Abstract
Background:
Recent neuroimaging studies have reported grey matter alterations in primary trigeminal neuralgia patients. However, few studies have focused on quantitative measurements of trigeminal nerves and the interaction between trigeminal nerve volume and brain morphology, particularly grey matter volume. In this study, we investigated the link between trigeminal nerves and grey matter volume changes in primary trigeminal neuralgia patients compared to healthy controls. Moreover, we explored the association of structure of trigeminal nerves and grey matter to collected pain clinical variables.
Methods:
Eighty participants (40 patients and 40 controls) were recruited for the study. All participants underwent MRI sessions and clinical pain assessment. Trigeminal nerve volume and whole brain grey matter volume were evaluated using quantitative imaging techniques. Sensory and affective pain rating indices were assessed using the visual analog scale and short-form McGill Pain Questionnaire. Mediation analysis was conducted to investigate the relationship between clinical pain variables and volumetric changes in trigeminal nerves and grey matter.
Results:
Decreased trigeminal nerve volume was detected in primary trigeminal neuralgia patients compared to controls. Additionally, reduced grey matter volume was found in several regions associated with pain in primary trigeminal neuralgia subjects, including the insula, secondary somatosensory cortex, hippocampus, dorsal anterior cingulate cortex, precuneus, and several areas of the temporal lobe. Mediation analysis revealed that decreased trigeminal nerve volume drove grey matter volume abnormality of the left insula, and further led to increased pain ratings.
Conclusion:
This study showed a predominantly direct effect of trigeminal nerve atrophy on clinical pain variables in primary trigeminal neuralgia patients, providing new insight into the pathophysiology of the disease.
Trial registration:
Keywords: Primary trigeminal neuralgia, trigeminal nerve, voxel-based morphometry, pain ratings
Introduction
Primary trigeminal neuralgia (primary TN) is a neuropathic pain disorder characterized by intermittent, lancinating attacks in one or more branches of the trigeminal nerve (TGN). These attacks can be evoked by either innocuous stimuli or occur spontaneously, with abrupt onsets and endings (1). Primary TN patients often experience paroxysmal increases in the frequency and duration of attacks as their disease progresses, with pain-free periods between bouts.
Unfortunately, the underlying etiology of TN has not been completely explored. Increasing evidence suggests that nearly 90% of primary TN cases exhibit neurovascular conflict (NVC), wherein the TGN has immediate contact with or is displaced by adjacent blood vessels (2). Microvascular decompression (MVD) is a procedure in which the offending blood vessel is separated and moved away from the TGN root by a small sponge or Teflon pad. MVD is by far the most effective treatment for primary TN, with upwards of 98% of patients reporting partial to complete pain relief immediately following surgery, and 68% reporting good to excellent pain relief at a 10-year follow-up (3–5).
However, NVC is not exclusive to TN patients; in neuroimaging studies, 25–49% of healthy individuals (6,7) and 14–39% of cadavers (8,9) also had this anatomical variation, despite no history of TN. Thus, it is not enough to simply define NVC and its severity with labels such as “suspected contact,” “contact,” and “nerve displacement and indentation.”
In recent years, a link between peripheral nerve injury and resulting brain structural and functional changes has been established (10). Several studies have reported altered grey matter volume (GMV) in primary TN (11–13). However, no study has investigated the link between TGN atrophy and potential changes of GMV in primary TN patients.
For this study, our team performed a quantitative measurement on the cisternal segment of TGN patients using Medical Image Processing, Analysis, and Visualization software (MIPAV) (14). Volumetric data of TGN was calculated to correlate with regional GMV in primary TN patients to determine if there were local or whole-brain alterations or potential correlation between the two. From there, mediation analysis was performed to determine if either TGN volume or regional GMV had a predominantly direct effect on the duration and intensity of orofacial pain, with the goal of elucidating an alternative imaging marker for predicting the characteristics of primary TN. Ultimately, by better defining traits associated with TGN and NVC in primary TN patients, this could assist neurosurgeons in making targeted therapeutic decisions to provide better outcomes for MVD.
Materials and methods
Participants
Eighty participants (40 primary TN patients and 40 healthy volunteer controls matched for age, gender, education and handedness) were recruited through the First Affiliated Hospital of Xi’an Jiaotong University. Primary TN diagnosis was confirmed by physicians using the International Classification of Headache Disorders (ICHD-3) (15). The disease duration for all the patients was more than 2 years. Inclusion criteria included unilateral pain within the TGN distribution; stabbing or electric-like paroxysms evoked by select triggers; and no neurological deficits or sensory loss. Exclusion criteria included secondary trigeminal neuralgia; other types of pain disorders; and major psychiatric disorders. The medication regimens for the primary TN patients are not uniform; most of the patients took carbamazepine for pain treatment, and a minority used oxcarbazepine and phenytoin, others took neurotrophic drugs such as mecobalamine and oryzanol, a few people even tried different types of Chinese medicine. This study was approved by the Research Ethics Review Board of the Institute of the First Affiliated Hospital of Xi’an Jiaotong University, with written informed consent obtained from all participants prior to the start of experimental procedures.
Clinical assessment
Quantitative assessment of orofacial pain was performed in primary TN patients under the supervision of a technician who was blinded to the experiment. Both the visual analog scale (VAS) and the short form of the McGill Pain Questionnaire (SF-MPQ) were applied; the former rating pain intensity from 0 (no pain) to 10 (the worst pain you can imagine), and the latter giving additional qualitative information on the “sensory” and “affective” dimensions of pain severity. Patients recorded their VAS pain ratings and SF-MPQ in a daily paper pain diary for 7 days, which was used to calculate the average pain rating and obtain sensory, affective, and total pain indices. Independent sample t-tests were conducted to determine the significance in pain ratings between primary TN patients and healthy controls.
Magnetic resonance imaging data acquisition
Magnetic resonance imaging (MRI) data were collected on a 3.0-T GE Signa HDxt scanner with an eight-channel phase-array head coil. Routine MRI sequences included axial T1 and T2, with an additional coronal T2 sequence to rule out any neurological diseases. High-resolution sequences were performed to facilitate the identification and characterization of NVC in participants as previously reported (14). Sequences included a three-dimensional fast imaging procedure employing steady state acquisition [3D-FIESTA, TR: 6.3 ms; TE: 2.4 ms; NEX: 2; FOV 18 cm × 18 cm; matrix 448 × 320; flip angle 60°; slice thickness: 0.8 mm; spacing: 0 mm] (Figure 1(a)), and time-of-flight magnetic resonance angiography [TOF-MRA, TR: 19 ms; TE: 2.8 ms; NEX: 1; FOV 18 cm × 18 cm; matrix 256 × 256; slice thickness: 0.8 mm; spacing: 0 mm] (Figure 1(b)). Following acquisition, images parallel to the cisternal segment of the TGNs were reconstructed to analyze NVC via a multiplanar reformation algorithm (Figure 1(c), (d)). Lastly, three-dimensional brain structural images were acquired using an axial fast spoiled gradient recalled sequence (3D-FSPGR, TR: 2300 ms; TE: 4.9 ms; FOV: 256 × 256 mm; matrix: 256 × 256; slice thickness: 1 mm; spacing: 0 mm; flip angle: 15.0°).
Figure 1.
