Abstract
Background
There is a lack of studies examining the long-term trend and survival of axillary surgery for breast cancer patients with sentinel node metastasis, especially for the patients with 3–5 node metastases.
Methods
Breast cancer patients with 1–5 sentinel node metastases from the Surveillance, Epidemiology, and End Results (SEER) database from 2000 to 2016. Our study presented the trend of axillary surgery and assessed the long-term survival of sentinel lymph node biopsy (SLNB) alone vs axillary lymph node dissection (ALND) for those patients.
Results
Of the 41,996 patients diagnosed with T1-2 breast cancer after lumpectomy and radiation included, 34,940 had 1-2 sentinel node metastases and 7056 had 3-5 sentinel node metastases. The percentage of patients undergoing SLNB alone increased from 22.4% in 2000 to 81.0% in 2016 for patients with 1–2 sentinel node metastases, and quadrupled from 5.2% in 2009 to 20.6% in 2016 for those with 3–5 sentinel node metastases. Completion of ALND did not benefit the long-term survival of 1–2 sentinel node metastasis patients (hazard ratio [HR] = 1.02, P = 0.539), but improved the long-term survival of 3–5 node metastasis patients (HR = 0.73, P < 0.001). Subgroup analysis demonstrated the inferiority of SLNB to ALND in all subgroups of 3–5 sentinel node metastases.
Conclusion
For patients with T1-2 breast cancer after lumpectomy and radiation, SLNB alone was an efficient and safe surgical choice for 1–2 sentinel node metastases but not for 3–5 sentinel node metastases. It is worth noting that for patients with 3–5 node metastasis, the proportion of omitted ALND quadrupled after 2009.
Keywords: Sentinel lymph node biopsy, Axillary lymph node dissection, Sentinel node metastasis, Overall survival
Highlights
-
•
Using SEER database, the research presents the long-term trend and survival of axillary surgery for breast cancer patients with 1-2 and 3-5 sentinel nodes metastasis.
-
•
For patients with T1-2 breast cancer after lumpectomy and radiation, SLNB alone was efficient for 1–2 sentinel node metastases but not for 3–5 sentinel node metastases.
-
•
Among patients with 3–5 node metastasis, the proportion of omitted ALND quadrupled from 2009 to 2016.
1. Introduction
Breast cancer becomes the most common female malignancy worldwide [1]. Sentinel lymph node biopsy (SLNB) and Axillary lymph node dissection (ALND) are the two classical and common axillary surgeries for operable breast cancer. ALND is an effective surgery for maintaining local lymph node control, but with the complications, such as lymphedema and decreased upper extremity motion [2]. SLNB is the substitute for ALND among patients with negative axilla, and complications caused by SLNB are negligible [3,4]. Therefore, SLNB has become the standard staging method and the use of ALND declined from 94% to 36% in women with no axillary nodal metastases from 1998 to 2004 [5].
Approximately half of sentinel node-positive patients do not have non-sentinel node metastases, and those patients may forgo completion ALND [6]. The Z0011 trial demonstrated the safety of SLNB alone in patients with 1–2 positive sentinel lymph nodes [7]. After the publication of the Z0011 trial, the axilla management for patients under the Z0011 eligibility criteria changed greatly from developed to developing countries [[8], [9], [10]]. Omission of ALND has become the standard practice and been recommended since 2013 [11]. However, the limitations of Z0011 were still questioned for failure to meet the accrual target, high ratio of lost follow-up, and lack of radiation treatment quality assurance [12,13]. The findings of the Z0011 trial need to be examined in real-world practice. Several studies analyzed the data from the population-based database and validated the outcome of the Z0011 trial from different perspectives [[14], [15], [16]]. These studies did not report long-term survival analysis or the trend of axilla management in the real practice. Moreover, it was unknown whether ALND could be avoided for the certain patients with 3–5 positive sentinel lymph nodes.
In this study, we used the data extracted from the population-based Surveillance, Epidemiology, and End Results (SEER) database. Our objectives were to examine the trends in axillary surgery and clinical outcomes of either SLNB alone or ALND among breast cancer patients with 1–2 or 3–5 sentinel node metastases.
2. Materials and methods
2.1. Database
The Surveillance, Epidemiology, and End Results (SEER) database (November 2020 submission) is a National Cancer Institute-sponsored program that comprised the cases from 2000 to 2018. We obtained patient data from 18 registries by SEER*Stat software, version 8.3.9 (http://seer.cancer.gov/about/). The SEER 18 registries include the data from Alaska, Connecticut, San Francisco and other 15 registries. The data from the SEER program were publicly available and deidentified and did not require the patient's informed consent. Approved by the ethics board of the Westchina Hospital, this study was deemed exempt from ethical approval.
