Abstract
Bacterial resistance to antibiotics threatens our progress in healthcare, modern medicine, food production and ultimately life expectancy. Antibiotic resistance is a global concern, which spreads rapidly across borders and continents due to rapid travel of people, animals and goods. Derivatives of metabolically stable pyrazole nucleus are known for their wide range of pharmacological properties, including antibacterial activities. This review highlights recent reports of pyrazole derivatives targeting different bacterial strains focusing on the drug-resistant variants. Pyrazole derivatives target different metabolic pathways of both Gram-positive and Gram-negative bacteria.
Keywords: : Acinetobacter baumannii, antibacterial, antibiotics, antimicrobials, bacterial resistance, drug-resistant, ESKAPE pathogens, MRSA, pyrazole
Graphical abstract
Microbial resistance & ESKAPE pathogens
The modern ‘antibiotic era’ started in the 1910s with the discovery of salvarsan, or compound 606, followed neosalvarsan to treat syphilis caused by Treponema pallidum [1]. Subsequent discovery of sulfonamides and Alexander Fleming’s codiscovery of penicillin saved millions of human lives worldwide. Discovery of several novel classes of new antibiotics after World War II made the 1950s–1970s the golden era of antibiotics [2]. At the beginning, Fleming and other scientists cautioned about the development of resistance to penicillin if not used judiciously [3]. Recently, unabated emergence of antibiotic-resistant bacteria is an unsolved global concern [4]. Drug-resistant infections cause >700,000 deaths worldwide each year, and the death toll is expected to reach 10 million annually by 2050 [5]. Without an imperative and coordinated action, we are heading toward an era in which minor infections or normal injuries may become lethal. The ESKAPE (Enterococcus faecium, Staphylococcus aureus, Klebsiella pneumoniae, Acinetobacter baumannii, Pseudomonas aeruginosa and Enterobacter species) bacteria cause two-thirds of the hospital-acquired infections in the United States, and these pathogens are unaffected by all the current antibiotics [6]. Finding new antibiotics with novel chemical compositions is an important strategy to address this problem [7]. The CDC recommends fighting antibiotic-resistance by developing new antibiotics for drug-resistant bacteria [8,9].
S. aureus is one of the most common types of germs that ~30% of people carry in their noses, and 2% of the population carry its drug-resistant variant, methicillin-resistant S. aureus (MRSA), which causes the highest number of invasive infections among all the antibiotic-resistant bacteria [10,11]. S. aureus can cause a variety of serious and fatal pathologies including bacteremia, endocarditis, pneumonia, osteomyelitis, skin infections and sepsis [12]. The failure of antibiotic therapy against S. aureus is due its ability to develop multidrug-resistant strains and adopt persistent nongrowing lifestyle [13]. Gram-positive enterococci are normal human microbiota capable of causing severe infections and vancomycin-resistant enterococci (VRE) are among the most dangerous pathogens [14]. Enterococci bacteria that are found in human intestines and in the female genital tract can cause serious infections. These bacteria are constantly finding new ways to neutralize the effects of antibiotics, and vancomycin-resistant enterococci (VRE) infections are becoming common [15]. MRSA causes the highest number of invasive infections among all antibiotic-resistant bacteria [10,11]. Both genera are among the WHO’s list of high-priority pathogens [16], and both are known to produce biofilms that contain antibiotic-insensitive persister cells [17,18].
Nevertheless, the WHO lists only Gram-negative bacteria as critical-priority pathogens. The outer membrane of these bacteria not only acts as a barrier to entry of antibiotics but is a virulence factor responsible for severe diseases [16]. The last four members of ESKAPE pathogens are Gram-negative bacteria. These and other Gram-negative bacteria are specifically difficult to treat with antibiotics because intracellular targets have to pass through the negatively charged phospholipid outer membrane and hydrophobic inner membrane. The presence of efflux pumps further makes the drug ineffective to treat Gram-negative pathogens [19,20]. Gram-negative bacterial resistance to antibiotics is often mediated by multiple gene-carrying plasmids, so treatment is mostly limited to second- and third-line antibiotics with high toxicity and poor efficacy [21]. K. pneumoniae can cause different types of healthcare-associated infections such as pneumonia and bloodstream infections. Klebsiella bacteria have developed most of the commonly used antibiotics including carbapenems [22]. A group of bacteria, Enterobacter species, are globally important pathogens that are becoming resistant to antibiotics, including carbapenem and colistin [23]. In recent years, emergence of extremely resistant and pandrug-resistant A. baumannii has alarmed the medical community [24–26]. In 2017, the WHO has released a list of 12 drug-resistant bacteria that pose the greatest threat to human health and for which new antibiotics are urgently needed. Carbapenem-resistant A. baumannii (CRAB) is on top of the list [27,28] and is associated with high morbidity and mortality; it has the potential to cause outbreaks and spread resistance [29]. P. aeruginosa is an opportunistic pathogen that is a leading cause of morbidity and mortality of cystic fibrosis patients [30]. It is challenging to find new compounds that can inhibit the growth of nonfermenting bacteria such as A. baumannii and P. aeruginosa that are often multidrug-resistant [31]. These two bacteria have a highly impermeable outer membrane that thwarts the uptake of most molecules including several antibiotics [32].
Pyrazole derivatives as therapeutic agents
Pyrazole or 1,2-diazole is a five-membered azaheterocycle, and substituted-pyrazoles are known for a wide spectrum of therapeutic activities [33]. This heterocycle is found as the cornerstone of several leading drugs, such as a potent antiinflammatory medicine, celecoxib [34]; tepoxalin, a nonsteroidal antiinflammatory agent for veterinary use [33]; the antiobesity drug rimonabant [35]; and the analgesic difenamizole [36], among several other therapeutic agents. A number of drugs containing pyrazole nucleus may be due to its decreased susceptibility to oxidative degradation by metabolic enzymes than other five-membered heterocycles. Pyrazole compounds have been reported as antimicrobial agents in a number of publications [37–39]. Pyrazole derivatives as anti-MRSA agents have been reported by our group [40–44] and others [45,46], whereas pyrazole compounds as antiacinetobacterial activity had not been developed until our more recent papers [40–44].
Two pyrazole-containing antibiotics, cefoselis (1) and ceftolozane (2), have been approved to treat bacterial infection (Figure 1). These two antibiotics have a zwitterion structure that helps to penetrate the outer membrane of Gram-negative bacteria. Cefoselis is a fourth-generation cephalosporin antibiotics that can cross the blood–brain barrier [47]. Ceftolozane (2) is zwitterionic fifth-generation cephalosporin antibiotic. It is one of the most widely used antibacterial agents to treat drug-resistant bacterial infection, including MRSA, enterobacterales and P. aeruginosa [48]. Ceftolozane (2) in combination with tazobactam, a bacterial β-lactamase inhibitor, sold under the brand name Zerbaxa is used to treat urinary tract infection (UTI), bacterial pneumonia and intraabdominal infection. The pyrazolium substituent of ceftolozane (2) is one of the key components to prevent the AmpC β-lactamase mediated hydrolysis of the lactam moiety [49].
Figure 1. . Approved pyrazole-containing antibiotics.
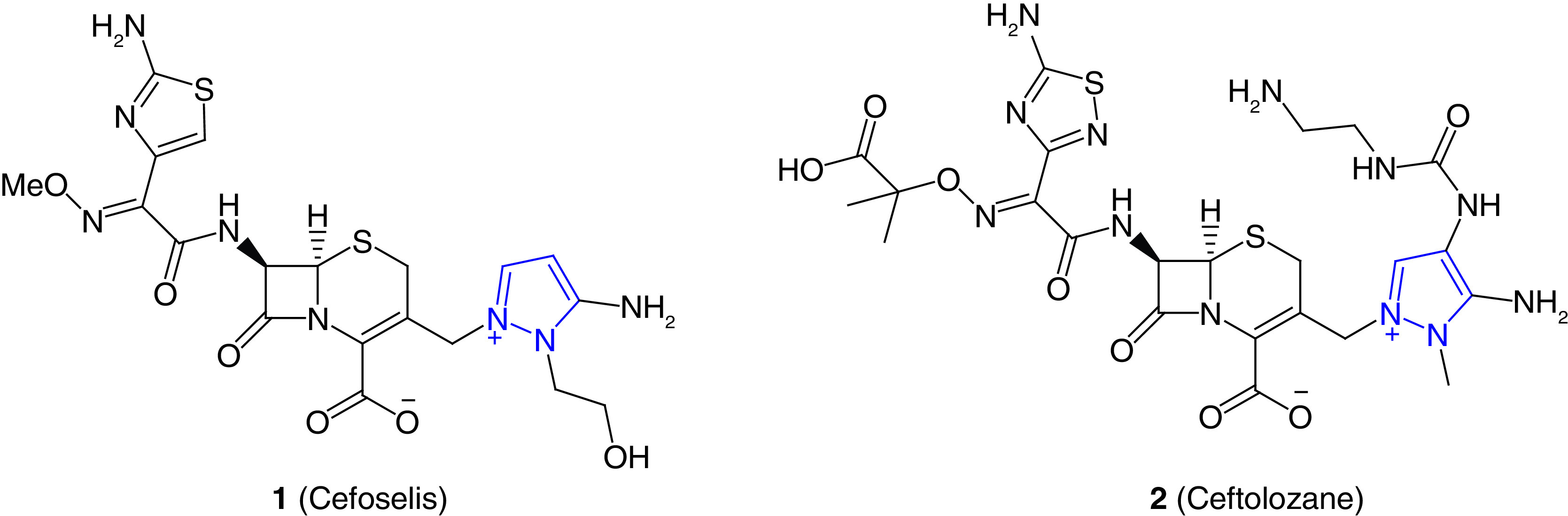
Structures of pyrazole containing antibiotics cefoselis (1) and ceftolozane (2) to treat bacterial infection.
In addition to preceding approved antibiotics, a large number of pyrazole derivatives have been reported as potent antibacterial agents. In this review, recent reports on pyrazoles as antibacterial agents are summarized. These pyrazole derivatives are classified based on their mode of action. If the mode of action has not been reported, the compounds are classified based on their structures.
Pyrazole-derived hydrazone as potent antibacterial agents
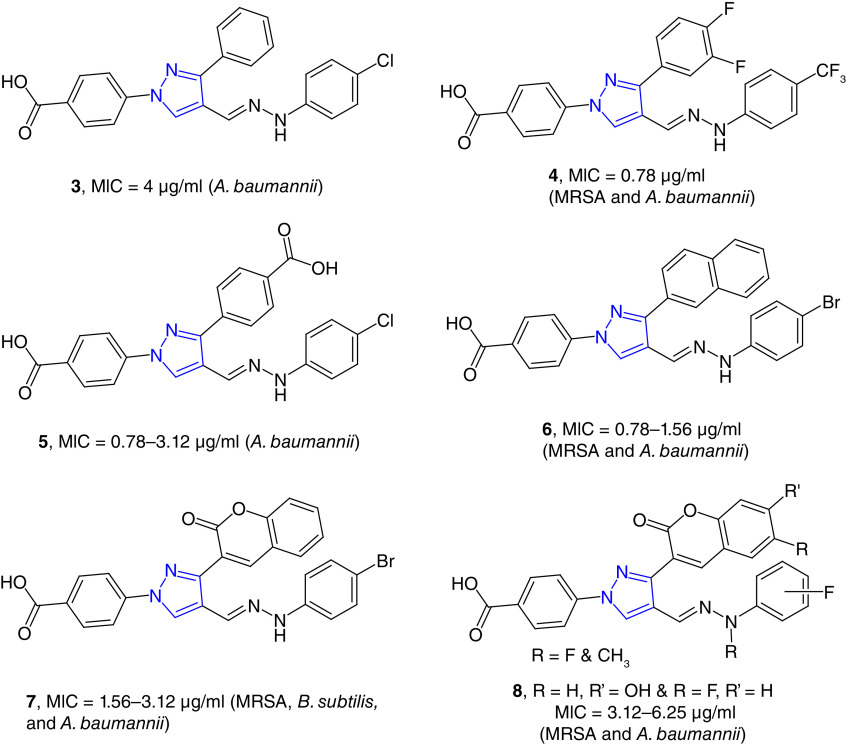
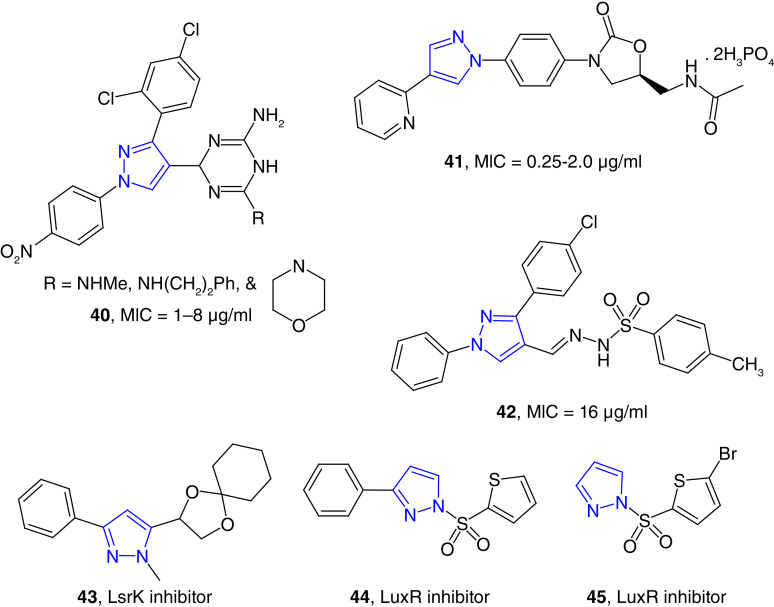
We have developed pyrazole-derived hydrazones as potent antimicrobial agents including drug-resistant strains (Figure 2) [50,51]. N-Benzoic acid derived pyrazole hydrazones (e.g., 3) is a potent growth inhibitor of A. baumannii with minimum inhibitory concentration (MIC) values as low as 4 μg/ml. These compounds were nontoxic to healthy human embryonic kidney cells (HEK-293) with 50% cytotoxic concentration (CC50) values >32 μg/ml [40]. Difluorophenyl substituted derivatives (4) showed broader potency against Gram-positive bacteria, and these compounds were less toxic to HEK-293 cells. Difluorophenyl-substituted compounds were also effective against A. baumannii strains with MIC values as low as 0.78 μg/ml [41]. These compounds were somewhat hydrophobic, to increase the hydrophilicity, we synthesized dibenzoic-acid-derived pyrazole hydrazone, and these compounds are potent and selective inhibitors of A. baumannii strains. These dibenzoic acid pyrazole derivatives are nontoxic to human healthy and cancer cell lines. The most potent compound in this series, 5, was studied for its possible toxicity studies in vivo murine model. This compound did not show any noticeable toxicity in mice based on the effect on 14 physiological markers of organ injury [52].