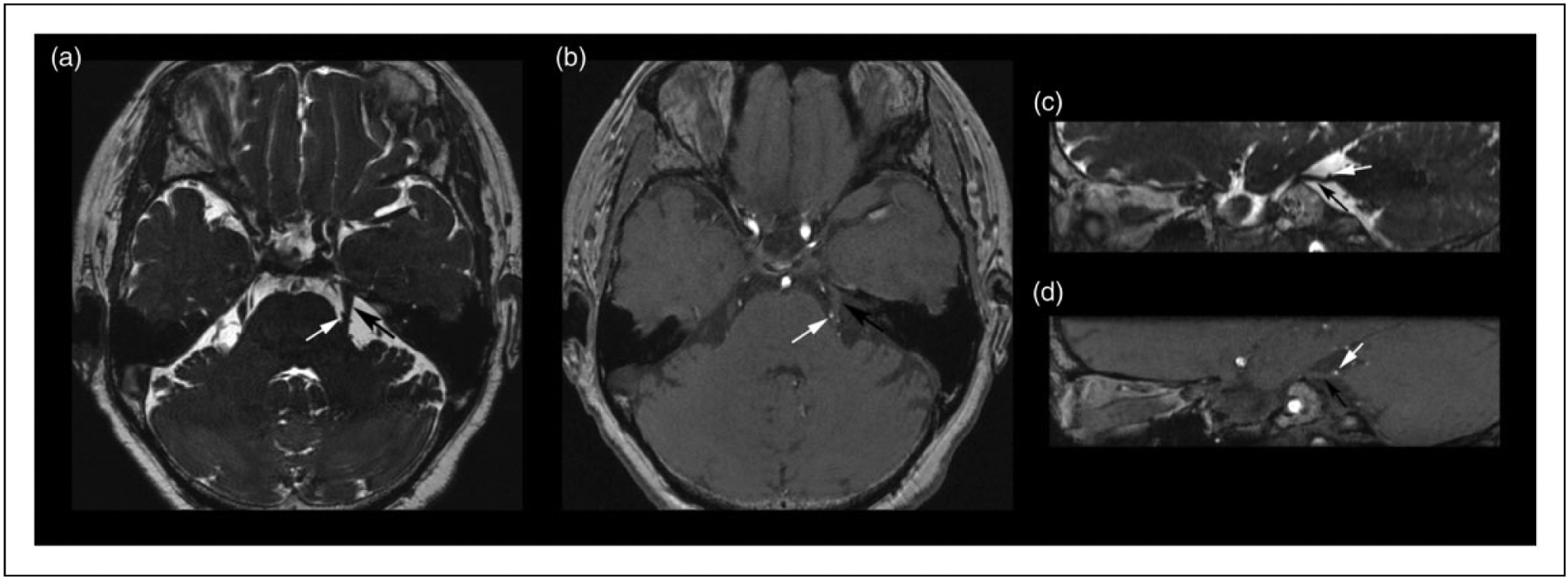
Demonstration of neurovascular compression in a TN patient with MRI multiplanar reconstruction technique. (a) Axial 3D-FIESTA and (b) 3D-TOF-MRA sequences exhibit an aberrant superior cerebellar artery (SCA, white arrow) compressing the medial surface of left TGN root (black arrow). (c) Sagittal 3D-FIESTA and (d) 3D-TOF-MRA images demonstrate indentation and deviation of the upper surface of the nerve root by the left SCA.
TN: trigeminal neuralgia; TGN: trigeminal nerve; 3D-FIESTA: three-dimensional fast imaging procedure employing steady state acquisition; 3D-TOF-MRA: three-dimensional time-of-flight magnetic resonance angiography.
MRI data processing and analysis
NVC detection and volume measurement of TGN.
3D-FIESTA and TOF-MRA images were transferred to a GE ADW4.4 workstation for post-processing and analysis. The presence or the absence of NVC in TN patients was detected by two observers with more than 10 years’ clinical experience in neuroradiology. Volumetric measurements of each TGN were performed using MIPAV software, which can perform 3D visualization and quantitative testing for raw data-sets. To obtain measurements of the TGN, it was first identified from the surrounding area starting from the location where the nerve enters the pons to a pre-determined boundary at the entrance of the Meckel’s cave. Each section of the TGN was manually outlined and the volume was automatically calculated using a 3D reconstruction method (Figure 2). Neuroradiologists followed the nerve pathway to distinguish TGN from vessels. Volumes of TGN were calculated independently by two observers, who were blinded to the TN diagnosis. For statistical analyses, we used the calculated mean value. The TGN volume of the patients’ ipsilateral side and contralateral side, and of the bilateral sides in the healthy controls, were compared with one-way ANOVA followed by LSD-t multiple comparison test using SPSS 18.0 (IBM Corporation, Armonk, NY, USA).
Figure 2.
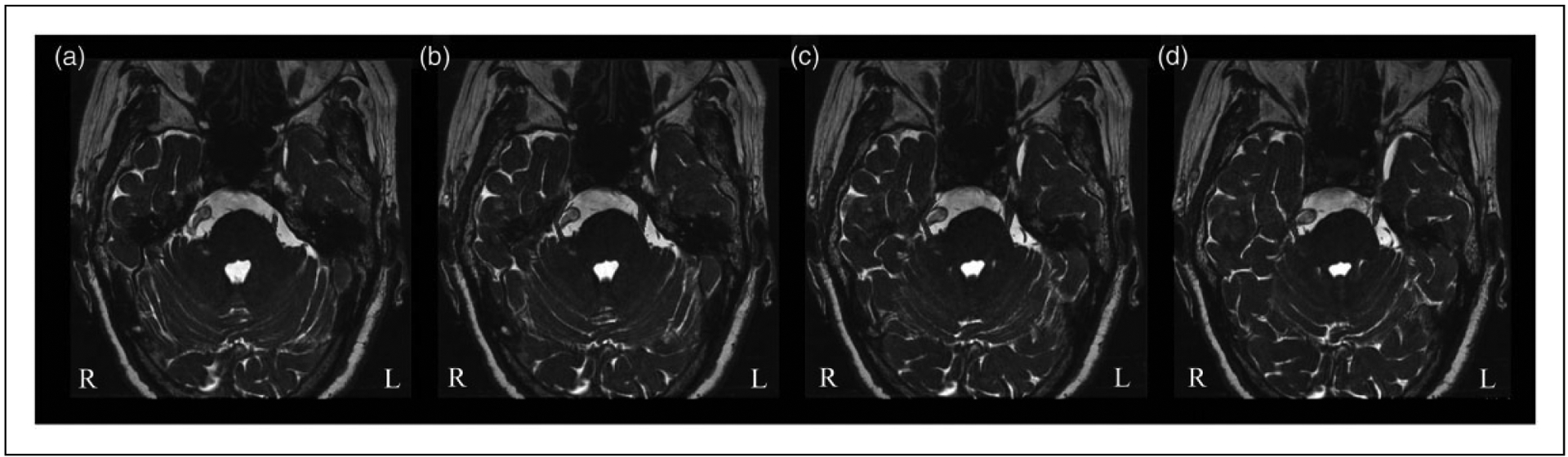
Quantitative measurement of the TGN volume in a primary TN patient. Axial 3D-FIESTA images depict the segmentation of bilateral TGN starting from Meckel’s cave to the entry point of the pons ((a)–(d)). In this patient, the right TGN is compressed by a tortuous and enlarged basilar artery, leading to the displacement and atrophy of the nerve, whereas the left TGN remains unaffected.
Whole brain voxel-based morphometry (VBM).