2.2. Study population
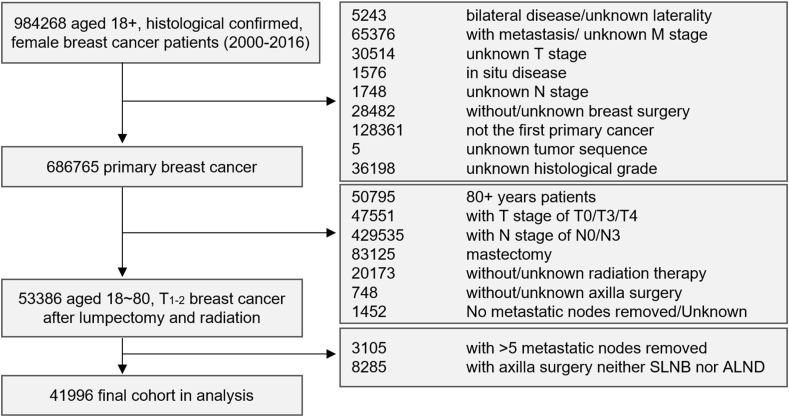
Between January 1, 2000, and December 31, 2016, a total of 984,268 patients aged older than 18 years were extracted from the SEER database. The selection diagram of the analyzed cohort is shown in Fig. 1. We included the patients before 2017 to ensure at least a two-year follow-up. Of these patients, 53,386 patients, aged 18–80, T1-2 with unilateral invasive breast cancer, who underwent lumpectomy and radiation were selected. The SEER database did not clearly define the type of axillary surgery. SLNB usually involved less than six axillary lymph nodes (ALNs) [17,18]. We in practice specified the type of axillary surgery according to the number of examined ALNs, as described by previous studies [15,16]: 1) the patients, with 1–5 examined ALNs, underwent SLNB; 2) the patients, with ≥10 examined ALNs, underwent ALND. We defined 1–5 positive regional nodes as sentinel node metastasis. Patients with 6–9 ALNs removed underwent SLNB-Plus surgery, which intermediated SLNB and ALND. Due to unclear and controversial classification, the 8285 patients with SLNB-Plus were excluded. Finally, 41,996 patients were enrolled, including 34,940 with 1–2 sentinel node metastases (83.20%, including 17,143 patients with SLNB alone and 17,797 with ALND) and 7056 with 3–5 sentinel node metastases (16.80%, including 680 patients with SLNB alone and 6376 with ALND).
Fig. 1.
Flow diagram for selection of the study cohort
A total of 41,996 patients were enrolled in this study.
2.3. Assembly of key variables
The following variables were assembled from SEER*Stat software: year of diagnosis, age, race, marital status, histological grade, ICD-O-3 histology, estrogen receptor (ER), progesterone receptor (PR), human epidermal growth factor receptor 2 (HER2) recode, primary tumor T stage, regional nodes positive, chemotherapy recode, radiation therapy, vital status recode, cause-specific death classification, and survival month. The ER and PR variables were merged as the hormone receptor (HoR) status.
2.4. Main outcome measure
Overall survival (OS) was defined as the survival time from the initial diagnosis of cancer to the death from any cause. The main outcome measure was 15-year overall survival. The end date for the follow-up was December 31, 2018, and the data were analyzed in October 2021.
2.5. Statistical analysis
The demographic and clinicopathological variables were compared across the two groups by the Pearson χ2 test. Kaplan–Meier curves and log-rank tests were generated to measure survival differences. Univariate and multivariate Cox hazard models were conducted to estimate the features associated with OS. Subgroup analyses presented the hazard ratios of SLNB versus ALND in the specific sub-cohort. All statistical analyses were accomplished by Stata statistical software, version 12.0 (StataCorp), and a significant difference was considered for two-sided P < 0.05.
3. Results
3.1. Patient description and trend in Axilla surgery
The baseline characteristics of all 41,996 T1-2 patients after lumpectomy and radiation are summarized in Table 1. The median follow-up time was 99 months. There were 34,940 patients with 1–2 sentinel node metastases (including 17,143 patients with SLNB alone and 17,797 with ALND) and 7056 patients with 3–5 sentinel node metastases (including 680 patients with SLNB alone and 6376 with ALND). Within the 1–2 sentinel node metastasis cohort, compared with the SLNB alone patients, the ALND patients were more likely to be younger (aged ≤50 years: 33.9% vs 24.2%, P < 0.001), have worse histological differentiation (40.0% vs 27.4% for poorly differentiated or undifferentiated, P < 0.001), have a lower HoR positive rate (HoR+: 78.6% vs 88.6%, P < 0.001), and have a higher proportion of two sentinel node metastases (33.3% vs 14.1%, P < 0.001). Within the 3–5 sentinel node metastasis cohort, the ALND patients were also more likely to be younger (aged ≤50 years: 35.5% vs 28.7%, P < 0.001) and had a higher proportion of 4–5 sentinel node metastases (50.6% vs 33.0%, P < 0.001).
Table 1.
Baseline characteristics of patients diagnosed with T1-2 breast cancer after lumpectomy and radiation from the SEER database 2000–2016.
