Abstract
Objective
To estimate associations of statin use with hospitalisation, intensive care unit (ICU) admission and mortality at 30 days among individuals with and without a positive test for SARS-CoV-2.
Design
Retrospective cohort study.
Setting
US Veterans Health Administration (VHA).
Participants
All veterans receiving VHA healthcare with ≥1 positive nasal swab for SARS-CoV-2 between 1 March 2020 and 10 March 2021 (cases; n=231 154) and a comparator group of controls comprising all veterans who did not have a positive nasal swab for SARS-CoV-2 but who did have ≥1 clinical lab test performed during the same time period (n=4 570 252).
Main outcomes
Associations of: (1) any statin use, (2) use of specific statins or (3) low-intensity/moderate-intensity versus high-intensity statin use at the time of positive nasal swab for SARS-CoV-2 (cases) or result of clinical lab test (controls) assessed from pharmacy records with hospitalisation, ICU admission and death at 30 days. We also examined whether associations differed between individuals with and without a positive test for SARS-CoV-2.
Results
Among individuals who tested positive for SARS-CoV-2, statin use was associated with lower odds of death at 30 days (OR 0.81 (95% CI 0.77 to 0.85)) but not with hospitalisation or ICU admission. Associations were similar comparing use of each specific statin to no statin. Compared with low-/moderate intensity statin use, high-intensity statin use was not associated with lower odds of ICU admission or death. Over the same period, associations of statin use with 30-day outcomes were significantly stronger among individuals without a positive test for SARS-CoV-2: hospitalisation OR 0.79 (95% CI 0.77 to 0.80), ICU admission OR 0.86 (95% CI 0.81 to 0.90) and death 0.60 (95% CI 0.58 to 0.62; p for interaction all <0.001).
Conclusions
Associations of statin use with lower adverse 30-day outcomes are weaker among individuals who tested positive for SARS-CoV-2 compared with individuals without a positive test, indicating that statins do not exert SARS-CoV-2 specific effects.
Keywords: COVID-19, epidemiology, internal medicine
Strengths and limitations of this study.
Large, well-characterised national (US) sample.
First study to formally assess and compare statin effects seen in SARS-CoV-2 infection using a negative control.
Observational design cannot exclude the possibility of residual confounding.
Did not capture hospitalisations or diagnoses occurring outside Veterans Health Administration.
Introduction
New cases of COVID-19/SARS-CoV-2 infection continue to occur at high rates in the USA and worldwide with few treatments available to decrease mortality. Statin use at the time of COVID-19 diagnosis has been associated with a lower risk of short-term mortality in observational studies1 and systematic reviews.2 Based on these early findings and their demonstrated effects on inflammation, oxidative stress and immune responses, statins have been proposed as a low-cost, accessible and effective treatment for COVID-19.3 However, an inverse association of statin use with mortality is not uniformly seen across observational studies of persons with COVID-19.4 5 Furthermore, preliminary findings from a randomised placebo-controlled trial of patients admitted to the ICU did not show a protective effect of atorvastatin 20 mg/day on 30-day mortality after COVID-19 diagnosis, among patients not taking statins prior to admission.6 These paradoxical findings may reflect the presence of residual confounding in observational studies. In addition, effects of statins on mortality after COVID-19 may differ across populations, for example, among individuals with or without cardiovascular disease (CVD), or specific to certain statins but not all medications in this class. Therefore, observational studies with comprehensive strategies to examine potential bias from unmeasured confounding—such as the use of negative control populations2—are needed to improve estimates of the potential causal effect of statin use at diagnosis on mortality after COVID-19.
To address these gaps, we used national data from the Veterans Health Administration (VHA) to quantify the independent association of statin use at diagnosis with adverse outcomes from COVID-19 at 30 days, including hospitalisation, intensive care unit (ICU) admission and mortality. We used the following strategies to mitigate or estimate bias: (1) directed-acyclic graphs to guide the choice of potential confounders; (2) comparison of associations among SARS-CoV-2 infected individuals (n=231 154) with associations among an uninfected comparator sample (n=4 570 252); and (3) a dose–response analysis comparing low-intensity or moderate-intensity statin use to high-intensity use. In additional analyses, we investigated associations of individual statins with 30-day outcomes after COVID-19 and evaluated the magnitude of the statin–mortality association in strata of sex, age, race, body mass index (BMI), clinical comorbidities and C reactive protein (CRP) level prior to diagnosis.
Methods
Study setting and population
The VHA—the largest integrated healthcare system in the USA—provides care to more than 7 million veterans at 170 medical centres and 1074 outpatient sites.7 We used data from the Corporate Data Warehouse, a data repository derived from VHA’s integrated electronic medical record, including a COVID-19 Shared Data Resource, which contains analytic variables for all enrollees tested for SARS-CoV-2.8
Selection of the SARS-CoV-2 positive cohort
We identified all enrollees with one or more positive nasal swabs for SARS-CoV-2 between 1 March 2020 and 10 March 2021. The index date was defined as the date the first positive test was performed. Most tests were performed in VA laboratories using US Food and Drug Administration approved RealTime (Abbott Laboratories) or Xpert-Xpress (Cepheid) SARS-CoV-2 assays. A small number were sent to outside laboratories.
Selection of the SARS-CoV-2 negative cohort
Individuals without a positive nasal swab for SARS-CoV-2 and with any clinical lab test available in the medical record between 1 March 2020 and 31 March 2021 were chosen as a comparison group. A negative nasal swab for SARS-CoV-2 was not required for inclusion. Participants without a positive nasal swab for SARS-CoV-2 were assigned an index month during the study period for which they had a lab result, and a random index date during the index month, which was used as the start of follow-up.
Exposure
Current statin use was defined as receipt of a statin prescription with a fill date prior to the index date and a quantity prescribed that would extend past the index date. Statin intensity was defined as low, moderate or high using definitions from the American Heart Association/American College of Cardiology guidelines on management of cholesterol9 and was calculated based on the specific statin and dosage prescribed. Prescribing data were available for the following specific statins: atorvastatin, fluvastatin, lovastatin, pitavastatin, pravastatin, rosuvastatin and simvastatin. We defined prior statin use as receipt of a statin prescription with a fill date that included the time period 6 months prior to the index date.
Covariates
We collected data on age, sex, race/ethnicity, VHA facility location and urban, rural or highly rural residence using a validated classification scheme that has been previously described.10 BMI was defined as weight in kg divided by (height in metres).2 Smoking status was classified as current, former or never based on VHA health factors data. If no smoking code was entered, the participant was classified as never smoked. At-risk drinking was defined using a score ≥3 for men and ≥4 for women on the Alcohol Use Disorders Identification Test consumption questions.11 Comorbidities (hypertension, CVD and heart failure) were identified using International Classification of Diseases (ICD)-9-Clinical Modification (CM) and ICD-10 codes entered after 1 October 1999, the date when VHA began using a universal electronic health record.12 We defined chronic kidney disease (CKD) by categories of estimated glomerular filtration rate13 using the most recent creatinine at least 3 days, but not more than 1 year, before the index date. For individuals with data available on CRP at least 14 days but not more than 6 months before the index date (n=27 630), we dichotomised CRP values as normal or elevated based on cut points provided for each assay at the testing site because a variety of assays for these biomarkers are used across the VA system.
Outcomes
In both groups, we collected data on 30-day hospitalisations, ICU admissions and deaths occurring through 10 March 2021. Deaths were verified by official sources including VHA Patient Treatment File, the Beneficiary Identification Records Locator Subsystem and VA/CMS Medicare Vital Status File; Social Security Administration Death Master File; death certificates; and VHA National Cemetery Administration.14
Statistical analyses
We summarised baseline characteristics for SARS-CoV-2 infected and uninfected participants, stratified by statin use at the index date. We used multiple imputation with 10 sets of imputations for analyses that included BMI or CKD due to approximately 20% missing values for each of these variables. We used DAGitty15 to generate a directed acyclic graph (DAG) to assist in variable selection. We fit separate logistic regression models for individuals with and without a positive swab for SARS-CoV-2, testing the association of statin use at index date with occurrence of hospitalisation, ICU admission and death, adjusting for the minimal sufficient covariate set to estimate the total effect of statin use according to our DAG (statin use ≥6 months prior to diagnosis, sex, age, race/ethnicity, BMI, tobacco use, facility location, index month, urban/rural status, eGFR and history of diabetes, hypertension, CVD, heart failure and alcohol use disorder) separately. Index month was included as a precision variable. Facility location was included because both patterns of statin use and COVID-19 outcomes are expected to differ by region in the USA. In combined models, we tested for the presence of multiplicative first-order interactions to determine whether the association between statins and odds of hospitalisation, ICU admission and death at 30 days differed between persons with and without a positive swab for SARS-CoV-2. We also controlled for prior statin use to approximate a comparison of incident users and non-users. In a sensitivity analysis, we examined associations of statin use at diagnosis with occurrence of hospitalisation, ICU admission and death in models that were not adjusted for statin use 6 months prior to diagnosis.
Among individuals with a positive swab for SARS-CoV-2, we fit logistic regression models examining associations of specific statins compared with no statin use with outcomes adjusted for sex, age, race/ethnicity, BMI, tobacco use, facility location, urban/rural status, eGFR and history of diabetes, hypertension, CVD, heart failure and alcohol use disorder, as well as models comparing low-intensity to moderate-intensity or high-intensity statin use. We evaluated the magnitude of the statin-mortality association in strata of sex, age, race, BMI, clinical comorbidities and prior CRP concentration and tested for first-order multiplicative interactions by using interaction terms in combined models.
Patient and public involvement
Patients or the public were not involved in the design, or conduct, or reporting, or dissemination plans of our research.