Imaging data were preprocessed and analyzed using the VBM12 tool-box implemented in the Statistical Parametric Mapping 12 software (SPM12; www.fil.ion.ucl.ac.uk/spm). Voxel-based morphometry is a fully automated technique for computational analysis of differences in regional GM, white matter (WM) or cerebral spinal fluid (CSF) volume. Images were first segmented into GM, WM, and CSF, and then spatially normalized with diffeomorphic anatomical registration through exponentiated Lie algebra (DARTEL) to the standardized Montreal Neurological Institute (MNI) templates (voxel size: 1 × 1 × 1 mm3) provided by SPM12. The normalized images were smoothed with an isotropic Gaussian kernel of 8 mm full-width at half maximum (FWHM). Total intracranial volume (TIV), whole-brain GMV, WM volume and CSF volume were calculated and averaged for each group. Group difference on voxel-wise GMV images with TIV as covariate were performed with a cluster forming statistical threshold of p < 0.001 uncorrected, and then corrected for multiple comparisons using false discovery rate (FDR) thresholded at p < 0.05 at the cluster level.
Correlations with clinical variables.
A two-tailed Spearman correlation analysis was performed to investigate the relationships between TGN volume and regional GMV in primary TN patients using SPSS. Additionally, mediation analysis was applied to determine if either TGN volume or whole brain GMV from patients had a direct effect on the clinical variables, such as disease duration, VAS pain scores, and SF-MPQ, including total, sensory, and affective pain rating indices (SF-MPQ-Total, SF-MPQ-Sensory, and SF-MPQ-Affective, respectively).
Results
Demographics and clinical characteristics of participants
Demographic and clinical details are provided in Table 1. The same number of males and females were enrolled in both the primary TN and healthy control groups (23 female, 17 male). Both groups displayed similar mean ages and educational backgrounds. In contrast to healthy controls, primary TN patients displayed significantly higher VAS and SF-MPQ index scores.
Table 1.
Demographics and clinical characteristics of study population.
| Primary TN patients | Healthy controls | ||
|---|---|---|---|
| Sex, n (%) | |||
| Female | 23 (30.3) | 23 (30.3) | N/A |
| Male | 17 (22.4) | 17 (22.4) | N/A |
| Age (years) | 55.76 ± 8.23 | 55.80 ± 8.09 | 0.985 |
| Education (years) | 12.58 ± 3.64 | 13.25 ± 4.17 | 0.837 |
| Disease characteristics | |||
| Pain laterality (left/right) | 17/23 | N/A | N/A |
| Pain duration (years) | 7.08 ± 5.29 | N/A | N/A |
| Attack frequency (times per day) | 5.93 ± 5.78 | N/A | N/A |
| Duration of attack (min) | 1.35 ± 0.74 | N/A | N/A |
| VAS Pain Rating | 5.83 ± 1.72 | N/A | N/A |
| SF-McGill Pain Questionnaire | |||
| SF-MPQ-Total | 13.32 ± 6.82 | N/A | N/A |
| SF-MPQ-Sensory | 8.13 ± 4.15 | N/A | N/A |
| SF-MPQ-Affective | 5.18 ± 2.73 | N/A | N/A |
| Emotional assessment | |||
| HAMA | 4.10 ± 3.37 | N/A | N/A |
| HAMD | 4.25 ± 4.38 | N/A | N/A |
| Brain VBM | |||
| TIV | 1.409 ± 0.116 | 1.445 ± 0.104 | 0.086 |
| GMV | 0.664 ± 0.050 | 0.679 ± 0.044 | 0.065 |
| WMV | 0.516 ± 0.055 | 0.530 ± 0.056 | 0.242 |
| CSF | 0.229 ± 0.034 | 0.235 ± 0.028 | 0.273 |
Demographics presented as () unless otherwise stated. Independent two-samples t test was conducted to compare clinical traits between primary TN patients and healthy controls.
VAS: visual analogue scale; SF-MPQ: short-form McGill pain questionnaire; HAMA: Hamilton anxiety rating scale; HAMD: Hamilton depression rating scale; TIV: total intracranial volume; GMV: gray matter volume; WMV: white matter volume; CSF: cerebral spinal fluid; N/A: not applicable.
NVC detection and TGN volumetric quantification
Most of the recruited subjects (36/40) displayed various degrees of NVC on the side affected by trigeminal neuralgia; among them, 22 patients presented major NVC (there were indentations or distortions of the responsible blood vessels compressing the affected nerves), and minor NVC was shown in a further 14 patients, leaving four patients without apparent NVC.
To ensure accuracy of TGN volumetric data collected by the two observers mentioned above, we investigated the Pearson correlation coefficients. Volumetric measurements of TGNs by both observers was 0.75 (p < 0.01). Intraclass correlation coefficients in the volumetric evaluation of the ipsilateral TGN in primary TN patients were 0.83 for the first observer and 0.90 for the second observer.
In healthy controls, no difference in volume was detected between the left versus right TGN (p > 0.05; left: 79.52 ± 6.97 mm3, right: 77.89 ± 6.06 mm3). This was calculated to be only a 2.1% difference in volume. As such, a bilateral average TGN volume was calculated for healthy controls and used for all analyses (78.78 ± 6.50 mm3) (Figure 3).
Figure 3.
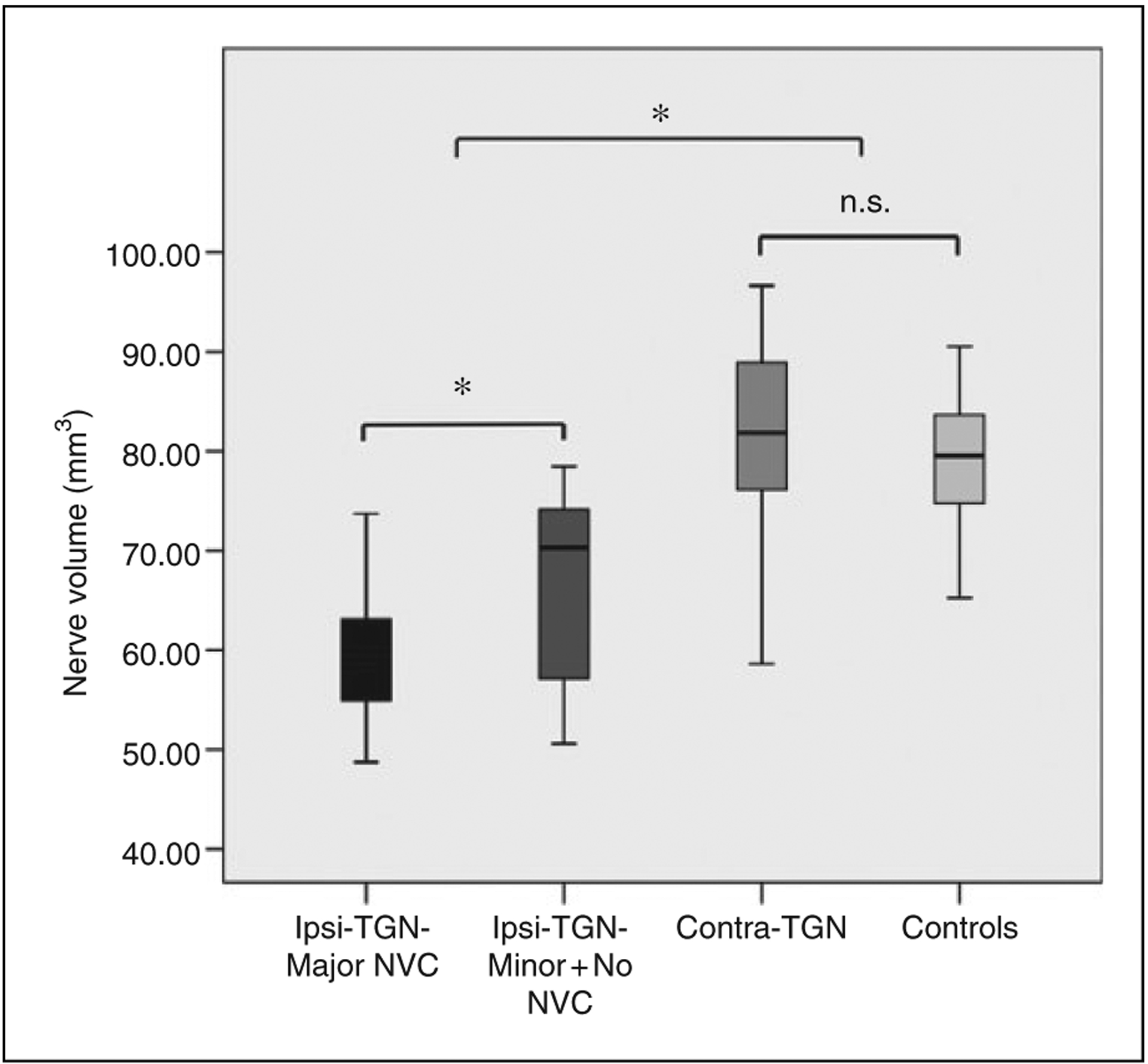
Comparisons of ipsilateral and contralateral TGN volume between primary TN patients and controls. Box-and-whisker plot demonstrating that the ipsilateral TGN volume was significantly lower compared to the contralateral TGN volume in primary TN patients and the mean volume of bilateral TGN in healthy controls (p < 0.05). Meanwhile, the ipsilateral TGN of patients with major NVC demonstrated decreased volume compared to those with minor (and without) NVC (p < 0.05).
