Highlights
-
•
MPFC GABA level increased concurrently with decreased DMN FC in FEP patients.
-
•
MPFC-DMN FC mediated the correlation of GABA and PANSS positive score changes.
-
•
PCC/PCu-DMN FC mediated the correlation of NAA and PANSS positive score changes.
Keywords: First-episode psychosis, Antipsychotic, Magnetic resonance spectroscopy, Functional magnetic resonance imaging, Neurometabolite, Longitudinal
Abstract
Background
Antipsychotic treatment has improved the disrupted functional connectivity (FC) and neurometabolites levels of the default mode network (DMN) in schizophrenia patients, but a direct relationship between FC change, neurometabolic level alteration, and symptom improvement has not been built. This study examined the association between the alterations in DMN FC, the changes of neurometabolites levels in the medial prefrontal cortex (MPFC), and the improvements in psychopathology in a longitudinal study of drug-naïve first-episode psychosis (FEP) patients.
Methods
Thirty-two drug-naïve FEP patients and 30 matched healthy controls underwent repeated assessments with the Positive and Negative Syndrome Scale (PANSS) and 3T proton magnetic resonance spectroscopy as well as resting-state functional magnetic resonance imaging. The levels of γ-aminobutyric acid, glutamate, N-acetyl-aspartate in MPFC, and the FC of DMN were measured. After 8-week antipsychotic treatment, 24 patients were re-examined.
Results
After treatment, the changes in γ-aminobutyric acid were correlated with the alterations of FC between the MPFC and DMN, while the changes in N-acetyl-aspartate were associated with the alterations of FC between the posterior cingulate cortex/precuneus and DMN. The FC changes of both regions were correlated with patients PANSS positive score reductions. The structural equation modeling analyses revealed that the changes of DMN FC mediated the relationship between the changes of neurometabolites and the symptom improvements of the patients.
Conclusions
The derived neurometabolic-functional changes underlying the clinical recovery provide insights into the prognosis of FEP patients. It is noteworthy that this is an exploratory study, and future work with larger sample size is needed to validate our findings.
1. Introduction
The aberrant organization of functional brain networks plays an important role in the pathology of schizophrenia (SCZ) (McCutcheon et al., 2020). Among them, the functional activity changes in the default mode network (DMN) have been most commonly reported in SCZ patients (Landin-Romero et al., 2015, O’Neill et al., 2019, Whitfield-Gabrieli and Ford, 2012). For example, the within-DMN functional connectivity (FC) decreased in first-episode psychosis (FEP) (Bastos-Leite et al., 2015, Camchong et al., 2011) and increased in medicated SCZ patients or their first-degree relatives (Whitfield-Gabrieli et al., 2009). Also, decreased anticorrelations between DMN and dorsolateral prefrontal cortex (DLPFC) were consistently found in the subjects at ultra-high risk for psychosis (Shim et al., 2010) and medicated SCZ patients or their relatives (Whitfield-Gabrieli et al., 2009, Liu et al., 2012). More importantly, longitudinal studies have suggested that antipsychotic medication might act on the DMN-related brain networks. For example, increased posterior cingulate cortex/precuneus (PCC/PCu) FC was found after 8-week risperidone monotherapy for drug-naïve FEP (DN-FEP) patients (Zong et al., 2019). Increased DMN-DLPFC anticorrelations were found in SCZ patients after 8 weeks of olanzapine treatment, compared to 4 weeks of treatment (Sambataro et al., 2010). Nevertheless, the underlying neurometabolic mechanism of the DMN-related FC changes as well as its relationship to the therapeutic effects in SCZ patients remain not fully understood.
The intrinsic brain functional activity is attributed to the gamma oscillations generated by the interplay between excitatory and inhibitory neurons (McCutcheon et al., 2020). Using combined functional magnetic resonance imaging (fMRI) and proton magnetic resonance spectroscopy (1H-MRS), the relationship between brain functional activity and neurometabolic concentrations could be evaluated non-invasively. Chen et al. found that the γ-aminobutyric acid (GABA) concentration in the medial prefrontal cortex (MPFC) was closely related to the within-DMN deactivation and the anticorrelation between DMN and DLPFC in healthy subjects (Du et al., 2018). In SCZ patients, disrupted correlations between the cognitive task-related functional responses and the local glutamate or GABA levels were constantly reported (Cadena et al., 2018, Kaminski et al., 2020, Overbeek et al., 2019). Moreover, the resting-state FC between DMN and hippocampus was found to be correlated with the hippocampal glutamatergic signal in DN-FEP patients, indicating the intrinsic coupling between the functional and neurometabolic signals at baseline (Nelson et al., 2020). Recently, a growing interest has been drawn to the therapeutic effect of pharmacological intervention on the neurometabolites concentrations of SCZ patients. Using 1H-MRS, it was shown that the elevated GABA concentration in the MPFC of DN-FEP patients was decreased after 4-week risperidone treatment (de la Fuente-Sandoval et al., 2018). The increased thalamic glutamate level was normalized after 6 and 26 weeks of treatment in DN-FEP patients (Bojesen et al., 2020). After 6 weeks of antipsychotic treatment, the SCZ patients showed a normalized relationship between the functional activation in the anterior cingulate cortex (ACC) and its regional glutamate concentration (Cadena et al., 2018). Nevertheless, the alterations of brain functional-neurometabolic signals in relation to the clinical recovery after pharmacological intervention for SCZ patients remain to be elucidated.
In this study, we aim to investigate the relationship between FC change, neurometabolites levels alteration, and symptom improvement of DN-FEP patients, before and after 8-week antipsychotic treatment. We hypothesized that the decreased FC of DMN in FEP patients would be enhanced after medication, which might be related to the alterations in neurometabolite and neurotransmitter concentrations, e.g., decreased GABA level within DMN after antipsychotic treatment (de la Fuente-Sandoval et al., 2018). Furthermore, we hypothesized that the coupled functional-neurometabolic changes would be related to patients’ clinical recovery after medication. A combined resting-state fMRI and 1H-MRS methodology was utilized for brain functional and neurometabolic imaging. This longitudinal design allows us to investigate the therapeutic effects of the neurobiological substrate in SCZ without the confounds of medication at baseline and illness chronicity.
2. Methods and materials
2.1. Participants
Thirty-two drug-naïve, right-handed FEP patients [mean age: 26.8 years (range: 19 to 40 years)] and 30 age-, sex-, and education-matched healthy controls (HCs) [mean age: 27.1 years (range: 20 to 39 years)] participated in this study. The patients were recruited from Shanghai Mental Health Center, and the HCs were recruited from the local community. Exclusion criteria included substance abuse, major medical/neurological conditions, other psychiatric or neurological diseases, pregnancy or breastfeeding, and MRI contraindications. All the patients were diagnosed using the Diagnostic and Statistical Manual of Mental Disorders, Fifth Edition (DSM-5) (American Psychiatric Association [APA], 2013) diagnostic criteria (Association, 2013) by an experienced psychiatrist. Inclusion criteria for patients include 1) fulfilled the Diagnostic and Statistical Manual of Mental Disorders, Fifth Edition (DSM-5) (American Psychiatric Association [APA], 2013) diagnostic criteria (Association, 2013) for schizophrenia, schizophreniform or schizoaffective disorder; 2) age between 18 and 40 years old; 3) no previous contact with health services for psychosis; 4) duration of untreated psychosis in <5 years; 5) provided informed consent. All control subjects underwent interviews with the Mini-International Neuropsychiatric Interview (MINI) plus v 7.0 (Sheehan, 1998) to rule out Axis I diagnosis. All participants underwent clinical assessment, resting-state fMRI, and 1H-MRS scans. The clinical symptoms were assessed using the Positive and Negative Syndrome Scale (PANSS) (Kay et al., 1987). After the baseline MR scans, all the patients were treated with second-generation antipsychotics based on clinical evaluation, and the drug dosage was determined based on the clinical judgment. After 8 weeks of medication, 24 patients received follow-up scans and clinical assessments. This study was approved by the Institutional Review Board of the Shanghai Mental Health Center. All participants provided written informed consents.
2.2. Image acquisition
All image data were collected on a 3T Siemens Verio MR Scanner (Siemens AG, Erlangen, Germany) using a 32-channel head coil. To minimize head motions, additional padding was placed around each subject’s head. The T1-weighted MR images were acquired for anatomical reference with a magnetization prepared rapid acquisition gradient-echo (MPRAGE) sequence (repetition time (TR) = 2530 ms, echo time (TE) = 3.65 ms, flip angle (FA) = 7°, field of view (FOV) = 256×256 mm2, matrix size = 256×256, slice number = 224, slice thickness = 1 mm, voxel size = 1.0 1.0 1.0 mm3). The single-voxel 1H-MRS data were acquired using a MEGA-PRESS spectral editing sequence (Mescher et al., 1998) (voxel size = 30×30×30 mm3, TR = 1500 ms, TE = 69 ms, number of averages = 128, edit pulse frequency = 1.90 ppm, edit pulse bandwidth = 45 Hz). The voxel was placed in front of the corpus callosum and centered on the interhemispheric fissure to optimally cover the MPFC region, as shown in Fig. 3A. The resting-state fMRI data were acquired using a gradient-echo echo-planar imaging (EPI) sequence (TR = 2000 ms, TE = 30 ms, FA = 90°, FOV = 220×220 mm2, matrix size = 64×64, slice number = 30, slice thickness = 4 mm, slice gap = 0 mm, voxel size = 3.4×3.4×4.0 mm3). All subjects were required to lay supine and remain awake without systematic thinking during the scan.