| Characteristics | Total (n = 41996) |
1-2 sentinel-node metastases |
3-5 sentinel-node metastases |
||||
|---|---|---|---|---|---|---|---|
| SLNB (n = 17143) |
ALND (n = 17797) |
P | SLNB (n = 680) |
ALND (n = 6376) |
P | ||
| n (%) | n (%) | n (%) | n (%) | n (%) | |||
| Year | <0.001 | <0.001 | |||||
| 2000–2005 | 14748 (35.12) | 3804 (22.19) | 8081 (45.41) | 201 (29.56) | 2662 (41.75) | ||
| 2006–2011 | 14066 (33.49) | 4490 (26.19) | 7007 (39.37) | 175 (25.74) | 2394 (37.55) | ||
| 2012–2016 | 13182 (31.39) | 8849 (51.62) | 2709 (15.22) | 304 (44.71) | 1320 (20.70) | ||
| Age | <0.001 | <0.001 | |||||
| 18–50 years | 12625 (30.06) | 4140 (24.15) | 6025 (33.85) | 195 (28.68) | 2265 (35.52) | ||
| 51–65 years | 18691 (44.51) | 7664 (44.71) | 7910 (44.45) | 313 (46.03) | 2804 (43.98) | ||
| 66–80 years | 10680 (25.43) | 5339 (31.14) | 3862 (21.70) | 172 (25.29) | 1307 (20.50) | ||
| Histology | <0.001 | 0.003 | |||||
| IDC | 34006 (80.97) | 13581 (79.22) | 14678 (82.47) | 525 (77.21) | 5222 (81.90) | ||
| ILC & Others | 7990 (19.03) | 3562 (20.78) | 3119 (17.53) | 155 (22.79) | 1154 (18.10) | ||
| Grade | <0.001 | 0.388 | |||||
| I | 7654 (18.23) | 3985 (23.25) | 2856 (16.05) | 85 (12.50) | 728 (11.42) | ||
| II | 19370 (46.12) | 8459 (49.34) | 7823 (43.96) | 307 (45.15) | 2781 (43.62) | ||
| III & IV | 14972 (35.65) | 4699 (27.41) | 7118 (40.00) | 288 (42.35) | 2867 (44.97) | ||
| T | <0.001 | 0.820 | |||||
| T1 | 25395 (60.47) | 11384 (66.41) | 10734 (60.31) | 313 (46.03) | 2964 (46.49) | ||
| T2 | 16601 (39.53) | 5759 (33.59) | 7063 (39.69) | 367 (53.97) | 3412 (53.51) | ||
| HoR | <0.001 | 0.090 | |||||
| Negative | 5944 (14.15) | 1600 (9.33) | 3083 (17.32) | 101 (14.85) | 1160 (18.19) | ||
| Positive | 34733 (82.71) | 15180 (88.55) | 13992 (78.62) | 554 (81.47) | 5007 (78.53) | ||
| Unknown | 1319 (3.14) | 363 (2.12) | 722 (4.06) | 25 (3.68) | 209 (3.28) | ||
| HER2 | <0.001 | <0.001 | |||||
| Negative | 15380 (36.62) | 9538 (55.64) | 3859 (21.68) | 303 (44.56) | 1680 (26.35) | ||
| Positive | 2163 (5.15) | 1102 (6.43) | 726 (4.08) | 45 (6.62) | 290 (4.55) | ||
| Unknown | 24453 (58.23) | 6503 (37.93) | 13212 (74.24) | 332 (48.82) | 4406 (69.10) | ||
| Node number of metastases | <0.001 | ||||||
| 1 | 26601 (63.34) | 14725 (85.90) | 11876 (66.73) | ||||
| 2 | 8339 (19.86) | 2418 (14.10) | 5921 (33.27) | ||||
| 3 | 3605 (8.58) | 456 (67.06) | 3149 (49.39) | <0.001 | |||
| 4 | 2112 (5.03) | 159 (23.38) | 1953 (30.63) | ||||
| 5 | 1339 (3.19) | 65 (9.56) | 1274 (19.98) | ||||
| Chemotherapy | <0.001 | <0.001 | |||||
| Yes | 30309 (72.17) | 10070 (58.74) | 13995 (78.64) | 570 (83.82) | 5674 (88.99) | ||
| No/Unknown | 11687 (27.83) | 7073 (41.26) | 3802 (21.36) | 110 (16.18) | 702 (11.01) | ||
Abbreviations: HoR, hormone receptor; IDC, invasive lobular carcinoma; ILC, invasive ductal carcinoma; T, primary tumor T stage.
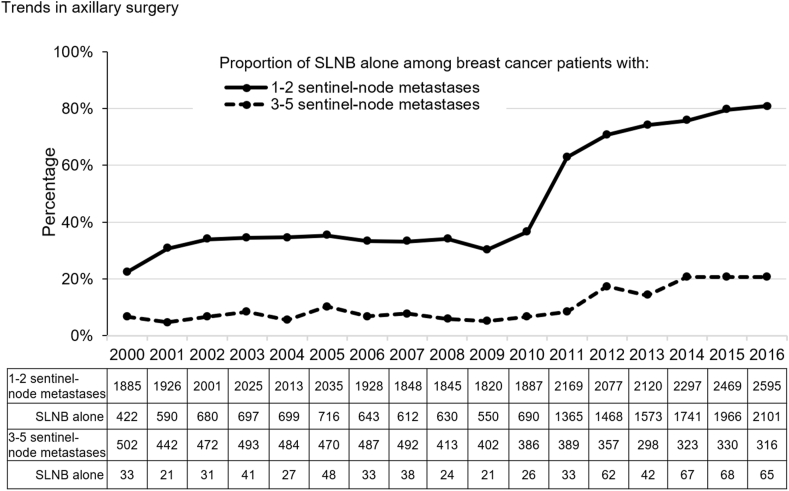
The trend in axillary surgery for sentinel node metastasis shown that for the 1–2 sentinel node metastasis cohort, the percentage of patients undergoing SLNB increased dramatically (from 22.4% in 2000 to 81.0% in 2016) (Fig. 2). Especially, the SLNB proportion doubled from 30.2% in 2009 to 62.9% in 2011. In contrast, the SLNB alone proportion in patients with 3–5 sentinel node metastases kept low until 2009 and then almost quadrupled from 5.2% in 2009 to 20.6% in 2016.
Fig. 2.
Trends in axillary surgery among breast cancer patients with sentinel node metastasis
The proportion of SLNB alone among breast cancer patients with 1–2 or 3–5 sentinel-node metastases.