*statistically significant.
n.s.: not significant; NVC: neurovascular compression.
The average volume of the ipsilateral TGN compared to the contralateral TGN in primary TN patients was significantly smaller (p < 0.05; 62.83 ± 8.92 mm3 vs. 81.37 ± 10.00 mm3) (Figure 3). This was calculated to be an average volumetric reduction of 22.8% from the ipsilateral to contralateral TGN. Meanwhile, the ipsilateral TGN of primary TN patients was statistically smaller than the average volume of bilateral TGNs in the control subjects (p < 0.05). When comparing the volume of the trigeminal nerve in patients with major NVC (22 patients) to those with minor (and without) NVC (18 patients), there was still significant volume difference between the two sub-groups (p < 0.05; 59.29 ± 6.51 mm3 vs. 67.14 ± 9.21mm3) (Figure 3). Additionally, the volume of the ipsilateral TGN was negatively correlated with the VAS scores (p < 0.05, Figure 4(a)) and total, sensory, and affective pain rating indices of SF-MPQ (p < 0.01, Figure 4(b), (c), (d)) in primary TN patients. Unfortunately, no association was found for ipsilateral TGN atrophy to the duration of disease.
Figure 4.
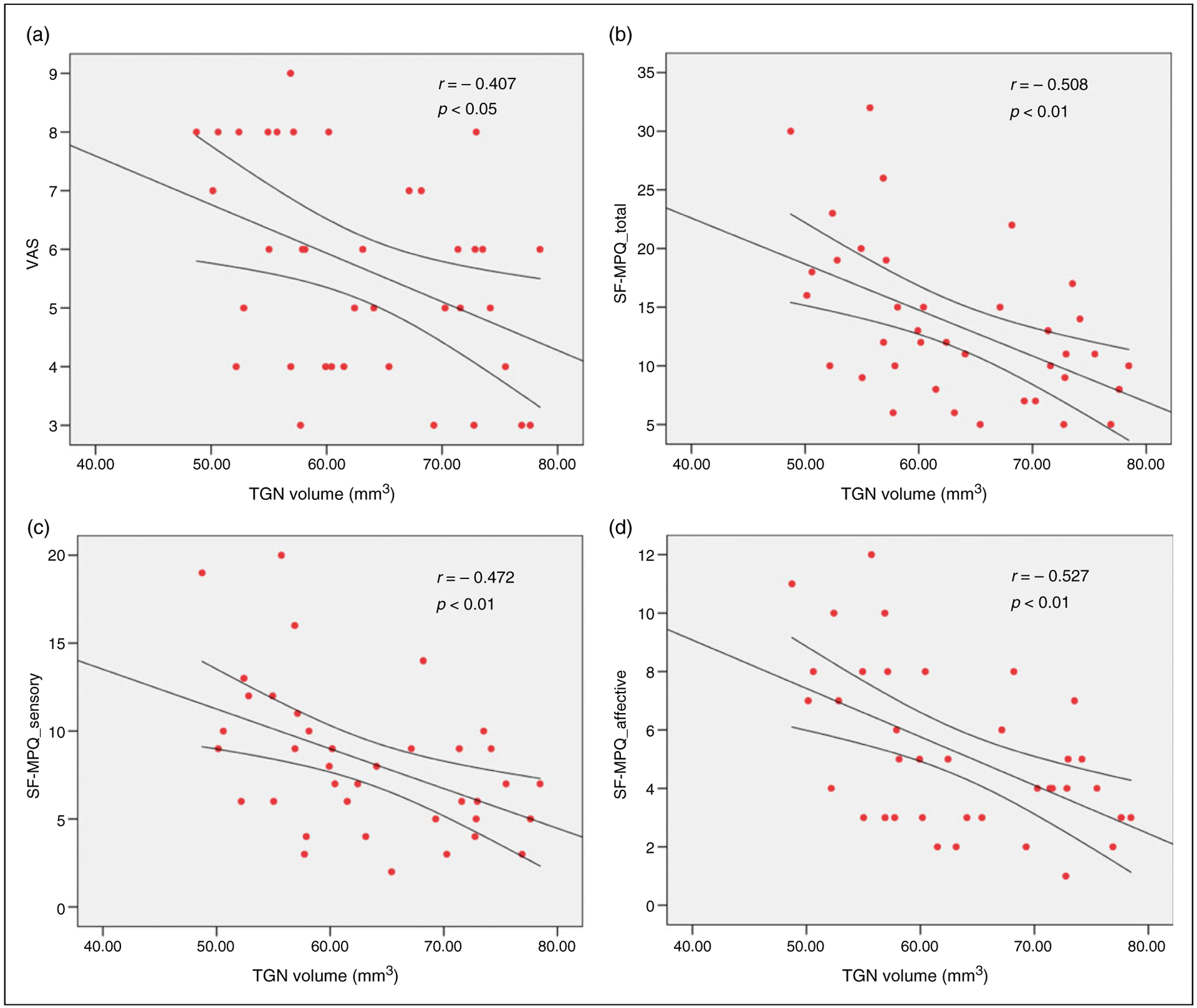
Correlation of ipsilateral TGN volume to different pain indices in primary TN patients. Ipsilateral TGN volume was negatively associated with pain ratings, including (a) the VAS, and (b) the total, (c) sensory, and (d) affective pain rating of SF-MPQ. VAS: visual analog scale; SF-MPQ: the short form of McGill Pain Questionnaire.
Comparisons of global tissue volume between primary TN patients and healthy controls
There were no statistical differences of total intracranial volume (p = 0.086), GMV (p = 0.065), WMV (p = 0.242), or CSF volume (p = 0.273) between primary TN patients and healthy controls (Table 1). The high-resolution T1 structural images of all subjects showed no morphological abnormalities or apparent motion artifacts.
Regional GMV changes between primary TN and healthy subjects
VBM analysis across the whole brain revealed GM atrophy in several regional clusters in primary TN patients compared to healthy controls. Figure 5 shows the locations of these clusters in MNI space, including the left insula, left secondary somatosensory cortex (S2), right hippocampus, right dorsal anterior cingulate cortex (dACC), bilateral precuneus lobe, and several regions of the temporal lobe (p < 0.05, FDR corrected). Table 2 lists the standard space coordinates, Brodmann’s area, extents, and t-values of these brain regions. In addition, a positive correlation was found between the GMV of the left insula and the volume of the ipsilateral TGN in the patients (r = 0.534, p < 0.01) (Figure 6); all other regional GMV changes did not reveal any significant correlations to ipsilateral TGN volume.
