Abstract
The intracellular protozoan Toxoplasma gondii is a widespread opportunistic parasite of humans and animals. Normally, T. gondii establishes itself within brain and skeletal muscle tissues, persisting for the life of the host. Initiating and sustaining strong T-cell-mediated immunity is crucial in preventing the emergence of T. gondii as a serious pathogen. The parasite induces high levels of gamma interferon (IFN-γ) during initial infection as a result of early T-cell as well as natural killer (NK) cell activation. Induction of interleukin-12 by macrophages is a major mechanism driving early IFN-γ synthesis. The latter cytokine, in addition to promoting the differentiation of Th1 effectors, is important in macrophage activation and acquisition of microbicidal functions, such as nitric oxide release. During chronic infection, parasite-specific T lymphocytes release high levels of IFN-γ, which is required to prevent cyst reactivation. T-cell-mediated cytolytic activity against infected cells, while easily demonstrable, plays a secondary role to inflammatory cytokine production. While part of the clinical manifestations of toxoplasmosis results from direct tissue destruction by the parasite, inflammatory cytokine-mediated immunopathologic changes may also contribute to disease progression.
INTRODUCTION
Life Cycle and General Aspects of Immunity
Toxoplasma gondii is an intracellular coccidian belonging to the phylum Apicomplexa. The parasite is globally distributed and can be found within many different species of mammals and birds. It is estimated that up to 5 × 108 people worldwide are infected with T. gondii. Sexual stages of the parasite occur within gut epithelial cells of the cat, and the products of gamete fusion, the oocysts, are shed in the feces. Once in contact with the atmosphere, the oocysts sporulate and become infective to other definitive or intermediate hosts. As in most coccidia, the sexual stages of Toxoplasma are highly specific, occurring in no other known hosts than those of feline species. Nevertheless, in contrast to other coccidia, Toxoplasma has evolved to infect a wide variety of vertebrate species, including humans. Indeed, this asexual stage of the parasite life cycle, unlike the sexual phase in cats, is notable for lack of both host and tissue specificity.
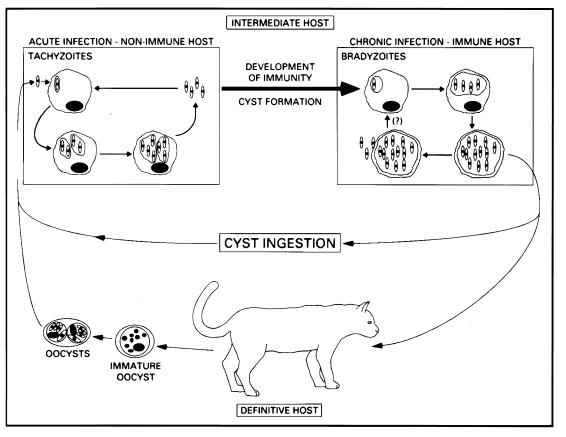
In the intermediate host, after infection of intestinal epithelial cells, the infective stages (oocysts or bradyzoites) transform into tachyzoites, which display rapid multiplication by endodyogeny within an intracellular parasitophorous vacuole. When the cells become packed with tachyzoites, the host cell plasma membrane ruptures and parasites are released into the extracellular milieu. The free tachyzoites can then infect virtually any nucleated cell they encounter, and they continue intracellular replication, spreading throughout host tissues (Fig. 1). If not controlled by the immune system, tachyzoites are highly virulent and cause a generalized toxoplasmosis which is always fatal (57). Indeed, many studies show that normally avirulent strains of T. gondii are highly virulent in T-lymphocyte-deficient animals (71, 133). Therefore, induction of T-cell-mediated immune responses and resistance to the tachyzoite stage is a key step in the T. gondii life cycle, determining the survival of the intermediate host and the parasite itself.
FIG. 1.
T. gondii life cycle. During acute infection, initiated by ingestion of cysts or oocysts, tachyzoites invade and proliferate within virtually any nucleated cell, resulting in host cell lysis and reinfection of more cells. Concurrent with the development of immunity, tachyzoites transform into slow-growing bradyzoites, which reside within cysts in tissues of the muscles and CNS. This chronic infection can persist for the life of the host, but in patients with immunodeficiency, cysts may rupture, leading to reinitiation of an acute infection. Within the gut of the cat, ingested parasites undergo differentiation into male and female gametes, which results in the formation of oocysts. These are shed in the feces and can remain infectious for many months.
After development of immunity, the tachyzoite stage is cleared from host tissues, and bradyzoites, the slowly multiplying, essentially dormant and harmless forms of the parasite, persist. The bradyzoites survive within cysts and are effectively isolated from the host immune system by the cyst wall, which is composed mainly of host tissue-derived products (Fig. 2A). The ability of bradyzoites to escape the host immune response and persist in a quiescent form within the host is therefore another key event in the T. gondii life cycle. The bradyzoites are infective for either definitive or intermediate hosts and are largely responsible for parasite transmission to different species of mammals and birds (Fig. 1).
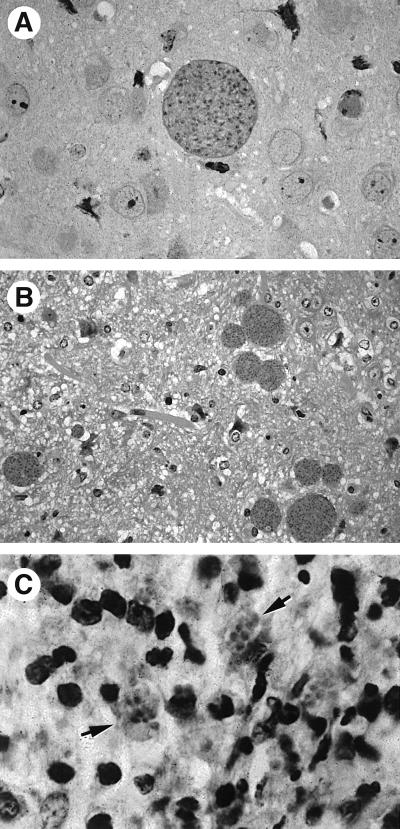
FIG. 2.
(A) Cyst in an immunocompetent mouse chronically infected with T. gondii. (B and C) Satellite cysts (B) and clusters of free tachyzoites (arrows) (C) in the brains of chronically infected mice subsequently treated with anti-TNF-α or anti-IFN-γ MAb. These sections were stained with hematoxylin and eosin.
Although bradyzoites are apparently harmless, sequestered within dormant cysts, a persistent immunity to T. gondii is required to avoid reemergence of the tachyzoite stage and accompanying pathologic changes. Indeed, the latter is often observed in chronically infected immunocompromised hosts (139, 162, 213). Bradyzoites are found in proportionately larger numbers in the central nervous system (CNS); indeed, cyst reactivation most often occurs in the brain. This fact is well illustrated by the high incidence of encephalitis induced by T. gondii as a major cause of morbidity and mortality in patients with AIDS (139, 162).
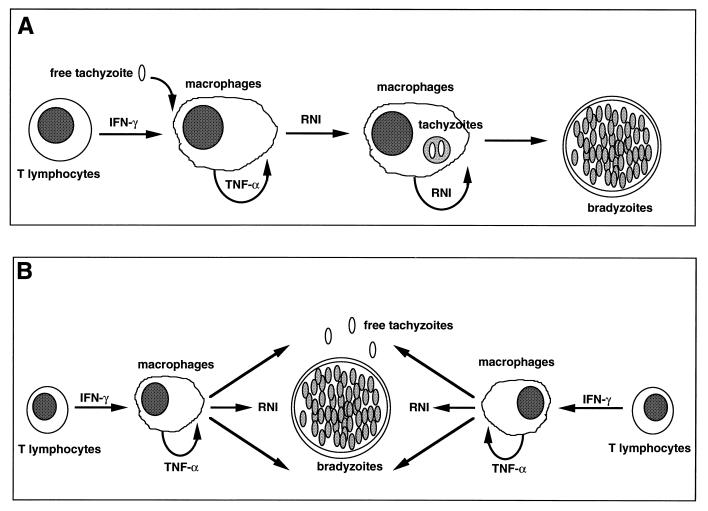
Two hypotheses have been put forward regarding control of parasite replication during chronic toxoplasmosis. According to the first, the host immune response actively induces tachyzoite transformation into the bradyzoite stage and is crucial in maintaining T. gondii in the latter developmental form (Fig. 3A). Indeed, recent in vitro studies indicate that NO, an important effector molecule produced by activated macrophages, induces both parasite stasis and expression of a subset of bradyzoite-specific antigens (Ag) (14, 208).
FIG. 3.
Alternative hypotheses for the control of T. gondii tachyzoite replication in tissues of immunocompetent hosts. (A) The cellular immune response plays an active role in driving encystment of the parasite. Recent evidence suggests that production of RNI may promote tachyzoite-bradyzoite transformation. (B) Initiation of cyst formation occurs independently of host immunity. Once cysts are established, a low rate of conversion of bradyzoites to tachyzoites occurs continuously, but these reemergent parasites are effectively controlled by components of type 1 cytokine-based immunity such as RNI.
The second hypothesis suggests that an immune response controls tachyzoite replication but does not, in itself, exert an effect on bradyzoites, which are essentially harmless to the host (Fig. 3B). Instead, it is suggested that parasites are continually released from cysts in chronically infected hosts, resulting in constant boosting of the immune system. Tachyzoite replication itself is controlled by the immune system in immunocompetent animals. Strong evidence for this model comes from the finding that in animals chronically infected with T. gondii, treatment with neutralizing doses of antibody (Ab) against either gamma interferon (IFN-γ) or tumor necrosis factor alpha (TNF-α) results in the emergence of free tachyzoites and an increased number of cysts (Fig. 2B and C).
It is noteworthy that in nature, mice are a major intermediate host of T. gondii. This would suggest that the mouse immune response is well adapted, in evolutionary terms, to cope optimally with this particular parasite. Of further note, the life cycle of the parasite in mice closely resembles that in humans. Toxoplasma is simple to maintain in vitro as tachyzoites and in vivo as either tachyzoites or bradyzoites. Thus, T. gondii infection in inbred mice has become a major model to elucidate the basis of protective immunity against intracellular pathogens in general and to examine the regulation of immunopathologic changes elicited by such infectious agents (62, 67, 200). The knowledge acquired from these studies has provided, and should continue to provide, new insights into designing more effective vaccines based on induction of cell-mediated immunity (CMI), as well as new strategies for immunotherapy of opportunistic infections in immunodeficient hosts.
Importance of T-Cell-Mediated Immunity in Resistance to T. gondii
One of the most distinctive immunologic features of T. gondii infection is the strong and persistent CMI elicited by the parasite, resulting in host protection against rapid tachyzoite growth and consequent pathologic changes. Another interesting aspect of Toxoplasma-triggered immunity is that it is normally harmless to the host. Thus, in contrast to many other parasites and several experimental models of toxoplasmosis, T. gondii under normal conditions fails to elicit significant immunopathologic changes in immunocompetent hosts and is usually accompanied by symptoms no more severe than fever, fatigue, and lymphadenopathy.
A series of early studies have pointed away from Ab and toward T cells as the major effectors of resistance to T. gondii. First, passive transfer of serum from chronically infected animals to naive recipients fails to protect the latter against virulent-parasite challenge (56). In addition, mice treated with antibodies against the μ heavy chain of immunoglobulin M (IgM) can control infection despite a lack of B lymphocytes and antibodies (177). Consistent with these observations is the fact that T. gondii lacks an extracellular stage, in contrast to some trypanosomatids such as Trypanosoma brucei, against which Ab have been shown to play a crucial protective role (127, 185). However, it is important to note that some studies indicate the importance of IgA as an element of mucosal immunity to oral infection with Toxoplasma cysts (30). Antibodies of this isotype may be important in avoiding reinfection with T. gondii, and therefore induction of IgA is a major strategy for vaccine development.
Studies showing the importance of T cells in resistance against T. gondii are nonequivocal. Thus, athymic nude mice, which lack functional T cells, are extremely susceptible to both virulent and avirulent parasite strains (71, 133). More importantly, adoptive transfer of immune T cells (in particular the CD8+ subset) to naive mice protects animals against challenge with virulent T. gondii strains (56, 168, 220, 221). Immunogenetic studies also point to a major influence of major histocompatibility complex (MHC) class I and II on resistance and susceptibility to the parasite, consistent with the idea that T lymphocytes are crucial in determining the outcome of infection (15, 16, 147, 149).
Virtually all mouse strains develop a strong Th1 immune response to T. gondii, regardless of whether they possess resistant or susceptible MHC haplotypes (61, 70). Thus, cytokines such as IFN-γ and TNF-α (which activate macrophage functions) are important for controlling tachyzoite replication during both acute and chronic phases of infection (61, 68, 110, 192, 213). In contrast, interleukin-10 (IL-10) and IL-12 appear to be crucial at the initial phase of infection and less important during chronic toxoplasmosis (75, 76).
While IL-12 is clearly important in initiating a strong and effective CMI against T. gondii tachyzoites, IL-10 appears to modulate both IL-12 and IFN-γ synthesis in vivo, avoiding an excessive immune response that could cause extensive inflammation and host tissue damage (76, 163). Thus, IL-10 and IL-12 are two major antagonists involved in regulating IFN-γ synthesis during the initial phase of infection. Whereas NK cells and CD4+ and CD8+ T lymphocytes appear to be major sources of IFN-γ at the early stages of infection αβ T lymphocytes are the dominant source of this cytokine during the chronic phase (58, 61, 70, 71, 75, 99, 117, 210).
In this paper, we review studies that have elucidated the molecular and cellular basis underlying the induction of T-cell-mediated immunity to T. gondii. We also discuss the effector mechanisms of parasite-triggered immunity, examining both beneficial and detrimental host effects that are brought into play during the encounter of this opportunistic pathogen with its mammalian host.
INITIATION AND REGULATION OF T-CELL RESPONSES DURING T. GONDII INFECTION
Role of Macrophages and Natural Killer Cells
In addition to inducing an effective parasite-specific T-cell-mediated immune response, it is important to note that T. gondii infection elicits strong nonspecific, T-cell-independent immunity. Indeed, the latter response plays an important role in influencing the development of parasite-specific T cells. The nonspecific aspect is revealed by studies showing that T. gondii infection limits coinfection by nonrelated pathogens such as Listeria monocytogenes and Schistosoma mansoni, infection with certain viruses, and development of certain tumors (69, 90, 143, 183). In addition, early studies demonstrated the ability of T. gondii to activate cells from the innate compartment of the immune system, such as macrophages and NK cells (85, 86). Further studies demonstrated that nonspecific activation occurs at early stages of infection, and is a T-cell-independent phenomenon resulting in IFN-γ synthesis by NK cells and leading to macrophage microbicidal function (62, 203). This early activation of the immune system appears to play two main roles during infection with T. gondii. The first is to limit tachyzoite replication at a time prior to recruitment of T-cell-mediated immunity. The second is to direct the development of an appropriate T-cell response by driving the differentiation of Th precursor (Thp) cells into Th1 effector cells. These considerations suggest that T. gondii tachyzoites may possess adjuvant-like activity that causes generalized potentiation of CMI.
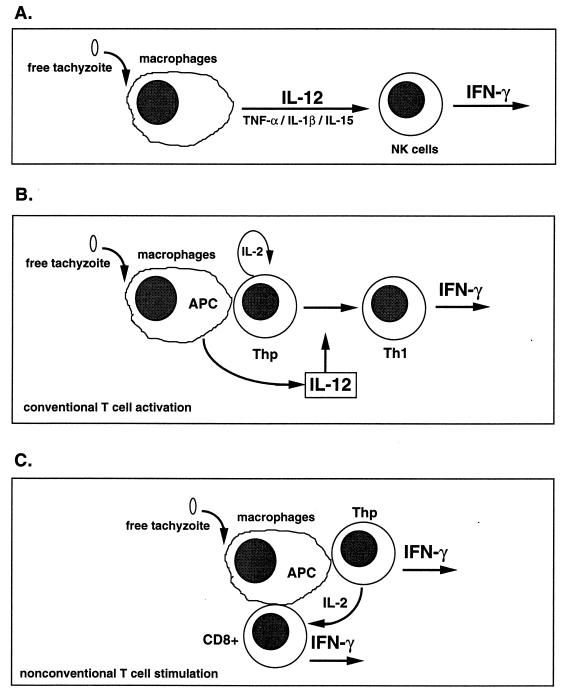
More recent studies have focused on elucidating the cytokine circuits, host cell populations, and parasite molecules involved in the adjuvanticity of tachyzoites. The use of mice with severe combined immunodeficiency (scid mice), which lack both T and B cells, and more recently the use of gene knockout (KO) mice have shed light on these processes (8, 45). NK cells have been identified as a major source of IFN-γ (203). The cytokine IL-12 appears to be the central mediator initiating the synthesis of NK cell IFN-γ, whereas other monokines, such as TNF-α, IL-1β and IL-15, potentiate the effects of IL-12 on NK cells (27, 71, 97, 100, 102, 105) (Fig. 4A). A recent study also shows the importance of costimulatory molecules such as CD28 in stimulating IFN-γ synthesis by murine NK cells (102). A similar system has recently been shown to be operative when human NK cells and monocytes are cultured in the presence of soluble tachyzoite Ag (STAg) (124).
FIG. 4.
Alternative pathways for induction of T-cell-independent and T-cell-dependent IFN-γ synthesis. (A) The T-cell-independent pathway is driven largely by IL-12, as well as TNF-α, IL-1β, and IL-15. These proinflammatory cytokines induce NK cell production of IFN-γ, which can promote macrophage activation and acquisition of microbiostatic activity, as well as Th1-cell differentiation. (B) Presentation of parasite peptide by a professional Ag-presenting cell (APC) such as a macrophage or dendritic cell to a Thp cell. This results in Th1 differentiation in the presence of T-cell-derived IL-2 and IL-12 produced by the Ag-presenting cell. (C) A less well-characterized but potentially very important pathway of early IFN-γ production, in which T. gondii is able to directly activate T cells in a manner that does not require IL-12.
The above-described cytokine circuit appears to be functional in vivo as well as in vitro. Thus, upon infection with T. gondii, scid mice produce high levels of IFN-γ and other proinflammatory cytokines such as IL-12 (97). Similar studies with scid mice show that in vivo neutralization of IL-12 or IFN-γ enhances their susceptibility to acute T. gondii infection (75). Conversely, administration of recombinant IL-12 prolongs the survival of scid animals, an effect which is abolished by Ab-mediated NK cell or IFN-γ depletion (71).
Enhanced susceptibility is also the outcome when immunocompetent mice are treated at early stages of infection with anti-IL-12, anti-IFN-γ, anti-TNF-α, or anti-NK cell Ab (75, 99, 110). Furthermore, in vivo treatment with either recombinant IL-12, IFN-γ, TNF-α, IL-1α, or IL-1β has a protective effect against T. gondii infection (28, 71, 75). For the case of recombinant IL-12 (rIL-12), protection was curtailed by treatment with either anti-NK cell or anti-IFN-γ Ab. The latter finding suggests that IL-12 may function through induction of NK cell IFN-γ; this result finds support from the observation that IFN-γ KO mice are susceptible to T. gondii despite maintaining an ability to produce IL-12 (192).