Results
SARS-CoV-2 infected participants were 60.9 years old (±16.5) on average, and 10% (n=23 974) were female. Thirty per cent (69,263) had an active statin prescription at enrolment. During the 30 days after diagnosis, 14% (32 490) of SARS-CoV-2 infected participants were hospitalised, 3% (6140) were admitted to the ICU and 5% (12 111) died. SARS-COV-2 uninfected participants were 61.6 years old (±16.7) on average, and 13% (577,718) were female. Thirty per cent (1 389 364) had an active statin prescription at enrolment. During the 30 days after the index date, 2% (91 604) were hospitalised, 0.2% (9298) were admitted to the ICU and 0.4% died (n=19 298). Statin users were more likely to be of white race/ethnicity, have BMI of 30 kg/m2 or greater, be former smokers and reside in a rural zip code regardless of SARS-CoV-2 test result. Not surprisingly, statin use was higher among cardiometabolic conditions but lower in alcohol use disorder. A higher proportion of statin users were receiving high-potency therapy among participants testing positive for SARS-CoV-2 (table 1).
Table 1.
Characteristics of VHA veterans with and without a positive respiratory swab for SARS-CoV-2 (1 March 2020–10 March 2021), stratified by presence of an active statin prescription at enrolment
| Overall | No positive respiratory swab for SARS-CoV-2 | ≥1 positive respiratory swab for SARS-CoV-2 | ||||||||
| n=4 801 406 | No statin prescription | Active statin prescription | No statin prescription | Active statin prescription | ||||||
| n=3 180 888 | n=1 389 364 | n=161 891 | n=69 263 | |||||||
| Age, years | 61.6 | ±16.7 | 58.3 | ±17.7 | 69.3 | ±10.8 | 57.8 | ±17.5 | 68.0 | ±11.4 |
| Age category, years | ||||||||||
| 19–39 | 661 777 | 14% | 613 885 (19) | 19% | 15 272 (1) | 1% | 31 645 (20) | 20% | 975 (1) | 1% |
| 40–49 | 482 871 | 10% | 402 201 | 13% | 54 467 | 4% | 22 718 | 14% | 3485 | 5% |
| 50–59 | 728 340 | 15% | 516 328 | 16% | 172 006 | 12% | 29 099 | 18% | 10 907 | 16% |
| 60–69 | 993 105 | 21% | 600 530 | 19% | 346 361 | 25% | 28 785 | 18% | 17 429 | 25% |
| 70–79 | 1 408 065 | 29% | 735 949 | 23% | 610 855 | 44% | 33 303 | 21% | 27 958 | 40% |
| 80+ | 525 548 | 11% | 311 010 | 10% | 189 825 | 14% | 16 204 | 10% | 8509 | 12% |
| Sex at birth, female | 601 692 | 13% | 509 443 | 16% | 68 275 | 5% | 20 598 | 13% | 3376 | 5% |
| Race/ethnicity | ||||||||||
| White | 3 335 105 | 69% | 2 122 989 | 67% | 1 055 742 | 76% | 106 448 | 66% | 49 926 | 72% |
| Black | 860 829 | 18% | 582 091 | 18% | 226 384 | 16% | 38 080 | 24% | 14 274 | 21% |
| Hispanic | 333 593 | 7% | 230 848 | 7% | 79 866 | 6% | 17 770 | 11% | 5109 | 7% |
| Other | 542 562 | 11% | 430 377 | 14% | 94 575 | 7% | 13 410 | 8% | 4200 | 6% |
| Body mass index, kg/m2 | 30.2 | ±6.09 | 29.8 | ±6.04 | 30.8 | ±6.06 | 30.9 | ±6.35 | 31.9 | ±6.26 |
| Body mass index category, kg/m² | ||||||||||
| <18.5 | 28 116 | 1% | 20 717 | 1% | 6230 | 1% | 951 | 1% | 218 | 0% |
| 18.5–24.9 | 553 988 | 17% | 379 204 | 20% | 152 657 | 14% | 16 249 | 15% | 5878 | 11% |
| 25–29.9 | 1 107 238 | 35% | 687 781 | 36% | 367 799 | 34% | 34 949 | 32% | 16 709 | 30% |
| 30–34.9 | 869 628 | 27% | 507 893 | 26% | 313 277 | 29% | 30 944 | 29% | 17 514 | 32% |
| 35–39.9 | 399 754 | 13% | 224 651 | 12% | 149 833 | 14% | 15 734 | 15% | 9536 | 17% |
| ≥40 | 208 950 | 7% | 113 491 | 6% | 80 708 | 8% | 9077 | 8% | 5674 | 10% |
| Active statin prescription 6 months prior to enrolment | 1 375 009 | 29% | 259 070 | 8% | 1 046 850 | 75% | 17 020 | 11% | 52 069 | 75% |
| Never | 1 747 387 | 36% | 1 338 452 | 42% | 328 440 | 24% | 62 782 | 39% | 17 713 | 26% |
| Former | 1 729 275 | 36% | 984 318 | 31% | 651 438 | 47% | 58 321 | 36% | 35 198 | 51% |
| Current | 1 323 044 | 28% | 857 133 | 27% | 408 908 | 29% | 40 651 | 25% | 16 352 | 24% |
| Urban/rural/highly rural zip code | ||||||||||
| Highly rural | 57 047 | 1% | 34 211 | 1% | 20 620 | 1% | 1360 | 1% | 856 | 1% |
| Rural | 1 561 076 | 33% | 975 607 | 31% | 518 394 | 37% | 43 690 | 27% | 23 385 | 34% |
| Urban | 3 172 176 | 66% | 2 163 063 | 68% | 847 474 | 61% | 116 643 | 72% | 44 996 | 65% |
| Unknown | 9407 | 0% | 7022 | 0% | 2298 | 0% | 61 | 0% | 26 | 0% |
| Estimated glomerular filtration rate, mL/min/1.73 m² | ||||||||||
| ≥90 | 938 310 | 27% | 654 399 | 31% | 235 893 | 20% | 36 230 | 32% | 11 788 | 19% |
| 60–89 | 1 718 393 | 49% | 1 034 287 | 49% | 599 357 | 50% | 54 598 | 48% | 30 151 | 48% |
| 45–59 | 520 635 | 15% | 268 392 | 13% | 226 556 | 19% | 13 799 | 12% | 11 888 | 19% |
| 30–44 | 212 116 | 6% | 100 776 | 5% | 100 175 | 8% | 5626 | 5% | 5539 | 9% |
| 15–29 | 58 464 | 2% | 27 223 | 1% | 27 527 | 2% | 1906 | 2% | 1808 | 3% |
| <15 or dialysis | 25 765 | 1% | 12 803 | 1% | 10 449 | 1% | 1503 | 1% | 1010 | 2% |
| Diabetes | 1 482 197 | 31% | 679 909 | 21% | 716 920 | 52% | 44 364 | 27% | 41 004 | 59% |
| Hypertension | 2 874 378 | 60% | 1 551 529 | 49% | 1 176 398 | 85% | 86 382 | 53% | 60 069 | 87% |
| Cardiovascular disease | 1 749 197 | 36% | 857 912 | 27% | 794 940 | 57% | 53 635 | 33% | 42 710 | 62% |
| Heart failure | 352 710 | 7% | 144 971 | 5% | 183 128 | 13% | 12 388 | 8% | 12 223 | 18% |
| Alcohol use disorder | 909 010 | 19% | 629 005 | 20% | 238 333 | 17% | 31 212 | 19% | 10 460 | 15% |
| Statin prescribed | ||||||||||
| None | 3 341 657 | 70% | 3 179 903 | 100% | 0 | 0% | 161 754 | 100% | 0 | 0% |
| Atorvastatin | 872 981 | 18% | 829 795 | 60% | 43 186 | 62% | ||||
| Fluvastatin | 364 | <1% | 348 | 0% | 16 | 0% | ||||
| Lovastatin | 15 375 | <1% | 14 751 | 1% | 624 | 1% | ||||
| Pitavastatin | 801 | <1% | 748 | <1% | 53 | <1% | ||||
| Pravastatin | 123 779 | 3% | 118 039 | 8% | 5740 | 8% | ||||
| Rosuvastatin | 173 943 | 4% | 165 066 | 12% | 8877 | 13% | ||||
| Simvastatin | 270 806 | 6% | 260 039 | 19% | 10 767 | 16% | ||||
| High-potency statin (vs low or moderate potency)* | 616 824 | 42% | 0 | <1% | 585 224 | 42% | 0 | <1% | 31 600 | 46% |
| Mean hsCRP in the prior 6 months, mg/L† | 17.3 | ±136 | 16.4 | ±151 | 18.2 | ±120 | 19.9 | ±49.6 | 21.0 | ±52.9 |
| hsCRP in the prior 6 months ≥2 mg/L† | 390 796 | 41% | 217 408 | 40% | 145 787 | 42% | 17 407 | 44% | 10 194 | 45% |
| Mean hsCRP at or after the index date, mg/L‡ | 29.3 | ±54.1 | 22.2 | ±46.6 | 25.6 | ±50.8 | 57.8 | ±70.8 | 65.2 | ±71.1 |
| hsCRP at or after the index date ≤2 mg/L‡ | 125 178 | 52% | 61 501 | 46% | 34 630 | 49% | 18 260 | 75% | 10 787 | 79% |
| Outcomes | ||||||||||
| Hospital admission within 30 days | 124 094 | 3% | 61 651 | 2% | 29 953 | 2% | 20 280 | 13% | 12 210 | 18% |
| ICU admission within 30 days | 15 438 | <1% | 5710 | <1% | 3588 | <1% | 3754 | 2% | 2386 | 3% |
| Death within 30 days | 31 409 | 1% | 13 074 | <1% | 6224 | <1% | 7815 | 5% | 4296 | 6% |
Data are presented as mean±SD for continuous variables and n (%) for categorical variables.
P values for global differences in participant characteristics across categories of COVID-19 diagnosis and prior statin use all <0.001.
*Based on estimated % low-density lipoprotein-cholesterol reduction.
†Up to 14 days prior to index date (overall n=958 343).
‡Overall n=224 930.
VHA, Veterans Health Administration.