Figure 5.
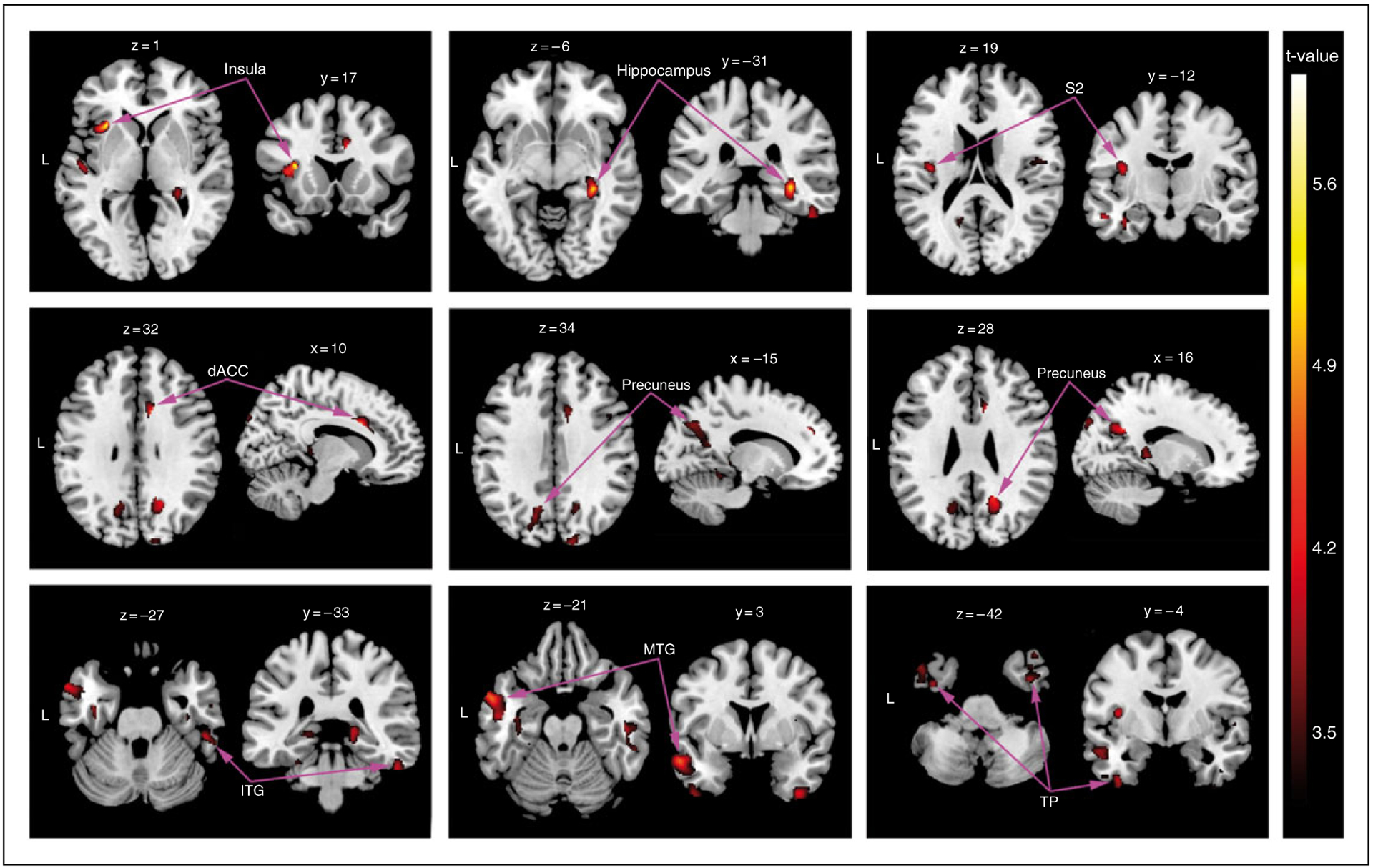
VBM analysis of GMV. VBM analysis revealed significantly decreased GMV clusters in primary TN patients compared to control subjects. In particular, GMV atrophy was observed in the left insula, left S2, right hippocampus, right dACC, bilateral precuneus, and several portions of the temporal lobe (p < 0.05, FDR corrected). Images are shown on the MNI template in neurological convention (x, y, and z values refer to MNI coordinates) with the gradient color bar corresponding to t-values of the clusters. VBM: voxel-based morphometry; MNI: Montreal Neurological Institute; GMV: grey matter volume; S2: secondary somatosensory cortex; dACC: dorsal anterior cingulate cortex; FDR: false discovery rate.
Table 2.
Regions of reduced grey matter volume in primary TN patients compared to healthy controls.
| Anatomical regions | Side | Brodmann’s area | Voxels in cluster | MNI coordinates | Peak voxel t-value | ||
|---|---|---|---|---|---|---|---|
| x | y | z | |||||
| Insula | L | 13 | 248 | −38 | 17 | 1 | 5.60 |
| Hippocampus | R | 36 | 361 | 30 | −31 | −6 | 5.14 |
| Somatosensory cortex 2 | L | 40 | 139 | −38 | −12 | 19 | 4.18 |
| Anterior cingulate cortex | R | 24 | 112 | 10 | 16 | 32 | 4.25 |
| Inferior temporal gyrus | R | 20 | 215 | 52 | −33 | −27 | 4.03 |
| Middle temporal gyrus | L | 21 | 439 | −57 | 3 | −21 | 4.68 |
| Temporal pole | L | 38 | 205 | −39 | −4 | −42 | 3.93 |
| Temporal pole | R | 38 | 171 | 38 | 0 | 46 | 4.41 |
| Precuneus lobe | L | 7 | 256 | −15 | −68 | 34 | 3.74 |
| Precuneus lobe | R | 7 | 175 | 16 | −56 | 28 | 4.36 |
Data are corrected for multiple comparisons using FDR with threshold at p < 0.05.
Figure 6.