The T-cell-independent system is potentiated by IL-2, and in certain immunodeficiency states, this is critical to host survival. Thus, in β2-microglobulin KO animals, which lack CD8+ T lymphocytes, an enormous expansion of NK cells occurs in response to infection, and these cells are responsible for controlling tachyzoite proliferation via synthesis of IFN-γ (43). This NK cell expansion appears to be dependent on CD4+ lymphocytes, probably through the production of IL-2 itself (209). More importantly, continuous treatment of AIDS patients with IL-2 leads to enhancement of NK cell IFN-γ synthesis, compensating (at least in part) for HIV-induced CD4+ T-lymphocyte deficiency (124).
The early burst of IFN-γ and other proinflammatory cytokines is crucial in shaping the adaptive immune response which develops against T. gondii (67). First, IFN-γ triggers, or potentiates, the synthesis of chemokines such as the macrophage gene induced by IFN-γ (MIG) and IFN-γ-inducible protein 10, which are important in T-lymphocyte recruitment (1, 62). In addition, IFN-γ synergizes with IL-12 in driving the differentiation of Thp cells toward the Th1 phenotype (Fig. 4B). Thus, IFN-γ enhances IL-12 synthesis by macrophages exposed to tachyzoite products (71, 75, 141), induces expression of the IL-12 receptor on T cells (108), and inhibits IL-4, an important cytokine for differentiation of Thp to the Th2 phenotype (197). Engagement of the IL-12 receptor also activates the phosphorylation of signal transducer and activator of transcription 4 (STAT-4), a transcription factor involved in Th1 lymphocyte differentiation (6, 108, 197).
Interestingly, the Bcl-3 oncoprotein, a member of the IκB family which functions as a positive regulator of NF-κB activity, plays a critical role in the development of T-cell-mediated immunity and resistance to T. gondii (55). Most Bcl-3 KO mice infected with T. gondii do not survive beyond 1 month, whereas 100% of infected wild-type or heterozygote animals survive for over 6 months. While STAg-stimulated splenocytes (or purified T cells) from Bcl-3 KO mice produce normal levels of IFN-γ during early (7 days) infection, the ability to produce this cytokine (after 12 days of infection) is impaired. Cytotoxic T-lymphocyte (CTL) activity against infected target cells, but not NK cytolytic activity, is also defective in these animals. Together, these findings suggest that the Bcl-3 KO mice possess an intrinsic T-cell defect responsible for blocking the development of Th1 cells, resulting in compromised adaptive, rather than natural, immunity to T. gondii.
Because of the importance of IL-12 in the development of CMI, several groups are characterizing signalling pathways involved in IL-12 induction by microbial products. Briefly, IL-12 is a heterodimer cytokine composed of two chains, p35 and p40, linked by a disulfide bond. Whereas the p40 chain is tightly regulated in macrophages and can be induced with different microbial stimuli, the p35 chain is constitutively expressed by many different types of cells and tissues (12, 225). The signalling pathways involved in the induction of the p40 chain have been partially characterized. First, several studies have demonstrated the requirement of two signals for optimal IL-12 (p40) synthesis by macrophages. The first is a priming signal provided by IFN-γ, and the second comes from microbial products themselves, such as lipopolysaccharide or STAg (58, 71, 75, 141).
Recent studies have identified the IFN-γ consensus sequence binding protein (ICSBP) as an important element in IL-12(p40) responsiveness to IFN-γ (95, 189). The ICSBP gene is normally induced by IFN-γ, and mice lacking a functional ICSBP gene are highly deficient in IL-12 synthesis both in vivo and in vitro, although they produce normal levels of IL-12- independent IFN-γ upon mitogen stimulation. More importantly, the ICSBP KO animals are highly susceptible to T. gondii, although they express normal levels of other proinflammatory cytokines, such as IL-1, TNF-α, and IL-6 (189). This result indicates that ICSBP activity is required for IL-12 production exclusively. In contrast, activation of reactivating kinase (or p38) from the mitogen-activated protein kinase-activated protein 2 pathway appears to be necessary for the induction of several monokines, including IL-1, TNF-α, and IL-12(p40) itself (11, 22, 129). Molecular analysis of the IL-12(p40) gene promoter suggests a functional role for an NF-κB half-site, a nucleotide sequence matched at 8 of 10 nucleotides forming the consensus NF-κB sequence (141). In addition, the ets-2 element is present in the promoter region (156). The ets molecules form a large family of transcription factors, some of which are known to be important for the regulation of other inflammatory cytokine genes, such as TNF-α. The induction of IL-12(p40) is modulated by cyclic AMP (22), consistent with early reports showing suppressive effects of prostaglandins on expression of this cytokine.
A central paradox has been raised by these experiments: If IFN-γ is required for the induction of macrophage IL-12 synthesis and if IFN-γ production requires IL-12, which of these cytokines is produced first during infection? Of relevance to this issue, it has recently been found that mouse dendritic cells produce high levels of IL-12 in response to T. gondii stimulation, even in the absence of IFN-γ (175). In addition, STAg-induced IL-12 synthesis occurs independently of CD40-CD40L interactions. This observation suggests lack of a requirement for T-cell signalling to dendritic cells through CD40-CD40L interaction (175), the latter of which has been shown to be necessary in several other systems (39, 198, 204). However, as mentioned above, mice without a functional ICSBP gene are deficient in IL-12 synthesis and are highly susceptible to T. gondii infection, although their macrophage effector functions remain normal (189). Thus, this study suggests that IFN-γ is necessary for optimal production of IL-12 and development of CMI in vivo. It is also important to note that in contrast to Th precursor cells, resting NK cells express the IL-12 receptor and can therefore respond to IL-12 even in the absence of IFN-γ.
Thus, during T-cell differentiation, it is likely that the NK cells provide the initial IFN-γ source, which functions to further enhance IL-12 synthesis initiated by microbial stimuli. Nevertheless, recent evidence also indicates that Toxoplasma displays the capability of activating T cells in an IL-12-independent manner (Fig. 4C) (191). This may also relate to studies in humans and mice (described below), which suggest that the parasite can act as a T-cell-specific and possibly a Vβ-specific mitogen (41, 211).
Thus, during the earliest stages of infection, Toxoplasma triggers several components of the innate immune system (Fig. 4). Macrophages, NK cells, and dendritic cells and neutrophils (144, 175) respond by releasing cytokines such as IL-12, TNF-α, and IFN-γ. Within this milieu of proinflammatory cytokines, components of the acquired immune system important for control of infection, namely, T lymphocytes, recognize the appropriate combination of parasite Ag, MHC, and costimulatory molecules. Viewed in this regard, it is clear that innate immune responses exert a strong influence on developing T-cell immunity, and it should not be surprising that T. gondii drives an extremely powerful Th1 response.
Early T-Lymphocyte Activation
During the early stages of Toxoplasma infection, as described above, macrophages and NK cells become activated and produce cytokines, such as IL-12, which drive the development of a strong T-cell-mediated response. An additional component of the above-described response is the ability of T. gondii to induce T-lymphocyte proliferation and IFN-γ production during early acute infection. Studies in both humans and mice reveal that both γδ and αβ T lymphocytes display functional activity during the first days of infection, which may also contribute to the adjuvant properties observed during infection with the parasite. The molecular basis for how T. gondii drives such a large initial T-cell response is not known, but it may relate in part to superantigen-like properties displayed by tachyzoites in vitro.
γδ T lymphocytes.
Through the use of T-cell receptor (TCR) KO mice and depleting monoclonal Ab mAb, γδ T cells are now known to play a protective role against several bacterial and protozoan pathogens (32, 118, 150, 151, 182, 226). The protective effects of γδ T cells are often (although not always [25, 226]) associated with early disease stages rather than later in infection or during reinfection models. Indeed, γδ-T-cell numbers are elevated during early infection with bacterial pathogens such as Listeria monocytogenes (165). These findings have led to the general view that γδ T cells find their major role as members of the armamentarium providing the “first line of defense” against infection.
In T. gondii-infected mice, increased numbers of γδ T lymphocytes have been detected in the spleen and peritoneal cavity within 7 days of infection with PLK and Beverley parasite strains, and the cells secrete IFN-γ and TNF-α in response to in vitro stimulation with the parasite (91, 93, 117). As such, γδ T cells may play a role in early macrophage activation; indeed, in vivo MAb-mediated γδ T cell depletion results in depressed levels of macrophage NO production (93). The γδ T lymphocytes display protective activity, as demonstrated by adoptive transfer, MAb depletion, and TCR KO experiments (91, 117).
Elevated γδ T-lymphocyte levels also occur in humans with acute toxoplasmosis. In these cases, preferential expansion of Vδ2+ γδ T cells has been reported (49, 188). In patients with acute congenital toxoplasmosis, Vδ2+ cells display nonresponsiveness to in vitro stimulation with either parasite or anti-CD3 stimulation in vitro, as do αβ T cells (84). While Vδ2+ function is regained during later infection, as measured by proliferation and IFN-γ secretion, αβ T cells remain largely nonresponsive. These data raise the novel possibility that γδ T lymphocytes contribute to protection during chronic stages of human congenital infection.
In vitro studies indicate that parasite stimulation of peripheral blood γδ T cells induces preferential Vγ9+ Vδ2+ T-lymphocyte expansion (210). The latter population produces IFN-γ, IL-2, and TNF-α and displays MHC-unrestricted cytotoxicity toward infected target cells. T cells expressing the same Vγ9 TCR component respond to other pathogens such as Plasmodium falciparum and Mycobacterium tuberculosis (78, 112). With regard to Ag specificity, Vγ9+ Vδ2+ T lymphocytes recognize a series of nonprotein low-molecular-weight mycobacterial and synthetic compounds (32, 34, 194, 224). These simple molecules, some of which are phosphorylated metabolites, are widely expressed by eukaryotic cells, raising the possibility that similar γδ cell-stimulating molecules are expressed by T. gondii.
Interestingly, γδ T cells have also been implicated in the induction of macrophage heat shock protein, possibly through IFN-γ production, during early T. gondii infection. Thus, treatment with anti-γδ T-cell MAb blocks hsp65 induction during infection with the Beverley strain (91, 159). Expression of hsp65 correlates with protection against T. gondii (160). The significance of the latter finding can possibly be explained by recent results suggesting that hsp65 induction prevents macrophages from undergoing a programmed cell death response after parasite infection (92). Thus, it is noteworthy that the highly virulent strain, RH, fails to induce macrophage hsp65, and host cells undergo apoptosis, whereas infection with the low-virulence strain, Beverley, induces hsp65 and triggering for programmed cell death does not occur. A causal linkage between expression of hsp65 and prevention of apoptosis was suggested by the finding that an hsp65 antisense probe inhibited the relative proportion of cells undergoing programmed cell death (92).
Thus, T lymphocytes expressing the γδ TCR appear to be important during Toxoplasma infection. This is probably a result of their ability to produce type 1 cytokines which exert microbicidal effects on the parasite, as well as promoting the development of a strong inflammatory cytokine response. Nevertheless, little is yet known about the Ag specificity of this T-cell subset during T. gondii infection, nor is anything known about their requirement for recognizing processed peptide (or possibly nonpeptide) ligand in the context of the MHC and other accessory molecules. These issues, which are basic to the general biology of γδ T lymphocytes, should provide investigators with a challenging avenue of inquiry for several years to come.
αβ T lymphocytes.
Infection with T. gondii also provides a direct and potent stimulus for αβ T-lymphocyte activation, and as a result, T-cell-derived IFN-γ production occurs early during acute infection. In part, early T-cell production of IFN-γ may be explained by the ability of host Ag-presenting cells to activate T lymphocytes in a milieu of inflammatory cytokines. While such an early type 1 cytokine response may be expected to serve a protective role for the host, under certain conditions, T. gondii-induced inflammatory cytokines may contribute to the pathologic findings of the disease.
In vivo evidence for early αβ-T-cell activation comes from a variety of reports. αβ-T-cell production of IFN-γ during acute infection (7 days postinoculation) has been linked to host protection during intraperitoneal infection with the cystogenic ME49 parasite strain (75). In addition, CD8+ intraepithelial lymphocytes isolated 11 days after oral infection are able to adoptively transfer protection in an IFN-γ-dependent manner (19). CD4-mediated pathologic findings have also been reported in 7-day orally infected C57BL/6 mice (131) and during acute infection of IL-10 KO mice (discussed below) (76, 163).
While classical processing and presentation of antigenic peptide in the context of MHC class I or II and the appropriate costimulatory molecules undoubtedly plays a major role in this early T-cell activity (47), there is evidence to suggest that in mice, although not in humans, T. gondii possesses superantigen-like properties that may contribute to early T-cell activation. Culture of spleen cells from noninfected mice with tachyzoite lysate or irradiated parasites results in a vigorous T-cell proliferative response and secretion of IFN-γ. Interestingly, in extended (7-day) cultures, CD8+ T lymphocytes preferentially expand in number relative to CD4+ cells, and Vβ5+ lymphocytes are overrepresented among the T cells (41). In vivo studies also suggest an early Vβ5+-cell expansion during acute murine toxoplasmosis (122). However, in the in vivo situation, Vβ5+ cells, although initially responding, become anergic to ex vivo stimulation with parasite Ag or TCR MAb (40, 122).
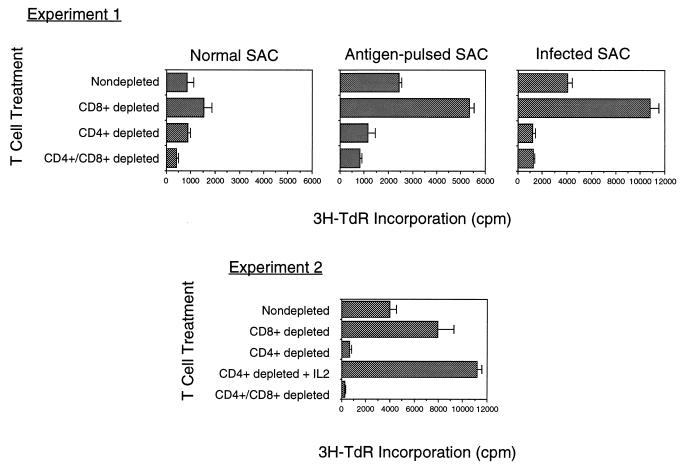
The cellular basis of preferential CD8+ expansion in vitro is not known, but several models can be invoked based on the following observations. First, as shown in Fig. 5, coincubation of infected Ag-presenting cells with purified CD4+ cells induces strong proliferation, demonstrating that the latter cell type is not inherently nonresponsive to parasite stimulation (42). Second, purified CD8+ T lymphocytes do not proliferate in response to infected Ag-presenting cells unless supplied with exogenous IL-2 (Fig. 5). These results indicate that CD4+ cells may initially be triggered to release IL-2, which helps drive CD8+ proliferation and ultimately results in outgrowth of CD8+ T cells. In addition to active suppression of CD4+ cells by CD8+-derived lymphokines, it is possible that CD4+, but not CD8+, T cells undergo activation-induced cell death, as is reported to occur during Trypanosoma cruzi infection (50). Such a phenomenon may have as its basis divergent apoptosis-inducing signals among CD4+- and CD8+-T-cell populations (239). Interestingly, several reports indicate that during human toxoplasmosis, CD8+ T cells increase in number relative to CD4+ T lymphocytes—a finding which suggests a clinical correlate to the in vitro murine studies (94, 140).
FIG. 5.
Nonimmune CD4+ and CD8+ T lymphocytes proliferate in response to T. gondii and Ag-presenting cells. T lymphocytes from normal C57BL/6 mice (purified by passage over anti-mouse Ig columns) were subjected to treatment with rabbit complement and anti-CD4 MAb (to obtain CD8+ T cells) or anti-CD8 MAb (to obtain CD4+ T cells). The resulting populations were cultured with irradiated splenic adherent cells (SAC) that were either untreated, preincubated with T. gondii soluble Ag extract, or preinfected with RH strain tachyzoites. For some CD8+ populations, recombinant murine IL-2 was included. Proliferation was measured by incorporation of [3H]thymidine (3H-TdR), added 72 h after culture initiation.
Recent studies on human peripheral blood T lymphocytes from previously unexposed donors also indicates that T. gondii acts as a strong T-cell mitogen, inducing high levels of proliferation and IFN-γ secretion (211). However, unlike the situation in mice, human T cells show no consistent TCR Vβ skewing after in vitro stimulation. Moreover, while MHC class II is required in the response, paraformaldehyde fixation of the Ag-presenting cells prior to incubation with parasite Ag abrogates the activity, indicating a requirement for Ag processing. Thus, present data suggests that although T. gondii is a powerful stimulator of early human T-cell responses, unlike the case in mice, the human response resembles more a classical Ag-driven rather than a superantigen-driven phenomenon.
Modulation of T-cell activation.
While early production of inflammatory cytokines is required for the subsequent parasite-induced protective T-cell-mediated immune response, if uncontrolled this response can lead to severe immunopathologic changes and even to death. Thus, under normal circumstances, the strength of the host CMI triggered by T. gondii must be tightly regulated to limit infection, on the one hand, and to avoid immunopathologic changes, on the other. Elucidation of the molecular mechanisms underlying regulation of the protective immune response has provided important clues for understanding the basis of successful long-term interaction between parasites and their vertebrate host. At the same time that macrophages induce strong type 1 cytokines, these cells also promote the regulation of CMI through production of IL-10 and transforming growth factor β (TGF-β), which regulate the expression and function of IL-12 and other monokines (62). A recent study also suggests that IL-4 may display a similar regulatory role during acute toxoplasmosis (179). In addition, upon stimulation with microbial products, macrophages produce high levels of NO, which displays potent antiproliferative effects on cells from the lymphocytic lineage (62).
Role of regulatory cytokines.
The cytokine IL-10 was first identified by its ability to inhibit IFN-γ synthesis by Th1 lymphocytes and was shown to be produced by Th2 cells (53). Similar effects of IL-10 were also observed on in vitro IFN-γ synthesis by STAg-stimulated NK cells from scid mice (203). Follow-up studies indicated that IL-10 is produced by a large variety of cells in addition to Th2 lymphocytes, including B cells and macrophages. Indeed, the latter cell population, itself, appears to be the main target of IL-10 suppressive action (153). Thus, IL-10 inhibits the synthesis of a wide variety of proinflammatory monokines by macrophages and is therefore an important modulator of macrophage effector functions against different pathogens, including T. gondii (54, 74). The main method by which IL-10 inhibits IFN-γ synthesis by NK and Th1 lymphocytes is through inhibition of macrophage IL-12 synthesis (37, 96).