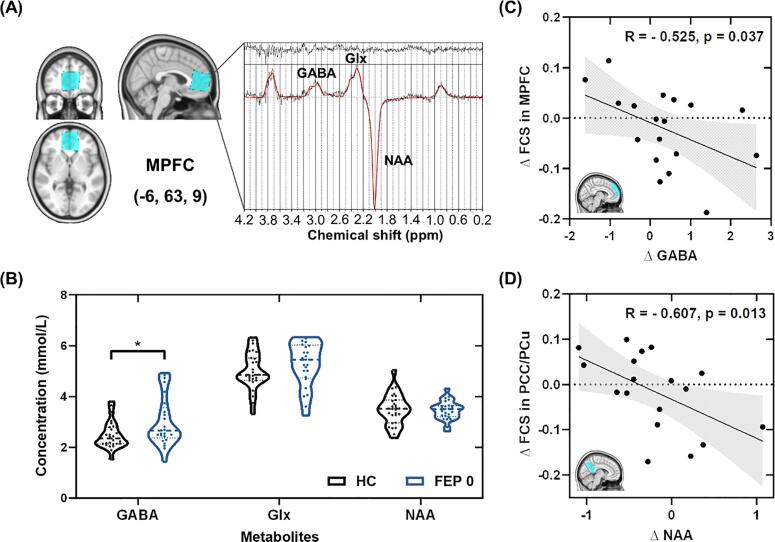
Fig. 3.
The coupled changes of neurometabolites concentrations and FCS in the DMN. (A) The placement of the 1H-MRS voxel (blue) along with the spectrum fitted by LCModel. (B) Comparisons of GABA, Glx, and NAA concentrations between HC and FEP 0 groups. (C) The changes of GABA level in the MPFC were negatively correlated with the alterations of FCS in the MPFC of the patients. (D) The changes of NAA level in the MPFC were negatively correlated with the alterations of FCS in the PCC/PCu of the patients. denotes the changes before and after treatment. Abbreviations: FCS, functional connectivity strength; DMN, default mode network; HC, healthy control; FEP 0, first-episode psychosis patients at baseline; FEP 1, first-episode psychosis patients at 8-week follow-up; MPFC, medial prefrontal cortex; PCC/PCu, posterior cingulate cortex/precuneus; GABA, gamma-aminobutyric acid; Glx, glutamate and glutamine; NAA, N-acetyl-aspartate. * p < 0.05. (For interpretation of the references to colour in this figure legend, the reader is referred to the web version of this article.)
2.3. 1H-MRS data processing
The 1H-MRS data were quantified using LCModel software (http://s-provencher.com/pages/lcmodel.shtml; RRID: SCR_014455) (Provencher, 1993). The unsuppressed water signal was used for eddy-current correction and as the reference for metabolite quantification. For quality control, only concentrations with quantification Cramer-Rao lower bounds (CRLB) < 20% were included for further analysis. Detailed information for spectral quality and voxel tissue composition were compared among the three groups. As summarized in Supplementary Table S1, no significant between-group differences were detected. The corrections of partial volume effects were performed for all the data (see Supplementary Methods).
2.4. FMRI data processing
2.4.1. FMRI data preprocessing
The fMRI data were preprocessed using the Data Processing Assistant for Resting-State fMRI Toolbox (DPARSFhttp://rfmri.org/DPARSF; RRID:SCR_002372) (Yan and Zang, 2010). The first 10 frames were removed to alleviate the impact of the subjects’ inadaptation to the scanning environment, and the slice-timing correction was performed to correct the interleaved acquisitions. The acquisition time delay was corrected by realigning the remaining 170 volumes to the first volume. Head motion correction, spatial normalization to the Montreal Neurological Institute (MNI) space with a resampled resolution of 3×3×3 mm3, and band-pass filtering (0.01–0.1 Hz) were then performed. The nuisance signals, including Friston 24 head motion parameters (Friston et al., 1996), 5 principal components of the time courses extracted from white matter (WM) and cerebral spinal fluid (CSF), as well as the global signal, were regressed out from each voxel’s time series. The analysis without global signal regression was also performed to show the robustness of the results, as illustrated in Supplementary Fig. S1 and Fig. S2. Spatial smoothing with a 6-mm full-width half-maximum Gaussian kernel was applied to all fMRI images. To minimize the influence of head motion, we excluded the participants with over 0.25 mm mean framewise displacement (FD) (Power et al., 2012), or with over 2.5 mm translation or 2.5° rotation in any direction. The fMRI data of two healthy control subjects, one FEP patient at baseline and two patients at 8 weeks post treatment were excluded due to excessive head motions. A detailed flowchart of the data inclusion is provided in Supplementary Fig. S3.
2.4.2. Identification of the DMN
The DMN was defined following the previous literature (Whitfield-Gabrieli et al., 2009), in which four DMN seed regions were used, including MPFC (-1, 47, −4), PCC (-5, −49, 40), left lateral parietal (-45, −67, 36) and right lateral parietal (45, −67, 36) peak foci, each represented with a 6-mm sphere. The DMN seeds were created based on the fslmaths command of FSL (https://fsl.fmrib.ox.ac.uk/fsl/; RRID: SCR_002823). For each seed, Pearson’s correlation coefficients were calculated between its mean time series and those of the other voxels in the brain. The coefficient maps were obtained for each subject individually and converted into z-maps using Fisher z-transformation. By averaging the z-maps from all four seeds, we obtained one mean z-map for each subject, which reflects the widespread connectivity with the DMN regions. To determine the DMN regions, one-sample t-tests were performed for the average z-maps of each group, and the results were corrected for multiple comparisons using false discovery rate (FDR) correction at p < 0.001. The DMN map of the HC group was binarized as the DMN mask and used for subsequent analyses. Graphical representation of the above analyses can be found in Supplementary Fig. S4A.
2.4.3. Identification of the anticorrelation networks with DMN
Since the anticorrelated networks for different DMN seeds are strikingly different (Uddin et al., 2009), the anticorrelation analyses were separately performed for the z-maps of MPFC and PCC seed, which are two functional hubs of the DMN (Fransson and Marrelec, 2008, Uddin et al., 2009). To identify the anticorrelated networks of each seed, one-sample t-tests were performed for the z-maps. The anticorrelation mask of each seed was determined from the FDR (p < 0.001) correction results. Graphical representation of the above analyses is provided in Supplementary Fig. S5A.
2.4.4. Calculation of FCS
To quantitatively measure the FC with DMN, the functional connectivity strength (FCS) was calculated as the average FC within a given cluster to the voxels within the DMN. For cluster , the FCS was defined as (Liang et al., 2013):
| (1) |
for , where is the number of voxels within the DMN mask, denotes the Fisher z-transformed correlation coefficient between the mean fMRI time series of a cluster and that of each voxel within the DMN mask. Graphical representation of the calculation of FCS within and anticorrelation with DMN can be found in Supplementary Fig. S4C and Fig. S5C, respectively.
2.5. Statistical analyses
2.5.1. Group comparisons of functional connectivity
To identify the regions that showed the most significant differences in FC between HCs and DN-FEP patients, two-sample t-tests were performed on the average z-maps within the DMN mask as well as the z-maps of MPFC seed and PCC seed within the anticorrelation networks. To investigate the longitudinal alterations of the FC, paired-sample t-tests were similarly performed on the z-maps between FEP patients at baseline and at 8 weeks within the DMN and anticorrelation masks. Clusters that showed significant between-group differences were corrected for multiple comparisons using AlphaSim correction (p < 0.001). All the above analyses were implemented in a MATLAB-based REsting-State fMRI data analysis Toolkit (REST, http://www.restfmri.net; RRID: SCR_009641).
2.5.2. Group comparisons of neurometabolic levels and clinical characteristics
The group comparisons were performed using SPSS 19 (https://www.ibm.com/products/spss-statistics; RRID: SCR_019096). All data have been tested for normality using Kolmogorov-Smirnov tests. For normally distributed measures, two-tailed two-sample t-tests were utilized to compare the group differences at baseline; paired t-tests were used to compare the patients’ longitudinal data. For non-normally-distributed measures, between-group differences were evaluated by the Mann-Whitney U tests; the longitudinal data were tested using Wilcoxon signed-rank tests. The outliers were identified using the function ‘outlierTest’ from the ‘car’ package in R software (Fox and Weisberg, 2018), which reports the Bonferroni corrected p-values for the extreme observations. If p < 0.05, it is considered as an outlier and was removed.