Among SARS-CoV-2 positive individuals, statin use was associated with lower odds of death at 30 days (OR 0.81 (95% CI 0.77 to 0.85)), but not with hospitalisation or ICU admission. Adjustment for receipt of statin 6 months prior to baseline attenuated the magnitude of the association of statin use at diagnosis with all outcomes (figure 1, tables 2 and 3). Associations with outcomes were similar for individual statins (table 4). Compared with low/moderate intensity statin, high-intensity statin use was associated with higher odds of hospitalisation (1.06 (95% CI 1.01 to 1.10)) but not with ICU admission or death (table 5). Associations of statin use with hospitalisation differed across strata of sex, age, race (black vs non-black) and eGFR (eg, OR for hospitalisation in black participants 0.98 (95% CI 0.92 to 1.03), OR for hospitalisation in non-black participants 0.92 (95% CI 0.89 to 0.95), p for interaction=0.022). Associations of statin use with ICU admission differed across strata of sex and ethnicity (Latinx vs not Latinx) (eg, OR for ICU admission in Latinx participants 0.77 (95% CI 0.62 to 0.95), OR for ICU admissioni in non-Latinx participants 0.94 (95% CI 0.89 to 1.00), p for interaction=0.044). Associations of statin use with mortality differed across strata of age, race/ethnicity (white vs non-white and black vs non-black) and BMI (eg, OR for mortality in black participants: 0.83 (95% CI 0.76 to 0.92), OR for mortality in non-black participants: 0.77 (95% CI 0.74 to 0.81), p for interaction=0.006). Associations did not differ across strata of prevalent diabetes, hypertension or CVD (online supplemental figures 1–3).
Figure 1.
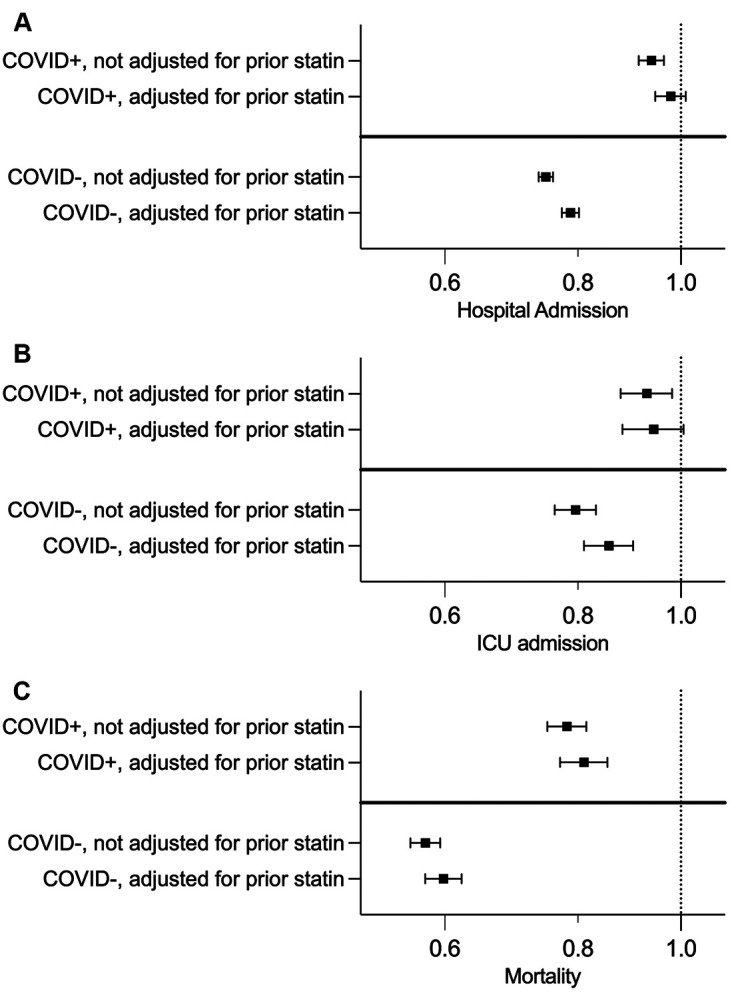
ORs and 95% CIs for associations of statin use at study enrolment with: (A) hospitalisation, (B) ICU admission and (C) death at 30 days before and after adjustment for statin use 6 months prior to diagnosis among VHA veterans with and without a positive respiratory swab for SARS-CoV-2. All analyses are adjusted for sex, age, race/ethnicity, BMI, tobacco use, facility location, urban/rural status, EGFR and history of diabetes, hypertension, cardiovascular disease, heart failure and alcohol use disorder. BMI, body mass index; ICU, intensive care unit; VHA, Veterans Health Administration.
Table 2.
ORs from logistic regression models testing the association of active statin prescription at enrolment with adverse 30-day outcomes among VHA veterans with and without a positive respiratory swab for SARS-CoV-2, including adjustment for prior statin use
| No positive swab, n=4 568 689 | ≥1 positive swab, n=2 31 017 | |||||||||||
| Hospital admission | ICU admission | Death | Hospital admission | ICU admission | Death | |||||||
| OR | 95% CI | OR | 95% CI | OR | 95% CI | OR | 95% CI | OR | 95% CI | OR | 95% CI | |
| Active statin prescription at enrolment | 0.79 | 0.77 to 0.80 | 0.86 | 0.81 to 0.90 | 0.60 | 0.58 to 0.62 | 0.98 | 0.95 to 1.01 | 0.94 | 0.88 to 1.01 | 0.81 | 0.77 to 0.85 |
| Statin prescription 6 months prior to enrolment | 0.91 | 0.89 to 0.93 | 0.88 | 0.83 to 0.93 | 0.93 | 0.9 to 0.97 | 0.93 | 0.9 to 0.96 | 0.97 | 0.91 to 1.04 | 0.94 | 0.89 to 0.99 |
| Sex at birth, female | 0.73 | 0.71 to 0.75 | 0.73 | 0.67 to 0.8 | 0.63 | 0.58 to 0.69 | 0.75 | 0.71 to 0.79 | 0.74 | 0.65 to 0.84 | 0.56 | 0.50 to 0.64 |
| Age category, years | ||||||||||||
| 19–39 | 1.16 | 1.12 to 1.19 | 0.61 | 0.54 to 0.69 | 0.26 | 0.22 to 0.30 | 0.61 | 0.57 to 0.65 | 0.6 | 0.51 to 0.71 | 0.15 | 0.11 to 0.21 |
| 40–49 | 0.97 | 0.94 to 1.01 | 0.81 | 0.73 to 0.91 | 0.46 | 0.40 to 0.54 | 0.75 | 0.70 to 0.80 | 0.68 | 0.59 to 0.79 | 0.38 | 0.30 to 0.48 |
| 50–59 | ref | ref | ref | ref | ref | ref | ref | ref | ref | ref | ref | ref |
| 60–69 | 1.01 | 0.99 to 1.03 | 1.16 | 1.08 to 1.25 | 1.95 | 1.81 to 2.10 | 1.29 | 1.23 to 1.34 | 1.32 | 1.21 to 1.46 | 2.86 | 2.55 to 3.20 |
| 70–79 | 0.81 | 0.79 to 0.83 | 1.00 | 0.92 to 1.08 | 2.6 | 2.41 to 2.79 | 1.43 | 1.37 to 1.49 | 1.49 | 1.36 to 1.64 | 5.93 | 5.32 to 6.61 |
| ≥80 | 0.96 | 0.93 to 0.99 | 0.95 | 0.87 to 1.04 | 5.06 | 4.68 to 5.46 | 1.81 | 1.71 to 1.91 | 1.62 | 1.45 to 1.82 | 13.86 | 12.37 to 15.53 |
| White (vs not white) | 1.21 | 1.14 to 1.28 | 1.23 | 1.02 to 1.49 | 1.1 | 0.95 to 1.27 | 0.88 | 0.81 to 0.97 | 0.74 | 0.61 to 0.91 | 0.87 | 0.74 to 1.04 |
| Black vs(not Black) | 1.49 | 1.40 to 1.58 | 1.53 | 1.26 to 1.85 | 1.05 | 0.90 to 1.22 | 1.36 | 1.24 to 1.50 | 1.10 | 0.89 to 1.35 | 0.78 | 0.66 to 0.94 |
| Hispanic (vs not Hispanic) | 1.07 | 1.04 to 1.10 | 1.23 | 1.13 to 1.35 | 1.07 | 0.99 to 1.14 | 1.16 | 1.10 to 1.22 | 1.03 | 0.92 to 1.15 | 1.13 | 1.04 to 1.24 |
| Body mass index category, kg/m² | ||||||||||||
| <18.5 | 1.35 | 1.29 to 1.42 | 1.51 | 1.31 to 1.73 | 2.48 | 2.33 to 2.65 | 1.14 | 1.01 to 1.29 | 1.35 | 1.06 to 1.72 | 1.81 | 1.59 to 2.07 |
| 18.5–24.9 | ref | ref | ref | ref | ref | ref | ref | ref | ref | ref | ref | ref |
| 25–29.9 | 0.75 | 0.73 to 0.76 | 0.70 | 0.65 to 0.74 | 0.54 | 0.52 to 0.56 | 0.81 | 0.78 to 0.85 | 0.89 | 0.82 to 0.97 | 0.73 | 0.68 to 0.79 |
| 30–34.9 | 0.67 | 0.65 to 0.68 | 0.62 | 0.57 to 0.66 | 0.42 | 0.40 to 0.44 | 0.76 | 0.73 to 0.80 | 0.88 | 0.81 to 0.96 | 0.69 | 0.64 to 0.74 |
| 35–39.9 | 0.63 | 0.61 to 0.65 | 0.57 | 0.52 to 0.62 | 0.37 | 0.35 to 0.40 | 0.76 | 0.72 to 0.80 | 0.85 | 0.76 to 0.94 | 0.64 | 0.59 to 0.70 |
| ≥40 | 0.65 | 0.63 to 0.67 | 0.59 | 0.53 to 0.65 | 0.43 | 0.39 to 0.46 | 0.87 | 0.82 to 0.93 | 1.03 | 0.91 to 1.16 | 0.80 | 0.72 to 0.88 |
| Tobacco use | ||||||||||||
| Never | ref | ref | ref | ref | ref | ref | ref | ref | ref | ref | ref | ref |
| Former | 1.25 | 1.23 to 1.28 | 1.12 | 1.06 to 1.19 | 1.09 | 1.04 to 1.13 | 1.11 | 1.08 to 1.15 | 1.10 | 1.02 to 1.17 | 1.18 | 1.12 to 1.24 |
