Abstract
The exfoliative (epidermolytic) toxins of Staphylococcus aureus are the causative agents of the staphylococcal scalded-skin syndrome (SSSS), a blistering skin disorder that predominantly affects children. Clinical features of SSSS vary along a spectrum, ranging from a few localized blisters to generalized exfoliation covering almost the entire body. The toxins act specifically at the zona granulosa of the epidermis to produce the characteristic exfoliation, although the mechanism by which this is achieved is still poorly understood. Despite the availability of antibiotics, SSSS carries a significant mortality rate, particularly among neonates with secondary complications of epidermal loss and among adults with underlying diseases. The aim of this article is to provide a comprehensive review of the literature spanning more than a century and to cover all aspects of the disease. The epidemiology, clinical features, potential complications, risk factors, susceptibility, diagnosis, differential diagnoses, investigations currently available, treatment options, and preventive measures are all discussed in detail. Recent crystallographic data on the toxins has provided us with a clearer and more defined approach to studying the disease. Understanding their mode of action has important implications in future treatment and prevention of SSSS and other diseases, and knowledge of their specific site of action may provide a useful tool for physiologists, dermatologists, and pharmacologists.
Staphylococcal scalded skin syndrome (SSSS) is the term used for a collection of blistering skin diseases induced by the exfoliative (epidermolytic) toxins (ETs) of Staphylococcus aureus. It primarily affects neonates and young children (Fig. 1), although adults with underlying diseases are also susceptible (Fig. 2). The severity of SSSS varies from a few blisters in localized SSSS to severe exfoliation affecting almost the whole body surface in generalized SSSS. Because of the relative rarity of the disease and ease of treatment, SSSS has not received as much attention as it deserves by either clinicians or researchers. Staphylococcal infections are on the increase in all age groups worldwide, and show an increasing resistance to conventional antibiotics; despite the availability of a wide range of antibiotics, these infections still carry a significant morbidity and mortality, particularly among adults. Furthermore, although the condition was described over a century ago, our understanding of it began only 25 years ago, when the toxins were first discovered. Even now, their mechanism of action is still not certain. However, recent exciting data from computer modelling and three-dimensional crystallography of the toxins has provided us with a clearer and more defined approach to understanding the pathologic processes of the disease. Elucidating the mechanism of action of the toxins holds exciting prospects for understanding the normal physiology of the skin, targeting drugs to very specific regions of the skin, and developing antitoxins and toxoids that may soon play a vital role in the treatment and prevention of SSSS. The aim of this review is to provide both the clinician and the researcher with our current understanding—from sources worldwide and spanning more than a century—of the epidemiology, clinical features, diagnosis, differential diagnoses, investigations, treatment and prevention of the disease. The review also discusses the organisms responsible for SSSS, probable mechanisms of action of the ETs, and their potential role in diseases other than SSSS. It is hoped that the information provided will (i) help the clinician to make a correct diagnosis of SSSS by using the available routine investigations and to manage the disease appropriately, (ii) discourage repetitive research by bringing researchers up to date with the current knowledge of the disease, and (iii) encourage collaboration between various research groups working on SSSS around the world.
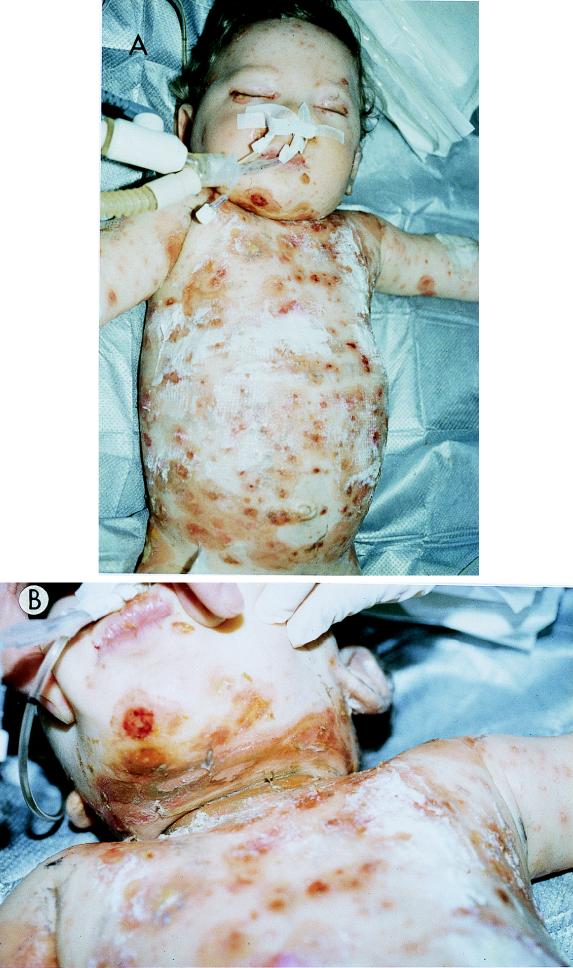
FIG. 1.
Generalized SSSS in a previously well neonate. The neonate (A and B) shows the characteristic well-demarcated erythematous superficial exfoliation with areas of skin sparing. Courtesy of J. Etienne, Faculte de Medecine RTH Laennec, Laboratoire de Bacteriologie, Centre National de Reference Des Toxemies a Staphylocoques, France.
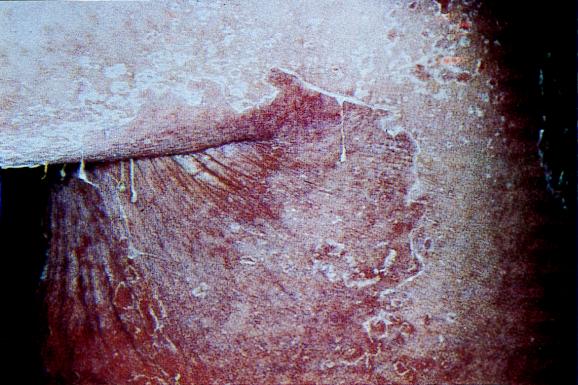
FIG. 2.
Generalized SSSS in a 77-year-old adult with renal impairment. The photograph shows extensive exfoliation of the trunk and arm. Although the condition is rare in adults, the observed lesions are similar to those in neonates. Reprinted from reference 103 with permission of the publisher.
HISTORICAL PERSPECTIVES
The clinical features of SSSS were first described over a century ago by a German physician, Baron Gotfried Ritter von Rittershain, who observed 297 such cases among young children in a 10-year period while working in a Czechoslovakian foundling asylum; he called it dermatitis exfoliativa neonatorum but was unable to determine its cause (275). The term “Ritter’s disease,” named after its describer, is still used when generalized SSSS occurs in neonates. “Pemphigus neonatorum” describes a milder, self-limiting disease of infants that produces a few blisters (162). S. aureus was first isolated in 1891 from a patient with pemphigus neonatorum by Almquist (7), who called the organism Micrococcus pemphigi neonatorum, and then by Winternitz in 1898 (281).
The disease received little attention until 1956, when Lyell described a skin eruption that resembled scalded skin and called it toxic epidermal necrolysis (TEN) (159). He speculated that the condition might be due to a circulating toxin that damages the skin and causes necrosis, although he was not aware that he was describing two distinct, unrelated conditions. In 1967, however, Lyell reviewed 128 cases that fitted his diagnostic criteria of TEN and found that almost one-quarter—all children under 10 years of age—presented predominantly with a staphylococcal infection (160). A link between staphylococcal infection in children and exfoliation was also observed by Lowney et al. (158).
It soon became apparent that the exfoliation associated with S. aureus infection occurred specifically within the midepidermis whereas exfoliation that was not associated with staphylococcal infection occurred at the dermoepidermal junction (158, 161). As a result, the former condition was termed SSSS and the latter was termed TEN. The etiology and pathogenesis of SSSS are very different from those of TEN, and although they may be clinically difficult to distinguish, correct diagnosis is essential because the treatment for the two conditions is different (236).
By this time, S. aureus had also been implicated in bullous impetigo (60, 200), staphylococcal scarlantiniform rash (74, 253), and outbreaks of Ritter’s disease (30, 200, 221). While a circulating staphylococcal exotoxin was widely accepted to cause SSSS (158, 229), it was not until the elegant demonstration by Melish and Glasgow that injection of newborn mice with group II staphylococci isolated from patients with localized SSSS such as bullous impetigo or from patients with generalized SSSS resulted in exfoliation that SSSS was appropriately termed (170).
CLINICAL FEATURES
SSSS is primarily a disease of neonates and children (48, 96, 174), and white children may be more prone to SSSS than black children, according to a study of 75 cases published more than 2 decades ago (211). The clinical features of SSSS vary from localized blisters to severe exfoliation affecting over 90% of the entire body surface. In localized forms of SSSS, also known as bullous impetigo, tropical bullous impetigo, measles pemphigoid (162), and bullous varicella (174), characteristic fragile, thin-roofed, flaccid bullae are formed, which rupture easily to release fluid that varies from a thin, cloudy, amber liquid to purulent, opaque, white or yellow pus (174). This is the mildest form of the disease, in which the surrounding skin remains normal and there are no systemic symptoms or signs (100). In neonates, the lesions are found mostly on the perineum, periumbilical area, or both, while in older children, the lesions are found most often on the extremities (174).
In the generalized forms of SSSS, widespread involvement of the entire skin surface can occur but the mucous membranes are usually spared (172, 208). The disease usually follows a localized infection of the upper respiratory tract, inner ear, conjunctiva, or umbilical stump (24), although rare cases of SSSS caused by staphylococci isolated from patients with pneumonia, septic arthritis (174), pyomyositis (284), and maternal breast abscesses (213) have been reported. In adults, in whom isolated cases tend to occur, bacteria are reported to enter via catheterization, abscesses, septic arthritis, infection of arteriovenous shunts, and parenteral infections, although in many cases, no primary localized staphylococcal infection is found (48). In neonates, the age of onset is usually between 3 and 16 days, and only one case of SSSS has been reported to occur within 24 h of birth, where a term infant, delivered by emergency cesarian section to a 20-year-old woman who presented with clinical signs of septicemia, developed SSSS. S. aureus was isolated from the baby’s blood and urine (157). Patients with generalized SSSS initially develop fever, malaise, and lethargy, with poor feeding and irritability, followed by a generalized, tender erythematous rash, which generally begins on the head and neck and spreads to the rest of the body within a few days (110, 114, 193). The rash is more marked in flexural creases. Soon thereafter, large, fragile, thinroofed blisters appear, which rapidly rupture on the slightest pressure (with a positive Nikolsky sign), resulting in large sheets of epidermis sloughing off to leave extensive areas of raw, denuded, varnish-like skin. These patients have poor temperature control, can lose extensive amounts of fluids, and may develop secondary infection; such secondary complications of epidermal loss significantly contribute to mortality in neonates and young children (110, 135). They may also develop primary staphylococcal or secondary sepsis and may present with hypotension, tachycardia, neutropenia, and/or respiratory distress (78, 157).
Staphylococcal scarlet fever, also called scarlatiniform erythroderma/rash, was first described in the 1920s and until recently was generally considered to be a milder or abortive form of SSSS (24, 172, 174, 266), although very little literature exists to confirm this association. Patients usually develop a generalized tender erythroderma with a roughened, sandpaper-like texture, associated with fever. This is followed by thick flakes developing within a few days and the entire skin desquamating over the next week (174). Unlike generalized SSSS, the scarlatiniform eruption is not associated with the formation of bullae or exfoliation and is very difficult to differentiate from other infectious erythrodermal causes such as toxic shock syndrome (TSS) and streptococcal scarlet fever. TSS is a multisystemic disease, caused by staphylococcal toxic shock syndrome toxin 1 (TSST-1) and other superantigens, that may rapidly progress to death (242). It occurs mainly in menstruating women and is associated with tampon use, although it has been found in nonmenstruating women, postmenopausal women, children, and men (79, 90). Recently, Lina et al. (154) analyzed 60 strains of S. aureus isolated from children with suspected SSSS. They demonstrated that although the strains isolated from patients with localized or generalized exfoliation produced the ETs and caused a positive Nikolsky sign when injected into neonatal mice, only 1 of the 17 strains (6%) isolated from patients with staphylococcal scarlet fever produced ET and the other 16 produced TSST-1, enterotoxins, or both. Therefore, it is likely that most cases of staphylococcal scarlet fever are a clinical manifestation of mild TSS, with the enterotoxins (40) occasionally mimicking this condition. The few cases of staphylococcal scarlet fever caused by the ETs that Lina et al. (154) have reported may be a mild form of SSSS previously described by Melish et al. (172), where a transient positive Nikolsky sign can be demonstrated in most patients. It should also be noted that TSS occasionally coexists with SSSS (42, 269).
STAPHYLOCOCCUS AUREUS
S. aureus remains one of the most common causes of community- and hospital-acquired localized and systemic infections; indeed, large epidemiological studies, including the National Nosocomial Infections Surveillance in the United States, consistently show that the incidence of S. aureus infections has been increasing steadily over the past few decades in all age groups (20, 206), including neonates (122). Despite the recent explosion in antibiotic development, S. aureus infections still carry a significantly high morbidity and mortality in children and adults (122, 141, 169). Isolates of S. aureus are able to produce a range of more than 30 different extracellular proteins, most of which play a direct role in pathogenicity or enhance virulence (112). S. aureus is probably the most frequently implicated organism in bacterial illness, although the mechanisms by which infection is established is not always known (5).
Carriers of S. aureus
Nasal carriage of S. aureus occurs in 35% of the normal population (54, 191) but varies according to age (19) and race (176). Carriage rates of S. aureus are higher in certain subpopulations such as patients suffering from atopic dermatitis (8, 152), contact dermatitis (190), psoriasis (164), and cutaneous T-cell lymphoma (115). These carriers can be responsible for SSSS outbreaks (108). In neonates, organisms are also common on the skin, eyes, perineum (51), wound sites (194), circumcision wounds (11), and umbilical stumps (282). The hospital remains one of the common sources of S. aureus, mainly due to inadequate adherence to infection control practices, such as barrier nursing care, hand-washing, and cleaning stethoscopes between patients (123, 155). About 30% of neonates are colonized by S. aureus strains within a week of birth (51, 183, 241), although some studies have shown carriage rates of 60 to 90% of newborn babies discharged from hospitals, particularly in areas where the use of antiseptic umbilical cord care is discouraged and during staphylococcal epidemics (105, 239, 240). In hospitals, neonatal colonization is more likely to originate from nursery attendants than mothers (51, 183, 241), who, in turn, are more likely to be responsible for outbreaks.
A study published more than two decades ago showed that ventilation shafts and other inanimate objects such as stethoscope bells, ophthalmoscope and otoscope handles, laundry carts, and nursery magazines in hospitals may also harbour ET-producing strains and were responsible for prolonged epidemics of staphylococcal infections in neonatal nurseries (123). More recently, a Nigerian study revealed that healthy animals may also be carriers of ET-producing S. aureus strains, with 3.9% of animal S. aureus strains producing ETs (4). However, their importance as reservoirs of SSSS is not known.
Outbreaks of SSSS
Occasional isolated cases of SSSS can occur after organisms colonize the infant from the maternal vaginal flora acquired during delivery (157). There has been one recent reported case of a breast-fed infant developing SSSS from S. aureus that possibly originated from the mother’s breast abscess (213). However, more commonly, as a consequence of cross-infection, SSSS infections tend to occur in outbreak clusters (29, 51, 52). During the 1980s, several papers were published showing that the use of antiseptics such as chlorhexidine on neonatal umbilical stumps delayed the time to cord separation (26, 142, 227, 272). Because of the resulting increase in community midwives’ workload due to the delay in cord separation, as well as rare isolated cases of hexachlorophane toxicity causing spongiform myelinopathy after percutaneous absorption (10, 237), hospitals have gradually stopped using antiseptic umbilical cord care. This move has occurred despite previous studies demonstrating that the use of antiseptics for cord care is essential for preventing staphylococcal infection and cross-infection in hospitals (47, 244).
As a consequence of stopping antiseptic cord care, many clinicians have observed an increase in the rate of staphylococcal colonization and infections among neonates (6, 251, 276). Furthermore, several studies have demonstrated that reimplementation of antiseptic cord care resulted in a decrease in neonatal staphylococcal colonization and infections (168, 239, 240, 246). Therefore, many clinicians are very concerned that if hospitals do not start reimplementing the antiseptic cord care policy, the number of neonatal staphylococcal infections is likely to increase.
Over the decades, many outbreaks have been reported, often with dozens of babies affected over several months and usually due to several asymptomatic carriers of ET-producing S. aureus strains (49, 51, 52, 55, 95). Nursery-associated outbreaks usually manifest themselves during the first month, after the baby has been discharged from hospital (29, 174). Along with the recent pressure to increase day surgery and encourage early discharges from hospitals, outbreaks such as those associated with SSSS are being dissipated into the community, where infections are often treated by the primary health care team—an observation made almost two decades ago (174). As a result, the hospital carrier of the offending organism, usually nursery attendants in close contact with the infants, such as midwives and pediatricians (29, 51, 52, 55, 95), will continue to spread the infection over months until the outbreak is acknowledged and the carrier is removed from work. Recently, for example, four babies aged 6 to 8 days were found to have blistering rashes characteristic of SSSS during a 6-week period. An inquiry and retrospective case finding revealed that 36 babies had been infected with an ET-producing strain of S. aureus. Swabs from suspect health care personnel revealed the source, a pediatrician who was suspended from duty until shown to be free of staphylococcal colonization (29).
Exfoliative Toxin-Producing S. aureus Strains
Most S. aureus strains giving rise to SSSS in Europe and the United States belong to phage group II, particularly types 71 and 55/71 (50, 170, 199), which account for 7 to 25% of human S. aureus strains isolated in the United Kingdom (54). In Japan, most strains causing SSSS belong to phage groups other than II (132, 186, 230). The only study published on the ETs in Africa revealed that almost 60% of toxin-producing staphylococcal strains were nontypeable, with phage group II strains accounting for only 18% (4). It should be noted that only 31 to 40% of phage group II S. aureus strains produce ETs (85, 128, 279).
Unfortunately, due to the lack of a suitable and standardized in vitro assay, there is very little literature available on the epidemiology of ET-producing S. aureus strains. One prospective study of 944 S. aureus isolates from 577 dermatological patients of all age groups revealed a 5.1% carriage rate of ET-producing strains, although only 32% of these patients presented with any symptoms of SSSS, indicating that a large proportion were simply asymptomatic carriers (71). Another epidemiological survey revealed that 164 (33%) of 500 pregnant women attending an antenatal clinic carried S. aureus in the nose, axilla, and/or perineum. Of the 184 S. aureus strains isolated, 5 produced ET as determined by the neonatal-mouse assay (54). Other studies reported toxin production in 6% of 2,632 S. aureus strains isolated from hospitalized patients of all ages (203), 4.4% of 194 strains isolated from human diarrhea and wounds (4), 3.9% of 666 strains isolated from apparently healthy animals (4), 19% of 100 S. aureus strains isolated from the mouths of children with dental disease (179), and 40% of S. aureus isolated from superficial skin infections (17).
