasmodium berghei ANKA 2.34 strain and mutant parasites as previously described (Kou et al., 2016). Animal handling was conducted according to a China Medical University Animal Care and Use Committee approved protocol. Female Anopheles stephensi mosquitoes were fed a 10% (w/v) glucose solution and reared at 25 °C and 50–80 % humidity with a 12:12 h light:dark period under standard laboratory conditions.
2.3. Generation of transgenic parasites
Targeted deletion of the pbpp5 gene was accomplished by double-crossover homologous recombination using the vector PbGEM-301794 (kindly provided by plasmoGEM; http://plasmogem.sanger.ac.uk/). Primers used for transgenic plasmid construction are listed in Supplementary Table S1. To generate the plasmids for the pbs47 (PlasmoDB accession number PBANKA_1359700) knockout, DNA fragments of 962 bp upstream and 766 bp downstream sequences were amplified from P. berghei genomic DNA (gDNA) using primers in Supplementary Table S1. To knock out pbs48/45 (PlasmoDB accession number PBANKA_1359600), DNA fragments of 538 bp upstream and 779 bp downstream sequences were amplified (Supplementary Table S1). The amplified fragments were digested with HindIII/PstI and KpnI/NotI, respectively, and subsequently cloned into the pL0034 plasmid, which contains the hdhfr cassette conferring resistance to pyrimethamine, to generate the pL0034-pbs47 and pL0034-pbs48/45 plasmids (van Dijk et al., 1995). Before transfection, the PbGEM-30194 plasmid was linearized using NotI, and the pL0034-pbs47 and pL0034-pbs48/45 plasmids were linearized with SacII and KpnI. To generate episomally expressed full-length PbPP5 protein tagged with green fluorescent protein (GFP) at the C-terminus, specific primers PbPP5full lenthB1F and PbPP5full lengthB2RV3 were used to amplify the open reading frame (ORF) of PbPP5, and PbPP55’UTRF and PbPP55’UTRR primers were used to amplify the 5’ untranslated region (UTR) region (−1892 ~ −2 bp) of the pbpp5 gene (Supplementary Table S1). Gateway BP (attachment bacterial site (attB) and attachment phage site (attP)) recombination reactions of PCR product with pDONR™ 221 and pDONR™ P4-P1R (Invitrogen) were performed to generate pEN12-PbPP5full length and pENTR41-PbPP5–5’UTR plasmids. A Gateway MultiSite LR (attachment left site (attL) and attachment right site (attR)) recombination reaction was performed with pEN12-PbPP5full length, pENTR41-PbPP5–5’UTR, pENT23-GFP and pCHDR-3/4 plasmids to generate a pCHDR-PbPP5full length-GFP plasmid (van Dooren et al., 2005) according to the manufacturer’s instructions. Transfection, selection and parasite cloning were performed as previously described (Janse et al., 2006a,b). Infected blood was collected to confirm the correct integration by diagnostic PCR (Supplementary Table S1).
2.4. Expression of recombinant PbPP5 and immunization
The recombinant PbPP5 protein used in the current study was expressed using the Pichia expression system in the host strain GS115 his4 (auxotrophic for histidine) (Genecreate, Inc.). Briefly, the PP2Ac domain of PbPP5 (amino acid (aa) positions 407–711) was amplified, cloned into the expression vector pPIC9K plasmid and transformed into the GS115 strain. Expression of the recombinant PbPP5yPP2Ac (rPbPP5 yPP2Ac) protein was induced by adding 200 μl of 5% methanol into the yeast peptone (YP) culture medium at 30oC for 24 h. The rPbPP5yPP2Ac protein was purified using a Ni-NTA column, dialyzed in 20 mM Tris-HCl (pH 7.5) and 1 mM DTT, and concentrated using Amicon®Ultra 0.5 (Millipore, USA) to yield 2 mg/ml of protein concentration.
For immunization of mice, 6–8 weeks old female BALB/c mice (n = 10) were initially immunized by s.c. injection with 50 μg of rPbPP5yPP2Ac protein emulsified in FCA (Sigma), followed by two booster injections at 2 week intervals with the same amount of protein in incomplete Freund’s adjuvant. Mice in the control group (n = 10) were injected with PBS using the same immunization procedure. Final bleeding was done 2 weeks after the last immunization. Blood was collected from the tail vein of each mouse and allowed to clot at room temperature. The antiserum titer against PbPP5 was analyzed by ELISA as previously described (Zheng et al., 2016). Briefly, a 96-well plate was coated with the rPbPP5yPP2Ac protein at 10 μg/mL overnight at 4°C, and then blocked with 3% BSA in 1 × PBS and 0.05% Tween 20 (PBS-T) for 2 h at room temperature. Mouse antiserum was diluted in PBS containing 10% calf serum albumin (pH 7.2), added to the wells, and incubated for 2 h at 37 °C. After two washes with PBS-T, horse radish peroxidase (HRP)-conjugated goat anti-mouse IgG antibodies (Thermo Scientific) diluted 1:5000 was added and incubated for 2 h at 37 °C. After seven washes with PBS-T, the chromogenic substrate was added and developed for 5 min. The reaction was stopped by adding 50 μl of 2 mM H2SO4 to each well. The plate was immediately read with a plate reader at 490 nm.
2.5. Indirect immunofluorescence assay (IFA)
IFA was carried out on different developmental stages of P. berghei as previously described (Tonkin et al., 2004). Briefly, 100 μl of P. berghei-infected tail blood were collected into a 1.5 ml tube, and washed three times with PBS. Cells were fixed with 4% paraformaldehyde and 0.0075% electron microscope (EM) grade glutaraldehyde in PBS for 30 min. Fixed cells were either subjected to permeabilization with 0.1% Triton X-100 for 5 min on ice or without permeabilization, followed by PBS washing and rinsing with 0.1 mg/ml of sodium borohydride (NaBH4)/PBS for 10 min, and blocked with 5% skimmed milk for 30 min at 37°C. After blocking, cells were incubated with mouse anti-PbPP5 sera (1:500), mouse or rabbit anti-GFP monoclonal antibody (mAb) (abcam, USA), or rabbit anti-GAPDH (abcam, USA) at 37°C for 1 h. After washing three times in PBS, the slides were incubated with the secondary antibodies (AlexaFluor-goat anti-mouse 488 and/or anti-rabbit 594) (Invitrogen) at 1:500 for 1 h at 37°C. Parasite nuclei were counter-stained with 1 μg/mL of DAPI (Thermo scientific, USA). Cells were washed three times with PBS and mounted with ProLong® Gold anti-fade reagent (Thermo scientific, USA). After that, the cells were settled on a slide, covered by coverslips and visualized under a Nikon ECLIPSE 80i microscope.
2.6. Western blot
The purification of schizonts, gametocytes and ookinetes was performed as previously described (Sinden et al., 1985; Beetsma et al., 1998; Janse et al., 2006b). After collection, parasite-infected cells were treated with 0.15% saponin (Sigma) in PBS for 8 min on ice and pelleted through centrifugation at 4°C. Parasite pellets were washed several times with PBS containing protease inhibitors (PBS-PI) and proteins were extracted with PBS-PI containing 2% SDS and 0.1% Triton-X100 for 30 min at room temperature. Equal amounts of parasite antigens (10 μg) were electrophoresed on a 12% SDS-PAGE gel and transferred to a 0.22 μm polyvinylidene fluoride (PVDF) membrane (Bio-Rad). The membrane was incubated with mouse anti-PbPP5 antiserum (1:500), anti-GFP mAb (9F9. F9, abcam), rabbit polyclonal anti-GAPDH antibodies (1:2000, abcam), or anti-Hsp70 mAb (5A5, abcam) in Tris-buffered saline containing 0.1% Tween 20 (TBS-T) for 3 h at room temperature, washed three times in TBS-T, and then incubated for 2 h at 37°C with HRP-conjugated goat anti-mouse IgG (H+L) antibodies (Thermo Scientific) diluted at 1:25,000 in TBS-T. Proteins on the blot were visualized with a Pierce ECL Western Blotting Kit (Thermo Scientific) on Tanon 4200 (Tanon). The relative molecular masses of proteins were estimated with reference to PageRuler Prestained Protein Ladder (Thermo Scientific).
2.7. Protein phosphatase assay
The phosphatase activity of the PbPP5yPP2Ac protein was assayed by using the ProFluor® Ser/Thr PPase Assay kit (Promega, USA). Briefly, concentrations of PbPP5yPP2Ac and His-tag protein as the control (Zhu et al., 2017) were determined by the Bradford method using BSA as the standard (TaKaRa, Japan). The rPbPP5yPP2Ac and the His protein in 25 μl of phosphatase dilution solution were added into each well. An equal volume of peptide solution was added into all wells, mixed for 15 s, and incubated for 10 min at room temperature. Then, 25 μl of protease were added into each well and the plate was incubated at room temperature for 90 min. Finally, 25 μl of stabilizer solution were added into each well and the plate was read on a Biomek 2000 laboratory automation workstation (Beckman Coulter, Inc., Fullerton, CA, USA). Fluorescence emission from the product was measured with a multiwell plate reader (Cytofluor II; Applied Biosystems, Foster City, CA, USA) with excitation at 485 nm (20 nm bandwidth) and emission at 530 nm (30 nm bandwidth). Inhibition of the rPbPP5yPP2Ac by several PP1/PP2A inhibitors from the Screen-Well Phosphatase Inhibitor Library (BML-2384, Enzo Life Science), including cantharidic acid, cantharidin and endothall (Supplementary Table S2), was performed as described above.