Figure 2. . Pyrazole-derived hydrazones as potent antimicrobial agents.
Naphthyl-substituted pyrazole-derived hydrazones (e.g., 6) were potent growth inhibitors of Gram-positive strains and A. baumannii with MIC values in the range of 0.78–1.56 μg/ml. These compounds were effective against S. aureus and A. baumannii biofilms. Naphthyl derivative (6) was bactericidal for S. aureus and bacteriostatic for A. baumannii strains. The antibacterial mode of action of these compounds was due to their ability to disrupt the bacterial cell wall [53]. 3-Coumarinyl substituted compounds (e.g., 7) were found to be the potent growth inhibitors of MRSA and A. baumannii strains. These compounds were nontoxic to human cell line in vitro and to mice in vivo [42]. Substitution on the coumarin moiety decreased the potency of the hydrazones (8) [54].
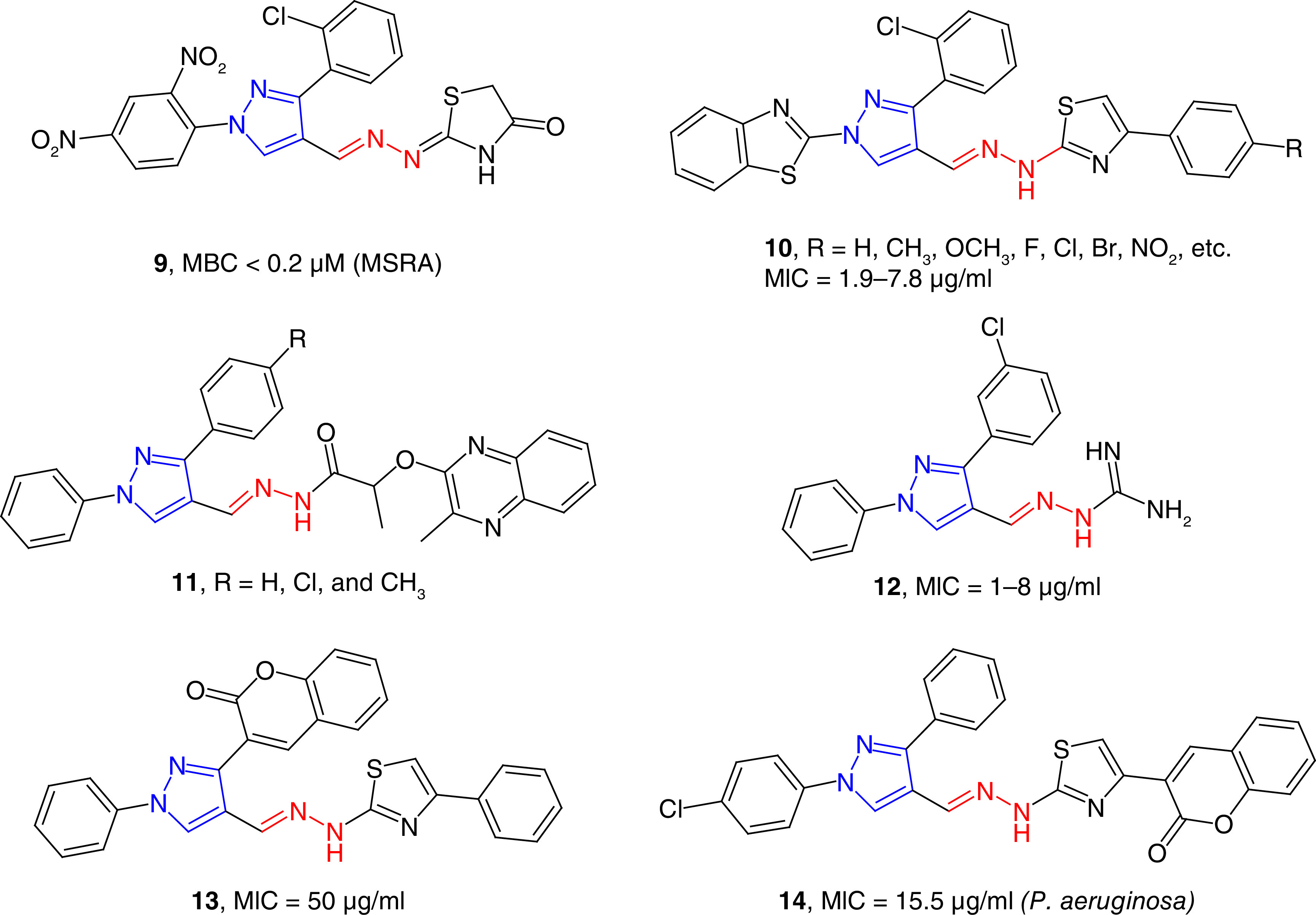
Several other groups have reported the synthesis and antibacterial studies of pyrazole-derived hydrazones (e.g., 9) for the potential treatment of drug-resistant bacterial infections (Figure 3). In a series of pyrazole-thiazole derivatives, some of the compounds were found to be potent growth inhibitors of MRSA with minimum bactericidal concentration (MBC) values of <0.2 μM. Molecular docking studies have revealed that the possible molecular targets were topoisomerase II and topoisomerase IV for its potent activity [55]. Gondru et al. reported the synthesis of pyrazole-thiazole hybrids (10) containing the hydrazone moiety as potent antimicrobial agents [56]. These compounds showed antibacterial activity with the MIC/MBC spectrum of 1.9/7.8 μg/ml to 3.9/7.8 μg/ml. These compounds were also effective against S. aureus and Klebsiella planticola strains with an IC50 value of 11.8 μM. K. planticola, also known as Raoultella planticola is a Gram-negative bacterium that cause a wide range of anomalies, including conjunctivitis. R. planticola is a rare but emerging pathogen [57]. Hydrazide derivatives (e.g., 11) of pyrazole were found to be better than the positive controls in inhibiting the growth of Gram-positive and Gram-negative bacteria [58]. One of these compounds inhibited the growth of Neisseria gonorrhoeae with an MIC value of 3.9 μg/ml, which was twofold more potent than the positive control, gentamicin. These results are exciting because N. gonorrhoeae has emerged as broad-spectrum antibiotic-resistant pathogen and recently reached the status of ‘superbug’ [59]. Aminoguanidine-derived 1,3-diphenyl pyrazoles (12) have been found as potent antimicrobial agents against several strains of bacteria with MIC values in the range of 1–8 μg/ml [60]. These activities were equivalent to approved drugs moxifloxacin and gatifloxacin for inhibiting the S. aureus strains. Compounds 12 showed better activity (MIC = 1 μg/ml) than the positive control moxifloxacin (MIC = 2 μg/ml) against Escherichia coli 1924 strain. These compounds were equally effective against several multidrug-resistant clinical isolates of S. aureus with MIC values ranging from 1 to 32 μg/ml. E. coli is a Gram-negative bacterium that is a part of gut microbiota. Some strains of E. coli are known to cause foodborne diseases and other different anomalies including UTIs [61,62]. In a series of pyrazole-triazole derived hydrazides, some compounds were potent growth inhibitors of S. aureus, E. coli and P. aeruginosa strains with the MIC80 values of 2–8 μg/ml, which were comparable to the standard, ciprofloxacin [63]. Pyrimidine and pyrazole clubbed hydrazone derivatives have been reported as moderate inhibitors of S. aureus and P. aeruginosa strains [64]. Coumarin-attached pyrazole-derived thiazole hydrazones (e.g., 13) have been reported as broad-spectrum antibacterial agents with moderate potency against the tested strains [65]. Trisubstituted pyrazoles containing coumarin-thiazole moiety have been reported as moderate growth inhibitors of bacterial strains. Compound 14 has shown the most potent activity in the series, with MIC values as low as 15.5 μg/ml [66].
Figure 3. . Pyrazole-containing hydrazones as antibacterial agents.