Several studies suggest that induction of macrophage IL-10 synthesis enables different pathogens, including T. gondii, to avoid the induction of an excessively strong CMI and thus to allow the parasite to persist and establish chronic infection in the vertebrate host (98, 155, 201, 206). To assess the role of IL-10 in the regulation of type 1 cytokine responses in vivo, as well as in the establishment of a host-parasite equilibrium, we infected IL-10 KO mice with the avirulent ME49 strain of T. gondii. Surprisingly, the IL-10 KO mice showed enhanced susceptibility to infection, with all the mice dying by day 14 postinfection. Interestingly, mortality was delayed by depletion of CD4+ T lymphocytes, indicating a role for these cells in promoting early death. Increased mortality was associated with elevated levels of IL-12 and IFN-γ in serum relative to those in uninfected IL-10 KO or infected wild-type mice (76). Importantly, although the parasite burden was similar or decreased in the IL-10 KO mice, the animals displayed enhanced pathologic changes in the liver and, to a lesser extent, the lung tissue. Taken together, our results and similar studies by others suggest that the increased mortality of IL-10 KO mice is due to an abnormally high inflammatory cytokine response during acute toxoplasmosis rather than to uncontrolled parasite replication (76, 163). These findings indicate that IL-10 is an important physiological regulator of proinflammatory cytokine induction and that simultaneous induction of IL-10 during CMI initiation is of crucial importance in avoiding an overwhelming type 1 cytokine response.
The cytokine IL-4 has been previously shown to inhibit certain macrophage functions and to potentiate the effect of IL-10 on macrophages (166, 202). In addition, IL-4 plays a major role in controlling the development of CMI is through its effects on Thp cells, resulting in STAT 6 induction and T-cell differentiation to the Th2 phenotype (130, 174, 223). Thus, IL-4 KO mice are also more susceptible to acute T. gondii infection than are wild-type animals (179). Similar to the IL-10 KO studies, the enhanced mortality in IL-4 KO mice is accompanied by increased expression of IFN-γ and lower tissue parasitism. Although the mechanism is not yet clear, this study also suggests that IL-4, by potentiating IL-10 effects and/or antagonizing Thp-cell differentiation toward the Th1 phenotype, is another important cytokine preventing the induction of an overwhelming parasite-induced Th1 response.
Nevertheless, it should be noted that a clear view on the role of IL-4 during toxoplasmosis has yet to emerge. While some studies suggest a role for this cytokine in avoiding pathologic changes (as discussed above), others suggest that absence of this mediator may be beneficial to the host in surviving acute infection (228). Part of this confusion may be due to the use of different parasite strains, and it would also seem to indicate that the line between protective and pathologic immune responses is easily crossed.
In addition to IL-4 and IL-10, TGF-β is an important regulator of macrophage activation (227). This cytokine has been shown to influence macrophage effector function (e.g., NO production) against several protozoan parasites (9, 207). Furthermore, TGF-β potentiates the effects of IL-10 on macrophages and inhibits IL-12-induced IFN-γ synthesis by NK cells (73, 98, 166). Interestingly, the latter activity appears to operate at the level of inhibition of function, rather than synthesis, since in vivo treatment with TGF-β blocks the protective effects of rIL-12 during T. gondii infection in scid mice. While this study suggests that by inhibiting NK-cell IFN-γ synthesis, TGF-β may be a potent regulator of CMI development, this hypothesis has not yet been tested in immunocompetent mice. Nevertheless, studies with the Leishmania model show that in vivo treatment of genetically resistant mice with TGF-β favors Thp-cell differentiation toward the Th2 instead of the expected Th1 phenotype, resulting in enhanced susceptibility to the parasite (9). Therefore, several independent studies indicate that TGF-β treatment enhances susceptibility to infection with several distinct microbial pathogens by regulating the development of protective CMI.
Role of nitric oxide.
Another important mechanism by which macrophages regulate the immune response during experimental acute toxoplasmosis is through generation of NO (109). After priming with IFN-γ, macrophages exposed to microbial products or TNF-α produce high levels of reactive nitrogen intermediates (RNI). These compounds were first recognized for their importance as effector molecules responsible for microbicidal and microbiostatic functions displayed by activated murine macrophages (see below). Experiments performed both in vivo and in vitro also reveal an immunosuppressive activity associated with RNI, in particular during the early phase of infection with T. gondii (23).
Thus, during acute murine toxoplasmosis, T cells are highly impaired in the ability to produce IL-2 and proliferate upon stimulation with either parasite Ag or mitogen (23). IFN-γ synthesis is only partially suppressed (87). Interestingly, this CD4+-T-cell-suppressive effect is macrophage mediated and is inhibited by neutralization of either endogenous TNF-α or IFN-γ. Both cytokines are required for induction of optimal levels of inducible NO synthase (iNOS) and RNI (109). In addition, synthetic inhibitors of iNOS partially overcome this immunosuppressive effect (23, 87). The importance of NO as an immunoregulatory molecule is supported by findings in animals infected with T. gondii and treated with iNOS inhibitors (87). Thus, treatment with these synthetic compounds results in increased cyst numbers and an intensified inflammatory reaction in the CNS. Although not completely understood, recent studies indicate that the regulatory effects of RNI on T lymphocytes is due at least in part to the induction of apoptosis (145).
Recent findings in Toxoplasma-infected iNOS KO mice are relevant to these studies. Thus, orally infected iNOS KO mice survive early infection with decreased inflammation in the small intestine and increased survival relative to wild-type controls (123). This result suggests that some of the pathologic changes associated with acute infection involve NO-mediated effects. Indeed, these findings are reiterated in a model of T. gondii tachyzoite Ag toxicity in d-galactosamine-sensitized mice, where the lethality of the pathogen is associated with high NO levels in serum (144).
Nevertheless, iNOS KO animals succumb during chronic infection with increased parasite burden and inflammation in the brain (123, 190). Thus, in chronic infection, NO may act to down-regulate host pathologic changes that would otherwise be induced by presence of the parasite. Therefore, our view is that although parasite-induced RNI during acute infection can be detrimental, as the host enters the chronic stage of disease, NO emerges as an important regulatory molecule involved in minimizing the immunopathologic changes induced by the parasite.
EFFECTOR FUNCTIONS MEDIATED BY PARASITE-SPECIFIC CD4+ AND CD8+ T CELLS
Acquired immunity induced by T. gondii is characterized by strong CD4+ and CD8+ activity. Thus, through infection of cells, or Ag shedding, parasite peptides are efficiently presented to parasite-specific T lymphocytes. The lymphocytes that differentiate in the environment of peptide Ag and type 1 cytokines display CTL activity (in particular with respect to CD8+ effectors) and the ability to produce large amounts of IFN-γ. Production of the latter cytokine and other proinflammatory mediators is necessary for immunity, but evidence suggests that such cytokines must be tightly regulated to avoid pathologic changes.
Parasite Antigen Entry into MHC Class I and II Pathways of Presentation
Toxoplasma is a strong inducer of Ag-specific CD4+ and CD8+ T lymphocytes, indicating that parasite peptides are efficiently targeted to the appropriate cellular pathways of Ag presentation during infection. In general, processing for MHC class I presentation requires Ag entry into the cytoplasm, followed by proteolytic generation of peptides by proteasomes, transporter associated with antigen presentation-mediated transport across the endoplasmic reticulum, association with MHC class I heavy chain and β2-microglobulin, and exocytosis to the cell surface (237). Presentation in the context of MHC class II molecules generally requires soluble Ag endocytosis, proteolysis within the phagolysosome, trafficking to MHC class II-containing endosomes, association with MHC class II, and transport to the cell surface (35, 232).
For T. gondii Ag, it is not clear where peptide loading onto MHC occurs. In one model, Ag-MHC interaction could occur at the cell surface, as a result of either Ag secretion by extracellular tachyzoites or deposition on the cell surface during invasion. This model would require proteolytic degradation of parasite Ag outside of host cells and would additionally require that parasite Ag displace already bound peptide. In an alternate model, presentation of parasite Ag would depend upon phagocytosis or receptor-mediated uptake of dead or dying tachyzoites or soluble parasite Ag. Parasites entering the cell in this manner are trafficked through the endosomal-lysomal pathway. Although this route classically favors MHC class II presentation, it is now clear that peptides can access the cytosolic presentation pathway by this route (111, 154). In this regard, murine bone marrow macrophages are extremely efficient at processing and presenting to soluble Ag cytolytic CD8+ T cells in a class I-restricted manner (42, 47).
A final model would involve the transport of Ag from within the parasitophorous vacuole to the cytosolic and possibly endocytic presentation pathway. The parasitophorous vacuole is believed to function as a molecular sieve, allowing free diffusion of molecules smaller than 1,300 to 1,900 Da between the host cytoplasm and parasitophorous vacuolar space (196). This property alone would exclude macromolecular Ag from accessing the host cell cytoplasm but instead suggests that antigenic peptides are formed within the parasitophorous vacuole and that this is followed by passive diffusion into the host cell cytoplasm. Nevertheless, intact antigenic proteins could also potentially be actively transported across the parasitophorous vacuole membrane into the host cell cytoplasm for subsequent trafficking to the conventional MHC presentation pathways.
Cytolytic T-Cell Activity
Generation of T lymphocytes possessing parasite-specific cytolytic activity is a characteristic of both human and murine infection. In humans, both CD4+ and CD8+ T cells with cytolytic activity have been isolated from seropositive donors. For the most part, studies with mice provide evidence for only CD8+ cytolytic activity. The ability of these T-cell subsets to simultaneously produce the protective cytokine IFN-γ and exhibit cytolytic function has obscured the issue of whether the latter activity plays a role during infection. Nevertheless, the finding that CD8+, but not CD4+, T cells are more efficient at transferring immunity has led to the suggestion that CTL activity contributes to protection. However, recent studies in mice defective for cytolysis suggest that this function plays at best a secondary role with respect to IFN-γ production.
CD8+ CTL.
CTL are best known for their ability to kill virus-infected and transformed target cells. It is now clear that several intracellular protozoans are also effective at stimulating CD8+ CTL function against infected target cells, in part through their ability to traffic antigenic peptides into the MHC class I presentation pathway. Vaccination of mice with Plasmodium berghei and P. falciparum sporozoites generates circumsporozoite-specific CD8+ T cells capable of killing Ag-specific target cells and transferring protection to naive recipients (128, 181). Infection with Trypanosoma cruzi likewise results in the presentation of parasite-derived peptide with cell surface MHC class I glycoproteins, in turn inducing CD8+ CTL effector function (59, 164). The ability of these parasites to stimulate CTL function is most probably related to their capacity of evading the lysomal pathway during cell invasion, residing instead either directly in the cytoplasm (T. cruzi) or within a parasitophorous vacuole (Plasmodium). This may also explain why Leishmania spp., which find their niche within the macrophage phagolysosome, are relatively poor at inducing CD8+ CTL function (167, 230).
For the case of T. gondii, injection of the attenuated strain, ts-4, generates CD8+-T-cell effectors in mice. Together with CD4+ T lymphocytes, these cells confer strong protective immunity to subsequent challenge with the highly virulent RH strain (66). In addition to releasing IFN-γ in response to parasite Ag, CD8+ cells display strong MHC class I-restricted CTL activity toward infected target cells (42, 82, 212). Oral infection also results in a population of CD8α/β TCR-positive intraepithelial lymphocytes which are capable of transferring protection and which display the in vitro activities of IFN-γ secretion and cytolytic activity toward infected enterocytes (19, 29). HLA class I-restricted CD8+ CTL activity has been found in the peripheral blood of humans with acute toxoplasmosis, and CD8+ CTL clones can be cultured from peripheral blood lymphocytes of chronically infected patients (152, 173, 234). Thus, in both humans and mice, Toxoplasma infection provides a potent stimulus for the generation of CD8+ effectors capable of lysing parasite-infected target cells.
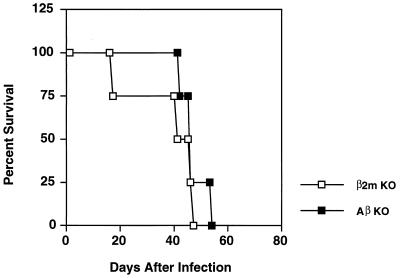
Experiments in β2-microglobulin KO mice, which lack peripheral CD8+ T lymphocytes, reveal that absence of the latter effectors, or possibly lack of MHC class I expression itself, drives preferential expansion of NK cells in response to ts-4 vaccination (43, 45). This result, which is also found when the class I KO strain is infected with lymphocytic choriomeningitis virus (209), underscores the importance of CD8+ effector cells in the normal host response to infection. Thus, when class I Ag-presenting cells are crippled by inactivation of β2-microglobulin, the murine host responds with a remarkable overproduction of NK cells, which provide partial protection through IFN-γ production (43). Nevertheless, we have also found that β2-microglobulin-deficient mice, while surviving acute infection, succumb during chronic infection with the cystogenic Toxoplasma strain, ME49 (Fig. 6).
FIG. 6.
Both β2-microglobulin and Aβ KO mice, which lack MHC class I-restricted CD8+ and class II-restricted CD4+ T lymphocytes, respectively, are susceptible to normally nonlethal T. gondii infection. Animals were infected by intraperitoneal injection of 20 cysts of the low-virulence ME49 parasite strain, and survival was monitored. Strain C57BL/6 mice (wild-type counterparts to the KO strains) do not begin to succumb to infection until approximately 100 days postinfection (48).
Although CD8+ T cells are major effectors of immunity during T. gondii infection, surprisingly little is known about the identity of the parasite Ag presented in the context of MHC class I during natural infection. However, the major surface protein expressed on the surface of tachyzoites, SAG-1 (or p30), appears capable of driving murine CD8+ responses when administered in the context of adjuvants such as Quil A (120). After immunization, CD8+ clones can be isolated; they display cytolytic activity against infected cells and release IFN-γ in response to SAG-1 in vitro (116, 119). Moreover, the clones are capable of conferring immunity to challenge infection in adoptive transfer experiments (119).
Although SAG-1 can elicit protective CD8 responses when presented in the context of MHC class I, it is clear that other parasite proteins are also recognized by CD8+ cells during infection. In this regard, when soluble tachyzoite extract is subjected to biochemical fractionation, the major cytolytic activity is contained within a fraction which does not include the SAG-1 protein when tested with splenocyte effectors from Toxoplasma-immune mice (42). In addition, peptides which sensitize human targets for CTL lysis have been isolated by acid elution from a T. gondii-infected B-cell lymphoma (3). Amino acid analysis of the peptides reveals that they are not derived from protein of any of the cloned genes of the parasite, including SAG-1. Thus, while SAG-1 can act as a target of CD8+ T cells during infection, other T. gondii Ag are likewise able to elicit CTL responses. The identity of these other Ag is, as yet, unknown.
Generation of CD8+ effectors requires the presence of CD4+ T lymphocytes during vaccination with ts-4, since removal of the latter cell type with depleting MAb results in failure to generate protective CD8 activity (70) and since removal of CD4+ cells abrogates Ag-driven CD8 proliferation (Fig. 5). Nevertheless, MHC class II (Aβ) KO mice, which lack class II-restricted CD4+ T cells, maintain the ability to generate CD8 effector cells displaying CTL activity and IFN-γ production. The resolution to this apparent paradox is that a population of CD4+ NK1.1+ (NK1) T cells provides helper function for CD8 generation (44, 46). This novel T-cell subpopulation functions independently of MHC class II but is selected during thymic ontogeny by the class I-like molecule, CD1 (10). NK1 T cells produce IL-2 early during the period of ts-4 vaccination, and this activity provides an essential helper activity for generation of CD8 effector cells.
Nevertheless, although Aβ KO mice survive acute ME49 infection, the animals succumb during early chronic infection (Fig. 6). Possibly, the reason why the KO animals survive acute infection is that CD8+ effectors, generated through NK1 T-cell IL-2 production, provide protection during acute infection. However, during chronic infection, both CD8+ and conventional MHC class II-restricted CD4+ T cells are required to prevent cyst reactivation (61), and in the absence of the latter population, the Aβ KO strain would fail to survive.
NK1 T cells have gained considerable attention recently because of their ability to release large amounts of cytokines, in particular IL-4, without the need for priming Ag. As such, these cells are believed to be positioned at the earliest stages of immune response induction and may be important in influencing the pattern of cytokines that subsequently develops (10). It is becoming increasingly clear that NK1 T cells are capable of producing other cytokines, such as IFN-γ, when appropriately stimulated (31). The data from the T. gondii studies show that IL-2 secretion is also an important functional activity of these cells.
CD4+ CTL.
While CD4+ lymphocytes are not generally considered major cytotoxic effectors, several reports indicate that Toxoplasma infection in humans results in generation of CTL of the helper T-cell phenotype (24, 36, 152, 173). Indeed, some groups report that CD4+ CTL are easier to isolate in vitro than CD8+ CTL, although the explanation for this phenomenon is presently unknown (173). Interestingly, human CTL lines display a predominant Vβ7 TCR usage, possibly indicating the presence of an immunodominant Ag (152).
With regard to the Ag specificity of human CD4+ T cells, an IFN-γ-secreting, DPw4-restricted clone was generated which possesses specificity for the rhoptry 2 (ROP-2) protein of T. gondii (88, 187). The ROP-2 protein itself possess three potential T-cell epitopes as predicted by computer algorithms (186). When these peptides were synthesized and tested in vitro, it was found T cells from a large proportion of donors seropositive for Toxoplasma responded to at least one synthetic peptide. The ROP-2 protein is therefore likely to be a major Ag recognized during the human T-lymphocyte response to the parasite.
Importance of CTL activity versus IFN-γ production.
Infection with T. gondii provides a strong stimulus for CTL activity in mice (with respect to CD8+ T cells) and humans (with respect to both CD8+ and CD4+ lymphocytes). Nevertheless, these cells are also well known for their ability to simultaneously produce high levels of IFN-γ in response to the parasite. The requirement for the latter cytokine in immunity to T. gondii (see below) has made it difficult to evaluate the contribution of CTL activity in resistance and susceptibility to the parasite.
Nevertheless, the recent construction of perforin KO mice allowed us to evaluate the role of perforin-dependent cytotoxicity in protective immunity to T. gondii (48, 229). Although the KO strain fails to develop detectable CTL or NK lytic activity after ts-4 vaccination, the animals are fully capable of resisting challenge infection with the highly virulent RH parasite strain. In addition, we found that the murine KO strain is able to survive acute ME49 infection although the same parasite strain is lethal in IFN-γ KO mice (45, 48, 192). Therefore, perforin-mediated CTL activity does not appear to be required for resistance to acute infection. While it is possible that other cytolytic mechanisms are involved (e.g., Fas/Fas ligand- and TNF-α-triggered cell death [115, 137, 239]), this seems unlikely since no cytolytic activity can be detected in the perforin KO strain, and, indeed, perforin-dependent cytolysis is generally considered the major mechanism of CTL activity (33, 171).