| Current | 2.02 | 1.98 to 2.06 | 1.76 | 1.66 to 1.87 | 1.67 | 1.60 to 1.74 | 1.39 | 1.35 to 1.44 | 1.29 | 1.20 to 1.39 | 1.24 | 1.17 to 1.32 |
| Urban/rural/highly rural residence | ||||||||||||
| Highly rural | 0.68 | 0.64 to 0.73 | 0.98 | 0.82 to 1.18 | 0.92 | 0.81 to 1.05 | 0.58 | 0.50 to 0.66 | 0.74 | 0.54 to 1.00 | 1.16 | 0.98 to 1.38 |
| Rural | 0.74 | 0.73 to 0.75 | 0.74 | 0.70 to 0.78 | 0.89 | 0.87 to 0.92 | 0.70 | 0.68 to 0.72 | 0.88 | 0.82 to 0.93 | 1.03 | 0.99 to 1.08 |
| Urban | ref | ref | ref | ref | ref | ref | ref | ref | ref | ref | ref | ref |
| Unknown | 0.25 | 0.18 to 0.35 | 0.35 | 0.13 to 0.94 | 0.80 | 0.51 to 1.25 | 0.27 | 0.10 to 0.75 | 0.55 | 0.08 to 3.99 | 1.77 | 0.73 to 4.30 |
| Diabetes | 1.18 | 1.16 to 1.20 | 1.26 | 1.21 to 1.32 | 1.41 | 1.36 to 1.45 | 1.30 | 1.27 to 1.34 | 1.26 | 1.19 to 1.34 | 1.37 | 1.31 to 1.43 |
| Hypertension | 1.22 | 1.19 to 1.24 | 1.30 | 1.22 to 1.39 | 1.09 | 1.04 to 1.14 | 1.30 | 1.25 to 1.35 | 1.29 | 1.19 to 1.41 | 0.96 | 0.90 to 1.02 |
| Cardiovascular disease | 2.04 | 2.01 to 2.08 | 2.69 | 2.55 to 2.84 | 1.88 | 1.81 to 1.95 | 1.84 | 1.79 to 1.90 | 2.08 | 1.95 to 2.23 | 1.24 | 1.18 to 1.30 |
| Heart failure | 2.13 | 2.09 to 2.16 | 2.34 | 2.22 to 2.46 | 2.51 | 2.42 to 2.59 | 1.64 | 1.59 to 1.70 | 1.53 | 1.43 to 1.63 | 1.31 | 1.25 to 1.38 |
| Alcohol use disorder | 1.12 | 1.10 to 1.14 | 1.03 | 0.97 to 1.09 | 0.78 | 0.75 to 0.82 | 0.75 | 0.72 to 0.78 | 0.86 | 0.79 to 0.93 | 0.68 | 0.64 to 0.73 |
| Estimated glomerular filtration rate, mL/min/1.73 m² | ||||||||||||
| ≥90 | ref | ref | ref | ref | ref | ref | ref | ref | ref | ref | ref | ref |
| 60–89 | 0.80 | 0.79 to 0.82 | 0.80 | 0.76 to 0.85 | 0.60 | 0.57 to 0.62 | 0.90 | 0.87 to 0.93 | 0.96 | 0.88 to 1.04 | 1.10 | 1.02 to 1.18 |
| 45–59 | 0.84 | 0.81 to 0.86 | 0.84 | 0.78 to 0.91 | 0.71 | 0.67 to 0.74 | 0.99 | 0.94 to 1.03 | 1.00 | 0.91 to 1.10 | 1.44 | 1.33 to 1.56 |
| 30–44 | 0.93 | 0.90 to 0.96 | 0.92 | 0.84 to 1.01 | 0.99 | 0.93 to 1.05 | 1.10 | 1.04 to 1.16 | 1.14 | 1.02 to 1.29 | 1.83 | 1.68 to 1.99 |
| 15–29 | 1.15 | 1.10 to 1.20 | 1.14 | 1.01 to 1.29 | 2.07 | 1.94 to 2.21 | 1.31 | 1.21 to 1.42 | 1.32 | 1.14 to 1.53 | 2.65 | 2.36 to 2.97 |
| <15 or dialysis | 1.60 | 1.52 to 1.69 | 1.77 | 1.56 to 2.00 | 3.07 | 2.85 to 3.32 | 1.46 | 1.33 to 1.60 | 1.51 | 1.29 to 1.77 | 2.48 | 2.16 to 2.85 |
Models additionally adjusted for index month and geographic location by Veterans Integrated Service Network location.
Table 3.
ORs from logistic regression models testing the association of active statin prescription at enrolment with adverse 30-day outcomes among VHA veterans with and without a positive respiratory swab for SARS-CoV-2, without adjustment for prior statin use
| No positive swab, n=4 568 689 | ≥1 positive swab, n=231 017 | |||||||||||
| Hospital admission | ICU admission | Death | Hospital admission | ICU admission | Death | |||||||
| OR | 95% CI | OR | 95% CI | OR | 95% CI | OR | 95% CI | OR | 95% CI | OR | 95% CI | |
| Active statin prescription at enrolment | 0.75 | 0.74 to 0.76 | 0.8 | 0.76 to 0.83 | 0.58 | 0.56 to 0.59 | 0.94 | 0.91 to 0.96 | 0.93 | 0.88 to 0.98 | 0.78 | 0.75 to 0.82 |
| Sex at birth, female | 0.73 | 0.71 to 0.75 | 0.73 | 0.67 to 0.81 | 0.63 | 0.58 to 0.69 | 0.75 | 0.71 to 0.79 | 0.74 | 0.65 to 0.84 | 0.57 | 0.5 to 0.65 |
| Age category, years | ||||||||||||
| 19–39 | 1.16 | 1.13 to 1.2 | 0.61 | 0.54 to 0.69 | 0.26 | 0.22 to 0.3 | 0.61 | 0.58 to 0.66 | 0.6 | 0.51 to 0.71 | 0.15 | 0.11 to 0.21 |
| 40–49 | 0.98 | 0.95 to 1.01 | 0.82 | 0.73 to 0.92 | 0.47 | 0.4 to 0.54 | 0.75 | 0.71 to 0.8 | 0.68 | 0.59 to 0.79 | 0.38 | 0.3 to 0.48 |
| 50–59 | ref | ref | ref | ref | ref | ref | ref | ref | ref | ref | ref | ref |
| 60–69 | 1.01 | 0.98 to 1.03 | 1.16 | 1.08 to 1.25 | 1.95 | 1.81 to 2.1 | 1.28 | 1.23 to 1.34 | 1.32 | 1.21 to 1.45 | 2.86 | 2.55 to 3.2 |
| 70–79 | 0.81 | 0.79 to 0.83 | 0.99 | 0.92 to 1.07 | 2.59 | 2.41 to 2.78 | 1.43 | 1.36 to 1.49 | 1.49 | 1.36 to 1.64 | 5.92 | 5.31 to 6.6 |
| ≥80 | 0.96 | 0.93 to 0.99 | 0.95 | 0.87 to 1.04 | 5.06 | 4.68 to 5.46 | 1.81 | 1.71 to 1.91 | 1.62 | 1.45 to 1.82 | 13.86 | 12.37 to 15.53 |
| White (vs not white) | 1.2 | 1.14 to 1.28 | 1.23 | 1.02 to 1.49 | 1.1 | 0.95 to 1.27 | 0.88 | 0.81 to 0.97 | 0.75 | 0.61 to 0.91 | 0.87 | 0.74 to 1.04 |
| Black vs (not Black) | 1.49 | 1.4 to 1.58 | 1.53 | 1.26 to 1.86 | 1.05 | 0.9 to 1.22 | 1.37 | 1.24 to 1.5 | 1.1 | 0.89 to 1.35 | 0.78 | 0.66 to 0.94 |
| Hispanic (vs not Hispanic) | 1.07 | 1.04 to 1.1 | 1.23 | 1.13 to 1.35 | 1.06 | 0.99 to 1.14 | 1.16 | 1.1 to 1.22 | 1.03 | 0.92 to 1.15 | 1.13 | 1.04 to 1.24 |
| Body mass index category, kg/m² | ||||||||||||
| <18.5 | 1.35 | 1.29 to 1.42 | 1.51 | 1.32 to 1.74 | 2.49 | 2.33 to 2.65 | 1.14 | 1.02 to 1.29 | 1.35 | 1.06 to 1.72 | 1.82 | 1.59 to 2.07 |
| 18.5–24.9 | ref | ref | ref | ref | ref | ref | ref | ref | ref | ref | ref | ref |
| 25–29.9 | 0.75 | 0.73 to 0.76 | 0.7 | 0.65 to 0.74 | 0.54 | 0.52 to 0.56 | 0.81 | 0.78 to 0.85 | 0.89 | 0.82 to 0.97 | 0.73 | 0.68 to 0.79 |
| 30–34.9 | 0.67 | 0.65 to 0.68 | 0.61 | 0.57 to 0.66 | 0.42 | 0.4 to 0.44 | 0.76 | 0.73 to 0.8 | 0.88 | 0.81 to 0.96 | 0.68 | 0.64 to 0.74 |
| 35–39.9 | 0.63 | 0.61 to 0.64 | 0.57 | 0.52 to 0.62 | 0.37 | 0.35 to 0.4 | 0.76 | 0.72 to 0.8 | 0.85 | 0.76 to 0.94 | 0.64 | 0.59 to 0.7 |
| ≥40 | 0.65 | 0.63 to 0.67 | 0.58 | 0.53 to 0.65 | 0.43 | 0.39 to 0.46 | 0.87 | 0.81 to 0.92 | 1.03 | 0.91 to 1.16 | 0.79 | 0.72 to 0.88 |
| Tobacco use | ||||||||||||
| Never | ref | ref | ref | ref | ref | ref | ref | ref | ref | ref | ref | ref |
| Former | 1.25 | 1.22 to 1.27 | 1.12 | 1.05 to 1.19 | 1.09 | 1.04 to 1.13 | 1.11 | 1.08 to 1.15 | 1.1 | 1.02 to 1.17 | 1.18 | 1.12 to 1.24 |