Pyrazole with other heterocycles
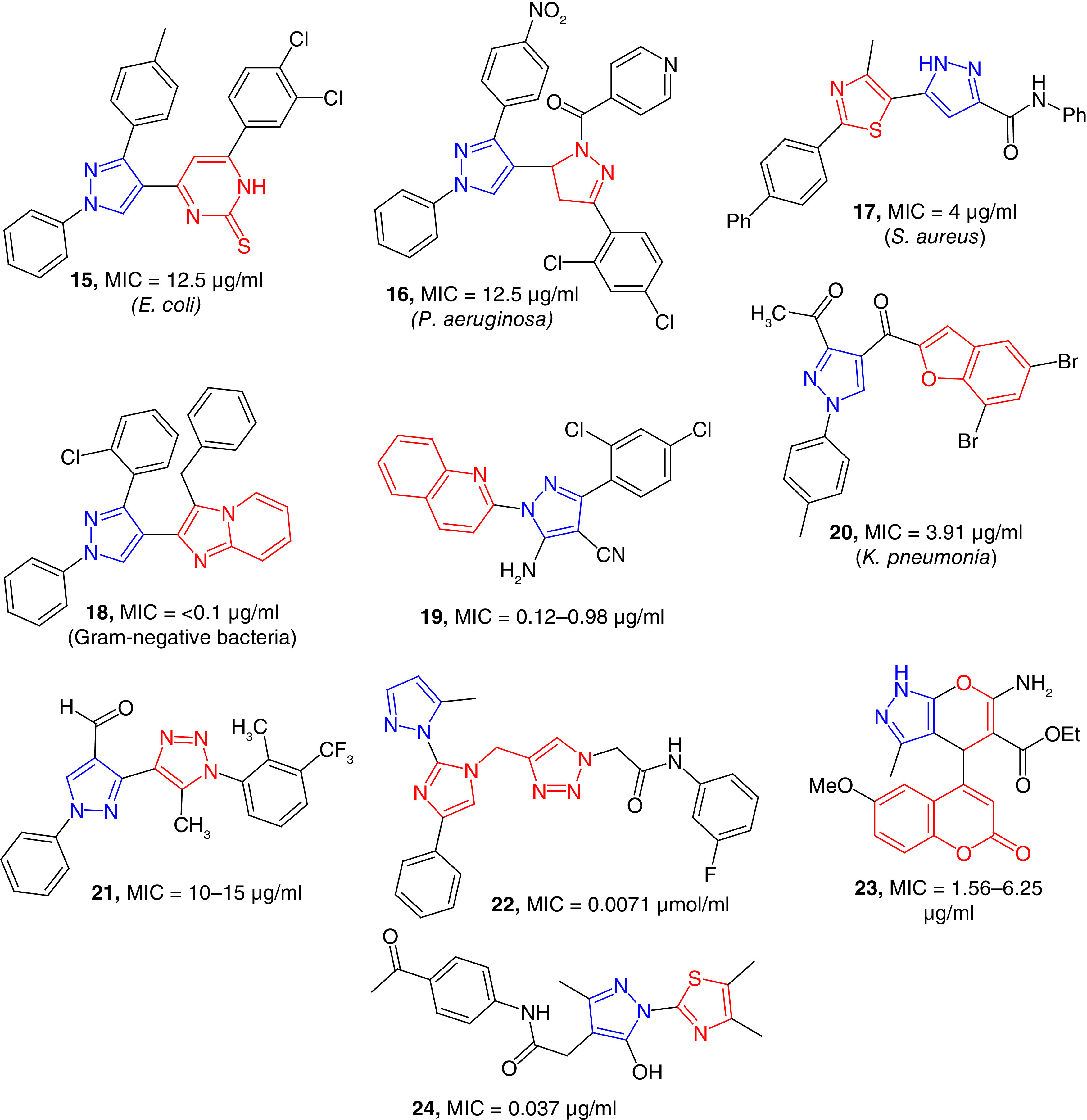
A series of pyrazole-pyrimidinethiones (e.g., 15) has been reported, and these compounds found to be potent inhibitor of E. coli with an MIC value of 12.5 μg/ml (Figure 4). Some of the compounds of this series were moderate inhibitors of S. aureus and Streptococcus pyogenes [67]. S. pyogenes is a human-specific Gram-positive bacterial pathogen that can cause a wide variety of infections. Improper treatment of S. pyogenes can cause postinfection acute rheumatic fever and glomerulonephritis [68]. Pyrazoline-clubbed pyrazole derivatives (16) have been reported as antimicrobial agents, and some of these compounds are potent against P. aeruginosa. Another series of pyrazoline-attached pyrazole derivatives have been found as moderate growth inhibitors of S. aureus bacteria [69]. These compounds were moderate growth inhibitors of other bacterial strains such as E. coli and S. aureus. Santosh et al. have reported the synthesis and antimicrobial studies of a library of 23 tethered thiazolo-pyrazole derivatives (e.g., 17) as potent anti-MRSA agents with MIC values as low as 4 μg/ml. Compound 17 has better toxicity profile than the standard antibiotics [70]. Thiazolidinone-clubbed pyrazoles have been reported as moderate antibacterial agents with an MIC value of 16 μg/ml against E. coli [71]. Imidazo-pyridine substituted pyrazole derivatives (18) have been reported as potent broad-spectrum antibacterial agents. These compounds were better than ciprofloxacin studied in vitro against two Gram-positive and four Gram-negative bacterial strains. The minimum bactericidal concentration (MBC) values were <1 μg/ml except for MRSA [72]. Compound 18 was potent against Gram-negative strains including E. coli, K. pneumoniae, P. aeruginosa and Salmonella typhimurium. Salmonella is a Gram-negative, rod-shaped intracellular pathogen that can cause symptoms ranging from diarrhea to lethal typhoid [73]. Quinoline-substituted pyrazole derivatives (e.g., 19) have been reported as potent antimicrobial agents with MIC values in the range of 0.12–0.98 μg/ml [74]. Compound 19 was potent against S. aureus, Staphylococcus epidermidis and B. subtilis. S. epidermidis is one of the most common commensal bacteria of human skin. This bacterium is among the most common sources of infection in in-dwelling medical devices that causes variety of problems including UTIs and endocarditis [75]. Compound 19 was also potent against Gram-negative strains, it was fourfold potent than gentamycin in inhibiting the growth of Shigella flexneri, a Gram-negative bacterium causing diarrhea in humans. Pyrazole-nucleus-containing benzofuran substitution (20) were found to be potent growth inhibitors of S. aureus, S. mutans, E. coli and K. pneumonia bacteria with the MIC values of 7.81, 15.6, 15.6 and 3.91 μg/ml, respectively. These values were better than the standard antibiotics used as positive controls in the study [76]. Pyrazole nucleus clubbed with oxadiazole derivatives have been reported as moderate growth inhibitors of different strains of bacteria [77]. In a series of pyrazole-triazole hybrids (21), some of the compounds were potent inhibitors of Gram-positive and Gram-negative bacterial strains with MIC values in the range of 10–15 μg/ml compared with the MIC values in the range of 2–6 μg/ml of the positive control ciprofloxacin [78]. A series of novel pyrazole-imidazole-triazole hybrids (e.g., 22) have been reported as potent growth inhibitors of S. aureus, E. coli and P. aeruginosa strains with MIC values in the low μmol/ml range. Furthermore, the binding affinity of these compounds with DNA gyrase was predicted as their mode of action [79]. Coumarin-substituted and pyran-fused derivatives (e.g., 23) of pyrazole have been reported as potent growth inhibitors of bacteria including S. aureus and P. aeruginosa strains with MIC values in the range of 1.56–6.25 μg/ml. The potent compound 23 was better than the positive control in inhibiting S. aureus strains [80]. A semiautomated high-throughput screening (HTS) platform have been used to screen more than 125,000 molecules to find potent antimicrobial agents. Eight compounds (e.g., 24) containing the pyrazole-thiazole hybrid structure showed potent activity against ΔTolC E. coli with MIC values as low as 0.037 μg/ml, which was significantly more potent than the positive controls erythromycin and levofloxacin. The limitation of these compounds for further antibiotic development was their toxicity for HEK-293 cells [81]. Dayankar et al. has reported a series of pyrazolyl triazole and one of the intermediates is a potent growth inhibitor of Micrococcus luteus with MIC/MBC values of 3.9/7.8 μg/ml [82]. Some of these pyrazole-triazole hybrids moderately active against Salmonella typhi and Salmonella paratyphi. Nevertheless, these potent compounds containing pyrazole-thiazole scaffold is a good starting point for the lead optimization. The pyrazole nucleus in conjugation with other heterocycles have resulted potent compounds with antimicrobial properties against different bacterial strains including ESKAPE pathogens.
Figure 4. . Pyrazole-clubbed heterocycles as potent antibacterial agents.
Pyrazole-derived aniline derivatives antibacterial agents
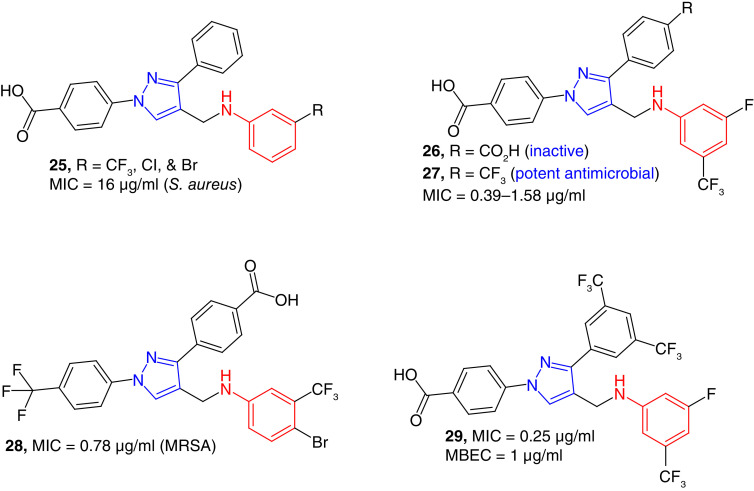
We have found aniline-derived pyrazoles as growth inhibitors of S. aureus. 3-phenyl pyrazoles (e.g., 25, Figure 5) were moderate growth inhibitors of S. aureus with MIC values as low as 16 μg/ml [43]. The nature of substituents on the N-phenyl ring changed the antimicrobial properties of the compounds significantly. As discussed earlier, dibenzoic-acid-derived hydrazones were narrow-spectrum antimicrobial agents with specific activity against A. baumannii [83], and aniline derivatives (26) of these compounds did not show any activity against the tested bacterial strains [84]. Replacing the carboxylic acid functional group on the 3-phenyl ring resulted the formation of potent antibacterial compounds (e.g., 27). These trifluorophenyl-substituted compounds (e.g., 27) were potent growth inhibitors of S. aureus including MRSA strains with MIC values as low as 0.39 μg/ml. Potent compounds of this series were also effective against S. epidermidis with an MIC value of 1.56 μg/ml. These compounds were very potent against B. subtilis and Enterococcus faecalis. The MIC values of several of these compounds against vancomycin-resistant E. faecalis (VRE) were as low as 1.56 μg/ml. These results are very significant as E. faecalis, a Gram-positive enterococcus, causes a variety of nosocomial infections, which are very difficult to treat by conventional antibiotics [85]. These potent compounds were also active against bacterial biofilms. Compound 27 was 25 times more active than the positive control vancomycin against catheter-associated biofilms. This potent compound was very effective against MRSA strains with an MIC value as low as 2 μg/ml [84]. N-(trifluoromethylphenyl) derivatives (e.g., 28) were also found to be potent growth inhibitors of Gram-positive bacterial strains with MIC values as low as 0.78 μg/ml for MRSA strains. Compound 28 was also effective against the clinical isolates of antibiotic resistant S. aureus and enterococci strains. These compounds were relatively nontoxic to HEK-293 cells. In mode of action studies, these compounds caused the bactericidal effect in multiple pathways including the inhibition of cell wall, protein and nucleic acid synthesis [86]. After finding the trifluoromethyl phenyl derivative (27 and 28), bistrifluoromethyl compounds (e.g., 29) were discovered. As expected, these compounds were potent antimicrobial agents. The potent compound 29 was a growth inhibitor Gram-positive bacteria with MIC values as low as 0.25 μg/ml. This compound was also potent against S. aureus biofilm with minimum biofilm eradication concentration (MBEC) value of 1 μg/ml. Nevertheless, this series of compounds were less tolerant to HEK-293 cell line [87].
Figure 5. . Pyrazole-derived anilines as potent anti-Gram-positive bacterial agents.
MRSA: Methicillin-resistant Staphylococcus aureus.
Fused-pyrazole derivatives as antibacterial agents
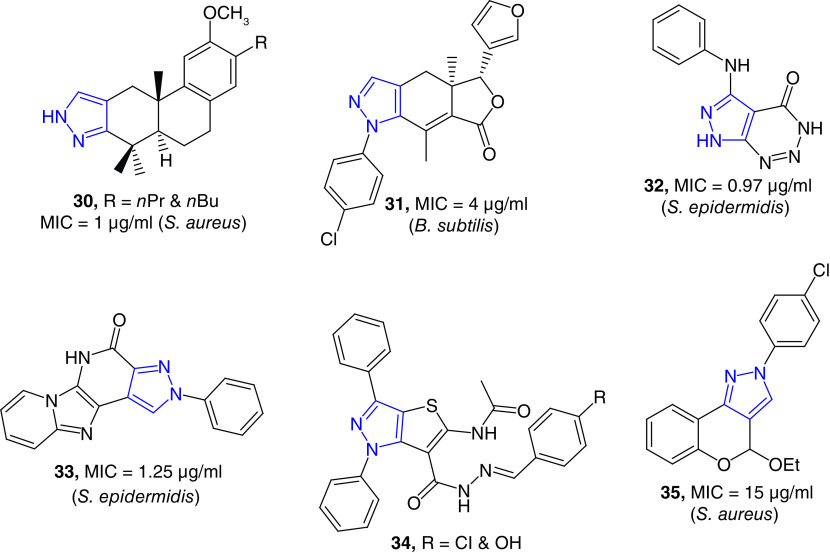
Pyrazole-fused diterpenoid have been reported as potent growth inhibitors of S. aureus strains with MIC values as low as 0.71 μg/ml (Figure 6). In a series of derivatives, two compounds (30) were most potent against S. aureus Newman strain. These compounds were even better against the antibiotic-resistant variants. These potent compounds did not show any obvious toxicity to human fibroblast (HAF) cells at MIC concentrations [88]. These pyrazole-derived diterpenoids are the rare examples of pyrazole-fused natural product derivatives as potent antimicrobial agents. Fraxinellone, a degraded limonoid natural product, is known for its wide range of pharmacological properties [89]. Synthesis and antibacterial studies of a series of novel N-phenylpyrazole-fused fraxinellone have been reported. One of these hybrid compounds (31) was a potent growth inhibitor of B. subtilis with an MIC value of 4 μg/ml [90]. Several aromatic fused-pyrazole derivatives have been reported as potent antibacterial agents. A series of triazine-fused pyrazole derivatives have been reported as potent growth inhibitors of multidrug-resistant bacterial strains [91]. The most active compound, 32, was a potent growth inhibitor of S. epidermidis and Enterobacter cloacae with MIC of 0.97 and 0.48 μg/ml, respectively. These MIC values were better than that of the positive control, tetracycline [91]. E. cloacae complex is a group of bacteria that are emerging as broad-spectrum antibiotic resistant nosocomial pathogens, including the emergence of last-resort carbapenem-resistant strains [92]. Singh et al. have reported the synthesis and antimicrobial studies of pyrazolopyridinone-fused imidazopyridines, These compounds showed good to excellent antibacterial properties with MIC values as low as 0.39 μg/ml. Compound 33 showed better activity against S. epidermidis than the positive control. Some of these compounds were also effective against S. aureus and P. aeruginosa [93]. Thiophene-fused and other heterocycle-fused compounds (e.g., 34) have been reported antibacterial agents with moderate activity [94]. Pyran-fused pyrazole derivatives have been reported as antibacterial agents. Compound 35 has shown moderate activity against S. aureus with an MIC value of 15 μg/ml [95].
Figure 6. . Fused-pyrazole derivatives as potent antibacterial agents.