3.2. Survival analysis and multivariate analysis
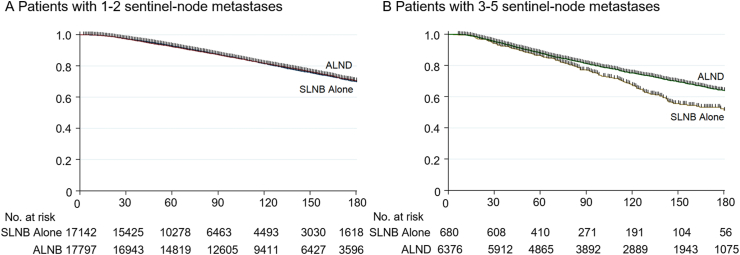
For the patients with 1–2 sentinel node metastasis, the SLNB alone group had a similar 15-year OS to the ALND group (70.0% vs 70.5%, log-rank test P = 0.953), as shown in Fig. 3. For the patients with 3–5 sentinel node metastases, the SLNB alone and ALND groups showed comparable five-year OS rates (86.3% vs 88.0%, log-rank test P = 0.182). Interestingly, the survival difference between the two groups showed clearly after five years, and ALND exhibited a better 15-year OS than the SLNB alone cohort (51.4% vs 64.1%, log-rank test P < 0.001).
Fig. 3.
Kaplan–Meier plots of the 15-year overall survival among breast cancer patients with sentinel node metastasis
The overall survival comparison of SLNB alone vs ALND among breast cancer patients with 1–
2 sentinel-node metastases(A), and with 3–5 sentinel-node metastases(B).
Abbreviations: ALND, axillary lymph node dissection; OS, overall survival; SLNB, sentinel lymph node biopsy.
To balance the biased baseline, we conducted univariate and multivariate Cox proportional hazards analysis among patients with 1–2 or 3–5 sentinel node metastases respectively. The factors that were statistically significant in the univariate analysis (Table S1) were included in the multivariate analysis (Table 2), including age, race, marital status, grade, tumor size, HoR status, number of node metastases, axillary surgery type and chemotherapy. After the balancing, ALND presented no statistical significance for survival benefit over SLNB (hazard ratio [HR] 1.02; 95% CI, 0.96–1.07; P = 0.539) for patients with 1–2 sentinel node metastasis, which is consistent with the above survival analysis. By comparison, ALND was demonstrated to be an independent favorable variable over SLNB in OS (HR 0.73; 95% CI, 0.63–0.85; P < 0.001) for patients with 3–5 sentinel node metastases.
Table 2.
Multivariate Cox regression model analysis of the OS.
| Characteristics | 1-2 sentinel-node metastases SLNB vs ALND |
3-5 sentinel-node metastases SLNB vs ALND |
||
|---|---|---|---|---|
| HR (95% CI) | P | HR (95% CI) | P | |
| Age | ||||
| 18–50 years | Reference | Reference | ||
| 51–65 years | 1.25 (1.16–1.33) | <0.001 | 1.24 (1.11–1.40) | <0.001 |
| 66–80 years | 3.06 (2.85–3.29) | <0.001 | 2.66 (2.34–3.01) | <0.001 |
| Race | ||||
| White | Reference | Reference | ||
| Black | 1.37 (1.27–1.48) | <0.001 | 1.33 (1.16–1.52) | <0.001 |
| Others | 0.84 (0.76–0.94) | 0.002 | 0.82 (0.67–0.99) | 0.043 |
| Marital status | ||||
| Married | Reference | Reference | ||
| Not married | 1.29 (1.23–1.36) | <0.001 | 1.22 (1.11–1.34) | <0.001 |
| Unknown | 1.10 (0.95–1.29) | 0.214 | 0.67 (0.48–0.93) | 0.018 |
| Grade | ||||
| I | Reference | Reference | ||
| II | 1.32 (1.22–1.43) | <0.001 | 1.33 (1.11–1.58) | 0.002 |
| III & IV | 1.81 (1.67–1.97) | <0.001 | 1.80 (1.50–2.15) | <0.001 |
| T | ||||
| T1 | Reference | Reference | ||
| T2 | 1.50 (1.43–1.59) | <0.001 | 1.37 (1.24–1.50) | <0.001 |
| HoR | ||||
| Negative | Reference | Reference | ||
| Positive | 0.71 (0.66–0.77) | <0.001 | 0.71 (0.63–0.80) | <0.001 |
| Unknown | 0.88 (0.77–0.99) | 0.038 | 0.61 (0.47–0.79) | <0.001 |
| Node number of metastases | ||||
| 1 | Reference | |||
| 2 | 1.15 (1.08–1.22) | <0.001 | ||
| 3 | Reference | |||
| 4 | 1.32 (1.19–1.47) | <0.001 | ||
| 5 | 1.37 (1.21–1.54) | <0.001 | ||
| Axilla Surgery | ||||
| SLNB | Reference | Reference | ||
| ALND | 1.02 (0.96–1.07) | 0.539 | 0.73 (0.63–0.85) | <0.001 |
| Chemotherapy | ||||
| Yes | Reference | Reference | ||
| No/Unknown | 1.43 (1.34–1.52) | <0.001 | 1.40 (1.23–1.60) | <0.001 |
Abbreviations: HoR, hormone receptor; IDC, invasive lobular carcinoma; ILC, invasive ductal carcinoma; OS, overall survival; T, primary tumor T stage.
3.3. Subgroup analysis
To clarify the effects of axillary surgery among the subgroup patients, we conducted multivariable Cox analysis within the subgroups according to the three variables (age, HoR status, and the number of metastatic nodes) (Table S2). All three variables were independent risk factors in the survival analysis and key factors in clinical practice. Among 1–2 sentinel node metastasis patients, SLNB showed comparable outcomes with ALND in most subgroups, regardless of the five-year or 15-year follow-up. The exception was ALND showed a protective effect on five-year OS in the subgroups of 18–50 years and two positive sentinel nodes. Among the most subgroups of 3–5 sentinel node metastasis patients, the survival benefit induced by ALND was not statistically significant in the five-year follow-up but showed significance in the 15-year follow-up.