2.8. Phenotypic analysis of Δpbpp5 parasites during parasite development
To study the functions of pbpp5 during development, 10 mice were pre-treated with phenylhydrazine. Two groups of five mice each were injected i.p. with 1 × 106 Δpbpp5- and WT P. berghei-infected RBCs (iRBCs), respectively. Parasitemia was monitored daily by Giemsa-stained tail blood smears. On day 3 p.i., gametocytemia (mature gametocytes per 100 RBCs), gametocyte sex ratio, exflagellating male gametocytes, and interactions between male and female gametes were determined. For the exflagellation assay, 10 μl of gametocyte-infected blood were obtained from the tail vein and mixed immediately with 90 μl of complete ookinete culture medium. The mixture was placed under a Vaseline-coated coverslip at 25°C for 15 min, the exflagellation centers were counted under a phase contrast microscope in 30 fields. The numbers of male gametocytes forming or not forming exflagellation centers were counted. An exflagellation center is defined as an exflagellating male gametocyte with more than four tightly associated red blood cells (Bialojan and Takai, 1988). For quantification of male-female interactions, 10 min after induction of gamete formation, the cell suspension was placed in a Vaseline-coated coverslip and observed for a period of 20 min under a phase contrast microscope. Attachment of males to females was scored if the male had active interaction/attachment with the female for more than 3 s. Ookinete formation was evaluated by adding 10 μl of iRBCs into 90 μl of ookinete culture medium and incubating at 19°C for 24 h. Cultured ookinetes were labeled with a mouse anti-Pbs21 monoclonal antibody (1:500) and enumerated under a fluorescence microscope. Cross-fertilization experiments with different parasite lines defective in either male or female gametogenesis were performed as previously described (Ponzi et al., 2009; Boisson et al., 2011). Briefly, gametes from the Δpbpp5 line were cross-fertilized with gametes of parasite lines that produced only fertile female gametes (Δp48/45) (van Dijk et al., 2001) or fertile male gametes (Δp47) (Khan et al., 2005). All the fertilization/ookinete maturation assays were done in triplicate from independent experiments.
To determine the effect of Δpbpp5 on sporogonic development in mosquitoes, A. stephensi mosquitoes (~100/mouse) starved for 6 h were allowed to feed for 30 min on three mice infected 3 days earlier with either the WT P. berghei or Δpbpp5. Fully engorged mosquitoes were maintained at 19–22°C and in 50–80% relative humidity. Ten days after feeding, up to 40 mosquitoes were dissected in each group. The midguts of mosquitos were removed and stained with 0.5% mercurochrome (Sigma–Aldrich). Oocysts were counted to determine the prevalence (number of infected mosquitoes) and intensity of infection (number of oocysts per midgut). Mosquitoes were dissected on day 18 post blood feeding to determine the presence of salivary gland sporozoites.
2.9. Exflagellation and ookinete conversion inhibition assay
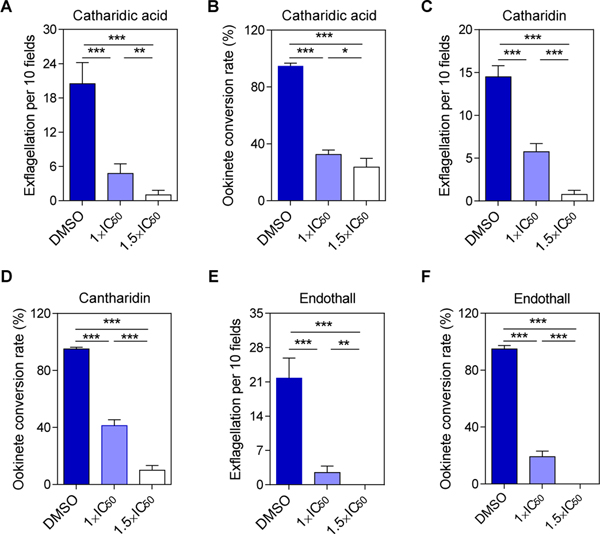
The inhibitory effects of cantharidic acid, cantharidin and endothall on P. berghei exflagellation and ookinete conversion were evaluated in vitro. All three compounds were prepared as 10 mM stock solutions in DMSO. They were further diluted in ookinete culture medium to a final concentration of 1× and 1.5× of their respective half maximal inhibitory concentration (IC50) determined for the rPbPP5yPP2Ac recombinant enzyme: cantharidic acid (1×IC50=122.2 nM, 1.5×IC50=183.3 nM), cantharidin (1×IC50=74.3 nM, 1.5×IC50=111.5 nM) and endothall (1×IC50=365.5 nM, 1.5×IC50=548.3 nM). They were used for in vitro exflagellation and ookinete conversion assays as described above.Ookinete culture medium containing 0.1% DMSO was used as a control. All experiments were performed in triplicate with each replicate using the parasite-infected blood of a different mouse.
2.10. Statistical analyses
Statistical comparison between groups (IgG levels, parasitemia, gametocytemia, and ookinete numbers) was done by Student’s t tests using the GraphPad Prism software. The intensity of infection (oocysts/midgut) was analyzed using the Mann–Whitney U test, while infection prevalence was assessed using the Fisher’s exact test.
3. Results
3.1. Sequence analysis of PbPP5
The pbpp5 gene encodes a protein of 711 aa. Similar to its P. falciparum orthologue PfPP5, PbPP5 contains a C-terminal phosphatase domain (PP2Ac, 418–694 aa) and an extended N-terminal domain containing three TPR motifs (TPR1, 186–219 aa; TPR2, 278–311 aa; and TPR3, 312–345 aa), a typical feature of PPPs (Supplementary Fig. S1A). Searching the motif database revealed 12 putative N-glycosylation sites, nine casein kinase II phosphorylation sites, 13 protein kinase C phosphorylation sites, and one tyrosine kinase phosphorylation site (Supplementary Fig. S1B). Additional biological evidence is required to validate these predictions. PbPP5 was aligned with other known PP5 protein family members, which showed significant homology in the TPR domain and the PP2Ac domain (Supplementary Fig. S1C). A phylogenetic tree constructed from the alignment showed that PbPP5 was clustered with other protozoan PP5s (Supplementary Fig. S1D).
3.2. PbPP5 enzyme activity and sensitivity to PP1/PP2A inhibitors
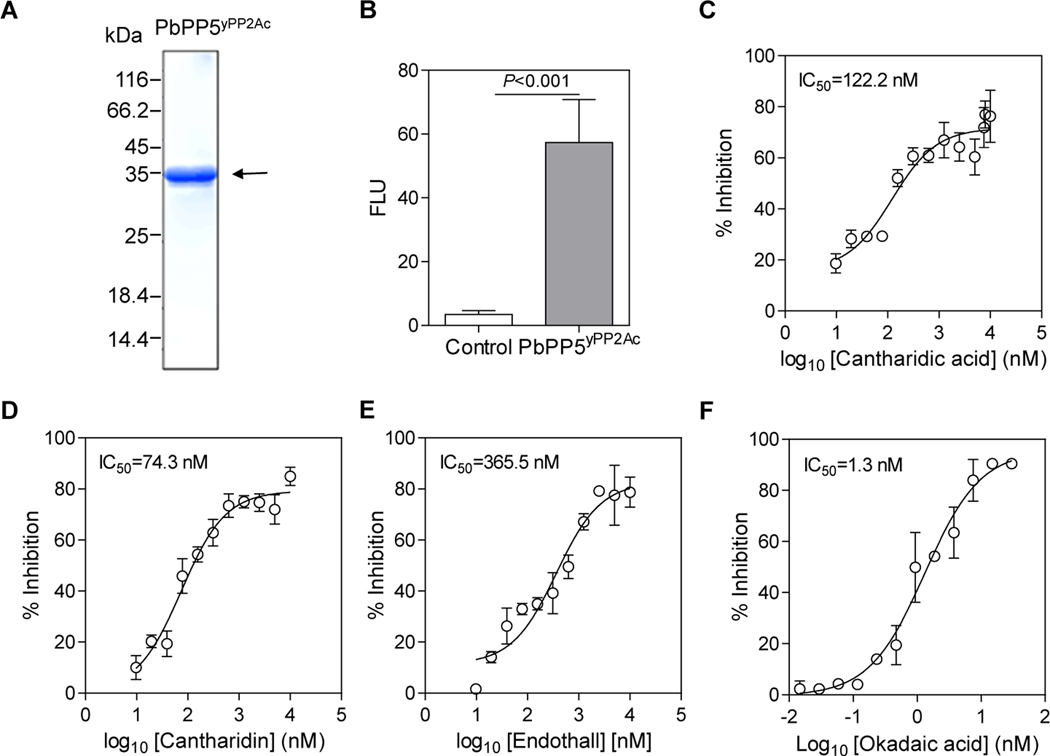
To examine the catalytic activity of PbPP5, the PP2Ac domain of PbPP5 protein was expressed in yeast as a His-tag fusion protein and was affinity-purified to almost homogeneity (Fig. 1A). The band size of the rPbPP5yPP2Ac protein on the SDS-PAGE gel is around 36 kDa, consistent with the predicted size (Fig. 1A). The catalytic activity of rPbPP5yPP2Ac protein was compared with the control His-tag protein from the pET32a (+) vector expressed and purified from Escherichia coli (Zhu et al., 2017) using phosphorylated S/T PPase R110 (Promega) as the substrate. As shown in Fig. 1B, rPbPP5 exhibited obvious phosphatase activity compared with the control (P < 0.001). Furthermore, the phosphatase activity of the rPbPP5 was sensitive to PP1/PP2A inhibitors, with IC50 values for cantharidic acid, cantharidin, endothall and okadaic acid of 122.2, 74.3, 365.5, and 1.3 nM, respectively (Fig. 1C–F). This result is in good accordance with the inhibitory effect of okadaic acid on recombinant PfPP5 (Dobson et al., 2001).