DNA gyrase inhibitors
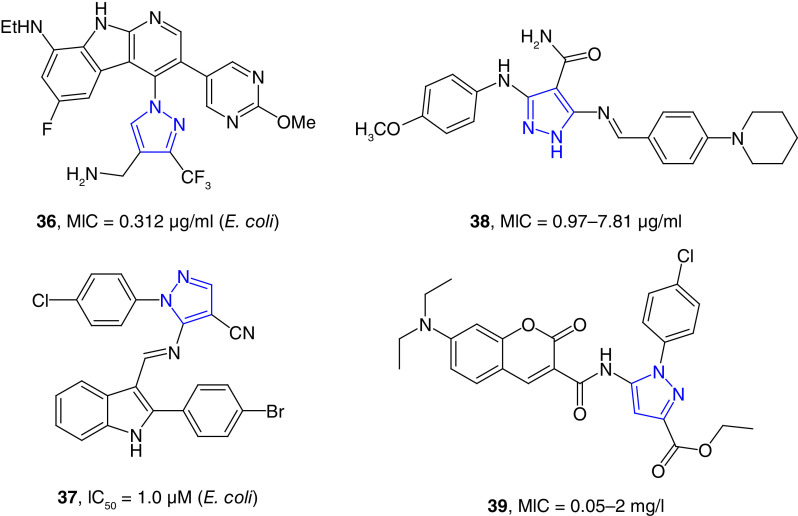
DNA gyrase is an ATP-dependent enzyme classified as topoisomerase II that is important for DNA replication, transcription, and chromosome segregation. This enzyme is essential for all bacteria for their growth, and it is an important target for antibacterial drug development. DNA gyrase is the protein target of fluoroquinolone class of antibiotics that are widely used as broad-spectrum antibiotics [96]. Pyrazole derivatives as DNA gyrase inhibitors have been reviewed recently [97]. Tan et al. have reported the synthesis and broad-spectrum antimicrobial properties of aza-indole-derived pyrazole derivatives (e.g., 36, Figure 7) as potent DNA gyrase and topoisomerase IV inhibitors [98]. The broad-spectrum compounds were discovered by extensive synthesis and SAR studies of potent anti-Gram-positive pyrazole-substituted aza-indole derivative reported by AstraZeneca [99]. The lead compound, 36, was a potent growth inhibitor of several Gram-negative bacterial strains, including E. coli, K. pneumoniae, A. baumannii and P. aeruginosa with MIC values in the range of 0.31–1.56 μg/ml. This lead compound has balanced physicochemical properties showing high aqueous solubility and desirable pharmacokinetic (PK) features. In vivo studies showed that this compound has bactericidal effect in neutropenic mouse thigh infection model [98]. In another study, a series of indole-attached imines of pyrazole (e.g., 37) were found to be potent drug-resistant antibacterial agents. These compounds were tested for their potency against S. aureus, Listeria monocytogenes, E. coli and Salmonella strains. Compound 37 was potent growth inhibitor of drug-resistant strains of E. coli with IC50 values as low as 1.0 μM. Molecular docking studies have shown that the bactericidal properties of these compounds could be due to their potent DNA gyrase inhibition properties [100]. Hassan et al. reported the synthesis of Schiff-based tethered pyrazole derivatives (e.g., 38) as potent dual dihydrofolate reductase (DHFR) and DNA gyrase inhibitor with IC50 values as low as 3.98 μM. Molecular docking studies of the potent compounds against DHFR and DNA gyrase exhibited potent binding affinity with different types of interactions, including hydrogen bonding, arene–arene and arene–cations bonds. Several of these compounds were potent growth inhibitors of Gram-positive and Gram-negative bacterial strains with the MIC values in the range of 0.97 to 62.5 μg/ml, which were much better than the positive control antibiotic tetracycline. Furthermore, these compounds were effective against multidrug-resistant strains of S. aureus, E. coli and P. aeruginosa [101]. A number of coumarin attached pyrazole derivatives has been reported as antibacterial agents. Compound 39 inhibited the growth of S. aureus with an MIC value of 1 mg/l. This compound inhibited the growth of L. monocytogenes, a Gram-positive bacterium that caused food borne disease listeriosis, with an MIC value of 0.5 mg/l. Compound 39 showed the most remarkable activity against Salmonella with an MIC value of 0.05 mg/l. These MIC values were better than the positive controls novobiocin and ciprofloxacin. Compound 39 was a potent inhibitor of Topo II and Topo 1V with IC50 values of 0.25 and 8 mg/l, respectively [102].
Figure 7. . Pyrazole-derived DNA gyrase inhibitors.
In silico studies on newly designed pyrazole compounds based on drug molecules led to the prediction of S. aureus DNA gyrase inhibitors. Among the predicted compounds, four pyrazole derivatives were synthesized and tested for their potency against Gram-positive and Gram-negative bacterial strains. These compound showed moderate antibacterial activity with MIC values as low as 12.5 μg/ml [103]. These results indicated that several pyrazole derivatives are potent DNA gyrase inhibitors, and these compounds were active against both Gram-positive and Gram-negative bacteria.
Inhibitors of DHFR, protein synthesis & quorum-sensing inhibitors
DHFR is a molecular target to develop drugs to treat several anomalies such as cancer, malaria and bacterial infections. Trimethoprim (TMP) is a classical DHFR inhibitor used as antibiotics to treat UTIs [104]. Piao et al. have reported a series of pyrazole-thioxothiazolidinone hybrids (e.g., 40) as potent antimicrobial agents (Figure 8). In vitro studies have shown that some of these compounds are potent growth inhibitors of Streptococcus mutans, an anaerobic Gram-positive bacteria found in oral cavity and a significant contributor to tooth decay, with an MIC value of 1 μg/ml. These compounds were potent against MRSA with an MIC value of 1 μg/ml, which was equivalent to the positive control moxifloxacin and better than several approved antibiotics such as oxacillin [105]. Further structure–activity relationship (SAR) studies let to the discovery of dihydrotriazine substituted pyrazole derivatives (40) as potent broad-spectrum antibacterial agents. One of these compounds inhibited the growth of MRSA and E. coli strains with MIC values as low as 1 μg/ml, which was comparable or better than several standard antibiotics. Molecular docking studies predicted the inhibition of DHFR as the possible mode of inhibition, which was further validated by enzyme inhibition studies [106].
Figure 8. . Bacterial dihydrofolate reductase inhibitor (40), protein synthesis inhibitor (41), β-lactamase inhibitor (42), and quorum sensing inhibitors (43, 44 and 45).
Several antibiotics, such as tetracyclines, macrolide, aminoglycosides, chloramphenicol and oxazolidinones, inhibit bacterial protein synthesis [107]. Some pyrazole-containing compounds have also been reported as the inhibitors of bacterial protein synthesis. Luo et al. reported a series of pyrazole-derived oxazolidinone compounds (e.g., 41) as potent antimicrobial compounds. In an extensive SAR studies, the compounds with N-heterocyclic substituents at the pyrazole ring found to be better antibacterial agents. The potent compound 41 inhibited the growth of MRSA strains with the MIC values in the range of 0.25–2.0 μg/ml, which was better than the widely used oxazolidinone antibiotic linezolid. In vivo mice model studies showed that this compound significantly improved the survival of MRSA-infected murine models. Furthermore, this potent compound has demonstrated high bioavailability and would be predicted to have less bone marrow suppression [108]. This result shows the significance of pyrazole in treating the MRSA infection because it has improved the anti-MRSA and pharmacological properties of linezolid skeleton. Molecular docking studies revealed that the mode of action was the binding with the 50S ribosomal subunit of bacteria to prevent protein synthesis [108].
β-Lactamases are the enzymes that hydrolyze the β-lactam ring of penicillins, cephalosporins, carbapenems and monobactams. These hydrolytic enzymes are broadly classified as serine-β-lactamases and zinc-dependent hydrolases. These enzymes are the major determinant of β-lactam antibiotics effectiveness in Gram-negative bacteria. Different classes of compounds have been found as potent β-lactamase inhibitors [109,110]. By using structure-guided approach, a series of pyrazole-derived aryl sulfonyl hydrazones (e.g., 42) was synthesized to target metallo-β-lactamases (MBLs). Several of these compounds showed potent inhibition of MBLs at low μM concentration. These compounds showed promising antibacterial activity against drug-resistant clinical isolates of K. pneumoniae. In a synergistic studies, these compounds resensitized resistant K. pneumoniae (K5) strain for meropenem and cephalexin antibiotics. The most active compound, 42, was well tolerated in animal model studies [111].
Quorum sensing (QS) is a bacterial cell–cell communication strategy of gene expression that acts as a group to regulate pathogenic processes such as biofilm formation, tolerance to antibiotics and expression of virulence factors. QS is regulated by small organic compounds called autoinducer. Oligopeptide and N-acyl homoserine lactone are the most common autoinducers in Gram-positive and Gram-negative bacteria, respectively [112]. LsrK kinase, a carbohydrate family of kinases, is one of the key enzymes to activate QS in both Gram-positive and Gram-negative bacteria [113]. Collina et al. designed a series of heterocycles to find potent LsrK inhibitors. SAR studies of these compounds led to the discovery of LsrK inhibitor (43) with IC50 values as low as 100 μM. In this study, it was confirmed that the pyrazole nucleus was essential for LsrK inhibition [114]. Vibriosis caused by vibrios bacteria causes an estimated 80,000 illnesses and 100 deaths each year in the US. Vibriosis or cholera is considered rare in the US and other industrialized countries, but it is one of the major diseases in the developing and underdeveloped world [115]. In vibrios, QS is regulated by a master regulator called LuxR protein, which is conserved in all pathogenic vibrios [116]. Screening a library of compounds followed by the synthesis of 50 sulfonamide derivatives to examine the SAR effects on QS in vivo led to the discovery several compounds as potent LuxR inhibitors. Among these compounds, the pyrazole-derived sulfonamide (44) was the most potent inhibitor with an IC50 value of submicromolar concentration [117]. Bromothiophene-derived pyrazole (45) has been found to be a potent vibrio QS inhibitor at nanomolar concentration. This small molecule inhibited Vibrio harveyi LuxR homologues, which is a well-conserved master transcriptional regulator for QS in Vibrio species [118].
Cell wall synthesis inhibition & cell wall disrupting agents
MurB Inhibitors & Bacterial Cystathionine γ-lyase (bCSE) inhibitors
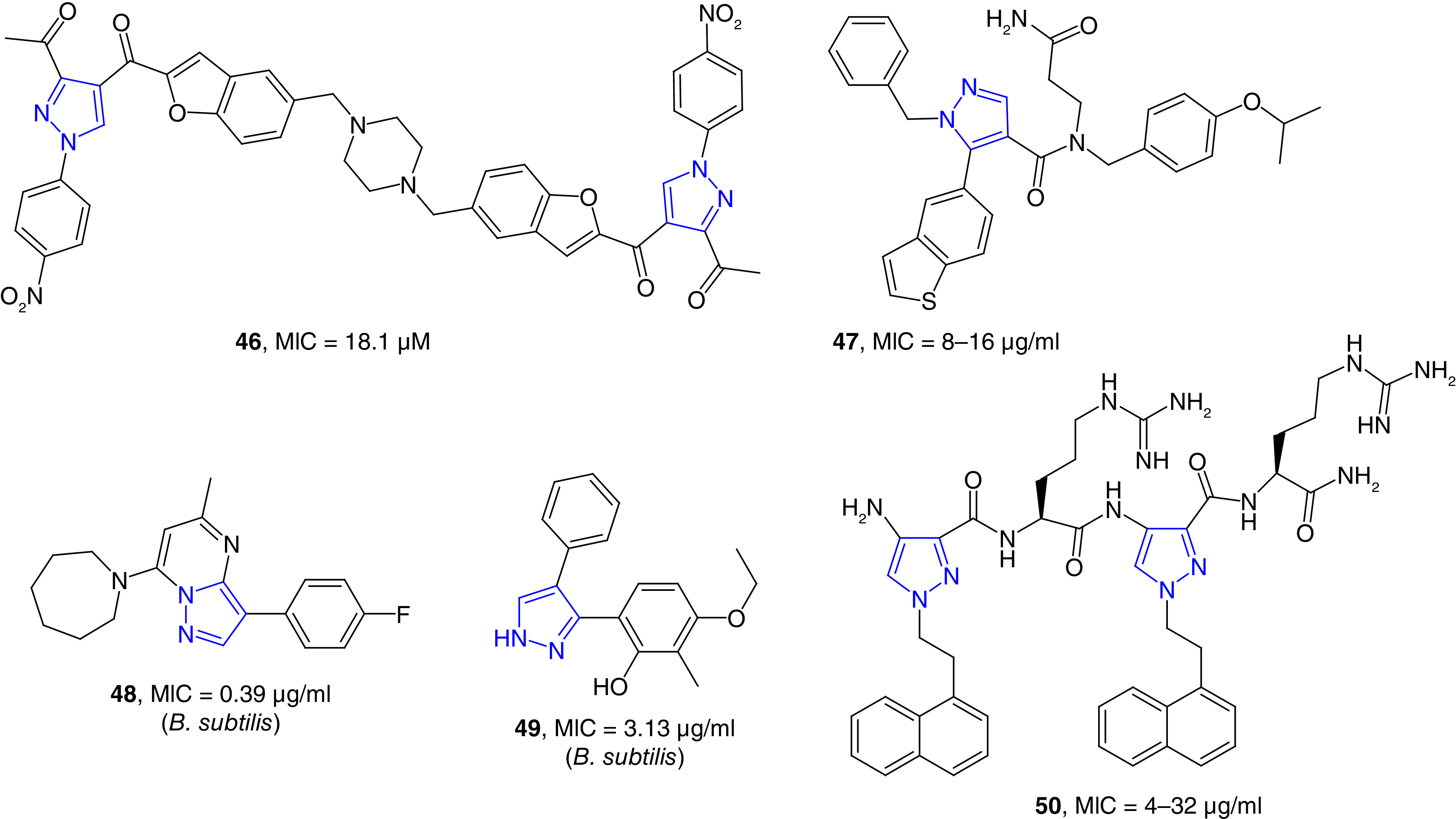
MurB, UND-N-acetylenolpyruvylglucosamine reductase, is an enzyme that participate in cytoplasmic steps of bacterial cell wall biosynthesis [119]. Novel piperazine bis(pyrazole-benzofuran) hybrid compounds found to be potent inhibitors of MurB enzyme. Compound 46 inhibited the MurB with an IC50 value of 3.1 μM (Figure 9). This compound inhibited the growth of MRSA strain with the MIC value of 18.1 μM and this compound was effective against the biofilms of S. aureus, S. mutans and E. coli strains. The antibiofilm activities of these compounds were better than the reference drug ciprofloxacin [120].