3.4. Sensitivity analysis
As described in the Methods section, we practically defined SLNB and ALND. To avoid the possibility of unintentional bias from the definition, sensitivity analyses were conducted (Table S3). In this part, SLNB was redefined from one to five regional lymph nodes removed, and ALND was defined as more than five or nine axillary lymph nodes removed. The analysis demonstrated that the definition for axillary surgery did not make a difference for the conclusion. The exceptional cases were that when SLNB was defined as three regional nodes removed, there was no statistical significance between SLNB and ALND for the patients with 3 sentinel node metastasis patients. Even in this situation, a statistical trend for the superiority of ALND was shown.
4. Discussion
We analyzed the data of 41,996 T1-2 patients with lumpectomy and radiation from 2000 to 2016, and the median follow-up time was 99 months. This study was a large population-based analysis to retrospectively examine the trends in axillary surgery and clinical outcomes of either SLNB alone or ALND among breast cancer patients with 1–2 or 3–5 sentinel-node metastases. The percentage of patients undergoing SLNB alone increased from 22.4% in 2000 to 81.0% in 2016 for patients with 1–2 sentinel node metastases and quadrupled from 5.2% in 2009 to 20.6% in 2016 for those with 3–5 sentinel node metastases. Omitting ALND did not affect the long-term survival of 1–2 sentinel node metastasis patients but damaged the long-term survival of 3–5 node metastasis patients. Subgroup analysis consistently demonstrated the inferiority of SLNB to ALND in all subgroups of 3–5 sentinel node metastases. Thus, SLNB is an efficient and safe surgical choice for 1–2 sentinel node metastases but not for 3–5 sentinel node metastases.
It has been known that breast cancer biology, rather than the extent of surgery, is a major determinant of recurrence [19,20]. Initially, SLNB was demonstrated as the standard staging method for breast cancer patients with clinically negative axilla [3]. Furthermore, the Z0011 and IBCSG 23-01 trials provided the support for the change in clinical practice for the patients with minimal lymph node involvement [7,21]. The axilla management for patients under Z0011 eligibility criteria changed greatly from developed to developing countries [[8], [9], [10]]. For example, Yao et al. reported that the SLND alone in patients meeting the Z0011 eligibility criteria increased from 23% in 2009 to 56% in 2011 [22]. Our study showed a dramatic change in axillary surgery for sentinel node metastases 1–2 from 2000 to 2016, and the SLNB proportion doubled within three years from 30.2% in 2009 to 62.9% in 2011. It is worth noting that for patients with 3–5 node metastasis who did not meet the Z0011 eligibility criteria, the SLNB alone proportion was quite low until 2009 and then almost quadrupled from 5.2% in 2009 to 20.6% in 2016. The increasing trend of SLNB alone for 3–5 node metastasis started in 2009 and seemed to be influenced by the Z0011 trial, although the efficacy and safety were not well demonstrated then. Whether the conclusion from the Z0011 trial could be extrapolated to the management of patients with more positive nodes should be precisely examined.
The proponents of completion ALND argued that the total number of involved nodes provides important prognostic information and guides the subsequent treatment [23,24]. Early studies reported that the total number of involved nodes is important prognostic information and that an increasing number of positive nodes portends worse survival [25]. However, whether the improved survival was due to axillary clearance itself or to adjuvant treatment for lymph node involvement was unknown. Orr et al. performed a Bayesian meta-analysis of six randomized controlled trials and revealed a 5.4% survival benefit associated with ALND for clinically node-negative patients [26]. However, the value of this systematic review was controversial because no patients in the six trials were treated with adjuvant therapy, in contrast to current clinical practice. From the perspective of opponents, approximately 50% of patients with positive sentinel nodes were found to have no other nodal metastases [6,27]. Perhaps, many patients underwent unnecessary ALND, with no additional therapeutic benefit or staging information. Several studies validated the outcome of the Z0011 trial from different perspectives, while long-term survival analysis was not reported [[14], [15], [16]]. We analyzed 41,996 patients and found that omitting ALND did not affect the long-term survival of 1–2 sentinel node metastasis patients but damaged the survival of 3–5 node metastasis patients.
From our results, the number of positive lymph nodes was critical in the choice of axillary surgery. In other words, the de-escalation of SLNB alone seemed rational for 1–2 sentinel node metastasis and escalation of ALND for 3–5 sentinel node metastasis. For breast cancer, the treatment paradigm has shifted from “maximum tolerable” to “minimum effective” since the 1970s [28]. From our findings, the initial five-year survival was comparable between the SLNB alone and ALND cohorts for the 3–5 node metastasis, and the protective benefit induced by ALND was then appeared to be significant after the initial five-year period. Thus, radiation plus adjuvant systemic therapy perhaps was inadequate for 3–5 node metastasis from a long-term view. Breast cancer, particularly HoR positive breast cancer, was a disease with a long natural history [29,30]. ALND could improve the long-term survival via the better axillary clearance. Similarly, Bilimoria et al. found that for the patients with macroscopic disease, the completion ALND seemed to decrease the axillary recurrence and death, although without statistical significance [16].
As our study showed, the proportion of omitting ALND in patients with 3–5 sentinel node metastases increased to 20.6% in 2016, while omitting ALND would damage long-term survival. There was a similar trend to omit ALND in women with T3-4 disease [31]. The eligibility criteria and extrapolation of the Z0011 trial should be considered. It was once controversial whether the results of the Z0011 trial were applicable for high-risk patients, such as young age patients. Mamtani and Chung et al. presented that for young or triple-negative patients, there was no difference in survival between those who had ALND or not [32,33]. In contrast, the number of positive lymph nodes was critical in the choice of axillary surgery, and the Z0011 conclusion could not be extrapolated to patients with 3–5 node metastasis, and other patients such as those with positive palpable nodes, those forgoing whole-breast irradiation, or patients receiving neoadjuvant therapy [7].