| Current | 2.02 | 1.98 to 2.05 | 1.76 | 1.66 to 1.87 | 1.66 | 1.6 to 1.74 | 1.39 | 1.35 to 1.44 | 1.29 | 1.2 to 1.39 | 1.24 | 1.17 to 1.32 |
| Urban/rural/highly rural residence | ||||||||||||
| Highly rural | 0.68 | 0.64 to 0.73 | 0.98 | 0.82 to 1.18 | 0.92 | 0.81 to 1.05 | 0.57 | 0.5 to 0.66 | 0.74 | 0.54 to 1 | 1.16 | 0.98 to 1.38 |
| Rural | 0.74 | 0.73 to 0.75 | 0.74 | 0.7 to 0.78 | 0.89 | 0.86 to 0.92 | 0.7 | 0.68 to 0.72 | 0.87 | 0.82 to 0.93 | 1.03 | 0.99 to 1.08 |
| Urban | ref | ref | ref | ref | ref | ref | ref | ref | ref | ref | ref | ref |
| Unknown | 0.25 | 0.18 to 0.35 | 0.35 | 0.13 to 0.94 | 0.8 | 0.51 to 1.25 | 0.27 | 0.1 to 0.74 | 0.55 | 0.08 to 3.98 | 1.75 | 0.72 to 4.27 |
| Diabetes | 1.17 | 1.15 to 1.19 | 1.25 | 1.2 to 1.31 | 1.4 | 1.36 to 1.45 | 1.3 | 1.26 to 1.33 | 1.26 | 1.19 to 1.33 | 1.36 | 1.3 to 1.42 |
| Hypertension | 1.21 | 1.19 to 1.24 | 1.29 | 1.21 to 1.38 | 1.09 | 1.04 to 1.13 | 1.3 | 1.25 to 1.35 | 1.29 | 1.18 to 1.41 | 0.96 | 0.9 to 1.02 |
| Cardiovascular disease | 2.03 | two to 2.07 | 2.67 | 2.53 to 2.82 | 1.87 | 1.8 to 1.94 | 1.84 | 1.78 to 1.89 | 2.08 | 1.94 to 2.23 | 1.24 | 1.18 to 1.3 |
| Heart failure | 2.12 | 2.08 to 2.16 | 2.33 | 2.21 to 2.45 | 2.5 | 2.41 to 2.59 | 1.64 | 1.59 to 1.7 | 1.53 | 1.43 to 1.63 | 1.31 | 1.25 to 1.38 |
| Alcohol use disorder | 1.12 | 1.1 to 1.14 | 1.03 | 0.97 to 1.09 | 0.78 | 0.75 to 0.82 | 0.75 | 0.73 to 0.78 | 0.86 | 0.79 to 0.93 | 0.68 | 0.64 to 0.73 |
| Estimated glomerular filtration rate, mL/min/1.73 m² | ||||||||||||
| ≥90 | ref | ref | ref | ref | ref | ref | ref | ref | ref | ref | ref | ref |
| 60–89 | 0.8 | 0.79 to 0.82 | 0.8 | 0.75 to 0.85 | 0.6 | 0.57 to 0.62 | 0.9 | 0.86 to 0.93 | 0.96 | 0.88 to 1.04 | 1.09 | 1.02 to 1.18 |
| 45–59 | 0.84 | 0.81 to 0.86 | 0.84 | 0.78 to 0.91 | 0.71 | 0.67 to 0.74 | 0.98 | 0.94 to 1.03 | 1 | 0.91 to 1.09 | 1.44 | 1.33 to 1.56 |
| 30–44 | 0.93 | 0.9 to 0.96 | 0.92 | 0.84 to 1.01 | 0.99 | 0.93 to 1.05 | 1.1 | 1.04 to 1.16 | 1.14 | 1.02 to 1.29 | 1.83 | 1.68 to 1.99 |
| 15–29 | 1.14 | 1.09 to 1.2 | 1.14 | 1.01 to 1.29 | 2.07 | 1.94 to 2.21 | 1.31 | 1.21 to 1.42 | 1.32 | 1.14 to 1.53 | 2.65 | 2.36 to 2.97 |
| <15 or dialysis | 1.6 | 1.52 to 1.69 | 1.77 | 1.56 to 2.01 | 3.08 | 2.85 to 3.32 | 1.46 | 1.33 to 1.6 | 1.51 | 1.29 to 1.77 | 2.48 | 2.16 to 2.85 |
ICU, intensive care unit; VHA, Veterans Health Administration.
Table 4.
ORs from logistic regression models testing the association of specific statins compared with no statin with adverse 30-day outcomes among VHA veterans with a positive respiratory swab for SARS-CoV-2, n=231 017
| Hospital admission | ICU admission | Death | |||||||
| OR | 95% CI | P value | OR | 95% CI | P value | OR | 95% CI | P value | |
| No statin | ref | ref | ref | ||||||
| Atorvastatin | 0.98 | 0.95 to 1.01 | 0.136 | 0.96 | 0.9 to 1.02 | 0.194 | 0.8 | 0.76 to 0.84 | <0.001 |
| Fluvastatin | 1.47 | 0.45 to 4.82 | 0.524 | 1.79 | 0.23 to 13.8 | 0.577 | 0.55 | 0.07 to 4.43 | 0.575 |
| Lovastatin | 0.73 | 0.57 to 0.93 | 0.012 | 0.48 | 0.26 to 0.9 | 0.022 | 0.64 | 0.45 to 0.91 | 0.013 |
| Pitavastatin | 0.45 | 0.16 to 1.26 | 0.128 | 0.66 | 0.09 to 4.8 | 0.679 | 0.82 | 0.25 to 2.68 | 0.738 |
| Pravastatin | 0.93 | 0.86 to 1 | 0.045 | 0.94 | 0.81 to 1.1 | 0.443 | 0.78 | 0.7 to 0.87 | <0.001 |
| Rosuvastatin | 0.81 | 0.76 to 0.86 | <0.001 | 0.82 | 0.72 to 0.93 | 0.002 | 0.72 | 0.65 to 0.79 | <0.001 |
| Simvastatin | 0.91 | 0.86 to 0.97 | 0.001 | 0.91 | 0.8 to 1.02 | 0.107 | 0.77 | 0.71 to 0.84 | <0.001 |
| Sex at birth, female | 0.75 | 0.71 to 0.8 | <0.001 | 0.74 | 0.65 to 0.84 | <0.001 | 0.57 | 0.5 to 0.65 | <0.001 |
| Age category, years | |||||||||
| 19–39 | 0.61 | 0.58 to 0.66 | <0.001 | 0.6 | 0.51 to 0.71 | <0.001 | 0.15 | 0.11 to 0.21 | <0.001 |
| 40–49 | 0.75 | 0.71 to 0.8 | <0.001 | 0.68 | 0.59 to 0.79 | <0.001 | 0.38 | 0.3 to 0.48 | <0.001 |
| 50–59 | ref | ref | ref | ||||||
| 60–69 | 1.28 | 1.23 to 1.34 | <0.001 | 1.32 | 1.21 to 1.45 | <0.001 | 2.85 | 2.55 to 3.2 | <0.001 |
| 70–79 | 1.43 | 1.36 to 1.49 | <0.001 | 1.49 | 1.36 to 1.64 | <0.001 | 5.92 | 5.31 to 6.6 | <0.001 |
| ≥80 | 1.81 | 1.71 to 1.91 | <0.001 | 1.62 | 1.45 to 1.82 | <0.001 | 13.86 | 12.37 to 15.54 | <0.001 |
| White (vs not white) | 0.88 | 0.81 to 0.97 | 0.007 | 0.75 | 0.61 to 0.91 | 0.004 | 0.88 | 0.74 to 1.04 | 0.121 |
| Black (vs not black) | 1.36 | 1.24 to 1.5 | <0.001 | 1.1 | 0.89 to 1.35 | 0.39 | 0.78 | 0.66 to 0.94 | 0.007 |
| Hispanic (vs not Hispanic) | 1.16 | 1.1 to 1.22 | <0.001 | 1.03 | 0.92 to 1.15 | 0.633 | 1.13 | 1.04 to 1.24 | 0.007 |
| Body mass index category, kg/m² | |||||||||
| <18.5 | 1.15 | 1.02 to 1.29 | 0.025 | 1.35 | 1.06 to 1.73 | 0.015 | 1.82 | 1.59 to 2.08 | <0.001 |
| 18.5–24.9 | ref | ref | ref | ||||||
| 25–29.9 | 0.81 | 0.78 to 0.85 | <0.001 | 0.89 | 0.82 to 0.97 | 0.006 | 0.73 | 0.68 to 0.79 | <0.001 |
| 30–34.9 | 0.76 | 0.73 to 0.8 | <0.001 | 0.88 | 0.81 to 0.97 | 0.006 | 0.68 | 0.64 to 0.74 | <0.001 |
| 35–39.9 | 0.76 | 0.72 to 0.8 | <0.001 | 0.85 | 0.76 to 0.94 | 0.002 | 0.64 | 0.59 to 0.7 | <0.001 |
| ≥40 | 0.87 | 0.81 to 0.92 | <0.001 | 1.03 | 0.91 to 1.16 | 0.675 | 0.79 | 0.72 to 0.88 | <0.001 |
| Tobacco use | |||||||||
| Never | ref | ||||||||
| Former | 1.11 | 1.08 to 1.15 | <0.001 | 1.1 | 1.02 to 1.17 | 0.01 | 1.18 | 1.12 to 1.24 | <0.001 |
| Current | 1.39 | 1.35 to 1.44 | <0.001 | 1.29 | 1.2 to 1.39 | <0.001 | 1.24 | 1.17 to 1.32 | <0.001 |
| Urban/rural/highly rural residence | |||||||||
| Highly rural | 0.57 | 0.5 to 0.66 | <0.001 | 0.74 | 0.54 to 1 | 0.051 | 1.16 | 0.97 to 1.38 | 0.096 |
| Rural | 0.7 | 0.68 to 0.72 | <0.001 | 0.88 | 0.82 to 0.93 | <0.001 | 1.04 | 0.99 to 1.08 | 0.14 |
| Urban | ref | ref | ref | ||||||