Figure 9. . Cell wall synthesis inhibitors and cell wall disrupting agents.
Undecaprenyl pyrophosphate synthase inhibitors
Undecaprenyl pyrophosphate synthase (UppS) catalyzes the reaction to synthesize undecaprenyl pyrophosphate by the condensation of a pyrophosphate (FPP) with eight isopentenyl pyrophosphate. UppS is an essential enzyme to synthesize peptidoglycan of bacterial cell wall [121]. Several inhibitors UppS of S. aureus were discovered from an encoded library technology. The binding of a potent compound to the hydrophobic substrate site was confirmed through cocrystallagraphy studies. Compound 47 inhibited the growth of S. aureus strains with an MIC value of 8 μg/ml (Figure 9). This compound was also a moderate growth inhibitor of two pathogenic Gram-negative bacterial strains; Haemophilus influenzae and Moraxella catarrhalis [122]. M. catarrhalis has gained recent attention for respiratory tract infection [123]. Screening a collection of 142,000 small molecules led to the discovery of a pyrazole-fused derivative (48) as potent UppS inhibitor with IC50 values as low as 0.05 μM. In this study, another pyrazole derivative (49) was also discovered as potent UppS inhibitor. These compounds effectively inhibited the growth of B. subtilis 168 with MIC values ranging from 0.1 to 12 μg/ml. These compounds were moderately active against S. aureus and did not show any activity against Gram-negative strains. Nevertheless, the fused-pyrazole compound (48) showed potent UppS inhibition at nanomolar concentration without off-target effects on membrane potential [124].
Cell wall disrupting agents
Several cell wall disrupting agents, such as daptomycin, polymixins and gramicidins, are currently used to treat various bacterial infections. The structure of the bacterial membrane is relatively conserved, and thus antibiotic-disrupting the bacterial membrane is considered to be less likely drug resistance than that of other classes of antibiotics [125]. Bang et al. have reported the first chemical synthesis of ultra-short pyrazole incorporated arginine-based peptidomimetics as potent antimicrobial agents. By systematic tuning of peptide length and hydrophobicity, compound 50 has been found as the most potent pyrazole containing peptidomimetics (Figure 9). This pyrazole-containing peptide inhibited the growth of S. aureus and P. aeruginosa strains with MIC values of 4 and 8 μg/ml, respectively. This compound was 2–4 times more potent against drug-resistant bacterial strains than melittin, the main component of honey bee venom. Fluorescence spectroscopy and transmission electron microscopy studies revealed the possible mechanism of action of these compounds, which caused cell wall disruption leading to cytosol leakage and eventual cell lysis [126].
Pyrazole derivatives as photodynamic antimicrobial agents
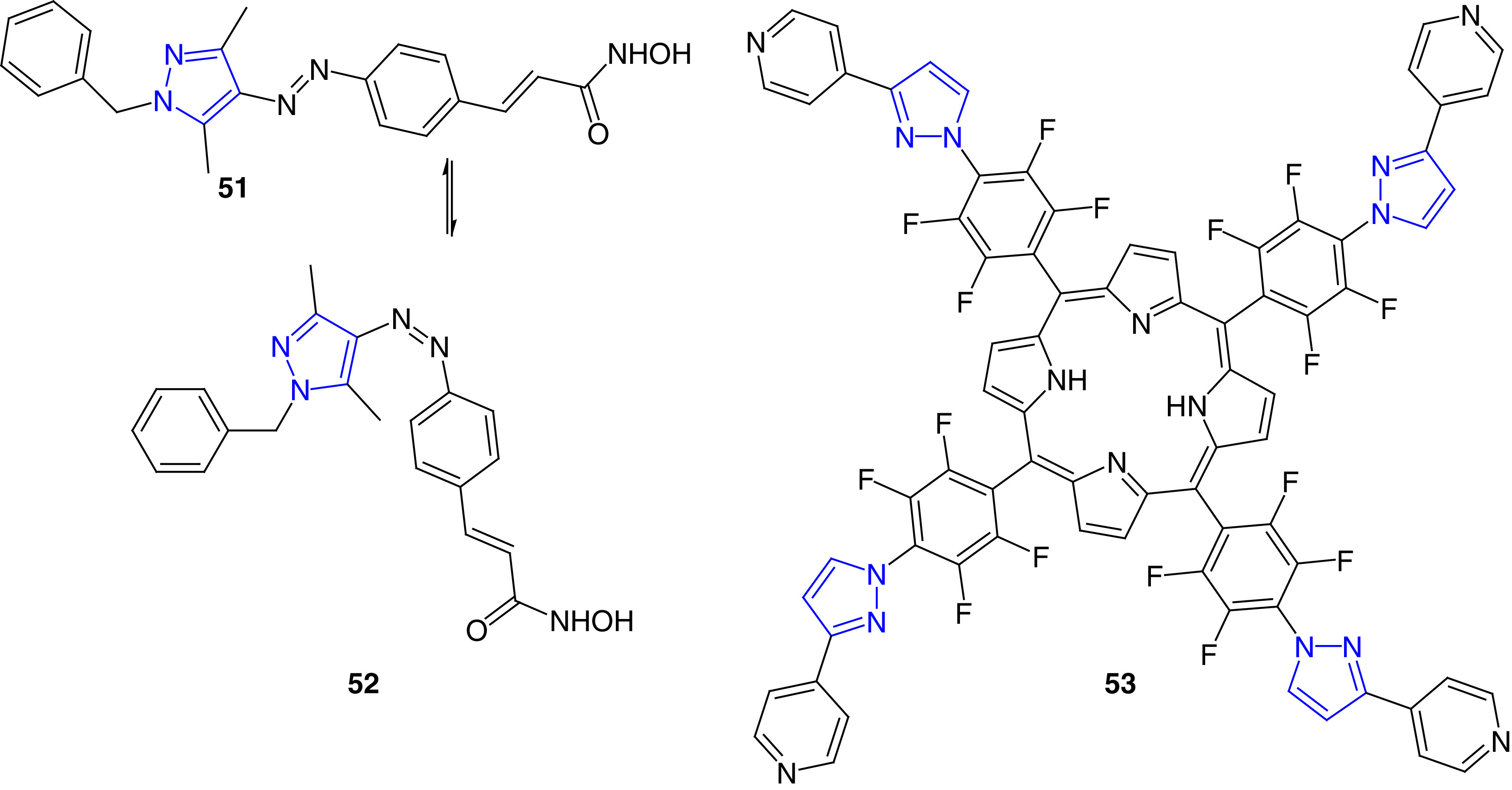
Antimicrobial photodynamic therapy (aPDT) is emerging a promising alternative technique to treat microbial infections [127,128]. Photodynamic therapy involves a combination of a photosensitizer (PS), oxygen and light to eliminate metabolically active cells or microorganism [128]. Some PS compounds as antimicrobial agents have been reported in recent years (Figure 10). Fuchter et al. have reported arylazopyrazole derivatives (51 and 52) as photopharmacological agents, and these compounds may have the potential to treat bacterial infections [129]. These compounds were potent growth inhibitors of amido-hydrolases, homologues to human histone deacetylases (HDACs). In this study, two classes of photoswitches (azobenzenes and arylazopyrazoles) were studied; the arylazopyrazoles were found to be better intrinsic photoswitches with nM IC50 values. Although no antimicrobial properties were reported in this study, due to potent amidohydrolase inhibition activities, these molecules have the potential for the development of new antimicrobial agents and modalities [129]. Lourenco et al. reported the synthesis and antimicrobial studies of pyrazole-attached porphyrins and chlorins as photosensitizers. These compounds (e.g., 53) were potent against E. coli bacteria both in planktonic and biofilm context. Compound 53 caused photoinactivation of E. coli at 2.0 μM concentration after 30 min of red light irradiation. This compound caused higher levels of 1O2 in bacteria to cause cell death [130].
Figure 10. . Arylazopyrazole derivatives as photopharmacological agents.
Miscellaneous pyrazole derivative
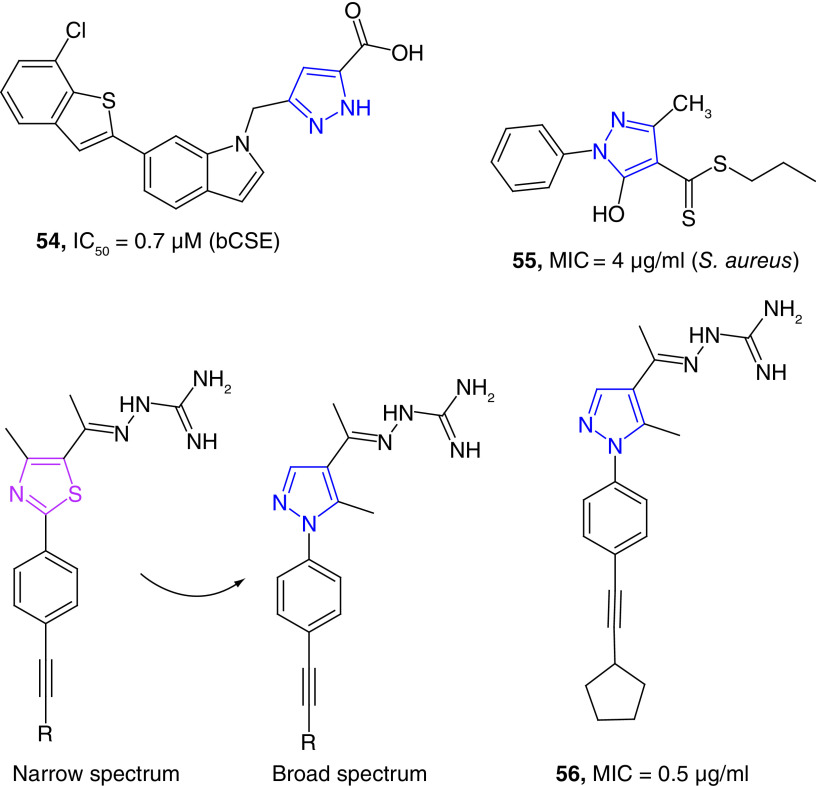
A series of small molecules have been tested for their bacterial cystathionine γ-lyase (bCSE) inhibition activity. The pyrazole derivative (54) has been as the most potent inhibitor of S. aureus CSE (SaCSE) and P. aeruginosa CSE (PaCSE) with the IC50 values of 0.7 and 1.2 μM respectively (Figure 11) [131]. Inhibition of CSE is significant because this enzyme is the key to produce hydrogen sulfide (H2S), which protects bacteria against oxidative stress [132]. A carbodithionate derivative (55) has been reported as a bacteriostatic agent against MRSA strains with an MIC value of 4 μg/ml. This molecule is moderately active against E. faecium with an MIC value of 16 μg/ml. In vivo studies further showed the potential of these compounds by rescuing Caenorhabditis elegans from S. aureus infection. Whole-genome sequencing of treated S. aureus and screening of a S. aureus promoter-lux array were used to find the mechanism of action of this potent compound (55). Twenty analogues of the lead compound (55) were further synthesized and tested for their potency against different bacterial strains. Some of these compounds were found to be potent antimicrobial agents with MIC values as low as 0.5 μg/ml [133]. These pyrazole derivatives (e.g., 55) were potential regulators of global transcriptional factor MgrA [134], an oxidation-sensing mechanism used by S. aureus to neutralize reactive oxygen species. The MgrA protein acts as both virulence factor and a regulator of antibiotic resistance in S. aureus [135].