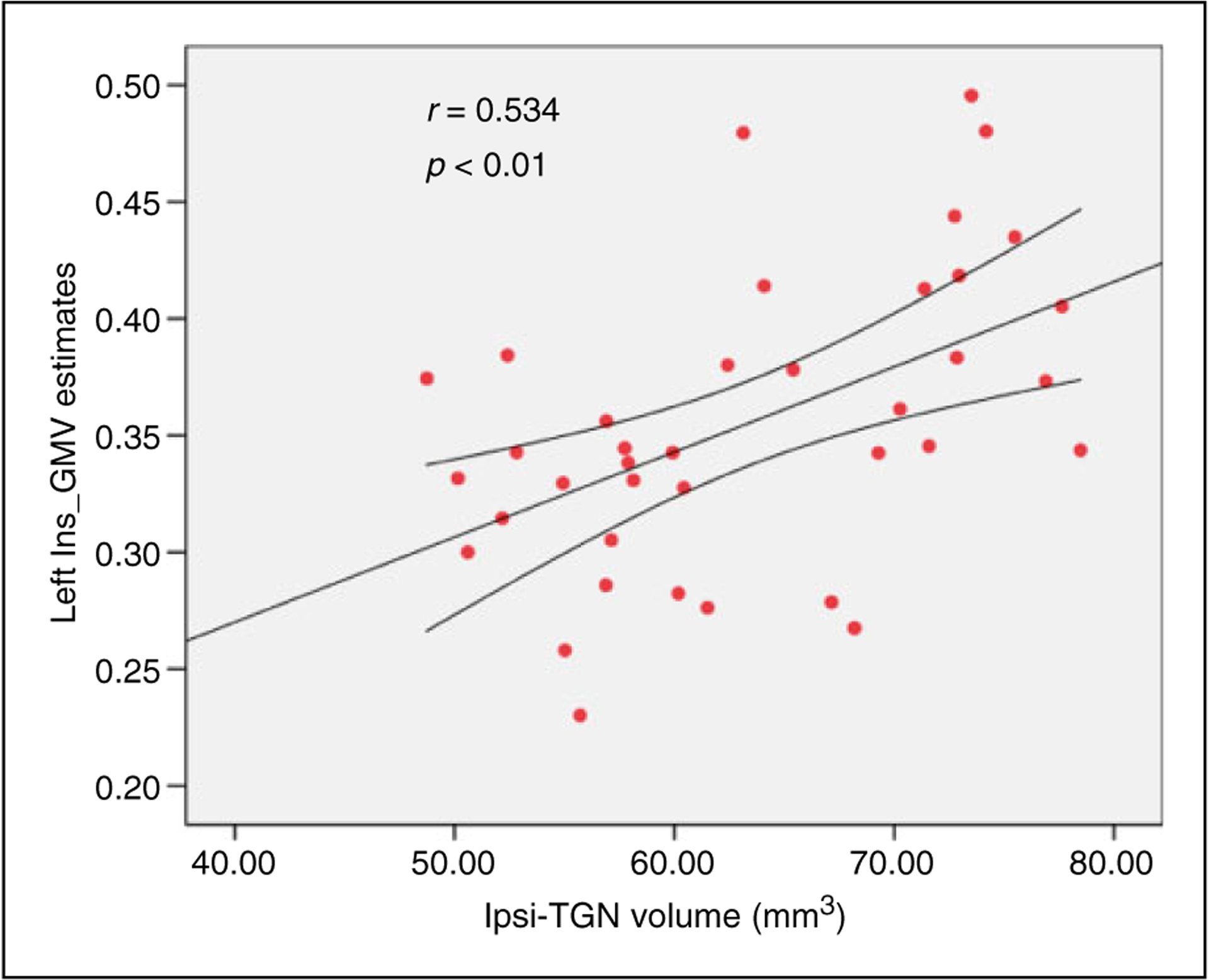
Association of ipsilateral TGN volume to the GMV of the left insula. The positive correlation was detected between the atrophic ipsilateral TGN and left insula GMV in the primary TN patients (p < 0.01).
Correlation of TGN volume and regional GMV to pain severity
When we took ipsilateral TGN volume as the independent variable, pain severity as the outcome variable (VAS), and left insular GMV as the mediator variable in the mediation analysis in the patient group, the t-value was −1.965 and p-value was 0.0473. On the contrary, when left insular GMV was regarded as the independent variable, VAS as the outcome variable, and ipsilateral TGN volume as the mediator variable, the t-value was −0.814 and p-value was 0.401 (Figure 7). These results showed a predominantly direct effect of the ipsilateral TGN on pain severity in primary TN patients, not the left insular GMV.
Figure 7.
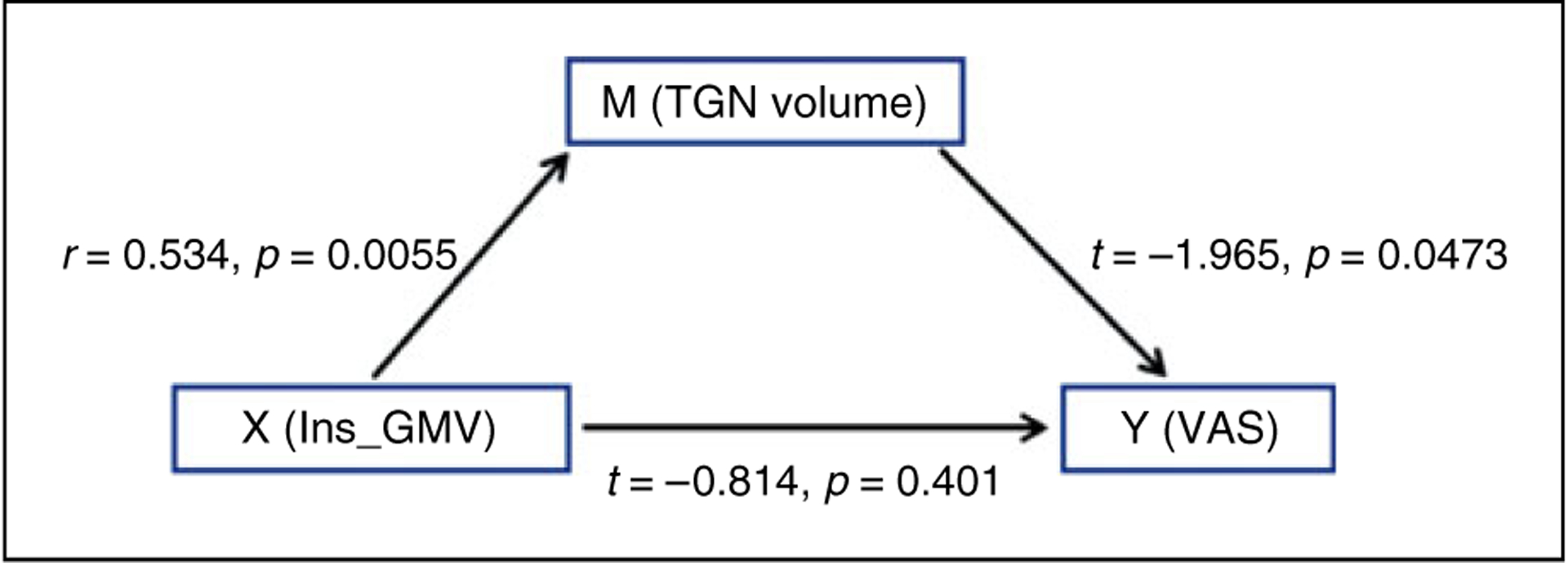
The link between ipsilateral TGN volume, GMV of the left insula, and pain rating index. The mediation analysis showed that the trigeminal nerve morphology, not the left insular GMV, had a predominantly direct effect on the pain severity in primary TN patients.
Discussion
This study investigated the morphological abnormalities of affected TGN and brain GM in primary TN patients, and examined the association between localized cranial nerve injury and brain structural anomaly. In addition, we correlated the peripheral and central structural changes to the duration and severity of orofacial pain. To our knowledge, this is the first reported study documenting the relationship between cranial nerve damage and structural changes in the brain, and one of the few studies linking quantitative measurement of TGN to different dimensions of pain in TN.
Previous MRI studies have identified morphological changes of the affected TGN in TN patients; however, most of these reports focused on the qualitative characteristics of NVC, including the category of responsible vessel, and contact location and severity (16,17). It is well documented that NVC is common on the affected TGNs in primary TN patients. Counterintuitive to this, NVC is frequently observed in healthy volunteers, demonstrating that qualitative assessment of NVC is not enough to act as a diagnostic component for primary TN (18,19). Atrophy rarely occurs on the unaffected TGN in classical TN patients or on bilateral TGNs in healthy subjects; however, it is prevalent on the affected TGN of classical TN individuals and is undoubtedly a potential factor in the underlying etiology of TN (20). In our study, the affected TGN had decreased volume compared to the unaffected nerve, which paralleled a previous report that quantified TGN atrophy in TN patients (21). In addition, the TGN volumetric differences observed between TN patients and healthy controls corroborate prior observations recorded at surgery (22) and from histopathology samples (8). Furthermore, the ipsilateral TGN of patients with major NVC demonstrated decreased volume compared to those with minor (and without) NVC, indicating high impact of severe NVC on the atrophic changes of the affected TGN. Our study has overcome several of the limiting factors that were observed in earlier studies, including small sample sizes, images acquired using radiography or a 1.5T MR scanner, failure to measure pain characteristics of their patient population (23), and using a younger cohort of subjects with fewer age-related diseases (20).