| Unknown | 0.27 | 0.1 to 0.74 | 0.011 | 0.55 | 0.08 to 3.96 | 0.549 | 1.75 | 0.72 to 4.25 | 0.22 |
| Diabetes | 1.29 | 1.26 to 1.33 | <0.001 | 1.26 | 1.19 to 1.33 | <0.001 | 1.36 | 1.3 to 1.42 | <0.001 |
| Hypertension | 1.3 | 1.25 to 1.35 | <0.001 | 1.29 | 1.18 to 1.41 | <0.001 | 0.96 | 0.9 to 1.02 | 0.149 |
| Cardiovascular disease | 1.84 | 1.78 to 1.89 | <0.001 | 2.08 | 1.94 to 2.23 | <0.001 | 1.24 | 1.18 to 1.3 | <0.001 |
| Heart failure | 1.64 | 1.58 to 1.69 | <0.001 | 1.53 | 1.43 to 1.63 | <0.001 | 1.31 | 1.25 to 1.38 | <0.001 |
| Alcohol use disorder | 0.75 | 0.73 to 0.78 | <0.001 | 0.86 | 0.79 to 0.93 | <0.001 | 0.69 | 0.64 to 0.73 | <0.001 |
| Estimated glomerular filtration rate, mL/min/1.73 m² | |||||||||
| ≥90 | ref | ref | ref | ||||||
| 60–89 | 0.9 | 0.87 to 0.93 | <0.001 | 0.96 | 0.88 to 1.04 | 0.273 | 1.1 | 1.02 to 1.18 | 0.018 |
| 45–59 | 0.99 | 0.94 to 1.03 | 0.519 | 1 | 0.91 to 1.1 | 0.941 | 1.44 | 1.33 to 1.56 | <0.001 |
| 30–44 | 1.1 | 1.04 to 1.16 | 0.001 | 1.15 | 1.02 to 1.29 | 0.024 | 1.83 | 1.68 to 1.99 | <0.001 |
| 15–29 | 1.31 | 1.21 to 1.42 | <0.001 | 1.32 | 1.14 to 1.53 | <0.001 | 2.65 | 2.36 to 2.97 | <0.001 |
| <15 or dialysis | 1.45 | 1.32 to 1.59 | <0.001 | 1.51 | 1.29 to 1.77 | <0.001 | 2.48 | 2.16 to 2.84 | <0.001 |
Models additionally adjusted for month of diagnosis and geographic location by Veterans Integrated Service Network location; not adjusted for the presence of an active statin prescription 6 months prior to enrolment.
ICU, intensive care unit; VHA, Veterans Health Administration.
Table 5.
ORs from logistic regression models testing the association of low or moderate potency versus high-potency active statin prescription at enrolment with adverse 30-day outcomes among VHA veterans with a positive respiratory swab for SARS-CoV-2, n=69 263
| Hospital admission | ICU admission | Death | |||||||
| OR | 95% CI | P value | OR | 95% CI | P value | OR | 95% CI | P value | |
| High-potency statin | 1.06 | 1.01 to 1.1 | 0.011 | 1.05 | 0.96 to 1.15 | 0.258 | 0.97 | 0.91 to 1.04 | 0.407 |
| Sex at birth, female | 0.89 | 0.8 to 1 | 0.041 | 0.95 | 0.75 to 1.19 | 0.634 | 0.52 | 0.4 to 0.68 | <0.001 |
| Age category, years | |||||||||
| 19–39 | 0.82 | 0.63 to 1.06 | 0.123 | 0.46 | 0.22 to 0.98 | 0.045 | 0.1 | 0.01 to 0.73 | 0.023 |
| 40–49 | 0.74 | 0.64 to 0.86 | <0.001 | 0.55 | 0.38 to 0.79 | 0.001 | 0.45 | 0.27 to 0.75 | 0.002 |
| 50–59 | ref | ref | ref | ||||||
| 60–69 | 1.3 | 1.2 to 1.4 | <0.001 | 1.24 | 1.06 to 1.45 | 0.009 | 2.45 | 2.02 to 2.96 | <0.001 |
| 70–79 | 1.47 | 1.36 to 1.58 | <0.001 | 1.47 | 1.25 to 1.72 | <0.001 | 4.42 | 3.67 to 5.32 | <0.001 |
| ≥80 | 1.95 | 1.78 to 2.15 | <0.001 | 1.69 | 1.4 to 2.04 | <0.001 | 9.54 | 7.84 to 11.6 | <0.001 |
| White (vs not white) | 0.81 | 0.69 to 0.96 | 0.012 | 0.75 | 0.52 to 1.08 | 0.118 | 0.85 | 0.64 to 1.11 | 0.221 |
| Black vs (not Black) | 1.31 | 1.1 to 1.55 | 0.002 | 1.12 | 0.77 to 1.63 | 0.545 | 0.8 | 0.6 to 1.06 | 0.125 |
| Hispanic (vs not Hispanic) | 1.12 | 1.02 to 1.22 | 0.013 | 0.91 | 0.75 to 1.1 | 0.33 | 1.2 | 1.04 to 1.38 | 0.014 |
| Body mass index category, kg/m² | |||||||||
| <18.5 | 1.04 | 0.79 to 1.38 | 0.766 | 1.19 | 0.71 to 1.98 | 0.511 | 1.45 | 1.02 to 2.05 | 0.039 |
| 18.5–24.9 | ref | ref | ref | ||||||
| 25–29.9 | 0.81 | 0.75 to 0.86 | <0.001 | 0.89 | 0.77 to 1.02 | 0.095 | 0.78 | 0.7 to 0.87 | <0.001 |
| 30–34.9 | 0.78 | 0.73 to 0.84 | <0.001 | 0.92 | 0.8 to 1.07 | 0.272 | 0.78 | 0.7 to 0.87 | <0.001 |
| 35–39.9 | 0.78 | 0.71 to 0.85 | <0.001 | 0.9 | 0.76 to 1.06 | 0.188 | 0.75 | 0.67 to 0.85 | <0.001 |
| ≥40 | 0.86 | 0.77 to 0.95 | 0.002 | 1.08 | 0.89 to 1.3 | 0.452 | 0.91 | 0.78 to 1.06 | 0.221 |
| Tobacco use | |||||||||
| Never | ref | ref | ref | ||||||
| Former | 1.18 | 1.12 to 1.25 | <0.001 | 1.16 | 1.03 to 1.3 | 0.013 | 1.29 | 1.18 to 1.4 | <0.001 |
| Current | 1.36 | 1.28 to 1.44 | <0.001 | 1.36 | 1.19 to 1.55 | <0.001 | 1.21 | 1.09 to 1.34 | <0.001 |
| Urban/rural/highly rural residence | |||||||||
| Highly rural | 0.59 | 0.47 to 0.73 | <0.001 | 0.93 | 0.61 to 1.42 | 0.728 | 1.4 | 1.08 to 1.82 | 0.011 |
| Rural | 0.68 | 0.65 to 0.72 | <0.001 | 0.87 | 0.79 to 0.96 | 0.006 | 1.05 | 0.97 to 1.12 | 0.233 |
| Urban | ref | ref | ref | ||||||
| Unknown | 0.17 | 0.02 to 1.26 | 0.083 | 1.69 | 0.22 to 12.83 | 0.612 | 1.41 | 0.31 to 6.48 | 0.662 |
| Diabetes | 1.29 | 1.23 to 1.35 | <0.001 | 1.16 | 1.05 to 1.27 | 0.003 | 1.31 | 1.22 to 1.41 | <0.001 |
| Hypertension | 1.28 | 1.18 to 1.39 | <0.001 | 1.38 | 1.14 to 1.67 | 0.001 | 0.98 | 0.85 to 1.11 | 0.704 |
| Cardiovascular disease | 1.71 | 1.62 to 1.8 | <0.001 | 1.96 | 1.75 to 2.21 | <0.001 | 1.25 | 1.14 to 1.36 | <0.001 |
| Heart failure | 1.68 | 1.6 to 1.77 | <0.001 | 1.58 | 1.44 to 1.74 | <0.001 | 1.33 | 1.23 to 1.43 | <0.001 |
| Alcohol use disorder | 0.66 | 0.62 to 0.71 | <0.001 | 0.81 | 0.71 to 0.94 | 0.004 | 0.69 | 0.61 to 0.77 | <0.001 |
| Estimated glomerular filtration rate, mL/min/1.73 m² | |||||||||
| ≥90 | ref | ref | ref | ||||||
| 60–89 | 0.98 | 0.92 to 1.05 | 0.625 | 1.05 | 0.91 to 1.2 | 0.538 | 1.14 | 1.01 to 1.29 | 0.041 |
| 45–59 | 1.08 | one to 1.16 | 0.05 | 1.05 | 0.9 to 1.24 | 0.53 | 1.58 | 1.37 to 1.82 | <0.001 |
| 30–44 | 1.21 | 1.1 to 1.32 | <0.001 | 1.2 | one to 1.44 | 0.055 | 2 | 1.72 to 2.33 | <0.001 |
| 15–29 | 1.53 | 1.35 to 1.72 | <0.001 | 1.37 | 1.09 to 1.72 | 0.007 | 3.19 | 2.69 to 3.78 | <0.001 |
| <15 or dialysis | 1.64 | 1.42 to 1.9 | <0.001 | 1.95 | 1.52 to 2.5 | <0.001 | 3.01 | 2.44 to 3.73 | <0.001 |
Models additionally adjusted for month of diagnosis and geographic location by Veterans Integrated Service Network location; not adjusted for the presence of an active statin prescription 6 months prior to enrolment.