EXFOLIATIVE TOXINS
Although the presence of a soluble toxin in the pathogenesis of SSSS was the subject of speculation as far back as the 1950s (159), the first clue of its existence came only after Melish and Glasgow showed that sterile fluid obtained from intact bullae and from phage group II S. aureus culture medium was able to produce a positive Nikolsky sign in neonatal mice (170). Even intraperitoneal inoculation resulted in exfoliation, indicating that the toxin had its specific target in the skin. Within a year, the active component in the supernatant was isolated, purified, characterized, termed exfoliatin (125), and confirmed by others (13, 171). Soon thereafter, it was realized that at least two different ET serotypes existed (131). The second serotype was isolated and characterized as a heat-labile toxin that has a similar molecular weight to the original toxin. This second ET serotype was able to elicit a positive Nikolsky sign in the neonatal-mouse bioassay; the discoverers termed this toxin ETB (132).
Serotypes
At present, at least two serologically distinct toxins, ETA and ETB, can cause disease in humans and are produced by a small but significant proportion of S. aureus strains (205). Although they differ in some physicochemical properties, both toxins produce the dermatological effects described above in neonatal mice. The toxins are species specific, affecting humans, monkeys, mice, and hamsters but not rats, rabbits, dogs, hedgehogs, voles, guinea pigs, chickens, or frogs (24, 68, 125). Both toxins have been identified and fully sequenced; they have 40% sequence homology (21, 145, 196). ETA and ETB consist of 242 and 246 amino acids, respectively, with molecular masses of 26,950 and 27,274 kDa (145). The gene for ETA is chromosomal, while that for ETB is plasmid located (91); both genes have been fully sequenced and cloned into plasmid vectors for protein expression in Escherichia coli (116, 224). ETA is heat stable and retains its exfoliative activity even after being heated at 60°C for 30 min (112, 125). Initial work with EDTA, a metal ion chelator, suggested that ETA but not ETB required a metal ion for activity (113). However, this is now uncertain. Comparison of the primary amino acid sequences revealed that amino acid residues 93 to 107 of ETA were significantly homologous to a region of rat intestinal and placental calcium-binding protein and supported the evidence that ETA but not ETB may require calcium for activity (53). On the other hand, calcium-binding motifs have not been identified in the two recently determined crystallographic structures of ETA (44, 270) (see below).
The prevalence of different ET serotypes shows a significant geographical distribution. In the United Kingdom and Ireland, 32% of 116 S. aureus isolates from patients with suspected SSSS produced ETA, 27% produced both ETA and ETB, and 12% produced ETB only, with neither toxin being detected in 34 strains (56). A large epidemiological study of dermatological patients in Germany reported that 98% of the ET-producing strains produced ETA (71). A French study found that most cases of childhood SSSS were associated with ETA alone (89%) and that only a few were due to both ETA and ETB (8%) or ETB alone (4%) (203, 279). Similarly, a Nigerian study reported that ETA accounted for 91.1% of the 34 toxin-producing strains isolated from a range of human and animal sources (4). In the United States, 40% of S. aureus isolates from SSSS patients produced ETA, 36% produced both, and 16% produced ETB only (174). On the other hand, ETB seems to be the predominant toxin in Japan, with two studies reporting ETB, ETA and ETB, and ETA production rates of 52, 23, and 25% of 61 strains (186) and 35, 37, and 21% of 43 strains isolated from patients with SSSS (133), respectively.
Two other serologically different staphylococcal ETs that affect animals have also been reported (232, 233, 263). Staphylococcus hyicus is known to cause exudative epidermitis in piglets younger than 1 month; the infection is characterized by exudation, exfoliation, and vesicle formation with skin erosion (121, 144). The responsible toxin, termed S. hyicus exfoliative toxin (ShET), has been isolated and purified from culture supernatants of S. hyicus recently (232). This toxin, a monomer with a molecular mass of 27 kDa, does not cross-react with ETA or ETB in immunodiffusion tests and causes exfoliation in piglets less than 1 month old and in 1-day-old chickens but not in older pigs, 15-day-old chickens, mice, or guinea pigs (263). While still in its infancy, work on ShET has already shed light on possible receptors for the ETs (see below) and may provide a more acceptable model for studying the mechanism of action of the toxins. A serologically different ET isolated and purified from a horse strain of S. aureus was termed ETC by the authors on the basis of its similarities to the human ETs (233). ETC is a heat-labile, 27,000-kDa toxin that can produce intraepidermal splitting in both neonatal mice and chickens. However, the role of these toxins in human SSSS is still to be determined.
Site of Action
Most of the work on ETs has been done with ETA and the neonatal-mouse model. The toxins are made during the postexponential phase of bacterial growth (24) and excreted from colonizing staphylococci before being absorbed into the systemic circulation (173). The toxins reach the zona granulosa of the epidermis by diffusing through dermal capillaries. Work with 125I-labelled ET injected into neonatal mice has shown that apart from the skin, there is no significant binding of the toxins to any other body organ (94).
Histological studies have shown that addition of ETs to confluent keratinocyte cultures isolated from pieces of human skin obtained from plastic surgery or mouse organotypic skin cultures results in the disappearance of small vesicles that are usually present between the cells (153, 166); this is followed by formation of intercellular fluid-filled gaps in the granulosa-spinosum interface, which eventually leads to the characteristic midepidermal splitting seen in SSSS (Fig. 3). Furthermore, the biological actions of ETA and ETB are histologically indistinguishable (97). Cytolysis or necrolysis does not occur; an inflammatory response or cell degeneration has not been observed in the neighboring region (58, 68, 109, 285). Although S. aureus is sometimes seen in localized forms of SSSS, it is only rarely seen in generalized SSSS (27).
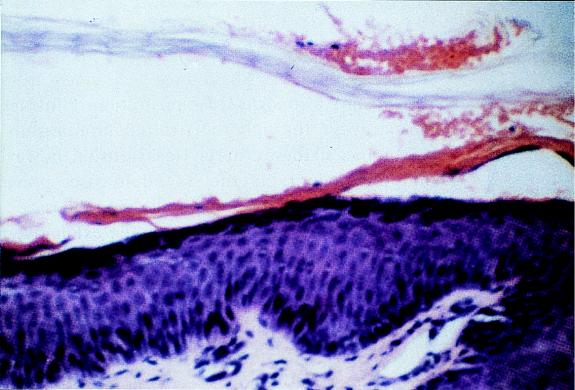
FIG. 3.
Histological characteristics of skin splitting of patients with SSSS. A photomicrograph of a skin biopsy specimen from the adult depicted in Fig. 2 shows epidermal splitting at the granular layer of the epidermis. Hematoxylin and eosin stain; magnification, ×200. Reprinted from reference 103 with permission of the publisher.
Recently, immunoperoxidase staining on histological specimens by Gentilhomme et al. (97) revealed that cells that bordered both the upper and lower edges of the cleavage site caused by the ET were positive for keratohyalin granules. Electron microscopy of ferritin-labelled toxin and light microscopy of fluorescein thiocarbamyl toxin were previously used to show that the toxin bound intensely to keratohyalin granules in the stratum granulosum of the epidermis and also that ETB had a particularly high affinity toward profilaggrin (360 kDa) and filaggrin (30 kDa) in epidermal extracts (247, 248). However, there is no evidence suggesting that the toxins have an intracellular mechanism of action. Profilaggrin is synthesized during the pathway of keratinocyte development and incorporated into keratohyalin granules, where it is hydrolyzed to intermediate filaggrins and then to amino acids in the stratum corneum (24). The authors speculated that ET binding to these intracellular granular proteins may lead to disruption of intercellular cohesion because the keratohyalin granules are linked to the keratin intermediate filaments, which extend to desmosomes at the cell surface. These observations fitted well with previous electron micrographic observations suggesting that desmosomes, which link adjacent cells, were disrupted (172). However, several histological studies have demonstrated that even though intercellular edema can be demonstrated, the desmosomes are initially intact and desmosomal disruption is a secondary event, occurring only as the edema progresses (70, 109, 153). Along with the above observation, some authors (62, 285) also noted that adding the toxin to mouse epidermis resulted in the development of intracytoplasmic clearing along the cell membrane at the granulosa-spinosum interface, followed by disappearance of the cell membranes. It was speculated that the toxins may act by weakening cell membranes and making them more fragile and that the desmosome disruption was a secondary event (62). Therefore, the role of the desmosome in the epidermolysis seen in SSSS is still unclear, and it is not likely to be the primary site of action of the toxins.
A more promising primary site of toxin action may be the putative receptor first identified in mice (223). Water-insoluble sodium dodecyl sulfate fractions of neonatal and adult mouse epidermal extracts containing various gangliosides were incubated with ETA for 3 h and then subcutaneously injected into neonatal mice. ETA incubated with neonatal (but not adult) mouse epidermis fractions was unable to induce exfoliation, indicating that an ET-inhibitory substance may be present in neonatal epidermis (223). This ability was not lost after trypsin or pronase digestion, heating at 100°C for 30 min, or neuraminidase treatment, thereby suggesting that the receptor was not a protein but possibly an amino sugar. The presence of the receptor in neonatal but not adult epidermis may explain the increased susceptibility of neonates to SSSS, and the authors speculated that the identified receptor may be masked or changed as the neonate evolves into an adult (223).
More recently, ETA and ETB were shown to bind GM4-like glycolipid extracted from the skin of suckling mice (264). Furthermore, ShET from S. hyicus bound to skin extracted from 1-day-old chickens but not to skin extracted from adult chickens. Exfoliation in the respective neonatal animal models was abolished when the ETs and ShET were preincubated with GM4-like, but not GM1-, GM2-, or GM3-like, glycolipids from the skin of 1-day-old chickens and suckling mice, respectively. Their results strongly suggest that the GM4-like glycolipids that are present in susceptible neonatal but not adult or nonsusceptible animals are possible receptors for the toxins and would account for the age and species specificity of the toxins. Furthermore, the majority of adults who develop SSSS have an underlying disease which may alter the normal physiology of adult skin, making it susceptible to exfoliative toxins (48). Inflammation, for example, will induce the expression of major histocompatibility complex (MHC) class II molecules on the surface of keratinocytes (245). However, despite these recent successes, three decades of intensive research have been unable to elucidate the exact mechanisms by which the toxins induce exfoliation.
Putative Mechanism of Action
Initial attempts to determine the mechanisms involved in exfoliation were made by studying the enzyme pattern of S. aureus isolated from clinical cases of localized and extensive SSSS. It was initially suggested that the potent dermonecrotic effect of delta-hemolysin may be responsible, but this theory was soon rejected after the discovery of the exfoliative toxins (96). Histological studies on the epidermal effects of ETs led to speculations that the initial appearance followed by the disappearance of intercellular vesicles may be due to the release of proteolytic lysosomal enzymes by nearby cells (153). Lysosome-like lamellar bodies inside adjacent cells are known to release lipids and enzymes into the surrounding extracellular space (92, 102); proteases will produce a similar nonspecific disruption of the extracellular matrix to some extent (24, 252). Subsequent histochemical studies did not identify any difference between enzyme reactions in ET-injected, saline-injected, and uninjected specimens of mouse epidermis (62). Native ET was also shown to have no intrinsic arylsulfatase, β-galactosidase, or cathepsin activity (these enzymes are normally present in lysosomes) (278). The toxins did not have any enzymatic activity toward known nonspecific substrates, including casein (134, 218), and using sensitivity plates (15, 21), and their activity was unaffected by any of a wide range of metabolic inhibitors tested (24, 249). Even attempts at crystallizing either ETA (289) or ETB (180) to determine their three-dimensional structure, which might shed light on their mechanism of action, met with little success because of difficulty in obtaining stable crystals.
Takiuchi et al. (262) were the first group to provide evidence that ETs may act as proteases. Epidermis from 1- to 3-day-old mice (jla-ddY) was incubated with 200 μg of partially purified ET, ET boiled for 40 min at 100°C, epidermis alone, or ET alone as negative controls. The mixtures were incubated at 37°C for 12 h, and the supernatant was then tested for caseinolytic activity after removing the epidermis by centrifuging at 1,000 × g for 15 min. Incubation of ET with epidermis induced a fourfold increase in caseinolytic activity compared to the three other negative controls; this activity was inhibited by α2-macroglobulin, indicating the activation of proteases or inhibition of protease inhibitors in the epidermis. Contamination with other proteases is unlikely, because ETA or epidermis alone did not demonstrate any caseinolytic activity. However, the authors did not identify the protease in the supernatant or provide any evidence supporting that ETA was the protease induced.
A major step forward followed after both ETs were shown to have significant primary amino acid sequence homology (25%) to the staphylococcal V8 protease, particularly in the region containing the protease active site (53). V8 protease is a member of the trypsin-like serine protease family; it has an unusual specificity for cleaving the carboxyl-terminal side of acidic amino acid residues and does not possess any disulfide bridges (64). The active site in question consists of a catalytic triad of serine, histidine, and aspartic acid and forms the active site of all known eukaryotic trypsin-like serine proteases. The high degree of sequence conservation around these regions was speculated to preserve the active site of possible protease activity of the toxins. It was also shown that phenylmethylsulfonyl fluoride (PMSF), a specific serine protease inhibitor, was able to delay exfoliation by the ETA in neonatal mice; however, inhibition was not achieved because higher doses of PMSF were lethal to the animals (53).
At the same time, Bailey and Smith (22) observed that radiolabelled diisopropyl phosphorofluoridate (i2Pr2P-F), which has the ability to covalently modify the active site of known serine proteases such as V8 protease and chymotrypsin, was also able to label the Ser-195 of the putative active site of ETA, supporting the theory proposed by Dancer et al. that the ETs may act as serine proteases. However, it should be noted that i2Pr2P-F could label only 6% of the total ETA (22), suggesting that the active site of the toxin was not easily accessible to i2Pr2P-F.
Soon thereafter, several papers appeared which further supported this hypothesis. Two groups independently reported that site-directed replacement of the Ser-195 active site of the catalytic triad of ETA resulted in a complete loss of biological activity when excess amounts were injected into newborn mice (210, 214).
Redpath et al. used oligonucleotide site-directed mutagenesis to replace the active-site Ser-195 of ETA with glycine and showed that injection of 1 and 10 μg of wild-type ETA into 3-day-old Sha-Sha mice gave a positive Nikolsky sign within 2.5 h whereas the mutated ETA had no effect at doses up to 100 μg, indicating that Ser-195 is essential for the biological activity of ETA (214). Prevost et al. (210) also performed a similar experiment, replacing the Ser-195 with cysteine, and showed that a positive Nikolsky sign was obtained in neonatal mice with as little as 0.1 μg of wild-type ETA but not with up to 300 μg of mutated ETA.
Research on the ETs was hampered by the lack of a suitable model. Neonatal and hairless mice still remain the “gold standard” for demonstrating the toxins as causes of exfoliation. However, this model, as well as isolated epidermis from various sources, does not allow for accurate, quantitative, and reproducible studies of toxin activity (69). A search for an in vitro model for the toxins soon began in an attempt to develop a rapid method to routinely identify the toxins and possibly aid in elucidating their mechanism of action. Bailey and Redpath (23) tried to determine whether the ETs might have an esterolytic activity on the basis that proteases are always esterases and serine proteases tend to have a higher catalytic efficiency toward esters (76). They demonstrated that both ETA and ETB have very low but significant N-t-butoxycarbonyl l-glutamic acid α-phenyl (boc-l-Glu-O-phenyl) esterase activity, which was not present in a mutant form of ETA where the Ser-195 of the putative catalytic site was replaced by glycine (23). Furthermore, the pH dependence of both ETA and ETB in this reaction was typical of the histidine-associated pKa found with all serine proteases. On the other hand, Jaulhac et al. (118) modified the original neonatal-mouse model (262) and demonstrated an induction in proteolytic activity by using a supernatant of the epidermis. An epidermal extract was prepared by grinding neonatal-mouse epidermis in liquid nitrogen, suspending it in buffer, and subjecting it to high-speed centrifugation. The supernatant was then incubated with ETA or ETB and different protein substrates, and the appearance of soluble peptides was estimated by the Lowry titration assay. Significant proteolytic activity in the supernatant with ETA and ETB (but not with biologically inactive toxin mutants) was induced after 20 h (118).
Many authors have argued that the proteolytic activity observed by both Takiuchi et al. (262) and Jaulhac et al. (118) was due to initial protease contamination of the toxin preparation (24, 44). However, this is very unlikely, because a threefold induction in supernatant proteolytic activity was obtained compared to the native toxin preparation or epidermis alone, and this activity was no different from the negative controls. Jaulhac et al. (118) also showed that mutants of ETA had no proteolytic activity. This provides strong evidence that ETA is the serine protease in the supernatant, although it is still possible that altering the active site of the toxins also resulted in destruction of their ability to release or activate serine protease(s) from epidermal cells. Furthermore, the authors demonstrated proteolytic activity against whole bovine casein, β-casein, and α-casein but not κ-casein or bovine serum albumin (118). Preliminary purification of one of the digested peptides by high-performance liquid chromatography also revealed that ETA might cleave a peptide bond between glutamic acid and valine, although the authors did not search for other cleaved peptides (118). Their results suggested that the toxin might cleave the peptide bond between a glutamic acid and a hydrophobic amino acid; this has been supported by recent structural findings (see below).
Probably the strongest evidence supporting the toxins as serine proteases comes from some recent spectacular studies on computer models and crystallographic data. By using the structures of known glutamate-specific trypsin-like serine proteases to model the three-dimensional structure of ETA and other serine proteases, a 15 to 20% sequence homology to other known glutamate-specific serine proteases (including V8 protease), particularly in the region of the putative catalytic triad, was demonstrated (25). The computer model predicted that ETA consisted of two domains, S1 and S2, each made up of six antiparallel β-strands that form a β-barrel common to all members of the trypsin family, although the toxin had a larger N-terminal portion than the other members. The model also demonstrated that Thr-190 and His-213 (chymotrypsin numbering), which form the core elements of the S1 pocket, were unconditionally conserved in all serine proteases that are glutamate specific as well as in the ETs. However, because both toxins differed from the others in that they had a Lys-216 protruding into the S1 pocket, it was thought that its positive charge would help stabilize binding to a negatively charged substrate, such as glutamic acid.
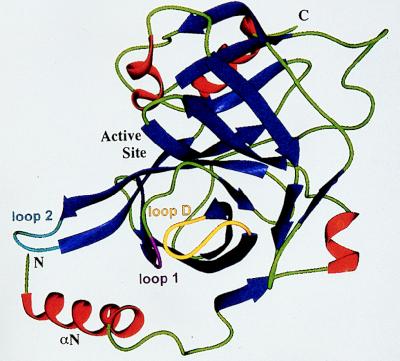
Crystallographic data from two independent studies confirmed all these speculations and provided even more insight into the structure and function of the toxins. Vath et al. (270) reported that both toxins were unique in that they possessed a highly charged N-terminal α-helix (αN helix) containing four positive and four negative charges (Fig. 4). This αN helix interacts with loop 2, which forms part of the S1 pocket, in such a way as to partially block the pocket entrance. Furthermore, the Pro-192–Gly-193 peptide bond is flipped 180° compared to that in the other glutamate-specific proteases. This allows the glycine to form a hydrogen bond with Asp-164 of loop D of the S2 pocket and the proline to form a hydrogen bond with the Ser-195 of the active site, thereby preventing any activity by the toxin. The authors proposed that binding of the highly charged αN helix to a specific epidermal receptor results in a conformational change which opens the S1 pocket and flips the proline-glycine peptide bond by 180° to allow the proline to form a hydrogen bond with Asp-164 instead of the glycine. This conformational change then allows the active site to cleave the carboxyl-terminal side of glutamic acid.
FIG. 4.
Ribbon diagram of the structural element of ETA. Helices are in red; β strands are in blue; loops 1, 2, and D are in magenta, cyan, and yellow, respectively. Reprinted from reference 270 with permission of the publisher.