Figure 11. . Miscellaneous example of pyrazole derivatives as potent antibacterial agents.
In a unique study on the importance of pyrazole nucleus, Seleem et al. reported the synthesis and antimicrobial properties of pyrazole derivatives as broad-spectrum antibacterial agents [136]. In this study, the narrow antibacterial spectrum of phenyl thiazole derivatives was expanded by replacing the thiazole nucleus with a pyrazole ring without compromising the pharmacokinetics features of the resultant compounds. The promising derivatives (e.g., 56) showed better activity than that of vancomycin against vancomycin- and linezolid-resistant MRSA strains with MIC values as low as 0.5 μg/ml. Some of these derivatives were potent against carbapenem-resistant A. baumannii (CRAB), K. pneumoniae and E. coli strains. Furthermore, these compounds were effective against clearing intracellular Gram-positive and Gram-negative pathogenic bacteria present in murine macrophage (J774) cells [136]. Finally, several pyrazole-derived metal complexes were found to be moderate growth inhibitors of bacterial strains [137].
Conclusion
Pyrazole derivatives have been approved to treat different type of diseases including bacterial infections. Cefoselis and ceftolozane are two pyrazole-derived antibiotics, which have been approved to treat different bacterial infections. A large number of pyrazole derivatives are in different stages of drug development to treat microbial infections.
Future perspective
Pyrazole derivatives have been found as potent antimicrobial agents targeting different molecular pathways of bacteria. It has been found that replacing other heterocycles with pyrazole increases the potency and the spectrum of the resultant compounds. Thus, replacing other heterocycles such as thiazole with pyrazole could generate potent antibacterial agents. The mechanism of action for most of the pyrazole derivatives are not known or are not reported. Therefore, it is difficult to determine the exact role of pyrazole for their antimicrobial properties. More mechanistic studies of pyrazole antibacterials will help to design better compounds. Metabolic stability of the pyrazole nucleus makes it one of the important heterocycles for novel antibiotic drug development.
Executive summary.
Microbial resistance to drugs is a global concern.
ESKAPE pathogens cause majority of nosocomial drug-resistant infections.
Pathogenic bacteria have developed resistance to existing antibiotics, and pandrug resistance bacteria are becoming common.
We are moving toward a post-antibiotic era in which minor infections and injuries may be lethal.
Major pharmaceutical companies began to abandon the discovery of novel antibiotics in the 1980s.
A few antibacterials are in the clinical pipeline, and less is known about their preclinical pipelines.
Pyrazole-derivatives are being used to treat various types of diseases, including bacterial infections.
A number of pyrazole-based small molecules have been reported as potent antibacterial agents.
Different scaffolds containing pyrazole nucleus are known to show potent antimicrobial properties.
These compounds show bactericidal or bacteriostatic properties by inhibiting different metabolic pathways including DNA gyrase inhibition, inhibition of protein synthesis and peptidoglycan synthesis inhibitions.
These pyrazole-containing compounds are also known as cell wall disrupting agents, photosensitizer and reactive oxygen species generators.
Footnotes
Financial & competing interests disclosure
This review was made possible by Arkansas INBRE Summer Research Grant (SRG) supported by a grant from the National Institute of General Medical Sciences (NIGMS), P20 GM103429 from the National Institutes of Health. An ABI mini grant was also helpful to write this paper. The author has no other relevant affiliations or financial involvement with any organization or entity with a financial interest in or financial conflict with the subject matter or materials discussed in the manuscript apart from those disclosed.
No writing assistance was utilized in the production of this manuscript.
References
Papers of special note have been highlighted as: • of interest; •• of considerable interest
- 1.Williams K. The introduction of ‘chemotherapy’ using arsphenamine – the first magic bullet. J. Royal Soc. Med. 102(8), 343–348 (2009). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Davies J. Where have all the antibiotics gone? Can. J. Infect. Dis. Med. Microbiol. 17(5), 287–290 (2006). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Aminov RI. A brief history of the antibiotic era: lessons learned and challenges for the future. Front. Microbiol. 1, 134–134 (2010). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Friedrich MJ. WHO survey reveals misconceptions about antibiotic resistance. JAMA 315(3), 242–242 (2016). [Google Scholar]
- 5.Mullard A. Pharmaceutical firms commit US$1 billion to antibiotic development. Nat. Rev. Drug Discov. 19(9), 575–576 (2020). [DOI] [PubMed] [Google Scholar]
- 6.Santajit S, Indrawattana N. Mechanisms of antimicrobial resistance in ESKAPE pathogens. Biomed. Res. Int. 2016, 2475067–2475067 (2016). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Boucher HW, Talbot GH, Bradley JS et al. Bad bugs, no drugs: no ESKAPE! An update from the Infectious Diseases Society of America. Clin. Infect. Dis. 48(1), 1–12 (2009). [DOI] [PubMed] [Google Scholar]
- 8.Centers for Disease Control and Prevention. Antibiotic/antimicrobial resistance (2016). https://www.cdc.gov/drugresistance/index.html
- 9.Centers for Disease Control and Prevention. Antibiotic/antimicrobial resistance (AR/AMR) (2019). https://www.cdc.gov/drugresistance/index.html
- 10.De Matos PDM, De Oliveira TLR, Cavalcante FS et al. Molecular markers of antimicrobial resistance in methicillin-resistant Staphylococcus aureus SCCmec IV presenting different genetic backgrounds. Microb. Drug Resist. (NY) 22(8), 700–706 (2016). [DOI] [PubMed] [Google Scholar]
- 11.Hu XL, Li D, Shao L et al. Triazole-linked glycolipids enhance the susceptibility of MRSA to beta-lactam antibiotics. ACS Med. Chem. Lett. 6(7), 793–797 (2015). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Centers for Disease Control and Prevention. Methicillin-resistant Staphylococcus aureus (MRSA) (2019). https://www.cdc.gov/mrsa/index.html
- 13.Song S, Wood TK. Combatting persister cells with substituted indoles. Front. Microbiol. 11, 1565 (2020). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Cetinkaya Y, Falk P, Mayhall CG. Vancomycin-resistant enterococci. Clin. Microbiol. Rev. 13(4), 686–707 (2000). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Centers for Disease Control and Prevention. Vancomycin-resistant enterococci (VRE) in healthcare settings (2019). https://www.cdc.gov/hai/organisms/vre/vre.html
- 16.Breijyeh Z, Jubeh B, Karaman R. Resistance of Gram-negative bacteria to current antibacterial agents and approaches to resolve it. Molecules (Basel) 25(6), 1340 (2020). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Bradley CA. Disrupting MRSA ‘persisters’. Nat. Rev. Drug Discov. 17(6), 394–394 (2018). [DOI] [PubMed] [Google Scholar]
- 18.Berditsch M, Afonin S, Reuster J et al. Supreme activity of gramicidin S against resistant, persistent and biofilm cells of staphylococci and enterococci. Sci Rep 9(1), 17938 (2019). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Silver LL. A Gestalt approach to Gram-negative entry. Bioorg. Med. Chem. 24(24), 6379–6389 (2016). [DOI] [PubMed] [Google Scholar]
- 20.Soto SM. Role of efflux pumps in the antibiotic resistance of bacteria embedded in a biofilm. Virulence 4(3), 223–229 (2013). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Fitzpatrick MA. Real-world antibiotic needs for resistant Gram-negative infections. Lancet Infect. Dis. 20(10), 1108–1109 (2020). [DOI] [PubMed] [Google Scholar]
- 22.Gonzalez-Ferrer S, Penaloza HF, Budnick JA et al. Finding order in the chaos: outstanding questions in Klebsiella pneumoniae pathogenesis. Infect. Immun. 89(4), e00693 (2021). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Zong Z, Feng Y, Mcnally A. Carbapenem and colistin resistance in enterobacter: determinants and clones. Trends Microbiol. 29(6), 473–476 (2021). [DOI] [PubMed] [Google Scholar]
- 24.Inchai J, Liwsrisakun C, Theerakittikul T et al. Risk factors of multidrug-resistant, extensively drug-resistant and pandrug-resistant Acinetobacter baumannii ventilator-associated pneumonia in a medical intensive care unit of university hospital in Thailand. J. Infect. Chemother. 21(8), 570–574 (2015). [DOI] [PubMed] [Google Scholar]
- 25.Vourli S, Frantzeskaki F, Meletiadis J et al. Synergistic interactions between colistin and meropenem against extensively drug-resistant and pandrug-resistant Acinetobacter baumannii isolated from ICU patients. Int. J. Antimicrob. Agents 45(6), 670–671 (2015). [DOI] [PubMed] [Google Scholar]
- 26.Smani Y, Dominguez-Herrera J, Pachon J. Rifampin protects human lung epithelial cells against cytotoxicity induced by clinical multi and pandrug-resistant Acinetobacter baumannii. J. Infect. Dis. 203(8), 1110–1119 (2011). [DOI] [PubMed] [Google Scholar]
- 27.World Health Organization. Global priority list of antibiotic-resistant bacteria to guide research, discovery, and development of new antibiotics (2017). [Google Scholar]
- 28.Willyard C. The drug-resistant bacteria that pose the greatest health threats. Nature 543(7643), 15 (2017). [DOI] [PubMed] [Google Scholar]
- 29.World Health Organization. In: Guidelines for the Prevention and Control of Carbapenem-Resistant Enterobacteriaceae, Acinetobacter baumannii and Pseudomonas aeruginosa in Health Care Facilities. Geneva, 76 (2017). [PubMed] [Google Scholar]
- 30.Moradali MF, Ghods S, Rehm BHA. Pseudomonas aeruginosa lifestyle: a paradigm for adaptation, survival, and persistence. Front. Cellular Infect. Microbiol. 7(39), 1–29 (2017). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Su SC, Vaneechoutte M, Dijkshoorn L et al. Identification of non-fermenting Gram-negative bacteria of clinical importance by an oligonucleotide array. J. Med. Microbiol. 58(Pt 5), 596–605 (2009). [DOI] [PubMed] [Google Scholar]
- 32.Tommasi R, Brown DG, Walkup GK et al. ESKAPEing the labyrinth of antibacterial discovery. Nat. Rev. Drug Discov. 14(8), 529–542 (2015). [DOI] [PubMed] [Google Scholar]; • Important bacterial pathogens.
- 33.Naim MJ, Alam O, Nawaz F et al. Current status of pyrazole and its biological activities. J. Pharm. Bioallied Sci. 8(1), 2–17 (2016). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Steinbach G, Lynch PM, Phillips RKS et al. The effect of celecoxib, a cyclooxygenase-2 inhibitor, in familial adenomatous polyposis. New Engl. J. Med. 342(26), 1946–1952 (2000). [DOI] [PubMed] [Google Scholar]
- 35.Hampp C, Hartzema AG, Kauf TL. Cost-utility analysis of rimonabant in the treatment of obesity. Value Health 11(3), 389–399 (2008). [DOI] [PubMed] [Google Scholar]
- 36.Kameyama T, Nabeshima T. Effects of 1,3-diphenyl-5-(2-dimethylaminopropionamide)-pyrazole[difenamizole] on a conditioned avoidance-response. Neuropharmacology 17(4–5), 249–256 (1978). [DOI] [PubMed] [Google Scholar]
- 37.Chen LW, Wang PF, Tang DJ et al. Metronidazole containing pyrazole derivatives potently inhibit tyrosyl-tRNA synthetase: design, synthesis, and biological evaluation. Chem. Biol. Drug Des. 88(4), 592–598 (2016). [DOI] [PubMed] [Google Scholar]
- 38.Hafez HN, El-Gazzar AR, Al-Hussain SA. Novel pyrazole derivatives with oxa/thiadiazolyl, pyrazolyl moieties and pyrazolo[4,3-d]-pyrimidine derivatives as potential antimicrobial and anticancer agents. Bioorg. Med. Chem. Lett. 26(10), 2428–2433 (2016). [DOI] [PubMed] [Google Scholar]
- 39.Nayak N, Ramprasad J, Dalimba U. New INH-pyrazole analogs: design, synthesis and evaluation of antitubercular and antibacterial activity. Bioorg. Med. Chem. Lett. 25(23), 5540–5545 (2015). [DOI] [PubMed] [Google Scholar]
- 40.Allison D, Delancey E, Ramey H et al. Synthesis and antimicrobial studies of novel derivatives of 4-(4-formyl-3-phenyl-1H-pyrazol-1-yl)benzoic acid as potent anti-Acinetobacter baumannii agents. Bioorg. Med. Chem. Lett. 27(3), 387–392 (2017). [DOI] [PMC free article] [PubMed] [Google Scholar]; •• First report on pyrazole derivatives as anti-Acinetobacter baumannii agents.