Our study demonstrated that the mean volume of the ipsilateral TGN in primary TN patients was significantly associated with the sensory and emotional aspects of pain. It is widely acknowledged that sustained and pulsatile NVC has a higher likelihood of inducing different pathological alterations in the TGN, such as demyelination, dysmyelination, creating residual myelin debris, and sometimes axonal impairment for severe cases (24). These microstructural deficits likely lead to atrophy of the affected TGN and evoke ectopic impulse discharges, amplified and synchronized by crosstalk between axons and crossed after discharge, which can trigger pain paroxysms (24). Meanwhile, these TGN pathologic changes may be secondary to ischemia of the root entry zone (REZ) due to focal endoneural vascular injury, which tends to induce episodes of TN. This may explain why we found the association between nerve atrophy and pain severity. As was shown in our results, negative correlations of TGN deficit to different aspects of the orofacial pain suggest multidimensional nociceptive abnormalities in primary TN. Patients diagnosed with trigeminal neuralgia have an increased risk of developing psychiatric comorbidities, including anxiety and depression (25). Because emotional disturbances are closely related to decreased quality of life, chronification of pain, and poor response to therapy (26), TN needs to be evaluated in a comprehensive manner.
VBM analysis detected extensive GMV reductions in select regions of primary TN patients compared to controls, mirroring other reports on TN. These studies suggest that GMV atrophy in these regions represents cortical adaption to the intermittent and stabbing pain perception (13,27,28). Obermann et al. explored decreased GMV in several pain-related regions similar to our study, such as the S2, insula, ACC, and parahippocampus (12). In addition to these regions, our results revealed GMV atrophy in multiple temporal subregions in primary TN patients, including the bilateral temporal pole, right inferior temporal gyrus (ITG), and left middle temporal gyrus (MTG). Although decreased GMV of the temporal lobe is not commonly detected in chronic pain states, it has been observed more frequently in primary TN patients (11–13). A possible interpretation is that anticipation of the severe pain recruits the limbic system including hippocampus, temporal pole, and ITG to modulate aversive memory recognition (29,30), which, in turn, supports the involvement of the temporal lobe in the anticipation of sporadic and lancinating orofacial pain.
Our data suggests that the structural integrity of the ipsilateral TGN may present as an important factor in predicting the development and severity of primary TN, but until now there has been little focus on primary TN in conjunction with TGN deficits and brain morphological changes. In this study, positive correlation was detected between the volume of the ipsilateral TGN and left insula GMV in primary TN patients. Previous research provided evidence for a central component involved in the pathophysiology of primary TN (31) and regarded regional cerebral atrophy as a possible consequence of the peripheral pain impulse caused by NVC (12). Interestingly, sensory-motor cortical plasticity following peripheral nerve injury can occur throughout the brain in non-human primates (32). Although several histological, neurophysiological, and neuroimaging studies (24,33,34) consistently confirmed the primary role of demyelination near REZ to ensure that alterations of specific cortical areas are not secondary, future studies similar to ours might be carried out in patients with a very short duration of the disease.
Finally, because of the association between the atrophic ipsilateral TGN and volume loss of the left insula, it was essential to determine which one had the leading role in the fierce orofacial pain. With the results of mediation analysis among the three factors, we detected a predominantly direct effect of TGN volume on VAS ratings. Although previous studies had demonstrated a close relationship of regional cerebral GMV abnormalities to duration and severity of the pain (12,27), it was most likely that the trigeminal nerve abnormalities drove GMV changes, and further had impact on the pain characteristics.
Several limitations in this study have to be disclosed. First, several anti-epileptic drugs (i.e. carbamazepine) taken by TN patients could influence the brain structure, thus this would be an important confounder of the study. However, medication regimens for TN patients are not uniform and are sometimes irregular in China; a few subjects even took several types of Chinese medicine, so it is impossible to regress out the medication effect on every TN patient. In addition, only 15 TN patients received MRI scanning at 1 year after MVD surgery because many patients lived far away from our hospital and they were reluctant to come back for re-examinations after prominent pain relief. Actually, the volume of the ipsilateral TGN increased about 20% post-operatively compared to that at the time point of pre-operation, and GMV in the left insula also experienced 4% elevation or so in the follow-up patients, and the corresponding figure is listed in the supplementary data.
Taken together, our study observed atrophy of the ipsilateral TGN and brain GM in primary TN patients. We not only demonstrate a correlation of the ipsilateral TGN to the left insula GMV, indicating a potential link between the cranial nerve and cerebral cortex, but we show that volumetric measurement of the TGN is associated with multiple pain dimensions in TN patients. Above all, these findings complement the investigation of TGN and whole brain GMV changes in primary TN patients, which provide more information on the delicate alterations in the nervous system and suggest a better imaging biomarker for monitoring the therapeutic effects of this disease.
Supplementary Material
Key findings.
Marked atrophy was detected in the affected trigeminal nerves in patients with trigeminal neuralgia.
Volume reduction was shown in the insula, hippocampus, and several sub-regions of the temporal lobe in patients with trigeminal neuralgia.
There is an association between the affected trigeminal nerve and grey matter volume of the insula in the patient group.
Decreased TGN volume has a predominantly direct effect on the increased pain rating indices.
Acknowledgements
We thank Dr. Faxiu Bao and Fengli Liang for their assistance with recruiting patients, as well as Dr. Michael Keaser for his advice on VBM-related analyses.
Funding
The authors disclosed receipt of the following financial support for the research, authorship, and/or publication of this article: This work was supported in part by the National Natural Science Foundation of China (No. 81301207), Natural Science Foundation of Shaanxi Province (No. 2018JM7026), Fundamental Research Funds for the Central Universities in Xi’an Jiaotong University (No. xjj2018272), the Clinical Research Award of the First Affiliated Hospital of Xi’an Jiaotong University, China (No. XJTU1AFCRF-2015-028), and the Opening Project of Key Laboratory of Shaanxi Province for Craniofacial Precision Medicine Research, College of Stomatology, Xi’an Jiaotong University (No. 2016LHM-KFKT001).
Footnotes
Declaration of conflicting interests
The authors declared no potential conflicts of interest with respect to the research, authorship, and/or publication of this article.