Cavarelli et al. (44) confirmed most aspects of the above model with their own crystallographic data (44). Their free-energy simulations showed that the proline-glycine flip requires very little energy, less than 3 kCal per mol, and that there were no steric hindrances to prevent the proline-glycine flip from occurring. The possibility of binding to a specific molecule that induces a conformational change leading to toxin activation had been proposed before (112); this event is not novel, since thrombin (98, 99) and the hepatitis C virus NS3 protease also demonstrate such a phenomenon (130). However, the highly charged N-terminal region of the toxins have significant sequence homology to that of trypsinogen. It is therefore possible that cleavage (by an as yet unknown mechanism) of, for example, the first few N-terminal amino acids of ETA and ETB is sufficient (as in trypsinogen) to induce the conformational change that activates the toxins (53). However, it should be noted that larger deletions of 10 or 20 amino acids in this region reduce toxin activity (44).
In vitro enzymatic studies by the same authors (44) with synthetic substrates confirmed previous work by Bailey and Redpath (23) showing that the toxins could hydrolyze the carboxyl terminal of glutamic acid of the ester boc-l-Glu-O-phenyl. These authors also showed that the toxins were unable to hydrolyze boc-l-Asp-O-phenyl, indicating the glutamic acid specificity of the S1 pocket. This activity, as well as the ability to produce exfoliation in neonatal mice, was lost when any of the three amino acids constituting the putative catalytic triad of ETA was replaced by another amino acid, confirming their importance in the observed activity.
However, there is some evidence, speculated as far back as 1969, that ETs might have intrinsic lipase activity, since analysis of clinical isolates of S. aureus from patients with localized or extensive SSSS revealed that a significant proportion of the strains had lipolytic activity toward a range of lipids (12, 261). Soon thereafter, Wiley and Rogolsky (278) demonstrated that incubation of purified ET with either egg yolk emulsion or bovine brain sphingomyelin resulted in a dose-dependent release of phosphate, suggesting that ETs have intrinsic phospholipase activity (278). However, it should be noted that other investigators were unable to detect any lipase activity toward triacylglycerol or phospholipids (210).
More recently, a triacylglycerol acylhydrolase of Mucor miehei (37) has been speculated to have structural homology to serine proteases, with His-257, Asp-203, and Ser-144 forming the catalytic triad of the lipase. As with serine proteases, lipase activity could be reduced with the serine protease inhibitor PMSF. A chemically analogous but structurally different aspartate-histidine-serine catalytic triad has also been found in human pancreatic lipases which hydrolyze triglycerides into diglycerides and subsequently into monoglycerides and free fatty acids (280). Regarding the ETs, ETA also has the sequence Gly-Asn-Ser195-Gly-Ser-Gly, which resembles the Gly-X1-Ser-X2-Gly consensus sequence of all known mammalian and microbial lipases (201). More recently, on the basis of the Lys-216 residue unique to both the ETs, computer models for the toxins were used to demonstrate that the Ser-195 active site of the toxins might act on lipids by cleaving phosphodiester bonds in a similar manner to alkaline phosphatase (25). The model showed that a phosphonyl group of a phosphodiester, instead of a peptide, would fit into the active site of the toxin and that Lys-216, along with His-213, would serve to stabilize the negative charge on the phosphonyl group. Similarly, lipases with a serine protease catalytic triad may also have an active site that is covered by a protein region displaced during the activation process (37, 41, 280).
Although the ETs might have no inherent lipase activity, it is possible that enzyme activity is induced only after activation in the epidermis. For example, the human pancreatic lipase in its native form has little enzyme activity, and data from its three-dimensional structure suggest that the serine residue representing the catalytic site is covered by a short one-turn α-helix surface loop which undergoes a conformational change to expose the active site at a lipid-aqueous interface (280).
Recently, preliminary work was presented in which an octapeptide derived from ETB prevented the formation of a precipitation line between ETB and anti-ETB serum. Furthermore, a subcutaneous injection of 2 mg of the peptide resulted in a positive Nikolsky sign in neonatal mice 16 h later (225). This work has not been published in a peer-reviewed journal; it is unlikely that such a small peptide could simulate the whole toxin molecule, and the positive Nikolsky sign may be due to a toxic rather than an exfoliative effect.
In summary, current evidence suggests that ETs induce exfoliation by possessing an atypical glutamic acid-specific trypsin-like serine protease activity which may be activated only locally by an as yet unknown mechanism. The GM4 gangliosides, toward which the ETs have a strong affinity, are unlikely to be the activating factors, since such binding abolishes exfoliation in the neonatal-animal models. It is, however, possible that binding to cell surface GM4 gangliosides results in internalization of the toxin by keratinocytes to reach keratohyalin granules; during this journey, the toxin is activated either by partial cleavage by an intracellular protease or by attachment to coreceptor to induce the exfoliation seen. On the other hand, the possibility of ETs acting as lipases, as well as their potential ability to activate or release other serine proteases from surrounding cells, still exists. In the endless search to identify the complex mechanism of action of the toxins, simpler explanations should not be forgotten. A very specific substrate in the epidermis, the requirement of specific microenvironment conditions for toxin activation, or physical disruption of the intercellular matrix by excess fluid-filled vesicles are only a few such possibilities.
SUPERANTIGENIC ACTIVITY
Whether staphylococcal ETs possess superantigenic activity is currently under considerable debate. Superantigens are a group of bacterial and viral proteins that can activate a large number of T-cell clones simultaneously. They have been reviewed recently (137). Conventional antigens require digestion by antigen-presenting cells (APCs) followed by surface presentation of a small peptide fragment complexed to an MHC protein in order to activate a small proportion (less than 0.1%) of T cells bearing specific T-cell receptors (245). In contrast, superantigens can bypass this process of intracellular processing (80, 81) and can bind directly to the MHC molecule on the surface of the APC outside the antigen-binding groove (59). This complex can then cross-link with the variable Vβ region of the β chain of the T-cell receptor (77, 124), resulting in polyclonal CD4+- and CD8+-T-cell activation (up to 20% of all T cells) that is characteristic of superantigens (245).
S. aureus produces several exotoxins that also possess superantigenic activity, including TSST-1 (2, 209) and the staphylococcal enterotoxins (SE), including SEA, SEB, SEC1-3, SED, and SEE (77, 109, 137, 198, 234, 260); new toxins continue to emerge (259). While the ETs are globular proteins with molecular masses of 24 to 30 kDa, similar to the masses of other superantigens, the degree of primary sequence and structural homology such as that seen between other superantigens is poor (165). Initial reports indicated that the ETs had mitogenic activity toward murine T lymphocytes (181), and ETA has been shown to preferentially stimulate Vβ2 human (46) and Vβ3 murine T cells (106).
The main argument against the ETs being superantigens arises from work by Fleischer and Bailey (82). Cloning ETA into a non-toxin-producing strain of S. aureus by using a shuttle vector, these investigators showed that purified recombinant ETA (confirmed by ETA-specific rabbit antiserum on Western blots), and even crude concentrated extract of the recombinant toxin, was unable to stimulate human or murine T cells even at concentrations of 10 μg/ml (83). On the other hand, 10 ng of commercially available ETA, as well as TSST-1 and SEB, per ml could stimulate activity that was consistent with superantigens (46, 124). The authors speculated that because superantigens exert their effects at concentrations that are not detectable by conventional biochemical methods, previous demonstration of superantigenic activity with ET was probably due to contamination by other staphylococcal enterotoxins (82, 83), as was demonstrated with protein A (235). They also mentioned that another group of researchers (107) could not precipitate MHC class II molecules with commercial ETA, possibly because the toxin did not bind to the molecule. Similarly, ETA and ETB purified from S. aureus strains could stimulate spleen cells of transgenic mutant mice deficient in the expression of MHC class II antigens (45), as well as MHC class II deficient macrophages, but did not bind to class II molecules (28). Further arguments against superantigenic activity presented by Cavarelli et al. (44) are as follows: (i) the characteristic symptoms of cytokines (including rash, fever, and hypotension) induced by the action on lymphocytes of other superantigens such as TSST-1 and the enterotoxins is not seen with the ETs; (ii) histological examination of skin from patients with SSSS does not show any recruitment of immune system cells; and (iii) none of the other known superantigens possess the speculated hydrolytic activity of the ETs.
On the other hand, Vath et al. (270) cloned ETA into a superantigen-free strain of S. aureus and showed that the purified toxin had mitogenic activity toward T cells. The authors argued that since superantigenic activity could be demonstrated only after cloning ETA into the superantigen-free strain of S. aureus, ETA must be the source of the superantigenic activity and contamination by other superantigens was unlikely. Also, recombinant ETA with a mutated active site, Ser195Cys-ETA, was shown to possess a similar mitogenic activity, indicating that the mitogenic activity was separate from the exfoliative activity of the ETs. This dual action is also seen with the separate emetic activity and mitogenicity of the enterotoxins (33) and with the lethality and mitogenicity of TSST-1 (188). The ETs also activate lipopolysaccharide-responsive murine macrophages to produce tumor necrosis factor alpha and high levels of interleukin-6 (IL-6) (compared to TSST-1 and SE) and nitric oxide (which was not produced by either TSST-1 or the SE) resulting in contact-dependent cytotoxicity of transformed embryo fibroblast cells, although the level of killing was lower than that for TSST-1 and SE (84).
Furthermore, SSSS does share many clinical features with other superantigen-mediated syndromes, including the tender erythematous rash, tachycardia, hypotension, fever, and shock (29, 114, 157, 174, 245). Since S. aureus in SSSS produces ETs at a site distant from the exfoliation (174), it is very likely that these systemic symptoms, particularly the characteristic rash, are a direct result of the toxins.
Exfoliative Toxins and the Skin
Recently, ETs were shown to stimulate the skin associated lymphoid tissue (SALT) by demonstrating that the toxins activated murine splenic T cells in the presence of Langerhans’ cells and MHC class II-bearing keratinocytes to produce a range of cytokines, including IL-1 and tumor necrosis factor alpha, capable of stimulating other accessory and immune system cells (268). Furthermore, binding of ETs to Langerhans’ cells resulted in dose-dependent depletion of these cells (as measured by the number of ATPase-positive cells per square millimeter) in cultured mouse skin, presumably due to migration of the cells to draining lymph nodes, where they may act as APCs for T lymphocytes (202). SALT is part of the immune system that deals with antigenic challenges common to the skin and includes antigen-presenting Langerhans’ cells, keratinocytes, and T-cell subsets with skin-homing receptors (258). Despite the presence of MHC class II molecules, which are induced on the cell surface during inflammatory processes (273), keratinocytes are unable to act as conventional APC and seem to function only in the presence of superantigens (268). Activation of SALT by ETs may therefore result in the erythematous rash associated with SSSS. For example, there is some evidence that the ability of the superantigen TSST-1 to activate cutaneous lymphocyte-associated antigens may be responsible for the rash and desquamation seen in TSS (149), just as the superantigens of group A streptococci might play a role in the development of the characteristic cutaneous swelling, erythema, and desquamation seen in streptococcal infections (254). However, the lack of immune cells on histological specimens from patients with SSSS cannot be easily explained and requires more research. It may be that the very specific (possibly intracellular) site of action of the toxins does not allow them to exert superantigenic activity in terms of duration or location or that activation of the toxins to proteases, which probably requires a structural change (25, 44, 270), results in loss of superantigenic activity at the site of action.
The ability of the ETs to activate the SALT subpopulation of the immune system has important consequences for several dermatological conditions, including cutaneous T-cell lymphoma and atopic dermatitis. ETA is capable of inducing the proliferation of Vβ2.1-bearing cutaneous T-cell lymphoma cells in vitro, and this proliferative response is enhanced by adding IL-1. This fits in with observations showing that activation of SALT by ETA results in IL-1 release (268), and IL-1, along with IL-6, plays an important role in enhancing the activity of other superantigens (136).
Patients with atopic dermatitis have a higher staphylococcal colonization rate than does the general population (8, 152). Antibiotic treatment of patients with atopic dermatitis has resulted in significant clinical improvement of the condition (150), suggesting that staphylococci play a role in the pathogenesis of the disease. Recently, it has been shown that a large proportion of S. aureus strains isolated from patients with atopic dermatitis release superantigens, including enterotoxins, TSST-1, and ETs (147, 167). It has been speculated that superantigens bind to surface MHC class II molecules found on keratinocytes during inflammation and thereby activate polyclonal T cells locally (257).
Exfoliative Toxins and Other Human Diseases
The Vβ-expansion of T cells characteristic of superantigens has also been demonstrated in other conditions, where the role of staphylococcal toxins is still unclear. Kawasaki disease, also known as mucocutaneous lymph node syndrome, is a multisystem disorder in children that is characterized by fever, rash, conjunctivitis, mucosal inflammation, erythematous peeling of the hands and feet, and, rarely, coronary artery aneurysms (147, 175). A selective expansion of Vβ2- and Vβ8-expressing T cells has been detected in the blood of patients with Kawasaki disease (1), and, while TSST-1 has been detected in a proportion of these patients (146, 147, 148), the potential role of the ETs has not been studied. Other conditions where expansion of Vβ-expressing T cells, often by staphylococcal superantigens, has been demonstrated include hematogenously acquired staphylococcal nephritis (138, 271), staphylococcal septic arthritis (38, 290), various autoimmune diseases (137, 184), rheumatoid arthritis (197), multiple sclerosis (39, 256), contact sensitivity (228), and guttate and chronic plaque psoriasis (151, 288); another possible role for the expansion of Vβ-expressing T cells is in the gradual decline in the number of CD4+ T cells in human immunodeficiency virus infection (143). Unfortunately, many of the studies on the superantigenic effects of ETs in various diseases have used commercially prepared toxins, which, as many authors have pointed out, may be contaminated by other superantigens. Future studies should take this factor into consideration and use appropriate precautions to eliminate the problem.
Several other conditions have demonstrated a significant association with staphylococcal toxins; sudden infant death syndrome (SIDS) is probably one of the most important (31). S. aureus strains producing multiple toxins have been isolated from infants with SIDS (265). In addition, S. aureus is the most common organism isolated from 2- to 4-month-old infants, being found in almost 50% of the infants (222). This age group is also associated with the highest incidence of SIDS (31). Lee et al. (145) have shown that bacterial isolates, particularly S. aureus and Escherichia coli, from infants with SIDS were lethal to gnotobiotic rats. Therefore, some authors have proposed that common bacterial toxins absorbed through the respiratory tract might be involved (182). Furthermore, staphylococcal infection in combination with influenza virus infection was shown to greatly increase the production of SE, resulting in an exaggerated inflammatory response in ferrets (117). This activity might explain the higher prevalence of SIDS in winter (43). Although TSST-1 was demonstrated in renal tubular cells of some infants with SIDS but not control infants (189), the role of the ETs in SIDS has, unfortunately, never been studied. It is possible that an abnormal inflammatory response due to the superantigenic properties of the ETs, which may be produced locally in the nasopharynx and then systemically absorbed (189), coupled with enhancement by concurrent viral infection will result in sudden death in infants.
SUSCEPTIBILITY AND SEVERITY
A whole range of host and organism factors govern the development and severity of SSSS, and many of these are not fully understood. Furthermore, it is still not clear why neonates are more susceptible to SSSS than adults. Initially, anatomical differences in the skin were believed to be the reason, based mainly on the fact that the toxins induced exfoliation in neonatal but not adult mice and that, in humans, the condition was seen primarily in neonates. However, this premise has now been refuted for several reasons: (i) healthy adults of the hairless mice strain will develop SSSS when injected with ET (126); (ii) adult mice can also develop localized SSSS at the site of toxin injection (93) and can even develop generalized SSSS if they are immunosuppressed with drugs such as prednisolone and azathioprine (277); (iii) SSSS cases have been reported in healthy human adults and in adults with underlying disease (48, 194); and (iv) intradermal injection of purified ET into the forearm of a healthy human adult will result in blister formation characteristic of SSSS (127, 277). Nevertheless, anatomical differences are the most likely candidates in determining species susceptibility (69).
Poor renal clearance of the toxins by neonates and by adults with impaired renal function is a major risk factor for developing SSSS and may partly explain why neonates may be more susceptible to developing SSSS. Adult mice excrete one-third of a test dose of intravenous ETA within 3 h compared to 1/15 of a test dose in newborn mice (94). As a consequence, toxin levels reach a higher peak in the newborn and decline more slowly. Furthermore, nephrectomized (but not hepatectomized) adult mice develop generalized SSSS when challenged with ET. However, renal function alone cannot explain why injection of very high doses of ET into adult mice, which would be expected to more than compensate for the rapid renal clearance, does not result in exfoliation (94).
Antibody status may also have a protective function, and this has been shown in mice, in which inoculation of small doses of toxigenic S. aureus, for example, resulted in exfoliation only in neonatal mice that had not previously been immunized with purified extracts of ET (173). A large epidemiological study looking at the antibody status of patients over the whole range of SSSS spectrum was also performed (173). Anti-toxin antibodies were found in 88% of infant cord blood samples. These findings reflect passive maternal antibodies, which, in turn, may protect newborn infants. This finding is further supported by the observation that infants involved in neonatal-nursery outbreaks tend to have the localized rather than the generalized form of the disease. In addition, work with mice showed that maternal antibodies play an important role in protecting offspring from subcutaneous challenges of ETA (163).
The large epidemiological study also showed that antibody levels fell to 30% in patients between 3 and 24 months of age and then rose steadily to 50% in those older than 10 years and to 91% in those older than 40 years. Furthermore, the same authors noted that 13 (62%) of 21 and 15 (100%) of 15 patients with localized SSSS had anti-toxin antibodies in acute-phase (5 days) and convalescent-phase (14 days) sera, respectively, in contrast to 0 of 10 and 5 of 5 patients with generalized SSSS (173). The authors concluded that while antibodies may not always protect against exfoliation, they may serve to localize the infection around the port of entry of the responsible S. aureus strain. In support of this, the blisters of localized SSSS, such as bullous impetigo, contain S. aureus while the blisters of generalized SSSS, where ETs are produced by S. aureus at a distant site, tend to be sterile (70). Although the study involved ETA only, the overall conclusions are unlikely to be affected by excluding ETB, and at least two other studies have confirmed the above findings (173).
Protective antibodies may also explain why only a proportion of nasal carriers of toxin-producing S. aureus are infected. However, it is possible that the strains harbored by these carriers are not active producers of the toxins. The antibody theory also provides an explanation for observations that breast-feeding is protective in neonates.
While adult SSSS is very uncommon, more than 30 cases have been reported (this is likely to be a gross underestimation, since the disease was linked to the ETs only in 1970) and have been reviewed recently (48). Of the 32 reported cases, 21 (66%) were in males, and the ages of the patients ranged from 19 to 91 years; more than half of the patients were older than 60 years. Most of the adults with SSSS had an underlying disorder such as poor renal function (63); immunosuppression with immunosuppressive drugs; infections such as HIV and AIDS (63); malignancy including Hodgkin’s and non-Hodgkin’s lymphomas, metastatic breast carcinoma, and acute and chronic myeloid leukaemias; and other less common causes such as chronic alcohol abuse, heroin misuse, diabetes mellitus, cachexia, and rheumatic fever (96, 173). Another review of adult SSSS found that 78% of patients had factors suggesting possible impaired immunity such as those described above (32). Furthermore, renal impairment was found in 73% of those who had their renal function tested, and many had frank renal failure.