Despite the finding that control of acute infection does not require CTL activity, the perforin KO strain is slightly more susceptible than wild-type animals during chronic ME49 infection (48). Thus, the KO mice harbor approximately three times the number of cysts as wild-type mice, and the animals succumb to long-term chronic infection at a slightly accelerated rate. Therefore, CTL function appears to play a role in host protection during the later stages of infection, possibly contributing to the prevention of cyst reactivation or, alternatively, limiting the number of parasites initially encysting within tissues of the CNS.
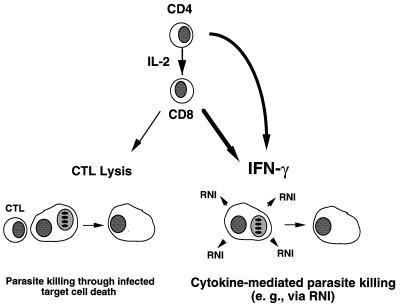
Nevertheless, ME49-infected CD8 KO (48) and β2-microglobulin KO (Fig. 6) animals die much more rapidly during chronic infection than do perforin-deficient mice; indeed, the absolute requirement for IFN-γ to survive chronic infection is well established (61, 192, 213). Together, these findings confirm the importance of CD8+ T cells during infection, and they establish that although CTL activity can contribute to control of infection, the ability to produce IFN-γ is most probably the key characteristic of this major effector population (Fig. 7).
FIG. 7.
Dual role of CD8+ T lymphocytes as effectors of immunity to T. gondii. Priming of resting CD8+ cells, which requires parasite Ag, CD4+ T cells, and IL-2, results in anti-parasite effectors. These cells display MHC class I-restricted cytolytic activity toward Toxoplasma-infected target cells and release high levels of IFN-γ in response to parasite stimulation. Based upon studies in IFN-γ and perforin KO mouse strains, cytokine secretion is the major effector activity of CD8+ cells in acute and vaccine models of infection. IFN-γ acts, at least in part, through its ability to induce macrophage microbicidal functions such as NO production. Nevertheless, CTL function appears to play a secondary functional role in protection during chronic infection, as measured by increased mortality and incidence of brain cysts in perforin KO animals.
Our findings may have parallel with studies on susceptibility of perforin KO mice to cytopathic and noncytopathic viruses. Thus, control of Semliki Forest virus and vesicular stomatitis virus (both of which are cytopathic) does not require cytolytic function, but clearance of the noncytopathic lymphocytic choriomeningitis virus is dependent upon this mechanism (113, 114, 229). This difference may reflect an inability of CTL to control a rapidly spreading pathogen, possibly a result of limited direct contact between effectors and targets. Soluble factors, namely, cytokines, which can act over distance and on multiple targets would be more important in such infections. For chronic ME49 infection, where a small number of tachyzoites would be present, cytolytic function may play a proportionately larger role in control of infection (48), similar to the macrophage microbicidal function outlined in Fig. 3B.
Production of Type 1 Cytokines
As discussed above, infection with T. gondii induces strong CMI, characterized by a highly polarized Th1-cell response (62, 67, 200). The acute phase of infection is marked by elevated levels of IFN-γ and IL-12, as well as other proinflammatory cytokines such as TNF-α, granulocyte-macrophage colony-stimulating factor, IL-6, and IL-1. Nevertheless, IL-10, an anti-inflammatory cytokine, is also induced at this stage of infection. Macrophages and/or dendritic cells are generally considered the major source of proinflammatory cytokines, as well as the anti-inflammatory mediator IL-10, during acute T. gondii infection. In addition, neutrophils, like macrophages, are capable of producing both proinflammatory and anti-inflammatory cytokines during early infection (144, 180). In the first days following inoculation with T. gondii, NK cells and T lymphocytes provide a major source of IFN-γ. Perhaps not surprisingly, given the cross-regulatory properties of type 1 and type 2 cytokines (199), infection is associated with low levels of IL-4 and IL-5 (75).
As the chronic stage of infection progresses in mice, levels of proinflammatory cytokines such as IL-1, IL-6, TNF-α, and IFN-γ decrease (as measured by presence of gene transcripts in the brain) while the level of the anti-inflammatory cytokine IL-10 increases (68). Interestingly, IL-4 can also be briefly detected early in chronic disease (days 10 to 15 after infection), but its expression rapidly falls to background levels (103). During the chronic phase, both CD4+ and CD8+ T lymphocytes are required to prevent reactivation of toxoplasmosis (61). When the latter cells are stimulated in vitro with parasite Ag, they produce high levels of both IFN-γ and IL-2. Unlike acute-stage disease, NK cells do not appear to contribute significantly to cytokine production during the persistant period of infection (75). This general pattern of a polarized type 1 cytokine response associated with chronic toxoplasmosis has also been reported in human patients (63, 142).
In susceptible mouse strains (such as C57BL/6), a moderate inflammation characterized by the presence of gene transcripts for IFN-γ, TNF-α, granulocyte-macrophage colony-stimulating factor, IL-6, IL-1, and IL-10, is found in the CNS of chronically infected animals (68, 103, 104). Interestingly, the inflammatory infiltrate appears to be dominated largely by CD8+ and CD4+ T lymphocytes (101, 193). The moderate inflammatory process may gradually increase in severity (depending upon the mouse strain and parasite dose), resulting in a high rate of mortality over a period of 2 to 6 months. Both in vitro and in vivo studies show that cytokines such as IFN-γ and TNF-α are key mediators in triggering the effector functions against T. gondii during both acute and chronic stages of infection. However, if uncontrolled, this T. gondii-induced CMI response can lead to host tissue damage, pathologic changes, and, sometimes, death.
Protective Activities of Type 1 Cytokines
Cytokines produced by T lymphocytes, such as IL-2, IFN-γ and TNF, trigger important effector mechanisms mediated by other cells of the immune system. As described above, IL-2 produced by CD4+ T lymphocytes is an important growth factor for generating CD8+-lymphocyte effector functions, namely, CTL activity and IFN-γ production. In addition, IL-2 can enhance NK cell expansion, lytic activity, and IFN-γ synthesis initiated by IL-12 (38, 125).
The cytokine IFN-γ is central in resistance to T. gondii at both acute and chronic stages of infection, as demonstrated by cytokine depletion, cytokine repletion, and gene knockout studies (70, 192, 214, 218). Although IFN-γ may play multiple roles in resistance to the parasite, macrophage activation is generally believed to be the critical effector function (2, 62). As discussed above, macrophage activation results in iNOS gene induction and synthesis of moderate levels of RNI (109). In general, RNI are produced as by-products during degradation of arginine into citrulline by iNOS. Synthesis of RNI is highly potentiated by certain microbial products, as well as by TNF-α. Indeed, pathogen-derived factors themselves may enhance RNI production indirectly, by triggering macrophage TNF-α synthesis (109). Nevertheless, results with T. gondii-infected TNF receptor KO mice, showing that NO levels are normal, suggest that the requirement for TNF-triggering is not absolute (235).
In vivo experiments are highly consistent with a role for inflammatory cytokines in resistance to T. gondii. Thus, MAb neutralization of endogenous IL-12, TNF-α, or IFN-γ leads to 100% mortality during the acute phase of infection with an avirulent parasite strain (75, 110). Similarly, neutralization of TNF-α or IFN-γ leads to decreased CNS-associated iNOS gene expression, reactivation of chronic toxoplasmosis, and death due to toxoplasmic encephalitis (TE) (68).
The importance of iNOS activity in resistance to T. gondii has recently been confirmed by infecting iNOS KO mice with an avirulent strain of T. gondii. At approximately 21 to 30 days postinfection, iNOS KO mice begin to succumb to severe TE associated with large numbers of cysts and tachyzoites, a result demonstrating the importance of endogenous NO in the control of chronic infection (190). Consistent with this finding are experiments with animals lacking the transcription factor IFN regulatory factor 1 (IRF-1), which is essential for IFN-γ iNOS induction (121). Although the IRF-1 KO animals are more susceptible to infection with T. gondii than are wild-type animals, they maintain an early line of defense, which is independent of iNOS activity.
While iNOS KO and IRF-1 KO mouse strains do not succumb until a time normally associated with cyst formation and initiation of chronic infection, neither IL-12 KO nor IFN-γ KO animals are capable of surviving beyond 10 days after T. gondii infection (218). These results together make it clear that in addition to iNOS induction, other IFN-γ-dependent mechanisms are important for controlling tachyzoite replication at both the acute and chronic stages of disease. Candidate mechanisms would be release of reactive oxygen intermediates (ROI) (161), tryptophan degradation (157, 170), or a novel mechanism that has yet to be characterized.
The role of iNOS-induced RNI as an effector mechanism of human macrophages is questionable (157, 158). However, in vitro studies indicate that IFN-γ-induced tryptophan degradation, resulting in tachyzoite growth cessation, is an important antiparasite mechanism in both human macrophages and fibroblasts (157, 170). Indeed, IFN-γ triggers the same degradative pathway in many other cell types in both human and murine systems (89). The release of ROI and generation of leukotrienes by IFN-γ-activated human macrophages has also been implicated in the control of tachyzoite replication (134, 135, 161, 236). Nevertheless, while these activities can exert a microbicidal or microbiostatic activity in vitro, their functional significance during clinical toxoplasmosis is not known.
IFN-γ has other important effects on the immune system that may contribute to resistance against T. gondii. Thus, IFN-γ is an important factor for CTL differentiation (238), as well as for promotion of upregulated MHC expression (136). Both activities would favor CTL effector function. Moreover, B-cell synthesis of specific IgG isotypes, namely, IgG1 in humans and IgG2a in mice, is driven in part by IFN-γ (52). Indeed, the parasite-specific humoral response during T. gondii infection is dominated by IgG1 and IgG2a isotypes in humans and mice, respectively (83, 106). These isotypes can play an important role in resistance to different pathogens through mechanisms such as complement fixation, opsonization, or Ab-dependent cell cytotoxicity. Nevertheless, it seems unlikely that these IFN-γ-influenced effects on components of acquired immunity would be operative at the early time of T. gondii infection during which IFN-γ KO and IL-12 KO animals succumb. First, such acquired responses would be expected to require longer to develop. Second, as already described, neither CTL nor antibodies appear to contribute significantly to early immunity to the parasite. Thus, elucidation of alternative mechanisms of IFN-γ-dependent protection is an important goal for the future.
ROLE OF T-CELL-MEDIATED IMMUNITY IN THE PATHOLOGIC CHANGES DUE TO TOXOPLASMOSIS
Toxoplasma infection rapidly overcomes hosts with impaired T-cell function and diminished ability to produce type 1 cytokines. The parasite itself is a strong stimulus of this type of immunity, perhaps reflecting an advantage in keeping the host alive during infection. In recent years, it has become clear that type I and II cytokine responses must be tightly regulated for optimal control of infection and that cytokine imbalances resulting from loss of control can play a role in the pathologic changes associated with toxoplasmosis. Nevertheless, it is difficult to experimentally separate pathologic changes caused by the parasite through direct tissue destruction from more systemic damage caused by parasite-induced cytokines.
Acute Infection
The main pathologic findings associated with acute toxoplasmosis are lymphadenopathy and fever (57), which occur simultaneously with parasite-induced activation of the immune system and concurrent release of high levels of proinflammatory cytokines. In most patients, acute toxoplasmosis is benign and will evolve to the asymptomatic stage within a few weeks of infection.
Nevertheless, congenital transmission of Toxoplasma, occurring when a pregnant female is undergoing the acute phase of a primary infection, can result in severe disease in the infant. This important topic has been expertly reviewed by others (176). Briefly, the pathological consequences for the fetus are dependent upon the trimester during which transmission occurs, and will vary in severity from mild ocular disease to death (4, 176). In general, infection occurring during the early stages of pregnancy poses more of a health risk to the fetus than that occurring later, although the risk of transmission during maternal infection increases at later stages of pregnancy. The fetal pathologic findings are thought to be related to uncontrolled parasite replication in tissues and organs (4, 176). However, recent studies indicate that induction of a strong type 1 cytokine response at the fetal-maternal interface may also result in rejection of the fetus (233). Thus, such a response could contribute to spontaneous abortion during acute toxoplasmosis in a pregnant female.
The concept that T-cell-derived cytokines may promote pathologic changes during Toxoplasma infection finds an experimental basis in recent studies on inflammatory mediator production during infection in mice. During oral infection, the susceptible C57BL/6 mouse strain develops a severe gut inflammatory reaction, characterized by severe necrosis of the villi and mucosal cells of the small intestine (131). The pathologic changes are reversed by administration of either anti-IFN-γ or anti-CD4 MAb. Indeed, these two MAb also delay the onset of death in lethally infected animals (5, 131). While the function of IFN-γ in mediating this disease process is not known, it has recently been demonstrated this cytokine induces Fas-dependent apoptosis of Peyer’s patch T cells in orally infected mice (132). Additionally, depletion of another inflammatory cytokine capable of triggering apoptosis, TNF (239), delays the death of mice undergoing acute infection with the highly virulent RH strain (144).
The above findings are reminiscent of the behavior of infected IL-10 KO animals. As described above, these mice, like orally infected C57BL/6 animals, appear to develop uncontrolled type 1 cytokine pathologic changes mediated by T lymphocytes (76, 163). In addition, recent studies in which d-galactosamine was used as a model to study mechanisms of low-dose endotoxicity suggest that granulocytes can contribute to the inflammatory pathologic changes triggered by Toxoplasma infection (144).
Thus, several lines of evidence suggest that Toxoplasma-induced inflammatory responses can contribute to the pathologic findings associated with acute infection in mice. T lymphocytes, in particular the CD4+ subset, as well as granulocytes, contribute to the response. In some circumstances, the parasite is capable of triggering a catastrophic inflammatory cytokine storm which the animals cannot survive. It is unclear whether similar pathologic changes are involved in clinical manifestations of human toxoplasmosis. Nevertheless, septic shock due to Toxoplasma has been described in patients infected with human immunodeficiency virus (HIV), suggesting that an uncontrolled inflammatory cytokine response may underlie this response (138).
Chronic Infection
In contrast to acute disease, most of the pathologic findings associated with chronic toxoplasmosis in humans are thought to be caused by lack of appropriate T-cell immunity rather than an excessively vigorous response. Immunogenetic studies have demonstrated the influence of MHC loci on the development of TE, implicating T-cell involvement in resistance (13, 16, 216, 217). Thus, the Ld gene has been shown to confer resistance to the development of disease (15), suggesting that CD8+ responses restricted by this MHC class I molecule play a protective role, through either IFN-γ production or CTL activity (48). MHC class II-restricted CD4+ T lymphocytes are similarly implicated by the finding that inbred mice carrying a mutation in the class II Aβ locus display increased cyst numbers (16). The human MHC has also recently been linked to the development of TE in AIDS patients (see below). Together, these genetic studies reinforce the importance of MHC class I- and II-restricted T-cell interactions in control of chronic disease.
Depletion of T cells in the setting of chronic infection rapidly leads to reactivation of infection and death (61). The observation that TE is accompanied by a large T-lymphocyte infiltrate may therefore indicate that these cells are recruited to provide a protective function with respect to disease (101, 193). A recent report indicates that CD4+ cells in the infected brain appear to produce several cytokines, including IL-2, IL-10, TNF-α, IFN-γ, and IL-4. The CD8+-T-cell subset produces a similar cytokine spectrum, except that IL-4 is not produced but, instead, IL-1β is synthesized (193). Thus, whether the protective effects of CD4+ and CD8+ cells result from production of proinflammatory or anti-inflammatory cytokines, or a balance of both, is at present unclear. In contrast to findings suggesting that CD4+ cells are required to prevent the reactivation of chronic toxoplasmosis, it has been reported that CD4+ depletion can limit pathologic changes during this stage of disease (5, 107). These contradictory findings may possibly be explained by the fact that inflammatory cytokines possess both beneficial and harmful effects on the host and that the outcome of infection is dependent upon tight regulation of these mediators.
Nevertheless, in general the fact remains that during T. gondii infection, TNF-α and IFN-γ appear to be important for control rather than exacerbation of chronic disease (Fig. 2) (61, 68, 213). Additionally, IL-6 KO mice display increased susceptibility to TE as measured by increased cyst number and areas of necrosis and tachyzoite-associated inflammation (219). The KO animals also have smaller numbers of inflammatory mononuclear cell infiltrates. Thus, IL-6 may mediate protection against TE by inducing the accumulation of inflammatory cells, which act to limit infection. This scenario provides a dramatic contrast to disease caused by another apicomplexan parasite, Plasmodium falciparum. Thus, cerebral malaria, which contributes to a large proportion of Plasmodium-induced fatalities, results from the lethal action of inflammatory cytokines and NO in the infected brain (79, 80).
Progression of chronic toxoplasmosis in mice correlates with rising levels of IL-10 mRNA in the CNS (68). Since the latter cytokine modulates macrophage-mediated tachyzoite killing, production of anti-inflammatory mediators may contribute to TE susceptibility (74). An alternative, but not necessarily mutually exclusive, view is that higher levels of IL-10 later during chronic infection could play a role in down-regulating the inflammatory response once the cyst burden has decreased and, in this way, could be beneficial for the host (18). In addition to IL-10, an early transient IL-4 response occurs in the brains of cyst-susceptible C57BL/10 mice (103). Furthermore, mice with a targeted inactivation of the IL-4 gene, while more susceptible to acute oral infection, display increased resistance to the establishment of tissue cysts (179). The implication of the latter result is that IL-4 acts to down-regulate CD4+-T cell-mediated type 1 cytokine-mediated pathologic changes during acute infection, as occurs in the gut of susceptible mice after oral infection (131). Nevertheless, in the chronically infected brain, IL-4 (like IL-10) functions to down-regulate protective IFN-γ, thereby promoting susceptibility to TE.
HIV-Related Pathology
In Toxoplasma-infected AIDS patients, or patients receiving immunosuppressive therapy, the parasite may reemerge, causing multiple CNS lesions with encephalopathy and focal neurological lesions (139, 162, 184). Up to 30% of HIV-positive patients infected with T. gondii will develop encephalitis caused by the parasite (146). Nevertheless, in recent years, the incidence of TE in the HIV-positive population has been decreasing, probably due to the use of the antibiotic trimethoprim-sulfamethoxazole as prophylaxis against both Pneumocystis carinii and T. gondii (17, 26, 209a).
Although an intense inflammatory process, characterized by the presence of mononuclear and polymorphonuclear cells, is observed during TE in HIV-positive patients (169), the major cause of CNS lesions is most likely to be uncontrolled parasite replication itself rather than immunopathologic changes. This hypothesis is supported by experimental models involving neutralizing MAb to IFN-γ or TNF-α, or depletion of T lymphocytes (61, 68, 213). Further support for T-cell involvement in preventing the development of TE comes from studies indicating that expression of HLA-DQ3 confers susceptibility to TE during AIDS progression (222). As revealed during HIV infection, the enhanced susceptibility to T. gondii is thought to be due mainly to decreased numbers of CD4+ T lymphocytes, but it may also reflect a decrease in IL-12 synthesis or a Th-cell switch from the Th1 to Th0 phenotype (63, 142).