The concept of axillary radiation for patients with positive nodes developed quickly, while the role of axillary radiation for patients with 3–5 sentinel node metastases was still unclear. Most patients receive adjuvant systemic therapy and/or whole breast irradiation, which benefited the survival significantly [34,35]. The EORTC 10981–22023 AMAROS trial enrolled 4806 patients with positive sentinel nodes and showed that axillary radiotherapy had the similar and comparable regional control with ALND [35]. The finding resulted in some patients with positive sentinel node undergoing axillary radiation instead of ALND after 2014. However, 95% patients enrolled in EORTC 10981–22023 AMAROS trial had 1-2 sentinel node metastases, and the patients with 3–5 sentinel node metastases were underrepresented. Besides, the trials of MA.20 and EORTC 22922/10925 showed that the extended regional nodal irradiation (including internal mammary and medial supraclavicular lymph nodes) did not improve overall survival, although decreased breast-cancer recurrence [36,37].
We would like to acknowledge the limitations of our study and provide further suggestions. First, detailed information on radiation therapy, such as high-tangential or nodal irradiation, was not described in detail in the SEER database. However, the roles of specific radiation fields in the Z0011 results remain unclear. Second, the unbalanced baseline between the SLNB alone and ALND groups existed, and we conducted multivariate Cox analysis to avoid imbalance across the groups. Third, some important clinical and treatment-level information were unknown, including detailed adjuvant treatment and physical status. Fourth, the limitation of using OS as primary endpoint was that the selection bias might existed and the difference of regional control was neglected. In the future, better prediction methods and well-designed trials would be expected. Several nomograms were available to predict non-sentinel nodal involvement, which offers an approach other than completion ALND to guide subsequent treatment [6,23]. Ongoing trials may also address the controversy about the eligibility of omitting or selected ALND in different situations [[38], [39], [40]]. For example, SERC study, a multicenter randomized phase-3 trial, included Z0011 non-eligible patients, of whom 43 patients had more than two involved sentinel nodes in the newest publication (20 in ALND arm vs 23 in only SLNB arm) [39]. These ongoing prospective randomized trials would unveil the importance of ALND for the Z0011 non-eligible patients.
5. Conclusions
Our research reported that the proportion of ALND omission increased for patients with sentinel node metastasis from 2000 to 2016. For T1-2 patients after lumpectomy and radiation, omitting ALND did not affect the long-term survival of 1–2 sentinel node metastasis patients but damaged the long-term survival of 3–5 node metastasis patients. It is worth noting that the proportion of omitted ALND quadrupled from 2009 to 2016 for patients with 3–5 node metastasis.
Funding
None.
Declaration of competing interest
The authors declare that they have no known competing financial interests or personal relationships that could have appeared to influence the work reported in this paper.
Footnotes
Supplementary data to this article can be found online at https://doi.org/10.1016/j.breast.2022.02.014.
Contributor Information
Zongchao Gou, Email: fdchao@126.com.
Luoting Yu, Email: yuluot@scu.edu.cn.
Appendix A. Supplementary data
The following is the Supplementary data to this article:
References
- 1.Sung H., Ferlay J., Siegel R.L., et al. Global cancer statistics 2020: GLOBOCAN estimates of incidence and mortality worldwide for 36 cancers in 185 countries. CA A Cancer J Clin. 2021;71(3):209–249. doi: 10.3322/caac.21660. [DOI] [PubMed] [Google Scholar]
- 2.Lucci A., McCall L.M., Beitsch P.D., et al. Surgical complications associated with sentinel lymph node dissection (SLND) plus axillary lymph. Node Dissection Compared With SLND Alone in the American College of Surgeons Oncology Group Trial Z0011. 2016;25(24):3657–3663. doi: 10.1200/JCO.2006.07.4062. https://doi.org/101200/JCO2006074062. [DOI] [PubMed] [Google Scholar]
- 3.Canavese G., Catturich A., Vecchio C., et al. Sentinel node biopsy compared with complete axillary dissection for staging early breast cancer with clinically negative lymph nodes: results of randomized trial. Ann Oncol. 2009;20(6):1001–1007. doi: 10.1093/ANNONC/MDN746. [DOI] [PubMed] [Google Scholar]
- 4.Giuliano A.E., Haigh P.I., Brennan M.B., et al. Prospective observational study of sentinel lymphadenectomy without further axillary dissection in patients with sentinel node-negative breast cancer. J Clin Oncol. 2000;18(13):2553–2559. doi: 10.1200/JCO.2000.18.13.2553. [DOI] [PubMed] [Google Scholar]
- 5.Rescigno J., Zampell J.C., Axelrod D. Patterns of axillary surgical care for breast cancer in the era of sentinel lymph node biopsy. Ann Surg Oncol. 2009;16(3):687–696. doi: 10.1245/S10434-008-0195-5. [DOI] [PubMed] [Google Scholar]