There have been only two reports of apparently healthy immunocompetent young adults developing SSSS, and both cases were due to ETB (185); it is not known whether this is merely a coincidence or whether the ETB subtype is more pathogenic. Another epidemiological study (185) did note that although both toxins were equally responsible for localized SSSS such as bullous impetigo, ETB was more frequently isolated from children with generalized SSSS than was ETA alone (20 of 24 and 2 of 24, respectively). Recently, a case of SSSS was reported in an immunocompetent 73-year-old man who had very mild renal impairment and who was taking ibuprofen (103). Nonsteroidal anti-inflammatory drugs have been previously reported to predispose to SSSS (129), possibly by a combined effect of inhibiting prostaglandin-mediated renal vasodilation and inhibiting neutrophil function. However, as the authors correctly point out, because such drugs are so widely used, this association may be purely coincidental.
DIFFERENTIAL DIAGNOSIS
While the mortality rate from SSSS in neonates and children is low, it is important to make a correct diagnosis in order to initiate early and appropriate treatment, as well as to prevent medicolegal implications, since the disease may result in allegations of burns by neglect (160) or child abuse (231). Differential diagnoses of generalized SSSS in neonates are few and include drug- or virus-mediated TEN, burns, epidermolysis bullosa, bullous erythema multiforme, listeriosis, syphilis, diffuse cutaneous mastocytosis, and graft-versus-host rejection (65). Chemical burns from petrol, paraffin, boric acid, ethylene, acrylonitrile fumigant spray, tribromofluorene, and monosulfiram, which act at the dermoepidermal junction, must also be excluded (162). TEN, also known as Lyell’s syndrome after the author who first described it (159), is probably the most important differential diagnosis of SSSS, since their treatments are very different (236). TEN is an uncommon but life-threatening cutaneous disorder that occurs as frequently in children as in adults (215). More than 100 different drugs, including antibiotics and anti-inflammatory drugs, and occasionally immunizations and infections such as with members of the family Herpesviridiae, have been implicated in TEN (236). TEN occurs 1 to 3 weeks after drug ingestion, although reexposure to the offending drug may result in a more rapid and very severe presentation, with involvement of multiorgan systems including the liver, kidneys, gut, and lungs (212, 236). The dermatological features are characterized by an initial prodrome of pyrexia, malaise, anorexia, pharyngitis, and a tender, morbilliform rash followed by epidermal exfoliation in sheets (236). As in SSSS, fragile blisters are also seen in TEN and the Nikolsky sign is positive (18, 236). However, unlike SSSS, eroding mucosal lesions of the mouth, conjunctiva, trachea, bronchi, esophagus, anus, and vagina (18) are almost always present in TEN, as demonstrated in one study of 87 patients (215), and can be differentiated histologically from SSSS lesions (see below).
Localized SSSS such as bullous impetigo is usually characterized by blisters that dry up without producing any marked crusting (162, 178). On the other hand, in streptococcal impetigo there is copious exudation from raw surfaces that dries into a characteristic thick, dirty yellow crust, which soon re-forms if removed. Furthermore, glomerulonephritis is a known complication of the latter but not the former condition (162, 174). However, staphylococcal impetigo and streptococcal impetigo are often clinically indistinguishable, and antibiotic treatment should cover both groups of organisms.
The initial generalized erythematous rash seen in SSSS may be confused with streptococcal scarlet fever, but the very tender skin, exfoliation, lack of a strawberry tongue, and possible perioral and periorbital crusting seen in SSSS should confirm the diagnosis (174). Congenital epidermolysis bullosa can present with exfoliation of the skin upon slightest mechanical pressure, but, unlike SSSS, it presents from birth. Other congenital bullous diseases include acrodermatitis enteropathica and bullous ichthyosis. Occasionally, bacterial (staphylococci, streptococci, E. coli, or, rarely, Haemophilus spp.) (207), fungal (Candida spp.), and viral (herpesviruses, vaccinia virus, varicella-zoster virus) infections resemble SSSS. Isolated cases of diffuse cutaneous mastocytosis (195), Kawasaki disease (79), psoriasis (217), and TSS (66) have been reported to mimic SSSS.
DIAGNOSIS AND INVESTIGATIONS
Most cases of SSSS are diagnosed on clinical grounds and are easily treated with antibiotics, which rapidly eliminate the staphylococci producing the toxin. Laboratory investigations are required only if the clinical findings are equivocal or when outbreaks occur. Because the condition is the result of exotoxins which may be produced by staphylococci at a distant site, the blister fluid in generalized SSSS tends to be sterile whereas the fluid in localized bullous impetigo will contain S. aureus (70, 158, 208). Staphylococci producing ET can usually be cultured from the nares, conjunctiva, or nasopharynx (50, 158, 229). Blood cultures are usually negative because the organisms are frequently noninvasive, particularly in children (49, 114, 157, 208, 211). In one study, only 3% of children had a positive blood culture (101), in contrast to 20 (62.5%) of 32 adults (48). Of note, children with renal disease tend to have bacteremia with generalized SSSS rather than the localized form (34). Bacteriological studies of the skin are not usually useful in differentiating SSSS from TEN, since the skin of patients with generalized SSSS is usually sterile and that of patients with TEN is often colonized by S. aureus (67, 236). Indeed, up to half of the deaths in patients with TEN are due to secondary bacterial infection of the denuded skin (18).
Phage typing is not routinely available in hospital laboratories. In the United Kingdom, bacterial swabs of S. aureus can be sent to the Public Health Laboratory at Colindale, London, for phage typing, despite this test having poor reproducibility and requiring special reagents and a large number of tests (95). Many authors assume that the presence of characteristic skin lesions, including a positive Nikolsky sign, along with the isolation of phage group II S. aureus from a distal site is sufficient for the diagnosis of SSSS (for a review, see reference 72). However, it is now generally agreed that the production of ET is not associated with any particular phage type, since phage types other than group II will also produce the ETs and only a proportion of phage II strains produce ETs (56, 85, 174). For example, Falk and King (72) reported the case of a 43-year-old schizophrenic who presented with fever, erythema, and exfoliation with a positive Nikolsky sign but no mucosal involvement and whose routine skin swabs contained S. aureus phage type 71. He was treated for SSSS until a blister biopsy revealed a diagnosis of TEN. Phage typing is currently not acceptable for confirming the diagnosis of SSSS since it is neither sensitive nor specific (56, 174, 185).
A biopsy of the blister is one of the most definitive diagnostic tests in SSSS. One study revealed a positive blister biopsy result with intraepidermal cleavage in all 30 adults with SSSS (48). Blister biopsy is also essential in ruling out other bullous dermatoses (42, 75, 269). Histological testing will clearly exclude conditions involving subepidermal separation, including erythema multiforme and TEN, bullous lichen planus, polymorphic light eruptions, graft-versus-host rejection, and noninflammatory processes such as bullous amyloidosis, Wilson’s disease, drug ingestion, epidermolysis bullosa acquisita, and porphyria (75). To rapidly differentiate TEN from SSSS, a Tzanck preparation from a freshly denuded area will show large polygonal epithelial cells with large nuclei and no inflammatory cells in SSSS and only a few rounded or cuboidal epithelial cells with many inflammatory cells in TEN (9, 73).
Histologically, SSSS needs to be distinguished from other intraepidermal conditions, including TSS and nutritional disorders. TSS is usually diagnosed clinically (90), with or without laboratory tests to detect TSST-1 and other superantigens (178). Histological tests for the diagnosis of TSS are only occasionally required and show spongiosis, scattered necrotic keratinocytes, and scattered neutrophils, with a perivascular and interstitial mixed-cell infiltrate and dilatation of the superficial plexus and edema in the dermis (111). Nutritional deficiency syndromes, including pellagra, zinc deficiency, biotin deficiency, essential fatty acid deficiency, and the glucagonoma syndromes (necrolytic migratory erythema), may present with erythematous, scaly, and vesicular eruptions (3). All have a histologically similar picture, with intercellular edema in the upper half of the stratum spinosum that may be severe enough to cause intraepidermal vesiculation (3). Along with SSSS, subcorneal pustular dermatosis and pemphigus foliaceus are two other causes of subcorneal blistering; they can be distinguished from SSSS by the presence of a large number of neutrophils in the blister and by distinct immunohistochemical findings respectively (75). Lesions from bullous impetigo are characterized by stratum granulosum epidermal cleavage with a local infiltrate of staphylococci and polymorphonuclear leucocytes (174).
If further confirmation is required, the Public Health Laboratory in the United Kingdom may attempt to detect the ETs by using the Ouchterlony method. This method is known to be crude, unreliable, and time-consuming with poor sensitivity and is unsuitable for batch testing of large samples (139). Furthermore, our work (139) and work with diagnostic kits for TSST-1 (219, 238) have shown that protein A in culture supernatants is produced by more than 90% of S. aureus strains (88, 89), resulting in high rates of false-positive results due to nonspecific binding to the detecting antibodies (36, 255).
While several techniques have been proposed in the past to study the effects of ETs, few have been developed. Subcutaneous injection of small amounts of the partially purified toxin into neonatal (<5-day-old) Sha-Sha, BALB/c, CD-1, TA, ICR, Swiss-Webster, or Park mice (56, 57, 170) or adult hairless mice (14, 126) results in a positive Nikolsky sign 2 to 120 min after inoculation, although the adult hairless-mouse model is less sensitive. While such mouse models remain the gold standard for confirming the diagnosis of SSSS, they are clearly not suitable for routine rapid identification of ETs in hospital laboratories. Gel immunoprecipitation (Mancini test) is expensive and has limited sensitivity (16). Radioimmunological assays (RIA) are more recent tests developed for detecting the toxins (288), since they can detect ETA at 100 pg/ml by using 125I (173); these tests are very sensitive and quantitative but are too expensive and time-consuming for routine use, and they require the handling of radioactive materials. Simple enzyme-linked immunosorbent assays (204) are not as sensitive as RIA, but a double-antibody procedure, in which a secondary rat polyclonal antibody is used, has attained the sensitivity of RIA (156). Other methods developed include slide latex agglutination (187), electrosyneresis and DNA hybridization with oligodeoxynucleotide probes (204, 216), and genomic DNA fingerprinting after SmaI digestion of DNA extracted from S. aureus strains (95). Unfortunately, most of these tests have been developed for research purposes and are not suitable as routine tests in hospital laboratories because they tend to be time-consuming, laborious, and expensive.
More recently, PCR with specific ETA and ETB primers has been used to identify the toxin genes in suspected strains of S. aureus (120, 226). For example, Sakurai et al. correctly identified all 30 ETA-, 20 ETB-, and 6 ETA- and ETB-producing strains within 3 h (226). Thus, this technique is rapid and sensitive and has been used to confirm the diagnosis of SSSS in suspected clinical cases (288). Random amplified polymorphic DNA analysis, which is simple and rapid to perform, has also been used to demonstrate that the S. aureus strain isolated from a mother’s breast abscess was identical to the strain causing SSSS in her breast-feeding infant (213).
TREATMENT
Although SSSS can be very severe, it is easily managed with antibiotics to eradicate the offending toxin-producing S. aureus strain and appropriate nursing care to prevent and/or treat any secondary symptoms. Neonates must be isolated from other babies and strict barrier precautions must be applied to prevent cross-infection (79). A focal source of infection, which may be absent in more than half the patients (48), must be sought and treated to prevent further spread of the disease. This includes blood, wound, eye exudates, and throat swabs initially, followed by osteomyelitis, septic arthritis, and occult foci if the source is not obvious (174). Potential carriers of toxin-producing strains must also be identified and treated to prevent cross-transmission, which may result in outbreaks. This involves culturing nasal swabs for S. aureus from all personnel who have handled the infected baby (79).
Mild forms of SSSS such as bullous impetigo should be managed with cleaning and drying of the lesions with compresses and administration of oral antibiotics that are effective against penicillin-resistant staphylococci as well as streptococci. Methicillin, nafcillin, dicloxacillin, cloxacillin, and flucloxacillin have all been used to treat S. aureus, and other proposed regimens include cloxacillin plus tobramycin, and erythromycin (48). Multidrug-resistant strains are rarely encountered in phage group II S. aureus (48, 279). One recent study of 61 ET-producing S. aureus strains revealed that 7% were resistant to tetracycline, 5% were resistant to gentamicin, and 2% were resistant to chloramphenicol. None of the strains were resistant to methicillin, cephalosporins, or vancomycin (186). Another epidemiological study of 82 methicillin-resistant S. aureus (MRSA) strains isolated from outbreaks in neonatal intensive-care units in Japan revealed that only 1 strain produced ETA and none produced ETB (287). However, the same authors also reported a case involving a 6-month-old infant with SSSS due to a methicillin-resistant ETB-producing phage II S. aureus strain in Japan, who was successfully treated with minocycline (287).
Topical antibiotics are often not effective and should be avoided due to unpredictable absorption through denuded skin; even localized forms of SSSS such as bullous impetigo should be treated with oral antibiotics, which have been shown to be more effective than topical antibiotics (61). Steroids are contraindicated (157, 208) on the basis of both experimental (171) and clinical (220) evidence.
Severe forms require more aggressive treatment with intravenous antistaphylococcal antibiotics and extra care of denuded skin to prevent secondary infection and fluid losses and to maintain body temperature, especially in neonates. Additional broad-spectrum antibiotics should be considered if secondary skin infection is suspected, and they should cover Pseudomonas species (110). Intravenous crystalloids should be used to replace fluids, and an incubator should be used to maintain body temperature. Sterile wrapping and minimal handling may be used to decrease conductive heat, fluid losses, and the risk of secondary infections. Semipermeable dressings, such as those used to cover graft donor sites, have been used to provide a barrier against evaporative fluid losses (177). Topical applications such as silver sulfadiazine, which are sometimes used for burns and TEN, are not recommended for SSSS because of enhanced systemic absorption through denuded skin (86). Because the complications of severe SSSS resemble those of severe burns, many clinicians prefer treating such patients in specialized burns units (243). A few severe cases of SSSS have benefited from extracorporeal detoxification involving sorption detoxification and separation plasmapheresis (250).
Within 2 to 3 days, fever usually subsides, the erythema begins to resolve, and no new bullae form (157). Desquamation of the skin occurs 3 to 5 days after onset, and complete resolution occurs in most cases, usually within 2 to 3 weeks, with no permanent sequalae (100, 114, 157). Serious complications, including lung abscesses, staphylococcal pneumonia, and osteomyelitis, have been reported (87, 104). Mild emollients may be used if the skin becomes very dry during the healing process (114).
The prognosis of SSSS in children who are appropriately treated is good, with a mortality of less than 5% (48, 96, 174). Nevertheless, some children will die despite antibiotic therapy (211). In contrast, mortality rates in adults are very high, usually due to underlying diseases, despite aggressive treatment. One review of adult SSSS reported that 19 (59%) of 32 patients reported in the literature died despite antibiotic therapy (48); all but 2 of those who died had severe underlying disease, and the prognosis was not necessarily better in those with localized forms of SSSS.
PREVENTION
Hospitals remain the most important source of S. aureus, and simple barrier nursing techniques are still considered to be the most effective method of preventing transmission and outbreaks of infection. Using MRSA as a parallel example, reinforcement of hygiene measures has consistently led to a decrease in MRSA colonization and infection rates (35, 119, 155, 185). Simple measures such as hand-washing, minimal handling, cleaning objects such as stethoscopes and thermometers before use, and avoiding unnecessary invasive catheterization all contribute to prevention of cross-infection (119). Such aseptic technique should be encouraged among all health care professionals and reenforced at all levels.
Any patient developing SSSS should be immediately isolated. Anyone else at risk of developing SSSS who may have been exposed to ET-producing S. aureus strains will benefit from prompt administration of prophylactic anti-staphylococcal antibiotics, which, in mice, have shown to abort exfoliation if given early enough (172). Furthermore, carriers of toxin-producing strains should be rapidly identified and treated to prevent outbreaks. It is important to emphasize that any one outbreak may have more than one carrier (49, 52, 55). Unfortunately, a rapid diagnostic kit for SSSS is not yet available. As a consequence, there is often a delay in diagnosis as well as identification of carriers. The organism has to be isolated and sent to the Public Health Laboratory for phage typing and then possibly for culturing to produce the ETs, which are then tested for by the Ouchterlony method (140).
FUTURE DIRECTIVES AND CONCLUDING REMARKS
Research into SSSS is now reaching an exciting era. Elucidation of the three-dimensional structure of the ETs has given us an insight into the mode of action of the toxins. The precise mechanisms by which they split the epidermis will be determined in the very near future, and their ability to target to a very specific region of the epidermis can be exploited in many ways (140). In particular, the targeting domain of the toxins may be used to deliver drugs to precise intraepidermal locations for the treatment of dermatological conditions such as neoplasms, as has already been demonstrated with Corynebacterium diphtheriae and Pseudomonas toxins (283). Furthermore, if the ETs are confirmed to be superantigens, they may play a wider role in a whole range of dermatological and other diseases.
Similarly, there have been studies showing that antitoxin antibodies will protect neonatal mice from lethal doses of ET, even if the antitoxin is administered late during the course of the disease (127). Because antibiotics are targeted to the offending organism, exfoliation will continue for 24 to 36 h after antibiotic administration. Development of an effective antitoxin based on the structure and function of the toxin may be invaluable in halting the progression of exfoliation in severe cases of generalized SSSS, particularly in adults, in whom mortality rates are high (140). Although the rarity of the disease would not support large-scale immunization, the use of toxoids in immunization against SSSS may also play a role for at-risk groups such as neonates with immune deficiency or adults with cancer or renal failure.
The need for a rapid diagnostic kit for SSSS can no longer be ignored. Current methods of diagnosis are neither sensitive nor specific, and delay in diagnosing the disease will result in increased morbidity and mortality, particularly among adults. Most hospital laboratories have facilities to detect a wide range of microbial toxins, and the ETs should be no exception. Rapid detection of the ETs is important not only in diagnosing SSSS but also in detecting carriers and screening at-risk patients for ET-producing S. aureus carriage to prevent the development of SSSS.
Clinicians should also be aware of the need for increased surveillance of staphylococcal infections in general. The pressure to discharge babies early from hospitals, along with the recent trend to stop using antiseptic neonatal umbilical cord care, is likely to increase the prevalence of staphylococcal infections in the community. Therefore, it might be useful to develop a central reporting system, whereby primary-care physicians report to the local hospital any neonatal infection in the community so that possible outbreaks dissipated into the community would be rapidly recognized and contained and emerging trends such as antibiotic resistance would be quickly detected and dealt with.
It is hoped that the relative rarity of the disease will not lull researchers and clinicians into a false sense of security and that the recent exciting developments in the field will strengthen efforts to conquer the disease once and for all.