Because several proinflammatory monokines are able to enhance HIV-1 replication in vitro (51, 126, 172), we decided to investigate the effects of T. gondii infection on HIV-1 replication in vitro and in vivo. As might be predicted, in vitro stimulation with tachyzoite products enhances viral replication in primary human macrophages (7). Similarly, Toxoplasma infection of transgenic mice containing multiple copies of HIV-1 proviral DNA triggers the expression of HIV-1-specific mRNA through induction of NF-κB (60). These findings suggest a double-barreled impact of HIV infection on chronic Toxoplasma infection. Thus, T. gondii is capable of enhancing HIV-1 replication within reservoir host cells; simultaneously, HIV-1 itself undermines acquired immunity to the parasite, promoting reactivation of chronic toxoplasmosis.
Ocular Toxoplasmosis
Congenital ocular toxoplasmosis is another disease manifestation which occurs late during the chronic stage of infection (176). In most instances, ocular lesions are thought to be caused by tissue damage due to parasite proliferation. Indeed, studies in the mouse model show that in vivo neutralization of TNF-α or IFN-γ, or in vivo treatment with iNOS inhibitors, also results in an intense uveitis that is associated with tachyzoite emergence and increased cyst number (64, 87).
While most cases of ocular toxoplasmosis originate from congenital transmission, this disease manifestation can occur during normally acquired infection. In this regard, epidemiological studies performed on a patient population in Erexim (situated in southern Brazil) indicate that the frequency of acquired ocular toxoplasmosis at this location is extremely high (77). Comparative studies on patients with acquired and congenital ocular disease indicate that the latter group displays very weak delayed-type hypersensitivity, as well as low T-cell proliferation and Th1 cytokine production in response to T. gondii (178). Indeed, other studies have also found that congenitally infected patients in general possess lower αβ-T-cell responsiveness later in life (84, 148).
These results are consistent with the hypothesis that congenital ocular disease may be a consequence of immunological tolerance developed by the infected fetus, manifesting later in life as an inability to control parasite growth. The data also raise the possibility that acquired ocular disease occurs as a result of an excessive inflammatory reaction elicited by parasite Ag in the eye tissues. Nevertheless, during this and other forms of Toxoplasma-associated pathologic changes (with only a few exceptions [76, 131, 144]), it is difficult to establish a detrimental role for inflammatory cytokines, since these mediators must be present to prevent lethal parasitemia.
CONCLUSIONS AND FUTURE DIRECTIONS
A wealth of data point to the major role of T lymphocytes and type 1 cytokines during experimental T. gondii infection. In the clinical setting, this is dramatically confirmed by the emergence of Toxoplasma as a major opportunistic infection during the progression of AIDS. Much progress has been made in recent years in determining how early innate responses, namely, production of cytokines such as IL-12 and TNF-α, drive the development of the strong Th1 response to Toxoplasma. While this response is crucial to the control of infection, it is becoming increasingly appreciated that an excessively vigorous response induced by the parasite can lead to pathologic changes in the host. Therefore, it is important for the host and parasite to maintain tight control of the inflammatory response. Within the teleological context of the host-parasite relationship, Toxoplasma may seek to induce strong protective host immunity, because without such a response, the parasite rapidly overcomes the host, resulting in host death and consequently in a minimal chance for the parasite to transmit itself to a new host.
Recent studies have revealed several distinct clonal Toxoplasma lineages, defined by genetic polymorphism, which differ in virulence and epidemiological patterns of occurrence (205). Variation in the spectrum of Ag expressed by the parasite is also known to occur among different Toxoplasma isolates (231). Moreover, it is probable that strain- or isolate-specific variation exerts an effect on host control of infection (215). Defining the immunological basis of such differences at the cellular and molecular level will probably be an important issue in the future.
Several distinct cell types are important in triggering and sustaining the type 1 cytokine response (e.g., CD4+ and CD8+ T lymphocytes, γδ T cells, and non-T cells such as macrophages, dendritic cells, and neutrophils), and it is therefore probable that distinct parasite Ag are involved in activation of the different cell types. Identification and cloning of these parasite molecules will be an important effort in the next several years, hopefully leading to development of vaccines capable of preventing the serious manifestations of toxoplasmosis, and, in general, deepening our knowledge of how opportunistic pathogens interact with their hosts.
ACKNOWLEDGMENTS
We thank G. Yap for a critical review of the manuscript.
R.T.G. is supported by a Biotechnology Career Fellowship from the Rockefeller Foundation and a grant from the Brazilian Government (CNPq 522.056/95-4). E.Y.D. is supported by the USDA Hatch Act and Animal Health and Disease Research Program (Cornell University College of Veterinary Medicine) and the National Institutes of Health (AI-40540).
REFERENCES
- 1.Amichay D, Gazzinelli R T, Karupiah G, Moench T, Sher A, Farber J. The genes for the chemokines MuMig and Crg-2 are induced in protozoan and viral infections in response to IFN-γ with patterns of tissue expression that suggest nonredundant roles in vivo. J Immunol. 1996;157:4511–4520. [PubMed] [Google Scholar]
- 2.Anderson S E, Remington J S. Effect of normal and activated human macrophages on Toxoplasma gondii. J Exp Med. 1974;139:1154–1174. doi: 10.1084/jem.139.5.1154. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Aosai F, Yang T-H, Ueda M, Yano A. Isolation of naturally processed peptides from a Toxoplasma gondii-infected human B lymphoma cell line that are recognized by cytotoxic T lymphocytes. J Parasitol. 1994;80:260–266. [PubMed] [Google Scholar]
- 4.Apt W B. Toxoplasmosis in developing countries. Parasitol Today. 1985;1:44–46. doi: 10.1016/0169-4758(85)90113-9. [DOI] [PubMed] [Google Scholar]
- 5.Araujo F G. Depletion of CD4+ T cells but not inhibition of the protective activity of IFN-γ prevents cure of toxoplasmosis mediated by drug therapy in mice. J Immunol. 1992;149:3003–3007. [PubMed] [Google Scholar]
- 6.Bacon C M, Petricoin E F, Ortaldo J R, Rees R C, Larner A C, Johnston J A, O’Shea J J. IL-12 induces tyrosine phosphorylation and activation of STAT 4 in human lymphocytes. Proc Natl Acad Sci USA. 1995;92:7307–7311. doi: 10.1073/pnas.92.16.7307. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Bala S, Englund G, Kovacs J, Wahl L, Martin M, Sher A, Gazzinelli R T. Toxoplasma gondii soluble products induce cytokine secretion by macrophages and potentiate in vitro replication of a monotropic strain of HIV. J Eukaryot Microbiol. 1994;41:7S. [PubMed] [Google Scholar]
- 8.Bancroft G J, Schreiber R D, Unanue E R. Natural immunity: a T-cell-independent pathway of macrophage activation defined in the scid mouse. Immunol Rev. 1991;124:5–24. doi: 10.1111/j.1600-065x.1991.tb00613.x. [DOI] [PubMed] [Google Scholar]
- 9.Barral-Netto M, Barral A, Brownell C E, Skeiky Y A W, Ellingsworth L R, Twardzik D R, Reed S G. Transforming growth factor-β in leishmanial infection: a parasite escape mechanism. Science. 1992;257:545–548. doi: 10.1126/science.1636092. [DOI] [PubMed] [Google Scholar]
- 10.Bendelac A, Rivera M N, Park S H, Roark J H. Mouse CD1-specific NK1 T cells: development, specificity, and function. Annu Rev Immunol. 1997;15:535–562. doi: 10.1146/annurev.immunol.15.1.535. [DOI] [PubMed] [Google Scholar]
- 11.Beyaert R, Cuenda A, Berghe W V, Plaisance S, Lee J C, Haegeman G, Cohen P, Fiers W. The p38/RK mitogen-activated protein kinase pathway regulates interleukin-6 synthesis in response to tumor necrosis factor. EMBO J. 1996;15:1914–1923. [PMC free article] [PubMed] [Google Scholar]
- 12.Biron C A, Gazzinelli R T. Effects of IL-12 on immune responses to microbial infections: a key mediator in regulating disease outcome. Curr Opin Immunol. 1995;77:485–496. doi: 10.1016/0952-7915(95)80093-x. [DOI] [PubMed] [Google Scholar]
- 13.Blackwell J M, Roberts C, Alexander J. Influence of genes within the MHC on mortality and brain cyst development in mice infected with Toxoplasma gondii: kinetics of immune regulation in BALB H-2 congenic mice. Parasite Immunol. 1993;15:317–324. doi: 10.1111/j.1365-3024.1993.tb00616.x. [DOI] [PubMed] [Google Scholar]
- 14.Bohne W, Heesemann J, Gros U. Reduced replication of Toxoplasma gondii is necessary for induction of bradyzoite-specific antigens: a possible role for nitric oxide in triggering stage conversion. Infect Immun. 1994;62:1761–1767. doi: 10.1128/iai.62.5.1761-1767.1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Brown C R, Hunter C A, Estes R A, Beckmann E, Forman J, David C, Remington J S, Mcleod R. Definitive identification of a gene that confers resistance against Toxoplasma cyst burden and encephalitis. Immunology. 1995;85:419–428. [PMC free article] [PubMed] [Google Scholar]
- 16.Brown C R, McLeod R. Class I MHC genes and CD8+ T cells determine cyst number in Toxoplasma gondii infection. J Immunol. 1990;145:3438–3441. [PubMed] [Google Scholar]
- 17.Bucher H C, Griffith L, Guyatt G H, Opravil M. Meta-analysis of prophylactic treatments against Pneumocystis carinii pneumonia and toxoplasma encephalitis in HIV-infected patients. J Acquired Immune Defic Syndr Hum Retrovirol. 1997;15:104–114. doi: 10.1097/00042560-199706010-00002. [DOI] [PubMed] [Google Scholar]
- 18.Burke J A, Roberts C W, Hunter C A, Murray M, Alexander J. Temporal differences in the expression of mRNA for IL-10 and IFN-γ in the brains and spleens of C57BL/6 mice infected with Toxoplasma gondii. Parasite Immunol. 1994;16:305–314. doi: 10.1111/j.1365-3024.1994.tb00353.x. [DOI] [PubMed] [Google Scholar]
- 19.Buzoni-Gatel D, Lepage A C, Dimier-Poisson I H, Bout D T, Kasper L H. Adoptive transfer of gut intraepithelial lymphocytes protects against murine infection with Toxoplasma gondii. J Immunol. 1997;158:5883–5889. [PubMed] [Google Scholar]
- 20.Reference deleted.
- 21.Reference deleted.
- 22.Camargo, M. M., A. Clifton, I. C. Almeida, L. R. Travassos, and R. T. Gazzinelli. 1997. Induction of IL-12 (p40) by microbial products requires activation of the p38/RK MAP kinase and is regulated by cyclic AMP. Mem. Inst. Oswaldo Cruz 92(Suppl.):141.
- 23.Candolfi E, Hunter C A, Remington J S. Mitogen- and antigen-specific proliferation of T cells in murine toxoplasmosis is inhibited by reactive nitrogen intermediates. Infect Immun. 1994;62:1995–2001. doi: 10.1128/iai.62.5.1995-2001.1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Canessa A, Pistoia V, Roncella S, Merli A, Melioli G, Terragna A, Ferrarini M. An in vitro model for Toxoplasma infection in man. Interaction between CD4+ monoclonal T cells and macrophages results in killing of trophozoites. J Immunol. 1988;140:3580–3588. [PubMed] [Google Scholar]
- 25.Carding S R, Allan W, Kyes S, Hayday A, Bottomly K, Doherty P C. Late dominance of the inflammatory process in murine influenza by γδ+ T cells. J Exp Med. 1990;172:1225–1231. doi: 10.1084/jem.172.4.1225. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Carr A, Tindall B, Brew B J, Marriott D J, Harkness J L, Penny R, Cooper D A. Low-dose trimethoprim-sulfamethoxazole prophylaxis for toxoplasmic encephalitis in patients with AIDS. Ann Intern Med. 1992;117:106–111. doi: 10.7326/0003-4819-117-2-106. [DOI] [PubMed] [Google Scholar]
- 27.Carson W E, Ross M E, Baiocchi R A, Marien M J, Boiani N, Grabstein K, Caligiuri M. Endogenous production of interleukin-15 by activated human monocytes is critical for optimal production of interferon-γ by natural killer cells in vitro. J Clin Investig. 1995;96:2578–2582. doi: 10.1172/JCI118321. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Chang H R, Grau G E, Pechere J C. Role of TNF and IL-1 in infections with Toxoplasma gondii. Immunology. 1990;69:33–37. [PMC free article] [PubMed] [Google Scholar]
- 29.Chardes T, Buzoni-Gatel D, Lepage A, Bernard F, Bout D. Toxoplasma gondii oral infection induces specific cytotoxic CD8α/β+ Thy-1+ gut intraepithelial lymphocytes, lytic for parasite-infected enterocytes. J Immunol. 1994;153:4596–4603. [PubMed] [Google Scholar]
- 30.Chardes T, Bourguin I, Mevelec M N, Dubremetz J F, Bout D. Antibody response to Toxoplasma gondii in the sera, intestinal secretions, and milk from orally infected mice and characterization of target antigens. Infect Immun. 1990;58:1240–1246. doi: 10.1128/iai.58.5.1240-1246.1990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Chen H, Paul W E. Cultured NK1.1+CD4+ T cells produce large amounts of IL-4 and IFN-γ upon activation by anti-CD3 or CD1. J Immunol. 1997;159:2240–2249. [PubMed] [Google Scholar]
- 32.Chien Y, Jores R, Crowley M P. Recognition by γδ T cells. Annu Rev Immunol. 1996;14:511–532. doi: 10.1146/annurev.immunol.14.1.511. [DOI] [PubMed] [Google Scholar]
- 33.Clark W R, Walsh C M, Glass A A, Hayashi F, Matloubian M, Ahmed R. Molecular pathways of CTL-mediated cytotoxicity. Immunol Rev. 1995;146:33–44. doi: 10.1111/j.1600-065x.1995.tb00682.x. [DOI] [PubMed] [Google Scholar]
- 34.Constant P, Davodeau F, Peyrat M A, Poquet Y, Puzo G, Bonneville M, Fournie J J. Stimulation of human γδ T cells buy nonpeptidic mycobacterial ligands. Science. 1994;264:267–270. doi: 10.1126/science.8146660. [DOI] [PubMed] [Google Scholar]
- 35.Cresswell P. Antigen presentation. Getting peptides into MHC class II molecules. Curr Biol. 1994;4:541–543. doi: 10.1016/s0960-9822(00)00119-6. [DOI] [PubMed] [Google Scholar]
- 36.Curiel T J, Krug E C, Purner M B, Poignard P, Berens R L. Cloned human CD4+ cytotoxic T lymphocytes specific for Toxoplasma gondii lyse tachyzoite-infected target cells. J Immunol. 1993;151:2024–2031. [PubMed] [Google Scholar]
- 37.D’Andrea A, Aste-Amezaga M, Valiante N, Ma X, Kubin M, Trinchieri G. Interleukin 10 (IL-10) inhibits human lymphocyte interferon-γ production by suppressing natural killer cell stimulatory factor/IL-12 synthesis in accessory cells. J Exp Med. 1993;178:1041–1048. doi: 10.1084/jem.178.3.1041. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.D’Andrea A M, Rengarajau M, Valiante N, Chemini J, Kubin M, Aste-Amezaga M, Chan S H, Kobayashi M, Young D, Nickbarg R, Chizzonite R, Wolf S F, Trinchieri G. Production of natural killer cell stimulatory factor (NKSF/IL-12) by peripheral blood mononuclear cells. J Exp Med. 1992;176:1387–1397. doi: 10.1084/jem.176.5.1387. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.DeKruyff R H, Gieni R S, Umetsu D T. Antigen-driven, but not lipopolysaccharide-driven, IL-12 production in macrophages requires triggering of CD40. J Immunol. 1997;158:359–366. [PubMed] [Google Scholar]
- 40.Denkers E Y, Caspar P, Heiny S, Sher A. Toxoplasma gondii induces specific nonresponsiveness in lymphocytes bearing the Vβ5 chain of the mouse T cell receptor. J Immunol. 1996;156:1089–1094. [PubMed] [Google Scholar]
- 41.Denkers E Y, Caspar P, Sher A. Toxoplasma gondii possesses a superantigen activity that selectively expands murine T cell receptor Vβ5-bearing CD8+ lymphocytes. J Exp Med. 1994;180:985–995. doi: 10.1084/jem.180.3.985. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Denkers E Y, Gazzinelli R T, Hieny S, Caspar P, Sher A. Bone marrow macrophages process exogenous Toxoplasma gondii peptides for recognition by parasite-specific cytolytic T lymphocytes. J Immunol. 1993;150:517–526. [PubMed] [Google Scholar]
- 43.Denkers E Y, Gazzinelli R T, Martin D, Sher A. Emergence of NK1.1+ cells as effectors of immunity to Toxoplasma gondii in MHC class I-deficient mice. J Exp Med. 1993;178:1465–1472. doi: 10.1084/jem.178.5.1465. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Denkers E Y, Scharton-Kersten T, Barbieri S, Caspar P, Sher A. A role for CD4+NK1.1+ T lymphocytes as MHC class II independent helper cells in the generation of CD8+ effector function against intracellular infection. J Exp Med. 1996;184:131–139. doi: 10.1084/jem.184.1.131. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Denkers E Y, Scharton-Kersten T, Gazzinelli R T, Yap G, Charest H, Sher A. Cell-mediated immunity to Toxoplasma gondii: redundant and required mechanisms as revealed by studies in gene knockout mice. In: Kaufmann S H E, editor. Medical intelligence unit: host response to intracellular pathogens. R. G. Austin, Tex: Landes Co.; 1997. pp. 167–181. [Google Scholar]
- 46.Denkers E Y, Sher A. Role of natural killer cells and NK1+ T cells in regulating cell-mediated immunity during Toxoplasma gondii infection. Biochem Soc Trans. 1997;25:699–703. doi: 10.1042/bst0250699. [DOI] [PubMed] [Google Scholar]
- 47.Denkers E Y, Sher A, Gazzinelli R T. CD8+ T cell interactions with Toxoplasma gondii: implications for processing of antigen for class I-restricted recognition. Res Immunol. 1993;144:51–56. doi: 10.1016/s0923-2494(05)80099-9. [DOI] [PubMed] [Google Scholar]
- 48.Denkers E Y, Yap G, Scharton-Kersten T, Charest H, Butcher B, Caspar P, Heiny S, Sher A. Perforin-mediated cytolysis plays a limited role in host resistance to Toxoplasma gondii. J Immunol. 1997;159:1903–1908. [PubMed] [Google Scholar]
- 49.De Paoli P, Basaglia G, Gennari D, Crovatto M, Modolo M L, Santini G. Phenotypic profile and functional characteristics of human gamma and delta T cells during murine acute toxoplasmosis. J Clin Microbiol. 1992;30:729–731. doi: 10.1128/jcm.30.3.729-731.1992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.DosReis G A, Fonseca M E F, Lopes M F. Programmed T-cell death in experimental Chagas disease. Parasitol Today. 1995;11:390–394. doi: 10.1016/0169-4758(95)80011-5. [DOI] [PubMed] [Google Scholar]
- 51.Fauci A S. Multifactorial nature of human immunodeficiency virus disease: implications for therapy. Science. 1993;262:1011–1018. doi: 10.1126/science.8235617. [DOI] [PubMed] [Google Scholar]
- 52.Finkelman F D, Holmes J, Katona I M, Urban J F, Beckmann M P, Park L S, Shooley K A, Coffman R L, Mosmann T R, Paul W E. Lymphokine control of in vivo immunoglobulin isotype selection. Annu Rev Immunol. 1990;8:303–333. doi: 10.1146/annurev.iy.08.040190.001511. [DOI] [PubMed] [Google Scholar]
- 53.Fiorentino D F, Bond M W, Mosmann T R. Two types of mouse T helper T cell clones. IV: Th2 clones secrete a factor that inhibits cytokine production of Th1 clones. J Exp Med. 1989;170:2081–2095. doi: 10.1084/jem.170.6.2081. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Fiorentino D F, Zlotnick A, Mosmann T, Howard M, O’Garra A. IL-10 inhibits cytokine production by activated macrophages. J Immunol. 1991;147:3815–3822. [PubMed] [Google Scholar]
- 55.Franzoso G, Carlson L, T. S-K, Shores E W, Epstein S, Grinberg A, Tran T, Shacter E, Leonardi A, Anver M, Love P, Sher A, Siebenlist U. Critical roles for the Bcl-3 oncoprotein in T cell-mediated immunity, splenic microarchitecture, and germinal center reactions. Immunity. 1997;6:479–490. doi: 10.1016/s1074-7613(00)80291-5. [DOI] [PubMed] [Google Scholar]
- 56.Frenkel J K. Adoptive immunity to intracellular infection. J Immunol. 1967;98:1309–1319. [PubMed] [Google Scholar]
- 57.Frenkel J K. Pathophysiology of toxoplasmosis. Parasitol Today. 1988;4:273–278. doi: 10.1016/0169-4758(88)90018-x. [DOI] [PubMed] [Google Scholar]
- 58.Gajewski T F, Fitch F W. Anti-proliferative effect of IFN-γ in immunoregulation. I. IFN-γ inhibits the proliferation of Th2 but not Th1 murine helper T lymphocyte clones. J Immunol. 1988;140:4245–4252. [PubMed] [Google Scholar]
- 59.Garg N, Nunes M P, Tarleton R L. Delivery by Trypanosoma cruzi of proteins into the MHC class I antigen processing and presentation pathway. J Immunol. 1997;158:3293–3302. [PubMed] [Google Scholar]
- 60.Gazzinelli R, Sher A, Cheever A, Gerstberger S, Martin M A, Dickie P. Infection of human immunodeficiency virus transgenic mice with Toxoplasma gondii stimulates proviral transcription in macrophages in vivo. J Exp Med. 1997;183:1645–1655. doi: 10.1084/jem.183.4.1645. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Gazzinelli R, Xu Y, Hieny S, Cheever A, Sher A. Simultaneous depletion of CD4+ and CD8+ T lymphocytes is required to reactivate chronic infection with Toxoplasma gondii. J Immunol. 1992;149:175–180. [PubMed] [Google Scholar]
- 62.Gazzinelli R T, Amichay D, Scharton-Kersten T, Grunvald E, Farber J M, Sher A. Role of macrophage-derived cytokines in the induction and regulation of cell mediated immunity to Toxoplasma gondii. Curr Top Microbiol Immunol. 1996;219:127–140. doi: 10.1007/978-3-642-51014-4_12. [DOI] [PubMed] [Google Scholar]
- 63.Gazzinelli R T, Bala S, Stevens R, Baseler M, Wahl L, Kovacs J, Sher A. HIV infection suppresses Type 1 lymphokine and IL-12 responses to Toxoplasma gondii but fails to inhibit the synthesis of other parasite-induced monokines. J Immunol. 1995;155:1565–1574. [PubMed] [Google Scholar]
- 64.Gazzinelli R T, Brezin A, Li Q, Nussenblatt R B, Chan C C. Toxoplasma gondii: acquired ocular toxoplasmosis in the murine model, protective role of TNF-α and IFN-γ. Exp Parasitol. 1994;78:217–229. doi: 10.1006/expr.1994.1022. [DOI] [PubMed] [Google Scholar]
- 65.Reference deleted.