2.5.3. Correlation analyses of the functional and neurometabolic changes
For the correlation analyses between the longitudinal changes of FCS and neurometabolic levels as well as between the longitudinal changes of FCS and clinical scores, we used the MPFC and PCC/PCu as the regions of interest (ROIs) for the calculation of regional FCS. The rationale is these ROIs are the two major functional hubs in the DMN (Fransson and Marrelec, 2008, Uddin et al., 2009) and the clusters showing significant between-group differences at baseline were identified within these two regions. The ROIs were delineated based on the AAL atlas, which included the medial superior frontal gyrus, posterior cingulate gyrus and precuneus, as shown in Fig. 3C and Fig. 3D (Tzourio-Mazoyer et al., 2002). The correlations were evaluated by partial correlation analyses with age and sex as covariates.
2.5.4. Structural equation modeling
The relationship among the changes of neurometabolites in MPFC, the FCS of DMN, and the recovery of patients' clinical symptoms was evaluated using structural equation modeling (SEM). Based on the correlation analyses results that the MPFC FCS changes were correlated with both the MPFC GABA alterations and patients’ PANSS positive symptom recovery, while the PCC/PCu FCS changes were associated with both the MPFC NAA changes and patients PANSS positive symptom recovery, we assumed that the FCS changes might serve as mediators between neurometabolic changes and clinical symptom recovery. Therefore, an MPFC FCS mediation model (i.e., GABA change MPFC FCS change PANSS positive score change) and a PCC/PCu FCS mediation model (i.e., NAA change PCC/PCu FCS change PANSS positive score change) were built and shown in Fig. 4. The goodness-of-fit of the model was assessed using the test, goodness-of-fit index (GFI), and Akaike information criterion (AIC). A non-significant value and a GFI value above 0.9 indicated a good fit (Kline, 2015). The AIC was applied to penalize overfitting, with a minimal value being the preferred model (Akaike, 1974). The significance of the mediation effect, i.e., indirect effect, was evaluated by bootstrapping (5000 iterations) confidence intervals. The mediation effect was considered significant if zero does not fall into the bias-corrected bootstrap 95% confidence interval (Cheung and Lau, 2008).
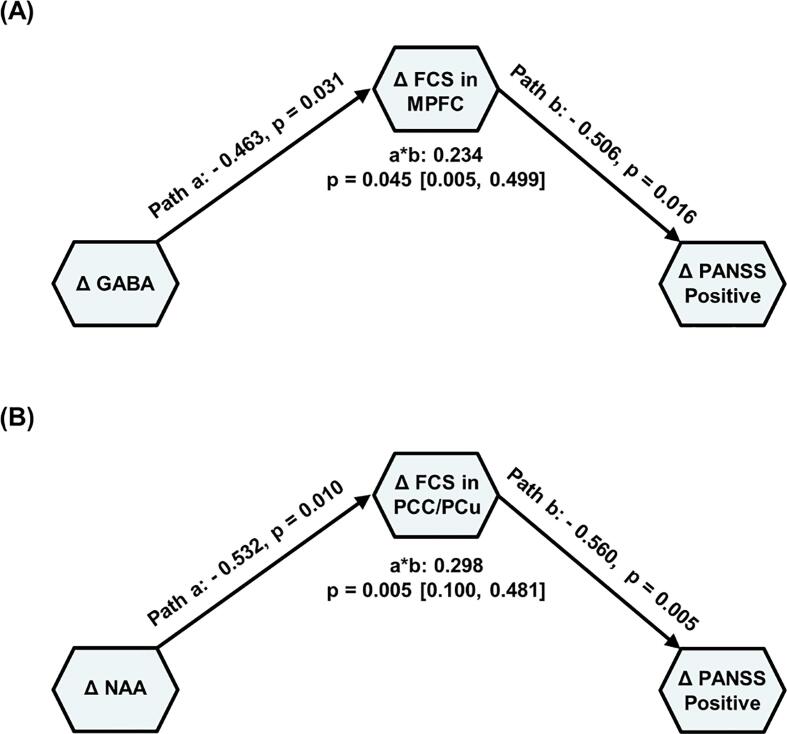
Fig. 4.
Mediation analysis of longitudinal changes of neurometabolites concentrations, FCS, and PANSS symptom scores in the FEP patients. (A) The association between the changes of GABA concentration in the MPFC and the recovery of PANSS positive symptom score was mediated by the FCS between MPFC and DMN. (B) The association between the changes of NAA concentration in the MPFC and the recovery of PANSS positive symptom score was mediated by the FCS between PCC/PCu and DMN. Standardized path coefficients are labeled along each path. The path a refers to the effect of neurometabolic concentration on DMN FCS; the path b refers to the effect of DMN FCS on PANSS symptom scores, controlling for neurometabolic level; indirect effect (product a*b) refers to the amount of mediation that the FCS of DMN had on the neurometabolic concentration – PANSS symptom relationship. denotes the changes before and after treatment. Abbreviations: FCS, functional connectivity strength; PANSS, Positive and Negative Syndrome Scale; FEP, first-episode psychosis patients; MPFC, medial prefrontal cortex; PCC/PCu, posterior cingulate cortex/precuneus; GABA, gamma-aminobutyric acid; NAA, N-acetyl-aspartate. * p < 0.05, ** p < 0.01.
3. Results
3.1. Clinical characteristics
Clinical characteristics are summarized in Table 1 for all participants. There were no significant differences between the DN-FEP patients and HCs in sex, age, and education (Table 1). After 8 weeks (62.9 8.0 days) of treatment, 24 patients were re-examined and all patients’ PANSS scores significantly decreased.
Table 1.
Demographic and Clinical Characteristics of Participants.
| Measure | HC (n = 30) | FEP 0 (n = 32) | FEP 1 (n = 24) | p value (HC vs. FEP 0) | p value (HC vs. FEP 1) | p value (FEP 0 vs. FEP 1) |
|---|---|---|---|---|---|---|
| Age (Years) | 27.1 (4.3) | 26.8 (6.1) | 27.0 (5.8) | 0.823a | 0.962a | |
| Sex (Female/Male) | 15/15 | 16/16 | 12/12 | 1.000b | 1.000b | |
| Education (Years) | 14.3 (2.4) | 13.0 (3.2) | 13.4 (3.1) | 0.310c | 0.822c | |
| Handedness (Right/Left) | 30/0 | 32/0 | 24/0 | 1b | 1b | |
| Current tobacco use (yes/no) | 8/22 | 3/29 | 2/22 | 0.057b | 0.157b | |
| Current cannabis use (yes/no) | 0/30 | 0/32 | 0/24 | 1b | 1b | |
| DUP (Months) | NA | 7.2 (12.4) | 6.7 (12.2) | |||
| PANSS Total | NA | 73.8 (18.2) | 48.6 (14.1) | < 0.001d | ||
| PANSS Positive | NA | 20.3 (5.3) | 10.9 (3.6) | < 0.001d | ||
| PANSS Negative | NA | 16.9 (6.1) | 12.7 (5.0) | < 0.001d | ||
| PANSS General | NA | 36.7 (9.9) | 24.7 (6.8) | < 0.001d | ||
| CGI | NA | 4.8 (0.9) | 3.0 (1.1) | < 0.001d | ||
| CPZ eq (mg/day) | NA | NA | 335.4 (140.2) |
Note: Values are presented as mean (SD). Abbreviations: HC, healthy control; FEP 0, drug-naïve first-episode psychosis; FEP 1, first-episode psychosis patients at 8 weeks post treatment; DUP, duration of untreated psychosis; PANSS, Positive and Negative Syndrome Scale; CGI, clinical global impression; CPZ eq, chlorpromazine equivalence.
Independent two-sample t-test.
Chi-square test.
Mann-Whitney U test.
Wilcoxon signed-rank test.