REFERENCES
- 1.Abe J, Kotzin B L, Jujo K, Melish M E, Glode M P, Kohsaka T, Leung D Y. Selective expansion of T cells expressing T-cell receptor variable regions Vβ2− and Vβ8 in Kawasaki disease. Proc Natl Acad Sci USA. 1992;89:4066–4070. doi: 10.1073/pnas.89.9.4066. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Acharya K R, Passalacqua E F, Jones E Y, Harlos K, Stuart D I, Brehm R D, Tranter H S. Structural basis of superantigen action inferred from crystal structure of toxic-shock syndrome toxin-1. Nature. 1994;367:94–97. doi: 10.1038/367094a0. [DOI] [PubMed] [Google Scholar]
- 3.Ackerman A B. Histologic diagnosis of inflammatory skin disease. Philadelphia, Pa: Lea & Febiger; 1978. p. 169. [Google Scholar]
- 4.Adesiyun A A, Lenz W, Schaal K P. Exfoliative toxin-production by Staphylococcus aureus strains isolated from animals and human beings in Nigeria. Microbiologica. 1991;14:357–362. [PubMed] [Google Scholar]
- 5.Adlam C, Anderson J C, Arbuthnott J P, Easmon C S F, Noble W C. Staphylococci and staphylococcal infections. Vol. 1. London, United Kingdom: Academic Press, Ltd.; 1983. pp. 357–384. [Google Scholar]
- 6.Allen K D, Ridgeway E J, Parsons L A. Hexachlorophane powder and neonatal staphylococcal infection. J Hosp Infect. 1994;27:29–33. doi: 10.1016/0195-6701(94)90065-5. [DOI] [PubMed] [Google Scholar]
- 7.Almquist E. Pemphigus neonatorum, backteriologisch und epidemiologisch beleuchtet. Z Hyg Infectionskr. 1891;10:253. [Google Scholar]
- 8.Aly R, Maibach H I, Shinefield H R. Microbial flora of atopic dermatitis. Arch Dermatol. 1977;113:780–782. [PubMed] [Google Scholar]
- 9.Amon R B, Dimond R L. Toxic epidermal necrolysis: rapid differentiation between staphylococcal and drug-induced disease. Arch Dermatol. 1975;111:1433–1437. doi: 10.1001/archderm.111.11.1433. [DOI] [PubMed] [Google Scholar]
- 10.Anderson J M, Cockburn F, Forfar J Q, Harkness R A, Kelly R W, Kilshaw B. Neonatal spongiform myelinopathy after restricted application of hexochlorophane skin disinfectant. J Clin Pathol. 1981;34:25–29. doi: 10.1136/jcp.34.1.25. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Annunziato D, Goldblum L M. Staphylococcal scalded skin syndrome. Am J Dis Child. 1978;132:1187–1188. doi: 10.1001/archpedi.1978.02120370035008. [DOI] [PubMed] [Google Scholar]
- 12.Arbuthnott J P, Gemmell C G, Kent J, Lyell A. Hemolysin and enzyme patterns of coagulase-positive staphylococci isolated from toxic epidermal necrolysis, Ritter’s disease, and impetigo contagiosa. J Med Microbiol. 1969;2:479–487. doi: 10.1099/00222615-2-4-479. [DOI] [PubMed] [Google Scholar]
- 13.Arbuthnott J P, Kent J, Lyell A, Gemmell C G. Toxic epidermal necrolysis produced by an extracellular product of Staphylococcus aureus. Br J Dermatol. 1971;85:145–149. doi: 10.1111/j.1365-2133.1971.tb07200.x. [DOI] [PubMed] [Google Scholar]
- 14.Arbuthnott J P, Kent J, Noble W C. The response of hairless mice to staphylococcal epidermolytic toxin. Br J Dermatol. 1973;88:481–485. doi: 10.1111/j.1365-2133.1973.tb15454.x. [DOI] [PubMed] [Google Scholar]
- 15.Arbuthnott J P, Billcliffe B, Thompson W D. Isoelectric focusing studies of staphylococcal epidermolytic toxin. FEBS Lett. 1974;46:92–95. doi: 10.1016/0014-5793(74)80342-x. [DOI] [PubMed] [Google Scholar]
- 16.Arbuthnott J P, Billcliffe B. Qualitative and quantitative methods for detecting staphylococcal epidermolytic toxin. J Med Microbiol. 1976;9:191–201. doi: 10.1099/00222615-9-2-191. [DOI] [PubMed] [Google Scholar]
- 17.Arbuthnott J P. Characterisation of the epidermolytic toxins of Staphylococcus aureus. In: MacDonald A, Smith G, editors. The staphylococci. Proceedings of the Alexander Ogsten Centennial Conference. Aberdeen, United Kingdom: Aberdeen University Press; 1981. pp. 109–118. [Google Scholar]
- 18.Avakian R, Flowers F P, Araujo O E, Ramos-Caro F A. Toxic epidermal necrolysis: a review. J Am Acad Dermatol. 1991;31:301–302. doi: 10.1016/0190-9622(91)70176-3. [DOI] [PubMed] [Google Scholar]
- 19.Ayliffe G A J, Brightwell K M, Collins B J, Lowbury E J L, Goonatilake P C L, Etheridge R A. Studies of hospital infection in Birmingham region. J Hyg Camb. 1977;79:229–314. doi: 10.1017/s0022172400053110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Ayliffe G A. The progressive intercontinental spread of methicillin-resistant Staphylococcus aureus. Clin Infect Dis. 1997;24(Suppl. 1):S74–S79. doi: 10.1093/clinids/24.supplement_1.s74. [DOI] [PubMed] [Google Scholar]
- 21.Bailey C J, DeAzavedo J, Arbuthnott J P. A comparative study of two serotypes of epidermolytic toxins from Staphylococcus aureus. Biochim Biophys Acta. 1980;624:111–120. doi: 10.1016/0005-2795(80)90230-5. [DOI] [PubMed] [Google Scholar]
- 22.Bailey C J, Smith T P. The reactive serine residue of epidermolytic toxin A. Biochem J. 1990;269:535–537. doi: 10.1042/bj2690535. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Bailey C J, Redpath M B. The esterolytic activity of epidermolytic toxins. Biochem J. 1992;284:177–180. doi: 10.1042/bj2840177. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Bailey C J, Lockhart B P, Redpath M B, Smith T P. The epidermolytic (exfoliative) toxins of Staphylococcus aureus. Med Microbiol Immunol. 1995;184:53–61. doi: 10.1007/BF00221387. [DOI] [PubMed] [Google Scholar]
- 25.Barbosa J A R G, Saldanha J W, Garratt R C. Novel features of serine protease active sites and specificity pockets: sequence analysis and modelling studies of glutamate-specific endopeptidases and epidermolytic toxins. Protein Eng. 1996;9:591–601. doi: 10.1093/protein/9.7.591. [DOI] [PubMed] [Google Scholar]
- 26.Barr J. The umbilical cord: to treat or not to treat? Midwives Chron Nursing Notes. 1984;97:224–226. [PubMed] [Google Scholar]
- 27.Beers B, Wilson B. Adult staphylococcal scalded skin syndrome. Int J Dermatol. 1990;29:428. doi: 10.1111/j.1365-4362.1990.tb03829.x. [DOI] [PubMed] [Google Scholar]
- 28.Beharka A A, Armstrong J W, Iandolo J J, Chapes S K. Binding and activation of major histocompatibility complex class II-deficient macrophages by staphylococcal exotoxins. Infect Immun. 1994;62:3907–3915. doi: 10.1128/iai.62.9.3907-3915.1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Bell F G, Fenton P A. Early hospital discharge and cross-infection. Lancet. 1993;342:120. doi: 10.1016/0140-6736(93)91325-g. [DOI] [PubMed] [Google Scholar]
- 30.Benson P F, Rankin G L S, Rippey J. An outbreak of exfoliative dermatitis of the newborn (Ritter’s disease) due to Staphylococcus aureus, phage type 55/71. Lancet. 1962;i:999–1002. doi: 10.1016/s0140-6736(62)92036-6. [DOI] [PubMed] [Google Scholar]
- 31.Blackwell C C, Saadi A T, Raza M W, Wier D M, Busuttil A. The potential role of bacterial toxins in sudden infant death syndrome. Int J Leg Med. 1993;105:333–338. doi: 10.1007/BF01222118. [DOI] [PubMed] [Google Scholar]
- 32.Blanc M F, Janier M. Staphylococcies exfoliantes (SSSS) de l’adulte. Ann Dermatol Vererol. 1986;113:833–843. [PubMed] [Google Scholar]
- 33.Bohach G A, Fast D J, Nelson R D, Schlievert P M. Staphylococcal and streptococcal pyrogenic toxins involved in toxin shock syndromes and related diseases. Crit Rev Microbiol. 1990;17:251–272. doi: 10.3109/10408419009105728. [DOI] [PubMed] [Google Scholar]
- 34.Borchers S L, Gomez E C, Isseroff R R. Generalised staphylococcal scalded skin syndrome in an anephric boy undergoing haemodialysis. Arch Dermatol. 1984;120:912–918. [PubMed] [Google Scholar]
- 35.Boyce J M. Methicillin-resistant Staphylococcus aureus in hospitals and long-term facilities: microbiology, epidemiology, and preventative measures. Infect Control Hosp Epidemiol. 1992;13:725–737. doi: 10.1086/648346. [DOI] [PubMed] [Google Scholar]
- 36.Boyer M D P, Reis K J. Bacterial Fc receptors. Bio/Technology. 1987;5:697–703. [Google Scholar]
- 37.Brady L, Brzozowski A M, Derewenda Z S, Dodson E, Dodson G, Tolley S, Turkenburg J P, Christensen L, Huge-Jensen B H, Norskov L, Thim L, Menge U. A serine protease triad forms the catalytic centre of a triacylglycerol lipase. Nature (London) 1990;343:767–770. doi: 10.1038/343767a0. [DOI] [PubMed] [Google Scholar]
- 38.Bremmell T, Tarkowski A. Preferential induction of septic arthritis and mortality by superantigen-producing staphylococci. Infect Immun. 1995;63:4185–4187. doi: 10.1128/iai.63.10.4185-4187.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Brocke S, Veromaa T, Weissman I L, Gijbels K, Steinman L. Infection and multiple sclerosis: a possible role for superantigens? Trends Microbiol. 1994;2:250–254. doi: 10.1016/0966-842x(94)90630-0. [DOI] [PubMed] [Google Scholar]
- 40.Brook M G, Bannister B A. Staphylococcal enterotoxins in scarlet fever complicating chicken pox. Postgrad Med J. 1991;67:1013–1014. doi: 10.1136/pgmj.67.793.1013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Brzozowski A M, Derewenda U, Derewenda Z S, Dodson G G, Lawson D M, Turkenburg J P, Bjorking F, Huge-Jensen B, Patkar S A, Thim L. A model for interfacial activation in lipases from the structure of a fungal lipase-inhibitor complex. Nature. 1991;351:491–494. doi: 10.1038/351491a0. [DOI] [PubMed] [Google Scholar]
- 42.Buslau M, Biermann H, Shah P M. Gram-positive septic-shock with bullae. Intraepidermal splitting as an indication of toxin effect. Hautarzt. 1996;47:783–789. doi: 10.1007/s001050050510. . (In German.) [DOI] [PubMed] [Google Scholar]
- 43.Carpenter R G, Gardener A. Studies of medical and population subjects, publication 45. London, United Kingdom: Her Majesty’s Stationary Office; 1967. pp. 23–31. [Google Scholar]
- 44.Cavarelli J, Prevost G, Bourguet W, Moulinier L, Chevrier B, Delagoutte B, Bilwes A, Mourey L, Rifai S, Piemont Y, Moras D. The structure of Staphylococcus aureus epidermolytic toxin A, an atypic serine protease, at 1.7Å resolution. Structure. 1997;5:813–824. doi: 10.1016/s0969-2126(97)00235-9. [DOI] [PubMed] [Google Scholar]
- 45.Chapes S, Hoynowski S M, Woods K M, Armstrong J W, Beharka A A, Iandolo J J. Staphylococcus-mediated T-cell activation and spontaneous natural killer cell activity in the absence of major histocompatibility complex class II molecules. Infect Immun. 1994;61:4013–4016. doi: 10.1128/iai.61.9.4013-4016.1993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Choi Y, Kotzin B, Herron L, Callahan J, Marrack P, Kappler J. Interaction of Staphylococcus aureus toxin superantigens with human T cells. Proc Natl Acad Sci USA. 1990;86:8941–8945. doi: 10.1073/pnas.86.22.8941. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Corner B D, Crowther S T, Eades S M. Control of staphylococcal infection in a maternity hospital. Br Med J. 1960;1:1927–1929. doi: 10.1136/bmj.1.5190.1927. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Cribier B, Piemont Y, Grosshans E. Staphylococcal scalded skin syndrome in adults: a clinical review illustrated with a case. J Am Acad Dermatol. 1984;30:319–324. doi: 10.1016/s0190-9622(94)70032-x. [DOI] [PubMed] [Google Scholar]
- 49.Curran J P, Al-Salihi F L. Neonatal staphylococcal scalded skin syndrome: massive outbreak due to an unusual phage type. Pediatrics. 1980;66:285–290. [PubMed] [Google Scholar]
- 50.Dajani A S. The scalded skin syndrome: relation to phage group II staphylococci. J Infect Dis. 1972;125:548. doi: 10.1093/infdis/125.5.548. [DOI] [PubMed] [Google Scholar]
- 51.Dancer S J, Simmons N A, Poston S M, Noble W C. Outbreak of staphylococcal scalded skin syndrome among neonates. J Infect. 1988;16:87–103. doi: 10.1016/s0163-4453(88)96249-4. [DOI] [PubMed] [Google Scholar]
- 52.Dancer S J, Poston S M, East J, Simmons N A, Noble W C. An outbreak of pemphigus neonatorum. J Infect. 1990;20:73–82. doi: 10.1016/s0163-4453(90)92434-m. [DOI] [PubMed] [Google Scholar]
- 53.Dancer S J, Garratt R, Saldanha J, Jhoti H, Evans R. The epidermolytic toxins are serine proteases. FEBS Lett. 1990;268:129–132. doi: 10.1016/0014-5793(90)80990-z. [DOI] [PubMed] [Google Scholar]
- 54.Dancer S J, Noble W C. Nasal, axillary, and perineal carriage of Staphylococcus aureus among women: identification of strains producing epidermolytic toxin. J Clin Pathol. 1991;44:681–684. doi: 10.1136/jcp.44.8.681. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Dave J, Reith S, Nash J Q, Marples R R, Dulake C. A double outbreak of exfoliative-toxin producing strains of Staphylococcus aureus in a maternity unit. Epidemiol Infect. 1994;112:103–114. doi: 10.1017/s0950268800057460. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.De Azavedo A, Arbuthnott J P. Prevalence of epidermolytic toxin in clinical isolates of Staphylococcus aureus. J Med Microbiol. 1981;14:341–344. doi: 10.1099/00222615-14-3-341. [DOI] [PubMed] [Google Scholar]
- 57.De Azavedo A, Arbuthnott J P. Assays for epidermolytic toxins of Staphylococcus aureus. Methods Enzymol. 1988;165:333–337. doi: 10.1016/s0076-6879(88)65049-x. [DOI] [PubMed] [Google Scholar]
- 58.Dedobbeleer G, Achten G. Staphylococcal scalded skin syndrome. An ultrastructural study. J Cutaneous Pathol. 1975;2:91–96. doi: 10.1111/j.1600-0560.1975.tb00212.x. [DOI] [PubMed] [Google Scholar]
- 59.Dellabona P, Peccoud J, Kappler J, Marrack P, Benoist C, Mathis D. Superantigens interact with MHC class II molecules outside of the antigen groove. Cell. 1990;62:1115–1121. doi: 10.1016/0092-8674(90)90388-u. [DOI] [PubMed] [Google Scholar]
- 60.Dillon H C. Impetigo contagiosa: suppurative and non-suppurative complications. Am J Dis Child. 1968;115:530–541. doi: 10.1001/archpedi.1968.02100010532002. [DOI] [PubMed] [Google Scholar]
- 61.Dillon H C. Topical and systemic therapy for pyodermas. J Dermatol. 1980;19:443–451. doi: 10.1111/j.1365-4362.1980.tb05895.x. [DOI] [PubMed] [Google Scholar]
- 62.Dimond R L, Wolff H H, Braun-Fulco O. The staphylococcal scalded skin syndrome. An experimental histochemical and electron microscopic study. Br J Dermatol. 1977;96:483–492. doi: 10.1111/j.1365-2133.1977.tb07150.x. [DOI] [PubMed] [Google Scholar]
- 63.Donahus D, Robinson B, Goldberg N S. Staphylococcal scalded skin syndrome in a woman with chronic renal failure exposed to human immunodeficiency virus. CUTIS. 1991;47:317. [PubMed] [Google Scholar]
- 64.Drapeau G R. The primary structure of Staphylococcus aureus. Can J Biochem. 1978;56:534–544. doi: 10.1139/o78-082. [DOI] [PubMed] [Google Scholar]
- 65.Easterley N B, Solomon L M. Neonatal dermatology II: blistering and scaling dermatoses. J Paediatr. 1970;77:1075–1088. doi: 10.1016/s0022-3476(70)80100-7. [DOI] [PubMed] [Google Scholar]
- 66.Elbaum D J, Wood C, Abuabara F, Morhenn V B. Bullae in a patient with toxic shock syndrome. J Am Acad Dermatol. 1984;10:267–272. doi: 10.1016/s0190-9622(84)70035-1. [DOI] [PubMed] [Google Scholar]
- 67.Elias P M, Arndt K A, Peck G C. Scalded skin syndrome in adults. N Engl J Med. 1973;288:582. doi: 10.1056/nejm197303152881117. [DOI] [PubMed] [Google Scholar]
- 68.Elias P M, Fritsch P, Dahl M V, Wolff K. Staphylococcal toxic epidermal necrolysis: pathogenesis and studies on the subcellular site of action of exfoliatin. J Investig Dermatol. 1975;65:501–512. doi: 10.1111/1523-1747.ep12610196. [DOI] [PubMed] [Google Scholar]
- 69.Elias P M, Fritsch P, Mittermayer H. Staphylococcal toxic epidermal necrolysis: species and tissue susceptibility and resistance. J Investig Dermatol. 1976;66:80. doi: 10.1111/1523-1747.ep12481412. [DOI] [PubMed] [Google Scholar]
- 70.Elias P M, Fritsch P, Epstein E H., Jr Staphylococcal scalded skin syndrome. Arch Dermatol. 1977;113:207–219. doi: 10.1001/archderm.113.2.207. [DOI] [PubMed] [Google Scholar]
- 71.Elsner P, Hartman A A. Epidemiology of ETA- and ETB-producing staphylococci in dermatological patients. Zentbl Bacteriol Mikrobiol Hyg Ser A. 1988;268:534. [Google Scholar]
- 72.Falk D K, King L E. Criteria for the diagnosis of staphylococcal scalded skin syndrome in adults. CUTIS. 1983;31:421–424. [PubMed] [Google Scholar]
- 73.Feder H M, Hoss D M, Dimond R L. Toxic epidermal necrolysis. N Engl J Med. 1996;334:922–923. doi: 10.1056/NEJM199604043341414. [DOI] [PubMed] [Google Scholar]
- 74.Feldman C A. Staphylococcal scarlet fever. N Engl J Med. 1962;267:877–878. doi: 10.1056/NEJM196210252671709. [DOI] [PubMed] [Google Scholar]
- 75.Feldman S R. Bullous dermatoses associated with systemic disease. Dermatol Clin. 1993;11:597–609. [PubMed] [Google Scholar]
- 76.Ferscht A R. Enzyme structure and mechanism. 2nd ed. New York, N.Y: W. H. Freeman & Co.; 1985. [Google Scholar]