- 66.Gazzinelli R T, Denkers E Y, Hakim F T, Sher A. Immunologic control of Toxoplasma gondii infection by CD8+ lymphocytes: a model for class I restricted recognition of intracellular parasites. In: Sitkovsky M, Henkart P, editors. Cytotoxic cells: generation, triggering, effector functions, methods. Boston, Mass: Birkhauser Press; 1993. pp. 370–377. [Google Scholar]
- 67.Gazzinelli R T, Denkers E Y, Sher A. Host resistance to Toxoplasma gondii: model for studying the selective induction of cell-mediated immunity by intracellular parasites. Infect Agents Dis. 1993;2:139–149. [PubMed] [Google Scholar]
- 68.Gazzinelli R T, Eltoum I, Wynn T A, Sher A. Acute cerebral toxoplasmosis is induced by in vivo neutralization of TNF-α and correlates with the down-regulated expression of inducible nitric oxide synthase and other markers of macrophage activation. J Immunol. 1993;151:3672–3681. [PubMed] [Google Scholar]
- 69.Gazzinelli R T, Hakim F T, Hieny S, Shearer G M, Sher A. Opportunistic infections and retrovirus-induced immunodeficiency: studies of acute and chronic infections with Toxoplasma gondii in mice infected with LP-BM5 murine leukemia viruses. Infect Immun. 1991;60:4394–4401. doi: 10.1128/iai.60.10.4394-4401.1992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Gazzinelli R T, Hakim F T, Hieny S, Shearer G M, Sher A. Synergistic role of CD4+ and CD8+ T lymphocytes in IFN-γ production and protective immunity induced by an attenuated T. gondii vaccine. J Immunol. 1991;146:286–292. [PubMed] [Google Scholar]
- 71.Gazzinelli R T, Hieny S, Wynn T, Wolf S, Sher A. IL-12 is required for the T-cell independent induction of IFN-γ by an intracellular parasite and induces resistance in T-cell-deficient hosts. Proc Natl Acad Sci USA. 1993;90:6115–6119. doi: 10.1073/pnas.90.13.6115. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Reference deleted.
- 73.Gazzinelli R T, Oswald I P, Hieny S, James S, Sher A. The microbicidal activity of interferon-γ-treated macrophages against Trypanosoma cruzi involves an l-arginine-dependent, nitrogen oxide-mediated mechanism inhibitable by interleukin-10 and transforming growth factor-β. Eur J Immunol. 1992;22:2501–2506. doi: 10.1002/eji.1830221006. [DOI] [PubMed] [Google Scholar]
- 74.Gazzinelli R T, Oswald I P, James S, Sher A. IL-10 inhibits parasite killing and nitrogen oxide production by IFN-γ activated macrophages. J Immunol. 1992;148:1792–1796. [PubMed] [Google Scholar]
- 75.Gazzinelli R T, Wysocka M, Hayashi S, Denkers E Y, Hieny S, Caspar P, Trinchieri G, Sher A. Parasite-induced IL-12 stimulates early IFN-γ synthesis and resistance during acute infection with Toxoplasma gondii. J Immunol. 1994;153:2533–2543. [PubMed] [Google Scholar]
- 76.Gazzinelli R T, Wysocka M, Hieny S, Scharton-Kersten T, Cheever A, Kuhn R, Muller W, Trinchieri G, Sher A. In the absence of endogenous IL-10, mice acutely infected with Toxoplasma gondii succumb to a lethal immune response dependent upon CD4+ T cells and accompanied by overproduction of IL-12, IFN-γ, and TNF-α. J Immunol. 1996;157:798–805. [PubMed] [Google Scholar]
- 77.Glasner P D, Silveira C, Kruzon-Moran D, Martins M C, Burnier M, Silveira S, Camargo M E, Nussenblatt R A, Kaslow R A, Belfort J. An unusually high prevalence of ocular toxoplasmosis in Southern Brazil. Am J Ophthamol. 1992;114:136–144. doi: 10.1016/s0002-9394(14)73976-5. [DOI] [PubMed] [Google Scholar]
- 78.Goerlich R, Hacker G, Pfeffer K, Heeg K, Wagner H. Plasmodium falciparum merozoites primarily stimulate the Vγ9 subset of human γδ T cells. Eur J Immunol. 1991;21:2613–2616. doi: 10.1002/eji.1830211045. [DOI] [PubMed] [Google Scholar]
- 79.Grau G E, Farjardo L F, Piguet P F, Allet B, Lambert P H, Vassali P. Tumor necrosis factor (cachectin) as an essential mediator in murine cerebral malaria. Science. 1987;237:1210–1212. doi: 10.1126/science.3306918. [DOI] [PubMed] [Google Scholar]
- 80.Grau G E, Heremans H, Piguet P-F, Poiuntaire P, Lambert P-H, Billiau A, Vassalli P. Monoclonal antibody against interferon-γ can prevent experimental cerebral malaria and its associated overproduction of tumor necrosis factor. Proc Natl Acad Sci USA. 1989;86:5572–5574. doi: 10.1073/pnas.86.14.5572. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Reference deleted.
- 82.Hakim F T, Gazzinelli R T, Denkers E, Hieny S, Shearer G M, Sher A. CD8+ T cells from mice vaccinated against Toxoplasma gondii are cytotoxic for parasite-infected or antigen-pulsed host cells. J Immunol. 1991;147:2310–2316. [PubMed] [Google Scholar]
- 83.Handman E, Remington J S. Antibody response to Toxoplasma antigens in mice infected with strains of different virulence. Infect Immun. 1980;40:215–225. doi: 10.1128/iai.29.1.215-220.1980. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Hara T, Ohashi S, Yamashita Y, Abe T, Hisaeda H, Himeno K, Good R A, Takeshita K. Human Vδ2+ γδ T-cell tolerance to foreign antigens of Toxoplasma gondii. Proc Natl Acad Sci USA. 1996;93:5136–5140. doi: 10.1073/pnas.93.10.5136. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Hauser W E, Sharma S D, Remington J S. Natural killer cells induced by acute and chronic Toxoplasma infection. Cell Immunol. 1982;69:330–346. doi: 10.1016/0008-8749(82)90076-4. [DOI] [PubMed] [Google Scholar]
- 86.Hauser W E, Tsai V. Acute Toxoplasma infection of mice induces spleen NK cells that are cytotoxic for T. gondii in vitro. J Immunol. 1986;136:313–319. [PubMed] [Google Scholar]
- 87.Hayashi S, Chan C C, Gazzinelli R, Roberge F G. Contribution of nitric oxide to the host parasite equilibrium in toxoplasmosis. J Immunol. 1996;154:1476–1481. [PubMed] [Google Scholar]
- 88.Herion P, Hernandez-Pando R, Dubremetz J F, Saavedra R. Subcellular localization of the 54-kDa antigen of Toxoplasma gondii. J Parasitol. 1993;79:216–222. [PubMed] [Google Scholar]
- 89.Heyes M P, Saito K, Crowley J S. Quinolinic acid and kynurenine pathway in inflammatory and non-inflammatory neurological disease. Brain. 1992;115:1249–1273. doi: 10.1093/brain/115.5.1249. [DOI] [PubMed] [Google Scholar]
- 90.Hibbs J B, Lambert L H, Remington J S. Resistance to murine tumours conferred by chronic infection with intracellular protozoa. J Infect Dis. 1971;124:587–592. doi: 10.1093/infdis/124.6.587. [DOI] [PubMed] [Google Scholar]
- 91.Hisaeda H, Nagasawa H, Maeda K, Maekawa Y, Ishikawa H, Ito Y, Good R A, Himeno K. γδ T cells play an important role in hsp65 expression and acquiring protective immune responses against infection with Toxoplasma gondii. J Immunol. 1995;154:244–251. [PubMed] [Google Scholar]
- 92.Hisaeda H, Sakai T, Ishikawa H, Maekawa Y, Yasutomo K, Good R A, Himeno K. Heat shock protein 65 induced by γδ T cells prevents apoptosis of macrophages and contributes to host defense in mice infected with Toxoplasma gondii. J Immunol. 1997;159:2375–2381. [PubMed] [Google Scholar]
- 93.Hisaeda H, Sakai T, Maekawa Y, Ishikawa H, Yasutomo K, Himeno K. Mechanisms of HSP65 expression induced by γδ T cells in murine Toxoplasma gondii infection. Pathobiology. 1996;64:198–203. doi: 10.1159/000164048. [DOI] [PubMed] [Google Scholar]
- 94.Hohlfeld P, Forestier F, Marion S, Thulliez P, Marcon P, Daffos F. Toxoplasma gondii infection during pregnancy: T lymphocyte subpopulations in mothers and fetuses. Pediatr Infect Dis J. 1990;9:878–881. doi: 10.1097/00006454-199012000-00004. [DOI] [PubMed] [Google Scholar]
- 95.Holtschke T, Lohler J, Kanno J, Fehr T, Giese N, Rosenbauer F, Lou J, Knobeloch K, Gabriele L, Waring J F, Bachmann M F, Zinkernagel R M, Morse III H M, Ozato K, Horak I. Immunodeficiency and chronic myelogenous leukemia-like syndrome in mice with a targeted mutation of the ICSBP gene. Cell. 1996;87:307–317. doi: 10.1016/s0092-8674(00)81348-3. [DOI] [PubMed] [Google Scholar]
- 96.Hsieh C S, Macatonia S E, Tripp C S, Wolf S, O’Garra A, Murphy K M. Development of Th1 CD4+ T cells through IL-12 produced by Listeria-induced macrophages. Science. 1993;260:547–549. doi: 10.1126/science.8097338. [DOI] [PubMed] [Google Scholar]
- 97.Hunter C A, Abrams J S, Beaman M H, Remington J S. Cytokine mRNA in the CNS of SCID mice infected with Toxoplasma gondii: importance of T-cell independent regulation of resistance to T. gondii. Infect Immun. 1993;61:4038–4044. doi: 10.1128/iai.61.10.4038-4044.1993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 98.Hunter C A, Bermudez L, Beernink H, Waegell W, Remington J S. Transforming growth factor-beta inhibits interleukin-12-induced production of interferon-gamma by natural killer cells: a role for transforming growth factor-beta in regulation of T cell-independent resistance to Toxoplasma gondii. Eur J Immunol. 1995;25:994–1000. doi: 10.1002/eji.1830250420. [DOI] [PubMed] [Google Scholar]
- 99.Hunter C A, Candolfi E, Subauste C, Van Cleave V, Remington J S. Studies on the role of IL-12 in murine toxoplasmosis. Immunology. 1995;84:16–21. [PMC free article] [PubMed] [Google Scholar]
- 100.Hunter C A, Chizzonite R, Remington J S. IL-1β is required for IL-12 to induce production of IFN-γ by NK cells. J Immunol. 1995;155:4347–4354. [PubMed] [Google Scholar]
- 101.Hunter C A, Litton M J, Remington J S, Abrams J S. Immunocytochemical detection of cytokines in the lymph nodes and brains of mice resistant or susceptible to toxoplasmic encephalitis. J Infect Dis. 1994;170:939–945. doi: 10.1093/infdis/170.4.939. [DOI] [PubMed] [Google Scholar]
- 102.Hunter C A, Neyer L E, Gabriel K E, Kennedy M K, Grabstein K H, Linsley P S, Remington J S. The role of the CD28/B7 interaction in regulation of NK cell responses during infection with Toxoplasma gondii. J Immunol. 1997;158:2285–2293. [PubMed] [Google Scholar]
- 103.Hunter C A, Roberts C W, Alexander J. Kinetics of cytokine mRNA production in the brains of mice with progressive toxoplasmic encephalitis. Eur J Immunol. 1992;22:2317–2322. doi: 10.1002/eji.1830220921. [DOI] [PubMed] [Google Scholar]
- 104.Hunter C A, Roberts C W, Murray M, Alexander J. Detection of cytokine mRNA in the brains of mice with toxoplasmic encephalitis. Parasite Immunol. 1992;14:405–413. doi: 10.1111/j.1365-3024.1992.tb00015.x. [DOI] [PubMed] [Google Scholar]
- 105.Hunter C A, Subauste C S, Van Cleave V H, Remington J S. Production of gamma interferon by natural killer cells from Toxoplasma gondii-infected SCID mice: regulation by interleukin-10, interleukin-12, and tumor necrosis factor alpha. Infect Immun. 1994;62:2818–2824. doi: 10.1128/iai.62.7.2818-2824.1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 106.Huskinson J, Stepick-Biek P N, Araujo F G, Thulliez P, Suzuki Y, Remington J S. Toxoplasma antigens recognized by immunoglobulin G subclasses during acute and chronic infection. J Clin Microbiol. 1989;27:2031–2038. doi: 10.1128/jcm.27.9.2031-2038.1989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 107.Israelski D M, Araujo F G, Conley F K, Suzuki Y, Sharma S, Remington J S. Treatment with anti-L3T4 (CD4) monoclonal antibody reduces the inflammatory response in toxoplasmic encephalitis. J Immunol. 1989;142:954–958. [PubMed] [Google Scholar]
- 108.Jacobson N G, Szabo S J, Weber-Nordt R M, Zhong Z, Schreiber R D, Darnell J E, Murphy K M. Interleukin-12 signaling in T helper type 1 cells involves tyrosine phosphorylation of signal transducer and activator of transcription (STAT) s and STAT 4. J Exp Med. 1995;181:1755–1762. doi: 10.1084/jem.181.5.1755. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 109.James S L. Role of nitric oxide in parasitic infections. Microbiol Rev. 1995;59:533–547. doi: 10.1128/mr.59.4.533-547.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 110.Johnson L L. A protective role for endogenous tumor necrosis factor in Toxoplasma gondii infection. Infect Immun. 1992;60:1979–1985. doi: 10.1128/iai.60.5.1979-1983.1992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 111.Joiner K A, Fuhrman S A, Miettinen H M, Kasper L H, Mellman I. Toxoplasma gondii: fusion competence of parasitophorous vacuoles in Fc receptor-transfected fibroblasts. Science. 1990;249:641–646. doi: 10.1126/science.2200126. [DOI] [PubMed] [Google Scholar]
- 112.Kabelitz D, Bender A, Prospero T, Wesselborg S, Pechhold K. The primary response of human γδ+ T cells to Mycobacterium tuberculosis is restricted to Vγ9+ bearing cells. J Exp Med. 1991;173:1331–1338. doi: 10.1084/jem.173.6.1331. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 113.Kagi D, Ledermann B, Burki K, Seiler P, Odermatt B, Olsen K, Podack E, Zinkernagel R, Hengartner H. Cytotoxicity mediated by T cells and natural killer cells is greatly impaired in perforin-deficient mice. Nature. 1994;369:31–37. doi: 10.1038/369031a0. [DOI] [PubMed] [Google Scholar]
- 114.Kagi D, Seiler P, Pavlovic J, Ledermann B, Burki K, Zinkernagal R M, Hengartner H. The roles of perforin- and Fas-dependent cytotoxicity in protection against cytopathic and noncytopathic viruses. Eur J Immunol. 1995;25:3256–3262. doi: 10.1002/eji.1830251209. [DOI] [PubMed] [Google Scholar]
- 115.Kagi D, Vignaux F, Ledermann B, Burki K, Depraetere V, Nagata S, Hengartner H, Golstein P. Fas and perforin pathways as major mechanisms of T cell-mediated cytotoxicity. Science. 1994;265:528–530. doi: 10.1126/science.7518614. [DOI] [PubMed] [Google Scholar]
- 116.Kasper L H, Khan I A, Ely K H, Buelow R, Boothroyd J C. Antigen-specific (p30) mouse CD8+ T cells are cytotoxic against Toxoplasma gondii-infected peritoneal macrophages. J Immunol. 1992;148:1493–1498. [PubMed] [Google Scholar]
- 117.Kasper L H, Matsuura T, Fonseka S, Arruda J, Channon J Y, Khan I A. Induction of γδ T cells during acute murine infection with Toxoplasma gondii. J Immunol. 1996;157:5521–5527. [PubMed] [Google Scholar]
- 118.Kaufmann S H E, Ladel C H. Role of T cell subsets in immunity against intracellular bacteria: experimental infections of knock-out mice with Listeria monocytogenes and Mycobacterium bovis BCG. Immunobiology. 1994;191:509–519. doi: 10.1016/S0171-2985(11)80457-2. [DOI] [PubMed] [Google Scholar]
- 119.Khan I, Ely K H, Kasper L H. Antigen-specific CD8+ T cell clone protects against acute Toxoplasma gondii infection in mice. J Immunol. 1994;152:1856–1860. [PubMed] [Google Scholar]
- 120.Khan I A, Ely K H, Kasper L H. A purified parasite antigen (p30) mediates CD8+ T cell immunity against fatal Toxoplasma gondii infection in mice. J Immunol. 1991;147:3501–3506. [PubMed] [Google Scholar]
- 121.Khan I A, Matsuura T, Fonseka S, Kasper L H. Production of nitric oxide (NO) is not essential for protection against acute Toxoplasma gondii infection in IRF-1−/− mice. J Immunol. 1996;156:636–643. [PubMed] [Google Scholar]
- 122.Khan I A, Matsuura T, Kasper L H. Activation-mediated CD4+ T cell unresponsiveness during acute Toxoplasma gondii infection in mice. Int Immunol. 1996;8:887–896. doi: 10.1093/intimm/8.6.887. [DOI] [PubMed] [Google Scholar]