3.2. Functional connectivity with the DMN
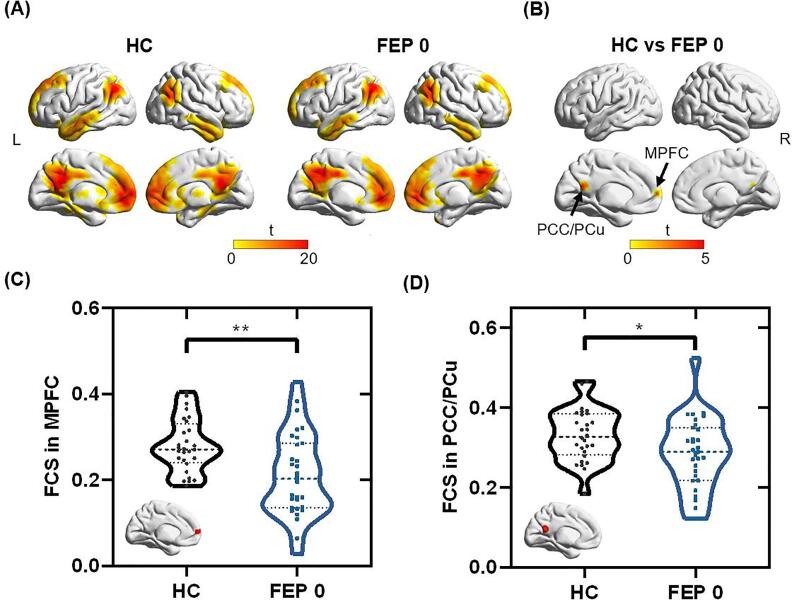
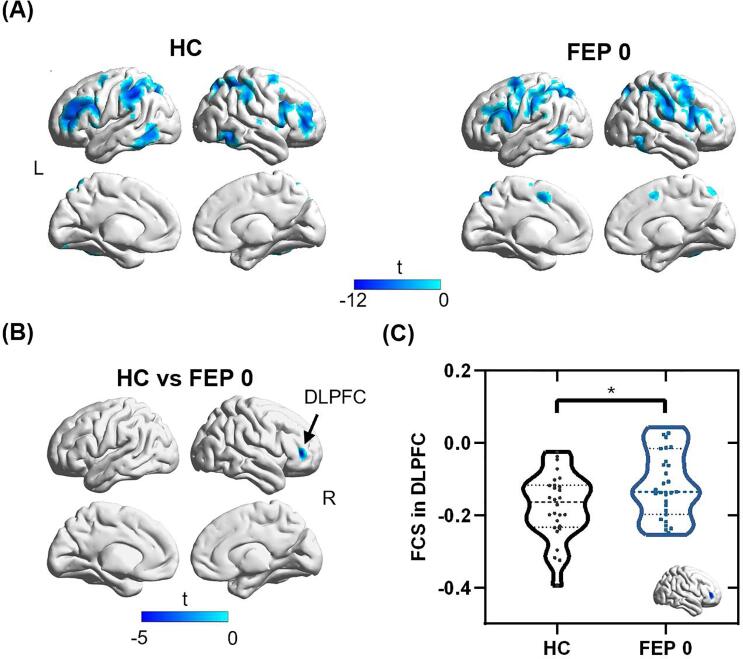
The DMN regions of HCs and DN-FEP patients are shown in Fig. 1A. There are 2 clusters within DMN that showed significantly decreased FC with DMN in DN-FEP patients (see Fig. 1B), with one cluster in the MPFC region (peak MNI coordinate: [-6, 63, 9]), and the other one in the PCC/PCu region (peak MNI coordinate: [-12, −48, 18]). The DN-FEP patients demonstrated significantly lower FCS than HCs in both clusters (MPFC cluster: t = 3.348, p = 0.002; PCC/PCu cluster: t = 2.228, p = 0.030). There was no significant difference in anticorrelation with PCC between HCs and DN-FEP patients. The regions showing anticorrelations with MPFC are exhibited in Fig. 2A. Compared to HCs, a cluster in DLPFC (peak MNI coordinates: [42, 42, 6]) was identified with significantly decreased anticorrelation (i.e., negative connectivity) with MPFC in the DN-FEP patients (t = - 2.520, p = 0.015), as shown in Fig. 2B. For the FC comparison between the FEP patients at baseline and at 8 weeks, there is one cluster within the DMN that showed increased FC in the MPFC region (peak MNI coordinate: [-12, 60, -18], t = - 4.725, p < 0.001). A cluster in the DLPFC (peak MNI coordinate: [-36, 39, 12], t = 4.567, p < 0.001) showed increased anticorrelation with the MPFC, and a cluster in the middle temporal gyrus (peak MNI coordinate: [-39, - 63, 6], t = - 4.391, p < 0.001) showed decreased anticorrelation with the MPFC. The detailed cluster information is listed in Supplementary Table S2 and S3.
Fig. 1.
Comparisons of FC within DMN between HC and FEP 0 groups. (A) FC maps within DMN in HC and FEP 0 groups. (B) Two clusters in the MPFC and PCC/PCu showed significantly reduced FCs in the FEP 0 group compared to the HC group. (C) The FCS in the MPFC decreased in the FEP 0 group compared to the HC group (p = 0.002). (D)The FCS in PCC/PCu decreased in FEP 0 group compared to the HC group (p = 0.030). Abbreviations: FCS, functional connectivity strength; DMN, default mode network; MPFC, medial prefrontal cortex; PCC/PCu, posterior cingulate cortex/precuneus; FC, functional connectivity; HC, healthy control; FEP 0, first-episode psychosis patients at baseline. Two-tailed two-sample t-tests were used for the comparison. * p < 0.05, ** p < 0.01.
Fig. 2.
Comparisons of anticorrelations (i.e., negative connectivity) with MPFC between HC and FEP 0 groups. (A) Anticorrelation maps with MPFC in HC and FEP 0 groups. (B) One cluster in DLPFC showed significantly reduced anticorrelation or increased connectivity in FEP 0 group compared to the HC group. (C) The FEP 0 group showed increased FCS in the DLPFC compared to the HC group (p = 0.015). Abbreviations: FCS, functional connectivity strength; HC, healthy control; FEP 0, first-episode psychosis patients at baseline; DMN, default mode network; DLPFC, dorsolateral prefrontal cortex. Two-tailed two-sample t-tests were used for the comparison. * p < 0.05.
3.3. Neurometabolites levels in the MPFC
The 1H-MRS voxel placement and the correspondingly acquired and fitted spectra are shown in Fig. 3A. The GABA level in the MPFC of DN-FEP patients increased significantly compared to the controls (p = 0.034), as shown in Fig. 3B. No significant difference was found between the DN-FEP patients and healthy control groups in NAA or Glx, as presented in Supplementary Table S4.
For GABA levels of MPFC, the differences between FEP patients and HCs were not significant after 8-week antipsychotic treatment (p = 0.821). No differences in NAA and Glx levels were found between FEP patients at baseline and after 8-week medication. The detailed results are provided in Supplementary Table S4.
3.4. Associations of MPFC neurometabolites levels and DMN connectivity
At baseline, no significant correlation was found between neurometabolites and DMN FCS in the MPFC, PCC, or DLPFC cluster in FEP patients or HCs (Supplementary Table S5). After treatment, the DMN FCS values for the MPFC region were significantly correlated with the GABA levels in the MPFC voxel (r = - 0.611, p = 0.004) in the patients. More interestingly, the changes of GABA concentrations were negatively correlated with the changes of FCS in the MPFC of FEP patients (r = - 0.525, p = 0.037) (Fig. 3C), while the changes of NAA concentrations were negatively correlated with the changes of FCS in the PCC/PCu of the patients (r = - 0.607, p = 0.013) post treatment (Fig. 3D).
3.5. Functional-neurometabolic signal changes in association with symptom recovery of FEP patients
The longitudinal alterations of FCS in the PCC/PCu were negatively correlated with the decreases in patients PANSS positive (r = - 0.540, p = 0.031) and PANSS general psychopathology subscales (r = - 0.551, p = 0.027), while the increases in FCS of MPFC were negatively correlated with the decreases of PANSS positive scores (r = - 0.531, p = 0.034) in the patients. More detailed correlation results can be found in Supplementary Table S6. The changes of neurometabolites were not significantly correlated with symptom recovery of FEP patients across the 8-week treatment, as shown in Supplementary Table S7.
3.6. Mediation analyses
To evaluate the relationship between the longitudinal changes of neurometabolites concentrations and FCS changes, as well as their contributions to patients’ clinical recovery, we applied the SEM analysis. As shown in Fig. 4A, the changes of FCS between MPFC and DMN mediated the relationship between the changes of GABA concentration in the MPFC and positive symptom recovery of the FEP patients (p = 0.045; bias-corrected bootstrapping confidence interval [0.005 0.499]). Moreover, the changes of FCS between PCC/PCu and DMN mediated the relationship between the changes of NAA concentrations in the MPFC and positive symptom recovery of the FEP patients (p = 0.005; bias-corrected bootstrapping confidence interval [0.100 0.481]), as shown in Fig. 4B. The models were well fit for both the MPFC FCS mediation model ( = 0.583, p = 0.445; GFI = 0.978; AIC = 10.583) and the PCC/PCu FCS mediation model ( = 0.013, p = 0.910; GFI = 0.999; AIC = 10.013).
4. Discussion
In this study, we investigated the concurrent alterations of neurometabolites levels and FC in DMN, as well as their contributions to the recovery of clinical symptoms after 8 weeks of pharmacological intervention for DN-FEP patients. Our findings confirmed the hypothesis about an intrinsic linkage between the DMN functional and neurometabolic signal changes during medication therapy, which is related to the clinical recovery of FEP patients.