- 6.Van Zee K.J., Manasseh D.M.E., Bevilacqua J.L.B., et al. A nomogram for predicting the likelihood of additional nodal metastases in breast cancer patients with a positive sentinel node biopsy. Ann Surg Oncol. 2003;10(10):1140–1151. doi: 10.1245/ASO.2003.03.015. [DOI] [PubMed] [Google Scholar]
- 7.Giuliano A.E., Ballman K.V., McCall L., et al. Effect of axillary dissection vs no axillary dissection on 10-year overall survival among women with invasive breast cancer and sentinel node metastasis: the ACOSOG Z0011 (Alliance) randomized clinical trial. JAMA, J Am Med Assoc. 2017;318(10):918–926. doi: 10.1001/jama.2017.11470. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Tsao M.W., Cornacchi S.D., Hodgson N., et al. A population-based study of the effects of a regional guideline for completion axillary lymph node dissection on axillary surgery in patients with breast cancer. Ann Surg Oncol. 2016;23(10):3354–3364. doi: 10.1245/S10434-016-5310-4. [DOI] [PubMed] [Google Scholar]
- 9.Gondos A., Jansen L., Heil J., et al. Time trends in axilla management among early breast cancer patients: persisting major variation in clinical practice across European centers. Acta Oncol. 2016;55(6):712–719. doi: 10.3109/0284186X.2015.1136751. https://doi.org/103109/0284186X20151136751. [DOI] [PubMed] [Google Scholar]
- 10.Fisher R.A., Muthuswamy K., Petrou F., et al. Worldwide impact of the American college of surgeons oncology group Z0011 trial on rates of axillary lymph node dissection: a systematic review. Eur J Surg Oncol. 2018;44(6):900. doi: 10.1016/J.EJSO.2018.02.165. [DOI] [Google Scholar]
- 11.Goldhirsch A., Winer E.P., Coates A.S., et al. Personalizing the treatment of women with early breast cancer: highlights of the St Gallen international expert consensus on the primary therapy of early breast cancer 2013. Ann Oncol Off J Eur Soc Med Oncol. 2013;24(9):2206–2223. doi: 10.1093/ANNONC/MDT303. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Gatzemeier W., Bruce Mann G. Which sentinel lymph-node (SLN) positive breast cancer patient needs an axillary lymph-node dissection (ALND) – ACOSOG Z0011 results and beyond. Breast. 2013;22(3):211–216. doi: 10.1016/J.BREAST.2013.02.001. [DOI] [PubMed] [Google Scholar]
- 13.Leitch A.M., McCall L., Beitsch P., et al. Factors influencing accrual to ACOSOG Z0011, a randomized phase III trial of axillary dissection vs. observation for sentinel node positive breast cancer. J Clin Oncol. 2006;24(18_suppl):601. doi: 10.1200/JCO.2006.24.18_SUPPL.601. https://doi.org/101200/jco20062418_suppl601. 601. [DOI] [Google Scholar]
- 14.Yi M., Giordano S.H., Meric-Bernstam F., et al. Trends in and outcomes from sentinel lymph node biopsy (SLNB) alone vs. SLNB with axillary lymph node dissection for node-positive breast cancer patients: experience from the SEER database. Ann Surg Oncol. 2010;17(SUPPL. 3) doi: 10.1245/s10434-010-1253-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Jung J., Kim B.H., Kim J., et al. Validating the ACOSOG Z0011 trial result: a population-based study using the SEER database. Cancers. 2020;12(4):1–12. doi: 10.3390/cancers12040950. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Bilimoria K.Y., Bentrem D.J., Hansen N.M., et al. Comparison of sentinel lymph node biopsy alone and completion axillary lymph node dissection for node-positive breast cancer. J Clin Oncol. 2009;27(18):2946–2953. doi: 10.1200/JCO.2008.19.5750. [DOI] [PubMed] [Google Scholar]
- 17.Wang J., Mittendorf E.A., Sahin A.A., et al. Outcomes of sentinel lymph node dissection alone vs. axillary lymph node dissection in early stage invasive lobular carcinoma: a retrospective study of the Surveillance, Epidemiology and End Results (SEER) database. PLoS One. 2014;9(2):1–7. doi: 10.1371/journal.pone.0089778. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Li S., Liu F., Chen K., et al. The extent of axillary surgery is associated with breast cancer-specific survival in T1-2 breast cancer patients with 1 or 2 positive lymph nodes. Med (United States) 2016;95(14):1–7. doi: 10.1097/MD.0000000000003254. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Lowery A.J., Kell M.R., Glynn R.W., Kerin M.J., Sweeney K.J. Locoregional recurrence after breast cancer surgery: a systematic review by receptor phenotype. Breast Cancer Res Treat. 2012;133(3):831–841. doi: 10.1007/S10549-011-1891-6. [DOI] [PubMed] [Google Scholar]
- 20.Perou C.M., Sorlie T., Eisen M.B., et al. Molecular portraits of human breast tumours. Nature. 2000;406(6797):747–752. doi: 10.1038/35021093. [DOI] [PubMed] [Google Scholar]
- 21.Galimberti V., Cole B.F., Viale G., et al. Axillary dissection versus no axillary dissection in patients with breast cancer and sentinel-node micrometastases (IBCSG 23-01): 10-year follow-up of a randomised, controlled phase 3 trial. Lancet Oncol. 2018;19(10):1385–1393. doi: 10.1016/S1470-2045(18)30380-2. [DOI] [PubMed] [Google Scholar]
- 22.Yao K., Liederbach E., Pesce C., Wang C.H., Winchester D.J. Impact of the American college of surgeons oncology group Z0011 randomized trial on the number of axillary nodes removed for patients with early-stage breast cancer. J Am Coll Surg. 2015;221(1):71–81. doi: 10.1016/J.JAMCOLLSURG.2015.02.035. [DOI] [PubMed] [Google Scholar]