- 123.Khan I A, Schwartzman J D, Matsuura T, Kasper L H. A dichotomous role for nitric oxide during acute Toxoplasma gondii infection in mice. Proc Natl Acad Sci USA. 1997;94:13955–13960. doi: 10.1073/pnas.94.25.13955. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 124.Khatri V P, Fehniger T A, Baiocchi R A, Yu F, Shah M H, Schiller D S, Gould M, Gazzinelli R T, Bernstein Z P, Caligiuri M A. Ultra low dose IL-2 therapy promotes a type 1 cytokine profile in vivo in patients with AIDS and AIDS-associated malignancies. J Clin Investig. 1998;101:1373–1378. doi: 10.1172/JCI2038. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 125.Kobayashi M, Fitz L, Ryan M, Hewik R M, Clark S C, Chan S, Louden R, Sherman F, Perussia B, Trinchieri G. Identification and purification of natural killer cell stimulatory factor (NKSF), a cytokine with multiple biological effects on human lymphocytes. J Exp Med. 1989;170:827–845. doi: 10.1084/jem.170.3.827. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 126.Koyanagi Y, O’Brian W A, Zhao J Q, Golde D W, Gasson J C, Chen I S Y. Cytokines alter production of HIV-1 from primary mononuclear phagocytes. Science. 1988;241:1673–1675. doi: 10.1126/science.241.4873.1673. [DOI] [PubMed] [Google Scholar]
- 127.Krettli A U, Brenner Z. Protective effects of specific antibodies in Trypanosoma cruzi infection. J Immunol. 1976;116:755–760. [PubMed] [Google Scholar]
- 128.Kumar S, Miller L M, Quakyi I A, Keister D B, Houghten R A, Maloy W L, Moss B, Berzofsky J A, Good M F. Cytotoxic T cells specific for the circumsporozoite protein of Plasmodium falciparum. Nature. 1988;334:258–260. doi: 10.1038/334258a0. [DOI] [PubMed] [Google Scholar]
- 129.Lee J, Laydon J T, McDonnell P C, Gallagher T F, Kumar S, Green D, McNulty D, Blumenthal M J, Heys J R, Landvatter S W, Strickler J E, Maclaughlin M M, Siemens I R, Fisher S M, Livi G P, White J R, Adams J L, Young P R. A protein kinase involved in the regulation of inflammatory cytokine biosynthesis. Nature. 1994;372:739–746. doi: 10.1038/372739a0. [DOI] [PubMed] [Google Scholar]
- 130.Le Gros G, Ben-Sasson S Z, Seder R, Finkelman F D, Paul W E. Generation of interleukin 4 (IL-4)-producing cells in vivo and in vitro: IL-2 and IL-4 are required for in vitro generation of IL-4-producing cells. J Exp Med. 1990;172:921–929. doi: 10.1084/jem.172.3.921. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 131.Liesenfeld O, Kosek J, Remington J S, Suzuki Y. Association of CD4+ T cell-dependent, IFN-γ-mediated necrosis of the small intestine with genetic susceptibility of mice to peroral infection with Toxoplasma gondii. J Exp Med. 1996;184:597–607. doi: 10.1084/jem.184.2.597. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 132.Liesenfeld O, Kosek J C, Suzuki Y. Gamma interferon induces Fas-dependent apoptosis of Peyer’s patch T cells in mice following peroral infection with Toxoplasma gondii. Infect Immun. 1997;65:4682–4689. doi: 10.1128/iai.65.11.4682-4689.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 133.Lindberg R E, Frenkel J K. Toxoplasmosis in nude mice. J Parasitol. 1977;63:219–221. [PubMed] [Google Scholar]
- 134.Locksley R M, Fankhauser J, Henderson W R. Alteration of leukotriene release by macrophages ingesting Toxoplasma gondii. Proc Natl Acad Sci USA. 1985;82:6922–6927. doi: 10.1073/pnas.82.20.6922. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 135.Locksley R M, Klebanoff S J. Oxygen-dependent microbicidal systems of phagocytes against intracellular protozoa. J Cell Biochem. 1983;22:173–180. doi: 10.1002/jcb.240220306. [DOI] [PubMed] [Google Scholar]
- 136.Loh J E, Chang C H, Fodor W L, Flavell R A. Dissection of the interferon gamma-MHC class II signal transduction pathway reveals that type I and type II interferon systems share common signalling component(s) EMBO J. 1992;11:1351–1363. doi: 10.1002/j.1460-2075.1992.tb05180.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 137.Lowin B, Hahne M, Mattmann C, Tschopp J. Cytolytic T-cell cytotoxicity is mediated through perforin and Fas lytic pathways. Nature. 1994;370:650–652. doi: 10.1038/370650a0. [DOI] [PubMed] [Google Scholar]
- 138.Lucet J-C, Bailly M-P, Bedos J-P, Wolff M, Gachot B, Vachon F. Septic shock due to toxoplasmosis in patients with the human immunodeficiency virus. Chest. 1993;104:1054–1058. doi: 10.1378/chest.104.4.1054. [DOI] [PubMed] [Google Scholar]
- 139.Luft B J, Brooks R G, Conley F K, McCabe R E, Remington J S. Toxoplasmic encephalitis in patients with acquired immune response deficiency syndrome. JAMA. 1984;252:913–917. [PubMed] [Google Scholar]
- 140.Luft B J, Kansas G, Engleman E G, Remington J S. Functional and quantitative alterations in T lymphocyte subpopulations in acute toxoplasmosis. J Infect Dis. 1984;150:761–767. doi: 10.1093/infdis/150.5.761. [DOI] [PubMed] [Google Scholar]
- 141.Ma X, Chow J M, Gri G, Carra G, Gerosa F, Wolf S F, Dzialo R, Trinchieri G. The interleukin-12 p40 promoter is primed by interferon-γ in monocytic cells. J Exp Med. 1996;183:147–157. doi: 10.1084/jem.183.1.147. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 142.Maggi E, Mazzetti M, Ravina A, Annunziato F, Carli M D, Piccini M P, Manetti R, Carbonari M, Pesce A M, Del Prete G, Romagnani S. Ability of HIV to promote a Th1 to Th0 shift and to replicate preferentially in Th2 and Th0 cells. Science. 1994;265:244–248. doi: 10.1126/science.8023142. [DOI] [PubMed] [Google Scholar]
- 143.Mahmoud A A F, Warren K S, Strickland G T. Acquired resistance to infection with Schistosoma mansoni induced by Toxoplasma gondii. Nature. 1976;263:56–57. doi: 10.1038/263056a0. [DOI] [PubMed] [Google Scholar]
- 144.Marshall A J, Denkers E Y. Toxoplasma gondii triggers granulocyte-dependent, cytokine-mediated lethal shock in d-galactosamine sensitized mice. Infect Immun. 1998;66:1325–1333. doi: 10.1128/iai.66.4.1325-1333.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 145.Martins, G. A., M. G. A. Cardoso, J. C. S. Aliberti, and J. S. Silva. 1995. Modulation of apoptosis by nitric oxide in Trypanosoma cruzi infected mice. Mem. Inst. Oswaldo Cruz 90(Suppl.):171.
- 146.McCabe R, Remington J S. Toxoplasmosis: the time has come. N Eng J Med. 1988;380:313–315. doi: 10.1056/NEJM198802043180509. [DOI] [PubMed] [Google Scholar]
- 147.McLeod R, Brown C, Mack D. Immunogenetics influence outcome of Toxoplasma gondii infection. Res Immunol. 1993;144:61–65. doi: 10.1016/s0923-2494(05)80101-4. [DOI] [PubMed] [Google Scholar]
- 148.McLeod R, Mack D R, Boyer K, Mets M, Roizen N, Swisher C, Patel D, Beckmann E, Vitullo D, Johnson D, Meier P. Phenotypes and functions of lymphocytes in congenital toxoplasmosis. J Lab Clin Med. 1990;116:623–635. [PubMed] [Google Scholar]
- 149.McLeod R, Skamene E, Brown C R, Eisenhauer P B, Mack D M. Genetic regulation of early survival and cyst number after peroral Toxoplasma gondii infection of AxB/BxA recombinant inbred and B10 congenic mice. J Immunol. 1989;143:3031–3034. [PubMed] [Google Scholar]
- 150.Mixter P F, Camerini V, Stone B J, Miller V L, Kronenberg M. Mouse T lymphocytes that express a γδ T-cell antigen receptor contribute to resistance to Salmonella infection in vivo. Infect Immun. 1994;62:4618–4621. doi: 10.1128/iai.62.10.4618-4621.1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 151.Mombaerts P, Arnoldi J, Russ F, Tonegawa S, Kaufmann S H E. Different roles for αβ and γδ T cells in immunity against an intracellular pathogen. Nature. 1993;365:53–56. doi: 10.1038/365053a0. [DOI] [PubMed] [Google Scholar]
- 152.Montoya J G, Lowe K E, Clayberger C, Moody D, Do D, Remington J S, Talib S, Subauste C S. Human CD4+ and CD8+ T lymphocytes are both cytotoxic to Toxoplasma gondii-infected cells. Infect Immun. 1996;64:176–181. doi: 10.1128/iai.64.1.176-181.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 153.Moore K W, O’Garra A, Malefyt R W, Vieira P, Mosmann T R. Interleukin-10. Annu Rev Immunol. 1993;11:165–190. doi: 10.1146/annurev.iy.11.040193.001121. [DOI] [PubMed] [Google Scholar]
- 154.Mordue D G, Sibley L D. Intracellular fate of vacuoles containing Toxoplasma gondii is determined at the time of formation and depends upon the mechanism of entry. J Immunol. 1997;159:4452–4459. [PubMed] [Google Scholar]
- 155.Mosmann T R, Moore K W. The role of IL-10 in crossregulation of Th1 and Th2 responses. In: Ash C, Gallagher R B, editors. Immunoparasitology today. Cambridge, United Kingdom: Elsevier Trade Journals; 1991. pp. A49–A53. [DOI] [PubMed] [Google Scholar]
- 156.Murphy T, Cleveland M, Kulezka P, Magram J, Murphy K. Regulation of interleukin 12 p40 expression through an NF-κB half-site. Mol Cell Biol. 1995;15:5258–5267. doi: 10.1128/mcb.15.10.5258. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 157.Murray H W, Szuro-Sudol A, Wellner D, Oca M J, Granger A M, Libby D M, Rothermel C D, Rubin B Y. Role of tryptophan degradation in respiratory burst-independent antimicrobial activity of gamma interferon-stimulated human macrophages. Infect Immun. 1989;57:845–849. doi: 10.1128/iai.57.3.845-849.1989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 158.Murray H W, Teitelbaum R. l-Arginine-dependent reactive nitrogen intermediates and the antimicrobial effect of activated human mononuclear phagocytes. J Infect Dis. 1992;165:513–517. doi: 10.1093/infdis/165.3.513. [DOI] [PubMed] [Google Scholar]
- 159.Nagasawa H, Hisaeda H, Maekawa Y, Fujioka H, Ito Y, Aikawa M. γδ T cells play a crucial role in the expression of 65000 MW heat-shock protein in mice immunized with Toxoplasma antigen. Immunology. 1994;83:347–352. [PMC free article] [PubMed] [Google Scholar]
- 160.Nagasawa H, Oka M, Maedi K-M, Jian-Guo C, Hisaeda H, Ito Y, Good R A, Himeno K. Induction of a heat shock protein closely correlates with protection against Toxoplasma gondii infection. Proc Natl Acad Sci USA. 1992;89:3155–3158. doi: 10.1073/pnas.89.7.3155. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 161.Nathan C, Nogueira N, Juangbhanich C, Ellis J, Cohn Z. Activation of macrophages: in vivo and in vitro correlation between hydrogen peroxide release and killing of Trypanosoma cruzi. J Exp Med. 1979;149:1056–1066. doi: 10.1084/jem.149.5.1056. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 162.Navia B A, Petito C K, Gold J W M, Cho E S, Jordon B D, Price J W. Cerebral toxoplasmosis complicating the acquired immune deficiency syndrome: clinical and neuropathological findings in 27 patients. Ann Neurol. 1986;19:224–238. doi: 10.1002/ana.410190303. [DOI] [PubMed] [Google Scholar]
- 163.Neyer L E, Grunig G, Fort M, Remington J S, Rennick D, Hunter C A. Role of interleukin-10 in regulation of T-cell-dependent and T-cell-independent mechanisms of resistance to Toxoplasma gondii. Infect Immun. 1997;65:1675–1682. doi: 10.1128/iai.65.5.1675-1682.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 164.Nickell S P, Stryker G A, Arevalo C. Isolation from Trypanosoma cruzi-infected mice of CD8+, MHC-restricted cytotoxic T cells that lyse parasite-infected target cells. J Immunol. 1993;150:1446–1457. [PubMed] [Google Scholar]
- 165.Ohga S, Yoshikai Y, Takeda Y, Hiromatsu K, Nomoto K. Sequential appearance of γδ- and αβ-bearing T cells in the peritoneal cavity during an i.p. infection with Listeria monocytogenes. Eur J Immunol. 1990;20:533–538. doi: 10.1002/eji.1830200311. [DOI] [PubMed] [Google Scholar]
- 166.Oswald I P, Gazzinelli R T, Sher A, James S L. IL-10 synergizes with IL-4 and transforming growth factor-β to inhibit macrophage cytotoxic activity. J Immunol. 1992;148:3578–3582. [PubMed] [Google Scholar]
- 167.Overath P, Harbecke D. Course of Leishmania infection in beta 2-microglobulin-deficient mice. Immunol Lett. 1993;37:13–17. doi: 10.1016/0165-2478(93)90126-m. [DOI] [PubMed] [Google Scholar]
- 168.Parker S J, Roberts C W, Alexander J. CD8+ T cells are the major lymphocyte subpopulation involved in the protective immune response to Toxoplasma gondii in mice. Clin Exp Immunol. 1991;84:207–212. doi: 10.1111/j.1365-2249.1991.tb08150.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 169.Patsouris E, Kretzchmar H A, Stavrou D, Mehraein P. Cellular composition and distribution of gliomesenchymal nodules in the CNS of AIDS patients. Clin Neuropathol. 1993;12:130–137. [PubMed] [Google Scholar]
- 170.Pfefferkorn E R. Interferon-γ blocks the growth of Toxoplasma gondii in human fibroblasts by inducing the host cells to degrade tryptophan. Proc Natl Acad Sci USA. 1984;81:908–912. doi: 10.1073/pnas.81.3.908. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 171.Podak E. Excecution and suicide: cytotoxic lymphocytes enforce Draconian laws through separate molecular pathways. Curr Opin Immunol. 1995;7:11–16. doi: 10.1016/0952-7915(95)80023-9. [DOI] [PubMed] [Google Scholar]
- 172.Poli G, Kinter A, Justement J S, Kehrl J H, Bressler P, Stanley S, Fauci A S. Tumor necrosis factor-a functions in an autocrine manner in the induction of human immunodeficiency virus expression. Proc Natl Acad Sci USA. 1990;87:782–785. doi: 10.1073/pnas.87.2.782. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 173.Purner M B, Berens R L, Nash P B, van Linden A, Ross E, Kruse C, Krug E C, Curiel T J. CD4-mediated and CD8-mediated cytotoxic and proliferative immune response to Toxoplasma gondii in seropositive humans. Infect Immun. 1996;64:4330–4338. doi: 10.1128/iai.64.10.4330-4338.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 174.Quelle F W, Shimoda K, Thierfelder W, Fisher C, Kim A, Ruben S M, Cleveland J L, Pierce J H, Keegan A D, Nelms K. Cloning of murine STAT6 and human STAT6, Stat proteins that are tyrosine phosphorylated in response to IL-4 and IL-3 but not required for mitogenesis. Mol Cell Biol. 1995;15:3336–3343. doi: 10.1128/mcb.15.6.3336. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 175.Reis e Sousa C, Hieny S, Scharton-Kersten T, Jankovic D, Charest H, Germain R N, Sher A. In vivo microbial stimulation induces rapid CD40L-independent production of IL-12 by dendritic cells and their re-distribution to T cell areas. J Exp Med. 1997;186:1819–1829. doi: 10.1084/jem.186.11.1819. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 176.Remington J S, Desmonts G. Toxoplasmosis. In: Remington J S, Klein J O, editors. Infectious diseases of the fetus and newborn infant. Philadelphia, Pa: The W. B. Saunders Co.; 1990. pp. 89–195. [Google Scholar]
- 177.Reyes L, Frenkel J K. Specific and nonspecific mediation of protective immunity to Toxoplasma gondii. Infect Immun. 1987;55:856–863. doi: 10.1128/iai.55.4.856-863.1987. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 178.Rizzo, L. V., J. H. Yamamoto, C. Silveira, J. Kalil, R. B. Nussenblatt, E. Cunha-Neto, R. Belfort, and R. T. Gazzinelli. Patients with acquired toxoplasmosis can be discriminated from patients with congenital toxoplasmosis based on their T cell activity but not their T cell receptor (Vβ family) repertoire. Submitted for publication.