- 41.Zakeyah AA, Whitt J, Duke C et al. Synthesis and antimicrobial studies of hydrazone derivatives of 4-[3-(2,4-difluorophenyl)-4-formyl-1H-pyrazol-1-yl]benzoic acid and 4-[3-(3,4-difluorophenyl)-4-formyl-1H-pyrazol-1-yl]benzoic acid. Bioorg. Med. Chem. Lett. 28(17), 2914–2919 (2018). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Whitt J, Duke C, Sumlin A et al. Synthesis of hydrazone derivatives of 4-[4-formyl-3-(2-oxochromen-3-yl)pyrazol-1-yl]benzoic acid as potent growth inhibitors of antibiotic-resistant Staphylococcus aureus and Acinetobacter baumannii. Molecules 24(11), 2051 (2019). [DOI] [PMC free article] [PubMed] [Google Scholar]; • Coumarin-attached pyrazole derivatives as antimicrobial agents.
- 43.Brider J, Rowe T, Gibler DJ et al. Synthesis and antimicrobial studies of azomethine and N-arylamine derivatives of 4-(4-formyl-3-phenyl-1H-pyrazol-1-yl)benzoic acid as potent anti-methicillin-resistant Staphylococcus aureus agents. Med. Chem. Res. 25(11), 2691–2697 (2016). [Google Scholar]
- 44.Whitt J, Duke C, Ali MA et al. Synthesis and antimicrobial studies of 4-[3-(3-fluorophenyl)-4-formyl-1h-pyrazol-1-yl]benzoic acid and 4-[3-(4-fluorophenyl)-4-formyl-1h-pyrazol-1-yl]benzoic acid as potent growth inhibitors of drug-resistant bacteria. ACS Omega 4(10), 14284–14293 (2019). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Ahn M, Gunasekaran P, Rajasekaran G et al. Pyrazole derived ultra-short antimicrobial peptidomimetics with potent anti-biofilm activity. Eur. J. Med. Chem. 125, 551–564 (2017). [DOI] [PubMed] [Google Scholar]
- 46.Khan MF, Alam MM, Verma G et al. The therapeutic voyage of pyrazole and its analogs: a review. Eur. J. Med. Chem. 120, 170–201 (2016). [DOI] [PubMed] [Google Scholar]
- 47.Ohtaki K, Matsubara K, Fujimaru S et al. Cefoselis, a β-lactam antibiotic, easily penetrates the blood–brain barrier and causes seizure independently by glutamate release. J. Neural Transmission 111(12), 1523–1535 (2004). [DOI] [PubMed] [Google Scholar]
- 48.Yusuf E, Bax HI, Verkaik NJ, Van Westreenen M. An update on eight ‘new’ antibiotics against multidrug-resistant Gram negative bacteria. J. Clin. Med. 10(5), 1068 (2021). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Murano K, Yamanaka T, Toda A et al. Structural requirements for the stability of novel cephalosporins to AmpC β-lactamase based on 3D-structure. Bioorg. Med. Chem. 16(5), 2261–2275 (2008). [DOI] [PubMed] [Google Scholar]
- 50.Alam MA. US10596153B2 (2020).
- 51.Alam MA. WO2017205814A1 (2017).
- 52.Delancey E, Allison D, Kc HR et al. Synthesis of 4,4′-(4-formyl-1h-pyrazole-1,3-diyl)dibenzoic acid derivatives as narrow spectrum antibiotics for the potential treatment of Acinetobacter Baumannii infections. Antibiotics 9(10), 650 (2020). [DOI] [PMC free article] [PubMed] [Google Scholar]; •• Rare example to narrow-spectrum antibiotics specific activity against A. baumannii.
- 53.Alnufaie R, Alsup N, Kc HR et al. Design and synthesis of 4-[4-formyl-3-(2-naphthyl)pyrazol-1-yl]benzoic acid derivatives as potent growth inhibitors of drug-resistant Staphylococcus aureus. J. Antibiot. 73(12), 818–827 (2020). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Alnufaie R, Raj Kc H, Alsup N et al. Synthesis and antimicrobial studies of coumarin-substituted pyrazole derivatives as potent anti-Staphylococcus aureus agents. Molecules 25(12), 2758 (2020). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Ebenezer O, Singh-Pillay A, Koorbanally NA, Singh P. Antibacterial evaluation and molecular docking studies of pyrazole–thiosemicarbazones and their pyrazole–thiazolidinone conjugates. Molec. Diversity 25(1), 191–204 (2021). [DOI] [PubMed] [Google Scholar]
- 56.Gondru R, Sirisha K, Raj S et al. Design, synthesis, in vitro evaluation and docking studies of pyrazole-thiazole hybrids as antimicrobial and antibiofilm agents. ChemistrySelect 3(28), 8270–8276 (2018). [Google Scholar]
- 57.Atıcı S, Alp Ünkar Z, Öcal Demir S et al. A rare and emerging pathogen: Raoultella planticola identification based on 16S rRNA in an infant. J. Infect. Public Health 11(1), 130–132 (2018). [DOI] [PubMed] [Google Scholar]
- 58.El Shehry MF, Abbas SY, Farrag AM et al. Design, synthesis and biological evaluation of quinoxaline N-propionic and O-propionic hydrazide derivatives as antibacterial and antifungal agents. Med. Chem. Res. 27(10), 2287–2296 (2018). [Google Scholar]
- 59.Mcsheffrey GG, Gray-Owen SD. Chapter 82 – Neisseria gonorrhoeae. In: Molecular Medical Microbiology (2nd Edition). Tang Y-W, Sussman M, Liu D, Poxton I, Schwartzman J. (Eds). Academic Press Boston, USA, 1471–1485 (2015). [Google Scholar]
- 60.Li Y-R, Li C, Liu J-C et al. Synthesis and biological evaluation of 1,3-diaryl pyrazole derivatives as potential antibacterial and anti-inflammatory agents. Bioorg. Med. Chem. Lett. 25(22), 5052–5057 (2015). [DOI] [PubMed] [Google Scholar]
- 61.Garretto A, Miller-Ensminger T, Ene A et al. Genomic survey of E. coli from the bladders of women with and without lower urinary tract symptoms. Front. Microbiol. 11(2094), 1–13 (2020). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Percival SL, Williams DW. Chapter Six – Escherichia coli. In: Microbiology of Waterborne Diseases (2nd Edition). Percival SL, Yates MV, Williams DW, Chalmers RM, Gray NF. (Eds). Academic Press London, UK, 89–117 (2014). [Google Scholar]
- 63.PS K S, Subhashini N. Microwave assisted green synthesis of pyrazole, 1,2,3-triazole based novel benzohydrazones and their antibacterial activities. J. Heterocycl. Chem. 55(2), 508–516 (2018). [Google Scholar]
- 64.Kamal R, Kumar V, Bhardwaj V et al. Synthesis, characterization and in vitro antimicrobial evaluation of some novel hydrazone derivatives bearing pyrimidinyl and pyrazolyl moieties as a promising heterocycles. Med Chem Res 24(6), 2551–2560 (2015). [Google Scholar]
- 65.Gondru R, Banothu J, Thatipamula RK et al. 3-(1-phenyl-4-((2-(4-arylthiazol-2-yl)hydrazono)methyl)-1H-pyrazol-3-yl)-2H-chromen-2-ones: one-pot three component condensation, in vitro antimicrobial, antioxidant and molecular docking studies. RSC Adv. 5(42), 33562–33569 (2015). [Google Scholar]
- 66.Harikrishna N, Isloor AM, Ananda K et al. 1,3,4-trisubstituted pyrazole bearing a 4-(chromen-2-one) thiazole: synthesis, characterization and its biological studies. RSC Adv. 5(54), 43648–43659 (2015). [Google Scholar]
- 67.Desai NC, Joshi SB, Jadeja KA. A one-pot multicomponent Biginelli reaction for the preparation of novel pyrimidinthione derivatives as antimicrobial agents. J. Heterocyc. Chem. 57(2), 791–795 (2020). [Google Scholar]
- 68.Alves-Barroco C, Rivas-García L et al. Tackling multidrug resistance in streptococci – from novel biotherapeutic strategies to nanomedicines. Front.Microbiol. 11(2487), 1–21 (2020). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Harikrishna N, Isloor AM, Ananda K et al. Synthesis, and antitubercular and antimicrobial activity of 1′-(4-chlorophenyl)pyrazole containing 3,5-disubstituted pyrazoline derivatives. New J. Chem. 40(1), 73–76 (2016). [Google Scholar]
- 70.Patel B, Zunk M, Grant G, Rudrawar S. Design, synthesis and bioactivity evaluation of novel pyrazole linked phenylthiazole derivatives in context of antibacterial activity. Bioorg. Med. Chem. Lett. 39, 127853 (2021). [DOI] [PubMed] [Google Scholar]
- 71.Bhat M, Poojary B, Kalal BS et al. Synthesis and evaluation of thiazolidinone–pyrazole conjugates as anticancer and antimicrobial agents. Future Med. Chem. 10(9), 1017–1036 (2018). [DOI] [PubMed] [Google Scholar]
- 72.Ebenezer O, Awolade P, Koorbanally N, Singh P. New library of pyrazole–imidazo[1,2-α]pyridine molecular conjugates: synthesis, antibacterial activity and molecular docking studies. Chem. Biol. Drug Des. 95(1), 162–173 (2020). [DOI] [PubMed] [Google Scholar]
- 73.Jantsch J, Chikkaballi D, Hensel M. Cellular aspects of immunity to intracellular Salmonella enterica. Immunol. Rev. 240(1), 185–195 (2011). [DOI] [PubMed] [Google Scholar]
- 74.El Shehry MF, Ghorab MM, Abbas SY et al. Quinoline derivatives bearing pyrazole moiety: synthesis and biological evaluation as possible antibacterial and antifungal agents. Eur. J. Med. Chem. 143, 1463–1473 (2018). [DOI] [PubMed] [Google Scholar]
- 75.Chabi R, Momtaz H. Virulence factors and antibiotic resistance properties of the Staphylococcus epidermidis strains isolated from hospital infections in Ahvaz, Iran. Trop. Med. Health 47(1), 56 (2019). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Sanad SMH, Hanna DH, Mekky AEM. Regioselective synthesis of novel antibacterial pyrazole-benzofuran hybrids: 2D NMR spectroscopy studies and molecular docking. J. Molecul. Struct. 1188, 214–226 (2019). [Google Scholar]
- 77.Karad SC, Purohit VB, Avalani JR et al. Design, synthesis, and characterization of a fluoro substituted novel pyrazole nucleus clubbed with 1,3,4-oxadiazole scaffolds and their biological applications. RSC Adv. 6(47), 41532–41541 (2016). [Google Scholar]
- 78.Bhat M, Nagaraja GK, Kayarmar R et al. Design, synthesis and characterization of new 1,2,3-triazolyl pyrazole derivatives as potential antimicrobial agents via a Vilsmeier–Haack reaction approach. RSC Adv. 6(64), 59375–59388 (2016). [Google Scholar]
- 79.Punia S, Verma V, Kumar D et al. Facile synthesis, antimicrobial evaluation and molecular docking studies of pyrazole-imidazole-triazole hybrids. J. Molecul. Struct. 1223, 129216 (2021). [Google Scholar]
- 80.Chougala BM, Samundeeswari S, Holiyachi M et al. Synthesis, characterization and molecular docking studies of substituted 4-coumarinylpyrano[2,3-c]pyrazole derivatives as potent antibacterial and anti-inflammatory agents. Eur. J. Med. Chem. 125, 101–116 (2017). [DOI] [PubMed] [Google Scholar]
- 81.Ivanenkov YA, Yamidanov RS, Osterman IA et al. 2-Pyrazol-1-yl-thiazole derivatives as novel highly potent antibacterials. J. Antibiot. 72(11), 827–833 (2019). [DOI] [PubMed] [Google Scholar]
- 82.Dayakar C, Kumar BS, Sneha G et al. Synthesis, pharmacological activities and molecular docking studies of pyrazolyltriazoles as anti-bacterial and anti-inflammatory agents. Bioorg. Med. Chem. 25(20), 5678–5691 (2017). [DOI] [PubMed] [Google Scholar]
- 83.Delancey E, Allison D, Kc HR et al. Synthesis of 4,4′-(4-formyl-1h-pyrazole-1,3-diyl)dibenzoic acid derivatives as narrow spectrum antibiotics for the potential treatment of Acinetobacter baumannii infections. Antibiotics 9(10), 650 (2020). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Hansa RKC, Khan MMK, Frangie MM et al. 4-4-(anilinomethyl)-3-[4-(trifluoromethyl)phenyl]-1H-pyrazol-1-ylbenzoic acid derivatives as potent anti-Gram-positive bacterial agents. Eur. J. Med. Chem. 219, 113402 (2021). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Kau AL, Martin SM, Lyon W et al. Enterococcus faecalis tropism for the kidneys in the urinary tract of C57BL/6J mice. Infect. Immun. 73(4), 2461–2468 (2005). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Alkhaibari IS, Kc HR, Roy S et al. Synthesis of 3,5-bis(trifluoromethyl)phenyl-substituted pyrazole derivatives as potent growth inhibitors of drug-resistant bacteria. Molecules 26(16), 5083 (2021). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Alkhaibari I, Raj Kc H, Roy S et al. Design, synthesis, and antimicrobial activity of N-(trifluoromethyl)phenyl substituted pyrazole derivatives. RSC Med. Chem. 12, 1690–1697 (2021). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Yu L-G, Ni T-F, Gao W et al. The synthesis and antibacterial activity of pyrazole-fused tricyclic diterpene derivatives. Eur. J. Med. Chem. 90, 10–20 (2015). [DOI] [PubMed] [Google Scholar]