- 77.Fields B A, Malchiodi E L, Li H, Ysern X, Stauffacher C V, Schlievert P M. Crystal structure of a T-cell receptor beta-chain complexed with a superantigen. Nature. 1996;384:188–192. doi: 10.1038/384188a0. [DOI] [PubMed] [Google Scholar]
- 78.Finch R. Skin and soft-tissue infections. Lancet. 1988;i:164–168. doi: 10.1016/s0140-6736(88)92730-4. [DOI] [PubMed] [Google Scholar]
- 79.Finch R G, Whitby M. Toxic-shock syndrome. J R Coll Physicians London. 1985;19:219. [PMC free article] [PubMed] [Google Scholar]
- 80.Fleischer B, Schrezenmeier H. T cell stimulation by staphylococcal enterotoxins: clonally variable response and requirement for major histocompatibility complex class II molecules on accessory or target cells. J Exp Med. 1988;167:1697–1707. doi: 10.1084/jem.167.5.1697. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Fleischer B, Schrezenmeier H, Conradt P. T lymphocyte activation by staphylococcal enterotoxins: role of class II molecules and T cell surface structures. Cell Immunol. 1989;120:92–101. doi: 10.1016/0008-8749(89)90177-9. [DOI] [PubMed] [Google Scholar]
- 82.Fleischer B, Bailey C J. Recombinant epidermolytic (exfoliative) toxin A of Staphylococcus aureus is not a superantigen. Med Microbiol Immunol. 1992;180:273–278. doi: 10.1007/BF00191548. [DOI] [PubMed] [Google Scholar]
- 83.Fleischer B, Gerlach D, Fuhrmann A, Schmidt K H. Superantigens and pseudosuperantigens of gram-positive cocci. Med Microbiol Immunol. 1995;184:1–8. doi: 10.1007/BF00216783. [DOI] [PubMed] [Google Scholar]
- 84.Fleming S D, Iandolo J J, Chapes S K. Murine macrophage activation by staphylococcal exotoxins. Infect Immun. 1991;59:4049–4055. doi: 10.1128/iai.59.11.4049-4055.1991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Fleurette J, Ritter J. Prevalence des souches de Staphylococcus aureus productrices d’exfoliatine dans le groupe bacteriopagique II. Ann Microbiol. 1980;1131B(2):175–183. [PubMed] [Google Scholar]
- 86.Fligner C L, Jack R, Twiggs G A, Raisys V A. Hyperosmolality induced by propylene glycol—a complication of silver sulfadiazine therapy. JAMA. 1985;253:1606. [PubMed] [Google Scholar]
- 87.Forfar J O, Bale C L, Elias-Jones T F. Staphylococcal infection of the newborn. Br Med J. 1953;2:170–174. doi: 10.1136/bmj.2.4829.170. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Forsgren A. Significance of protein A production by staphylococci. Infect Immun. 1970;2:672–673. doi: 10.1128/iai.2.5.672-673.1970. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.Forsgren A, Nordstrom L, Phillipson L, Szoquist B. Protein A mutants of Staphylococcus aureus. J Bacteriol. 1971;107:245–250. doi: 10.1128/jb.107.1.245-250.1971. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90.Freedman J D, Beers D J. Expanding perspectives on the toxic shock syndrome. Adv Intern Med. 1991;36:363. [PubMed] [Google Scholar]
- 91.Freer J, Arbuthnott J P. Toxins of Staphylococcus aureus. Pharmacol Ther. 1983;19:55–106. doi: 10.1016/0163-7258(82)90042-0. [DOI] [PubMed] [Google Scholar]
- 92.Freinkel R K, Traczyk T N. A method for partial purification of lamellar granules from fetal rat epidermis. J Investig Dermatol. 1981;77:478–482. doi: 10.1111/1523-1747.ep12497735. [DOI] [PubMed] [Google Scholar]
- 93.Fritsch P. Staphylogene toxische epidermale Nekrolyse. Z Haut-Geschlechtstkr. 1975;50:5. [Google Scholar]
- 94.Fritsch P, Elias P, Varga J. The fate of staphylococcal exfoliatin in newborn and adult mice. Br J Dermatol. 1976;95:275–284. doi: 10.1111/j.1365-2133.1976.tb07015.x. [DOI] [PubMed] [Google Scholar]
- 95.Furue M, So K, Ogasa U, Sugiyama H, Ohtake N, Sakurai S, Tamaki K. Outbreak of staphylococcal scalded skin syndrome evaluated by DNA fingerprinting. Lancet. 1995;345:1308. doi: 10.1016/s0140-6736(95)90957-5. [DOI] [PubMed] [Google Scholar]
- 96.Gemmell C G. Staphylococcal scalded skin syndrome. J Med Microbiol. 1995;43:318–327. doi: 10.1099/00222615-43-5-318. [DOI] [PubMed] [Google Scholar]
- 97.Gentilhomme E, Faure M, Piemont Y, Binder P, Thivolet J. Action of staphylococcal exfoliative toxins on epidermal cell cultures and organotypic skin. J Dermatol. 1990;17:526–532. doi: 10.1111/j.1346-8138.1990.tb01689.x. [DOI] [PubMed] [Google Scholar]
- 98.Gershkovich A A. Inducing conformational changes in thrombin by ligands as a way of regulating its activity. Biochemistry (Moscow) 1996;61:817–824. . (In Russian.) [PubMed] [Google Scholar]
- 99.Gershkovich A A, Kara D I, Kibirev V K. Synthesis and study of a novel inhibitor of thrombin containing no side chain at the P1 position of the polypeptide chain. Semin Thromb Hemostasis. 1996;22:343–345. doi: 10.1055/s-2007-999029. . (In Russian.) [DOI] [PubMed] [Google Scholar]
- 100.Ginsburg C M. Staphylococcal toxin syndromes. Pediatr Infect Dis J. 1991;10:319–321. [PubMed] [Google Scholar]
- 101.Goldberg N S, Ahmed T, Robinson B, Ascensao H, Horwitz H. Staphylococcal scalded skin syndrome mimicking acute graft-versus-host disease in a bone marrow transplant recipient. Arch Dermatol. 1989;15:385–389. [PubMed] [Google Scholar]
- 102.Grayson S, Johnson-Winegar A W, Wintroub B U, Isserott R R, Epstein E H, Elias P M. Lamellar body-enriched fractions from neonatal mice: preparative techniques and partial characterisation. J Investig Dermatol. 1984;85:289–299. doi: 10.1111/1523-1747.ep12276826. [DOI] [PubMed] [Google Scholar]
- 103.Hardwick N, Parry C M, Sharpe G R. Staphylococcal scalded skin syndrome in an adult: influence of immune and renal factors. Br J Dermatol. 1995;132:468–471. doi: 10.1111/j.1365-2133.1995.tb08686.x. [DOI] [PubMed] [Google Scholar]
- 104.Hardyment A F. Control of infections of newborn infants. Pediatr Clin North Am. 1958;5:287–300. doi: 10.1016/s0031-3955(16)30650-2. [DOI] [PubMed] [Google Scholar]
- 105.Hargiss C, Larson E. The epidemiology of Staphylococcus aureus in a newborn nursery from 1970 through 1976. Pediatrics (Springfield) 1978;61:348–353. [PubMed] [Google Scholar]
- 106.Herman A, Croteau G, Sekaley R P, Kappler J, Marrack P. HLA-DR alleles differ in their ability to present staphylococcal enterotoxins to T cells. J Exp Med. 1990;172:709–717. doi: 10.1084/jem.172.3.709. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 107.Hermann T, Acolla R S, MacDonald H R. Different staphylococcal enterotoxins bind preferentially to distinct MHC class II isotypes. Eur J Immunol. 1989;19:2171–2174. doi: 10.1002/eji.1830191131. [DOI] [PubMed] [Google Scholar]
- 108.Hoegger P H, Elsner P. Transmission of exfoliatin-producing Staphylococcus aureus by an asymptomatic carrier. Pediatr Infect Dis J. 1988;7:344. [PubMed] [Google Scholar]
- 109.Hoffmann M L, Jablanski L M, Crum K K, Hackett S P, Chi Y L, Stauffacher C V, Stevens D L, Bohach G A. Predictions of T-cell receptor- and major histocompatibility complex-binding sites on staphylococcal enterotoxin C1. Infect Immun. 1994;62:3396–3407. doi: 10.1128/iai.62.8.3396-3407.1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 110.Hoffmann R, Lohner M, Bohm N, Schaefer H E, Letitis J. Staphylococcal scalded skin syndrome and consecutive septicaemia in a preterm infant. Pathol Res Pract. 1994;190:77–81. doi: 10.1016/S0344-0338(11)80499-1. [DOI] [PubMed] [Google Scholar]
- 111.Hurwitz R M, Ackerman A B. Cutaneous pathology of the toxic shock syndrome. Am J Dermatopathol. 1985;7:563. doi: 10.1097/00000372-198512000-00011. [DOI] [PubMed] [Google Scholar]
- 112.Iandolo J J. Genetic analysis of extracellular toxins of Staphylococcus aureus. Annu Rev Microbiol. 1989;43:375–402. doi: 10.1146/annurev.mi.43.100189.002111. [DOI] [PubMed] [Google Scholar]
- 113.Inaoki M. Experimental studies on the mechanism of action of staphylococcal epidermolytic toxin A utilizing recombinant toxin. Nippon Hifuka Gakkai Zasshi. 1990;100:1405–1414. . (In Japanese.) [PubMed] [Google Scholar]
- 114.Itani O, Crump R, Minoumi F, Tunnessen W W. Picture of the month: staphylococcal scalded skin syndrome. Am J Dis Child. 1992;146:424–425. [PubMed] [Google Scholar]
- 115.Jackow C M, Cather J C, Hearne V, Asano A T, Musser J M, Duvic M. Association of erythrodermic cutaneous T-cell lymphoma, superantigen-positive Staphylococcus aureus, and oligoclonal T-cell receptor V beta gene expansion. Blood. 1997;89:32–40. [PubMed] [Google Scholar]
- 116.Jackson M P, Iandolo J J. Cloning and expression of exfoliative toxin B gene from Staphylococcus aureus. J Bacteriol. 1986;166:574–580. doi: 10.1128/jb.166.2.574-580.1986. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 117.Jakeman K J, Rushton D I, Smith H, Sweet C. Exacerbation of bacterial toxicity to infant ferrets by influenza virus: possible role in sudden infant death syndrome. J Infect Dis. 1991;163:35–40. doi: 10.1093/infdis/163.1.35. [DOI] [PubMed] [Google Scholar]
- 118.Jaulhac B, Piemont Y, Prevost G. Staphylococcal exfoliative toxins induce proteolytic activity when combined with epidermis. In: Mollby R, Flock J I, Nord C E, Christensson B, editors. Staphylococci and staphylococcal infections. Proceedings of the 7th International Symposium. Stuttgart, Germany: Gustav Fischer Verlag; 1994. pp. 280–282. [Google Scholar]
- 119.Jernigan J A, Titus M G, Groschel D H, Getchell-White S, Farr B M. Effectiveness of contact isolation during a hospital outbreak of methicillin-resistant Staphylococcus aureus. Am J Epidemiol. 1996;143:496–504. doi: 10.1093/oxfordjournals.aje.a008770. [DOI] [PubMed] [Google Scholar]
- 120.Johnson W M, Tyler S D, Ewan E P, Ashton F E, Pollard D R, Rosee K R. Detection of genes for enterotoxins, exfoliative toxins, and toxic shock syndrome toxin-1 in Staphylococcus aureus by the polymerase chain reaction. J Clin Microbiol. 1991;29:426–430. doi: 10.1128/jcm.29.3.426-430.1991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 121.Jones L D. Observation of exudative epidermitis. Vet Med. 1961;56:95–103. [Google Scholar]
- 122.Kallman J, Kihlstrom E, Sjoberg L, Schollin J. Increase in staphylococci in neonatal septicaemia: a fourteen-year study. Acta Paediatr. 1997;86:533–538. doi: 10.1111/j.1651-2227.1997.tb08926.x. [DOI] [PubMed] [Google Scholar]
- 123.Kaplan M H, Chmel H, Hseih H, Staphens A, Brinsko V. Importance of exfoliative toxin A producing Staphylococcus aureus strains isolated from clustered epidemics of neonatal pustulosis. J Clin Microbiol. 1986;23:83–91. doi: 10.1128/jcm.23.1.83-91.1986. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 124.Kappler J, Kotzin B, Herron L, Gelfand E W, Bigler R D, Boylston A, Carrel S, Posnett D N, Choi Y. V beta-specific stimulation of human T cells by staphylococcal toxins. Science. 1989;244:811–813. doi: 10.1126/science.2524876. [DOI] [PubMed] [Google Scholar]
- 125.Kapral F A, Miller M M. Product of Staphylococcus aureus responsible for the scalded skin syndrome. Infect Immun. 1971;4:541–545. doi: 10.1128/iai.4.5.541-545.1971. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 126.Kapral F A, Miller M M. Skin lesions produced by Staphylococcus aureus exfoliatin in hairless mice. Infect Immun. 1972;6:877–879. doi: 10.1128/iai.6.5.877-879.1972. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 127.Kapral F A. Staphylococcus aureus: some host-parasite interactions. Ann N Y Acad Sci USA. 1974;236:267–275. doi: 10.1111/j.1749-6632.1974.tb41497.x. [DOI] [PubMed] [Google Scholar]
- 128.Kapral F A. Staphylococcus exfoliatin. In: Schlessinger D, editor. Microbiology—1975. Washington, D.C: American Society for Microbiology; 1975. pp. 263–266. [Google Scholar]
- 129.Khuong M A, Chosidow O, El-Solh N, Wechsler J, Piemont Y, Roujeau J C, Revuz J. Staphylococcal scalded skin syndrome in an adult: possible influence of non-steroidal anti-inflammatory drugs. Dermatologica. 1993;186:153–154. doi: 10.1159/000247328. [DOI] [PubMed] [Google Scholar]
- 130.Kim J L, Morgenstern K A, Lin C, Fox T, Dwyer M D, Landro J A, Chambers S P, Markland W, Lepre C A, O’Malley E T, Harbeson S L, Rice C M, Murcko M A, Caron P R, Thomson J A. Crystal structure of the hepatitis C virus NS3 protease domain complexed with a synthetic NS4A cofactor peptide. Cell. 1996;87:343–355. doi: 10.1016/s0092-8674(00)81351-3. [DOI] [PubMed] [Google Scholar]
- 131.Kondo I, Sakurai S, Sarai Y. Purification of exfoliatin produced by Staphylococcus aureus of bacteriophage group 2 and its physico-chemical properties. Infect Immun. 1975;8:156–164. doi: 10.1128/iai.8.2.156-164.1973. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 132.Kondo I, Sakurai S, Sarai Y. New type of exfoliatin obtained from staphylococcal strains belonging to phage groups other than group II, isolated from patients with impetigo and Ritter’s disease. Infect Immun. 1974;10:851–861. doi: 10.1128/iai.10.4.851-861.1974. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 133.Kondo I, Sakurai S, Sarai Y, Fukati S. Two serotypes of exfoliatin and their distribution in staphylococcal strains isolated from patients with scalded skin syndrome. J Clin Microbiol. 1975;1:397–400. doi: 10.1128/jcm.1.5.397-400.1975. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 134.Kondo I. Staphylococcal-exfoliatin A and B. In: Kato I, editor. Saikin dokuso kenkyu. Tokyo, Japan: Kyouritsu Press; 1976. pp. 82–95. [Google Scholar]
- 135.Korting H C. Critical commentary to staphylococcal scalded skin syndrome and consecutive septicaemia in a preterm infant. Pathol Res Pract. 1994;190:82–83. doi: 10.1016/S0344-0338(11)80499-1. [DOI] [PubMed] [Google Scholar]
- 136.Kotb M, Majumdar G, Tomai M, Beachey E H. Accessory cell-independent stimulation of human T cells by streptococcal M protein superantigen. J Immunol. 1990;145:1332–1336. [PubMed] [Google Scholar]
- 137.Kotb M. Bacterial pyrogenic exotoxins as superantigens. Clin Microbiol Rev. 1995;8:411–426. doi: 10.1128/cmr.8.3.411. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 138.Koyama A, Kobayashi M, Yamaguchi N, Yamagata K, Takano K, Nakajima M, Irie F, Goto M, Iragashi M, Litsuka I. Glomerulonephritis associated with MRSA infection: a possible role of bacterial superantigen. Kidney Int. 1995;47:207–216. doi: 10.1038/ki.1995.25. [DOI] [PubMed] [Google Scholar]
- 139.Ladhani S, Joannou C, Cameron J, Poston S, Garrett R, Robbie S, Evans R W. Proceedings of the Eighth European Conference on Bacterial Protein Toxins. Stuttgart, Germany: Gustav Fischer Verlag; 1998. The epidermolytic toxins in the staphylococcal scalded skin syndrome; pp. 81–82. [Google Scholar]
- 140.Ladhani S, Evans R W. Staphylococcal scalded skin syndrome. Arch Dis Child. 1998;78:85–88. doi: 10.1136/adc.78.1.85. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 141.Lautenschlager S, Herzog C, Zimmerli W. Course and outcome of bacteraemia due to Staphylococcus aureus: evaluation of different clinical case definitions. Clin Infect Dis. 1993;16:567–573. doi: 10.1093/clind/16.4.567. [DOI] [PubMed] [Google Scholar]
- 142.Lawrence C R. Effect of two different methods of umbilical cord care on its separation time. Midwives Chron Nursing Notes. 1982;95:204–205. [PubMed] [Google Scholar]
- 143.Lawrence J, Hodtsev A S, Posnett D N. Superantigen implicated in dependence of HIV-1 replication in T cells on TCR Vβ expression. Nature. 1992;358:255–259. doi: 10.1038/358255a0. [DOI] [PubMed] [Google Scholar]
- 144.L’Ecuyer C, Jercho K. Exudative epidermitis in pigs: etiological studies and pathology. Can J Comp Med Vet Sci. 1966;30:94–101. [PMC free article] [PubMed] [Google Scholar]
- 145.Lee C Y, Schmidt J J, Johnson-Winger A D, Spero L, Iandolo J J. Sequence determination and comparison of exfoliative toxin A and toxin B genes from Staphylococcus aureus. J Bacteriol. 1987;169:3904–3909. doi: 10.1128/jb.169.9.3904-3909.1987. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 146.Lee S, Barson A J, Drucker D B, Morris J A, Telford D R. Lethal challenges of gnotobiotic weanling rats with bacterial isolates from cases of sudden infant death syndrome (SIDS) J Clin Pathol. 1987;40:1393–1396. doi: 10.1136/jcp.40.12.1393. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 147.Leung D Y, Harbeck R, Bina P, Reiser R F, Yang E, Norris D F, Hanifin J M, Scampson H A. Presence of IgE antibodies to staphylococcal exotoxins on the skin of patients with atopic dermatitis: evidence for a new group of allergens. J Clin Investig. 1993;92:1374–1380. doi: 10.1172/JCI116711. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 148.Leung D Y, Meissner C, Fulton D, Schlievert P M. The potential role of bacterial superantigens in the pathogenesis of Kawasaki syndrome. J Clin Immunol. 1995;15(Suppl. 6):11S–17S. doi: 10.1007/BF01540888. [DOI] [PubMed] [Google Scholar]