Fig. 1.
The catalytic activity of rPbPP5yPP2Ac (yeast system expressed Protein phosphatase 2A homologues, catalytic domain (PP2Ac )) domain of recombinant Plasmodium berghei protein phosphatase 5) and its sensitivity to protein phosphatase-1 and −2A (PP1/PP2A ) inhibitors. (A) Expression of the rPbPP5yPP2Ac protein. The rPbPP5yPP2Ac was purified from yeast and analyzed by SDS-PAGE under reducing conditions. Molecular weight markers are shown. The arrow indicates the expected size of rPbPP5 protein (~36 kDa). (B) Phosphatase activity of rPbPP5. Control, His-tag protein. ***, P<0.001. FLU, fluorescence light units. (C-F) Dose-response curves of different PP1/PP2A inhibitors: cantharidic acid (C), cantharidin (D), endothall (E), and okadaic acid (F). Each point represents the mean, and values are given as means and S.D. from three biological replicates.
3.3. PbPP5 expression and localization
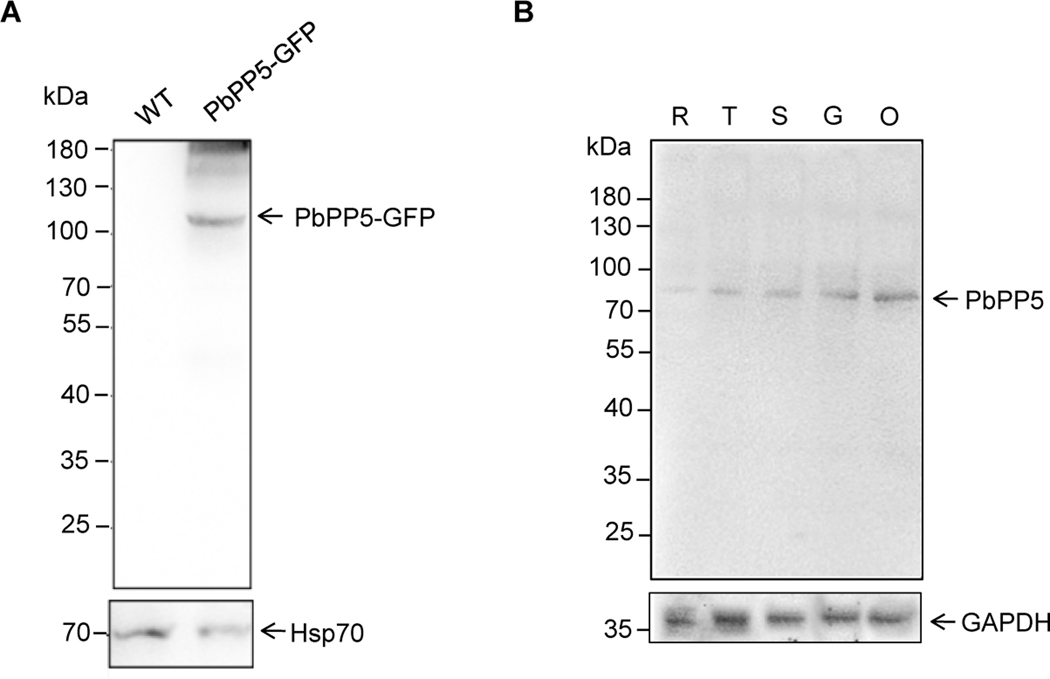
To investigate the localization of PbPP5, we generated a parasite line with episomally-expressed PbPP5 fused with GFP at its C-terminus (PbPP5-GFP) driven by its own promoter. The expression of PbPP5-GFP was confirmed by Western blot analysis of parasite lysates of mixed blood stages using the anti-GFP antibody, which showed an ~110 kDa protein band that is consistent with the predicted molecular size of the fusion protein (Fig. 2A). Additionally, we raised polyclonal mouse antibodies against rPbPP5, and the antisera recognized the rPbPP5 protein in a Western blot (data not shown). To study PbPP5 protein expression in different developmental stages, proteins extracted from different stages of the WT parasites were separated by SDS-PAGE and probed with the polyclonal anti-PbPP5 antisera. Whereas the mouse pre-immune sera did not identify any specific protein bands in a parallel Western blot (data not shown), a protein band with a molecular mass of ~82 kDa was detected in each of the developmental stages examined by the anti-PbPP5 antisera, and the protein band corresponds to the size of the endogenous PbPP5 (Fig. 2B). It is noteworthy that the PbPP5 protein was substantially more abundant in sexual stages than in asexual stages.
Fig. 2.
Stage-specific expression of Plasmodium berghei protein phosphatase 5 (PbPP5). (A) Western blot of whole cell lysates of different stage PbPP5-GFP parasites with monoclonal anti-GFP antibody. (B) Western blot of wild type (WT) parasite cell lysates with anti-PbPP5 antisera. R, ring stage; T, trophozoite stage; S, schizont stage; G, gametocyte stage; O, ookinete stage. The positions of the pre-stained molecular mass markers are indicated. Western blots with mouse anti-PbHSP70 antisera or rabbit polyclonal anti-GAPDH antibody is shown as loading controls. HSP, heat shock protein.