- 89.Bailly C, Vergoten G. Fraxinellone: from pesticidal control to cancer treatment. Pesticide Biochem. Physiol. 168, 104624 (2020). [DOI] [PubMed] [Google Scholar]
- 90.Guo Y, Wang X, Qu L et al. Design, synthesis, antibacterial and insecticidal activities of novel N-phenylpyrazole fraxinellone hybrid compounds. RSC Adv. 7(19), 11796–11802 (2017). [Google Scholar]
- 91.Hassan AS, Moustafa GO, Askar AA et al. Synthesis and antibacterial evaluation of fused pyrazoles and Schiff bases. Syn. Commun. 48(21), 2761–2772 (2018). [Google Scholar]
- 92.Annavajhala MK, Gomez-Simmonds A, Uhlemann A-C. Multidrug-resistant enterobacter cloacae complex emerging as a global, diversifying threat. Front Microbiol 10(44), (2019). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93.Devi N, Jana AK, Singh V. Assessment of novel pyrazolopyridinone fused imidazopyridines as potential antimicrobial agents. Karbala Int. J. Modern Sci. 4(1), 164–170 (2018). [Google Scholar]
- 94.Abdel Reheim M, Baker SM. Synthesis, characterization and in vitro antimicrobial activity of novel fused pyrazolo[3,4-c]pyridazine, pyrazolo[3,4-d]pyrimidine, thieno[3,2-c]pyrazole and pyrazolo[3′,4′:4,5]thieno[2,3-d]pyrimidine derivatives. Chem. Cent. J. 11(1), 112–112 (2017). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95.Gurunanjappa P, Nagamallu R, Kariyappa AK. Synthesis and antimicrobial activity of novel fused pyrazoles. Int. J. Pharm. Pharmaceut. Sci. 7, 379–381 (2014). [Google Scholar]
- 96.Khan T, Sankhe K, Suvarna V et al. DNA gyrase inhibitors: progress and synthesis of potent compounds as antibacterial agents. Biomed. Pharmacother. 103, 923–938 (2018). [DOI] [PubMed] [Google Scholar]
- 97.Harish K, Kushal Kumar B, Anju G. Synthetic methods and antimicrobial perspective of pyrazole derivatives: an insight. Anti-Infective Agents 18(3), 207–223 (2020). [Google Scholar]
- 98.Hu Y, Shi H, Zhou M et al. Discovery of pyrido[2,3-b]indole derivatives with Gram-negative activity targeting both DNA gyrase and topoisomerase IV. J. Med. Chem. 63(17), 9623–9649 (2020). [DOI] [PubMed] [Google Scholar]
- 99.Manchester JI, Dussault DD, Rose JA et al. Discovery of a novel azaindole class of antibacterial agents targeting the ATPase domains of DNA gyrase and topoisomerase IV. Bioorg. Med. Chem. Lett. 22(15), 5150–5156 (2012). [DOI] [PubMed] [Google Scholar]
- 100.Liu H, Chu Z-W, Xia D-G, Cao H-Q, Lv X-H. Discovery of novel multi-substituted benzo-indole pyrazole schiff base derivatives with antibacterial activity targeting DNA gyrase. Bioorg. Chem. 99, 103807 (2020). [DOI] [PubMed] [Google Scholar]
- 101.Hassan AS, Askar AA, Naglah AM et al. Discovery of new Schiff bases tethered pyrazole moiety: design, synthesis, biological evaluation, and molecular docking study as dual targeting DHFR/DNA gyrase inhibitors with immunomodulatory activity. Molecules 25(11), 2593 (2020). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 102.Liu H, Ren Z-L, Wang W et al. Novel coumarin-pyrazole carboxamide derivatives as potential topoisomerase II inhibitors: design, synthesis and antibacterial activity. Eur. J. Med. Chem. 157, 81–87 (2018). [DOI] [PubMed] [Google Scholar]
- 103.Shubhangi Kumar N, Kanagaraj R et al. Modeling molecular interactions of propounded pyrazole based drug candidates against bacterial DNA gyrase: validation by syntheses and biological studies. J. Molecul. Struct. 1195, 435–450 (2019). [Google Scholar]
- 104.Wróbel A, Arciszewska K, Maliszewski D, Drozdowska D. Trimethoprim and other nonclassical antifolates an excellent template for searching modifications of dihydrofolate reductase enzyme inhibitors. J. Antibiot. 73(1), 5–27 (2020). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 105.Zhi-Yu W, Jia-Chun L, Wen Z et al. Synthesis and antimicrobial evaluation of (Z)-5-((3-phenyl-1H-pyrazol-4-yl)methylene)-2-thioxothiazolidin-4-one derivatives. Med. Chem. 12(8), 751–759 (2016). [DOI] [PubMed] [Google Scholar]
- 106.Zhang T-Y, Zheng C-J, Wu J et al. Synthesis of novel dihydrotriazine derivatives bearing 1,3-diaryl pyrazole moieties as potential antibacterial agents. Bioorg. Med. Chem. Lett. 29(9), 1079–1084 (2019). [DOI] [PubMed] [Google Scholar]
- 107.Bhattacharjee MK. Chemistry of Antibiotics and Related Drugs. [Google Scholar]
- 108.Yang T, Chen G, Sang Z et al. Discovery of a teraryl oxazolidinone compound (S)-N-((3-(3-fluoro-4-(4-(pyridin-2-yl)-1H-pyrazol-1-yl)phenyl)-2-oxooxazolidin-5-yl)methyl)acetamide phosphate as a novel antimicrobial agent with enhanced safety profile and efficacies. J. Med. Chem. 58(16), 6389–6409 (2015). [DOI] [PubMed] [Google Scholar]
- 109.Bush K. Past and present perspectives on β-lactamases. Antimicrob. Agents Chemother. 62(10), e01076–01018 (2018). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 110.González-Bello C, Rodríguez D, Pernas M et al. β-lactamase inhibitors to restore the efficacy of antibiotics against superbugs. J. Med. Chem. 63(5), 1859–1881 (2020). [DOI] [PubMed] [Google Scholar]
- 111.Shaaban MM, Ragab HM, Akaji K et al. Design, synthesis, biological evaluation and in silico studies of certain aryl sulfonyl hydrazones conjugated with 1,3-diaryl pyrazoles as potent metallo-β-lactamase inhibitors. Bioorg. Chem. 105, 104386 (2020). [DOI] [PubMed] [Google Scholar]
- 112.Silpe JE, Bassler BL. A host-produced quorum-sensing autoinducer controls a phage lysis-lysogeny decision. Cell 176(1), 268–280.e213 (2019). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 113.Zhang Y, Zagnitko O, Rodionova I et al. The FGGY carbohydrate kinase family: insights into the evolution of functional specificities. PLoS Comput. Biol. 7(12), e1002318 (2011). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 114.Stotani S, Gatta V, Medarametla P et al. DPD-inspired discovery of novel LsrK kinase inhibitors: an opportunity to fight antimicrobial resistance. J. Med. Chem. 62(5), 2720–2737 (2019). [DOI] [PubMed] [Google Scholar]
- 115.Centers for Disease Control and Prevention. Vibrio species causing vibriosis (2021). https://www.cdc.gov/vibrio/index.html
- 116.Rajput A, Kumar M. In silico analyses of conservational, functional and phylogenetic distribution of the LuxI and LuxR homologs in Gram-positive bacteria. Sci. Rep. 7(1), 6969 (2017). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 117.Newman JD, Shah P, Chopra J et al. Thiophenesulfonamides are specific inhibitors of quorum sensing in pathogenic Vibrios. bioRxiv (2021). [Google Scholar]
- 118.Kim BS, Jang SY, Bang Y-J et al. QStatin, a selective inhibitor of quorum sensing in Vibrio species. mBio 9(1), e02262–02217 (2018). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 119.Naqvi KF, Patin D, Wheatley MS et al. Identification and partial characterization of a novel UDP-N-acetylenolpyruvoylglucosamine reductase/UDP-N-acetylmuramate: ʟ-alanine ligase fusion enzyme from Verrucomicrobium spinosum DSM 4136T. Front. Microbiol. 7(362), (2016). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 120.Mekky AEM, Sanad SMH. Novel bis(pyrazole-benzofuran) hybrids possessing piperazine linker: synthesis of potent bacterial biofilm and MurB inhibitors. Bioorg Chem 102, 104094 (2020). [DOI] [PubMed] [Google Scholar]
- 121.Guo RT, Ko TP, Chen AP et al. Crystal structures of undecaprenyl pyrophosphate synthase in complex with magnesium, isopentenyl pyrophosphate, and farnesyl thiopyrophosphate: roles of the metal ion and conserved residues in catalysis. J. Biol. Chem. 280(21), 20762–20774 (2005). [DOI] [PubMed] [Google Scholar]
- 122.Concha N, Huang J, Bai X et al. Discovery and characterization of a class of pyrazole inhibitors of bacterial undecaprenyl pyrophosphate synthase. J. Med. Chem. 59(15), 7299–7304 (2016). [DOI] [PubMed] [Google Scholar]
- 123.Shaikh SBU, Ahmed Z, Arsalan SA, Shafiq S. Prevalence and resistance pattern of Moraxella catarrhalis in community-acquired lower respiratory tract infections. Infect. Drug Resist. 8, 263–267 (2015). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 124.Czarny TL, Brown ED. A small-molecule screening platform for the discovery of inhibitors of undecaprenyl diphosphate synthase. ACS Infect. Dis. 2(7), 489–499 (2016). [DOI] [PubMed] [Google Scholar]
- 125.Steinbuch KB, Fridman M. Mechanisms of resistance to membrane-disrupting antibiotics in Gram-positive and Gram-negative bacteria. Medchemcomm 7(1), 86–102 (2016). [Google Scholar]
- 126.Ahn M, Gunasekaran P, Rajasekaran G et al. Pyrazole derived ultra-short antimicrobial peptidomimetics with potent anti-biofilm activity. Eur. J. Med. Chem. 125, 551–564 (2017). [DOI] [PubMed] [Google Scholar]
- 127.Hamblin MR Upconversion in photodynamic therapy: plumbing the depths. Dalton Trans. 47(26), 8571–8580 (2018). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 128.Rapacka-Zdończyk A, Woźniak A, Michalska K et al. Factors determining the susceptibility of bacteria to antibacterial photodynamic Inactivation. Front. Med. 8(617), (2021). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 129.Weston CE, Krämer A, Colin F et al. Toward photopharmacological antimicrobial chemotherapy using photoswitchable amidohydrolase inhibitors. ACS Infect. Dis. 3(2), 152–161 (2017). [DOI] [PubMed] [Google Scholar]
- 130.Santos I, Gamelas SRD, Vieira C et al. Pyrazole-pyridinium porphyrins and chlorins as powerful photosensitizers for photoinactivation of planktonic and biofilm forms of E. coli. Dyes Pigments 193, 109557 (2021). [Google Scholar]
- 131.Shatalin K, Nuthanakanti A, Kaushik A et al. Inhibitors of bacterial H2S biogenesis targeting antibiotic resistance and tolerance. Science 372(6547), 1169–1175 (2021). [DOI] [PMC free article] [PubMed] [Google Scholar]; •• Among the most important pyrazole compounds as potent antibacterial agents.
- 132.Luhachack L, Nudler E. Bacterial gasotransmitters: an innate defense against antibiotics. Curr. Opinion Microbiol. 21, 13–17 (2014). [DOI] [PubMed] [Google Scholar]
- 133.Majed H, Johnston T, Kelso C et al. Structure–activity relationships of pyrazole-4-carbodithioates as antibacterials against methicillin-resistant Staphylococcus aureus. Bioorg. Med. Chem. Lett. 28(22), 3526–3528 (2018). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 134.Johnston T, Van Tyne D, Chen RF et al. Propyl-5-hydroxy-3-methyl-1-phenyl-1H-pyrazole-4-carbodithioate (HMPC): a new bacteriostatic agent against methicillin-resistant Staphylococcus aureus. Sci. Rep. 8(1), 7062 (2018). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 135.Chen PR, Bae T, Williams WA et al. An oxidation-sensing mechanism is used by the global regulator MgrA in Staphylococcus aureus. Nat. Chem. Bio. 2(11), 591–595 (2006). [DOI] [PubMed] [Google Scholar]
- 136.Hammad A, Abutaleb NS, Elsebaei MM et al. From phenylthiazoles to phenylpyrazoles: broadening the antibacterial spectrum toward carbapenem-resistant bacteria. J. Med. Chem. 62(17), 7998–8010 (2019). [DOI] [PubMed] [Google Scholar]
- 137.Wang J-X, Zhu Z-R, Bai F-Y et al. Molecular design and the optimum synthetic route of the compounds with multi-pyrazole and its derivatives and the potential application in antibacterial agents. Polyhedron 99, 59–70 (2015). [Google Scholar]