- 179.Roberts C W, Ferguson D J P, Jebbari H, Satoskar A, Bluethmann H, Alexander J. Different roles for interleukin-4 during the course of Toxoplasma gondii infection. Infect Immun. 1996;64:897–904. doi: 10.1128/iai.64.3.897-904.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 180.Romani L, Mencacci A, Cenci E, Spaccapelo R, Del Sero G, Nicoletti I, Trinchieri G, Bistoni F, Puccetti P. Neutrophil production of IL-12 and IL-10 in candidiasis and efficacy of IL-12 therapy in neutropenic mice. J Immunol. 1997;158:5349–5356. [PubMed] [Google Scholar]
- 181.Romero P, Maryanski J L, Corradin G, Nussenzweig R S, Nussenzweig V, Zavala F. Cloned cytotoxic T cells recognize an epitope in the circumsporozoite protein and protect against malaria. Nature. 1989;341:323–326. doi: 10.1038/341323a0. [DOI] [PubMed] [Google Scholar]
- 182.Rosat J P, MacDonald H R, Louis J A. A role for γδ+ T cells during experimental infection of mice with Leishmania major. J Immunol. 1993;150:550–555. [PubMed] [Google Scholar]
- 183.Ruskins J, Remington J S. Immunity and intracellular infection: resistance to bacteria in mice infected with a protozoan. Science. 1968;160:72–74. doi: 10.1126/science.160.3823.72. [DOI] [PubMed] [Google Scholar]
- 184.Ruskins J, Remington J S. Toxoplasmosis in the compromised host. Ann Intern Med. 1976;84:193–203. doi: 10.7326/0003-4819-84-2-193. [DOI] [PubMed] [Google Scholar]
- 185.Russo D C, Williams D J, Grab D J. Mechanisms for elimination of potentially lytic complement-fixing variable surface glycoprotein-antibody complexes in Trypanosoma brucei. Parasitol Res. 1994;80:487–492. doi: 10.1007/BF00932695. [DOI] [PubMed] [Google Scholar]
- 186.Saavedra R, Becerril M A, Dubeaux C, Lippens R, De Vos M J, Herion P, Bollen A. Epitopes recognized by human T lymphocytes in the ROP2 protein antigen of Toxoplasma gondii. Infect Immun. 1996;64:3858–3862. doi: 10.1128/iai.64.9.3858-3862.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 187.Saavedra R, de Meuter F, Decourt J-L, Herion P. Human T cell clone identifies a potentially protective 54-kDa protein antigen of Toxoplasma gondii cloned and expressed in Escherichia coli. J Immunol. 1991;147:1975–1982. [PubMed] [Google Scholar]
- 188.Scalise F, Gerli R, Castelluci G, Spinozzi F, Fabietti G M, Crupi S, Sensi L, Britta R, Vaccaro R, Bertotto A. Lymphocytes bearing the γδ T-cell receptor in acute toxoplasmosis. Immunology. 1992;76:668–670. [PMC free article] [PubMed] [Google Scholar]
- 189.Scharton-Kersten T, Contursi C, Masumi A, Sher A, Ozato K. ICSBP-deficient mice display impaired resistance to intracellular infection due to a primary defect in IL-12 p40 induction. J Exp Med. 1997;186:1523–1534. doi: 10.1084/jem.186.9.1523. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 190.Scharton-Kersten T, Yap G, Magram J, Sher A. Inducible nitric oxide is essential for host control of persistent but not acute infection with the intracellular pathogen Toxoplasma gondii. J Exp Med. 1997;185:1–13. doi: 10.1084/jem.185.7.1261. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 191.Scharton-Kersten T M, Caspar P, Sher A, Denkers E Y. Toxoplasma gondii: evidence for interleukin-12-dependent and -independent pathways of IFN-γ production induced by an attenuated parasite strain. Exp Parasitol. 1996;84:102–114. doi: 10.1006/expr.1996.0096. [DOI] [PubMed] [Google Scholar]
- 192.Scharton-Kersten T M, Wynn T A, Denkers E Y, Bala S, Showe L, Grunvald E, Hieny S, Gazzinelli R T, Sher A. In the absence of endogenous IFN-γ mice develop unimpaired IL-12 responses to Toxoplasma gondii while failing to control acute infection. J Immunol. 1996;157:4045–4054. [PubMed] [Google Scholar]
- 193.Schluter D, Kaefer N, Hof H, Wiestler O D, Deckert-Schluter M. Expression pattern and cellular origin of cytokines in the normal and Toxoplasma gondii-infected murine brain. Am J Pathol. 1997;150:1021–1035. [PMC free article] [PubMed] [Google Scholar]
- 194.Schoel B, Sprenger S, Kaufmann S H E. Phosphate is essential for stimulation of Vγ9Vδ2 T lymphocytes by low molecular weight ligand. Eur J Immunol. 1994;24:1886–1892. doi: 10.1002/eji.1830240826. [DOI] [PubMed] [Google Scholar]
- 195.Reference deleted.
- 196.Schwab J C, Beckers C J M, Joiner K A. The parasitophorous vacuole membrane surrounding intracellular Toxoplasma gondii functions as a molecular sieve. Proc Natl Acad Sci USA. 1994;91:509–513. doi: 10.1073/pnas.91.2.509. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 197.Seder R A, Gazzinelli R T, Sher A, Paul W E. IL-12 acts directly on CD4+ T cells to enhance priming for IFN-γ production and diminishes IL-4 inhibition of such priming. Proc Natl Acad Sci USA. 1993;90:10188–10194. doi: 10.1073/pnas.90.21.10188. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 198.Seder R A, Kelsall B L, Jankovic D. Different roles for IL-12 in the maintenance of immune responses in infectious versus autoimmune disease. J Immunol. 1996;157:2745–2748. [PubMed] [Google Scholar]
- 199.Sher A, Coffman R L. Regulation of immunity to parasites by T cells and T cell-derived cytokines. Annu Rev Immunol. 1992;10:385–410. doi: 10.1146/annurev.iy.10.040192.002125. [DOI] [PubMed] [Google Scholar]
- 200.Sher A, Denkers E Y, Gazzinelli R T. Induction and regulation of host cell-mediated immunity by Toxoplasma gondii. Ciba Found Symp. 1995;195:95–109. doi: 10.1002/9780470514849.ch7. [DOI] [PubMed] [Google Scholar]
- 201.Sher A, Fiorentino D F, Caspar P, Pearce E, Mosmann T. Production of IL-10 by CD4+ lymphocytes correlates with down-regulation of Th1 cytokine synthesis in helminth infection. J Immunol. 1991;144:2713–2716. [PubMed] [Google Scholar]
- 202.Sher A, Gazzinelli R T, Oswald I P, Clerici M, Kullberg M, Pearce E J, Berzovsky J A, Mosmann T R, James S L, Morse III H R, Shearer G M. Role of T cell derived cytokines in the downregulation of immune responses in parasitic and retroviral infection. Immunol Rev. 1992;127:183–204. doi: 10.1111/j.1600-065x.1992.tb01414.x. [DOI] [PubMed] [Google Scholar]
- 203.Sher A, Oswald I O, Hieny S, Gazzinelli R T. Toxoplasma gondii induces a T-independent IFN-γ response in NK cells which requires both adherent accessory cells and TNF-α. J Immunol. 1993;150:3982–3989. [PubMed] [Google Scholar]
- 204.Shu U, Kiniwa M, Wu C Y, Malizewski C, Vezzio N, Hakimi J, Gately M, Delespesse G. Activated T cells induce interleukin-12 production by monocytes via CD40-CD40 ligand interaction. Eur J Immunol. 1995;25:1125–1128. doi: 10.1002/eji.1830250442. [DOI] [PubMed] [Google Scholar]
- 205.Sibley L D, Howe D K. Genetic basis of pathogenicity in toxoplasmosis. Curr Top Microbiol Immunol. 1996;216:4–15. doi: 10.1007/978-3-642-51014-4_1. [DOI] [PubMed] [Google Scholar]
- 206.Silva J S, Morrissey P J, Grabstein K H, Mohler K M, Anderson D, Reed S G. Interleukin-10 and interferon-γ regulation of experimental Trypanosoma cruzi infection. J Exp Med. 1992;175:539–545. doi: 10.1084/jem.175.1.169. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 207.Silva J S, Twardzik D R, Reed S G. Regulation of Trypanosoma cruzi infections in vitro and in vivo by transforming growth factor-β (TGF-β) J Exp Med. 1991;174:539–545. doi: 10.1084/jem.174.3.539. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 208.Soete M, Dubremetz J F. Toxoplasma gondii: kinetics of stage-specific protein expression during tachyzoite-bradyzoite conversion in vitro. Curr Top Microbiol Immunol. 1996;219:76–80. [PubMed] [Google Scholar]
- 209.Su H C, Orange J S, Fast L D, Chan A T, Simpson S J, Terhorst C, Biron C A. IL-2-dependent NK cell responses discovered in virus-infected β2-microglobulin-deficient mice. J Immunol. 1994;153:5674–5681. [PubMed] [Google Scholar]
- 209a.Subauste, C. S. Personal communication.
- 210.Subauste C S, Chung J Y, Do D, Koniaris A H, Hunter C A, Montoya J G. Preferential activation and expansion of human peripheral blood γδ T cells in response to Toxoplasma gondii in vitro and their cytokine production and cytotoxic activity against T. gondii-infected cells. J Clin Invest. 1995;96:610–619. doi: 10.1172/JCI118076. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 211.Subauste C S, Fuh F, de Waal Malefyt R, Remington J S. αβ T cell response to Toxoplasma gondii in previously unexposed individuals. J Immunol. 1998;160:3403–3411. [PubMed] [Google Scholar]
- 212.Subauste C S, Koniaris A H, Remington J S. Murine CD8+ cytotoxic T lymphocytes lyse Toxoplasma gondii-infected cells. J Immunol. 1991;147:3955–3959. [PubMed] [Google Scholar]
- 213.Suzuki Y, Conley F K, Remington J S. Importance of endogenous IFN-γ for the prevention of toxoplasmic encephalitis in mice. J Immunol. 1989;143:2045–2050. [PubMed] [Google Scholar]
- 214.Suzuki Y, Conley F K, Remington J S. Treatment of toxoplasmic encephalitis in mice with recombinant gamma interferon. Infect Immun. 1990;58:3050–3055. doi: 10.1128/iai.58.9.3050-3055.1990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 215.Suzuki Y, Joh K. Effect of the strain of Toxoplasma gondii on the development of toxoplasmic encephalitis in mice treated with antibody to interferon-gamma. Parasitol Res. 1994;80:125–130. doi: 10.1007/BF00933779. [DOI] [PubMed] [Google Scholar]
- 216.Suzuki Y, Joh K, Kwon O-C, Yang Q, Conley F K, Remington J S. MHC class I gene(s) in the D/L region but not the TNF-α gene determines development of toxoplasmic encephalitis in mice. J Immunol. 1994;153:4649–4654. [PubMed] [Google Scholar]
- 217.Suzuki Y, Joh K, Orellana M A, Conley F K, Remington J S. A gene(s) within the H-2D region determines the development of toxoplasmic encephalitis in mice. Immunology. 1991;74:732–739. [PMC free article] [PubMed] [Google Scholar]
- 218.Suzuki Y, Orellana M A, Schreiber R D, Remington J S. Interferon-γ: The major mediator of resistance against Toxoplasma gondii. Science. 1988;240:516–518. doi: 10.1126/science.3128869. [DOI] [PubMed] [Google Scholar]
- 219.Suzuki Y, Rani S, Liesenfeld O, Kojima T, Lim S, Nguyen T A, Dalrymple S A, Murray R, Remington J S. Impaired resistance to the development of toxoplasmic encephalitis in interleukin-6-deficient mice. Infect Immun. 1997;65:2339–2345. doi: 10.1128/iai.65.6.2339-2345.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 220.Suzuki Y, Remington J S. Dual regulation of resistance against Toxoplasma gondii infection by Lyt-2+ and Lyt-1+, L3T4+ T cells in mice. J Immunol. 1988;140:3943–3946. [PubMed] [Google Scholar]
- 221.Suzuki Y, Remington J S. The effect of anti-IFN-γ antibody on the protective effect of lyt-2+ immune T cells against toxoplasmosis in mice. J Immunol. 1990;144:1954–1956. [PubMed] [Google Scholar]
- 222.Suzuki Y, Wong S Y, Grumet F C, Fessel J, Montoya J G, Zolopa A R, Portmore A, Schumacher-Perdreau F, Schrappe M, Koppen S, Ruf B, Brown B W, Remington J S. Evidence for genetic regulation of susceptibility to toxoplasmic encephalitis in AIDS patients. J Infect Dis. 1996;173:265–268. doi: 10.1093/infdis/173.1.265. [DOI] [PubMed] [Google Scholar]
- 223.Swain S L, Weinberg A D, English M, Huston G. IL-4 directs the development of Th2-like helper effectors. J Immunol. 1990;145:3796–3806. [PubMed] [Google Scholar]
- 224.Tanaka Y, Morita C, Tanaka Y, Nieves E, Brenner M, Bloom B. Natural and synthetic non-peptide antigens recognized by human gamma-delta T cells. Nature. 1995;375:155–158. doi: 10.1038/375155a0. [DOI] [PubMed] [Google Scholar]
- 225.Trinchieri G. Interleukin-12: a proinflammatory cytokine with immuno-regulatory functions that bridge innate resistance and antigen-specific adaptive immunity. Annu Rev Immunol. 1995;13:252–276. doi: 10.1146/annurev.iy.13.040195.001343. [DOI] [PubMed] [Google Scholar]
- 226.Tsuji M, Mombaerts P, Lefrancois L, Nussenzweig R S, Zavala F, Tonegawa S. γδ T cells contribute to immunity against the liver stages of malaria in αβ T-cell-deficient mice. Proc Natl Acad Sci USA. 1994;91:345–349. doi: 10.1073/pnas.91.1.345. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 227.Tsunawaki S, Sporn M, Ding A, Nathan C F. Deactivation of macrophages by transforming growth factor-β. Nature. 1988;344:260–263. doi: 10.1038/334260a0. [DOI] [PubMed] [Google Scholar]
- 228.Villard O, Candolfi E, Despringe J L, Derouin F, Marcellin L, Viville S, Kien T. Protective effect of low doses of an anti-IL-4 monoclonal antibody in a murine model of acute toxoplasmosis. Parasite Immunol. 1995;17:233–236. doi: 10.1111/j.1365-3024.1995.tb01020.x. [DOI] [PubMed] [Google Scholar]
- 229.Walsh C M, Matloubian M, Liu C C, Ueda R, Kurahara C G, Chritensen J L, Huang M T, Young J D, Ahmed R, Clark W R. Immune function in mice lacking the perforin gene. Proc Natl Acad Sci USA. 1994;23:10854–10858. doi: 10.1073/pnas.91.23.10854. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 230.Wang Z E, Reiner S L, Hatam F, Heinzel F P, Bouvier J, Turck C W, Locksley R M. Targeted activation of CD8 cells and infection of beta 2-microglobulin-deficient mice fail to confirm a primary protective role for CD8 cells in experimental leishmaniasis. J Immunol. 1993;151:2077–2086. [PubMed] [Google Scholar]
- 231.Ware P L, Kasper L H. Strain-specific antigens of Toxoplasma gondii. Infect Immun. 1987;55:778–783. doi: 10.1128/iai.55.3.778-783.1987. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 232.Watts C. Capture and processing of exogenous antigen for presentation on MHC molecules. Annu Rev Immunol. 1997;15:821–850. doi: 10.1146/annurev.immunol.15.1.821. [DOI] [PubMed] [Google Scholar]
- 233.Wegmann T G, Lin H, Guilbert L, Mosmann T R. Bidirectional cytokine interactions in the maternal-fetal relationship: is successful pregnancy a TH2 phenomenon? Immunol Today. 1993;14:353–356. doi: 10.1016/0167-5699(93)90235-D. [DOI] [PubMed] [Google Scholar]
- 234.Yano A, Aosai F, Ohta M, Hasekura H, Sugane K, Hayashi S. Antigen presentation by Toxoplasma gondii-infected cells to CD4+ proliferative T cells and CD8+ cytotoxic cells. J Parasitol. 1989;75:411–416. [PubMed] [Google Scholar]
- 235.Yap G S, Scharton-Kersten T, Charest H, Sher A. Decreased resistance of TNF receptor p55- and p75-deficient mice to chronic toxoplasmosis despite normal activation of inducible nitric oxide synthase in vivo. J Immunol. 1998;160:1340–1345. [PubMed] [Google Scholar]
- 236.Yong E C, Chi E Y, Henderson W R. Toxoplasma gondii alters eicosanoid release by human mononuclear phagocytes: role of leukotrienes in interferon-γ-induced antitoxoplasma activity. J Exp Med. 1994;180:1637–1648. doi: 10.1084/jem.180.5.1637. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 237.York I A, Rock K L. Antigen processing and presentation by the class I major histocompatibility complex. Annu Rev Immunol. 1996;14:369–396. doi: 10.1146/annurev.immunol.14.1.369. [DOI] [PubMed] [Google Scholar]
- 238.Zanovello P, Vallerani E, Biasi G, Landolfo S, Colavo D. Monoclonal antibody against IFN-γ inhibits Moloney murine sarcoma virus-specific cytotoxic T cell differentiation. J Immunol. 1988;140:1341–1344. [PubMed] [Google Scholar]
- 239.Zheng L, Fisher G, Miller R E, Peschon J, Lynch D H, Lenardo M J. Induction of apoptosis in mature T cells by tumor necrosis factor. Nature. 1995;377:348–351. doi: 10.1038/377348a0. [DOI] [PubMed] [Google Scholar]