The reduced FC of DMN in FEP patients has been consistently reported in previous studies (Bastos-Leite et al., 2015, Dong et al., 2018, Fan et al., 2020), in agreement with our findings. After 8-week antipsychotic medication, we found increased FC between the MPFC cluster and DMN, similar to the findings in the previous study (Sambataro et al., 2010). The animal model studies showed that the second-generation antipsychotics such as olanzapine increased the extracellular level of dopamine in the PFC (Gessa et al., 2000, Ichikawa et al., 2001), which modulated the GABAergic inputs to pyramidal cells (Seamans and Yang, 2004) and contributed to the enhancement of coupling between DMN and frontal-parietal control network (Dang et al., 2012). An alternative mechanism underlying the treatment effect on DMN FC might be attributed to the indirect regulation via the increase of dopamine level in the anticorrelated networks (Bertolino et al., 2004, Sambataro et al., 2010). In support of this, we found that the reduced anticorrelation between DMN and DLPFC in the FEP patients was normalized after 8 weeks of antipsychotic treatment. Furthermore, we showed the association between the changes of the DMN connectivity with MPFC and PCC/PCu and the improvement in positive symptom scores in the FEP patients after 8-week medication. These findings are in line with previous reports that showed the FC changes between PCC/PCu and MPFC were correlated with the positive symptom improvement in FEP patients after 8-week risperidone monotherapy (Zong et al., 2019). Our results consolidated the medication effects on both within-DMN FC and anticorrelation between DMN and DLPFC as well as their associations with clinical recovery in FEP patients.
Besides the FC changes, elevated GABA level in the MPFC of DN-FEP patients was found in our work. Deficits in GABAergic neurotransmission have been implicated in the pathophysiology of SCZ (Marín, 2012). Postmortem studies have shown the reductions of GABA-synthesizing enzyme and GABAergic interneurons in the prefrontal and cingulate cortices of SCZ patients (Marín, 2012, Volk et al., 2001). The elevated GABA signal we found is concordant with previous findings in unmedicated SCZ patients and subjects at ultra-high risk for psychosis (Cen et al., 2020, de la Fuente-Sandoval, 2015, Kegeles et al., 2012). Furthermore, the differences of GABA levels between DN-FEP and HC groups were not significant after 8-week treatment, in line with the previous results that showed no differences in the MPFC GABA concentration between medicated SCZ and HC groups (Marenco et al., 2016, Rowland et al., 2016).
More interestingly, the change of MPFC GABA signal was negatively correlated with the alteration of its FCS with DMN after treatment. The GABAergic neurotransmission has been closely involved in the generation of synchronized gamma oscillations (Cardin et al., 2009, Uhlhaas and Singer, 2010), which were correlated with the hemodynamic responses (Niessing et al., 2005). Previous studies of healthy subjects suggested that the regional GABA levels were associated with the FC or functional activation (Gu et al., 2019, Kapogiannis et al., 2013). For example, Northoff et al. showed that the ACC GABA concentration mediated the negative fMRI signal changes of DMN during emotional processing task (Northoff et al., 2007), and Kapogiannis et al. found that the GABA level in PCC/PCu was negatively correlated with the resting-state FC of DMN (Kapogiannis et al., 2013). Similar to our results shown in Supplementary Fig. S6, Chen et al. found that the GABA level in MPFC was negatively correlated with the MPFC-DLPFC anticorrelation in HCs (Du et al., 2018).
Besides the regional neurometabolic-functional signal coupling, the neurometabolic levels may also contribute to the functional activity in distant but functionally connected regions. Interestingly, we found that changes of NAA concentration in MPFC were negatively correlated with the changes of PCC/PCu FCS within DMN. This finding suggests that the alteration of NAA concentration is associated with the long-range connection changes in the DMN of FEP patients. NAA is an amino acid synthesized in neuronal mitochondria (Moffett et al., 2007). In the cytosol, NAA acts as a precursor for the enzymatic synthesis of N-acetyl-aspartyl-glutamate (NAAG), which regulates glutamate and dopamine release (Xi et al., 2002, Zhao et al., 2001). Glutamate, as the major excitatory neurotransmitter, is involved in the long-range modulation of the BOLD responses. Previous studies have found that glutamate in ACC could modulate the functional activation in posterior DMN (Overbeek et al., 2019). In FEP patients, the coupling between ACC glutamate and posterior DMN response was disrupted (Overbeek et al., 2019), along with aberrant striatal dopamine synthesis and release (Howes et al., 2012). The dysregulation of striatum dopamine release has been associated with NAA reductions in the prefrontal cortex (Bertolino et al., 2000, MOFFETT et al., 2007). Since the pharmacological treatments act to deplete striatum dopamine levels or block dopamine receptors, we speculate that the treatment-related changes of NAA levels may facilitate the normalization of glutamatergic long-range projections to distant but functionally connected regions.
Furthermore, using the SEM approach, we found that the alteration of DMN FCS in MPFC influenced a path from the alterations of MPFC GABA concentration to the recovery of PANSS positive symptoms. Deficits of inhibitory GABAergic interneurons would lead to the excitatory/inhibitory imbalance of neural circuits (Marín, 2012). In SCZ, several genes that regulate the glutamate-GABA system, e.g., GRIA1, GABRA3, and GBRB3, were primarily associated with the DMN dysconnectivity, indicating the intrinsic linkage between the neurotransmitters and FC (Meda et al., 2014). The abnormalities in GABAergic function mediated the prefrontal hypodopaminergia, which might contribute to patients’ clinical symptoms (Di Pietro and Seamans, 2007). Current results firstly showed that the relationship between FEP patients’ positive symptoms recovery and the altered GABAergic signal in MPFC was mediated by the restoration of its FC with DMN.
On the other hand, we showed that the NAA alteration was associated with the changes of the FCS between PCC/PCu and DMN, which contributed to the recovery of patients’ positive symptoms. It’s shown that the D2 receptor antagonism of risperidone plays a key role in alleviating patients' positive symptoms (Zong et al., 2015). Previous studies have suggested a potential linkage between dopamine signaling and DMN modulation (Minzenberg et al., 2011, Nagano-Saito et al., 2009). As mentioned above, NAA is involved in the regulation of dopamine release (Xi et al., 2002, Zhao et al., 2001). Taken together, we speculate that MPFC NAA changes may underlie the alterations of DMN connectivity and the recovery of positive symptoms through its complex interactions with dopamine signaling.
Recently, the aberrant coupling between the FC and glutamate concentration of the salience network (SN) has been reported in SCZ patients (Limongi et al., 2020, Maximo et al., 2021, McCutcheon et al., 2021). The relationship between Glx levels in the dorsal ACC and FC in the dorsal ACC, insula, and lateral parietal cortex was weaker or absent in medication-naive FEP patients (Maximo et al., 2021). Glutamate concentration in the dorsal ACC was associated with weaker inhibitory connections in the FEP group than in the HC group (Limongi et al., 2020). Besides, the increases in glutamate levels induced by riluzole were correlated with the increases in connectivity localized to the SN (McCutcheon et al., 2021). A triple-network model, composed of DMN, SN, and central executive network (CEN), has been proposed to underlie a range of psychiatric disorders including schizophrenia (Han et al., 2019, Liang et al., 2021, Menon, 2011, Supekar et al., 2019). For example, the decreased functional activity of SN has been found to be associated with increased FC between DMN and CEN (Manoliu et al., 2014). Our results provided insights into the neurometabolic-functional signal changes within DMN of FEP patients before and after treatment. Further studies integrating the DMN, SN, and CEN are in merit to reveal the biochemical-functional interactions among these networks using combined MRS and fMRI measures.
Strengths of the present study include the longitudinal study design, and the combination of fMRI and 1H-MRS, which makes it possible to investigate the functional-neurometabolic mechanism of therapeutic effect. The inclusion of DN-FEP patients could rule out the effects of medication at baseline and illness chronicity. Some limitations should, however, be considered. First, the measured GABA signal should be interpreted with caution since it included the macromolecule signals (Behar et al., 1994). Second, the current resting-state scan of 6 min is relatively short, and a longer scanning time may improve the test-retest reliability of the results. Third, the possibility that the observed changes may be caused by other factors could not be completely excluded because of the lack of a patient group receiving placebos, which has not been conducted in this study due to ethical issues. Also, we excluded the subjects with substance abuse in order to avoid the potential impact of comorbid substance use disorders, which might limit the generalisability of the findings. Finally, the lack of follow-up scans in the HC group is a limitation of our study, which could confound the interpretation of the medication effects. We could not exclude the possibility that the observed functional and neurometabolic changes might be caused by the experiential changes (e.g., the adaptation to the scanning environment) instead of the purely therapeutic effect. The purpose of this study is to examine whether the DMN functional and neurometabolic abnormalities in DN-FEP patients were improved after 8-week antipsychotic treatment. A more solid design of the study is to include follow-up scans for the control group and examine the group-by-time interaction using two-way analysis of variance with a repeated measures design as in previous literature (Li et al., 2016, Sambataro et al., 2010). Also, the sample size of our study is relatively small due to the difficulty in patient recruitment. For stable estimates in mediation analyses, a larger sample size is in need (Schönbrodt and Perugini, 2013). Although the bias-corrected bootstrapping technique was used to help with the analyses, our current sample size is still at the lower bound of possibility (Fritz and Mackinnon, 2007). Therefore, our findings are rather exploratory and should be interpreted with caution. Future studies with larger sample sizes and with follow-up control groups are in need to consolidate our findings.