- 149.Leung D Y, Gately M, Trumble A, Ferguson-Darnell B, Schlievert P M, Picker L J. Bacterial superantigens induce T cell expression of the skin-selective homing receptor, the cutaneous lymphocyte-associated antigen, via stimulation of interleukin 12 production. J Exp Med. 1995;181:747–753. doi: 10.1084/jem.181.2.747. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 150.Lever R, Hadley K, Downey D, Mackie R. Staphylococcal colonisation in atopic dermatitis and the effect of topical mupirocin therapy. Br J Dermatol. 1988;119:189–198. doi: 10.1111/j.1365-2133.1988.tb03201.x. [DOI] [PubMed] [Google Scholar]
- 151.Lewis H M, Baker B S, Bokth S, Powles A V, Garioch J J, Valdimarsson H, Fry L. Restricted T-cell receptor Vβ gene usage in the skin of patients with guttate and chronic plaque psoriasis. Br J Dermatol. 1993;129:514–520. doi: 10.1111/j.1365-2133.1993.tb00477.x. [DOI] [PubMed] [Google Scholar]
- 152.Leyden J J, Marples H I, Kligman A M. Staphylococcus aureus in the lesions of atopic dermatitis. Br J Dermatol. 1974;90:525–530. doi: 10.1111/j.1365-2133.1974.tb06447.x. [DOI] [PubMed] [Google Scholar]
- 153.Lillibridge C D, Melish M E, Glasgow L A. Site of action of exfoliative toxin in the staphylococcal scalded skin syndrome. Pediatrics. 1972;50:728–738. [PubMed] [Google Scholar]
- 154.Lina G, Gillet Y, Vandenesch F, Jones M E, Floret D, Etienne J. Toxin involvement in staphylococcal scalded skin syndrome. Clin Infect Dis. 1997;25:1369–1373. doi: 10.1086/516129. [DOI] [PubMed] [Google Scholar]
- 155.Lingnau W, Allerberger F. Control of an outbreak of methicillin-resistant Staphylococcus aureus (MRSA) by hygienic measures in a general intensive care unit. Infect. 1994;22(Suppl. 2):S135–S139. doi: 10.1007/BF01793578. [DOI] [PubMed] [Google Scholar]
- 156.Lockhart B P, Bailey C J. ELISA estimation of the binding of the epidermal toxin to neonatal mouse skin. Toxicon. 1993;31:569–576. doi: 10.1016/0041-0101(93)90112-v. [DOI] [PubMed] [Google Scholar]
- 157.Loughead J L. Congenital staphylococcal scalded skin syndrome: report of a case. Pediatr Infect Dis J. 1992;10:319–321. [PubMed] [Google Scholar]
- 158.Lowney E D, Baublis J V, Kreye G M, Harrell E R, McKenzie A R. The scalded skin syndrome in small children. Arch Dermatol. 1967;95:359–369. [PubMed] [Google Scholar]
- 159.Lyell A. Toxic epidermal necrolysis: an eruption resembling scalding of the skin. Br J Dermatol. 1956;68:355–361. doi: 10.1111/j.1365-2133.1956.tb12766.x. [DOI] [PubMed] [Google Scholar]
- 160.Lyell A. A review of toxic epidermal necrolysis in Britain. Br J Dermatol. 1967;79:662–671. doi: 10.1111/j.1365-2133.1967.tb11434.x. [DOI] [PubMed] [Google Scholar]
- 161.Lyell A, Dick H M, Alexander J O. Outbreak of toxic epidermal necrolysis associated with staphylococci. Lancet. 1969;i:787–789. doi: 10.1016/s0140-6736(69)92061-3. [DOI] [PubMed] [Google Scholar]
- 162.Lyell A. Toxic epidermal necrolysis (the scalded skin syndrome): a reappraisal. Br J Dermatol. 1979;100:69–86. doi: 10.1111/j.1365-2133.1979.tb03571.x. [DOI] [PubMed] [Google Scholar]
- 163.Machida K. Immunological investigations on pathogenesis of staphylococcal scalded skin syndrome. J Clin Pathol. 1995;43:547–556. . (In Japanese.) [PubMed] [Google Scholar]
- 164.Marples R R, Heaton C L, Kligman A M. Staphylococcus aureus in psoriasis. Arch Dermatol. 1973;107:568–570. [PubMed] [Google Scholar]
- 165.Marrack P, Kappler J. The staphylococcal enterotoxins and their relatives. Science. 1990;248:705–711. doi: 10.1126/science.2185544. [DOI] [PubMed] [Google Scholar]
- 166.McCallum H M. Action of staphylococcal epidermolytic toxin on mouse skin in organ culture. Br J Dermatol. 1972;86(Suppl. 8):40–41. doi: 10.1111/j.1365-2133.1972.tb01607.x. [DOI] [PubMed] [Google Scholar]
- 167.McFadden J P, Noble W C, Camp R D R. Superantigenic exotoxin-secreting potential of staphylococci isolated from atopic eczematous skin. Br J Dermatol. 1993;128:631–632. doi: 10.1111/j.1365-2133.1993.tb00257.x. [DOI] [PubMed] [Google Scholar]
- 168.Meeberg A, Schoyen R. Bacterial colonisation and neonatal infections. Acta Paediatr Scand. 1985;74:366–371. doi: 10.1111/j.1651-2227.1985.tb10985.x. [DOI] [PubMed] [Google Scholar]
- 169.Mehtar S. The continuing problem of “Hospital Staphylococci”: why? J Chemother. 1994;6(Suppl. 4):25–40. [PubMed] [Google Scholar]
- 170.Melish M E, Glasgow L A. The staphylococcal scalded skin syndrome: development of an experimental model. N Engl J Med. 1970;282:1114–1119. doi: 10.1056/NEJM197005142822002. [DOI] [PubMed] [Google Scholar]
- 171.Melish M E, Glasgow L A, Turner M D. The staphylococcal scalded skin syndrome: isolation and partial characterisation of the exfoliative toxin. Br J Dermatol. 1972;125:129–140. doi: 10.1093/infdis/125.2.129. [DOI] [PubMed] [Google Scholar]
- 172.Melish M E, Glasgow L A, Turner M D, Lillibridge C B. The staphylococcal epidermolytic toxin: its isolation, characterization and site of action. Ann N Y Acad Sci. 1974;236:317–342. doi: 10.1111/j.1749-6632.1974.tb41501.x. [DOI] [PubMed] [Google Scholar]
- 173.Melish M E, Chen F S, Sprouse S, Stuckey M, Murata M S. Epidermolytic toxin Staphylococcal infection: toxin levels and host response. In: Jeljaszewicz J, editor. Staphylococci and staphylococcal infections. Stuttgart, Germany: Gustav Fischer Verlag; 1981. pp. 287–298. [Google Scholar]
- 174.Melish M E. Staphylococci, streptococci and the skin: review of impetigo and staphylococcal scalded skin syndrome. Semin Dermatol. 1982;1:101–109. [Google Scholar]
- 175.Melish M E. Kawasaki syndrome: a 1992 update. Pediatr Dermatol. 1992;9:335–337. doi: 10.1111/j.1525-1470.1992.tb00620.x. [DOI] [PubMed] [Google Scholar]
- 176.Millan S J, Baldwin J N, Rhein M S, Weiser H H. Studies on the incidence of coagulase positive staphylococci in a normal unconfined population. Am J Public Health. 1960;50:791–798. doi: 10.2105/ajph.50.6_pt_1.791. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 177.Mirabile R, Wiser M, Barot L R, Brown A S. Staphylococcal scalded skin syndrome. Plast Reconstr Surg. 1986;77:752–756. doi: 10.1097/00006534-198605000-00009. [DOI] [PubMed] [Google Scholar]
- 178.Miwa K, Fukuyama M, Kunitomo T, Igarashi H. Rapid assay for detection of toxic shock syndrome toxin-1 from human sera. J Clin Microbiol. 1994;32:539–542. doi: 10.1128/jcm.32.2.539-542.1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 179.Miyake Y, Iwai T, Sugai M, Miura K, Suginaka H, Nagasaka N. Incidence and characterisation of Staphylococcus aureus from the tongues of children. J Dent Res. 1991;70:1045–1047. doi: 10.1177/00220345910700070501. [DOI] [PubMed] [Google Scholar]
- 180.Moras D, Thierry J-C, Cavarelli J, Piemont Y. Preliminary crystallographic data for exfoliative toxin B from Staphylococcus aureus. J Mol Biol. 1984;175:89–91. doi: 10.1016/0022-2836(84)90448-0. [DOI] [PubMed] [Google Scholar]
- 181.Morlock B A, Spero L, Johnson A D. Mitogenic activity of staphylococcal exfoliative toxin. Infect Immun. 1980;30:381–384. doi: 10.1128/iai.30.2.381-384.1980. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 182.Morris J A, Haran D, Smith A. Hypothesis: common bacterial toxins are a possible cause of sudden infant death syndrome. Med Hypotheses. 1987;22:211–222. doi: 10.1016/0306-9877(87)90145-9. [DOI] [PubMed] [Google Scholar]
- 183.Mortimer E A, Wolinsky E, Rammelkamp C H. The transmission of staphylococci by the hands of personnel. In: Maibach H I, Hildick-Smith G, editors. Skin bacteria and their role in infection. New York, N.Y: McGraw-Hill Book Co.; 1965. pp. 46–61. [Google Scholar]
- 184.Mourad W, Al-Daccak R, Chatila T, Geha R S. Staphylococcal superantigens as inducers of signal transduction in MHC class-II-positive cells. Semin Immunol. 1993;5:47–55. doi: 10.1006/smim.1993.1007. [DOI] [PubMed] [Google Scholar]
- 185.Mulligan M E, Murray-Leisure K A, Ribner B S, Standiford H C, John H F, Korvick J A, Kauffman C A, Yu V L. Methicillin-resistant Staphylococcus aureus: a consensus review of the microbiology, pathogenesis, and epidemiology with implications for prevention and treatment. Am J Med. 1993;94:313–328. doi: 10.1016/0002-9343(93)90063-u. [DOI] [PubMed] [Google Scholar]
- 186.Murono K, Fujita K, Yoshioka H. Microbiological characteristics of exfoliative toxin-producing Staphylococcus aureus. Pediatr Infect Dis J. 1988;7:313–315. doi: 10.1097/00006454-198805000-00003. [DOI] [PubMed] [Google Scholar]
- 187.Murono K, Fujita K, Yoshioka H. Detection of staphylococcal exfoliative toxin by slide latex hemagglutination. J Clin Microbiol. 1988;26:271–274. doi: 10.1128/jcm.26.2.271-274.1988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 188.Murray D L, Earhart C A, Mitchell D T, Ohlandorf D H, Novick R P, Schlievert P M. Localization of biologically important regions on toxic-shock syndrome toxin 1. Infect Immun. 1996;64:371–374. doi: 10.1128/iai.64.1.371-374.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 189.Newbould M J, Malam J, McIllmurray J M, Morris J A, Telford D R, Barson A J. Immunohistological localisation of staphylococcal toxic shock syndrome toxin (TSST-1) antigen in sudden infant death syndrome. J Clin Pathol. 1989;42:935–939. doi: 10.1136/jcp.42.9.935. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 190.Nilsson E, Henning C, Hjorleifsson M. Density of the microflora in hand eczema before and after topical treatment with a potent corticosteroid. J Am Acad Dermatol. 1986;15:192–197. doi: 10.1016/s0190-9622(86)70155-2. [DOI] [PubMed] [Google Scholar]
- 191.Noble W C. The micrococci. London, United Kingdom: Lloyd-Luke; 1981. pp. 152–181. [Google Scholar]
- 192.Notermans S, Timmermans P, Nagel J. Interaction of staphylococcal protein A in enzyme-linked immunosorbent assays for detecting staphylococcal antigens. J Immunol Methods. 1982;55:35–41. doi: 10.1016/0022-1759(82)90074-6. [DOI] [PubMed] [Google Scholar]
- 193.Ochsendorf F R, Schofer H, Milbradt R. Diagnostik des Lyell syndroms: SSSS oder TEN? Dtsch Med Wochenschr. 1988;133:860–863. doi: 10.1055/s-2008-1067735. [DOI] [PubMed] [Google Scholar]
- 194.Opal S M, Johnson-Winegar A D, Cross A S. Staphylococcal scalded skin syndrome in two immunocompetent adults caused by exfoliatin B-producing Staphylococcus aureus. J Clin Microbiol. 1988;26:1283–1286. doi: 10.1128/jcm.26.7.1283-1286.1988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 195.Oranje A P, Soekanto W, Sukardi A, Vuzévski V D, van der Willigen A, Afiani H M. Diffuse cutaneous mastocytosis mimicking staphylococcal scalded skin syndrome: report of three cases. Pediatr Dermatol. 1991;8:147–151. doi: 10.1111/j.1525-1470.1991.tb00306.x. [DOI] [PubMed] [Google Scholar]
- 196.O’Toole P, Foster T J. Nucleotide sequence of the epidermolytic toxin A gene of Staphylococcus aureus. J Bacteriol. 1987;169:3910–3915. doi: 10.1128/jb.169.9.3910-3915.1987. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 197.Paliard X, West S G, Lafferty J A, Clements J R, Kappler J W, Marrack P, Kotzin B L. Evidence for the effects of a superantigen in rheumatoid arthritis. Science. 1991;253:325–329. doi: 10.1126/science.1857971. [DOI] [PubMed] [Google Scholar]
- 198.Papageorgiou A C, Acharya K R, Shapiro R, Passalacqua E F, Brehm R D, Tranter H S. Crystal structure of the superantigen enterotoxin C2 from Staphylococcus aureus reveals a zinc-binding site. Structure. 1995;3:769–779. doi: 10.1016/s0969-2126(01)00212-x. [DOI] [PubMed] [Google Scholar]
- 199.Parker M T, Tomlinson A J H, Williams R E O. Impetigo contagiosa: the association of certain types of Staphylococcus aureus and of Streptococcus pyogenes with superficial skin infections. J Hyg Camb. 1955;53:458–473. doi: 10.1017/s0022172400000966. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 200.Parker M T, Williams R E O. Further observations on the bacteriology of impetigo and pemphigus neonatorum. Acta Paediatr Scand. 1961;50:101–112. doi: 10.1111/j.1651-2227.1961.tb08028.x. [DOI] [PubMed] [Google Scholar]
- 201.Persson B, Bengtsson-Olivecrona G, Enerback S, Olivecrona T. Structural features of lipoprotein lipase: lipase family relationships, binding interactions, non-equivalence of lipase co-factors, vitellogenin similarities and functional subdivision of lipoprotein lipase. Eur J Biochem. 1989;179:39–45. doi: 10.1111/j.1432-1033.1989.tb14518.x. [DOI] [PubMed] [Google Scholar]
- 202.Pickard S, Shankar G, Burnham K. Langerhan cell depletion by staphylococcal superantigens. Immunology. 1994;83:568–572. [PMC free article] [PubMed] [Google Scholar]
- 203.Piemont Y, Monteil H. Mise en evidence par electrosynerese des deux serotypes d’exfoliatine produits par Staphylococcus aureus. Ann Microbiol (Inst Pasteur) 1983;134A:169–175. [PubMed] [Google Scholar]
- 204.Piemont Y, Monteil H. Rapid detection and quantitation of exfoliative toxin in staphylococcal cultures by electrosyneresis and enzyme-linked immunosorbent assay. In: Jeljaszewicz J, editor. The staphylococci. Stuttgart, Germany: Gustav Fischer Verlag; 1985. pp. 271–272. [Google Scholar]
- 205.Piemont Y, Rifai S, Monteil H. Les exfoliatines de Staphylococcus aureus. Bull Inst Pasteur. 1988;86:263–296. [Google Scholar]
- 206.Pittet D, Wenzel R P. Nosocomial bloodstream infections: secular trends in rates, mortality, and contribution to total hospital deaths. Arch Intern Med. 1995;155:1177–1184. doi: 10.1001/archinte.155.11.1177. [DOI] [PubMed] [Google Scholar]
- 207.Plaut M E, Mirani M. Toxic epidermal necrolysis due to E. coli. JAMA. 1972;219:1631–1632. doi: 10.1001/jama.219.12.1629c. [DOI] [PubMed] [Google Scholar]
- 208.Pollack S. Staphylococcal scalded skin syndrome. Pediatr Rev. 1996;17:18. doi: 10.1542/pir.17-1-18. [DOI] [PubMed] [Google Scholar]
- 209.Prasad G S, Earhart C A, Murray D L, Novick R P, Schlievert P M, Ohlendorf D H. Structure of toxic-shock syndrome toxin-1. Biochemistry. 1993;32:13761–13766. doi: 10.1021/bi00213a001. [DOI] [PubMed] [Google Scholar]
- 210.Prevost G, Rifai S, Chaix L, Piemont Y. Functional evidence that the Ser-195 residue of staphylococcal exfoliative toxin is essential for biological activity. Infect Immun. 1991;59:3337–3339. doi: 10.1128/iai.59.9.3337-3339.1991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 211.Rasmussen J E. Toxic epidermal necrolysis: a review of 75 cases in children. Arch Dermatol. 1975;111:658–659. doi: 10.1001/archderm.111.9.1135. [DOI] [PubMed] [Google Scholar]
- 212.Rasmussen J E. Erythema multiforme: should anyone care about standards of care? Arch Dermatol. 1995;131:726–729. doi: 10.1001/archderm.131.6.726. [DOI] [PubMed] [Google Scholar]
- 213.Raymond J, Bingen E, Brahimi N, Bergeret M, Lepercq J, Badoual J, Gendrel D. Staphylococcal scalded skin syndrome in a neonate. Eur J Clin Microbiol Infect Dis. 1997;16:453–454. doi: 10.1007/BF02471909. [DOI] [PubMed] [Google Scholar]
- 214.Redpath M B, Foster T J, Bailey C J. The role of serine protease active site in the mode of action of epidermolytic toxin of S. aureus. FEMS Microbiol Lett. 1991;81:151–156. doi: 10.1016/0378-1097(91)90295-l. [DOI] [PubMed] [Google Scholar]
- 215.Revuz J, Penso D, Roujeau J C, Guillaume J C, Payne C R, Wechsler J, Touraine R. Toxic epidermal necrolysis: clinical findings and prognostic factors in 87 patients. Arch Dermatol. 1987;123:1166–1170. doi: 10.1001/archderm.123.9.1160. [DOI] [PubMed] [Google Scholar]
- 216.Rifai S, Barbancon V, Prevost G, Piemont Y. Synthetic exfoliative toxin A and B DNA probes for detection of toxigenic Staphylococcus aureus strains. J Clin Microbiol. 1989;27:504–506. doi: 10.1128/jcm.27.3.504-506.1989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 217.Rogers S E, McKee P H. Toxic epidermal necrolysis in two patients with pustular psoriasis. Br J Dermatol. 1977;96:323. doi: 10.1111/j.1365-2133.1977.tb06146.x. [DOI] [PubMed] [Google Scholar]
- 218.Rogolsky M, Wiley B B, Keyhani M, Glasgow L A. Interaction of staphylococcal exfoliative toxin with concanavalin A. Infect Immun. 1974;10:1260–1265. doi: 10.1128/iai.10.6.1260-1265.1974. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 219.Rosten P M, Bartlett K H, Chow A W. Detection of toxic shock syndrome toxin-1 in vivo and in vitro by noncompetitive enzyme-linked immunosorbent assay. J Clin Microbiol. 1987;25:327–332. doi: 10.1128/jcm.25.2.327-332.1987. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 220.Rudolph R I, Schwartz W, Leyden J J. Treatment of staphylococcal toxic epidermal necrolysis. Arch Dermatol. 1974;111:1135–1139. [PubMed] [Google Scholar]