- 23.Katz A., Smith B.L., Golshan M., et al. Nomogram for the prediction of having four or more involved nodes for sentinel lymph node-positive breast cancer. J Clin Oncol. 2008;26(13):2093–2098. doi: 10.1200/JCO.2007.11.9479. [DOI] [PubMed] [Google Scholar]
- 24.Truong P.T., Lee J., Kader H.A., Speers C.H., Olivotto I.A. Locoregional recurrence risks in elderly breast cancer patients treated with mastectomy without adjuvant radiotherapy. Eur J Cancer. 2005;41(9):1267–1277. doi: 10.1016/j.ejca.2005.02.027. [DOI] [PubMed] [Google Scholar]
- 25.Cabanes P.A., Salmon R.J., Vilcoq J.R., et al. Value of axillary dissection in addition to lumpectomy and radiotherapy in early breast cancer. The Breast Carcinoma Collaborative Group of the Institut Curie. Lancet (London, England) 1992;339(8804):1245–1248. doi: 10.1016/0140-6736(92)91591-U. [DOI] [PubMed] [Google Scholar]
- 26.Orr R.K. The impact of prophylactic axillary node dissection on breast cancer survival--a Bayesian meta-analysis. Ann Surg Oncol. 1999;6(1):109–116. doi: 10.1007/S10434-999-0109-1. [DOI] [PubMed] [Google Scholar]
- 27.Abdessalam S.F., Zervos E.E., Prasad M., et al. Predictors of positive axillary lymph nodes after sentinel lymph node biopsy in breast cancer. Am J Surg. 2001;182(4):316–320. doi: 10.1016/S0002-9610(01)00719-X. [DOI] [PubMed] [Google Scholar]
- 28.Veronesi U., Stafyla V., Luini A., Veronesi P. Breast cancer: from “maximum tolerable” to “minimum effective” treatment. Front Oncol. 2012;2 doi: 10.3389/FONC.2012.00125. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Colleoni M., Sun Z., Price K.N., et al. Annual hazard rates of recurrence for breast cancer during 24 Years of follow-up: results from the international breast cancer study group trials I to V. J Clin Oncol. 2016;34(9):927–935. doi: 10.1200/JCO.2015.62.3504. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Pan H., Gray R.G., Davies C., et al. Predictors of recurrence during years 5-14 in 46,138 women with ER+ breast cancer allocated 5 years only of endocrine therapy (ET) J Clin Oncol. 2016;34(15_suppl):505. https://doi.org/101200/JCO20163415_suppl505. 505. doi:10.1200/JCO.2016.34.15_SUPPL.505. [Google Scholar]
- 31.Morris M.C., Lee T.C., Johnston M.E., et al. National trend of axillary management in clinical T3/T4 N0 patients having breast conserving therapy. J Surg Res. 2020;255:361–370. doi: 10.1016/J.JSS.2020.05.073. [DOI] [PubMed] [Google Scholar]
- 32.Chung A., Gangi A., Mirocha J., Giuliano A. Applicability of the ACOSOG Z0011 criteria in women with high-risk node-positive breast cancer undergoing breast conserving surgery. Ann Surg Oncol. 2015;22(4):1128–1132. doi: 10.1245/S10434-014-4090-Y. [DOI] [PubMed] [Google Scholar]
- 33.Mamtani A., Patil S., Van Zee K.J., et al. Age and receptor status do not indicate the need for axillary dissection in patients with sentinel lymph node metastases. Ann Surg Oncol. 2016;23(11):3481–3486. doi: 10.1245/S10434-016-5259-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.F B., A S., B J., et al. Twenty-year follow-up of a randomized trial comparing total mastectomy, lumpectomy, and lumpectomy plus irradiation for the treatment of invasive breast cancer. N Engl J Med. 2002;347(16):1233–1241. doi: 10.1056/NEJMOA022152. [DOI] [PubMed] [Google Scholar]
- 35.Donker M., van Tienhoven G., Straver M.E., et al. Radiotherapy or surgery of the axilla after a positive sentinel node in breast cancer (EORTC 10981-22023 AMAROS): a randomised, multicentre, open-label, phase 3 non-inferiority trial. Lancet Oncol. 2014;15(12):1303–1310. doi: 10.1016/S1470-2045(14)70460-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Poortmans P.M., Weltens C., Fortpied C., et al. Internal mammary and medial supraclavicular lymph node chain irradiation in stage I–III breast cancer (EORTC 22922/10925): 15-year results of a randomised, phase 3 trial. Lancet Oncol. 2020;21(12):1602–1610. doi: 10.1016/S1470-2045(20)30472-1. [DOI] [PubMed] [Google Scholar]
- 37.Whelan T.J., Olivotto I.A., Parulekar W.R., et al. Regional nodal irradiation in early-stage breast cancer. N Engl J Med. 2015;373(4):307–316. doi: 10.1056/NEJMOA1415340/SUPPL_FILE/NEJMOA1415340_DISCLOSURES.PDF. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Goyal A., Mann B., Thompson A.M. vol. 36. 2018. (POSNOC: positive Sentinel Node—adjuvant therapy alone versus adjuvant therapy plus clearance or axillary radiotherapy). https://doi.org/101200/JCO20183615_supplTPS600. 15_suppl):TPS600-TPS600. doi:10.1200/JCO.2018.36.15_SUPPL.TPS600. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Houvenaeghel G., Cohen M., Raro P., et al. Sentinel node involvement with or without completion axillary lymph node dissection: treatment and pathologic results of randomized SERC trial. npj Breast Cancer. 2021;7(1):1–8. doi: 10.1038/s41523-021-00336-3. 2021 71. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Henke G., Knauer M., Ribi K., et al. Tailored axillary surgery with or without axillary lymph node dissection followed by radiotherapy in patients with clinically node-positive breast cancer (TAXIS): study protocol for a multicenter, randomized phase-III trial. Trials. 2018;19(1):1–28. doi: 10.1186/S13063-018-3021-9/FIGURES/2. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.