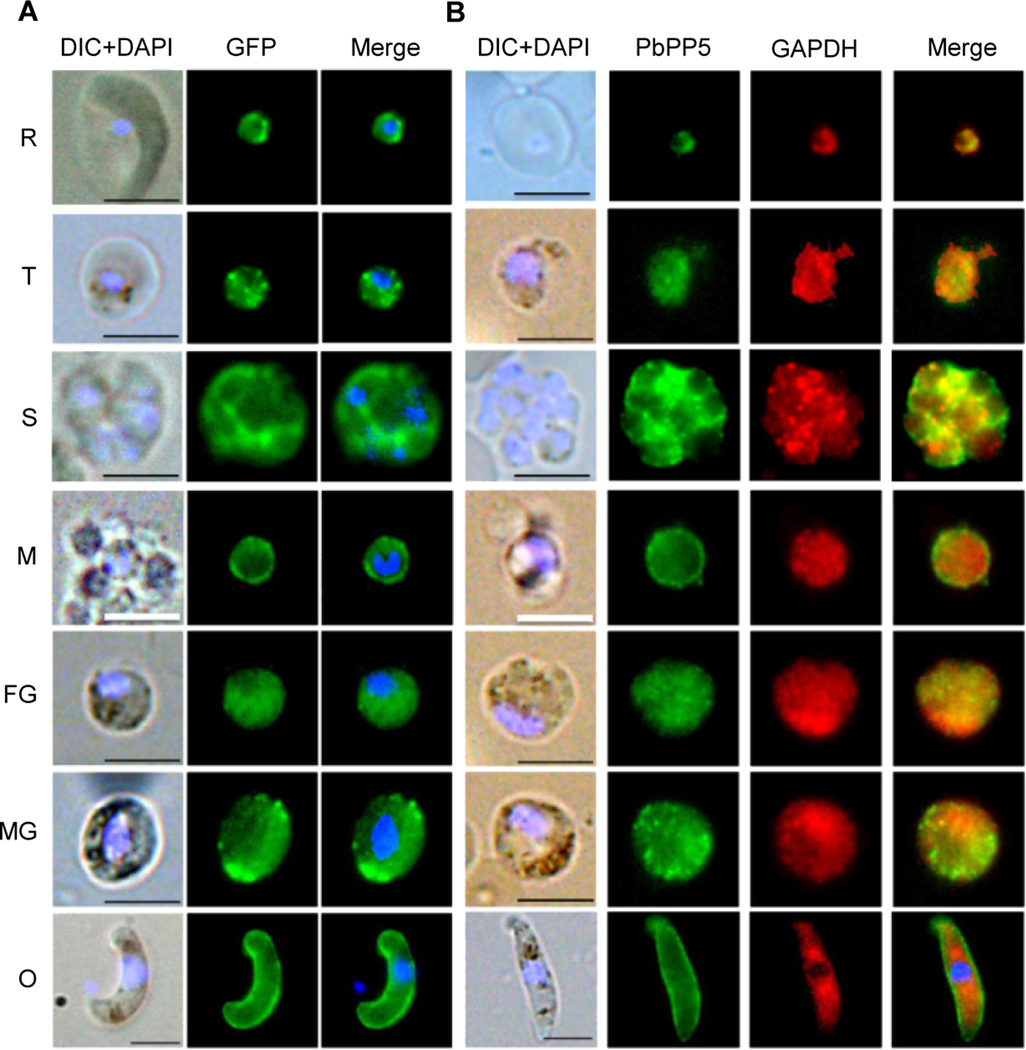
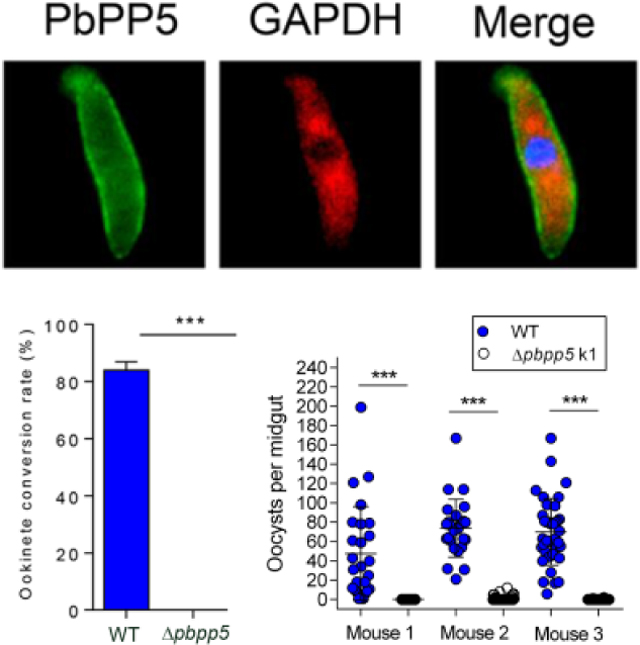
We used both WT and PbPP5-GFP parasites to determine the localization of PbPP5 protein during blood stage development of the parasite. First, using mouse-anti-PbPP5 antisera and rabbit anti-GFP antibody, we showed significant overlap of the fluorescent signals, indicating that PbPP5-GFP was correctly localized in the parasites (Supplementary Fig. S2). The fluorescence signals for GFP at ring, trophozoite, schizont and gametocyte stages were diffused in parasite cytosol, and some signals were associated with the membranes (Fig. 3A). The localization patterns were similar to that of the PbPP5 in WT parasites detected with the anti-PbPP5 antisera (Fig. 3B). Co-localization analysis showed that the fluorescent signals for PbPP5 and GAPDH (a cytosolic marker) partially overlapped, suggesting some of the PbPP5 proteins were cytosolic (Fig. 3B). Interestingly, in free merozoites and zygotes - ookinetes, the fluorescent signals were more intense at the rim of the parasites, which were reminiscent of membrane localization (Fig. 3B, Supplementary Fig. S2). Yet, when the zygote to ookinete stage parasites were examined under non-permeabilized conditions, PbPP5 was not detectable, suggesting that PbPP5 was concentrated beneath the parasite plasma membrane (Supplementary Fig. S3).
Fig. 3.
Subcellular localizations of Plasmodium berghei protein phosphatase 5 (PbPP5). (A) IFA of Plasmodium berghei blood stages to localize GFP-tagged PbPP5 proteins using mouse anti-GFP antibody. (B) Co-localization of the endogenous PbPP5 with anti-PbPP5 sera and anti-GAPDH antibodies. Different stages were observed under a Nikon fluorescence microscope with differential interference contrast (DIC), with the Alexa 488 channel (green) for detection of PbPP5, Alexa 594 channel (red) for detection of GAPDH, and with the DAPI channel (blue) to visualize DNA. Alexa 488, Alexa 594 and DAPI images were merged to show concordance. Note that exposure times for different images were different, and were not meant for quantitative comparison. Anti-GAPDH antibody was used as a marker for the parasite cytoplasm. For all the images, the black scale bar = 5 μm, and the white scale bar = 2.5 μm. R, ring stage; T, trophozoite stage; S, schizont stage.
3.4. PbPP5 deletion and function during asexual stages
To study the function of PbPP5 during the parasite’s intraerythrocytic cycle, we generated pbpp5 knockout (Δpbpp5) parasite lines. After homologous recombination, the pbpp5 open reading frame (ORF) was replaced by the hdhfr selectable cassette (Supplementary Fig. S4). Correct allelic replacement was confirmed by diagnostic PCR (Supplementary Fig. S4B). Furthermore, Western blot analysis using specific antibodies against PbPP5 in mixed blood stage parasites confirmed deletion of pbpp5, where the PbPP5 protein of ~82.5 kDa was observed in WT but not the Δpbpp5 parasites (Supplementary Fig. S4C). We selected two clones, Δpbpp5 clones k1 and k3, from two independent transfection experiments, for phenotypic analysis. In mice infected by i.v. injection of approximately the same numbers of WT, Δpbpp5 k1, and Δpbpp5 k3 parasites, we did not observe noticeable differences in daily parasitemias among the three groups between days 3 and 20 (Supplementary Fig. S5A), indicating that PbPP5 is dispensable for asexual blood stages of P. berghei. Furthermore, pbpp5 deletion did not appear to affect the growth phenotype of the parasites as the survival curves of BALB/c mice infected with the WT parasites and the two Δpbpp5 lines were comparable (Supplementary Fig. S5B).
3.5. Functions of PbPP5 during sexual development
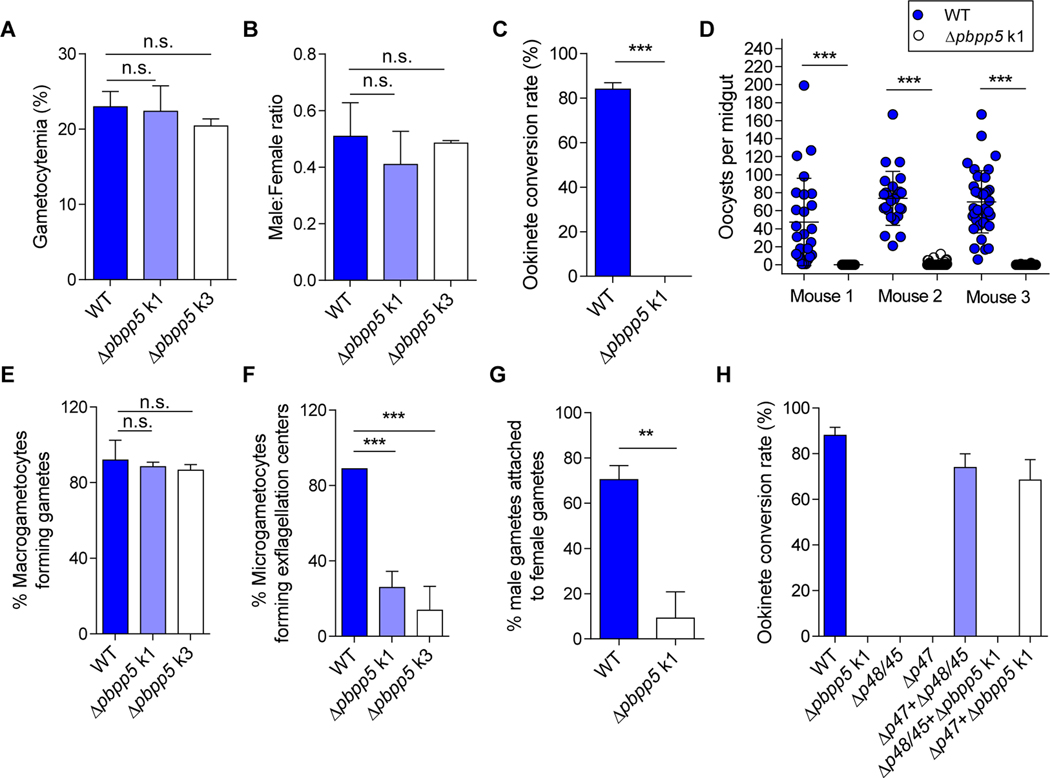
We next explored whether PbPP5 plays any rolesin sexual development of the parasite. Compared with the WT parasite, the pbpp5 deletion lines did not show a defect in gametocytogenesis, as the day 3 gametocytemias were similar among the three parasite lines (P > 0.05, t test; Fig. 4A). In addition, although sex ratios of gametocytes were slightly reduced in the Δpbpp5 lines, the difference was not significant (P > 0.05, t test; Fig. 4B). However, when day 3 infected blood was used for in vitro culture of ookinetes, there was a complete lack of ookinetes in Δpbpp5 parasites (Fig. 4C).
Fig. 4.