ICU, intensive care unit; VHA, Veterans Health Administration.
bmjopen-2021-058363supp001.pdf (346.4KB, pdf)
bmjopen-2021-058363supp002.pdf (354.9KB, pdf)
bmjopen-2021-058363supp003.pdf (352.1KB, pdf)
Compared with persons with SARS-CoV-2 infection, OR for all three outcomes were significantly lower in persons without SARS-CoV-2 infection, as reflected by p<0.001 for the interaction term of SARS-CoV-2*statin use in all three models. Among SARS-COV-2 negative individuals, statin use was associated with lower odds of hospitalisation (OR 0.79 (95% CI 0.77 to 0.80)), ICU admission (OR 0.86 (95% CI 0.81 to 0.90)) and death at 30 days (OR 0.60 (95% CI 0.58 to 0.62)) (table 2).
Discussion
In this cohort of US Veterans with (n=231 154) and without (n=4 570 252) a positive respiratory swab for SARS-CoV-2, statin use was independently associated with lower odds of death at 30 days compared with no statin use, but this association over a similar time period was significantly stronger among veterans without a positive respiratory swab for SARS-CoV-2. Among individuals with and without a positive respiratory swab for SARS-CoV-2, adjusting for prior statin use attenuated the association of statin use with all outcomes; however, in every case, the magnitude of the association remained substantially greater among individuals without a diagnosis of COVID-19. Associations were similar for specific statins, and receipt of high-potency statin was not associated with lower odds of any outcome compared with moderate and low potency, except for a small difference in the odds of hospitalisation. Associations were not significantly different in strata of prevalent diabetes, hypertension or CVD. Furthermore, the lack of a gradient of effect with statin potency also does not support a potential causal benefit of statin use. Taken together, these results suggest that while statin use is associated with lower mortality among individuals with a positive swab for SARS-CoV-2, the benefit is actually smaller for than it is for those without evidence of SARS-CoV-2 infection and does not support a possible anti-COVID effect of statin treatment. It is important to note, however, that the current study does not demonstrate a harmful effect of statin use among individuals with COVID-19, only that statins may not exert a SARS-CoV-2-specific protective effect and/or that positive findings in previous observational studies may be due to residual confounding. Current findings therefore do not support statin cessation among individuals with COVID-19.
Use of negative controls is an important technique to detect confounding or other sources of bias in epidemiological studies16 that has gone underused in the era of COVID-19 research. An instructive example is the association of pneumonia or influenza vaccination with all-cause mortality seen in elderly individuals despite rigorous control for confounding by factors related to overall health status.17 Using negative controls, Jackson et al18 examined the association of vaccination with a negative control outcome: mortality prior to influenza season. They found a stronger association with mortality during the period prior to influenza season compared with during or after, a biologically implausible result that was attributed by the authors to preferential receipt of vaccines by healthy individuals. This source of bias is now recognised in studies of this topic.19 While the use of a negative control outcome is not precisely analogous to the methods used in the current study, the example can inform interpretation of the current findings.
Several recent systematic reviews and meta-analyses have examined the association of prior statin use with short-term outcomes after COVID-19.2 20–25 Many of these reported an inverse association of statin use at diagnosis with mortality. For example, statin use was associated with a lower hazard of death (HR 0.72 (95% CI 0.69 to 0.75)) in a large population-based study of English patients with diabetes independent of age and comorbid CVD.26 In a recent nationwide US study of hospitalised individuals (n=10 541), outpatient statin, either alone or with blood pressure-lowering medications, was associated with lower odds of in-hospital death (OR 0.59 (95% CI 0.50 to 0.69)). The magnitude of the association of statin use at diagnosis with mortality reported in these and other analyses is quite similar to the OR in the current report among individuals with COVID-19 in models that were not adjusted for prior statin use (OR for death at 30 days 0.78 (95% CI 0.75 to 0.82)), likely reflecting similar strategies for confounder adjustment. The lower COVID-19 mortality risk among statin users, however, is not a universal finding. In fact, among French hospitalised patients with diabetes, statin use at diagnosis was associated with higher odds of death at 28 days (OR 1.46 (95% CI 1.08 to 1.95)).27 Reasons for these disparate findings are unclear but may be due in part to differences in timing, as early in the pandemic, treatments such as dexamethasone and remdesivir were not widely used. Consistent with this, in the French cohort mortality was about 21% at 28 days, considerably higher than our overall 30-day mortality rate of about 7%. No prior study to our knowledge has examined outcomes following statin use comparing SARS-CoV-2 infected and uninfected statin users.
We did not examine in-hospital statin continuation in the current analysis—a question that remains unaddressed—but instead focused on the association between statin use prior to COVID-19 diagnosis and outcomes, where use of this medication would not have been confounded by the onset of COVID-19. Methodological issues (most importantly residual confounding by indication and heterogeneity of the populations studied) limit the conclusions that can be drawn from earlier observational studies of statin continuation at hospitalisation. Masana et al28 examined associations of statin use with in-hospital mortality in a cohort of hospitalised Spanish patients with a positive test for SARS-CoV-2 comparing statin non-users, users who continued statins during hospitalisation and users who stopped statins during hospitalisation. Overall, 25.7% of non-users died, while 19.8% of continued users died and 17.4% of stoppers died. In that analysis, matching was used to account for differences in preadmission characteristics; however, the authors were not able to account for characteristics (eg, severity of COVID-19 illness, perceived prognosis, goals of care, etc) that might impact the decision to stop statin therapy at the time of admission. In a meta-analysis, Permana et al21 examined associations of preadmission statin use and in-hospital statin use among patients hospitalised after a positive test for SARS-CoV-2, which is a related question. In-hospital but not preadmission statin use was associated with a lower risk of mortality; however, these preadmission and in-hospital study populations differed in characteristics such as age and sex that are strongly associated with adverse COVID-19 outcomes, limiting direct comparisons between the groups. Given the many possible determinants of statin cessation or continuation following the diagnosis of COVID-19 potentially related to adverse outcomes that would be difficult to extract from medical records (electronic or otherwise), the question of whether to cease or initiate statins following COVID-19 diagnosis will be best determined by a clinical trial.
We noted several differences in outcomes associated with statin use by certain characteristics such as sex, age and race (online supplemental figures 1-3). As our main analysis did not show evidence of a lower risk of outcomes associated with statin use confined to COVID-19 infected participants, these interactions likely reflect associations independent of presence of this infection and therefore reflecting effect modification between statin use, stratum variables and outcomes of interest.
Our study has several strengths, most importantly a large, well-characterised national sample. To our knowledge, this is the largest observational study of prior statin use and adverse outcomes from SARS-CoV-2 in the USA (n=4 801 406) as well as the first to formally assess and compare statin effects seen in SARS-CoV-2 infection using a negative control (non-infected statin users). Second, we used several methods designed to mitigate or quantify bias due to unmeasured confounding. We: (1) constructed a DAG to estimate the minimal sufficient adjustment set to estimate the total effect of statin use on 30-day outcomes; (2) compared associations in SARS-CoV-2 infected individuals and an uninfected comparator sample; and (3) conducted dose–response analyses using statin potency to reflect dose. In addition, most VHA enrollees receive medical care and medications without cost, which likely decreases the contribution of unmeasured financial factors to differences in the quality of care received and most importantly to receipt of statin medications. Our results should be considered within the context of several limitations. The VHA population is generally older, with lower income and socioeconomic status29 than the US population as a whole, and our findings may not be generalisable to non-VHA populations. Additionally, the proportion of women was low (13%); however, although women comprised only a small proportion of the sample, the number of female participants (n=601 765) is adequate for robust statistical inference. We were also unable to capture hospitalisations or some outpatient prescriptions that occurred outside VHA. This is an important source of potential bias should propensity to seek outside care be associated with likelihood of receiving a statin, although VHA users are asked to provide notification within 72 hours of an outside hospital admission, and when possible are transferred to a VHA facility, which would then be captured in the VHA electronic health record. Given the timing of this study, we were unable to evaluate mediating or moderating effects of vaccination use due to very limited vaccination coverage of our population by the index date. No data were available on prescription adherence; however, statin discontinuation rates have previously shown to be low in VHA patients relative to discontinuation of other lipid-lowering medications.30 The comparison of all-cause mortality is in our opinion the best outcome by which to assess whether statin use benefitted patients with versus without SARS-CoV-2 infection. The comparison of admission to hospital or ICU is of less value given that the reasons for hospitalisation likely differed greatly by presence of infection but, nevertheless, are of value in demonstrating that no apparent benefit is seen that might not be reflected in overall mortality. Finally, not all individuals in the comparator group were tested for SARS-CoV-2, so we were unable to exclude the possibility that some SARS-CoV-2 positive participants with asymptomatic or mild disease were misclassified as SARS-CoV-2 negative. We elected to include individuals without SARS-CoV-2 tests because individuals with indications for SARS-CoV-2 testing may represent a particular (and sicker) population than the general group of VA enrollees as a whole. Furthermore, based on the current results, inclusion of individuals with undiagnosed COVID-19 in the SARS-CoV-2 negative comparator group would be expected to attenuate observed differences in the associations of statin use with adverse outcomes between the SARS-CoV-2 infected and negative comparator groups. It is unlikely that exclusion of participants with undiagnosed COVID-19 from the comparator group would have resulted in a reduction in the observed negative association between statin use and mortality, as this would have required an opposite association to be present between undiagnosed COVID-19 infection and mortality, a possibility for which there is little reason or evidence to support.
Conclusions
In conclusion, statin use is associated with lower odds of 30-day mortality both among US Veterans with or without a positive respiratory swab for SARS-CoV-2 indicating that statins may not exert COVID-19 specific beneficial effects.