- 221.Rycheck R R, Taylor P M, Gezon H M. Epidemic staphylococcal pyoderma associated with Ritter’s disease and the appearance of phage type 3b/71. N Engl J Med. 1963;269:332–337. doi: 10.1056/NEJM196308152690702. [DOI] [PubMed] [Google Scholar]
- 222.Saadi A T, Blackwell C C, Raza M W, James V S, Stewart J, Elton R A, Weir D M. Factors enhancing adherence of toxigenic Staphylococcus aureus to epithelial cells and their possible role in sudden infant death syndrome. Epidemiol Infect. 1993;110:507–517. doi: 10.1017/s0950268800050937. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 223.Sakurai S, Kondo I. A possible receptor substance for staphylococcal exfoliatin isolated from mice. Jpn J Med Sci Biol. 1979;32:85–88. [PubMed] [Google Scholar]
- 224.Sakurai S, Suzuki H, Kondo I. Cloning of the gene coding for staphylococcal exfoliative toxin A and its expression in Escherichia coli. FEMS Microbiol Lett. 1987;42:63–67. [Google Scholar]
- 225.Sakurai S, Suzuki H, Youhizawa Y, Mochizuki S, Kondo I. Studies on toxicity and immunogenicity of staphylococcal exfoliative toxin. In: Mollby R, Flock J I, Nord C E, Christensson B, editors. Staphylococci and staphylococcal infections. Proceedings of the 7th International Symposium. Stuttgart, Germany: Gustav Fischer Verlag; 1994. pp. 283–285. [Google Scholar]
- 226.Sakurai S, Suzuki H, Machida K. Rapid identification by polymerase chain reaction of staphylococcal exfoliative toxin serotype A and B genes. Microbiol Immunol. 1995;39:379–386. doi: 10.1111/j.1348-0421.1995.tb02216.x. [DOI] [PubMed] [Google Scholar]
- 227.Salariya E M, Kowbus N M. Variable umbilical cord care. Midwifery. 1988;4:70–76. doi: 10.1016/s0266-6138(88)80004-4. [DOI] [PubMed] [Google Scholar]
- 228.Saloga J, Enk A H, Becker D, Mohammadzadeh M, Spieles S, Bellinghausen I, Leung D Y, Gelfand E W, Knop J. Modulation of contact sensitivity responses by bacterial superantigen. J Investig Dermatol. 1995;105:220–224. doi: 10.1111/1523-1747.ep12317503. [DOI] [PubMed] [Google Scholar]
- 229.Samuels M J. Toxic epidermal necrolysis: a report of 42 cases. Br J Dermatol. 1967;79:672–677. doi: 10.1111/j.1365-2133.1967.tb11435.x. [DOI] [PubMed] [Google Scholar]
- 230.Sarai Y, Nakahara H, Ishikawa T, Kondo I, Futaki S. A bacteriological study on children with staphylococcal toxic epidermal necrolysis in Japan. Dermatologica. 1977;154:161–167. doi: 10.1159/000251125. [DOI] [PubMed] [Google Scholar]
- 231.Sarsfield J K. Battering: dangers of a backlash. Br Med J. 1974;2:57. doi: 10.1136/bmj.2.5909.57-b. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 232.Sato H, Tanabe T, Kuramoto M, Tanaka K, Hashimoto T, Saito H. Isolation of exfoliative toxin from Staphylococcus hyicus subsp. hyicus and its exfoliative activity in the piglet. Vet Microbiol. 1991;27:263–275. doi: 10.1016/0378-1135(91)90153-7. [DOI] [PubMed] [Google Scholar]
- 233.Sato H, Matsumori Y, Tanabe T, Saito H, Shimizu A, Akira A, Kawano J. A new type of staphylococcal exfoliative toxin isolated from a Staphylococcus aureus strain isolated from a horse with phlegmon. Infect Immun. 1994;62:3780–3785. doi: 10.1128/iai.62.9.3780-3785.1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 234.Schad E M, Zaitseva I, Zaitsev V N, Dohlsten M, Kalland T, Schlievert P M, Ohlendorf D H, Svensson L A. Crystal structure of the superantigen staphylococcal enterotoxin type A. EMBO J. 1995;14:3292–3301. doi: 10.1002/j.1460-2075.1995.tb07336.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 235.Schrezenmeier H, Fleischer B. Mitogenic activity of staphylococcal protein A is due to contaminating staphylococcal enterotoxins. J Immunol Methods. 1987;105:133–137. doi: 10.1016/0022-1759(87)90423-6. [DOI] [PubMed] [Google Scholar]
- 236.Schwartz R A. Toxic epidermal necrolysis. CUTIS. 1997;59:123–128. [PubMed] [Google Scholar]
- 237.Scopes J W, Eykyn S, Phillips I. Staphylococcal infection in the newborn. Lancet. 1974;2:1392. doi: 10.1016/s0140-6736(74)92268-5. [DOI] [PubMed] [Google Scholar]
- 238.See R H, Adilman S, Bartlett K H, Chow A W. Colony immunoblot assay for the detection of staphylococcal toxic shock syndrome 1 (TSST-1) with anti-TSST-1 F(ab′)2 fragments. J Clin Microbiol. 1989;27:2050–2053. doi: 10.1128/jcm.27.9.2050-2053.1989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 239.Seeberg S, Brinkoff B. Epidemiology and control of staphylococcal pyoderma among newborn infants: evaluation of a method for routine care with a 4 percent chlorhexidine detergent solution. J Hosp Infect. 1984;5:121–136. doi: 10.1016/0195-6701(84)90116-6. [DOI] [PubMed] [Google Scholar]
- 240.Seeberg S, Brinkoff B, John E, Kjellmer I. Prevention and control of neonatal proderma with chlorhexidine. Acta Paediatr Scand. 1984;73:498–504. doi: 10.1111/j.1651-2227.1984.tb09961.x. [DOI] [PubMed] [Google Scholar]
- 241.Shaffer T E, Baldwin J N, Wheeler W E. Staphylococcal infection in nurseries. In: Levine S Z, editor. Advances in pediatrics. Vol. 10. Chicago, Ill: Year Book; 1958. pp. 243–281. [Google Scholar]
- 242.Shands K N, Schmid G P, Dan B B, Guidotti R J, Hargrett N T, Anderson R L, Hill D L, Broome C V, Band J D, Fraser D W. Toxic-shock syndrome in menstruating women: its association with tampon use and Staphylococcus aureus and the clinical features in 52 cases. N Engl J Med. 1980;303:1436. doi: 10.1056/NEJM198012183032502. [DOI] [PubMed] [Google Scholar]
- 243.Sheridan R L, Briggs S E, Remensnyder J P, Gagnon S W, Doody D P, Ryan D P, Tompkins R G. The burns unit as a resource for the management of acute non-burn conditions in children. J Burn Care Rehabil. 1995;16:62–64. doi: 10.1097/00004630-199501000-00012. [DOI] [PubMed] [Google Scholar]
- 244.Simpson K, Tozer R C. Prevention of staphylococcal sepsis in a maternity hospital by means of hexochlorophane. Br Med J. 1960;1:315–317. doi: 10.1136/bmj.1.5169.315. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 245.Skov L, Baadsgaard O. Superantigens: do they have a role in skin diseases? Arch Dermatol. 1995;131:829–832. doi: 10.1001/archderm.131.7.829. [DOI] [PubMed] [Google Scholar]
- 246.Smales O. A comparison of umbilical cord treatment in the control of superficial infection. N Z Med J. 1988;101:453–455. [PubMed] [Google Scholar]
- 247.Smith T P, Bailey C J. Epidermolytic toxin from Staphylococcus aureus binds to filaggrins. FEBS Lett. 1986;194:309–312. doi: 10.1016/0014-5793(86)80107-7. [DOI] [PubMed] [Google Scholar]
- 247a.Smith T P, John D A, Bailey C J. The binding of epidermolytic toxin from Staphylococcus aureus to mouse epidermal tissue. Histochem J. 1987;19:137–149. doi: 10.1007/BF01695138. [DOI] [PubMed] [Google Scholar]
- 248.Smith T P, John D A, Bailey C J. Epidermolytic toxin binds to components in the epidermis of a resistant species. Eur J Cell Biol. 1989;49:341–349. [PubMed] [Google Scholar]
- 249.Smith T P, Bailey C J. Activity requirements of epidermolytic toxins from Staphylococcus aureus studied by an in vitro assay. Toxicon. 1990;28:675–683. doi: 10.1016/0041-0101(90)90256-7. [DOI] [PubMed] [Google Scholar]
- 250.Starikov A V. Experience in using extracorporeal detoxication in the intensive therapy of autoimmune pathology. Likars’ka Sprava. 1995;9–12:155–157. . (In Russian.) [PubMed] [Google Scholar]
- 251.Stark V, Harrison S P. Staphylococcus aureus colonization of the newborn in a Darlington Hospital. J Hosp Infect. 1992;21:205–211. doi: 10.1016/0195-6701(92)90077-y. [DOI] [PubMed] [Google Scholar]
- 252.Stephen J, Pietrowski R A. Bacterial toxins. Washington, D.C: American Society for Microbiology; 1986. pp. 8–10. [Google Scholar]
- 253.Stevens F A. Occurrence of Staphylococcus aureus infection with scarlantiniform rash. JAMA. 1927;88:1957–1958. [Google Scholar]
- 254.Stevens D L, Tanner M H, Winship J, Swarts C, Ries K M, Schlievert P M, Kaplan E. Severe group A streptococcal infections associated with a toxic shock-like syndrome and scarlet fever toxin A. N Engl J Med. 1989;321:1–7. doi: 10.1056/NEJM198907063210101. [DOI] [PubMed] [Google Scholar]
- 255.Stewart G A, Varro R, Stanworth D R. The influence of enzymatic cleavage and chemical modification of human and rabbit IgG on their reactivity with staphylococcal protein A. Immunology. 1978;35:785–791. [PMC free article] [PubMed] [Google Scholar]
- 256.Stinnisen P, Vandevyver C, Raus J, Zhang J. Superantigen reactivity of gamma delta cell clones isolated from patients with multiple sclerosis and controls. Cell Immunol. 1995;166:227–235. doi: 10.1006/cimm.1995.9975. [DOI] [PubMed] [Google Scholar]
- 257.Strange P, Skov L, Baadsgaard O. Interferon gamma-treated keratinocytes activate T cells in the presence of superantigens: involvement of major histocompatibility complex class II molecules. J Investig Dermatol. 1994;102:150–154. doi: 10.1111/1523-1747.ep12371753. [DOI] [PubMed] [Google Scholar]
- 258.Streilein J W. Skin associated lymphoid tissue. In: Norris D A, editor. Immune mechanisms in cutaneous disease. New York, N.Y: Marcel Dekker Inc.; 1989. pp. 73–94. [Google Scholar]
- 259.Su Y C, Wong A C L. Identification and purification of a new staphylococcal enterotoxin, H. Appl Environ Microbiol. 1995;61:1438–1443. doi: 10.1128/aem.61.4.1438-1443.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 260.Swaminathan S, Furey W, Pletcher J, Sax M. Crystal structure of staphylococcal enterotoxin B, a superantigen. Nature. 1992;359:801–806. doi: 10.1038/359801a0. [DOI] [PubMed] [Google Scholar]
- 261.Tait S C. The hydrolysis of lipids and esters by Staphylococcus aureus. D.Phil. thesis. Paisley, United Kingdom: Paisley College of Technology; 1973. [Google Scholar]
- 262.Takiuchi I, Kawamura I, Teramoto T, Higuchi D. Staphylococcal exfoliative toxin induces caseinolytic activity. J Infect Dis. 1987;156:508–509. doi: 10.1093/infdis/156.3.508. [DOI] [PubMed] [Google Scholar]
- 263.Tanabe T, Sato H, Kuramoto M, Saito H. Purification of exfoliative toxin produced by Staphylococcus hyicus and its antigenicity. Infect Immun. 1993;61:2973–2977. doi: 10.1128/iai.61.7.2973-2977.1993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 264.Tanabe T, Sato H, Ueda K, Chihara H, Watanabe T, Nakano K, Saito H, Maehara N. Possible receptor for exfoliative toxins produced by Staphylococcus hyicus and Staphylococcus aureus. Infect Immun. 1995;63:1591–1594. doi: 10.1128/iai.63.4.1591-1594.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 265.Telford D R, Morris J A, Hughes P, Conway A R, Lee S, Barson A J, Drucker D B. The nasopharyngeal flora in sudden infant death syndrome. J Infect. 1989;18:125–130. doi: 10.1016/s0163-4453(89)91094-3. [DOI] [PubMed] [Google Scholar]
- 266.Todd J K. Staphylococcal toxin syndromes. Annu Rev Med. 1985;36:1017–1019. doi: 10.1146/annurev.me.36.020185.002005. [DOI] [PubMed] [Google Scholar]
- 267.Tokura Y, Heald P W, Yan S L, Edelson R L. Stimulation of cutaneous T-cell lymphoma cells with superantigenic staphylococcal toxins. J Investig Dermatol. 1992;98:33–37. doi: 10.1111/1523-1747.ep12494184. [DOI] [PubMed] [Google Scholar]
- 268.Tokura Y, Yagi J, O’Malley M, Lewis J, Takigawa M, Edelson R, Tigelaar R E. Superantigenic staphylococcal exotoxins induce T-cell proliferation in the presence of langerhan cells or class II-bearing keratinocytes to produce T-cell activating cytokines. J Investig Dermatol. 1994;102:31–38. doi: 10.1111/1523-1747.ep12371727. [DOI] [PubMed] [Google Scholar]
- 269.Toyota E, Mitake H, Mikame Y, Shichi I, Morita S, Morishita T, Kasahara M, Kobayashi Y. A case of TSS complicated with SSSS in an adult with liver cirrhosis. J Jpn Assoc Infect Dis. 1994;68:1421–1427. doi: 10.11150/kansenshogakuzasshi1970.68.1421. . (In Japanese.) [DOI] [PubMed] [Google Scholar]
- 270.Vath G M, Earhart C A, Rago J V, Kim M H, Bohach G A, Schlievert P M, Ohlendorf D H. The structure of the superantigen exfoliative toxin A suggests a novel regulation as a serine protease. Biochemistry. 1997;36:1559–1566. doi: 10.1021/bi962614f. [DOI] [PubMed] [Google Scholar]
- 271.Verba V, Tarkowski A. Participation of V beta 4(+)-, V beta 7(+)-, and V beta 11(+)-T lymphocytes in haematogenously acquired Staphylococcus aureus nephritis. Scand J Immunol. 1996;44:261–266. doi: 10.1046/j.1365-3083.1996.d01-309.x. [DOI] [PubMed] [Google Scholar]
- 272.Verber I G, Pagan F S. What cord care—if any? Arch Dis Child. 1993;68:594–596. doi: 10.1136/adc.68.5_spec_no.594. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 273.Volc-Platzer B, Majdix O, Knapp K, Wolff K, Hinterberger W, Lechner K, Stingl G. Evidence of HLA-DR antigen biosynthesis by human keratinocytes in disease. J Exp Med. 1984;159:1784–1789. doi: 10.1084/jem.159.6.1784. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 274.Voller A, Bidwell D, Bartlett A. Enzyme-linked immunosorbent assay. In: Rose N R, Friedman H, editors. Manual of clinical immunology. Washington, D.C: American Society for Microbiology; 1980. pp. 359–371. [Google Scholar]
- 275.Von Rittershain G R. Die exfoliative dermatitis jungener senglinge. Z Kinderheilkd. 1878;2:3–23. [Google Scholar]
- 276.Watkinson M, Dyas A. Staphylococcus aureus still colonises the untreated neonatal umbilicus. J Hosp Infect. 1992;21:131–136. doi: 10.1016/0195-6701(92)90032-h. [DOI] [PubMed] [Google Scholar]
- 277.Wiley B B, Allman S, Rogolsky M, Norden C W, Glasgow L A. Staphylococcal scalded skin syndrome: potentiation by immunosuppression in mice: toxin-mediated exfoliation in a healthy adult. Infect Immun. 1974;9:636–640. doi: 10.1128/iai.9.4.636-640.1974. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 278.Wiley B B, Rogolsky M S. Phospholipase activity associated with electrfocused Staphylococcal exfoliative toxin. In: Jeljaszewicz J, editor. The staphylococci. Stuttgart, Germany: Gustav Fischer Verlag; 1985. pp. 295–300. [Google Scholar]
- 279.Willard D, Monteil H, Piemont Y, Assi R, Messer J, Lavillaureix J, Minck R, Gauder R. L’exfoliatine dans les staphylococcies neonatales. Nouv Presse Med. 1982;11:3769–3771. [PubMed] [Google Scholar]
- 280.Winkler F K, D’Arcy A, Hunziker W. Structure of human pancreatic lipase. Nature. 1990;343:771–774. doi: 10.1038/343771a0. [DOI] [PubMed] [Google Scholar]
- 281.Winternitz R. Ein Beitrag zur Kenntnis der Dermatitis exfoliativa neonatorum (Ritter) Arch Dermatol Syphilis. 1898;44:397–416. [Google Scholar]
- 282.Wolinsky, E., P. J. Lipsitz, E. A. Mortimer, and C. H. Rammelkamp. Acquisition of staphylococci in newborns: direct versus indirect transmission. Lancet ii:620–622. [DOI] [PubMed]
- 283.Woodworth T G, Nichols J C. Recombinant fusion toxins—a new class of targeted biologic therapeutics. Cancer Treat Res. 1995;68:145–160. doi: 10.1007/978-1-4615-3076-3_8. [DOI] [PubMed] [Google Scholar]
- 284.Wong G W, Oppenheimer S J, Evans R M, Leung S S, Cheng J C. Pyomyositis and staphylococcal scalded skin syndrome. Acta Paediatr. 1993;82:113–115. doi: 10.1111/j.1651-2227.1993.tb12536.x. [DOI] [PubMed] [Google Scholar]
- 285.Wuepper K D, Dimond R L, Knutson D D. Studies of the mechanism of epidermal injury by a staphylococcal epidermolytic toxin. J Investig Dermatol. 1975;65:191–200. doi: 10.1111/1523-1747.ep12598130. [DOI] [PubMed] [Google Scholar]
- 286.Wuepper K D, Baker D H, Dimond R L. Measurement of the staphylococcal epidermolytic toxin: a comparison of bioassay, radial immunodiffusion, and radioimmunoassay. J Investig Dermatol. 1976;67:526–530. doi: 10.1111/1523-1747.ep12664548. [DOI] [PubMed] [Google Scholar]
- 287.Yokota S, Imagawa T, Kataakura S, Mitsuda T, Arai T. Staphylococcal scalded skin syndrome caused by exfoliative toxin B-producing methicillin-resistant Staphylococcus aureus. Eur J Paediatr. 1996;155:722. doi: 10.1007/BF01957163. [DOI] [PubMed] [Google Scholar]
- 288.Yokote R, Tokura Y, Furukawa F, Takigawa M. Susceptible responsiveness to bacterial superantigens in peripheral blood mononuclear cells from patients with psoriasis. Arch Dermatol Res. 1995;287:443–447. doi: 10.1007/BF00373426. [DOI] [PubMed] [Google Scholar]
- 289.Yoo C S, Wang B C, Sax M. Preliminary crystallographic data for Staphylococcus aureus exfoliative toxin. J Mol Biol. 1978;124:421–423. doi: 10.1016/0022-2836(78)90307-8. [DOI] [PubMed] [Google Scholar]
- 290.Zhao Y X, Ljungdahl A, Olsson T, Tarkowski A. In situ hybridisation analysis synovial and systemic cytokine messenger RNA expression in superantigen-mediated staphylococcal arthritis. Arthritis Rheum. 1996;39:959–967. doi: 10.1002/art.1780390613. [DOI] [PubMed] [Google Scholar]