Functional analysis of Plasmodium berghei protein phosphatase 5 (PbPP5) during sexual development. (A) Gametocytemia of wild type (WT) and Δpbpp5 parasites at day 3 p.i. (B) Male/female gametocyte ratios of WT and Δpbpp5 parasites at day 3 p.i. (C) Absence of ookinete formation in the Δpbpp5 parasites. The conversion rate is the percentage of Pbs21-positive parasites (stained by anti-Pbs21 monoclonal antibody) that had successfully differentiated into elongated ‘banana-shaped’ ookinetes. (D) Absence of oocysts in midguts of mosquitoes infected with Δpbpp5 k1 parasites compared with WT at day 10 post feeding. Circles correspond to oocyte numbers in individual mosquitoes. The panel represents one of the two or more infections performed with three infected mice per parasite clone and per infection. The number of mosquitoes analyzed per infection: WT, n = 29, 26, 39; Δpbpp5 k1, n = 33, 36, 40. (E) The formation of macrogametes. (F) The interaction of Δpbpp5 male gametes with red blood cells (exflagellation centers). Results were obtained from five independent experiments for the comparison between the WT and Δpbpp5 parasites. Two infected mice were analyzed per parasite clone. (G) The interaction of Δpbpp5 male gametes with female gametes. (H) Ookinete conversion after crossing Δpbpp5 parasites with Δp47 and Δp45/48 parasites. For all experiments, WT parasites were used as the control and three mice were used for each group. Results were obtained from at least two independent experiments. n.s., not significant; *, P<0.05; **, P<0.01; ***, P<0.001.
To substantiate the in vitro findings, mosquitoes were fed on mice infected with either WT or Δpbpp5 k1 parasites and oocyst development was examined on day 10 after feeding. Pbpp5 deletion almost resulted in a complete blockade of subsequent sporogonic development. In mosquitoes fed on three Δpbpp5-infected mice, infection prevalences were 0% (0/33), 30.6% (11/36), and 7.5% (3/40) compared with 100% (26/26), 100% (26/26) and 100% (37/37) among mosquitoes fed on WT P. berghei-infected mice (Fig. 4D). The mean oocyst density was 0, 1.4, and 0.1 oocysts/midgut in mosquitoes fed on the three Δpbpp5 k1 parasite-infected mice, significantly lower than that of 47.5, 80.8, and 69.9 oocysts/midgut in mosquitoes fed on the three WT P. berghei-infected mice (P < 0.001, Mann–Whitney U test; Fig. 4D). Subsequent dissection of ~35 mosquitoes 18 days after feeding on Δpbpp5-infected mice did not reveal salivary gland sporozoites.
To identify which step(s) during the development of ookinetes was defective in the Δpbpp5 parasites, we first compared the gametogenesis process. Whereas the formation of macrogametes appeared normal in the pbpp5 KO lines (Fig. 4E), the proportion of microgametocytes forming exflagellation centers in the Δpbpp5 parasite lines was severely reduced: 13.8 – 25% exflagellating Δpbpp5 male gametocytes in the Δpbpp5 lines compared with 88.9% in WT parasites (P < 0.001, t test; Fig. 4F). To investigate whether the male gametes lacking expression of Δpbpp5 could attach to female gametes, we analyzed the interactions between male and female gametes at 30 min after induction of gametogenesis under a light microscope. The results revealed that the proportions of male gametes attached to female gametes were drastically reduced in Δpbpp5 parasite lines (mean = 9.26%) compared with those in WT parasites (mean = 70.42%) (P <0.01, t test; Fig. 4G).
To ascertain that the block in ookinete formation resulted from defective males, we performed genetic crosses with parasite lines defective in either males or females (Boisson et al., 2011). For this purpose, we generated Δpbs48/45 and Δpbs47 parasite lines that are defective in male and female gametes, respectively (Supplementary Fig. S6), using the previously described method (van Dijk et al., 2010). Crossing of Δpbpp5 with the pbs48/45 deletion mutant showed no rescue of the phenotype, whereas crossing with the pbs47 deletion parasite resulted in 78.4% ookinete formation compared with 88.0% in the WT (Fig. 4H). These results confirmed that PbPP5 was vital for the sexual development with a major effect on male gamete fertility.
3.6. Effect of phosphatase inhibitors on sexual development
To assess the potential of phosphatase inhibitors for blocking malaria transmission, we determined the activities of three PP1/PP2A inhibitors on blocking exflagellation and ookinete conversion. Previous studies identified that okadaic acid not only inhibits PP1/PP2A, but also PP2B and PP6 (Bialojan and Takai, 1988; Cohen, 1989; Takai et al., 1992; Prickett and Brautigan, 2006), and thus was excluded from the in vitro exflagellation and ookinete conversion inhibition assay.Cantharidic acid, cantharidin and endothall all inhibited exflagellation and ookinete conversion in a concentration-dependent manner (Fig. 5). The specific PP2A inhibitor endothall completely blocked exflagellation of male gametocytes and ookinete conversion at the 1.5×IC50 concentration of the recombinant rPbPP5yPP2Ac protein (Fig. 5E, F).
Fig. 5.
Effects of protein phosphatase-1 and −2A (PP1/PP2A) inhibitors on gametocyte exflagellation (A, C, E) and ookinete formation (B, D, F). Inhibitors used include cantharidic acid (A, B), cantharidin (C, D) and endothall (E, F). Concentrations used were at 1× and 1.5× of the half maximal inhibitory concentration (IC50) of each individual drug on the recombinant rPbPP5yPP2Ac (yeast system expressed Protein phosphatase 2A (PP2Ac) homologues, catalytic domain) domain of recombinant Plasmodium berghei protein phosphatase 5) enzyme activity. The conversion rate is the percentage of Plasmodium berghei surface protein 21 (Pbs21) -positive parasites that had successfully differentiated into elongated ‘banana-shaped’ ookinetes. *, P<0.05; **, P<0.01; ***, P<0.001.
4. Discussion
Reversible protein phosphorylation is crucial for multiple cellular processes. The protein kinases and phosphatases that catalyze protein phosphorylation and dephosphorylation are well-identified drug targets in various diseases (Cher et al., 2010; Haslbeck et al., 2015; Chen et al., 2017). Similarly, the malaria parasite kinome and phosphatome have been subjects of both bioinformatic and functional studies (Ward et al., 2004; Wilkes and Doerig, 2008; Tewari et al., 2010; Solyakov et al., 2011; Guttery et al., 2014). A recent functional study of the P. berghei phosphatome showed approximately half of the 30 predicted phosphatases were essential for asexual intraerythrocytic development (Guttery et al., 2014). However, the functions for phosphatases in sexual stage development are not fully understood. Among these essential phosphatases, PP5 plays important roles in protozoan parasites such as Toxoplasma, Eimeria, Leishmania and Trypanosoma (Liu et al., 2005; Golden et al., 2008; Jones et al., 2008; Han et al., 2012; Zhou et al., 2013; Figueras et al., 2014; Norris-Mullins et al., 2014; Feng et al., 2015; Wang et al., 2015). This makes PP5 protein a potential target for novel antimalarial and antiparasitic drugs.
An intriguing finding on the predominant expression of PbPP5 during sexual development and yet essential function of this gene during asation, which is consistent with the recent reports showing that both pbpp5 and pfpp5 are dispensable in blood stages (Bushell et al., 2017; Zhang et al., 2018). This discrepancy between the studies of Guttery et al. (2014) and those of Bushell et al. (2017), which is consistent with the recent reports showing that both pbpp5 and pfpp5 are dispensable in blood stages (Bushell et al., 2017; Zhang et al., 2018). This discrepancy between the studies of Guttery et al. (2014) and those of Bushell et al. (2017) and our own may be due to the use of different recombination arms. Guttery et al. (2014) used relatively short recombination arms, which may have resulted in reduced recombination efficiency during genetic manipulation. Based on these and our studies, we conclude that pbpp5 is not essential for asexual blood stages, although we do not know whether this is due to complementation of other phosphatases expressed in asexual blood stages. Thus, the functions of PbPP5 in asexual blood stages warrant further studies to determine its targets during asexual stages and whether the pbpp5 deletion line is more sensitive to phosphatase inhibitors. Interestingly, phenotypic analysis showed that Δpbpp5 parasites exhibited severe defects in male gamete formation and subsequent interactions with the female gametes, leading to a strong reduction in ookinete formation in vitro and oocyst formation in vivo. The defect in male gametogenesis was further corroborated through genetic crosses with either the female-defective Δpbs47 or male-defective Δpbs48/45 lines. Whether the function of PP5 in male gametogenesis and fertility is conserved in P. falciparum awaits further investigation. Moreover, strong expression of PbPP5 in ookinetes and possibly sporozoites suggests that PbPP5 may have additional functions in these stages, but these are not possible to dissect with the tools used in this study.