5. Conclusions
In conclusion, this study showed the GABA level increased concurrently with decreased FC in the DMN of DN-FEP patients. After 8-week antipsychotic medication, the changes of the FCS between the MPFC and DMN mediated the association between the changes of GABA concentration in the MPFC and the improvement in positive symptom scores of FEP patients. Moreover, the changes of FCS between the PCC/PCu and DMN mediated the relationship between the changes of NAA concentration in the MPFC and the improvement in positive symptom scores of FEP patients. Our results provide novel insights into the understanding of the neurometabolic mechanisms of the DMN-related FC changes as well as their contributions to the pharmacological and therapeutic effects in DN-FEP patients using noninvasive multimodal neuroimaging.
CRediT authorship contribution statement
Wenli Li: Conceptualization, Methodology, Formal analysis, Software, Investigation. Jiale Xu: Software, Investigation, Formal analysis. Qiong Xiang: Resources, Data curation. Kaiming Zhuo: Resources, Data curation. Yaoyu Zhang: Supervision. Dengtang Liu: Resources, Funding acquisition, Data curation, Investigation. Yao Li: Conceptualization, Resources, Supervision, Methodology.
Declaration of Competing Interest
The authors declare that they have no known competing financial interests or personal relationships that could have appeared to influence the work reported in this paper.
Acknowledgments
This work was supported by the National Natural Science Foundation of China [Grant No. 81871083, 82171496]; Key Program of Multidisciplinary Cross Research Foundation of Shanghai Jiao Tong University [YG2021ZD28, YG2017ZD13]; Shanghai Jiao Tong University Scientific and Technological Innovation Funds [2019QYA12]; Medical innovation research project of science and technology innovation action plan of Shanghai Science and Technology Committee [20y11906300]; Key Program of SMHC Clinical Research Center [CRC2017ZD03] and Shanghai Clinical Research Center for Mental Health [19MC1911100].
Footnotes
Supplementary data to this article can be found online at https://doi.org/10.1016/j.nicl.2022.102970.
Contributor Information
Dengtang Liu, Email: liudengtang@smhc.org.cn.
Yao Li, Email: yaoli@sjtu.edu.cn.
Appendix A. Supplementary data
The following are the Supplementary data to this article:
References
- Akaike H. A new look at the statistical model identification. IEEE Trans Automat Contr. 1974;19(6):716–723. [Google Scholar]
- Association, A.P., 2013. Diagnostic and statistical manual of mental disorders (DSM-5®). American Psychiatric Pub. [DOI] [PubMed]
- Bastos-Leite A.J., Ridgway G.R., Silveira C., Norton A., Reis S., Friston K.J. Dysconnectivity within the default mode in first-episode schizophrenia: a stochastic dynamic causal modeling study with functional magnetic resonance imaging. Schizophr Bull. 2015;41(1):144–153. doi: 10.1093/schbul/sbu080. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Behar K.L., Rothman D.L., Spencer D.D., Petroff O.A.C. Analysis of macromolecule resonances in 1H NMR spectra of human brain. Magn Reson Med. 1994;32(3):294–302. doi: 10.1002/mrm.1910320304. [DOI] [PubMed] [Google Scholar]
- Bertolino A., et al. The relationship between dorsolateral prefrontal neuronal N-acetylaspartate and evoked release of striatal dopamine in schizophrenia. Neuropsychopharmacology. 2000;22:125–132. doi: 10.1016/S0893-133X(99)00096-2. [DOI] [PubMed] [Google Scholar]
- Bertolino A., Caforio G., Blasi G., De Candia M., Latorre V., Petruzzella V., Altamura M., Nappi G., Papa S., Callicott J.H., Mattay V.S., Bellomo A., Scarabino T., Weinberger D.R., Nardini M. Interaction of COMT Val108/158 Met genotype and olanzapine treatment on prefrontal cortical function in patients with schizophrenia. Am J Psychiatry. 2004;161(10):1798–1805. doi: 10.1176/ajp.161.10.1798. [DOI] [PubMed] [Google Scholar]
- Bojesen K.B., et al. Treatment response after 6 and 26 weeks is related to baseline glutamate and GABA levels in antipsychotic-naive patients with psychosis. Psychol Med. 2020;50:2182–2193. doi: 10.1017/S0033291719002277. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cadena E.J., White D.M., Kraguljac N.V., Reid M.A., Maximo J.O., Nelson E.A., Gawronski B.A., Lahti A.C. A Longitudinal Multimodal Neuroimaging Study to Examine Relationships Between Resting State Glutamate and Task Related BOLD Response in Schizophrenia. Front Psychiatry. 2018;9 doi: 10.3389/fpsyt.2018.00632. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Camchong J., MacDonald A.W., Bell C., Mueller B.A., Lim K.O. Altered functional and anatomical connectivity in schizophrenia. Schizophr Bull. 2011;37(3):640–650. doi: 10.1093/schbul/sbp131. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cardin J.A., Carlén M., Meletis K., Knoblich U., Zhang F., Deisseroth K., Tsai L.-H., Moore C.I. Driving fast-spiking cells induces gamma rhythm and controls sensory responses. Nature. 2009;459(7247):663–667. doi: 10.1038/nature08002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cen H., Xu J., Yang Z., Mei L.i., Chen T., Zhuo K., Xiang Q., Song Z., Wang Y., Guo X., Wang J., Jiang K., Xu Y., Li Y., Liu D. Neurochemical and brain functional changes in the ventromedial prefrontal cortex of first-episode psychosis patients: A combined functional magnetic resonance imaging—proton magnetic resonance spectroscopy study. Aust N Z J Psychiatry. 2020;54(5):519–527. doi: 10.1177/0004867419898520. [DOI] [PubMed] [Google Scholar]
- Cheung G.W., Lau R.S. Testing mediation and suppression effects of latent variables – Bootstrapping with structural equation models. Organ Res Methods. 2008;11(2):296–325. [Google Scholar]
- Dang L.C., O'Neil J.P., Jagust W.J. Dopamine supports coupling of attention-related networks. J Neurosci. 2012;32(28):9582–9587. doi: 10.1523/JNEUROSCI.0909-12.2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- de la Fuente-Sandoval C., et al. Cortico-Striatal GABAergic and Glutamatergic Dysregulations in Subjects at Ultra-High Risk for Psychosis Investigated with Proton Magnetic Resonance Spectroscopy. Int J Neuropsychopharmacol. 2015;19(3):yv105. doi: 10.1093/ijnp/pyv105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- de la Fuente-Sandoval C., et al. Prefrontal and Striatal Gamma-Aminobutyric Acid Levels and the Effect of Antipsychotic Treatment in First-Episode Psychosis Patients. Biol Psychiatry. 2018;83:475–483. doi: 10.1016/j.biopsych.2017.09.028. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pietro N., Seamans J. Dopamine and serotonin interactions in the prefrontal cortex: insights on antipsychotic drugs and their mechanism of action. Pharmacopsychiatry. 2007;40(S 1):S27–S33. doi: 10.1055/s-2007-992133. [DOI] [PubMed] [Google Scholar]
- Dong D., Wang Y., Chang X., Luo C., Yao D. Dysfunction of Large-Scale Brain Networks in Schizophrenia: A Meta-analysis of Resting-State Functional Connectivity. Schizophr Bull. 2018;44(1):168–181. doi: 10.1093/schbul/sbx034. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Du F., et al. Regional GABA Concentrations Modulate Inter-Network Resting-State Functional Connectivity. Biol Psychiatry. 2018;83:S160. doi: 10.1093/cercor/bhy059. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fox J., Weisberg S. An R companion to applied regression. Sage. 2018 Publications. [Google Scholar]
- Fransson P., Marrelec G. The precuneus/posterior cingulate cortex plays a pivotal role in the default mode network: Evidence from a partial correlation network analysis. Neuroimage. 2008;42(3):1178–1184. doi: 10.1016/j.neuroimage.2008.05.059. [DOI] [PubMed] [Google Scholar]
- Friston K.J., Williams S., Howard R., Frackowiak R.S.J., Turner R. Movement-related effects in fMRI time-series. Magn Reson Med. 1996;35(3):346–355. doi: 10.1002/mrm.1910350312. [DOI] [PubMed] [Google Scholar]