Supplementary Material
Footnotes
Twitter: @pandoralucrezia
Contributors: PLW conceived the project, designed the overall research plan and wrote the first draft of the manuscript. EL analysed the data and reviewed/edited the manuscript; LAB, LT-P and SEK contributed to the conception of the work and reviewed/edited the manuscript; AK contributed to design/interpretation of the analyses and reviewed/edited the manuscript; AP and GD contributed to the design/interpretation of the analyses and reviewed/edited the manuscript. EJB conceived the project, designed the overall research plan and reviewed/edited the manuscript. PLW and EJB are the guarantors of this work, as such they accept full responsibility for the finished work and/or the conduct of the study, had access to the data, and controlled the decision to publish.
Funding: The work was funded by VA Clinical Science Research & Development (COVID19-8990-19).
Competing interests: None declared.
Provenance and peer review: Not commissioned; externally peer reviewed.
Supplemental material: This content has been supplied by the author(s). It has not been vetted by BMJ Publishing Group Limited (BMJ) and may not have been peer-reviewed. Any opinions or recommendations discussed are solely those of the author(s) and are not endorsed by BMJ. BMJ disclaims all liability and responsibility arising from any reliance placed on the content. Where the content includes any translated material, BMJ does not warrant the accuracy and reliability of the translations (including but not limited to local regulations, clinical guidelines, terminology, drug names and drug dosages), and is not responsible for any error and/or omissions arising from translation and adaptation or otherwise.
Data availability statement
No data are available. Access to VA electronic health records data is limited to researchers with active, VA appointments and an IRB-approved protocol.
Ethics statements
Patient consent for publication
Not applicable.
Ethics approval
This study involves human participants and was approved by VA Puget Sound Health Care System Institutional Review Board approved this study. The approval number is #01897. Requirement for informed consent was waived by the IRB.
References
- 1.Daniels LB, Ren J, Kumar K, et al. Relation of prior statin and anti-hypertensive use to severity of disease among patients hospitalized with COVID-19: findings from the American heart association's COVID-19 cardiovascular disease registry. PLoS One 2021;16:e0254635. 10.1371/journal.pone.0254635 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Pal R, Banerjee M, Yadav U. Statin use and clinical outcomes in patients with COVID-19: an updated systematic review and meta-analysis. Postgrad Med J 2021. [Epub ahead of print: 04 Feb 2021]. 10.1136/postgradmedj-2020-139172 [DOI] [PubMed] [Google Scholar]
- 3.Castiglione V, Chiriacò M, Emdin M, et al. Statin therapy in COVID-19 infection. Eur Heart J Cardiovasc Pharmacother 2020;6:258–9. 10.1093/ehjcvp/pvaa042 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Butt JH, Gerds TA, Schou M, et al. Association between statin use and outcomes in patients with coronavirus disease 2019 (COVID-19): a nationwide cohort study. BMJ Open 2020;10:e044421. 10.1136/bmjopen-2020-044421 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Yetmar ZA, Challener DW, Tleyjeh IM, et al. Association between chronic statin use and 30-day mortality in hospitalized patients with COVID-19. Mayo Clin Proc Innov Qual Outcomes 2021;5:442–6. 10.1016/j.mayocpiqo.2021.02.002 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Rubin R. Could statins do more than lower cholesterol in patients with COVID-19? JAMA 2021;325:2424. 10.1001/jama.2021.8201 [DOI] [PubMed] [Google Scholar]
- 7.U.S. department of Veterans Affairs: VHA, 2020. Available: https://www.va.gov/health/ [Accessed 28 Sep 2020].
- 8.CDW . COVID-19 shared data resource (intranet-only resource), 2020. Available: https://vhacdwdwhweb100.vha.med.va.gov/phenotype/index.php/COVID-19:Shared_Data_Resource [Accessed 28 Sep 2020].
- 9.Grundy SM, Stone NJ, Bailey AL, et al. 2018 AHA/ACC/AACVPR/AAPA/ABC/ACPM/ADA/AGS/APhA/ASPC/NLA/PCNA guideline on the management of blood cholesterol: a report of the American College of Cardiology/American heart association Task force on clinical practice guidelines. Circulation 2019;139:e1082–143. 10.1161/CIR.0000000000000625 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.West AN, Lee RE, Shambaugh-Miller MD, et al. Defining "rural" for veterans' health care planning. J Rural Health 2010;26:301–9. 10.1111/j.1748-0361.2010.00298.x [DOI] [PubMed] [Google Scholar]
- 11.Bush K, Kivlahan DR, McDonell MB, et al. The audit alcohol consumption questions (AUDIT-C): an effective brief screening test for problem drinking. ambulatory care quality improvement project (ACQUIP). alcohol use disorders identification test. Arch Intern Med 1998;158:1789–95. 10.1001/archinte.158.16.1789 [DOI] [PubMed] [Google Scholar]
- 12.Quan H, Sundararajan V, Halfon P, et al. Coding algorithms for defining comorbidities in ICD-9-CM and ICD-10 administrative data. Med Care 2005;43:1130–9. 10.1097/01.mlr.0000182534.19832.83 [DOI] [PubMed] [Google Scholar]
- 13.Young BA, Katz R, Boulware LE, et al. Risk factors for rapid kidney function decline among African Americans: the Jackson heart study (JHS). Am J Kidney Dis 2016;68:229–39. 10.1053/j.ajkd.2016.02.046 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Sohn M-W, Arnold N, Maynard C, et al. Accuracy and completeness of mortality data in the Department of Veterans Affairs. Popul Health Metr 2006;4:2. 10.1186/1478-7954-4-2 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Textor J, van der Zander B, Gilthorpe MS, et al. Robust causal inference using directed acyclic graphs: the R package 'dagitty'. Int J Epidemiol 2016;45:1887–94. 10.1093/ije/dyw341 [DOI] [PubMed] [Google Scholar]
- 16.Lipsitch M, Tchetgen Tchetgen E, Cohen T. Negative controls: a tool for detecting confounding and bias in observational studies. Epidemiology 2010;21:383–8. 10.1097/EDE.0b013e3181d61eeb [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Rivetti D, Jefferson T, Thomas R, et al. Vaccines for preventing influenza in the elderly. Cochrane Database Syst Rev 2006;3:CD004876. 10.1002/14651858.CD004876.pub2 [DOI] [PubMed] [Google Scholar]
- 18.Jackson LA, Jackson ML, Nelson JC, et al. Evidence of bias in estimates of influenza vaccine effectiveness in seniors. Int J Epidemiol 2006;35:337–44. 10.1093/ije/dyi274 [DOI] [PubMed] [Google Scholar]
- 19.Levett-Jones T. Vaccines for preventing influenza in the elderly: a cochrane review summary. Int J Nurs Stud 2020;109:103372. 10.1016/j.ijnurstu.2019.06.003 [DOI] [PubMed] [Google Scholar]
- 20.Hariyanto TI, Kurniawan A. Statin and outcomes of coronavirus disease 2019 (COVID-19): A systematic review, meta-analysis, and meta-regression. Nutr Metab Cardiovasc Dis 2021;31:1662–70. 10.1016/j.numecd.2021.02.020 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Permana H, Huang I, Purwiga A, et al. In-Hospital use of statins is associated with a reduced risk of mortality in coronavirus-2019 (COVID-19): systematic review and meta-analysis. Pharmacol Rep 2021;73:769–80. 10.1007/s43440-021-00233-3 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Hariyanto TI, Kurniawan A. Statin therapy did not improve the in-hospital outcome of coronavirus disease 2019 (COVID-19) infection. Diabetes Metab Syndr 2020;14:1613–5. 10.1016/j.dsx.2020.08.023 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Wu K-S, Lin P-C, Chen Y-S, et al. The use of statins was associated with reduced COVID-19 mortality: a systematic review and meta-analysis. Ann Med 2021;53:874–84. 10.1080/07853890.2021.1933165 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Vahedian-Azimi A, Mohammadi SM, Heidari Beni F, et al. Improved COVID-19 ICU admission and mortality outcomes following treatment with statins: a systematic review and meta-analysis. Arch Med Sci 2021;17:579–95. 10.5114/aoms/132950 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Onorato D, Pucci M, Carpene G, et al. Protective effects of statins administration in European and North American patients infected with COVID-19: a meta-analysis. Semin Thromb Hemost 2021;47:392–9. 10.1055/s-0040-1722307 [DOI] [PubMed] [Google Scholar]
- 26.Holman N, Knighton P, Kar P, et al. Risk factors for COVID-19-related mortality in people with type 1 and type 2 diabetes in England: a population-based cohort study. Lancet Diabetes Endocrinol 2020;8:823–33. 10.1016/S2213-8587(20)30271-0 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Cariou B, Goronflot T, Rimbert A, et al. Routine use of statins and increased COVID-19 related mortality in inpatients with type 2 diabetes: results from the CORONADO study. Diabetes Metab 2021;47:101202. 10.1016/j.diabet.2020.10.001 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Masana L, Correig E, Rodríguez-Borjabad C, et al. Effect oF statin therapy oN SARS-CoV-2 infection-related. Eur Heart J Cardiovasc Pharmacother 2020. 10.1093/ehjcvp/pvaa128. [Epub ahead of print: 02 Nov 2020] (published Online First: 2020/11/03). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Kondo K, Low A, Everson T, et al. Health disparities in veterans: a map of the evidence. Med Care 2017;55 Suppl 9 Suppl 2:S9–15. 10.1097/MLR.0000000000000756 [DOI] [PubMed] [Google Scholar]
- 30.Hiatt JG, Shamsie SG, Schectman G. Discontinuation rates of cholesterol-lowering medications: implications for primary care. Am J Manag Care 1999;5:437–44. [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
bmjopen-2021-058363supp001.pdf (346.4KB, pdf)
bmjopen-2021-058363supp002.pdf (354.9KB, pdf)
bmjopen-2021-058363supp003.pdf (352.1KB, pdf)
Data Availability Statement
No data are available. Access to VA electronic health records data is limited to researchers with active, VA appointments and an IRB-approved protocol.