In malaria parasites, the complex pathways of gametogenesis are still not clear, but important pieces of the puzzle have emerged. It has been shown that redundant protein phosphatases and kinases play important roles in controlling gametogenesis (Guttery et al., 2012a, 2014; Fang et al., 2017). In both P. berghei and P. falciparum, male gametocyte exflagellation is also completely blocked in parasites lacking mitogen-activated kinase-2, a gametocyte-specific mitogen-activated protein kinase (MAPK), which is not expressed in asexual stage parasites (Dorin et al., 1999; Tewari et al., 2005). The phenotypic similarity between Δmapk2 and Δpbpp5 knockout lines may imply that these two proteins might be in the same pathway. Although a direct link between PP5 and kinases in regulating gametogenesis has not yet been established, PfPP5 is predicted to interact with several regulatory proteins (Pandey et al., 2014). Interestingly, in mammalian cells, PP5 suppressed apoptosis of pancreatic islets and β-cells by a mechanism that involved p38 MAPK regulation (Fransson et al., 2014), suggesting a potential universal link between PP5 and MAPK. Analogously, a correlation between kinase, the GSK3α levels and spermatogenesis was noted (Bhattacharjee et al., 2018) and a phosphatase CDC14A was found to affect male fertility (Imtiaz et al., 2018). To elucidate the signaling pathways during sexual development, future work should determine the protein-protein interaction network, and substrates of the kinases and phosphatases.
Consistent with a previous study (Guttery et al., 2014), analysis of PbPP5 protein expression confirmed its ubiquitous expression during blood stages, albeit more predominant expression was noticed in sexual stages. The subcellular location of PP5 proteins varies in mammalian and protozoan parasites (Chen et al., 1994; Lindenthal and Klinkert, 2002). Even in P. berghei, our data demonstrated that PbPP5 retained a diffused cytosolic localization in rings, trophozoites, schizonts and gametocytes, whereas it was concentrated at or beneath the parasite plasma membrane in merozoite and ookinete stages. From the localization study, it is possible that PP5 might be associated with the inner membrane complex (IMC) in the zoite stages, as our protein pull-down work using the PP5-GFP parasites detected proteins present in the IMC and glideosome (data not shown). The differential localizations of PbPP5 in these stages suggest it may have different substrates and functions. Previous reports identifying the interacting proteins of protein phosphatases by using human keratinocyte (HaCaT) cells revealed that PP2Ac interacted with the regulatory subunit (MYPT1) of myosin phosphatase (Becsi et al., 2014). In Plasmodium, myosin is a component of the glideosome, and it is located beneath the plasma membrane, where it powers the motility of invasive stages of the parasite life cycle including merozoite, ookinete and sporozoite (Green et al., 2017). The predominant expression of PbPP5 in ookinete stages and its membrane-associated localization pattern in merozoites and ookinetes suggest that this protein may also participate in parasite mobility other than gamete development, a question open for further investigation.
Efforts to develop new drugs have identified gametocytes as a good drug target to interrupt malaria transmission (Ross and Brancucci, 2018). Here we determined that the PP1/PP2A competitive inhibitors cantharidic acid, cantharidin, endothall and okadaic acid exhibit specific inhibition of the catalytic activity of recombinant PbPP5 (Dounay and Forsyth, 2002). These inhibitors also have significant inhibitory effects on exflagellation and ookinete conversion. Endothall, which has been reported to exhibit greater selectivity for PP2A, could completely block sexual development at 548.3 nM. The structural similarity of Plasmodium PP5s to other PP5 proteins suggests that newly identified inhibitors of PP5 proteins such as Ro 90–7501, aurothioglucose, and N-oleoyldopamine may also be effective on PbPP5 (Hong et al., 2017). Due to the sequence similarity between human and parasite PP5 proteins, drug development efforts targeting PP5 need to emphasize selectivity of the compounds on parasites and mammalian cells. Furthermore, due to the structural similarity of the catalytic sites of PPP family phosphatases and the common competitive inhibition mechanism the inhibitors share, the specificity of compounds toward PP5 needs to be verified by observing their inhibitory effects on the other phosphatase members of the PPP family (Swingle et al., 2004; Bertini et al., 2009). The current study may serve as the starting point for designing effective transmission blocking drugs for malaria parasites. Taken together, this study identified a critical role of the protein phosphatase PbPP5 for male gamete fertility in the rodent parasite P. berghei, and further emphasizes signaling pathways during gametogenesis as potential targets for designing drugs targeting parasite transmission.
Supplementary Material
Highlights.
Recombinant Plasmodium berghei PP5 (rPbPP5) had phosphatase activity and was sensitive to PP1/PP2A inhibitors
PbPP5 was localized in the parasite cytoplasm and under the plasma membrane in some stages
A Pbpp5 gene knockout severely reduced male gamete formation and fertility
PP1/PP2A inhibitors reduced microgametocyte exflagellation and ookinete conversion
Acknowledgements
We would like to thank Ms Jun Liu for technical support (China Medical University, China), the PlasmoGEM team (Wellcome Trust Sanger Institute, UK) for providing the PlasmoGEM vectors. All animal experiments were conducted according to the guidelines and regulations issued by the China Medical University. This work was supported by grants from National Natural Science Foundation of China (Grant no. 81429004) and the National Institutes of Health, USA (R01AI099611). The authors declare that they have no competing interests.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- Becker W, Kentrup H, Klumpp S, Schultz JE, Joost HG, 1994. Molecular cloning of a protein serine/threonine phosphatase containing a putative regulatory tetratricopeptide repeat domain. J Biol Chem 269, 22586–22592. [PubMed] [Google Scholar]
- Becsi B, Dedinszki D, Gyemant G, Mathe C, Vasas G, Lontay B, Erdodi F, 2014. Identification of protein phosphatase interacting proteins from normal and UVA-irradiated HaCaT cell lysates by surface plasmon resonance based binding technique using biotin-microcystin-LR as phosphatase capturing molecule. J Photochem Photobiol B 138, 240–248. [DOI] [PubMed] [Google Scholar]
- Beetsma AL, van de Wiel TJ, Sauerwein RW, Eling WM, 1998. Plasmodium berghei ANKA: purification of large numbers of infectious gametocytes. Exp Parasitol 88, 69–72. [DOI] [PubMed] [Google Scholar]
- Bertini I, Calderone V, Fragai M, Luchinat C, Talluri E, 2009. Structural basis of serine/threonine phosphatase inhibition by the archetypal small molecules cantharidin and norcantharidin. J Med Chem 52, 4838–4843. [DOI] [PubMed] [Google Scholar]
- Bhatt S, Weiss DJ, Cameron E, Bisanzio D, Mappin B, Dalrymple U, Battle KE, Moyes CL, Henry A, Eckhoff PA, Wenger EA, Briet O, Penny MA, Smith TA, Bennett A, Yukich J, Eisele TP, Griffin JT, Fergus CA, Lynch M, Lindgren F, Cohen JM, Murray CL, Smith DL, Hay SI, Cibulskis RE, Gething PW, 2015. The effect of malaria control on Plasmodium falciparum in Africa between 2000 and 2015. Nature 526, 207–211. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bhattacharjee R, Goswami S, Dey S, Gangoda M, Brothag C, Eisa A, Woodgett J, Phiel C, Kline D, Vijayaraghavan S, 2018. Isoform specific requirement for GSK3alpha in sperm for male fertility. Biol Reprod. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bialojan C, Takai A, 1988. Inhibitory effect of a marine-sponge toxin, okadaic acid, on protein phosphatases.Specificity and kinetics. Biochem J 256, 283–290. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Billker O, Dechamps S, Tewari R, Wenig G, Franke-Fayard B, Brinkmann V, 2004. Calcium and a calcium-dependent protein kinase regulate gamete formation and mosquito transmission in a malaria parasite. Cell 117, 503–514. [DOI] [PubMed] [Google Scholar]
- Billker O, Shaw MK, Margos G, Sinden RE, 1997. The roles of temperature, pH and mosquito factors as triggers of male and female gametogenesis of Plasmodium berghei in vitro. Parasitology 115 ( Pt 1), 1–7. [DOI] [PubMed] [Google Scholar]