- Fritz M.S., MacKinnon D.P. Required sample size to detect the mediated effect. Psychol Sci. 2007;18(3):233–239. doi: 10.1111/j.1467-9280.2007.01882.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gessa G.L., et al. Dissociation of haloperidol, clozapine, and olanzapine effects on electrical activity of mesocortical dopamine neurons and dopamine release in the prefrontal cortex. Neuropsychopharmacology. 2000;22:642–649. doi: 10.1016/S0893-133X(00)00087-7. [DOI] [PubMed] [Google Scholar]
- Gu H., Hu Y., Chen X.i., He Y., Yang Y. Regional excitation-inhibition balance predicts default-mode network deactivation via functional connectivity. Neuroimage. 2019;185:388–397. doi: 10.1016/j.neuroimage.2018.10.055. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Han W., Sorg C., Zheng C., Yang Q., Zhang X., Ternblom A., Mawuli C.B., Gao L., Luo C., Yao D., Li T., Liang S., Shao J. Low-rank network signatures in the triple network separate schizophrenia and major depressive disorder. Neuroimage Clin. 2019;22:101725. doi: 10.1016/j.nicl.2019.101725. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Howes O.D., Kambeitz J., Kim E., Stahl D., Slifstein M., Abi-Dargham A., Kapur S. The nature of dopamine dysfunction in schizophrenia and what this means for treatment: meta-analysis of imaging studies. Arch Gen Psychiatry. 2012;69(8) doi: 10.1001/archgenpsychiatry.2012.169. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ichikawa J., Ishii H., Bonaccorso S., Fowler W.L., O’Laughlin I.A., Meltzer H.Y. 5-HT(2A) and D(2) receptor blockade increases cortical DA release via 5-HT(1A) receptor activation: a possible mechanism of atypical antipsychotic-induced cortical dopamine release. J Neurochem. 2001;76(5):1521–1531. doi: 10.1046/j.1471-4159.2001.00154.x. [DOI] [PubMed] [Google Scholar]
- Kaminski J., Gleich T., Fukuda Y.u., Katthagen T., Gallinat J., Heinz A., Schlagenhauf F. Association of Cortical Glutamate and Working Memory Activation in Patients With Schizophrenia: A Multimodal Proton Magnetic Resonance Spectroscopy and Functional Magnetic Resonance Imaging Study. Biol Psychiatry. 2020;87(3):225–233. doi: 10.1016/j.biopsych.2019.07.011. [DOI] [PubMed] [Google Scholar]
- Kapogiannis D., Reiter D.A., Willette A.A., Mattson M.P. Posteromedial cortex glutamate and GABA predict intrinsic functional connectivity of the default mode network. Neuroimage. 2013;64:112–119. doi: 10.1016/j.neuroimage.2012.09.029. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kay S.R., Fiszbein A., Opler L.A. The positive and negative syndrome scale (PANSS) for schizophrenia. Schizophr Bull. 1987;13(2):261–276. doi: 10.1093/schbul/13.2.261. [DOI] [PubMed] [Google Scholar]
- Kegeles L.S., et al. Elevated prefrontal cortex γ-aminobutyric acid and glutamate-glutamine levels in schizophrenia measured in vivo with proton magnetic resonance spectroscopy. Arch Gen Psychiatry. 2012;69:449–459. doi: 10.1001/archgenpsychiatry.2011.1519. [DOI] [PubMed] [Google Scholar]
- Kline R.B. Principles and practice of structural equation modeling. Guilford. 2015 publications. [Google Scholar]
- Landin-Romero R., McKenna P.J., Salgado-Pineda P., Sarró S., Aguirre C., Sarri C., Compte A., Bosque C., Blanch J., Salvador R., Pomarol-Clotet E. Failure of deactivation in the default mode network: a trait marker for schizophrenia? Psychol. Med. 2015;45(6):1315–1325. doi: 10.1017/S0033291714002426. [DOI] [PubMed] [Google Scholar]
- Li F., Lui S.u., Yao L.i., Hu J., Lv P., Huang X., Mechelli A., Sweeney J.A., Gong Q. Longitudinal Changes in Resting-State Cerebral Activity in Patients with First-Episode Schizophrenia: A 1-Year Follow-up Functional MR Imaging Study. Radiology. 2016;279(3):867–875. doi: 10.1148/radiol.2015151334. [DOI] [PubMed] [Google Scholar]
- Liang S., Wang Q., Greenshaw A.J., Li X., Deng W., Ren H., Zhang C., Yu H., Wei W., Zhang Y., Li M., Zhao L., Du X., Meng Y., Ma X., Yan C.-G., Li T. Aberrant triple-network connectivity patterns discriminate biotypes of first-episode medication-naive schizophrenia in two large independent cohorts. Neuropsychopharmacology. 2021;46(8):1502–1509. doi: 10.1038/s41386-020-00926-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liang X., Zou Q., He Y., Yang Y. Coupling of functional connectivity and regional cerebral blood flow reveals a physiological basis for network hubs of the human brain. Proc Natl Acad Sci U S A. 2013;110(5):1929–1934. doi: 10.1073/pnas.1214900110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Limongi R., Jeon P., Mackinley M., Das T., Dempster K., Théberge J., Bartha R., Wong D., Palaniyappan L. Glutamate and Dysconnection in the Salience Network: Neurochemical, Effective Connectivity, and Computational Evidence in Schizophrenia. Biol Psychiatry. 2020;88(3):273–281. doi: 10.1016/j.biopsych.2020.01.021. [DOI] [PubMed] [Google Scholar]
- Marenco S., Meyer C., Kuo S., van der Veen J.W., Shen J., DeJong K., Barnett A.S., Apud J.A., Dickinson D., Weinberger D.R., Berman K.F. Prefrontal GABA Levels Measured With Magnetic Resonance Spectroscopy in Patients With Psychosis and Unaffected Siblings. Am J Psychiatry. 2016;173(5):527–534. doi: 10.1176/appi.ajp.2015.15020190. [DOI] [PubMed] [Google Scholar]
- Marín O. Interneuron dysfunction in psychiatric disorders. Nat Rev Neurosci. 2012;13(2):107–120. doi: 10.1038/nrn3155. [DOI] [PubMed] [Google Scholar]
- Maximo J.O., et al. Salience network glutamate and brain connectivity in medication-naive first episode patients – A multimodal magnetic resonance spectroscopy and resting state functional connectivity MRI study. Neuroimage Clin. 2021;32 doi: 10.1016/j.nicl.2021.102845. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McCutcheon R.A., Pillinger T., Rogdaki M., Bustillo J., Howes O.D. Glutamate connectivity associations converge upon the salience network in schizophrenia and healthy controls. Transl Psychiatry. 2021;11(1) doi: 10.1038/s41398-021-01455-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McCutcheon R.A., Reis Marques T., Howes O.D. Schizophrenia-An Overview. JAMA. Psychiatry. 2020;77(2):201. doi: 10.1001/jamapsychiatry.2019.3360. [DOI] [PubMed] [Google Scholar]
- Meda S.A., Ruano G., Windemuth A., O'Neil K., Berwise C., Dunn S.M., Boccaccio L.E., Narayanan B., Kocherla M., Sprooten E., Keshavan M.S., Tamminga C.A., Sweeney J.A., Clementz B.A., Calhoun V.D., Pearlson G.D. Multivariate analysis reveals genetic associations of the resting default mode network in psychotic bipolar disorder and schizophrenia. Proc Natl Acad Sci U S A. 2014;111(19):E2066–E2075. doi: 10.1073/pnas.1313093111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Menon V. Large-scale brain networks and psychopathology: a unifying triple network model. Trends Cogn Sci. 2011;15(10):483–506. doi: 10.1016/j.tics.2011.08.003. [DOI] [PubMed] [Google Scholar]
- Mescher M., Merkle H., Kirsch J., Garwood M., Gruetter R. Simultaneous in vivo spectral editing and water suppression. NMR in Biomedicine. 1998;11(6):266–272. doi: 10.1002/(sici)1099-1492(199810)11:6<266::aid-nbm530>3.0.co;2-j. [DOI] [PubMed] [Google Scholar]
- Minzenberg M.J., Yoon J.H., Carter C.S. Modafinil modulation of the default mode network. Psychopharmacology (Berl) 2011;215(1):23–31. doi: 10.1007/s00213-010-2111-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- MOFFETT J., ROSS B., ARUN P., MADHAVARAO C., NAMBOODIRI A. N-Acetylaspartate in the CNS: from neurodiagnostics to neurobiology. Prog Neurobiol. 2007;81(2):89–131. doi: 10.1016/j.pneurobio.2006.12.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nagano-Saito A., Liu J., Doyon J., Dagher A. Dopamine modulates default mode network deactivation in elderly individuals during the Tower of London task. Neurosci Lett. 2009;458(1):1–5. doi: 10.1016/j.neulet.2009.04.025. [DOI] [PubMed] [Google Scholar]