References
- 1.Bennetto L, Patel NK and Fuller G. Trigeminal neuralgia and its management. BMJ 2007; 334: 201–205. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Maarbjerg S, Wolfram F, Gozalov A, et al. Significance of neurovascular contact in classical trigeminal neuralgia. Brain 2015; 138: 311–319. [DOI] [PubMed] [Google Scholar]
- 3.Barker FG 2nd, Jannetta PJ, Bissonette DJ, et al. The long-term outcome of microvascular decompression for trigeminal neuralgia. N Engl J Med 1996; 334: 1077–1083. [DOI] [PubMed] [Google Scholar]
- 4.Linskey ME, Ratanatharathorn V and Penãgaricano J. A prospective cohort study of microvascular decompression and Gamma Knife surgery in patients with trigeminal neuralgia. J Neurosurg 2008; 109: 160–172. [DOI] [PubMed] [Google Scholar]
- 5.Miller JP, Acar F, Hamilton BE, et al. Radiographic evaluation of trigeminal neurovascular compression in patients with and without trigeminal neuralgia: Clinical article. J Neurosurg 2009; 110: 627–632. [DOI] [PubMed] [Google Scholar]
- 6.Adamczyk M, Bulski T,J, et al. Trigeminal nerve-artery contact in people without trigeminal neuralgia: MR study. Med Sci Monit 2007; 13: 38–43. [PubMed] [Google Scholar]
- 7.Kakizawa Y, Seguchi T, Kodama K, et al. Anatomical study of the trigeminal and facial cranial nerves with the aid of 3.0-tesla magnetic resonance imaging. J Neurosurg 2008; 108: 483–490. [DOI] [PubMed] [Google Scholar]
- 8.Hamlyn PJ. Neurovascular relationships in the posterior cranial fossa, with special reference to trigeminal neuralgia. 2. Neurovascular compression of the trigeminal nerve in cadaveric controls and patients with trigeminal neuralgia: Quantification and influence of method. Clin Anat 1997; 10: 380–388. [DOI] [PubMed] [Google Scholar]
- 9.Ramesh VG and Premkumar G. An anatomical study of the neurovascular relationships at the trigeminal root entry zone. J Clin Neurosci 2009; 16: 934–936. [DOI] [PubMed] [Google Scholar]
- 10.Taylor KS, Anastakis DJ and Davis KD. Cutting your nerve changes your brain. Brain 2009; 132: 3122–3133. [DOI] [PubMed] [Google Scholar]
- 11.Wang Y, Cao D-y, Remeniuk B, et al. Altered brain structure and function associated with sensory and affective components of classic trigeminal neuralgia. Pain 2017; 158: 1561–1570. [DOI] [PubMed] [Google Scholar]
- 12.Obermann M, Rodriguez-Raecke R, Naegel S, et al. Gray matter volume reduction reflects chronic pain in trigeminal neuralgia. Neuroimage 2013; 74: 352–358. [DOI] [PubMed] [Google Scholar]
- 13.Li M, Yan J, Li S, et al. Reduced volume of gray matter in patients with trigeminal neuralgia. Brain Imaging Behav 2017; 11: 486–492. [DOI] [PubMed] [Google Scholar]
- 14.Wang Y, Li D, Bao F, et al. Microstructural abnormalities of the trigeminal nerve correlate with pain severity and concomitant emotional dysfunctions in idiopathic trigeminal neuralgia: A randomized, prospective, double-blind study. Magn Reson Imaging 2016; 34: 609–616. [DOI] [PubMed] [Google Scholar]
- 15.Headache Classification Committee of the International Headache Society. The International Classification of Headache Disorders, 3rd edition (beta version). Cephalalgia 2013; 33: 629–808. [DOI] [PubMed] [Google Scholar]
- 16.Leal PRL, Hermier M, Souza MA, et al. Visualization of vascular compression of the trigeminal nerve with high-resolution 3T MRI: A prospective study comparing preoperative imaging analysis to surgical findings in 40 consecutive patients who underwent microvascular decompression for trigeminal neuralgia. Neurosurgery 2011; 69: 15–26. [DOI] [PubMed] [Google Scholar]
- 17.Lorenzoni J, David P and Levivier M. Patterns of neurovascular compression in patients with classic trigeminal neuralgia: A high-resolution MRI-based study. Eur J Radiol 2012; 81: 1851–1857. [DOI] [PubMed] [Google Scholar]
- 18.Peker SA and Necmettin Pamir M Vascular compression of the trigeminal nerve is a frequent finding in asymptomatic individuals: 3-T MR imaging of 200 trigeminal nerves using 3D CISS sequences. Acta Neurochir 2009; 151: 1081–1088. [DOI] [PubMed] [Google Scholar]
- 19.Desouza DD, Hodaie M and Davis KD. Abnormal trigeminal nerve microstructure and brain white matter in idiopathic trigeminal neuralgia. Pain 2014; 155: 37–44. [DOI] [PubMed] [Google Scholar]
- 20.Erbay SH, Bhadelia RA, O’Callaghan M, et al. Nerve atrophy in severe trigeminal neuralgia: Noninvasive confirmation at MR imaging – initial experience 1. Radiology 2006; 238: 689–692. [DOI] [PubMed] [Google Scholar]
- 21.Leal PRL, Barbier C, Hermier M, et al. Atrophic changes in the trigeminal nerves of patients with trigeminal neuralgia due to neurovascular compression and their association with the severity of compression and clinical outcomes: Clinical article. J Neurosurg 2014; 120: 1484–1495. [DOI] [PubMed] [Google Scholar]
- 22.Sindou M, Howeidy T and Acevedo G. Anatomical observations during microvascular decompression for idiopathic trigeminal neuralgia (with correlations between topography of pain and site of the neurovascular conflict). Prospective study in a series of 579 patients. Acta Neurochir 2002; 144: 1–13. [DOI] [PubMed] [Google Scholar]
- 23.Hořínek D, Brezova V, Nimsky C, et al. The MRI volumetry of the posterior fossa and its substructures in trigeminal neuralgia: A validated study. Acta Neurochir 2009; 151: 669–675. [DOI] [PubMed] [Google Scholar]
- 24.Devor M, Govrin-Lippmann R and Rappaport ZH. Mechanism of trigeminal neuralgia: An ultrastructural analysis of trigeminal root specimens obtained during microvascular decompression surgery. J Neurosurg 2002; 96: 532–543. [DOI] [PubMed] [Google Scholar]
- 25.Wu T-H, Hu L-Y, Lu T, et al. Risk of psychiatric disorders following trigeminal neuralgia: A nationwide population-based retrospective cohort study. J Headache Pain 2015; 16: 64. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Maizels M, Smitherman TA and Penzien DB. A review of screening tools for psychiatric comorbidity in headache patients. Headache 2006; 46: S98–S109. [DOI] [PubMed] [Google Scholar]
- 27.Gustin SM, Peck CC, Wilcox SL, et al. Different pain, different brain: Thalamic anatomy in neuropathic and non-neuropathic chronic pain syndromes. J Neurosci 2011; 31: 5956–5964. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.DeSouza DD, Davis KD and Hodaie M. Reversal of insular and microstructural nerve abnormalities following effective surgical treatment for trigeminal neuralgia. Pain 2015; 156: 1112–1123. [DOI] [PubMed] [Google Scholar]
- 29.Bauch EM, Rausch VH and Bunzeck N. Pain anticipation recruits the mesolimbic system and differentially modulates subsequent recognition memory. Hum Brain Map 2014; 35: 4594–4606. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Tillfors M, Furmark T, Marteinsdottir I, et al. Cerebral blood flow during anticipation of public speaking in social phobia: A PET study. Biol Psych 2002; 52: 1113–1119. [DOI] [PubMed] [Google Scholar]
- 31.Obermann M, Yoon MS, Ese D, et al. Impaired trigeminal nociceptive processing in patients with trigeminal neuralgia. Neurology 2007; 69: 835–841. [DOI] [PubMed] [Google Scholar]
- 32.Kaas JH. Plasticity of sensory and motor maps in adult mammals. Annu Rev Neurosci 1991; 14: 137–167. [DOI] [PubMed] [Google Scholar]
- 33.Love S and Coakham H. Trigeminal neuralgia: Pathology and pathogenesis. Brain 2001; 124: 2347–2360. [DOI] [PubMed] [Google Scholar]
- 34.Antonini G, Di Pasquale A, Cruccu G, et al. Magnetic resonance imaging contribution for diagnosing symptomatic neurovascular contact in classical trigeminal neuralgia: A blinded case-control study and meta-analysis. Pain 2014; 155: 1464–1471. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