- Boisson B, Lacroix C, Bischoff E, Gueirard P, Bargieri DY, Franke-Fayard B, Janse CJ, Menard R, Baldacci P, 2011. The novel putative transporter NPT1 plays a critical role in early stages of Plasmodium berghei sexual development. Mol Microbiol 81, 1343–1357. [DOI] [PubMed] [Google Scholar]
- Bushell E, Gomes AR, Sanderson T, Anar B, Girling G, Herd C, Metcalf T, Modrzynska K, Schwach F, Martin RE, Mather MW, McFadden GI, Parts L, Rutledge GG, Vaidya AB, Wengelnik K, Rayner JC, Billker O, 2017. Functional Profiling of a Plasmodium Genome Reveals an Abundance of Essential Genes. Cell 170, 260–272 e268. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen MX, McPartlin AE, Brown L, Chen YH, Barker HM, Cohen PT, 1994. A novel human protein serine/threonine phosphatase, which possesses four tetratricopeptide repeat motifs and localizes to the nucleus. EMBO J 13, 4278–4290. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen YL, Hung MH, Chu PY, Chao TI, Tsai MH, Chen LJ, Hsiao YJ, Shih CT, Hsieh FS, Chen KF, 2017. Protein phosphatase 5 promotes hepatocarcinogenesis through interaction with AMP-activated protein kinase. Biochem Pharmacol 138, 49–60. [DOI] [PubMed] [Google Scholar]
- Cher C, Tremblay MH, Barber JR, Chung Ng S, Zhang B, 2010. Identification of chaulmoogric acid as a small molecule activator of protein phosphatase 5. Appl Biochem Biotechnol 160, 1450–1459. [DOI] [PubMed] [Google Scholar]
- Chinkers M, 1994. Targeting of a distinctive protein-serine phosphatase to the protein kinase-like domain of the atrial natriuretic peptide receptor. Proc Natl Acad Sci U S A 91, 11075–11079. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cohen P, 1989. The structure and regulation of protein phosphatases. Annu Rev Biochem 58, 453–508. [DOI] [PubMed] [Google Scholar]
- Cui L, Mharakurwa S, Ndiaye D, Rathod PK, Rosenthal PJ, 2015. Antimalarial Drug Resistance: Literature Review and Activities and Findings of the ICEMR Network. Am J Trop Med Hyg 93, 57–68. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dobson S, Kar B, Kumar R, Adams B, Barik S, 2001. A novel tetratricopeptide repeat (TPR) containing PP5 serine/threonine protein phosphatase in the malaria parasite, Plasmodium falciparum. BMC Microbiol 1, 31. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Doerig C, Billker O, Haystead T, Sharma P, Tobin AB, Waters NC, 2008. Protein kinases of malaria parasites: an update. Trends Parasitol 24, 570–577. [DOI] [PubMed] [Google Scholar]
- Doerig C, Meijer L, 2007. Antimalarial drug discovery: targeting protein kinases. Expert Opin Ther Targets 11, 279–290. [DOI] [PubMed] [Google Scholar]
- Dorin D, Alano P, Boccaccio I, Ciceron L, Doerig C, Sulpice R, Parzy D, Doerig C, 1999. An atypical mitogen-activated protein kinase (MAPK) homologue expressed in gametocytes of the human malaria parasite Plasmodium falciparum. Identification of a MAPK signature. J Biol Chem 274, 29912–29920. [DOI] [PubMed] [Google Scholar]
- Dounay AB, Forsyth CJ, 2002. Okadaic acid: the archetypal serine/threonine protein phosphatase inhibitor.Curr Med Chem 9, 1939–1980. [DOI] [PubMed] [Google Scholar]
- Fang H, Klages N, Baechler B, Hillner E, Yu L, Pardo M, Choudhary J, Brochet M, 2017. Multiple short windows of calcium-dependent protein kinase 4 activity coordinate distinct cell cycle events during Plasmodium gametogenesis. Elife 6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Feng L, Sun P, Li Z, Liu M, Sun S, 2015. Knockdown of PPP5C inhibits growth of hepatocellular carcinoma cells in vitro. Appl Biochem Biotechnol 175, 526–534. [DOI] [PubMed] [Google Scholar]
- Figueras MJ, Echeverria PC, Angel SO, 2014. Protozoan HSP90-heterocomplex: molecular interaction network and biological significance. Curr Protein Pept Sci 15, 245–255. [DOI] [PubMed] [Google Scholar]
- Fransson L, Rosengren V, Saha TK, Grankvist N, Islam T, Honkanen RE, Sjoholm A, Ortsater H, 2014. Mitogen-activated protein kinases and protein phosphatase 5 mediate glucocorticoid-induced cytotoxicity in pancreatic islets and beta-cells. Mol Cell Endocrinol 383, 126–136. [DOI] [PubMed] [Google Scholar]
- Golden T, Aragon IV, Rutland B, Tucker JA, Shevde LA, Samant RS, Zhou G, Amable L, Skarra D, Honkanen RE, 2008. Elevated levels of Ser/Thr protein phosphatase 5 (PP5) in human breast cancer. Biochim Biophys Acta 1782, 259–270. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Graves PM, Choi L, Gelband H, Garner P, 2018. Primaquine or other 8-aminoquinolines for reducing Plasmodium falciparum transmission. Cochrane Database Syst Rev 2, CD008152. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Green JL, Wall RJ, Vahokoski J, Yusuf NA, Ridzuan MAM, Stanway RR, Stock J, Knuepfer E,Brady D, Martin SR, Howell SA, Pires IP, Moon RW, Molloy JE, Kursula I, Tewari R, Holder AA, 2017. Compositional and expression analyses of the glideosome during the Plasmodium life cycle reveal an additional myosin light chain required for maximum motility. J Biol Chem 292, 17857–17875. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Guttery DS, Ferguson DJ, Poulin B, Xu Z, Straschil U, Klop O, Solyakov L, Sandrini SM, Brady D, Nieduszynski CA, Janse CJ, Holder AA, Tobin AB, Tewari R, 2012a. A putative homologue of CDC20/CDH1 in the malaria parasite is essential for male gamete development. PLoS Pathog 8, e1002554. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Guttery DS, Poulin B, Ferguson DJ, Szoor B, Wickstead B, Carroll PL, Ramakrishnan C, Brady D, Patzewitz EM, Straschil U, Solyakov L, Green JL, Sinden RE, Tobin AB, Holder AA, Tewari R, 2012b. A unique protein phosphatase with kelch-like domains (PPKL) in Plasmodium modulates ookinete differentiation, motility and invasion. PLoS Pathog 8, e1002948. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Guttery DS, Poulin B, Ramaprasad A, Wall RJ, Ferguson DJ, Brady D, Patzewitz EM, Whipple S, Straschil U, Wright MH, Mohamed AM, Radhakrishnan A, Arold ST, Tate EW, Holder AA, Wickstead B, Pain A, Tewari R, 2014. Genome-wide functional analysis of Plasmodium protein phosphatases reveals key regulators of parasite development and differentiation. Cell Host Microbe 16, 128–140. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Han X, Xu B, Beevers CS, Odaka Y, Chen L, Liu L, Luo Y, Zhou H, Chen W, Shen T, Huang S, 2012. Curcumin inhibits protein phosphatases 2A and 5, leading to activation of mitogen-activated protein kinases and death in tumor cells. Carcinogenesis 33, 868–875. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Haslbeck V, Drazic A, Eckl JM, Alte F, Helmuth M, Popowicz G, Schmidt W, Braun F, Weiwad M, Fischer G, Gemmecker G, Sattler M, Striggow F, Groll M, Richter K, 2015. Selective activators of protein phosphatase 5 target the auto-inhibitory mechanism. Biosci Rep 35. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hinds TD Jr., Sanchez ER, 2008. Protein phosphatase 5. Int J Biochem Cell Biol 40, 2358–2362. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hong TJ, Park K, Choi EW, Hahn JS, 2017. Ro 90–7501 inhibits PP5 through a novel, TPR-dependent mechanism. Biochem Biophys Res Commun 482, 215–220. [DOI] [PubMed] [Google Scholar]
- Imtiaz A, Belyantseva IA, Beirl AJ, Fenollar-Ferrer C, Bashir R, Bukhari I, Bouzid A, Shaukat U, Azaiez H, Booth KT, Kahrizi K, Najmabadi H, Maqsood A, Wilson EA, Fitzgerald TS, Tlili A, Olszewski R, Lund M, Chaudhry T, Rehman AU, Starost MF, Waryah AM, Hoa M, Dong L,Morell RJ, Smith RJH, Riazuddin S, Masmoudi S, Kindt KS, Naz S, Friedman TB, 2018. CDC14A phosphatase is essential for hearing and male fertility in mouse and human. Hum Mol Genet 27, 780–798. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Janse CJ, Franke-Fayard B, Mair GR, Ramesar J, Thiel C, Engelmann S, Matuschewski K, van Gemert GJ, Sauerwein RW, Waters AP, 2006a. High efficiency transfection of Plasmodium berghei facilitates novel selection procedures. Mol Biochem Parasitol 145, 60–70. [DOI] [PubMed] [Google Scholar]
- Janse CJ, Ramesar J, Waters AP, 2006b. High-efficiency transfection and drug selection of genetically transformed blood stages of the rodent malaria parasite Plasmodium berghei. Nat Protoc 1, 346–356. [DOI] [PubMed] [Google Scholar]
- Jones C, Anderson S, Singha UK, Chaudhuri M, 2008. Protein phosphatase 5 is required for Hsp90 function during proteotoxic stresses in Trypanosoma brucei. Parasitol Res 102, 835–844. [DOI] [PubMed] [Google Scholar]