- Nelson E.A., et al. Hippocampal Dysconnectivity and Altered Glutamatergic Modulation of the Default Mode Network–a Combined Resting State Connectivity and Magnetic Resonance Spectroscopy Study in Schizophrenia. Biol Psychiatry Cogn Neurosci. 2020 doi: 10.1016/j.bpsc.2020.04.014. Neuroimaging. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Niessing Jörn, Ebisch B., Schmidt K.E., Niessing M., Singer W., Galuske R.A.W. Hemodynamic signals correlate tightly with synchronized gamma oscillations. Science. 2005;309(5736):948–951. doi: 10.1126/science.1110948. [DOI] [PubMed] [Google Scholar]
- Northoff G., Walter M., Schulte R.F., Beck J., Dydak U., Henning A., Boeker H., Grimm S., Boesiger P. GABA concentrations in the human anterior cingulate cortex predict negative BOLD responses in fMRI. Nat Neurosci. 2007;10(12):1515–1517. doi: 10.1038/nn2001. [DOI] [PubMed] [Google Scholar]
- Fan F., Tan S., Huang J., Chen S., Fan H., Wang Z., Li C.R., Tan Y. Functional disconnection between subsystems of the default mode network in schizophrenia. Psychol Med. 2020;13:1–11. doi: 10.1017/S003329172000416X. [DOI] [PubMed] [Google Scholar]
- Liu H., Kaneko Y., Ouyang X., Li L., Hao Y., Chen E.Y., Jiang T., Zhou Y., Liu Z. Schizophrenic patients and their unaffected siblings share increased resting-state connectivity in the task-negative network but not its anticorrelated task-positive network. Schizophr Bull. 2012;38(2):285–294. doi: 10.1093/schbul/sbq074. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Manoliu A., Riedl V., Zherdin A., Mühlau M., Schwerthöffer D., Scherr M., Peters H., Zimmer C., Förstl H., Bäuml J., Wohlschläger A.M., Sorg C. Aberrant dependence of default mode/central executive network interactions on anterior insular salience network activity in schizophrenia. Schizophr Bull. 2014;40(2):428–437. doi: 10.1093/schbul/sbt037. [DOI] [PMC free article] [PubMed] [Google Scholar]
- O’Neill A., Mechelli A., Bhattacharyya S. Dysconnectivity of Large-Scale Functional Networks in Early Psychosis: A Meta-analysis. Schizophr Bull. 2019;45(3):579–590. doi: 10.1093/schbul/sby094. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Overbeek G., Gawne T.J., Reid M.A., Salibi N., Kraguljac N.V., White D.M., Lahti A.C. Relationship Between Cortical Excitation and Inhibition and Task-Induced Activation and Deactivation: A Combined Magnetic Resonance Spectroscopy and Functional Magnetic Resonance Imaging Study at 7T in First-Episode Psychosis. Biol Psychiatry Cogn Neurosci Neuroimaging. 2019;4(2):121–130. doi: 10.1016/j.bpsc.2018.10.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Power J.D., Barnes K.A., Snyder A.Z., Schlaggar B.L., Petersen S.E. Spurious but systematic correlations in functional connectivity MRI networks arise from subject motion. Neuroimage. 2012;59(3):2142–2154. doi: 10.1016/j.neuroimage.2011.10.018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Provencher S.W. Estimation of metabolite concentrations from localized in vivo proton NMR spectra. Magn Reson Med. 1993;30(6):672–679. doi: 10.1002/mrm.1910300604. [DOI] [PubMed] [Google Scholar]
- Rowland L.M., Krause B.W., Wijtenburg S.A., McMahon R.P., Chiappelli J., Nugent K.L., Nisonger S.J., Korenic S.A., Kochunov P., Hong L.E. Medial frontal GABA is lower in older schizophrenia: a MEGA-PRESS with macromolecule suppression study. Mol Psychiatry. 2016;21(2):198–204. doi: 10.1038/mp.2015.34. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sambataro F., Blasi G., Fazio L., Caforio G., Taurisano P., Romano R., Di Giorgio A., Gelao B., Lo Bianco L., Papazacharias A., Popolizio T., Nardini M., Bertolino A. Treatment with Olanzapine is Associated with Modulation of the Default Mode Network in Patients with Schizophrenia. Neuropsychopharmacology. 2010;35(4):904–912. doi: 10.1038/npp.2009.192. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schönbrodt F.D., Perugini M. At what sample size do correlations stabilize? J Res Pers. 2013;47(5):609–612. [Google Scholar]
- Seamans J.K., Yang C.R. The principal features and mechanisms of dopamine modulation in the prefrontal cortex. Prog Neurobiol. 2004;74(1):1–58. doi: 10.1016/j.pneurobio.2004.05.006. [DOI] [PubMed] [Google Scholar]
- Sheehan D. MINI-Mini International neuropsychiatric interview-english version 5.0. 0-DSM-IV. J Clin Psychiatry. 1998;59:34–57. [PubMed] [Google Scholar]
- Shim G., Oh J.S., Jung W.H., Jang J.H., Choi C.-H., Kim E., Park H.-Y., Choi J.-S., Jung M.H., Kwon J.S. Altered resting-state connectivity in subjects at ultra-high risk for psychosis: an fMRI study. Behav. Brain Funct. 2010;6(1) doi: 10.1186/1744-9081-6-58. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Supekar K., Cai W., Krishnadas R., Palaniyappan L., Menon V. Dysregulated Brain Dynamics in a Triple-Network Saliency Model of Schizophrenia and Its Relation to Psychosis. Biol Psychiatry. 2019;85(1):60–69. doi: 10.1016/j.biopsych.2018.07.020. [DOI] [PubMed] [Google Scholar]
- Tzourio-Mazoyer N., Landeau B., Papathanassiou D., Crivello F., Etard O., Delcroix N., Mazoyer B., Joliot M. Automated anatomical labeling of activations in SPM using a macroscopic anatomical parcellation of the MNI MRI single-subject brain. Neuroimage. 2002;15(1):273–289. doi: 10.1006/nimg.2001.0978. [DOI] [PubMed] [Google Scholar]
- Uddin L.Q., Clare Kelly A.M., Biswal B.B., Xavier Castellanos F., Milham M.P. Functional connectivity of default mode network components: correlation, anticorrelation, and causality. Hum Brain Mapp. 2009;30(2):625–637. doi: 10.1002/hbm.20531. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Uhlhaas P.J., Singer W. Abnormal neural oscillations and synchrony in schizophrenia. Nat Rev Neurosci. 2010;11(2):100–113. doi: 10.1038/nrn2774. [DOI] [PubMed] [Google Scholar]
- Volk D.W., Austin M.C., Pierri J.N., Sampson A.R., Lewis D.A. GABA transporter-1 mRNA in the prefrontal cortex in schizophrenia: decreased expression in a subset of neurons. Am J Psychiatry. 2001;158(2):256–265. doi: 10.1176/appi.ajp.158.2.256. [DOI] [PubMed] [Google Scholar]
- Whitfield-Gabrieli S., Ford J.M. Default mode network activity and connectivity in psychopathology. Annu Rev Clin Psychol. 2012;8(1):49–76. doi: 10.1146/annurev-clinpsy-032511-143049. [DOI] [PubMed] [Google Scholar]
- Whitfield-Gabrieli S., Thermenos H.W., Milanovic S., Tsuang M.T., Faraone S.V., McCarley R.W., Shenton M.E., Green A.I., Nieto-Castanon A., LaViolette P., Wojcik J., Gabrieli J.D.E., Seidman L.J. Hyperactivity and hyperconnectivity of the default network in schizophrenia and in first-degree relatives of persons with schizophrenia. Proc Natl Acad Sci U S A. 2009;106(4):1279–1284. doi: 10.1073/pnas.0809141106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Xi Z.-X., Baker D.A., Shen H., Carson D.S., Kalivas P.W. Group II metabotropic glutamate receptors modulate extracellular glutamate in the nucleus accumbens. J Pharmacol Exp Ther. 2002;300(1):162–171. doi: 10.1124/jpet.300.1.162. [DOI] [PubMed] [Google Scholar]
- Yan C., Zang Y. DPARSF: a MATLAB toolbox for“ pipeline” data analysis of resting-state fMRI. Front Syst Neurosci. 2010;4:13. doi: 10.3389/fnsys.2010.00013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhao J., Ramadan E., Cappiello M., Wroblewska B., Bzdega T., Neale J.H. NAAG inhibits KCl-induced [3H]-GABA release via mGluR3, cAMP, PKA and L-type calcium conductance. Eur J Neurosci. 2001;13(2):340–346. [PubMed] [Google Scholar]
- Zong X., et al. N-acetylaspartate reduction in the medial prefrontal cortex following 8 weeks of risperidone treatment in first-episode drug-naive schizophrenia patients. Sci Rep. 2015;5:9109. doi: 10.1038/srep09109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zong X., Hu M., Pantazatos S.P., Mann J.J., Wang G., Liao Y., Liu Z.C., Liao W., Yao T., Li Z., He Y., Lv L., Sang D., Tang J., Chen H., Zheng J., Chen X. A Dissociation in Effects of Risperidone Monotherapy on Functional and Anatomical Connectivity Within the Default Mode Network. Schizophr Bull. 2019;45(6):1309–1318. doi: 10.1093/schbul/sby175. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.