- Kiszewski AE, 2011. Blocking Plasmodium falciparum Malaria Transmission with Drugs: The Gametocytocidal and Sporontocidal Properties of Current and Prospective Antimalarials. Pharmaceuticals (Basel) 4, 44–68. [Google Scholar]
- Kou X, Zheng W, Du F, Liu F, Wang M, Fan Q, Cui L, Luo E, Cao Y, 2016. Characterization of a Plasmodium berghei sexual stage antigen PbPH as a new candidate for malaria transmission-blocking vaccine. Parasit Vectors 9, 190. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kutuzov MA, Andreeva AV, 2008. Protein Ser/Thr phosphatases of parasitic protozoa. Mol Biochem Parasitol 161, 81–90. [DOI] [PubMed] [Google Scholar]
- Letunic I, Bork P, 2018. 20 years of the SMART protein domain annotation resource. Nucleic Acids Res 46, D493–D496. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lindenthal C, Klinkert MQ, 2002. Identification and biochemical characterisation of a protein phosphatase 5 homologue from Plasmodium falciparum. Mol Biochem Parasitol 120, 257–268. [DOI] [PubMed] [Google Scholar]
- Liu F, Iqbal K, Grundke-Iqbal I, Rossie S, Gong CX, 2005. Dephosphorylation of tau by protein phosphatase 5: impairment in Alzheimer’s disease. J Biol Chem 280, 1790–1796. [DOI] [PubMed] [Google Scholar]
- Liu Z, Miao J, Cui L, 2011. Gametocytogenesis in malaria parasite: commitment, development and regulation. Future Microbiol 6, 1351–1369. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lucet IS, Tobin A, Drewry D, Wilks AF, Doerig C, 2012. Plasmodium kinases as targets for new-generation antimalarials. Future Med Chem 4, 2295–2310. [DOI] [PubMed] [Google Scholar]
- McRobert L, Taylor CJ, Deng W, Fivelman QL, Cummings RM, Polley SD, Billker O, Baker DA, 2008. Gametogenesis in malaria parasites is mediated by the cGMP-dependent protein kinase. PLoS Biol 6, e139. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Miliu A, Lebrun M, Braun-Breton C, Lamarque MH, 2017. Shelph2, a bacterial-like phosphatase of the malaria parasite Plasmodium falciparum, is dispensable during asexual blood stage. PLoS One 12, e0187073. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Norris-Mullins B, Vacchina P, Morales MA, 2014. Catalytic activity of a novel serine/threonine protein phosphatase PP5 from Leishmania major. Parasite 21, 25. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pandey R, Mohmmed A, Pierrot C, Khalife J, Malhotra P, Gupta D, 2014. Genome wide in silico analysis of Plasmodium falciparum phosphatome. BMC Genomics 15, 1024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Patzewitz EM, Guttery DS, Poulin B, Ramakrishnan C, Ferguson DJ, Wall RJ, Brady D, Holder AA, Szoor B, Tewari R, 2013. An ancient protein phosphatase, SHLP1, is critical to microneme development in Plasmodium ookinetes and parasite transmission. Cell Rep 3, 622–629. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ponzi M, Siden-Kiamos I, Bertuccini L, Curra C, Kroeze H, Camarda G, Pace T, Franke-Fayard B, Laurentino EC, Louis C, Waters AP, Janse CJ, Alano P, 2009. Egress of Plasmodium berghei gametes from their host erythrocyte is mediated by the MDV-1/PEG3 protein. Cell Microbiol 11, 1272–1288. [DOI] [PubMed] [Google Scholar]
- Prickett TD, Brautigan DL, 2006. The alpha4 regulatory subunit exerts opposing allosteric effects on protein phosphatases PP6 and PP2A. J Biol Chem 281, 30503–30511. [DOI] [PubMed] [Google Scholar]
- Ross A, Brancucci NM, 2018. On a mission to block transmission. Elife 7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sinden RE, Hartley RH, Winger L, 1985. The development of Plasmodium ookinetes in vitro: an ultrastructural study including a description of meiotic division. Parasitology 91 ( Pt 2), 227–244. [DOI] [PubMed] [Google Scholar]
- Solyakov L, Halbert J, Alam MM, Semblat JP, Dorin-Semblat D, Reininger L, Bottrill AR, Mistry S, Abdi A, Fennell C, Holland Z, Demarta C, Bouza Y, Sicard A, Nivez MP, Eschenlauer S, Lama T, Thomas DC, Sharma P, Agarwal S, Kern S, Pradel G, Graciotti M, Tobin AB, Doerig C, 2011. Global kinomic and phospho-proteomic analyses of the human malaria parasite Plasmodium falciparum. Nat Commun 2, 565. [DOI] [PubMed] [Google Scholar]
- Swingle MR, Honkanen RE, Ciszak EM, 2004. Structural basis for the catalytic activity of human serine/threonine protein phosphatase-5. J Biol Chem 279, 33992–33999. [DOI] [PubMed] [Google Scholar]
- Takai A, Murata M, Torigoe K, Isobe M, Mieskes G, Yasumoto T, 1992. Inhibitory effect of okadaic acid derivatives on protein phosphatases. A study on structure-affinity relationship. Biochem J 284 ( Pt 2), 539–544. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tewari R, Dorin D, Moon R, Doerig C, Billker O, 2005. An atypical mitogen-activated protein kinase controls cytokinesis and flagellar motility during male gamete formation in a malaria parasite. Mol Microbiol 58, 1253–1263. [DOI] [PubMed] [Google Scholar]
- Tewari R, Straschil U, Bateman A, Bohme U, Cherevach I, Gong P, Pain A, Billker O, 2010. The systematic functional analysis of Plasmodium protein kinases identifies essential regulators of mosquito transmission. Cell Host Microbe 8, 377–387. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tonkin CJ, van Dooren GG, Spurck TP, Struck NS, Good RT, Handman E, Cowman AF, McFadden GI, 2004. Localization of organellar proteins in Plasmodium falciparum using a novel set of transfection vectors and a new immunofluorescence fixation method. Mol Biochem Parasitol 137, 13–21. [DOI] [PubMed] [Google Scholar]
- van Dijk MR, Janse CJ, Thompson J, Waters AP, Braks JA, Dodemont HJ, Stunnenberg HG, van Gemert GJ, Sauerwein RW, Eling W, 2001. A central role for P48/45 in malaria parasite male gamete fertility. Cell 104, 153–164. [DOI] [PubMed] [Google Scholar]
- van Dijk MR, van Schaijk BC, Khan SM, van Dooren MW, Ramesar J, Kaczanowski S, van Gemert GJ, Kroeze H, Stunnenberg HG, Eling WM, Sauerwein RW, Waters AP, Janse CJ, 2010. Three members of the 6-cys protein family of Plasmodium play a role in gamete fertility. PLoS Pathog 6, e1000853. [DOI] [PMC free article] [PubMed] [Google Scholar]
- van Dijk MR, Waters AP, Janse CJ, 1995. Stable transfection of malaria parasite blood stages. Science 268, 1358–1362. [DOI] [PubMed] [Google Scholar]
- van Dooren GG, Marti M, Tonkin CJ, Stimmler LM, Cowman AF, McFadden GI, 2005.Development of the endoplasmic reticulum, mitochondrion and apicoplast during the asexual life cycle ofPlasmodium falciparum. Mol Microbiol 57, 405–419. [DOI] [PubMed] [Google Scholar]
- Wang J, Zhu J, Dong M, Yu H, Dai X, Li K, 2015. Inhibition of protein phosphatase 5 (PP5) suppresses survival and growth of colorectal cancer cells. Biotechnol Appl Biochem 62, 621–627. [DOI] [PubMed] [Google Scholar]
- Ward P, Equinet L, Packer J, Doerig C, 2004. Protein kinases of the human malaria parasite Plasmodium falciparum: the kinome of a divergent eukaryote. BMC Genomics 5, 79. [DOI] [PMC free article] [PubMed] [Google Scholar]
- WHO, 2018. World Malaria Report 2017. [Google Scholar]
- Wilkes JM, Doerig C, 2008. The protein-phosphatome of the human malaria parasite Plasmodium falciparum.BMC Genomics 9, 412. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wu Y, Sinden RE, Churcher TS, Tsuboi T, Yusibov V, 2015. Development of malaria transmission-blocking vaccines: from concept to product. Adv Parasitol 89, 109–152. [DOI] [PubMed] [Google Scholar]
- Zhang M, Wang C, Otto TD, Oberstaller J, Liao X, Adapa SR, Udenze K, Bronner IF, Casandra D, Mayho M, Brown J, Li S, Swanson J, Rayner JC, Jiang RHY, Adams JH, 2018. Uncovering the essential genes of the human malaria parasite Plasmodium falciparum by saturation mutagenesis. Science 360. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zheng W, Kou X, Du Y, Liu F, Yu C, Tsuboi T, Fan Q, Luo E, Cao Y, Cui L, 2016. Identification of three ookinete-specific genes and evaluation of their transmission-blocking potentials in Plasmodium berghei. Vaccine 34, 2570–2578. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhou BH, Wang HW, Zhao ZS, Liu M, Yan WC, Zhao J, Zhang Z, Xue FQ, 2013. A novel serine/threonine protein phosphatase type 5 from second-generation merozoite of Eimeria tenella is associated with diclazuril-induced apoptosis. Parasitol Res 112, 1771–1780. [DOI] [PubMed] [Google Scholar]
- Zhu X, He Y, Liang Y, Kaneko O, Cui L, Cao Y, 2017. Tryptophan-rich domains of Plasmodium falciparum SURFIN4.2 and Plasmodium vivax PvSTP2 interact with membrane skeleton of red blood cell. Malar J 16, 121. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.